CONTENTS
| 1. Executive summary | 7 |
| 2. Target audience | 11 |
| 3. Assessment of quality of evidence and grading of strength of recommendations | 12 |
| 4. Fetal growth restriction: background, definition, etiology, and risks | 13 |
| 4.1. Background | 13 |
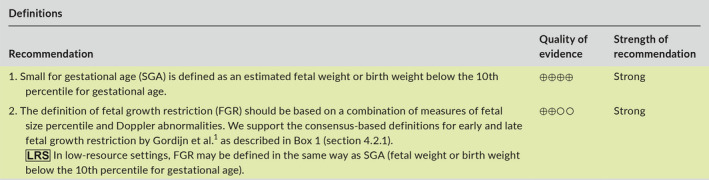
| 4.2. Terminology and definitions | 13 |
| 4.2.1. Consensus‐based definition of placenta‐related fetal growth restriction | 13 |
| 4.2.2. Early‐ versus late‐onset fetal growth restriction | 13 |
| 4.3. Etiology of fetal growth restriction | 16 |
| 4.4. Risks associated with fetal growth restriction | 16 |
| 4.5. Recommendations | 17 |
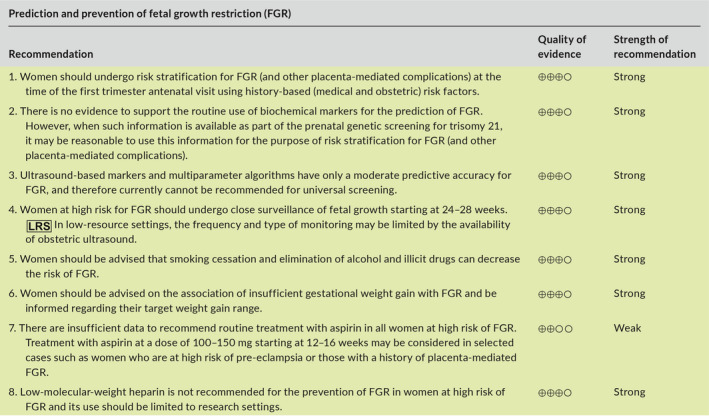
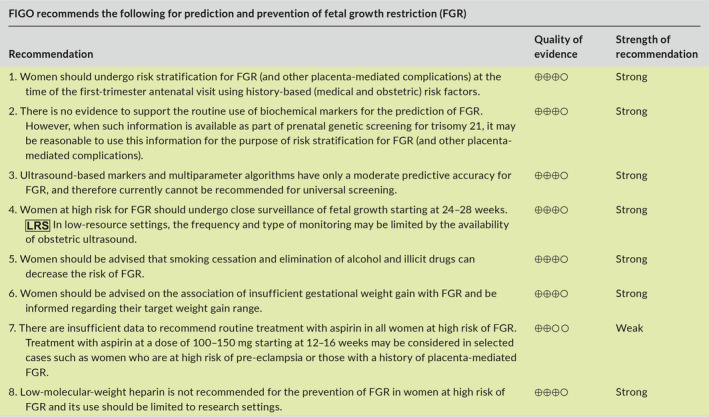
| 5. Early prediction and prevention of fetal growth restriction | 18 |
| 5.1. History‐based risk factors | 18 |
| 5.2. Biochemical markers | 18 |
| 5.3. Ultrasound markers | 19 |
| 5.4. Prediction models | 19 |
| 5.5. Prevention of fetal growth restriction in high‐risk populations | 20 |
| 5.5.1. Lifestyle modifications | 20 |
| 5.5.2. Medical interventions | 20 |
| 5.6. Recommendations | 21 |
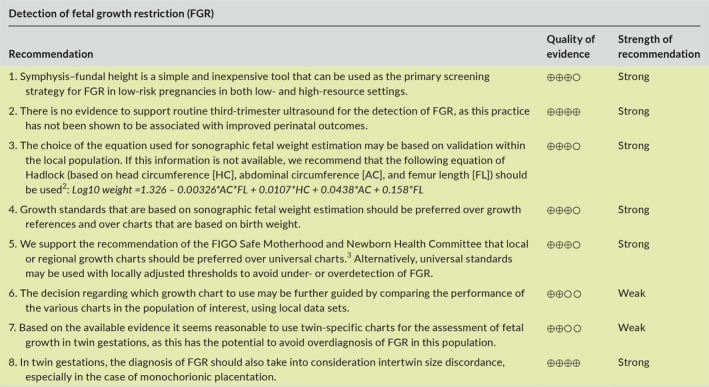
| 6. Detection of fetal growth restriction | 22 |
| 6.1. Symphysis–fundal height | 22 |
| 6.2. Sonographic fetal weight estimation | 22 |
| 6.3. Is there a role for routine third‐trimester ultrasound to assess fetal growth? | 22 |
| 6.4. Which growth chart should be used to determine fetal weight percentile? | 23 |
| 6.4.1. Growth references versus growth standards | 23 |
| 6.4.2. Charts based on birth weight versus sonographic fetal weight estimation | 23 |
| 6.4.3. Universal versus customized charts | 24 |
| 6.4.4. Description of commonly available charts | 24 |
| 6.4.5. How to choose the best chart | 25 |
| 6.5. How to assess fetal growth in twin gestations | 26 |
| 6.6. Recommendations | 27 |
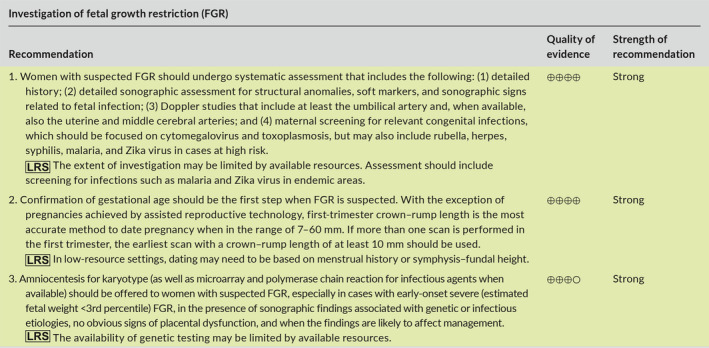
| 7. What kind of investigations should be performed when fetal growth restriction is suspected? | 28 |
| 7.1. Detailed history | 28 |
| 7.2. Detailed anatomy scan | 28 |
| 7.3. Doppler studies | 28 |
| 7.4. Additional testing | 28 |
| 7.5. Recommendations | 29 |
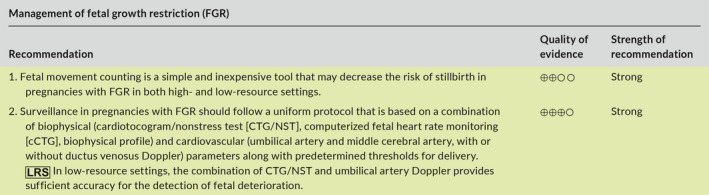
| 8. Management of pregnancies with fetal growth restriction | 30 |
| 8.1. Monitoring | 30 |
| 8.1.1. Fetal movement counting | 30 |
| 8.1.2. Fetal heart rate monitoring | 30 |
| 8.1.3. Computerized fetal heart rate monitoring | 32 |
| 8.1.4. Ultrasound measurement of amniotic fluid volume | 33 |
| 8.1.5. Biophysical profile scoring | 33 |
| 8.1.6. Umbilical artery Doppler | 33 |
| 8.1.7. Cerebral artery Doppler | 33 |
| 8.1.8. Ductus venosus Doppler | 34 |
| 8.1.9. Surveillance strategy | 34 |
| 8.2. Timing of delivery | 35 |
| 8.2.1. Gestational age‐related risks in fetal growth restriction | 35 |
| 8.2.2. Gestational age‐related management strategy | 35 |
| 8.2.3. Absolute delivery criteria for fetal growth restriction (independent of gestational age) | 35 |
| 8.2.4. Relative delivery criteria for fetal growth restriction (adjusted for gestational age) | 36 |
| 8.3. Mode of delivery and intrapartum considerations | 36 |
| 8.4. Medical interventions | 37 |
| 8.4.1. Antenatal corticosteroids | 37 |
| 8.4.2. Magnesium sulfate for neuroprotection | 37 |
| 8.4.3. Treatments under investigation | 37 |
| 8.5. Recommendations | 38 |
| 9. Postpartum assessment and counselling for future pregnancies | 40 |
| 9.1. Infant follow‐up | 40 |
| 9.2. Maternal follow‐up | 40 |
| 9.3. Counselling regarding future pregnancies | 40 |
| 9.3.1. Risk of recurrence based on severity and onset | 40 |
| 9.3.2. Risk of recurrence based on placental histopathology | 41 |
| 9.3.3. Role of thrombophilia screening | 41 |
| 9.3.4. Preconception counselling and management of future pregnancies | 41 |
| 9.4. Recommendations | 43 |
| 10. Summary and future research directions | 44 |
| 11. References | 45 |
1. EXECUTIVE SUMMARY
Fetal growth restriction (FGR) is defined as the failure of the fetus to meet its growth potential due to a pathological factor, most commonly placental dysfunction. Worldwide, FGR is a leading cause of stillbirth, neonatal mortality, and short‐ and long‐term morbidity. Ongoing advances in clinical care, especially in definitions, diagnosis, and management of FGR, require efforts to effectively translate these changes to the wide range of obstetric care providers. This article highlights agreements based on current research in the diagnosis and management of FGR, and the areas that need more research to provide further clarification of recommendations.
The purpose of this article is to provide a comprehensive summary of available evidence along with practical recommendations concerning the care of pregnancies at risk of or complicated by FGR, with the overall goal to decrease the risk of stillbirth and neonatal mortality and morbidity associated with this condition. To achieve these goals, FIGO (the International Federation of Gynecology and Obstetrics) brought together international experts to review and summarize current knowledge of FGR.
This summary is directed at multiple stakeholders, including healthcare providers, healthcare delivery organizations and providers, FIGO member societies, and professional organizations. Recognizing the variation in the resources and expertise available for the management of FGR in different countries or regions, this article attempts to take into consideration the unique aspects of antenatal care in low‐resource settings (labelled “LRS” in the recommendations). This was achieved by collaboration with authors and FIGO member societies from low‐resource settings such as India, Sub‐Saharan Africa, the Middle East, and Latin America.
Aspects of FGR addressed in this article include prediction, diagnosis, investigation, management, and postpartum counselling. The main recommendations are given below and are summarized in Table 1 (section 8) and in the management algorithms for high‐resource settings (Figure 1a) and low‐resource settings (Figure 1b) (section 4).
TABLE 1.
Recommendations for monitoring, timing, and mode of delivery in cases with suspected fetal growth restriction.
| Findings | Risk of stillbirth | Suggested monitoring a | Timing and mode of delivery b |
|---|---|---|---|
| SGA (EFW at 3rd–9th percentile, normal fluid and Doppler studies) | Low |
|
|
| Uncomplicated FGR at <3rd percentile (normal fluid and Doppler studies) | Low |
|
|
|
FGR with mild abnormalities:
|
Low |
|
|
| FGR with umbilical artery AEDV/REDV |
|
||
| FGR with abnormal ductus venosus Doppler |
|
|
Abbreviations: AEDV/REDV, absent or reversed diastolic velocity in the umbilical artery; BPP, biophysical profile; CPR, cerebroplacental ratio; DV, ductus venosus; FGR, fetal growth restriction; MCA, middle cerebral artery; NST, nonstress test; OR, odds ratio; PI, pulsatility index; PIV, pulsatility index for veins; SGA, small for gestational age; UA, umbilical artery; UtA, uterine artery.
Monitoring should be based on integration of multiple modalities (Doppler, BPP, NST).
Absolute indications for delivery at any gestational age and birth weight combination that are considered to be viable include: BPP or NST abnormalities or severe pre‐eclampsia with uncontrolled hypertension or end‐organ damage (section 8.2.3). In addition, timing of delivery should be individualized based on factors such as parental decision regarding threshold for intervention.
There is lack of evidence on the appropriate test to predict the risk of fetal deterioration and on the optimal monitoring strategy in cases of uncomplicated SGA fetuses, especially at term. Given this, there are differences in practice in various regions of the world regarding use of BPP/NST for fetal monitoring in this context, and some of the authors of these guidelines do not use BPP or NST for monitoring of fetuses with uncomplicated SGA as long as Doppler studies are normal. We suggest that the decision regarding use of BPP/NST should be based on local practices, the risk profile of the local population, and the available resources in each particular setting.
Timing should be individualized based on local neonatal outcomes. Before 26 weeks, careful and shared decision making with the parents and neonatology team is recommended.
FIGURE 1A.

Approach to screening, diagnosis, and management of fetal growth restriction in high‐resource settings. Abbreviations: FGR, fetal growth restriction; NST, nonstress test; PCR, polymerase chain reaction; SFH, symphysis–fundal height.
FIGURE 1B.
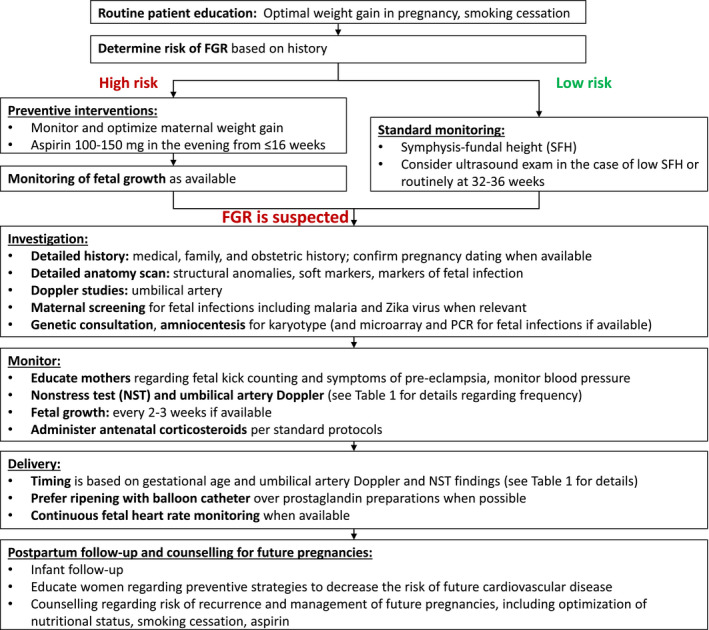
Approach to screening, diagnosis, and management of fetal growth restriction in low‐resource settings. Abbreviations: FGR, fetal growth restriction; NST, nonstress test; PCR, polymerase chain reaction; SFH, symphysis–fundal height.


2. TARGET AUDIENCE
This article is directed at multiple stakeholders with the intention of bringing attention to the assessment of fetal growth, with a particular focus on the screening, diagnosis, and management of FGR, which is a leading cause of stillbirth and neonatal mortality and morbidity. This article proposes to standardize and provide guidance for the screening, prevention, diagnosis, and management of FGR.
The intended target audience includes:
Healthcare providers: all those qualified to care for pregnant women (obstetricians, maternal‐fetal medicine specialists, general practitioners, midwives, nurses, advance practice clinicians, radiologists, sonographers, pediatricians, and neonatologists).
Healthcare delivery organizations and providers: governments, federal and state legislators, healthcare management organizations, health insurance organizations, international development agencies, and nongovernmental organizations.
Professional organizations: international, regional, and national professional organizations of obstetricians and gynecologists, obstetric ultrasound, family practitioners, pediatricians, neonatologists, and worldwide national organizations dedicated to the care of pregnant women and their offspring.
3. ASSESSMENT OF QUALITY OF EVIDENCE AND GRADING OF STRENGTH OF RECOMMENDATIONS
In assessing the quality of evidence and grading of strength of recommendations, the article follows the terminology proposed by the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group. 5 This system uses consistent language and graphical descriptions for the strength and quality of the recommendations and the evidence on which they are based.
Recommendations are classified as strong or conditional (weak) (Table S1). 6 The strength of recommendation is dependent not only on the quality of evidence, but also on factors such as risk–benefit, cost, resource allocation, values, and preferences. Thus, some recommendations may be based on low‐quality evidence but still represent a benefit that outweighs the risks and burdens, and therefore may be strongly recommended.
The overall quality of evidence was assessed for each of the recommendations and expressed using four levels of quality: very low, low, moderate, and high (Table S2). 7 Considerations for quality of evidence include primarily the study design and methodology. As such, evidence based on randomized controlled trials is considered high‐quality evidence, observational studies provide moderate or low quality of evidence, and all others are very low. However, other parameters must be considered while assessing the level of evidence: risk of bias, study limitations, consistency of results, precision, publication bias, indirectness of evidence, and scarcity of evidence. For the quality of evidence, cross‐filled circles are used: ⊕○○○ denotes very low‐quality evidence; ⊕⊕○○ low quality; ⊕⊕⊕○ moderate quality; and ⊕⊕⊕⊕ high‐quality evidence.
4. FETAL GROWTH RESTRICTION: BACKGROUND, DEFINITION, ETIOLOGY, AND RISKS
4.1. Background
FGR is a common pregnancy complication that worldwide is a leading cause of stillbirth, neonatal mortality, and short‐ and long‐term neonatal morbidity. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 The definition, diagnosis, and optimal management of FGR have generated controversy as clinicians strive for more harmonized care.
The purpose of this article is to provide a summary of the available evidence and provide recommendations regarding the early prediction and prevention, diagnosis, investigation, monitoring, and timing of delivery of pregnancies complicated by FGR, with the overall goal to decrease the risk of stillbirth and neonatal mortality and morbidity associated with this pregnancy complication. Given the variation in resources and expertise available for the assessment and monitoring of pregnancies complicated by FGR in different countries or regions, we have included, in addition to the standard of care or “best” recommendations, specific recommendations for low‐resource settings, which are marked as  in the recommendation tables. Management algorithms for women in high‐resource and low‐resource settings are summarized in Figure 1a,b, respectively.
in the recommendation tables. Management algorithms for women in high‐resource and low‐resource settings are summarized in Figure 1a,b, respectively.
4.2. Terminology and definitions
FGR is defined as the failure of the fetus to meet its growth potential due to a pathological factor, most commonly placental dysfunction. Clinically, this is reflected by a drop in fetal size percentiles over the course of gestation. However, fetal growth potential is difficult to determine, and serial assessments of fetal size to detect a drop in fetal weight percentile are usually not available. Instead, care providers most commonly have only a “snapshot” of fetal weight estimation at a given point in time. Therefore, in clinical practice, small for gestational age (SGA), defined as estimated fetal weight (EFW) or abdominal circumference below a certain threshold such as the 10th or 3rd percentile, is most commonly used to suspect FGR.
The use of SGA as a proxy for FGR has several limitations that need to be recognized. First, most SGA fetuses are constitutionally healthy small fetuses, whose smallness is merely the result of their predetermined growth potential (i.e. false‐positive diagnosis of FGR). Second, some growth‐restricted fetuses, depending on their original growth potential and timing of insult, may remain above the percentile threshold described above and may thus not be SGA (i.e. false‐negative diagnosis of FGR). Third, the use of SGA as a proxy for FGR is limited by the accuracy of sonographic fetal weight estimation, which has an estimation error of up to ±15%–20%. Finally, the diagnosis of SGA is highly dependent on the growth chart being used, which can therefore have a considerable effect on the proportion of fetuses or infants flagged as SGA in a given population.
It should be noted that there is inconsistency in the literature regarding the terminology described above, where some use the term FGR to describe a fetus with an estimated weight below the 10th percentile for gestational age and the term SGA to describe an infant with birth weight below the 10th percentile for gestational age. However, for the purpose of this article, the term SGA is used to indicate an EFW or birth weight below the 10th percentile for gestational age, and the term FGR to refer to a small fetus that has failed to achieve its growth potential because of a pathologic process.
4.2.1. Consensus‐based definition of placenta‐related FGR
The major member societies of FIGO follow a definition using the 10th percentile as a means of diagnosing an SGA fetus, which then leads to further testing, assessment, and follow‐up. There are proposals to address the limitations of this definition, but their validity regarding reduction in adverse outcomes needs to be tested. For example, in an attempt to overcome some of the limitations described above, a consensus‐based definition for placenta‐mediated FGR has been proposed via a Delphi procedure. 1 To decrease the likelihood of false‐positive and false‐negative diagnosis of FGR, the consensus definition was based on a combination of measures of fetal size (fetal weight estimation and abdominal circumference) and abnormal Doppler findings in the umbilical, uterine, and middle cerebral arteries, as described in Box 1. The implementation of this definition is limited by the lack of a recommendation on which growth chart should be used to define the 10th and 3rd percentiles for EFW and fetal abdominal circumference. In addition, further research is needed to correlate this definition with adverse perinatal outcomes.
Box 1. Consensus‐based definitions for early and late fetal growth restriction.
|
Early‐onset FGR (<32 weeks)
|
Late‐onset FGR (≥32 weeks)
|
Abbreviations: AC, fetal abdominal circumference; AREDV, absent or reversed end‐diastolic velocity; CPR, cerebroplacental ratio; EFW, estimated fetal weight; PI, pulsatility index; UA, umbilical artery; UtA, uterine artery. Adapted from Gordijn et al. 1
4.2.2. Early‐ versus late‐onset FGR
It has been suggested that FGR should be broadly classified, based on gestational age at the time of diagnosis, into early‐onset FGR (<32 weeks) and late‐onset FGR (≥32 weeks). The rationale underlying this classification is based on differences between these two phenotypes of FGR in severity, natural history, Doppler findings, association with hypertensive complications, placental findings, and management. 16 , 17 , 18
Early‐onset FGR has a prevalence of 0.5%–1%, is usually more severe, and is more likely to be associated with abnormal umbilical artery Doppler than late‐onset FGR. The underlying placental pathology is frequently similar to that observed in cases of early‐onset pre‐eclampsia (maternal vascular malperfusion), which explains the strong association of early‐onset FGR with pre‐eclampsia. Therefore, early‐onset FGR is usually easier to detect, and the natural history tends to follow a predictable sequence of Doppler changes in the umbilical artery and ductus venosus. The main challenge in cases of early‐onset FGR is management (i.e. timing of delivery), by attempting to determine the optimal balance between the opposing risks of stillbirth and prematurity. 19
Late‐onset FGR is more common than early‐onset FGR with a prevalence of 5%–10%. In contrast to early‐onset FGR, it is usually milder, is less likely to be associated with pre‐eclampsia, and is usually associated with normal umbilical artery Doppler. Therefore, the main challenge with regard to late‐onset FGR is diagnosis, while management (i.e. delivery) is relatively simple given that the diagnosis is commonly made during the late‐preterm or term periods, where the risks associated with delivery are relatively small. The diagnosis of late‐onset FGR mainly relies on adaptive changes in the cerebral circulation (“redistribution” or “brain‐sparing effect”), which is reflected by low resistance to flow in the middle cerebral artery thereby generating a low cerebroplacental ratio, as described in section 8.1.7. Given that the umbilical artery and ductus venosus Doppler studies are usually normal in cases of late‐onset FGR, the natural history in these cases is less predictable and there is a risk of sudden decompensation and stillbirth. 16 , 19
4.3. Etiology of fetal growth restriction
FGR is often the result of one or more maternal, placental, or fetal disorders that interfere with the normal mechanisms regulating fetal growth. 20 , 21 The most common etiologies of FGR are listed in Box 2. It is important to note that there is often confusion in the literature between “etiologies” (or pathogenetic pathways) and “risk factors” for FGR. For example, although maternal conditions such as chronic hypertension, kidney disease, systemic lupus erythematosus, and long‐standing diabetes are often listed as “maternal etiologies” for FGR, these conditions should probably be viewed instead as maternal risk factors for abnormal placentation that may result in placenta‐mediated FGR.
Box 2. Common etiologies for fetal growth restriction.
|
Given that maternal nutrition and fetal growth are closely related, 22 , 23 maternal undernutrition is an important cause of FGR worldwide. 24 , 25 , 26 The impact of maternal undernutrition on fetal growth depends on its timing and severity. 20 To date, maternal interventions in dietary advice and modifications have lacked significant success in preventing FGR. While the mechanisms by which maternal anemia contribute to FGR are unclear, both impaired nutrient transport to the fetus 27 and abnormal placental adaptation to low maternal hemoglobin 28 have been suggested as potential mechanisms.
Abnormal placentation is a common cause of FGR, 29 which is often diagnosed by ultrasound Doppler studies 30 and typical histopathological placental findings. 31 , 32 , 33
Chromosomal abnormalities have been suggested to contribute to up to 5% of FGR cases; triploidy and trisomy 13 and 18 are important considerations in early‐onset FGR and the risk of many aneuploidies is higher in the presence of structural fetal anomalies. 34 , 35 , 36 In 1%–6% of cases of FGR with normal karyotype, submicroscopic (micro) duplications/deletions can be found using chromosomal microarray analysis, 35 even when FGR is an apparently isolated finding. 37 FGR is also more prevalent in fetuses with structural malformations, and the risk increases when multiple anomalies are present. 38
FGR is related to intrauterine infection in up to 5% of cases. 20 , 39 Viral agents such as rubella, cytomegalovirus, HIV, and Zika are common causes of infection‐related FGR. 40 , 41 , 42 , 43 , 44 Protozoan infections like toxoplasmosis and malaria are another important cause, especially in endemic areas. 45 , 46 The main mechanism involved in the pathogenesis of FGR in these cases is a decline in cell population. 20 Finally, maternal exposure to teratogens such as radiation, 47 illicit drugs, 48 , 49 and alcohol 50 is another important etiology for FGR.
4.4. Risks associated with fetal growth restriction
The main short‐ and long‐term risks associated with FGR are listed in Box 3. It is associated with both fetal and obstetric complications. The most devastating complication is stillbirth, 51 , 52 , 53 and there is a well‐established inverse relationship between weight percentile and the risk of stillbirth, 54 , 55 , 56 , 57 which is more pronounced in the early preterm period than at term. 58 FGR is an important cause of iatrogenic preterm birth, 59 as early delivery remains the main and perhaps only strategy for the prevention of stillbirth in cases of severe FGR. 16 , 60 FGR is also an independent risk factor for spontaneous preterm birth. 61 Other obstetric complications associated with FGR include pre‐eclampsia and placental abruption, as the pathophysiology of these conditions is often closely related. 29 , 30 , 62 , 63 , 64 , 65 , 66
Box 3. Risks associated with fetal growth restriction.
|
Despite ongoing improvements in neonatal care, FGR is associated with increased neonatal mortality and short‐term morbidity. The risk of perinatal mortality in term FGR is reported to be five‐ to 10‐fold higher than in appropriately grown neonates. 57 , 61 , 67 The severity of FGR, Doppler abnormalities, and associated prematurity are independent predictors of neonatal complications. 68 Among preterm infants, the co‐presence of FGR further increases the risk of certain prematurity‐related complications such as respiratory morbidity, intraventricular hemorrhage, necrotizing enterocolitis, and metabolic disorders. 57 Among term infants, FGR increases the risks of low cord artery pH, 69 low Apgar score, 69 and neonatal complications such as hypoglycemia, hypothermia, and jaundice. 70 , 71 , 72
Growth‐restricted infants are also at risk of long‐term complications including neurodevelopmental impairment 11 , 73 , 74 , 75 , 76 , 77 , 78 and noncommunicable diseases. 15 , 79 , 80 , 81 , 82 This is discussed in greater detail in section 9.1 (Infant follow‐up).
4.5. Recommendations

5. EARLY PREDICTION AND PREVENTION OF FETAL GROWTH RESTRICTION
Early prediction of FGR is important as it can identify women at high risk of FGR who may benefit from preventive interventions and close monitoring during pregnancy. Box 4 lists the most common risk factors for FGR. While the predictive value of individual risk factors is low, clinical prediction models that are based on combinations of the risk factors outlined below can considerably improve the prediction of FGR. One important limitation of most of the studies on early prediction of FGR is the lack of a gold standard for the antenatal or postnatal diagnosis of FGR. As such, there is wide variation among studies regarding the outcomes being predicted, including either SGA (birth weight below the 10th or 3rd percentile) or adverse perinatal outcomes that are associated with (but are not specific to) FGR. As many SGA infants are constitutionally small and healthy, differentiating between healthy small fetuses and those that are small due to FGR is critically important. As a rule, the prediction of early‐onset severe FGR is better than of late‐onset FGR.
Box 4. Risk factors for fetal growth restriction.
|
Abbreviations: FGR, fetal growth restriction; PlGF, placental growth factor; PAPP‐A, pregnancy‐associated plasma protein‐A; AFP, alpha‐fetoprotein.
aRefers to placental dimension (short‐based thick placenta) and texture (calcifications, echogenic cystic lesions).
5.1. History‐based risk factors
Several maternal factors influence fetal growth and the risk of FGR: advanced maternal age, racial/ethnic origin (e.g. South Asian), consanguinity, low body mass index, nulliparity, use of recreational drugs and alcohol, assisted reproductive technology, and medical disorders such as chronic hypertension, diabetes mellitus, and autoimmune conditions (Box 4). 83 , 84 , 85 , 86 , 87 , 88 , 89 Cigarette smoking is a common risk factor for FGR and reduces birth weight by an average of 200 g in a dose–response manner. 90 In a cohort of 33 602 pregnancies, maternal characteristics predicted 37% of women who subsequently delivered SGA neonates (birth weight <5th percentile) at a false‐positive rate of 10%. 83
Some risk factors for FGR are especially relevant in low‐resource countries. In a recent review from Africa, the main risk factors reported were low maternal nutritional status, HIV infection, malaria, and hypertensive diseases. Based on these findings, the authors concluded that to a large extent FGR in Africa is preventable through established interventions for malaria, HIV, and maternal undernutrition. 42 In addition, exposure during pregnancy and lactation to toxic environmental chemicals and heavy metals has become a growing problem, especially in low‐resource countries. 91
5.2. Biochemical markers
At this point there is no role for routine screening with serum biomarkers for FGR. However, when biochemical markers are available as part of prenatal genetic screening for trisomy 21, it may be reasonable to use this information for the purpose of risk stratification for FGR.
