Abstract
Vertebrate beta-catenin plays a key role as a transducer of canonical-Wnt signals. We earlier reported that, similar to beta-catenin, the cytoplasmic signaling pool of p120-catenin-isoform1 is stabilized in response to canonical-Wnt signals. To obtain a yet broader view of the Wnt-pathway’s impact upon catenin proteins, we focused upon plakophilin3 (plakophilin-3; Pkp3) as a representative of the plakophilin-catenin subfamily. Promoting tissue integrity, the plakophilins assist in linking desmosomal cadherins to intermediate filaments at desmosome junctions, and in common with other catenins they perform additional functions including in the nucleus. In this report, we test whether canonical-Wnt pathway components modulate Pkp3 protein levels. We find that in common with beta-catenin and p120-catenin-isoform1, Pkp3 is stabilized in the presence of a Wnt-ligand or a dominant-active form of the LRP6 receptor. Pkp3’s levels are conversely lowered upon expressing destruction-complex components such as GSK3β and Axin, and in further likeness to beta-catenin and p120-isoform1, Pkp3 associates with GSK3beta and Axin. Finally, we note that Pkp3-catenin trans-localizes into the nucleus in response to Wnt-ligand and stimulates an accepted Wnt reporter. These findings fit an expanded model where context-dependent Wnt-signals or pathway components modulate Pkp3-catenin levels. Future studies will be needed to assess potential gene regulatory, cell adhesive, or cytoskeletal effects.
Keywords: Plakophilin-3 catenin / plakophilin3-catenin / Pkp-3, Wnt signaling pathway, destruction complex, desmosome junction / desmosomal junction, nucleus, signaling pool
INTRODUCTION
The organization of most animal tissues requires the adhesive and signaling contributions of catenins, each of which functions at both cell-cell junctions and in the nucleus. For example, beta-catenin indirectly links actin microfilaments to the cytoplasmic domains of classic cadherins [1], and it also transduces Wnt signals to the nucleus [2,3]. In analogy, the plakophilin-catenins help to link desmosomal (desmocollin or desmoglein) cadherins to the intermediate filament cytoskeleton [4], and while less studied, they additionally have nuclear roles as referenced below. Also of interest is that p120-catenin and related subfamily members regulate small-GTPases which engage in multiple processes including cytoskeletal control [5,6].
Vertebrate armadillo-domain catenins are classified into the beta-, p120- and plakophilin-catenin-subfamilies [7–9]. The beta-catenin-subfamily includes beta-catenin and plakoglobin/ gamma-catenin; the p120-catenin subfamily contains p120-, δ-, p0071- and ARVCF-catenin; and the plakophilin/ Pkp-subfamily possesses Pkp1-, Pkp2- and Pkp3-catenin.
beta-Catenin and its associated Wnt pathway have central roles in development, regeneration, homeostasis and disease. We tested whether the plakophilin3-catenin – hereafter referred to as Pkp3 - might likewise respond to Wnt pathway activity and components. Wnt activity stabilizes beta’s signaling pool, whereupon a proportion enters the nucleus to de-repress Wnt/ beta-catenin (TCF/ LEF) target genes [3,10].
We previously reported that Pkp3 enters the nucleus to bind the ETV1 transcription factor, activating genes involved in the synthesis of the neurotransmitter dopamine [11]. Additional groups have likewise examined nuclear plakophilins [12–14], with for example, plakophilin2/ Pkp2 associating with RNA Pol III [13] and plakophilin1/ Pkp1 with single-stranded DNA [14]. Knowledge of the direct gene targets of nuclear plakophilin-catenins is limited, with at least some Pkp3:ETV1 targets appearing to be insensitive to canonical-Wnt activation [11].
Intriguingly, in independent work we showed that p120-catenin-isoform1 responds to canonical-Wnt signals and components with consequent nuclear outcomes [15]. At the same time we provided some evidence for the Wnt-responsiveness of ARVCF1-catenin and δ-catenin, with the latter being independently supported [16]. To test whether Wnt pathway responsiveness further extends to the plakophilin-catenin subfamily, we examined Pkp3 [17]. Together with recent reports of downstream (nuclear) beta-catenin effects being modulated by Pkp1 and Pkp2 [18,19], our work here suggests that upstream Wnt pathway signals or components positively regulate Pkp3 protein levels. We thus expect that vertebrate Wnt signals have the capacity to act upon multiple catenins. This would widen the number of available Wnt-driven outcomes in accordance with the cellular or developmental context.
MATERIALS AND METHODS
Embryo Manipulations
Xenopus laevis embryos were obtained, fertilized and microinjected as described [20–26]. Xenopus laevis procedures were conducted to minimize animal discomfort as approved by the UT MDACC Institutional Animal Care and Use Committee (ACUF Protocol # 09-93-05717) and UTHealth’s Animal Welfare Committee (Protocol # AWC-19-0081). Embryos were microinjected at the 1–2-cell stage with capped mRNAs synthesized in vitro (mMessage mMachine, ThermoFisher Scientific), and then harvested at the indicated embryonic stages (between 9–12) for immuno-blotting [27]. All pCS2-based constructs were linearized using NotI prior to in vitro transcription. The single pSP64 construct was linearized with EcoR1.
cDNA constructs
pCS2 plasmids were generated via PCR or traditionally with the inserts Flag-Pkp3 (human Pkp3a / variant 1; NCBI Reference Sequence: NM_007183.3), Myc-xPkp3 (Xenopus laevis Pkp3 S homeolog/ NCBI Reference Sequence: NM_001090955.1) [28], Myc-betaTrCP [15], Myc-δ-catenin [29], and Flag-hTrim24. Kindly provided as indicated were Myc-Axin (PS Klein, U Pennsylvania), Trim24 (M Barton, UT MDACC), HA-GSK3beta–pcDNA (Addgene), Myc-hLRP6 deletion E1–4-pCS2 (GJ Rosman, Fred Hutchinson Cancer Center), and XWnt8 in psp64T. PCR-generated deletion constructs on human Pkp3 were placed into the pCMV-Flag vector. All constructs were sequence verified.
Mammalian cell culture and transfection
We employed HaCaT, MDA-231, HEK293, HEK293T and A431 cell lines obtained from the ATCC, the UT MDACC Characterized Cell Line Core Facility, or the Korean Cell Line Bank (Korea), with each authenticated as mycoplasma-free. Cells were grown at 37°C/ 5% CO2 in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS and penicillin/ streptomycin. HaCaT, 293T and A431 cells were transiently transfected with the constructs indicated (Lipofectamine 2000/3000; manufacturer’s protocol for six-well dishes). All siRNAs were from Invitrogen and previously validated against transcripts encoding Axin1/2 or LRP5/6 [15,30]. After incubations for 48–72hours, cell lysates were collected using RIPA buffer (Thermo Fisher), and proteins detected via immuno-blotting/ ECL (Thermo Fisher). For immunofluorescence, transfected cells were fixed and imaged following treatment, as described below. The Wnt3a peptide used for treatment was purchased from R&D systems (#5036-WN-010).
