Abstract
Objective
Brain‐injured patients who are unresponsive at the bedside (ie, vegetative state/unresponsive wakefulness syndrome – VS/UWS) may present brain activity similar to patients in minimally conscious state (MCS). This peculiar condition has been termed “non‐behavioural MCS” or “MCS*”. In the present study we aimed to investigate the proportion and underlying brain characteristics of patients in MCS*.
Methods
Brain 18F‐fluorodeoxyglucose Positron Emission Tomography (FDG‐PET) was acquired on 135 brain‐injured patients diagnosed in prolonged VS/UWS (n = 48) or MCS (n = 87). From an existing database, relative metabolic preservation in the fronto‐parietal network (measured with standardized uptake value) was visually inspected by three experts. Patients with hypometabolism of the fronto‐parietal network were labelled “VS/UWS”, while its (partial) preservation either confirmed the behavioural diagnosis of “MCS” or, in absence of behavioural signs of consciousness, suggested a diagnosis of “MCS*”. Clinical outcome at 1‐year follow‐up, functional connectivity, grey matter atrophy, and regional brain metabolic patterns were investigated in the three groups (VS/UWS, MCS* and MCS).
Results
67% of behavioural VS/UWS presented a partial preservation of brain metabolism (ie, MCS*). Compared to VS/UWS patients, MCS* patients demonstrated a better outcome, global functional connectivity and grey matter preservation more compatible with the diagnosis of MCS. MCS* patients presented lower brain metabolism mostly in the posterior brain regions compared to MCS patients.
Interpretation
MCS* is a frequent phenomenon that is associated with better outcome and better brain preservation than the diagnosis of VS/UWS. Complementary exams should be provided to all unresponsive patients before taking medical decisions. ANN NEUROL 2021;90:89–100
Behavioral tests currently remain the first‐line assessments to evaluate the level of consciousness in patients with disorders of consciousness (DOC) for obvious reasons (eg, practicability, cost, accessibility). The most sensitive scale developed to disentangle the minimally conscious state (MCS; show reproducible but inconsistent conscious behaviours, such as command following or visual pursuit. 1 , 2 ) from the vegetative state/unresponsive wakefulness syndrome (VS/UWS; presence of eye‐opening and reflexive behaviours 3 ) is the Coma Recovery Scale‐Revised (CRS‐R 4 , 5 ). 1 , 2 The MCS was further subcategorized in MCS+ and MCS‐ based on the preservation of language‐related behaviours. 6 A correct diagnosis is crucial since it has important implications in terms of prognosis, treatment, pain management and ethical considerations (eg, end‐of‐life decisions 7 ). Bedside assessments relying on patients' responsiveness may be impeded by various potential neurological deficits such as aphasia, paresis, blindness, deafness or vigilance fluctuation. Therefore, in the past years, researchers have put a lot of effort into the development of brain‐computer interfaces to detect non‐behavioural evidence of consciousness. 8 , 9 The landmark papers of Owen et al. 10 and Monti et al. 11 convey the notion that covert consciousness might be present in patients who remain unresponsive at the bedside (VS/UWS), as clearly demonstrated by neuroimaging‐based and task‐specific residual brain activity. This led to the conceptualization of ‘cognitive motor dissociation’ (CMD), which defines patients who present appropriate cortical responses to active paradigms (ie, command following) despite the absence of behavioural command following. 12 The term ‘higher‐order cortex motor dissociation’ (HMD) defines patients who lack behavioural and brain imaging evidence of language comprehension (ie, without clinical or neuroimaging response to command) but exhibit contingent brain responses to passive stimulation (eg, sounds and/or language). 9 Both conditions are associated with a better prognosis than patients who fail to demonstrate cortical responses to external stimuli. 9 In addition to CMD and HMD, the term ‘non‐behavioural MCS’ or MCS* is used for patients who show no evidence of consciousness at the bedside (ie, VS/UWS), yet with neuroimaging or neurophysiological data showing residual brain activity compatible with the diagnosis of MCS. 13 Note that the term MCS* encompasses patients with CMD and HMD and also corresponds to the cortically mediated state (CMS), type 3a (ie, behaviourally in a VS/UWS but in a MCS/CMS based on functional brain‐imaging). 14
According to a recent meta‐analysis of electroencephalography (EEG) and functional magnetic resonance imaging (fMRI) studies, using active paradigms, about 15% of patients diagnosed as unresponsive at the bedside are able to covertly follow commands by modulating their brain activity. 15 However, such procedures may underestimate the level of consciousness due to a substantial false negative rate. 16 Regarding passive paradigms, to date, there are not enough studies for statistical evaluation of their accuracy. 15 Beside active and passive paradigms, resting‐state fluorodeoxyglucose positron emission tomography (FDG‐PET) assessments seem more sensitive than active fMRI to aid clinical diagnosis of DOC patients. 17 Studies with FDG‐PET confirm the importance of the fronto‐parietal network for consciousness by highlighting the correlation between behavioural metrics and metabolism preservation within this network. It has been shown that UWS who retain some degree of fronto‐parietal metabolic preservation have better outcomes than those with fronto‐parietal hypometabolism. 17 Thus, FDG‐PET is a promising tool to improve diagnostic accuracy in this clinically challenging population; however previous studies relied on complex analytic procedures to disentangle MCS from VS/UWS patients. In this work, we use visual inspection to categorize DOC patients based on their brain metabolism. Our cross‐sectional multimodal study investigated differences in outcome, grey matter integrity, functional connectivity and regional brain metabolism between VS/UWS, MCS* and MCS patients. We hypothesize that MCS* represents a distinct clinical entity compared to VS/UWS and MCS in terms of outcome, cerebral atrophy and brain activity.
Methods
Participants
From our existing database of FDG‐PET collected between 2012 and 2018, we excluded patients under 16 years old, acute patients (<28 days post‐onset at the time of the PET), patients with less than five CRS‐R assessments performed in a limited time window (as this is the recommended number of CRS‐R for an accurate diagnosis 18 ), patients with a diagnosis of coma or of emergence from MCS, locked‐in syndrome or an unclear diagnosis (eg, experienced neuropsychologists could not reach a clinical consensus diagnosis; Fig 1).
FIGURE 1.
