Abstract
Background
Haemolytic uraemic syndrome (HUS) is a common cause of acquired kidney failure in children and rarely in adults. The most important risk factor for development of HUS is a gastrointestinal infection by Shiga toxin‐producing Escherichia coli (STEC). This review addressed the interventions aimed at secondary prevention of HUS in patients with diarrhoea who were infected with a bacteria that increase the risk of HUS.
Objectives
Our objective was to evaluate evidence regarding secondary preventative strategies for HUS associated with STEC infections. In doing so, we sought to assess the effectiveness and safety of interventions as well as their potential to impact the morbidity and death associated with this condition.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 12 November 2020 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
Studies were considered based on the methods, participants, and research goals. Only randomised controlled trials were considered eligible for inclusion. The participants of the studies were paediatric and adult patients with diarrhoeal illnesses due to STEC. The primary outcome of interest was incidence of HUS.
Data collection and analysis
We used standard methodological procedures as recommended by Cochrane. Summary estimates of effect were obtained using a random‐effects model, and results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes. Confidence in the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results
We identified four studies (536 participants) for inclusion that investigated four different interventions including antibiotics (trimethoprim‐sulfamethoxazole), anti‐Shiga toxin antibody‐containing bovine colostrum, Shiga toxin binding agent (Synsorb Pk: a silicon dioxide‐based agent), and a monoclonal antibody against Shiga toxin (urtoxazumab). The overall risk of bias was unclear for selection, performance and detection bias and low for attrition, reporting and other sources of bias.
It was uncertain if trimethoprim‐sulfamethoxazole reduced the incidence of HUS compared to no treatment (47 participants: RR 0.57, 95% CI 0.11‐2.81, very low certainty evidence). Adverse events relative to this review, need for acute dialysis, neurological complication and death were not reported.
There were no incidences of HUS in either the bovine colostrum group or the placebo group. It was uncertain if bovine colostrum caused more adverse events (27 participants: RR 0.92, 95% CI 0.42 to 2.03; very low certainty evidence). The need for acute dialysis, neurological complications or death were not reported.
It is uncertain whether Synsorb Pk reduces the incidence of HUS compared to placebo (353 participants: RR 0.93, 95% CI 0.39 to 2.22; very low certainty evidence). Adverse events relevant to this review, need for acute dialysis, neurological complications or death were not reported.
One study compared two doses of urtoxazumab (3.0 mg/kg and 1.0 mg/kg) to placebo. It is uncertain if either 3.0 mg/kg urtoxazumab (71 participants: RR 0.34, 95% CI 0.01 to 8.14) or 1.0 mg/kg urtoxazumab (74 participants: RR 0.95, 95% CI 0.79 to 1.13) reduced the incidence of HUS compared to placebo (very low certainty evidence). Low certainty evidence showed there may be little or no difference in the number of treatment‐emergent adverse events with either 3.0 mg/kg urtoxazumab (71 participants: RR 1.00, 95% CI 0.84 to 1.18) or 1.0 mg/kg urtoxazumab (74 participants: RR 0.95, 95% CI 0.79 to 1.13) compared to placebo. There were 25 serious adverse events reported in 18 patients: 10 in the placebo group, and 9 and 6 serious adverse events in the 1.0 mg/kg and 3.0 mg/kg urtoxazumab groups, respectively. It is unclear how many patients experienced these adverse events in each group, and how many patients experienced more than one event. It is uncertain if either dose of urtoxazumab increased the risk of neurological complications or death (very low certainty evidence). Need for acute dialysis was not reported.
Authors' conclusions
The included studies assessed antibiotics, bovine milk, and Shiga toxin inhibitor (Synsorb Pk) and monoclonal antibodies (Urtoxazumab) against Shiga toxin for secondary prevention of HUS in patients with diarrhoea due to STEC. However, no firm conclusions about the efficacy of these interventions can be drawn given the small number of included studies and the small sample sizes of those included studies. Additional studies, including larger multicentre studies, are needed to assess the efficacy of interventions to prevent development of HUS in patients with diarrhoea due to STEC infection.
Plain language summary
Interventions for prevention of haemolytic uraemic syndrome in patients
What is the issue?
Haemolytic uraemic syndrome (HUS) is a serious illness that primarily affects children and can have severe side effects such as anaemia (low red blood cell counts), kidney damage, brain damage, and death in some cases. HUS most commonly occurs as a complication of diarrhoeal illness caused by a particular form of Escherichia coli (E. coli) bacteria called Shiga toxin‐producing E. coli (STEC). Despite the severity of this illness, there are currently no standard practices for treating these patients.
What did we do?
We summarized the available evidence that was collected to date on methods for preventing HUS in patients diagnosed with STEC‐associated diarrhoea (loose bowel movements). We searched the literature for past studies looking at different treatments aimed at preventing HUS in children with diarrhoeal illness and summarized the findings in our review,
What did we find? Four studies randomising 536 patients were included. These studies looked at four different preventative treatments including antibiotic therapy, anti‐Shiga toxin antibody‐containing bovine colostrum, Shiga toxin binding agent (Synsorb Pk: a silicon dioxide‐based agent) and a monoclonal antibody against Shiga toxin (urtoxazumab). The included studies had small number of participants and the results did not favour any one intervention to reduce the progression of the disease to HUS in patients who were infected with STEC.
Conclusions
No conclusion on the best method for preventing HUS in patients with STEC‐associated diarrhoea can be drawn from this data; more studies with a larger group of patients is required before any recommendation can be made.
Summary of findings
Background
Description of the condition
Haemolytic uraemic syndrome (HUS) is a serious condition caused by abnormal destruction of red blood cells and kidney damage and diagnosed clinically as a triad of microangiopathic haemolytic anaemia, thrombocytopenia and acute kidney injury (Fakhouri 2017; Mele 2014). HUS most commonly occurs secondary to infections with about 90% cases developing after diarrhoeal disease due to Shiga toxin‐producing Escherichia coli (STEC) (Jokiranta 2017). Shigella dysenteriae is another common cause of HUS. Children are most commonly affected (Mody 2015; Talarico 2016), however cases of adults with HUS have been reported (Gould 2011; Mele 2014). Available evidence suggest endothelial cell damage as a primary event in the pathogenesis of HUS, mediated by Shiga‐toxin in case of STEC infection and complement activation in atypical and secondary causes of HUS (Corrigan 2001; Fakhouri 2017). Additionally, Shiga toxin‐producing organisms infect the gastrointestinal tract, and induce diarrhoea that may progress to haemorrhagic colitis (Melton‐Celsa 2014).
This review focuses on HUS associated with diarrhoeal disease due to STEC. The annual incidence of STEC HUS in the U.S. and Europe is estimated to be between 1.9 and 2.9 cases per 100,000 children, three to five years of age (Majowicz 2014; Ylinen 2020). The incidence in Latin American is estimated to be 10 times higher than other continents with an incidence between 10 and 17 cases per 100,000 children less than five years of age (Rivas 2014). Most infections are due to STECs that belong to serogroup O157, although other serotypes are also implicated in HUS. Incubation periods last anywhere from one to 12 days and symptoms can include nausea, vomiting, cramping, abdominal pain, and watery diarrhoea that then turns bloody within two to three days (Bell 1994; Keir 2015; Riley 1983). Progression to HUS typically occurs 7 to 10 days after the onset of symptoms. It is estimated that 10% to 15% of cases of diarrhoeal illness due to STEC infection will progress to HUS. Of these patients affected by HUS, 30% will go on to develop serious complications such as end‐stage kidney disease and neurological sequelae and death (Garg 2003; Keir 2015; Rowe 1998; Siegler 1994).
Description of the intervention
Prevention of diarrhoea‐associated HUS can be in the form of primary or secondary prevention. Primary prevention relies on identifying and modifying predisposing risk factors for STEC infection, such as food safety, handwashing, and waste disposal. Secondary prevention relies on taking actions to reduce the risk of developing HUS once the predisposing disease, in this case, infectious diarrhoea, has been diagnosed. Some examples of intervention for secondary prevention of HUS include aggressive hydration, antibiotics, monoclonal antibodies against Shiga toxin and Shiga toxin binding proteins (i.e. Synsorb Pk) (Grisaru 2017; Thomas 2013).
How the intervention might work
Use of antibiotics to treat STEC infection to prevent HUS is debatable (Fakhouri 2017). The Centers for Disease Control and Prevention and American Gastroenterology Association both recommend against the use of antibiotics to treat STEC to prevent HUS, due to concerns that antibiotic can potentially increase risk of HUS after STEC infection (CDC 2018a; Riddle 2016). These recommendations are mostly based on findings from observational studies and one randomised controlled trial (RCT) that did not show any increased or protective effect of antibiotics with relation to HUS (Freedman 2016;Proulx 1992; Thomas 2013; Wong 2000). There is some evidence in favour of the use of antibiotics. During the 2011 outbreak of HUS in Germany, an observational study assessed the use of azithromycin in children with STEC infections found that treatment with azithromycin was associated with a lower frequency of long‐term STEC carriage (Nitschke 2012). Monoclonal antibodies against Shiga toxin are another potential novel approach for clinical detection of the toxin (Skinner 2016). Anti‐Shiga toxin monoclonal antibodies have been investigated as potential treatments in animal models and in healthy volunteers, yet the evidence for their efficacy is still inconclusive (Bitzan 2009; Dowling 2005; Lopez 2010a; Mejias 2016; Melton‐Celsa 2014). Monoclonal antibodies against Shiga toxins 1 and 2 may be used as a preventative strategy in preventing the onset of HUS (Melton‐Celsa 2014; Thomas 2013). Synsorb Pk is a silicon dioxide‐based compound containing the trisaccharide part of Gb3 that serves as binding protein to prevent the absorption of Shiga toxin from the gastrointestinal system (Armstrong 1991; Trachtman 2003). In addition to Synsorb Pk, several other Shiga toxin receptor analogues have been developed including STARFISH, Daisy, SUPER TWIG, Gb3 polymers, Ac‐PPPtet, and probiotic with a Shiga toxin binder (Melton‐Celsa 2014). These developments can potentially prevent HUS by neutralizing the action of Shiga‐toxins (Melton‐Celsa 2014).