Immuno-precipitation and immuno-blot assays
Immuno-precipitation and immuno-blotting employed commercial monoclonal antibodies directed against Myc (9E10), HA (12CA5), Flag (M2) and plakophilin3 (23E3/4, Progen 651113). For immuno-blots, Pkp3 (500 pg) was co-injected with various titrations of RNA (either β-gal or Xenopus wnt8, axin1, gsk3β, or human LRP6∆1–4) along with 1 ng membrane-RFP RNA [31], which serves as a lineage tracer. 10 embryos were collected at stage 11–12 [27], to make protein lysates as previously described [32]. One embryo equivalent of lysate was run in each well of a 10% SDS-PAGE gel. Protein was transferred onto a nitrocellulose membrane that was incubated at room temperature in KPL block (SeraCare, Milford MA) for one hour at 4°C. Blots were incubated with rabbit anti-Pkp3 antibody (1:1000), mouse anti-Myc antibody (1:5000), or rabbit anti-glyceraldehyde 3-phosphate dehydrogenase antibody (anti-GAPDH) (1:1000, Santa Cruz) overnight at 4°C. Blots were washed with TBST, incubated in goat anti-rabbit or goat anti-mouse IgG horseradish peroxidase secondary antibody (1:3000; BioRad) for 1 hour at room temperature, and washed again with TBST prior to imaging with BioRad ChemiDoc XRS+ (BioRad) using SuperSignal West Pico PLUS Chemiluminescent Substrate (ThermoFisher Scientific). The adjusted density (AU) for the positive and negative regulators in Xenopus experiments (Fig 1B) were measured against the upper Pkp3 band recognized by the anti-Pkp3 antibody. Statistical significance was established using a two-tailed T-test. For immuno-precipitations, equal quantities of protein or equal numbers of cells at confluence were isolated from 6-well tissue culture dishes that had been transfected with 0.5–2ug of the indicated plasmid DNA (10–15ug in the case of 100mm dishes). Mammalian cell lysates were prepared using RIPA buffer (1% NP40; ThermoFisher Scientific), inclusive of Halt™ Protease and Phosphatase inhibitor cocktail (ThermoFisher Scientific). Lysates from mammalian cells and procedures for immuno-precipitations were performed as described [21,33].
Figure 1. Pkp3-catenin responds to components of the canonical-Wnt pathway.
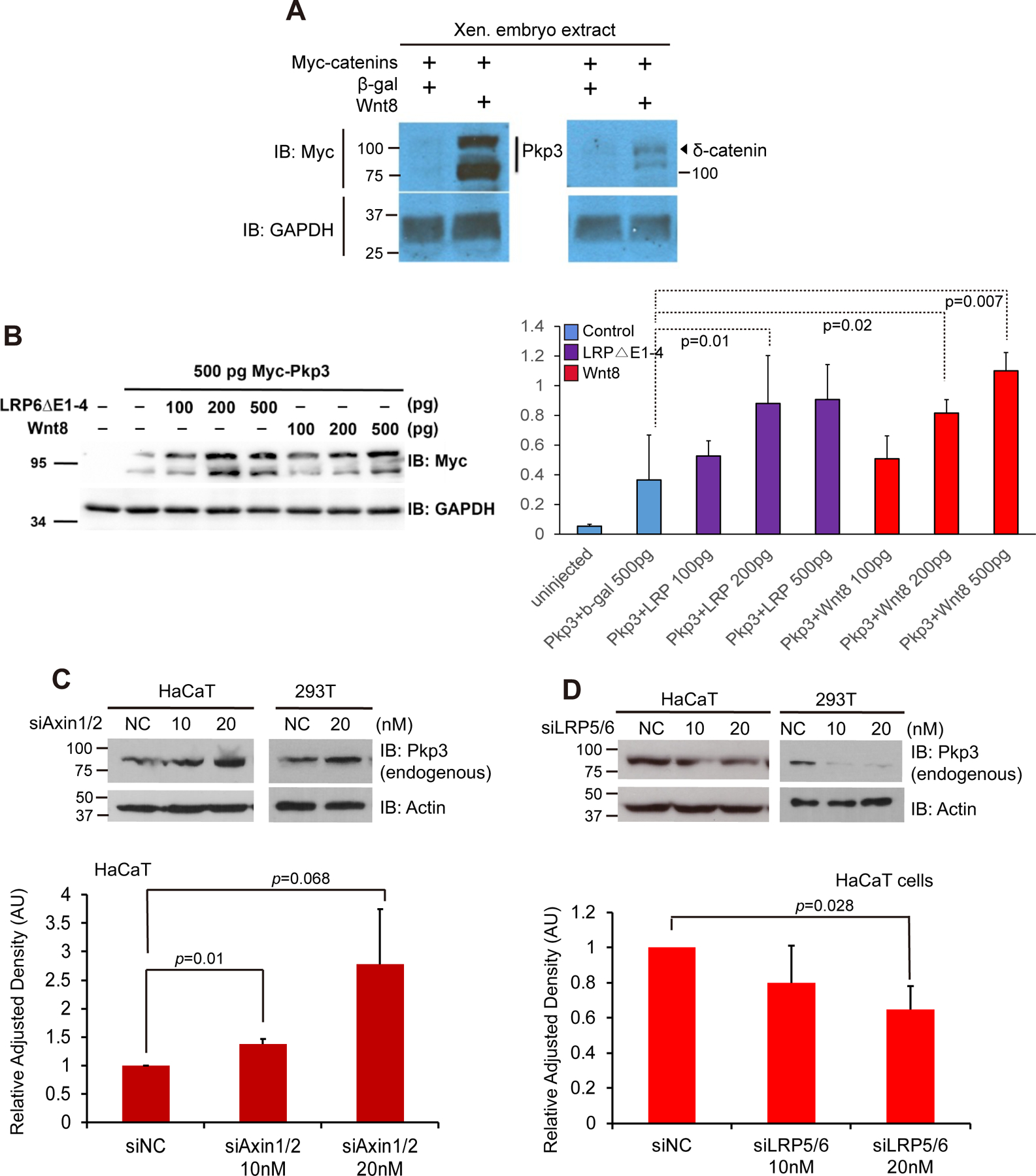
(A) In vitro transcribed mRNA encoding Myc-tagged Pkp3-catenin (1 ng) or Myc-tagged δ-catenin (1 ng) microinjected with mRNAs encoding Wnt8 (1 ng) or β-gal (1 ng; negative control) into one-cell stage embryos. Gastrulating embryos (stages 10–11) were harvested followed by immuno-blotting of their lysates. Catenin-protein detection occurred using anti-Myc antibodies, with GAPDH serving as an internal loading control. (B) Myc-pkp3 mRNA (500 pg) was microinjected into each blastomere of one-cell stage embryos with the indicated mRNA levels of wnt8, LRP6△E1–4 or β -gal (500 pg; negative control) varying from 100 to 500 pg. Statistical significance was found when comparing β-gal (lane 2) to LRP6△E1–4 at 200 pg p=.01 (lane 4), and wnt8 at both 200 pg p=0.02 (lane 7), and 500 pg p=0.007 (lane 8). GAPDH was used as a loading control. Bars along the sides of immuno-blots refers to the observed SDS-PAGE mobility of the corresponding molecular-weight standards. Statistical significance was established using a two-tailed T-test. Myc-Pkp3 and GAPDH were visualized using antibodies directed against Myc or GAPDH. (C) HaCaT and 293T cells were seeded in six-well plates, followed by the combined siRNA mediated depletion of Axin1 and Axin2. NC denotes negative-control siRNA. Endogenous Pkp3 was monitored using the 23E3/4 monoclonal antibody. Lower panel: employing densitometry and ImageJ software, endogenous Pkp3 levels were quantitated and normalized relative to Actin loading control. (D) HaCaT and 293T cells were transfected (48 hours) with siRNAs [15] to deplete LRP5 and LRP6. Endogenous Pkp3 was monitored using the 23E3/4 monoclonal antibody. Lower panel: using densitometry and ImageJ software, endogenous Pkp3 levels were quantitated and normalized to the actin loading control. Experiments were repeated at least three times with consistent results.
