Abstract
Objective
To determine the potential efficacy of ubrogepant for acute treatment of migraine based on historical experience with triptans.
Background
Although triptans have improved migraine treatment, their efficacy and tolerability may limit their utility in some individuals. Ubrogepant is a small‐molecule, oral calcitonin gene–related peptide receptor antagonist approved by the Food and Drug Administration for acute treatment of migraine in adults.
Methods
This post hoc analysis of pooled data from the pivotal trials ACHIEVE I and II, identically designed, randomized, double‐blind, phase 3, single‐attack trials of ubrogepant in adults with a history of migraine with/without aura, examined the efficacy and tolerability of ubrogepant 50 mg versus placebo based on participants’ historical experience with triptans: triptan responder, triptan‐insufficient responder, and triptan naïve. Co‐primary efficacy endpoints were pain freedom and absence of most bothersome migraine‐associated symptom (MBS) 2 h post initial dose. Adverse events (AEs) within historical triptan experience subgroups were evaluated.
Results
In the pooled analysis population (n = 1799), 682 (placebo, n = 350; ubrogepant 50 mg, n = 332), 451 (placebo, n = 223; ubrogepant, n = 228), and 666 (placebo, n = 339; ubrogepant, n = 327) participants were triptan responders, triptan‐insufficient responders, and triptan‐naïve, respectively. Response rates on co‐primary efficacy endpoints were higher for ubrogepant versus placebo across all groups. Treatment‐by‐subgroup interaction p values based on odds ratios for pain freedom (p = 0.290) and absence of MBS (p = 0.705) indicated no significant impact of historical triptan experience on ubrogepant efficacy. AE incidence for ubrogepant did not differ appreciably across historical triptan experience subgroups.
Conclusions
Ubrogepant efficacy and tolerability did not differ for the acute treatment of migraine in participants classified as triptan responders, triptan‐insufficient responders, and triptan‐naïve based on their historical experience with triptans.
Keywords: ACHIEVE, calcitonin gene–related peptide receptor antagonist, migraine, pain freedom, pain relief, triptan
Abbreviations
- AE
adverse event
- CGRP
calcitonin gene–related peptide
- MBS
most bothersome migraine‐associated symptom
- mITT
modified intent‐to‐treat
- OR
odds ratio
- RD
risk difference
- RR
risk ratio
- SAE
serious adverse event
- SD
standard deviation
- TEAE
treatment‐emergent adverse event
INTRODUCTION
Migraine is a highly prevalent disease that has a negative impact on numerous aspects of an individual's life. 1 , 2 Options for acute treatment of migraine include triptans (serotonin 5‐HT1B/1D receptor agonists), ditans (serotonin 5‐HT1F receptor agonists), nonsteroidal anti‐inflammatory drugs, dihydroergotamine, and combination analgesics; many of these fail to meet treatment goals. 3 , 4 , 5 As such, it is estimated that over 95% of people with migraine taking an orally administered acute prescription medication for headache have at least one unmet acute treatment need. 5
Although the availability of triptans has improved the acute treatment of many people with migraine, use of triptans can be limited by insufficient efficacy and poor tolerability in some individuals. 6 In a study of current and past triptan users, the most common reasons for discontinuation cited by the 25% who discontinued use were lack of a therapeutic effect and side effects. 6 Based on their vasoconstrictive actions mediated by the 5‐HT1B receptor, the use of triptans is contraindicated for individuals with cardiovascular disease, specifically individuals with coronary artery disease or a history of stroke, peripheral vascular disease, or chronically uncontrolled hypertension, and caution of triptan use may be warranted in individuals with other cardiovascular risk factors. 7 , 8 , 9 In addition, frequent use of triptans or analgesics has the potential to cause medication overuse headache. 10 , 11 Thus, to enable more individuals to achieve migraine freedom and reduce migraine‐related disability, acute treatment options with different safety and tolerability profiles are needed, specifically for individuals not adequately managed by triptans.
