Abstract
Increasing evidence on the impact of the different wavelengths of sunlight on the skin demonstrates the need for tailored recommendations of sunscreen according to skin phototype and dermatoses, which is now possible due to advances in the filters and formulations of sunscreens. A selective literature search was performed by an international expert panel, focusing on the type of sunscreen to recommend for photoaging, skin cancers, photodermatoses, pigmentary disorders and skin inflammatory disorders. Protection against ultraviolet (UV)B is especially important for light skin as there is a high risk of sunburn, DNA damage and skin cancers. Darker skin may be naturally better protected against UVB but is more prone to hyperpigmentation induced by visible light (VL) and UVA. Protection against UVA, VL and infrared A can be helpful for all skin phototypes as they penetrate deeply and cause photoaging. Long‐wave UVA1 plays a critical role in pigmentation, photoaging, skin cancer, DNA damage and photodermatoses. Adapting the formulation and texture of the sunscreen to the type of skin and dermatoses is also essential. Practical recommendations on the type of sunscreen to prescribe are provided to support the clinician in daily practice.
Introduction
General measures of photoprotection include seeking shade when outdoors, application of sunscreen, and wearing protective clothing, hats and sunglasses. Although sunscreen is only one measure, it remains essential in many circumstances. Studies have shown that users should be encouraged to apply appropriate amounts of sunscreen to obtain the recommended 2 mg/cm2 concentration (sun protection factor [SPF] test conditions), which can be achieved following the teaspoon rule 1 or reapplication within an hour. 2 As an example, one teaspoon of sunscreen is the recommended amount for covering the face. The SPF of a sunscreen is a universal quantitative index of protection against sunburn from ultraviolet UVB. However, long‐wave UVA (UVA1; 340–400 nm) is known to play an important role in pigmentation, photoaging, skin cancer, DNA damage and photodermatoses. 3 , 4 Furthermore, there is increasing evidence on the role of visible light (VL) in pigmentary disorders in dark‐skinned subjects and immediate erythema in light‐skinned individuals. 5 , 6 , 7 Adapting the formulation and texture of the sunscreen to account for skin phototype (SPT) and any associated dermatoses is also essential. However, when it comes to choosing a sunscreen, most people rely only on the SPF. 8
An international panel of 12 experts convened in July 2020 to develop practical recommendations to support clinicians/ dermatologists on the type of sunscreen to prescribe depending on skin phototype and dermatoses. The panel reviewed and discussed the current available literature. When appropriate literature was scarce, personal experiences were discussed and all the recommendations were based on the consensus of the group. Oral and injectable photoprotective agents are outside the scope of these recommendations.
Skin phototypes
Skin phototype can be classified using the Fitzpatrick phototype classification or by using the evaluation of individual typology angle (ITA), which is more precise but requires colorimetry measurements (see Fig. 1). 9 , 10 , 11 Different SPT responds differently to the sun, but most individuals can benefit from using daily photoprotection. 12 , 13 A recent review of the literature showed that the regurlar use of sunscreen will not compromise the vitamin D status in healthy individuals. 14 However, in mid to high latitudes, regular use of high‐SPF sunscreen might theoretically compromise vitamin D levels and thus might require a vitamin D supplementation. 15 , 16 Secondly, although percutaneous absorption of organic UV filters has been reported, no well‐documented systemic side‐effects have been reported to be caused by the use of sunscreens; in fact, the authors of those studies clearly specified that the results do not indicate that individuals should refrain from the use of sunscreen. 17 , 18 Compared to light skin, dark skin has a higher quantity of melanin distributed in the upper layers of the epidermis and a higher eumelanin/ pheomelanin ratio. After UVB exposure, DNA damage is mainly observed in the upper layers of the epidermis in dark skin, while in light skin it also affects the basal layers where the stem cells are located. Moreover, DNA repair is more efficient in dark skin than in light skin. 19 Thus, protection against UVB is more important for individuals with light skin as there is a higher risk of sunburn, DNA damage and the development of skin cancers. Protection against UVA/UVA1 is essential as it penetrates deeper into the skin compared to UVB and causes photoaging. 14 , 20 Importantly, UVA1 affects all skin types and optimal protection against UVA1 is thus essential for all individuals. 21 Although darker skin is naturally better protected against UVB, it is more prone to hyperpigmentation induced by VL and UVA. 5 , 6 , 7 , 22 Providing optimal protection against UVA1 and VL is thus beneficial in dark‐skinned individuals. VL (specifically high‐energy violet light [HEV]) protection with tinted sunscreens containing iron oxides and/or pigmentary titanium dioxide is important for dark‐skinned individuals, and these products should preferably be colour matched to the constitutive skin colour of the user to maximize compliance. 23 There is a need for clear and practical recommendations to highlight the importance of regular sunscreen use even in darker skin types to prevent pigmentation and photoaging. The spectral absorption profile of the sunscreen should be chosen depending on the SPT (Fig. 1). 24
Figure 1.
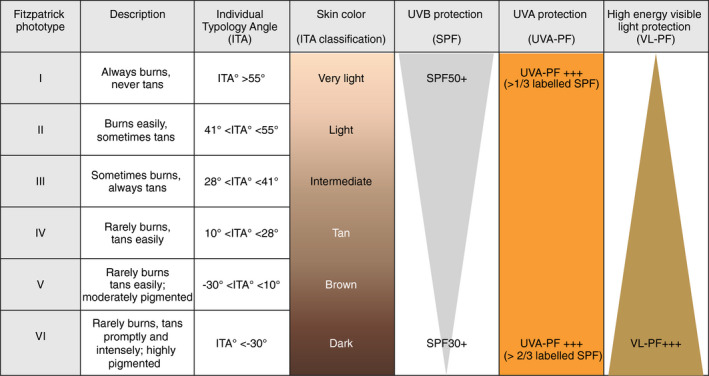
Spectral absorption profiles of sunscreens suitable for different skin phototypes. This figure represents the absorption profile of sunscreen recommended for healthy individuals with different skin phototypes for the preventation of skin cancers and photoaging. The latitude of where the individual lives should also be taken in consideration. Individuals with skin conditions (such as photodermatoses or pigmentary disorders) should follow the specific recommendations described in Table 1. ITA individual typology angle, SPF sun protection factor, UVA‐PF ultraviolet A, VL visible light, PF protection factor.