Why it is important to do this review
Treatment strategies for HUS have been discussed in a prior Cochrane review (Michael 2009); however, no Cochrane review has focused on secondary prevention of diarrhoea‐associated HUS. Other non‐Cochrane reviews have focused on selective interventions (Freedman 2016; Grisaru 2017; Thomas 2013) and need an update. We therefore aim to synthesize up to date evidence regarding secondary preventative strategies for diarrhoea‐associated HUS.
Objectives
To assess the effectiveness and safety of intervention for secondary prevention of morbidity and death from diarrhoea‐associated HUS in children and adults, compared to placebo or no secondary intervention use.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) examining secondary prevention strategies of diarrhoea‐associated HUS were included. We included randomised studies if the randomisation was conducted at the individual or the cluster level. We also considered cross‐over RCTs eligible for inclusion. We excluded all observational studies such as cohort, case‐control, case series, and case reports.
Types of participants
We included evidence from studies that focused on paediatric and adult patients with diarrhoea who are at risk of developing HUS, such as those infected with STEC including both O157 and non‐O157 serogroups. We included studies with participants at risk of developing diarrhoea‐associated HUS regardless of a particular setting, educational status, gender, race, geographic location, or socioeconomic status of the participants.
We excluded studies with patients that are at risk of non‐diarrhoea‐associated HUS such as those associated with Streptococcus pneumoniae infections, disorders of complement regulation, ADAMTS13 deficiency, cancer, organ transplant and pregnancy. This is because pathophysiology of non‐diarrhoea‐associated HUS is thought to be different than diarrhoea‐associated HUS and it is hard to predict occurrence of HUS in non‐diarrhoea‐associated cases (Fakhouri 2017).
Types of interventions
We included evidence from studies that evaluated the following interventions used to prevent diarrhoea‐associated HUS:
Antibiotics
Anti‐Shiga toxin monoclonal antibodies
Shiga toxin binding protein (i.e. Synsorb Pk)
Aggressive hydration.
We included studies regardless of the type of antibiotics used, mode of delivery of intervention (oral versus intravascular/intramuscular), or frequency of intervention.
Eligible comparison groups included placebo and standard of care conditions. We included studies with multiple treatment arms such as factorial design trials, as long as the study reports contrasted in a way whereby the only difference between two groups was the intervention.
We excluded studies that evaluated interventions delivered after the diagnosis of HUS, given that these interventions were outside the scope of this review. We also excluded studies in which the intervention was provided as a primary form of prevention for diarrhoea itself, as these interventions were also outside the scope of the review.
Types of outcome measures
Primary outcomes
-
Incidence of HUS in patients with diarrhoea
HUS was defined as a triad of microangiopathic haemolytic anaemia, thrombocytopenia, and acute kidney injury that happened within three weeks of the diarrhoeal episode. The laboratory evidence included anaemia with microangiopathic changes such as presence of schistocytes, burr cells, or helmet cells on peripheral blood smear and kidney injury evidenced by haematuria, proteinuria or elevated creatinine or blood urea nitrogen (CDC 1996). We also included cases of thrombotic thrombocytopenic purpura (TTP) after a diarrhoeal episode. The definition of TTP includes the triad of HUS plus central nervous system involvement and fever. If the definition of the primary outcomes was not provided explicitly in the study, we contacted the authors for further information. If no information was available on how the HUS was defined, we still included the data from that study but planned a sensitivity analysis to assess if the inclusion/exclusion of the study altered our findings and conclusions.
Secondary outcomes
Oligoanuric kidney failure defined as urine output < 0.5 mL/kg/hour
Need for acute kidney replacement therapy
Need for prolonged dialysis for one to three months post HUS acute phase, or develop dialysis‐dependent kidney failure needing kidney transplant
Death (any cause)
Adverse events or any serious acute phase complications such as bowel perforation or obstruction, peritonitis, sepsis, cardiac injury, or pancreatitis
Need for blood transfusion and platelet transfusions
Incidence of neurological complications (e.g. stroke, seizures).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 12 November 2020 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website under CKT Register of Studies.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete RCTs to investigators known to be involved in previous studies.
Grey literature from the Conference Proceeding Citation Index database (hosted on Web of Science).
Manually searched reference lists of potentially included studies and previous reviews on topic.
Data collection and analysis
Selection of studies
The search strategy was used to identify titles and abstracts of studies that were potentially relevant to the review. The titles and abstracts identified in the search were screened independently by two authors who selected potentially eligible titles to progress to the next stage of full text review. Any conflict between the two authors was resolved by discussion or contacting a senior author. Two authors independently assessed retrieved full text articles and made a determination about the study’s eligibility for inclusion in the review. If there was no consensus on inclusion/exclusion of a study between two authors, a third author was consulted for a final decision.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were to be translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used for the purposes of effect size estimation. Where relevant outcomes were only published in earlier versions, these earlier versions were used for the purposes of effect size estimation. Any discrepancy between published versions was highlighted in the text of the review.
For eligible studies, at least two authors extracted the data and any discrepancies in extracted data were resolved based on discussion with a third author.
A codebook was used to define and describe all the variables abstracted from included studies. We abstracted the information on the following variables: study type, study site, baseline mortality and morbidity, inclusion/exclusion criteria, details of the intervention (e.g. type, route, frequency) risk of bias, attrition, coverage of intervention, characteristics of participants (e.g. age, race, gender, socioeconomic status), place of living (home versus facility), and outcome data.
When information regarding any of the above was unclear, we attempted to contact the authors of the original reports to provide further details. If authors had not performed an analysis for a particular variable, we asked authors to perform that analysis or provide the original dataset so that we could perform the analysis for that outcome.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the Cochrane risk of bias assessment tool for randomised trials (Higgins 2011) (see Appendix 2). All risk of bias items were coded as high, low, or unclear risk of bias, with additional textual support for each item.
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third author.
Measures of treatment effect
For dichotomous outcomes (incidence of HUS, need for acute dialysis, dialysis‐dependent kidney failure) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment, the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales were used.
Unit of analysis issues
We planned to consider the data from individual and cluster RCTs in the same meta‐analysis. However, none of the included studies used a cluster randomised trial design. We had planned to use cluster‐adjusted values when reported by authors. If authors did not appropriately adjust for their cluster designs, we would have conducted our own adjustments by inflating the standard error (SE) of the effect size estimate by multiplying it by the square root of design effect as described by the Cochrane handbook (Higgins 2011). If the design effect could not have been estimated for a primary study (e.g. if the cluster sizes, intra‐class correlation coefficients, or both were not reported), a design effect from similar study would have been considered.
For studies using cross‐over designs, we planned to only include data from the first phase of the study prior to the first cross‐over. However, no eligible studies using a cross‐over design were ultimately included.
Dealing with missing data
Attrition is an important factor in RCTs and differential loss to follow‐up may lead to biased results. We therefore extracted information on attrition and reported missing data, including dropouts and reasons for dropout as reported by authors. We contacted authors if data were missing, there were no reasons provided for missing data, or both. When authors reported data for completers as well as controlling for dropout (e.g. imputed using regression methods), we extracted the latter. We also contacted authors to obtain data if a study did not report data for a primary or secondary outcome of this review.
Data were included based on an intention‐to‐treat analysis, i.e., all participants randomised to each group in the analyses were analysed based on initial allocation, regardless of whether or not they received the allocated intervention.
Assessment of heterogeneity
We first assessed heterogeneity by visual inspection of the forest plot. We quantified statistical heterogeneity using the I² statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). A guide to the interpretation of I² values was as follows.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I² depends on the magnitude and direction of treatment effects, the total amount of observed heterogeneity, and the strength of evidence for heterogeneity (e.g. P‐value from the Chi² test, value of τ, confidence interval for I²) (Higgins 2011).
Clinical heterogeneity was described in terms of the different types, durations, and frequencies of the included interventions. Methodological heterogeneity was described in terms of the prevalence of individual versus cluster‐randomised trials. No meta‐analysis was performed in this review, so inferences were made about the statistical heterogeneity.
Assessment of reporting biases
If 10 or more studies were included in the meta‐analysis, we planned to investigate reporting bias such as publication bias using funnel plots. We intended to assess funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we then planned to perform additional analyses to investigate it by using an Egger regression test to quantify the magnitude of asymmetry, and a trim and fill analysis to assess the potential effect of funnel plot asymmetry on the estimated mean effect size. There were not enough included studies to conduct these assessments of reporting bias.
Data synthesis
We planned to combine data from individual trials for meta‐analysis when the interventions, patient groups, and outcomes were sufficiently similar (as determined by consensus). We planned to synthesize effect sizes in the meta‐analysis using a random effects model, using the restricted maximum likelihood estimator for the random‐effects variance component. Because none of the included studies were conceptually similar in terms of interventions, patient groups, and outcomes, no meta‐analyses were performed.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses. Again, however, we were unable to conduct any subgroup analyses given that no meta‐analyses were performed.
STEC versus other causes of diarrhoea‐associated HUS
Children (< 18 years) versus adults (≥ 18 years)
Outbreak settings versus non‐outbreak settings
Hospital setting versus community‐based studies versus mixed/undefined settings
Low and middle‐income countries versus high‐income countries.
