Abstract
Background
The current COVID-19 pandemic is causing significant morbidity and death worldwide and produces significant socio-economic losses.
Objective
To assess the cost–benefit relation of implementing point-of-care COVID-19 antigen testing (POCT) in emergency rooms (ER) of German hospitals.
Methods
A deterministic decision-analytic model simulated the incremental costs of using the Sofia® SARS Antigen FIA test compared to those of using clinical judgement alone to confirm or exclude COVID-19 in adult patients in German ER, prior to hospitalization. Direct and indirect costs, with and without subsequent RT-PCR confirmation, were evaluated from the hospital perspective.
Results
With respect to ER patients, in base-case analysis, considering a COVID-19 prevalence of 15.6% and a hospitalization rate among COVID-19 suspects of 10.1%, POCT testing reduces average costs of hospitalized patients by €213 per tested patient if nasopharyngeal swabs of patients suspected to have COVID-19 are also sent to external labs for RT-PCR testing.
In probabilistic sensitivity analysis, under all reasonable assumptions, implementing the Sofia® SARS Antigen FIA saves on average about €210 as compared to applying the clinical-judgement-only strategy. The major part of cost savings, €159 or 75.9%, is due to the POC test´s high specificity resulting in a 21-fold lower proportion of unnecessary bed blocking at the first day of hospitalization.
Conclusions
Using highly specific rapid COVID-19 tests in COVID-19 suspects at German ER, despite of their sub-optimal sensitivity, may significantly reduce hospital expenditure.
Keywords: Cost-benefit analysis, Point-of-care, Antigen testing, Real-time reverse transcriptase polymerase chain reaction (RT-PCR), SARS-CoV-2, COVID-19
Introduction
The severe acute respiratory syndrome COVID-19, caused by coronavirus 2 (SARS-CoV-2), first appeared in December 2019 in Wuhan, China, with an accumulation of pneumonia and has since spread across the globe.1 Clinical features of the disease, known as COVID-19, include fever, headache, and cough, but more severe symptoms such as shortness of breath and respiratory failure have also been reported.2 As of April 30, 2021, around 151 million cases and more than 3.2 million deaths have been registered in 210 countries and territories worldwide.3
The rapid escalation of the situation caused the World Health Organization to declare a pandemic on March 11, 2020.4 Since then, the continued human-to-human transmission of SARS-CoV-2 has created tremendous challenges for healthcare systems and public health laboratories. Accurate and rapid identification of those infected with SARS-CoV-2 is therefore key to immediate clinical care and to containing the spread of the virus. The current reference test used to establish SARS-CoV infection worldwide is the real-time reverse transcriptase polymerase chain reaction (RT-PCR). These assays have nearly perfect sensitivity and specificity and are therefore well suited as “gold standard” for the diagnosis of clinically ill patients. However, utilization of RT-PCT tests for immediate COVID-19 in hospitals raises substantial challenges: As they require RNA extraction, are dependent on availability of PCR reagents and have a relatively long turnaround time, RT-PCR tests are often performed in batches in clinical laboratories outside the hospital, necessitating specimen transport. Therefore, they usually require a time-lag of one day before the report of the test result becomes available. In Germany currently 71.5% of all hospitals have eliminated their in-house laboratories.5 Thus, to ensure the correct diagnosis, nasopharyngeal swabs or other respiratory specimen of patients suspected of having COVID-19 must usually be sent to external labs for centralized RT-PCR testing.
In contrast, lateral flow assay (LFA) SARS-CoV-2 antigen tests can be performed at point of care, provide results within 15–30 min and are inexpensive. Numerous SARS-CoV-2 POC antigen tests are currently available, offering the potential for rapid identification of those individuals in the emergency setting who are not only infected, but infectious and are therefore at greatest risk of spreading the infection. For methodological reasons, the detection limit for SARS‐CoV‐2 RNA material out of clinical samples tested by RT‐PCR is always lower than the detection limit for SARS‐Cov‐2 antigen. Whilst RT-PCR results may still show positive signals for up to several weeks after reaching peak cycle threshold (Ct) values, the detectability of even the best performing antigen test deteriorates with decreasing viral load.6 However, if patients visit ER before the end of the first weeks of symptoms when pharyngeal virus shedding is very high and infected individuals are likely to be most infectious, sensitivity of high-quality antigen tests is only slightly reduced and can help to filter out the infectious persons.7 Consequently, POCT may help to prevent the hospital – if COVID-19 suspects have to be hospitalized due to the severity of symptoms - from isolating such patients and blocking a second bed in the patients´ rooms at the hospital ward unnecessarily.
