Abstract
Imbalances of blood biomarkers are associated with disease, and biomarkers may also vary non-pathologically across population groups. We described variation in concentrations of biomarkers of one-carbon metabolism, vitamin status, inflammation including tryptophan metabolism, and endothelial and renal function among cancer-free older adults. We analyzed 5167 cancer-free controls aged 40–80 years from 20 cohorts in the Lung Cancer Cohort Consortium (LC3). Centralized biochemical analyses of 40 biomarkers in plasma or serum were performed. We fit multivariable linear mixed effects models to quantify variation in standardized biomarker log-concentrations across four factors: age, sex, smoking status, and body mass index (BMI). Differences in most biomarkers across most factors were small, with 93% (186/200) of analyses showing an estimated difference lower than 0.25 standard-deviations, although most were statistically significant due to large sample size. The largest difference was for creatinine by sex, which was − 0.91 standard-deviations lower in women than men (95%CI − 0.98; − 0.84). The largest difference by age was for total cysteine (0.40 standard-deviation increase per 10-year increase, 95%CI 0.36; 0.43), and by BMI was for C-reactive protein (0.38 standard-deviation increase per 5-kg/m2 increase, 95%CI 0.34; 0.41). For 31 of 40 markers, the mean difference between current and never smokers was larger than between former and never smokers. A statistically significant (p < 0.05) association with time since smoking cessation was observed for 8 markers, including C-reactive protein, kynurenine, choline, and total homocysteine. We conclude that most blood biomarkers show small variations across demographic characteristics. Patterns by smoking status point to normalization of multiple physiological processes after smoking cessation.
Subject terms: Biomarkers, Epidemiology
Introduction
Imbalances of blood biomarkers are associated with several diseases. For example, perturbations in one-carbon metabolism may lead to cardiovascular disease1, cancer2–4, and other diseases5, 6. Deficiencies of vitamins A, D and E are associated with increased risk of type II diabetes and multiple immune system disorders7. Accordingly, measurements of blood biomarkers have been studied extensively in relation to illness8–13, but their variation among healthy individuals is less commonly described.
The Lung Cancer Cohort Consortium (LC3) was initially established to provide evidence on the potential importance of biomarkers of one-carbon metabolism in lung cancer etiology among 20 prospective cohorts. The project measured markers of B-vitamins and fat-soluble vitamins, functional biomarkers such as one-carbon metabolites, inflammation markers including tryptophan metabolites of the kynurenine pathway, and markers of renal and endothelial function. Previous analyses of the LC3 primarily assessed the relationship of these markers with lung cancer risk8–13, while one study analyzed control participants to describe the variation in biomarkers across geographical regions14. Lung cancer risk was found to be positively associated with levels of vitamin B129, vitamin B613 and components of tryptophan metabolism15. Among cancer-free control participants, analyses by geography showed higher levels of vitamins in the USA due to widespread vitamin supplementation and food-fortification practices, while one-carbon and tryptophan metabolites were highest in Asian populations14.
Beyond patterns by geography, circulating levels of biomarkers can also vary within healthy populations across demographic and behavioral factors such as age16, 17, smoking history18, 19, and sex20. The large and diverse LC3 dataset offers an important opportunity to comprehensively describe biomarker variation among cancer-free older adults, but the prior analysis of control participants focused only on geographical variation. Here, we analyzed the LC3 control participants to describe variation in biomarkers of one-carbon metabolism, vitamin status, inflammation including tryptophan metabolites of the kynurenine pathway, and renal and endothelial function. We examined key demographic and behavioral factors, specifically age, sex, smoking status, and body mass index (BMI).
Methods
Study sample
We analyzed data from 20 cohorts in the Lung Cancer Cohort Consortium (LC3), including 11 US cohorts, 4 Nordic cohorts, 4 Asian cohorts, and 1 Australian cohort (see Table 1 footnote for details)8, 11–14. In each cohort, biomarkers were measured in plasma or serum samples from lung cancer cases and matched controls. Here, our aim was to describe patterns of blood biomarker status in cancer-free older adults, so we restricted analysis to the matched controls. These controls were matched individually to the cases by sex, date of blood collection, date of birth, smoking status in 5 categories (never smokers, former smokers with < 10 or ≥ 10 quit-years, and current smokers with < 15 or ≥ 15 cigarettes per day), and (for US cohorts only) race/ethnicity. All cohort participants provided written, informed consent.
Table 1.
Descriptive characteristics of 5167 control subjects in the Lung Cancer Cohort Consortium.
| Characteristic | N (%) |
|---|---|
| Geographical region | |
| USA | 2259 (43.7%) |
| Nordic | 803 (15.6%) |
| Asian | 1747 (33.8%) |
| Australian | 358 (6.9%) |
| Age, years | |
| 49 or younger | 473 (9.1%) |
| 50 to 59 | 1448 (28.0%) |
| 60 to 69 | 2324 (45.0%) |
| 70 to 80 | 922 (17.9%) |
| Sex | |
| Male | 2810 (54.4%) |
| Female | 2357 (45.6%) |
| Body mass index | |
| Less than 18.5 | 121 (2.3%) |
| 18.5 to < 25 | 2608 (50.5%) |
| 25 to < 30 | 1802 (34.9%) |
| 30 or higher | 636 (12.3%) |
| Smoking status | |
| Never | 1264 (24.5%) |
| Former | 1453 (28.1%) |
| Current | 2450 (47.4%) |
| Race/ethnicity | |
| White | 2947 (57.0%) |
| Black | 247 (4.8%) |
| Asian | 1812 (35.1%) |
| Other | 161 (3.1%) |
The study population includes cancer-free control subjects from the Lung Cancer Cohort Consortium, which comprises 20 cohorts from the USA (Campaign Against Cancer and Stroke and Campaign Against Cancer and Heart Disease (CLUE, N = 171), American Cancer Society Cancer Prevention Study-II Nutrition Cohort (CPS-II, N = 179), Health Professionals Follow-Up Study (HPFS, N = 130), Multiethnic Cohort (MEC, N = 148), Nurses’ Health Study (NHS, N = 328), New York University Women’s Health Study (NYUWHS, N = 167), Physicians’ Health Study (PHS, N = 76), Prostate Lung Colorectal and Ovarian Cancer Screening Trial (PLCO, N = 440), Southern Community Cohort Study (SCCS, N = 209), Women’s Health Initiative (WHI, N = 228), Women’s Health Study (WHS, N = 183)), Nordic countries (Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC, N = 200), Trøndelag Health Study (HUNT, N = 174), Northern Sweden Health and Disease Study Cohort (NSHDS, N = 230), Malmö Diet and Cancer Study (MDCS, N = 199)), Asia (Shanghai Men’s Health Study (SMHS, N = 419), Shanghai Women’s Health Study (SWHS,N = 417), Shanghai Cohort Study (SCS, N = 502), Singapore Chinese Health Study (SCHS, N = 409)), and Australia (Melbourne Collaborative Cohort Study (MCCS, N = 358)). Details regarding the data from individual cohorts have been published previously7.
