Abstract
Background
Hypothermia-treated and intubated infants with moderate or severe hypoxic-ischemic encephalopathy (HIE) usually receive morphine for sedation and analgesia (SA) during therapeutic hypothermia (TH) and endotracheal ventilation. Altered drug pharmacokinetics in this population increases the risk of drug accumulation. Opioids are neurotoxic in preterm infants. In term infants undergoing TH, the long-term effects of morphine exposure are unknown. We examined the effect of opioid administration during TH on neurodevelopmental outcome and time to extubation after sedation ended.
Methods
In this prospectively collected population-based cohort of 282 infants with HIE treated with TH (2007–2017), the cumulative opioid dose of morphine and equipotent fentanyl (10–60 µg/kg/h) administered during the first week of life was calculated. Clinical outcomes and concomitant medications were also collected. Of 258 survivors, 229 underwent Bayley-3 neurodevelopmental assessments of cognition, language and motor function at 18–24 months. Multivariate stepwise linear regression analysis was used to examine the relation between cumulative opioid dose and Bayley-3 scores. Three severity-groups (mild-moderate-severe) were stratified by early (<6 h) amplitude-integrated electroencephalography (aEEG) patterns.
Findings
The cumulative dose of opioid administered as SA during TH was median (IQR) 2121 µg/kg (1343, 2741). Time to extubation was independent of SA dose (p > 0.2). There was no significant association between cumulative SA dose and any of the Bayley-3 domains when analysing the entire cohort or any of the aEEG severity groups.
Interpretation
Higher cumulative opioid doses in TH-treated infants with HIE was not associated with worse Bayley-3 scores at 18–24 months of age.
Funding
The Bristol cooling program was funded by the Children's Medical Research Charity SPARKS managing donations for our research from the UK and US, the UK Moulton Foundation, the Lærdal Foundation for Acute Medicine in Norway and the Norwegian Research Council (JKG).
Research in context.
Evidence before this study
Neonatal hypoxic-ischaemic encephalopathy (HIE) is usually caused by insufficient cerebral perfusion and oxygen supply around the time of birth. Infants diagnosed with moderate or severe HIE are treated with therapeutic hypothermia (TH) for 3 days, which reduces brain injury and improves long-term outcome. During TH, infants are cooled to 33.5 °C core temperature and undergo sedation and analgesia (SA) with morphine to relieve discomfort from cooling and endotracheal ventilation. There are concerns whether morphine may be harmful to the term-born injured brain. In preterm infants, prolonged morphine exposure is associated with poor neurodevelopmental outcomes. In experimental models of HIE, the effects of opioid exposure on the brain are inconclusive. On one hand, opioids cause neuronal death in the cerebellum and on the other, they stimulate microglia-mediated brain repair following injury. In infants with HIE treated with TH, the long-term effects of morphine exposure in high doses on neurodevelopmental outcomes are unknown.
Added value of this study
We examined the dose-dependent relationship between opioid-exposure during TH and Bayley-3 developmental scores at 18–24 months in 282 infants over an 11-year period. Morphine was the most commonly used opioid, though 11% of the infants also received fentanyl. We show an annual increase in the dose of opioids given due to a change in clinical practice. Asphyxia severity and the duration of ventilation remained largely unchanged. Overall, at 18–24 months, we found no adverse effects of morphine exposure in high doses during TH on neurodevelopmental outcome.
Implications of all the available evidence
We found no adverse effects of continuous morphine and fentanyl delivery on developmental scores at 18–24 months. There was no relation between opioid dose received and time to extubation after rewarming. As data on fentanyl in larger cohorts are still lacking, we recommend the use of morphine. More reliable assessments of stress and discomfort during TH are needed to support clinical decision-making.
Alt-text: Unlabelled box
Introduction
Infants with hypoxic-ischaemic encephalopathy (HIE) treated with therapeutic hypothermia (TH) require sedation and analgesia (SA) to ameliorate the discomfort and pain from cooling, intubation and mechanical ventilation [1]. Morphine is the most frequently used analgesic drug in this population [2]. To date, there are no standardized dosing guidelines for SA during TH. Out of four early randomized controlled cooling trials [[3], [4], [5], [6]] only the European hypothermia trial recommended a standard dose of morphine at 100 µg/kg every 4 h or combined with fentanyl at equipotent doses. There are no cohort studies with sufficient sample size that have examined the dose-dependent effect of neonatal morphine exposure on neurodevelopmental outcome in term-born asphyxiated infants treated with TH.
Morphine exposure is associated with adverse neurodevelopmental outcome in preterm and very preterm infants. In very preterm infants (median gestational weeks (GW) = 27.4), morphine dose (median cumulative morphine dose 1905 µg/kg) administered from birth to term-equivalent age was associated with smaller cerebella at term-equivalent age and poorer motor and cognitive outcomes at 18 months [7]. In the NEOPAIN trial of preterm infants [8], those treated with morphine had 7% smaller head circumferences and lower body weights (−4%) at school age. Morphine-exposed individuals also exhibited longer latencies in decision-making, but with no effect on the intelligence quotient or academic achievement [9]. Neonatal pain is also potentially harmful to the developing brain. For example, in very preterm infants (24–32 GW), the number of painful procedures during intensive care correlated with smaller cerebral cortex [10], cerebellum[11] and subcortical structures [12] at school-age.
Opioid-receptors are widespread in the brain and participate in regulating neurodevelopment. From our own work, in a global hypoxic-ischaemic model in newborn pigs, lack of SA during 24 h of post-insult TH completely abolished the neuroprotective effect of cooling [13]. The cooled unanaesthetised pigs had increased heart rate (HR) and four times the cortisol levels as a sign of stress, as compared to cooled anaesthetised pigs [14]. SA during TH improved neuroprotection in this model. In another study, 24 h of fentanyl infusion in healthy newborn pigs without HIE induced cell death in the cerebellar granular cell layer [15], while other regions were spared [16]. Differences between species in developmental milestones and opioid receptor type and distribution makes it challenging to assess opioid neurotoxicity in human newborns compared to other species.