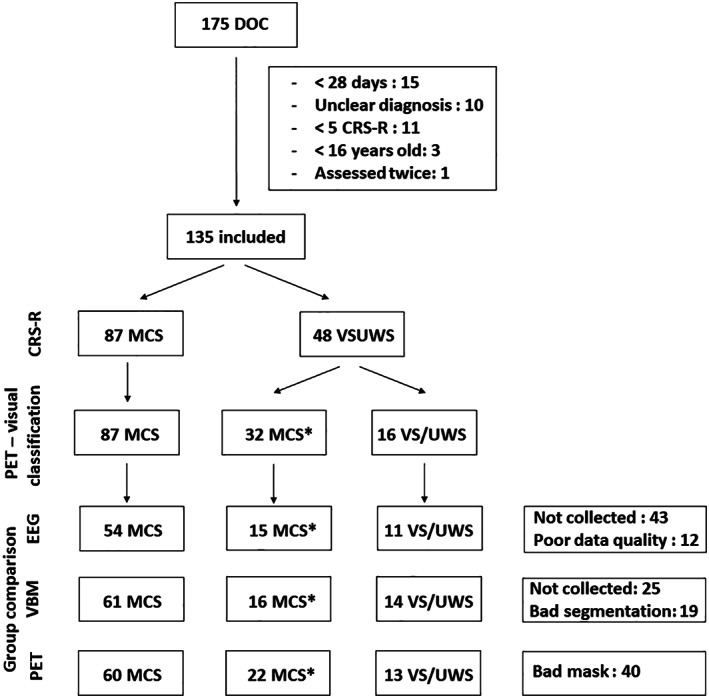
Study flowchart. CRS‐R: Coma Recovery Scale‐Revised; DOC: disorders of consciousness; EEG: electroencephalography; MCS: minimally conscious state; PET: positron emission tomography; VS/UWS: vegetative state/unresponsive wakefulness syndrome; VBM: voxel‐based morphometry.
Patients underwent repeated behavioural assessments, a cerebral FDG‐PET, a structural MRI and/or a high‐density resting‐state EEG recording within a 10‐day period. The flowchart of the study is illustrated in Fig 1. Individual data is reported in supplementary material 1.
For the FDG‐PET and EEG analyses, we included a group of 33 age‐matched healthy controls (range 19–70 years old, 15 women). We included a group of 36 age‐matched healthy subjects (range 20–75 years old, 13 women) as controls for the structural MRI.
The study was approved by the Ethics Committee of the Faculty of Medicine of the University of Liege. Written informed consent was obtained from healthy controls and patients' legal representatives. This cross‐sectional study follows the STROBE statement. 19
Clinical Assessments
All CRS‐R assessments were performed by trained and experienced clinicians. Particular behaviours such as resistance to manual eye opening 20 or cries were noted. Clinical outcomes were collected (death, VS/UWS, MCS or EMCS) via structured telephone interviews with the patients' physician or relatives 1 year after the FDG‐PET. This phone interview is based on the six subscales of the CRS‐R. 21 Examples of questions are “Is your relative able to respond to a simple command? For instance, can your relative squeeze your hand, close his/her eyes, stick out his/her tongue, move his/her legs when you ask him/her to do so? If yes: What are the command(s)? What frequency: sometimes or always?”. Outcomes were grouped in three categories: (1) patients who died, (2) patients who maintained a VS/UWS diagnosis or worsened their diagnosis (ie, “poor outcome”), and (3) patients who remained in a MCS or improved their diagnosis such as emerging from MCS (ie, “good outcome”).
Demographic and clinical differences between VS/UWS, MCS* and MCS groups were assessed using a Chi‐Square test for categorical variables (ie, gender and aetiology; traumatic versus non‐traumatic), an ANOVA (and two‐tailed t‐tests for post‐hoc analyses) for continuous variables (ie, age and time since injury) and a Kruskal‐Wallis test (and Wilcoxon signed rank tests for post‐hoc analyses) for ordinal variables (ie, CRS‐R diagnosis). A Bonferroni correction was applied to correct for multiple comparisons (three comparisons – p < 0.017). For the one‐year outcome, we used a Chi‐Square test to assess whether the three diagnostic groups had different outcomes.
Visual FDG‐SUV Classification
The FDG‐PET (Gemini TF CT scanner, Philips Medical Systems) was performed approximately 30 minutes after intravenous injection of 5–10 mCi (185–370 MBq) FDG in a resting state, awake, eyes open condition in a dark and quiet room.
The FDG‐PET standardised uptake value (SUV) was calculated to approximate the cerebral metabolic rate of glucose: at single subject level. The brain FDG‐PET SUV of each patient with an unequivocal and reliable bedside diagnosis of VS/UWS or MCS was visually inspected by three experts in the analyses of FDG‐PET of DOC patients. They were blinded to the clinical diagnosis and they categorized each patient as VS/UWS or MCS based on the SUV level in the fronto‐parietal network. Discrepancies were discussed to reach a common consensus FDG‐PET diagnosis for all patients. The degree of agreement between the three experts was calculated using the Cohen's kappa (K). Clinical VS/UWS patients were subsequently classified as MCS* 13 if they were categorized as MCS based on the SUV. 22 The study cohort was thus divided into three groups: (1) VS/UWS patients (clinical diagnosis and FDG‐PET diagnosis of VS/UWS); (2) MCS* patients (clinical diagnosis of VS/UWS but FDG‐PET diagnosis of MCS), and (3) MCS patients (clinical and FDG‐PET diagnosis of MCS) (Fig 2).
FIGURE 2.
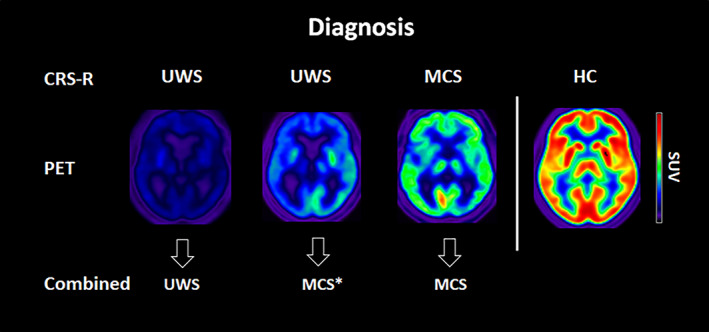
Examples of representative SUV for VS/UWS, MCS* and MCS patients and HC. Red colour represents a high brain metabolism (max SUV = 12), while blue represents low metabolism. CRS‐R = Coma Recovery Scale‐Revised; HC: healthy controls; MCS: minimally conscious state; SUV: standardized uptake value; VS/UWS: vegetative state/unresponsive wakefulness syndrome. [Color figure can be viewed at www.annalsofneurology.org]
EEG
EEG data acquisition and processing were similar to our previous study. 23 Resting‐state high‐density EEG data (256 channels, EGI, Geodesics) was acquired at rest with eyes open for a minimum of 30 minutes, during the FDG uptake period.
Data analysis was performed using EEGLAB (version 13.5.1; www.sccn.ucsd.edu/eeglab). The preprocessing and analyses performed were similar to the one published here. 23 EEG data were down‐sampled to 250 Hz and band‐pass filtered between 1 and 45 Hz. Facial and neck electrodes (n = 83) were excluded from the analysis, as well as bad channels and epochs containing evident noise, which were discarded by visual inspection. Subsequently, Independent Component Analysis was carried out to remove diffuse contributions of ocular, muscular and cardiac sources. Bad channels were interpolated using spline interpolation, and data were re‐referenced to the average reference. For each EEG channel, the power spectral densities were estimated using pwelch, a spectral multitaper approach with five Slepian tapers, as implemented in Fieldtrip 24 (version 20170723; www.fieldtriptoolbox.org/).
