Abstract
Objective
ACPA-positive RA is associated with distinct HLA-DR alleles and with antibodies targeting citrullinated α-enolase. We screened α-enolase for tentative T cell epitopes and focused the present study on aa326–340. Both the frequency and quality of T cell responses were studied in blood and synovial fluid of RA patients.
Methods
Binding of native and citrullinated α-eno326–340 HLA-DRB1*04:01 was studied by in vitro competition assays and by DSC, and T cell responses by in vitro culture. HLA-DRB1*04:01 tetramers were used for assessing ex vivo frequency and phenotype of α-enolase-specific T cells. Cross-reactivity, i.e. whether the same T cells could recognize both peptides, was addressed utilizing RA patient samples and HLA-DRB1*04:01-transgenic mice.
Results
Frequencies of T cells recognizing native eno326–340 were similar in blood and synovial fluid, whereas the frequency of cit-eno326–340 T cells was elevated in synovial fluid. The cit-eno-specific, but not native-eno-specific T cells in blood displayed a memory CD45RO phenotype indicating previous exposure to citrullinated enolase. In vitro assays revealed functional responses to both peptides in patient samples and immunized HLA-DRB1*04:01-IE-transgenic mice. Cross-reactivity to the two peptides was observed but was not universal.
Conclusions
HLA-DRB1*04:01-restricted CD4 T cells recognizing native and cit-eno326–340 peptides are part of the normal circulating T cell repertoire. We observed more T cells specific for the citrullinated peptide with a memory phenotype in the circulation, which supports the concept that such T cells might be activated outside the joints and subsequently recruited and possibly re-activated in inflamed joints.
Keywords: Rheumatoid arthritis, CD4+ T cells, MHC class II tetramer, ACPA, alpha-enolase
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic, systemic inflammatory disease that can be divided into subsets based on the presence of antibodies to citrullinated proteins (ACPAs) [1]. Antibody targets represent candidate autoantigens for T cell responses [2–5]. This is further substantiated by the observation that ACPA-positive RA is strongly associated with HLA-DR-shared epitope alleles [6–8]. Previous studies of peripheral blood from ACPA-positive RA patients have demonstrated auto-reactive T cells against several citrullinated self-proteins [9–13]. In this context α-enolase represents an interesting autoantigen as the presence of autoantibodies against citrullinated α-enolase is preferentially linked to HLA-DRB1*04 [14].
α-enolase is an ubiquitous protein but is also abundantly expressed in the inflamed synovium [4] and antibodies to the immunodominant B cell epitope of citrullinated α-enolase (CEP-1) are present in approximately 40% of ACPA-positive patients [14, 15]. These autoantibodies are highly specific for RA [4, 14] and enriched in synovial fluid [16].
CD4+ T cells play an important role in RA by providing B cell help and secreting proinflammatory cytokines, which may contribute to the perpetuation of the inflammation in RA. A major hurdle in research aimed at characterizing T cells that specifically recognize potential RA-specific autoantigens is the fact that autoantigen-specific T cells, as shown in different disease settings, are generally present in very low frequencies [17–21]. Through recent improvements in the field of MHC class II tetramers it is now possible to look specifically for rare antigen-specific CD4+ T cells directly ex vivo without the need of in vitro expansion [13, 22–24], utilizing a bead enrichment method. Herein we have utilized this technology to study a-enolase specific T cells in blood and synovial fluid of RA patients.
MATERIAL AND METHODS
Patients and healthy control subjects
RA patients were recruited under the auspices of the Karolinska University Hospital Rheumatology clinic, the Benaroya Research Institute (BRI) rheumatic disease registry, and the BRI immune-mediated disease registry. Control subjects were recruited from the BRI IMD registry or the Uppsala blood bank (table S1). Informed consent was obtained from everyone based on local ethical permits. All patients were diagnosed as having RA by a rheumatologist in accordance with the 1987 American College of Rheumatology criteria [25]. All subjects recruited in this study had at least one copy of the HLA-DRB1*04:01 allele. Peripheral blood mononuclear cells (PBMC), obtained from heparinized blood, and synovial fluid mononuclear cells (SFMC) were prepared by centrifugation over Ficoll-Hypaque gradients. Frozen samples were cryopreserved in liquid nitrogen in 10% DMSO and 90% heat-inactivated FBS. Frozen samples were utilized for studies carried out at KI and fresh samples were used for all analyses carried out at BRI. Synovial biopsy was obtained through ultrasound guided arthroscopy from one RA patient. Synovial tissue was digested via incubation with Collagenase A (Roche) and DNAse (Roche). The tissue was then sieved through a 100μm filter and washed before staining.
Peptides and selection
Peptides used for intracellular cytokine staining were synthesized by ProImmune. Peptides used for all other purposes were synthesized by Genscript. For peptide binding assays increasing concentrations of each non-biotinylated test peptide were incubated in competition with 0.01μM biotinylated influenza-HA306–318 peptide in wells coated with HLA-DRB1*04:01 protein as previously described [26]. Peptide binding curves were fitted by non-linear regression with a sigmoidal dose response curve model using Prism software (version 5.0, GraphPad Software Inc.).
Tetramers
Recombinant DRB1*04:01 protein was produced as previously described [27]. The biotinylated monomer was loaded with either influenza-HA306–318 or the α-enolase peptide of interest by incubating in the presence of n-octyl-β-D-glucopyranoside and Pefabloc SC (Sigma-Aldrich). Peptide loaded monomers were subsequently conjugated to tetramers using either R-PE streptavidin (Invitrogen) or APC streptavidin (BD).
In vitro detection of influenza-hemagglutinin (HA) and α-enolase-specific T cells by HLA class II tetramers
For tetramer assays PBMCs from healthy controls or a synovial biopsy from a DRB1*04:01 RA subject were cultured in RPMI-1640 + 10% pooled human serum with 10μg/ml of either HA306–318 or the α-enolase peptides. Interleukin-2 (IL-2) (Proleukin, Novartis) was added on day 6. After 14 days cells were stained according to table S2. The data was analyzed by FlowJo software version 9.3.3. or higher (Treestar Inc.).
Ex vivo detection of influenza-hemagglutin and α-enolase-specific T cells by HLA class II tetramers
PBMC and SFMC samples from RA patients were labeled according to table S2 as previously described [27]. Samples were run on a BD LSRII flow cytometer or Beckman Coulter Gallios, and data was analyzed using FlowJo software version 9.3.3. or higher. The frequency of antigen-specific cells was calculated as the total number of Tmr+ cells in the bound fraction divided by the total number of CD4+ T cells. A cut-off of 1 per 1x106 CD4+ T cells was applied.