The placenta releases multiple factors into maternal circulation from the early stages of pregnancy, and first‐trimester serum levels of some of these factors have been shown to be associated with subsequent placenta‐mediated complications. 92 , 93 Low levels of pregnancy‐associated plasma protein‐A (PAPP‐A), a placental glycoprotein produced by the syncytiotrophoblast layer, have been associated with adverse pregnancy outcomes including SGA. A meta‐analysis including 32 studies and 175 240 pregnancies found that PAPP‐A levels below the 5th percentile had a moderate association with birth weight below the 10th percentile (OR 2.08, positive predictive value of 18%), while the association was stronger for PAPP‐A levels below the 1st percentile (OR 3.4; positive predictive value of 28%). 94 Thus, although women with low PAPP‐A are at increased risk for FGR, the majority of these women will have a normal pregnancy outcome, especially as an isolated biomarker in healthy women. However, a low PAPP‐A level is often considered an indication for closer monitoring of fetal growth. 95 Elevated second‐trimester maternal serum levels of alpha‐fetoprotein are thought to reflect abnormal placental permeability and are associated with increased risk of placenta‐mediated complications including FGR and stillbirth. 96 , 97 The combination of low PAPP‐A in the first trimester and high alpha‐fetoprotein in the second trimester is particularly predictive of severe FGR. 98 Elevated human chorionic gonadotropin (hCG) levels greater than 2.5 MoM in the second trimester, alone or combined with high alpha‐fetoprotein levels, are also associated with an increased risk of SGA. 99
Angiogenic factors play a key role in the regulation of placental vascular development. 100 Placental growth factor (PlGF) is a proangiogenic factor highly expressed in the syncytiotrophoblast and the maternal endothelium. Impaired placentation is associated with reduced placental production of this protein. Low first‐trimester PlGF levels have been shown to be associated with adverse pregnancy outcome including pre‐eclampsia and SGA. 101 , 102 , 103 , 104 In a case–control study of 296 pregnancies with SGA and 609 controls, the detection rate of low PlGF for SGA at a false‐positive rate of 5% and 10% was 15% and 21%, respectively. The combined use of PlGF and PAPP‐A increased the detection rate to 19% and 27%, respectively. 103 A multicenter screening study found that the detection rate of a combined screening by maternal factors, fetal biometry, and serum PlGF and alpha‐fetoprotein at 19–24 weeks for the delivery of SGA infants below the 5th percentile at less than 32, 32–36, and greater than or equal to 37 weeks of gestation was 100%, 76%, and 38%, respectively, at a false‐positive rate of 10%. 96
Findings are less consistent for soluble fms‐like tyrosine kinase‐1 (sFlt‐1), an antiangiogenic factor released from the placenta that results in maternal endothelial dysfunction characteristic of pre‐eclampsia. 105 Although maternal serum sFlt‐1 levels are known to be elevated in pre‐eclamptic pregnancies, a large case–control study demonstrated that high levels of sFlt‐1 at 10–14 weeks were actually associated with a slightly reduced risk of SGA (OR 0.92; 95% CI, 0.88–0.96). 101 Therefore, the sFlt‐1:PlGF ratio test used to diagnose pre‐eclampsia should not be used in the first trimester as a screening test for FGR. 106
5.3. Ultrasound markers
Several ultrasound‐based markers have been shown to be predictive of FGR, including uterine artery Doppler, placental morphology, and placental volumes. However, given their modest predictive accuracy, they cannot be recommended for universal screening for FGR.
Increased uterine artery resistance largely reflects a failure of extravillous cytotrophoblast invasion and transformation of the spiral arteries and is associated with the development of pre‐eclampsia and FGR due to maternal vascular malperfusion of the placenta. 107
First‐ and second‐trimester abnormal uterine artery Doppler waveforms, defined as mean pulsatility index above the 95th percentile, have been shown to be associated with FGR. 108 , 109 , 110 In a large prospective cohort study of 4610 nulliparous women, uterine artery pulsatility index at 11+0 to 13+6 weeks predicted 60% of preterm and 17% of term SGA infants at a false‐positive rate of 10%. 111 Although uterine artery Doppler shows promise, especially for the prediction of early‐onset FGR, current evidence does not support routine screening with uterine artery Doppler for FGR in low‐ or high‐risk pregnancies. 112
Sonographic evaluation of the placenta is a routine part of the obstetric ultrasound examination. A method for systematic two‐dimensional (2D) placental ultrasound examination has been described, often in combination with other parameters 30 , 113 , 114 Abnormal placental morphology is defined by placental dimensions, shape, texture, and cord insertion. Placental shape is considered abnormal when the placental thickness is above 4 cm or greater than 50% of placental length. Placental texture is defined as normal when it is homogenous, and abnormal when the placenta is heterogeneous and contains multiple echogenic cystic lesions or has a jelly‐like appearance with turbulent uteroplacental flow. 115 , 116 Placental cord insertion is defined as central (>2 cm from placental disc margin), marginal (within 2 cm of margin), or velamentous (inserting into the surrounding membranes). 114 In a cohort of 60 high‐risk women with abnormal uterine artery Doppler, women with abnormal placental shape at 19–23 weeks had higher odds of FGR (OR 4.7) than women with normal placental shape. 108 However, the use of 2D placental imaging has significant limitations, including difficulty in assessing nonanterior placentas and a wide variability in the morphology of normal placentas. Furthermore, there are no large‐scale prospective studies validating the use of this modality for prediction of FGR. 114
Improvements in ultrasonographic imaging provide a tool for estimating placental volume using three‐ and four‐dimensional scanning techniques. Placental volume has been proposed as a marker for various obstetric complications related to defective placental function, including FGR. 117 , 118 A systematic review estimating the value of first‐trimester 3D placental volume for the prediction of SGA found a detection rate of 24.7% at a 10% false‐positive rate. 119 Another parameter is the placental quotient, defined as the ratio of the placental volume to the fetal crown–rump length. The placental quotient was reported to have a high negative predictive value for perinatal complications but was not very useful when used for screening of SGA in a low‐risk population, with a sensitivity of 27.1%. 120 The discriminatory ability of placental volume alone for SGA appears to be modest, but may be integrated into a multivariable screening model. However, the use of 3D placental volume as a routine screening tool for FGR is limited by the need for proper equipment and training required to obtain these measurements in a reproducible manner.
5.4. Prediction models
Currently there is no single screening test sufficiently predictive of FGR to recommend routine clinical use. Investigations are underway to combine various tests, but such prediction models have not been sufficiently validated in terms of outcomes studies and therefore must be considered investigative protocols at this time. In a prospective cohort of 4970 women, the combination of first‐trimester maternal serum PAPP‐A, beta hCG, maternal blood pressure, and uterine artery Doppler performed in the first trimester had a detection rate of 73% for early SGA (<34 weeks) but only 32% for late SGA (≥34 weeks). 19 A different model that included maternal characteristics, first‐trimester blood pressure, uterine artery pulsatility index, PlGF, and sFlt‐1 was evaluated in a larger cohort of 9150 women and achieved a detection rate of 86% for early‐onset FGR and 66% for late‐onset FGR, both at a false‐positive rate of 10%. 19 , 121 In the second trimester, the SCOPE consortium examined 5606 healthy nulliparous women with singleton pregnancies and found that the combination of clinical risk factors, 15‐week biomarkers (53 biomarkers were used), and 20‐week ultrasound (fetal biometry and Doppler studies of the umbilical and uterine arteries) had only a moderate detection rate for SGA below the 10th percentile, with a positive predictive value of 32% and a negative predictive value of 91%. 122
5.5. Prevention of fetal growth restriction in high‐risk populations
5.5.1. Lifestyle modifications
Ideally, all women should plan their pregnancies, adopting a healthy lifestyle and optimizing any medical conditions and their body mass index. The preconception period provides an opportunity for health promotion with the aim of reducing accepted risk factors, including those associated with FGR. 123
Insufficient gestational weight gain has been associated with an increased risk of FGR, especially in women with low body mass index (BMI, calculated as weight in kilograms divided by height in meters squared). 124 Recognizing that these associations are only based on observational data, we still believe that it would be reasonable to recommend monitoring of weight gain and informing women of the target weight gain range, as recommended by the 2009 Institute of Medicine guidelines. 125 These guidelines recommend a total gestational weight gain of 12.5–18 kg (28–40 lb) for underweight women (BMI <18.5); 11.5–16 kg (25–35 lb) for the normal weight group (BMI 18.5–24.9); 7–11.5 kg (15–25 lb) for overweight women (BMI 25.0–29.9); and 5–9 kg (11–20 lb) for obese women (BMI ≥30). 126
Substance use, including smoking, alcohol, and illicit drugs, is associated with low birth weight and increased perinatal morbidity and mortality. 90 Interventions to promote smoking cessation during pregnancy have been shown to result in a reduction in low birth weight (RR 0.81) and an increase in mean birth weight (+33 g). 127 Women should be advised that smoking cessation at any point in gestation is of benefit, and that the greatest benefit is associated with cessation before 15 weeks of pregnancy. 128 The risk of SGA with alcohol intake is increased with as little as one drink per day. 129
5.5.2. Medical interventions
Most studies on early prevention of placental complications have focused on pre‐eclampsia, with the results often being extrapolated to FGR due to the common pathophysiology. However, to date, other than lifestyle modifications, no medical interventions to prevent FGR have been clearly established.
Aspirin is recommended for women at increased risk of pre‐eclampsia, but there is some evidence that it may also reduce the risk of FGR 130 , 131 In a recent meta‐analysis of 45 trials that included 20 909 women at high risk of pre‐eclampsia, the administration of aspirin starting at less than or equal to 16 weeks of pregnancy reduced the risk of FGR by nearly half (RR 0.56; 95% CI, 0.44–0.70), with higher dosages of aspirin associated with a greater reduction, favoring a dose of 100–150 mg. 132 A second individual patient data meta‐analysis also supported earlier initiation of aspirin for the prevention of FGR, with an RR of 0.76 (95% CI, 0.61–0.94) for women randomized before 16 weeks versus an RR of 0.95 (95% CI, 0.84–1.08) for women randomized at 16 weeks or beyond. 131 One randomized trial found that evening but not morning administration of aspirin is associated with reduction in the rate of pre‐eclampsia and FGR. 133 However, it should be emphasized that most of the available data on aspirin come from studies that focused on the prevention of pre‐eclampsia as the primary outcome in women at high risk of pre‐eclampsia, with the prevention of FGR considered only as a secondary outcome. Furthermore, in the largest trial to date on the use of aspirin for the prevention of pre‐eclampsia (ASPRE trial), aspirin was not associated with a reduction in the risk of SGA below the 10th, 5th, or 3rd percentile. 130 However, we believe that given the safety of aspirin and the overlap in the risk factors and pathogenesis of pre‐eclampsia and FGR, it is reasonable to recommend aspirin to women at high risk of FGR, using the same regimen of aspirin used for women at high risk of pre‐eclampsia. Most international guidelines recommend 100–150 mg aspirin to prevent FGR in women at high risk. 134
The adjunct role of heparin in combination with aspirin to prevent placenta‐mediated complications in high‐risk situations was originally attributed to its anticoagulant properties and the speculative prevention of placental thrombosis. However, in vitro and in vivo data suggest heparins may have other biological properties including anti‐inflammatory, complement inhibition, and proangiogenic activities. 135 , 136 , 137 , 138 A study‐level meta‐analysis of six trials including 848 women showed that low‐molecular‐weight heparin (LMWH) was associated with a reduction in the composite outcome of pre‐eclampsia, birth weight below the 10th percentile, placental abruption, or pregnancy loss after 20 weeks (RR 0.52; 95% CI, 0.32–0.86) with similar risk reduction for SGA below the 10th and 5th percentiles. However, the higher‐quality trials suggest no treatment effect, 139 and a subsequent individual patient data meta‐analysis looking at the same composite outcome found no beneficial effect of LMWH treatment (RR 0.64; 95% CI, 0.36–1.11). 140 Likewise, the enoxaparin for pre‐eclampsia and intrauterine growth restriction (EPPI) trial included women at high risk for placenta‐mediated complications (with a high proportion of women with prior FGR) and showed no difference in the rate of the composite outcome (pre‐eclampsia or SGA <5th percentile) between treated and nontreated women. 141 Therefore, based on the most up‐to‐date evidence, LMWH cannot be recommended for the prevention of FGR in women at high risk of placenta‐mediated complications. Its use for the prevention of FGR should therefore be limited to research settings, for example in women already on aspirin who are found to have abnormal levels of angiogenic markers prior to fetal viability. 142
5.6. Recommendations
6. DETECTION OF FETAL GROWTH RESTRICTION
Detection of FGR is based on the identification of a fetus that is smaller than expected for gestational age, through either physical examination (symphysis–fundal height, SFH) or ultrasound.
6.1. Symphysis–fundal height
Measurement of SFH using a tape is a simple, inexpensive, and widely used strategy to screen for FGR. 143 , 144 , 145 , 146 SFH is measured with the woman in a supine position using a nonelastic metric tape after she has emptied her bladder. To decrease the interobserver variability, a standardized technique for measuring SFH should be followed. 144 , 145 SFH is defined as the distance from the upper border of the symphysis pubis bone to the top of the uterine fundus. 145 SFH measured in centimeters between 24 and 38 weeks of gestation approximates the gestational age. 147 Numerous local charts are currently used worldwide, 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 with the recent addition of an international standard for SFH based on serial measurements. 145 However, the accuracy of SFH measurement in predicting SGA (EFW <10th percentile) is limited, and there are no randomized controlled trials that compare SFH measurement with serial ultrasound evaluation of fetal biometry. 157 In a meta‐analysis of 34 observational studies, SFH was reported to have a sensitivity of 58% and a specificity of 87% for predicting birth weight below the 10th percentile. There was marked heterogeneity between studies, mainly due to the use of different SFH charts. 158 A single SFH measurement at 32–34 weeks of pregnancy has been reported to be approximately 65%–85% sensitive and 96% specific for detecting FGR. 143 It is important to acknowledge that factors such as maternal obesity, uterine leiomyomas, and polyhydramnios may further limit the accuracy of SFH as a screening tool. 144 , 159
6.2. Sonographic fetal weight estimation
Sonographic fetal biometry is the cornerstone for detection of fetal growth disorders. Standard fetal biometry includes assessment of head circumference (HC), biparietal diameter, abdominal circumference (AC), and femur length (FL). Measurement of these biometric indices should be obtained by an experienced individual and in a standardized manner, as has been previously described. 160 Fetal weight is estimated based on various combinations of the four biometric indices described above, using one of many published equations. 161 , 162 , 163 , 164 , 165 The accuracy of most equations falls within the range of ±10%, and the error has been shown to be greater at the extremes of birth weight, and to be affected by factors such as fetal sex, presentation, and plurality (greater in twin gestations). 162 , 163 , 164 , 166 , 167 , 168 , 169 , 170 , 171 Several studies have compared the accuracy of various equations. Most studies concluded that equations that are based on 3–4 biometric indices (rather than only 1–2 indices) provide the most consistent and accurate results. A recent systematic review 165 found that the Hadlock equation, based on three indices (HC, AC, and FL: Log10 weight = 1.326 − 0.00326*AC*FL + 0.0107*HC + 0.0438*AC + 0.158*FL), 2 provided the greatest accuracy. Since the accuracy of the various equations may vary between different populations, it may be reasonable for radiologists, sonographers, or care providers to choose an equation that has been validated within their local population and within the gestational age range in which it will be used. However, if such information is not available—a very frequent scenario—it seems reasonable to use the Hadlock equation as described above.
6.3. Is there a role for routine third‐trimester ultrasound to assess fetal growth?
In many countries, measurement of SFH is the primary screening tool for FGR in low‐risk pregnancies and ultrasound measurement of fetal biometry is performed only when indicated on the basis of risk factors or abnormal SFH. 134 , 143 , 172 , 173 , 174 However, this approach fails to identify the majority of FGR infants, 146 a concerning finding given that undetected FGR is associated with increased risk of adverse perinatal outcome and stillbirth. 53 , 175
An alternative approach is to perform a routine third‐trimester ultrasound for fetal weight estimation. However, a strategy for routine third‐trimester ultrasound in low‐risk pregnancies is not supported by available data and cannot be recommended. 176 , 177 , 178
A meta‐analysis of 13 trials assessed the effect of routine sonographic weight estimation at more than 24 weeks of gestation on pregnancy outcomes in both unselected and low‐risk pregnancies. 178 The authors found no association between routine sonographic EFW and adverse pregnancy outcomes including perinatal mortality, preterm birth, induction of labor, or cesarean section. In a recent randomized controlled trial of women with uncomplicated pregnancies, the use of serial (every 4 weeks) third‐trimester ultrasound was superior to routine care in the detection of a composite outcome of fetal growth or amniotic fluid abnormalities (RR 3.43; 95% CI, 1.64–7.17). 179 However, it is important to note that the incidence of maternal or fetal morbidity was not significantly different between the groups. Similar results were reported by others. 180 In contrast, the Pregnancy Outcome Prediction (POP) study prospectively assessed 3977 women and compared the detection of SGA (birth weight <10th percentile) by routine ultrasound versus clinically indicated ultrasound in the third trimester. 181 The detection rate of SGA was nearly tripled in the routine ultrasound group (57% vs 20%). The risk of neonatal morbidity was increased only in the subset of SGA fetuses with fetal abdominal circumference growth velocity in the lowest decile (RR 3.9; 95% CI, 1.9–8.1), emphasizing the importance of combined analysis of fetal biometry and fetal growth velocity for better detection of fetuses at risk. 182 Furthermore, it has been suggested that the prediction of FGR based on routine third‐trimester ultrasound can be improved by integrating EFW with additional biomarkers. A combined screening model that included maternal characteristics, third‐trimester EFW and placental Doppler, and biochemical markers (PlGF and estriol) achieved better performance than EFW alone in the detection of FGR (77% vs 64%) at a 10% false‐positive rate. 183
There are many conceptual explanations to support third‐trimester ultrasound as it can assist in the diagnosis of clinically significant findings other than FGR, including fetal malpresentation, 184 disorders of amniotic fluid, and fetal anomalies, 185 , 186 especially when combined with Doppler measurements and biochemical markers. 95 , 187 , 188 , 189 However, there is no evidence that this information improves outcomes when performed routinely in low‐risk pregnancies.
6.4. Which growth chart should be used to determine fetal weight percentile?
The interpretation of sonographic EFW depends on gestational age and is commonly classified as appropriate for gestational age, SGA, or large for gestational age, based on the calculation of EFW percentile using one of the many available growth charts. The choice of growth chart has been shown to have a considerable impact on the proportion of fetuses classified as either SGA or large for gestational age. 190 , 191 Over the past several years there has been an ongoing debate regarding the optimal growth chart that should be used, and numerous studies have compared the performance of a wide variety of charts in different populations with conflicting results. Prior to further discussion of specific charts, it is important to clarify the terminology and the types of charts that are currently available.
6.4.1. Growth references versus growth standards
Growth references are descriptive charts that provide information on the distribution of weight of all newborns in a given population, and as such they include both normal and complicated pregnancies. Although growth references are useful as they provide information on the overall distribution of birth weight in the population, their use for the purpose of antenatal detection of FGR may be challenging as they are affected by the rate of pathologies in the population. For example, in populations with a high rate of large fetuses (e.g. due to a high rate of obesity and diabetes), the reference would be shifted upward. Similarly, in populations with a high rate of FGR (e.g. due to a high rate of malnutrition), the reference would be shifted downward.
For that reason, it may be reasonable to prefer growth standards over growth references for the antenatal detection of FGR. Growth standards are prescriptive charts that are based only on low‐risk or uncomplicated pregnancies, and as such provide information on what is the optimal fetal growth. There is variation between different growth standards with regard to the definition of “low‐risk” pregnancies; while some standards excluded women with pre‐existing medical conditions and pregnancy complications, others also excluded women below or above certain height or weight, women with suboptimal nutrition, low socioeconomic status, exposure to air pollution, high altitude etc. Since growth standards include only low‐risk uncomplicated pregnancies, their distribution is usually narrower (i.e. the 10th and 90th percentiles are closer to the mean) compared with growth references.
One important and practical aspect regarding the use of reference versus standard charts relates to the weight percentile threshold that should be used to trigger further evaluation for FGR. When using a growth reference, it is reasonable to use the 10th percentile for that purpose, as a considerable proportion of infants below the10th percentile will be affected by pathology. In the case of growth standard, however, using the same threshold of the 10th percentile would, per definition, identify 10% of the low‐risk pregnancies as suspected for FGR, which is not practical. Therefore, when using a growth standard, a lower threshold—such as the 5th or 3rd percentile—should be used to indicate further evaluation for FGR.
6.4.2. Charts based on birth weight versus sonographic fetal weight estimation
A second important distinction is between growth charts that are based on birth weight versus those that are based on sonographic EFW. Birth weight‐based charts rely on cross‐sectional data of infant birth weights across the full range of gestational ages, usually obtained from large databases. Different types of regression techniques are then used to calculate the mean and various percentiles of birth weight across gestation. These charts are commonly used as they are easy to develop. However, their main limitation is that infants born prematurely (before 37 weeks) are more likely to be affected by placental dysfunction and to be growth restricted. Therefore, these charts are likely to underestimate the optimal weight of fetuses during the preterm period, which in turn may lead to an underdiagnosis of FGR before 37 weeks. This is illustrated in Figure 2, where the birth weight‐based chart of Alexander (USA) 192 is compared with several ultrasound‐based charts.
FIGURE 2.
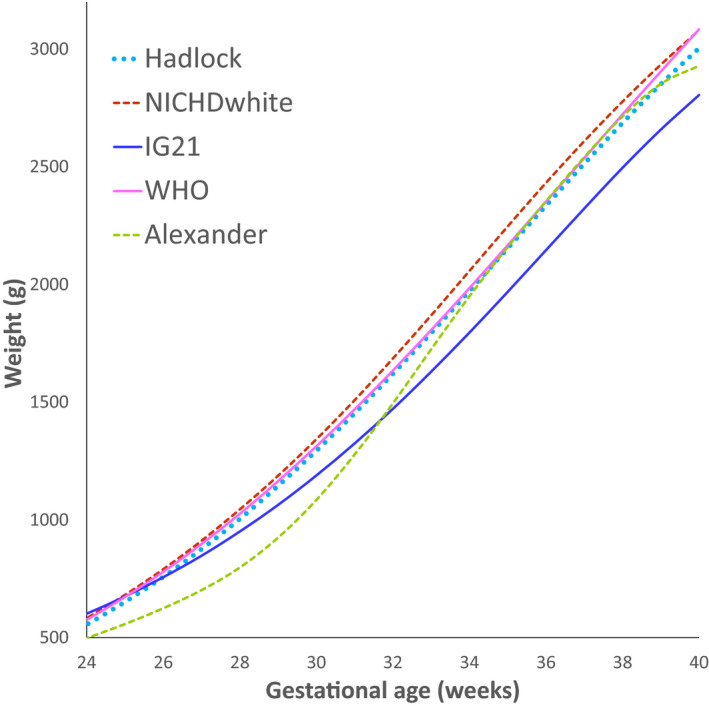
Comparison of the 10th percentile curves of common growth charts. Key: Hadlock: ultrasound‐based chart 193 ; NICHD, National Institute of Child Health and Human Development chart 198 ; IG21, Intergrowth‐21st chart 196 ; WHO, World Health Organization chart 197 Alexander: birth weight‐based chart. 192 [Colour figure can be viewed at wileyonlinelibrary.com]
Therefore, it seems reasonable to prefer growth charts that are based on sonographic EFW over those that are based on birth weight. Ultrasound‐based growth charts are more difficult and expensive to develop, as they are usually based on data from prospective longitudinal studies where women undergo several sonographic weight estimations during pregnancy. However, these charts do not share the limitation of birth weight‐based charts, described above, and are thus more likely to reflect the optimal fetal growth throughout pregnancy (Figure 2). Another reason why ultrasound‐based charts should be preferred is that the measure used during pregnancy to assess fetal growth is sonographic EFW; it is therefore more appropriate to compare it to charts based on the same measure (i.e. sonographic EFW) rather than to charts based on birth weight. Some of the commonly used ultrasound based charts are presented in Figure 2. 193
6.4.3. Universal versus customized charts
One final distinction is between universal and customized growth charts, which represent a spectrum of approaches towards the similarity of the genetic growth potential of different fetuses across the world. At one end of this spectrum there are universal charts that are based on the assumption that under optimal conditions, all fetuses are expected to have the same growth potential, irrespective of their country of origin or race and that the only reason for the differences currently observed between different countries or races are purely due to environmental factors, such as malnutrition and environmental toxins. These ultrasound‐based charts are developed through multicenter, multinational, prospective longitudinal studies, where data on sonographic fetal growth from multiple countries are pooled into a single international universal chart. The best examples of such universal charts are the recently published Intergrowth‐21st 194 , 195 , 196 and World Health Organization (WHO) charts. 197
Others, however, believe that the variation in fetal growth between countries and races is not solely the result of environmental factors. Instead, it is suggested that genetic variation in growth potential contributes to the observed differences in fetal growth between race groups, and that race‐specific charts should therefore be preferred over universal charts. Examples of such race‐specific charts are the National Institute of Child Health and Human Development (NICHD) charts which include separate charts for white, black, Hispanic, and Asian women, 198 and the recently published PRB/NICHD customized standard for African American women. 199
According to the third approach, growth charts should be adjusted not only for maternal race but also for other physiologic factors that are thought to determine fetal growth potential, such as maternal height, weight, parity, and fetal sex. One such example is the Gestation Related Optimal Weight (GROW) software for customized growth percentiles. 200 , 201
At the other end of the spectrum is the individualized growth assessment (IGA) approach, which is based on estimation of the growth potential of the individual fetus, calculated from the second‐trimester growth velocity of that fetus. These estimates are used to generate individualized trajectories that are used to interpret fetal growth during the third trimester (https://igap.research.bcm.edu). 202 , 203 , 204 While compelling, this approach requires earlier ultrasound exams during pregnancy, as well as appropriate software, and is therefore challenging at present for the purpose of FGR screening in the general population, and especially in low‐resource settings.
6.4.4. Description of commonly available charts
The 10th percentile curves of some of the charts described above are compared in Figure 2. The Hadlock chart (1991), one of the most commonly used growth charts, is an ultrasound‐based standard. It is based on a cohort of 392 low‐risk, primarily white women from Texas. The Alexander chart (1996) is based on over 3 million singleton live births in the USA and is included as an example of a birth weight‐based reference to illustrate their limitation, which is the underestimation of optimal fetal growth during the preterm period.
The goal of the Intergrowth‐21st project (2014) was to develop a universal ultrasound‐based prescriptive growth chart. This was a prospective longitudinal study of 4321 low‐risk women from eight centers located in eight high‐ and middle‐income countries. 194 The study had strict inclusion and exclusion criteria to ensure that participants were not exposed to environmental factors known to affect fetal growth, and it therefore aimed to reflect optimal fetal growth. Based on predetermined criteria, the authors concluded that the differences between participants from different countries in measures of skeletal growth (crown–rump length and head circumference) were similar enough to justify pooling the data, and they therefore generated a single universal chart. No information was provided on the differences between countries with respect to measures such as fetal weight estimation and abdominal circumference, which are used in clinical practice to detect FGR and are known to be associated with adverse perinatal outcomes. Interestingly, there were considerable differences in birth weight between infants from different countries, even in this highly selected group of women free from the negative influence of environmental factors known to affect fetal growth. For example, the mean birth weight at term in India was 2.9 kg, which was approximately 600 g lower than the mean birth weight in the UK (3.5 kg). 195 These differences have led some to question the validity of the pooled chart and of the hypothesis that underlies the Intergrowth‐21st project. 205 , 206 As demonstrated in Figure 2, the 10th percentile of the Intergrowth‐21st chart is significantly lower throughout gestation than most other ultrasound‐based standards.
At around the same time, the results of the NICHD growth study (2015) were published. 198 The overall design of this study was similar to that of the Intergrowth‐21st study. It was a prospective longitudinal study of 2334 low‐risk women from 12 centers in the USA. The authors found substantial differences in fetal weight between different race groups, and therefore developed separate race‐specific growth charts for white, black, Hispanic, and Asian women. The 10th percentile of the NICHD growth chart for white women is included as an example in Figure 2.
The WHO fetal growth charts were published in 2017. 197 Similar to the Intergrowth‐21st project, this study aimed to develop a prescriptive universal chart to extend the previously published WHO child growth standard 207 to the fetal period. The design of this study was also similar to that of Intergrowth‐21st—a prospective longitudinal study of 1387 low‐risk women from 10 centers in 10 high‐ and middle‐income countries. Despite this, the results of the WHO study differed from those of Intergrowth‐21st in two aspects. First, the 10th percentile of the WHO chart is considerably higher than the Intergrowth‐21st chart, and is in fact almost identical to the 10th percentile of the Hadlock standard (Figure 2). Second, unlike Intergrowth‐21st, the investigators of the WHO study found substantial differences in fetal growth between the various countries, and concluded that “…populations, even under optimal nutritional conditions and environment, vary and that fetal growth varies and should be considered when the WHO fetal growth charts or any growth references are applied”. 208 They expressed concern that use of a universal chart carries a risk of misclassification of FGR, 209 , 210 , 211 and recommended that their chart should be adjusted in each country to the local population.
The benefit of customized charts remains a matter of debate. The GROW software incorporates certain factors that are believed to determine fetal growth potential (maternal race, height, weight, parity, and fetal sex) to calculate the predicted optimal (customized) weight at 40 weeks for each individual fetus. 200 , 201 The customized fetal growth curve is then determined retrospectively, based on a proportionality growth function derived from the ultrasound‐based Hadlock standard. 193 The use of customized charts is appealing, especially in the setting of ethnically mixed populations where their use has been shown to decrease over‐ and underestimation of FGR rates in certain race groups. 212 A large number of studies investigated the association of customized charts with adverse pregnancy outcomes compared with other birth weight‐ and ultrasound‐based charts, with conflicting results. Several studies found that customized charts performed better at predicting stillbirth and adverse neonatal outcomes, 66 , 201 , 211 , 213 , 214 , 215 , 216 while others found no benefit and concluded that the benefit reported by others is merely because they are based on an ultrasound‐based chart and are thus more likely to reflect optimal fetal growth, while the act of customization has a minimal contribution to the stronger association with adverse outcome. 217 , 218 , 219 Another criticism is that the GROW approach assumes that all fetuses follow the same growth trajectory (which is derived from the Hadlock chart)—an assumption that may not be true. Finally, it has been suggested that the required adjustment for multiple factors may be too complex for low‐resource countries and, in that setting, a simple adjustment to only one factor—mean birth weight at 40 weeks in the local population—is as predictive for adverse perinatal outcomes as the fully customized GROW charts. 220 It may thus be reasonable for care providers to compare the performance of customized growth charts in their population with that of noncustomized charts (as discussed below), especially in regions or countries with a mixed population where the benefit of customization is expected to be greatest.
6.4.5. How to choose the best chart
The conflicting results and conclusions regarding the growth charts described above have led to an ongoing debate about the best approach (i.e. universal versus customized charts), as well as to considerable confusion among care providers over which chart they should be using in their local population.
