Abstract
Background
Fecal calprotectin (FC) is a non‐invasive marker of gut inflammation which is frequently used to guide therapeutic decisions in patients with inflammatory bowel diseases (IBD). Each step of FC measurement can influence the results, leading to misinterpretations and potentially impacting the management of IBD patients. To date, there is high heterogeneity between FC measurements and no current method is universally accepted as a standard.
Aims
Our aim was to provide clear position statementsabout the pre‐analytical and the analytical phases of FC measurement to homogenize FC levels and to minimize variability and risk of misinterpretation through aninternational consensus.
Materials & Methods
Fourteen physicians with expertise in the field of IBD and FC from 11 countries attended a virtual international consensus meeting on July 17th, 2020. A systematic literature was conducted and the literature evidence was shared and discussedamong the participants. Statements were formulated, discussed, and voted. Statements were considered approved if all participants agreed.
Results
Nine statements were formulated and approved. Based on the available evidence, quantitative tests should be preferred for measuring FC. Furthermore, FC measurement, if possible, should always be performed with the same method and factors influencing FC levels should be taken into account when interpreting the results.
Discussion
FC has an increasingly important role in the management of patients with IBD. However, large multicenter studies should be conducted to define the reproducibility and to confirm the diagnostic accuracy of the available FC tests.
Conclusion
FC concentrations guide clinicians' treatment decisions. Our statements have a relevant impact in daily practice and could be applied in clinical trials to standardize FC measurement.
Keywords: fecal calprotectin, inflammatory bowel disease, measurement, standardization
Key Summary
Summarise the established knowledge on this subject.
‐ FC is a surrogate non‐invasive marker of gut inflammation.
‐ FC is closely correlated with endoscopic and histological activity of disease.
‐ High variability exists between FC measurements.
‐ There is no globally accepted cut‐off of FC.
What are the significant findings of this study?
‐ Stool consistency can influence FC extraction.
‐ Quantitative tests are recommended for FC measurement.
‐ Serial FC measurement in an individual patient should be performed with the same FC test.
‐ Interpretation of FC measurement results should include the evaluation of factors that may influence the test.
INTRODUCTION
Crohn's disease (CD) and ulcerative colitis (UC) are chronic inflammatory bowel diseases (IBD) with a remitting and relapsing course requiring periodic follow‐up. 1 , 2 Endoscopy is the gold standard for IBD monitoring as it directly visualizes the mucosa to assess the presence of inflammation. 3 However, endoscopy is expensive, time‐consuming, can cause discomfort to patients and requires bowel preparation. 4 , 5 , 6 To overcome these limitations, the use of fecal calprotectin (FC) has been proposed. 7 FC is considered a surrogate non‐invasive marker of the infiltration of neutrophils in the intestinal mucosa and an increase in FC levels is associated with intestinal inflammation. 8 FC plays an increasingly crucial role in the management of IBD patients. It is closely correlated with endoscopic and histological activity of disease and allows to distinguish IBD from irritable bowel syndrome (IBS), assesses disease activity and response to therapy, and predicts disease recurrence. 7 , 9 , 10 Despite its proven clinical utility, high variability exists between FC measurements, preventing the definition of a globally accepted cut‐off for the interpretation of FC results. 11 , 12 FC measurement consists of two main phases, a pre‐analytical and an analytical phase. 8 The pre‐analytical phase involves timing of stool sampling, collection, storage, and FC extraction. 8 , 13 The test to be used and the threshold for result interpretation must be decided during the analytical phase. 8 , 13 Each step can influence FC results and contribute to the heterogeneity of FC measurement. 8 It is important to emphasize that an error in this process can cause impaired assessment of disease activity, impacting the medical decision‐making process and leading to an over‐ or under‐treatment. To date, no standardization regarding FC measurement is available. Thus, following a comprehensive literature review, 8 we aimed to provide clear position statements about the pre‐analytical and the analytical phases of FC measurement to homogenize FC levels and to minimize variability and risk of misinterpretation through an international consensus.
METHODS
A virtual consensus meeting was organized on July 17th, 2020 to define the optimal method for measuring FC in order to reduce the heterogeneity and homogenize the measurements. Fourteen physicians with expertise in the field of IBD and FC (FDA, DTR, PGK, FM, BS, TK, PO, PB, LP, EL, ED, SG, SD, and LPB) from eleven countries worldwide (Argentina, Belgium, Brazil, England, France, Germany, Italy, Japan, Portugal, Spain, United States) attended the meeting. A systematic search of the scientific literature was conducted in the PubMed/MEDLINE, EMBASE [Excerpta Medica Database], and Cochrane databases up to January 2020 to identify all studies reporting data on FC measurement. Results from the literature search were recently published in extenso. 8 The literature evidence was shared and discussed among the participants. Subsequently, statements were formulated and discussed. Statements were considered approved if all participants agreed. If a statement was not unanimously accepted, it was re‐discussed and amended until all participants approved it. All experts were involved in drafting the manuscript and approved its final version.
RESULTS
All statements on the pre‐analytical and the analytical phases of FC measurements are shown in Table 1.
TABLE 1.
Statements for pre‐analytical and analytical phases of fecal calprotectin measurement
| Statement 1 | Feces should be collected in a dedicated clean container without additives to avoid any accidental contamination. |
| Statement 2 | The most appropriate timing for stool sampling is unclear. |
| Statement 3 | The analysis of a single stool sample is usually sufficient for FC measurement. |
| Statement 4 | Stool consistency can influence FC extraction. |
| Statement 5 | Stool storage at room temperature is preferably limited to 3 days, although up to one week is still acceptable. If the sample cannot be processed within 7 days, feces should be stored in a freezer at −20 C° for an optimal conservation. |
| Statement 6 | Quantitative tests are recommended for FC measurement. The ELISA tests and automated ELISA tests have an accurate analytical performance and should be preferred. Point of care tests and home tests represent valid alternatives to ELISA tests. |
| Statement 7 | There is insufficient evidence to support the use of one specific FC test over another. |
| Statement 8 | The tests for FC measurement are not interchangeable because there is a high variability between the different methods. If possible, serial FC measurement in an individual patient should be performed with the same FC test. |
| Statement 9 | Interpretation of FC measurement results should include the evaluation of factors that may influence the test. |
Abbreviations: ELISA, enzyme‐linked immunosorbent assay; FC, fecal calprotectin.
Pre‐analytical phase
Statement 1: Feces should be collected in a dedicated clean container without additives to avoid any accidental contamination.
The most common method of fecal sampling is with a dedicated container that is equipped with a spoon on the lid. This allows patients to handle the stool in a hygienically safe and convenient way. The spoon usually collects less than 5 g of feces, while the containers can generally contain up to 60 g of feces. 13 Of note, the amount needed for the analysis is definitely lower, about 100 mg. 12 , 14 , 15 A crucial aspect of this step is to avoid sample contamination with liquids (e.g., water or urine) in order to prevent sample dilution, but to reassure patients that if they are passing liquid stool, it should still be collected. Lasson et al. found that stool consistency was significantly correlated with FC levels (median r = 0.68, range: −0.68 to 0.87; p = 0.01), noting that the more liquid the stools, the higher the FC concentrations. 16 It is important to underline that the presence of liquid stools is not a contraindication to the FC assay as it is the most frequent situation in patients with active disease. However, stool consistency should be taken into account when interpreting the results. To date, it is not known whether the impact of liquid stools on FC values is due to technical issues related to greater difficulty in collecting liquid stool, to issues with sampling heterogeneity, or to the increased disease activity in these patients.
