Abstract
GLP-1 receptor agonists (GLP-1RAs) and SGLT-2 inhibitors (SGLT-2is) are novel antidiabetic medications associated with considerable cardiovascular benefits therapying treatment of diabetic patients. GLP-1 exhibits atherosclerosis resistance, whereas SGLT-2i acts to ameliorate the neuroendocrine state in the patients with chronic heart failure. Despite their distinct modes of action, both factors share pathways by regulating the central nervous system (CNS). While numerous preclinical and clinical studies have demonstrated that GLP-1 can access various nuclei associated with energy homeostasis and hedonic eating in the CNS via blood–brain barrier (BBB), research on the activity of SGLT-2is remains limited. In our previous studies, we demonstrated that both GLP-1 receptor agonists (GLP-1RAs) liraglutide and exenatide, as well as an SGLT-2i, dapagliflozin, could activate various nuclei and pathways in the CNS of Sprague Dawley (SD) rats and C57BL/6 mice, respectively. Moreover, our results revealed similarities and differences in neural pathways, which possibly regulated different metabolic effects of GLP-1RA and SGLT-2i via sympathetic and parasympathetic systems in the CNS, such as feeding, blood glucose regulation and cardiovascular activities (arterial blood pressure and heart rate control). In the present article, we extensively discuss recent preclinical studies on the effects of GLP-1RAs and SGLT-2is on the CNS actions, with the aim of providing a theoretical explanation on their mechanism of action in improvement of the macro-cardiovascular risk and reducing incidence of diabetic complications. Overall, these findings are expected to guide future drug design approaches.
Keywords: autonomic nervous system, diabetic complications, brain nuclei, cardiovascular diseases, diabetes, obesity
Introduction
Type 2 diabetes (T2D) is currently a severe global public health issue, owing to the huge burden associated with management of microvascular and macrovascular complications.1 These complications may not only impair a patient’s physical or social functions, thereby reducing the quality of life, but also cause death. The pathogenesis of diabetes comprises various functional changes in multi-organs in response to systemic energy disarrangement, which were previously described as “ominous octet” by Professor Defronzo.2 In recent years, there have been demands for re-classification of diabetes into various categories, namely severe autoimmune diabetes (SAID), severe insulin deficient diabetes (SIDD), severe insulin resistance diabetes (SIRD), mild obese related diabetes, and mild age-related diabetes (MARD), based on the ages of incidence, body mass index (BMI), hemoglobulin A1C (HbA1c), β-cell function, insulin resistance, and the positivity of glutamic acid decarboxylase autoantibody (GADA).3 Generally, this re-classification was aimed at ensuring accurate stratification for diabetic complications and guiding personalized treatments. Previous studies have shown that the control level of glycemia is positive with reduced incidence of microvascular diseases, such as diabetic kidney diseases and diabetic retinopathy, although it has a weak relationship with improvement of macrovascular diseases. A previous meta-analysis4 found that application of rosiglitazone, a thiazolidinedione (TZD) diabetic drug, could significantly increase the risk of myocardial infarction and death from cardiovascular causes, a concept that has subsequently been proven to be incorrect. However, these events forever changed the notion of the developing antidiabetic medications to endure tests with cardiovascular safety. Interestingly, multiple clinical trials have proved that the new medications, like glucagon-like peptide 1 receptor agonists (GLP-1RAs) and sodium glucose co-transporter 2 inhibitors (SGLT-2is), not only guarantees cardiovascular safety by lowering glucose levels but also have the ability to improve clinical outcomes in diabetic patients with cardiovascular diseases.5,6 Thus, new T2D pharmacotherapy guidelines have recommended the use of GLP-1RAs for prevention and treatment of obese patients with risks of atherosclerotic cardiovascular diseases, whereas SGLT-2is has been proposed for patients with a risk of chronic heart failure.7 Indications for both classes of drugs may vary, owing to different modes and mechanisms of action in T2D. Since regulation of the CNS is a critical pathway affected by these drugs, this review focused on studies describing similarities and differences by GLP-1RAs and SGLT-2is on the CNS.
Differences and Similarities Associated with the Biology and Physiology of GLP-1RA and SGLT-2i in Glucose Metabolism and Beyond
GLP-1 is mainly expressed in two sites in the body, namely intestinal L cells and brainstem. The GLP-1 receptor (GLP-1R), which belongs to class B, G protein-coupled receptor, is expressed in a wide range of tissues in the whole body, including α, β cells of the pancreatic islets, gastrointestinal tract, lung, skin, cardiovascular system, kidney, nodose ganglion neurons of the vagal nerve, as well as the hypothalamus and brainstem in the CNS.8,9 Generally, released GLP-1 from intestinal L cells bind onto canonical receptors in the pancreatic islet, after oral glucose load, or may indirectly signal the hepatic vagal branch within intraportal vein, thereby promoting glucose-dependent insulin secretion from the pancreatic β-cells, concurrently causing inhibition of glucagon secretion, and enhancing insulin gene transcription.10,11 A rapid decay in circulation, coupled with less than 2 minutes of half-life of GLP-1 by dipeptidyl peptidase-IV (DPP-IV), caused researchers to develop GLP-1R agonists or DPP-IV inhibitors aimed at maintaining the effects of GLP-1 in pharmacological or physiological concentration, respectively. The first extensively used agonist of GLP-1R was exendin-4, a 53% homologous peptide extracted from the venom of a Gila monster (Heloderma suspectum), while its potent antagonist is exendin 9–39.12 Functionally, GLP-1RAs has several effects, including lowering of glucose dependence, reducing appetite and body weight, anti-atherosclerosis, neural-protection, natriuresis, and bone osteogenesis, among others. These roles of GLP-1RAs have been extensively reviewed.13,14 Numerous researches have reported that the function of incretin axis is impaired in T2D or metabolic syndrome, a phenomenon that causes insufficient GLP-1 production, or disrupted GLP-1 action.15,16
SGLT-2, a member of the human SGLT (SLC5) gene family, has been shown to play various functions such as active cotransport for sugars, anions, vitamins, and short-chain fatty acids. The biology of the human SGLTs has also extensively reviewed elsewhere.17 Apart from glucose transport and maintenance of a physiological state, SGLTs have also been linked to sodium, water, and urea transport, as well as cellular depolarization. In fact, they frequently serve as glucose sensors in critical organs throughout the whole-body, including the brain.18 Previous studies have shown that this role may influence multifunctioning of specific neurons responsible for hormone secretion, wake and sleep, as well as appetite, among other activities.19–22 Moreover, SGLT-2 was reportedly upregulated in renal proximal tubule of T2D patients; other conditions,23 including the cancer secretory diarrhea, glucose galactose malabsorption and so on. Other studies have also demonstrated that SGLT-2 was overexpressed in the proximal tubule of the kidney, in patients with T2D and metabolic syndrome, partially due to sympathetic overactivity.24
The two classes of drugs exhibit similarities with regards to their physiology and biology, as evidenced by their ability to lower the blood glucose in a glucose-level-dependent manner. This property may be attributed to a physiology that is independent of insulin action. Both drugs control energy balance thereby causing a deficient. Specifically, GLP-1RAs inhibit energy intake, concurrently elevating energy expenditure, whereas SGLT-2is simultaneously promote energy secretion, and utilization at the same time. Secondly, both drugs can reduce the mass of the adipose tissue, thereby improving lipid toxicity and lowering body weight. Thirdly, both drugs modulate metabolic parameters, such as blood pressure, and uric acid, and hence exert multiple metabolic benefits to various organs in the body, including the heart, brain, liver, pancreas, and kidney. This benefit adds strength to management of diabetic complications, and could effectively suppress development of diabetic “ominous octet”.
Clinical Effects of GLP-1RAs and SGLT-2is on Cardiovascular Diseases
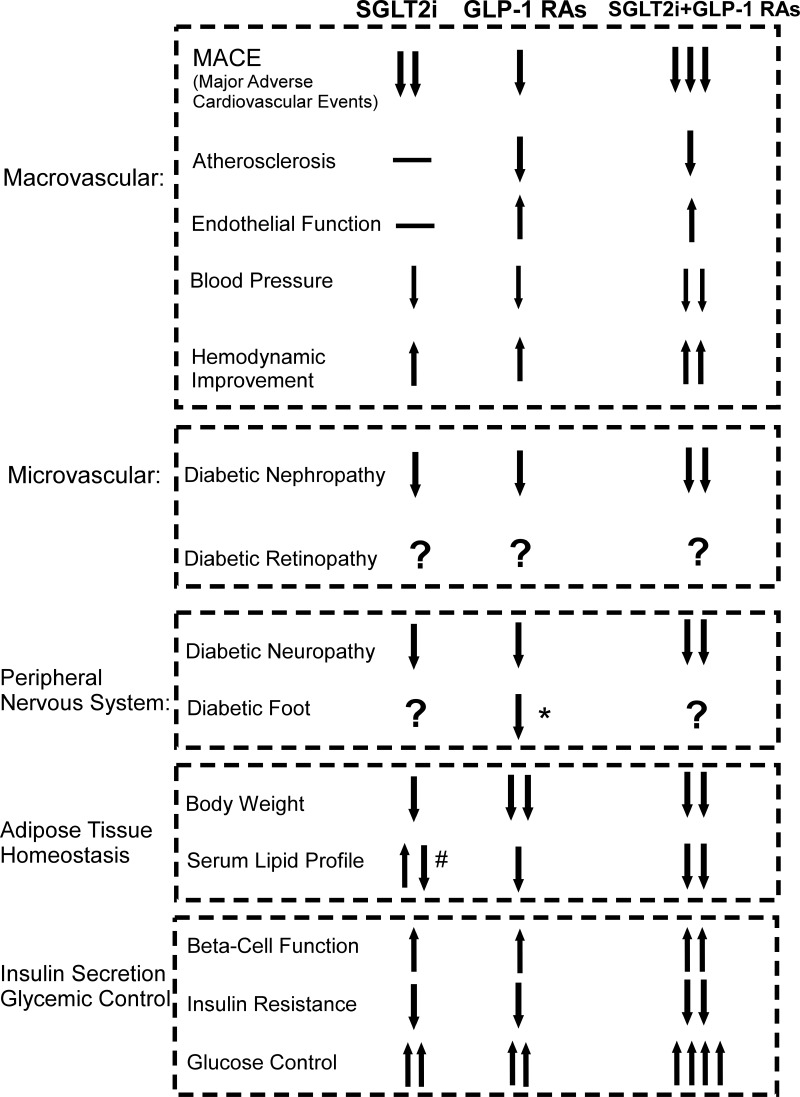
In the past 20 years, several changes have been made in treatment of diabetes. Between 1990 and 2000, results from clinical trials like the Diabetes Control and Complications Trial (DCCT), UK Prospective Diabetes Study (UKPDS), suggested that controlling intensive blood-glucose levels could reduce the incidence of microvascular complications, such as diabetic retinopathy, diabetic nephropathy, diabetic neuropathy. However, control of blood glucose alone was not effective in managing macrovascular complications.25–27 In 2008, the Food and Drug Administration (FDA) strongly warned that clinical application of antidiabetic medicine needed to be tested for cardiovascular safety, following a report that rosiglitazone could potentially exacerbate the risk of myocardial infarction and death. Before 2016, a series of clinical trials had explored this issue and showed no additional cardiovascular risk of available antidiabetic medications. However, there was no evidence that using those conventional antidiabetic drugs resulted in significant cardiovascular benefits. At the beginning of 2016, results from several clinical trials demonstrated that novel drugs, such as GLP-1RAs and SGLT-2is, showed efficacy in reducing the incidence of cardiovascular events in an unprecedented manner. A comparison of the clinical efficacies of GLP-1RAs and SGLT-2is o is summarized in Figure 1. As the figure shows, both GLP-1RAs and SGLT-2is could reduce the MACE, with the SGLT-2is treatment seem to be nominally associated with lower risk of heart failure and total mortality, whereas with GLP-1RAs seem to be associated with lower risks of stroke and peripheral artery disease.28 SGLT-2is reduced mortality and admission to hospital for heart failure more than GLP-1RAs, and GLP-1 RAs reduced non-fatal stroke more than SGLT-2is.29 In multiple clinical trials, both drugs showed reduced significantly on cardiovascular risk factors like blood pressure, body weight.30–33 However, the application of SGLT-2i in some studies has been reported to significantly increase total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), non-high density lipoprotein cholesterol (non-HDL-C), and high-density lipoprotein cholesterol (HDL-C) and decrease triglyceride (TG).34 Improved renal function, diabetic neuropathy and pancreatic β-cell functions were seen in administration of both two drugs.35–38 Due to the current limited studies and related sample size, the risks of complication of diabetic foot in SGLT-2is is not clear, but are not with GLP-1RA.39,40 In addition, there were no strong clinical evidences showing the application of either GLP-1RA or SGLT-2i has the positive effect on the diabetic retinopathy.41,42 Nonetheless, it is noticeable that there were emerging clinical studies reported that combination of GLP-1RAs and SGLT-2is could amplify many merits with monotherapy alone, like enhanced glucose control, body weight reduction, improved hemodynamics, and cardiovascular benefits and so on.43–45 Currently, GLP-1RAs, including liraglutide, and exenatide, as well as SGLT2is like canagliflozin, dapagliflozin and empagliflozin, have been approved for clinical use in patients with T2DM across many countries. Consequently, these novel antidiabetic medications have been proven to be safe, and efficacious in multiorgan metabolic benefits.
