1. INTRODUCTION
The immune dysregulation triggered by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection has been hypothesized as a causal pathway for the increasingly reported oral manifestations associated with coronavirus diseases (COVID‐19), especially the ones of fungal origin. 1 , 2 , 3 As a result of this, we aim to report according to the CARE guidelines, three COVID‐19 cases who sought teleconsultations from our private practice (Cairo, Egypt) from July to September 2020. In addition, we have performed a literature search in Ovid MEDLINE®, EMBASE, Cochrane Library, and Epistemonikos from inception until November 30, 2020, with a combination of keywords (COVID‐19 or SARS‐CoV‐2) and oral candidiasis.
2. PATIENT 1
A 70‐year‐old female patient with a history of geriatric depression, peripheral neuropathy, urinary incontinence, chronic constipation, and vascular disease had sought a teleconsultation on September 15, 2020, due to severe burning sensation and dysphagia. Twenty days earlier, the patient started to suffer from diarrhea with no apparent underlying cause; therefore, she had been admitted to the hospital after confirming her SARS‐CoV‐2 infection through polymerase chain reaction (PCR) testing. During her 15‐day stay at the primary care, the patient was given azithromycin (Zithromax), levofloxacin (Uniloxam), rivaroxaban (Xarelto), and lactoferrin (Pravotin). After her respiratory and gastrointestinal symptoms were controlled, the patient was released from the hospital and sent for home isolation, although her PCR result remained positive.
Three days after hospital release, the patient started to feel throbbing pain related to her tongue and oropharynx. On September 15, 2020, her son sent us images of the recently grown white spots in her mouth (Figure 1). On examining her intra‐oral images, we have found white membranous patches spread over the tongue dorsum, mouth floor, soft palate, oropharynx region, and to a lesser extent the buccal mucosa. Her son was instructed to assist telediagnosis by being guided to hold a sterile gauze and to scrub her tongue dorsum. The tongue coating was friable and not bleeding; however, the patient reported bitter taste while eating probably due to bleeding. To manage her oral candidal infection, the patient was given a topical antifungal, nystatin (Micostatin), four times/day and a local antibacterial mouthwash, chlorhexidine 0.2%, twice daily. Alongside the oral symptoms, the patient was reported to have got vaginal candidal infection meanwhile. Her oral symptoms had resolved completely within ten days.
FIGURE 1.
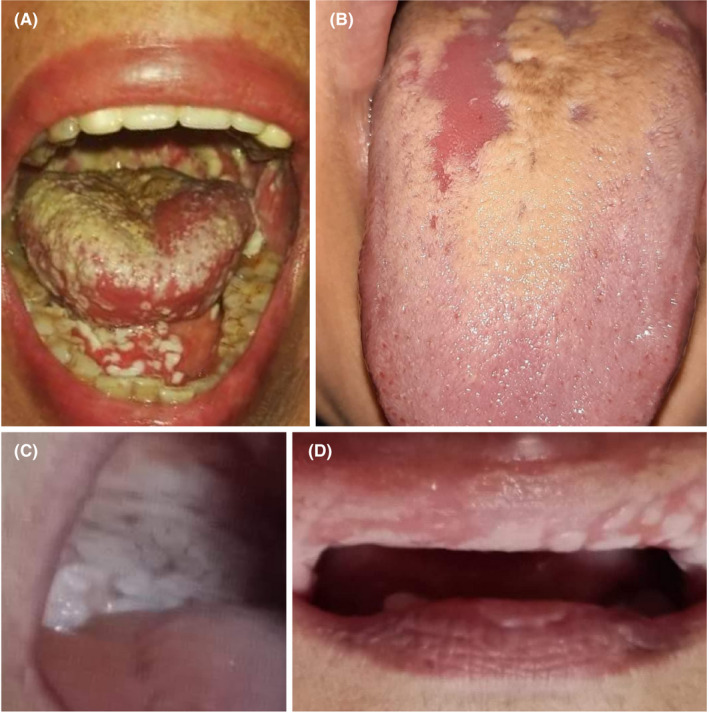
Oral candidiasis of COVID‐19 patients (Cairo, Egypt). (A) White membranous patches spread over the tongue dorsum, mouth floor, and soft palate of Case No. 1. (B) Erythematous candidiasis over the tongue dorsum of Case No. 2. (C) and (D) White membranous patches extended over the soft palate and labial mucosa, respectively
3. PATIENT 2
A previously healthy 25‐year‐old female dentist was quarantined because SARS‐CoV‐2 conformingly infected some members of her household. A few days later, the patient started to feel fatigue, headache, anosmia, and ageusia; therefore, she was instructed to follow a medical protocol composed of moxifloxacin, pantoprazole (Zurcal), and multivitamins. Two weeks after losing taste and smell, the patient had a severe burning sensation, and she immediately sought a teleconsultation and sent us her intra‐oral images (Figure 1). The images revealed a typical presentation of erythematous candidiasis over the tongue dorsum. The patient was managed by topical antifungal only, miconazole (Daktarin Gel) four times/day. The candida pain has degraded within four days without further complications.
