Abstract
Background
Hydrogen sulphide (H2S) is considered as the third member of the gasotransmitter family, along with nitric oxide (NO) and carbon monoxide. H2S has been reported to induce angiogenesis by promoting the growth, migration and tube-like structure formation of endothelial cells. Those studies were conducted in conditions of cell culture, mouse Matrigel plug assay model, rat wound healing model or rat hindlimb ischaemia model. Recent in vivo studies showed the physiological importance of H2S in muscle angiogenesis. However, the importance of endogenous H2S for brain angiogenesis during development remains unknown. We therefore aimed at determining the role of H2S in brain vascular development.
Methods and results
Both knockdown and knockout of H2S-producing enzymes, cystathionine β-synthase (cbs) and cystathionine γ-lyase (cth), using morpholino oligonucleotides and clustered regularly interspaced short palindromic repeats/Cas9-mediated mutation, impaired brain vascular development of larval zebrafish. Incubation with the slow-releasing H2S donor GYY4137 alleviated the defects of brain vascular development in cbs and cth morphants. Quantitative analysis of the midbrain vascular network showed that H2S enhances angiogenesis without affecting the topological structure of the brain vasculature. Mechanically, nitric oxide synthase 2a (nos2a) expression and NO production were decreased in both cbs and cth morphants. Overexpression of nos2a by coinjection of cbs or cth MO with full-length zebrafish nos2a mRNA alleviated the brain vascular developmental defects in cbs and cth morphants.
Conclusion
We conclude that H2S promotes brain developmental angiogenesis via the NOS/NO pathway in zebrafish.
Keywords: brain, stroke, vascular malformation
Introduction
Since found in the mammalian brain in 1989,1 hydrogen sulphide (H2S) has been attracting continuous attention on its physiological and pathophysiological roles in the central nervous system (CNS). Along with nitric oxide (NO) and carbon monoxide, H2S has been considered to be the third member of the gasotransmitter family.2 Cystathionine β-synthase (CBS) and cystathionine γ-lyase (CTH) are two main H2S-producing enzymes from cysteine and homocysteine in mammalian tissues.3 CBS is abundantly expressed in the brain,4 especially in the hippocampus and the cerebellum.5 In developing mouse brains, H2S is preferentially produced in radial glia/astrocyte lineages.6 7 The expression of CTH is rather widely distributed among peripheral tissues including the liver, pancreas, uterus and intestine.8 Western blotting experiments detected CTH rather than CBS in cerebral microvessels of newborn pigs9 and CTH is the main H2S-producing enzyme in blood vessels.10
In the CNS, the well-studied functions of H2S include the modulation of neurotransmission4 and neuroprotection.11 In the peripheral vascular system, H2S is reported to induce vasorelaxation12 and angiogenesis.13–15 In vitro studies have demonstrated that exogenous H2S at physiologically relevant doses induced angiogenesis by promoting the growth, migration and tube-like structure formation of vascular endothelial cells (ECs),13 14 while in situ studies showed that neovascularisation is promoted by H2S in the mouse Matrigel plug assay model,13 rat wound healing model14 and rat hindlimb ischaemia model.15 Recently, two in vivo studies have shown the importance of H2S in muscle angiogenesis.16 17 However, the importance of endogenous H2S for brain angiogenesis during development remains unknown.
The zebrafish is a well-established vertebrate model for in vivo study of vascular development.18 Its accessible, small and transparent embryo facilitates high resolution in vivo imaging of the brain vasculature.19 In the present study, we used larval zebrafish as a model and found that endogenous H2S plays an important role in the brain vascular development by promoting angiogenesis, and this effect is mainly mediated through the nitric oxide synthase (NOS)/NO pathway.
Methods
Detailed methods are available in the online supplemental material.
svn-2020-000584supp001.pdf (7.4MB, pdf)
In vivo confocal imaging
In vivo long-term serial confocal imaging of the midbrain vasculature was conducted in the same larva during 3–5 days post fertilisation (dpf). Larvae were embedded in 1.2% low-melting agarose (Invitrogen, 16520050) without anaesthetic at room temperature. After being imaged, the 3 or 4—dpf larvae were dug out for husbandry till subsequent imaging of the same larva was performed. Imaging was carried out with an Olympus Fluoview 1000 confocal microscope (Olympus, Japan). XLumplfl 20× (W/IR; NA, 0.95) objective lenses were used. The Z-step of imaging was 3 µm. Raw images were processed with ImageJ. Quantitative analysis of the midbrain vasculature morphology was conducted following the processes previously described.19
Whole-mount in situ hybridization
Zebrafish whole-mount in situ hybridization was performed as previously described.20 21 Probes for the cbs and cth mRNAs were synthesised and labelled with digoxigenin (Roche, Mannheim, Germany). Embryos were incubated with corresponding probes (1 ng/µL) at 68°C overnight. Antidigoxigenin AP-conjungated antibody (1:5000, Roche 11093274910) was used to detect digoxigenin and was further stained with nitro-blue-tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate (NBT/BCIP) solution (Roche, 11681451001).
