Abstract
Seasonal changes in climate are accompanied by shifts in carbon allocation and phenological changes in woody angiosperms, the timing of which can have broad implications for species distributions, interactions and ecosystem processes. During critical transitions from autumn to winter and winter to spring, physiological and anatomical changes within the phloem could impose a physical limit on the ability of woody angiosperms to transport carbon and signals. There is a paucity of the literature that addresses tree (floral or foliar) phenology, seasonal phloem anatomy and seasonal phloem physiology together, so our knowledge of how carbon transport could fluctuate seasonally, especially in temperate climates is limited. We review phloem phenology focussing on how sieve element anatomy and phloem sap flow could affect carbon availability throughout the year with a focus on winter. To investigate whether flow is possible in the winter, we construct a simple model of phloem sap flow and investigate how changes to the sap concentration, pressure gradient and sieve plate pores could influence flow during the winter. Our model suggests that phloem transport in some species could occur year-round, even in winter, but current methods for measuring all the parameters surrounding phloem sap flow make it difficult to test this hypothesis. We highlight outstanding questions that remain about phloem functionality in the winter and emphasize the need for new methods to address gaps in our knowledge about phloem function.
Keywords: Carbon transport, dormancy, phenology, sap viscosity, winter
How does sugar movement change seasonally and can sugars be moved during the winter when temperate trees are largely dormant? We review the current knowledge of seasonal changes to the sugar-conducting tissue, the phloem, and use a simple model to facilitate a discussion of whether or not the phloem could be more active than previously assumed in some species.
Introduction
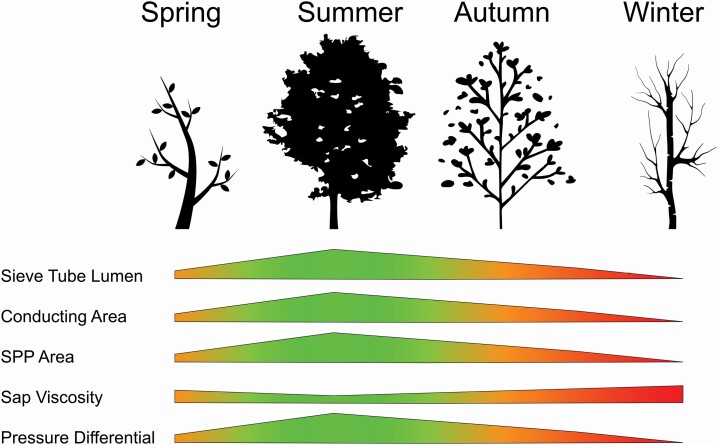
In temperate environments, perennial plants exhibit cyclical shifts in resource allocation from the growth of flowers and leaves in the spring to leaf senescence and carbon sequestration during fall and early winter (Brüggemann et al. 2011; Palacio et al. 2018). These large changes in allocation require remobilization of carbon, water and nutrients in the vascular system and should be influenced by seasonal changes in vascular function (Fig. 1; Lechowicz 1984; Savage 2021). The phloem is particularly important during these periods of seasonal change because it transports carbon, nutrients and signalling molecules such as hormones, small RNAs and electrolytes (Thompson and Schulz 1999; Ruiz-Medrano et al. 2001; Kehr and Buhtz 2008) that can influence phenology directly (e.g. trigger senescence and flowering) or indirectly (e.g. impact circadian rhythm and apical dominance; Chincinska et al. 2013; Van den Ende 2014; Kim et al. 2018; Kim 2019, 2019; Kumar et al. 2019). Sugar itself can also serve as a signal for processes such as flowering (Horacio and Martinez-Noel 2013; Li 2016; Cho et al. 2018; Kim 2019). Therefore, understanding the capacity for signal and carbon movement by the phloem during the winter-spring transition is vital to being able to understand spring growth. Considering that there are limited data documenting seasonal anatomical changes to phloem physiology (Savage 2020), the extent that phloem transport function is reduced or compromised in the winter for many species is unclear.
Figure 1.
The effects of changes in anatomical and physiological parameters on phloem sap flow during the year. Polygon width indicates the relative change to the parameter listed in the left column and colours indicate relative effect on sap flow where orange is a moderate flow, green is optimal flow and red is little to no flow. SPP = Sieve plate pore.
Historically, seasonal studies of anatomy have been used to infer changes in phloem functionality (for a review see Evert 2006). Many studies have shown that while the classical view presented in even some recent plant anatomy textbooks is that carbon transport stops in the winter (e.g. Crang et al. 2018), anatomical studies suggest that there may be variation in species ability to transport carbon throughout the winter months (Evert 2006). Some species retain completely open and potentially active sugar-conducting conduits (sieve tubes; Tucker and Evert 1969), while others retain a reduced number of open sieve tubes or seal their sieve tubes with the polysaccharide callose either for the winter or permanently (Zamski and Zimmermann 1979; Fisher 1983; Evert 2006). In the late winter or early spring, new phloem tissue is produced by the cambium before xylogenesis (Tucker and Evert 1969; Lavrič et al. 2017) and in some species there are open sieve tubes adjacent to the cambium before division begins (Davis and Evert 1970; Aloni and Peterson 1997). In general, phloem development and re-activation is prioritized over xylem development in the early spring and new phloem in the stem is always produced before leaf-out (for review of vascular phenology, Savage and Chiune 2021).
Despite the prevalent assumption that sieve elements are often closed in the winter, there could be benefits to retaining some phloem function all winter to allow for transport of signals and resources throughout the plant body. For example, the phloem could support changes in carbon allocation leading to starch accumulation in floral buds as seen in Prunus and Olea during the winter (De la Rosa and Rallo 2000; Fadón et al. 2018). It could also support other types of growth including development of leaf primordia which continue to grow slowly throughout the winter in some species (Gordon et al. 2006). Overall, the greatest demand on the phloem is during peak growth in the early spring. During this time, the size of floral tissue in buds can increase four 100-fold (Savage 2019). Phloem transport capacity in the spring may be especially important for seasonally precocious species that undergo floral bud burst during the persistent threat of winter frost before there are leaves on the plant.
There is limited research that has attempted to directly link phloem anatomy with phloem function in the spring (Aloni and Peterson 1991) partly because the methods necessary to do so are limited. There has been a renewed interest in developing and optimizing methods for studying in situ phloem transport (Savage et al. 2013; Knoblauch et al. 2015; Ray and Savage 2020). Recent efforts have improved our ability to overcome problems with wounding and manipulation (Knoblauch et al. 2014; Howell et al. 2020), but studying the phloem during winter in temperate climates presents an added challenge because most trees do not have leaves and the source-sink dynamics utilized by many phloem physiological methods are reduced.
In this review, we consider what is known about how changes in phloem anatomy and plant physiology could impact transport of carbon throughout a plant in the winter and its potential implications for spring growth. Transport in sieve tubes is driven by a pressure differential created by local osmotic gradients in source and sink tissues (Münch 1930). We will consider how linear sap velocity (ν) is influenced by several key components: the pressure gradient (Δp), sieve tube lumen area (A) and the tube resistance (R):
| (1) |
We will discuss the ramifications of changes to hydraulic resistance and the source-sink differential, highlighting how the composition of the phloem sap, sieve element structure and sap viscosity could change seasonally with a focus on the winter. Our goal is to demonstrate that carbon transport in the winter is likely dynamic and there may be species-specific differences that could have implications for spring growth. Our analysis includes an in-depth discussion of the literature and a consideration of how methodology has shaped our perception of phloem physiology.