Sensitivity analysis
We planned the following sensitivity analyses to explore the influence of the following factors on the estimated mean effect sizes.
Repeating the analysis excluding unpublished studies
Repeating the analysis excluding studies with high risk of bias on sequence generation
Repeating the analysis excluding any small sample size studies.
We also planned to conduct sensitivity analyses to investigate the effect of missing data.
5% to 10% missing data
10% to 20% missing data
20% or more missing data.
Again, however, no sensitivity analyses were conducted given that no meta‐analyses were performed.
Summary of findings and assessment of the certainty of the evidence
We present the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schunemann 2011b). We presented the following outcomes in the 'Summary of findings' tables.
Incidence of HUS in patients with diarrhoea
Adverse events
Need for acute dialysis
Incidence of neurological complications
Death (any cause)
Results
Description of studies
Results of the search
We searched Cochrane Kidney and Transplant's Specialised Register and identified 23 reports. Figure 1 provides the PRISMA flow diagram for selection of studies. Four studies were included (Huppertz 1999; Lopez 2010; Proulx 1992; Rowe 1995) and four studies were excluded (Caletti 2011; HUS‐SYNSORB Pk 1998; NCT02205541; Pape 2009). There are three ongoing studies (NCT03275792; NCT04132375; SHIGATEC 2011) and one study is awaiting classification (McLaine 1995). These four studies will be evaluated in a future update of this review. See Figure 1.
1.
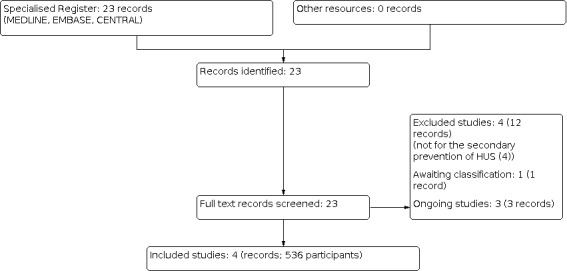
Study flow diagram.
Included studies
See Characteristics of included studies.
Four studies (Huppertz 1999; Proulx 1992; Rowe 1995, Lopez 2010) randomising 536 patients were included. These four studies assessed four different interventions. These studies were then categorized according to the intervention assessed for the secondary prevention of HUS:
Antibiotic therapy (trimethoprim/sulfamethoxazole)
Anti‐Shiga toxin antibody containing bovine colostrum
Oral Shiga toxin binding agent (Synsorb Pk)
Humanized monoclonal antibody (urtoxazumab).
Antibiotic therapy (trimethoprim/sulfamethoxazole)
Proulx 1992 was a parallel RCT that assessed the use of antibiotic therapy for E. coli O157:H7 enteritis. The study included 47 children with proven E. coli O157:H7 enteritis. Participants were randomised to one of the two treatment groups. The intervention group received 4/20 mg/kg of oral trimethoprim‐sulfamethoxazole twice daily for five days. The control group received no treatment. The primary outcomes assessed were incidence of HUS, duration of diarrhoeal symptoms, and faecal excretion of pathogen. No funding source was specified by the authors.
Anti‐Shiga toxin antibody containing bovine colostrum
Huppertz 1999 was a parallel RCT that assessed the use of anti‐Shiga toxin containing antibodies bovine colostrum for treatment of diarrhoea‐associated with diarrheogenic Shiga toxin‐producing E. coli expressing intimin and haemolysin. The study included 30 children with diarrhoea whose stool cultures yielded E. coli containing gene eae which encodes intimin, in addition to Stx1, Stx2, or both or enterohaemorrhagic E. coli (EHEC)‐haemolysin. The included children were randomised to one of the two treatment groups. The intervention group received 7 grams of bovine colostrum preparation given orally before meals three times/day for 14 days. The comparison group received placebo preparation that was similar in chemical composition and identical in appearance to the bovine colostrum treatment. The primary outcomes assessed were bovine colostrum oral tolerance, diarrhoeal stool frequency reduction, and faecal excretion of E. coli and occurrence of HUS. The study also included two patients who already had HUS. No funding source was specified by the authors.
Oral Shiga toxin binding agent (Synsorb Pk)
Rowe 1995 was a parallel RCT that assessed the use of Synsorb Pk for the prevention of HUS in children with STEC. Synsorb Pk is silicon‐based oral Shiga toxin‐binding agent that competitively inhibits the absorption of Shiga toxin from the gut. The study included 364 children diagnosed with E. coli O157, other verotoxin‐producing E. coli (VTEC) infection, close contact with individuals diagnosed with HUS or VTEC infection, and symptoms consistent with VTEC infection. Participants were randomised to one of the two treatment groups. The intervention group received 500 mg/kg of Synsorb Pk mixed in baby food given orally divided into 2 doses/day for 7 days. The placebo group received equal volumes of corn meal. The primary outcome assessed was development of HUS at day 7 of treatment. No funding source was specified by the authors.
Humanised monoclonal antibody (urtoxazumab)
Lopez 2010 evaluated the pharmacokinetics and safety of urtoxazumab in adults and children. Urtoxazumab is a humanised monoclonal IgG subclass IgG1 against the B subunit of against Shiga toxin (Stx) 2. The first phase of the study included otherwise healthy adults aged 19 to 65 years. The adults were given a single IV 100 mL dose of urtoxazumab at four different dosage levels (0.1, 0.3, 1.0, 3.0 mg/kg/body weight). The control group received placebo. Safety, tolerability and pharmacokinetic were measured after the administration of the medication until day 7. The paediatric part of the study was a parallel RCT and included 109 children, aged from one to 15 years who had diarrhoea for less than 3 days and tested positive for STEC infection. The study excluded children with chronic diseases and the children who had already developed the HUS. The paediatric study was conducted in two parts. In the first part of the study, children were divided into two cohorts. In the first cohort, participants were randomised to receive IV infusion of 1.0 mg/kg urtoxazumab and the control group received placebo. The second cohort received IV infusion of 3.0 mg/kg urtoxazumab and the control group received placebo. Safety and pharmacokinetic outcomes were measured. Once the safety was established, further 85 children were recruited into cohorts of 1.0 mg/kg and 3.0 mg/kg of urtoxazumab. The primary outcomes assessed were adverse events and efficacy outcomes. The authors identified the Program of Fundamental Studies in Health Science of the Organization for Pharmaceutical Safety and Research of Japan as a funding source.
Excluded studies
See Characteristics of excluded studies
None of the four excluded studies gave the interventions for the secondary prevention of HUS. Two gave the intervention during episodes of HUS (HUS‐SYNSORB Pk 1998; NCT02205541); one gave the intervention after HUS developed (Caletti 2011); and the intervention was given as a treatment for HUS (Pape 2009).
Studies awaiting classification
McLaine 1995 was only available as an abstract and no full text publication has been identified. This study compared Synsorb Pk to placebo, however no details on population, dose or outcomes were available.
Ongoing studies
Three ongoing studies were identified and will be assessed in a future update of this review (NCT03275792; NCT04132375; SHIGATEC 2011). These studies plan to compare the following.
Infusion of 40 mL/kg of 0.9% normal saline IV over 60 minutes, 0.9% normal saline with 5% dextrose at 150% of standard maintenance volume compared to standard emergency department care (NCT03275792)
IV dose of 4 mg/kg INM004 (anti‐Stx hyperimmune equine immunoglobulin F[ab']2 fragments) and a 2nd IV dose of 4 mg/kg of INM004 compared to placebo (NCT04132375)
Shigamabs infused over one hour as a single infusion, Shigamabs. Two doses (1 and 3 mg/kg) are being tested in 2 sequential cohorts of children. Shigamabs is infused over one hour as a single infusion (SHIGATEC 2011)
Risk of bias in included studies
The overall risk of bias in regard to allocation and selection across all four studies was generally unclear due to lack of discussion regarding allocation methods. The overall risk of bias in regard to blinding, performance and detection was generally high and unclear. We determined that the overall risk of bias in terms of incomplete outcome data and attrition was low across all four studies. Finally, we found that the overall risk of bias in regard to selective reporting and other potential biases was low for all four studies. Figure 2 show the risk of bias in the included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Proulx 1992 was judged to be at low risk of bias for random sequence generation, while Huppertz 1999, Lopez 2010 and Rowe 1995 were judged to have unclear risk of bias.
Allocation concealment
The risk of allocation bias was judged to be unclear for all four studies due to a lack of discussion of efforts to conceal allocation.
Blinding
Performance bias
Huppertz 1999 was judged to be a low risk of performance bias, Proulx 1992 and Rowe 1995 were judged to be at high risk of performance bias, and Lopez 2010 was determined to be at unclear risk of performance bias.
Detection bias
Huppertz 1999; Lopez 2010; Rowe 1995 were judged to be at unclear risk of detection bias while Proulx 1992 was judged to be at high risk of detection bias/
Incomplete outcome data
All four studies were judged to be at low risk of attrition bias.
Selective reporting
All four studies were judged to be a low risk of reporting bias.
