Abstract
Essential oils are potential antimicrobial alternatives and their applications in animal feeds are limited due to their fast absorption in the upper gastrointestinal tract. This study investigated the effects of encapsulated cinnamaldehyde (CIN) at 50 mg/kg or 100 mg/kg on the growth performance, organ weights, meat quality, intestinal morphology, jejunal gene expression, nutrient digestibility, and ileal and cecal microbiota. A total of 320 male day-old broiler Cobb-500 chicks were randomly allocated to four treatments with eight pens per treatment (10 birds per pen): 1) basal diet (negative control, NC); 2) basal diet supplemented with 30 mg/kg avilamycin premix (positive control, PC); 3) basal diet with 50 mg/kg encapsulated CIN (EOL); 4) basal diet with 100 mg/kg encapsulated CIN (EOH). Despite birds fed EOH tended to increase (P = 0.05) meat pH at 24 h, all pH values were normal. Similar to PC group, meats from birds fed EOL and EOH showed a reduced (P < 0.05) Warner–Bratzler force shear (WBFS) compared to the NC group. The highest villus to crypt ratios (VH/CD; P < 0.05) were observed in broilers fed either EOL or EOH, with an average of 14.67% and 15.13% in the duodenum and 15.13% and 13.58% in the jejunum, respectively. For jejunal gene expressions, only six out of the 11 studied genes showed statistically significant differences among the dietary treatments. Gene expressions of cationic amino acid transporter 1 (CAT-1) and neutral amino acid transporter 1 (B0AT-1) were upregulated in EOH-fed birds compared to PC and NC-fed birds (P < 0.05), respectively; while the expression of proliferating cell nuclear antigen (PCNA) was downregulated in EOL-fed birds when compared to NC birds (P < 0.05). Nonetheless, the expressions of cadherin 1 (CDH-1), zonula occludens 1 (ZO-1), and maltase-glucoamylase (MG) were all upregulated (P < 0.05) in EOH-fed birds compared to PC-fed birds. The apparent ileal digestibility (AID) of dry matter, crude protein, crude fat and of all 18 tested amino acids increased in EOL-fed birds (P < 0.01). Additionally, relative abundances (%) of ileal Proteobacteria decreased, while ileal and cecal Lactobacillus increased in EOH-fed birds (P < 0.05). In conclusion, dietary encapsulated CIN improved meat quality and gut health by reducing meat WBFS, increasing VH/CD in intestines, jejunal gene expressions, AID of nutrients and beneficial ileal and cecal microbiota composition.
Keywords: antimicrobial alternatives, broiler chickens, encapsulated essential oils, gut health, meat quality
INTRODUCTION
Poultry products are essential components of a well-balanced human diet due to their nutritional richness in highly digestible proteins, B-group vitamins, and minerals (Marangoni et al., 2015). In Canada, it has been reported that broiler meats accounted for a large portion of total poultry products with over 130,000 metric tons in 2019 (Bedford, 2019). However, broiler could be a reservoir of pathogens such as Eimeria spp., Clostridium perfringens, nontyphoidal Salmonella enterica serovars, and extraintestinal pathogenic Escherichia coli (ExPEC) (Craven et al., 2001; Bergeron et al., 2012; Györke et al., 2013). These pathogens can be transmitted horizontally from feces or feathers to healthy birds through contaminated feeds, drinking water or beddings, and vertically from infected maternal breeders to their offspring (Liljebjelke et al., 2005). Additionally, due to the increased qualitative aspects of poultry meats, advanced management of poultry industry requires feed additives to promote growth performance, reduce incidences of infections, and improve meat quality (da Silva et al., 2017). Traditionally, antimicrobials were supplemented in feed as sub-therapeutic antimicrobial growth promoters (AGP) to prevent infections and improve performance (Wellenreiter et al., 2000). However, the overuse and misuse of antimicrobials have been linked to the development of antimicrobial resistances (Ventola, 2015). Thus, Chicken Farmers of Canada (CFC) eliminated category I antibiotics in 2014 and the preventive use of Category II antibiotics in 2018 and proposed to stop the use of category III antibiotics by end of 2020 (Chicken Farmers of Canada, 2020). However, withdrawal of AGP in feed may have adverse effects on broiler chicken such as reduction in performance and may pose food safety issues (Kumar et al., 2018). Consequently, it is necessary to explore antimicrobial alternatives such as probiotics, prebiotics, organic acids, and plant extracts for improving chicken production and health (Casewell et al., 2003; Osman et al., 2013).
Essential oils (EOs) are aromatic and volatile liquids extracted mainly from plants by steam distillation (Preedy, 2015). It has been reported that EOs from star anise oil, ginko biloba, and oregano had beneficial impacts on enhancing nutrient utilization, immunity, and liver antioxidant status in broilers (Galal et al., 2016; Ding et al., 2017; Ren et al., 2018). Encapsulated cinnamaldehyde and citral alone or in combination were shown to reduce necrotic enteritis (NE), improve chicken growth performance as bacitracin, and beneficially alter cecal microbiota compositions (Yang et al., 2020). However, limited studies were conducted on the effects of cinnamon oil on performance, meat quality, and intestinal histology and microbiota of broilers. Cinnamaldehyde (CIN; from Cinnamonmum) has been used for food flavorings and medications for many years without receiving much attention of their potential effects as antimicrobial alternatives on broilers (Burt, 2004; Nabavi et al., 2015). CIN powder had shown antimicrobial activities against some other pathogenic bacteria including Listeria monocytogenes and Bacillus cereus in laboratory media and rice cakes (Hong et al., 2013). It also demonstrated high antimicrobial efficacy against C. perfringens, S. typhimurium DT104, E. coli O157: H7, and enterotoxigenic Escherichia coli (ETEC) with little inhibition towards Lactobacillus and Bifidobacterium in vitro (Si et al., 2006; Si et al., 2009). However, CIN should not be applied directly to broilers as feed additives due to their instability during feed processing and gastric transition (Tian et al., 2016). In this study, the CIN was encapsulated by the soy protein polysaccharide reaction products to maintain its stability during prolonged storage, feed processing, gastric transitions (Yang et al., 2015). It was hypothesized that encapsulated CIN can promote growth performance, gut health, and meat quality of broiler chickens. The objective of this study was to evaluate the effects of encapsulated CIN at 50 mg/kg or 100 mg/kg on growth performance, organ weight, meat quality and gut health of broilers.
MATERIALS AND METHODS
Preparation of Encapsulated Materials
CIN (catalogue no. W228613; ≥95% purity; $828/5 kg) was purchased from Sigma Aldrich Chemical Co. (St. Louis, MO) and encapsulated separately in a soy-derived product called soy protein-soy polysaccharide Maillard reaction product (SPPMP) by emulsification and spray drying technologies (20% CIN in the capsules) (Yang et al., 2020). Surmax 100 Premix (avilamycin premix, 100 g/kg) was purchased from Elanco Canada Co. Ltd (Guelph, Ontario, Canada).
Experimental Design
A total of 320 1-d-old male Cobb 500 broiler chickens obtained from a local hatchery in Manitoba (Carleton Hatchery, Grunthal, Manitoba) were housed in 32 pens with 10 birds per pen (University of Manitoba, Winnipeg, MB, Canada). The pens were randomly allocated to four dietary treatments (8 pens/treatment): 1) basal diet as negative control (NC); 2) basal diet with 30 mg/kg avilamycin premix as positive control (PC); 3) basal diet with encapsulated CIN at 50 mg/kg (EOL); 4) basal diet with encapsulated CIN at 100 mg/kg (EOH). The birds were fed a starter diet from d 1 to d 14, a grower diet from d 15 to d 28, and a finisher diet from d 29 to d 41. The diets were provided as mash form and formulated (Table 1) according to the nutritional recommendation by Cobb 500 guidelines (Cobb-Vantress Inc., 2012) and prepared in Glenlea Research Station (Manitoba, Canada), which were described by a previous study (Mogire et al., 2021). For diets in PC, EOL and EOH groups, antibiotic and EOs were added by replacing equal amounts of corn.
Table 1.
Ingredient compositions and nutrient contents of starter (d 1–14), grower (d 15–28), and finisher (d 29–41) diets for broiler chickens (g/kg, as-fed basis, otherwise indicated)
| Ingredients | Inclusion in basal diet | ||
|---|---|---|---|
| Starter | Grower | Finisher | |
| Corn | 522.29 | 529.38 | 563.00 |
| Soybean meal | 305.00 | 261.00 | 225.00 |
| Corn gluten meal | 35.00 | 35.00 | 35.00 |
| Wheat | 25.00 | 30.00 | 30.00 |
| Canola meal | 25.00 | 30.00 | 30.00 |
| Soy oil | 22.60 | 43.80 | 45.80 |
| Corn DDGS | 20.00 | 30.00 | 30.00 |
| Limestone | 15.00 | 13.00 | 13.00 |
| Vitamin premixa | 10.00 | 10.00 | 10.00 |
| 21% Monocalcium phosphate | 9.00 | 7.00 | 5.00 |
| Mineral premixb | 5.00 | 5.00 | 5.00 |
| 99% dl-methionine | 2.65 | 2.37 | 2.05 |
| Lysine-HCl | 2.25 | 2.46 | 2.31 |
| Threonine | 0.71 | 0.49 | 0.34 |
| Xylanase 8000Gc | 0.20 | 0.20 | 0.20 |
| Phytase 5000Gd | 0.30 | 0.30 | 0.30 |
| Calculated composition | |||
| ME (kcal/kg) | 3000.00 | 3150.00 | 3200.00 |
| CP | 223.00 | 208.00 | 194.00 |
| Ca | 8.60 | 7.40 | 7.00 |
| Total P | 6.00 | 5.40 | 5.20 |
| SID Lys | 11.8 | 11.0 | 10.0 |
| SID Met | 6.80 | 5.50 | 5.00 |
| SID Met + Cys | 8.80 | 8.40 | 7.70 |
| SID Thr | 7.80 | 7.40 | 6.70 |
a Provided per kilogram of diet: vitamin A, 8,255 IU; vitamin D3, 3,000 IU; vitamin E, 30 IU; vitamin B12, 0.013 mg; vitamin K3, 2.0 mg; niacin, 41.2 mg; choline, 1300.5 mg; folic acid, 1.0 mg; biotin, 0.25 mg; pyridoxine, 4.0 mg; thiamine, 4.0 mg; calcium pantothenic acid, 11.0 mg; riboflavin, 6.0 mg.
b Provided per kilogram of diet: manganese, 70.0 mg; zinc, 80.0 mg; iron, 80.0 mg; iodine, 0.5 mg; copper, 10 mg; selenium, 0.3 mg.
c Xylanase 8000 G: 8,000 U/g; Danisco Animal Nutrition, Marlborough, United Kingdom.
d Quantum blue 5000 G: 5,000 FTU/g; AB Vista, Plantation, FL, United States.