Pressure Gradient
Movement of osmolytes into and out of the sieve tubes can occur either by active transport in the apoplast, or by active or passive transport in the symplast (Gamalei 1989; Lalonde et al. 2003; Rennie and Turgeon 2009). As osmolytes are loaded into sieve elements (the individual cells that make up the sieve tubes), high osmolyte concentration or low water potential draws water in, creating positive pressure to push the phloem sap to areas of low pressure where osmolytes are unloaded (sinks; Münch 1930). Because the pressure gradient that drives phloem sap flow is influenced by local processes in the source and sink tissue, understanding seasonal changes in phloem transport requires knowledge about source and sink activity, osmolyte concentration and composition, membrane permeability and enzyme activity in the phloem and adjacent cells.
Source and sink activity
Studies of photosynthesis, growth, non-structural carbohydrate allocation and enzyme activity have shown dynamic shifts in source and sink activity during and between seasons (Gruber et al. 2013; Jyske and Hölttä 2015). In the fall, low photosynthetic rates can slow carbon transport by decreasing the sugars available for loading into the phloem sap (Ho 1976), which could impact pressure in the source tissue. However, phloem pressure does not have to change directly in proportion to changes in carbon fixation. In leaves after carbon is fixed, sucrose must be synthesized before sugars are loaded into sieve tubes. Anything that impacts the rate of carbohydrate synthesis could decouple photosynthetic rates and phloem transport. For example, at low temperatures, the activity of sucrose phosphate synthase, the enzyme that catalyses sucrose synthase, increases resulting in higher sucrose concentrations in Spinacia leaves (Guy et al. 1992). Similar complications may arise in the winter in species that can fix carbon when conditions become more favourable but are unlikely to matter once deciduous species become endodormant (Ogren 1997).
If a pressure gradient is maintained in the winter, it needs to be supported by different sources and sinks than during the growing season. Potential sinks that can drive flow include respiration, seed or fruit filling and leaf or flower growth which have different phenologies (Patrick 1997). In winter, developing flowers or leaves and respiration are the most likely active sinks but they should be weak. Stem respiration rates are greatly reduced in the winter (Ceschia et al. 2002; Damesin 2003), while leaf and floral buds appear to be varied in their winter growth and development. Bud growth often occurs in the final dormancy stage, ecodormancy, thus buds are more likely to be a sink near the end of winter (Faust et al. 1997; Horvath et al. 2003). As previously mentioned, some species appear to accumulate starch during the winter (De la Rosa and Rallo 2000; Fadón et al. 2018), creating a potential sink, but whether the carbon required for this process is supplied locally or transported in the vascular system remains unclear. It seems likely that sink strength decreases considerably in early winter and begins to increase in late winter as metabolism and growth begin in the buds.
Throughout the year, the relative proportion of sugars in the phloem sap fluctuates as carbon is allocated between different sources and sinks (Fisher 1983; Gruber et al. 2013; Jyske and Hölttä 2015). Phloem sugar concentrations within an individual tree may even vary when sampled at different heights during the same season (Pate 1998), suggesting that the winter dynamics of sugar transport may also vary by sampling location within an individual. The primary cause of changes in phloem sap composition during spring is the conversion of starch into soluble sugars through re-mobilization of stored carbon in stems, trunks and roots. In autumn and winter, phloem sap sugar concentration sampled by stylectomy increased to concentrations as high as double that of the spring and summer (Fisher 1983).
Osmolyte composition
Species that produce flowers or leaves early in spring before freezing danger has passed need mechanisms to protect them from damage (Lineberger and Steponkus 1980;Peters and Keller 2009; Charrier et al. 2013) and the composition of the phloem sap may assist in the cryoprotection of sieve tubes. The contents of the phloem sap are complex, consisting of sugars, amino acids, proteins and small RNAs. Sugars (sucrose, glucose, fructose, mannose, galactose and raffinose) are present in the highest concentrations and likely confer some freezing tolerance (Dinant et al. 2010; Hijaz and Killiny 2014; Hijaz et al. 2016). For example, sucrose typically makes up the largest fraction of the phloem sap, and could provide cryoprotection for some species. The freezing point depression of 17 % sucrose (w/v), the optimal sucrose concentration for flow within the phloem (Jensen et al. 2013), is ~1.1 °C (Lide 2009). If the phloem sap sugar concentration were doubled, the freezing point would be depressed 3.37 °C (Lide 2009), possibly explaining the phloem transport range for Salix viminalis (−4 °C; Weatherley and Watson 1969). Many species can transport carbon at lower temperatures (Salix exigua −13 °C; Fisher 1983) indicating that there must be other forms of cryprotection in the phloem. Sieve elements can supercool when plunged in liquid nitrogen, though the process of supercooling appears to be reliant on hardening at cool temperatures prior to the onset of winter (Froelich et al. 2011). It is often assumed that if ice forms inside sieve tubes it is lethal, but it is also possible that there could be the formation of amorphous, uncrystallized ice allowing for vitrification of the tissue in some species (Hirsh et al. 1985; Debenedetti 1996).
Raffinose is another phloem osmolyte that confers osmoprotection (dos Santos et al. 2011), cryoprotection (Bachmann et al. 1994; Stushnoff et al. 1998; Pennycooke et al. 2003; Peters and Keller 2009), and inhibits the tendency of sucrose solutions to crystalize at low temperatures (Caffrey et al. 1988; Lipavská et al. 2000). The proportion of raffinose to sucrose in the phloem increases in autumn (Bachmann et al. 1994) and under cold conditions (Hinesley et al. 1992) and in frost resistant species (Parker 1963; Sakai and Larcher 1987; Lintunen et al. 2018), in response to changes in photoperiod and temperature (Wiemken and Ineichen 1993). The ratio of raffinose to other phloem sugars is highest at colder latitudes in both angiosperms and gymnosperms in Europe (Lintunen et al. 2018). Raffinose is present in minor leaf veins (Haritatos et al. 2000) of polymer-trapping species (Rennie and Turgeon 2009), some apoplastic-loading species, conifers (Hinesley et al. 1992), and can be made from sucrose as a response to abiotic stress (ElSayed et al. 2014). It is possible raffinose could also be made from sucrose within the phloem sap rather than being transported into the sieve tubes. It is not clear how the presence of raffinose affects the viscosity of the phloem sap at low temperatures, but modelling suggests that raffinose and similar sugars found in the phloem sap are more favourable to viscosity than sucrose (Lang 1978). Also, sucrose and raffinose solutions in glycine of the same concentration have similar viscosities (Ali et al. 2019).
Given its cryoprotective effects, raffinose could be a major contributor to the pressure gradient during temperate winters, as it appears to allow the phloem sap to remain at a viscosity conducive to flow longer than predicted for a simple sucrose solution. Raffinose has more carbon than sucrose, so fewer raffinose molecules are made for the same amount of carbon. If we assume a discrete level of carbon, having a greater proportion of raffinose in the phloem sap than sucrose would decrease its osmotic potential and therefore reduce source pressure. As a result, phloem sap flow could be altered as the temperature drops if sugar concentrations were modified in the source and sink sap. It is possible that during the winter genera with a portion of their sieve tubes open contain more raffinose in their phloem sap than species that completely occlude their sieve tubes, but we are not aware of any studies that have investigated the phloem sap contents and phloem overwintering strategies. Species that retain open and seemingly active sieve tubes in the winter should be studied to better understand the contents of the phloem sap and their contribution in sieve element cryoprotection.
