Abstract
Background
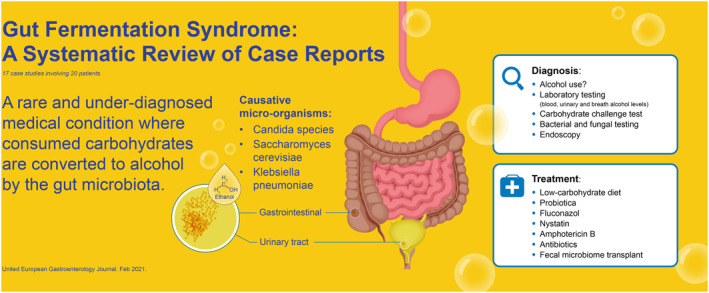
The gut fermentation syndrome (GFS), also known as the endogenous alcohol fermentation syndrome or auto brewery syndrome, is a rare and underdiagnosed medical condition where consumed carbohydrates are converted to alcohol by the microbiota in the gastrointestinal or urinary tract. The symptoms of GFS can have severe impact on patients' wellbeing and can have social and legal consequences. Unfortunately, not much is reported about GFS. The aim of this systematic review was to assess the evidence for GFS, causal micro‐organisms, diagnostics, and possible treatments.
Methods
A protocol was developed prior to initiation of the systematic review (PROSPERO 207182).
We performed a literature search for clinical studies on 1 September 2020 using PubMed and Embase. We included all clinical studies, including case reports that described the GFS.
Results
In total, 17 case reports were included, consisting of 20 patients diagnosed with GFS. The species that caused the GFS included Klebsiella pneumoniae, Candida albicans, C. glabrata, Saccharomyces cerevisiae, C. intermedia, C. parapsilosis, and C. kefyr.
Conclusions
GFS is a rare but underdiagnosed disease in daily practice. The disease is mostly reported by Saccharomyces and Candida genera, and some cases were previously treated with antibiotics. Studies in Nonalcoholic Fatty Liver disease suggest a bacterial origin of endogenous alcohol‐production, which might also be causal micro‐organisms in GFS. Current treatments for GFS include antibiotics, antifungal medication, low carbohydrate diet, and probiotics. There might be a potential role of fecal microbiota transplant in the treatment of GFS.
Keywords: auto‐brewery syndrome, Candida albicans, drunkenness disease, endogenous alochol fermentation syndrome, gut fermentation syndrome, microbiota, nonalcoholic steatohepatitis, nonalcoholic fatty liver disease, Saccharomyces cerevisiae
BACKGROUND
The consumption of alcoholic beverages is as old as human history and dates back to early civilizations such as ancient Egypt and ancient China. 1 The distillation of alcohol (الكحل, al‐Kuhl) can be attributed to early scientists from the Islamic world. 2 Ethanol‐containing alcoholic beverages are one of the most widely used and accepted recreational drugs worldwide. 3 Excessive consumption of alcoholic beverages has negative medical and social consequences. However, some individuals might suffer from these consequences without consuming any alcohol. These unfortunate individuals suffer from the so‐called gut fermentation syndrome (GFS), also known as the endogenous alcohol fermentation syndrome, gut fermentation syndrome, or auto‐brewery syndrome. 4 We suggest to refer this disease as gut‐fermentation syndrome in future literature. GFS is a rare and underrecognized medical condition. Consumed carbohydrates are metabolized to alcohol by fungi and/or bacteria in the gastrointestinal tract. 5 Fungi are not commonly present in the upper gastrointestinal tract, but may be present in the colon as part of the commensal microbiome. There are some fungi that are known that produces ethanol such as fungi from the Candida and Saccharomyces genera. 6 Recently, also the role of bacteria including Klebsiella and Escherichia in intestinal alcohol production have become apparent. 7 , 8
Ethanol formation by ethanol‐producing micro‐organisms
The fermentation process of glucose has several steps before ethanol can be formed in fungi and bacteria. First, glucose is transporter into the cell by the hexose transporter, and subsequently phosphorylated by hexokinase to glucose‐6‐phosphate. This will be converted to fructose‐6‐phosphate by phosphohexo‐isomerase. Fructose‐6‐phosphate is phosphorylated by phosphofructokinase‐2 to fructose 1,6‐bisphosphate, which subsequently converted to glyceraldehyde‐3‐phosphate by aldolase. The latter is converted to 1,3‐biphosphoglycerate by glyceraldehyde‐3‐phosphate dehydrogenase. This 1,3‐biphosphoglycerate will be converted to pyruvate in multiple steps. Pyruvate is converted to acetaldehyde by pyruvate decarboxylase, which is subsequently converted to the final product ethanol by alcohol dehydrogenase (see Figure 1).
FIGURE 1.
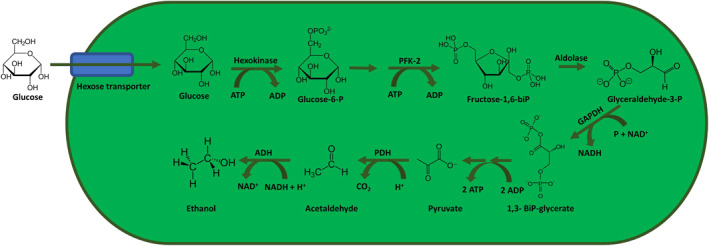
Fungal and bacterial fermentation process of the metabolism of glucose to ethanol. ADH, alcohol dehydrogenase; ADP, adenosine‐diphosphate; ATP, adenosine‐triphosphate; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; NAD+, nicotinamide adenine dinucleotide; P, phosphate; PFK‐2, phosphofructokinase‐2; PHD, pyruvate decarboxylase
Data from literature
A study from the United Arab Emirates found that in 1557 participants of different nationalities, ages, and sexes, the median endogenous ethanol level was 0.04 mg/dl (IQR 0.01–0.09 mg/dl). 9 One study found that residents from different nationalities in Saudi Arabia had a mean endogenous blood alcohol level of 0.14 mg/dl (range: 0–1.53 mg/dl). 10 This is line with other studies that assessed endogenous alcohol production. 11 Recently, it was reported that high alcohol‐producing K. pneumoniae (HiAlc Kpn) occurs in a large percentage of individuals with nonalcoholic fatty liver disease (NAFLD) in a Chinese cohort. 7 Transfer of HiAlc Kpn into mice resulted in NAFLD, suggesting increased levels of this bacterial strain might be one cause of NAFLD in humans.