Figure 1.
An overview and comparison of major effects of GLP-1RAs and SGLT-2is. The figure summarizes major clinical effects of both antidiabetic drugs on the energy homeostasis and complication management, with regards to macrovascular, microvascular, peripheral nervous system, adipose tissue homeostasis, and insulin secretion and glycemic control. *GLP-1RAs significantly reduce diabetic foot ulcer- related amputations. #SGLT2is significantly increase total cholesterol, LDL-C, non-HDL-C, and HDL-C and decrease triglyceride.
Abbreviations: MACE, major adverse cardiovascular events; SGLT-2i, sodium glucose co-transporter-2 inhibitors; GLP-1RAs, glucagon like peptide-1 receptor agonists.
Initial approval of the first GLP-1RA, Exenatide, in 2006, followed by that of GLP-1RAs was swiftly followed up by randomized controlled trials (RCT) whose results revealed their role in treating T2D.46 Specifically, long-term GLP-1RAs administration was found to effectively lower glucose levels, tolerability, and safety. The first study to report GLP-1RA’s efficacy in cardiovascular disease was ELIXA (2015). Here, lixisenatide exerted no significant benefits relative to the placebo, with regards to primary composite endpoint of cardiovascular death, myocardial infarction, stroke, or hospitalization in unstable angina. Moreover, treatment groups revealed no significant increase in incidences of severe hypoglycemia, pancreatitis, pancreatic neoplasms, or allergic reactions relative to the placebo.47 In 2016, initial results from the LEADER trial revealed that Liraglutide had cardiovascular benefits, and significantly lowered the risk of primary composite outcomes of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke by 13% risk compared to the placebo.6 However, unlike SGLT-2i, liraglutide did not significantly reduce the incidence of hospitalization for patients with heart failure, which was a predominant benefit with empagliflozin,48 although the drug was associated with delayed cardiovascular benefits, possibly due to the modification of progression of atherosclerotic cardiovascular disease. Additionally, patients treated with liraglutide exhibited a significant loss of weight, as well as reduction in systolic blood pressure and proteinuria reduction. Trials that have examined efficacy of GLP-1RA therapies include Evaluation of Lixisenatide in Acute Coronary Syndrome [ELIXA] trial,47 Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results [LEADER] trial,6 Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes [SUSTAIN-6],49 oral administration of semaglutide in the Trial Investigating the Cardiovascular Safety of Oral Semaglutide in Subjects With Type 2 Diabetes [PIONEER-6],50 once-weekly exenatide in the Exenatide Study of Cardiovascular Event Lowering [EXSCEL] trial,51 albiglutide in the Harmony Outcomes trial,52 and dulaglutide in the Researching Cardiovascular Events With a Weekly Incretin in Diabetes [REWIND] trial,53 among others. Current progress in clinical trials and outcomes of GLP-1RA have been extensively reviewed elsewhere,54,55 with results of these trials indicating that GLP-1RA therapy does not only guarantee cardiovascular safety but also has considerable benefits. Thus, recent ADA and CDC guidelines have recommended use of GLP-1RAs for treatment of T2D patients with high risk of atherosclerosis and coronary heart disease.
SGLT-2is have been also shown to lower the levels of blood sugar in a glucose-dependent manner. Particularly, they mediate a decrease in the level of blood glucose during hyperglycemia, and diminish or cease the action when the level of blood glucose is below normal. This effect is not a common phenomenon in conventional antidiabetic drugs, such as sulfonylureas., Besides, SGLT-2i, including empagliflozin, canagliflozin, dapagliflozin, have also showed excellent cardiovascular safety.48,56,57 For SGLT-2 inhibitors, the major completed CVOTs including empagliflozin in the Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients [EMPA-REG OUTCOME], canagliflozin in the Canagliflozin Cardiovascular Assessment Study [CANVAS Program], and dapagliflozin in the Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 [DECLARE-TIMI 58] trial. Results from such clinical trials have been summarized elsewhere.54,55 Functionally, these classes of drugs strongly and selectively inhibit reabsorption of glucose by renal SGLT-2, thereby resulting in glucosuria, and rearrangement of bodily hemodynamics, such as reduction of arterial blood pressure, depletion of interstitial fluid rather than blood volume that improves both preload and afterload in heart failure, and neuroendocrine factors like the RAAS system.58,59 Based on FDA specifications, it is evident that patients treated with canagliflozin, dapagliflozin, empagliflozin exhibit a 24-hour glucosuria of about 100, 70, and 64g, respectively, which correspondingly cause about 400, 280, and 256Kcal/d of energy loss.60–63 Additionally, a recent meta-analysis revealed that all SGLT-2is reportedly cause a weight loss of about 1.5–2kg in obese T2D patients, relative to placebo, and this effect is dose dependent.33,64,65 Clinical data from 4 years of follow-up showed that bodyweight reduction is maintained under SGLT2i administration.66–68 On the other hand, results of bodily fluid displacement through kidney demonstrated that SGLT-2is can significantly lower blood pressure, reduce both preload and afterload of the heart without stimulating sympathetic nervous system and changing the heart rate, indicating that it can improve the outcomes of chronic heart failure in patients with T2D. Interestingly, SGLT-2is distinctly suppresses macrovascular events like 3P-MACE (ie, 3-point major adverse cardiovascular events including cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke) earlier than GLP-1RAs in T2D. Furthermore, results from other studies, such as the Credence trial, have demonstrated that SGLT-2is can alleviate diabetic nephropathy, suggesting that it has renal safety benefits and can prevent complications associated with T2D therapy.35 Therefore, SGLT-2is is highly recommended for treating T2D patients with a risk of heart failure and diabetic retinopathy. The unique pharmacological properties and complementary action of the 2 drugs have enhanced their efficacy in prevention of CVD, thereby making them efficacious in treatment of T2D and its complications.44,69,70
The Relationship Between Central Energy Mechanism and Cardiovascular Regulation
Correlation Between Energy Metabolism Pathways and Cardiovascular Control
Energy and cardiovascular regulation represent critical partial targets controlled by the autonomic nervous system in the CNS. Apart from sharing common neural components, substrates and circuits, both systems also interact as cause and effect. One common result of energy imbalance is the metabolic syndrome,71 while its landmark pathophysiology change is insulin resistance and adipose tissue inflammation. Previous studies have associated both factors with increased body mass, atherosclerosis, and other cardiovascular diseases.71 Besides, some SGLTs in CNS have been shown to act as glucose sensors, by counter-regulating hypoglycemia.72 For example, a previous study found that secondary activation of the SNS could elevate blood pressure, possibly via neural signals within the hypothalamus, and this action was driven by the central actions of leptin as well as activation of neurotrophic factors in the brain.73 Chronic heart failure is mainly caused by sustained activation of the SNS, while RAAS and compensated increase in other neuroendocrine factors have been associated with cardiac remodeling and fibrosis.
Results from some in vitro studies have also reported a link between energy homeostasis with cardiovascular activity. For example, Orexin, a neuropeptide that is commonly expressed within the hypothalamus, was associated with food intake, energy homeostasis, and consciousness, among others. Studies using electrophysiology and cell patch clamp demonstrated that orexin could activate the PVN neurons, subsequently increasing the heart rate, and elevating blood pressure via projection to IML of spinal cord.74 On the other hand, leptin, an adipokine, was found to interact with orexin during energy homeostasis, thereby influencing cardiovascular activity.75,76 The urocortin system, which is extensively expressed in the CNS and plays a role in HPA axis as well as counter-regulation response to hypoglycemia,77 and energy homeostasis,78 was recently reported to have potential in regulating cardiovascular activity.79 Previous studies have also shown that estrogens, which centrally affect glucose homeostasis, could activate estrogen receptor α (Erα) thereby contributing to cardiometabolic protection.80–82 These data affirm that neuropeptides or hormones that influence energy and metabolism could be playing critical roles in ANS, and have functional convergences in cardiovascular control.
The Independences Between Energy Metabolism and Cardiovascular Control
Energy control by the CNS, including areas and pathways, take charge of physiological energy homeostasis and psychological rewarding motivational activities.71,83 The nuclei and pathways responsible for energy homeostasis are mainly distributed in the brainstem and hypothalamus, as well as the nucleus of tractus solitarius (NTS), and parabrachial nucleus (PBN), among others.84 Hedonic feeding in the CNS mainly occurs through the mesolimbic and corticolimbic pathways. Specifically, the mesolimbic pathway represents “wanting” or motivation for foods, which comprise nuclei of midbrain like ventral tegmental area (VTA), substantia nigra (SN), and striatum, as well as nucleus accumbens (NAc). On the other hand, corticolimbic pathway denotes “liking” area for feeding, which have a complex shape, owing to presence of multiple links that originate from the hippocampus, prefrontal cortex, and amygdala.85 Dopamine, endocannabinoid, and endogenous opioids, among others, play a critical role as mediators for the rewarding effects of energy balance. Previous studies have shown that GLP-1RAs not only modulate the activities of specific neurons within energy metabolism, including PVN, ARC, LH, and NTS of classic homeostasis areas but also influence those of NAc, VTA, cortex in hedonic regions, thereby contributing to energy deficient through reduced food intake, and enhanced energy expenditure, as well as alleviation of the metabolic syndrome.86
In cardiovascular regulation, a similar sensing system exists in areas that lack impermeable BBB. Specifically, these structures are termed circumventricular organs, and act to receive signals from circulation, like angiotensin II (Ang II), nitric oxide (NO), atrial natriuretic peptide (ANP), vasopressin (AVP), and endothelin.87 Previous studies have also shown that hypothalamus activities can also influence cardiovascular activity.88,89 For example, PVN plays a significant role in elevating sympathetic-driven hypertension, while some studies have demonstrated that disequilibrium of GABA and AVP neuron transduction in PVN may play a role in the pathogenesis of hypertension.90–92 Other studies have also shown that ARC also has a significant impact on blood pressure regulation.93–95 Moreover, expression of proinflammatory cytokines, like NF-κB,96 and TNF,97 in the hypothalamus was found to significantly affect the Renin-angiotensin-aldosterone system (RAAS) and cause hypertension in many murine models. Notably, administration of anti-inflammatory agents or substances that reduce reactive oxygen species (ROS) or improve NO pathways alleviated the observed hypertension.98–101 Salt intake has also been associated with a change in both activity of hypothalamic intraneuronal salt-inducible kinase 1 (SIK1)-Na+/K+ ATPase,102 and PVN glutamate, angiotensin type 1 receptor (AT1R).103 Results from studies using rodents revealed that activation of AMPK in hypothalamus was associated with activation of the sympathetic nerve and hypertension.104,105 Recent advances in hypothalamus signaling in response to hypertension have been extensively reviewed.106 NTS of brainstem is responsible for the initiation and integration of various reflexes that control circulation.88,107 Besides, the two functionally different regions in VLM: the rostral VLM represents “pressor area”,107,108 whereas the caudal VLM is a “depressor area”.88,109–111 The physiology of the ANS and the cardiovascular control have been extensively reviewed elsewhere.88,112 To date, however, only a handful of studies have described functional localization of SGLT-2 and its associated interventions.19,20
Central Nervous Pathways Associated with GLP-1RAs’ Treatment Outcomes
Application of GLP-1RAs is associated with a reduction in major adverse cardiovascular events, microvascular diseases, injury of peripheral nervous system, and improved glucose control (Figure 1). GLP-1’s metabolic cardiovascular benefits can be attributed to a reduction in body weight, improved glucose and lipids metabolism, preserved pancreatic function and direct cardiovascular protection.