Statement 2: The most appropriate timing for stool sampling is unclear.
Several studies investigated the most appropriate timing for stool sampling. Calafat and colleagues enrolled 18 patients with acute UC flares to assess the intra‐individual variations in within‐day FC values. 17 All patients were asked to collect up to four stool samples within 24 h. In most cases, the highest FC value was obtained with the second stool sample of the day (44%) and a high variability between the different samples was found (median coefficient of variation = 40%). 17 Similarly, Kristensen et al. examined the FC values of three stool samples (morning, evening, and next morning) from 50 IBD patients, reporting a high coefficient of variation (39.4%, 95% CI 31.1%–47.7%). 18 Two other studies enrolling patients with active disease evaluated the variability of FC concentrations during the day, reporting variations of 52% and 61% between measurements. 11 , 16 It was noted that the longer the time between bowel movements, the higher the FC values, suggesting that the first sample in the morning could be preferred as it guaranteed the longest interval in patients without night evacuations. 16 A prospective observational cohort study including 45 patients with IBD (of which 21 with clinically active disease) showed that variability in FC concentrations ranged from 64% to 77% based on time of collection (morning, afternoon, evening). 19 Interestingly, if samples were stratified according to time of collection and FC threshold, a relatively small variability rate (7%) was found analyzing the first feces in the morning with a cut‐off of 250 µg/g. 19 The available evidence is not sufficient to recommend the correct timing of FC measurement and further studies on large populations are needed to address this topic. It is important to emphasize that within‐day variability is less relevant when considering a cut‐off which is significantly higher than the values generally used in clinical practice to define disease activity (>300 µg/g). For now, patients' convenience may drive the collection timing. Given this variability, patients should be advised to collect stool samples approximately at the same time in successive FC measurements.
Statement 3: The analysis of a single stool sample is usually sufficient for FC measurement.
The need to repeat the FC assay is heavily debated. A single‐center prospective study recruiting 98 CD patients in clinical remission assessed the FC variability on three consecutive days. 15 Consistency between FC levels was high as evidenced by the intra‐class correlation (ICC) value (0.84, 95% CI: 0.79–0.89). On the other hand, a relevant day‐to‐day variability was described in UC patients with a median coefficient of variation of 40.8% between two consecutive days. 16 An observational case‐control study by Cremer and colleagues enrolling patients with active and inactive disease confirmed the intra‐individual variability of FC concentrations between two different bowel movements within 1–6 days period, reporting a median variation coefficient of 36%. 12 At the same time, the FC amount in different punches of the same stool sample was also analyzed, finding a low median intra‐stool variability (17%). Another study including 63 patients with active CD found a high variability among the samples collected on two consecutive days (kappa 0.355; p < 0.0002). 20 Interestingly, the variability between measurements was greater with increasing FC levels. Furthermore, only in 6 cases (9%) a contrasting value (remission vs. disease activity, using a FC threshold of 200 mg/L) between the first and second measurement was found. These data are of crucial importance as they indicate that variability rarely affects therapeutic decisions. Consequently, despite the intra‐individual variability between FC measurements, repetition of FC assessment on two consecutive days is not to be recommended. 20 Instead, it may be prudent to repeat the procedure within a few weeks in case of borderline values that could influence the therapeutic decision, or in the suspicion of measurement inaccuracy and factors invalidating the result.
Statement 4: Stool consistency can influence FC extraction.
The extraction phase allows to separate the calprotectin from the feces. 8 This procedure requires that feces are first weighed and then positioned inside a buffer to obtain protein extraction. Weighing can be manually performed, but currently it is preferable to use devices that are specific to each measuring kit in order to collect a predefined amount of feces and to reduce sample manipulation. 8 There is high variability among commercially available extractors. Whitehead et al. found different FC values with three different extractors (Roche extraction device, Schebo device, Immunodiagnostik device) compared to manual extraction method and the lowest FC values were detected by analyzing liquid feces, suggesting how stool consistency could influence test results. 21 Similarly, Juricic et al. compared two extractors (EliA™ Calprotectin EliA SEK device and fCAL®Turbo Calex®Cap “N”), detecting significant differences in FC values based on stool consistency (27.0, 77.0, and 277.0 mg/kg vs. 103.5, 249.0, and 203.5 mg/kg in hard, normal, and liquid feces respectively, p < 0.001). 22 Liquid stools have been associated with both increase and decrease in FC levels and should therefore be avoided to prevent measurement errors. To overcome this technical issue, feces should be diluted in the extraction buffer and eventually centrifuged allowing the detection of sedimented particles. In addition, the use of a mix of four extracts from the same fecal sample showed to be well correlated with the standard extraction of a single punch (r 2 = 0.8517), indicating how this technique could homogenize the sample to be analyzed and reduce the intra‐individual variability. 23
Statement 5: Stool storage at room temperature is preferably limited to 3 days, although up to 1 week is still acceptable. If the sample cannot be processed within 7 days, feces should be stored in a freezer at −20 C° for an optimal conservation.
Stool storage is an essential step in FC measurement as the delay between stool sampling and analysis can lead to sample deterioration and to invalidate FC results. Røseth et al. investigated the temperature impact on stool storage in 111 patients (33 healthy subjects, 40 hospital controls, 21 CD, and 17 UC). 24 No significant difference in FC concentrations was found between fresh samples and those stored at room temperature (+20°C) for 7 days, suggesting that FC was resistant to intestinal proteolysis for up to one week. On the other hand, a study including patients with active UC showed that FC levels remained stable at room temperature for up to 3 days, while after 7 days a mean reduction of 28% was reported (p < 0.01; 95% CI 0.10–0.47). 16 Padoan et al. compared the stability of the samples stored at room temperature with those stored at 4°C. After one week, a greater sample deterioration was detected in samples at room temperature (p = 0.004), suggesting that temperature could alter FC concentrations. 25 Stool freezing at minus 20°C has been proposed to limit the progressive sample deterioration. Oyaert et al. assessed the effect of freezing on six extraction assays, revealing that in most cases (4/6, 66.6%) no reduction in FC occurred after freezing at minus 20°C. 26 Finally, Pelkmans et al. compared FC results in fresh fecal extracts and in aliquots frozen at minus 20°C of the same sample, confirming stability and comparability of the obtained values. 27 Based on these data, it would be preferable to process and analyze the stool samples as soon as possible. The physical characteristics of calprotectin allow it to be stable at room temperature for 3 days in optimal conditions and up to one week in acceptable conditions with a minimal reduction in FC concentrations. In case of further delay between collection and analysis, the unprocessed stool sample should be frozen to avoid its deterioration or sampling should be repeated.
Analytical phase
Statement 6: Quantitative tests are recommended for FC measurement. The enzyme‐linked immunosorbent assay (ELISA) tests and automated ELISA tests have an accurate analytical performance and should be preferred. Point of care (POC) tests and home tests represent valid alternatives to ELISA tests.
