Abstract
Infection caused by carbapenem-resistant hypervirulent Klebsiella pneumoniae (CR-hvKP) has become a tricky health care threat in China and KPC-2 enzyme is a main factor mediating resistance to carbapenems of K. pneumoniae. Here, we report the characterization of the genetic environment of the blaKPC-2 gene in CR-hvKP clinical isolates from South China. Forty-five non-duplicated CR-hvKP isolates collected in Jiangxi Province from 2018 to 2019 were analyzed. Each of them were multidrug-resistant due to the presence not only of blaKPC-2 gene but also of other resistance determinants, including Metallo-β-lactamases (NDM-1), extended-spectrum β-lactamases (TEM-1, CTX-M-14, SHV-1), and plasmid-mediated quinolone resistance determinants (qnrS, aac(6′)-Ib-cr). After plasmid analyses of PCR-based replicon typing (PBRT), mapping PCR, amplicon sequencing, and whole-genome sequencing (WGS) were used to analyze the genetic environment of the blaKPC-2 gene. PCR analysis of pLVPK-like plasmids, Southern Blot, and mouse lethality assay were used to characterize the virulence phenotype of K. pneumoniae. Multilocus sequence typing (MLST) analysis showed ST11 CR-hvKP was the predominant clone. In conclusion, this is the first analysis of diverse genetic structures blaKPC-2 gene in CR-hvKP isolates from south China. Both the NTEKPC-I on the IncF plasmids and pLVPK-like virulence plasmids make contributions to the formation of CR-hvKP especially ST11 which need more attention.
Keywords: carbapenem resistance, hypervirulent Klebsiella pneumoniae, NTEKPC-2, IncFII-like plasmids, ST11
Introduction
Recently, carbapenem-resistant hypervirulent Klebsiella pneumoniae (CR-hvKP) have become important pathogens of morbidity and mortality among hospital-acquired and long-term care-associated infections (Gu et al., 2018; Zhang et al., 2020). Regardless which plasmids-associated mechanisms underlying the formation of CR-hvKPs clones, the acquisition of carbapenem resistance plasmids by the hypervirulent K. pneumoniae (hvKP) strains or the acquisition of virulence plasmids by the carbapenem-resistant K. pneumoniae (CRKP) strains, carbapenem resistance plasmids are the main actors (Wyres et al., 2019).
KPC-2, the most common variant of KPC carbapenemase enzymes, is a main factor mediating resistance to carbapenems of K. pneumoniae (Shen et al., 2016). Transmission of the KPC gene, blaKPC, can be mediated by different molecular mechanisms such as the mobility of small genetic elements, horizontal transfer of plasmids, and the clonal spread (Munoz-Price and Quinn, 2009). In most countries and regions, such as Europe (Naas et al., 2008) and the United States (Chen et al., 2013), blaKPC−2 is mainly located on Tn4401 transposon. However, blaKPC-bearing non-Tn4401 elements (NTEKPC) were first reported on the plasmid pKP048 from a Chinese clinical K. pneumoniae isolate in 2009 (Shen et al., 2009). Then, NTEKPC was not only reported in different provinces in China (Li et al., 2016; Wang et al., 2016; Fu et al., 2019), but also in other countries such as Brazil (Cerdeira et al., 2017, 2019) and Singapore (Octavia et al., 2019). In a review about molecular and genetic decoding in carbapenemase-producing Klebsiella pneumoniae, NTEKPC has been divided into three groups (NTEKPC I, NTEKPC II, and NTEKPC III) on the basis of the genes adjacent to blaKPC (Chen et al., 2014).
The characteristics of this genetic structure, which mobilizes blaKPC in CR-hvKP strains within and among different clones or plasmids, is unknown, and understanding it would provide insight into diffusion processes and their evolutionary history. This study aimed to present the genetic environment of blaKPC−2 in CR-hvKP isolates in Jiangxi Province using a series of PCR assays and whole-genome sequencing.
Methods and Materials
Bacterial Isolates and Antimicrobial Susceptibility Testing
A total of 45 non-duplicated CR-hvKP clinical isolates were collected from 11 prefecture-level cities in Jiangxi Province including Nanchang, Jingdezhen, Ganzhou, Jiujiang, Xinyu, Pingxiang, Yingtan, Ji'an, Yichun, Shangrao, and Fuzhou, from January 2018 to December 2019. Among them 38 CR-hvKP clinical isolates were collected from 513 non-duplicated CRKP clinical isolates in the First Affiliated Hospital of Nanchang University. K. pneumoniae isolates were identified by an automated Vitek II system (bioMerieux, Balmes-les-Grottes, France) and were further verified with 16S rRNA gene sequencing. According to the latest definition of hvKP (Xu et al., 2019; Zhang et al., 2020), we chose the CRKP strains carrying the pLVPK-like virulence plasmid as CR-hvKP strains in this study. Antibiotic susceptibilities were determined by the disk diffusion method on Mueller-Hinton agar according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (Humphries et al., 2018). K. pneumoniae ATCC 700603 and Escherichia coli ATCC 25922 were used as quality control.