The FIGO Safe Motherhood and Newborn Health Committee recently published a position paper on the choice of reference charts for fetal growth and size at birth. 3 In that paper, the committee reviewed in detail the commonly available charts and the available data on their predictive accuracy. The main conclusions were as follows: (1) local or regional charts are likely to be best to identify the 10th percentile of infants at highest risk, given that universal charts such as Intergrowth‐21st are likely to under detect SGA fetuses in high‐resource countries and, at the same time, over detect SGA in low‐ and middle‐income countries; (2) as an alternative, universal standards such as Intergorwth‐21st and WHO may be used with locally adjusted thresholds (e.g. 3rd or 5th percentile in low‐ or middle‐income countries versus 15th or 20th percentile in high‐income countries) to avoid under or overdetection of SGA; and (3) when assessing fetal size antenatally by ultrasound, fetal (i.e. ultrasound‐based) charts should be used rather than birth weight‐based charts. We fully endorse and support these recommendations.
Furthermore, we as well as others, 160 , 208 believe that the decision on which chart to use can be further based on a comparison of performance of the various charts in the population of interest, using a local data set. This can be achieved by the following approaches: (1) statistical validation: finding the chart that matches best the distribution of fetal weight in low‐risk pregnancies in the local population. That is, identifying the chart that when applied to the local population yields weight percentiles that follow a normal distribution centered at approximately the 50th percentile, and identifies approximately 10% of the low‐risk population as being below the 10th percentile and above the 90th percentile, and approximately 5% of the population as being below the 5th percentile and above the 95th percentile. An example of this approach is provided in Figure 3; (2) outcome‐based validation: finding the chart for which the diagnosis of SGA has the best predictive value for adverse outcomes related to FGR.. 211 , 221 While this approach seems compelling, interpretation of the predictive value of the different charts for adverse outcomes may be challenging, as there is a trade‐off between detection rate and false positive rate for adverse outcomes. 221 Thus, charts that are shifted upward (e.g. Hadlock, WHO) would have a higher detection rate but also a high false‐positive rate, while charts that are shifted downward (e.g. Intergrowth‐21st) would have a lower false‐positive rate but would also have a lower detection rate for SGA fetuses at risk of adverse outcomes (Figure 4). Finding the chart that provides the best balance between these two measures requires careful consideration and should be based on a clear definition of the goals of screening.
FIGURE 3.
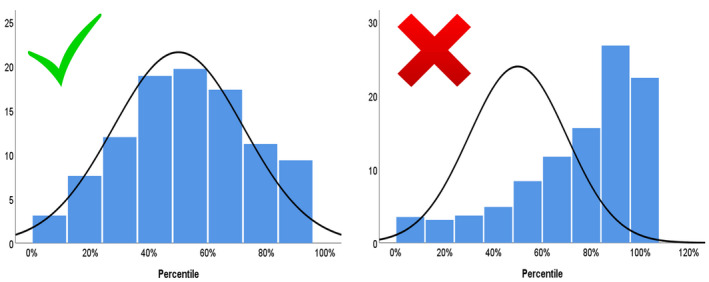
Illustration of the statistical validation of two charts in a local population. The left chart shows a good match to the population of interest: the distribution of fetal weight percentiles based on this chart follows a normal distribution that is centered at the 50th percentile, with approximately 10% of the population below the 10th and above the 90th percentile. The right chart shows a poor fit for the population of interest as it is skewed to the right: it overdiagnoses fetuses as large for gestational age and underdiagnoses small‐for‐gestational‐age fetuses. [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 4.
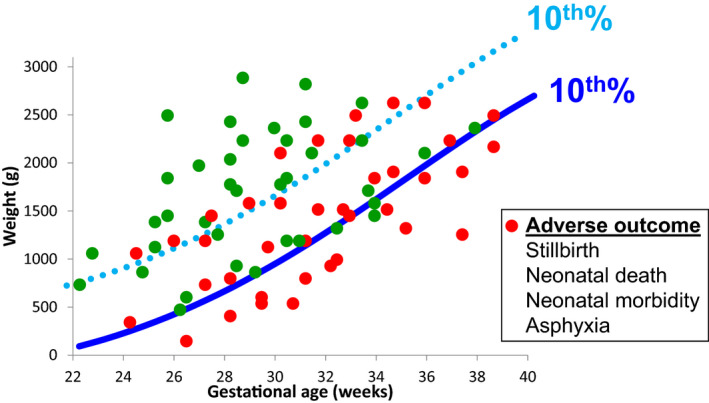
Illustration of the impact of the growth chart chosen on the trade‐off between detection rate and false‐positive rate of fetuses at risk of adverse outcome. Charts that are shifted upward (light blue dotted line) will have a higher detection rate for pregnancies at risk of adverse outcomes (red circles) but would also have a higher false‐positive rate (i.e. identify normal pregnancies [green circle] as being at risk). In contrast, charts that are shifted downward (dark blue solid line) will have a lower false‐positive rate (i.e. identify fewer normal pregnancies [green circle] as being at risk) but will also have a lower detection rate for pregnancies at risk of adverse outcomes (red circles). [Colour figure can be viewed at wileyonlinelibrary.com]
6.5. How to assess fetal growth in twin gestations
Twin fetuses grow more slowly than singletons, starting from 28–32 weeks of gestation onward. 222 , 223 , 224 , 225 At term, approximately 30%–50% of twins would be identified as SGA (EFW <10th percentile) using singleton growth standards. 223 , 226 , 227 The mechanisms underlying the relative smallness of twins remain unclear. While some believe that this represents a pathological phenomenon due to failure of the uteroplacental circulation to meet the demands of two fetuses (i.e. twins are more likely to be growth restricted due to the same mechanism responsible for FGR in singletons), 224 , 228 , 229 , 230 others suggest that this represents an early benign physiological adaptation of twins to the “crowded” intrauterine environment in an effort to delay the onset of labor (by decreasing uterine distension) and gain maturation at the expense of size. 231 One important implication of this question relates to the growth standard that should be used in twins. If the slower growth of twins represents FGR, it would be reasonable to use singleton growth standards to identify the small twin fetus that, like SGA singletons, may be at increased risk for perinatal mortality and morbidity. However, if the relative smallness of twins is due to a benign adaptive mechanism, it may be preferable to use twin‐specific growth charts 223 , 232 , 233 , 234 , 235 to avoid overdiagnosis of FGR in twin gestations, 63 , 227 which is associated with increased use of resources, ultrasound exams, interventions, and patient anxiety.
Most current guidelines do not provide clear recommendations as to which type of charts should be used to monitor the growth of twins, 143 , 236 , 237 while other guidelines specifically recommend the use of singleton‐based charts 238 or twin‐specific charts. 239 As a result, singleton‐based standards are used by default in most centers to assess the growth of twins. However, recent data provide support to the hypothesis that the relative smallness of twins is a benign adaptive mechanism and, therefore, for the use of twin‐specific charts. For example, several studies suggest that the slower growth of twins is the result of differences in programming that is determined as early as the first trimester.. 229 , 240 , 241 , 242 , 243 In addition, it was found that the use of twin‐specific (versus singleton‐based) charts was associated with a marked decrease in the rate of twins classified as SGA, without affecting the detection rate of stillbirth, suggesting that twin‐specific charts can be used safely. 227 , 244 , 245 Similar findings were reported in studies that investigated the association between the type of chart used (twin versus singleton charts) and other outcomes such as perinatal complications and long‐term morbidity. 246 , 247 Studies that investigated placental pathology findings reported that SGA twins (based on singleton charts) are less likely to have placental histopathological evidence of placental insufficiency when compared with SGA singletons. 248 , 249 In another recent study on the association between SGA and pre‐eclampsia, it was found that in contrast to singletons, the diagnosis of SGA in twins based on singleton charts was not associated with a greater risk of pre‐eclampsia, while the association of SGA in twins diagnosed using twin‐specific charts had the same magnitude of association with pre‐eclampsia to that observed between SGA and pre‐eclampsia in singletons. 63 Overall, these findings provide support to the hypothesis that the relative smallness of twins is less likely to be the result of placental insufficiency and, thus, less likely to reflect true growth restriction. Based on that, we believe that it seems reasonable to use twin‐specific charts for the assessment of fetal growth in twin gestations, as this has the potential to avoid overdiagnosis of FGR and the consequences associated with this diagnosis. 246 This approach is supported by the recent guidelines of the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG). 239 Of note, the diagnosis of FGR in twin gestations should also take into consideration intertwin size discordance, especially in the case of monochorionic placentation. 250
6.6. Recommendations
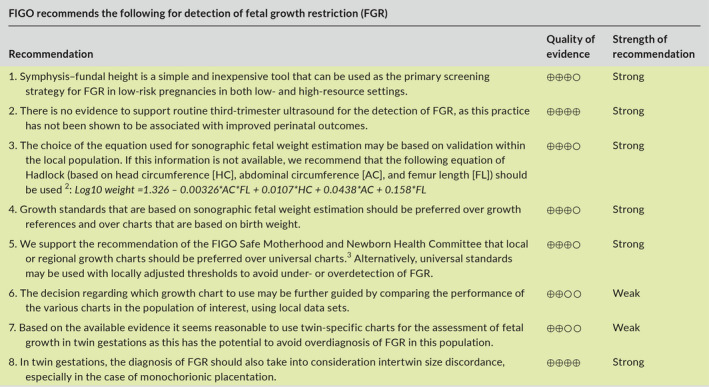
7. WHAT KIND OF INVESTIGATIONS SHOULD BE PERFORMED WHEN FETAL GROWTH RESTRICTION IS SUSPECTED?
Once FGR is suspected, a systematic investigation should be performed aimed at identifying the underlying etiology for fetal smallness, with the most important reasons being constitutional SGA, placental dysfunction, and fetal conditions such as genetic or infectious disorders. Establishing the most likely etiology is essential to allow for proper counseling, surveillance, and interventions. The investigation should consist of detailed history, evaluation of screening test results for trisomy 21 and biochemical markers, detailed sonographic assessment for structural anomalies and Doppler studies, and additional testing directed at genetic or infectious etiologies when they are suspected.
7.1. Detailed history
A detailed maternal and family history is essential to correctly identify the etiology of FGR. This should include information on maternal age, racial/ethnic group, height and weight, nutritional status, socioeconomic status, medications, cigarette smoking and use of recreational drugs, chronic medical conditions, personal or family history suggestive of thrombophilia, genetic disorders or consanguinity, obstetric history including birth weight of previous children, and confirmation of pregnancy dating by first‐trimester ultrasound. 143
Advanced maternal age has been associated with FGR, with risk increasing for women over the age of 35 years. 251 , 252 Maternal social issues, low income, and domestic violence during pregnancy have been shown to be associated with low birth weight. 253 , 254 Poor nutritional status due to conditions such as celiac disease 255 and eating disorders is a potentially treatable cause of FGR. 256 , 257 Maternal smoking is an important and potentially modifiable risk factor for FGR. 258 , 259
History should also address the risk of congenital fetal infection with cytomegalovirus, toxoplasmosis, syphilis, Zika virus, and varicella‐zoster virus. Relevant questions include a history of febrile disease or rash in pregnancy or the periconceptional period, recent travel history to endemic areas (e.g. for Zika virus), and frequent exposure to young children (cytomegalovirus) or to domestic animals (toxoplasmosis).
Accurate dating of pregnancy is essential for the correct interpretation of estimated fetal size and to avoid a false diagnosis of FGR. Determining gestational age based on menstrual history is often unreliable. 260 , 261 Therefore, with the exception of pregnancies achieved by assisted reproductive technology, the crown–rump length measured at the time of first‐trimester ultrasound is the most accurate method to date pregnancy, and establishes gestational age with a precision of 5 days in 95% of cases. 262 , 263 , 264 , 265 Crown–rump length is most accurate for the purpose of dating when in the range of 7–60 mm. 266 , 267 Therefore, confirmation of gestational age based on first‐trimester ultrasound (when available) should be the first step when FGR is suspected. If more than one scan is performed in the first trimester, the earliest scan with a crown–rump length of at least 10 mm should be used. 268
7.2. Detailed anatomy scan
Detailed anatomy scan should be routinely performed when FGR is suspected, especially in cases of early‐onset severe FGR. The presence of major structural anomalies, soft sonographic markers, or disorders of amniotic fluid (e.g. polyhydramnios) may raise the possibility of chromosomal, subchromosomal, or single gene abnormalities as the cause of FGR. 269 , 270 The presence of very shortened fetal long bones (shorter than –2SD and especially –4SD below the mean) should raise the possibility of skeletal dysplasia and indicates targeted genetic assessment. 271 , 272 , 273 Attention should also be given to findings that are associated with congenital infections, especially in women with a relevant history, as described above. Examples of such sonographic findings include small head circumference, ventriculomegaly, brain or liver calcifications, periventricular hyperechogenicity, cortical brain malformations, echogenic bowel, hydrops, or placentomegaly. 40 , 274
7.3. Doppler studies
Doppler assessment is an integral part of the diagnostic process and management of FGR. The presence of abnormal Doppler findings in the uterine, umbilical, or middle cerebral arteries is highly suggestive of placental dysfunction as the underlying etiology of FGR. A more detailed description of the different types of Dopplers studies and their application in monitoring and timing of delivery in pregnancies complicated by FGR is provided in section 8 (Management of FGR).
It should be noted that umbilical artery Doppler findings may be normal in the early stages of placental FGR. Therefore, normal umbilical artery Doppler studies do not rule out placental dysfunction, and therefore serial monitoring is recommended in all cases of suspected FGR. 275 , 276 At the same time, abnormal umbilical artery Doppler is not pathognomonic of placental dysfunction, as certain genetic conditions (e.g. triploidy) may mimic early‐onset placental FGR, including the presence of abnormal umbilical artery Doppler, most likely due to concomitant placental insufficiency secondary to the abnormal placental karyotype. 34 , 277 , 278 , 279 In contrast to umbilical artery Doppler, uterine artery Doppler is less likely to be abnormal among fetuses with FGR and abnormal karyotype, and should therefore be considered to be more specific for primary placental FGR, especially in the presence of abnormal angiogenic markers in maternal blood. 34 , 107 , 280
7.4. Additional testing
Screening for congenital infections should be offered when FGR is suspected, especially in cases of early‐onset FGR or when infection is possible based on history of ultrasound findings. Testing should be focused on cytomegalovirus and toxoplasmosis, but may also include rubella, varicella, and syphilis in cases at high risk for these infections. Testing for Zika virus and malaria should also be considered in the relevant travel history or location context. However, it should be noted that interpretation of serology results may be challenging due to limited specificity and cross‐reactivity of some of the assays, especially when baseline serology results prior to pregnancy or from early pregnancy are not available. 281 When fetal infection is highly suspected based on serology results or clinical findings, further testing should be offered by means of amniocentesis for the detection of viral DNA in the amniotic fluid using polymerase chain reaction. In these cases, amniocentesis should be delayed until after 21 weeks of gestation and at least 6–8 weeks following the estimated onset of maternal infection to minimize the risk of false‐negative results. 274 , 282
Genetic consultation and genetic testing by amniocentesis should be offered to women with FGR, especially in cases of early‐onset or severe FGR (<3rd percentile), co‐presence of sonographic findings (such as structural anomalies, soft markers, or polyhydramnios), and the absence of obvious signs of placental dysfunction such as abnormal uterine or umbilical artery Doppler. In addition, women should be counselled about the risk of a genetic etiology even in the presence of “isolated” FGR (i.e. without associated fetal anomalies). 37 , 270 , 283 , 284 , 285 A recent meta‐analysis of 10 studies found that in cases of isolated FGR, chromosomal microarray had an incremental yield of 4% (95% CI, 1%–6%) over karyotyping: 17 of 376 fetuses with isolated FGR and normal karyotype had significant findings in microarray, most commonly 22q11.2 duplication, Xp22.3 deletion, and 7q11.23 deletion. The incremental yield of microarray over karyotyping was even higher at 10% (95% CI, 6%–14%) in the presence of associated fetal malformations. 35 Based on these data, it seems reasonable to offer amniocentesis with karyotype and microarray analysis (when available) to women with FGR, with the decision based on factors such as ultrasound findings, gestational age, lack of evidence of placental dysfunction, and whether the results of the amniocentesis would affect management. These data also suggest that the temptation to substitute amniocentesis by noninvasive prenatal testing (NIPT) using cell‐free fetal DNA analysis in this context should be strongly resisted.
7.5. Recommendations
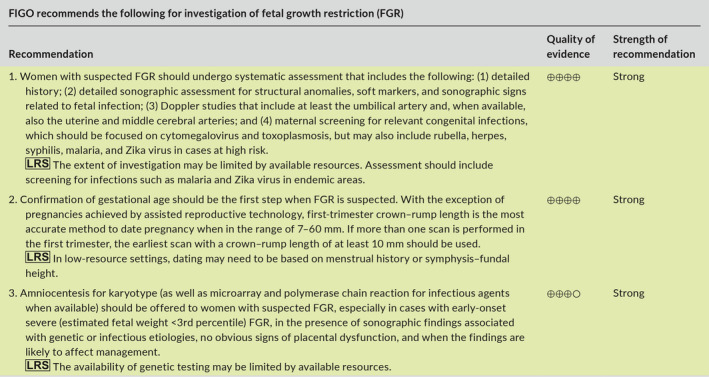
8. MANAGEMENT OF PREGNANCIES WITH FETAL GROWTH RESTRICTION
Management of pregnancies with FGR depends in part on the results of the investigation described in section 7. In cases of fetal abnormalities (genetic or infectious) the management (expectant versus pregnancy termination) should be individualized based on the nature of the disorder, the expected prognosis, gestational age, parental wishes, and local policies.
The most common underlying etiology for FGR is placental dysfunction. In early‐onset FGR (<32 weeks), increased resistance in umbilical artery Doppler is the primary rate‐limiting step to subsequent deterioration of cardiovascular and biophysical parameters. 286 , 287 , 288 , 289 , 290 , 291 , 292 The primary management challenge arises from the risk of fetal deterioration and stillbirth in pregnancies undergoing surveillance versus the neonatal morbidity and mortality associated with preterm delivery. 68 , 293 , 294 , 295 , 296 , 297 In late‐onset FGR (≥32 weeks), cardiovascular deterioration in response to fetal hypoxia is predominantly confined to the cerebral circulation with little umbilical artery Doppler changes. 289 , 291 , 292 , 298 Pregnancies complicated by late‐onset FGR are major contributors to adverse perinatal outcome attributable to FGR because of misdiagnosis and challenges in detecting deterioration during fetal surveillance. 299 , 300
There is no effective antenatal treatment for placental dysfunction and therefore once FGR has been identified, the principal management steps are institution of fetal surveillance and determination of appropriate thresholds for delivery. Perinatal outcome in early‐onset FGR is improved when pregnancies are managed in a high‐level fetal medicine and neonatology unit utilizing a uniform management protocol. 301 Likewise, the optimal management setting for late‐onset FGR is a unit that has access and experience in interpretation of surveillance tests, together with an appropriate level neonatal unit. The recommendations for monitoring, timing and mode of delivery, and potential treatments for placenta‐mediated FGR are described below and summarized in Table 1 and Figure 5.
FIGURE 5.
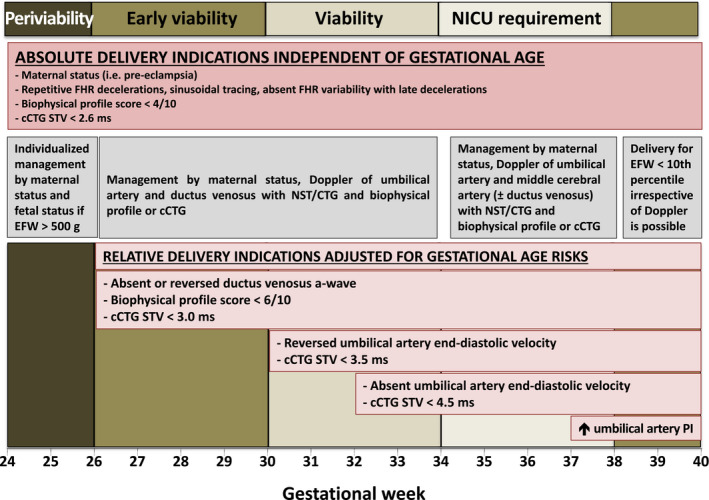
Delivery criteria for fetal growth restriction. Delivery criteria are based on monitoring with umbilical artery, ductus venosus, and middle cerebral artery Doppler at specified gestational ages with traditional nonstress testing or computerized CTG (cCTG) if available. Abbreviations: NICU, neonatal intensive care unit; FHR, fetal heart rate; CTG, cardiotocogram; STV, short‐term variation; ms, milliseconds; EFW, estimated fetal weight; PI, pulsatility index. [Colour figure can be viewed at wileyonlinelibrary.com]
8.1. Monitoring
The primary goal of fetal monitoring is prevention of stillbirth by detection of fetal deterioration that precedes irreversible compromise. To achieve this goal, monitoring tests need to be accurate in identifying fetal risks that favor delivery and, for pregnancies where delivery thresholds are not met, follow‐up monitoring needs to be frequent enough to provide a safety net against unanticipated deterioration or stillbirth. Fetal surveillance tests include maternal monitoring of fetal movements, cardiotocography, ultrasound evaluation of amniotic fluid volume and fetal activity, and Doppler ultrasound of the fetal arterial and venous circulations. With progressive compromise, abnormal fetal activity and fetal heart rate patterns are observed, independent of gestational age at diagnosis of FGR. 288 , 292 , 302 , 303 , 304 In contrast, cardiovascular manifestations of fetal compromise are driven by placental blood flow resistance in the umbilical artery, and therefore differ significantly between early‐ and late‐onset FGR. 286 , 289 , 290 , 291 , 298 , 299 The surveillance tests that have been evaluated in the management of FGR pregnancies are described below.
8.1.1. Fetal movement counting
Fetal activity is established from the first trimester onward and as gestational age advances becomes organized into coordinated behavioral states. Progressive fetal hypoxemia is accompanied by a reduction of fetal activity that can be perceived most accurately by the mother when she is lying down and paying focused attention to fetal movements. 305 Decreased fetal movement is often defined as less than 10 movements in 2 hours during focused maternal counting. 306 Although reports on whether quality improvement tools to promote awareness and management of reduced fetal movements can effectively decrease the risk of stillbirth have been conflicting, most of these interventions were focused on unselected populations rather than in pregnancies with suspected FGR. 307 , 308 , 309 Given that fetal movement counting is a simple and inexpensive tool that may provide a safety net between scheduled outpatient monitoring visits, it seems reasonable to use movement counting as an adjunct to monitoring in FGR. The mother should be provided with clear behavioral instructions and confirmatory monitoring should be performed for patients presenting with decreased fetal movements. 305 , 308
8.1.2. Fetal heart rate monitoring
Fetal heart rate monitoring is universally recommended to monitor pregnancies complicated with FGR. 134 , 310 , 311 , 312 Antepartum cardiotocography (CTG), also known as a nonstress test (NST), can be performed as a standalone evaluation or in conjunction with measurement of amniotic fluid volume (modified biophysical profile), or a five‐component biophysical profile (BBP).
Some heart rate characteristics reflect fetal oxygenation, gestational age, and maturational state of the nervous and cardiovascular systems. The normal heart rate baseline is between 110 and 160 beats per minute (bpm) and decreases with advancing gestation. Periodic accelerations of fetal heart rate (FHR) usually coincide with fetal movements, are observed from the early second trimester, and increase in magnitude and duration with advancing gestation. These are defined as increases in FHR over the baseline of at least 15 bpm and 15 seconds’ duration. Two or more of these accelerations define a “reactive” pattern. Recognizing that the frequency of reactivity increases from 50% at 24–28 weeks to 85% at 28–32 weeks of gestation, criteria of greater than or equal to 10 bpm amplitude and greater than 10 seconds’ duration are recommended at earlier gestational ages. 313 , 314 , 315 , 316 A “nonreactive” FHR pattern is one that does not display accelerations over an observation period of 40 minutes. In addition to reactivity, FHR patterns display “variability”—the average oscillations in the FHR signal, evaluated in bpm in 1‐minute windows. Reduced variability appears later than absent reactivity in the process of progressive fetal hypoxia. It reflects reduced sympathetic–parasympathetic activity, secondary to diminished brainstem oxygenation.
The FHR pattern reflects fetal oxygenation and acid‐base status at the time of evaluation but does not predict deterioration in FGR. In unselected pregnancies the rate of stillbirth in the week following a reactive CTG/NST is 1.9/1000 (negative predictive rate of 99.8%). 317 Without any additional information, the empirically recommended minimum frequency is twice weekly CTG/NST. The frequency may be increased when evaluation of amniotic fluid or Doppler parameters indicate a more advanced degree of fetal compromise and delivery criteria have not yet been met. A nonreactive CTG/NST has low specificity for hypoxia and requires additional tests to determine fetal status and distinguish FHR pattern variations caused by fetal behavior, while reduced variability is a much stronger predictor of central nervous system hypoxia.
8.1.3. Computerized fetal heart rate monitoring
Some professional societies recommend computerized fetal heart rate monitoring (cCTG) as the preferred modality to analyze CTG/NST tracings. 134 , 308 Inconsistency in visual assessment, particularly FHR variability, is a major contributor to interobserver variations in interpretation of CTG/NST tracings. 318 cCTG evaluates FHR parameters such as baseline, accelerations, decelerations, and variability in an objective and quantifiable way. The Sonicaid cCTG system (Huntleigh Healthcare, Cardiff, UK) provides the parameter “short‐term variation” (STV) in milliseconds, while others quantify variability in a more traditional way in bpm. 319 In contrast to visual FHR analysis, cCTG decreases observer variability and allows longitudinal numerical analysis of variability. 320
FHR variability increases with gestational age; after 29 weeks of gestation, below 4.0 ms or below 3.0 ms meet criteria for reduced or very low STV, respectively. 321 Before 29 weeks of gestation, STV below 3.5 ms is considered reduced, and below 2.6 ms is considered very low. STV below 3 ms has a 77% positive predictive value for fetal acidemia. 322 , 323
Similar to CTG/NST, cCTG does not predict fetal deterioration. In early‐onset FGR the daily risks for abnormal STV are 4%–5% but are unpredictable by additional monitoring tests. Accordingly, CTG/NST or cCTG monitoring needs to be performed more frequently than Doppler assessments. In patients receiving inpatient monitoring, a minimum frequency of daily cCTG/CTG/NST is recommended. 320
8.1.4. Ultrasound measurement of amniotic fluid volume
Professional societies do not recommend inclusion of isolated amniotic fluid volume assessment into management decisions for FGR. A decrease in amniotic fluid volume can occur as a result of fetal oliguria in response to progressive placental dysfunction and hypoxia, as well as rupture of membranes. 287 , 288 , 324 Accordingly, additional evaluation is required to determine the significance of decreased amniotic fluid volume. Oligohydramnios can be defined as an ultrasound measured four‐quadrant amniotic fluid index below or equal to 5 cm, or a maximum vertical amniotic fluid pocket below or equal to 2 cm. 325 Use of the latter reduces overdiagnosis of oligohydramnios and is preferred. Oligohydramnios is associated with an increased rate of intrapartum FHR abnormalities, need for cesarean section, and low 5‐minute Apgar scores, but not acidosis at birth. 326
8.1.5. Biophysical profile scoring
Biophysical profile (BPP) scoring is not universally recommended as the primary surveillance tool for FGR and is predominantly utilized in Canada and North America where the concept was first developed for fetal surveillance in the later part of the third trimester. The modified BPP refers to combined use of the CTG/NST as a short‐term indicator of fetal acid‐base balance and the maximum amniotic fluid pocket as an indicator of long‐term placental function. 327 The five‐component BPP comprises fetal breathing movements, gross body movements, and tone, in addition to CTG/NST and maximum amniotic fluid pocket, and therefore includes four indicators of short‐term acid‐base balance. 301
The modified BPP is considered abnormal when either the CTG/NST is nonreactive, or the maximum amniotic fluid pocket is below 2 cm. The most common reason for an abnormal modified BPP is a nonreactive CTG/NST, requiring additional ultrasound observation to complete a five‐component BPP and determine fetal acid‐base balance. The BPP is scored over a 30‐minute ultrasound observation period of the fetus. Fetal breathing movements are considered present if one or more episodes of 30 seconds of breathing or hiccups are observed. Fetal body movement is present when three or more discrete body or limb movements are observed. Fetal tone is present when one or more episodes of extension and flexion of the fetal extremities are observed. Each component of the BPP receives a score of 2 for its presence and 0 for its absence. Scores of 8–10, 6, and 4 or less are considered normal, equivocal, and abnormal, respectively.
In unselected pregnancies, the rate of stillbirth in the week following a normal modified or five‐component BPP is 0.8/1000 (negative predictive rate >99.9%). FGR fetuses show a sequential loss of heart rate reactivity, breathing movements, gross body movement, and tone with decrease in pH. 287 , 288 , 301 , 302 In FGR pregnancies, an abnormal BPP (score of 4 or less) is associated with an umbilical artery pH of less than 7.20, with sensitivity increasing to 100% at a score of 0/10. 301 , 302 , 319
The BPP is a more accurate predictor of fetal acid‐base status at the time of testing than CTG/NST, with a similar accuracy as cCTG. Therefore, a five‐component BPP can be used to clarify fetal acid‐base status when a nonreactive CTG/NST is obtained. The frequency of BPP testing is guided by the same principles as timing of fetal heart rate testing.
8.1.6. Umbilical artery Doppler
Umbilical artery Doppler is universally recommended for monitoring of FGR because it assesses the hemodynamic aspect of placental dysfunction. 134 , 143 , 308 , 310 It is estimated that approximately one‐third of the villous circulation needs to be damaged before a decrease in umbilical artery end‐diastolic velocity occurs. Absent or reversed umbilical artery end‐diastolic velocity corresponds to malperfusion of 50%–70% of the villous vascular tree. 328 Because elevated villous blood flow resistance is predominantly associated with the placental pathology found in early‐onset FGR, umbilical artery Doppler does not reliably predict outcome in late‐onset FGR. 329 , 330 , 331
The umbilical artery Doppler waveform can be quantified using the pulsatility index, or by visual classification of end‐diastolic velocity as absent (AEDV) or reversed (REDV). With increasing degrees of placental blood flow resistance, an abnormal umbilical artery waveform is defined as either having an elevated pulsatility index, AEDV, or REDV. The degree of placental blood flow resistance elevation is the primary factor determining the rate of clinical progression and the associated risk for fetal deterioration and stillbirth in early‐onset‐FGR. 286 , 289 , 291 , 292 When the umbilical artery pulsatility index is elevated but end‐diastolic forward flow is still present, the median time interval to additional surveillance abnormalities is 2 weeks. Once AEDV occurs, cardiovascular deterioration advances after a median of 5 days and the weighted odds ratio for stillbirth is 3.6 (2.3–5.6). 286 , 291 , 332 When REDV occurs, the median interval for further fetal deterioration is 2 days and the weighted odds ratio for stillbirth is 7.3 (4.6–11.4). 291 , 331
In patients with normal umbilical artery Doppler, the recommended frequency to repeat Doppler monitoring ranges from weekly to every other week. However, when AEDV develops, Doppler surveillance is recommended at minimum twice weekly, and for REDV at least three times weekly unless delivery criteria have been met.