Before exclusions, numbers of control participants in each cohort ranged from 81 (Physicians’ Health Study) to 513 (Shanghai Cohort Study). Across the consortium, samples were collected between 1974 and 2010. Some cohorts included almost exclusively current and former smokers (for example the Southern Community Cohort Study included 73% current and 20% former smokers) while in others, never smokers were intentionally over-sampled (Women’s Health Initiative, 100% never smokers). Blood samples were drawn at baseline when participants were required to be free of any cancer (except non-melanoma skin cancer). Controls were required to be free of cancer at the time of diagnosis of the matched case, and the median time between blood collection and diagnosis of the index cancer case was 7.0 years (interquartile range 3.3 to 11.0 years).
Individuals with extreme values of covariates (e.g. continuous covariates such as BMI and age) can have high influence on estimated coefficients. To avoid this, we excluded individuals with BMI outside the range of 17–40 kg/m2 (N = 138) and subsequently excluded individuals with age at blood draw outside the range of 40–80 years (N = 84). Therefore, from 5389 individuals, 222 were excluded and the final study sample included 5167 participants.
Biomarker measurements
Biomarker measurements in serum or plasma were performed at the BEVITAL laboratory in Bergen, Norway using mass spectrometry based and microbiological methods as described in Supplementary Table 1. Samples in each cohort were divided into batches of approximately 86 samples. All samples in a single batch were derived from the same cohort and a batch adjustment was included in analyses (see below). Between-batch coefficients of variation (CVs) ranged from 2 to 15%, with the exception of methionine sulfoxide which had a CV of 29%14.
We divided the 40 biomarkers into 4 categories: (1) one-carbon metabolism markers (methionine, methionine-sulfoxide, total homocysteine, cystathionine, total cysteine, serine, glycine, choline, betaine, dimethylglycine, and sarcosine), (2) vitamins including B-vitamins (pyridoxal 5′-phosphate (PLP), pyridoxal (PL), 4-pyridoxic acid (PA), riboflavin, nicotinamide, folate and cobalamin and methylmalonic acid), and fat-soluble vitamins (vitamin A measured as all trans-retinol metabolites, 25OH-vitamin D, alpha-tocopherol, gamma-tocopherol), (3) tryptophan metabolites of the kynurenine pathway (tryptophan, kynurenine, kynurenic acid, anthranilic acid, 3-hydroxykynurenine, xanthurenic acid, 3-hydroxyanthranilic acid, quinolinic acid) and inflammation markers (C-reactive protein (CRP), total neopterin, kynurenine/tryptophan ratio (KTR), Par index ), and (4) markers of endothelial or renal function (arginine, homoarginine, asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), and creatinine).
Blood samples were stored for different lengths of time in each cohort, but there was also substantial variation in storage time within cohorts (e.g. NSHDS samples were stored for between 0 and 21 years). A storage time adjustment was included in the analysis (see below). Information on blood collection dates by cohort is provided in Supplementary Table 2.
Statistical analysis
We calculated the PAr index, which serves as a measure of vitamin B6 catabolism, as the ratio of pyridoxic acid to the sum of pyridoxal and PLP concentrations13. We calculated the kynurenine-tryptophan ratio, which serves as a measure of cellular immune activation, by dividing kynurenine concentrations by tryptophan concentrations15. Concentrations of total vitamin D were calculated by adding the concentrations of 25(OH)D2 to seasonally adjusted concentrations of 25(OH)D3. Because 25(OH)D3 is strongly affected by exposure to UVB radiation, we performed seasonal adjustments using Bayesian hierarchical regression models21. Seasonally adjusted 25(OH)D3 was estimated by adding or subtracting the estimated deviation from the mean concentration according to the day of blood draw. We applied a logarithmic base-2 transformation to all biomarker measurements and ratios. We then standardized all values by centering on the mean and dividing by the standard deviation.
To compare biomarker values across our variables of interest (age, sex, smoking status, and BMI), we used multivariable linear mixed effects models. We fit one model for each biomarker with the log-transformed, standardized biomarker value as the outcome. Each model included fixed effects for age (continuous), sex (female vs. male), smoking status (never, former, current), BMI (continuous), and random effects for batch. Models were adjusted using fixed effects for cohort, race/ethnicity, and freezer storage time. Using this approach, each model coefficient (noted as β) represents the mean change in the log-biomarker, in standard-deviation units, associated with a 1-unit change in the independent variable. For age and BMI, we re-scaled these coefficients to represent the effect of a 10-year increase in age and a 5-unit increase in BMI. While we adjusted for race/ethnicity, we did not report these results because racial/ethnic differences in our dataset mostly occur across geographical regions, and this was the subject of a prior manuscript14.
Finally, we quantified proportions of vitamin deficiency for riboflavin, PLP, folate, cobalamin, vitamin A, alpha-tocopherol and vitamin D across demographic categories, using previously established cutpoints for vitamin deficiency14. To investigate associations with time since quitting smoking, we restricted to cohorts that provided data on quitting time among former smokers, then assessed whether the difference in biomarker measurements between former smokers and never smokers attenuated as time since quitting increased.
We performed multiple sensitivity analyses. For the modeling approach, we compared the primary analysis results to results from three different changes to the model. The first excluded the fixed effect for cohort, the second excluded the random effect for batch, and the third excluded the fixed effect for storage time (Supplementary Methods). We also assessed the effect of excluding single-sex cohorts on the coefficients for sex, the effect of excluding outlying values of the log-biomarkers on all results, and the assumption of linear relationships between each biomarker and age/BMI.
All analyses were done in R versions 3.5.3, 3.6, and 4.0. Mixed effects models were fit using the lme4 package.
Ethical approval
The protocol of the Lung Cancer Cohort Consortium was approved by the Ethics Committee of the International Agency for Research on Cancer (Project number 11-13). The recruitment, data collection, and follow-up of the participating cohorts was approved by local institutional review boards. This study involved no additional contact or intervention with participants. This research was performed in accordance with the Declaration of Helsinki.