PCR Detection of Resistance Genes, Virulence Genes, and Plasmid Replicon Types
Single PCR was used to analyze quinolone resistance genes [aac(6′)-Ib-cr, qnrA, qnrB, qnrS, and qepA], ESBLs genes (blaCTX−M, blaSHV, blaTEM), carbapenemase genes (blaKPC, blaNDM, blaOXA−48, blaIMP, blaVIM), and pLVPK-related loci (rmpA, iutA, rmpA2), as previously reported (Liao et al., 2020). To confirm the existence of the pLVPK-like plasmid, specific primers of repA, sopB, Lv049 were also designed based on a previous study (Zhao et al., 2019). Plasmid incompatibility was analyzed by using PCR-based replicon typing (PBRT)-KIT 2.0 (DIATHEVA, Italy) (Zhou et al., 2020). A PCR mapping approach was carried out to compare the genetic context of the blaKPC−2 gene in all the CR-hvKP isolates with the NTEKPC in plasmid pKP048 (Shen et al., 2009). All the PCR products were purified and sequenced, and their sequences were compared with the reference sequences stored in the GenBank nucleotide database.
PFGE and MLST
S1 Nuclease Pulsed Field Gel Electrophoresis (S1-PFGE) and Southern blotting were used to determine the location of virulence genes. Hybridization were performed with the DIG-High Prime DNA Labeling and Detection Starter Kit II with of the probe rmpA2 (Roche, Basel, Switzerland) (Li et al., 2020). All the CR-hvKP isolates were subjected to PFGE after digestion with XbaI. The molecular marker was Salmonella serotype Braenderup strain H9812. The cluster cutoff line at 80% similarity was used to analyze genetic relatedness. MLST of Klebsiella pneumoniae was performed online (http://bigsdb.pasteur.fr/klebsiella/klebsiella.html), as previously described (Liao et al., 2020).
Whole-Genome Sequencing and Analysis
Given the PCR mapping analysis, we chose one isolate, respectively, from each different NTEKPC pattern for WGS. Genome sequences were obtained using a combination of Illumina Miseq (150 bp paired-end), and they were assembled with SPAdes version 3.9.1. An average sequencing depth of ×64 was achieved for the genomes. Genomics analysis was performed as described in a previous study (Octavia et al., 2019).
Nucleotide Sequence Accession Numbers
The genome sequences of 12 CR-hvKP strains were submitted to GenBank under the bioproject number PRJNA672246.
Mouse Lethality Assay
Determination of the virulence of K. pneumoniae in mouse lethality tests and the medium lethal dose (LD50, expressed as colony-forming units) was performed as previously described (Yu et al., 2008). In short, a graded dose of 101-107 CFU of each strain in 10-fold serial dilutions in 0.1 ml of normal saline was injected intraperitoneally into mice (four mice for each dose of inoculum). The survival rate of all the vaccinated mice was recorded daily in 2 weeks. The hvKP strain NTUH-K2044 and the classic K. pneumoniae strain ATCC700603 were used as controls of high and low virulence strains, respectively. The interpretation of virulence was referred to reference (Siu et al., 2012).
Ethics Statement
The study has been approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University. Patients participating in the study were anonymous, as a result of the retrospective study, so informed consent was not obtained.
Results
Prevalence of ESBLs Genes and Quinolone Resistance Genes Among CR-hvKP Clinical Isolates
A total of 45 carbapenem-resistant hypervirulent K. pneumoniae isolates were selected from clinical specimens including 19 from sputum, 19 from blood, 4 from urine, 1 from pus, 1 from a deep vein catheter, and 1 from ascites. As shown in Supplementary Table 1, almost all the CR-hvKP isolates were resistant to 18 antibiotics commonly used in clinical treatment, except for some isolates that were sensitive to sulfamethoxazole, tobramycin, and amikacin. As shown in Table 1, all of the CR-hvKP isolates were found to carry carbapenemase gene blaKPC−2, three of which were found to co-carry carbapenemase gene blaNDM−1. Almost all of the CR-hvKP isolates were found to carry at least one ESBLs gene (blaCTX−M, blaSHV, blaTEM) and most of them were found to carry quinolone resistance gene qnrS and aac(6′)-Ib-cr.
Table 1.