Interestingly, endogenous GLP-1 can only exist for less than 3 minutes in systematic circulation, while its effects can only be felt locally. Therefore, there can be a massive distinction in central actions between physiological concentration of endogenous GLP-1 with GLP-1RAs’ pharmacological levels. However, whether endogenous GLP-1 exerts similar effects to the classic hormone is debatable.113 As previously mentioned, endogenous GLP-1 can be expressed in gastrointestinal L cells, and the brain,114 which is produced physiologically relevant to be within NTS.115,116 Previous studies have hypothesized that local activation of GLP-1R may be relayed, to the NTS of brainstem, and vagal nerve, as signals and cause this transmission.10,117–119 This is partially due to limited overlapped distribution of the GLP-1 with GLP-1R neurons in the NTS, but with the terminals of vagal afferents from the gastrointestinal tract. GLP-1 and GLP-1R neurons have been extensively reviewed elsewhere.120 The NTS can project to extensive brain nuclei,86,121–123 or to the autonomic column of spinal cords.124,125 Although previous research evidences have indicated that there is only one unique GLP-1R throughout the body, signaling between peripheral and central GLP-1R may vary.126 Particularly, central GLP-1R signaling may largely encode meal information, visceral organ signals relayed by vagal afferent transferred to the NTS, and circulation of limited GLP-1 to the ARC as well as other circumventricular organs,86,127 whereas peripheral signaling may be transduced through local GLP-1R-induced neural-hormonal reflexes which influence insulin release or gastrointestinal motility.10 Endogenous GLP-1 causes peripheral food intake effects, including delay gastric emptying as well as reduction in gastrointestinal secretions and gastric mobility.128 This disparity may be obvious between physiological GLP-1 and GLP-1RA, owing to the fact that GLP-1RA has a higher chance of accessing the CNS. A study by Brierley et al129 found that pre-proglucagon neurons in NTS (PPGNTS) encode satiation in mice, which receives vagal signaling of gastrointestinal distension. However, PPGNTS neurons predominantly received vagal input from oxytocin-receptor-expressing vagal neurons, rather than GLP-1 receptors. In addition, PPGNTS neurons did not significantly suppress eating via GLP-1RA, although concurrent activation of the PPGNTS neuron had a higher effect in suppressing eating than semaglutide alone. The authors concluded that the central and peripheral GLP-1 systems play a role in eating suppression via independent gut–brain circuits. Regardless of these findings, it is known that physiologically, the central and peripheral mechanism commonly co-exist and function synergistically, and are complementary to each other.130–132 A combination of central and peripheral GLP-1 signaling may eventually cause net satiation, and anorexigenic effects as well as reduction in body weight, improved glucose and lipids metabolism, and increased energy negative balance.
GLP-1RAs were developed to not only prevent degradation by DPP-IV but to also enhance levels of GLP-1 analog in circulation. Thus, the mechanism of action employed by GLP-1RAs differs from that of endogenous GLP-1. To date, GLP-1RAs’ central action have been demonstrated by either penetrated leaky BBB in hypothalamus and brainstem or activation of abdominal vagal GLP-1R afferents. The primary type of action has also been documented. Previous studies have demonstrated that in humans, vagal afferents mediate the effects of exogenous GLP-1 on food intake, gastric emptying, as well as insulin and glucagon secretion.133 In addition, GLP-1RAs were found to influence acute satiation or short-term process.134 Recently, Fortin et al135 evaluated the effects of liraglutide in both NTS AAV-shRNA-driven Glp1r knockdown and AP-lesioned animals, and used fluorescence in situ hybridization to detect Glp1r transcripts in NTS GABAergic neurons. Their inhibition, using chemogenetics, resulted in attenuated food intake- and body weight-reducing effects by liraglutide. Taken together, their findings demonstrate that NTS GLP-1Rs contributes to anorectic potential of liraglutide and highlights a phenotypically distinct (GABAergic) population of neurons within the NTS that express GLP-1R are involved in the mediation of liraglutide signaling. However, their results are in contrast with those of Adams et al,136 who found that liraglutide modulated appetite and body weight through GLP-1R-expressing glutamatergic neurons. Moreover, Secher et al137 found that liraglutide did not actually upregulate preproglucagon (PPG) mRNA in the hindbrain, while reduction in the body weight of rats was independent of GLP-1R in the vagal nerve, area postrema, and PVN. Moreover, peripheral injection of fluorescently-labeled liraglutide in mice revealed presence of the drug in the circumventricular organs, whereas labeled liraglutide bound neurons within ARC and other discrete sites in the hypothalamus. Liraglutide seems to interact with POMC and NPY neurons in ARC. In a recent study, which demonstrated that GLP-1RA caused elevated heart rate (HR), it was clear that this increase was not mediated by NTS PPG neurons in that Exendin-4 also did not activate PPG neurons.138 In fact, their findings revealed that Ex-4-induced tachycardia persisted following ablation of PPG neurons of NTS, while Ex-4 did not induce expression of the neuronal activity marker c-Fos in PPG neurons. Moreover, inhibition or ablation of PPG neurons did not alter the resting HR in mice, although chemogenetic activation of the PPG neurons resulted in an increase. A recent study by Gabery et al,139 using Semaglutide, revealed that GLP-1RAs could directly access multiple brain nuclei, including the brainstem, septal nucleus, and hypothalamus, but did not cross the BBB. It only interacted with the brain through the circumventricular organs and several select sites adjacent to the ventricles. Particularly, Semaglutide induced central c-Fos activation in 10 brain areas, including hindbrain areas that it directly targets, as well as secondary regions without direct GLP-1R interaction, such as the lateral parabrachial nucleus (LPB). On the other hand, Baraboi et al140 used a c-Fos mRNA assay to reveal that GLP-1RAs could activate multiple brain nuclei, in a dose- and vagal-dependent manner. In our previous study,141 we used a c-Fos antibody to detect brain activation by GLP-1Ras. Indirect evaluation and comparison between the central action of Liraglutide and Exenatide revealed that GLP-1RAs could significantly induced c-Fos expression in caudal NTS of SD rats relative to controls in which we detected sparse c-Fos expression. Our results further revealed multiple nuclei, with significant upregulation of c-Fos relative to the control group. This expression was evident in ARC, PVN, periaqueductal gray (PAG), AP (area postrema), LPB, and IML of spinal cords, but not in the hippocampus, cortex, basal ganglia, suggesting that GLP-1RAs may be activating the CNS via multiple neuroendocrine pathways. Intriguingly, our results revealed that elevation in glucose levels in the first hour after exenatide administration in SD rats. After excluding occasionality, we hypothesized that this may be due to the excitability of SNS by GLP-1RA, especially exenatide, in line with the findings of multiple preclinical studies that showed that Exendin may acutely activate SNS, and this effect independent of insulinotropic and hypothalamus-pituitary-adrenal axis activation can be blocked by GLP-1 antagonist.142,143 Furthermore, this effect has been found to be dose-dependent.140 Previous studies have also corroborated our findings, as evidenced by the fact that GLP-1RA may only exert heart action via canonical GLP-1R in atrial but not ventricular myocardium, owing to a lack of canonical GLP-1R expressions in the ventricular myocardium.144–146 In fact, results from both in vivo and in vitro studies have shown that GLP-1RA, endogenous GLP-1, GLP-1 metabolites, or DPP-IV inhibitors may have distinct targets beyond canonical GLP-1R in the cyto-protection, which enable them to play important roles in improvement of endothelial function, increasing coronary blood flow, and modification of myocardial motility, among others. A summary of the cardiac effect for different GLP-1s can be found in a review.147 Although peripheral GLP-1 plays various essential cardioprotective roles, such as direct influence on cardiac electrophysiology, regulation of blood lipids, anti-atherosclerosis and anti-inflammation (see55), the central actions of GLP-1RAs cannot be overlooked. For example, Jessen et al132 demonstrated that central administration of GLP-1 increased neuronal activity in brain regions (PVN and NTS), thereby mediating autonomic nervous system, as well as activation of HPA axis and stimulation of circulating levels of corticosterone and epinephrine. On the other hand, Yamamoto et al148 found that GLP-1R activation could induce distinct c-fos expression in the adrenal medulla and neurons in autonomic control sites, including PVN, LH, PBL, NTS, and RVLM in rat brain, the medullary catecholamine neurons of brainstem can provide input to sympathetic preganglionic neurons, and GLP-1R agonists rapidly activated tyrosine hydroxylase transcription in brainstem catecholamine neurons like area postrema (AP).149 Moreover, Baggio et al150 established cardiomyocyte conditional GLP-1R (Glp1rCM-/-) and HCN4 knockout mice models, and found that treatment with both Liraglutide and lixisenatide elevated HR in vivo in the wild types, and attenuated in Glp1rCM-/- mice, which could be blocked by a β-adrenoceptor antagonist. However, the chronotropic effect exposure to GLP-1RA were not significant when the heart tissues, including atrium were isolated. Their findings suggested that the increase in HR may centrally depend on activation of the autonomic nervous system, while cardiac GLP-1R is not essential. Moreover, Griffioen et al151 reported that GLP-1R activation suppressed HR variability, and this effect was supported by the findings of the inhibition on release of both excitatory glutamatergic and inhibitory glycinergic neurotransmitters to the preganglionic parasympathetic cardiac vagal neurons; Furthermore, Holt et al,138 intra-peritoneally injected exendin-4 to the spinal cord of rats and detected an increase HR although this did not influence the arterial blood pressure, which is independent of PPG neurons activation in NTS, and can be blocked by a β-adrenoceptor antagonist. Results from long-term clinical trials have shown that GLP-1RA still shows cardiovascular safety in patients regardless of activation SNS and increase HR.152,153 Another possible mechanism of action may be attributed to its cardioprotective effects, that play a role in regulating the parasympathetic nervous system. Basalay et al154 analyzed a myocardial infarction mouse model and found that both remote ischemic pre- or per- conditioning reduced infarction size by 50%. However, this effect was abolished by a GLP-1 antagonist, subdiaphragmatic vagotomy, or M3 muscarinic receptor blockade, via a mechanism similar to GLP-1R signaling, manifested by alteration in phosphorylation of AKT and STAT3. On the other hand, Chen et al155 evaluated the role of exendin-4 in a myocardial infarction SD rat model, and assessed the effects of exendin-4 on atrial electrophysiology, atrial fibrosis and PI3K/AKT signaling. Their results suggested that GLP-1RA could inhibit atrial arrhythmogenesis, and improve conducting properties as well as fibrosis in myocardial infarction-induced heart failure. Similarly, Verouhis et al156 evaluated the endothelial function by flow-mediated dilatation (FMD), in human subjects, at various conditions including remote ischemic conditioning (RIC) with GLP-1 antagonist, and found significantly lower FMD compared with the RIC alone. These results suggested that RIC protects against endothelial ischemia-reperfusion injury via a GLP-1 receptor-mediated mechanism in humans. Taken together, these evidences suggest that GLP-1R signaling can exert cardio-protection traits, while the autonomic nervous system could be a critical regulator of this effect.