Furthermore, rapid assessment of infectious COVID-19 is highly relevant to the management of scarce economic resources also for another reason. Since 1 January 2004, hospital costs in Germany are based on the German diagnosis-related groups (G-DRG) system, which assigns each COVID-19 case to the category E79C). This imposes a fixed “base rate” of payment for 13 days of treatment. If the hospital treatment exceeds the so-called “mean length of stay”, i.e., 6.9 days (as calculated mathematically by the DRG Institute for Hospital Reimbursement (InEK) using case-related data from its contracted hospitals8), then the G-DRG rate paid as reimbursement by the statutory health insurance (SHI) usually does not cover the costs incurred by the hospital. Accordingly, when treating COVID-19 patients covered by the SHI, hospitals should try to keep the duration of hospital stays as short as possible.9
According to the most recent guidelines of the German Robert Koch Institute (RKI) isolation of an immunocompetent patient can be stopped and discharge be started only if - although viral load on swabs decreases as symptoms resolve10 - at least 14 days have passed since the onset of the first symptoms, a lasting improvement in the acute COVID-19 symptoms has been present for > 48 h and a RT-PCR (preferable recommended) or an antigen test is negative.11 Again, as the negative result of a POCT test is usually available one day earlier than that of the RT-PCR costs may be saved from the hospital´s perspective by a respectively earlier discharge. The aim of our calculations was to examine whether routine implementation of POCT in COVID-19 suspects visiting an ER leads to directly measurable economic advantages from the hospital perspective, taking as an example the Sofia® SARS Antigen FIA test under the assumption that all nasopharyngeal swabs of COVID-19 suspects are sent to external labs for RT-PCR testing. Using its performance characteristics, we compared the economic outcomes to those that occurred when conventional clinical judgement alone was used to confirm or exclude SARS-CoV-2 in patients deemed to have a combination of symptoms so serious as to warrant hospitalization. The hypothetical savings would come about thanks to earlier patient classification, in anticipation of a RT-PCR result, available only one day later.
Materials and methods
Test system
The Sofia® SARS Antigen Fluorescent Immunoassay (FIA) is a point-of-care system based on lateral flow technology that uses monoclonal antibodies labelled with Europium as a fluorescent tag. The assay uses SARS CoV-2 specific epitopes of the nucleocapsid protein as target. The tip of a nasal or nasopharyngeal swab is dispensed in a solution that disrupt the viral membrane in order to inactivate the virus and to release the nucleocapsid protein into the solution for subsequent detection with the assay. After pipetting of 120 µl of the solution by a fixed-volume pipette, its contents will be dispensed into the sample well of a cassette and inserted into the Sofia® analyzer. The analyzer performs incubation, then measurement of the fluorescent signal, and calculates the qualitative result using assay specific algorithms. The final result is available in 15 min.
Model approach
Our model is parametrized by data on sensitivity and specificity of the Sofia® SARS Antigen FIA compared to the conventional clinical approach. With respect to POCT, two scenarios are considered: In the first, all COVID-19 patients coming to the ER of a hospital during the current COVID-19 pandemic are tested with the Sofia, after using a nasopharyngeal swab. Depending on the severity of symptoms, a patient is hospitalized or discharged from the ER. In case of hospitalization, the patient is isolated from the moment of presumptive diagnosis, given a positive Sofia test result, upon resolution of fever and respiratory symptoms, but in any case at least for 14 days after first onset of symptoms. Given the high specificity, but only moderate sensitivity of the Sofia (98.9% and 80.0%,12 see in Online Supplement for details), additional RT-PCR testing of the patient´s samples is always required in patients whose test is scored negative. As RT-PCR testing in an external laboratory, where the patients´ samples have to be sent in addition, ideally has both a sensitivity and a specificity of up to 100%, this would clarify whether or not the disease is due to SARS-CoV-2 and also false negative Sofia results could be corrected. According to the current German guidelines, however, antigen test results in COVID-19 suspects must always be confirmed by RT-PCR, even positive antigen test results.13
Due to the increased risk of thromboembolism associated with COVID-19 disease, a course of antithrombotic prevention, using low molecular weight heparin at half the therapeutic dose, is immediately started in all COVID-19 suspects admitted to the hospital.14
In the alternative scenario (versus Sofia® SARS Antigen FIA), i.e.in the conventional clinical approach, the decision as to whether the present respiratory symptoms are caused by COVID-19 is made using symptom-based judgement, without rapid pre-testing. Thus, if hospitalization were required, the decision to isolate a COVID-19 suspect is only based on that clinical decision. In any case, a clinical sample in the form of a nasopharyngeal swab is taken from all COVID-19 suspects deemed to require hospitalization, to be sent out for RT-PCR testing.
If the patient is not to be hospitalized but discharged and sent home directly from the ER, SHI is charged for the costs of routine diagnostics (chest X-ray, routine laboratory values, physical examination, etc.) as well as the costs of POCT, the latter following the corresponding ambulatory doctors fee schedule, position number 32791.15
Thus, these patients are not considered in our analysis. If a COVID-19 suspect is ultimately hospitalized the costs of the Sofia testing have to paid by the hospital itself. In contrast, the costs of the externally performed RT-PCR that are directly billed to the hospital by the external laboratory are usually balanced by the reimbursement the hospital receives for performing a RT-PCR according to the German Hospital Finance Act (Krankenhausfinanzierungsgesetz, KHG).16 Accordingly, initial RT-PCR testing, the swabs of which are taken in the ER, does not appear as a cost factor in our model.