Main molecular features of all the CR-hvKP isolates.
| Isolates | LD50(cfu) | Virulence genes | Carbapenemase genes | Other durg resistance genes | NTEKPC plasmid replicon type |
|---|---|---|---|---|---|
| Kp1 | 5.4 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 |
CTX-M-14, SHV-1, qnrS aac(6′)-Ib-cr |
IncFII(k) |
| Kp2 | 1.2 × 105 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 |
CTX-M-14, qnrS aac(6′)-Ib-cr |
IncFII(k) |
| Kp3 | 3.7 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 |
CTX-M-14, SHV-1, TEM-1, qnrS aac(6′)-Ib-cr |
– |
| Kp4 | 5.5 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 |
CTX-M-14, SHV-1, TEM-1, qnrS aac(6′)-Ib-cr |
– |
| Kp5 | 1.1 × 105 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 |
CTX-M-14, SHV-1, TEM-1, qnrS aac(6′)-Ib-cr |
IncFII(k) |
| Kp6 | 5.7 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 |
CTX-M-14, SHV-1, TEM-1, qnrS aac(6′)-Ib-cr |
– |
| Kp7 | 3.2 × 105 | rmpA, silS, iutA, rmpA2 | KPC-2, NDM-1 | CTX-M-14, SHV-1, TEM-1, qnrS | IncFII(k) |
| Kp8 | 7.8 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 |
CTX-M-14, SHV-1, TEM-1, qnrS aac(6′)-Ib-cr |
IncFII(k) |
| Kp9 | 7.7 × 105 | rmpA, silS, iutA, rmpA2 | NDM-1 |
CTX-M-14, SHV-1, TEM-1, qnrS aac(6′)-Ib-cr |
– |
| Kp10 | 8.1 × 105 | rmpA, silS, iutA, rmpA2 | KPC-2, NDM-1 |
CTX-M-14, SHV-1, TEM-1, qnrS aac(6′)-Ib-cr |
IncFII(k) |
| Kp11 | 2.1 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 |
CTX-M-14, SHV-1, qnrS aac(6′)-Ib-cr |
IncFII(k) |
| Kp12 | 3.3 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 |
CTX-M-14, TEM-1, qnrS aac(6′)-Ib-cr |
IncFII(k) |
| Kp13 | 6.5 × 103 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 |
CTX-M-14, SHV-1, TEM-1, qnrS aac(6′)-Ib-cr |
IncFII(k) |
| Kp14 | 4.2 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 |
CTX-M-14, TEM-1, qnrS aac(6′)-Ib-cr |
IncFII(k) |
| Kp15 | 7.9 × 105 | terW, silS, iutA, rmpA2 | KPC-2 |
CTX-M-14, TEM-1, qnrS aac(6′)-Ib-cr |
IncFII(k) |
| Kp16 | 7.6 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 |
CTX-M-14, TEM-1, qnrS aac(6′)-Ib-cr |
IncH1B/IncFrepB |
| Kp17 | 4.5 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2,VIM-2 |
CTX-M-14, SHV-1, TEM-1, qnrS aac(6′)-Ib-cr |
IncFII(k) |
| Kp18 | 8.5 × 105 | terW, silS, rmpA2 | KPC-2 | CTX-M-14, SHV-1, TEM-1 | IncFII(k) |
| Kp19 | 7.7 × 103 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 |
SHV-1, TEM-1, qnrS aac(6′)-Ib-cr- |
– |
| Kp20 | 7.7 × 104 | terW, silS, iutA, rmpA2 | KPC-2 |
SHV-1, TEM-1, qnrS aac(6′)-Ib-cr- |
– |
| Kp21 | 6.1 × 103 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 | – | IncFII(k) |
| Kp22 | 6.4 × 105 | terW, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, SHV-1, TEM-1, qnrS | IncFII(k) |
| Kp23 | 7.2 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, SHV-1 | IncFII(k) |
| Kp24 | 8.8 × 105 | terW, iutA, rmpA2 | KPC-2 | CTX-M-14, SHV-1, TEM-1 | IncFII(k) |
| Kp25 | 6.9 × 105 | terW, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, SHV-1, TEM-1 | IncFII(k) |
| Kp26 | 8.9 × 105 | terW, iutA, rmpA2 | KPC-2 | CTX-M-14, SHV-1, TEM-1, qnrS | IncFII(k) |
| Kp27 | 6.2 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, SHV-1, TEM-1, qnrS | IncFII(k) |
| Kp28 | 6.4 × 105 | terW, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, TEM-1, qnrS | IncH1B/IncFrepB |
| Kp29 | 5.7 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, SHV-1, TEM-1, qnrS | IncFII(k) |
| Kp30 | 6.2 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, SHV-1, TEM-1, qnrS | IncFII(k) |
| Kp31 | 3.8 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, qnrS | – |
| Kp32 | 3.9 × 105 | terW, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, SHV-1, TEM-1, qnrS | – |
| Kp33 | 6.8 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, SHV-1, qnrS | IncFII(k) |
| Kp34 | 3.9 × 103 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 | TEM-1 | IncFII(k) |
| Kp35 | 6.3 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, SHV-1, TEM-1, qnrS | IncFII(k) |
| Kp36 | 8.3 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, SHV-1, qnrS | IncFII(k) |
| Kp37 | 8.7 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, SHV-1, TEM-1 | – |
| Kp38 | 3.4 × 105 | terW, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, SHV-1, TEM-1, qnrS | IncFII(k) |
| Kp39 | 4.7 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, SHV-1, qnrS | IncFII(k) |
| Kp40 | 5.6 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, qnrS | IncFII(k) |
| Kp41 | 2.6 × 104 | rmpA, terW, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, TEM-1, qnrS | – |
| Kp42 | 1.4 × 105 | terW, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, TEM-1, qnrS | IncFII(k) |
| Kp43 | 2.7 × 105 | rmpA, terW, silS, rmpA2 | KPC-2 | SHV-1, qnrS | – |
| Kp44 | 4.4 × 104 | terW, silS, iutA, rmpA2 | KPC-2 | SHV-1, qnrS | IncFII(k) |
| Kp45 | 2.9 × 105 | rmpA, silS, iutA, rmpA2 | KPC-2 | CTX-M-14, TEM-1, qnrS | IncFII(k) |
Virulence Assessment of CR-hvKP Clinical Isolates
In this scenario, PCR analysis of pLVPK-like plasmids revealed that all the CR-hvKP isolates were found to carry the pLVPK-like plasmid. These results were also verified by S1-PFGE and Southern blotting as shown in Supplementary Figure 1. However, PCR analysis of virulence genes revealed that not every isolate was found to carry the complete pLVPK-related loci (rmpA, iutA, rmpA2). The mouse lethality assay also proved that all the CR-hvKP isolates having the 50% lethal dose (LD50) of <105 CFU were hypervirulent, and were a little lower virulent than the hvKP strain NTUH-K2044 having the LD50 of <102 CFU, while classic K. pneumoniae strain ATCC700603 had the LD50 of more than 107 CFU.
