Abstract
Objective
Reviews on the relationship of low-energy sweeteners (LES) with body weight (BW) have reached widely differing conclusions. To assess possible citation bias, citation analysis was used to quantify the relevant characteristics of cited articles, and explore citation patterns in relation to review conclusions.
Design
A systematic search identified reviews published from January 2010 to March 2020. Different characteristics (for example, type of review or research, journal impact factor, conclusions) were extracted from the reviews and cited articles. Logistic regression was used to estimate likelihood of articles with particular characteristics being cited in reviews. A qualitative network analysis linked reviews sub-grouped by conclusions with the types of articles they cited.
Main outcome measures
(OR; 95% CI) for likelihood that articles with particular characteristics were cited as evidence in reviews.
Results
From 33 reviews identified, 183 different articles were cited (including other reviews). Narrative reviews were 62% less likely to be cited than systematic reviews with meta-analysis (OR 0.38; 0.16 to 0.86; p=0.03). Likelihood of being cited was higher for evidence on children than adults (OR 2.27; 1.59 to 3.25; p<0.0001), and with increased journal impact factor (OR 1.15; 1.00 to 1.31; p=0.04). No other factors were statistically significant in the main analysis, and few factors were significant in subgroup analyses. Network analysis showed that reviews concluding a beneficial relationship of LES with BW cited mainly randomised controlled trials, whereas reviews concluding an adverse relationship cited mainly observational studies.
Conclusions
Overall reference to the available evidence across reviews appears largely arbitrary, making citation bias likely. Differences in the conclusions of individual reviews map onto different types of evidence cited. Overall, inconsistent and selective use of the available evidence may account for the diversity of conclusions in reviews on LES and BW.
Trial registration number
Prior to data analysis, the protocol was registered with the Open Science Framework (https://osf.io/9ghws).
Keywords: weight management, nutritional treatment
Summary box.
What this paper adds
Network analyses show that reviews concluding a beneficial relationship of LES with BW cite mainly randomised controlled trials, whereas reviews concluding an adverse relationship cite mainly observational studies.
The selection and importance given to different types of prior evidence across reviews seem arbitrary and citation bias is probable.
The lack of consistent use of available evidence is likely to account for the divergent conclusions of reviews on this topic.
Introduction
The relationship of low-energy sweeteners (LES) with body weight (BW) has been widely discussed.1 2 Replacing sugars (monosaccharides and disaccharides) with LES has been argued to benefit BW control by decreasing net calorie intake while satisfying a desire for sweetness.1 In contrast, it has also been argued that LES may promote weight gain by having the opposite effects: causing dysregulation of appetite and metabolism, and promoting intake of sweet-tasting, energy-containing foods.2 Widely differing views are evident in recent narrative and systematic reviews in relation to LES and BW, with some reviews concluding a beneficial effect or association,3–5 others a detrimental effect or association,6 7 and others again that the evidence is too limited or inconsistent to conclude either way.8–10 Given that the same evidence base is available to all reviewers, these different views presumably arise from the selection and importance given to different types of evidence, and potential citation bias in the reviews.
Citation bias can be defined as ‘the citation or non-citation of research findings, depending on the nature and direction of the results’.11 Song et al 12 concluded that citation bias is especially known to occur for positive or significant results. Furthermore, non-systematic narrative reviews were particularly susceptible to biased citation leading to misleading conclusions.12 Citation bias is not unknown in nutrition research, for example, in relation to dietary fatty acid intake and the risk of cardiovascular disease (CVD). Urlings et al 13 investigated citation bias in the literature concerning dietary trans fatty acids and serum cholesterol. They concluded that several factors such as statistically significant results, sample size and journal impact factor (JIF) were important determinants for selective citation.13 Leng used a network analysis to assess the patterns of citations in reviews published before 1984 of the early randomised controlled trials (RCT) on cholesterol lowering diets for prevention of coronary heart disease.14 That analysis provided evidence of selective citation of RCTs, especially in the reviews supporting dietary intervention in secondary prevention of CVD.14 This also shows that citation bias is not a new phenomenon.14
In relation to LES and BW, citation analysis could help to understand the basis for differing conclusions from reviews. Mela et al 15 specifically raised selective citation as a general issue in the interpretation and reporting of research on LES. However, there has been no objective analysis of citation patterns or bias in the literature on LES. The aim of this analysis was therefore to assess the citation pattern in reviews on the relationship of LES with BW-related outcomes. The main analysis evaluated whether variation in the overall pattern of articles cited in reviews was quantitatively associated to specific characteristics of the cited articles, such as article type, conclusions, population, sample size, authorship, JIF and years between the review and the cited (original) article. Network analysis was used to assess qualitative relationships between individual review conclusions and the nature of cited articles.
Methods
The methods were originally described in a study protocol registered online prior to undertaking any analyses (https://osf.io/9ghws). Any later additions, deviations and modifications from that original protocol are noted below. The protocol is available in online supplemental material (including online supplemental tables S1, S2 and S3).
bmjnph-2020-000210supp001.pdf (55.3KB, pdf)
bmjnph-2020-000210supp002.pdf (126.4KB, pdf)
Search strategy and article selection
A systematic literature search was conducted in March 2020 using the Web of Science Core Collection. The aim was to transparently identify a comprehensive, representative and unbiased selection of reviews assessing the relationship between LES exposure and outcomes related to BW or obesity risk. Search terms included ‘low-energy sweetener(s)’ and related terms for exposure, ‘body weight’, ‘overweight’, ‘obesity’ and ‘adiposity’ for outcome, together with different types of publication, for example, ‘narrative review’, ‘systematic review’, ‘meta-analysis’, ‘scientific report’ and ‘perspective’. For a complete list of search terms, see online supplemental table S1. Articles were included if they were published in English, in refereed scientific journals (ie, excluding ‘grey’ literature). Any other potentially eligible reviews subsequently identified (for example, from reference lists in the reviews returned by our search) were not included, because this could cause an over-representation of articles cited within the network, whereas eligible articles outside the network would be ignored.13 As a modification of the registered protocol, the search was limited to publications within the preceding 10 years to better reflect current rather than historical practices.
Screening of review articles was done in two steps: (1) screening of title and abstract to identify potentially relevant articles, (2) full-text screening of identified articles from step 1 to confirm that relevant articles meeting the inclusion criteria were correctly identified, and that data for the required outcome measures were reported. The screening was done independently by two reviewers (DM and MN). Any uncertainties or disagreements were resolved by a third reviewer (AR). The eligibility of reviews was determined according to the predefined inclusion and exclusion criteria (online supplemental table S2). Each review was re-classified as one or more ‘evidence assessment units’ (EAU—see below) for analysis. If a single review contained multiple independent analyses and conclusions based on different evidence sets (for example, RCT vs observational evidence, evidence for adults vs children), these were treated as separate EAUs in the citation analysis. Children were defined as a study population with mean age under 18 years.
For clarity, the following terminology is used:
Review: a published review identified by the systematic literature search. A review is a single publication, consisting of one or more EAUs.
EAU: a single review or, where present, each of the independent evidence assessments within a review, such as wholly separate sections for evidence or meta-analyses on children or adults, with independent conclusions. For the purpose of the citation analysis, each EAU in any single review publication was treated as if they were separate publications, because each EAU cited different evidence sets and could differ in conclusions.
Cited article: any publication cited as evidence for the LES–BW relationship in a review (EAU). Cited articles could be original research or earlier reviews (including those identified for the present citation analysis).
Inclusion criteria for articles cited in reviews
The included reviews were screened for any articles cited as evidence of possible effects or associations of LES exposure and human BW-related outcomes. Obesity or BW-related outcomes of interest included BW, body mass index, population risk of obesity or weight gain and other outcomes commonly used as indicators of relative BW or fatness (fat mass, percent body fat, waist circumference, skinfold thickness, adiposity). This was done independently by two reviewers (DM and MN). Cited articles were included or excluded from the analysis depending on the context in which they were used. Cited articles were included when they were an explicit part of the empirical evidence-base used for drawing conclusions on the effect or association of LES and BW-related outcomes. Citations describing BW outcomes in animal studies were only included where they were used in this same context, and integrated into the narrative on BW or obesity risk in humans. Citations were excluded if they were only used in other contexts such as:
Introductory descriptions of the general topic area or current public health guidance.
Evidence limited to potential underlying mechanisms or hypotheses, for example, appetite control, energy intake or expenditure, adipogenesis, diet quality and so on.
Cited but not used in quantitative or qualitative evidence assessments in the systematic review or meta-analysis.
Animal studies clearly used in the context of narrative text on effects in animals.
Evidence limited to visceral fat mass or ectopic fat as outcomes.
Evidence limited to other health outcomes including metabolic syndrome.
Part of an inventory (simple listing or description) of papers in a database or cited in other reviews.
Obvious errors in citations within reviews (for example, clear reference name and description in text linked to incorrect number in reference list, double-citing of the same paper in a reference list, mistakes in cited author or journal name and so on) were corrected where possible. However, if there was not an unambiguous resolution (for example, citation could not be matched to any clear source), these were treated as missing data.
Data extraction
Data on a number of characteristics were extracted from each EAU and cited article (table 1 and table 2). This information was independently reviewed and subsequently agreed by two authors (MN and DM), and a third author (AR) consulted where needed to reach a consensus. A number of guiding decision rules were applied to ensure greater consistency and transparency in the independent assessor judgements on the relevant citations and data for extraction. The most important guiding rules are described below. See online supplemental information for additional decision rules.
Table 1.
Characteristics of included evidence assessment units (n=51*)
| n (%) | |
| Author’s conclusion | |
| Decrease BW/more beneficial | 11 (22) |
| Neutral (no directional effect or association) | 7 (14) |
| Increase BW/less beneficial | 7 (14) |
| No conclusion directly relevant to the LES–BW relationship | 0 |
| Evidence is insufficient to draw a conclusion | 26 (51) |
| Unable to draw a conclusion from the paper | 0 |
| Statistical significance† | |
| Decrease BW/more beneficial | 2 (4) |
| Neutral (no directional effect or association) | 3 (6) |
| Increase BW/less beneficial | 3 (6) |
| No conclusion directly relevant to the LES–BW relationship | 0 |
| Evidence is insufficient to draw a conclusion | 0 |
| Unable to draw a conclusion from the paper | 2 (4) |
| Missing data | 1 (2) |
| Type | |
| Narrative review | 26 (51) |
| Systematic review with meta-analysis | 11 (22) |
| Systematic review without meta-analysis | 14 (27) |
| Population | |
| Adults | 6 (12) |
| Children | 14 (27) |
| Both | 31 (61) |
| Funding source | |
| Non-profit organisation‡ | 25 (49) |
| For profit organisation | 0 |
| Both profit and non-profit | 0 |
| Not stated/stated as no funding received | 26 (51) |
| Affiliation of the corresponding author | |
| University | 44 (86) |
| Government | 4 (8) |
| Non-profit organisation | 1 (2) |
| Industry | 2 (4) |
| Other | 0 |
| Affiliation of the first author | |
| University | 46 (90) |
| Government | 2 (4) |
| Non-profit organisation | 1 (2) |
| Industry | 2 (4) |
| Other | 0 |
| Median (IQR) | |
| Number of authors | 3 (2–6) |
| Journal impact factor, current (2018) | 4.17 (3.57–5.78) |
| Journal impact factor, last 5 years | 4.81 (3.43–7.45) |
| Number of relevant cited articles | 9 (6–13) |
| Number of review authors publications in the section concerning BW | 0 (0–0) |
*From a total number of 33 included reviews. Where a review publication contained independent analyses and conclusions for randomised controlled trials and observational evidence, or adults and children, those were treated as separate evidence assessment units. This is the case for 18 papers, resulting in 51 evidence assessment units from the 33 reviews.