Results
Baseline characteristics of the study sample are presented in Table 1, and stratified characteristics by cohort are presented in Supplementary Table 3. Geographically, 43.7% of the population was from the USA, with smaller proportions from Asia (33.8%), Europe (15.6%), and Australia (6.9%). Of all participants, 54% were male, and 57% were of white race/ethnicity. The mean age was 61.7 years with a standard deviation of 8.3. The majority of the sample was comprised of current smokers (47.4%) and former smokers (28.1%). Storage duration of serum/plasma samples had a modest impact on log biomarker concentrations. Among all biomarkers, the median change in log-concentration was 0.01 standard deviations per year stored, and the maximum change was 0.04 standard deviations per year stored (Supplementary Table 4).
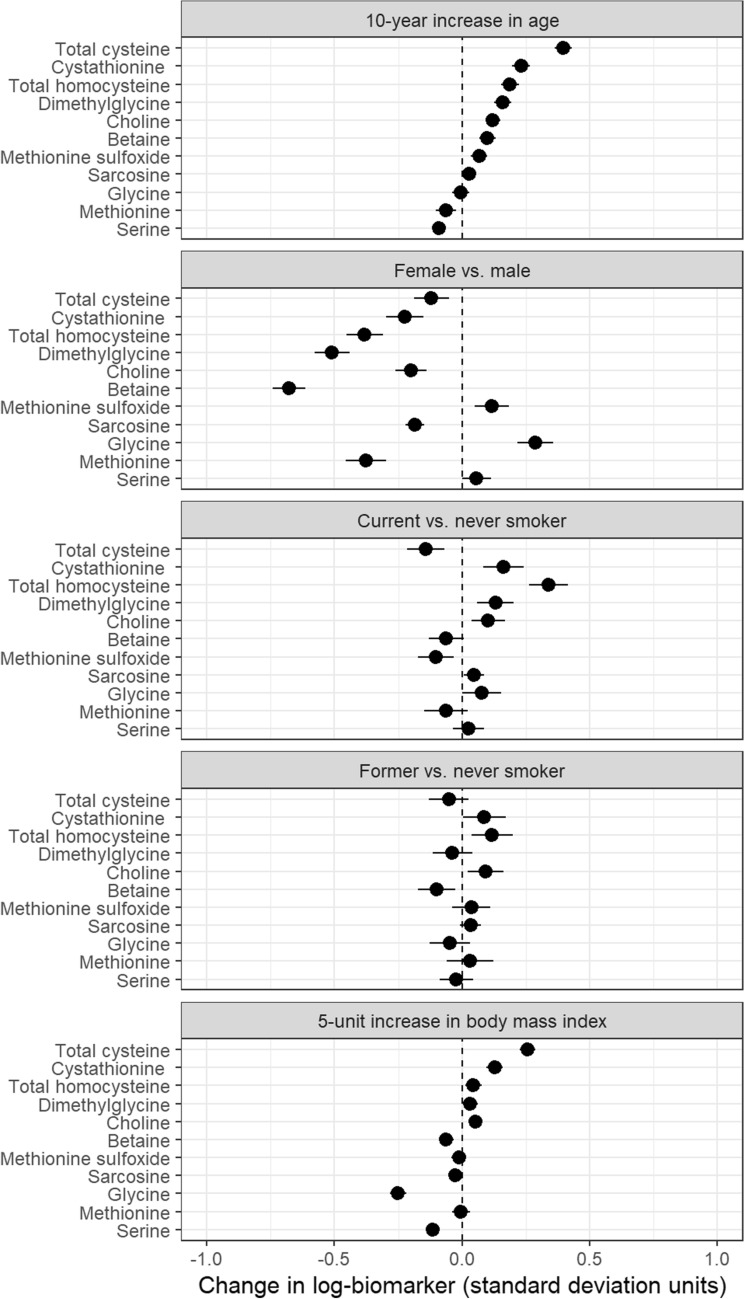
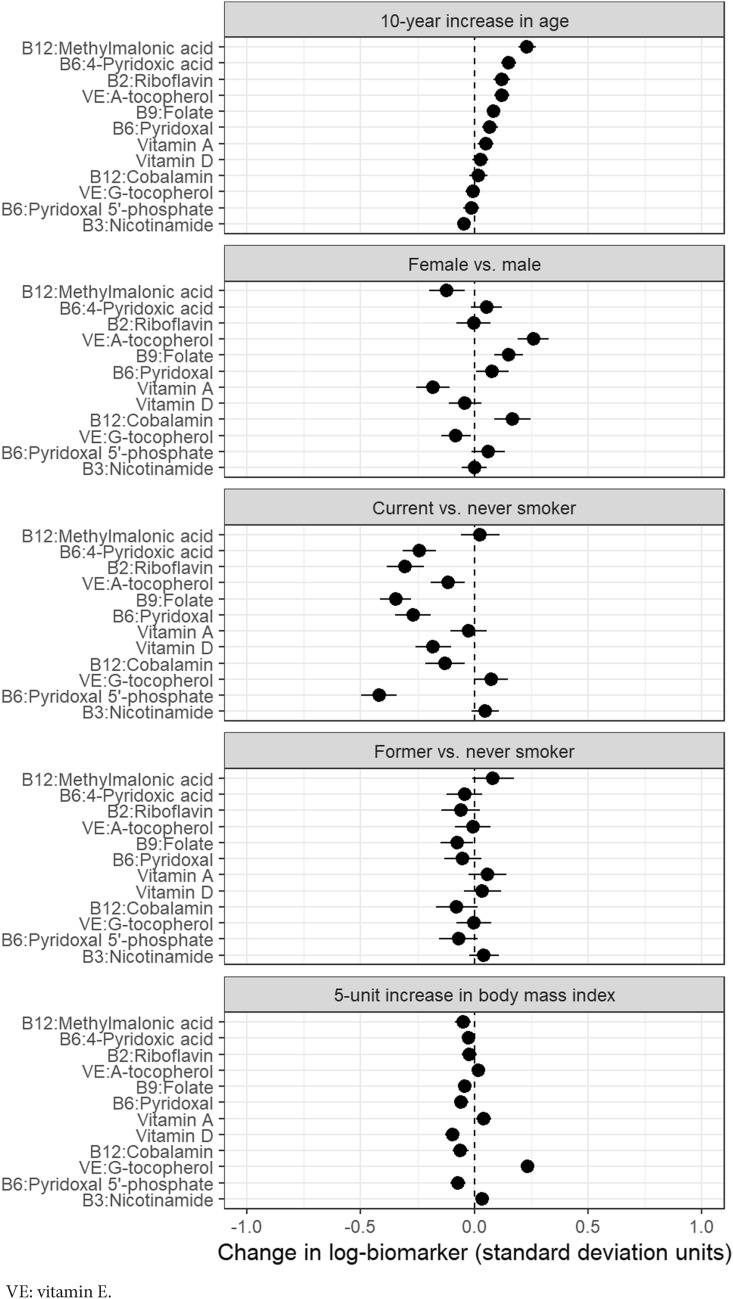
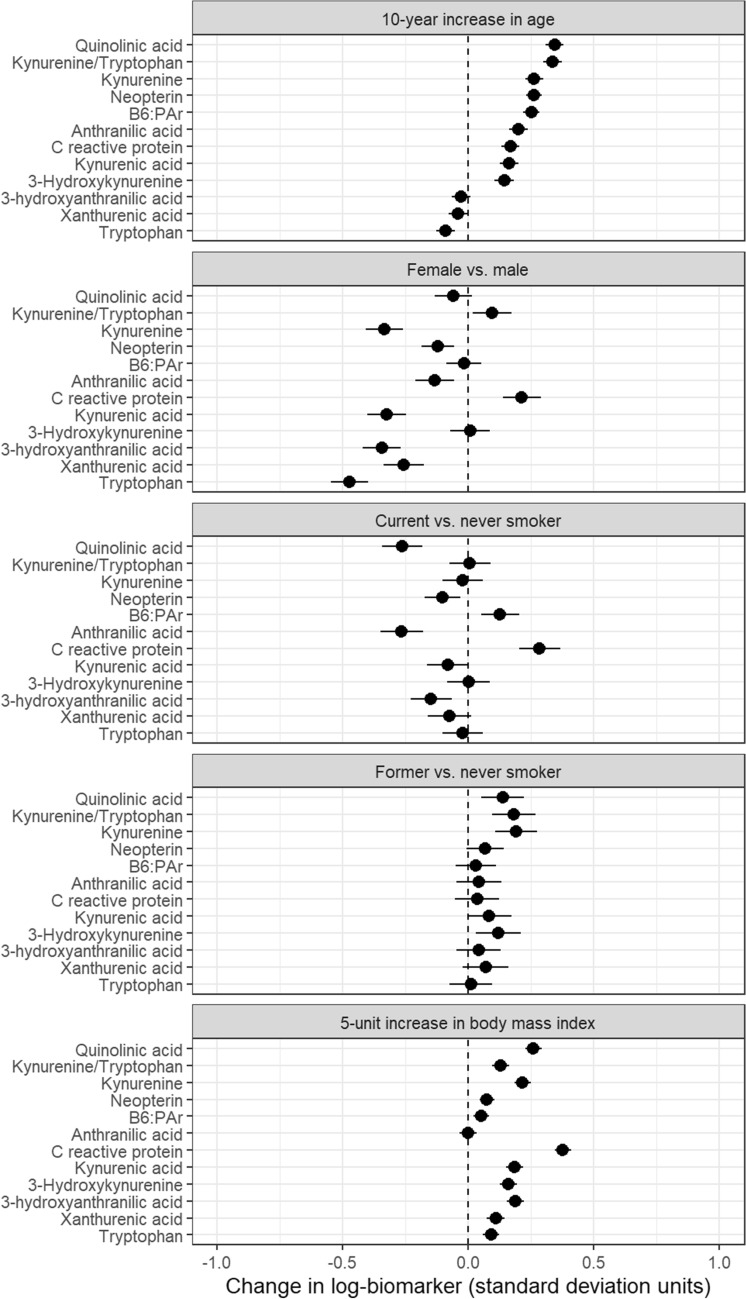
