To the Editor,
Vaccination seems the most effective public health tools to contrast the spreading of Coronavirus disease‐19 (COVID‐19) pandemic. To date, the European Medicines Agency (EMA) authorized three anti‐SARS‐CoV‐2 vaccines. The Pfizer‐BioNTech and the Moderna vaccines contain messenger RNA (mRNA) encapsulated in lipid nanoparticles, which encodes the SARS‐CoV‐2 viral spike (S) protein, inducing both antibody and cell‐mediated responses. The AstraZeneca vaccine is based on a viral vector that uses a modified version of the chimpanzee adenovirus to provide instructions for synthesizing SARS‐CoV‐2 protein S. The vaccine series consists of two doses administered intramuscularly (Pfizer‐BioNTech: 21 days apart; Moderna: 28 days apart; AstraZeneca: 28–84 days apart).
During clinical approval studies and early post‐marketing phases, mucous‐cutaneous adverse reactions have been rarely observed. Among hypersensitivity reactions, immediate reactions (anaphylaxis, urticaria‐angioedema syndrome) were more frequently observed than delayed reactions (maculo‐papular eruptions). 1 , 2
The anti‐SARS‐CoV‐2 vaccines contain excipients with known sensitizing potential: Pfizer‐BioNTech vaccine contains polyethylene glycol‐2000, Moderna vaccine polyethylene glycol‐2000 and tromethamine and AstraZeneca vaccine polysorbate 80. 3
Considering this, before receiving anti‐SARS‐CoV‐2 vaccination, an adequate medical history is mandatory to detect possible risk factors and, consequently, to minimize the incidence of adverse reactions. Furthermore, it is recommended to administer the vaccine by trained healthcare personnel in adequate medical settings in presence of emergency drugs and an observation period. 3 , 4
In General Hospital of Perugia and in Local Health Unit 1, Umbria Region, Italy, 5574 healthcare professional received the first dose of Pfizer‐BioNTech vaccine. Six subjects (0.11%) without previous drug hypersensitivity or polyethylene glycol reactions developed mucous‐cutaneous adverse reactions, summarized in Table 1.
TABLE 1.
Patients characteristic and adverse mucous‐cutaneous reactions in 6 patients after the first dose of Pfizer‐BioNTech vaccine
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Gender | Female | Female | Male | Female | Female | Female |
| Age | 24 | 31 | 28 | 58 | 44 | 54 |
| Personal atopy | Allergic rhinitis | Allergic rhinitis | Allergic rhinitis | Allergic rhinitis and asthma | Allergic rhinitis | Allergic rhinitis, atopic dermatitis |
| Allergy history | – | – | – | – | – | Contact allergy (nichel sulphate, fragrances) |
| Type of reaction | Generalized acute urticaria | Angioedema (tongue, gums) | Generalized acute urticaria | Flushing of the face | Flushing of the face | Angioedema (tongue, lips) |
| Time of onset | 5 min | 24 h | 5 min | 30 min | 20 min | 10 min |
| Treatment | Betametasone sodium phosphate (IV) | – | – | – | – | – |
These patients underwent an allergologic workup with Pfizer‐BioNTech vaccine as suggested by EAACI 4 and German allergy centres. 5 In absence of standardized methodology for this vaccine testing, we referred to Italian 6 and EAACI recommendations. 7 Unusable vaccine residues, regain from the vaccine campaign, were used under sterile conditions within 6 hours from reconstitution and according to the storage conditions. Skin prick test (SPT) with neat vaccine (reading: 20 min) and intradermal test (IDT) vaccine dilution 1/100 (readings: 20 min, 24 h) were performed. SPT resulted always negative, but IDT induced, 12 hours after, an erythematosus, oedematous and infiltrated asymptomatic reaction in all patients. A 1/1000 dilution test induced the same reaction in all patients (Figure 1A). We followed the patients daily until resolution, and the IDT reactions persisted for 2 days. All patients then received the second dose of vaccine without relapses.
FIGURE 1.