The pharmacokinetics and pharmacodynamics of opioids and their metabolites are temperature-dependent. In asphyxiated infants treated with TH, morphine metabolism is reduced because of cooling and hypoxic-ischaemic liver and renal injury [17,18]. Prolonged SA during TH may lead to toxic accumulation of the sedative and its metabolites, as reported from a single site in the TOBY trial [19]. Morphine receptor affinity is reduced by 80% at 30 °C as compared to 37 °C in the guinea pig ilium [20]. If this is translatable to cooled infants, 3.5 °C reduction in body temperature would lower the potency of morphine by 40%.
The long-term effects into childhood and adolescence of morphine exposure in term-born cooled asphyxiated infants are unknown. Given the adverse effects of SA on the developing brain in experimental studies and preterm infants, we hypothesized that higher cumulative SA doses during cooling and rewarming will impair cognitive ability and language in late infancy. We investigated whether there is a dose-dependent effect of SA on Bayley-3 scores at 18–24 months in a cohort of 282 cooled asphyxiated infants. During normothermia, high doses of opioids are a respiratory depressant. We investigated whether high SA dose delayed extubation after rewarming.
Methods
Cohort
We analysed a prospectively collected population-based cohort of 361 (suppl. 1) term infants (GW ≥36 weeks) born between 01/01/2007 and 31/12/2019. Ethical approval was obtained from the NHS South West-Central Bristol Research Ethics Committee (CH/2009/3091), the Health Research Authority and was sponsored by the University of Bristol. The anonymized cohort study had waiver of consent. Infants were either inborn or transferred to St. Michael's Hospital, Bristol, UK for whole-body cooling (active cooling to 33.5 °C for 72 h with servo-controlled equipment, CritiCool, Mennen Medical, Israel). St. Michael's hospital is a level 4 NICU and one of two regional cooling centres among eight hospitals with 30 000 annual deliveries in the Southwest of England. Inclusion criteria for TH followed the TOBY protocol [21]: in infants ≥36weeks with reduced consciousness and A) at least one of the following: Apgar10min ≤5, need for assisted ventilation 10 min after birth, pH <7.0 or base excess (BE) ≤−16 mmol/L within the first hour after birth; B) clinical presentation of moderate (HIE-2) or severe encephalopathy (HIE-3); or C) moderately or severely abnormal amplitude-integrated electroencephalography (aEEG) background voltage pattern within 6 h of birth or aEEG-confirmed seizures lasting ≥3 min within 1 h with any background pattern. All infants <6 h old who fulfilled these entry criteria, including other comorbid diagnoses such as postnatal collapse, were offered TH [22]. Twenty-two infants born 2014–2017 with mild encephalopathy (HIE-1) were removed from the dataset. Infants born between 01/01/2018 and 31/12/2019 were excluded from the main analyses and are only presented in Fig. 1 (n = 57). Neurodevelopmental outcome was not yet available for these infants, leaving 282 patients for the main analysis, of which 258 survived.
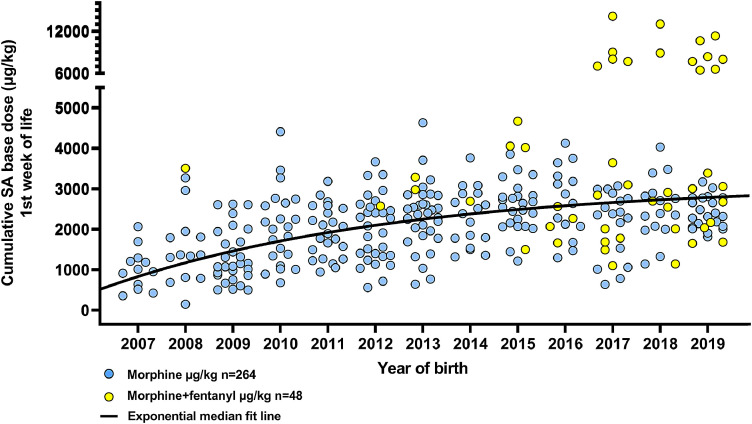
Fig. 1.
Cumulative SA µg/kg administered during the first week of life shows a monotonous increase over years. Additional data from surviving infants born in 2018–2019 (n = 57) are included to show the progression of morphine and fentanyl use over time. N = 25 infants received additional fentanyl in 2007 to 2017, n = 23 received additional fentanyl in 2018 to 2019. Deceased infants are excluded from figure (n = 29).
The Bristol TH protocol [23] includes starting passive cooling early (<1 h) and routinely intubating cooled infants. aEEG was monitored before and during TH until after rewarming. Infants received pre-emptive morphine-sulphate with a loading dose of 50 µg/kg followed by a continuous infusion rate of 20 µg/kg/h. The dose was adjusted to clinical needs. In 28 patients additional opioids (25 fentanyl-citrate, 3 remifentanil) were administered (boli or IV continuous infusions). Pain was assessed by the bedside health professionals using clinical experience, facial pain score [2] and heart rate changes as a marker for stress or discomfort. A standard escalating protocol for anticonvulsant drugs was followed during the study period: phenobarbital, phenytoin, clonazepam, lidocaine or midazolam [23].
At 18–24 months of age, survivors were scheduled for follow-up in the cooling programme with a senior neurodevelopmental physiotherapist (SJ) for assessment of motor, cognition and language skills with Bayley-3 Scales of Infant and Toddler Development 3rd edition [24] (mean 100, standard deviation 15). Bayley-3 scores <85 in any domain are defined as adverse outcome in this study. The examiner was blinded to information on clinical treatment and results of investigations (e.g. MRI) at the time of examination. Scores <85 in any domain are indicative of adverse outcome. Other clinical examination and information at ≥2 years were undertaken for 29 infants without Bayley-3 examination.
Data collection
We collected information on drugs administered up to the eighth day of life. All authors had access to the data. Morphine-sulphate doses were converted to morphine-base doses by a factor of 0.853. Fentanyl-citrate doses were converted to fentanyl-base by a factor of 0.673. We calculated the cumulative morphine dose µg/kg administered as IV or bolus during the first week of life. The cumulative SA dose was calculated by converting fentanyl and remifentanil to morphine equipotent doses by a factor x40 and x80 respectively and added to the morphine dose. Cumulative SA therefore includes all morphine, fentanyl and remifentanil doses administered. We also collected muscle relaxants, inotropic and anticonvulsant drugs and total duration of SA use and duration of intubation. As a marker of stress, mean HR was registered during stable periods at the following intervals: 18–24 h, 42–48 h, 66–72 h and 84–90 h after commencing active cooling, and at 12–18 h after cooling was stopped. We have previously shown that inotropic drugs increase HR, and that ≥2 boli of atropine increase HR and morphine dose administered [25].
