Abstract
Introduction
Microscopic colitis is a chronic inflammatory bowel disease characterised by normal or almost normal endoscopic appearance of the colon, chronic watery, nonbloody diarrhoea and distinct histological abnormalities, which identify three histological subtypes, the collagenous colitis, the lymphocytic colitis and the incomplete microscopic colitis. With ongoing uncertainties and new developments in the clinical management of microscopic colitis, there is a need for evidence‐based guidelines to improve the medical care of patients suffering from this disorder.
Methods
Guidelines were developed by members from the European Microscopic Colitis Group and United European Gastroenterology in accordance with the Appraisal of Guidelines for Research and Evaluation II instrument. Following a systematic literature review, the Grading of Recommendations Assessment, Development and Evaluation methodology was used to assess the certainty of the evidence. Statements and recommendations were developed by working groups consisting of gastroenterologists, pathologists and basic scientists, and voted upon using the Delphi method.
Results
These guidelines provide information on epidemiology and risk factors of microscopic colitis, as well as evidence‐based statements and recommendations on diagnostic criteria and treatment options, including oral budesonide, bile acid binders, immunomodulators and biologics. Recommendations on the clinical management of microscopic colitis are provided based on evidence, expert opinion and best clinical practice.
Conclusion
These guidelines may support clinicians worldwide to improve the clinical management of patients with microscopic colitis.
Keywords: budesonide, diarrhoea, inflammatory bowel disease, microscopic colitis
1. INTRODUCTION
Microscopic colitis (MC) is an increasingly recognised inflammatory bowel disease associated with significant symptom burden and an impaired health‐related quality of life (HRQoL). The clinical course of MC is variable, with chronic or recurrent mild to severe symptoms persisting for months to years. The prevalence of MC varies substantially between geographical regions. The two major histological subtypes are collagenous colitis (CC) and lymphocytic colitis (LC), but incomplete forms may occur (incomplete MC [MCi]). The diagnosis of MC relies on the histological examination of colonic biopsies and requires dedicated gastroenterologists, endoscopists and histopathologists.
Several review articles have been published on various diagnostic and therapeutic aspects of MC. 3 , 4 , 5 , 136 , 140 In 2012, the European Microscopic Colitis Group (EMCG) proposed their first recommendations for the diagnosis and treatment of MC. 6 In 2013, MC was included in the European consensus on the histopathology of inflammatory bowel disease published on behalf of the European Society of Pathology and the European Crohn's and Colitis Organisation. 7 According to this particular guideline, MC is defined as a ‘clinical pathological entity characterised by chronic watery (non‐bloody) diarrhoea, a normal or almost normal endoscopic appearance of the colon, and a distinct histologic pattern of collagenous colitis or lymphocytic colitis'. This includes that other causes for chronic diarrhoea such as infections or other exogenous factors have been ruled out by clinical routine procedures. More recently, the Spanish Microscopic Colitis Group and the American Gastroenterology Associations have published first evidence‐based statements and recommendations using Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology, which is now considered as the standard tool for the development of clinical practice guides. 8 , 9
With persistent uncertainties and new developments in the clinical management of MC, the United European Gastroenterology (UEG) and EMCG identified the need to develop updated clinical practical guidelines in order to increase awareness for MC and support clinicians to improve clinical care of MC patients in daily routine practice.
2. METHODOLOGY
2.1. The guideline working group
All members of EMCG were asked to participate and an open invitation was placed on the UEG website for several months prior to the first group meeting held in Vienna in October 2018. Finally, the entire group consisted of 32 physicians and researchers from 14 European countries, including gastroenterologists, pathologists and basic scientists with expertise in scientific methodology, evidence‐based medicine and clinical and therapeutic management of MC. A total of five working groups were established (1: epidemiology, risk factors; 2: pathogenesis; 3: clinical manifestation, quality of life; 4: diagnosis, monitoring; 5: treatment), each consisting of a working group leader and 5–7 group members. A steering committee was established consisting of the two coordinators (S.M., A.Mü.) and the working group leaders (D.G., Y.Z., G.E.T., A.M.K.F., S.W). First, a list of topics and research questions to be covered by the guidelines was created by the steering committee based upon discussions with the working group members on their relevance and their potential impact on clinical practice. The final list of research questions was formatted into the patient, intervention, control, outcome framework, when appropriate.
Literature search and assessment of evidence
A formal systematic review of the literature was carried out for each research question using MEDLINE (accessed via PubMed), EMBASE electronic databases and the Cochrane Database of Systematic Reviews (Cochrane Library) and the Cochrane Central Register of Controlled Trials from inception until July 2019, with no restriction of languages and periodically updated. The search strategy and the process of study selection categorised per research question can be found in online Supporting Information Appendix a. A review of the citations to identify potentially relevant articles was also carried out. This included systematic reviews and other documents offering a critical synthesis of the scientific literature, as well as randomised clinical trials, whenever possible.
Data on epidemiology, pathogenesis, clinical manifestations, diagnosis and treatment of MC were critically reviewed and meta‐analyses conducted, when applicable. The working groups followed the GRADE methodology (https://www.gradeworkinggroup.org/) to assess the quality of evidence of statements/recommendations, and classified the recommendations for the different clinical scenarios into four final categories: strong recommendation for an intervention (implying to do it), weak recommendation for an intervention (implying to probably do it), weak against an intervention (implying to probably not do it) and strong against an intervention (implying not to do it). The strength of recommendation (GR: strong or weak) using the GRADE approach was only given for studies on the accuracy of diagnostic procedures and on the assessment of the treatment efficacy.
The level of evidence (LE) was classified in four categories: high, moderate, low or very low quality, based on the strict assessment of the quality of the evidence. The quality of the evidence could be downgraded as a result of limitations in the study design or in its implementation, imprecision of estimates, variability in the results, indirectness of the evidence or publication bias; or upgraded because of a very large magnitude of effects, a dose‐response gradient or if all the plausible biases would reduce an apparent treatment effect. Moreover, the recommendations were also based on some other factors, such as desirable and undesirable consequences of alternative management strategies, variability in values and preferences and the use of resources (costs). The results of data extraction and quality of the evidence assessments are summarised in Supporting Information Appendix B.
2.2. Evolution of statements/recommendations
Based on the literature review and assessment of evidence, the working groups drafted initial statements and recommendations, which subsequently underwent a voting process by the entire guideline group using the Delphi method. The participants judged the statement/recommendation based on a 5‐point Likert scale (1: strongly disagree; 2: disagree; 3: neutral; 4: agree; 5: strongly agree), and suggested modifications or even new ones. Following this process, the statements and recommendations were revised by the working groups. They were modified if necessary and voted on again during a final face‐to‐face consensus meeting held in Barcelona in October 2019. Statements and recommendations were approved if 75% or more of the participants agreed with it (Likert score of 4 or 5; 75%–94%: consensus, 95%–100%: strong consensus). Each statement and recommendation is accompanied by the LE (high, moderate, low, very low), grade of recommendation, result of the vote (percentage agreement) at the consensus meeting and discussion of the corresponding evidence. The guideline group formulated a total of 39 statements and recommendations (Table 1).
TABLE 1.
Summary of UEG/EMCG statements and recommendations for MC
| Section and number | Statement/recommendation | Level of evidence | Grade of recommendation | Voting |
|---|---|---|---|---|
| Section 1 | Epidemiology and risk factors | |||
| Section 1.1 | The pooled overall incidence rate of MC is estimated to be 11.4 (95% CI: 9.2–13.6) cases per 100,000 person‐years. The incidence of CC and LC ranges from 0.6 to 16.4 cases per 100,000 person‐years and from 0.6 to 16.0 cases per 100,000 person‐years, respectively. | High | NA | 100% |
| Section 1.2 | The pooled overall prevalence of MC is estimated to be 119 (95% CI: 73–66) per 100,000 persons, with an overall prevalence of 50.1 per 100,000 person‐year for CC and 61.7 per 100,000 persons for LC. | High | NA | 94% |
| Section 1.3 | The pooled frequency of MC in patients with chronic watery diarrhoea is 12.8% (95% CI: 10–16), with significant heterogeneity (I 2 = 93.6%). | Moderate | NA | 100% |
| Section 1.4 | Former, but especially current smoking is associated with an increased risk of both CC and LC. | Moderate | NA | 100% |
| Section 1.5 | The risk of developing CC or LC is higher in women than in men. | High | NA | 100% |
| Section 1.6 | There is insufficient evidence to evaluate the influence of smoking cessation on the disease course. | Low | NA | 78% |
| Section 1.7 | Chronic or frequent use of PPI, NSAID or SSRI is associated with an increased risk of MC. However, this does not imply a causal relationship. | Low | NA | 94% |
| Section 1.8 | We suggest to consider withdrawal of any drugs with a suspected chronological relationship between drug introduction and onset of diarrhoea. | Very low | Weak in favour | 97% |
| Section 1.9 | MC does not increase the risk of colorectal cancer or adenoma. A special surveillance colonoscopy program is not recommended. | Low | Strong in favour | 100% |
| Section 2 | Pathogenesis | |||
| Section 2.1 | Pathogenesis of MC is complex and multifactorial. It may include luminal factors, immune dysregulation and genetic predisposition. | Low | NA | 100% |
| Section 3 | Clinical manifestation | |||
| Section 3.1 | The most common symptom of MC is chronic watery, nonbloody diarrhoea, which is frequently associated with concomitant symptoms including faecal urgency, nocturnal stools and faecal incontinence. nocturnal stools and faecal incontinence. | Moderate | NA | 97% |
| Section 3.2 | MC diagnosis should be ruled out in patients fulfilling the criteria for functional bowel disease, especially in presence of MC risk factors and/or in absence of IBS‐therapy response. | Moderate | NA | 93% |
| Section 3.3 | Health‐related quality of life is impaired in patients with MC, depending on the activity and severity of the disease and concomitant comorbidities. | Moderate | NA | 100% |
| Section and number | Statement/recommendation | Level of evidence | Grade of recommendation | Voting |
|---|---|---|---|---|
| Section 3.4 | In the absence of a formally validated metric of disease activity, disease activity and clinical remission in MC should be assessed by the Hjortswang criteria (clinical remission: mean of <3 stools/day and a mean <1 water stool/day during a 1‐week registration). | Moderate | NA | 100% |
| Section 4 | Diagnosis | |||
| Section 4.1 | Endoscopic findings are recognised with increased frequency in patients with MC, however they are nonspecific. | Low | NA | 95% |
| Section 4.2 | The histopathologic criteria of CC are a thickened subepithelial collagenous band ≥10 μm combined with an increased inflammatory infiltrate in lamina propria. The criteria apply to haematoxylin and eosin‐stained slides. | Moderate | NA | 89% |
| Section 4.3 | The histopathologic criteria of LC are an increased number of intraepithelial lymphocytes ≥20 per 100 surface epithelial cells combined with an increased inflammatory infiltrate in lamina propria and a not significantly thickened collagenous band (<10 μm). The criteria apply to haematoxylin and eosin‐stained slides. | Moderate | NA | 100% |
| Section 4.4 | Incomplete MC comprises incomplete CC (defined by a thickened subepithelial collagenous band >5 μm but <10 μm) and incomplete LC (defined by >10 IELs but <20 IELs and a normal collagenous band). Both types show a mild inflammatory infiltrate in the lamina propria. The criteria apply to haematoxylin and eosin‐stained slides. | Low | NA | 95% |
| Section 4.5 | We recommend ileocolonoscopy with biopsies from at least the right and left colon. | High | Strong in favour | 100% |
| Section 4.6 | We recommend against histological monitoring in patients with MC. | Very low | Strong in favour | 100% |
| Section 4.7 | Faecal calprotectin is not useful to exclude or monitor MC. | Moderate | NA | 100% |
| Section 4.8 | We recommend screening for coeliac disease in patients with MC. | High | Strong in favour | 100% |
| Section 4.9 | Testing for bile acid diarrhoea is not part of routine diagnostic workup in MC. | Low | NA | 83% |
| Section 4.10 | Testing for bile acid diarrhoea can be considered in patients who experience nonresponse to budesonide treatment. | Low | Strong in favour | 82% |
| Section 5 | Treatment | |||
| Section 5.1.1 | We recommend using oral budesonide to induce remission in patients with CC. | Moderate | Strong in favour | 100% |
| Section 5.1.2 | We recommend using oral budesonide to induce remission in patients with LC. | Low | Strong in favour | 100% |
| Section 5.2.1 | Oral budesonide is effective to maintain remission in patients with CC. | Moderate | Strong in favour | 94% |
| Section 5.2.2 | We suggest using oral budesonide to maintain remission in patients with LC. | Very low | Weak in favour | 84% |
| Section 5.3.1 | There is no increased risk of serious adverse events with budesonide in MC. | Low | NA | 100% |
| Section 5.3.2 | The risk of osteoporotic bone fractures seems not be increased in budesonide treated MC patients, although prolonged use might be associated with a decrease of bone mineral density. | Low | NA | 97% |
| Section 5.4 | We recommend against treatment with mesalazine in patients with MC for induction of remission. There are no studies for maintenance. | Low | Strong against | 94% |
| Section 5.5 | There is not enough evidence to recommend bismuth subsalicylate in patients with MC. | Very low | Strong against | 92% |
| Section 5.6 | There is not enough evidence to recommend the use of loperamide in MC. Given the documented effect in patients with chronic diarrhoea, the expert's opinion favours the use of this drug in mild disease. | Very low | Strong in favour | 100% |
| Section 5.7 | In patients with MC and bile acid diarrhoea we suggest treatment with bile acid binders. | Very low | Weak in favour | 100% |
| Section 5.8 | There is not enough evidence to recommend antibiotics for treatment of MC. | Very low | Strong against | 100% |
| Section 5.9 | We recommend against use of probiotics for treatment of MC. | Low | Strong against | 100% |
| Section 5.10 | We recommend against the use of prednisolone or other corticoste roids than budesonide for the treatment of MC. | Low | Strong against | 100% |
| Section 5.11 | We recommend treatment with thiopurines, anti‐TNF drugs or vedolizumab in selected patients with MC who fail to respond to budesonide to induce and maintain clinical remission. We recommend against the use of methotrexate in patients with MC. | Low | Strong in favour | 97% |
| Section 5.12 | Surgery can be considered in selected MC patients as last option if all medical therapy fails. | Very low | Weak in favour | 100% |
Abbreviations: CC, collagenous colitis; CI, confidence interval; EMCG, European Microscopic Colitis Group; LC, lymphocytic colitis; IELs, intraepithelial lymphocytes; IBS, irritable bowel syndrome; MC, microscopic colitis; PPI, proton pump inhibitor; TNF, tumour necrosis factor; NSAID, nonsteroidal anti‐inflammatory drugs; SSRI, selective serotonin reuptake inhibitor; NA, not applicable; UEG, United European Gastroenterology.
2.3. Epidemiology and risk factors of MC
What is the incidence of MC?
Statement 1.1: The pooled overall incidence rate of MC is estimated to be 11.4 (95% confidence interval [CI]: 9.2–13.6) cases per 100,000 person‐years. The incidence of CC and LC ranges from 0.6 to 16.4 cases per 100,000 person‐years and from 0.6 to 16.0 cases per 100,000 person‐years, respectively.
LE: high; GR: not applicable; agreement: 100%, strong consensus.
Summary of evidence: Epidemiological studies have documented an increasing incidence of MC in western countries. An overall pooled incidence rate of 11.4 (95% CI: 9.2–13.6, I 2 = 99.72%) cases of MC per 100,000 person‐years was calculated based on studies providing population‐based data. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 Several studies from North America 20 , 27 and Europe 14 , 16 , 17 , 18 , 25 , 26 , 29 reported variations in incidence rates over a 10‐years‐time period in the same region. They all showed an increasing incidence in the early years, which has reached a plateau. 32 The pooled incidence rate for CC 10 , 11 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 26 , 27 , 28 , 29 , 30 , 31 , 33 , 34 , 35 , 36 was 4.9 (95% CI: 4.2–5.7, I 2 = 98.3%) cases per 100,000 person‐years. The pooled LC incidence rate was 5.0 (95% CI: 4.0–6.1, I 2 98.75%) cases per 100,000 person‐years. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 Geographic variations in the incidence of MC have been reported; however, the limited number of studies from Southern Europe compared to Northern Europe and the lack of direct comparative studies from different countries for the same time period does hinder firm conclusions on this matter.
The MC incidence is higher in the elderly. A previous meta‐analysis showed the median patients' age at the time of diagnosis was over 60 years old (CC: 64.9, CI: 57.03–72.78; LC: 62.2, CI: 54.0–70.4 years). 32 However, up to 25% of patients diagnosed with CC were less than 45 years 33 and cases of CC have even been described in children. 37 , 38 , 39 , 40
What is the prevalence of MC?
Statement 1.2: The pooled overall prevalence of MC is estimated to be 119 (95% CI: 73–166) per 100,000 persons, with an overall prevalence of 50.1 per 100,000 person‐year for CC and 61.7 per 100,000 persons for LC.
LE: high; GR: NA; agreement: 94%, consensus.
Summary of evidence: Five population‐based studies from Spain, 21 , 41 North America 20 , 27 and Sweden 30 have assessed the prevalence of MC and provided a wide range from 47.5 to 219 cases per 100,000 persons. These studies were pooled to provide an overall MC prevalence of 119.4 (95% CI: 72.9–165.9, I 2 = 97.08%) cases per 100,000 persons. For CC, the pooled prevalence was estimated to be 50.1 (95% CI: 13.69–76.5, I 2 = 98.37%) cases per 100,000 persons. 20 , 21 , 27 , 30 , 33 , 41 The estimated pooled prevalence of LC was 61.7 (95% CI: 48.2–75.3, I 2 = 80.56%) per 100,000 persons. 20 , 21 , 27 , 30 , 41 Some studies reported that increasing age was a risk factor for developing MC, 20 , 33 , 41 with a 5.25 (95% CI: 3.81–7.24) times higher probability of MC in people over 65 years of age. 41
What is the frequency of MC in chronic diarrhoea?
Statement 1.3: The pooled frequency of MC in patients with unexplained chronic watery diarrhoea is 12.8% (95% CI: 10–16), with significant heterogeneity (I 2 = 93.6%).
LE: moderate; GR: NA; agreement: 100%, strong consensus.
