Summary
Amblyopia (lazy eye) is a neurodevelopmental disorder of vision with no ocular pathology. The loss of vision in the amblyopic eye is assumed to be the main deficit in amblyopia, which has resulted in visual acuity (VA) being the primary outcome measure for treatment. Here we used a binocular orientation combination task to quantitatively assess the binocular status by measuring the binocular balance. We set out to determine whether amblyopes who reach the acuity-based end point have a residual binocular imbalance. Our results suggest that even amblyopes who have regained normal acuity have residual binocular deficits over a wide range of spatial frequencies. A further control study suggests that these binocular deficits could not be explained by any residual contrast sensitivity deficits of the amblyopic eye. Consequently, amblyopia is not the primary problem and VA is not the appropriate end point measure.
Subject areas: Biological sciences, neuroscience, sensory neuroscience
Graphical abstract
Highlights
-
•
Treated amblyopes have normal visual acuity
-
•
Treated amblyopes have residual binocular deficits across spatial frequencies
-
•
Visual acuity is not the appropriate end point measure in amblyopia treatment
Biological sciences; Neuroscience; Sensory neuroscience
Introduction
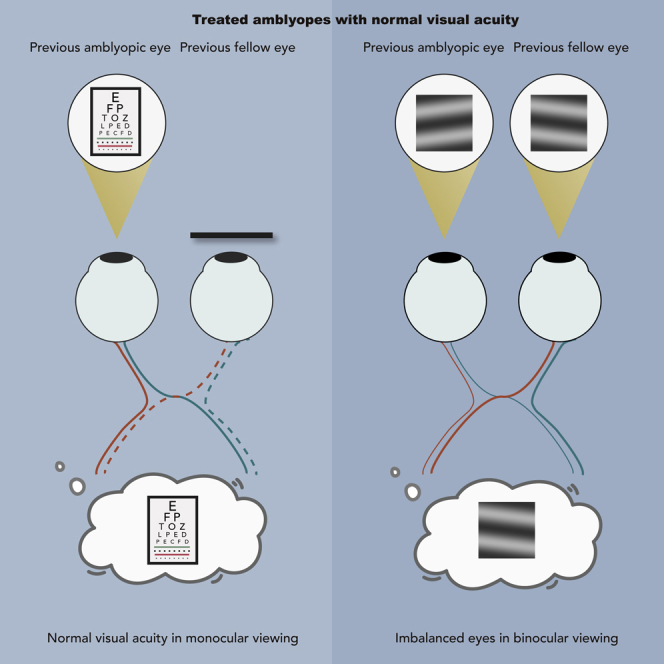
For over 200 years our understanding of amblyopia and its accepted treatment has been based on two fundamental premises: the loss of vision in one eye is the primary problem and the best way of assessing the response to treatment is by assessing the recovery of visual acuity (VA) of the affected eye (Birch, 2013; Loudon and Simonsz, 2005). This led to the universal use of patching therapy and preoccupation with the monocular letter acuity of the amblyopic eye under the assumption that improved acuity in the amblyopic eye would lead to improved binocularity. While it is recognized that even treated amblyopes have deficient stereopsis, a more important question relevant to the issue outlined above is whether improving the VA in an amblyopic eye leads to at least a rudimentary combination of information between the two eyes. This is because VA is measured monocularly with the untested eye covered by an opaque patch in clinical practice. While in real-world activities, we see the world with two eyes. Whether the normal VA of the previously amblyopic eye is used during binocular viewing depends on whether the two eyes work together. If there is measurable inhibition, even if the VA of amblyopic eyes is improved, the utilization of this when both eyes are open will be limited.
A fresh perspective on amblyopia and its treatment was provided by the pioneering anatomical and physiological studies of Hubel and Wiesel (Wiesel and Hubel, 1963a, 1963b; Hubel and Wiesel, 1968) on the development of the visual cortex of cats and monkeys. On the basis of their studies of selectively visually deprived animals they suggested that amblyopia may arise from unbalanced visual inputs to the two eyes in early postnatal development. For instance, early monocular visual deprivation during an early critical period shifts the ocular dominance of cells in the visual cortex toward the nondeprived eye in monkeys (Hubel et al., 1976, 1977; LeVay et al., 1980) and kittens (Hubel and Wiesel, 1970; Olson and Freeman, 1975; Shatz and Stryker, 1978). These amblyopic animal model studies indicate that amblyopia is a monocular manifestation of the consequences of a prior imbalance between the two eyes. This has also been further strengthened by Mitchell et al., who showed that short exposures of binocular inputs allowed normal development of VA in the two eyes in kittens (Mitchell et al., 2011). These previous animal studies supports balanced binocular treatment strategies for human amblyopia (for review, see (Mitchell and Duffy, 2014)). Such binocular treatment strategies can ensure that the improvement of monocular vision is functionally realized during binocular viewing. For instance, dichoptic training not only reduces patients' interocular suppression and improves their stereopsis (Hess et al., 2011), but also benefits the amblyopic eye's VA (Hess and Thompson, 2013) and contrast sensitivity (Li et al., 2015). Furthermore, restoring binocular vision will result in real benefits for everyday vision, impacting binocular tasks which will include, postural stability (Wu and Lee, 2015), reading performance (Johansson et al., 2014), driving performance (McKnight et al., 1991), depth perception (Kelly et al., 2016), fine motion control (Grant et al., 2007; Webber et al., 2016; Loftus et al., 2004; Adrian et al., 2019), and sport performance (Heinen and Vinken, 2011). In short, these previous studies highlight the importance of binocular status in amblyopia development and treatment.
An acid test of whether improved acuity in the amblyopic eye leads to improved use of that eye-based information in normal binocular viewing is the focus of this study. For this reason, we focus particularly on the binocular balance at high spatial frequencies, as this is most relevant to the acuity improvement. In this study, we examine the binocular status of a group of amblyopes who have undertaken the present standard-of-care treatment and achieved normal letter acuity in their amblyopic eyes. The binocular measure we use is a rudimentary one that reflects how information from the two eyes is combined at the site of binocular combination early in visual processing. The treatment for these patients would be regarded as having been 100% successful in so far as the letter acuity in their amblyopic eye is now within normal limits (i.e., 0.1 logMAR or better), which is what the present patching therapy aims to accomplish. According to this present approach, these patients have been cured of their “amblyopia”. We show that this acuity improvement does not result in a better combination of information between the two eyes. The rudimentary combination of visual information between the eyes of these “cured amblyopes” is defective, particularly at high spatial frequencies that would be most relevant to their acuity gains. This residual rudimentary binocular deficit greatly limits the real-world performance of amblyopes even after they have been considered “successfully” treated by the current standard of care.
