Abstract
Since the early 20th century, progress in cancer therapies has significantly improved disease prognosis. Nonetheless, cancer treatments are often associated with side effects that can negatively affect patient well-being and disrupt the course of treatment. Among the main side effects, taste impairment is associated with depression, malnutrition, and morbid weight loss. Although relatively common, taste disruption associated with cancer therapies remains poorly understood. Here, we review the current knowledge related to the molecular mechanisms underlying taste maintenance and disruption in the context of cancer therapies.
Keywords: chemotherapy, Hedgehog signaling, Notch signaling, organoids, radiotherapy, Wnt signaling
A multitude of medical conditions and treatments are associated with altered (dysgeusia) or loss (ageusia) of taste, ranging from infection to cancer therapies. Regardless of the agent(s) causing taste alteration, this disruption is highly troubling to patients and often detrimental to their well-being and quality of life. Taste loss causes lack of appetite, which in turn can lead individuals to isolate socially and ultimately to depression (Leventhal 1959; Maes et al. 2002). The depth of psychological distress is well illustrated by Ethna MacCarthy-Leventhal, M.D., who described her “mouth blindness” after she was treated with radiotherapy for pharyngeal cancer: “What is it like to lose your sense of taste? To know that the most luscious fruit is a cinder, and its juice an acid liquid flavoured with bicarbonate of soda or copper, or that a Whitstable oyster is no more appetizing than a slug?” (Leventhal 1959). Physiologically, taste disruption impedes daily nutrient intake leading to malnutrition and dangerous weight loss (Ravasco et al. 2005; Ruo Redda and Allis 2006; Hutton et al. 2007; Mahdavi et al. 2007; Ogama et al. 2010; Deshpande et al. 2018), as Dr. MacCarty-Leventhal goes on to say: “If, by a mighty effort, the ‘cinders’ are forced down with copious fluid, the consequences are acute indigestion or vomiting. The patient is not hungry anyway, and it is easier to starve” (Leventhal 1959). Importantly, this distressing side effect can lead to poorer treatment outcomes, and in extreme cases, death may ensue (Bolze et al. 1982; Chencharick and Mossman 1983; Nelson 1998; Hutton et al. 2007; Zabernigg et al. 2012; Rathod et al. 2015).
The sense of taste, among our basic senses, seems particularly prone to perturbation. Like skin, taste bud cells continually and rapidly regenerate from adult stem cells, and disruption of taste function is thought broadly to be due to interruption of the renewal process. But given the complex regulation of renewing epithelia, the specific insults to this process in the taste epithelium by individual disease-causing agents or drugs are likely quite diverse, yet nonetheless result in a grossly similar impact on taste function. For example, inflammation can cause taste dysfunction (Cohn et al. 2010; Steinbach et al. 2011; Feng et al. 2014, 2015; Kaufman et al. 2018, 2020), and most recently, taste loss, distinct from loss of smell, has been reported as a symptom of SARS-CoV2 infection, that is, COVID-19 (Agyeman et al. 2020; Cooper et al. 2020; Parma et al. 2020; Niklassen et al. 2021). Middle ear infection also can lead to distorted taste; nerve fibers that convey taste information from the anterior tongue to the brainstem travel through the middle ear and are exposed to the infected/inflamed environment (Landis et al. 2005; Sano et al. 2007; Goyal et al. 2009), damaging nerve fibers’ ability to transmit taste information, and/or to support taste bud maintenance (Oakley 1993; Miura et al. 2004; Oakley and Witt 2004).
Cancer therapies represent the largest category of taste-altering agents. A large majority of patients with head-and-neck cancer (HNC) are treated with targeted ionizing radiation that results in ageusia within weeks, followed by dysgeusia that can persist for months or years (Bolze et al. 1982; Chencharick and Mossman 1983; Nelson 1998; Maes et al. 2002; Ray-Chaudhuri et al. 2013; Irune et al. 2014). Additionally, an extensive list of chemotherapeutics used to treat a host of cancers lead to taste distortion or loss in large proportions of patients (e.g., see Vigarios et al. 2017). Although the impact of cancer treatment on taste function is well recognized by clinicians and healthcare providers, little progress has been made on approaches to mitigate or prevent this debilitating side effect. This is in large part because it is difficult to ameliorate loss of taste if we understand so little mechanistically of how taste is maintained and how it is disrupted. Further, as there are so many different drugs and treatments that result in taste dysfunction, it is likely that maintenance of taste function is perturbed in a myriad of ways, depending upon the specific drug or treatment, yet a generally distorted sense of taste is the common complaint. Here we present examples of where we better understand how identifiable processes in taste bud renewal are regulated and discuss how specific cancer treatments perturb molecular regulators of discrete processes in taste regeneration. These studies reveal how the taste system can be “broken” by specific cancer treatments, shedding light on how we might devise approaches to “fix” taste dysfunction for patients.
Taste bud cells are continuously and reliably renewed
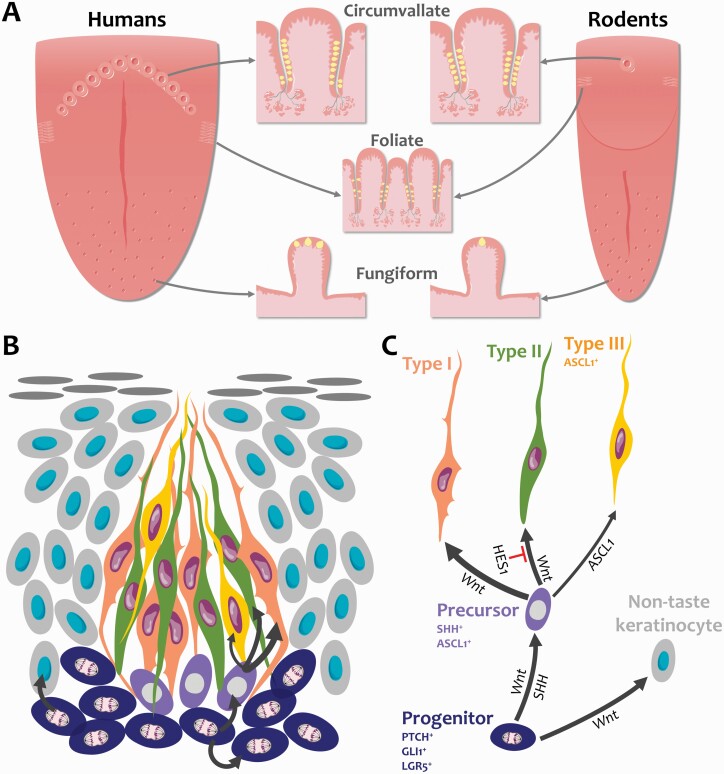
In mammals, taste buds are found on the tongue housed in epithelial specializations termed gustatory or taste papillae (Figure 1A). Fungiform taste papillae are distributed on the anterior tongue, and in rodents, each contains a single taste bud while multiple taste buds can be found in a single human fungiform papilla. Foliate and circumvallate taste papillae are larger, more complex structures that house hundreds of taste buds and are situated posteriorly. In rodents, the foliate papillae comprise inward-folding epithelial trenches on each side of the posterior tongue, while a single circumvallate papilla sits posteriorly at the lingual midline (Figure 1A). Additionally, taste buds are found in the soft palate and upper respiratory tract epithelium (Henkin and Christiansen 1967; Purves and Williams 2001). Each bud, regardless of location, is made up of a collection of roughly 50–100 cells that historically have been categorized into three morphological types: I, II, and III. Over the past decades, these morphological types have been further refined to reveal their correspondence to functional and molecularly distinct subsets that detect sweet, bitter, umami, sour, and salt stimuli, as well as a glial-like population. Briefly, subsets of Type II cells make up roughly 15–20% of taste bud cells, and are sensitive to sweet, bitter, or umami stimuli, based on the G-protein-coupled taste receptor expressed by an individual cell (Adler et al. 2000; Clapp et al. 2001; Nelson et al. 2001; Kim et al. 2003; Zhang et al. 2003; Oka et al. 2013), whereas Type III taste cells are sour detectors and are the least common taste cell type (Huang et al. 2006, 2008; Ma et al. 2007; Kataoka et al. 2008; Ohtubo and Yoshii 2011; Oka et al. 2013; Lewandowski et al. 2016; Teng et al. 2019; Wilson et al. 2019). An additional taste cell population that responds to salt (sodium) has been identified in fungiform taste buds, which does not fit criteria of either Type II or III cells in terms of morphology or molecular markers (Nomura et al. 2020). Finally, Type I cells are thought to function as support cells within buds (Pumplin et al. 1997; Lawton et al. 2000; Bartel et al. 2006; Yang et al. 2020), and this population makes up roughly half of the differentiated cells in each taste bud. For a more detailed account of functional and morphological characteristics of these taste cell populations, please see the review by Finger and Barlow (2021).
