Abstract
Background and Aims
Many inflammatory bowel disease [IBD] patients in remission have persisting symptoms, compatible with irritable bowel syndrome [IBS-type symptoms]. We aimed to compare the effectiveness of gut-directed hypnotherapy vs standard medical treatment [SMT] for IBS-type symptoms in IBD patients.
Methods
In this multicentre, randomized, controlled, open-label trial, patients aged 12–65 years with IBD in clinical remission [global assessment] and biochemical remission [faecal calprotectin ≤100 µg/g, or ≤200 µg/g without inflammation at endoscopy] with IBS according to Rome III criteria were randomized to hypnotherapy or SMT. Primary outcome was the proportion with ≥50% reduction on a visual analog scale for symptom severity, as measured with the Irritable Bowel Syndrome Severity Scoring System [IBS-SSS] at week 40 [i.e. 6 months after finishing the intervention], compared to baseline. Secondary outcomes included total IBS-SSS score, quality of life, adequate relief, IBS-related cognitions, and depression and anxiety scores.
Results
Eighty patients were included, of whom 70 received at least one session of the allocated treatment and were included in the modified intention-to-treat-population. Seven patients were excluded because of missing baseline data required for the primary outcome. The primary outcome was met in nine [27%] of 33 patients randomized to SMT and nine [30%] of 30 patients randomized to hypnotherapy [p = 0.81]. Adequate relief was reported in 60% and 40% of subjects, respectively. Exploratory analyses of secondary outcomes revealed no apparent differences between the two treatment groups.
Conclusions
Hypnotherapy was not superior to SMT in the treatment of IBS-type symptoms in IBD patients. Both treatment strategies are reasonable options from a clinical perspective.
Keywords: Crohn’s disease, ulcerative colitis, inflammatory bowel disease, irritable bowel syndrome, IBS-like symptoms, IBS-type symptoms, hypnotherapy, gut-directed hypnotherapy
1. Introduction
Inflammatory bowel diseases [IBD], including the main phenotypes Crohn’s disease and ulcerative colitis, are often characterized by alternating periods of active inflammation and remission. A substantial number of IBD patients in remission report persisting gastrointestinal symptoms, such as abdominal pain and diarrhoea.1 These symptoms commonly mimic irritable bowel syndrome [IBS] and are thus referred to as IBS-type or IBS-like symptoms. In a systematic review of 11 studies including 1197 IBD patients in remission, the pooled prevalence of IBS-type symptoms was 35%.1
Despite this high prevalence of IBS-type symptoms in patients with quiescent IBD, there is a lack of evidence on the efficacy of therapeutic strategies. To our knowledge, only retrospective and small prospective studies have been performed to date. Results from these studies suggest that fibre supplements, tricyclic antidepressants, mindfulness and osteopathy may provide some relief of symptoms.2–5
Gut-directed hypnotherapy is considered one of the most effective treatments for IBS in both children and adults, with response rates ranging from 24% to 85%.6–8 Furthermore, preliminary data suggest that hypnotherapy may also be beneficial for IBD patients.8,9 In two studies in ulcerative colitis patients, hypnotherapy was associated with a reduced risk of clinical relapse10 and a decrease in inflammatory markers in patients with active disease.11 To date, the effectiveness of hypnotherapy for treating IBD patients with IBS-type symptoms is unknown. Therefore, the aim of this study was to investigate the effectiveness of hypnotherapy for treating patients with quiescent IBD with IBS-type symptoms in comparison to standard medical treatment [SMT] for IBS.
2. Methods
2.1. Study design
We conducted this open, multicentre, randomized controlled trial at the outpatient clinic of four hospitals in the Netherlands from September 2012 to December 2016. The study consisted of a screening phase [weeks −2 to 0], a treatment phase [weeks 0 to 12] and a follow-up phase [weeks 12 to 40]. The trial protocol was reviewed and approved by the Medical Ethics Committees of all participating hospitals and is registered in the Netherlands Trial Register [www.trialregister.nl] with identification number NL3261. This study has been designed according to recommendations by the Design of Treatment Trials Committee of the Rome Foundation.12
2.2. Population
We included patients aged 12–65 years consecutively with an established diagnosis of Crohn’s disease or ulcerative colitis based on endoscopy and histology. All patients had normal levels of inflammatory markers and had a normal faecal calprotectin [FC] level. The latter was defined as an FC level ≤100 µg/g or an FC level ≤200 µg/g combined with no macroscopic signs of inflammation on endoscopy. All patients were in clinical remission according to global assessment by the treating physician, who was not blinded to the inflammatory markers. The relevant physicians could use any other diagnostic modality necessary at their own discretion to conclude that candidates were indeed in clinical remission. All included participants fulfilled the Rome III criteria for IBS.13 All subjects reported pain or discomfort at least 2 days a week, which was confirmed using a diary 2 weeks before randomization. Participants were allowed to continue all medication that was used before inclusion. When necessary, treatment adaptations of anti-inflammatory or immune modulating agents were allowed to be made by the treating physician during participation.
We excluded patients with other concomitant organic gastrointestinal abnormalities besides IBD, patients with an intestinal stenosis and patients who previously had undergone more than one gastrointestinal surgery for IBD. Furthermore, patients with significant comorbidities [e.g. malignancy, instable cardiovascular disease] were excluded. Patients were not allowed to receive concomitant [conventional, complementary or alternative] treatment by another healthcare professional for gastrointestinal symptoms. Also, patients who had previously received hypnotherapy and patients with an intellectual disability or insufficient knowledge of the Dutch language to participate in hypnotherapy were excluded.