Animals and Management
All procedures involving birds in this experiment were approved by the Animal Care and Welfare Committee of University of Manitoba according to animal use protocol (# F18-024). The birds were weighed on day 1 and distributed into floor pens of identical size (81.5 inches × 59 inches = 4,808.5 square inches) in a deep litter system with a wood shaving floor. The size of floor pen was 2 m2, and 4 cm of low floor straw were provided for chickens. Chickens were allowed ad libitum access to feed and drinking water (water cups were 6.5 inches in diameter and 2.25 inches deep) during the experiment. The temperature was maintained at 31°C from d 1 to d 3, 30 °C from d 3 to d 7, 28 °C from d 7 to d 14, 25 °C from d 21 to d 28, 24 °C from d 28 to d 35, and 22 °C from d 35 to d 41. The lighting program during the study was as follows: 24L:0D from d 0 to d 3, 22L:2D from d 4 to d 7 and 18L:6D in the period from d 8 to d 39 and 23L:1D in the period of d 40 to d 41. The birds were handled according to the guidelines of the Canadian Council on Animal Care (CCAC, 2009).
Growth Performance and Organ Weights
All individual birds were weighed on day 1 and allotted with similar initial body weight (BW; 48.3 ± 3.3g) in a randomized complete-block (RCB) design. Feed intake and BW were recorded weekly throughout d 1–41 to calculate average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR). Besides, any injured, malformed, or suffering bird was euthanized by carbon dioxide (CO2) and confirmed by cervical dislocation. Euthanized birds were recorded to calculate mortality (%). On d 41, a total of 32 birds (one bird per pen) were sacrificed to collect heart, liver, spleen, and bursa. The relative organ weights (%) were calculated as follows:
Intestinal Morphology
Intestinal sections were collected from duodenum (0.5 cm, the middle of the descending duodenum), jejunum (1.5 cm, midway between entry of bile ducts and Meckel’s diverticulum), and ileum (0.5 cm, mid-ileum at the Meckel’s diverticulum) on d 41 from 32 random-selected euthanized birds (1 bird per pen) for morphology. The collected sections were flushed gently using ice-cold physiological saline solution, dehydrated with alcohol, fixed in 10% neutral buffered formalin, and embedded in paraffin. Approximately 6 µm of each section was cut, mounted on slides, deparaffinized in xylene, rehydrated and stained with hematoxylin and eosin (H&E) for histology analysis (Fischer et al., 2008). Villus height (VH) and crypt depth (CD) were measured by Carl Zeiss MicroImaging equipped with a computer-assisted morphometric system (Carl Zeiss Ltd, Göttingen, Germany) and calculated villus/crypt ratio (VH/CD).
Gene Expression
The jejunum from 32 random-selected euthanized birds (one bird per pen) were flushed with phosphate buffered saline (PBS) and stored in liquid nitrogen immediately in 15 mL tubes.
Total RNA was extracted from jejuna using Trizol reagents according to manufacturer’s protocol. The extracted RNA concentrations were measured by a Nanodrop 2000 Spectrophotometer (Thermo Fisher Scientific Inc., Ottawa, ON, Canada). The RNA quality was visually checked by 1% agarose gel electrophoresis. Complementary DNA (cDNA) was synthesized from RNA using iScriptTM cDNA Synthesis kit (Bio-Rad, Mississauga, ON, Canada). Quantitative Real-time PCR (RT-PCR) of genes, including cationic amino acid transporter (CAT-1), neutral amino acid transporter (B0AT-1), glutamate transporters excitatory amino acid carrier 1 (EAAC-1), cysteine/glutamate antiporter (xCT), sodium-dependent glucose transporter 1 (SGLT-1), peptide transporter 1 (PepT-1), maltase-glucoamylase (MG), zonula occludens 1 (ZO-1), cadherin 1 (CDH-1), claudin 1 (CLDN-1), and proliferating cell nuclear antigen (PCNA) were determined by iQ SYBR Green Supermix (Bio-Rad) in a CFX Connect Real-Time PCR Detection System (Bio-Rad). The detailed primer information was described previously (Lu et al., 2020). The cycling conditions were 95 °C for 3 min, 40 cycles at 95 °C for 20 s, 60 °C for 30 s and 72 °C for 30 s. Relative gene expressions were calculated using the 2−ΔΔCT method (Livak et al., 2001).
Apparent Ileum Nutrient Digestibility
An indigestible analytical marker, 0.3% chromium oxide (Cr2O3), was added to the mash feed at the last 4 d of the trial for analysis of apparent ileal digestibility (AID) of dry matter (DM), crude protein (CP), crude fat (CF), and amino acids (AA). Briefly, approximately 1 kg of diets in each treatment were collected and kept in a cold room at 4 °C. Ileal digesta were collected from four birds per pen (pool digesta) on d 41 and then freeze-dried. The dried digesta samples were kept in airtight bags and stored at 22 °C for further analysis. Before analyzing, the dried digesta and feedstuff were finely ground by a grinder (CBG5 Smart Grind; Applica Consumer Products, Inc., Shelton, CT, USA).
The DM was measured by Official Methods of Analysis (AOAC, 2000; procedure # 934.01), CP was analyzed by a Leco NS 2000 Nitrogen Analyzer (Leco Corporation, St. Joseph, MI, USA) and calculated based on nitrogen content (CP = nitrogen × 6.25), and CF was determined using ANKOM Extraction System. Samples for AA analysis were prepared by acid hydrolysis according to the method of AOAC (2006; procedure # 994.12). Samples for methionine and cysteine analysis were oxidized with performic acid (AOAC, 2006; procedure # 985.18) before acid hydrolysis. Samples for tryptophan analysis were determined by the method of Commission Directive (2000) after hydrolyzing with barium hydroxide octahydrate for 20 h at 110 °C. The AA were analyzed using an Amino Acid analyzer (SYKAM, Germany). The Cr content was measured by inductively coupled plasma spectrometer (VarianInc., Palo Alto, CA, USA):
where ND is the nutrient concentration in digesta; CF is the chromium concentration in feed; NF is the nutrient concentration in diet; CD is the chromium concentration in digesta.
Genomic DNA Extraction and 16S Ribosomal RNA Gene Sequencing
Ileal and cecal digesta were collected from four birds per pen (pooled digesta) at d 41 and stored in −80 °C for genomic DNA extraction using QIAamp Fast DNA Stool Mini Kit (QIAGEN, Toronto, Canada). The quantity and quality of DNA were determined by Nanodrop 2.0 and 1.0% agarose gel electrophoresis, respectively. The sequencing of the 16S ribosomal RNA gene (16S rRNA) were prepared according to Illumina 16S Metagenomics Sequencing Library Preparation Guide Rev. B and sequenced using a MiSeq instrument (Illumina). Briefly, the amplicon library of V3-V4 hypervariable region (444 bp) was amplified and sequencing libraries were prepared as previously described (Yang et al., 2021). A 600-cycle v3 reagent kit (Illumina, MS1023003) was used to sequence after pooling equimolar quantities of each sample together. The sequencing data was analyzed by Quantitative Insights Into Microbial Ecology 2 (QIIME 2) (Bolyen et al., 2018). Briefly, 300 bp paired-end reads were processed with DADA2 to denoise reads, remove chimeric sequences and singletons, join paired-ends and de-replicate sequences to produce unique amplicon sequence variants (ASVs) (Callahan et al., 2016). Taxonomic classification of the resulting feature table was performed with VSEARCH and the Greengenes 99% OTU sequences as reference (McDonald et al., 2012; Rognes et al., 2016). ASVs were discarded if they had fewer than 10 instances across all samples, were present in fewer than two samples, or were not assigned taxonomy at the phylum level. Multiple sequence alignment of ASV representative sequences was performed with MAFFT and a rooted phylogenetic tree constructed with FastTree (Katoh et al., 2009; Price et al., 2009). Core diversity analysis was performed using a sampling depth of 10,000 sequences to plot taxonomic relative abundances, calculate alpha-diversity metrics, and generate dissimilarity matrices based on Bray-Curtis, Jaccard, and UniFrac distances, which were used for principal coordinate (PCoA) analyses.
Meat Quality
The birds were fed finisher diet until d 49 for collecting breast meat (Pectoralis major muscle) for meat quality analysis as described previously (Lu et al., 2020). The breasts from euthanized birds (four birds per pen) were carefully split, deboned, and trimmed off extra-muscular fat and connective tissues without damaging the exposed surface. The split breasts were placed on white Styrofoam trays (foam meat tray, 8.25 × 5.75 × 1, Pack. All Manufacturing Inc, Rockland, ON, Canada) containing soaking pads and covered with oxygen permeable polyvinyl chloride films (PVC; 037242 PUR Value Polyvinylchloride Standard Meat Films, AGL, Richmond Hill, Ontario, Canada). The trays with breast samples were placed on a retail display cabinet (Model MI, Husmann) at 2 °C under LED lighting (light emitting diodes; Acuity Brands Dimmable Rigid 30-LED Light Strip Board HTG S7 - 94v-0 – 4000k) with an intensity about 1240 lx. The cabinet was rotated every 24 h to minimize temperature and lighting variations of the machine.
White striping (WS) and woody breast (WB) were evaluated visually and scored by a well-trained technician at a processing plant. The WS was scored as normal (0; no distinct white lines), moderate (1; visible white lines with <1 mm thick), and severe (2; large white lines 1–2 mm thick) as previously described (Kuttappan et al., 2012). The WB was scored as normal (0; fillets are flexible), mild (1; hard in the cranial region but flexible), moderate (2; hard throughout but flexible in mid to caudal region), and severe (3; extremely hard and rigid throughout from cranial region to caudal tip) based on tactile evaluation (Tijare et al., 2016).