Growing evidence shows that different FC values can be associated with different outcomes in IBD patients, including endoscopic activity or remission and histological remission of disease. 8 , 9 , 28 , 29 Thus, it is of extreme relevance for clinicians to quantify FC concentrations. For this reason, quantitative tests are recommended over qualitative tests as the latter only express a positive or negative result according to the range and cut‐off provided by the manufacturer without indicating the FC value. The ELISA test is the most frequently used quantitative test for FC measurement and has been used for other purposes for almost 30 years. 24 , 30 ELISA tests have an accurate analytical performance but are time‐consuming as they are commonly performed as a batch‐like procedure every 1 or 2 weeks. 8 , 31 , 32 Automated ELISA tests are currently available allowing to analyze the samples at any time, even individually. Importantly, the diagnostic accuracy of these tests is equivalent to the traditional ELISA method. 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 Recently, point of care rapid tests and home tests have been developed. POC tests use specific lateral flow immunoassays. 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 Patients provide a stool sample which is collected, and an extract of the sample is prepared for analysis by a healthcare professional. Subsequently, the extracted sample is inserted into a reader and the quantitative or semi‐quantitative results are displayed on a connected computer within 30 min. POC tests are highly correlated with traditional ELISA tests 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 and their direct and indirect costs are lower than traditional ELISA tests. 30 The home tests allow to measure FC directly at home. It is imperative that patients have a smartphone compatible with the measurement test and that they download an application to perform data analysis. 51 In addition to collecting the feces, patients carry out the extraction process and after placing the feces in the specific reading cassette, they proceed with the sample analysis through the camera of their smartphone. Finally, the result is forwarded to the patient's healthcare team to remotely monitor FC levels. The home tests provide semi‐quantitative results and are sufficiently correlated with both standard ELISA and POC tests. 52 , 53 , 54 , 55 , 56 , 57 , 58 Interestingly, the correlation is high when FC values ≤500 μg/g are taken into consideration (agreement interval of 560, 455, and 458 μg/g with IBDoc®, QuantonCal®, and CalproSmartTM respectively), while the agreement is low when FC concentrations are >500 μg/g (agreement interval of 1160, 2392, and 1310 μg/g with IBDoc®, QuantonCal®, and CalproSmartTM respectively). 57 , 59 This aspect suggests that home tests can be used in daily clinical practice as threshold values ≤500 μg/g generally guide the therapeutic decisions. 60 , 61 It should be emphasized that the use of home tests requires high patient compliance and adequate training to avoid reduced test adherence or measurement errors. 55 , 62
Statement 7: There is insufficient evidence to support the use of one specific FC test over another.
Multiple tests are available for FC monitoring and several studies have compared their operating characteristics in order to identify the optimal method to be used in clinical practice. A study by Mirsepasi‐Lauridsen et al. compared the accuracy of 3 ELISA tests (EK‐CAL®, CALPROTM, and HK325®) in diagnosing IBD. 63 CALPROTM ELISA test achieved higher rates of specificity compared to the other tests but the study was neither designed nor powered to demonstrate the superiority of one method over the others. A head‐to‐head comparative trial evaluated the characteristics of 3 FC home tests. 59 No difference in terms of agreement with ELISA tests was found, but fewer reading errors (defined as an image of the test cassette not leading to quantitative result) were found with the IBDoc® compared with CalproSmartTM and QuantonCal® (1.9% vs. 5.8% and 4.8%, p < 0.05 for both comparisons). Moreover, a prospective cohort study investigated the performance of 6 tests (3 POC tests, 2 ELISA tests, and one automated ELISA test) for the diagnosis and follow‐up of 62 patients with suspected or confirmed diagnosis of IBD. 64 The tests showed a sensitivity of 75%–83% and a specificity of 68%–95% for the diagnosis of IBD considering a FC cut‐off of 50 μg/g. High sensitivity and specificity were also found in predicting moderate‐severe endoscopic disease activity in both CD and UC patients. As regards to mild disease activity, acceptable performance values were identified in patients with UC, while low sensitivity values were reported in CD patients. Overall, all assays were significantly correlated (p < 0.05) and no method proved to be superior to the others. Further comparative studies on large IBD populations are needed to assess whether a specific ELISA test, POC test, or home test has better diagnostic accuracy compared to the others and should be preferred.
Statement 8: The tests for FC measurement are not interchangeable because there is a high variability between the different methods. If possible, serial FC measurement in an individual patient should be performed with the same FC test.
A head‐to‐head trial compared the performance of two ELISA tests (CalproTM and Calprest®) in assessing disease activity in 116 patients with UC. 65 Both tests accurately predicted endoscopic disease activity defined as Mayo endoscopic score (MES) of 2–3 (AUC = 0.79 for CalproTM and AUC = 0.80 for Calprest®). However, different FC cut‐offs were identified (148 mg/kg for Calprest® and 208 mg/kg for CalproTM) and a difference of up to 30% in FC levels was found between the tools when FC values between 200 and 1000 mg/kg were analyzed. Similarly, Oyaert and colleagues investigated the accuracy of 6 automated ELISA tests in distinguishing between functional and organic bowel disorders. 66 A total of 105 patients with IBD, other gastroenterological diseases, or rheumatological conditions were enrolled, and all stool samples were extracted with the same device to reduce the variability risk. All tests showed a sensitivity of 100% and a specificity ranging from 58% to 78% at the manufacturer threshold of 50 μg/g. The areas under the curves (AUC) were very high (from 0.974 to 0.998) for all tests and no statistically significant difference was found. On the other hand, total imprecision values ranged from 1.5% to 23.3% and the difference between the tests increased with increasing FC concentrations. For this reason, it is legitimate to assume that the tests currently available to measure FC are not interchangeable. For patient follow‐up, preferentially, the same assay should be used. It would be desirable to have a reference stool sample for FC at the initiation of a new treatment which can be compared with the endoscopic evaluation at baseline, ensuring the reliability of the biochemical finding. This could allow for the evaluation of FC trend and could be used for patient monitoring, predicting any recurrence of disease and facilitating interpretation of the results.
Statement 9: Interpretation of FC measurement results should include the evaluation of factors that may influence the test.
FC is a marker of gut inflammation, but it is not specific for IBD as some gastrointestinal diseases, medication, and lifestyle can alter its concentrations. 8 Increased FC values have been found in patients with IBS, colon polyps, colonic diverticular disease, colon cancer, gastrointestinal bleeding, gastrointestinal infections, microscopic colitis, proctitis after radiation therapy, pouchitis, rheumatologic diseases, and liver cirrhosis (Table 2). 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 In addition, some medications such as non‐steroidal anti‐inflammatory drugs (NSAIDs) and proton pump inhibitors (PPI) have been associated with increased FC levels (up to 520 µg/g in NSAIDs users and 150 µg/g in PPI users). 78 , 79 , 80 , 81 , 82 Prolonged use of NSAIDs can lead to NSAID‐induced enteropathy which is characterized by intestinal erosions and ulcers. 82 For this reason, NSAIDs should be discontinued at least two weeks before FC measurement. 82 The rationale for the increase in FC after PPI treatment is unknown, however it may be related to the inhibition of gastric acid production, resulting in intestinal bacterial overgrowth. 78 , 83 Similarly, PPIs should be suspended 4 weeks prior to FC measurement, allowing for adequate drug wash out and preventing any measurement bias. 80 Furthermore, patients' age, including younger (<9 years) and older patients (>65 years), body mass index (obesity), physical inactivity, perianal disease, presence of an ostomy, and bowel preparation have been reported as factors associated with increased FC values. 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 FC is strongly correlated with disease activity in pregnant women with IBD and is frequently used for monitoring these patients. 92 However, a trend of progressive reduction in FC levels was found during pregnancy of IBD women suggesting that the immune changes occurring in pregnant women could be associated with a beneficial effect on disease activity. 93 Finally, interpretation of FC values should take into consideration all factors that may influence the FC results. In daily clinical practice, physicians should evaluate the alignment between FC concentrations and endoscopic and histologic data. The correlation between these findings could support the reliability of the fecal test. On the other hand, if no correlation is found or if there are factors influencing FC levels, FC measurement should be repeated after a few weeks.
