Abstract
Before the introduction of tyrosine kinase inhibitors (TKIs), the overall survival of patients with advanced or metastatic gastrointestinal stromal tumors (GISTs) was 10 to 20 months because of the lack of approved therapies. In the last 20 years, a treatment algorithm for patients with advanced GISTs, which includes imatinib, sunitinib, and regorafenib as first‐, second‐, and third‐line therapies, respectively, has been established. Recently, 2 new TKIs have been approved: ripretinib for fourth‐line therapy and avapritinib as first‐line therapy in patients harboring platelet‐derived growth factor receptor α (PDGFRA) exon 18 D842V mutations. Additionally, there are several experimental therapies under investigation that could advance individualized patient care. All of these therapies have varying efficacies and safety profiles that warrant an updated treatment landscape review. This review article summarizes the efficacy and safety data currently available for conventional TKIs along with recently approved and experimental therapies.
Keywords: avapritinib, gastrointestinal stromal tumor, imatinib, ripretinib, toxicity, treatment landscape, tyrosine kinase inhibitors
Short abstract
With evolving treatment options and effective toxicity management, patients with advanced gastrointestinal stromal tumors are living longer than ever before. Recently approved targeted therapies and the investigation of experimental treatment options have the potential to alter the current treatment algorithm and encourage personalized patient care.
Introduction
Although they are rare, gastrointestinal stromal tumors (GISTs) are the most common sarcoma of the digestive tract and are frequently found in the stomach or small intestines, but they can arise anywhere in the gastrointestinal tract. 1 , 2 , 3 The estimated annual incidence of GISTs ranges from 6.8 to 15 cases per million individuals. 1 , 3 , 4 The majority of GISTs harbor activating mutations in KIT (approximately 69%‐83%) or platelet‐derived growth factor receptor α (PDGFRA; approximately 5%‐10%). 5 , 6 , 7 The ~15% of GISTs without KIT/PDGFRA mutations are heterogenous and are called wild type. 5 The most common KIT mutation occurs in exon 11 (~66%), 8 whereas the most common PDGFRA mutation occurs in exon 18. 6 These primary mutations are mutually exclusive such that primary tumors have either a KIT mutation or a PDGFRA mutation but not both. However, patients may have more than 1 mutation in the same gene because they develop secondary resistance mutations while on treatment. With the advent of next‐generation sequencing, it may become possible to monitor or assess the development of secondary resistance mutations in circulating tumor cells without the need for tumor biopsy. 9 , 10 This complicated and heterogeneous mutational landscape makes curative treatment of relapsed/refractory GISTs very difficult.
The first line of treatment for patients with localized GISTs is surgical resection, but patients may experience recurrent disease even with complete resection. The risk of recurrence is based on several factors, including location, size, and mitotic activity. 11 Systemic intravenous chemotherapy is ineffective against GISTs with response rates < 10%. 12 Before the introduction of targeted inhibitors, the median overall survival (mOS) for patients with advanced or metastatic GISTs was 10 to 20 months. 13 , 14 For these reasons, tyrosine kinase inhibitors (TKIs) became the standard of care for patients with advanced GISTs. These inhibitors, however, differ in their efficacies against certain mutations and often become ineffective because of the development of secondary resistance mutations. Traditional TKIs—imatinib, sunitinib, and regorafenib—also have varying safety profiles. The recent approval of 2 new TKIs with unique mechanisms of action and favorable safety profiles will likely alter the current treatment algorithm and provide more treatment choices (Fig. 1). Therefore, an update on the current treatment landscape is warranted. In this review, we discuss the safety and efficacy of approved therapies and detail the newer approved therapies (ripretinib and avapritinib) and experimental therapies.
Figure 1.
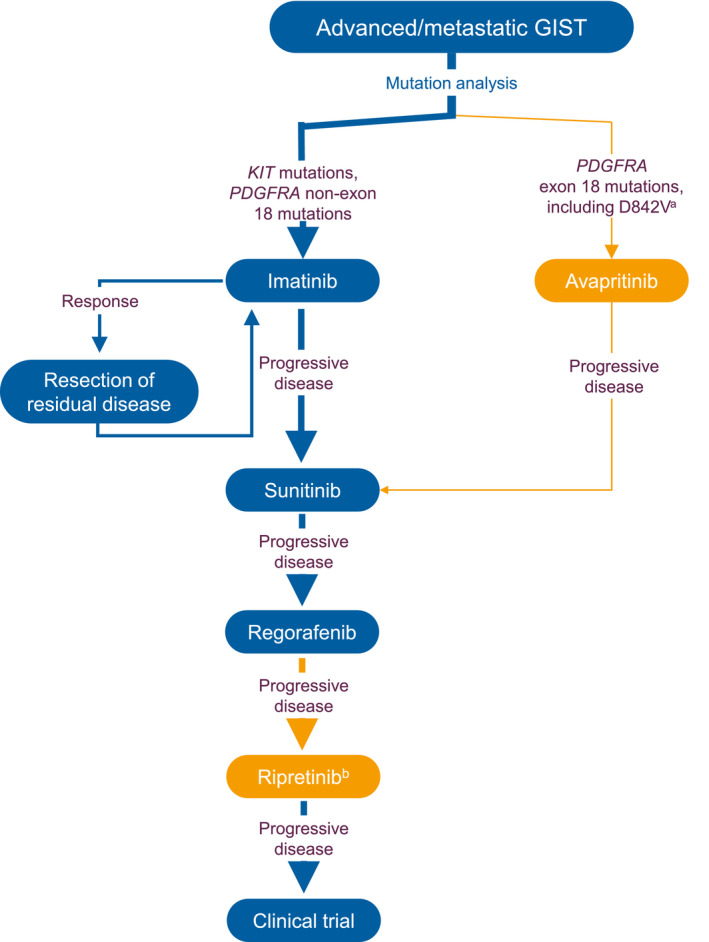
Treatment algorithm for patients with advanced GISTs. Blue indicates the conventional treatment algorithm; orange indicates recently approved therapies. aAvapritinib is approved only for PDGFRA exon 18 D842V mutations in Europe. bRipretinib is not yet approved in Europe. GIST indicates gastrointestinal stromal tumor; PDGFRA, platelet‐derived growth factor receptor α.