Photoaging
Sunscreens protect against wrinkles and uneven pigmentation, including actinic lentigines, in Caucasian, East Asian and South Asian skin. In a randomized controlled trial in 46 adults of mean age 63 years old with a previous diagnosis of skin cancer and/or actinic keratoses, the percentage of solar elastosis were 30.1% after 24 months application of SPF29 UVB/UVA (short wavelength UVA2) sunscreen vs 39.4% in the placebo group (P = 0.0001). 25 In 903 adults under 55 years old, daily use of broad‐spectrum SPF15+ sunscreen for 4.5 years slowed down the process of photoaging. 26 Compared to discretionary sunscreen users, daily SPF15+ sunscreen users had 24% less skin wrinkling and coarse skin (relative odds 0.76; 95%CI 0.50–0.98). 26 Daily use of a broad‐spectrum sunscreen (SPF30) in 32 subjects for 52 weeks significantly improved clinical signs of photoaging, e.g. crow’s feet, fine lines, mottled pigmentation, discrete pigmentation and evenness of skin tone. 27
In Caucasian skin, wrinkles generally appear 10 to 20 years earlier than in Asian skin, while Asian and dark‐skinned individuals are more prone to actinic lentigines and hyperpigmentation. 28 , 29 In 14 elderly Japanese people, changes in the number of spots and skin tone uniformity were negatively correlated with the amount of sunscreen used over 18 months. 30 In 216 Indian subjects, significant improvement in the density of pigmented spots and skin radiance was observed after 12 weeks of sunscreen use (SPF50, UVA‐PA+++) compared to baseline (P < 0.001). 31 These studies have been performed in people with different skin phototypes and living at different latitudes as a daily photoprotection may be more beneficial for those living in locations with higher UVR irradiances.
Protection against infrared A (IRA) is also required to prevent photoaging. In a vehicle‐controlled, randomized study in 30 healthy volunteers, the application of SPF30 sunscreen supplemented with an antioxidant cocktail protected human skin against changes induced by IRA radiation. 32 Until new sunscreens are developed to protect against IRA, broad‐spectrum sunscreens containing topical antioxidants could provide the best protection.
The role of VL in skin aging is less clear, and there has been no demonstration of skin wrinkling induced by VL. However, the use of a broad‐spectrum UVB/UVA‐VL sunscreen (containing pigments) for 60 days significantly decreased the hyperpigmented area of actinic lentigo compared to the use of sunscreens containing only UV filters. 33
Skin cancers
While there is good evidence that both UVB and UVA promote melanoma development in fair‐skinned individuals, a recent systematic review suggests that UV exposure may not be an important risk factor for melanoma development in people with skin of colour. 34 Sunscreen use cannot completely prevent melanoma, but the majority of melanoma (around 70%) have a high mutational burden and UV signature. 35 A randomized trial in Australia over 4.5 years with an 8‐year follow‐up period showed a reduction of the rate of melanoma in those randomly assigned to daily sunscreen use (hazard ratio [HR], 0.50; 95% CI, 0.24 to 1.02; P < 0.051). The reduction in invasive melanomas was substantial (HR, 0.27; 95% CI, 0.08 to 0.97) compared with that for preinvasive melanomas (HR, 0.73; 95% CI, 0.29 to 1.81). 36
A strong positive correlation has been observed between incidence of melanoma and keratinocyte cancers and a history of sunburn in childhood, presence of atypical naevi, light skin, freckles, red hair, photoaging, sunbed use and family history of skin cancer. 37 , 38 Both physical barriers and sunscreens can partially prevent UVB effects on naevi. 39
Both UVB and UVA cause DNA damage and immune suppression, which play crucial roles in skin carcinogenesis, and both UV types are also involved in skin carcinomas. 40 Basal cell carcinomas (BCC) 41 , 42 and squamous cell carcinomas (SCC) 43 , 44 have higher mutational burden than melanoma and also exhibit a strong UV signature. 41 , 42 , 43 , 44 A Cochrane report concluded that low‐quality evidence was unable to demonstrate whether sunscreen was effective in preventing keratinocyte cancer (BCC and SCC). 45 A randomized controlled trial in 1621 subjects with 1383 followed up for 4.5 years, 55% of whom had light skin, provided low‐quality limited evidence that daily use of SPF15+ sunscreen resulted in a small reduction of SCC and no difference in BCC incidence compared to the discretionary sunscreen group. 46 After an 8‐year follow‐up, regular sunscreen use possibly reduced the number of cases of SCC (RR 0.65, 95% CI 0.45 to 0.94) but not BCC; 45 an explanation could be that BCC may take many years to develop compared to SCC. 47 , 48
Limited, low‐quality evidence has been obtained from short‐term randomized trials suggesting that regular use of sunscreens protects against the development of solar keratoses/ actinic keratosis (AK). 49 , 50 A 24‐month prospective, case–control study provided limited evidence that regular use of sunscreen (SPF > 50, high UVA‐PF) may help prevent the development of further AK and invasive SCC in immune‐compromised organ transplant recipients. 51 , 52
Equivocal results obtained on the effect of sunscreen on AK and keratinocyte carcinomas may be due to the use of older less well‐balanced sunscreens, poor adherence and improper application of sunscreen, or poor study design with insufficient numbers of fair‐skinned and older people to be able to detect differences. Education is needed on the proper amount and frequency of application and the importance of UVA protection in addition to a high‐SPF sunscreen.
Photodermatoses
The three most common categories of photodermatoses are immunologically mediated (polymorphous light eruption [PMLE], chronic actinic dermatitis, solar urticaria/solar angioedema), drug‐ and chemical‐induced photodermatoses and photoaggravated dermatoses (e.g. lupus erythematosus and dermatomyositis). The fourth category, DNA repair‐deficient photodermatoses are beyond the scope of this publication. 53
In a study of 1080 photosensitive patients from four US academic dermatology clinics over a 10‐year period, PMLE was the most common photodermatosis. 54 Furthermore, it was observed that PMLE was more common in the Black racial group, while phototoxic drug eruption, cutaneous porphyrias and solar urticaria were more common in Whites. 54
It is now well‐documented that photodermatoses significantly affect quality of life (QoL).
A recent systematic analysis showed that one‐third of adult and child patients with photosensitivity experience very or extremely large impact on QoL, with effects on clothing choices, anxiety and depression. 55 Therefore, effective photoprotection measures are essential for these patients.
Polymorphous light eruption
PMLE can be induced by UVB or UVA depending on the patient. 56 Broad‐spectrum sunscreens are essential but not always sufficient alone; a widely used effective treatment is narrowband (NB)‐UVB hardening (in certain latitudes, it may be beneficial to avoid high‐SPF sunscreen in winter for photoadaptation). Other treatments include hydroxychloroquine and oral antioxidants. 57
Chronic actinic dermatitis
Chronic actinic dermatitis, presenting with lichenified eruption in a photodistribution pattern, is more often caused by UVB than UVA. 58 , 59 Aside from photoprotection, other treatments include NB‐UVB hardening, topical calcineurin inhibitors, azathioprine, mycophenolate mofetil, methotrexate, cyclosporine and dupilumab (IL4/IL13 inhibitor). 60
Solar urticaria
Solar urticaria/solar angioedema is induced by UVB/UVA/VL; the use of tinted sunscreens is required for those with VL as the action spectrum. 61 Other treatments include antihistamines, UVA/UVA1 rush hardening, cyclosporine and omalizumab. 62 , 63 , 64
Drug‐induced phototoxicity and photoallergy
Drug‐induced phototoxicity from the interaction of topical or systemic agents with UVA 65 manifests as an exaggerated sunburn reaction with rapid onset. This is not to be mistaken with photoallergy, which generally has delayed onset at 24–48 h after sun exposure, and requires only a minimal concentration of the photoallergen to induce the lesions in photosensitized individuals. Photosensitivity induced by systemic drugs has been documented in 5% to 16% of patients referred to photodermatology centres. 66 , 67 , 68 Phototoxicity and photoallergy can result in postinflammatory hyperpigmentation (PIH), especially in dark‐skinned individuals. Non‐steroidal anti‐inflammatory agents (topical and systemic) are a common cause of drug‐induced phototoxicity.