Calcitonin gene–related peptide (CGRP) is an endogenous proinflammatory and pronociceptive neuropeptide that has been shown to have an important role in the pathogenesis of migraine. 12 Small‐molecule CGRP receptor antagonists (gepants) have demonstrated efficacy for the acute treatment of migraine in phase 3 clinical trials. 13 , 14 , 15 , 16 Ubrogepant is a small‐molecule, oral CGRP receptor antagonist approved by the US Food and Drug Administration for the acute treatment of migraine with or without aura in adults. In two identically designed pivotal clinical trials (ACHIEVE I and II), treatment with ubrogepant was associated with significantly greater rates of pain freedom and absence of most bothersome migraine‐associated symptom (MBS) at 2 h compared with placebo. 13 , 14
The goal of this post hoc analysis of pooled data from the ACHIEVE trials was to determine the impact, if any, of an individual's previous response to triptans on the efficacy and tolerability of ubrogepant versus placebo. Because of the different pharmacology of ubrogepant and triptans, 17 , 18 we hypothesized that the treatment response to ubrogepant would not be predicted by the participants’ historical response to triptans. This work was presented in abstract form at the 61st Annual Scientific Meeting of the American Headache Society (July 11–14, 2019; Philadelphia, Pennsylvania, USA). 19
METHODS
Trial design
Two phase 3, randomized, double‐blind, multicenter, single‐attack trials (ACHIEVE I [NCT02828020] and ACHIEVE II [NCT02867709]) evaluated the clinical efficacy and safety of ubrogepant versus placebo for acute treatment of migraine. The trials were conducted from July 22, 2016 to February 26, 2018, and randomized at least one participant to treatment at 188 US sites. Each trial was approved by a local or central institutional review board at each site. Written informed consent was obtained from each participant prior to enrollment in each trial.
Eligibility criteria and methods of the ACHIEVE trials have been reported previously. 13 , 14 Briefly, adults aged 18–75 years with a history of migraine, with or without aura, were randomized 1:1:1 to receive placebo, ubrogepant 50 mg, or ubrogepant 100 mg in ACHIEVE I and placebo, ubrogepant 25 mg, or ubrogepant 50 mg in ACHIEVE II to treat a single attack (Figure 1). The randomization schedule was generated by a computer, contained a block size of 6, and used an automated interactive web‐response system. At screening, participants were categorized into three groups based on previous experience with triptans (triptan responder, triptan‐insufficient responder, and triptan naïve), and at randomization, randomization was stratified by previous experience with triptans and current use of preventive medication for migraine (yes/no). Participants had up to 60 days to treat a single qualifying migraine attack of moderate or severe intensity at home.
FIGURE 1.
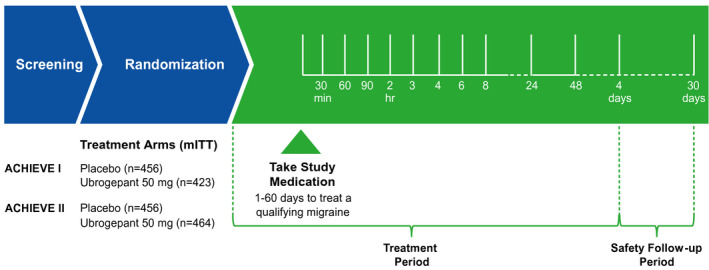
Trial design for ACHIEVE I and ACHIEVE II trials. mITT, modified intent‐to‐treat. Ubrogepant 25 and 100 mg dose groups were not available in both trials and not included in this pooled analysis
Outcome measures
Co‐primary efficacy endpoints were pain freedom at 2 h post initial dose and absence of MBS at 2 h post initial dose. Pain freedom was defined as a reduction in headache severity from moderate/severe at baseline to no pain at 2 h after the initial dose. Participants reported their MBS (photophobia, phonophobia, or nausea) immediately before taking the initial dose for a qualifying migraine attack, and subsequently recorded the presence or absence of that symptom at 2 h after the dose. Pain relief at 2 h after the initial dose was a secondary endpoint, defined as the reduction of a moderate/severe migraine headache to a mild headache or no headache. Efficacy assessments were based on information recorded by the participant in an electronic diary.
In each trial, historical triptan response data were collected via physician interview. Triptan responders included those who reported currently using a triptan or had used a triptan in the past 6 months and achieved pain freedom at 2 h post dose on more than half of the occasions it was taken or, in the past, had a response to a triptan, but no longer uses a triptan for another reason. Triptan‐insufficient responders included those currently using a triptan or who used a triptan in the past 6 months and did not achieve pain freedom at 2 h post dose on more than half of the occasions it was taken, or no longer used a triptan due to lack of efficacy and/or side effects, or never used a triptan due to warnings, precautions, or contraindications (e.g., cardiovascular disease, specifically in individuals with coronary artery disease, a history of stroke, peripheral vascular disease, or chronically uncontrolled hypertension). 7 Triptan‐naïve participants reported no prior exposure to triptans, excluding those contraindicated. Adverse events (AEs), coded by system organ class and preferred term using the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Medical Dictionary for Regulatory Activities, version 20.1 (www.meddra.org), were recorded up to 30 days after the last dose of study medication.
