Abstract
Signaling through chemokine receptor, C-X-C chemokine receptor type 4 (CXCR4) regulates essential processes in normal physiology, including embryogenesis, tissue repair, angiogenesis, and trafficking of immune cells. Tumors co-opt many of these fundamental processes to directly stimulate proliferation, invasion, and metastasis of cancer cells. CXCR4 signaling contributes to critical functions of stromal cells in cancer, including angiogenesis and multiple cell types in the tumor immune environment. Studies in animal models of several different types of cancers consistently demonstrate essential functions of CXCR4 in tumor initiation, local invasion, and metastasis to lymph nodes and distant organs. Data from animal models support clinical observations showing that integrated effects of CXCR4 on cancer and stromal cells correlate with metastasis and overall poor prognosis in >20 different human malignancies. Small molecules, Abs, and peptidic agents have shown anticancer efficacy in animal models, sparking ongoing efforts at clinical translation for cancer therapy. Investigators also are developing companion CXCR4-targeted imaging agents with potential to stratify patients for CXCR4-targeted therapy and monitor treatment efficacy. Here, pre-clinical studies demonstrating functions of CXCR4 in cancer are reviewed.
Keywords: chemokine, CXCL12, CXCR4 antagonist, CXCR4 inhibitor, radioimaging, tumor
1 |. INTRODUCTION:
CXCR4 AND CANCER
C-X-C chemokine receptor type 4 (CXCR4), also known as CD184, is a member of the seven-transmembrane (G-protein coupled) receptor family, the largest class of cell surface receptors and the targets of ∼35% of all approved drugs.1 CXCR4 signaling critically regulates essential processes in normal physiology, including embryogenesis, tissue repair, and hematopoiesis. Homozygous deletion of CXCR4 in mice causes embryonic lethality due to widespread defects, affecting vascularization of the gastrointestinal tract, generation of B lymphocytes and myeloid cells, formation of the cerebellum, and ventriculoseptal defects in the heart.2 Conditional knockout of CXCR4 in mice reveals even more functions in development, such as myogenesis, innervation of limbs, and formation of renal vasculature.3,4 In adults, high expression of CXCR4 occurs in bone marrow (BM) and multiple cell types in the immune system, with modest levels in most other tissues and organs. CXCR4 on hematopoietic stem cells (HSC) controls homing and retention of these cells in the BM, a function that has been targeted therapeutically to mobilize these cells into the circulation for recovery and transplantation.5 Expression of CXCR4 on more differentiated immune cells controls homeostatic trafficking for immune surveillance and host defense. CXCR4 on T lymphocytes also serves as a co-receptor for some strains of human immunodeficiency virus. Essential functions of CXCR4 in immunity contribute to pathogenesis of multiple common diseases, including cancer, autoimmunity, atherosclerosis, and neurodegeneration.6–9
Beyond regulation of immunity, CXCR4 on cancer cells directly enhances multiple steps in tumor initiation, growth, and metastasis. Müller et al. first discovered that CXCR4 promotes organ-specific patterns of breast cancer (BC) metastasis to lung, liver, bone, and brain. These sites all express high levels of C-X-C motif chemokine 12 (CXCL12, also known as stromal cell-derived factor 1), the chemokine ligand for CXCR4.10 Blocking CXCR4 with neutralizing Abs markedly reduced metastatic BC in mice, highlighting CXCR4 as a therapeutic target to block or prevent metastasis. Subsequent studies expanded functions of CXCR4 on cancer cells to local growth of tumors in orthotopic mouse models of BC and established high levels of CXCR4 as a marker of poor prognosis in patients with BC.11–13 CXCR4 is reported to enhance local tumor growth, invasion, and metastasis in >20 different cancers,6 pointing to CXCR4 as a general driver of human malignancies. Functions of CXCR4 in cancer typically result from up-regulation of a normal, non-mutated receptor. However, congenital or de novo mutations that truncate the intracellular C-terminus of CXCR4, resulting in defective internalization and amplified signaling, occur in warts, hypogammaglobulinemia, immunodeficiency, and myelokathexis (abnormal retention of neutrophils in bone marrow) (WHIM) syndrome.14 Truncation of the CXCR4 C-terminus also occurs as a somatic mutation in the hematologic malignancy, Waldenström’s macroglobulinemia (WM) as described in Section 2.15 Discoveries about CXCR4 also sparked a new area of research about chemokine receptors in cancer. Ongoing studies continue to link multiple chemokine receptors to hallmark features of cancer, including proliferation, survival, and metabolism.16
Environments in primary tumors and metastases highlight interconnections between cancer and stromal cells that activate CXCR4 with multiple direct and indirect effects on tumor progression. Using BC as an example, carcinoma-associated fibroblasts (CAFs) in a primary breast tumor secrete chemokine CXCL12,17–19 driven in part by cytokine signals from cancer cells in a feed-forward loop that directly enhance survival, proliferation, and invasion of malignant cells.20 CXCL12 secreted by CAFs also selects BC cells primed to metastasize to bone, another environment rich in CXCL12.21 Hypoxia, a feature common to both primary and metastatic tumors, drives transcription of both CXCL12 and CXCR4 to further stimulate tumor growth and metastasis.22–24 CXCR4 is expressed on both immunosuppressive and effector populations of immune cells, so signaling through this receptor regulates the balance of pro- and antitumor leukocytes recruited to a tumor. CXCR4 promotes tumor angiogenesis by recruiting endothelial progenitor cells to a tumor and/or amplifying pro-angiogenic effects of molecules such as vascular endothelial growth factor (VEGF).25,26 In response to chemotactic gradients of CXCL12, CXCR4 promotes local invasion, intravasation, and extravasation of cancer cells from the vasculature into environments with high levels of CXCL12. Within the BM, CXCR4 enables disseminated cancer cells to displace HSC from CXCL12-rich vascular niches, an environment that establishes dormancy and protects malignant cells from chemotherapy.27,28 These findings highlight integrated, multifaceted functions of CXCR4 in tumor environments and emphasize both challenges and opportunities to improve cancer therapy by targeting CXCR4.
2 |. ACTIVATING MUTATIONS IN CXCR4:
WHIM SYNDROME AND WM
Both WHIM syndrome and WM share mutations in CXCR4 that truncate the intracellular C-terminus of the receptor. Such mutations prevent ligand-induced internalization of CXCR4, resulting in higher levels of cell surface receptor and amplified signaling. Mutant CXCR4 enhances and prolongs activation of AKT and extracellular signal-regulated kinases (ERK), as well as reducing apoptosis.29 Truncated CXCR4, or amino acid mutations that mimic effects of truncation, impairs stability of the immunologic synapse between T cells and APCs.30 In mouse and zebrafish models, expressing truncated CXCR4 in HSCs increases bone marrow engraftment while impairing release of leukocytes (lymphocytes and neutrophils) into the peripheral circulation.31–33 These phenotypes reproduce clinical abnormalities in patients with WHIM syndrome. Immune defects caused by truncated CXCR4 account for increased frequencies of bacterial and viral infections in patients with WHIM syndrome. Infection with human papilloma virus (HPV) leads not only to warts but also to squamous cell carcinomas, such as cervical and head and neck cancers.34 Similar truncating mutations in CXCR4 occur in ∼30% of patients with WM, an indolent lymphoma marked by expansion of immunoglobulin (Ig) M-producing lymphoplasmacytic cells in BM.35–37 Mutations in CXCR4 confer resistance to ibrutinib, the only drug approved for WM.38 Effects of amplified CXCR4 in WHIM syndrome and WM point to key signaling pathways and processes that contribute to tumor-promoting effects of CXCR4 in other malignancies.38
3 |. CXCR4 SIGNALING AND CANCER STEM CELLS
Regulation of CXCR4 signaling occurs at multiple levels (Fig. 1). Chemokine CXCL12 was the first ligand identified for CXCR4.39 For many years, investigators regarded CXCL12 as the only ligand for CXCR4, based in large part on the nearly identical phenotypes of mice with genetic knockout of either CXCL12 or CXCR4.40 However, recent studies have revealed unexpected complexity and diversity of ligands for CXCR4. Alternative splicing generates six biochemically distinct isoforms of CXCL12 (α, β, γ, δ, ε, and ϕ). CXCL12-γ has an extended C-terminus with a high number of positively charged amino acids that confer nanomolar binding affinity to glycosaminoglycans.41–44 CXCL12-γ normally is expressed predominantly in heart and brain,43 but transcripts for all isoforms have been identified in human BCs.44 Various isoforms of CXCL12 vary in kinetics, amplitude, preferential activation of β-arrestin versus G protein signaling outputs, and outputs such as chemotaxis.41,43,45,46 Notably, CXCL12-γ increases metastasis in BC and castration-resistant prostate cancer (PCa) in part by increasing frequencies of cancer stem cells.41,47
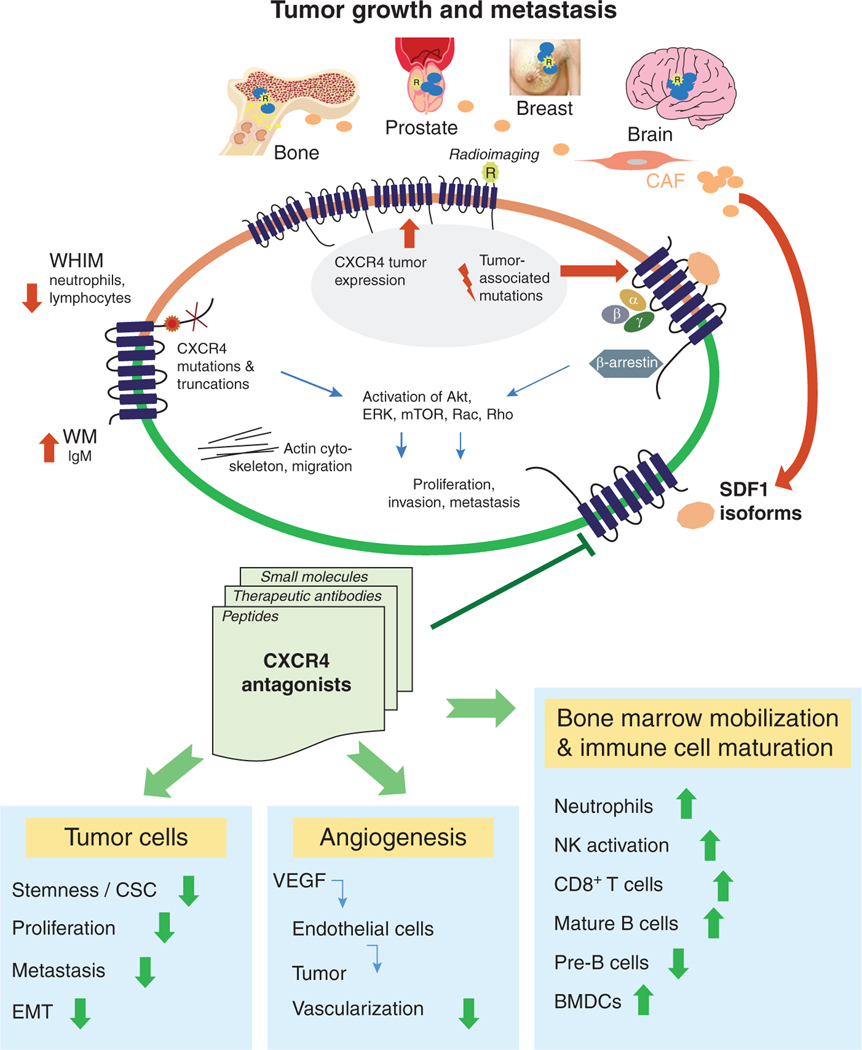
FIGURE 1. CXCR4 signaling promotes tumor growth and organ-specific metastasis.
CXCR4 signaling promotes tumor growth in primary tumors from sites including prostate, breast, and brain. Various isoforms of CXCL12 produced by carcinoma-associated fibroblasts in a primary tumor environment activates CXCr4 signaling through G protein and β-arrestin-dependent pathways. CXCR4 activates Akt, ERK, mTOR, Rac, Rho, and other effector molecules to drive survival, proliferation, and actin assembly for migration and invasion. Truncating mutations in CXCR4 as present in WHIM syndrome increase signaling through the receptor and reduced circulating neutrophils and lymphocytes. CXCL12-CXCR4 signaling promotes metastasis to sites including lung, liver, brain, and bone. Inhibitors of CXCR4 reverse aggressive features of cancer cells, angiogenesis, and abnormalities in circulating leukocytes. Targeted imaging agents can detect expression of CXCR4, potentially informing selection of patients and measurements of treatment efficacy
CXCL12 exists as monomers and dimers that also produce divergent CXCR4 signaling responses, likely dependent upon cell type and environmental context.48,49 The nuclear protein high mobility group box 1 released from necrotic cells forms a heterocomplex with CXCL12 that induces conformational changes in CXCR4 distinct from those produced by CXCL12 alone.50 In addition, extracellular ubiquitin, CXCL14, and Mϕ migration inhibitory factor (MIF) activate aspects of CXCR4 signaling in at least some cell types, likely through allosteric effects on the receptor.51–54 The extent to which different single ligands or combinations of agonists determine activation of specific CXCR4 signaling pathways and/or functions requires further investigation under physiologic and pathophysiologic conditions.
Formation of CXCR4 dimers, higher order homo-oligomers, and heterocomplexes with other receptors provide additional regulation of CXCR4 receptor dynamics and signaling. Although potentially dependent upon cell type, multiple techniques generally show that CXCR4 exists as a homodimer even in the absence of ligand.55–58 Homodimers of CXCR4 may occur due to confinement of the receptor within lipid rafts.59 Ligand binding increases oligomers of CXCR4 that internalize more efficiently than monomers, regulating intracellular signaling and subsequent desensitization.60–62 CXCR4 forms heterocomplexes with other chemokine receptors (CXCR7, now designated as atypical chemokine receptor 3 [ACKR3], C-C chemokine receptor type 2 [CCR2], and CCR5), and the T cell receptor [TCR]).56,63–69 ACKR3 binds CXCL12 with higher affinity than CXCR4, so cells co-expressing both receptors exhibit modified signaling outputs and functions including migration.70–73 Heterocomplexes with other chemokine receptors also cross-sensitize CXCR4 to inhibitors of the binding partner, making CXCR4 signaling and functions vulnerable to cross-inhibition.74 Similarly, CXCR4-TCR heterodimers cross-regulate cytokine responses and chemotaxis of T lymphocytes by engaging distinct downstream signaling molecules.75 Particularly in tumor environments where cells encounter multiple ligands and inhibitors, homodimers, and heterodimers of CXCR4 potentially increase plasticity of signaling and open potential opportunities to target multiple receptors and signaling pathways with a single drug.
As a seven-transmembrane receptor, CXCR4 signals through G proteins. CXCR4 and other chemokine receptors predominantly activate Gαi, resulting in release of intracellular calcium, activation of pathways including MAPK/ERK and PI3K/AKT/mammalian target of rapamycin (mTOR), and inhibition of adenyl cyclase and cyclic adenosine monophosphate.76 However, CXCR4 may signal through other Gα proteins, such as Gα 13.77 CXCL12 binding to CXCR4 causes phosphorylation of the receptor and recruitment of adapter protein β-arrestin 2, causing receptor internalization and activation of arrestin-dependent signaling to ERK. A recent study suggests biased antagonists that selectively block CXCR4-dependent signaling to G proteins but not β-arrestin 2 can overcome tolerance to chronic inhibition of this receptor.78 Activation of ERK, AKT, and mTOR promotes proliferation, survival, and protein synthesis, all of which enhance tumor initiation and metastasis. CXCR4 activates small guanosine triphosphatases such as Rac1 and Rho to remodel the actin cytoskeleton, an essential step in cell motility and chemotaxis. CXCR4/CXCL12 signaling activates integrins necessary for cell adhesion to the extracellular matrix (ECM) and endothelial cells, although CXCL12 also may allosterically activate integrins independent of CXCR4.79 Functions of these downstream effectors could collectively or independently promote CXCR4-mediated tumor progression and metastasis.