†From evidence assessment units with meta-analysis (n=11).
‡Of the 25 EAUs with support from non-profit sources, 4 were supported by primarily industry-funded non-profit organisations and the rest by grants primarily from government, independent foundations and universities.
BW, body weight; IQR, Interquartile range; LES, low-energy sweeteners; n, sample size.
Table 2.
Characteristics of included cited articles (n=183) in the total set of 51 evidence assessment units reported in 33 reviews
| n (%) | |
| Main message of cited article | |
| Decrease BW/more beneficial | 32 (17) |
| Neutral (no directional effect or association) | 39 (21) |
| Increase BW/less beneficial | 54 (30) |
| No conclusion directly relevant to the LES–BW relationship | 26 (14) |
| Evidence insufficient to draw a conclusion | 20 (11) |
| Unable to draw a conclusion from the paper | 9 (5) |
| Missing data | 3 (2) |
| Cited article type | |
| Randomised controlled trial | 51(28) |
| Observational study | 72 (40) |
| Animal | 13 (7) |
| Other | 1 (1) |
| Systematic review with meta-analysis | 16 (9) |
| Systematic review without meta-analysis | 9 (5) |
| Narrative review | 18 (10) |
| Missing data | 3 (2) |
| Cited article population | |
| Adults | 85 (46) |
| Children | 49 (27) |
| Both | 32 (17) |
| Missing data | 17 (9) |
| Median (IQR) | |
| Sample size | |
| Randomised controlled trials | 50 (25–155) |
| Observational studies | 2760 (781–15 984) |
| Number of authors | 5 (3–7) |
| Journal impact factor, current (2018) | 3.97 (3.05–6.57) |
| Journal impact factor, last 5 years | 4.51 (3.33–7.67) |
| Years since cited article was published | 5 (2–10) |
BW, body weight; IQR, Interquartile range; LES, low-energy sweeteners; n, sample size.
bmjnph-2020-000210supp003.pdf (63.7KB, pdf)
If an EAU included a quantitative meta-analysis, both the authors’ stated conclusion and conclusion from the statistical analysis were recorded. For cited articles, the conclusion was classified on the basis of the main stated message. Data for cited articles were drawn from the abstract if possible, and the full texts only accessed where needed.
An EAU was classified as systematic (as opposed to a narrative) if the authors described a replicable systematic approach to the identification and selection of the literature, regardless of whether more formal tools or criteria for a formal systematic review were applied (for example, quality or bias assessment). Cited articles were classified as either RCT, observational study, animal study, systematic review (with or without meta-analysis) or narrative review.
Population (adults, children or both), number of authors, current JIF and JIF from the last 5 years were extracted for both EAUs and cited articles, and sample size extracted for the latter. Years since the cited paper was published was obtained by subtracting a cited article’s publication year from the year the review was published. Funding source, affiliation of corresponding and first author, number of relevant cited articles and number of these being self-citations were only extracted from EAUs.
Statistical analysis
The pre-planned main analysis assessed the likelihood of articles being cited in reviews, based on the characteristics of the cited articles. EAUs and their cited articles were combined in a citation matrix, and logistic mixed-effects regression used to quantify the association of characteristics of cited articles with likelihood of being cited. Random effects were included to account for multiple entries of the same cited articles in different EAUs. Both univariate and multivariate models (including adjustment for number of authors, JIF and years since cited article was published) were fitted. ORs with 95% CIs were reported. The criterion for statistical significance was p<0.05.
A post hoc subgroup analysis was conducted based on the EAU conclusions and type of review, using the same procedures as the main analysis. An additional post hoc analysis included only studies cited five times or more.
A network analysis linked the cited articles to their citing EAUs, graphically illustrated with articles and EAUs as dots (nodes) connected by arrows (edges). The network was based on articles cited five times or more in EAUs, in order to simplify visual interpretation and remove potentially trivial citations. The network analysis was further divided into subgroups based on the conclusion of each EAU. This approach resulted in four different networks, corresponding to EAUs concluding a beneficial, neutral or adverse relationship of LES with BW, or that there was insufficient evidence to draw a conclusion.
All statistical analyses were performed in R V.3.6.1.16
Results
Included articles
Out of 153 potentially eligible reviews identified from the systematic search (figure 1), 33 reviews met the criteria and were included in the analysis.2–10 17–40 Of these, 16 reviews had two independent sections (EAUs) separately reviewing evidence from RCT and observational studies.3–5 8 17 19 21 26 29–31 33 34 37 39 40 Two reviews had two independent sections (EAUs) separately assessing evidence from adults and children,20 38 whereas the remaining 15 reviews did not have such independent sections, and were thus treated as single EAUs. Thus, a total of 51 EAUs were available and included in the analysis (table 1). A total of 183 cited articles were identified from the included EAUs (table 2).2–4 10 17 19 29 30 33 36–38 41–204 Several of the included EAUs were also cited as evidence by other EAUs.2–4 10 17 19 29 30 33 36–38
Figure 1.
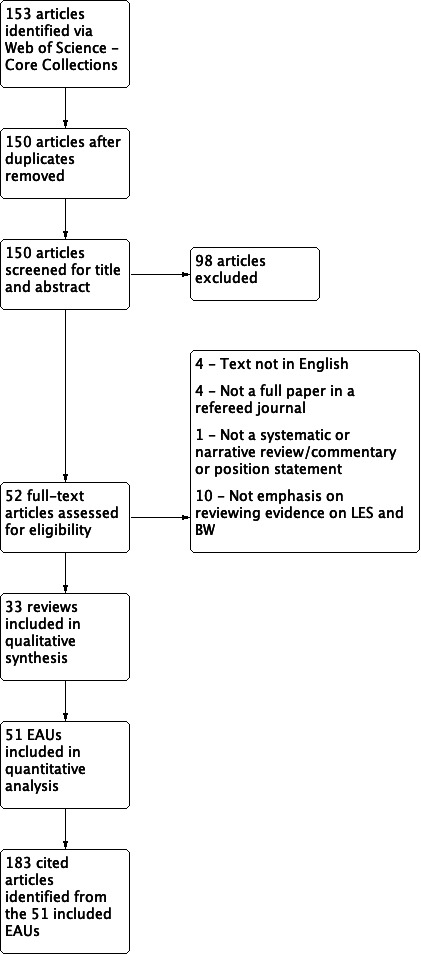
Flow diagram. If a single review contained multiple independent analyses and conclusions based on different evidence sets (randomised controlled trial vs observational evidence, evidence for adults vs children), these were treated as separate evidence assessment units in the analysis. BW, body weight; EAUs, evidence assessment units; LES, low-energy sweeteners.
About half of the EAUs (n=25) concluded that there was an effect or association of LES and BW, either beneficial, neutral or adverse, whereas the other half (n=26) concluded that the evidence was insufficient to draw a conclusion. Half of the EAUs were narrative reviews (n=26), 22% were systematic review with meta-analysis (n=11) and 27% systematic review without meta-analysis (n=14).
The majority (68%) of the 183 cited articles concluded that there was a relationship (beneficial, neutral or adverse) of LES with BW outcomes (n=125) (table 2). The majority of the cited articles were original research (n=136), either RCT, observational or animal, while 43 of the cited articles were reviews, either narrative or systematic with or without meta-analysis. Notably, however, almost 15% of the cited articles contained no relevant conclusion (n=26), mainly because they included no relevant data or analyses. The article type of one article could not be classified.
For a small number of the cited articles (n=9), it was not possible to discern any conclusion regarding LES, even though they contained potentially relevant data (for example, where LES were not really a focus of the research).
ORs for likelihood of being cited
Only a small number of characteristics of the cited articles were significantly associated with likelihood of being cited (table 3). On average, an article was 62% less likely to be cited if it was a narrative review compared with a systematic review with meta-analysis. Articles on children were 127% more likely to be cited than articles on adults. An article was 15% more likely to be cited for every twofold increase in the JIF. No other statistically significant associations were seen. However, an article was 64% more likely to be cited when it did not contain any conclusion directly relevant to the relationship of LES with BW (p=0.08). Adjusting for number of authors, JIF and years since cited study was published did not alter the results (online supplemental table S4).
Table 3.
ORs for the likelihood of an article being cited, based on univariate analyses of 183 articles cited in 51 evidence assessment units from 33 reviews
| n (%) | OR (95% CI) | P value | |
| Main message of cited articles | |||
| Neutral (no directional effect or association) | 39 (21) | 1 (ref) | |
| No conclusion directly relevant to the LES–BW relationship | 26 (14) | 1.64 (0.95 to 2.84) | 0.08 |
| Decrease BW/more beneficial | 32 (17) | 1.31 (0.76 to 2.27) | 0.33 |
| Increase BW/less beneficial | 54 (30) | 1.11 (0.68 to 1.85) | 0.68 |
| Unable to draw a conclusion from the article | 9 (5) | 1.11 (0.43 to 2.50) | 0.81 |
| Evidence is insufficient to draw a conclusion | 20 (11) | 1.05 (0.53 to 2.00) | 0.87 |
| Cited article type | |||
| Systematic review with meta-analysis | 16 (9) | 1 (ref) | |
| Systematic review without meta-analysis | 9 (5) | 0.85 (0.36 to 1.90) | 0.70 |
| Randomised controlled trial | 51 (28) | 0.82 (0.48 to 1.46) | 0.48 |
| Observational study | 72 (39) | 0.65 (0.39 to 1.16) | 0.13 |
| Animal | 13 (7) | 0.63 (0.27 to 1.38) | 0.26 |
| Narrative review | 18 (10) | 0.38 (0.16 to 0.86) | 0.03 |
| Other | 1 (1) | 0.22 (0.00 to 2.67) | 0.44 |
| Cited article population‡ | |||
| Adults | 85 (46) | 1 (ref) | |
| Children | 49 (27) | 2.27 (1.59 to 3.25) | <0.0001 |
| Both | 32 (17) | 1.01 (0.60 to 1.63) | 0.98 |
| Sample size*,§ | 124 (68) | 1.00 (0.83 to 1.21) | 1.00 |
| Number of authors | 181 (99) | 1.05 (1.00 to 1.10) | 0.06 |
| Journal impact factor, current (2018)† | 179 (98) | 1.15 (1.00 to 1.31) | 0.04 |
| Journal impact factor, last 5 years† | 178 (97) | 1.13 (0.98 to 1.30) | 0.08 |
| Years since cited article was published | 183 (100) | 1.00 (0.97 to 1.02) | 0.73 |
Logistic mixed-effects regression. All analyses are additionally adjusted for overdispersion.