Immunofluorescence and nuclear localization assay
Immunostaining of HEK293 cells was completed using an established protocol [34]. Briefly, 24 hours after treatment with Wtn3a-peptide (50μg/ml), cells were fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton X-100 solution prior to staining. Immunostaining of HEK293 cells employed a commercial DAPI stain and monoclonal antibody directed against Flag (Sigma-Aldrich). Images were acquired using a Nikon A1 confocal microscope with a 63x, 1.4 numerical aperture oil immersion objective (BRSB Microscopy Facility at Department of Genetics, UT MD Anderson Cancer Center). Nuclear and cytosolic regions of cells were distinguished using DAPI staining, and selected in ImageJ. The mean fluorescence intensity of each region was recorded and used to create a Nuclear:Cytosolic localization ratio for each cell analyzed. Data was analyzed using GraphPad Prism. Statistical significance was determined using the Student’s t test, using a two-tailed test that assumes unequal variances. Significance was assigned at p < 0.05.
TOP-flash/ FOP-flash assay of canonical Wnt activity
HEK293 cells were transfected with constructs encoding Renillia luciferase and either the TOP-flash artificial reporter (3x TCF binding sites upstream of luciferase reporter; experimental), or FOP-flash (3x mutated TCF binding sites; negative control) [35]. To test the impact of Pkp3 on canonical Wnt signaling, cells were additionally transfected with Flag-tagged Pkp3. Expression of Pkp3 was confirmed with a monoclonal Pkp3-antibody (Invitrogen). Luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega - Cat. # E1910) using a BMG Labtech Fluostar Omega plate reader. Firefly luciferase luminescence was normalized to Renilla luciferase luminescence for each condition/experiment. Significance was assigned at p < 0.05.
RESULTS
Members of the plakophilin/ Pkp-catenin subfamily are more evolutionarily distant from beta-catenin than are p120-catenin subfamily members. Since some p120-catenin subfamily members respond to components of the Wnt pathway [15], we tested whether this might extend further to the plakophilin-catenins. Indeed, co-expression of Wnt8 ligand in Xenopus embryos dramatically increased the level of epitope-tagged Pkp3, here barely detectable in the absence of ligand (Fig 1A). While not the focus of this study, we similarly observed a response of epitope-tagged δ-catenin to Wnt pathway activation (Fig 1A) consistent with prior reports [15,16]. We next tested additional components of the canonical-Wnt pathway. Similar to known responses of β-catenin and p120-catenin-isoform1 [15,36], positive regulators such as Wnt8-ligand and the constitutively-active co-receptor mutant LRP6△E1–4 were examined in Xenopus embryos (Fig 1B). Further supporting the findings of Fig 1A, Wnt8-ligand expression increased Pkp3 levels in a dose-dependent manner. In transducing canonical Wnt signals, LRP5/6 acts as a co-receptor of Frizzled receptors [37]. We made use of the constitutively-active mutant LRP6 ΔE1–4 [28], co-expressing it with Myc-tagged Pkp3 (Fig 1B). Consistent with our Wnt8-ligand findings, LRP6 ΔE1–4 produced a dose dependent increase in Pkp3 protein levels (Fig 1B).
To complement our embryo findings that reflect the responses of exogenous Pkp3, we evaluated knock-down of Axin or LRP5/6 upon endogenous Pkp3 in mammalian HaCaT and HEK293T cells. This provided us the opportunity to compare findings from independent experimental systems. Thus, using siRNAs that had been previously characterized [15], we tested whether depleting Axin or LRP5/6 would conversely increase the levels of endogenous Pkp3. Analogous to prior findings for beta-catenin and p120-isoform1 [15,36], depletion of Axin increased endogenous Pkp3 levels in a dose-dependent manner (Fig 1C and Supplemental Fig 1A). Correspondingly, when we depleted LRP5/6, which as noted is a positive modulator of Wnt signaling, Pkp3 levels were lowered (Fig. 1D and Supplemental Fig 1B). Overall, Pkp3 responds to positive- and negative-acting Wnt-pathway components in a manner analogous to the established responses of beta-catenin and p120-isoform1.
