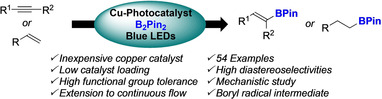
Abstract
The photocatalytic hydroboration of alkenes and alkynes is reported. The use of newly‐designed copper photocatalysts with B2Pin2 permits the formation a boryl radical, which is used for hydroboration of a large panel of alkenes and alkynes. The hydroborated products were isolated in high yields, with excellent diastereoselectivities and a high functional group tolerance under mild conditions. The hydroboration reactions were developed under continuous flow conditions to demonstrate their synthetic utility. The reaction mechanism was studied and suggested an oxidation reaction between an in situ formed borate and the Cu‐photocatalyst in its excited state for the boryl radical formation.
Keywords: boryl radical, continuous flow, copper, hydroboration, photocatalysis
We report herein the photocatalytic hydroboration of alkenes and alkynes using newly‐designed copper photocatalysts with B2Pin2. The hydroborated products were obtained under mild conditions in high yields, excellent diastereoselectivities, a high functional group tolerance and developed under continuous flow conditions to demonstrate their synthetic utility. Finally, the reaction mechanism was also studied.
Introduction
Organoboron compounds are linchpins in organic synthesis and focused particular attention over the last decades. These strategic compounds found applications as versatile building blocks in several important transformations (e.g. Suzuki–Miyaura cross‐coupling reactions, allylation reactions…) [1] and to some extend in medicinal chemistry (e.g. Velcade and Dutogliptin). Among the developed reactions to build up these strategic building blocks, [2] the celebrated hydroboration reaction, discovered by H. C. Brown in 1956, [3] is probably the most popular one. It became a textbook reaction in organic synthesis, that found applications in complex natural products synthesis or in the elaboration of marketed drugs (e.g. Nebivolol). Over the last ten years, major advances were made thanks to the development of efficient methods catalyzed by noble metals [4] and more recently by non‐noble metals, [5] to promote the addition of borohydride species on alkenes and alkynes.
Alternatively to these traditional approaches, the use of boryl radicals recently emerged as a promising approach to broaden the scope of possible transformations to build up borylated residues. [6] Key contributions in the field from Curran, Malacria, Lacôte and Fensternbank [7] demonstrated the high potential of the NHC‐borane species to generate the corresponding boryl radical from its reaction with a radical initiator (e.g. AIBN or peroxide). The addition of a boryl radical to an unsaturated C−C bond is a very recent research area, pioneered by Lalevée, [8] Curran and Taniguchi, [9] and further extended by others.[ 6 , 10 ] Very recently, an important milestone was reached, demonstrating the possible generation of these NHC‐boryl radical using photoredox catalysis with iridium complex (Scheme 1). This approach was applied to the defluoroborylation of gem‐difluorinated alkenes, [11] α‐trifluoromethylated styrenes[ 11a , 12 ] and perfluoroarenes. [11a] With respect to the hydroboration reaction, the inverse hydroboration of imines was reported by Xie and Zhu, [13] while the hydroboration of α,α‐diarylethenes and β,β‐diarylacrylates was described by Xiang, Chen and Yang. [12a] Very recently, Curran reported the 1,4‐hydroboration of electron‐deficient aromatic rings. [14] Importantly, the generation of boryl radicals from a diboron species according to a B−B cleavage, particularly from the readily available B2Pin2, is scarce and restricted to few examples.[ 6 , 15 ]
Scheme 1.
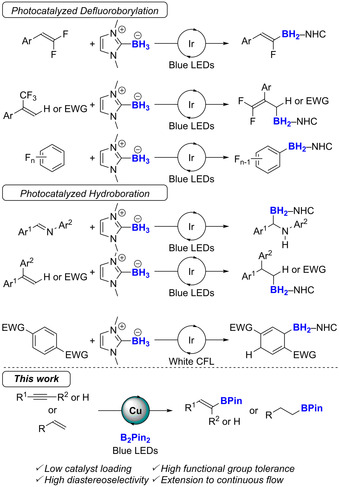
Photocatalytic generation of boryl radicals: past and present work.