TABLE 2.
Factors associated with increased fecal calprotectin concentration
| Gastrointestinal diseases | Range of FC increase (µg/g) |
|---|---|
| Colorectal neoplasia 70 | 57–133 |
| Colon polyps 68 | 1–117.7 |
| Colonic diverticular disease 69 | <15–60 |
| Bacterial and viral gastrointestinal infections 72 | 0–994 |
| Gastrointestinal bleeding 71 | <20–429 |
| Liver cirrhosis 73 | 21–357 |
| Irritable bowel syndrome 67 | 16–294 |
| Microscopic colitis 74 | 130–480 |
| Proctitis after radiation therapy 75 | 50–270 |
| Pouchitis 76 | 55–110 |
| Medication | |
| Non‐steroidal anti‐inflammatory drugs 82 | 5–520 |
| Proton pump inhibitors 79 | 50–150 |
| Lifestyle | |
| Obesity 90 | 5–185 |
| Physical inactivity 87 | 25–60 |
| Other | |
| Age < 9 years 85 | 18–213 |
| Age > 65 years 85 | 14–118 |
| Bowel preparation for colonoscopy 84 | 51–17,379 |
| Rheumatologic diseases 77 | 14–513 |
| Perianal disease 91 | 207–1705 |
| Stoma 88 | <150–1130 |
Abbreviation: FC, fecal calprotectin.
RESEARCH GAPS
Although FC has an increasingly important role in the management of patients with IBD, some research questions need to be further addressed. The standardization of FC measurement could allow to reduce the heterogeneity between the studies and to obtain more reliable data. First, it is necessary to define whether the timing of stool collection can affect the measurement results, clarifying whether the repetition of the FC measurement is useful or not (Figure). Second, the best strategy for stool storage at room temperature should be investigated and a comparative study evaluating FC concentrations in stool samples analyzed within 3 or 7 days should be conducted to address this question. Third, it is necessary to define how to deal with liquid stools as this is the most frequent situation in IBD patients, defining the best approach for stool collection and homogenization. Fourthly, large multicenter studies should be conducted to define the reproducibility and to confirm the diagnostic accuracy of the available FC tests. Fifthly, to date, there is no generally accepted FC cut‐off to distinguish activity or disease remission. The homogenization of the FC assay could allow clinicians to identify and validate a threshold that predicts disease activity, guiding therapeutic decisions and representing a new treat‐to‐target for IBD patients.
FIGURE 1.
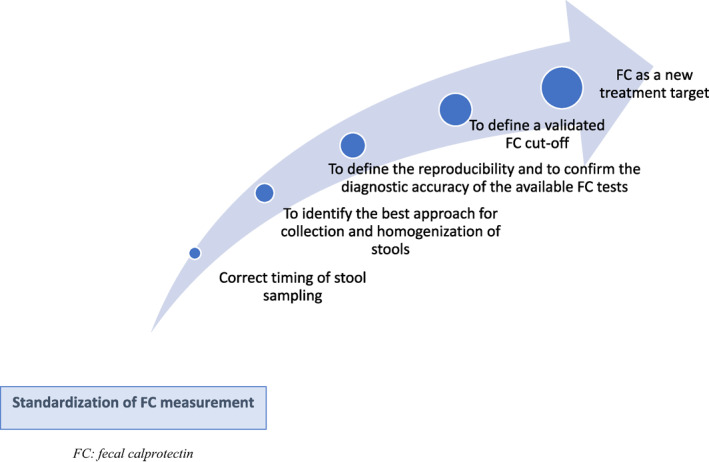
Research gaps that could be filled with standardization of fecal calprotectin measurement
CONCLUSION
FC is increasingly recognized as a treatment target for UC and CD and is commonly used in daily practice and clinical trials for the management of IBD patients. Several pre‐analytical and analytical variables can influence the FC measurement. We summarize available data and propose recommendations concerning FC measurement in order to standardize the method. The main limitation of our statements is the absence of a validated methodology to reach consensus such as the Delphi method. To the best of our knowledge, this is the first international consensus addressing this topic. A uniformly accepted protocol for FC measurement could allow to reduce inter‐assay variability and increase measurement reliability. Furthermore, large studies using a standardized method are needed to identify a FC cut‐off, predicting disease activity and guiding therapeutic decisions.
CONFLICT OF INTEREST
Ferdinando D’Amico declares no conflict of interest. David T. Rubin has received grant support from Takeda; and has served as a consultant for Abbvie, Abgenomics, Allergan Inc., Biomica, Boehringer Ingelheim Ltd., Bristol‐Myers Squibb, Celgene Corp/Syneos, Check‐cap, Dizal Pharmaceuticals, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, GlaxoSmithKline Services, Ichnos Sciences S.A., InDex Pharmaceuticals, Janssen Pharmaceuticals, Lilly, Narrow River Mgmt, Pfizer, Prometheus Laboratories, Reistone, Shire, Takeda, and Techlab Inc. Fernando Magro has served as a speaker and received honoraria from Merck Sharp & Dohme, Abbvie, Vifor, Falk, Laboratorios Vitoria, Ferring, Hospira, and Biogen. Britta Siegmund has served as Consultant for Abbvie, Boehringer, Celgene, Falk, Janssen, Lilly, Pfizer, Prometheus, Takeda and received speaker's fees from Abbvie, CED Service GmbH, Falk, Ferring, Janssen, Novartis, Takeda (BS served as representative of theCharité). Subrata Ghosh declares consulting fees from Pfizer, Janssen, AbbVie, Takeda, Bristol‐Myers Squibb, Receptos, Celgene, Gilead, Eli Lilly and Boehringer Ingelheim and speaker fees from AbbVie, Janssen, Takeda, Ferring, Shield, and Falk Pharma; outside of the submitted work. Taku Kobayashi has served as a speaker, a consultant or an advisory board member for AbbVie, Ajinomoto Pharma, Asahi Kasei Medical, Astellas, Alfresa Pharma, Celltrion, Covidien, EA Pharma, Eisai, Eli Lilly, Ferring Pharmaceuticals, Gilead Sciences, Janssen, JIMRO, Kyorin Pharmaceutical, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Nippon Kayaku, Pfizer, Takeda Pharmaceutical, Thermo Scientific, Zeria Pharmaceutical, and received research funding from AbbVie, Alfresa Pharma, Asahi Kasei Medical, EA Pharma, Kyorin Pharmaceutical, Mochida Pharmaceutical, Nippon Kayaku, Otsuka Holdings, Sekisui Medical, Thermo Fisher Scientific, Zeria Pharmaceutical. Paulo Gustavo Kotze reports personal fees from Abbvie, Janssen, Pfizer and Takeda; research grants from Pfizer and Takeda. Pablo A. Olivera received consulting fees from Abbvie, Takeda, and Janssen and lecture fees from Takeda and Janssen. Peter Bossuyt received has received financial support for research from AbbVie, Mundipharma, Pfizer, Janssen, Amgen, and Mylan; lecture fees from AbbVie, Takeda, Pfizer, and Janssen; advisory board fees from Abbvie, Takeda, Hospira, Janssen, Celltrion, BMS, Roche, Arena, MSD, Mundipharma, Roche, Pfizer, Sandoz, and Pentax. Lieven Pouillon received advisory board fees from Janssen and Takeda; presentation fees from Abbvie and Ferring; and personal fees from Abbvie, Ferring, Norgine, and Takeda. Edouard Louis reports grants from European Commission for BIOCYCLE Research Programme; grants and personal fees from Abbvie, Takeda, Pfizer and Janssen and personal fees from MSD, Ferring, Falk, and Celgene. Eugeni Domènech has served as a speaker and has received research and educational funding and advisory fees from MSD, AbbVie, Takeda, Kern Pharma, Pfizer, Janssen, Celgene, Adacyte Therapeutics, Otsuka Pharmaceuticals, Ferring, Shire Pharmaceuticals, Tillots, Thermofisher, Grifols, and Gebro. Silvio Danese has served as a speaker, consultant, and advisory board member for Schering‐Plough, AbbVie, Actelion, Alphawasserman, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson and Johnson, Millenium Takeda, MSD, Nikkiso Europe GmbH, Novo Nordisk, Nycomed, Pfizer, Pharmacosmos, UCB Pharma and Vifor. Laurent Peyrin‐Biroulet has served as a speaker, consultant and advisory board member for Merck, Abbvie, Janssen, Genentech, Mitsubishi, Ferring, Norgine, Tillots, Vifor, Hospira/Pfizer, Celltrion, Takeda, Biogaran, Boerhinger‐Ingelheim, Lilly, HAC‐ Pharma, Index Pharmaceuticals, Amgen, Sandoz, For‐ward Pharma GmbH, Celgene, Biogen, Lycera, Samsung Bioepis, Theravance.