Figure 1.
Anatomy of the tongue and taste cell renewal. (A) Taste buds (yellow) are embedded in taste papillae in the tongue epithelium. The number of taste buds in fungiform papillae, which are located in the anterior two-thirds of the tongue, is species-dependent; rodent fungiform papillae each contain a single taste bud while multiple taste buds can be found in a human fungiform papilla. Hundreds of taste buds line the trenches (epithelial invaginations) of foliate and circumvallate papillae in the posterior tongue. In human circumvallate papillae, taste buds are mostly found in the inner wall of the trench. Foliate papillae lie laterally while circumvallate papillae are organized in a central V-shaped formation. Rodents possess only a single circumvallate papilla. (B) Taste buds are made of 50–100 cells that are continually replaced throughout life. Progenitor cells (dark blue) reside along the basement membrane outside taste buds and actively divide to self-renew and produce taste cells and non-taste keratinocytes (light gray) that surround taste buds. Following mitosis, taste-fated lingual progenitors enter taste buds and specify into post-mitotic SHH+ taste precursor cells (magenta). Precursor cells then differentiate into most prevalent Type I glial-like cells (tangerine), Type II sweet/bitter/umami receptor cells (green), and least common Type III sour receptor cells (yellow). (C) Fate decision is regulated by the Wnt pathway, Hedgehog, and Notch signaling. The Wnt/β-catenin pathway controls all steps of taste and non-taste cell renewal, while SHH instructs progenitors to differentiate into taste cells. Notch signaling represses Type II cell fate via HES1 and transcriptionally represses ASCL1 to control Type III taste cell differentiation. Illustrations are modified from Servier Medical Art licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com/).
All taste cells, regardless of function, continually turn over throughout life. In textbooks, each taste cell is reported to live for 10 days, such that every ~10 days all cells within each bud are new. However, this metric is based on early birth dating studies where median lifespan of 10 days was reported for taste bud cells; in fact, many cells were found to live as long as 4 weeks, which was the duration of the study (Beidler and Smallman 1965). More recent work has confirmed a longer lifespan specifically for Type II and III taste bud cells. In particular, Type II cells have a populational half-life of 8 days, whereas long-lived Type II cells were evident at 40 days. Overall, Type III cells are more long-lived with a populational half-life of 24 days, and many surviving for up to 40 days (Perea-Martinez et al. 2013). Because of technical limitations, assessing the longevity of Type I cells has been difficult. Nonetheless, a population of short-lived taste cells has been identified, which do not express markers of Type II or III taste cells, and thus may be Type I cells (Hamamichi et al. 2006; Perea-Martinez et al. 2013). These findings suggest that the receptor cells detecting sweet, bitter, umami, salt, and sour have significantly longer lifespans than the glial-like cell population; these Type I cells appear to renew at a pace similar to the non-taste epithelial cells of the tongue, which turn over every 3–6 days (Hume and Potten 1976; Hill 1988; Potten et al. 2002).
Both taste bud cells and non-taste epithelia are generated from adult stem cells resident in taste papillae. These basal keratinocytes are located at the basement membrane and divided to replace themselves as well as produce both taste and non-taste daughter cells (Figure 1B,C). In the circumvallate papilla, roughly 80% of progenitors are actively proliferating (Nguyen et al. 2012) to accommodate the rapid replacement of the much more abundant non-taste epithelial cells, which surround taste buds and make up the epithelial barrier of the tongue, as well as maintain steady production of the smaller populations of longer-lived taste bud cells.
As taste progenitors adjacent to buds produce taste-fated daughter cells, these cells exit the cell cycle, enter taste buds and express the gene product Sonic Hedgehog (SHH) 12–24 h after their final division (Miura et al. 2006). SHH+ cells have an ovoid morphology with round nuclei and reside in the basal compartment of taste buds (Figure 1B), consistent with the location and shape of Type IV cells classically defined using ultrastructural criteria (Yoshie et al. 1990; Yang et al. 2020). Although Type IV cells have been suggested to be taste progenitors, SHH+ cells do not comprise a proliferating population but rather are post-mitotic. Using molecular genetic lineage tracing in mice, we have shown that SHH+ cells differentiate into any of the taste bud cell types (Figure 1B,C) with frequencies consistent with the proportion of each type resident in buds and to some extent their relative lifespans, that is, Type I > Type II > Type III (Miura et al. 2014). Thus, we have termed these SHH+ cells “post-mitotic taste precursor cells.”
In sum, lingual progenitors must maintain proliferation and daughter cell generation to balance taste versus non-taste lineages with vastly different lifespans as well as generate the proper ratio of the different taste cell types again with different longevities. How each of these lineage decisions is deployed must be tightly regulated in order to maintain taste function; and each step of this lineage generation is likely susceptible to perturbation by subsets of different cancer treatments. Below we discuss the impact of radiotherapy and specific chemotherapies on taste function in patients in the context of identified molecular and cellular regulators of taste cell renewal elucidated using animal models.
How is taste cell homeostasis impacted by cancer therapies?
Radiation therapies for HNCs
About 900,000 new cases of HNCs are reported annually worldwide, representing more than 5% of all cancers and leading to ~500,000 deaths (Bray et al. 2018). HNCs are treated with radiotherapy alone or in association with surgery and/or chemotherapy. Radiation therapy reduces tumor size by inducing DNA damage in dividing cells, leading to cell death (Surova and Zhivotovsky 2013; Wang 2019). The use of radiation in medical applications is more than a century old. The first documented use of radiotherapy to treat cancer dates back to 1896 and targeted radiotherapy for HNC was made possible with the development of new X-ray irradiators in the 1920s and 1930s (Lederman 1981; Holsti 1995; Gianfaldoni et al. 2017). Efficacy of head-and-neck radiotherapy significantly improved in the second half of the 20th century with technological innovation and new treatment protocols (Cognetti et al. 2008). Patients receive daily fractionated X-ray doses of 1–2 Gy administered for up to 7 weeks (Deloch et al. 2016) via a variety of protocols, including volumetric modulated arc therapy, intensity-modulated radiation therapy, image-guided radiation therapy, and radiosurgery (Cognetti et al. 2008; Deloch et al. 2016; De Felice et al. 2018). These paradigms are designed to specifically target tumor sites and spare healthy tissues from radiation damage. However, HNC patients treated with radiotherapy suffer taste dysfunction associated with taste pore loss, suggestive of taste bud degeneration (Just et al. 2005; Deshpande et al. 2018). Noticeably, taste impairment is often accompanied by xerostomia (dry mouth) and mucositis (oral inflammation, ulceration, and blistering) resulting in difficulty chewing and swallowing, which contributes to reduced quality of life in patients (Bolze et al. 1982; Chencharick and Mossman 1983; Nelson 1998; Ray-Chaudhuri et al. 2013). Nonetheless, the extent of salivary dysfunction and of taste loss are not necessarily correlated (Temmel et al. 2005), suggesting that dysgeusia originates from direct effects on taste tissues. Importantly, taste disruption is prolonged in the months and years following the end of treatment suggesting that depleted taste buds do not fully regenerate after injury (Bolze et al. 1982; Chencharick and Mossman 1983; Nelson 1998; Maes et al. 2002; Ray-Chaudhuri et al. 2013; Irune et al. 2014; Deshpande et al. 2018; Barbosa da Silva et al. 2019; Chen et al. 2019). In addition to perturbed taste bud regeneration, authors have posited that gustatory nerve damage and improper reinnervation may contribute to long-term taste distortion (Nelson 1998; Sandow et al. 2006; Barlow 2015; Deshpande et al. 2018). Because actively dividing cells are significantly affected by radiation, it has been assumed that like tumor cells, proliferating taste progenitor cells are primarily impacted by radiation, altering their ability to generate new taste cells. Mouse models have been instrumental to explore the mechanisms underlying radiation-mediated taste disruption. Exposing the head-and-neck region of mice to a single 8 Gy dose of radiation results in a dramatic reduction in proliferation of taste progenitors. Depletion of the progenitor population is associated with augmented cell death, resulting in a transient drop followed by a rapid recovery in the number of differentiated taste cells (Nguyen et al. 2012) and sensitivity to sweet (Jewkes et al. 2017). Although these studies provide exhaustive data about the behavior of irradiated taste progenitors in mice, a single dose of radiation does not mirror conventional fractionated radiotherapy for patients. Studies employing fractionated irradiation paradigms in mice report longer-lasting drops in proliferation and taste bud cell count (Dorr et al. 1994, 1996; Gaillard et al. 2019) compared with single-dose irradiation (Nguyen et al. 2012). Surprisingly, progenitor loss upon fractionated irradiation does not seem to result from cell death; we proposed that lower repeated doses may induce progenitors to prematurely differentiate or activate autophagy and senescence pathways (Gaillard et al. 2019). Altogether, these findings using rodent models show that the dose regimen is critical to trigger long-term taste loss, supporting variable degrees of taste impairment in patients who received different radiation doses (Chen et al. 2019).