Brain connectivity was measured using debiased weighted phase lag index (dwPLI) between each pair of electrodes for delta (1‐4 Hz), theta (4.1–8 Hz), alpha (8.1‐13 Hz), beta (13.1‐20 Hz) and higher (ie, beta2 and gamma; 20.1–45 Hz 25 , 26 , 27 , 28 ) frequency bands using ‘wPLI function’ as implemented in Fieldtrip. 23 For each subject, the dwPLI values across all channel pairs were used to construct symmetric 173 × 173 dwPLI connectivity matrices for each frequency band. Brain network analysis was carried out using graph‐theory measures implemented in the Brain Connectivity Toolbox, 29 to assess altered topological network properties. To avoid biases associated with using a single threshold, we determined a range of thresholds using ‘connection densities (D)’ ranging from 90% to 10% in steps of 2.5%. The dwPLI matrix was thresholded and binarized (values below threshold were set to 0 and values above threshold were set to 1) for every D. The thresholded and binarized matrix for every value of D was represented as a network with the electrodes as nodes and non‐zero values as edges or connections. 23 The network topological properties measured include the degree (ie, denotes the total number of connections for each node), and the participation coefficient (ie, network integration at the global (whole brain) and regional (single electrode) level). 23 Brain regional connectivity and graph‐theory measures were quantified for the three groups and healthy controls for four brain regions of interest as proposed elsewhere 30 (ie, frontal, parietal, temporal and occipital – see below the ROIs channel locations).
Group differences for relative power, mean whole‐brain connectivity, degree and participation coefficient were assessed using two‐sample t‐tests with Bonferroni corrections for multiple comparisons (groups, frequency bands and brain regions).
We also performed exploratory comparisons between MCS* and MCS+ and MCS‐ patients' groups with regards to relative power, mean whole‐brain connectivity, degree and participation coefficients.
Structural MRI with Voxel‐Based Morphometry (VBM)
Structural MRI data were obtained using T1‐weighted 3D gradient echo sequences (120 slices, repetition time 2.3 seconds, echo time 2.47 ms, voxel size 1 × 1 × 1.2 mm3, flip angle 9°, field of view 256 × 256 mm2).
To investigate brain structural damages, T1‐MPRAGE images were analyzed using VBM analysis using the SPM12 and VBM8 toolboxes (www.fil.ion.ucl.ac.uk/spm, Structural Brain Mapping Group, Christian Gaser, Department of Psychiatry, University of Jena, Germany). The images were segmented into grey matter, white matter and cerebrospinal fluid using the standard segmentation module of SPM12. We used very light regularization (0.0001) for bias correction, 0.15 of Markov Random Field (MRF) clean‐up and 1 mm sampling distance. Tissue classes were set as “Native+Dartel imported”. After segmentation, the images were spatially normalized using a DARTEL‐based method to construct a study‐specific template from T1 images of DOC patients and healthy controls. 31 , 32 , 33 The modulated normalized grey matter images were then smoothed with an 8‐mm full‐width half‐maximum (FWHM) isotropic Gaussian kernel. As DOC patients have greater cerebral atrophy and brain damage, we created a grey matter mask by averaging all subjects (DOC and controls) and used this as explicit mask in the GLM design.
To assess group‐level differences, a full factorial design of four‐level generalized linear model (GLM) was modelled for VS/UWS, MCS*, MCS and healthy controls. Unequal independent variance and total intracranial volume were included as covariates in the design. Group differences in grey matter volume were investigated with a t‐contrast. Results were considered significant at false discovery rate (FDR) corrected cluster level p < 0.05.
As exploratory analyses, the differences in grey matter atrophy between MCS* versus MCS+ and MCS‐ patients' groups were also investigated.
FDG‐PET
Data were preprocessed and analyzed following the procedure published here. 17 Data were reoriented and spatially normalized to a stereotaxic space and smoothed using a 14 mm full width at half maximum Gaussian kernel. To overcome the problem of big deformations due to brain lesions as well as the fact that SPM has a default template based on H15 2O data, the normalization was performed using a customized template using the procedure described by Phillips et al. 34 Statistical analyses were performed using Statistical Parametric Mapping (SPM12; www. fil.ion.ucl.ac.uk/spm). The resulting set of voxel values for each contrast, constituting a statistical parametric map of the t‐statistics (SPM{t}), was transformed to the unit normal distribution (SPM{Z}) and thresholded at voxel‐wise p < 0.05 FDR‐corrected at the whole‐brain level.
For the metabolic index of the best preserved hemisphere (MIBH), in sum, individual images were registered to a common template in Montreal Neurological Institute (MNI) space by affine and non‐linear transformation using Advanced Normalization Tools (ANTs version 2.0.3). Images were then segmented into left cortex, right cortex and extracerebral tissue and normalized on the metabolism of the extracerebral tissue in reference to controls. Lastly, metabolic activity was scaled by setting the mean activity of extracerebral regions to an index value of 1 (all values are comprised from 0 to 1). The index was computed as the highest mean metabolic activity of the two hemispheres (for more details regarding this procedure see 35 ). The cut‐of for the MIBH was set‐up at 3.18), as described by Stender et al. 35
We identified regional glucose uptake and MIBH differences: (1) MCS* patients and healthy controls, (2) VS/UWS and MCS* patients, (3) MCS* and MCS patients.
As our visual inspection was based on the preservation of brain metabolism in the fronto‐parietal network, we obtained a quantitative metabolic measure from the same areas, by computing the metabolic index in fronto‐parietal ROI's as per the Automated Anatomical Labeling (AAL atlas; left and right frontal middle gyri, frontal inferior gyri opercularis and triangular, parietal inferior gyri and angular gyri).
Group differences were assessed using two‐sample t‐tests with Bonferroni corrections for multiple comparisons.
As exploratory analyses, differences in regional brain metabolism between MCS* versus MCS+ and MCS‐ patients' groups were also investigated.
Results
Clinical Data
From the 135 patients who met the inclusion criteria, 87 patients were behaviourally diagnosed in MCS (64% ‐ 24 MCS‐ and 63 MCS+) and 48 in VS/UWS (36%) based on repeated CRS‐R (Fig 1 and Table 1). A congruent SUV diagnosis between the three experts was met in 93% (n = 125) and for the remaining 7% (n = 10) a consensus was reached. The degree of agreement between the experts is considered to be almost perfect (Kappa of 0.86). Out of the 48 patients clinically unresponsive, 16 (33%) presented a global brain metabolism compatible with their diagnosis (VS/UWS) and 32 (67%) presented a mismatch between their behaviour and (partial) residual brain metabolism within the fronto‐parietal network (MCS*). Table 1 illustrates the demographic and clinical characteristics of the three groups.