8.1.7. Cerebral artery Doppler
The majority of professional societies now recommend middle cerebral artery Doppler for monitoring in late‐onset FGR. Concurrent measurement of the umbilical artery and middle cerebral artery pulsatility index allows calculation of the cerebroplacental Doppler ratio. Both the cerebroplacental ratio and middle cerebral artery pulsatility index decrease as a hemodynamic response to fetal hypoxemia and therefore reflect placental dysfunction, even in those pregnancies where the villous blood flow resistance is not elevated enough to produce an abnormal umbilical artery pulsatility index. Approximately 20% of term SGA fetuses with normal umbilical artery Doppler have a decreased middle cerebral artery pulsatility index, which is associated with a higher rate of cesarean section for intrapartum distress, poor neonatal transition, and adverse developmental outcome. 333 , 334 , 335 The cerebroplacental Doppler ratio is more closely related to fetal hypoxia than its individual components, 336 but has a similar predictive accuracy for perinatal death, fetal distress, or poor neonatal transition as the umbilical artery pulsatility index. 337
Cardiovascular deterioration in late‐onset FGR is characterized by abnormal cerebral artery Doppler. Therefore, an important role of middle cerebral artery Doppler is to provide an estimate of perinatal risk in patients with normal umbilical artery Doppler. 292 , 331 Because of the higher risk for adverse outcome within 1 week of a decrease in middle cerebral artery pulsatility index, it is recommended to utilize at least twice weekly surveillance in this setting.
8.1.8. Ductus venosus Doppler
The few professional societies that recommend ductus venosus Doppler evaluation specify that it should be performed in specialized centers that have expertise in the comprehensive perinatal management of early‐onset FGR. 312 The relative forward flow in atrial systole in the ductus venosus decreases with worsening placental function or reduced fetal cardiac function, leading to an increase in the pulsatility index for veins, absent, or reversal of the a‐wave. 286 , 288 , 291 , 292 , 338
Abnormal ductus venosus Doppler is primarily observed in early‐onset FGR and can provide an estimate of fetal acid‐base balance and the risk of stillbirth. The odds ratio of absent or reversed atrial systolic velocity for an umbilical artery pH less than 7.20 at birth is 4.4 (1.2–17.2). 339 , 340 The weighted odds ratio of absent or reversed ductus venosus atrial systolic velocity for fetal death is 11.6 (6.3–19.7). 331
Abnormal ductus venosus Doppler also predicts fetal decompensation to an abnormal BPP, reduced variability on cCTG, or stillbirth. In fetuses with elevated ductus venosus pulsatility index for veins but forward flow during atrial systole, the median interval to progressive venous Doppler deterioration can be as short as 2 days. 291 In patients that do not yet meet delivery criteria, ductus venosus Doppler is recommended at minimum twice weekly in patients with AEDV and three times weekly when REDV is observed. 286 , 291 , 292 , 341 When ductus venosus Doppler indices increase as a new finding, the frequency of monitoring needs to be increased further.
8.1.9. Surveillance strategy
Monitoring in FGR pregnancies is intended to prevent fetal compromise or stillbirth, and the choice of tests and their timing is heavily influenced by gestational age. A robust plan is essential, since expectant management with ongoing monitoring, particularly in the setting of early‐onset FGR, can result in a three to five‐fold increased stillbirth rate when compared with immediate delivery, depending on the degree of cardiovascular compromise that is tolerated before triggering delivery. 294 , 342 , 343 The optimal monitoring frequency in FGR has not been determined due to the varying circumstances of gestational age and severity of FGR. A combination of surveillance modalities is needed to accurately determine fetal acid‐base status at the time of testing, as well as allowing anticipation of future deterioration. 289 , 290 , 291 , 292 , 298 , 344 The accurate prediction of fetal acid‐base status is required to prevent unnecessary intervention and nonindicated delivery. The anticipation of deterioration informs subsequent monitoring intervals that provide a safety net against unanticipated fetal acidosis and asphyxia. The combination of biophysical (CTG/NST, cCTG, BPP) and cardiovascular parameters (umbilical artery, middle cerebral artery, and ductus venosus Doppler) is considered a robust approach for FGR surveillance. Among these modalities, the combination of CTG/NST and umbilical artery Doppler is universally recommended.
There is good evidence that umbilical artery Doppler offers sufficient information to determine monitoring frequency in early‐onset FGR. Although middle cerebral artery Doppler could provide additional information in those late‐onset FGR pregnancies with normal umbilical artery Doppler, this practice has not been evaluated. 292 , 328 , 329 Based on observational data in term FGR with normal umbilical artery Doppler, the approximate interval to stillbirth in patients with abnormal middle cerebral artery Doppler is 4 days, suggesting the need for twice weekly CTG/NST monitoring. In the absence of further evidence on the clinical benefit of middle cerebral artery Doppler, twice weekly CTG/NST monitoring in FGR after 32 weeks of gestation in patients with normal umbilical artery Doppler provides the same safety net (Table 1).
When umbilical artery pulsatility index is elevated, weekly Doppler is suggested, and when there is AEDV or REDV, more frequent assessment is recommended (Table 1). In early‐onset FGR with AEDV or REDV, the risk of stillbirth increases when the ductus venosus Doppler or the CTG/NST patterns become abnormal. 292 , 319 , 340 However, there is currently no evidence that adjusting the timing of monitoring based on ductus venosus Doppler improves outcome. In patients with AEDV the stillbirth rate is 0%–1% when at least once daily CTG/NST, cCTG, or BPP are performed with predefined delivery criteria. 341 , 342 When monitoring is continued to allow for an increase in the ductus venosus pulsatility index for veins, the stillbirth rate is 2%, and 11% of deliveries occur for abnormal STV, 19% for an abnormal BPP, and 22% for FHR decelerations. 304 , 342 When monitoring is continued in anticipation of reversal of the ductus venosus a‐wave velocity, the stillbirth rate is 4%, and 20% of deliveries occur for abnormal STV, 29% for an abnormal BPP, and 31% for FHR decelerations (Table 1). 342 , 345 This indicates that with ongoing monitoring the risk of FHR abnormalities or an abnormal BPP requiring delivery cannot be predicted by the ductus venosus Doppler. 319 , 346 Based on the regional pattern of practice, this indicates that in patients who are admitted for AEDV, the minimum frequency of CTG/NST or BPP should be daily and more frequent with REDV (Table 1). 319
8.2. Timing of delivery
The timing of delivery in FGR is determined by gestational age, severity of FGR, findings of fetal monitoring tests, and maternal factors such as pre‐eclampsia (Table 1 and Figure 5). Delivery indications can be considered as absolute if they are independent of gestational age, and relative if the threshold to deliver based on the surveillance findings varies across gestational age.
8.2.1. Gestational age‐related risks in fetal growth restriction
With advancing gestational age there are several important changes in the relative risks of delivery versus ongoing surveillance that define the delivery thresholds.
From 24–28 weeks of gestation each day of pregnancy prolongation results in an estimated 2% decrease in neonatal death, as well as major neonatal complications including bronchopulmonary dysplasia, high‐grade intraventricular hemorrhage, and surgical necrotizing enterocolitis. The impact of prematurity, neonatal weight below 500 g, challenging resuscitation, and decreased tolerance for low Apgar scores results in average neonatal survival rates below 50% and intact survival below 50% until 26 weeks. 68 , 298 , 345 , 346
Between 28 and 30 weeks of gestation the daily increment in survival is approximately 0.7%. After 30 weeks, neonatal survival rates exceed 90%, 68 , 296 , 297 and there is a significant decrease in major neonatal complications from approximately 35% at 30 weeks to less than 10% at 34 weeks, as well as a decrease in the risk of neurodevelopmental delay for neonates delivered after this time. FGR infants delivered prior to 30 weeks have a three‐fold higher rate of developmental abnormalities and an up to eight‐fold increased rate of cerebral palsy. 10 , 295
From 34–38 weeks of gestation neonates are more likely to require admission to the intensive care nursery but have reduced risks of major neonatal complications. 347 , 348 In SGA fetuses that remain undelivered after 38 weeks, the risk of stillbirth doubles every week and reaches 60/10 000 for pregnancies that continue beyond the due date. 349 , 350
8.2.2. Gestational age‐related management strategy
The balance between fetal and neonatal risks defines the predominant management strategy at different gestational epochs. Accordingly, the goal of management shifts from gaining fetal viability at 26 weeks to a graded improvement in survival, neonatal morbidity, and neurodevelopment by delaying delivery until 34–36 weeks. The increase in stillbirth rate in undelivered fetuses increasingly favors delivery from 36 weeks onward.
Timing of delivery in FGR has been evaluated in three randomized trials. The growth restriction intervention trial (GRIT) randomized pregnancies that had abnormal fetal biometry and umbilical artery Doppler studies performed as part of clinical management into immediate delivery after completion of a course of steroids versus delivery when the managing physician was no longer comfortable with conservative management. 294 , 295 The monitoring protocol and delivery criteria were not specified. The trial demonstrated that, in the absence of specific criteria, either management approach resulted in the same perinatal outcome. Delaying delivery increased the risk of stillbirth, while earlier delivery resulted in a higher degree of prematurity‐related complications that either led to neonatal death or an increased risk of developmental delay.
The disproportionate intrauterine growth intervention trial at term (DIGITAT) randomized SGA fetuses by several biometry criteria, independent of the umbilical artery Doppler pattern, to induction or expectant monitoring between 36 and 41 weeks of gestation. 347 The study demonstrated that, while elective induction did not affect neonatal or obstetric outcomes, deliveries prior to 38 weeks resulted in a higher rate of admissions to the neonatal intensive care unit.
These trials demonstrate that the relative risk for neonatal complications requires definitive delivery indications until 38 weeks of gestation. After that time, delivery for indication of FGR is likely to prevent stillbirth in ongoing pregnancies. The continuous decrease in neonatal risks requires that delivery indications at early gestational ages occur at a higher threshold for fetal risks than after 30–32 weeks.
8.2.3. Absolute delivery criteria for fetal growth restriction (independent of gestational age)
Absolute delivery criteria are findings associated with important health risks to the mother or fetus, and therefore require delivery without consideration of gestational age (Figure 5).
The fetal biophysical variables are strongly influenced by oxygen tension in the regulatory centers. A 30‐minute BBP score of 0 or 2, or a 60‐minute score of 4 indicates a prelabor fetal pH of less than 7.20 and requires delivery to prevent fetal demise.
Repetitive FHR decelerations, a sinusoidal heart rate, absent variability with recurrent late decelerations, or bradycardia predict fetal acidemia and poor perinatal outcome and require delivery if the causative stimulus cannot be removed. When cCTG is used, a short‐term variation below 2.6 ms is below the 5th percentile irrespective of gestational age and requires delivery for its strong association with fetal acidemia.
Maternal pre‐eclampsia with severe features complicates up to 30% of FGR pregnancies, with a higher proportion in early‐onset FGR. In the absence of effective treatment other than delivery, pre‐eclampsia with uncontrolled severe hypertension, HELLP syndrome (hemolysis, elevated liver enzyme levels, and low platelets), or other evidence of end‐organ damage (e.g. oliguria or acute renal injury other than proteinuria, pulmonary edema, or eclampsia) requires delivery (Figure 5).
8.2.4. Relative delivery criteria for fetal growth restriction (adjusted for gestational age)
The trial of umbilical and fetal flow in Europe (TRUFFLE) evaluated two monitoring strategies and specific delivery criteria in early‐onset FGR, with survival without neurodevelopmental impairment at 2 years of age as the primary outcome. 343 Monitoring with cCTG and umbilical artery Doppler was universal in all patients, while ductus venosus Doppler was only added in two study arms. Patients were randomized to one of three specific delivery criteria: (1) abnormal cCTG STV; (2) mild ductus venosus abnormalities; and (3) severe ductus venosus abnormalities with absence or reversal of the a‐wave. Because patients with ductus venosus Doppler monitoring also had FHR monitoring, safety net delivery criteria based on cCTG were also applied in these groups. These included STV below 2.6 ms irrespective of gestational age and below 3.0 ms from 29 weeks onward. In addition, umbilical artery Doppler findings were utilized as relative delivery criteria from 30 weeks onward for REDV and 32 weeks onward for AEDV. The choice of these thresholds is supported by a recent meta‐analysis that found that in undelivered FGR pregnancies, umbilical artery REDV has a 19% stillbirth rate, which exceeds mortality for neonates delivered from 30 weeks onward, while AEDV carries a 6.8% stillbirth risk, which favors delivery due to lower neonatal mortality from 32 weeks onward (Table 1 and Figure 1). 332
The TRUFFLE study demonstrated that a predefined management strategy produces better outcomes than expected in all FGR pregnancies. 343 The primary endpoint was less frequently observed in patients randomized to deliver for late ductus venosus abnormality. Overall, cCTG was the most frequent trigger for delivery. In the three arms, delivery was based on abnormal STV in 11%–51% of participants, and visually apparent FHR decelerations led to delivery in 22%–31% of participants. While the strategy of awaiting absent or reversed ductus venosus a‐wave to determine delivery produced the better study outcome, it is noteworthy that the stillbirth rate increased four‐fold compared with patients who were monitored with cCTG and umbilical artery Doppler. In addition, an absent ductus venosus a‐wave only triggered delivery in 10% of participants in this study arm. The frequency of delivery decisions based on FHR abnormalities emphasizes the importance of concurrently monitoring growth‐restricted fetuses with more than one modality.
Because cCTG is not universally available, most healthcare providers need to rely on traditional CTG/NST monitoring. While BPP has not been studied in randomized intervention trials in FGR, it is an established monitoring tool to verify fetal status in patients with a nonreactive tracing. In FGR, an abnormal BPP predicts abnormal arterial pH with a similar accuracy to cCTG and is an independent delivery trigger at a comparable frequency to cCTG. 304 , 319 Therefore, it is recommended that in FGR fetuses with nonreassuring CTG/NST not yet meeting the criteria for delivery, a BPP is completed to establish fetal status. If the expertise is not available to perform a BPP, prolongation of the CTG/NST or increase in testing frequency may be required to determine if delivery is necessary.
Optimal delivery criteria for FGR presenting after 32 weeks of gestation have not been evaluated in a randomized trial and are based on expert consensus. Table 1 and Figure 5 summarize the management approaches and recommendations for delivery. When local neonatal outcomes are consistently more favorable for FGR neonates, relative delivery indications may be applied at earlier gestational ages than indicated. For example, improved neonatal survival may justify delivery for REDV from 30 weeks onward.
8.3. Mode of delivery and intrapartum considerations
FGR in itself is not an indication for cesarean section. However, primary cesarean section may be considered in selected cases of severe FGR where the likelihood of successful vaginal delivery is low.
Fetuses with placenta‐mediated FGR are less likely to tolerate the stress associated with labor and are at increased risk of requiring intrapartum urgent cesarean section for nonreassuring FHR tracing. Therefore, in certain cases of FGR, a trial of labor is highly unlikely to be successful and might be associated with fetal risks to the extent that a primary cesarean section should be preferred. This depends on multiple factors including gestational age, severity of FGR, Doppler changes, associated pre‐eclampsia, parity, cervical Bishop score, and patient preference (Table 1).
In cases of early‐onset FGR the main goal is to prolong pregnancy and maximize fetal maturation by means of expectant management under close monitoring until there is evidence of late Doppler changes in the umbilical artery (AEDV or REDV), ductus venosus alterations, or FHR abnormalities. Therefore, at the point when delivery is indicated in cases of severe early‐onset FGR, the fetus might already be experiencing some degree of hypoxia or acidosis, 291 in which case the likelihood of the fetus tolerating labor is low and the rate of cesarean section has been reported to be greater than 80%. 351 In addition, labor induction in general is less likely to be successful during the preterm period. 352 , 353 For these reasons, primary cesarean section is usually the preferred option when delivery is indicated in cases of severe early‐onset FGR. 354
In contrast, late‐onset FGR is usually less severe and fetal hypoxia or acidosis are less likely to be present at the time when delivery is indicated. Indeed, in the DIGITAT trial the rate of vaginal delivery was greater than 80% in pregnancies induced for SGA with normal umbilical artery Doppler after 36 weeks of gestation. 347 This observation suggests that most term SGA fetuses with normal umbilical artery Doppler can tolerate labor and that the presence of late‐onset FGR in the absence of additional factors does not preclude induction of labor. Several studies have tried to individualize the decision regarding mode of delivery through the development of models for the prediction of urgent cesarean section in women with late‐onset SGA undergoing labor induction. The factors that were most predictive of urgent cesarean section were gestational age, severity of SGA (EFW <3rd percentile), cerebral Doppler (middle cerebral artery and cerebroplacental ratio), and Bishop score. 355 , 356 For example, in a large cohort study of 509 women undergoing labor induction for late‐onset SGA, the predictive model had a positive predictive value of 36% and a negative predictive value of 89% for urgent cesarean section for nonreassuring fetal state. 355 Thus, although this information can be helpful for patient counselling regarding mode of delivery and may reassure women with none of these risk factors of the high likelihood of a successful trial of labor (nearly 90%), the positive predictive value of these models (i.e. a risk of cesarean section in the range of 30%–40%) is not high enough to preclude a trial of labor even when these risk factors are present.
The optimal approach for cervical ripening in women undergoing induction for FGR remains unclear. In a recent meta‐analysis of 12 trials on cervical ripening in pregnancies complicated by SGA or FGR, the authors concluded that mechanical methods (such as balloon catheters) seem to be associated with a lower risk of cesarean section and intrapartum complications compared with alternatives such as dinoprostone. 357 Given these data, it seems reasonable to prefer balloon catheter over prostaglandin preparations, when possible, for cervical ripening in pregnancies with suspected FGR. If prostaglandin agents are used, a reversible method (e.g. dinoprostone vaginal insert) should be preferred.
During labor, continuous FHR monitoring is recommended. Delivery should take place at an institution with the appropriate level of neonatal care for the gestational age and the anticipated management needs of the neonate.
It is recommended that the placenta is sent for histopathological evaluation after delivery. Ideally this should be done in accordance with the Amsterdam workshop consensus statement. 358 High‐quality evaluation of the placental pathology is not only likely to increase the precision of the diagnosis but also provides information on the risks of recurrence. 18 , 359 , 360
8.4. Medical interventions
8.4.1. Antenatal corticosteroids
The efficacy of antenatal corticosteroids in cases of FGR has been questioned, based on reports of elevated endogenous cortisol levels in this population when compared with normally grown fetuses. 361 , 362 , 363 , 364 In addition, the unique cardiovascular, hormonal, and metabolic changes characteristic of growth‐restricted fetuses 276 , 365 , 366 , 367 , 368 , 369 have raised concerns that exposure to exogenous steroids may produce potentially harmful cardiovascular and metabolic effects in these already compromised fetuses. Indeed, exposure to corticosteroids has been shown to result in Doppler changes in growth‐restricted fetuses such as transient increase in diastolic flow in the umbilical artery 370 , 371 , 372 , 373 and the middle cerebral artery, 374 , 375 , 376 which have been attributed to peripheral vasodilatation or an increase in cardiac output and circulatory stress. 376 , 377 Despite this, recent data support the efficacy and safety of antenatal corticosteroids in the subgroup of SGA fetuses, 378 , 379 which should be administered when delivery is anticipated, ideally within 1–7 days before birth. 380 When administered in cases of severe FGR with late Doppler changes, an inpatient setting is advised where the fetus can be closely monitored. Finally, it is important to recognize that the “improvement” in umbilical artery Doppler that is often seen following administration of antenatal corticosteroids is transient, and is thought to be the result of vasodilation of the fetoplacental arterial tree and increased fetal cardiac output rather than a true decrease in placental resistance. 381 Therefore, these transient changes should not be interpreted as an improvement in fetal status and should not affect the management plan. Of note, the absence of any change in end‐diastolic flow in response to antenatal corticosteroids is a concern and predicts subsequent fetal deterioration. 372
8.4.2. Magnesium sulfate for neuroprotection
Administration of magnesium sulfate to women at risk of preterm birth has been shown to have a neuroprotective role, with a decrease in the risk of perinatal mortality, cerebral palsy, and gross motor dysfunction. 382 , 383 Possible mechanisms thought to be involved in the beneficial effects of magnesium sulfate include reducing intracellular calcium levels, stabilizing blood pressure, normalizing cerebral blood flow, blocking the effects of excitatory neurotransmitters such as glutamate, and antioxidant and anti‐inflammatory effects. 384 , 385 However, the optimal protocol for the administration of magnesium sulfate for the purpose of neuroprotection remains unclear and available protocols vary with regard to the timing of administration, upper gestational age limit, dose, duration, and need for repeat doses. 386 , 387 , 388 , 389
The observation that term FGR infants have higher cord blood magnesium levels compared with normally grown infants raises the theoretical concern that maternal administration of magnesium sulfate in cases of FGR might result in toxic magnesium levels in the fetus. 390 , 391 However, there are currently no data on the efficacy and safety of magnesium sulfate in FGR fetuses that can support or refute these theoretical concerns. Therefore, there is currently no evidence in favor or against recommending administration of magnesium sulfate for neuroprotection in women at risk of preterm birth with suspected FGR. 379 We believe that, at the current time, it is reasonable to extrapolate the efficacy of magnesium sulfate to specific subgroups of pregnancies, including those complicated by FGR, especially given that FGR is an independent risk factor for cerebral palsy.
8.4.3. Treatments under investigation
Several novel therapies aiming to improve poor placentation and uterine blood flow are being explored, some of which are described below. However, there are currently no proven treatments for FGR, and any of the therapies currently under investigation should be evaluated only in an appropriately regulated research setting. 392
Phosphodiesterase type‐5 inhibitors, such as sildenafil citrate, potentiate nitric oxide availability, lead to vasodilatation,, 393 , 394 and can improve umbilical artery and middle cerebral artery Doppler. 395 However, in the recently published STRIDER trial, which randomized 135 women with early‐onset FGR to 25 mg sildenafil three times daily or placebo, sildenafil did not prolong pregnancy or improve pregnancy outcomes. 396 More recently, a similar randomized trial was halted prematurely due to lack of benefit along with concerns that sildenafil may cause neonatal pulmonary hypertension. 397
Another approach is to target the uteroplacental circulation with maternal vascular endothelial growth factor gene therapy, thereby improving local vasodilatation and angiogenesis. 392 Clinically, vector delivery into the uterine arteries can be achieved with intervention radiology. This approach is currently being investigated in the ongoing EVERREST trial. 398 Protein pump inhibitors have been shown in vitro to decrease sFlt‐1 and soluble endoglin and improve markers of endothelial dysfunction. However, in a recent randomized trial involving 120 women with preterm pre‐eclampsia, esomeprazole did not improve pregnancy outcomes. 399 Pravastatin has been shown to have anti‐inflammatory, antioxidant, and proangiogenic properties. 400 , 401 However, in a recently published randomized trial of 94 women with early‐onset pre‐eclampsia, the administration of 40 mg pravastatin daily did not lower maternal sFlt‐1 levels or prolong pregnancy when compared with placebo. 402 Other novel potential therapies include nanoparticles and microRNAs that deliver drugs locally to the uterine arterial endothelium or trophoblasts, to improve uterine blood flow and placental function.
8.5. Recommendations
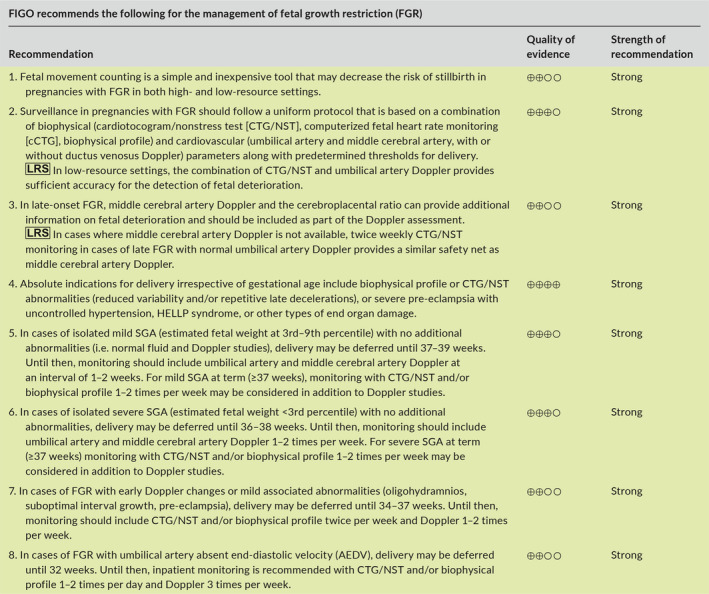
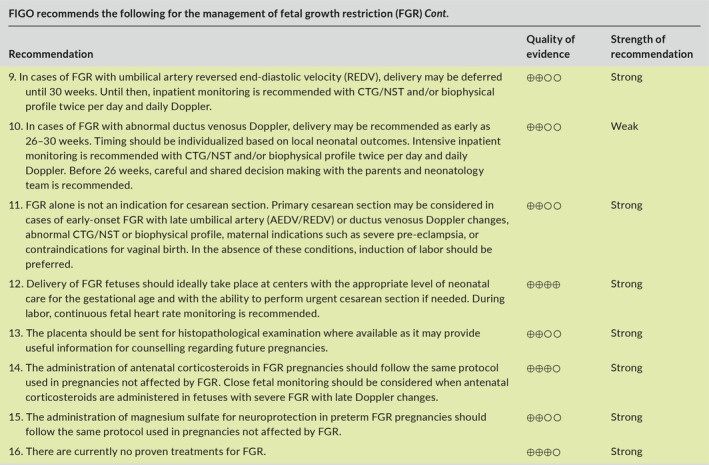
9. POSTPARTUM ASSESSMENT AND COUNSELLING FOR FUTURE PREGNANCIES
9.1. Infant follow‐up
Growth‐restricted infants are at increased risk of both immediate and long‐term complications, and therefore require closer follow‐up than normally grown infants in the first years of life.
Growth‐restricted infants have lower survival rates compared with those appropriate for gestational age. 403 Although this may be attributed in part to prematurity that is often associated with FGR, birth weight has been shown to be an independent prognostic factor for neonatal mortality, irrespective of gestational age. 404 In a population‐based cohort study, the mortality rate of term FGR neonates was approximately five‐fold higher compared with appropriate for gestational age neonates (0.3% vs 0.06%). 405
FGR can affect postnatal growth. In cases of mild FGR, infants tend to achieve normal height during the first year of life. 406 In cases affected by severe FGR, however, height in the late teens is lower than those born appropriate for gestational age (169.9 ± 1.5 vs 175.4 ± 0.8 cm; p < 0.0001 for boys; and 159.4 ± 1.3 vs 163.1 ± 0.8 cm; p < 0.0005 for girls). 407
FGR infants are also at increased risk of adverse long‐term neurodevelopmental outcomes. A systematic review of this topic found that FGR infants are at higher risk of poor neurodevelopmental outcomes measured up to 3 years of age; however, high levels of heterogeneity in primary outcomes were reported in the studies included in the review. 12 Of note, adverse neurodevelopmental outcomes may at least partly be related to coexisting increased prematurity rates. 408
In line with the developmental origins of health and disease hypothesis, FGR has been associated, in both animal and human studies, with an increased risk of future noncommunicable diseases including obesity, diabetes, hypertension, and cardiovascular disease. 409 , 410 , 411 The risk is especially high in those infants who experience rapid catch‐up growth in the first few years of life. 412 , 413 The mechanisms underlying these associations are not entirely clear. However, fetal programming by means of epigenetic changes as well as direct organ damage are thought to play a role. 414 Ongoing studies are investigating the optimal follow‐up and prevention strategies to decrease the risk of these complications. 415 , 416
9.2. Maternal follow‐up
It is well established that women with a history of pregnancy complicated by FGR or other placenta‐mediated complications such as pre‐eclampsia are at an increased risk of future cardiovascular disease, especially in the presence of early‐onset disease. In a population‐based study that included more than 100 000 pregnancies and provided maternal follow‐up for 15–19 years, delivery of a low birth weight infant was associated with increased maternal risk of ischemic heart disease or death (aHR 1.9; 95% CI, 1.5–2.4). 417 Moreover, a combination of FGR, pre‐eclampsia, and preterm delivery amplified the risk of disease seven‐fold. For a detailed review of the evidence supporting these associations, their underlying mechanisms, and recommendations on maternal follow‐up and prevention strategies please refer to the recently published FIGO postpregnancy initiative on long‐term maternal implications of pregnancy complications and follow‐up considerations. 4
9.3. Counselling regarding future pregnancies
The most frequent and relevant question that care providers are being asked by couples whose prior pregnancy was complicated by FGR relates to the likelihood of a similar complication in subsequent pregnancies. The answer to this question is often difficult and depends on several factors, namely the underlying etiology, severity and timing of onset, and the presence or absence of modifiable risk factors (e.g. maternal medical conditions or smoking). In cases of placenta‐mediated FGR, the results of the placental histopathological examination may provide valuable information that can assist care providers in counselling patients regarding the risk of recurrence, role of further investigation, and potential preventive interventions in subsequent pregnancies.
9.3.1. Risk of recurrence based on severity and onset
Most of the data on the risk of recurrence of placenta‐mediated complications come from studies evaluating hypertensive complications of pregnancy. In a recent individual patient data meta‐analysis of 22 studies, the overall risk of recurrence of hypertensive complications was 21%, and was higher in women who experienced early‐onset hypertensive complications. 418 Data on the recurrence of FGR are limited. 419 , 420 , 421 , 422 In a population‐based study, the overall recurrence rate of FGR in women who gave birth to an infant with a birth weight below the 10th percentile was 24%, compared with 6% in women without a history of FGR (OR 3.9; 95% CI, 3.7–4.0). The risk of recurrence was related to the severity of FGR, and was nearly six‐fold when the infant birth weight was below the 5th percentile (OR 5.7; 95% CI, 5.4–6.0). 423 Thus, couples with FGR in the first pregnancy can be reassured that the overall chance of recurrence in subsequent pregnancies is less than 25%. However, interpretation of the data is limited by the lack of distinction between constitutionally SGA infants and infants who were truly growth restricted, as much of the association described in that study may be driven by the recurrence of constitutional SGA. Therefore, counselling regarding the risk of recurrence should be further refined based on the risk factors of the individual patient, severity of FGR as reflected by timing of onset and Doppler findings, the co‐presence of pre‐eclampsia, and placental histopathological findings.
9.3.2. Risk of recurrence based on placental histopathology
The results of the placental histopathological examination are important for two main reasons. First, they may assist care providers in counselling couples regarding the most likely etiology of FGR, especially when the clinical presentation and Doppler findings were inconclusive. Second, placental findings may provide valuable information regarding the risk of recurrence, as certain types of placental pathologies are associated with a relatively high recurrence rate. The main types of placental pathologies, the clinical phenotypes associated with these pathologies, and their estimated risks of recurrence are summarized in Table 2. 424 , 425 , 426
TABLE 2.
