The isolation and characterization of the Anderson–Evans-type tungstoantimonate [SbVWVI 6O24]7− with a T-shaped sodium–serinol complex, [Na5(H2O)18{(HOCH2)2CHNH3}2]7+, as counter-cation is reported.
Keywords: polyoxometalate, POM, polyoxotungstate, POT, serinol, crystal structure, organic-inorganic hybrid
Abstract
A novel tungstoantimonate, [Na5(H2O)18{(HOCH2)2CHNH3}2][SbVWVI 6O24] (SbW6 ), was synthesized from an aqueous solution and structurally characterized by single-crystal X-ray diffraction, which revealed C2/c symmetry. The structure contains two serinol [(HOCH2)2CHNH3]+ and five Na+ cations, which are octahedrally surrounded by 18 water molecules, and one [SbVWVI 6O24]7− anion. The serinol molecules also play a critical role in the synthesis by acting as a mild buffering agent. Each of the WVI and SbV ions is six-coordinated and displays a distorted octahedral motif. A three-dimensional supramolecular framework is formed via hydrogen-bonding interactions between the tungstoantimonates and cations. Powder X-ray diffraction, elemental analysis, thermogravimetric analysis and IR spectroscopy were performed on SbW6 to prove the purity, to identify the water content and to characterize the vibrational modes of the crystallized phase.
Introduction
Polyoxometalates (POMs) are known as early transition metal–oxygen clusters (Pope, 1983 ▸; Gumerova & Rompel, 2020 ▸). They are assembled from {MO x } polyhedra, with x = 4–7 and M being commonly MoV/VI, WV/VI, VIV/V, NbV or TaV as addenda ions, through sharing of corners and edges (Pope, 1983 ▸). POMs can be considered as either isopolyanions [Mm O y ] q−, which feature only one metallic M ion (MoV/VI, WV/VI, VIV/V, NbV or TaV), or heteropolyanions [XrMm O y ] q−, which additionally contain a heteroelement X (Pope, 1983 ▸). POMs display a wide range of crucial applications, ranging from catalysis (Wang & Yang, 2015 ▸), materials science (Cherevan et al., 2020 ▸) and molecular magnetism (Clemente-Juan et al., 2012 ▸), to bio- and nanotechnology (Rhule et al., 1998 ▸; Bijelic et al., 2018 ▸, 2019 ▸), as well as macromolecular crystallography (Bijelic & Rompel, 2015 ▸, 2017 ▸, 2018 ▸).
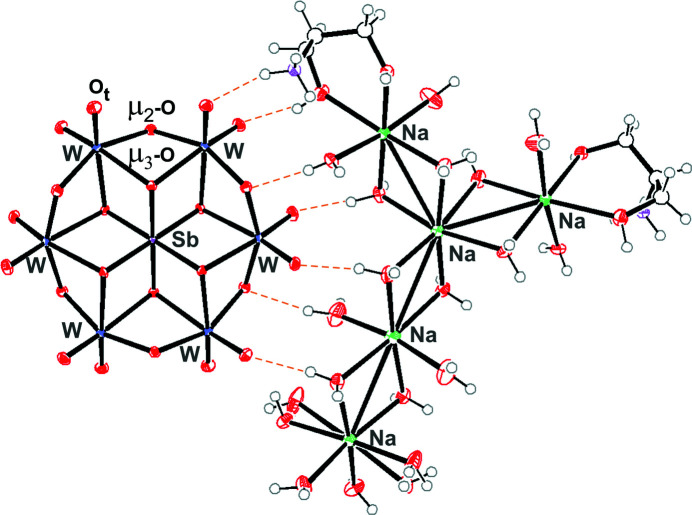
The Anderson–Evans polyoxoanion has the general formula [H y (XO6)M 6O18] n− , where y = 0–6, n = 2–8, M = addenda ion (MoVI or WVI) and X = central heteroion in oxidation states from +2 to +7 (Blazevic & Rompel, 2016 ▸). Its structure consists of six corner- and edge-shared {MoO6} or {WO6} octahedra, which surround the {XO}6 octahedron (Evans, 1948 ▸). In the structure, there exist three differently coordinated oxygen ions (Fig. 1 ▸): six triple-bridged oxygen ions (μ3-O) that connect the heteroion and two addenda ions, six double-bridged oxygen ions (μ2-O) that connect two addenda ions and lastly two terminal oxygen ions (Ot) per addenda ion (Evans, 1948 ▸; Pope, 1983 ▸). The oxidation state of a heteroion plays a significant role in the protonation mode of the triple-bridged oxygen ions (μ3-O) in the Anderson–Evans archetype, according to which they can be divided into three groups. The first, i.e. [Xn + M 6O24](12–n)− (n = 5–7), referred to as ‘type A’, is a deprotonated structure that exists when it contains heteroions with a high oxidation state (e.g. TeVI or IVII). The second, i.e. [Xn +(OH)6 M 6O18](6–n)− (n = 2–4), referred to as ‘type B’, is protonated on the six μ3-O ions, with each side having three