TABLE 1.
Demographic and Clinical Characteristics of the UVS/WS, MCS* and MCS Groups
| VS/UWS (n = 16) | MCS* (n = 32) | MCS (n = 87) | Statistics | |
|---|---|---|---|---|
| Gender | 8 men (50%) | 18 men (56%) | 54 men (62%) | Group: Chi2= 3.075—p = 0.213 |
| Aetiology | ||||
| TBI (%) | 1 (6%) | 12 (37.5%) | 41 (47%) | Group: Chi2 = 9.518—p = 0.009 |
| Stroke | 1 | 7 | 20 | UWS = MCS*—p = 0.022 |
| Anoxia | 11 | 8 | 18 | MCS* = MCS—p = 0.349 |
| Mixed | 3 | 3 | 7 | UWS < MCS—p = 0.002 |
| Meningitis | 0 | 2 | 1 | |
| Age—mean ± SD (min‐max) | 51 ± 12 years old (26–74) | 43 ± 15 years old (16–73) | 43 ± 14 years old (18–79) | F = 1.55, p = 0.217 |
| TSO—mean ± SD (min‐max) | 15.5 ± 14.0 months (1.0–57.0) | 15.2 ± 11.6 months (0.9–67.8) | 23.7 ± 20.2 months (1.0–159.5) | F = 1.61, p = 0.205 |
| CRS‐R total score—median ± IQR (min‐max) | 5 ± 2 (2–6) | 6 ± 2 (4–8) | 12 ± 3 (4–21) |
Group: Chi2 = 75.77—p < 0.001 UWS = MCS*—p = 0.084 MCS* < MCS—p < 0.001 UWS < MCS—p < 0.001 |
Post‐hoc tests were corrected for multiple comparisons and significance was set at p < 0.017.
Abbreviations: IQR, interquartile range; MCS, minimally conscious state; TBI, traumatic brain injury; TSO, time since onset; VSUWS, vegetative state/unresponsive wakefulness syndrome.
We found group differences for the aetiology and the CRS‐R total scores. Post‐hoc analyses revealed that traumatic brain injury (TBI) was more frequent in the MCS group compared to the VS/UWS group (p = 0.002). Differences in aetiology between MCS* compared to VS/UWS (p = 0.022) or MCS patients (p = 0.349) could not be considered significant when corrected for multiple comparisons. The CRS‐R total scores were lower for both MCS* and VS/UWS patients compared to MCS patients, while there was no difference between MCS* and VS/UWS patients (Table 1).
Atypical behaviours were observed in five MCS* patients. One presented resistance to eye‐opening, two showed localizations to sounds, and two had contextualized tears during the CRS‐R assessments. No VS/UWS patients presented atypical behaviours.
Outcomes at one‐year follow‐up were available for 94 patients (70%), 12 out of 16 VS/UWS, 23 out of 32 MCS* and 59 out of 87 MCS patients. There was no difference between available outcomes (n = 94) and missing data (n = 41) for diagnosis (p = 0·417), age (p = 0·690), time since injury (p = 0·602) nor aetiology (p = 0·453). Six VS/UWS patients died, six remained in VS/UWS, and none recovered to MCS or EMCS. Ten MCS* patients died, six remained clinically unresponsive, five recovered to MCS and two improved to EMCS. Fifteen MCS patients died, five regressed to VS/UWS, 35 remained in MCS and four evolved to EMCS (Fig 3). When looking at the difference between poor (ie, dead or VS/UWS) and good prognosis (ie, MCS or EMCS), we found that VS/UWS patients had worse outcome than MCS* patients (χ2=4·57; p = 0·033) and MCS patients (χ2=17·60; p < 0·001), and that MCS* patients had worse outcome than MCS patients (χ2=855; p = 0·003).
FIGURE 3.
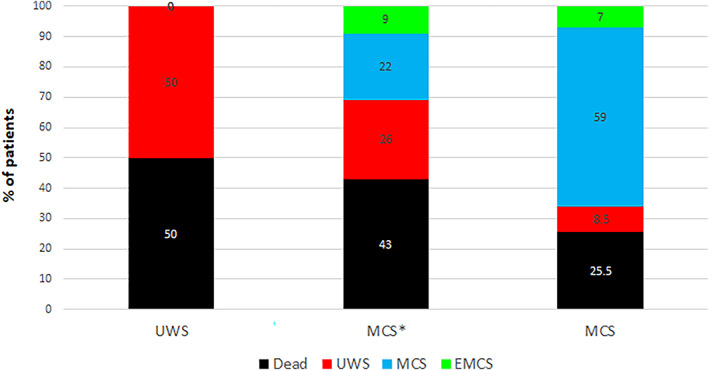
Patients' outcomes. The percentage of patients who died (in black), who remained in or worsened to VS/UWS (in red), who improved to or remained in MCS (in blue) and who emerged from MCS (in green) are presented for the three groups, VS/UWS, MCS* and MCS. Patients in MCS* presented a better outcome (ie, recovery of signs of consciousness) than VS/UWS patients but worse outcome than MCS patients. EMCS: emergence from the minimally conscious state; MCS: minimally conscious state; VS/UWS: vegetative state/unresponsive wakefulness syndrome. [Color figure can be viewed at www.annalsofneurology.org]
EEG
Ninety‐two patients had a high‐density EEG, out of which 12 (13%) had to be excluded due to poor data quality (ie, important movement artefacts), and thus 80 patients were included in the analyses (Fig 1). Patients in MCS* (n = 15) compared to VS/UWS (n = 11) had higher theta, alpha and lower delta power for the whole brain. The other comparisons (altered beta power, as well as regional power measures for MCS* versus VS/UWS, and MCS* versus MCS) did not reach significance after correction for multiple comparisons. No difference in the power spectrum was found between patients in MCS (n = 54) and patients in MCS* for any bandwidth or any scalp region.
Additionally, MCS* patients had higher functional connectivity (dwPLI) compared to VS/UWS patients in the alpha band at the whole brain level and in the left hemisphere. In addition, we found a lower connectivity in the delta band at the whole brain level and in the left hemisphere in VS/UWS compared to MCS* patients (Fig 4A). Compared to MCS*, MCS patients had a higher connectivity in the theta band in the left hemisphere.
FIGURE 4.