Genetic Linkage of blakpc−2 Among the CR-hvKP Isolates
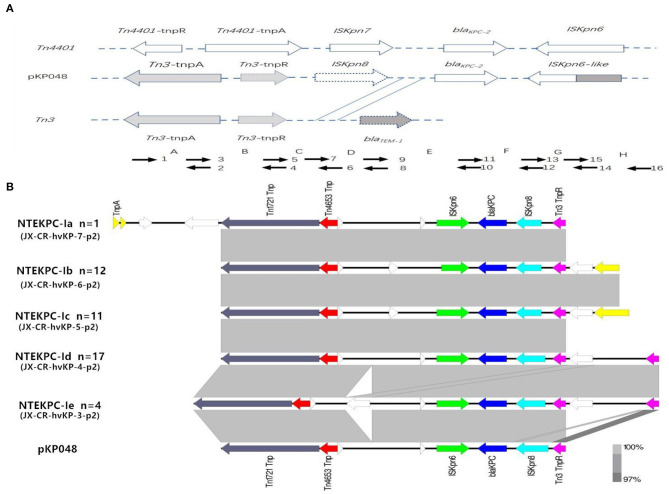
The analysis of the genetic environment of blaKPC−2 genes showed five distinct NTE patterns compared with the classic Tn3-based structure in the plasmid pKP048. All the different NTE patterns were classified as NTEKPC-I. In detail, the distribution of different NTE patterns among these clinical CR-hvKP strains was shown in the Figure 1.
Figure 1.
Schematic representation of the genetic structures surrounding the blaKPC-2 gene in the CR-hvKP isolates. (A) Classic Tn3-based structure in plasmid pKP048; Short arrowheads with numbers were used for PCR mapping. (B) Five distinct NTE patterns. Gene blaKPC−2 is represented by a blue arrow, and the remaining genes are color coded. NTEKPC-Ib, NTEKPC-Ic, and NTEKPC-Id were the most common genetic structures in the CR-hvKP isolates.
NTEkpc-I Plasmids Diversity
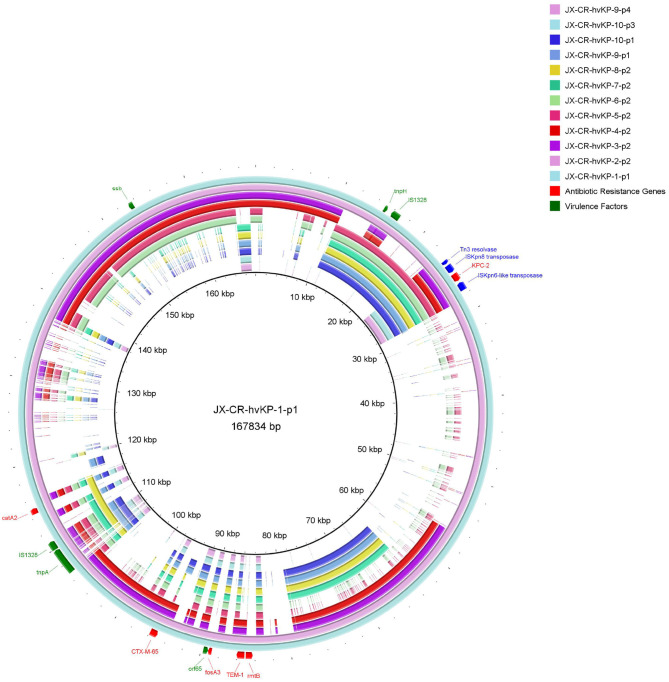
Almost all the different NTE patterns carrying blaKPC-2 were shown to be carried on the IncF plasmids. Interestingly untyped plasmid replicons were detected in 11 CR-hvKP isolates (Table 1). As shown in Figure 2, these NTEKPC plasmids also had diversity of gene structure. Plasmid gene structure differences existed in the same ST (ST11) clinical K. pneumoniae strains. JX-CR-hvKP-9-p1 and JX-CR-hvKP-9-p4 from the same isolate had the totally different plasmid gene structure, the same goes for JX-CR-hvKP-10-p1 and JX-CRhvKP-10-p3. Interestingly JX-CR-hvKP-6-p2 and JX-CR-hvKP-5-p2 from ST23 CR-hvKP had the same plasmid gene structure, while JX-CR-hvKP-8-p2, JX-CR-hvKP-7-p2, JX-CR-hvKP-9-p1, JX-CR-hvKP-10-p1 from ST11 CR-hvKP also had highly similar plasmid gene structure.