Hydraulic Resistance
Phloem hydraulic resistance within sieve tubes (R) can be affected by several variables that change seasonally including conduit anatomy and sap viscosity. Some of the resistance to flow in a sieve tube comes from the sieve element lumen (Rlumen), which can be expressed as:
| (2) |
where η is the sap viscosity, L is the path length from source to sink and r is the radius of the sieve tube lumen. Equation (2) demonstrates that anatomy (sieve tube radius) has a major effect on flow rate due to the fourth power relationship, i.e. narrower sieve tubes are significantly more resistant to flow than wider ones. Longer path lengths can also increase the resistance in this model, but sieve tube elements taper in diameter as a function of distance from base of the trunk to the tips of branches, which minimizes resistance conferred by conduit radius along the flow path (Petit and Crivellaro 2014; Liesche et al. 2016; Savage et al. 2017).
At least half of the resistance in sieve tubes is caused by the sieve plates, which occur at the ends of each sieve element (Savage et al. 2017; Stanfield et al. 2019). Jensen (2012), modified Equation (2) for a single sieve element, adding terms to account for the resistance of the sieve plate pore radii by using the mean pore diameter and standard deviation:
| (3) |
where N is the total number of pores in a sieve plate, rp is the radius of the sieve plate, , and , where is the length of the pore lumen, i.e. the plate thickness, is the average pore radius and is the standard deviation. As such, the resistance for an entire sieve tube is the sum of the resistances for each sieve element () that are stacked to form a sieve tube.
Sieve element anatomy and cross-sectional area
There are several ways that sieve element anatomy changes during the year. First, sieve elements produced in a single growing season are not always uniform in diameter. Elements produced during the fall are narrower and have a higher resistance to flow than those produced in the spring (Evert 2006; Prislan et al. 2013). It is not clear if this pattern is advantageous or is a result of developmental ties between the phloem and xylem (Jyske and Hölttä 2015; Ray and Jones 2018). Second, sieve plate resistance is increased when callose is deposited on the sieve plates (Mullendore et al. 2010; Jensen et al. 2012), a process that occurs seasonally in some species (Evert 2006). Plants with callose-occluded sieve plates either re-activate them by removing callose in the spring or differentiating new sieve tubes in the late winter and early spring. If callose only partially covers pores, it may reduce the sieve plate pore diameter, but still allow flow to occur. It is unclear if callose is present in sieve tubes under normal conditions because most anatomical studies of sieve tubes in woody trees use methods that cause mechanical damage eliciting a callose response and fix the collected tissue too slowly resulting in callose artefact (Esau and Cheadle 1961; Evert and Derr 1964). Clearly more work focussed on callose and its presence seasonally within the phloem is needed (Montwé et al. 2019).
The total phloem conducting area changes seasonally and likely varies by the ability to keep sieve elements open in the winter. In species that retain open sieve tubes throughout the year, transport capacity would be similar in the winter and summer months, whereas in species that occlude all sieve tubes during the winter, all flow would stop during winter. Those species that retain a small number of seemingly open sieve elements, however, would have less conducting area during the winter and likely a lower capacity for carbon transport in early spring (Savage 2020). Seasonal anatomical changes are likely coordinated with sink and source strength, ensuring flow when it is necessary. Smaller diameter sieve tubes produced at the end of the season may allow flow to continue in the winter by facilitating easier build-up of pressure to drive flow. Research specifically focussed on such changes is needed to better contextualize the seasonal changes of anatomy in the sieve tubes and its implications for the winter-spring transition.
Changes in viscosity
Viscosity increases exponentially with concentration in sucrose solutions indicating that sap concentration could easily impact resistance to flow in sieve tubes (Morison 2002; Sevanto 2014). Considering the viscosity in sucrose solutions also increases at lower temperatures and with higher concentrations of solutes (Telis et al. 2007), viscosity should build up during the transition from fall into winter when phloem sap osmolyte concentrations increase (Fisher 1983). If this increase is high, viscosity could be a limiting factor for movement of signalling molecules as well as carbon in the winter, similar to what may theoretically occur in the phloem during drought (Sevanto et al. 2013; Sevanto 2014; Gaylord et al. 2015).
The Effects of Winter on Phloem Sap Flow
To directly illustrate how seasonal changes in sieve tube anatomy, callose deposition and changes in source and sink activity impact phloem sap flow, we modelled phloem transport velocity using a simplified model and published values for Quercus rubra (Savage et al. 2017). We calculated theoretical flow rates for a single sieve tube within a 27 m tall individual assuming that the sieve tube is the length of the tree. We chose this species because all of the parameters required to calculate resistance, including the number of sieve elements per metre, sieve pore area and diameter and sap flow using a pipe-flow model have been previously measured (Münch 1930; Huber et al. 1937; Savage et al. 2017). Our model considers the viscosity of an aqueous sucrose solution within a single sieve tube and demonstrates the effects on flow that result from changes in sap viscosity, the pressure gradient, and sieve plate callose occlusion at a variety of temperatures.
We calculated viscosities for an aqueous sucrose solution at a range of temperatures experienced by trees in temperate climates using the relation from Mathlouthi (1995), linear sap velocity using Equation (1) and resistance using Equation (3), which accounts for both the sieve plate pores and sieve element lumen. To account for the widening of sieve elements from the tree apex towards the base of the trunk (Savage et al. 2017), we divided our 27 m tall tree into three 9-m segments calculating the resistance first for a single sieve element within each segment and then for the sum of resistances for all the sieve elements within that segment. The sum of each segment was added together to calculate the resistance for an entire sieve tube as in Clerx (2020). Finally, to convert values from volumetric sap flow to the more commonly used linear sap velocity, we divided the volumetric sap velocity by the area of a circular sieve element of radius ~24 µm, the radius of a sieve tube at approximately the midpoint of the tree (see Equation (1)). This assumption ignores the widening of sieve elements from branch tip to tree base and represents only a single point measurement, though the maximal velocity in the model (~100 µm s−1; Fig. 2) is near what has been reported for Q. rubra (Huber et al. 1937).
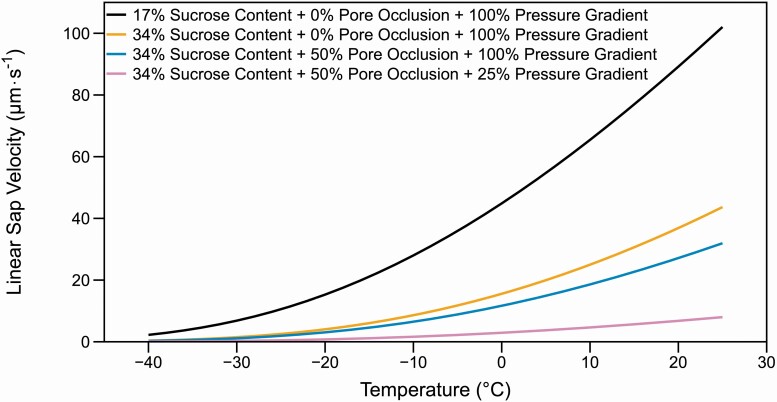
Figure 2.