Author’s contribution
Ferdinando D'Amico, wrote the first draft and created table and figure, discussed the statements and contributed to the final manuscript. David T Rubin, critically reviewed the content of the paper, Paulo Gustavo Kotze, critically reviewed the content of the paper, discussed the statements and contributed to the final manuscript. Fernando Magro, critically reviewed the content of the paper, discussed the statements and contributed to the final manuscript. Britta Siegmund, critically reviewed the content of the paper, discussed the statements and contributed to the final manuscript. Taku Kobayashi, critically reviewed the content of the paper, discussed the statements and contributed to the final manuscript. Pablo A. Olivera, critically reviewed the content of the paper, discussed the statements and contributed to the final manuscript. Peter Bossuyt, critically reviewed the content of the paper, discussed the statements and contributed to the final manuscript. Lieven Pouillon, critically reviewed the content of the paper, discussed the statements and contributed to the final manuscript. Edouard Louis, critically reviewed the content of the paper, discussed the statements and contributed to the final manuscript. Eugeni Domènech, critically reviewed the content of the paper, discussed the statements and contributed to the final manuscript. Subrata Ghosh, critically reviewed the content of the paper, discussed the statements and contributed to the final manuscript. Silvio Danese, conceived the study, critically reviewed the content of the paper, discussed the statements and contributed to the final manuscript. discussed the statements and contributed to the final manuscript. Laurent Peyrin‐Biroulet, conceived the study, critically reviewed the content of the paper, discussed the statements and contributed to the final manuscript.
ACKNOWLEDGMENTS
We thank Materia Prima for organizing the international virtual meeting and supporting the paper. This study was funded by Amgen via Materia Prima.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article
REFERENCES
- 1. Torres J, Mehandru S, Colombel J‐F, Peyrin‐Biroulet L. Crohn's disease. The Lancet. 2017;389:1741–55. [DOI] [PubMed] [Google Scholar]
- 2. Ungaro R, Mehandru S, Allen PB, Peyrin‐Biroulet L, Colombel J‐F. Ulcerative colitis. The Lancet. 2017;389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982–1018. [DOI] [PubMed] [Google Scholar]
- 4. Motaganahalli S, Beswick L, Con D, van Langenberg DR. Faecal calprotectin delivers on convenience, cost reduction and clinical decision‐making in inflammatory bowel disease: a real‐world cohort study. Intern Med J. 2019;49:94–100. [DOI] [PubMed] [Google Scholar]
- 5. Mukewar S, Costedio M, Wu X, Bajaj N, Lopez R, Brzezinski A, et al. Severe adverse outcomes of endoscopic perforations in patients with and without IBD. Inflamm Bowel Dis. 2014;20:2056–66. [DOI] [PubMed] [Google Scholar]
- 6. Shafer LA, Walker JR, Waldman C, Yang C, Michaud V, Bernstein CN, et al. Factors associated with anxiety about colonoscopy: the preparation, the procedure, and the anticipated findings. Dig Dis Sci. 2018;63:610–8. [DOI] [PubMed] [Google Scholar]
- 7. Mumolo MG, Bertani L, Ceccarelli L, Laino G, Fluri GD, Albano E, et al. From bench to bedside: fecal calprotectin in inflammatory bowel diseases clinical setting. World J Gastroenterol. 2018;24:3681–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. D’Amico F, Nancey S, Danese S, Peyrin‐Biroulet L. A practical guide for faecal calprotectin measurement: myths and realities. J Crohns Colitis. 2021;15(1):152–61. [DOI] [PubMed] [Google Scholar]
- 9. D’Amico F, Bonovas S, Danese S, Peyrin‐Biroulet L. Review article: faecal calprotectin and histologic remission in ulcerative colitis. Aliment Pharmacol Ther. 2020;51:689–98. [DOI] [PubMed] [Google Scholar]
- 10. Guardiola J, Lobatón T, Rodríguez‐Alonso L, Ruiz‐Cerulla A, Arajol C, Loayza C, et al. Fecal level of calprotectin identifies histologic inflammation in patients with ulcerative colitis in clinical and endoscopic remission. Clin Gastroenterol Hepatol. 2014;12:1865–70. [DOI] [PubMed] [Google Scholar]
- 11. Toyonaga T, Kobayashi T, Nakano M, Saito E, Umeda S, Okabayashi S. Usefulness of fecal calprotectin for the early prediction of short‐term outcomes of remission‐induction treatments in ulcerative colitis in comparison with two‐item patient‐reported outcome. PloS One. 2017;12:e0185131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cremer A, Ku J, Amininejad L, Bouvry M‐R, Brohet F, Liefferinckx C, et al. Variability of faecal calprotectin in inflammatory bowel disease patients: an observational case‐control study. J Crohns Colitis. 2019;13:1372–9. [DOI] [PubMed] [Google Scholar]
- 13. Guardiola J, Lobatón T, Cerrillo E, Ferreiro‐Iglesias R, Gisbert JP, Domènech E, et al. Recommendations of the Spanish Working Group on Crohn's Disease and Ulcerative Colitis (GETECCU) on the utility of the determination of faecal calprotectin in inflammatory bowel disease. Gastroenterol Hepatol. 2018;41:514–29. [DOI] [PubMed] [Google Scholar]
- 14. Anon. Calprotectin information for patients | your test for IBS/IBD. Calprotectin. 2020. https://www.calprotectin.co.uk/about‐calprotectin/information‐for‐patients/. Accessed 21 July 2020. [Google Scholar]
- 15. Naismith GD, Smith LA, Barry SJE, Munro JI, Laird S, Rankin K, et al. A prospective single‐centre evaluation of the intra‐individual variability of faecal calprotectin in quiescent Crohn's disease. Aliment Pharmacol Ther. 2013;37:613–21. [DOI] [PubMed] [Google Scholar]
- 16. Lasson A, Stotzer P‐O, Öhman L, Isaksson S, Sapnara M, Strid H. The intra‐individual variability of faecal calprotectin: a prospective study in patients with active ulcerative colitis. J Crohns Colitis. 2015;9:26–32. [DOI] [PubMed] [Google Scholar]
- 17. Calafat M, Cabré E, Mañosa M, Lobatón T, Marín L, Domènech E. High within‐day variability of fecal calprotectin levels in patients with active ulcerative colitis. Inflamm Bowel Dis. 2015;21:1072–6. [DOI] [PubMed] [Google Scholar]