Figure 2.
Gene map of twelve NTEKPC plasmids harbored by CR-hvKP strains. JX-CR-hvKP-5-p2 and JX-CR-hvKP-6-p2 are from ST23 CR-hvKP, and the others are from ST11 CR-hvKP. The circular map was generated using the BLAST Ring Image Gnerator. A sequence comparison showed the polymorphism of blaKPC plasmids in CR-hvKP.
Molecular Characteristics
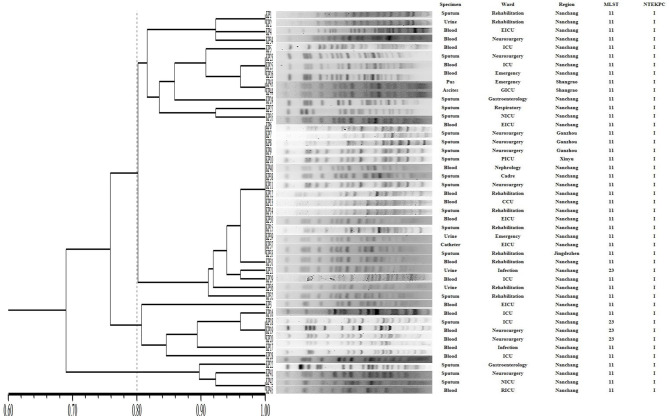
The PFGE-based fingerprints of the CR-hvKP isolates displayed three different clusters (named A–C) using a similarity cutoff value of 80% (Figure 3), including cluster A (34/45, 75.6%), cluster B (7/45, 15.6%), and cluster C (4/45, 8.8%). The MLST analysis distinguished two different STs. The most prevalent ST in CR-hvKP isolates was ST11 (41/45, 91.1%), followed by ST23 (4/45, 8.9%).
Figure 3.
Pulsed-field gel electrophoresis (PFGE) patterns, NTEKPC patterns, and STs among 45 clinical CR-hvKP isolates. NICU, neurosurgery ICU; GICU, gastroenterology ICU; RICU, respiratory ICU; PICU, Pediatric ICU; EICU, Emergency ICU.
Discussion
Over the past few decades, hvKP has emerged worldwide, causing invasive infections since the first clinical hvKP report was published in 1986 (Russo and Marr, 2019). Although initially reported hvKP isolates were usually antimicrobial sensitive, clonal complexes of hypervirulent (hvKP) and carbapenem-resistant (CR) strains are non-overlapping (Liao et al., 2020). In this study, we collected 45 CR-hvKP strains causing nosocomial infections, including 41 ST11 CRKP acquiring the pLVPK like plasmid, 4 ST23 hvKP acquiring the carbapenemase plasmid. It was consistent with the evolution of CR-hvKP in a Chinese multicenter and molecular epidemiological analysis that the KPC-2-producing ST11 clone was the common type of CR-hvKP isolates (Zhang et al., 2020).
PCR analysis of repA, sopB, Lv049 and Southern Blot revealed that all the CR-hvKP carrying pLVPK-like virulence plasmids were identified in this study. PCR analysis of pLVPK-related loci (rmpA, iutA, rmpA2) revealed that some virulence genes such as rmpA, terW were lost in a few CR-hvKP isolates. Maybe some virulence genes such as rmpA, terW were lost at the time of pLVPK-like plasmid transferring. It was consistent with the findings in a previous study about core genome allelic profiles of clinical Klebsiella pneumoniae strains based on multi-locus sequence typing scheme for hypervirulence analysis (Lan et al., 2020). Moreover, mouse lethality assay revealed that all the CR-hvKP isolates had a 50% lethal dose (LD50) of <105 CFU, while classic K. pneumoniae (cKP) strain ATCC700603 had the LD50 of more than 107 CFU. To our best knowledge, at present, the mouse lethality assay is the most standard method to differentiate hvKP from cKP (Russo and MacDonald, 2020).