Intracellular cytokine staining
Functional T cell assays were performed as previously described [11]. Cells were treated with LIVE/DEAD Fixable Green Dead Cell Stain (Invitrogen) and stained according to table S2. Intracellular cytokine staining (ICS) was performed using Cytofix/Cytoperm Kit (BD) according to the manufacturers instructions. Cells were run on a Beckman Coulter CyAn. Analyses were performed with FlowJo software, version 7.5.1 (Treestar Inc.).
Murine Assays
HLA-DR04:01-IE transgenic mice on a class II deficient C57Bl/6 (I-Abo/o) background were obtained from Taconic and were housed under specific pathogen-free conditions. Mice were immunized with 100μg of peptide and spleenocytes harvested day 14 were used for studying recall responses as previously reported [13]. All animal work was approved by the BRI Animal Care and Use Committee (ACUC) and animals were housed in the BRI AAALAC-accredited animal facility.
Statistics
Statistical tests used for this paper include Fischer exact test, Wilcoxon matched pairs ranked test and Mann-Whitney tests. Spearman’s rank correlation coefficient was used to analyze correlations. Analyses were performed using prism software (version 5.0). Values <0.05 were considered significant.
RESULTS
Alpha-enolase326–340 can be presented by HLA-DRB1*04:01 in both its native and citrullinated form
Overlapping peptides covering the entire length of α-enolase were screened for HLA-DRB1*04:01 binding. Among the peptides identified to contain binding epitopes for HLA-DRB1*04:01, three were successfully used to generate HLA-DRB1*04:01 tetramers. The sequences for these are shown in figure 1A and supplementary figure S1A-B. We subsequently performed in vitro culture experiments with the candidate peptides to validate whether T cells recognizing the different peptide-MHC complexes were indeed present in HLA-matched patient samples. We focused our study on the peptide pair eno326–340 and cit-eno326–340, because these specificities were reproducibly seen in the pilot experiments. As shown in figure 1B both peptides bound with equal avidity to the HLA-DRB1*04:01 molecule.
Figure 1. Peptide sequence, binding to HLA-DRB1*04:01 and tetramer staining.
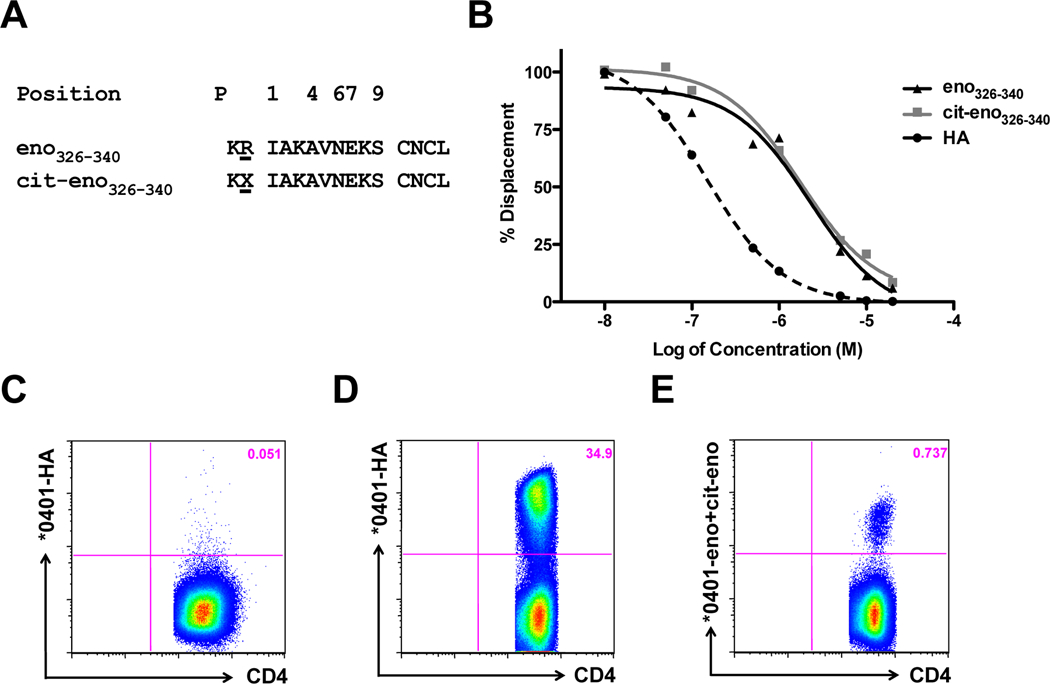
(A) Amino acid sequence from α-enolase aa326–340 in the native (eno326–340) and citrullinated form (cit-eno326–340). Numbers indicate the tentative binding position for P1, P4, P6, P7 and P9. (B) Binding affinity to HLA-DRB1*04:01, as determined by europium-based peptide competition assay, was done for the native arginine and the citrullinated version of α-enolase. HA: hemagglutinin306–318 antigen as a positive control. (C-E) PBMC from DRB1*04:01 healthy subjects were stimulated with peptides, cultured for 14 days and stained with tetramers. (C) Negative control, cells were stimulated with eno326–340 and stained with HA306–318 tetramer. (D+E) Cells were stimulated with HA306–318 (D) or eno326–340 and cit-eno326–340 peptides (E) and stained with corresponding tetramers.
In vitro detection of antigen-specific T cells by tetramer
To substantiate that antigen-specific T cells can be found in HLA-DRB1*04:01-positive samples and to validate the autoantigen-loaded tetramers, PBMCs from healthy subjects were stimulated with either influenza-HA or α-enolase peptides. After 14 days of culture, during which both naïve and memory T cells specific for the respective peptides can expand, we stained with tetramers for the presence of antigen-specific T cells. Hereby we could demonstrate the presence CD4+ T cells specific for both HA (figure 1D) and the two α-enolase peptides (figure 1E).
Frequencies of autoantigen-specific T cells
Studying expanded cell populations involves in vitro culture steps, which can alter phenotypes and does not allow for the enumeration of the frequency of cells at the time of sampling. To avoid these complications we continued to study antigen-specific cells directly ex vivo in the peripheral blood and synovial fluid of our study subjects.
HA306–318 was used as a control tetramer (figure 2A). HA-specific T cells were found in similar numbers and frequency in the peripheral blood of healthy controls, RA peripheral blood, and in RA synovial fluid (figure 2B, supplementary figure S2).
Figure 2. Frequency and characterization of tetramer-positive cells.