Summary of evidence: The frequency of MC in patients with chronic or intermittent watery diarrhoea and a macroscopically normal (or near normal) colon has been evaluated in several studies. 17 , 18 , 21 , 26 , 27 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 Based on studies with a moderate or high quality, and a sample size of ≥100 patients, 17 , 18 , 21 , 26 , 27 , 42 , 43 , 45 , 46 , 47 , 49 , 52 , 54 , 56 , 59 , 60 the pooled overall frequency of MC was estimated to be 12.8% (95% CI: 9.9–15.9, I 2 = 93.6%). The pooled frequency of CC and LC was 4.96% (95% CI: 3.6–6.5, I 2 = 85.2%) 17 , 18 , 21 , 26 , 27 , 42 , 43 , 45 , 47 , 49 , 52 , 54 , 56 , 60 and 8.2% (95% CI: 6.0–10.8, I 2 = 92.0%), 17 , 18 , 21 , 26 , 27 , 42 , 43 , 45 , 47 , 49 , 52 , 54 , 56 , 60 respectively (see also Supporting Information Appendix d). The data showed high heterogeneity and are not directly comparable, considering the different geographical and genetic background, different definitions of chronic watery diarrhoea used, the lack of clearly described diagnostic criteria for MC and diagnostic work‐up before colonoscopy.
Is smoking a risk factor for MC?
Statement 1.4: Former, but especially current smoking is associated with an increased risk for both CC and LC.
LE: moderate; GR: NA; agreement: 100%, strong consensus.
Summary of evidence: The prevalence of current smoking in MC patients ranged from 15.3% to 40.7% (CC: 13.6%–37.1%, LC: 13.2%–26.0%) compared to 5.0 28.2% in non‐MC control groups. 28 , 43 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 In a recent meta‐analysis, 83 current smokers had a significantly increased risk of MC compared with never smokers (odds ratio [OR]: 2.99, 95% CI: 2.15–4.15). 83 Current smoking was more strongly associated with CC than LC (OR: 5.5, 95% CI: 3.4–8.9, OR: 2.96, 95% CI: 2.0–4.3, respectively). 83 Former smoking was also associated with an increased risk (OR: 1.6, 95% CI: 1.4–1.9). 83 However, interstudy heterogeneity was high or moderate for all analyses. Smoking status was often assessed by self‐administered questionnaires or review of medical records, and a homogeneous definition of smoking was lacking.
Is female gender a risk factor for MC?
Statement 1.5: The risk of developing CC or LC is higher in women than in men.
LE: high; GR: NA; agreement: 100%, strong consensus.
Summary of evidence: The incidence of MC is higher in women than in men, as reported in a previous meta‐analysis published in 2015. 32 Actually, subgroup analyses on the incidence of MC by sex were possible in 19 studies. 10 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 26 , 27 , 28 , 29 , 30 Female sex was significantly associated with MC (pooled OR: 2.52, 95% CI: 2.28–2.79, I 2 = 89%), with no differences between studies from Northern Europe (pooled OR: 2.48, 95% CI: 2.22–2.78, I 2 = 90%), Southern Europe (pooled OR: 2.53, 95% CI: 1.63–3.94, I 2 = 62%) and North America (pooled OR: 2.77, 95% CI: 2.02–3.81, I 2 = 37%). Subgroup analyses of CC (n = 18 studies) 10 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 23 , 26 , 27 , 28 , 29 , 30 , 33 , 35 , 36 and LC (n = 15 studies) 10 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 23 , 26 , 27 , 28 , 29 , 30 reproduced these results, with a pooled OR of 3.24 (95% CI: 3.03–3.47, I 2 = 35%) in CC and 2.06 (95% CI: 1.84–2.31, I 2 = 78%) in LC (see also Supporting Information Appendix D). The proportion of females among MC populations have been described in the range of 52%–86% (see Supporting Information Material, p. 28). In the three largest studies from Sweden, 12 Denmark 14 and the Netherlands, 29 the average proportion of females was approximately 72%.
Does smoking cessation influence the disease course of MC?
Statement 1.6: There is insufficient evidence to evaluate the influence of smoking cessation on the disease course.
LE: low; GR: NA; agreement: 78%, consensus.
Summary of evidence: No studies directly evaluated the effect of smoking cessation on the disease course. In one study, the risk of developing MC declined significantly over time (p = 0.017), leading to an attenuated risk after 5 years after smoking cessation. 73 However, compared to smokers, former smokers do not have a significantly lower risk of MC (OR: 1.44, 95% CI: 0.76–2.72). 73 , 74 , 75 , 76 , 73 , 74 , 75 , 76 , 73 , 74 , 75 , 76 , 73 , 74 , 75 , 76 In two studies, current smokers developed MC more than one decade earlier than former or never smokers. 77 , 84 The majority of the studies showed no differences in terms of clinical presentation response to treatment, spontaneous remission rates and disease recurrence or need for maintenance treatment 73 , 75 , 77 , 78 , 81 , 84 , 85 , 86 , 87 , 88 , 89 (see also Suporting Information Appendix D). Only in a post‐hoc analysis of pooled data from two randomised controlled trials (RCTs) was current smoking associated with a decreased ability to achieve clinical remission with corticosteroid treatment (OR: 0.31, 95% CI: 0.10–0.98). 90
Is drug use associated with a significant increased risk of MC?
Statement 1.7: Chronic or frequent use of proton pump inhibitors (PPIs), nonsteroidal anti‐inflammatory drugs (NSAIDs) or selective serotonin reuptake inhibitors (SSRIs) is associated with an increased risk of MC. However, this does not imply a causal relationship.
LE: low; GR: NA; agreement: 94%, consensus.
Summary of evidence: Drug‐induced MC was addressed by retrospective case‐control studies 54 , 81 , 82 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 showing an association with the use of NSAIDs, PPIs and SSRIs. PPI use was strongly associated with MC (OR: 2.95, 95% CI: 1.82–4.80, I 2 = 99%), 91 , 92 , 93 , 94 , 95 , 96 , 91 , 92 , 93 , 94 , 95 , 96 , 91 , 92 , 93 , 94 , 95 , 96 , 91 , 92 , 93 , 94 , 95 , 96 , 98 , 99 , 100 especially when used continuously for 4–12 months (OR: 4.69, 95% CI: 3.58–6.13). 98 Exposure to NSAIDs was also associated with an increased risk of MC (OR: 2.40, 95% CI: 1.99–2.89, I 2 = 88%). 54 , 82 , 91 , 92 , 93 , 94 , 95 , 97 , 98 , 99 The combined use with PPIs might further increase this risk. 98 MC was also associated with SSRI exposure (OR: 2.98, 95% CI: 2.35–3.78, I 2 = 90%) 54 , 81 , 82 , 91 , 92 , 93 , 95 , 96 , 98 , 99 (see also Supporting Information Appendix D). It should be stressed that different criteria for ‘drug exposure' were applied and different reference populations were considered. Moreover, the studies lack information on the evolution of clinical symptoms after drug exposure, withdrawal or re‐challenge, hindering assessment of causality.
Should any drug, potentially related to MC onset, been withdrawn?
Recommendation 1.8: We suggest to consider withdrawal of any drugs with a suspected chronological relationship between drug introduction and onset of diarrhoea.
LE: very low; GR: weak in favour; agreement: 97%, strong consensus.
Summary of evidence: In total, 62 case reports and 13 case‐control studies 97 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 describing drug‐induced MC were analysed to calculate the so‐called ‘imputability score' describing the likelihood of a causal relationship between drug exposure and MC. PPIs were the most reported drugs in relation to MC. Resolution of diarrhoea and histological normalisation after PPI withdrawal has been reported in four cases using omeprazole, 156 , 157 in 16 cases using lansoprazole 111 , 112 , 113 , 119 , 123 , 124 , 129 , 138 , 141 , 142 , 146 , 150 , 153 , 154 , 160 and in one case using esomeprazole. 157 For rabeprazole, only one case of clinical improvement without histological control has been published. 139 In 10 cases 111 , 112 , 138 , 153 , 154 , 156 , 157 , 160 switch to another PPI did not result in recurrence of diarrhoea, which contradicts the presumption of a class effect of PPI. One case‐control study clearly demonstrated that current and recent use of NSAIDs and PPIs were associated with an increased risk of MC, when compared to never and past use, especially in the case of continuous exposure for 4–12 months 98 This observation underlines the clinical relevance of a suspected chronological relationship between drug use an onset of MC.
Do MC patients require a special program for colonoscopy surveillance to rule out colorectal cancer (CRC) compared to general population?
Recommendation 1.9: MC does not increase the risk of CRC or adenoma. A special surveillance colonoscopy program is not recommended.
LE: low; GR: strong in favour; agreement: 100%, strong consensus.
Summary of evidence: Only a few studies examined whether persistent chronic inflammation in MC is associated with an increased risk of CRC or adenomas. 60 , 71 , 80 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 The meta‐analysis of five case‐control studies showed that MC was associated with a reduced risk for CRC or adenoma compared to controls (pooled OR: 0.65, 95% CI: 0.33–1.28, I 2 = 19% and OR: 0.49, 95% CI: 0.30–0.81, I 2 = 92%, respectively). In a larger retrospective cohort of 547 MC patients (171 CC and 376 LC), CRC was detected in five CC (2.82%) and five LC patients (1.33%). 163 MC was negatively associated with the risk for CRC and adenoma (OR: 0.34, 95% CI: 0.16–0.73, p = 0.006 and OR: 0.52, 95% CI: 0.50–0.76, p < 0.001, respectively), during a mean follow‐up of 4.63 years 163
2.4. Pathogenesis of MC
Statement 2.1: Pathogenesis of MC is complex and multifactorial. It may include luminal factors, immune dysregulation and genetic predisposition.
LE: low; GR: NA; agreement: 100%, strong consensus.
Summary of evidence: The mechanisms involved in the development of MC are poorly understood and the LE is scarce. It is not in the scope of this guideline to provide in‐depth information on this subject. The current knowledge of the factors involved is briefly summarised in Supporting Information Appendix C.
2.5. Clinical manifestation and quality of life
What are the most common symptoms in MC?
Statement 3.1: The most common symptom in MC is chronic watery, nonbloody diarrhoea, which is frequently associated with concomitant symptoms including faecal urgency, nocturnal stools and faecal incontinence.
LE: moderate; GR: NA; agreement: 97%, strong consensus.
Summary of evidence: The predominant symptom of MC is chronic watery, nonbloody diarrhoea, which was reported by 84%–100% of patients in 22 studies. In one third of the cases, the onset of diarrhoea was acute in nature, 170 , 171 , 172 , 173 and according to a European prospective registry 174 it persists for 6 months before diagnosis in 43%. Symptoms such as stool frequency, stool consistency and overall duration of diarrhoea are reported in a number of the studies, including a large Danish study of 539 patients, 13 in which an average of 6–7 bowel movements per day was reported. Common concomitant symptoms included faecal urgency (55%), nocturnal stools (35.3%) and faecal incontinence (26.3%). Less frequent complaints with varying prevalences among studies are abdominal pain, weight loss and bloating. 172 , 173 , 175 A Swedish study from 2004 involving 199 patients with LC 173 reported a median weight loss of 5 (4–8) kg; however, early studies might have included a selected population, as the awareness for MC was lower.
Should MC be ruled out in patients fulfilling the criteria for functional bowel disease with diarrhoea predominant subtype?
Statement 3.2: MC diagnosis should be ruled out in patients fulfilling the criteria for functional bowel disease, especially in presence of MC risk factors and/or in absence of irritable bowel syndrome (IBS)‐therapy response.
LE: moderate; GR: NA; agreement: 93%, consensus.
Summary of available evidence: MC shares similar symptoms and endoscopic results with functional bowel disorders, especially in diarrhoea‐dominant IBS and chronic functional diarrhoea. 176 , 177 , 178 , 179 In two meta‐analyses, the identification of underlying MC diagnosis was reported in 9% (95% CI: 4.5%–14.9%) among patients exhibiting diarrhoea‐predominant functional disorders. 176 , 178 However, not all studies employed the currently accepted diagnostic criteria for MC, and different criteria for defining functional bowel disorders were used, contributing to the high heterogeneity of the results.
Is the patient's HRQoL impaired by MC?
Statement 3.3: HRQoL is impaired in patients with MC, depending on the activity and severity of the disease and concomitant comorbidities.
LE: moderate; GR: NA; agreement: 100%, strong consensus.
Summary of evidence: MC can severely impact HRQoL, with baseline HRQoL being lower than that of patients with other intestinal and proctological disorders. 180 Impaired HRQoL was demonstrated in both active CC and LC, including impact on function in daily living, disease‐related worry and well‐being. 2 , 8 , 181 , 182 , 183 However, HRQoL can also be impaired in patients with MC achieving clinical remission. 89 , 184 , 185
In a population‐based study, 116 patients with active CC had an impaired HRQoL compared with a background population, whereas patients in remission scored similar. 186 HRQoL was impaired in those with a mean of ≥3 stools/day or a mean of ≥1 watery stool/day. Therefore, it was proposed that remission in CC should be defined as a mean of less than 3 stools/day and a mean less than 1 watery stool/day during a 1‐week registration. 187
In a case‐control study including 212 MC patients, all four HRQoL dimensions (symptom burden, social function, disease‐related worry, general well‐being) were impaired in patients with active CC and LC. 184 In a cross‐sectional survey of 151 MC patients, 52 (34.4%) reported IBS‐type symptoms and had higher levels of anxiety, depression and somatisation and impaired quality of life. 179 In another cross‐sectional survey of 129 patients with a new diagnosis of MC, fatigue severity resulted to be associated with IBS‐type symptoms, psychological comorbidity and impaired quality of life, with a negative correlation in HRQoL measures. 188 In a cross‐sectional study including 158 female MC patients, those with coexisting IBS‐like symptoms (55%) experienced worse psychological well‐being than those without. Also, smoking and PPI were associated with gastrointestinal symptoms and impaired psychological well‐being in MC patients. 89 HRQoL was evaluated in five RCTs including CC patients 189 , 190 , 191 , 192 , 193 , 194 and in two RCTs including LC patients. 192 , 193 , 194 , 195 In all seven RCTs, HRQoL was markedly altered at baseline in both CC and LC patients, and improved after budesonide treatment. 196 , 197 , 198
Are there established metrics to measure disease activity and clinical remission in MC?
Statement 3.4: In the absence of a formally validated metric of disease activity, disease activity and clinical remission in MC should be assessed by the Hjortswang criteria (clinical remission: mean of less than 3 stools/day and a mean less than 1 water stool/day during a 1‐week registration).
LE: moderate; GR: NA; agreement: 100%, strong consensus.
Summary of evidence: In the absence of a reliable biomarker, the definition of disease activity is based on clinical disease activity. Various definitions for relapse or clinical remission have been used in clinical trials on MC, mainly based on stool frequency 191 , 199 , 200 , 201 , 202 , 203 , 204 and stool weight. 200 , 202 A reduction of the mucosal inflammation or thinning of the collagen layer has also been used to assess histopathological response in trials, 195 , 199 , 200 , 201 , 199 , 200 , 201 but the correlation between histology and clinical symptoms is weak. 205
In a Swedish population‐based survey, CC patients with a mean of less than 3 stools/day and a mean of less than 1 watery stool/day during a 1‐week symptom registration had no or only mild impact on their HRQoL and were, hence, defined as being in remission. 187
In contrast, CC patients with either ≥3 stools/day or ≥1 watery stool/day had a significant impact on their HRQoL and were, thus, defined as having active disease. This definition is often referred to as the ‘Hjortswang criteria' for disease activity.
An MC Disease Activity Index (MCDAI) has been proposed based on the same methodological principles as was once used for the development of the Crohn's Disease Activity Index. 206 A total of 162 MC patients completed a symptom questionnaire and the HRQoL questionnaire Inflammatory Bowel Disease Questionnaire (IBDQ). 180 A single investigator scored a physician global assessment (PGA) of disease severity on a 10‐point scale based on the patients' survey results. Multiple linear regressions identified the following symptoms to best predict the PGA: number ofunformed stools daily, presence of nocturnal stools, abdominal pain, weight loss, faecal urgency and faecal incontinence. These symptoms were then combined in a weighted formula to create the MCDAI. The MCDAI was moderately associated with the IBDQ (r = ‐0.62, p < 0.001).
Neither the ‘Hjortswang criteria' nor the MCDAI have undergone formal prospective validation and they do not fulfil the new requirements from the Food and Drug Administration for a patient reported outcome in clinical trials. 207 However, the ‘Hjortswang criteria' has been used in seven published clinical studies, of which three were RCTs, 193 , 195 , 203 which represents a real‐life external and prospective validation of the score in clinical practice.
2.6. Diagnosis of MC
What is the endoscopic appearance of MC?
Statement 4.1: Endoscopic findings are recognised with increased frequency in patients with MC; however, they are nonspecific.
LE: low; GR: NA; agreement: 95%, strong consensus.
Summary of evidence: Overall, 80 informative articles including 1582 patients on endoscopic findings in MC were identified, including 756 patients with CC, 779 patients with LC and 47 patients with MC. 19 , 23 , 166 , 208 Macroscopically visible lesions or alterations were reported in 38.8% of patients in various parts of the colon, including isolated linear ulcerations, pseudomembranes, irregular vascular patterns, mucosal lacerations, erythema, oedema, nodularity and surface textural alterations.
Although a larger number of publications exist for CC, the number of published CC and LC patients is very similar. 208 Therefore, no conclusive statement can be made as to whether or not endoscopic findings (and which) may be more common in one or the other histological subtype.
What are the criteria for the histological diagnosis of CC?
Statement 4.2: The histopathologic criteria of CC are a thickened subepithelial collagenous band ≥10 mm combined with an increased inflammatory infiltrate in the lamina propria. The criteria apply to haematoxylin and eosin (HE)‐stained slides.
LE: moderate; GR: NA; agreement: 89%, consensus.
