Abstract
Background
Overweight (Ow) and obesity among adults and children increases the risk of metabolic consequences. Metabolic syndrome (MS) and impaired glucose metabolism are well-known risk factors for cardiovascular diseases and type 2 diabetes. The aim of this study was to evaluate the prevalence of MS and impaired glucose metabolism among Ow and obese (Ob) children and adolescents (aged 10–17 years) in Lithuania, and to evaluate the associations between insulin resistance (IR) indices and anthropometric parameters as well as metabolic disturbances.
Methods
The study population consisted of 344 OwOb children and adolescents of all pubertal stages. Oral glucose tolerance tests (OGTTs), IR and β cell function indices, lipid profile, and anthropometric parameters of all subjects were analyzed. MS was defined according to the International Diabetes Federation consensus guidelines.
Results
MS was found in 21.3% of the OwOb children and adolescents, and 12.1% had impaired glucose metabolism (6.9% with impaired fasting glucose, 4.5% with impaired glucose tolerance, and 0.6% with type 2 diabetes). IR was directly related to body mass index and waist circumference, waist-to-height and waist-to-hip ratios, and sum of skin-fold thicknesses. Children with MS were more insulin-resistant, had higher odds ratio for prediabetes and had a more disturbed lipid profile than subjects without MS. Moreover, total cholesterol and low-density lipoprotein cholesterol levels were significantly lower in the more mature OwOb adolescents.
Conclusion
MS and lipid profile disturbances are common in OwOb children and adolescents. MS is directly associated with IR. Therefore, OwOb children and adolescents should be carefully followed up for metabolic abnormalities during late childhood as these can persist into adulthood.
Keywords: Obesity, Overweight, Insulin resistance, Lipid profile, Metabolic syndrome, Children, Adolescents, Waist circumference
Introduction
The cluster of symptoms defined as metabolic syndrome (MS) is known as a risk factor for the development of type 2 diabetes (T2D) and cardiovascular diseases (CVD) in children and adults [1]. Some studies have shown direct links between childhood overweight (Ow) and adult metabolic disturbances and cardiovascular risks [1]. Insulin resistance (IR) has also been reported as a key risk factor for CVD and T2D [2].
The prevalence of MS varies in different populations according to age, gender, and ethnic origin, and also depends on the diagnostic criteria used [3]. According to International Diabetes Federation (IDF) criteria [4], the prevalence of MS in Ow adults ranges from 25 to 43.8% [5].
According to IDF diagnostic criteria, the prevalence of MS varies from 1.9% in Brazilian to 20.5% in Chinese Ow children and adolescents [6, 7, 8, 9, 10, 11]. The prevalence of MS in obese (Ob) children is reported to be considerably higher, from 3.7% in Chinese to 44.0% in US children and adolescents [6, 7, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25].
The prevalence of impaired glucose metabolism varies from 18.1% in the UK to 32% in Kuwait in Ow adults [5, 26, 27], and from 7.0% in Spanish to 12.6% in German OwOb children and adolescents [5, 28, 29].
Luksiene et al. [30] found a prevalence of MS in Lithuanian men and women (35–64 years of age) of 29.7 and 35.1%, respectively. A recently published study showed that the prevalence of Ow and obesity in children and adolescents (7–17 years of age) in Lithuania is 12.6 and 4.1%, respectively [31]. To date, there are no data on metabolic disturbances in OwOb children and adolescents in Lithuania.
The aim of this study was to determine the prevalence of MS and impaired glucose metabolism (impaired fasting glucose [IFG], impaired glucose tolerance [IGT], and T2D) in OwOb Lithuanian children and adolescents aged 10–17 years and to evaluate the associations with IR and metabolic disturbances.
Materials and Methods
Materials
The study population included 344 Ow (body mass index standard deviation score [BMI-SDS] 1.0–1.99 for gender and age), Ob (BMI-SDS 2.0–2.99 for gender and age), and morbidly Ob (BMI-SDS ≥3.0 for gender and age) prepubertal and pubertal (stages 1–5) children aged 10–17 years from the Kaunas region, Lithuania. Children and adolescents involved in the school survey and/or consulted for Ow/obesity at the Department of Endocrinology, Hospital of Lithuanian University of Health Sciences, were recruited for the study over a 3-year period (from January 2012 to April 2014).
The measurements were performed with standardized equipment. Height was measured to the nearest 0.1 cm with a portable SECA stadiometer (Seca®214). Weight was measured to the nearest 0.1 kg using portable SECA electronic scales (Seca®813). BMI was calculated by using the standard equation: BMI = weight (kg)/height (m2). Waist circumference (WC), hip, middle thigh and middle arm on the left side, to the nearest 0.1 cm, were measured with nonelastic tape.
Blood pressure was measured in a sitting position after the child had been relaxing for 5–10 min. Measurements were taken twice at intervals of 2–3 min with an automatic device with a special pediatric cuff. The lowest value was used in the analysis. Evaluation of arterial hypertension was defined according to the American Academy of Pediatrics updated guidelines for children and adolescents [32].
Puberty staging for girls was determined by pubarche and breast stage according to Emmanuel and Bokor [33]. In boys, the pubertal stage was determined by pubarche and testicular volume (<4, 4–8, 9–12, 15–20, and >20 mL represented stages 1–5, respectively), using a Prader orchidometer.
BMI and WC measurements were converted to Z scores according to International Obesity Task Force references for BMI [34] and Polish references for WC in boys and girls aged 5–19 years [35].
Laboratory Measurements and Evaluation
All study participants underwent a clinical examination and an oral glucose tolerance test (OGTT). All blood samples for analysis were collected after a ≥8-h fast. A standard OGTT was performed with oral 1.75 g/kg glucose challenge (max. 75 g) following blood sampling for glycemia and insulin concentrations analysis at 0, 30, and 120 min after glucose load. A fasting lipid profile was assessed by a standard procedure in the biochemistry lab by enzyme-linked immunosorbent assay (ELISA; Instrumentation Laboratory, Lexington, KY, USA); the intra-/interassay coefficient of variation (CV) for total cholesterol (TCh), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), and triglycerides was 1.8/1.8%, 1.2/2.6%, 3.2/2.0%, and 1.2/2.3%, respectively. Serum insulin concentration was measured in a certified clinical lab by radioimmune assay (RIA; DIAsource, Belgium); the intra-/interassay CV was 1.8/6.3%.
Impaired Glucose Metabolism
Impaired glucose metabolism included IFG, IGT, and T2D diagnosed according to International Society for Pediatric and Adolescent Diabetes (ISPAD) criteria [36]:
IFG: fasting plasma glucose 5.6–6.9 mmol/L;
IGT: 2-h postload glucose 7.8–11.1 mmol/L;
T2D: OGTT: fasting plasma glucose ≥7.0 mmol/L and/or 2-h postload glucose ≥11.1 mmol/L; randomly fixed on 2 different occasions if asymptomatic or once if presenting symptoms of T2D: fasting plasma glucose ≥7.0 mmol/L and/or casual plasma glucose ≥11.1 mmol/L.
Metabolic Syndrome
MS was diagnosed according to the IDF consensus for children and adolescents [4].
Children Aged 10–16 Years. Obesity according to WC ≥90th percentile (or adult cut-off if lower) plus any 2 of the following:
Triglycerides: ≥1.7 mmol/L;
HDL: <1.03 mmol/L;
Blood pressure: ≥130 mm Hg systolic or ≥85 mm Hg diastolic;
Fasting plasma glucose: ≥5.6 mmol/L or known T2D.
Adolescents Aged >16 Years. Existing IDF criteria for adults were used, i.e., central obesity (BMI >30 or WC >94 cm in males and >80 cm in females) plus any 2 of the following:
Increased triglycerides: ≥1.7 mmol/L, or specific treatment for this lipid abnormality;
Reduced HDL: <1.03 mmol/L in males and <1.29 mmol/L in females, or specific treatment for this lipid abnormality;
Increased blood pressure: systolic ≥130 mm Hg, or diastolic ≥85 mm Hg, or treatment of previously diagnosed hypertension;
Raised fasting plasma glucose: ≥5.6 mmol/L or previously diagnosed T2D.