Phenotypes and risk of recurrence associated with specific types of placental pathologies.
| Placental pathology | Incidence | Common placental findings | Pathophysiology | Phenotype | Risk of recurrence | Recommendations for investigation and prevention in next pregnancy | Refs |
|---|---|---|---|---|---|---|---|
| Maternal vascular malperfusion (MVM) | Common | Decidual arteriopathy, agglutinated villi, increased syncytial knots, intervillous fibrin deposition, villous infarcts | Placental malperfusion due to shallow trophoblast invasion and failure of remodeling of spiral arteries | Early‐ or late‐onset FGR, pre‐eclampsia, placental abruption | 10%–25% |
Screening for antiphospholipid antibodies may be considered in selected cases of severe early‐onset FGR, when placental examination shows features of severe MVM such as especially central or multiple areas of villous infarction Consider aspirin in subsequent pregnancy, especially if associated with pre‐eclampsia |
173, 425 |
| Fetal vascular malperfusion (FVM) | Relatively common | Avascular villi, chorionic plate or stem villous thrombi, obstructive lesions of umbilical cord |
Most common cause: chronic/intermittent cord obstruction due to cord compression, entanglement, or hypercoiling. Possible association with hereditary thrombophilia |
FGR, fetal CNS injury, stillbirth | Low | Consider screening of the infant or the mother for hereditary thrombophilia | 454, 455, 456 |
| Chronic inflammation | |||||||
| Villitis of unknown etiology (VUE) | Relatively common (5%–10% of term placentas) | Chronic T‐cell mediated inflammation of villous stroma | Maternal graft versus host response to fetal antigens in the placenta | Late‐onset FGR, abnormal neurodevelopmental outcome, stillbirth | 10%–50% | 457, 458, 459, 460, 461, 462, 463, 464 | |
| Chronic histiocytic intervillositis | Rare | Maternal histiocytic infiltrate in the intervillous space | Recurrent miscarriages, recurrent severe early‐onset FGR, stillbirth | 70%–100% |
Suggested interventions include prednisone, hydroxychloroquine, aspirin, low‐molecular‐weight heparin Associated with increased levels of serum alpha‐fetoprotein and alkaline phosphatase |
461, 463, 465, 466, 467, 468, 469 | |
| Massive perivillous fibrinoid deposition (maternal floor infarction) | Rare | Large amounts of fibrinoid matrix surrounding villi | Unclear | Recurrent miscarriages, recurrent severe early‐onset FGR, stillbirth | 40%–60% |
Consider screening for antiphospholipid antibodies, hereditary thrombophilia Anecdotal reports of treatment with aspirin, heparin, and IVIG |
426, 463, 470 |
Abbreviations: CNS, central nervous system; FGR, fetal growth restriction; IVIG, intravenous immunoglobulins.
9.3.3. Role of thrombophilia screening
Whether women who experienced placenta‐mediated pregnancy complications should be screened for antiphospholipid syndrome is a matter of debate. Although the consensus criteria for antiphospholipid syndrome include premature birth before 34 weeks for severe pre‐eclampsia or features consistent with placental insufficiency including birth weight below the 10th percentile, 427 the association of antiphospholipid (aPL) antibodies with these conditions is relatively weak and conflicting, especially for FGR. 428 , 429 , 430 In addition, although some care providers recommend treatment with LMWH during pregnancy to women with aPL syndrome and previous preterm birth for placenta‐mediated complications, this practice is mostly extrapolated from women with aPL syndrome and recurrent pregnancy loss, where there is some evidence in favor of LMWH. 431 , 432 , 433 However, the only trial on LMWH in women with aPL syndrome and prior placenta‐related complications (FRUIT trial) found no evidence that LMWH improves outcomes in these cases. 434 Given the above, there is insufficient evidence to justify routine screening for aPL antibodies in women with prior FGR. 435 However, screening for aPL antibodies is recommended in women with a history of thromboembolism or recurrent pregnancy loss (or ≥1 late fetal loss), and may be considered in selected cases of women with a history of severe FGR associated with severe early‐onset pre‐eclampsia, when placental examination shows features of severe maternal vascular malperfusion, especially central or multiple areas of villous infarction that are due to multiple spiral artery thromboses.
Management of women already diagnosed with antiphospholipid syndrome based on a history of placenta‐mediated complications is also under debate. Based on the evidence from the FRUIT trial described above, some only recommend treatment with aspirin in this setting, 436 while others recommend either surveillance or LMWH during the antepartum and postpartum periods. 437 Based on available evidence we only recommend treatment with aspirin, and suggest that LMWH be considered only in selected cases, such as for women who have experienced recurrent complications despite aspirin treatment (aspirin failure).
The findings are clearer for inherited thrombophilias. Most prospective studies found no significant association between inherited thrombophilia and placenta‐mediated complications. 438 , 439 , 440 , 441 , 442 , 443 Furthermore, the TIPPS and FRUIT trials found no benefit of LMWH in women with thrombophilia and a history of placenta‐mediated pregnancy complications. 444 , 445 These findings were confirmed by a recent individual patient data meta‐analysis that found no benefit of LMWH in decreasing the risk of recurrence of placenta‐mediated complications, including in women with thrombophilia. 140 Therefore, there is no indication for routine screening for inherited thrombophilia in women with prior FGR. 446 , 447
9.3.4. Preconception counselling and management of future pregnancies
Given the considerable risk of recurrence of FGR, efforts should be made to decrease this risk in future pregnancies. 448 Modifiable risk factors for FGR such as smoking or poor nutritional status should be identified as early as possible and managed accordingly, as discussed in section 5.5.1.
There is some evidence that administration of aspirin can reduce the risk of FGR. However, as described in section 5.5.2 most available data focused on the prevention of pre‐eclampsia as the primary outcome in women at high risk of pre‐eclampsia, with the prevention of FGR being considered a secondary outcome. Data on the prevention of recurrence of FGR in women with a history of FGR are limited. 449 Therefore, some recommend that aspirin should be considered in women with past FGR only if they have risk factors for pre‐eclampsia at the time of the next pregnancy. 450 However, we believe that given the safety of aspirin and the overlap in pathogenesis of pre‐eclampsia and FGR, it is reasonable to recommend aspirin to women with a history of placenta‐mediated FGR in the previous pregnancy, using the same regimen of aspirin used for the prevention of pre‐eclampsia. This recommendation is shared by most professional societies. 134
Data on the role of LMWH to prevent recurrence of placenta‐mediated complications including FGR are conflicting, and this topic is reviewed in section 5.5.2. Based on available data, LMWH therapy should not be used in women with a past history of FGR except in a research setting.
Given the association of insufficient gestational weight gain with FGR, we recommend monitoring of weight gain and informing women about their target weight gain range, as described in section 5.5.1. Other interventions, such as bed rest or nutritional supplements are of unproven benefit and should not be routinely offered. 451 , 452 The risk of recurrence can be further stratified in early pregnancy by means of prenatal screening with biochemical markers (PAPP‐A, beta hCG, alpha‐fetoprotein, and PlGF) as well as by uterine artery Doppler, as described in section 5. Due to the increased risk of recurrence, pregnant women with a history of FGR in a previous pregnancy should be managed in a high‐risk pregnancy clinic and should receive closer antenatal surveillance, including close monitoring of fetal growth and maternal blood pressure. 453
9.4. Recommendations
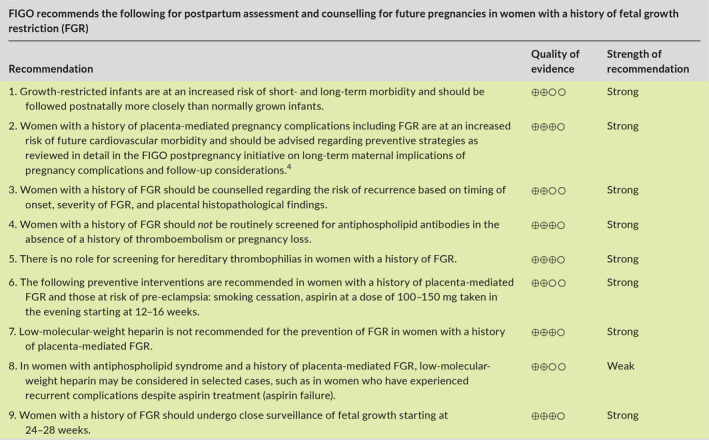
10. SUMMARY AND FUTURE RESEARCH DIRECTIONS
FGR is an important cause of stillbirth, neonatal mortality, and short‐ and long‐term neonatal morbidity. Early prediction and preventive strategies, timely diagnosis, and management using a standardized protocol to determine the proper monitoring and timing of delivery can decrease the risk of stillbirth and improve perinatal outcomes in pregnancies complicated by FGR.
Future research should focus on the development of new fetal assessment tools that may improve the accuracy of the prediction of fetal deterioration and thus further optimize timing of delivery of FGR fetuses, as well as on novel treatments that may improve placental function in cases of placenta‐mediated FGR and thereby deferring delivery in cases of early‐onset FGR.
Supporting information
Table S1‐S2
Funding Information
Publication of this Supplement was funded by FIGO.
Conflicts of Interest
The authors have no conflicts of interest. Dr Romero contributed to this work as part of his official duties as an employee of the United States Federal Government.
REFERENCES
- 1. Gordijn SJ, Beune IM, Thilaganathan B, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol. 2016;48(3):333‐339. [DOI] [PubMed] [Google Scholar]
- 2. Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements–a prospective study. Am J Obstet Gynecol. 1985;151(3):333‐337. [DOI] [PubMed] [Google Scholar]
- 3. Visser GHA, Nicholson WK, Barnea ER, et al. FIGO position paper on reference charts for fetal growth and size at birth: Which one to use? Int J Gynecol Obstet. 2020; 10.1002/ijgo.13500 . [DOI] [PubMed] [Google Scholar]
- 4. Sheiner E, Kapur A, Retnakaran R, et al. FIGO (International Federation of Gynecology and Obstetrics) postpregnancy initiative: long‐term maternal implications of pregnancy complications‐follow‐up considerations. Int J Gynecol Obstet. 2019;147(Suppl 1):1‐31. [DOI] [PubMed] [Google Scholar]
- 5. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schunemann HJ, Jaeschke R, Cook DJ, et al. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med. 2006;174:605‐614. [DOI] [PubMed] [Google Scholar]
- 7. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401‐406. [DOI] [PubMed] [Google Scholar]
- 8. Pels A, Beune IM, van Wassenaer‐Leemhuis AG, Limpens J, Ganzevoort W. Early‐onset fetal growth restriction: a systematic review on mortality and morbidity. Acta Obstet Gynecol Scand. 2020;99(2):153‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baschat AA, Viscardi RM, Hussey‐Gardner B, Hashmi N, Harman C. Infant neurodevelopment following fetal growth restriction: relationship with antepartum surveillance parameters. Ultrasound Obstet Gynecol. 2009;33(1):44‐50. [DOI] [PubMed] [Google Scholar]
- 10. Baschat AA. Neurodevelopment following fetal growth restriction and its relationship with antepartum parameters of placental dysfunction. Ultrasound Obstet Gynecol. 2011;37(5):501‐514. [DOI] [PubMed] [Google Scholar]
- 11. Baschat AA. Neurodevelopment after fetal growth restriction. Fetal Diagn Ther. 2014;36(2):136‐142. [DOI] [PubMed] [Google Scholar]
- 12. Levine TA, Grunau RE, McAuliffe FM, Pinnamaneni R, Foran A, Alderdice FA. Early childhood neurodevelopment after intrauterine growth restriction: a systematic review. Pediatrics. 2015;135(1):126‐141. [DOI] [PubMed] [Google Scholar]
- 13. Murray E, Fernandes M, Fazel M, Kennedy SH, Villar J, Stein A. Differential effect of intrauterine growth restriction on childhood neurodevelopment: a systematic review. BJOG. 2015;122(8):1062‐1072. [DOI] [PubMed] [Google Scholar]
- 14. Melamed N, Asztalos E, Murphy K, et al. Neurodevelopmental disorders among term infants exposed to antenatal corticosteroids during pregnancy: a population‐based study. BMJ Open. 2019;9(9):e031197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crispi F, Miranda J, Gratacos E. Long‐term cardiovascular consequences of fetal growth restriction: biology, clinical implications, and opportunities for prevention of adult disease. Am J Obstet Gynecol. 2018;218(2S):S869‐S879. [DOI] [PubMed] [Google Scholar]
- 16. Figueras F, Gratacos E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage‐based management protocol. Fetal Diagn Ther. 2014;36(2):86‐98. [DOI] [PubMed] [Google Scholar]
- 17. Mifsud W, Sebire NJ. Placental pathology in early‐onset and late‐onset fetal growth restriction. Fetal Diagn Ther. 2014;36(2):117‐128. [DOI] [PubMed] [Google Scholar]
- 18. Aviram A, Sherman C, Kingdom J, Zaltz A, Barrett J, Melamed N. Defining early vs late fetal growth restriction by placental pathology. Acta Obstet Gynecol Scand. 2019;98(3):365‐373. [DOI] [PubMed] [Google Scholar]
- 19. Crovetto F, Crispi F, Scazzocchio E, et al. First‐trimester screening for early and late small‐for‐gestational‐age neonates using maternal serum biochemistry, blood pressure and uterine artery Doppler. Ultrasound Obstet Gynecol. 2014;43(1):34‐40. [DOI] [PubMed] [Google Scholar]
- 20. Maulik D. Fetal growth restriction: the etiology. Clin Obstet Gynecol. 2006;49(2):228‐235. [DOI] [PubMed] [Google Scholar]
- 21. Nardozza LM, Caetano AC, Zamarian AC, et al. Fetal growth restriction: current knowledge. Arch Gynecol Obstet. 2017;295(5):1061‐1077. [DOI] [PubMed] [Google Scholar]
- 22. Hiersch L, Yogev Y. Pregnancy: impact of maternal nutrition on intrauterine fetal growth. World Rev Nutr Diet. 2018;117:151‐164. [DOI] [PubMed] [Google Scholar]
- 23. Yogev Y, Hiersch L. Pregnancy: impact of maternal nutrition on intrauterine fetal growth. World Rev Nutr Diet. 2014;109:101‐108. [DOI] [PubMed] [Google Scholar]
- 24. Ghaly A, Maki Y, Nygard K, Hammond R, Hardy DB, Richardson BS. Maternal nutrient restriction in guinea pigs leads to fetal growth restriction with increased brain apoptosis. Pediatric Res. 2019;85(1):105‐112. [DOI] [PubMed] [Google Scholar]
- 25. Liu Y, Li H, Sha Q, et al. Effects of maternal undernutrition on the growth, development and antioxidant status of ovine placentome subtypes during late pregnancy. Theriogenology. 2018;110:96‐102. [DOI] [PubMed] [Google Scholar]
- 26. Papathakis PC, Singh LN, Manary MJ. How maternal malnutrition affects linear growth and development in the offspring. Mol Cell Endocrinol. 2016;435:40‐47. [DOI] [PubMed] [Google Scholar]
- 27. Rahman MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low‐ and middle‐income countries: systematic review and meta‐analysis. Am J Clin Nutr. 2016;103(2):495‐504. [DOI] [PubMed] [Google Scholar]
- 28. Stangret A, Wnuk A, Szewczyk G, Pyzlak M, Szukiewicz D. Maternal hemoglobin concentration and hematocrit values may affect fetus development by influencing placental angiogenesis. J Matern Fetal Neonatal Med. 2017;30(2):199‐204. [DOI] [PubMed] [Google Scholar]
- 29. Burton GJ, Jauniaux E. Pathophysiology of placental‐derived fetal growth restriction. Am J Obstet Gynecol. 2018;218(2S):S745‐S761. [DOI] [PubMed] [Google Scholar]
- 30. Kingdom JC, Audette MC, Hobson SR, Windrim RC, Morgen E. A placenta clinic approach to the diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2018;218(2S):S803‐S817. [DOI] [PubMed] [Google Scholar]
- 31. Zur RL, Kingdom JC, Parks WT, Hobson SR. The Placental Basis of Fetal Growth Restriction. Obstet Gynecol Clin North Am. 2020;47(1):81‐98. [DOI] [PubMed] [Google Scholar]
- 32. Gluck O, Schreiber L, Marciano A, Mizrachi Y, Bar J, Kovo M. Pregnancy outcome and placental pathology in small for gestational age neonates in relation to the severity of their growth restriction. J Matern Fetal Neonatal Med. 2019;32(9):1468‐1473. [DOI] [PubMed] [Google Scholar]
- 33. Bos M, Harris‐Mostert E, van der Meeren LE, et al. Clinical outcomes in chronic intervillositis of unknown etiology. Placenta. 2020;91:19‐23. [DOI] [PubMed] [Google Scholar]
- 34. Snijders RJ, Sherrod C, Gosden CM, Nicolaides KH. Fetal growth retardation: associated malformations and chromosomal abnormalities. Am J Obstet Gynecol. 1993;168(2):547‐555. [DOI] [PubMed] [Google Scholar]
- 35. Borrell A, Grande M, Pauta M, Rodriguez‐Revenga L, Figueras F. Chromosomal microarray analysis in fetuses with growth restriction and normal karyotype: a systematic review and meta‐analysis. Fetal Diagn Ther. 2018;44(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 36. Sagi‐Dain L, Peleg A, Sagi S. Risk for chromosomal aberrations in apparently isolated intrauterine growth restriction: a systematic review. Prenatal Diagn. 2017;37(11):1061‐1066. [DOI] [PubMed] [Google Scholar]
- 37. Borrell A, Grande M, Meler E, et al. Genomic microarray in fetuses with early growth restriction: a multicenter study. Fetal Diagn Ther. 2017;42(3):174‐180. [DOI] [PubMed] [Google Scholar]
- 38. Khoury MJ, Erickson JD, Cordero JF, McCarthy BJ. Congenital malformations and intrauterine growth retardation: a population study. Pediatrics. 1988;82(1):83‐90. [PubMed] [Google Scholar]
- 39. Longo S, Borghesi A, Tzialla C, Stronati M. IUGR and infections. Early Hum Dev. 2014;90(Suppl 1):S42‐44. [DOI] [PubMed] [Google Scholar]
- 40. Crino JP, Driggers RW. Ultrasound findings associated with antepartum viral infection. Clin Obstet Gynecol. 2018;61(1):106‐121. [DOI] [PubMed] [Google Scholar]
- 41. Leruez‐Ville M, Ville Y. Fetal cytomegalovirus infection. Best Pract Res Clin Obstet Gynaecol. 2017;38:97‐107. [DOI] [PubMed] [Google Scholar]
- 42. Accrombessi M, Zeitlin J, Massougbodji A, Cot M, Briand V. What do we know about risk factors for fetal growth restriction in Africa at the time of sustainable development goals? A Scoping Review. Paediatr Perinat Epidemiol. 2018;32(2):184‐196. [DOI] [PubMed] [Google Scholar]
- 43. Platt DJ, Miner JJ. Consequences of congenital Zika virus infection. Curr Opin Virol. 2017;27:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mandelbrot L. Fetal varicella – diagnosis, management, and outcome. Prenatal Diagn. 2012;32(6):511‐518. [DOI] [PubMed] [Google Scholar]
- 45. Seitz J, Morales‐Prieto DM, Favaro RR, Schneider H, Markert UR. Molecular principles of intrauterine growth restriction in plasmodium falciparum infection. Front Endocrinol. 2019;10:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Umbers AJ, Aitken EH, Rogerson SJ. Malaria in pregnancy: small babies, big problem. Trends Parasitol. 2011;27(4):168‐175. [DOI] [PubMed] [Google Scholar]
- 47. Yoon I, Slesinger TL. Radiation exposure in pregnancy. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 48. Ganapathy V. Drugs of abuse and human placenta. Life Sci. 2011;88(21–22):926‐930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Holbrook BD, Rayburn WF. Teratogenic risks from exposure to illicit drugs. Obstet Gynecol Clin North Am. 2014;41(2):229‐239. [DOI] [PubMed] [Google Scholar]
- 50. Carter RC, Jacobson JL, Molteno CD, Dodge NC, Meintjes EM, Jacobson SW. Fetal alcohol growth restriction and cognitive impairment. Pediatrics. 2016;138(2):e20160775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Basso O, Wilcox AJ, Weinberg CR. Birth weight and mortality: causality or confounding? Am J Epidemiol. 2006;164(4):303‐311. [DOI] [PubMed] [Google Scholar]
- 52. Flenady V, Koopmans L, Middleton P, et al. Major risk factors for stillbirth in high‐income countries: a systematic review and meta‐analysis. Lancet. 2011;377(9774):1331‐1340. [DOI] [PubMed] [Google Scholar]
- 53. Gardosi J, Madurasinghe V, Williams M, Malik A, Francis A. Maternal and fetal risk factors for stillbirth: population based study. BMJ. 2013;346:f108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pilliod RA, Cheng YW, Snowden JM, Doss AE, Caughey AB. The risk of intrauterine fetal death in the small‐for‐gestational‐age fetus. Am J Obstet Gynecol. 2012;207(4):318.e311‐318e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kady SM, Gardosi J. Perinatal mortality and fetal growth restriction. Best Pract Res Clin Obstet Gynaecol. 2004;18(3):397‐410. [DOI] [PubMed] [Google Scholar]
- 56. Bukowski R, Hansen NI, Willinger M, et al. Fetal growth and risk of stillbirth: a population‐based case‐control study. PLoS Med. 2014;11(4):e1001633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999;340(16):1234‐1238. [DOI] [PubMed] [Google Scholar]
- 58. Hiersch L, Lipworth H, Kingdom J, Barrett J, Melamed N. Identification of the optimal growth chart and threshold for the prediction of antepartum stillbirth. Arch Gynecol Obstet. 2020;1‐10. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 59. Temming LA, Dicke JM, Stout MJ, et al. Early second‐trimester fetal growth restriction and adverse perinatal outcomes. Obstet Gynecol. 2017;130(4):865‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Figueras F, Gratacos E. An integrated approach to fetal growth restriction. Best Pract Res Clin Obstet Gynaecol. 2017;38:48‐58. [DOI] [PubMed] [Google Scholar]
- 61. Lackman F, Capewell V, Richardson B, daSilva O, Gagnon R. The risks of spontaneous preterm delivery and perinatal mortality in relation to size at birth according to fetal versus neonatal growth standards. Am J Obstet Gynecol. 2001;184(5):946‐953. [DOI] [PubMed] [Google Scholar]
- 62. Maulik D, Frances Evans J, Ragolia L. Fetal growth restriction: pathogenic mechanisms. Clin Obstet Gynecol. 2006;49(2):219‐227. [DOI] [PubMed] [Google Scholar]
- 63. Proctor LK, Kfouri J, Hiersch L, et al. Association between hypertensive disorders and fetal growth restriction in twin compared with singleton gestations. Am J Obstet Gynecol. 2019;221(3):251.e1‐251.e8. [DOI] [PubMed] [Google Scholar]
- 64. Ananth CV, Oyelese Y, Prasad V, Getahun D, Smulian JC. Evidence of placental abruption as a chronic process: associations with vaginal bleeding early in pregnancy and placental lesions. Eur J Obstet Gynecol Reprod Biol. 2006;128(1–2):15‐21. [DOI] [PubMed] [Google Scholar]
- 65. Hiersch L, Shinar S, Melamed N, et al. Recurrent placenta‐mediated complications in women with three consecutive deliveries. Obstet Gynecol. 2017;129(3):416‐421. [DOI] [PubMed] [Google Scholar]
- 66. Gardosi J, Francis A. Adverse pregnancy outcome and association with small for gestational age birthweight by customized and population‐based percentiles. Am J Obstet Gynecol. 2009;201(1):28.e1‐8. [DOI] [PubMed] [Google Scholar]
- 67. Pallotto EK, Kilbride HW. Perinatal outcome and later implications of intrauterine growth restriction. Clin Obstet Gynecol. 2006;49(2):257‐269. [DOI] [PubMed] [Google Scholar]
- 68. Baschat AA, Cosmi E, Bilardo CM, et al. Predictors of neonatal outcome in early‐onset placental dysfunction. Obstet Gynecol. 2007;109(2 Pt 1):253‐261. [DOI] [PubMed] [Google Scholar]
- 69. Cavallaro A, Veglia M, Svirko E, Vannuccini S, Volpe G, Impey L. Using fetal abdominal circumference growth velocity in the prediction of adverse outcome in near‐term small‐for‐gestational‐age fetuses. Ultrasound Obstet Gynecol. 2018;52(4):494‐500. [DOI] [PubMed] [Google Scholar]
- 70. Sharma D, Farahbakhsh N, Shastri S, Sharma P. Intrauterine growth restriction ‐ part 2. J Matern Fetal Neonatal Med. 2016;29(24):4037‐4048. [DOI] [PubMed] [Google Scholar]
- 71. Sharma D, Shastri S, Sharma P. Intrauterine growth restriction: antenatal and postnatal aspects. Clin Med Insights Pediatr. 2016;10:67‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Flamant C, Gascoin G. Short‐term outcome and small for gestational age newborn management [in French]. J Gynecol Obstet Biol Reprod (Paris). 2013;42(8):985‐995. [DOI] [PubMed] [Google Scholar]
- 73. Figueras F, Oros D, Cruz‐Martinez R, et al. Neurobehavior in term, small‐for‐gestational age infants with normal placental function. Pediatrics. 2009;124(5):e934‐941. [DOI] [PubMed] [Google Scholar]
- 74. Eixarch E, Meler E, Iraola A, et al. Neurodevelopmental outcome in 2‐year‐old infants who were small‐for‐gestational age term fetuses with cerebral blood flow redistribution. Ultrasound Obstet Gynecol. 2008;32(7):894‐899. [DOI] [PubMed] [Google Scholar]
- 75. Savchev S, Sanz‐Cortes M, Cruz‐Martinez R, et al. Neurodevelopmental outcome of full‐term small‐for‐gestational‐age infants with normal placental function. Ultrasound Obstet Gynecol. 2013;42(2):201‐206. [DOI] [PubMed] [Google Scholar]
- 76. Figueras F, Cruz‐Martinez R, Sanz‐Cortes M, et al. Neurobehavioral outcomes in preterm, growth‐restricted infants with and without prenatal advanced signs of brain‐sparing. Ultrasound Obstet Gynecol. 2011;38(3):288‐294. [DOI] [PubMed] [Google Scholar]
- 77. Sanz‐Cortes M, Figueras F, Bargallo N, Padilla N, Amat‐Roldan I, Gratacos E. Abnormal brain microstructure and metabolism in small‐for‐gestational‐age term fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol. 2010;36(2):159‐165. [DOI] [PubMed] [Google Scholar]
- 78. Padilla N, Falcon C, Sanz‐Cortes M, et al. Differential effects of intrauterine growth restriction on brain structure and development in preterm infants: a magnetic resonance imaging study. Brain Res. 2011;1382:98‐108. [DOI] [PubMed] [Google Scholar]
- 79. Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49(2):270‐283. [DOI] [PubMed] [Google Scholar]
- 80. Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341(8850):938‐941. [DOI] [PubMed] [Google Scholar]
- 81. Crispi F, Figueras F, Cruz‐Lemini M, Bartrons J, Bijnens B, Gratacos E. Cardiovascular programming in children born small for gestational age and relationship with prenatal signs of severity. Am J Obstet Gynecol. 2012;207(2):121.e1‐9. [DOI] [PubMed] [Google Scholar]
- 82. Valsamakis G, Kanaka‐Gantenbein C, Malamitsi‐Puchner A, Mastorakos G. Causes of intrauterine growth restriction and the postnatal development of the metabolic syndrome. Ann N Y Acad Sci. 2006;1092:138‐147. [DOI] [PubMed] [Google Scholar]
- 83. Poon LC, Karagiannis G, Staboulidou I, Shafiei A, Nicolaides KH. Reference range of birth weight with gestation and first‐trimester prediction of small‐for‐gestation neonates. Prenatal Diagn. 2011;31(1):58‐65. [DOI] [PubMed] [Google Scholar]
- 84. Odibo AO, Nelson D, Stamilio DM, Sehdev HM, Macones GA. Advanced maternal age is an independent risk factor for intrauterine growth restriction. Am J Perinatol. 2006;23(5):325‐328. [DOI] [PubMed] [Google Scholar]
- 85. Han Z, Mulla S, Beyene J, Liao G, McDonald SD; Knowledge Synthesis Group . Maternal underweight and the risk of preterm birth and low birth weight: a systematic review and meta‐analyses. Int J Epidemiol. 2011;40(1):65‐101. [DOI] [PubMed] [Google Scholar]
- 86. Jaddoe VW, Bakker R, Hofman A, et al. Moderate alcohol consumption during pregnancy and the risk of low birth weight and preterm birth. The Generation R Study. Ann Epidemiol. 2007;17(10):834‐840. [DOI] [PubMed] [Google Scholar]
- 87. McCowan LM, Dekker GA, Chan E, et al. Spontaneous preterm birth and small for gestational age infants in women who stop smoking early in pregnancy: prospective cohort study. BMJ. 2009;338:b1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gouin K, Murphy K, Shah PS. Knowledge Synthesis Group on Determinants of Low Birth W, Preterm B: Effects of cocaine use during pregnancy on low birthweight and preterm birth: systematic review and metaanalyses. Am J Obstet Gynecol. 2011;204(4):340.e1‐12. [DOI] [PubMed] [Google Scholar]
- 89. Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta‐analysis. Obstet Gynecol. 2004;103(3):551‐563. [DOI] [PubMed] [Google Scholar]
- 90. Andres RL, Day MC. Perinatal complications associated with maternal tobacco use. Semin Neonatol. 2000;5(3):231‐241. [DOI] [PubMed] [Google Scholar]