Statistical analyses
Data from the placebo and ubrogepant 50 mg groups of ACHIEVE I and II were pooled for the current analysis. The ubrogepant 100 mg dose (ACHIEVE I) and 25 mg dose (ACHIEVE II) were not included in the pooled analysis, as each dose was only included in a single study. 13 , 14 All authors had full access to the data from both studies. Efficacy analyses were based on the modified intent‐to‐treat (mITT) population (all randomized participants who received at least one dose of investigational product, recorded a baseline migraine headache severity measurement, and had at least one post dose migraine headache severity or migraine‐associated symptom measurement at or before the 2‐h time point). Tolerability analyses were based on the safety population: all participants who received at least one dose of investigational product. Data were summarized with descriptive statistics (mean, standard deviation [SD], number, and percent). The last observation carried forward approach was used to impute missing values at 2 h after the initial dose. All p values were nominal p values, and the significance threshold was 5%. Odds ratios (ORs) with 95% CIs and p values were calculated based on a logistic regression model with treatment group, historical triptan response, historical triptan response by treatment interaction, use of medication for migraine prevention, and baseline headache severity as explanatory variables; for MBS, underlying symptom was included as an additional variable. The estimated risk ratio (RR) and corresponding 95% CI and p values were estimated from a generalized linear mixed model (GLMM), which assumes a binary distribution for the response and uses a log link function. It included treatment group, historical triptan response, historical triptan response by treatment interaction, use of medication for migraine prevention, and baseline headache severity as explanatory variables; for MBS, underlying symptom was included as an additional variable. In addition, the estimated risk difference (RD) and corresponding 95% CI and p values were estimated from a similar GLMM model that assumes normal distribution of the response and uses an identity link function. Analyses were conducted with SAS version 9.4 (SAS Institute Inc, Cary, North Carolina, USA).
RESULTS
Participants
Overall, 1327 participants in ACHIEVE I and 1355 participants in ACHIEVE II were included in the mITT population. The pooled analysis population contained 1799 participants from the two treatment arms common across both studies (placebo, n = 912; ubrogepant 50 mg, n = 887). In the pooled analysis population, 682 (placebo, n = 350; ubrogepant 50 mg, n = 332), 451 (placebo, n = 223; ubrogepant, n = 228), and 666 (placebo, n = 339; ubrogepant, n = 327) participants were triptan responders, triptan‐insufficient responders, and triptan‐naïve, respectively.
In the pooled analysis population, approximately 25% of participants in each treatment group were classified as triptan‐insufficient responders (Table 1). Of these, most (approximately 80% in each treatment group) reported insufficient efficacy associated with triptan use. The proportions of participants with insufficient responder status due to tolerability issues and contraindications to triptans were approximately 17% and 3%, respectively, in each treatment group. Baseline demographics were comparable between the pooled placebo and ubrogepant 50 mg groups in each of the historical triptan experience subgroups, and were consistent with the typical migraine population (Table 2).
TABLE 1.
Classification of historical triptan response (mITT population)
| Pooled ACHIEVE participants, n (%) | ||
|---|---|---|
| Placebo | Ubrogepant 50 mg | |
| (n = 912) | (n = 887) | |
| Triptan responder | 350 (38.4) | 332 (37.4) |
| Triptan‐insufficient responder a | 223 (24.5) | 228 (25.7) |
| Insufficient efficacy | 174 (78.0) | 185 (81.1) |
| Insufficient tolerability | 38 (17.0) | 37 (16.2) |
| Contraindications | 7 (3.1) | 5 (2.2) |
| Triptan naive | 339 (37.2) | 327 (36.9) |
Abbreviation: mITT, modified intent‐to‐treat.
Data missing for n = 2 placebo (ACHIEVE I) and n = 2 placebo and n = 1 ubrogepant 50 mg (ACHIEVE II).
TABLE 2.
Participant characteristics (mITT population)
| Pooled ACHIEVE participants | ||
|---|---|---|
| Placebo | Ubrogepant 50 mg | |
| (n = 912) | (n = 887) | |
| Triptan responder, N | 350 | 332 |
| Age, mean (SD), y | 44.4 (12.5) | 44.0 (11.4) |
| Female, n (%) | 318 (90.9) | 307 (92.5) |
| White, n (%) | 312 (89.1) | 303 (91.3) |
| BMI, mean (SD), kg/m2 | 28.3 (6.3) | 29.2 (7.3) |
| Moderate/high CV risk, n (%) | 27 (7.7) | 31 (9.3) |
| Triptan‐insufficient responder, N | 223 | 228 |
| Age, mean (SD), y | 40.1 (10.7) | 38.4 (10.7) |
| Female, n (%) | 206 (92.4) | 214 (93.9) |
| White, n (%) | 192 (86.1) | 189 (82.9) |
| BMI, mean (SD), kg/m2 | 30.1 (7.9) | 31.1 (8.4) |
| Moderate/high CV risk, n (%) | 32 (14.3) | 24 (10.5) |
| Triptan naive, N | 339 | 327 |
| Age, mean (SD), y | 38.4 (11.2) | 38.2 (12.7) |
| Female, n (%) | 285 (84.1) | 282 (86.2) |
| White, n (%) | 250 (73.8) | 236 (72.2) |
| BMI, mean (SD), kg/m2 | 31.5 (8.2) | 30.9 (7.7) |
| Moderate/high CV risk, n (%) | 33 (9.7) | 37 (11.3) |
Abbreviations: BMI, body mass index; CV, cardiovascular; mITT, modified intent‐to‐treat; SD, standard deviation.