Written informed consent was obtained from all patients. For patients aged <18 years, written informed consent was also obtained from both parents.
2.3. Randomization and masking
Participants were randomly allocated to either hypnotherapy or SMT using computerized block-randomization with a 1:1 ratio. Random block sizes [with a maximum of six] were used. Randomization was stratified according to age group [12–18 or 18–65 years] and diagnosis [Crohn’s disease or ulcerative colitis]. Due to the nature of study treatment, masking of study treatment was not possible.
2.4. Interventions: hypnotherapy
Hypnotherapy consisted of six sessions of approximately 50 min per session over a period of 12 weeks and was distinctively performed by two certified hypnotherapists, who were trained in the treatment protocol. The protocol was based on that previously used by our research group14 and the Manchester approach,15 and consisted of exercises for general relaxation, stress control, control of abdominal pain and gut and immune functioning, and suggestions to improve self-esteem. It was permitted to adapt contents and order of the protocol to the participant’s interests and specific issues that could come up during therapy. Patients received a digital audio recording containing five hypnosis exercises that was previously designed for adolescents with IBS.14 Furthermore, each patient received one personalized audio recording. The same protocol was used for all participants.
In the first session, therapists took a full history, explained gut-directed hypnotherapy, introduced the mind–body connection, performed breathing exercises while the patients were instructed to imagine a warm healing feeling flowing from the hands into the abdomen, and introduced progressive relaxation according to the method of Jacobson. Patients were asked to listen to one of the digital audio recordings or to practise self-hypnosis once a day. Furthermore, patients were instructed to practise conscious breathing exercises several times throughout the day. In the second session, ‘the safe/favourite place exercise’ was introduced that focused on relaxation, increased self-control, and enhanced sleep and energy. Patients were instructed to visualize a colour that symbolized health. In the third session, ‘the hot air balloon exercise’ was introduced. This exercise focused on the reduction of stress and worry and the promotion of calm, comfortable and confident feelings. During the fourth session, the exercise ‘the beach without worries’ was introduced which promoted calm, comfortable and confident feelings, and included visualizations of a healthy gut and a healthy immune system. During the fifth session, ‘the slide’ exercise was introduced. During hypnosis, the patient received suggestions of going down a slide, which was used to promote a reduction of stress, worry and pain and to introduce a calm, comfortable and confident feeling. During this exercise, the gut was also imagined as a slide. In the final session, an evaluation of the past weeks was made. Furthermore, an exercise was performed consisting of elements of the previous sessions. Patients were instructed to continue listening to the hypnosis exercises daily.
2.5. Interventions: standard medical treatment
SMT consisted of six sessions of approximately 50 min per session over a period of 12 weeks and was performed by a research physician with experience in treating functional gastrointestinal disorders at the outpatient clinic. The protocol was based on, but not limited to, recommendations regarding the management of IBS from the Dutch multidisciplinary guideline ‘Diagnosis and Management of Irritable Bowel Syndrome’.16 Treatment could consist of various modalities, including dietary advice according to MacDermott et al.,17 antispasmodics, analgesics, fibre supplementation, laxatives, low-dose antidepressants [e.g. amitriptyline once daily 10–20 mg] and anti-diarrhoeal drugs.
In the first session, symptoms and provoking factors were explored. Furthermore, any previous treatments and their respective efficacy were discussed. Also, the impact of symptoms on patients’ [quality of] life was examined. Education, reassurance, and general lifestyle and dietary advice were given when considered necessary. Based on the participants’ individual preference, a treatment plan was made. During the following sessions, the effect of the initiated treatments was evaluated and further treatment options were discussed and initiated when necessary. If the patient perceived benefit from the initiated treatment, it could be continued up to [at least] week 40.
2.6. Outcome assessment
Outcomes were measured at baseline [week 0], after treatment [week 12], and 26 and 40 weeks after inclusion [i.e. 3 and 6 months after the end of treatment] using participant self-administered paper questionnaires.
2.6.1. Primary outcome
In our pre-specified primary analysis, the primary outcome was the proportion of participants with a reduction of ≥50% at week 40 compared to baseline of the 100-mm visual analog scale [VAS] for pain of the Irritable Bowel Syndrome Severity Scoring System [IBS-SSS] questionnaire.18
Rome III criteria for IBS were used for inclusion. Thus, patients could also be included if they suffered from abdominal discomfort, rather than abdominal pain. In the IBS-SSS questionnaire, there is an distinction between abdominal pain and abdominal distension/tightness. Consequently, a proportion [n = 3] of the participants did not report any abdominal pain at baseline on the IBS-SSS questionnaire, preventing the primary endpoint [a reduction of ≥50% of abdominal pain] from being met. For these participants, it was decided to use a reduction of ≥50% at week 40 compared to baseline of the 100-mm VAS for distension/tightness of the IBS-SSS questionnaire instead as the primary endpoint.18 There was no minimum severity of abdominal pain or discomfort for inclusion.
2.6.2. Secondary outcomes
Secondary outcomes were total IBS-SSS score, adequate relief, generic and disease-specific quality of life, treatment expectation and preference. Adequate relief was assessed at weeks 12, 26 and 40 by asking the participant a single binary question [yes/no] for four consecutive weeks whether he/she had experienced adequate relief of IBS-related abdominal pain or discomfort.