Insulin Resistance
IR was evaluated using the homeostasis model assessment of insulin resistance (HOMA-IR): fasting plasma glucose in mmol/L × fasting insulin in mIU/L/22.5 [37]; homeostasis model assessment of β cell function (HOMA-B): 20 × fasting insulin in µU/mL/fasting glucose in mmol/L-3; and quantitative insulin sensitivity index (QUICKI): 1/(loginsulin in µU/mL + logglucose in mg/dL) [38]. Insulin secretion was assessed using the insulinogenic index (IGI): δinsulin (0–30 min)/δglucose (0–30 min) [38]. Using the standard formulae [39, 40, 41], insulin sensitivity (IS) indices (modified by Gutt et al. [41]) were calculated.
For further analysis, the study population was divided into groups according to weight status: Ow, Ob, and morbidly Ob. IR status was defined by HOMA-IR tertiles; individuals with HOMA-IR <2.96 were attributed to the first tertile, HOMA-IR between 2.96 and 4.46 to the second, and HOMA-IR >4.46 to the third.
The prevalence of lipid profile abnormalities in triglycerides and HDL was evaluated according to IDF guidelines for MS in children and adolescents [4]. TCh and LDL concentrations were evaluated according to the US National Heart, Lung and Blood Institute (NHLBI) criteria for dyslipidemias in adolescents [42].
Statistical Analyses
Statistical analyses were performed using SPSS software 20.0. ANOVA was used to compare means between groups with and without MS, adjusted for BMI-SDS, gender, and pubertal stage. One-way between-group ANOVA with the post hoc Turkey test was used for comparing HOMA-IR tertile groups. Statistical significance was set at p < 0.05. Cutoff values for HOMA-IR were calculated by receiver-operating characteristics (ROC) analysis.
To compare our results with those of other studies, we performed a target search (scoping review) in PubMed, using the following key words: children, adolescents, overweight, obesity, and metabolic syndrome. The initial search highlighted 1,658 articles. In the literature review, we included articles similar to our study with regard to the age and weight status of subjects, diagnostic criteria for MS according to IDF, glucose metabolism evaluation according to ISPAD, and biochemical parameters.
Results
The study population included children and adolescents of all pubertal stages, 43.9% were males, mean age was 13.5 ± 2.0 years, and mean BMI and BMI-SDS 29.8 ± 4.4 and 2.54 ± 0.5, respectively. The detailed characteristics of the study population by gender and weight status are presented in Table 1.
Table 1.
Comparison of characteristics of the study population between males and females by weight status
| Overweight |
Obesea |
|||||
|---|---|---|---|---|---|---|
| males (16.9%) | females (83.1%) p value | males (49.3%) | females (50.7%) | p value | ||
| Subjects | n = 11 | n = 49 | n = 97 (Ob); n = 43 (mOb) | n = 128 (Ob); n = 16 (mOb) | ||
| Age, years | 12.4±2.2 | 14.3±2.1 | 0.015 | 13.2±2.0 | 13.5±2.0 | 0.234 |
| BMI-SDSb | 1.86±0.1 | 1.71±0.2 | 0.031 | 2.81±0.4 | 2.61±0.4 | <0.0001 |
| WC-SDS*** | 1.02±0.3 | 1.27±0.4 | 0.049 | 2.05±0.4 | 2.02±0.3 | 0.436 |
| WC, cm | 76.4±4.5 | 78.7±5.4 | 0.212 | 94.2±10.3 | 89.8±8.8 | <0.0001 |
| WHtR | 0.48±0.03 | 0.48±0.03 | 0.896 | 0.57±0.05 | 0.55±0.04 | 0.0001 |
| WHpR | 0.84±0.06 | 0.79±0.05 | 0.013 | 0.91±0.17 | 0.82±0.05 | <0.0001 |
| SSFT, mm | 71.5±14.3 | 81.4±15.9 | 0.076 | 104.4±21.9 | 106.4±22.6 | 0.469 |
Ob, obese; mOb, morbidly obese; BMI-SDS, body mass index standard deviation score; WC-SDS, waist circumference standard deviation score; WHtR, waist-to-height ratio; WHpR, waist-to-hip ratio; SSFT, sum of skin-fold thicknesses.
Included Ob and mOb participants.
Evaluated according to IOTF standards [34].
c Evaluated according to the Polish reference for WC [35].
The time point of recruitment did not influence the study results: BMI-SDS (2.48 ± 0.5 vs. 2.55 ± 0.6 vs. 2.57 ± 0.5 in 2012 vs. 2013 vs. 2014 groups, p = 0.07 between 2012 and 2013 groups; p = 0.87 between 2013 and 2014 groups, respectively), WC-SDS (1.96 ± 0.5 vs. 1.82 ± 0.6 vs. 1.81 ± 0.5 in 2012 vs. 2013 vs. 2014 groups, p = 0.32 between 2012 and 2013 groups; p = 0.093 between 2013 and 2014 groups, respectively) and HOMA-IR (4.05 ± 1.9 vs. 4.22 ± 2.2 vs. 4.74 ± 1.7 in 2012 vs. 2013 vs. 2014 groups, p = 0.26 between 2012 and 2013 groups; p = 0.32 between 2013 and 2014 groups, respectively) did not differ by season and year of recruitment.
Metabolic Syndrome
We found a prevalence of MS of 21.3% in OwOb children and adolescents. BMI had a direct impact on the prevalence of MS (10.7, 22.8, and 25.9% in Ow, Ob, and morbidly Ob children and adolescents, respectively; p = 0.046 between Ow and Ob). MS prevalence in OwOb boys was 25.0% compared with 18.4% in girls (p > 0.5).
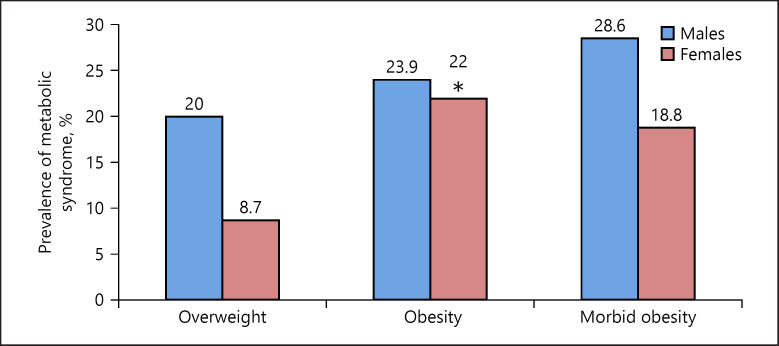
The distribution of MS between genders in relation to weight status is presented in Figure 1. MS prevalence was significantly higher in Ob girls than in Ow girls (p = 0.048), but did not increase further in morbidly Ob girls. In males, the occurrence of MS was not related to weight status (Fig. 1). Adjusted for age, the odds ratio (OR) for MS in Ob versus Ow females was 3.95 (95% CI 1.25–12.49, p = 0.02), but morbid obesity did not further increase the risk of MS (OR 2.59, 95% CI 0.50–13.41, p = 0.26). OR for MS in males was not significant.
Fig. 1.
Prevalence of metabolic syndrome for males and females by weight status. Values are in percentages. * p < 0.05 in comparison with overweight females. Overweight: BMI-SDS 1.0–1.99; obesity: BMI-SDS 2.0–2.99; morbid obesity: BMI-SDS ≥3.0. BMI-SDS, body mass index standard deviation scoreevaluated according to IOTF standards [34].
MS was significantly more prevalent in children and adolescents with HOMA-IR in the third tertile than in those in the first tertile (32.4 and 7.5%, p < 0.0001, respectively). Adjusted for age, pubertal stage, and BMI-SDS, the fasting glucose and insulin levels, HOMA-IR, HOMA-B, TCh, and LDL were significantly higher in children with MS than in their counterparts without MS (Table 2).
Table 2.