Discovery of GLP-1 and its receptor distribution in the CNS was rapidly followed by identification of GLP-1RAs, which is capable of accessing or influencing multiple brain nuclei or areas associated with regulation of energy metabolism. GLP-1RA may influence the activity of two opposite subgroup neurons within ARC, which are related to food intake and body weight, containing proopiomelanocortin/cocaine- and amphetamine-regulated transcript (POMC/CART) and neuropeptide Y/agouti related peptide (NPY/AgRP) neurons;137,157 In PVN,158,159 GLP-1RA particularly, has been shown to interact with neuropeptides, such as AVP, oxytocin, and CRH release, thereby regulating eating and stress; BNST,160,161 which is associated with suppression of food intake and stress response; LPB,162 which reportedly mediates food intake and reward; hippocampus,163 which influences cognition performance for food intake in rodents; NTS,164 a major critical knot for afferent signals of gastro-intestine and satiety; VTA165,166 and NAc,167–169 which process reward for food and drug behavior; amygdala170,171 that influences glucose homeostasis and food reward; as well as HPA-axis172,173 which could affect stress and SNS.174 Previous studies have reported the comprehensive effects of GLP-1RA on the CNS, which go beyond the levels of blood glucose modulation, but are associated with numerous metabolic aspects such as food intake and preference,127,175 water intake,176 energy expenditure,177 body weight reduction,178 pancreatic function,132,179,180 hypertension,181 stress,182 addiction behaviors,183,184 as well as neurodegenerative disorders, such as Alzheimer and Parkinson diseases.185 These metabolic effects have proved versatile central actions of GLP-1RAs, and have been associated with direct or indirect potential secondary benefits like reduced risks of dyslipidemia, anti-atherosclerosis, modulation of cardiovascular activity, and proteinuria, as well as influencing clinical outcomes in T2D patients.
Central Pathways Associated with SGLT-2is’ Treatment Outcomes
To date, only a handful of studies have reported the effects of SGLT-2is on the CNS, mainly due to complexity of SGLTs family, and a lack of research elucidating the exact roles of SGLT-2 in the CNS. One issue to be solved may be the differential expression patterns of SGLT-2 in CNS between human and rodents, although some studies have attempted to describe central distribution and the related function.18,72 So far, preclinical investigations on the relationships between CNS and SGLT-2i are scarce, possibly because energy regulation and cardiovascular control by ANS share many common pathways, neural circuits, and nuclei. Therefore, we reviewed available literature describing the versatile effects of SGLT-2i on CNS, with the aim of establishing the actual role played by SNS and central physiology of SGLT-2i with regards to the mechanism of cardiovascular benefits. Although the kidney overexpresses SGLT-2, and the antidiabetic medication SGLT-2is largely acts on this target, we found evidences of its expression in other tissues apart from the kidney across many databases (such as PubMed), which may also have critical functions essential for cardiac protections. Knockout of SLC5A2, which encodes the protein of SGLT-2 in rodents, reportedly caused polyuria, hyperphagia, increased glucosuria, and decreased aldosterone, and increased circulation renin.186,187 Besides, previous studies have also emphasized the emerging roles of obesity and metabolic syndrome in pathogenesis of SNS overactivity and cardiovascular diseases, as well as upregulation of SGLT-2 and its activity.24,188–190 In diabetic patients, an increase in filtered glucose of renal proximal tubule was found to enhance SGLT-2 activity, which subsequently worsened glycemic control and promoted Na+ loading following impaired blood pressure control.191 Therefore, we discuss the current evidences discussing the relationship between SGLT-2 inhibition and SNS activity, as well as the possible role of central SGLT-2.
The acknowledged mechanism of SGLT-2 action involves glucosuria and osmotic diuresis, which modifies the overall hemodynamics. However, the reason for which fluid deplete seldom induce RAAS and HR alterations should be attributed to the sympathetic inhibition by SGLT-2i with unidentified pathway.24 Erdogan et al192 found that Dapagliflozin decreased seizure activity in rats with pentylenetetrazol (PTZ) –but induced epileptic seizure. This may be because the anti-seizure effects reduced glucose availability and suppressed sodium transport across neuronal membranes, thereby conferring a stabilizing effect against abnormal depolarization. Since only limited body weight reductions have been previously reported following SGLT2i treatment, hyperphagia is reportedly one of the causes of this limited weight loss. Consequently, Chiba et al193 found that the oxygen consumption and brown adipose tissue (BAT) expression of ucp1, a thermogenesis-related gene, was significantly downregulated 18 hours after dapagliflozin treatment relative to the control group. In addition, Dapagliflozin significantly suppressed norepinephrine (NE) turnover in BAT and downregulated c-fos in the rostral raphe pallidus nucleus (rRPa). Moreover, the authors observed a decrease in glycogen contents and upregulation of phosphoenolpyruvate carboxykinase, 6 hours after Dapagliflozin treatment, while these changes could be abolished via common hepatic branch vagotomy. Overall, their findings proved the sympathetic inhibition via CNS of SGLT-2i. Furthermore, Tahara et al194 found that diabetic rats exhibited hyperphagia and elevated plasma levels of the appetite-stimulating hormones neuropeptide Y and ghrelin. However, treatment with Ipragliflozin induced significant weight loss and reduced plasma levels of appetite-stimulating hormones without affecting food intake, while reduced arteriovenous difference in postprandial glucose levels was improved by ipragliflozin. These results suggest that regulation of appetite-related hormones may be a critical mechanism associated with SGLT-2i-mediated regulation of body weight and energy. Moreover, Matthews et al195 reported that administration of Dapagliflozin to neurogenic hypertensive Schlager (BPH/2J) mice caused a reduction in blood pressure and prevented weight gain. The authors also found that chemical sympathetic denervation achieved by systemic administration of 6-hydroxy-dopamine (6-OHDA) reduced body weight and heightened SNS innervation in white adipose tissue (WAT). Notably, two weeks of Dapagliflozin treatment increased SNS innervation in WAT of hypertensive mice, and was accompanied by a non-significant upregulation of Ucp1 and Pgc-1α genes, which are markers of beiging. The authors found no significant differences in expression levels of the inflammatory mediators Il-6 and Tnf-α in WAT of Dapagliflozin-treated mice. Metabolic syndrome is often associated with disruption of circadian rhythm of systemic hemodynamics and cardiovascular disease. A study by Rahman et al196 found that luseogliflozin, a selective SGLT2 inhibitor, significantly increased the difference in the low frequency component of systolic blood pressure between the dark and light period in SHRcp rats. Moreover, higher locomotor activity was recorded during the dark relative to the light period following luseogliflozin treatment. In our previous study,197 we used c-Fos and SGLT-2 antibodies and detected SGLT-2 expression across multiple areas in the CNS, which belongs to autonomic nervous system, including lateral septum, amygdala, hypothalamus, PGA, locus caeruleus (LC), and NTS. Notably, c-Fos was significantly upregulated after intragavage of Dapagliflozin in most autonomic areas like amygdala, PVN, PGA, LC, and NTS, and this pattern was associated with both lower systolic and diastolic blood pressure without significant alteration to the heart rate 2 hours after administration of Dapagliflozin. These findings suggested that SGLT-2i may be causing hypotensive effects via the CNS, although its exact role is yet to be elucidated. These findings affirm that SGLT-2is may be regulating energy homeostasis and cardiovascular control via the central pathway, which is an important underlying mechanism with beneficial clinical implications. However, it is not known whether these mechanisms are direct, central actions of SGLT-2is may be one of the essential links to the clinical improvement of RAAS and sympathetic activation in cardiovascular conditions like chronic heart failure in T2D.
Conclusions
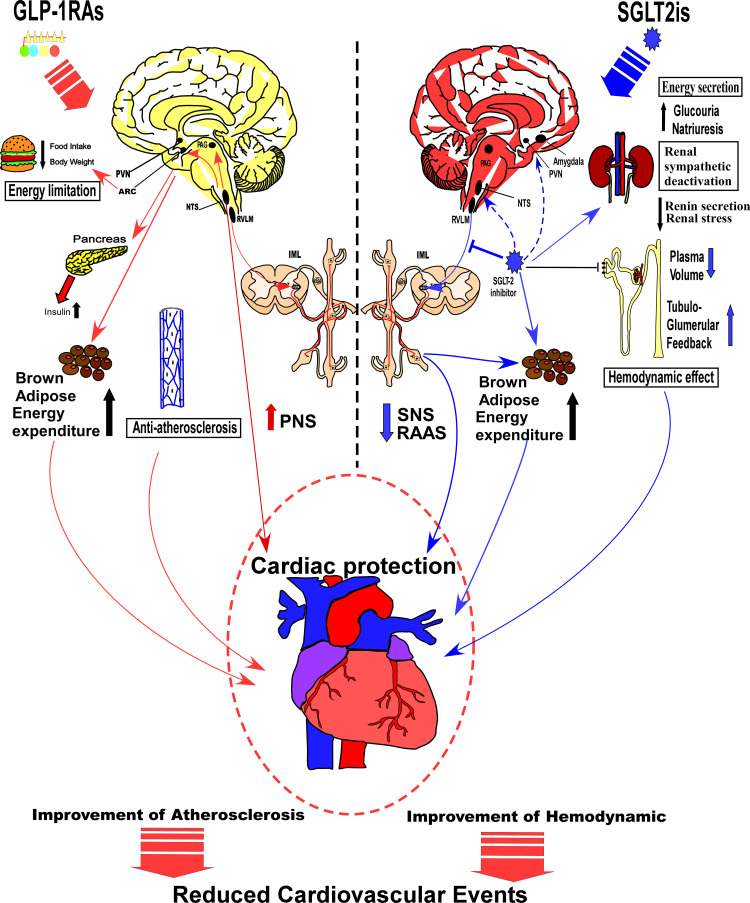
Energy and cardiovascular regulation represent the two crucial functions of the autonomic nervous system, while GLP-1RAs and SGLT-2is signaling may generate different effects on the central nervous system. Particularly, GLP-1RAs influence the energy setpoint of the hypothalamus, especially with regards to fat intake, meal size, pancreatic function, energy expenditure, and body weight, thereby providing either a direct or indirect protection to the heart and its vessels. On the other hand, SGLT-2is influence glucose sensing or absorption in specific neurons, and suppresses the sympathetic nervous system, thus regulating the cardiovascular activity, lowering energy expenditure, and maintaining glucose homeostasis in the pancreas and kidney (Figure 2). A variation in the underlying mechanisms of action by the two drugs has paved the way for the different clinical effects and benefits. Consequently, GLP-1RAs are currently recommended for treatment of T2D patients with higher risk of coronary heart diseases, owing to its anti-atherosclerosis and tube benefits. On the other hand, SGLT-2is causes early benefit on heart failure than GLP-1RAs, shows improved hemodynamics and sympathetic inhibition, and is frequently applied for treatment of T2D patients with higher risks of progression to severe heart failure. Nonetheless, there is large convergence in the effects of GLP-1RAs and SGLT-2is on the central nervous system. In the peripheral nervous system, both drugs affect the cardiac function, via SNS and parasympathetic nervous system (PNS). In organs and tissue, both of them play a role on energy expenditure of brown adipose tissue via the SNS, whereas in the brain stem, middle brain and forebrain, the relative nuclei may be attributed to modulation of the SNS and PNS, which may be the converging point of action for both drugs. Overall, GLP-1RAs and SGLT-2is not only share a common central nervous mechanism but also have an individual neuronal pathway, which are mutually complementary, and play a crucial role in effectively treating T2D patients.
Figure 2.