The theoretical effects of sucrose concentration, sieve plate pore occlusion and pressure gradient changes on linear sap flow in the phloem. Under optimal conditions, such as in the summer, phloem sap concentration is around 17 % sucrose, sieve plate pores are not occluded and the pressure gradient is unchanged (black line). Linear sap velocity is reduced if sucrose concentration is doubled as may happen in the winter (orange line). Further decreases in linear sap velocity occur if the sieve plate pores become partially occluded with callose (blue line) in the late fall or winter. The lowest linear sap velocities occur when the pressure differential is also reduced (25 % of normal) as may occur when leaves are not present during late fall and winter (purple line).
We note that these equations, while used in many complex modelling studies (Jensen et al. 2013; Knoblauch et al. 2016; Savage et al. 2017; Clerx et al. 2020) are only applicable under specific conditions: they consider a single sieve tube that is continuous along the length of the tree that is loaded at the branch tip, widens from branch tip to tree base, and is unloaded at the trunk base. The resistance equation considers an empty pipe (no organelles) with no lateral water movement along the flow path. This model also assumes a sieve tube with impermeable walls, which may not be the case for the sieve tubes (Phillips and Dungan 1993; Sevanto 2014; Stanfield et al. 2017). While simple, this model allows us to illustrate the factors that impact flow within the phloem and understand the factors that may allow some species to undergo the transition to spring more quickly than others.
The sap concentration of Q. rubra, was reported as 17.1 % in the summer (Münch 1930; Jensen et al. 2013) and we used that as our base value for viscosity calculations (Fig. 2, black line). We considered the additive effect of three possible changes to the phloem in the winter and their combined effect on velocity. First, phloem viscosity likely increases due to lower temperatures and an increase in phloem sap sugar concentrations. In Salix, sugar content of the phloem sap doubles in the winter (Fisher 1983). If similar sap concentration changes occur in Q. rubra, the sucrose concentration would be near 34 % in the winter (Fig. 2 orange line). Next, compounding with the increase to the sap concentration, we added a reduction in the size of the sieve plate pores due to callose accumulation (Fig. 2 blue line). We used a 50 % reduction in the sieve plate pore radius of all sieve plate pores throughout the phloem but note that the degree to which the sieve plates become occluded in functional winter sieve elements during the winter is not documented. Finally, with the loss of leaves in autumn, the pressure gradient within sources and sinks is likely greatly altered. Here we used a 75 % reduction in the pressure gradient (Fig. 2 purple line), but again note that more seasonal studies are necessary to determine the pressure gradient within trees during the winter.
Our model suggests that sap flow within a sieve tube can continue below freezing if any one variable considered by the model is reduced in value, but the compounding effects to all variables appear to reduce the capacity for sap flow below freezing temperatures. At 0 °C, for example, the calculated velocity considering an increased sap concentration, partially occluded sieve plate pores, and a reduced pressure gradient is ~3 µm/s, when compared with ~45 µm/s at the same temperature with open sieve elements and a ‘normal’ pressure gradient within the sieve tube. Our model supports the dominance of the pressure gradient in limiting sap velocity (Fig. 2). Our model suggests flow is possible well below 0 °C if the pressure gradient is maintained. Thus, local flow could occur over shorter distances if a pressure gradient could be generated, for example within a portion of a single branch, rather than between the tips of the branches and base of the tree. Flow could also occur in regions of the plant body where wider diameter sieve tubes occur such as in a tree trunk, where sieve elements are wider when compared with distal stems (Savage et al. 2017; Clerx et al. 2020), especially if there are differences in early versus late sieve element diameter, a factor not included in our model.
Our model also suggests that in order to restore flow, even in temperatures below zero, trees need only to alter their sink activity. When put into context with the various overwintering strategies, this model suggests that retaining some open sieve tubes may allow species to quickly re-activate in the spring because flow could be restored by expanding leaves or flowers in buds acting as sinks. Species that must differentiate and mature new sieve tubes to resume phloem function in the spring may not be able to transport a high enough quantity of resources to support growth early in the spring. Alternatively, species could restart phloem flow if the xylem becomes a carbon sink. Sucrose may, for example, be moved into the xylem to reduce the likelihood of spring embolism within the xylem (Zwieniecki et al. 2015; Konrad et al. 2019).
It is likely that many different strategies are utilized to facilitate phloem sap flow in late winter and are currently unrecognized due to our limited knowledge of species-specific phloem overwintering and phenology. Acer negundo, for example, flowers precociously early in the season and does not appear to occlude or otherwise close sieve tubes in the winter (Tucker and Evert 1969), while Populus tremuloides, which also flowers precociously often before A. negundo appears to produce new phloem from overwintered, partially differentiated phloem ‘mother cells’ (Davis and Evert 1968). Broad seasonal studies that measure phloem flow while documenting phloem overwintering strategies and phenology are necessary to fully understand the advantages and disadvantages to open sieve elements, but those studies will require methods that allow for seasonally repeated measures on large numbers of species or individuals.
Outstanding Questions about Phloem Seasonality
Before plants can invest in significant new growth in the spring, they need to have a partially functional vascular system, but the timing and mechanics of the phloem’s transition from winter to spring is still understudied. Our knowledge of seasonal changes to phloem physiology is limited to a small number of studies documenting phloem functional area, sieve tube pressure and sap flow change during the winter and spring. One reason for this gap in knowledge is long-recognized methodological challenges that arise from studying the phloem, a pressurized, membrane-bound system (Knoblauch and van Bel 1998). Further limitations in studying phloem seasonally arise from a high level of among-species-variation in seasonal responses and the need for time-series data. To determine whether seasonal changes in the phloem have implications for spring phenology, we need to answer three key questions, outlined below. With each question we consider challenges and potential future direction for expanding our knowledge in these areas.
How much does sieve element conducting area change seasonally?
A critical part of understanding seasonal changes in flow is determining the area of conducting sieve tubes in the phloem. In our model, differences in the conducting area would influence the volumetric sap flow in the phloem (product of sap velocity and conducting area). While not explicitly explored in our model, the total conducting area directly affects the sap volume that can be conducted through the plant body. Current methods for identifying functional sieve tubes using TEM or light microscopy can be time consuming and complex to interpret (Knoblauch and Oparka 2012). More targetted approaches with a higher specificity to active sieve tubes are necessary to get enough replication of time-series data to examine seasonal changes in phloem transport area. Transgenic expression of fluorescent proteins specific to sieve tube walls is used in Arabidopsis (Yang and Russell 1990; Thompson and Wolniak 2008). This method allows for rapid assessment of sieve tube area but does not indicate whether conduits are functional and would, therefore, require a subsequent screening using aniline blue or immunogold staining and detection with TEM to confirm functionality (Prislan et al. 2013). The recent discovery of a monoclonal antibody, LM26, that binds to the epitope on a pectin specific to sieve tube cell walls of herbaceous crop species, also appears promising (Torode et al. 2018). This epitope appears to be present in the sieve tube walls of two species of Populus only at times when the sieve tubes have been previously described as actively conducting (Ray and Savage 2020). Immunostaining to detect the LM26 epitope is relatively straightforward and can be conducted on fresh or fixed tissue and completed in less than a day, which could allow for analysis of more samples and more accurate measurements of active sieve tubes. The LM26 antibody may become even more powerful if combined with Fourier Transformed Infrared Spectroscopy (FTIR), which involves the analysis of infrared absorption to identify individual chemical components of a sample. In plants, FTIR has been used to identify cell wall components in Phaseolus (Alonso-Simón et al. 2004) and was recently used to map the sucrose gradients of frozen cross-sections in cereal crops and Arabidopsis at resolutions of near 12 µm (Guendel et al. 2018). If applied to woody plants using plunge frozen microcores, FTIR spectroscopy could be used to discern the sugar composition of actively transporting sieve elements. Next steps should test the applicability of these techniques to larger samples sizes and a wider variety of species and growth forms. The FTIR work should also be replicated in conjunction with techniques that can identify active sieve elements, such as the sap flow measurements to confirm the results of the FTIR technique can provide reliable results specific to sieve tubes and to compare those results to those of other more established methods such as stylectomy. If such confirmational studies suggest that FTIR is able to discern the sugar contents within the sieve tubes, it could be used to analyse the seasonal change in the phloem sap sugar content across entire cross-sections.