Other potential sources of bias
There were no other potential sources of bias in any of the four studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings 1. Antibiotics versus no treatment for secondary prevention of HUS.
| Trimethoprim‐sulfamethoxazole versus no treatment for secondary prevention of HUS in patients with diarrhoea due to Shiga toxin‐producing E. coli | |||||
| Patient or population: patients with diarrhoea due to Shiga toxin‐producing E. coli Intervention: trimethoprim‐sulfamethoxazole Comparison: no treatment | |||||
| Outcomes | No. of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with no treatment | Risk with antibiotics | ||||
| Incidence of HUS Follow up: 10 days |
47 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 0.57 (0.11 to 2.81) | 160 per 1,000 | 69 fewer per 1,000 (142 fewer to 290 more) |
| Adverse events | Not reported | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| Need for acute dialysis | Not reported | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| Neurological complications | Not reported | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| Death (any cause) | Not reported | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). HUS: haemolytic uraemic syndrome; CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded once for risk of bias: The risk of bias was high for blinding of participants and personnel
2 Downgraded twice for imprecision: very wide confidence intervals crossing the line of no effect and few events
Summary of findings 2. Bovine colostrum versus placebo for secondary prevention of HUS.
| Bovine colostrum versus placebo for secondary prevention of HUS in patients with diarrhoea due to Shiga toxin‐producing E. coli | |||||
| Patient or population: patients with diarrhoea due to Shiga toxin‐producing E. coli Intervention: bovine colostrum Comparison: placebo | |||||
| Outcomes | No. of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placebo | Risk with bovine colostrum | ||||
| Incidence of HUS Follow up: 21 days |
27 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 | not estimable | No events | No events |
| Adverse events Follow up: 21 days |
27 (1) | ⊕⊝⊝⊝ VERY LOW 1 3 | RR 0.92 (0.42 to 2.03) | 500 per 1,000 | 40 fewer per 1,000 (290 fewer to 515 more) |
| Need for acute dialysis | Not reported | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| Neurological complications | Not reported | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| Death (any cause) | Not reported | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). HUS: haemolytic uraemic syndrome; CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded once for risk of bias: Selection and detection bias were unclear
2 Downgraded twice for imprecision: No events in the intervention and control group
3 Downgraded twice for imprecision: very wide confidence intervals crossing the line of no effect and few events
Summary of findings 3. Synsorb Pk versus placebo for secondary prevention of HUS.
| Synsorb Pk versus placebo for secondary prevention of HUS in patients with diarrhoea due to Shiga toxin‐producing E. coli | |||||
| Patient or population: patients with diarrhoea due to Shiga toxin‐producing E. coli Intervention: Synsorb Pk Comparison: placebo | |||||
| Outcomes | No. of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placebo | Risk with Synsorb Pk | ||||
| Incidence of HUS Follow up: 7 days |
353 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 0.93 (0.39 to 2.22) | 56 per 1,000 | 4 fewer per 1,000 (34 fewer to 68 more) |
| Adverse events | Not reported | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| Need for acute dialysis | Not reported | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| Neurological complications | Not reported | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| Death (any cause) | Not reported | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). HUS: haemolytic uraemic syndrome; CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded once for risk of bias: high risk of performance bias and unclear risk of selection and detection bias
2 Downgraded twice for imprecision: The number of events was small in both groups and the CI of the summary estimate was large
Summary of findings 4. Urtoxazumab (3.0 mg/kg) versus placebo for secondary prevention of HUS.
| Urtoxazumab (3.0 mg/kg) versus placebo for secondary prevention of HUS | |||||
| Patient or population: paediatric patients with diarrhoea due to Shiga toxin‐producing E. coli Intervention: urtoxazumab (3.0 mg/kg) Comparison: placebo | |||||
| Outcomes | No. of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placebo | Risk with 3.0 mg/kg urtoxazumab | ||||
| Incidence of HUS Follow up: 7 days |
71 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 0.34 (0.01 to 8.14) | 28 per 1,000 | 18 fewer per 1,000 (27 fewer to 198 more) |
| Adverse events ‐ treatment emergent Follow up: to 56 days |
71 (1) | ⊕⊕⊝⊝ LOW 1 3 | RR 1.00 (0.84 to 1.18) | 889 per 1,000 | 0 fewer per 1,000 (142 fewer to 160 more) |
| Adverse events ‐ serious Follow‐up: to 56 days |
71 (1) | ⊕⊕⊝⊝ LOW 1 4 | risk ratio could not be calculated4 | ‐ | ‐ |
| Need for acute dialysis | Not reported | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| Neurological complications Follow up: to 56 days |
71 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 2.06 (0.20 to 21.68) | 28 per 1,000 | 29 more per 1,000 (22 fewer to 574 more) |
| Death (any cause) Follow up: to 56 days |
71 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 0.34 (0.01 to 8.14) | 1/36** | No events |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** Event rate derived from the raw data. A 'per thousand' rate is non‐informative in view of the scarcity of evidence and zero events in the treatment group HUS: haemolytic uraemic syndrome; CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded once for risk of bias: The risk of selection, performance and detection bias were all unclear
2 Downgraded twice for imprecision: The number of events were small in the intervention and control group and the summary estimate had wide confidence intervals
3 Downgraded once for imprecision because the CI included 1
4 Downgraded once for imprecision: The study reported 25 serious adverse events in 18 patients: 10 in the placebo group, and 9 and 6 serious adverse events in the 1.0 mg/kg and 3.0 mg/kg urtoxazumab groups, respectively. It is unclear how many patients experienced these adverse events in each group, and how many patients experienced more than one event. So risk ratio could not be calculated in this scenario
Summary of findings 5. Urtoxazumab (1.0 mg/kg) versus placebo for secondary prevention of HUS.
| Urtoxazumab (1.0 mg/kg) versus placebo for secondary prevention of HUS | |||||
| Patient or population: paediatric patients with diarrhoea due to Shiga toxin‐producing E. coli Intervention: urtoxazumab 1.0 mg/kg Comparison: placebo | |||||
| Outcomes | No. of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placebo | Risk with 1.0 mg/kg urtoxazumab | ||||
| Incidence of HUS Follow up: 56 days |
74 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 0.95 (0.06 to 14.59) | 28 per 1,000 | 1 fewer per 1,000 (26 fewer to 378 more) |
| Adverse events ‐ treatment emergent Follow up: 56 days |
74 (1) | ⊕⊕⊝⊝ LOW 1 3 | RR 0.95 (0.79 to 1.13) | 889 per 1,000 | 44 fewer per 1,000 (187 fewer to 116 more) |
| Adverse events ‐ serious Follow up: 56 days |
74 (1) | ⊕⊕⊝⊝ LOW 1 4 | ‐‐ | ‐‐ | ‐‐ |
| Need for acute dialysis | Not reported | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| Neurological complications Follow up: 56 days |
74 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 2.84 (0.31 to 26.08) | 28 per 1,000 | 51 more per 1,000 (19 fewer to 697 more) |
| Death (any cause) Follow up: 56 days |
74 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 0.95 (0.06 to 14.59) | 28 per 1,000 | 1 fewer per 1,000 (26 fewer to 378 more) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). HUS: haemolytic uraemic syndrome; CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded once for risk of bias: The risk of selection, performance and detection bias were all unclear
2 Downgraded twice for imprecision: The number of events were small in the intervention and control group and the summary estimate had wide confidence intervals
3 Downgraded once for imprecision because the CI included 1.
4 Downgraded once for imprecision: The study reported 25 serious adverse events in 18 patients: 10 in the placebo group, and 9 and 6 serious adverse events in the 1.0 mg/kg and 3.0 mg/kg urtoxazumab groups, respectively. It is unclear how many patients experienced these adverse events in each group, and how many patients experienced more than one event. So risk ratio could not be calculated in this scenario.
Trimethoprim‐sulfamethoxazole versus no treatment
Proulx 1992 compared trimethoprim‐sulfamethoxazole with no treatment and randomised a total of 47 participants. There were 2/22 (9%) patients with HUS in the trimethoprim‐sulfamethoxazole group compared to 4/25 (16%) in the control group. See Table 1.
Incidence of HUS
It is uncertain whether trimethoprim‐sulfamethoxazole reduces the incidence of HUS compared to no treatment (Analysis 1.1: RR 0.57; 95 % CI 0.11 to 2.81; very low certainty evidence) and was downgraded due to imprecision and risk of bias.
1.1. Analysis.

Comparison 1: Trimethoprim/sulfamethoxazole versus control, Outcome 1: Incidence of HUS
Other outcomes
This study did not report adverse events relevant to this review, need for acute dialysis, neurological complications or death.
The authors reported the data on effect of antibiotics on duration of symptoms compared to control. The data showed inconclusive evidence on whether trimethoprim‐sulfamethoxazole had any influence on duration of symptoms for bloody stools, diarrhoea, abdominal pain, vomiting and fever.
Bovine colostrum versus placebo
Huppertz 1999 compared bovine colostrum to placebo and randomised a total of 27 participants. See Table 2.
Incidence of HUS
There were no incidences of HUS in either the treatment or control groups. The evidence was graded very low and was downgraded due to risk of bias and imprecision.
Adverse events
It is uncertain whether bovine colostrum causes more adverse events compared to placebo (Analysis 2.2: RR 0.92, 95% CI 0.42 to 2.03; very low certainty evidence) and was downgraded based on risk of bias and imprecision. Symptoms considered adverse were determined to be poor appetite, abdominal colic, and occasional vomiting.
2.2. Analysis.

Comparison 2: Bovine colostrum versus control, Outcome 2: Adverse events
Other outcomes
This study did not report need for acute dialysis, neurological complications or death.
The authors did, however, report on the effect of bovine colostrum on median stool frequency. The median stool frequency decreased from three stools/day to one stool/day in the intervention group whereas there was no change in stool frequency (three stools/day) in the control group.
Synsorb Pk versus placebo
Rowe 1995 compared Synsorb Pk with placebo and randomised a total of 353 participants. See Table 3.
Incidence of HUS
It is uncertain whether Synsorb Pk reduces the incidence of HUS compared to placebo (Analysis 3.1: RR 0.93, 95% CI 0.39 to 2.22; very low certainty evidence) and was downgraded due to imprecision and risk of bias.
3.1. Analysis.

Comparison 3: Synsorb Pk versus control, Outcome 1: Incidence of HUS
Other outcomes
This study did not report adverse events relevant to this review, need for acute dialysis, neurological complications or death.