Summary of evidence: The original histological criteria of CC have not been contested but elaborated by few others. 209 The most characteristic feature is a thickened subepithelial collagenous band exceeding 10 mm. 210 , 211 , 212 , 213 , 214 The band often has an irregular deeper edge and may contain entrapped capillaries, red blood cells and inflammatory cells. Focal damage of the surface epithelium, including detachment from the basement membrane, flattening and mucindepletion, 205 , 210 , 212 , 215 , 216 , 217 , 218 , 219 , 220 as well as an increased number of intraepithelial lymphocytes (IELs) is seen. 210 , 211 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 This should be combined with an inflammatory infiltrate in lamina propria of mild to moderate degree, dominated by plasma cells and lymphocytes, but also includes eosinophils, 205 , 210 , 213 , 214 , 215 , 216 , 217 , 213 , 214 , 215 , 216 , 217 , 223 , 224 , 225 mast cells 213 and, more rarely, neutrophils. 212 , 214 , 215 , 216 , 219 , 220 , 221 , 219 , 220 , 221 , 219 , 220 , 221 Paneth cell metaplasia 205 , 210 , 221 , 224 and occasionally cryptitis can be seen. 212 , 216 , 220 , 221 , 224 , 227 The biopsies should be orientated vertically, since tangential sectioning can simulate a thickened collagenous band. 228
The histologic criteria are based on HE‐stained sections. Supplementary stains, such as Van Gieson, Masson Trichrome or Sirius red, 219 , 220 , 229 might be helpful since the collagenous band is highlighted. The interobserver reproducibility of the histological diagnose of CC is good. 230 , 231
What are the criteria for the histological diagnosis of LC?
Statement 4.3: The histopathologic criteria of LC are an increased number of IELs ≥20 per 100 surface epithelial cells combined with an increased inflammatory infiltrate in the lamina propria and a not significantly thickened collagenous band (<10 mm). The criteria apply to HE‐stained slides.
LE: moderate; GR: NA; agreement: 100%, strong consensus.
Summary of evidence: LC was originally named in 1989, 217 although described under the name MC in 1980. 232 The criteria were based on HE‐stained slides. 217 The most characteristic feature of LC is an increased number of IELs in the surface epithelium ≥20 per 100 epithelial cells. 1 , 3 , 4 , 7 , 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 , 242 , 243 , 244 Counting should be performed in the surface epithelium, and areas in close relation to lymphoid aggregates in the lamina propria should be avoided. 1 Focal and mild damage of the surface epithelium, including flattening, mucin depletion and vacuolisation, is seen, although not as prominently as in CC. 1 , 3 , 167 , 218 , 220 , 236 , 237 , 240 , 242 , 245 , 246 , 247 , 248 This should be combined with an inflammatory infiltrate in lamina propria of a mild to moderate degree, dominated by plasma cells and lymphocytes, 3 , 4 , 167 , 217 , 218 , 220 , 233 , 236 , 238 , 239 , 241 , 242 , 246 , 247 , 248 , 249 , 250 , 251 , 252 , 253 , 254 but might also include fewer eosinophils and neutrophils. 3 , 217 , 220 , 248 , 250 , 254 , 255 occasionally, cryptitis 217 , 220 , 221 , 249 , 252 , 256 or Paneth cells metaplasia is seen. 221 , 236 , 241 , 250 , 252
Supplementary immunohistochemical staining might be helpful, especially in borderline cases, since highlighting the lymphocytes makes counting easier. 3 , 6 , 136 , 244 , 257 This might lead to over diagnosing and it has been suggested to use higher cut‐off values when counting is performed on CD3‐stained slides. 258
What are the criteria for the histological diagnosis of MCi?
Statement 4.4: MCi comprises incomplete CC (CCi; defined by a thickened subepithelial collagenous band >5 μm but <10 μm) and incomplete LC (LCi; defined by >10 IELs but <20 IELs and a normal collagenous band). Both types show a mild inflammatory infiltrate in the lamina propria. The criteria apply to HE‐stained slides.
LE: low; GR: NA; agreement: 95%, strong consensus.
Summary of evidence: Patients with symptoms of MC not completely fulfilling the histological criteria of CC or LC can be classified as CCi or LCi. 1 , 6 , 237 Different terms have been used, including MC not otherwise specified, 224 , 259 , 260 MC type undesignated, 261 borderline LC 217 and paucicellular LC. 251 , 262 Although the clinical characteristics of MC and MCi seem indistinguishable, 13 , 263 , 264 one study reports that a greater proportion of patients with MCi experience spontaneous remission. 263 In CCi, the subepithelial collagenous band is more than 5 mm but less than 10 mm. In LCi, greater than 10 but less than 20 IELs are required. The inflammatory infiltrate in lamina propria is usually mild but comprises identical cell types, as in CC and LC.
In borderline cases, it is recommended to use a supplementary special stain or an immunohistochemical staining procedure in addition to HE stains. 265
Where should biopsies be taken in patients with suspected MC?
Recommendation 4.5: We recommend ileocolonoscopy with biopsies from at least the right and left side of the colon.
LE: high; GR: strong in favour; agreement: 100%, strong consensus.
Summary of evidence: Studies including a high number of patients with simultaneous biopsies taken from the right and left colon show characteristic histological changes of MC in both sides in 95%–98%. 13 , 23 , 263 Similarly, smaller studies have found high concordance. 18 , 45 , 46 , 205 , 211 , 266 , 267 , 268 , 269 , 270 Studies without a strict biopsy protocol reported a lower number of diagnostic biopsies from the left colon. 214 , 219 , 229 , 243 Biopsies exclusively from the rectum are not sufficient. 10 , 214 , 215 , 219 , 220
However, since a full ileocolonoscopy is indicated for virtually all patients with chronic diarrhoea, it is recommended to take biopsies from the right and left side of the colon.
It may be advisable to send these in separately labelled containers as the number of inflammatory cells in normal surface epithelium and lamina propria is higher in the right colon. 233 , 271 Similarly, the normal collagenous band has been reported to be thicker in the sigmoid colon and rectum. 226 , 227 Especially in borderline cases, this may help the pathologists know that the biopsies are from, for example, the left side where the cellularity is usually lower, because this would support the diagnosis if the pathologist is in doubt. For these reasons, expert opinion among the pathologists participating in this guideline tended towards separate containers, although there is no firm evidence to support this.
Is histological monitoring necessary in patients with MC?
Recommendation 4.6: We recommend against histological monitoring in patients with MC.
LE: very low; GR: strong in favour; agreement: 100%, strong consensus.
Summary of evidence: Histology of postdiagnostic disease activity has been described, but histological assessment of remission and relapse is not standardised 171 , 195 , 199 , 203 , 215 , 241 , 247 , 263 , 272 , 273 , 274 , 275 , 276 and correlation between clinical disease activity and histologic features is only weak. 171 , 195 , 199 , 203 , 215 , 241 , 247 , 263 , 272 , 273 , 274 , 275 , 276
Conversion between CC and LC occurs in some studies. 263 , 273 , 275 In a study of 283 patients, histological features persisted in postdiagnostic biopsies for up to 1 year in 77% with CC, 64% with LC and 45% in MCi, of whom 6%, 9% and 18% converted to a different subtype, respectively. Histological features normalised in approximately 10% and persisted beyond the first year in a significant number of patients, including those in whom diarrhoea had resolved and not recurred. 263
Is faecal calprotectin useful in MC?
Statement 4.7: Faecal calprotectin is not useful to exclude or monitor MC.
LE: moderate; GR: NA; agreement: 100%, strong consensus.
Summary of evidence: Small studies have demonstrated that faecal calprotectin was slightly, although significantly, higher in those with MC as compared to patients without organic cause of diarrhoea 277 and IBS. 278 The predictive value was low due to a large overlap. Wildt et al. 279 demonstrated that faecal calprotectin was increased in some but not all 21 patients with active CC and overlapped between patients with active and quiescent disease and normal controls. Further studies demonstrated overlapping values of other faecal biomarkers, including faecal eosinophil protein and eosinophil cationic protein, 63 faecal lactoferrin, 279 , 280 alpha‐1‐antitryptin, 281 and tryptase, eosinophil protein X and myeloperoxidase. 282 More studies on faecal biomarkers in MC including calprotectin are clearly needed.
Should patients with MC be tested for coeliac disease?
Recommendation 4.8: We recommend screening for coeliac disease in patients with MC.
LE: high; GR: strong in favour; agreement: 100%, strong consensus.
Summary of evidence: One large prospective study demonstrated an incidence of celiac disease in 3.3% of patients with MC versus 0.4% in controls. 283 Incidence rates were between 2% and 4% in large cohort studies, 13 , 284 a case‐control study 76 and one pathology registry including 3456 MC patients having undergone both gastroscopy and lower endoscopy with biopsy. 285 These estimates are larger than in the background populations, although lower than reported in numerous retrospective studies, mostly older case series and incomplete cohorts. 28 , 88 , 163 , 166 , 170 , 172 , 173 , 286 , 287 , 288 , 289 , 290 Coeliac disease was mainly diagnosed by biochemical testing rather than histology and most studies screened only approximately half of the patients. Development of MC was not associated with intake of gluten. 291
Should patients with MC be tested for bile acid diarrhoea?
Statement 4.9: Testing for bile acid diarrhoea is not part of routine diagnostic work‐up in patients with MC.
LE: low; GR: NA; agreement: 83%, consensus.
Recommendation 4.10: Testing for bile acid diarrhoea can be considered in patients who experience nonresponse to budesonide treatment.
LE: low; GR: strong in favour; agreement: 82%, consensus.
Summary of evidence: Symptoms of MC and bile acid diarrhoea are indistinguishable, and the two conditions coexist. 13 , 292 , 293 The diagnosis of bile acid diarrhoea relies on radiolabelled 75 selenium homotaurocholic acid taurine (SeHCAT) testing. SeHCAT for was performed in 181 of 539 patients included in a large incidence cohort, and retention (<10%) was reduced in 125. 13 Small case series reporting a high incidence of bile acid diarrhoea were probably biased by referral. 292 , 293 Active CC was associated with a reduced ileal bile acid reuptake and normalisation of disease activity increased retention and normalised bile acid synthesis. 294 Whether this bile acid diarrhoea is a consequence of inflammation in the right colon or even terminal ileum or merely a coexisting disease per se remains to be explored. Expression of the main bile acid receptor was reduced in biopsies from the colon of patients with MC. 295 MC was not associated with prior cholecystectomy. 296
2.6.1. Treatment
Is oral budesonide effective in inducing remission of CC?
Recommendation 5.1.1: We recommend using oral budesonide to induce remission in patients with CC.
LE: moderate; GR: strong in favour; agreement: 100%, strong consensus.
Summary of evidence:
2.7. Clinical response
A meta‐analysis conducted in 2017 197 included four randomised placebo‐controlled trials with a total of 161 CC patients. 199 , 200 , 201 , 203 After 6–8 weeks of treatment, pooled analysis revealed 81% (62/77) of patients treated with budesonide 9 mg/day achieved a clinical response compared to 36% (30/84) of patients treated with placebo (relative risk [RR]: 2.98, 95% CI: 1.14–7.75; random‐effects). This analysis was statistically significant for heterogeneity (p = 0.001, I 2 = 81%). After excluding an outlier with an unusually high response rate to placebo, 203 the I 2 statistic decreased to 0% and the respective clinical response rates were 81% (38/47) and 17% (8/47) (RR: 4.56, 95% CI: 2.43–8.55). Secondary end points in that study 203 included assessing clinical remission at 8 weeks according to the Hjortswang criteria of disease activity (mean <3 stools/day, with <1 watery stool/day). The inclusion of this study in the meta‐analysis using these data resulted in a pooled clinical remission rate of 81% (62/77) for budesonide compared to 26% (22/84) with placebo (RR: 3.10, 95% CI: 1.8–5.3; random effects). There was no significant heterogeneity (p = 0.186, I 2 = 37.7%; Supporting Information Appendix D).
2.8. Histological response
The pooled analysis of histological response of the four studies 197 included a total of 161 patients with histological remission occurring in 60/77 (78%) and 27/84 (32%) of patients receiving budesonide and placebo, respectively (RR: 2.68, 95% CI: 1.37–5.24), which did demonstrate a statistically significant response.
2.9. Quality of life
In one study, 201 the validated Gastrointestinal Quality of Life Index (GIQLI) was used to measure quality of life at baseline and after 6 weeks of treatment with budesonide or placebo. A complete quality of life assessment was calculated for 29 trial participants (budesonide: n = 17; placebo: n = 12). The mean baseline GIQLI score was 67 in the budesonide group and 86 in the placebo group. After 6 weeks of treatment, the mean GIQLI score remained unchanged in the placebo group (86–88) but increased significantly in the budesonide group (67–92; p < 0.001).
Is oral budesonide effective in inducing remission of LC?
Recommendation 5.1.2: We recommend using oral budesonide to induce remission in patients with LC.
LE: low; GR: strong in favour; agreement: 100%, strong consensus.
Summary of evidence:
3. Clinical response
A pooled analysis for clinical response in three studies 192 , 195 , 297 shows a statistically significant benefit for budesonide over placebo. Clinical remission was noted in 84% (43/51) of budesonide patients and 43% (19/44) of placebo patients (RR: 1.89, 95% CI: 1.3–2.7), without heterogeneity (I 2 = 0%) (see also Supporting Information Appendix D).
3.1. Histological response
The pooled analysis for histological response showed a statistically significant benefit for budesonide over placebo. Histological response was noted in 78% of budesonide patients compared to 33% of placebo patients (two studies; 39 participants; RR: 2.44, 95% CI: 1.13–5.28, I 2 = 0%). 196
3.2. Quality of life
The 36‐item Short Form Health Survey scores at baseline were reduced compared to normal values for both the physical and mental domains. In the budesonide group, the mean physical sum score increased from 42.0 at baseline to 49.7 after 6 weeks of treatment, while the mean mental sum score was unchanged, with a value of 46.5 at baseline and 46.9 after 6 weeks 192 In the placebo group, the mean physical sum score increased from 44.1 at baseline to 48.0 after 6 weeks of treatment, while the mean mental sum score was unchanged, with a value of 49.0 at baseline and 49.1 after 6 weeks. 192
Is oral budesonide effective for maintaining remission of CC?
Recommendation 5.2.1: We recommend using oral budesonide to maintain remission in patients with CC.
LE: moderate; GR: strong in favour; agreement: 94%, consensus.
Summary of evidence:
3.3. Maintenance of clinical response
In three studies, 191 , 193 , 272 patients with CC who had achieved a clinical response with open‐label budesonide were randomised to continuous treatment with budesonide or placebo. A pooled analysis of the three studies showed that 68% (57/84) of patients receiving budesonide maintained remission at their respective study endpoints, whereas only 20% (18/88) of patients receiving placebo maintained remission (RR: 3.30, 95% CI: 2.13–5.09). 197 At the end of 6 months, more patients assigned to budesonide than placebo had maintained their clinical response (75% vs. 25%). Results from two randomised clinical trials showed that maintenance therapy with budesonide 6 mg daily over 6 months resulted in a lower risk of clinical relapse (RR: 0.34, 95% CI: 0.19–0.6). 197 A lower dose of budesonide (3 mg daily alternating with 6 mg daily) over 12 months showed similar efficacy in maintaining clinical response (see also Supporting Information Appendix D). In a retrospective study on 75 patients with CC, only 20% required budesonide doses of 6 mg/day or more to maintain clinical remission. 85
3.4. Maintenance of histological response
In two studies, 191 , 272 25 patients assigned to budesonide with a maintained clinical response underwent a follow‐up colonoscopy or sigmoidoscopy at the end of 6 months of treatment. Of these, 19 patients had also maintained their histological response, representing 48% (19/40) of the initial patient cohort randomised to budesonide. In comparison, 19 patients assigned to placebo with a maintained clinical response also underwent a follow‐up colonoscopy or sigmoidoscopy at the end of 6 months of treatment. Six of these patients, representing 15% (6/40) of the initial patient cohort randomised to placebo, had a maintained histological response. The pooled RR for maintenance of histological response was 3.17 (95% CI: 1.44–6.95). This was not significant for heterogeneity (p = 0.60, I 2 = 0%). 197
Is oral budesonide effective for maintaining remission of LC?
Recommendation 5.2.2: We suggest using oral budesonide to maintain remission in patients with LC.
LE: very low; GR: weak in favour; agreement: 84%, consensus.
Summary of evidence: There is no RCT assessing the efficacy of budesonide to maintain remission in LC. However, given the similarity of this disease with CC, budesonide has been used to maintain remission in LC in clinical practice. The opinion of the experts favours the use of this drug in the maintenance of clinical remission in LC.
Is budesonide a safe drug in the treatment of MC?
Statement 5.3.1: There is no increased risk of serious adverse events with budesonide in MC.
LE: low; GR: NA; agreement: 100%, strong consensus.
Summary of evidence: Five of seven RCTs of CC reported the proportion of patients experiencing at least one adverse event. 191 , 193 , 201 , 203 , 272 Pooled adverse event data, regardless of whether the study was an induction or maintenance trial, showed no statistically significant difference in adverse event rates between budesonide and placebo. 197 Forty‐nine percent (68/140) of patients given budesonide and 42% (63/150) of patients given placebo experienced at least one adverse event (five studies, 290 patients; RR: 1.18, 95% CI: 0.92–1.51). Seven percent (10/140) and 7% (11/150) of patients administered budesonide and placebo, respectively, withdrew due to adverse events (five studies, 290 patients; RR: 0.97, 95% CI: 0.43–2.17). Serious adverse events were rare, with 1% (1/84) of patients receiving budesonide and 1% (1/91) of patients receiving placebo experiencing one (four studies, 175 patients; RR: 1.11, 95% CI: 0.15–8.01).
Adverse events were reported in two RCTs of LC. 192 , 195 In one study, six adverse events occurred in two patients (10%) in the budesonide group, compared to nine adverse events in three patients (15%) in the placebo group (RR: 0.63, 95% CI: 0.12–3.41). 192 In another RCT, 47.4% (9/19) in the budesonide group and 42.1% (8/19) in the placebo group presented adverse events. 195
Is prolonged use of oral budesonide in MC associated with an increased risk of osteoporosis?
Statement 5.3.2: The risk of osteoporotic bone fractures seems not be increased in budesonide‐treated MC patients, although prolonged use might be associated with a decrease of bone mineral density.
LE: low; GR: NA; agreement: 97%, strong consensus.
Summary of evidence: Data on the effect of longterm budesonide on bone mineral density mainly come from its use in other diseases. A mean dose of budesonide of 8.5 mg/day (range: 6 9 mg/day) for 2 years induced more alterations in bone mineral density (loss >2% per year) than not receiving corticosteroid treatment in patients with Crohn's disease in remission. 298 However, in a case‐control study, treatment with budesonide at a dose of around 3 mg/day was not associated with an increased risk of fracture. 299 Oral budesonide (6 mg/day for 3 years) plus ursodeoxycholic acid to treat patients with primary biliary cirrhosis was also associated with a decrease in bone mass density, with no relation to the stage of liver disease. 300
One study in MC patients (n = 50) showed no significant differences in bone mineral density compared to a control group (n = 49) of similar age and sex: 58% osteoporosis and osteopenia in MC versus 39% in the control group. 79 However, the sample size was insufficient and the statistical power low. The cumulative dose of budesonide was associated with lower bone mineral density and T‐score in the hip, with a cut‐off of 2500 mg of budesonide to predict osteopenia. The markers of bone formation pro‐N‐terminal peptide procollagen type 1 and bone alkaline phosphatase were lower in patients with MC than in controls, suggesting an osteoblast dysfunction due to the systemic effect of budesonide or to the disease itself. In a recent case‐control study, 301 there was no increase in osteoporotic fractures in general, but a modest isolated effect of budesonide on the risk of spinal fractures was observed, mainly in younger patients.
