Abstract
HER3 is a pseudo-kinase member of the EGFR family having a role in both tumor progression and drug resistance. Although HER3 was discovered more than 30 years ago, no therapeutic interventions have reached clinical approval to date. Since the evidence of the importance of HER3 is accumulating, increased amount of preclinical and clinical trials with HER3 targeting agents are emerging. In this review article, we discuss the most recent HER3 biology in tumorigenic events and drug resistance, and overview the current and emerging strategies to target HER3.
Keywords: HER3, Cancer, Cell signaling/ protein tyrosine kinases, Drug targets/protein kinase & phosphatase drug targets
INTRODUCTION
The human epidermal growth factor receptor (HER) proteins are a family of receptor tyrosine kinases that play a role in both normal and tumor cell biology. The family consists of four highly homologous members epidermal growth factor receptor (EGFR [ERBB1/HER1]), HER2 (ERBB2), HER3 (ERBB3) and HER4 (ERBB4), consisting of a ligand-binding extracellular domain, a transmembrane domain, an intracellular kinase domain, and a C-terminal tail (1). The family members (except HER2) are generally activated through extracellular ligand binding inducing a conformational change, followed by homo- or heterodimerization among the family members, eventually leading to the activation of an intracellular signaling cascade (2). The cellular responses include increased cell survival and proliferation, explaining why aberrant EGFR family signaling is strongly connected with oncogenic events (1).
When inactive, the EGFR receptors exist in a monomeric tethered conformation, but upon ligand binding, the receptor changes into its extended form, exposing a dimerization arm (2,3). Contact with another open conformation receptor permits formation of a receptor dimer, inducing a further conformational change in the intracellular domain of the receptor complex. This conformational change leads to a transphosphorylation event, where the donor receptor introduces multiple phosphorylations into the C-terminal tail of the acceptor receptor, allowing attachment and activation of downstream signaling cascade (3). The paradigm of EGFR family receptors existing solely in a monomeric form prior to ligand binding has been challenged by suggesting that EGFR can be found in an inactive dimerized form before ligand stimulus (4). The signal inactivation happens via dephosphorylation, receptor internalization, and proteolysis or recycling of the receptor (5).
EGFR binds to at least seven ligands including EGF, transforming growth factor-alpha, heparin-binding EGF-like growth factor, betacellulin, amphiregulin, epiregulin, and epigen (6). Neuregulins (NRG) 1–4 are the ligands for HER3 and HER4 (7). Unlike the other three EGFR family members, HER2 has no known ligands and is found constitutively in an open conformation with exposed dimerization loop and needs a ligand-bound heterodimerization partner to signal (8). The variety in both ligands and dimerization partners provides diversity in the downstream signaling response (9).
HER3: The oddball of the EGFR family
HER3 is a unique EGFR family member with no or little intracellular tyrosine kinase activity. Compared with the other EGFR family members, HER3 diverges at critical residues in the kinase domain locking it in an inactive-like conformation (10). Although HER3 has been reported to have some kinase activity, it is suggested to be 1000-fold weaker than the kinase activity of the fully activated EGFR (10,11). Since HER3 is unable to form homodimers, its activation depends on heterodimerization with another receptor in order to induce the downstream C-terminal phosphorylation events (12).
The HER3 gene localizes in the long arm of chromosome 12 (12q13.2), encoding a 180 kDa protein (13,14). The extracellular domain of HER3 is divided into four subdomains (I–IV): subdomains I and III are leucine-rich β-helical areas responsible for the ligand binding, whereas subdomains II and IV are cysteine-rich regions (15). Subdomain II also contains a dimerization arm necessary for the interaction with other receptors. The transmembrane domain is followed by an intracellular domain enclosing a flexible juxtamembrane region, kinase domain, and the C-terminal tail. In absence of a ligand, binding between subdomains II and IV keeps HER3 in an inactive state (16). Upon ligand binding, the dimerization partner’s kinase domain trans-phosphorylates the tyrosine residues in the C-terminal tail of HER3 (17).
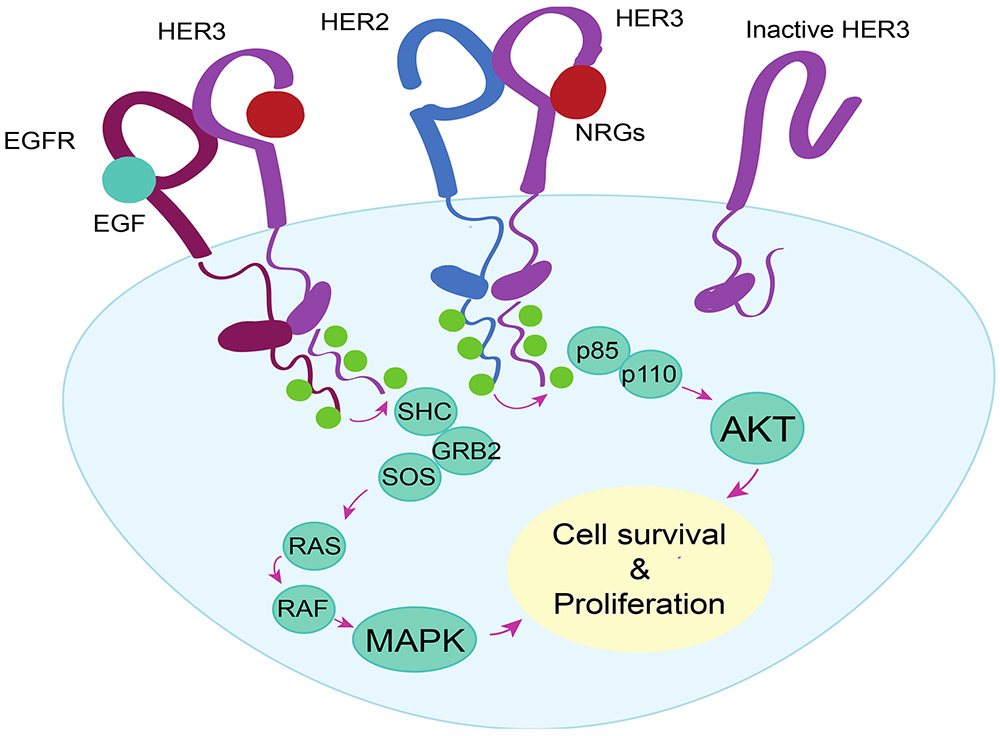
The preferable dimerization partners for HER3 are EGFR and HER2, followed by lower affinity to HER4 (Figure 1). HER3 also dimerizes with some non-EGFR family receptors, including mesenchymal epithelial transition factor (MET) receptor and fibroblast growth factor receptor 2 (FGFR2) (18,19). Six of 11 HER3 tyrosine phosphorylation sites are direct recruiters of phosphoinositide-3-kinase (PI3K), making HER3 a strong activator for PI3K / protein kinase B (AKT) signaling, important for cancer cell survival (20-22). HER3 also activates mitogen-activated protein kinase (MAPK) signaling, stimulating cell proliferation. Other suggested effectors of HER3 include Janus kinases (JAKs) and activators of transcription and proto-oncogene tyrosine-protein kinase SRC signaling pathways involved in signal transduction and increased cell proliferation (23,24).
Figure 1: HER3 dimerization and signaling cascade.
Upon ligand binding, HER3 preferentially dimerizes with EGFR or HER2 inducing a conformational change in the receptor pair. The conformational change leads into transphosphorylation event in the intracellular kinase tail, where the C-terminal tail of HER3 acts as an acceptor for multiple phosphorylations. This induces activation of signaling cascades promoting cell survival and proliferation. EGFR: Epidermal growth factor receptor, EGF: Epidermal growth factor, HER2: Human epidermal growth factor receptor 2, HER3: Human epidermal growth factor receptor 3, NRG: Neuregulin, p85: 85kDa regulator subunit of phosphoinositide 3-kinase, p110: 110kDa catalytic subunit of phosphoinositide 3-kinase, AKT: protein kinase B, SHC: SHC-transforming protein 1, GRB2: growth factor receptor bound protein 2, SOS: Son of sevenless, RAS: RAS GTPase, RAF: Raf kinase, MAPK: Mitogen-activated protein kinase.
HER3 SIGNALING IN CANCER DEVELOPMENT AND PROGRESSION
HER3 overexpression in cancer
In contrast to other EGFR family members, HER3 is not oncogenic when overexpressed alone (25). However, ubiquitous HER3 expression is detected in various cancers including breast, ovarian, colon, gastric, lung, cutaneous, and pancreatic cancers (26,27). High HER3 expression is also linked to disease progression and/or poor prognosis in many cancer types (28,29).
Although HER3 does not cause tumorigenesis on its own, HER2:HER3 heterodimers possess the highest transforming capability among all the possible EGFR family dimers (30,31). The superior oncogenic capability of the dimer pair makes HER3 critical for HER2-mediated tumorigenesis in multiple tumor types. HER3 overexpression is a frequent event, especially in HER2-positive breast cancers, and mice expressing neu (rodent HER2) transgene exhibit elevated HER3 expression (32,33). In breast cancer cell lines, HER3 was shown to be critical for maintaining cell viability, while EGFR was dispensable (34). In addition, inhibition of HER3 revokes HER2-dependent tumorigenesis in transgenic mammary tumor models (35). HER3 has also been implicated in the pathogenesis of non-small cell lung cancer (NSCLC), many of which have EGFR mutations (36-38).
Ovarian cancers often express high levels of HER3, and HER3 expression has been associated with poor survival (39,40). HER3 was deemed essential for ovarian cancer cell proliferation in vitro and in vivo, and the activation of HER3 was mediated by NRG1 autocrine signaling (41). NRG mRNA was detected in 83 % of ovarian carcinomas, and addition of ectopic NRG1 was stimulating the growth of several ovarian cancer cell lines (42). Additionally, NRG driven HER3 activation or NRG activating fusions have been reported in multiple other tumor types including pancreatic, head and neck, colorectal, lung, and prostate cancers (43-51), but NRG is also secreted into the tumor microenvironment by cancer-associated fibroblasts (CAFs) (51,52). Furthermore, NRG inhibition was suppressing tumor growth in preclinical models of pancreatic cancer (52), demonstrating the importance of NRG as a HER3 activating oncogenic factor.
Rare oncogenic HER3 mutations have been reported. Recurrent somatic HER3 mutations are found in 11 % of colon and gastric cancers and are associated with malignant transformation (53). Although these mutations transform cells in a ligand-independent manner, the oncogenic activity is dependent on co-expression with HER2. shRNA mediated HER3 knockdown delayed tumor growth in HER3 mutant tumors. Also in breast cancer several HER3 mutations (F94L, G284R, D297Y, T355I, and E1261A) were shown to have gain of function properties (54). HER3 mutations caused increased HER2:HER3 heterodimerization and made the cells resistant to the HER2-targeting drug lapatinib. Overall, the prognostic value of the mutations and their role in tumor development or progression is still not well understood.
HER3 in cancer progression
HER3 expression is connected with disease progression and metastatic events in various cancer types. In breast cancer, HER3 expression is linked to increased intravasation and metastasis, and higher HER3 expression was found in metastatic breast cancer (MBC) samples compared with primary tumor samples (55). In another breast cancer study, 30% of the primary tumors were shown to express HER3, whereas the expression was 60% in the matched metastatic samples, suggesting that HER3 expression is linked to metastatic events (56). In a meta-analysis from multiple malignant tumor types it was confirmed, that HER3 expression led to worse overall survival and 1.6-fold higher death risk than in HER3 negative patients (57).
In NSCLC, HER3 messenger RNA (mRNA) expression was associated with increased metastatic rate and decreased survival (58). In a more recent study, EGFR and HER3 protein levels were analyzed from primary tumors, brain metastases, and circulating tumor cells (CTCs) (27). Over 50% of the primary tumors had EGFR expression and ~80% expressed HER3; the numbers for brain metastases were 60% and 90%, again highlighting the importance of HER3 in the disease progression. Codetection with EGFR/HER3 was successful in CTCs from the blood of 67% of the patients.