How does the phloem pressure gradient change seasonally?
Our model suggests that the pressure gradient in the phloem may play a critical role in regulating seasonal changes in sap flow. A large reduction in the pressure gradient during winter is likely for winter deciduous species, because the leaves are the primary source of sugar production most of the year. We assumed a 25 % reduction in the phloem pressure gradient, but the actual value is unknown. There is currently limited data on phloem pressure (Hammel 1968; Wright and Fisher 1980; Lee 1981; Knoblauch et al. 2016; Savage et al. 2017) and less research that relates to seasonality (except see Mencuccini et al. 2013). Sieve tube pressure has been measured with three techniques: sap-feeding insects using stylectomy, pressure sensors and more recently with pico gauges (Hammel 1968; Wright and Fisher 1980; Lee 1981; Knoblauch et al. 2014, 2016; Savage et al. 2017). Pico gauges are currently the most promising technique for directly measuring phloem turgor pressure and they work in some woody species (Knoblauch et al. 2016; Savage et al. 2017). More widespread use of these gauges could yield insights into the seasonal dynamics of sources and sinks, but they require very finely pulled pipettes and the ability to visualize their insertion into the sieve elements, making this technique difficult to implement at the sample sizes necessary for survey-level studies. Future studies should apply the pico gauge technique to more species and investigate the diurnal and seasonal trends of sieve tube turgor with a focus on winter. This could provide crucial empirical data about the location and strength of the sources and sinks during times when leaves are not present.
How does the sap flow rate change seasonally?
The clearest way to understand seasonal changes in phloem function is to measure or visualize flow rate directly. Our model suggests that sap flow can continue below freezing temperatures only if the pressure gradient is able to be maintained and if the phloem sap concentration remains relatively close to that for optimal flow (17 %) rather than doubling as has been documented in Salix (Wright and Fisher 1980). The ideal approach to answer this question would be directly measuring sap flow during seasonal studies. Unfortunately, many common methods require highly specialized equipment (Magnetic Resonance Imaging, isotope studies, microCT) that in some cases must be customized to the specific individual being studied. While these techniques have provided essential information on phloem function (Windt et al. 2006; Homan et al. 2007; Devaux et al. 2009; Brüggemann et al. 2011; Brodersen and Roddy 2016), they are not amenable to the large sample numbers (and large sampling areas) required for measuring seasonal changes in phloem function in multiple species. Other methods that utilize phloem-mobile fluorophores may allow for greater replication, but currently exhibit variation in their species-specificity, which may be related to phloem loading type (Savage et al. 2013; Knoblauch et al. 2015). Because there is currently no technique that would allow for a wide-scale screening of winter sap flow across species, future work should focus on testing the validity of using different anatomical techniques to assess function. If future research could demonstrate a clear connection between seasonal changes in phloem function and anatomy, it would be possible to better judge differences in phloem function across species.
Conclusions
Carbon and nutrients are mobilized in the phloem along with a host of signalling molecules that support the seasonal pulses of growth and senescence (van Bel et al. 2013). Though seasonal carbon allocation is well studied, anatomical and physiological changes to the phloem are not well documented. In this paper, we highlight the dynamic nature of phloem transport and the diversity of factors that could influence phloem transport in the winter and during the winter-spring transition from seasonal changes in source and sink activity to changes in sieve tube anatomy. We use a simple model to demonstrate the importance of phloem pressure in determining flow at low temperatures and consider the implications for different overwintering strategies for the timing of phloem re-activation in the spring. Future work should focus on gathering more time-series data on phloem anatomy and physiology and overcoming methodological barriers to collecting this type of data. In a time when plant phenology is changing in response to climate change, it is critical that we develop a more comprehensive perspective on seasonal changes in plant physiology and resource allocation.
Acknowledgements
We would like to acknowledge two anonymous reviewers for insightful comments that greatly improved the manuscript.
Plants, Ecosystems & Climate. Chief Editor: Mary Heskel
Sources of Funding
Funding was provided by National Science Foundation Integrative Organismal Systems 1656318 (J.A.S.) and National Science Foundation CAREER 1942916 (J.A.S.).
Conflict of Interest
None declared.
Contributions by the Authors
D.M.R. and J.A.S. conceived the original idea. D.M.R. led the writing with substantial contributions from J.A.S. All authors contributed to discussions and subsequent writing of the manuscript.
Data Availability
The annotated R code and related data used to make the phloem model can be accessed from DRYAD (doi:10.5061/dryad.rbnzs7hbg).