Therefore, pathological colonization of alcohol‐producing fungi and bacteria in the gastrointestinal, but also the urinary tract, can lead to overproduction of endogenous alcohol and may lead to symptoms of GFS in exceptional cases. Symptoms include decreased social inhibition, decreased peripheral vision, ataxia, nausea, and slurred speech, similar to those of excessive alcoholic consumption. These symptoms can have severe impact on patients' wellbeing and can have social and legal consequences. Unfortunately, not much is known about GFS, and its existence is not known to many physicians. Therefore, the aim of this systematic review was to assess the evidence for GFS, including diagnostics and treatment options.
METHODS
Data sources and searches
We systematic searched PubMed and Embase from inception up to September 2020 for any clinical evidence for GFS. This electronic search strategy was augmented by a manual examination of references cited in articles, recent reviews, editorials, and meta‐analyses. No restrictions were imposed on the language, study period, or sample size.
Study selection and outcome definition
Two investigators (AB and CJM) independently screened titles and abstracts, identified duplicates, reviewed full articles, and determined their eligibility. Any discrepancies were resolved by reaching a consensus regarding the inclusion or exclusion of a trial between the two researchers.
Study eligibility criteria
The population‐intervention‐comparator‐outcomes‐study design framework was used to identify eligible cases. Details of the criteria established a priori were as follows.
-
‐
Population: only human patients, with no restrictions on age or other demographics.
-
‐
Intervention and comparator: any intervention that was used for GFS was included in this study. No comparator was required.
-
‐Outcomes: only patients were included that had at least one of the two following criteria.
- a. Unexplained high levels of blood alcohol concentrations or breath alcohol levels.
- b. A positive carbohydrate challenge test (i.e., ethanol level increased after carbohydrate intake).
We included studies if patients were positive for these criteria and were diagnosed with GFS. Any outcome of natural course or therapy must be included in the study.
-
‐
Study design: all study designs were included. Letters to the editor were included, if all other criteria for inclusion were satisfied.
Data extraction and quality assessment
The data compiled a standardized form to extract the following study characteristics: study design, number of patients, age, BMI, previous antibiotic treatment, symptoms, differential diagnosis, micro‐organism, and all given treatments. Case reports are known for increased risk of bias. To assess the quality of the eligible studies, we chose the framework for appraisal, synthesis, and application of evidence suggested by Murad et al. 12 based on the domains of selection, ascertainment, causality, and reporting. We have additionally assessed whether undisclosed alcohol consumption could have been present. This was based on whether studies reported strict monitoring of patients during diagnostics. This framework was based on the Newcastle‐Ottawa Quality Assessment Scale. This study was reported according to the PRISMA statement.
RESULTS
Search findings
The literature search conducted on 1 September 2020 identified 821 studies. We identified three additional studies through searching of references. After removal of duplicates, 724 studies remained for review of titles and abstracts. After review, we selected 22 studies that described GFS for full‐text review. In total, we included 17 case reports involving 20 patients. The flowchart of this review can be found in Figures 2and 3. We excluded five studies. One study was a review and four studies did not describe GFS properly.
FIGURE 2.
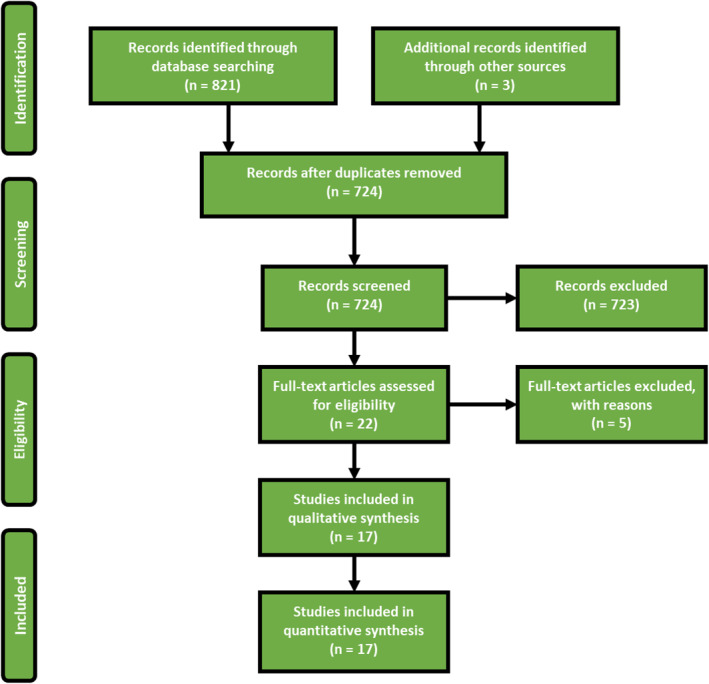
Flowchart of this study. In total, 17 studies were included
FIGURE 3.