HER2 and HER3 protein levels were evaluated in colorectal cancer patients with liver metastasis (59). Whereas high HER2 levels were found only from 8% of the primary liver tumors, high HER3 levels were found from 75% of the metastatic samples, again suggesting a role for HER3 in the metastatic process and cancer progression. HER3 is also connected to worse outcomes in pancreatic cancer (60). Furthermore, HER3 is highly expressed in cutaneous tumors and was reported to act as an indicator for poor prognosis in melanoma (61,62). In contrast to other tumor types, high HER3 expression was associated with better survival in bladder cancers (63). Researchers suggested that this could be explained by increased expression of soluble form of HER3 (sHER3), which is overexpressed in bladder cancer (63). sHER3 is a 85 kDa truncated and secreted form of HER3 (64), which is reported as a negative regulator of HER2, HER3, and HER4 (64,65).
Although classically HER3 signals from the membrane, occasional nuclear localization of the full-length HER3 has been reported and nuclear HER3 expression has been connected to better overall survival in uveal melanomas (66-68). Functionally nuclear HER3 increased the mRNA level expression of cyclin D1, suggesting it might act as a transcriptional activator (66). However, the mechanisms and role of nuclear HER3 in cancer remain to be studied further.
HER3 AS A DRIVER OF DRUG RESISTANCE
HER3 as a mediator of resistance to targeted therapies
HER3 expression acts as a bypass mechanism for various targeted therapies, and elevated HER3 signaling confers resistance to multiple therapeutic agents. Since HER3 dimerizes with receptors other than EGFR including HER2 and MET receptor, HER3 can confer resistance to EGFR-targeting therapies via dimerization with partners other than EGFR (69). Early on it was shown that HER2:HER3-mediated signaling associates with EGFR tyrosine kinase inhibitor (TKI) gefitinib resistance in head and neck cancer and in breast cancer (70,71). Later it was shown that HER3-ligand, NRG1, and the following increase in HER2:HER3 dimerization confers resistance to EGFR-directed antibody cetuximab in colorectal cancer (72). Similarly, an increase in EGFR:HER3 dimerisation was found in the majority of the residual cancer burden (RCB) of cetuximab/panitumumab resistant breast cancer patients (73). Other anti-HER TKIs such as osimertinib may also induce HER3 upregulation as part of the resistance mechanism (74,75). This NRG1 driven EGFR inhibitor resistance was reverted using HER3 selective antibody patritumab. Interestingly, circulating NRG1 levels were a better indicator for patritumab efficacy than HER3 mRNA expression (37). In another study, MET amplification caused gefitinib resistance via increased HER3/PI3K signaling, and MET amplification was detected in 22% of lung cancer patients bearing tumors resistant to gefitinib or erlotinib (18).
As with EGFR inhibitors, HER3 is known to confer resistance to HER2-targeted therapies. Trastuzumab (herceptin) is a monoclonal HER2-directed neutralizing antibody used mainly in HER2-positive breast cancer (76,77). Although several resistance mechanisms to trastuzumab exist, bypass activation of PI3K/AKT and SRC are the major molecular mechanisms behind therapy escape, and the bypass signaling can be driven by HER3 (78,79). It was suggested that heterotrimer formation between HER2, HER3, and insulin-growth factor receptor 1 (IGF1R) are the major inducer of AKT- and SRC-driven trastuzumab resistance in breast cancer cells (24). In the same study, knockdown of HER3 decreased the phosphorylation activity of AKT and SRC signaling and re-sensitized the cells to trastuzumab, suggesting that dual blocking of HER2 and HER3 is needed to prevent the survival signaling. Additionally, stimulation with NRG1 induced trastuzumab resistance in HER2 overexpressing breast cancer cells (80).
Lapatinib is a dual TKI of EGFR and HER2 used against HER2-positive MBC. Lapatinib treatment was shown to induce feedback upregulation of both mRNA and protein levels in breast cancer cell lines, and HER3 knockdown restored the drug sensitivity in lapatinib-resistant cells (81). Another study showed that lapatinib-resistant breast cancer cells were not dependent on HER2:HER3 signaling, but were relying on NRG1-driven HER3:EGFR dimerization (82). In fact, lapatinib can induce the symmetrical HER2:HER3 dimer which may have unexpected effects on tumor cell proliferation (83).
Since HER3 is a substantial activator of PI3K/AKT survival signaling, it confers resistance also to other targeted therapies. PI3K/AKT inhibitors are known to cause feedback upregulation of HER3 by relieving AKT/FoxO dependent suppression of HER3 (84), leading to reduced efficacy of PI3K/AKT inhibitors. NRG1 induces resistance to ALK inhibitors and BRAF-V600E inhibitor, vemurafenib (85,86). Furthermore, transcriptional HER3 activation was connected to resistance to MAPK and RAF kinase inhibitors both in melanomas and thyroid cancer, and most recently HER3 amplification was described as a clinical bypass mechanism for MET inhibitors in NSCLC (87-89).
HER3 in resistance to hormonal therapy, chemotherapy, and radiation therapy
HER3 expression is also connected to resistance to hormonal therapies. HER3 plays a critical role in the phosphorylation of HER2 in breast cancer cells, and downregulation of HER3 reversed anti-estrogen receptor (ER) tamoxifen resistance in breast cancer cell lines (90). Furthermore, breast cancer patients bearing tumors coexpressing HER2 and HER3 are more prone to develop tamoxifen resistance measured by disease-free survival (91,92). Increased activity of EGFR, HER2, and HER3 was also connected to resistance to ER agonist fulvestrant (93). Fulvestrant treatment enhanced the HER3 expression and phosphorylation in breast cancer cells in an NRG1-dependent manner, and this was suggested as a resistance mechanism for fulvestrant in breast cancer (94). In triple-negative breast cancer (TNBC) patients, high HER3/EGFR protein expression (but not HER3 or EGFR alone) conferred worse 10-year survival after chemotherapy. Interestingly, high HER3/EGFR was associated with worse survival following adjuvant chemotherapy, when compared to patients who did not receive adjuvant chemotherapy (95).
In castration-resistant prostate cancer (CRPC), EGFR:HER3 dimers caused androgen receptor therapy resistance via increased PI3K/AKT signaling. Blocking HER3 with small interfering RNA abolished the growth of these cells, suggesting that HER3 was responsible for the increased PI3K/AKT expression (96). Recently it was also shown that NRG1 secreted by the stromal cells promotes antiandrogen resistance in CRPC (51). Autocrine NRG1/HER3 signaling, measured by NRG1 qPCR and HER3 phosphorylation status, was shown to induce therapy resistance in mouse models of prostate cancer. Blockade of NRG1/HER3 with monoclonal antibodies was re-sensitizing tumors to hormone deprivation in vitro and in vivo. Androgen deprivation therapy (ADT) was shown to increase the amount of NRG1 positive cancer associated fibroblasts also in prostate cancer patients, measured by immunohistochemistry and protein analysis. These studies in breast cancer and CRPC suggest that HER3 as well as NRG1 protein levels could be a useful biomarker for the use and withdrawal of hormonal therapies in cancer.
HER2/HER3 coexpression and PI3K/AKT signaling is connected to increased resistance for several chemotherapeutic agents including 5-fluorouracil, paclitaxel, camptothecin, and etoposide in breast cancer cells (97). HER3 expression was also reported to cause paclitaxel resistance in HER2 positive breast cancer cells by enhanced expression of AKT and survivin (98). Another DNA-damaging agent, doxorubicin, induced NRG upregulation and HER3-mediated AKT signaling in ovarian cancer cells and dual use of doxorubicin with HER3 inhibition increased apoptosis in the chemoresistant cells (99).
Additional studies link HER3 with resistance to radiation therapy. Ionizing radiation (IR) increases the phosphorylation of EGFR, HER2, HER3, and HER4, and silencing of HER3 reduced the cancer cell viability after treatment with IR in vitro and in vivo mouse models (100,101).
HER3-TARGETING THERAPIES AND CLINICAL TRIALS
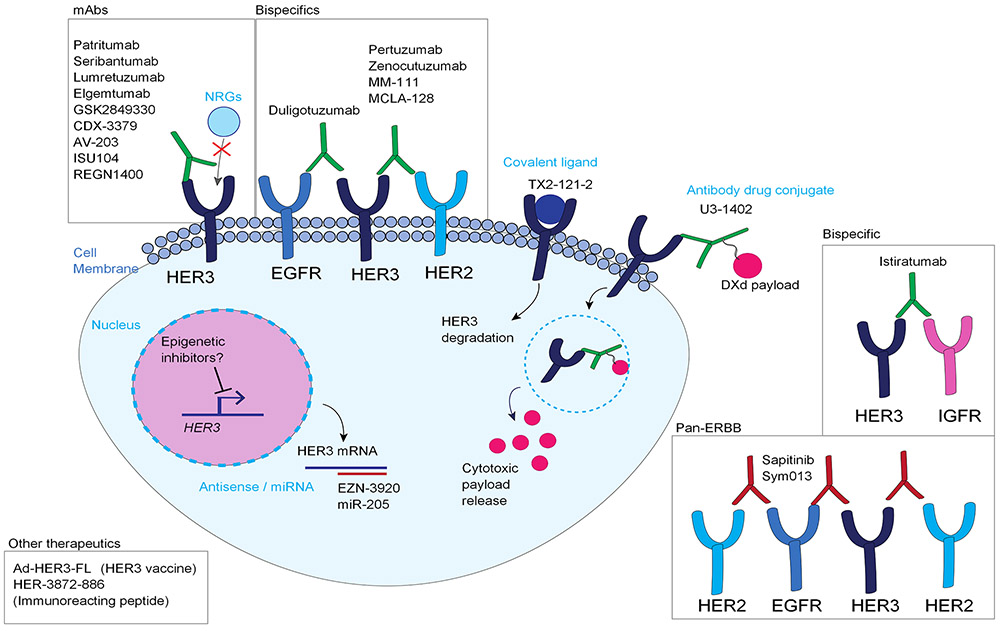
Monoclonal antibodies (mAbs) and small molecule TKIs have been essential in targeting EGFR and HER2 in various tumor types; however, because of the impaired kinase activity, HER3 was long ignored as a therapeutic target. Recently, HER3 has come more into focus as the importance of HER3 in tumor progression and drug resistance has emerged (Figure 2).
Figure 2: Therapeutic strategies to target HER3.
The most popular strategies to target HER3 have been monoclonal and bispecific antibodies, as well as pan-HER strategies. Emerging strategies to target HER3 include antibody drug conjugates, HER3-targeting vaccines, and different ways to affect HER3 degradation either in mRNA or protein level. Epigenetic inhibitors could potentially be useful for inhibiting HER3 gene expression, although the mechanisms and feasibility of these strategies will have to be further validated. EGFR: Epidermal growth factor receptor, HER2: Human epidermal growth factor receptor 2, HER3: Human epidermal growth factor receptor 3, NRG: Neuregulin, mAbs: Monoclonal antibodies, IGFR: Insulin-like growth factor receptor. DXd: DX-8951 derivative.