Generic quality of life was assessed at baseline, and weeks 12 and 40 in all age groups using the short-form 36 [SF-36] health-related quality of life questionnaire.19 Disease-specific quality of life was assessed using the Impact III questionnaire20 or Inflammatory Bowel Disease Questionnaire [IBDQ],21 for participants aged 12–18 or 18–65 years, respectively. In patients aged ≥18 years, the presence of dysfunctional IBS-related cognitions was assessed using the Cognitive Scale for Functional Bowel Disorders [CS-FBD] at baseline and weeks 12 and 40.22 Anxiety and depression were assessed using the anxiety and depression subscales of the SCL-90-R questionnaire in all patients at baseline, week 12 and week 40.23 Baseline depression and anxiety SCL-90-R scores were compared with reference scores of the general population.24
Treatment expectation and preference were determined at baseline using a self-constructed questionnaire. Participants were asked to rate their expected improvement after treatment on an 11-point scale [0 = no improvement; 10 = complete recovery]. Treatment preference was ranked on a five-point scale [−2 = strong preference for hypnotherapy, 0 = no preference, 2 = strong preference for SMT].
2.7. Statistical analysis
All participants who attended at least one session of the allocated treatment were included in the modified intention to treat [ITT] population, excluding participants with missing baseline IBS-SSS scores required for assessment of the primary outcome. Analyses were performed in the modified ITT population, unless otherwise specified. Baseline and outcome variables were summarized using mean and standard deviation [SD] for continuous, normally distributed variables median and interquartile range [IQR] for non-normally distributed continuous data, and using counts and percentages for categorical variables. The primary analysis concerned the comparison of the proportion of participants with at least a 50% reduction in symptom severity at week 40 vs baseline between the two study treatment groups, using a chi-squared test and expressed in an absolute difference in proportions with 95% confidence interval [CI].
Additionally, we conducted sensitivity analyses for the primary outcome, including all participants as randomized, considering all subjects excluded from the modified ITT population as not meeting the primary outcome, and in the per-protocol population, including all subjects who attended all six sessions of the allocated treatment.
Secondary outcomes were compared using Student’s t-tests, Mann–Whitney U tests or chi-squared test, as appropriate. For adequate relief, we analysed the proportion of participants at each time point who reported adequate relief for at least 50% of the weeks. Analyses of other secondary outcomes were exploratory using two-sided 95% CIs. No adjustments for multiple comparisons were made and secondary outcome analyses should therefore be interpreted as exploratory.
Significance was set at a two-sided p < 0.05. All analyses were performed using SPSS version 25 [IBM]. Missing data were analysed according to the last observation carried forward principle. Multiple scenarios were analysed to explore the effect of alternative handling of missing data/excluded subjects.
2.8. Sample size calculation
We hypothesized that hypnotherapy would be superior to SMT. Based on results from previous studies on the effectiveness of hypnotherapy in IBS,6,25,26 we estimated that 75% of the participants in the hypnotherapy group would reach the primary endpoint compared to 40% of the participants in the SMT group at week 40 [i.e. 6 months after the end of treatment]. A chi-squared test with a two-sided α of 0.05 will have 80% power to detect the expected difference between treatment arms when the sample size in each group is 36. With an estimated dropout rate of 10%, we aimed to include a total of 80 participants [40 in each arm].
3. Results
3.1. Screening and enrollment
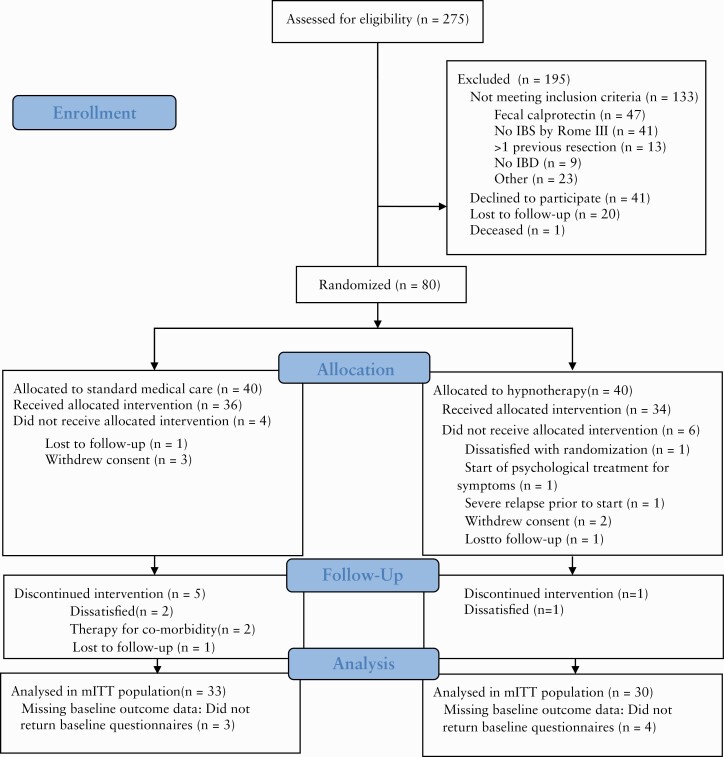
A total of 275 patients were screened for eligibility within the study period, of whom 133 did not meet inclusion criteria, 41 declined to participate and 21 could not be included because of other reasons [Figure 1]. Thus, 80 patients were randomized [hypnotherapy: 40], of whom 70 attended at least one session of the allocated treatment. Of these, seven patients did not return baseline questionnaires despite repeated requests. Thus, in total, 63 patients were included in the modified ITT population, of whom four had baseline FC levels of 100–200 µg/g. There were no significant differences between both the population as randomized and the modified ITT population, with respect to sex, diagnosis or age at inclusion [data not shown]. Six patients in the modified ITT population discontinued the allocated treatment prematurely, of whom five were allocated to SMT. In total, 57 participants were included in the per-protocol population [hypnotherapy: 29].