Early Approved Targeted Therapies
Imatinib, First‐Line Therapy
Imatinib mesylate was approved for the treatment of Philadelphia chromosome–positive chronic myelogenous leukemia because of its effective targeting of the Bcr‐Abl tyrosine kinase. 15 This compound also inhibits KIT and PDGFRA activity by binding to the adenosine triphosphate–binding pocket and preventing substrate phosphorylation and downstream signaling. 16 , 17 , 18 After 1 month of treatment with 400 mg of imatinib once daily, 1 patient with an advanced GIST demonstrated histologic and radiologic evidence of anticancer activity (decreased tumor cell density and tumor volume). 19 In the initial phase 1 study, lower doses (400 mg once daily, 300 mg twice daily, and 400 mg twice daily) were well tolerated, but 500 mg twice daily resulted in dose‐limiting toxicities (DLTs), including dyspnea, edema, and severe nausea. 20 In the randomized phase 2 trial, 53.7% of patients treated with 400 or 600 mg once daily achieved partial responses (PR), and 27.9% had stable disease (SD) after 9 months of treatment. 21 However, 13.6% of patients developed early resistance to imatinib therapy. Follow‐up revealed an mOS of 57 months, regardless of the treatment regimen. 22 In the phase 3 trial that compared 400 mg once daily and 400 mg twice daily, patients had mOS times of 55 and 51 months, respectively; the overall response rate was 45% for either dosing strategy (Table 1). 23 Of the 117 patients who progressed on low‐dose imatinib (400 mg once daily) and crossed over to a high dose (400 mg twice daily), 31% achieved an objective response or SD. At the 10‐year follow‐up, the overall survival rate was 19.4% for patients receiving 400 mg once daily and 21.5% for patients receiving 400 mg twice daily. 28 Data from the 10‐year follow‐up also indicated that age (<60 years), the size of the largest lesion (smaller), and KIT mutations (exon 11) were associated with a better prognosis. In a large meta‐analysis, patients with a KIT exon 9 mutation demonstrated an increased progression‐free survival benefit from 400 mg twice daily versus 400 mg once daily (P = .017). 29
TABLE 1.
Pivotal Trial Efficacy Outcomes for Approved Tyrosine Kinase Inhibitors for the Treatment of GISTs 23 , 24 , 25 , 26 , 27
| Drug | Population | Overall Response Rate, % (95% CI) c | PFS, Median (95% CI), mo | OS, Median (95% CI), mo |
|---|---|---|---|---|
| Imatinib a | 1L GIST | 45 | 18 (16‐21) | 55 (47‐62) |
| Avapritinib b | PDGFRA D842V‐mutant GIST | 93 (77‐99) | NE | NE |
| Sunitinib | 2L GIST | 7 | 5.5 (2.6‐6.5) | NE |
| Regorafenib | 3L GIST | 4.5 | 4.8 (4.1‐5.8) | NR |
| Ripretinib | ≥4L GIST | 9.4 (4.2‐17.7) | 6.3 (4.6‐6.9) | 15.1 (12.3‐15.1) |
Abbreviations: CI, confidence interval; GIST, gastrointestinal stromal tumor; L, line; NE, not estimable; NR, not reported; OS, overall survival; PDGFRA, platelet‐derived growth factor receptor α; PFS, progression‐free survival.
Data for a 400‐mg dose once daily.
Data for a 300‐mg dose.
95% CI for overall response rate was not reported for imatinib, sunitinib, and regorafenib.
In dose‐comparison studies, high‐dose imatinib was more likely to result in dose reductions and discontinuations. 23 , 30 In the phase 3 study, 43% of patients in the low‐dose arm and 63% in the high‐dose arm experienced grade 3 to 5 toxicities. 23 These severe toxicities included anemia, cardiac toxicity, gastrointestinal toxicity, and hemorrhage. There were 2 and 9 deaths in the low‐dose and high‐dose arms, respectively, that were possibly related to treatment, with a contribution from the tumor far more likely. Specifically, 4 patients in the high‐dose arm died because of gastrointestinal hemorrhage. 23
Patients who respond well to imatinib therapy are often reevaluated for surgical intervention (Fig. 1). 31 If progression is focal, local treatment of the progressing lesion (ie, surgery or liver‐directed ablation) and continuation of the same TKI dose are an option. If diffuse progression occurs, patients may opt to cross over to 400 mg of imatinib twice daily. 31 , 32 Although crossing over to the higher dose can be beneficial for patients with GISTs harboring a KIT exon 9 mutation, the potential increase in adverse events (AEs) often leads to the need for a dose reduction. 29 , 33 Patients may stop responding to imatinib because of the development of secondary mutations acquired during treatment. 34 , 35 Approximately 12% of GISTs harbor primary resistance to imatinib, and approximately 40% of patients develop secondary resistance. 36 In these cases, the next option is to switch to the approved second‐line therapy, sunitinib.