Canonical-Wnt ligands promote the segregation of negative modulators such as GSK3beta and Axin away from beta-catenin, enabling beta-catenin’s signaling pool to rise. Otherwise, GSK3beta activity assists in the destruction of beta-catenin (Fig 2A). As might be postulated from our above results, we found that depletion of the negative-regulator GSK3beta, through using a mix of two established shRNAs, led to raised endogenous levels of Pkp3 (Fig 2B). Similarly, when GSK was inhibited by incubating cells in the presence of lithium chloride, endogenous Pkp3 levels increased in a dose-dependent manner (Fig 2C). Fig 2C’s bottom panel reflects quantification after normalization to the actin loading control. Complementing such findings, decreased levels of Pkp3 were instead observed upon expressing negative modulators of the Wnt pathway such as GSK3beta and Axin (Fig 2D). Collectively, our data suggest that Pkp3-catenin is responsive to Wnt pathway components, analogous to the sensitivity of beta-catenin [3] and p120-catenin-isoform1 [15].
Figure 2. GSK3β modulates Pkp3-catenin protein levels.
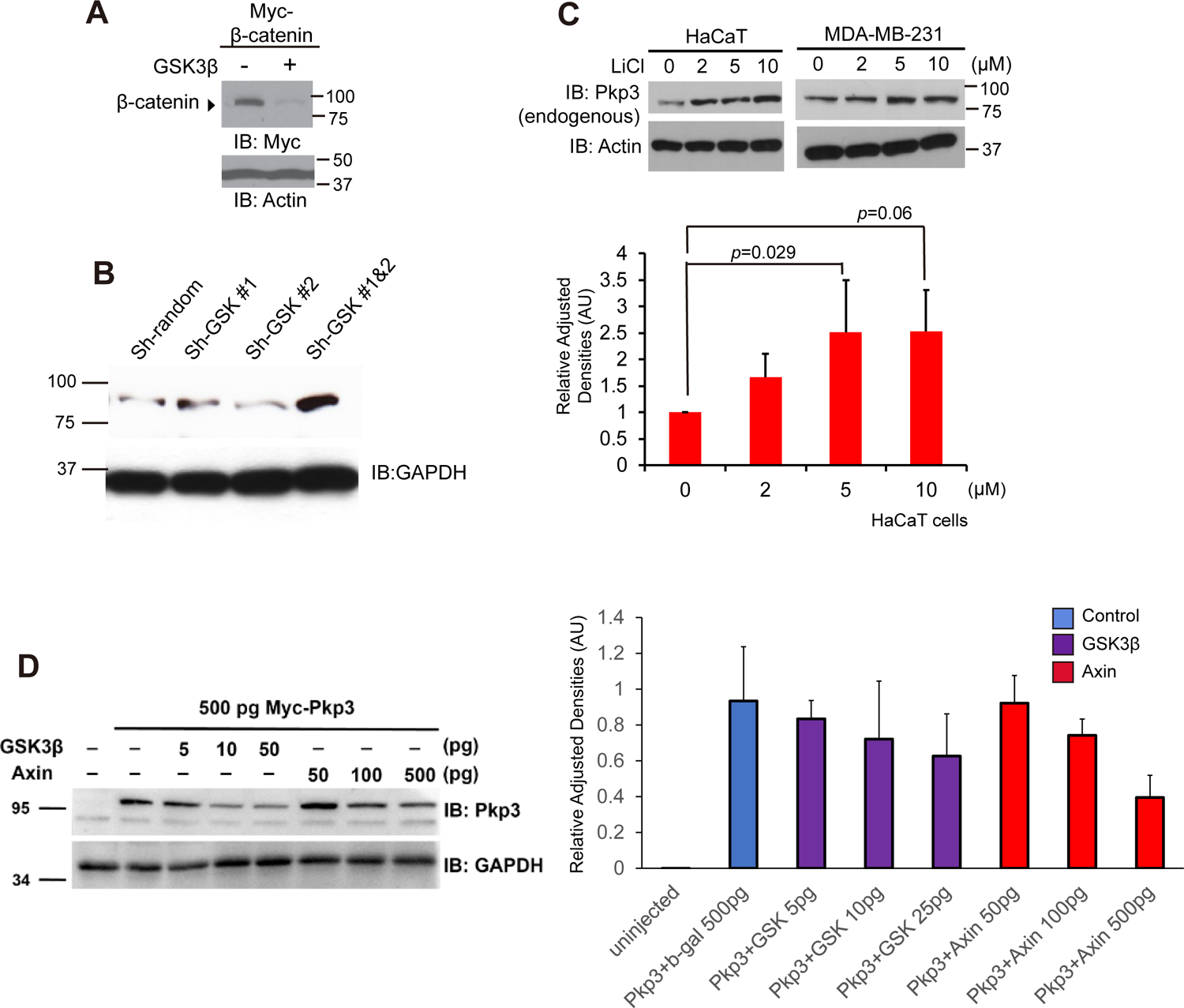
(A) beta-Catenin mRNA (1 ng) was co-injected with GSK3beta (500 pg) into embryos at the one-to-two-cell stage. Gastrulae extracts (stages 11–12) were immuno-blotted with anti-Myc antibody. Actin serves as an internal loading control. (B) HaCaT cells were transfected with the indicated shRNA constructs directed against transcripts of GSK. Endogenous Pkp3 and GAPDH levels were assessed via immuno-blotting. (C) HaCaT and MDA-231 cells were incubated with LiCl for 6 hours at the indicated concentrations, followed by immuno-blotting for endogenous Pkp3 (23E3/4 mAb). Endogenous Pkp3 levels are normalized to Actin in the lower panel. (D) Myc-pkp3 mRNA (500 pg) was microinjected into each blastomere of one-cell stage embryos with the indicated mRNA levels of gsk3β, axin1, or β-gal (500 pg; negative control), varying from 5 to 500 pg. Although no significance was found when compared to the negative-control β-gal (lane 2), there is a general trend of loss of Pkp3 expression upon increasing doses of negative regulators of the Wnt pathway, gsk3β or axin1. GAPDH was used as a loading control. Bars along the sides of immuno-blots refers to the observed SDS-PAGE mobility of the corresponding molecular-weight standard(s). Statistical significance was established using a two-tailed T-test.
β-catenin’s degradation is promoted through association with destruction components of the Wnt-pathway such as GSK3β, Axin and β-TrCP. We thus tested whether Flag-Pkp3 associates with GSK3β. Indeed, upon their expression even at levels barely detectable in whole cell lysates, we clearly and reproducibly observed their co-association (Fig 3A lane 1 versus 2; negative-control Trim24 panel is to right). We next assessed Pkp3’s association with Axin, which scaffolds components of the destruction complex. As for GSK3beta, Axin was resolved in association with Pkp3 following the enrichment provided by Pkp3 immuno-precipitation (Fig 3B). We likewise confirmed that exogenous Pkp3-catenin associates with β-TrCP (Fig 3C lanes 1 versus 2). β-TrCP is the E3 substrate recognition subunit of SCFβ-TrCP E3, known to bind β-catenin and p120-catenin-isoform1[15,38,39]. As expected, a small fraction of endogenous Pkp3-catenin was likewise found in immuno-precipitates of β-TrCP (Fig 3D). Similar to β-catenin and p120-catenin-isoform1, our Xenopus embryo and cell-line findings (Figs 1–3) suggest that Pkp3-catenin responds to upstream Wnt-ligand stimulation and to destruction complex components. Further, immuno-precipitations indicate that GSK3, Axin and β-TrCP co-associate with Pkp3.
Figure 3. Pkp3-catenin associates with components of the canonical-Wnt destruction complex.
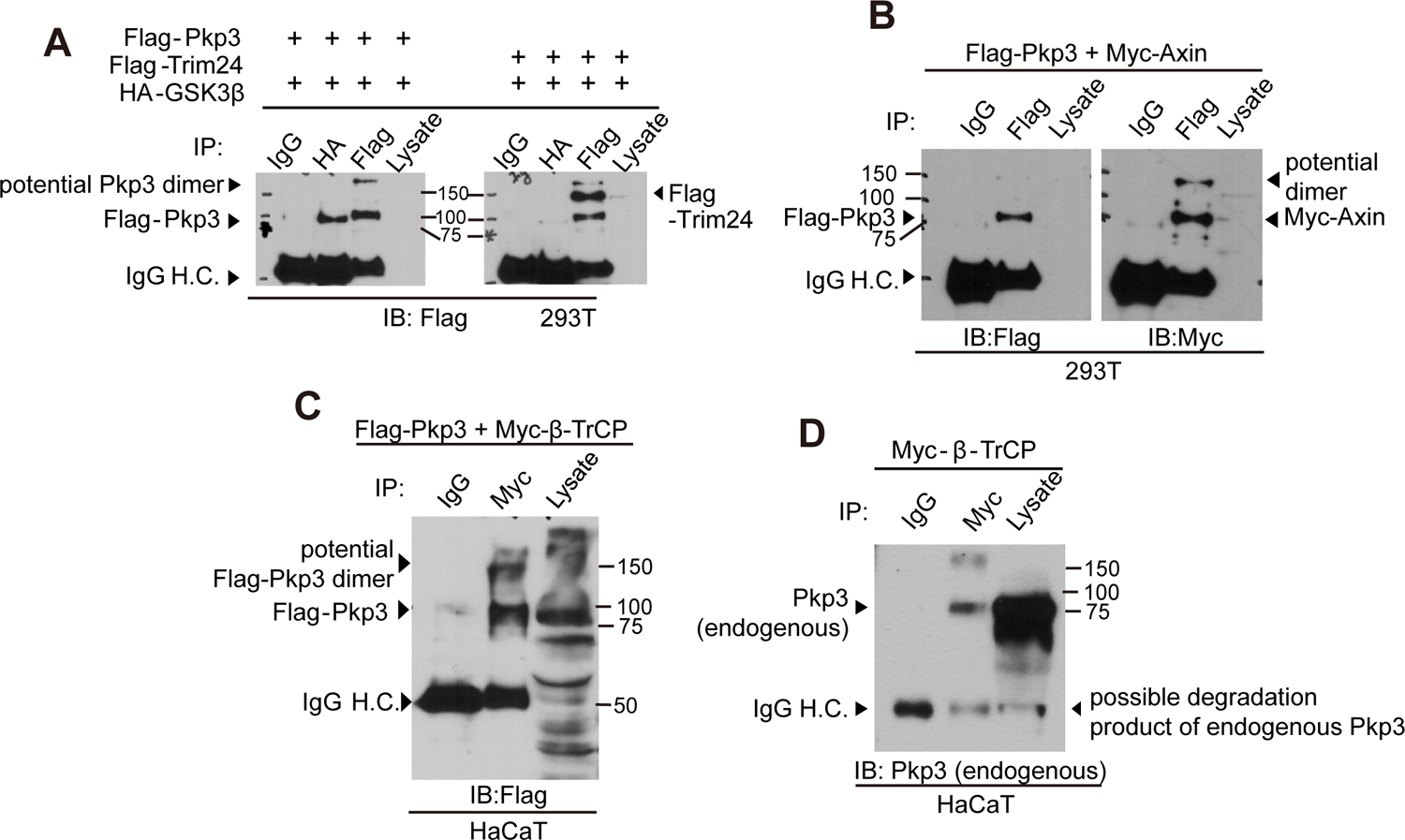
(A) 293T cells in 100mm dishes were transfected with Flag-Pkp3 (8μg) and HA-GSK3beta (5 μg). The Pkp3:GSK3β association was resolved via anti-HA or anti-Flag immuno-precipitation/ immuno-blot. (B) Flag-Pkp3 (2μg) and Myc-Axin (2μg) were co-transfected in 293T cells. Immuno-precipitates (IP) of Flag-Pkp3 were immuno-blotted [19] with anti-Flag or -Myc antibodies to detect Pkp3 or Axin. (C) Flag-Pkp3 (3μg) and Myc-β-TrCP (3μg) were co-transfected into HaCaT cells. IPs were performed with anti-Myc antibody to precipitate β-TrCP, followed by immuno-blotting with anti-Flag antibody. (D) Endogenous Pkp3 coprecipitates with Myc-β-TrCP from HaCaT-cell extracts.