Additional costs from the hospital perspective are the so called “opportunity costs” that might occur as long as a COVID-19 suspect is uneccesarily kept in isolation (see details below). This occurs in the cases of false-positive clinical judgement or a false-positive POCT. Under the premise that most COVID-19 patients are accommodated in a twin-bedded room and that hospital wards in Germany during COVID-19 pandemic are working at nearly full capacity, the economic losses caused by blocking the second bed are incurred by the hospital itself.
If a patient is isolated due to erroneous clinical judgement (no SARS-CoV-2 infection present) or false positive POCT, the isolation can be ended as soon as the report of the negative laboratory RT-PCR result is available the next day. It is assumed that the administration of low-molecular-weight heparin is continued until discharge if SARS-CoV-2 infection is confirmed by external PCR. In the case of a negative PCR result, that medication is dropped immediately. Thus, patients falsely suspected of having COVID-19, by whatever means, end up being isolated and receiving antithrombotic prevention for one day.
According to the current CDC guidelines,17 no studies have yet found evidence that clinically recovered adults with persisting viral RNA have transmitted SARS-CoV-2 to others. This has led to the recommendation that discontinuing isolation prior to discharge should rely on a symptom-based rather than test-based strategy. The German RKI, however, requests not only that isolation in hospital should end no earlier than 14 days after onset of symptoms, it also requests a negative test result, preferably RT-PCR.11 As the median duration of hospital stay in Germany is currently 10 days18 it can be expected that, by performing a POCT, patients can be discharged one day earlier than forseen by the DRG, saving the assumed delay that external RT-PCR testing imposes. As the hospital receives a fixed DRG flat rate in any case, this would result in an economic benefit to the hospital.
Our model also takes into account the effects of COVID-19 transmission to unvaccinated health care workers by COVID-19 sufferers who have gone undetected and not been isolated, due to false clinical judgement or a false-negative POCT result. For this we have incorporated a secondary attack rate. Although data are insufficient to precisely define the duration of exposure time that constitutes a significant transmission risk, even exposure to an infected individual for less than 15 min over a 24-h period, especially during performance of an aerosol generating procedure, may be sufficient19 for transmission to occur. The measured effect is sick days for hospital workers, the costs of which, under the German system, is borne by the hospital. For purposes of simplification, in our model only one health care worker is assigned to an unisolated patient, and the infection risk weighted by the probability of being effectively vaccinated.
In a modified approach we assume a positive POCT does not need confirmation by a RT-PCR. In this case those who were tested false positive are isolated for the whole duration of hospitalization and intensified antithrombotic preventive therapy is offered unnecessarily.
Model structure
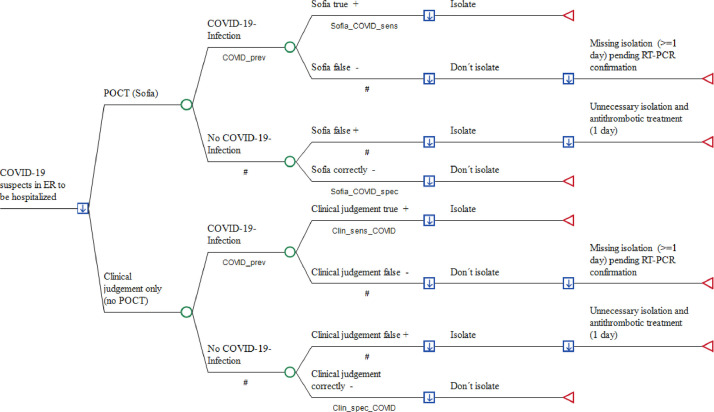
The decision tree simulates the outcomes of three management strategies in the ER of a German hospital in a hypothetical cohort of 1000 adult patients attending the ER with acute moderate-to-severe respiratory infection and suspicion of COVID-19. Costs from the hospital perspective were compared, as described above: (1) empiric clinical investigation with RT-PCR, but without POC antigen COVID-19 testing (POCT) and (2) POCT and mandatory RT-PCR testing, or (3) RT-PCR testing only when the POCT was negative, used to guide the decision as to whether a patient - if hospitalization is required due to signs of severe lower respiratory infection - requires strict isolation. As POCT for those patients who are sent home from ER is paid by the local KV and external RT-PCR is not required in such mild cases, the decision tree is restricted to patients due for hospitalization.
Total costs of outcomes were simulated for each study arm including (1) medical cost of POCT with the Sofia® SARS Antigen FIA which has been authorized for use by the German Paul-Ehrlich-Institut (PEI), the German Federal Institute for Vaccines and Biomedicines, (2) medical costs of external RT-PCR testing if performed prior to hospitalization, (3) opportunity costs due to blocking a twin-bed reimbursement for one day of hospital stay, (4) reimbursement per day of hospital stay within the fixed payment DRG period and (5) sick pay costs at the expense of the hospital if staff members are secondarily infected by hospitalized but unrecognized COVID-19 patients (Fig. 1 ).