protons. The B-type POMs are usually present when the heteroion has a low oxidation state (e.g. NiII or CoII) (Blazevic & Rompel, 2016 ▸). The third group, called ‘mixed type’, is a combination of the two types mentioned above, as it has protonated μ3-O ions only on one side (Gumerova et al., 2019 ▸). Therefore, it is referred to as one-side protonated with one known polyoxotungstate example, [CrIII(OH)3WVI 6O21]6–, so far (Gumerova et al., 2019 ▸). Interestingly, there are some platinum-based compounds, with the general formula [H n PtIV M VI 6O24](8–n)− (M = W or Mo, where 1 < n < 6; Lee et al., 2004 ▸), which exhibit protonation degrees of their μ3-O ions ranging from 1 to 6 and n is not necessarily an integer.
Figure 1.
Displacement ellipsoid plot of SbW6 with the hydrogen-bond interactions between the anion and the counter-cation complex highlighted (orange dashed line). Displacement ellipsoids are displayed at the 50% probability level. Colour code: W blue, Sb pink, Na green, C grey, N purple, O red and H white.
The majority of Anderson–Evans-type clusters have a planar hexagonal configuration with
 m (D
3d
) point symmetry, which is known as the α-isomer (Anderson, 1937 ▸). Another configuration of the Anderson–Evans-type cluster shows a bent structure of 2mm (C
2v
) point symmetry and is called the β-isomer (Lindqvist, 1959 ▸). An example of the β-isomer was presented by the Ogawa group (Ogawa et al., 1988 ▸), i.e. [SbV(OH)2MoVI
6O22]5− and is one of the few reported structures to date (Lee & Sasaki, 1994 ▸; Zhang et al., 2017 ▸; Li & Wei, 2021 ▸). The Anderson–Evans compounds can be modified in several ways: (i) by variation of the heteroion in the central position; (ii) by combination with various inorganic and organic cations, and (iii) by covalent attachment of one or two alkoxo ligands.
m (D
3d
) point symmetry, which is known as the α-isomer (Anderson, 1937 ▸). Another configuration of the Anderson–Evans-type cluster shows a bent structure of 2mm (C
2v
) point symmetry and is called the β-isomer (Lindqvist, 1959 ▸). An example of the β-isomer was presented by the Ogawa group (Ogawa et al., 1988 ▸), i.e. [SbV(OH)2MoVI
6O22]5− and is one of the few reported structures to date (Lee & Sasaki, 1994 ▸; Zhang et al., 2017 ▸; Li & Wei, 2021 ▸). The Anderson–Evans compounds can be modified in several ways: (i) by variation of the heteroion in the central position; (ii) by combination with various inorganic and organic cations, and (iii) by covalent attachment of one or two alkoxo ligands.
In recent decades, some unsubstituted tungstoantimonates, which have only one heteroion, SbIII/V, have been synthesized and structurally characterized (Table 1 ▸), but the majority of Sb-containing polyoxotungstates (POTs) contain additional heteroions, such as 3d or 4f metals (Tanuhadi et al., 2018 ▸, 2020 ▸). Only three Sb-POTs of the Anderson–Evans archetype, namely, K5Na2[SbVWVI 6O24]·12H2O (Lee & Sasaki, 1987 ▸), K5.5H1.5[SbVWVI 6O24]·6H2O (Naruke & Yamase, 1992 ▸) and Na7[SbVWVI 6O24]·24H2O (Mukhacheva et al., 2017 ▸), have been reported so far. Interestingly, the relative luminescence yield from the O→W LCMT transition of K5.5H1.5[SbVWVI 6O24]·6H2O was higher than that of Ln-containing Na9[GdIII(WVI 5O18)2]·18H2O under the same conditions (Naruke & Yamase, 1992 ▸). The high luminescence yield of [SbVWVI 6O24]7− is attractive for potential photochemical applications in the future.