From monoclonal antibodies to antibody-drug conjugates
Since HER3 has only minimal kinase activity, HER3-directed antibodies have been the most pursued strategy to target HER3 so far. Various HER3-directed mAbs have been under preclinical and clinical development (Table 1). Most of these agents have been tested in solid tumor types for safety, tolerability, and preliminary efficacy in phase 1 studies. Seribantumab, lumertuzumab, and patritumab showed the most promise in clinical trials so far, progressing up to phase 2 and phase 3 studies. Although preclinical mouse studies demonstrate the importance of HER3 in cardiovascular development (102,103), no significant cardiovascular effects have been observed when anti-HER3 antibodies have been evaluated as single agents or in combination with erlotinib or trastuzumab (104-107). In the phase I trial of patritumab, no dose limiting toxicities were observed and no maximum tolerated dose was reached (104). The most common treatment related toxicities were mild and included fatigue, diarrhea and nausea. Although most of the mAbs demonstrated favorable toxicity profiles, the single agent activity of the HER3 mAbs has been limited, and development for most of the HER3 antibodies for clinical use has been discontinued. However, recent advances in the development of bispecific (EGFR/HER3, HER3/IGF1, HER2/HER3) antibodies and antibody-drug conjugates (ADCs) have created new hope for HER3 targeting. Bispecific antibodies generally inhibit the kinase by blocking the ligand-binding site (108,109). ADCs are monoclonal antibodies conjugated to cytotoxic agents via synthetic linkers and have been shown to induce receptor endocytosis and degradation, as well as cancer cell death (110,111). Allosteric HER3 antibodies, which do not block NRG-1 binding, and can bind HER3 even in the presence of NRG-1, maybe more effective than antibodies that block NRG-1 binding, but have yet to enter clinical development (112).
Table 1:
Status of HER3-targeting agents in preclinical and clinical trials.
| Drug type | Drug name | Mechanism of Action |
Developer | Highest clinical trial phase |
Indication (cancer type) |
Key trial numbers |
References |
|---|---|---|---|---|---|---|---|
| Antibodies | Patritumab (U3-1287) | HER3 mAb | Daiichi Sankyo | Phase III | NSCLC, breast, head & neck |
NCT02134015 NCT02134015 NCT01211483 |
(104,120) |
| Seribantumab (MM-121) | HER3 mAb | Merrimack Pharmaceuticals / Elevation Oncology | Phase II | NSCLC, breast, ovarian, advanced |
NCT00994123 NCT01209195 NCT01451632 NCT04383210 |
(156) | |
| Lumretuzumab (RO5479599, RG7116) | HER3 mAb | Genentech/Roche | Phase Ib/II | NSCLC, breast solid tumors |
NCT01918254 NCT02204345 NCT01482377 |
(157) | |
| Elgemtumab (LJM716) | HER3 mAb | Morphosys/Novartis | Phase I/II | Breast, gastric, head & neck, solid |
NCT02143622 NCT01602406 NCT02167854 |
(158) | |
| GSK2849330 | HER3 mAb | GlaxoSmithKline | Phase I | Solid tumors | NCT01966445 | (159) | |
| CDX-3379 (KTN3379) | HER3 mAb | Celldex Therapeutics | Phase II | Head & neck, advanced tumors |
NCT02473731 NCT02014909 |
(160) | |
| AV-203 | HER3 mAb | Aveo Oncology | Phase I | Solid tumors | NCT01603979 | (161) | |
| ISU104 | HER3 mAb | ISU Abxis | Phase I | Solid tumors | NCT03552406 | ||
| REGN1400 | HER3 mAb | Regeneron Pharmaceuticals | Phase I | Metastatic cancers | NCT01727869 | (162) | |
| TK-A3, TK-A4 | HER3 mAb | Takis Biotech | Preclinical | (163) | |||
| MP-EV20 | HER3 mAb | Mediapharma | Preclinical | (164) | |||
| 1A5-3D4 | HER3 mAb | Preclinical | (165) | ||||
| 9F7-F11, 16D3-C1 | HER3 mAb | GamaMabs Pharma | Preclinical | (131) | |||
| NG33 | HER3 mAb | Preclinical | (150) | ||||
| A5, F4 | HER3 mAb | Preclinical | (150) | ||||
| HMBD-001 (10D1F) | HER3 mAb | Hummingbird Bioscience | Preclinical | (166) | |||
| huHER3-8 | HER3 mAb | Preclinical | (167) | ||||
| ADCs | U3-1402 | HER3-ADC | Daiichi Sankyo | Phase II | Breast, NSCLC, colorectal |
NCT04479436 NCT03260491 NCT02980341 |
(123,168) |
| EV20/MMAF, EV20-Sap | HER3-ADC | Mediapharma | Preclinical | (128,129) | |||
| HER3-ADC 9F7-F11 | HER3-ADC | Preclinical | (169) | ||||
| Bi-specific antibodies | Zenocutuzumab (MCLA-128) | HER2/HER3 bispecific | Merus | Phase I/II | Solid tumors | NCT02912949 | (170) |
| Sym013 | EGFR/HER2/ HER3 mAb mixture | Symphogen | Phase I/II | Advanced epithelial malignancies | NCT02906670 | ||
| Duligotuzumab (MEHD7954A) | EGFR/HER3 bispecific | Genentech/Roche | Phase II | Head & neck, advanced tumors |
NCT01911598 NCT01986166 |
(137) | |
| Istiratumab (MM-141) | HER3/IGF1R bispecific | Merrimack Pharmaceuticals | Phase II | Advanced solid, colorectal, Pancreatic, head & neck |
NCT01733004 NCT02538627 NCT02399137 |
(171) | |
| SI-B001 | EGFR/HER3 bispecific | Biokin Pharma | Phase I | Locally advanced or metastatic epithelial tumors | NCT04603287 | (172) | |
| MM-111 | HER2/HER3 bispecific | Merrimack Pharmaceuticals | Phase I | HER2 / heregulin positive, breast |
NCT01097460 NCT00911898 NCT01304784 |
(108) | |
| scDb Fc (scDb hu225x3-43-Fc) | EGFR/HER3 bispecific | Preclinical | (109) | ||||
| Antisense oligonucleotide | EZN-3920 | HER3 mRNA antagonist | Enzon Pharmaceuticals | Preclinical | (152) | ||
| micro-RNAs | miR-450b-3p | Inhibits HER3 expression | Preclinical | (173) | |||
| miR-205 | Inhibits HER3 expression | Preclinical | (174) |
ADC, antibody drug conjugate; EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor; IGF1R, insulin-like growth factor receptor; mAb, monoclonal antibody; miR, micro-RNA; NSCLC, non-small cell lung cancer.
Seribantumab (MM-121) is a fully human IgG2 monoclonal antibody binding to HER3 while blocking the NRG ligand binding and the ligand-dependent downstream activity of HER3 (113). In preclinical studies, seribantumab reduced HER3 activity and growth of xenograft tumors (41,113). Seribantumab reached several phase 1 and phase 2 studies and it was tried either as a single agent or in combination with EGFR-inhibiting antibodies, chemotherapies, or PI3K inhibitors (107,114). NRG ligand levels were shown to correlate with seribantumab response (107). The furthest phase 2 studies in combination with paclitaxel or exemestane (aromatase inhibitor) in ovarian cancer and breast cancer did not reach the clinical endpoint of progression-free survival (PFS); however, retrospective analysis showed that there was survival benefit in the NRG-high patient group (115,116). In EGFR-dependent tumors, there was a limited activity for combination of seribantumab and cetuximab with or without irinotecan chemotherapy (117). Similarly, HER3-specific mAb lumretuzumab was combined in a phase 1b study with EGFR-inhibiting cetuximab or erlotinib, and even though the toxicity was acceptable, clinical activity was modest in HER3-positive solid tumors (118). Lumretuzumab was evaluated in MBC together with paclitaxel and pertuzumab, but the combination was associated with high incidence of diarrhea and narrow therapeutic window, and the clinical trial was discontinued (119). Recently a phase 2 clinical trial with single agent seribantumab was initiated in solid tumors with NRG1 fusions (NCT04383210).
Patritumab (U3-1287) is a fully humanized HER3-targeting antibody targeted toward the extracellular domain of HER3, which blocks HER3 ligand binding (104). Patritumab suppressed proliferation and survival of cancer cells in in vitro and in vivo xenograft models (120). It was also effective as a combination with anti-EGFR–targeting antibodies in both wild-type EGFR tumor models and models resistant to the first-generation EGFR inhibitors. Circulating NRG ligand was a predictive biomarker for patritumab efficacy in NSCLC patients (121,122). In HER2-positive breast cancer, patritumab together with trastuzumab and paclitaxel was reported to have an overall response rate (ORR) of 39 % (105). In the further clinical studies assessing the efficacy of patritumab (including a phase 3 study in NSCLC together with erlotinib) the drug failed to meet the efficacy criteria (NCT02134015). However, as a continuation, the novel HER3 antibody-drug conjugate U3-1402 was constructed using patritumab as the antibody component (110).
Patritumab deruxtecan (HER3-DXd; U3-1402) is a HER3-directed antibody-drug conjugate composed of patritumab, a cleavable tetrapeptide-based linker, and a topoisomerase 1 inhibitor (exatecan derivative, DXd) payload (123). Patritumab deruxtecan was shown to have preclinical efficacy in NSCLC cells resistant to EGFR inhibitors, as well as colorectal cancer xenografts, and it was shown to have superior efficacy compared with patritumab alone (110,123). Patritumab deruxtecan efficacy is associated with high baseline HER3 expression (110). It was recently reported that Patritumab deruxtecan induces antitumor immune response through DXd-induced cell damage and immune activation. Patritumab deruxtecan sensitizes HER3 expressing tumors for anti–PD-1 checkpoint blockade in vitro and in vivo, suggesting that it could be beneficial to combine Patritumab deruxtecan with immunotherapy agents (124). Preliminary results from a phase 1/2 study in MBC showed that Patritumab deruxtecan has a manageable safety profile and there was an ORR of 42.9% in heavily pretreated patients (125,126). In another phase 1 study in metastatic EGFR-mutant and EGFR TKI-resistant NSCLC, Patritumab deruxtecan led to a response rate of 25% (127). Importantly, clinical efficacy was observed in cancers with diverse EGFR TKI resistance mechanisms as HER3 is not a known resistance mechanism to EGFR TKIS. Additionally, two EV20 derived HER3-specific ADCs, EV20-Sap and EV20/MMAF, recently reported (128,129). EV20-Sap was shown to be effective in preclinical models of melanoma, whereas EV20/MMAF showed preclinical activity in melanoma and breast cancer (130). Also 9F7-F11 derived HER3-ADC was recently reported (131). So far, no clinical studies with these agents have been reported.
Pan-HER strategies
Since tumors often express more than one EGFR family member and the family members are known to induce resistance to single-HER strategies, pan-HER therapies have been developed to overcome compensation mechanisms. Pan-HER therapies are either antibody mixtures, bispecific antibodies directed to multiple antigens, or TKIs targeting more than one EGFR family member. Although most of the pan-HER strategies have focused on co-targeting EGFR and HER2, some strategies attempting to co-target HER3 have recently emerged.
Pan-HER (Sym013) is a mixture of six antibodies targeting EGFR, HER2, and HER3, and it was shown to reduce cancer cell growth in vitro and in vivo (132). Interestingly, pan-HER was effective even in cells with acquired resistance to cetuximab, trastuzumab, or pertuzumab, or in cells additionally stimulated with EGFR family ligands (132). In a mechanistic study, pan-HER prevented the EGFR family dimer formation and blocked the switch in HER dependencies (133). A phase 1/2 study with Sym013 was initially launched, but clinical development was subsequently discontinued so the toxicity profile remains unknown (NCT02906670).
Bispecific antibodies can target two distinct tumor-associated antigens and could thus overcome some of the problems of redundant kinase activity. Bispecific antibodies have been developed to simultaneously block either EGFR/HER3, HER2/HER3, or HER3/IGF-1R signaling. Duligotuzumab (MEDH7945A) is an EGFR/HER3-directed bispecific antibody that has two identical binding sites binding to the extracellular domain of either EGFR or HER3 (134). Duligotuzumab exhibited antitumor activity in both in vitro and in vivo models and overcame EGFR inhibitors and radiation resistance in preclinical models (135,136). Although duligotuzumab demonstrated an acceptable safety profile and it showed some clinical activity together with cisplatin/5-fluorouracil in a phase 2b study in head and neck cancers, it failed to provide clinical benefit in another study in metastatic colorectal cancer (137,138). No further clinical activity with the compound has been reported. Recently, a pharmacokinetic predictive study with another EGFR/HER3-bispecific antibody SI-B001 was reported (139). Bispecific antibody IgG3-43 was shown to prevent the growth and cancer stem cell expansion in TNBC cells, demonstrating that some preclinical activities in the field of EGFR/HER3 bispecifics are still ongoing (109).