Fig. 1.
Point-of-Care antigen testing (POCT) versus the conventional approach in COVID-19 suspects prior to hospitalization
Legend to Fig. 1:A decision node (square) indicates a choice facing the decision maker or the consequences of a decision. Branches from a chance node (circles) represent the possible outcomes of an event; terminal nodes (triangles) denote the endpoints of a scenario and are assigned the costs of a prior series of actions and events. The arrows in the decision notes pointing downwards demonstrate that the optimal path of the model is that with the lowest total cost. ER: Emergency room; POCT: Point-of-Care antigen testing; RT-PCR: Reverse Transcriptase-PCR; COVID_prev: Prevalence of COVID-19 [reference 3 (Supplement)], Sofia_COVID_sens: Real life sensitivity of Sofia test [reference 12 (Supplement)]: Sofia_Covid_spec: Real life specificity of Sofia testing [reference 12 (Supplement)]; Clin_sens_COVID: Sensitivity of diagnosing SARS-CoV-2 infection [reference 8 (Supplement)]; Clin_spec_COVID: Probability of correctly excluding SARS-CoV-2 [reference 8 (Supplement)].
#: Complementary probability (all probabilities of chance node's branches to sum to 1.0); +: positive; -: negative.
We used TreeAge Software (TreeAge Inc. Williamstown MA, USA) for model building and analysis and examined our inputs over a wide range in sensitivity analyses to identify influential factors that would alter the base-case findings. Firstly, univariate sensitivity analysis was performed using all variables to examine the extent to which our calculations are affected by varying selected assumptions. Variation was done using either a) the lower and upper bounds of a parameter´s standard deviation or b) those of its 95% confidence interval. Where these are not applicable, our model simply causes parameter values to vary by ± 20% of the base-case value according to international practice, unless stated otherwise.
Furthermore, and in order to capture the interactions between multiple inputs, we provide a probabilistic sensitivity analyses (PSA) by assigning an appropriate statistical (probability) distribution for all parameters, randomly drawn in a 2nd order Monte-Carlo simulation (n = 1000). All costs are reported in 2021 Euros (€).
Model input
The figures for the other epidemiological, labaraotoy and economic parameters are listed in Table 1 ; their origins are described in detail in the Online Supplement.
Table 1.
Input for cost–benefit analysis.
| Variables Category | Variable Name | Distribution * | Value (Base Case) | Relative Change (Range) | Reference |
|---|---|---|---|---|---|
| Prevalence of COVID-19 | COVID_prev | PERT | 0.156 | 0.079–0.412 | [3 (Supplement)] |
| Additional revenue per day due earlier discharge | cRev_day_POCT | uniform | €323.91 | ±20% (€259.13–€388.69) | Calculated using data from the Institut für das Entgeltsystem im Krankenhaus (InEK) [20 (Supplement)] |
| Real life specificity of Sofia testing | Sofia_COVID_spec | uniform | 0.989 | 95% CI (0.958–0.998) | [12 (Supplement)] |
| Opportunity costs due to blocking twin bed | cOpp_POCT | uniform | €690.92 | ±20% (€522.74–€829.1) | Calculated from InEK data [20 (Supplement)] |
| Probability of correctly excluding SARS-CoV-2 | Clin_spec_COVID | PERT | 0.683 | 95% CI (0.60–0.758) | [8 (Supplement)] |
| Sensitivity of diagnosing SARS-CoV-2 infection | Clin_sens_COVID | PERT | 0.806 | 95% CI (0.729–869) | [8 (Supplement)] |
| Costs of enoxaparin per day | cAntithromb_day | uniform | €7.09 | ±20% (€5.67–€8.51) | Rote Liste [Red List] 2021 |
| Costs of Sofia SARS-CoV FIA® | cSofia_COVID | uniform | €12 | ±20% (€9.6–€14.40) | As declared by manufacturer |
| Real life sensitivity of Sofia test | Sofia_COVID_sens | PERT | 0.80 | 95% CI (0.644–0.909) | [12 (Supplement)] |
| Secondary cases due to one unknown COVI-19 case | sec_COVID | PERT | 0.025 | 95% CI (0.013–0.05) | [17 (Supplement)] |
| Costs of productivity loss per day | cPL_day | uniform | €167.58 | ±20% (€134.06–€201.1) | Calculated from [27 (Supplement)] |
| Number of days of health care workers out of work due to COVID-19 | sick_days | uniform | 15 | +12 (27) | [25 (Supplement)] |
| Probability that hospitalization is required | pHosp | PERT | 0.1010 | 95% CI (0.097–0.1050) | [14 (Supplement)] |
| Costs of RT-PCR performed in external laboratory | cRT-PCR_ext | uniform | €42.74 | +20% (€51.29) | Nationwide laboratory inquiry |
in probabilistic sensitivity analysis.