Urtoxazumab versus placebo
Lopez 2010 compared two doses of urtoxazumab (3.0 mg/kg and 1.0 mg/kg) to placebo and randomised 107 participants. See Table 4 and Table 5
Incidence of HUS
It is uncertain whether either 3.0 mg/kg urtoxazumab (Analysis 4.1.1 (71 participants): RR 0.34, 95% CI 0.01 to 8.14; very low certainty evidence) or 1.0 mg/kg urtoxazumab (Analysis 4.1.2 (74 participants): RR 0.95, 95% CI 0.79 to 1.13; very low certainty evidence) reduced the incidence of HUS compared to placebo. The GRADE ratings were downgraded due to imprecision and risk of bias.
4.1. Analysis.

Comparison 4: Urtoxazumab versus control, Outcome 1: Incidence of HUS
Adverse events
Treatment‐emergent adverse events (non‐serious)
Low certainty evidence showed there may be little or no difference in the number of treatment‐emergent adverse events with either 3.0 mg/kg urtoxazumab (Analysis 4.1.1 (71 participants): RR 1.00, 95% CI 0.84 to 1.18) or 1.0 mg/kg urtoxazumab (Analysis 4.1.2 (74 participants): RR 0.95, 95% CI 0.79 to 1.13) compared to placebo. The GRADE ratings for these outcomes were downgraded based on risk of bias and imprecision.
Serious adverse events
Lopez 2010 reported a total of 25 serious adverse events in 18 patients: 10 in the placebo group, and 9 and 6 serious adverse events in the 1.0 mg/kg and 3.0 mg/kg urtoxazumab groups, respectively. It is unclear how many patients experienced these adverse events in each group, and how many patients experienced more than one event. It should be noted that only four serious adverse events were considered by Lopez 2010 to be remotely related to urtoxazumab. The serious adverse events included hypokalaemia, intussusception, and HUS in the 1.0 mg/kg urtoxazumab group and HUS in the placebo group.
Neurological complications
It is uncertain whether either 3.0 mg/kg urtoxazumab (Analysis 4.3.1 (71 participants): RR 2.06, 95% CI 0.20 to 21.68; very low certainty evidence) or 1.0 mg/kg urtoxazumab (Analysis 4.3.2 (74 participants): RR 2.84, 95% CI 0.31 to 26.08; very low certainty evidence) increased the risk of neurological complications compared to placebo. The GRADE ratings for these outcomes were downgraded due to imprecision and risk of bias.
4.3. Analysis.

Comparison 4: Urtoxazumab versus control, Outcome 3: Neurological complications
Death (any cause)
It is uncertain whether either 3.0 mg/kg urtoxazumab (Analysis 4.4.1 (71 participants): RR 0.34, 95% CI 0.01 to 8.14; very low certainty evidence) or 1.0 mg/kg urtoxazumab (Analysis 4.4.2 (74 participants): RR 0.95, 95% CI 0.06 to 14.59; very low certainty evidence) compared to placebo. The GRADE ratings for these outcomes were downgraded for risk of bias and imprecision.
4.4. Analysis.

Comparison 4: Urtoxazumab versus control, Outcome 4: Death (any cause)
Other outcomes
Need for acute dialysis was not reported.
Discussion
Summary of main results
This systematic review assessed interventions for secondary prevention of HUS in patients with gastrointestinal infection due to STEC. We identified four studies that examined four different interventions including antibiotics, anti‐Shiga toxin antibodies containing bovine colostrum, a Shiga toxin binding agent (Synsorb Pk) and monoclonal antibodies (urtoxazumab) against Shiga toxin‐2. The included studies reported a range of outcomes. However, the sample sizes were small, and given the small number of identified studies, no firm conclusions can be drawn at this time about the efficacy of these intervention for secondary prevention of HUS in patients with STEC. We identified a number of ongoing studies that could add significant contributions to the field in the future.
Overall completeness and applicability of evidence
The included studies that addressed four different interventions all had small sample sizes. The number of events was low for most of the outcomes in the included studies, resulting in wide 95% CIs around the summary estimates. The study with the largest sample size (Rowe 1995) included 364 children and tested Synsorb Pk versus placebo for the prevention of HUS in children with verotoxin‐producing E. coli (VTEC) gastroenteritis. This study was available only in abstract form and the study has not as yet been published in a peer review journal. We tried to reach out to authors to obtain more details about the study however could not establish contact. Nonetheless, the number of events was low for incidence of HUS, hence no firm conclusions can be drawn from the summary estimate.
The second largest study (Lopez 2010) addressed pharmacokinetics of urtoxazumab in adults and its efficacy and safety in prevention of HUS and adverse events in 109 children. The number of events was low for incidence of HUS in the intervention and control group, and the 95% CI around the estimate was wide. The study reported treatment‐emergent (non‐serious) adverse event and serious adverse events. More than 85% of the patient population experienced at least one adverse event and these adverse events were present all three study groups, including the placebo group. There was no significant difference in prevalence of non‐serious adverse events between any of the study group and the placebo. The most commonly reported adverse events were related to blood and lymphatic system disorders followed by infections and infestations and gastrointestinal disorders. Authors further described that among all the adverse events reported in the study about four patients had an adverse event thought to be related to the study medication. Three of these were mild in intensity and one was moderate. The mild adverse events included fever, headache and erythema, while the moderate event was vomiting.
This study also reported 25 serious events with almost equal proportion in all the study groups including the control group (Lopez 2010). A serious adverse event is defined by FDA 2016 as an adverse event in human drug trials that lead to any untoward medical occurrence that at any dose can results in death, is life threatening, and require hospitalisations, results in persistent or significant disability, may have caused congenital anomaly and require intervention to prevent permanent impairment and damage. Lopez 2010 describe that in their study 4/25 serious adverse events were considered to be remotely related to the study drug. This included hypokalaemia, intussusception and HUS (two cases, one in urtoxazumab 1 mg/kg/dose group and one case in the placebo group). There were two deaths reported, one in the urtoxazumab 1 mg/kg/dose group and other in the placebo group. Overall, the study did not show any convincing evidence of increased incidence of treatment‐related (non‐serious) adverse events or serious adverse events in the study groups versus the control groups.
Quality of the evidence
There were varying degrees of risk of bias among the four included studies. In Huppertz 1999 the risk of selection and detection bias was unclear, but the risk of performance, attrition, and reporting bias was low. The GRADE quality of evidence from this study was considered very low due to risk of bias and small sample size. In Lopez 2010 the risk of selection, performance, and detection bias was unclear, but the risk of attrition and reporting bias was low. The GRADE quality of evidence from this study was rated very low for incidence of HUS and low for adverse events. In Proulx 1992, the risk of performance and detection bias was high, the risk of selection bias was unclear, while the risk of attrition and reporting bias was low. The GRADE quality of evidence was rated very low. Finally, in Rowe 1995, the risk of performance bias was high, the risk of selection and detection bias was unclear, and the risk of attrition and reporting bias was low. The GRADE quality of evidence was rated as very low due to concern for risk of bias and low imprecision in the summary estimate due to low number of events in the intervention and control groups.
Potential biases in the review process
We followed the standard guidelines of Cochrane Kidney and Transplant Register. We performed a comprehensive search of multiple databases. Data were extracted by two authors using standard Cochrane standard data extraction forms. The risk of bias assessment was conducted in accordance with the Cochrane Handbook. We intentionally did not conduct a meta‐analysis to quantitatively synthesize effect size given that the small number of included studies that examined different types of interventions for different patient populations and different outcome measures. We did, however, identify several ongoing studies examining secondary prevention of HUS on primary outcomes of interest; future updates to this review may permit meta‐analyses given the accrual of additional evidence from these ongoing studies.
Agreements and disagreements with other studies or reviews
These results from this review differ from those of past Cochrane Reviews. In Michael 2009, methods for treating HUS and TTP were evaluated by conducting a meta‐analysis of RCTs assessing the efficacy of different forms of treatment. The authors concluded that plasma exchange with fresh frozen plasma was the most effective treatment of these conditions. In this review, we attempted to evaluate methods for secondary prevention of HUS in paediatric patients who had contracted a diarrhoeal illness due to STEC.Thomas 2013 conducted a non‐Cochrane systematic review looking at the prevention of diarrhoea‐associated HUS and included animal and human studies. The authors of this review found three RCTs in humans, two that are reported in our review as well (Proulx 1992; Rowe 1995); and, one additional study that focused on the primary prevention of STEC infection rather than secondary prevention of HUS after the STEC infection. We report the similar findings from the two included studies that were included in both the reviews (Proulx 1992; Rowe 1995) and included two additional studies (Huppertz 1999; Lopez 2010).
Authors' conclusions
Implications for practice.
We identified four different studies that assessed various interventions including the use of antibiotics, anti‐Shiga toxin antibodies containing bovine colostrum, and Shiga toxin binding agent (Synsorb Pk) and monoclonal antibodies (Urtoxazumab) in the secondary prevention of HUS in children with E. coli O157:H7 enteritis. However, no conclusions regarding the implications for practice can be drawn at this time due to the small number of RCTs in this field of research and the relatively small sample sizes of those few existing trials.
Implications for research.
The available studies target four different interventions that could be used to prevent secondary occurrence of HUS in patients with STEC. One small study tested a well‐known antibiotic (trimethoprim‐sulfamethoxazole) and showed no significant effect for secondary prevention of HUS in patients with STEC (Proulx 1992). Data from observational studies showed that use of antibiotics in patients with STEC might increase the risk of HUS hence the recommendation against their use by CDC and American gastroenterology association (CDC 2018a; Riddle 2016; Wong 2000; Wong 2012). Any future efforts to test antibiotics for prevention of HUS should start with determination of their safety profile in animal studies before a human trial is conducted on this intervention for prevention of HUS in patients with STEC.