Chemotherapies and targeted therapies
Dating back to the early 1900s, alkylating agents were developed as chemotherapies to induce DNA damage and subsequent cell death in quickly dividing cancer cells, but started to be widely used in the 1960s (DeVita and Chu 2008). Since the turn of the century, targeted therapies have been the center of attention with the promise of minimizing undesired side effects by developing small molecules and antibodies that target mutated or upregulated signaling pathways in cancers (Dobosz and Dzieciatkowski 2019; Bedard et al. 2020). Although more specific than chemotherapeutics, targeted therapies disrupt oncogenic molecular programs that may also be required for the maintenance of taste cell renewal, resulting in ageusia and dysgeusia.
Inhibition of the SHH signaling pathway
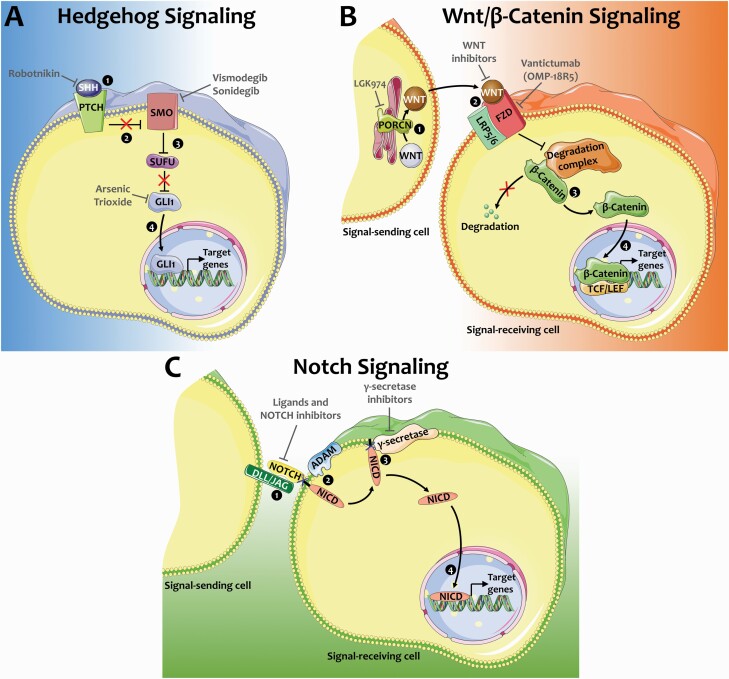
The Hedgehog (Hh) pathway is a key regulator of the development and homeostasis of multiple tissues (Varjosalo and Taipale 2008; Petrova and Joyner 2014). In the absence of Hh ligand, protein patched homolog (PTCH) binds the transmembrane protein Smoothened (SMO) inhibiting activation of the pathway. When SHH binds PTCH, SMO is released from PTCH inhibition, turning on transcription of Hh target genes including the GLI1 transcription factor and PTCH (Figure 2A); the latter then feeds back to reduce Hh signaling via resumed inhibition of SMO (Varjosalo and Taipale 2008; Petrova and Joyner 2014).
Figure 2.
Signaling pathways targeted in cancer therapies. Hedgehog, Wnt/β-catenin, and Notch signaling are developmental pathways that are activated in multiple forms of cancer, rendering them promising therapeutic targets. (A) Hedgehog signaling pathway. In the absence of SHH, PTCH receptor inhibits SMO. SUFU sequesters GLI1 in the cytoplasm resulting in inactive Hh signaling. SHH binding to PTCH receptor ① lifts its inhibition of SMO ②. Activated SMO sequesters SUFU ③, resulting in translocation of GLI1 to the nucleus ④ and transcription of target genes. Targeted cancer therapeutics include Robotnikin that binds and inhibits SHH, SMO inhibitors Vismodegib and Sonidegib, and GLI1 inhibitor arsenic trioxide. (B) Wnt/β-catenin signaling pathway. PORCN palmitoylates WNTs in the endoplasmic reticulum of signal-sending cells ①. Secreted WNTs bind to FZD/LRP receptor complex on the surface of signal-receiving cells ②, resulting in dismantling of the degradation complex and release of β-catenin rather than its degradation ③. β-catenin then translocated to the nucleus and binds to T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors to induce the transcription of target genes ④. Anticancer drug LGK974 averts the activation of WNTs via inhibition of PORCN, while WNT inhibitors prevent binding to the receptors and vantictumab inhibits FZD proteins. (C) Notch signaling pathway. Interaction of delta-like (DLL) and jagged (JAG) ligands present on the surface of signal-sending cells with NOTCH receptors anchored in the membrane of signal-receiving cells ① results in extracellular S2 site cleavage by ADAM metalloproteases ②. Subsequent intramembrane S3 site cleavage by γ-secretases releases Notch intracellular domain (NICD) ③ that translocates to the nucleus and activates the transcription of target genes ④. Anticancer therapeutics have been developed to inhibit Notch ligands and receptors, as well as γ-secretases. Illustrations are modified from Servier Medical Art licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com/).
The Hh pathway is also activated in multiple types of cancer, including basal cell carcinoma (BCC), medulloblastoma, rhabdomyosarcoma, prostate, and lung cancers (Rubin and de Sauvage 2006). Inhibitors of SHH, SMO, and GLI1 have been tested to disrupt the Hh pathway in cancer tissues (Figure 2A; Carpenter and Ray 2019). Small molecule inhibitors of SMO, Vismodegib and Sonidegib, are currently used for the treatment of BCC (Carpenter and Ray 2019) and are undergoing multiple clinical trials to treat other types of malignancies (Girardi et al. 2019). Dysgeusia is among the most prevalent side effects reported in patients treated with these SMO antagonists (LoRusso et al. 2011; Sekulic et al. 2012; Tang et al. 2012; Rodon et al. 2014; Basset-Seguin et al. 2015; Le Moigne et al. 2016) and occasionally leads to treatment discontinuation (Basset-Seguin et al. 2015). Noticeably, gustatory function recovers when the treatment is halted (Tang et al. 2012). Dysgeusia appears within weeks of BCC treatment initiation, suggesting that taste bud maintenance is disrupted (Tang et al. 2012).
Expression analysis data from rodents have long supported a role for the Hh pathway in adult taste cell renewal. In adult mouse tongue, SHH is expressed by post-mitotic precursor (Type IV) cells within taste buds, whereas PTCH and GLI1 are expressed by taste progenitors outside of taste buds (Figure 1; Miura et al. 2001). This arrangement led to the testable idea that signaling from SHH+ precursor cells regulates renewal of taste receptor cells from taste progenitors, but left open the specific aspects of renewal that might depend on Hh signaling (Miura et al. 2001, 2004, 2014; Liu et al. 2013). Genetic gain-of-function (GOF) and loss-of-function (LOF) studies have revealed the pathway is required for taste bud cell differentiation from lingual progenitors, independent of an impact on progenitor proliferation (Castillo et al. 2014; Ermilov et al. 2016; Castillo-Azofeifa et al. 2017, 2018).
Similar to results from Hh pathway LOF studies, taste buds progressively shrink and are lost within 2–3 weeks in mice treated with a SMO inhibitor (Kumari et al. 2015, 2017, 2018; Castillo-Azofeifa et al. 2017); further, all taste cell types are depleted (Yang et al. 2015; Kumari et al. 2017), consistent with SHH promotion of differentiation of Type I, II, and III taste cells (Castillo et al. 2014). In addition, gustatory signals in the seventh cranial nerve are virtually abolished within ~2 weeks of treatment (Kumari et al. 2015, 2017, 2018). Similar to patients, drug withdrawal results in taste function recovery via taste bud regeneration and restoration of gustatory nerve signals (Kumari et al. 2017). The mechanisms of action of SMO inhibitors in the taste system remain under debate, specifically whether SMO inhibition hinders proliferation or differentiation of taste tissues. Proliferation was unchanged in taste papillae of mice treated for up to 21 days with one SMO inhibitor (Castillo-Azofeifa et al. 2017), while it was reduced in other reports using a different antagonist (Kumari et al. 2015, 2017, 2018). However, genetic constitutive expression of SHH in lingual progenitors induces taste bud differentiation (Castillo et al. 2014), supporting a model where SMO antagonists cause taste loss by inhibiting taste cell differentiation rather than progenitor proliferation.