Results
In the base-case analysis, utilizing the Sofia® SARS Antigen FIA test in COVID-19 patients is on average €212.57 less costly per eventually hospitalized patient, compared to the conventional clinical approach (see Table 2 a), although all POCT results, negatives as well as positives in the ER, will be re-checked by external PCR. Included in this amount is a cost saving of €20.36 in absolute terms per tested patient in favor of the hospital. The costs for the initial RT-PCR ordered by the ER are not considered here, since the incurred laboratory costs – in contrast to the POCT – are de facto reimbursed to the hospital at the expense of the SHI.
Table 2.
Results of the base-case analysis (with and without confirmation by external RT-PCR).
| Base-Case Analysis | Comparators | Mean Cost Per Patient (€) | Incremental Cost (€) * | Absolute Cost Savings (€) |
|---|---|---|---|---|
| a) with confirmation by external RT-PCR | ||||
| COVID-19 patients prior to hospitalisation | Sofia SARS Antigen FIA® | −20.36 | 0 | −20.36 |
| Conventional approach | 192.21 | 212.57 | ||
| b) without confirmation by external RT-PCR | ||||
| Base-Case Analysis | Comparators | Mean Cost Per Patient (€) | Incremental Cost (€)* | Absolute Cost Savings (€) |
|---|---|---|---|---|
| COVID-19 patients prior to hospitalisation | Sofia SARS Antigen FIA® | 37.96 | 0 | – |
| Conventional approach | 192.21 | 154.25 |
Incremental cost denotes the increase in total costs resulting from using the conventional approach alone versus POCT.
The amount of cost saving is, above all, dependent on the specificity of clinical judgement. Reducing the base case value of 68.3 to60.0% (worst case) results in a further cost savings of €48.90 on top of the €212.57, whilst an increase to 75.8% diminishes the saving by to €169.38. This is revealed by our univariate sensitivity analysis, in which all variables included in the decision analysis are changed between plausible extremes ranges (Table 3 ). Decreasing by 20% the opportunity costs of blocking a twin bed reduces the amount of cost saving by €43.19. The principal advantage of the Sofia® SARS Antigen FIA — namely of excluding a COVID-19 infection, with high specificity — is the third important component. However, even when assuming a decrease in specificity of the Sofia to the lower bound estimate of the 95% confidence interval, i.e. by 3.19% from the base case value of 98.9%, no reversion of the relative cost savings occurring by utilizing the Sofia takes place, the cost savings decrease only to €194.19.
Table 3.
Tornado diagram* (Point-of-Care COIVD-19 antigen testing versus the conventional clinical approach).
| Variable Name | Variable Description | Lowest value | Basecase value | Highest value | Saving (€) at lowest value | Saving (€) at highest value | Spread Ƭ | Risk%¥ | Cum Risk% |
|---|---|---|---|---|---|---|---|---|---|
| Clin_spec_COVID | Probability of correctly excluding SARS-CoV-2 | 0.60 | 0.683 | 0.758 | −261.47 | −168.38 | 93.08 | 0.53 | 0.53 |
| cOpp_POCT | Opportunity costs due to blocking twin bed | 522.74 | 690.92 | 829.10 | −169.13 | −248.26 | 79.12 | 0.38 | 0.90 |
| Sofia_COVID_spec | Real life specificity of Sofia testing | 0.958 | 0.989 | 0.998 | −194.19 | −217.93 | 23.74 | 0.03 | 0.94 |
| COVID_prev | Prevalence of SARS-CoV-2 | 0.079 | 0.156 | 0.412 | −230.58 | −207.15 | 23.43 | 0.03 | 0.97 |
| cRev_day_POCT | Additional revenue per day due to POCT | 259.13 | 323.91 | 388.69 | −204.48 | −220.65 | 16.17 | 0.02 | 0.99 |
| Sofia_COVID_sens | Real life sensitivity of Sofia test | 0.644 | 0.80 | 0.909 | −204.91 | −217.92 | 13.01 | 0.01 | 1.00 |
| cSofia_COVID | Costs of Sofia test | 9.60 | 12.00 | 14.40 | −215.27 | −209.87 | 5.40 | 0.00 | 1.00 |
| cRT_PCR_ext | Costs of RT-PCR in external laboratory | 42.74 | 42.74 | 51.29 | −213.64 | −212.57 | 1.07 | 0.00 | 1.00 |
| Clin_sens_COVID | Sensitivity of diagnosing SARS-CoV-2 infection | 0.729 | 0.806 | 0.869 | −212.96 | −212.09 | 0.87 | 0.00 | 1.00 |
| cAntithromb_day | Costs of enaxaparin per day | 5.67 | 7.09 | 8.51 | −212.20 | −212.94 | 0.73 | 0.00 | 1.00 |
| sec_COVID | Secondary cases due to one unknown COVID-19 case | 0.013 | 0.025 | 0.050 | −212.61 | −212.61 | 0.06 | 0.00 | 1.00 |
| sick_days | Number of days of HCW out of work due to COVID-19 | 15.00 | 15.00 | 27.00 | −212.57 | −212.60 | 0.03 | 0.00 | 1.00 |
| cPL_day | Costs of productivity loss per day | 134.06 | 167.58 | 201.10 | −212.56 | −212.58 | 0.01 | 0.00 | 1.00 |
| pVacc_eff_COVID_HCW | Probability of effectively vaccinated health care workers | 0.6355 | 0.6360 | 0.6363 | −212.57 | −212.57 | 0.00 | 0.00 | 1.00 |
| pHosp | Probability that hospitalization is required | 0.097 | 0.1010 | 0.1050 | −212.57 | −212.57 | 0.00 | 0.00 | 1.00 |
One-way sensitivity analyses of all model variables arranged in order, with the variable with the biggest impact at the top and the variable with the smallest impact at the bottom.