The amino-terminal region of β-catenin as well as p120-catenin-isoform1 harbor a primary sequence region (“destruction box”) that in the absence of canonical-Wnt signals promotes association with GSK3β, CK1α, β-TrCP and Axin [40,41]. We constructed deletion constructs of Pkp3-isoformA to begin mapping Pkp3’s association with and sensitivity to GSK3β (Figs 4A and 4B; WCL = whole cell lysate). As opposed to the N-terminal construct “a” of Pkp3, we found that construct “b” - largely composed of the Armadillo domain, associates with GSK3beta. GSK3beta also associates with construct “c” missing Pkp3’s C-terminus, but since “c” expressed itself at low levels, its interaction was difficult to detect except upon prolonged exposures. While future work will be needed to resolve sub-regions or possibly even defined residues within Pkp3 that are required for Pkp3’s interaction with GSK3beta, CK1alpha or β-TrCP, we expect that Pkp3’s Armadillo domain participates in conferring its sensitivity to destruction complex components in the absence of Wnt signals.
Figure 4. Mapping of Pkp3-catenin in relation to GSK3beta.
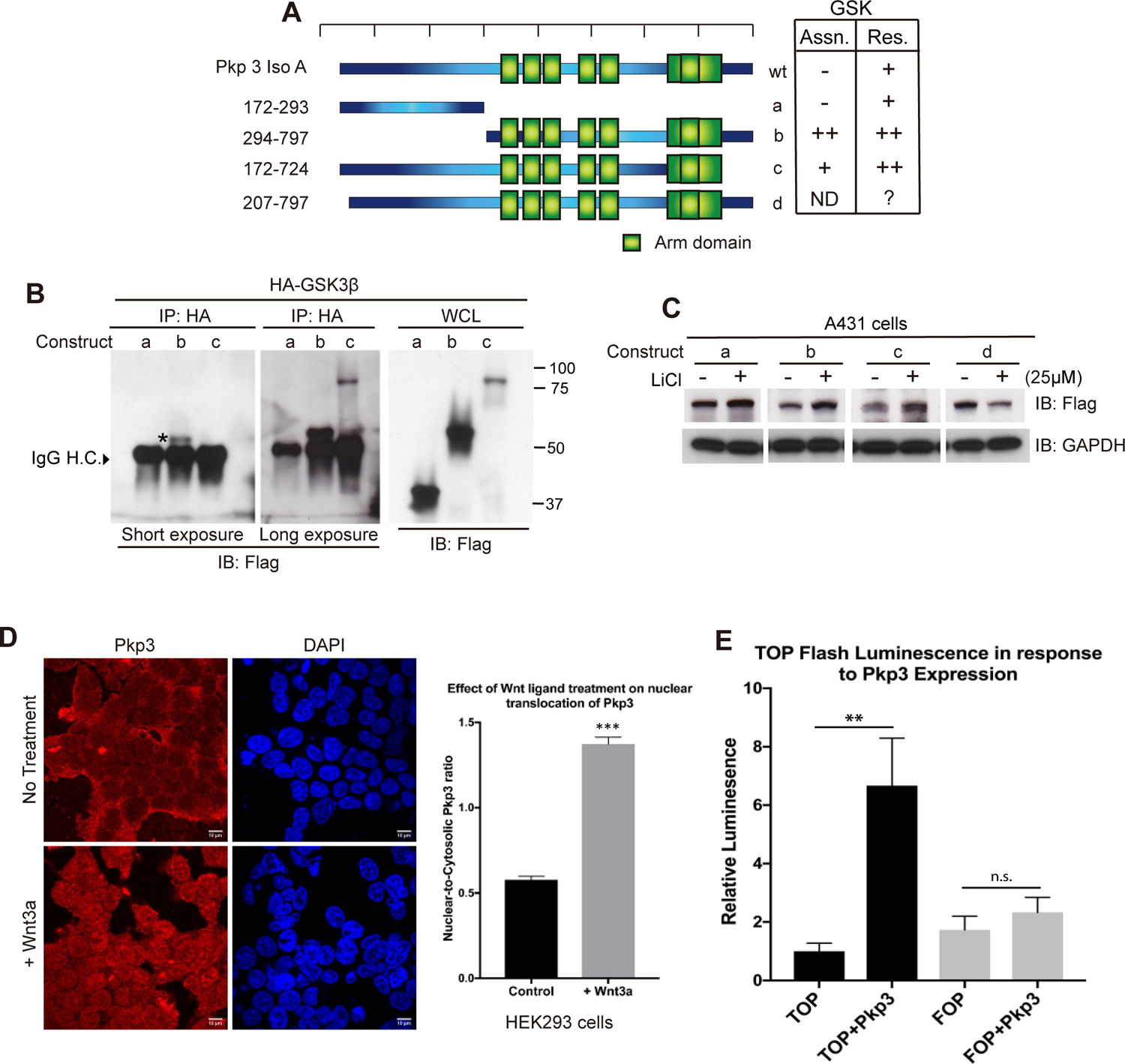
(A) Cartoon depicting Flag-tagged deletion constructs of Pkp3-isoformA (a-d). The chart summarizes construct association with GSK3beta as well as response to GSK3beta based upon data from panels B and C and similar experiments. (B) Deletion constructs of Flag-tagged Pkp3-catenin were co-transfected with HA-GSK3β in HaCaT cells. Immuno-precipitation (IP) of HA-GSK3beta was followed by anti-Flag immuno-blotting to resolve co-associated Pkp3. (C) Flag-tagged Pkp3 constructs were expressed in A431 cells, and their response monitored upon treatment with lithium chloride. The SDS-PAGE mobilities for constructs (a-c) are shown in Panel B, while (d) not shown in Panel B, migrates at ~65 kDa). Nuclear localization of Flag-tagged Pkp3 in HEK293 cells following treatment with Wnt3a peptide (50 μg/ml) for 24 hours. The nuclear:cytosolic ratio of Pkp3-catenin notably increased relative to that of untreated controls (1.373 ± 0.041 versus 0.5778 ± 0.020; n = 30 cells/ condition; *** = p < 0.0001). (E) Exogenous expression of Pkp3 increases activity of the TOP-/FOP-flash canonical-Wnt reporter system. Relative to controls, HEK293 cells expressing Flag-tagged Pkp3 produced a 6.67-fold (±1.62) increase in luciferase activity (p=0.0006). Significance was determined using a One-way ANOVA. Error bars represent SEM.