Cutaneous Porphyrias
The action spectrum of cutaneous porphyrias lies in the visible range at 400–410 nm (Soret band) requiring physical photoprotection and sun avoidance. 69 For areas not covered by clothing, a sunscreen with VL photoprotection (tinted sunscreen) is recommended. Treatments for porphyria cutanea tarda include phlebotomy and low‐dose hydroxychloroquine. Erythropoietic protoporphyria can be effectively treated with afamelanotide (alpha‐melanocyte stimulating hormone analog), which requires an implant every 60 days. 70
Photoaggravated dermatoses
The action spectrum for both lupus erythematosus and dermatomyositis lies in the UVB and UVA range. 71 Regular use of a well‐balanced UVB/UVA sunscreen is mandatory.
Pigmentary disorders
Solar‐induced pigmentation
As well as UVB, sunscreens used for treating or preventing hyperpigmentary disorders must also cover UVA1 and VL. 5 , 6 , 24 , 72 , 73 VL induces hyperpigmentation in SPT III to VI subjects, but not in SPT II subjects. VL‐induced pigmentation is more intense and more prolonged compared to that induced by UVA1. Furthermore, VL and UVA1 have a synergistic effect. 5 , 7 , 73 , 74 Blue‐violet light (HEV) is responsible for hyperpigmentation induced by VL and can be prevented by inorganic sunscreens containing iron oxides. 6 , 75 , 76 More effective filters against VL/HEV could provide even better protection in the future.
Melasma
Melasma requires a comprehensive therapeutic approach including the use of broad‐spectrum, tinted sunscreen all year‐round as it involves UVB, UVA and HEV wavelengths. 77 The regular use of sunscreen (SPF50+, UVA‐PF28) for 12 months was found to be effective in preventing melasma relapses in pregnant women (2.7% new cases vs 53% in usual conditions). 78 Sunscreen (SPF19 and PA+++) 3 times daily for 12 weeks improved the melasma area severity index (MASI) and Melasma Quality of Life Index in 100 South Asian melasma patients. 79 In a randomized controlled trial in 40 Caucasian melasma patients, tinted sunscreen containing iron oxides for VL protection provided better protection against melasma relapses than the same sunscreen without VL protection. 80 Furthermore, sunscreen protecting against UV and VL enhanced the depigmenting efficacy of hydroquinone compared with UV‐only sunscreen in the treatment of melasma. 81
Postinflammatory hyperpigmentation
Ambient light is sufficient to promote PIH in dark skin. 82 Opaque dressings are recommended for at least 15 days after the inflammation has subsided. 83 If not possible, broad‐spectrum tinted sunscreen (including HEV protection) is mandatory. A split‐face study in 30 patients with SPT IV demonstrated that use of broad‐spectrum SPF60+ sunscreen containing anti‐inflammatory agents (licochalcone‐A and glycyrrhetinate) reduced the incidence of PIH at one week after laser treatment. 84
Lichen planus pigmentosus/ Riehl melanosis
The role of UV exposure on the pathogenesis of lichen planus pigmentosus/Riehl melanosis remains uncertain and a definite aetiology (e.g. photosensitive drug, lupus erythematosus and contact dermatitis) should be excluded. 85
Vitiligo
Vitiligo patients have decreased risk of developing skin cancer, especially melanoma. 86 , 87 Although sunburn is a provoking factor for vitiligo, 88 , 89 repigmentation of vitiligo lesions is almost impossible without UV exposure (natural or using phototherapy booths, lamps or lasers). 90 , 91 Vitiligo patients should be advised to regularly expose their lesional skin to the sun until vitiligo lesions become pink, after which a high‐SPF broad‐spectrum sunscreen is advised to prevent sunburn. 90 , 91
Skin inflammatory disorders
Rosacea
Caucasians with light sun‐sensitive skin are at highest risk of rosacea. 92 The daily use of broad‐spectrum sunscreens is recommended since both UV radiation and heat are potential triggers of initiation and aggravation of erythema and telangiectasia in rosacea patients by dysregulation of the innate and adaptive immune system. 92 , 93 Sunscreens containing dimethicone, cyclomethicone, or both to mitigate facial irritation 94 and repair the skin barrier may be advisable.
Acne
As UV radiation, especially UVA, increases the thickness of the stratum corneum 95 and alters the skin microbiome inducing a dysibiosis, 96 it may worsen the presence of retentional lesions (closed comedones) causing inflammation and flare ups of acne during the autumn.
No studies have shown whether sunscreen use is beneficial for acne lesions, but studies have shown that UVA can induce PIH on acne skin (irritation, excoriations and treatment adverse effects), especially in dark skin types (SPT IV to VI) and severe inflammatory acne grade III to V (GEA grading). 97 , 98 Inorganic sunscreens, while they may be non‐comedogenic, tend to be chalky in consistency. Therefore, mist formulas of organic sunscreens with a water or light liquid base and non‐greasy textures have higher cosmetic acceptability and lead to better adherence for teenagers with acne‐prone skin. 99 To minimize phototoxicity induced by acne medications, topical treatments should be applied in the evening and systemic treatments taken during the evening meal.
Atopic dermatitis
UVA1 phototherapy is usually beneficial for acute atopic dermatitis (AD), while treatment with UVB can reduce Staphylococcus aureus colonization of AD lesions. 100 , 101 High temperatures on the skin surface and sweating, due to the action of IRA, may worsen erythema or itching of eczematous lesions in AD, and UV may affect skin barrier functions. Photoaggravation of AD may occur in some patients. 102 Photosensitive AD, which typically presents with a photodistributed rash and involvement of non‐sun‐exposed skin, should be suspected if dermatitis worsens despite the use of photoprotection or local treatments. 103 Photobiologic evaluation is necessary for patients with photosensitive AD 102 ; this may include phototesting to determine the minimal erythema dose to UVB and UVA, and photopatch testing to exclude photocontact allergies. Sunscreens should not be used on weeping and moist lesions or scared lesions resulting from severe scratching. 104
Psoriasis
Most psoriasis is improved by sun exposure, and phototherapy is a well‐demonstrated approach for treating psoriatic lesions. Photoaggravation of psoriasis can occur in around 5 to 24% of cases, particularly in light‐skinned individuals. 105 Sunburn can provoke Koebnerization of psoriasis. 105
Conclusions
Increasing evidence on the impact of the different wavelengths of sunlight on the skin demonstrates the need for a tailored prescription of sunscreen according to SPT and dermatoses, which has been made possible due to advances in the filters and formulations of sunscreens. The recommendations of the expert panel are summarized in Table 1.
Table 1.