Results
Experiment 1: Binocular imbalance measured with a fixed contrast of 50% in the pAE/nDE
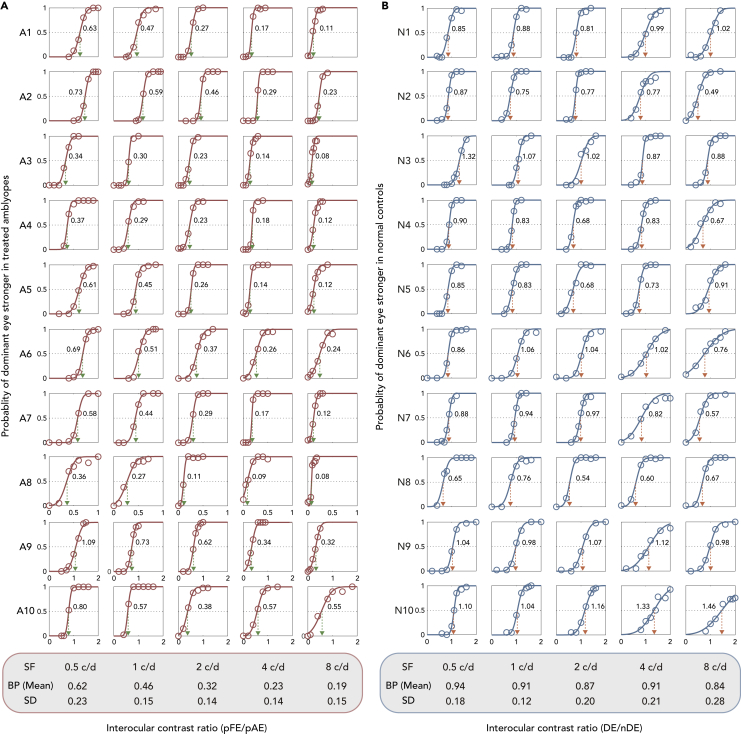
Normal observers (n = 10) had balance points (BPs) of 0.94 ± 0.18, 0.91 ± 0.12, 0.87 ± 0.20, 0.91 ± 0.21, and 0.84 ± 0.28 (mean ± SD) at five spatial frequencies (0.5, 1, 2, 4, and 8 c/d), respectively (see Figure 2A). Treated amblyopes (n = 10) had BPs of 0.62 ± 0.23, 0.46 ± 0.15, 0.32 ± 0.14, 0.23 ± 0.14, and 0.19 ± 0.15 (mean ± SD) at five spatial frequencies (0.5, 1, 2, 4, and 8 c/d), respectively (see Figure 2B). For some controls and all treated amblyopes, the BPs decreased as spatial frequency increased. This indicates the presence of a greater binocular imbalance at higher spatial frequencies, particularly prominent in treated amblyopes.
Figure 2.
Binocular combination of treated amblyopes (n = 10) and normal controls (n = 10) from Experiment 1
The relationship between the probability of dominant eye being stronger and interocular contrast ratio (pFE/pAE or DE/nDE) was plotted for all the subjects.
(A) The crossing of red dotted line and the horizontal black line represents the balance point (BP), which is denoted by the green dotted arrow lines.
(B) The crossing of blue dotted line and the horizontal black line represents the balance point (BP), which is denoted by the orange dotted arrow lines.
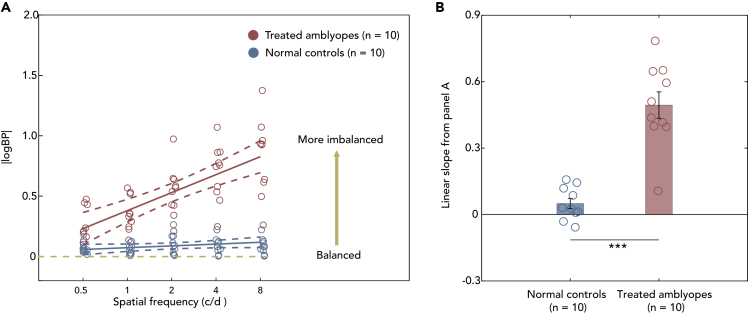
BPs were converted into absolute values of log10(BP), i.e., |logBP| (see Figure 3A). Two-way mixed ANOVA was conducted (between-subject factor: subject group, within-subject factor: spatial frequency). It showed a main effect for subject group, spatial frequency and interaction (all p's < 0.001). For post-hoc, pairwise multiple comparisons (all p's < 0.001 with Bonferroni correction) were conducted in the treated amblyopia group between 0.5 and 1 c/d (Cohen's d = 1.77), 2 c/d (Cohen's d = 3.75), 4 c/d (Cohen's d = 5.26), and 8 c/d (Cohen's d = 5.39). The effect size (shown in Table S1, Cohen's d) is most notable between |logBP| at 0.5 c/d and higher frequencies such as 4 and 8 c/d but less so with 1 c/d.
Figure 3.
Different binocular imbalances in treated amblyopes (n = 10) and normal controls (n = 10)
(A) The relationship between the averaged |logBP| (i.e., the absolute values of log10(BP)) and spatial frequency. Red points indicate treated amblyopes; blue points indicate normal controls. The solid line indicates the best linear fit, AND the dotted line is expressed as a proportion with relative 95% confidence interval. The horizontal yellow dashed line represents idealized binocular balanced eyes.
(B) Mean slopes of the linear regression analysis of individual's |logBP| vs. logSF function. The slopes were calculated by linear regression analysis for each subject and the mean values (±SEM) for each group were shown. Blue bar represents the averaged slope of the normal observers, red bar represents the averaged slope of the treated amblyopes. ∗∗∗ represents the results of “p < 0.001” according the independent t test for two groups. Error bars represents standard error (SE).