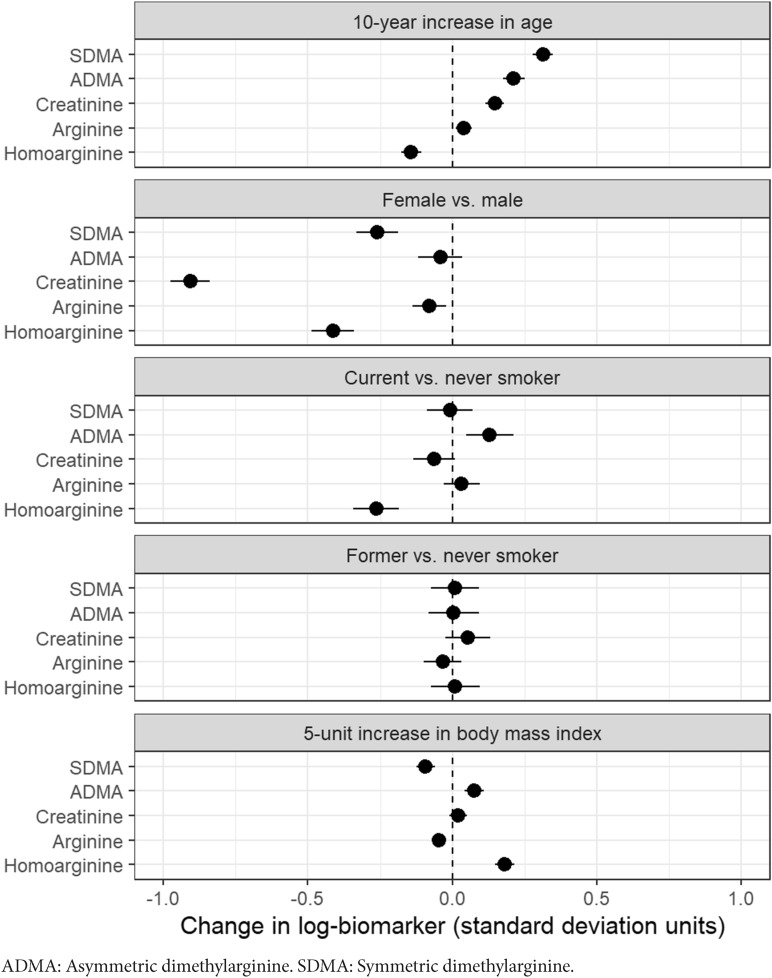
Adjusted differences in standardized means of log-biomarker values are shown by age, sex, smoking status, and BMI for one-carbon metabolites in Fig. 1, for vitamins in Fig. 2, for kynurenine pathway metabolites and inflammation markers in Fig. 3, and for markers of renal and endothelial function in Fig. 4. Across all analyses, 93% (186/200) of analyses showed a difference lower than 0.25 standard-deviations, though most of these small differences were statistically significant due to large sample size. For 31 of 40 markers, the mean difference compared with never smokers was larger for current than for former smokers.
Figure 1.
Differences in biomarkers of one-carbon metabolism by age, sex, smoking status, and BMI, in units of log-biomarker standard deviations, among 5167 control subjects in the Lung Cancer Cohort Consortium. Biomarkers are listed in order of the age coefficient.
Figure 2.