Literature Cited
- Ali A, Bidhuri P, Malik NA, Uzair S. 2019. Density, viscosity, and refractive index of mono-, di-, and tri-saccharides in aqueous glycine solutions at different temperatures. Arabian Journal of Chemistry 12:1684–1694. [Google Scholar]
- Aloni R, Peterson CA. 1991. Seasonal changes in callose levels and fluorescein translocation in the phloem of Vitis Vinifera L. IAWA Journal 12:223–234. [Google Scholar]
- Aloni R, Peterson CA. 1997. Auxin promotes dormancy callose removal from the phloem of Magnolia kobus and callose accumulation and earlywood vessel differentiation in Quercus robur. Journal of Plant Research 110:37. [DOI] [PubMed] [Google Scholar]
- Alonso-Simón A, Encina AE, García-Angulo P, Álvarez JM, Acebes JL. 2004. FTIR spectroscopy monitoring of cell wall modifications during the habituation of bean (Phaseolus vulgaris L.) callus cultures to dichlobenil. Plant Science 167:1273–1281. [Google Scholar]
- Bachmann M, Matile P, Keller F. 1994. Metabolism of the raffinose family oligosaccharides in leaves of Ajuga reptans. Plant Physiology. 105:1335–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen CR, Roddy AB. 2016. New frontiers in the three-dimensional visualization of plant structure and function. American Journal of Botany 103:1–5. [DOI] [PubMed] [Google Scholar]
- Brüggemann, N, Gessler A, Kayler Z, Keel SG, Badeck F, Barthel M, Pascal Boeckx, NBuchmann, EBrugnoli, JEsperschütz, OGavrichkova, JGhashghaie, NGomez-Casanovas, CKeitel, AKnohl, DKuptz, SPalacio, YSalmon, Y Uchida, and Bahn M. 2011. Carbon Allocation and Carbon Isotope Fluxes in the Plant-soil-atmosphere Continuum: a Review. Biogeosciences 8:3457–3489. [Google Scholar]
- Caffrey M, Fonseca V, Leopold AC. 1988. Lipid-sugar interactions: relevance to anhydrous biology. Plant Physiology 86:754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceschia E, Damesin C, Lebaube S, Pontailler J-Y, Dufrêne E. 2002. Spatial and seasonal variations in stem respiration of beech trees (Fagus sylvatica). Annals of Forest Science 59:801–812. [Google Scholar]
- Charrier G, Cochard H, Améglio T. 2013. Evaluation of the impact of frost resistances on potential altitudinal limit of trees. Tree Physiology 33:891–902. [DOI] [PubMed] [Google Scholar]
- Chincinska I, Gier K, Krügel U, et al.. 2013. Photoperiodic regulation of the sucrose transporter StSUT4 affects the expression of circadian-regulated genes and ethylene production. Frontiers in Plant Science 4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho L-H, Pasriga R, Yoon J, Jeon J-S, An G. 2018. Roles of sugars in controlling flowering time. Journal of Plant Biology 61:10. [Google Scholar]
- Clerx LE, Rockwell FE, Savage JA, Holbrook NM. 2020. Ontogenetic scaling of phloem sieve tube anatomy and hydraulic resistance with tree height in Quercus rubra. American Journal of Botany 107:852–863. [DOI] [PubMed] [Google Scholar]
- Crang R, Lyons-Sobaski S, Wise R. 2018. Phloem. In: Crang R, Lyons-Sobaski S, Wise R, eds. Plant anatomy: a concept-based approach to the structure of seed plants. Cham: Springer International Publishing, 247–275. [Google Scholar]
- Damesin C. 2003. Respiration and photosynthesis characteristics of current-year stems of Fagus sylvatica: from the seasonal pattern to an annual balance. New Phytologist 158:465–475. [DOI] [PubMed] [Google Scholar]
- Davis JD, Evert RF. 1968. Seasonal development of the secondary phloem in Populus tremuloides. Botanical Gazette 129:1–8. [Google Scholar]
- Davis JD, Evert RF. 1970. Seasonal cycle of phloem development in woody vines. Botanical Gazette 131:128–138. [Google Scholar]
- De la Rosa R, Rallo L. 2000. Olive floral bud growth and starch content during winter rest and spring budbreak. HortScience 35:1223–1227. [Google Scholar]
- Debenedetti PG. 1996. Metastable liquids: concepts and principles. Princeton, NJ: Princeton University Press. [Google Scholar]
- Devaux M, Ghashghaie J, Bert D, Lambrot C, Gessler A, Bathellier C, Ogee J, Loustau D. 2009. Carbon stable isotope ratio of phloem sugars in mature pine trees throughout the growing season: comparison of two extraction methods. Rapid Communications in Mass Spectrometry 23:2511–2518. [DOI] [PubMed] [Google Scholar]
- Dinant S, Bonnemain JL, Girousse C, Kehr J. 2010. Phloem sap intricacy and interplay with aphid feeding. Comptes Rendus Biologies 333:504–515. [DOI] [PubMed] [Google Scholar]
- dos Santos TB, Budzinski IG, Marur CJ, Petkowicz CL, Pereira LF, Vieira LG. 2011. Expression of three galactinol synthase isoforms in Coffea arabica L. and accumulation of raffinose and stachyose in response to abiotic stresses. Plant Physiology and Biochemistry 49:441–448. [DOI] [PubMed] [Google Scholar]
- ElSayed AI, Rafudeen MS, Golldack D. 2014. Physiological aspects of raffinose family oligosaccharides in plants: protection against abiotic stress. Plant Biology (Stuttgart, Germany) 16:1–8. [DOI] [PubMed] [Google Scholar]
- Esau K, Cheadle VI. 1961. An evaluation of studies on ultrastructure of sieve plates. Proceedings of the National Academy of Sciences of the United States of America 47:1716–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evert RF. 2006. Esau’s plant anatomy: meristems, cells, and tissues of the plant body: their structure, function, and development. Hoboken, NJ: Wiley-Interscience. [Google Scholar]
- Evert RF, Derr WF. 1964. Callose substance in sieve elements. American Journal of Botany 51:552–559. [Google Scholar]
- Fadón E, Herrero M, Rodrigo J. 2018. Dormant flower buds actively accumulate starch over winter in sweet cherry. Frontiers in Plant Science 9:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust M, Erez A, Rowland LJ, Wang SY, Norman HA. 1997. Bud dormancy in perennial fruit trees: physiological basis for dormancy induction, maintenance, and release. HortScience 32:623–629. [Google Scholar]
- Fisher DB. 1983. Year-round collection of willow sieve-tube exudate. Planta 159:529–533. [DOI] [PubMed] [Google Scholar]
- Froelich DR, Mullendore DL, Jensen KH, Ross-Elliott TJ, Anstead JA, Thompson GA, Pélissier HC, Knoblauch M. 2011. Phloem ultrastructure and pressure flow: sieve-element-occlusion-related agglomerations do not affect translocation. The Plant Cell 23:4428–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamalei Y. 1989. Structure and function of leaf minor veins in trees and herbs—a taxonomic review. Trees 3:96–110. [Google Scholar]
- Gaylord ML, Kolb TE, McDowell NG. 2015. Mechanisms of piñon pine mortality after severe drought: a retrospective study of mature trees. Tree Physiology 35:806–816. [DOI] [PubMed] [Google Scholar]
- Gordon D, Damiano C, DeJong TM. 2006. Preformation in vegetative buds of Prunus persica: factors influencing number of leaf primordia in overwintering buds. Tree Physiology 26:537–544. [DOI] [PubMed] [Google Scholar]
- Gruber A, Pirkebner D, Oberhuber W. 2013. Seasonal dynamics of mobile carbohydrate pools in phloem and xylem of two alpine timberline conifers. Tree Physiology 33:1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guendel A, Rolletschek H, Wagner S, Muszynska A, Borisjuk L. 2018. Micro imaging displays the sucrose landscape within and along its allocation pathways. Plant Physiology 178:1448–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy CL, Huber JL, Huber SC. 1992. Sucrose phosphate synthase and sucrose accumulation at low temperature. Plant Physiology 100:502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel HT. 1968. Measurement of turgor pressure and its gradient in the phloem of oak. Plant Physiology 43:1042–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haritatos E, Medville R, Turgeon R. 2000. Minor vein structure and sugar transport in Arabidopsis thaliana. Planta 211:105–111. [DOI] [PubMed] [Google Scholar]