Microinjection
Zebrafish embryos were microinjected with 8 ng cbs morpholino oligonucleotide (MO), 0.5 ng cth MO, or equivalent Ctrl MO at one-cell stage. The MOs were purchased from Gene Tools (Philomath, Oregon, USA), and the sequences are as follows.
cbs MO: 5′- CTGGCATGGTTTACCCTGACTATCA-3′
cth MO: 5′- GGCTGAGCTGTCGTTCTGCATCTCT-3′
Ctrl MO: 5′- CCTCTTACCTCAGTTACAATTTATA-3′
CRISPR/Cas9-mediated mutation of zebrafish cbs or cth
Mutations of zebrafish cbs or cth genes were induced by using the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 system as previously reported.22–24 Single guide RNA (sgRNA) sequences (cbs: 5′-GATGCTGACGATGTCTTCAA-3′, cth: 5′-CGTTGGTTCAGAGCCCGAGC-3′) were designed to target the sequences of mature cbs and cth. The paired oligonucleotides which contained the cbs or cth sgRNA sequence were annealed and cloned into a pT7-gRNA plasmid. Then, cbs sgRNA and cth sgRNA were synthesised with the MAXIscript T7 kit (Ambion, AM1312M). The synthesised sgRNA was purified with the mirVana miRNA isolation kit (Ambion, AM1560).25 Coinjection of 600 pg Cas9 nuclease (NEB, M0386M) and 80 pg cbs or cth gRNA into zebrafish embryos were conducted at one-cell stage. The cbs or cth gene mutations in F0 embryos were examined by sequencing analysis after in vivo confocal imaging of the brain vasculature. The imaging data of F0 embryos carrying cbs or cth mutations were analysed.
The cbs or cth heterozygous mutant lines were generated by Nanjing XinJia Medical Technology Co. The two lines were separately outcrossed with the Tg(Flk1:eGFP) line. The crossed lines were further incrossed to observe the vascular development in homozygous mutant larvae at 3 and 5 dpf larvae. Genotyping was conducted after in vivo confocal imaging of the brain vasculature. The imaging data of embryos carrying cbs or cth homozygous mutations were analysed.
Drug treatment
GYY4137 (SIGMA, SML0100) was dissolved in zebrafish culture medium at 1 dpf (2000 µM for cbs MO group and 1000 µM for cth MO group). The medium was refreshed once a day and phenotypes were characterised at 3–5 dpf.
Measurement of NO production
Total NO production in the zebrafish larvae was examined with the total NO assay kit (Beyotime Institute of Biotechnology, S0023). It measured the concentration of nitrate and nitrite which are stable metabolites of NO.
Data analysis
All data were represented as mean±SEM. Statistical analysis was performed by using unpaired two-tailed Student’s t-test or One-way analysis of variance.
Results
Endogenous H2S is necessary for the brain vascular development in zebrafish
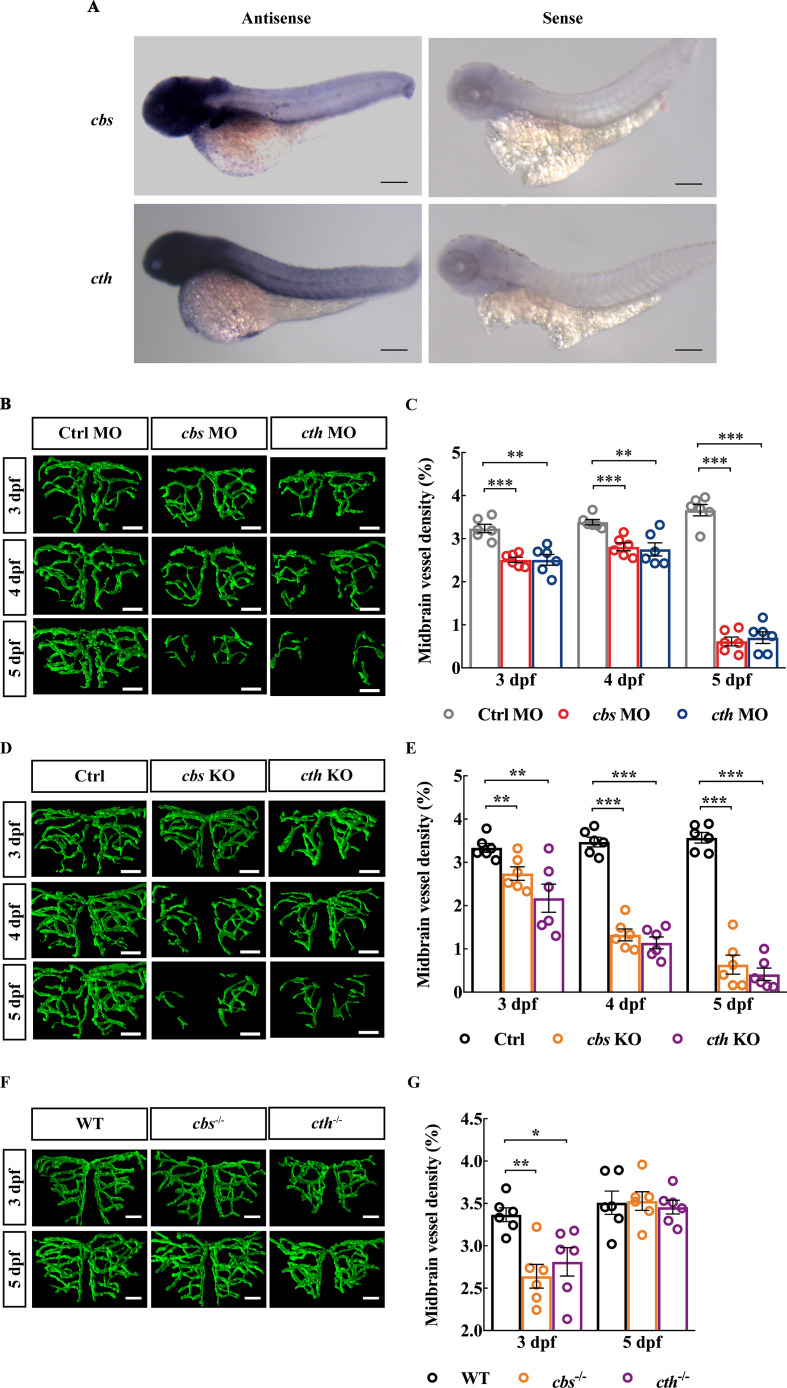
To examine the expression pattern of cbs and cth in zebrafish, we conducted whole-mount in situ hybridisation in larval zebrafish at 3 dpf and found that both cbs and cth were ubiquitously expressed in the zebrafish brain (figure 1A). To examine whether H2S is involved in brain vascular development, we first downregulated the expression of cbs and cth using MO-based knockdown. The expression of cbs and cth in zebrafish larvae were efficiently downregulated by the MOs (online supplemental figure