- 18. Kristensen V, Malmstrøm GH, Skar V, Røseth A, Moum B. Clinical importance of faecal calprotectin variability in inflammatory bowel disease: intra‐individual variability and standardisation of sampling procedure. Scand J Gastroenterol. 2016;51:548–55. [DOI] [PubMed] [Google Scholar]
- 19. Du L, Foshaug R, Huang VW, Kroeker KI, Dieleman LA, Halloran BP, et al. Within‐stool and within‐day sample variability of fecal calprotectin in patients with inflammatory bowel disease. J Clin Gastroenterol. 2018;52:235–40. [DOI] [PubMed] [Google Scholar]
- 20. Moum B, Jahnsen J, Bernklev T. Fecal calprotectin variability in Crohnʼs disease. Inflamm Bowel Dis. 2010;16:1091–2. [DOI] [PubMed] [Google Scholar]
- 21. Whitehead SJ, French J, Brookes MJ, Ford C, Gama R. Between‐assay variability of faecal calprotectin enzyme‐linked immunosorbent assay kits. Ann Clin Biochem. 2013;50:53–61. [DOI] [PubMed] [Google Scholar]
- 22. Juricic G, Brencic T, Tesija‐Kuna A, Njegovan M, Honovic L. Faecal calprotectin determination: impact of preanalytical sample treatment and stool consistency on within‐ and between‐method variability. Biochem Med (Zagreb). 2019;29:010707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pansart C, Roblin X, Paul S. Preanalytical heterogeneity in fecal calprotectin measurement needs to Be considered for tight control. Clin Gastroenterol Hepatol. 2020;18:524–525. [DOI] [PubMed] [Google Scholar]
- 24. RØseth AG, Fagerhol MK, Aadland E, Schjønsby H. Assessment of the neutrophil dominating protein calprotectin in feces: a methodologic study. Scand J Gastroenterol. 1992;27:793–8. [DOI] [PubMed] [Google Scholar]
- 25. Padoan A, D’Incà R, Scapellato ML, De Bastiani R, Caccaro R, Mescoli C, et al. Improving IBD diagnosis and monitoring by understanding preanalytical, analytical and biological fecal calprotectin variability. Clin Chem Lab Med. 2018;56:1926–35. [DOI] [PubMed] [Google Scholar]
- 26. Oyaert M, Van den Bremt S, Boel A, Bossuyt X, Van Hoovels L. Do not forget about pre‐analytics in faecal calprotectin measurement! Clin Chim Acta. 2017;473:124–6. [DOI] [PubMed] [Google Scholar]
- 27. Pelkmans LPJ, de Groot MJM, Curvers J. Analytical performance and clinicopathologic correlation of four fecal calprotectin methods. Am J Clin Pathol. 2019;152:392–8. [DOI] [PubMed] [Google Scholar]
- 28. Theede K, Holck S, Ibsen P, Ladelund S, Nordgaard‐Lassen I, Nielsen AM. Level of fecal calprotectin correlates with endoscopic and histologic inflammation and identifies patients with mucosal healing in ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13:1929–36.e1. [DOI] [PubMed] [Google Scholar]
- 29. Reinisch W, Panaccione R, Bossuyt P, Baert F, Armuzzi A, Hébuterne X, et al. Association of biomarker cutoffs and endoscopic outcomes in crohn's disease: a post hoc analysis from the CALM study. Inflamm Bowel Dis. 2020;26(10):1562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Waugh N, Cummins E, Royle P, Kandala NB, Shyangdan D, Arasaradnam R. Faecal calprotectin testing for differentiating amongst inflammatory and non‐inflammatory bowel diseases: systematic review and economic evaluation. Health Technol Assess. 2013;17:xv–xix, 1–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tibble J, Teahon K, Thjodleifsson B, Roseth A, Sigthorsson G, Bridger S. A simple method for assessing intestinal inflammation in Crohn's disease. Gut. 2000;47:506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22. [DOI] [PubMed] [Google Scholar]
- 33. De Sloovere MMW, De Smet D, Baert FJ, Debrabandere J, Vanpoucke HJM. Analytical and diagnostic performance of two automated fecal calprotectin immunoassays for detection of inflammatory bowel disease. Clin Chem Lab Med. 2017;55:1435–46. [DOI] [PubMed] [Google Scholar]
- 34. Nilsen T, Sunde K, Hansson L‐O, Havelka AM, Larsson A. A novel turbidimetric immunoassay for fecal calprotectin optimized for routine chemistry analyzers. J Clin Lab Anal. 2017;31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kraemer A, Bulgakova T, Schukina O, Kharitidis A, Kharitonov A, Korostovtseva E. Automated fecal biomarker profiling ‐ a convenient procedure to support diagnosis for patients with inflammatory bowel diseases. Clin Lab. 2020;66. [DOI] [PubMed] [Google Scholar]
- 36. Radillo O, Pascolo L, Martelossi S, Dal Bo S, Ventura A. Fecal calprotectin: diagnostic accuracy of the immunochromatographic CalFast assay in a pediatric population. J Clin Lab Anal. 2016;30:500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kittanakom S, Shajib MS, Garvie K, Turner J, Brooks D, Odeh S. Comparison of fecal calprotectin methods for predicting relapse of pediatric inflammatory bowel disease. Can J Gastroenterol Hepatol. 2017;2017:1450970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Okuyama Y, Doi Y, Matsuyama N, Uchino M, Yamamoto T. A novel sol particle immunoassay for fecal calprotectin in inflammatory bowel disease patients. Clin Chim Acta. 2016;456:1–6. [DOI] [PubMed] [Google Scholar]
- 39. Mandic‐Havelka A, Nilsen T, Sunde K, Norell M, O Hansson L, Larsson A. Turbidimetric determination of fecal calprotectin using two table top chemistry analyzers: Mindray BS‐200E and Cobas® c111. Clin Lab. 2017;63:907–13. [DOI] [PubMed] [Google Scholar]
- 40. Hiraoka S, Takashima S, Inokuchi T, Nakarai A, Takahara M, Harada K, et al. The novel latex agglutination turbidimetric immunoassay system for simultaneous measurements of calprotectin and hemoglobin in feces. Intest Res. 2019;17:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rodriguez A, Yokomizo L, Christofferson M, Barnes D, Khavari N, Park K. Correlation of rapid point‐of‐care vs send‐out fecal calprotectin monitoring in pediatric inflammatory bowel disease. Wjgpt. 2017;8:127–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schulz C, Wex T, von Arnim U, Malfertheiner P. Validation of two calprotectin rapid tests in daily routine. Clin Lab. 2016;62:1249–54. [DOI] [PubMed] [Google Scholar]
- 43. Bin‐Nun A, Booms C, Sabag N, Mevorach R, Algur N, Hammerman C. Rapid fecal calprotectin (FC) analysis: point of care testing for diagnosing early necrotizing enterocolitis. Am J Perinatol. 2015;32:337–42. [DOI] [PubMed] [Google Scholar]