Breast pH values at 24 h and 96 h post-slaughter were recorded by a waterproof meter (HI 99163, HANNA Instruments, Carrollton, TX). Meat color was measured at three locations by a colorimeter (Chroma Meter CR-410, Minolta Canada Inc., Mississauga, ON) using CIELAB systems for determining lightness (L*), redness (a*), and yellowness (b*). Dripping loss (%) was measured as previously described (Wang et al., 2016). Briefly, a small piece (approximately 5 g) of each breast sample on the caudal portion of the breast was cut and weighed. The pieces were hung perpendicularly to the ground in a flat-bottomed volumetric flask without touching the surface of the flask. The samples were suspended at 2 °C and final weights were measured after 48 h to calculate dripping loss (%):
Myofibrillar fragmentation index (MFI) was measured as previously described (Culler et al., 1978). Firstly, a 4 g of breast sample was minced at 2 °C, suspended in 40 mL of cold MFI buffer (100 mM KCk, 20 mM KPO4, 2 mM MgCl2, 2 mM EGTA, 1 mM NaN3, pH 7.0), and homogenized for 30 s. The homogenate was transferred into a 50 mL sterilized tube to centrifuge at 2 °C with a speed of 1000 × g for 15 min (Thermo Scientific Sorvall RC6 plus Centrifuge). After centrifugation, the pellet was re-suspended by 10 mL cold MFI buffer and mixed by a vortex mixer. The mixture was poured through a strainer to remove connective tissues. Then, the protein concentration of each suspension was determined using bovine serum albumin (BSA) as the standard. Briefly, 0.25 mL suspension was added in tubes with 0.75 mL MFI buffer and 4 mL of biuret reagent. Then the suspension was placed in the dark for 30 min at room temperature. Simultaneously, serial dilutions of BSA were made to generate a standard curve by measuring the optical density (OD) at 540 nm (OD540 nm) using a spectrophotometer (GENESYS 30 visible spectrophotometer). The protein concentration of each suspension was determined by OD540nm measurements using the BSA standard curve. Finally, based on the protein concentration, the suspension was diluted to 8 mL of 0.5 mg protein per mL solution and mixed well. The MFI was calculated after the OD540 nm determination (Culler et al., 1978):
After the measurements of WS, WB, pH, color, dripping loss (%), and MFI, the rest of the breast samples were cut into five pieces, vacuum packed (6″ × 10″ FlairPak Vacuum Pouch, Flair Flexible Packaging Corporation, Canada/USA), and stored in a −40 °C freezer for analyzing cooking loss (%) and Warner–Bratzler Shear Force (WBSF). Frozen breast samples were thawed overnight in a cold room at 2 °C, sealed in plastic bags, and cooked in a water bath at 85 °C. A thermometer was inserted immediately in breast samples to monitor the internal temperatures until they reached 75–78°C and the cooking times were recorded. Then breast samples were moved from the water bath to the cold room (2 °C) for 2 h cooling. The cooled samples were weighted to calculate cooking loss (%):
The cooked samples were placed in the cold room at 2 °C overnight and were moved to room temperature for 30 min to measure WBSF by an analyzer (TA-XT Plus, Texture Technologies). Before analysis, the analyzer was calibrated with a 2 kg weight using a 10 kg loading cell. Then five rectangular strips (2–4 cm long, 1 cm wide, 1 cm height) were cut along the fiber by a ruler and a knife. The strips were placed in the analyzer with fiber perpendicular to the blade to record WBSF values (kg).
Data Analysis
The experiment was analyzed as complete random design (CRD) and pens were considered as experimental unit. The growth performance, organ weight, intestinal morphology, ileal digestibility, and gene expressions obtained in each treatment were evaluated using PROC ANOVA followed by the Tukey’s multiple comparison test (SAS 9.4) with the model: Yij = µ + Ti + eij, where µ is the total means, Ti is the fixed treatment effects, eij is residual of the model. The relative abundance of microbial taxa and diversity, and meat quality including meat color (a*, b*, L*), pH, purge loss, cooking loss, MFI, and shear force were analyzed using PROC MIXED followed by the Tukey’s multiple comparison test (SAS 9.4). Chi-square analysis was analyzed using PROC FREQ to check differences in the distribution of severity scores in WS and WB. A P-value of 0.05 was used to declare significance.
RESULTS
Growth Performance, Organ Weights
Results demonstrated that encapsulated CIN at 50 mg/kg or 100 mg/kg had no significant effects on BW, ADFI, and FCR compared to the controls for either each growing stage or for the overall feeding trial (Table 2). Similarly, no significant differences were noted between treatments for the weights of heart, liver, spleen, and bursa.
Table 2.
Effects of encapsulated cinnamaldehyde at either 50 mg/kg or 100 mg/kg in feed on growth performance of broiler chickens
| Itemsa | Treatmentsb | SEMc | P-value | |||
|---|---|---|---|---|---|---|
| NC | PC | EOL | EOH | |||
| Starter (d 1–14) | ||||||
| BW (14 d, g) | 514.83 | 501.14 | 505.03 | 497.36 | 4.802 | 0.63 |
| ADG, g | 33.32 | 32.34 | 32.62 | 32.07 | 0.337 | 0.61 |
| ADFI, g | 44.99 | 46.07 | 46.63 | 45.42 | 0.401 | 0.51 |
| FCR, g/g | 1.35 | 1.43 | 1.43 | 1.42 | 0.015 | 0.19 |
| Grower (d 15–28) | ||||||
| BW (28 d, g) | 1749.33 | 1784.13 | 1728.14 | 1696.89 | 15.793 | 0.26 |
| ADG, g | 88.18 | 91.64 | 87.37 | 85.68 | 1.067 | 0.25 |
| ADFI, g | 118.64 | 119.03 | 118.70 | 116.37 | 1.152 | 0.85 |
| FCR, g/g | 1.35 | 1.30 | 1.36 | 1.36 | 0.011 | 0.12 |
| Finisher (d 29–41) | ||||||
| BW (41 d, g) | 3274.39 | 3319.32 | 3305.02 | 3185.67 | 34.625 | 0.54 |
| ADG, g | 116.54 | 118.09 | 121.3 | 114.52 | 2.146 | 0.74 |
| ADFI, g | 185.56 | 182.63 | 186.18 | 179.37 | 2.348 | 0.75 |
| FCR, g/g | 1.60 | 1.55 | 1.54 | 1.57 | 0.015 | 0.54 |
| Overall (d 1–41) | ||||||
| ADG, g | 76.57 | 77.88 | 77.54 | 74.70 | 0.825 | 0.54 |
| ADFI, g | 110.06 | 108.87 | 110.66 | 108.12 | 0.982 | 0.81 |
| FCR, g/g | 1.44 | 1.40 | 1.43 | 1.45 | 0.010 | 0.32 |
| Morality, % | 16.25 | 10 | 12.5 | 10 | - | - |
a BW, body weight; ADFI, average daily feed intake; ADG, average daily gain; FCR, feed conversion ratio.
b NC, negative control, birds fed with basal diet; PC, positive control, birds fed with 30 mg/kg avilamycin premix; EOL, birds fed 50 mg/kg encapsulated cinnamaldehyde; EOH, birds fed 100 mg/kg encapsulated cinnamaldehyde.
c SEM, standard error of the mean.
Intestinal Morphology, Jejunal Gene Expression, and Ileal Digestibility
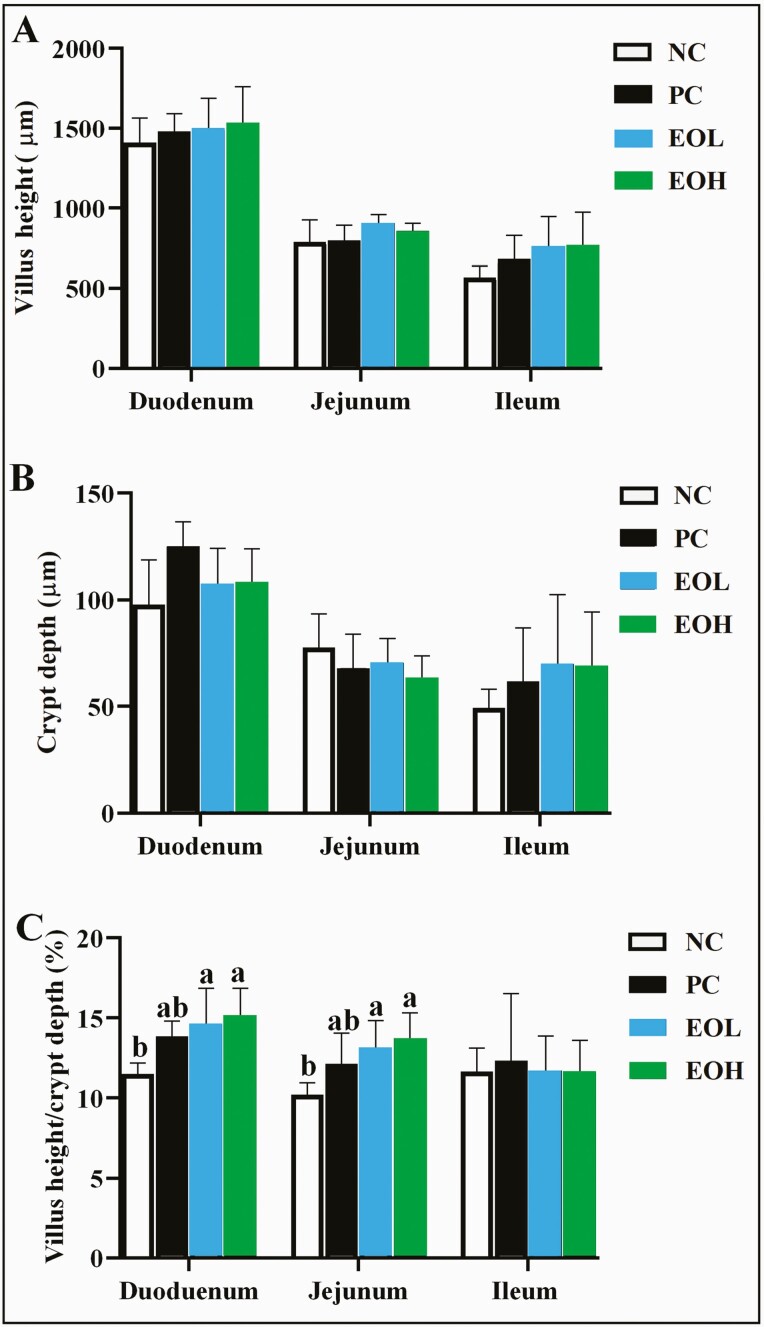
Feeding EOL or EOH to birds, had no significant effect on VH and CD of duodenum jejunum and ileum of d 41 old broilers. However, EOL and EOH feeding increased (P < 0.05) VH/CD compared to NC and PC birds, with average increases by EOL and EOH respectively being 14.67% and 15.13% in the duodena and 15.13% and 13.58% in the jejuna (Figure 1).