Comparison of characteristics between overweight / obese children with MS and without MS (adjusted for gender, BMI-SDS, and pubertal stage)
| With MS | Without MS | p value | |
|---|---|---|---|
| BMI-SDSa | 2.68±0.5 | 2.51±0.6 | <0.0001 |
| WC-SDSb | 2.08±0.5 | 1.86±0.5 | <0.0001 |
| WC, cm | 95.0±10.8 | 88.4±10.0 | <0.0001 |
| WHtR | 0.56±0.06 | 0.54±0.05 | <0.0001 |
| WHpR | 0.86±0.07 | 0.85±0.14 | <0.0001 |
| SSFT, mm | 108.2±19.1 | 99.8±24.3 | <0.0001 |
| Glycemia | |||
| At 0 min, mmol/L | 5.24±0.7 | 5.04±0.4 | 0.019 |
| At 30 min, mmol/L | 7.79±1.5 | 7.73±1.4 | 0.201 |
| At 120 min, mmol/L | 5.73±1.2 | 5.67±1.2 | 0.017 |
| Insulin | |||
| At 0 min, mIU/L | 22.9±11.2 | 16.6±8.1 | <0.0001 |
| At 30 min, mIU/L | 148.2±85.2 | 117.8±72.0 | 0.002 |
| At 120 min, mIU/L | 84.8±72.1 | 67.6±56.5 | 0.026 |
| HOMA-IR | 5.31±2.9 | 3.78±1.9 | <0.0001 |
| QUICKI | 0.132±0.008 | 0.139±0.011 | <0.0001 |
| HOMA-B | 82.8±40.8 | 63.4±31.2 | <0.0001 |
| I/G_30 | 18.8±9.6 | 15.3±8.9 | <0.0001 |
| ISI | 49.1±22.3 | 69.4±39.9 | <0.0001 |
| ISIMatsuda | 2.71±1.2 | 3.85±2.2 | <0.0001 |
| ISICederholm | 33.0±10.4 | 34.9±11.5 | 0.012 |
| ISIGutt | 32.2±9.4 | 35.3±10.6 | 0.009 |
| TCh, mmol/L | 4.64±1.0 | 4.48±0.8 | <0.0001 |
| LDL, mmol/L | 2.95±0.8 | 2.77±0.8 | 0.001 |
| TG/HDL ratio | 1.85±1.2 | 0.85±0.7 | <0.0001 |
| LDL/HDL ratio | 3.05±1.0 | 2.52±1.7 | 0.020 |
Values are mean ± SD. MS, metabolic syndrome; BMI-SDS, body mass index standard deviation score; WC-SDS, waist circumference standard deviation score; WHtR, waist-to-height ratio; WHpR, waist-to-hip ratio; SSFT, sum of skin-fold thicknesses; HOMA-IR, homeostasis model assessment of insulin resistance; QUICKI, quantitative insulin sensitivity index; HOMA-B, homeostasis model assessment of β cell function; ISI, insulin sensitivity index; I/G_30, insulin/glucose at 30 min of OGTT;OGTT, oral glucose tolerance test; ISIMatsuda, Matsuda whole-body ISI; ISICederholm, Cederholm peripheral ISI; ISIGutt, Gutt ISI; TCh, total cholesterol; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; TG, triglycerides.
Evaluated according to IOTF standards [34].
Evaluated according to the Polish reference for WC [35].
Adjusted for BMI, pubertal stage, and height, systolic blood pressure was significantly higher in boys than in girls (121.6 ± 15.3 and 118.7 ± 14.6 mm Hg, respectively; p < 0.0001), but diastolic blood pressure was significantly higher in girls than in boys (74.0 ± 9.2 and 73.2 ± 8.6 mm Hg, respectively; p = 0.003). MS comorbidity analysis showed that 74.3% of individuals with MS had arterial hypertension, 45.7% had increased triglycerides, 25.7% had increased LDL, and 65.7% had decreased HDL. MS in OwOb children and adolescents significantly increased the risk for arterial hypertension (OR 5.64, 95% CI 3.11–10.25, p < 0.0001). Increased systolic or diastolic blood pressure was found in 60.0 and 41.4% of OwOb subjects with MS, respectively.
IR and Glucose Metabolism
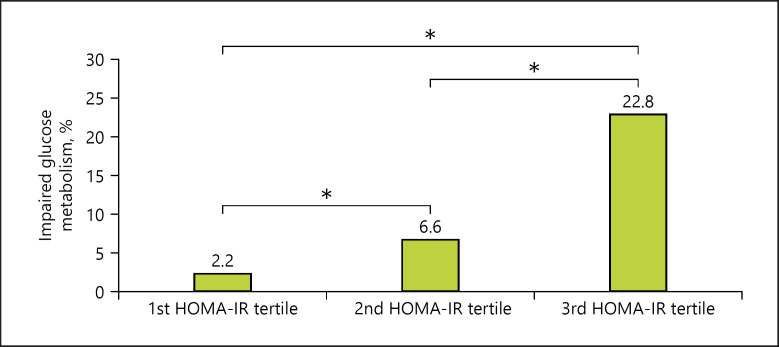
Analyses of IR revealed that children and adolescents in the third HOMA-IR tertile had a significantly higher prevalence of impaired glucose metabolism than those in the first and second HOMA-IR tertiles (Fig. 2).
Fig. 2.
Prevalence of impaired glucose metabolism in overweight / obese males and females with different HOMA-IR tertiles. * p < 0.001. HOMA-IR, homeostasis model assessment of insulin resistance. 1st HOMA-IR tertile: <2.96; 2nd HOMA-IR tertile: 2.96–4.46; 3rd HOMA-IR tertile: >4.46.
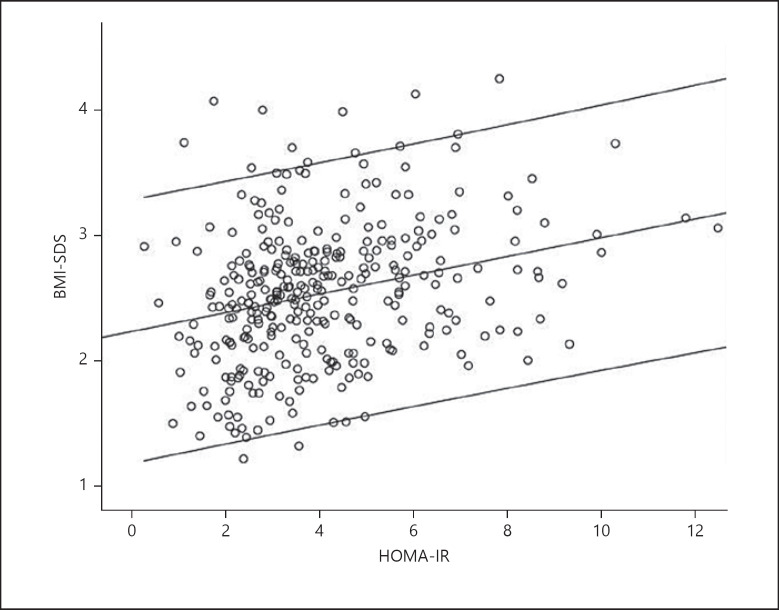
HOMA-IR was significantly directly related to BMI-SDS (Fig. 3), WC-SDS (r = 0.36, p < 0.001), sum of skin-fold thicknesses (r = 0.34, p < 0.001), and triglyceride levels (r = 0.21, p < 0.001).
Fig. 3.
HOMA-IR correlation with BMI-SDS in overweight / obese children and adolescents (mean, 95% confidence interval [CI]); r = 0.30, p < 0.0001.
Impaired glucose metabolism was detected in 12.1% of OwOb children and adolescents; 6.9% had IFG, 4.5% had IGT, and 0.6% were diagnosed as having T2D. IGT was more frequent in boys than in girls (7.1 vs. 2.7%, p < 0.05) but girls had a higher prevalence of IFG (8.2 vs. 5.0%, p < 0.05).