- 91. Di Renzo GC, Conry JA, Blake J, et al. International Federation of Gynecology and Obstetrics opinion on reproductive health impacts of exposure to toxic environmental chemicals. Int J Gynecol Obstet. 2015;131(3):219‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Smith GC. First trimester origins of fetal growth impairment. Semin Perinatol. 2004;28(1):41‐50. [DOI] [PubMed] [Google Scholar]
- 93. Smith GCS. Universal screening for foetal growth restriction. Best Pract Res Clin Obstet Gynaecol. 2018;49:16‐28. [DOI] [PubMed] [Google Scholar]
- 94. Morris RK, Bilagi A, Devani P, Kilby MD. Association of serum PAPP‐A levels in first trimester with small for gestational age and adverse pregnancy outcomes: systematic review and meta‐analysis. Prenatal Diagn. 2017;37(3):253‐265. [DOI] [PubMed] [Google Scholar]
- 95. Gaccioli F, Aye I, Sovio U, Charnock‐Jones DS, Smith GCS. Screening for fetal growth restriction using fetal biometry combined with maternal biomarkers. Am J Obstet Gynecol. 2018;218(2S):S725‐S737. [DOI] [PubMed] [Google Scholar]
- 96. Lesmes C, Gallo DM, Gonzalez R, Poon LC, Nicolaides KH. Prediction of small‐for‐gestational‐age neonates: screening by maternal serum biochemical markers at 19–24 weeks. Ultrasound Obstet Gynecol. 2015;46(3):341‐349. [DOI] [PubMed] [Google Scholar]
- 97. Smith GC, Shah I, White IR, Pell JP, Crossley JA, Dobbie R. Maternal and biochemical predictors of antepartum stillbirth among nulliparous women in relation to gestational age of fetal death. BJOG. 2007;114(6):705‐714. [DOI] [PubMed] [Google Scholar]
- 98. Proctor LK, Toal M, Keating S, et al. Placental size and the prediction of severe early‐onset intrauterine growth restriction in women with low pregnancy‐associated plasma protein‐A. Ultrasound Obstet Gynecol. 2009;34(3):274‐282. [DOI] [PubMed] [Google Scholar]
- 99. Benn PA, Horne D, Briganti S, Rodis JF, Clive JM. Elevated second‐trimester maternal serum hCG alone or in combination with elevated alpha‐fetoprotein. Obstet Gynecol. 1996;87(2):217‐222. [DOI] [PubMed] [Google Scholar]
- 100. Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology (Bethesda). 2009;24:147‐158. [DOI] [PubMed] [Google Scholar]
- 101. Smith GC, Crossley JA, Aitken DA, et al. Circulating angiogenic factors in early pregnancy and the risk of preeclampsia, intrauterine growth restriction, spontaneous preterm birth, and stillbirth. Obstet Gynecol. 2007;109(6):1316‐1324. [DOI] [PubMed] [Google Scholar]
- 102. Erez O, Romero R, Espinoza J, et al. The change in concentrations of angiogenic and anti‐angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small‐for‐gestational age. J Matern Fetal Neonatal Med. 2008;21(5):279‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Poon LC, Zaragoza E, Akolekar R, Anagnostopoulos E, Nicolaides KH. Maternal serum placental growth factor (PlGF) in small for gestational age pregnancy at 11(+0) to 13(+6) weeks of gestation. Prenatal Diagn. 2008;28(12):1110‐1115. [DOI] [PubMed] [Google Scholar]
- 104. Cowans NJ, Stamatopoulou A, Matwejew E, von Kaisenberg CS, Spencer K. First‐trimester placental growth factor as a marker for hypertensive disorders and SGA. Prenatal Diagn. 2010;30(6):565‐570. [DOI] [PubMed] [Google Scholar]
- 105. Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms‐like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt‐1:PlGF ratio in women with suspected preeclampsia. New Engl J Med. 2016;374(1):13‐22. [DOI] [PubMed] [Google Scholar]
- 107. Levytska K, Higgins M, Keating S, et al. Placental pathology in relation to uterine artery doppler findings in pregnancies with severe intrauterine growth restriction and abnormal umbilical artery doppler changes. Am J Perinatol. 2017;34(5):451‐457. [DOI] [PubMed] [Google Scholar]
- 108. Toal M, Keating S, Machin G, et al. Determinants of adverse perinatal outcome in high‐risk women with abnormal uterine artery Doppler images. Am J Obstet Gynecol. 2008;198(3):330.e1‐7. [DOI] [PubMed] [Google Scholar]
- 109. Lesmes C, Gallo DM, Saiid Y, Poon LC, Nicolaides KH. Prediction of small‐for‐gestational‐age neonates: screening by uterine artery Doppler and mean arterial pressure at 19–24 weeks. Ultrasound Obstet Gynecol. 2015;46(3):332‐340. [DOI] [PubMed] [Google Scholar]
- 110. Cnossen JS, Morris RK, ter Riet G, et al. Use of uterine artery Doppler ultrasonography to predict pre‐eclampsia and intrauterine growth restriction: a systematic review and bivariable meta‐analysis. CMAJ. 2008;178(6):701‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Drouin O, Boutin A, Paquette K, et al. First‐trimester uterine artery doppler for the prediction of SGA at birth: the great obstetrical syndromes study. J Obstet Gynaecol Can. 2018;40(12):1592‐1599. [DOI] [PubMed] [Google Scholar]
- 112. Rodriguez A, Tuuli MG, Odibo AO. First‐, second‐, and third‐trimester screening for preeclampsia and intrauterine growth restriction. Clin Lab Med. 2016;36(2):331‐351. [DOI] [PubMed] [Google Scholar]
- 113. Moloney A, Hladunewich M, Manly E, et al. The predictive value of sonographic placental markers for adverse pregnancy outcome in women with chronic kidney disease. Pregnancy Hypertens. 2020;20:27‐35. [DOI] [PubMed] [Google Scholar]
- 114. Viero S, Chaddha V, Alkazaleh F, et al. Prognostic value of placental ultrasound in pregnancies complicated by absent end‐diastolic flow velocity in the umbilical arteries. Placenta. 2004;25(8–9):735‐741. [DOI] [PubMed] [Google Scholar]
- 115. Proctor LK, Whittle WL, Keating S, Viero S, Kingdom JC. Pathologic basis of echogenic cystic lesions in the human placenta: role of ultrasound‐guided wire localization. Placenta. 2010;31(12):1111‐1115. [DOI] [PubMed] [Google Scholar]
- 116. Porat S, Fitzgerald B, Wright E, Keating S, Kingdom JC. Placental hyperinflation and the risk of adverse perinatal outcome. Ultrasound Obstet Gynecol. 2013;42(3):315‐321. [DOI] [PubMed] [Google Scholar]
- 117. Florido J, Ocon O, del Castillo L, et al. Analysis of measurement process of placental volume in early pregnancy: an interobserver reliability study. J Perinat Med. 2014;42(5):559‐564. [DOI] [PubMed] [Google Scholar]
- 118. Burstein E, Sheiner E, Hershkovitz R. Three‐dimensional placental volume measurements between 11 and 13 weeks’ gestation. Am J Perinatol. 2009;26(2):169‐171. [DOI] [PubMed] [Google Scholar]
- 119. Farina A. Systematic review on first trimester three‐dimensional placental volumetry predicting small for gestational age infants. Prenatal Diagn. 2016;36(2):135‐141. [DOI] [PubMed] [Google Scholar]
- 120. Hafner E, Metzenbauer M, Hofinger D, et al. Comparison between three‐dimensional placental volume at 12 weeks and uterine artery impedance/notching at 22 weeks in screening for pregnancy‐induced hypertension, pre‐eclampsia and fetal growth restriction in a low‐risk population. Ultrasound Obstet Gynecol. 2006;27(6):652‐657. [DOI] [PubMed] [Google Scholar]
- 121. Crovetto F, Triunfo S, Crispi F, et al. Differential performance of first‐trimester screening in predicting small‐for‐gestational‐age neonate or fetal growth restriction. Ultrasound Obstet Gynecol. 2017;49(3):349‐356. [DOI] [PubMed] [Google Scholar]
- 122. McCowan LM, Thompson JM, Taylor RS, et al. Prediction of small for gestational age infants in healthy nulliparous women using clinical and ultrasound risk factors combined with early pregnancy biomarkers. PLoS One. 2017;12(1):e0169311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Grivell R, Dodd J, Robinson J. The prevention and treatment of intrauterine growth restriction. Best Pract Res Clin Obstet Gynaecol. 2009;23(6):795‐807. [DOI] [PubMed] [Google Scholar]
- 124. Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. Int J Gynecol Obstet. 2006;93(3):269‐274. [DOI] [PubMed] [Google Scholar]
- 125. Rasmussen KM, Yaktine AL, eds. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press (US); 2009. [PubMed] [Google Scholar]
- 126. Cedergren MI. Optimal gestational weight gain for body mass index categories. Obstet Gynecol. 2007;110(4):759‐764. [DOI] [PubMed] [Google Scholar]
- 127. Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2009(3):CD001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Tobacco and nicotine cessation during pregnancy: ACOG Committee opinion, number 807. Obstet Gynecol. 2020;135(5):e221‐e229. [DOI] [PubMed] [Google Scholar]
- 129. Mills JL, Graubard BI, Harley EE, Rhoads GG, Berendes HW. Maternal alcohol consumption and birth weight. How much drinking during pregnancy is safe? JAMA. 1984;252(14):1875‐1879. [PubMed] [Google Scholar]
- 130. Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. New Engl J Med. 2017;377(7):613‐622. [DOI] [PubMed] [Google Scholar]
- 131. Meher S, Duley L, Hunter K, Askie L. Antiplatelet therapy before or after 16 weeks’ gestation for preventing preeclampsia: an individual participant data meta‐analysis. Am J Obstet Gynecol. 2017;216(2):121‐128.e2. [DOI] [PubMed] [Google Scholar]
- 132. Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta‐analysis. Am J Obstet Gynecol. 2017;216(2):110‐120.e6. [DOI] [PubMed] [Google Scholar]
- 133. Ayala DE, Ucieda R, Hermida RC. Chronotherapy with low‐dose aspirin for prevention of complications in pregnancy. Chronobiol Int. 2013;30(1–2):260‐279. [DOI] [PubMed] [Google Scholar]
- 134. McCowan LM, Figueras F, Anderson NH. Evidence‐based national guidelines for the management of suspected fetal growth restriction: comparison, consensus, and controversy. Am J Obstet Gynecol. 2018;218(2S):S855‐S868. [DOI] [PubMed] [Google Scholar]
- 135. Sobel ML, Kingdom J, Drewlo S. Angiogenic response of placental villi to heparin. Obstet Gynecol. 2011;117(6):1375‐1383. [DOI] [PubMed] [Google Scholar]
- 136. Mousavi S, Moradi M, Khorshidahmad T, Motamedi M. Anti‐inflammatory effects of heparin and its derivatives: a systematic review. Adv Pharmacol Sci. 2015;2015:507151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Yinon Y, Ben Meir E, Margolis L, et al. Low molecular weight heparin therapy during pregnancy is associated with elevated circulatory levels of placental growth factor. Placenta. 2015;36(2):121‐124. [DOI] [PubMed] [Google Scholar]
- 138. Oberkersch R, Attorresi AI, Calabrese GC. Low‐molecular‐weight heparin inhibition in classical complement activation pathway during pregnancy. Thromb Res. 2010;125(5):e240‐245. [DOI] [PubMed] [Google Scholar]
- 139. Rodger MA, Carrier M, Le Gal G, et al. Meta‐analysis of low‐molecular‐weight heparin to prevent recurrent placenta‐mediated pregnancy complications. Blood. 2014;123(6):822‐828. [DOI] [PubMed] [Google Scholar]
- 140. Rodger MA, Gris J‐C, de Vries JIP, et al. Low‐molecular‐weight heparin and recurrent placenta‐mediated pregnancy complications: a meta‐analysis of individual patient data from randomised controlled trials. Lancet. 2016;388(10060):2629‐2641. [DOI] [PubMed] [Google Scholar]
- 141. Groom KM, McCowan LM, Mackay LK, et al. Enoxaparin for the prevention of preeclampsia and intrauterine growth restriction in women with a history: a randomized trial. Am J Obstet Gynecol. 2017;216(3):296.e1‐296.e14. [DOI] [PubMed] [Google Scholar]
- 142. McLaughlin K, Scholten RR, Parker JD, Ferrazzi E, Kingdom JCP. Low molecular weight heparin for the prevention of severe preeclampsia: where next? Br J Clin Pharmacol. 2018;84(4):673‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics and the Society forMaternal‐Fetal Medicine . ACOG practice bulletin no. 204: fetal growth restrictionsObstet Gynecol. 2019;133(2):e97‐e109. [DOI] [PubMed] [Google Scholar]
- 144. Morse K, Williams A, Gardosi J. Fetal growth screening by fundal height measurement. Best Pract Res Clin Obstet Gynaecol. 2009;23(6):809‐818. [DOI] [PubMed] [Google Scholar]
- 145. Papageorghiou AT, Ohuma EO, Gravett MG, et al. International standards for symphysis‐fundal height based on serial measurements from the Fetal Growth Longitudinal Study of the INTERGROWTH‐21st Project: prospective cohort study in eight countries. BMJ. 2016;355:i5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Sparks TN, Cheng YW, McLaughlin B, Esakoff TF, Caughey AB. Fundal height: a useful screening tool for fetal growth? J Matern Fetal Neonatal Med. 2011;24(5):708‐712. [DOI] [PubMed] [Google Scholar]
- 147. Knox AJ, Sadler L, Pattison NS, Mantell CD, Mullins P. An obstetric scoring system: its development and application in obstetric management. Obstet Gynecol. 1993;81(2):195‐199. [PubMed] [Google Scholar]
- 148. Azziz R, Smith S, Fabro S. The development and use of a standard symphysial‐fundal height growth curve in the prediction of small for gestational age neonates. Int J Gynecol Obstet. 1988;26(1):81‐87. [DOI] [PubMed] [Google Scholar]
- 149. Belizan JM, Villar J, Nardin JC, Malamud J, De Vicurna LS. Diagnosis of intrauterine growth retardation by a simple clinical method: measurement of uterine height. Am J Obstet Gynecol. 1978;131(6):643‐646. [DOI] [PubMed] [Google Scholar]
- 150. Calvert JP, Crean EE, Newcombe RG, Pearson JF. Antenatal screening by measurement of symphysis‐fundus height. Br Med J (Clin Res Ed). 1982;285(6345):846‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Challis K, Osman NB, Nystrom L, Nordahl G, Bergstrom S. Symphysis‐fundal height growth chart of an obstetric cohort of 817 Mozambican women with ultrasound‐dated singleton pregnancies. Trop Med Int Health. 2002;7(8):678‐684. [DOI] [PubMed] [Google Scholar]
- 152. Ebite LE, Ebeigbe PN, Igbigbi P, Akpuaka FC. Symphysiofundal height growth curve and growth velocity in pregnant women in a Nigerian community. J Obstet Gynaecol. 2009;29(7):605‐608. [DOI] [PubMed] [Google Scholar]
- 153. Hakansson A, Aberg A, Nyberg P, Schersten B. A new symphysis‐fundus height growth chart based on a well defined female population with ultrasound‐dated singleton pregnancies. Acta Obstet Gynecol Scand. 1995;74(9):682‐686. [DOI] [PubMed] [Google Scholar]
- 154. Jensen OH, Larsen S. Evaluation of symphysis‐fundus measurements and weighing during pregnancy. Acta Obstet Gynecol Scand. 1991;70(1):13‐16. [DOI] [PubMed] [Google Scholar]
- 155. Pay AS, Wiik J, Backe B, Jacobsson B, Strandell A, Klovning A. Symphysis‐fundus height measurement to predict small‐for‐gestational‐age status at birth: a systematic review. BMC Pregnancy Childbirth. 2015;15:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Pay ASD, Froen JF, Staff AC, Jacobsson B, Gjessing HK. Symphysis‐fundus measurement – the predictive value of a new reference curve. Tidsskr Nor Laegeforen. 2017;137(10):717‐720. [DOI] [PubMed] [Google Scholar]
- 157. Robert Peter J, Ho JJ, Valliapan J, Sivasangari S. Symphysial fundal height (SFH) measurement in pregnancy for detecting abnormal fetal growth. Cochrane Database Syst Rev. 2015(9):CD008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Goto E. Prediction of low birthweight and small for gestational age from symphysis‐fundal height mainly in developing countries: a meta‐analysis. J Epidemiol Community Health. 2013;67(12):999‐1005. [DOI] [PubMed] [Google Scholar]
- 159. Griffiths A, Pinto A, Margarit L. A survey of methods used to measure symphysis fundal height. J Obstet Gynaecol. 2008;28(7):692‐694. [DOI] [PubMed] [Google Scholar]
- 160. Salomon LJ, Alfirevic Z, Da Silva CF, et al. ISUOG Practice Guidelines: ultrasound assessment of fetal biometry and growth. Ultrasound Obstet Gynecol. 2019;53(6):715‐723. [DOI] [PubMed] [Google Scholar]
- 161. Melamed N, Yogev Y, Meizner I, Mashiach R, Bardin R, Ben‐Haroush A. Sonographic fetal weight estimation: which model should be used? J Ultrasound Med. 2009;28(5):617‐629. [DOI] [PubMed] [Google Scholar]
- 162. Nahum GG, Stanislaw H. Ultrasonographic prediction of term birth weight: how accurate is it? Am J Obstet Gynecol. 2003;188(2):566‐574. [DOI] [PubMed] [Google Scholar]
- 163. Stanislaw H, Nahum GG. Accuracy of birth weight prediction methods. J Reprod Med. 2008;53(3):238‐239; author reply 239. [PubMed] [Google Scholar]
- 164. Dudley NJ. A systematic review of the ultrasound estimation of fetal weight. Ultrasound Obstet Gynecol. 2005;25(1):80‐89. [DOI] [PubMed] [Google Scholar]
- 165. Milner J, Arezina J. The accuracy of ultrasound estimation of fetal weight in comparison to birth weight: a systematic review. Ultrasound. 2018;26(1):32‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Melamed N, Ben‐Haroush A, Meizner I, Mashiach R, Glezerman M, Yogev Y. Accuracy of sonographic weight estimation as a function of fetal sex. Ultrasound Obstet Gynecol. 2011;38(1):67‐73. [DOI] [PubMed] [Google Scholar]
- 167. Melamed N, Ben‐Haroush A, Meizner I, Mashiach R, Yogev Y, Pardo J. Accuracy of sonographic fetal weight estimation: a matter of presentation. Ultrasound Obstet Gynecol. 2011;38(4):418‐424. [DOI] [PubMed] [Google Scholar]
- 168. Melamed N, Yogev Y, Meizner I, Mashiach R, Pardo J, Ben‐Haroush A. Prediction of fetal macrosomia: effect of sonographic fetal weight‐estimation model and threshold used. Ultrasound Obstet Gynecol. 2011;38(1):74‐81. [DOI] [PubMed] [Google Scholar]
- 169. Melamed N, Yogev Y, Ben‐Haroush A, Meizner I, Mashiach R, Glezerman M. Does use of a sex‐specific model improve the accuracy of sonographic weight estimation? Ultrasound Obstet Gynecol. 2012;39(5):549‐557. [DOI] [PubMed] [Google Scholar]
- 170. Melamed N, Yogev Y, Linder N, et al. Role of fetal length in the prediction of fetal weight. J Ultrasound Med. 2012;31(5):687‐694. [DOI] [PubMed] [Google Scholar]
- 171. Melamed N, Ryan G, Windrim R, Toi A, Kingdom J. Choice of formula and accuracy of fetal weight estimation in small‐for‐gestational‐age fetuses. J Ultrasound Med. 2016;35(1):71‐82. [DOI] [PubMed] [Google Scholar]
- 172. Expert Panel on Women's Imaging , Shipp TD, Zelop CM, et al. ACR Appropriateness Criteria© growth disturbances‐risk of fetal growth restriction. J Am Coll Radiol. 2019;16(5S):S116‐S125. [DOI] [PubMed] [Google Scholar]
- 173. Lausman A, Kingdom J; Maternal Fetal Medicine C . Intrauterine growth restriction: screening, diagnosis, and management. J Obstet Gynaecol Can. 2013;35(8):741‐748. [DOI] [PubMed] [Google Scholar]
- 174. Bakalis S, Cao K, Johal N, Cuckow P, Pandya P. The value of the routine third trimester ultrasound scan in antenatal care: Problems with guidance and outdated data in a highly technological field. Eur J Obstet Gynecol Reprod Biol. 2020;245:51‐55. [DOI] [PubMed] [Google Scholar]
- 175. Lindqvist PG, Molin J. Does antenatal identification of small‐for‐gestational age fetuses significantly improve their outcome? Ultrasound Obstet Gynecol. 2005;25(3):258‐264. [DOI] [PubMed] [Google Scholar]
- 176. Ray CL, Grange G. Routine third trimester ultrasound in low risk pregnancy confers no benefit!: AGAINST: Arguments for a routine third trimester ultrasound: what the meta‐analysis does not show!. BJOG. 2016;123(7):1122. [DOI] [PubMed] [Google Scholar]
- 177. Thornton J. Routine third trimester ultrasound in low risk pregnancy confers no benefit!: FOR: The benefits of routine third‐trimester scanning are less clear cut. BJOG. 2016;123(7):1121. [DOI] [PubMed] [Google Scholar]
- 178. Bricker L, Medley N, Pratt JJ. Routine ultrasound in late pregnancy (after 24 weeks’ gestation). Cochrane Database Syst Rev. 2015(6):CD001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Ashimi Balogun O, Sibai BM, Pedroza C, Blackwell SC, Barrett TL, Chauhan SP. Serial third‐trimester ultrasonography compared with routine care in uncomplicated pregnancies: a randomized controlled trial. Obstet Gynecol. 2018;132(6):1358‐1367. [DOI] [PubMed] [Google Scholar]
- 180. Skrastad RB, Eik‐Nes SH, Sviggum O, et al. A randomized controlled trial of third‐trimester routine ultrasound in a non‐selected population. Acta Obstet Gynecol Scand. 2013;92(12):1353‐1360. [DOI] [PubMed] [Google Scholar]
- 181. Sovio U, White IR, Dacey A, Pasupathy D, Smith GCS. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study. Lancet. 2015;386(10008):2089‐2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Hiersch L, Melamed N. Fetal growth velocity and body proportion in the assessment of growth. Am J Obstet Gynecol. 2018;218(2S):pp. S700–S711.e1. [DOI] [PubMed] [Google Scholar]
- 183. Miranda J, Rodriguez‐Lopez M, Triunfo S, et al. Prediction of fetal growth restriction using estimated fetal weight vs a combined screening model in the third trimester. Ultrasound Obstet Gynecol. 2017;50(5):603‐611. [DOI] [PubMed] [Google Scholar]
- 184. De Castro H, Ciobanu A, Formuso C, Akolekar R, Nicolaides KH. Value of routine ultrasound examination at 35–37 weeks’ gestation in diagnosis of non‐cephalic presentation. Ultrasound Obstet Gynecol. 2020;55(2):248‐256. [DOI] [PubMed] [Google Scholar]
- 185. Ewigman BG, Crane JP, Frigoletto FD, LeFevre ML, Bain RP, McNellis D. Effect of prenatal ultrasound screening on perinatal outcome. RADIUS Study Group. New Engl J Med. 1993;329(12):821‐827. [DOI] [PubMed] [Google Scholar]
- 186. Ficara A, Syngelaki A, Hammami A, Akolekar R, Nicolaides KH. Value of routine ultrasound examination at 35–37 weeks’ gestation in diagnosis of fetal abnormalities. Ultrasound Obstet Gynecol. 2020;55(1):75‐80. [DOI] [PubMed] [Google Scholar]
- 187. Ciobanu A, Rouvali A, Syngelaki A, Akolekar R, Nicolaides KH. Prediction of small for gestational age neonates: screening by maternal factors, fetal biometry, and biomarkers at 35–37 weeks’ gestation. Am J Obstet Gynecol. 2019;220(5):486.e1‐486.e11. [DOI] [PubMed] [Google Scholar]
- 188. Martinez‐Portilla RJ, Caradeux J, Meler E, Lip‐Sosa DL, Sotiriadis A, Figueras F. Third‐trimester uterine‐artery Doppler for prediction of adverse outcome in late small‐for‐gestational‐age fetuses: systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2019;55(5):575‐585. [DOI] [PubMed] [Google Scholar]
- 189. Conde‐Agudelo A, Villar J, Kennedy SH, Papageorghiou AT. Predictive accuracy of cerebroplacental ratio for adverse perinatal and neurodevelopmental outcomes in suspected fetal growth restriction: systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2018;52(4):430‐441. [DOI] [PubMed] [Google Scholar]
- 190. Katz J, Wu LA, Mullany LC, et al. Prevalence of small‐for‐gestational‐age and its mortality risk varies by choice of birth‐weight‐for‐gestation reference population. PLoS One. 2014;9(3):e92074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Salomon LJ, Bernard JP, Duyme M, Buvat I, Ville Y. The impact of choice of reference charts and equations on the assessment of fetal biometry. Ultrasound Obstet Gynecol. 2005;25(6):559‐565. [DOI] [PubMed] [Google Scholar]
- 192. Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163‐168. [DOI] [PubMed] [Google Scholar]
- 193. Hadlock FP, Harrist RB, Martinez‐Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181(1):129‐133. [DOI] [PubMed] [Google Scholar]
- 194. Papageorghiou AT, Ohuma EO, Altman DG, et al. International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH‐21st Project. Lancet. 2014;384(9946):869‐879. [DOI] [PubMed] [Google Scholar]
- 195. Villar J, Papageorghiou AT, Pang R, et al. The likeness of fetal growth and newborn size across non‐isolated populations in the INTERGROWTH‐21st Project: the Fetal Growth Longitudinal Study and Newborn Cross‐Sectional Study. Lancet Diabetes Endocrinol. 2014;2(10):781‐792. [DOI] [PubMed] [Google Scholar]
- 196. Stirnemann J, Villar J, Salomon LJ, et al. International estimated fetal weight standards of the INTERGROWTH‐21(st) Project. Ultrasound Obstet Gynecol. 2017;49(4):478‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Kiserud T, Piaggio G, Carroli G, et al. The World Health Organization fetal growth charts: a multinational longitudinal study of ultrasound biometric measurements and estimated fetal weight. PLoS Med. 2017;14(1):e1002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Buck Louis GM, Grewal J, Albert PS, et al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. Am J Obstet Gynecol. 2015;213(4):449.e41‐449.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Tarca AL, Romero R, Gudicha DW, et al. A new customized fetal growth standard for African American women: the PRB/NICHD Detroit study. Am J Obstet Gynecol. 2018;218(2S):pp. S679–S691.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Gardosi J, Chang A, Kalyan B, Sahota D, Symonds EM. Customised antenatal growth charts. Lancet. 1992;339(8788):283‐287. [DOI] [PubMed] [Google Scholar]
- 201. Gardosi J, Francis A, Turner S, Williams M. Customized growth charts: rationale, validation and clinical benefits. Am J Obstet Gynecol. 2018;218(2S):S609‐S618. [DOI] [PubMed] [Google Scholar]
- 202. Deter RL. Individualized growth assessment predicts birth weight accurately. Ultrasound Obstet Gynecol. 1996;7(2):156‐157. [DOI] [PubMed] [Google Scholar]
- 203. Deter RL, Levytska K, Melamed N, Lee W, Kingdom JC. Classifying neonatal growth outcomes: use of birth weight, placental evaluation and individualized growth assessment. J Matern Fetal Neonatal Med. 2016;29(24):3939‐3949. [DOI] [PubMed] [Google Scholar]
- 204. Deter RL, Lee W, Yeo L, et al. Individualized growth assessment: conceptual framework and practical implementation for the evaluation of fetal growth and neonatal growth outcome. Am J Obstet Gynecol. 2018;218(2S):S656‐S678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Gardosi J. Fetal growth and ethnic variation. Lancet Diabetes Endocrinol. 2014;2(10):773‐774. [DOI] [PubMed] [Google Scholar]
- 206. Albert PS, Grantz KL. Fetal growth and ethnic variation. Lancet Diabetes Endocrinol. 2014;2(10):773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. WHO Multicentre Growth Reference Study Group . WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76‐85. [DOI] [PubMed] [Google Scholar]
- 208. Kiserud T, Benachi A, Hecher K, et al. The World Health Organization fetal growth charts: concept, findings, interpretation, and application. Am J Obstet Gynecol. 2018;218(2S):S619‐S629. [DOI] [PubMed] [Google Scholar]
- 209. Cheng Y, Leung TY, Lao T, Chan YM, Sahota DS. Impact of replacing Chinese ethnicity‐specific fetal biometry charts with the INTERGROWTH‐21(st) standard. BJOG. 2016;123(Suppl 3):48‐55. [DOI] [PubMed] [Google Scholar]
- 210. Poon LC, Tan MY, Yerlikaya G, Syngelaki A, Nicolaides KH. Birth weight in live births and stillbirths. Ultrasound Obstet Gynecol. 2016;48(5):602‐606. [DOI] [PubMed] [Google Scholar]
- 211. Anderson NH, Sadler LC, McKinlay CJD, McCowan LME. INTERGROWTH‐21st vs customized birthweight standards for identification of perinatal mortality and morbidity. Am J Obstet Gynecol. 2016;214(4):509.e1‐509.e7. [DOI] [PubMed] [Google Scholar]
- 212. Melamed N, Ray JG, Shah PS, Berger H, Kingdom JC. Should we use customized fetal growth percentiles in urban Canada? J Obstet Gynaecol Can. 2014;36(2):164‐170. [DOI] [PubMed] [Google Scholar]
- 213. Gardosi J. Customized charts and their role in identifying pregnancies at risk because of fetal growth restriction. J Obstet Gynaecol Can. 2014;36(5):408‐415. [DOI] [PubMed] [Google Scholar]
- 214. Francis A, Hugh O, Gardosi J. Customized vs INTERGROWTH‐21(st) standards for the assessment of birthweight and stillbirth risk at term. Am J Obstet Gynecol. 2018;218(2S):S692‐S699. [DOI] [PubMed] [Google Scholar]
- 215. Pritchard N, Lindquist A, Siqueira IDA, Walker SP, Permezel M. INTERGROWTH‐21st compared with GROW customized centiles in the detection of adverse perinatal outcomes at term. J Matern Fetal Neonatal Med. 2020;33(6):961‐966. [DOI] [PubMed] [Google Scholar]
- 216. Ego A, Subtil D, Grange G, et al. Customized versus population‐based birth weight standards for identifying growth restricted infants: a French multicenter study. Am J Obstet Gynecol. 2006;194(4):1042‐1049. [DOI] [PubMed] [Google Scholar]
- 217. Sovio U, Smith GCS. The effect of customization and use of a fetal growth standard on the association between birthweight percentile and adverse perinatal outcome. Am J Obstet Gynecol. 2018;218(2S):S738‐S744. [DOI] [PubMed] [Google Scholar]