Complementing the above interaction-mapping, we undertook functional mapping by testing which Pkp3 regions respond to the inhibition of GSK3. While the N-terminal Pkp3 construct “a” modestly responded to GSK3 inhibition with lithium chloride, larger relative increases were observed for the “b” and “c” constructs which contain Pkp3’s Armadillo domain (Fig 4C). We do not currently understand the response of construct “d” (question mark indicated in Fig 4A) that lacks a small N-terminal region (we had initially conjectured it might harbor a destruction motif); its sensitivity to lithium chloride is reversed relative to constructs “a”, “b” and “c”. We also added to our functional analysis a naturally-occurring splicing product of Pkp3 named Pkp3-isoformB (Iso B). The N-terminus of Pkp3-isoformB extends fifteen residues further than isoformA and differs in its first twenty-seven amino acids. The response of full-length Pkp3-isoformB to lithium chloride was similar to that of isoformA. Further, using small deletions or point mutations, we found no indication that residues present in the extreme N-terminus of Pkp3-isoformB encode a destruction motif (results not shown).
Considering our findings (Figs 1–4) that Pkp3 levels respond to Wnt-pathway components including Wnt-ligand, Axin, LRP5/6 and GSK3beta, our results here suggest that Pkp3 contains a region(s) within its larger Armadillo domain that enables it to associate with or respond to the actions of GSK3. The localization of this region appears not to mimic that of β-catenin or p120-catenin-isoform1, which contain destruction boxes at their respective N-termini [15,39]. Our evaluation here of Pkp3-catenin, together with earlier findings for β-catenin and p120-catenin-isoform1 [3,15], suggest that canonical-Wnt signals or components modulate the levels of catenins belonging to three distinct catenin sub-families.
Finally, we tested if endogenous Pkp3-catenin translocates into the nucleus in response to a Wnt-ligand, since in relation to the established response of beta-catenin, this is a defining characteristic of canonical Wnt-pathway activity. Indeed, upon incubating HEK293 cells in the presence of Wnt3a followed by immuno-fluorescence imaging, we observed a clear increase in the nuclear translocation of Flag-Pkp3-catenin (Fig 4D). Following treatment with the Wnt3a-ligand, the nuclear:cytosolic ratio of Flag-Pkp3 notably increased relative to that of untreated controls. A similar response was also seen with endogenous Pkp3 (Supplemental Fig 1C). In our previous work, we likewise reported Pkp3 in the nucleus. We found that Pkp3 binds the transcription factor ETV1 [11], although the few ETV1 gene targets we examined did not appear to be Wnt-responsive. Interestingly, in our study here, the use of a well characterized artificial reporter of canonical-Wnt acivity (TOP/FOP-flash) suggests instead that Pkp3 may indeed have a capacity to activate Wnt-pathway gene control regions in complex with TCF/ LEF [35] or via an unknown effect upon beta-catenin (Fig 4E). Future work will be needed to examine such potential downstream effects of Pkp3, for example, in comparison to beta-catenin or p120-catenin-isoform1. Thus, while each indicator points to Pkp3-catenin being regulated by upstream canonical Wnt components and signals (Figs 1–4; Supplemental Fig 1), future study will be required to establish the downstream regulatory impact - upon genes or other entities - of Pkp3 as a consequence of canonical-Wnt signals.
Discussion
Members of the plakophilin-catenin subfamily (Pkp1, Pkp2, Pkp3) are expressed most highly in vertebrate tissues subject to mechanical stress (e.g. ectoderm and heart muscle), promoting trans-cellular linkages of desmosome junctional complexes with intermediate filaments [4,28,42–49]. The plakophilins exhibit both shared and distinct patterns of expression and function [48], with Pkp3-catenin being present in all living layers of stratified epithelia. Pkp3 deficient mice exhibit hair follicle defects, spontaneous dermatitis and inflammatory responses [50]. In Xenopus, the disruption of Pkp3-catenin results in skin fragility and neural defects [11,28]. Knock-out of the related subfamily member Pkp2 produces cardiac damage and lethality [42], while phosphorylation of Pkp1 by RIPK4 is required in epidermal differentiation [44].
Pkp3 associates with the small-GTPase Rap1, and the depletion of Pkp3 results in aberrant desmosome assembly and adherens junction sealing [51,52]. Less is known about the functions of plakophilins in the cytosol or nucleus [53,54]. Pkp2 modulates the transcription of genes involved in intracellular calcium cycling [55], while Pkp1 and Pkp3 associate with RNA-binding proteins that modulate the transcripts of desmoplakin and Pkp2 [56]. Pkp3 further affects desmosome assembly through increasing transcriptional expression of desmocollin2 [57].
Suggesting another role of Pkp3 in the nucleus, we found previously that Pkp3 binds to the ETV1 transcription factor to regulate the expression of enzymes needed for the synthesis of dopamine [11]. In analogy to canonical-Wnt control of beta-catenin [3], we also revealed that the longest isoform of p120-catenin (isoform1) possesses N-terminal residues comparable to those in the “destruction box” of beta-catenin, that sensitizes it to Wnt pathway modulation [15]. We have thus proposed that vertebrate canonical-Wnt signals act through multiple catenins. In this report, we have addressed whether this concept might extend to the more distantly related Pkp3-catenin.
Indeed, we find that the same destruction-complex components engaged in the post-translational control of beta-catenin and p120-catenin-isoform1 likewise modulate Pkp3-catenin. Namely, negative-acting Axin and GSK3β decreased Pkp3 levels, while positive-acting LRP5/6 and Wnt8-ligand had the opposite effect. We also resolved Pkp3’s association with key destruction complex components such as GSK3β, Axin and βTrCP. In mapping studies using exogenous constructs, we observed that the Armadillo region of Pkp3-catenin is likely involved. Finally, the nuclear localization of Pkp3-catenin increased substantially when cells were incubated in the presence of Wnt-ligand and correlated with the activation of an accepted reporter of Wnt activity (TOP-/FOP-flash). Together, these are among hallmark features of canonical Wnt signaling as established for the structurally-related β-catenin.
Our combined findings take advantage of both knock-down and expression approaches, and both vertebrate embryo and mammalian cell line systems. They suggest as noted that the level of Pkp3’s signaling-pool is modulated by mechanisms generally similar to those acting upon beta-catenin and p120-catenin-isoform1, although for Pkp3, its central Armadillo domain (rather than an amino-terminal “destruction box”) appears more functionally relevant. In our previous report [15], two further members of the p120-catenin subfamily, namely δ- and ARVCF-catenin, were found to respond to destruction complex components. Taken together, we envisage that a number of vertebrate catenins are coordinately modulated via canonical-Wnt signals and components. Wnt-pathway relationships were also suggested recently for Pkp1 [18] and for Pkp2, with the latter indicated to provide negative Wnt-feedback [19].