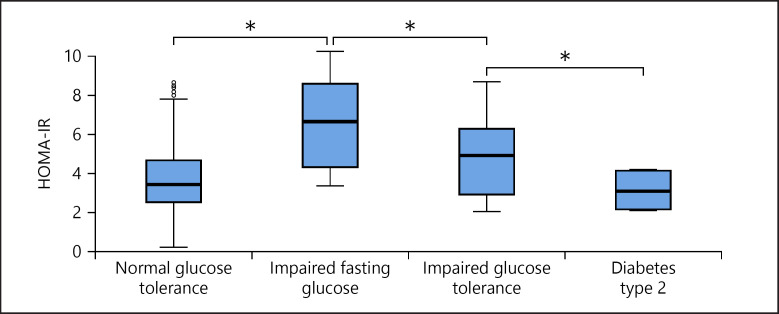
According to different glucose metabolism abnormalities, adjusting for age, gender, and pubertal stages, mean HOMA-IR was highest in children and adolescents with IFG and lowest in those with T2D (vs. subjects with normal glucose metabolism and IGT; p < 0.05 between all groups; Fig. 4).
Fig. 4.
HOMA-IR values according to glucose metabolism type in overweight / obese children and adolescents (mean, CI). * p < 0.05.
There was a significant direct correlation between insulin levels at all 3 time points of OGTT and sum of skin-fold thicknesses (r = 0.28, p < 0.001; r = 0.24, p < 0.001; and r = 0.16, p = 0.016, respectively).
Comparison of anthropometric parameters by the groups of HOMA-IR tertiles are presented in Table 3. Adjusting for BMI, pubertal stage, gender, and height, both systolic and diastolic blood pressure were significantly higher in children and adolescents with HOMA-IR in the third tertile than in those in the first tertile (Table 3).
Table 3.
Comparison of parameters between the overweight / obese children and adolescents from the first and third HOMA-IR tertiles
| 1st tertile | 3rd tertile | p value | |
|---|---|---|---|
| BMI-SDSa | 2.33±0.6 | 2.75±0.6 | <0.0001 |
| WC-SDSb | 1.68±0.5 | 2.11±0.4 | <0.0001 |
| WHpR | 0.85±0.07 | 0.88±0.2 | <0.0001 |
| WHtR | 0.53±0.05 | 0.56±0.05 | <0.0001 |
| SSFT, mm | 91.2±21.3 | 112.7±22.8 | <0.0001 |
| Glycemia | |||
| At 0 min, mmol/L | 4.92±0.3 | 5.28±0.5 | <0.0001 |
| At 30 min, mmol/L | 7.72±1.5 | 7.94±1.4 | 0.252 |
| At 120 min, mmol/L | 5.51±1.4 | 5.91±1.1 | <0.0001 |
| Insulin | |||
| At 0 min, mIU/L | 10.1±2.6 | 27.7±8.7 | <0.0001 |
| At 30 min, mIU/L | 84.2±46.5 | 165.3±89.0 | <0.0001 |
| At 120 min, mIU/L | 45.9±36.3 | 105.0±80.0 | <0.0001 |
| TCh, mmol/L | 4.52±0.8 | 4.57±0.9 | 0.001 |
| TG, mmol/L | 0.88±0.6 | 1.29±0.7 | <0.0001 |
| LDL, mmol/L | 2.77±0.8 | 2.88±0.8 | 0.002 |
| HDL, mmol/L | 1.21±0.3 | 1.14±0.3 | 0.012 |
| Systolic BP, mm Hgc | 116.8±13.4 | 120.8±16.4 | <0.0001 |
| Diastolic BP, mm Hgc | 72.3±8.7 | 75.5±9.5 | 0.002 |
Values are mean ± SD. BMI-SDS, body mass index standard deviation score; WC-SDS, waist circumference standard deviation score; WHtR, waist-to-height ratio; WHpR, waist-to-hip ratio; SSFT, sum of skinfold thicknesses; TCh, total cholesterol; TG, triglycerides; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; BP, blood pressure.
Evaluated according to IOTF standards [34].
Evaluated according to the Polish reference for WC [35].
Adjusted by height.
Comparing anthropometric measurements (BMI, BMI-SDS, WC, WC-SDS, sum of skin-fold thicknesses, systolic and diastolic blood pressure) and lipid profiles of the group with normal glucose metabolism and the group with impaired glucose metabolism did not reveal any significant differences.
Interestingly, BMI-SDS-adjusted glucose profile was similar in different pubertal stages in girls. Boys in pubertal stage 4 showed significantly higher fasting glucose than those in stages 1 and 2 (5.27 ± 0.5, 5.04 ± 0.3, and 4.99 ± 0.3, respectively; p = 0.034 between pubertal stages 1 and 4 and p = 0.021 between pubertal stages 2 and 4). The highest blood glucose at 2 h after glucose load was in the prepubertal boys, compared to in boys in pubertal stages 4 and 5 (6.09 ± 1.4, 5.73 ± 1.4, and 5.39 ± 1.15, respectively; p = 0.0011 between pubertal stages 1 and 4 and p = 0.013 between pubertal stages 1 and 5).
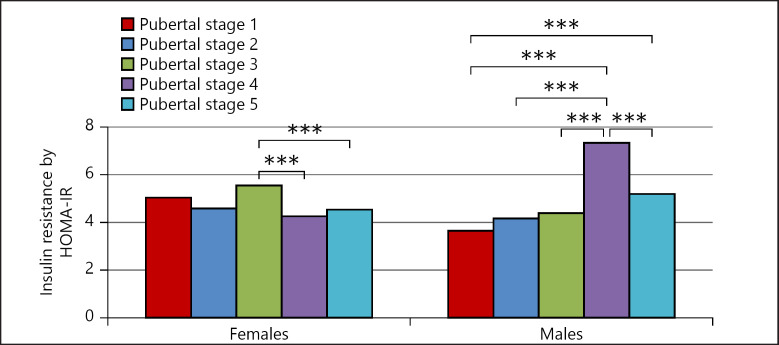
Comparing the IR by HOMA-IR between pubertal stages showed that IR in males was the highest in pubertal stage 4. Meanwhile, in females, IR decreased significantly during puberty progression from stage 3 to stages 4 and 5 (Fig. 5).
Fig. 5.
Insulin resistance according to pubertal stage and sex. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.005.
The OR for prediabetes (IFG and IGT) and impaired glucose metabolism in association with MS was 5.76 (95% CI 2.86–11.60, p < 0.0001) and 3.44 (95% CI 1.71–6.93, p < 0.0001), respectively. ROC analysis showed that HOMA-IR in association with MS for both genders had an area under the curve (AUC) of 0.675 (95% CI 0.62–0.76, p = 0.0001); HOMA-IR 3.12 had 82.4% sensitivity and 48.1% specificity. The OR for IR (HOMA-IR >3.16) in the presence of MS was 3.7 (95% CI 1.88–7.23, p = 0.0001).