Besides, the advent of photocatalysis using visible light expended possibilities to generate radicals and culminated in the discovery of new reaction manifolds.[ 16 , 17 ] As part of our program to develop new transformations using copper and copper‐photocatalysts as inexpensive and sustainable alternatives to noble metal‐based photocatalysts (Ir, Ru…), [18] we sought to develop an original and straightforward strategy to generate boryl radicals using the inexpensive and bench stable B2Pin2, under visible light irradiation. Herein, we present a copper‐photocatalyzed formation of a boryl radical (i.e. .BPin) from B2Pin2, for the hydroboration of alkynes and alkenes with high levels of chemo‐, regio‐ and stereoselectivities.
Results and Discussion
To address this novel reaction manifold, we conjectured that the cleavage of the B−B bond of the B2Pin2 could be readily achieved through the in situ formation of a borate intermediate [B2Pin2]OH− (A), in the presence of a Lewis base (e.g. HO−), according to an oxidation reaction (Scheme 2 A). Such a proposal, conceptually related to the oxidation of NHC‐borane, [7] might lead to the generation of the corresponding boryl radical. According to this scenario, a well‐designed Cu‐photocatalyst in its excited state would accept an electron from the borate species A. This oxidation followed by proton loss will promote the B−B bond cleavage and form radical anion B. Species B would be in equilibrium with a putative boryl radical C which could itself be in equilibrium with available Lewis bases, e.g., MeCN. [20] BPin radical C could add on an unsaturated C−C bond (e.g. alkene or alkyne) forming a new carbon centered radical D. DFT calculations predict the possible addition of boryl radicals to alkenes and alkynes to be kinetically and thermodynamically favorable. [19] The final product might be formed by two proposed paths from radical species D: (left path) SET from the reduced Cu‐photocatalyst, closing the photoredox catalytic cycle or (right path) by reacting with borate species A. The right path will regenerate radical anion B as the boryl radical precursor that can continue a chain reaction is globally favorable according to DFT calculations. [20]
Scheme 2.
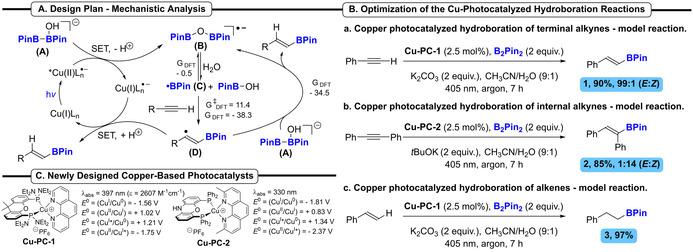
A) Design plan and mechanistic analysis with DFT‐calculated reaction energies in kcal mol−1 (R=Ph) for the proposed chain reaction. B) Development of the Cu‐photocatalyzed hydroboration reactions. C) Newly designed Cu‐photocatalysts Cu‐PC‐1 and Cu‐PC‐2. Potentials are reported vs. SCE.
To ascertain our hypothesis, we studied the reaction of phenylacetylene with B2Pin2 in the presence of a Cu‐photocatalyst, a base in a CH3CN/H2O mixture under blue light irradiation (405 nm LEDs). After an extensive set of optimization reactions, [20] we found that 2.5 mol % of the newly designed complex Cu‐PC‐1 in the presence of K2CO3 as a base in a 9:1 mixture of CH3CN/H2O furnished the hydroborated product 1 in an excellent 90 % yield and a complete E selectivity (Scheme 2 B, Eq. (a)). The hydroboration reaction with diphenyl acetylene was also developed using slightly modified conditions (Scheme 2 B, Eq. (b)). The use of the novel catalyst Cu‐PC‐2 (2.5 mol %) with tBuOK as a base under blue light irradiation gave the hydroborated product 2 in 85 % yield and a 14:1 E/Z selectivity. Finally, the optimized conditions used for the reaction with phenylacetylene permitted the hydroboration of styrene, providing the alkyl boronic ester 3 in 97 % yield (Scheme 2 B, Eq. (c)). In all cases, control experiments demonstrated that the reactions occurred exclusively in the presence of the Cu‐catalyst and light, precluding a reaction catalyzed by a Cu‐species resulting from a ligands reorganization. [20]
With these optimized protocols in hand, we explored the scope of the reactions (Scheme 3). First, a large panel of aromatic substituted terminal alkynes was tested. The reaction proved to be tolerant to a broad range of halogens and functional groups (electron‐donating and electron‐withdrawing), whatever the substitution pattern and the hydroborated products were isolated in good to excellent yields and perfect diastereoselectivities in favor of the E‐isomers (4–30). The reaction did not require the protection of alcohols and amines (9, 10 & 12). The compatibility of the method with various functional groups highlights this process as orthogonal to classical transformations to form C−C and C‐heteroatom bonds. Heterocyclic derivatives were also suitable substrates, giving the vinyl boronic esters 21 and 22 in good yields. Alkyl‐substituted alkynes were reacted and the corresponding hydroborated products (23–27) were obtained in good to excellent yields. Interestingly, the presence of a iodine substituent was well tolerated (28 & 29) as no reduction of the C−I bond was observed. [18b]
Scheme 3.
