Abstract
As vectors of disease, mosquitoes are a global threat to human health. The Anopheles mosquito is the deadliest mosquito species as the insect vector of the malaria-causing parasite, which kills hundreds of thousands every year. These mosquitoes are reliant on their sense of smell (olfaction) to guide most of their behaviors, and a better understanding of Anopheles olfaction identifies opportunities for reducing the spread of malaria. This review takes a detailed look at Anopheles olfaction. We explore a range of topics from chemosensory receptors, olfactory neurons, and sensory appendages to behaviors guided by olfaction (including host-seeking, foraging, oviposition, and mating), to vector management strategies that target mosquito olfaction. We identify many research areas that remain to be addressed.
Keywords: chemosensory receptors, host-seeking, malaria, olfactory neurons, sensory appendages, vector management
Introduction
There are ~3600 recognized species of mosquito (Diptera: Culicidae) belonging to 3 subfamilies: Anophelinae, Culicinae, and Toxorynchtinae (Harbach 2007, 2013) found on all continents except Antarctica. Despite popular impressions, not all mosquitoes are hematophagous (i.e., feed on blood). For example, adult Toxorynchtinae mosquitoes, which are the largest of all mosquitoes, feed only on nectar. Adult females of some Anophelinae (Anopheles) and Culicinae (e.g., Aedes and Culex) mosquitoes however do require a blood meal to complete egg development. Hematophagy in arthropods evolved independently multiple times from pre-existing associations with vertebrates or plants (Grimaldi and Engel 2005; Lehane 2005). What probably started as accidental feeding on vertebrates, over time led to morphological (e.g., mouthparts), physiological (e.g., salivary secretions, blood digestions), and behavioral (e.g., host preferences) adaptations to feed on blood (Graça-Souza et al. 2006; Mans 2011; Krenn and Aspöck 2012; Arcà and Ribeiro 2018).
A mosquito blood meal can come from a variety of vertebrate (e.g., birds, frogs, mammals, snakes) and occasionally invertebrate (e.g., earthworms, leaches) hosts (Clements 1999; Reeves et al. 2018; Wolff and Riffell 2018). Although some degree of plasticity in host preference exists, females of each blood-feeding mosquito species usually specialize on one host type. For some mosquitoes, the preferred hosts are humans. This requirement for blood and the close association with and preference for human hosts makes these mosquitoes capable of vectoring disease-causing pathogens. Several viruses and parasites, which affect millions of people globally each year, are vectored specifically by mosquitoes. For example, Culex mosquitoes vector West Nile virus, whereas Aedes mosquitoes vector viruses, which cause chikungunya, dengue fever, yellow fever, and Zika (Farajollahi et al. 2011; Lee et al. 2018; Weaver et al. 2018; Huang et al. 2019).
The deadliest of all anthropophilic (human preferring) mosquitoes are those of the genus Anopheles, capable of vectoring the malaria-causing Plasmodium parasite, which kills over 400 000 people each year (WHO 2019). Although different species of Anopheles might opportunistically feed on human and nonhuman hosts (Dekker et al. 2002; Bakker et al. 2020), malaria vectors such as Anopheles gambiae are predominantly anthropophilic and are highly attracted to and specialized to blood-feed on humans (Braks et al. 1997; Costantini et al. 1998; Pates et al. 2001). Species of Anopheles mosquitoes are found on every continent (except Antarctica), in over 100 countries. Their global distribution is limited by temperature, with declining survival below 15°C and above 35°C (Lyons et al. 2012, 2013).
Blood-seeking female mosquitoes must detect and interpret information about their environment, and thus rely on different sensory modalities and a finely tuned sensory system. Olfaction is an important sensory modality for adult female mosquitoes as it is involved in foraging, host-searching, and oviposition site selection. Most importantly, it is primarily through olfaction that female mosquitoes locate and recognize a specific host for blood-feeding, and potentially transmit diseases. Therefore, targeting mosquito olfaction can reduce the number of infectious bites and presents opportunities for effective interventions.
Substantial advances have been made over the past decade in understanding the mosquito olfactory system. Olfactory cues are detected by receptors on sensory appendages, which activate olfactory sensory neurons (OSNs) to carry the signals to the brain. Interpretation of those signals by the brain leads to olfactory guided behaviors, including host-seeking. Although it is often assumed that all mosquitoes rely on the same host-seeking mechanism (i.e., the molecular basis underlying these behaviors), there are probably marked differences among major subfamilies, which remain unexplored. Research on Anopheles mosquitoes has traditionally focused on vector competence and control to address the public health challenges associated with malaria transmission and eradication (Ferguson 2018; Greenwood et al. 2008). Combining current control efforts with a deeper understanding of vector biology, behavior, and the mechanisms underlying them has the potential to guide novel interventions (Shaw and Catteruccia 2019).
In this review, we synthesize the current state of knowledge and discuss recent advances in our understanding of olfaction in Anopheles mosquitoes from signal perception and processing of olfactory cues at the neuronal level to elicited behaviors at the organismal level. First, we describe the olfactory system in Anopheles mosquitoes: olfactory receptors, peripheral olfactory appendages, and higher olfactory centers (Figures 1 and 2; Table 1). Then, we discuss the role of olfaction in adults focusing on females, from foraging and mating, to host-searching, blood-feeding, and oviposition (Figure 3). Finally, we outline the role mosquito olfaction plays in malaria transmission (Figure 4) and highlight integrated vector management strategies involving olfaction. Our goal is to bring attention to recent developments and discoveries and highlight promising avenues for future work in Anopheles olfaction.
Figure 1.
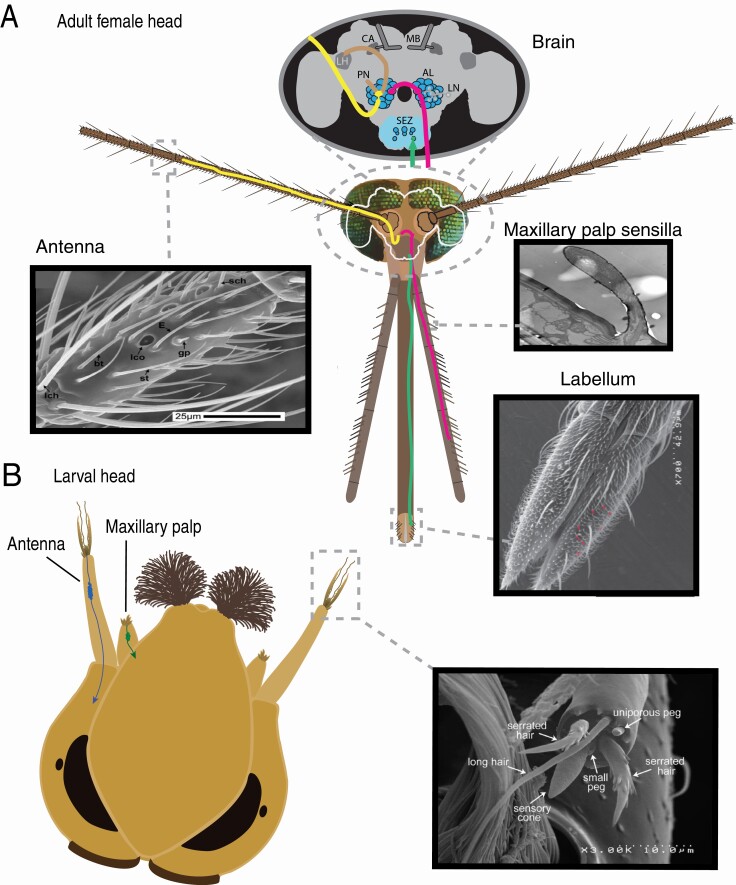
Organization of olfactory system in Anopheles mosquitoes. (A) The peripheral olfactory system in mosquitoes is composed of 3 sensory appendages/organs: a pair of antennae, a pair of maxillary palps, and one labella at the tip of the proboscis. Each of the sensory appendages is covered in specialized types of sensilla, which allow for detection of odors and lead to neuronal activation (see Figure 2 and text for more details). Olfactory neurons that originate in one of the sensory appendages (indicated by yellow, pink, and green lines in the mosquito head cartoon) innervate one of the 2 brain regions: the antennal lobes (AL) (from the antennae and the maxillary palps) and the subesophageal zone (SEZ) (from the labella). Olfactory local interneurons (LNs) shape the olfactory signal in the glomeruli of the antennal lobe that is transmitted to the olfactory projection neurons (PNs), which in turn project to higher olfactory centers: LH (lateral horn), CA (calyx) of the MB (mushroom bodies). The MB calyx is a dense dendritic region that collects olfactory inputs into an association (learning/memory) brain center and allows the insect to associate (remember) odors that coincide with pleasant or aversive stimuli. The LH is involved in influencing innate olfactory behaviors. The function of the neurons in the Anopheles lateral horn have yet to be investigated, but they probably help to organize olfactory information into biological meanings (such as foraging odors or oviposition odors) (Dolan et al. 2019; Jeanne et al. 2018; Jefferis et al. 2007). Scanning electron microscopy (SEM) images reprinted with permission: antenna (Pitts and Zwiebel 2006), maxillary palp (Lu et al. 2007), and labella (Saveer et al. 2018). Sensilla shown in antennal SEM image: bt, blunt trichoid, E, similar to type E trichoid, gp, grooved peg, lch, large cheatica, lco, large coeloconic, sch, small cheatica, st, sharp trichoid. (B) Anopheles larvae use antennae and maxillary palps for olfaction. The sensory cone of the larval antenna is innervated by the dendrites of olfactory neurons. This sensory organ has a finely ridged porous surface with vacuoles at the basal region. These vacuoles are probably used for detection of soluble chemicals. When the antenna is stimulated with odorants, the molecules may diffuse through the cuticular covering, and then pass through the vacuoles to the dendrites (Zacharuk et al. 1971). Toward the outer part of the antenna tip is a uniporous peg organ—a basiconic-like appendage presumed to be gustatory. The brain targets for larval sensory organs are currently unknown. The SEM image of the larval antennal tip is from (Xia et al. 2008), Copyright (2008) National Academy of Sciences.
Figure 2.
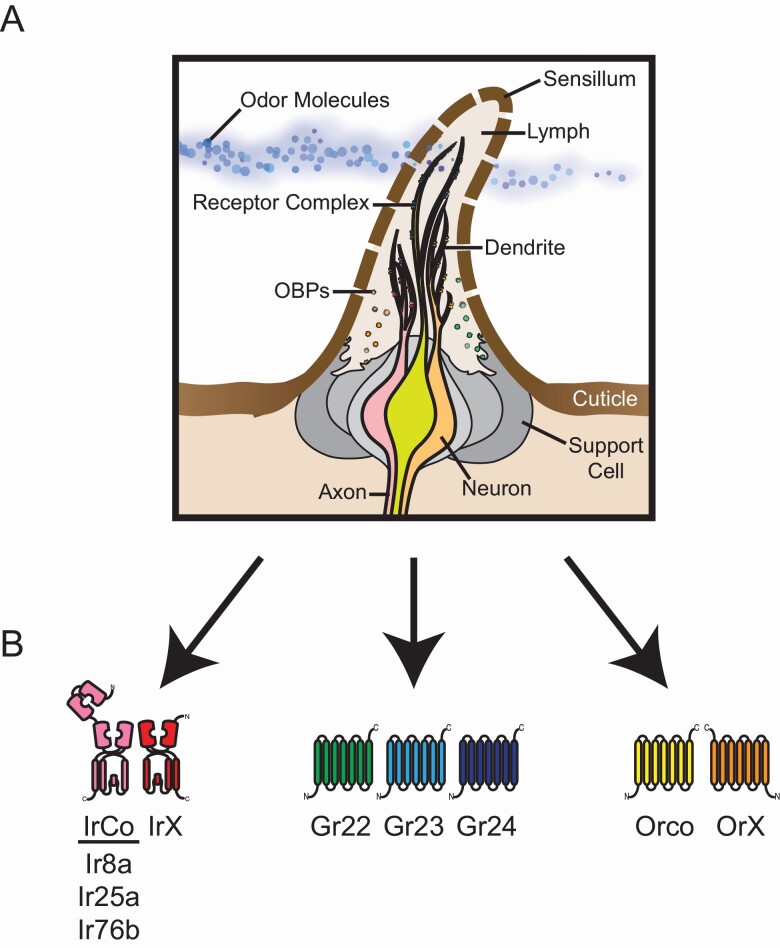
Anatomy of a sensillum and olfactory receptors in Anopheles mosquitoes. (A) Mosquito olfactory organs are covered in sensory hairs called sensilla. A cross-section of a typical sensillum is illustrated here. In Anopheles mosquitoes, each sensillum contains between 2 and 5 olfactory sensory neurons or OSNs (3 neurons are shown in this example). The dendrites of these neurons are found within the sensillum, whereas the axons send olfactory information to the mosquito brain. The neurons are surrounded by support cells (gray); these cells secrete the fluid or lymph filling the sensillum and produce OBPs. Odor molecules enter the sensillum through pores in the sensillar cuticle. OBPs may help in the transport of some odorants to the dendrites or act to sequester odor molecules. The dendrites express various olfactory receptor complexes. These complexes are ligand-gated ion channels that open when specific odor molecules bind to them, leading to the activation of the neurons. (B) Olfactory receptor complexes are composed of subunits from 3 broad classes or gene families: IRs, GRs, and ORs. Within each class, multiple subunits come together to form a functional ion channel. The IR complexes (left) consist of an IrX tuning receptor which confers ligand specificity on the neuron, in addition to one or more obligate coreceptors (Ir8a, Ir25a, and/or Ir76b). GR complexes (middle) consist of 3 subunits (Gr22, Gr23, and Gr24) which together sense carbon dioxide. OR complexes (right) consist of an OrX tuning receptor and the obligate coreceptor Orco.
Table 1.
Olfactory sensilla in sensory organs of adult Anopheles mosquitoes
| Sensory organ | Sensillar type | Sensillar class/subtype | Hypothesized function | Notes | References |
|---|---|---|---|---|---|
| Antenna | Sensilla chaetica | Mechanosensory | |||
| Sensilla ampullacea | Thermosensory | − Hypothesized to be small type of coeloconic-like sensilla | McIver (1982) | ||
| − Not well studied | |||||
| Coeloconic sensilla | Small coeloconics | Thermosensory | − Common to all mosquito genera | Greppi et al. (2020); McIver (1982) | |
| − Tunning receptor responding to cooling in the 3 coeloconic sensilla at the tip of the antenna identified | |||||
| Large coeloconics | Olfactory | − Specific to Anopheline mosquitoes | |||
| − Least abundant and studied of the antennal sensilla | |||||
| − Probably express IRs | |||||
| Trichoid sensilla | Up to 5 morphological types/classes suggested in certain Anopheles species | Olfactory | − Most abundant olfactory sensilla | Boo (1980); McIver (1982); Qiu et al. (2006b) | |
| − Unknown whether morphological class correlates with receptor expression and olfactory responses | |||||
| − Some morphologically similar sensilla can be distinguished physiologically after the female has taken a blood meal | |||||
| − Many express ORs; some may express IRs | |||||
| Grooved pegs | Subtype A | Olfactory and/or humidity sensing | − Not well studied | McIver (1982) | |
| Subtype B | − May express IRs | ||||
| Maxillary palps | Sensilla chaetica | Mechanosensory | |||
| Campaniform sensilla | Mechanosensory | ||||
| Capitate pegs | Olfactory | − Each peg has 3 neurons (A, B, and C) | McIver (1982) | ||
| − A neuron responds to CO2 and expresses GRs | |||||
| − B and C neurons express ORs | |||||
| Labella | T1 | Gustatory | − Sparse, largest sensilla on the labella | ||
| T2 | Olfactory | − About 30 short sensilla with 2 OSNs/sensillum: large amplitude A neuron and small amplitude B neuron | Kwon et al. (2006) a; Riabinina et al. (2016); Saveer et al. (2018) |
aNote reversed nomenclature of A and B neurons in Kwon et al. (2006).
Figure 3.
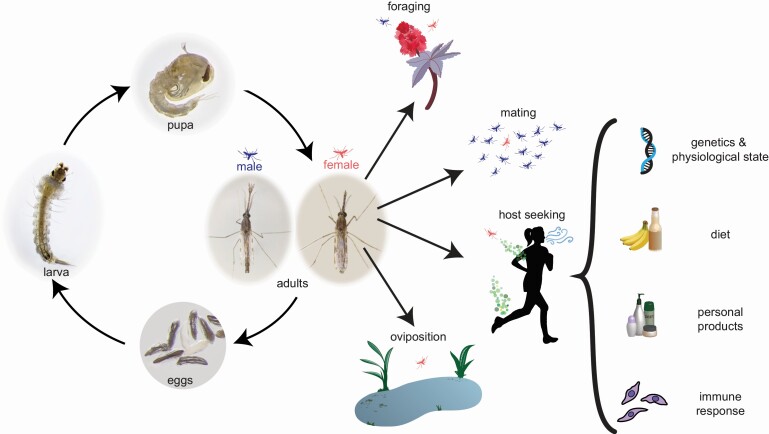
Life cycle of Anopheles mosquitoes and olfactory-guided behaviors of adults. Left: Anopheles mosquitoes go through 4 main developmental stages. Eggs (bottom) are laid individually in water and remain on the surface due to egg floats. After approximately 24 h, they hatch into larvae (left), which will feed on bacteria and detritus in the water as they grow and molt through 4 instars. This process takes about 1 week, depending on environmental conditions. With the final molt, larvae become pupae (top), which no longer feed but are still motile. Adults (right) emerge from pupae approximately 24 h later. Male mosquitoes are indicated in blue, female mosquitoes in light red. Right: Both male and female mosquitoes must forage for nectar from plants (top). Mosquitoes mate in swarms of males (second from top), into which females fly. Only females engage in host-seeking and blood feeding behaviors (second from bottom), as well as oviposition or egg-laying (bottom). Bracket: many factors affect the attractiveness of a human host to a female Anopheles mosquito, including genetic influences on skin flora and emitted odor volatiles, physiological state such as age and pregnancy, diet, the use of personal hygiene products and mosquito repellents, and immune response, including infection with the Plasmodium parasite (bottom).
Figure 4.
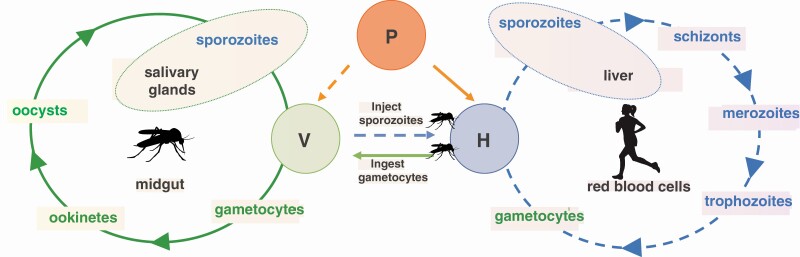
Malaria-causing Plasmodium needs both a female mosquito vector and a vertebrate host to complete its lifecycle. Different stages of Plasmodium parasite (orange) are infective to mosquito vectors (green) and human hosts (blue). A female mosquito needs to blood-feed at least twice to transmit malaria and she can only be infected following ingestion of the sexual gametocyte stage of Plasmodium when she bites an infected host (solid green lines and arrows). Once inside a mosquito, male and female Plasmodium gametocytes form motile zygotes (ookinetes), which invade the mosquito midgut wall. Ookinetes develop into oocysts, which eventually rupture releasing sporozoites, which invade the mosquito’s salivary glands. Sporozoites are the only stage of Plasmodium that can infect human hosts after a female mosquito injects them along with her saliva during a blood meal (dashed blue lines and arrows). Sporozoites invade the liver and mature into schizont, which release merozoites. Merozoites in turn invade red blood cells, where Plasmodium goes through repeated cycles of asexual reproduction. Merozoites produce immature ring stages, which develop into mature trophozoites and produce schizonts again (which will go through the cycle and infect new red blood cells). Small proportion of the immature ring stage trophozoites develop into male and female gametocytes (Plasmodium sexual stages). The human host now harbors the only Plasmodium stage infective to female mosquitoes taking a blood meal. The critical step for infection of both mosquito vectors and vertebrate hosts is highly dependent on mosquito olfaction. P, Plasmodium parasite; V, mosquito vector; H, human host.