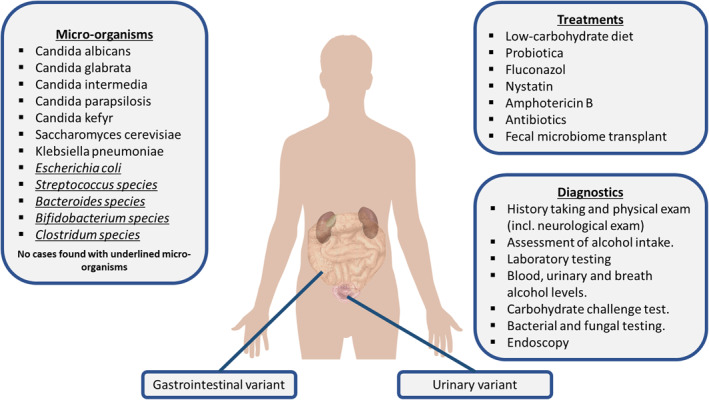
Overview of the gut‐fermentation syndrome. The underlined micro‐organisms were not described in the case reports but are known in literature for ethanol‐producing capabilities
Risk of bias in included studies
A summary of the risk of bias assessment is provided in Table 1. All studies were case reports. Seven studies 13 , 14 , 15 , 16 , 17 , 18 were judged as good, seven studies 19 , 20 , 21 , 22 , 23 , 24 were judged as fair and two studies 25 , 26 were poor in terms of risk of bias.
TABLE 1.
Summary of studies and risk of bias analysis
| Author | Design | Country | Year | Setting | Risk of bias judgment |
|---|---|---|---|---|---|
| Vandekerckhove 13 | CR | Belgium | 2020 | Hospital | Good |
| Kruckenberg 14 | CR | USA | 2020 | Hospital | Good |
| Akbaba 15 | CR | Turkey | 2020 | Hospital | Good |
| Yuan 7 | CR | China | 2019 | Hospital | Poor |
| Saverimuttu 16 | CR | USA | 2019 | Hospital | Good |
| Malik 5 | CR | UK | 2019 | Hospital | Good |
| Akhavan 19 | CR | USA | 2019 | Hospital | Fair |
| Ahmed 20 | CR | USA | 2018 | Hospital | Fair |
| Guo 21 | CR | China | 2018 | Hospital | Fair |
| Mishra 22 | CR | USA | 2017 | Hospital | Fair |
| Welch 23 | CR | USA | 2016 | Hospital | Fair |
| Cordell 25 | CR | USA | 2015 | Hospital | Poor |
| Cordell 24 | CR | USA | 2013 | Hospital | Fair |
| Jansson‐Nettelbladt 27 | CR | Sweden | 2006 | Hospital | Fair |
| Spinucci 17 | CR | Italy | 2006 | Hospital | Good |
| Dahshan 18 | CR | USA | 2001 | Hospital | Good |
| Kaji 26 | CR | Japan | 1984 | Hospital | Poor |
Clinical characteristics
The patients described in the included case reports had various initial symptoms at presentation. These symptoms include: slurred speech 17 , 19 , 20 , 23 , 26 (n = 5), fruity breath odor 18 , 26 , 27 (n = 3), walking difficulties 19 , 23 , 26 , 27 (n = 5), episodes of depression 5 , 25 (n = 2), seizures 16 , 26 (n = 2), vomiting 19 , 20 , 21 , 26 (n = 4), intoxicated feeling 5 , 13 , 17 , 18 , 21 , 24 , 25 (n = 7), and disorientation 17 , 18 , 19 (n = 3). Two cases had elevated liver function tests. 13 , 21 Two case reports 15 , 28 came to light during traffic alcohol tests, of which one was after an accident. 15 Eight cases 5 , 13 , 20 , 23 , 26 , 27 , 28 were initially suspected of GFS as main working diagnosis. Cordell et al. 29 found in a case‐control study that patients self‐reported a smelly breath odor. The median age of patients was 44 years old (range: 3–71) and 14 patients were male (70%). In 11 out of 20 case reports, comorbidities were present in the patient. The comorbidities included short bowel syndrome 13 , 18 , 27 (n = 3), type 2 diabetes mellitus 14 , 16 , 20 , 22 (n = 4), hypertension (n = 3) 15 , 24 , 25 , liver cirrhosis 14 (n = 1) and Crohn's disease 23 (n = 1). Kruckenberg et al. 14 described one patient that suffered from urinary GFS. This variant of GFS was caused by chronic glycosuria and urinary tract colonization of C. glabrata and S. cerevisiae. This patient was difficult to treat due to persisting glycosuria. Another comorbidity that has been described is the short‐bowel syndrome. Stagnation of digested food in the short bowel may cause favorable conditions for fungi and bacteria to grow. 30 Recent studies also found changes associated with gastric bypass Roux‐en‐Y surgery. 31 , 32 An important consideration in the diagnosis of the GFS is the possibility of undisclosed alcohol consumption. In eight case reports, 5 , 14 , 15 , 16 , 17 , 18 , 24 , 27 the patients were under strict supervision and monitoring, while under diagnostic evaluation. In nine case reports, 7 , 13 , 19 , 20 , 21 , 22 , 23 , 25 , 26 it was not clear whether patients were monitored, thus it is possible that undisclosed alcohol consumption still occurred.
Micro‐organisms associated with GFS
Eighteen out of 20 case reports described various micro‐organisms that caused the GFS. The species included K. pneumoniae, C. albicans, C. glabrata, S. cerevisiae, C. intermedia, C. parapsilosis, and C. kefyr. One study mentioned they found Pseudomonas bacteria in a duodenal aspirate. 15 Pseudomonas is rarely present in the microbiome; however, the coinfection of Pseudomonas and C. genera is relatively common. P. aeruginosa biofilm formation and phenazine production is strongly influenced by ethanol production by C. albicans. 33 However, in the case described by Akbaba et al. 15 no fungal organisms were found in the fungal culture. The diagnosis GFS was made after the carbohydrate challenge test was positive, without identifying a causal micro‐organism.