4. PATIENT 3
A 56‐year‐old female patient with a history of diabetes mellitus type 2 and rheumatoid arthritis had sought a teleconsultation due to dysphagia and abdominal pain. Seventeen days earlier, the patient was quarantined because her daughter, a physician, was conformingly infected by SARS‐CoV‐2 and isolated at home. The patient started to have a fever, dry cough, and burning sensation in the nose. Her oxygen saturation had dropped rapidly during the following two days; therefore, she was treated with oxygen tanks at home with close follow‐up by her daughter. For 15 days, the patient was treated with azithromycin (Zithromax). On the 18th day, the patient's daughter sent us images for recent white patches inside her mouth (Figure 1). On examining the intra‐oral images, white membranous patches extended over labial mucosa, and soft palate and tongue dorsum were found. The patient was managed by both systemic antifungal fluconazole (Flucoral), three times/day, and topical antifungal miconazole (Daktarin Gel) four times/day. The oral symptoms were resolved in 7 days.
On reviewing the currently growing evidence on oral candidiasis in COVID‐19 patients, we have found 63 cases reported in 8 studies: 5 case reports, one case‐series, one cross‐sectional study, and one open‐label trial. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 Fifty‐three cases (84.1%) were from the Middle East, seven (11.1%) from Europe, two (3.2%) from Latin America, and one (1.6%) from Africa (Table 1). The condition was more expressed in female patients (56.7%) than male patients (43.3%). Their mean age was 59.5 years old, with most reported cases (73.8%) were above the age of 50 years. While 91.9% of them had comorbidities, including cardiovascular, kidney, autoimmune and hormonal diseases, and malignancies, five cases were previously healthy, including three neonates who were tested positive 10–15 days after delivery. All cases were tested positive for SARS‐CoV‐2 by PCR, except one case which was confirmed by the positivity of the serological test. The vast majority of cases were hospitalized and treated by an array of medications, including broad‐spectrum antibiotics mostly azithromycin, corticosteroids, hydroxychloroquine sulfate, and vitamin D mainly for pediatric cases. The onset of oral candidiasis varied considerably among reported patients, and it ranged between 1 and 30 days since COVID‐19 symptoms’ emergence. Most of the reported cases had oral pseudomembranous candidiasis described as white plaque extending over tongue dorsum mainly and oral mucosa. Typical antifungal protocols had been followed where systemic fluconazole was prescribed for 39 cases and oral nystatin for 36 cases.
TABLE 1.