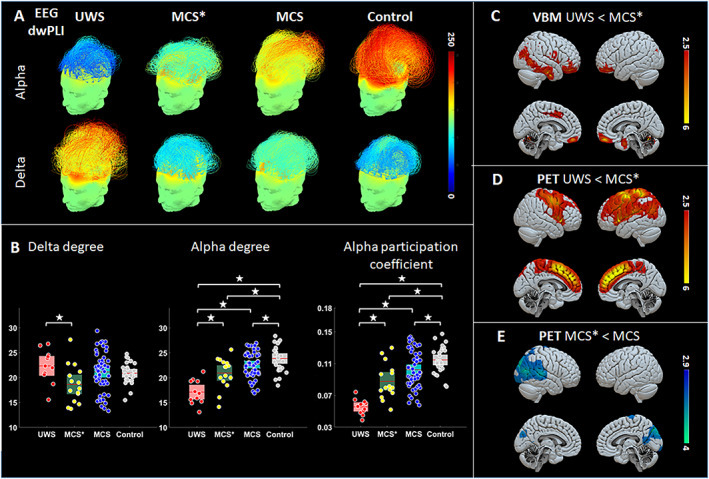
Neurophysiological and neuroimaging groups' results. EEG results showed a higher functional connectivity in alpha band and lower connectivity in the delta band ranging from VS/UWS to MCS* to MCS and controls, with blue colors representing low connectivity and red colors high connectivity (A). A higher alpha‐degree was found in MCS* compared to VS/UWS and in MCS compared to both MCS* and VS/UWS (B—left). At the whole‐brain level, a lower delta‐degree was found in MCS* compared to VS/UWS patients (B—middle). A higher alpha‐participation coefficient was found in MCS* compared to VS/UWS and in MCS compared to both MCS* and VS/UWS (B—right). VBM findings show that MCS* patients presented less cerebral atrophy in the frontal regions bilaterally, in the right temporal area, and in the insula compared to VS/UWS (C). FDG‐PET results show higher brain metabolism in the lateral and medial fronto‐parietal network in MCS* compared to VS/UWS patients (D). Lower brain metabolism in the right posterior parietal cortex is found in MCS* compared to MCS patients (E). EEG: electroencephalography; MCS: minimally conscious state; MCS*: minimally conscious state patients based on glucose metabolism; PET: positron emission tomography; rSTG: right supra temporal gyrus; VS/UWS: vegetative state/unresponsive wakefulness syndrome; VBM: voxel‐based morphometry. [Color figure can be viewed at www.annalsofneurology.org]
The network topological graph measures showed higher participation coefficient and degree in the alpha band. The degree was lower in the theta band in MCS* compared to VS/UWS patients at the whole brain level (Fig 4B). For the regional graph measures, the alpha participation coefficient in the frontal and parietal regions was higher in MCS* compared to VS/UWS patients. No other group comparisons reached significance after correction for multiple comparisons.
A detailed description of the results can be found in supplementary material 2.
Additionally, we looked at MCS‐ and MCS+ compared to MCS* patients' groups. We found that there was no difference for any of the EEG variables between MCS* and MCS‐. MCS+ compared to MCS* was found to have a higher mean connectivity in the theta band in the left hemisphere, and higher participation coefficient in the alpha band in the temporal area. Details of the results can be found in supplementary material 2.
VBM
Out of 135 patients, the MRI was not acquired for 25 patients. Ninety‐one patients were analyzed as 19 patients (17%) were excluded due to segmentation issues (Fig 1). Patients in MCS* (n = 16) presented higher grey matter volume than patients in VS/UWS (n = 14) in the inferior frontal gyrus bilaterally, the fusiform gyrus, the right temporal area (ventral and lateral) and the right insula (Fig 4C). There were no regions showing more grey matter volume in VS/UWS compared to MCS*, nor in MCS (n = 61) compared to MCS* patients. When comparing between VS/UWS and MCS patients, bilateral fronto‐parietal lateral and median regions were, as expected, more atrophic in VS/UWS patients.
A detailed description of the results can be found in supplementary material 2.
When dividing MCS into MCS‐ and MCS+, no difference in grey matter volume was found between patients from either subcategory and MCS* patients.
PET
Out of 135 patients' scans, 95 patients were included, while 40 patients (30%) had to be excluded due to normalisation issues (ie, < 75% preserved brain volume; Fig 1).
When comparing MCS* to VS/UWS patients, we found a higher brain metabolism in the fronto‐parietal lateral and median regions bilaterally (left > right; Fig 4D). Compared to patients in MCS (n = 22), patients in MCS* had lower brain metabolism in the right precuneus, the right supplementary motor area, the right superior temporal gyrus (STG) and the right visual cortex (Fig 4E). A detailed description of the results can be found in supplementary material 2.
When dividing MCS into MCS‐ and MCS+, no difference in brain metabolism was found between patients from either subcategory and MCS* patients.
The MIBH was significantly higher in MCS* patients (3·78 ± 0·71) than in VS/UWS patients (2·47 ± 0·28; p < 0·001), and lower than in MCS patients (4·59 ± 1·09; p = 0·002 – see Fig 5). At the single subject level, six MCS patients (10%), six MCS* (27%) and all VS/UWS patients had an index below 3.18. The outcome was available for four MCS* presenting an index below 3.18, one recovered to MCS, one remained in VS/UWS and two died. The outcome was known for three out of six MCS patients: two remained in MCS and one died. When dividing MCS into MCS‐ and MCS+, no difference between MCS* and MCS‐ was found (p = 0.697), while MCS+ had an index significantly higher than MCS* (p < 0.001).
FIGURE 5.
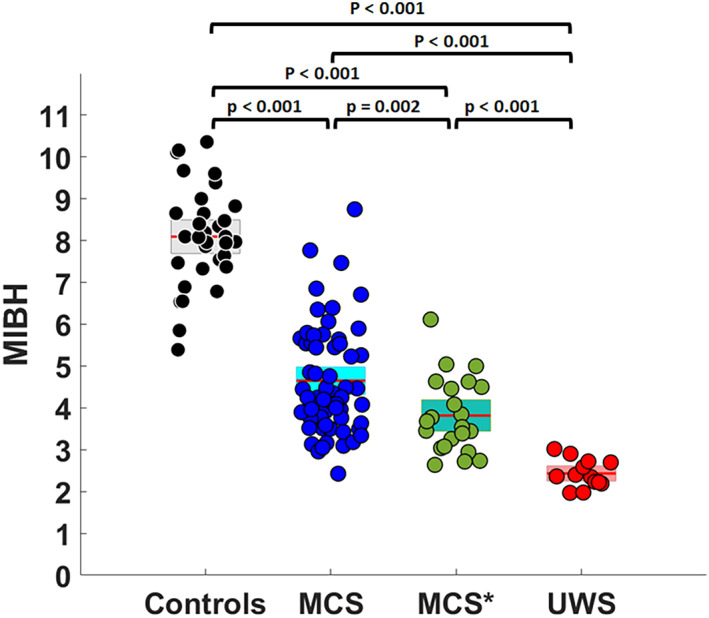
Metabolic index of the best preserved hemisphere. Metabolic index of the best preserved hemisphere (MIBH) as calculated in Stender et al., 2016 35 for each group, namely healthy controls, minimally conscious state (MCS), minimally conscious state star (MCS*) and vegetative state/unresponsive wakefulness syndrome (VS/UWS). [Color figure can be viewed at www.annalsofneurology.org]
When looking at the MI‐FPN, a cutoff of 2.7 was found to distinguish perfectly UWS from MCS* (individual data can be found in supplementary material 1). The MI‐FPN was significantly higher in MCS* patients (3.73 ± 0.69) than in VS/UWS patients (2.30 ± 0.21; p < 0·001), and lower than in MCS patients (4.37 ± 0·94; p = 0.014).