Sunitinib, Second‐Line Therapy
Sunitinib was approved in 2006 for patients with advanced GISTs after progression on imatinib. 37 Sunitinib inhibits multiple receptor tyrosine kinases, including PDGFRA and KIT. Like imatinib, sunitinib binds to the kinase adenosine triphosphate–binding pocket and locks it in the inactive conformation. 38 In an early trial, sunitinib provided a clinical benefit to 36% of patients who progressed on imatinib (PR, 7%; SD for ≥6 months, 29%). 39 The maximum tolerated dose was established at 50 mg once daily with a recommended schedule of 4 weeks on treatment followed by 2 weeks off after patients on higher doses experienced DLTs of fatigue, nausea, and vomiting. In a separate study, the median time to tumor progression in patients resistant or intolerant to imatinib was 27.3 weeks for patients receiving sunitinib and 6.4 weeks for patients receiving a placebo. 24 The overall objective response rates in the sunitinib and placebo groups were 7% (all PRs) and 0%, respectively (Table 1). 24 Similarly, a clinical benefit was observed in 53% of patients receiving sunitinib with a median progression‐free survival (mPFS) of 34 weeks. 40 Of patients receiving sunitinib, 20% reported serious AEs, whereas 5% of patients receiving a placebo did. 24 Common AEs (grade 3 or higher) were fatigue, diarrhea, palmar‐plantar erythrodysesthesia syndrome (PPES), hypertension, neutropenia, and lymphopenia; 4% of patients taking sunitinib developed hypothyroidism. 24
In another study, sunitinib treatment on alternative dosing schedules (continuous dosing at 37.5 mg once daily or other modifications) provided longer survival than the recommended dosing schedule (50 mg once daily, 4 weeks on, 2 weeks off). 41 Continuous dosing modifications of sunitinib included reductions to 25 or 12.5 mg once daily or an escalation to 50 mg once daily. 40 The mOS was 23.5 months for patients on an alternative dosing schedule and 11.1 months for patients on the recommended dosing schedule. 41 The investigators theorized that dose adjustments allowed for patients to avoid toxicities and continue treatment for longer; this emphasizes the need for dose maintenance and supportive care. The proportion of patients who discontinued sunitinib because of AEs was higher with the recommended schedule versus alternative schedules (34% vs 26%). Similarly to previous studies, the most common treatment‐related AEs were diarrhea, fatigue, and PPES. 41
Patients can also develop resistance to sunitinib therapy. Some patients develop mutations in the activation loop of the KIT gene and become resistant to further treatment 42 ; such mutations can shift the ratio of inactive KIT to active KIT and render sunitinib ineffective. 38 In the event of resistance to sunitinib, patients can begin the approved third‐line therapy, regorafenib.
Regorafenib, Third‐Line Therapy
Regorafenib is an oral, multitargeted TKI that acts against KIT and PDGFRA, among others. 43 In a phase 1 study in patients with solid tumors, the optimal dosing schedule for regorafenib was determined to be 160 mg once daily on a schedule of 3 weeks on treatment and 1 week off. 44 When administered in patients with advanced GISTs after the failure of imatinib and sunitinib, regorafenib demonstrated a clinical benefit in 75% of patients with an mPFS of 10 months. 45 In a phase 3 study, the mPFS was 4.8 months for patients receiving regorafenib and 0.9 months for patients receiving a placebo (P < .0001); patients who crossed over from the placebo to regorafenib had an mPFS of 5 months. 25 The overall response rate was 4.5% in the regorafenib group and 1.5% in the placebo group; all were PRs (Table 1). A best possible response of a PR or SD was observed in 76% of patients receiving regorafenib and in 35% of placebo patients. 25 Similarly, in a retrospective analysis of 50 patients with GISTs previously treated with at least 2 therapies, the mPFS was 7.7 months. 46 In a study that evaluated continuous regorafenib dosing (120 mg once daily), the mPFS was 8.7 months. 47
In the phase 2 study, the most common AEs of any grade were PPES, fatigue, hypertension, and diarrhea. 45 The most common grade 3 AEs were hypertension, PPES, and hypophosphatemia, and grade 4 AEs included hyperuricemia and thrombotic events. Six patients died during the study: 5 deaths were attributed to disease progression, and 1 was attributed to an unrelated illness. In the phase 3 study, all patients receiving regorafenib experienced an AE of any grade; 98.5% of these were determined to be related to the study drug. 25 Of these related AEs, 61% were grade 3 or higher and included hypertension, PPES, and diarrhea. Serious AEs were reported in 29% of patients receiving regorafenib and in 21% of patients receiving a placebo, and permanent discontinuations were similar between the 2 groups (regorafenib, 6.1%; placebo, 7.6%). There were 2 grade 5 AEs of cardiac arrest and hepatic failure related to regorafenib treatment. In a retrospective analysis, 46% of patients experienced grade 3 or 4 AEs of PPES, fatigue, hypertension, hepatotoxicity, diarrhea, and arthralgia. 46 Patients on the continuous schedule (120 mg once daily) demonstrated similar grade 3 or 4 AEs of PPES and fatigue, and 59% required dose reductions. 47
In the retrospective analysis, 20% discontinued regorafenib therapy because of AEs, but none of these patients had a prior dose reduction; this indicated a failure to effectively manage associated toxicities. 46 Research shows that supportive care and dose maintenance can extend the use of regorafenib in patients experiencing AEs. 48 Until recently, there were no approved treatment options for patients who progressed on regorafenib treatment after the failure of imatinib and sunitinib. In May 2020, the Food and Drug Administration approved the novel TKI ripretinib for use as a fourth‐line therapy in patients with advanced GISTs. 49
Novel Approved Targeted Therapies
Ripretinib, Fourth‐Line Therapy