Practical recommendations for clinicians compiled by the expert panel
| Recommendations | |
|---|---|
| Skin phototype | |
| All SPT can benefit from using daily sunscreen photoprotection. The type of photoprotection must be adapted to the skin phototype but also latitude and altitude. | |
| UVA protection is important for all skin types and is even more important than protection against UVB (SPF factor) for dark skin. | |
| For dark skin, sunscreen with SPF30+ and an SPF/UVA‐PF ratio of <1.5 is recommended. | |
| For light skin, SPF50+ and an SPF/UVA‐PF ratio of <3 is recommended. | |
| Tinted sunscreens containing pigments, particularly iron oxide, have a greater protective effect against VL (blue light) and are, therefore, highly recommended for the prevention and treatment of hyperpigmentation disorders, especially for intermediate and dark skin. | |
| Transparency is important to reduce white residues, especially for darker SPT, or coloured sunscreens matched to skin colour. | |
| Photoaging | |
| Daily use of sunscreen with a balanced UVB/UVA protection is very important to prevent photoaging all year‐round in all skin phototypes. | |
| Generally, an SPF of at least 30 (SPF15 may be adequate in higher latitudes in the winter) with good UVA and UVB protection, and IRA protection. † | |
| IRA protection is recommended (sunscreens and daily photoprotection). † | |
| The need for VL protection for the prevention of photoaging is not yet clear, but should be recommended to avoid actinic lentigines. | |
| Skin cancer | |
| Melanoma | Sunscreens with high‐SPF and good UVA protection, SPF50+ and an SPF/UVA‐PF ratio close to 1, are recommended for melanoma prevention in fair‐skinned individuals. |
| Photoprotection, including sunscreen with SPF50+ and an SPF/UVA ratio as close to 1 as possible, is especially important in childhood for preventing sunburn as this is a high‐risk factor for developing melanoma later in life. | |
| Basal cell carcinoma | Sunscreens with SPF50+ and an SPF/UVA‐PF ratio <3 are recommended. |
| Actinic keratosis and squamous cell carcinoma | For AK and SCC, a well‐balanced sunscreen with SPF50+ and with an SPF/UVA‐PF ratio <3 with protection is recommended. |
| Photodermatoses | |
| Polymorphous light eruption | Recommendations for PMLE include broad‐spectrum sunscreen with an SPF/UVA‐PF ratio close to 1. |
| Chronic actinic dermatitis | For chronic actinic dermatitis, high‐SPF broad‐spectrum sunscreen is essential. |
| Solar urticaria/solar angioedema | For solar urticaria/solar angioedema, antihistamines and sun avoidance remain the mainstay treatment. Broad‐spectrum sunscreens with UVB, UVA and VL photoprotection (tinted sunscreen) are recommended. |
| Drug‐induced phototoxicity | If drug‐induced phototoxicity, the causative phototoxic agent should be identified and avoided and broad‐spectrum sunscreens with SPF50+ and an SPF/UVA‐PF ratio as close to 1 as possible are recommended, in combination with corticosteroids (topical or systemic) for acute phototoxicity. |
| Cutaneous porphyrias | For cutaneous porphyrias, physical protection and sun avoidance are recommended in severe cases due to the difficulty of protecting against VL. In areas not covered by clothes, a sunscreen with VL photoprotection (tinted sunscreen) is recommended. |
| Lupus erythematosus and dermatomyositis | For both lupus erythematosus and dermatomyositis, broad‐spectrum sunscreen with an SPF/UVA‐PF ratio close to 1 is recommended. |
| Any lesions should be treated first to repair the skin barrier function before use of sunscreen in order to minimize systemic absorption and irritant reactions. | |
| SPF50+ sunscreen may not be necessary in the winter for people living in higher latitudes as this can prevent natural photoadaptation/hardening. | |
| Pigmentary disorders | |
| Broad‐spectrum sunscreen with SPF50+ and a balanced protection against UVA (UVB/UVA protection ratio as close to 1 as possible) is recommended for the prevention or treatment of pigmentary disorders. | |
| VL/HEV photoprotection with tinted sunscreen is recommended to prevent VL‐induced pigmentation in skin type III or higher. | |
| Melasma | Protection against VL/HEV is essential, in addition to broad‐spectrum and well‐balanced UVB/UVA protection, all year‐round for the treatment of melasma and prevention of relapses. |
| Postinflammatory hyperpigmentation | Photoprotection is recommended for at least 2 weeks before any procedure or inflammatory dermatoses. When possible, opaque dressing is the best option. In other cases, application of broad‐spectrum sunscreen with UVB, UVA and VL/HEV protection is recommended on the treated areas for at least 15 days (one month maximum) after inflammation has resolved in order to prevent PIH. |
| Lichen planus pigmentosus | Broad‐spectrum sunscreen is recommended for lichen planus pigmentosus all year‐round to prevent further aggravation. |
| Vitiligo | Vitiligo patients should be advised to regularly expose their lesional skin to UV radiation without sunscreen until their vitiligo lesions start becoming pink. When the vitiligo lesions are pink or repigmented, SPF50+ broad‐spectrum sunscreen is recommended to prevent sunburn that could cause Koebnerization. |
| Skin inflammatory disorders | |
| Rosacea | For rosacea, broad‐spectrum (UVB and UVA) photoprotection of SPF30+ with an SPF/UVA‐PF ratio <3, as well as protection against IRA and VL, is recommended. |
| Acne | Use of an SPF30+ broad‐spectrum sunscreen with good UVB and UVA protection, as well as VL protection (sun hats and shade), is strongly recommended for retentional acne with signs of PIH or for patients at high risk of PIH, e.g. Fitzpatrick skin type IV or higher, or if significant occupational, or recreational sun exposure. |
| For inflammatory or cystic acne, a mist formula of organic SPF30+ broad‐spectrum sunscreen is recommended since inorganic sunscreen can cause irritation (pain and burning), especially if being treated with isotretinoin. | |
| Although sunscreen containing zinc oxide may be recommended to decrease risks of phototoxicity of both topical and systemic acne drugs, transparency is important to reduce white residues, especially for darker skins types; teenagers with acne generally prefer a mist formula. | |
| Atopic dermatitis | Generally, sunscreen should not be applied for inflammatory disorders until any lesional skin has been treated and the inflammation has resolved in order to avoid systemic absorption and photosensitization reactions. |
| For AD, regular use of broad‐spectrum sunscreens is recommended for preventing photosensitivity. | |
| Some sunscreen compounds, e.g. benzophenones (not commonly found in sunscreens for children) and butyl methoxydibenzoylmethane, may cause allergic reactions and are best avoided. | |
| Broad‐spectrum sunscreens SPF30+ that do not contain organic UV filters but only contain inorganic UV filters are recommended. Generally, sunscreens for babies only contain inorganic filters (titanium dioxide and zinc oxide). Sunscreens are not recommended for infants younger than 6 months old. | |
| Psoriasis | Exposure to sun can be beneficial in psoriatic patients, except for erythroderma and pustular types. Sun exposure should however be limited and patients must avoid sunburn that could cause koebnerization. |
| Broad‐spectrum sunscreens SPF50+ combined with a high UVA protection is recommended for photoaggravated forms of psoriasis. | |
AD, atopic dermatitis; AK actinic keratosis; BCC basal cell carcinoma; HEV high‐energy violet light; IR infrared; ITA individual typology angle; KC keratinocyte cancer; PIH postinflammatory hyperpigmentation; PLE polypodium leucotomos extract; PMLE polymorphous light eruption; SCC squamous cell carcinoma; SPF sun protection factor; SPT skin phototype; UV ultraviolet; VL visible light.