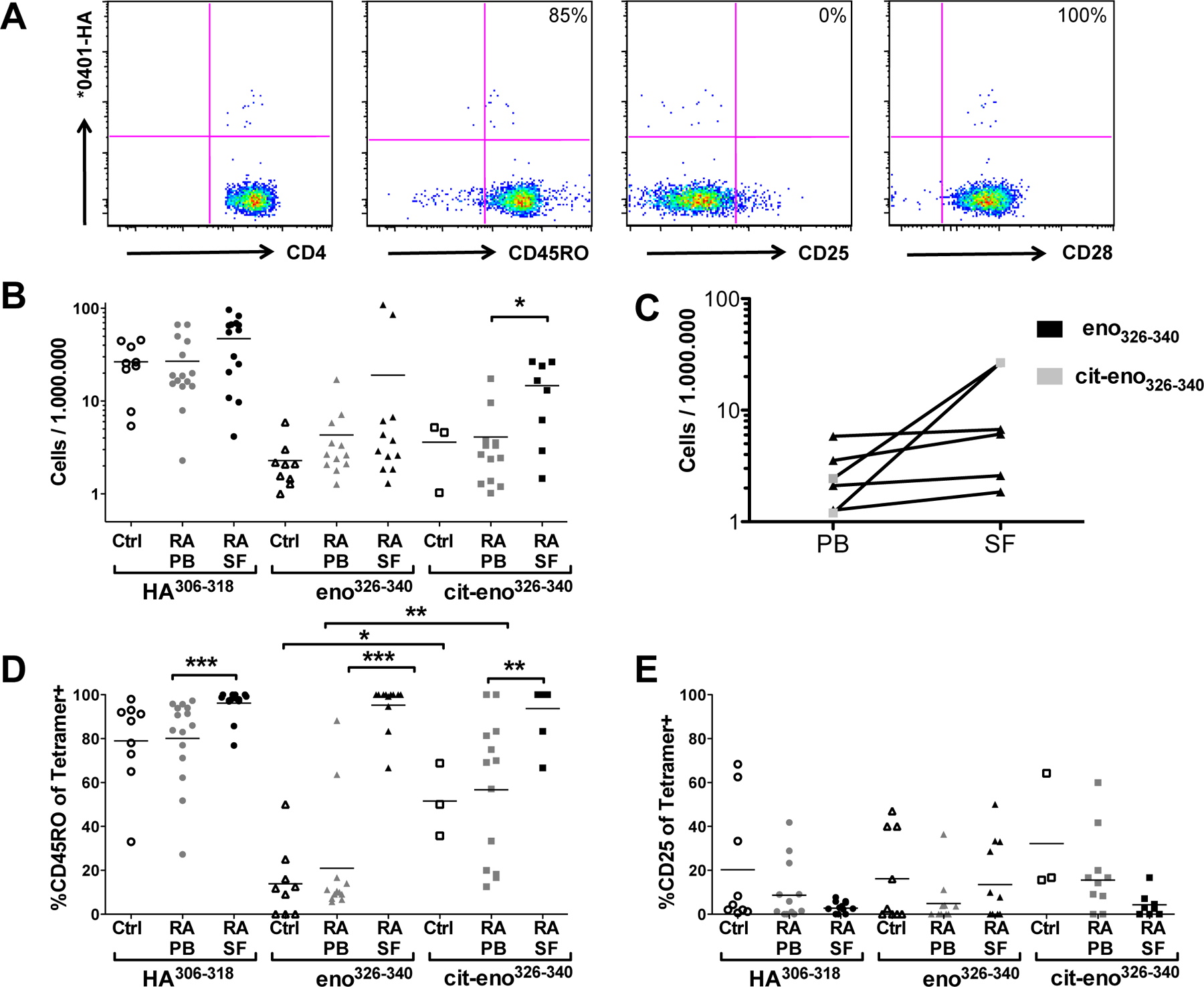
(A) Representative example of ex vivo analysis for flu specific T cells from SFMC of a DRB1*04:01 RA subject after staining with HA306–318 tetramer along with CD4, CD45RO, CD25 and CD28 antibodies. (B) The frequency of antigen-specific CD4+ T cells in blood from healthy controls (unfilled symbols) and RA-patients (grey symbols) and synovial fluid from RA-patients (black symbols). Plotted are positive cells per 1 x 106 CD4+ T cells. Cut-off for positivity is 1/1 x 106. (C) The frequency of antigen-specific CD4+ T cells in peripheral blood (PB) and synovial fluid (SF) in paired samples from RA patients is shown. Plotted are positive cells per 1 x 106 CD4+ T cells. Black: cells specific for eno326–340, grey: cit- eno326–340 specific cells. (D-E) Further characterization of tetramer positive cells from blood and synovial fluid. Depicted is the percentage of Tetramer-positive cells that are positive for CD45RO (D) or CD25 (E).
Native α-enolase specific CD4+ T cells were found in the blood of all healthy controls (10/10), in the blood of 12/18 investigated patients and in 13/18 of the investigated synovial fluid samples (supplementary figure S2). There was no significant difference in the frequencies of T cells recognizing native eno326–340 in RA blood as compared with synovial fluid, nor between peripheral blood of RA patients and healthy controls.
T cells specific for the citrullinated version of eno326–340 were present in the blood of 3/11 controls and in 13/21 RA patients and in 9/18 synovial fluids from RA joint effusions (figure S2). In contrast to the case for native enolase reactive T cells, there was an increased frequency of T cells reactive to the citrullinated enolase peptide in the synovial fluid samples as compared with peripheral blood, p<x (figure 2B). The same tendency was seen also by direct comparison in paired samples from synovial fluid and peripheral blood (figure 2C).
Phenotype of autoantigen-specific T cells
We next investigated the phenotypes of the tetramer-positive T cells. Peripheral blood T cells specific for cit-eno326–340 from RA patients displayed a higher proportion of CD45RO-positive (memory-type) T cells as compared to the native eno326–340-specific T cells, p<x (figure 2D). The expression of CD25, a cell surface marker that can either depict recently activated T cells or those of regulatory function, was generally low on the tetramer+ cells, although variable, with no bias toward a CD25high phenotype (figure 2E). We also investigated whether the tetramer+ T cells belonged to the proinflammatory CD4+CD28null phenotype, which can be enriched in the blood of some RA patients [28], We saw no differences, with most tetramer-positive T cells expressing CD28 both in peripheral blood and synovial fluid (data not shown).