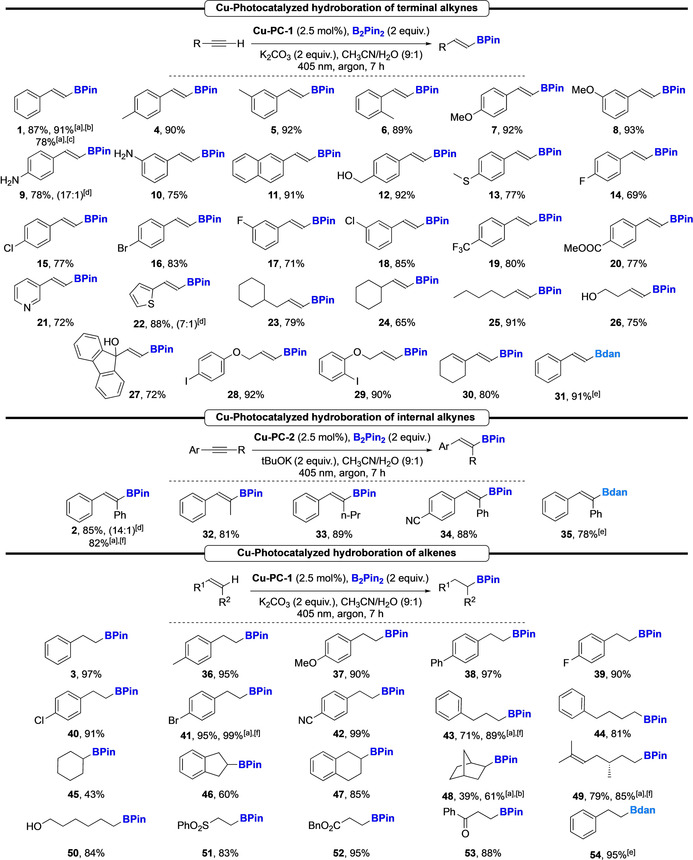
Applications to the hydroboration of alkynes and alkenes. [a] Reaction was performed in continuous flow, see supporting information for details. [b] 0.2 mmol scale. [c] 20 mmol scale. [d] E:Z ratio. [e] PinB–Bdan was used instead of B2Pin2. [f] 10 mmol scale.
The reaction was selective to the alkyne motif as no reaction on the olefin was observed when an enyne was reacted (30). With respect to the hydroboration of internal alkynes, the reaction proceeded well, with a complete diastereoselectivity (32–35) offering an interesting approach to versatile building blocks. Then, the reaction was developed on alkenes using the optimized conditions (Scheme 2 B, Eq. (c)). Various styrene derivatives were readily converted into the corresponding hydroborated products (36–42) in excellent yields and functional group tolerance. Then, alkyl substituted alkenes as well as cyclic ones were subjected to the standard conditions, affording the boronic esters 43–50 in moderate to excellent yields. The reaction on electron deficient alkenes (i.e. Michael acceptor) provided the β‐borylated sulfone, ester and ketone (51–53) in excellent yields. [21]
Interestingly, our methodology could be extended to the formation of the synthetically useful Bdan (1,8‐diaminonaphtylboramide) derivatives by using the PinB–Bdan reagent instead of B2Pin2. Under the standard reaction conditions, the synthesis of 31, 35 and 54 was achieved in excellent yields and stereoselectivities.