Prior antibiotics use at the onset of symptoms
Patients used antibiotics shortly before the onset of symptoms in seven case reports. 5 , 13 , 16 , 17 , 20 , 23 , 24 Spinucci et al. 17 described a case of GFS who had prolonged use amoxicillin‐clavulanic acid 1 g twice daily. Malik et al. 5 described a patient who suffered for years from memory loss, mental changes, and episodes of depression after using cephalexin 250 mg orally three times a day for 3 weeks. The patient remained asymptomatic after he was treated for S. cerevisiae and C. species. Welch et al. 23 described a 71‐year old Crohn's disease patient who had long‐term use of amoxicillin‐clavulanate 500/125 mg twice daily and metronidazole 500 mg thrice daily. A 47‐year‐old male was described by Vandekerckhove et al. 13 who was treated with amoxicillin‐clavulanic acid and moxifloxacin for a respiratory tract infection and who had a history of Roux‐en‐Y gastric bypass surgery. Saverimuttu et al. 16 described a case of 45‐year old male who received amoxicillin/clavulanic acid after extensive dental surgery prior to developing GFS symptoms. Ahmed et al. 20 described a 45‐year old obese, diabetic male patient who received two courses of antibiotics for deviated nasal septum and dental procedure. Cordell et al. 24 described a 61‐year old male who was treated with antibiotics after surgery for a broken foot. The subsequent years this patient developed unexplained episodes of intoxication.
Diagnostic evaluation
The complete evaluation of the GFS includes history taking, physical examination, laboratory testing, stool sampling with culture, a carbohydrate challenge test, and endoscopy with biopsies for culture. The evaluation of patients should be composed of complete history taking, including antibiotic use, alcohol intake, and unexplained episodes of intoxication. A general physical exam should have followed by a neurological exam. In the exam, special focus should be given to signs of liver abnormalities (e.g. liver enlargement, jaundice, spider naevi) and neurological deficits (e.g. slurred speech and walking difficulties) matching with alcohol intoxication. Laboratory testing includes complete blood count, electrolytes (sodium, potassium), kidney function tests (creatinine, blood urea nitrogen), liver function tests (alanine aminotransferase, alkaline phosphatase, bilirubin), endocrine functions (glucose, thyroid stimulating hormone), and vitamin status (especially vitamin B1 and B12). A fecal stool test can be used for fungal or bacterial growth. A carbohydrate challenge of 100–200 g glucose combined with blood alcohol concentration (BAC) and breath or plasma alcohol testing at intervals of 0, 4, 8, 16 and 24 h can be performed to diagnose the GFS. The t o measurement should be performed before administering glucose. It is important that the patients are strictly monitored during the test for any consumption of alcoholic beverages, which would otherwise severely bias the test. 4 An upper and lower GI‐tract endoscopy can be used to collect gastrointestinal secretions and biopsies for fungal and bacterial testing. These fungi and bacteria can then be tested for antifungal and antibiotic sensitivity testing. The diagnosis GFS can be made when the carbohydrate challenge test is positive and a causal micro‐organism have been cultured, and all other causes of symptoms have been excluded.
Treatment options
In five case reports, fluconazole 100 mg/day for 3 weeks and/or low‐carbohydrate diet was sufficient to treat the GFS. 16 , 18 , 19 , 23 , 27 However, in some patients switching to other medications was necessary because fluconazole was ineffective. These other treatments included nystatin, amphotericin, micafungin, itraconazole, voriconazole, metronidazole, or combinations thereof. Fluconazole was used empirically in some cases as treatment for GFS; however, it would be more ideal to treat patients based on antifungal and antibiotic sensitivity testing. Furthermore, one study described the successful treatment of the GFS with fecal microbiota transplant (FMT) after all other therapies have failed. 13 The summary of study outcomes can be found in Table 2.
TABLE 2.
Summary of results derived from 16 case reports that described GFS
| Author | Age | Sex | BMI | Symptoms | First differential diagnosis | Comorbidity | Prior AB use? | Possibility of undisclosed alcohol Consumption? | Micro‐organisms | Blood level of ethanol | Treatment | Cessation of symptoms? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yuan 7 | NA | NA | NA | NA | NASH | NA | NA | Yes | Klebsiella pneumoniae | 400 mg/L | Antifungal treatment ineffective. Antibiotics and diet more effective Fluconazole 100 mg + low carb diet, nystatin, 500, 000 IU, amphotericin B 100 mg, FMTOral fungal treatment | NA |
| Vandekerckhove 13 | 47 | M | NA | Intermittent episodes of drunkenness + ↑LFT | GFS | RY bypass | Yes | Yes | Candida glabrata | 34.7 mmol/L | Yes | |
| Kruckenberg 14 | 61 | F | NA | Screening | Hidden alcohol use | DM II, cirrhosis | No | No | C. glabrata S. cerevisiae (urinary) | 18 mg/dl | No | |
| Akbaba 15 | 38 | M | NA | Car accident while drunk | Alcohol abuse | HT, alcohol abuses, sleep disorder | No | No | Pseudomonas | 257.8 mg/dl | NA | NA |