There are currently no available sunscreen filters that protect against IRA. Until new sunscreens are developed, sunscreens with antioxidants as a strategy to protect against IRA are highly recommended.
Despite the significant advances that have been made during the past decade, better protection against UVA1, VL and IRA is still required. Active compounds used topically or systemically could provide a good adjunct to filters by enhancing DNA repair, minimizing oxidative stress, decreasing inflammation or restoring skin microbiota.
Acknowledgement
Editorial and writing assistance was provided by Helen Simpson, PhD, ISMPP CMPP™, of My Word Medical Writing and funded by La Roche Posay.
Funding sources: An international panel of 12 experts convened in July 2020 for a virtual meeting sponsored by La Roche Posay (L'Oréal).
Conflicts of interest: Dr. Passeron reports personal fees from La Roche Posay during the conduct of the study, personal fees from L'Oréal, personal fees from SVR, personal fees from Symrise, personal fees from Isis Pharma, personal fees from Bioderma, personal fees from Beiersdorf and personal fees from ISDIN, outside the submitted work. Dr. Lim reports a research grant from L'Oreal, personal fees from La Roche Posay, personal fees from Pierre Fabre, personal fees from ISDIN, personal fees from Ferndale and personal fees from Beiersdorf during the conduct of the study; grants from Incyte, grants from Pfizer and grants from PCORI, outside the submitted work; in addition, Dr. Lim has a pending patent on Visible light phototesting. Dr. Goh reports other support from La Roche Posay during the conduct of the study. Dr. Kang reports personal fees from L'Oréal during the conduct of the study. Dr. Ly has nothing to disclose. Dr. Morita has nothing to disclose. Dr. Ocampo Candiani reports personal fees from La Roche Posay during the conduct of the study and personal fees from Pierre Fabre outside the submitted work. Dr. Puig reports personal fees from La Roche Posay, during the conduct of the study; grants, personal fees and non‐financial support from Almirall, personal fees from Amgen, personal fees and non‐financial support from Avene, personal fees and non‐financial support from BMS, grants and personal fees from Cantabria, grants, personal fees and non‐financial support from ISDIN, grants from MSD, non‐financial support from Lilly, non‐financial support from Abbvie, personal fees and non‐financial support from Pierre Fabre, grants, personal fees and non‐financial support from La Roche Posay, personal fees from Pfizer, personal fees and non‐financial support from Sanofi, grants, personal fees and non‐financial support from Sunpharma, grants and personal fees from Roche, outside the submitted work. Dr. Schalka reports grants from L'Oreal, grants from ISDIN and grants from Beiersdorf, outside the submitted work. Dr. Wei has nothing to disclose. Dr. Dreno reports personal fees from La Roche Posay International during the conduct of the study. Dr. Krutmann reports personal fees from La Roche Posay during the conduct of the study; grants and personal fees from Amway, grants and personal fees from Beiersdorf, grants and personal fees from bitop, grants and personal fees from Blue Lagoon, grants and personal fees from Estee Lauder, grants and personal fees from Evonik, grants and personal fees from Galderma, grants and personal fees from Henkel, grants and personal fees from Horphag, grants and personal fees from ISDIN, grants and personal fees from Kiessling, grants and personal fees from Lancaster‐Coty, grants and personal fees from La Roche Posay, grants and personal fees from L'Oréal, grants and personal fees from Lycored, grants and personal fees from Mary Kay, grants and personal fees from Mibelle, grants and personal fees from Procter & Gamble, grants and personal fees from Repairogen, grants and personal fees from RepliCel, grants and personal fees from Skinceuticals, grants and personal fees from SkinMedica, an Allergan Company, grants and personal fees from Stada, grants and personal fees from Symrise, grants and personal fees from Unilever, grants and personal fees from Vichy, grants and personal fees from Walgreens Boots Alliance, outside the submitted work.
References
- 1. Isedeh P, Osterwalder U, Lim HW. Teaspoon rule revisited: proper amount of sunscreen application. Photodermatol Photoimmunol Photomed 2013; 29: 55–56. [DOI] [PubMed] [Google Scholar]
- 2. Petersen B, Wulf HC. Application of sunscreen–theory and reality. Photodermatol Photoimmunol Photomed 2014; 30: 96–101. [DOI] [PubMed] [Google Scholar]
- 3. Moyal DD, Fourtanier AM. Broad‐spectrum sunscreens provide better protection from the suppression of the elicitation phase of delayed‐type hypersensitivity response in humans. J Invest Dermatol 2001; 117: 1186–1192. [DOI] [PubMed] [Google Scholar]
- 4. Krutmann J, Passeron T, Gilaberte Y et al. Photoprotection of the future: challenges and opportunities. J Eur Acad Dermatol Venereol 2020; 34: 447–454. [DOI] [PubMed] [Google Scholar]
- 5. Mahmoud BH, Ruvolo E, Hexsel CL et al. Impact of long‐wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol 2010; 130: 2092–2097. [DOI] [PubMed] [Google Scholar]
- 6. Duteil L, Cardot‐Leccia N, Queille‐Roussel C et al. Differences in visible light‐induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res 2014; 27: 822–826. [DOI] [PubMed] [Google Scholar]
- 7. Kohli I, Chaowattanapanit S, Mohammad TF et al. Synergistic effects of long‐wavelength ultraviolet al and visible light on pigmentation and erythema. Br J Dermatol 2018; 178: 1173–1180. [DOI] [PubMed] [Google Scholar]
- 8. Hault K, Rönsch H, Beissert S, Knuschke P, Bauer A. Knowledge of outdoor workers on the effects of natural UV radiation and methods of protection against exposure. J Eur Acad Dermatol Venereol 2016; 30(Suppl 3): 34–37. [DOI] [PubMed] [Google Scholar]
- 9. Fitzpatrick TB. The validity and practicality of sun‐reactive skin types I through VI. Arch Dermatol 1988; 124: 869–871. [DOI] [PubMed] [Google Scholar]
- 10. Chardon A, Cretois I, Hourseau C. Skin colour typology and suntanning pathways. Int J Cosmet Sci 1991; 13: 191–208. [DOI] [PubMed] [Google Scholar]