Differences in biomarkers of vitamin status by age, sex, smoking status, and BMI, in units of log-biomarker standard deviations, among 5167 control subjects in the Lung Cancer Cohort Consortium. Biomarkers are listed in order of the age coefficient.
Figure 3.
Differences in biomarkers of the kynurenine pathway and inflammation by age, sex, smoking status, and BMI in units of log-biomarker standard deviations, among 5167 control subjects in the Lung Cancer Cohort Consortium. Biomarkers are listed in order of the age coefficient.
Figure 4.
Differences in biomarkers of renal and endothelial function by age, sex, smoking status, and BMI in units of log-biomarker standard deviations, among 5167 control subjects in the Lung Cancer Cohort Consortium. Biomarkers are listed in order of the age coefficient.
Age had a substantial impact on levels of most one-carbon metabolites (Fig. 1). Among all markers, the largest difference by age was for total cysteine (β = 0.40 standard-deviation increase per 10 years, 95%CI 0.36 to 0.43). Levels of one-carbon metabolites also varied strongly by sex, with markedly lower levels among women for betaine (β = − 0.68, 95%CI − 0.74 to − 0.61), dimethylglycine (β = − 0.51, 95%CI − 0.58 to − 0.44), total homocysteine (β = − 0.38, 95%CI − 0.45 to − 0.31), and methionine (β = − 0.38, 95%CI − 0.46 to − 0.30). Current smokers had higher levels of homocysteine than never smokers (β = 0.34, 95%CI 0.26 to 0.42). BMI did not have a strong influence on one carbon metabolite levels; the largest differences were β = 0.26 (for a 5-unit increase in BMI) for total cysteine (95%CI 0.23 to 0.29) and β = − 0.25 for glycine (95% CI − 0.28 to − 0.22).
For vitamin biomarkers, most levels increased with age and were higher for women (Fig. 2). Current smokers had lower levels of almost all vitamins compared with never smokers, particularly B vitamins (β = − 0.42, 95%CI − 0.50 to − 0.34 for vitamin B6/PLP, β = − 0.35, 95%CI − 0.41 to − 0.28 for vitamin B9/folate, and β = − 0.30, 95%CI − 0.38 to − 0.22 for vitamin B2/riboflavin). Differences between former and never smokers were much smaller, as were differences across BMI. One exception was vitamin E/gamma-tocopherol, which increased with BMI (β = 0.23 for a 5-unit increase in BMI, 95%CI 0.21 to 0.26).
With few exceptions, all kynurenine pathway and inflammation markers increased with age and BMI (Fig. 3). Among all the markers, the largest difference by BMI was for C-reactive protein (β = 0.38 standard-deviations per 5-kg/m2 increase, 95%CI 0.34 to 0.41). The largest differences were seen by sex, particularly for tryptophan (β = − 0.47 for women, 95%CI − 0.55 to − 0.40). Current smokers had lower levels of almost every component of the kynurenine pathway; conversely, former smokers showed a small increase in all kynurenines compared with never smokers. CRP was higher in current smokers (β = 0.28, 95%CI 0.20 to 0.37) while differences between former and never smokers were small for all inflammation markers.
Of all markers and characteristics, the largest difference was for creatinine by sex (β = − 0.91 for women, 95%CI − 0.98 to − 0.84; Fig. 4). Differences in creatinine by other variables were small. Markers of endothelial function showed inconsistent patterns by age and BMI, but were consistently decreased in women (e.g., β = − 0.41 for homoarginine, 95%CI − 0.49 to − 0.34). Some differences in endothelial function markers were seen between current and never smokers, but not between former and never smokers.
We measured the prevalence of deficiency for 7 vitamins (Supplementary Table 5). Deficiency was most frequent for PLP (16%) and vitamin D (9%), but otherwise uncommon (0.2% to 3.4%). Stratified analysis of vitamin deficiencies showed more frequent PLP deficiency in men than in women (20% vs. 11%, p < 0.001), among individuals with lower BMI (decreasing from 39% among underweight individuals to 14% among obese individuals, p < 0.001), and with current smoking (25% vs. 7–8% for never and former smokers, p < 0.001). For vitamin D, deficiency decreased with age (decreasing from 11% among age 40–49 to 5% among age 70–80, p < 0.001). However, the mean change in blood Vitamin D levels with increasing age was small (β = 0.03, 95%CI − 0.01; 0.06, Fig. 2). Supplementary Tables 6 and 7 show that intake of multivitamins increased with age, and that vitamin D deficiency was lower among multivitamin users. Vitamin D deficiency also varied by sex, BMI, smoking status, and (for USA participants) race/ethnicity.
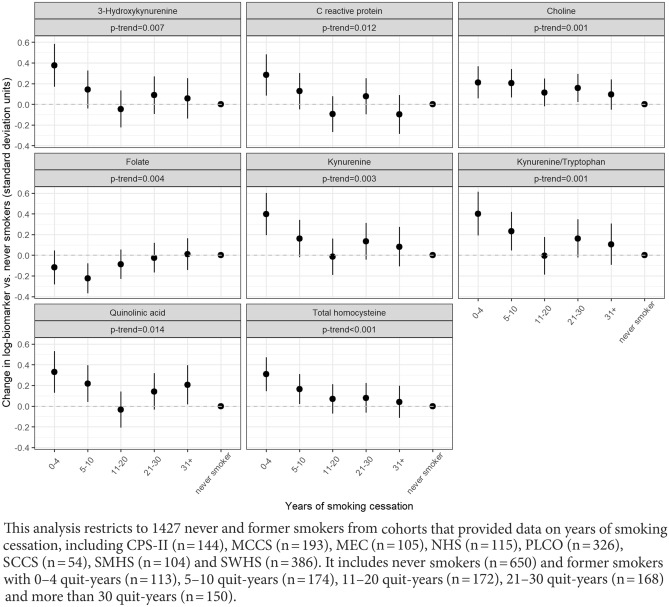
Trends in biomarker levels across time since smoking cessation were statistically significant (p < 0.05) for 8 of 40 biomarkers. Biomarkers with statistically significant trends are shown in Fig. 5, with the remainder shown in Supplementary Fig. 1. For some markers such as CRP, kynurenine, 3-hydroxykynurenine, and quinolinic acid, measurements appeared similar to those of never smokers within approximately 10–20 years of cessation (Fig. 5). For other markers, such as choline and total homocysteine, a steady trend was observed up to more than 30 years since quitting. This analysis was underpowered to detect biomarkers that normalize within 4 years of cessation, but visual inspection suggests that such markers might include kynurenic acid, pyridoxal, betaine, cystathionine, serine, and others (Supplementary Fig. 1).
Figure 5.