| Saverimuttu 16 | 45 | M | 35 | Recurrent seizures | Alcohol abuse | DM II | Yes | No | S. cerevisiae C. intermedia | 410 mg/dl | Fluconazole 100 mg + probiotica Intravenous micafungin | Yes |
| Malik 5 | 46 | M | 30 | Memory loss, mental changes and episodes of depression | GFS | None | Yes | No | Saccharomyces and C. fungi | 57 mg/dl | Fluconazole 150 mg, itraconazole 150 mg, micafungin 150 mg | Yes |
| Akhavan 19 | 25 | M | NA | Slurred speech, fatigue, stumbling, dizziness and nausea | Celiac disease, thyroid disease | None | No | Yes | NA | 30 mg/dl | Fluconazole 100 mg | Yes |
| Ahmed 20 | 45 | M | NA | Vomiting, edema, slurred speech, hallucinations and loss of consciousness, precipitated after meals. | GFS | Obese, DMII | Yes | Yes | S. cerevisiae C. intermedia | NA | Fluconazole + low carbohydrate diet | Yes |
| Guo 21 | 30 | M | NA | Recurrent unexplained GI discomfort, intoxication, ↑LFT | Alcohol abuse | None | No | Yes | C. parapsilosis | 311.2 mg/dl | Fluconazole 150 mg + Bifico, voriconazole 400 mg, nystatin 200 MU | Yes |
| Mishra 22 | 45 | M | NA | ↑ alcohol in traffic test | GFS | DMII, obese | No | Yes | Not found | NA | Fluconazole | Yes |
| Welch 23 | 71 | M | NA | Slurred speech and walking difficulties. | GFS | IBD(CD) | Yes | Yes | C. glabrata | 170 mg/dl | Low carbohydrate diet | Yes |
| Cordell 25 | 604232 | MFM | 33NA22 | 1. Episodes of drunkenness, depression 2. Episodes of drunkenness 3. Intoxication | 1. Alcohol abuse2. Alcohol abuse3. Alcohol abuse | HepC, HTNoneNone | NoNo | YesYesYes | 1. C. albicans, C. krusei2. S. cerevesiae, S. bulardii3. S. cerevesiae | 170 mg/dlNANA | 1. Low carbohydrate diet 2. Fluconazole + low carbohydrate diet. 3. Fluconazole 150 mg, nystatin, low carbohydrate diet | YesYesNo |
| Cordell 24 | 61 | M | NA | Intoxications | Hidden alcohol use | HT | Yes | No | S. cerevisiae | 120 mg/dl | Fluconazole 100 mg + nystatin 500, 000 IU | Yes |
| Jansson‐Nettelbladt 27 | 3 | F | NA | Fruity breath odor and walking difficulties | GFS | Small bowel malformation | No | No | C. kefyr S. cerevisiae | 15 mmol/L | Fluconazole 100 mg + low carbohydrate diet | Yes |
| Spinucci 17 | 44 | M | 16 | Mental confusion, disorientation and slurred speech. | Alcohol abuse | Chronic intestinal Pseudo‐obstruction | Yes | No | C. albicans S. cerevisiae | 24.9 mg/dl | Fluconazole 100 mg + low carbohydrate diet | Yes |
| Dahshan 18 | 13 | F | NA | Bizarre behavior, somnolence, disorientation and fruity breath odor | Alcohol abuse | Short bowel syndrome | No | No | C. glabrata S. cerevisiae | 250‐350 mg/dl | Fluconazole 100 mg | Yes |
| Kaji 26 | 2435 | FM | NA | 1. Seizures, nausea and vomiting.2. Fruity breath odor, slurred speech, blurred vision and walking difficulties | 1. GFS2. FS | NoneNone | NoNo | YesYes | C. albicans | 254 mg/dl | Low carbohydrate diet.Nystatin, metronidazole | Yes |
DISCUSSION
This is the first systematic review that has been written for the GFS. In this systematic review, 17 case reports have been described consisting out of 20 patients. The cases showed that GFS often is misdiagnosed and that it comes along with somatic and social suffering. Most patients were suspected for alcohol abuse even though patients denied using alcohol, which is a typical presentation for this disease. All patients were either treated with antifungal therapy and/or low carbohydrate diet. One patient was ultimately treated with FMT. 13 GFS is generally well‐treatable if the syndrome is recognized by physicians.
Excessive alcohol consumption is known to increase the risk of developing liver cirrhosis and fatty liver disease. 34 Gut dysbiosis (i.e., disruption of the normal gut microbiota) might also play an important role in the pathogenesis of liver disease. 35 It has been found by Hafez et al. 36 that patients with liver cirrhosis or diabetes mellitus have significant higher endogenous ethanol production. Patients with diabetes mellitus had a BAC of 4.85 mg/dl, while patients with liver cirrhosis had a BAC of 3.45 mg/dl. In patients who had both diabetes mellitus and liver cirrhosis the mean BAC was 10.88 mg/dl (max 22.3 mg/dl). The healthy adult males, who served as controls in the study of Hafez et al., had a BAC of 0.3 mg/dl. Furthermore, Yuan et al. 7 found the presence of high‐alcohol producing K. pneumoniae in patients with NAFLD in a Chinese cohort. In addition, it has been found that endogenous ethanol produced by K. pneumoniae induces mitochondrial dysfunction in NAFLD. 37 Moreover, Zhu et al. 38 reported higher abundance of alcohol‐producing Escherichia bacteria in nonalcoholic steatohepatitis (NASH) patients compared to normal and obese controls. Alcohol‐metabolizing enzymes were upregulated in livers of NASH patients, suggesting a potential role of alcohol in the pathogenesis of NASH. 38 , 39 Therefore, liver cirrhosis in GFS patients may therefore not be the risk factor, but the consequence of GFS. Furthermore, diabetes mellitus might cause the urinary variant of the GFS. Glycosuria causes high levels of glucose in the urinary tract, which is a suitable environment for alcohol‐producing species in the urinary tract. Proper diabetes management, besides antifungal or antibacterial medication, is therefore necessary for subgroup of patients. As shown in the case by Kruckenberg et al., 14 undertreated diabetes leads to difficult‐to‐treat GFS.