Ripretinib is a switch‐control TKI that broadly inhibits a spectrum of KIT and PDGFRA mutations through a dual mechanism of action. 50 Different from conventional TKIs, ripretinib specifically binds to both the switch pocket and the activation loop to lock the kinase in an inactive state and prevent downstream signaling and cell proliferation. The dual mechanism of action provides broad inhibition of KIT and PDGFRA kinase activity, including wild type and multiple primary and secondary mutations. Targeting both the switch pocket (KIT exons 13 and 14) and the activation loop (KIT exons 17 and 18) allows ripretinib to be effective against a variety of treatment‐resistant GISTs, as conventional inhibitors generally do not perform well against both types of mutations. 50 , 51
Ripretinib demonstrated promising efficacy in a phase 1 study in patients receiving 150 mg of ripretinib once daily as second‐line therapy (mPFS, 10.7 months), third‐line therapy (mPFS, 8.3 months), or fourth‐line therapy (mPFS, 5.5 months). 52 Additionally, a dose escalation to 150 mg twice daily provided additional clinical benefit for second‐line (mPFS, 5.6 months) and third‐/fourth‐line patients (mPFS, 3.7 months). 53 In the pivotal INVICTUS phase 3 trial, ripretinib at 150 mg once daily was evaluated in patients for whom treatment with at least imatinib, sunitinib, and regorafenib had failed. The mPFS and mOS for fourth‐line or higher patients receiving ripretinib were 6.3 and 15.1 months, respectively, whereas they were 1.0 and 6.6 months, respectively, for patients receiving a placebo. 26 The overall response rate was 9.4% for the ripretinib group (all PRs) and 0% for the placebo group (Table 1). 26
In addition to promising efficacy, ripretinib has a well‐tolerated safety profile. In the phase 1 study, most AEs were minor, and common events included alopecia, myalgia, nausea, fatigue, PPES, and muscle spasms. The safety profile was similar during the period of 150 mg once daily and the period of dose escalation (150 mg twice daily); this demonstrated that ripretinib was similarly well tolerated. 53 The most common AEs (grades 1 and 2) reported by ripretinib patients in the phase 3 study were alopecia, myalgia, nausea, fatigue, and PPES. 26 The majority of AEs were categorized as mild (grades 1 and 2), and the most common grade 3 or 4 event was increased lipase, which was observed in 4 patients. Only 6% experienced AEs that led to dose reductions, and 5% discontinued ripretinib because of AEs. 26 Among patients receiving ripretinib in the phase 3 study, 4.7% developed new cutaneous squamous cell carcinoma, and 2.4% developed melanoma. 49 Routine dermatologic evaluations are recommended for patients taking ripretinib. When patient‐reported outcome measures were assessed, patients receiving ripretinib reported improved quality of life and general functioning in comparison with patients who received a placebo. 54 When stratified by common AEs (alopecia and PPES), patient‐reported outcome measures remained stable over time, and this indicated that these AEs were manageable and did not negatively affect quality of life. 55
Ripretinib's well‐tolerated safety profile and broad‐spectrum efficacy against several mutations make it an attractive option for patients struggling with treatment‐related toxicities or resistance. Although it is currently approved for use as a fourth‐line or higher therapy in advanced GISTs, 49 it is also under investigation for use as a second‐line therapy in a randomized phase 3 study of ripretinib versus sunitinib. The results of the INTRIGUE study may have a significant impact on the current treatment algorithm for patients with advanced GISTs (Fig. 1). 56
Avapritinib, First‐Line Therapy for Patients With PDGFRA Exon 18 D842V Mutations
In January 2020, the Food and Drug Administration approved avapritinib for use as a first‐line therapy in patients with advanced GISTs harboring a PDGFRA exon 18 D842V mutation. 57 The approval of avapritinib further underscores the importance of mutational profiling in advanced GISTs because the PDGFRA exon 18 D842V mutation is highly resistant to other TKIs. 58 Mutations in the activation loop (eg, the PDGFRA exon 18 D842V mutation) result in a higher ratio of active kinases, and although other TKIs target the inactive forms of KIT and PDGFRA, avapritinib potently and selectively targets the active conformation. 59
In the phase 1 NAVIGATOR trial, the optimal dose was determined to be 300 mg once daily after patients experienced DLTs at higher doses. Among the patients receiving 300 mg of avapritinib once daily who had PDGFRA exon 18 D842V mutations, the overall response rate was 93% (complete response, 4%; PR, 89%; SD, 7%). 27 The data were not sufficiently mature to estimate mPFS and mOS (Table 1). Avapritinib was not effective in treating patients with a broad range of mutations in the phase 3 VOYAGER trial (mPFS, 4.2 months vs 5.6 months with regorafenib). 60
In addition to patients with PDGFRA exon 18 D842V mutations (n = 56), the safety population included patients with PDGFRA exon 18 non‐D842V mutations (n = 2), PDGFRA exon 14 mutations (n = 1), and KIT mutations (n = 23). 27 Most treatment‐related AEs were mild (grades 1 and 2), and the most common events were nausea, diarrhea, decreased appetite, and fatigue. At the once daily dose of 300 mg, the most common grade 3 AE was anemia. The appearance of cognitive difficulties was of special interest in these patients. In the safety population, 40% developed a cognitive difficulty (memory impairment, cognitive disorder, confused state, or encephalopathy). Additionally, 2 patients had intracranial bleeding considered possibly related to the study drug. In the safety population, 54% of patients discontinued treatment, but the proportion of patients who discontinued was lower in the PDGFRA exon 18 D842V mutation group (34%).
Investigational Therapies
Several alternative strategies were investigated in patients with advanced GISTs because, until the recent approval of ripretinib, there were no further treatment options for patients for whom the first 3 lines of treatment failed. These strategies include kinase inhibitors approved for other forms of cancer, immunotherapies, combination therapies, and alternating or cycling therapies; examples of these therapeutic strategies are provided in Table 2.
TABLE 2.