To showcase the synthetic utility and the versatility of our new methodologies we sought to develop a protocol under continuous flow conditions. Indeed, photocatalyzed processes often suffer from limitations regarding the increase of the reaction scale. [22] The developed protocols were applied to the hydroboration of 1, 2, 41, 43, and 49 with similar yields on 10 or 20 mmol scale. Interestingly, flow conditions applied to 43 and 48 allowed a significant increase of the reaction yields, from 71 % and 39 % to 89 % and 61 %, respectively.
Finally, to get a better understanding on the mechanism of the Cu‐photocatalyzed hydroboration reaction, control experiments were conducted (Scheme 4 A). First, the reaction of styrene or phenyl acetylene with H−BPin, instead of B2Pin2, gave no product, showing that H−Bpin is not a reactive species in the process [Eq. (1)]. Then, the addition of TEMPO (5 equiv) and BHT to the reaction of styrene with B2Pin2 was performed, giving no desired product in both reactions. However, we have been able to detect in both reactions the products resulting from the trapping of the putative boryl radical by TEMPO and BHT [Eq. (2)]. [20] These two reactions support the possible involvement of a boryl radical species during the process. The hydroboration of phenylpropyne was performed under standard conditions with either CD3CN or D2O as the co‐solvent [Eq. (3)]. The deuterated product was only observed when D2O was used instead of H2O, demonstrating that the H atom is coming from H2O. [23] However, taking into account the Bond Dissociation Energy (BDE) of both acetonitrile and water, a direct H abstraction from the solvent is unlikely, [24] hence these results could support a possible H abstraction from a transient borate species. Subsequently, we studied the kinetic isotopic effect (KIE, Scheme 4 B). Parallel reactions revealed a KIE=5.5, demonstrating that the H abstraction after the boryl radical addition to the alkyne is the rate determining step. The precise species responsible for the rate limiting H‐atom donation to the carbon‐centered radical is still unknown. Using DFT calculations, we obtained activation energies that were prohibitively high (>25 kcal mol−1) for H‐atom transfers from several boron candidates, however, the reaction is thermodynamically favored using borate A as shown in Scheme 2 A. Interestingly, the addition of HOBPin (2 equiv) to the reaction media provided a significant increase of the reaction rate. [20] This result confirms a plausible H abstraction from a transient boron species formed in the reaction media. Then, to demonstrate the involvement of a borate intermediate, reactions were carried out with the preformed K[B2Pin2]OtBu and K[B2Pin2]OH in the absence of base [Eq. (4)]. Pleasingly, the hydroborated product was isolated in 39 % and 66 % yield, respectively. These results highlight the involvement of the borate species along with its ability to react under our standard conditions. Moreover, the analysis of the B2Pin2:KOH mixture (1:1) in MeCN by cyclic voltammetry showcased an irreversible oxidation wave (E 1/2°=+0.25 V vs. SCE, Scheme 4 C). [20] This observation demonstrated the possibility of the borate species K[B2Pin2]OH to be oxidized by the catalyst in its excited state (vide supra) and support its involvement in the formation of the boryl radical (.BPin). Additional light ON–OFF reactions suggested that the formation of 1 occurred only in the presence of light. [20] To evaluate the impact of chain reactions on the mechanism, quantum yields (Φ) of the reactions were determined (Scheme 4 D). [25] The obtained values of 0.31, 0.18 and 0.48 corroborate a photocatalytic process that most likely includes short lived chain propagation as shown in Scheme 2 A. Nanosecond laser‐flash photolysis experiments of the copper photocatalyst Cu‐PC‐1 using a 355 nm laser pulse gave no clear transient absorption signals (data not shown). Combined with very weak emission spectra, these observations indicate that Cu‐PC‐1 has a short‐lived excited state (<10 ns). While the photocatalyst's short lifetime will limit the efficiency of electron transfer reactions with neighboring molecules, the possibility of a chain length increases the photon efficiency of this reaction.
Scheme 4.