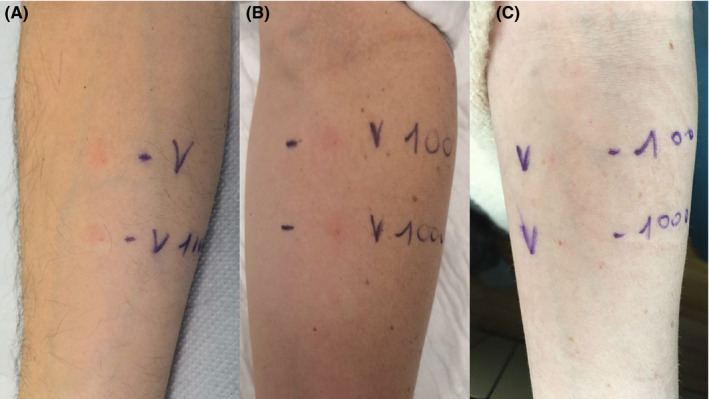
Intradermal test (IDT) with Pfizer‐BioNTech vaccine. Erythematosus, oedematous and infiltrated reaction at 24 h inpatient 1 (A) and in a healthcare volunteer who had received the first dose of Pfizer‐BioNTech vaccine (B). Negative IDT in a not‐vaccinated volunteer (C)
In order to verify these reactions, IDT with 1/1000 and 1/100 Pfizer‐BioNTech vaccine dilution was performed in six healthcare volunteers who had received the two doses of Pfizer‐BioNTech vaccine, in six healthcare volunteers who had received at least 2 weeks before only the first dose of Pfizer‐BioNTech vaccine and in six volunteers who did not receive Pfizer‐BioNTech vaccine. All the 18 volunteers did not refer previous allergy to vaccines or drugs containing polyethylene glycols. IDT induced the same reaction 12 h after in the 12 vaccinated volunteers (Figure 1B), while resulted negative in the six not‐vaccinated volunteers (Figure 1C). The six volunteers who had received only the first dose were then vaccinated with the second dose without problem. All patients and controls have provided an informed consent to perform these skin tests.
Even if the morphology of the IDT reactions could suggest a type IV a immune reaction that IDT reactions observed in patients and vaccinated volunteers could be a sign of desired cellular immune protection rather than an allergy to SARS‐CoV‐2 viral S protein or to vaccine components. This hypothesis was confirmed by the lack of relapses of mucous‐cutaneous adverse reactions after second vaccine administration.
It is impossible to draw conclusions about the utility of immediate readings of SPT and IDT to investigate anaphylaxis to Pfizer‐BioNTech vaccine, but for purely cutaneous reactions, they have not shown positive results in six patients and IDT has a high risk of positive delayed reactions due to cellular immune protection.
Further studies are needed to investigate the utility of SPT and IDT to investigate Pfizer‐BioNTech vaccine allergy and to better clarify the pathomechanism of the reactions observed to IDT.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
PATIENTS CONSENT STATEMENT
All patients and controls give their consent to the skin test and to the data publication.
ACKNOWLEDGEMENTS
None.
The article has not been previously published and is not currently submitted elsewhere.
REFERENCES
- 1. Shimabukuro T, Nair N. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer‐BioNTech COVID‐19 Vaccine. JAMA 2021;325(8):780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Center for Disease Control and Prevention. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer‐BioNTech COVID‐19 Vaccine — United States; December 14–23, 2020. https://www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm. Accessed February 17, 2021.
- 3. Caballero ML, Quirce S. Excipients as potential agents of anaphylaxis in vaccines: analyzing the formulations of the current authorized COVID‐19 vaccines. J Investig Allergol Clin Immunol. 2021;31(1):92–93. 10.18176/jiaci.0667 [DOI] [PubMed] [Google Scholar]
- 4. Sokolowska M, Eiwegger T, Ollert M, et al. EAACI statement on the diagnosis, management and prevention of severe allergic reactions to COVID‐19 vaccines. Allergy. 2021;76(6):1629–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Worm M, Bauer A, Wedi B, et al. Practical recommendations for the allergological risk assessment of the COVID‐19 vaccination ‐ a harmonized statement of allergy centers in Germany. Allergol Select. 2021;26(5):72‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stingeni L, Bianchi L, Tramontana M, et al. Skin tests in the diagnosis of adverse drug reactions. G Ital Dermatol Venereol. 2020;155(5):602‐621. [DOI] [PubMed] [Google Scholar]
- 7. Brockow K, Garvey LH, Aberer W, et al. Skin test concentrations for systemically administered drugs ‐ an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013;68(6):702‐712. [DOI] [PubMed] [Google Scholar]