The results show that binocular imbalance is least at low spatial frequency but more prominent at high spatial frequency in the treated amblyopes. This is not the case in normal observers. Therefore, spatial frequency-dependent binocular imbalances are present in the treated amblyopes. To better illustrate the spatial frequency-dependent binocular imbalance, we quantified the relationship between spatial frequency and |logBP| via linear regression for each subject group (see Figure 3B). Spatial frequencies were converted into log10 units (ex., log10(2) = 0.3). An independent t test found a significant difference of the slopes between the two groups (t (11.675) = 6.943, p < 0.001).
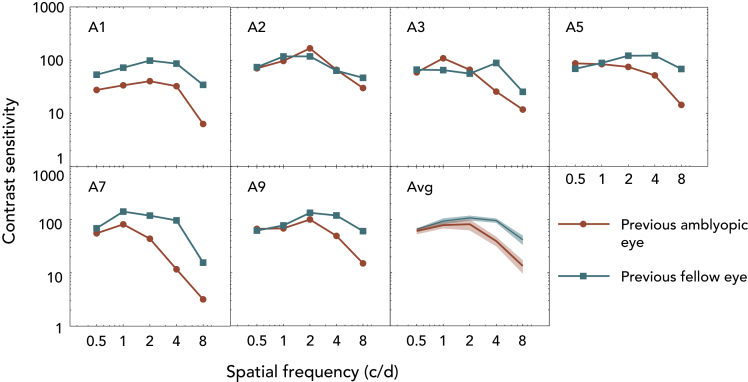
The finding that there still persists a binocular imbalance in treated amblyopes and that it increases as the spatial frequency of the stimuli increases is open to two different interpretations. First, although contrast thresholds are higher at higher spatial frequencies in normal subjects, this may be more exaggerated in amblyopes even if they have normal VA (Huang et al., 2007). Such deficits would reduce the suprathreshold level of the 50% base contrast at higher spatial frequencies that was used in experiment 1. This in turn could reduce the visibility (Hess and Bradley, 1980; Loshin and Levi, 1983) of the 50% base contrast at high spatial frequencies and could be the reason why greater imbalances were observed in experiment 1 for treated amblyopes at higher spatial frequencies (Figure 3). If this were indeed the case, it would support the proposition that the monocular loss is primary and the binocular loss is secondary. However, on the other hand, if the binocular loss is the primary anomaly and the monocular loss, the secondary consequence (Li et al., 2011) then correcting for suprathreshold base contrast (i.e., making all the spatial frequencies to be at a consistent suprathreshold contrast level) will have no impact on the binocular deficit, as reflected in the change in the BP with spatial frequency. Circumstantial support for the first explanation came from the additional finding that our treated amblyopes whose VA was normal in fact had residual contrast sensitivity deficits that were larger at high spatial frequencies (Figure 4). Experiment 2 directly addresses this issue by measuring the relationship between the BP and spatial frequency for base contrasts of comparable suprathreshold contrast.
Figure 4.
Contrast sensitivity of the previous amblyopic eye (pAE) and previous fellow eye (pFE).
CSFs of the six treated amblyopes and their average result. The red circle curves indicate the contrast sensitivity of pAE and the blue square curves indicate the contrast sensitivity of pFE. The average curves show the range of mean ± SEM.
Experiment 2: Binocular imbalance measured with a fixed relative suprathreshold contrast in the pAE
With the exception of subject A10 the binocular performance of the subjects in Experiment 1 were quite different from that of the normal controls, which may have resulted from the loss of contrast threshold at higher spatial frequencies. In order to factor out any causative influence of this contrast threshold loss at higher spatial frequencies, we matched the suprathreshold contrast of the pAE across spatial frequency in Experiment 2. Six of the nine subjects participated in Experiment 2 as the others could not be scheduled for personal reasons. By matching the suprathreshold contrast of the pAE across spatial frequency (see STAR Methods), we ensured that the subjects viewed gratings of a comparable suprathreshold level relative to the contrast threshold at all spatial frequencies. We then remeasured the BPs on six treated amblyopes.
To calculate the comparable suprathreshold contrast across all spatial frequency, we had to first measure the monocular contrast sensitivity of the treated amblyopes. The typical contrast sensitivity function was observed in all subjects, with thresholds dependent upon spatial frequency. This observation is corroborated by a two-way repeated measures ANOVA analysis (within-subject factors: eye, spatial frequency). It showed a main effect of the spatial frequency (p < 0.001). Moreover, main effects of eye and interaction were observed (p's < 0.05). We noted a small loss in contrast sensitivity at higher spatial frequencies that was not reflected in their recovered acuities, consistent with previous published results (Huang et al., 2007). Our individual adjustment ensured that we used a base contrast to factor out any influence of this threshold contrast loss at higher spatial frequencies.
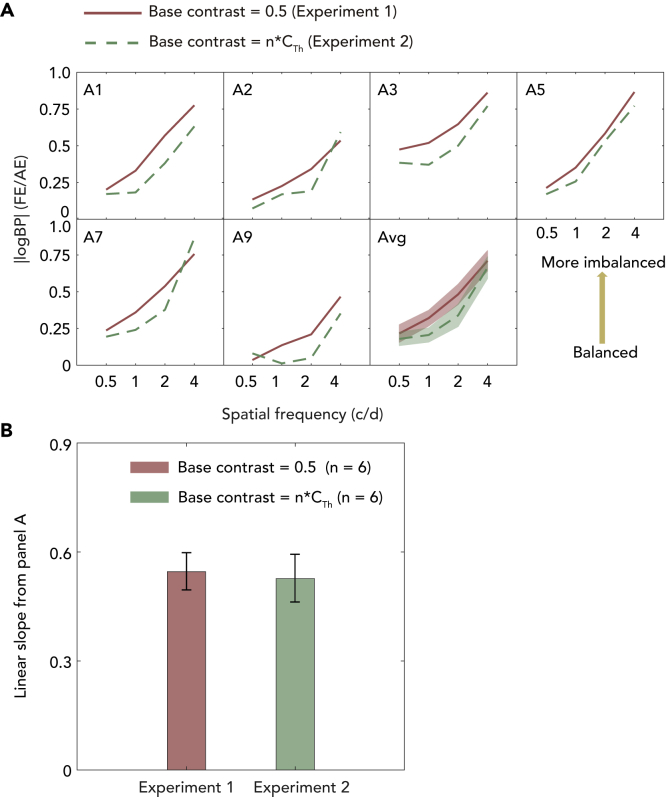
BPs were converted into absolute values of log10(BP) (see Figure 5A). A two-way repeated measures ANOVA (within-subject factors: experiment type and spatial frequency) showed a main effect of the experiment type (p = 0.001), spatial frequency (p < 0.001) and interaction (p = 0.026). The results indicate that binocular imbalance remains when the suprathreshold contrast of the grating is normalized across spatial frequencies in the pAE. We next calculated linear regression of the transformed BPs (i.e., |logBP|) as a function of logSF. Subsequently, we performed paired samples t test between the linear slopes across the two experiments (see Figure 5B) and found no significant difference (t (5) = 0.392, p = 0.711, Cohen's d = 0.25).