- 11. Del Bino S, Duval C, Bernerd F. Clinical and biological characterization of skin pigmentation diversity and its consequences on UV impact. Int J Mol Sci 2018; 19(9): 2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cestari T, Buster K. Photoprotection in specific populations: Children and people of color. J Am Acad Dermatol 2017; 76: S110–S121. [DOI] [PubMed] [Google Scholar]
- 13. Schalka S, Steiner D, Ravelli FN et al. Brazilian consensus on photoprotection. An Bras Dermatol 2014; 89(6 Suppl 1): 1–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Passeron T, Bouillon R, Callender V et al. Sunscreen photoprotection and vitamin D status. Br J Dermatol 2019; 181: 916–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Gruijl FR, Webb AR, Rhodes LE. Everyday sunscreen use may compromise vitamin D in temperate climes. Br J Dermatol 2020; 182: 1312–1313. [DOI] [PubMed] [Google Scholar]
- 16. Young AR, Passeron T. Everyday sunscreen use may compromise vitamin D in temperate climes: reply from authors. Br J Dermatol 2020; 182: 1313–1314. [DOI] [PubMed] [Google Scholar]
- 17. Matta MK, Zusterzeel R, Pilli NR et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA 2019; 321: 2082–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matta MK, Florian J, Zusterzeel R et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA 2020; 323: 256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miyamura Y, Coelho SG, Wolber R et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res 2007; 20: 2–13. [DOI] [PubMed] [Google Scholar]
- 20. Tewari A, Grage MM, Harrison GI, Sarkany R, Young AR. UVA1 is skin deep: molecular and clinical implications. Photochem Photobiol Sci 2013; 12: 95–103. [DOI] [PubMed] [Google Scholar]
- 21. Marionnet C, Nouveau S, Hourblin V et al. UVA1‐induced skin darkening is associated with molecular changes even in highly pigmented skin individuals. J Invest Dermatol 2017; 137: 1184–1187. [DOI] [PubMed] [Google Scholar]
- 22. Dlova NC, Akintilo LO, Taylor SC. Prevalence of pigmentary disorders: A cross‐sectional study in public hospitals in Durban, South Africa. Int J Womens Dermatol 2019; 5: 345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lyons AB, Trullas C, Kohli I, Hamzavi IH, Lim HW. Photoprotection beyond ultraviolet radiation: A review of tinted sunscreens. J Am Acad Dermatol 2020; 10.1016/j.jaad.2020.04.079. [DOI] [PubMed] [Google Scholar]
- 24. Moyal D. Need for a well‐balanced sunscreen to protect human skin from both Ultraviolet A and Ultraviolet B damage. Indian J Dermatol Venereol Leprol 2012; 78(Suppl 1): S24–30. [DOI] [PubMed] [Google Scholar]
- 25. Boyd AS, Naylor M, Cameron GS, Pearse AD, Gaskell SA, Neldner KH. The effects of chronic sunscreen use on the histologic changes of dermatoheliosis. J Am Acad Dermatol 1995; 33: 941–946. [DOI] [PubMed] [Google Scholar]
- 26. Hughes MC, Williams GM, Baker P, Green AC. Sunscreen and prevention of skin aging: a randomized trial. Ann Intern Med 2013; 158: 781–790. [DOI] [PubMed] [Google Scholar]
- 27. Randhawa M, Wang S, Leyden JJ, Cula GO, Pagnoni A, Southall MD. Daily use of a facial broad spectrum sunscreen over one‐year significantly improves clinical evaluation of photoaging. Dermatol Surg 2016; 42: 1354–1361. [DOI] [PubMed] [Google Scholar]
- 28. Nouveau‐Richard S, Yang Z, Mac‐Mary S et al. Skin ageing: a comparison between Chinese and European populations. A pilot study. J Dermatol Sci 2005; 40: 187–193. [DOI] [PubMed] [Google Scholar]
- 29. Vierkötter A, Hüls A, Yamamoto A et al. Extrinsic skin ageing in German, Chinese and Japanese women manifests differently in all three groups depending on ethnic background, age and anatomical site. J Dermatol Sci 2016; 83: 219–225. [DOI] [PubMed] [Google Scholar]
- 30. Mizuno M, Kunimoto K, Naru E, Kameyama K, Furukawa F, Yamamoto Y. The effects of continuous application of sunscreen on photoaged skin in Japanese elderly people ‐ the relationship with the usage. Clin Cosmet Investig Dermatol 2016; 9: 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sarkar R, Garg VK, Jain A et al. A randomized study to evaluate the efficacy and effectiveness of two sunscreen formulations on Indian skin types IV and V with pigmentation irregularities. Indian J Dermatol Venereol Leprol 2019; 85: 160–168. [DOI] [PubMed] [Google Scholar]
- 32. Grether‐Beck S, Marini A, Jaenicke T, Krutmann J. Effective photoprotection of human skin against infrared A radiation by topically applied antioxidants: results from a vehicle controlled, double‐blind, randomized study. Photochem Photobiol 2015; 91: 248–250. [DOI] [PubMed] [Google Scholar]
- 33. Martini APM, Maia Campos P. Influence of visible light on cutaneous hyperchromias: Clinical efficacy of broad‐spectrum sunscreens. Photodermatol Photoimmunol Photomed 2018; 34: 241–248. [DOI] [PubMed] [Google Scholar]
- 34. Lopes F, Sleiman MG, Sebastian K, Bogucka R, Jacobs EA, Adamson AS. UV exposure and the risk of cutaneous melanoma in skin of color: a systematic review. JAMA Dermatol 2020; 157: 213. [DOI] [PubMed] [Google Scholar]
- 35. Alexandrov LB, Nik‐Zainal S, Wedge DC et al. Signatures of mutational processes in human cancer. Nature 2013; 500: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow‐up. J Clin Oncol 2011; 29: 257–263. [DOI] [PubMed] [Google Scholar]
- 37. Boniol M, Autier P, Boyle P, Gandini S. Cutaneous melanoma attributable to sunbed use: systematic review and meta‐analysis. BMJ 2012; 345: e4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Vries E, Trakatelli M, Kalabalikis D et al. Known and potential new risk factors for skin cancer in European populations: a multicentre case–control study. Br J Dermatol 2012; 167(s2): 1–13. [DOI] [PubMed] [Google Scholar]
- 39. Carrera C, Puig‐Butillè JA, Aguilera P et al. Impact of sunscreens on preventing UVR‐induced effects in nevi: in vivo study comparing protection using a physical barrier vs sunscreen. JAMA Dermatol 2013; 149: 803–813. [DOI] [PubMed] [Google Scholar]
- 40. Liu‐Smith F, Jia J, Zheng Y. UV‐induced molecular signaling differences in melanoma and non‐melanoma skin cancer. Adv Exp Med Biol 2017; 996: 27–40. [DOI] [PubMed] [Google Scholar]
- 41. Chang J, Zhu GA, Cheung C, Li S, Kim J, Chang AL. Association between programmed death ligand 1 expression in patients with basal cell carcinomas and the number of treatment modalities. JAMA Dermatol 2017; 153: 285–290. [DOI] [PubMed] [Google Scholar]
- 42. Jayaraman SS, Rayhan DJ, Hazany S, Kolodney MS. Mutational landscape of basal cell carcinomas by whole‐exome sequencing. J Invest Dermatol 2014; 134: 213–220. [DOI] [PubMed] [Google Scholar]