Bold value indicates result is statistically significant with p<0.05 or lower.
*Sample size was base 10 log-transformed, so OR is the change per 10-fold change in study population.
†Journal impact factor was base 2 log-transformed, so OR is the change per twofold change in journal impact factor.
‡Data on population were only extracted for articles considering human subjects.
§Data on sample size were only extracted for primary evidence (ie, not for reviews).
BW, body weight; CI, confidence interval; LES, low-energy sweeteners; OR, odds ratio; ref, reference variable.
Statistically significant findings for the subgroup analyses are depicted in table 4 (full results are available in online supplemental tables S5–S12). Subgrouping by the direction of EAU conclusions (neutral, beneficial, adverse) showed limited relationships with the nature or conclusions of the cited articles. For EAUs concluding a beneficial or adverse effect or association of LES and BW, there were no significant associations with the main message (conclusions) of the cited articles (see online supplemental tables S5, S7). For EAUs concluding a neutral effect or association of LES and BW, articles from which it was not possible to draw a conclusion were 67% more likely to be cited than articles with neutral conclusions (p=0.03), and observational studies 45% more likely to be cited than systematic reviews with meta-analysis (p=0.03). For EAUs concluding that there was insufficient evidence to draw a conclusion, articles on children were 84% more likely to be cited than articles on adults (p<0.001). For systematic reviews without meta-analysis, articles on children were 66% more likely to be cited than articles on adults (p=0.002), and articles on both children and adults were 86% more likely to be cited (p=0.004) than articles only on adults.
Table 4.
Statistically significant findings for the subgroup analysis based on evidence assessment unit conclusions and type of review. Data from 51 evidence assessment units reported in 33 reviews
| OR (95% CI) | P value | |
| Evidence assessment units concluding a neutral effect or association of LES on BW (n=7) | ||
| Main message of cited articles | ||
| Neutral (no directional effect or association) | 1 (ref) | |
| Unable to draw a conclusion from the article | 1.67 (1.07 to 2.54) | 0.03 |
| Cited article type | ||
| Systematic review with meta-analysis | 1 (ref) | |
| Observational study | 1.45 (1.06 to 2.02) | 0.03 |
| Evidence assessment units concluding insufficient evidence to draw a conclusion about the effect of LES on BW (n=26) | ||
| Cited article type | ||
| Systematic review with meta-analysis | 1 (ref) | |
| Systematic review without meta-analysis | 1.97 (1.12 to 3.45) | 0.02 |
| Cited article population | ||
| Adults | 1 (ref) | |
| Children | 1.84 (1.43 to 2.37) | <0.001 |
| Cited article journal impact factor, current (2018)* | 1.10 (1.00 to 1.20) | 0.049 |
| Systematic reviews (evidence assessment units) without meta-analysis (n=14) | ||
| Cited article type | ||
| Systematic review with meta-analysis | 1 (ref) | |
| Randomised controlled trial | 0.61 (0.38 to 1.00) | 0.04 |
| Cited article population | ||
| Adults | 1 (ref) | |
| Both | 1.86 (1.20 to 2.82) | 0.004 |
| Children | 1.66 (1.20 to 2.29) | 0.002 |
Logistic mixed-effects regression. The analysis of neutral reviews is additionally adjusted for overdispersion.
Bold value indicates result is statistically significant with p<0.05 or lower.
*Journal impact factor was base 2 log-transformed, so OR is the change per twofold change in journal impact factor.
BW, body weight; CI, confidence interval; LES, low-energy sweeteners; n, sample size; OR, odds ratio; ref, reference variable.
Network analysis
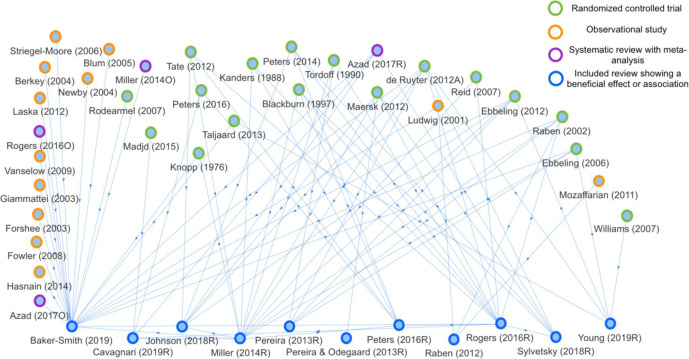
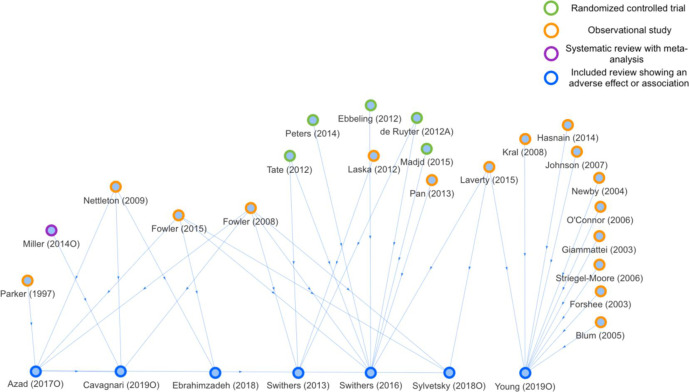
Figures 2 and 3, online supplemental figures 2 and 3 show the network analysis divided into subgroups based on the conclusions of the EAUs with cited articles coloured based on study type.
Figure 2.
Network analysis based on evidence assessmentunits concluding a beneficial effect or association of low-energy sweeteners with body weight (n=11). Cited articles (n=33) are marked based on the type of study.
Figure 3.
Network analysis based on evidence assessment units concluding an adverse effect or association of low-energy sweeteners with body weight (n=7). Cited articles (n=22) are marked based on the type of study.
bmjnph-2020-000210supp004.pdf (444.7KB, pdf)
bmjnph-2020-000210supp005.pdf (772.6KB, pdf)
EAUs concluding a beneficial effect or association (figure 2) cited mainly RCTs, with the exception of Baker-Smith et al,18 who cited a large number of observational studies. Conversely, EAUs reporting an adverse effect or association (figure 3) cited mostly observational studies. More observational studies than RCTs were cited in EAUs concluding a neutral effect or association of LES on BW (online supplemental figure S1). EAUs concluding that there was insufficient evidence to draw a conclusion (online supplemental figure S2) cited a mix of both RCTs and observational studies.
Online supplemental figures S3-S6 show network analysis divided into subgroups based on the conclusions of the EAUs with cited articles coloured based on conclusion of the article. EAUs concluding a beneficial effect or association of LES with BW tended to cite articles which also concluded a beneficial effect or association (online supplemental figure S3). Similarly, EAUs concluding an adverse effect or association tended to cite articles which also concluded an adverse effect or association of LES with BW (online supplemental figure S4). EAUs concluding a neutral effect or association (online supplemental figure S5) and EAUs reporting insufficient evidence to draw a conclusion (online supplemental figure S6) cited articles with various conclusions.
bmjnph-2020-000210supp006.pdf (618.9KB, pdf)
bmjnph-2020-000210supp007.pdf (408.2KB, pdf)
bmjnph-2020-000210supp008.pdf (449.7KB, pdf)
bmjnph-2020-000210supp009.pdf (784.1KB, pdf)
Comparing figure 2 with online supplemental figure S3 shows that most of the cited RCTs reported a beneficial effect of LES on BW. Similarly, comparing figure 3 with online supplemental figure S4 shows that most of the cited observational studies reported either a neutral or an adverse association of LES on BW.
Discussion
This study assessed the pattern of citations in reviews on the relationship of LES with BW-related outcomes. Surprisingly, across all reviews only a few consistent determinants of likelihood of citation were evident, favouring systematic reviews with meta-analysis, studies on children and publications in higher impact journals. In the overall data set, there was little clear quantitative association between the direction of review conclusions on the LES–BW relationship (neutral, adverse, beneficial) and the conclusions of the cited articles. The network analysis indicated that individual reviews concluding a beneficial relationship of LES with BW cited mainly RCTs, whereas the reviews concluding an adverse relationship cited mainly observational studies.
Taken together, this shows a very diverse and inconsistent pattern of citations, suggesting that the citation of evidence across reviews overall is somewhat arbitrary, which may contribute to the diversity in review conclusions. For individual reviews, conclusions mapped onto different patterns of cited evidence providing support for those conclusions.
This is the first citation analysis of its kind in relation to LES, and we believe it has several strengths. First, the approach was systematic and pre-planned for the search and selection of review articles, extraction of data and analyses. This reduced the potential for bias and strengthened the likely reproducibility of the study. Second, citations were only extracted from sections used as evidence for the LES–BW relationships and conclusions of the reviews. This approach ensured that only relevant articles cited as evidence were included.
The analysis also has a number of potential limitations. We were limited to using a database (Web of Science) from which it was possible to readily extract citations. The risk of only using one database is that relevant reviews may have been missed, although it would still provide an unbiased, reasonably comprehensive and representative sampling of reviews in this field. Future analyses may benefit from extraction of citations from several databases to extend the citation network with additional relevant reviews. Second, some of the guiding criteria and principles for the cited article selection and data extraction, as described in the methods, had to be operationalized by the authors specifically for this research, because there were no pre-existing recommended guidelines. Although this approach provided an objective and transparently replicable basis for decision-making, there is always some possible subjectivity and thus potential bias. This was minimised by requiring that the entire process of study selection and data extraction was agreed by two independent assessors against our defined criteria. Lastly, quality assessment of the included reviews was not undertaken, since this was not of primary interest in the present study. However, quality assessment of included reviews can potentially add an extra element, providing information about a possible association between the quality of reviews and citation of different types of primary studies. From a broader perspective, formulation of standard approaches and guidelines for citation analyses should be encouraged, similar to the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines’ for systematic reviews and meta-analyses.205
The present findings for LES and BW differ somewhat from other citation analyses in nutrition research. Leng14 examined how the first RCTs evaluating the efficacy of cholesterol-lowering diets in the secondary prevention of coronary heart disease were interpreted in reviews of the literature. They concluded that reviews supporting dietary interventions underutilised the available RCTs to a greater extent than other reviews. However, in contrast to the present study, their research used only network analysis as methodology and did not include a statistical analysis of the specific factors underlying citation of studies in reviews. Urlings et al found a higher likelihood of being cited for studies with statistically significant results in the literature of dietary trans fatty acids and serum cholesterol.13 Sample size, JIF and authority of the author were also important determinants of citation. One significant finding from our analysis was that OR for being cited was higher for articles on children compared with articles in adults. This was also evident in several of the subgroup analyses. However, a simple explanation for this consistent finding may be that the number of cited articles on children (n=49) was considerably smaller than the number of cited articles on adults (n=89), suggesting that the total available evidence for children is smaller, thus making those articles more frequently cited in reviews.