Note that the MCS* classification is primarily based on FDG‐PET. Group comparisons of brain metabolism among MCS* and other patient categories are therefore somewhat circular and should be interpreted with appropriate caution.
Discussion
In this multimodal cross‐sectional study, we investigated differences in outcome, EEG connectivity, grey matter atrophy, and regional brain metabolism between behavioural unresponsive patients with a hypometabolism in the fronto‐parietal network (VS/UWS) and behavioural unresponsive patients with a partial preservation of this network (MCS*), as well as with a group of MCS patients. We found a better outcome at one year for MCS* patients than VS/UWS patients. Electroencephalographically, MCS* patients had higher power in theta and alpha bands, lower power in delta band, higher alpha participation coefficient (ie, integration) and alpha degree (ie, connectivity) in the whole brain, frontal and parietal regions, compared to VS/UWS patients. We found less severe structural atrophy in the frontal, right temporal region, fusiform gyrus, and insula in MCS* patients compared to VS/UWS. Compared to MCS* patients, MCS patients presented higher glucose metabolism in the right posterior regions of the brain, and higher theta band connectivity in the left hemisphere, while no difference in grey matter atrophy was found. The MIBH was gradually higher from VS/UWS to MCS* to MCS patients.
Diagnosis of MCS*
When using information from resting‐state FDG‐PET to complement the diagnosis obtained from repeated behavioural assessments, the proportion of patients in VS/UWS dropped from 36% to 12%; the majority of VS/UWS patients demonstrated residual fronto‐parietal functional brain activity. This notion of (partially) preserved fronto‐parietal glucose uptake demonstrates that VS/UWS patients presenting a compatible brain metabolism are rarer than expected; and that careful multimodal evaluation of VS/UWS patients is warranted. We therefore stress the importance to perform at least five assessments using the CRS‐R18 and to evaluate patients' residual brain function with paraclinical assessments, as recently recommended by the American 36 and European 37 guidelines.
In comparison with Stender et al. (1) 38 and (2), 35 we observed a higher rate (67% here, versus 33% and 31%) of unresponsive patients who retained residual brain activity compatible with (minimal) consciousness. Of note, besides the samples being different, the methodologies of previous PET studies, 35 , 38 varies from the one used in the present study. First, previous studies did not use behavioural diagnoses based on at least five CRS‐R. In addition, neuroimaging diagnosis (FDG‐PET) was achieved visually after statistical comparison with healthy controls (SPM), using partial preservation of glucose uptake within the associative fronto‐parietal cortex bilaterally as yardstick for MCS. In contrast, we here inspected visually each patient’ SUV which reflects cerebral glucose uptake. The methodologies of these previous PET studies may have been more restrictive than the one used here, leading to a possible high rate of false negatives (ie, patients categorized as VS/UWS while they are in MCS*). On the other hand, our method may have led to a higher rate of false positives.
It should be noted that we only included chronic patients with an average of 15 months post‐injury. Given the recent literature on CMD in the acute setting, 9 , 39 the proportion of unresponsive patients in MCS* is likely to be significant as well in the early days or weeks post‐injury. Given the ethical and clinical implications of the diagnosis, the distinction between VS/UWS and MCS* is even more important in the (sub‐)acute stage. Therefore, there is a critical need to develop accessible tools to offer a better diagnosis and prognosis of DoC in the acute and sub‐acute settings.
Based on the FDG‐SUV findings, we might conclude that the proportion of VS/UWS patients presenting a compatible global hypometabolism is rather small. Therefore, our results challenge the definition of VS/UWS and highlight the need for a consensus regarding the neuroimaging requirements needed for the diagnosis of DOC patients.
Outcome
When looking at patients' outcomes, we noticed that all patients in VS/UWS remained in this state or died, while 31% of the patients in MCS* recovered at least some behavioural signs of consciousness (ie, MCS or EMCS). Hence, MCS* patients had more favorable outcomes than patients in VS/UWS. Most patients in MCS remained in this state, while a few relapsed to VS/UWS and a few emerged from the MCS. Looking at the rate of mortality, it seems equal between VS/UWS and MCS* patients; however, it should be noted that as MCS* patients were considered as unresponsive, this may have influenced end‐of‐life decisions. Prospective studies excluding patients who died following withdrawal of life support or end‐of‐life decisions should provide unbiased prognostic information for MCS* patients, and VS/UWS and MCS patients alike. Unfortunately, reason of death was not available for most of the outcomes.
Resting‐State Brain Imaging Assessments
EEG power spectrum analyses revealed an overall increase in theta and alpha power in MCS* compared to VS/UWS, and lower activity in the delta band, while no difference was found between MCS and MCS* groups. Theta and alpha power have been widely linked to consciousness recovery in many previous EEG studies on DOC (eg, 23 , 40 ). In addition, a higher connectivity in the alpha band and a reduced connectivity in the delta band was found in MCS* compared to VS/UWS patients, and this was shown in previous studies comparing MCS to VS/UWS patients. 23 These findings tend to show that the brain neurophysiological activity of MCS* patients is closer to what is usually observed in MCS compared to VS/UWS patients.
Grey matter differences between VS/UWS and MCS* patients were found in the right hemisphere, including the inferior and middle temporal gyri. These areas are known to be involved in multimodal sensory integration and visual perception (inferior temporal gyrus). 41 On the other hand, no difference in regional brain atrophy was found between MCS* and MCS patients, suggesting that MCS* patients have a brain morphometry that does not significantly differ from MCS patients.
Finally, regional PET analyses showed that patients in MCS presented higher brain metabolism in the motor region, the visual cortex and the precuneus, specifically in the right hemisphere, compared to MCS*. This (partial) preservation in the visual and motor cortices could explain why MCS patients are able to execute simple motor commands and behaviourally respond to external stimuli, whereas MCS* patients are physically and/or cognitively unable to. The precuneus is a critical hub for internal awareness, as shown in previous studies on VS/UWS, 42 deep sleep 43 and anesthesia. 44 More globally, the difference in brain metabolism preservation between MCS* and MCS in the right parietal cortex could highlight the presence of neglect. 45 Additionally, MCS* patients showed higher MIBH values than VS/UWS patients, suggesting again that these two groups differ in terms of brain preservation. Importantly, when looking at the MI‐FPN, a cut‐off between MCS* and VS/UWS (ie, index of 2.7) was found showing a perfect distinction between the two groups.