Pertuzumab is a HER2-targeting monoclonal antibody that blocks HER2/HER3 heterodimerization (34,140). In a phase 1 study of 21 patients with solid tumors, pertuzumab was well tolerated with a pharmacokinetic profile supporting 3-week dosing (141). Patients with HER2-positive breast cancer treated with combination therapy of pertuzumab with chemotherapy and trastuzumab had improved disease-free survival (142). In first-line patients with HER3-positive, HER2-low MBC, ORR was 56% after administration of lumretuzumab (500 mg) every 3 weeks, in combination with pertuzumab (840 mg loading dose [LD] followed by 420 mg) every 3 weeks, and paclitaxel (80 mg/m2) weekly (119). When the patients were given the same combination therapy without the LD of pertuzumab, ORR was 39% (119). However, this therapy demonstrated a small therapeutic range, with a high proportion of patients in the study experiencing Grade 3 diarrhea (119).
MM-111 is a bispecific antibody for HER2/HER3 that inhibits heregulin-induced HER3 activation and slows down the tumor growth in HER2-dependent preclinical models (108). The combination of MM-111 and trastuzumab or lapatinib further inhibits growth of HER2 overexpressing cells. MM-111 was tested in clinical trials as a single agent, together with chemotherapies, or together with other HER2-targeting therapies (143). MM-141 (istiratumab) was designed to target HER3 and IGF-1R, but it failed to show clinical benefit (144). Zenocutuzumab (MCLA-128) is a HER2/HER3 targeting antibody that showed the most promise in the clinical setting. It is currently being evaluated in solid tumors with NRG1 fusions in phase 1 studies, and promising interim results were recently published (NCT02912949).
Sapitinib (AZD8931) is a pan-EGFR–targeting inhibitor that inhibits the activation of EGFR, HER2, and HER3 (145). A phase 1/2 study in advanced breast cancer failed to reach the clinical endpoint (146). Sapitinib was tested in breast cancer together with hormonal therapy but there was increased toxicity with no additional benefit (147). Sapitinib led to a worse PFS compared to placebo in metastatic colorectal cancer, and no further clinical activities with sapitinib have been reported (148).
It is important to mention that also indirect targeting of HER3 through its dimerization partners can be considered as HER3 targeting strategies, but those approaches are outside the scope of this review.
Emerging treatment approaches
In addition to direct therapeutic strategies to target HER3, some approaches inhibit HER3 indirectly.
Proteasomal degradation has emerged as a new therapeutic modality, including proteolysis targeting chimera (PROTAC) inhibitors linked to a warhead that directs the drug target into cellular degradation (149). Although PROTAC for HER3 has not been reported, at least partial HER3 degradation can be achieved with monoclonal antibodies. The HER3-directed antibody NG33 was shown to induce HER3 degradation and inhibit the growth of HER2-driven cancer cells (150). HER3 was also degraded by a crosslinked form of trastuzumab binding to HER2. Only HER2 and HER3, but not EGFR, were pulled into degradation (111). Another unique approach to inhibit and degrade HER3 is TX2-121-1, a covalent ligand that binds to HER3 receptor and induces partial HER3 degradation by interfering with HER3 dimerization with HER2 and MET (151). HER3 mRNA degradation can be induced by antisense oligonucleotides or micro-RNAs, but this concept has not been tested in clinical trials to date (152).
Recently, a HER3-targeting vaccine (Ad-HER3-FL) was created. Ad-HER3-FL stimulates the production of HER3-specific T cells and antibodies in mouse models, suggesting that HER3 might be a good target for antitumor vaccines (153). In the same study, Ad-HER3-FL was also combined with anti-PD1, showing enhanced response compared to the vaccine alone. Along the same lines, an earlier study described an immunoreacting HER3 epitope (HER-3872-868), and this peptide was used to provoke antitumor immune responses in preclinical models of lung cancer and head and neck cancer (154). Although these new therapies modalities to target HER3 are promising, further preclinical and clinical evaluation is required.
CONCLUSIONS
HER3 is an exceptional EGFR family member that is not oncogenic alone, but can cooperate with other receptors to induce tumorigenesis, metastatic events, and drug resistance. HER3 is a compelling cancer therapeutic target, but so far, no HER3-directed therapies have been approved for clinical use. In contrast to EGFR and HER2, which have been broadly targeted with TKIs, HER3 has been mainly targeted with monoclonal or bispecific antibodies due to the minimal kinase activity, either blocking the ligand binding or heterodimerization with other receptors. In clinical trials, the safety profile of these antibodies has been acceptable, but the efficacy has been disappointing. It is unclear if the failure of the antibody therapies has been due to the use of wrong antibody epitopes, pharmacokinetic problems, or lack of biomarkers, but with some exceptions the clinical development of most of these agents has been discontinued. However, retrospective studies and a meta-analysis from HER3 mAbs in various tumor types suggest that NRG expression could be used as a predictor for HER3 mAb response in the future (37,122,155). It has also been suggested, that HER3 antibodies that don’t compete with the NRG binding site could be more effective in tumors with high NRG1 expression before antibody treatment.
The interest in targeting HER3 has persisted and new mechanisms to target HER3 have constantly emerged. New therapeutic strategies, including HER3 directed ADCs, are being investigated in a broad range of cancers. The potential advantage of ADCs over monoclonal antibodies could be that the cancer cells need to express HER3, but do not have to be fully dependent on HER3 to induce cell death. Other novel strategies to target HER3 include proteasomal degradation, antisense oligos, and most recently a HER3-targeted peptide vaccine. So far, these agents have shown efficacy in preclinical models, but the clinical safety and efficacy remains to be determined. The winning strategy to therapeutically target HER3 remains to be seen, but HER3 is as a promising drug target and the era of drugging the “undruggables” has already started.
Acknowledgements
This study was supported by the American Cancer Society (CRP-17-111-01-CDD to P.A.J.) and the National Cancer Institute (R35 CA220497 to P.A.J.). H.M.H. was supported by Sigrid Jusélius Foundation and The Finnish Cultural Foundation. Medical writing and editorial support were provided by Jennifer Meyering, MS, RN, CMPP of AlphaBioCom, LLC (King of Prussia, PA) and funded by Daiichi Sankyo, Inc.
Footnotes
Conflicts of Interest:
P.A.J. has received consulting fees from AstraZeneca, Boehringer-Ingelheim, Pfizer, Roche/Genentech, Takeda Oncology, ACEA Biosciences, Eli Lilly and Company, Araxes Pharma, Ignyta, Mirati Therapeutics, Novartis, LOXO Oncology, Daiichi Sankyo, Sanofi Oncology, Voronoi, SFJ Pharmaceuticals, Takeda Oncology, Transcenta, Silicon Therapeutics, Syndax, Nuvalent, Bayer, Esai and Biocartis; receives post-marketing royalties from DFCI owned intellectual property on EGFR mutations licensed to Lab Corp; has sponsored research agreements with AstraZeneca, Daichi-Sankyo, PUMA, Boehringer Ingelheim, Eli Lilly and Company, Revolution Medicines and Astellas Pharmaceuticals; and has stock ownership in LOXO Oncology and Gatekeeper Pharmaceuticals.
References
- 1.Sheng Q, Liu J. The therapeutic potential of targeting the EGFR family in epithelial ovarian cancer. Br J Cancer 2011;104(8):1241–5 doi 10.1038/bjc.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH, et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell 2002;110(6):775–87 doi 10.1016/s0092-8674(02)00963-7. [DOI] [PubMed] [Google Scholar]
- 3.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 2002;110(6):669–72 doi 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 4.Yu X, Sharma KD, Takahashi T, Iwamoto R, Mekada E. Ligand-independent dimer formation of epidermal growth factor receptor (EGFR) is a step separable from ligand-induced EGFR signaling. Mol Biol Cell 2002;13(7):2547–57 doi 10.1091/mbc.01-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomas A, Futter CE, Eden ER. EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol 2014;24(1):26–34 doi 10.1016/j.tcb.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freed DM, Bessman NJ, Kiyatkin A, Salazar-Cavazos E, Byrne PO, Moore JO, et al. EGFR Ligands Differentially Stabilize Receptor Dimers to Specify Signaling Kinetics. Cell 2017;171(3):683–95 e18 doi 10.1016/j.cell.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montero JC, Rodriguez-Barrueco R, Ocana A, Diaz-Rodriguez E, Esparis-Ogando A, Pandiella A. Neuregulins and cancer. Clin Cancer Res 2008;14(11):3237–41 doi 10.1158/1078-0432.CCR-07-5133. [DOI] [PubMed] [Google Scholar]
- 8.Burgess AW, Cho HS, Eigenbrot C, Ferguson KM, Garrett TP, Leahy DJ, et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell 2003;12(3):541–52 doi 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy SP, Hastings JF, Han JZ, Croucher DR. The Under-Appreciated Promiscuity of the Epidermal Growth Factor Receptor Family. Front Cell Dev Biol 2016;4:88 doi 10.3389/fcell.2016.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi F, Telesco SE, Liu Y, Radhakrishnan R, Lemmon MA. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci U S A 2010;107(17):7692–7 doi 10.1073/pnas.1002753107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jura N, Shan Y, Cao X, Shaw DE, Kuriyan J. Structural analysis of the catalytically inactive kinase domain of the human EGF receptor 3. Proc Natl Acad Sci U S A 2009;106(51):21608–13 doi 10.1073/pnas.0912101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger MB, Mendrola JM, Lemmon MA. ErbB3/HER3 does not homodimerize upon neuregulin binding at the cell surface. FEBS Lett 2004;569(1-3):332–6 doi 10.1016/j.febslet.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Kraus MH, Issing W, Miki T, Popescu NC, Aaronson SA. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proc Natl Acad Sci U S A 1989;86(23):9193–7 doi 10.1073/pnas.86.23.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sithanandam G, Anderson LM. The ERBB3 receptor in cancer and cancer gene therapy. Cancer Gene Ther 2008;15(7):413–48 doi 10.1038/cgt.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plowman GD, Whitney GS, Neubauer MG, Green JM, McDonald VL, Todaro GJ, et al. Molecular cloning and expression of an additional epidermal growth factor receptor-related gene. Proc Natl Acad Sci U S A 1990;87(13):4905–9 doi 10.1073/pnas.87.13.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho HS, Leahy DJ. Structure of the extracellular region of HER3 reveals an interdomain tether. Science 2002;297(5585):1330–3 doi 10.1126/science.1074611. [DOI] [PubMed] [Google Scholar]