S1)and H2S production were also significantly reduced in cbs and cth morphants (online supplemental figure S2). We performed in vivo long-term serial confocal imaging of the midbrain vasculature during 3–5 dpf in the same transgenic zebrafish Tg(Flk1:eGFP)26 larva, in which vascular ECs express enhanced green fluorescent protein (eGFP). Compared with embryos injected with a control MO (‘Ctrl MO’), both the cbs and cth morphants displayed impaired brain vascular development (figure 1B, online supplemental figure S3A), as evidenced by reduced midbrain vessel density during 3–5 dpf (figure 1C; p<0.01). Meanwhile, the brain size (indicated by the width of the optic tectum27) and cell apoptosis in cbs and cth morphants showed no significant difference compared with the Ctrl group at 3 dpf (online supplemental figure S4A, B, D and F), indicating that the impaired brain vascular development in cbs and cth morphants were not caused by the delayed brain development or neuron death. Moreover, cbs and cth mutations (online supplemental figure S5) created by coinjecting cbs or cth sgRNA and Cas9 nuclease also caused a significant decrease in the midbrain vessel density during 3–5 dpf in F0 embryos (figure 1D, E, online supplemental figure S3B; p< 0.01). Furthermore, cbs and cth homozygous mutants (online supplemental figure S6), obtained by incrossing the stable heterozygous mutant lines with the Tg(Flk1:eGFP) background, also displayed impaired brain vascular development at 3 dpf, with the relative normal vascular development at 5 dpf possibly due to the compensatory effect of the stable lines (figure 1F, G, online supplemental figure S3C; p< 0.05). Although brains of cbs and cth homomutants were slightly smaller at 3 dpf online supplemental figure S4C; p< 0.05), they did not show increased cell apoptosis (online supplemental figure S4E, G).
Figure 1.
Knockdown and knockout of cbs or cth impair brain vascular development of larval zebrafish. (A) In situ hybridisation of whole zebrafish larvae at 3 dpf showing the ubiquitous expression of cbs and cth in the brain (lateral view). (B and C) Effects of morpholino oligonucleotide-mediated cbs or cth knockdown on brain vascular development. (B) Representative midbrain vessel structures reconstructed from confocal images of cbs or cth morphants at 3–5 dpf of the same larva. (C) Summary of data. The experiments were repeated three times, and six embryos were examined for each group at each time. (D and E) Effects of cbs or cth knockout (F0) on brain vascular development. (D) Representative midbrain vessel structures reconstructed from confocal images of the same F0 mutant larva at 3–5 dpf. (E) Summary of data. Six embryos were examined for each group. (F and G) Effects of cbs or cth homomutants on brain vascular development. (F) Representative midbrain vessel structures reconstructed from confocal images of the same cbs or cth homomutant larva at 3 and 5 dpf. (G) Summary of data. Six embryos were examined for each group. Scale bar, 300 µm (A), 25 µm (B, D, F). Error bars, SEM. *P<0.05, **p<0.01, ***p<0.001 (unpaired two-tailed Student’s t-test).