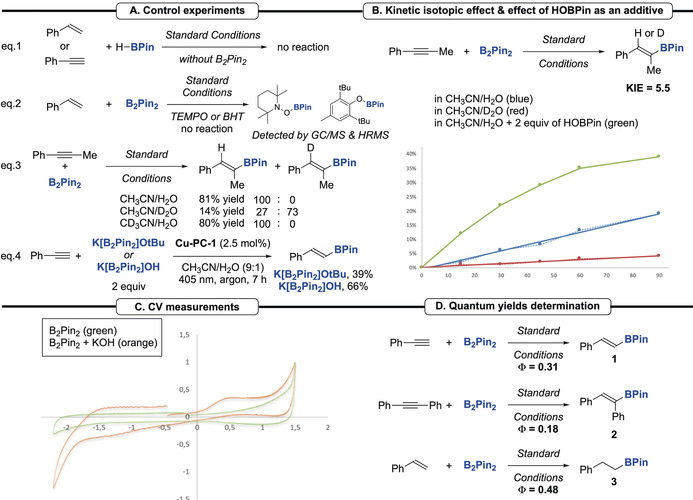
Mechanistic study. A) Control experiments. B) Kinetic isotopic effect and the kinetic effect of HOBPin as an additive. C) Cyclic voltammetry measurements. D) Quantum yields determination.
Conclusion
In conclusion, we reported herein the unprecedented photocatalytic hydroboration reactions using B2Pin2 as alternative to NHC‐borane species, by means of original copper photocatalysts. The reaction was applied to a broad range of alkynes and alkenes with an excellent functional group tolerance. The methodology was extended to continuous flow conditions to allow an easy scale up of the reaction. The mechanism of the transformation was studied and an oxidation of a transient borate species was suggested as the key step to form a possible boryl radical, involved in the hydroboration of the unsaturated C−C bond.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
This work was partially supported by Normandie Université (NU), the Région Normandie, the Centre National de la Recherche Scientifique (CNRS), Université de Rouen Normandie (URN), INSA Rouen Normandie, Labex SynOrg (ANR‐11‐LABX‐0029) and Innovation Chimie Carnot (I2C). M.Z. thanks the China Scholarship Council (CSC) for a doctoral fellowship. T.P. thanks the Institut Universitaire de France (IUF) for support and the Agence National pour la Recherche (ANR‐CE07‐0004‐1) for funding. M.F. and Y.G. acknowledge NSERC for funding in the form of a Discovery Grant (RGPIN‐2016‐06773) and a graduate scholarship, respectively. We are grateful for technical support from NanoQAM and to Gaussian and Compute Canada (http://www.computecanada.ca) for computational resources. Mrs. Rosy Valdez is warmly acknowledged for preliminary investigations regarding the batch to flow extension of the reactions. Dr. M. Durandetti is gratefully acknowledged for her help regarding CV measurements. Dr. T. Gallavardin is gratefully acknowledged for his help regarding UV/Vis measurements.
M. Zhong, Y. Gagné, T. O. Hope, X. Pannecoucke, M. Frenette, P. Jubault, T. Poisson, Angew. Chem. Int. Ed. 2021, 60, 14498.