Olfactory system in Anopheles
There are 3 peripheral olfactory appendages in Anopheles mosquitoes: the antennae, the maxillary palps, and the labella on the proboscis (Figure 1). All share some basic features also found in other insects: the appendages are covered in sensory hairs called sensilla; each sensillum is innervated by the dendrites of olfactory neurons expressing various combinations of olfactory receptors (Figure 2; see Olfactory receptors and coreceptors). When odorant molecules bind to these receptors, the neurons fire, sending an olfactory signal via their axons to the primary processing center(s) in the brain (see Higher olfactory centers). At the base of the sensilla, olfactory neurons are surrounded by support cells (also called accessory or auxiliary cells); these cells secrete the fluid filling the sensillum (lymph) and produce odorant binding proteins (OBPs). A direct role for OBPs in insect olfaction remains debateable (Larter et al. 2016; Xiao et al. 2019). OBPs are among the most abundant transcripts found in antennal tissues (Pitts et al. 2011b), and given their abundance, it is hypothesized they play a role in odor reception, most likely by binding to an odor molecule and transporting it to an odorant receptor (OR) (Venthur and Zhou 2018). In vitro studies of An. gambiae OBPs have identified OBPs that can bind a variety of odorants (Venthur and Zhou 2018). The most well characterized is AgOBP1, which based on in vitro assays, can bind the human odorant sulcatone, the repellent N, N-diethyl-meta-toluamide (DEET), and the animal odor indole (Biessmann et al. 2010; Murphy et al. 2013). RNAi knockdown of OBP1 leads to reduced electrical activity of whole antennae toward indole (Biessmann et al. 2010). However, it was not determined if OBP1 is expressed in sensilla that respond to these odorants, nor the effects of an OBP1 mutant on olfactory or behavioral responses. The role of OBPs in olfactory responses has been most rigorously examined in Drosophila melanogaster. The Drosophila OBP LUSH enhances reception to the pheromone cis-vaccenyl acetate, in support of a role for OBPs in the transport of certain odor molecules (Gomez-Diaz et al. 2013). However, a comprehensive expression and mutant examination of the most abundantly expressed OBPs in the Drosophila antennae indicated that these OBPs played little role, if any, in odor reception (Larter et al. 2016; Xiao et al. 2019). In these experiments, up to 4 obp gene mutants were combined together to eliminate OBP expression in a sensilla, followed by single sensillar electrophysiological recordings of the olfactory neuron responses to a wide panel of odorants. These rigorous experiments demonstrated that olfactory neurons in sensilla lacking OBPs functioned normally, with some exceptions in which olfactory neurons exhibited increased odor responses. These data suggest that OBPs may not play a major role in odor reception, and may actually function as “sponges” to reduce odor concentrations in the lymph (Larter et al. 2016; Xiao et al. 2019). Further research is needed to determine the relative contribution of OBPs to odor perception in Anopheles mosquitoes (Biessmann et al. 2010; Murphy et al. 2013; Venthur and Zhou 2018).
Odorant molecules enter the lymph through multiple pores on the sensilla, allowing them to reach the olfactory receptors enriched on olfactory neuron dendrites. Transport through the lymph to the olfactory receptor might be aided by binding to OBPs, or the odorant might travel from the pore via pore tubules (structures found in many insect sensilla that extend from the wall pore to the dendritic membrane) (Steinbrecht 1997; Larter et al. 2016). Here, we describe olfactory receptor function and topology, followed by the anatomy of the peripheral olfactory appendages of adult Anopheles mosquitoes. Larval olfaction is also addressed in this section (see Larval olfaction).
Olfactory receptors and coreceptors
In mosquitoes, each olfactory neuron primarily expresses 1 of 3 classes of olfactory receptors: odorant receptors (ORs), ionotropic receptors (IRs), or gustatory receptors (GRs) (Figure 2). These receptors function as transmembrane ligand-gated ion channels comprised of various combinations of subunits. The OR and IR receptor groups contain a highly conserved coreceptor(s) paired with a subunit(s) tuned to respond to specific odorant ligands. ORs require an obligate olfactory receptor coreceptor called Orco; IRs utilize at least 3 different coreceptors (Ir8a, Ir25a, and Ir76b). There are no known olfactory coreceptors for GRs in mosquitoes, suggesting that either olfactory GRs do not require coreceptors, many different GRs function as coreceptors, or that multiple tuning receptors complex together. Here, we provide a brief overview of olfactory receptors and focus on the structural facets elucidated directly from Anopheles species. In-depth description of the primary olfactory receptor families across mosquito species has been reviewed elsewhere (Sparks et al. 2018). Recent studies suggest the coexpression of coreceptors in olfactory neurons in Aedes and Drosophila (Task et al. 2020; Younger et al. 2020); if this pattern also extends to Anopheles olfactory neurons remains to be determined.
Of the 3 olfactory receptor families, ORs have been the most studied. Each OR channel/complex is thought to be a heterotetramer consisting of subunits of Orco and odorant tuning receptor, ORx (Pask et al. 2011). These subunits are 7-transmembrane (7-TM) proteins with the N- and C-termini positioned intracellularly and extracellularly, respectively. Orco coreceptor is a highly conserved type of OR subunit that is found across insect species, whereas the ORx subunits tend to be highly divergent both within and between species (Rinker, Zhou, et al. 2013). Anopheles gambiae Orco was originally identified as OR7 through functional studies and homology to the D. melanogaster Orco (originally named Or83b), with 78% sequence similarity (Hill et al. 2002; Vosshall and Hansson 2011). Insect ORs represent the most diverse group of ligand-gated channels identified so far in nature. Both overall structure and individual amino acids play an important role in OR function in anopheline mosquitoes. Comparison of OR sequences of Anopheles and D. melanogaster revealed 3 distinct motifs (A, B, and C) in the C-terminus region in 76 of 79 (96%) known anopheline OR proteins (Miller and Tu 2008). These regions are hypothesized to function as protein–protein interaction sites used for Orco/ORx channel pore formation.
The second class of olfactory receptors, IRs, are composed of various subunits of odor binding IRs (IRx) and one of 3 IR coreceptors (IRco) (Sparks et al. 2018). IRx/IRco channel composition has yet to be fully characterized in anopheline mosquitoes. Current predicted structures include complexes of 3 IRco subunits with one IRx subunit (a similar heterotetramer structure seen in ORs) in 1:1 ratios (Pitts et al. 2017; Sparks et al. 2018; Li et al. 2019). Based on the sequence alignment of An. sinensis, the predicted topology of IRs includes 4 transmembrane domains (M1, pore loop, M2, and M3), along with an additional extracellular ligand-binding domain (LBD) (Benton et al. 2009; Li et al. 2019).
Although ORs only have one coreceptor (Orco), there are currently 3 identified IRco subunits: IR8a, IR25a, and IR76b. The structure of IR coreceptors differs compared with IRxs. Specifically, IRcos have an additional amino-terminal domain that directly follows the LBD, similar in structure to ionotropic glutamate receptors (iGluRs) found in other animals. Based on sequence homology, IRs are hypothesized to have evolved from iGluRs (Abuin et al. 2011; Wang et al. 2018; Li et al. 2019). Unlike iGluRs, due to the loss of glutamate-binding residues, IRcos are no longer able to bind glutamate. IRs are more highly conserved across insect species compared with ORs and GRs (Pitts et al. 2017; Wang et al. 2018).
Compared with ORs and IRs, little is known about the structure of GRs in Anopheles mosquitoes. GRs are hypothesized to be the ancestral receptor type that ORs evolved from as arthropods began their transition from aquatic to terrestrial environments (Eyun et al. 2017; Yan et al. 2020). Structurally, GRs retain the 7-TM topology found in ORs. Based on sequence alignment, despite the close relationship between GRs and ORs, the 3 conserved C-terminal motifs found in ORs are absent in GRs (Miller and Tu 2008). There is currently no published data on resolved protein architectures, limiting our knowledge on insect GR structures.
Despite advances in understanding the structure–function relationship in anopheline olfactory receptors, specific 3D structures of the olfactory receptors are unresolved. While obtaining the structures of transmembrane proteins is difficult, recent breakthroughs with cryogenic electron microscopy (cryo-EM) are bridging that gap. For example, cryo-EM allowed for the structure of the fig wasp Apocrypta bakeri Orco to be solved in a homotetramer formation (Butterwick et al. 2018). The structure of an ORx/Orco OR complex remains to be determined.
Various additional receptor and signaling molecules play important roles in insect olfaction. For example, several classes of transmembrane receptors may be involved in olfactory signal transduction in anopheline mosquitoes. These include transient receptor potential (TRP) channels which might respond to repellents such as citronellal (Kwon et al. 2010) or catnip/nepetalactone (Melo et al. 2021), epithelial sodium channels (DEG/ENaC)/pickpocket channels (ppk) found to amplify olfactory responses to pheromones in Drosophila (Ng et al. 2019), and sensory neuron membrane proteins found to mediate pheromone responses in Drosophila and Lepidoptera (Benton et al. 2007; Cassau and Krieger 2021). Odorant clearance or removal in the lymph by OBPs or odorant degrading enzymes will also affect olfactory signaling (Leal 2013). Similarly, numerous intracellular molecules probably function to regulate olfactory signaling, such as arrestins (Merrill et al. 2005) and other G-protein signaling components like Gα s (Deng et al. 2011). The role of these signaling molecules in Anopheles olfaction is an important yet under-explored area of research.
Sensory appendages: antennae
Both male and female adult Anopheles antennae have 13 segments called flagellomeres; at the base of each antenna is the donut-like pedicel, containing the Johnston’s organ (the mosquito “ear”) (McIver 1982). Anopheles antennae are sexually dimorphic. Males use audition to identify a mate, and thus most of their antennal sensory structures appear to be devoted to hearing (they have many more auditory hairs or fibrillae, and larger pedicels). Male olfactory sensilla are located only on the 2 distal segments of the antenna (Riabinina et al. 2016). In contrast, females have fewer auditory hairs and many more olfactory sensilla, which are distributed throughout all but the most proximal flagellomere (flagellomere 1; McIver 1982), suggesting that olfaction is central to female physiology and behavior. There are 5 sensillar types on Anopheles antennae: sensilla chaetica, sensilla ampullacea, coeloconic sensilla, trichoid sensilla, and grooved pegs (McIver 1982). Three of these types are likely to be olfactory (Table 1). Both males and females appear to have all the same types of olfactory sensilla, but in different numbers and locations (Riabinina et al. 2016). For a comprehensive review of sensillar distributions in males and females, and detailed descriptions of sensillar types and subtypes in several Anopheles species, see (McIver 1982; Pitts and Zwiebel 2006; Qiu, van Loon, et al. 2006).
All Anopheles olfactory sensilla contain between 2 (in trichoid) and 5 (in large coeloconic) olfactory neurons (McIver 1982). The olfactory tuning receptor identity for most of these neurons is currently unknown. Olfactory neurons expressing the Orco coreceptor are found in trichoid sensilla (Pitts et al. 2004; Riabinina et al. 2016). Based on sensillar counts and genetic labeling of Orco+ cells, either most trichoid sensilla contain one Orco+ and one Orco− cell or else approximately half of trichoid sensilla contain 2 Orco+ cells each and the other trichoid sensilla express other receptors such as IRs (McIver 1982; Riabinina et al. 2016). It is currently difficult to distinguish these possibilities by histological means; new genetic reagents that label subsets of olfactory neurons might aid in this endeavor (as has been the case in D. melanogaster) (Couto et al. 2005; Fishilevich and Vosshall 2005; Kurtovic et al. 2007).
Little is known about IR expression in the antennae. Although the coreceptor Ir76b does not appear to overlap with Orco expression, the type(s) of sensilla housing Ir76b neurons were not identified (Pitts et al. 2017). Interestingly, Ir76b expression was later found to be closely associated with the expression of the ammonium transporter AgAmt, located in supporting cells surrounding Ir76b+ neurons. In An. coluzzii antennae, AgAmt was found in neurons and supporting cells in grooved peg and coeloconic sensilla, as well as in the palps and labella (Ye et al. 2020). It did not colocalize with Orco. This complex expression pattern prompts the question of AgAmt’s role in these neurons and supporting cells. Ye et al. (2020) found ammonia-sensitive neurons not only in coeloconic sensilla, but also, surprisingly, in the capitate peg sensilla in the palps. Recent mutation of AgAmt revealed another surprise: the antennae of these mutant mosquitoes retain the ability to respond to ammonia odors (Ye et al. 2021). These data suggest AgAmt is unlikely to directly mediate ammonia detection in mosquitoes and suggest the molecular mechanism responsible for ammonia detection remains to be identified.
Given that both male and female Anopheles appear to have the same sensillar types, and express many of the same olfactory receptors, this suggests that both sexes are capable of sensing or responding to the same odors. However, since females probably have more sensilla and more neurons of each type, they may have a lower threshold of detection (be more sensitive to low concentrations of odorants) (e.g., Lu et al. 2007; Schymura et al. 2010). Transcriptomics studies also raise intriguing open questions such as the function of olfactory receptors expressed only in one sex or the chemosensory function of GRs expressed in the antennae (Pitts et al. 2011a).
In general, there are 2 broad challenges to connecting olfactory neuron identity to function in the Anopheles antenna: the first is the lack of a stereotypical organization of neuron types as found in other insects such as Drosophila, and the second is the sheer number of sensilla, neurons, and olfactory receptor genes (McIver 1982; Hill et al. 2002; Riabinina et al. 2016). Although many olfactory receptors (especially in the OR family) have been functionally characterized in heterologous expression systems (Carey et al. 2010; Wang et al. 2010; Pitts et al. 2017), few have been connected to antennal location or even sensillar type (Schymura et al. 2010; Schultze et al. 2013, 2014; Karner et al. 2015). Conversely, many electrophysiological experiments have been done on mosquito antennae with little to no knowledge of the underlying molecular mechanisms/identities of the neurons being recorded (van den Broek and den Otter 1999, 2000; Meijerink et al. 2001; Qiu, van Loon, et al. 2006). Although currently existing techniques such as calcium imaging in the periphery (Afify et al. 2019) in combination with heterologous expression and histology can begin to address some of these issues, new genetic tools to label and manipulate specific cell types are sorely needed.
Sensory appendages: maxillary palps
Of the Anopheles olfactory appendages, the maxillary palps are the simplest and most well characterized. Both male and female palps consist of 5 segments; in the males, the 2 most distal segments are expanded into a club (McIver 1982). As in the antennae, both male and female palps have the same types of sensory structures (Table 1), but their distribution and numbers are sexually dimorphic (McIver 1982). The only chemosensory sensillar type on the palps is the capitate peg sensillum (cp). Each is innervated by 3 olfactory neurons designated cpA, cpB, and cpC based on their characteristic spontaneous spike amplitudes (largest in A to smallest in C) (Lu et al. 2007; McIver 1982). The cpA neuron expresses Grs and responds to carbon dioxide (Lu et al. 2007), whereas the cpB and cpC neurons express Orco and a tuning OR (AgOr8 and AgOr28, respectively) and respond to 1-ocent-3-ol and a variety of odorants (Pitts et al. 2004; Lu et al. 2007; Pitts et al. 2011a; Riabinina et al. 2016). The cpA neuron expresses a complex of 3 GRs (Gr22, Gr23, and Gr24) necessary for CO2 reception (Lu et al. 2007). Interestingly, recent work indicates that only Gr23 and Gr24 probably form the functional CO2 receptor, with Gr22 acting to modulate the Gr23/Gr24 response toward CO2 (Liu et al. 2020). In addition to CO2, the cpA neuron is also excited and inhibited by many odorants (Lu et al. 2007; Tauxe et al. 2013; Coutinho-Abreu et al. 2019), and some of these odor responses are mediated by Gr22 (Liu et al. 2020), and possibly by coexpression of IRs (Younger et al. 2020).
Despite thorough anatomical, molecular, and physiological characterization, some questions about the Anopheles maxillary palps remain. For example, transcriptional profiling (quantifying RNA expression using techniques such as qRT-PCR or RNAseq) of the palps in males and females revealed additional potential olfactory receptors, including nine IRs (3 found in both sexes, plus 2 female-specific IRs and 4 male-specific ones), and 2 additional GRs (one specific to each sex) (Pitts et al. 2011a). Since the only known chemosensory sensilla on the palps are the capitate pegs, this suggests that there may be some neurons that coexpress IRs with ORs or GRs; this idea is supported by recent findings in the palps of Aedes mosquitoes (Younger et al. 2020). Furthermore, a small proportion of Orco+ neurons in the palps apparently do not express either AgOr8 or AgOr28, suggesting that they may instead express different tuning receptors (Lu et al. 2007).
Finally, if the primary role of the palps is the integration of host cues, such as CO2 and octenol, as has been suggested (Lu et al. 2007), then what is the role of the palps in Anopheles male physiology/behavior? Interestingly, animals are not the only source of CO2 in the mosquito’s environment: plants also emit CO2 (Peach et al. 2019). One possibility is that males (and females) use CO2 in combination with other plant volatiles to choose a nectar source (see Role of olfaction in foraging). Whether males can detect these plant volatiles with their maxillary palps, and then integrate this information with CO2, has not, to our knowledge, been investigated.
Sensory appendages: labella
The mosquito proboscis consists of a fascicle or bundle of 6 stylets with a retractable sheath (the labium); the stylets include 2 maxillae, 2 mandibles, a labrum, and a hypopharynx (McIver 1982; Wahid et al. 2003). In females, the maxillae saw through the skin of the host, whereas the mandibles separate the tissue; in both males and females, the hypopharynx secretes saliva, and the labrum is the food canal for both nectar and blood (Wahid et al 2003; Choo et al. 2015). Of the 6 stylets, only the labrum has sensilla, which probably house gustatory neurons and possibly sensory neurons for other modalities (e.g., mechanosensory, thermosensory, and/or hygrosensory) (McIver 1982; Maekawa et al. 2011; Jové et al. 2020). The stylets are enclosed by a retractable and flexible labium, at the tip of which is the labellum, composed of 2 lobes (McIver 1982; Choo et al. 2015). When a female mosquito takes a blood meal, the stylets pierce the skin of the host; the labium bends out of the way, with the labellar lobes remaining on the surface of the skin (Choo et al. 2015). Interestingly, even though only female mosquitoes bite, male proboscises are morphologically quite similar to those of the females. Anopheles males have all 6 stylets, but their mandibles and maxillae are shorter than females’ and less adapted for piercing (Wahid et al. 2003). Of the various proboscis structures, the labellum is the only one to have been implicated in olfaction.
Like the other peripheral appendages, An. gambiae labella are covered in sensilla, including a small population of T2 olfactory sensilla (Table 1). Each T2 sensillum contains 2 olfactory neurons (Kwon et al. 2006; Riabinina et al. 2016; Saveer et al. 2018). There are around 60 olfactory neurons per labellar lobe, approximately 45 of which express Orco (Pitts et al. 2004; Riabinina et al. 2016). These numbers suggest that there may be approximately 15 T2 neurons, which express other olfactory receptors, such as IRs (see Olfactory receptors and coreceptors). As the identities of the ligand-binding receptors have not been mapped to the vast majority of the labellar olfactory neurons, this is a fruitful avenue of future research. To date, only one olfactory receptor, AgOr6, has been confirmed in labellar olfactory neurons (Kwon et al. 2006). RNAseq experiments have revealed that a number of ORs, IRs, GRs, and OBPs are expressed in the labella (Saveer et al. 2018). These experiments demonstrated that some OR transcripts enriched in the labella are lowly expressed or even absent in the other olfactory appendages, suggesting an interesting potential target for specific, close-range modulation of Anopheles olfactory behavior to prevent biting.
Although currently there is no receptor-to-neuron map of the OSNs in the labella, efforts have been made to characterize their functional profiles (Kwon et al. 2006; Saveer et al. 2018). Using the gustatory T1 sensilla as landmarks, the labellum was subdivided into 4 anatomical zones (numbered 1 through 4 from distal to proximal) (Saveer et al. 2018). Interestingly, OSNs in different zones appear to have different odorant response properties: for example, the A neurons in zone 3 are more narrowly tuned than the A neurons in the other zones (Saveer et al. 2018).
One present challenge is dissociating the relative behavioral contributions of labellar OSNs from olfactory neurons in the antennae and maxillary palps. For example, many of the strongest activators of labellar neurons, such as indole and acetophenone (Saveer et al. 2018), are known to also activate OSNs in the antennae (Qiu, van Loon, et al. 2006) and palps (Lu et al. 2007), respectively. Experiments in which the different olfactory appendages are ablated or inactivated, as has been done in the Culex proboscis (Choo et al. 2015), might help elucidate the behavioral significance of the labellar olfactory neurons.
Higher olfactory centers: general organization
OSNs in peripheral tissues serve as neuronal odorant detectors and function to directly convey olfactory signals into the brain. The insect brain organizes these neuronal signals into perceptions which guide behaviors. Recent work identified 2 An. coluzzii brain regions innervated by olfactory neurons: the antennal lobes (ALs) and the subesophageal zone (SEZ) (Riabinina et al. 2016).
A central dogma of insect olfactory neurons is that antennal and maxillary palp olfactory neurons that express the same type of tuning olfactory receptor converge their axons into a shared region of the brain called a glomerulus. The collection of all glomeruli makes up the AL. For example, there are ~10 olfactory receptor neurons that express the AgOR2 receptor across the Anopheles antennae, with roughly 0–2 AgOR2-expressing neurons in each flagellomere (Schymura et al. 2010). Although it remains to be experimentally verified, the axons for each of these neurons are expected to target the same glomerulus. This converging of olfactory neurons expressing the same olfactory receptor toward a common brain region serves to amplify the olfactory signals from the periphery as they enter the brain.