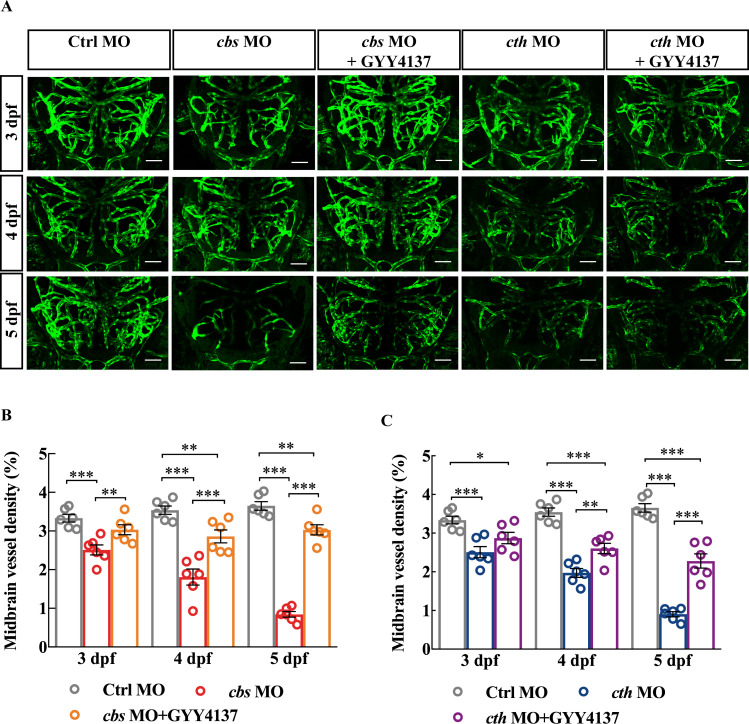
Next, we conducted rescue experiments on cbs and cth morphants by bath application of GYY4137 (200 µM), a slow-releasing H2S donor which is able to yield stable levels of H2S at about 5–10 µM at least for 7 days.28 Embryos injected with MOs were exposed to Hanks’ solution diluted with GYY4137 since 24 hours post fertilisation. GYY4137 treatment alleviated the brain vascular developmental defects in both cbs and cth morphants (figure 2). It is worth mentioning that administration of GYY4137 did not increase the midbrain vessel density in control fish (online supplemental figure S7). Taken together, these results indicate that endogenous H2S is important for the brain vascular development.
Figure 2.
Rescue effect of GYY4137 on the defects of brain vascular development in cbs and cth morphants. (A) Representative projected confocal images showing that GYY4137 treatment ameliorated the impaired brain vascular development in cbs and cth morphants. Confocal images were taken at 3–5 dpf of the same larva. (B) Summary of the rescue effect of GYY4137. Six embryos were examined for each group. Scale bar, 50 µm (A). Error bars, SEM. *P<0.05, **p<0.01, ***p<0.001 (one-way analysis of variance). MO, morpholino oligonucleotide.
H2S promotes brain angiogenesis rather than topological structure formation
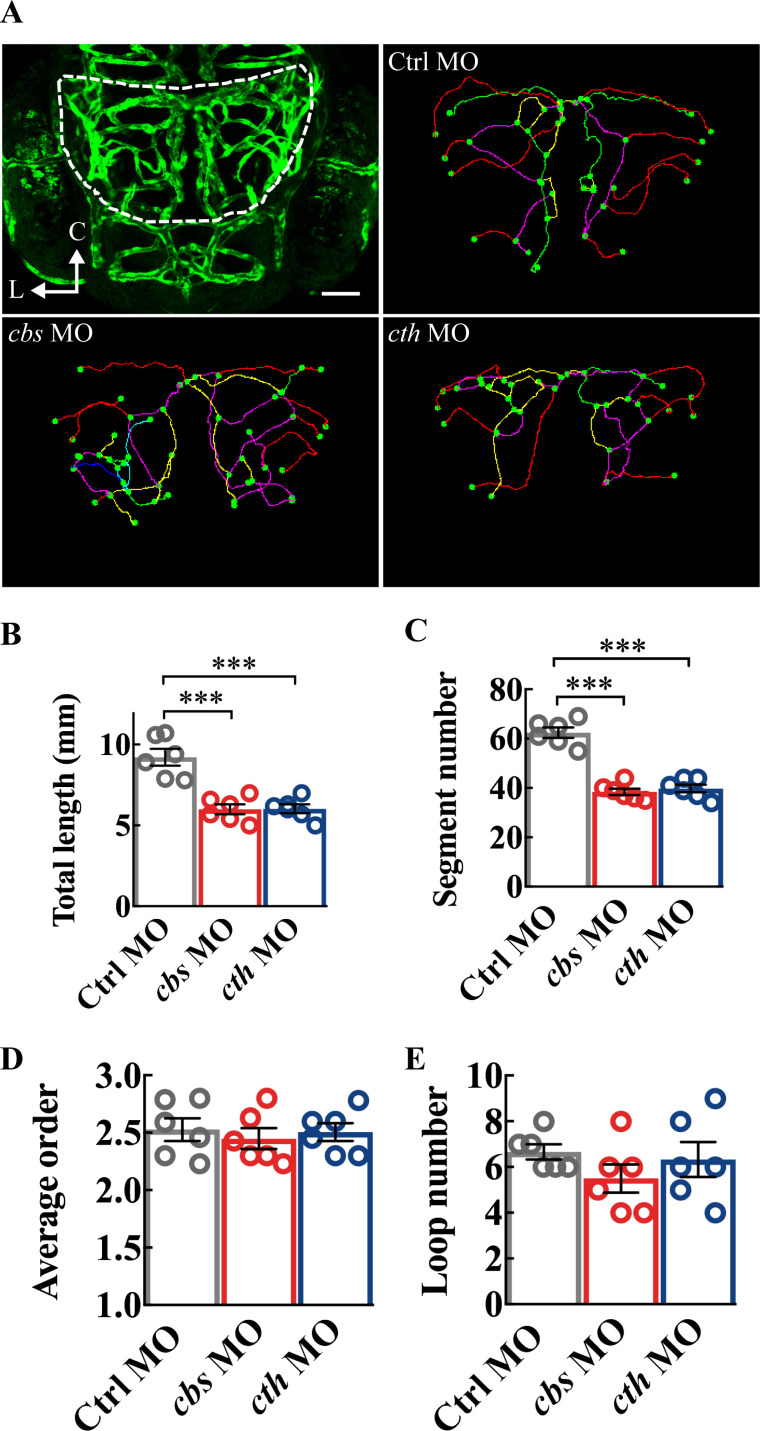
Maturation of vascular networks involves complicated processes including vasculogenesis, angiogenesis and followed with vessel formation, stabilisation, branching, pruning and specialisation.29 It can be generalised into two major aspects: vessel growth (via angiogenesis) and topological structure formation (ie, branching and pruning). To figure out the detailed roles of H2S, we thus analysed quantitatively the characteristics of the midbrain vasculature. We used the total length and vessel segment number to, respectively, quantify vessel elongation and new vessel addition through angiogenesis, and the average order of vessel segments and vessel loop number to quantify the complexity of the vascular network.19 Both the total length and vessel segment number were markedly decreased in 3-dpf cbs and cth morphants (figure 3A–C; p<0.001), while the average order and loop number had no significant change (figure 3A, D and E). These results indicate that H2S enhances angiogenesis without affecting the topological structure during brain vascular development in zebrafish.