Table 1. POTs with SbIII/V as the only heteroion [based on the Inorganic Crystal Structure Database (FIZ, Karlsruhe; http://www.fiz-informationsdienste.de/DB/icsd/www-recherche.htm) and the Cambridge Structural Database (CSD; Groom et al., 2016 ▸) in April 2021].
| POT | Type | References |
|---|---|---|
| K5Na2[SbVWVI 6O24] | Anderson | Lee & Sasaki (1987 ▸) |
| K5.5H1.5[SbVWVI 6O24] | Anderson | Naruke & Yamase (1992 ▸) |
| Na7[SbVWVI 6O24] | Anderson | Mukhacheva et al. (2017 ▸) |
| K6[H12SbV 6WVI 4O36] | Pseudo-Anderson–Evans dimer | Park et al. (1994 ▸) |
| (NH4)9[SbVWVI 18O60(OH)2] | Dawson | Zhang et al. (2010 ▸) |
| Na9[SbIIIWVI 9O33] | Keggin | Bösing et al. (1997 ▸) |
| K12[SbIII 2WVI 22O74(OH)2] | Krebs | Bösing et al. (1997 ▸) |
| [N(CH3)4]10Na12[Na2SbIII 8WVI 36O132(H2O)4] | Trimer based on lacunary Keggin | Bösing et al. (1997 ▸) |
| (H2en)8H6{[SbIII 2(WVIO2)2(B-β-SbIIIWVI 9O33)2][(WVIO2)2(WVIO3)2(B-β-SbIIIWVI 9O33)2]} (en = ethylenediamine) | Krebs | Xin et al. (2019 ▸) |
| K11Na16[H2(SbIIIWVI 9O33)(WVI 5O12)(SbIII 2WVI 29O103)] | Trimer based on lacunary Keggin | Tanuhadi et al. (2021 ▸) |
Serinol (C3H9NO2, 2-aminopropane-1,3-diol) is a very stable, highly water soluble, nontoxic, odourless, biodegradable compound which is widely used as a versatile starting material in organic synthesis and as an additive for materials applications, such as composite materials (Barbera et al., 2020 ▸; Andreessen & Steinbüchel, 2011 ▸). In POM synthesis, serinol can be seen as an alkoxylation ligand or a counter-cation or buffering agent due to the presence of an amino group. Considering that the Sb-centred Anderson–Evans POT has not yet been reported with organic counter-cations, we expand the compound class by applying serinol, which can coordinate in different ways to metals through the –NH2 and –HOCH2 groups and thus significantly affects both the structure and properties, in the synthesis in Sb5+–WO4 2− (with an Sb:W ratio of 1:6) systems. Here we report a novel Anderson–Evans Sb-centred POT, [Na5(H2O)18{(HOCH2)2CHNH3}2][SbVWVI 6O24] (SbW6 ), being the first example of [SbVWVI 6O24]7− crystallized with an organic counter-cation, which was synthesized from aqueous solution and has been fully characterized.
Experimental
Synthesis and crystallization
The reagents were used as purchased from Merck (Austria) and VWR (Austria) without further purification.