An olfactory glomerulus is formed by the olfactory circuits that innervate the glomerular region: 1) the axons of the OSNs which serve as the input to the circuit, 2) the dendrites of the olfactory projection neurons, which serve as the output for the circuit, and 3) the processes of the local neurons, which innervate only the AL glomeruli to help shape the olfactory signal that is transmitted from olfactory neuron to projection neuron. From a study examining dye-filled projection neurons in Anopheles mosquitoes (Ignell et al. 2005), it appears these projection neurons share features found in other insects such as Drosophila (Jefferis et al. 2007) and moths (Homberg et al. 1988) and primarily target 2 regions of the brain: the calyx of the mushroom bodies and the lateral horn (Figure 1).
Higher olfactory centers: ALs
The An. coluzzii AL is comprised of 67–70 glomeruli as determined by staining for a synaptic protein (bruchpilot, detected by the Drosophila nc82 antibody) (Riabinina et al. 2016), although variability between brain samples regarding the size and shape of glomeruli was noted (Riabinina et al. 2016; also see Anton and Rospars 2004). Variability of ALs might be due to technical issues of dissection, brain staining, and imaging, or they might reflect biological variability between individuals. The male AL contains a similar number of identifiable glomeruli as the female (67–68) but is smaller in size due to the proportionally fewer olfactory neurons of each class innervating and forming each glomerulus (Riabinina et al. 2016). The female AL measures ~170 000 µm3, which is roughly 1.9 times larger than the male AL at ~90 000 µm3 (Riabinina et al. 2016). Genetic labeling of Orco+ olfactory neurons revealed that approximately half of the AL glomeruli are innervated by Orco+ olfactory neurons (33 in males and females) (Riabinina et al. 2016). This finding suggests that the remaining glomeruli are probably innervated by IR neurons (IRNs) or by CO2-sensing GR neurons (GRNs). Note, the original investigation of the An. gambiae AL structure mis-classified a group of glomeruli as a Johnston’s organ center (Ghaninia et al. 2007); improved neurogenetic and staining methods in An. coluzzii identified this region as containing many glomeruli, as well as being innervated by Orco+ olfactory neurons (Riabinina et al. 2016).
Anopheles olfactory neurons that originate from the antenna project only to the ipsilateral AL (the AL on the same side as the antenna) (Riabinina et al. 2016). This contrasts D. melanogaster, in which an antennal olfactory neuron generally first targets the ipsilateral AL, and also sends a projection to the matching glomerulus on the contralateral AL (the AL on the other side of the brain) (Dobritsa et al. 2003). Anopheles olfactory neurons that originate from the maxillary palp, however, do target both ALs: the 3 olfactory neurons from the capitate peg sensilla target the ipsilateral AL, and also send contralateral projections to the matching glomeruli on the contralateral AL (Anton et al. 2003; Ghaninia et al. 2007; Riabinina et al. 2016).
The 2 Orco+ olfactory neurons in the capitate peg sensilla of the maxillary palps (Or8- and Or28-expressing neurons) target 2 posterior glomeruli in the Anopheles AL (Ghaninia et al. 2007; Riabinina et al. 2016). Dye filling experiments of the maxillary palp suggest that the third olfactory neuron in capitate peg sensilla, which expresses the Gr22/Gr23/Gr24 complex and is sensitive to CO2, may target up to 3 posterior glomeruli (Riabinina et al. 2016). It remains to be determined if subsets of the olfactory GRNs target one of the 3 glomeruli (and hence are organized into 3 different grouping of GRNs), or if the same olfactory neuron can target all 3 glomeruli. Recent evidence suggests that Gr22 is not required for detection of CO2, but instead enhances the ability of Gr23/Gr24 to respond to CO2 (Liu et al. 2020). This observation suggests that changes in expression of Gr22 might lead to changes in the ability of the neuron to respond to CO2 (and some other odorants), which might be further reflected by potentially differential targeting of AL glomeruli.
Higher olfactory centers: SEZ
The ~45 Orco+ olfactory neurons from the An. coluzzii labella do not target the AL, but instead target the SEZ of the brain (Riabinina et al. 2016). One report using dye labeling of the labella suggested some labellar olfactory neurons innervate the AL (Kwon et al. 2006), but further genetic labeling of Orco+ neurons and dye labeling could not verify these results (Riabinina et al. 2016). The SEZ region of an insect brain is often associated with gustatory behaviors (Wang et al. 2004; Miyazaki and Ito 2010). Genetic labeling of Orco+ olfactory neurons in An. coluzzii revealed the presence of 8 glomerular structures in the SEZ in both male and female brains (Riabinina et al. 2016). It remains to be determined if labellar olfactory neurons in other insects also target the SEZ region of the brain. The implications of olfactory innervation in a gustatory region suggest that olfactory and gustatory sensory information could be directly integrated by neurons within this brain area. Integration of olfactory and gustatory sensory modalities in the human brain gives rise to the perception of flavor, and an intriguing hypothesis is that Anopheles mosquitoes may be using gustatory and olfactory neurons on the labella to similarly examine the “flavor” of a host-landing site and use this to inform on its decision to bite. Similarly, it is possible that favorable host odors detected by the labella might be interpreted by the SEZ brain region as appetitive tastes.
Larval olfaction
In contrast to adult Anopheles, not much is known about olfaction in larvae and pupae. There is no current evidence for chemosensory-driven behavior in Anopheles pupae. Pupae do not forage and do not seem to have functional antennae or palps (Montell and Zwiebel 2016). Fully functioning adult sensory appendages need to develop inside their heads, whereas the larval olfactory appendages are removed by the last molt. Pupae appear to predominantly rely on visual cues to interact with their environment.
Anopheles larvae navigate their chemical world using olfactory receptors expressed in the antennae and maxillary palps (Pitts et al. 2004). Similar to other dipteran insects, the antennae of larval An. gambiae mosquitoes are bilaterally symmetrical, projecting anteromedially from the lateral surface of the head. Morphologically, the larval antenna is composed of chemosensory structures including a sensory cone and a peg organ (Figure 1B). These structures play a crucial role in olfaction and presumably gustation, respectively (Zacharuk et al. 1971).
The sensory cone is proposed to be the main olfactory organ in the larvae of An. gambiae and is innervated by ~12 bipolar neurons expressing Orco (Xia et al. 2008). The larval chemosensory structures of An. coluzzii are very similar between males and females (Riabinina et al. 2016). Notably, the median number of Orco+ cells identified in the male and female larval antennae is twice that of the maxillary palps (Riabinina et al. 2016). This pattern of expression suggests that in Anopheles, antennae might be more important than maxillary palps in larval odorant detection.
Larval antennae express 12 putative AgOrs and the obligate coreceptor, Orco (Carey et al. 2010; Wang et al. 2010; Xia et al. 2008). Further investigations of the 12 reported AgOrs identified 4 (AgOr37, AgOr40, AgOr52, and AgOr58) that are speculated to be larval specific (Xia et al. 2008), although some have been detected at low levels in adult tissues (Maguire et al. 2020). Recently, upregulation of AgOr52 in the male and female adult An. gambiae during mating was reported, suggesting that this OR might not be larval specific (Mozūraitis et al. 2020). Heterologous expression of the 12 candidate AgOrs using either Xenopus oocytes or Drosophila empty neuron system revealed some corresponding ligands that activate these receptors (notably indole and benzaldehyde), which activate AgOr2 and AgOr10 in the larvae and adult female An. gambiae (Xia et al. 2008; Carey et al. 2010; Wang et al. 2010). Nonetheless, the ligands for AgOr52 and AgOr58 have not yet been identified. Apart from the AgOrs, AgIr76b has been shown to be crucial for behavioral responses of Anopheles larvae to butylamine (Liu et al. 2010). The role of AgIrs in larval olfaction has been largely understudied.
Contrary to adult Anopheles mosquitoes that are free-living on land, the larvae inhabit confined aquatic environments such as puddles, pools of standing water, and drainage ditches (Muema et al. 2017). Given the nature of the larval habitat, to survive, larvae must find sufficient food resources while avoiding numerous dangers, including predators (e.g., Toxorhynchites larvae, dragonfly nymphs, and some aquatic Hemiptera) (Ohba et al. 2010), and toxic compounds.
Anopheles larvae have evolved chemosensory detectors of harmful odorants and display strong aversive behavioral responses to compounds such as acetophenone produced by Pseudomonas bacteria (a compound associated with mammalian disease; Xia et al. 2008), DEET (a potent adult mosquito repellent) (Xia et al. 2008), and a mosquito larval repellent VUAA1 (2-((4-ethyl-5-(pyridine-3-yl)-4H-1,2,4-triazol-3-yl)thio)-N-(4-ethylphenyl)acetamide) (Yang et al. 2020). A compound from Bacillus thuringiensis israelensis bacteria has also been shown to be highly toxic to mosquito larvae (Benelli et al. 2016). Additionally, compounds such as sulcatone and dimethyl disulfide (possibly produced by the bacteria or the larvae themselves) are present in water with high larval densities. It is unclear if /how some of those potentially toxic compounds are detected by the Anopheles larvae to cause aversive behaviors.
Olfactory responses may also allow larvae to navigate to and locate food resources. The larvae of An. gambiae can detect products derived from organic decay (of both plant and animal origin) including indole, 2-methylphenol, and 4-methylcyclohexanol (Xia et al. 2008). Although products of organic decay might indicate the presence of a food source to the mosquito larvae, there is also evidence that larvae have a symbiotic relationship with indole-producing bacteria such as Serratia and Pantoea (Villegas and Pimenta 2014). Little is known about how the detection of this bacteria-derived volatile influences larval behavior.
Electrophysiological recordings of the larval sensory cone have recently revealed the population response properties of larval olfactory neurons (Sun, Liu, Baker, et al. 2020). Larval olfactory neurons responded to a wide array of odorants, primarily thiazoles, alcohols, and heterocyclics, and different subsets of neurons could be identified that respond strongly to many of the key odorants described above. Future studies will probably link chemoreceptors to their distinct larval olfactory neurons and investigate how odor coding functions in this simpler system.
Role of olfaction in Anopheles adults
Role of olfaction in foraging
Both male and female mosquitoes rely on sugar-feeding from plant and other nectar sources (Foster 1995; Müller et al. 2010) for nutrition (Baker and Baker 1973; Foster 1995; Rivera-Pérez et al. 2017; Peach and Gries 2020) and energy, driving processes such as metabolism, flight, and mating (Foster 1995; Manda et al. 2007; Nyasembe and Torto 2014). During these sugar-foraging states, mosquitoes exhibit plant- and nectar-seeking behaviors guided by olfactory and gustatory cues to selectively locate and feed on carbohydrate sources of specific host plants (Impoinvil et al. 2004; Manda et al. 2007; Gouagna et al. 2010; Müller et al. 2010).
Plants and other sugar sources release volatile organic compounds (VOCs) including terpenes, benzenes, esters, aldehydes, and alcohols (Knudsen et al. 1993; Nyasembe and Torto 2014). Some of these VOCs influence olfaction-guided mosquito foraging, though their effects are often species specific and dependent on blend composition (Nyasembe et al. 2012; Nyasembe and Torto 2014). There is significant overlap in plant preference between both sexes, but differential responses and some distinct plant preferences exist (Healy and Jepson 1988; Manda et al. 2007; Müller et al. 2010; Nikbakhtzadeh et al. 2014). For example, An. gambiae s.s. females are more likely to fly upwind in the presence of certain plant odors and enter the plant-baited traps more frequently than males (Nikbakhtzadeh et al. 2014). Furthermore, some flowering plants are uniquely attractive to females (Müller et al. 2010). A complete characterization of sex-specific mosquito responses and preferences for plants and other carbohydrate sources is lacking.
Olfactory discrimination of suitable plants and nectar sources is species specific. Anopheles gambiae, Aedes aegypti, and Ae. ochraceus mosquitoes detect unique volatile compound classes from their preferred host plants (e.g., sesquiterpenes and alkenes), as well as general plant attractants (e.g., monoterpenes β-myrcene and (E)-β-ocimene) (Nyasembe et al. 2018). Although a complete profile of plant semiochemicals involved in olfaction-mediated nectar-seeking is lacking, several volatile compounds from plant headspaces that elicit responses in Anopheles species have been identified (Nyasembe and Torto 2014). For example, An. arabiensis responds to extracted volatiles from the flowering plant Achillea millefolium, with a cyclic or bicyclic monoterpene tentatively identified as the active compound (Healy and Jepson 1988). In An. gambiae s.s. females, 6 electroantennographic detection (EAD) active plant compounds (hexanal, limonene, (Z)- and (E)-linalool oxide, β-pinene, (Z)- and (E)-β-ocimene, and (E)-β-farnesene) are involved in olfaction-mediated attraction to several plant species (Nyasembe et al. 2012). Of those compounds, linalool oxide (detected by AgOr50 and AgOr20) (Carey et al. 2010; Wang et al. 2010) was more efficient in capturing female An. gambiae s.s. than the full 6 component blend under field conditions (Nyasembe et al. 2015; Jacob et al. 2018). Combining linalool oxide with plant monoterpenoids β‐pinene, β‐ocimene, and L-limonene can also increase the efficiency of trapping An. pharoensis, but not An. arabiensis or An. funestus (Jacob et al. 2018). Thus, some plant preferences are species specific and are probably influenced by ecological niche, season, and food source availability.
Another consideration in mosquito olfaction is how yeast and microbiota inhabiting plants may influence plant VOC emission and thus the cues received and interpreted by mosquitoes (Madden et al. 2018; Barredo and DeGennaro 2020; Klaps et al. 2020). Yeast-generated CO2 (≥200 ml CO2/min) is sufficient to attract female mosquitoes, including An. gambiae s.s. and An. arabiensis (Smallegange et al. 2010; Aldridge et al. 2016). Traps with CO2 also enhance mosquito attraction compared with plant inflorescences alone (Nyasembe, Tchouassi, et al. 2014; Nyasembe et al. 2015; Aldridge et al. 2016; Peach et al. 2019), suggesting that CO2 is also a potential cue for mosquitoes during nectar-seeking.
Although many VOCs eliciting behavioral and electrophysiological responses in mosquitoes have been identified, the context in which odors are interpreted is equally important. This context-dependent olfactory-mediated behavior depends on several factors, including volatile released ratios, blend composition, and the physiological state of the mosquito. Different plant species often vary in odor compound composition as well as released ratios of VOCs, and mosquitoes use both these qualitative and quantitative differences in host odor profiles to discriminate during plant-foraging (Nyasembe et al. 2012, 2018). Modifying blend ratios of the active components of plants alters the attractiveness to female An. gambiae s.s. mosquitoes, in some cases making the synthetic blend even more attractive than the natural plant host (Nyasembe et al. 2012). There is also evidence that attractive components can become repellent at different ratios (see Repellents and attractants of host-seeking mosquitoes) (Jacob et al. 2018), and many plant species attractive to An. gambiae also contain known repellents (Nyasembe and Torto 2014; Lutz et al. 2017).
There is also significant overlap in odor profiles between human volatiles attractive to mosquitoes and headspace volatiles found in plants foraged upon by mosquitoes. For example, 1-octen-3-ol, nonanal, butanoic acid, 2-methylpropionic acid, 2-methylbutanoic acid, 3-methylbutanoic acid, benzoic acid, hexanoic acid, (−)-α-pinene, benzaldehyde, and acetophenone are all odorants found either in the odor bouquet of inflorescences or in human skin or breath (Peach et al. 2019). The mechanisms of host discrimination remain unclear, but context of chemical cues combined with other sensory modalities probably allow mosquitoes to differentiate between plant and blood host.
Role of olfaction in mating
Anopheline mosquitoes are among various insect species that mate in swarms. During swarm formation, males aggregate at dusk using visual markers (Marchand 1983). Females then enter the swarm, pair with a mate, and leave in copula for in-flight mating to occur (Charlwood and Jones 1979). Olfaction appears to have a limited role in this process, which instead predominately involves visual and auditory cues.
Although males primarily rely on visual cues known as swarm markers to select sites for swarm formation, it is still unclear how female mosquitoes locate these swarms. In some species, there is evidence that male-produced aggregation pheromones may attract females (Cabrera and Jaffe 2007; Fawaz et al. 2014) and a recent study showed that An. arabiensis and An. gambiae male mosquitoes produce and release aggregation pheromones that attract both male and female mosquitoes to the swarm and enhance mating success (Mozūraitis et al. 2020). The identified 5-component blend of 3-hydroxy-2-butanone (acetoin), 6-methyl-5-hepten-2-one (sulcatone), octanal, nonanal, and decanal was released by An. gambiae and An. arabiensis males in significantly higher amounts during swarming. These compounds also elicited increased mating in 3 other Anopheline species (An. coluzzii, An. merus, and An. funestus) (Mozūraitis et al. 2020). All 5 of these compounds are found in human odor profiles, highlighting the emerging question of how olfaction-guided mosquito responses may differ in various contexts.
Role of olfaction in host-searching and blood-feeding
Once a female mosquito has obtained enough food resources, either before or after mating (Charlwood et al. 2003), she begins searching for a suitable host to blood-feed (Figure 3). This blood meal is crucial for egg development. Females orient themselves toward potential hosts by detecting and responding to various long-range (e.g., CO2 gas and volatile components of host body odor) and short-range (e.g., body heat, relative humidity, visual contrast) cues. The final decision to bite is made after landing and assessing host suitability using contact cues (e.g., skin moisture and surface tastants). Although different sensory modalities are involved in mosquito–host attraction (Bowen 1991; Raji and DeGennaro 2017), olfaction is arguably the most important, as it initiates the entire sequence of events and mediates mosquito–host interactions (Bowen 1991; Takken 1991; Takken and Knols 1999).
Role of olfaction in mosquito attraction to human scent
Since mosquitoes predominantly rely on olfaction while host-searching, variation in human scent affects mosquito attraction, making some humans more likely to be approached and bitten than others (Knols et al. 1995; Qiu, Smallegange, et al. 2006). Host-searching females exploit 3 sources of chemical cues of human scent: exhaled breath, body odor, and urine (Takken 1991). Human odor is a complex blend of hundreds of volatiles (including organic fatty acids, ketones, aldehydes, esters, and alcohols), which varies from person to person (Krotoszynski et al. 1977; Bernier et al. 2000; Curran, Rabin, Furton 2005; Curran, Rabin, Prada, et al. 2005; Qiu, Smallegange, et al. 2006; Dormont et al. 2013). This interindividual scent variation is influenced by genetics, diet and environmental factors, and use of personal/cosmetic products (Curran, Rabin, Furton 2005). Some elements of attraction to blood-feeding insects might be heritable in humans as shown in non-malarial vectors. Evidence from the volatiles emanating from identical twins highly correlates with their attractiveness to Ae. aegypti mosquitoes (Logan, Cook, et al. 2010; Fernández-Grandon et al. 2015).
Attractiveness to mosquitoes also changes with age, body size, and physiological state of the host. Anopheles gambiae attraction increases with host age probably because of increased skin surface area, resulting in more emission of volatile compounds (Busula, Verhulst, et al. 2017). As expected, more bites are recorded on young children and adults than on infants (Bryan and Smalley 1978; Carnevale et al. 1978). Furthermore, certain components of body odor (nonanal, dimethylsulphone, and benzothiazole) might also be specifically associated with aging (Gallagher et al. 2008). Moreover, pregnant women are more attractive to An. gambiae and An. arabiensis mosquitoes compared with nonpregnant ones during sleep. Heightened attractiveness during pregnancy might be linked to increased body temperature and increased release of skin volatiles (Lindsay et al. 2000; Ansell et al. 2002; Himeidan et al. 2004). Thus, human attractiveness to mosquitoes is dependent on our prevailing physiological states, in addition to body type/size.
Certain dietary items consumed by humans may guide females to locate and select those people as hosts. Aedes albopictus and An. gambiae females exhibit a strong affinity (activation, orientation, and landing) to people within 15 min of them drinking alcohol (Shirai et al. 2004; Lefevre et al. 2010). Similarly, increased attractiveness to An. stephensi and An. gambiae mosquitoes is observed within 3 h of those eating bananas (Paskewitz et al. 2018). Conversely, consumption of garlic or vitamin B supplements provides no protection against mosquito bites, as shown in Ae. aegypti and An. stephensi (Ives and Paskewitz 2005; Rajan et al. 2005). The effect of diet might be confounded with host physiological state (CO2 release, body temperature, and metabolic changes) and vector’s physiological responses (detection and encoding by the olfactory system).
Modification of human scent with personal care products (e.g., soap, perfume) can temporarily repel or attract mosquitoes. Some of these products incorporate synthetic and natural repellents (see Repellents and attractants of host-seeking mosquitoes) that ward off host-searching mosquitoes. For example, certain underarm deodorant compounds (e.g., isopropyl tetradecanoate) can repel An. coluzzii (Verhulst et al. 2016), whereas methyl dihydrojasmonate and lilial components of perfume fragrances effectively repel Culex quinquefasciatus mosquitoes (Zeng et al. 2018). These products can suppress or activate mosquito attraction via 2 modes of action. First, they can directly affect the mosquito sense of smell. Second, they can affect the skin microflora dynamics, which then modify human scent to repel or attract mosquitoes (Verhulst et al. 2016).