Figure 5.
Correlation in Binocular imbalance between different experiments (n = 6)
(A) Correlation in Binocular imbalance between different experiments (n = 6). The relationship between the |logBP| and spatial frequency for the six treated amblyopes and their average result. X axis represents Experiment 1 and y axis represents Experiment 2. The solid red curves indicate binocular imbalance in Experiment 1 (base contrast of the gratings shown to the pAE was set at 50%); dashed green curves indicate binocular imbalance in Experiment 2 (base contrast of the gratings shown to the pAE was the identical suprathreshold contrast across spatial frequency, i.e., 1/Contrast thresholdx c/d times of the contrast threshold of the pAE). The average curves show the range of mean ± SEM.
(B) Linear slopes of these six treated amblyopes calculated from panel A and compared with that calculated from experiment 1. Error bars represents standard error (SE).
A small sample size can cause a false negative error (type II error) because the probability of achieving statistical significance decreases as the sample size decreases. Since the null difference between Experiments 1 and 2 could be due to the small sample size (n = 6), we conducted a power analysis, which estimates the necessary sample size to achieve a statistical significance across two means. We calculated that we would need to recruit 450 more subjects to reach statistical significance for the slope difference found (power = 80% and 2-tailed significance level at α = 0.05). Therefore, while we cannot reject that there is a difference between the means of exp1 and 2, we conclude it is very small and meaningless.
Discussion
There are three noteworthy findings from this study. First, amblyopes who have been successfully treated and whose monocular VA has returned to normal levels in the amblyopic eye still exhibit a residual binocular deficit. Second, amblyopes who have recovered normal VA in the amblyopic eye as a result of treatment still have a residual contrast threshold deficit. Third, although these two residual deficits, one monocular, involving contrast thresholds and the other binocular, and involving interocular sensory balance, the latter is not a direct consequence of the former.
The residual binocular deficit in treated amblyopes
The binocular deficit involves an interocular sensory imbalance that is worse at higher spatial frequencies. It is, we believe, a reflection of the net imbalance in the inhibitory interocular network (Zhou et al., 2018) that underlines clinical suppression and whose site is at the very early input stage of area V1. Although it has been known that amblyopes, even after successful treatment, can have reduced stereopsis (Birch, 2013), the deficit we report is at a level in the pathway well before relative disparity is computed (Cumming and Parker, 1999, 2000; Neri et al., 2004) and represents a more fundamental deficit. Actually, in human studies, several models suggest that the suppression (interocular interaction) occurs prior to the disparity calculation (for example, the multi-pathway contrast gain-control model (Hou et al., 2013) and binocular integration model (May and Zhaoping, 2016)). There are also studies suggest that binocular imbalance (the interocular contrast ratio at where the two eyes are balanced in binocular combination/rivalry) is correlated with that of stereopsis in amblyopes (Han et al., 2018; He et al., 2018; Li et al., 2011). In addition, several anti-suppression training studies show that amblyopes' stereopsis improves as a result of reducing suppression (Vedamurthy et al., 2015; Hess et al., 2010a; Webber et al., 2020). We thus choose the binocular orientation combination task to quantify the binocular performance of treated amblyopes in our study. And our finding of a residual binocular deficit in treated amblyopes highlights the inadequacies of the current monocularly based patching therapy. If one reverses the current thinking on the etiology of this condition and assume (Hess and Thompson, 2015) that the primary deficit is binocular and the secondary consequence is monocular (i.e. amblyopia), our results becomes more understandable. Then, it is not therefore surprising that a resolution of the monocular acuity deficit does not by itself impact the binocular deficit.
The residual contrast threshold deficit
We confirm what has already been shown by (Huang et al., 2007), namely that amblyopes who have been successfully treated in terms of letter acuity can still have a residual contrast threshold deficit at high spatial frequencies. Amblyopes are known to have larger deficits for letter acuity than for grating acuity (McKee et al., 2003), but after treatment this appears to reverse and letter acuity normalizes before grating acuity. There is also evidence (e.g. (McKee et al., 2003)) that anisometropic amblyopes have generally worse contrast sensitivity than the strabismic subtype with equivalent VA losses, and that amblyopes with some recovery of binocular function have worse contrast sensitivity than those with no binocular function at all. Given that the subjects in the current experiments were all anisometropic and had only slightly reduced random dot stereoacuities (of 200–400 arc s), it is perhaps unsurprising that they exhibited a residual monocular contrast threshold deficit.
For the six observers who participated in Experiment 2, their residual contrast threshold deficits are larger at higher spatial frequency. Such a pattern is similar to the binocular imbalance pattern that we found in Experiment 1, i.e., clear binocular imbalance across spatial frequencies with more imbalance at mid to high frequencies than that at low frequency. Would the former account for the later? Namely, is it because that the amblyopic eye could not see the stimuli well due to poor monocular contrast sensitivity, and thus patients developed more binocular imbalance at high frequencies? Our observations in Experiment 2 does not support this hypothesis, as the binocular imbalance vs. SF pattern remained even when we tested with visibility-matched contrast of the previously amblyopic eye (the contrast of the inputs in the previously amblyopic eye was calibrated to be a consistent time of its contrast threshold on a per spatial frequency and per individual basis, i.e., low contrast at low frequency and high contrast at high frequency). Thus, any simple explanation based on a low-level monocular attenuation (Zhou et al., 2018) cannot explain the BP deficit that we report here which increased with spatial frequency.