Synthesis of [Na5(H2O)18{(HOCH2)2CHNH3}2][SbW6O24] (SbW6)
Na2WO4·2H2O (0.99 g, 3 mmol) and KSbV(OH)6 (0.13 g, 0.5 mmol) were mixed in a 6:1 ratio in H2O (12 ml), yielding a turbid solution. The solution was then acidified with aqueous HCl (1 M, 4.4 ml) and the pH was set at 4.0. Serinol [(HOCH2)2CHNH2; 0.18 g, 2 mmol] was then added and the pH was altered to 7.1. Under stirring and heating for 1 h, at 75 °C, the precipitate was dissolved, and the final solution was colourless. The pH after the reaction was 7.0. The solution was left for evaporation at room temperature, leading to colourless crystals suitable for single-crystal X-ray diffraction within 1 d (yield: 0.4 g, 60%, based on W). The pH of the Sb5+–WO4 2− solution was varied from 3.7 to 5.0; however, after the addition of serinol (0.18 g, 2 mmol), the final pH was in the range from 7.0 to 7.7 and, in all cases, crystals with the same unit cell were obtained. Other synthetic routes, such as reflux reaction and hydrothermal synthesis at 120 °C, for the same reaction mixture led to the same product. Elemental analysis found (calculated) for [Na5(H2O)18{(HOCH2)2CHNH3}2][SbVWVI 6O24] (%): C 3.18 (3.23), H 2.56 (2.53), N 1.29 (1.25), O 32.89 (32.97). FT–IR (cm−1): 3357 (s), 2952 (sh), 1610 (s), 1498 (s), 1464 (sh), 1373 (w), 1256 (w), 1099 (w), 1037 (s), 1018 (sh), 927 (s), 850 (s), 703 (sh), 632 (w), 640 (w), 563 (w), 420 (s), 349 (s), 310 (s).
IR spectroscopy
SbW6 was characterized by IR spectroscopy on a Bruker Vertex70 IR Spectrometer equipped with a single-reflection diamond-ATR unit in the range 4000–300 cm−1.
TGA measurements
Thermogravimetric analysis (TGA) was performed on a Mettler SDTA851e Thermogravimetric Analyzer under a nitrogen flow with a heating rate of 5 K min−1 in the region from 303 to 873 K.
Elemental analysis
The determination of C/H/N/O was carried out using an ‘EA 1108 CHNS-O’ elemental analyzer by Carlo Erba Instruments at the Mikroanalytisches Laboratorium, Faculty of Chemistry, University of Vienna.
Powder X-ray diffraction (PXRD)
PXRD was performed on a Bruker D8 Advance diffractometer, with Cu Kα radiation (λ = 1.54056 Å), a Lynxeye silicon strip detector and a SolX energy dispersive detector (variable slit aperture with 12 mm, 10° ≤ 2θ ≤ 50°).
Refinement
In Table 2 ▸, the crystallographic characteristics of SbW6 and the experimental conditions of data collection and refinement are reported. The positions of the H atoms of the water molecules were obtained by difference Fourier techniques and were refined with free isotropic displacement parameters and O—H distances restrained to 0.95 (2) Å. The disordered water molecule in the coordination sphere of atom Na1 was refined with two positions (O23 and O24), with free occupancy factors to a total of 100%. The H atoms of this disordered group had U iso(H) values set to 1.5U eq(O) of the parent atom. H atoms bound to N or C atoms were placed in idealized positions (N—H = 0.91 Å and C—H = 0.99 or 1.00 Å for CH2 and CH groups, respectively) and refined in riding modes, with U iso(H) values set to 1.5U eq(N) or to 1.2U eq(C).
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | [Na5(H2O)18(C3H10NO2)2][SbW6O24] |
| M r | 2232.32 |
| Crystal system, space group | Monoclinic, C2/c |
| Temperature (K) | 200 |
| a, b, c (Å) | 21.9761 (14), 13.9179 (9), 16.209 (1) |
| β (°) | 111.189 (2) |
| V (Å3) | 4622.5 (5) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 15.61 |
| Crystal size (mm) | 0.1 × 0.08 × 0.05 |
| Data collection | |
| Diffractometer | Bruker APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Bruker, 2016 ▸) |
| T min, T max | 0.542, 0.747 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 100214, 8841, 8213 |
| R int | 0.030 |
| (sin θ/λ)max (Å−1) | 0.770 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.013, 0.025, 1.15 |
| No. of reflections | 8841 |
| No. of parameters | 390 |
| No. of restraints | 23 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.60, −0.69 |
Results and discussion
The preparation of SbW6 was carried out at a WVI to SbV ratio of 6:1 and at a pH of 7.1. In the absence of serinol, at pH 7.5, protonated K5.5H1.5[SbVWVI 6O24]·6H2O (Naruke & Yamase, 1992 ▸), and at pH 4.5, unprotonated K5Na2[SbVWVI 6O24]·12H2O (Lee & Sasaki, 1987 ▸), were obtained.