Figure 1.
Effects of encapsulated cinnamaldehyde on villus height (A), crypt depth (B) and ratio of villus height and crypt depth (C) on duodenum, ileum, and jejunum in broilers. NC, negative control, birds fed with basal diet; PC, positive control, birds fed with 30 mg/kg avilamycin premix; EOL, birds fed 50 mg/kg encapsulated cinnamaldehyde; EOH, birds fed 100 mg/kg encapsulated cinnamaldehyde. Significant differences are indicated by letters (a, b).
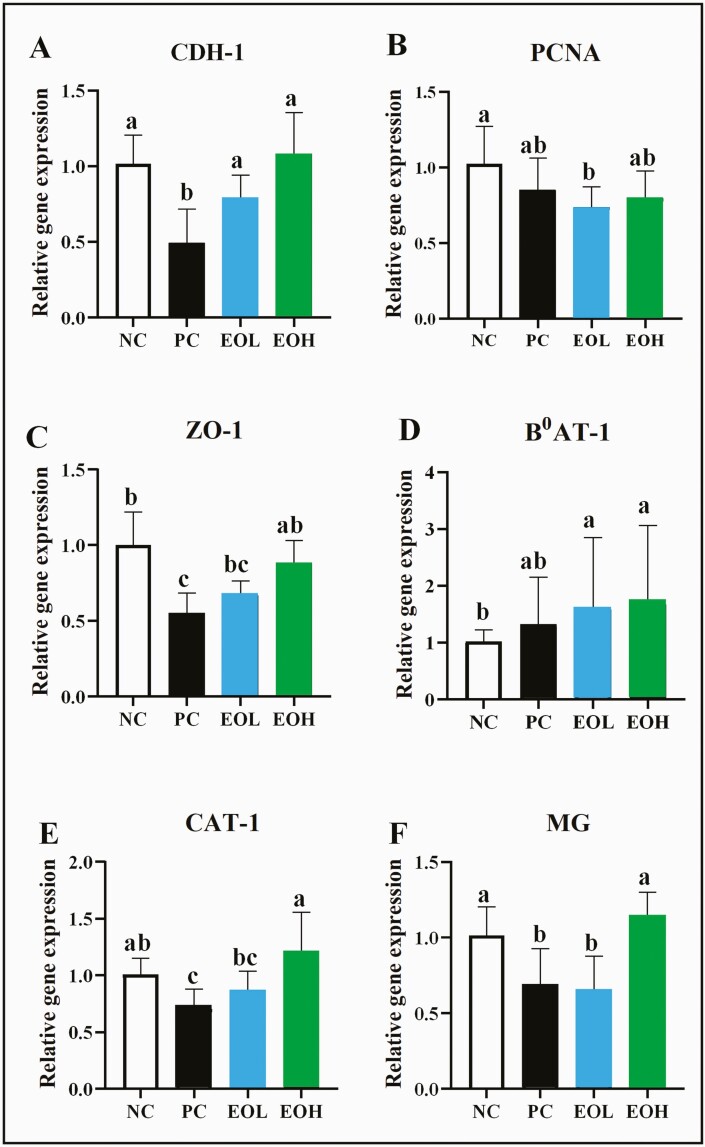
For jejunal gene expression, 6 of the 11 studied genes, including CDH-1, PCNA, ZO-1, B0AT-1, CAT-1, and MG showed significant (P < 0.05) effects of dietary treatments. Birds fed EOL or EOH increased (P < 0.05) expressions of CDH-1 and B0AT-1 compared to PC and NC, respectively. The PCNA expression was significantly downregulated (P < 0.05) with EOL supplemented diet compared to NC treatment. The ZO-1 expressions were significantly upregulated in EOH treatments compared to PC (P < 0.05) but not different from NC treatment. Nonetheless, EOH fed birds showed a higher expression (P < 0.05) of CAT-1 and MG compared to PC (Figure 2).
Figure 2.
Effects of encapsulated cinnamaldehyde on jejunal gene expressions of CDH-1 (A), PCNA (B), ZO-1 (C), B0AT-1 (D), CAT-1 (E) and MG (F). NC, negative control, birds fed with basal diet; PC, positive control, birds fed with 30 mg/kg avilamycin premix; EOL, birds fed 50 mg/kg encapsulated cinnamaldehyde; EOH, birds fed 100 mg/kg encapsulated cinnamaldehyde; CDH-1, cadherin 1; PCNA, proliferating cell nuclear antigen; ZO-1, zonula occludens 1; B0AT-1, neutral amino acid transporter; CAT-1, cationic amino acid transporter; MG, maltase-glucoamylase. Significant differences are indicated by letters (a, b, c).
The AID of DM, CP, CF, and 18 amino acids (alanine, arginine, aspartate, cysteine, glutamine, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylamine, proline, serine, threonine, tryptophan, tyrosine, and valine) were improved in birds fed EOL compared to NC birds (P < 0.05). However, when birds were fed EOH, no increases in AID of DM and CP were observed, and only eight amino acids (alanine, arginine, glutamine, isoleucine, leucine, phenylamine, tyrosine, and valine) showed a higher (P < 0.05) AID than NC birds. Additionally, as shown on Table 4, birds on PC showed an increased (P < 0.01) AID of CF and digestibility of 13 amino acids (alanine, arginine, cysteine, glutamine, glycine, histidine, isoleucine, leucine, lysine, phenylamine, threonine, tyrosine, and valine) compared to NC birds.
Table 4.
Effects of encapsulated cinnamaldehyde at either 50 mg/kg or 100 mg/kg in feed on dry matter, crude fat, crude protein, and amino acid digestibility
| Itema | Treatmentsb | SEMc | P value | |||
|---|---|---|---|---|---|---|
| NC | PC | EOL | EOH | |||
| DM | 71.26b | 71.96b | 76.21a | 69.39b | 0.514 | <0.01 |
| CF | 81.51b | 87.18a | 89.70a | 86.36a | 0.765 | <0.01 |
| CP | 76.88b | 79.83b | 84.03a | 79.14b | 0.632 | <0.01 |
| Ala | 78.12b | 84.78a | 87.25a | 82.94a | 0.905 | <0.01 |
| Arg | 79.51c | 87.27ab | 89.76a | 85.49b | 0.915 | <0.01 |
| Asp | 74.10bc | 79.15ab | 81.35a | 77.11ab | 0.933 | 0.03 |
| Cys | 70.25b | 79.00a | 80.15a | 73.32ab | 1.188 | <0.01 |
| Glu | 82.42c | 87.23ab | 88.94a | 86.13ab | 0.680 | <0.01 |
| Gly | 71.16c | 77.75ab | 80.20a | 75.10abc | 1.035 | 0.01 |
| His | 54.48bc | 62.91a | 67.68a | 59.54ab | 1.301 | <0.01 |
| Ile | 64.74c | 80.48ab | 82.47a | 78.87ab | 1.547 | <0.01 |
| Leu | 78.24c | 85.54ab | 89.12a | 84.67b | 0.867 | <0.01 |
| Lys | 82.60c | 87.88ab | 89.15a | 83.12bc | 0.791 | <0.01 |
| Met | 89.65b | 92.10ab | 94.38a | 90.38b | 0.546 | 0.01 |
| Phe | 77.12c | 84.62ab | 88.13a | 83.64b | 0.874 | <0.01 |
| Pro | 79.57b | 83.89ab | 86.36a | 81.92ab | 0.812 | 0.02 |
| Ser | 75.55b | 80.95ab | 83.11a | 79.10ab | 0.862 | 0.01 |
| Thr | 65.94b | 76.35a | 78.78a | 71.39ab | 1.374 | <0.01 |
| Trp | 78.26b | 80.70ab | 84.81a | 79.82ab | 0.869 | 0.04 |
| Tyr | 73.11c | 84.79ab | 88.45a | 83.16b | 1.212 | <0.01 |
| Val | 62.35c | 79.13ab | 84.27a | 77.32b | 1.701 | <0.01 |
a DM, dry matter; CF, crude fat; CP, crude protein.
b NC, negative control, birds fed with basal diet; PC, positive control, birds fed with 30 mg/kg avilamycin premix; EOL, birds fed 50 mg/kg encapsulated cinnamaldehyde; EOH, birds fed 100 mg/kg encapsulated cinnamaldehyde.
c SEM, standard error of the mean.