CXCR4 marks cancer stem cells (CSCs) in malignancies including breast, lung, esophagus, stomach, prostate, and glioblastoma,80–84 and CXCL12-CXCR4 signaling through pathways such as ERK leads to invasion and metastasis. CSCs, also referred to as tumor-initiating cells, define a dynamic sub-population of malignant cells with enhanced capabilities for self-renewal, tumor formation, metastasis, and differentiation into the full range of cancer cell types present in a primary or metastatic tumor. A variety of resistance mechanisms enable CSCs to survive standard chemotherapy drugs and radiation, making these cells a key cause of treatment failure and recurrent cancer. Expression of CXCR4 on CSCs is consistent with clinical data showing that upregulation of CXCR4 typically correlates with poor prognosis across malignancies. Up-regulation of CXCR4 through mechanisms including PI3K/AKT/mTOR signaling and transcription activated by ΔNP63α, the predominant isoform of TP63 in epithelium, increases abundance and phenotypes of CSCs.80 Activation of CXCR4 stimulates key functions of CSCs, including self-renewal, local invasion, and dissemination to secondary organs and tissues.85–87 In pancreatic cancer, CXCR4 demarks a highly metastatic subset of CSCs.88 Key functions of CXCR4 in CSCs suggest that inhibiting CXCR4 signaling may be an effective strategy to reduce or eliminate CSCs in multiple malignancies.
4 |. CXCR4 IN THE TUMOR MICROENVIRONMENT
The tumor microenvironment (TME) describes all components of a tumor mass, including malignant cells, ECM, immune cells, mesenchymal stem/stromal cells, fibroblasts, vasculature, metabolic products, and signaling molecules.89,90 The TME shapes different phases of cancer, from initiation to metastasis, with reciprocal interactions between cancer cells and surrounding cellular and biophysical components that establish conditions permissive or restrictive to tumor progression.90 The tumor immune microenvironment (TIME) defines the immunologic environment composed of infiltrating immune cells and their secreted products (cytokines, chemokines, growth factors, etc.). Chemokines expressed in the TIME regulate polarization of immune cells and the overall balance of immunosuppressive T-regulatory cells (Tregs), some subgroups of T helper 17 cells (Th17), myeloid derived suppressor cells (MDSCs), and M2 Mϕs versus effector NK and CD8+ T cells.91 Recruited leukocytes, along with endothelial cells and fibroblasts, make up the stromal cell compartment, and these stromal cells interact with tumor cells to modulate the TME, where both chemokines and chemokine receptors participate in this process.92 Emerging evidence identifies chemokine CXCL12 as one of the most important factors controlling recruitment of immunosuppressive cells to the TIME.91,93–95 CXCL12, particularly CXCL12-γ, binds to glycosaminoglycans (GAGs) on the surface of endothelial cells, forming chemotactic gradients that promote migration of leukocytes and cancer cells.96–98 In the following sections, we describe functions of CXCR4 in various components of tumor environments, with a particular emphasis on tumor immunity.
4.1 |. CXCR4 in endothelial cells
Endothelial cells express both CXCR4 and CXCL12, and this receptor-ligand interaction facilitates intravasation and extravasation of cancer cells as well as tumor angiogenesis through a process that involves integrin activation, resulting in enhanced adhesion of CXCR4 expressing cells to the endothelium during extravasation or intravasation.99–101 Tumor growth and metastasis require angiogenesis and increased vascularization; otherwise, tumor cells may replicate rapidly, but their growth and metastasis are restricted by inadequate blood supply.102 Cancer cells stimulate angiogenesis through the CXCR4/CXCL12 signaling pathway, leading to tumor neovascularization, growth, and progression to metastasis. Owing to potential clinical benefits of therapeutically manipulating tumor angiogenesis, the mechanisms controlling this process attracted scientists to focus on vascular research over the past two decades. Blood vessel formation by angiogenesis is a complex multistep process that critically requires tight control and coordination of endothelial cells. Angiogenesis is a process including ECM dissolution, endothelial cell mitoses, and new branches sprouting into lumens to form a complex vessel network.103,104 During the process, endothelial cells interact together in a process termed “tip-stalk cell selection.”103 The endothelial tip senses attractive and repulsive signals and extends filopodia, leading sprouts toward the angiogenic chemokines, whereas the stalk cells, trailing behind the tip cells, facilitate lumen formation and mitosis to support sprout elongation.105 Angiogenesis is orchestrated not only by VEGF, but also by CXCR4/CXCL12. CXCR4 facilitates angiogenesis by recruitment of endothelial progenitor cells or BM-derived accessory cells. VEGF promotes sprouting angiogenesis by inducing tip cell filopodia and serving as an attraction cue,106 whereas the CXCR4/CXCL12 axis stimulates tip cells and migration in neovascular sprouting.107 Thus, sprouting endothelial cells are hierarchically organized into leading tip cells and trailing stalk cells that exhibit very distinct and specialized cell behaviors. These studies indicate critical roles for CXCR4 in endothelial tip cell behavior in vitro,108–110 ex vivo, and in vivo.111
4.2 |. CXCR4 in bone marrow myeloid cells
CXCR4/CXCL12 signaling regulates trafficking and tissue localization of human HSC to hematopoietic organs.10,101 CXCR4 expressed on myeloid cells binds CXCL12 that is presented to the receptor by GAGs in the BM microenvironment.10,101 Once bound to ligand, CXCR4-expressing BM myeloid cells remain tethered to GAG-bound CXCL12 in the BM. CXCR4 antagonists break this tethered interaction and release CXCR4-expressing cells into the vascular system.112,113 Recently in an in vivo model of CXCR4 genetic knockout in myeloid cells, Yang et al. demonstrated that disruption of the CXCR4/CXCL12 axis in these cells led to increased neutrophil but not monocyte release from BM, and enhanced NK-mediated antitumor immune response, significantly reducing melanoma tumor growth. The observation from the GEM model that targeted deletion of CXCR4 in myeloid cells results in increased circulating neutrophils is in agreement with prior studies by Devi et al. who demonstrated that CXCR4 antagonism with the Food and Drug Administration (FDA)-approved small molecule plerixafor results in a statistically significant increase in neutrophils in peripheral blood due to the mobilization of neutrophils from a marginated pool in the lung and prevention of the return of neutrophils to bone marrow.114 Yang et al. did not observe pan leukocyte mobilization in the CXCR4myexΔ/Δ mice or with treatment with systemic treatment with the LY2510924 CXCR4 antagonist but did observe an increase in the total percentage of CD45+ cells in peripheral blood that were neutrophils or NK cells. Either genetic deletion of CXCR4 in myeloid cells or systemic inhibition of CXCR4 with LY2510924 increased the population of circulating neutrophils that release IL-18 to activate NK cells, resulting in enhanced antitumor immunity.115 Jaeger et al. suggested neutrophils may play a role as non-redundant regulatory cells ensuring terminal maturation of NK cells, which occurs not only in the BM but also in the periphery, where neutrophils can interact with NK cells.116 IL-18 can activate NK cells to release IFN-γ.117 Hence, neutrophils offer a significant source of IL-18, which increases the NK cell population and their activation in myeloid CXCR4 knockout mice.115 In contrast, plerixafor treatment of healthy control or patients with severe leukopenia in myelokathexis or WHIM syndrome resulted in ∼3-fold increase in neutrophils, monocytes, and lymphocytes in peripheral blood.118 In another study, treatment of 3 WHIM syndrome patients with plerixafor reduced myelofibrosis, panleukopenia, anemia, thrombocytopenia, and wart burden.112,113 Moreover delivery of the CXCR4 antagonist plerixafor after HSC transplant mobilized residual recipient cells into the circulation and enhanced the proliferation and engraftment of HSCs.119,120 Differences observed in response to either treatment with LY2510924 or myeloid CXCR4 deletion and treatment with plerixafor may be due to mechanisms by which these 3 ways of inhibiting CXCR4 take place.
4.3 |. CXCR4 in dendritic cells
Dendritic cells (DCs) are potent APCs in the immune system, especially for maturation and activation of T cells.121,122 Maturation of DCs is induced by cytokines such as TNF-α, LPS, IL-1β, and CD40 ligand. These cytokines induce expression of CXCR4 in murine BM-derived DCs (BMDCs) and skin Langerhans cells (LCs).123,124 BMDCs produce CXCL12 that interacts with DCs in an autocrine manner to promote DC maturation and survival.123,125 CXCR4 antagonist treatment results in reduction of mature, but not immature, BMDCs and LCs. CXCL12 enhances the proliferative response of BMDCs, which is suppressed by a CXCR4-antagonist. Thus, the CXCR4/CXCL12 signaling axis controls survival and maturation of DCs.126 High numbers of plasmacytoid DCs have been observed in human ovarian carcinoma, BC metastases, and lymphoma due to high levels of CXCL12 that attract DCs into the TME.127–129
4.4 |. CXCR4 in Tregs
Immune cell progenitors develop and differentiate in primary lymphoid organs such as BM, thymus, and fetal liver. CXCR4/CXCL12 signaling regulates thymocyte trafficking inside the thymus and leads to migration of T cells to lymph nodes (LNs).130,131 In a TME, the anti-tumor immune response changes drastically during tumor progression. Cancer progression is often accompanied by escape from the host immune system. CD4+CD25+ Tregs are increased and linked to compromised immune responses in patients with multiple solid cancers. Tregs are identified through expression of CD4, CD25, FOXP3, CD127, and CD45RA. Also, members of the CD28/cytotoxic T lymphocyte-associated Ag 4 (CTLA-4) and CD39/ENTPD1 families define a highly suppressive Treg population.132 CTLA-4 and programmed death-1 (PD-1) have been proposed as critical molecules in the generation of suppressive function of Tregs.133 Tregs suppress a range of immune cells134 and are associated with poor prognosis in solid cancers such as renal,135 ovarian,136 pancreatic,137 and liver.138 Tregs derived from cancer patients usually express a distinct profile of chemokine receptors such as CXCR4 and can migrate toward a gradient of CXCL12 produced in a TME.139,140 Antagonists of CXCR4 reduce infiltration of Foxp3 cells into a tumor and enhance responses to anti-PD-1 therapy.141
4.5 |. CXCR4 in CD8+ T cells
Adaptive immunity with a capacity to develop long-lived memory T cells counters cancers and infection. Memory CD8+ T cells are classified into two main subsets: central memory (CD44hi CD62Lhi) cells and effector memory (CD44hi CD62Llo) cells. Central memory cells, which preferentially home to secondary lymphoid organs, have longer life spans and greater capacity for homeostatic proliferation than do effector memory cells.142 CXCR4 promotes homeostatic self-renewal of central memory CD8+ T cells and maintains the CD8+ T cell memory pool.143 CXCR4/CXCL12 signaling directs leukocyte migration among blood, lymph, and tissues. In addition to its chemotactic functions, CXCR4 fine-tunes immune responses. During T cell activation by APCs, CXCR4 is recruited into the immunological synapse, where it delivers costimulatory signals.63
CXCL12 controls recruitment of immunosuppressive cells.91,93–95 The first evidence that CXCR4/CXCL12 regulates T-mediated immune response was more than a decade ago when Nomura et al. demonstrated that transducing CXCL12 into 2 murine immunogenic tumor cells (fibrosarcoma and ovarian cancer, Meth A and HM-1) increased infiltration of CD4+ and CD8+ T cells and antitumor immune responses.144 Dunussi-Joannopoulos then demonstrated that CXCL12 secreted at the tumor site by genetically modified murine tumor cells regulated the in vivo priming and effector phase of immune responses required for successful rejection of tumors, and supported development of long-lived tumor-specific CTL responses.145 The most updated model of melanoma demonstrated that CXCL12 has a bimodal effect on CXCR4-expressing T effector cell migration. Low CXCL12 serves as T cell chemoattractant, while high CXCL12 concentrations can repel T cells via a mechanism termed chemorepulsion or fugetaxis. This chemoattraction mechanism contributes to the physiological process of T cell migration from the thymus. Moreover, repelling T effector cells inside the TME may represent a mechanism by which high CXCL12-expressing tumors evade the immune system.146–148 CXCR4 activation by a recombinant CXCL12 agonist significantly reduced IL-23 and IL-12, cytokines that polarize to Th17 and Th1 states, respectively.149 These results show that CXCL12 is not only involved in attracting APCs and T cells but also in directing their anti-inflammatory properties, potentially explaining how tumors that produce high levels of CXCL12 evade the immune system.
Reflecting on the concept of fugetaxis leads one to ask how it is that recirculating T cells manage to migrate into BM or LN where there are fairly high concentrations of CXCL12. Indeed, CXCR4 expressing T cells recirculate back to the BM, and extravasate from the blood back into BM where they are retained. Here, the concentration of CXCL12 in the blood is lower than in the bone marrow, so T cells are able to migrate into the BM or LN tissue following the CXCL12 gradient. Then, a CXCL12/CXCR4 and integrin interaction keeps the T cells in the BM or LN. Egress from LN or BM does not involve a CXCL12 gradient but involves sphingosine-1-phosphate (S1P) interaction with its receptor S1PR1.150 Since the levels of S1P are lower in the BM and LN than in the blood, CD4 and CD8+ T cells can respond to a S1P concentration gradient and be recruited into the blood. However, this whole process is quite complicated and can be offset by CD69 that mediates retention of memory CD4+ T cells in the BM.150–153
4.6 |. CXCR4 in B cells
During infection, B cells respond by maturing and differentiating into plasma cells. CXCR4 signaling is involved in late B cell lymphopoiesis, whereby CXCR4 activation of MAPK signaling leads to development of small pre- and immature B cells that then stop dividing and initiate Igk recombination as well as expression of the B cell receptor.154 Plasma cells secrete Abs (Igs) that help T lymphocytes attack and kill targets. Plasma cells express CXCR4 and reside mainly in the BM and lamina propria of epithelia. As with myeloid cells, CXCR4/CXCL12 signaling holds B cells in the BM, and antagonism of this ligand-receptor interaction releases B cells into the peripheral blood. Intestinal plasma cells are IgA-producing cells and contribute to the maintenance of gut homeostasis. Plasma cells producing IgG Abs are highly abundant, and their numbers increase in response to infection, chronic inflammation, and autoimmune diseases.155 CXCR4 directs infiltration of IgG plasma cells and accumulation into inflamed mucosa. This process mediates pathogenesis by exacerbating mucosal inflammation.156 When plasma cells become cancerous, multiple myeloma (MM) develops. CXCR4/CXCL12 is a critical regulator of MM cell migration and homing through activation of PI3K and ERK/MAPK pathways, but not p38 MAPK.157 These data imply that CXCR4 might be a target for the abrogation of metastasis by MM cells. Moreover, CXCR4, MYD88, and ARID1A somatic mutations are often found in patients with WM as outlined in Section 2 of this review. CXCR4 mutations led to diminished B cell differentiation and a reduction in the mutant MYD88-mediated expression of tumor suppressors.158 Interestingly, CXCR4 and CCR7 are expressed at higher levels in stage IV than in stage 0 B cell chronic lymphocytic leukemia (B-CLL) patients.159 CXCR4 receptors play a key role in B-CLL and B cell acute lymphoblastic leukemia where they are involved in homing and in generating signals that yield these cancers resistant to ibrutinib.160–162 Recruitment of B cells into developing tumors promotes tumor progression, thus indirectly implicating a role for CXCL12 and CXCR4 due to expression of CXCR4 on B cells. Both recruitment of B-regulatory cells and antibodies produced by B cells have been associated with enhanced tumor growth and tumor progression.163–165
4.7 |. CXCR4/CXCL12 in TIME
The ability to evade immune responses constitutes a hallmark feature of cancer.166–168 In fact, persons with genetic immunodeficiency or chronic immunosuppression exhibit a higher incidence of cancer.169 For example, acquired immune deficiency syndrome patients are immune deficient and frequently develop lymphoma,170 while patients with prolonged immune suppression frequently develop cancers such as Merkel cell carcinoma where immune suppression is associated with poorer overall survival.171 The incidence of cancer is elevated in patients who are immune suppressed and in those who undergo organ transplant.169,172,173
Primary resistance to immunity principally occurs due to the lack of recognition by T cells because of absence of tumor antigens or defects in antigen presentation.174,175 The lack of immune effector cells, presence of immune suppressive cells, and polarization of immune cells in the TME play a fundamental role in shifting the balance from an immune active “hot” to immune suppressive “cold” TIME.176 In progressing cancers, tumors and associated TIME are not static, but interactions between cancer and immune/stromal cells evolve with tumor progression. As a result of this process, tumor heterogeneity is not just referred to as the heterogeneity of cancer cells/clones, but also includes heterogeneity of TME and TIME.166 CXCL12 is mainly secreted into the TME from CAFs that favor a tumor permissive microenvironment as shown in Fig. 2. In BC metastases, tumor-infiltrating lymphocyte counts and PD-L1 protein expression (5-fold lower, P = 0.0004 based on quantitation from full slides) were substantially lower than in primary tumors, and this was accompanied by significant down-regulation of messenger ribonucleic acid (mRNA) expression of chemotactic and immune activating cytokines along with reduced Ag presentation and up-regulation of immune suppressive mechanisms (recruitment of M2 Mϕs) demonstrating that metastases’ TIME were immunologically inert and thus “colder” than primary tumors.177
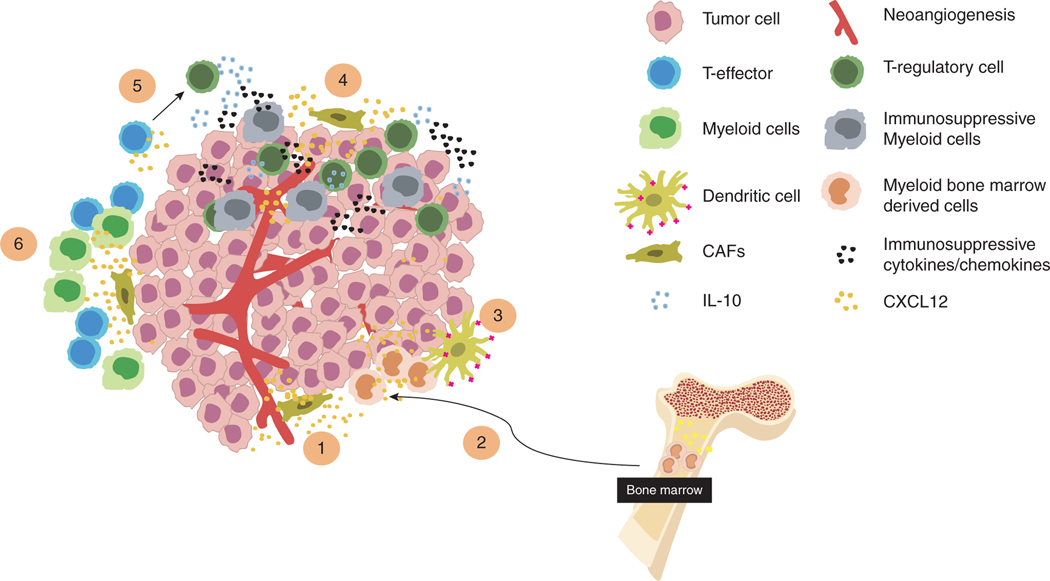
FIGURE 2. CXCR4 in the tumor microenvironment.