Selected Investigational Therapeutic Strategies for Advanced GISTs
| Therapeutic Agent | Indication | Efficacy | Safety |
|---|---|---|---|
| Targeted therapies | |||
| Cabozantinib 61 | KI used in thyroid cancer, renal cell carcinoma, and hepatocellular carcinoma; tested in third‐line GIST | PFS at 12 wk, 60%; mPFS, 6.0 mo; 80% of patients achieved a PR or SD | The most common treatment‐related AEs ≥ grade 3 were diarrhea, PPES, fatigue, and hypertension. |
| Sorafenib 62 | KI used in kidney, liver, and thyroid cancer; tested in ≥third‐line GIST | PR or SD achieved in 40% of patients; mPFS, 7.2 mo | Treatment‐related AEs were reported in 72% of patients; none of the patients discontinued. |
| Nilotinib 63 , 64 | KI used to treat Philadelphia chromosome CML; tested in ≥first‐line GIST | First line: PFS at 24 mo with nilotinib, 51.6%; with imatinib, 59.2% | First line: The study's rates of discontinuation due to AEs were 8.0% with nilotinib and 5.3% with imatinib; frequently reported AEs in the nilotinib arm were rash, nausea, and abdominal pain. |
| Second/third line: 4 of 12 patients achieved SD | Second/third line: The most common AEs were fatigue, anemia, and anorexia; 1 patient experienced grade 4 anemia. | ||
| Dasatinib 65 | KI used to treat CML and ALL; tested in second‐line GIST | mPFS, 2.9 mo; PR reported in 25% of patients | Serious AEs occurred in 24% of patients and included pleural effusion, nausea/vomiting, and muscle weakness. |
| Pazopanib 66 | KI used in advanced renal cell cancer and metastatic soft tissue sarcoma; tested in third‐line GIST | mPFS with pazopanib, 3.4 mo; mPFS with supportive care only, 2.3 mo | Grade 3 or higher AEs were reported in 72% of pazopanib‐treated patients; the most common was hypertension. |
| Ponatinib 67 | KI used to treat CML and ALL; tested in ≥third‐line GIST | CBR at ≥16 wk for patients with a primary KIT exon 11 mutation, 55%; without a primary KIT exon 11 mutation, 22% | Seventeen of 35 patients discontinued treatment; the most common AEs were rash, fatigue, myalgia, and dry skin. |
| Immunotherapy | |||
| Pembrolizumab 68 | Anti–PD‐1 antibody used to treat multiple forms of cancer | 6‐mo nonprogression rate, 11.1% | AEs were mild and included fatigue, diarrhea, and anemia. |
| Nivolumab + ipilimumab 69 | Anti–PD‐1 antibody used to treat multiple forms of cancer; tested in ≥second‐line GIST | mPFS with nivolumab only, 8 wk; with nivolumab + ipilimumab, 8.43 wk | Grade 3 fatigue (1 patient in the nivolumab‐only arm) and diarrhea (1 patient in the nivolumab + ipilimumab arm). |
| Combination therapy | |||
| Imatinib + peginterferon α‐2b 70 | Interferon given to promote antitumor activity; used to treat hepatitis C and melanoma; tested in ≥third‐line GIST | All 7 patients had a CR or PR; OS at 3.6‐y follow‐up, 100% | All patients experienced temporary low‐grade fever and flu‐like symptoms; grade 3 neutropenia (3 patients) and skin rash (2 patients). |
| Imatinib + buparlisib 71 | Phosphoinositide 3‐kinase inhibitor used experimentally to treat breast cancer; tested in third‐line GIST | No PR or CR; mPFS, 3.5 mo | Treatment‐related AEs were reported in 98.3% of patients; 45% of these patients had grade 3‐4 AEs; the most common were nausea and fatigue. |
| Imatinib + binimetinib 72 | MAPK inhibitor used to treat metastatic melanoma; tested in first‐line GIST | Of 38 patients, 26 had a PR; best objective response rate, 68.4% | Grade 3‐4 toxicities included CPK elevations, neutrophil decreases, rash, and anemia. |
| Cycling therapies | |||
| Imatinib/regorafenib (ALT‐GIST) 73 | Imatinib for 21‐25 d, 3‐ to 7‐d washout, regorafenib for 21 d, 7‐d washout; tested in first‐line GIST | Responses to imatinib only and the alternating therapy were similar; 1 patient on alternating therapy had a CR, 23 had a PR, and 15 had SD; PFS at 1 y, 86% | Seven patients on alternating therapy discontinued because of toxicity; 38% of patients on alternating therapy had serious AEs. |
| Sunitinib/regorafenib 74 | Sunitinib for 3 d, regorafenib for 4 d, no washout; tested in fourth‐line GIST | SD was achieved in 4 patients; mPFS, 1.9 mo; mOS, 10.8 mo | All patients experienced treatment‐related AEs, but the majority were mild (grades 1 and 2); 4 patients experienced grade 3 AEs of hypertension or PPES. |
| Imatinib rechallenge (RIGHT) 75 | Imatinib rechallenge for patients who progressed on imatinib and sunitinib; tested in third‐line GIST | mPFS with imatinib, 1.8 mo; with placebo, 0.9 mo | Grade 3 or higher toxicities included anemia, fatigue, and hyperbilirubinemia. |
Abbreviations: AE, adverse event; ALL, acute lymphocytic leukemia; ALT‐GIST, alternating‐regimen gastrointestinal stromal tumor; CBR, clinical benefit rate; CML, chronic myeloid leukemia; CPK, creatinine phosphokinase; CR, complete response; GIST, gastrointestinal stromal tumor; KI, kinase inhibitor; MAPK, mitogen‐activated protein kinase; mOS, median overall survival; mPFS, median progression‐free survival; OS, overall survival; PD‐1, programmed cell death protein 1; PFS, progression‐free survival; PPES, palmar‐plantar erythrodysesthesia syndrome; PR, partial response; SD, stable disease.
Other kinase inhibitors used in different types of cancers and tested in patients with advanced GISTs include cabozantinib, sorafenib, nilotinib, dasatinib, pazopanib, and ponatinib (Table 2). With the approval of ripretinib, it is unclear whether the use of these types of inhibitors will continue. However, promising preliminary results with cabozantinib may provide patients with an additional treatment option (Table 2). 61 The combination of imatinib and interferon showed promising efficacy in a small number of patients, whereas the combination of imatinib and phosphoinositide 3‐kinase inhibitor buparlisib had no effect as a third‐line therapy (Table 2). 70 , 71
Cycling therapies such as rapid alternation between imatinib and regorafenib or between sunitinib and regorafenib have demonstrated only modest benefits, but they may be options for individualized patient care. 73 , 74 Imatinib rechallenge as a third‐line therapy extended patients' progression‐free survival but was not as effective as regorafenib as a third‐line option (Table 2). 25 , 75 A comprehensive list of recent or ongoing clinical trials evaluating various therapeutic agents and/or strategies for the treatment of advanced GISTs can be found at ClinicalTrials.gov.