Efficacy across triptan subgroups
A greater proportion of ubrogepant 50 mg versus placebo participants achieved pain freedom (Figure 2A) and absence of MBS (Figure 2B) at 2 h post initial dose. For each historical triptan experience subgroup, a greater proportion of ubrogepant 50 mg versus placebo participants achieved pain freedom (Figure 2A; Table 3) and absence of MBS (Figure 2B; Table 3) at 2 h post initial dose. Treatment‐by‐subgroup interaction p values based on the OR, RR, and RD were nonsignificant for both pain freedom (p = 0.290, p = 0.248, and p = 0.622, respectively) and absence of MBS (p = 0.705, p = 0.636, and p = 0.772, respectively), demonstrating that there was no significant impact of triptan historical responder status on efficacy of ubrogepant. Similarly, a greater proportion of ubrogepant 50 mg versus placebo participants achieved the secondary endpoint of pain relief at 2 h post dose (Figure 2C). Treatment‐by‐subgroup interaction p values for pooled trial data demonstrated no significant impact of triptan historical experience status on pain relief based on the OR (p = 0.140) and RD (p = 0.106); based on RR, the p value was 0.038, but the direction of the RR was the same (RR of the outcome with ubrogepant was greater than with placebo) in all three subgroups.
FIGURE 2.
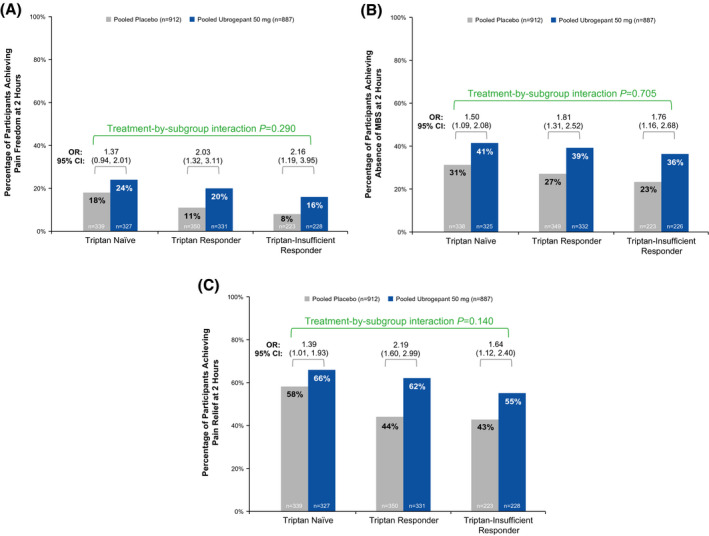
Efficacy outcome measures by triptan historical experience for (A) pain freedom at 2 h, (B) absence of MBS at 2 h, (C) pain relief at 2 h. MBS, most bothersome migraine‐associated symptom; OR, odds ratio
TABLE 3.
Primary efficacy endpoint outcomes by triptan historical experience (mITT population)
| Pooled ACHIEVE participants | ||
|---|---|---|
| Placebo | Ubrogepant 50 mg | |
| (n = 912) | (n = 887) | |
| Triptan naive | ||
| Pain freedom at 2 h, n/N (%) | 61/339 (18) | 78/327 (24) |
| Absence of MBS at 2 h, n/N (%) | 105/338 (31) | 132/325 (41) |
| Triptan responders | ||
| Pain freedom at 2 h, n/N (%) | 40/350 (11) | 67/331 (20) |
| Absence of MBS at 2 h, n/N (%) | 94/349 (27) | 129/332 (39) |
| Triptan‐insufficient responders | ||
| Pain freedom at 2 h, n/N (%) | 18/223 (8) | 37/228 (16) |
| Absence of MBS at 2 h, n/N (%) | 52/223 (23) | 81/226 (36) |
Abbreviations: MBS, most bothersome migraine‐associated symptom; mITT, modified intent‐to‐treat.