CAF-produced CXCL12 acts on different TME cells and regulates the recruitment of immune cells. (1) CXCR4/CXCL12 axis stimulates endothelial cells promoting neovascularization, tumor growth and metastatic progression. (2) CXCL12/CXCR4 axis regulates trafficking and tissue localization of human HSC in the BM. (3) CXCR4/CXCL12 axis recruits BM derived myeloid cells promoting DC maturation and survival. (4–6) CXCL12 recruits immunosuppressive cells and excludes effector cells designing a “colder” TME that impair immunotherapy response. CXCL12 also redirects the polarization of effector Th1 cells into CD4+CD25−Foxp3−IL 10high regulatory T cells
In the BM, CXCL12 plays a critical role in the regulation of hematopoietic stem and progenitor cells (HSPC) self-renewal, retention and differentiation, creating the so-called “HSPC niche.” CXCR4 overexpression in engineered therapeutic CD8+ T cells improves their functions and antitumor efficacy by redirecting and favoring their engraftment to the “memory niches” in BM. The BM microenvironment drives homeostatic expansion of IL-15–dependent engineered therapeutic CD8+ T cells and promotes differentiation of memory precursor-like cells with low expression of PD-1, resistance to apoptosis, and amplified capacity to generate polyfunctional cytokine-producing effector cells. Thus, following transfer to lymphoma-bearing mice, CXCR4-overexpressing engineered therapeutic CD8+ T cells showed a greater capacity for effector expansion, a memory precursor-like gene signature, increased responsiveness to IL-15, a nearly 2-fold increase in CD62Lhi effector T cells, and better antitumor capability, with a median survival of >42 days versus 31 days for controls.178
5 |. CXCR4 IN CANCER DEVELOPMENT AND PROGRESSION:
TUMOR EXAMPLES
The role of the CXCR4/CXCL12 axis is described for glioblastoma, PCa, and BC, where these pathways may modulate growth, epithelial to mesenchymal transition (EMT), CSC maintenance, metastasis, and reduced survival.
5.1 |. Gliomas and glioma stem cells
World Health Organization grade IV glioma, defined as glioblastoma multiforme (GBM), represents the most frequent and malignant primary astrocytoma, accounting for >60% of all brain tumors in adults.179,180 GBM continues to have a dismal prognosis with median survival of 14.6 months.181 GBM tumor cells commonly infiltrate healthy brain tissue,182,183 contributing to failures of current therapies. GBMs commonly have areas of necrosis and hypoxia, which increases expression of CXCL12, MIF, and CXCR4 to drive EMT through an MIF-CXCR4-AKT pathway in GBM cells.184,185 This same signaling pathway also causes GBM cells to form blood vascular channels, a process known as vascular mimicry.
Recently, the Cancer Genome Atlas categorized GBM into 4 subtypes based on gene expression patterns and clinical characteristics. CXCR4 is preferentially expressed in the mesenchymal subgroup, which contains frequent mutations in the NF1, phosphatase and tensin homolog (PTEN) and TP53 tumor suppressor genes. Patients in this subgroup have significant increases in survival after aggressive treatment. CXCR4/CXCL12 signaling regulates many other aspects of brain tumor biology, including resistance to radio- and chemotherapy, migration of cancer cells through the brain, angiogenesis, and recruitment of vascular progenitor cells.186,187 These properties suggest that CXCR4 antagonists may help control this disease. CXCR4 expression varies significantly among different subtypes of GBM and in glioblastoma stem-like cells (GSCs).188–192 Low expression of CXCR4 due to processes such as promoter hypermethylation might predict favorable overall survival.
GBM cells tend to migrate along blood vessels and white matter structures, both of which express high levels of CXCL12 in concert with other growth factors such as epidermal growth factor and platelet-derived growth factor. Migrating GBM cells show a reduced propensity to undergo apoptosis and proliferation and exhibit up-regulation of CXCR4.193,194 Since the highest levels of CXCR4 expression are associated with stemness, the poor prognosis of GBM may reflect increased CXCR4-positive GSCs. In addition, expression of CXCR4 increases in recurrent tumors compared with matched primary GBMs.182
Preclinical studies support addition of CXCR4 inhibitors to therapy in GBM. Knockdown or pharmacological inhibition of CXCR4 reduced GBM cell migration toward brain-derived endothelial cell monolayers,195 and knockdown of CXCR4 in GBM cells prior to intracranial injection in mice reduced tumor growth and perivascular invasion with increased survival. CXCR4 knockdown resulted in reduced local invasiveness.193 Similarly, these strategies increased apoptosis of cells treated with radiotherapy, prolonging median survival of mice with established GBM.196 Inhibition of CXCR4/CXCL12 signaling reduced the viability and number of GSCs197 and recruitment of glioma-associated Mϕ/microglial cells.198 In addition, the brain-penetrating CXCR4 antagonist PRX77561 inhibited growth of established tumors, local cell recruitment and inflammation in GBM. PRX77561 also induced differentiation of glioma cells.199 CXCR4 inhibitors may improve efficacy of anti-angiogenic therapy since CXCL12-CXCR4 signaling contributes to resistance to the anti-VEGF agent, bevacizumab.199 Combining PRX77561 with anti-angiogenic agents further improved control of tumor growth and overall survival.199 Similarly, the selective CXCR4 antagonist POL5551 VEGF reduced glioma growth and dissemination by inhibiting tumor cell migration and local invasion.193 POL5551, similarly to PRX177561 or plerixafor200 dose-dependently reduced hypoxia-and CXCL12-mediated migration. Combining POL5551 with a VEGF-blocking Ab significantly reduced invasion of GBM cells and vascular density to greater extent than single agents.193 Analysis of GSC and more malignant GBM cell content suggested that inhibition of CXCR4-regulated cell recruitment (tumoral stem and immune cells) accounted for improved efficacy of antiangiogenic therapy. CXCR4 antagonists also may improve treatment of brain metastases, particularly for CXCR4-positive primary tumors.
5.2 |. CXCR4/CXCL12 signaling and prostate cancer
PCa is the most common cancer in men and the second leading cause of cancer-related death among men in developed countries.201 The most common cause of mortality is not the primary tumor growth but rather its spread to other organs, predominantly bone.202 Understanding molecular mechanisms of metastasis is crucial in developing new therapy strategies.203 Recent advances in cancer biology have indicated the critical role that CXCR4/CXCL12 play in CSC renewal and metastasis of various cancers, including PCa.6,204
Some data report that PCa expresses 35x higher levels of CXCR4 than non-malignant prostate tissue, suggesting that CXCR4 expression could be a diagnostic biomarker for PCa.205,206 CXCR4 expression correlates significantly with LN or bone metastasis.207 Bone metastatic PCa cells express higher levels of CXCR4 than primary tumors, suggesting that CXCR4 expression may be a useful prognostic marker for metastasis in PCa.208,209 However, the clinical relevance of CXCR4 in PCa remains controversial, and its association with clinicopathological features remains inconclusive due to relatively small sample sizes in studies. A meta-analysis by Lee et al.210 reported that increased CXCR4 expression in PCa correlates with presence of metastasis but not tumor stage (of the TNM classification of malignant tumors, UICC TNM Project) of PCa.211 A meta-analysis by Chen, on the other hand, found significantly higher amounts of CXCR4 protein in T3–4 than in T1–2 stages of PCa, as well as being significantly associated with the presence of LN and bone metastasis.207 Darash-Yahana et al. reported that CXCR4 inhibitory Abs blocked CXCR4-dependent vascularization and growth of previously established tumors.212 Moreover, anti-CXCR4 Abs and peptide analogs disrupt tumor-stromal interactions and reduce intraosseous growth of established prostate cancer cells in mouse xenograft models of PCa.213–217 Taken together, inhibition of CXCR4/CXCL12 signaling could be a potentially beneficial addition to treatment in PCa.
Androgen deprivation treatment (ADT) is the most effective intervention for advanced and metastatic PCa. Although ∼80% patients initially respond to ADT, incurable castration-resistant PCa develops almost invariably.207,218,219 Importantly, androgen receptors continue to be critical for PCa growth and progression after ADT. It has been demonstrated that several putative consensus-binding sites for the oncogenic erythroblast transformation specific-related gene (ERG) transcription factor are present in the promoter region of CXCR4; thus, androgen-dependent regulation of ERG could induce CXCR4 expression in PCa cells. Findings also link TMPRSS2-ERG translocations and enhanced metastasis of tumor cells through CXCR4 function in PCa cells.220 Androgen-independent PCa cells commonly lose the PTEN tumor suppressor, resulting in metastasis.221,222 The pathways of CXCR4 and PTEN converge, leading to promotion and regulation of tumorigenesis, respectively. Loss of PTEN may permit CXCR4 to shift PCa to advanced disease.213 Reconstitution of PTEN in PTEN-negative PC3 cells reversed EMT and inhibited CXCR4-mediated migration and proliferation in PC3 cells, suggesting that loss of PTEN permits CXCR4-mediated functions in PCa cells.213 These data may indicate that targeting CXCR4 may improve treatment for PCa patients with resistance to androgen deprivation.
5.3 |. CXCR4/CXCL12 signaling in breast cancer
BC is the most common malignancy among females, diagnosed in nearly 1.7 million women and causing more than 40,000 deaths in the United States alone in 2019.201 CXCR4 is significantly overexpressed in BC, not only increasing metastasis but also promoting survival and proliferation of BC cells. The first evidence for this was the much smaller tumor mass resulting from subcutaneous inoculation in immunodeficient mice of low CXCR4-expressing MCF-7 BC cells than high CXCR4-expressing MDA-MB-231 cells. In syngeneic, immunocompetent mice, implanted tumor cells with RNAi-down-regulated expression of CXCR4 grew more slowly than cells with up-regulated or normal levels of CXCR4.12,223 CXCR4 facilitates growth of primary BC through mechanisms including angiogenesis as discussed, activation of numerous downstream signaling pathways (including PI3K/AKT, Src/ERK1–2, NF-κ B, STAT3, Notch, Wnt, and sonic hedgehog),224 and recruitment of immunosuppressive immune cells.225
Estrogen regulates tumorigenesis, metastasis, and drug resistance in estrogen receptor (ER)-positive BC, the most common subtype. Several studies have reported the relationship between hormone-dependent BC cell proliferation and CXCR4 signaling. CXCL12 was shown to be a target of ER action and to mediate the mitogenic effects of estradiol in ovarian and BC cells.226 Co-overexpression of ER coactivators and Her2/neu indicates poor prognosis: coactivators increase CXCL12 expression while Her2/neu stabilizes CXCR4.227 17beta-estradiol promotes CXCL12-mediated transactivation of the epidermal growth factor receptor (EGFR) transactivation, increasing proliferation of BC cells.228 CXCR4 activation transduces proliferative signals from ER to EGFR, whose inhibition reverts BC cell proliferation induced by activation of multiple receptors.228 CXCR4/CXCL12 and ERalpha/ERbeta form an autocrine signaling loop that dictates ER-dependent gene expression and growth of BC cells.229 Expression of CXCL12 in invasive BC correlates with ER status and patient prognosis.230 CXCR4 also mediates estrogen-independent tumorigenesis, metastasis, and resistance to endocrine therapy in human BC, although the study did not address overall survival.231 Thus, CXCR4 is a rational therapeutic target for ER-positive, estrogen-independent BC. More recently, it was demonstrated that estrogen stimulated CXCR4 transcription via ER-binding sites at the CXCR4 promoter, thus enhancing mediated cellular growth.232
Estrogen may also promote progression of ER-negative BC, by stimulating CAFs to secrete CXCL12, which can recruit MDSCs to the TME to exert tumor-promoting effects. ER-negative BC cells xenografted into ovariectomized mice, followed by continuous injection of estrogen, demonstrated high levels of CXCL12 and tumor-infiltrating MDSCs. Blocking CXCR4 with plerixafor prevented recruitment of MDSCs.233
CXCR4 promotes BC metastasis to organs, bone, liver, and lung, with high levels of CXCL12.217 The interaction between CXCR4 and CXCL12 makes BC cells move out of the circulation and into organs with high amounts of chemokines, thus forming metastases. CXCR4-high, but not CXCR4-low, MCF-7 human BC cells spontaneously metastasized to lung in severe combined immunodeficient mice.234 Bone metastatic BC cells in patients have increased CXCR4.235 CXCL12-producing adventitial reticular cells and other stromal cell types in bone and BM produce CXCL12, facilitating bone metastasis.236 Similarly, stromal cells in liver and lungs produce large amounts of CXCL12 to recruit BC cells and support metastases.237 Giving a peptide CXCR4 antagonist (CTCE-9908) to athymic mice prior to inoculation of GFP-MDA-MB-231 cells, while not reducing the incidence of metastasis, did decrease the metastatic burden (size of metastases) in all organs examined (lungs, bone, heart, liver, kidneys, pancreas, and spleen).235,238
A recent report found an association between tumoral CXCR4 expression in patients with locally advanced BC and increased metastases and rapid tumor progression. Moreover, high CXCR4 expression identified a group of patients with BM disseminated tumor cells at high risk for metastasis and death.239 POL5551, another peptide CXCR4 antagonist, in vitro had no direct effect on tumor cell viability, but reduced migration of BC cells. In 2 orthotopic models of triple-negative BC, POL5551 administered after formation of tumor xenografts had little inhibitory effect on primary tumor growth but significantly reduced distant metastasis. In mice after resection of the primary tumor, combination of POL5551 with the microtubule inhibitor eribulin additively reduced metastasis, and overall survival was longer than with single-agent eribulin.239
About 20–30% of BC patients with metastatic disease develop brain metastases.240–242 Disruption of the blood-brain barrier (BBB) is critical to the development of metastases.243,244 An in vitro study demonstrated that CXCL12 introduces BC cells into the brain by increasing vascular permeability. Several CXCR4 cofactors are involved in BC cell invasion. The CXCR4/CXCL12-mediated activation of PI3K/AKT and phosphorylation of focal adhesion kinase and Forkhead transcription factor FKHR-L1 were necessary for BC cell migration through the BBB.245
BC cells that metastasized to the lungs exhibited high CXCR4 expression compared with their parental cells, supporting the role of CXCR4 in organ directed-metastasis and invasion of BC. Neutralizing Abs to CXCR4 markedly inhibited metastases of BC to LNs and lung.246 Local LN involvement in BC patients has been widely investigated. Kato et al. analyzed CXCR4 expression in 79 surgically resected invasive ductal carcinomas and found that patients with high CXCR4 levels had more extensive metastasis to LNs than those with low levels.247 A recent meta-analysis13 of 13 studies (3865 participants) across the US, Canada, China, Japan, Korea, and Taiwan showed CXCR4 expression to be significantly associated with both LN status and distant metastasis and indicated poor overall and disease-free survival. Xu et al. analyzed 15 studies (3104 patients) from the US, Canada, Germany, France, China, and Japan, and found results consistent with those of Zhang, with both overall and disease-free survival significantly lower in BC patients with high CXCR4 expression than in those with low expression.248 CXCR4 thus appears to be an efficient prognostic factor for BC and promising target for therapy.