Statistics
In order to detect any potential confounders between recorded variables, we performed a multivariate stepwise linear regression (SPSS Statistics v.26.0) with Bayley-3 scores (cognition, language or motor) as dependent variable, and included the following independent variables: cumulative SA dose, year of birth, duration of cardiopulmonary resuscitation (CPR) in minutes, assisted ventilation (intubation or bagging) at 10 min, sex, being outborn, weight-centile, Apgar at 10 min, worst pH and BE at <1 h, HIE-grade, worst aEEG pattern at <6 h, number of inotropic drugs, number of anticonvulsant drugs, lactate dehydrogenase at 72 h, time to lactate <5 mmol/l and intubation duration. Variables in the final regression model were included at significance level < 0.05. In every regression performed, only 1–3 variables were significant; number of anticonvulsants used, worst aEEG pattern at <6 h and lactate dehydrogenase at 72 h. All cumulative SA doses >5500 µg/kg were set to 5500 µg/kg in the regression analysis. Because the effect of SA on Bayley 3 scores may be severity-dependent, we also conducted a stratified multivariate linear regression. The worst aEEG pattern <6 h of age is the best early outcome predictor among the entry criteria [27]. The study population was stratified into three severity subgroups: mild (CNV, CNVsz, DNV n = 109), moderate (BS n = 110) and severe (LV, FT n = 39). For standardised comparison between survivors and deceased infants, we compared the mean cumulative SA dose administered per hour during SA administration using the two-sample Wilcoxon Mann-Whitney test.
As above, to examine which clinical variables were associated with cumulative SA dose, a multivariate stepwise linear regression was conducted with cumulative SA dose as dependent variable, and the listed independent variables. To detect a change over the study period, univariate linear regression analysis was used with year as the independent variable. The results present which variables changed over time. Since the data were non-normally distributed, we used non-parametric analysis with Wilcoxon Mann-Whitney to detect significant differences in two-sample situations. Correlation between two continuous variables was tested with Kendell's correlation coefficient. Ranges are given in either interquartile ranges or 95% confidence intervals (CI). Figures and graphs were created in GraphPad Prism v.8.0.3. Missing values were replaced in n subjects by using the median value for the whole cohort regards Apgar10min (n = 10) and intubation duration (n = 2). For the following variables we used the median in the patients aEEG subgroup classification; LDH72h (n = 10), pH (n = 1), hours when the plasma lactate value was reduced to <5 mmol/L (n = 1) and for duration in CPR, we replaced with the median of those who received CPR (n = 1).
Role of the funding source
The funding source had no role in the study design, the data analysis or data interpretation.
Results
We included 282 infants born between 2007 and 2017 in the analysis. Table 1 displays the neonatal clinical characteristics. Twenty-four infants died, 21 in the neonatal period and 3 at the ages of 6 months, 22 months and 8 years. The median (IQR) cumulative SA dose was 1200 µg/kg (507, 2403), not significantly different from survivors when the dose of received SA was standardised per hour, median (IQR) 24.6 µg/kg/h (15.4, 34.5) in deceased vs 23.0 µg/kg/h (15.5, 31.1), p = 0.66 in survivors.
Table 1.
Clinical characteristics for n = 282 infants, born 01/01/2007–31/12/2017.
| Baseline characteristics 2007 to 2017 (n = 282) | |
| Outborn, number (%) | 159/282 (56.4) |
| Male, number (%) | 159/282 (56.4) |
| Gestational age in weeks, median (IQR) | 40.0 (38.3, 41.0) |
| CPR at birth, number (%) | 103/282 (36.5) |
| Duration (min) of CPR, median (IQR) | 0.0 (0.0, 2.3) |
| Needing ventilation (intubated or bagged) at 10 min, number (%) | 222/282 (78.7) |
| Birth weight in grams, median (IQR) | 3361 (2983, 3780) |
| Apgar 10 min, median (IQR) | 6 (4, 8) |
| Apgar 10 min ≤5, number (%) | 105/282 (37.2) |
| Worst pH within 1 h of birth, median (IQR) | 6.96 (6.85, 7.09) |
| Worst pH within 1 hour of birth <7.0, number (%) | 156/282 (55.3) |
| Worst base excess within 1 h of birth, median (IQR) | −16.0 (−12.6, −20.5) |
| Worst base excess within 1 h of birth ≤−16 mmol/L, number (%) | 138/282 (48.9) |
| Normal aEEG voltage*, number (%) | 9/282 (3.2) |
| Moderately abnormal aEEG voltage*, number (%) | 219/282 (77.7) |
| Severely abnormal aEEG voltage*, number (%) | 54/282 (19.1) |
| HIE grade 2, number (%) | 186/282 (66.0) |
| HIE grade 3, number (%) | 96/282 (34.0) |
| Death, number (%) | 24/282 (8.5) |
| Mechanical ventilation and SA during 1st week of life in survivors (n = 258) | |
| Intubation duration in hours, median (IQR) | 96 (86, 112) |
| Duration (h) end of active cooling to extubation, median (IQR) | 23 (14, 35) |
| Duration (h) end of SA delivery to extubation, median (IQR) | 7 (2, 16) |
| Sedation duration, median (IQR) | 86 (79, 97) |
| Cumulative morphine µg/kg, median (IQR) | 2052 (1291, 2628) |
| Additional fentanyl or remifentanil, number (%) | 28/258 (10.9) |
| Bolus, number (%) | 14/28 (50.0) |
| Continuous infusion, number (%) | 14/28 (50.0) |
| Cumulative non-converted fentanyl (µg/kg) median (IQR) | 7.5 (3.0, 126.1) |
| Cumulative converted SA µg/kg, median (IQR) | 2121 (1343, 2741) |
*Voltage classification of aEEG for entry-criteria (pattern classification groups). Normal aEEG voltage: Continuous Normal Voltage (CNV). Moderately abnormal aEEG voltage: continuous normal voltage with seizures (CNVsz) or Discontinuous Normal Voltage (DNV), or Burst Suppression (BS).
Severely abnormal aEEG voltage: Low Voltage (LV) or Flat Trace (FT).