The main structural elements of SbW6 are the Anderson–Evans [SbVWVI 6O24]7− anion and the complex [Na5(H2O)18{(HOCH2)2CHNH3}2]7+ cation, which are connected via hydrogen bonds between terminal (Ot) and bridging O atoms (μ2-O) of the polyanion and protons from the cationic complex (Fig. 1 ▸). Crystallographically centrosymmetric [SbVWVI 6O24]7− shows the characteristic Anderson–Evans A-type structure with a central {SbO6} octahedron surrounded by six edge-shared {WO6} octahedra that form a planar array of distorted octahedra (Fig. 1 ▸). The average W—Sb bond length is 3.26 Å. As is typical for all Anderson–Evans A-type structures, three different coordination modes of the O atoms are present in the structure: six triple-bridged oxygen ions (μ3-O) connect the heteroion and two W ions, six double-bridged oxygen ions (μ2-O) connect two W ions and two terminal oxygen (Ot) ions are connected to each of the six W ions (Fig. 1 ▸). The average distance for Sb—μ3-O is 1.98 Å, for W—μ3-O is 2.27 Å, for W—μ2-O is 1.94 Å and for W—Ot is 1.74 Å. The values are comparable with those of K5.5H1.5[SbVWVI 6O24] (Naruke & Yamase, 1992 ▸). For instance, the Sb—μ3-O bond length (1.98 Å) differs by only 0.03 Å from the others reported by Naruke & Yamase (1992 ▸) (2.01 Å). Applying bond valence sum (BVS) calculations (Brown & Altermatt, 1985 ▸), all the W ions in [SbVWVI 6O24]7− exhibit the +VI oxidation state (average calculated value of 6.01) and Sb shows the +V oxidation state (5.37). Based on BVS analysis and the number of counter-cations, it was concluded that the Anderson–Evans anion is not protonated and belongs to type A.
The counter-cation is composed of five octahedrally coordinated Na+ ions, which assemble in an elevated T-shape form, and two protonated serinol molecules that are coordinated to two Na+ ions via –HOCH2 groups. This group has crystallographically imposed twofold symmetry. One serinol ligand interacts through the –NH3 group with the terminal O atom of the POT anion, and the second interacts with the adjacent O atom in {NaO6} (Fig. 1 ▸).
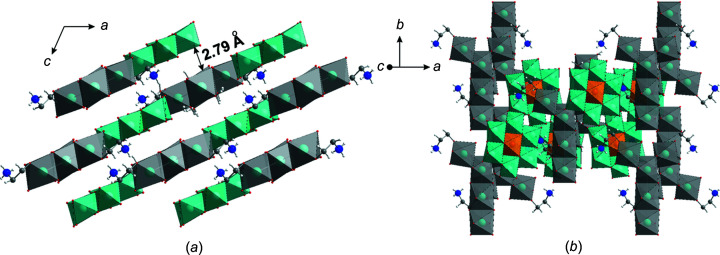
The three-dimensional (3D) structure of SbW6 consists of two-dimensional (2D) sheets formed of [SbVWVI 6O24]7− anions and complex [Na5(H2O)18{(HOCH2)2CHNH3}2]7+, cations connected via hydrogen bonds (Figs. 1 ▸ and 2 ▸). The distances between 2D layers are approximately 2.79 Å, which allows the formation of hydrogen bonds between the layers and creates cavities along the b axis (Fig. 2 ▸ a). This packing is different to that observed in K5.5H1.5[SbVWVI 6O24]·6H2O and K5Na2[SbVWVI 6O24]·12H2O, where the layers of anions alternate with layers or single polyhedra of counter-cations.
Figure 2.
The crystal packing of SbW6 , (a) viewed along the b axis and (b) viewed along the z axis. Colour code: {WO6} turquoise, {SbO6} orange, {Na(H2O)6} grey, C grey, N blue, O red and H black.