6 |. IMAGING CXCR4—BRIDGE TO PHARMACODYNAMICS IN VIVO
Given the aforementioned pivotal involvement of CXCR4/CXCL12 in various aspects of cancer, development of molecular imaging tools for CXCR4 offers the potential to identify patients with CXCR4-positive tumors for therapy and quantify pharmacodynamics of compounds. Excellent reviews summarizing the emergence of diverse CXCR4-targeted radiolabeled, fluorescently labeled and bimodal (“hybrid”) probes for in vivo imaging have been published.6,249–251 We present only the most promising classes of CXCR4-targeted imaging probes with potential clinical applications: small-molecule ligands based on plerixafor; CXCL12-derived disulfide-bridged 14 amino acid peptides based on T140; and FC-131 based cyclic pentapeptides. All three classes of imaging agents were adapted from compounds initially designed as potent inhibitors of HIV infection and subsequently investigated as therapeutic agents in oncology.
6.1 |. Bicylam tracers and small molecules
Plerixafor and the structurally related AMD3465 allow direct complexation of some metal ions, a feature exploited for radiolabeling with Cu,252,253 68Ga, or 99mTc.254–256 Both mouse and first-in-human studies with [64Cu]-plerixafor showed high levels of radiotracer accumulation in liver and BM due to endogenous expression of CXCR4 in these organs. Normal accumulation in these organs limit detection of primary and metastatic tumors in these sites and produces unwanted radiation doses.
6.2 |. T-140 based tracers
As described in “Targeting CXCR4 therapeutically” below, research in the 1990s yielded polyphemusin II analogs with anti-HIV activity and CXCR4 binding affinity: T22, T140, and then more stable analogs with improved CXCR4 affinity and anti-HIV potency, for example, FB-TN14003 and Ac-TZ14011.257 These second generation T140 analogs represent the targeting agent for CXCR4-targeted imaging probes (nuclear and/or fluorescent).255,258–265 Despite substantial differences in binding affinity (half maximal inhibitory concentration values in the range of 2–200 nM), preclinical evaluation of diverse T140-based imaging probes invariably showed reasonably specific uptake in CXCR4-expressing cancer cells in vitro and in vivo in mouse xenograft models. One feature, however, that is common to all T140-based molecular imaging agents developed so far, is their high accumulation in non-target tissues; this includes strong binding to blood cells and high uptake in liver, kidney, intestines, and spleen. These results suggest a contribution of CXCR4-mediated tracer uptake to background activity accumulation, particularly in the liver, and is in line with results with the different radiolabeled AMD analogs. Thus, although having undisputed potential as tools for in vitro characterization and quantification of CXCR4 expression, the in vivo characteristics of T140-based molecular imaging agents generally limit clinical translation. The only compound of this class that has been evaluated in a clinical setting so far is [68Ga]-1,4,7-triazacyclononane-N,N’,N”-triacetic acid-N-terminal 4-fluoro-benzoyl in glioma266 (see matching Bedside review for more details).
6.3 |. FC-131 based cyclic pentapeptides
Based on the insights and experience gained from development and optimization of T140 ligands, further downsizing the T140 pharmacophore to the essential amino acids led to discovery of the small cyclic pentapeptide FC-131 as a fully stable and highly potent CXCR4 antagonist.267 A proof-of-concept study using radio-iodinated FC-131 in a human small cell lung cancer mouse xenograft model demonstrated the general applicability of this class of CXCR4 antagonists for sensitive and specific in vivo detection of receptor expression.268 Further research to improve biodistribution of this class of peptide imaging agents for CXCR4 produced [68Ga]-Pentixafor. [68Ga]-Pentixafor269 shows high affinity and selectivity for human CXCR4, rapid renal rather than hepatobiliary excretion, and very low nonspecific background accumulation, thus allowing sensitive and high-contrast imaging of CXCR4-expressing tissues in vivo using positron emission tomography (PET). After its first successful application in patients with lymphoma,270 [68Ga]-Pentixafor-PET has now found widespread clinical applications in various hematological and solid cancers.271–273 Data from PET imaging studies in patients with adrenocortical carcinoma showed good correlations with mRNA and protein levels of CXCR4 in tumors and revealed that imaging with [68Ga]-Pentixafor could detect heterogeneous expression of CXCR4 among different metastases. Derivatives of Pentixafor also have been developed for targeted radiotherapy of CXCR4-positive tumors.274,275 In two patient-derived xenograft models of leukemia with different levels of CXCR4 expression, CXCR4-directed radiotherapy with [177Lu]-Pentixather efficiently reduced leukemic burden in spleen and bone marrow. This radiotherapy approach is to date being further explored in the clinic (see Bedside review).
7 |. TARGETING CXCR4 THERAPEUTICALLY
Multiple companies have and continue to develop approaches to target CXCR4, and hundreds of clinical trials have investigated safety profiles and efficacy in hematologic and solid cancers and other diseases. However, plerixafor remains the first and so far only approved CXCR4 antagonist. In 2008, the FDA approved plerixafor in combination with granulocyte-colony stimulating factor to mobilize HSCs to the peripheral blood for collection and subsequent autologous transplantation in adult patients with non-Hodgkin’s lymphoma and MM.276
Preclinical antitumor therapies using either plerixafor277 or structurally related small molecule CXCR4 antagonists such as the plerixafor analog AMD3465278,279 reduced tumor burden by ∼50% in mouse models of a variety of human hematological as well as solid cancers, primarily by effectively preventing distant organ metastasis.280 Plerixafor sensitized BC cells to radiotherapy in vitro and in vivo by increasing cell-cycle arrest and apoptosis281 Low-dose plerixafor also sensitized cells to low-dose taxol, substantially reducing colony formation and proliferation.282
While plerixafor remains the most studied CXCR4 antagonist, the drug lacks selectivity and also has a narrow safety profile. Plerixafor acts as allosteric agonist of ACKR3/CXCR7, the second receptor for CXCL12.283 In a Phase 1/2 trial in 2004, 8 of 40 enrolled HIV patients who received plerixafor from 2.5 to 160 μg/kg/h for 10 days by intravenous infusion discontinued treatment due to adverse events including 2 cases of ventricular ectopy, thrombocytopenia, and paresthesia.284 However, low-dose (4–8% of the dose used for mobilization of HSCs) plerixafor administered twice daily by self-administered injection has been well-tolerated in clinical trials for WHIM syndrome. Results from trials for patients with WHIM syndrome show declines in infections, stabilization of HPV-associated oropharyngeal squamous-cell carcinoma, and improved quality of life. Adverse events were mainly infections attributable to the underlying immunodeficiency.285,286
Several more clinically viable non-cyclam small molecule antagonists with high potency, improved selectivity, and better tolerability profiles were identified over the past 2 decades. When known, we highlight the current status of development for each agent in pre-clinical and clinical studies. Currently, the most advanced compound is the tetrahydroquinoline compound mavorixafor (X4P-001, X4 Pharmaceuticals, formerly AMD11070/AMD070 Genzyme Corp).287 A recent Phase 2 clinical trial with mavorixafor in patients with WHIM syndrome showed increases in total white blood cell counts, neutrophils, and lymphocytes.288 Mavorixafor also reduced frequencies of infections and warts. The company website for X4 Pharmaceuticals lists ongoing studies with additional CXCR4 antagonists X4P-002 and X4P-003, in preclinical models for brain cancers and primary immunodeficiencies, respectively. X4 Pharmaceuticals also previously studied X4–136 in ovarian cancer and melanoma models.289,290 The structures of these compounds have not been published. More recently, Emory University and Bristol-Myers Squibb disclosed newer generations of tetrahydroisoquinoline-containing CXCR4 antagonists with improved in vitro absorption, distribution, metabolism, excretion, and toxicity properties, such as TIQ-15 and analogs.291,292 The compounds were also optimized for higher selectivity against a panel of proteins known to interact with many nitrogen-based chemotypes, such as phenethyl amines, pyridines, piperazines, and piperidines similar to those found in the tetrahydroisoquinoline class.293 GMI-1359 (GlycoMimetics) is a rationally designed small molecule that simultaneously targets both E-selectin and CXCR4, discovered by applying a platform that produces mimetics of naturally occurring carbohydrates. The structure of GMI-1359 is undisclosed. Kureha Chemical Industries identified another promising candidate through a large screening campaign.294 The bisimidazole lead compound KRH-1639 was further optimized; however, the Phase 1 candidate KRH-3955 did not enter clinical trials.295 There have been other promising small molecule CXCR4 antagonists that have not advanced beyond late preclinical or early clinical stages. A detailed review about small molecule CXCR4 antagonists was published by Peng et al.296
In parallel with these small molecule approaches, other companies and research groups worked on peptide-based CXCR4 antagonists. Polyphemusin II is a self-defense β-sheet-like peptide isolated from the hemocytes of horseshoe crab species Tachypleus tridentatus and Limulus polyphemus. In 1992, Masuda et al. synthesized the first polyphemusin II analogs and examined antiviral activity against HIV type 1 in vitro.297 By binding to CXCR4, low concentrations of the 18-residue peptide T22 inhibited infection of human T cells with CXCR4-tropic strains of HIV type 1. Further optimization led to BL-8040 (BKT-140, 4F-benzoyl-TN14003, or T-140 as described in the section for imaging agents), a 14-mer peptide that binds CXCR4 with greater affinity than T22 and better biostability.298 BL-8040 is currently in clinical trials for acute myeloid leukemia and pancreatic cancer. The maximal tolerated dose of BL-8040 in man is 1.5 mg/kg subcutaneously daily.
Protein epitope mimetic technology applied to the β-sheet-like structure of polyphemusin II yielded POL3026 (Polyphor),299 followed by POL5551 and the clinical candidate POL6326 (balixafortide).300 In a mouse model of triple-negative BC, POL5551 nearly completely prevented distant metastases while having minimal effect of growth of orthotopic mouse 4T1 tumors.239 A Phase 1 trial combining balixafortide with eribulin in patients with metastatic BC showed objective responses in 38% and clinical benefit in 67% of heavily pretreated patients.301 Based on these data, the FDA granted fast track designation for balixafortide plus eribulin for patients with metastatic Her-negative BC and prior treatment with at least 2 chemotherapy drugs in the metastatic setting. A Phase 3 trial now is underway. Pharmacologic properties of balixafortide that enabled it to progress to a Phase 3 trial remain uncertain but may relate to modest binding affinity and half-life combined with greater selectivity for CXCR4.302 Both balixafortide and POL5551 also induce efficient stem cell mobilization.300,303
Other peptide inhibitors of CXCR4 include FC131, a cyclic pentapeptide derived from T22 and LY2510924 (Eli Lilly).304–306 LY2510924 has very high in vitro potency but a low maximum tolerated dose (∼20 mg subcutaneously daily) in people.307 This compound remains in clinical trials for solid tumors such as pancreatic cancer.308 E5, a synthetic peptide (Chinese Academy of Medical Sciences) inhibits migration and adhesion of leukemia cells in vitro, modestly extended survival when given as monotherapy in mice with leukemia, and significantly inhibited tumor growth when combined with paclitaxel or cyclophosphamide in a murine BC model.309,310
The design of other peptidic antagonists has been inspired by natural ligands of CXCR4, such as a 3-residue reverse order segment identified in CXCL12 that was similar to the inhibitory chemokine secreted by human herpes virus 8 vMIP-II. Further optimization delivered 3 peptides (R, S, and I) that nearly completed eliminated experimental lung metastases in an immunocompetent mouse model of melanoma.311 TCM Biotech is exploring PTX-9908 (CTCE-9908), a CXCL12 N-terminus–derived peptide that is a dimerized sequence of CXCL12 amino acids 1–8.312 A Phase 1/2 study in hepatocellular carcinoma is starting.
Therapeutic Abs represent a third large pool of CXCR4 antagonists. Several candidates reached clinical trials, for example, PF-06747143 (Pfizer),313 ulocuplumab (BMS-936564/MDX1338 from BMS/Medarex),314 LY2624587 (Eli Lilly),315 or the nanobody ALX-0651 (Ablynx).316 However, the Phase 2 trial of ulocuplumab in WM (NCT03225716) is the only active trial with a CXCR4-targeted Ab. Most of these Abs have been described as exhibiting Ab-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity, possible advantages for elimination of tumor cells. Others, such as ulocuplumab, induce apoptosis through a reactive oxygen species-dependent pathway. However, blocking CXCR4 on various subsets of leukocytes may limit antitumor immune responses. Moreover, permanent CXCR4 blockade by high affinity/slow off-rate Abs may impact normal, regular functions of the organism such as stem cell mobilization or tissue regeneration. For example, LY2624587 induces receptor internalization and down-regulation of CXCR4 density on the cell surface, which may impair trafficking of CXCR4-positive immune cells. While generally safe, anti-CXCR4 antibodies may produce short-term myelosuppression or abnormally elevated leukocyte counts.317,318 More recent work shows that AD-114, a human single domain antibody (so-called i-body) binds CXCR4 without mobilizing hematopoietic stem cells.319 Based on preclinical work showing reductions in collagen synthesis by fibroblasts from patients with idiopathic pulmonary fibrosis and fibrosis in a mouse model, AD-114 received FDA Orphan Drug Designation for idiopathic pulmonary fibrosis.319
In summary, several CXCR4 antagonists have progressed to clinical trials, raising hope that a second CXCR4 antagonist may become available to treat patients over a decade after FDA approval of plerixafor. These agents are described in more detail in the linked Bedside (clinical) review in this issue.
8 |. CONCLUSION
In the past 10 years, numerous investigations have been conducted on the role of the CXCR4/CXCL12 signaling pathway in solid tumors and hematologic malignancies. CXCR4 antagonists could be promising agents for treatment of local and metastatic disease because of direct effects against cancer cells and broader effects in TMEs. However, CXCR4-targeted therapy also may disrupt essential functions of this receptor in development and normal physiology. Since CXCR4 regulates trafficking and functions of both immunosuppressive and effector immune cells, CXCR4-targeted therapy has the potential to dampen host immune responses and decrease anticancer effects. Balancing these effects will require ongoing investigations in immunocompetent animal models of cancer, informed by live-animal imaging studies of pharmacodynamics of CXCR4-targeted therapy. Reciprocal feedback from clinical trials and animal models will help fulfill the promise of CXCR4-targeted therapy to improve outcomes for patients with cancer.
ACKNOWLEDGMENTS
Prof. Gary D. Luker acknowledges research funding from the United States National Institutes of Health (R01CA238042, R01CA196018, U01CA210152, R01CA238023, R33CA225549, and R37CA222563). Professor Ann Richmond acknowledges research funding from the United States National Institutes of Health (R01CA116021, R01CA34590, R01CA243326) and United States Veterans Administration (VA Senior Research Career Scientist Award and VA Merit Award). Professor Stefania Scala acknowledges research funding from the Italian Ministry of Health (RF-2018-12367026 and TRS-2016-00000341).