Trends in biomarker measurements among former smokers, by years of smoking cessation, for biomarkers with statistically significant trends (p < 0.05), among 1427 control subjects in selected cohorts from the Lung Cancer Cohort Consortium.
Sensitivity analyses for model parameterization had negligible effects on coefficients (Supplementary Figs. 2, 3, and 4). The exclusion of outlying values of log-biomarkers had small effects (Supplementary Fig. 5). Similarly, the exclusion of single sex cohorts had very small effects on sex coefficients (Supplementary Fig. 6). Assuming a linear relationship between log-biomarker concentrations and age or BMI was generally justified as shown by the adjusted variable plots (Supplementary Figs. 7 and 8). The values and confidence intervals shown in Figs. 1, 2, 3, and 4 are provided in Supplementary Table 8.
Discussion
In this study, we described variation in concentrations of 40 blood biomarkers across age, sex, smoking status, and BMI among cancer-free older adults. Most blood biomarkers of one-carbon metabolism, vitamin status, inflammation, and renal and endothelial function showed statistically robust but small variations across demographic and behavioral characteristics. There were important differences between men and women for several markers of one-carbon metabolism, some B vitamins, and components of the kynurenine pathway. Across multiple domains of physiological function, biomarker perturbations were stronger in current smokers than former smokers, illustrating the broadly positive influences of tobacco cessation.
Many of the biomarkers we studied were substantially altered in current smokers (compared with never smokers), whereas levels normalized with time since quitting smoking among former smokers. This observation suggests that changes in these biomarkers caused by smoking may resolve after smoking cessation. This pattern occurred broadly across multiple domains of analytes: for 31 out of 40 markers, the magnitude of difference between current and never smokers exceeded the difference between former and never smokers. These results are consistent with prior data for some individual markers, including vitamin E (alpha-tocopherol) and vitamin B1218, 22. Kynurenine pathway markers showed reductions among current smokers (vs. never smokers) but increases among former smokers, which is consistent with prior reports23–26. Decreased levels of kynurenines and neopterin in smokers might be attributable to decreased activity of cytokine IFN-gamma27.
Other studies have demonstrated that increased disease risk, particularly for lung cancer, can be reversed substantially with smoking cessation, and can even approach that of never smokers with sufficient quitting time28, 29. For example, levels of C-reactive protein revert to those of never-smokers after approximately 20 years of cessation30. Our data showed a similar result for CRP along with kynurenine, 3-hydroxykynurenine, and quinolinic acid. Some markers, such as choline and total homocysteine, may take even longer to normalize to the levels of never smokers, while others likely normalize within a few years. In general, our data suggest that this pattern of normalization after smoking cessation occurs across multiple physiological functions.
One-carbon metabolism is linked to B-vitamin status and is important for genome stability, with disturbances associated with tumorigenesis31. We found strong differences in some one-carbon metabolite levels between current and never smokers, which translated into higher prevalence of deficiency. We also observed lower levels of most one-carbon metabolites in women compared to men. This finding is consistent with some prior studies for total homocysteine32, betaine, and choline33, 34, though one study found the opposite pattern35.
Previous studies have addressed the role of several B-vitamins (B12, B9, B6), which are linked to one-carbon metabolism, in the development of lung cancer9, 13, 36. The fat-soluble vitamins A, D, and E are important for a variety of physiological functions relevant to potential associations between deficiencies and cancer 7. We found higher concentrations of most B-vitamins in women, although some studies showed the opposite pattern for PLP, folate32, cobalamin, and B634. Previously reported sex differences in riboflavin levels34, 37 were not present in our data. One possible explanation is that women used more multivitamin supplements than men in our dataset (Supplementary Tables 6 and 7). For BMI, increasing vitamin E (gamma-tocopherol) levels with higher BMI, and decreasing vitamin D levels, are consistent with prior studies38–40. However, prevalence of vitamin D deficiency was highest among underweight individuals, followed by obese individuals. Vitamin D deficiency also decreased with age, while prior population-based studies in the USA showed potential small increases in vitamin D deficiency (or decreases in mean levels) with age41, 42.
Inflammation plays a central role in the pathogenesis of many chronic diseases, and many of the kynurenines are neuroactive compounds with immunomodulatory effects23. We observed expected increases in components of the kynurenine pathway and inflammation with increasing age23, and lower levels of kynurenine in women are also consistent with prior data. Positive associations of BMI with kynurenines and inflammation markers are consistent with prior findings for middle-aged individuals, with the exception of anthranilic acid37, 43.
Lower levels of creatinine in women are well-established, are due to lower amounts of muscle mass, and form the basis for sex-specific clinical reference ranges44. Unfortunately, we were not able to convert our creatinine measurements to estimated glomerular filtration rate (eGFR) because creatinine was not measured by isotope dilution mass spectrometry. Among this group of markers, we also observed lower levels of SDMA and homoarginine in women. Previous studies confirm the positive association between BMI and the endothelial function marker, ADMA45.
Strengths of our study include centralized biomarker analysis with robust quality control, participation of cohorts from different regions, and large sample size. The primary limitation is that we analyzed matched controls from a lung cancer study. This means that the study participants were not representative of their original cohorts, although this should not affect the relationships with biomarker concentrations estimated in our study, since we controlled for matching variables in analyses. In addition, our results may have limited generalizability to younger adults, as our population had a mean age of 62 years. Another limitation is that we had limited data on multivitamin use, precluding adjustment in models. Finally, we did not adjust p-values for multiple comparisons because the objective of our analyses was descriptive.
In summary, we analyzed population variation by age, sex, smoking status, and body mass index for multiple domains of biomarkers and metabolites including one-carbon metabolism, vitamin status, inflammation, and renal and endothelial function. Most markers showed small but statistically robust differences across these characteristics, although some larger differences were observed. This emphasizes the importance of adjusting for standard confounding factors in studies of pathways related to these biomarkers. Across multiple domains of physiological function, we observed resolution of biomarker perturbations in former compared with current smokers, illustrating the positive physiological effects of tobacco cessation.