Therapeutic recommendations
The unavoidable conclusion is that letter acuity should not be the primary end point for assessing the outcome of treatment in amblyopia. First, as we and others (Huang et al., 2007) have shown, contrast threshold deficits can remain after letter acuity has been improved to normal limits. Second, a binocular deficit of a very fundamental nature, involving an interocular imbalance at high spatial frequencies, remains after the letter acuity deficit has been resolved through treatment. Given the fact that we see the world with two eyes, our study suggests that, in amblyopia treatment, and in future clinical trials for comparing different treatments, both the monocular VA and the binocular imbalance across spatial frequencies should be taken into account. Binocular treatment (Hess and Thompson, 2015; Hess et al., 2010b; Li et al., 2013), as a complementary treatment to the monocular patching, might result in more significant real-world benefits for amblyopes as both binocular vision (the ability to use both eyes at the same time in a single fused percept) and VA will be improved.
Limitations of study
We recruited ten treated amblyopes, who had residual binocular vision and were therefore able to fuse the images between the two eyes (nine patients with anisometropia, one patient with both anisometropia and strabismus). Although our sample size of strabismic amblyopes was small, we did not purposely limit our study to anisometropic amblyopia. The fewer strabismic amblyopes could be partially because some studies suggest that patching therapy by itself is less successful in strabismic amblyopia (Good, 1996; Attebo et al., 1998). On the other hand, this could also because our measure of binocular imbalance requires observers to have at least some residual binocular vision and be able to fuse the two eyes images, which is more achievable in anisometropic amblyopes (Holopigian et al., 1988). It remains to be seen whether the extent of binocular imbalance is different between treated strabismic amblyopia and anisometropic amblyopia.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| Matlab R2016b v9.1.0 | MathWorks | https://www.mathworks.com/products/matlab.html |
| Psychtoolbox extension v3.0.14 | Brainard, 1997; Pelli, 1997 | http://psychtoolbox.org/ |
| Psykinematix v2.0.1 GPU edition | Kyberversion | http://psykinematix.kybervision.net/ |
| IBM-SPSS v23.0 | International Business Machines Corporation | https://www.ibm.com/analytics/spss-statistics-software |
| Other | ||
| MacBook Pro 2017 | Apple, Inc. | https://www.apple.com/mac/ |
| ASUS monitor (PG279Q) | AsusTek Computer Inc. | https://www.asus.com/Displays-Desktops/Monitors/All-series/ |
| GOOVIS (AMOLED display) | NED Optics | https://goovis.en.alibaba.com/company_profile.html |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Jiawei Zhou (zhoujw@mail.eye.ac.cn).
Materials availability
This study did not generate new unique reagents.
Data and code availability
All data supporting findings of this work are provided within the manuscript (Figure 2) and its supplemental information section. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and subject details
Participants
Ten treated amblyopes (average age: 23.3 ± 4.2 years old) and ten adults (24 ± 1.1 years old) with normal or corrected to normal vision participated in this study. We used three criteria to define treated amblyopia: (1) visual acuity in the previous amblyopic eye achieving 0.1 logMAR or better, (2) the differences of visual acuity between the previous amblyopic eye and the previous fellow eye being no more than 2 lines, (3) the improvements persisting for at least three months after treatment, (4) have residual binocular vision and were able to fuse the two eyes. Clinical details of the treated amblyopes, including age, sex, refraction and corrected visual acuity are listed in Table S2. All of the subjects were naive as to the purpose of the experiment. Written informed consent was obtained. This study was approved by the Ethics Committee at Wenzhou Medical University and adhered to the tenets of the Declaration of Helsinki. For normal observers, a hole-in-the-card test (Dane and Dane, 2004) was used to determine the eye dominance.
Method details
Apparatus
The experiments were conducted on a MacBook Pro (13-in., 2017; Apple, Inc., Cupertino, CA, USA) running MATLAB R2016b (v9.1.0 MathWorks, Inc., Natick, MA, USA) with Psychtoolbox extension 3.0.14 (Brainard, 1997; Pelli, 1997) and Psykinematix (v2.0.1 GPU edition; KyberVision, Sendai, Miyagi, Japan). In Experiment 1, the stimuli were presented on gamma-corrected goggles (GOOVIS, AMOLED display, NED Optics, Shenzhen, China) with a resolution of 2560 × 1600, a refresh rate of 60 Hz and a maximal luminance of 150 cd/m2. Subjects viewed the display dichoptically with their refractive errors corrected if necessary. In Experiment 2, stimuli were presented via an ASUS monitor (ASUS PG279Q; AsusTek Computer Inc., Taipei, Taiwan), which has a resolution of 2560 × 1440 resolution and a refresh rate of 60 Hz. Subjects viewed the display monocularly with the untested eye covered by a black opaque patch in a dark room at a viewing distance of 60 cm.
Stimuli and design
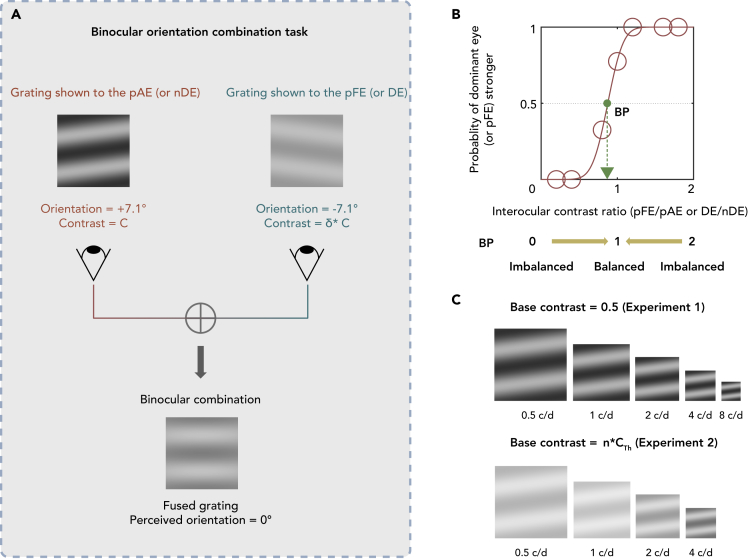
We used a binocular orientation combination paradigm (Wang et al., 2019) in this study. Sinusoidal gratings were shown to both eyes but at different contrasts and tilts (Figure 1A). Since the grating for each eye can have different contrasts, the ratio between these contrasts (i.e., interocular contrast ratio) could be diverse, ranging from almost 0 to just below 2 (Figure 1B). In this study, we presented the gratings at seven interocular contrast ratios. Throughout this paper we will use these four abbreviations to refer to each eye: pAE = previous amblyopic eye, pFE = previous fellow eye, nDE = non-dominant eye, DE = dominant eye. pAE and pFE belong to treated amblyopes, and nDE and DE belong to normal observers. pAE and nDE are considered equivalent as non-dominant eye; pFE and DE are considered equivalent as the dominant eye.