Among the 258 survivors, 229 had Bayley-3 assessment at 18–24 months. The remaining 29 were given a binary adverse or favourable outcome based on clinical examination carried out locally or by colleagues in other regions at ≥2 years. With respect to binary outcome, none were lost to follow-up, however, 29 were missing Bayley examination required for some analyses. Main clinical and biochemical variables did not differ between infants given a binary score and infants assessed with Bayley-3. Selected variables in infants with binary score versus Bayley-3 assessment respectively are: worst pH < 1 h (p = 0.86), aEEG at entry (p = 0.88), cumulative SA (p = 0.19, median (IQR) 2013 µg/kg (1280, 2340) versus 2158 µg/kg (1350, 2771)) and HIE-grade (p = 0.16, 56% HIE-2 versus 69% HIE-2). The mean Bayley-3 scores for cognition, language and motor have stayed unchanged over the 11 year duration of this study as shown by linear regression analysis with year as the independent variable (all p-values > 0.6).
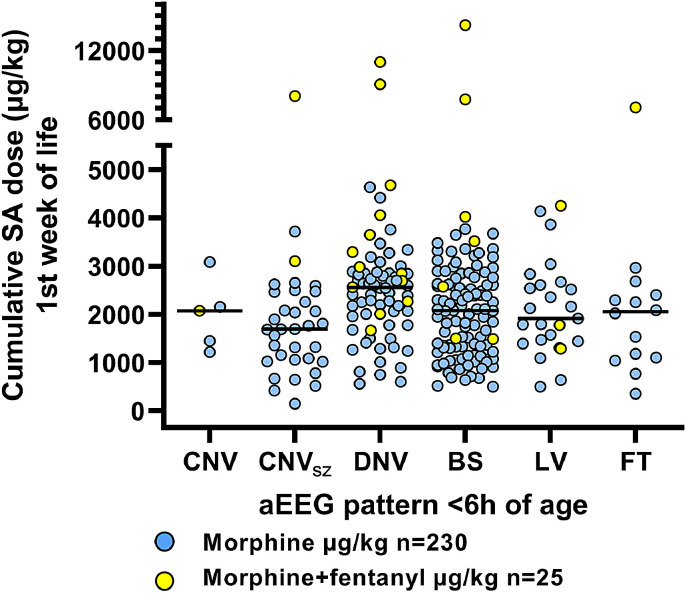
The cumulative SA dose administered during the first week of life was median (IQR) 2121 µg/kg (1343, 2741), with an annual monotonous increase of coefficient +216 µg/kg/year, 95%CI (160, 272) from 2007 to 2017 (Fig. 1). The duration of SA administration, median (IQR) 86 h (79, 97), remained unchanged across the study period: coefficient +0.036 h/year, 95%CI (−0.916, 0.988). The use of fentanyl (n = 25) and remifentanil (n = 3) has increased over the last 4 years (Fig. 1), from nearly no use up to 2014, to 40% of infants receiving additional sedatives by 2017. These infants (n = 28), received median (IQR) 1580 µg/kg morphine (958, 2536), with total median (IQR) SA dose 3045 µg/kg (2029, 4574). There was no difference between the morphine-only group and the group receiving other opioids in their pH at <1 h (p = 0.12, respectively median (IQR) 6.97 (6.86, 7.09) versus 7.0 (6.9, 7.12)), aEEG background pattern at <6 h (Fig. 2, p = 0.11), heart rate (HR) during cooling (p = 0.56), intubation duration (p = 0.95) or SA duration (p = 0.55). However, infants receiving fentanyl also received fewer anticonvulsive drugs (Fig. 3, p = 0.006) and had milder HIE-grade (p = 0.03). Fig. 4 illustrates the temporal relation between active cooling, rewarming, intubation and continuous IV morphine infusion during the first week of life. Eighty-seven% of infants were mechanically ventilated for the entire duration of TH, with median intubation duration of 95 h (84, 112), which is strongly correlated with sedation duration (p < 0.001). Intubation duration did not change over time. We did not observe the expected adverse effect of high SA doses like delayed extubation, neonatal abstinence syndrome (NAS) or urine retention.
Fig. 2.
Cumulative SA dose (µg/kg) distribution for each aEEG pattern classification category. Black line shows the median in each group. This distribution shows that cumulative SA dose is not associated with aEEG severity.
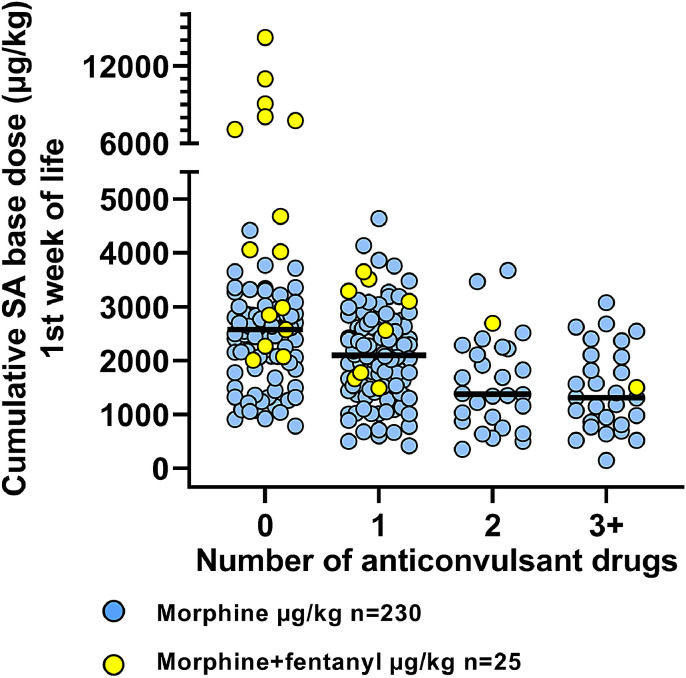
Fig. 3.
Data from 2007 to 2017. Infants with clinical or electrical seizures received from 1 to 5 number of different anticonvulsive drugs of which phenobarbital, clonazepam and midazolam also have a sedative effect. Infants with more anticonvulsants received less morphine (−530 µg/kg per anticonvulsant drug, p < 0•001).The black line shows the median. Multiple administrations of the same drug counts as one drug.