- 17.Prigent SA, Gullick WJ. Identification of c-erbB-3 binding sites for phosphatidylinositol 3'-kinase and SHC using an EGF receptor/c-erbB-3 chimera. EMBO J 1994;13(12):2831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316(5827):1039–43 doi 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 19.Kunii K, Davis L, Gorenstein J, Hatch H, Yashiro M, Di Bacco A, et al. FGFR2-amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer Res 2008;68(7):2340–8 doi 10.1158/0008-5472.CAN-07-5229. [DOI] [PubMed] [Google Scholar]
- 20.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 2000;19(13):3159–67 doi 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelman JA, Janne PA, Mermel C, Pearlberg J, Mukohara T, Fleet C, et al. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proc Natl Acad Sci U S A 2005;102(10):3788–93 doi 10.1073/pnas.0409773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suenaga A, Takada N, Hatakeyama M, Ichikawa M, Yu X, Tomii K, et al. Novel mechanism of interaction of p85 subunit of phosphatidylinositol 3-kinase and ErbB3 receptor-derived phosphotyrosyl peptides. J Biol Chem 2005;280(2):1321–6 doi 10.1074/jbc.M410436200. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Kern JA. Neuregulin-1 activates the JAK-STAT pathway and regulates lung epithelial cell proliferation. Am J Respir Cell Mol Biol 2002;27(3):306–13 doi 10.1165/rcmb.4850. [DOI] [PubMed] [Google Scholar]
- 24.Huang X, Gao L, Wang S, McManaman JL, Thor AD, Yang X, et al. Heterotrimerization of the growth factor receptors erbB2, erbB3, and insulin-like growth factor-i receptor in breast cancer cells resistant to herceptin. Cancer Res 2010;70(3):1204–14 doi 10.1158/0008-5472.CAN-09-3321. [DOI] [PubMed] [Google Scholar]
- 25.Zhang K, Sun J, Liu N, Wen D, Chang D, Thomason A, et al. Transformation of NIH 3T3 cells by HER3 or HER4 receptors requires the presence of HER1 or HER2. J Biol Chem 1996;271(7):3884–90. [PubMed] [Google Scholar]
- 26.Ocana A, Vera-Badillo F, Seruga B, Templeton A, Pandiella A, Amir E. HER3 overexpression and survival in solid tumors: a meta-analysis. J Natl Cancer Inst 2013;105(4):266–73 doi 10.1093/jnci/djs501. [DOI] [PubMed] [Google Scholar]
- 27.Scharpenseel H, Hanssen A, Loges S, Mohme M, Bernreuther C, Peine S, et al. EGFR and HER3 expression in circulating tumor cells and tumor tissue from non-small cell lung cancer patients. Sci Rep 2019;9(1):7406 doi 10.1038/s41598-019-43678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards KN, Zweidler-McKay PA, Van Roy N, Speleman F, Trevino J, Zage PE, et al. Signaling of ERBB receptor tyrosine kinases promotes neuroblastoma growth in vitro and in vivo. Cancer 2010;116(13):3233–43 doi 10.1002/cncr.25073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berghoff AS, Magerle M, Ilhan-Mutlu A, Dinhof C, Widhalm G, Dieckman K, et al. Frequent overexpression of ErbB--receptor family members in brain metastases of non-small cell lung cancer patients. APMIS 2013;121(12):1144–52 doi 10.1111/apm.12063. [DOI] [PubMed] [Google Scholar]
- 30.Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol 1996;16(10):5276–87 doi 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, et al. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J 1996;15(10):2452–67. [PMC free article] [PubMed] [Google Scholar]
- 32.Bieche I, Onody P, Tozlu S, Driouch K, Vidaud M, Lidereau R. Prognostic value of ERBB family mRNA expression in breast carcinomas. Int J Cancer 2003;106(5):758–65 doi 10.1002/ijc.11273. [DOI] [PubMed] [Google Scholar]
- 33.Siegel PM, Ryan ED, Cardiff RD, Muller WJ. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. EMBO J 1999;18(8):2149–64 doi 10.1093/emboj/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res 2008;68(14):5878–87 doi 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 35.Vaught DB, Stanford JC, Young C, Hicks DJ, Wheeler F, Rinehart C, et al. HER3 is required for HER2-induced preneoplastic changes to the breast epithelium and tumor formation. Cancer Res 2012;72(10):2672–82 doi 10.1158/0008-5472.CAN-11-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sholl L Molecular diagnostics of lung cancer in the clinic. Transl Lung Cancer Res 2017;6(5):560–9 doi 10.21037/tlcr.2017.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yonesaka K, Hirotani K, Kawakami H, Takeda M, Kaneda H, Sakai K, et al. Anti-HER3 monoclonal antibody patritumab sensitizes refractory non-small cell lung cancer to the epidermal growth factor receptor inhibitor erlotinib. Oncogene 2016;35(7):878–86 doi 10.1038/onc.2015.142. [DOI] [PubMed] [Google Scholar]
- 38.Yonesaka K, Iwama E, Hayashi H, Suzuki S, Kato R, Watanabe S, et al. Heregulin expression and its clinical implication for patients with EGFR-mutant non-small cell lung cancer treated with EGFR-tyrosine kinase inhibitors. Sci Rep 2019;9(1):19501 doi 10.1038/s41598-019-55939-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung YW, Kim S, Hong JH, Lee JK, Lee NW, Lee YS, et al. Overexpression of HER2/HER3 and clinical feature of ovarian cancer. J Gynecol Oncol 2019;30(5):e75 doi 10.3802/jgo.2019.30.e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanner B, Hasenclever D, Stern K, Schormann W, Bezler M, Hermes M, et al. ErbB-3 predicts survival in ovarian cancer. J Clin Oncol 2006;24(26):4317–23 doi 10.1200/JCO.2005.04.8397. [DOI] [PubMed] [Google Scholar]
- 41.Sheng Q, Liu X, Fleming E, Yuan K, Piao H, Chen J, et al. An activated ErbB3/NRG1 autocrine loop supports in vivo proliferation in ovarian cancer cells. Cancer Cell 2010;17(3):298–310 doi 10.1016/j.ccr.2009.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilmour LM, Macleod KG, McCaig A, Sewell JM, Gullick WJ, Smyth JF, et al. Neuregulin expression, function, and signaling in human ovarian cancer cells. Clin Cancer Res 2002;8(12):3933–42. [PubMed] [Google Scholar]
- 43.De Boeck A, Pauwels P, Hensen K, Rummens JL, Westbroek W, Hendrix A, et al. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression through paracrine neuregulin 1/HER3 signalling. Gut 2013;62(4):550–60 doi 10.1136/gutjnl-2011-301393. [DOI] [PubMed] [Google Scholar]
- 44.Drilon A, Somwar R, Mangatt BP, Edgren H, Desmeules P, Ruusulehto A, et al. Response to ERBB3-Directed Targeted Therapy in NRG1-Rearranged Cancers. Cancer Discov 2018;8(6):686–95 doi 10.1158/2159-8290.CD-17-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heining C, Horak P, Uhrig S, Codo PL, Klink B, Hutter B, et al. NRG1 Fusions in KRAS Wild-Type Pancreatic Cancer. Cancer Discov 2018;8(9):1087–95 doi 10.1158/2159-8290.CD-18-0036. [DOI] [PubMed] [Google Scholar]
- 46.Jones MR, Williamson LM, Topham JT, Lee MKC, Goytain A, Ho J, et al. NRG1 Gene Fusions Are Recurrent, Clinically Actionable Gene Rearrangements in KRAS Wild-Type Pancreatic Ductal Adenocarcinoma. Clin Cancer Res 2019;25(15):4674–81 doi 10.1158/1078-0432.CCR-19-0191. [DOI] [PubMed] [Google Scholar]
- 47.Jonna S, Feldman RA, Swensen J, Gatalica Z, Korn WM, Borghaei H, et al. Detection of NRG1 Gene Fusions in Solid Tumors. Clin Cancer Res 2019;25(16):4966–72 doi 10.1158/1078-0432.CCR-19-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shames DS, Carbon J, Walter K, Jubb AM, Kozlowski C, Januario T, et al. High heregulin expression is associated with activated HER3 and may define an actionable biomarker in patients with squamous cell carcinomas of the head and neck. PLoS One 2013;8(2):e56765 doi 10.1371/journal.pone.0056765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trombetta D, Graziano P, Scarpa A, Sparaneo A, Rossi G, Rossi A, et al. Frequent NRG1 fusions in Caucasian pulmonary mucinous adenocarcinoma predicted by Phospho-ErbB3 expression. Oncotarget 2018;9(11):9661–71 doi 10.18632/oncotarget.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson TR, Lee DY, Berry L, Shames DS, Settleman J. Neuregulin-1-mediated autocrine signaling underlies sensitivity to HER2 kinase inhibitors in a subset of human cancers. Cancer Cell 2011;20(2):158–72 doi 10.1016/j.ccr.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Z, Karthaus WR, Lee YS, Gao VR, Wu C, Russo JW, et al. Tumor Microenvironment-Derived NRG1 Promotes Antiandrogen Resistance in Prostate Cancer. Cancer Cell 2020;38(2):279–96 e9 doi 10.1016/j.ccell.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogier C, Colombo PE, Bousquet C, Canterel-Thouennon L, Sicard P, Garambois V, et al. Targeting the NRG1/HER3 pathway in tumor cells and cancer-associated fibroblasts with an anti-neuregulin 1 antibody inhibits tumor growth in pre-clinical models of pancreatic cancer. Cancer Lett 2018;432:227–36 doi 10.1016/j.canlet.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 53.Jaiswal BS, Kljavin NM, Stawiski EW, Chan E, Parikh C, Durinck S, et al. Oncogenic ERBB3 mutations in human cancers. Cancer Cell 2013;23(5):603–17 doi 10.1016/j.ccr.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Mishra R, Alanazi S, Yuan L, Solomon T, Thaker TM, Jura N, et al. Activating HER3 mutations in breast cancer. Oncotarget 2018;9(45):27773–88 doi 10.18632/oncotarget.25576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xue C, Liang F, Mahmood R, Vuolo M, Wyckoff J, Qian H, et al. ErbB3-dependent motility and intravasation in breast cancer metastasis. Cancer Res 2006;66(3):1418–26 doi 10.1158/0008-5472.CAN-05-0550. [DOI] [PubMed] [Google Scholar]
- 56.Da Silva L, Simpson PT, Smart CE, Cocciardi S, Waddell N, Lane A, et al. HER3 and downstream pathways are involved in colonization of brain metastases from breast cancer. Breast Cancer Res 2010;12(4):R46 doi 10.1186/bcr2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Q, Zhang R, Yan H, Zhao P, Wu L, Wang H, et al. Prognostic significance of HER3 in patients with malignant solid tumors. Oncotarget 2017;8(40):67140–51 doi 10.18632/oncotarget.18007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muller-Tidow C, Diederichs S, Bulk E, Pohle T, Steffen B, Schwable J, et al. Identification of metastasis-associated receptor tyrosine kinases in non-small cell lung cancer. Cancer Res 2005;65(5):1778–82 doi 10.1158/0008-5472.CAN-04-3388. [DOI] [PubMed] [Google Scholar]
- 59.Styczen H, Nagelmeier I, Beissbarth T, Nietert M, Homayounfar K, Sprenger T, et al. HER-2 and HER-3 expression in liver metastases of patients with colorectal cancer. Oncotarget 2015;6(17):15065–76 doi 10.18632/oncotarget.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Friess H, Yamanaka Y, Kobrin MS, Do DA, Buchler MW, Korc M. Enhanced erbB-3 expression in human pancreatic cancer correlates with tumor progression. Clin Cancer Res 1995;1(11):1413–20. [PubMed] [Google Scholar]