- Hijaz F, Killiny N. 2014. Collection and chemical composition of phloem sap from Citrus sinensis L. Osbeck (sweet orange). PLoS One 9:e101830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijaz F, Manthey JA, Van der Merwe D, Killiny N. 2016. Nucleotides, micro- and macro-nutrients, limonoids, flavonoids, and hydroxycinnamates composition in the phloem sap of sweet orange. Plant Signaling & Behavior 11:e1183084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinesley LE, Pharr DM, Snelling LK, Funderburk SR. 1992. Foliar raffinose and sucrose in four conifer species: relationship to seasonal temperature. Journal of the American Society for Horticultural Science 117:852–855. [Google Scholar]
- Hirsh AG, Williams RJ, Meryman HT. 1985. A novel method of natural cryoprotection: intracellular glass formation in deeply frozen Populus. Plant Physiology 79:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho LC. 1976. The relationship between the rates of carbon transport and of photosynthesis in tomato leaves. Journal of Experimental Botany 27:87–97. [Google Scholar]
- Homan NM, Windt CW, Vergeldt FJ, Gerkema E, Van As H. 2007. 0.7 and 3 T MRI and sap flow in intact trees: xylem and phloem in action. Applied Magnetic Resonance 32:157–170. [Google Scholar]
- Horacio P, Martinez-Noel G. 2013. Sucrose signaling in plants: a world yet to be explored. Plant Signaling & Behavior 8:e23316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath DP, Anderson JV, Chao WS, Foley ME. 2003. Knowing when to grow: signals regulating bud dormancy. Trends in Plant Science 8:534–540. [DOI] [PubMed] [Google Scholar]
- Howell AH, Peters WS, Knoblauch M. 2020. The diffusive injection micropipette (DIMP). Journal of Plant Physiology 244:153060. [DOI] [PubMed] [Google Scholar]
- Huber B, Schmidt E, Jahnel H. 1937. Untersuchungen über die Assimilatstrom. Tharandter Forstliches Jahrbuch 88:1017–1050. [Google Scholar]
- Jensen KH, Mullendore DL, Holbrook NM, Bohr T, Knoblauch M, Bruus H. 2012. Modeling the hydrodynamics of phloem sieve plates. Frontiers in Plant Science 3:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KH, Savage JA, Holbrook NM. 2013. Optimal concentration for sugar transport in plants. Journal of the Royal Society, Interface 10:20130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyske T, Hölttä T. 2015. Comparison of phloem and xylem hydraulic architecture in Picea abies stems. The New Phytologist 205:102–115. [DOI] [PubMed] [Google Scholar]
- Kehr J, Buhtz A. 2008. Long distance transport and movement of RNA through the phloem. Journal of Experimental Botany 59:85–92. [DOI] [PubMed] [Google Scholar]
- Kim J. 2019. Sugar metabolism as input signals and fuel for leaf senescence. Genes & Genomics 41:737–746. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim JH, Lyu JI, Woo HR, Lim PO. 2018. New insights into the regulation of leaf senescence in Arabidopsis. Journal of Experimental Botany 69:787–799. [DOI] [PubMed] [Google Scholar]
- Knoblauch M, Knoblauch J, Mullendore DL, et al.. 2016. Testing the Münch hypothesis of long distance phloem transport in plants. eLife 5:e15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch J, Mullendore DL, Jensen KH, Knoblauch M. 2014. Pico gauges for minimally invasive intracellular hydrostatic pressure measurements. Plant Physiology 166:1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch M, Oparka K. 2012. The structure of the phloem–still more questions than answers. The Plant Journal 70:147–156. [DOI] [PubMed] [Google Scholar]
- Knoblauch M, van Bel AJE. 1998. Sieve tubes in action. The Plant Cell 10:35–50. [Google Scholar]
- Knoblauch M, Vendrell M, de Leau E, Paterlini A, Knox K, Ross-Elliot T, Reinders A, Brockman SA, Ward J, Oparka K. 2015. Multispectral phloem-mobile probes: properties and applications. Plant Physiology 167:1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad W, Katul G, Roth-Nebelsick A, Jensen KH. 2019. Xylem functioning, dysfunction and repair: a physical perspective and implications for phloem transport. Tree Physiology 39:243–261. [DOI] [PubMed] [Google Scholar]
- Kumar R, Bishop E, Bridges WC, Tharayil N, Sekhon RS. 2019. Sugar partitioning and source-sink interaction are key determinants of leaf senescence in maize. Plant, Cell & Environment 42:2597–2611. [DOI] [PubMed] [Google Scholar]
- Lalonde S, Tegeder M, Throne-Holst M, Frommer WB, Patrick JW. 2003. Phloem loading and unloading of sugars and amino acids. Plant, Cell & Environment 26:37–56. [Google Scholar]
- Lang A. 1978. A model of mass flow in the phloem. Functional Plant Biology 5:535. [Google Scholar]
- Lavrič M, Eler K, Ferlan M, Vodnik D, Gričar J. 2017. Chronological sequence of leaf phenology, xylem and phloem formation and sap flow of Quercus pubescens from abandoned Karst Grasslands. Frontiers in Plant Science 8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechowicz MJ. 1984. Why do temperate deciduous trees leaf out at different times? Adaptation and ecology of forest communities. The American Naturalist 124:821–842. [Google Scholar]
- Lee DR. 1981. Synchronous pressure-potential changes in the phloem of Fraxinus americana L. Planta 151:304–308. [DOI] [PubMed] [Google Scholar]
- Li L, Sheen J. 2016. Dynamic and diverse sugar signaling. Current Opinion in Plant Biology 33:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lide DR. 2009. CRC handbook of chemistry and physics. London, UK: CRC Press. [Google Scholar]
- Liesche J, Pace MR, Xu Q, Li Y, Chen S. 2016. Height-related scaling of phloem anatomy and the evolution of sieve element end wall types in woody plants. The New Phytologist 214:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lineberger RD. 1980. Cryoprotection by glucose, sucrose, and raffinose to chloroplast thylakoids. Plant Physiology 65:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintunen A, Mayr S, Salmon Y, Cochard H, Hölttä T. 2018. Drivers of apoplastic freezing in gymnosperm and angiosperm branches. Ecology and Evolution 8:333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipavská H, Svobodová H, Albrechtová J. 2000. Annual dynamics of the content of non-structural saccharides in the context of structural development of vegetative buds of Norway spruce. Journal of Plant Physiology 157:365–373. [Google Scholar]
- Mathlouthi M, Reiser P (eds). 1995. Sucrose: properties and applications. Boston, MA USA: Springer US. [Google Scholar]
- Mencuccini M, Hölttä T, Sevanto S, Nikinmaa E. 2013. Concurrent measurements of change in the bark and xylem diameters of trees reveal a phloem-generated turgor signal. The New Phytologist 198:1143–1154. [DOI] [PubMed] [Google Scholar]
- Montwé D, Hacke U, Schreiber SG, Stanfield RC. 2019. Seasonal vascular tissue formation in four boreal tree species with a focus on callose deposition in the phloem. Frontiers in Forests and Global Change 2:58. [Google Scholar]
- Morison K. 2002. Viscosity equations for sucrose solutions: old and new 2002. In: Proceedings of the ninth APCChE congress and CHEMECA 2002. New Zealand: University of Canterbury, Canterbury, Christchurch, Paper No. 984. [Google Scholar]
- Mullendore DL, Windt CW, Van As H, Knoblauch M. 2010. Sieve tube geometry in relation to phloem flow. The Plant Cell 22:579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch E. 1930. Die Stoffbewegungen in der Pflanze. Jena, Germany: Gustav Fischer. [Google Scholar]
- Ogren E. 1997. Relationship between temperature, respiratory loss of sugar and premature dehardening in dormant Scots pine seedlings. Tree Physiology 17:47–51. [DOI] [PubMed] [Google Scholar]