- 218. Hutcheon JA, Walker M, Platt RW. Assessing the value of customized birth weight percentiles. Am J Epidemiol. 2011;173(4):459‐467. [DOI] [PubMed] [Google Scholar]
- 219. Hutcheon J. Do customized birth weight charts add anything but complexity to the assessment of fetal growth? J Obstet Gynaecol Can. 2014;36(2):107‐109. [DOI] [PubMed] [Google Scholar]
- 220. Mikolajczyk RT, Zhang J, Betran AP, et al. A global reference for fetal‐weight and birthweight percentiles. Lancet. 2011;377(9780):1855‐1861. [DOI] [PubMed] [Google Scholar]
- 221. Pritchard NL, Hiscock RJ, Lockie E, et al. Identification of the optimal growth charts for use in a preterm population: An Australian state‐wide retrospective cohort study. PLoS Med. 2019;16(10):e1002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Hiersch L, Okby R, Freeman H, et al. Differences in fetal growth patterns between twins and singletons. J Matern Fetal Neonatal Med. 2020;33(15):2546‐2555. [DOI] [PubMed] [Google Scholar]
- 223. Grantz KL, Grewal J, Albert PS, et al. Dichorionic twin trajectories: the NICHD Fetal Growth Studies. Am J Obstet Gynecol. 2016;215(2):221.e1‐221.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Blickstein I. Is it normal for multiples to be smaller than singletons? Best Pract Res Clin Obstet Gynaecol. 2004;18(4):613‐623. [DOI] [PubMed] [Google Scholar]
- 225. Stirrup OT, Khalil A, D'Antonio F, Thilaganathan B; Southwest Thames Obstetric Research Collaborative (STORK) . Fetal growth reference ranges in twin pregnancy: analysis of the Southwest Thames Obstetric Research Collaborative (STORK) multiple pregnancy cohort. Ultrasound Obstet Gynecol. 2015;45(3):301‐307. [DOI] [PubMed] [Google Scholar]
- 226. Gielen M, Lindsey PJ, Derom C, et al. Twin‐specific intrauterine ‘growth’ charts based on cross‐sectional birthweight data. Twin Res Hum Genet. 2008;11(2):224‐235. [DOI] [PubMed] [Google Scholar]
- 227. Mendez‐Figueroa H, Truong VTT, Pedroza C, Chauhan SP. Growth among twins: use of singleton versus twin‐specific growth nomograms. Am J Perinatol. 2018;35(2):184‐191. [DOI] [PubMed] [Google Scholar]
- 228. Bleker OP, Wolf H, Oosting J. The placental cause of fetal growth retardation in twin gestations. Acta Genet Med Gemellol. 1995;44(2):103‐106. [DOI] [PubMed] [Google Scholar]
- 229. Vatnick I, Schoknecht PA, Darrigrand R, Bell AW. Growth and metabolism of the placenta after unilateral fetectomy in twin pregnant ewes. J Dev Physiol. 1991;15(6):351‐356. [PubMed] [Google Scholar]
- 230. Liao AW, Brizot Mde L, Kang HJ, Assuncao RA, Zugaib M. Longitudinal reference ranges for fetal ultrasound biometry in twin pregnancies. Clinics. 2012;67(5):451‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Muhlhausler BS, Hancock SN, Bloomfield FH, Harding R. Are twins growth restricted? Pediatr Res. 2011;70(2):117‐122. [DOI] [PubMed] [Google Scholar]
- 232. Doom EC, Delbaere I, Martens G, Temmerman M. Birth weight for gestational age among Flemish twin population. Facts Views Vis Obgyn. 2012;4(1):42‐49. [PMC free article] [PubMed] [Google Scholar]
- 233. Dollberg S, Haklai Z, Mimouni FB, Gorfein I, Gordon ES. Birth weight standards in the live‐born population in Israel. Isr Med Assoc J. 2005;7(5):311‐314. [PubMed] [Google Scholar]
- 234. Min S‐J, Luke B, Gillespie B, et al. Birth weight references for twins. Am J Obstet Gynecol. 2000;182(5):1250‐1257. [DOI] [PubMed] [Google Scholar]
- 235. Ong S, Lim MN, Fitzmaurice A, Campbell D, Smith AP, Smith N. The creation of twin centile curves for size. BJOG. 2002;109(7):753‐758. [DOI] [PubMed] [Google Scholar]
- 236. FIGO Working Group on Good Clinical Practice in Maternal‐Fetal Medicine . Good clinical practice advice: Role of ultrasound in the management of twin pregnancy. Int J Gynecol Obstet. 2019;144(3):338‐339. [Google Scholar]
- 237. Committee on Practice Bulletins—Obstetrics; Society for Maternal‐Fetal Medicine . Practice bulletin no. 169: multifetal gestations: twin, triplet, and higher‐order multifetal pregnancies. Obstet Gynecol. 2016;128(4):e131‐e146. [DOI] [PubMed] [Google Scholar]
- 238. Morin L, Lim KN. 260‐Ultrasound in twin pregnancies. J Obstet Gynaecol Can. 2017;39(10):e398‐e411. [DOI] [PubMed] [Google Scholar]
- 239. Khalil A, Rodgers M, Baschat A, et al. ISUOG practice guidelines: role of ultrasound in twin pregnancy. Ultrasound Obstet Gynecol. 2016;47(2):247‐263. [DOI] [PubMed] [Google Scholar]
- 240. Alexander JM, Hammond KR, Steinkampf MP. Multifetal reduction of high‐order multiple pregnancy: comparison of obstetrical outcome with nonreduced twin gestations. Fertil Steril. 1995;64(6):1201‐1203. [DOI] [PubMed] [Google Scholar]
- 241. Begum G, Stevens A, Smith EB, et al. Epigenetic changes in fetal hypothalamic energy regulating pathways are associated with maternal undernutrition and twinning. FASEB J. 2012;26(4):1694‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Tsai PC, Van Dongen J, Tan Q, et al. DNA methylation changes in the IGF1R gene in birth weight discordant adult monozygotic twins. Twin Res Hum Genet. 2015;18(6):635‐646. [DOI] [PubMed] [Google Scholar]
- 243. Williams‐Wyss O, Zhang S, MacLaughlin SM, et al. Embryo number and periconceptional undernutrition in the sheep have differential effects on adrenal epigenotype, growth, and development. Am J Physiol Endocrinol Metab. 2014;307(2):E141‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Kalafat E, Sebghati M, Thilaganathan B, Khalil A; Southwest Thames Obstetric Research Collaborative . Predictive accuracy of Southwest Thames Obstetric Research Collaborative (STORK) chorionicity‐specific twin growth charts for stillbirth: a validation study. Ultrasound Obstet Gynecol. 2019;53(2):193‐199. [DOI] [PubMed] [Google Scholar]
- 245. Odibo AO, McDonald RE, Stamilio DM, Ural SH, Macones GA. Perinatal outcomes in growth‐restricted twins compared with age‐matched growth‐restricted singletons. Am J Perinatol. 2005;22(5):269‐273. [DOI] [PubMed] [Google Scholar]
- 246. Joseph KS, Fahey J, Platt RW, et al. An outcome‐based approach for the creation of fetal growth standards: do singletons and twins need separate standards? Am J Epidemiol. 2009;169(5):616‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Herlihy N, Odom E, Cohen N, Stroustrup A, Rebarber A, Fox NS. Long‐term outcomes of small for gestational age twins born at 34 weeks or later. Am J Perinatol. 2018;35(3):254‐261. [DOI] [PubMed] [Google Scholar]
- 248. Kibel M, Kahn M, Sherman C, et al. Placental abnormalities differ between small for gestational age fetuses in dichorionic twin and singleton pregnancies. Placenta. 2017;60:28‐35. [DOI] [PubMed] [Google Scholar]
- 249. Barber E, Weiner E, Feldstein O, et al. The differences in placental pathology and neonatal outcome in singleton vs. twin gestation complicated by small for gestational age. Arch Gynecol Obstet. 2018;298(6):1107‐1114. [DOI] [PubMed] [Google Scholar]
- 250. D'Antonio F, Odibo AO, Prefumo F, et al. Weight discordance and perinatal mortality in twin pregnancy: systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2018;52(1):11‐23. [DOI] [PubMed] [Google Scholar]
- 251. Lean SC, Derricott H, Jones RL, Heazell AEP. Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta‐analysis. PLoS One. 2017;12(10):e0186287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Yogev Y, Melamed N, Bardin R, Tenenbaum‐Gavish K, Ben‐Shitrit G, Ben‐Haroush A. Pregnancy outcome at extremely advanced maternal age. Am J Obstet Gynecol. 2010;203(6):558.e1‐7. [DOI] [PubMed] [Google Scholar]
- 253. Shamu S, Abrahams N, Temmerman M, Musekiwa A, Zarowsky C. A systematic review of African studies on intimate partner violence against pregnant women: prevalence and risk factors. PLoS One. 2011;6(3):e17591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Felker‐Kantor E, Wallace M, Theall K. Living in violence: Neighborhood domestic violence and small for gestational age births. Health Place. 2017;46:130‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Saccone G, Berghella V, Sarno L, et al. Celiac disease and obstetric complications: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214(2):225‐234. [DOI] [PubMed] [Google Scholar]
- 256. Yee LM, Silver RM, Haas DM, et al. Quality of periconceptional dietary intake and maternal and neonatal outcomes. Am J Obstet Gynecol. 2020;223(1):121.e1‐121.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet. 2013;382(9890):427‐451. [DOI] [PubMed] [Google Scholar]
- 258. Luke S, Kirby RS. Timing of maternal tobacco exposure, hypertension, and risk of singleton small‐for‐gestational age infants. Am J Perinatol. 2018;35(3):215‐219. [DOI] [PubMed] [Google Scholar]
- 259. Tong VT, England LJ, Rockhill KM, D'Angelo DV. Risks of preterm delivery and small for gestational age infants: effects of nondaily and low‐intensity daily smoking during pregnancy. Paediatr Perinat Epidemiol. 2017;31(2):144‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Mahendru AA, Daemen A, Everett TR, et al. Impact of ovulation and implantation timing on first‐trimester crown‐rump length and gestational age. Ultrasound Obstet Gynecol. 2012;40(6):630‐635. [DOI] [PubMed] [Google Scholar]
- 261. Saito M, Yazawa K, Hashiguchi A, Kumasaka T, Nishi N, Kato K. Time of ovulation and prolonged pregnancy. Am J Obstet Gynecol. 1972;112(1):31‐38. [DOI] [PubMed] [Google Scholar]
- 262. Hadlock FP, Shah YP, Kanon DJ, Lindsey JV. Fetal crown‐rump length: reevaluation of relation to menstrual age (5–18 weeks) with high‐resolution real‐time US. Radiology. 1992;182(2):501‐505. [DOI] [PubMed] [Google Scholar]
- 263. Daya S. Accuracy of gestational age estimation by means of fetal crown‐rump length measurement. Am J Obstet Gynecol. 1993;168(3 Pt 1):903‐908. [DOI] [PubMed] [Google Scholar]
- 264. Altman DG, Chitty LS. New charts for ultrasound dating of pregnancy. Ultrasound Obstet Gynecol. 1997;10(3):174‐191. [DOI] [PubMed] [Google Scholar]
- 265. Savitz DA, Terry JW Jr, Dole N, Thorp JM Jr, Siega‐Riz AM, Herring AH. Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. Am J Obstet Gynecol. 2002;187(6):1660‐1666. [DOI] [PubMed] [Google Scholar]
- 266. Bottomley C, Bourne T. Dating and growth in the first trimester. Best Pract Res Clin Obstet Gynaecol. 2009;23(4):439‐452. [DOI] [PubMed] [Google Scholar]
- 267. Piantelli G, Sacchini C, Coltri A, Ludovici G, Paita Y, Gramellini D. Ultrasound dating‐curve analysis in the assessment of gestational age. Clin Exp Obstet Gynecol. 1994;21(2):108‐118. [PubMed] [Google Scholar]
- 268. Butt K, Lim KI. Guideline no. 388‐determination of gestational age by ultrasound. J Obstet Gynaecol Can. 2019;41(10):1497‐1507. [DOI] [PubMed] [Google Scholar]
- 269. Agathokleous M, Chaveeva P, Poon LC, Kosinski P, Nicolaides KH. Meta‐analysis of second‐trimester markers for trisomy 21. Ultrasound Obstet Gynecol. 2013;41(3):247‐261. [DOI] [PubMed] [Google Scholar]
- 270. Sagi‐Dain L, Maya I, Reches A, et al. Chromosomal microarray analysis results from pregnancies with various ultrasonographic anomalies. Obstet Gynecol. 2018;132(6):1368‐1375. [DOI] [PubMed] [Google Scholar]
- 271. Papageorghiou AT, Fratelli N, Leslie K, Bhide A, Thilaganathan B. Outcome of fetuses with antenatally diagnosed short femur. Ultrasound Obstet Gynecol. 2008;31(5):507‐511. [DOI] [PubMed] [Google Scholar]
- 272. Krakow D, Rimoin DL. The skeletal dysplasias. Genet Med. 2010;12(6):327‐341. [DOI] [PubMed] [Google Scholar]
- 273. D'Ambrosio V, Vena F, Marchetti C, et al. Midtrimester isolated short femur and perinatal outcomes: a systematic review and meta‐analysis. Acta Obstet Gynecol Scand. 2019;98(1):11‐17. [DOI] [PubMed] [Google Scholar]
- 274. Khalil A, Sotiriadis A, Chaoui R, et al. ISUOG Practice Guidelines: role of ultrasound in congenital infection. Ultrasound Obstet Gynecol. 2020;56(1):128‐151. [DOI] [PubMed] [Google Scholar]
- 275. Baschat AA, Weiner CP. Umbilical artery doppler screening for detection of the small fetus in need of antepartum surveillance. Am J Obstet Gynecol. 2000;182(1 Pt 1):154‐158. [DOI] [PubMed] [Google Scholar]
- 276. Baschat AA. Pathophysiology of fetal growth restriction: implications for diagnosis and surveillance. Obstet Gynecol Surv. 2004;59(8):617‐627. [DOI] [PubMed] [Google Scholar]
- 277. Trudinger BJ, Cook CM. Umbilical and uterine artery flow velocity waveforms in pregnancy associated with major fetal abnormality. Br J Obstet Gynaecol. 1985;92(7):666‐670. [DOI] [PubMed] [Google Scholar]
- 278. Rochelson B, Schulman H, Farmakides G, et al. The significance of absent end‐diastolic velocity in umbilical artery velocity waveforms. Am J Obstet Gynecol. 1987;156(5):1213‐1218. [DOI] [PubMed] [Google Scholar]
- 279. Hata K, Hata T, Senoh D, Aoki S, Takamiya O, Kitao M. Umbilical artery blood flow velocity waveforms and associations with fetal abnormality. Gynecol Obstet Invest. 1989;27(4):179‐182. [DOI] [PubMed] [Google Scholar]
- 280. Griffin M, Seed PT, Webster L, et al. Diagnostic accuracy of placental growth factor and ultrasound parameters to predict the small‐for‐gestational‐age infant in women presenting with reduced symphysis‐fundus height. Ultrasound Obstet Gynecol. 2015;46(2):182‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Yinon Y, Farine D, No YMH. 240‐Cytomegalovirus infection in pregnancy. J Obstet Gynaecol Can. 2018;40(2):e134‐e141. [DOI] [PubMed] [Google Scholar]
- 282. Enders M, Daiminger A, Exler S, Ertan K, Enders G, Bald R. Prenatal diagnosis of congenital cytomegalovirus infection in 115 cases: a 5 years’ single center experience. Prenat Diagn. 2017;37(4):389‐398. [DOI] [PubMed] [Google Scholar]
- 283. Peng R, Yang J, Xie HN, Lin MF, Zheng J. Chromosomal and subchromosomal anomalies associated to small for gestational age fetuses with no additional structural anomalies. Prenat Diagn. 2017;37(12):1219‐1224. [DOI] [PubMed] [Google Scholar]
- 284. Brun S, Pennamen P, Mattuizzi A, et al. Interest of chromosomal microarray analysis in the prenatal diagnosis of fetal intrauterine growth restriction. Prenat Diagn. 2018;38(13):1111‐1119. [DOI] [PubMed] [Google Scholar]
- 285. Ma Y, Pei Y, Yin C, et al. Subchromosomal anomalies in small for gestational‐age fetuses and newborns. Arch Gynecol Obstet. 2019;300(3):633‐639. [DOI] [PubMed] [Google Scholar]
- 286. Ferrazzi E, Bozzo M, Rigano S, et al. Temporal sequence of abnormal Doppler changes in the peripheral and central circulatory systems of the severely growth‐restricted fetus. Ultrasound Obstet Gynecol. 2002;19(2):140‐146. [DOI] [PubMed] [Google Scholar]
- 287. Cosmi E, Ambrosini G, D'Antona D, Saccardi C, Mari G. Doppler, cardiotocography, and biophysical profile changes in growth‐restricted fetuses. Obstet Gynecol. 2005;106(6):1240‐1245. [DOI] [PubMed] [Google Scholar]
- 288. Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001;18(6):571‐577. [DOI] [PubMed] [Google Scholar]
- 289. Hecher K, Bilardo CM, Stigter RH, et al. Monitoring of fetuses with intrauterine growth restriction: a longitudinal study. Ultrasound Obstet Gynecol. 2001;18(6):564‐570. [DOI] [PubMed] [Google Scholar]
- 290. Unterscheider J, Daly S, Geary MP, et al. Predictable progressive Doppler deterioration in IUGR: does it really exist? Am J Obstet Gynecol. 2013;209(6):539.e1‐7. [DOI] [PubMed] [Google Scholar]
- 291. Turan OM, Turan S, Gungor S, et al. Progression of Doppler abnormalities in intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;32(2):160‐167. [DOI] [PubMed] [Google Scholar]
- 292. Crimmins S, Desai A, Block‐Abraham D, Berg C, Gembruch U, Baschat AA. A comparison of Doppler and biophysical findings between liveborn and stillborn growth‐restricted fetuses. Am J Obstet Gynecol. 2014;211(6):669.e1‐10. [DOI] [PubMed] [Google Scholar]
- 293. Bilardo CM, Wolf H, Stigter RH, et al. Relationship between monitoring parameters and perinatal outcome in severe, early intrauterine growth restriction. Ultrasound Obstet Gynecol. 2004;23(2):119‐125. [DOI] [PubMed] [Google Scholar]
- 294. Group GS . A randomised trial of timed delivery for the compromised preterm fetus: short term outcomes and Bayesian interpretation. BJOG. 2003;110(1):27‐32. [DOI] [PubMed] [Google Scholar]
- 295. Thornton JG, Hornbuckle J, Vail A, Spiegelhalter DJ, Levene M. GRIT study group. Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): multicentred randomised controlled trial. Lancet. 2004;364(9433):513‐520. [DOI] [PubMed] [Google Scholar]
- 296. Lees C, Marlow N, Arabin B, et al. Perinatal morbidity and mortality in early‐onset fetal growth restriction: cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE). Ultrasound Obstet Gynecol. 2013;42(4):400‐408. [DOI] [PubMed] [Google Scholar]
- 297. Sharp A, Jackson R, Cornforth C, et al. A prediction model for short‐term neonatal outcomes in severe early‐onset fetal growth restriction. Eur J Obstet Gynecol Reprod Biol. 2019;241:109‐118. [DOI] [PubMed] [Google Scholar]
- 298. Oros D, Figueras F, Cruz‐Martinez R, Meler E, Munmany M, Gratacos E. Longitudinal changes in uterine, umbilical and fetal cerebral Doppler indices in late‐onset small‐for‐gestational age fetuses. Ultrasound Obstet Gynecol. 2011;37(2):191‐195. [DOI] [PubMed] [Google Scholar]
- 299. Figueras F, Gardosi J. Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Am J Obstet Gynecol. 2011;204(4):288‐300. [DOI] [PubMed] [Google Scholar]
- 300. Chauhan SP, Rice MM, Grobman WA, et al. Neonatal morbidity of small‐ and large‐for‐gestational‐age neonates born at term in uncomplicated pregnancies. Obstet Gynecol. 2017;130(3):511‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Frusca T, Todros T, Lees C, Bilardo CM, Investigators T. Outcome in early‐onset fetal growth restriction is best combining computerized fetal heart rate analysis with ductus venosus Doppler: insights from the Trial of Umbilical and Fetal Flow in Europe. Am J Obstet Gynecol. 2018;218(2S):S783‐S789. [DOI] [PubMed] [Google Scholar]
- 302. Manning FA, Snijders R, Harman CR, Nicolaides K, Menticoglou S, Morrison I. Fetal biophysical profile score. VI. Correlation with antepartum umbilical venous fetal pH. Am J Obstet Gynecol. 1993;169(4):755‐763. [DOI] [PubMed] [Google Scholar]
- 303. Vintzileos AM, Fleming AD, Scorza WE, et al. Relationship between fetal biophysical activities and umbilical cord blood gas values. Am J Obstet Gynecol. 1991;165(3):707‐713. [DOI] [PubMed] [Google Scholar]
- 304. Baschat AA, Galan HL, Bhide A, et al. Doppler and biophysical assessment in growth restricted fetuses: distribution of test results. Ultrasound Obstet Gynecol. 2006;27(1):41‐47. [DOI] [PubMed] [Google Scholar]
- 305. Tuffnell DJ, Cartmill RS, Lilford RJ. Fetal movements; factors affecting their perception. Eur J Obstet Gynecol Reprod Biol. 1991;39(3):165‐167. [DOI] [PubMed] [Google Scholar]
- 306. Moore TR, Piacquadio K. A prospective evaluation of fetal movement screening to reduce the incidence of antepartum fetal death. Am J Obstet Gynecol. 1989;160(5 Pt 1):1075‐1080. [DOI] [PubMed] [Google Scholar]
- 307. Norman JE, Heazell AEP, Rodriguez A, et al. Awareness of fetal movements and care package to reduce fetal mortality (AFFIRM): a stepped wedge, cluster‐randomised trial. Lancet. 2018;392(10158):1629‐1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Tveit JV, Saastad E, Stray‐Pedersen B, et al. Reduction of late stillbirth with the introduction of fetal movement information and guidelines ‐ a clinical quality improvement. BMC Pregnancy Childbirth. 2009;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Saastad E, Winje BA, Stray Pedersen B, Froen JF. Fetal movement counting improved identification of fetal growth restriction and perinatal outcomes–a multi‐centre, randomized, controlled trial. PLoS One. 2011;6(12):e28482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Unterscheider J, O'Donoghue K, Malone FD. Guidelines on fetal growth restriction: a comparison of recent national publications. Am J Perinatol. 2015;32(4):307‐316. [DOI] [PubMed] [Google Scholar]
- 311. ACOG practice bulletin no. 204 summary: fetal growth restriction. Obstet Gynecol. 2019;133(2):390‐392. [DOI] [PubMed] [Google Scholar]
- 312. Vayssiere C, Sentilhes L, Ego A, et al. Fetal growth restriction and intra‐uterine growth restriction: guidelines for clinical practice from the French College of Gynaecologists and Obstetricians. Eur J Obstet Gynecol Reprod Biol. 2015;193:10‐18. [DOI] [PubMed] [Google Scholar]
- 313. Lavin JP Jr, Miodovnik M, Barden TP. Relationship of nonstress test reactivity and gestational age. Obstet Gynecol. 1984;63(3):338‐344. [PubMed] [Google Scholar]
- 314. Cousins LM, Poeltler DM, Faron S, Catanzarite V, Daneshmand S, Casele H. Nonstress testing at ≤32.0 weeks’ gestation: a randomized trial comparing different assessment criteria. Am J Obstet Gynecol. 2012;207(4):pp. 311, e1–7. [DOI] [PubMed] [Google Scholar]
- 315. Practice bulletin no. 145: antepartum fetal surveillance. Obstet Gynecol. 2014;124(1):182‐192. [DOI] [PubMed] [Google Scholar]
- 316. Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112(3):661‐666. [DOI] [PubMed] [Google Scholar]
- 317. Freeman RK, Anderson G, Dorchester W. A prospective multi‐institutional study of antepartum fetal heart rate monitoring. I. Risk of perinatal mortality and morbidity according to antepartum fetal heart rate test results. Am J Obstet Gynecol. 1982;143(7):771‐777. [DOI] [PubMed] [Google Scholar]
- 318. Grivell RM, Alfirevic Z, Gyte GM, Devane D. Antenatal cardiotocography for fetal assessment. Cochrane Database Syst Rev. 2012;(12):CD007863. [DOI] [PubMed] [Google Scholar]
- 319. Turan S, Turan OM, Berg C, et al. Computerized fetal heart rate analysis, Doppler ultrasound and biophysical profile score in the prediction of acid‐base status of growth‐restricted fetuses. Ultrasound Obstet Gynecol. 2007;30(5):750‐756. [DOI] [PubMed] [Google Scholar]
- 320. Pardey J, Moulden M, Redman CW. A computer system for the numerical analysis of nonstress tests. Am J Obstet Gynecol. 2002;186(5):1095‐1103. [DOI] [PubMed] [Google Scholar]
- 321. Wolf H, Arabin B, Lees CC, et al. Longitudinal study of computerized cardiotocography in early fetal growth restriction. Ultrasound Obstet Gynecol. 2017;50(1):71‐78. [DOI] [PubMed] [Google Scholar]
- 322. Serra V, Moulden M, Bellver J, Redman CW. The value of the short‐term fetal heart rate variation for timing the delivery of growth‐retarded fetuses. BJOG. 2008;115(9):1101‐1107. [DOI] [PubMed] [Google Scholar]
- 323. Lobmaier SM, Huhn EA, Pildner von Steinburg S, et al. Phase‐rectified signal averaging as a new method for surveillance of growth restricted fetuses. J Matern Fetal Neonatal Med. 2012;25(12):2523‐2528. [DOI] [PubMed] [Google Scholar]
- 324. Manning FA, Harman CR, Morrison I, Menticoglou SM, Lange IR, Johnson JM. Fetal assessment based on fetal biophysical profile scoring. IV. An analysis of perinatal morbidity and mortality. Am J Obstet Gynecol. 1990;162(3):703‐709. [DOI] [PubMed] [Google Scholar]
- 325. Nabhan AF, Abdelmoula YA. Amniotic fluid index versus single deepest vertical pocket as a screening test for preventing adverse pregnancy outcome. Cochrane Database Syst Rev. 2008(3):CD006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Chauhan SP, Sanderson M, Hendrix NW, Magann EF, Devoe LD. Perinatal outcome and amniotic fluid index in the antepartum and intrapartum periods: a meta‐analysis. Am J Obstet Gynecol. 1999;181(6):1473‐1478. [DOI] [PubMed] [Google Scholar]
- 327. Clark SL, Sabey P, Jolley K. Nonstress testing with acoustic stimulation and amniotic fluid volume assessment: 5973 tests without unexpected fetal death. Am J Obstet Gynecol. 1989;160(3):694‐697. [DOI] [PubMed] [Google Scholar]
- 328. Morrow RJ, Adamson SL, Bull SB, Ritchie JW. Effect of placental embolization on the umbilical arterial velocity waveform in fetal sheep. Am J Obstet Gynecol. 1989;161(4):1055‐1060. [DOI] [PubMed] [Google Scholar]
- 329. Chang TC, Robson SC, Spencer JA, Gallivan S. Prediction of perinatal morbidity at term in small fetuses: comparison of fetal growth and Doppler ultrasound. Br J Obstet Gynaecol. 1994;101(5):422‐427. [DOI] [PubMed] [Google Scholar]
- 330. Vergani P, Andreotti C, Roncaglia N, et al. Doppler predictors of adverse neonatal outcome in the growth restricted fetus at 34 weeks’ gestation or beyond. Am J Obstet Gynecol. 2003;189(4):1007‐1011. [DOI] [PubMed] [Google Scholar]
- 331. Bahado‐Singh RO, Kovanci E, Jeffres A, et al. The Doppler cerebroplacental ratio and perinatal outcome in intrauterine growth restriction. Am J Obstet Gynecol. 1999;180(3 Pt 1):750‐756. [DOI] [PubMed] [Google Scholar]
- 332. Caradeux J, Martinez‐Portilla RJ, Basuki TR, Kiserud T, Figueras F. Risk of fetal death in growth‐restricted fetuses with umbilical and/or ductus venosus absent or reversed end‐diastolic velocities before 34 weeks of gestation: a systematic review and meta‐analysis. Am J Obstet Gynecol. 2018;218(2S):pp. S774–S782.e21. [DOI] [PubMed] [Google Scholar]
- 333. Hershkovitz R, Kingdom JC, Geary M, Rodeck CH. Fetal cerebral blood flow redistribution in late gestation: identification of compromise in small fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol. 2000;15(3):209‐212. [DOI] [PubMed] [Google Scholar]
- 334. Severi FM, Bocchi C, Visentin A, et al. Uterine and fetal cerebral Doppler predict the outcome of third‐trimester small‐for‐gestational age fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol. 2002;19(3):225‐228. [DOI] [PubMed] [Google Scholar]
- 335. Cruz‐Martinez R, Figueras F, Oros D, et al. Cerebral blood perfusion and neurobehavioral performance in full‐term small‐for‐gestational‐age fetuses. Am J Obstet Gynecol. 2009;201(5):474.e1‐7. [DOI] [PubMed] [Google Scholar]
- 336. Arbeille P, Maulik D, Fignon A, et al. Assessment of the fetal PO2 changes by cerebral and umbilical Doppler on lamb fetuses during acute hypoxia. Ultrasound Med Biol. 1995;21(7):861‐870. [DOI] [PubMed] [Google Scholar]
- 337. Vollgraff Heidweiller‐Schreurs CA, van Osch IR, Heymans MW, et al. Cerebroplacental ratio in predicting adverse perinatal outcome: a meta‐analysis of individual participant data. BJOG. 2020; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 338. Bellotti M, Pennati G, De Gasperi C, Bozzo M, Battaglia FC, Ferrazzi E. Simultaneous measurements of umbilical venous, fetal hepatic, and ductus venosus blood flow in growth‐restricted human fetuses. Am J Obstet Gynecol. 2004;190(5):1347‐1358. [DOI] [PubMed] [Google Scholar]
- 339. Baschat AA, Guclu S, Kush ML, Gembruch U, Weiner CP, Harman CR. Venous Doppler in the prediction of acid‐base status of growth‐restricted fetuses with elevated placental blood flow resistance. Am J Obstet Gynecol. 2004;191(1):277‐284. [DOI] [PubMed] [Google Scholar]
- 340. Rizzo G, Capponi A, Talone PE, Arduini D, Romanini C. Doppler indices from inferior vena cava and ductus venosus in predicting pH and oxygen tension in umbilical blood at cordocentesis in growth‐retarded fetuses. Ultrasound Obstet Gynecol. 1996;7(6):401‐410. [DOI] [PubMed] [Google Scholar]
- 341. Turan OM, Turan S, Berg C, et al. Duration of persistent abnormal ductus venosus flow and its impact on perinatal outcome in fetal growth restriction. Ultrasound Obstet Gynecol. 2011;38(3):295‐302. [DOI] [PubMed] [Google Scholar]
- 342. Divon MY, Girz BA, Lieblich R, Langer O. Clinical management of the fetus with markedly diminished umbilical artery end‐diastolic flow. Am J Obstet Gynecol. 1989;161(6 Pt 1):1523‐1527. [DOI] [PubMed] [Google Scholar]
- 343. Lees CC, Marlow N, van Wassenaer‐Leemhuis A, et al. 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): a randomised trial. Lancet. 2015;385(9983):2162‐2172. [DOI] [PubMed] [Google Scholar]