One of the most important components of exhaled breath for mosquito attraction is CO2 gas, along with water vapor and VOCs. This long-range cue acts as a behavioral activator for mosquitoes and it synergizes responses to other host cues (Reeves 1951; Healy and Copland 1995; Mboera and Takken 1997; Takken and Knols 1999; Hinze et al. 2021). Since CO2 levels in human exhaled breath are about 100 times more than the atmospheric levels (Gillies 1980), female mosquitoes are behaviorally activated by detecting small changes in CO2 concentration (Webster et al. 2015). Detection of CO2 gas motivates females to find additional specific cues indicative of host presence. Responses to these other host-derived cues, which may or may not be attractive by themselves, can be enhanced by the addition of CO2. This synergy is exemplified by increased attractiveness to known components of human odor (lactic acid, 1-octen-3-ol, nonanal, and carboxylic acids) when mosquitoes are presented with CO2 (Dekker et al. 2005; Njiru et al. 2006; Qiu, van Loon, et al. 2006; Smallegange and Takken 2010; van Loon et al. 2015).
Mutant Ae. aegypti females that lack a functional orco olfactory coreceptor (and thus do not respond to many human odors) regain the attractiveness (but not the preference) to humans when CO2 is added (DeGennaro et al. 2013). Similar findings were reported in An. coluzzii mosquitoes using Orco-defective neurons or orco mutants (Maguire et al. 2020; Sun, Liu, Ye, et al. 2020). These findings suggest that CO2 is needed to trigger a host-seeking state in mosquitoes and to integrate multiple cues involved in host-searching (McMeniman et al. 2014; Potter 2014).
Once in closer vicinity to a host, CO2 becomes less important to mosquitoes in identifying host’s suitability. At this stage, body odor predominantly activates the olfactory receptors, located on all 3 sensory organs of the mosquito’s peripheral olfactory system (Montell and Zwiebel 2016) (see Sensory appendages). Carboxylic acids contained in sweat and VOCs above the skin are the main component of human odor. Alcohols, aldehydes, aromatic hydrocarbons, amines, esters, ketones, sulfides, and thiols are also present (Bernier et al. 2000; Dormont et al. 2013). These compounds, responsible for attraction in An. gambiae, are either naturally produced by gland secretions (i.e., sweat) or are a byproduct of bacterial growth on the skin surface (Braks et al. 2000). Of these sweat and skin odorants, several important Anopheles attractants include 1-octen-3-ol (octenol) (Lu et al. 2007), lactic acid (Braks and Takken 1999), ammonia (Smallegange et al. 2005; Qiu, van Loon, et al. 2006; Ye et al. 2020), nonanal, and carboxylic acids (e.g., hexenoic and octenoic acids) (Knols et al. 1997; Meijerink and van Loon 1999; Costantini et al. 2001). Interestingly, these studies found that these odorants, when tested alone, are minimally (if at all) attractive, yet become attractive in the presence of CO2; this reflects the ability of CO2 to activate olfactory host-seeking behaviors, and the synergy between CO2 and host-odors in olfactory attraction. Generally, acetates, alcohols, and ketones are detected by ORs, whereas acids and amines are detected by IRs.
Of the hundreds of known components of human scent, it is clear that only a specific fraction is crucial for host-seeking mosquitoes. Although some of those components elicit physiological and behavioral responses when presented on their own, it is the blend and concentration of those individual components that modulate the responses to host cues in mosquitoes, including An. gambiae (Dekker et al. 2002; Smallegange et al. 2005; Qiu et al. 2011; Majeed et al. 2016). This is supported by recent neurogenetic work in Ae. aegypti mosquitoes that examined the composition of volatile odorants from humans or animals (rat, dog, guinea pig, quail, sheep), and also the mosquito’s olfactory responses to these complex blends (Zhao et al. 2020). Odor profiles were overlapping among humans and nonhuman animals, with human odors enriched for sulcatone, geranylacetone, decanal, undecanal, and acetoin and showing lower relative abundances of hexanal and heptanal (Zhao et al. 2020). Interestingly, a single population of olfactory neurons were more strongly activated by human odor blends than animal odor blends, with decanal and undecanal being the odorants most strongly activating “human” olfactory neurons; conversely, a different olfactory neuron population was more strongly activated by non-human animal odors (Zhao et al. 2020). These data suggest that the brain of the Aedes mosquito can gauge the relative “humanness” of a complex odor blend and possibly use this to guide host-seeking behaviors. It will be critical to determine whether the Anopheles olfactory system shares this coding strategy.
Role of olfaction in the control and plasticity of host-searching and preference
The degree of plasticity in host preference must weigh the trade-off between obtaining a blood meal and avoiding defensive host behaviors as well as other selective pressures in the environment (host diversity, density, and distribution). Although it is widely accepted that An. gambiae mosquitoes show an extreme form of specialization for human hosts, they will readily bite other mammals if humans are unavailable (Lefevre et al. 2009).
It is likely that the organization of the mosquito nervous system underlies host preference. To date, a few studies have investigated the genetic basis of host preference by comparing the genetic makeup of closely related mosquito species with different host preferences. Although An. gambiae specifically prefer human hosts, An. quadriannulatus strongly prefer nonhuman hosts. This preference may be in part determined by the olfactory system. Comparison of the antennal transcriptome of the 2 species revealed differential enrichment of chemosensory genes in the Obp, Ir, and Or gene families (Rinker, Zhou, et al. 2013).
Interestingly, all Obp transcripts were more abundant in the antennae of An. gambiae, with the total reads per kilobase million (RPKM) of detectable Obps twice that of Obps found in An. quadriannulatus. In contrast to the Obps, the Irs and the Ors exhibited widespread variation in transcript abundances. Specifically, Irs varied significantly between the 2 species, with 27 of the 30 showing detectable differences in abundance. Although these species share the same suite of genes encoding ORs, they also showed major deviations in Or transcripts. Although there were no Ors whose antennal expression was specific to An. gambiae, 84% of tuning Ors showed significant differences in expression, with 16 Ors enriched more than 2-fold in one of the species (Rinker, Zhou, et al. 2013). These differences in chemosensory gene abundance in different Anopheles species might mediate host preference, for example by increasing the proportion or sensitivity of OSNs in the periphery that respond to human odors (in anthropophilic strains) or animal odors (in zoophilic strains).
Although the genetically predetermined preference for humans is established before pupal emergence, responsiveness to host odors depends on the mosquito’s age. Females generally refrain from host-seeking and biting ~1-day postemergence (Takken et al. 1998; Omondi et al. 2019). Once maturation is complete, females show a gradual increase in responsiveness to a host at 2–6 days old (Takken et al. 1998). This gradual change in the valence of human odor (from slightly aversive to attractive) as the female mosquito ages from days 1 to 4, is correlated with changes in Or expression. These selected Ors (e.g., Or1, Or2, Or75, which increase ~1.5- to 3-fold) respond to human odorants. During this period, the sensitivity of odorant receptor neurons tuned to human odorants also slightly increases (Omondi et al. 2019). These changes in olfactory sensitivity demonstrate an age-dependent response to host odors. The expression levels of Ae. aegypti chemoreceptors were also found to vary during different life stages (Hill et al. 2021).
Once fully mature and ready to blood-feed, mosquitoes host-seek according to an endogenous timing system. This “circadian clock” system synchronizes many physiological and behavioral processes to occur at a specific time of the day. Blood-feeding behavior in An. gambiae is under circadian regulation as shown under laboratory conditions using membrane feeders (Das and Dimopoulos 2008) and live hosts (Rund et al. 2013). Under natural conditions, An. gambiae mosquitoes are primarily nocturnal biters, exhibiting the highest frequency of biting behavior between 9 pm and midnight (Mathenge et al. 2001). There is a clear time-of-day modulation of olfactory sensitivities that is responsible for this pattern of Anopheles host-searching and biting activity. Specifically, olfactory responses to host odors are stronger a few hours after than before darkness (Rund et al. 2013). It remains to be determined if these changes in olfactory physiology gate the timing of biting behavior.
Blood-feeding as a critical step in disease transmission
In hematophagous mosquitoes, egg production is cyclic and is initiated by a blood meal, which contains necessary proteins for normal egg development and maturation. As shown in Ae. aegypti, major components of human blood such as gamma-globulins, albumin, and hemoglobin (Leeman et al. 2018) are crucial to allow hormonal activation of egg development and increased fecundity (Kogan 1990; Zhou et al. 2007). The same blood proteins are probably important for other vectors including Anopheles mosquitoes.
Following a blood meal, female mosquitoes show a reduction in host-seeking and biting behavior. The initial inhibition probably results from abdominal distension produced by blood ingestion, as demonstrated in Ae. aegypti mosquitoes (Klowden and Lea 1979). This initial short-term reduction in biting is followed by a suppression of host-seeking and biting for several days (Duvall et al. 2019). Aedes aegypti will generally not bite again until their next gonotrophic cycle (time between blood meal and oviposition). Similarly, An. gambiae mosquitoes experience this initial host-seeking and biting reduction 12 h after a blood meal (Klowden and Briegel 1994). Although their initial feeding suppression is probably similar to that of Ae. aegypti, the olfactory system may also cause a short-term suppression of host-seeking. Transcripts for individual tuning AgIrs and AgOrs in the antennae show a general pattern of depletion up to 12–48 h following a blood meal, which may reduce antennal odorant receptivity in vivo (Fox et al. 2001; Rinker, Pitts, et al. 2013).
After the initial suppression of host-seeking behavior, unlike Ae. aegypti females (Klowden 1981), An. gambiae host-seek and bite multiple times during a single gonotrophic cycle. The total caloric reserves, carried over from the larval stages, are considerably lower in teneral (newly emerged) Anophelines than in Culicines (Briegel 1990b). This difference in energy resource may explain how Ae. aegypti mosquitoes can complete a gonotrophic cycle with a single blood meal, whereas An. gambiae cannot. Moreover, this effect seems to be body size dependent. Small An. gambiae individuals need more blood meals than large ones (Lyimo and Takken 1993; Takken et al. 1998) (Briegel and Hörler 1993). Larger mosquitoes contain relatively more metabolic reserves at emergence, and they can produce and lay more eggs per gonotrophic cycle than smaller individuals (Briegel 1990a). Oviposition in Anopheles (see Role of olfaction in oviposition) takes place within 48–60 h after a blood meal (Briegel and Hörler 1993), which marks the end of the gonotrophic cycle. At this point, the female mosquito probably regains her olfactory responsivity to host odors and is ready to host-search again (Klowden 1981).
The need to obtain blood to complete egg development makes blood-feeding a critical step in disease transmission. Although blood-feeding itself probably relies more on gustation and mechanosensation than on olfaction (see Sensory appendages), it marks a transitional phase between plant, host, and oviposition site seeking states of an adult female. Each of these stages is characterized by olfactory changes that maximize the chances of mosquito success. The female mosquito’s reliance on olfaction is critical for her success, but this dependence can be exploited by other organisms. Specifically, the finely tuned olfactory system of host-seeking female Anopheles is vulnerable to parasite-induced changes in host attractiveness and behavioral manipulation by the malaria-causing Plasmodium (see Plasmodium host and Vector manipulation sections). Fortunately, mosquito olfactory sensitivity can also be exploited by humans in vector management and prevention of disease transmission (see Olfaction and vector management).
Role of olfaction in oviposition
After blood-feeding, female mosquitoes develop eggs and subsequently search for an oviposition site (Figure 3). Gravid (egg carrying) females use olfactory cues, among others (visual and tactile), to assess an oviposition substrate and ensure its suitability for their offspring (e.g., presence of food, mosquito immature stages, predators, and pathogens) (Bentley and Day 1989; Afify and Galizia 2015; Day 2016). Mosquito larvae feed on plant infusions and microorganisms that live on them (Merritt et al. 1992), and accordingly, cues of plant infusions and microorganisms have been shown to attract egg-laying mosquitoes. For example, females of An. arabiensis and An. coluzzii showed an oviposition preference to volatile extracts from certain grasses such as the antelope grass (Echinochloa pyramidalis) and hippo grass (Echinochloa stagnina) (Asmare et al. 2017). In addition, females of An. gambiae prefer ovipositing on substrates from their natural breeding sites that contain live microorganisms over sterilized substrates (Sumba et al. 2004). The presence and density of immature mosquito stages can be interpreted by the gravid female differently: limited numbers can indicate a suitable oviposition substrate, whereas higher densities of these stages can indicate competition (Afify and Galizia 2015; Day 2016). A good example for this is the oviposition preference of An. gambiae females for water that contains low densities of C. quinquefasciatus eggs. As the density of C. quinquefasciatus eggs increases in the water, this water becomes repellent to An. gambiae gravid females (Wachira et al. 2010). The developmental stage itself also seems to have an effect on oviposition preference; An. coluzzii gravid females prefer to lay eggs on water containing conspecific first-instar larvae but avoid laying eggs on water with fourth-instar larvae, possibly to limit chances of intraspecific competition or cannibalism (Mwingira et al. 2020). Gravid mosquitoes in these studies were not allowed to touch the test water (Wachira et al. 2010; Mwingira et al. 2020), suggesting that attraction/repellency toward mosquito immature stages is olfaction dependent. Gravid mosquitoes are also known to avoid oviposition sites that contains their predators. For example, An. gambiae s.s. females avoid laying eggs on water that contains the predatory fish Gambusia affinis and Carassius auratus (Chobu et al. 2015).
Mosquitoes use their sensory appendages to detect oviposition cues. Volatile compounds of different plant odors attract An. arabiensis gravid females and activate their antennae in electroantennogram experiments (Wondwosen et al. 2016, 2017, 2018). Oviposition repellent volatiles from overcrowded and resource-deprived An. coluzzii larvae (dimethyl disulfide, dimethyl trisulfide, and 6-methyl-5-hepten-2-one) also elicit an electrophysiological response in the antennae, maxillary palps, and labella of gravid An. coluzzii mosquitoes (Suh et al. 2016). Some An. gambiae ORs, such as AgOr10, strongly respond to oviposition attractants such as 3-methylindole (Carey et al. 2010). In support of an important role for OR signaling in oviposition, gravid An. coluzzii females mutant for orco demonstrated significant defects in attraction to olfactory cues associated with oviposition site selection (Sun, Liu, Ye, et al. 2020). Changes in sensitivity to oviposition and host cues are believed to be regulated across the mosquito gonotrophic cycle, to reflect changes in mosquito behavior. Electrophysiological responses in An. gambiae to oviposition odorants increase after blood-feeding and are accompanied by a decrease in response to host odorants (Qiu et al. 2013). This regulation of odorant sensitivity reflects changes in antennal chemosensory gene expression following blood-feeding (Rinker, Pitts, et al. 2013).
Anopheles olfaction and malaria transmission
The Plasmodium blood parasite, vectored by Anopheles mosquitoes, causes malaria that affects over 200 million people worldwide, with 400 000 deaths annually (WHO 2019). For successful transmission, this parasite depends on both its vertebrate host and mosquito vector to complete different phases of its lifecycle (Figure 4). Only P. ovale, P. malariae, P. knowlesi, P. falciparum, and P. vivax infect humans, with the latter 2 being the deadliest and most prevalent (CDC 2020).
There is regional variation in incidence and seasonality of malaria, with high prevalence in Sub-Saharan Africa and Asia. Climatic conditions, host susceptibility, as well as distribution and biological characteristic of vectors (e.g., dominant mosquito species, vectorial capacity) determine this variation (Kiszewski et al. 2004). In Africa, the An. gambiae complex (including An. gambiae s.s., An. coluzzii, and An. arabiensis) and An. funestus codominate, whereas An. darlingi dominates the Americas (Sinka et al. 2010, 2012; Autino et al. 2012; Kyalo et al. 2017; Irish et al. 2020). In the Asian-Pacific region, multiple mosquito species including An. stephensi and An. culicifacies complex are recorded as major malaria vectors (Sinka et al. 2012; Suwonkerd et al. 2013). Although there are ~480 formally recognized species of Anopheles, only 30–40 of them are capable of transmitting malaria under natural conditions and are thus considered dominant vector of malaria (Autino et al. 2012; Harbach 2013; CDC 2020).
Transmission of Plasmodium parasite is also dependent on the female mosquito’s physiology and her olfactory-guided host-searching abilities. A female bites at least twice to transmit malaria: first ingesting of the gametocyte stage of Plasmodium from an infected host and then passing the sporozoite stage to a new uninfected host (Figure 4). The time between these bites is ~12–14 days to allow sporozoites development in the Anopheles mosquito midgut and migration to the salivary glands (Shaw and Catteruccia 2019). To enhance this transmission, Plasmodium may manipulate both the vector’s propensity to bite and the host’s body odor to increase the chances of interaction between the vector and the host.
Plasmodium host odor modification for increased mosquito attraction
To increase the likelihood of a female biting an infected host, the Plasmodium parasite modifies the host’s odor to make the host more attractive to blood-seeking mosquitoes. This modification can include changes in the relative amounts of certain components of the host emanate (exhaled breath, specific body parts, and whole-body odors) (De Moraes et al. 2014; Berna et al. 2015; de Boer et al. 2017) and the production of novel components (Kelly et al. 2015; Emami et al. 2017).
Notable increases in aldehydes (e.g., heptanal, octanal, nonanal) (Robinson et al. 2018) and thioethers (Berna et al. 2015) and decreases in some ketones (e.g., 6-methyl-5-hepten-2-one) (de Boer et al. 2017) occur in host body odor and breath during malaria infection. These changes result in different volatile profiles of symptomatic (overall decrease in emission) and asymptomatic (overall increase in emission) malaria carriers compared with uninfected individuals (De Moraes et al. 2018). These malaria-induced changes in the host odor profile do not depend on the infection itself, but rather on the developmental stage of the Plasmodium parasite inside the host. Specifically, marked changes in host odor and mosquito attraction are observed between infectious (sexual gametocytes) and noninfectious (asexual schizont) stages of malaria in vertebrate hosts. Since the gametocyte stage of Plasmodium needs to complete its lifecycle in a mosquito (Figure 4), the parasite may increase the attractiveness of hosts that carry this infection stage. In dual-choice laboratory and semifield studies, gametocyte-carrying individuals (both human and nonhuman) attract more Anopheles mosquitoes compared with those carrying asexual stages of Plasmodium or those being malaria-free (Lacroix et al. 2005; Batista et al. 2014; De Moraes et al. 2014; Busula, Bousema, et al. 2017; Robinson et al. 2018). Therefore, malaria induces stage-specific changes in the host volatile profile to increase attraction of mosquitoes to hosts most likely carrying the infectious stage of malaria, ensuring transmission.
Additionally, certain volatiles such as terpenes in host odor and exhaled breath profiles are induced by Plasmodium itself (Kelly et al. 2015; Emami et al. 2017) or result from an interaction between host and parasite metabolic pathways (Berna et al. 2015; Kelly et al. 2015; de Boer et al. 2017; Schaber et al. 2018). Plasmodium-produced isoprenoid metabolic precursor, (E)-4-hydroxy-3-methyl-but2-enyl pyrophosphate (HMBPP), is responsible for increased release of CO2 gas, aldehydes, and monoterpenes (including α- and β-pinene) in cultured human red blood cells. Similarly, certain thioether breath components (e.g., (Z)-1-methylthio-1-propene and 1-methylthio-propane) increase from barely detectable to 50–100 times higher over the course of malaria infection, only to drastically decline just hours later upon antimalaria drug administration. Consequently, parasite modifications of the host emanate profiles can enhance mosquito attraction.
Moreover, changes in human odor profile during malaria infection are amplified by skin microflora, involved in the production of volatiles that mosquitoes respond to during host-searching (Braks et al. 1999; Busula, Verhulst, et al. 2017; de Boer et al. 2017). Malaria-associated compounds detected during early infection (e.g., 2-and 3-methylbutanal and 3-hydroxy-2-butanone) are produced by skin bacteria, and not by Plasmodium (de Boer et al. 2017). The release of these compounds by the skin bacteria may explain the long-lasting effects on host odor profile (and probably mosquito attraction) following immune challenge with malaria, even after gametocytes have been cleared from the blood (i.e., when the host is no longer infective) (de Boer et al. 2017).
Plasmodium vector manipulation for increased host-seeking and biting
Once a female Anopheles has blood fed on a Plasmodium-infected host, behavioral and physiological modifications may occur if she becomes infected by the parasite (Hurd 2003; Stanczyk et al. 2017). These modifications, dependent on the Plasmodium developmental stage the vector carries, are manifested as altered biting motivation and efficiency. Mosquitoes harboring the nontransmittable oocyte stage of Plasmodium reduced both the biting persistence and host attraction to humans (Anderson et al. 1999; Cator et al. 2013). Only when the transmittable sporozoite stage was present did increases in host attraction and the number of close interactions (landing, biting persistence, blood-feeding frequency, and meal size) occur in the females (Koella et al. 1998; Anderson et al. 1999; Cator et al. 2013; Smallegange et al. 2013). Consequently, chances of malaria transmission to new hosts would be higher by mosquitoes carrying the infective stages of parasite due to increased host attraction compared with those carrying the noninfective stages. These behavioral modifications by Plasmodium appear to be species specific as some Anopheles show no changes in host-seeking behavior (Vantaux et al. 2015; Nguyen et al. 2017). Thus, the sensory bias of infected mosquitoes (i.e., vector manipulation by the Plasmodium parasite) might not be universal. Other nonhost-seeking behavioral modification of the Plasmodium-infected mosquitoes is observed during foraging and nectar feeding, which suggests broad changes in the mosquito olfactory system (Nyasembe, Teal Peter, et al. 2014).