Risk%: This is a measure of how much of the total uncertainty is represented by the respective variable. The Risk% values sum to 1.0 across all the variables.
Highest cost saving minus lowest cost saving in €.
An increasing number of COVID-19 cases in the ER, i.e. a higher level of prevalence, hardly influences the economic outcome. Even under worst-case assumptions, where 41.2% of all patients with respiratory symptoms reporting to an ER turn out to be COVID-19 cases, a cost saving of €207.15 in favor of the hospital remains. Also, when the revenue costs for one hospital day gained by early release of a COVID-19 patient thanks to a negative POCT are lowered by 20%, the cost savings are only reduced by €8.09. If the sensitivity of the Sofia decreases from 80% to 64.4%, the lower bound of its 95% confidence interval, savings are even less diminished (by €7.66). Variations of all other parameters do not or do only marginally change the absolute amount of expenditures in favor of the hospital.
A modified approach, where the positive result of POCT is not retested by RT-PCR, results only in relative, but not in absolute cost savings (€154.25, see Table 2b). Patients who tested false positive by POCT would have been isolated unnecessarily and received antithrombotic prevention on average for 10 days. During this period, no other patient could be admitted to the second bed in the two-bed room and thus opportunity costs for each single day would occur at the expense of the hospital. Although the specificity of the antigen test testing in the ER is very high, one of those falsely isolated non-COVID-19 patients would burden the hospital with additional costs of €6282. Although only 1.1% of the 84.4% hospitalized non-COVID-19 patients would be tested false positive, the approach without RT-PCR re-testing would on average lead to additional costs per falsely tested patient of €69.1 compared to the re-testing approach. Therefore, the approach with re-testing of POCT by RT-PCR is clearly favorable not only from the clinical but also economic point of view.
In probabilistic sensitivity analysis (PSA), i.e., under all reasonable assumptions, performing POCT on each patient prior to hospitalization reduces the costs that occur when COVID-19 suspects are isolated based only on the conventional clinical approach, by €209.91 (see Table 4 ). Of note, testing with Sofia® SARS Antigen FIA is constantly less expensive than the purely clinical approach and on average even less expensive than in base analysis, even when a RT-PCR test is used to confirm or deny the preceding POCT result one day later.
Table 4.
Results of the probabilistic sensitivity analysis (Monte Carlo Simulation).
| Probabilistic Sensitivity Analysis | Comparators | Mean Cost Per Patient (€) | Standard Deviation (± SD) | Incremental Cost (€) * |
|---|---|---|---|---|
| COVID-19 patients prior to hospitalisation | Sofia® SARS Antigen FIA | −24.76 | 16.62 | 0 |
| Conventional approach | 185.15 | 30.58 | 209.91 |
Incremental cost denotes the increase in total costs resulting from using the conventional approach alone versus POCT.
The major portion of this savings figure is due to the fact that in PSA, where the results are based on random-sampling and therefore differ from those of the univariate analysis, the proportion of initial unnecessary bed blocking was more than twenty-one-fold higher (25.9 vs 1.2%) with conventional clinical judgement than with the Sofia® SARS Antigen FIA. As this mistake can be corrected only 1 day later, when the result of the RT-PCR is available, the cost difference between the two strategies, with respect to opportunity costs - weighted by the proportion of 81.5% of patients who were not infected with SARS-CoV-2, is €159.24 in favor of the Sofia test. Although the sensitivity of the Sofia is minimally lower than that of the purely clinical approach, the earlier discharge by obtaining a negative POCT result one day earlier that the result of the RT-PCR results in a cost saving of €50.57.