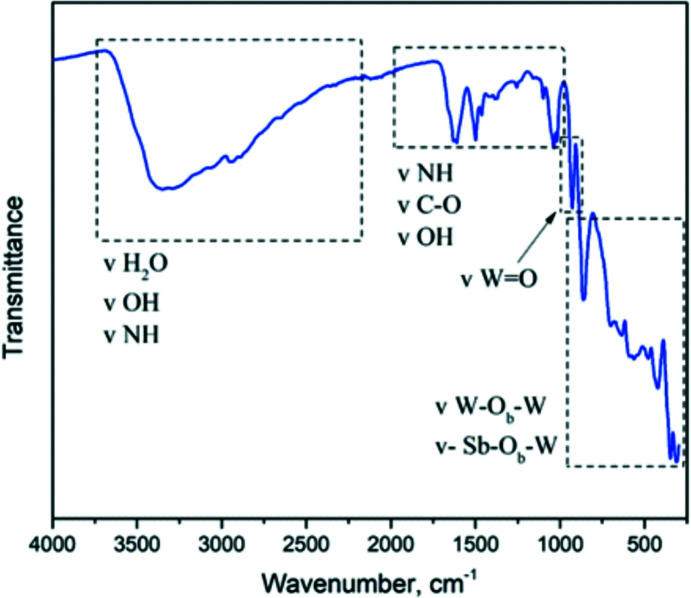
The IR spectrum of SbW6 (Fig. 3 ▸) is characteristic for Anderson–Evans POMs (Liu et al., 2015 ▸; Qu et al., 2012 ▸). The broad bands in the region between 2300 to 3750 cm−1 represent the vibrations of the –OH groups of H2O and the N—H bonds of the amine groups of serinol. In the area between 1610 and 1018 cm−1, the bands are attributed to the vibrations of C—H, C—O and again N—H in serinol. The bands at about 930 and 880 cm−1 are attributed to antisymmetric stretching vibrations of the terminal W=O bonds and Sb—O—W bridges (Ob), respectively. The bands at 640 and 563 cm−1 are associated with the asymmetric stretching of W—O—W bridges (Ob) and the bending vibrations of W—O—W, respectively. Lastly, the bands between 750 and 300 cm−1 are contributed by Sb—O—W vibrations (Liu et al., 2015 ▸).
Figure 3.
IR spectrum of SbW6 in the region from 4000 to 300 cm−1.
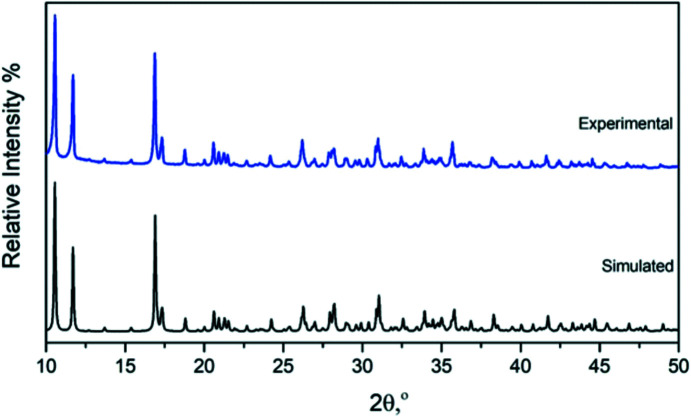
The powder XRD pattern of SbW6 (Fig. 4 ▸) was investigated at room temperature. The simulated powder diffraction pattern was based on the single-crystal structural data. The observed peak positions are in good alignment with the simulated patterns, which confirms that the POT structure had been solved accurately and that SbW6 consists of a single phase.
Figure 4.
Experimental (blue colour) and simulated (black colour) powder XRD pattern of SbW6 .