Bovine colostrum containing anti‐Shiga toxin antibodies is a potential therapy as colostrum has been used for protection against enteric pathogens (Brunser 1992; Mieten 1979). Use of bovine colostrum could be a cost‐effective method however the included study had a small sample size with no HUS events, so it is hard to make any suggestion to test this intervention in the future studies.
Use of oral Shiga toxin binding agent like Synsorb Pk seems to be an attractive intervention that needs further investigations. Unfortunately, the included study (Rowe 1995) was only available as an abstract so details of the intervention were not readily available. Synsorb Pk had been tested in a RCT for the treatment of HUS (Trachtman 2003) and even though it did not show an effect for treatment of HUS, it was found to be safe for use. An interesting observation from the available data from Rowe 1995 was that effect of Synsorb Pk seems to be prominent if the intervention was given within four days of diagnosis. This observation, along with lack of effect of Synsorb Pk for treatment of HUS, indicate that there is a short window period in the early days of the disease where oral Synsorb Pk could be helpful to prevent subsequent development of HUS. The early use is attached to early diagnosis of STEC‐associated diarrhoea. The early diagnosis can be potentially achieved with newly available, culture‐independent, rapid diagnostic techniques (Buchan 2013), hence an opportunity to start the intervention early. We suggest that this medication should be further tested in the future studies and these studies should focus on early recruitment of the participants to assess the preventive effect of Synsorb Pk for HUS in patients with STEC.
Intravenous use of monoclonal antibodies against Shiga toxin is a potential strategy to mitigate the effect of Shiga toxin to induce the development of HUS. Even though the only included study on this intervention (Lopez 2010) did not show an effect of urtoxazumab (a monoclonal antibody against Shiga toxin) on incidence of HUS however this study was not meant to define the efficacy of the intervention for secondary prevention of HUS but the pharmacokinetics and safety profile. Even though the number of adverse events, both serious and non‐serious adverse events, were common in study participants, they were proportionally distributed in the two study groups and control group and there was no statistically significant difference between the study groups and the placebo. There was no increased risk of serious or non‐serious adverse events in the intervention groups compared to the control group. We therefore think this intervention should be tested in further larger RCTs to assess its efficacy for secondary prevention of HUS in patients with STEC. This effort might require a multicentre potentially multi‐country study as the incidence of STEC‐associated HUS has been decreasing with better primary preventive strategies around safe food practices and early discovery and control of STEC‐related outbreaks (CDC 2018b).
We also found two other studies that assessed efficacy of monoclonal antibodies against Shiga toxin for prevention of HUS in patients with STEC (NCT04132375; SHIGATEC 2011). We hope to include the results of these studies in a future update.
We aimed to assess the volume expansion with aggressive hydration at the time of STEC infection for secondary prevention of HUS in these patients. We did not find any published clinical study that addressed this intervention. The data from observational studies and meta‐analysis of observational studies suggest that this intervention could be a potential intervention for secondary prevention of HUS in patients with STEC infection (Ardissino 2016; Grisaru 2017). This intervention should therefore be tested further in the future studies. We found an ongoing study (NCT03275792) addressing the secondary prevention of HUS in STEC infected patients and we hope to include the results of this study in the next update of this review.
What's new
| Date | Event | Description |
|---|---|---|
| 5 July 2021 | Amended | Minor edits |
History
Protocol first published: Issue 4, 2018 Review first published: Issue 7, 2021
Acknowledgements
We wish to thank the referees for their comments and feedback during the preparation of this protocol and the review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Trimethoprim/sulfamethoxazole versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Incidence of HUS | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Bovine colostrum versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Incidence of HUS | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
2.1. Analysis.

Comparison 2: Bovine colostrum versus control, Outcome 1: Incidence of HUS
Comparison 3. Synsorb Pk versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Incidence of HUS | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 4. Urtoxazumab versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Incidence of HUS | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1.1 Urtoxazumab 3.0 mg/kg versus placebo | 1 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.14] |
| 4.1.2 Urtoxazumab 1.0 mg/kg versus placebo | 1 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.06, 14.59] |
| 4.2 Adverse events: treatment emergent | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.2.1 Urtoxazumab 3.0 mg/kg versus placebo | 1 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.84, 1.18] |
| 4.2.2 Urtoxazumab 1.0 mg/kg versus placebo | 1 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.79, 1.13] |
| 4.3 Neurological complications | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.3.1 Urtoxazumab 3.0 mg/kg versus placebo | 1 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.20, 21.68] |
| 4.3.2 Urtoxazumab 1.0 mg/kg versus placebo | 1 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 2.84 [0.31, 26.08] |
| 4.4 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.4.1 Urtoxazumab 3.0 mg/kg versus placebo | 1 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.14] |
| 4.4.2 Urtoxazumab 1.0 mg/kg versus placebo | 1 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.06, 14.59] |
4.2. Analysis.

Comparison 4: Urtoxazumab versus control, Outcome 2: Adverse events: treatment emergent
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Huppertz 1999.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients meeting the entry criteria and still in the hospital at the time of the bacteriologic diagnosis were randomly allocated to receive either bovine colostrum or placebo administered double‐blind.." Comment: The authors do not provide any further details on how the randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | Comment: Authors do not provide any details of concealment of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "an innocuous preparation devoid of antibodies but similar in chemical composition and identical in appearance with bovine colostrum, served as placebo..." Comment: Most likely done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Even though the authors claimed that it was a double‐blind study, no details were provided for blinding of the assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two patients were lost to follow‐up in the intervention group and one in the control group |
| Selective reporting (reporting bias) | Low risk | Comment: Authors seems to report all the relevant outcomes |
| Other bias | Low risk | Study also included two patients with HUS at the time of recruitment. These patients were not considered for outcome assessment in this review |
Lopez 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "STEC‐infected pediatric patients were sequentially enrolled in double‐blind randomised fashion into one of two cohorts of 12 patients each." Comment: The authors do not provide any further details on how the randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | Comment: Authors do not provide any details of concealment of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The patients in the first cohort were stratified to receive an i.v. infusion of either placebo or 1.0 mg/kg urtoxazumab (1:2 ratio) in a total volume of 10 ml/kg." Comment: Unclear whether placebo appeared different from study drug preparation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Even though the authors claimed that it was a double‐blind study, no details were provided for blinding of the assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: The authors report outcomes for all patients enrolled at the start of the study |
| Selective reporting (reporting bias) | Low risk | Comment: Authors seems to report all the relevant outcomes |
| Other bias | Low risk | No other risk of bias was noted |
Proulx 1992.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly selected, according to a computer‐generated list of random numbers, to receive either a standard dose of TMP‐SMX twice daily for 5 days or to receive no treatment." |
| Allocation concealment (selection bias) | Unclear risk | Comment: Authors do not provide any details of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "No placebo was available, neither patients nor treating physicians were unaware of the treatment." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "No placebo was available, neither patients nor treating physicians were unaware of the treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No patients were lost to follow up." |
| Selective reporting (reporting bias) | Low risk | Comment: Authors seem to report all relevant outcomes |
| Other bias | Low risk | No other risk of bias was noted |
Rowe 1995.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible subjects received 500 mg/kg of Synsorb‐Pk mixed in baby food divided BID for 7 days or an equal volume of corn meal." Comment: Authors do not provide any further details on how the randomisation was done. Also, full text was not available for further evaluation |
| Allocation concealment (selection bias) | Unclear risk | Comment: Authors do not provide any details of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Eligible subjects received 500 mg/kg of Synsorb‐Pk mixed in baby food divided BID for 7 days or an equal volume of corn meal." Comment: The control seems to be different from the intervention group in appearance and taste |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: No details were provided on blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 subjects were excluded after randomisation, 7 from the treatment group and 4 from the control group. Overall loss to follow up was 3.1% |
| Selective reporting (reporting bias) | Low risk | Comment: Authors seem to report all relevant outcomes |
| Other bias | Low risk | No other risk of bias was noted |
AKI ‐ acute kidney injury; HUS ‐ haemolytic uraemic syndrome; M/F ‐ male/female; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Caletti 2011 | The intervention was given after the HUS was developed and not as a secondary prevention |
| HUS‐SYNSORB Pk 1998 | The intervention was given during an episode of HUS and not as a secondary prevention |
| NCT02205541 | The study aims to give the intervention during an episode of HUS and not as secondary prevention |
| Pape 2009 | The intervention was given as a treatment for HUS and not as a secondary prevention |
HUS ‐ haemolytic uraemic syndrome
Characteristics of studies awaiting classification [ordered by study ID]
McLaine 1995.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
HUS ‐ haemolytic uraemic syndrome; RCT ‐ randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT03275792.
| Study name | Shiga toxin producing Escherichia coli (STEC) volume expansion |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date | May 2019 |
| Contact information | Stephen Freedman, MDCM, MSc, 403‐955‐7740, stephen.freedman@ahs.ca Karen Lowerison, 403‐955‐3186, karen.lowerison@ahs.ca |
| Notes |
|
NCT04132375.
| Study name | Phase 2/3 study to evaluate PK, safety & efficacy of INM004 in STEC positive pediatric patients for prevention of HUS |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date | July 17, 2019 |
| Contact information | Mariana Colanna, Bioch, + 54 9 1161718697, mcolonna@inmunova.com Santiago Sanguineti, PhD, +54 9 11 4564‐3625, ssanguineti@inmunova.com |
| Notes | Funding source: not reported |
SHIGATEC 2011.
| Study name | Shigatec: a phase II study assessing monoclonal antibodies against Shiga toxin 1 and 2 in Shiga toxin‐producing E. coli‐infected children |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date | November 2010 |
| Contact information | No contact information listed |
| Notes | The data were reported for 23 patients from low dose arm. No data were available from the control group. The study is ongoing and no final results are available |
AKI ‐ acute kidney injury; CKD ‐ chronic kidney disease; ED ‐ emergency department; HCT ‐ haematocrit; HUS ‐ haemolytic uraemic syndrome; IV ‐ intravenous; LDL ‐ lactate dehydrogenase; M/F ‐ male/female; PCR ‐ polymerase chain reaction; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; STEC ‐ Shiga toxin producing Escherichia coli
Contributions of authors
Draft the protocol: AI, TS, ETS
Study selection: AI, SPM, DMU
Extract data from studies: AI, SPM, DMU
Enter data into RevMan: AI, SPM, DMU
Carry out the analysis: AI
Interpret the analysis: AI, ETS, OG, DH
Draft the final review: AI, SPM, DMU, ETS
Disagreement resolution: OG, ETS, DH
Update the review: AI
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
All authors declare they do not have any conflict of interest. None of the authors listed in this review have any present or past affiliations or other involvement in any organization or entity with an interest in the outcome of the review. There have been no reported relationships present during the past 36 months, including, but not restricted to, financial remuneration for lectures, consultancy, travel or authorship of a study that might be included in this review.