Discussion
Newer real-time POC tests such as the Sofia® SARS Antigen FIA, which can claim specificity of nearly 99%, come close to laboratory RT-PCR testing in their ability to very rapidly and reliably exclude the presence in a patient of transmissible COVID-19. Therefore, they offer the potential to avoid unnecessary isolation that occurs extensively under the conventional clinical approach. The COVID-19 situation, which forces snap clinical decisions, does not work in favor of the conventional approach. There have been complex attempts to better predict the presence of COVID-19 by creating artificial intelligence (AI) programs which process clinical data as well as imaging techniques. Xia et al.20 describe that when considering 52 clinical and laboratory coefficients, e.g., disseminated intravascular coagulation, d-dimer, procalcitonin, enlarged lymph nodes or rhabdomyolysis together with CXR features, sensitivity increased to 94% and specificity to 75%. However, the complex information required is hardly available in the setting of an ER before deciding whether a possible COVID-19 patient should be hospitalized or not.
In real life studies, POCT with the Sofia to detect the COVID-19 virus in symptomatic patients shows sensitivity nearly identical to that of the empirical clinical approach. However, little information on the onset of symptoms among study participants was available to the researchers there, and an unknown percentage of the patients included may have been tested later than 7 days following the start of symptoms, when sensitivity of the antigen test is known to decrease, again due to decreasing viral load over time. This may at least partially explain the striking discrepancy of more than 15% between the values in the pivotal studies of the manufacturer and the few evaluation studies used for our economic analysis. Another cause may be inappropriate preanalytics, e.g., pipetting swab material into viral transport media rather than performing the POCT immediately as required by the manufacturer´s operation procedures.
Nevertheless, the key to achieving the calculated cost saving of €209.91 per patient by implementing a POC antigen COVID-19 test from the hospital's perspective lies in the time lag between taking the swabs in the ER, which in most cases get sent to an external laboratory, and receiving the RT-PCR report one day later. Each time a patient is wrongly assumed to be suffering from a SARS-CoV-2 infection; hospital capacity is reduced, leading to corresponding revenue loss in terms of one day of opportunity costs for the hospital. Performing the Sofia test on the spot results in significantly fewer false assumptions made regarding the presence of COVID-19 patients and the rate of unnecessary bed blocking on the first day of hospitalization is twenty-one nine-fold lower when compared to the conventional clinical approach.
Thus, in PSA of our model, the routine implementation of a POCT for COVID-19 suspects being moved from the ER for admission to a German hospital ward is consistently less expensive than the conventional symptom-based judgement for which the RT-PCR testing results is available only after a delay of 1 day. Of note, this ranking is not dependent on changes in the prevalence of COVID-19 in such patients, as long as during the ongoing COVID-19 “third wave” the COVID-19 prevalence in ER patients does not exceed and remain above 41.2%. Of note, we did not consider the number of those patients with severe COVID-19 who needed respiratory support (oxygen with or without subsequent invasive mechanical ventilation) at the beginning of hospital stay. As the 28-day mortality of those patients may be reduced by administering anti-inflammatory treatment with dexamethasone,21 a prompt and reliable diagnosis of SARS-CoV-2 in those patients is necessary. In their cost-effectiveness model, Ricks et al.22 found that a POCT-led strategy averted more deaths and entailed lower costs than did RT-PCR testing, given that RT-PCR testing was performed in fewer than 85% of cases, with the remainder managed through clinical judgement alone ($140,000 versus $150,000 per death averted).
However, in contrast to our model, where a sensitivity of clinical judgement was estimated to be nearly the same as that of the POCT (80.6% versus 80.0%), the authors stipulated a broad range of uncertainty for the sensitivity of clinical judgement, starting with low 45% (range 45–99%), whilst sensitivity of POCT was a priori set at 80%.
Our study has some limitations that must be kept in mind when interpreting its results. As always, the general limitation of a single-center economic model that cannot depict the reality of utilization of bed capacity of every hospital deserves consideration, as does the local SARS-CoV-2 infection prevalence among exposed health care workers. Therefore, to validate our estimates, prospective cost studies, preferably with a multicenter study design, are required. Furthermore, our calculations refer only to hospitals that must send samples to an external laboratory for COVID-19 testing and wait for the report. Hospitals that have a laboratory department at their disposal that already conducts high quality RT-PCR tests whilst the patients are waiting in the ER, even during weekends and at night, will probably not benefit by COVID-19 POCT. It is important, however, that, although test results must be quickly provided, the cycle threshold (Ct) values, which inversely correlate with the number of virus present in the sample, must be reported so as to reliably indicate infectiousness of a COVID-19 suspect.
Conclusions
The utilization of the Sofia® SARS Antigen FIA test, as representative of high quality POC antigen tests, is likely to reduce hospital-related costs in cases of suspected COVID-19 in German emergency departments. As such, POCT can reduce costs from the hospital´s perspective and allows resources to be allocated for other precautions. Prospective clinical studies should be undertaken to further evaluate its economic advantages in the immediate future.
Ethical considerations
Ethical approval was not necessary as only publicly available secondary data were used.
Conflicts of interest
R.D. received a fee for speaking at a microbiological congress supported by Quidel Inc.
A.N. declares no conflict of interest.
Funding
This research received no external funding.