Figure 1.
Study flow and disposition of patients.
3.2. Demographics and baseline characteristics
Baseline characteristics of the modified ITT population are provided in Table 1. The vast majority of patients [92%] were adults. Demographics and baseline characteristics were balanced between the two treatment arms. Three patients did not report any abdominal pain at baseline on the IBS-SSS questionnaire.
Table 1.
Baseline characteristics of patients in the modified ITT population
| SMT [n = 33] | HT [n = 30] | |
|---|---|---|
| Age group | ||
| • 12–18 years [n, %] | 2 [6%] | 3 [10%] |
| • 18–65 years [n, %] | 31 [94%] | 27 [90%] |
| Age, years [mean, SD] | 35.7 [11.9] | 32.8 [13.0] |
| Males [n,%] | 4 [12%] | 7 [23%] |
| Smoking [n, %] | 6 [18%] | 7 [23%] |
| Body mass index [kg/m2, mean, SD] | 23.5 [4.1] | 23.0 [4.7] |
| Duration of IBD [years, median, IQR] | 8 [2–12] | 5 [2–10] |
| Faecal calprotectin [µg/g, median, IQR] | 32[8–53] | 39 [20–61] |
| IBS-subtype [n, %] | ||
| • Diarrhoea-predominant IBS [IBS-D] | 15 [45%] | 15 [50%] |
| • Constipation-predominant IBS [IBS-C] | 6 [18%] | 3 [10%] |
| • Mixed-type IBS [IBS-M] | 2 [6%] | 2 [7%] |
| • Unsubtyped IBS [IBS-U] | 10 [30%] | 10 [33%] |
| Duration of IBS-type symptoms [years, median, IQR] | 9 [3–15] | 8 [3–16] |
| Crohn’s disease subgroup [n, %]: | 19 [58%] | 16 [53%] |
| Age at diagnosis [n] | ||
| • A1: 0 to <10 years | 3 | 4 |
| • A2: 17–40 years | 15 | 11 |
| • A3: >40 years | 1 | 1 |
| Location [n] | ||
| • L1: ileal disease | 6 | 7 |
| • L2: ileocolonic disease | 7 | 7 |
| • L3: colonic disease | 6 | 2 |
| • L4: upper gastrointestinal disease | 2 | 3 |
| Behaviour [n] | ||
| • B1: non-stricturing, non-penetrating | 16 | 14 |
| • B2: structuring | 1 | 2 |
| • B3: penetrating | 2 | 0 |
| • P: perianal disease | 3 | 1 |
| Ulcerative colitis subgroup [n, %]: | 14 [42%] | 14 [47%] |
| Extent [n] | ||
| • E1: proctitis | 5 | 1 |
| • E2: left-sided disease | 5 | 5 |
| • E3: extensive disease | 4 | 8 |
SMT, standard medical treatment; HT, hypnotherapy; SD, standard deviation; IBD, inflammatory bowel disease; IQR, interquartle range; IBS, irritable bowel syndrome.
3.3. Primary outcome/analysis
The numbers with a reduction of ≥50% on the IBS-SSS VAS for symptom [i.e. pain or distension/tightness] severity at week 40 compared to baseline values amounted to nine [27%] patients randomized to SMT and nine [30%] patients randomized to hypnotherapy [difference hypnotherapy vs SMT: 3%, 95% CI: −19 to 24%, p = 0.81]. In the sensitivity analyses, no differences were observed between groups. Also, in the per-protocol population, the proportions of participants meeting the primary endpoint did not differ significantly between the two study treatment groups [hypnotherapy 9/29, 31% vs SMT 9/28, 32%; difference: −1%, 95% CI: −24% to 22%, p = 0.93]. Exclusion of the three participants who did not report any abdominal pain at baseline on the IBS-SSS questionnaire did not affect the primary outcome.