Inhibition of Wnt/β-catenin pathway components
Wnt/β-catenin is a major developmental pathway that controls embryonic development and tissue homeostasis (Clevers 2006; Nusse and Clevers 2017). WNTs comprise a family of 19 secreted ligands that are processed in signal-producing cells by PORCN, a palmitoleoyl transferase (Nusse and Clevers 2017). Secreted WNTs bind to Frizzled (FZD) receptors and low-density lipoprotein receptor-related protein (LRP) co-receptors at the cell membrane of signal-receiving cells (Figure 2B). In the absence of ligand, the degradation complex keeps cytosolic β-catenin levels low. Specifically, GSK3β phosphorylates β-catenin, leading to the latter’s degradation. Upon WNT binding to FZD, the destruction complex is dismantled, and β-catenin accumulates and translocates to the nucleus where it complexes with transcription factors to activate Wnt target gene expression (Figure 2B).
Dysregulation of the Wnt/β-catenin pathway is associated with multiple types of cancer, including colorectal, pancreatic, and liver cancers (Jung and Park 2020), making it a target of interest for therapeutic applications. Different approaches to inhibit (1) the secretion of Wnt ligands, (2) ligand/receptor interaction, or (3) intracellular signaling are currently in clinical trials (Figure 2B; Takebe et al. 2015; Shaw et al. 2019; Jung and Park 2020; Zhong and Virshup 2020). Similar to SMO antagonists, dysgeusia is one of the most reported side effects in patients administered Wnt signaling inhibitors (Davis 1961; Jimeno et al. 2017; Moore et al. 2019). However, the mechanisms underlying dysgeusia in these patients remain unknown.
Wnt signaling is prevalent in mouse taste tissues (Gaillard and Barlow 2011; Suzuki et al. 2012; Gaillard et al. 2015; Xu et al. 2017), providing insights into potential mechanisms of taste impairment in patients treated with Wnt pathway inhibitors. Subsets of adult taste progenitor cells, SHH+ post-mitotic taste precursors, and Type I, II, and III taste cells are each WNT-responsive (Gaillard and Barlow 2011; Xu et al. 2017), suggesting the pathway may regulate progenitor cell proliferation and taste cell differentiation at several steps of the renewal process. Both GOF and LOF genetic studies have confirmed this prediction.
Genetic stabilization of the Wnt signaling effector β-catenin in lingual progenitors (GOF) promotes proliferation and specification of taste progenitors. Notably, β-catenin GOF biases the production of endogenous and ectopic taste cells toward Type I and II cells fate, suggesting the Wnt pathway is an instructive signal for taste cell lineage establishment (Gaillard et al. 2015). Wnt/β-catenin is also essential to maintain taste cell homeostasis. β-catenin deletion in lingual progenitors (LOF) is associated with diminished proliferation and depletion of the progenitor population. In turn, differentiation of all three taste cell types is impeded resulting in taste bud decline and decreased taste perception (Gaillard et al. 2017; Xu et al. 2017). In lieu of direct investigations of the cause of taste loss in patients receiving Wnt inhibitors, the β-catenin LOF data in mice suggest that these inhibitors may disrupt taste cell homeostasis by primarily impacting taste progenitors. In addition, β-catenin LOF resulted in a more dramatic phenotype in the anterior taste field than the posterior taste papillae (Gaillard et al. 2017), suggesting that dysgeusia may result from an imbalance in taste field inputs to taste perception upon disruption by Wnt signaling inhibitors in cancer patients. Importantly, expression of targeted Wnt pathway components in cancer and healthy tissues should be well understood to design drugs that are more specific to cancer cells and minimize side effects. For example, a function-blocking antibody vantictumab (OMP-18R5) targets FZD1, 2, 5, 7, and 8 (Gurney et al. 2012), three of which are expressed in taste buds and taste progenitors of adult mice (Suzuki et al. 2012), supporting taste impairment observed in patients treated with vantictumab (Davis et al. 2020).
Targeting the Notch pathway in cancer treatment
Other signaling pathways are associated with the development of various cancers. For instance, the Notch pathway is abnormally active in forms of lung, liver, brain, and breast cancers (Takebe et al. 2015; Siebel and Lendahl 2017). A wide array of drugs has been developed to inhibit Notch signaling and reduce tumorigenesis (Takebe et al. 2015). Gamma-secretase inhibitors (GSI) tested in clinical trials aim to broadly block the Notch pathway by inhibiting the cleavage of Notch receptors and subsequent intracellular signaling (Figure 2C). Unsurprisingly, GSI treatments result in dysgeusia in cancer patients (Lee et al. 2015; Massard et al. 2018; Even et al. 2020); The Notch pathway is active in taste tissues of mice (Seta et al. 2003, 2006, 2011; Hsu et al. 2020) and zebra fish (Kapsimali et al. 2011) and has been implicated in sour cell fate decision by controlling the development and differentiation of Type III cells via transcriptional repression of Ascl1 (Seta et al. 2003, 2006, 2011; Kapsimali et al. 2011; Kito-Shingaki et al. 2014; Hsu et al. 2020). In addition, a Notch target gene, Hes1, restricts the development of Type II taste cells as HES1-null mice produce more Type II cells in late mouse embryos and new-born pups (Ota et al. 2009). Taste cell balance is also shifted in taste organoids treated with GSI; γ-secretase inhibition results in more Type II and III taste cells and fewer Type I cells (Ren et al. 2017). Altogether, mouse data support the importance of targeting Notch pathway elements that are more prevalent in cancer tissues than healthy cells to minimize taste disruption.
Taste organoids as a high-throughput drug screening tool
We reviewed different cancer therapies that cause dysgeusia and highlighted the importance of characterizing the function of potential molecular targets in taste bud cell renewal to develop drugs with reduced side effects. In addition to radiotherapy and targeted therapies inhibiting the Wnt, Hh, and Notch pathways, a multitude of therapies target other critical developmental signals. For example, more than 25 tyrosine kinase inhibitors (TKIs) inhibit a variety of protein tyrosine kinases activated in certain cancers (Jiao et al. 2018; Wing Tung Ho et al. 2019). Taste impairment has been reported for a number of them, including in the treatment of metastatic renal cell carcinoma (van der Werf et al. 2017; Vigarios et al. 2017). The advent of next-generation genomic and proteomic tools, including single-cell RNA sequencing and proteomics, offers incredible opportunities to explore the expression of this multitude of targets at single-cell resolution in healthy and diseased tissues. However, animal models remain a costly and time-consuming tool to screen the side effects of so many drugs in development. Further, to date, no immortalized taste cell line is available. Alternatively, taste organoids have become common in taste biology (Ren et al. 2014, 2017, 2020; Aihara et al. 2015; Qin et al. 2018; Guo et al. 2019; Matsumoto et al. 2019; Takai et al. 2019; Feng et al. 2020; Lin et al. 2021) and offer high-throughput drug screening possibilities. Murine taste organoids can be generated in multi-well plates from single cells isolated from whole taste papillae or from discrete taste stem cell populations such as Lgr5+ progenitor cells (Figure 1C; Ren et al. 2014, 2017). Single taste stem cells incubated in the right culture media proliferate and produce taste cell-replete organoids within 10 days (Ren et al. 2014, 2017). Quick generation of taste organoids in large numbers makes these organoids a promising model to address the effect of a multitude of injuries (Feng et al. 2020), including cancer therapies on taste bud cell renewal (Ren et al. 2017; Guo et al. 2019). Taste organoids allow screening of cancer therapies for impacts on taste stem cell proliferation and survival, taste cell differentiation and function (e.g., calcium imaging), and could be co-cultured with gustatory neurons to reproduce in vivo nerve inputs in organoid generation, as reported in other systems (Pastula et al. 2016; Chukwurah et al. 2019).