Figure 3.
Structure analysis of the midbrain vasculature in cbs and cth morphants. (A) Image of a 3-dpf larva showing the midbrain position delineated with dashed lines (upper left), and representative midbrain vasculature centerlines of 3-dpf larvae of Ctrl morpholino oligonucleotide (MO; upper right), cbs MO (lower left), and cth MO (lower right). C, caudal; L, lateral. (B–E) Summary of changes in the total vessel length (B), vessel segment number (C), weighted average segment Strahler order (D) and internal vessel loop number (E) of the midbrain vasculature in groups of Ctrl MO, cbs MO and cth MO. Six embryos were examined for each group. Error bars, SEM. ***P<0.001 (unpaired two-tailed Student’s t-test).
NOS/NO pathway is involved in the proangiogenic effect of H2S
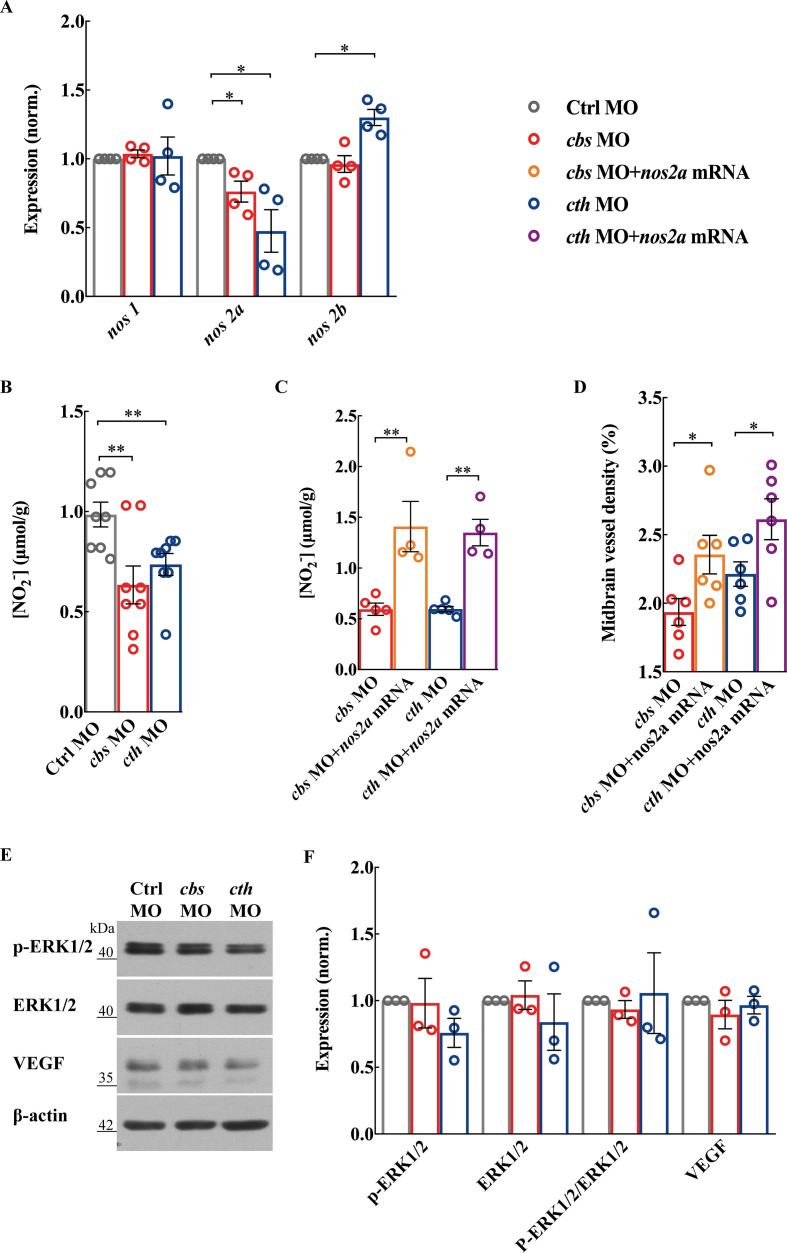
NO is formed from the reaction of L-arginine with O2, a reaction catalysed by NOS. Three NOS isoforms have been identified in mammals30: neuronal NOS (NOS1), inducible NOS (NOS2) and endothelial NOS (NOS3). While in the zebrafish genome, there are one nos1 homolog gene and two nos2 homolog genes (nos2a and nos2b).31 We found that there was a decrease in nos2a expression in both cbs and cth morphants, but an increase in nos2b in cth morphants which might be due to a compensatory effect (figure 4A; p<0.05). Meanwhile, the expression of nos1 was not affected (figure 4A). Consistently, NO production was also decreased in both cbs and cth morphants (figure 4B; p<0.01). We then performed rescue experiments by coinjection of cbs or cth MO with full-length zebrafish nos2a mRNA, and found that the decreased NO production and brain vascular defects caused by cbs or cth knockdown were significantly alleviated (figure 4C, D; p<0.01 for NO production, p<0.05 for vascular density). It is noteworthy that the expression of ERK1/2 (p44/42 mitogen-activated protein kinase), phosphorylated ERK1/2 and vascular endothelial growth factor (VEGF) was not significantly changed in both cbs and cth morphants (figure 4E, F). Taken together, these results suggest that the NOS/NO signalling pathway plays an important role in the proangiogenic effect of H2S in brain vascular development.
Figure 4.