Supplementary Information
Author contributions
H.A.R., H.Z. and M.J. conceived of the study and its design. M.J., P.B., R.L.M., G.G.G., A.L., K.G., M.J., M.S., J.M., Q.C., W.J.B., A.A.A., A.Z.J., V.L.S., Y.W., X.O.S., W.Z., J.M.Y., W.P.K., S.J.W., D.A., N.D.F., H.W.Y., K.V., H.D.S., X.Z., J.M.G., L.L.M., L.R.W., C.C., R.P., D.M. and A.F. provided study data. H.Z. and H.A.R. completed statistical analyses. H.Z., M.J., P.M.U., O.M., F.G., H.A.R., R.L.M., K.G., A.L., E.P.S., RP, X.O.S., V.L.S. and S.J.W. interpreted the results. H.Z. and H.A.R. drafted the manuscript. H.Z., M.J., P.M.U., Ø.M., R.L.M., G.G.G., J.M., M.S., A.L., E.P.S., K.G., M.J., N.D.F., W.Y.H., C.C., R.P., V.L.S., Y.W., L.L.M., L.R.W., S.J.W., D.A., Q.C., W.J.B., A.A.A., A.Z.J., X.O.S., W.Z., J.M.Y., W.P.K., K.V., H.D.S., Z.X., J.M.G., A.F., D.M., P.B., F.G., and H.A.R. reviewed the draft manuscript and approved the final version. Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Funding
The Lung Cancer Cohort Consortium (LC3) was supported by the National Institutes of Health/National Cancer Institute (Grant numbers 1U1CA155340 and U19 CA203654) and National Health and Medical Research Council (NHMRC) (Grant ID: 1050198). We thank all cohorts and all cohort participants for making this research possible. MCCS cohort recruitment was funded by VicHealth and Cancer Council Victoria. The MCCS was further augmented by Australian National Health and Medical Research Council Grants 209057, 396414 and 1074383 and by infrastructure provided by Cancer Council Victoria. Cases and their vital status were ascertained through the Victorian Cancer Registry and the Australian Institute of Health and Welfare, including the National Death Index and the Australian Cancer Database (ACD). New South Wales (NSW) cancer registry data were obtained via the ACD with the assistance of the NSW Ministry of Health.The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. The WHI authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. The Health Professionals Follow-up Study (HPFS) and Nurses’ Health Study (NHS) thank the cohort participants and staff for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The HPFS and NHS were supported by the NCI at the NIH (Grant numbers UM1 CA186107, P50 CA127003, P01 CA87969, and U01 CA167552). The Multiethnic Cohort Study cohort acknowledges partial funding from National Institutes of Health Grant U01 CA164973. Cancer incidence data for the CLUE cohort were provided by the Maryland Cancer Registry, Center for Cancer Prevention and Control, Maryland Department of Health, with funding from the State of Maryland and the Maryland Cigarette Restitution Fund. The collection and availability of cancer registry data is also supported by the Cooperative Agreement NU58DP006333, funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services. The ATBC Study is supported by the Intramural Research Program of the U.S. National Cancer Institute, National Institutes of Health, Department of Health and Human Services.The NYUWHS is supported by Grants U01 CA182934 and P30 CA16087 from the National Cancer Institute and by Grant P30 ES000260 from the National Institute of Environmental Health Sciences. Funding for the PHS is through Grants CA097193, CA34944, CA40360, HL2649 0, and HL34595 from the National Institutes of Health, Bethesda, MD. The Singapore Chinese Health Study and the Shanghai Cohort Study acknowledge partial funding National Institutes of Health Grants R01CA144034 and UM1CA182876. The Shanghai Women’s Health Study (UM1 CA182910) and Shanghai Men’s Health Study (UM1 CA173640) were supported by Grants from US National Cancer Institute.
Data availability
Data from the Lung Cancer Cohort Consortium are not publicly available because approval for analysis must be obtained from each participating cohort. Researchers who are interested in analyzing the LC3 data are encouraged to contact the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-93214-8.
References
- 1.Guasch-Ferré M, et al. Plasma metabolites from choline pathway and risk of cardiovascular disease in the PREDIMED (Prevention with Mediterranean Diet) study. J. Am. Heart Assoc. 2017;6:e006524. doi: 10.1161/JAHA.117.006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim YI. Folate and colorectal cancer: An evidence-based critical review. Mol. Nutr. Food Res. 2007;51:267–292. doi: 10.1002/mnfr.200600191. [DOI] [PubMed] [Google Scholar]
- 3.Fanidi A, et al. A prospective study of one-carbon metabolism biomarkers and cancer of the head and neck and esophagus. Int. J. Cancer. 2015;136:915–927. doi: 10.1002/ijc.29051. [DOI] [PubMed] [Google Scholar]
- 4.Johansson M, et al. One-carbon metabolism and prostate cancer risk: Prospective investigation of seven circulating B vitamins and metabolites. Cancer Epidemiol. Biomarkers Prev. 2009;18:1538–1543. doi: 10.1158/1055-9965.EPI-08-1193. [DOI] [PubMed] [Google Scholar]
- 5.Bailey LB, et al. Biomarkers of nutrition for development-folate review. J. Nutr. 2015;145:1636S–1680S. doi: 10.3945/jn.114.206599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li K, Wahlqvist ML, Li D. Nutrition, one-carbon metabolism and neural tube defects: A review. Nutrients. 2016;8:741. doi: 10.3390/nu8110741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albahrani AA, Greaves RF. Fat-soluble vitamins: Clinical indications and current challenges for chromatographic measurement. Clin. Biochem. Rev. 2016;37:27–47. [PMC free article] [PubMed] [Google Scholar]
- 8.Fanidi A, et al. Circulating folate, vitamin B6, and methionine in relation to lung cancer risk in the Lung Cancer Cohort Consortium (LC3) J. Natl. Cancer Inst. 2018;110:57–67. doi: 10.1093/jnci/djx119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fanidi A, et al. Is high vitamin B12 status a cause of lung cancer? Int. J. Cancer. 2019;145:1499–1503. doi: 10.1002/ijc.32033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gylling B, et al. One-carbon metabolite ratios as functional B-vitamin markers and in relation to colorectal cancer risk. Int. J. Cancer. 2019;144:947–956. doi: 10.1002/ijc.31606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larose TL, et al. Circulating cotinine concentrations and lung cancer risk in the Lung Cancer Cohort Consortium (LC3) Int. J. Epidemiol. 2018;47:1760–1771. doi: 10.1093/ije/dyy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller DC, et al. Circulating high sensitivity C reactive protein concentrations and risk of lung cancer: Nested case-control study within Lung Cancer Cohort Consortium. BMJ. 2019;364:k4981. doi: 10.1136/bmj.k4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuo H, et al. Vitamin B6 catabolism and lung cancer risk: Results from the Lung Cancer Cohort Consortium (LC3) Ann. Oncol. 2019;30:478–485. doi: 10.1093/annonc/mdz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Midttun O, et al. Circulating concentrations of biomarkers and metabolites related to vitamin status, one-carbon and the kynurenine pathways in US, Nordic, Asian, and Australian populations. Am. J. Clin. Nutr. 2017;105:1314–1326. doi: 10.3945/ajcn.116.151241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuang SC, et al. Circulating biomarkers of tryptophan and the kynurenine pathway and lung cancer risk. Cancer Epidemiol. Biomarkers Prev. 2014;23:461–468. doi: 10.1158/1055-9965.EPI-13-0770. [DOI] [PubMed] [Google Scholar]