A surprising result was the limited evidence for quantitative relationships between conclusions of reviews and the citing of articles with corresponding conclusions. A possible explanation for this can be that, in the absence of a quantitative (meta-) analysis, review authors selectively interpret some of the articles they cite in reviews. This is supported by the surprisingly large percentage (almost 15%) of cited articles which had no relevant conclusion at all in relation to LES and BW. Subgroup analysis showed that articles which contained information about the relationship of LES with BW, but from which it was not possible to draw a conclusion, were significantly more likely to be cited in reviews concluding a neutral effect or association. Thus, variable interpretations of the LES–BW relationship may arise from differences in the qualitative ‘weights’ authors assign to different specific parts of the same cited evidence base, possibly influenced by their prior beliefs or published views on this relationship. It can also arise if qualitative conclusions are being influenced by other less direct evidence, such as mechanistic studies (which were excluded from this analysis). Across systematic reviews, important differences may arise from choices relating to setting inclusion and exclusion criteria, prior to (or even while) performing the review (see below).
Taken together, these findings show a very arbitrary pattern of citation across the overall body of reviews, which could be explained by selective choice and interpretation of cited articles by review authors. A well-known weakness of narrative reviews is the lack of a systematic search process, leaving space for selective and potentially biased citing of evidence. However, systematic reviews (with or without meta-analysis) are also potentially subject to citation bias through subjective choices made in the inclusion and exclusion criteria, and how studies are grouped or compared. In the case of the LES–BW relationship, recent systematic reviews have differed markedly in, for example, arbitrary restrictions on the types of LES exposures included, duration of RCTs or follow-up of prospective cohort studies, differences in comparators (water or sugar, for example) and so on, all leading to conclusions being based on quite different evidence sets.8 Over half of the EAUs concluded that evidence was insufficient to draw a conclusion about the relationship of LES with BW. It is a subjective judgement as to when evidence is believed to be sufficient to draw a conclusion within a specific area. However, the network analysis showed that differing conclusions are qualitatively associated with differing degrees of citation of RCTs vs observational evidence. If both types of evidence are cited and assigned equal weight in the overall interpretation, it is perhaps not surprising if authors deem it impossible to draw a conclusion.
Several approaches are suggested to address citation bias in future reviews of this topic. First, it has been recommended that new research is always placed in the context of the totality of different types of evidence, with consideration of their relative strengths and weaknesses.15 Looking at the totality of evidence will per definition lead to avoidance of citation bias. Furthermore, it can potentially close anticipated gaps in the literature, and focus the resources on the true gaps of evidence. Citation of the totality of evidence in reviews must be encouraged independent of the direction of the results. Arguably, this is what systematic reviews already should do, so perhaps greater emphasis needs to be given to better justification and consensus on the criteria for nature and quality of evidence. Second, when concluding that evidence is insufficient to draw a conclusion, more attention should be given to elaborating the specific needs for research that would resolve the gaps. This also raises the question of whether the topic really suffers from gaps in the evidence itself, or gaps in the consistent understanding and use of the existing evidence. These approaches are suggested to limit the number of reviews concluding simply that ‘evidence is insufficient to draw a conclusion’, and to focus instead on where there is consensus and remaining gaps in the literature on the effects of LES in relation to BW.
One of the more interesting findings of this study is from the network analyses showing that reviews concluding a beneficial relationship of LES with BW cited mainly RCTs, whereas reviews concluding an adverse relationship cited mainly observational studies. These findings represent a possible source of citation bias in the included reviews. However, from this analysis it is not possible to identify whether differences in cited articles arise due to ‘neutral’ processes used to select the literature or whether this is potentially (with intent or not) biased by review authors’ view of the relationship of LES with BW. Variation in the literature cited across reviews can only be explained by a few characteristics of the cited articles, suggesting that citation across reviews assessing the relationship of LES with BW is overall inconsistent and arbitrary. Inconsistent use of the available evidence may allow and account for the diversity of conclusions in the currently available reviews on LES and BW. Replication of the current analyses with further expansion or more types of analyses would be useful to confirm or refute the observations and suggested explanations given here.
Footnotes
Contributors: The project was initiated by DM. All authors (MN, DM, AR and CR) made substantial contributions to the development of idea and methods. The protocol was drafted by MN with support from DM, AR and CR. Extraction of data was done by MN and DM and discussed with AR, where needed. The manuscript draft was written by MN, and carefully revised by DM, AR and CR. All authors were involved in both the analysis and interpretation of the data. MN and CR were responsible for the statistical analyses. The final manuscript was approved by all authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: AR has received financial support from Unilever and International Sweeteners Association (ISA) and project co-coordinator of the EU Horizon 2020 project SWEET, grant number 884293. DM is an advisor to project SWEET and a former employee of Unilever.
Provenance and peer review: Not commissioned; externally peer reviewed by Kees de Graaf, Wageningen, the Netherlands (kees.degraaf@wur.nl) and Peter Rogers, United Kingdom (peter.rogers@bristol.ac.uk).
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available from UCPH upon request to the first author, e-mail: mino@nexs.ku.dk. Protocol is available at https://osf.io/9ghws.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Rogers PJ. The role of low-calorie sweeteners in the prevention and management of overweight and obesity: evidence v. conjecture. Proc Nutr Soc 2018;77:230–8. 10.1017/S0029665117004049 [DOI] [PubMed] [Google Scholar]
- 2. Swithers SE. Artificial sweeteners produce the Counterintuitive effect of inducing metabolic derangements. Trends Endocrinol Metab 2013;24:431–41. 10.1016/j.tem.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rogers PJ, Hogenkamp PS, de Graaf C, et al. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int J Obes 2016;40:381–94. 10.1038/ijo.2015.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller PE, Perez V. Low-Calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr 2014;100:765–77. 10.3945/ajcn.113.082826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cavagnari BM. Non-caloric sweeteners and body weight. Medicina 2019;79:115–22. [PubMed] [Google Scholar]
- 6. Ebrahimzadeh V, Ardalan MR, Mahdavi AM, et al. A review of the health hazards of artificial sweeteners: are they safe? Prog Nutr 2018;20:36–43. 10.23751/pn.v20i2-S.5901 [DOI] [Google Scholar]
- 7. Swithers SE. Not-so-healthy sugar substitutes? Curr Opin Behav Sci 2016;9:106–10. 10.1016/j.cobeha.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hunter SR, Reister EJ, Cheon E, et al. Low calorie sweeteners differ in their physiological effects in humans. Nutrients 2019;11:2717 10.3390/nu11112717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Durán Agüero S, Angarita Dávila L, Escobar Contreras MC, et al. Noncaloric sweeteners in children: a controversial theme. Biomed Res Int 2018;2018:1–7. 10.1155/2018/4806534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wiebe N, Padwal R, Field C, et al. A systematic review on the effect of sweeteners on glycemic response and clinically relevant outcomes. BMC Med 2011;9:1–18. 10.1186/1741-7015-9-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JPT, Thomas J, Chandler J. Cochrane Handbook for systematic reviews of interventions. 2nd ed. Oxford: The Cochrane Collaboration and John Wiley & Sons Ltd, 2019: 3–694 p. [Google Scholar]
- 12. Song F, Parekh, S, Hooper L, et al. Dissemination and publication of research findings: an updated review of related biases. Health Technol Assess 2010;14:1–220. 10.3310/hta14080 [DOI] [PubMed] [Google Scholar]
- 13. Urlings MJE, Duyx B, Swaen GMH, et al. Citation bias in the literature on dietary trans fatty acids and serum cholesterol. J Clin Epidemiol 2019;106:88–97. 10.1016/j.jclinepi.2018.10.008 [DOI] [PubMed] [Google Scholar]
- 14. Leng RI. A network analysis of the propagation of evidence regarding the effectiveness of fat-controlled diets in the secondary prevention of coronary heart disease (CHD): selective citation in reviews. PLoS One 2018;13:e0197716. 10.1371/journal.pone.0197716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mela DJ, McLaughlin J, Rogers PJ. Perspective: Standards for Research and Reporting on Low-Energy (“Artificial”) Sweeteners. Adv Nutr 2020;00:1–8. 10.1093/advances/nmz137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. RStudio Team . RStudio: Intehrated development for R. PBC, Boston, MA: RStudio, 2020. [Google Scholar]
- 17. Azad MB, Abou-Setta AM, Chauhan BF, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. Can Med Assoc J 2017;189:E929–39. 10.1503/cmaj.161390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baker-Smith CM, de Ferranti SD, Cochran WJ, et al. The use of nonnutritive sweeteners in children. Pediatrics 2019;144:e20192765. 10.1542/peds.2019-2765 [DOI] [PubMed] [Google Scholar]