Micro‐organisms
Five case reports 5 , 13 , 16 , 17 , 23 described recent antibiotics use before or at onset of symptoms. The use of antibiotics might affect the microbiome, and would allow for colonization of alcohol‐producing species. The micro‐organisms that are described in this study were usually from the Saccharomyces and Candida genera. K. pneumoniae was also found in gastric and jejunal samples in the case presented by Saverimuttu et al. 16 However, in their case, K. pneumoniae was still present in the microbiome after successful treatment of Candida intermedia with micafungin. Symptoms did not reoccur after treatment. Other potential microbial genera that are capable of producing endogenous ethanol are Escherichia, 8 Streptococcus, 40 Bacteroides, 41 Bifidobacterium, 42 and Clostridium. 43 Identification of these micro‐organisms in patients suspected of GFS might perhaps be the causal organisms, which needs adequate therapy. However, till so far no such cases have been published.
Limitations
Unfortunately, no case control studies have been performed for GFS. As GFS is a rare disease that is often misdiagnosed or unrecognized, a low amount of reports have been published. Case reports are often considered a weak source of evidence due to the high risk of potential bias. However, the case reports that have been published for the GFS did contain a comprehensive amount of valuable data that shed some light on the diagnosis and management of the GFS. In eight case reports, the diagnosis GFS was made based on positive carbohydrate challenge test and/or positive cultures under controlled monitoring of patients. However, in nine case reports, it was not clear whether patients were diagnosed under controlled conditions.
Clinical implications
Physicians should be aware of this rare but unpleasant diagnosis. As shown in some of the case reports, patients were misdiagnosed as alcohol abusers. Some even received psychiatric treatment for detoxification, which was unsuccessful. Physicians should specially be aware if a patient was previously treated with antibiotics and presents with symptoms of alcohol intoxication but denies any use. This also differentiates between GFS patients and individuals who consumed excessive alcohol. Individuals who consumed too much alcohol, would not have an increase of the ethanol level after the carbohydrate challenge test. Fluconazole combined with low‐carbohydrate diet was effective in five case reports; however, some patients needed additional antifungal treatment. The decision for antifungal or antibiotic treatment should be based on the cultured micro‐organism, preferably based on sensitivity testing. One study showed that FMT might also be effective for therapy‐resistant GFS. Recently, Vandekerckhove et al. 13 reported the only case that described treatment with FMT. They showed that FMT can be a successful treatment for GFS when all other antifungal treatments have failed. Transplanting a novel microbiome might be interesting strategy for GFS as the main cause lies in the patients’ microbiome. Currently, FMT is being reported for various other diseases, and it may have a good potential to be part of GFS treatment. 44 More research is needed for this rare and underrecognized medical condition.
Legal implications
In two case reports, the abnormally high blood ethanol levels had legal consequences for the patient. 15 , 22 Excessive use of alcohol while driving is legally forbidden worldwide. The legal consequences range from a financial fine to imprisonment when death occurred during a car accident. Patients with GFS might cause a car accident or are found to have high levels of ethanol during routine breath alcohol screening, without consuming alcohol. Recently, in the Netherlands, a male patient diagnosed with GFS was acquitted from charges after he caused an accident while intoxicated by endogenous ethanol without having consumed alcohol. 45 This stresses out the importance of early recognition of this rare condition. However, there is a critical note as there is no test to differentiate between endogenous and exogenous ethanol levels. So, theoretically, high BAC might still be caused by excessive alcohol consumption in patients with GFS. This is important to consider in legal cases. Physicians should take patients with unexplained episodes of intoxication serious, and that might prevent patients to cause(deadly) car accidents.
CONCLUSION
GFS is a rare, and often a misunderstood and unrecognized condition that physicians should consider or be aware of. The literature only consists out of case reports, no high level evidence studies have been performed regarding prevalence and treatment. The disease is mostly caused by Saccharomyces and Candida genera, and some cases were previously treated with antibiotics. In a few case reports, risk factors such as diabetes mellitus, liver cirrhosis, and prior intestinal operations have been identified. Diagnosis can be made by adequate history taking and carbohydrate challenge test. Current treatments include antifungal medication, low carbohydrate diet, and probiotics. There might be a future role of FMT in the treatment of GFS. Low‐grade GFS should be considered and studied in NASH as well.
CONFLICT OF INTERESTS
The authors have no conflict of interests to declare.