Edited (no change to conclusions)
References
References to studies included in this review
Huppertz 1999 {published data only}
- Huppertz HI, Rutkowski S, Busch DH, Eisebit R, Lissner R, Karch H. Bovine colostrum ameliorates diarrhea in infection with diarrheagenic Escherichia coli, shiga toxin-producing E. Coli, and E. coli expressing intimin and hemolysin. Journal of Pediatric Gastroenterology & Nutrition 1999;29(4):452-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lopez 2010 {published data only}
- Lopez EL, Contrini MM, Glatstein E, Gonzalez Ayala S, Santoro R, Allende D, et al. Safety and pharmacokinetics of urtoxazumab, a humanized monoclonal antibody, against Shiga-like toxin 2 in healthy adults and in pediatric patients infected with Shiga-like toxin-producing Escherichia coli. Antimicrobial Agents & Chemotherapy 2010;54(1):239-43. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Proulx 1992 {published data only}
- Proulx F, Turgeon JP, Delage G, Lafleur L, Chicoine L. Randomized, controlled trial of antibiotic therapy for Escherichia coli O157:H7 enteritis. Journal of Pediatrics 1992;121(2):299-303. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rowe 1995 {published data only}
- Rowe P, Orrbine E, Armstrong G, Wells G, McLaine P, Canadian Pediatric Kidney Disease Research Centre (CPKDRC) - Synsorb Study Group. Design and methodology of a randomized trial of Synsorb PK for the prevention of hemolytic uremic syndrome (HUS) [abstract no: P225]. Pediatric Nephrology 1995;9(6):C111. [CENTRAL: CN-00509445] [Google Scholar]
- Rowe PC, Milner R, Orrbine E, Klassen TP, Mackenzie AM, Wells GA, et al. A phase II randomized controlled trial of Synsorb PK for the prevention of hemolytic uremic syndrome in children with verotoxin-producing E. coli (VTEC) gastroenteritis [abstract no: 1684]. Pediatric Research 1997;41(4 Suppl):283. [CENTRAL: CN-01658206] [Google Scholar]
References to studies excluded from this review
Caletti 2011 {published data only}
- Caletti MG, Missoni M, Vezzani C, Grignoli M, Piantanida JJ, Repetto HA, et al. Effect of diet, enalapril, or losartan in post-diarrheal hemolytic uremic syndrome nephropathy. Pediatric Nephrology 2011;26(8):1247-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HUS‐SYNSORB Pk 1998 {published data only}
- De Petris L, Patrick J, Christen E, Trachtman H. Urinary podocyte mRNA excretion in children with D+HUS: a potential marker of long-term outcome. Renal Failure 2006;28(6):475-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mackenzie AM, Lebel P, Orrbine E, Rowe PC, Hyde L, Chan F, et al. Sensitivities and specificities of premier E. coli O157 and premier EHEC enzyme immunoassays for diagnosis of infection with verotxin (Shiga-like toxin)-producing Escherichia coli. The SYNSORB Pk Study investigators. Journal of Clinical Microbiology 1998;36(6):1608-11. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P, Acheson D, Chitrakar R, Cnaan A, Gibbs K, Hirschman GH, et al. Basic fibroblast growth factor among children with diarrhea-associated hemolytic uremic syndrome. Journal of the American Society of Nephrology 2002;13(3):699-707. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thurman JM, Marians R, Emlen W, Wood S, Smith C, Akana H, et al. Alternative pathway of complement in children with diarrhea-associated hemolytic uremic syndrome. Clinical Journal of the American Society of Nephrology: CJASN 2009;4(12):1920-4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtman H, Christen E, Cnaan A, Acheson DW, HUS-SYNSORB Pk Investigators. Failure of an oral shiga toxin binding agent to ameliorate diarrheal-related hemolytic uremic syndrome: results of a randomized clinical trial [abstract no: F-FC027]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):6A. [CENTRAL: CN-00448052] [Google Scholar]
- Trachtman H, Christen E, Cnaan A, Patrick J, Mai V, Mishra J, et al. Urinary neutrophil gelatinase-associated lipocalcin in D+HUS: a novel marker of renal injury. Pediatric Nephrology 2006;21(7):989-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Trachtman H, Christen E, Patrick J, Devarajan P. Urinary excretion of neutrophil gelatinase-associated lipocalcin (NGAL) in diarrhea-associated hemolytic uremic syndrome (D+HUS) [abstract no: TH-PO901]. Journal of the American Society of Nephrology 2005;16(Abstracts):316A. [CENTRAL: CN-00601943] [Google Scholar]
- Trachtman H, Cnaan A, Christen E, Gibbs K, Zhao S, Acheson DW, et al. Effect of an oral Shiga toxin-binding agent on diarrhea-associated hemolytic uremic syndrome in children: a randomized controlled trial. JAMA 2003;290(10):1337-44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT02205541 {published data only}
- Garnier A. Eculizumab in shiga-toxin related hemolytic and uremic syndrome pediatric patients - ECULISHU (ECULISHU). www.clinicaltrials.gov/ct2/show/NCT02205541 (first received 31 July 2014).
Pape 2009 {published data only}
- Pape L, Ahlenstiel T, Kreuzer M, Drube J, Froede K, Franke D, et al. Early erythropoietin reduced the need for red blood cell transfusion in childhood hemolytic uremic syndrome: a randomized prospective pilot trial. Pediatric Nephrology 2009;24(5):1061-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pape L, Ehrich J, Ahlenstiel T. Early erythropoietin for preventing red blood cell transfusion in childhood hemolytic uremic syndrome [abstract no: P006]. Pediatric Nephrology 2008;23(9):1598. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
McLaine 1995 {published data only}
- McLaine P. The use of synsorb PK for preventing hemolytic uremic syndrome (HUS) [abstract]. Pediatric Nephrology 1995;9(6):C30. [CENTRAL: CN-00401899] [Google Scholar]
References to ongoing studies
NCT03275792 {unpublished data only}
- Freedman S. Shiga toxin producing Escherichia coli (STEC) volume expansion. www.clinicaltrials.gov/show/NCT03275792 (first received 8 September 2017).
NCT04132375 {unpublished data only}
- Sanguineti S. Phase 2/3 study to evaluate PK, safety and efficacy of INM004 in STEC positive pediatric patients for prevention of HUS. www.clinicaltrials.gov/show/NCT04132375 (first received 18 October 2019).
SHIGATEC 2011 {published data only}
- Bitzan M, Mellmann A, Karch H, Reymond D. SHIGATEC: a phase II study evaluating shigamabs in STEC-infected children [abstract]. Zoonoses & Public Health 2012;59(Suppl 1):18. [EMBASE: 70928816] [Google Scholar]
- Lopez EL, Cleary T, Reymond D. SHIGATEC Trial: a phase II study assessing monoclonal antibodies against shiga toxin 1 and 2 in shiga toxin-producing E. coli-infected children [abstract no: 609]. In: Infectious Diseases Society of America Annual Meeting; 2011 Oct 20-23; Boston, USA. 2011.
- Taylor CM, Bitzan M, Reymond D. Shigatec: a phase II study assessing monoclonal antibodies against shiga toxin 1 and 2 in Shiga toxin-producing E. coli-infected children [abstract no: PS1-THU-069]. Pediatric Nephrology 2011;26(9):1595. [CENTRAL: CN-01657421] [Google Scholar]
Additional references
Ardissino 2016
- Ardissino G, Tel F, Possenti I, Testa S, Consonni D, Paglialonga F, et al. Early volume expansion and outcomes of hemolytic uremic syndrome. Pediatrics 2016;137(1):e20152153. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Armstrong 1991
- Armstrong GD, Fodor E, Vanmaele R. Investigation of Shiga-like toxin binding to chemically synthesized oligosaccharide sequences. Journal of Infectious Diseases 1991;164(6):1160-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bell 1994
- Bell BP, Goldoft M, Griffin PM, Davis MA, Gordon DC, Tarr PI, et al. A multistate outbreak of Escherichia coli O157:H7-associated bloody diarrhea and hemolytic uremic syndrome from hamburgers. The Washington experience. JAMA 1994;272(17):1349-53. [MEDLINE: ] [PubMed] [Google Scholar]
Bitzan 2009
- Bitzan M. Treatment options for HUS secondary to Escherichia coli O157:H7. Kidney International - Supplement 2009;(112):S62-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brunser 1992
- Brunser O, Espinoza J, Figueroa G, Araya M, Spencer E, Hilpert H, et al. Field trial of an infant formula containing anti-rotavirus and anti-Escherichia coli milk antibodies from hyperimmunized cows. Journal of Pediatric Gastroenterology & Nutrition 1992;15(1):63-72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Buchan 2013
- Buchan BW, Olson WJ, Pezewski M, Marcon MJ, Novicki T, Uphoff TS, et al. Clinical evaluation of a real-time PCR assay for identification of Salmonella, Shigella, Campylobacter (Campylobacter jejuni and C. coli), and shiga toxin-producing Escherichia coli isolates in stool specimens. Journal of Clinical Microbiology 2013;51(12):4001-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 1996
- Centers for Disease Control and Prevention. Hemolytic uremic syndrome, post-diarrheal (HUS). 1996 case definition. wwwn.cdc.gov/nndss/conditions/hemolytic-uremic-syndrome-post-diarrheal/case-definition/1996/ (accessed 5 February 2018).