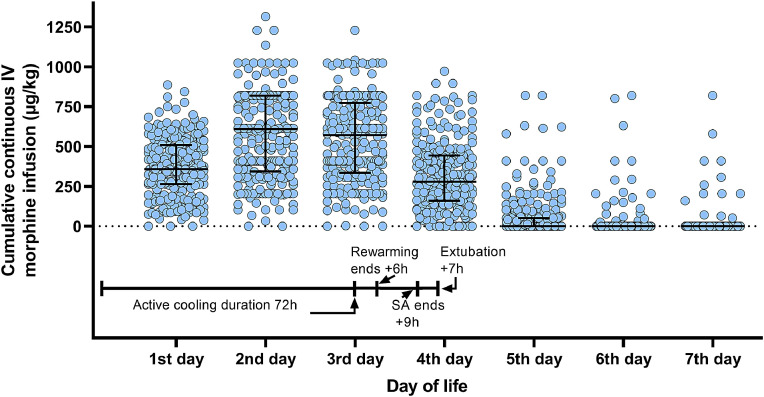
Fig. 4.
Timeline over the first week of life shows scatter plot of cumulative continuous IV morphine infusion administered each day, excluding deceased infants and those receiving fentanyl or remifentanil. Lines show median and interquartile range.
We examined which clinical factors were associated with the cumulative SA dose administered. Among the early clinical variables (<6 h of age), the cumulative SA dose was only significantly associated with year of birth (coefficient +229 µg/kg/year, 95%CI (170, 288)). aEEG pattern <6 h of age was not associated with cumulative SA dose (p = 0.5, Fig. 2). After allowing clinical variables obtained after 6 h of age into the regression analysis, year of birth was still the most significant variable, followed by number of anticonvulsant drugs administered (Fig. 3) (coefficient −372 µg/kg/drug, 95%CI (−525, −219)) and longer intubation duration (coefficient +6.66 µg/kg/h, 95%CI (1.8, 11.5)).
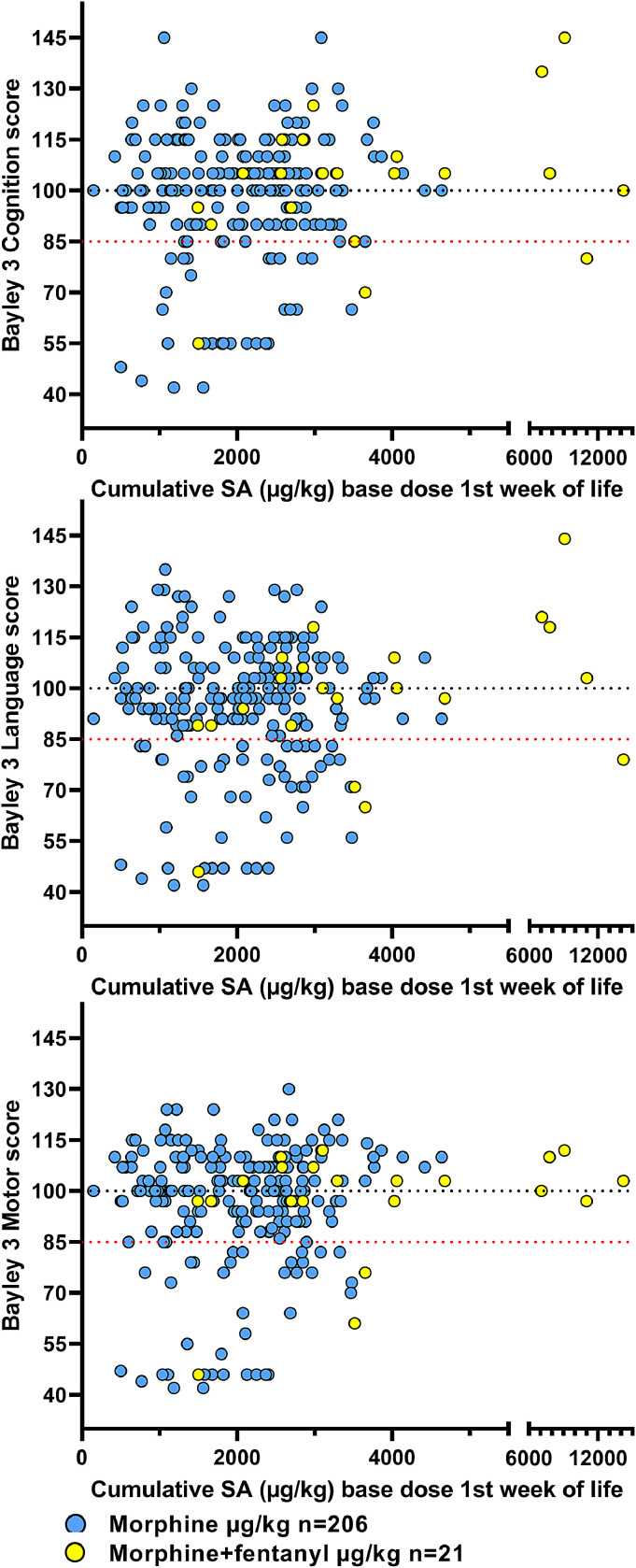
Stepwise linear regression analysis of each Bayley-3 domain as the dependent variable was conducted in the entire cohort and in each of the 3 aEEG-severity subgroups. There was no significant association between cumulative SA dose and Bayley-3 scores in any of the Bayley-3 domains (Fig. 5). The strongest clinical predictors of adverse Bayley-3 scores in our cohort are further explored in a logistic regression model presented in our reference [26].
Fig. 5.
Individual Bayley-3 scores at 18–24 months in cognition, language and motor domain. The black dotted line shows the predicted mean of the score (100), and the red dotted line shows the cut off value (85) for adverse outcome used. Scores below the lowest testable value were obtained by ranking the raw scores in each domain [26].