Conclusions
The traditional treatment algorithm for advanced GISTs has been imatinib as first‐line therapy, sunitinib as second‐line therapy, and regorafenib as third‐line therapy. Now, however, the GIST treatment landscape is evolving, and patients with PDGFRA exon 18 D842V mutations have the option of receiving avapritinib as first‐line therapy. Additionally, patients who progress on imatinib, sunitinib, and regorafenib now have the option of receiving ripretinib as fourth‐line therapy (Fig. 1). With ripretinib's broad efficacy and favorable safety profile, this therapy has the potential to alter the current treatment algorithm, as it is being investigated as a second‐line therapy versus sunitinib in a randomized study (Fig. 1). Although these are the currently approved therapies, there are several alternative strategies being investigated that could further advance individualized care of patients with advanced GISTs. With all therapies, however, the effective management of AEs and supportive care are crucial to ensuring the longevity of treatment and the maximum clinical benefit. With evolving treatment options and effective toxicity management, patients with advanced GISTs are living longer than ever before.
Funding Support
The University of Texas MD Anderson Cancer Center is supported by the National Institutes of Health (grant P30 CA016672).
Conflict of Interest Disclosures
Shreyaskumar Patel reports personal fees from Deciphera and Bayer and grant funding to his institution from Blueprint Medicines and Deciphera outside the submitted work. Peter Reichardt reports personal fees from Bayer, Clinigen, BMS, Roche, MSD, Deciphera, Novartis, PharmaMar, Pfizer, Lilly, and Amgen outside the submitted work and is chairman of the German Sarcoma Foundation.
Patel S, Reichardt P. An updated review of the treatment landscape for advanced gastrointestinal stromal tumors. Cancer. 2021. 10.1002/cncr.33630
Medical writing and editorial support was provided by Lauren Hanlon, PhD (AlphaBioCom, LLC, King of Prussia, Pennsylvania); this support was funded by Deciphera Pharmaceuticals, LLC (Waltham, Massachusetts).
References
- 1. Patel N, Benipal B. Incidence of gastrointestinal stromal tumors in the United States from 2001‐2015: a United States cancer statistics analysis of 50 states. Cureus. 2019;11:e4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731‐1741. [DOI] [PubMed] [Google Scholar]
- 3. Soreide K, Sandvik OM, Soreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population‐based cohort studies. Cancer Epidemiol. 2016;40:39‐46. [DOI] [PubMed] [Google Scholar]
- 4. Ma GL, Murphy JD, Martinez ME, Sicklick JK. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population‐based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Szucs Z, Thway K, Fisher C, et al. Molecular subtypes of gastrointestinal stromal tumors and their prognostic and therapeutic implications. Future Oncol. 2017;13:93‐107. [DOI] [PubMed] [Google Scholar]
- 6. Corless CL, Schroeder A, Griffith D, et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol. 2005;23:5357‐5364. [DOI] [PubMed] [Google Scholar]
- 7. Martin‐Broto J, Martinez‐Marin V, Serrano C, et al. Gastrointestinal stromal tumors (GISTs): SEAP‐SEOM consensus on pathologic and molecular diagnosis. Clin Transl Oncol. 2017;19:536‐545. [DOI] [PubMed] [Google Scholar]
- 8. Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813‐3825. [DOI] [PubMed] [Google Scholar]
- 9. Li B, Bai J, Zhu L, et al. Application of next generation sequencing (NGS) in gastrointestinal stromal tumor (GIST). J Clin Oncol. 2020;38(suppl):e13675. [Google Scholar]
- 10. Serrano C, Vivancos A, Lopez‐Pousa A, et al. Clinical value of next generation sequencing of plasma cell‐free DNA in gastrointestinal stromal tumors. BMC Cancer. 2020;20:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rutkowski P, Nowecki ZI, Michej W, et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol. 2007;14:2018‐2027. [DOI] [PubMed] [Google Scholar]
- 12. DeMatteo RP, Heinrich MC, El‐Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI‐571. Hum Pathol. 2002;33:466‐477. [DOI] [PubMed] [Google Scholar]
- 13. Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002;3:655‐664. [DOI] [PubMed] [Google Scholar]
- 14. DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen MH, Williams G, Johnson JR, et al. Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous leukemia. Clin Cancer Res. 2002;8:935‐942. [PubMed] [Google Scholar]
- 16. Dagher R, Cohen M, Williams G, et al. Approval summary: imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res. 2002;8:3034‐3038. [PubMed] [Google Scholar]
- 17. Quek R, George S. Update on the treatment of gastrointestinal stromal tumors (GISTs): role of imatinib. Biologics. 2010;4:19‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tuveson DA, Willis NA, Jacks T, et al. STI571 inactivation of the gastrointestinal stromal tumor c‐KIT oncoprotein: biological and clinical implications. Oncogene. 2001;20:5054‐5058. [DOI] [PubMed] [Google Scholar]
- 19. Joensuu H, Roberts PJ, Sarlomo‐Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052‐1056. [DOI] [PubMed] [Google Scholar]
- 20. van Oosterom AT, Judson I, Verweij J, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet. 2001;358:1421‐1423. [DOI] [PubMed] [Google Scholar]
- 21. Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472‐480. [DOI] [PubMed] [Google Scholar]
- 22. Blanke CD, Demetri GD, von Mehren M, et al. Long‐term results from a randomized phase II trial of standard‐ versus higher‐dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620‐625. [DOI] [PubMed] [Google Scholar]
- 23. Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626‐632. [DOI] [PubMed] [Google Scholar]
- 24. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329‐1338. [DOI] [PubMed] [Google Scholar]