References
- 1.For selected reviews, see:
- 1a. Carreras J., Caballero A., Pérez P. J., Chem. Asian J. 2019, 14, 329–343; [DOI] [PubMed] [Google Scholar]
- 1b. Fyfe J. W. B., Watson A. J. B., Chem 2017, 3, 31–55; [Google Scholar]
- 1c. Miyaura N., Suzuki A., Chem. Rev. 1995, 95, 2457–2483; [Google Scholar]
- 1d. Xu L., Zhang S., Li P., Chem. Soc. Rev. 2015, 44, 8848–8858; [DOI] [PubMed] [Google Scholar]
- 1e. Lennox A. J. J., Lloyd-Jones G. C., Chem. Soc. Rev. 2014, 43, 412–443; [DOI] [PubMed] [Google Scholar]
- 1f. Duret G., Quinlan R., Bisseret P., Blanchard N., Chem. Sci. 2015, 6, 5366–5382; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1g. Ollivier C., Renaud P., Chem. Rev. 2001, 101, 3415–3434; [DOI] [PubMed] [Google Scholar]
- 1h. Namirembe S., Morken J. P., Chem. Soc. Rev. 2019, 48, 3464–3474. For the importance of boron-containing building blocks in medicinal chemistry, see: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1i. Roughley S. D., Jordan A. M., J. Med. Chem. 2011, 54, 3451–3479. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a. Friese F. W., Studer A., Chem. Sci. 2019, 10, 8503–8518; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2b. Neeve E. C., Geier S. J., Mkhalid I. A. I., Westcott S. A., Marder T. B., Chem. Rev. 2016, 116, 9091–9161; [DOI] [PubMed] [Google Scholar]
- 2c. Ishiyama T., Miyaura N., Chem. Rec. 2004, 3, 271–280; [DOI] [PubMed] [Google Scholar]
- 2d. Mkhalid I. A. I., Barnard J. H., Marder T. B., Murphy J. M., Hartwig J. F., Chem. Rev. 2010, 110, 890–931; [DOI] [PubMed] [Google Scholar]
- 2e. Ros A., Fernández R., Lassaletta J. M., Chem. Soc. Rev. 2014, 43, 3229–3243; [DOI] [PubMed] [Google Scholar]
- 2f. Snieckus V., Chem. Rev. 1990, 90, 879–933. [Google Scholar]
- 3. Brown H. C., Tetrahedron 1961, 12, 117–138. [Google Scholar]
- 4.
- 4a. Burgess K., Ohlmeyer M. J., Chem. Rev. 1991, 91, 1179–1191; [Google Scholar]
- 4b. Crudden C. M., Edwards D., Eur. J. Org. Chem. 2003, 4695–4712; [Google Scholar]
- 4c. Vogels C. M., Westcott S. A., Curr. Org. Chem. 2005, 9, 687–699; [Google Scholar]
- 4d. Chen J.-B., Whiting A., Synthesis 2018, 50, 3843–3861. [Google Scholar]
- 5. Obligacion J. V., Chirik P. J., Nat. Rev. Chem. 2018, 2, 15–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taniguchi T., Eur. J. Org. Chem. 2019, 6308–6319. [Google Scholar]
- 7.For a review, see:
- 7a. Curran D. P., Solovyev A., Makhlouf Brahmi M., Fensterbank L., Malacria M., Lacôte E., Angew. Chem. Int. Ed. 2011, 50, 10294–10317; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 10476–10500; [Google Scholar]
- 7b. Ueng S.-H., Brahmi M. M., Derat E., Fensterbank L., Lacôte E., Malacria M., Curran D. P., J. Am. Chem. Soc. 2008, 130, 10082–10083; [DOI] [PubMed] [Google Scholar]
- 7c. Ueng S.-H., Solovyev A., Yuan X., Geib S. J., Fensterbank L., Lacôte E., Malacria M., Newcomb M., Walton J. C., Curran D. P., J. Am. Chem. Soc. 2009, 131, 11256–11262. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. Lalevée J., Blanchard N., Chany A.-C., Tehfe M.-A., Allonas X., Fouassier J.-P., J. Phys. Org. Chem. 2009, 22, 986–993; [Google Scholar]
- 8b. Lalevée J., Tehfe M.-A., Allonas X., Fouassier J.-P., Macromolecules 2008, 41, 9057–9062. [Google Scholar]
- 9.