Discussion
The long-term effects of morphine exposure in term-born cooled asphyxiated infants are unknown. Our hypothesis was that higher cumulative SA doses during the first week of life could impair cognitive ability and language in infancy. We found no evidence for an adverse dose-dependent effect of morphine and fentanyl exposure on Bayley-3 scores in cognition, language or motor function at 18–24 months of age. The infants who died did not receive more opioids than the survivors and there was no overdosing of deceased infants. We had a concern for confounding regarding the less severe infants with milder encephalopathy and potentially stronger clinical expression of discomfort, thereby receiving higher cumulative SA doses. These are also the infants with expected good outcome. To reduce this confounding, we included known severity variables and outcome predictors, such as early aEEG, number of anticonvulsant drugs needed and LDH at 72 h. Because the coefficient for cumulative SA dose in this multivariate linear regression alternated between being positive and negative, this indicated that even if such confounders were to be present, their effect would be small.
Recently published studies in cooled asphyxiated infants support our general findings. In a secondary retrospective analysis of the RCT NICHD-trial [28] from 2005, infants were treated with normothermia or TH. SA was collected as a categorical variable without dose or duration and they report no effect of SA on Bayley-2 MDI-scores at 18–24 months. Similarly, in the secondary analysis of the MARBLE study no effect of similar categorically collected SA on Bayley-3 scores at 20–24 months was reported [29].
In our study, we collected hourly data of ventilation and all drug administration for seven days and not just the duration of cooling and rewarming to include infants with delayed extubation due to disease severity.
Sedation was stopped at a median of 7 h after rewarming and infants were extubated at a median of 9 h after end of sedation. There was no relationship between cumulative SA-dose and time at extubation as shown in Fig. 4.
Longer intubation durations required longer SA duration, and mechanical ventilation is one of the main reasons for delivering SA during cooling. Infants with seizures who received sedative anticonvulsant drugs, received lower cumulative SA dose. Seizures are common in an asphyxia cohort; phenobarbital was given to 60%, clonazepam to 14% and midazolam to 5% and the sedative effect of these drugs are likely to reduce the need for SA. Very severely asphyxiated infants are more encephalopathic, expressing less clinical signs of stress, discomfort and pain and therefore need less SA. A subgroup of the patients were paralysed and needing paralysis resulted in higher SA doses. This is due to clinical practice in paralysed patients and adds to the variability in SA administration.
The choice of morphine only or morphine and fentanyl as the opioid used reflects a change in practice among medical staff toward the end of the recruitment-period. There was no change in the protocol sedation guidelines. Morphine safety-data are available from a few studies which report plasma levels in relation to outcome. Our most recent SA data on surviving infants born in 2018–2019 (n = 57) show that the use of fentanyl (n = 23) in addition to morphine is continuing (Fig. 1) and in 50% of the patients. Infants who received additional fentanyl or remifentanil are evenly distributed among the range of aEEG severity patterns (Fig. 2) and Bayley 3 scores in all domains (Fig. 5).
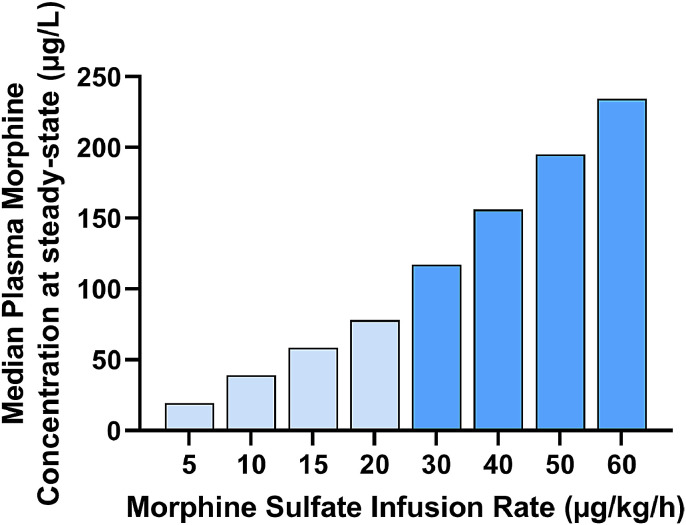
In a prospective pharmacokinetic study of 20 term-born infants, morphine was administered during TH for SA, plasma concentrations were measured to create a simulated pharmacokinetic model. At an infusion rate of 5 µg/kg/h, 81% of neonates achieved target morphine concentrations of 10–40 ng/ml. At an infusion rate of 10 µg/kg/h, 54% were in the target zone, while 46% had morphine concentrations >40 ng/ml [17]. Because the infusion rates in our cohort often are much higher, we extrapolated from the Frymoyer et al. pharmacokinetic model the steady-state morphine plasma concentrations for infusion rates of 30 to 60 µg/kg/h (Fig. 6). The predicted median morphine concentrations ranged from almost 120 to 240 ng/ml, which is much higher than the proposed target concentration. If this was true, it is surprising we found no delay in extubation after weaning of SA. It is unknown whether the reduced receptor affinity seen experimentally during cooling [20] is translatable to humans.
Fig. 6.
Estimated median morphine plasma concentrations at steady-state for various hourly infusion rates, for birthweight=3500 g using clearance (L/h) = 0.765 as reported by Frymoyer [17]. A proposed therapeutic target plasma concentration is 10–100 µg/L (light blue bars). Periodically, infusion rates as high as 60 µg/kg/h were given in our cohort (medium blue bars).
Even though we found no effect on neurodevelopmental outcome at 18–24 months, the increasing use of morphine and fentanyl during TH is concerning. It is still unknown whether opioids may impair higher order cognitive functions later in childhood (executive functions e.g. goal orientation, adaptive behaviour, etc.) which can be tested at early school age. In a RCT of preterm infants (median 29 GW) randomized to SA with morphine 100 µg/kg + 10 µg/kg/h (n = 73), or placebo (n = 77), no effect on intelligence, visual motor integration or behaviour was found at 5-year follow up [30]. A significant negative effect was found in a subtest of the intelligence test, which assessed response inhibition (a component of executive functions), however this was negated when retested at 8–9-years [31].
In our study, exact doses of morphine, fentanyl and remifentanil were collected for 7 days. This is a unique database because of increasing SA use over the years. The limitations of our study are that we were unable to measure the morphine plasma concentrations during TH and that we did not have a reliable pain score for cooled infants as all facial pain scores were recorded as zero. From 2014, the patients being offered TH also incorrectly included 22 with HIE-1. We have removed these from the analysis so all children fulfilled the TOBY entry criteria. We presupposed that the cumulative SA dose most precisely reflects drug exposure, as it incorporates both dosage and duration. However, it is unknown whether the peak plasma concentration or the plasma concentration over time (area under the curve) is the most toxic for the developing brain. It is also an unanswered question whether cooling reduces opioid receptor binding and hence drug potency. Further research with long term follow-up is necessary before concluding that high opioid doses are safe for term born infants.