The nitric oxide synthase (NOS)/nitric oxide (NO) pathway is involved in cbs and cth knockdown-induced defects of brain vascular development. (A and B) Effects of cbs and cth knockdown on the NOS/NO signalling pathway. Summary of nos1, nos2a and nos2b RNA expression (A) and total NO production (B) in 3-days post fertilisation (dpf) embryos. (C and D) Rescue effect of nos2a mRNA on the brain vascular developmental defects in cbs and cth morphants. Summary of increased total NO production (C) and midbrain vessel density (D) in 3-dpf embryos coinjected with cbs or cth MO and nos2a mRNA in comparison with those injected with cbs or cth MO. Six embryos were examined for each group. (E and F) No effect of cbs and cth knockdown on the protein expression of p-ERK1/2, ERK1/2 and VEGF. Representative blots (E) and summary (F) of Western blotting data. Error bars, SEM. *P<0.05, **p<0.01 (unpaired two-tailed Student’s t-test).
Discussion
H2S has been shown to exert potent proangiogenic effects in vitro and in pathological models.14 15 32–35 Recently, two in vivo studies showed the importance of H2S in muscle angiogenesis.16 17 By in vivo confocal imaging of zebrafish larvae, our study reveals the role of endogenous H2S in developmental angiogenesis of the brain.
The signalling pathways involved in the proangiogenic effect of exogenous H2S on peripheral vessels has been well studies with in vitro preparations.14 15 32–34 The proangiogenic effect of H2S is mediated via: (1) EC-related angiogenic properties through a KATP channel/MAPK pathway14; (2) upregulation of VEGF expression and release from cells36 37; (3) stimulation of the NOS/NO signalling pathway. In the present study, we examined ERK1/2 phosphorylation and VEGF expression and found no change of these signals in cbs and cth morphants. NO and H2S share many similar regulatory roles including promotion of angiogenesis, vasodilation, attenuation of apoptosis and antioxidant actions.32 38 H2S is reported to exert proangiogenic functions via both NO-dependent and NO-independent mechanisms.32 34 Exogenously administered H2S increases NOS expression33 and phosphorylation34 in cultured ECs and stimulates the production of NO. In zebrafish larvae, expression of nos2a was mainly detected in the head, eyes and gut regions,31 consistent with the expression pattern of cbs and cth we observed. In the present study, depletion of H2S resulted in a reduction in nos2a expression and NO production. Injection of full-length zebrafish nos2a mRNA alleviated the brain vascular developmental defects in cbs and cth morphants. Thus, H2S promotes brain angiogenesis via the NOS/NO pathway in zebrafish.