- 16.Chaleckis R, Murakami I, Takada J, Kondoh H, Yanagida M. Individual variability in human blood metabolites identifies age-related differences. Proc. Natl. Acad. Sci. U. S. A. 2016;113:4252–4259. doi: 10.1073/pnas.1603023113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galan P, et al. Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur. J. Clin. Nutr. 2005;59:1181–1190. doi: 10.1038/sj.ejcn.1602230. [DOI] [PubMed] [Google Scholar]
- 18.Bashar SK, Mitra AK. Effect of smoking on vitamin A, vitamin E, and other trace elements in patients with cardiovascular disease in Bangladesh: A cross-sectional study. Nutr. J. 2004;3:18. doi: 10.1186/1475-2891-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu F, et al. Cigarette smoking behaviour and blood metabolomics. Int. J. Epidemiol. 2016;45:1421–1432. doi: 10.1093/ije/dyv330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krumsiek J, et al. Gender-specific pathway differences in the human serum metabolome. Metabolomics. 2015;11:1815–1833. doi: 10.1007/s11306-015-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller DC, et al. No association between circulating concentrations of vitamin D and risk of lung cancer: An analysis in 20 prospective studies in the Lung Cancer Cohort Consortium (LC3) Ann. Oncol. 2018;29:1468–1475. doi: 10.1093/annonc/mdy104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tungtrongchitr R, et al. Relationship of tobacco smoking with serum vitamin B12, folic acid and haematological indices in healthy adults. Public Heal. Nutr. 2003;6:675–681. doi: 10.1079/PHN2003483. [DOI] [PubMed] [Google Scholar]
- 23.Theofylaktopoulou D, et al. A community-based study on determinants of circulating markers of cellular immune activation and kynurenines: The Hordaland Health Study. Clin. Exp. Immunol. 2013;173:121–130. doi: 10.1111/cei.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schennach H, et al. Factors influencing serum neopterin concentrations in a population of blood donors. Clin. Chem. 2002;48:643–645. doi: 10.1093/clinchem/48.4.643. [DOI] [PubMed] [Google Scholar]
- 25.Diamondstone LS, et al. Factors influencing serum neopterin and β2-microglobulin levels in a healthy diverse population. J. Clin. Immunol. 1994;14:368–374. doi: 10.1007/BF01546321. [DOI] [PubMed] [Google Scholar]
- 26.Sulo G, et al. Neopterin and kynurenine-tryptophan ratio as predictors of coronary events in older adults, the Hordaland Health Study. Int. J. Cardiol. 2013;168:1435–1440. doi: 10.1016/j.ijcard.2012.12.090. [DOI] [PubMed] [Google Scholar]
- 27.Gonçalves RB, et al. Impact of smoking on inflammation: Overview of molecular mechanisms. Inflamm. Res. 2011;60:409–424. doi: 10.1007/s00011-011-0308-7. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida K, et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature. 2020;578:266–272. doi: 10.1038/s41586-020-1961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tse LA, et al. Smoking cessation sharply reduced lung cancer mortality in a historical cohort of 3185 Chinese silicotic workers from 1981 to 2014. Br. J. Cancer. 2018;119:1557–1562. doi: 10.1038/s41416-018-0292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EFM. Systemic effects of smoking. Chest. 2007;131:1557–1566. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- 31.Locasale JW. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dierkes J, et al. Factors explaining the difference of total homocysteine between men and women in the European investigation into cancer and nutrition Potsdam study. Metabolism. 2001;50:640–645. doi: 10.1053/meta.2001.23286. [DOI] [PubMed] [Google Scholar]
- 33.Awwad HM, Kirsch SH, Geisel J, Obeid R. Measurement of concentrations of whole blood levels of choline, betaine, and dimethylglycine and their relations to plasma levels. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014;957:41–45. doi: 10.1016/j.jchromb.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 34.Bertoia ML, et al. Plasma homocysteine, dietary B vitamins, betaine, and choline and risk of peripheral artery disease. Atherosclerosis. 2014;235:94–101. doi: 10.1016/j.atherosclerosis.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao X, Randell E, Zhou H, Sun G. Higher serum choline and betaine levels are associated with better body composition in male but not female population. PLoS ONE. 2018;13:e0193114. doi: 10.1371/journal.pone.0193114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansson M, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303:2377–2385. doi: 10.1001/jama.2010.808. [DOI] [PubMed] [Google Scholar]
- 37.Theofylaktopoulou D, et al. Vitamins B2 and B6 as determinants of kynurenines and related markers of interferon-γ-mediated immune activation in the community-based Hordaland Health Study. Br. J. Nutr. 2014;112:1065–1072. doi: 10.1017/S0007114514001858. [DOI] [PubMed] [Google Scholar]
- 38.Oregon State University . Obese People Need More Vitamin E, But Actually Get Less. ScienceDaily; 2015. [Google Scholar]
- 39.White E, et al. Correlates of serum alpha- and gamma-tocopherol in the Women’s Health Initiative. Ann. Epidemiol. 2001;11:136–144. doi: 10.1016/S1047-2797(00)00189-7. [DOI] [PubMed] [Google Scholar]
- 40.Lagunova Z, Porojnicu AC, Lindberg F, Hexeberg S, Moan J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009;29:3713–3720. [PubMed] [Google Scholar]
- 41.de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III) Am. J. Kidney Dis. 2007;50:69–77. doi: 10.1053/j.ajkd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Ginde AA, Liu MC, Camargo CA. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch. Int. Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Timpson NJ, et al. C-reactive protein levels and body mass index: Elucidating direction of causation through reciprocal Mendelian randomization. Int. J. Obes. 2011;35:300–308. doi: 10.1038/ijo.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones CA, et al. Serum creatinine levels in the US population: Third National Health and Nutrition Examination Survey. Am. J. Kidney Dis. 1998;32:992–999. doi: 10.1016/S0272-6386(98)70074-5. [DOI] [PubMed] [Google Scholar]
- 45.Schulze F, et al. Determination of a reference value for N(G), N(G)-dimethyl-L-arginine in 500 subjects. Eur. J. Clin. Invest. 2005;35:622–626. doi: 10.1111/j.1365-2362.2005.01561.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the Lung Cancer Cohort Consortium are not publicly available because approval for analysis must be obtained from each participating cohort. Researchers who are interested in analyzing the LC3 data are encouraged to contact the corresponding author.