Is mesalazine effective in MC?
Recommendation 5.4: We recommend against treatment with mesalazine in patients with MC for induction of remission. There are no studies for maintenance.
LE: low; GR: strong against; agreement: 94%, consensus.
Summary of evidence: Mesalazine has been shown in placebo‐controlled, randomised studies to lack efficacy and to be inferior to treatment with budesonide in CC 203 and LC. 195 Remission rates were 80%, 44% and 38% after 8 weeks of treatment with budesonide, mesalazine and placebo, respectively, in patients with CC, 203 and 79%, 63% and 42%, respectively, in patients with LC. 195 These findings are supported by real‐life experience in larger cohorts reporting clinical response to mesalazine in 4/28 with CC, 1/9 with LC and 1/6 with MCi 13 in 15 of 33 with LC 170 and in 12 of 31 with CC. 173 Others case series reported response to mesalazine in about half of patients with CC and LC. 272 , 302 , 303 , 304 By contrast, mesalazine was effective in almost all patients in an open‐label mesalazine ±cholestyramine trial. 305
Is there a role for bismuth subsalicylate in MC?
Recommendation 5.5: There is not enough evidence to recommend bismuth subsalicylate in patients with MC.
LE: very low; GR: strong against; agreement: 92%, consensus.
Summary of evidence: The effect of treatment with bismuth subsalicylate for 8 weeks was studied in one open‐label study with 13 patients with LC or CC. 274 Clinical remission was reported in 11 and histological abnormalities resolved in nine of 13. An effect of bismuth in 10 of 55 patients with LC (45.5%) and in 21 of 76 patients with CC (63.6%) was reported in a retrospectively collected case series. 302 A total of 23% of 22 patients with LC identified retrospectively reported cessation of diarrhoea, 288 but the histological criteria were 10 IELs per 100 epithelial cells.
Is there a role for loperamide in MC?
Recommendation 5.6: There is not enough evidence to recommend the use of loperamide in MC. Given the documented effect in patients with chronic diarrhoea, the expert's opinion favours the use of this drug in mild disease.
LE: very low; GR: strong in favour; agreement: 100%, strong consensus.
Summary of evidence: Two large retrospective case series reported response or remission in 49 of 69 patients with CC 173 and in 47 of 67 patients with LC. 170 A large retrospective cohort of 539 patients with MC reported a subjective effect of loperamide in 46/77 with MC. 13 Several cohorts or smaller series reported complete or near complete relief of diarrhoea in 18 57% patients with MC treated with loperamide. 288 , 304 Loperamide has proven efficacious and safe in several randomised, placebo‐controlled trials in patients with chronic diarrhoea, in particular abolishing faecal incontinence. 306 , 307 , 308 , 309
Are bile acid binding agents effective in MC?
Recommendation 5.7: In patients with MC and bile acid diarrhoea we suggest treatment with bile acid binders.
LE: very low; GR: weak in favour; agreement: 100%, strong consensus.
Summary of evidence: A large, prospective cohort study demonstrated that bile acid diarrhoea diagnosed with SeHCAT coexists with MC with an estimated prevalence of approximately 14%, and 84 of 167 patients treated with cholestyramine reported subjective cessation of diarrhoea. 13 This concurs with two large case series reporting the effect of cholestyramine in 26 of 44 patients with CC 170 and in 26 of 46 patients with LC. 173 An open‐label controlled trial demonstrated a very high response rate to cholestyramine, 305 as did Ung et al. 293 in CC patients both with and without concurrent bile acid diarrhoea. An effect of cholestyramine was also reported in further small case series. 273 , 288 , 302 Thus, the available data indicates that bile acid diarrhoea coexists with MC in a substantial number of patients, and that cholestyramine could be efficacious in patients with coexisting MC and bile acid diarrhoea.
Is there a role for antibiotics in MC?
Recommendation 5.9: There is not enough evidence to recommend antibiotics for treatment of MC.
LE: very low; GR: strong against; agreement: 100%, strong consensus.
Summary of evidence: Antibiotics for inducing and maintaining remission in MC have not been investigated in controlled trials. Only a few retrospective case series have reported the outcomes of MC after antibiotic treatment. In a retrospective series of 161 CC patients, various antibiotics (metronidazole, erythromycin and penicillin) showed response rates of up to 60%. 170 In another retrospective cohort series of 199 patients with LC, 14/23 and 2/5 responded to metronidazole and norfloxacin. 173 In both studies, no information about response definition, concomitant treatment, dosing or relapse rate were reported. Finally, in a large consecutive cohort of 539 patients with MC, 6/33 patients had response to antibiotics; however, effect measurement was not defined, and treatment duration and antibiotics of choice not reported. 13
Is there a role for probiotics in MC?
Recommendation 5.10: We recommend against use of probiotics for treatment of MC.
LE: low; GR: strong against; agreement: 100%, strong consensus.
Summary of evidence: Only one placebo‐controlled trial examining probiotics against placebo has been published. In an induction study with sample size = 29, Lactobacillus acidophilus and Bifidobacterium animalis subs Lactis were not superior to placebo. 194 In another randomised but open‐labelled trial, the effect of the probiotic VSL#3 versus mesalazine was examined. Twenty‐four patients fulfilled the study. In the VSL#3 group, a significant reduction in stool weight at 8 weeks was demonstrated (p = 0.03) but no change was seen in stool frequency. 310
Is there a role for prednisolone in MC?
Recommendation 5.11: We recommend against the use of prednisolone or other corticosteroids than budesonide for the treatment of MC.
LE: low; GR: strong against; agreement: 100%, strong consensus.
Summary of evidence: Only one placebo‐controlled trial with prednisolone exists. Treatment duration was very short, sample size low (12 patients) and prednisolone was without significant effect. 202 In one open small trial and in several retrospective cohort studies, a positive effect of prednisolone has been reported; however, relapse rates were high. 170 , 173 , 288 , 311 , 312 An open‐label retrospective study investigated beclomethasone dipropionate as a synthetic corticosteroid with topical colonic release in 30 patients with MC showing a response rate of 80% and remission rate of 67%. 313
Is there a role for immunomodulators and biologics in the treatment of patients with MC?
Recommendation 5.12: We recommend treatment with thiopurines, anti‐tumour necosis factor (TNF) drugs or vedolizumab in selected patients with MC who fail to respond to budesonide to induce and maintain clinical remission. We recommend against the use of methotrexate in patients with MC.
LE: low; GR: strong; agreement: 97%, strong consensus.
Summary of evidence: Azathioprine/6‐mercaptopurine. The effect of thiopurines in MC has been evaluated in several retrospective case series including from 9 to 49 MC patients who usually were steroid‐dependent or ‐refractory. The reported long‐term response rates allowing corticosteroid discontinuation ranged from 28% to 89%. 314 , 315 , 316
A retrospective analysis of 49 patients (43 on azathioprine and six on mercaptopurine) demonstrated complete or partial response in 43% and 22%, respectively, whereas cessation of therapy because of adverse events occurred in 17 patients (35%). 317
3.5. Methotrexate
Methotrexate was evaluated in a retrospective analysis including 19 MC patients, of whom 16 (84%) showed complete or partial clinical response. 318 Another series of 12 patients reported complete response in seven, partial response in two patients and no response in three patients. 317 Only one study has prospectively evaluated the effect of methotrexate in patients intolerant or refractory to budesonide. Here, none of the nine included patients achieved clinical remission. 319
3.6. Biologics
Anti‐TNF agents in MC have been studied in small case series 320 , 321 and single cases. 322 , 323 , 324 In four MC patients with severe symptoms refractory to standard medical therapies, infliximab or adalimumab lead to long‐term clinical remission in three cases (two with adalimumab and one with infliximab). One patient on adalimumab had an early loss of response and was referred for colectomy. 320 Münch et al. 321 reported three CC patients receiving adalimumab as a third‐line therapy. Two achieved clinical remission at Week 6, while one had to discontinue due to side effects, despite clinical response. The largest series included 18 patients (16 CC, two LC) treated with adalimumab or infliximab. 323 At Week 12, nine patients achieved remission and six were responders.
Vedolizumab has been studied in an international case series of 11 patients (five LC, six CC) who failed to respond to other therapies including anti‐TNF agents. 325 After three infusions, clinical remission was observed in five patients (two LC and three CC), of whom three remained well with maintenance therapy during a median duration of 13 months. Other case series reported successful use of vedolizumab to induce remission of MC. 326 , 327 , 328
Is there a role for surgery in MC?
Recommendation 5.13: Surgery can be considered in selected patients as last option if all medical therapy fails.
LE: very low; GR: weak; agreement: 100%, strong consensus.
Summary of evidence: Scientific evidence on surgical treatment in MC comes only from a few case reports. 329 , 330 , 331 , 332 One case series published in 1995 reported on nine female CC patients who failed to respond to medical therapies (none of them received budesonide, immunomodulators or biologics). An ileostomy was performed in eight patients and a sigmoidostomy in one patient. Postoperatively, diarrhoea ceased in all patients; however, clinical symptoms recurred after restoration of intestinal continuity.
A case report in 2000 described a CC patient who was treated successfully by total proctocolectomy and ileal pouch anal anastomosis. 332 In two case reports of CC patients not responding to budesonide 331 or adalimumab, 330 symptoms improved after temporary loop ileostomy, but recurred after restoration of bowel continuity. One case report described a CC patient undergoing colectomy after adalimumab failure, but no outcome has been reported. 320
3.6.1. Therapeutic management of MC
Based upon the available evidence and expert opinion, a therapeutic algorithm for MC is proposed (Figure 1). This algorithm is supported by a high level of agreement among the guideline group (strongly agree 64.3%, agree 35.7%). For patients with active MC oral budesonide, which is currently the only licenced drug for treatment of MC, should be the medical therapy of choice. In case of chronic active disease, long‐term treatment with oral budesonide with the lowest possible dose for as long as needed is advised. The question of budesonide withdrawal should be discussed with the patient and decided on an individual basis. In case of long‐term budesonide treatment, supplementation with calcium/vitamin D and monitoring of bone mineral density may be considered on an individual basis, especially in patients with additional risk factors for osteoporosis. Loperamide may be used on demand if needed. In budesonide‐refractory patients and in patients requiring budesonide more than 6 mg/day to maintain clinical remission, alternative medical therapies including immunomodulators or biologics should be considered.
FIGURE 1.
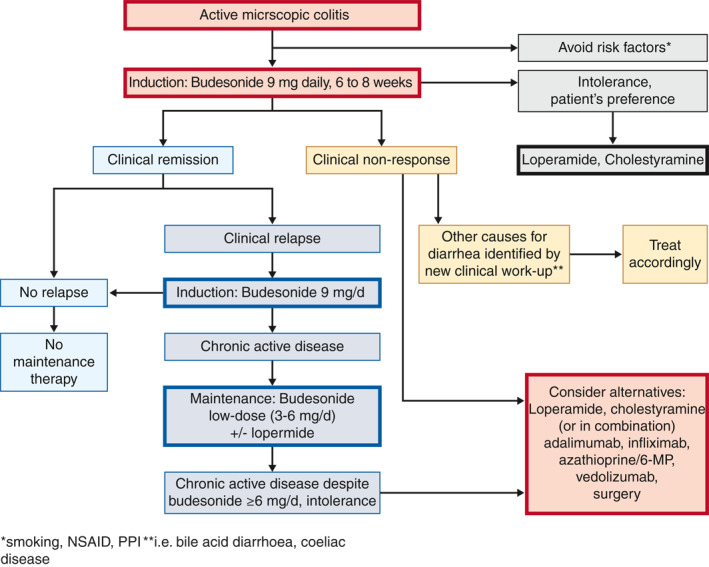
Therapeutic algorithm for microscopic colitis in clinical practice. *Smoking, NSAID, PPI; **for example bile acid diarrhoea, coeliac disease. NSAID, nonsteroidal anti‐inflammatory drugs; PPI, proton pump inhibitor
4. CONCLUSIONS AND FUTURE PERSPECTIVES
These EMCG/UEG guidelines provide evidenced‐based statements and recommendations for essential aspects of the clinical management of MC. The main objective and potential of these guidelines is to increase awareness for a presumably under‐recognised medical condition and to improve medical care and patient outcomes. Extensive dissemination of these guidelines is needed to facilitate widespread use and implementation in clinical practice. Several unmet needs have been identified, including a better understanding of the natural course and pathophysiological mechanisms of disease, reliable noninvasive biomarkers, validated instruments for assessment of disease activity and new treatment modalities. These gaps should be addressed by high‐quality basic research and well‐designed clinical trials.
CONFLICT OF INTERESTS
There are no industry or government relationships to report: Johan Bohr, Mauro D'Amato, JE, Anne‐Marie Kanstrup Fiehn, Jouzas Kupcinskas, Giovanni Latella, Ivan Lyutakov, Gilles Macaigne, Fernando Magro, Emese Mihaly, Lars Kristian Munck, Árpád V. Patai, Karolina Skonieczna‐Zydecka, Bas Verhaegh. Advisory boards, consulting: Stephan Miehlke: Dr Falk Pharma, Tillotts; Yamile Zabana: Tillotts, Janssen, FAES; Fernando Fernandez‐Banares: Tillotts, Thermofisher; Henrik Hjortswang: Abbvie, Janssen, Pfizer, Takeda, Tillotts; Anastasios Koulaouzidis: Tillotts, Dr Falk Pharma; Wojciech Marlicz Sanprobi; Ahmed Madisch: Dr Falk Pharma; Ole Bonderup: Tillotts; Gian E. Tontini: Aorta SRL, CapsoVision Inc; Andreas Münch: Ferring, Tillotts, Dr Falk Pharma; Signe Wildt: Tillotts, Takeda. Research grants/clinical trial funding: Ole Bonderup: Tillotts; Fernando Fernandez‐Banares: Dr Falk Pharma; Henrik Hjortswang: Abbvie, Ferring, Tillotts, Biomedal; Andreas Münch: Ferring. Speaker's bureau: Stephan Miehlke: Dr Falk Pharma/Falk Foundation; Yamile Zabana: Abbvie, MSD, Ferring; Danila Guagnozzi: Allergan; Ole Bonderup: Dr Falk Pharma/Falk Foundation, Tillotts; Henrik Hjortswang: Abbvie, Tillotts; Anastasios Koulaouzidis: Dr Falk Pharma; Ahmed Madisch: Dr Falk Pharma/Falk Foundation; Wojciech Marlicz: Alfasigma; Andreas Münch: Tillotts.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The steering committee (S. M., A. Mü., D. G., Y. Z., G. E. T., A. M. K., S. W.) organised the working groups and designed the preliminary list of topics to be covered. All authors systematically reviewed the literature and drafted the statements and recommendations and provided Grading of Recommendations Assessment, Development and Evaluation evaluations. All authors and members of the consensus group voted on the statements and recommendations. The steering committee then drafted the initial manuscript, which was reviewed, revised and approved by all authors and members of the consensus group. Subsequently it was made available to all members for final comments prior to submission for publication. Collaborators: Nadine Steubesand and Franziska Dambon, Clinical Guideline Services, Kiel, Germany. These guidelines have been developed with reasonable care and with the best of knowledge available to the authors at the time of preparation. They are intended to assist healthcare professionals and allied healthcare professionals as an educational tool to provide information that may support them in providing care to patients. Patients or other community members using these guidelines shall do so only after consultation with a health professional and shall not mistake these guidelines as professional medical advice. These guidelines must not substitute seeking professional medical and health advice from a health professional. These guidelines may not apply to all situations and should be interpreted in the light of specific clinical situations and resource availability. It is up to every clinician to adapt these guidelines to local regulations and to each patient's individual circumstances and needs. The information in these guidelines shall not be relied upon as being complete, current or accurate, nor shall it be considered as inclusive of all proper treatments or methods of care or as a legal standard of care. United European Gastroenterology (UEG) makes no warranty, express or implied, in respect of these guidelines and cannot be held liable for any damages resulting from the application of these guidelines, in particular for any loss or damage (whether direct or indirect) resulting from a treatment based on the guidance given herein. UEG shall not be held liable to the utmost extent permissible according to the applicable laws for any content available on such external websites, which can be accessed by using the links included herein. These guidelines were developed with the support of a UEG Activity Grant. The European Microscopic Colitis Group administered all aspects of the meetings without other external funding sources.