Declaration of Competing Interest
AF reports personal fees as a scientific consultant for Takeda Pharmaceuticals unrelated to this work. All other authors have nothing to declare.
Acknowledgments
Acknowledgments
We thank our funders, clinical collaborators and parents of patients for their support in carrying out treatment and for prospective data collection of infants undergoing Therapeutic Hypothermia as standard of care at St Michael's Hospital, Bristol since December 2006.
Authors’ contributions
All authors fulfil the four ICMJE criteria with 1: Substantial contribution to the conception, design and analysis of the work (MT, JKG, LW) and acquisition and interpretation of data (MT, JKG, LW, ESB, AF, SJ, EC). 2: Drafting the work or revising it critically for important intellectual content (MT, JKG, LW, AF, DAM, EC). 3: Final approval of the version to be submitted (MT, JKG, LW, AF, DAM, EC, SJ, ESB) and 4: Agreement to be accountable for all aspects of the work (MT, JKG, LW, AF, DAM, EC, SJ, ESB).
Funding
We thank the NHS and our Universities and funders in UK and Norway: SPARKS, The Moulton Foundation, The Norwegian Research Council, The Neonatal Society UK and charitable donations to cooling therapy. The staff maintaining the database were supported by the UK children's charity SPARKS and a linked external charitable donation. Equipment used to cool the patients and the aEEG machines to monitor the brain were supported by the Moulton foundation, the Lærdal foundation and charitable donations. Using the MRI-scanners were supported by grants, the NHS and the University of Bristol. Clinical and research staff were supported by the NHS, the University of Bristol and the University of Oslo through their salaries.
Data sharing statement
The datasets analysed in the current study are not publicly available due to restricted access until Apr1st 2023, when further information about the datasets will be made available from the corresponding author on reasonable request.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100892.
Appendix. Supplementary materials
References
- 1.Szakmar E., Jermendy A., El-Dib M. Respiratory management during therapeutic hypothermia for hypoxic-ischemic encephalopathy. J Perinatol. 2019;39(6):763–773. doi: 10.1038/s41372-019-0349-2. [DOI] [PubMed] [Google Scholar]
- 2.McPherson C., Miller S.P., El-Dib M., Massaro A.N., Inder T.E. The influence of pain, agitation, and their management on the immature brain. Pediatr Res. 2020 doi: 10.1038/s41390-019-0744-6. https://www.nature.com/articles/s41390-019-0744-6.pdf /01/03 ed. 2020 Jan 2; Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gluckman P.D., Wyatt J.S., Azzopardi D., Ballard R., Edwards A.D., Ferriero D.M. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–670. doi: 10.1016/S0140-6736(05)17946-X. /02/22 ed. 2005 Feb 19. [DOI] [PubMed] [Google Scholar]
- 4.Shankaran S., Laptook A.R., Ehrenkranz R.A., Tyson J.E., McDonald S.A., Donovan E.F. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584. doi: 10.1056/NEJMcps050929. /10/14 ed. 2005 Oct 13. [DOI] [PubMed] [Google Scholar]
- 5.Azzopardi D.V., Strohm B., Edwards A.D., Dyet L., Halliday H.L., Juszczak E. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–1358. doi: 10.1056/NEJMoa0900854. /10/03 ed. 2009 Oct 1. [DOI] [PubMed] [Google Scholar]
- 6.Simbruner G., Mittal R.A., Rohlmann F., Muche R. Vol. 126. 2010. pp. e771–e778. (Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. pediatrics. 2010/09/22 ed). Oct. [DOI] [PubMed] [Google Scholar]
- 7.Zwicker J.G., Miller S.P., Grunau R.E., Chau V., Brant R., Studholme C. Smaller cerebellar growth and poorer neurodevelopmental outcomes in very preterm infants exposed to neonatal morphine. J Pediatr. 2016;172:81–87. doi: 10.1016/j.jpeds.2015.12.024. /01/15 ed. 2016 Maye2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anand K.J., Hall R.W., Desai N., Shephard B., Bergqvist L.L., Young T.E. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363(9422):1673–1682. doi: 10.1016/S0140-6736(04)16251-X. /05/26 ed. 2004 May 22. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson S.A., Ward W.L., Paule M.G., Hall R.W., Anand K.J.S. A pilot study of preemptive morphine analgesia in preterm neonates: effects on head circumference, social behavior, and response latencies in early childhood. Neurotoxicol Teratol. 2012;34(1):47–55. doi: 10.1016/j.ntt.2011.10.008. Feb. [DOI] [PubMed] [Google Scholar]
- 10.Ranger M., Chau C.M., Garg A., Woodward T.S., Beg M.F., Bjornson B. Neonatal pain-related stress predicts cortical thickness at age 7 years in children born very preterm. PLoS One. 2013;8(10):e76702. doi: 10.1371/journal.pone.0076702. 2013/11/10 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranger M., Zwicker J.G., Chau C.M., Park M.T., Chakravarthy M.M., Poskitt K. Neonatal pain and infection relate to smaller cerebellum in very preterm children at school age. J Pediatr. 2015;167(2):292–298. doi: 10.1016/j.jpeds.2015.04.055. /05/20 ed. 2015 Auge1. [DOI] [PubMed] [Google Scholar]
- 12.Chau C.M.Y., Ranger M., Bichin M., Park M.T.M., Amaral R.S.C., Chakravarty M. Hippocampus, Amygdala, and Thalamus volumes in very preterm children at 8 years: neonatal pain and genetic variation. Front Behav Neurosci. 2019;13:51. doi: 10.3389/fnbeh.2019.00051. 2019/04/04 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thoresen M., Satas S., Loberg E.M., Whitelaw A., Acolet D., Lindgren C. Twenty-four hours of mild hypothermia in unsedated newborn pigs starting after a severe global hypoxic-ischemic insult is not neuroprotective. Pediatr Res. 2001;50(3):405–411. doi: 10.1203/00006450-200109000-00017. 2001/08/24 edSep. [DOI] [PubMed] [Google Scholar]