CDC 2018a
- Centers for Disease Control and Prevention. E. coli (Escherichia coli). Resources for clinicians and laboratories. Guidance to healthcare providers and clinical laboratories. www.cdc.gov/ecoli/clinicians.html (Date last accessed: 24 March 2021).
CDC 2018b
- Centers for Disease Control and Prevention. FoodNet:FAST: HUS. wwwn.cdc.gov/FoodNetFast/HUS (Date last accessed: 24 March 2021).
Corrigan 2001
- Corrigan JJ Jr, Boineau FG. Hemolytic-uremic syndrome [Erratum in: Pediatr Rev 2002 Jan;23(1): 1 p]. Pediatrics in Review 2001;22(11):365-9. [MEDLINE: ] [PubMed] [Google Scholar]
Dowling 2005
- Dowling TC, Chavaillaz PA, Young DG, Melton-Celsa A, O'Brien A, Thuning-Roberson C, et al. Phase 1 safety and pharmacokinetic study of chimeric murine-human monoclonal antibody c alpha Stx2 administered intravenously to healthy adult volunteers. Antimicrobial Agents & Chemotherapy 2005;49(5):1808-12. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fakhouri 2017
- Fakhouri F, Zuber J, Fremeaux-Bacchi V, Loirat C. Haemolytic uraemic syndrome. Lancet 2017;390(10095):681-96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
FDA 2016
- FDA. What is a serious adverse event? www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event (Date last accessed: 24 March 2021).
Freedman 2016
- Freedman SB, Xie J, Neufeld MS, Hamilton WL, Hartling L, Tarr PI, et al. Shiga toxin-producing Escherichia coli Infection, antibiotics, and risk of developing hemolytic uremic syndrome: a meta-analysis. Clinical Infectious Diseases 2016;62(10):1251-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garg 2003
- Garg AX, Suri RS, Barrowman N, Rehman F, Matsell D, Rosas-Arellano MP, et al. Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: a systematic review, meta-analysis, and meta-regression. JAMA 2003;290(10):1360-70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gould 2011
- Gould LH, Jordan JG, Dunn J, Apostol M, Griffin PM, Emerging Infections Program FoodNet Working Group. Postdiarrheal hemolytic uremic syndrome in persons aged 65 and older in foodnet sites, 2000-2006. Journal of the American Geriatrics Society 2011;59(2):366-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grisaru 2017
- Grisaru S, Xie J, Samuel S, Hartling L, Tarr PI, Schnadower D, et al. Associations between hydration status, intravenous fluid administration, and outcomes of patients infected with Shiga toxin-producing Escherichia coli: a systematic review and meta-analysis. JAMA Pediatrics 2017;171(1):68-76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Jokiranta 2017
- Jokiranta TS. HUS and atypical HUS. Blood 2017;129(21):2847-56. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keir 2015
- Keir LS. Shiga toxin associated hemolytic uremic syndrome. Hematology - Oncology Clinics of North America 2015;29(3):525-39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lopez 2010a
- Lopez EL, Contrini MM, Glatstein E, Gonzalez Ayala S, Santoro R, Allende D, et al. Safety and pharmacokinetics of urtoxazumab, a humanized monoclonal antibody, against Shiga-like toxin 2 in healthy adults and in pediatric patients infected with Shiga-like toxin-producing Escherichia coli. Antimicrobial Agents & Chemotherapy 2010;54(1):239-43. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Majowicz 2014
- Majowicz SE, Scallan E, Jones-Bitton A, Sargeant JM, Stapleton J, Angulo FJ, et al. Global incidence of human Shiga toxin-producing Escherichia coli infections and deaths: a systematic review and knowledge synthesis. Foodborne Pathogens & Disease 2014;11(6):447-55. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mejias 2016
- Mejias MP, Hiriart Y, Lauche C, Fernandez-Brando RJ, Pardo R, Bruballa A, et al. Development of camelid single chain antibodies against Shiga toxin type 2 (Stx2) with therapeutic potential against hemolytic uremic syndrome (HUS). Scientific Reports 2016;6:24913. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mele 2014
- Mele C, Remuzzi G, Noris M. Hemolytic uremic syndrome. Seminars in Immunopathology 2014;36(4):399-420. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Melton‐Celsa 2014
- Melton-Celsa AR, O'Brien AD. New therapeutic developments against Shiga toxin-producing Escherichia coli. Microbiology Spectrum 2014;2(5). [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Michael 2009
- Michael M, Elliott EJ, Ridley GF, Hodson EM, Craig JC. Interventions for haemolytic uraemic syndrome and thrombotic thrombocytopenic purpura. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD003595. [DOI: 10.1002/14651858.CD003595.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mieten 1979
- Mietens C, Kleinhorst H, Hilpert H, Gerber H, Amster H, Pahud JJ. Treatment of infantile E. coli gastroenteritis with specific bovine anti-E. coli milk immunoglobulins. European Journal of Pediatrics 1979;132(4):239-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mody 2015
- Mody RK, Gu W, Griffin PM, Jones TF, Rounds J, Shiferaw B, et al. Postdiarrheal hemolytic uremic syndrome in United States children: clinical spectrum and predictors of in-hospital death. Journal of Pediatrics 2015;166(4):1022-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nitschke 2012
- Nitschke M, Sayk F, Härtel C, Roseland RT, Hauswaldt S, Steinhoff J, et al. Association between azithromycin therapy and duration of bacterial shedding among patients with Shiga toxin-producing enteroaggregative Escherichia coli O104:H4. JAMA 2012;307(10):1046-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Riddle 2016
- Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. American Journal of Gastroenterology 2016;111(5):602-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Riley 1983
- Riley LW, Remis RS, Helgerson SD, McGee HB, Wells JG, Davis BR, et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. New England Journal of Medicine 1983;308(12):681-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rivas 2014
- Rivas M, Chinen I, Miliwebsky E, Masana M. Risk factors for Shiga toxin-producing Escherichia coli-associated human diseases. Microbiology Spectrum 2014;2(5). [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rowe 1998
- Rowe PC, Orrbine E, Lior H, Wells GA, Yetisir E, Clulow M, et al. Risk of hemolytic uremic syndrome after sporadic Escherichia coli O157:H7 infection: results of a Canadian collaborative study. Investigators of the Canadian Pediatric Kidney Disease Research Center. Journal of Pediatrics 1998;132(5):777-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Siegler 1994
- Siegler RL, Pavia AT, Christofferson RD, Milligan MK. A 20-year population-based study of postdiarrheal hemolytic uremic syndrome in Utah. Pediatrics 1994;94(1):35-40. [MEDLINE: ] [PubMed] [Google Scholar]
Skinner 2016
- Skinner C, Patfield S, Khalil R, Kong Q, He X. New monoclonal antibodies against a novel subtype of Shiga toxin 1 produced by Enterobacter cloacae and their use in analysis of human serum. Msphere 2016;1(1). [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Talarico 2016
- Talarico V, Aloe M, Monzani A, Miniero R, Bona G. Hemolytic uremic syndrome in children. Minerva Pediatrica 2016;68(6):441-55. [MEDLINE: ] [PubMed] [Google Scholar]
Thomas 2013
- Thomas DE, Elliott EJ. Interventions for preventing diarrhea-associated hemolytic uremic syndrome: systematic review. BMC Public Health 2013;13:799. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trachtman 2003
- Trachtman H, Cnaan A, Christen E, Gibbs K, Zhao S, Acheson DW, et al. Effect of an oral Shiga toxin-binding agent on diarrhea-associated hemolytic uremic syndrome in children: a randomized controlled trial. JAMA 2003;290(10):1337-44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wong 2000
- Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. New England Journal of Medicine 2000;342(26):1930-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2012
- Wong CS, Mooney JC, Brandt JR, Staples AO, Jelacic S, Boster DR, et al. Risk factors for the hemolytic uremic syndrome in children infected with Escherichia coli O157:H7: a multivariable analysis. Clinical Infectious Diseases 2012;55(1):33-41. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ylinen 2020
- Ylinen E, Salmenlinna S, Halkilahti J, Jahnukainen T, Korhonen L, Virkkala T, et al. Hemolytic uremic syndrome caused by Shiga toxin-producing Escherichia coli in children: incidence, risk factors, and clinical outcome. Pediatric Nephrology 2020;35(9):1749-59. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Imdad 2018
- Imdad A, Syed T, Gomez‐Duarte OG, Tanner‐Smith EE, Huang D. Interventions for preventing diarrhoea‐associated haemolytic uraemic syndrome. Cochrane Database of Systematic Reviews 2018, Issue 4. Art. No: CD012997. [DOI: 10.1002/14651858.CD012997] [DOI] [PMC free article] [PubMed] [Google Scholar]