Lipid Profile
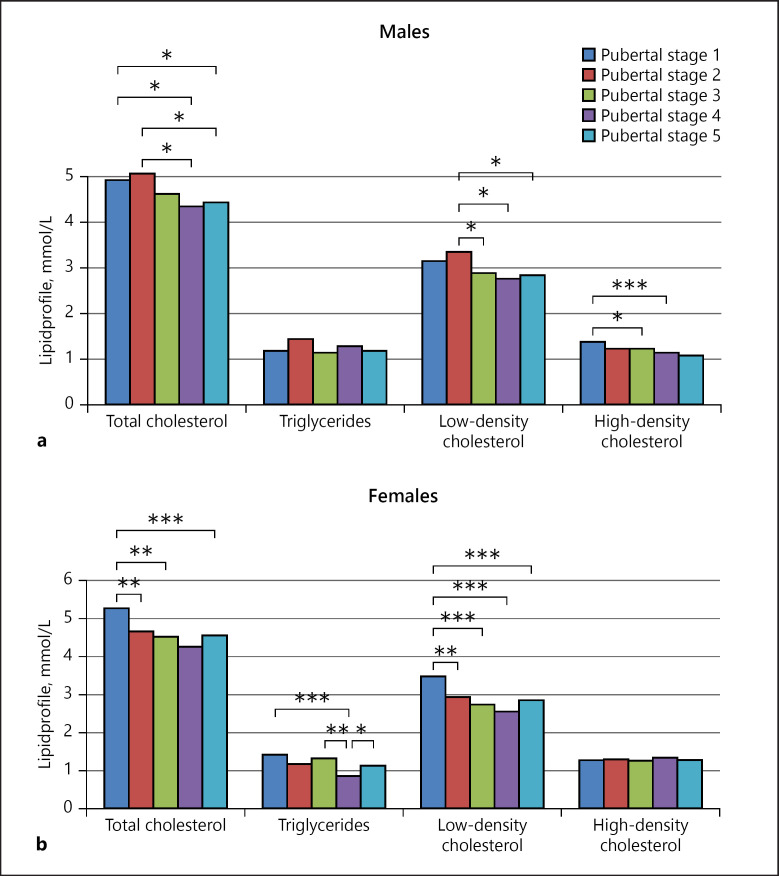
Decreased HDL and increased triglyceride, TCh, and LDL levels were found in 37.8, 13.4, 20.6, and 18.6% of the OwOb children and adolescents, respectively. Lipid profile analyses showed a significant decrease in TCh and LDL levels along with puberty progression in both genders (Fig. 6). TCh was highest during pubertal stages 1 and 2, and HDL was highest in prepubertal boys (Fig. 6a). Meanwhile, in girls, TCh, LDL, and triglycerides were significantly higher before puberty and decreased with advancing pubertal stages (Fig. 6b).
Fig. 6.
Lipid profile in overweight / obese subjects according to pubertal stage. a Lipid profile in males. * p < 0.05, ** p < 0.005. b Lipid profile in females. * p < 0.05, ** p < 0.005.
TCh and low LDL levels as well as triglycerides/HDL and LDL/HDL ratios were significantly higher in children with MS than in those without MS (Table 2). TCh, LDL, and triglyceride levels were significantly higher and HDL significantly lower in those in the third HOMA-IR tertile than in those in the first tertile (Table 3).
The OR for hypertriglyceridemia in association with MS was 16.77 (95% CI 8.56–32.85, p < 0.0001), and with decreased HDL 6.95 (95% CI 3.91–12.35, p < 0.0001), respectively.
Discussion
One-fifth of the OwOb children and adolescents in our study met the criteria for the presence of MS, which is associated with reduced insulin sensitivity, dyslipidemia, and a higher prevalence of impaired glucose metabolism. This is the first large-scale study in Lithuania to report on metabolic status in OwOb children and adolescents. To the best of our knowledge, it is also the first study analyzing glucose metabolism and lipid profile according to sex and pubertal stage.
MS Prevalence in OwOb Children and Adolescents
Dramatically increasing over the last 2 decades, the prevalence of obesity has now flattened out in developed countries [43]. The prevalence of Ow and obesity among children and adolescents in Lithuania is one of the lowest in Europe [44]. Data on the prevalence of MS depend on the criteria used and vary across countries. In general, in our study, the prevalence of MS in a pediatric OwOb population was similar to that reported by previous publications [8, 12, 13, 14, 24, 25, 45, 46, 47, 48]. However, Druet et al. [8] documented a lower prevalence of MS (15.9%) in Ob French children aged 9–13 years with a higher prevalence of obesity in France than Lithuania at the time [44].
A recent study by Jung et al. [49] reported that BMI has the best predictive power to identify MS and its components in adolescents. In our study, the OwOb boys had a higher prevalence of MS than the girls, in agreement with the data in Laurson et al. [50]. This might partly be explained by gender differences in the definition of MS between boys and girls for HDL cutoff values. Ervin [51] highlighted a higher prevalence of greater WC and low HDL in females, with a weaker association between BMI status and MS than in males, suggesting the importance of obesity but also other underlying genetic and environmental factors in the development of MS. Girls experience a greater increase in fat mass throughout childhood and puberty than boys, and this is even more pronounced in heavier (than in leaner) girls [52, 53]; this might, in part, explain the higher risk for developing MS in Ob females than in Ow females. Interestingly, in our study, morbid obesity (vs. Ow) in females does not increase the risk for MS. It has been suggested that adipose tissue has a limited maximal capacity to increase in mass and to store lipids. Adipocyte hyperplasia may present a mechanism for healthy fat storage capacity [54]. This adipose tissue expandability appears to be a more important determinant of obesity-associated metabolic problems than the absolute amount of adipose tissue. It is hypothesized that when a point of maximal expansion of adipose tissue is reached, metabolic complications occur due to the ectopic deposition of excess lipid in nonadipose organs, causing IR via lipid-induced toxicity [55]. Moreover, adipose tissue macrophage infiltration correlates directly with adipocyte size but not with a subject's weight [56]. The resulting changes appear to induce profound consequences for basal systemic inflammation and IS, which are the basal mechanisms underlying MS [57].
MS and IR
The IDF definition of MS does not include IR. However, some studies have highlighted the importance of IR as an independent risk factor that may contribute to the development of CVD [58]. However, data on IR and MS in several previous studies are conflicting [58, 59]. The most recent National Health and Nutrition Examination Survey (NHANES, 1999–2010) showed a low sensitivity of MS to identify adolescents with IGT [60]. On the other hand, according to the Adult Treatment Panel III (ATP-III) classification, individuals with IGT have significantly higher rates of MS [60]. Juárez-López et al. [2] reported that IR is associated with a higher prevalence of the components of MS.
We found a significantly higher insulin concentration, higher IR indices, and lower IS indices in children and adolescents with MS than in those without MS, not only at baseline but also at all time points of OGTT. Although within the normal range, the mean level of glucose at baseline and the glucose load after 2 h were also significantly higher in subjects with MS; only glycemia at 30 min of OGTT during the first insulin secretion phase did not reach significance. These findings may partially be influenced by puberty being a challenging period for glucose metabolism and IR. Pubertal IR is associated with lower insulin receptor sensibility or other cofactors, such as dyslipidemia, and upregulates compensatory insulin secretion to be able to keep the glucose concentration at the same peak level at 30 min of OGTT. Moreover, in the presence of Ow or obesity, pubertal IR may contribute to the eventual development of MS. Similar findings were reported in a Finnish pediatric study where fasting insulin levels were found to be higher in the children who later developed MS [61]. Similarly, in Druet et al. [8], it was found that the likelihood of MS increased strongly along with IR.
In our study, children with MS were not only more insulin-resistant, they also had a 3-fold higher prevalence of impaired glucose metabolism than those without MS. Although this association is partly a consequence of the definition of MS itself, as MS involves IFG and T2D, at the same time, it points to a significant link between MS and IR.
IR and Puberty
Individuals in the third HOMA-IR tertile were found to have significantly higher insulin levels at all time points of OGTT, and the glucose concentration was significantly higher at the baseline and 2 h after glucose load. This may reflect a compensatory secretion of insulin due to increasing IR to maintain normal glycemia at 30 min of OGTT. Assuming that the compensatory scope is insufficient, this may explain the higher frequency of impaired glucose metabolism in the higher HOMA-IR tertiles. We also found a different glucose profile in girls and in boys at different pubertal stages, which may be related to the differences in IS caused by the different levels of gender-related sex hormones [62].
It is worth noting that the prevalence of IGT was higher in female children and adolescents, but the prevalence of IFG was higher in males. George et al. [63] also reported a higher prevalence of IGT in Ob females than in Ob males [63]. This strengthens the previous finding that girls are more insulin-resistant than boys in all pubertal stages [64].
IR and Obesity Levels
Kurtoğlu et al. [65] did not find any significant difference in BMI between the groups with and without IR. In contrast, our study results showed a direct relationship between weight status and IR, with BMI-SDS, WC-SDS, and sum of skin-fold thicknesses scores significantly higher in the children in the third HOMA-IR tertile, i.e., showing a considerable influence of fat accumulation on the development of IR. Moreover, Pastucha et al. [12] in their study found a relatively high incidence of IR in Ob children, and raised the question of whether the existing diagnostic criteria do not falsely exclude some cases of MS.