A mechanistic comparison of central and peripheral pathways related to the treatment outcomes by GLP-1RAs and SGLT-2is. As showing in the left side of the figure, GLP-1RAs influences energy setpoint of the hypothalamus, and may limit food intake, decrease body weight, promote insulin secretion and energy expenditure by increasing adipose tissue browning. They can also increase PNS activity, and exert effects of anti-atherosclerosis, which could benefit cardiovascular system. As the right side of the figure showing, SGLT-2is influence glucose absorption and inhibit energy expenditure. They also promote renal tubule-glomerular feedback, decrease the blood volume, and reduce the renal stress, which interacts with SNS and RAAS. Besides, SGLT-2is may activate specific sensing neurons and certain neural pathways in the CNS, thereby suppressing the SNS, regulating cardiovascular activity.
Abbreviations: PNS, parasympathetic nervous system; SNS, sympathetic nervous system; RAAS, renin-angiotensin- aldosterone system.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389(10085):2239–2251. doi: 10.1016/S0140-6736(17)30058-2 [DOI] [PubMed] [Google Scholar]
- 2.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–795. doi: 10.2337/db09-9028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361–369. doi: 10.1016/S2213-8587(18)30051-2 [DOI] [PubMed] [Google Scholar]
- 4.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761 [DOI] [PubMed] [Google Scholar]
- 5.Abdul-Ghani M, Del Prato S, Chilton R, DeFronzo RA. SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG OUTCOME Study. Diabetes Care. 2016;39(5):717–725. doi: 10.2337/dc16-0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl1):S111–s124. doi: 10.2337/dc21-S009 [DOI] [PubMed] [Google Scholar]
- 8.Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci U S A. 1992;89(18):8641–8645. doi: 10.1073/pnas.89.18.8641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorens B, Porret A, Bühler L, Deng SP, Morel P, Widmann C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9-39) an antagonist of the receptor. Diabetes. 1993;42(11):1678–1682. doi: 10.2337/diab.42.11.1678 [DOI] [PubMed] [Google Scholar]
- 10.Nishizawa M, Nakabayashi H, Uehara K, Nakagawa A, Uchida K, Koya D. Intraportal GLP-1 stimulates insulin secretion predominantly through the hepatoportal-pancreatic vagal reflex pathways. Am J Physiol Endocrinol Metab. 2013;305(3):E376–387. doi: 10.1152/ajpendo.00565.2012 [DOI] [PubMed] [Google Scholar]
- 11.Gautier JF, Choukem SP, Girard J. Physiology of incretins (GIP and GLP-1) and abnormalities in type 2 diabetes. Diabetes Metab. 2008;34(Suppl 2):S65–72. doi: 10.1016/S1262-3636(08)73397-4 [DOI] [PubMed] [Google Scholar]
- 12.Göke R, Fehmann HC, Linn T, et al. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J Biol Chem. 1993;268(26):19650–19655. doi: 10.1016/S0021-9258(19)36565-2 [DOI] [PubMed] [Google Scholar]
- 13.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006 [DOI] [PubMed] [Google Scholar]
- 14.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054 [DOI] [PubMed] [Google Scholar]
- 15.Aulinger BA, Vahl TP, Prigeon RL, D’Alessio DA, Elder DA. The incretin effect in obese adolescents with and without type 2 diabetes: impaired or intact? Am J Physiol Endocrinol Metab. 2016;310(9):E774–781. doi: 10.1152/ajpendo.00496.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holst JJ, Vilsbøll T, Deacon CF. The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol. 2009;297(1–2):127–136. doi: 10.1016/j.mce.2008.08.012 [DOI] [PubMed] [Google Scholar]
- 17.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91(2):733–794. [DOI] [PubMed] [Google Scholar]
- 18.Koekkoek LL, Mul JD, la Fleur SE. Glucose-sensing in the reward system. Front Neurosci. 2017;11:716. doi: 10.3389/fnins.2017.00716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu AS, Hirayama BA, Timbol G, et al. Functional expression of SGLTs in rat brain. Am J Physiol Cell Physiol. 2010;299(6):C1277–1284. doi: 10.1152/ajpcell.00296.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu AS, Hirayama BA, Timbol G, et al. Regional distribution of SGLT activity in rat brain in vivo. Am J Physiol Cell Physiol. 2013;304(3):C240–C247. doi: 10.1152/ajpcell.00317.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzàlez JA, Reimann F, Burdakov D. Dissociation between sensing and metabolism of glucose in sugar sensing neurones. J Physiol. 2009;587(1):41–48. doi: 10.1113/jphysiol.2008.163410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Malley D, Reimann F, Simpson AK, Gribble FM. Sodium-coupled glucose cotransporters contribute to hypothalamic glucose sensing. Diabetes. 2006;55(12):3381–3386. doi: 10.2337/db06-0531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scafoglio C, Hirayama BA, Kepe V, et al. Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci U S A. 2015;112(30):E4111–4119. doi: 10.1073/pnas.1511698112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elliott RH, Matthews VB, Rudnicka C, Schlaich MP. Is it time to think about the sodium glucose co-transporter 2 sympathetically? Nephrology (Carlton). 2016;21(4):286–294. doi: 10.1111/nep.12620 [DOI] [PubMed] [Google Scholar]
- 25.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–865. doi: 10.1016/S0140-6736(98)07037-8 [DOI] [PubMed] [Google Scholar]
- 26.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]
- 28.Lugner M, Sattar N, Miftaraj M, et al. Cardiorenal and other diabetes related outcomes with SGLT-2 inhibitors compared to GLP-1 receptor agonists in type 2 diabetes: nationwide observational study. Cardiovasc Diabetol. 2021;20(1):67. doi: 10.1186/s12933-021-01258-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573. doi: 10.1136/bmj.m4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrikou E, Tsioufis C, Andrikou I, Leontsinis I, Tousoulis D, Papanas N. GLP-1 receptor agonists and cardiovascular outcome trials: an update. Hellenic J Cardiol. 2019;60(6):347–351. [DOI] [PubMed] [Google Scholar]
- 31.Boyle JG, Livingstone R, Petrie JR. Cardiovascular benefits of GLP-1 agonists in type 2 diabetes: a comparative review. Clin Sci (Lond). 2018;132(15):1699–1709. doi: 10.1042/CS20171299 [DOI] [PubMed] [Google Scholar]
- 32.Kario K, Okada K, Kato M, et al. 24-hour blood pressure-lowering effect of an SGLT-2 inhibitor in patients with diabetes and uncontrolled nocturnal hypertension: results from the Randomized, Placebo-Controlled SACRA Study. Circulation. 2018;139(18):2089–2097. doi: 10.1161/CIRCULATIONAHA.118.037076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira MJ, Eriksson JW. Emerging role of SGLT-2 inhibitors for the treatment of obesity. Drugs. 2019;79(3):219–230. doi: 10.1007/s40265-019-1057-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sánchez-García A, Simental-Mendía M, Millán-Alanís JM, Simental-Mendía LE. Effect of sodium-glucose co-transporter 2 inhibitors on lipid profile: a systematic review and meta-analysis of 48 randomized controlled trials. Pharmacol Res. 2020;160:105068. doi: 10.1016/j.phrs.2020.105068 [DOI] [PubMed] [Google Scholar]
- 35.Ninčević V, Omanović Kolarić T, Roguljić H, Kizivat T, Smolić M, Bilić Ćurčić I. Renal benefits of SGLT 2 inhibitors and GLP-1 receptor agonists: evidence supporting a paradigm shift in the medical management of type 2 diabetes. Int J Mol Sci. 2019;20(23):5831. doi: 10.3390/ijms20235831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali AM, Mari A, Martinez R, et al. Improved beta cell glucose sensitivity plays predominant role in the decrease in HbA1c with Cana and Lira in T2DM. J Clin Endocrinol Metab. 2020;105(10):3226–3233. doi: 10.1210/clinem/dgaa494 [DOI] [PubMed] [Google Scholar]
- 37.Mehta K, Behl T, Kumar A, Uddin MS, Zengin G, Arora S. Deciphering the neuroprotective role of glucagon-like Peptide-1 agonists in diabetic neuropathy: current perspective and future directions. Curr Protein Pept Sci. 2021;22(1):4–18. doi: 10.2174/1389203721999201208195901 [DOI] [PubMed] [Google Scholar]
- 38.Dorsey-Treviño EG, González-González JG, Alvarez-Villalobos N, et al. Sodium-glucose cotransporter 2 (SGLT-2) inhibitors and microvascular outcomes in patients with type 2 diabetes: systematic review and meta-analysis. J Endocrinol Invest. 2020;43(3):289–304. doi: 10.1007/s40618-019-01103-9 [DOI] [PubMed] [Google Scholar]
- 39.Chang HY, Singh S, Mansour O, Baksh S, Alexander GC. Association between sodium-glucose cotransporter 2 inhibitors and lower extremity amputation among patients with type 2 diabetes. JAMA Intern Med. 2018;178(9):1190–1198. doi: 10.1001/jamainternmed.2018.3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhatariya K, Bain SC, Buse JB, et al. The impact of liraglutide on diabetes-related foot ulceration and associated complications in patients with type 2 diabetes at high risk for cardiovascular events: results from the LEADER Trial. Diabetes Care. 2018;41(10):2229–2235. doi: 10.2337/dc18-1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avgerinos I, Karagiannis T, Malandris K, et al. Glucagon-like peptide-1 receptor agonists and microvascular outcomes in type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2019;21(1):188–193. doi: 10.1111/dom.13484 [DOI] [PubMed] [Google Scholar]
- 42.Gaborit B, Julla JB, Besbes S, et al. Glucagon-like peptide 1 receptor agonists, diabetic retinopathy and angiogenesis: the angiosafe type 2 Diabetes Study. J Clin Endocrinol Metab. 2020;105(4):e1549–e1560. doi: 10.1210/clinem/dgz069 [DOI] [PubMed] [Google Scholar]
- 43.Patoulias D, Stavropoulos K, Imprialos K, et al. Glycemic efficacy and safety of glucagon-like peptide-1 receptor agonist on top of sodium-glucose co-transporter-2 inhibitor treatment compared to sodium-glucose co-transporter-2 inhibitor alone: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2019;158:107927. doi: 10.1016/j.diabres.2019.107927 [DOI] [PubMed] [Google Scholar]
- 44.Lajara R. Combination therapy with SGLT-2 inhibitors and GLP-1 receptor agonists as complementary agents that address multi-organ defects in type 2 diabetes. Postgrad Med. 2019;131(8):555–565. doi: 10.1080/00325481.2019.1670017 [DOI] [PubMed] [Google Scholar]
- 45.Ikonomidis I, Pavlidis G, Thymis J, et al. Effects of glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and their combination on endothelial glycocalyx, arterial function, and myocardial work index in patients with type 2 diabetes mellitus after 12-month treatment. J Am Heart Assoc. 2020;9(9):e015716. doi: 10.1161/JAHA.119.015716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aroda VR. A review of GLP-1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab. 2018;20(Suppl 1):22–33. doi: 10.1111/dom.13162 [DOI] [PubMed] [Google Scholar]
- 47.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257. doi: 10.1056/NEJMoa1509225 [DOI] [PubMed] [Google Scholar]
- 48.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 49.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141 [DOI] [PubMed] [Google Scholar]
- 50.Bain SC, Mosenzon O, Arechavaleta R, et al. Cardiovascular safety of oral semaglutide in patients with type 2 diabetes: rationale, design and patient baseline characteristics for the PIONEER 6 trial. Diabetes Obes Metab. 2019;21(3):499–508. doi: 10.1111/dom.13553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–1239. doi: 10.1056/NEJMoa1612917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–1529. [DOI] [PubMed] [Google Scholar]
- 53.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130. [DOI] [PubMed] [Google Scholar]
- 54.McKee A, Al-Khazaali A, Albert SG. Glucagon-like peptide-1 receptor agonists versus sodium-glucose cotransporter inhibitors for treatment of T2DM. J Endocr Soc. 2020;4(5):bvaa037. doi: 10.1210/jendso/bvaa037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma A, Verma S. Mechanisms by which glucagon-like-peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors reduce cardiovascular risk in adults with type 2 diabetes mellitus. Can J Diabetes. 2020;44(1):93–102. doi: 10.1016/j.jcjd.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 56.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 57.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 58.Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20(3):479–487. doi: 10.1111/dom.13126 [DOI] [PubMed] [Google Scholar]
- 59.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752–772. doi: 10.1161/CIRCULATIONAHA.116.021887 [DOI] [PubMed] [Google Scholar]
- 60.Takebayashi K, Inukai T. Effect of sodium glucose cotransporter 2 inhibitors with low SGLT2/SGLT1 selectivity on circulating glucagon-like peptide 1 levels in type 2 diabetes mellitus. J Clin Med Res. 2017;9(9):745–753. doi: 10.14740/jocmr3112w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.INVOKANA® (canagliflozin) tablets, for oral use. Titusville, NJ, USA: Janssen Pharmaceuticals, Inc; 2013. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/204042s026lbl.pdf. Accessed June 25, 2021.