Ileal and Cecal Microbiota
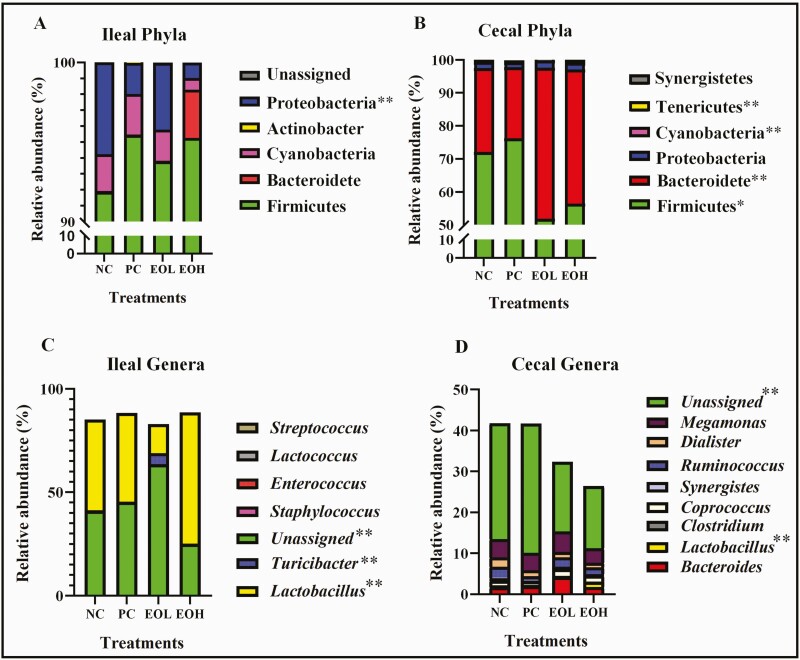
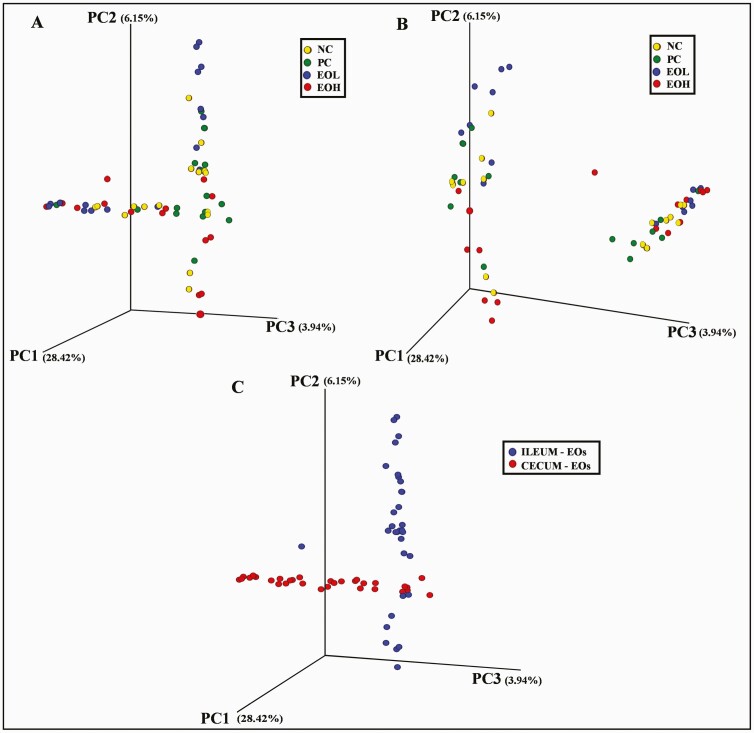
Four bacterial phyla (Firmicutes, Bacteroidetes, Proteobacteria, and Cyanobacteria) with greater than 1% relative abundance were observed in both ileal and cecal digesta (Figure 3). A lower relative abundance of Proteobacteria was observed in ileal digesta of EOH-fed birds compared to NC birds (P < 0.01) while a higher proportion of Bacteroidetes were observed in cecal digesta of EOL- and EOH-fed birds than in those of NC and PC birds (P < 0.01). At the genus level, ileal and cecal digesta from EOH-fed birds showed the highest relative abundance of Lactobacillus population (P < 0.01) compared to other treatments, but a higher relative abundance of Turicibacter was observed in ileal digesta in EOL-fed birds (P < 0.01) than those in NC, PC and EOH groups. No significant differences were observed between treatments for alpha diversity (richness and diversity) including Chao1, Shannon, and Simpson indices (Table 5). Principal Coordinates Analysis (PCoA) of the microbiota according to weighted UniFrac phylogenetic distances showed that the majority of samples from ileal (blue) and cecal (red) digesta clustered separately (P < 0.01). In ileal samples, EOL was separated from NC (P < 0.05), PC (P < 0.05), and EOH (P < 0.01). In cecal samples, EOL was separated from NC (P < 0.05) and PC (P < 0.05). However, there were no significant differences between NC, PC, and EOH in both ileal and cecal samples (Figure 4).
Figure 3.
Relative abundance of ileal phyla (A) and major (>1% relative abundance) genera (C) and cecal phyla (B) and major (>1% relative abundance) genera (D) in birds treated with 30 mg/kg avilamycin or encapsulated cinnamaldehyde. NC, negative control, birds fed with basal diet; PC, positive control, birds fed with 30 mg/kg avilamycin premix; EOL, birds fed 50 mg/kg encapsulated cinnamaldehyde; EOH, birds fed 100 mg/kg encapsulated cinnamaldehyde. Asterisks indicate significant statistically differences (one asterisk means a significance level of 0.05; two asterisks mean a significance level of 0.01).
Table 5.
Summary of alpha-diversity measurements of microbiota in ileum and caecum of broilers treated with avilamycin premix or cinnamaldehyde
| α-diversity | Gut segments | Treatmentsa | SEMb | P value | |||
|---|---|---|---|---|---|---|---|
| NC | PC | EOL | EOH | ||||
| Observed OTUs | Ileum | 32.14 | 32.86 | 32.13 | 34.14 | 1.490 | 0.97 |
| Cecum | 100.50 | 89.17 | 92.75 | 110.29 | 4.952 | 0.82 | |
| Chao1 | Ileum | 33.29 | 37.75 | 38.14 | 34.60 | 1.854 | 0.79 |
| Cecum | 100.14 | 93.63 | 94.57 | 110.13 | 6.200 | 0.78 | |
| Shannon | Ileum | 4.38 | 4.50 | 4.39 | 4.73 | 0.079 | 0.43 |
| Cecum | 6.27 | 6.28 | 6.03 | 6.12 | 0.071 | 0.82 | |
| Simpson | Ileum | 0.94 | 0.94 | 0.94 | 0.95 | 0.003 | 0.39 |
| Cecum | 0.98 | 0.98 | 0.98 | 0.98 | 0.001 | 0.91 |
a NC, negative control, birds fed with basal diet; PC, positive control, birds fed with 30 mg/kg avilamycin premix; EOL, birds fed 50 mg/kg encapsulated cinnamaldehyde; EOH, birds fed 100 mg/kg encapsulated cinnamaldehyde.
b SEM, standard error of the mean.
Figure 4.
The 3D principal coordinate analysis (PCoA) graph shows the variation among distance matrixes (weighted UniFrac) of ileal (A) and cecal (B) microbiota alone or together (C) in birds treated with avilamycin premix or encapsulated cinnamaldehyde. Percentages shown are percentages of variation explained by the PC1 (28.42%), PC2 (6.15%), and PC3 (3.94%). NC, negative control, birds fed with basal diet; PC, positive control, birds fed with 30 mg/kg avilamycin premix; EOL, birds fed 50 mg/kg encapsulated cinnamaldehyde; EOH, birds fed 100 mg/kg encapsulated cinnamaldehyde; EOs, essential oils.
Meat Quality
No treatment effects were found on cooking time (min), purge loss, dripping loss, pH values (96 h), color (L*, a*, b*), white striping (WS), and woody meat (WB). Despite meat pH at 24 h post slaughter broilers fed EOH tended to be higher (P = 0.05) than other treatments, all pH values were at the normal range. However, birds fed in PC and EOH treatments yielded (P < 0.05) the WBSF (kg) in breast meat compared to NC birds (Table 3).
Table 3.
Effects of encapsulated cinnamaldehyde at either 50 mg/kg or 100 mg/kg in feed on breast meat quality of broilers
| Variablesb | NCa | PC | EOL | EOH | SEMc | P-value |
|---|---|---|---|---|---|---|
| WBSF (kg) | 1.95a | 1.46b | 1.59ab | 1.33b | 0.16 | 0.04 |
| Cooking loss (%) | 23.11 | 23.63 | 23.24 | 23.04 | 0.85 | 0.96 |
| Cooking time (min) | 37.52 | 37.96 | 35.85 | 35.8 | 1.47 | 0.31 |
| pH 24 h | 6.12a | 6.18ab | 6.20ab | 6.21b | 0.078 | 0.05 |
| pH 96 h | 5.41 | 5.61 | 5.56 | 5.52 | 0.316 | 0.54 |
| Dripping loss (%) | 1.34 | 1.26 | 1.29 | 0.78 | 0.37 | 0.69 |
| L* | 58.68 | 59.86 | 58.49 | 59.62 | 0.57 | 0.23 |
| a* | 11.52 | 10.91 | 11.56 | 11.09 | 0.24 | 0.14 |
| b* | 18.69 | 19.06 | 17.56 | 18.02 | 0.45 | 0.09 |
| MFI (mg/mL) | 39.54 | 37.99 | 40.28 | 37.54 | 0.91 | 0.69 |
| WS % (n) | ||||||
| WS scores | 0.19 | 0.32 | 0.33 | 0.27 | 0.06 | 0.52 |
| Normal | 95.24 (20) | 90.91 (20) | 87.10 (27) | 89.29 (25) | – | 0.88 |
| Moderate | 4.76 (1) | 9.09 (2) | 12.9 (4) | 10.71 (3) | – | – |
| Severe | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.00 (0) | – | – |
| WB % (n) | ||||||
| WB scores | 0.08 | 0.27 | 0.35 | 0.34 | 0.08 | 0.28 |
| Normal | 100 (21) | 86.36 (19) | 80.65 (25) | 85.71 (24) | – | 0.38 |
| Mild | 0.00 (0) | 13.64 (3) | 16.13 (5) | 14.29 (4) | – | – |
| Moderate | 0.00 (0) | 0.00 (0) | 3.23 (1) | 0.00 (0) | – | – |
| Severe | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.00 (0) | – | – |
a NC, negative control, birds fed with basal diet; PC, positive control, birds fed with 30 mg/kg avilamycin premix; EOL, birds fed 50 mg/kg encapsulated cinnamaldehyde; EOH, birds fed 100 mg/kg encapsulated cinnamaldehyde.
bWBSF, Warner−Bratzler shear force (kg); WS, white striping; WB, woody breast; .L*, lightness; a*, redness; b*, yellowness; MFI, Myofibril Fragmentation Index.
c SEM, standard error of the mean.
DISCUSSION
A recent study found that dietary supplementations of encapsulated CIN (100 mg/kg) improved growth performance, reduced gut lesions caused by Eimeria spp. and C. perfringens, and modulated cecal microbiota in broiler chickens that received coccidiosis vaccine (Yang et al., 2020). In the present study, no vaccination was applied and a lower dosage of CIN (50 mg/kg) was selected in addition to the 100 mg/kg to evaluate their effects on performance and gut health parameters including some organ’s weight, meat quality, intestinal morphology, jejunal gene expression, and ileal nutrient digestibility. Avilamycin (30 mg/kg), which is another common antibiotic besides bacitracin (55 mg/kg) used in broiler chicken production to prevent NE lesions caused by C. perfringens, was supplemented in diets as the PC (CFIA, 2020).