Characteristics of COVID‐19 Patients with Oral Candidiasis; Reported January—November 2020
| Study, Location | Number | Gender | Age | Medical History | Confirmation | COVID−19 Treatment | Onset | Description | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| Amorim dos Santos et al. 2020; Brasilia (Brazil) | 1 | Male | 67 | Coronary disease, systemic hypertension, kidney disease, and kidney transplant which led him to take immunosuppressants and pharmacological prophylaxis. | Confirmed by PCR |
ICU admission, and orotracheal intubation. Hydroxychloroquine sulfate (HCQ), ceftriaxone sodium, and azithromycin. |
24 days after hospital admission. | Persistent white plaque on the tongue dorsum. | Intravenous fluconazole and oral nystatin. |
| Baraboutis et al. 2020; Athens (Greece) | 2 | N/A | N/A | No risk factors like known immunosuppression or recent antimicrobial use. | Confirmed by PCR | N/A | 7–10 days after symptoms emergence. | Unexpected “oral candidiasis.” One of them resembled esophageal candidiasis. | N/A |
| Cantini et al. 2020; Tuscany (Italy) | 1 | N/A | > 50 | N/A | Confirmed by PCR | Baricitinib | N/A | Oral candidiasis was recorded as an adverse event for Baricitinib as COVID−19 medication. | N/A |
| Corchuelo et al. 2020; Cali (Colombia) | 1 | Female | 40 | Lymphadenopathy managed by azithromycin. Headache managed by ibuprofen. | Confirmed by serological test (+IgG) | N/A | N/A | Mild oral candidiasis infection at the level of the posterior tongue. | Oral nystatin |
| Díaz Rodríguez et al. 2020; Madrid (Spain) | 1 | Female | 78 | N/A | Confirmed by PCR. | N/A | N/A | Lesions on the tongue, palate, and commissure compatible with pseudomembranous candidiasis and angular cheilitis were observed. | Oral nystatin |
| Dima et al. 2020; Timisoara (Romania) | 3 |
1 Female, 2 Male |
0 (neonates) | Previously healthy | Confirmed by PCR. | Vitamin D | During hospitalization | Oral candidiasis was accompanied by diaper erythema. | Nystatin |
| Riad et al. 2020; Gharbia (Egypt) | 1 | Female | 47 | Mild hypothyroidism managed by levothyroxine. | Confirmed by PCR | Azithromycin, linezolid, and ceftriaxone | A few days after anosmia and amblygeustia. | Multiple medium‐sized pseudomembranous structures were detected with white plaques scattered over the dorsal surface of the tongue. | N/A |
| Salehi et al. 2020; Tehran (Iran) | 53 |
30 Female, 23 Male |
11 (< 50 y), 42 (≥ 50 y) |
28 cardiovascular diseases, 20 diabetes mellitus, 11 chronic kidney diseases, 5 hematological malignancies. |
Confirmed by PCR | 49 broad‐spectrum antibiotics, 25 corticosteroids, 26 ICU admission, 16 mechanical ventilation | Mean interval between COVID−19 diagnosis and candidiasis emergence was 8 days (range 1–30 days). | In total, 65 Candida isolates causing OPC were recovered from 53 patients. C albicans (46/65; 70.7%) was the most prevalent yeast species. |
21 (fluconazole), 17 (fluconazole and nystatin), 13 (nystatin), 1 (caspofungin). |
| Total | 63 |
34 Female, 26 Male, 3 Missed |
Mean age: 59.5 years (3 missed). |
56 comorbidities, 5 previously healthy, 2 missed. |
62 confirmed by PCR, 1 confirmed by serological test |
The majority are hospitalized and treated by HCQ, antibiotics, corticosteroids | Within one month since COVID−19 symptoms emergence. | Pseudomembranous oral candidiasis related to tongue and oral mucosa, and oropharyngeal candidiasis |
39 fluconazole, 36 nystatin, 1 caspofungin, 4 missed. |
Abbreviation: N/A, not reported by the investigators.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Dry mouth (xerostomia) is a risk factor for various oral infections, and in this report, two of our patients suffered from difficulty of swallowing (dysphagia), thus indicating xerostomia which had been frequently reported as an oral manifestion of COVID‐19. 12 It had also been suggested that xerostomia could be one of the early symptoms of COVID‐19 or due to medical comorbidities and drug reactions. 13 Moreover, older patients may have difficulty in maintaining good oral health in the form of physical disabilities or psychological illnesses. It has been recently reported that Candida colonization was significantly associated with cognitive impairment, multimorbiditiy, and reduced oral hygiene capacity. 14
In conclusion, oral candidiasis has been consistently recorded in severely affected COVID‐19 patients, especially the ones with predisposing comorbidities and antibiotics intake, either justified or unjustified. Older age and female gender seemed to be the most prominent demographic risk factors for this opportunistic infection, which tends to have late‐onset and requires an immediate therapeutic intervention either systemically or topically to stop it from progression into lethal candidemia.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ETHICAL STATEMENT
The study was conducted according to the guidelines of the Declaration of Helsinki, and it was exempted from ethical approval due to its observational nature and the use of publicly accessible data.