The subgroup of triptan‐insufficient responders included participants with prior insufficient efficacy, insufficient tolerability, or contraindications to triptan use; prior insufficient efficacy was the most commonly reported reason for triptan‐insufficient response (placebo, n = 174/223 [78.0%]; ubrogepant 50 mg, n = 185/228 [81.1%]). Within this subgroup of triptan‐insufficient responders based on insufficient efficacy, this post hoc analysis demonstrated that participants randomized to ubrogepant 50 mg versus placebo showed significantly greater rates of pain freedom (15% vs. 6%; p = 0.011), absence of MBS (35% vs. 21%; p = 0.005), and pain relief at 2 h (55% vs. 40%; p = 0.015; Figure 3), demonstrating that ubrogepant was effective in the subgroup of participants with insufficient efficacy with triptans.
FIGURE 3.
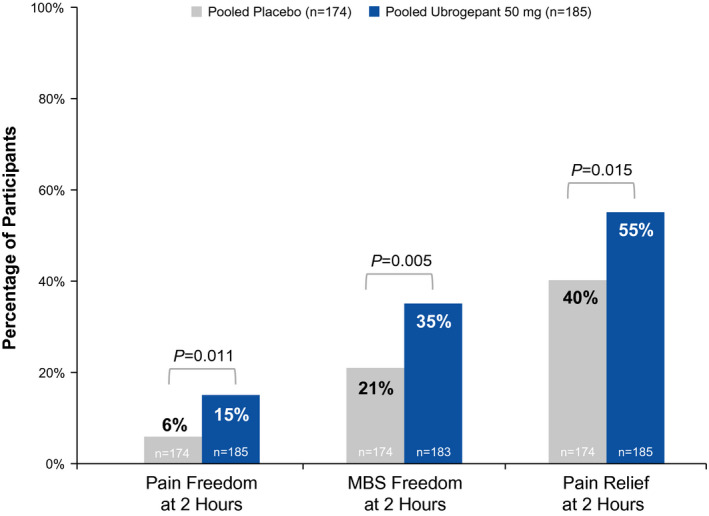
Summary of efficacy outcome measures at 2 h in subgroup of triptan‐insufficient responders with insufficient efficacy. MBS, most bothersome migraine‐associated symptom
Tolerability
Adverse events recorded for the pooled safety population (placebo, n = 984; ubrogepant 50 mg, n = 954) are summarized in Table 4. The incidence of treatment‐emergent AEs (TEAEs) and treatment‐related TEAEs did not differ appreciably across historical triptan experience subgroups. The highest percentage of participants experiencing a treatment‐related TEAE in the pooled ubrogepant 50 mg treatment group was found in the triptan‐insufficient responders (10.4%), whereas the highest percentage in the placebo group was found in the triptan‐naïve subgroup (9.7%). No serious AEs (SAEs) were reported in any subgroup.
TABLE 4.
Summary of adverse events reported within 48 h after initial or optional second dose (safety population)
| Pooled ACHIEVE participants | ||
|---|---|---|
| Placebo | Ubrogepant 50 mg | |
| (n = 984) | (n = 954) | |
| Triptan responders | 372 | 354 |
| All TEAEs, n (%) | 37 (9.9) | 38 (10.7) |
| Treatment‐related TEAEs | 21 (5.6) | 26 (7.3) |
| All serious AEs, n (%) | 0 | 0 |
| Triptan‐insufficient responders | 239 | 240 |
| All TEAEs, n (%) | 29 (12.1) | 37 (15.4) |
| Treatment‐related TEAEs | 14 (5.9) | 25 (10.4) |
| All serious AEs, n (%) | 0 | 0 |
| Triptan naive | 373 | 360 |
| All TEAEs, n (%) | 47 (12.6) | 32 (8.9) |
| Treatment‐related TEAEs | 36 (9.7) | 18 (5.0) |
| All serious AEs, n (%) | 0 | 0 |
Abbreviations: AE, adverse event; TEAE, treatment‐emergent adverse event.
DISCUSSION
We analyzed pooled data from two multicenter, single‐attack, phase 3 trials, ACHIEVE I and ACHIEVE II, for ubrogepant 50 mg versus placebo in adults with a history of migraine to evaluate the efficacy of ubrogepant in three subgroups of participants with varied experiences with triptans. This analysis demonstrated no significant difference in the magnitude of the ubrogepant treatment effect based on historical triptan responder status for any of the outcomes analyzed. Within the subgroup of triptan‐insufficient responders due to insufficient efficacy, ubrogepant 50 mg demonstrated significant efficacy versus placebo for both co‐primary endpoints and pain relief at 2 h post initial dose. These findings suggest that individuals who experience a migraine attack can respond to ubrogepant treatment regardless of their previous experience with triptans.