Abbreviations:
- ACKR3
atypical chemokine receptor 3
- ADT
androgen deprivation treatment
- AKT
RAC-alpha serine/threonine-protein kinase 1/PKB
- BBB
blood-brain barrier
- BC
breast cancer
- BM
bone marrow
- BMDC
BM-derived dendritic cells
- CAF
carcinoma-associated fibroblasts
- CCR
C-C chemokine receptor
- CSC
cancer stem cell
- CTL
cytotoxic T lymphocyte
- CTLA-4
cytotoxic T lymphocyte-associated antigen 4
- Cu
copper
- CXCL
C-X-C motif chemokine
- CXCR
C-X-C chemokine receptor type
- DC
dendritic cell
- ECM
extracellular Matrix
- EGFR
epidermal growth factor receptor
- EMT
epithelial-mesenchymal transition
- ER
estrogen receptor
- ERG
erythroblast transformation specific-related gene
- ERK
extracellular signal-regulated kinase
- FDA
Food and Drug Administration
- Ga
gallium
- GAG
glycosaminoglycan
- GBM
glioblastoma multiforme
- GSC
glioma stem(-like) cells
- h
human
- HPV
human papilloma virus
- HSC
hematopoietic stem cells
- HSPC
hematopoietic stem and progenitor cells
- LC
Langerhans cells
- LN
lymph node
- m
murine
- MDSC
myeloid-derived suppressor cell
- MIF
Mϕ migration inhibitory factor
- MM
multiple myeloma
- mTOR
mammalian target of rapamycin
- PCa
prostate cancer
- PD-1
programmed death-1
- PET
positron emission tomography
- PI3K
phosphatidylinositol-3-kinase
- PTEN
phosphatase and tensin homolog
- RNA
ribonucleic acid
- S1P
sphingosine-1-phosphate
- Tc
technetium
- Th17
T helper 17 cell
- TIME
tumor immune microenvironment
- TME
tumor microenvironment
- Treg
T-regulatory cells
- VEGF
vascular endothelial growth factor
- WHIM
warts, hypogammaglobulinemia, immunodeficiency, and myelokathexis
- WM
Waldenström’s macroglobulinemia
Footnotes
DISCLOSURE
Prof. Gary D. Luker and Dr. Claudio Festuccia receive research funding from Polyphor. H.J. Wester is shareholder of Scintomics GmbH, which in turn is shareholder of Pentixapharm.
REFERENCES
- 1.Sriram K, Insel PA. G protein-coupled receptors as targets for approved drugs: how many targets and how many drugs? Mol Pharmacol. 2018;93(4):251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10(4):463–471. [DOI] [PubMed] [Google Scholar]
- 3.Odemis V, Lamp E, Pezeshki G, et al. Mice deficient in the chemokine receptor CXCR4 exhibit impaired limb innervation and myogenesis. Mol Cell Neurosci. 2005;30(4):494–505. [DOI] [PubMed] [Google Scholar]
- 4.Takabatake Y, Sugiyama T, Kohara H, et al. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol. 2009;20(8):1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devine SM, Vij R, Rettig M, et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood. 2008;112(4):990–998. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee S, Behnam Azad B, Nimmagadda S. The intricate role of CXCR4 in cancer. Adv Cancer Res. 2014;124:31–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doring Y, Pawig L, Weber C, Noels H. The CXCL12/CXCR4 chemokine ligand/receptor axis in cardiovascular disease. Front Physiol. 2014;5:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H, Wang R. A focus on CXCR4 in Alzheimer’s disease. Brain Circulation. 2017;3(4):199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teixido J, Martinez-Moreno M, Diaz-Martinez M, Sevilla-Movilla S. The good and bad faces of the CXCR4 chemokine receptor. Int J Biochem Cell Biol. 2018;95:121–131. [DOI] [PubMed] [Google Scholar]
- 10.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanon M, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Pan Y, Wei Y, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6(5):459–469. [DOI] [PubMed] [Google Scholar]
- 12.Smith M, Luker K, Garbow J, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64(23): 8604–8612. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Ni C, Chen W, et al. Expression of CXCR4 and breast cancer prognosis: a systematic review and meta-analysis. BMC Cancer. 2014;14:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez PA, Gorlin RJ, Lukens JN, et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34(1):70–74. [DOI] [PubMed] [Google Scholar]
- 15.Treon SP, Cao Y, Xu L, Yang G, Liu X, Hunter ZR. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom macroglobulinemia. Blood. 2014;123(18):2791–2796. [DOI] [PubMed] [Google Scholar]
- 16.Lacalle RA, Blanco R, Carmona-Rodriguez L, Martin-Leal A, Mira E, Manes S. Chemokine receptor signaling and the hallmarks of cancer. Int Rev Cell Mol Biol. 2017;331:181–244. [DOI] [PubMed] [Google Scholar]
- 17.Allinen M, Beroukhim R, Cai L, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6(1): 17–32. [DOI] [PubMed] [Google Scholar]
- 18.Orimo A, Gupta P, Sgroi D, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–348. [DOI] [PubMed] [Google Scholar]
- 19.Orimo A, Weinberg R. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5(15):1597–1601. [DOI] [PubMed] [Google Scholar]
- 20.Kojima Y, Acar A, Eaton E, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107(46):20009–20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Jin X, Malladi S, et al. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell. 2013;154:1060–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin F, Brockmeier U, Otterbach F, Metzen E. New insight into the SDF-1/CXCR4 axis in a breast carcinoma model: hypoxia-induced endothelial SDF-1 and tumor cell CXCR4 are required for tumor cell intravasation. Mol Cancer Res. 2012;10(8):1021–1031. [DOI] [PubMed] [Google Scholar]
- 23.Pan J, Mestas J, Burdick MD, et al. Stromal derived factor-1 (SDF-1/CXCL12) and CXCR4 in renal cell carcinoma metastasis. Mol Cancer. 2006;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schioppa T, Uranchimeg B, Saccani A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198(9): 1391–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Falco E, Porcelli D, Torella AR, et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104(12):3472–3482. [DOI] [PubMed] [Google Scholar]
- 26.Liang Z, Brooks J, Willard M, et al. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem Biophys Res Commun. 2007;359(3):716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price TT, Burness ML, Sivan A, et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci Transl Med. 2016;8(340):340ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiozawa Y, Pedersen E, Havens A, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121(4): 1298–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao Y, Hunter ZR, Liu X, et al. The WHIM-like CXCR4(S338X) somatic mutation activates AKT and ERK, and promotes resistance to ibrutinib and other agents used in the treatment of Waldenstrom’s macroglobulinemia. Leukemia. 2015;29(1):169–176. [DOI] [PubMed] [Google Scholar]
- 30.Kallikourdis M, Trovato AE, Anselmi F, et al. The CXCR4 mutations in WHIM syndrome impair the stability of the T-cell immunologic synapse. Blood. 2013;122(5):666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balabanian K, Brotin E, Biajoux V, et al. Proper desensitization of CXCR4 is required for lymphocyte development and peripheral compartmentalization in mice. Blood. 2012;119(24):5722–5730. [DOI] [PubMed] [Google Scholar]
- 32.Kawai T, Choi U, Cardwell L, et al. WHIM syndrome myelokathexis reproduced in the NOD/SCID mouse xenotransplant model engrafted with healthy human stem cells transduced with C-terminus-truncated CXCR4. Blood. 2007;109(1):78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walters KB, Green JM, Surfus JC, Yoo SK, Huttenlocher A. Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome. Blood. 2010;116(15):2803–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cipriani NA, Blair E, Taxy JB. WHIM syndrome and oral squamous cell carcinoma. 2010:105–108. [DOI] [PubMed] [Google Scholar]
- 35.Cao Y, Hunter Z, Liu X, et al. Somatic activating mutations in CXCR4 are common in patients with Waldenstrom’s macroglobulinemia, and their expression in WM cells promotes resistance to ibrutinib. Blood. 2013;122(21):4424. [Google Scholar]
- 36.Castillo JJ, Moreno DF, Arbelaez MI, Hunter ZR, Treon SP. CXCR4 mutations affect presentation and outcomes in patients with Waldenstrom macroglobulinemia: a systematic review. Expert Rev Hematol. 2019;12(10):873–881. [DOI] [PubMed] [Google Scholar]
- 37.Poulain S, Roumier C, Venet-Caillault A, et al. Genomic landscape of CXCR4 mutations in waldenstrom macroglobulinemia. Clin Cancer Res. 2016;22(6):1480–1488. [DOI] [PubMed] [Google Scholar]
- 38.Castillo JJ, Xu L, Gustine JN, et al. CXCR4 mutation subtypes impact response and survival outcomes in patients with Waldenstrom macroglobulinaemia treated with ibrutinib. Br J Haematol. 2019;187(3):356–363. [DOI] [PubMed] [Google Scholar]
- 39.Oberlin E, Amara A, Bachelerie F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382(6594):833–835. [DOI] [PubMed] [Google Scholar]
- 40.Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635–638. [DOI] [PubMed] [Google Scholar]
- 41.Jung Y, Cackowski FC, Yumoto K, et al. CXCL12gamma promotes metastatic castration-resistant prostate cancer by inducing cancer stem cell and neuroendocrine phenotypes. Cancer Res. 2018;78(8):2026–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laguri C, Sadir R, Rueda P, et al. The novel CXCL12gamma isoform encodes an unstructured cationic domain which regulates bioactivity and interaction with both glycosaminoglycans and CXCR4. PLoS One. 2007;2(10):e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu L, Cecil J, Peng S, et al. Identification and expression of novel isoforms of human stromal cell-derived factor 1. Gene. 2006;374: 174–179. [DOI] [PubMed] [Google Scholar]
- 44.Zhao S, Chang SL, Linderman JJ, Feng FY, Luker GD . A comprehensive analysis of CXCL12 isoforms in breast cancer(1,2.). Transl Oncol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cavnar SP, Ray P, Moudgil P, et al. Microfluidic source-sink model reveals effects of biophysically distinct CXCL12 isoforms in breast cancer chemotaxis. Integr Biol (Camb). 2014;6(5):564–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang S, Cavnar S, Takayama S, Luker G, Linderman J. Cell, isoform, and environment factors shape gradients and modulate chemotaxis. PLoS One. 2015;10(4):e0123450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ray P, Stacer A, Fenner J, et al. CXCL12-γ in primary tumors drives breast cancer metastasis. Oncogene. 2015;34(16):2043–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drury L, Ziarek J, Gravel S, et al. Monomeric and dimeric CXCL12 inhibit metastasis through distinct CXCR4 interactions and signaling pathways. Proc Natl Acad Sci USA. 2011;108:17655–17660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ray P, Lewin S, Mihalko L, et al. Secreted CXCL12 (SDF-1) forms dimers under physiologic conditions. Biochem J. 2012;442: 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schiraldi M, Raucci A, Munoz LM, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209(3): 551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Collins PJ, McCully ML, Martinez-Munoz L, et al. Epithelial chemokine CXCL14 synergizes with CXCL12 via allosteric modulation of CXCR4. Faseb J. 2017;31(7):3084–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rajasekaran D, Groning S, Schmitz C, et al. Macrophage migration inhibitory factor-CXCR4 receptor interactions: evidence for partial allosteric agonism in comparison with CXCL12 chemokine. J Biol Chem. 2016;291(30):15881–15895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saini V, Marchese A, Majetschak M. CXC chemokine receptor 4 is a cell surface receptor for extracellular ubiquitin. J Biol Chem. 2010;285(20):15566–15576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saini V, Staren DM, Ziarek JJ, et al. The CXC chemokine receptor 4 ligands ubiquitin and stromal cell-derived factor-1alpha function through distinct receptor interactions. J Biol Chem. 2011;286(38):33466–33477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Babcock GJ, Farzan M, Sodroski J. Ligand-independent dimerization of CXCR4, a principal HIV-1 coreceptor. J Biol Chem. 2003;278(5):3378–3385. [DOI] [PubMed] [Google Scholar]
- 56.Luker K, Gupta M, Luker G. Imaging chemokine receptor dimerization with firefly luciferase complementation. FASEB J. 2009;23(3): 823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toth P, Ren D, Miller R. Regulation of CXCR4 receptor dimerization by the chemokine SDF-1alpha and the HIV-1 coat protein gp120: a fluorescence resonance energy transfer (FRET) study. J Pharmacol Exp Ther. 2004;310(1):8–17. [DOI] [PubMed] [Google Scholar]
- 58.Wu B, Chien EY, Mol CD, et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330(6007):1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J, He L, Combs CA, Roderiquez G, Norcross MA. Dimerization of CXCR4 in living malignant cells: control of cell migration by a synthetic peptide that reduces homologous CXCR4 interactions. Mol Cancer Ther. 2006;5(10):2474–2483. [DOI] [PubMed] [Google Scholar]
- 60.Ge B, Lao J, Li J, Chen Y, Song Y, Huang F. Single-molecule imaging reveals dimerization/oligomerization of CXCR4 on plasma membrane closely related to its function. Sci Rep. 2017;7(1):16873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lao J, He H, Wang X, et al. Single-molecule imaging demonstrates ligand regulation of the oligomeric status of CXCR4 in living cells. J Phys Chem B. 2017;121(7):1466–1474. [DOI] [PubMed] [Google Scholar]
- 62.Vila-Coro A, Rodriguez-Frade J, Martin De Ana A, Moreno-Ortiz M, Martinez-A C, Mellado M. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J. 1999;13(13):1699–1710. [PubMed] [Google Scholar]
- 63.Contento RL, Molon B, Boularan C, et al. CXCR4-CCR5: a couple modulating T cell functions. Proc Natl Acad Sci U S A. 2008;105(29):10101–10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Decaillot F, Kazmi M, Lin Y, Ray-Saha S, Sakmar T, Sachdev P. CXCR7/CXCR4 heterodimer constitutively recruits (beta)-arrestin to enhance cell migration. J Biol Chem. 2011;286(37):32188–32197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar A, Humphreys TD, Kremer KN, et al. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity. 2006;25(2):213–224. [DOI] [PubMed] [Google Scholar]
- 66.Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113(24):6085–6093. [DOI] [PubMed] [Google Scholar]
- 67.Percherancier Y, Berchiche Y, Slight I, et al. Bioluminescence resonance energy transfer reveals ligand-induced conformational changes in CXCR4 homo- and heterodimers. J Biol Chem. 2005;280(11):9895–9903. [DOI] [PubMed] [Google Scholar]
- 68.Sierro F, Biben C, Martinez-Munoz L, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci U S A. 2007;104(37):14759–14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sohy D, Parmentier M, Springael J. Allosteric transinhibition by specific antagonists in CCR2/CXCR4 heterodimers. 2007:30062–30069. [DOI] [PubMed] [Google Scholar]
- 70.Balabanian K, Lagane B, Infantino S, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280(42):35760–35766. [DOI] [PubMed] [Google Scholar]
- 71.Burns J, Summers B, Wang Y, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203(9):2201–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coggins NL, Trakimas D, Chang SL, et al. CXCR7 controls competition for recruitment of beta-arrestin 2 in cells expressing both CXCR4 and CXCR7. PLoS One. 2014;9(6):e98328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Del Molino Del Barrio I, Wilkins GC, Meeson A, Ali S, Kirby JA. Breast cancer: an examination of the potential of ACKR3 to modify the response of CXCR4 to CXCL12. Int J Mol Sci. 2018;19(11):3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sohy D, Yano H, de Nadai P, et al. Hetero-oligomerization of CCR2, CCR5, and CXCR4 and the protean effects of “selective” antagonists. J Biol Chem. 2009;284(45):31270–31279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kremer KN, Clift IC, Miamen AG, et al. Stromal cell-derived factor-1 signaling via the CXCR4-TCR heterodimer requires phospholipase C-beta3 and phospholipase C-gamma1 for distinct cellular responses. J Immunol. 2011;187(3):1440–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. 2007;1768(4):952–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tan W, Martin D, Gutkind J. The Galpha13-Rho signaling axis is required for SDF-1-induced migration through CXCR4. J Biol Chem. 2006;281(51):39542–39549. [DOI] [PubMed] [Google Scholar]