Search query
PubMed
((("Auto‐brewery"[All Fields] AND (((((((("syndrom"[All Fields] OR "syndromal"[All Fields]) OR "syndromally"[All Fields]) OR "syndrome"[MeSH Terms]) OR "syndrome"[All Fields]) OR "syndromes"[All Fields]) OR "syndrome s"[All Fields]) OR "syndromic"[All Fields]) OR "syndroms"[All Fields])) OR ("auto"[All Fields] AND (("breweries"[All Fields] OR "brewery"[All Fields]) OR "brewery s"[All Fields]))) OR (("gut"[Journal] OR "gut"[All Fields]) AND (((((((((((((((((("ferment"[All Fields] OR "fermentabilities"[All Fields]) OR "fermentability"[All Fields]) OR "fermentable"[All Fields]) OR "fermentate"[All Fields]) OR "fermentated"[All Fields]) OR "fermentates"[All Fields]) OR "fermentation"[MeSH Terms]) OR "fermentation"[All Fields]) OR "fermentations"[All Fields]) OR "fermentative"[All Fields]) OR "fermentatively"[All Fields]) OR "fermentator"[All Fields]) OR "fermented"[All Fields]) OR "fermenter"[All Fields]) OR "fermenters"[All Fields]) OR "fermenting"[All Fields]) OR "fermention"[All Fields]) OR "ferments"[All Fields]) AND (((((((("syndrom"[All Fields] OR "syndromal"[All Fields]) OR "syndromally"[All Fields]) OR "syndrome"[MeSH Terms]) OR "syndrome"[All Fields]) OR "syndromes"[All Fields]) OR "syndrome s"[All Fields]) OR "syndromic"[All Fields]) OR "syndroms"[All Fields]))) OR (((((((("endogen"[All Fields] OR "endogene"[All Fields]) OR "endogeneous"[All Fields]) OR "endogeneously"[All Fields]) OR "endogenes"[All Fields]) OR "endogenic"[All Fields]) OR "endogenous"[All Fields]) OR "endogenously"[All Fields]) AND (((("ethanol"[MeSH Terms] OR "ethanol"[All Fields]) OR "ethanols"[All Fields]) OR "ethanol s"[All Fields]) OR "ethanolic"[All Fields]) AND (((((((("syndrom"[All Fields] OR "syndromal"[All Fields]) OR "syndromally"[All Fields]) OR "syndrome"[MeSH Terms]) OR "syndrome"[All Fields]) OR "syndromes"[All Fields]) OR "syndrome s"[All Fields]) OR "syndromic"[All Fields]) OR "syndroms"[All Fields]))
Embase
'auto‐brewery syndrome' OR ('auto brewery' AND ('syndrome'/exp OR syndrome)) OR 'gut fermentation syndrome' OR (('gut'/exp OR gut) AND ('fermentation'/exp OR fermentation) AND ('syndrome'/exp OR syndrome)) OR ′endogenous ethanol fermentation syndrome' OR (endogenous AND ('ethanol'/exp OR ethanol) AND ('fermentation'/exp OR fermentation) AND ('syndrome'/exp OR syndrome))
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Khaderi SA. Introduction: Alcohol and Alcoholism. Clin Liver Dis. 2019;23(1):1–10. [DOI] [PubMed] [Google Scholar]
- 2. Amr S, Tbakhi A. Abu Bakr Muhammad Ibn Zakariya Al Razi (Rhazes): philosopher, physician and alchemist. Ann Saudi Med. 2007;27(4):305–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatr. 2018;5(12):987‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Painter K, Cordell BJ, Sticco KL. Auto‐Brewery Syndrome (Gut Fermentation). StatPearls Publishing LLC; 2020. [PubMed] [Google Scholar]
- 5. Malik F, Wickremesinghe P, Saverimuttu J. Case report and literature review of auto‐brewery syndrome: probably an underdiagnosed medical condition. BMJ Open Gastroenterol. 2019;6(1):e000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bivin WS, Heinen BN. Production of ethanol from infant food formulas by common yeasts. J Appl Bacteriol. 1985;58(4):355–7. [DOI] [PubMed] [Google Scholar]
- 7. Yuan J, Chen C, Cui J, Lu J, Yan C, Wei X, et al. Fatty liver disease caused by high‐alcohol‐producing Klebsiella pneumoniae . Cell Metabol. 2019;30(4):675–88. [DOI] [PubMed] [Google Scholar]
- 8. Dawes EA, Foster SM. The formation of ethanol in Escherichia coli. Biochim Biophys Acta. 1956;22(2):253–65. [DOI] [PubMed] [Google Scholar]
- 9. Al‐Awadhi A, Wasfi IA, Al Reyami F, Al‐Hatali Z. Autobrewing revisited: endogenous concentrations of blood ethanol in residents of the United Arab Emirates. Sci Justice. 2004;44(3):149–52. [DOI] [PubMed] [Google Scholar]
- 10. Afify M, Ragab A, Al‐Mazroua M. Endogenous ethanol production levels in Saudi Arabia residents. J Alcohol Drug Depend. 2015;3:211. [Google Scholar]
- 11. Logan BK, Jones AW. Endogenous ethanol 'auto‐brewery syndrome' as a drunk‐driving defence challenge. Med Sci Law. 2000;40(3):206–15. [DOI] [PubMed] [Google Scholar]
- 12. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid‐Based Med. 2018;23(2):60–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vandekerckhove E, Janssens F, Tate D, De Looze D. Treatment of gut fermentation syndrome with fecal microbiota transplantation. Ann Intern Med. 2020;173:855. [DOI] [PubMed] [Google Scholar]
- 14. Kruckenberg KM, DiMartini AF, Rymer JA, Pasculle AW, Tamama K. Urinary auto‐brewery syndrome: a case report. Ann Intern Med. 2020;172(10):702–4. [DOI] [PubMed] [Google Scholar]
- 15. Akbaba M. A medicolegal approach to the very rare auto‐brewery (endogenous alcohol fermentation) syndrome. Traffic Inj Prev. 2020;21(5):295–7. [DOI] [PubMed] [Google Scholar]
- 16. Saverimuttu J, Malik F, Arulthasan M, Wickremesinghe P. A case of auto‐brewery syndrome treated with micafungin. Cureus. 2019;11(10):e5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spinucci G, Guidetti M, Lanzoni E, Pironi L. Endogenous ethanol production in a patient with chronic intestinal pseudo‐obstruction and small intestinal bacterial overgrowth. Eur J Gastroenterol Hepatol. 2006;18(7):799–802. [DOI] [PubMed] [Google Scholar]
- 18. Dahshan A, Donovan K. Auto‐brewery syndrome in a child with short gut syndrome: case report and review of the literature. J Pediatr Gastroenterol Nutr. 2001;33(2):214–5. [DOI] [PubMed] [Google Scholar]