Data showed no significant improvement of BW, ADG, and FCR by inclusion of either CIN (50 or 100 mg/kg) or 30 mg/kg avilamycin in feeds. The result was inconsistent with a previous study (Yang et al., 2020) which may be due to the raising conditions since it has been reported that growth performance of broilers could be altered by different broiler-housing conditions (Mesa et al., 2017). The high mortality rate in the present study could be ascribed to a high rate of culling birds due to injuries according to the guidelines (CCAC, 2009). High mortality could be explained by high BW (>3,000 g in average at d 41) in this study which could have increased the incidences of injuries (Martins et al., 2016). Notably, birds fed EOL and EOH had a relative lower mortality than those in NC. In poultry barns, enteric diseases of broilers including coccidiosis and NE could be the main cause of high mortality (Christaki et al., 2004; Cooper et al., 2013). In this study, the lower mortality in birds fed EOL and EOH may suggest that enteric infections could have been controlled by supplementation of CIN at 50 or 100 mg/kg, when compared to the previous study (Yang et al., 2020).
Any abnormal changes to organ weights including heart, liver, spleen, and bursa are indicators of chicken health disorders (Bowes and Julian, 1988). No changes in bursa and spleen weights may suggest no disorders were generated after feeding CIN since bursa and spleen could get larger due to inflammation (Cazaban et al., 2015). No differences in liver and heart weights could suggest that CIN may not be toxic to birds since toxicity could be one of major causes of abnormal liver and heart weight gains (Zaefarian et al., 2019), which was also consistent with results from a previous study when birds were fed with CIN powder (Najafi and Torki, 2010). However, toxic assessment is required in the further study.
Intestinal morphology, gene expression, nutrient digestibility, and gut microbiota, are four major parameters to reflect gut health. In this study, the higher VH/CD in duodena and jejuna detected in broilers fed EOL and EOH indicated potential of CIN in improving nutrient digestion, absorption, and gut barrier function. Higher VH/CD could explain increased AID of DM, CP, CF, and AAs when birds were fed EOL or EOH. The improvement of AID in broiler chickens was also reported in a previous study when birds were fed with mixtures of oregano, CIN, and pepper oils (Hernandez et al., 2004). Interestingly, lower AID of DM, CP, and AAs such as lysine and tryptophan were shown in birds fed EOH compared to EOL. This may be due to high concentrations of chemical compounds (aldehyde) in EOH, which can block lysine and tryptophan residuals from digestive enzymes (Rawel et al., 2002). Another interesting finding in the current study was that mRNA expressions of some proteins for nutrient absorption, gut barrier integrity, and DNA repair were altered by EOL or EOH. In this study, higher mRNA expressions for B0AT-1 (compared to NC) and CAT-1 (compared to PC) in birds fed EOH were observed, suggesting improvements of neutral amino acids and cationic amino acids absorption (Gilbert et al., 2007). Additionally, higher MG expressions in birds fed EOH compared to PC and EOL indicated higher maltase-glucoamylase expressions for carbohydrate digestion (Diaz-Sotomayor et al., 2013). Since lower AID of DM was observed in birds fed EOH compared to EOL in this study, upregulated the mRNA expression of MG in birds fed EOH could be a compensatory feedback to maximize carbohydrate digestion (Ebrahimi et al., 2015). Tight junctions are multi-protein complexes that regulate ion and water transportations and prevent entry of harmful substances such as pathogens and endotoxins (Pitman and Blumberg, 2000). A recent study on IPEC-J2 cells in our lab reported that thymol oil could enhance intestinal barrier function by increasing the gene expression of ZO-1 (Omonijo et al., 2019). The present study suggested that birds fed EOH could improve gut barrier function when compared to birds fed PC. Additionally, lower mRNA expressions of PCNA may suggest alleviated pathogenic inflammations in birds fed EOL. This is because the mRNA expression of PCNA was higher in animals with inflammations compared to those without inflammations (Manohar and Acharya, 2015). For ileal microbiota, lower phylum Proteobacteria and higher genus Lactobacillus indicated that broilers fed EOH may possess ability to control the growth of pathogenic bacteria. This is because phylum Proteobacteria includes pathogenic genera such as Salmonella and Campylobacter, which are associated with inflammatory disorders in hosts (Moon et al., 2018), while many species in genus Lactobacillus such as L. acidophilus are beneficial bacteria (Azad et al., 2018). For cecal microbiota, higher phylum Bacteroidetes suggested increased carbohydrate degradation and propionate synthesis via succinate pathway in birds supplemented with CIN, and higher genus Lactobacillus demonstrated improvements of beneficial bacterial populations (Glendinning et al., 2019). The current results differ from the previous study in which that genus Lactobacillus was found to be more abundant in the cecum of broilers on citral, but not on CIN compared to birds fed a basal diet (Yang et al., 2020). Additionally, no significant differences of alpha diversity suggest that broilers fed EOL or EOH had minor effects on richness and diversity of microbiota. The PCoA results showed that diversity of ileal microbiota was significantly different from cecal microbiota, which is consistent with a recent study (Rios-Covian et al., 2017).
Increased AID, improved intestinal morphology, enhanced expressions of nutrient transporters, and altered ileal and cecal microbiota in birds fed EOL or EOH did not seem to promote growth performance in this study. This may because other factors such as genetics, management, and environment can affect growth performance of broilers (Craig et al., 2016). Additionally, in comparison to the recommendation in Cobb 500 guidelines (Cobb-Vantress Inc., 2012), the FCR values in each treatment was lower than the provided in the recommendation, indicating the growth performance in the present study was reaching the optimum genetic potentials and there is little room for improvement.
In addition to the importance of bird growth performance and gut health, post-slaughtering meat quality is essential in ensuring consumers’ satisfaction and could be judged by parameters including color, pH, water holding capacity (WHC), and scores of myopathies (Baracho et al., 2006). Since muscle myopathies such as WS and WB are frequently observed in heavy birds (>3,500 g live weight), four birds per pen were fed finisher diet until d 49 for investigating effects of CIN on breast meat quality (Kuttappan et al., 2013; Mogire et al., 2021). Because the fast-growing birds may be exposed to heat and pre-slaughtering stress in current intensive poultry farming system that often reduces meat quality (Sandercock et al., 2009), we tested the effects of CIN on breast meat quality of broiler chickens in the present study. Meat color is a critical parameter affecting consumer selection of deboned and skinless raw meats in markets (Qiao et al., 2001). In this study, all meat color parameters were normal and no color changes were found in breast meat from broilers fed EOL and EOH compared to NC and PC. This may be due to antioxidant compounds (aldehyde) in CIN, which could maintain meat color by preventing further oxidation of lipids and myoglobins due to air exposure (Kanani et al., 2017). Additionally, no changes on dripping loss (%) and cooking loss (%) may suggest that feeding birds with EOL or EOH did not have adverse effects on WHC of meat during storage, thawing and cooking, reflecting no alternations to meat juiciness (Bowker, 2017). Despite the observation of a higher pH value in EOH compared to NC at 24 h post slaughter, no pH differences among treatments were detected at 96 h. This result was in line with lack of alternations in meat color, cooking loss (%) and dripping loss (%) among treatments since any abnormal meat pH could cause alterations of these parameters (Mir et al., 2017). Interestingly, a similar study did not detect changes in pH, cooking loss (%) and dripping loss (%) in broiler chickens fed cinnamic bark powder at 200 mg/kg (Logaranjani, 2014). Myopathies on breast meat, such as WB and WS, reflect histological stress including lipolysis, fibrosis, necrosis, and myo-degeneration (Kuttappan et al., 2012; Kuttappan et al., 2013). In this study, we did not observe any differences of WB and WS scores among treatments. This may be due to better management and relatively lower stock density at the research barn used in the current study, compared to commercial farms (Kuttappan et al., 2016). Thus, a future study with higher bird numbers and a density comparable to commercial farms is required to test effects of CIN on WB and WS in broiler chickens. Additionally, the WBSF and MFI are another two meat quality parameters in predicting tenderness (Lyon and Lyon, 1990). The lower WBSF in birds treated with EOH compared to NC, suggested that CIN may have potential to enhance meat tenderness, which is consistent with the results obtained in a previous study when birds were fed with CIN (Gomathi et al., 2018).
In conclusion, this study indicated that encapsulated CIN in broiler feed could promote meat tenderness by reducing WBSF and gut health by improving AID, intestinal morphology and microbiota, and expressions of nutrient transporters. The impacts of CIN on growth performance were not significant probably due to reaching their optimum performance. Future studies are necessary to conduct a complete assessment to investigate the toxicity, safety, and economic impacts of encapsulated CIN in broiler chickens.
ACKNOWLEDGMENTS
The authors appreciate Jason Bourcier and Gemmar Maramot at the Glenlea Research Station, University of Manitoba for their help on the chicken trial. The authors also thank Dr. Paula Azevedo for the help with the manuscript preparation. The author was financially supported by Agriculture and Agri-Food Canada through the Genomics Research and Development Initiative on Antimicrobial Resistance (GRDI-AMR) mitigation project (PSS# 1858; J-001262) of the Government of Canada and the Start-Up Grant (C. Yang, 46561) from the University of Manitoba. Equipment used for analyses in this study was generously funded by the Canada Foundation for Innovation (CFI).
Conflict of interest statement. None declared.
LITERATURE CITED
- Association of official analytical chemists. 2000. 17th ed. Washington (DC): USA Official Method of Analysis.
- Association of official analytical chemists. 2006. 18th ed. Arlington (VA): USA Official Method of Analysis.
- Azad, M. A. K., Sarker M., Li T., and Yin J.. . 2018. Probiotic species in the modulation of gut microbiota: an overview. Biomed Res. Int. 2018:9478630. doi: 10.1155/2018/9478630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracho, M., Camargo G., Lima A., Mentem J., Moura D., Moreira J., and Nääs I.. . 2006. Variables impacting poultry meat quality from production to pre-slaughter: a review. Rev. Bras. Cienc. Avic. 8:201–212. doi: 10.1590/S1516-635X2006000400001 [DOI] [Google Scholar]
- Bedford, E. 2019. Poultry industry in Canada – statistics & facts. Accessed August 2019. Available from https://www.statista.com/topics/5441/poultry-industry-in-canada/.