- 44. Lobatón T, López‐García A, Rodríguez‐Moranta F, Ruiz A, Rodríguez L, Guardiola J. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn's disease. Journal of Crohn's and Colitis. 2013;7:e641–e651. [DOI] [PubMed] [Google Scholar]
- 45. Kolho K, Turner D, Veereman‐Wauters G, Sladek M, de Ridder L, Shaoul R, et al. Rapid test for fecal calprotectin levels in children with Crohn disease. J Pediatr Gastroenterol Nutr. 2012;55:436–9. [DOI] [PubMed] [Google Scholar]
- 46. Damms A, Bischoff SC. Validation and clinical significance of a new calprotectin rapid test for the diagnosis of gastrointestinal diseases. Int J Colorectal Dis. 2008;23:985–92. [DOI] [PubMed] [Google Scholar]
- 47. Otten CMT, Kok L, Witteman BJM, Baumgarten R, Kampman E, Moons KGM. Diagnostic performance of rapid tests for detection of fecal calprotectin and lactoferrin and their ability to discriminate inflammatory from irritable bowel syndrome. Clin Chem Lab Med. 2008;46:1275–80. [DOI] [PubMed] [Google Scholar]
- 48. Sydora MJ, Sydora BC, Fedorak RN. Validation of a point‐of‐care desk top device to quantitate fecal calprotectin and distinguish inflammatory bowel disease from irritable bowel syndrome. Journal of Crohn's and Colitis. 2012;6:207–14. [DOI] [PubMed] [Google Scholar]
- 49. Wassell J, Wallage M, Brewer E. Evaluation of the Quantum Blue(R) rapid test for faecal calprotectin. Ann Clin Biochem. 2012;49:55–8. [DOI] [PubMed] [Google Scholar]
- 50. Fukunaga S, Kuwaki K, Mitsuyama K, Takedatsu H, Yoshioka S, Yamasaki H. Detection of calprotectin in inflammatory bowel disease: fecal and serum levels and immunohistochemical localization. Int J Mol Med. 2018;41:107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. D’Amico F, Netter P, Baumann C, Veltin M, Zallot C, Aimone‐Gastin I, et al. Setting up a virtual calprotectin clinic in inflammatory bowel diseases: literature review and nancy experience. J Clin Med. 2020;9:2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hejl J, Theede K, Møllgren B, Madsen KV, Heidari A, Steig A, et al. Point of care testing of fecal calprotectin as a substitute for routine laboratory analysis. Practical Laboratory Medicine. 2018;10:10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Elkjaer M, Burisch J, Voxen Hansen V, Deibjerg Kristensen B, Slott Jensen J‐K, Munkholm P. A new rapid home test for faecal calprotectin in ulcerative colitis. Aliment Pharmacol Ther. 2010;31:323–330. [DOI] [PubMed] [Google Scholar]
- 54. Piekkala M, Alfthan H, Merras‐Salmio L, Puustinen Wikström A, Heiskanen K, Jaakkola T, et al. Fecal calprotectin test performed at home: a prospective study of pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2018;66:926–31. [DOI] [PubMed] [Google Scholar]
- 55. Bello C, Roseth A, Guardiola J, Reenaers C, Ruiz‐Cerulla A, Van Kemseke C, et al. Usability of a home‐based test for the measurement of fecal calprotectin in asymptomatic IBD patients. Dig Liver Dis. 2017;49:991–6. [DOI] [PubMed] [Google Scholar]
- 56. Vinding KK, Elsberg H, Thorkilgaard T, Belard E, Pedersen N, Elkjaer M. Fecal calprotectin measured by patients at home using smartphones‐A new clinical tool in monitoring patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:336–44. [DOI] [PubMed] [Google Scholar]
- 57. Heida A, Knol M, Kobold AM, Bootsman J, Dijkstra G, van Rheenen PF. Agreement between home‐based measurement of stool calprotectin and ELISA results for monitoring inflammatory bowel disease activity. Clin Gastroenterol Hepatol. 2017;15:1742–9. [DOI] [PubMed] [Google Scholar]
- 58. Wei S‐C, Tung C‐C, Weng M‐T, Wong J‐M. Experience of patients with inflammatory bowel disease in using a home fecal calprotectin test as an objective reported outcome for self‐monitoring. Intest Res. 2018;16:546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Haisma S‐M, Galaurchi A, Almahwzi S, Adekanmi Balogun JA, Muller Kobold AC, van Rheenen PF. Head‐to‐head comparison of three stool calprotectin tests for home use. PloS One. 2019;14:e0214751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Colombel J‐F, Panaccione R, Bossuyt P, Lukas M, Baert F, Vaňásek T. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779–89. [DOI] [PubMed] [Google Scholar]
- 61. Colombel J‐F, D’haens G, Lee W‐J, Petersson J, Panaccione R. Outcomes and strategies to support a treat‐to‐target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2020;14:254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McCombie A, Walmsley R, Barclay M, Ho C, Langlotz T, Regenbrecht H, et al. A noninferiority randomized clinical trial of the use of the smartphone‐based health applications IBDsmart and IBDoc in the care of inflammatory bowel disease patients. Inflamm Bowel Dis. 2020;26:1098–109. [DOI] [PubMed] [Google Scholar]
- 63. Mirsepasi‐Lauridsen HC, Bachmann Holmetoft U, Ingdam Halkjær S, Angeliki Krogfelt K, Munk Petersen A. Comparison of three commercial fecal calprotectin ELISA test kits used in patients with Inflammatory Bowel Disease. Scand J Gastroenterol. 2016;51:211–7. [DOI] [PubMed] [Google Scholar]
- 64. Labaere D, Smismans A, Van Olmen A, Christiaens P, D’Haens G, Moons V, et al. Comparison of six different calprotectin assays for the assessment of inflammatory bowel disease. United European Gastroenterol J. 2014;2:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Goll R, Heitmann R, Moe ØK, Carlsen K, Florholmen J. Head to head comparison of two commercial fecal calprotectin kits as predictor of Mayo endoscopic sub‐score and mucosal TNF expression in ulcerative colitis. PloS One. 2019;14:e0224895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Oyaert M, Boel A, Jacobs J, Van den Bremt S, De Sloovere M, Vanpoucke H. Analytical performance and diagnostic accuracy of six different faecal calprotectin assays in inflammatory bowel disease. Clin Chem Lab Med. 2017;55:1564–73. [DOI] [PubMed] [Google Scholar]
- 67. Carrasco‐Labra A, Lytvyn L, Falck‐Ytter Y, Surawicz CM, Chey WD. AGA technical review on the evaluation of functional diarrhea and diarrhea‐predominant irritable bowel syndrome in adults (IBS‐D). Gastroenterology. 2019;157:859–80. [DOI] [PubMed] [Google Scholar]