MS, Puberty, and the Lipid Profile
OwOb children are prone to have dyslipidemia and MS [66]. Compared to the study by Mellerio et al. [67] that involved adolescents with normal weight in whom triglycerides levels increased with age and other lipid fractions showed modest changes during puberty, in our study, TCh in both genders and LDL in girls only was significantly higher in prepubertal children and decreased with the progression of puberty. These results are similar to the study of Pinhas-Hamiel et al. [68], who found lower TCh and LDL levels in Ob adolescents at later stages of puberty, possibly related to the increased secretion of sex steroids and pubertal spurt.
However, TCh and LDL levels were significantly higher in children with MS than in those without MS. Moreover, increased triglyceride and decreased HDL levels were associated with MS. The significantly higher LDL/HDL ratio in children with MS than in non-MS individuals is similar to the findings of Burns et al. [69], suggesting that this ratio might be a good predictive marker for MS and IR in young people. It could also play a role in the early detection of risk factors for CVD as LDL-cholesterol is known to have a strong association with coronary artery calcification in adults [70].
Similar to the data published by Vukovic et al. [71], lipid levels were found to be significantly higher in the most insulin-resistant children and adolescents.
This study has some limitations. It was a cross-sectional study that included Ow and Ob children and adolescents, but no comparison with normal-weight children was made. Longitudinal follow-up is necessary to establish the cutoffs for risk factors, such as IR, during different stages of puberty, to correct the diagnostic criteria for MS in children and adolescents, i.e., IFG and T2D, but also IR, and to evaluate the associations between risk factors and metabolic consequences.
Conclusions
In summary, our findings support a strong association between MS and IR. We found MS in one-fifth of the OwOb children and adolescents, i.e., those who were more Ob and less sensitive to insulin. Impaired glucose metabolism was found in 12.1% of OwOb children and adolescents, with IGT more frequently in boys and IFG in girls. Children with IFG were the most insulin-resistant.
Every sixth OwOb child or adolescent (aged 10–17 years) had lipid metabolism abnormalities. Our results confirm that Ow/obesity-related early metabolic disturbances and risk factors for CVD and T2D were found in children and adolescents, and these may be predictive of metabolic status in adulthood. Therefore, OwOb children should be followed up for early metabolic derangements from childhood onwards.
Statement of Ethics
All children and their parents signed an informed consent form approved by the Lithuanian Bioethics Committee (BE-2-1, 2009-01-22; P-13-24, 2013-03-22). Study also registered as EudraCT No. 2011-006352-36.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The research was supported by Research Council of Lithuania grant No. MIP-039/2013, Research Foundation for Lithuanian University of Health Sciences, and Swedish governmental university hospital grants (ALF).
Author Contributions
N.S. contributed to the study design, clinical evaluation of the cohort, and the acquisition, analysis, and interpretation of data; performed statistical analyses; and drafted the manuscript. R.Val. carried out the radiology and clinical evaluation. A.V. carried out the laboratory tests, was involved in data analysis and interpretation. K.A.W. contributed to the study design, data analysis, and interpretation of results. R.Ver. designed, initiated, and coordinated the study, and also helped to draft the manuscript. All authors made an intellectual contribution to the manuscript, revised it critically, and approved the final version for submission.
Acknowledgment
The authors are grateful to the team of Pediatric endocrinology, especially nurses, from Department of Endocrinology, Hospital of Lithuanian University of Health Science for project assistance; to Jurgita Grigiene from International relations and study center, Lithuanian University of Health Science, for English editing, and to all participants and their parents for attendance to the study.
References
- 1.Gurka MJ, Ice CL, Sun SS, Deboer MD. A confirmatory factor analysis of the metabolic syndrome in adolescents: an examination of sex and racial/ethnic differences. Cardiovasc Diabetol. 2012 Oct;11((1)):128. doi: 10.1186/1475-2840-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juárez-López C, Klünder-Klünder M, Medina-Bravo P, Madrigal-Azcárate A, Mass-Díaz E, Flores-Huerta S. Insulin resistance and its association with the components of the metabolic syndrome among obese children and adolescents. BMC Public Health. 2010 Jun;10((1)):318. doi: 10.1186/1471-2458-10-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011 May;9((1)):48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, et al. IDF Consensus Group The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes. 2007 Oct;8((5)):299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd LJ, Langley-Evans SC, McMullen S. Childhood obesity and risk of the adult metabolic syndrome: a systematic review. Int J Obes. 2012 Jan;36((1)):1–11. doi: 10.1038/ijo.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tavares Giannini D, Caetano Kuschnir MC, Szklo M. Metabolic syndrome in overweight and obese adolescents: a comparison of two different diagnostic criteria. Ann Nutr Metab. 2014;64((1)):71–9. doi: 10.1159/000362568. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Yin J, Xu L, Cheng H, Zhao X, Xiang H, et al. Prevalence of metabolic syndrome in a cohort of Chinese schoolchildren: comparison of two definitions and assessment of adipokines as components by factor analysis. BMC Public Health. 2013 Mar;13((1)):249. doi: 10.1186/1471-2458-13-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Druet C, Dabbas M, Baltakse V, Payen C, Jouret B, Baud C, et al. Insulin resistance and the metabolic syndrome in obese French children. Clin Endocrinol (Oxf) 2006 Jun;64((6)):672–8. doi: 10.1111/j.1365-2265.2006.02526.x. [DOI] [PubMed] [Google Scholar]
- 9.Liu W, Lin R, Liu A, Du L, Chen Q. Prevalence and association between obesity and metabolic syndrome among Chinese elementary school children: a school-based survey. BMC Public Health. 2010 Dec;10((1)):780. doi: 10.1186/1471-2458-10-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim H, Xue H, Wang Y. Association between obesity and metabolic co-morbidities among children and adolescents in South Korea based on national data. BMC Public Health. 2014 Mar;14((1)):279. doi: 10.1186/1471-2458-14-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haroun D, Mechli R, Sahuri R, AlKhatib S, Obeid O, El Mallah C, et al. Metabolic syndrome among adolescents in Dubai, United Arab Emirates, is attributable to the high prevalence of low HDL levels: a cross-sectional study. BMC Public Health. 2018 Nov;18((1)):1284. doi: 10.1186/s12889-018-6215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pastucha D, Filipcikova R, Horakova D, Radova L, Marinov Z, Malincikova J, Kocvrlich M, Horak S, Bezdickova M, Dobias M. The incidence of metabolic syndrome in obese Czech children: the importance of early detection of insulin resistance using homeostatic indexes HOMA-IR and QUICKI. Physiological research / Academia Scientiarum Bohemoslovaca. 2013;62((3)):277–283. doi: 10.33549/physiolres.932438. [DOI] [PubMed] [Google Scholar]