3.4. Secondary outcomes
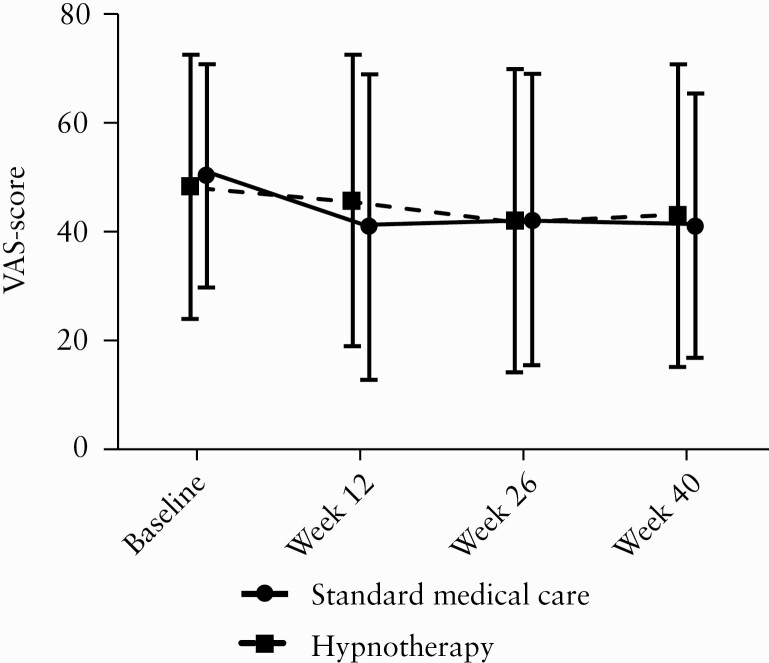
Exploratory analyses showed no significant differences between IBS-SSS VAS score for symptom severity or total IBS-SSS score at any time point [Figure 2, and Supplementary Figure 1]. Due to missing data, adequate relief rates 6 months after treatment were available for 60 subjects. Adequate relief rates were similar between groups, with 40% randomized to hypnotherapy and 60% randomized to SMT reporting adequate relief 6 months after treatment [Supplementary Figure 2]. In addition, no apparent differences with respect to generic or disease-specific health-related quality of life were observed between the two treatment groups [Supplementary Tables 1 and 2]. Individual Impact III scores over time of the five subjects <18 years are depicted in Supplementary Figure 3. Baseline depression and anxiety SCL-90-R scores were significantly higher compared to the general population, indicating higher levels of depression and anxiety. SCL-90-R depression and anxiety scores and dysfunctional abdominal pain-related cognition scores over time between groups appeared to be similar [Supplementary Tables 3 and 4].
Figure 2.
Mean and standard deviation of IBS-SSS VAS scores for symptom severity [pain, distension/tightness] at multiple time points in the modified ITT population. No difference was found between groups.
4. Discussion
In this study, we compared the effectiveness of hypnotherapy with SMT for IBD patients in remission but with persisting gastrointestinal symptoms consistent with IBS. Based on previous studies and our own experience with hypnotherapy as a treatment for functional gastrointestinal disorders,6,7,27,28 we hypothesized that hypnotherapy would be superior to SMT in the treatment of IBS-type symptoms in IBD patients. However, no difference was observed between the two treatments with respect to the primary outcome. Furthermore, most secondary endpoints, including generic and disease-specific quality of life, did not show a significant benefit of one intervention over the other.
We found that hypnotherapy was not superior to SMT in the management of IBD patients in remission with IBS-type symptoms, which is in contrast to data on the efficacy of hypnotherapy in IBS [in subjects without IBD].29 This could indicate that at least a significant proportion of IBS-type symptoms in IBD patients does not represent the same entity as ‘true’ IBS that occurs frequently in the general population.30 A potential alternative explanation for IBS-type symptoms in IBD is low-grade inflammation. In support of this, two studies have shown higher FC levels in patients with quiescent IBD with IBS compared to asymptomatic IBD patients.31,32 However, other studies found no such association.33–38 Furthermore, a high prevalence [29%] of IBS-type symptoms was found in ulcerative colitis patients with ‘deep remission’, defined as normal histology on biopsies from colon and rectum.38 Also, if IBS-type symptoms do result from inflammation, anti-inflammatory treatment should presumably be effective. However, subgroup analysis of the SONIC trial showed that potent anti-inflammatory therapy is probably ineffective in symptomatic IBD patients without inflammation.39 For the present study, patients were excluded if FC levels were >100 µg/g, or >200 µg/g in the absence of macroscopic signs of inflammation on endoscopy. This threshold for FC is much lower than the FC levels observed by Keohane et al. in Crohn’s disease patients (mean 415 µg/g, standard error [SE] 80) and ulcerative colitis patients [mean 591 µg/g, SE 173] with IBS-type symptoms considered in clinical remission, which led to the hypothesis of low-grade inflammation.31 Although the optimal threshold for FC to define IBD remission remains to be elucidated, we believe, based on our inclusion and exclusion criteria, that most if not all IBS-type symptoms in our study cannot be attributed to ongoing macroscopic inflammation. In support of this, the proportion of subjects meeting the primary endpoint and adequate relief rates from either hypnotherapy or SMT was not related to baseline FC levels.
Some of our findings suggest that IBS-type symptoms in IBD are similar to IBS that occurs in the general population. First, our study population consisted mainly of female patients, consistent with the female preponderance of ‘true’ IBS, and in line with previous studies showing that IBS-type symptoms are more common in female IBD patients.34,37,40 Furthermore, similar to IBS, high levels of anxiety and depression were observed, with SCL-90-R anxiety and depression subscale scores similar to those observed in IBS patients.41 Moreover, the level of dysfunctional abdominal pain-related cognitions, which have been thought to play an important role in ‘true IBS’,42 was higher than in the general Dutch population and similar to levels reported in Dutch subjects with ‘true IBS’.43
Another potential explanation for the low proportion of subjects randomized to hypnotherapy meeting the primary endpoint could be that the studied population is more somatically orientated than IBS patients without concomitant IBD. During hypnotherapy sessions, many patients still considered the possibility that their abdominal pain was caused by ongoing inflammation, which may have reduced their motivation to listen to the hypnosis exercises on a daily basis. We are not aware of any studies that have compared somatic vigilance between subjects with IBD and concomitant IBS-type symptoms and subjects with ‘true’ IBS. It would be of interest to study whether this could moderate the effectiveness of hypnotherapy and other interventions. Adaptation of the hypnosis protocol, including suggestions for influencing pain cognitions, may improve the effectiveness of hypnotherapy in IBD patients with IBS-type symptoms.