Limitations
Several limitations should be acknowledged. First, this is a retrospective one‐site study. For the outcome, we cannot exclude an element of self‐fulfilling prophecy, as the behavioural status of MCS* (ie, unresponsive at the bedside) might have impacted patient care and outcome. In addition, the rate of missing data at 12‐month follow‐up might also have impacted the results. Future longitudinal studies should investigate the behavioural outcome of consciousness along with its neuroimaging counterparts within the same subjects. In addition, the absence of difference when we sub‐categorized MCS into MCS+ and MCS‐ might be due to the small sample in each subgroup. The same comment can be made regarding the lack of significant difference in aetiologies between groups. Due to the limited number of patients in each subgroup, the absence of significant results should not be interpreted as evidence of similarity between groups. It should also be noted that some of the patients' data included in this study were analyzed in previous studies. However, none of the data from Stender et al. 17 , on which our hypothesis was based, were reused in the present study.
Conclusion
When combining appropriate and repeated behavioral assessments with FDG‐PET, the proportion of true VS/UWS patients (ie, without behavioral or cerebral signs of residual consciousness) in the whole DOC population is rather small (12%). Taken together, our results show that patients in MCS* have a better outcome than VS/UWS patients, exhibit brain structure and activity that is more compatible with MCS patients (who show behavioural signs of minimal awareness), than VS/UWS patients. These paraclinical findings suggest that MCS* patients represent a truly distinct diagnostic group in terms of brain function and integrity. Our study also confirms the urgent need to reframe the terminology of patients with covert consciousness detected by various paradigms (eg, CMD, HMD, CMS, MCS*).
Added value of these findings
Our findings, based on visual inspection of patients' brain metabolism, provide evidence that non‐behavioral MCS or MCS* patients represent a non‐negligible proportion of patients behaviorally unresponsive at the bedside, while VS/UWS patients with a congruent brain metabolism are rather rare. We found that unresponsive patients with an incongruent brain metabolism (ie, partial preservation of brain metabolism in the fronto‐parietal network) have a 50% chance to regain behavioral signs of consciousness at one‐year follow‐up.
In addition, our resting state electrophysiological and neuroimaging findings demonstrate a clear difference in brain activity and brain structure between patients in VS/UWS and in MCS*, as well as some differences between patients in MCS* and in MCS, suggesting that MCS* patients represent a truly distinct diagnostic group in terms of brain function and integrity.
Taken together, our results highlighted that the proportion of VS/UWS patients presenting a mismatch between their behaviour and their brain function (measured at rest) is much higher (64%) compared to previous studies using active or passive paradigms (10–15%). Therefore, when a patient is diagnosed in VS/UWS at the bedside, resting state brain imaging assessments should be performed to detect if residual brain activity compatible with consciousness is present.
Author Contributions
Aurore Thibaut, Olivia Gosseries and Steven Laureys contributed to the conception and design of the study; Aurore Thibaut, Rajanikant Panda, Johan Stender, Olivia Gosseries, Leandro R. D. Sanz, Charlotte Martial, Camille Chatelle, Charlène Aubinet, Estelle A. C. Bonin, Alice Barra, Marie‐Michèle Briand, Benedetta Cecconi and Sarah Wannez contributed to the acquisition and analysis of data; Aurore Thibaut, Rajanikant Panda, Leandro R. D. Sanz, Jitka Annen, Lionel Naccache, Olivia Gosseries contributed to drafting the text and preparing the figures.
Potential Conflicts of Interest
Nothing to report.
Supporting information
Appendix S1: Supporting Information
Acknowledgements
The study was supported by the University and University Hospital of Liege, the Belgian National Funds for Scientific Research (FRS‐FNRS), the European Union's Horizon 2020 Framework Programme for Research and Innovation under the Specific Grant Agreement No. 945539 (Human Brain Project SGA3), the European Space Agency (ESA) and the Belgian Federal Science Policy Office (BELSPO) in the framework of the PRODEX Programme, “Fondazione Europea di Ricerca Biomedica”, the BIAL Foundation, the Mind Science Foundation and the European Commission, the fund Generet, the King Baudouin Foundation, AstraZeneca Foundation, and DOCMA project [EU‐H2020‐MSCA–RISE–778234].
We thank the whole teams of the intensive care, MRI and PET departments of the University Hospital of Liege, especially Didier Ledoux, Paul Massion, Claire Bernard, Roland Hustinx and Jean‐Flory Luaba Tshibanda. We also thank all the volunteers, the patients and their families. R.P., C.A., B.C. and L.S. are research fellows, A.T. is a post‐doctoral fellow, O.G. is research associate and S.L. is research director at the F.R.S‐FNRS. B.C. is supported by the GIGA Doctoral School for Health Sciences, ULiège. M.M.B. is supported by the Canadian Institute of Health Research (CIHR) and the Fonds de recherche du Québec – Santé (FRQS).
Aurore Thibaut and Rajanikant Panda have equally contributed.
Steven Laureys and Olivia Gosseries share the senior position.