Changes in olfaction, which ultimately result in these modified behaviors, originate from altered sensitivities and detection thresholds of the sensory appendages (Cator et al. 2013; Stanczyk et al. 2019), and changes in protein expression in the brain (Lefevre et al. 2007; Emami et al. 2017). The changes in olfactory sensitivities, and thus behavioral responses to host cues, are species specific and seem to depend on mosquito–Plasmodium pairings. This pattern is exemplified by differential antennal and maxillary palp responses of malaria infected and uninfected species of Anopheles to human volatile extracts including known mosquito attractants (e.g., lactic acid, 1-octen-3-ol, benzothiazole) (Cator et al. 2013; Grant et al. 2013; Stanczyk et al. 2019). Moreover, physiological responses to some of those odorants can decrease at the noninfective oocyte stage, but increase once the sporozoites invade the salivary glands of the females to make them infectious (Cator et al. 2013). Similarly, differential expression of several proteins involved in metabolism and neuronal function is observed in the head proteome of An. gambiae infected with the sporozoite (i.e., infective) stage of P. berghei (Lefevre et al. 2007). Upregulation of genes with modulatory functions in neural synapses can also occur after ingesting a blood meal containing a Plasmodium-produced compound (Emami et al. 2017). Together, these findings suggest that the presence of Plasmodium in female mosquitoes may lead to changes in gene expression, olfactory sensitivity, and behavior to favor malaria parasite transmission.
It is still unclear if the changes in mosquito behavior and olfactory sensitivities result from direct manipulation of the vector by Plasmodium or if they are associated with an immune response and overall physiological adjustment to infection. Similar behavioral effects and altered olfactory sensitivity experienced by a Plasmodium-infected mosquito can be induced by immune challenge by other microorganisms. Direct injection of heat-killed Escherichia coli bacteria in An. stephensi, after a blood meal from an uninfected host, mirrors the behavioral and neurophysiological modifications of infection with Plasmodium (Cator et al. 2013). Similarly, even if An. gambiae females are not infected after ingesting a Plasmodium-containing blood meal, they respond less to certain odorants, indicating modification of olfactory organ sensitivity following immune challenge (Stanczyk et al. 2019). These data suggest that changes in the mosquito olfactory system might not be exclusively induced by Plasmodium.
Olfaction and vector management
Integrated vector management (IVM) is the rational use of multiple resources and strategies for the purpose of vector control. This concept includes the use of different strategies of intervention, including chemical (e.g., indoor residual spraying) and biological methods (e.g., using natural enemies) instead of a single-intervention approach to successfully control insect vectors (Beier et al. 2008). In this review, we focus on strategies exploiting mosquito olfaction to reduce vector–human interaction to decrease chances of disease transmission. These strategies include the use of odorants to attract or repel host-seeking mosquitoes, disrupt mosquito mating, and prevent mosquito oviposition (Wooding et al. 2020).
Repellents and attractants of host-seeking mosquitoes
To successfully prevent mosquito biting, a push/pull strategy can be used. This strategy takes advantage of mosquito dependence on olfaction to find humans for blood-feeding, and uses repellents to “push” mosquitoes away from humans or their dwellings and “pull” them with attractants toward alternative hosts or toward traps to kill them (Cook et al. 2007).
Mosquito repellents are a group of odorants that prevent mosquitoes from locating their hosts (e.g., humans) and hence disrupt blood-feeding. Repellents can be applied directly on the skin or clothes (topical repellents) or used to protect an area or space from biting mosquitoes (spatial repellents) (Debboun et al. 2014). There are 2 types of topical repellents: 1) plant-based repellents such as lemongrass oil, eugenol (extracted from clove oil), nepetalactone (extracted from catnip), and para-methane 3-8, diol (PMD; extracted from lemon–eucalyptus essential oil), and 2) synthetic repellents such as DEET, Picaridin, and IR3535 (Debboun et al. 2014).
Plant-based repellents have been shown to work against Anopheles mosquitoes (Maia and Moore 2011; Debboun et al. 2014; Afify and Potter 2020). Nepetalactone (the active repellent ingredient in catnip), for example, appears to repel many insects, including An. coluzzii mosquitoes (Melo et al. 2021). Interestingly, nepetalactone may act by directly stimulating TRPA1 receptors: TRPA1 directly activates heterologously expressed Ae. aegypti and Drosophila TRPA1 receptors, and Ae. aegypti and Drosophila TRPA1 mutants are no longer repelled by nepetalactone (Melo et al. 2021). It remains to be determined if nepetalactone also activates Anopheles TRPA1 receptors to mediate repellency to catnip in Anopheles mosquitoes. Nonetheless, most natural repellents evaporate quickly after application and therefore offer relatively short protection time (Logan, Stanczyk, et al. 2010; Maia and Moore 2011).
On the other hand, synthetic repellents like DEET are less volatile (Spencer et al. 1979), making DEET the gold standard in long-lasting mosquito repellents (Debboun et al. 2014). There are different hypotheses on repellents’ mode of action. A repellent could act directly on the mosquitoes by activating chemoreceptors on their sensory appendages to elicit a repellent behavior (Boeckh et al. 1996; Ditzen et al. 2008; Syed and Leal 2008; Afify et al. 2019; Afify and Potter 2020) or by modulating OR activity in response to attractive odorants (Bohbot and Dickens 2010; Pellegrino et al. 2011). Repellents could also act at a physical level by reducing the volatility of odorants on human skin and preventing them from reaching the mosquito (Syed and Leal 2008; Afify et al. 2019). Mosquito repellents might also work in more than one of these modes of action (Bohbot et al. 2011). The modes of action for mosquito repellents are species specific (Afify and Potter 2020). In An. coluzzii, plant-based repellents have been shown to strongly activate chemoreceptors on the antennae, probably leading to a repellent olfactory signal that drives repellent behaviors. In contrast, synthetic repellents (DEET, Picaridin, IR3535) probably do not activate An. coluzzii chemoreceptors as a mechanism for mediating repulsion as close-range olfactory behavioral assays with these repellents revealed no olfactory repulsion (Afify and Potter 2020). Odor-dependent activity monitoring of Orco-positive olfactory neurons instead suggests that these synthetic repellents may function primarily to mask the olfactory responses triggered by attractive odorants (Afify et al. 2019). The likely olfactory mechanism is by decreasing the volatility of these odorants (Syed and Leal 2008; Afify et al. 2019). The search for safe, efficient, and long-lasting new repellents can benefit from recent advances in understanding how currently used repellents function.
Repellents can also be used to prevent mosquito biting in a 3D space (spatial or area repellents). These spatial repellents are either volatile enough to release in the air at ambient conditions or require active aerosolization or applying heat to volatilize the active ingredients (e.g., mosquito coils, candles, and torches) (Debboun et al. 2014). Spatial repellents can be plant based (like some topical repellents) (Maia and Moore 2011) or synthetic (like pyrethroids) (Debboun et al. 2014). Pyrethroids are frequently used insecticides with high knockdown activity that also repel mosquitoes at sublethal doses (Debboun et al. 2014). Laboratory studies showed that some pyrethroids repel Anopheles mosquitoes on contact (irritant effect), whereas others have a noncontact repellent effect, suggesting that they work through olfaction (Chareonviriyaphap et al. 1997; Dusfour et al. 2009; Kawada et al. 2014). In field studies, pyrethroid compounds have been shown to reduce house entrance and increase house exiting of Anopheles mosquitoes, suggesting that they can play a significant role in malaria vector control (Grieco et al. 2000; Kawada et al. 2008).
To lure mosquitoes toward traps, attractive odorants involved in host finding, such as CO2 (Mboera et al. 2000; Rueda et al. 2001; Cooper et al. 2004; Njiru et al. 2006), ammonia (Njiru et al. 2006), and 1-octen-3-ol (Rueda et al. 2001; Cooper et al. 2004; Njiru et al. 2006), have been used to successfully capture Anopheles mosquitoes in field trials. Although these attractants can work individually, the combination of multiple attractants has been shown to increase their effectiveness in attracting Anopheles mosquitoes (Rueda et al. 2001; Cooper et al. 2004; Smallegange et al. 2005; Njiru et al. 2006). However, an odorant blend as attractive as a human host has not yet been identified.
The attraction of mosquitoes to various people can differ (see Role of olfaction in mosquito attraction to human scent), and an approach to identify new insect repellents has been to identify human odorants that reduce attraction and to utilize these chemicals as insect repellents (Logan et al. 2008; Logan, Stanczyk, et al. 2010). A number of human-derived odorants were identified to reduce An. gambiae mosquito attraction, including decanal, octanal, 6-methyl-hepten-2-one (also known as sulcatone), and geranylacetone, and mixtures of these odorants provided strong protection in arm-in-cage biting assays (Logan, Stanczyk, et al. 2010). Since these chemicals are typically found on human skin, they are more likely to be safe for human use and could provide promising alternatives to plant-derived repellents.
The response to attractive odorants has been shown to be concentration dependent in Anopheles. High concentrations of the attractants 1-octen-3-ol and benzaldehyde were found to be repellent to An. coluzzii (Afify and Potter 2020). In addition, higher concentrations of these odorants activate more antennal olfactory receptor neurons than lower concentrations, suggesting that high concentrations of normally attractive odorants may activate repellent-sensing neurons or globally activate many olfactory neurons leading to a repellent-like effect (Afify and Potter 2020).
Vector management: mating disruption
The knowledge about the olfactory aspect of mosquito mating is limited (see Role of olfaction in mating). Aggregation pheromones play a role in Ae. aegypti mating (Fawaz et al. 2014) and have also been recently identified in Anopheles (Mozūraitis et al. 2020). These aggregation pheromones could be used to attract mosquitoes into traps or disrupt mosquito mating (Vaníčková et al. 2017). Male Anopheles mosquitoes show some attraction to human odorants, suggesting that these volatiles may play a role in swarm formation and mating (Foster and Takken 2004). In addition, male Anopheles mosquitoes are more attracted to nectar volatiles than to human odorants (Foster and Takken 2004). These volatiles can be used to attract and capture male mosquitoes before swarming and thereby disrupt or decrease mating (Foster and Hancock 1994).
Vector management: oviposition site selection disruption
A push/pull approach can also be applied using mosquito oviposition cues. Oviposition repellents can prevent Anopheles mosquito egg laying around human dwellings, and oviposition attractants can be used to direct gravid mosquitoes into traps (Seenivasagan et al. 2019; Schoelitsz et al. 2020). Additionally, oviposition cues can be used for mosquito control in an autodissemination approach, where oviposition attractant is used to lure gravid mosquitoes into traps treated with the insect growth regulator pyriproxyfen. These gravid mosquitoes subsequently transfer the insect growth regulator to their natural oviposition sites, where the pyriproxyfen kills mosquito larvae. The benefit of this approach is that it enables broader coverage of pyriproxyfen and treats harder to reach mosquito habitats. This method was first developed to control container-breeding mosquitoes, such as Ae. aegypti (Itoh et al. 1994; Devine et al. 2009), and was later successfully tested in semifield studies against Anopheles mosquitoes (Lwetoijera et al. 2014, 2019; Mbare et al. 2014). The use of oviposition cues in push/pull and autodissemination approaches can complement other olfaction-based methods to efficiently limit Anopheles mosquito biting and malaria transmission.
Conclusion
To be a vector of malaria, the Anopheles mosquito must first locate, identify, and distinguish a human in its environment. If an Anopheles female mosquito bites a Plasmodium-infected person, it too can become infected, develop Plasmodium parasites, and spread this infection to other humans with her next bite. Before infection spreads, the female mosquito cycles through a number of behaviors such as foraging, mating, and ovipositing. As discussed in this review, each of these steps involves olfaction. We suggest that interfering with Anopheles olfaction is a potent strategy for modifying mosquito behavior, with each behavioral step representing an opportunity to target olfaction and reduce the spread of disease.
The current revolution in genetic engineering, fostered by the application of CRISPR/Cas9, will herald in a wave of new discoveries regarding Anopheles olfaction. These new technologies will allow the generation of targeted mutations or genetic knock-ins to capture olfactory expression patterns. In this review, we identified many open questions that we expect will be addressed by applying such new methods. CRISPR/Cas9 has also enabled the development of gene drives methods that can be used to decimate entire mosquito populations (Adolfi et al. 2020; Carballar-Lejarazú et al. 2020; Simoni et al. 2020). The decision to enact such measures requires rigorous research and debate. Gene-drive mechanisms could also be used to target the Anopheles olfactory system, and hence alter olfactory host-seeking behaviors.
The world is full of odors, and Anopheles mosquitoes, like most insects, have evolved sensitive olfactory systems to turn volatile chemicals into perceptions of the external world. Ultimately, the mosquito brain combines information from other sensory systems (such as hearing, taste, vision, thermoreception) to make optimal behavioral decisions. In the years to come, investigation of Anopheles olfaction will play a central role in the emerging field of mosquito sensory biology.
Authors’ note
The An. gambiae mosquito (of the An. gambiae species complex) can be categorized into an S form (An. gambiae ss) and M form (Anopheles coluzzii) (della Torre et al. 2001; Coetzee et al. 2013). These 2 forms are morphologically indistinguishable and require molecular techniques for identification (Powell et al. 2014). The M and S forms exhibit a range of hybridization depending on the geographical region examined; for example, in the Cameroon, reproductive isolation is complete (Wondji et al. 2005), whereas in The Gambia, M/S hybrids have been found in frequencies nearing 17% (Caputo et al. 2008). Since molecular methods are required to distinguish M versus S forms, many studies may not necessarily report which An. gambiae form (M or S) was under investigation. In this review, the specific form is specified if known (An. gambiae s.s. for S form and Anopheles coluzzii for M form). If a distinction is not made in the review (An. gambiae), either form may have been the species used in the study.
Acknowledgments
We thank D. Poinapen for comments on an earlier version of this manuscript.
Funding
This work was supported by grants from the Department of Defense to C.J.P. (W81XWH-17-PRMRP), the National Institutes of Health (NIH) to C.J.P. (National Institue of Allergy and Infectious Diseases R01Al137078), the Johns Hopkins Malaria Research Institute Pilot Fund to C.J.P., Natural Science and Engineering Research Council Postdoctoral Fellowship (NSERC-PDF) to J.K.K, and a Johns Hopkins Malaria Research Institute Postdoctoral Fellowship to S.M. We thank the Johns Hopkins Malaria Research Institute and Bloomberg Philanthropies for their support.
Conflict of interest
The authors declare no conflict of interest.
References
- Abuin L, Bargeton B, Ulbrich MH, Isacoff EY, Kellenberger S, Benton R. 2011. Functional architecture of olfactory ionotropic glutamate receptors. Neuron. 69(1):44–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolfi A, Gantz VM, Jasinskiene N, Lee HF, Hwang K, Terradas G, Bulger EA, Ramaiah A, Bennett JB, Emerson JJ, et al. 2020. Efficient population modification gene-drive rescue system in the malaria mosquito Anopheles stephensi. Nat Commun. 11(1):5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afify A, Betz JF, Riabinina O, Lahondère C, Potter CJ. 2019. Commonly used insect repellents hide human odors from Anopheles mosquitoes. Curr Biol. 29(21):3669–3680.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afify A, Galizia CG. 2015. Chemosensory cues for mosquito oviposition site selection. J Med Entomol. 52(2):120–130. [DOI] [PubMed] [Google Scholar]
- Afify A, Potter CJ. 2020. Insect repellents mediate species-specific olfactory behaviours in mosquitoes. Malar J. 19(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge RL, Britch SC, Allan SA, Tsikolia M, Calix LC, Bernier UR, Linthicum KJ. 2016. Comparison of volatiles and mosquito capture efficacy for three carbohydrate sources in a yeast-fermentation CO2 generator. J Am Mosq Control Assoc. 32(4):282–291. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Koella JC, Hurd H. 1999. The effect of Plasmodium yoelii nigeriensis infection on the feeding persistence of Anopheles stephensi Liston throughout the sporogonic cycle. Proc Biol Sci. 266(1430):1729–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell J, Hamilton KA, Pinder M, Walraven GE, Lindsay SW. 2002. Short-range attractiveness of pregnant women to Anopheles gambiae mosquitoes. Trans R Soc Trop Med Hyg. 96(2):113–116. [DOI] [PubMed] [Google Scholar]
- Anton S, Rospars JP. 2004. Quantitative analysis of olfactory receptor neuron projections in the antennal lobe of the malaria mosquito, Anopheles gambiae. J Comp Neurol. 475(3):315–326. [DOI] [PubMed] [Google Scholar]
- Anton S, van Loon JJ, Meijerink J, Smid HM, Takken W, Rospars JP. 2003. Central projections of olfactory receptor neurons from single antennal and palpal sensilla in mosquitoes. Arthropod Struct Dev. 32(4):319–327. [DOI] [PubMed] [Google Scholar]
- Arcà B, Ribeiro JM. 2018. Saliva of hematophagous insects: a multifaceted toolkit. Curr Opin Insect Sci. 29:102–109. [DOI] [PubMed] [Google Scholar]
- Asmare Y, Hill SR, Hopkins RJ, Tekie H, Ignell R. 2017. The role of grass volatiles on oviposition site selection by Anopheles arabiensis and Anopheles coluzzii. Malar J. 16(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autino B, Noris A, Russo R, Castelli F. 2012. Epidemiology of malaria in endemic areas. Mediterr J Hematol Infect Dis. 4(1):e2012060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker HG, Baker I. 1973. Amino-acids in nectar and their evolutionary significance. Nature. 241:543–545.4693956 [Google Scholar]
- Bakker JW, Loy DE, Takken W, Hahn BH, Verhulst NO. 2020. Attraction of mosquitoes to primate odours and implications for zoonotic Plasmodium transmission. Med Vet Entomol. 34(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barredo E, DeGennaro M. 2020. Not Just from Blood: Mosquito Nutrient Acquisition from Nectar Sources. Trends Parasitol. 36(5):473–484. [DOI] [PubMed] [Google Scholar]
- Batista EP, Costa EF, Silva AA. 2014. Anopheles darlingi (Diptera: Culicidae) displays increased attractiveness to infected individuals with Plasmodium vivax gametocytes. Parasit Vectors. 7:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier JC, Keating J, Githure JI, Macdonald MB, Impoinvil DE, Novak RJ. 2008. Integrated vector management for malaria control. Malar J. 7(Suppl 1):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benelli G, Jeffries CL, Walker T. 2016. Biological control of mosquito vectors: Past, present, and future. Insects. 7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley MD, Day JF. 1989. Chemical ecology and behavioral aspects of mosquito oviposition. Annu Rev Entomol. 34:401–421. [DOI] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. 2009. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 136(1):149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Vosshall LB. 2007. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature. 450(7167):289–293. [DOI] [PubMed] [Google Scholar]
- Berna AZ, McCarthy JS, Wang RX, Saliba KJ, Bravo FG, Cassells J, Padovan B, Trowell SC. 2015. Analysis of breath specimens for biomarkers of Plasmodium falciparum infection. J Infect Dis. 212(7):1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier UR, Kline DL, Barnard DR, Schreck CE, Yost RA. 2000. Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for the yellow fever mosquito (Aedes aegypti). Anal Chem. 72(4):747–756. [DOI] [PubMed] [Google Scholar]
- Biessmann H, Andronopoulou E, Biessmann MR, Douris V, Dimitratos SD, Eliopoulos E, Guerin PM, Iatrou K, Justice RW, Kröber T, et al. 2010. The Anopheles gambiae odorant binding protein 1 (AgamOBP1) mediates indole recognition in the antennae of female mosquitoes. PLoS One. 5(3):e9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckh J, Breer H, Geier M, Hoever F-P, Krüger B-W, Nentwig G, Sass H. 1996. Acylated 1,3-aminopropanols as repellents against bloodsucking arthropods. Pestic Sci. 48:359–373. [Google Scholar]
- Bohbot JD, Dickens JC. 2010. Insect repellents: modulators of mosquito odorant receptor activity. PLoS One. 5(8):e12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot JD, Fu L, Le TC, Chauhan KR, Cantrell CL, Dickens JC. 2011. Multiple activities of insect repellents on odorant receptors in mosquitoes. Med Vet Entomol. 25(4):436–444. [DOI] [PubMed] [Google Scholar]
- Boo KS. 1980. Fine structure of the antennal sensory hairs in female Anopheles stephensi. Z Parasitenkd. 61(2):161–171. [DOI] [PubMed] [Google Scholar]
- Bowen MF. 1991. The sensory physiology of host-seeking behavior in mosquitoes. Annu Rev Entomol. 36:139–158. [DOI] [PubMed] [Google Scholar]
- Braks MA, Anderson RA, Knols BG. 1999. Infochemicals in mosquito host selection: human skin microflora and Plasmodium parasites. Parasitol Today. 15(10):409–413. [DOI] [PubMed] [Google Scholar]
- Braks M, Cork A, Takken W. 1997. Olfactometer studies on the attraction of Anopheles gambiae sensu stricto (Diptera: Culicidae) to human sweat. In: Sommeijer MJ, Francke PJ, editors. Proceedings of the Section Experimental and Applied Entomology of the Netherlands Entomological Society, Amsterdam, The Netherlands. Available from: https://research.wur.nl/en/publications/olfactometer-studies-on-the-attraction-of-anopheles-gambiae-sensu. [Google Scholar]
- Braks MAH, Scholte EJ, Takken W, Dekker T. 2000. Microbial growth enhances the attractiveness of human sweat for the malaria mosquito, Anopheles gambiae sensu stricto (Diptera: Culicidae). Chemoecology. 10:129–134. [Google Scholar]
- Braks MAH, Takken W. 1999. Incubated human sweat but not fresh sweat attracts the malaria mosquito Anopheles gambiae sensu stricto. J Chem Ecol. 25:663–672. [Google Scholar]
- Briegel H. 1990a. Fecundity, metabolism, and body size in Anopheles (Diptera: Culicidae), vectors of malaria. J Med Entomol. 27(5):839–850. [DOI] [PubMed] [Google Scholar]
- Briegel H. 1990b. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J Insect Physiol. 36:165–172. [Google Scholar]
- Briegel H, Hörler E. 1993. Multiple blood meals as a reproductive strategy in Anopheles (Diptera: Culicidae). J Med Entomol. 30(6):975–985. [DOI] [PubMed] [Google Scholar]
- Bryan JH, Smalley ME. 1978. The use of ABO blood groups as markers for mosquito biting studies. Trans R Soc Trop Med Hyg. 72(4):357–360. [DOI] [PubMed] [Google Scholar]
- Busula AO, Bousema T, Mweresa CK, Masiga D, Logan JG, Sauerwein RW, Verhulst NO, Takken W, de Boer JG. 2017. Gametocytemia and Attractiveness of Plasmodium falciparum-Infected Kenyan Children to Anopheles gambiae Mosquitoes. J Infect Dis. 216(3):291–295. [DOI] [PubMed] [Google Scholar]
- Busula AO, Verhulst NO, Bousema T, Takken W, de Boer JG. 2017. Mechanisms of Plasmodium-enhanced attraction of mosquito vectors. Trends Parasitol. 33(12):961–973. [DOI] [PubMed] [Google Scholar]
- Butterwick JA, Del Mármol J, Kim KH, Kahlson MA, Rogow JA, Walz T, Ruta V. 2018. Cryo-EM structure of the insect olfactory receptor Orco. Nature. 560(7719):447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera M, Jaffe K. 2007. An aggregation pheromone modulates lekking behavior in the vector mosquito Aedes aegypti (Diptera: Culicidae). J Am Mosq Control Assoc. 23(1):1–10. [DOI] [PubMed] [Google Scholar]
- Caputo B, Nwakanma D, Jawara M, Adiamoh M, Dia I, Konate L, Petrarca V, Conway DJ, della Torre A. 2008. Anopheles gambiae complex along The Gambia river, with particular reference to the molecular forms of An. gambiae s.s. Malar J. 7:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballar-Lejarazú R, Ogaugwu C, Tushar T, Kelsey A, Pham TB, Murphy J, Schmidt H, Lee Y, Lanzaro GC, James AA. 2020. Next-generation gene drive for population modification of the malaria vector mosquito, Anopheles gambiae. Proc Natl Acad Sci U S A. 117(37):22805–22814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AF, Wang G, Su CY, Zwiebel LJ, Carlson JR. 2010. Odorant reception in the malaria mosquito Anopheles gambiae. Nature. 464(7285):66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale P, Frezil JL, Bosseno MF, Le Pont F. 1978. Etude de l’agressivite d’anopheles gambiae a en fonction de l’age et du sexe des sujets humains. Bull l’Organ mond Sante. 56:147–154. [PMC free article] [PubMed] [Google Scholar]
- Cassau S, Krieger J. 2021. The role of SNMPs in insect olfaction. Cell Tissue Res. 383(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cator LJ, George J, Blanford S, Murdock CC, Baker TC, Read AF, Thomas MB. 2013. ‘Manipulation’ without the parasite: altered feeding behaviour of mosquitoes is not dependent on infection with malaria parasites. Proc Biol Sci. 280(1763):20130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . 2020. Malaria biology. Available from: https://www.cdc.gov/malaria/about/biology/index.html [accessed 12 November 2020].