- 68. Pezzilli R, Barassi A, Morselli Labate AM, Finazzi S, Fantini L, Gizzi G, et al. Fecal calprotectin levels in patients with colonic polyposis. Dig Dis Sci. 2008;53:47–51. [DOI] [PubMed] [Google Scholar]
- 69. Tursi A, Brandimarte G, Elisei W, Giorgetti GM, Inchingolo CD, Aiello F. Faecal calprotectin in colonic diverticular disease: a case‐control study. Int J Colorectal Dis. 2009;24:49–55. [DOI] [PubMed] [Google Scholar]
- 70. Tibble J, Sigthorsson G, Foster R, Sherwood R, Fagerhol M, Bjarnason I. Faecal calprotectin and faecal occult blood tests in the diagnosis of colorectal carcinoma and adenoma. Gut. 2001;49:402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vavricka SR, Heinrich H, Buetikofer S, Breitenmoser F, Burri E, Schneider‐Yin X, et al. The Vampire Study: significant elevation of faecal calprotectin in healthy volunteers after 300 ml blood ingestion mimicking upper gastrointestinal bleeding. United European Gastroenterol J. 2018;6:1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shastri YM, Bergis D, Povse N, Schäfer V, Shastri S, Weindel M, et al. Prospective multicenter study evaluating fecal calprotectin in adult acute bacterial diarrhea. Am J Med. 2008;121:1099–106. [DOI] [PubMed] [Google Scholar]
- 73. Gundling F, Schmidtler F, Hapfelmeier A, Schulte B, Schmidt T, Pehl C, et al. Fecal calprotectin is a useful screening parameter for hepatic encephalopathy and spontaneous bacterial peritonitis in cirrhosis. Liver Int. 2011;31:1406–15. [DOI] [PubMed] [Google Scholar]
- 74. Conroy S, Hale MF, Cross SS, Swallow K, Sidhu RH, Sargur R, et al. Unrestricted faecal calprotectin testing performs poorly in the diagnosis of inflammatory bowel disease in patients in primary care. J Clin Pathol. 2018;71:316–22. [DOI] [PubMed] [Google Scholar]
- 75. Hille A, Rave‐Fränk M, Christiansen H, Herrmann MKA, Kertesz T, Hermann RM, et al. Faecal calprotectin and lactoferrin values during irradiation of prostate cancer correlate with chronic radiation proctitis: results of a prospective study. Scand J Gastroenterol. 2009;44:939–46. [DOI] [PubMed] [Google Scholar]
- 76. Yamamoto T, Shimoyama T, Bamba T, Matsumoto K. Consecutive monitoring of fecal calprotectin and lactoferrin for the early diagnosis and prediction of pouchitis after restorative proctocolectomy for ulcerative colitis. Am J Gastroenterol. 2015;110:881–7. [DOI] [PubMed] [Google Scholar]
- 77. Fauny M, D’Amico F, Bonovas S, Netter P, Danese S, Loeuille D, et al. Faecal calprotectin for the diagnosis of bowel inflammation in patients with rheumatological diseases: a systematic review. J Crohns Colitis. 2020;14:688–93. [DOI] [PubMed] [Google Scholar]
- 78. Lundgren D, Eklöf V, Palmqvist R, Hultdin J, Karling P. Proton pump inhibitor use is associated with elevated faecal calprotectin levels. A cross‐sectional study on subjects referred for colonoscopy. Scand J Gastroenterol. 2019;54:152–7. [DOI] [PubMed] [Google Scholar]
- 79. Poullis A, Foster R, Mendall MA, Shreeve D, Wiener K. Proton pump inhibitors are associated with elevation of faecal calprotectin and may affect specificity. Eur J Gastroenterol Hepatol. 2003;15:573–4.author reply 574. [DOI] [PubMed] [Google Scholar]
- 80. Cohen M. Proton pump inhibitors may cause elevation in faecal calprotectin levels. Br J Gen Pract. 2016;66:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tibble JA, Sigthorsson G, Foster R, Scott D, Fagerhol MK, Roseth A, et al. High prevalence of NSAID enteropathy as shown by a simple faecal test. Gut. 1999;45:362–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID‐induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172–8. [DOI] [PubMed] [Google Scholar]
- 83. Jackson MA, Goodrich JK, Maxan M‐E, Freedberg DE, Abrams JA, Poole AC, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kolho K‐L, Alfthan H, Hämäläinen E. Effect of bowel cleansing for colonoscopy on fecal calprotectin levels in pediatric patients. J Pediatr Gastroenterol Nutr. 2012;55:751–3. [DOI] [PubMed] [Google Scholar]
- 85. Joshi S, Lewis SJ, Creanor S, Ayling RM. Age‐related faecal calprotectin, lactoferrin and tumour M2‐PK concentrations in healthy volunteers. Ann Clin Biochem. 2010;47:259–63. [DOI] [PubMed] [Google Scholar]
- 86. Mindemark M, Larsson A. Ruling out IBD: estimation of the possible economic effects of pre‐endoscopic screening with F‐calprotectin. Clin Biochem. 2012;45:552–5. [DOI] [PubMed] [Google Scholar]
- 87. Poullis A, Foster R, Shetty A, Fagerhol MK, Mendall MA. Bowel inflammation as measured by fecal calprotectin. Cancer Epidemiol Biomarkers Prev. 2004;13:279–84. [DOI] [PubMed] [Google Scholar]
- 88. Bouri S, Segal J, Oke S, Gabe S, Hart A. PWE‐009 the accuracy of faecal calprotectin measurement from stoma effluent in predicting crohn’s disease activity. Gut. 2018;67. A72–A72. [Google Scholar]
- 89. Kotze LMdS, Nisihara RM, Marion SB, Cavassani MF, Kotze PG. FECAL CALPROTECTIN: levels for the ethiological diagnosis in Brazilian patients with gastrointestinal symptoms. Arq Gastroenterol. 2015;52:50–4. [DOI] [PubMed] [Google Scholar]
- 90. Kant P, Fazakerley R, Hull MA. Faecal calprotectin levels before and after weight loss in obese and overweight subjects. Int J Obes. 2013;37:317–9. [DOI] [PubMed] [Google Scholar]
- 91. Stevens TW, D'Haens GR, Duijvestein M, Bemelman WA, Buskens CJ, Gecse KB. Diagnostic accuracy of faecal calprotectin in patients with active perianal fistulas. United European Gastroenterol J. 2019;7:496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kammerlander H, Nielsen J, Kjeldsen J, Knudsen T, Gradel KO, Friedman S, et al. Fecal calprotectin during pregnancy in women with moderate‐severe inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:839–48. [DOI] [PubMed] [Google Scholar]
- 93. Kim ES, Tarassishin L, Eisele C, Barre A, Nair N, Rendon A. Longitudinal changes in fecal calprotectin levels among pregnant women with and without inflammatory bowel disease and their babies. Gastroenterology. 2020:S0016‐5085(20)35532‐3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article