Within the past decades, the fight against cancer has been marked by significant progress with early detection and development of state-of-the-art technologies and protocols that maximize cure and survival rates. As much as modern therapies are targeted, side effects remain common and are occasionally detrimental to the course of the treatment. For instance, patients can experience taste impairment that is so unbearable that treatment must be paused. Thus, there is a need for more comprehensive investigations of potential side effects of anticancer therapies on the peripheral taste system upstream of clinical trials. The recent developments in taste organoid culture will speed up the process, and combined with in vivo and next-generation molecular tools, will help design more effective therapies.
Footnotes
Submitted to Chemical Senses Special Issue “Clinical science in the chemical senses: mechanisms, perception, behaviors and disorders”. Editors: Sanne Boesveldt and Johan Lundstrom
Funding
This work was supported by grants from the National Institutes of Health/National Institute for Deafness and Other Communication Disorders to D.G. [R21DC016131] and to L.A.B. [R01DC012383, R01DC018489] and the National Cancer Institute to L.A.B. [R21CA236480].
Conflict of interest
The authors declare no competing interests.
References
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. 2000. A novel family of mammalian taste receptors. Cell. 100(6):693–702. [DOI] [PubMed] [Google Scholar]
- Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori-Asenso R. 2020. Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc. 95(8):1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aihara E, Mahe MM, Schumacher MA, Matthis AL, Feng R, Ren W, Noah TK, Matsu-ura T, Moore SR, Hong CI, et al. . 2015. Characterization of stem/progenitor cell cycle using murine circumvallate papilla taste bud organoid. Sci Rep. 5:17185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa da Silva JL, Doty RL, Miyazaki J, Borges R, Pinna FR, Voegels RL, Fornazieri MA. 2019. Gustatory disturbances occur in patients with head and neck cancer who undergo radiotherapy not directed to the oral cavity. Oral Oncol. 95:115–119. [DOI] [PubMed] [Google Scholar]
- Barlow LA. 2015. Progress and renewal in gustation: new insights into taste bud development. Development. 142(21):3620–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DL, Sullivan SL, Lavoie EG, Sévigny J, Finger TE. 2006. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 497(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basset-Seguin N, Hauschild A, Grob JJ, Kunstfeld R, Dréno B, Mortier L, Ascierto PA, Licitra L, Dutriaux C, Thomas L, et al. . 2015. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): a pre-planned interim analysis of an international, open-label trial. Lancet Oncol. 16(6):729–736. [DOI] [PubMed] [Google Scholar]
- Bedard PL, Hyman DM, Davids MS, Siu LL. 2020. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet. 395(10229):1078–1088. [DOI] [PubMed] [Google Scholar]
- Beidler LM, Smallman RL. 1965. Renewal of cells within taste buds. J Cell Biol. 27(2):263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolze MS, Fosmire GJ, Stryker JA, Chung CK, Flipse BG. 1982. Taste acuity, plasma zinc levels, and weight loss during radiotherapy: a study of relationships. Radiology. 144(1):163–169. [DOI] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68(6):394–424. [DOI] [PubMed] [Google Scholar]
- Carpenter RL, Ray H. 2019. Safety and tolerability of sonic hedgehog pathway inhibitors in cancer. Drug Saf. 42(2):263–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo D, Seidel K, Salcedo E, Ahn C, de Sauvage FJ, Klein OD, Barlow LA. 2014. Induction of ectopic taste buds by SHH reveals the competency and plasticity of adult lingual epithelium. Development. 141(15):2993–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Azofeifa D, Losacco JT, Salcedo E, Golden EJ, Finger TE, Barlow LA. 2017. Sonic hedgehog from both nerves and epithelium is a key trophic factor for taste bud maintenance. Development. 144(17):3054–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Azofeifa D, Seidel K, Gross L, Golden EJ, Jacquez B, Klein OD, Barlow LA. 2018. SOX2 regulation by hedgehog signaling controls adult lingual epithelium homeostasis. Development 145(14):dev.164889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WC, Tsai MS, Tsai YT, Lai CH, Lee CP, Chen MF. 2019. Long-term taste impairment after intensity-modulated radiotherapy to treat head-and-neck cancer: correlations with glossectomy and the mean radiation dose to the oral cavity. Chem Senses. 44(5):319–326. [DOI] [PubMed] [Google Scholar]
- Chencharick JD, Mossman KL. 1983. Nutritional consequences of the radiotherapy of head and neck cancer. Cancer. 51(5):811–815. [DOI] [PubMed] [Google Scholar]
- Chukwurah E, Osmundsen A, Davis SW, Lizarraga SB. 2019. All together now: modeling the interaction of neural with non-neural systems using organoid models. Front Neurosci. 13:582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Stone LM, Margolskee RF, Kinnamon SC. 2001. Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci. 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. 2006. Wnt/β-catenin signaling in development and disease. Cell. 127(3):469–480. [DOI] [PubMed] [Google Scholar]
- Cognetti DM, Weber RS, Lai SY. 2008. Head and neck cancer: an evolving treatment paradigm. Cancer. 113(7 Suppl):1911–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn ZJ, Kim A, Huang L, Brand J, Wang H. 2010. Lipopolysaccharide-induced inflammation attenuates taste progenitor cell proliferation and shortens the life span of taste bud cells. BMC Neurosci. 11:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KW, Brann DH, Farruggia MC, Bhutani S, Pellegrino R, Tsukahara T, Weinreb C, Joseph PV, Larson ED, Parma V, et al. . 2020. COVID-19 and the chemical senses: supporting players take center stage. Neuron. 107(2):219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JD. 1961. Electronic drinkometer and recorder. J Exp Anal Behav. 4:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SL, Cardin DB, Shahda S, Lenz HJ, Dotan E, O’Neil BH, Kapoun AM, Stagg RJ, Berlin J, Messersmith WA, et al. . 2020. A phase 1b dose escalation study of Wnt pathway inhibitor vantictumab in combination with nab-paclitaxel and gemcitabine in patients with previously untreated metastatic pancreatic cancer. Invest New Drugs. 38(3):821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice F, Polimeni A, Valentini V, Brugnoletti O, Cassoni A, Greco A, de Vincentiis M, Tombolini V. 2018. Radiotherapy controversies and prospective in head and neck cancer: a literature-based critical review. Neoplasia. 20(3):227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloch L, Derer A, Hartmann J, Frey B, Fietkau R, Gaipl US. 2016. Modern radiotherapy concepts and the impact of radiation on immune activation. Front Oncol. 6:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande TS, Blanchard P, Wang L, Foote RL, Zhang X, Frank SJ. 2018. Radiation-related alterations of taste function in patients with head and neck cancer: a systematic review. Curr Treat Options Oncol. 19(12):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVita VT Jr, Chu E. 2008. A history of cancer chemotherapy. Cancer Res. 68:8643–8653. [DOI] [PubMed] [Google Scholar]
- Dobosz P, Dzieciatkowski T. 2019. The intriguing history of cancer immunotherapy. Front Immunol. 10:2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr W, Emmendörfer H, Haide E, Kummermehr J. 1994. Proliferation equivalent of ‘accelerated repopulation’ in mouse oral mucosa. Int J Radiat Biol. 66(2):157–167. [DOI] [PubMed] [Google Scholar]
- Dorr W, Emmendörfer H, Weber-Frisch M. 1996. Tissue kinetics in mouse tongue mucosa during daily fractionated radiotherapy. Cell Prolif. 29(9):495–504. [DOI] [PubMed] [Google Scholar]