- 43. Martincorena I, Roshan A, Gerstung M et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 2015; 348: 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pickering CR, Zhou JH, Lee JJ et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Cancer Res 2014; 20: 6582–6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sánchez G, Nova J, Rodriguez‐Hernandez AE et al. Sun protection for preventing basal cell and squamous cell skin cancers. Cochrane Database Syst Rev 2016;7:Cd011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Green A, Williams G, Neale R et al. Daily sunscreen application and betacarotene supplementation in prevention of basal‐cell and squamous‐cell carcinomas of the skin: a randomised controlled trial. Lancet 1999; 354: 723–729. [DOI] [PubMed] [Google Scholar]
- 47. van der Pols JC, Williams GM, Pandeya N, Logan V, Green AC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev 2006; 15: 2546–2548. [DOI] [PubMed] [Google Scholar]
- 48. Olsen CM, Wilson LF, Green AC et al. Cancers in Australia attributable to exposure to solar ultraviolet radiation and prevented by regular sunscreen use. Aust N Z J Public Health 2015; 39: 471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med 1993; 329: 1147–1151. [DOI] [PubMed] [Google Scholar]
- 50. Naylor MF, Boyd A, Smith DW, Cameron GS, Hubbard D, Neldner KH. High sun protection factor sunscreens in the suppression of actinic neoplasia. Arch Dermatol 1995; 131: 170–175. [PubMed] [Google Scholar]
- 51. Ulrich C, Jürgensen JS, Degen A et al. Prevention of non‐melanoma skin cancer in organ transplant patients by regular use of a sunscreen: a 24 months, prospective, case‐control study. Br J Dermatol 2009; 161(Suppl 3): 78–84. [DOI] [PubMed] [Google Scholar]
- 52. Blomberg M, He SY, Harwood C et al. Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol 2017; 177: 1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Choi D, Kannan S, Lim HW. Evaluation of patients with photodermatoses. Dermatol Clin 2014; 32: 267–275, vii. [DOI] [PubMed] [Google Scholar]
- 54. Hamel R, Mohammad TF, Chahine A et al. Comparison of racial distribution of photodermatoses in USA academic dermatology clinics: A multicenter retrospective analysis of 1080 patients over a 10‐year period. Photodermatol Photoimmunol Photomed 2020; 36: 233–240. [DOI] [PubMed] [Google Scholar]
- 55. Rutter KJ, Ashraf I, Cordingley L, Rhodes LE. Quality of life and psychological impact in the photodermatoses: a systematic review. Br J Dermatol 2020; 182: 1092–1102. [DOI] [PubMed] [Google Scholar]
- 56. Gruber‐Wackernagel A, Byrne SN, Wolf P. Polymorphous light eruption: clinic aspects and pathogenesis. Dermatol Clin 2014; 32: 315–334, viii. [DOI] [PubMed] [Google Scholar]
- 57. Tanew A, Radakovic S, Gonzalez S, Venturini M, Calzavara‐Pinton P. Oral administration of a hydrophilic extract of Polypodium leucotomos for the prevention of polymorphic light eruption. J Am Acad Dermatol 2012; 66: 58–62. [DOI] [PubMed] [Google Scholar]
- 58. Paek SY, Lim HW. Chronic actinic dermatitis. Dermatol Clin 2014; 32: 355–361, viii‐ix. [DOI] [PubMed] [Google Scholar]
- 59. Menagé HD, Harrison GI, Potten CS, Young AR, Hawk JL. The action spectrum for induction of chronic actinic dermatitis is similar to that for sunburn inflammation. Photochem Photobiol 1995; 62: 976–979. [DOI] [PubMed] [Google Scholar]
- 60. Patel N, Konda S, Lim HW. Dupilumab for the treatment of chronic actinic dermatitis. Photodermatol Photoimmunol Photomed. 2020; 36: 398–400. [DOI] [PubMed] [Google Scholar]
- 61. Haylett AK, Koumaki D, Rhodes LE. Solar urticaria in 145 patients: Assessment of action spectra and impact on quality of life in adults and children. Photodermatol Photoimmunol Photomed 2018; 34: 262–268. [DOI] [PubMed] [Google Scholar]
- 62. Kieselova K, Santiago F, Henrique M. Incapacitating solar urticaria: successful treatment with omalizumab. An Bras Dermatol 2019; 94: 331–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jiang AJ, Lim HW. Phototherapy in the evaluation and management of photodermatoses. Dermatol Clin 2020; 38: 71–77. [DOI] [PubMed] [Google Scholar]
- 64. Vollono L, Bianchi L, Piccolo A, Mazzilli S, Campione E, Diluvio L. Good things come to those who wait: Successful response of solar urticaria to omalizumab after 1 year of treatment. Photodermatol Photoimmunol Photomed 2020; 36: 408–411. [DOI] [PubMed] [Google Scholar]
- 65. Kim WB, Shelley AJ, Novice K, Joo J, Lim HW, Glassman SJ. Drug‐induced phototoxicity: A systematic review. J Am Acad Dermatol 2018; 79: 1069–1075. [DOI] [PubMed] [Google Scholar]
- 66. Fotiades J, Soter NA, Lim HW. Results of evaluation of 203 patients for photosensitivity in a 7.3‐year period. J Am Acad Dermatol 1995; 33: 597–602. [DOI] [PubMed] [Google Scholar]
- 67. Crouch RB, Foley PA, Baker CS. Analysis of patients with suspected photosensitivity referred for investigation to an Australian photodermatology clinic. J Am Acad Dermatol 2003; 48: 714–720. [DOI] [PubMed] [Google Scholar]
- 68. Wong SN, Khoo LS. Analysis of photodermatoses seen in a predominantly Asian population at a photodermatology clinic in Singapore. Photodermatol Photoimmunol Photomed 2005; 21: 40–44. [DOI] [PubMed] [Google Scholar]
- 69. Schulenburg‐Brand D, Katugampola R, Anstey AV, Badminton MN. The cutaneous porphyrias. Dermatol Clin 2014; 32: 369–384, ix. [DOI] [PubMed] [Google Scholar]
- 70. Langendonk JG, Balwani M, Anderson KE et al. Afamelanotide for erythropoietic protoporphyria. N Engl J Med 2015; 373: 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. O'Gorman SM, Murphy GM. Photoaggravated disorders. Dermatol Clin 2014; 32: 385–398, ix. [DOI] [PubMed] [Google Scholar]
- 72. Marionnet C, Tricaud C, Bernerd F. Exposure to non‐extreme solar UV daylight: spectral characterization, effects on skin and photoprotection. Int J Mol Sci 2014; 16: 68–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ramasubramaniam R, Roy A, Sharma B, Nagalakshmi S. Are there mechanistic differences between ultraviolet and visible radiation induced skin pigmentation? Photochem Photobiol Sci 2011; 10: 1887–1893. [DOI] [PubMed] [Google Scholar]
- 74. Narla S, Kohli I, Hamzavi IH, Lim HW. Visible light in photodermatology. Photochem Photobiol Sci 2020; 19: 99–104. [DOI] [PubMed] [Google Scholar]