Both native and cit-eno326–340 elicit functional T cell responses
The presence of memory T cells specific for citrullinated-α-enolase in RA patients suggests they have seen their cognate antigen in vivo. To investigate any functional differences of α-enolase recognition we stimulated PBMC from RA patients with the two α-enolase peptides. After 5 days we measured surface expression of CD40L, as a marker for antigen-recognition, as well as intracellular levels of IFN-γ, IL-17A and TNF. We detected immune responses to both peptides, with some variation in the degree of CD40L upregulation and cytokine pattern (figure 3A). This response was HLA-DR restricted as it could be blocked by antibodies against HLA-DR, but not by antibodies against HLA-DQ or HLA-DP (data not shown). As seen in figure 3A, some patients had T cells favoring a cit-eno response, others preferentially responded to the native peptide, and some patients responded similarly to both peptides.
Figure 3. Functional T cell assay and murine studies.
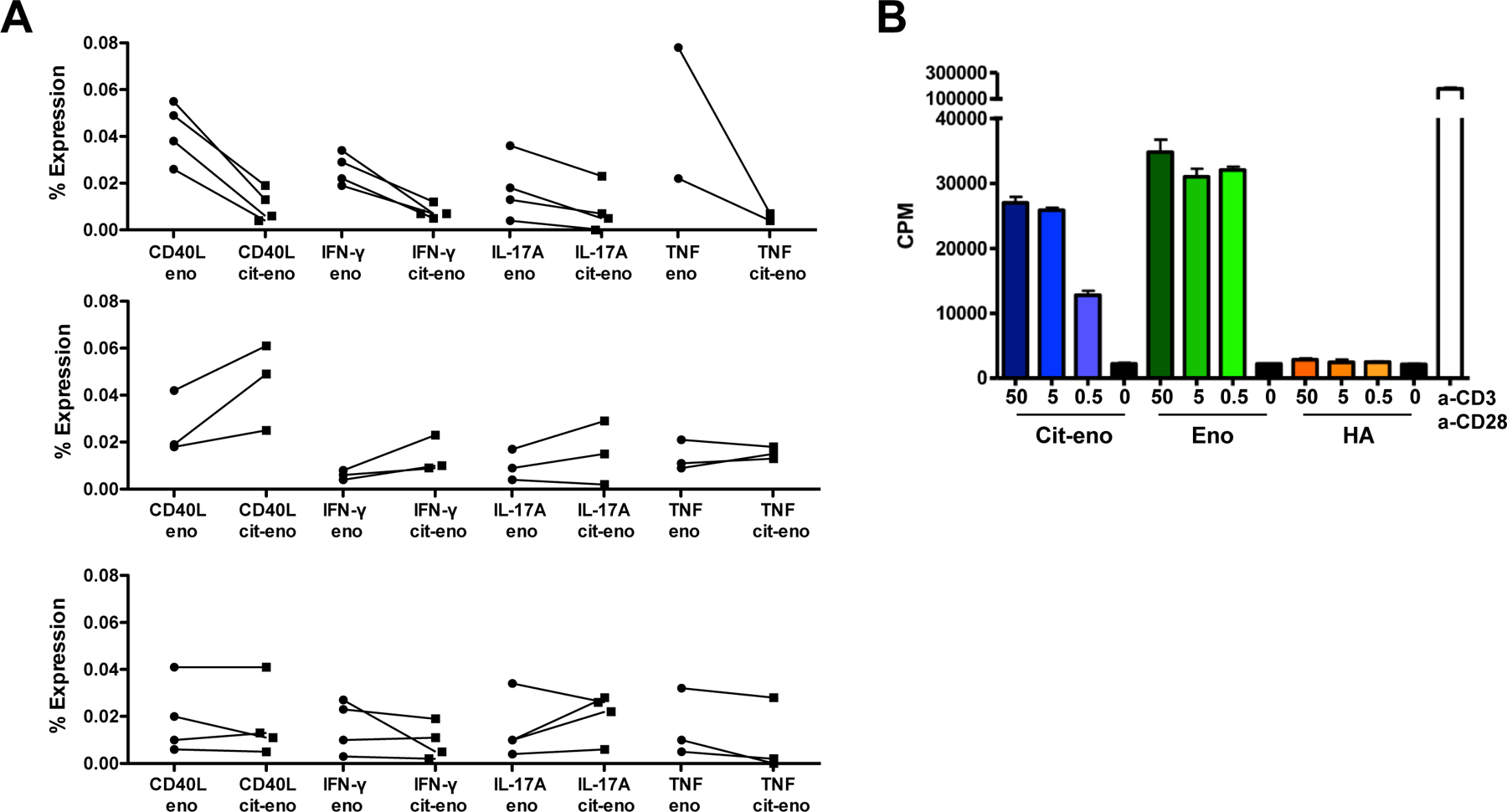
(A) PBMC were stimulated with eno326–340 or cit-eno326–340 peptides for 5 days. -17A and TNF. We detected immunePatients were then divided into 3 groups (tertiles) based on response pattern: generally more stimulation with eno326–340peptide (upper panel), generally more stimulation with cit-eno326–340 peptide (centered panel) and patients with same or mixed stimulation (lower panel). Visualized is the % of expression on CD4+ T cells. (B) HLA-DRB1*04:01-IE transgenic mice were immunized with eno326–340 peptide, restimulated with either eno326–340 or cit-eno326–340 peptide and proliferation was measured.
Our data suggests that the T cells from some of our patients fail to distinguish the two versions of the α-enolase peptide. To dissect this further, we immunized HLA-DRB1*04:01-IE transgenic mice with the native eno326–340 peptide and examined their recall response to both enolase peptides by proliferation assays. Mice immunized with eno326–340 showed a similar proliferation response irrespective if they were restimulated with eno326–340 or cit-eno326–340 (figure 3B), suggesting cross-recognition between the peptides in a setting of active immunization. As we have reported previously, also the opposite is true, i.e. mice immunized with the citrullinated peptide display recall responses to both versions of the peptide (13).
T cells can cross-recognize native and cit-eno326–340
To further elucidate if an individual T cells could recognize both forms of the α-enolase peptide we manufactured our α-enolase tetramers in two different flourophores to allow co-staining of samples and used these tetramers for ex vivo stainings of T cells obtained from three synovial fluids of DRB1*04:01 positive RA patients. One of these samples contained T cells reactive to enolase and in this case, all stained cells were positive for both tetramers (figure 4).
Figure 4. Tetramer stainings in synovial fluid.