- 344. Baschat AA. Integrated fetal testing in growth restriction: combining multivessel Doppler and biophysical parameters. Ultrasound Obstet Gynecol. 2003;21(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 345. Wallenstein MB, Birnie KL, Arain YH, et al. Failed endotracheal intubation and adverse outcomes among extremely low birth weight infants. J Perinatol. 2016;36(2):112‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Genzel‐Boroviczeny O, Hempelman J, Zoppelli L, Martinez A. Predictive value of the 1‐min Apgar score for survival at 23–26 weeks gestational age. Acta Paediatr. 2010;99(12):1790‐1794. [DOI] [PubMed] [Google Scholar]
- 347. Boers KE, Vijgen SM, Bijlenga D, et al. Induction versus expectant monitoring for intrauterine growth restriction at term: randomised equivalence trial (DIGITAT). BMJ. 2010;341:c7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Boers KE, van Wyk L, van der Post JAM, et al. Neonatal morbidity after induction vs expectant monitoring in intrauterine growth restriction at term: a subanalysis of the DIGITAT RCT. Am J Obstet Gynecol. 2012;206(4):344.e1‐7. [DOI] [PubMed] [Google Scholar]
- 349. Trudell AS, Cahill AG, Tuuli MG, Macones GA, Odibo AO. Risk of stillbirth after 37 weeks in pregnancies complicated by small‐for‐gestational‐age fetuses. Am J Obstet Gynecol. 2013;208(5):376.e1‐7. [DOI] [PubMed] [Google Scholar]
- 350. van Wyk L, Boers KE, van der Post JA, et al. Effects on (neuro)developmental and behavioral outcome at 2 years of age of induced labor compared with expectant management in intrauterine growth‐restricted infants: long‐term outcomes of the DIGITAT trial. Am J Obstet Gynecol. 2012;206(5):406.e1‐7. [DOI] [PubMed] [Google Scholar]
- 351. Poulain P, Palaric JC, Milon J, et al. Absent end diastolic flow of umbilical artery Doppler: pregnancy outcome in 62 cases. Eur J Obstet Gynecol Reprod Biol. 1994;53(2):115‐119. [DOI] [PubMed] [Google Scholar]
- 352. Melamed N, Yogev Y, Hadar E, Hod M, Ben‐Haroush A. Preinduction cervical ripening with prostaglandin E2 at preterm. Acta Obstet Gynecol Scand. 2008;87(1):63‐67. [DOI] [PubMed] [Google Scholar]
- 353. Sievert RA, Kuper SG, Jauk VC, Parrish M, Biggio JR, Harper LM. Predictors of vaginal delivery in medically indicated early preterm induction of labor. Am J Obstet Gynecol. 2017;217(3):375.e1‐375.e7. [DOI] [PubMed] [Google Scholar]
- 354. Cruz‐Lemini M, Crispi F, Van Mieghem T, et al. Risk of perinatal death in early‐onset intrauterine growth restriction according to gestational age and cardiovascular Doppler indices: a multicenter study. Fetal Diagn Ther. 2012;32(1–2):116‐122. [DOI] [PubMed] [Google Scholar]
- 355. Figueras F, Savchev S, Triunfo S, Crovetto F, Gratacos E. An integrated model with classification criteria to predict small‐for‐gestational‐age fetuses at risk of adverse perinatal outcome. Ultrasound Obstet Gynecol. 2015;45(3):279‐285. [DOI] [PubMed] [Google Scholar]
- 356. Garcia‐Simon R, Figueras F, Savchev S, Fabre E, Gratacos E, Oros D. Cervical condition and fetal cerebral Doppler as determinants of adverse perinatal outcome after labor induction for late‐onset small‐for‐gestational‐age fetuses. Ultrasound Obstet Gynecol. 2015;46(6):713‐717. [DOI] [PubMed] [Google Scholar]
- 357. Familiari A, Khalil A, Rizzo G, et al. Adverse intrapartum outcome in pregnancies complicated by small for gestational age and late fetal growth restriction undergoing induction of labor with Dinoprostone, Misoprostol or mechanical methods: a systematic review and meta‐analysis. Eur J Obstet Gynecol Reprod Biol. 2020;252:455‐467. [DOI] [PubMed] [Google Scholar]
- 358. Khong TY, Mooney EE, Ariel I, et al. Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med. 2016;140(7):698‐713. [DOI] [PubMed] [Google Scholar]
- 359. Khong TY, Ting M, Gordijn SJ. Placental pathology and clinical trials: Histopathology data from prior and study pregnancies may improve analysis. Placenta. 2017;52:58‐61. [DOI] [PubMed] [Google Scholar]
- 360. Levy M, Alberti D, Kovo M, et al. Placental pathology in pregnancies complicated by fetal growth restriction: recurrence vs. new onset. Arch Gynecol Obstet. 2020;301(6):1397‐1404. [DOI] [PubMed] [Google Scholar]
- 361. Economides DL, Nicolaides KH, Linton EA, Perry LA, Chard T. Plasma cortisol and adrenocorticotropin in appropriate and small for gestational age fetuses. Fetal Ther. 1988;3(3):158‐164. [DOI] [PubMed] [Google Scholar]
- 362. Shams M, Kilby MD, Somerset DA, et al. 11Beta‐hydroxysteroid dehydrogenase type 2 in human pregnancy and reduced expression in intrauterine growth restriction. Hum Reprod. 1998;13(4):799‐804. [DOI] [PubMed] [Google Scholar]
- 363. McTernan CL, Draper N, Nicholson H, et al. Reduced placental 11beta‐hydroxysteroid dehydrogenase type 2 mRNA levels in human pregnancies complicated by intrauterine growth restriction: an analysis of possible mechanisms. J Clin Endocrinol Metab. 2001;86(10):4979‐4983. [DOI] [PubMed] [Google Scholar]
- 364. Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol. 2008;35(7):730‐743. [DOI] [PubMed] [Google Scholar]
- 365. Beltrand J, Verkauskiene R, Nicolescu R, et al. Adaptive changes in neonatal hormonal and metabolic profiles induced by fetal growth restriction. J Clin Endocrinol Metab. 2008;93(10):4027‐4032. [DOI] [PubMed] [Google Scholar]
- 366. Rizzo G, Capponi A, Cavicchioni O, Vendola M, Arduini D. Low cardiac output to the placenta: an early hemodynamic adaptive mechanism in intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;32(2):155‐159. [DOI] [PubMed] [Google Scholar]
- 367. Turan S, Miller J, Baschat AA. Integrated testing and management in fetal growth restriction. Semin Perinatol. 2008;32(3):194‐200. [DOI] [PubMed] [Google Scholar]
- 368. Hubinont C, Nicolini U, Fisk NM, Tannirandorn Y, Rodeck CH. Endocrine pancreatic function in growth‐retarded fetuses. Obstet Gynecol. 1991;77(4):541‐544. [PubMed] [Google Scholar]
- 369. Ville Y, Proudler A, Kuhn P, Nicolaides KH. Aldosterone concentration in normal, growth‐retarded, anemic, and hydropic fetuses. Obstet Gynecol. 1994;84(4):511‐514. [PubMed] [Google Scholar]
- 370. Wallace EM, Baker LS. Effect of antenatal betamethasone administration on placental vascular resistance. Lancet. 1999;353(9162):1404‐1407. [DOI] [PubMed] [Google Scholar]
- 371. Senat MV, Ville Y. Effect of steroids on arterial Doppler in intrauterine growth retardation fetuses. Fetal Diagn Ther. 2000;15(1):36‐40. [DOI] [PubMed] [Google Scholar]
- 372. Simchen MJ, Alkazaleh F, Adamson SL, et al. The fetal cardiovascular response to antenatal steroids in severe early‐onset intrauterine growth restriction. Am J Obstet Gynecol. 2004;190(2):296‐304. [DOI] [PubMed] [Google Scholar]
- 373. Robertson MC, Murila F, Tong S, Baker LS, Yu VY, Wallace EM. Predicting perinatal outcome through changes in umbilical artery Doppler studies after antenatal corticosteroids in the growth‐restricted fetus. Obstet Gynecol. 2009;113(3):636‐640. [DOI] [PubMed] [Google Scholar]
- 374. Wijnberger LD, Bilardo CM, Hecher K, Stigter RH, Visser GH. Effect of antenatal glucocorticoid therapy on arterial and venous blood flow velocity waveforms in severely growth‐restricted fetuses. Ultrasound Obstet Gynecol. 2004;23(6):584‐589. [DOI] [PubMed] [Google Scholar]
- 375. Mulder EJ, de Heus R, Visser GH. Antenatal corticosteroid therapy: short‐term effects on fetal behaviour and haemodynamics. Semin Fetal Neonatal Med. 2009;14(3):151‐156. [DOI] [PubMed] [Google Scholar]
- 376. Piazze J, Dillon KC, Cerekja A. Betamethasone effects on umbilical arteries and ductus venosus Doppler velocity waveforms in growth‐restricted fetuses. J Matern Fetal Neonatal Med. 2012;25(7):1179‐1182. [DOI] [PubMed] [Google Scholar]
- 377. McMillen IC, Adams MB, Ross JT, et al. Fetal growth restriction: adaptations and consequences. Reproduction. 2001;122(2):195‐204. [DOI] [PubMed] [Google Scholar]
- 378. Melamed N, Pittini A, Barrett J, et al. Antenatal corticosteroids and outcomes of small‐for‐gestational‐age neonates. Obstet Gynecol. 2016;128(5):1001‐1008. [DOI] [PubMed] [Google Scholar]
- 379. Ting JY, Kingdom JC, Shah PS. Antenatal glucocorticoids, magnesium sulfate, and mode of birth in preterm fetal small for gestational age. Am J Obstet Gynecol. 2018;218(2S):S818‐S828. [DOI] [PubMed] [Google Scholar]
- 380. Melamed N, Shah J, Soraisham A, et al. Association between antenatal corticosteroid administration‐to‐birth interval and outcomes of preterm neonates. Obstet Gynecol. 2015;125(6):1377‐1384. [DOI] [PubMed] [Google Scholar]
- 381. Cahill LS, Whitehead CL, Hobson SR, et al. Effect of maternal betamethasone administration on feto‐placental vascular resistance in the mousedagger. Biol Reprod. 2019;101(4):823‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Rouse DJ, Hirtz DG, Thom E, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. New Engl J Med. 2008;359(9):895‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2009(1):CD004661. [DOI] [PubMed] [Google Scholar]
- 384. Marret S, Doyle LW, Crowther CA, Middleton P. Antenatal magnesium sulphate neuroprotection in the preterm infant. Semin Fetal Neonatal Med. 2007;12(4):311‐317. [DOI] [PubMed] [Google Scholar]
- 385. Costantine MM, Drever N. Antenatal exposure to magnesium sulfate and neuroprotection in preterm infants. Obstet Gynecol Clin North Am. 2011;38(2):351‐366, xi. [DOI] [PubMed] [Google Scholar]
- 386. American College of Obstetricians and Gynecologists Committee on Obstetric Practice; Society for Maternal‐Fetal Medicine . Committee Opinion No. 455: Magnesium sulfate before anticipated preterm birth for neuroprotection. Obstet Gynecol. 2010;115(3):669‐671. [DOI] [PubMed] [Google Scholar]
- 387. Reeves SA, Gibbs RS, Clark SL. Magnesium for fetal neuroprotection. Am J Obstet Gynecol. 2011;204(3):202.e1‐4. [DOI] [PubMed] [Google Scholar]
- 388. Raju TN, Mercer BM, Burchfield DJ, Joseph GF Jr. Periviable birth: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal‐Fetal Medicine, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. Am J Obstet Gynecol. 2014;210(5):406‐417. [DOI] [PubMed] [Google Scholar]
- 389. Magee L, Sawchuck D, Synnes A, von Dadelszen P; Magnesium Sulphate For Fetal Neuroprotection Consensus Committee; Maternal Fetal Medicine Committee . SOGC Clinical Practice Guideline. Magnesium sulphate for fetal neuroprotection. J Obstet Gynaecol Can. 2011;33(5):516‐529. [DOI] [PubMed] [Google Scholar]
- 390. Mallard C, Loeliger M, Copolov D, Rees S. Reduced number of neurons in the hippocampus and the cerebellum in the postnatal guinea‐pig following intrauterine growth‐restriction. Neuroscience. 2000;100(2):327‐333. [DOI] [PubMed] [Google Scholar]
- 391. Sasaki J, Fukami E, Mimura S, Hayakawa M, Kitoh J, Watanabe K. Abnormal cerebral neuronal migration in a rat model of intrauterine growth retardation induced by synthetic thromboxane A(2). Early Hum Dev. 2000;58(2):91‐99. [DOI] [PubMed] [Google Scholar]
- 392. Groom KM, David AL. The role of aspirin, heparin, and other interventions in the prevention and treatment of fetal growth restriction. Am J Obstet Gynecol. 2018;218(2S):S829‐S840. [DOI] [PubMed] [Google Scholar]
- 393. Oyston C, Stanley JL, Oliver MH, Bloomfield FH, Baker PN. Maternal administration of sildenafil citrate alters fetal and placental growth and fetal‐placental vascular resistance in the growth‐restricted ovine fetus. Hypertension. 2016;68(3):760‐767. [DOI] [PubMed] [Google Scholar]
- 394. Dilworth MR, Andersson I, Renshall LJ, et al. Sildenafil citrate increases fetal weight in a mouse model of fetal growth restriction with a normal vascular phenotype. PLoS One. 2013;8(10):e77748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Dastjerdi MV, Hosseini S, Bayani L. Sildenafil citrate and uteroplacental perfusion in fetal growth restriction. J Res Med Sci. 2012;17(7):632‐636. [PMC free article] [PubMed] [Google Scholar]
- 396. Sharp A, Cornforth C, Jackson R, et al. Maternal sildenafil for severe fetal growth restriction (STRIDER): a multicentre, randomised, placebo‐controlled, double‐blind trial. Lancet Child Adolesc Health. 2018;2(2):93‐102. [DOI] [PubMed] [Google Scholar]
- 397. Pels A, Derks J, Elvan‐Taspinar A, et al. Maternal sildenafil vs placebo in pregnant women with severe early‐onset fetal growth restriction: a randomized clinical trial. JAMA Netw Open. 2020;3(6):e205323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Spencer R, Ambler G, Brodszki J, et al. EVERREST prospective study: a 6‐year prospective study to define the clinical and biological characteristics of pregnancies affected by severe early onset fetal growth restriction. BMC Pregnancy Childbirth. 2017;17(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Cluver CA, Hannan NJ, van Papendorp E, et al. Esomeprazole to treat women with preterm preeclampsia: a randomized placebo controlled trial. Am J Obstet Gynecol. 2018;219(4):388.e1‐388.e17. [DOI] [PubMed] [Google Scholar]
- 400. Bauer AJ, Banek CT, Needham K, et al. Pravastatin attenuates hypertension, oxidative stress, and angiogenic imbalance in rat model of placental ischemia‐induced hypertension. Hypertension. 2013;61(5):1103‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Costantine MM, Tamayo E, Lu F, et al. Using pravastatin to improve the vascular reactivity in a mouse model of soluble fms‐like tyrosine kinase‐1‐induced preeclampsia. Obstet Gynecol. 2010;116(1):114‐120. [DOI] [PubMed] [Google Scholar]
- 402. Ahmed A, Williams DJ, Cheed V, et al. Pravastatin for early‐onset pre‐eclampsia: a randomised, blinded, placebo‐controlled trial. BJOG. 2020;127(4):478‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Griffin IJ, Lee HC, Profit J, Tancedi DJ. The smallest of the small: short‐term outcomes of profoundly growth restricted and profoundly low birth weight preterm infants. J Perinatol. 2015;35(7):503‐510. [DOI] [PubMed] [Google Scholar]
- 404. Malin GL, Morris RK, Riley R, Teune MJ, Khan KS. When is birthweight at term abnormally low? A systematic review and meta‐analysis of the association and predictive ability of current birthweight standards for neonatal outcomes. BJOG. 2014;121(5):515‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Ray JG, Park AL, Fell DB. Mortality in infants affected by preterm birth and severe small‐for‐gestational age birth weight. Pediatrics. 2017;140(6):e20171881. [DOI] [PubMed] [Google Scholar]
- 406. Karlberg J, Albertsson‐Wikland K. Growth in full‐term small‐for‐gestational‐age infants: from birth to final height. Pediatr Res. 1995;38(5):733‐739. [DOI] [PubMed] [Google Scholar]
- 407. Paz I, Seidman DS, Danon YL, Laor A, Stevenson DK, Gale R. Are children born small for gestational age at increased risk of short stature? Am J Dis Child. 1993;147(3):337‐339. [DOI] [PubMed] [Google Scholar]
- 408. De Jesus LC, Pappas A, Shankaran S, et al. Outcomes of small for gestational age infants born at <27 weeks’ gestation. J Pediatr. 2013;163(1):55‐60.e1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Longo S, Bollani L, Decembrino L, Di Comite A, Angelini M, Stronati M. Short‐term and long‐term sequelae in intrauterine growth retardation (IUGR). J Matern Fetal Neonatal Med. 2013;26(3):222‐225. [DOI] [PubMed] [Google Scholar]
- 410. Carmody JB, Charlton JR. Short‐term gestation, long‐term risk: prematurity and chronic kidney disease. Pediatrics. 2013;131(6):1168‐1179. [DOI] [PubMed] [Google Scholar]
- 411. Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early‐life conditions on adult health and disease. New Engl J Med. 2008;359(1):61‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Bhargava SK, Sachdev HS, Fall CH, et al. Relation of serial changes in childhood body‐mass index to impaired glucose tolerance in young adulthood. New Eng J Med. 2004;350(9):865‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. New Eng J Med. 2005;353(17):1802‐1809. [DOI] [PubMed] [Google Scholar]
- 414. Eriksson JG. Developmental Origins of Health and Disease ‐ from a small body size at birth to epigenetics. Ann Med. 2016;48(6):456‐467. [DOI] [PubMed] [Google Scholar]
- 415. Heindel JJ, Vandenberg LN. Developmental origins of health and disease: a paradigm for understanding disease cause and prevention. Curr Opin Pediatr. 2015;27(2):248‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Jaddoe VWV. Translational challenges for the developmental origins of health and disease: time to fulfill the promises for innovative prevention strategies. J Dev Orig Health Dis. 2019;10(3):260‐262. [DOI] [PubMed] [Google Scholar]
- 417. Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001;357(9273):2002‐2006. [DOI] [PubMed] [Google Scholar]
- 418. van Oostwaard MF, Langenveld J, Schuit E, et al. Recurrence of hypertensive disorders of pregnancy: an individual patient data metaanalysis. Am J Obstet Gynecol. 2015;212(5):624.e1‐17. [DOI] [PubMed] [Google Scholar]
- 419. Hoffman HJ, Bakketeig LS. Heterogeneity of intrauterine growth retardation and recurrence risks. Semin Perinatol. 1984;8(1):15‐24. [PubMed] [Google Scholar]
- 420. Patterson RM, Gibbs CE, Wood RC. Birth weight percentile and perinatal outcome: recurrence of intrauterine growth retardation. Obstet Gynecol. 1986;68(4):464‐468. [PubMed] [Google Scholar]
- 421. Bratton SL, Shoultz DA, Williams MA. Recurrence risk of low birthweight deliveries among women with a prior very low birthweight delivery. Am J Perinatol. 1996;13(3):147‐150. [DOI] [PubMed] [Google Scholar]
- 422. Bakketeig LS, Hoffman HJ, Jacobsen G, Hagen JA, Storvik BE. Intrauterine growth pattern by the tendency to repeat small‐for‐gestational‐age births in successive pregnancies. Acta Obstet Gynecol Scand Suppl. 1997;165:3‐7. [PubMed] [Google Scholar]
- 423. Ananth CV, Kaminsky L, Getahun D, Kirby RS, Vintzileos AM. Recurrence of fetal growth restriction in singleton and twin gestations. J Matern Fetal Neonatal Med. 2009;22(8):654‐661. [DOI] [PubMed] [Google Scholar]
- 424. Redline RW. Placental pathology: a systematic approach with clinical correlations. Placenta. 2008;29(Suppl A):S86‐S91. [DOI] [PubMed] [Google Scholar]
- 425. Redline RW. Classification of placental lesions. Am J Obstet Gynecol. 2015;213(4 Suppl):S21‐28. [DOI] [PubMed] [Google Scholar]
- 426. Redline RW. The clinical implications of placental diagnoses. Semin Perinatol. 2015;39(1):2‐8. [DOI] [PubMed] [Google Scholar]
- 427. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295‐306. [DOI] [PubMed] [Google Scholar]
- 428. Gleicher N, Pratt D, Dudkiewicz A. The association of antiphospholipid antibodies with pregnancies complicated by fetal growth restriction. Obstet Gynecol. 1992;79(4):637‐638. [PubMed] [Google Scholar]
- 429. Lynch A, Marlar R, Murphy J, et al. Antiphospholipid antibodies in predicting adverse pregnancy outcome. A prospective study. Ann Intern Med. 1994;120(6):470‐475. [DOI] [PubMed] [Google Scholar]
- 430. Pattison NS, Chamley LW, McKay EJ, Liggins GC, Butler WS. Antiphospholipid antibodies in pregnancy: prevalence and clinical associations. Br J Obstet Gynaecol. 1993;100(10):909‐913. [DOI] [PubMed] [Google Scholar]
- 431. Empson M, Lassere M, Craig JC, Scott JR. Recurrent pregnancy loss with antiphospholipid antibody: a systematic review of therapeutic trials. Obstet Gynecol. 2002;99(1):135‐144. [DOI] [PubMed] [Google Scholar]
- 432. Ziakas PD, Pavlou M, Voulgarelis M. Heparin treatment in antiphospholipid syndrome with recurrent pregnancy loss: a systematic review and meta‐analysis. Obstet Gynecol. 2010;115(6):1256‐1262. [DOI] [PubMed] [Google Scholar]
- 433. Mak A, Cheung MW, Cheak AA, Ho RC. Combination of heparin and aspirin is superior to aspirin alone in enhancing live births in patients with recurrent pregnancy loss and positive anti‐phospholipid antibodies: a meta‐analysis of randomized controlled trials and meta‐regression. Rheumatology (Oxford). 2010;49(2):281‐288. [DOI] [PubMed] [Google Scholar]
- 434. van Hoorn ME, Hague WM, van Pampus MG, Bezemer D, de Vries JI, Investigators F. Low‐molecular‐weight heparin and aspirin in the prevention of recurrent early‐onset pre‐eclampsia in women with antiphospholipid antibodies: the FRUIT‐RCT. Eur J Obstet Gynecol Reprod Biol. 2016;197:168‐173. [DOI] [PubMed] [Google Scholar]
- 435. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists . Practice bulletin no. 132: antiphospholipid syndrome. Obstet Gynecol. 2012;120(6):1514‐1521. [DOI] [PubMed] [Google Scholar]
- 436. Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78(10):1296‐1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Bates SM, Greer IA, Pabinger I, Sofaer S, Hirsh J. Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):844S‐886S. [DOI] [PubMed] [Google Scholar]
- 438. Howley HE, Walker M, Rodger MA. A systematic review of the association between factor V Leiden or prothrombin gene variant and intrauterine growth restriction. Am J Obstet Gynecol. 2005;192(3):694‐708. [DOI] [PubMed] [Google Scholar]
- 439. Franchi F, Cetin I, Todros T, et al. Intrauterine growth restriction and genetic predisposition to thrombophilia. Haematologica. 2004;89(4):444‐449. [PubMed] [Google Scholar]
- 440. Dizon‐Townson D, Miller C, Sibai B, et al. The relationship of the factor V Leiden mutation and pregnancy outcomes for mother and fetus. Obstet Gynecol. 2005;106(3):517‐524. [DOI] [PubMed] [Google Scholar]
- 441. Said JM, Higgins JR, Moses EK, et al. Inherited thrombophilia polymorphisms and pregnancy outcomes in nulliparous women. Obstet Gynecol. 2010;115(1):5‐13. [DOI] [PubMed] [Google Scholar]
- 442. Murphy RP, Donoghue C, Nallen RJ, et al. Prospective evaluation of the risk conferred by factor V Leiden and thermolabile methylenetetrahydrofolate reductase polymorphisms in pregnancy. Arterioscler Thromb Vasc Biol. 2000;20(1):266‐270. [DOI] [PubMed] [Google Scholar]
- 443. Silver RM, Zhao Y, Spong CY, et al. Prothrombin gene G20210A mutation and obstetric complications. Obstet Gynecol. 2010;115(1):14‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Rodger MA, Hague WM, Kingdom J, et al. Antepartum dalteparin versus no antepartum dalteparin for the prevention of pregnancy complications in pregnant women with thrombophilia (TIPPS): a multinational open‐label randomised trial. Lancet. 2014;384(9955):1673‐1683. [DOI] [PubMed] [Google Scholar]
- 445. de Vries JI, van Pampus MG, Hague WM, Bezemer PD, Joosten JH, Investigators F. Low‐molecular‐weight heparin added to aspirin in the prevention of recurrent early‐onset pre‐eclampsia in women with inheritable thrombophilia: the FRUIT‐RCT. J Thromb Haemost. 2012;10(1):64‐72. [DOI] [PubMed] [Google Scholar]
- 446. Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO. VTE, thrombophilia, antithrombotic therapy, and pregnancy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e691S‐e736S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins‐Obstetrics . ACOG practice bulletin no. 197: inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132(1):e18‐e34. [DOI] [PubMed] [Google Scholar]
- 448. Berghella V. Prevention of recurrent fetal growth restriction. Obstet Gynecol. 2007;110(4):904‐912. [DOI] [PubMed] [Google Scholar]
- 449. Wallenburg HC, Rotmans N. Prevention of recurrent idiopathic fetal growth retardation by low‐dose aspirin and dipyridamole. Am J Obstet Gynecol. 1987;157(5):1230‐1235. [DOI] [PubMed] [Google Scholar]
- 450. ACOG Committee Opinion No. 743: low‐dose aspirin use during pregnancy. Obstet Gynecol. 2018;132(1):e44‐e52. [DOI] [PubMed] [Google Scholar]
- 451. Horvath A, Koletzko B, Szajewska H. Effect of supplementation of women in high‐risk pregnancies with long‐chain polyunsaturated fatty acids on pregnancy outcomes and growth measures at birth: a meta‐analysis of randomized controlled trials. Br J Nutr. 2007;98(2):253‐259. [DOI] [PubMed] [Google Scholar]
- 452. Gulmezoglu AM, Hofmeyr GJ. Bed rest in hospital for suspected impaired fetal growth. Cochrane Database Syst Rev. 2000(2):CD000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453. Morris RK, Malin G, Robson SC, Kleijnen J, Zamora J, Khan KS. Fetal umbilical artery Doppler to predict compromise of fetal/neonatal wellbeing in a high‐risk population: systematic review and bivariate meta‐analysis. Ultrasound Obstet Gynecol. 2011;37(2):135‐142. [DOI] [PubMed] [Google Scholar]
- 454. de Laat MW, van der Meij JJ, Visser GH, Franx A, Nikkels PG. Hypercoiling of the umbilical cord and placental maturation defect: associated pathology? Pediatr Dev Pathol. 2007;10(4):293‐299. [DOI] [PubMed] [Google Scholar]
- 455. Redline RW, Ravishankar S. Fetal vascular malperfusion, an update. APMIS. 2018;126(7):561‐569. [DOI] [PubMed] [Google Scholar]
- 456. Saleemuddin A, Tantbirojn P, Sirois K, et al. Obstetric and perinatal complications in placentas with fetal thrombotic vasculopathy. Pediatr Dev Pathol. 2010;13(6):459‐464. [DOI] [PubMed] [Google Scholar]
- 457. Redline RW, Abramowsky CR. Clinical and pathologic aspects of recurrent placental villitis. Hum Pathol. 1985;16(7):727‐731. [DOI] [PubMed] [Google Scholar]
- 458. Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum Pathol. 2007;38(10):1439‐1446. [DOI] [PubMed] [Google Scholar]
- 459. Kim MJ, Romero R, Kim CJ, et al. Villitis of unknown etiology is associated with a distinct pattern of chemokine up‐regulation in the feto‐maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti‐fetal graft‐versus‐host disease. J Immunol. 2009;182(6):3919‐3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460. Kim CJ, Romero R, Chaemsaithong P, Kim JS. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S53‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461. Nowak C, Joubert M, Jossic F, et al. Perinatal prognosis of pregnancies complicated by placental chronic villitis or intervillositis of unknown etiology and combined lesions: about a series of 178 cases. Placenta. 2016;44:104‐108. [DOI] [PubMed] [Google Scholar]
- 462. Derricott H, Jones RL, Heazell AE. Investigating the association of villitis of unknown etiology with stillbirth and fetal growth restriction – a systematic review. Placenta. 2013;34(10):856‐862. [DOI] [PubMed] [Google Scholar]
- 463. Chen A, Roberts DJ. Placental pathologic lesions with a significant recurrence risk – what not to miss!. APMIS. 2018;126(7):589‐601. [DOI] [PubMed] [Google Scholar]
- 464. Boog G. Chronic villitis of unknown etiology. Eur J Obstet Gynecol Reprod Biol. 2008;136(1):9‐15. [DOI] [PubMed] [Google Scholar]
- 465. Boyd TK, Redline RW. Chronic histiocytic intervillositis: a placental lesion associated with recurrent reproductive loss. Hum Pathol. 2000;31(11):1389‐1396. [PubMed] [Google Scholar]
- 466. Mekinian A, Costedoat‐Chalumeau N, Masseau A, et al. Chronic histiocytic intervillositis: outcome, associated diseases and treatment in a multicenter prospective study. Autoimmunity. 2015;48(1):40‐45. [DOI] [PubMed] [Google Scholar]
- 467. Hannaford P, Mittal N, Sethna F, Dahlstrom JE. Recurrent chronic intervillositis: the diagnostic challenge – a case report and review of the literature. J Obstet Gynaecol Can. 2019;41(3):344‐347. [DOI] [PubMed] [Google Scholar]
- 468. Bos M, Nikkels P, Cohen D, et al. Towards standardized criteria for diagnosing chronic intervillositis of unknown etiology: a systematic review. Placenta. 2018;61:80‐88. [DOI] [PubMed] [Google Scholar]
- 469. Contro E, deSouza R, Bhide A. Chronic intervillositis of the placenta: a systematic review. Placenta. 2010;31(12):1106‐1110. [DOI] [PubMed] [Google Scholar]
- 470. Andres RL, Kuyper W, Resnik R, Piacquadio KM, Benirschke K. The association of maternal floor infarction of the placenta with adverse perinatal outcome. Am J Obstet Gynecol. 1990;163(3):935‐938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2