- Ermilov AN, Kumari A, Li L, Joiner AM, Grachtchouk MA, Allen BL, Dlugosz AA, Mistretta CM. 2016. Maintenance of taste organs is strictly dependent on epithelial hedgehog/GLI signaling. PLoS Genet. 12(11):e1006442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even C, Lassen U, Merchan J, Le Tourneau C, Soria JC, Ferte C, Ricci F, Diener JT, Yuen E, Smith C, et al. . 2020. Safety and clinical activity of the Notch inhibitor, crenigacestat (LY3039478), in an open-label phase I trial expansion cohort of advanced or metastatic adenoid cystic carcinoma. Invest New Drugs. 38(2):402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Achoute L, Margolskee RF, Jiang P, Wang H. 2020. Lipopolysaccharide-induced inflammatory cytokine expression in taste organoids. Chem Senses. 45(3):187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Huang L, Wang H. 2014. Taste bud homeostasis in health, disease, and aging. Chem Senses. 39(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Jyotaki M, Kim A, Chai J, Simon N, Zhou M, Bachmanov AA, Huang L, Wang H. 2015. Regulation of bitter taste responses by tumor necrosis factor. Brain Behav Immun. 49:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Barlow LA. 2021. Cellular diversity and regeneration in taste buds. Curr Opin Physiol. 20:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard D, Barlow LA. 2011. Taste bud cells of adult mice are responsive to Wnt/β-catenin signaling: implications for the renewal of mature taste cells. Genesis. 49(4):295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard D, Bowles SG, Salcedo E, Xu M, Millar SE, Barlow LA. 2017. β-catenin is required for taste bud cell renewal and behavioral taste perception in adult mice. PLoS Genet. 13(8):e1006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard D, Shechtman LA, Millar SE, Barlow LA. 2019. Fractionated head and neck irradiation impacts taste progenitors, differentiated taste cells, and Wnt/β-catenin signaling in adult mice. Sci Rep. 9(1):17934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard D, Xu M, Liu F, Millar SE, Barlow LA. 2015. β-Catenin signaling biases multipotent lingual epithelial progenitors to differentiate and acquire specific taste cell fates. PLoS Genet. 11(5):e1005208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfaldoni S, Gianfaldoni R, Wollina U, Lotti J, Tchernev G, Lotti T. 2017. An overview on radiotherapy: from its history to its current applications in dermatology. Open Access Maced J Med Sci. 5(4):521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi D, Barrichello A, Fernandes G, Pereira A. 2019. Targeting the Hedgehog pathway in cancer: current evidence and future perspectives. Cells. 8:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal A, Singh PP, Dash G. 2009. Chorda tympani in chronic inflammatory middle ear disease. Otolaryngol Head Neck Surg. 140(5):682–686. [DOI] [PubMed] [Google Scholar]
- Guo Q, Chen S, Rao X, Li Y, Pan M, Fu G, Yao Y, Gao X, Tang P, Zhou Y, et al. . 2019. Inhibition of SIRT1 promotes taste bud stem cell survival and mitigates radiation-induced oral mucositis in mice. Am J Transl Res. 11(8):4789–4799. [PMC free article] [PubMed] [Google Scholar]
- Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, et al. . 2012. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci USA. 109(29):11717–11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamichi R, Asano-Miyoshi M, Emori Y. 2006. Taste bud contains both short-lived and long-lived cell populations. Neuroscience. 141(4):2129–2138. [DOI] [PubMed] [Google Scholar]
- Henkin RI, Christiansen RL. 1967. Taste localization on the tongue, palate, and pharynx of normal man. J Appl Physiol. 22(2):316–320. [DOI] [PubMed] [Google Scholar]
- Hill MW. 1988. Influence of age on the morphology and transit time of murine stratified squamous epithelia. Arch Oral Biol. 33(4):221–229. [DOI] [PubMed] [Google Scholar]
- Holsti LR. 1995. Development of clinical radiotherapy since 1896. Acta Oncol. 34(8):995–1003. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Seta Y, Matsuyama K, Kataoka S, Nakatomi M, Toyono T, Gunjigake KK, Kuroishi KN, Kawamoto T. 2020. Mash1-expressing cells may be relevant to type III cells and a subset of PLCβ2-positive cell differentiation in adult mouse taste buds. Cell Tissue Res. 383(2):667–675. [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Tränkner D, Ryba NJ, Zuker CS. 2006. The cells and logic for mammalian sour taste detection. Nature. 442(7105):934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Stimac R, Roper SD. 2008. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J Physiol. 586(12):2903–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume WJ, Potten CS. 1976. The ordered columnar structure of mouse filiform papillae. J Cell Sci. 22(1):149–160. [DOI] [PubMed] [Google Scholar]
- Hutton JL, Baracos VE, Wismer WV. 2007. Chemosensory dysfunction is a primary factor in the evolution of declining nutritional status and quality of life in patients with advanced cancer. J Pain Symptom Manage. 33(2):156–165. [DOI] [PubMed] [Google Scholar]
- Irune E, Dwivedi RC, Nutting CM, Harrington KJ. 2014. Treatment-related dysgeusia in head and neck cancer patients. Cancer Treat Rev. 40(9):1106–1117. [DOI] [PubMed] [Google Scholar]
- Jewkes BC, Barlow LA, Delay ER. 2017. Effect of radiation on sucrose detection thresholds of mice. Chem Senses. 43(1):53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Q, Bi L, Ren Y, Song S, Wang Q, Wang YS. 2018. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol Cancer. 17(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno A, Gordon M, Chugh R, Messersmith W, Mendelson D, Dupont J, Stagg R, Kapoun AM, Xu L, Uttamsingh S, et al. . 2017. A first-in-human phase I study of the anticancer stem cell agent Ipafricept (OMP-54F28), a decoy receptor for Wnt ligands, in patients with advanced solid tumors. Clin Cancer Res. 23(24):7490–7497. [DOI] [PubMed] [Google Scholar]
- Jung YS, Park JI. 2020. Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp Mol Med. 52(2):183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just T, Pau HW, Bombor I, Guthoff RF, Fietkau R, Hummel T. 2005. Confocal microscopy of the peripheral gustatory system: comparison between healthy subjects and patients suffering from taste disorders during radiochemotherapy. Laryngoscope. 115(12):2178–2182. [DOI] [PubMed] [Google Scholar]
- Kapsimali M, Kaushik AL, Gibon G, Dirian L, Ernest S, Rosa FM. 2011. Fgf signaling controls pharyngeal taste bud formation through miR-200 and Delta-Notch activity. Development. 138(16):3473–3484. [DOI] [PubMed] [Google Scholar]
- Kataoka S, Yang R, Ishimaru Y, Matsunami H, Sévigny J, Kinnamon JC, Finger TE. 2008. The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem Senses. 33(3):243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A, Choo E, Koh A, Dando R. 2018. Inflammation arising from obesity reduces taste bud abundance and inhibits renewal. PLoS Biol. 16(3):e2001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A, Kim J, Noel C, Dando R. 2020. Taste loss with obesity in mice and men. Int J Obes (Lond). 44(3):739–743. [DOI] [PubMed] [Google Scholar]
- Kim MR, Kusakabe Y, Miura H, Shindo Y, Ninomiya Y, Hino A. 2003. Regional expression patterns of taste receptors and gustducin in the mouse tongue. Biochem Biophys Res Commun. 312(2):500–506. [DOI] [PubMed] [Google Scholar]
- Kito-Shingaki A, Seta Y, Toyono T, Kataoka S, Kakinoki Y, Yanagawa Y, Toyoshima K. 2014. Expression of GAD67 and Dlx5 in the taste buds of mice genetically lacking Mash1. Chem Senses. 39(5):403–414. [DOI] [PubMed] [Google Scholar]
- Kumari A, Ermilov AN, Allen BL, Bradley RM, Dlugosz AA, Mistretta CM. 2015. Hedgehog pathway blockade with the cancer drug LDE225 disrupts taste organs and taste sensation. J Neurophysiol. 113(3):1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A, Ermilov AN, Grachtchouk M, Dlugosz AA, Allen BL, Bradley RM, Mistretta CM. 2017. Recovery of taste organs and sensory function after severe loss from Hedgehog/Smoothened inhibition with cancer drug sonidegib. Proc Natl Acad Sci USA. 114(48):E10369–E10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A, Yokota Y, Li L, Bradley RM, Mistretta CM. 2018. Species generalization and differences in Hedgehog pathway regulation of fungiform and circumvallate papilla taste function and somatosensation demonstrated with sonidegib. Sci Rep. 8(1):16150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis BN, Beutner D, Frasnelli J, Hüttenbrink KB, Hummel T. 2005. Gustatory function in chronic inflammatory middle ear diseases. Laryngoscope. 115(6):1124–1127. [DOI] [PubMed] [Google Scholar]