- Bergeron, C. R., Prussing C., Boerlin P., Daignault D., Dutil L., Reid-Smith R. J., Geroge G. Z., and Manges A. R.. . 2012. Chicken as reservoir for extraintestinal pathogenic Escherichia coli in humans, Canada. Emerg. Infect. Dis. 18(Suppl. 3):415. doi: 10.3201/eid1803.111099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen, E., Rideout J. R., Dillon M. R., Bokulich N. A., Abnet C., Al-Ghalith G. A., Alexander H., Alm E. J., Arumugam M., and Asnicar F.. . 2018. QIIME 2: reproducible, interactive, scalable, and extensible microbiome data science. 2167–9843: PeerJ Preprints. doi: 10.7287/peerj.preprints.27295v1 [DOI] [PMC free article] [PubMed]
- Bowes, V. A., and Julian R. J.. . 1988. Organ weights of normal broiler chickens and those dying of sudden death syndrome. Can. Vet. J. 29:153–156. [PMC free article] [PubMed] [Google Scholar]
- Bowker, B. 2017. Developments in our understanding of water-holding capacity. In: Petracci, M., C. Berri, editor. Poultry quality evaluation. Sawston (UK): Woodhead Publishing. 77–113. [Google Scholar]
- Burt, S. 2004. Essential oils: their antibacterial properties and potential applications in foods - a review. Int. J. Food Microbiol. 94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022 [DOI] [PubMed] [Google Scholar]
- Callahan, B. J., McMurdie P. J., Rosen M. J., Han A. W., Johnson A. J., and Holmes S. P.. . 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13:581–583. doi: 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Council on Animal Care. 2009. CCAC guidelines on: the care and the use of farm animals in research, teaching, and testing. https://ccac.ca/Documents/Standards/Guidelines/Farm_Animals.pdf. Accessed 2018.
- Canadian Food Inspection Agency. 2020. Avilamycin (AVI) - medicating ingredient brochure. https://inspection.canada.ca/animal-health/livestock-feeds/medicating-ingredients/avilamycin/eng/1518556003460/1518556073546. Accessed April 2020.
- Casewell, M., Friis C., Marco E., McMullin P., and Phillips I.. . 2003. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 52:159–161. doi: 10.1093/jac/dkg313 [DOI] [PubMed] [Google Scholar]
- Cazaban, C., Masferrer N. M., Pascual R. D., Espadamala M. N., Costa T., and Gardin Y.. . 2015. Proposed bursa of fabricius weight to body weight ratio standard in commercial broilers. Poult. Sci. 94(Suppl. 9):2088–2093. doi: 10.3382/ps/pev230 [DOI] [PubMed] [Google Scholar]
- Chicken Farmers of Canada. 2020. Category III reduction - everything you need to know. https://www.chickenfarmers.ca/category-3-reduction/. Accessed April 2020.
- Christaki, E. P., Florou-Paneri I., Giannenas M., Papazahariadou M., Botsoglou N. A., and Spais A. B.. . 2004. Effect of a mixture of herbal extracts on broiler chickens infected with Eimeria tenella. Anim. Res. 53:137–144. doi: 10.1051/animres:2004006 [DOI] [Google Scholar]
- Cobb-Vantress Inc. 2012. Cobb 500 broiler performance and nutrition supplement. Siloam Springs (AR): Cobb-Vantress Inc. [Google Scholar]
- Cooper, K. K., Songer J. G., and Uzal F. A.. . 2013. Diagnosing clostridial enteric disease in poultry. J. Vet. Diagn. Invest. 25:314–327. doi: 10.1177/1040638713483468 [DOI] [PubMed] [Google Scholar]
- Craig, W. T., Ilkka L., and Ilias K.. . 2016. Breeding for efficiency in the broiler chicken: a review. Agron. Sustain. Dev. 36:66. doi: 10.1007/s13593-016-0398-2 [DOI] [Google Scholar]
- Craven, S. E., Cox N. A., Stern N. J., and Mauldin J. M.. . 2001. Prevalence of Clostridium perfringens in commercial broiler hatcheries. Avi. Dis. 45(Suppl. 4):1050–1053. doi: 10.2307/1592887 [DOI] [PubMed] [Google Scholar]
- Culler, R. D., Smith G. C., and Cross H. R.. . 1978. Relationship of myofibril fragmentation index to certain chemical, physical and sensory characteristics of bovine longissimus muscle. J. Food Sci. 43 (Suppl. 4):1177–1180. doi: 10.1111/j.1365-2621.1978.tb15263.x [DOI] [Google Scholar]
- Diaz-Sotomayor, M., Quezada-Calvillo R., Avery S. E., Chacko S. K., Yan L. K., Lin A. H., Ao Z. H., Hamaker B. R., and Nichols B. L.. . 2013. Maltase-glucoamylase modulates gluconeogenesis and sucrase-isomaltase dominates starch digestion glucogenesis. J. Pediatr. Gastroenterol. Nutr. 57:704–712. doi: 10.1097/MPG.0b013e3182a27438 [DOI] [PubMed] [Google Scholar]
- Ding, X., Yang C. W., and Yang Z. B.. . 2017. Effects of star anise (Illicium verum Hook. f.), essential oil, and leavings on growth performance, serum, and liver antioxidant status of broiler chickens. J. Appl. Poult. Res. 26:459–466. doi: 10.3382/japr/pfx014 [DOI] [Google Scholar]
- Ebrahimi, R., Faseleh Jahromi M., Liang J. B., Soleimani Farjam A., Shokryazdan P., and Idrus Z.. . 2015. Effect of dietary lead on intestinal nutrient transporters mRNA expression in broiler chickens. Biomed Res. Int. 2015:149745. doi: 10.1155/2015/149745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, A. H., Jacobson K. A., Rose J., and Zeller R.. . 2008. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008:pdb.prot4986. doi: 10.1101/pdb.prot4986 [DOI] [PubMed] [Google Scholar]
- Galal, A. A. A. E., El-Araby I. E., Hassanin O., and El-Said Omar A.. . 2016. Positive impact of oregano essential oil on growth performance, humoral immune responses and chicken interferon alpha signalling pathway in broilers. Adv. Anim. Vet. Sci. 4:57–65. doi: 10.14737/journal.aavs/2016/4.1.57.65 [DOI] [Google Scholar]
- Gilbert, E. R., Li H., Emmerson D. A., K. E.Webb, Jr, and Wong E. A.. . 2007. Developmental regulation of nutrient transporter and enzyme mRNA abundance in the small intestine of broilers. Poult. Sci. 86:1739–1753. doi: 10.1093/ps/86.8.1739 [DOI] [PubMed] [Google Scholar]
- Glendinning, L., Watson K. A., and Watson M.. . 2019. Development of the duodenal, ileal, jejunal and caecal microbiota in chickens. Anim. Microbiome 1:17. doi: 10.1186/s42523-019-0017-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomathi, G., Senthilkumar S., Natarajan A., Amutha R., and Purushothaman M. R.. . 2018. Effect of dietary supplementation of cinnamon oil and sodium butyrate on carcass characteristics and meat quality of broiler chicken. Vet. World 11:959–964. doi: 10.14202/vetworld.2018.959-964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke, A., Pop L., and Cozma V.. . 2013. Prevalence and distribution of Eimeria species in broiler chicken farms of different capacities. Parasite 20:50. doi: 10.1051/parasite/2013052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández, F., Madrid J., García V., Orengo J., and Megías M. D.. . 2004. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poult. Sci. 83:169–174. doi: 10.1093/ps/83.2.169 [DOI] [PubMed] [Google Scholar]
- Hong, Y. J., Bae Y. M., Moon B., and Lee S. Y.. . 2013. Inhibitory effect of cinnamon powder on pathogen growth in laboratory media and oriental-style rice cakes (sulgidduk). J. Food Prot. 76:133–138. doi: 10.4315/0362-028X.JFP-12-241 [DOI] [PubMed] [Google Scholar]
- Kanani, P. B., Daneshyar M., Aliakbarlu J., and Hamian F.. . 2017. Effect of dietary turmeric and cinnamon powders on meat quality and lipid peroxidation of broiler chicken under heat stress condition. Vet. Res. Forum 8:163–169. [PMC free article] [PubMed] [Google Scholar]
- Katoh, K., Asimenos G., and Toh H.. . 2009. Multiple alignment of DNA sequences with MAFFT. Methods Mol. Biol. 537:39–64. doi: 10.1007/978-1-59745-251-9_3 [DOI] [PubMed] [Google Scholar]
- Kumar, S., Chen C., Indugu N., Werlang G. O., Singh M., Kim W. K., and Thippareddi H.. . 2018. Effect of antibiotic withdrawal in feed on chicken gut microbial dynamics, immunity, growth performance and prevalence of foodborne pathogens. PLoS One. 13:e0192450. doi: 10.1371/journal.pone.0192450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttappan, V. A., Hargis B. M., and Owens C. M.. . 2016. White striping and woody breast myopathies in the modern poultry industry: a review. Poult. Sci. 95:2724–2733. doi: 10.3382/ps/pew216 [DOI] [PubMed] [Google Scholar]
- Kuttappan, V. A., Lee Y. S., Erf G. F., Meullenet J. F., McKee S. R., and Owens C. M.. . 2012. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poult. Sci. 91:1240–1247. doi: 10.3382/ps.2011-01947 [DOI] [PubMed] [Google Scholar]
- Kuttappan, V. A., Shivaprasad H. L., Shaw D. P., Valentine B. A., Hargis B. M., Clark F. D., McKee S. R., and Owens C. M.. . 2013. Pathological changes associated with white striping in broiler breast muscles. Poult Sci. 92:331–338. doi: 10.3382/ps.2012-02646 [DOI] [PubMed] [Google Scholar]
- Liljebjelke, K. A., Hofacre C. L., Liu T., White D. G., Ayers S., Young S., and Maurer J. J.. . 2005. Vertical and horizontal transmission of salmonella within integrated broiler production system. Foodborne Pathog. Dis. 2:90–102. doi: 10.1089/fpd.2005.2.90 [DOI] [PubMed] [Google Scholar]
- Livak, K. J., and Schmittgen T. D.. . 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Logaranjani, G. 2014. Cinnamon (Cinnamomum zeylanicum) as a substitute for antibiotic in broiler ration. M.V.Sc., Thesis, Tamil Nadu Veterinary and Animal Sciences University Chennai: 1–79. [Google Scholar]