This study first provides key insights into the horizontal transfer of the NTEKPC and IncF plasmids, which appears to be a potential element driving the molecular diversification in ST11 CR-hvKP isolates. Transposon elements are believed to be responsible for the rapid spread of blaKPC (Chen et al., 2011; Zhang et al., 2012). In China, a different genetic organization of the blaKPC locus from the “traditional” Tn4401 was detected by Shen et al. (2009). The genetic locus located on the plasmid pKP048 contains a Tn3-based transposon and a partial Tn4401 segment, ISKpn8, and an ISKpn6-like element (Shen et al., 2009). Afterward, various mutations in the surrounding environment of the blaKPC−2 gene were detected, and most of which were mainly caused by the insertion of a truncated blaTEM gene sequence between ISKpn8 and blaKPC gene with different sizes (Yang et al., 2013; Li et al., 2016). In this study, the KPC structures of all the CR-hvKP isolates should be separated into the NTEKPC-I group because of the blaTEM absence (NTEKPC-I) (Figure 1) (Chen et al., 2014). PCR and WGS analysis showed that there were five different NTEKPC-I patterns in these CR-hvKP strains. They had high similarity to the NTEKPC on the earliest plasmid pKP048. An increasing number of CR-hvKP in Jiangxi Province revealed that NTEKPC-I had strong dissemination ability and good stability (Liao et al., 2020; Li et al., 2020). It is noteworthy that NTEKPCs are primarily found in non-ST258 K. pneumoniae or other non-K. pneumoniae species (Chen et al., 2014).
The pandemic spread of blaKPC−2 among Klebsiella pneumoniae ST11 in China is mainly related to the horizontal transfer mediated by incompatibility group F (IncF) plasmids (Chi et al., 2019; Fu et al., 2019). PCR-based replicon type and representative strain WGS analysis revealed that almost all the different NTE patterns carrying blaKPC−2 in this study were also shown to be carried on the IncF plasmids. We suppose that there was also a close correlation between NTEKPC-I and IncF plasmids in CR-hvKP, whereby ST11 CR-hvKP is a seemingly good colonizer to capture IncF plasmids. The gene map of twelve NTEKPC plasmids showed that there are gene structure differences among ST11 CR-hvKP strains in Jiangxi Province, which is consistent with the finding that the diversity of the plasmids of genetically related K. pneumoniae strains harboring the beta-lactamase gene blaKPC-2 existed in the Netherlands from 2014 to 2019 (Hendrickx et al., 2020). Interestingly JX-CR-hvKP-6-p2 and JX-CR-hvKP-5-p2 from ST23 CR-hvKP had the same plasmid gene structure without any plasmid replicon type. It appeared to be that the NTEKPC plasmids lost their plasmid replicon after entering ST23 hvKP, while JX-CR-hvKP-8-p2, JX-CR-hvKP-7-p2, JX-CR-hvKP-9-p1, and JX-CR-hvKP-10-p1 from ST11 CR-hvKP had highly similar plasmid gene structure. This indicates that the resistance/virulence hybrid plasmids in JX-CR-hvKP-9 and JX-CR-hvKP-10 formed by the fusion of NTEKPC plasmids and pLVPK-like virulence plasmids. The hypothesis must be validated by further experiments.
The PFGE patterns show that all the CR-hvKP isolates were assigned to three clusters based on >80% pattern similarity. It is consistent with a previous study in China that the ST11 genomes were highly heterogeneous and clustered into at least three major lineages based on single nucleotide polymorphism (SNP) analysis (Dong et al., 2018).
The study has certain limitations, including its retrospective nature and a relatively small study population. Therefore, there may be selection bias, which limits the general application of study results to other areas. Consequently, a further study that includes more patients, especially for ST23 hvKp isolates, is needed.
Conclusion
In conclusion, this is the first analysis of the diverse genetic structures of the blaKPC−2 gene in CR-hvKP isolates from South China. Both the NTEKPC-I on the IncF plasmids and pLVPK-like virulence plasmids make contributions to the formation of CR-hvKP, especially ST11, which need more attention.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: GenBank, PRJNA672246.