- 13.Lafortuna CL, Adorni F, Agosti F, De Col A, Sievert K, Siegfried W, et al. Prevalence of the metabolic syndrome among extremely obese adolescents in Italy and Germany. Diabetes Res Clin Pract. 2010 Apr;88((1)):14–21. doi: 10.1016/j.diabres.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999-2002. J Pediatr. 2008 Feb;152((2)):165–70. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Santoro N, Amato A, Grandone A, Brienza C, Savarese P, Tartaglione N, et al. Predicting metabolic syndrome in obese children and adolescents: look, measure and ask. Obes Facts. 2013;6((1)):48–56. doi: 10.1159/000348625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atabek ME, Selver Eklioglu B, Akyürek N. Which metabolic syndrome criteria best predict non-alcoholic fatty liver disease in children? Eat Weight Disord. 2014 Dec;19((4)):495–501. doi: 10.1007/s40519-014-0129-0. [DOI] [PubMed] [Google Scholar]
- 17.Vukovic R, Zdravkovic D, Mitrovic K, Milenkovic T, Todorovic S, Vukovic A, et al. Metabolic syndrome in obese children and adolescents in Serbia: prevalence and risk factors. J Pediatr Endocrinol Metab. 2015 Jul;28((7-8)):903–9. doi: 10.1515/jpem-2014-0533. [DOI] [PubMed] [Google Scholar]
- 18.Sewaybricker LE, Antonio MA, Mendes RT, Barros Filho Ade A, Zambon MP. Metabolic syndrome in obese adolescents: what is enough? Rev Assoc Med Bras (1992) 2013;59((1)):64–71. doi: 10.1590/s0104-42302013000100013. [DOI] [PubMed] [Google Scholar]
- 19.Rank M, Siegrist M, Wilks DC, Langhof H, Wolfarth B, Haller B, et al. The cardio-metabolic risk of moderate and severe obesity in children and adolescents. J Pediatr. 2013 Jul;163((1)):137–42. doi: 10.1016/j.jpeds.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 20.İnanç BB. Metabolic syndrome in school children. J Clin Res Pediatr Endocrinol. 2013;5((2)):140–1. doi: 10.4274/Jcrpe.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makkes S, Renders CM, Bosmans JE, van der Baan-Slootweg OH, Seidell JC. Cardiometabolic risk factors and quality of life in severely obese children and adolescents in The Netherlands. BMC Pediatr. 2013 Apr;13((1)):62. doi: 10.1186/1471-2431-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park J, Hilmers DC, Mendoza JA, Stuff JE, Liu Y, Nicklas TA. Prevalence of metabolic syndrome and obesity in adolescents aged 12 to 19 years: comparison between the United States and Korea. J Korean Med Sci. 2010 Jan;25((1)):75–82. doi: 10.3346/jkms.2010.25.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurtoglu S, Akin L, Kendirci M, Hatipoglu N, Elmali F, Mazicioglu M. The absence of insulin resistance in metabolic syndrome definition leads to underdiagnosing of metabolic risk in obese patients. Eur J Pediatr. 2012 Sep;171((9)):1331–7. doi: 10.1007/s00431-012-1724-6. [DOI] [PubMed] [Google Scholar]
- 24.Chedjou-Nono E, Sap S, Choukem SP, Ngosso Tetanye I, Nebongo D, Koki Ndombo O. Cardiometabolic profile of obese children in a sub-Saharan African setting: a cross-sectional study. BMC Pediatr. 2017 May;17((1)):129. doi: 10.1186/s12887-017-0880-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masquio DC, de Piano-Ganen A, Oyama LM, Campos RM, Santamarina AB, de Souza GI, et al. The role of free fatty acids in the inflammatory and cardiometabolic profile in adolescents with metabolic syndrome engaged in interdisciplinary therapy. J Nutr Biochem. 2016 Jul;33:136–44. doi: 10.1016/j.jnutbio.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Wilmot EG, Edwardson CL, Biddle SJ, Gorely T, Henson J, Khunti K, et al. Prevalence of diabetes and impaired glucose metabolism in younger ‘at risk’ UK adults: insights from the STAND programme of research. Diabet Med. 2013 Jun;30((6)):671–5. doi: 10.1111/dme.12173. [DOI] [PubMed] [Google Scholar]
- 27.Alattar A, Al-Majed H, Almuaili T, Almutairi O, Shaghouli A, Altorah W. Prevalence of impaired glucose regulation in asymptomatic Kuwaiti young adults. Medical principles and practice : international journal of the Kuwait University. Health Science Centre. 2012;21((1)):51–5. doi: 10.1159/000330024. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Capape M, Alonso M, Colino E, Mustieles C, Corbaton J, Barrio R. Frequency of the metabolic syndrome in obese Spanish pediatric population. European journal of endocrinology / European Federation of Endocrine Societies. 2006;155((2)):313–319. doi: 10.1530/eje.1.02206. [DOI] [PubMed] [Google Scholar]
- 29.Körner A, Wiegand S, Hungele A, Tuschy S, Otto KP, l'Allemand-Jander D, et al. APV initiative. German Competence Net Obesity Longitudinal multicenter analysis on the course of glucose metabolism in obese children. Int J Obes. 2013 Jul;37((7)):931–6. doi: 10.1038/ijo.2012.163. [DOI] [PubMed] [Google Scholar]
- 30.Luksiene DI, Baceviciene M, Tamosiūnas A, Cerniauskiene LR, Margeviciene L, Reklaitiene R. Prevalence of the metabolic syndrome diagnosed using three different definitions and risk of ischemic heart disease among Kaunas adult population. Medicina (Kaunas) 2010;46((1)):61–9. [PubMed] [Google Scholar]
- 31.Smetanina N, Albaviciute E, Babinska V, Karinauskiene L, Albertsson-Wikland K, Petrauskiene A, et al. Prevalence of overweight/obesity in relation to dietary habits and lifestyle among 7-17 years old children and adolescents in Lithuania. BMC Public Health. 2015 Oct;15((1)):1001. doi: 10.1186/s12889-015-2340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. SUBCOMMITTEE ON SCREENING AND MANAGEMENT OF HIGH BLOOD PRESSURE IN CHILDREN Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017 Sep;140((3)):e20171904. doi: 10.1542/peds.2017-1904. [DOI] [PubMed] [Google Scholar]
- 33.Emmanuel M, Bokor BR. In: StatPearls: Treasure Island (FL); 2019. Tanner Stages. [PubMed] [Google Scholar]
- 34.Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. 2012 Aug;7((4)):284–94. doi: 10.1111/j.2047-6310.2012.00064.x. [DOI] [PubMed] [Google Scholar]
- 35.Nawarycz LO, Krzyzaniak A, Stawińska-Witoszyńska B, Krzywińska-Wiewiorowska M, Szilagyi-Pagowska I, Kowalska M, et al. Percentile distributions of waist circumference for 7-19-year-old Polish children and adolescents. Obes Rev. 2010 Apr;11((4)):281–8. doi: 10.1111/j.1467-789X.2009.00694.x. [DOI] [PubMed] [Google Scholar]
- 36.Craig ME, Hattersley A, Donaghue K, International Society for Pediatric and Adolescent Diabetes ISPAD Clinical Practice Consensus Guidelines 2006-2007. Definition, epidemiology and classification. Pediatr Diabetes. 2006 Dec;7((6)):343–51. doi: 10.1111/j.1399-5448.2006.00216.x. [DOI] [PubMed] [Google Scholar]
- 37.Soonthornpun S, Setasuban W, Thamprasit A, Chayanunnukul W, Rattarasarn C, Geater A. Novel insulin sensitivity index derived from oral glucose tolerance test. J Clin Endocrinol Metab. 2003 Mar;88((3)):1019–23. doi: 10.1210/jc.2002-021127. [DOI] [PubMed] [Google Scholar]
- 38.Singh B, Saxena A. Surrogate markers of insulin resistance: A review. World J Diabetes. 2010 May;1((2)):36–47. doi: 10.4239/wjd.v1.i2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radikova Z. Assessment of insulin sensitivity/resistance in epidemiological studies. Endocr Regul. 2003 Sep;37((3)):189–94. [PubMed] [Google Scholar]
- 40.Lorenzo C, Haffner SM, Stančáková A, Kuusisto J, Laakso M. Fasting and OGTT-derived measures of insulin resistance as compared with the euglycemic-hyperinsulinemic clamp in nondiabetic Finnish offspring of type 2 diabetic individuals. J Clin Endocrinol Metab. 2015 Feb;100((2)):544–50. doi: 10.1210/jc.2014-2299. [DOI] [PubMed] [Google Scholar]