- 19. Brown RJ, de Banate MA, Rother KI. Artificial sweeteners: a systematic review of metabolic effects in youth. Int J Pediatr Obes 2010;5:305–12. 10.3109/17477160903497027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bruyère O, Ahmed SH, Atlan C, et al. Review of the nutritional benefits and risks related to intense sweeteners. Arch Public Health 2015;73:1–10. 10.1186/s13690-015-0092-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fernstrom JD. Non-Nutritive sweeteners and obesity. Annu Rev Food Sci Technol 2015;6:119–36. 10.1146/annurev-food-022814-015635 [DOI] [PubMed] [Google Scholar]
- 22. Ferreira AVM, Generoso SV, Teixeira AL. Do low-calorie drinks 'cheat' the enteral-brain axis? Curr Opin Clin Nutr Metab Care 2014;17:465–70. 10.1097/MCO.0000000000000082 [DOI] [PubMed] [Google Scholar]
- 23. Gardner C. Non-Nutritive sweeteners: evidence for benefit vs. risk. Curr Opin Lipidol 2014;25:80–4. 10.1097/MOL.0000000000000034 [DOI] [PubMed] [Google Scholar]
- 24. Gardner C, Wylie-Rosett J, Gidding SS, et al. Nonnutritive sweeteners: current use and health perspectives: a scientific statement from the American heart association and the American diabetes association. Circulation 2012;126:509–19. 10.1161/CIR.0b013e31825c42ee [DOI] [PubMed] [Google Scholar]
- 25. Green CH, Syn W-K. Non-Nutritive sweeteners and their association with the metabolic syndrome and non-alcoholic fatty liver disease: a review of the literature. Eur J Nutr 2019;58:1785–800. 10.1007/s00394-019-01996-5 [DOI] [PubMed] [Google Scholar]
- 26. Johnson RK, Lichtenstein AH, Anderson CAM, et al. Low-Calorie sweetened beverages and cardiometabolic health: a science Advisory from the American heart association. Circulation 2018;138:e126–40. 10.1161/CIR.0000000000000569 [DOI] [PubMed] [Google Scholar]
- 27. Karalexi MA, Mitrogiorgou M, Georgantzi GG, et al. Non-Nutritive sweeteners and metabolic health outcomes in children: a systematic review and meta-analysis. J Pediatr 2018;197:128–33. 10.1016/j.jpeds.2018.01.081 [DOI] [PubMed] [Google Scholar]
- 28. Lohner S, Toews I, Meerpohl JJ. Health outcomes of non-nutritive sweeteners: analysis of the research landscape. Nutr J 2017;16:1–21. 10.1186/s12937-017-0278-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pereira MA. Diet beverages and the risk of obesity, diabetes, and cardiovascular disease: a review of the evidence. Nutr Rev 2013;71:433–40. 10.1111/nure.12038 [DOI] [PubMed] [Google Scholar]
- 30. Pereira MA, Odegaard AO. Artificially sweetened Beverages—Do they influence cardiometabolic risk? Curr Atheroscler Rep 2013;15:1–6. 10.1007/s11883-013-0375-z [DOI] [PubMed] [Google Scholar]
- 31. Peters JC, Beck J. Low calorie sweetener (LCS) use and energy balance. Physiol Behav 2016;164:524–8. 10.1016/j.physbeh.2016.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raben A, Richelsen B. Artificial sweeteners: a place in the field of functional foods? focus on obesity and related metabolic disorders. Curr Opin Clin Nutr Metab Care 2012;15:597–604. 10.1097/MCO.0b013e328359678a [DOI] [PubMed] [Google Scholar]
- 33. Reid AE, Chauhan BF, Rabbani R, et al. Early exposure to Nonnutritive sweeteners and long-term metabolic health: a systematic review. Pediatrics 2016;137:e20153603. 10.1542/peds.2015-3603 [DOI] [PubMed] [Google Scholar]
- 34. Serra-Majem L, Raposo A, Aranceta-Bartrina J, et al. Ibero⁻American consensus on low- and No-Calorie sweeteners: safety, nutritional aspects and benefits in food and beverages. Nutrients 2018;10:818. 10.3390/nu10070818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shankar P, Ahuja S, Sriram K. Non-Nutritive sweeteners: review and update. Nutrition 2013;29:1293–9. 10.1016/j.nut.2013.03.024 [DOI] [PubMed] [Google Scholar]
- 36. Sylvetsky A, Rother KI, Brown R. Artificial sweetener use among children: epidemiology, recommendations, metabolic outcomes, and future directions. Pediatr Clin North Am 2011;58:1467–80. 10.1016/j.pcl.2011.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sylvetsky AC, Rother KI. Nonnutritive sweeteners in weight management and chronic disease: a review. Obesity 2018;26:635–40. 10.1002/oby.22139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Toews I, Lohner S, Küllenberg de Gaudry D, et al. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ 2019;364:k4718–13. 10.1136/bmj.k4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Young J, Conway EM, Rother KI, et al. Low‐calorie sweetener use, weight, and metabolic health among children: a mini‐review. Pediatr Obes 2019;14:1–7. 10.1111/ijpo.12521 [DOI] [PubMed] [Google Scholar]
- 40. Archibald AJ, Dolinsky VW, Azad MB. Early-Life exposure to non-nutritive sweeteners and the developmental origins of childhood obesity: global evidence from human and rodent studies. Nutrients 2018;10:194. 10.3390/nu10020194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Swithers SE, Ogden SB, Laboy AF, et al. Saccharin pre-exposure enhances appetitive flavor learning in pre-weanling rats. Dev Psychobiol 2012;54:818–24. 10.1002/dev.21047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chia CW, Shardell M, Tanaka T, et al. Chronic low-calorie sweetener use and risk of abdominal obesity among older adults: a cohort study. PLoS One 2016;11:e0167241. 10.1371/journal.pone.0167241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Crichton G, Alkerwi Ala'a, Elias M. Diet soft drink consumption is associated with the metabolic syndrome: a two sample comparison. Nutrients 2015;7:3569–86. 10.3390/nu7053569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Imamura F, O'Connor L, Ye Z, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015;351:h3576. 10.1136/bmj.h3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Borges MC, Louzada ML, de Sá TH, et al. Artificially sweetened beverages and the response to the global obesity crisis. PLoS Med 2017;14:e1002195. 10.1371/journal.pmed.1002195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bortsov AV, Liese AD, Bell RA, et al. Sugar-Sweetened and diet beverage consumption is associated with cardiovascular risk factor profile in youth with type 1 diabetes. Acta Diabetol 2011;48:275–82. 10.1007/s00592-010-0246-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gu X, Tucker KL. Dietary intakes of the US child and adolescent population and their adherence to the current dietary guidelines: trends from 1999 to 2012. Faseb J 2017;31:29.1.27682203 [Google Scholar]
- 48. Han E, Powell LM. Consumption patterns of sugar-sweetened beverages in the United States. J Acad Nutr Diet 2013;113:43–53. 10.1016/j.jand.2012.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu LL, Lawrence JM, Davis C, et al. Prevalence of overweight and obesity in youth with diabetes in USA: the search for diabetes in youth study. Pediatr Diabetes 2010;11:4–11. 10.1111/j.1399-5448.2009.00519.x [DOI] [PubMed] [Google Scholar]
- 50. Pan A, Malik VS, Hao T, et al. Changes in water and beverage intake and long-term weight changes: results from three prospective cohort studies. Int J Obes 2013;37:1378–85. 10.1038/ijo.2012.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Agúero SD, Batten EB, Noel M. Association between non-nutritive sweeteners and obesity risk among university students in Latin America. Rev Med Chil 2015;143:367–73. [DOI] [PubMed] [Google Scholar]
- 52. Durán Agüero S, Vásquez Leiva A, Morales Illanes G, Agüero SD, Leiva AV, Illanes GM, et al. [ASSOCIATION BETWEEN STEVIA SWEETENER CONSUMPTION AND NUTRITIONAL STATUS IN UNIVERSITY STUDENTS]. Nutr Hosp 2015;32:362–6. 10.3305/nh.2015.32.1.8961 [DOI] [PubMed] [Google Scholar]
- 53. Appleton KM, Conner MT. Body weight, body-weight concerns and eating styles in habitual heavy users and non-users of artificially sweetened beverages. Appetite 2001;37:225–30. 10.1006/appe.2001.0435 [DOI] [PubMed] [Google Scholar]
- 54. Azad MB, Sharma AK, de Souza RJ, et al. Association between artificially sweetened beverage consumption during pregnancy and infant body mass index. JAMA Pediatr 2016;170:662–70. 10.1001/jamapediatrics.2016.0301 [DOI] [PubMed] [Google Scholar]
- 55. Bellisle F, Altenburg de Assis MA, Fieux B, et al. Use of ‘light’ foods and drinks in French adults: biological, anthropometric and nutritional correlates. J Hum Nutr Diet 2001;14:191–206. 10.1046/j.1365-277X.2001.00289.x [DOI] [PubMed] [Google Scholar]
- 56. Bouchard DR, Ross R, Janssen I. Coffee, tea and their additives: association with BMI and waist circumference. Obes Facts 2010;3:345–52. 10.1159/000322915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen L, Appel LJ, Loria C, et al. Reduction in consumption of sugar-sweetened beverages is associated with weight loss: the premier trial. Am J Clin Nutr 2009;89:1299–306. 10.3945/ajcn.2008.27240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Drewnowski A, Rehm CD. The use of low-calorie sweeteners is associated with self-reported prior intent to lose weight in a representative sample of US adults. Nutr Diabetes 2016;6:e202. 10.1038/nutd.2016.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. French SA, Sherwood NE, JaKa MM, et al. Physical changes in the home environment to reduce television viewing and sugar-sweetened beverage consumption among 5- to 12-year-old children: a randomized pilot study. Pediatr Obes 2016;11:e12–15. 10.1111/ijpo.12067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. García-Meseguer MJ, Burriel FC, García C. Consumption of non caloric sweeteners in University population. Ann Nutr Metab 2013;63:1103. [Google Scholar]
- 61. Duran Agüero S, Oñate G, Haro Rivera P. Consumption of non-nutritive sweeteners and nutritional status in 10-16 year old students. Arch Argent Pediatr 2014;112:207–14. 10.5546/aap.2014.207 [DOI] [PubMed] [Google Scholar]
- 62. Geraldo A, Pinto-e-Silva M. Factors associated with diet soda consumption by employees of public universities in São Paulo state (Brazil). Obes Facts 2013;6:150. [Google Scholar]
- 63. Hammersley R, Reid M, Ballantyne C, et al. Obese women partially compensate for sucrose added to the diet, without weight gain, over 28 days. Proc Nutr Soc 2011;70:E384. 10.1017/S0029665111004691 [DOI] [Google Scholar]
- 64. Kanders BS, Lavin PT, Kowalchuk MB, et al. An evaluation of the effect of aspartame on weight loss. Appetite 1988;11:73–84. 10.1016/S0195-6663(88)80050-3 [DOI] [PubMed] [Google Scholar]
- 65. Kassi E, Landis G, Pavlaki A. Long-Term effects of Stevia rebaudiana on glucose and lipid profile, adipocytokines, markers of inflammation and oxidation status in patients with metabolic syndrome. Endocr Abstr 2016;41:EP545. [Google Scholar]
- 66. Kim EJ, Kim MY, Kim J-S. Effects of fructooligosaccharides intake on body weight, lipid profiles, and calcium status among Korean college students. Faseb J 2011;25:771.4. [Google Scholar]
- 67. Ledoux TA, Watson K, Barnett A, et al. Components of the diet associated with child adiposity: a cross-sectional study. J Am Coll Nutr 2011;30:536–46. 10.1080/07315724.2011.10720000 [DOI] [PubMed] [Google Scholar]