References
- 1. Langner C, Aust D, Ensari A, et al. Histology of microscopic colitis‐review with a practical approach for pathologists. Histopathology. 2015;66:613–26. [DOI] [PubMed] [Google Scholar]
- 2. Miehlke S, Verhaegh B, Tontini GE, et al. Microscopic colitis: pathophysiology and clinical management. Lancet Gastroenterol Hepatol. 2019;4:305–14. [DOI] [PubMed] [Google Scholar]
- 3. Munch A, Langner C. Microscopic colitis: clinical and pathologic perspectives. Clin Gastroenterol Hepatol. 2015;13:228–36. [DOI] [PubMed] [Google Scholar]
- 4. Pardi DS. Diagnosis and management of microscopic colitis. Am J Gastroenterol. 2017;112:78–85. [DOI] [PubMed] [Google Scholar]
- 5. Pardi DS, Kelly CP. Microscopic colitis. Gastroenterology. 2011;140:1155–65. [DOI] [PubMed] [Google Scholar]
- 6. Munch A, Aust D, Bohr J, et al. Microscopic colitis: current status, present and future challenges: statements of the European Microscopic Colitis Group. J Crohns Colitis. 2012;6:932–45. [DOI] [PubMed] [Google Scholar]
- 7. Magro F, Langner C, Driessen A, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. 2013;7:827–51. [DOI] [PubMed] [Google Scholar]
- 8. Fernandez‐Banares F, Casanova MJ, Arguedas Y, et al. Current concepts on microscopic colitis: evidence‐based statements and recommendations of the Spanish Microscopic Colitis Group. Aliment Pharmacol Ther. 2016;43:400–26. [DOI] [PubMed] [Google Scholar]
- 9. Pardi DS, Tremaine WJ, Carrasco‐Labra A. American Gastroenterological Association Institute technical review on the medical management of microscopic colitis. Gastroenterology. 2016;150:247–74. [DOI] [PubMed] [Google Scholar]
- 10. Agnarsdottir M, Gunnlaugsson O, Orvar KB, et al. Collagenous and lymphocytic colitis in Iceland. Dig Dis Sci. 2002;47:1122–8. [DOI] [PubMed] [Google Scholar]
- 11. Andrews CN, Beck PL, Wilsack L, et al. Evaluation of endoscopist and pathologist factors affecting the incidence of microscopic colitis. Can J Gastroenterol. 2012;26:515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bergman D, Clements MS, Khalili H, et al. A nation‐wide cohort study of the incidence of microscopic colitis in Sweden. Aliment Pharmacol Ther. 2019;49:1395–400. [DOI] [PubMed] [Google Scholar]
- 13. Bjornbak C, Engel PJ, Nielsen PL, et al. Microscopic colitis: clinical findings, topography and persistence of histopathological subgroups. Aliment Pharmacol Ther. 2011;34:1225–34. [DOI] [PubMed] [Google Scholar]
- 14. Bonderup OK, Wigh T, Nielsen GL, et al. The epidemiology of microscopic colitis: a 10‐year pathology‐based nationwide Danish cohort study. Scand J Gastroenterol. 2015;50:393–8. [DOI] [PubMed] [Google Scholar]
- 15. Daferera N, Almer S, Münch A. P634 incidence of microscopic colitis 2008–2011 in central Ostergotland, Sweden. Evidence for an increase?. J Crohns Colitis. 2013;7:S265. [Google Scholar]
- 16. Davidson S, Sjoberg K, Engel PJH, et al. Microscopic colitis in Denmark and Sweden: incidence, putative risk factors, histological assessment and endoscopic activity. Scand J Gastroenterol. 2018;53:818–24. [DOI] [PubMed] [Google Scholar]
- 17. Fernandez‐Banares F, Salas A, Esteve M, et al. Evolution of the incidence of collagenous colitis and lymphocytic colitis in Terrassa, Spain: a population‐based study. Inflamm Bowel Dis. 2011;17:1015–20. [DOI] [PubMed] [Google Scholar]
- 18. Fernandez‐Banares F, Salas A, Forne M, et al. Incidence of collagenous and lymphocytic colitis: a 5‐year population‐based study. Am J Gastroenterol. 1999;94:418–23. [DOI] [PubMed] [Google Scholar]
- 19. Fumery M, Kohut M, Gower‐Rousseau C, et al. Incidence, clinical presentation, and associated factors of microscopic colitis in northern France: a population‐based study. Dig Dis Sci. 2017;62:1571–9. [DOI] [PubMed] [Google Scholar]
- 20. Gentile NM, Khanna S, Loftus EV Jr, et al. The epidemiology of microscopic colitis in Olmsted County from 2002 to 2010: a population‐based study. Clin Gastroenterol Hepatol. 2014;12:838–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guagnozzi D, Lucendo AJ, Angueira‐Lapena T, et al. Prevalence and incidence of microscopic colitis in patients with diarrhoea of unknown aetiology in a region in central Spain. Dig Liver Dis. 2012;44:384–8. [DOI] [PubMed] [Google Scholar]
- 22. Heron T, Walsh S, Mowat C. Microscopic colitis in Tayside: clinical features, associations, and behaviour. Gut. 2005;54:A84. [Google Scholar]
- 23. Kane JS, Rotimi O, Ford AC. Macroscopic findings, incidence and characteristics of microscopic colitis in a large cohort of patients from the United Kingdom. Scand J Gastroenterol. 2017;52:988–94. [DOI] [PubMed] [Google Scholar]
- 24. Lewis NR, Archer T, Kaye P. PWE–061 epidemiology of microscopic colitis in Nottingham: a contemporary cohort study. Gut. 2017;66:A156. [Google Scholar]
- 25. Moore M, Coleman HG, Allen PB, et al. Microscopic colitis: a population‐based case series over a 9‐year period in Northern Ireland. Colorectal Dis. 2018;20:1020–7. [DOI] [PubMed] [Google Scholar]
- 26. Olesen M, Eriksson S, Bohr J, et al. Microscopic colitis: a common diarrhoeal disease. An epidemiological study in Orebro, Sweden, 1993–1998. Gut. 2004;53:346–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pardi DS, Loftus EV Jr., Smyrk TC, et al. The epidemiology of microscopic colitis: a population based study in Olmsted County, Minnesota. Gut. 2007;56:504‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thorn M, Sjoberg D, Ekbom A, et al. Microscopic colitis in Uppsala health region, a population‐based prospective study 2005–2009. Scand J Gastroenterol. 2013;48:825‐30. [DOI] [PubMed] [Google Scholar]
- 29. Verhaegh BP, Jonkers DM, Driessen A, et al. Incidence of microscopic colitis in the Netherlands. A nationwide population‐based study from 2000 to 2012. Dig Liver Dis. 2015;47:30–6. [DOI] [PubMed] [Google Scholar]
- 30. Wickbom A, Bohr J, Eriksson S, et al. Stable incidence of collagenous colitis and lymphocytic colitis in Orebro, Sweden, 1999–2008: a continuous epidemiologic study. Inflamm Bowel Dis. 2013;19:2387–93. [DOI] [PubMed] [Google Scholar]
- 31. Williams JJ, Kaplan GG, Makhija S, et al. Microscopic colitis‐defining incidence rates and risk factors: a population‐based study. Clin Gastroenterol Hepatol. 2008;6:35–40. [DOI] [PubMed] [Google Scholar]
- 32. Tong J, Zheng Q, Zhang C, et al. Incidence, prevalence, and temporal trends of microscopic colitis: a systematic review and meta‐analysis. Am J Gastroenterol. 2015;110:265–76. [DOI] [PubMed] [Google Scholar]
- 33. Bohr J, Tysk C, Eriksson S, et al. Collagenous colitis in Orebro, Sweden, an epidemiological study 1984–1993. Gut. 1995;37:394‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raclot G, Queneau P, Ottignon Y. Incidence of collagenous colitis—a retrospective study in the east of France. Gastroenterology. 1994;106:A23. [Google Scholar]
- 35. Rajan J, Noble C, Anderson C. The epidemiology and clinical features of collagenous colitis in Lothian. Gut. 2005;54:A99–100. [Google Scholar]
- 36. Vigren L, Olesen M, Benoni C, et al. An epidemiological study of collagenous colitis in southern Sweden from 2001–2010. World J Gastroenterol. 2012;18:2821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. El‐Matary W, Girgis S, Huynh H, et al. Microscopic colitis in children. Dig Dis Sci. 2010;55:1996–2001. [DOI] [PubMed] [Google Scholar]
- 38. Gremse DA, Boudreaux CW, Manci EA. Collagenous colitis in children. Gastroenterology. 1993;104:906–9. [DOI] [PubMed] [Google Scholar]
- 39. Liu X, Xiao SY, Plesec TP, et al. Collagenous colitis in children and adolescents: study of 7 cases and literature review. Mod Pathol. 2013;26:881–7. [DOI] [PubMed] [Google Scholar]
- 40. Vanderhoof JA, Goble K, Young RJ. Collagenous colitis in a 4‐year‐old child: response to budesonide. J Pediatr Gastroenterol Nutr. 2010;50:688–90. [DOI] [PubMed] [Google Scholar]
- 41. Fernandez‐Banares F, Zabana Y, Aceituno M, et al. Prevalence and natural history of microscopic colitis: a population‐based study with long‐term clinical follow‐up in Terrassa, Spain. J Crohns Colitis. 2016;10:805–11. [DOI] [PubMed] [Google Scholar]
- 42. Batista L, Ruiz L, Zabana Y, et al. P240 microscopic colitis is the most frequent diagnosis of patients with watery chronic diarrhoea and macroscopically normal colonoscopy in a context of clinical practice. J Crohns Colitis. 2018;12:S221. [Google Scholar]
- 43. Cotter TG, Binder M, Smyrk T, et al. Sa1410 optimization of a scoring system to predict microscopic colitis in a cohort of patients with chronic diarrhea. Gastroenterology. 2016;150:S308. [DOI] [PubMed] [Google Scholar]
- 44. da Silva JG, De Brito T, Cintra Damiao AO, et al. Histologic study of colonic mucosa in patients with chronic diarrhea and normal colonoscopic findings. J Clin Gastroenterol. 2006;40:44–8. [DOI] [PubMed] [Google Scholar]
- 45. Erdem L, Yildirim S, Akbayir N, et al. Prevalence of microscopic colitis in patients with diarrhea of unknown etiology in Turkey. World J Gastroenterol. 2008;14:4319–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fine KD, Seidel RH, Do K. The prevalence, anatomic distribution, and diagnosis of colonic causes of chronic diarrhea. Gastrointest Endosc. 2000;51:318–26. [DOI] [PubMed] [Google Scholar]
- 47. Gado AS, Ebeid BA, El Hindawi AA, et al. Prevalence of microscopic colitis in patients with chronic diarrhea in Egypt: a single‐center study. Saudi J Gastroenterol. 2011;17:383–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Garg PK, Singh J, Dhali GK, et al. Microscopic colitis is a cause of large bowel diarrhea in Northern India. J Clin Gastroenterol. 1996;22:11–5. [DOI] [PubMed] [Google Scholar]
- 49. Gu HX, Zhi FC, Huang Y, et al. Microscopic colitis in patients with chronic diarrhea and normal colonoscopic findings in Southern China. Int J Colorectal Dis. 2012;27:1167–73. [DOI] [PubMed] [Google Scholar]
- 50. Hatemi AI, Senates E, Dobrucali A, et al. Collagenous colitis: a retrospective survey of patients with chronic diarrhea. Hepatogastroenterology. 2011;58:1963–7. [DOI] [PubMed] [Google Scholar]
- 51. Hotouras A, Collins P, Speake W, et al. Diagnostic yield and economic implications of endoscopic colonic biopsies in patients with chronic diarrhoea. Colorectal Dis. 2012;14:985–8. [DOI] [PubMed] [Google Scholar]
- 52. Kagueyama FM, Nicoli FM, Bonatto MW, et al. Importance of biopsies and histological evaluation in patients with chronic diarrhea and normal colonoscopies. Arq Bras Cir Dig. 2014;27:184–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Larsson JK, Sjoberg K, Vigren L, et al. Chronic non‐bloody diarrhoea: a prospective study in Malmo, Sweden, with focus on microscopic colitis. BMC Res Notes. 2014;7:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Macaigne G, Lahmek P, Locher C, et al. Microscopic colitis or functional bowel disease with diarrhea: a French prospective multicenter study. Am J Gastroenterol. 2014;109:1461–70. [DOI] [PubMed] [Google Scholar]
- 55. Miraglia S, Luigiano C, Siringo S, et al. P.12.14 the paucicellular lymphocytic colitis is the most frequent type of microscopic colitis in a city of southern Italy: preliminary results of a 6‐month pilot study. Dig Liver Dis. 2013;45:S177. [Google Scholar]
- 56. Misra V, Misra SP, Dwivedi M, et al. Microscopic colitis in patients presenting with chronic diarrhea. Indian J Pathol Microbiol. 2010;53:15–9. [DOI] [PubMed] [Google Scholar]
- 57. Shah RJ, Fenoglio‐Preiser C, Bleau BL, et al. Usefulness of colonoscopy with biopsy in the evaluation of patients with chronic diarrhea. Am J Gastroenterol. 2001;96:1091–5. [DOI] [PubMed] [Google Scholar]
- 58. Shaw A, Hall J, Ravi S. Random colonic biopsies for chronic diarrhoea—a numbers needed to investigate approach. Int J Surg. 2016;36:S61. [Google Scholar]
- 59. Sidhu PS, Khan F, Hebden J, et al. PWE‐187 colonic biopsies to detect microscopic colitis in patients with diarrhoea and “normal” colonoscopy: worth the effort? Gut 2012;61:A372. [Google Scholar]
- 60. Tontini GE, Pastorelli L, Spina L, et al. Microscopic colitis and colorectal neoplastic lesion rate in chronic nonbloody diarrhea: a prospective, multicenter study. Inflamm Bowel Dis. 2014;20:882–91. [DOI] [PubMed] [Google Scholar]
- 61. Trembling PM, Nicol F, Hoare J. PTU‐259 routine biopsy during lower gi endoscopy is of diagnostic value for patients with chronic diarrhoea. Gut 2015;64:A175–76. [Google Scholar]
- 62. Villafuerte‐Galvez J, Sotelo‐Olivera MI, Cok J, et al. Colonoscopic findings in Peruvian patients with chronic diarrhea. PLoS One. 2012;7:e46690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wagner M, Sjoberg K, Vigren L, et al. Elevated fecal levels of eosinophil granule proteins predict collagenous colitis in patients referred to colonoscopy due to chronic non‐bloody diarrhea. Scand J Gastroenterol. 2016;51:835–41. [DOI] [PubMed] [Google Scholar]
- 64. Arcana Lopez R, Frisancho Velarde O, Chacaltana A. Etiology of chronic diarrhea in the elderly in hospital Edgardo Rebagliati, Lima‐Peru. Rev Gastroenterol Peru. 2012;32:366–70. [PubMed] [Google Scholar]
- 65. Carmona‐Sanchez R, Tostado‐Fernandez F, Esmer‐Sanchez D. Usefulness of colonoscopy with biopsy for the study of patients with chronic diarrhea. Revista de Gastroenterologıa de Mexico. 2007;72:349–54. [PubMed] [Google Scholar]
- 66. Channaiah D, Mohammad K, Kini R, et al. Yield of colonoscopy with biopsy in the evaluation of chronic diarrhea. Indian J Gastroenterol. 2017;36:A76. [Google Scholar]
- 67. Essid M, Kallel S, Brahim EB, et al. Prevalence of microscopic colitis to the course of the chronic diarrhea: about 150 cases. Tunis Med. 2005;83:284–7. [PubMed] [Google Scholar]
- 68. Gonzalez N, Guerra L, Sanguinetti A, et al. Prevalence of microscopic colitis in a group of patients from Montevideo, Uruguay. Acta Gastroenterol Latinoam. 2019;40:216–20. [PubMed] [Google Scholar]
- 69. Marshall J, Singh R, Diaz‐Arias A. Chronic, unexplained diarrhea: are biopsies necessary if colonoscopy is normal?. Am J Gastroenterol. 1995;90:372–6. [PubMed] [Google Scholar]
- 70. Matsubara Y, Ohta T, Maemoto A. Abnormal colonic biopsy findings in chronic diarrhea patients with almost normal endoscopic findings. Gastroenterol Endosc. 2014;56:1563–9. [Google Scholar]
- 71. Sethi S. Outcomes in patients undergoing colonoscopy to investigate chronic diarrhea. J Gastroenterol Hepatol. 2012;27:43. [Google Scholar]
- 72. Valle Mansilla JL, Leon Barua R, Recavarren Arce S, et al. Microscopic colitis in patients with chronic diarrhea. Rev Gastroenterol Peru. 2002;22:275–8. [PubMed] [Google Scholar]
- 73. Burke KE, Ananthakrishnan AN, Lochhead P, et al. Smoking is associated with an increased risk of microscopic colitis: results from two large prospective cohort studies of US women. J Crohns Colitis. 2018;12:559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Larsson JK, Sonestedt E, Ohlsson B, et al. The association between the intake of specific dietary components and lifestyle factors and microscopic colitis. Eur J Clin Nutr. 2016;70:1309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Roth B, Gustafsson RJ, Jeppsson B, et al. Smoking‐ and alcohol habits in relation to the clinical picture of women with microscopic colitis compared to controls. BMC Wom Health. 2014;14:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Verhaegh BPM, Pierik MJ, Goudkade D, et al. Early life exposure, lifestyle, and comorbidity as risk factors for microscopic colitis: a case‐control study. Inflamm Bowel Dis. 2017;23:1040–6. [DOI] [PubMed] [Google Scholar]
- 77. Vigren L, Sjoberg K, Benoni C, et al. Is smoking a risk factor for collagenous colitis?. Scand J Gastroenterol. 2011;46:1334–9. [DOI] [PubMed] [Google Scholar]
- 78. Wickbom A, Nyhlin N, Montgomery SM, et al. Family history, comorbidity, smoking and other risk factors in microscopic colitis: a case‐control study. Eur J Gastroenterol Hepatol. 2017;29:587–94. [DOI] [PubMed] [Google Scholar]
- 79. Wildt S, Munck LK, Becker S, et al. Risk of osteoporosis in microscopic colitis. Postgrad Med. 2018;130:348–54. [DOI] [PubMed] [Google Scholar]
- 80. Yen EF, Pokhrel B, Du H, et al. Current and past cigarette smoking significantly increase risk for microscopic colitis. Inflamm Bowel Dis. 2012;18:1835–41. [DOI] [PubMed] [Google Scholar]
- 81. Fernandez‐Banares F, de Sousa MR, Salas A, et al. Epidemiological risk factors in microscopic colitis: a prospective case‐control study. Inflamm Bowel Dis. 2013;19:411–7. [DOI] [PubMed] [Google Scholar]