Figure 1.
The binocular orientation combination task
(A) An illustration of the binocular orientation paradigm. Two sinusoidal gratings with equal and opposite orientation of 7.1° relative to the horizontal axis were dichoptically presented to the two eyes. The orientation of the fused grating would be 0° relative to horizontal position when the two eyes were balanced. The contrast of the grating presented to the pAE (or nDE) was fixed at 50% and contrast of the grating presented to the pFE (or DE) varied between 0 and 100% with the distinct set of seven interocular contrast ratios (between 0 and 2) for each subject. pAE = previous amblyopic eye, pFE = previous fellow eye, DE = dominant eye, nDE = non-dominant eye.
(B) An illustration of the psychometric function. The binocular perceived orientation of the fused grating is plotted as a function of the interocular contrast ratio (pFE/pAE eye or DE/nDE). We used a cumulative Gaussian distribution function to fit this curve. The green point is the point of interocular contrast ratio where the proportion of the dominant eye is stronger at 50% (i.e., equal contribution between the eyes).
(C) During the Experiment 1, the contrast of the grating presented to the pAE/nDE was fixed at 50%. During the Experiment 2, the contrast of the grating presented to the pAE was fixed at the same perceived contrast across spatial frequency (i.e., 0.5, 1, 2 and 4 c/d for the pAE).
The contrast of the grating presented to pAE/nDE (i.e., non-dominant eye) was fixed at 50%. Seven interocular contrast ratios (between 0 and 2) were used. Therefore, the contrast of the grating presented to the pFE/DE (i.e., dominant eye) was varied between 0 and 100%. To remove positional bias, we used two orientation configurations of the gratings. In one configuration, the orientation was +7.1° in pAE/nDE and −7.1° in the pFE/DE. In the other configuration, the orientation was −7.1° in pAE/nDE and +7.1° in pFE/DE. Each condition (stimuli configuration and interocular contrast ratio) was repeated 20 times (i.e., 40 trials per interocular contrast ratio). In one experimental block, there were 280 trials (2 configurations x 7 interocular contrast ratios x 20 repetitions). We randomized the interocular contrast ratios and configurations in each block. Practice trials were conducted to ensure that subjects understood the task.
Only six of the ten subjects in Experiment 1 participated in Experiment 2 as the others were unable to participate due to issues with their schedules. We remeasured the balance point of subset of the treated amblyopes (n = 6) at 0.5, 1, 2 and 4 c/d. However, the contrast of the gratings presented to pAE was different than that in Experiment 1 and unique for each subject. To illustrate, the subjects were presented with stimuli of a normalized suprathreshold contrast (i.e., relative to the contrast threshold) across spatial frequency to pAE. To achieve this base normalized suprathreshold contrast for pAE (Figure 1C), we first measured the contrast threshold (1/contrast sensitivity) of each eye (pAE) at different spatial frequencies. During this measurement, the untested eye was occluded with a black opaque patch. The contrast of the stimuli during the contrast sensitivity measurement was changed via a 2- down-1- up adaptive staircase procedure, which ended at the sixth reversal point. In particular, the contrast of the stimuli was decreased proportionally by 50% before the first reversal, subsequently decreased by 12.5% when subjects properly performed two consecutive trials and increased proportionally by 25% when subjects performed one trial in error. Each staircase procedure was repeated for three times and averaged the last five reversal points of each repetition to acquire the threshold.
To establish the appropriate suprathreshold contrast across spatial frequency, we set the base contrast for pAE with the formula below, where the denominator represents the highest contrast threshold (assuming that the treated amblyopes have worst contrast sensitivity at higher spatial frequencies):
| (Equation 1) |
In this formula, base contrastx c/d is the base contrast of the pAE at 0.5, 1, 2 or 4 c/d; contrast thresholdx c/d is the contrast threshold of the pAE at 0.5, 1, 2 or 4 c/d; n was set as 1/. This ensured that for all the spatial frequencies we remeasured in Experiment 2, the base contrast of these stimuli for the pAE was of comparable visibility.
Procedure
Each trial of the orientation task had two phases. During the alignment phase, the subjects were asked to adjust the coordinates of images and achieve a perfect fusion of images shown to both eyes. Then there was the test phase during which the gratings for the orientation task were displayed indefinitely until the subjects responded.
During contrast threshold measurement, the stimulus was a horizontally oriented Gabor (±7.1°) with a sigma size of 2° and equal or opposite orientation tilts relative toward the horizontal position and shown to the tested eye for 117ms. Subjects were instructed to press a keyboard to register their response as to whether they perceived grating to be rotated clockwise or anticlockwise relative to the horizontal. In addition, an adaptive staircase procedure was used and an auditory feedback was provided.
Quantification and statistical analysis
Data analysis
We used a cumulative Gaussian distribution function to approximate the balance point, where both eyes contribute equally in binocular combination. The balance point of 1 indicates perfect binocular balance. Conversely, balance points deviating from 1 indicate binocular imbalance. The balance point was transformed into the absolute value of log10 units (|logBP|). Therefore, a value of 0 indicates perfect binocular balance. The larger the |logBP|, the larger the binocular imbalance.
Statistical analysis
All analyses were performed using IBM-SPSS 23.0 (IBM Inc., Armonk, NY, United States). Data were visualized using MATLAB and checked for normality before analysis with Shapiro-Wilk test (p > 0.05 indicates normal distribution). We used a mixed Analysis of variance (ANOVA), with subject group as a between-subject factor and spatial frequency as a within-subject factor for results of Experiment 1. Moreover, a two-way repeated measures ANOVA was used for results of Experiment 2 (spatial frequency and eye as within factors). Significance level was established at 0.05 (ex. p < 0.05 indicates rejection of the null hypothesis). Moreover, Pearson correlation, paired sample t test and Wilcoxon signed rank test were conducted for more detailed analyses.