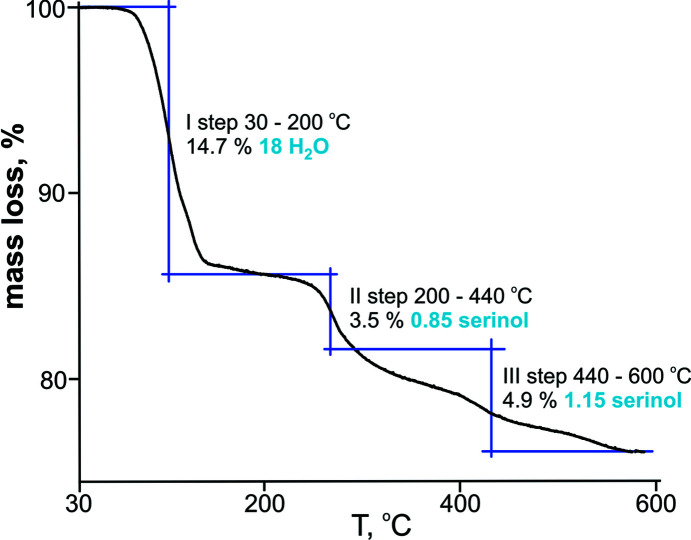
The exact number of water molecules was determined using TGA. The curve (Fig. 5 ▸) shows three weight-loss steps during the heating process from 30 to 600 °C. The first weight loss of 14.7% in the temperature range 30–200 °C corresponds to all water molecules from the Na+ coordinating spheres. The second and third step correspond in total to 8.4% and the loss of two serinol molecules.
Figure 5.
Thermogravimetric curve of SbW6 .
The composition of the counter-cation has remarkable effects on the crystal packing and thus on the physical properties of Anderson–Evans POMs (Blazevic & Rompel, 2016 ▸). The success in synthesizing SbW6 shows that the Sb-centred Anderson–Evans POT is a versatile building block, which can be modified by organic counter-cations into high-dimensional architectures. SbW6 is the first reported K+-free salt with an organic counter-cation, and it has much higher water solubility and can expand the areas of its application in aqueous solution.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2053229621006239/ky3204sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2053229621006239/ky3204Isup2.hkl
Chemical scheme for the title compound. DOI: 10.1107/S2053229621006239/ky3204sup3.pdf
CCDC reference: 2079597
Acknowledgments
The authors are grateful to Ass.-Prof. Dr. P. Unfried for support with the TGA and to Ao.Univ.-Prof. Mag. Dr. K. Richter for PXRD measurements at the Department of Inorganic Chemistry, Faculty of Chemistry, University of Vienna. We thank Elias Tanuhadi, MSc, for valuable discussions concerning this work.
Funding Statement
This work was funded by Austrian Science Fund grants P33927 and P33089 to Nadiia I. Gumerova and Annette Rompel; Erasmus+ grant 1016/2020 to Kleanthi Sifaki.
References
- Anderson, J. S. (1937). Nature, 140, 850.
- Andreessen, B. & Steinbüchel, A. (2011). AMB Express, 1, article No. 12. [DOI] [PMC free article] [PubMed]
- Barbera, V., Leonardi, G., Valerio, A. M., Rubino, L., Sun, S., Famulari, A., Galimberti, M., Citterio, A. & Sebastiano, R. (2020). ACS Sustainable Chem. Eng. 8, 9356–9366.
- Bijelic, A., Aureliano, M. & Rompel, A. (2018). Chem. Commun. 54, 1153–1169. [DOI] [PMC free article] [PubMed]
- Bijelic, A., Aureliano, M. & Rompel, A. (2019). Angew. Chem. Int. Ed. 58, 2980–2999. [DOI] [PMC free article] [PubMed]
- Bijelic, A. & Rompel, A. (2015). Coord. Chem. Rev. 299, 22–38. [DOI] [PMC free article] [PubMed]
- Bijelic, A. & Rompel, A. (2017). Acc. Chem. Res. 50, 1441–1448. [DOI] [PMC free article] [PubMed]
- Bijelic, A. & Rompel, A. (2018). ChemTexts, 4, article No. 10. [DOI] [PMC free article] [PubMed]
- Blazevic, A. & Rompel, A. (2016). Coord. Chem. Rev. 307, 42–64.
- Bösing, M., Loose, I., Pohlmann, H. & Krebs, B. (1997). Chem. Eur. J. 3, 1232–1237.
- Brandenburg, K. (2006). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Brown, I. D. & Altermatt, D. (1985). Acta Cryst. B41, 244–247.