- 41.Gutt M, Davis CL, Spitzer SB, Llabre MM, Kumar M, Czarnecki EM, et al. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res Clin Pract. 2000 Mar;47((3)):177–84. doi: 10.1016/s0168-8227(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 42.Sarah de Ferranti JWN. Definition and screening for dyslipidaemia in children. UpToDate. 2013 [Google Scholar]
- 43.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014 Aug;384((9945)):766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janssen I, Katzmarzyk PT, Boyce WF, Vereecken C, Mulvihill C, Roberts C, et al. Health Behaviour in School-Aged Children Obesity Working Group Comparison of overweight and obesity prevalence in school-aged youth from 34 countries and their relationships with physical activity and dietary patterns. Obes Rev. 2005 May;6((2)):123–32. doi: 10.1111/j.1467-789X.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 45.Sangun Ö, Dündar B, Köşker M, Pirgon Ö, Dündar N. Prevalence of metabolic syndrome in obese children and adolescents using three different criteria and evaluation of risk factors. J Clin Res Pediatr Endocrinol. 2011;3((2)):70–6. doi: 10.4274/jcrpe.v3i2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rizzo AC, Goldberg TB, Silva CC, Kurokawa CS, Nunes HR, Corrente JE. Metabolic syndrome risk factors in overweight, obese, and extremely obese Brazilian adolescents. Nutr J. 2013 Jan;12((1)):19. doi: 10.1186/1475-2891-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jamoussi H, Mahjoub F, Sallemi H, Berriche O, Ounaissa K, Amrouche C, et al. Metabolic syndrome in Tunisian obese children and adolescents. Tunis Med. 2012 Jan;90((1)):36–40. [PubMed] [Google Scholar]
- 48.Bustos P, Saez K, Gleisner A, Ulloa N, Calvo C, Asenjo S. Metabolic syndrome in obese adolescents. Pediatr Diabetes. 2010 Feb;11((1)):55–60. doi: 10.1111/j.1399-5448.2009.00531.x. [DOI] [PubMed] [Google Scholar]
- 49.Jung C, Fischer N, Fritzenwanger M, Figulla HR. Anthropometric indices as predictors of the metabolic syndrome and its components in adolescents. Pediatr Int. 2010 Jun;52((3)):402–9. doi: 10.1111/j.1442-200X.2009.02973.x. [DOI] [PubMed] [Google Scholar]
- 50.Laurson KR, Welk GJ, Eisenmann JC. Diagnostic performance of BMI percentiles to identify adolescents with metabolic syndrome. Pediatrics. 2014 Feb;133((2)):e330–8. doi: 10.1542/peds.2013-1308. [DOI] [PubMed] [Google Scholar]
- 51.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: united States, 2003-2006. Natl Health Stat Report. 2009 May;((13)):1–7. [PubMed] [Google Scholar]
- 52.Biro FM, Huang B, Morrison JA, Horn PS, Daniels SR. Body mass index and waist-to-height changes during teen years in girls are influenced by childhood body mass index. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2010;46((3)):245–250. doi: 10.1016/j.jadohealth.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Demerath EW, Schubert CM, Maynard LM, Sun SS, Chumlea WC, Pickoff A, et al. Do changes in body mass index percentile reflect changes in body composition in children? Data from the Fels Longitudinal Study. Pediatrics. 2006 Mar;117((3)):e487–95. doi: 10.1542/peds.2005-0572. [DOI] [PubMed] [Google Scholar]
- 54.Zeve D, Tang W, Graff J. Fighting fat with fat: the expanding field of adipose stem cells. Cell Stem Cell. 2009 Nov;5((5)):472–81. doi: 10.1016/j.stem.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Virtue S, Vidal-Puig A. It's not how fat you are, it's what you do with it that counts. PLoS Biol. 2008 Sep;6((9)):e237. doi: 10.1371/journal.pbio.0060237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smorlesi A, Frontini A, Giordano A, Cinti S. The adipose organ: white-brown adipocyte plasticity and metabolic inflammation. Obes Rev. 2012 Dec;13(Suppl 2):83–96. doi: 10.1111/j.1467-789X.2012.01039.x. [DOI] [PubMed] [Google Scholar]
- 57.Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013 Dec;2013:139239. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurtoglu S, Akin L, Kendirci M, Hatipoglu N, Elmali F, Mazicioglu M. The absence of insulin resistance in metabolic syndrome definition leads to underdiagnosing of metabolic risk in obese patients. Eur J Pediatr. 2012 Sep;171((9)):1331–7. doi: 10.1007/s00431-012-1724-6. [DOI] [PubMed] [Google Scholar]
- 59.Yin J, Li M, Xu L, Wang Y, Cheng H, Zhao X, et al. Insulin resistance determined by Homeostasis Model Assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. Diabetol Metab Syndr. 2013 Nov;5((1)):71. doi: 10.1186/1758-5996-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeBoer MD, Gurka MJ. Low sensitivity of the metabolic syndrome to identify adolescents with impaired glucose tolerance: an analysis of NHANES 1999-2010. Cardiovasc Diabetol. 2014 Apr;13((1)):83. doi: 10.1186/1475-2840-13-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raitakari OT, Porkka KV, Rönnemaa T, Knip M, Uhari M, Akerblom HK, et al. The role of insulin in clustering of serum lipids and blood pressure in children and adolescents. The Cardiovascular Risk in Young Finns Study. Diabetologia. 1995 Sep;38((9)):1042–50. doi: 10.1007/BF00402173. [DOI] [PubMed] [Google Scholar]
- 62.Moran A, Jacobs DR, Jr, Steinberger J, Hong CP, Prineas R, Luepker R, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999 Oct;48((10)):2039–44. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 63.George L, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Surrogate estimates of insulin sensitivity in obese youth along the spectrum of glucose tolerance from normal to prediabetes to diabetes. J Clin Endocrinol Metab. 2011 Jul;96((7)):2136–45. doi: 10.1210/jc.2010-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Aa MP, Fazeli Farsani S, Knibbe CA, de Boer A, van der Vorst MM. Population-Based Studies on the Epidemiology of Insulin Resistance in Children. J Diabetes Res. 2015;2015:362375. doi: 10.1155/2015/362375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kurtoğlu S, Hatipoğlu N, Mazıcıoğlu M, Kendirici M, Keskin M, Kondolot M. Insulin resistance in obese children and adolescents: HOMA-IR cut-off levels in the prepubertal and pubertal periods. J Clin Res Pediatr Endocrinol. 2010;2((3)):100–6. doi: 10.4274/jcrpe.v2i3.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casavalle PL, Lifshitz F, Romano LS, Pandolfo M, Caamaño A, Boyer PM, et al. Prevalence of dyslipidemia and metabolic syndrome risk factor in overweight and obese children. Pediatr Endocrinol Rev. 2014 Dec;12((2)):213–23. [PubMed] [Google Scholar]
- 67.Mellerio H, Alberti C, Druet C, Capelier F, Mercat I, Josserand E, et al. Novel modeling of reference values of cardiovascular risk factors in children aged 7 to 20 years. Pediatrics. 2012 Apr;129((4)):e1020–9. doi: 10.1542/peds.2011-0449. [DOI] [PubMed] [Google Scholar]
- 68.Pinhas-Hamiel O, Lerner-Geva L, Copperman NM, Jacobson MS. Lipid and insulin levels in obese children: changes with age and puberty. Obesity (Silver Spring) 2007 Nov;15((11)):2825–31. doi: 10.1038/oby.2007.335. [DOI] [PubMed] [Google Scholar]
- 69.Burns SF, Lee SJ, Arslanian SA. Surrogate lipid markers for small dense low-density lipoprotein particles in overweight youth. J Pediatr. 2012 Dec;161((6)):991–6. doi: 10.1016/j.jpeds.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hannon TS, Bacha F, Lee SJ, Janosky J, Arslanian SA. Use of markers of dyslipidemia to identify overweight youth with insulin resistance. Pediatr Diabetes. 2006 Oct;7((5)):260–6. doi: 10.1111/j.1399-5448.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 71.Vukovic R, Mitrovic K, Milenkovic T, Todorovic S, Soldatovic I, Sipetic-Grujicic S, et al. Insulin-sensitive obese children display a favorable metabolic profile. Eur J Pediatr. 2013 Feb;172((2)):201–6. doi: 10.1007/s00431-012-1867-5. [DOI] [PubMed] [Google Scholar]