Additional resources
Our study has not generated or contributed to a new website/forum and it is not part of a clinical trial.
Acknowledgments
This work was supported by the National Natural Science Foundation of China Grant NSFC 31970975 to JZ, the NSERC grant 228103, and an ERA-NET Neuron grant (JTC2015) to RFH. The sponsor or funding organizations had no role in the design or conduct of this research.
Author contributions
S.C., S.M., R.H., and J.Z. conceived the experiments. S.C., Z.C., Y.X., X.Y., and L.W. performed the experiments. S.C., S.M., Y.M., and J.Z. analyzed and interpreted the data, and wrote the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Declaration of interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Published: July 23, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102727.
Contributor Information
Robert F. Hess, Email: robert.hess@mcgill.ca.
Jiawei Zhou, Email: zhoujw@mail.eye.ac.cn.
Supplemental information
References
- Adrian J., Le Brun J., Miller N.R., Sahel J.A., Saillant G., Bodaghi B. Implications of monocular vision for racing drivers. PLoS One. 2019;14:e0226308. doi: 10.1371/journal.pone.0226308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attebo K., Mitchell P., Cumming R., Smith W., Jolly N., Sparkes R. Prevalence and causes of amblyopia in an adult population. Ophthalmology. 1998;105:154–159. doi: 10.1016/s0161-6420(98)91862-0. [DOI] [PubMed] [Google Scholar]
- Birch E.E. Amblyopia and binocular vision. Prog. Retin. Eye Res. 2013;33:67–84. doi: 10.1016/j.preteyeres.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard D.H. The psychophysics Toolbox. Spat. Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Cumming B.G., Parker A.J. Binocular neurons in V1 of awake monkeys are selective for absolute, not relative, disparity. J. Neurosci. 1999;19:5602–5618. doi: 10.1523/JNEUROSCI.19-13-05602.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming B.G., Parker A.J. Local disparity not perceived depth is signaled by binocular neurons in cortical area V1 of the Macaque. J. Neurosci. 2000;20:4758–4767. doi: 10.1523/JNEUROSCI.20-12-04758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dane A., Dane S. Correlations among handedness, eyedness, monocular shifts from binocular focal point, and nonverbal intelligence in university mathematics students. Percept. Mot. Skills. 2004;99:519–524. doi: 10.2466/pms.99.2.519-524. [DOI] [PubMed] [Google Scholar]
- Good W.V. Factors affecting the outcome of children treated for amblyopia. Surv. Ophthalmol. 1996;40:422–423. doi: 10.1016/s0039-6257(96)80080-4. [DOI] [PubMed] [Google Scholar]
- Grant S., Melmoth D.R., Morgan M.J., Finlay A.L. Prehension deficits in amblyopia. Invest. Ophthalmol. Vis. Sci. 2007;48:1139–1148. doi: 10.1167/iovs.06-0976. [DOI] [PubMed] [Google Scholar]
- Han C., He Z.J., Ooi T.L. On sensory eye dominance revealed by binocular integrative and binocular competitive stimuli. Invest. Ophthalmol. Vis. Sci. 2018;59:5140–5148. doi: 10.1167/iovs.18-24342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z.J., Ooi T.L., Su Y.R. Perceptual mechanisms underlying amodal surface integration of 3-D stereoscopic stimuli. Vis. Res. 2018;143:66–81. doi: 10.1016/j.visres.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen T., Vinken P.M. Monocular and binocular vision in the performance of a complex skill. J. Sports Sci. Med. 2011;10:520–527. [PMC free article] [PubMed] [Google Scholar]
- Hess R.F., Bradley A. Contrast coding in amblyopia is only minimally impaired above threshold. Nature. 1980;287:463–464. doi: 10.1038/287463a0. [DOI] [PubMed] [Google Scholar]
- Hess R.F., Mansouri B., Thompson B. A binocular approach to treating amblyopia: antisuppression therapy. Optom. Vis. Sci. 2010;87:697–704. doi: 10.1097/OPX.0b013e3181ea18e9. [DOI] [PubMed] [Google Scholar]
- Hess R.F., Mansouri B., Thompson B. A new binocular approach to the treatment of Amblyopia in adults well beyond the critical period of visual development. Restor. Neurol. Neurosci. 2010;28:793. doi: 10.3233/RNN-2010-0550. [DOI] [PubMed] [Google Scholar]
- Hess R.F., Mansouri B., Thompson B. Restoration of binocular vision in amblyopia. Strabismus. 2011;19:110–118. doi: 10.3109/09273972.2011.600418. [DOI] [PubMed] [Google Scholar]
- Hess R.F., Thompson B. New insights into amblyopia: binocular therapy and noninvasive brain stimulation. J. AAPOS. 2013;17:89–93. doi: 10.1016/j.jaapos.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Hess R.F., Thompson B. Amblyopia and the binocular approach to its therapy. Vis. Res. 2015;114:4–16. doi: 10.1016/j.visres.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Holopigian K., Blake R., Greenwald M.J. Clinical suppression and amblyopia. Invest. Ophthalmol. Vis. Sci. 1988;29:444–451. [PubMed] [Google Scholar]
- Hou F., Huang C.B., Liang J., Zhou Y., Lu Z.L. Contrast gain-control in stereo depth and cyclopean contrast perception. J. Vis. 2013;13:3. doi: 10.1167/13.8.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Tao L., Zhou Y., Lu Z.L. Treated amblyopes remain deficient in spatial vision: a contrast sensitivity and external noise study. Vis. Res. 2007;47:22–34. doi: 10.1016/j.visres.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Hubel D.H., Wiesel T.N. Receptive fields and functional architecture of monkey striate cortex. J. Physiol. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D.H., Wiesel T.N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D.H., Wiesel T.N., Levay S. Functional architecture of area 17 in normal and monocularly deprived macaque monkeys. Cold Spring Harb. Symp. Quant. Biol. 1976;40:581–589. doi: 10.1101/sqb.1976.040.01.054. [DOI] [PubMed] [Google Scholar]