- 82. Guagnozzi D, Lucendo AJ, Angueira T, et al. Drug consumption and additional risk factors associated with microscopic colitis: case‐control study. Rev Esp Enferm Dig. 2015;107:347–53. [PubMed] [Google Scholar]
- 83. Jaruvongvanich V, Poonsombudlert K, Ungprasert P. Smoking and risk of microscopic colitis: a systematic review and meta‐analysis. Inflamm Bowel Dis. 2019;25:672–8. [DOI] [PubMed] [Google Scholar]
- 84. Fernandez‐Banares F, de Sousa MR, Salas A, et al. Impact of current smoking on the clinical course of microscopic colitis. Inflamm Bowel Dis. 2013;19:1470–6. [DOI] [PubMed] [Google Scholar]
- 85. Fernandez‐Banares F, Piqueras M, Guagnozzi D, et al. Collagenous colitis: requirement for high‐dose budesonide as maintenance treatment. Dig Liver Dis. 2017;49:973–7. [DOI] [PubMed] [Google Scholar]
- 86. Gentile NM, Khanna S, Kammer PP, et al. Outcomes of microscopic colitis and smoking: a population‐based study. Gastroenterology. 2013;144:S439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Munch A, Tysk C, Bohr J, et al. Smoking status influences clinical outcome in collagenous colitis. J Crohns Colitis. 2016;10:449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. O'Toole A, Coss A, Holleran G, et al. Microscopic colitis: clinical characteristics, treatment and outcomes in an Irish population. Int J Colorectal Dis. 2014;29:799–803. [DOI] [PubMed] [Google Scholar]
- 89. Roth B, Bengtsson M, Ohlsson B. Diarrhoea is not the only symptom that needs to be treated in patients with microscopic colitis. Eur J Intern Med. 2013;24:573–8. [DOI] [PubMed] [Google Scholar]
- 90. Miehlke S, Hansen JB, Madisch A, et al. Risk factors for symptom relapse in collagenous colitis after withdrawal of short‐term budesonide therapy. Inflamm Bowel Dis. 2013;19:2763–7. [DOI] [PubMed] [Google Scholar]
- 91. Bonderup OK, Fenger‐Gron M, Wigh T, et al. Drug exposure and risk of microscopic colitis: a nationwide Danish case‐control study with 5751 cases. Inflamm Bowel Dis. 2014;20:1702–7. [DOI] [PubMed] [Google Scholar]
- 92. Bonderup OK, Nielsen GL, Dall M, et al. Significant association between the use of different proton pump inhibitors and microscopic colitis: a nationwide Danish case‐control study. Aliment Pharmacol Ther. 2018;48:618–25. [DOI] [PubMed] [Google Scholar]
- 93. Fernandez‐Banares F, Esteve M, Espinos JC, et al. Drug consumption and the risk of microscopic colitis. Am J Gastroenterol. 2007;102:324–30. [DOI] [PubMed] [Google Scholar]
- 94. Keszthelyi D, Jansen SV, Schouten GA, et al. Proton pump inhibitor use is associated with an increased risk for microscopic colitis: a case‐control study. Aliment Pharmacol Ther. 2010;32:1124–8. [DOI] [PubMed] [Google Scholar]
- 95. Masclee GM, Coloma PM, Kuipers EJ, et al. Increased risk of microscopic colitis with use of proton pump inhibitors and non‐steroidal anti‐inflammatory drugs. Am J Gastroenterol. 2015;110:749–59. [DOI] [PubMed] [Google Scholar]
- 96. Pascua MF, Kedia P, Weiner MG, et al. Microscopic colitis and medication use. Clin Med Insights Gastroenterol. 2010;2010:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Riddell RH, Tanaka M, Mazzoleni G. Non‐steroidal anti‐inflammatory drugs as a possible cause of collagenous colitis: a case‐control study. Gut. 1992;33:683–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Verhaegh BP, de Vries F, Masclee AA, et al. High risk of drug‐induced microscopic colitis with concomitant use of NSAIDs and proton pump inhibitors. Aliment Pharmacol Ther. 2016;43:1004–13. [DOI] [PubMed] [Google Scholar]
- 99. Yen EF, Yoo J, Ture A, et al. Medication exposure and the risk of microscopic colitis: results from a prospective US trial. Gastroenterology. 2017;152:S194. [Google Scholar]
- 100. Harma C, Havelet M, Dean T, et al. Lymphocytic colitis and proton pump inhibitor use: a case‐control study. J Gastroenterol Hepatol. 2011;26:68–83.21175796 [Google Scholar]
- 101. Al‐Ghamdi MY, Malatjalian DA, Veldhuyzen van Zanten S. Causation: recurrent collagenous colitis following repeated use of NSAIDs. Can J Gastroenterol. 2002;16:861–2. [DOI] [PubMed] [Google Scholar]
- 102. Beaugerie L, Luboinski J, Brousse N, et al. Drug induced lymphocytic colitis. Gut. 1994;35:426–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Beaugerie L, Pardi DS. Review article: drug‐induced microscopic colitis—proposal for a scoring system and review of the literature. Aliment Pharmacol Ther. 2005;22:277–84. [DOI] [PubMed] [Google Scholar]
- 104. Beaugerie L, Patey N, Ranitidine BN. Diarrhoea, and lymphocytic colitis. Gut. 1995;37:708–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Begaud B, Evreux J, Jouglard J, et al. Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France. Therapie. 1985;40:111–8. [PubMed] [Google Scholar]
- 106. Berrebi D, Sautet A, Flejou JF, et al. Ticlopidine induced colitis: a histopathological study including apoptosis. J Clin Pathol. 1998;51:280–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bouaniche M, Chassagne P, Landrin I, et al. Colite lymphocytaire au Cyclo 3 Fort® . La Revue de Medecine Interne. 1996;17:776–8. [DOI] [PubMed] [Google Scholar]
- 108. Bouchet‐Laneuw F, Deplaix P, Dumollard JM, et al. Chronic diarrhea following ingestion of Tardyferon associated with lymphocytic colitis. Gastroenterol Clin Biol. 1997;21:83–4. [PubMed] [Google Scholar]
- 109. Bouvet C, Bellaiche G, Slama R, et al. Lymphocytic colitis and villous atrophy after treatment with ticlopidine. Gastroenterol Clin Biol. 1998;22:1117‐8. [PubMed] [Google Scholar]
- 110. Brigot C, Courillon‐Mallet A, Roucayrol AM, et al. Lymphocytic colitis and ticlopidine. Gastroenterol Clin Biol. 1998;22:361–2. [PubMed] [Google Scholar]
- 111. Capurso G, Marignani M, Attilia F, et al. Lansoprazole‐induced microscopic colitis: an increasing problem? Results of a prospective case‐series and systematic review of the literature. Dig Liver Dis. 2011;43:380–5. [DOI] [PubMed] [Google Scholar]
- 112. Chande N, Driman DK. Microscopic colitis associated with lansoprazole: report of two cases and a review of the literature. Scand J Gastroenterol. 2007;42:530–3. [DOI] [PubMed] [Google Scholar]
- 113. Chiba M, Sugawara T, Tozawa H, et al. Lansoprazole‐associated collagenous colitis: diffuse mucosal cloudi‐ness mimicking ulcerative colitis. World J Gastroenterol. 2009;15:2166–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Dharancy S, Dapvril V, Dupont‐Evrard F, et al. Colite lymphocytaire et atrophie villositaire ileale secondaires a` la prise de Cyclo 3 Fort. Gastroenterol Clin Biol. 2000:24–134. [PubMed] [Google Scholar]
- 115. Duncan HD, Talbot IC, Silk DB. Collagenous colitis and cimetidine. Eur J Gastroenterol Hepatol. 1997;9:819–20. [DOI] [PubMed] [Google Scholar]
- 116. Fathallah N, Chatti S, Azouz MM. Lymphocytic colitis associated with oxetorone consumption. Gastroenterol Clin Biol. 2010;34:154–5. [DOI] [PubMed] [Google Scholar]
- 117. Feurle GE, Bartz KO, Schmitt‐Graff A. Lymphocytic colitis, induced by ticlopidine. Z Gastroenterol. 1999;37:1105–8. [PubMed] [Google Scholar]
- 118. Fuste L, Arevalo D, Gomez M, et al. Lymphocytic colitis during treatment with ticlopidine. Gastroenterol Hepatol. 2000;23:363–4. [PubMed] [Google Scholar]
- 119. Ghilain JM, Schapira M, Maisin JM, et al. Lymphocytic colitis associated with lansoprazole treatment. Gastroenterol Clin Biol. 2000;24:960–2. [PubMed] [Google Scholar]
- 120. Giardiello FM, Hansen FC 3rd, Lazenby AJ, et al. Collagenous colitis in setting of nonsteroidal anti‐inflammatory drugs and antibiotics. Dig Dis Sci. 1990:257–60. [DOI] [PubMed] [Google Scholar]
- 121. Gugenberger C, Donner P, Naami A, et al. Persistent diarrhea and loss of weight during therapy with leflunomide. Dtsch Med Wochenschr. 2008;133:1730–2. [DOI] [PubMed] [Google Scholar]
- 122. Gwillim EC, Bowyer BA. Duloxetine‐induced lymphocytic colitis. J Clin Gastroenterol. 2012;46:717–8. [DOI] [PubMed] [Google Scholar]
- 123. Hilmer SN, Heap TR, Eckstein RP, et al. Microscopic colitis associated with exposure to lansoprazole. Med J Aust. 2006;184:185–6. [DOI] [PubMed] [Google Scholar]
- 124. Kitagawa T, Sato K, Yokouchi Y, et al. A case of lansoprazole‐associated collagenous colitis with longitudinal ulcer. J Gastrointestin Liver Dis. 2013;22:9. [PubMed] [Google Scholar]
- 125. Kusnik B, Stolte M. Lymphocytic colitis under treatment with duloxetine. Z Gastroenterol. 2010;48:693–5. [DOI] [PubMed] [Google Scholar]
- 126. Larzilliere I, Gargot D, Zleik T, et al. Microscopic colitis and ticlid. Gastroenterol Clin Biol. 1999;23:795–6. [PubMed] [Google Scholar]
- 127. Lim C, Macaigne G, Boivin JF, et al. Stalevo‐associated lymphocytic colitis. Gastroenterol Clin Biol. 2008;32:698–9. [DOI] [PubMed] [Google Scholar]
- 128. Linares Torres P, Fidalgo Lopez I, Castanon Lopez A, et al. Lymphocytic colitis as a cause of chronic diarrhea: possible association with carbamazepine. Atención Primaria. 2000;25:366–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Macaigne G, Boivin JF, Simon P, et al. Lansoprazole‐associated collagenous colitis. Gastroenterol Clin Biol. 2001;25:1030. [PubMed] [Google Scholar]
- 130. Macaigne G, Boivin JF, Chayette C, et al. Oxetorone‐associated lymphocytic colitis. Gastroenterol Clin Biol. 2002;26:537. [PubMed] [Google Scholar]
- 131. Macaigne G, Ozon N, Dikov D, et al. Colite lymphocytaire associée à la prise de Piasclédine® . Gastroenterol Clin Biol. 2004;28:412–3. [DOI] [PubMed] [Google Scholar]
- 132. Maroy B. Lymphocytic colitis probably due to etifoxine. A case with relapse after reintroduction. Therapie. 2009;64:137–8. [DOI] [PubMed] [Google Scholar]
- 133. Maroy B. Acute lymphocytic colitis due to carbamazepine. Gastroenterol Clin Biol. 2010;34:155–6. [DOI] [PubMed] [Google Scholar]
- 134. Mennecier D, Saloum T, Roycourt AM, et al. Chronic diarrhea and lymphocytic colitis associated with Daflon therapy. Gastroenterol Clin Biol. 1999;23:1101–2. [PubMed] [Google Scholar]
- 135. Mennecier D, Thiolet C, Bredin C, et al. Lymphocytic colitis after ingestion of Rustacea flavonoid extract. Presse Med. 2001;30:1063. [PubMed] [Google Scholar]
- 136. Menon R, Ng C. Sertraline‐induced microscopic colitis. Psychosomatics. 2015;56:316–7. [DOI] [PubMed] [Google Scholar]
- 137. Milman N, Kraag G. NSAID‐induced collagenous colitis. J Rheumatol. 2010;37:2432–3. [DOI] [PubMed] [Google Scholar]
- 138. Mukherjee S. Diarrhea associated with lansoprazole. J Gastroenterol Hepatol. 2003;18:602–3. [DOI] [PubMed] [Google Scholar]
- 139. Murasawa M, Sakurada T, Oishi D, et al. Collagenous colitis associated with rabeprazole in a peritoneal dialysis patient. Perit Dial Int. 2015;35:588–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Nielsen JA, Steephen A, Lewin M. Angiotensin‐II inhibitor (olmesartan)‐induced collagenous sprue with resolution following discontinuation of drug. World J Gastroenterol 2013. 19:6928–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Nomura E, Kagaya H, Uchimi K, et al. Linear mucosal defects: a characteristic endoscopic finding of lansoprazole‐associated collagenous colitis. Endoscopy 2010; 42(Suppl 2):E9–E10. [DOI] [PubMed] [Google Scholar]
- 142. Ozeki T, Ogasawara N, Izawa S, et al. Protein‐losing enteropathy associated with collagenous colitis cured by withdrawal of a proton pump inhibitor. Intern Med. 2013;52:1183–7. [DOI] [PubMed] [Google Scholar]
- 143. Pelizza L, Melegari M. Clozapine‐induced microscopic colitis: a case report and review of the literature. J Clin Psychopharmacol. 2007;27:571–4. [DOI] [PubMed] [Google Scholar]
- 144. Piche T, Raimondi V, Schneider S, et al. Acarbose and lymphhoctiic colitis. Lancet. 2000;356:1246. [DOI] [PubMed] [Google Scholar]
- 145. Pierrugues R, Saingra B. Lymphocytic colitis and Cyclo 3 fort: 4 new cases. Gastroenterol Clin Biol. 1996;20:916–7. [PubMed] [Google Scholar]
- 146. Rammer M, Kirchgatterer A, Hobling W, et al. Lansoprazole‐associated collagenous colitis: a case report. Z Gastroenterol. 2005;43:657–60. [DOI] [PubMed] [Google Scholar]
- 147. Rassiat E, Michiels C, Sgro C, et al. Lymphocytic colitis due to Modopar. Gastroenterol Clin Biol. 2000;24:852–3. [PubMed] [Google Scholar]
- 148. Rosa I, Nahon S, Cohen C, et al. Ticlopidine‐induced lymphocytic colitis. Ann Med Interne. 1999;150:437–9. [PubMed] [Google Scholar]
- 149. Salter TG, Williams MD. Antidepressant‐associated microscopic colitis: a case report and literature review. Psychosomatics. 2017;58:307–12. [DOI] [PubMed] [Google Scholar]
- 150. Sawada K, Fujiya M, Itabashi K, et al. Collagenous colitis appeared after 6‐year administration of lansoprazole. Clin J Gastroenterol. 2010;3:18–21. [DOI] [PubMed] [Google Scholar]
- 151. Swine C, Cornette P, Van Pee D, et al. Ticlopidine, diarrhea and lymphocytic colitis. Gastroenterol Clin Biol. 1998;22:475–6. [PubMed] [Google Scholar]
- 152. Thiolet C, Bredin C, Rimlinger H, et al. Lymphocytic colitis following administration of Cyclo 3 fort. Presse Med. 2003;32:1323–4. [PubMed] [Google Scholar]
- 153. Thomson RD, Lestina LS, Bensen SP, et al. Lansoprazole‐associated microscopic colitis: a case series. Am J Gastroenterol. 2002;97:2908–13. [DOI] [PubMed] [Google Scholar]
- 154. Umeno J, Esaki M, Nuki Y, et al. Letter: lansoprazole consumption is more common in Japanese patients with collagenous colitis. Aliment Pharmacol Ther. 2013;38:208–9. [DOI] [PubMed] [Google Scholar]
- 155. Verschueren P, Vandooren AK, Westhovens R. Debilitating diarrhoea and weight loss due to colitis in two RA patients treated with leflunomide. Clin Rheumatol. 2005;24:87–90. [DOI] [PubMed] [Google Scholar]
- 156. Wilcox GM, Mattia A. Collagenous colitis associated with lansoprazole. J Clin Gastroenterol. 2002;34:164–6. [DOI] [PubMed] [Google Scholar]
- 157. Wilcox GM, Mattia AR. Microscopic colitis associated with omeprazole and esomeprazole exposure. J Clin Gastroenterol. 2009;43:551–3. [DOI] [PubMed] [Google Scholar]
- 158. Yagi K, Nakamura A, Sekine A, et al. Nonsteroidal anti‐inflammatory drug‐associated colitis with a histology of collagenous colitis. Endoscopy. 2001;33:629–32. [DOI] [PubMed] [Google Scholar]
- 159. Chauveau E, Prignet JM, Carloz E, et al. Lymphocytic colitis likely attributable to use of vinburnine (Cervoxan). Gastroenterol Clin Biol. 1998;22:362. [PubMed] [Google Scholar]
- 160. Simsek Z, Alagozlu H, Tuncer C, et al. Lymphocytic colitis associated with lansoprazole treatment. Curr Ther Res Clin Exp. 2007;68:360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Coyne JD. Microscopic colitis occurring in association with hyperplastic polyps and tubulovillous adenomas: observations in 10 cases. J Clin Pathol. 2014;67:919–20. [DOI] [PubMed] [Google Scholar]
- 162. Fernàndez‐Bañares F, Salas A, Zabana A, et al. Risk of colorectal adenomas in patients with microscopic colitis: a case‐control study. United European Gastroenterol J. 2016;4:A434. [Google Scholar]
- 163. Kao KT, Pedraza BA, McClune AC, et al. Microscopic colitis: a large retrospective analysis from a health maintenance organization experience. World J Gastroenterol. 2009;15:3122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Levy A, Borren NZ, Maxner B, et al. Cancer risk in microscopic colitis: a retrospective cohort study. BMC Gastroenterol. 2019;19:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. McPhaul C, Sonnenberg A, Genta R. Low prevalence of colon polyps in patients with diarrhea and microscopic colitis. Am J Gastroenterol. 2013;108:S164. [Google Scholar]
- 166. Mellander MR, Ekbom A, Hultcrantz R, et al. Microscopic colitis: a descriptive clinical cohort study of 795 patients with collagenous and lymphocytic colitis. Scand J Gastroenterol. 2016;51:556–62. [DOI] [PubMed] [Google Scholar]
- 167. Mills LR, Schuman BM, Thompson WO. Lymphocytic colitis. A definable clinical and histologi‐cal diagnosis. Dig Dis Sci. 1993;38:1147–51. [DOI] [PubMed] [Google Scholar]
- 168. Sonnenberg A, Genta RM. Low prevalence of colon polyps in chronic inflammatory conditions of the colon. Am J Gastroenterol. 2015;110:1056–61. [DOI] [PubMed] [Google Scholar]
- 169. Chan JL, Tersmette AC, Offerhaus GJ, et al. Cancer risk in collagenous colitis. Inflamm Bowel Dis. 1999;5:40–3. [DOI] [PubMed] [Google Scholar]
- 170. Bohr J, Tysk C, Eriksson S, et al. Collagenous colitis: a retrospective study of clinical presentation and treatment in 163 patients. Gut. 1996;39:846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Calabrese C, Gionchetti P, Liguori G, et al. Clinical course of microscopic colitis in a single‐center cohort study. J Crohns Colitis. 2011;5:218–21. [DOI] [PubMed] [Google Scholar]