- Palacio S, Camarero JJ, Maestro M, Alla AQ, Lahoz E, Montserrat-Martí G. 2018. Are storage and tree growth related? Seasonal nutrient and carbohydrate dynamics in evergreen and deciduous Mediterranean oaks. Trees 32:777–790. [Google Scholar]
- Parker J. 1963. Cold resistance in woody plants. Botanical Review 29:123–201. [Google Scholar]
- Pate J. 1998. d13C analysis of phloem sap carbon: novel means of evaluating seasonal water stress and interpreting carbon isotope signatures of foliage and trunk wood of Eucalyptus globulus. Oecologia 117:11. [DOI] [PubMed] [Google Scholar]
- Patrick JW. 1997. PHLOEM UNLOADING: sieve element unloading and post-sieve element transport. Annual Review of Plant Physiology and Plant Molecular Biology 48:191–222. [DOI] [PubMed] [Google Scholar]
- Pennycooke JC, Jones ML, Stushnoff C. 2003. Down-regulating alpha-galactosidase enhances freezing tolerance in transgenic petunia. Plant Physiology 133:901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Keller F. 2009. Frost tolerance in excised leaves of the common bugle (Ajuga reptans L.) correlates positively with the concentrations of raffinose family oligosaccharides (RFOs). Plant, Cell & Environment 32:1099–1107. [DOI] [PubMed] [Google Scholar]
- Petit G, Crivellaro A. 2014. Comparative axial widening of phloem and xylem conduits in small woody plants. Trees 28:915–921. [Google Scholar]
- Phillips RJ, Dungan SR. 1993. Asymptotic analysis of flow in sieve tubes with semi-permeable walls. Journal of Theoretical Biology 162:465–485. [Google Scholar]
- Prislan P, Gričar J, de Luis M, Smith KT, Čufar K. 2013. Phenological variation in xylem and phloem formation in Fagus sylvatica from two contrasting sites. Agricultural and Forest Meteorology 180:142–151. [Google Scholar]
- Ray DM, Jones CS. 2018. Scaling relationships and vessel packing in petioles. American Journal of Botany 105:1–10. [DOI] [PubMed] [Google Scholar]
- Ray DM, Savage JA. 2020. Immunodetection of cell wall pectin galactan opens up new avenues for phloem research. Plant Physiology 183:1435–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie EA, Turgeon R. 2009. A comprehensive picture of phloem loading strategies. Proceedings of the National Academy of Sciences of the United States of America 106:14162–14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Medrano R, Xoconostle-Cázares B, Lucas WJ. 2001. The phloem as a conduit for inter-organ communication. Current Opinion in Plant Biology 4:202–209. [DOI] [PubMed] [Google Scholar]
- Sakai A, Larcher W. 1987. Frost survival of plants. Berlin, Heidelberg: Springer Verlag. [Google Scholar]
- Savage JA. 2019. A temporal shift in resource allocation facilitates flowering before leaf out and spring vessel maturation in precocious species. American Journal of Botany 106:113–122. [DOI] [PubMed] [Google Scholar]
- Savage JA. 2020. It’s all about timing—or is it? Exploring the potential connection between phloem physiology and whole plant phenology. American Journal of Botany 107:1–4. [DOI] [PubMed] [Google Scholar]
- Savage JA, Beecher SD, Clerx L, Gersony JT, Knoblauch J, Losada JM, Jensen KH, Knoblauch M, Holbrook NM. 2017. Maintenance of carbohydrate transport in tall trees. Nature Plants 3:965–972. [DOI] [PubMed] [Google Scholar]
- Savage JA, Chiune I. 2021. Coordination of spring vascular and organ phenology in deciduous angiosperms growing in seasonally cold climates. New Phytologist 230:1700–1715. [DOI] [PubMed] [Google Scholar]
- Savage JA, Zwieniecki MA, Holbrook NM. 2013. Phloem transport velocity varies over time and among vascular bundles during early cucumber seedling development. Plant Physiology 163:1409–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevanto S. 2014. Phloem transport and drought. Journal of Experimental Botany 65:1751–1759. [DOI] [PubMed] [Google Scholar]
- Sevanto S, McDowell NG, Dickman LT, Pangle R, Pockman WT. 2013. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant, Cell & Environment 37:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield RC, Hacke UG, Laur J. 2017. Are phloem sieve tubes leaky conduits supported by numerous aquaporins? American Journal of Botany 104:719–732. [DOI] [PubMed] [Google Scholar]
- Stanfield RC, Schulte PJ, Randolph KE, Hacke UG. 2019. Computational models evaluating the impact of sieve plates and radial water exchange on phloem pressure gradients. Plant, Cell & Environment 42:466–479. [DOI] [PubMed] [Google Scholar]
- Stushnoff C, Seufferheld M, Creegan T. 1998. Oligosaccharides as endogenous cryoprotectants in woody plants. In: Li PH, Chen THH, eds. Plant cold hardiness in woody plants, Boston, MA, USA 301–309. [Google Scholar]
- Telis VRN, Telis-Romero J, Mazzotti HB, Gabas AL. 2007. Viscosity of aqueous carbohydrate solutions at different temperatures and concentrations. International Journal of Food Properties 10:185–195. [Google Scholar]
- Thompson GA, Schulz A. 1999. Macromolecular trafficking in the phloem. Trends in Plant Science 4:354–360. [DOI] [PubMed] [Google Scholar]
- Thompson MV, Wolniak SM. 2008. A plasma membrane-anchored fluorescent protein fusion illuminates sieve element plasma membranes in Arabidopsis and tobacco. Plant Physiology 146:1599–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torode TA, O’Neill R, Marcus SE, Cornuault V, Pose S, Lauder RP, Kračun SK, Rydahl MG, Andersen MCF, Willats WGT, Braybrook SA, Townsend BJ, Clausen MH, Knox JP. 2018. Branched pectic galactan in phloem-sieve-element cell walls: implications for cell mechanics. Plant Physiology 176:1547–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker CM, Evert RF. 1969. Seasonal development of the secondary phloem in Acer negundo. American Journal of Botany 56:275–284. [Google Scholar]
- van Bel AJE, Helariutta Y, Thompson GA, et al.. 2013. Phloem: the integrative avenue for resource distribution, signaling, and defense. Frontiers in Plant Science 4:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Ende W. 2014. Sugars take a central position in plant growth, development and, stress responses. A focus on apical dominance. Plant Physiology 5:1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherley PE, Watson BT. 1969. Some low-temperature effects on sieve tube translocation in Salix viminalis. Annals of Botany 33:845–853. [Google Scholar]
- Wiemken V, Ineichen K. 1993. Effect of temperature and photoperiod on the raffinose content of spruce roots. Planta 190:387–392. [Google Scholar]
- Windt CW, Vergeldt FJ, de Jager PA, van As H. 2006. MRI of long-distance water transport: a comparison of the phloem and xylem flow characteristics and dynamics in poplar, castor bean, tomato and tobacco. Plant, Cell & Environment 29:1715–1729. [DOI] [PubMed] [Google Scholar]
- Wright JP, Fisher DB. 1980. Direct measurement of sieve tube turgor pressure using severed aphid stylets. Plant Physiology 65:1133–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang NS, Russell D. 1990. Maize sucrose synthase-1 promoter directs phloem cell-specific expression of Gus gene in transgenic tobacco plants. Proceedings of the National Academy of Sciences of the United States of America 87:4144–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamski E, Zimmermann MH. 1979. Sieve tube longevity in white ash Fraxinus americana studied with a new histochemical test for the identification of sugar. Canadian Journal of Botany 57:650–656. [Google Scholar]
- Zwieniecki MA, Tixier A, Sperling O. 2015. Temperature-assisted redistribution of carbohydrates in trees. American Journal of Botany 102:1216–1218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The annotated R code and related data used to make the phloem model can be accessed from DRYAD (doi:10.5061/dryad.rbnzs7hbg).