Ethics Statement
The animal study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
L-GW and T-xX did strain characterization and participated in manuscript writing. ZX and DL conceived the study and performed data analysis. WL did the whole-genome sequencing and comparative genomics and participated in manuscript writing. YL and DW wrote the paper. Q-SH collected the clinical data and analyzed the data. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by the National Natural Science Foundation of China (81560323 and 81860368), the Education Department of Jiangxi Province, China (GJJ160029), the Jiangxi Science and Technology Department in China (20181BAB205002, 20202ACBL206023, and 20202ZDB01016), the Health and Family Planning Commission of Jiangxi Province (20188006 and 2018A330), the National Mega-project for Innovative Drugs (2019ZX09721001), and Anti-infective drug research (2019BJZDS003).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.622280/full#supplementary-material
References
- Cerdeira L. T., Cunha M., Francisco G. R., Bueno M., Araujo B. F., Ribas R. M., et al. (2017). IncX3 plasmid harboring a non-Tn4401 genetic element (NTEKPC) in a hospital-associated clone of KPC-2-producing Klebsiella pneumoniae ST340/CG258. Diagn. Microbiol. Infect. Dis. 89, 164–167. 10.1016/j.diagmicrobio.2017.06.022 [DOI] [PubMed] [Google Scholar]
- Cerdeira L. T., Lam M., Wyres K. L., Wick R. R., Judd L. M., Lopes R., et al. (2019). Small IncQ1 and col-like plasmids harboring blaKPC-2 and non-Tn4401 elements (NTEKPC-IId) in high-risk lineages of klebsiella pneumoniae CG258. Antimicrob. Agents Chemother. 63:e02140–18. 10.1128/AAC.02140-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Chavda K. D., Melano R. G., Jacobs M. R., Levi M. H., Bonomo R. A., et al. (2013). Complete sequence of a bla(KPC-2)-harboring IncFII(K1) plasmid from a Klebsiella pneumoniae sequence type 258 strain. Antimicrob. Agents Chemother. 57, 1542–1545. 10.1128/AAC.02332-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Mathema B., Chavda K. D., DeLeo F. R., Bonomo R. A., Kreiswirth B. N. (2014). Carbapenemase-producing Klebsiella pneumoniae: molecular and genetic decoding. Trends Microbiol. 22, 686–696. 10.1016/j.tim.2014.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Hu F., Xu X., Liu Y., Wu W., Zhu D., et al. (2011). High prevalence of KPC-2-type carbapenemase coupled with CTX-M-type extended-spectrum beta-lactamases in carbapenem-resistant Klebsiella pneumoniae in a teaching hospital in China. Antimicrob. Agents Chemother. 55, 2493–2494. 10.1128/AAC.00047-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X., Hu G., Xu H., Li X., Xiao T., Zhou Y., et al. (2019). Genomic analysis of A KPC-2-producing klebsiella pneumoniae ST11 outbreak from a teaching hospital in Shandong Province, China. Infect. Drug Resist. 12, 2961–2969. 10.2147/IDR.S221788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N., Zhang R., Liu L., Li R., Lin D., Chan E. W., et al. (2018). Genome analysis of clinical multilocus sequence Type 11 Klebsiella pneumoniae from China. Microb. Genom. 4:e000149. 10.1099/mgen.0.000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu P., Tang Y., Li G., Yu L., Wang Y., Jiang X. (2019). Pandemic spread of bla KPC-2 among Klebsiella pneumoniae ST11 in China is associated with horizontal transfer mediated by IncFII-like plasmids. Int. J. Antimicrob. Agents 54, 117–124. 10.1016/j.ijantimicag.2019.03.014 [DOI] [PubMed] [Google Scholar]
- Gu D., Dong N., Zheng Z., Lin D., Huang M., Wang L., et al. (2018). A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect. Dis. 18, 37–46. 10.1016/S1473-3099(17)30489-9 [DOI] [PubMed] [Google Scholar]
- Hendrickx A., Landman F., de Haan A., Borst D., Witteveen S., van Santen-Verheuvel M. G., et al. (2020). Plasmid diversity among genetically related Klebsiella pneumoniae blaKPC-2 and blaKPC-3 isolates collected in the Dutch national surveillance. Sci. Rep. 10:16778. 10.1038/s41598-020-73440-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries R. M., Ambler J., Mitchell S. L., Castanheira M., Dingle T., Hindler J. A., et al. (2018). CLSI Methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J. Clin. Microbiol. 56:e01934–17. 10.1128/JCM.01934-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan P., Shi Q., Zhang P., Chen Y., Yan R., Hua X., et al. (2020). Core genome allelic profiles of clinical klebsiella pneumoniae strains using a random forest algorithm based on multilocus sequence typing scheme for hypervirulence analysis. J. Infect. Dis. 221, S263–S271. 10.1093/infdis/jiz562 [DOI] [PubMed] [Google Scholar]