- Lawton DM, Furness DN, Lindemann B, Hackney CM. 2000. Localization of the glutamate-aspartate transporter, GLAST, in rat taste buds. Eur J Neurosci. 12(9):3163–3171. [DOI] [PubMed] [Google Scholar]
- Le Moigne M, Saint-Jean M, Jirka A, Quéreux G, Peuvrel L, Brocard A, Gaultier A, Khammari A, Darmaun D, Dréno B. 2016. Dysgeusia and weight loss under treatment with vismodegib: benefit of nutritional management. Support Care Cancer. 24(4):1689–1695. [DOI] [PubMed] [Google Scholar]
- Lederman M. 1981. The early history of radiotherapy: 1895-1939. Int J Radiat Oncol Biol Phys. 7(5):639–648. [DOI] [PubMed] [Google Scholar]
- Lee SM, Moon J, Redman BG, Chidiac T, Flaherty LE, Zha Y, Othus M, Ribas A, Sondak VK, Gajewski TF, et al. . 2015. Phase 2 study of RO4929097, a gamma-secretase inhibitor, in metastatic melanoma: SWOG 0933. Cancer. 121(3):432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal EM. 1959. Post-radiation mouth blindness. Lancet. 274:1138–1139. [DOI] [PubMed] [Google Scholar]
- Lewandowski BC, Sukumaran SK, Margolskee RF, Bachmanov AA. 2016. Amiloride-insensitive salt taste is mediated by two populations of type III taste cells with distinct transduction mechanisms. J Neurosci. 36(6):1942–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Lu C, Ohmoto M, Choma K, Margolskee RF, Matsumoto I, Jiang P. 2021. R-spondin substitutes for neuronal input for taste cell regeneration in adult mice. Proc Natl Acad Sci USA 118:e2001833118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HX, Ermilov A, Grachtchouk M, Li L, Gumucio DL, Dlugosz AA, Mistretta CM. 2013. Multiple Shh signaling centers participate in fungiform papilla and taste bud formation and maintenance. Dev Biol. 382(1):82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoRusso PM, Rudin CM, Reddy JC, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Chang I, Darbonne WC, et al. . 2011. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 17(8):2502–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Yang R, Thomas SM, Kinnamon JC. 2007. Qualitative and quantitative differences between taste buds of the rat and mouse. BMC Neurosci. 8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes A, Huygh I, Weltens C, Vandevelde G, Delaere P, Evers G, Van den Bogaert W. 2002. De Gustibus: time scale of loss and recovery of tastes caused by radiotherapy. Radiother Oncol. 63(2):195–201. [DOI] [PubMed] [Google Scholar]
- Mahdavi R, Faramarzi E, Mohammad-Zadeh M, Ghaeammaghami J, Jabbari MV. 2007. Consequences of radiotherapy on nutritional status, dietary intake, serum zinc and copper levels in patients with gastrointestinal tract and head and neck cancer. Saudi Med J. 28(3):435–440. [PubMed] [Google Scholar]
- Massard C, Azaro A, Soria JC, Lassen U, Le Tourneau C, Sarker D, Smith C, Ohnmacht U, Oakley G, Patel BKR, et al. . 2018. First-in-human study of LY3039478, an oral Notch signaling inhibitor in advanced or metastatic cancer. Ann Oncol. 29(9):1911–1917. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Ohishi A, Iwatsuki K, Yamazaki K, Takayanagi S, Tsuji M, Aihara E, Utsumi D, Tsukahara T, Tominaga M, et al. . 2019. Transient receptor potential vanilloid 4 mediates sour taste sensing via type III taste cell differentiation. Sci Rep. 9(1):6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura H, Kato H, Kusakabe Y, Tagami M, Miura-Ohnuma J, Ninomiya Y, Hino A. 2004. A strong nerve dependence of sonic hedgehog expression in basal cells in mouse taste bud and an autonomous transcriptional control of genes in differentiated taste cells. Chem Senses. 29(9):823–831. [DOI] [PubMed] [Google Scholar]
- Miura H, Kusakabe Y, Harada S. 2006. Cell lineage and differentiation in taste buds. Arch Histol Cytol. 69(4):209–225. [DOI] [PubMed] [Google Scholar]
- Miura H, Kusakabe Y, Sugiyama C, Kawamatsu M, Ninomiya Y, Motoyama J, Hino A. 2001. Shh and Ptc are associated with taste bud maintenance in the adult mouse. Mech Dev. 106(1–2):143–145. [DOI] [PubMed] [Google Scholar]
- Miura H, Scott JK, Harada S, Barlow LA. 2014. Sonic hedgehog-expressing basal cells are general post-mitotic precursors of functional taste receptor cells. Dev Dyn. 243(10):1286–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KN, Gunderson CC, Sabbatini P, McMeekin DS, Mantia-Smaldone G, Burger RA, Morgan MA, Kapoun AM, Brachmann RK, Stagg R, et al. . 2019. A phase 1b dose escalation study of ipafricept (OMP54F28) in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer. Gynecol Oncol. 154(2):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson GM. 1998. Biology of taste buds and the clinical problem of taste loss. Anat Rec. 253(3):70–78. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. 2001. Mammalian sweet taste receptors. Cell. 106(3):381–390. [DOI] [PubMed] [Google Scholar]
- Nguyen HM, Reyland ME, Barlow LA. 2012. Mechanisms of taste bud cell loss after head and neck irradiation. J Neurosci. 32(10):3474–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklassen AS, Draf J, Huart C, Hintschich C, Bocksberger S, Trecca EMC, Klimek L, Le Bon SD, Altundag A, Hummel T. 2021. COVID-19: recovery from chemosensory dysfunction. a multicentre study on smell and taste. Laryngoscope. 131(5):1095–1100. [DOI] [PubMed] [Google Scholar]
- Nomura K, Nakanishi M, Ishidate F, Iwata K, Taruno A. 2020. All-electrical Ca2+-independent signal transduction mediates attractive sodium taste in taste buds. Neuron. 106:816–829.e6. [DOI] [PubMed] [Google Scholar]
- Nusse R, Clevers H. 2017. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 169(6):985–999. [DOI] [PubMed] [Google Scholar]
- Oakley B. 1993. The gustatory competence of the lingual epithelium requires neonatal innervation. Brain Res Dev Brain Res. 72(2):259–264. [DOI] [PubMed] [Google Scholar]
- Oakley B, Witt M. 2004. Building sensory receptors on the tongue. J Neurocytol. 33(6):631–646. [DOI] [PubMed] [Google Scholar]
- Ogama N, Suzuki S, Umeshita K, Kobayashi T, Kaneko S, Kato S, Shimizu Y. 2010. Appetite and adverse effects associated with radiation therapy in patients with head and neck cancer. Eur J Oncol Nurs. 14(1):3–10. [DOI] [PubMed] [Google Scholar]
- Ohtubo Y, Yoshii K. 2011. Quantitative analysis of taste bud cell numbers in fungiform and soft palate taste buds of mice. Brain Res. 1367:13–21. [DOI] [PubMed] [Google Scholar]
- Oka Y, Butnaru M, von Buchholtz L, Ryba NJ, Zuker CS. 2013. High salt recruits aversive taste pathways. Nature. 494(7438):472–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota MS, Kaneko Y, Kondo K, Ogishima S, Tanaka H, Eto K, Kondo T. 2009. Combined in silico and in vivo analyses reveal role of Hes1 in taste cell differentiation. PLoS Genet. 5(4):e1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma V, Ohla K, Veldhuizen MG, Niv MY, Kelly CE, Bakke AJ, Cooper KW, Bouysset C, Pirastu N, Dibattista M, et al. ; GCCR Group Author . 2020. More than smell-COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses. 45(7):609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastula A, Middelhoff M, Brandtner A, Tobiasch M, Höhl B, Nuber AH, Demir IE, Neupert S, Kollmann P, Mazzuoli-Weber G, et al. . 2016. Three-dimensional gastrointestinal organoid culture in combination with nerves or fibroblasts: a method to characterize the gastrointestinal stem cell niche. Stem Cells Int. 2016:3710836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea-Martinez I, Nagai T, Chaudhari N. 2013. Functional cell types in taste buds have distinct longevities. PLoS One. 8(1):e53399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova R, Joyner AL. 2014. Roles for Hedgehog signaling in adult organ homeostasis and repair. Development. 141(18):3445–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Booth D, Cragg NJ, O’Shea JA, Tudor GL, Booth C. 2002. Cell kinetic studies in murine ventral tongue epithelium: cell cycle progression studies using double labelling techniques. Cell Prolif. 35(Suppl 1):16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin DW, Yu C, Smith DV. 1997. Light and dark cells of rat vallate taste buds are morphologically distinct cell types. J Comp Neurol. 378(3):389–410. [DOI] [PubMed] [Google Scholar]
- Purves D, Williams SM. 2001. The organization of the taste system. In: Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia AS, McNamara JO, Williams SM, editors. Neuroscience. 2nd ed. Sunderland (MA): Sinauer Associates. Available from: https://www.ncbi.nlm.nih.gov/books/NBK11018/. [Google Scholar]