- Chareonviriyaphap T, Roberts DR, Andre RG, Harlan HJ, Manguin S, Bangs MJ. 1997. Pesticide avoidance behavior in Anopheles albimanus, a malaria vector in the Americas. J Am Mosq Control Assoc. 13(2):171–183. PMID: 9249657. [PubMed] [Google Scholar]
- Charlwood JD, Jones MDR. 1979. Mating behaviour in the mosquito, Anopheles gambiae s.l. save. Physiol Entomol. 4:111–120. [Google Scholar]
- Charlwood JD, Pinto J, Sousa CA, Ferreira C, Petrarca V, Rosario Vdo E. 2003. ‘A mate or a meal’ – pre-gravid behaviour of female Anopheles gambiae from the islands of São Tomé and Príncipe, West Africa. Malar J. 2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobu M, Nkwengulila G, Mahande AM, Mwang’onde BJ, Kweka EJ. 2015. Direct and indirect effect of predators on Anopheles gambiae sensu stricto. Acta Trop. 142:131–137. [DOI] [PubMed] [Google Scholar]
- Choo YM, Buss GK, Tan K, Leal WS. 2015. Multitasking roles of mosquito labrum in oviposition and blood feeding. Front Physiol. 6:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements AN. 1999. The biology of mosquitoes. Oxford: CAB International. [Google Scholar]
- Coetzee M, Hunt RH, Wilkerson R, Della Torre A, Coulibaly MB, Besansky NJ. 2013. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa. 3619:246–274. [PubMed] [Google Scholar]
- Cook SM, Khan ZR, Pickett JA. 2007. The use of push-pull strategies in integrated pest management. Annu Rev Entomol. 52:375–400. [DOI] [PubMed] [Google Scholar]
- Cooper RD, Frances SP, Popat S, Waterson DG. 2004. The effectiveness of light, 1-octen-3-ol, and carbon dioxide as attractants for anopheline mosquitoes in Madang Province, Papua New Guinea. J Am Mosq Control Assoc. 20(3):239–242. [PubMed] [Google Scholar]
- Costantini C, Birkett MA, Gibson G, Ziesmann J, Sagnon NF, Mohammed HA, Coluzzi M, Pickett JA. 2001. Electroantennogram and behavioural responses of the malaria vector Anopheles gambiae to human-specific sweat components. Med Vet Entomol. 15(3):259–266. [DOI] [PubMed] [Google Scholar]
- Costantini C, Sagnon NF, della Torre A, Diallo M, Brady J, Gibson G, Coluzzi M. 1998. Odor-mediated host preferences of West African mosquitoes, with particular reference to malaria vectors. Am J Trop Med Hyg. 58(1):56–63. [DOI] [PubMed] [Google Scholar]
- Coutinho-Abreu IV, Sharma K, Cui L, Yan G, Ray A. 2019. Odorant ligands for the CO2 receptor in two Anopheles vectors of malaria. Sci Rep. 9(1):2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto A, Alenius M, Dickson BJ. 2005. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 15(17):1535–1547. [DOI] [PubMed] [Google Scholar]
- Curran AM, Rabin SI, Furton KG. 2005. Analysis of the uniqueness and persistence of human scent. Forensic Sci Commun. 7(2). [Google Scholar]
- Curran AM, Rabin SI, Prada PA, Furton KG. 2005. Comparison of the volatile organic compounds present in human odor using SPME-GC/MS. J Chem Ecol. 31(7):1607–1619. [DOI] [PubMed] [Google Scholar]
- Das S, Dimopoulos G. 2008. Molecular analysis of photic inhibition of blood-feeding in Anopheles gambiae. BMC Physiol. 8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JF. 2016. Mosquito oviposition behavior and vector control. Insects. 7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer JG, Robinson A, Powers SJ, Burgers SLGE, Caulfield JC, Birkett MA, Smallegange RC, van Genderen PJJ, Bousema T, Sauerwein RW, et al. 2017. Odours of Plasmodium falciparum-infected participants influence mosquito-host interactions. Sci Rep. 7(1):9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moraes CM, Stanczyk NM, Betz HS, Pulido H, Sim DG, Read AF, Mescher MC. 2014. Malaria-induced changes in host odors enhance mosquito attraction. Proc Natl Acad Sci USA. 111(30): 11079–11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moraes CM, Wanjiku C, Stanczyk NM, Pulido H, Sims JW, Betz HS, Read AF, Torto B, Mescher MC. 2018. Volatile biomarkers of symptomatic and asymptomatic malaria infection in humans. Proc Natl Acad Sci USA. 115(22):5780–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debboun M, Frances S, Strickman D. 2014. Insect repellents handbook. Boca Raton (FL): CRC Press. [Google Scholar]
- Deng Y, Zhang W, Farhat K, Oberland S, Gisselmann G, Neuhaus EM. 2011. The stimulatory Gα(s) protein is involved in olfactory signal transduction in Drosophila. PLoS One. 6(4):e18605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGennaro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, Goldman C, Jasinskiene N, James AA, Vosshall LB. 2013. Orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature. 498(7455):487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker T, Geier M, Cardé RT. 2005. Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J Exp Biol. 208(Pt 15):2963–2972. [DOI] [PubMed] [Google Scholar]
- Dekker T, Steib B, Cardé RT, Geier M. 2002. L-lactic acid: a human-signifying host cue for the anthropophilic mosquito Anopheles gambiae. Med Vet Entomol. 16(1):91–98. [DOI] [PubMed] [Google Scholar]
- della Torre A, Fanello C, Akogbeto M, Dossou-yovo J, Favia G, Petrarca V, Coluzzi M. 2001. Molecular evidence of incipient speciation within Anopheles gambiae s.s. in West Africa. Insect Mol Biol. 10(1):9–18. [DOI] [PubMed] [Google Scholar]
- Devine GJ, Perea EZ, Killeen GF, Stancil JD, Clark SJ, Morrison AC. 2009. Using adult mosquitoes to transfer insecticides to Aedes aegypti larval habitats. Proc Natl Acad Sci USA. 106(28):11530–11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen M, Pellegrino M, Vosshall LB. 2008. Insect odorant receptors are molecular targets of the insect repellent DEET. Science. 319(5871):1838–1842. [DOI] [PubMed] [Google Scholar]
- Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. 2003. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron. 37(5):827–841. [DOI] [PubMed] [Google Scholar]
- Dolan MJ, Frechter S, Bates AS, Dan C, Huoviala P, Roberts RJ, Schlegel P, Dhawan S, Tabano R, Dionne H, et al. 2019. Neurogenetic dissection of the Drosophila lateral horn reveals major outputs, diverse behavioural functions, and interactions with the mushroom body. Elife. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormont L, Bessière JM, Cohuet A. 2013. Human skin volatiles: a review. J Chem Ecol. 39(5):569–578. [DOI] [PubMed] [Google Scholar]
- Dusfour I, Achee NL, Roberts DR, Grieco JP. 2009. Contact irritancy and spatial repellency behaviors in Anopheles albimanus Wiedemann (Diptera: Culicidae) collected in Orange Walk, Belize, CA. J Vector Ecol. 34(2):232–237. doi: 10.1111/j.1948-7134.2009.00031.x.20836827. [DOI] [PubMed] [Google Scholar]
- Duvall LB, Ramos-Espiritu L, Barsoum KE, Glickman JF, Vosshall LB. 2019. Small-molecule agonists of Ae. aegypti neuropeptide Y receptor block mosquito biting. Cell. 176(4):687–701.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami SN, Lindberg BG, Hua S, Hill SR, Mozuraitis R, Lehmann P, Birgersson G, Borg-Karlson AK, Ignell R, Faye I. 2017. A key malaria metabolite modulates vector blood seeking, feeding, and susceptibility to infection. Science. 355(6329):1076–1080. [DOI] [PubMed] [Google Scholar]
- Eyun SI, Soh HY, Posavi M, Munro JB, Hughes DST, Murali SC, Qu J, Dugan S, Lee SL, Chao H, et al. 2017. Evolutionary history of chemosensory-related gene families across the Arthropoda. Mol Biol Evol. 34(8):1838–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajollahi A, Fonseca DM, Kramer LD, Marm Kilpatrick A. 2011. “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect Genet Evol. 11(7):1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawaz EY, Allan SA, Bernier UR, Obenauer PJ, Diclaro JW 2nd. 2014. Swarming mechanisms in the yellow fever mosquito: aggregation pheromones are involved in the mating behavior of Aedes aegypti. J Vector Ecol. 39(2):347–354. [DOI] [PubMed] [Google Scholar]
- Ferguson NM. 2018. Challenges and opportunities in controlling mosquito-borne infections. Nature. 559(7715):490–497. [DOI] [PubMed] [Google Scholar]
- Fernández-Grandon GM, Gezan SA, Armour JA, Pickett JA, Logan JG. 2015. Heritability of attractiveness to mosquitoes. PLoS One. 10(4):e0122716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishilevich E, Vosshall LB. 2005. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol. 15(17):1548–1553. [DOI] [PubMed] [Google Scholar]
- Foster WA. 1995. Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol. 40:443–474. [DOI] [PubMed] [Google Scholar]
- Foster WA, Hancock RG. 1994. Nectar-related olfactory and visual attractants for mosquitoes. J Am Mosq Control Assoc. 10(2 Pt 2):288–296. [PubMed] [Google Scholar]
- Foster WA, Takken W. 2004. Nectar-related vs. human-related volatiles: behavioural response and choice by female and male Anopheles gambiae (Diptera: Culicidae) between emergence and first feeding. Bull Entomol Res. 94(2):145–157. [DOI] [PubMed] [Google Scholar]
- Fox AN, Pitts RJ, Robertson HM, Carlson JR, Zwiebel LJ. 2001. Candidate odorant receptors from the malaria vector mosquito Anopheles gambiae and evidence of down-regulation in response to blood feeding. Proc Natl Acad Sci USA. 98(25):14693–14697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Wysocki CJ, Leyden JJ, Spielman AI, Sun X, Preti G. 2008. Analyses of volatile organic compounds from human skin. Br J Dermatol. 159(4):780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaninia M, Hansson BS, Ignell R. 2007. The antennal lobe of the African malaria mosquito, Anopheles gambiae - innervation and three-dimensional reconstruction. Arthropod Struct Dev. 36(1):23–39. [DOI] [PubMed] [Google Scholar]
- Gillies MT. 1980. The role of carbon dioxide in host-finding by mosquitoes (Diptera: Culicidae): a review. Bull Entomol Res. 70:525–532. [Google Scholar]
- Gomez-Diaz C, Reina JH, Cambillau C, Benton R. 2013. Ligands for pheromone-sensing neurons are not conformationally activated odorant binding proteins. PLoS Biol. 11(4):e1001546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouagna LC, Poueme RS, Dabiré KR, Ouédraogo JB, Fontenille D, Simard F. 2010. Patterns of sugar feeding and host plant preferences in adult males of An. gambiae (Diptera: Culicidae). J Vector Ecol. 35(2):267–276. [DOI] [PubMed] [Google Scholar]
- Graça-Souza AV, Maya-Monteiro C, Paiva-Silva GO, Braz GR, Paes MC, Sorgine MH, Oliveira MF, Oliveira PL. 2006. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem Mol Biol. 36(4):322–335. [DOI] [PubMed] [Google Scholar]
- Grant AJ, Muskavitch MA, O’Connell RJ. 2013. Malaria infection does not affect the sensitivity of peripheral receptor neurons in Anopheles stephensi. Parasit Vectors. 6:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BM, Fidock DA, Kyle DE, Kappe SH, Alonso PL, Collins FH, Duffy PE. 2008. Malaria: progress, perils, and prospects for eradication. J Clin Invest. 118(4):1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greppi C, Laursen WJ, Budelli G, Chang EC, Daniels AM, van Giesen L, Smidler AL, Catteruccia F, Garrity PA. 2020. Mosquito heat seeking is driven by an ancestral cooling receptor. Science. 367(6478):681–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco JP, Achee NL, Andre RG, Roberts DR. 2000. A comparison study of house entering and exiting behavior of Anopheles vestitipennis (Diptera: Culicidae) using experimental huts sprayed with DDT or deltamethrin in the southern district of Toledo, Belize, CA. J Vector Ecol. 25(1):62–73. [PubMed] [Google Scholar]
- Grimaldi DA, Engel MS. 2005. Evolution of insects. New York: Cambridge University Press. [Google Scholar]
- Harbach RE. 2007. The culicidae (Diptera): a review of taxonomy, classification and phylogeny. Zootaxa. 1668:591–638. [Google Scholar]
- Harbach RE. 2013. Mosquito taxonomic inventory. Avaiable from: http://mosquito-taxonomic-inventory.info/ [accessed 24 September 2020].