The proportion of subjects randomized to SMT meeting the primary endpoint was also lower than expected, with only 27% of the patients reaching the primary endpoint of a decrease of 50% in symptom severity score. Nevertheless, the majority of these patients reported adequate relief, suggesting that standard IBS management may be efficacious in reducing persisting abdominal symptoms in some IBD patients.
Our inclusion and exclusion criteria may potentially have led to the selection of a subgroup of patients with IBS-type symptoms. As mentioned above, FC levels in IBD patients with IBS-type symptoms have been reported to be much higher than our strict threshold for exclusion.31 Indeed, in our study, 47 potentially eligible IBD patients with suspected IBS-type symptoms were excluded based on increased FC levels. However, as there is no universally accepted threshold for where symptoms from active IBD end and IBS-type symptoms begin, it could also be argued that IBD patients with IBS-type symptoms and substantially elevated FC levels have been misclassified as being in remission.31
In the present study, we strived to maintain clinical equipoise with respect to the patient perspective, considering that patients’ expectations of the effectiveness of a specific treatment can affect the outcome of a study.44 For this purpose, the study was explained to potentially eligible subjects as a comparison of two treatment strategies, rather than a comparison of an intervention with a control group. At baseline, there was no difference in expected improvement between treatment arms, suggesting that clinical equipoise was maintained, although most patients had a baseline preference for hypnotherapy.
The strenghts of our study include the prospective, randomized approach, and use of a strict definition of IBS-type symptoms in accordance with prevailing diagnostic criteria. Our study has also some limitations. First, we experienced a high drop-out rate and analyses were hampered by missing data. Furthermore, remission of IBD was confirmed by endoscopy in only a small proportion of patients, as endoscopy was not necessary for inclusion if FC levels were ≤100 µg/g. Also, no imaging was routinely performed to exclude the presence of upper gastrointestinal Crohn’s disease or a stenosis as a cause of IBS-type symptoms. Moreover, our study could not be blinded due to the study design and hypnotherapy was not compared to a placebo, which is a common limitation in trials using psychological interventions. Another limitation is the relatively small sample size [based on a large expected difference in efficacy between groups]. However, no trend towards superiority of any of the treatments was observed, suggesting that an increased sample size would probably not have affected the outcome. Also, we did not use the current [Rome IV] diagnostic criteria for IBS, because they were not yet available at the time of study initiation, which led to the inclusion of three subjects who did not abdominal pain at baseline.
Our results suggest that the effectiveness of SMT and hypnotherapy for IBS-type symptoms in IBD patients is comparable. Indeed, both treatments provided adequate relief in a substantial proportion of patients. The effectiveness may depend on a patient’s preference. A discussion of both treatment options, and a shared decision based on treatment preferences may improve the outcome.
In conclusion, in this randomized controlled trial, hypnotherapy was not superior to SMT in the treatment of IBS-type symptoms in patients with IBD in remission. Based on adequate relief rates, both treatments appear to be reasonable therapeutic options that can be applied in clinical practice. Further studies are needed to assess the optimal therapeutic strategy in this specific population.
Funding
This study was supported by a grant from ZonMw [grant no. 171101009], The Netherlands Organization for Health Research and Development. The funding source was not involved in study design, data collection/analysis/interpretation, or writing of the manuscript.
Conflict of Interest
N.dB. has served as a speaker for AbbVie and MSD. He has served as consultant and/or principal investigator for TEVA Pharma BV and Takeda. He has received a [unrestricted] research grant from Dr. Falk, TEVA Pharma BV, Takeda and MLDS. All outside the submitted work. G.D. has served as advisor for Abbvie, Ablynx, Active Biotech AB, Agomab Therapeutics, Allergan, Alphabiomics, Amakem, Amgen, AM Pharma, Applied Molecular Therapeutics, Arena Pharmaceuticals, AstraZeneca, Avaxia, Biogen, Bristol Meiers Squibb/Celgene, Boehringer Ingelheim, Celltrion, Cosmo, DSM Pharma, Echo Pharmaceuticals, Eli Lilly, Engene, Exeliom Biosciences, Ferring, Dr. Falk Pharma, Galapagos, Genentech/Roche, Gilead, Glaxo Smith Kline, Gossamerbio, Pfizer, Immunic, Johnson and Johnson, Kintai Therapeutics, Lycera, Medimetrics, Takeda, Medtronic, Mitsubishi Pharma, Merck Sharp Dome, Mundipharma, Nextbiotics, Novonordisk, Otsuka, Photopill, ProciseDx, Prodigest, Prometheus laboratories/Nestle, Progenity, Protagonist, RedHill, Robarts Clinical Trials, Salix, Samsung Bioepis, Sandoz, Seres/Nestec/Nestle, Setpoint, Shire, Teva, Tigenix, Tillotts, Topivert, Versant and Vifor. G.D. has received speaker fees from Abbvie, Biogen, Ferring, Galapagos/Gilead, Johnson and Johnson, Merck Sharp Dome, Mundipharma, Norgine, Pfizer, Samsung Bioepis, Shire, Millenium/Takeda, Tillotts and Vifor. All outside the submitted work. The other authors declare that they have no conflicts of interest.