- 9a. Watanabe T., Hirose D., Curran D. P., Taniguchi T., Chem. Eur. J. 2017, 23, 5404–5409; [DOI] [PubMed] [Google Scholar]
- 9b. Watanabe T., Geib S. J., Curran D. P., Taniguchi T., J. Org. Chem. 2017, 82, 13034–13042; [DOI] [PubMed] [Google Scholar]
- 9c. Shimoi M., Watanabe T., Maeda K., Curran D. P., Taniguchi T., Angew. Chem. Int. Ed. 2018, 57, 9485–9490; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 9629–9634. [Google Scholar]
- 10.For recent examples, see:
- 10a. Jin J.-K., Zheng W.-X., Xia H.-M., Zhang F.-L., Wang Y.-F., Org. Lett. 2019, 21, 8414–8418; [DOI] [PubMed] [Google Scholar]
- 10b. Liu X., Lin E. E., Chen G., Li J.-L., Liu P., Wang H., Org. Lett. 2019, 21, 8454–8458; [DOI] [PubMed] [Google Scholar]
- 10c. Shimoi M., Maeda K., Geib S. J., Curran D. P., Taniguchi T., Angew. Chem. Int. Ed. 2019, 58, 6357–6361; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 6423–6427; [Google Scholar]
- 10d. Huang Y.-S., Wang J., Zheng W.-X., Zhang F.-L., Yu Y.-J., Zheng M., Zhou X., Wang Y.-F., Chem. Commun. 2019, 55, 11904–11907; [DOI] [PubMed] [Google Scholar]
- 10e. Ren S.-C., Zhang F.-L., Xu A.-Q., Yang Y., Zheng M., Zhou X., Fu Y., Wang Y.-F., Nat. Commun. 2019, 10, 1934; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10f. Jin J.-K., Zhang F.-L., Zhao Q., Lu J.-A., Wang Y.-F., Org. Lett. 2018, 20, 7558–7562. [DOI] [PubMed] [Google Scholar]
- 11.
- 11a. Xu W., Jiang H., Leng J., Ong H. W., Wu J., Angew. Chem. Int. Ed. 2020, 59, 4009–4016; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 4038–4045; [Google Scholar]
- 11b. Chen G., Wang L., Liu X., Liu P., Adv. Synth. Catal. 2020, 362, 2990–2996. [Google Scholar]
- 12.
- 12a. Xia P.-J., Song D., Ye Z.-P., Hu Y.-Z., Xiao J.-A., Xiang H.-Y., Chen X.-Q., Yang H., Angew. Chem. Int. Ed. 2020, 59, 6706–6710; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 6772–6776; [Google Scholar]
- 12b. Qi J., Zhang F.-L., Jin J.-K., Zhao Q., Li B., Liu L. X., Wang Y.-F., Angew. Chem. Int. Ed. 2020, 59, 12876–12884; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 12976–12984. [Google Scholar]
- 13. Zhou N., Yuan X.-A., Zhao Y., Xie J., Zhu C., Angew. Chem. Int. Ed. 2018, 57, 3990–3994; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 4054–4058. [Google Scholar]
- 14. Dai W., Geib S. J., Curran D. P., J. Am. Chem. Soc. 2020, 142, 6261–6267. [DOI] [PubMed] [Google Scholar]
- 15.
- 15a. Wang G., Zhang H., Zhao J., Li W., Cao J., Zhu C., Li S., Angew. Chem. Int. Ed. 2016, 55, 5985–5989; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 6089–6093; [Google Scholar]
- 15b. Xu R., Lu G.-P., Cai C., New J. Chem. 2018, 42, 16456–16459. [Google Scholar]
- 16.For selected reviews, see:
- 16a. Prier C. K., Rankic D. A., MacMillan D. W. C., Chem. Rev. 2013, 113, 5322–5363; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16b. Twilton J., Le C. C., Zhang P., Shaw M. H., Evans R. W., MacMillan D. W. C., Nat. Rev. Chem. 2017, 1, 0052; [Google Scholar]
- 16c. Skubi K. L., Blum T. R., Yoon T. P., Chem. Rev. 2016, 116, 10035–10074; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16d. Schultz D. M., Yoon T. P., Science 2014, 343, 985–985; [DOI] [PMC free article] [PubMed] [Google Scholar]; Yoon T. P., Ischay M. A., Du J., Nat. Chem. 2010, 2, 527–532; [DOI] [PubMed] [Google Scholar]
- 16e. Romero N. A., Nicewicz D. A., Chem. Rev. 2016, 116, 10075–10166; [DOI] [PubMed] [Google Scholar]
- 16f. Davies J., Morcillo S. P., Douglas J. J., Leonori D., Chem. Eur. J. 2018, 24, 12154–12163; [DOI] [PubMed] [Google Scholar]
- 16g. Narayanam J. M. R., Stephenson C. R. J., Chem. Soc. Rev. 2010, 40, 102–113. [DOI] [PubMed] [Google Scholar]
- 17.For selected reviews regarding the development of Cu-photocatalyzed reactions, see:
- 17a. Reiser O., Acc. Chem. Res. 2016, 49, 1990–1996; [DOI] [PubMed] [Google Scholar]
- 17b. Paria S., Reiser O., ChemCatChem 2014, 6, 2477–2483; [Google Scholar]
- 17c. Hossain A., Bhattacharyya A., Reiser O., Science 2019, 364, eaav9713; [DOI] [PubMed] [Google Scholar]
- 17d. Hernandez-Perez A. C., Collins S. K., Acc. Chem. Res. 2016, 49, 1557–1565; [DOI] [PubMed] [Google Scholar]
- 17e. Zhong M., Pannecoucke X., Jubault P., Poisson T., Beilstein J. Org. Chem. 2020, 16, 451–481; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17f. Larsen C. B., Wenger O. S., Chem. Eur. J. 2018, 24, 2039–2058; [DOI] [PubMed] [Google Scholar]
- 17g. Wenger O. S., J. Am. Chem. Soc. 2018, 140, 13522–13533. [DOI] [PubMed] [Google Scholar]
- 18.