- 68. Leon AS, Hunninghake DB, Bell C, et al. Safety of long-term large doses of aspartame. Arch Intern Med 1989;149:2318–24. 10.1001/archinte.1989.00390100120026 [DOI] [PubMed] [Google Scholar]
- 69. Maki KC, Curry LL, Carakostas MC, et al. The hemodynamic effects of rebaudioside a in healthy adults with normal and low-normal blood pressure. Food Chem Toxicol 2008;46:S40–6. 10.1016/j.fct.2008.04.040 [DOI] [PubMed] [Google Scholar]
- 70. Maki KC, Curry LL, Reeves MS, et al. Chronic consumption of rebaudioside a, a steviol glycoside, in men and women with type 2 diabetes mellitus. Food and Chemical Toxicology 2008;46:S47–53. 10.1016/j.fct.2008.05.007 [DOI] [PubMed] [Google Scholar]
- 71. Vazquez-Duran M, Castillo Martinez L, Orea Tejeda A. Effect of decreasing the consumption of sweetened caloric and non-caloric beverages on weight, body composition and blood pressure in young adults. Eur J Prev Cardiol 2013;1:S120. [Google Scholar]
- 72. Ali F. Consumption of artificial sweeteners in pregnancy increased overweight risk in infants. Arch Dis Child Educ Pract Ed 2017;102:277. 10.1136/archdischild-2017-312618 [DOI] [PubMed] [Google Scholar]
- 73. Markey O, Le Jeune J, Lovegrove JA. Energy compensation following consumption of sugar-reduced products: a randomized controlled trial. Eur J Nutr 2016;55:2137–49. 10.1007/s00394-015-1028-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pereira MA. Sugar-Sweetened and artificially-sweetened beverages in relation to obesity risk. Adv Nutr 2014;5:797–808. 10.3945/an.114.007062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Porikos K, Hesser M, VANITALLIE T. Caloric regulation in normal-weight men maintained on a palatable diet of concentional foods☆. Physiol Behav 1982;29:293–300. 10.1016/0031-9384(82)90018-X [DOI] [PubMed] [Google Scholar]
- 76. Raben A, Møller AC, Vasilaras TH, et al. A randomized 10 week trial of sucrose vs artificial sweeteners on body weight and blood pressure after 10 weeks. Obes Res 2001;3:86S. [Google Scholar]
- 77. Reyna NY, Cano C, Bermúdez VJ. Sweeteners and beta-glucans improve metabolic and anthropometrics variables in well controlled type 2 diabetic patients. Am J Ther 2003;10:438–43. 10.1097/00045391-200311000-00010 [DOI] [PubMed] [Google Scholar]
- 78. Serra‐Majem L, Ribas L, Inglès C, et al. Cyclamate consumption in Catalonia, Spain (1992): relationship with the body mass index. Food Addit Contam 1996;13:695–703. 10.1080/02652039609374455 [DOI] [PubMed] [Google Scholar]
- 79. Shin DH, Lee JH, Kang MS, et al. Glycemic effects of rebaudioside A and erythritol in people with glucose intolerance. Diabetes Metab J 2016;40:283–9. 10.4093/dmj.2016.40.4.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sørensen LB, Vasilaras TH, Astrup A, et al. Sucrose compared with artificial sweeteners: a clinical intervention study of effects on energy intake, appetite, and energy expenditure after 10 wk of supplementation in overweight subjects. Am J Clin Nutr 2014;100:36–45. 10.3945/ajcn.113.081554 [DOI] [PubMed] [Google Scholar]
- 81. Stellman SD, Garfinkel L. Artificial sweetener use and one-year weight change among women. Prev Med 1986;15:195–202. 10.1016/0091-7435(86)90089-7 [DOI] [PubMed] [Google Scholar]
- 82. Van Wymelbeke V, Béridot-Thérond M-E, de La Guéronnière V, et al. Influence of repeated consumption of beverages containing sucrose or intense sweeteners on food intake. Eur J Clin Nutr 2004;58:154–61. 10.1038/sj.ejcn.1601762 [DOI] [PubMed] [Google Scholar]
- 83. de Ruyter JC, Olthof MR, Seidell JC, et al. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med 2012;367:1397–406. 10.1056/NEJMoa1203034 [DOI] [PubMed] [Google Scholar]
- 84. Vázquez-Durán M, Orea-Tejeda A, Castillo-Martínez L, et al. A randomized control trial for reduction of caloric and non-caloric sweetened beverages in young adults: effects in weight, body composition and blood pressure. Nutr Hosp 2016;33:1372–8. 10.20960/nh.797 [DOI] [PubMed] [Google Scholar]
- 85. Wulaningsih W, Van Hemelrijck M, Tsilidis KK, et al. Investigating nutrition and lifestyle factors as determinants of abdominal obesity: an environment-wide study. Int J Obes 2017;41:340–7. 10.1038/ijo.2016.203 [DOI] [PubMed] [Google Scholar]
- 86. Cancer Prevention study II . The American cancer Society prospective study. Stat Bull Metrop Insur Co 1992;73:21–9. [PubMed] [Google Scholar]
- 87. Gatenby SJ, Aaron JI, Jack VA, et al. Extended use of foods modified in fat and sugar content: nutritional implications in a free-living female population. Am J Clin Nutr 1997;65:1867–73. 10.1093/ajcn/65.6.1867 [DOI] [PubMed] [Google Scholar]
- 88. Gostner A, Schäffer V, Theis S, et al. Effects of isomalt consumption on gastrointestinal and metabolic parameters in healthy volunteers. Br J Nutr 2005;94:575–81. 10.1079/BJN20051510 [DOI] [PubMed] [Google Scholar]
- 89. Njike VY, Faridi Z, Shuval K, et al. Effects of sugar-sweetened and sugar-free cocoa on endothelial function in overweight adults. Int J Cardiol 2011;149:83–8. 10.1016/j.ijcard.2009.12.010 [DOI] [PubMed] [Google Scholar]
- 90. Bleich SN, Wang YC, Wang Y, et al. Increasing consumption of sugar-sweetened beverages among US adults: 1988–1994 to 1999–2004. Am J Clin Nutr 2009;89:372–81. 10.3945/ajcn.2008.26883 [DOI] [PubMed] [Google Scholar]
- 91. Odegaard AO, Choh AC, Czerwinski SA, et al. Sugar-Sweetened and diet beverages in relation to visceral adipose tissue. Obesity 2012;20:689–91. 10.1038/oby.2011.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Catenacci VA, Pan Z, Thomas JG, et al. Low/No calorie sweetened beverage consumption in the National weight control registry. Obesity 2014;22:2244–51. 10.1002/oby.20834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Phelan S, Lang W, Jordan D, et al. Use of artificial sweeteners and fat-modified foods in weight loss maintainers and always-normal weight individuals. Int J Obes 2009;33:1183–90. 10.1038/ijo.2009.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ebbeling CB, Feldman HA, Chomitz VR, et al. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med 2012;367:1407–16. 10.1056/NEJMoa1203388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bellisle F, Drewnowski A, Anderson GH, et al. Sweetness, satiation, and satiety. J Nutr 2012;142:1149S–54. 10.3945/jn.111.149583 [DOI] [PubMed] [Google Scholar]
- 96. Hu FB, Malik VS. Sugar-Sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiol Behav 2010;100:47–54. 10.1016/j.physbeh.2010.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr 2009;89:1–14. 10.3945/ajcn.2008.26792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mattes RD, Shikany JM, Kaiser KA, et al. Nutritively sweetened beverage consumption and body weight: a systematic review and meta-analysis of randomized experiments. Obes Rev 2011;12:346–65. 10.1111/j.1467-789X.2010.00755.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Naismith DJ, Rhodes C. Adjustment in energy intake following the covert removal of sugar from the diet. J Hum Nutr Diet 1995;8:167–75. 10.1111/j.1365-277X.1995.tb00309.x [DOI] [Google Scholar]
- 100. Fowler SPG. Low-Calorie sweetener use and energy balance: results from experimental studies in animals, and large-scale prospective studies in humans. Physiol Behav 2016;164:517–23. 10.1016/j.physbeh.2016.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Romo-Romo A, Gómez-Díaz RA, Brito-Córdova GX, et al. Non-Nutritive sweeteners: evidence on their association with metabolic diseases and potential effects on glucose metabolism and appetite. RIC 2017;69:129–38. 10.24875/RIC.17002141 [DOI] [PubMed] [Google Scholar]
- 102. Anderson GH, Foreyt J, Sigman-Grant M, et al. The use of low-calorie sweeteners by adults: impact on weight management. J Nutr 2012;142:1163s–9. 10.3945/jn.111.149617 [DOI] [PubMed] [Google Scholar]
- 103. Abou-Donia MB, El-Masry EM, Abdel-Rahman AA, et al. Splenda alters gut microflora and increases intestinal P-glycoprotein and cytochrome P-450 in male rats. J Toxicol Environ Health A 2008;71:1415–29. 10.1080/15287390802328630 [DOI] [PubMed] [Google Scholar]
- 104. Davidson TL, Swithers SE. A Pavlovian approach to the problem of obesity. Int J Obes 2004;28:933–5. 10.1038/sj.ijo.0802660 [DOI] [PubMed] [Google Scholar]
- 105. Foreyt J, Kleinman R, Brown RJ, et al. The use of low-calorie sweeteners by children: implications for weight management. J Nutr 2012;142:1155S–62. 10.3945/jn.111.149609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Feijó FdeM, Ballard CR, Foletto KC, et al. Saccharin and aspartame, compared with sucrose, induce greater weight gain in adult Wistar rats, at similar total caloric intake levels. Appetite 2013;60:203–7. 10.1016/j.appet.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 107. Foletto KC, Melo Batista BA, Neves AM, et al. Sweet taste of saccharin induces weight gain without increasing caloric intake, not related to insulin-resistance in Wistar rats. Appetite 2016;96:604–10. 10.1016/j.appet.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 108. Swithers SE, Davidson TL. A role for sweet taste: calorie predictive relations in energy regulation by rats. Behav Neurosci 2008;122:161–73. 10.1037/0735-7044.122.1.161 [DOI] [PubMed] [Google Scholar]
- 109. Grotz VL, Pi-Sunyer X, Porte D, et al. A 12-week randomized clinical trial investigating the potential for sucralose to affect glucose homeostasis. Regul Toxicol Pharmacol 2017;88:22–33. 10.1016/j.yrtph.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 110. Pase MP, Himali JJ, Beiser AS, et al. Sugar- and artificially sweetened beverages and the risks of incident stroke and dementia. Stroke 2017;48:1139–46. 10.1161/STROKEAHA.116.016027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sylvetsky AC, Blau JE, Rother KI. Understanding the metabolic and health effects of low-calorie sweeteners: methodological considerations and implications for future research. Rev Endocr Metab Disord 2016;17:187–94. 10.1007/s11154-016-9344-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Frey GH. Use of aspartame by apparently healthy children and adolescents. J Toxicol Environ Health 1976;2:401–15. 10.1080/15287397609529442 [DOI] [PubMed] [Google Scholar]
- 113. Kuzma JN, Cromer G, Hagman DK, et al. No difference in ad libitum energy intake in healthy men and women consuming beverages sweetened with fructose, glucose, or high-fructose corn syrup: a randomized trial. Am J Clin Nutr 2015;102:1373–80. 10.3945/ajcn.115.116368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Reid M, Hammersley R, Duffy M, et al. Effects on obese women of the sugar sucrose added to the diet over 28 D: a quasi-randomised, single-blind, controlled trial. Br J Nutr 2014;111:563–70. 10.1017/S0007114513002687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chantelau EA, Gösseringer G, Sonnenberg GE, et al. Moderate intake of sucrose does not impair metabolic control in pump-treated diabetic out-patients. Diabetologia 1985;28:204–7. 10.1007/BF00282233 [DOI] [PubMed] [Google Scholar]