- Healy TP, Copland MJ. 1995. Activation of Anopheles gambiae mosquitoes by carbon dioxide and human breath. Med Vet Entomol. 9(3):331–336. [DOI] [PubMed] [Google Scholar]
- Healy TP, Jepson PC. 1988. The location of floral nectar sources by mosquitoes: the long-range responses of Anopheles arabiensis Patton (Diptera: Culicidae) to Achillea millefolium flowers and isolated floral odour. Bull Entomol Res. 78:651–657. [Google Scholar]
- Hill CA, Fox AN, Pitts RJ, Kent LB, Tan PL, Chrystal MA, Cravchik A, Collins FH, Robertson HM, Zwiebel LJ. 2002. G protein-coupled receptors in Anopheles gambiae. Science. 298(5591):176–178. [DOI] [PubMed] [Google Scholar]
- Hill SR, Taparia T, Ignell R. 2021. Regulation of the antennal transcriptome of the dengue vector, Aedes aegypti, during the first gonotrophic cycle. BMC Genomics. 22(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himeidan YE, Elbashir MI, Adam I. 2004. Attractiveness of pregnant women to the malaria vector, Anopheles arabiensis, in Sudan. Ann Trop Med Parasitol. 98(6):631–633. [DOI] [PubMed] [Google Scholar]
- Hinze A, Lantz J, Hill SR, Ignell R. 2021. Mosquito host seeking in 3D using a versatile climate-controlled wind tunnel system. Front Behav Neurosci. 15:643693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg U, Montague RA, Hildebrand JG. 1988. Anatomy of antenno-cerebral pathways in the brain of the sphinx moth Manduca sexta. Cell Tissue Res. 254(2):255–281. [DOI] [PubMed] [Google Scholar]
- Huang YS, Higgs S, Vanlandingham DL. 2019. Arbovirus-mosquito vector–host interactions and the impact on transmission and disease pathogenesis of arboviruses. Front Microbiol. 10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd H. 2003. Manipulation of medically important insect vectors by their parasites. Annu Rev Entomol. 48:141–161. [DOI] [PubMed] [Google Scholar]
- Ignell R, Dekker T, Ghaninia M, Hansson BS. 2005. Neuronal architecture of the mosquito deutocerebrum. J Comp Neurol. 493(2):207–240. [DOI] [PubMed] [Google Scholar]
- Impoinvil DE, Kongere JO, Foster WA, Njiru BN, Killeen GF, Githure JI, Beier JC, Hassanali A, Knols BG. 2004. Feeding and survival of the malaria vector Anopheles gambiae on plants growing in Kenya. Med Vet Entomol. 18(2):108–115. [DOI] [PubMed] [Google Scholar]
- Irish SR, Kyalo D, Snow RW, Coetzee M. 2020. Updated list of Anopheles species (Diptera: Culicidae) by country in the Afrotropical Region and associated islands. Zootaxa. 4747(3):zootaxa.4747.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Kawada H, Abe A, Eshita Y, Rongsriyam Y, Igarashi A. 1994. Utilization of bloodfed females of Aedes aegypti as a vehicle for the transfer of the insect growth regulator pyriproxyfen to larval habitats. J Am Mosq Control Assoc. 10(3):344–347. [PubMed] [Google Scholar]
- Ives AR, Paskewitz SM; Inter-L&S 101; Biology Interest Groups; Entomology Class 201 . 2005. Testing vitamin B as a home remedy against mosquitoes. J Am Mosq Control Assoc. 21(2):213–217. [DOI] [PubMed] [Google Scholar]
- Jacob JW, Tchouassi DP, Lagat ZO, Mathenge EM, Mweresa CK, Torto B. 2018. Independent and interactive effect of plant- and mammalian- based odors on the response of the malaria vector, Anopheles gambiae. Acta Trop. 185:98–106. [DOI] [PubMed] [Google Scholar]
- Jeanne JM, Fişek M, Wilson RI. 2018. The organization of projections from olfactory glomeruli onto higher-order neurons. Neuron. 98(6):1198–1213.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis GS, Potter CJ, Chan AM, Marin EC, Rohlfing T, Maurer CR Jr., Luo L. 2007. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell. 128(6):1187–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jové V, Gong Z, Hol FJH, Zhao Z, Sorrells TR, Carroll TS, Prakash M, McBride CS, Vosshall LB. 2020. Sensory discrimination of blood and floral nectar by Aedes aegypti mosquitoes. Neuron. 108:1163–1180.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karner T, Kellner I, Schultze A, Breer H, Krieger J. 2015. Co-expression of six tightly clustered odorant receptor genes in the antenna of the malaria mosquito Anopheles gambiae. Front Ecol Evol. 3:8. [Google Scholar]
- Kawada H, Ohashi K, Dida GO, Sonye G, Njenga SM, Mwandawiro C, Minakawa N. 2014. Insecticidal and repellent activities of pyrethroids to the three major pyrethroid-resistant malaria vectors in western Kenya. Parasit Vectors. 7(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H, Temu EA, Minjas JN, Matsumoto O, Iwasaki T, Takagi M. 2008. Field evaluation of spatial repellency of metofluthrin-impregnated plastic strips against Anopheles gambiae complex in Bagamoyo, coastal Tanzania. J Am Mosq Control Assoc. 24(3):404–409. [DOI] [PubMed] [Google Scholar]
- Kelly M, Su CY, Schaber C, Crowley JR, Hsu FF, Carlson JR, Odom AR. 2015. Malaria parasites produce volatile mosquito attractants. mBio. 6:e00235-00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiszewski A, Mellinger A, Spielman A, Malaney P, Sachs SE, Sachs J. 2004. A global index representing the stability of malaria transmission. Am J Trop Med Hyg. 70(5):486–498. [PubMed] [Google Scholar]
- Klaps J, Lievens B, Álvarez-Pérez S. 2020. Towards a better understanding of the role of nectar-inhabiting yeasts in plant-animal interactions. Fungal Biol Biotechnol. 7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klowden M. 1981. Initiation and termination of host-seeking inhibition in Aedes aegypti during oöcyte maturation. J Insect Physiol. 27:799–803. [DOI] [PubMed] [Google Scholar]
- Klowden MJ, Briegel H. 1994. Mosquito gonotrophic cycle and multiple feeding potential: contrasts between Anopheles and Aedes (Diptera: Culicidae). J Med Entomol. 31(4):618–622. [DOI] [PubMed] [Google Scholar]
- Klowden MJ, Lea AO. 1979. Abdominal distention terminates subsequent host-seeking behaviour of Aedes aegypti following a blood meal. J Insect Physiol. 25(7):583–585. [DOI] [PubMed] [Google Scholar]
- Knols BG, de Jong R, Takken W. 1995. Differential attractiveness of isolated humans to mosquitoes in Tanzania. Trans R Soc Trop Med Hyg. 89(6):604–606. [DOI] [PubMed] [Google Scholar]
- Knols BGJ, vanLoon JJA, Cork A, Robinson RD, Adam W, Meijerink J, DeJong R, Takken W. 1997. Behavioural and electrophysiological responses of the female malaria mosquito Anopheles gambiae (Diptera: Culicidae) to Limburger cheese volatiles. Bull Entomol Res. 87:151–159. [Google Scholar]
- Knudsen JT, Tollsten L, Bergström LG. 1993. Floral scents – a checklist of volatile compounds isolated by head-space techniques. Phytochemistry. 33:253–280. [Google Scholar]
- Koella JC, Sørensen FL, Anderson RA. 1998. The malaria parasite, Plasmodium falciparum, increases the frequency of multiple feeding of its mosquito vector, Anopheles gambiae. Proc Biol Sci. 265(1398):763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan PH. 1990. Substitute blood meal for investigating and maintaining Aedes aegypti (Diptera: Culicidae). J Med Entomol. 27(4):709–712. [DOI] [PubMed] [Google Scholar]
- Krenn HW, Aspöck H. 2012. Form, function and evolution of the mouthparts of blood-feeding Arthropoda. Arthropod Struct Dev. 41(2):101–118. [DOI] [PubMed] [Google Scholar]
- Krotoszynski B, Gabriel G, O’Neil H. 1977. Characterization of human expired air: a promising investigative and diagnostic technique. J Chromatogr Sci. 15(7):239–244. [DOI] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ. 2007. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 446(7135):542–546. [DOI] [PubMed] [Google Scholar]
- Kwon HW, Lu T, Rützler M, Zwiebel LJ. 2006. Olfactory responses in a gustatory organ of the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 103(36):13526–13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Kim SH, Ronderos DS, Lee Y, Akitake B, Woodward OM, Guggino WB, Smith DP, Montell C. 2010. Drosophila TRPA1 channel is required to avoid the naturally occurring insect repellent citronellal. Curr Biol. 20(18):1672–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyalo D, Amratia P, Mundia CW, Mbogo CM, Coetzee M, Snow RW. 2017. A geo-coded inventory of anophelines in the Afrotropical Region south of the Sahara: 1898–2016. Wellcome Open Res. 2:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix R, Mukabana WR, Gouagna LC, Koella JC. 2005. Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol. 3(9):e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larter NK, Sun JS, Carlson JR. 2016. Organization and function of Drosophila odorant binding proteins. Elife. 5:e20242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal WS. 2013. Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol. 58:373–391. [DOI] [PubMed] [Google Scholar]
- Lee H, Halverson S, Ezinwa N. 2018. Mosquito-borne diseases. Prim Care. 45(3):393–407. [DOI] [PubMed] [Google Scholar]
- Leeman M, Choi J, Hansson S, Storm MU, Nilsson L. 2018. Proteins and antibodies in serum, plasma, and whole blood-size characterization using asymmetrical flow field-flow fractionation (AF4). Anal Bioanal Chem. 410(20):4867–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre T, Gouagna LC, Dabiré KR, Elguero E, Fontenille D, Renaud F, Costantini C, Thomas F. 2009. Beyond nature and nurture: phenotypic plasticity in blood-feeding behavior of Anopheles gambiae s.s. when humans are not readily accessible. Am J Trop Med Hyg. 81(6):1023–1029. [DOI] [PubMed] [Google Scholar]
- Lefevre T, Gouagna LC, Dabiré KR, Elguero E, Fontenille D, Renaud F, Costantini C, Thomas F. 2010. Beer consumption increases human attractiveness to malaria mosquitoes. PLoS One. 5(3):e9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre T, Thomas F, Schwartz A, Levashina E, Blandin S, Brizard JP, Le Bourligu L, Demettre E, Renaud F, Biron DG. 2007. Malaria Plasmodium agent induces alteration in the head proteome of their Anopheles mosquito host. Proteomics. 7(11):1908–1915. [DOI] [PubMed] [Google Scholar]
- Lehane M. 2005. The biology of blood-sucking in insects. Cambridge (UK): Cambridge University Press. [Google Scholar]
- Li J, Chen Q, Man Y, Pei D, Wu W. 2019. Variant ionotropic receptors are expressed in the antennae of Anopheles sinensis (Diptera: Culicidae). Biochem Genet. 57(4):571–582. [DOI] [PubMed] [Google Scholar]
- Lindsay S, Ansell J, Selman C, Cox V, Hamilton K, Walraven G. 2000. Effect of pregnancy on exposure to malaria mosquitoes. Lancet. 355(9219):1972. [DOI] [PubMed] [Google Scholar]
- Liu C, Pitts RJ, Bohbot JD, Jones PL, Wang G, Zwiebel LJ. 2010. Distinct olfactory signaling mechanisms in the malaria vector mosquito Anopheles gambiae. PLoS Biol. 8:e1000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Ye Z, Baker A, Sun H, Zwiebel LJ. 2020. Gene editing reveals obligate and modulatory components of the CO2 receptor complex in the malaria vector mosquito, Anopheles coluzzii. bioRxiv. doi: 10.1101/2020.05.13.094995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan JG, Birkett MA, Clark SJ, Powers S, Seal NJ, Wadhams LJ, Mordue Luntz AJ, Pickett JA. 2008. Identification of human-derived volatile chemicals that interfere with attraction of Aedes aegypti mosquitoes. J Chem Ecol. 34(3):308–322. [DOI] [PubMed] [Google Scholar]
- Logan JG, Cook JI, Stanczyk NM, Weeks EN, Welham SJ, Mordue Luntz AJ. 2010. To bite or not to bite! A questionnaire-based survey assessing why some people are bitten more than others by midges. BMC Public Health. 10:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan JG, Stanczyk NM, Hassanali A, Kemei J, Santana AE, Ribeiro KA, Pickett JA, Mordue Luntz AJ. 2010. Arm-in-cage testing of natural human-derived mosquito repellents. Malar J. 9:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Qiu YT, Wang G, Kwon JY, Rutzler M, Kwon HW, Pitts RJ, van Loon JJ, Takken W, Carlson JR, et al. 2007. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr Biol. 17(18):1533–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz EK, Lahondère C, Vinauger C, Riffell JA. 2017. Olfactory learning and chemical ecology of olfaction in disease vector mosquitoes: a life history perspective. Curr Opin Insect Sci. 20:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwetoijera D, Harris C, Kiware S, Dongus S, Devine GJ, McCall PJ, Majambere S. 2014. Effective autodissemination of pyriproxyfen to breeding sites by the exophilic malaria vector Anopheles arabiensis in semi-field settings in Tanzania. Malar J. 13:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwetoijera D, Kiware S, Okumu F, Devine GJ, Majambere S. 2019. Autodissemination of pyriproxyfen suppresses stable populations of Anopheles arabiensis under semi-controlled settings. Malar J. 18(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyimo EO, Takken W. 1993. Effects of adult body size on fecundity and the pre-gravid rate of Anopheles gambiae females in Tanzania. Med Vet Entomol. 7(4):328–332. [DOI] [PubMed] [Google Scholar]
- Lyons CL, Coetzee M, Chown SL. 2013. Stable and fluctuating temperature effects on the development rate and survival of two malaria vectors, Anopheles arabiensis and Anopheles funestus. Parasit Vectors. 6:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons CL, Coetzee M, Terblanche JS, Chown SL. 2012. Thermal limits of wild and laboratory strains of two African malaria vector species, Anopheles arabiensis and Anopheles funestus. Malar J. 11:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden AA, Epps MJ, Fukami T, Irwin RE, Sheppard J, Sorger DM, Dunn RR. 2018. The ecology of insect–yeast relationships and its relevance to human industry. Proc Biol Sci. 285:20172733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa E, Aonuma H, Nelson B, Yoshimura A, Tokunaga F, Fukumoto S, Kanuka H. 2011. The role of proboscis of the malaria vector mosquito Anopheles stephensi in host-seeking behavior. Parasit Vectors. 4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire SE, Afify A, Goff LA, Potter CJ. 2020. A feedback mechanism regulates odorant receptor expression in the malaria mosquito, Anopheles gambiae. bioRxiv. doi: 10.1101/2020.2007.2023.218586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia MF, Moore SJ. 2011. Plant-based insect repellents: a review of their efficacy, development and testing. Malar J. 10(Suppl 1):S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed S, Hill SR, Birgersson G, Ignell R. 2016. Detection and perception of generic host volatiles by mosquitoes modulate host preference: context dependence of (R)-1-octen-3-ol. R Soc Open Sci. 3(11):160467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manda H, Gouagna LC, Foster WA, Jackson RR, Beier JC, Githure JI, Hassanali A. 2007. Effect of discriminative plant-sugar feeding on the survival and fecundity of Anopheles gambiae. Malar J. 6:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans BJ. 2011. Evolution of vertebrate hemostatic and inflammatory control mechanisms in blood-feeding arthropods. J Innate Immun. 3(1):41–51. [DOI] [PubMed] [Google Scholar]
- Marchand RP. 1983. Field observations on swarming and mating in Anopheles gambiae mosquitoes in Tanzania. Neth J Zool. 34:367. [Google Scholar]
- Mathenge EM, Gimnig JE, Kolczak M, Ombok M, Irungu LW, Hawley WA. 2001. Effect of permethrin-impregnated nets on exiting behavior, blood feeding success, and time of feeding of malaria mosquitoes (Diptera: Culicidae) in western Kenya. J Med Entomol. 38(4):531–536. [DOI] [PubMed] [Google Scholar]
- Mbare O, Lindsay SW, Fillinger U. 2014. Pyriproxyfen for mosquito control: female sterilization or horizontal transfer to oviposition substrates by Anopheles gambiae sensu stricto and Culex quinquefasciatus. Parasit Vectors. 7:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mboera LEG, Knols BGJ, Braks MAH, Takken W. 2000. Comparison of carbon dioxide-baited trapping systems for sampling outdoor mosquito populations in Tanzania. Med Vet Entomol. 14:257–263. [DOI] [PubMed] [Google Scholar]
- Mboera LEG, Takken W. 1997. Carbon dioxide chemotropism in mosquitoes (Diptera: Culicidae) and its potential in vector surveillance and management programmes. Med Vet Entomol. 7:355–368. [Google Scholar]
- McIver SB. 1982. Sensilla mosquitoes (Diptera: Culicidae). J Med Entomol. 19(5):489–535. [DOI] [PubMed] [Google Scholar]
- McMeniman CJ, Corfas RA, Matthews BJ, Ritchie SA, Vosshall LB. 2014. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell. 156(5):1060–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijerink J, Braks MA, Van Loon JJ. 2001. Olfactory receptors on the antennae of the malaria mosquito Anopheles gambiae are sensitive to ammonia and other sweat-borne components. J Insect Physiol. 47(4-5):455–464. [DOI] [PubMed] [Google Scholar]
- Meijerink J, van Loon JJ. 1999. Sensitivities of antennal olfactory neurons of the malaria mosquito, Anopheles gambiae, to carboxylic acids. J Insect Physiol. 45(4):365–373. [DOI] [PubMed] [Google Scholar]
- Melo N, Capek M, Arenas OM, Afify A, Yilmaz A, Potter CJ, Laminette PJ, Para A, Gallio M, Stensmyr MC. 2021. The irritant receptor TRPA1 mediates the mosquito repellent effect of catnip. Curr Biol. 31:1988–1994.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill CE, Sherertz TM, Walker WB, Zwiebel LJ. 2005. Odorant-specific requirements for arrestin function in Drosophila olfaction. J Neurobiol. 63(1):15–28. [DOI] [PubMed] [Google Scholar]
- Merritt RW, Dadd RH, Walker ED. 1992. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu Rev Entomol. 37:349–376. [DOI] [PubMed] [Google Scholar]
- Miller R, Tu Z. 2008. Odorant receptor c-terminal motifs in divergent insect species. J Insect Sci. 8(1–10):10.20345291 [Google Scholar]
- Miyazaki T, Ito K. 2010. Neural architecture of the primary gustatory center of Drosophila melanogaster visualized with GAL4 and LexA enhancer-trap systems. J Comp Neurol. 518(20):4147–4181. [DOI] [PubMed] [Google Scholar]
- Montell C, Zwiebel LJ. 2016. Mosquito sensory systems. In: Raikhel AS, editor. Progress in mosquito research. London: Academic Press Ltd-Elsevier Science Ltd. p. 293–328. [Google Scholar]
- Mozūraitis R, Hajkazemian M, Zawada JW, Szymczak J, Pålsson K, Sekar V, Biryukova I, Friedländer MR, Koekemoer LL, Baird JK, et al. 2020. Male swarming aggregation pheromones increase female attraction and mating success among multiple African malaria vector mosquito species. Nat Ecol Evol. 4(10):1395–1401. [DOI] [PubMed] [Google Scholar]
- Muema JM, Bargul JL, Njeru SN, Onyango JO, Imbahale SS. 2017. Prospects for malaria control through manipulation of mosquito larval habitats and olfactory-mediated behavioural responses using plant-derived compounds. Parasit Vectors. 10(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller GC, Beier JC, Traore SF, Toure MB, Traore MM, Bah S, Doumbia S, Schlein Y. 2010. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malar J. 9:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EJ, Booth JC, Davrazou F, Port AM, Jones DN. 2013. Interactions of Anopheles gambiae odorant-binding proteins with a human-derived repellent: implications for the mode of action of n,n-diethyl-3-methylbenzamide (DEET). J Biol Chem. 288(6):4475–4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwingira VS, Spitzen J, Mboera LEG, Torres-Estrada JL, Takken W. 2020. The influence of larval stage and density on oviposition site-selection behavior of the afrotropical malaria mosquito Anopheles coluzzii (Diptera: Culicidae). J Med Entomol. 57(3):657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng R, Salem SS, Wu ST, Wu M, Lin HH, Shepherd AK, Joiner WJ, Wang JW, Su CY. 2019. Amplification of Drosophila olfactory responses by a DEG/ENaC channel. Neuron. 104(5):947–959.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PL, Vantaux A, Hien DF, Dabiré KR, Yameogo BK, Gouagna LC, Fontenille D, Renaud F, Simard F, Costantini C, et al. 2017. No evidence for manipulation of Anopheles gambiae, An. coluzzii and An. arabiensis host preference by Plasmodium falciparum. Sci Rep. 7(1):9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikbakhtzadeh MR, Terbot JW 2nd, Otienoburu PE, Foster WA. 2014. Olfactory basis of floral preference of the malaria vector Anopheles gambiae (Diptera: Culicidae) among common African plants. J Vector Ecol. 39(2):372–383. [DOI] [PubMed] [Google Scholar]
- Njiru BN, Mukabana WR, Takken W, Knols BG. 2006. Trapping of the malaria vector Anopheles gambiae with odour-baited MM-X traps in semi-field conditions in western Kenya. Malar J. 5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyasembe VO, Tchouassi DP, Kirwa HK, Foster WA, Teal PE, Borgemeister C, Torto B. 2014. Development and assessment of plant-based synthetic odor baits for surveillance and control of malaria vectors. PLoS One. 9(2):e89818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyasembe VO, Tchouassi DP, Mbogo CM, Sole CL, Pirk C, Torto B. 2015. Linalool oxide: generalist plant based lure for mosquito disease vectors. Parasit Vectors. 8:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyasembe VO, Tchouassi DP, Pirk CWW, Sole CL, Torto B. 2018. Host plant forensics and olfactory-based detection in Afro-tropical mosquito disease vectors. PLoS Negl Trop Dis. 12(2):e0006185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyasembe VO, Teal PE, Mukabana WR, Tumlinson JH, Torto B. 2012. Behavioural response of the malaria vector Anopheles gambiae to host plant volatiles and synthetic blends. Parasit Vectors. 5:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyasembe VO, Teal Peter EA, Sawa P, Tumlinson James H, Borgemeister C, Torto B. 2014. Plasmodium falciparum infection increases Anopheles gambiae attraction to nectar sources and sugar uptake. Curr Biol. 24:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyasembe VO, Torto B. 2014. Volatile phytochemicals as mosquito semiochemicals. Phytochem Lett. 8:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba SY, Kawada H, Dida GO, Juma D, Sonye G, Minakawa N, Takagi M. 2010. Predators of Anopheles gambiae sensu lato (Diptera: Culicidae) larvae in wetlands, western Kenya: confirmation by polymerase chain reaction method. J Med Entomol. 47(5):783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omondi AB, Ghaninia M, Dawit M, Svensson T, Ignell R. 2019. Age-dependent regulation of host seeking in Anopheles coluzzii. Sci Rep. 9(1):9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pask GM, Jones PL, Rützler M, Rinker DC, Zwiebel LJ. 2011. Heteromeric Anopheline odorant receptors exhibit distinct channel properties. PLoS One. 6(12):e28774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskewitz S, Irwin P, Konwinski N, Larson S. 2018. Impact of consumption of bananas on attraction of Anopheles stephensi to humans. Insects. 9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pates HV, Takken W, Stuke K, Curtis CF. 2001. Differential behaviour of Anopheles gambiae sensu stricto (Diptera: Culicidae) to human and cow odours in the laboratory. Bull Entomol Res. 91(4):289–296. [DOI] [PubMed] [Google Scholar]