- 18a. Brégent T., Bouillon J.-P., Poisson T., Org. Lett. 2020, 22, 7688–7693; [DOI] [PubMed] [Google Scholar]
- 18b. Poutrel P., Ivanova M. V., Pannecoucke X., Jubault P., Poisson T., Chem. Eur. J. 2019, 25, 15262–15266; [DOI] [PubMed] [Google Scholar]
- 18c. Nitelet A., Thevenet D., Schiavi B., Hardouin C., Fournier J., Tamion R., Pannecoucke X., Jubault P., Poisson T., Chem. Eur. J. 2019, 25, 3262–3266; [DOI] [PubMed] [Google Scholar]
- 18d. Ivanova M. V., Bayle A., Besset T., Pannecoucke X., Poisson T., Chem. Eur. J. 2017, 23, 17318–17338; [DOI] [PubMed] [Google Scholar]
- 18e. Ivanova M. V., Bayle A., Besset T., Pannecoucke X., Poisson T., Angew. Chem. Int. Ed. 2016, 55, 14141–14145; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 14347–14351; [Google Scholar]
- 18f. Ivanova M. V., Bayle A., Besset T., Poisson T., Pannecoucke X., Angew. Chem. Int. Ed. 2015, 54, 13406–13410; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 13604–13608; [Google Scholar]
- 18g. Bayle A., Cocaud C., Nicolas C., Martin O. R., Poisson T., Pannecoucke X., Eur. J. Org. Chem. 2015, 3787–3792; [Google Scholar]
- 18h. Belhomme M.-C., Bayle A., Poisson T., Pannecoucke X., Eur. J. Org. Chem. 2015, 1719–1726; [Google Scholar]
- 18i. Belhomme M.-C., Poisson T., Pannecoucke X., Org. Lett. 2013, 15, 3428–3431. [DOI] [PubMed] [Google Scholar]
- 19.All calculations were performed using Gaussian 16 with B3LYP/6–311+g(2d,2p)//CPCM(ACN). See supporting information for details.
- 20.See supporting information for details.
- 21.During the preparation of this manuscript a photocatalytic hydroboration of acrylamide with NHC-borane was reported, see: Zhu C., Dong J., Liu X., Gao L., Zhao Y., Xie J., Li S., Zhu C., Angew. Chem. Int. Ed. 2020, 59, 12817–12821; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 12917–12921. [Google Scholar]
- 22. Cambié D., Bottecchia C., Straathof N. J. W., Hessel V., Noël T., Chem. Rev. 2016, 116, 10276–10341. [DOI] [PubMed] [Google Scholar]
- 23.Note that control experiments showed that the deuteration did not occur after the formation of the product, see supporting information for details.
- 24.BDE H-OH=119 kcal mol−1 and BDE H-CH2CN=92 kcal mol−1.
- 25.The quantum yields were determined according to the reported procedures, see:
- 25a. Cismesia M. A., Yoon T. P., Chem. Sci. 2015, 6, 5426–5434; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25b. Ma J., Schäfers F., Daniliuc C., Bergander K., Strassert C. A., Glorius F., Angew. Chem. Int. Ed. 2020, 59, 9639–9645; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 9726–9732. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