- 25. Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet. 2013;381:295‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blay JY, Serrano C, Heinrich MC, et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double‐blind, randomised, placebo‐controlled, phase 3 trial. Lancet Oncol. 2020;21:923‐934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heinrich MC, Jones RL, von Mehren M, et al. Avapritinib in advanced PDGFRA D842V‐mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open‐label, phase 1 trial. Lancet Oncol. 2020;21:935‐946. [DOI] [PubMed] [Google Scholar]
- 28. Casali PG, Zalcberg J, Le Cesne A, et al. Ten‐year progression‐free and overall survival in patients with unresectable or metastatic GI stromal tumors: long‐term analysis of the European Organisation for Research and Treatment of Cancer, Italian Sarcoma Group, and Australasian Gastrointestinal Trials Group intergroup phase III randomized trial on imatinib at two dose levels. J Clin Oncol. 2017;35:1713‐1720. [DOI] [PubMed] [Google Scholar]
- 29. Gastrointestinal Stromal Tumor Meta‐Analysis Group . Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta‐analysis of 1,640 patients. J Clin Oncol. 2010;28:1247‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verweij J, Casali PG, Zalcberg J, et al. Progression‐free survival in gastrointestinal stromal tumours with high‐dose imatinib: randomised trial. Lancet. 2004;364:1127‐1134. [DOI] [PubMed] [Google Scholar]
- 31. Gastrointestinal Stromal Tumors (GISTs) . Version 1.2021. National Comprehensive Cancer Network. Published October 30, 2020. Accessed November 23, 2020. https://www.nccn.org/professionals/physician_gls/pdf/gist.pdf [Google Scholar]
- 32. Casali PG, Abecassis N, Aro HT, et al. Gastrointestinal stromal tumours: ESMO‐EURACAN clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2018;29:iv68‐iv78. [DOI] [PubMed] [Google Scholar]
- 33. Zalcberg JR, Verweij J, Casali PG, et al. Outcome of patients with advanced gastro‐intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer. 2005;41:1751‐1757. [DOI] [PubMed] [Google Scholar]
- 34. Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005;11:4182‐4190. [DOI] [PubMed] [Google Scholar]
- 35. Heinrich MC, Corless CL, Blanke CD, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24:4764‐4774. [DOI] [PubMed] [Google Scholar]
- 36. Van Glabbeke M, Verweij J, Casali PG, et al. Initial and late resistance to imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: a European Organisation for Research and Treatment of Cancer–Italian Sarcoma Group–Australasian Gastrointestinal Trials Group study. J Clin Oncol. 2005;23:5795‐5804. [DOI] [PubMed] [Google Scholar]
- 37. Rock EP, Goodman V, Jiang JX, et al. Food and Drug Administration drug approval summary: sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist. 2007;12:107‐113. [DOI] [PubMed] [Google Scholar]
- 38. Gajiwala KS, Wu JC, Christensen J, et al. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci U S A. 2009;106:1542‐1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Demetri GD, Heinrich MC, Fletcher JA, et al. Molecular target modulation, imaging, and clinical evaluation of gastrointestinal stromal tumor patients treated with sunitinib malate after imatinib failure. Clin Cancer Res. 2009;15:5902‐5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45:1959‐1968. [DOI] [PubMed] [Google Scholar]
- 41. Reichardt P, Kang YK, Rutkowski P, et al. Clinical outcomes of patients with advanced gastrointestinal stromal tumors: safety and efficacy in a worldwide treatment‐use trial of sunitinib. Cancer. 2015;121:1405‐1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nishida T, Takahashi T, Nishitani A, et al. Sunitinib‐resistant gastrointestinal stromal tumors harbor cis‐mutations in the activation loop of the KIT gene. Int J Clin Oncol. 2009;14:143‐149. [DOI] [PubMed] [Google Scholar]
- 43. Ferraro D, Zalcberg J. Regorafenib in gastrointestinal stromal tumors: clinical evidence and place in therapy. Ther Adv Med Oncol. 2014;6:222‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mross K, Frost A, Steinbild S, et al. A phase I dose‐escalation study of regorafenib (BAY 73‐4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:2658‐2667. [DOI] [PubMed] [Google Scholar]
- 45. George S, Wang Q, Heinrich MC, et al. Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: a multicenter phase II trial. J Clin Oncol. 2012;30:2401‐2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chamberlain F, Farag S, Williams‐Sharkey C, et al. Toxicity management of regorafenib in patients with gastro‐intestinal stromal tumour (GIST) in a tertiary cancer centre. Clin Sarcoma Res. 2020;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schvartsman G, Wagner MJ, Amini B, et al. Treatment patterns, efficacy and toxicity of regorafenib in gastrointestinal stromal tumour patients. Sci Rep. 2017;7:9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grothey A, George S, van Cutsem E, Blay JY, Sobrero A, Demetri GD. Optimizing treatment outcomes with regorafenib: personalized dosing and other strategies to support patient care. Oncologist. 2014;19:669‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Prescribing information for Qinlock. Deciphera Pharmaceuticals, LLC. Revised May 2020. Accessed October 25, 2020. https://qinlockhcp.com/Content/files/qinlock‐prescribing‐information.pdf [Google Scholar]
- 50. Smith BD, Kaufman MD, Lu WP, et al. Ripretinib (DCC‐2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug‐resistant KIT and PDGFRA variants. Cancer Cell. 2019;35:738‐751.e9. [DOI] [PubMed] [Google Scholar]
- 51. Serrano C, Marino‐Enriquez A, Tao DL, et al. Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib‐resistant gastrointestinal stromal tumours. Br J Cancer. 2019;120:612‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Janku F, Abdul Razak AR, Chi P, et al. Switch control inhibition of KIT and PDGFRA in patients with advanced gastrointestinal stromal tumor: a phase I study of ripretinib. J Clin Oncol. 2020;38:3294‐3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Janku F, Chi P, Heinrich MC, et al. Ripretinib intra‐patient dose escalation (IPDE) following disease progression provides clinically meaningful progression‐free survival (PFS) in gastrointestinal stromal tumor (GIST) in phase I study. Ann Oncol. 2020;31(suppl 4):1623MO.32979513 [Google Scholar]