Studies demonstrating the proangiogenic effect of endogenous H2S were mostly conducted through manipulating CTH,14 17 39 and the cellular target of H2S is believed to be ECs as stated above. However, our study found that CBS-derived and CTH-derived H2S both played vital roles in brain developmental angiogenesis. Indeed, in the brain, H2S production in the astrocyte is almost 10 times as much as that in other brain cell types,6 making the astrocyte an important target for H2S functioning and CBS a significant source of H2S in the brain. We speculate that H2S promotes brain angiogenesis by functioning on ECs through direct and indirect ways. The cellular mechanisms of H2S promoting brain angiogenesis require further study.
Currently, numerous animal and cellular studies have showed that H2S impacts ischaemic stroke outcomes due to its neuroprotective abilities.40 Taking advantage of slow-releasing H2S donors, H2S is emerging as a potential therapy for cerebral ischaemia. Our study reveals the role of endogenous H2S in brain angiogenesis, making H2S-based therapies for cerebral ischaemia even more promising.
Footnotes
Contributors: YW contributed to study design and interpretation of experimental data. WJ contributed to experiment performance and manuscript drafting. CL and MD contributed to analysis of experimental data. FW and XR contributed to manuscript revision. YF contributed to interpretation of experimental data. JD contributed to study design and final revision of the manuscript.
Funding: The authors are indebted to Bing Xu and Yu Zhang for help with research design and technical supports. This work was supported by a grant from the Natural Science Foundation of China (31770800, 91849104, 81870274, 81571329).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. No additional unpublished data are available.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
All animal use and handling procedures were approved by Institute of Neuroscience, Chinese Academy of Sciences.
References
- 1. Goodwin LR, Francom D, Dieken FP, et al. Determination of sulfide in brain tissue by gas dialysis/ion chromatography: postmortem studies and two case reports. J Anal Toxicol 1989;13:105–9. 10.1093/jat/13.2.105 [DOI] [PubMed] [Google Scholar]
- 2. Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? Faseb J 2002;16:1792–8. 10.1096/fj.02-0211hyp [DOI] [PubMed] [Google Scholar]
- 3. Kimura H. Hydrogen sulfide: from brain to gut. Antioxid Redox Signal 2010;12:1111–23. 10.1089/ars.2009.2919 [DOI] [PubMed] [Google Scholar]
- 4. Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 1996;16:1066–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robert K, Vialard F, Thiery E, et al. Expression of the cystathionine beta synthase (CBS) gene during mouse development and immunolocalization in adult brain. J Histochem Cytochem 2003;51:363–71. 10.1177/002215540305100311 [DOI] [PubMed] [Google Scholar]
- 6. Lee M, Schwab C, Yu S, et al. Astrocytes produce the antiinflammatory and neuroprotective agent hydrogen sulfide. Neurobiol Aging 2009;30:1523–34. 10.1016/j.neurobiolaging.2009.06.001 [DOI] [PubMed] [Google Scholar]
- 7. Enokido Y, Suzuki E, Iwasawa K, et al. Cystathionine beta-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. Faseb J 2005;19:1854–6. 10.1096/fj.05-3724fje [DOI] [PubMed] [Google Scholar]
- 8. Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids 2011;41:113–21. 10.1007/s00726-010-0510-x [DOI] [PubMed] [Google Scholar]
- 9. Leffler CW, Parfenova H, Basuroy S, et al. Hydrogen sulfide and cerebral microvascular tone in newborn pigs. Am J Physiol Heart Circ Physiol 2011;300:H440–7. 10.1152/ajpheart.00722.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kabil O, Vitvitsky V, Xie P, et al. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid Redox Signal 2011;15:363–72. 10.1089/ars.2010.3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu L-F, Lu M, Hon Wong PT, et al. Hydrogen sulfide: neurophysiology and neuropathology. Antioxid Redox Signal 2011;15:405–19. 10.1089/ars.2010.3517 [DOI] [PubMed] [Google Scholar]
- 12. Yang G, Wu L, Jiang B, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008;322:587–90. 10.1126/science.1162667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cai W-J, Wang M-J, Moore PK, et al. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res 2007;76:29–40. 10.1016/j.cardiores.2007.05.026 [DOI] [PubMed] [Google Scholar]
- 14. Papapetropoulos A, Pyriochou A, Altaany Z, et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A 2009;106:21972–7. 10.1073/pnas.0908047106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang M-J, Cai W-J, Li N, et al. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid Redox Signal 2010;12:1065–77. 10.1089/ars.2009.2945 [DOI] [PubMed] [Google Scholar]