- 116. Forshee RA, Storey ML. Total beverage consumption and beverage choices among children and adolescents. Int J Food Sci Nutr 2003;54:297–307. 10.1080/09637480120092143 [DOI] [PubMed] [Google Scholar]
- 117. Seferidi P, Millett C, Laverty AA. Sweetened beverage intake in association to energy and sugar consumption and cardiometabolic markers in children. Pediatr Obes 2018;13:195–203. 10.1111/ijpo.12194 [DOI] [PubMed] [Google Scholar]
- 118. Sylvetsky AC. Metabolic effects of Low‐Calorie sweeteners: a brief review. Obesity 2018;26:S25–31. 10.1002/oby.22252 [DOI] [PubMed] [Google Scholar]
- 119. Foods Standards Australia & New Zealand . Intense Sweeteners [Internet], 2019. Available: www.foodstandards.gov.au/consumer/additives/Pages/Sweeteners.aspx
- 120. Chen C. Non-calorie artificial sweeteners affect body weight: a meta-analysis of randomised controlled trials. Challenges to evidence-based heal care Cochrane Abstr 24th Cochrane Colloq, 2016. [Google Scholar]
- 121. Zheng M, Allman-Farinelli M, Heitmann BL, et al. Liquid versus solid energy intake in relation to body composition among Australian children. J Hum Nutr Diet 2015;28 Suppl 2:70–9. 10.1111/jhn.12223 [DOI] [PubMed] [Google Scholar]
- 122. Sylvetsky AC, Welsh JA, Brown RJ, et al. Low-Calorie sweetener consumption is increasing in the United States. American Journal of Clinical Nutrition 2012;96:640–6. 10.3945/ajcn.112.034751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Frank GKW, Oberndorfer TA, Simmons AN, et al. Sucrose activates human taste pathways differently from artificial sweetener. Neuroimage 2008;39:1559–69. 10.1016/j.neuroimage.2007.10.061 [DOI] [PubMed] [Google Scholar]
- 124. Katan MB, de Ruyter JC, Kuijper LDJ, et al. Impact of masked replacement of sugar-sweetened with sugar-free beverages on body weight increases with initial BMI: secondary analysis of data from an 18 month double-blind trial in children. PLoS One 2016;11:e0159771. 10.1371/journal.pone.0159771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2012;346:e7492. 10.1136/bmj.e7492 [DOI] [PubMed] [Google Scholar]
- 126. O'Connor TM, Yang S-J, Nicklas TA. Beverage intake among preschool children and its effect on weight status. Pediatrics 2006;118:e1010–8. 10.1542/peds.2005-2348 [DOI] [PubMed] [Google Scholar]
- 127. Scharf RJ, DeBoer MD. Sugar-Sweetened beverages and children's health. Annu Rev Public Health 2016;37:273–93. 10.1146/annurev-publhealth-032315-021528 [DOI] [PubMed] [Google Scholar]
- 128. Bucher Della Torre S, Keller A, Laure Depeyre J, et al. Sugar-Sweetened Beverages and Obesity Risk in Children and Adolescents: A Systematic Analysis on How Methodological Quality May Influence Conclusions. J Acad Nutr Diet 2016;116:638–59. 10.1016/j.jand.2015.05.020 [DOI] [PubMed] [Google Scholar]
- 129. Vos MB, Kaar JL, Welsh JA, et al. Added sugars and cardiovascular disease risk in children: a scientific statement from the American heart association. Circulation 2017;135:E1017–34. 10.1161/CIR.0000000000000439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Williams CL, Strobino BA, Brotanek J. Weight control among obese adolescents: a pilot study. Int J Food Sci Nutr 2007;58:217–30. 10.1080/09637480701198083 [DOI] [PubMed] [Google Scholar]
- 131. Zhu Y, Olsen SF, Mendola P, et al. Maternal consumption of artificially sweetened beverages during pregnancy, and offspring growth through 7 years of age: a prospective cohort study. Int J Epidemiol 2017;46:1499–508. 10.1093/ije/dyx095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Berkey CS, Rockett HRH, Field AE, et al. Sugar-added beverages and adolescent weight change. Obes Res 2004;12:778–88. 10.1038/oby.2004.94 [DOI] [PubMed] [Google Scholar]
- 133. Blum JW, Jacobsen DJ, Donnelly JE. Beverage consumption patterns in elementary school aged children across a two-year period. J Am Coll Nutr 2005;24:93–8. 10.1080/07315724.2005.10719449 [DOI] [PubMed] [Google Scholar]
- 134. Giammattei J, Blix G, Marshak HH. Television watching and soft drink consumption - Associations with obesity in 11-to 13-year-old schoolchildren. Arch Pediatr Adolesc Med 2003;157:882–6. [DOI] [PubMed] [Google Scholar]
- 135. Hasnain SR, Singer MR, Bradlee ML, et al. Beverage intake in early childhood and change in body fat from preschool to adolescence. Childhood Obesity 2014;10:42–9. 10.1089/chi.2013.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Johnson L, Mander AP, Jones LR, et al. Is sugar-sweetened beverage consumption associated with increased fatness in children? Nutrition 2007;23:557–63. 10.1016/j.nut.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 137. Katzmarzyk P, Broyles S, Champagne C, et al. Relationship between soft drink consumption and obesity in 9–11 years old children in a Multi-National study. Nutrients 2016;8:770 10.3390/nu8120770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kral TVE, Stunkard AJ, Berkowitz RI, et al. Beverage consumption born at different risk of patterns of children obesity. Obesity 2008;16:1802–8. 10.1038/oby.2008.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Laverty AA, Magee L, Monteiro CA, et al. Sugar and artificially sweetened beverage consumption and adiposity changes: national longitudinal study. Int J Behav Nutr Phys Act 2015;12:1–10. 10.1186/s12966-015-0297-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. The Lancet 2001;357:505–8. 10.1016/S0140-6736(00)04041-1 [DOI] [PubMed] [Google Scholar]
- 141. Newby PK, Peterson KE, Berkey CS, et al. Beverage consumption is not associated with changes in weight and body mass index among low-income preschool children in North Dakota. J Am Diet Assoc 2004;104:1086–94. 10.1016/j.jada.2004.04.020 [DOI] [PubMed] [Google Scholar]
- 142. Rodearmel SJ, Wyatt HR, Stroebele N, et al. Small changes in dietary sugar and physical activity as an approach to preventing excessive weight gain: the America on the move family study. Pediatrics 2007;120:e869–79. 10.1542/peds.2006-2927 [DOI] [PubMed] [Google Scholar]
- 143. Striegel-Moore RH, Thompson D, Affenito SG, et al. Correlates of beverage intake in adolescent girls: the National heart, lung, and blood Institute growth and health study. J Pediatr 2006;148:183–7. 10.1016/j.jpeds.2005.11.025 [DOI] [PubMed] [Google Scholar]
- 144. Sylvetsky AC, Jin Y, Mathieu K, et al. Low-Calorie sweeteners: disturbing the energy balance equation in adolescents? Obesity 2017;25:2049–54. 10.1002/oby.22005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Taljaard C, Covic NM, van Graan AE, et al. Effects of a multi-micronutrient-fortified beverage, with and without sugar, on growth and cognition in South African schoolchildren: a randomised, double-blind, controlled intervention. Br J Nutr 2013;110:2271–84. 10.1017/S000711451300189X [DOI] [PubMed] [Google Scholar]
- 146. Blackburn GL, Kanders BS, Lavin PT, et al. The effect of aspartame as part of a multidisciplinary weight-control program on short- and long-term control of body weight. Am J Clin Nutr 1997;65:409–18. 10.1093/ajcn/65.2.409 [DOI] [PubMed] [Google Scholar]
- 147. Ferri LAF, Alves-Do-Prado W, Yamada SS, et al. Investigation of the antihypertensive effect of oral crude stevioside in patients with mild essential hypertension. Phytother. Res. 2006;20:732–6. 10.1002/ptr.1944 [DOI] [PubMed] [Google Scholar]
- 148. Field AE, Sonneville KR, Falbe J, et al. Association of sports drinks with weight gain among adolescents and young adults. Obesity 2014;22:2238–43. 10.1002/oby.20845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Fowler SP, Williams K, Resendez RG, et al. Fueling the obesity epidemic? artificially sweetened beverage use and long-term weight gain. Obesity 2008;16:1894–900. 10.1038/oby.2008.284 [DOI] [PubMed] [Google Scholar]
- 150. Fowler SPG, Williams K, Hazuda HP. Diet soda intake is associated with long-term increases in waist circumference in a Biethnic cohort of older adults: the San Antonio longitudinal study of aging. J Am Geriatr Soc 2015;63:708–15. 10.1111/jgs.13376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Gearon E, Peeters A, Hodge A, et al. The role of dietary and physical activity behaviours in educational differences in weight gain among Australian adults – the Melbourne Collaborative cohort study. Obes Res Clin Pract 2014;8:35–6. 10.1016/j.orcp.2014.10.065 [DOI] [Google Scholar]
- 152. Haines J, Neumark-Sztainer D, Wall M, et al. Personal, behavioral, and environmental risk and protective factors for adolescent overweight. Obesity 2007;15:2748–60. 10.1038/oby.2007.327 [DOI] [PubMed] [Google Scholar]
- 153. Hsieh M-H, Chan P, Sue Y-M, et al. Efficacy and tolerability of oral stevioside in patients with mild essential hypertension: a two-year, randomized, placebo-controlled study. Clin Ther 2003;25:2797–808. 10.1016/S0149-2918(03)80334-X [DOI] [PubMed] [Google Scholar]
- 154. Lana A, Lopez-Garcia E, Rodríguez-Artalejo F. Consumption of soft drinks and health-related quality of life in the adult population. Eur J Clin Nutr 2015;69:1226–32. 10.1038/ejcn.2015.103 [DOI] [PubMed] [Google Scholar]
- 155. Madjd A, Taylor MA, Delavari A, et al. Effects on weight loss in adults of replacing diet beverages with water during a hypoenergetic diet: a randomized, 24-wk clinical trial. Am J Clin Nutr 2015;102:1305–12. 10.3945/ajcn.115.109397 [DOI] [PubMed] [Google Scholar]
- 156. Maersk M, Belza A, Stødkilde-Jørgensen H, et al. Sucrose-Sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr 2012;95:283–9. 10.3945/ajcn.111.022533 [DOI] [PubMed] [Google Scholar]
- 157. Nettleton JA, Lutsey PL, Wang Y, et al. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the multi-ethnic study of atherosclerosis (MESA). Diabetes Care 2009;32:688–94. 10.2337/dc08-1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Parker DR, Gonzalez S, Derby CA, et al. Dietary factors in relation to weight change among men and women from two southeastern new England communities. Int J Obes 1997;21:103–9. 10.1038/sj.ijo.0800373 [DOI] [PubMed] [Google Scholar]