- Bruker (2015). APEX3 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2016). SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Cherevan, A. S., Nandan, S. P., Roger, I., Liu, R., Streb, C. & Eder, D. (2020). Adv. Sci. 7, article No. 1903511. [DOI] [PMC free article] [PubMed]
- Clemente-Juan, J. M., Coronado, E. & Gaita-Ariño, A. (2012). Chem. Soc. Rev. 41, 7464–7478. [DOI] [PubMed]
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Evans, H. T. Jr (1948). J. Am. Chem. Soc. 70, 1291–1292.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Gumerova, N. I., Caldera Fraile, T., Roller, A., Giester, G., Pascual-Borràs, M., Ohlin, C. A. & Rompel, A. (2019). Inorg. Chem. 58, 106–113. [DOI] [PMC free article] [PubMed]
- Gumerova, N. I. & Rompel, A. (2020). Chem. Soc. Rev. 49, 7568–7601. [DOI] [PubMed]
- Hübschle, C. B., Sheldrick, G. M. & Dittrich, B. (2011). J. Appl. Cryst. 44, 1281–1284. [DOI] [PMC free article] [PubMed]
- Lee, U., Joo, H.-C. & Park, K.-M. (2004). Acta Cryst. E60, i55–i57.
- Lee, U. & Sasaki, Y. (1994). Bull. Korean Chem. Soc. 15, 37–45.
- Lee, U. K. & Sasaki, Y. (1987). Bull. Korean Chem. Soc. 8, 1–3.
- Li, Q. & Wei, Y. (2021). Chem. Commun. 57, 3865–3868. [DOI] [PubMed]
- Lindqvist, I. (1959). Ark. Kemi. 2, 323.
- Liu, W., Lin, Z., Bassil, B. S., Al-Oweini, R. & Kortz, U. (2015). CHIMIA Int. J. Chem. 69, 537–540. [DOI] [PubMed]
- Mukhacheva, A. A., Abramov, P. A. & Sokolov, M. N. (2017). Curr. Inorg. Chem. 7, 4–7.
- Naruke, H. & Yamase, T. (1992). Acta Cryst. C48, 597–599.
- Ogawa, A., Yamato, H., Lee, U., Ichida, H., Kobayashi, A. & Sasaki, Y. (1988). Acta Cryst. C44, 1879–1881.
- Park, K. M., Ozawa, Y. & Lee, U. (1994). J. Korean Chem. Soc. 3, 359–365.
- Pope, M. (1983). In Heteropoly and Isopoly Oxometalates. Berlin: Springer.
- Qu, X., Yang, Y., Zhang, F. & Yu, X. (2012). Struct. Chem. 23, 1867–1872.
- Rhule, J. T., Hill, C. L., Judd, D. A. & Schinazi, R. F. (1998). Chem. Rev. 98, 327–358. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. C71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Spek, A. L. (2020). Acta Cryst. E76, 1–11. [DOI] [PMC free article] [PubMed]
- Tanuhadi, E., Al-Sayed, E., Novitchi, G., Roller, A., Giester, G. & Rompel, A. (2020). Inorg. Chem. 59, 8461–8467. [DOI] [PMC free article] [PubMed]
- Tanuhadi, E., Gumerova, N. I., Prado-Roller, A., Mautner, A. & Rompel, A. (2021). Inorg. Chem. 60, 8917–8923. [DOI] [PMC free article] [PubMed]
- Tanuhadi, E., Roller, A., Giester, G., Kampatsikas, I. & Rompel, A. (2018). Dalton Trans. 47, 15651–15655. [DOI] [PMC free article] [PubMed]
- Wang, S.-S. & Yang, G.-Y. (2015). Chem. Rev. 115, 4893–4962. [DOI] [PubMed]
- Xin, X., Ma, Y., Hou, L., Wang, Y., Xue, X., Lin, J. & Han, Z. (2019). Inorg. Chem. 58, 9567–9571. [DOI] [PubMed]
- Zhang, J., Huang, Y., Hao, J. & Wei, Y. (2017). Inorg. Chem. Front. 4, 1215–1218.
- Zhang, Y.-Y., Liu, S., Yu, C., Tang, Q., Liang, D., Zhang, C., Ma, F., Li, S., Zhang, W. & Tan, R. (2010). Inorg. Chem. Commun. 13, 1418–1420.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2053229621006239/ky3204sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2053229621006239/ky3204Isup2.hkl
Chemical scheme for the title compound. DOI: 10.1107/S2053229621006239/ky3204sup3.pdf
CCDC reference: 2079597