Across all three outcome measures, the placebo response rates were higher in triptan‐naïve participants than in the two triptan‐experienced subgroups (triptan responders and triptan‐insufficient responders). This has an interesting parallel when considering participants in CGRP pathway monoclonal antibody studies who were naïve to prior preventive therapies. 20 , 21 Accordingly, this greater placebo response resulted in the triptan‐naïve subgroup exhibiting a numerically lower OR versus placebo compared with the other triptan subgroups. However, treatment‐by‐subgroup interaction p values from the logistic regression model, which accounts for all data, found that this pattern was not significant for any of the efficacy outcome measures evaluated. Increased placebo responses in treatment‐naïve participants have been observed in other clinical trials of acute treatment of migraine 22 , 23 , 24 and, although the reasons for this consistent finding are unclear, they may be attributable to a number of factors. It is possible that the diagnosis of migraine was less robustly established in these triptan‐naïve participants and, therefore, this did not lead to prior prescription of a standard migraine‐specific treatment, or that expectation of a new treatment in those who have not had migraine‐specific treatments amplifies the placebo response. For the purpose of drug development trials, this issue raises the possibility of an increased risk of negative trials in this subpopulation, as the treatment effect size may be smaller than that in triptan‐experienced patients with migraine.
The analysis of tolerability demonstrated that the incidence of TEAEs in participants randomized to ubrogepant did not vary appreciably with historical triptan status, and no SAEs were reported. Furthermore, comparable ubrogepant tolerability profiles were observed in triptan‐insufficient responders (including the poor tolerability subgroup) and in participants who reported no previous tolerability concerns with triptans, suggesting that intolerance of triptans does not affect the likelihood of experiencing a TEAE with ubrogepant.
Oral triptans are widely used for the acute treatment of migraine; however, they may fail to meet treatment goals because of partial effectiveness, poor tolerability, or contraindications. 6 , 25 , 26 Discontinuation of triptan use is common in people with migraine, with a pharmacy claims study showing that almost 54% of triptan users received no consecutive refill of their index triptan. 27 Lack of efficacy and tolerability issues are the most commonly reported reasons for triptan discontinuation or switching. 6 Our data suggest that small‐molecule CGRP receptor antagonists such as ubrogepant may represent an effective alternative treatment option for those who fail to achieve adequate efficacy with triptans or experience triptan‐associated tolerability issues.
Our analysis of the ACHIEVE I and II trials subdivided all pooled data into three subgroups based on participants’ historical experience with triptans: triptan responder, triptan‐insufficient responder, and triptan naïve. Participants with contraindications to triptans were grouped in the triptan‐insufficient responder subgroup because of their inability to use triptans as an acute treatment option. The strengths of this analysis include the large population of people with migraine across both trials. With this large overall trial population, we were able to subdivide our population into sizable groups based on historical triptan experience, although no formal sample size power estimation was included in this post hoc analysis. Despite the large overall population, however, sample sizes for some of the secondary triptan‐insufficient responder subgroups (e.g., tolerability and contraindications) were too small to evaluate the significance of the treatment effect in these groups. The ACHIEVE trials were single‐attack trials; thus, conclusions cannot be drawn regarding the impact of triptan experience history on repeated use of ubrogepant in this population. No active comparator was included in the ACHIEVE trials, and no direct comparison of efficacy between triptans and ubrogepant could be made. Furthermore, there was no adjustment for multiple comparisons in the post hoc analyses, and participants’ historical response to triptans was self‐reported and thus may have been impacted by recall bias.
CONCLUSION
Ubrogepant may be effective and well tolerated for the acute treatment of migraine in participants with an insufficient response to triptans. Treatment effects did not differ significantly among the three historical triptan use subgroups, although triptan‐naïve patients exhibited higher placebo responses, which may result in attenuated treatment effects. The results suggest that an insufficient response to triptans (due to lack of efficacy or intolerance) is not associated with an insufficient response to ubrogepant (due to lack of efficacy or intolerance), and AEs with triptans do not indicate AEs with ubrogepant. Ubrogepant, with a mechanism of action distinct from that of triptans, may enable more individuals with migraine to move toward migraine freedom.