- 159. Peters JC, Beck J, Cardel M, et al. The effects of water and non-nutritive sweetened beverages on weight loss and weight maintenance: a randomized clinical trial. Obesity 2016;24:297–304. 10.1002/oby.21327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Smith JD, Hou T, Hu FB, et al. A comparison of different methods for evaluating diet, physical activity, and long-term weight gain in 3 prospective cohort studies. J Nutr 2015;145:2527–34. 10.3945/jn.115.214171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Tate DF, Turner-McGrievy G, Lyons E, et al. Replacing caloric beverages with water or diet beverages for weight loss in adults: main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr 2012;95:555–63. 10.3945/ajcn.111.026278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Benton D. Can artificial sweeteners help control body weight and prevent obesity? Nutr Res Rev 2005;18:63–76. 10.1079/NRR200494 [DOI] [PubMed] [Google Scholar]
- 163. Ebbeling CB, Feldman HA, Osganian SK, et al. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics 2006;117:673–80. 10.1542/peds.2005-0983 [DOI] [PubMed] [Google Scholar]
- 164. Garavaglia MB, Rodríguez García V, Zapata ME, et al. Non-Nutritive sweeteners: children and adolescent consumption and food sources. Arch Argent Pediatr 2018;116:186–91. 10.5546/aap.2018.eng.186 [DOI] [PubMed] [Google Scholar]
- 165. Knopp RH, Brandt K, Arky RA. Effects of aspartame in young persons during weight reduction. J Toxicol Environ Health 1976;2:417–28. 10.1080/15287397609529443 [DOI] [PubMed] [Google Scholar]
- 166. Laska MN, Murray DM, Lytle LA, et al. Longitudinal associations between key dietary behaviors and weight gain over time: transitions through the adolescent years. Obesity 2012;20:118–25. 10.1038/oby.2011.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Millichap JG, Yee MM. The diet factor in attention-deficit/hyperactivity disorder. Pediatrics 2012;129:330–7. 10.1542/peds.2011-2199 [DOI] [PubMed] [Google Scholar]
- 168. Raben A, Vasilaras TH, Møller AC, et al. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr 2002;76:721–9. 10.1093/ajcn/76.4.721 [DOI] [PubMed] [Google Scholar]
- 169. Swithers SE, Martin AA, Clark KM, et al. Body weight gain in rats consuming sweetened liquids. Effects of caffeine and diet composition. Appetite 2010;55:528–33. 10.1016/j.appet.2010.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Sylvetsky AC, Jin Y, Clark EJ, et al. Consumption of low-calorie sweeteners among children and adults in the United States. J Acad Nutr Diet 2017;117:441–8. 10.1016/j.jand.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Vanselow MS, Pereira MA, Neumark-Sztainer D, et al. Adolescent beverage habits and changes in weight over time: findings from project EAT. Am J Clin Nutr 2009;90:1489–95. 10.3945/ajcn.2009.27573 [DOI] [PubMed] [Google Scholar]
- 172. Colditz GA, Willett WC, Stampfer MJ, et al. Patterns of weight change and their relation to diet in a cohort of healthy women. Am J Clin Nutr 1990;51:1100–5. 10.1093/ajcn/51.6.1100 [DOI] [PubMed] [Google Scholar]
- 173. de la Hunty A, Gibson S, Ashwell M. A review of the effectiveness of aspartame in helping with weight control. Nutr Bulletin 2006;31:115–28. 10.1111/j.1467-3010.2006.00564.x [DOI] [Google Scholar]
- 174. Duffey KJ, Steffen LM, Van Horn L, et al. Dietary patterns matter: diet beverages and cardiometabolic risks in the longitudinal coronary artery risk development in young adults (CARDIA) study. Am J Clin Nutr 2012;95:909–15. 10.3945/ajcn.111.026682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Mozaffarian D, Hao T, Rimm EB, et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404. 10.1056/NEJMoa1014296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Raben A, Møller B, Flint A. Increased postprandial glycaemia, insulinemia, and lipidemia after 10 weeks’ sucrose-rich diet compared to an artificially sweetened diet: a randomised controlled trial. Food Nutr Res 2011;55:1–13. 10.3402/fnr.v55i0.5961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Reid M, Hammersley R, Duffy M. Effects of sucrose drinks on macronutrient intake, body weight, and mood state in overweight women over 4 weeks. Appetite 2010;55:130–6. 10.1016/j.appet.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 178. Reid M, Hammersley R, Hill AJ, et al. Long-Term dietary compensation for added sugar: effects of supplementary sucrose drinks over a 4-week period. Br J Nutr 2007;97:193–203. 10.1017/S0007114507252705 [DOI] [PubMed] [Google Scholar]
- 179. Schulze MB, Manson JE, Ludwig DS. Sugar-Sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–34. 10.1001/jama.292.8.927 [DOI] [PubMed] [Google Scholar]
- 180. Sørensen LB, Raben A, Stender S, et al. Effect of sucrose on inflammatory markers in overweight humans. Am J Clin Nutr 2005;82:421–7. 10.1093/ajcn/82.2.421 [DOI] [PubMed] [Google Scholar]
- 181. Stellman S, Garfinkel L. Patterns of artificial sweetener use and weight change in an American cancer society prospective study. Appetite 1988;11:85–91 10.1016/0195-6663(88)90048-7 [DOI] [PubMed] [Google Scholar]
- 182. French S, Rosenberg M, Wood L, et al. Soft drink consumption patterns among Western Australians. J Nutr Educ Behav 2013;45:525–32. 10.1016/j.jneb.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 183. Ruanpeng D, Thongprayoon C, Cheungpasitporn W, et al. Sugar and artificially sweetened beverages linked to obesity: a systematic review and meta-analysis. QJM 2017;110:513–20. 10.1093/qjmed/hcx068 [DOI] [PubMed] [Google Scholar]
- 184. Collison KS, Makhoul NJ, Zaidi MZ, et al. Interactive effects of neonatal exposure to monosodium glutamate and aspartame on glucose homeostasis. Nutr Metab 2012;9:58. 10.1186/1743-7075-9-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Collison KS, Makhoul NJ, Zaidi MZ, et al. Gender dimorphism in aspartame-induced impairment of spatial cognition and insulin sensitivity. PLoS One 2012;7:e31570. 10.1371/journal.pone.0031570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Dhingra R, Sullivan L, Jacques PF, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007;116:480–8. 10.1161/CIRCULATIONAHA.107.689935 [DOI] [PubMed] [Google Scholar]
- 187. Fagherazzi G, Vilier A, Sartorelli DS, et al. Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the Etude Epidémiologique auprès des femmes de la Mutuelle Générale de l'Education Nationale–European prospective investigation into cancer and nutrition cohort. Am J Clin Nutr 2013;97:517–23. 10.3945/ajcn.112.050997 [DOI] [PubMed] [Google Scholar]
- 188. Kuk JL, Brown RE. Aspartame intake is associated with greater glucose intolerance in individuals with obesity. Appl Physiol Nutr Metab 2016;41:795–8. 10.1139/apnm-2015-0675 [DOI] [PubMed] [Google Scholar]
- 189. Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis risk in Communities study. Circulation 2008;117:754–61. 10.1161/CIRCULATIONAHA.107.716159 [DOI] [PubMed] [Google Scholar]
- 190. Nakagawa Y, Nagasawa M, Yamada S, et al. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS One 2009;4:e5106. 10.1371/journal.pone.0005106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. O’Connor L, Imamura F, Lentjes MAH, et al. Prospective associations and population impact of sweet beverage intake and type 2 diabetes, and effects of substitutions with alternative beverages. Diabetologia 2015;58:1474–83. 10.1007/s00125-015-3572-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Pepino MY, Bourne C. Non-Nutritive sweeteners, energy balance, and glucose homeostasis. Curr Opin Clin Nutr Metab Care 2011;14:391–5. 10.1097/MCO.0b013e3283468e7e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014;514:181–6. 10.1038/nature13793 [DOI] [PubMed] [Google Scholar]
- 194. Swithers SE, Baker CR, Davidson TL. General and persistent effects of high-intensity sweeteners on body weight gain and caloric compensation in rats. Behav Neurosci 2009;123:772–80. 10.1037/a0016139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Swithers SE, Laboy AF, Clark K, et al. Experience with the high-intensity sweetener saccharin impairs glucose homeostasis and GLP-1 release in rats. Behav Brain Res 2012;233:1–14. 10.1016/j.bbr.2012.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Swithers SE, Sample CH, Davidson TL. Adverse effects of high-intensity sweeteners on energy intake and weight control in male and obesity-prone female rats. Behav Neurosci 2013;127:262–74. 10.1037/a0031717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. de Koning L, Malik VS, Kellogg MD, et al. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation 2012;125:1735–41. 10.1161/CIRCULATIONAHA.111.067017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. de Koning L, Malik VS, Rimm EB, et al. Sugar-Sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr 2011;93:1321–7. 10.3945/ajcn.110.007922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. de Ruyter JC, Olthof MR, Kuijper LDJ, et al. Effect of sugar-sweetened beverages on body weight in children: design and baseline characteristics of the double-blind, randomized intervention study in kids. Contemp Clin Trials 2012;33:247–57. 10.1016/j.cct.2011.10.007 [DOI] [PubMed] [Google Scholar]
- 200. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non‐nutritive sweetened beverages on weight loss during a 12‐week weight loss treatment program. Obesity 2014;22:1415–21. 10.1002/oby.20737 [DOI] [PubMed] [Google Scholar]
- 201. Porikos KP, Booth G, Van Itallie TB. Effect of covert nutritive dilution on the spontaneous food intake of obese individuals: a pilot study. Am J Clin Nutr 1977;30:1638–44. 10.1093/ajcn/30.10.1638 [DOI] [PubMed] [Google Scholar]
- 202. Porikos KP, Pi-Sunyer FX. Regulation of food intake in human obesity: studies with caloric dilution and exercise. Clin Endocrinol Metab 1984;13:547–61. 10.1016/S0300-595X(84)80037-7 [DOI] [PubMed] [Google Scholar]
- 203. Tordoff MG, Alleva AM. Effect of drinking soda sweetened with aspartame or high-fructose corn syrup on food intake and body weight. Am J Clin Nutr 1990;51:963–9. 10.1093/ajcn/51.6.963 [DOI] [PubMed] [Google Scholar]
- 204. Davidson TL, Martin AA, Clark K, et al. Intake of high-intensity sweeteners alters the ability of sweet taste to signal caloric consequences: implications for the learned control of energy and body weight regulation. Q J Exp Psychol 2011;64:1430–41. 10.1080/17470218.2011.552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjnph-2020-000210supp001.pdf (55.3KB, pdf)
bmjnph-2020-000210supp002.pdf (126.4KB, pdf)
bmjnph-2020-000210supp003.pdf (63.7KB, pdf)
bmjnph-2020-000210supp004.pdf (444.7KB, pdf)
bmjnph-2020-000210supp005.pdf (772.6KB, pdf)
bmjnph-2020-000210supp006.pdf (618.9KB, pdf)
bmjnph-2020-000210supp007.pdf (408.2KB, pdf)
bmjnph-2020-000210supp008.pdf (449.7KB, pdf)
bmjnph-2020-000210supp009.pdf (784.1KB, pdf)
Data Availability Statement
Data are available from UCPH upon request to the first author, e-mail: mino@nexs.ku.dk. Protocol is available at https://osf.io/9ghws.