- Li D., Liao W., Huang H. H., Du F. L., Wei D. D., Mei Y. F., et al. (2020). Emergence of hypervirulent ceftazidime/avibactam-resistant Klebsiella pneumoniae isolates in a Chinese tertiary hospital. Infect. Drug Resist. 13, 2673–2680. 10.2147/IDR.S257477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zou M. X., Wang H. C., Dou Q. Y., Hu Y. M., Yan Q., et al. (2016). An outbreak of infections caused by a Klebsiella pneumoniae ST11 clone coproducing Klebsiella pneumoniae Carbapenemase-2 and RmtB in a Chinese teaching hospital. Chin. Med. J. 129, 2033–2039. 10.4103/0366-6999.189049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W., Wang L., Li D., Du F. L., Long D., Liu Y., et al. (2020). High Prevalence of 16s rRNA methylase genes among carbapenem-resistant hypervirulent Klebsiella pneumoniae isolates in a Chinese tertiary hospital. Microb. Drug Resist. 27, 44–52. 10.1089/mdr.2019.0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Price L. S., Quinn J. P. (2009). The spread of Klebsiella pneumoniae carbapenemases: a tale of strains, plasmids, and transposons. Clin. Infect. Dis. 49, 1739–1741. 10.1086/648078 [DOI] [PubMed] [Google Scholar]
- Naas T., Cuzon G., Villegas M. V., Lartigue M. F., Quinn J. P., Nordmann P. (2008). Genetic structures at the origin of acquisition of the beta-lactamase bla KPC gene. Antimicrob. Agents Chemother. 52, 1257–1263. 10.1128/AAC.01451-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Octavia S., Kalisvar M., Venkatachalam I., Ng O. T., Xu W., Sridatta P., et al. (2019). Klebsiella pneumoniae and Klebsiella quasipneumoniae define the population structure of blaKPC-2Klebsiella: a 5 year retrospective genomic study in Singapore. J. Antimicrob. Chemother. 74, 3205–3210. 10.1093/jac/dkz332 [DOI] [PubMed] [Google Scholar]
- Russo T. A., MacDonald U. (2020). The Galleria mellonella infection model does not accurately differentiate between hypervirulent and classical Klebsiella pneumoniae. mSphere 5:e00850–19. 10.1128/mSphere.00850-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo T. A., Marr C. M. (2019). Hypervirulent Klebsiella pneumoniae. Clin. Microbiol. Rev. 32:e00001–19. 10.1128/CMR.00001-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P., Wei Z., Jiang Y., Du X., Ji S., Yu Y., et al. (2009). Novel genetic environment of the carbapenem-hydrolyzing beta-lactamase KPC-2 among Enterobacteriaceae in China. Antimicrob. Agents Chemother. 53, 4333–4338. 10.1128/AAC.00260-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P., Zhang Y., Li G., Jiang X. (2016). Characterization of the genetic environment of the blaKPC-2 gene among Klebsiella pneumoniae isolates from a Chinese Hospital. Braz. J. Infect. Dis. 20, 384–388. 10.1016/j.bjid.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu L. K., Yeh K. M., Lin J. C., Fung C. P., Chang F. Y. (2012). Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect. Dis. 12, 881–887. 10.1016/S1473-3099(12)70205-0 [DOI] [PubMed] [Google Scholar]
- Wang L. H., Wei D. D., Wan L. G., Yu Y., Deng Q., Liu Y. (2016). Diversity of the genetic environment of the blaKPC-2 gene among Klebsiella pneumoniae clinical isolates in a Chinese hospital. Microb. Drug Resist. 22, 15–21. 10.1089/mdr.2014.0281 [DOI] [PubMed] [Google Scholar]
- Wyres K. L., Wick R. R., Judd L. M., Froumine R., Tokolyi A., Gorrie C. L., et al. (2019). Distinct evolutionary dynamics of horizontal gene transfer in drug resistant and virulent clones of Klebsiella pneumoniae. PLoS Genet. 15:e1008114. 10.1371/journal.pgen.1008114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Fu Y., Fang Y., Xu H., Kong H., Liu Y., et al. (2019). High prevalence of KPC-2-producing hypervirulent Klebsiella pneumoniae causing meningitis in Eastern China. Infect. Drug Resist. 12, 641–653. 10.2147/IDR.S191892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Ye L., Guo L., Zhao Q., Chen R., Luo Y., et al. (2013). A nosocomial outbreak of KPC-2-producing Klebsiella pneumoniae in a Chinese hospital: dissemination of ST11 and emergence of ST37, ST392 and ST395. Clin. Microbiol. Infect. 19, E509–515. 10.1111/1469-0691.12275 [DOI] [PubMed] [Google Scholar]
- Yu W. L., Ko W. C., Cheng K. C., Lee C. C., Lai C. C., Chuang Y. C. (2008). Comparison of prevalence of virulence factors for Klebsiella pneumoniae liver abscesses between isolates with capsular K1/K2 and non-K1/K2 serotypes. Diagn. Microbiol. Infect. Dis. 62, 1–6. 10.1016/j.diagmicrobio.2008.04.007 [DOI] [PubMed] [Google Scholar]
- Zhang X., Lü X., Zong Z. (2012). Enterobacteriaceae producing the KPC-2 carbapenemase from hospital sewage. Diagn. Microbiol. Infect. Dis. 73, 204–206. 10.1016/j.diagmicrobio.2012.02.007 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Jin L., Ouyang P., Wang Q., Wang R., Wang J., et al. (2020). Evolution of hypervirulence in carbapenem-resistant Klebsiella pneumoniae in China: a multicentre, molecular epidemiological analysis. J. Antimicrob. Chemother. 75, 327–336. 10.1093/jac/dkz446 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhang X., Torres V., Liu H., Rocker A., Zhang Y., et al. (2019). An outbreak of carbapenem-resistant and hypervirulent Klebsiella pneumoniae in an intensive care unit of a major teaching hospital in Wenzhou, China. Front. Public. Health 7:229. 10.3389/fpubh.2019.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Zhang K., Chen W., Chen J., Zheng J., Liu C., et al. (2020). Epidemiological characteristics of carbapenem-resistant Enterobacteriaceae collected from 17 hospitals in Nanjing district of China. Antimicrob. Resist. Infect. Control. 9:15. 10.1186/s13756-019-0674-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: GenBank, PRJNA672246.