- 78.Hitchinson B, Eby JM, Gao X, et al. Biased antagonism of CXCR4 avoids antagonist tolerance. Sci Signal. 2018;11(552):eaat2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fujita M, Davari P, Takada YK, Takada Y. Stromal cell-derived factor-1 (CXCL12) activates integrins by direct binding to an allosteric ligand-binding site (site 2) of integrins without CXCR4. Biochem J. 2018;475(4):723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DeCastro AJ, Cherukuri P, Balboni A, DiRenzo J. DeltaNP63alpha transcriptionally activates chemokine receptor 4 (CXCR4) expression to regulate breast cancer stem cell activity and chemotaxis. Mol Cancer Ther. 2015;14(1):225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fujita T, Chiwaki F, Takahashi RU, et al. Identification and characterization of CXCR4-positive gastric cancer stem cells. PLoS One. 2015;10(6):e0130808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jung MJ, Rho JK, Kim YM, et al. Upregulation of CXCR4 is functionally crucial for maintenance of stemness in drug-resistant non-small cell lung cancer cells. Oncogene. 2013;32(2):209–221. [DOI] [PubMed] [Google Scholar]
- 83.Wang X, Cao Y, Zhang S, et al. Stem cell autocrine CXCL12/CXCR4 stimulates invasion and metastasis of esophageal cancer. Oncotarget. 2017;8(22):36149–36160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng X, Xie Q, Li S, Zhang W. CXCR4-positive subset of glioma is enriched for cancer stem cells. Oncol Res. 2011;19(12):555–561. [DOI] [PubMed] [Google Scholar]
- 85.Eckert F, Schilbach K, Klumpp L, et al. Potential role of CXCR4 targeting in the context of radiotherapy and immunotherapy of cancer. Front Immunol. 2018;9:3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peitzsch C, Cojoc M, Kurth I, Dubrovska A. Implications of CXCR4/CXCL12 interaction for cancer stem cell maintenance and cancer progression. In: Babashah S, ed. Cancer Stem Cells: Emerging Concepts and Future Perspectives in Translational Oncology. Cham, Switzerand: Springer; 2015:89–130. [Google Scholar]
- 87.Zheng X, Xie Q, Li S, Zhang W. CXCR4-positive subset of glioma is enriched for cancer stem cells. Oncol Res. 2010;19(12):555–561. [DOI] [PubMed] [Google Scholar]
- 88.Hermann P, Huber S, Herrier T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–323. [DOI] [PubMed] [Google Scholar]
- 89.Junttila MR, de Sauvage FJ. Influence of tumour microenvironment heterogeneity on therapeutic response. Nature. 2013;501(7467):346–354. [DOI] [PubMed] [Google Scholar]
- 90.Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–68. [DOI] [PubMed] [Google Scholar]
- 91.Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ganju RK, Brubaker SA, Meyer J, et al. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273(36):23169–23175. [DOI] [PubMed] [Google Scholar]
- 93.Guo F, Wang Y, Liu J, Mok SC, Xue F, Zhang W. CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene. 2016;35:816–826. [DOI] [PubMed] [Google Scholar]
- 94.Scala S. Molecular pathways: targeting the CXCR4-CXCL12 axis-untapped potential in the tumor microenvironment. Clin Cancer Res. 2015;21(19):4278–4285. [DOI] [PubMed] [Google Scholar]
- 95.Susek KH, Karvouni M, Alici E, Lundqvist A. The role of CXC chemokine receptors 1–4 on immune cells in the tumor microenvironment. Front Immunol. 2018;9:2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kufareva I, Salanga CL, Handel TM. Chemokine and chemokine receptor structure and interactions: implications for therapeutic strategies. Immunol Cell Biol. 2015;93(4):372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sadir R, Imberty A, Baleux F, Lortat-Jacob H. Heparan sulfate/heparin oligosaccharides protect stromal cell-derived factor-1 (SDF-1)/CXCL12 against proteolysis induced by CD26/dipeptidyl peptidase IV. J Biol Chem. 2004;279(42):43854–43860. [DOI] [PubMed] [Google Scholar]
- 99.Grabovsky V, Feigelson S, Chen C, et al. Subsecond induction of alpha4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions. J Exp Med. 2000;192(4): 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peled A, Kollet O, Ponomaryov T, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95(11):3289–3296. [PubMed] [Google Scholar]
- 101.Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283(5403):845–848. [DOI] [PubMed] [Google Scholar]
- 102.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12(9):551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–887. [DOI] [PubMed] [Google Scholar]
- 104.Ribatti D, Crivellato E. “Sprouting angiogenesis”, a reappraisal. Dev Biol. 2012;372(2):157–165. [DOI] [PubMed] [Google Scholar]
- 105.Jakobsson L, Franco CA, Bentley K, et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12(10):943–953. [DOI] [PubMed] [Google Scholar]
- 106.Grunewald M, Avraham I, Dor Y, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124(1):175–189. [DOI] [PubMed] [Google Scholar]
- 107.Butler JM, Guthrie SM, Koc M, et al. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005;115(1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Anderson EM, Mooney DJ. The combination of vascular endothelial growth factor and stromal cell-derived factor induces superior angiogenic sprouting by outgrowth endothelial cells. J Vasc Res. 2015;52(1):62–69. [DOI] [PubMed] [Google Scholar]
- 109.Strasser GA, Kaminker JS, Tessier-Lavigne M. Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood. 2010;115(24):5102–5110. [DOI] [PubMed] [Google Scholar]
- 110.Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393(6685):591–594. [DOI] [PubMed] [Google Scholar]
- 111.Unoki N, Murakami T, Nishijima K, Ogino K, van Rooijen N, Yoshimura N. SDF-1/CXCR4 contributes to the activation of tip cells and microglia in retinal angiogenesis. Invest Ophthalmol Vis Sci. 2010;51(7):3362–3371. [DOI] [PubMed] [Google Scholar]
- 112.McDermott DH, De Ravin SS, et al. Severe congenital neutropenia resulting from G6PC3 deficiency with increased neutrophil CXCR4 expression and myelokathexis. Blood. 2010;116(15):2793–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Netelenbos T, Zuijderduijn S, Van Den Born J, et al. Proteoglycans guide SDF-1-induced migration of hematopoietic progenitor cells. J Leukoc Biol. 2002;72(2):353–362. [PubMed] [Google Scholar]
- 114.Devi S, Wang Y, Chew WK, et al. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J Exp Med. 2013;210(11):2321–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang J, Kumar A, Vilgelm AE, et al. Loss of CXCR4 in myeloid cells enhances antitumor immunity and reduces melanoma growth through NK cell and FASL mechanisms. Cancer Immunol Res. 2018;6(10):1186–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jaeger BN, Donadieu J, Cognet C, et al. Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis. J Exp Med. 2012;209(3):565–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takeda K, Tsutsui H, Yoshimoto T, et al. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8(3):383–390. [DOI] [PubMed] [Google Scholar]
- 118.Dale DC, Bolyard AA, Kelley ML, et al. The CXCR4 antagonist plerixafor is a potential therapy for myelokathexis, WHIM syndrome. Blood. 2011;118(18):4963–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Green MM, Chao N, Chhabra S, et al. Plerixafor (a CXCR4 antagonist) following myeloablative allogeneic hematopoietic stem cell transplantation enhances hematopoietic recovery. J Hematol Oncol. 2016;9(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kang Y, Chen BJ, Deoliveira D, Mito J, Chao NJ. Selective enhancement of donor hematopoietic cell engraftment by the CXCR4 antagonist AMD3100 in a mouse transplantation model. PLoS One. 2010;5(6):e11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. [DOI] [PubMed] [Google Scholar]
- 122.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5(8): 617–628. [DOI] [PubMed] [Google Scholar]
- 123.Kabashima K, Sugita K, Shiraishi N, Tamamura H, Fujii N, Tokura Y. CXCR4 engagement promotes dendritic cell survival and maturation. Biochem Biophys Res Commun. 2007;361(4):1012–1016. [DOI] [PubMed] [Google Scholar]
- 124.Sallusto F, Schaerli P, Loetscher P, et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28(9):2760–2769. [DOI] [PubMed] [Google Scholar]
- 125.Pablos JL, Amara A, Bouloc A, et al. Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin. Am J Pathol. 1999;155(5):1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105(10):3793–3801. [DOI] [PubMed] [Google Scholar]
- 127.Curiel TJ, Cheng P, Mottram P, et al. Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res. 2004;64(16):5535–5538. [DOI] [PubMed] [Google Scholar]
- 128.Gadalla R, Hassan H, Ibrahim SA, et al. Tumor microenvironmental plasmacytoid dendritic cells contribute to breast cancer lymph node metastasis via CXCR4/SDF-1 axis. Breast Cancer Res Treat. 2019;174(3):679–691. [DOI] [PubMed] [Google Scholar]
- 129.Lee CG, Das B, Lin TL, et al. A rare fraction of drug-resistant follicular lymphoma cancer stem cells interacts with follicular dendritic cells to maintain tumourigenic potential. Br J Haematol. 2012;158(1):79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. [DOI] [PubMed] [Google Scholar]
- 131.Schulz O, Hammerschmidt SI, Moschovakis GL, Forster R. Chemokines and chemokine receptors in lymphoid tissue dynamics. Annu Rev Immunol. 2016;34:203–242. [DOI] [PubMed] [Google Scholar]
- 132.Whiteside TL. Induced regulatory T cells in inhibitory microenvironments created by cancer. Expert Opin Biol Ther. 2014;14(10): 1411–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Whiteside TL. What are regulatory T cells (Treg) regulating in cancer and why? Semin Cancer Biol. 2012;22(4):327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Santagata S, Napolitano M, D’Alterio C, et al. Targeting CXCR4 reverts the suppressive activity of T-regulatory cells in renal cancer. Oncotarget. 2017;8(44):77110–77120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Polimeno M, Napolitano M, Costantini S, et al. Regulatory T cells, interleukin (IL)-6, IL-8, vascular endothelial growth factor (VEGF), CXCL10, CXCL11, epidermal growth factor (EGF) and hepatocyte growth factor (HGF) as surrogate markers of host immunity in patients with renal cell carcinoma. BJU Int. 2013;112(5):686–696. [DOI] [PubMed] [Google Scholar]
- 136.Fialova A, Partlova S, Sojka L, et al. Dynamics of T-cell infiltration during the course of ovarian cancer: the gradual shift from a Th17 effector cell response to a predominant infiltration by regulatory T-cells. Int J Cancer. 2013;132(5):1070–1079. [DOI] [PubMed] [Google Scholar]
- 137.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12(18):5423–5434. [DOI] [PubMed] [Google Scholar]
- 138.Fu J, Xu D, Liu Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328–2339. [DOI] [PubMed] [Google Scholar]
- 139.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117(5):1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yan M, Jene N, Byrne D, et al. Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast Cancer Res. 2011;13(2):R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.D’Alterio C, Buoncervello M, Ierano C, et al. Targeting CXCR4 potentiates anti-PD-1 efficacy modifying the tumor microenvironment and inhibiting neoplastic PD-1. J Exp Clin Cancer Res. 2019;38(1):432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31(6):859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chaix J, Nish SA, Lin WH, et al. Cutting edge: cXCR4 is critical for CD8+ memory T cell homeostatic self-renewal but not rechallenge self-renewal. J Immunol. 2014;193(3):1013–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nomura T, Hasegawa H, Kohno M, Sasaki M, Fujita S. Enhancement of anti-tumor immunity by tumor cells transfected with the secondary lymphoid tissue chemokine EBI-1-ligand chemokine and stromal cell-derived factor-1alpha chemokine genes. Int J Cancer. 2001;91(5):597–606. [DOI] [PubMed] [Google Scholar]
- 145.Dunussi-Joannopoulos K, Zuberek K, Runyon K, et al. Efficacious immunomodulatory activity of the chemokine stromal cell-derived factor 1 (SDF-1): local secretion of SDF-1 at the tumor site serves as T-cell chemoattractant and mediates T-cell-dependent antitumor responses. Blood. 2002;100(5):1551–1558. [PubMed] [Google Scholar]
- 146.Poznansky MC, Olszak IT, Foxall R, Evans RH, Luster AD, Scadden DT. Active movement of T cells away from a chemokine. Nat Med. 2000;6(5):543–548. [DOI] [PubMed] [Google Scholar]
- 147.Vianello F, Olszak IT, Poznansky MC. Fugetaxis: active movement of leukocytes away from a chemokinetic agent. J Mol Med (Berl). 2005;83(10):752–763. [DOI] [PubMed] [Google Scholar]
- 148.Vianello F, Papeta N, Chen T, et al. Murine B16 melanomas expressing high levels of the chemokine stromal-derived factor-1/CXCL12 induce tumor-specific T cell chemorepulsion and escape from immune control. J Immunol. 2006;176(5):2902–2914. [DOI] [PubMed] [Google Scholar]
- 149.Meiron M, Zohar Y, Anunu R, Wildbaum G, Karin N. CXCL12 (SDF-1alpha) suppresses ongoing experimental autoimmune encephalomyelitis by selecting antigen-specific regulatory T cells. J Exp Med. 2008;205(11):2643–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Di Rosa F, Gebhardt T. Bone marrow T cells and the integrated functions of recirculating and tissue-resident memory T cells. Front Immunol. 2016;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tindemans I, Joosse ME, Samsom JN. Dissecting the heterogeneity in T-cell mediated inflammation in IBD. Cells. 2020;9:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hosomi S, Oshitani N, Kamata N, et al. Increased numbers of immature plasma cells in peripheral blood specifically overexpress chemokine receptor CXCR3 and CXCR4 in patients with ulcerative colitis. Clin Exp Immunol. 2011;163(2):215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Benechet AP, Menon M, Xu D, et al. T cell-intrinsic S1PR1 regulates endogenous effector T-cell egress dynamics from lymph nodes during infection. Proc Natl Acad Sci USA. 2016;113(8):2182–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mandal M, Okoreeh MK, Kennedy DE, et al. CXCR4 signaling directs Igk recombination and the molecular mechanisms of late B lymphopoiesis. Nat Immunol. 2019;20(10):1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schroder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1996;93(1): 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Uo M, Hisamatsu T, Miyoshi J, et al. Mucosal CXCR4+ IgG plasma cells contribute to the pathogenesis of human ulcerative colitis through FcgammaR-mediated CD14 macrophage activation. Gut. 2013;62(12):1734–1744. [DOI] [PubMed] [Google Scholar]
- 157.Alsayed Y, Ngo H, Runnels J, et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood. 2007;109(7):2708–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hunter ZR, Xu L, Yang G, et al. Transcriptome sequencing reveals a profile that corresponds to genomic variants in Waldenstrom macroglobulinemia. Blood. 2016;128(6):827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ghobrial IM, Bone ND, Stenson MJ, et al. Expression of the chemokine receptors CXCR4 and CCR7 and disease progression in B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma. Mayo Clin Proc. 2004;79(3):318–325. [DOI] [PubMed] [Google Scholar]
- 160.Burger JA, Kipps TJ. Chemokine receptors and stromal cells in the homing and homeostasis of chronic lymphocytic leukemia B cells. Leuk Lymphoma. 2002;43(3):461–466. [DOI] [PubMed] [Google Scholar]
- 161.van den Berk LC, van der Veer A, et al. Disturbed CXCR4/CXCL12 axis in paediatric precursor B-cell acute lymphoblastic leukaemia. Br J Haematol. 2014;166(2):240–249. [DOI] [PubMed] [Google Scholar]
- 162.de Rooij MF, Kuil A, Kraan W, et al. Ibrutinib and idelalisib target B cell receptor- but not CXCL12/CXCR4-controlled integrin-mediated adhesion in Waldenstrom macroglobulinemia. Haematologica. 2016;101(3):e111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Affara NI, Ruffell B, Medler TR, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25(6):809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Andreu P, Johansson M, Affara NI, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17(2):121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gunderson AJ, Coussens LM. B cells and their mediators as targets for therapy in solid tumors. Exp Cell Res. 2013;319(11):1644–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. [DOI] [PubMed] [Google Scholar]