- 54. Heinrich MC, George S, Zalcberg J, et al. Quality of life (QoL) and self‐reported function with ripretinib in ≥4th‐line therapy for patients with gastrointestinal stromal tumors (GIST): analyses from INVICTUS. J Clin Oncol. 2020;38(suppl):11535. [Google Scholar]
- 55. George S, Heinrich MC, Zalcberg J, et al. Safety profile of ripretinib, including impact of alopecia, and palmar‐plantar erythrodysesthesia syndrome (PPES) on patient‐reported outcomes (PROs), in ≥ fourth‐line advanced gastrointestinal stromal tumors (GIST): analyses from INVICTUS. J Clin Oncol. 2020;38(suppl):11539. [Google Scholar]
- 56. Nemunaitis J, Bauer S, Blay JY, et al. Intrigue: phase III study of ripretinib versus sunitinib in advanced gastrointestinal stromal tumor after imatinib. Future Oncol. 2020;16:4251‐4264. [DOI] [PubMed] [Google Scholar]
- 57. Prescribing information for Ayvakit. Blueprint Medicines Corporation. Published 2020. Accessed October 25, 2020. https://www.blueprintmedicines.com/uspi/AYVAKIT.pdf [Google Scholar]
- 58. Cassier PA, Fumagalli E, Rutkowski P, et al. Outcome of patients with platelet‐derived growth factor receptor alpha–mutated gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Clin Cancer Res. 2012;18:4458‐4464. [DOI] [PubMed] [Google Scholar]
- 59. Evans EK, Gardino AK, Kim JL, et al. A precision therapy against cancers driven by KIT/PDGFRA mutations. Sci Transl Med. 2017;9:eaao1690. [DOI] [PubMed] [Google Scholar]
- 60. Blueprint Medicines announces top‐line results from phase 3 VOYAGER trial of avapritinib versus regorafenib in patients with advanced gastrointestinal stromal tumor. Blueprint Medicines Corporation. Published April 28, 2020. Accessed January 26, 2021. http://ir.blueprintmedicines.com/news‐releases/news‐release‐details/blueprint‐medicines‐announces‐top‐line‐results‐phase‐3‐voyager [Google Scholar]
- 61. Schoffski P, Mir O, Kasper B, et al. Activity and safety of cabozantinib in patients with gastrointestinal stromal tumor after failure of imatinib and sunitinib: EORTC phase II trial 1317 CaboGIST. J Clin Oncol. 2019;37(suppl):11006. [Google Scholar]
- 62. Kefeli U, Benekli M, Sevinc A, et al. Efficacy of sorafenib in patients with gastrointestinal stromal tumors in the third‐ or fourth‐line treatment: a retrospective multicenter experience. Oncol Lett. 2013;6:605‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Blay JY, Shen L, Kang YK, et al. Nilotinib versus imatinib as first‐line therapy for patients with unresectable or metastatic gastrointestinal stromal tumours (ENESTg1): a randomised phase 3 trial. Lancet Oncol. 2015;16:550‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cauchi C, Somaiah N, Engstrom PF, et al. Evaluation of nilotinib in advanced GIST previously treated with imatinib and sunitinib. Cancer Chemother Pharmacol. 2012;69:977‐982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schuetze SM, Bolejack V, Thomas DG, et al. Association of dasatinib with progression‐free survival among patients with advanced gastrointestinal stromal tumors resistant to imatinib. JAMA Oncol. 2018;4:814‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mir O, Cropet C, Toulmonde M, et al. Pazopanib plus best supportive care versus best supportive care alone in advanced gastrointestinal stromal tumours resistant to imatinib and sunitinib (PAZOGIST): a randomised, multicentre, open‐label phase 2 trial. Lancet Oncol. 2016;17:632‐641. [DOI] [PubMed] [Google Scholar]
- 67. Heinrich MC, von Mehren M, Demetri G, et al. A phase 2 study of ponatinib in patients (pts) with advanced gastrointestinal stromal tumors (GIST) after failure of tyrosine kinase inhibitor (TKI) therapy: initial report. J Clin Oncol. 2014;32(suppl):10506. [Google Scholar]
- 68. Toulmonde M, Penel N, Adam J, et al. Use of PD‐1 targeting, macrophage infiltration, and IDO pathway activation in sarcomas: a phase 2 clinical trial. JAMA Oncol. 2018;4:93‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Singh AS, Chmielowski B, Hecht JR, et al. A randomized phase 2 study of nivolumab monotherapy versus nivolumab combined with ipilimumab in patients with metastatic or unresectable gastrointestinal stromal tumor (GIST). J Clin Oncol. 2018;36(suppl):55. [Google Scholar]
- 70. Chen LL, Chen X, Choi H, et al. Exploiting antitumor immunity to overcome relapse and improve remission duration. Cancer Immunol Immunother. 2012;61:1113‐1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gelderblom H, Jones RL, George S, et al. Imatinib in combination with phosphoinositol kinase inhibitor buparlisib in patients with gastrointestinal stromal tumour who failed prior therapy with imatinib and sunitinib: a phase 1b, multicentre study. Br J Cancer. 2020;122:1158‐1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chi P, Qin L‐X, Kelly CM, et al. A phase II study of MEK162 (binimetinib [BINI]) in combination with imatinib in patients with untreated advanced gastrointestinal stromal tumor (GIST). J Clin Oncol. 2020;38(suppl):11508. [Google Scholar]
- 73. Yip D, Zalcberg J, Blay JY, et al. ALT‐GIST: randomized phase II trial of imatinib alternating with regorafenib versus imatinib alone for the first‐line treatment of metastatic gastrointestinal stromal tumor (GIST). J Clin Oncol. 2019;37(suppl):11023. [Google Scholar]
- 74. Serrano C, Leal A, Kuang Y, et al. Phase I study of rapid alternation of sunitinib and regorafenib for the treatment of tyrosine kinase inhibitor refractory gastrointestinal stromal tumors. Clin Cancer Res. 2019;25:7287‐7293. [DOI] [PubMed] [Google Scholar]
- 75. Kang YK, Ryu MH, Yoo C, et al. Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHT): a randomised, placebo‐controlled, phase 3 trial. Lancet Oncol. 2013;14:1175‐1182. [DOI] [PMC free article] [PubMed] [Google Scholar]