- 61.Wimmer E, Kraehn-Senftleben G, Issing WJ. HER3 expression in cutaneous tumors. Anticancer Res 2008;28(2A):973–9. [PubMed] [Google Scholar]
- 62.Reschke M, Mihic-Probst D, van der Horst EH, Knyazev P, Wild PJ, Hutterer M, et al. HER3 is a determinant for poor prognosis in melanoma. Clin Cancer Res 2008;14(16):5188–97 doi 10.1158/1078-0432.CCR-08-0186. [DOI] [PubMed] [Google Scholar]
- 63.Memon AA, Gilliver SC, Borre M, Sundquist J, Sundquist K, Nexo E, et al. Soluble HER3 predicts survival in bladder cancer patients. Oncol Lett 2018;15(2):1783–8 doi 10.3892/ol.2017.7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee H, Maihle NJ. Isolation and characterization of four alternate c-erbB3 transcripts expressed in ovarian carcinoma-derived cell lines and normal human tissues. Oncogene 1998;16(25):3243–52 doi 10.1038/sj.onc.1201866. [DOI] [PubMed] [Google Scholar]
- 65.Lee H, Akita RW, Sliwkowski MX, Maihle NJ. A naturally occurring secreted human ErbB3 receptor isoform inhibits heregulin-stimulated activation of ErbB2, ErbB3, and ErbB4. Cancer Res 2001;61(11):4467–73. [PubMed] [Google Scholar]
- 66.Brand TM, Iida M, Luthar N, Wleklinski MJ, Starr MM, Wheeler DL. Mapping C-terminal transactivation domains of the nuclear HER family receptor tyrosine kinase HER3. PLoS One 2013;8(8):e71518 doi 10.1371/journal.pone.0071518. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Trocme E, Mougiakakos D, Johansson CC, All-Eriksson C, Economou MA, Larsson O, et al. Nuclear HER3 is associated with favorable overall survival in uveal melanoma. Int J Cancer 2012;130(5):1120–7 doi 10.1002/ijc.26118. [DOI] [PubMed] [Google Scholar]
- 68.Reif R, Adawy A, Vartak N, Schroder J, Gunther G, Ghallab A, et al. Activated ErbB3 Translocates to the Nucleus via Clathrin-independent Endocytosis, Which Is Associated with Proliferating Cells. J Biol Chem 2016;291(8):3837–47 doi 10.1074/jbc.M115.686782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gala K, Chandarlapaty S. Molecular pathways: HER3 targeted therapy. Clin Cancer Res 2014;20(6):1410–6 doi 10.1158/1078-0432.CCR-13-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Erjala K, Sundvall M, Junttila TT, Zhang N, Savisalo M, Mali P, et al. Signaling via ErbB2 and ErbB3 associates with resistance and epidermal growth factor receptor (EGFR) amplification with sensitivity to EGFR inhibitor gefitinib in head and neck squamous cell carcinoma cells. Clin Cancer Res 2006;12(13):4103–11 doi 10.1158/1078-0432.CCR-05-2404. [DOI] [PubMed] [Google Scholar]
- 71.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 2007;445(7126):437–41 doi 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J, et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med 2011;3(99):99ra86 doi 10.1126/scitranslmed.3002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tao JJ, Castel P, Radosevic-Robin N, Elkabets M, Auricchio N, Aceto N, et al. Antagonism of EGFR and HER3 enhances the response to inhibitors of the PI3K-Akt pathway in triple-negative breast cancer. Sci Signal 2014;7(318):ra29 doi 10.1126/scisignal.2005125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mancini M, Gal H, Gaborit N, Mazzeo L, Romaniello D, Salame TM, et al. An oligoclonal antibody durably overcomes resistance of lung cancer to third-generation EGFR inhibitors. EMBO Mol Med 2018;10(2):294–308 doi 10.15252/emmm.201708076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Romaniello D, Marrocco I, Belugali Nataraj N, Ferrer I, Drago-Garcia D, Vaknin I, et al. Targeting HER3, a Catalytically Defective Receptor Tyrosine Kinase, Prevents Resistance of Lung Cancer to a Third-Generation EGFR Kinase Inhibitor. Cancers (Basel) 2020;12(9) doi 10.3390/cancers12092394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999;17(9):2639–48 doi 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 77.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 2002;20(3):719–26 doi 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 78.Zhang S, Huang WC, Li P, Guo H, Poh SB, Brady SW, et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med 2011;17(4):461–9 doi 10.1038/nm.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chandarlapaty S, Sakr RA, Giri D, Patil S, Heguy A, Morrow M, et al. Frequent mutational activation of the PI3K-AKT pathway in trastuzumab-resistant breast cancer. Clin Cancer Res 2012;18(24):6784–91 doi 10.1158/1078-0432.CCR-12-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang L, Li Y, Shen E, Cao F, Li L, Li X, et al. NRG1-dependent activation of HER3 induces primary resistance to trastuzumab in HER2-overexpressing breast cancer cells. Int J Oncol 2017;51(5):1553–62 doi 10.3892/ijo.2017.4130. [DOI] [PubMed] [Google Scholar]
- 81.Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sanchez V, Chakrabarty A, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci U S A 2011;108(12):5021–6 doi 10.1073/pnas.1016140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xia W, Petricoin EF 3rd, Zhao S, Liu L, Osada T, Cheng Q, et al. An heregulin-EGFR-HER3 autocrine signaling axis can mediate acquired lapatinib resistance in HER2+ breast cancer models. Breast Cancer Res 2013;15(5):R85 doi 10.1186/bcr3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Claus J, Patel G, Autore F, Colomba A, Weitsman G, Soliman TN, et al. Inhibitor-induced HER2-HER3 heterodimerisation promotes proliferation through a novel dimer interface. Elife 2018;7 doi 10.7554/eLife.32271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chakrabarty A, Sanchez V, Kuba MG, Rinehart C, Arteaga CL. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci U S A 2012;109(8):2718–23 doi 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilson FH, Johannessen CM, Piccioni F, Tamayo P, Kim JW, Van Allen EM, et al. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell 2015;27(3):397–408 doi 10.1016/j.ccell.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prasetyanti PR, Capone E, Barcaroli D, D'Agostino D, Volpe S, Benfante A, et al. ErbB-3 activation by NRG-1beta sustains growth and promotes vemurafenib resistance in BRAF-V600E colon cancer stem cells (CSCs). Oncotarget 2015;6(19):16902–11 doi 10.18632/oncotarget.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abel EV, Basile KJ, Kugel CH 3rd, Witkiewicz AK, Le K, Amaravadi RK, et al. Melanoma adapts to RAF/MEK inhibitors through FOXD3-mediated upregulation of ERBB3. J Clin Invest 2013;123(5):2155–68 doi 10.1172/JCI65780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Montero-Conde C, Ruiz-Llorente S, Dominguez JM, Knauf JA, Viale A, Sherman EJ, et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov 2013;3(5):520–33 doi 10.1158/2159-8290.CD-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Recondo G, Bahcall M, Spurr LF, Che J, Ricciuti B, Leonardi GC, et al. Molecular Mechanisms of Acquired Resistance to MET Tyrosine Kinase Inhibitors in Patients with MET Exon 14-Mutant NSCLC. Clin Cancer Res 2020;26(11):2615–25 doi 10.1158/1078-0432.CCR-19-3608. [DOI] [PubMed] [Google Scholar]
- 90.Liu B, Ordonez-Ercan D, Fan Z, Edgerton SM, Yang X, Thor AD. Downregulation of erbB3 abrogates erbB2-mediated tamoxifen resistance in breast cancer cells. Int J Cancer 2007;120(9):1874–82 doi 10.1002/ijc.22423. [DOI] [PubMed] [Google Scholar]
- 91.Tovey S, Dunne B, Witton CJ, Forsyth A, Cooke TG, Bartlett JM. Can molecular markers predict when to implement treatment with aromatase inhibitors in invasive breast cancer? Clin Cancer Res 2005;11(13):4835–42 doi 10.1158/1078-0432.CCR-05-0196. [DOI] [PubMed] [Google Scholar]
- 92.Tovey SM, Witton CJ, Bartlett JM, Stanton PD, Reeves JR, Cooke TG. Outcome and human epidermal growth factor receptor (HER) 1-4 status in invasive breast carcinomas with proliferation indices evaluated by bromodeoxyuridine labelling. Breast Cancer Res 2004;6(3):R246–51 doi 10.1186/bcr783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Frogne T, Benjaminsen RV, Sonne-Hansen K, Sorensen BS, Nexo E, Laenkholm AV, et al. Activation of ErbB3, EGFR and Erk is essential for growth of human breast cancer cell lines with acquired resistance to fulvestrant. Breast Cancer Res Treat 2009;114(2):263–75 doi 10.1007/s10549-008-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hutcheson IR, Goddard L, Barrow D, McClelland RA, Francies HE, Knowlden JM, et al. Fulvestrant-induced expression of ErbB3 and ErbB4 receptors sensitizes oestrogen receptor-positive breast cancer cells to heregulin beta1. Breast Cancer Res 2011;13(2):R29 doi 10.1186/bcr2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ogden A, Bhattarai S, Sahoo B, Mongan NP, Alsaleem M, Green AR, et al. Combined HER3-EGFR score in triple-negative breast cancer provides prognostic and predictive significance superior to individual biomarkers. Sci Rep 2020;10(1):3009 doi 10.1038/s41598-020-59514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mikhailova M, Wang Y, Bedolla RG, Krishnegowda NK, Kreisberg JI, Ghosh PM. Role of the receptor tyrosine kinase HER3 in the progression of prostate cancer to an androgen independent state. Cancer Res 2005;65(9):1033. [Google Scholar]
- 97.Knuefermann C, Lu Y, Liu B, Jin W, Liang K, Wu L, et al. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene 2003;22(21):3205–12 doi 10.1038/sj.onc.1206394. [DOI] [PubMed] [Google Scholar]
- 98.Wang S, Huang X, Lee CK, Liu B. Elevated expression of erbB3 confers paclitaxel resistance in erbB2-overexpressing breast cancer cells via upregulation of Survivin. Oncogene 2010;29(29):4225–36 doi 10.1038/onc.2010.180. [DOI] [PubMed] [Google Scholar]
- 99.Bezler M, Hengstler JG, Ullrich A. Inhibition of doxorubicin-induced HER3-PI3K-AKT signalling enhances apoptosis of ovarian cancer cells. Mol Oncol 2012;6(5):516–29 doi 10.1016/j.molonc.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yan Y, Hein AL, Greer PM, Wang Z, Kolb RH, Batra SK, et al. A novel function of HER2/Neu in the activation of G2/M checkpoint in response to gamma-irradiation. Oncogene 2015;34(17):2215–26 doi 10.1038/onc.2014.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He G, Di X, Yan J, Zhu C, Sun X, Zhang S. Silencing human epidermal growth factor receptor-3 radiosensitizes human luminal A breast cancer cells. Cancer Sci 2018;109(12):3774–82 doi 10.1111/cas.13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Camprecios G, Lorita J, Pardina E, Peinado-Onsurbe J, Soley M, Ramirez I. Expression, localization, and regulation of the neuregulin receptor ErbB3 in mouse heart. J Cell Physiol 2011;226(2):450–5 doi 10.1002/jcp.22354. [DOI] [PubMed] [Google Scholar]
- 103.Odiete O, Hill MF, Sawyer DB. Neuregulin in cardiovascular development and disease. Circ Res 2012;111(10):1376–85 doi 10.1161/CIRCRESAHA.112.267286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.LoRusso P, Janne PA, Oliveira M, Rizvi N, Malburg L, Keedy V, et al. Phase I study of U3-1287, a fully human anti-HER3 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res 2013;19(11):3078–87 doi 10.1158/1078-0432.CCR-12-3051. [DOI] [PubMed] [Google Scholar]