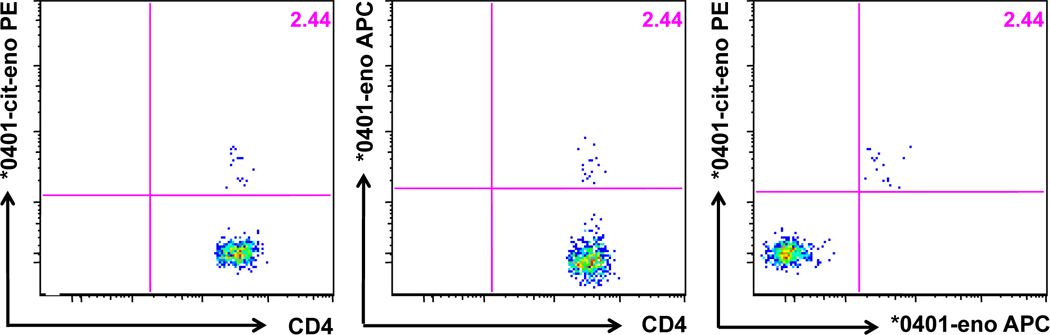
Ex vivo staining from a synovial fluid of a HLA-DRB1*04:01 RA patient. Eno326–340-Tmr was conjugated to APC and cit-eno326–340-Tmr was conjugated to PE.
We continued to investigate this cross-recognition by in vitro assays, where more Tmr-positive events can be expected. We performed in vitro restimulation experiments with T cells from peripheral blood of several DRB1*04:01 positive RA patients using both the native and citrullinated enolase peptides as well as the control HA peptide. Dual-color Tmr stainings were then performed on these in vitro expanded cells. As a positive control, in vitro expanded cells stimulated with HA306–318 were stained simultaneously with the two different labeled HA-tetramers. As depicted in figure 5, two types of reactivity patterns were found. Some patients had many double-positive T cells (figure 5A), while other patients had mainly T cells that specifically recognized only one of the peptides without cross-recognition (figure 5B).
Figure 5. Dual-tetramer staining with PE and APC-Tmr in peripheral blood.
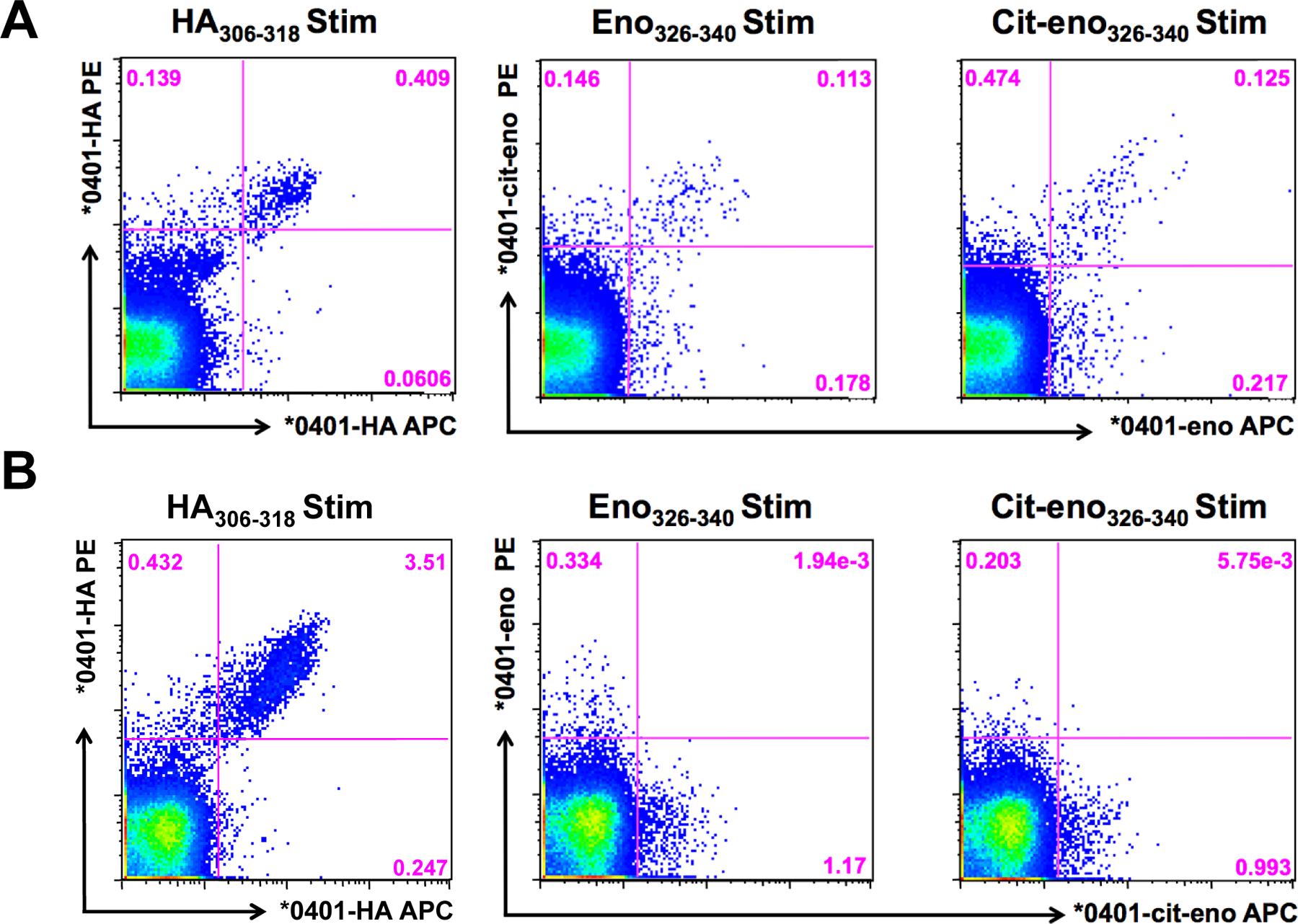
PBMC were expanded for 14 days with either HA306–318, eno326–340 or cit-eno326–340 and stained with corresponding tetramers. Two examples from different HLA-DRB1*04:01 RA patients.
Alpha-enolase-specific T cells in synovial tissue
As synovial fluid and synovial tissue are two discrete compartments of the rheumatic joint, we wanted to investigate whether autoantigen-specific T cells were also present in the tissue. To this end, we digested a synovial biopsy specimen from an HLA-DRB1*04:01 RA patient, and performed in vitro tetramer staining following in vitro stimulation with both peptides. Hereby, T cells specific for eno326–340 and cit-eno326–340 could be visualized (figure 6).