AUTHOR CONTRIBUTIONS
Abanoub Riad: Writing‐original draft. Esraa Gomaa: Data curation; investigation. Barbora Hockova: Formal analysis; writing‐review and editing. Miloslav Klugar: Supervision.
PATIENTS’ CONSENT
All the investigated patients agreed to use their clinical data for academic purposes while concealing their identifying personal data.
ACKNOWLEDGMENTS
Work on this research of AR and MK is supported by a grant number LTC20031—“Towards an International Network for Evidence‐based Research in Clinical Health Research in the Czech Republic”. AR is supported by Masaryk University grants (MUNI/IGA/1543/2020) and (MUNI/A/1608/2020)
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Iranmanesh B, Amiri R, Zartab H, Aflatoonian M. Oral manifestations of COVID‐19 disease: a review article. Dermatol Ther. 2020;34(1):e14578. 10.1111/dth.14578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riad A, Klugar M, Krsek M. COVID‐19‐related oral manifestations: early disease features? Oral Dis. 2020. 10.1111/odi.13516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hocková B, Riad A, Valky J, et al. Oral complications of ICU patients with COVID‐19: case‐series and review of two hundred ten cases. J Clin Med. 2021;10(4):581. 10.3390/jcm10040581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amorim dos Santos J, Normando AGC, Carvalho da Silva RL, et al. Oral mucosal lesions in a COVID‐19 patient: New signs or secondary manifestations? Int J Infect Dis. 2020;97:326‐328. 10.1016/j.ijid.2020.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D. Baricitinib therapy in COVID‐19: A pilot study on safety and clinical impact. J Infect. 2020;81(2):318‐356. 10.1016/j.jinf.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baraboutis IG, Gargalianos P, Aggelonidou E, Adraktas A. Initial real‐life experience from a designated COVID‐19 centre in Athens, Greece: a proposed therapeutic algorithm. SN Compr Clin Med. 2020;2(6):689‐693. 10.1007/s42399-020-00324-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corchuelo J, Ulloa FC. Oral manifestations in a patient with a history of asymptomatic COVID‐19: case report. Int J Infect Dis. 2020;100:154‐157. 10.1016/j.ijid.2020.08.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Díaz Rodríguez M, Jimenez Romera A, Villarroel M. Oral manifestations associated with COVID‐19. Oral Dis. 2020. 10.1111/odi.13555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dima M, Enatescu I, Craina M, Petre I, Iacob ER, Iacob D. First neonates with severe acute respiratory syndrome coronavirus 2 infection in Romania. Medicine (Baltimore). 2020;99(33):e21284. 10.1097/MD.0000000000021284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Riad A, Gad A, Hockova B, Klugar M. Oral candidiasis in non‐severe COVID‐19 patients: call for antibiotic stewardship. Oral Surg. 2020. 10.1111/ors.12561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salehi M, Ahmadikia K, Mahmoudi S, et al. Oropharyngeal candidiasis in hospitalised COVID‐19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses. 2020;63(8):771‐778. 10.1111/myc.13137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fantozzi PJ, Pampena E, Di Vanna D, et al. Xerostomia, gustatory and olfactory dysfunctions in patients with COVID‐19. Am J Otolaryngol ‐ Head Neck Med Surg. 2020;41(6):102721. 10.1016/j.amjoto.2020.102721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katz J. Prevalence of dry mouth in COVID‐19 patients with and without Sicca syndrome in a large hospital center. Irish Journal of Medical Science (1971‐). 2021;1‐3. 10.1007/s11845-020-02480-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kottmann HE, Derman SHM, Noack MJ, Barbe AG. The underestimated problem of oral Candida colonization—An observational pilot study in one nursing home. Clin Exp Dent Res. 2019;5(6):683‐691. 10.1002/cre2.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