- Hubel D.H., Wiesel T.N., Levay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Johansson J., Pansell T., Ygge J., Seimyr G. Monocular and binocular reading performance in subjects with normal binocular vision. Clin. Exp. Optom. 2014;97:341–348. doi: 10.1111/cxo.12137. [DOI] [PubMed] [Google Scholar]
- Kelly K.R., Jost R.M., Dao L., Beauchamp C.L., Leffler J.N., Birch E.E. Binocular iPad game vs patching for treatment of amblyopia in children: a randomized clinical trial. JAMA Ophthalmol. 2016;134:1402–1408. doi: 10.1001/jamaophthalmol.2016.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVay S., Wiesel T.N., Hubel D.H. The development of ocular dominance columns in normal and visually deprived monkeys. J. Comp. Neurol. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Li J., Spiegel D.P., Hess R.F., Chen Z., Chan L.Y., Deng D., Yu M., Thompson B. Dichoptic training improves contrast sensitivity in adults with amblyopia. Vis. Res. 2015;114:161–172. doi: 10.1016/j.visres.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Li J., Thompson B., Deng D., Chan L.Y., Yu M., Hess R.F. Dichoptic training enables the adult amblyopic brain to learn. Curr. Biol. 2013;23:R308–R309. doi: 10.1016/j.cub.2013.01.059. [DOI] [PubMed] [Google Scholar]
- Li J., Thompson B., Lam C.S., Deng D., Chan L.Y., Maehara G., Woo G.C., Yu M., Hess R.F. The role of suppression in amblyopia. Invest. Ophthalmol. Vis. Sci. 2011;52:4169–4176. doi: 10.1167/iovs.11-7233. [DOI] [PubMed] [Google Scholar]
- Loftus A., Servos P., Goodale M.A., Mendarozqueta N., Mon-Williams M. When two eyes are better than one in prehension: monocular viewing and end-point variance. Exp. Brain Res. 2004;158:317–327. doi: 10.1007/s00221-004-1905-2. [DOI] [PubMed] [Google Scholar]
- Loshin D.S., Levi D.M. Suprathreshold contrast perception in functional amblyopia. Doc. Ophthalmol. 1983;55:213–236. doi: 10.1007/BF00140810. [DOI] [PubMed] [Google Scholar]
- Loudon S.E., Simonsz H.J. The history of the treatment of amblyopia. Strabismus. 2005;13:93–106. doi: 10.1080/09273970590949818. [DOI] [PubMed] [Google Scholar]
- May K.A., Zhaoping L. Efficient coding theory predicts a tilt aftereffect from viewing untilted patterns. Curr. Biol. 2016;26:1571–1576. doi: 10.1016/j.cub.2016.04.037. [DOI] [PubMed] [Google Scholar]
- McKee S.P., Levi D.M., Movshon J.A. The pattern of visual deficits in amblyopia. J. Vis. 2003;3:380–405. doi: 10.1167/3.5.5. [DOI] [PubMed] [Google Scholar]
- McKnight A.J., Shinar D., Hilburn B. The visual and driving performance of monocular and binocular heavy-duty truck drivers. Accid. Anal. Prev. 1991;23:225–237. doi: 10.1016/0001-4575(91)90002-m. [DOI] [PubMed] [Google Scholar]
- Mitchell D.E., Duffy K.R. The case from animal studies for balanced binocular treatment strategies for human amblyopia. Ophthalmic Physiol. Opt. 2014;34:129–145. doi: 10.1111/opo.12122. [DOI] [PubMed] [Google Scholar]
- Mitchell D.E., Kennie J., Duffy K.R. Preference for binocular concordant visual input in early postnatal development remains despite prior monocular deprivation. Vis. Res. 2011;51:1351–1359. doi: 10.1016/j.visres.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Neri P., Bridge H., Heeger D.J. Stereoscopic processing of absolute and relative disparity in human visual cortex. J. Neurophysiol. 2004;92:1880–1891. doi: 10.1152/jn.01042.2003. [DOI] [PubMed] [Google Scholar]
- Olson C.R., Freeman R.D. Progressive changes in kitten striate cortex during monocular vision. J. Neurophysiol. 1975;38:26–32. doi: 10.1152/jn.1975.38.1.26. [DOI] [PubMed] [Google Scholar]
- Pelli D.G. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat. Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Shatz C.J., Stryker M.P. Ocular dominance in layer IV of the cat's visual cortex and the effects of monocular deprivation. J. Physiol. 1978;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedamurthy I., Nahum M., Bavelier D., Levi D.M. Mechanisms of recovery of visual function in adult amblyopia through a tailored action video game. Sci. Rep. 2015;5:8482. doi: 10.1038/srep08482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., He Z., Liang Y., Chen Y., Gong L., Mao Y., Chen X., Yao Z., Spiegel D.P., Qu J. The binocular balance at high spatial frequencies as revealed by the binocular orientation combination task. Front. Hum. Neurosci. 2019;13:106. doi: 10.3389/fnhum.2019.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber A.L., Schmid K.L., Baldwin A.S., Hess R.F. Suppression rather than visual acuity loss limits stereoacuity in amblyopia. Invest. Ophthalmol. Vis. Sci. 2020;61:50. doi: 10.1167/iovs.61.6.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber A.L., Wood J.M., Thompson B. Fine motor skills of children with amblyopia improve following binocular treatment. Invest. Ophthalmol. Vis. Sci. 2016;57:4713–4720. doi: 10.1167/iovs.16-19797. [DOI] [PubMed] [Google Scholar]
- Wiesel T.N., Hubel D.H. Effects of visual deprivation on morphology and physiology of cells in the cat's lateral geniculate body. J. Neurophysiol. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Wiesel T.N., Hubel D.H. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wu K.T., Lee G.S. Influences of monocular and binocular vision on postural stability. J. Vestib. Res. 2015;25:15–21. doi: 10.3233/VES-150540. [DOI] [PubMed] [Google Scholar]
- Zhou J., Reynaud A., Yao Z., Liu R., Feng L., Zhou Y., Hess R.F. Amblyopic suppression: passive attenuation, enhanced dichoptic masking by the fellow eye or reduced dichoptic masking by the amblyopic eye? Invest. Ophthalmol. Vis. Sci. 2018;59:4190–4197. doi: 10.1167/iovs.18-24206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting findings of this work are provided within the manuscript (Figure 2) and its supplemental information section. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.