- 169.Tota JE, Engels EA, Madeleine MM, et al. Risk of oral tongue cancer among immunocompromised transplant recipients and human immunodeficiency virus-infected individuals in the United States. Cancer. 2018;124(12):2515–2522. [DOI] [PubMed] [Google Scholar]
- 170.Hernandez-Ramirez RU, Qin L, Lin H, et al. Association of immunosuppression and HIV viraemia with non-Hodgkin lymphoma risk overall and by subtype in people living with HIV in Canada and the USA: a multicentre cohort study. Lancet HIV. 2019;6(4):e240–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Paulson KG, Park SY, Vandeven NA, et al. Merkel cell carcinoma: current US incidence and projected increases based on changing demo-graphics. J Am Acad Dermatol. 2018;78(3):457–463 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296(23): 2823–2831. [DOI] [PubMed] [Google Scholar]
- 173.Sheil AG, Mahoney JF, Horvath JS, et al. Cancer following renal transplantation. Aust N Z J Surg. 1979;49(6):617–620. [DOI] [PubMed] [Google Scholar]
- 174.Gettinger S, Choi J, Hastings K, et al. Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov. 2017;7(12):1420–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Syn NL, Teng MWL, Mok TSK, Soo RA. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18(12):e731–e41. [DOI] [PubMed] [Google Scholar]
- 176.Haanen JBAG. Converting cold into hot tumors by combining immunotherapies. Cell. 2017;170(6):1055–1056. [DOI] [PubMed] [Google Scholar]
- 177.Szekely B, Bossuyt V, Li X, Wali VB, et al. Immunological differences between primary and metastatic breast cancer. Ann Oncol. 2018;29(11):2232–2239. [DOI] [PubMed] [Google Scholar]
- 178.Khan AB, Carpenter B, Santos ESP, et al. Redirection to the bone marrow improves T cell persistence and antitumor functions. J Clin Invest. 2018;128(5):2010–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Johnson DR, Chang SM. Recent medical management of glioblastoma. Adv Exp Med Biol. 2012;746:26–40. [DOI] [PubMed] [Google Scholar]
- 180.Rock K, McArdle O, Forde P, et al. A clinical review of treatment outcomes in glioblastoma multiforme–the validation in a non-trial population of the results of a randomised Phase III clinical trial: has a more radical approach improved survival?. Br J Radiol. 2012;85(1017):e729–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 182.Tabouret E, Tchoghandjian A, Denicolai E, et al. Recurrence of glioblastoma after radio-chemotherapy is associated with an angiogenic switch to the CXCL12-CXCR4 pathway. Oncotarget. 2015;6(13):11664–11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fareh M, Turchi L, Virolle V, et al. The miR 302–367 cluster drastically affects self-renewal and infiltration properties of gliomainitiating cells through CXCR4 repression and consequent disruption of the SHH-GLI-NANOG network. Cell Death Differ. 2012;19(2): 232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Guo X, Xu S, Gao X, et al. Macrophage migration inhibitory factor promotes vasculogenic mimicry formation induced by hypoxia via CXCR4/AKT/EMT pathway in human glioblastoma cells. Oncotarget. 2017;8(46):80358–80372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Simons D, Grieb G, Hristov M, et al. Hypoxia-induced endothelial secretion of macrophage migration inhibitory factor and role in endothelial progenitor cell recruitment. J Cell Mol Med. 2011;15(3):668–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Komatani H, Sugita Y, Arakawa F, Ohshima K, Shigemori M. Expression of CXCL12 on pseudopalisading cells and proliferating microvessels in glioblastomas: an accelerated growth factor in glioblastomas. Int J Oncol. 2009;34(3):665–672. [DOI] [PubMed] [Google Scholar]
- 187.Zagzag D, Lukyanov Y, Lan L,, et al. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest. 2006;86(12):1221–1232. [DOI] [PubMed] [Google Scholar]
- 188.Bruyere C, Mijatovic T, Lonez C, et al. Temozolomide-induced modification of the CXC chemokine network in experimental gliomas. Int J Oncol. 2011;38(5):1453–1464. [DOI] [PubMed] [Google Scholar]
- 189.Fluh C, Hattermann K, Mehdorn HM, Synowitz M, Held-Feindt J. Differential expression of CXCR4 and CXCR7 with various stem cell markers in paired human primary and recurrent glioblastomas. Int J Oncol. 2016;48(4):1408–1416. [DOI] [PubMed] [Google Scholar]
- 190.Liu C, Pham K, Luo D, et al. Expression and functional heterogeneity of chemokine receptors CXCR4 and CXCR7 in primary patient-derived glioblastoma cells. PLoS One. 2013;8(3):e59750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pan Y, Ma S, Cao K, et al. Therapeutic approaches targeting cancer stem cells. J Cancer Res Ther. 2018;14(7):1469–1475. [DOI] [PubMed] [Google Scholar]
- 192.Sehgal A, Keener C, Boynton AL, Warrick J, Murphy GP. CXCR-4, a chemokine receptor, is overexpressed in and required for proliferation of glioblastoma tumor cells. J Surg Oncol. 1998;69(2):99–104. [DOI] [PubMed] [Google Scholar]
- 193.Gagner JP, Sarfraz Y, Ortenzi V, et al. Multifaceted C-X-C chemokine receptor 4 (CXCR4) inhibition interferes with anti-vascular endothelial growth factor therapy-induced glioma dissemination. Am J Pathol. 2017;187(9):2080–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yadav VN, Zamler D, Baker GJ, et al. CXCR4 increases in-vivo glioma perivascular invasion, and reduces radiation induced apoptosis: a genetic knockdown study. Oncotarget. 2016;7(50):83701–83719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rao S, Sengupta R, Choe EJ, et al. CXCL12 mediates trophic interactions between endothelial and tumor cells in glioblastoma. PLoS One. 2012;7(3):e33005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chiblak S, Tang Z, Lemke D, et al. Carbon irradiation overcomes glioma radioresistance by eradicating stem cells and forming an antiangiogenic and immunopermissive niche. JCI Insight. 2019;4(2):e123837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Calinescu AA, Yadav VN, Carballo E, et al. Survival and proliferation of neural progenitor-derived glioblastomas under hypoxic stress is controlled by a CXCL12/CXCR4 autocrine-positive feedback mechanism. Clin Cancer Res. 2017;23(5):1250–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mercurio L, Ajmone-Cat MA, Cecchetti S, et al. Targeting CXCR4 by a selective peptide antagonist modulates tumor microenvironment and microglia reactivity in a human glioblastoma model. J Exp Clin Cancer Res. 2016;35:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gravina GL, Mancini A, Marampon F, et al. The brain-penetrating CXCR4 antagonist, PRX177561, increases the antitumor effects of bevacizumab and sunitinib in preclinical models of human glioblastoma. J Hematol Oncol. 2017;10(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Barone A, Sengupta R, Warrington NM, et al. Combined VEGF and CXCR4 antagonism targets the GBM stem cell population and synergistically improves survival in an intracranial mouse model of glioblastoma. Oncotarget. 2014;5(20):9811–9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 202.Jung SJ, Kim CI, Park CH, et al. Correlation between chemokine receptor CXCR4 expression and prognostic factors in patients with prostate cancer. Korean J Urol. 2011;52(9):607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Goto Y, Kurozumi A, Enokida H, Ichikawa T, Seki N. Functional significance of aberrantly expressed microRNAs in prostate cancer. Int J Urol. 2015;22(3):242–252. [DOI] [PubMed] [Google Scholar]
- 204.Gladson CL, Welch DR. New insights into the role of CXCR4 in prostate cancer metastasis. Cancer Biol Ther. 2008;7(11):1849–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ponomaryov T, Peled A, Petit I, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106(11):1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mochizuki H, Matsubara A, Teishima J, et al. Interaction of ligand-receptor system between stromal-cell-derived factor-1 and CXC chemokine receptor 4 in human prostate cancer: a possible predictor of metastasis. Biochem Biophys Res Commun. 2004;320(3):656–663. [DOI] [PubMed] [Google Scholar]
- 207.Chen Q, Zhong T. The association of CXCR4 expression with clinicopathological significance and potential drug target in prostate cancer: a meta-analysis and literature review. Drug Des Devel Ther. 2015;9:5115–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Domanska UM, Boer JC, Timmer-Bosscha H, et al. CXCR4 inhibition enhances radiosensitivity, while inducing cancer cell mobilization in a prostate cancer mouse model. Clin Exp Metastasis. 2014;31(7): 829–839. [DOI] [PubMed] [Google Scholar]
- 209.Gravina GL, Mancini A, Muzi P, et al. CXCR4 pharmacogical inhibition reduces bone and soft tissue metastatic burden by affecting tumor growth and tumorigenic potential in prostate cancer preclinical models. Prostate. 2015;75(12):1227–1246. [DOI] [PubMed] [Google Scholar]
- 210.Lee JY, Kang DH, Chung DY, et al. Meta-analysis of the relationship between CXCR4 expression and metastasis in prostate cancer. World J Mens Health. 2014;32(3):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Paner GP, Stadler WM, Hansel DE, Montironi R, Lin DW, Amin MB. Updates in the eighth edition of the tumor-node-metastasis staging classification for urologic cancers. Eur Urol. 2018;73(4):560–569. [DOI] [PubMed] [Google Scholar]
- 212.Darash-Yahana M, Pikarsky E, Abramovitch R, et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. 2004;18(11):1240–1242. [DOI] [PubMed] [Google Scholar]
- 213.Chetram MA, Odero-Marah V, Hinton CV. Loss of PTEN permits CXCR4-mediated tumorigenesis through ERK1/2 in prostate cancer cells. Mol Cancer Res. 2011;9(1):90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Muller G, Lipp M. Signal transduction by the chemokine receptor CXCR5: structural requirements for G protein activation analyzed by chimeric CXCR1/CXCR5 molecules. Biol Chem. 2001;382(9): 1387–1397. [DOI] [PubMed] [Google Scholar]
- 215.Porvasnik S, Sakamoto N, Kusmartsev S, et al. Effects of CXCR4 antagonist CTCE-9908 on prostate tumor growth. Prostate. 2009;69(13):1460–1469. [DOI] [PubMed] [Google Scholar]
- 216.Sun YX, Schneider A, Jung Y, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20(2):318–329. [DOI] [PubMed] [Google Scholar]
- 217.Xu C, Zhao H, Chen H, Yao Q. CXCR4 in breast cancer: oncogenic role and therapeutic targeting. Drug Des Dev Ther. 2015;9:4953–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Leuprolide Study Group. Leuprolide versus diethylstilbestrol for metastatic prostate cancer. N Engl J Med. 1984;311(20):1281–1286. [DOI] [PubMed] [Google Scholar]
- 219.Etheridge T, Damodaran S, Schultz A, et al. Combination therapy with androgen deprivation for hormone sensitive prostate cancer: a new frontier. Asian J Urol. 2019;6(1):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cai J, Kandagatla P, Singareddy R, et al. Androgens induce functional CXCR4 through ERG factor expression in TMPRSS2-ERG fusion-positive prostate cancer cells. Transl Oncol. 2010;3(3): 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Festuccia C, Gravina GL, Muzi P, et al. Bicalutamide increases phospho-Akt levels through Her2 in patients with prostate cancer. Endocr Relat Cancer. 2007;14(3):601–611. [DOI] [PubMed] [Google Scholar]
- 222.Gravina GL, Biordi L, Martella F, et al. Epigenetic modulation of PTEN expression during antiandrogenic therapies in human prostate cancer. Int J Oncol. 2009;35(5):1133–1139. [DOI] [PubMed] [Google Scholar]
- 223.Lapteva N, Yang AG, Sanders DE, Strube RW, SY Chen. CXCR4 knockdown by small interfering RNA abrogates breast tumor growth in vivo. Cancer Gene Ther. 2005;12(1):84–89. [DOI] [PubMed] [Google Scholar]
- 224.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16(11):2927–2931. [DOI] [PubMed] [Google Scholar]
- 225.Seo EH, Namgung JH, Oh CS, Kim SH, Lee SH. Association of chemokines and chemokine receptor expression with monocytic-myeloid-derived suppressor cells during tumor progression. Immune Netw. 2018;18(3):e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hall JM, Korach KS. Stromal cell-derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Mol Endocrinol. 2003;17(5): 792–803. [DOI] [PubMed] [Google Scholar]
- 227.Kishimoto H, Wang Z, Bhat-Nakshatri P, Chang D, Clarke R, Naksha-tri H. The p160 family coactivators regulate breast cancer cell proliferation and invasion through autocrine/paracrine activity of SDF-1alpha/CXCL12. Carcinogenesis. 2005;26(10):1706–1715. [DOI] [PubMed] [Google Scholar]
- 228.Pattarozzi A, Gatti M, Barbieri F, et al. 17beta-estradiol promotes breast cancer cell proliferation-inducing stromal cell-derived factor-1-mediated epidermal growth factor receptor transactivation: reversal by gefitinib pretreatment. Mol Pharmacol. 2008;73(1):191–202. [DOI] [PubMed] [Google Scholar]
- 229.Sauve K, Lepage J, Sanchez M, Heveker N, Tremblay A. Positive feedback activation of estrogen receptors by the CXCL12-CXCR4 pathway. Cancer Res. 2009;69(14):5793–5800. [DOI] [PubMed] [Google Scholar]
- 230.Kobayashi T, Tsuda H, Moriya T, et al. Expression pattern of stromal cell-derived factor-1 chemokine in invasive breast cancer is correlated with estrogen receptor status and patient prognosis. Breast Cancer Res Treat. 2010;123(3):733–745. [DOI] [PubMed] [Google Scholar]
- 231.Rhodes LV, Short SP, Neel NF, et al. Cytokine receptor CXCR4 mediates estrogen-independent tumorigenesis, metastasis, and resistance to endocrine therapy in human breast cancer. Cancer Res. 2011;71(2):603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Boudot A, Kerdivel G, Habauzit D, et al. Differential estrogen-regulation of CXCL12 chemokine receptors, CXCR4 and CXCR7, contributes to the growth effect of estrogens in breast cancer cells. PLoS One. 2011;6(6):e20898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ouyang L, Chang W, Fang B, Qin J, Qu X, Cheng F. Estrogen-induced SDF-1alpha production promotes the progression of ER-negative breast cancer via the accumulation of MDSCs in the tumor microenvironment. Sci Rep. 2016;6:39541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dewan MZ, Ahmed S, Iwasaki Y, Ohba K, Toi M, Yamamoto N. Stromal cell-derived factor-1 and CXCR4 receptor interaction in tumor growth and metastasis of breast cancer. Biomed Pharmacother. 2006;60(6):273–276. [DOI] [PubMed] [Google Scholar]
- 235.Richert MM, Vaidya KS, Mills CN, et al. Inhibition of CXCR4 by CTCE-9908 inhibits breast cancer metastasis to lung and bone. Oncol Rep. 2009;21(3):761–767. [PubMed] [Google Scholar]
- 236.Sugiyama T, Nagasawa T. Bone marrow niches for hematopoietic stem cells and immune cells. Inflamm Allergy Drug Targets. 2012;11(3):201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cabioglu N, Sahin AA, Morandi P, et al. Chemokine receptors in advanced breast cancer: differential expression in metastatic disease sites with diagnostic and therapeutic implications. Ann Oncol. 2009;20(6):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Huang EH, Singh B, Cristofanilli M, et al. A CXCR4 antagonist CTCE-9908 inhibits primary tumor growth and metastasis of breast cancer. J Surg Res. 2009;155(2):231–236. [DOI] [PubMed] [Google Scholar]
- 239.Xiang J, Hurchla MA, Fontana F, et al. CXCR4 protein epitope mimetic antagonist POL5551 disrupts metastasis and enhances chemotherapy effect in triple-negative breast cancer. Mol Cancer Ther. 2015;14(11):2473–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Cheng X, Hung MC. Breast cancer brain metastases. Cancer Metastasis Rev. 2007;26(3–4):635–643. [DOI] [PubMed] [Google Scholar]
- 241.Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist. 2007;12(7):884–898. [DOI] [PubMed] [Google Scholar]
- 242.Sharma M, Abraham J. CNS metastasis in primary breast cancer. Expert Rev Anticancer Ther. 2007;7(11):1561–1566. [DOI] [PubMed] [Google Scholar]
- 243.Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24(12):719–725. [DOI] [PubMed] [Google Scholar]
- 244.Yonemori K, Tsuta K, Ono M, et al. Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type breast cancer but not HER2/neu-positive breast cancer. Cancer. 2010;116(2):302–308. [DOI] [PubMed] [Google Scholar]
- 245.Lee BC, Lee TH, Avraham S, Avraham HK. Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1alpha in breast cancer cell migration through human brain microvascular endothelial cells. Mol Cancer Res. 2004;2(6): 327–338. [PubMed] [Google Scholar]