Figure 6. Enolase-specific T cells in synovial tissue.
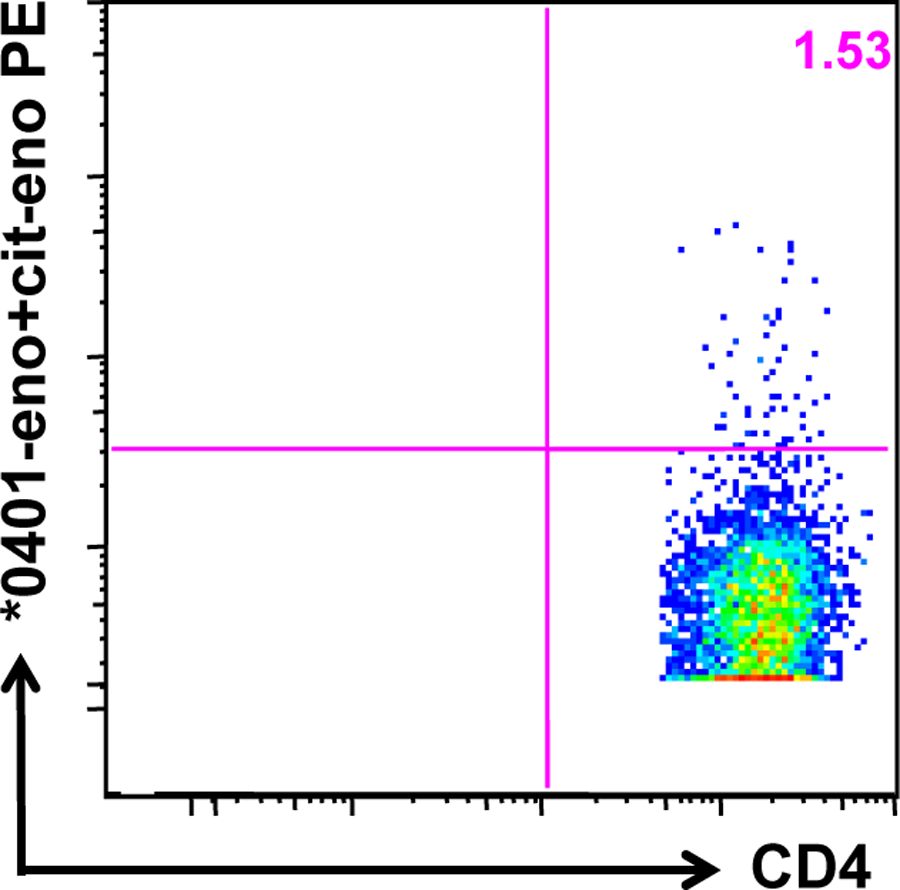
A synovial biopsy was obtained from a DRB1*04:01 RA patient. The tissue was enzymatically digested and the cells were stimulated with eno326–340 and cit-eno326–340, cultured for 14 days and stained with the tetramers.
DISCUSSION
Direct investigation of human antigen-specific CD4+ T cells has become feasible in recent years due to improvements in tetramer technology, allowing for enumeration of frequencies and ex vivo phenotyping of even rare T cells e.g. in the setting of autoimmune diseases [29]. Tissue staining is still not available for HLA class II tetramers, but the RA joint can be interrogated via cell suspensions by sampling synovial fluid and digesting synovial tissue.
In the present study we have focused on parallel studies of CD4+ T cells from the blood and inflamed joints of RA patients to assess whether auto-reactive T cells are enriched and their phenotypes change at the site of inflammation. Our primary material was synovial fluid, where α-enolase reactive T cells were readily found, but as a proof of principle, we could also demonstrate auto-reactive CD4+ T cells in synovial tissue. We chose α-enolase as our model autoantigen in this study because it represents one of the major autoantigens recognized by autoantibodies in RA [4, 14, 30], and because the response to citrullinated α-enolase is strongly associated with a specific HLA-DR allotype DRB1*04:01 [14]. We further focused our study on eno326–340, a peptide that binds to HLA-DRB1*04:01 in both its native as well as its citrullinated form.
Two findings in this study are of particular interest. First, we showed a skewing towards a memory phenotype in circulating T cells that recognized the citrullinated α-enolase peptide compared to those that recognized the native version. Secondly, even though both α-enolase reactive T cell subsets in synovial fluid were of the memory phenotype, we found a higher frequency of citrullinated α-enolase T cells from inflamed RA joints.
Our findings indicate that T cells reactive to both the native and citrullinated α-enolase peptides are part of the normal circulating T cell repertoire. The observation that RA patients have more circulating memory T cells to cit-α-enolase is compatible with the notion that the primary activation of auto-reactive T cells may take place outside the joints, for example in the gums [31, 32] or in the lungs [8, 33, 34]. These memory T cells may subsequently migrate to joints in response to some ‘local insult’, where they may be reactivated since extracellular citrullination is abundant during any kind of inflammation. These effector T cells may then contribute to the maintenance of an inflammatory milieu.
Our study also addresses the question of whether TCRs recognizing these α-enolase peptides are cross-reactive or specific for either the native or citrullinated form. In this context, it is essential to note that the post-translationally modified arginine is in a position that is just outside the binding grove of the HLA-DRB1*04:01 molecule (P-1), which explains why binding to this HLA-DR molecule is similar for the two peptides. Provided the position of the citrullinated residue, it’s not surprising that some cross-reactivity is seen for some T cell receptors. The partial cross-reactivity we detected could be explained by the existence of three different types of TCRs. One type of TCR recognizes both peptides, irrespective of the charge difference between the positive arginine and neutral citrulline, and two other TCR types only recognize either the arginine or the citrulline at P-1. It will be of interest to investigate in further detail to which extent cross-reactive and single-reactive T cells give different responses (e.g. cytokine secretion or proliferation) in response to the native or citrullinated peptide [35–37].
Obviously, there are also some limitations in our study; we only investigated one autoantigen. Additionally, all RA patients studied have chronic disease, and all of them are immunosuppressed, which could affect the frequency and functionality of the T cells [13]. However, our cohort is thus representative of real life scenario (typical) patients. In fact, our data on peripheral blood are in good agreement with other studies using both in vitro and ex vivo approaches [38] [13]. Concerning data from synovial fluid cells, there are two previous reports that have utilized tetramer-technology to enumerate antigen-reactive T cells, both in the setting of Lyme arthritis. Similar to our results, both of these reports found a higher frequency of antigen-(borrelia)-reactive T cells in synovial fluid as compared to peripheral blood [39, 40].