- Peach DAH, Gries G. 2020. Mosquito phytophagy – sources exploited, ecological function, and evolutionary transition to haematophagy. Entomol Exp Appl. 168:120–136. [Google Scholar]
- Peach DAH, Gries R, Zhai H, Young N, Gries G. 2019. Multimodal floral cues guide mosquitoes to tansy inflorescences. Sci Rep. 9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino M, Steinbach N, Stensmyr MC, Hansson BS, Vosshall LB. 2011. A natural polymorphism alters odour and DEET sensitivity in an insect odorant receptor. Nature. 478(7370):511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RJ, Derryberry SL, Zhang Z, Zwiebel LJ. 2017. Variant ionotropic receptors in the malaria vector mosquito Anopheles gambiae tuned to amines and carboxylic acids. Sci Rep. 7:40297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RJ, Fox AN, Zwiebel LJ. 2004. A highly conserved candidate chemoreceptor expressed in both olfactory and gustatory tissues in the malaria vector Anopheles gambiae. Proc Natl Acad Sci USA. 101(14):5058–5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RJ, Rinker DC, Jones PL, Rokas A, Zwiebel LJ. 2011a. Transcriptome profiling of chemosensory appendages in the malaria vector Anopheles gambiae reveals tissue- and sex-specific signatures of odor coding. BMC Genomics. 12:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RJ, Rinker DC, Jones PL, Rokas A, Zwiebel LJ. 2011b. Transcriptome profiling of chemosensory appendages in the malaria vector Anopheles gambiae reveals tissue- and sex-specific signatures of odor coding. BMC Genomics. 12:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RJ, Zwiebel LJ. 2006. Antennal sensilla of two female anopheline sibling species with differing host ranges. Malar J. 5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter CJ. 2014. Stop the biting: targeting a mosquito’s sense of smell. Cell. 156(5):878–881. [DOI] [PubMed] [Google Scholar]
- Powell JR, Besansky NJ, della Torre A, Petrarca V. 2014. Mario Coluzzi (1938–2012). Malar J. 13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu YT, Gort G, Torricelli R, Takken W, van Loon JJ. 2013. Effects of blood-feeding on olfactory sensitivity of the malaria mosquito Anopheles gambiae: application of mixed linear models to account for repeated measurements. J Insect Physiol. 59(11):1111–1118. [DOI] [PubMed] [Google Scholar]
- Qiu YT, Smallegange RC, VAN Loon JJ, Takken W. 2011. Behavioural responses of Anopheles gambiae sensu stricto to components of human breath, sweat and urine depend on mixture composition and concentration. Med Vet Entomol. 25(3):247–255. [DOI] [PubMed] [Google Scholar]
- Qiu YT, Smallegange RC, Van Loon JJ, Ter Braak CJ, Takken W. 2006. Interindividual variation in the attractiveness of human odours to the malaria mosquito Anopheles gambiae s.s. Med Vet Entomol. 20(3):280–287. [DOI] [PubMed] [Google Scholar]
- Qiu YT, van Loon JJ, Takken W, Meijerink J, Smid HM. 2006. Olfactory coding in antennal neurons of the malaria mosquito, Anopheles gambiae. Chem Senses. 31(9):845–863. [DOI] [PubMed] [Google Scholar]
- Rajan TV, Hein M, Porte P, Wikel S. 2005. A double-blinded, placebo-controlled trial of garlic as a mosquito repellant: a preliminary study. Med Vet Entomol. 19(1):84–89. [DOI] [PubMed] [Google Scholar]
- Raji JI, DeGennaro M. 2017. Genetic analysis of mosquito detection of humans. Curr Opin Insect Sci. 20:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves WC. 1951. Field studies on carbon dioxide as a possible host simulant to mosquitoes. Proc Soc Exp Biol Med. 77(1):64–66. [DOI] [PubMed] [Google Scholar]
- Reeves LE, Holderman CJ, Blosser EM, Gillett-Kaufman JL, Kawahara AY, Kaufman PE, Burkett-Cadena ND. 2018. Identification of Uranotaenia sapphirina as a specialist of annelids broadens known mosquito host use patterns. Commun Biol. 1:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabinina O, Task D, Marr E, Lin CC, Alford R, O’Brochta DA, Potter CJ. 2016. Organization of olfactory centres in the malaria mosquito Anopheles gambiae. Nat Commun. 7:13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinker DC, Pitts RJ, Zhou X, Suh E, Rokas A, Zwiebel LJ. 2013. Blood meal-induced changes to antennal transcriptome profiles reveal shifts in odor sensitivities in Anopheles gambiae. Proc Natl Acad Sci USA. 110(20):8260–8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinker DC, Zhou X, Pitts RJ, Rokas A, Zwiebel LJ; AGC Consortium . 2013. Antennal transcriptome profiles of anopheline mosquitoes reveal human host olfactory specialization in Anopheles gambiae. BMC Genomics. 14:749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Pérez C, Clifton ME, Noriega FG. 2017. How micronutrients influence the physiology of mosquitoes. Curr Opin Insect Sci. 23:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A, Busula AO, Voets MA, Beshir KB, Caulfield JC, Powers SJ, Verhulst NO, Winskill P, Muwanguzi J, Birkett MA, et al. 2018. Plasmodium-associated changes in human odor attract mosquitoes. Proc Natl Acad Sci USA. 115(18):E4209–E4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda LM, Harrison BA, Brown JS, Whitt PB, Harrison RL, Gardner RC. 2001. Evaluation of 1-octen-3-ol, carbon dioxide, and light as attractants for mosquitoes associated with two distinct habitats in North Carolina. J Am Mosq Control Assoc. 17(1):61–66. [PubMed] [Google Scholar]
- Rund SS, Bonar NA, Champion MM, Ghazi JP, Houk CM, Leming MT, Syed Z, Duffield GE. 2013. Daily rhythms in antennal protein and olfactory sensitivity in the malaria mosquito Anopheles gambiae. Sci Rep. 3:2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saveer AM, Pitts RJ, Ferguson ST, Zwiebel LJ. 2018. Characterization of chemosensory responses on the labellum of the malaria vector mosquito, Anopheles coluzzii. Sci Rep. 8(1):5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaber CL, Katta N, Bollinger LB, Mwale M, Mlotha-Mitole R, Trehan I, Raman B, Odom John AR. 2018. Breathprinting reveals malaria-associated biomarkers and mosquito attractants. J Infect Dis. 217(10): 1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoelitsz B, Mwingira V, Mboera LEG, Beijleveld H, Koenraadt CJM, Spitzen J, van Loon JJA, Takken W. 2020. Chemical mediation of oviposition by Anopheles mosquitoes: a push–pull system driven by volatiles associated with larval stages. J Chem Ecol. 46(4):397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze A, Breer H, Krieger J. 2014. The blunt trichoid sensillum of female mosquitoes, Anopheles gambiae: odorant binding protein and receptor types. Int J Biol Sci. 10(4):426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze A, Pregitzer P, Walter MF, Woods DF, Marinotti O, Breer H, Krieger J. 2013. The co-expression pattern of odorant binding proteins and olfactory receptors identify distinct trichoid sensilla on the antenna of the malaria mosquito Anopheles gambiae. PLoS One. 8(7):e69412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schymura D, Forstner M, Schultze A, Kröber T, Swevers L, Iatrou K, Krieger J. 2010. Antennal expression pattern of two olfactory receptors and an odorant binding protein implicated in host odor detection by the malaria vector Anopheles gambiae. Int J Biol Sci. 6(7):614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seenivasagan T, Sharma A, Yadav R, Tyagi V, Singh R, Sukumaran D. 2019. Plant infusions mediate oviposition of malaria, dengue and filariasis vectors: push–pull approach for vector surveillance and control. J Biopest. 12:95–103. [Google Scholar]
- Shaw WR, Catteruccia F. 2019. Vector biology meets disease control: using basic research to fight vector-borne diseases. Nat Microbiol. 4(1):20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai Y, Funada H, Seki T, Morohashi M, Kamimura K. 2004. Landing preference of Aedes albopictus (Diptera: Culicidae) on human skin among ABO blood groups, secretors or nonsecretors, and ABH antigens. J Med Entomol. 41(4):796–799. [DOI] [PubMed] [Google Scholar]
- Simoni A, Hammond AM, Beaghton AK, Galizi R, Taxiarchi C, Kyrou K, Meacci D, Gribble M, Morselli G, Burt A, et al. 2020. A male-biased sex-distorter gene drive for the human malaria vector Anopheles gambiae. Nat Biotechnol. 38(9):1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka ME, Bangs MJ, Manguin S, Rubio-Palis Y, Chareonviriyaphap T, Coetzee M, Mbogo CM, Hemingway J, Patil AP, Temperley WH, et al. 2012. A global map of dominant malaria vectors. Parasit Vectors. 5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka ME, Rubio-Palis Y, Manguin S, Patil AP, Temperley WH, Gething PW, Van Boeckel T, Kabaria CW, Harbach RE, Hay SI. 2010. The dominant Anopheles vectors of human malaria in the Americas: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 3:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallegange RC, Qiu YT, van Loon JJ, Takken W. 2005. Synergism between ammonia, lactic acid and carboxylic acids as kairomones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae). Chem Senses. 30(2):145–152. [DOI] [PubMed] [Google Scholar]
- Smallegange RC, Schmied WH, van Roey KJ, Verhulst NO, Spitzen J, Mukabana WR, Takken W. 2010. Sugar-fermenting yeast as an organic source of carbon dioxide to attract the malaria mosquito Anopheles gambiae. Malar J. 9:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallegange RC, Takken W. 2010. Host-seeking behaviour of mosquitoes: responses to olfactory stimuli in the laboratory. In: Takken W, Knols BGJ, editors. Olfaction in vector-host interactions. Wageningen (The Netherlands): Wageningen Academy Publication. p. 143–180. [Google Scholar]
- Smallegange RC, van Gemert GJ, van de Vegte-Bolmer M, Gezan S, Takken W, Sauerwein RW, Logan JG. 2013. Malaria infected mosquitoes express enhanced attraction to human odor. PLoS One. 8(5):e63602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks JT, Botsko G, Swale DR, Boland LM, Patel SS, Dickens JC. 2018. Membrane proteins mediating reception and transduction in chemosensory neurons in mosquitoes. Front Physiol. 9:1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TS, Hill JA, Feldmann RJ, Maibach HI. 1979. Evaporation of diethyltoluamide from human skin in vivo and in vitro. J Invest Dermatol. 72(6):317–319. [DOI] [PubMed] [Google Scholar]
- Stanczyk NM, Brugman VA, Austin V, Sanchez-Roman Teran F, Gezan SA, Emery M, Visser TM, Dessens JT, Stevens W, Smallegange RC, et al. 2019. Species-specific alterations in Anopheles mosquito olfactory responses caused by Plasmodium infection. Sci Rep. 9(1):3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczyk NM, Mescher MC, De Moraes CM. 2017. Effects of malaria infection on mosquito olfaction and behavior: extrapolating data to the field. Curr Opin Insect Sci. 20:7–12. [DOI] [PubMed] [Google Scholar]
- Steinbrecht RA. 1997. Pore structures in insect olfactory sensilla: a review of data and concepts. Int J Insect Morphol Embryol. 26:229–245. [Google Scholar]
- Suh E, Choe DH, Saveer AM, Zwiebel LJ. 2016. Suboptimal larval habitats modulate oviposition of the malaria vector mosquito Anopheles coluzzii. PLoS One. 11(2):e0149800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumba LA, Guda TO, Deng AL, Hassanali A, Beier JC, Knols BGJ. 2004. Mediation of oviposition site selection in the African malaria mosquito Anopheles gambiae (Diptera: Culicidae) by semiochemicals of microbial origin. Int J Trop Insect Sci. 24. [Google Scholar]
- Sun H, Liu F, Baker A, Zwiebel LJ. 2020. Neuronal odor coding in the larval sensory cone of Anopheles coluzzii: complex responses from a simple system. Available from: https://ssrn.com/abstract=3733600 or doi: 10.2139/ssrn.3733600. [DOI]
- Sun H, Liu F, Ye Z, Baker A, Zwiebel LJ. 2020. Mutagenesis of the orco odorant receptor co-receptor impairs olfactory function in the malaria vector Anopheles coluzzii. Insect Biochem Mol Biol. 127:103497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwonkerd W, Ritthison W, Ngo CT, Tainchum K, Bangs MJ, Chareonviriyaphap T.. 2013. Vector biology and malaria transmission in Southeast Asia. In: Manguin S editor. Anopheles mosquitoes – new insights into malaria vectors. IntechOpen. Available from: https://www.intechopen.com/books/anopheles-mosquitoes-new-insights-into-malaria-vectors/vector-biology-and-malaria-transmission-in-southeast-asia [Google Scholar]
- Syed Z, Leal WS. 2008. Mosquitoes smell and avoid the insect repellent DEET. Proc Natl Acad Sci USA. 105(36):13598–13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takken W. 1991. The role of olfaction in host-seeking of mosquitoes: a review. Int J Trop Insect Sci. 12:287–295. [Google Scholar]
- Takken W, Klowden MJ, Chambers GM. 1998. Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): the disadvantage of being small. J Med Entomol. 35(5):639–645. [DOI] [PubMed] [Google Scholar]
- Takken W, Knols BG. 1999. Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu Rev Entomol. 44:131–157. [DOI] [PubMed] [Google Scholar]
- Task D, Lin C-C, Afify A, Li H, Vulpe A, Menuz K, Potter CJ. 2020. Widespread polymodal chemosensory receptor expression in Drosophila olfactory neurons. bioRxiv. doi: 10.1101/2020.2011.2007.355651. [DOI] [Google Scholar]
- Tauxe GM, MacWilliam D, Boyle SM, Guda T, Ray A. 2013. Targeting a dual detector of skin and CO2 to modify mosquito host seeking. Cell. 155(6):1365–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek IV, den Otter CJ. 1999. Olfactory sensitivities of mosquitoes with different host preferences (Anopheles gambiae s.s., An. arabiensis, An. quadriannulatus, An. m. atroparvus) to synthetic host odours. J Insect Physiol. 45(11):1001–1010. [DOI] [PubMed] [Google Scholar]
- van den Broek IVF, den Otter CJ. 2000. Odour sensitivity of antennal olfactory cells underlying grooved pegs of Anopheles gambiae s.s. and An. quadriannulatus. Entomol Exp Appl. 96:167–175. [Google Scholar]
- Vaníčková L, Canale A, Benelli G. 2017. Sexual chemoecology of mosquitoes (Diptera, Culicidae): current knowledge and implications for vector control programs. Parasitol Int. 66(2):190–195. [DOI] [PubMed] [Google Scholar]
- van Loon JJ, Smallegange RC, Bukovinszkiné-Kiss G, Jacobs F, De Rijk M, Mukabana WR, Verhulst NO, Menger DJ, Takken W. 2015. Mosquito attraction: crucial role of carbon dioxide in formulation of a five-component blend of human-derived volatiles. J Chem Ecol. 41(6):567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantaux A, de Sales Hien D, Yaméogo B, Dabiré K, Thomas F, Cohuet A, Lefevre T. 2015. Host-seeking behaviors of mosquitoes experimentally infected with sympatric field isolates of the human malaria parasite Plasmodium falciparum: No evidence for host manipulation. Front Ecol Evol. 3:86. [Google Scholar]
- Venthur H, Zhou JJ. 2018. Odorant receptors and odorant-binding proteins as insect pest control targets: a comparative analysis. Front Physiol. 9:1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst NO, Weldegergis BT, Menger D, Takken W. 2016. Attractiveness of volatiles from different body parts to the malaria mosquito Anopheles coluzzii is affected by deodorant compounds. Sci Rep. 6:27141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas LM, Pimenta PF. 2014. Metagenomics, paratransgenesis and the Anopheles microbiome: a portrait of the geographical distribution of the anopheline microbiota based on a meta-analysis of reported taxa. Mem Inst Oswaldo Cruz. 109(5):672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall LB, Hansson BS. 2011. A unified nomenclature system for the insect olfactory coreceptor. Chem Senses. 36(6):497–498. [DOI] [PubMed] [Google Scholar]
- Wachira SW, Ndung’u M, Njagi PG, Hassanali A. 2010. Comparative responses of ovipositing Anopheles gambiae and Culex quinquefasciatus females to the presence of Culex egg rafts and larvae. Med Vet Entomol. 24(4):369–374. [DOI] [PubMed] [Google Scholar]
- Wahid I, Sunahara T, Mogi M. 2003. Maxillae and mandibles of male mosquitoes and female autogenous mosquitoes (Diptera: Culicidae). J Med Entomol. 40(2):150–158. [DOI] [PubMed] [Google Scholar]
- Wang G, Carey AF, Carlson JR, Zwiebel LJ. 2010. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 107(9):4418–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Si FL, He ZB, Chen B. 2018. Genome-wide identification, characterization and classification of ionotropic glutamate receptor genes (iGluRs) in the malaria vector Anopheles sinensis (Diptera: Culicidae). Parasit Vectors. 11(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. 2004. Taste representations in the Drosophila brain. Cell. 117(7):981–991. [DOI] [PubMed] [Google Scholar]
- Weaver SC, Charlier C, Vasilakis N, Lecuit M. 2018. Zika, chikungunya, and other emerging vector-borne viral diseases. Annu Rev Med. 69:395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster B, Lacey ES, Cardé RT. 2015. Waiting with bated breath: opportunistic orientation to human odor in the malaria mosquito, Anopheles gambiae, is modulated by minute changes in carbon dioxide concentration. J Chem Ecol. 41(1):59–66. [DOI] [PubMed] [Google Scholar]
- WHO . 2019. World malaria report 2019. Geneve, Switzerland: World Health Organization. [Google Scholar]
- Wolff GH, Riffell JA. 2018. Olfaction, experience and neural mechanisms underlying mosquito host preference. J Exp Biol. 221:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondji C, Frédéric S, Petrarca V, Etang J, Santolamazza F, Della Torre A, Fontenille D. 2005. Species and populations of the Anopheles gambiae complex in Cameroon with special emphasis on chromosomal and molecular forms of Anopheles gambiae s.s. J Med Entomol. 42(6):998–1005. [DOI] [PubMed] [Google Scholar]
- Wondwosen B, Birgersson G, Seyoum E, Tekie H, Torto B, Fillinger U, Hill SR, Ignell R. 2016. Rice volatiles lure gravid malaria mosquitoes, Anopheles arabiensis. Sci Rep. 6:37930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondwosen B, Birgersson G, Tekie H, Torto B, Ignell R, Hill SR. 2018. Sweet attraction: sugarcane pollen-associated volatiles attract gravid Anopheles arabiensis. Malar J. 17(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondwosen B, Hill SR, Birgersson G, Seyoum E, Tekie H, Ignell R. 2017. A(maize)ing attraction: gravid Anopheles arabiensis are attracted and oviposit in response to maize pollen odours. Malar J. 16(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooding M, Naudé Y, Rohwer E, Bouwer M. 2020. Controlling mosquitoes with semiochemicals: a review. Parasit Vectors. 13(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Wang G, Buscariollo D, Pitts RJ, Wenger H, Zwiebel LJ. 2008. The molecular and cellular basis of olfactory-driven behavior in Anopheles gambiae larvae. Proc Natl Acad Sci USA. 105(17):6433–6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Sun JS, Carlson JR. 2019. Robust olfactory responses in the absence of odorant binding proteins. Elife. 8:e51040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Jafari S, Pask G, Zhou X, Reinberg D, Desplan C. 2020. Evolution, developmental expression and function of odorant receptors in insects. J Exp Biol. 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Demares F, Norris EJ, Jiang S, Bernier UR, Bloomquist JR. 2020. Bioactivities and modes of action of VUAA1. Pest Manag Sci. [DOI] [PubMed] [Google Scholar]
- Ye Z, Liu F, Ferguson ST, Baker A, Pitts RJ, Zwiebel LJ. 2021. Mutagenesis of the ammonium transporter AcAmt reveals a reproductive role and a novel ammonia-sensing mechanism in the malaria vector mosquito Anopheles coluzzii. bioRxiv. doi: 10.1101/2021.2001.2029.428879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Liu F, Sun H, Barker M, Pitts RJ, Zwiebel LJ. 2020. Heterogeneous expression of the ammonium transporter AgAmt in chemosensory appendages of the malaria vector, Anopheles gambiae. Insect Biochem Mol Biol. 120:103360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger MA, Herre M, Ehrlich AR, Gong Z, Gilbert ZN, Rahiel S, Matthews BJ, Vosshall LB. 2020. Non-canonical odor coding ensures unbreakable mosquito attraction to humans. bioRxiv. doi: 10.1101/2020.2011.2007.368720. [DOI] [Google Scholar]
- Zacharuk RY, Yin LR, Blue SG. 1971. Fine structure of the antenna and its sensory cone in larvae of Aedes aegypti (L.). J Morphol. 135(3):273–297. [DOI] [PubMed] [Google Scholar]
- Zeng F, Xu P, Tan K, Zarbin PHG, Leal WS. 2018. Methyl dihydrojasmonate and lilial are the constituents with an “off-label” insect repellence in perfumes. PLoS One. 13(6):e0199386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Zung JL, Kriete AL, Iqbal A, Younger MA, Matthews BJ, Merhof D, Thiberge S, Strauch M, McBride CS. 2020. Chemical signatures of human odour generate a unique neural code in the brain of Aedes aegypti mosquitoes. bioRxiv. doi: 10.1101/2020.2011.2001.363861. [DOI] [Google Scholar]
- Zhou G, Kohlhepp P, Geiser D, Frasquillo Mdel C, Vazquez-Moreno L, Winzerling JJ. 2007. Fate of blood meal iron in mosquitoes. J Insect Physiol. 53(11):1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]