CONFLICT OF INTEREST
Andrew M. Blumenfeld has served on advisory boards for, consulted for, and/or been a speaker or contributing author for AbbVie, Alder, Amgen, Biohaven, Lilly, Novartis, Teva, Theranica, and Zoscano. He has received grant support from AbbVie and Amgen. Peter J. Goadsby reports, over the last 36 months, grants and personal fees from Amgen and Eli Lilly and Company; a grant from Celgene; personal fees from AbbVie, Aeon Biopharma, Alder Biopharmaceuticals, Autonomic Technologies Inc., Biohaven Pharmaceuticals Inc., Clexio, electroCore LLC, eNeura, Epalex, Impel Neuropharma, MedicoLegal work, Massachusetts Medical Society, MundiPharma, Novartis, Oxford University Press, Santara Therapeutics, Teva Pharmaceuticals, Trigemina Inc., Up‐to‐Date, WL Gore, and Wolters Kluwer; and a patent for magnetic stimulation for headache assigned to eNeura without fee. David W. Dodick reports the following conflicts within the past 12 months: consulting: AbbVie, AEON, Alder, Amgen, Biohaven, Clexio, Cerecin, Ctrl M, Eli Lilly, eNeura, Equinox, Impel, Linpharma, Lundbeck, Pieris, Promius, Nocira, Novartis, Revance, Theranica, Upjohn (Division of Pfizer), WL Gore, XoC, and Zosano. Honoraria: Cambridge University Press, CME Outfitters, Curry Rockefeller Group, DeepBench, Global Access Meetings, KLJ Associates, Majallin LLC, Medlogix Communications, Miller Medical Communications, Oxford University Press, Southern Headache Society (MAHEC), WebMD Health/Medscape, and Wolters Kluwer. Research Support: American Migraine Foundation, Henry Jackson Foundation, National Institutes of Health, Patient Centered Outcomes Research Institute (PCORI), Sperling Foundation, and US Department of Defense. Stock Options/Shareholder/Board of Directors/Patent: Aural Analytics (options), Ctrl M (shares), Epien (options/board), ExSano (options), Healint (options), King‐Devick Technologies (options/board), Matterhorn (shares/board), Nocira (options), Ontologics (shares/board), Palion (options), Precon Health (options/board), Second Opinion/Mobile Health (options), and Theranica (options). Patent 17189376.1‐1466:vTitle: Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis. Susan Hutchinson has served on advisory boards for AbbVie, Alder, Amgen, Biohaven, electroCore, Eli Lilly, Novartis, Teva, Theranica, and Upsher‐Smith. She is on the speakers bureau for AbbVie, Amgen, Biohaven, electroCore, Eli Lilly, Novartis, and Teva. Chengcheng Liu, Michelle Finnegan, and Joel M. Trugman are employees of AbbVie and may hold AbbVie stock. Armin Szegedi was an employee of AbbVie at the time of study conduct and data analysis, and may hold AbbVie stock.
AUTHOR CONTRIBUTIONS
Study concept and design: Peter J. Goadsby, David W. Dodick, Chengcheng Liu, Michelle Finnegan, Joel M. Trugman, Armin Szegedi. Analysis and interpretation of data: Andrew M. Blumenfeld, Peter J. Goadsby, David W. Dodick, Susan Hutchinson, Chengcheng Liu, Michelle Finnegan, Joel M. Trugman, Armin Szegedi. Revising the manuscript for intellectual content: Andrew M. Blumenfeld, Peter J. Goadsby, David W. Dodick, Susan Hutchinson, Chengcheng Liu, Michelle Finnegan, Joel M. Trugman, Armin Szegedi. Final approval of the completed manuscript: Andrew M. Blumenfeld, Peter J. Goadsby, David W. Dodick, Susan Hutchinson, Chengcheng Liu, Michelle Finnegan, Joel M. Trugman, Armin Szegedi.
CLINICAL TRIAL REGISTRATION
ClinicalTrials.gov identifiers: ACHIEVE I, NCT02828020; ACHIEVE II, NCT02867709.
INSTITUTIONAL REVIEW BOARD APPROVAL
The trials were approved by a local or central institutional review board at each participating institution.
ACKNOWLEDGMENTS
Writing and editorial assistance was provided to the authors by Cory R. Hussar, PhD, of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and was funded by AbbVie. Armin Szegedi was an employee of AbbVie at the time of the study.