CONCLUSIONS
We identified and characterized auto-reactive CD4+ T cells specific for the RA candidate autoantigen α-enolase from the periphery, synovial fluid, and synovial tissue of RA patients. Importantly, citrulline-α-enolase specific T cells were more often of a memory phenotype in the circulation and were enriched in the synovial fluid compared to those recognizing the native variant of the peptide. This study highlights the need to look at native and citrullinated -α-enolase T cell epitopes in future studies dissecting RA initiation and propagation.
Supplementary Material
S1 Other enolase peptides tested.
(A+B) Amino acid sequence from α-enolase aa26–40 (A) and α-enolase aa241–255 (B) in the native (eno26–40 and eno241–255) and citrullinated forms (cit-eno26–40 and cit-eno241–255). Numbers indicate the tentative binding position for P1, P4, P6, P7 and P9. (C-E) PBMC from a DRB1*04:01 RA patient were stimulated with eno26–40 (C), eno241–255 (D), cit-eno26–40 (E) or cit-eno241–255 (F), cultured for 14 days and stained with corresponding tetramers.
S2 Patients tested in ex vivo stainings.
Percentage of positive tested patients for the different peptides in peripheral blood of control subjects (white bars), peripheral blood of RA patients (grey bars) and synovial fluid of RA patients (black bars). The numbers of positive tested individuals/totally tested individuals is depicted on top of the bars.
Table S1 Patient characteristics
Table S2 Antibodies used
ACKNOWLEDGMENTS
The authors thank the staff and patients at the Rheumatology Clinic of Karolinska University Hospital, especially Eva Jemseby, Gull-Britt Almgren and Julia Boström for organizing sampling, storage, and administration of biomaterial, and Annika van Vollenhoven for excellent cell sorting. We would also like to thank the Virginia Mason Clinic’s Rheumatology group and the Translational Research Program at the BRI in Seattle.
FUNDING
Work performed at KI was supported by grants from the Margaretha af Ugglas Foundation, the Swedish Association against Rheumatism, the Swedish Medical Association, the King Gustaf-V-80-year Foundation, the Swedish Research Council, the EU FP7-project Masterswitch (HEALTH-F2–2008-223404), the IMI JU funded project BTCure 115142–2 and Boehringer Ingelheim Fonds. Work performed at BRI was supported by NIAID 5U19 AI050864, NIMS 5 R01 AR037296, and NIAID UO1 AI101981.
REFERENCES
- 1.Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet 2009. February 21; 373(9664):659–672. [DOI] [PubMed] [Google Scholar]
- 2.Auger I, Sebbag M, Vincent C, Balandraud N, Guis S, Nogueira L, et al. Influence of HLA-DR genes on the production of rheumatoid arthritis-specific autoantibodies to citrullinated fibrinogen. Arthritis and rheumatism 2005. November; 52(11):3424–3432. [DOI] [PubMed] [Google Scholar]
- 3.Burkhardt H, Sehnert B, Bockermann R, Engstrom A, Kalden JR, Holmdahl R. Humoral immune response to citrullinated collagen type II determinants in early rheumatoid arthritis. Eur J Immunol 2005. May; 35(5):1643–1652. [DOI] [PubMed] [Google Scholar]
- 4.Kinloch A, Tatzer V, Wait R, Peston D, Lundberg K, Donatien P, et al. Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis research & therapy 2005; 7(6):R1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snir O, Widhe M, von Spee C, Lindberg J, Padyukov L, Lundberg K, et al. Multiple antibody reactivities to citrullinated antigens in sera from patients with rheumatoid arthritis: association with HLA-DRB1 alleles. Ann Rheum Dis 2009. May; 68(5):736–743. [DOI] [PubMed] [Google Scholar]
- 6.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis and rheumatism 1987. November; 30(11):1205–1213. [DOI] [PubMed] [Google Scholar]
- 7.Huizinga TW, Amos CI, van der Helm-van Mil AH, Chen W, van Gaalen FA, Jawaheer D, et al. Refining the complex rheumatoid arthritis phenotype based on specificity of the HLA-DRB1 shared epitope for antibodies to citrullinated proteins. Arthritis and rheumatism 2005. November; 52(11):3433–3438. [DOI] [PubMed] [Google Scholar]
- 8.Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis and rheumatism 2006. January; 54(1):38–46. [DOI] [PubMed] [Google Scholar]
- 9.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. The New England journal of medicine 2011. December 8; 365(23):2205–2219. [DOI] [PubMed] [Google Scholar]
- 10.von Delwig A, Locke J, Robinson JH, Ng WF. Response of Th17 cells to a citrullinated arthritogenic aggrecan peptide in patients with rheumatoid arthritis. Arthritis and rheumatism 2010. January; 62(1):143–149. [DOI] [PubMed] [Google Scholar]
- 11.Snir O, Rieck M, Gebe JA, Yue BB, Rawlings CA, Nepom G, et al. Identification and functional characterization of T cells reactive to citrullinated vimentin in HLA-DRB1*0401-positive humanized mice and rheumatoid arthritis patients. Arthritis and rheumatism 2011. October; 63(10):2873–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Law SC, Street S, Yu CH, Capini C, Ramnoruth S, Nel HJ, et al. T-cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA-DRB1 shared epitope alleles. Arthritis research & therapy 2012; 14(3):R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James E, Rieck M, Pieper J, Gebe JA, Yue BB, Tatum M, et al. Citrulline specific Th1 cells are increased in rheumatoid arthritis and their frequency is influenced by disease duration and therapy. Arthritis Rheumatol 2014. March 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahdi H, Fisher BA, Kallberg H, Plant D, Malmstrom V, Ronnelid J, et al. Specific interaction between genotype, smoking and autoimmunity to citrullinated alpha-enolase in the etiology of rheumatoid arthritis. Nature genetics 2009. December; 41(12):1319–1324. [DOI] [PubMed] [Google Scholar]
- 15.Avouac J, Gossec L, Dougados M. Diagnostic and predictive value of anti-cyclic citrullinated protein antibodies in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis 2006. July; 65(7):845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snir O, Widhe M, Hermansson M, von Spee C, Lindberg J, Hensen S, et al. Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients. Arthritis and rheumatism 2010. January; 62(1):44–52. [DOI] [PubMed] [Google Scholar]
- 17.Oling V, Marttila J, Ilonen J, Kwok WW, Nepom G, Knip M, et al. GAD65- and proinsulin-specific CD4+ T-cells detected by MHC class II tetramers in peripheral blood of type 1 diabetes patients and at-risk subjects. J Autoimmun 2005. November; 25(3):235–243. [DOI] [PubMed] [Google Scholar]