β-catenin is a central player in the canonical-Wnt pathway, providing essential contributions during embryogenesis [58]. A key question is what biological advantage might be conferred by the existence of multiple Wnt-responsive pools of catenins. β-catenin and p120-catenin associate with classic cadherins at adherence junctions, while Pkp3-catenin binds desmosomal cadherins at desmosomal junctions. Members of the p120-subfamily of catenins further modulate small-GTPases (e.g. RhoA and Rac1)[5,21], and this property might extend to the plakophilin-catenins [59]. Finally, each catenin enters the nucleus to modulate gene activity [2]. Thus, the down-stream functions of Pkp3 may be modulated by components of the Wnt pathway, integrated with Wnt effects upon additional catenins [15,36]. That is, depending on which combination of catenins or catenin-isoforms are present in a chosen cell type or tissue, seemingly similar Wnt-signals may generate distinct outcomes. In all cases, we provide here evidence that Wnt pathway components have a direct impact upon a member of the plakophilin-catenin subfamily, Pkp3. Future studies will be needed to probe the down-stream nature of Wnt-pathway effects upon Pkp3-catenin, such as upon gene activity/transcription factors, or possibly on cell-adhesive or cytoskeletal complexes.
Supplementary Material
Highlights.
Pkp3-catenin protein levels are modulated by canonical-Wnt pathway components.
Pkp3-catenin associates with destruction-complex components of the Wnt pathway.
Armadillo-domain region of Pkp3-catenin involved in Wnt-pathway responsiveness.
In summary, even Pkp-/ plakophilin-catenin subfamily members are Wnt-sensitive.
Speculatively, Wnt signals affect multiple catenin subfamilies for networked effects.
Acknowledgements
We thank those individuals and laboratory groups who provided cDNAs and other reagents as noted in Materials & Methods. For helpful commentary we thank Malgosia Kloc, Bridget DeLay, Mark Corkins, Vanja Stankic and Jae-Il Park. For Nikon A1 confocal microscopy, we thank Adriana Paulucci-Holthauzen of the Department of Genetics Imaging Core UT MDACC.
Funding sources
Much appreciated funding: NIH/ NIMH R01 MH 115717 (PDM); NIH/NIGMS 1 RO1 GM 107079 (PDM); Ashbel Smith Professorship Award (PDM); NIH/NIGMS 3 RO1 GM107079-S (JZ); National Research Foundation of Korea, Ministry of Science, ICT & Future Planning NRF-2015R1C1A1A02036506 (JYH); K01DK092320, R03DK118771 and R01DK115655 (RKM); AB was generously supported by a Schissler Foundation Fellowship. DNA-sequencing was facilitated by NIH/ NCI Core Grant CA-16672 (UT MDACC). Use of the A1-Nikon microscope (confocal images) was made possible via the UT MDACC Department of Genetics NIH Instrumentation Grant 1S10OD024976.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ARVCF: Armadillo Repeat gene deleted in Velo-Cardio-Facial syndrome
Conflict of Interest Statement.
The authors have no conflict of interests to report.
References
- 1.Garcia MA, Nelson WJ, Chavez N (2018) Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb Perspect Biol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCrea PD, Gottardi CJ (2016) Beyond beta-catenin: prospects for a larger catenin network in the nucleus. Nat Rev Mol Cell Biol 17: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nusse R, Clevers H (2017) Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 169: 985–999. [DOI] [PubMed] [Google Scholar]
- 4.Green KJ, Jaiganesh A, Broussard JA (2019) Desmosomes: Essential contributors to an integrated intercellular junction network. F1000Res 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, et al. (2000) Inhibition of RhoA by p120 catenin. Nat Cell Biol 2: 637–644. [DOI] [PubMed] [Google Scholar]
- 6.Kourtidis A, Ngok SP, Anastasiadis PZ (2013) p120 catenin: an essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression. Prog Mol Biol Transl Sci 116: 409–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCrea PD, Park JI (2007) Developmental functions of the P120-catenin sub-family. Biochim Biophys Acta 1773: 17–33. [DOI] [PubMed] [Google Scholar]
- 8.Zhao ZM, Reynolds AB, Gaucher EA (2011) The evolutionary history of the catenin gene family during metazoan evolution. BMC Evol Biol 11: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gul IS, Hulpiau P, Saeys Y, van Roy F (2017) Evolution and diversity of cadherins and catenins. Exp Cell Res 358: 3–9. [DOI] [PubMed] [Google Scholar]
- 10.Gottardi CJ, Gumbiner BM (2004) Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol 167: 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munoz WA, Lee M, Miller RK, Ahmed Z, Ji H, et al. (2014) Plakophilin-3 catenin associates with the ETV1/ER81 transcription factor to positively modulate gene activity. PLoS One 9: e86784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonne S, van Hengel J, Nollet F, Kools P, van Roy F (1999) Plakophilin-3, a novel armadillo-like protein present in nuclei and desmosomes of epithelial cells. J Cell Sci 112 (Pt 14): 2265–2276. [DOI] [PubMed] [Google Scholar]
- 13.Mertens C, Hofmann I, Wang Z, Teichmann M, Sepehri Chong S, et al. (2001) Nuclear particles containing RNA polymerase III complexes associated with the junctional plaque protein plakophilin 2. Proc Natl Acad Sci U S A 98: 7795–7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sobolik-Delmaire T, Reddy R, Pashaj A, Roberts BJ, Wahl JK 3rd (2010) Plakophilin-1 localizes to the nucleus and interacts with single-stranded DNA. J Invest Dermatol 130: 2638–2646. [DOI] [PubMed] [Google Scholar]
- 15.Hong JY, Park JI, Cho K, Gu D, Ji H, et al. (2010) Shared molecular mechanisms regulate multiple catenin proteins: canonical Wnt signals and components modulate p120-catenin isoform-1 and additional p120 subfamily members. J Cell Sci 123: 4351–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh M, Kim H, Yang I, Park JH, Cong WT, et al. (2009) GSK-3 phosphorylates delta-catenin and negatively regulates its stability via ubiquitination/proteosome-mediated proteolysis. J Biol Chem 284: 28579–28589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt A, Langbein L, Pratzel S, Rode M, Rackwitz HR, et al. (1999) Plakophilin 3--a novel cell-type-specific desmosomal plaque protein. Differentiation 64: 291–306. [DOI] [PubMed] [Google Scholar]
- 18.Miyazaki K, Yoshizaki K, Arai C, Yamada A, Saito K, et al. (2016) Plakophilin-1, a Novel Wnt Signaling Regulator, Is Critical for Tooth Development and Ameloblast Differentiation. PLoS One 11: e0152206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niell N, Larriba MJ, Ferrer-Mayorga G, Sanchez-Perez I, Cantero R, et al. (2018) The human PKP2/plakophilin-2 gene is induced by Wnt/beta-catenin in normal and colon cancer-associated fibroblasts. Int J Cancer 142: 792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montross WT, Ji H, McCrea PD (2000) A beta-catenin/engrailed chimera selectively suppresses Wnt signaling. J Cell Sci 113 ( Pt 10): 1759–1770. [DOI] [PubMed] [Google Scholar]
- 21.Fang X, Ji H, Kim SW, Park JI, Vaught TG, et al. (2004) Vertebrate development requires ARVCF and p120 catenins and their interplay with RhoA and Rac. J Cell Biol 165: 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sive HL, Grainger RM, Harland RM (2007) Dejellying Xenopus laevis Embryos. CSH Protoc 2007: pdb prot4731. [DOI] [PubMed] [Google Scholar]