- 16. Das A, Huang GX, Bonkowski MS, et al. Impairment of an Endothelial NAD+-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell 2018;173:74–89. 10.1016/j.cell.2018.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Longchamp A, Mirabella T, Arduini A, et al. Amino Acid Restriction Triggers Angiogenesis via GCN2/ATF4 Regulation of VEGF and H2S Production. Cell 2018;173:117–29. 10.1016/j.cell.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weinstein B. Vascular cell biology in vivo: a new piscine paradigm? Trends Cell Biol 2002;12:439–45. 10.1016/S0962-8924(02)02358-9 [DOI] [PubMed] [Google Scholar]
- 19. Chen Q, Jiang L, Li C, et al. Haemodynamics-driven developmental pruning of brain vasculature in zebrafish. PLoS Biol 2012;10:e1001374. 10.1371/journal.pbio.1001374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wienholds E, Kloosterman WP, Miska E, et al. Microrna expression in zebrafish embryonic development. Science 2005;309:310–1. 10.1126/science.1114519 [DOI] [PubMed] [Google Scholar]
- 21. Yu P-chun, Gu S-ye, Bu J-wen, et al. Trpc1 is essential for in vivo angiogenesis in zebrafish. Circ Res 2010;106:1221–32. 10.1161/CIRCRESAHA.109.207670 [DOI] [PubMed] [Google Scholar]
- 22. Li J, Zhang B-bing, Ren Y-gang, et al. Intron targeting-mediated and endogenous gene integrity-maintaining knockin in zebrafish using the CRISPR/Cas9 system. Cell Res 2015;25:634–7. 10.1038/cr.2015.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rossi A, Kontarakis Z, Gerri C, et al. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 2015;524:230–3. 10.1038/nature14580 [DOI] [PubMed] [Google Scholar]
- 24. Shah AN, Davey CF, Whitebirch AC, et al. Rapid reverse genetic screening using CRISPR in zebrafish. Nat Methods 2015;12:535–40. 10.1038/nmeth.3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu B, Zhang Y, Du X-F, et al. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res 2017;27:882–97. 10.1038/cr.2017.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roman BL, Pham VN, Lawson ND, et al. Disruption of ACVRL1 increases endothelial cell number in zebrafish cranial vessels. Development 2002;129:3009–19. [DOI] [PubMed] [Google Scholar]
- 27. Näslund J. A simple non-invasive method for measuring gross brain size in small live fish with semi-transparent heads. PeerJ 2014;2:e586. 10.7717/peerj.586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee ZW, Zhou J, Chen C-S, et al. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS One 2011;6:e21077. 10.1371/journal.pone.0021077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee HS, Han J, Bai H-J, et al. Brain angiogenesis in developmental and pathological processes: regulation, molecular and cellular communication at the neurovascular interface. Febs J 2009;276:4622–35. 10.1111/j.1742-4658.2009.07174.x [DOI] [PubMed] [Google Scholar]
- 30. Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J 1994;298:249–58. 10.1042/bj2980249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lepiller S, Franche N, Solary E, et al. Comparative analysis of zebrafish nos2a and nos2b genes. Gene 2009;445:58–65. 10.1016/j.gene.2009.05.016 [DOI] [PubMed] [Google Scholar]
- 32. Holwerda KM, Karumanchi SA, Lely AT. Hydrogen sulfide: role in vascular physiology and pathology. Curr Opin Nephrol Hypertens 2015;24:170–6. 10.1097/MNH.0000000000000096 [DOI] [PubMed] [Google Scholar]
- 33. Bir SC, Kolluru GK, McCarthy P, et al. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1α and vascular endothelial growth factor-dependent angiogenesis. J Am Heart Assoc 2012;1:e004093. 10.1161/JAHA.112.004093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coletta C, Papapetropoulos A, Erdelyi K, et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A 2012;109:9161–6. 10.1073/pnas.1202916109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Madurga A, Golec A, Pozarska A, et al. The H2S-generating enzymes cystathionine β-synthase and cystathionine γ-lyase play a role in vascular development during normal lung alveolarization. Am J Physiol Lung Cell Mol Physiol 2015;309:L710–24. 10.1152/ajplung.00134.2015 [DOI] [PubMed] [Google Scholar]
- 36. Kan J, Guo W, Huang C, et al. S-Propargyl-Cysteine, a novel water-soluble modulator of endogenous hydrogen sulfide, promotes angiogenesis through activation of signal transducer and activator of transcription 3. Antioxid Redox Signal 2014;20:2303–16. 10.1089/ars.2013.5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holwerda KM, Burke SD, Faas MM, et al. Hydrogen sulfide attenuates sFlt1-induced hypertension and renal damage by upregulating vascular endothelial growth factor. J Am Soc Nephrol 2014;25:717–25. 10.1681/ASN.2013030291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res 2014;114:730–7. 10.1161/CIRCRESAHA.114.300505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tao B-B, Liu S-Y, Zhang C-C, et al. Vegfr2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal 2013;19:448–64. 10.1089/ars.2012.4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jia J, Li J, Cheng J. H2S-based therapies for ischaemic stroke: opportunities and challenges. Stroke Vasc Neurol 2019;4:63–6. 10.1136/svn-2018-000194 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2020-000584supp001.pdf (7.4MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. No additional unpublished data are available.