- 14.Tooley J.R., Satas S., Porter H., Silver I.A., Thoresen M. Head cooling with mild systemic hypothermia in anesthetized piglets is neuroprotective. Ann Neurol. 2003;53(1):65–72. doi: 10.1002/ana.10402. /01/02 ed. 2003 Jan. [DOI] [PubMed] [Google Scholar]
- 15.Sabir H., Dingley J., Scull-Brown E., Chakkarapani E., Thoresen M. Fentanyl induces cerebellar internal granular cell layer apoptosis in healthy newborn pigs. Front Neurol. 2018;9:294. doi: 10.3389/fneur.2018.00294. 2018/05/17 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabir H., Bishop S., Cohen N., Maes E., Liu X., Dingley J. Neither xenon nor fentanyl induces neuroapoptosis in the newborn pig brain. Anesthesiology. 2013;119(2):345–357. doi: 10.1097/ALN.0b013e318294934d. /04/18 ed. 2013 Aug. [DOI] [PubMed] [Google Scholar]
- 17.Frymoyer A., Bonifacio S.L., Drover D.R., Su F., Wustoff C.J., Van Meurs K.P. Decreased morphine clearance in neonates with hypoxic ischemic encephalopathy receiving hypothermia. J Clin Pharmacol. 2016;57(1):64–76. doi: 10.1002/jcph.775. /05/27 ed. 2017 Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favié L.M.A., Groenendaal F., van den Broek M.P.H., Rademaker C.M.A., de Haan T.R., van Straaten H.L.M. Pharmacokinetics of morphine in encephalopathic neonates treated with therapeutic hypothermia. PloS One. 2019;14(2) doi: 10.1371/journal.pone.0211910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roka A., Melinda K.T., Vasarhelyi B., Machay T., Azzopardi D., Szabo M. Elevated morphine concentrations in neonates treated with morphine and prolonged hypothermia for hypoxic ischemic encephalopathy. Pediatrics. 2008;121(4):e844–e849. doi: 10.1542/peds.2007-1987. /04/03 ed. 2008 Apr. [DOI] [PubMed] [Google Scholar]
- 20.Puig M.M., Warner W., Tang C.K., Laorden M.L., Turndorf H. Effects of temperature on the interaction of morphine with opioid receptors. BJA Br J Anaesth. 1987;59(11):1459–1464. doi: 10.1093/bja/59.11.1459. Nov 1. [DOI] [PubMed] [Google Scholar]
- 21.Azzopardi D., Brocklehurst P., Edwards D., Halliday H., Levene M., Thoresen M. The TOBY Study. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr. 2008;8:17. doi: 10.1186/1471-2431-8-17. /05/02 ed. 2008 Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smit E., Liu X., Jary S., Cowan F., Thoresen M. Cooling neonates who do not fulfil the standard cooling criteria - short- and long-term outcomes. Acta Paediatr. 2014;104(2):138–145. doi: 10.1111/apa.12784. /08/29 ed. 2015 Feb. [DOI] [PubMed] [Google Scholar]
- 23.Vol. 15. 2021. http://thoresen.org.uk/wp-content/uploads/Hypothermia_Bristol_updated_2015.pdf (Hypothermia as a neuroprotective intervention for neonatal encephalopathy). Mar 22. [Google Scholar]
- 24.Albers C.A., Grieve A.J. Test Review: Bayley, N. (2006). Bayley Scales of Infant and Toddler Development, Third Edition. San Antonio, TX: Harcourt Assessment. J Psychoeduc Assess. 2016 https://journals.sagepub.com/doi/10.1177/0734282906297199 Aug 19 [cited 2020 Nov 26]; Available from. [Google Scholar]
- 25.Gill H., Thoresen M., Smit E., Davis J., Liu X., Dingley J. Vol. 85. Resuscitation; 2014. pp. 1394–1398. (Sedation management during therapeutic hypothermia for neonatal encephalopathy: atropine premedication for endotracheal intubation causes a prolonged increase in heart rate). /07/27 ed. 2014 Oct. [DOI] [PubMed] [Google Scholar]
- 26.Thoresen M., Jary S., Walløe L., Karlsson M., Martinez-Biarge M., Chakkarapani E. MRI combined with early clinical variables are excellent outcome predictors for newborn infants undergoing therapeutic hypothermia after perinatal asphyxia. Lancet EClinicalMedicine. 2021;36 doi: 10.1016/j.eclinm.2021.100885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skranes J.H., Lohaugen G., Schumacher E.M., Osredkar D., Server A., Cowan F.M. Amplitude-integrated electroencephalography improves the identification of infants with Encephalopathy for therapeutic hypothermia and predicts neurodevelopmental outcomes at 2 years of age. J Pediatr. 2017;187:34–42. doi: 10.1016/j.jpeds.2017.04.041. /05/28 ed. 2017 Aug. [DOI] [PubMed] [Google Scholar]
- 28.Natarajan G., Shankaran S., Laptook A.R., McDonald S.A., Pappas A., Hintz S.R. Association between sedation-analgesia and neurodevelopment outcomes in neonatal hypoxic-ischemic encephalopathy. J Perinatol. 2018;38(8):1060–1067. doi: 10.1038/s41372-018-0126-7. /05/26 ed. 2018 Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liow N., Montaldo P., Lally P.J., Teiserskas J., Bassett P., Oliveira V. Preemptive morphine during therapeutic hypothermia after neonatal encephalopathy: a secondary analysis. Ther Hypothermia Temp Manag. 2019 doi: 10.1089/ther.2018.0052. /02/27 ed. 2019 Feb 26; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Graaf J., van Lingen R.A., Simons S.H., Anand K.J., Duivenvoorden H.J., Weisglas-Kuperus N. Long-term effects of routine morphine infusion in mechanically ventilated neonates on children's functioning: five-year follow-up of a randomized controlled trial. Pain. 2011;152(6):1391–1397. doi: 10.1016/j.pain.2011.02.017. /03/16 ed. 2011 Jun. [DOI] [PubMed] [Google Scholar]
- 31.de Graaf J., van Lingen R.A., Valkenburg A.J., Weisglas-Kuperus N., Groot Jebbink L., Wijnberg-Williams B. Vol. 154. 2013. Does neonatal morphine use affect neuropsychological outcomes at 8 to 9 years of age? pp. 449–458. (Pain). 2013/01/29 edMar. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.