- Qin Y, Sukumaran SK, Jyotaki M, Redding K, Jiang P, Margolskee RF. 2018. Gli3 is a negative regulator of Tas1r3-expressing taste cells. PLoS Genet. 14(2):e1007058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathod S, Livergant J, Klein J, Witterick I, Ringash J. 2015. A systematic review of quality of life in head and neck cancer treated with surgery with or without adjuvant treatment. Oral Oncol. 51(10):888–900. [DOI] [PubMed] [Google Scholar]
- Ravasco P, Monteiro-Grillo I, Marques Vidal P, Camilo ME. 2005. Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck. 27(8):659–668. [DOI] [PubMed] [Google Scholar]
- Ray-Chaudhuri A, Shah K, Porter RJ. 2013. The oral management of patients who have received radiotherapy to the head and neck region. Br Dent J. 214(8):387–393. [DOI] [PubMed] [Google Scholar]
- Ren W, Aihara E, Lei W, Gheewala N, Uchiyama H, Margolskee RF, Iwatsuki K, Jiang P. 2017. Transcriptome analyses of taste organoids reveal multiple pathways involved in taste cell generation. Sci Rep. 7(1):4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Lewandowski BC, Watson J, Aihara E, Iwatsuki K, Bachmanov AA, Margolskee RF, Jiang P. 2014. Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo. Proc Natl Acad Sci USA. 111(46):16401–16406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Liu Q, Zhang X, Yu Y. 2020. Age-related taste cell generation in circumvallate papillae organoids via regulation of multiple signaling pathways. Exp Cell Res. 394(2):112150. [DOI] [PubMed] [Google Scholar]
- Rodon J, Tawbi HA, Thomas AL, Stoller RG, Turtschi CP, Baselga J, Sarantopoulos J, Mahalingam D, Shou Y, Moles MA, et al. . 2014. A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors. Clin Cancer Res. 20(7):1900–1909. [DOI] [PubMed] [Google Scholar]
- Rubin LL, de Sauvage FJ. 2006. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 5(12):1026–1033. [DOI] [PubMed] [Google Scholar]
- Ruo Redda MG, Allis S. 2006. Radiotherapy-induced taste impairment. Cancer Treat Rev. 32(7):541–547. [DOI] [PubMed] [Google Scholar]
- Sandow PL, Hejrat-Yazdi M, Heft MW. 2006. Taste loss and recovery following radiation therapy. J Dent Res. 85(7):608–611. [DOI] [PubMed] [Google Scholar]
- Sano M, Ito K, Suzukawa K, Kaga K, Yamasoba T. 2007. Influence of chronic middle ear diseases on gustatory function: an electrogustometric study. Otol Neurotol. 28(1):44–47. [DOI] [PubMed] [Google Scholar]
- Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, Solomon JA, Yoo S, Arron ST, Friedlander PA, et al. . 2012. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 366(23):2171–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seta Y, Oda M, Kataoka S, Toyono T, Toyoshima K. 2011. Mash1 is required for the differentiation of AADC-positive type III cells in mouse taste buds. Dev Dyn. 240(4):775–784. [DOI] [PubMed] [Google Scholar]
- Seta Y, Seta C, Barlow LA. 2003. Notch-associated gene expression in embryonic and adult taste papillae and taste buds suggests a role in taste cell lineage decisions. J Comp Neurol. 464(1):49–61. [DOI] [PubMed] [Google Scholar]
- Seta Y, Stoick-Cooper CL, Toyono T, Kataoka S, Toyoshima K, Barlow LA. 2006. The bHLH transcription factors, Hes6 and Mash1, are expressed in distinct subsets of cells within adult mouse taste buds. Arch Histol Cytol. 69(3):189–198. [DOI] [PubMed] [Google Scholar]
- Shaw HV, Koval A, Katanaev VL. 2019. Targeting the Wnt signalling pathway in cancer: prospects and perils. Swiss Med Wkly. 149:w20129. [DOI] [PubMed] [Google Scholar]
- Siebel C, Lendahl U. 2017. Notch signaling in development, tissue homeostasis, and disease. Physiol Rev. 97(4):1235–1294. [DOI] [PubMed] [Google Scholar]
- Steinbach S, Proft F, Schulze-Koops H, Hundt W, Heinrich P, Schulz S, Gruenke M. 2011. Gustatory and olfactory function in rheumatoid arthritis. Scand J Rheumatol. 40(3):169–177. [DOI] [PubMed] [Google Scholar]
- Surova O, Zhivotovsky B. 2013. Various modes of cell death induced by DNA damage. Oncogene. 32(33):3789–3797. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Tsunekawa H, Obara N, Irie K, Shibata S. 2012. Expression and activation of β-catenin in developing and denervated taste buds. Dent J Health Sci Univ Hokkaido 31:63–72. [Google Scholar]
- Takai S, Watanabe Y, Sanematsu K, Yoshida R, Margolskee RF, Jiang P, Atsuta I, Koyano K, Ninomiya Y, Shigemura N. 2019. Effects of insulin signaling on mouse taste cell proliferation. PLoS One. 14(11):e0225190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, Yang SX, Ivy SP. 2015. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 12(8):445–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JY, Mackay-Wiggan JM, Aszterbaum M, Yauch RL, Lindgren J, Chang K, Coppola C, Chanana AM, Marji J, Bickers DR, et al. . 2012. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 366(23):2180–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmel AF, Quint C, Schickinger-Fischer B, Hummel T. 2005. Taste function in xerostomia before and after treatment with a saliva substitute containing carboxymethylcellulose. J Otolaryngol. 34(2):116–120. [DOI] [PubMed] [Google Scholar]
- Teng B, Wilson CE, Tu YH, Joshi NR, Kinnamon SC, Liman ER. 2019. Cellular and neural responses to sour stimuli require the proton channel Otop1. Curr Biol. 29(21):3647–3656.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre LA, Jemal A. 2018. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68(6):394–424. [DOI] [PubMed] [Google Scholar]
- van der Werf A, Rovithi M, Langius JAE, de van der Schueren MAE, Verheul HMW. 2017. Insight in taste alterations during treatment with protein kinase inhibitors. Eur J Cancer. 86:125–134. [DOI] [PubMed] [Google Scholar]
- Varjosalo M, Taipale J. 2008. Hedgehog: functions and mechanisms. Genes Dev. 22(18):2454–2472. [DOI] [PubMed] [Google Scholar]
- Vigarios E, Epstein JB, Sibaud V. 2017. Oral mucosal changes induced by anticancer targeted therapies and immune checkpoint inhibitors. Support Care Cancer. 25(5):1713–1739. [DOI] [PubMed] [Google Scholar]
- Wang JYJ. 2019. Cell death response to DNA damage. Yale J Biol Med. 92(4):771–779. [PMC free article] [PubMed] [Google Scholar]
- Wilson CE, Vandenbeuch A, Kinnamon SC. 2019. Physiological and behavioral responses to optogenetic stimulation of PKD2L1+ type III taste cells. eNeuro. 6(2):e0107-19.2019: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing Tung Ho V, Yue Tan H, Wang N, Feng Y. 2019. Cancer management by tyrosine kinase inhibitors: efficacy, limitation, and future strategies.In: Ren H, editor. Tyrosine kinases as druggable targets in cancer. London (UK): intechopen. pp. 1–31. doi: 10.5772/intechopen.79884. [DOI]
- Xu M, Horrell J, Snitow M, Cui J, Gochnauer H, Syrett CM, Kallish S, Seykora JT, Liu F, Gaillard D, et al. . 2017. WNT10A mutation causes ectodermal dysplasia by impairing progenitor cell proliferation and KLF4-mediated differentiation. Nat Commun. 8:15397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Cong WN, Yoon JS, Egan JM. 2015. Vismodegib, an antagonist of hedgehog signaling, directly alters taste molecular signaling in taste buds. Cancer Med. 4(2):245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Dzowo YK, Wilson CE, Russell RL, Kidd GJ, Salcedo E, Lasher RS, Kinnamon JC, Finger TE. 2020. Three-dimensional reconstructions of mouse circumvallate taste buds using serial blockface scanning electron microscopy: I. Cell types and the apical region of the taste bud. J Comp Neurol. 528(5):756–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshie S, Wakasugi C, Teraki Y, Fujita T. 1990. Fine structure of the taste bud in guinea pigs. I. Cell characterization and innervation patterns. Arch Histol Cytol. 53(1):103–119. [DOI] [PubMed] [Google Scholar]
- Zabernigg A, Giesinger JM, Pall G, Gamper EM, Gattringer K, Wintner LM, Sztankay MJ, Holzner B. 2012. Quality of life across chemotherapy lines in patients with cancers of the pancreas and biliary tract. BMC Cancer. 12:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. 2003. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 112(3):293–301. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Virshup DM. 2020. Wnt signaling and drug resistance in cancer. Mol Pharmacol. 97(2):72–89. [DOI] [PubMed] [Google Scholar]