- 62.FARXIGA® (dapagliflozin) tablets, for oral use. Wilmington, DE, USA: AstraZeneca, Inc; 2014. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202293s015lbl.pdf. Accessed June 25, 2021.
- 63.JARDIANCE® (empagliflozin) tablets, for oral use. Ridgefield, CT, USA: Boehringer Ingelheim Pharmaceuticals, Inc.; 2014. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/204629s008lbl.pdf. Accessed June 25, 2021.
- 64.Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes Obes Metab. 2016;18(8):783–794. doi: 10.1111/dom.12670 [DOI] [PubMed] [Google Scholar]
- 65.Cai X, Yang W, Gao X, et al. The association between the dosage of SGLT2 inhibitor and weight reduction in type 2 diabetes patients: a meta-analysis. Obesity (Silver Spring). 2018;26(1):70–80. doi: 10.1002/oby.22066 [DOI] [PubMed] [Google Scholar]
- 66.Bolinder J, Ljunggren Ö, Johansson L, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab. 2014;16(2):159–169. doi: 10.1111/dom.12189 [DOI] [PubMed] [Google Scholar]
- 67.Bailey CJ, Morales Villegas EC, Woo V, Tang W, Ptaszynska A, List JF. Efficacy and safety of dapagliflozin monotherapy in people with Type 2 diabetes: a randomized double-blind placebo-controlled 102-week trial. Diabet Med. 2015;32(4):531–541. doi: 10.1111/dme.12624 [DOI] [PubMed] [Google Scholar]
- 68.Del Prato S, Nauck M, Durán-Garcia S, et al. Long-term glycaemic response and tolerability of dapagliflozin versus a sulphonylurea as add-on therapy to metformin in patients with type 2 diabetes: 4-year data. Diabetes Obes Metab. 2015;17(6):581–590. doi: 10.1111/dom.12459 [DOI] [PubMed] [Google Scholar]
- 69.Deol H, Lekkakou L, Viswanath AK, Pappachan JM. Combination therapy with GLP-1 analogues and SGLT-2 inhibitors in the management of diabesity: the real world experience. Endocrine. 2017;55(1):173–178. doi: 10.1007/s12020-016-1125-0 [DOI] [PubMed] [Google Scholar]
- 70.Saroka RM, Kane MP, Busch RS, Watsky J, Hamilton RA. SGLT-2 inhibitor therapy added to GLP-1 agonist therapy in the management of T2DM. Endocr Pract. 2015;21(12):1315–1322. doi: 10.4158/EP15877.OR [DOI] [PubMed] [Google Scholar]
- 71.Gong M, Wen S, Nguyen T, Wang C, Jin J, Zhou L. Converging relationships of obesity and hyperuricemia with special reference to metabolic disorders and plausible therapeutic implications. Diabetes Metab Syndr Obes. 2020;13:943–962. doi: 10.2147/DMSO.S232377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Szablewski L. Glucose transporters in brain: in health and in Alzheimer’s disease. J Alzheimers Dis. 2017;55(4):1307–1320. doi: 10.3233/JAD-160841 [DOI] [PubMed] [Google Scholar]
- 73.Lim K, Jackson KL, Sata Y, Head GA. Factors responsible for obesity-related hypertension. Curr Hypertens Rep. 2017;19(7):53. doi: 10.1007/s11906-017-0750-1 [DOI] [PubMed] [Google Scholar]
- 74.Shirasaka T, Kunitake T, Takasaki M, Kannan H. Neuronal effects of orexins: relevant to sympathetic and cardiovascular functions. Regul Pept. 2002;104(1–3):91–95. doi: 10.1016/S0167-0115(01)00352-4 [DOI] [PubMed] [Google Scholar]
- 75.Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes. 1997;46(12):2040–2043. doi: 10.2337/diab.46.12.2040 [DOI] [PubMed] [Google Scholar]
- 76.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31(1 Pt 2):409–414. doi: 10.1161/01.HYP.31.1.409 [DOI] [PubMed] [Google Scholar]
- 77.Zhou L, Podolsky N, Sang Z, et al. The medial amygdalar nucleus: a novel glucose-sensing region that modulates the counterregulatory response to hypoglycemia. Diabetes. 2010;59(10):2646–2652. doi: 10.2337/db09-0995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Squillacioti C, Pelagalli A, Liguori G, Mirabella N. Urocortins in the mammalian endocrine system. Acta Vet Scand. 2019;61(1):46. doi: 10.1186/s13028-019-0480-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chatzaki E, Kefala N, Drosos I, Lalidou F, Baritaki S. Do urocortins have a role in treating cardiovascular disease? Drug Discov Today. 2019;24(1):279–284. doi: 10.1016/j.drudis.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 80.Guivarc’h E, Buscato M, Guihot AL, et al. Predominant role of nuclear versus membrane estrogen receptor α in arterial protection: implications for estrogen receptor α modulation in cardiovascular prevention/safety. J Am Heart Assoc. 2018;7(13). doi: 10.1161/JAHA.118.008950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gourdy P, Guillaume M, Fontaine C, et al. Estrogen receptor subcellular localization and cardiometabolism. Mol Metab. 2018;15:56–69. doi: 10.1016/j.molmet.2018.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ropero AB, Eghbali M, Minosyan TY, Tang G, Toro L, Stefani E. Heart estrogen receptor alpha: distinct membrane and nuclear distribution patterns and regulation by estrogen. J Mol Cell Cardiol. 2006;41(3):496–510. doi: 10.1016/j.yjmcc.2006.05.022 [DOI] [PubMed] [Google Scholar]
- 83.Wen S, Wang C, Gong M, Zhou L. An overview of energy and metabolic regulation. Sci China Life Sci. 2019;62(6):771–790. doi: 10.1007/s11427-018-9371-4 [DOI] [PubMed] [Google Scholar]
- 84.Waterson MJ, Horvath TL. Neuronal regulation of energy homeostasis: beyond the hypothalamus and feeding. Cell Metab. 2015;22(6):962–970. doi: 10.1016/j.cmet.2015.09.026 [DOI] [PubMed] [Google Scholar]
- 85.Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kanoski SE, Hayes MR, Skibicka KP. GLP-1 and weight loss: unraveling the diverse neural circuitry. Am J Physiol Regul Integr Comp Physiol. 2016;310(10):R885–895. doi: 10.1152/ajpregu.00520.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carlson SH, Wyss JM. Neurohormonal regulation of the sympathetic nervous system: new insights into central mechanisms of action. Curr Hypertens Rep. 2008;10(3):233–240. doi: 10.1007/s11906-008-0044-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68(10):988–1001. doi: 10.1016/S0025-6196(12)62272-1 [DOI] [PubMed] [Google Scholar]
- 89.Cechetto DF, Saper CB. Neurochemical organization of the hypothalamic projection to the spinal cord in the rat. J Comp Neurol. 1988;272(4):579–604. doi: 10.1002/cne.902720410 [DOI] [PubMed] [Google Scholar]
- 90.Yi SS, Kim HJ, Do SG, et al. Arginine vasopressin (AVP) expressional changes in the hypothalamic paraventricular and supraoptic nuclei of stroke-prone spontaneously hypertensive rats. Anat Cell Biol. 2012;45(2):114–120. doi: 10.5115/acb.2012.45.2.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim YB, Kim YS, Kim WB, et al. GABAergic excitation of vasopressin neurons: possible mechanism underlying sodium-dependent hypertension. Circ Res. 2013;113(12):1296–1307. doi: 10.1161/CIRCRESAHA.113.301814 [DOI] [PubMed] [Google Scholar]
- 92.Choe KY, Han SY, Gaub P, et al. High salt intake increases blood pressure via BDNF-mediated downregulation of KCC2 and impaired baroreflex inhibition of vasopressin neurons. Neuron. 2015;85(3):549–560. doi: 10.1016/j.neuron.2014.12.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kawabe T, Kawabe K, Sapru HN. Cardiovascular responses to chemical stimulation of the hypothalamic arcuate nucleus in the rat: role of the hypothalamic paraventricular nucleus. PLoS One. 2012;7(9):e45180. doi: 10.1371/journal.pone.0045180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kawabe T, Kawabe K, Sapru HN. Effect of barodenervation on cardiovascular responses elicited from the hypothalamic arcuate nucleus of the rat. PLoS One. 2012;7(12):e53111. doi: 10.1371/journal.pone.0053111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kawabe T, Kawabe K, Sapru HN. Tonic γ-aminobutyric acid-ergic activity in the hypothalamic arcuate nucleus is attenuated in the spontaneously hypertensive rat. Hypertension. 2013;62(2):281–287. doi: 10.1161/HYPERTENSIONAHA.113.01132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-κBin the paraventricular nucleus. Hypertension. 2012;59(1):113–121. doi: 10.1161/HYPERTENSIONAHA.111.182154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sriramula S, Xia H, Xu P, Lazartigues E. Brain-targeted angiotensin-converting enzyme 2 overexpression attenuates neurogenic hypertension by inhibiting cyclooxygenase-mediated inflammation. Hypertension. 2015;65(3):577–586. doi: 10.1161/HYPERTENSIONAHA.114.04691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yuan N, Zhang F, Zhang LL, et al. SOD1 gene transfer into paraventricular nucleus attenuates hypertension and sympathetic activity in spontaneously hypertensive rats. Pflugers Arch. 2013;465(2):261–270. doi: 10.1007/s00424-012-1173-0 [DOI] [PubMed] [Google Scholar]
- 99.Coleman CG, Wang G, Faraco G, et al. Membrane trafficking of NADPH oxidase p47(phox) in paraventricular hypothalamic neurons parallels local free radical production in angiotensin II slow-pressor hypertension. J Neurosci. 2013;33(10):4308–4316. doi: 10.1523/JNEUROSCI.3061-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Northcott CA, Billecke S, Craig T, et al. Nitric oxide synthase, ADMA, SDMA, and nitric oxide activity in the paraventricular nucleus throughout the etiology of renal wrap hypertension. Am J Physiol Heart Circ Physiol. 2012;302(11):H2276–2284. doi: 10.1152/ajpheart.00562.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou YB, Sun HJ, Chen D, et al. Intermedin in paraventricular nucleus attenuates sympathetic activity and blood pressure via nitric oxide in hypertensive rats. Hypertension. 2014;63(2):330–337. doi: 10.1161/HYPERTENSIONAHA.113.01681 [DOI] [PubMed] [Google Scholar]
- 102.Huang BS, White RA, Leenen FH. Possible role of brain salt-inducible kinase 1 in responses to central sodium in Dahl rats. Am J Physiol Regul Integr Comp Physiol. 2012;303(2):R236–245. doi: 10.1152/ajpregu.00381.2011 [DOI] [PubMed] [Google Scholar]
- 103.Gabor A, Leenen FH. Central mineralocorticoid receptors and the role of angiotensin II and glutamate in the paraventricular nucleus of rats with angiotensin II-induced hypertension. Hypertension. 2013;61(5):1083–1090. doi: 10.1161/HYPERTENSIONAHA.111.00797 [DOI] [PubMed] [Google Scholar]
- 104.Harlan SM, Guo DF, Morgan DA, Fernandes-Santos C, Rahmouni K. Hypothalamic mTORC1 signaling controls sympathetic nerve activity and arterial pressure and mediates leptin effects. Cell Metab. 2013;17(4):599–606. doi: 10.1016/j.cmet.2013.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tanida M, Yamamoto N, Shibamoto T, Rahmouni K. Involvement of hypothalamic AMP-activated protein kinase in leptin-induced sympathetic nerve activation. PLoS One. 2013;8(2):e56660. doi: 10.1371/journal.pone.0056660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carmichael CY, Wainford RD. Hypothalamic signaling mechanisms in hypertension. Curr Hypertens Rep. 2015;17(5):39. doi: 10.1007/s11906-015-0550-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reis DJ. The brain and hypertension: reflections on 35 years of inquiry into the neurobiology of the circulation. Circulation. 1984;70(5 Pt 2):Iii31–45. [PubMed] [Google Scholar]