- 75. Duteil L, Esdaile J, Maubert Y et al. A method to assess the protective efficacy of sunscreens against visible light‐induced pigmentation. Photodermatol Photoimmunol Photomed 2017; 33: 260–266. [DOI] [PubMed] [Google Scholar]
- 76. Schalka S, de Paula Corrêa M, Sawada LY, Canale CC, de Andrade TN. A novel method for evaluating sun visible light protection factor and pigmentation protection factor of sunscreens. Clin Cosmet Investig Dermatol 2019; 12: 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Passeron T, Picardo M. Melasma, a photoaging disorder. Pigment Cell Melanoma Res 2018; 31: 461–465. [DOI] [PubMed] [Google Scholar]
- 78. Lakhdar H, Zouhair K, Khadir K et al. Evaluation of the effectiveness of a broad‐spectrum sunscreen in the prevention of chloasma in pregnant women. J Eur Acad Dermatol Venereol 2007; 21: 738–742. [DOI] [PubMed] [Google Scholar]
- 79. Sarkar R, Ghunawat S, Narang I, Verma S, Garg VK, Dua R. Role of broad‐spectrum sunscreen alone in the improvement of melasma area severity index (MASI) and Melasma Quality of Life Index in melasma. J Cosmet Dermatol 2019; 18: 1066–1073. [DOI] [PubMed] [Google Scholar]
- 80. Boukari F, Jourdan E, Fontas E et al. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: a prospective randomized comparative trial. J Am Acad Dermatol 2015; 72: 189–90.e1. [DOI] [PubMed] [Google Scholar]
- 81. Castanedo‐Cazares JP, Hernandez‐Blanco D, Carlos‐Ortega B, Fuentes‐Ahumada C, Torres‐Álvarez B. Near‐visible light and UV photoprotection in the treatment of melasma: a double‐blind randomized trial. Photodermatol Photoimmunol Photomed 2014; 30: 35–42. [DOI] [PubMed] [Google Scholar]
- 82. Park JY, Park JH, Kim SJ et al. Two histopathological patterns of postinflammatory hyperpigmentation: epidermal and dermal. J Cutan Pathol 2017; 44: 118–124. [DOI] [PubMed] [Google Scholar]
- 83. Song HS, Park JY, Kim SJ, Kang HY. In vivo time‐sequential histological study focused on melanocytes: suggestion of golden time for intervention to prevent post‐laser pigmentary changes. J Eur Acad Dermatol Venereol 2016; 30: 306–310. [DOI] [PubMed] [Google Scholar]
- 84. Wanitphakdeedecha R, Phuardchantuk R, Manuskiatti W. The use of sunscreen starting on the first day after ablative fractional skin resurfacing. J Eur Acad Dermatol Venereol 2014; 28: 1522–1528. [DOI] [PubMed] [Google Scholar]
- 85. Kumarasinghe SPW, Pandya A, Chandran V et al. A global consensus statement on ashy dermatosis, erythema dyschromicum perstans, lichen planus pigmentosus, idiopathic eruptive macular pigmentation, and Riehl's melanosis. Int J Dermatol 2019; 58: 263–272. [DOI] [PubMed] [Google Scholar]
- 86. Paradisi A, Tabolli S, Didona B, Sobrino L, Russo N, Abeni D. Markedly reduced incidence of melanoma and nonmelanoma skin cancer in a nonconcurrent cohort of 10,040 patients with vitiligo. J Am Acad Dermatol 2014; 71: 1110–1116. [DOI] [PubMed] [Google Scholar]
- 87. Bae JM, Ju HJ, Lee RW et al. Evaluation for skin cancer and precancer in patients with vitiligo treated with long‐term narrowband UV‐B phototherapy. JAMA Dermatol 2020; 156: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dunlap R, Wu S, Wilmer E et al. Pigmentation traits, sun exposure, and risk of incident vitiligo in women. J Invest Dermatol 2017; 137: 1234–1239. [DOI] [PubMed] [Google Scholar]
- 89. Vrijman C, Hosseinpour D, Bakker JG et al. Provoking factors, including chemicals, in Dutch patients with vitiligo. Br J Dermatol 2013; 168: 1003–1011. [DOI] [PubMed] [Google Scholar]
- 90. Passeron T. Medical and maintenance treatments for vitiligo. Dermatol Clin 2017; 35: 163–170. [DOI] [PubMed] [Google Scholar]
- 91. Mohammad TF, Al‐Jamal M, Hamzavi IH et al. The Vitiligo Working Group recommendations for narrowband ultraviolet B light phototherapy treatment of vitiligo. J Am Acad Dermatol 2017; 76: 879–888. [DOI] [PubMed] [Google Scholar]
- 92. Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Research 2018; 7: 1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Steinhoff M, Vocanson M, Voegel JJ, Hacini‐Rachinel F, Schäfer G. Topical Ivermectin 10 mg/g and oral doxycycline 40 mg modified‐release: current evidence on the complementary use of anti‐inflammatory rosacea treatments. Adv Ther 2016; 33: 1481–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. van Zuuren EJ. Rosacea. N Engl J Med 2017; 377: 1754–1764. [DOI] [PubMed] [Google Scholar]
- 95. Pearse AD, Gaskell SA, Marks R. Epidermal changes in human skin following irradiation with either UVB or UVA. J Invest Dermatol 1987; 88: 83–87. [DOI] [PubMed] [Google Scholar]
- 96. Burns EM, Ahmed H, Isedeh PN et al. Ultraviolet radiation, both UVA and UVB, influences the composition of the skin microbiome. Exp Dermatol 2019; 28: 136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Poli F. Acne on pigmented skin. Int J Dermatol 2007; 46(Suppl 1): 39–41. [DOI] [PubMed] [Google Scholar]
- 98. Goh CL, Noppakun N, Micali G et al. Meeting the challenges of acne treatment in Asian patients: a review of the role of dermocosmetics as adjunctive therapy. J Cutan Aesthet Surg 2016; 9: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Goodman G. Cleansing and moisturizing in acne patients. Am J Clin Dermatol 2009; 10(Suppl 1): 1–6. [DOI] [PubMed] [Google Scholar]
- 100. Ortiz‐Salvador JM, Pérez‐Ferriols A. Phototherapy in atopic dermatitis. Adv Exp Med Biol 2017; 996: 279–286. [DOI] [PubMed] [Google Scholar]
- 101. Patrizi A, Raone B, Ravaioli GM. Safety and efficacy of phototherapy in the management of eczema. Adv Exp Med Biol 2017; 996: 319–331. [DOI] [PubMed] [Google Scholar]
- 102. Simonsen AB, Koppelhus U, Sommerlund M, Deleuran M. Photosensitivity in atopic dermatitis complicated by contact allergy to common sunscreen ingredients. Contact Dermatitis 2016; 74: 56–58. [DOI] [PubMed] [Google Scholar]
- 103. Rutt VL, Reed KX, Liu X, Richard EG, Purcell SM. Photosensitive atopic dermatitis exacerbated by UVB exposure. Cutis 2017; 100: 180–184. [PubMed] [Google Scholar]
- 104. Katoh N, Ohya Y, Ikeda M et al. Clinical practice guidelines for the management of atopic dermatitis 2018. J Dermatol 2019; 46: 1053–1101. [DOI] [PubMed] [Google Scholar]
- 105. Wolf P, Weger W, Patra V, Gruber‐Wackernagel A, Byrne SN. Desired response to phototherapy vs photoaggravation in psoriasis: what makes the difference? Exp Dermatol 2016; 25: 937–944. [DOI] [PubMed] [Google Scholar]