- 18.Veldman C, Eming R, Wolff-Franke S, Sonderstrup G, Kwok WW, Hertl M. Detection of low avidity desmoglein 3-reactive T cells in pemphigus vulgaris using HLA-DR beta 1*0402 tetramers. Clin Immunol 2007. March; 122(3):330–337. [DOI] [PubMed] [Google Scholar]
- 19.Raddassi K, Kent SC, Yang J, Bourcier K, Bradshaw EM, Seyfert-Margolis V, et al. Increased frequencies of myelin oligodendrocyte glycoprotein/MHC class II-binding CD4 cells in patients with multiple sclerosis. Journal of immunology 2011. July 15; 187(2):1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nepom GT. MHC class II tetramers. Journal of immunology 2012. March 15; 188(6):2477–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity 2013. February 21; 38(2):373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scriba TJ, Purbhoo M, Day CL, Robinson N, Fidler S, Fox J, et al. Ultrasensitive detection and phenotyping of CD4+ T cells with optimized HLA class II tetramer staining. Journal of immunology 2005. November 15; 175(10):6334–6343. [DOI] [PubMed] [Google Scholar]
- 23.Wambre E, DeLong JH, James EA, LaFond RE, Robinson D, Kwok WW. Differentiation stage determines pathologic and protective allergen-specific CD4+ T-cell outcomes during specific immunotherapy. J Allergy Clin Immunol 2012. February; 129(2):544–551, 551 e541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 2007. August; 27(2):203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988. March; 31(3):315–324. [DOI] [PubMed] [Google Scholar]
- 26.Hill CM, Liu A, Marshall KW, Mayer J, Jorgensen B, Yuan B, et al. Exploration of requirements for peptide binding to HLA DRB1*0101 and DRB1*0401. J Immunol 1994. March 15; 152(6):2890–2898. [PubMed] [Google Scholar]
- 27.Novak EJ, Liu AW, Nepom GT, Kwok WW. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. The Journal of clinical investigation 1999. December; 104(12):R63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fasth AE, Cao D, van Vollenhoven R, Trollmo C, Malmstrom V. CD28nullCD4+ T cells--characterization of an effector memory T-cell population in patients with rheumatoid arthritis. Scandinavian journal of immunology 2004. Jul-Aug; 60(1–2):199–208. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, James EA, Sanda S, Greenbaum C, Kwok WW. CD4+ T cells recognize diverse epitopes within GAD65: implications for repertoire development and diabetes monitoring. Immunology 2013. March; 138(3):269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundberg K, Kinloch A, Fisher BA, Wegner N, Wait R, Charles P, et al. Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis and rheumatism 2008. October; 58(10):3009–3019. [DOI] [PubMed] [Google Scholar]
- 31.Bagaitkar J, Daep CA, Patel CK, Renaud DE, Demuth DR, Scott DA. Tobacco smoke augments Porphyromonas gingivalis-Streptococcus gordonii biofilm formation. PLoS One 2011; 6(11):e27386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scher JU, Ubeda C, Equinda M, Khanin R, Buischi Y, Viale A, et al. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis and rheumatism 2012. October; 64(10):3083–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makrygiannakis D, Hermansson M, Ulfgren AK, Nicholas AP, Zendman AJ, Eklund A, et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis 2008. October; 67(10):1488–1492. [DOI] [PubMed] [Google Scholar]
- 34.Reynisdottir G, Karimi R, Joshua V, Olsen H, Hensvold AH, Harju A, et al. Structural lung changes and local anti-citrulline immunity are early features of anti citrullinated-proteins antibodies positive rheumatoid arthritis. Arthritis and rheumatism 2013. October 21. [Google Scholar]
- 35.Faith A, Akdis CA, Akdis M, Joss A, Wymann D, Blaser K. An altered peptide ligand specifically inhibits Th2 cytokine synthesis by abrogating TCR signaling. Journal of immunology 1999. February 1; 162(3):1836–1842. [PubMed] [Google Scholar]
- 36.Gebe JA, Novak EJ, Kwok WW, Farr AG, Nepom GT, Buckner JH. T cell selection and differential activation on structurally related HLA-DR4 ligands. Journal of immunology 2001. September 15; 167(6):3250–3256. [DOI] [PubMed] [Google Scholar]
- 37.Grakoui A, Donermeyer DL, Kanagawa O, Murphy KM, Allen PM. TCR-independent pathways mediate the effects of antigen dose and altered peptide ligands on Th cell polarization. Journal of immunology 1999. February 15; 162(4):1923–1930. [PubMed] [Google Scholar]
- 38.Snir O, Rieck M, Gebe JA, Yue BB, Rawlings CA, Nepom G, et al. Identification and functional characterization of T cells reactive to citrullinated-vimentin in HLA-DRB1*0401 humanized mice and RA patients. Arthritis and rheumatism 2011. May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer AL, Trollmo C, Crawford F, Marrack P, Steere AC, Huber BT, et al. Direct enumeration of Borrelia-reactive CD4 T cells ex vivo by using MHC class II tetramers. Proc Natl Acad Sci U S A 2000. October 10; 97(21):11433–11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kannian P, Drouin EE, Glickstein L, Kwok WW, Nepom GT, Steere AC. Decline in the frequencies of Borrelia burgdorferi OspA161 175-specific T cells after antibiotic therapy in HLA-DRB1*0401-positive patients with antibiotic-responsive or antibiotic-refractory lyme arthritis. Journal of immunology 2007. November 1; 179(9):6336–6342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1 Other enolase peptides tested.
(A+B) Amino acid sequence from α-enolase aa26–40 (A) and α-enolase aa241–255 (B) in the native (eno26–40 and eno241–255) and citrullinated forms (cit-eno26–40 and cit-eno241–255). Numbers indicate the tentative binding position for P1, P4, P6, P7 and P9. (C-E) PBMC from a DRB1*04:01 RA patient were stimulated with eno26–40 (C), eno241–255 (D), cit-eno26–40 (E) or cit-eno241–255 (F), cultured for 14 days and stained with corresponding tetramers.
S2 Patients tested in ex vivo stainings.
Percentage of positive tested patients for the different peptides in peripheral blood of control subjects (white bars), peripheral blood of RA patients (grey bars) and synovial fluid of RA patients (black bars). The numbers of positive tested individuals/totally tested individuals is depicted on top of the bars.
Table S1 Patient characteristics
Table S2 Antibodies used