- 108.Morrison SF, Ernsberger P, Milner TA, Callaway J, Gong A, Reis DJ. A glutamate mechanism in the intermediolateral nucleus mediates sympathoexcitatory responses to stimulation of the rostral ventrolateral medulla. Prog Brain Res. 1989;81:159–169. [DOI] [PubMed] [Google Scholar]
- 109.Catelli JM, Giakas WJ, Sved AF. GABAergic mechanisms in nucleus tractus solitarius alter blood pressure and vasopressin release. Brain Res. 1987;403(2):279–289. doi: 10.1016/0006-8993(87)90065-5 [DOI] [PubMed] [Google Scholar]
- 110.Sawchenko PE, Swanson LW. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science (New York, NY). 1981;214(4521):685–687. doi: 10.1126/science.7292008 [DOI] [PubMed] [Google Scholar]
- 111.Day TA. Control of neurosecretory vasopressin cells by noradrenergic projections of the caudal ventrolateral medulla. Prog Brain Res. 1989;81:303–317. [DOI] [PubMed] [Google Scholar]
- 112.Galosy RA, Clarke LK, Vasko MR, Crawford IL. Neurophysiology and neuropharmacology of cardiovascular regulation and stress. Neurosci Biobehav Rev. 1981;5(1):137–175. [DOI] [PubMed] [Google Scholar]
- 113.D’Alessio D. Is GLP-1 a hormone: whether and when? J Diabetes Investig. 2016;7 Suppl 1(Suppl1):50–55. doi: 10.1111/jdi.12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Graham DL, Durai HH, Trammell TS, et al. A novel mouse model of glucagon-like peptide-1 receptor expression: a look at the brain. J Comp Neurol. 2020;528(14):2445–2470. doi: 10.1002/cne.24905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403(2):261–280. doi: [DOI] [PubMed] [Google Scholar]
- 116.Zheng H, Cai L, Rinaman L. Distribution of glucagon-like peptide 1-immunopositive neurons in human caudal medulla. Brain Struct Funct. 2015;220(2):1213–1219. doi: 10.1007/s00429-014-0714-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krieger JP, Arnold M, Pettersen KG, Lossel P, Langhans W, Lee SJ. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes. 2016;65(1):34–43. doi: 10.2337/db15-0973 [DOI] [PubMed] [Google Scholar]
- 118.Krieger JP, Langhans W, Lee SJ. Vagal mediation of GLP-1’s effects on food intake and glycemia. Physiol Behav. 2015;152(Pt B):372–380. doi: 10.1016/j.physbeh.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 119.Diepenbroek C, Quinn D, Stephens R, et al. Validation and characterization of a novel method for selective vagal deafferentation of the gut. Am J Physiol Gastrointest Liver Physiol. 2017;313(4):G342–g352. doi: 10.1152/ajpgi.00095.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vrang N, Larsen PJ. Preproglucagon derived peptides GLP-1, GLP-2 and oxyntomodulin in the CNS: role of peripherally secreted and centrally produced peptides. Prog Neurobiol. 2010;92(3):442–462. doi: 10.1016/j.pneurobio.2010.07.003 [DOI] [PubMed] [Google Scholar]
- 121.Katsurada K, Maejima Y, Nakata M, et al. Endogenous GLP-1 acts on paraventricular nucleus to suppress feeding: projection from nucleus tractus solitarius and activation of corticotropin-releasing hormone, nesfatin-1 and oxytocin neurons. Biochem Biophys Res Commun. 2014;451(2):276–281. doi: 10.1016/j.bbrc.2014.07.116 [DOI] [PubMed] [Google Scholar]
- 122.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153(2):647–658. doi: 10.1210/en.2011-1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Renner E, Puskás N, Dobolyi A, Palkovits M. Glucagon-like peptide-1 of brainstem origin activates dorsomedial hypothalamic neurons in satiated rats. Peptides. 2012;35(1):14–22. doi: 10.1016/j.peptides.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 124.Llewellyn-Smith IJ, Marina N, Manton RN, Reimann F, Gribble FM, Trapp S. Spinally projecting preproglucagon axons preferentially innervate sympathetic preganglionic neurons. Neuroscience. 2015;284:872–887. doi: 10.1016/j.neuroscience.2014.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang D, Lv G. Therapeutic potential of spinal GLP-1 receptor signaling. Peptides. 2018;101:89–94. doi: 10.1016/j.peptides.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 126.Tudurí E, Nogueiras R. Insulinotropic actions of GLP-1: how much in the brain and how much in the periphery? Endocrinology. 2017;158(7):2071–2073. doi: 10.1210/en.2017-00410 [DOI] [PubMed] [Google Scholar]
- 127.Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J Clin Invest. 2014;124(6):2456–2463. doi: 10.1172/JCI72434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Umapathysivam MM, Lee MY, Jones KL, et al. Comparative effects of prolonged and intermittent stimulation of the glucagon-like peptide 1 receptor on gastric emptying and glycemia. Diabetes. 2014;63(2):785–790. doi: 10.2337/db13-0893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brierley DI, Holt MK, Singh A, et al. Central and peripheral GLP-1 systems independently suppress eating. Nat Metab. 2021;3(2):258–273. doi: 10.1038/s42255-021-00344-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Trapp S, Richards JE. The gut hormone glucagon-like peptide-1 produced in brain: is this physiologically relevant? Curr Opin Pharmacol. 2013;13(6):964–969. doi: 10.1016/j.coph.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011;152(8):3103–3112. doi: 10.1210/en.2011-0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jessen L, Smith EP, Ulrich-Lai Y, et al. Central nervous system GLP-1 receptors regulate islet hormone secretion and glucose homeostasis in male rats. Endocrinology. 2017;158(7):2124–2133. doi: 10.1210/en.2016-1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Plamboeck A, Veedfald S, Deacon CF, et al. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am J Physiol Gastrointest Liver Physiol. 2013;304(12):G1117–1127. doi: 10.1152/ajpgi.00035.2013 [DOI] [PubMed] [Google Scholar]
- 134.Rüttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150(3):1174–1181. doi: 10.1210/en.2008-1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fortin SM, Lipsky RK, Lhamo R, et al. GABA neurons in the nucleus tractus solitarius express GLP-1 receptors and mediate anorectic effects of liraglutide in rats. Sci Transl Med. 2020;12(533):eaay8071. doi: 10.1126/scitranslmed.aay8071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Adams JM, Pei H, Sandoval DA, Seeley RJ, Chang RB, Liberles SD. Olson DP: liraglutide modulates appetite and body weight through glucagon-like peptide 1 receptor-expressing glutamatergic neurons. Diabetes. 2018;67(8):1538–1548. doi: 10.2337/db17-1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124(10):4473–4488. doi: 10.1172/JCI75276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Holt MK, Cook DR, Brierley DI, et al. PPG neurons in the nucleus of the solitary tract modulate heart rate but do not mediate GLP-1 receptor agonist-induced tachycardia in mice. Mol Metab. 2020;39:101024. doi: 10.1016/j.molmet.2020.101024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gabery S, Salinas CG, Paulsen SJ, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6). doi: 10.1172/jci.insight.133429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baraboi ED, St-Pierre DH, Shooner J, Timofeeva E, Richard D. Brain activation following peripheral administration of the GLP-1 receptor agonist exendin-4. Am J Physiol Regul Integr Comp Physiol. 2011;301(4):R1011–1024. doi: 10.1152/ajpregu.00424.2010 [DOI] [PubMed] [Google Scholar]
- 141.Wen S, Nguyen T, Xiao WZ, et al. Comparative study on anorexigenic effect of glucagon-like peptide-1 receptor agonists in rats. Sheng Li Xue Bao. 2019;71(4):514–526. [PubMed] [Google Scholar]
- 142.Pérez-Tilve D, González-Matías L, Aulinger BA, et al. Exendin-4 increases blood glucose levels acutely in rats by activation of the sympathetic nervous system. Am J Physiol Endocrinol Metab. 2010;298(5):E1088–1096. doi: 10.1152/ajpendo.00464.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barragán JM, Rodríguez RE, Eng J, Blázquez E. Interactions of exendin-(9-39) with the effects of glucagon-like peptide-1-(7-36) amide and of exendin-4 on arterial blood pressure and heart rate in rats. Regul Pept. 1996;67(1):63–68. doi: 10.1016/S0167-0115(96)00113-9 [DOI] [PubMed] [Google Scholar]
- 144.Kim M, Platt MJ, Shibasaki T, et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19(5):567–575. doi: 10.1038/nm.3128 [DOI] [PubMed] [Google Scholar]
- 145.Pyke C, Knudsen LB. The glucagon-like peptide-1 receptor--or not? Endocrinology. 2013;154(1):4–8. doi: 10.1210/en.2012-2124 [DOI] [PubMed] [Google Scholar]
- 146.Richards P, Parker HE, Adriaenssens AE, et al. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014;63(4):1224–1233. doi: 10.2337/db13-1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ussher JR, Drucker DJ. Cardiovascular actions of incretin-based therapies. Circ Res. 2014;114(11):1788–1803. doi: 10.1161/CIRCRESAHA.114.301958 [DOI] [PubMed] [Google Scholar]
- 148.Yamamoto H, Lee CE, Marcus JN, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110(1):43–52. doi: 10.1172/JCI0215595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yamamoto H, Kishi T, Lee CE, et al. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23(7):2939–2946. doi: 10.1523/JNEUROSCI.23-07-02939.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Baggio LL, Ussher JR, McLean BA, et al. The autonomic nervous system and cardiac GLP-1 receptors control heart rate in mice. Mol Metab. 2017;6(11):1339–1349. doi: 10.1016/j.molmet.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Griffioen KJ, Wan R, Okun E, et al. GLP-1 receptor stimulation depresses heart rate variability and inhibits neurotransmission to cardiac vagal neurons. Cardiovasc Res. 2011;89(1):72–78. doi: 10.1093/cvr/cvq271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Verma S, Bhatt DL, Bain SC, et al. Effect of liraglutide on cardiovascular events in patients with type 2 diabetes mellitus and polyvascular disease: results of the LEADER Trial. Circulation. 2018;137(20):2179–2183. doi: 10.1161/CIRCULATIONAHA.118.033898 [DOI] [PubMed] [Google Scholar]
- 153.Rizzo M, Nikolic D, Patti AM, et al. GLP-1 receptor agonists and reduction of cardiometabolic risk: potential underlying mechanisms. Biochim Biophys Acta Mol Basis Dis. 2018;1864(9 Pt B):2814–2821. doi: 10.1016/j.bbadis.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 154.Basalay MV, Mastitskaya S, Mrochek A, et al. Glucagon-like peptide-1 (GLP-1) mediates cardioprotection by remote ischaemic conditioning. Cardiovasc Res. 2016;112(3):669–676. doi: 10.1093/cvr/cvw216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen J, Xu S, Wang L, et al. Exendin-4 inhibits atrial arrhythmogenesis in a model of myocardial infarction-induced heart failure via the GLP-1 receptor signaling pathway. Exp Ther Med. 2020;20(4):3669–3678. doi: 10.3892/etm.2020.9089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Verouhis D, Saleh N, Settergren M, Sörensson P, Gourine A, Pernow J. Remote ischemic conditioning protects against endothelial ischemia-reperfusion injury via a glucagon-like peptide-1 receptor-mediated mechanism in humans. Int J Cardiol. 2019;274:40–44. doi: 10.1016/j.ijcard.2018.09.061 [DOI] [PubMed] [Google Scholar]