Author Contributions
D.R.H. contributed to the conception and design of the study, to the acquisition, analysis and interpretation of data, and to the drafting of the article. A.M.V. and M.A.B. contributed to the conception and design of the study, to the acquisition, analysis and interpretation of data. and to revising the article critically for important intellectual content. P.C.S., N.M., S.R., N.K.B., T.G.M., C.F. and G.R.D. contributed to the acquisition and interpretation of data and to revising the article critically for important intellectual content. All authors approved the final version of the manuscript.
Data Availability
The data underlying this article cannot be shared publicly given the privacy of the individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1. Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2012;107:1474–82. [DOI] [PubMed] [Google Scholar]
- 2. Iskandar HN, Cassell B, Kanuri N, et al. Tricyclic antidepressants for management of residual symptoms in inflammatory bowel disease. J Clin Gastroenterol 2014;48:423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berrill JW, Sadlier M, Hood K, Green JT. Mindfulness-based therapy for inflammatory bowel disease patients with functional abdominal symptoms or high perceived stress levels. J Crohns Colitis 2014;8:945–55. [DOI] [PubMed] [Google Scholar]
- 4. Piche T, Pishvaie D, Tirouvaziam D, et al. Osteopathy decreases the severity of IBS-like symptoms associated with Crohn’s disease in patients in remission. Eur J Gastroenterol Hepatol 2014;26:1392–8. [DOI] [PubMed] [Google Scholar]
- 5. Hallert C, Kaldma M, Petersson BG. Ispaghula husk may relieve gastrointestinal symptoms in ulcerative colitis in remission. Scand J Gastroenterol 1991;26:747–50. [DOI] [PubMed] [Google Scholar]
- 6. Vlieger AM, Menko-Frankenhuis C, Wolfkamp SC, Tromp E, Benninga MA. Hypnotherapy for children with functional abdominal pain or irritable bowel syndrome: a randomized controlled trial. Gastroenterology 2007;133:1430–6. [DOI] [PubMed] [Google Scholar]
- 7. Miller V, Carruthers HR, Morris J, Hasan SS, Archbold S, Whorwell PJ. Hypnotherapy for irritable bowel syndrome: an audit of one thousand adult patients. Aliment Pharmacol Ther 2015;41:844–55. [DOI] [PubMed] [Google Scholar]
- 8. Peters SL, Muir JG, Gibson PR. Review article: Gut-directed hypnotherapy in the management of irritable bowel syndrome and inflammatory bowel disease. Aliment Pharmacol Ther 2015;41:1104–15. [DOI] [PubMed] [Google Scholar]
- 9. Moser G. The role of hypnotherapy for the treatment of inflammatory bowel diseases. Expert Rev Gastroenterol Hepatol 2014;8:601–6. [DOI] [PubMed] [Google Scholar]
- 10. Keefer L, Taft TH, Kiebles JL, Martinovich Z, Barrett TA, Palsson OS. Gut-directed hypnotherapy significantly augments clinical remission in quiescent ulcerative colitis. Aliment Pharmacol Ther 2013;38:761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mawdsley JE, Jenkins DG, Macey MG, Langmead L, Rampton DS. The effect of hypnosis on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Am J Gastroenterol 2008;103:1460–9. [DOI] [PubMed] [Google Scholar]
- 12. Irvine EJ, Whitehead WE, Chey WD, et al. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology 2006;130:1538–51. [DOI] [PubMed] [Google Scholar]
- 13. Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006;130:1377–90. [DOI] [PubMed] [Google Scholar]
- 14. Rutten JM, Vlieger AM, Frankenhuis C, et al. Gut-directed hypnotherapy in children with irritable bowel syndrome or functional abdominal pain (syndrome): a randomized controlled trial on self exercises at home using CD versus individual therapy by qualified therapists. BMC Pediatr 2014;14:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gonsalkorale WM. Gut-directed hypnotherapy: the Manchester approach for treatment of irritable bowel syndrome. Int J Clin Exp Hypn 2006;54:27–50. [DOI] [PubMed] [Google Scholar]
- 16. Woutersen-Koch H, Smout AJ, Flik CE, et al. Multidisciplinaire richtlijn prikkelbaredarmsyndroom. Ned Tijdschr Geneeskd 2013;156:A4584/1–4. [PubMed] [Google Scholar]
- 17. MacDermott RP. Treatment of irritable bowel syndrome in outpatients with inflammatory bowel disease using a food and beverage intolerance, food and beverage avoidance diet. Inflamm Bowel Dis 2007;13:91–6. [DOI] [PubMed] [Google Scholar]
- 18. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11:395–402. [DOI] [PubMed] [Google Scholar]
- 19. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 20. Otley A, Smith C, Nicholas D, et al. The IMPACT questionnaire: a valid measure of health-related quality of life in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2002;35:557–63. [DOI] [PubMed] [Google Scholar]
- 21. Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989;96:804–10. [PubMed] [Google Scholar]
- 22. Toner BB, Stuckless N, Ali A, Downie F, Emmott S, Akman D. The development of a cognitive scale for functional bowel disorders. Psychosom Med 1998;60:492–7. [DOI] [PubMed] [Google Scholar]