- 19. Akhavan BJ, Ostrosky‐Zeichner L, Thomas EJ. Drunk without drinking: a case of auto‐brewery syndrome. ACG Case Rep J. 2019;6(9):e00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahmed S, Wickremesinghe P, Kopetz V, Sarkar S. A rare diagnosis of gut fermentation/auto‐brewery syndrome in the setting of diabetes and obesity. Am J Clin Pathol. 2018;150:S2. [Google Scholar]
- 21. Guo X, Zhang W, Ma J, Liu Z, Hu D, Chen J, et al. The case study of one patient with gut fermentation syndrome: case report and review of the literature. Int J Clin Exp Med. 2018;11(4):4324–9. [Google Scholar]
- 22. Mishra A, Seril DN. Suspected gut fermentation syndrome (auto‐brewery syndrome): 2519. Am Coll Gastroenterol. 2017;112: S1373. [Google Scholar]
- 23. Welch BT, Coelho Prabhu N, Walkoff L, Trenkner SW. Auto‐brewery syndrome in the setting of long‐standing Crohn's disease: a case report and review of the literature. J Crohns Colitis. 2016;10(12):1448–50. [DOI] [PubMed] [Google Scholar]
- 24. Cordell B, McCarthy J. A case study of gut fermentation syndrome (auto‐brewery) with Saccharomyces cerevisiae as the causative organism. Int J Clin Med. 2013;04:309–12. [Google Scholar]
- 25. Cordell B, Kanodia A. Auto‐brewery as an emerging syndrome: three representative case studies. J Clin Med Case Rep. 2015;2:5. [Google Scholar]
- 26. Kaji H, Asanuma Y, Yahara O, Shibue H, Hisamura M, Saito N, et al. Intragastrointestinal alcohol fermentation syndrome: report of two cases and review of the literature. J Forensic Sci Soc. 1984;24(5):461–71. [DOI] [PubMed] [Google Scholar]
- 27. Jansson‐Nettelbladt E, Meurling S, Petrini B, Sjölin J. Endogenous ethanol fermentation in a child with short bowel syndrome. Acta Paediatr. 2006;95(4):502–4. [DOI] [PubMed] [Google Scholar]
- 28. Mishra A, Seril DN. Suspected gut fermentation syndrome (auto‐brewery syndrome). Am J Gastroenterol. 2017;112:S1373. [Google Scholar]
- 29. Cordell BJ, Kanodia A, Miller GK. Case‐control research study of auto‐brewery syndrome. Glob Adv Health Med. 2019;8:2164956119837566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dibaise JK, Young RJ, Vanderhoof JA. Enteric microbial flora, bacterial overgrowth, and short‐bowel syndrome. Clin Gastroenterol Hepatol. 2006;4(1):11–20. [DOI] [PubMed] [Google Scholar]
- 31. Steinert RE, Rehman A, Souto Lima EJ, Agamennone V, Schuren FHJ, Gero D, et al. Roux‐en‐Y gastric bypass surgery changes fungal and bacterial microbiota in morbidly obese patients—a pilot study. PloS One. 2020;15(7):e0236936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Furet J‐P, Kong L‐C, Tap J, Poitou C, Basdevant A, Bouillot J‐L, et al. Differential adaptation of human gut microbiota to bariatric surgery‐induced weight loss: links with metabolic and low‐grade inflammation markers. Diabetes. 2010;59(12):3049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen AI, Dolben EF, Okegbe C, et al. Candida albicans ethanol stimulates Pseudomonas aeruginosa WspR‐controlled biofilm formation as part of a cyclic relationship involving phenazines. PLoS Pathog. 2014;10(10):e1004480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seitz HK, Bataller R, Cortez‐Pinto H, Gao B, Gual A, Lackner C, et al. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4(1):16. [DOI] [PubMed] [Google Scholar]
- 35. Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13(7):412–25. [DOI] [PubMed] [Google Scholar]
- 36. Hafez E, Hamad M, Fouad M, Abdel‐Lateff A. Auto‐brewery syndrome: ethanol pseudo‐toxicity in diabetic and hepatic patients. Hum Exp Toxicol. 2017;36(5):445–50. [DOI] [PubMed] [Google Scholar]
- 37. Chen X, Zhang Z, Li H, et al. Endogenous ethanol produced by intestinal bacteria induces mitochondrial dysfunction in non‐alcoholic fatty liver disease. J Gastroenterol Hepatol. 2020;35:2009–19. [DOI] [PubMed] [Google Scholar]
- 38. Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–9. [DOI] [PubMed] [Google Scholar]
- 39. Baker SS, Baker RD, Liu W, Nowak NJ, Zhu L. Role of alcohol metabolism in non‐alcoholic steatohepatitis. PloS One. 2010;5(3):e9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mickelson MN. Glucose degradation, molar growth yields, and evidence for oxidative phosphorylation in Streptococcus agalactiae . J Bacteriol. 1972;109(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Frantz JC, McCallum RE. Growth yields and fermentation balance of Bacteroides fragilis cultured in glucose‐enriched medium. J Bacteriol. 1979;137(3):1263‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Amaretti A, Bernardi T, Tamburini E, Zanoni S, Lomma M, Matteuzzi D , et al. Kinetics and metabolism of Bifidobacterium adolescentis MB 239 growing on glucose, galactose, lactose, and galactooligosaccharides. Appl Environ Microbiol. 2007;73(11):3637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weimer PJ, Zeikus JG. Fermentation of cellulose and cellobiose by Clostridium thermocellum in the absence of Methanobacterium thermoautotrophicum . Appl Environ Microbiol. 1977;33(2):289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bakker GJ, Nieuwdorp M. Fecal microbiota transplantation: therapeutic potential for a multitude of diseases beyond Clostridium difficile . Microbiol Spectr. 2017;5(4):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Acquittal for man with auto brewery syndrome for driving while drunk (Dutch); 2020. https://www.rechtspraak.nl/Organisatie‐en‐contact/Organisatie/Rechtbanken/Rechtbank‐Gelderland/Nieuws/Paginas/Vrijspraak‐voor‐man‐met‐auto‐brouwerij‐syndroom‐voor‐rijden‐onder‐invloed‐van‐alcohol.aspx. Accessed 16 Sep 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