- 157.He Z, Gao Y, Lieu L, et al. Direct and indirect effects of liraglutide on hypothalamic POMC and NPY/AgRP neurons - Implications for energy balance and glucose control. Mol Metab. 2019;28:120–134. doi: 10.1016/j.molmet.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu J, Conde K, Zhang P, et al. Enhanced AMPA receptor trafficking mediates the anorexigenic effect of endogenous glucagon-like peptide-1 in the paraventricular hypothalamus. Neuron. 2017;96(4):897–909.e895. doi: 10.1016/j.neuron.2017.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Abtahi S, Howell E, Salvucci JT, Bastacky JMR, Dunn DP, Currie PJ. Exendin-4 antagonizes the metabolic action of acylated ghrelinergic signaling in the hypothalamic paraventricular nucleus. Gen Comp Endocrinol. 2019;270:75–81. doi: 10.1016/j.ygcen.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Williams DL, Lilly NA, Edwards IJ, Yao P, Richards JE, Trapp S. GLP-1 action in the mouse bed nucleus of the stria terminalis. Neuropharmacology. 2018;131:83–95. doi: 10.1016/j.neuropharm.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zheng H, Reiner DJ, Hayes MR, Rinaman L. Chronic suppression of glucagon-like peptide-1 receptor (GLP1R) mRNA translation in the rat bed nucleus of the stria terminalis reduces anxiety-like behavior and stress-induced hypophagia, but prolongs stress-induced elevation of plasma corticosterone. J Neurosci. 2019;39(14):2649–2663. doi: 10.1523/JNEUROSCI.2180-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Alhadeff AL, Baird JP, Swick JC, Hayes MR, Grill HJ. Glucagon-like peptide-1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed. Neuropsychopharmacology. 2014;39(9):2233–2243. doi: 10.1038/npp.2014.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hsu TM, Hahn JD, Konanur VR, Lam A, Kanoski SE. Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology. 2015;40(2):327–337. doi: 10.1038/npp.2014.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Alhadeff AL, Grill HJ. Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am J Physiol Regul Integr Comp Physiol. 2014;307(4):R465–470. doi: 10.1152/ajpregu.00179.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang XF, Liu JJ, Xia J, Liu J, Mirabella V, Pang ZP. Endogenous glucagon-like peptide-1 suppresses high-fat food intake by reducing synaptic drive onto mesolimbic dopamine neurons. Cell Rep. 2015;12(5):726–733. doi: 10.1016/j.celrep.2015.06.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Howell E, Baumgartner HM, Zallar LJ, Selva JA, Engel L, Currie PJ. Glucagon-like peptide-1 (GLP-1) and 5-hydroxytryptamine 2c (5-HT(2c)) receptor agonists in the ventral tegmental area (VTA) inhibit ghrelin-stimulated appetitive reward. Int J Mol Sci. 2019;20(4):889. doi: 10.3390/ijms20040889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sørensen G, Reddy IA, Weikop P, et al. The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice. Physiol Behav. 2015;149:262–268. doi: 10.1016/j.physbeh.2015.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hernandez NS, O’Donovan B, Ortinski PI, Schmidt HD. Activation of glucagon-like peptide-1 receptors in the nucleus accumbens attenuates cocaine seeking in rats. Addict Biol. 2019;24(2):170–181. doi: 10.1111/adb.12583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pierce-Messick Z, Pratt WE. Glucagon-like peptide-1 receptors modulate the binge-like feeding induced by µ-opioid receptor stimulation of the nucleus accumbens in the rat. Neuroreport. 2020;31(18):1283–1288. doi: 10.1097/WNR.0000000000001545 [DOI] [PubMed] [Google Scholar]
- 170.Parker JA, McCullough KA, Field BC, et al. Glucagon and GLP-1 inhibit food intake and increase c-fos expression in similar appetite regulating centres in the brainstem and amygdala. Int J Obes (Lond). 2013;37(10):1391–1398. doi: 10.1038/ijo.2012.227 [DOI] [PubMed] [Google Scholar]
- 171.Anderberg RH, Anefors C, Bergquist F, Nissbrandt H, Skibicka KP. Dopamine signaling in the amygdala, increased by food ingestion and GLP-1, regulates feeding behavior. Physiol Behav. 2014;136:135–144. doi: 10.1016/j.physbeh.2014.02.026 [DOI] [PubMed] [Google Scholar]
- 172.Gil-Lozano M, Romaní-Pérez M, Outeiriño-Iglesias V, et al. Corticotropin-releasing hormone and the sympathoadrenal system are major mediators in the effects of peripherally administered exendin-4 on the hypothalamic-pituitary-adrenal axis of male rats. Endocrinology. 2014;155(7):2511–2523. doi: 10.1210/en.2013-1718 [DOI] [PubMed] [Google Scholar]
- 173.Gil-Lozano M, Romaní-Pérez M, Outeiriño-Iglesias V, et al. Effects of prolonged exendin-4 administration on hypothalamic-pituitary-adrenal axis activity and water balance. Am J Physiol Endocrinol Metab. 2013;304(10):E1105–1117. doi: 10.1152/ajpendo.00529.2012 [DOI] [PubMed] [Google Scholar]
- 174.Farr OM, Upadhyay J, Rutagengwa C, et al. Longer-term liraglutide administration at the highest dose approved for obesity increases reward-related orbitofrontal cortex activation in response to food cues: implications for plateauing weight loss in response to anti-obesity therapies. Diabetes Obes Metab. 2019;21(11):2459–2464. doi: 10.1111/dom.13827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zanchi D, Depoorter A, Egloff L, et al. The impact of gut hormones on the neural circuit of appetite and satiety: a systematic review. Neurosci Biobehav Rev. 2017;80:457–475. doi: 10.1016/j.neubiorev.2017.06.013 [DOI] [PubMed] [Google Scholar]
- 176.Winzeler B, da Conceição I, Refardt J, Sailer CO, Dutilh G, Christ-Crain M. Effects of glucagon-like peptide-1 receptor agonists on fluid intake in healthy volunteers. Endocrine. 2020;70(2):292–298. doi: 10.1007/s12020-020-02394-2 [DOI] [PubMed] [Google Scholar]
- 177.Beiroa D, Imbernon M, Gallego R, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63(10):3346–3358. doi: 10.2337/db14-0302 [DOI] [PubMed] [Google Scholar]
- 178.Liao T, Zhang SL, Yuan X, et al. Liraglutide lowers body weight set point in DIO rats and its relationship with hypothalamic microglia activation. Obesity (Silver Spring). 2020;28(1):122–131. doi: 10.1002/oby.22666 [DOI] [PubMed] [Google Scholar]
- 179.Davis EM, Sandoval DA. Glucagon-like peptide-1: actions and influence on pancreatic hormone function. Compr Physiol. 2020;10(2):577–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kumari P, Nakata M, Zhang BY, Otgon-Uul Z, Yada T. GLP-1 receptor agonist liraglutide exerts central action to induce β-cell proliferation through medulla to vagal pathway in mice. Biochem Biophys Res Commun. 2018;499(3):618–625. doi: 10.1016/j.bbrc.2018.03.199 [DOI] [PubMed] [Google Scholar]
- 181.Katsurada K, Nakata M, Saito T, et al. Central glucagon-like peptide-1 receptor signaling via brainstem catecholamine neurons counteracts hypertension in spontaneously hypertensive rats. Sci Rep. 2019;9(1):12986. doi: 10.1038/s41598-019-49364-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Terrill SJ, Maske CB, Williams DL. Endogenous GLP-1 in lateral septum contributes to stress-induced hypophagia. Physiol Behav. 2018;192:17–22. doi: 10.1016/j.physbeh.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jerlhag E. GLP-1 signaling and alcohol-mediated behaviors; preclinical and clinical evidence. Neuropharmacology. 2018;136(Pt B):343–349. doi: 10.1016/j.neuropharm.2018.01.013 [DOI] [PubMed] [Google Scholar]
- 184.Hayes MR, Schmidt HD. GLP-1 influences food and drug reward. Curr Opin Behav Sci. 2016;9:66–70. doi: 10.1016/j.cobeha.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kellar D, Craft S. Brain insulin resistance in Alzheimer’s disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol. 2020;19(9):758–766. doi: 10.1016/S1474-4422(20)30231-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jurczak MJ, Lee HY, Birkenfeld AL, et al. SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta-cell function. Diabetes. 2011;60(3):890–898. doi: 10.2337/db10-1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jurczak MJ, Saini S, Ioja S, et al. SGLT2 knockout prevents hyperglycemia and is associated with reduced pancreatic β-cell death in genetically obese mice. Islets. 2018;10(5):181–189. doi: 10.1080/19382014.2018.1503027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Asferg CL, Nielsen SJ, Andersen UB, et al. Relative atrial natriuretic peptide deficiency and inadequate renin and angiotensin II suppression in obese hypertensive men. Hypertension. 2013;62(1):147–153. doi: 10.1161/HYPERTENSIONAHA.111.00791 [DOI] [PubMed] [Google Scholar]
- 189.Grassi G, Seravalle G, Cattaneo BM, et al. Sympathetic activation in obese normotensive subjects. Hypertension. 1995;25(4 Pt 1):560–563. [DOI] [PubMed] [Google Scholar]
- 190.Tanaka M, Itoh H. Hypertension as a metabolic disorder and the novel role of the gut. Curr Hypertens Rep. 2019;21(8):63. doi: 10.1007/s11906-019-0964-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Umino H, Hasegawa K, Minakuchi H, et al. High basolateral glucose increases sodium-glucose cotransporter 2 and reduces sirtuin-1 in renal tubules through glucose transporter-2 detection. Sci Rep. 2018;8(1):6791. doi: 10.1038/s41598-018-25054-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Erdogan MA, Yusuf D, Christy J, et al. Highly selective SGLT2 inhibitor dapagliflozin reduces seizure activity in pentylenetetrazol-induced murine model of epilepsy. BMC Neurol. 2018;18(1):81. doi: 10.1186/s12883-018-1086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chiba Y, Yamada T, Tsukita S, et al. Dapagliflozin, a sodium-glucose co-transporter 2 inhibitor, acutely reduces energy expenditure in BAT via neural signals in mice. PLoS One. 2016;11(3):e0150756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tahara A, Kondo Y, Takasu T, Tomiyama H. Effects of the SGLT2 inhibitor ipragliflozin on food intake, appetite-regulating hormones, and arteriovenous differences in postprandial glucose levels in type 2 diabetic rats. Biomed Pharmacother. 2018;105:1033–1041. doi: 10.1016/j.biopha.2018.06.062 [DOI] [PubMed] [Google Scholar]
- 195.Matthews JR, Herat LY, Magno AL, Gorman S, Schlaich MP, Matthews VB. SGLT2 inhibitor-induced sympathoexcitation in white adipose tissue: a novel mechanism for beiging. Biomedicines. 2020;8(11):514. doi: 10.3390/biomedicines8110514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rahman A, Fujisawa Y, Nakano D, Hitomi H, Nishiyama A. Effect of a selective SGLT2 inhibitor, luseogliflozin, on circadian rhythm of sympathetic nervous function and locomotor activities in metabolic syndrome rats. Clin Exp Pharmacol Physiol. 2017;44(4):522–525. doi: 10.1111/1440-1681.12725 [DOI] [PubMed] [Google Scholar]
- 197.Nguyen T, Wen S, Gong M, et al. Dapagliflozin activates neurons in the central nervous system and regulates cardiovascular activity by inhibiting SGLT-2 in mice. Diabetes Metab Syndr Obes. 2020;13:2781–2799. doi: 10.2147/DMSO.S258593 [DOI] [PMC free article] [PubMed] [Google Scholar]