Funding information
This study was sponsored by Allergan (prior to its acquisition by AbbVie)
REFERENCES
- 1. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496‐505. [DOI] [PubMed] [Google Scholar]
- 2. Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343‐349. [DOI] [PubMed] [Google Scholar]
- 3. Bigal M, Rapoport A, Aurora S, Sheftell F, Tepper S, Dahlof C. Satisfaction with current migraine therapy: experience from 3 centers in US and Sweden. Headache. 2007;47(4):475‐479. [DOI] [PubMed] [Google Scholar]
- 4. Lipton RB, Buse DC, Serrano D, Holland S, Reed ML. Examination of unmet treatment needs among persons with episodic migraine: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53(8):1300‐1311. [DOI] [PubMed] [Google Scholar]
- 5. Lipton RB, Munjal S, Buse DC, et al. Unmet acute treatment needs from the 2017 Migraine in America Symptoms and Treatment Study. Headache. 2019;59(8):1310‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wells RE, Markowitz SY, Baron EP, et al. Identifying the factors underlying discontinuation of triptans. Headache. 2014;54(2):278‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dodick D, Lipton RB, Martin V, et al. Consensus statement: cardiovascular safety profile of triptans (5‐HT agonists) in the acute treatment of migraine. Headache. 2004;44(5):414‐425. [DOI] [PubMed] [Google Scholar]
- 8. American Headache Society . The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59(1):1‐18. [DOI] [PubMed] [Google Scholar]
- 9. Ahn AH, Basbaum AI. Where do triptans act in the treatment of migraine? Pain. 2005;115(1‐2):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diener HC, Dodick D, Evers S, et al. Pathophysiology, prevention, and treatment of medication overuse headache. Lancet Neurol. 2019;18(9):891‐902. [DOI] [PubMed] [Google Scholar]
- 11. Tajti J, Majlath Z, Szok D, Csati A, Vecsei L. Drug safety in acute migraine treatment. Expert Opin Drug Saf. 2015;14(6):891‐909. [DOI] [PubMed] [Google Scholar]
- 12. Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies ‐ successful translation from bench to clinic. Nat Rev Neurol. 2018;14(6):338‐350. [DOI] [PubMed] [Google Scholar]
- 13. Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the treatment of migraine. N Engl J Med. 2019;381(23):2230‐2241. [DOI] [PubMed] [Google Scholar]
- 14. Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant versus placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II randomized clinical trial. JAMA. 2019;322(19):1887‐1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double‐blind, placebo‐controlled trial. Lancet. 2019;394(10200):737‐745. [DOI] [PubMed] [Google Scholar]
- 16. Lipton RB, Croop R, Stock EG, et al. Rimegepant, an oral calcitonin gene‐related peptide receptor antagonist, for migraine. N Engl J Med. 2019;381(2):142‐149. [DOI] [PubMed] [Google Scholar]
- 17. Moore E, Fraley ME, Bell IM, et al. Characterization of ubrogepant: a potent and selective antagonist of the human calcitonin gene‐related peptide receptor. J Pharmacol Exp Ther. 2020;373(1):160‐166. [DOI] [PubMed] [Google Scholar]
- 18. Goadsby PJ. The pharmacology of headache. Prog Neurobiol. 2000;62(5):509‐525. [DOI] [PubMed] [Google Scholar]
- 19. Blumenfeld AM, Goadsby PJ, Dodick DW, et al. Ubrogepant is effective for the acute treatment of migraine in patients for whom triptans are ineffective [abstract IOR02]. Headache. 2019;59(suppl. 1):19.30367821 [Google Scholar]
- 20. Goadsby PJ, Paemeleire K, Broessner G, et al. Efficacy and safety of erenumab (AMG334) in episodic migraine patients with prior preventive treatment failure: a subgroup analysis of a randomized, double‐blind, placebo‐controlled study. Cephalalgia. 2019;39(7):817‐826. [DOI] [PubMed] [Google Scholar]
- 21. Ruff DD, Ford JH, Tockhorn‐Heidenreich A, et al. Efficacy of galcanezumab in patients with episodic migraine and a history of preventive treatment failure: results from two global randomized clinical trials. Eur J Neurol. 2020;27(4):609‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lipton RB, Schmidt P, Diener HC. Post hoc subanalysis of two randomized, controlled phase 3 trials evaluating diclofenac potassium for oral solution: impact of migraine‐associated nausea and prior triptan use on efficacy. Headache. 2017;57(5):756‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ho TW, Olesen J, Dodick DW, Kost J, Lines C, Ferrari MD. Antimigraine efficacy of telcagepant based on patient's historical triptan response. Headache. 2011;51(1):64‐72. [DOI] [PubMed] [Google Scholar]
- 24. Knievel K, Buchanan AS, Lombard L, et al. Lasmiditan for the acute treatment of migraine: subgroup analyses by prior response to triptans. Cephalalgia. 2020;40(1):19‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holland S, Fanning KM, Serrano D, Buse DC, Reed ML, Lipton RB. Rates and reasons for discontinuation of triptans and opioids in episodic migraine: results from the American Migraine Prevalence and Prevention (AMPP) study. J Neurol Sci. 2013;326(1–2):10‐17. [DOI] [PubMed] [Google Scholar]
- 26. Messali A, Owens G, Bloudek L, Kori S, Cole A, Chia J. Health care resource utilization following initiation of a triptan: a retrospective claims analysis. J Manag Care Spec Pharm. 2014;20(4):368‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katic BJ, Rajagopalan S, Ho TW, Chen YT, Hu XH. Triptan persistency among newly initiated users in a pharmacy claims database. Cephalalgia. 2011;31(4):488‐500. [DOI] [PubMed] [Google Scholar]