- 246.Jiang J, Thyagarajan-Sahu A, Loganathan J, et al. BreastDefend prevents breast-to-lung cancer metastases in an orthotopic animal model of triple-negative human breast cancer. Oncol Rep. 2012;28(4):1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kato M, Kitayama J, Kazama S, Nagawa H. Expression pattern of CXC chemokine receptor-4 is correlated with lymph node metastasis in human invasive ductal carcinoma. Breast Cancer Res. 2003;5(5): R144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Xu TP, Shen H, Liu LX, Shu YQ. The impact of chemokine receptor CXCR4 on breast cancer prognosis: a meta-analysis. Cancer Epidemiol. 2013;37(5):725–731. [DOI] [PubMed] [Google Scholar]
- 249.Weiss ID, Jacobson O. Molecular imaging of chemokine receptor CXCR4. Theranostics. 2013;3(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Nayak TR, Hong H, Zhang Y, Cai W. Multimodality imaging of CXCR4 in cancer: current status towards clinical translation. Curr Mol Med. 2013;13(10):1538–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Fakhari A, Aghanejad A, Jalilian AR, Gharepapagh E. Recent developments in targeted imaging of CXCR4-chemokine receptor. J Radioanal Nucl Chem. 2018;317(1):1–14. [Google Scholar]
- 252.Weiss ID, Jacobson O, Kiesewetter DO, et al. Positron emission tomography imaging of tumors expressing the human chemokine receptor CXCR4 in mice with the use of 64Cu-AMD3100. Mol Imaging Biol. 2012;14(1):106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jacobson O, Weiss ID, Szajek L, Farber JM, Kiesewetter DO. 64Cu-AMD3100–a novel imaging agent for targeting chemokine receptor CXCR4. Bioorg Med Chem. 2009;17(4):1486–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Aghanejad A, Jalilian AR, Fazaeli Y, et al. Synthesis and evaluation of Ga-67-AMD3100; A novel imaging agent for targeting chemokine receptor CXCR4. Nucl Med Biol. 2014;41(7):640–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhang X, You L, Chen S, et al. Development of a novel (99m) Tc-labeled small molecular antagonist for CXCR4 positive tumor imaging. J Labelled Compd Radiopharm. 2018;61(5):438–446. [DOI] [PubMed] [Google Scholar]
- 256.De Silva RA, Peyre K, Pullambhatla M, Fox JJ, Pomper MG, Nimmagadda S. Imaging CXCR4 expression in human cancer xenografts: evaluation of monocyclam 64Cu-AMD3465. J Nucl Med. 2011;52(6):986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tamamura H, Hiramatsu K, Kusano S, et al. Synthesis of potent CXCR4 inhibitors possessing low cytotoxicity and improved biostability based on T140 derivatives. Org Biomol Chem. 2003;1(21):3656–3662. [DOI] [PubMed] [Google Scholar]
- 258.George GP, Stevens E, Aberg O, et al. Preclinical evaluation of a CXCR4-specific (68)Ga-labelled TN14003 derivative for cancer PET imaging. Bioorg Med Chem. 2014;22(2):796–803. [DOI] [PubMed] [Google Scholar]
- 259.Hanaoka H, Mukai T, Tamamura H, et al. Development of a 111In-labeled peptide derivative targeting a chemokine receptor, CXCR4, for imaging tumors. Nucl Med Biol. 2006;33(4):489–494. [DOI] [PubMed] [Google Scholar]
- 260.Jacobson O, Weiss ID, Kiesewetter DO, Farber JM, Chen X. PET of tumor CXCR4 expression with 4–18F-T140. J Nucl Med. 2010;51(11):1796–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Jacobson O, Weiss ID, Szajek LP, et al. PET imaging of CXCR4 using copper-64 labeled peptide antagonist. Theranostics. 2011;1:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Jacobson O, Weiss ID, Szajek LP, et al. Improvement of CXCR4 tracer specificity for PET imaging. J Control Release. 2012;157(2):216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kuil J, Buckle T, Yuan H, et al. Synthesis and evaluation of a bimodal CXCR4 antagonistic peptide. Bioconjug Chem. 2011;22(5): 859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Oishi S, Masuda R, Evans B, et al. Synthesis and application of fluorescein- and biotin-labeled molecular probes for the chemokine receptor CXCR4. ChemBioChem. 2008;9(7):1154–1158. [DOI] [PubMed] [Google Scholar]
- 265.Yan X, Niu G, Wang Z, et al. Al[18F]NOTA-T140 peptide for noninvasive visualization of CXCR4 expression. Mol Imaging Biol. 2016;18(1):135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wang Z, Zhang M, Wang L, et al. Prospective study of (68)Ga-NOTA-NFB: radiation dosimetry in healthy volunteers and first application in glioma patients. Theranostics. 2015;5(8):882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Fujii N, Oishi S, Hiramatsu K, et al. Molecular-size reduction of a potent CXCR4-chemokine antagonist using orthogonal combination of conformation- and sequence-based libraries. Angew Chem Int Ed Engl. 2003;42(28):3251–3253. [DOI] [PubMed] [Google Scholar]
- 268.Dijkgraaf I, Demmer O, Schumacher U, et al. CXCR4 receptor targeting for in-vivo imaging of metastases. Eur J Nucl Med Mol Imaging. 2008;35:S318-S. [Google Scholar]
- 269.Gourni E, Demmer O, Schottelius M, et al. PET of CXCR4 expression by a 68Ga-labeled highly specific targeted contrast agent. J Nucl Med. 2011;52(11):1803–1810. [DOI] [PubMed] [Google Scholar]
- 270.Wester HJ, Keller U, Schottelius M, et al. Disclosing the CXCR4 expression in lymphoproliferative diseases by targeted molecular imaging. Theranostics. 2015;5(6):618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Buck AK, Stolzenburg A, Hanscheid H, et al. Chemokine receptor - directed imaging and therapy. Methods (San Diego, Calif). 2017;130:63–71. [DOI] [PubMed] [Google Scholar]
- 272.Kircher M, Herhaus P, Schottelius M, et al. CXCR4-directed theranostics in oncology and inflammation. Ann Nucl Med. 2018;32(8): 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Walenkamp AME, Lapa C, Herrmann K, Wester HJ. CXCR4 ligands: the next big hit?. J Nucl Med. 2017;58(Suppl 2):77s–82s. [DOI] [PubMed] [Google Scholar]
- 274.Schottelius M, Osl T, Poschenrieder A, et al. [177]Lu-pentixather: preclinical and first patient results with a highly promising CXCR4-directed endoradiotherapeutic agent. J Nucl Med. 2015;56(supplement 3):339.25678490 [Google Scholar]
- 275.Habringer S, Lapa C, Herhaus P, et al. Dual targeting of acute leukemia and supporting niche by CXCR4-directed theranostics. Theranostics. 2018;8(2):369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Brave M, Farrell A, Ching Lin S, et al. FDA review summary: mozobil in combination with granulocyte colony-stimulating factor to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation. Oncology. 2010;78(3–4):282–288. [DOI] [PubMed] [Google Scholar]
- 277.Taromi S, Kayser G, Catusse J, et al. CXCR4 antagonists suppress small cell lung cancer progression. Oncotarget. 2016;7(51): 85185–85195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Bodart V, Anastassov V, Darkes MC, et al. Pharmacology of AMD3465: a small molecule antagonist of the chemokine receptor CXCR4. Biochem Pharmacol. 2009;78(8):993–1000. [DOI] [PubMed] [Google Scholar]
- 279.Ling X, Spaeth E, Chen Y, et al. The CXCR4 antagonist AMD3465 regulates oncogenic signaling and invasiveness in vitro and prevents breast cancer growth and metastasis in vivo. PLoS One. 2013;8(3):e58426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ramsey DM, McAlpine SR. Halting metastasis through CXCR4 inhibition. Bioorg Med Chem Lett. 2013;23(1):20–25. [DOI] [PubMed] [Google Scholar]
- 281.Zhou KX, Xie LH, Peng X, et al. CXCR4 antagonist AMD3100 enhances the response of MDA-MB-231 triple-negative breast cancer cells to ionizing radiation. Cancer Lett. 2018;418:196–203. [DOI] [PubMed] [Google Scholar]
- 282.Reeves PM, Abbaslou MA, Kools FRW, Poznansky MC. CXCR4 blockade with AMD3100 enhances Taxol chemotherapy to limit ovarian cancer cell growth. Anticancer Drugs. 2017;28(9):935–942. [DOI] [PubMed] [Google Scholar]
- 283.Kalatskaya I, Berchiche YA, Gravel S, Limberg BJ, Rosenbaum JS, Heveker N. AMD3100 is a CXCR7 ligand with allosteric agonist properties. Mol Pharmacol. 2009;75(5):1240–1247. [DOI] [PubMed] [Google Scholar]
- 284.Hendrix CW, Collier AC, Lederman MM, et al. Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection. J Acquir Immune Defic Syndr. 2004;37(2):1253–1262. [DOI] [PubMed] [Google Scholar]
- 285.McDermott DH, Liu Q, Velez D, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014;123(15):2308–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.McDermott DH, Pastrana DV, Calvo KR, et al. Plerixafor for the treatment of WHIM syndrome. N Engl J Med. 2019;380(2):163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Mosi RM, Anastassova V, Cox J, et al. The molecular pharmacology of AMD11070: an orally bioavailable CXCR4 HIV entry inhibitor. Biochem Pharmacol. 2012;83(4):472–479. [DOI] [PubMed] [Google Scholar]
- 288.Dale DC, Firkin FC, Bolyard AA, et al. Results of a phase 2 trial of an oral CXCR4 antagonist mavorixafor for treatment of WHIM syndrome. Blood. 2020; blood.2020007197. 10.1182/blood.2020007197. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Starbuck K, McGray R, Masoumi-Moghaddam S, Francois A, Odunsi K, Zsiros E. Abstract 956: novel oral small molecule CXCR4 inhibitor improves activity of immune checkpoint blockade in ovarian cancer mouse model. Cancer Res. 2017;77(13 Supplement):956. [Google Scholar]
- 290.Saxena R, Wang Y, Mier JW, Abstract 1749: CXCR4 inhibition modulates tumor microenvironment and robustly inhibits growth of B16-OVA melanoma. Cancer Res. 2018;78:1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Jecs E, Miller EJ, Wilson RJ, et al. Synthesis of novel tetrahydroisoquinoline CXCR4 antagonists with rigidified side-chains. ACS Med Chem Lett. 2017;9(2):89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Truax VM, Zhao H, Katzman BM, et al. Discovery of tetrahydroisoquinoline-based CXCR4 antagonists. ACS Med Chem Lett. 2013;4(11):1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Tahirovic YA, Truax VM, Wilson RJ, et al. Discovery of N-alkyl piperazine side chain based CXCR4 antagonists with improved drug-like properties. ACS Med Chem Lett. 2018;9(5):446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ichiyama K, Yokoyama-Kumakura S, Tanaka Y, et al. A duodenally absorbable CXC chemokine receptor 4 antagonist, KRH-1636, exhibits a potent and selective anti-HIV-1 activity. Proc Natl Acad Sci USA. 2003;100(7):4185–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Murakami T, Kumakura S, Yamazaki T, et al. The novel CXCR4 antagonist KRH-3955 is an orally bioavailable and extremely potent inhibitor of human immunodeficiency virus type 1 infection: comparative studies with AMD3100. Antimicrob Agents Chemother. 2009;53(7):2940–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Peng D, Cao B, Zhou YJ, Long YQ. The chemical diversity and structure-based evolution of non-peptide CXCR4 antagonists with diverse therapeutic potential. Eur J Med Chem. 2018;149:148–169. [DOI] [PubMed] [Google Scholar]
- 297.Masuda M, Nakashima H, Ueda T, et al. A novel anti-HIV synthetic peptide, T-22 ([Tyr5,12,Lys7]-polyphemusin II). Biochem Biophys Res Commun. 1992;189(2):845–850. [DOI] [PubMed] [Google Scholar]
- 298.Tamamura H, Xu Y, Hattori T, et al. A low-molecular-weight inhibitor against the chemokine receptor CXCR4: a strong anti-HIV peptide T140. Biochem Biophys Res Commun. 1998;253(3):877–882. [DOI] [PubMed] [Google Scholar]
- 299.DeMarco SJ, Henze H, Lederer A, et al. Discovery of novel, highly potent and selective beta-hairpin mimetic CXCR4 inhibitors with excellent anti-HIV activity and pharmacokinetic profiles. Bioorg Med Chem. 2006;14(24):8396–8404. [DOI] [PubMed] [Google Scholar]
- 300.Karpova D, Bräuninger S, Wiercinska E, et al. Mobilization of hematopoietic stem cells with the novel CXCR4 antagonist POL6326 (balixafortide) in healthy volunteers-results of a dose escalation trial. J Transl Med. 2017;15(2):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Pernas S, Martin M, Kaufman PA, et al. Balixafortide plus eribulin in HER2-negative metastatic breast cancer: a phase 1, single-arm, dose-escalation trial. Lancet Oncol. 2018;19(6):812–824. [DOI] [PubMed] [Google Scholar]
- 302.Douglas G, Gambino G, Lemercier G, Longuet S, Remus T, Zimmermann J. Pharmacology, ADME and selectivity profile of the next generation CXCR4 antagonist balixafortide. J Clin Oncol. 2018;36:e14553. [Google Scholar]
- 303.Karpova D, Dauber K, Spohn G, et al. The novel CXCR4 antagonist POL5551 mobilizes hematopoietic stem and progenitor cells with greater efficiency than Plerixafor. Leukemia. 2013;27:2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Peng SB, Zhang X, Paul D, et al. Identification of LY2510924, a novel cyclic peptide CXCR4 antagonist that exhibits antitumor activities in solid tumor and breast cancer metastatic models. Mol Cancer Ther. 2015;14(2):480–490. [DOI] [PubMed] [Google Scholar]
- 305.Tamamura H, Hiramatsu K, Ueda S, et al. Stereoselective synthesis of [L-Arg-L/D-3-(2-naphthyl)alanine]-type (E)-alkene dipeptide isosteres and its application to the synthesis and biological evaluation of pseudopeptide analogues of the CXCR4 antagonist FC131. J Med Chem. 2005;48(2):380–391. [DOI] [PubMed] [Google Scholar]
- 306.Thiele S, Mungalpara J, Steen A, Rosenkilde MM, Vabeno J. Determination of the binding mode for the cyclopentapeptide CXCR4 antagonist FC131 using a dual approach of ligand modifications and receptor mutagenesis. Br J Pharmacol. 2014;171(23):5313–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Hainsworth JD, Reeves JA, Mace JR, et al. A randomized, open-label phase 2 study of the CXCR4 inhibitor LY2510924 in combination with sunitinib versus sunitinib alone in patients with metastatic renal cell carcinoma (RCC). Target Oncol. 2016;11(5):643–653. [DOI] [PubMed] [Google Scholar]
- 308.O’Hara MH, Messersmith W, Kindler H, et al. Safety and pharmacokinetics of CXCR4 peptide antagonist, LY2510924, in combination with durvalumab in advanced refractory solid tumors. J Pancreat Cancer. 2020;6(1):21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Guo H, Ge Y, Li X, et al. Targeting the CXCR4/CXCL12 axis with the peptide antagonist E5 to inhibit breast tumor progression. Signal Transduct Target Ther. 2017;2:17033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Li X, Guo H, Yang Y, et al. A designed peptide targeting CXCR4 displays anti-acute myelocytic leukemia activity in vitro and in vivo. Sci Rep. 2014;4:6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Portella L, Vitale R, De Luca S, et al. Preclinical development of a novel class of CXCR4 antagonist impairing solid tumors growth and metastases. PLoS One. 2013;8(9):e74548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Faber A, Roderburg C, Wein F, et al. The many facets of SDF-1alpha, CXCR4 agonists and antagonists on hematopoietic progenitor cells. J Biomed Biotechnol. 2007;2007(3):26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Kashyap MK, Amaya-Chanaga CI, Kumar D, et al. Targeting the CXCR4 pathway using a novel anti-CXCR4 IgG1 antibody (PF-06747143) in chronic lymphocytic leukemia. J Hematol Oncol. 2017;10(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Kuhne MR, Mulvey T, Belanger B, et al. BMS-936564/MDX-1338: a fully human anti-CXCR4 antibody induces apoptosis in vitro and shows antitumor activity in vivo in hematologic malignancies. Clin Cancer Res. 2013;19(2):357–366. [DOI] [PubMed] [Google Scholar]
- 315.Peng SB, Zhang X, Paul D, et al. Inhibition of CXCR4 by LY2624587, a fully humanized anti-CXCR4 antibody induces apoptosis of hematologic malignancies. PLoS One. 2016;11(3):e0150585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Jahnichen S, Blanchetot C, Maussang D, et al. CXCR4 nanobodies (VHH-based single variable domains) potently inhibit chemotaxis and HIV-1 replication and mobilize stem cells. Proc Natl Acad Sci U S A. 2010;107(47):20565–20570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Becker PS, Foran JM, Altman JK, et al. Targeting the CXCR4 pathway: safety, tolerability and clinical activity of ulocuplumab (BMS-936564), an anti-CXCR4 antibody, in relapsed/refractory acute myeloid leukemia. Blood. 2014;124(21):386. [Google Scholar]
- 318.Cuesta-Mateos C, Alcaraz-Serna A, Somovilla-Crespo B, Munoz-Calleja C. Monoclonal antibody therapies for hematological malignancies: not just lineage-specific targets. Front Immunol. 2017;8:1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Griffiths K, Habiel DM, Jaffar J, et al. Anti-fibrotic effects of CXCR4-targeting i-body AD-114 in preclinical models of pulmonary fibrosis. Sci Rep. 2018;8(1):3212. [DOI] [PMC free article] [PubMed] [Google Scholar]