- 172. Koskela RM, Niemela SE, Karttunen TJ, et al. Clinical characteristics of collagenous and lymphocytic colitis. Scand J Gastroenterol. 2004;39:837–45. [DOI] [PubMed] [Google Scholar]
- 173. Olesen M, Eriksson S, Bohr J, et al. Lymphocytic colitis: a retrospective clinical study of 199 Swedish patients. Gut. 2004;53:536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Verhaegh B, Münch B, Cebula W, et al. Demographic and prognosis of incident patients with microscopic colitis‐first results of the European pro‐MC collaboration, a Link Award project. United European Gastroenterol J. 2017;5 (8):1138–50. [Google Scholar]
- 175. Chande N, Driman DK, Reynolds RP. Collagenous colitis and lymphocytic colitis: patient characteristics and clinical presentation. Scand J Gastroenterol. 2005;40:343–7. [DOI] [PubMed] [Google Scholar]
- 176. Guagnozzi D, Arias A, Lucendo AJ. Systematic review with meta‐analysis: diagnostic overlap of microscopic colitis and functional bowel disorders. Aliment Pharmacol Ther. 2016;43:851–62. [DOI] [PubMed] [Google Scholar]
- 177. Hilpusch F, Johnsen PH, Goll R, et al. Microscopic colitis: a missed diagnosis among patients with moderate to severe irritable bowel syndrome. Scand J Gastroenterol. 2017;52:173–7. [DOI] [PubMed] [Google Scholar]
- 178. Kamp EJ, Kane JS, Ford AC. Irritable bowel syndrome and microscopic colitis: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2016;14:659–68. [DOI] [PubMed] [Google Scholar]
- 179. Kane JS, Irvine AJ, Derwa Y, et al. High prevalence of irritable bowel syndrome‐type symptoms in microscopic colitis: implications for treatment. Therap Adv Gastroenterol. 2018;11:1756284818783600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Cotter TG, Binder M, Loftus EV Jr., et al. Development of a microscopic colitis disease activity Index: a prospective cohort study. Gut. 2018;67:441–6. [DOI] [PubMed] [Google Scholar]
- 181. Gentile N, Yen EF. Prevalence, pathogenesis, diagnosis, and management of microscopic colitis. Gut Liver. 2018;12:227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. O'Toole A. Optimal management of collagenous colitis: a review. Clin Exp Gastroenterol. 2016;9:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Bohr J, Wickbom A, Hegedus A, et al. Diagnosis and management of microscopic colitis: current perspectives. Clin Exp Gastroenterol. 2014;7:273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Nyhlin N, Wickbom A, Montgomery SM, et al. Long‐term prognosis of clinical symptoms and health‐related quality of life in microscopic colitis: a case‐control study. Aliment Pharmacol Ther. 2014;39:963–72. [DOI] [PubMed] [Google Scholar]
- 185. Roth B, Ohlsson B. Gastrointestinal symptoms and psychological well‐being in patients with microscopic colitis. Scand J Gastroenterol. 2013;48:27–34. [DOI] [PubMed] [Google Scholar]
- 186. Hjortswang H, Tysk C, Bohr J, et al. Health‐related quality of life is impaired in active collagenous colitis. Dig Liver Dis. 2011;43:102–9. [DOI] [PubMed] [Google Scholar]
- 187. Hjortswang H, Tysk C, Bohr J, et al. Defining clinical criteria for clinical remission and disease activity in collagenous colitis. Inflamm Bowel Dis. 2009;15:1875–81. [DOI] [PubMed] [Google Scholar]
- 188. Kane JS, Irvine AJ, Derwa Y, et al. Fatigue and its associated factors in microscopic colitis. Therap Adv Gastroenterol. 2018;11:1756284818799599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Madisch A, Heymer P, Voss C, et al. Oral budesonide therapy improves quality of life in patients with collagenous colitis. Int J Colorectal Dis. 2005;20:312–6. [DOI] [PubMed] [Google Scholar]
- 190. Madisch A, Miehlke S, Eichele O, et al. Boswellia serrata extract for the treatment of collagenous colitis. A double‐blind, randomized, placebo‐controlled, multi‐center trial. Int J Colorectal Dis. 2007;22:1445–51. [DOI] [PubMed] [Google Scholar]
- 191. Miehlke S, Madisch A, Bethke B, et al. Oral budesonide for maintenance treatment of collagenous colitis: a randomized, double‐blind, placebo‐controlled trial. Gastroenterology. 2008;135:1510–6. [DOI] [PubMed] [Google Scholar]
- 192. Miehlke S, Madisch A, Karimi D, et al. Budesonide is effective in treating lymphocytic colitis: a randomized double‐blind placebo‐controlled study. Gastroenterology. 2009;136:2092–100. [DOI] [PubMed] [Google Scholar]
- 193. Munch A, Bohr J, Miehlke S, et al. Low‐dose budesonide for maintenance of clinical remission in collagenous colitis: a randomised, placebo‐controlled, 12‐month trial. Gut. 2016;65:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Wildt S, Munck LK, Vinter‐Jensen L, et al. Probiotic treatment of collagenous colitis: a randomized, double‐blind, placebo‐controlled trial with Lactobacillus acidophilus and Bifidobacterium animalis subsp. Lactis . Inflamm Bowel Dis. 2006;12:395–401. [DOI] [PubMed] [Google Scholar]
- 195. Miehlke S, Aust D, Mihaly E, et al. Efficacy and safety of budesonide, vs mesalazine or placebo, as induction therapy for lymphocytic colitis. Gastroenterology. 2018;155:1795–804. [DOI] [PubMed] [Google Scholar]
- 196. Chande N, Al Yatama N, Bhanji T, et al. Interventions for treating lymphocytic colitis. Cochrane Database Syst Rev. 2017;7:CD006096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Kafil TS, Nguyen TM, Patton PH, et al. Interventions for treating collagenous colitis. Cochrane Database Syst Rev. 2017;11:CD003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Sebastian S, Wilhelm A, Jessica L, et al. Budesonide treatment for microscopic colitis: systematic review and meta‐analysis. Eur J Gastroenterol Hepatol. 2019;31:919–27. [DOI] [PubMed] [Google Scholar]
- 199. Baert F, Schmit A, D'Haens G, et al. Budesonide in collagenous colitis: a double‐blind placebo‐controlled trial with histologic follow‐up. Gastroenterology. 2002;122:20–5. [DOI] [PubMed] [Google Scholar]
- 200. Bonderup OK, Hansen JB, Birket‐Smith L, et al. Budesonide treatment of collagenous colitis: a randomised, double blind, placebo controlled trial with morphometric analysis. Gut. 2003;52:248–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Miehlke S, Heymer P, Bethke B, et al. Budesonide treatment for collagenous colitis: a randomized, double‐blind, placebo‐controlled, multicenter trial. Gastroenterology. 2002;123:978–84. [DOI] [PubMed] [Google Scholar]
- 202. Munck LK, Kjeldsen J, Philipsen E, et al. Incomplete remission with short‐term prednisolone treatment in collagenous colitis: a randomized study. Scand J Gastroenterol. 2003;38:606–10. [DOI] [PubMed] [Google Scholar]
- 203. Miehlke S, Madisch A, Kupcinskas L, et al. Budesonide is more effective than mesalamine or placebo in short‐term treatment of collagenous colitis. Gastroenterology. 2014;146:1222–30. [DOI] [PubMed] [Google Scholar]
- 204. Miehlke S, Madisch A, Voss C, et al. Long‐term follow‐up of collagenous colitis after induction of clinical remission with budesonide. Aliment Pharmacol Ther. 2005;22:1115–9. [DOI] [PubMed] [Google Scholar]
- 205. Goff JS, Barnett JL, Pelke T, et al. Collagenous colitis: histopathology and clinical course. Am J Gastroenterol. 1997;92:57–60. [PubMed] [Google Scholar]
- 206. Irvine EJ, Feagan B, Rochon J, et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn's Relapse Prevention Trial Study Group. Gastroenterology. 1994;106:287–96. [DOI] [PubMed] [Google Scholar]
- 207. Minsk ABJ and Cohen DR. FDA issues final guidance on patient‐reported outcome measures used to support labeling claims.Silver Spring: US Department of Health and Human Services; 2010. [Google Scholar]
- 208. Marlicz W, Skonieczna‐Zydecka K, Yung DE, et al. Endoscopic findings and colonic perforation in microscopic colitis: a systematic review. Dig Liver Dis. 2017;49:1073–85. [DOI] [PubMed] [Google Scholar]
- 209. Lindstrom CG. ‘Collagenous colitis' with watery diarrhoea–a new entity?. Pathol Eur. 1976;11:87–9. [PubMed] [Google Scholar]
- 210. Abdo A, Raboud J, Freeman HJ, et al. Clinical and histological predictors of response to medical therapy in collagenous colitis. Am J Gastroenterol. 2002;97:1164–8. [DOI] [PubMed] [Google Scholar]
- 211. Armes J, Gee DC, Macrae FA, et al. Collagenous colitis: jejunal and colorectal pathology. J Clin Pathol. 1992;45:784–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Baert F, Wouters K, D'Haens G, et al. Lymphocytic colitis: a distinct clinical entity? A clinicopathological confrontation of lymphocytic and collagenous colitis. Gut. 1999;45:375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Rubio CA. A simple method to evaluate the thickness of collagen in collagenous colitis. Scand J Gastroenterol. 2000;35:223–4. [DOI] [PubMed] [Google Scholar]
- 214. Tanaka M, Mazzoleni G, Riddell RH. Distribution of collagenous colitis: utility of flexible sigmoidoscopy. Gut. 1992;33:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Carpenter HA, Tremaine WJ, Batts KP, et al. Sequential histologic evaluations in collagenous colitis. Correlations with disease behavior and sampling strate‐gy. Dig Dis Sci. 1992;37:1903–9. [DOI] [PubMed] [Google Scholar]
- 216. Jessurun J, Yardley JH, Giardiello FM, et al. Chronic colitis with thickening of the subepithelial collagen layer (collagenous colitis): histopathologic findings in 15 patients. Hum Pathol. 1987;18:839–48. [DOI] [PubMed] [Google Scholar]
- 217. azenby AJ, Yardley JH, Giardiello FM, et al. Lymphocytic (“microscopic”) colitis: a comparative histopathologic study with particular reference to collagenous colitis. Hum Pathol. 1989;20:18–28. [DOI] [PubMed] [Google Scholar]
- 218. Mosnier JF, Larvol L, Barge J, et al. Lymphocytic and collagenous colitis: an immunohistochemical study. Am J Gastroenterol. 1996;91:709–13. [PubMed] [Google Scholar]
- 219. Offner FA, Jao RV, Lewin KJ, et al. Collagenous colitis: a study of the distribution of morphological abnormalities and their histological detection. Hum Pathol. 1999;30:451–7. [DOI] [PubMed] [Google Scholar]
- 220. Veress B, Lofberg R, Bergman L. Microscopic colitis syndrome. Gut. 1995;36:880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Ayata G, Ithamukkala S, Sapp H, et al. Prevalence and significance of inflammatory bowel disease‐like morphologic features in collagenous and lymphocytic colitis. Am J Surg Pathol. 2002;26:1414–23. [DOI] [PubMed] [Google Scholar]
- 222. Goranzon C, Kumawat AK, Hultgren‐Hornqvist E, et al. Immunohistochemical characterization of lymphocytes in microscopic colitis. J Crohns Colitis. 2013;7:e434–442. [DOI] [PubMed] [Google Scholar]
- 223. Levy AM, Yamazaki K, Van Keulen VP, et al. Increased eosinophil infiltration and degranulation in colonic tissue from patients with collagenous colitis. Am J Gastroenterol. 2001;96:1522–8. [DOI] [PubMed] [Google Scholar]
- 224. Falodia S, Makharia GK, Sateesh J, et al. Spectrum of microscopic colitis in a tertiary care centre in India. Trop Gastroenterol. 2007;28:121–5. [PubMed] [Google Scholar]
- 225. Foerster A, Fausa O. Collagenous colitis. Pathol Res Pract. 1985;180:99–106. [DOI] [PubMed] [Google Scholar]
- 226. Lee E, Schiller LR, Vendrell D, et al. Subepithelial collagen table thickness in colon specimens from patients with microscopic colitis and collagenous colitis. Gastroenterology. 1992;103:1790–6. [DOI] [PubMed] [Google Scholar]
- 227. Wang HH, Owings DV, Antonioli DA, et al. Increased subepithelial collagen deposition is not specific for collagenous colitis. Mod Pathol. 1988;1:329–35. [PubMed] [Google Scholar]
- 228. Lazenby AJ, Yardley JH, Giardiello FM, et al. Pitfalls in the diagnosis of collagenous colitis: experience with 75 cases from a registry of collagenous colitis at the Johns Hopkins hospital. Hum Pathol. 1990;21:905–10. [DOI] [PubMed] [Google Scholar]
- 229. Narabayashi K, Murano M, Egashira Y, et al. Endoscopic and histopathological evaluation of collagenous colitis. Digestion. 2012;85:136–40. [DOI] [PubMed] [Google Scholar]
- 230. Fiehn AM, Bjornbak C, Warnecke M, et al. Observer variability in the histopathologic diagnosis of microscopic colitis and subgroups. Hum Pathol. 2013;44:2461–6. [DOI] [PubMed] [Google Scholar]
- 231. Limsui D, Pardi DS, Smyrk TC, et al. Observer variability in the histologic diagnosis of microscopic colitis. Inflamm Bowel Dis. 2009;15:35–8. [DOI] [PubMed] [Google Scholar]
- 232. Read NW, Krejs GJ, Read MG, et al. Chronic diarrhea of unknown origin. Gastroenterology. 1980;78:264–71. [PubMed] [Google Scholar]
- 233. Carmack SW, Lash RH, Gulizia JM, et al. Lymphocytic disorders of the gastrointestinal tract: a review for the practicing pathologist. Adv Anat Pathol. 2009;16:290–306. [DOI] [PubMed] [Google Scholar]
- 234. Chetty R, Govender D. Lymphocytic and collagenous colitis: an overview of so‐called microscopic colitis. Nat Rev Gastroenterol Hepatol. 2012;9:209–18. [DOI] [PubMed] [Google Scholar]
- 235. Fernandez‐Banares F, Salas A, Esteve M. Pitfalls and errors in the diagnosis of collagenous and lymphocytic colitis. J Crohns Colitis. 2008;2:343–7. [DOI] [PubMed] [Google Scholar]
- 236. Geboes K. Lymphocytic, collagenous and other microscopic colitides: pathology and the relationship with idiopathic inflammatory bowel diseases. Gastroenterol Clin Biol. 2008;32:689–94. [DOI] [PubMed] [Google Scholar]
- 237. Guagnozzi D, Landolfi S, Vicario M. Towards a new paradigm of microscopic colitis: incomplete and variant forms. World J Gastroenterol. 2016;22:8459–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Langner C. Colorectal normal histology and histopathologic findings in patients with chronic diarrhea. Gastroenterol Clin N Am. 2012;41:561–80. [DOI] [PubMed] [Google Scholar]
- 239. Liszka L, Woszczyk D, Pajak J. Histopathological diagnosis of microscopic colitis. J Gastroenterol Hepatol. 2006;21:792–7. [DOI] [PubMed] [Google Scholar]
- 240. Mahajan D, Goldblum JR, Xiao SY, et al. Lymphocytic colitis and collagenous colitis: a review of clinicopathologic features and immunologic abnormalities. Adv Anat Pathol. 2012;19:28–38. [DOI] [PubMed] [Google Scholar]
- 241. Shaz BH, Reddy SI, Ayata G, et al. Sequential clinical and histopathological changes in collagenous and lymphocytic colitis over time. Mod Pathol. 2004;17:395–401. [DOI] [PubMed] [Google Scholar]
- 242. Stoicescu A, Becheanu G, Dumbrava M, et al. Microscopic colitis—a missed diagnosis in diarrhea‐predominant irritable bowel syndrome. Maedica (Buchar). 2012;7:3–9. [PMC free article] [PubMed] [Google Scholar]
- 243. Thijs WJ, van Baarlen J, Kleibeuker JH, et al. Microscopic colitis: prevalence and distribution throughout the colon in patients with chronic diarrhoea. Neth J Med. 2005;63:137–40. [PubMed] [Google Scholar]
- 244. Zabana Y, Ferrer C, Aceituno M, et al. Advances for improved diagnosis of microscopic colitis in patients with chronic diarrhoea. Gastroenterol Hepatol. 2017;40:107–16. [DOI] [PubMed] [Google Scholar]
- 245. Jaskiewicz K, Rzepko R, Adrych K, et al. Microscopic colitis in routine colonoscopies. Dig Dis Sci. 2006;51:241–4. [DOI] [PubMed] [Google Scholar]
- 246. Mohamed N, Marais M, Bezuidenhout J. Microscopic colitis as a missed cause of chronic diarrhea. World J Gastroenterol. 2011;17:1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Mullhaupt B, Guller U, Anabitarte M, et al. Lymphocytic colitis: clinical presentation and long term course. Gut. 1998;43:629–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Setia N, Alpert L, van der Sloot KW, et al. Lymphocytic colitis: pathologic predictors of response to therapy. Hum Pathol. 2018;78:1–7. [DOI] [PubMed] [Google Scholar]
- 249. Bo‐Linn GW, Vendrell DD, Lee E, et al. An evaluation of the significance of microscopic colitis in patients with chronic diarrhea. J Clin Invest. 1985;75:1559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Fasoli R, Talbot I, Reid M, et al. Microscopic colitis: can it be qualitatively and quantitatively characterized?. Ital J Gastroenterol. 1992;24:393–6. [PubMed] [Google Scholar]
- 251. Goldstein NS, Bhanot P. Paucicellular and asymp‐tomatic lymphocytic colitis: expanding the clinicopathologic spectrum of lymphocytic colitis. Am J Clin Pathol. 2004;122:405–11. [DOI] [PubMed] [Google Scholar]
- 252. Patil DT, Odze RD. Biopsy diagnosis of colitis: an algorithmic approach. Virchows Arch. 2018;472:67–80. [DOI] [PubMed] [Google Scholar]
- 253. Rahman MA, Raihan AS, Ahamed DS, et al. Symptomatic overlap in patients with diarrhea predominant irritable bowel syndrome and microscopic colitis in a sub group of Bangladeshi population. Bangladesh Med Res Counc Bull. 2012;38:33–8. [DOI] [PubMed] [Google Scholar]