- 23.Sive HL, Grainger RM, Harland RM (2007) Inducing Ovulation in Xenopus laevis. CSH Protoc 2007: pdb prot4734. [DOI] [PubMed] [Google Scholar]
- 24.Sive HL, Grainger RM, Harland RM (2010) Microinjection of Xenopus embryos. Cold Spring Harb Protoc 2010: pdb ip81. [DOI] [PubMed] [Google Scholar]
- 25.Sive HL, Grainger RM, Harland RM (2007) Xenopus laevis Egg Collection. CSH Protoc 2007: pdb prot4736. [DOI] [PubMed] [Google Scholar]
- 26.Sive HL, Grainger RM, Harland RM (2007) Xenopus laevis In Vitro Fertilization and Natural Mating Methods. CSH Protoc 2007: pdb prot4737. [DOI] [PubMed] [Google Scholar]
- 27.Nieuwkoop P, Faber J (1994) Normal Table of Xenopus laevis (Daudin): Garland Publishing Inc. [Google Scholar]
- 28.Munoz WA, Kloc M, Cho K, Lee M, Hofmann I, et al. (2012) Plakophilin-3 is required for late embryonic amphibian development, exhibiting roles in ectodermal and neural tissues. PLoS One 7: e34342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu D, Sater AK, Ji H, Cho K, Clark M, et al. (2009) Xenopus delta-catenin is essential in early embryogenesis and is functionally linked to cadherins and small GTPases. J Cell Sci 122: 4049–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan W, Choi SC, Wang H, Qin Y, Volpicelli-Daley L, et al. (2008) Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science 321: 1350–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davidson LA, Marsden M, Keller R, Desimone DW (2006) Integrin alpha5beta1 and fibronectin regulate polarized cell protrusions required for Xenopus convergence and extension. Curr Biol 16: 833–844. [DOI] [PubMed] [Google Scholar]
- 32.Kim SW, Fang X, Ji H, Paulson AF, Daniel JM, et al. (2002) Isolation and characterization of XKaiso, a transcriptional repressor that associates with the catenin Xp120(ctn) in Xenopus laevis. J Biol Chem 277: 8202–8208. [DOI] [PubMed] [Google Scholar]
- 33.Kim SW, Park JI, Spring CM, Sater AK, Ji H, et al. (2004) Non-canonical Wnt signals are modulated by the Kaiso transcriptional repressor and p120-catenin. Nat Cell Biol 6: 1212–1220. [DOI] [PubMed] [Google Scholar]
- 34.Lee M, Ji H, Furuta Y, Park JI, McCrea PD (2014) p120-catenin regulates REST and CoREST, and modulates mouse embryonic stem cell differentiation. J Cell Sci 127: 4037–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, et al. (1997) Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88: 789–799. [DOI] [PubMed] [Google Scholar]
- 36.Park JI, Kim SW, Lyons JP, Ji H, Nguyen TT, et al. (2005) Kaiso/p120-catenin and TCF/beta-catenin complexes coordinately regulate canonical Wnt gene targets. Dev Cell 8: 843–854. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald BT, He X (2012) Frizzled and LRP5/6 receptors for Wnt/beta-catenin signaling. Cold Spring Harb Perspect Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marikawa Y, Elinson RP (1998) beta-TrCP is a negative regulator of Wnt/beta-catenin signaling pathway and dorsal axis formation in Xenopus embryos. Mech Dev 77: 75–80. [DOI] [PubMed] [Google Scholar]
- 39.Liu C, Kato Y, Zhang Z, Do VM, Yankner BA, et al. (1999) beta-Trcp couples beta-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci U S A 96: 6273–6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yost C, Torres M, Miller JR, Huang E, Kimelman D, et al. (1996) The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev 10: 1443–1454. [DOI] [PubMed] [Google Scholar]
- 41.Stamos JL, Weis WI (2013) The beta-catenin destruction complex. Cold Spring Harb Perspect Biol 5: a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grossmann KS, Grund C, Huelsken J, Behrend M, Erdmann B, et al. (2004) Requirement of plakophilin 2 for heart morphogenesis and cardiac junction formation. J Cell Biol 167: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moriarty MA, Ryan R, Lalor P, Dockery P, Byrnes L, et al. (2012) Loss of plakophilin 2 disrupts heart development in zebrafish. Int J Dev Biol 56: 711–718. [DOI] [PubMed] [Google Scholar]
- 44.Lee P, Jiang S, Li Y, Yue J, Gou X, et al. (2017) Phosphorylation of Pkp1 by RIPK4 regulates epidermal differentiation and skin tumorigenesis. EMBO J 36: 1963–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.South AP (2004) Plakophilin 1: an important stabilizer of desmosomes. Clin Exp Dermatol 29: 161–167. [DOI] [PubMed] [Google Scholar]
- 46.Tucker DK, Stahley SN, Kowalczyk AP (2014) Plakophilin-1 protects keratinocytes from pemphigus vulgaris IgG by forming calcium-independent desmosomes. J Invest Dermatol 134: 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keil R, Rietscher K, Hatzfeld M (2016) Antagonistic Regulation of Intercellular Cohesion by Plakophilins 1 and 3. J Invest Dermatol 136: 2022–2029. [DOI] [PubMed] [Google Scholar]
- 48.Bass-Zubek AE, Godsel LM, Delmar M, Green KJ (2009) Plakophilins: multifunctional scaffolds for adhesion and signaling. Curr Opin Cell Biol 21: 708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J, Radice GL (2010) A new perspective on intercalated disc organization: implications for heart disease. Dermatol Res Pract 2010: 207835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sklyarova T, Bonne S, D’Hooge P, Denecker G, Goossens S, et al. (2008) Plakophilin-3-deficient mice develop hair coat abnormalities and are prone to cutaneous inflammation. J Invest Dermatol 128: 1375–1385. [DOI] [PubMed] [Google Scholar]
- 51.Todorovic V, Koetsier JL, Godsel LM, Green KJ (2014) Plakophilin 3 mediates Rap1-dependent desmosome assembly and adherens junction maturation. Mol Biol Cell 25: 3749–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sobolik-Delmaire T, Katafiasz D, Wahl JK 3rd (2006) Carboxyl terminus of Plakophilin-1 recruits it to plasma membrane, whereas amino terminus recruits desmoplakin and promotes desmosome assembly. J Biol Chem 281: 16962–16970. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt A, Jager S (2005) Plakophilins--hard work in the desmosome, recreation in the nucleus? Eur J Cell Biol 84: 189–204. [DOI] [PubMed] [Google Scholar]
- 54.Hatzfeld M (2007) Plakophilins: Multifunctional proteins or just regulators of desmosomal adhesion? Biochim Biophys Acta 1773: 69–77. [DOI] [PubMed] [Google Scholar]
- 55.Cerrone M, Noorman M, Lin X, Chkourko H, Liang FX, et al. (2012) Sodium current deficit and arrhythmogenesis in a murine model of plakophilin-2 haploinsufficiency. Cardiovasc Res 95: 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fischer-Keso R, Breuninger S, Hofmann S, Henn M, Rohrig T, et al. (2014) Plakophilins 1 and 3 bind to FXR1 and thereby influence the mRNA stability of desmosomal proteins. Mol Cell Biol 34: 4244–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gurjar M, Raychaudhuri K, Mahadik S, Reddy D, Atak A, et al. (2018) Plakophilin3 increases desmosome assembly, size and stability by increasing expression of desmocollin2. Biochem Biophys Res Commun 495: 768–774. [DOI] [PubMed] [Google Scholar]
- 58.Grigoryan T, Wend P, Klaus A, Birchmeier W (2008) Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev 22: 2308–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keil R, Wolf A, Huttelmaier S, Hatzfeld M (2007) Beyond regulation of cell adhesion: local control of RhoA at the cleavage furrow by the p0071 catenin. Cell Cycle 6: 122–127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