- Lu, P., Choi J., Yang C., Mogire M., Liu S., Lahaye L., Adewole D., Rodas-Gonzalez A., and Yang C.. . 2020. Effects of antibiotic growth promoter and dietary protease on growth performance, apparent ileal digestibility, intestinal morphology, meat quality, and intestinal gene expression in broiler chickens: a comparison. J. Anim. Sci. 98:skaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon, C. E., and Lyon B. G.. . 1990. The relationship of objective shear values and sensory tests to changes in tenderness of broiler breast meat. Poult Sci. 69:1420–1427. doi: 10.3382/ps.0691420 [DOI] [Google Scholar]
- Manohar, K., and Acharya N.. . 2015. Characterization of proliferating cell nuclear antigen (PCNA) from pathogenic yeast Candida albicans and its functional analyses in S. cerevisiae. BMC Microbiol. 15:257. doi: 10.1186/s12866-015-0582-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangoni, F., Corsello G., Cricelli C., Ferrara N., Ghiselli A., Lucchin L., and Poli A.. . 2015. Role of poultry meat in a balanced diet aimed at maintaining health and wellbeing: an Italian consensus document. Food Nutr. Res. 59:27606. doi: 10.3402/fnr.v59.27606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins, B. B., Martins M. R. F. B., Mendes A. A., and Fernandes B. C. S., and Aguiar E. F.. . 2016. Footpad dermatitis in broilers: differences between strains and gender. Braz J Poult Sci. 18:461–466. doi: 10.1590/1806-9061-2015-0105 [DOI] [Google Scholar]
- McDonald, D., Price M. N., Goodrich J., Nawrocki E. P., DeSantis T. Z., Probst A., Andersen G. L., Knight R., and Hugenholtz P.. . 2012. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. Isme J. 6:610–618. doi: 10.1038/ismej.2011.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa, D., Muniz E., Souza A., and Geffroy B.. . 2017. Broiler-Housing conditions affect the performance. Braz. J. Poult. Sci. 19:263–272. doi: 10.1590/1806-9061-2016-0346 [DOI] [Google Scholar]
- Mir, N. A., Rafiq A., Kumar F., Singh V., and Shukla V.. . 2017. Determinants of broiler chicken meat quality and factors affecting them: a review. J. Food Sci. Technol. 54:2997–3009. doi: 10.1007/s13197-017-2789-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogire, M., Choi J., Lu P., Yang C., Liu S., Adewole D., Rodas-Gonzalez A., and Yang C.. . 2021. Effects of red-osier dogwood extracts on growth performance, intestinal digestive, and absorptive functions, and meat quality of broiler chickens. Can. J. Anim. Sci. In press. doi: 10.1139/CJAS-2020-0191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, C. D., Young W., Maclean P. H., Cookson A. L., and Bermingham E. N.. . 2018. Metagenomic insights into the roles of Proteobacteria in the gastrointestinal microbiomes of healthy dogs and cats. Microbiologyopen 7:e00677. doi: 10.1002/mbo3.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi, S. F., Di Lorenzo A., Izadi M., Sobarzo-Sánchez E., Daglia M., and Nabavi S. M.. . 2015. Antibacterial effects of cinnamon: from farm to food, cosmetic and pharmaceutical industries. Nutrients 7:7729–7748. doi: 10.3390/nu7095359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi, P., and Torki M.. . 2010. Performance, blood metabolites and immune competence of broiler chicks fed diets included essential oils of medicinal herbs. J Anim Vet Adv. 9:1164–1168. doi: 10.3923/javaa.2010.1164.1168 [DOI] [Google Scholar]
- Omonijo, F. A., Liu S., Hui Q., Zhang H., Lahaye L., Bodin J. C., Gong J., Nyachoti M., and Yang C.. . 2019. Thymol improves barrier function and attenuates inflammatory responses in porcine intestinal epithelial cells during lipopolysaccharide (LPS)-induced inflammation. J. Agric. Food Chem. 67:615–624. doi: 10.1021/acs.jafc.8b05480 [DOI] [PubMed] [Google Scholar]
- Osman, K. M., and Elhariri M.. . 2013. Antibiotic resistance of Clostridium perfringens isolates from broiler chickens in Egypt. Rev. Sci. Tech. 32:841–850. doi: 10.20506/rst.32.2.2212 [DOI] [PubMed] [Google Scholar]
- Pitman, R. S., and Blumberg R. S.. . 2000. First line of defense: the role of the intestinal epithelium as an active component of the mucosal immune system. J. Gastroenterol. 35:805–814. doi: 10.1007/s005350070017 [DOI] [PubMed] [Google Scholar]
- Preedy, V. R., editor. 2015. Essential oils in food preservation, flavor and safety. Cambridge (MA): Academic Press. [Google Scholar]
- Price, M. N., Dehal P. S., and Arkin A. P.. . 2009. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 26:1641–1650. doi: 10.1093/molbev/msp077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao, M., Fletcher D. L., Smith D. P., and Northcutt J. K.. . 2001. The effect of broiler breast meat color on pH, moisture, water-holding capacity, and emulsification capacity. Poult. Sci. 80:676–680. doi: 10.1093/ps/80.5.676 [DOI] [PubMed] [Google Scholar]
- Rawel, H. M., Czajka D., Rohn S., and Kroll J.. . 2002. Interactions of different phenolic acid and flavonoids with soy proteins. Int. J. Biol. Macromol. 30:137–150. doi: 10.1016/S0141-8130(02)00016-8 [DOI] [PubMed] [Google Scholar]
- Ren, X. J., Yang Z. B., Ding X., and Yang C. W.. . 2018. Effects of Ginkgo biloba leaves (Ginkgo biloba) and Ginkgo biloba extract on nutrient and energy utilization of broilers. Poult. Sci. 97:1342–1351. doi: 10.3382/ps/pex445 [DOI] [PubMed] [Google Scholar]
- Rios-Covian, D., Salazar N., Gueimonde M., and de Los Reyes-Gavilan C. G.. . 2017. Shaping the metabolism of intestinal bacteroides population through diet to improve human health. Front. Microbiol. 8:376. doi: 10.3389/fmicb.2017.00376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognes, T., Flouri T., Nichols B., Quince C., and Mahé F.. . 2016. VSEARCH: a versatile open source tool for metagenomics. Peerj 4:e2584. doi: 10.7717/peerj.2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandercock, D. A., Barker Z. E., Mitchell M. A., and Hocking P. M.. . 2009. Changes in muscle cell cation regulation and meat quality traits are associated with genetic selection for high body weight and meat yield in broiler chickens. Genet. Sel. Evol. 41:8. doi: 10.1186/1297-9686-41-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si, W., Gong J., Tsao R., Zhou T., Yu H., Poppe C., Johnson R., and Du Z.. . 2006. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J. Appl. Microbiol. 100:296–305. doi: 10.1111/j.1365-2672.2005.02789.x [DOI] [PubMed] [Google Scholar]
- Si, W., Ni X., Gong J., Yu H., Tsao R., Han Y., and Chambers J. R.. . 2009. Antimicrobial activity of essential oils and structurally related synthetic food additives towards Clostridium perfringens. J. Appl. Microbiol. 106:213–220. doi: 10.1111/j.1365-2672.2008.03994.x [DOI] [PubMed] [Google Scholar]
- da Silva, D. C. F., de Arruda A. M. V., and Gonçalves A. A.. . 2017. Quality characteristics of broiler chicken meat from free-range and industrial poultry system for the consumers. J. Food Sci. Technol. 54:1818–1826. doi: 10.1007/s13197-017-2612-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, W. L., Lei L. L., Zhang Q., and Li Y.. . 2016. Physical stability and antimicrobial activity of encapsulated cinnamaldehyde by selfemulsifying nanoemulsion. J. Food Process Eng. 39:462–471. doi: 10.1111/jfpe.12237 [DOI] [Google Scholar]
- Tijare, V. V., Yang F. L., Kuttappan V. A., Alvarado C. Z., Coon C. N., and Owens C. M.. . 2016. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult. Sci. 95:2167–2173. doi: 10.3382/ps/pew129. [DOI] [PubMed] [Google Scholar]
- Ventola, C. L. 2015. The antibiotic resistance crisis: part 1: causes and threats. P T 40:277–283. [PMC free article] [PubMed] [Google Scholar]
- Wang, S., Zhang L., Li J., Cong J., Gao F., and Zhou G.. . 2016. Effects of dietary marigold extract supplementation on growth performance, pigmentation, antioxidant capacity and meat quality in broiler chickens. Asian-Australas J. Anim. Sci. 30:71–77. doi: 10.5713/ajas.16.0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenreiter, R. H., Mowrey D. H., Stobbs L. A., and d’Assonville J. A.. . 2000. Effects of avilamycin on performance of broiler chickens. Vet. Ther. 1:118–124. [PubMed] [Google Scholar]
- Yang, Y., Cui S., Gong J., Miller S. S., Wang Q., and Hua Y.. . 2015. Stability of citral in oil-in-water emulsions protected by a soy protein–polysaccharide Maillard reaction product. Food Res. Int. 69:357–363. doi: 10.1016/j.foodres.2015.01.006 [DOI] [Google Scholar]
- Yang, C., Kennes Y. M., Lepp D., Yin X., Wang Q., Yu H., Yang C., Gong J., and Diarra M. S.. . 2020. Effects of encapsulated cinnamaldehyde and citral on the performance and cecal microbiota of broilers vaccinated or not vaccinated against coccidiosis. Poult. Sci. 99:936–948. doi: 10.1016/j.psj.2019.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C., M. A. Rehman., X. Yin., C. D. Carrilloc., Q. Wang., C. Yang., J. Gong., and M. S. Diarra. 2021. Antimicrobial resistance phenotype and genotype of generic Escherichia coli from encapsulated cinnamaldehye and citral fed-broiler chicken. J. Food Prot. In press. doi: 10.4315/JFP-21-033 [DOI] [PubMed] [Google Scholar]
- Zaefarian, F., Abdollahi M. R., Cowieson A., and Ravindran V.. . 2019. Avian liver: the forgotten organ. Animals. 9:63. doi: 10.3390/ani9020063 [DOI] [PMC free article] [PubMed] [Google Scholar]