References
- 1.Zhou P., Yang X.L., Wang X.G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO director-general's opening remarks at the media briefing on covid-19. 11 March 2020:https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19 —11-march-2020.
- 3.Vetter P., Vu D.L., L'Huillier A.G., Schibler M., Kaiser L., Jacquerioz F. Clinical features of covid-19. BMJ. 2020;369:m1470. doi: 10.1136/bmj.m1470. pp. 1-2. [DOI] [PubMed] [Google Scholar]
- 4.COVID-19 deaths worldwide per million population as of April 30, 2021, by country:https://www.statista.com/statistics/1104709/coronavirus-deaths-worldwide-per-million-inhabitants/.
- 5.Bettenführende Fachabteilungen; Wiesbaden, Germany: 2019. Federal Statistical Office of Germany (Statistisches Bundesamt, Wiesbaden) – DESTATIS. Gesundheit. Grunddaten der Krankenhäuser; Fachserie 12, Reihe 6.1.1. Krankenhäuser 2017, 2.8. [Google Scholar]
- 6.Bruning A.H.L., Leeflang M.M.G., Vos J.M.B.W., et al. Rapid tests for COVID-19, respiratory syncytial virus, and other respiratory viruses: a systematic review and meta-analysis. Clin Infect Dis. 2017;65:1026–1032. doi: 10.1093/cid/cix461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wölfel R., Corman V.M., Guggemos W., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 8.Multiple choices. Available online:http://drg.unimuenster.de/index.php?option=com_webgrouper&Itemid=112&view=webgrouper [accessed 20 April 2021].
- 9.Vogl M. Assessing DRG cost accounting with respect to resource allocation and tariff calculation: the case of Germany. Health Econ Rev. 2012;2(15):1–12. doi: 10.1186/2191-1991-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magleby R., Westblade L.F., Trzebucki A., et al. Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis. 2020;ciaa851:1–9. doi: 10.1093/cid/ciaa851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robert Koch Institute. COVID-19: entlassungskriterien aus der Isolierung (as of 31 March 2021): https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Entlassmanagement.html.
- 12.Pray I.W., Ford L., Cole D., et al. Performance of an antigen-based test for asymptomatic and symptomatic SARS-CoV-2 testing at two university campuses - Wisconsin, September-October 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1642–1647. doi: 10.15585/mmwr.mm695152a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robert Koch Institute. Hinweise zur Testung von Patienten auf Infektion mit dem neuartigen Coronavirus SARS-CoV-2 - Zur Bewertung der Ergebnisse aus AG-Testen (as of 12 March 2021):https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Vorl_Testung_nCoV.html;jsessionid=0DABCE15E5CF14EE43D11785FCF2BCA7.internet062?nn=13490888#doc13490982bodyText7
- 14.AWMF-Register-Nr. 113/001S3-Leitlinie - Empfehlungen zur stationären Therapie von Patienten mit COVID-19 (as of 23 February 2021)
- 15.Physicians Fee Schedule [Einheitlicher Bewertungsmaßstab (EBM). Available at: www.kbv.de/html/13259.php?srt=relevance&stp=fulltext&q=32791&s=Suchen. [accesssed 15 April 2021]
- 16.Gesetz zur wirtschaftlichen Sicherung der Krankenhäuser und zur Regelung der Krankenhauspflegesätze (Krankenhausfinanzierungsgesetz - KHG). § 26 Zusatzentgelt für Testungen auf das Coronavirus SARS-CoV-2 im Kranken-haus [Law on the economic security of hospitals and on the regulation of hospital care rates (Hospital Financing Act - KHG). Section 26 Additional fee for tests for the SARS-CoV-2 coronavirus in hospitals].
- 17.CDC. Interim Guidance on Duration of Isolation and Precautions for Adults with COVID-19 Updated Feb. 13, 2021:https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.htm.
- 18.Tolksdorf K., Buda S., Schuler E., Wieler L.H., Haas W. Eine höhere Letalität und lange Beatmungsdauer unterscheiden COVID-19 von schwer verlaufenden Atemwegsinfektionen in Grippewellen. Epid Bull. 2020;41:3–10. [Google Scholar]
- 19.CDC. Interim U.S. Guidance for Risk Assessment and Work Restrictions for Healthcare Personnel with Potential Exposure to SARS-CoV-2. Updated Mar. 11, 2021.
- 20.Xia Y., Chen W., Ren H., et al. A rapid screening classifier for diagnosing COVID-19. Int J Biol Sci. 2021;17:539–548. doi: 10.7150/ijbs.53982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricks S., Kendall E.A., Dowdy D.W., Sacks J.A., Schumacher S.G., Arinaminpathy N. Quantifying the potential value of antigen-detection rapid diagnostic tests for COVID-19: a modelling analysis. BMC Med. 2021;19(75):1–13. doi: 10.1186/s12916-021-01948-z. [DOI] [PMC free article] [PubMed] [Google Scholar]