- 105.Mukai H, Saeki T, Aogi K, Naito Y, Matsubara N, Shigekawa T, et al. Patritumab plus trastuzumab and paclitaxel in human epidermal growth factor receptor 2-overexpressing metastatic breast cancer. Cancer Sci 2016;107(10):1465–70 doi 10.1111/cas.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nishio M, Horiike A, Murakami H, Yamamoto N, Kaneda H, Nakagawa K, et al. Phase I study of the HER3-targeted antibody patritumab (U3-1287) combined with erlotinib in Japanese patients with non-small cell lung cancer. Lung Cancer 2015;88(3):275–81 doi 10.1016/j.lungcan.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 107.Sequist LV, Gray JE, Harb WA, Lopez-Chavez A, Doebele RC, Modiano MR, et al. Randomized Phase II Trial of Seribantumab in Combination with Erlotinib in Patients with EGFR Wild-Type Non-Small Cell Lung Cancer. Oncologist 2019;24(8):1095–102 doi 10.1634/theoncologist.2018-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McDonagh CF, Huhalov A, Harms BD, Adams S, Paragas V, Oyama S, et al. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol Cancer Ther 2012;11(3):582–93 doi 10.1158/1535-7163.MCT-11-0820. [DOI] [PubMed] [Google Scholar]
- 109.Rau A, Lieb WS, Seifert O, Honer J, Birnstock D, Richter F, et al. Inhibition of Tumor Cell Growth and Cancer Stem Cell Expansion by a Bispecific Antibody Targeting EGFR and HER3. Mol Cancer Ther 2020;19(7):1474–85 doi 10.1158/1535-7163.MCT-19-1095. [DOI] [PubMed] [Google Scholar]
- 110.Koganemaru S, Kuboki Y, Koga Y, Kojima T, Yamauchi M, Maeda N, et al. U3-1402, a Novel HER3-Targeting Antibody-Drug Conjugate, for the Treatment of Colorectal Cancer. Mol Cancer Ther 2019;18(11):2043–50 doi 10.1158/1535-7163.MCT-19-0452. [DOI] [PubMed] [Google Scholar]
- 111.Wymant JM, Sayers EJ, Muir D, Jones AT. Strategic Trastuzumab Mediated Crosslinking Driving Concomitant HER2 and HER3 Endocytosis and Degradation in Breast Cancer. J Cancer 2020;11(11):3288–302 doi 10.7150/jca.32470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Le Clorennec C, Bazin H, Dubreuil O, Larbouret C, Ogier C, Lazrek Y, et al. Neuregulin 1 Allosterically Enhances the Antitumor Effects of the Noncompeting Anti-HER3 Antibody 9F7-F11 by Increasing Its Binding to HER3. Mol Cancer Ther 2017;16(7):1312–23 doi 10.1158/1535-7163.MCT-16-0886. [DOI] [PubMed] [Google Scholar]
- 113.Schoeberl B, Kudla A, Masson K, Kalra A, Curley M, Finn G, et al. Systems biology driving drug development: from design to the clinical testing of the anti-ErbB3 antibody seribantumab (MM-121). NPJ Syst Biol Appl 2017;3:16034 doi 10.1038/npjsba.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abramson VG, Supko JG, Ballinger T, Cleary JM, Hilton JF, Tolaney SM, et al. Phase Ib Study of Safety and Pharmacokinetics of the PI3K Inhibitor SAR245408 with the HER3-Neutralizing Human Antibody SAR256212 in Patients with Solid Tumors. Clin Cancer Res 2017;23(14):3520–8 doi 10.1158/1078-0432.CCR-16-1764. [DOI] [PubMed] [Google Scholar]
- 115.Liu JF, Ray-Coquard I, Selle F, Poveda AM, Cibula D, Hirte H, et al. Randomized Phase II Trial of Seribantumab in Combination With Paclitaxel in Patients With Advanced Platinum-Resistant or -Refractory Ovarian Cancer. J Clin Oncol 2016;34(36):4345–53 doi 10.1200/JCO.2016.67.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Higgins MJ, Doyle C, Paepke S, Azaro A, Martin M, Semiglazov V, et al. A randomized, double-blind phase II trial of exemestane plus MM-121 (a monoclonal antibody targeting ErbB3) or placebo in postmenopausal women with locally advanced or metastatic ER+/PR+, HER2-negative breast cancer. Journal of Clinical Oncology 2014;32(15_suppl):587- doi 10.1200/jco.2014.32.15_suppl.587.24419113 [DOI] [Google Scholar]
- 117.Cleary JM, McRee AJ, Shapiro GI, Tolaney SM, O'Neil BH, Kearns JD, et al. A phase 1 study combining the HER3 antibody seribantumab (MM-121) and cetuximab with and without irinotecan. Invest New Drugs 2017;35(1):68–78 doi 10.1007/s10637-016-0399-7. [DOI] [PubMed] [Google Scholar]
- 118.Meulendijks D, Jacob W, Voest EE, Mau-Sorensen M, Martinez-Garcia M, Taus A, et al. Phase Ib Study of Lumretuzumab Plus Cetuximab or Erlotinib in Solid Tumor Patients and Evaluation of HER3 and Heregulin as Potential Biomarkers of Clinical Activity. Clin Cancer Res 2017;23(18):5406–15 doi 10.1158/1078-0432.CCR-17-0812. [DOI] [PubMed] [Google Scholar]
- 119.Schneeweiss A, Park-Simon TW, Albanell J, Lassen U, Cortes J, Dieras V, et al. Phase Ib study evaluating safety and clinical activity of the anti-HER3 antibody lumretuzumab combined with the anti-HER2 antibody pertuzumab and paclitaxel in HER3-positive, HER2-low metastatic breast cancer. Invest New Drugs 2018;36(5):848–59 doi 10.1007/s10637-018-0562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Freeman DJ OS, Bready J, Sun J-K, Radisnky R, Hettmann T. . U3–1287 (AMG 888), a fully human anti-HER3 mAb, demonstrates in vitro and in vivo efficacy in the FaDu model of human squamous cell carcinoma of the head and neck (SCCHN). Mol Cancer Ther 2011(10). [Google Scholar]
- 121.Shimizu T, Yonesaka K, Hayashi H, Iwasa T, Haratani K, Yamada H, et al. Phase 1 study of new formulation of patritumab (U3-1287) Process 2, a fully human anti-HER3 monoclonal antibody in combination with erlotinib in Japanese patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol 2017;79(3):489–95 doi 10.1007/s00280-016-3231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yonesaka K, Hirotani K, von Pawel J, Dediu M, Chen S, Copigneaux C, et al. Circulating heregulin level is associated with the efficacy of patritumab combined with erlotinib in patients with non-small cell lung cancer. Lung Cancer 2017;105:1–6 doi 10.1016/j.lungcan.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 123.Yonesaka K, Takegawa N, Watanabe S, Haratani K, Kawakami H, Sakai K, et al. An HER3-targeting antibody-drug conjugate incorporating a DNA topoisomerase I inhibitor U3-1402 conquers EGFR tyrosine kinase inhibitor-resistant NSCLC. Oncogene 2019;38(9):1398–409 doi 10.1038/s41388-018-0517-4. [DOI] [PubMed] [Google Scholar]
- 124.Haratani K, Yonesaka K, Takamura S, Maenishi O, Kato R, Takegawa N, et al. U3-1402 sensitizes HER3-expressing tumors to PD-1 blockade by immune activation. J Clin Invest 2020;130(1):374–88 doi 10.1172/JCI126598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kogawa T, Yonemori K, Masuda N, Takahashi S, Takahashi M, Iwase H, et al. Single agent activity of U3-1402, a HER3-targeting antibody-drug conjugate, in breast cancer patients: Phase 1 dose escalation study. Journal of Clinical Oncology 2018;36(15_suppl):2512- doi 10.1200/JCO.2018.36.15_suppl.2512. [DOI] [Google Scholar]
- 126.Yonemori K, Shimomura A, Yasojima H, Masuda N, Aogi K, Takahashi M, et al. A phase I/II trial of olaparib tablet in combination with eribulin in Japanese patients with advanced or metastatic triple-negative breast cancer previously treated with anthracyclines and taxanes. Eur J Cancer 2019;109:84–91 doi 10.1016/j.ejca.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 127.Yu CSB HA, Gold K, Hayashi H, Johnson M, Koczywas M, Murakami H, Nishio M, Steuer C, Su W, Yang J, Karam S, Qi Z, Qiu Y, Chen S, Yu C, Jänne PA. Abstract LBA62: Efficacy and safety of patritumab deruxtecan (U3-1402), a novel HER3 directed antibody drug conjugate, in patients (pts) with EGFR-mutated (EGFRm) NSCLC. Annals of Oncology 2020;31:S1142–S215. [Google Scholar]
- 128.Capone E, Giansanti F, Ponziani S, Lamolinara A, Iezzi M, Cimini A, et al. EV20-Sap, a novel anti-HER-3 antibody-drug conjugate, displays promising antitumor activity in melanoma. Oncotarget 2017;8(56):95412–24 doi 10.18632/oncotarget.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Capone E, Lamolinara A, D'Agostino D, Rossi C, De Laurenzi V, Iezzi M, et al. EV20-mediated delivery of cytotoxic auristatin MMAF exhibits potent therapeutic efficacy in cutaneous melanoma. J Control Release 2018;277:48–56 doi 10.1016/j.jconrel.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 130.Gandullo-Sanchez L, Capone E, Ocana A, Iacobelli S, Sala G, Pandiella A. HER3 targeting with an antibody-drug conjugate bypasses resistance to anti-HER2 therapies. EMBO Mol Med 2020;12(5):e11498 doi 10.15252/emmm.201911498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lazrek Y, Dubreuil O, Garambois V, Gaborit N, Larbouret C, Le Clorennec C, et al. Anti-HER3 domain 1 and 3 antibodies reduce tumor growth by hindering HER2/HER3 dimerization and AKT-induced MDM2, XIAP, and FoxO1 phosphorylation. Neoplasia 2013;15(3):335–47 doi 10.1593/neo.121960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jacobsen HJ, Poulsen TT, Dahlman A, Kjaer I, Koefoed K, Sen JW, et al. Pan-HER, an Antibody Mixture Simultaneously Targeting EGFR, HER2, and HER3, Effectively Overcomes Tumor Heterogeneity and Plasticity. Clin Cancer Res 2015;21(18):4110–22 doi 10.1158/1078-0432.CCR-14-3312. [DOI] [PubMed] [Google Scholar]
- 133.Ellebaek S, Brix S, Grandal M, Lantto J, Horak ID, Kragh M, et al. Pan-HER-An antibody mixture targeting EGFR, HER2 and HER3 abrogates preformed and ligand-induced EGFR homo- and heterodimers. Int J Cancer 2016;139(9):2095–105 doi 10.1002/ijc.30242. [DOI] [PubMed] [Google Scholar]
- 134.Schaefer G, Haber L, Crocker LM, Shia S, Shao L, Dowbenko D, et al. A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell 2011;20(4):472–86 doi 10.1016/j.ccr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 135.De Pauw I, Wouters A, Van den Bossche J, Deschoolmeester V, Baysal H, Pauwels P, et al. Dual Targeting of Epidermal Growth Factor Receptor and HER3 by MEHD7945A as Monotherapy or in Combination with Cisplatin Partially Overcomes Cetuximab Resistance in Head and Neck Squamous Cell Carcinoma Cell Lines. Cancer Biother Radiopharm 2017;32(7):229–38. [PubMed] [Google Scholar]
- 136.Huang S, Li C, Armstrong EA, Peet CR, Saker J, Amler LC, et al. Dual targeting of EGFR and HER3 with MEHD7945A overcomes acquired resistance to EGFR inhibitors and radiation. Cancer Res 2013;73(2):824–33 doi 10.1158/0008-5472.CAN-12-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jimeno A, Machiels JP, Wirth L, Specenier P, Seiwert TY, Mardjuadi F, et al. Phase Ib study of duligotuzumab (MEHD7945A) plus cisplatin/5-fluorouracil or carboplatin/paclitaxel for first-line treatment of recurrent/metastatic squamous cell carcinoma of the head and neck. Cancer 2016;122(24):3803–11 doi 10.1002/cncr.30256. [DOI] [PubMed] [Google Scholar]
- 138.Hill AG, Findlay MP, Burge ME, Jackson C, Alfonso PG, Samuel L, et al. Phase II Study of the Dual EGFR/HER3 Inhibitor Duligotuzumab (MEHD7945A) versus Cetuximab in Combination with FOLFIRI in Second-Line RAS Wild-Type Metastatic Colorectal Cancer. Clin Cancer Res 2018;24(10):2276–84 doi 10.1158/1078-0432.CCR-17-0646. [DOI] [PubMed] [Google Scholar]