- 23. Derogatis L. SCL-90-R: Administration, Scoring of Procedures Manual-II for the R (evised) Version and Other Instruments of the Psychopathology Rating Scale Series. Clinical Psychometric Research Incorporated; 1992. [Google Scholar]
- 24. Arrindell WA, Ettema JHM.. SCL-90. Handleiding bij een multidimensionele psychopathologie-indicator. Swets & Zeitlinger; 2003. [Google Scholar]
- 25. Gonsalkorale WM, Miller V, Afzal A, et al. Long term benefits of hypnotherapy for irritable bowel syndrome. Gut 2003;52:1623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Webb AN, Kukuruzovic R, Catto-Smith AG, et al. Hypnotherapy for treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2007:CD005110. [DOI] [PubMed] [Google Scholar]
- 27. Schaefert R, Klose P, Moser G, Häuser W. Efficacy, tolerability, and safety of hypnosis in adult irritable bowel syndrome: systematic review and meta-analysis. Psychosom Med 2014;76:389–98. [DOI] [PubMed] [Google Scholar]
- 28. Vlieger AM, Rutten JM, Govers AM, Frankenhuis C, Benninga MA. Long-term follow-up of gut-directed hypnotherapy vs. standard care in children with functional abdominal pain or irritable bowel syndrome. Am J Gastroenterol 2012;107:627–31. [DOI] [PubMed] [Google Scholar]
- 29. Palsson OS. Hypnosis treatment of gastrointestinal disorders: a comprehensive review of the empirical evidence. Am J Clin Hypn 2015;58:134–58. [DOI] [PubMed] [Google Scholar]
- 30. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012;10:712–721.e4. [DOI] [PubMed] [Google Scholar]
- 31. Keohane J, O’Mahony C, O’Mahony L, O’Mahony S, Quigley EM, Shanahan F. Irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease: a real association or reflection of occult inflammation? Am J Gastroenterol 2010;105:1788, 1789–94; quiz 1795. [DOI] [PubMed] [Google Scholar]
- 32. Jelsness-Jørgensen LP, Bernklev T, Moum B. Calprotectin is a useful tool in distinguishing coexisting irritable bowel-like symptoms from that of occult inflammation among inflammatory bowel disease patients in remission. Gastroenterol Res Pract 2013;2013:620707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diederen K, Hoekman DR, Hummel TZ, et al. The prevalence of irritable bowel syndrome-type symptoms in paediatric inflammatory bowel disease, and the relationship with biochemical markers of disease activity. Aliment Pharmacol Ther 2016;44:181–8. [DOI] [PubMed] [Google Scholar]
- 34. Jonefjäll B, Strid H, Ohman L, Svedlund J, Bergstedt A, Simren M. Characterization of IBS-like symptoms in patients with ulcerative colitis in clinical remission. Neurogastroenterol Motil 2013;25:756–e578. [DOI] [PubMed] [Google Scholar]
- 35. Keszthelyi D, Jonkers DM, Hamer HM, Masclee AA. Letter: the role of sub-clinical inflammation and TRPV1 in the development of IBS-like symptoms in ulcerative colitis in remission. Aliment Pharmacol Ther 2013;38:560–1. [DOI] [PubMed] [Google Scholar]
- 36. Boztepe B, Sezgin O, Altintas E, et al. P176. Irritable bowel syndrome frequency in Inflammatory Bowel Disease during both clinical and deep remission and its association with fecal calprotectin. J Crohns Colitis 2015;: S164–5. [Google Scholar]
- 37. Berrill JW, Green JT, Hood K, Campbell AK. Symptoms of irritable bowel syndrome in patients with inflammatory bowel disease: examining the role of sub-clinical inflammation and the impact on clinical assessment of disease activity. Aliment Pharmacol Ther 2013;38:44–51. [DOI] [PubMed] [Google Scholar]
- 38. Henriksen M, Høivik ML, Jelsness-Jørgensen LP, Moum B; IBSEN Study Group . Irritable bowel-like symptoms in ulcerative colitis are as common in patients in deep remission as in inflammation: results from a population-based study [the IBSEN Study]. J Crohns Colitis 2018;12:389–93. [DOI] [PubMed] [Google Scholar]
- 39. Colombel JF, Sandborn WJ, Reinisch W, et al. ; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- 40. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol 2014;6:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tack J, Broekaert D, Fischler B, et al. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut 2005;55:1095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thijssen AY, Jonkers DM, Leue C, et al. Dysfunctional cognitions, anxiety and depression in irritable bowel syndrome. J Clin Gastroenterol 2010;44:e236–41. [DOI] [PubMed] [Google Scholar]
- 43. van der Veek PPJ, van Rood YR, Masclee AAM. Symptom severity but not psychopathology predicts visceral hypersensitivity in irritable bowel syndrome. Clin Gastroenterol Hepatol 2008;6:321–8. [DOI] [PubMed] [Google Scholar]
- 44. Krell HV, Leuchter AF, Morgan M, Cook IA, Abrams M. Subject expectations of treatment effectiveness and outcome of treatment with an experimental antidepressant. J Clin Psychiatry 2004;65:1174–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly given the privacy of the individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.