- 254. Singh P, Das P, Jain AK, et al. Microscopic colitis in children with chronic diarrhea. J Pediatr Gastroenterol Nutr. 2013;57:240–4. [DOI] [PubMed] [Google Scholar]
- 255. Fine KD, Lee EL, Meyer RL. Colonic histopathology in untreated celiac sprue or refractory sprue: is it lymphocytic colitis or colonic lymphocytosis?. Hum Pathol. 1998;29:1433–40. [DOI] [PubMed] [Google Scholar]
- 256. Wang N, Dumot JA, Achkar E, et al. Colonic epithelial lymphocytosis without a thickened subepithelial collagen table: a clinicopathologic study of 40 cases supporting a heterogeneous entity. Am J Surg Pathol. 1999;23:1068–74. [DOI] [PubMed] [Google Scholar]
- 257. Chang F, Deere H, Vu C. Atypical forms of microscopic colitis: morphological features and review of the literature. Adv Anat Pathol. 2005;12:203–11. [DOI] [PubMed] [Google Scholar]
- 258. Fiehn AK, Clausen LN, Engel U, et al. Is revision of cutoff values needed when using CD3 immunohistochemical staining in histopathologic diagnosis of lymphocytic colitis?. Hum Pathol. 2019;84:115–23. [DOI] [PubMed] [Google Scholar]
- 259. Fraser AG, Warren BF, Chandrapala R, et al. Microscopic colitis: a clinical and pathological review. Scand J Gastroenterol. 2002;37:1241–5. [DOI] [PubMed] [Google Scholar]
- 260. Warren BF, Edwards CM, Travis SP. ‘Microscopic colitis': classification and terminology. Histopathology. 2002;40:374–6. [DOI] [PubMed] [Google Scholar]
- 261. Kitchen PA, Levi AJ, Domizio P, et al. Microscopic colitis: the tip of the iceberg?. Eur J Gastroenterol Hepatol. 2002;14:1199–204. [DOI] [PubMed] [Google Scholar]
- 262. Fernandez‐Banares F, Casalots J, Salas A, et al. Paucicellular lymphocytic colitis: is it a minor form of lymphocytic colitis? A clinical pathological and immunological study. Am J Gastroenterol. 2009;104:1189–98. [DOI] [PubMed] [Google Scholar]
- 263. Rasmussen J, Engel PJ, Wildt S, et al. The temporal evolution of histological abnormalities in microscopic colitis. J Crohns Colitis. 2016;10:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Sonnenberg A, Genta RM. Lymphocytic and collagenous colitis: epidemiologic differences and similarities. Dig Dis Sci. 2013;58:2970–5. [DOI] [PubMed] [Google Scholar]
- 265. Fiehn AM, Engel U, Holck S, et al. CD3 immunohistochemical staining in diagnosis of lymphocytic colitis. Hum Pathol. 2016;48:25–31. [DOI] [PubMed] [Google Scholar]
- 266. Macaigne G, Lahmek P, Locher C, et al. Over 90% of cases of microscopic colitis can be diagnosed by performing a short colonoscopy. Clin Res Hepatol Gastroenterol. 2017;41:333–40. [DOI] [PubMed] [Google Scholar]
- 267. Matteoni CA, Wang N, Goldblum JR, et al. Flexible sigmoidoscopy for the detection of microscopic colitis. Am J Med. 2000;108:416–8. [DOI] [PubMed] [Google Scholar]
- 268. Prior A, Lessells AM, Whorwell PJ. Is biopsy necessary if colonoscopy is normal?. Dig Dis Sci. 1987;32:673–6. [DOI] [PubMed] [Google Scholar]
- 269. Shale MJ, Walters JR, Westaby D. Adequacy of flexible sigmoidoscopy with biopsy for diarrhea in patients under age 50 without features of proximal disease. Gastrointest Endosc. 2011;73:757–64. [DOI] [PubMed] [Google Scholar]
- 270. Zins BJ, Tremaine WJ, Carpenter HA. Collagenous colitis: mucosal biopsies and association with fecal leukocytes. Mayo Clin Proc. 1995;70:430–3. [DOI] [PubMed] [Google Scholar]
- 271. Paski SC, Wightman R, Robert ME, et al. The importance of recognizing increased cecal inflammation in health and avoiding the misdiagnosis of nonspecific colitis. Am J Gastroenterol. 2007;102:2294–9. [DOI] [PubMed] [Google Scholar]
- 272. Bonderup OK, Hansen JB, Teglbjaerg PS, et al. Long‐term budesonide treatment of collagenous colitis: a randomised, double‐blind, placebo‐controlled trial. Gut. 2009;58:68–72. [DOI] [PubMed] [Google Scholar]
- 273. Fernandez‐Banares F, Salas A, Esteve M, et al. Collagenous and lymphocytic colitis. evaluation of clinical and histological features, response to treatment, and long‐term follow‐up. Am J Gastroenterol. 2003;98:340–7. [DOI] [PubMed] [Google Scholar]
- 274. Fine KD, Lee EL. Efficacy of open‐label bismuth subsalicylate for the treatment of microscopic colitis. Gastroenterology. 1998;114:29–36. [DOI] [PubMed] [Google Scholar]
- 275. Vigren L, Olesen M, Benoni C, et al. Are collagenous and lymphocytic colitis different aspects of the same disease?. Scand J Gastroenterol. 2012;47:1448–53. [DOI] [PubMed] [Google Scholar]
- 276. Yagi K, Endo S, Nakamura A, et al. Clinical course of drug‐induced collagenous colitis and histological changes after drug withdrawal in a Japanese case series. Eur J Gastroenterol Hepatol. 2012;24:1105–9. [DOI] [PubMed] [Google Scholar]
- 277. Batista L, Ruiz L, Ferrer C, et al. Usefulness of fecal calprotectin as a biomarker of microscopic colitis in a cohort of patients with chronic watery diarrhoea of functional characteristics. Dig Liver Dis. 2019;51:1646–51. [DOI] [PubMed] [Google Scholar]
- 278. von Arnim U, Wex T, Ganzert C, et al. Fecal calprotectin: a marker for clinical differentiation of microscopic colitis and irritable bowel syndrome. Clin Exp Gastroenterol. 2016;9:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Wildt S, Nordgaard‐Lassen I, Bendtsen F, et al. Metabolic and inflammatory faecal markers in collagenous colitis. Eur J Gastroenterol Hepatol. 2007;19:567–74. [DOI] [PubMed] [Google Scholar]
- 280. Fine KD, Ogunji F, George J, et al. Utility of a rapid fecal latex agglutination test detecting the neutrophil protein, lactoferrin, for diagnosing inflammatory causes of chronic diarrhea. Am J Gastroenterol. 1998;93:1300–5. [DOI] [PubMed] [Google Scholar]
- 281. Strygler B, Nicar MJ, Santangelo WC, et al. a1‐Anti‐trypsin excretion in stool in normal subjects and in patients with gastrointestinal disorders. Gastroenterology. 1990;99:1380–7. [DOI] [PubMed] [Google Scholar]
- 282. Lettesjo H, Hansson T, Peterson C, et al. Detection of inflammatory markers in stools from patients with irritable bowel syndrome and collagenous colitis. Scand J Gastroenterol. 2006;41:54–9. [DOI] [PubMed] [Google Scholar]
- 283. Green HD, Beaumont RN, Thomas A, et al. Genome‐wide association study of microscopic colitis in the UK biobank confirms immune‐related pathogenesis. J Crohns Colitis. 2019;13:1578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Stewart M, Andrews CN, Urbanski S, et al. The association of coeliac disease and microscopic colitis: a large population‐based study. Aliment Pharmacol Ther. 2011;33:1340–9. [DOI] [PubMed] [Google Scholar]
- 285. Sonnenberg A, Turner KO, Genta RM. Associations of microscopic colitis with other lymphocytic disorders of the gastrointestinal tract. Clin Gastroenterol Hepatol. 2018;16:1762–7. [DOI] [PubMed] [Google Scholar]
- 286. Fernandez‐Banares F, Esteve M, Farre C, et al. Predisposing HLA‐DQ2 and HLA‐DQ8 haplotypes of coeliac disease and associated enteropathy in microscopic colitis. Eur J Gastroenterol Hepatol. 2005;17:1333–8. [DOI] [PubMed] [Google Scholar]
- 287. Matteoni CA, Goldblum JR, Wang N, et al. Celiac disease is highly prevalent in lymphocytic colitis. J Clin Gastroenterol. 2001;32:225–7. [DOI] [PubMed] [Google Scholar]
- 288. Pardi DS, Ramnath VR, Loftus EV Jr., et al. Lymphocytic colitis: clinical features, treatment, and outcomes. Am J Gastroenterol. 2002;97:2829–33. [DOI] [PubMed] [Google Scholar]
- 289. Svensson M, Bergman D, Olen O, et al. Validating microscopic colitis (MC) in Swedish pathology registers. Scand J Gastroenterol. 2018;53:1469–75. [DOI] [PubMed] [Google Scholar]
- 290. Vigren L, Tysk C, Strom M, et al. Celiac disease and other autoimmune diseases in patients with collagenous colitis. Scand J Gastroenterol. 2013;48:944–50. [DOI] [PubMed] [Google Scholar]
- 291. Liu PH, Lebwohl B, Burke KE, et al. Correction: dietary gluten intake and risk of microscopic colitis among US women without celiac disease: a prospective cohort study. Am J Gastroenterol. 2019;114:837. [DOI] [PubMed] [Google Scholar]
- 292. Femandez‐Banares F, Esteve M, Espinos JC, et al. Bile acid malabsorption (BAM) in patients with functional chronic diarrhea: response to cholestyramine. Gastroenterology. 2000;118:A885. [Google Scholar]
- 293. Ung KA, Gillberg R, Kilander A, et al. Role of bile acids and bile acid binding agents in patients with collagenous colitis. Gut. 2000;46:170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Bajor A, Kilander A, Galman C, et al. Budesonide treatment is associated with increased bile acid absorption in collagenous colitis. Aliment Pharmacol Ther. 2006;24:1643–9. [DOI] [PubMed] [Google Scholar]
- 295. Torres J, Palmela C, Gomes de Sena P, et al. Farnesoid X receptor expression in microscopic colitis: a potential role in disease etiopathogenesis. GE Port J Gastroenterol. 2018;25:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Laing AW, Pardi DS, Loftus EV Jr., et al. Microscopic colitis is not associated with cholecystectomy or appendectomy. Inflamm Bowel Dis. 2006;12:708–11. [DOI] [PubMed] [Google Scholar]
- 297. Pardi DS, Loftus EV, Tremaine WJ, et al. T1193 a randomized, double‐blind, placebo‐controlled trial of budesonide for the treatment of active lymphocytic colitis. Gastroenterology. 2009;136:A519–A520. [Google Scholar]
- 298. Cino M, Greenberg GR. Bone mineral density in Crohn's disease: a longitudinal study of budesonide, prednisone, and nonsteroid therapy. Am J Gastroenterol. 2002;97:915–21. [DOI] [PubMed] [Google Scholar]
- 299. Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with different types of oral corticosteroids and effect of termination of corticosteroids on the risk of fractures. Calcif Tissue Int. 2008;82:249–57. [DOI] [PubMed] [Google Scholar]
- 300. Rautiainen H, Farkkila M, Neuvonen M, et al. Pharmacokinetics and bone effects of budesonide in primary biliary cirrhosis. Aliment Pharmacol Ther. 2006;24:1545–52. [DOI] [PubMed] [Google Scholar]
- 301. Reilev M, Hallas J, Thomsen Ernst M, et al. Long‐term oral budesonide treatment and risk of osteoporotic fractures in patients with microscopic colitis. Aliment Pharmacol Ther. 2020;51:644–51. [DOI] [PubMed] [Google Scholar]
- 302. Colussi D, Salari B, Stewart KO, et al. Clinical characteristics and patterns and predictors of response to therapy in collagenous and lymphocytic colitis. Scand J Gastroenterol. 2015;50:1382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Fiedler LM, George J, Sachar DB, et al. Treatment responses in collagenous colitis. Am J Gastroenterol. 2001;96:818–21. [DOI] [PubMed] [Google Scholar]
- 304. Jobse P, Flens MJ, Loffeld RJ. Collagenous colitis: description of a single centre series of 83 patients. Eur J Intern Med. 2009;20:499–502. [DOI] [PubMed] [Google Scholar]
- 305. Calabrese C, Fabbri A, Areni A, et al. Mesalazine with or without cholestyramine in the treatment of microscopic colitis: randomized controlled trial. J Gastroenterol Hepatol. 2007;22:809–14. [DOI] [PubMed] [Google Scholar]
- 306. Allison MC, Sercombe J, Pounder RE. A double‐blind crossover comparison of lidamidine, loperamide and placebo for the control of chronic diarrhoea. Aliment Pharmacol Ther. 1988;2:347–51. [DOI] [PubMed] [Google Scholar]
- 307. Barbezat GO, Clain JE, Halter F. A double‐blind trial of loperamide in the treatment of chronic diarrhoea. S Afr Med J. 1979;55:502–3. [PubMed] [Google Scholar]
- 308. Mainguet P, Fiasse R. Double‐blind placebo‐controlled study of loperamide (Imodium) in chronic diarrhoea caused by ileocolic disease or resection. Gut. 1977;18:575–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Sun WM, Read NW, Verlinden M. Effects of loperamide oxide on gastrointestinal transit time and anorectal function in patients with chronic diarrhoea and faecal incontinence. Scand J Gastroenterol. 1997;32:34–8. [DOI] [PubMed] [Google Scholar]
- 310. Rohatgi S, Ahuja V, Makharia GK, et al. VSL#3 induces and maintains short‐term clinical response in patients with active microscopic colitis: a two‐phase randomised clinical trial. BMJ Open Gastroenterol. 2015;2:e000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Gentile NM, Abdalla AA, Khanna S, et al. Outcomes of patients with microscopic colitis treated with corticosteroids: a population‐based study. Am J Gastroenterol. 2013;108:256–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Sloth H, Bisgaard C, Grove A. Collagenous colitis: a prospective trial of prednisolone in six patients. J Intern Med. 1991;229:443–6. [DOI] [PubMed] [Google Scholar]
- 313. Corte T, Janssens E, D'Hondt A, et al. Beclomethasone dipropionate in microscopic colitis: results of an exploratory open‐label multicentre study (COLCO). United European Gastroenterol J. 2019;7:1183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Vennamaneni SR, Bonner GF. Use of azathioprine or 6‐mercaptopurine for treatment of steroid‐dependent lymphocytic and collagenous colitis. Am J Gastroenterol. 2001;96:2798–9. [DOI] [PubMed] [Google Scholar]
- 315. Munch A, Fernandez‐Banares F, Munck LK. Azathioprine and mercaptopurine in the management of patients with chronic, active microscopic colitis. Aliment Pharmacol Ther. 2013;37:795–8. [DOI] [PubMed] [Google Scholar]
- 316. Pardi DS, Loftus EV Jr, Tremaine WJ, et al. Treatment of refractory microscopic colitis with azathioprine and 6‐mercaptopurine. Gastroenterology. 2001;120:1483–4. [DOI] [PubMed] [Google Scholar]
- 317. Cotter TG, Kamboj AK, Hicks SB, et al. Immune modulator therapy for microscopic colitis in a case series of 73 patients. Aliment Pharmacol Ther. 2017;46:169–74. [DOI] [PubMed] [Google Scholar]
- 318. Riddell J, Hillman L, Chiragakis L, et al. Collagenous colitis: oral low‐dose methotrexate for patients with difficult symptoms: long‐term outcomes. J Gastroenterol Hepatol. 2007;22:1589–93. [DOI] [PubMed] [Google Scholar]
- 319. Munch A, Bohr J, Vigren L, et al. Lack of effect of methotrexate in budesonide‐refractory collagenous colitis. Clin Exp Gastroenterol. 2013;6:149–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Esteve M, Mahadevan U, Sainz E, et al. Efficacy of anti‐TNF therapies in refractory severe microscopic colitis. J Crohns Colitis. 2011;5:612–8. [DOI] [PubMed] [Google Scholar]
- 321. Münch A, Ignatova S, Strom M. Adalimumab in budesonide and methotrexate refractory collagenous colitis. Scand J Gastroenterol. 2012;47:59–63. [DOI] [PubMed] [Google Scholar]
- 322. Anderson RJ, Makins R. Successful use of adalimu‐mab in patient with treatment‐refractory microscopic colitis. BMJ Case Rep. 2016;2016:bcr2016215639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Daferera N, Hjortswang H, Ignatova S, et al. Single‐centre experience with anti‐tumour necrosis factor treatment in budesonide‐refractory microscopic colitis patients. United European Gastroenterol J. 2019;7:1234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Pola S, Fahmy M, Evans E, et al. Successful use of infliximab in the treatment of corticosteroid dependent collagenous colitis. Am J Gastroenterol. 2013;108:857–8. [DOI] [PubMed] [Google Scholar]
- 325. Riviere P, Munch A, Michetti P, et al. Vedolizumab in refractory microscopic colitis: an international case series. J Crohns Colitis. 2019;13:337–40. [DOI] [PubMed] [Google Scholar]
- 326. Casper M, Zimmer V, Hubschen U, et al. Vedolizumab for refractory collagenous colitis: another piece of the puzzle. Dig Liver Dis. 2018;50:1099–100. [DOI] [PubMed] [Google Scholar]
- 327. Cushing KC, Mino‐Kenudson M, Garber J, et al. Vedolizumab as a novel treatment for refractory collagenous colitis: a case report. Am J Gastroenterol. 2018;113:632–3. [DOI] [PubMed] [Google Scholar]
- 328. Jennings JJ, Charabaty A. Vedolizumab‐induced remission in 3 patients with refractory microscopic colitis: a tertiary care center case series. Inflamm Bowel Dis. 2019;25:e97. [DOI] [PubMed] [Google Scholar]
- 329. Daferera N, Kumawat AK, Hultgren‐Hornquist E, et al. Fecal stream diversion and mucosal cytokine levels in collagenous colitis: a case report. World J Gastroenterol. 2015;21:6065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Jarnerot G, Tysk C, Bohr J, et al. Collagenous colitis and fecal stream diversion. Gastroenterology. 1995;109:449–55. [DOI] [PubMed] [Google Scholar]
- 331. Munch A, Soderholm JD, Wallon C, et al. Dynamics of mucosal permeability and inflammation in collagenous colitis before, during, and after loop ileostomy. Gut. 2005;54:1126–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Williams RA, Gelfand DV. Total proctocolectomy and ileal pouch anal anastomosis to successfully treat a patient with collagenous colitis. Am J Gastroenterol. 2000;95:2147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