- 139.Xue J, Kong D, Yao Y, Yang L, Yao Q, Zhu Y, et al. Prediction of Human Pharmacokinetics and Clinical Effective Dose of SI-B001, an EGFR/HER3 Bi-specific Monoclonal Antibody. J Pharm Sci 2020. doi 10.1016/j.xphs.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 140.Thomas G, Chardes T, Gaborit N, Mollevi C, Leconet W, Robert B, et al. HER3 as biomarker and therapeutic target in pancreatic cancer: new insights in pertuzumab therapy in preclinical models. Oncotarget 2014;5(16):7138–48 doi 10.18632/oncotarget.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Agus DB, Gordon MS, Taylor C, Natale RB, Karlan B, Mendelson DS, et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol 2005;23(11):2534–43 doi 10.1200/JCO.2005.03.184. [DOI] [PubMed] [Google Scholar]
- 142.von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med 2017;377(2):122–31 doi 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Richards D, Braiteh F, Anthony S, Edenfield W, Hellerstedt B, Raju R, et al. Abstract 496P: A Phase 1 Study of MM-111; A Bispecific HER2/HER3 Antibody Fusion Protein, Combined with Multiple Treatment Regimens in Patients with Advanced HER2 Positive Solid Tumors. Annals of Oncology 2012;23:ix170. [Google Scholar]
- 144.Kundranda M, Gracian AC, Zafar SF, Meiri E, Bendell J, Algul H, et al. Randomized, double-blind, placebo-controlled phase II study of istiratumab (MM-141) plus nab-paclitaxel and gemcitabine versus nab-paclitaxel and gemcitabine in front-line metastatic pancreatic cancer (CARRIE). Ann Oncol 2020;31(1):79–87 doi 10.1016/j.annonc.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 145.Hickinson DM, Klinowska T, Speake G, Vincent J, Trigwell C, Anderton J, et al. AZD8931, an equipotent, reversible inhibitor of signaling by epidermal growth factor receptor, ERBB2 (HER2), and ERBB3: a unique agent for simultaneous ERBB receptor blockade in cancer. Clin Cancer Res 2010;16(4):1159–69 doi 10.1158/1078-0432.CCR-09-2353. [DOI] [PubMed] [Google Scholar]
- 146.José Baselga RH, Maria Vidal Losada, Tatiana Vidaurre, Ana Lluch, Katerina Petrakova, Helen Mann, Serban Ghiorghiu, Mary Stuart, Dónal Landers, Kenneth Thress, Teresa Klinowska and Javier Cortes. Abstract LB-146: A phase II randomized placebo-controlled study of AZD8931, an inhibitor of EGFR, HER2, and HER3 signaling, plus paclitaxel (P) vs P alone in patients (pts) with low HER2-expressing advanced breast cancer (BC) (THYME). Cancer Research 2013;73(8). [Google Scholar]
- 147.Johnston S, Basik M, Hegg R, Lausoontornsiri W, Grzeda L, Clemons M, et al. Inhibition of EGFR, HER2, and HER3 signaling with AZD8931 in combination with anastrozole as an anticancer approach: Phase II randomized study in women with endocrine-therapy-naive advanced breast cancer. Breast Cancer Res Treat 2016;160(1):91–9 doi 10.1007/s10549-016-3979-5. [DOI] [PubMed] [Google Scholar]
- 148.Adams R, Brown E, Brown L, Butler R, Falk S, Fisher D, et al. Inhibition of EGFR, HER2, and HER3 signalling in patients with colorectal cancer wild-type for BRAF, PIK3CA, KRAS, and NRAS (FOCUS4-D): a phase 2-3 randomised trial. Lancet Gastroenterol Hepatol 2018;3(3):162–71 doi 10.1016/S2468-1253(17)30394-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sun X, Gao H, Yang Y, He M, Wu Y, Song Y, et al. PROTACs: great opportunities for academia and industry. Signal Transduct Target Ther 2019;4:64 doi 10.1038/s41392-019-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gaborit N, Abdul-Hai A, Mancini M, Lindzen M, Lavi S, Leitner O, et al. Examination of HER3 targeting in cancer using monoclonal antibodies. Proc Natl Acad Sci U S A 2015;112(3):839–44 doi 10.1073/pnas.1423645112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xie T, Lim SM, Westover KD, Dodge ME, Ercan D, Ficarro SB, et al. Pharmacological targeting of the pseudokinase Her3. Nat Chem Biol 2014;10(12):1006–12 doi 10.1038/nchembio.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu Y, Zhang Y, Wang M, Li Q, Qu Z, Shi V, et al. Downregulation of HER3 by a novel antisense oligonucleotide, EZN-3920, improves the antitumor activity of EGFR and HER2 tyrosine kinase inhibitors in animal models. Mol Cancer Ther 2013;12(4):427–37 doi 10.1158/1535-7163.MCT-12-0838. [DOI] [PubMed] [Google Scholar]
- 153.Osada T, Morse MA, Hobeika A, Diniz MA, Gwin WR, Hartman Z, et al. Vaccination targeting human HER3 alters the phenotype of infiltrating T cells and responses to immune checkpoint inhibition. Oncoimmunology 2017;6(6):e1315495 doi 10.1080/2162402X.2017.1315495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kumai T, Ohkuri T, Nagato T, Matsuda Y, Oikawa K, Aoki N, et al. Targeting HER-3 to elicit antitumor helper T cells against head and neck squamous cell carcinoma. Sci Rep 2015;5:16280 doi 10.1038/srep16280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ocana A, Diez-Gonzalez L, Esparis-Ogando A, Montero JC, Amir E, Pandiella A. Neuregulin expression in solid tumors: prognostic value and predictive role to anti-HER3 therapies. Oncotarget 2016;7(29):45042–51 doi 10.18632/oncotarget.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schoeberl B, Pace EA, Fitzgerald JB, Harms BD, Xu L, Nie L, et al. Therapeutically targeting ErbB3: a key node in ligand-induced activation of the ErbB receptor-PI3K axis. Sci Signal 2009;2(77):ra31 doi 10.1126/scisignal.2000352. [DOI] [PubMed] [Google Scholar]
- 157.Mirschberger C, Schiller CB, Schraml M, Dimoudis N, Friess T, Gerdes CA, et al. RG7116, a therapeutic antibody that binds the inactive HER3 receptor and is optimized for immune effector activation. Cancer Res 2013;73(16):5183–94 doi 10.1158/0008-5472.CAN-13-0099. [DOI] [PubMed] [Google Scholar]
- 158.Garner AP, Bialucha CU, Sprague ER, Garrett JT, Sheng Q, Li S, et al. An antibody that locks HER3 in the inactive conformation inhibits tumor growth driven by HER2 or neuregulin. Cancer Res 2013;73(19):6024–35 doi 10.1158/0008-5472.CAN-13-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Alsaid H, Skedzielewski T, Rambo MV, Hunsinger K, Hoang B, Fieles W, et al. Non invasive imaging assessment of the biodistribution of GSK2849330, an ADCC and CDC optimized anti HER3 mAb, and its role in tumor macrophage recruitment in human tumor-bearing mice. PLoS One 2017;12(4):e0176075 doi 10.1371/journal.pone.0176075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee S, Greenlee EB, Amick JR, Ligon GF, Lillquist JS, Natoli EJ Jr., et al. Inhibition of ErbB3 by a monoclonal antibody that locks the extracellular domain in an inactive configuration. Proc Natl Acad Sci U S A 2015;112(43):13225–30 doi 10.1073/pnas.1518361112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Meetze K, Vincent S, Tyler S, Mazsa EK, Delpero AR, Bottega S, et al. Neuregulin 1 expression is a predictive biomarker for response to AV-203, an ERBB3 inhibitory antibody, in human tumor models. Clin Cancer Res 2015;21(5):1106–14 doi 10.1158/1078-0432.CCR-14-2407. [DOI] [PubMed] [Google Scholar]
- 162.Zhang L, Castanaro C, Luan B, Yang K, Fan L, Fairhurst JL, et al. ERBB3/HER2 signaling promotes resistance to EGFR blockade in head and neck and colorectal cancer models. Mol Cancer Ther 2014;13(5):1345–55 doi 10.1158/1535-7163.MCT-13-1033. [DOI] [PubMed] [Google Scholar]
- 163.Malm M, Frejd FY, Stahl S, Lofblom J. Targeting HER3 using mono- and bispecific antibodies or alternative scaffolds. MAbs 2016;8(7):1195–209 doi 10.1080/19420862.2016.1212147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sala G, Rapposelli IG, Ghasemi R, Piccolo E, Traini S, Capone E, et al. EV20, a Novel Anti-ErbB-3 Humanized Antibody, Promotes ErbB-3 Down-Regulation and Inhibits Tumor Growth In Vivo. Transl Oncol 2013;6(6):676–84 doi 10.1593/tlo.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang Q, Zhang X, Shen E, Gao J, Cao F, Wang X, et al. The anti-HER3 antibody in combination with trastuzumab exerts synergistic antitumor activity in HER2-positive gastric cancer. Cancer Lett 2016;380(1):20–30 doi 10.1016/j.canlet.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 166.Thakkar D, Sancenon V, Taguiam MM, Guan S, Wu Z, Ng E, et al. 10D1F, an Anti-HER3 Antibody that Uniquely Blocks the Receptor Heterodimerization Interface, Potently Inhibits Tumor Growth Across a Broad Panel of Tumor Models. Mol Cancer Ther 2020;19(2):490–501 doi 10.1158/1535-7163.MCT-19-0515. [DOI] [PubMed] [Google Scholar]
- 167.Kugel CH 3rd, Hartsough EJ, Davies MA, Setiady YY, Aplin AE. Function-blocking ERBB3 antibody inhibits the adaptive response to RAF inhibitor. Cancer Res 2014;74(15):4122–32 doi 10.1158/0008-5472.CAN-14-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hashimoto Y, Koyama K, Kamai Y, Hirotani K, Ogitani Y, Zembutsu A, et al. A Novel HER3-Targeting Antibody-Drug Conjugate, U3-1402, Exhibits Potent Therapeutic Efficacy through the Delivery of Cytotoxic Payload by Efficient Internalization. Clin Cancer Res 2019;25(23):7151–61 doi 10.1158/1078-0432.CCR-19-1745. [DOI] [PubMed] [Google Scholar]
- 169.Bourillon L, Bourgier C, Gaborit N, Garambois V, Lles E, Zampieri A, et al. An auristatin-based antibody-drug conjugate targeting HER3 enhances the radiation response in pancreatic cancer. Int J Cancer 2019;145(7):1838–51 doi 10.1002/ijc.32273. [DOI] [PubMed] [Google Scholar]
- 170.De Nardis C, Hendriks LJA, Poirier E, Arvinte T, Gros P, Bakker ABH, et al. A new approach for generating bispecific antibodies based on a common light chain format and the stable architecture of human immunoglobulin G1. J Biol Chem 2017;292(35):14706–17 doi 10.1074/jbc.M117.793497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fitzgerald JB, Johnson BW, Baum J, Adams S, Iadevaia S, Tang J, et al. MM-141, an IGF-IR- and ErbB3-directed bispecific antibody, overcomes network adaptations that limit activity of IGF-IR inhibitors. Mol Cancer Ther 2014;13(2):410–25 doi 10.1158/1535-7163.MCT-13-0255. [DOI] [PubMed] [Google Scholar]
- 172.Xue J, Kong D, Yao Y, Yang L, Yao Q, Zhu Y, et al. Prediction of Human Pharmacokinetics and Clinical Effective Dose of SI-B001, an EGFR/HER3 Bi-specific Monoclonal Antibody. J Pharm Sci 2020;109(10):3172–80 doi 10.1016/j.xphs.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 173.Zhao Z, Li R, Sha S, Wang Q, Mao W, Liu T. Targeting HER3 with miR-450b-3p suppresses breast cancer cells proliferation. Cancer Biol Ther 2014;15(10):1404–12 doi 10.4161/cbt.29923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lyu H, Huang J, He Z, Liu B. Targeting of HER3 with Functional Cooperative miRNAs Enhances Therapeutic Activity in HER2-Overexpressing Breast Cancer Cells. Biol Proced Online 2018;20:16 doi 10.1186/s12575-018-0081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]