References
- 1. Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria [Internet]. Neurology 2002;58:349–353. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11839831. [DOI] [PubMed] [Google Scholar]
- 2. Schnakers C, Chatelle C, Vanhaudenhuyse A, et al. The nociception coma scale: a new tool to assess nociception in disorders of consciousness [Internet]. Pain 2010;148:215–219. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19854576. [DOI] [PubMed] [Google Scholar]
- 3. Laureys S, Celesia GG, Cohadon F, et al. Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med 2010;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seel RT, Sherer M, Whyte J, et al. Assessment scales for disorders of consciousness: evidence‐based recommendations for clinical practice and research [Internet]. Arch Phys Med Rehabil 2010;91:1795–1813. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21112421. [DOI] [PubMed] [Google Scholar]
- 5. Giacino JT, Kalmar K, Whyte J. The JFK coma recovery scale‐revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 2004;85:2020–2029. [DOI] [PubMed] [Google Scholar]
- 6. Thibaut A, Bodien YG, Laureys S, Giacino JT. Minimally conscious state “plus”: diagnostic criteria and relation to functional recovery. J Neurol 2020;267:1245–1254. 10.1007/s00415-019-09628-y. [DOI] [PubMed] [Google Scholar]
- 7. Demertzi A, Ledoux D, Bruno MA, et al. Attitudes towards end‐of‐life issues in disorders of consciousness: a European survey [Internet]. J Neurol 2011;258:1058–1065. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21221625. [DOI] [PubMed] [Google Scholar]
- 8. Cruse D, Chennu S, Chatelle C, et al. Bedside detection of awareness in the vegetative state: a cohort study [Internet]. Lancet 2011;378:2088–2094. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22078855. [DOI] [PubMed] [Google Scholar]
- 9. Edlow BL, Chatelle C, Spencer CA, et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain 2017;140:2399–2414. 10.1093/brain/awx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Owen AM, Coleman MR, Boly M, et al. Detecting awareness in the vegetative state [Internet]. Science 2006;313:1402. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16959998. [DOI] [PubMed] [Google Scholar]
- 11. Monti MM, Vanhaudenhuyse A, Coleman MR, et al. Willful modulation of brain activity in disorders of consciousness [Internet]. N Engl J Med 2010;362:579–589. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20130250. [DOI] [PubMed] [Google Scholar]
- 12. Schiff ND. Cognitive motor dissociation following severe brain injuries. JAMA Neurol 2015;72:1413–1415. [DOI] [PubMed] [Google Scholar]
- 13. Gosseries O, Zasler ND, Laureys S. Recent advances in disorders of consciousness: focus on the diagnosis. Brain Inj 2014;28:1141–1150. [DOI] [PubMed] [Google Scholar]
- 14. Naccache L. Minimally conscious state or cortically mediated state? Brain 2018;141:949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kondziella D, Friberg CK, Frokjaer VG, et al. Preserved consciousness in vegetative and minimal conscious states: systematic review and meta‐analysis. J Neurol Neurosurg Psychiatry 2016;87:485–492. [DOI] [PubMed] [Google Scholar]
- 16. Hauger SL, Schanke AK, Andersson S, et al. The clinical diagnostic utility of electrophysiological techniques in assessment of patients with disorders of consciousness following acquired brain injury: a systematic review. J Head Trauma Rehabil 2017;32:185–196. [DOI] [PubMed] [Google Scholar]
- 17. Stender J, Gosseries O, Bruno MA, et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study [Internet]. Lancet 2014;384:514–522. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24746174. [DOI] [PubMed] [Google Scholar]
- 18. Wannez S, Heine L, Thonnard M, et al. The repetition of behavioral assessments in diagnosis of disorders of consciousness. Ann Neurol 2017;81:883–889. [DOI] [PubMed] [Google Scholar]
- 19. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–1499. 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 20. van Ommen HJ, Thibaut A, Vanhaudenhuyse A, et al. Resistance to eye opening in patients with disorders of consciousness. J. Neurol 2018;265:1376–1380. 10.1007/s00415-018-8849-0. [DOI] [PubMed] [Google Scholar]
- 21. Wolff A, Estraneo A, Noé E, et al. International validation of the phone outcome questionnaire for patients with disorders of consciousness. International Brain Injury Association World Congress, 2019. [Google Scholar]
- 22. Stender J, Gosseries O, Bruno M‐A, et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study [Internet]. Lancet 2014;6736:8–16. Available from: http://www.thelancet.com/journals/a/article/PIIS0140-6736(14)60042-8/fulltext%5Cnhttp://linkinghub.elsevier.com/retrieve/pii/S0140673614600428. [DOI] [PubMed] [Google Scholar]
- 23. Chennu S, Annen J, Wannez S, et al. Brain networks predict metabolism, diagnosis and prognosis at the bedside in disorders of consciousness. Brain 2017;140:2120–2132. [DOI] [PubMed] [Google Scholar]
- 24. Babadi B, Brown EN. A review of multitaper spectral analysis. IEEE Trans Biomed Eng 2014;61:1555–1564. [DOI] [PubMed] [Google Scholar]
- 25. Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience 2006;137:1087–1106. [DOI] [PubMed] [Google Scholar]
- 26. Axmacher N, Henseler MM, Jensen O, et al. Cross‐frequency coupling supports multi‐item working memory in the human hippocampus. Proc Natl Acad Sci U S A 2010;107:3228–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cohen MX, Elger CE, Ranganath C. Reward expectation modulates feedback‐related negativity and EEG spectra. Neuroimage 2007;35:968–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cohen MX, Elger CE, Fell J. Oscillatory activity and phase‐amplitude coupling in the human medial frontal cortex during decision making. J Cogn Neurosci 2009;21:390–402. [DOI] [PubMed] [Google Scholar]
- 29. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010;52:1059–1069. [DOI] [PubMed] [Google Scholar]
- 30. Schartner M, Seth A, Noirhomme Q, et al. Complexity of multi‐dimensional spontaneous EEG decreases during propofol induced general anaesthesia. PLoS One 2015;10:e0133532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guldenmund P, Soddu A, Baquero K, et al. Structural brain injury in patients with disorders of consciousness: a voxel‐based morphometry study. Brain Inj 2016;30:343–352. [DOI] [PubMed] [Google Scholar]
- 32. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007;38:95–113. [DOI] [PubMed] [Google Scholar]
- 33. Di Perri C, Thibaut A, Heine L, et al. Towards new methods of diagnosis in disorders of consciousness – Authors' reply. Lancet Neurol 2016;15:1115–1116. [DOI] [PubMed] [Google Scholar]
- 34. Phillips CL, Bruno MA, Maquet P, et al. “Relevance vector machine” consciousness classifier applied to cerebral metabolism of vegetative and locked‐in patients [Internet]. Neuroimage 2011;56:797–808. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20570741. [DOI] [PubMed] [Google Scholar]
- 35. Stender J, Mortensen KNN, Thibaut A, et al. The minimal energetic requirement of sustained awareness after brain injury. Curr Biol 2016;26:1494–1499. [DOI] [PubMed] [Google Scholar]
- 36. Giacino J, Katz D, Schiff N, et al. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology 2018;91:450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kondziella D, Bender A, Diserens K, et al. European academy of neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur. J. Neurol 2020;27:741–756. [DOI] [PubMed] [Google Scholar]
- 38. Stender J, Gosseries O, Bruno M‐A, et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study. [Internet]. Lancet 2014;384:514–522. Available from: http://www.sciencedirect.com/science/article/pii/S0140673614600428. [DOI] [PubMed] [Google Scholar]
- 39. Claassen J, Doyle K, Matory A, et al. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med 2019;380:2497–2505. [DOI] [PubMed] [Google Scholar]
- 40. Sitt JD, King JR, El Karoui I, et al. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain 2014;137:2258–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Herath P, Kinomura S, Roland PE. Visual recognition: evidence for two distinctive mechanisms from a PET study. Hum Brain Mapp 2001;12:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ, et al. Default network connectivity reflects the level of consciousness in non‐communicative brain‐damaged patients [Internet]. Brain 2010;133 (Pt 1):161–171. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20034928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Horovitz SG, Braun AR, Carr WS, et al. Decoupling of the brain's default mode network during deep sleep. Proc Natl Acad Sci U S A 2009;106:11376–11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia [Internet]. Science 2008;322:876–880. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18988836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li K, Malhotra PA. Spatial neglect. Pract Neurol 2015;15:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


