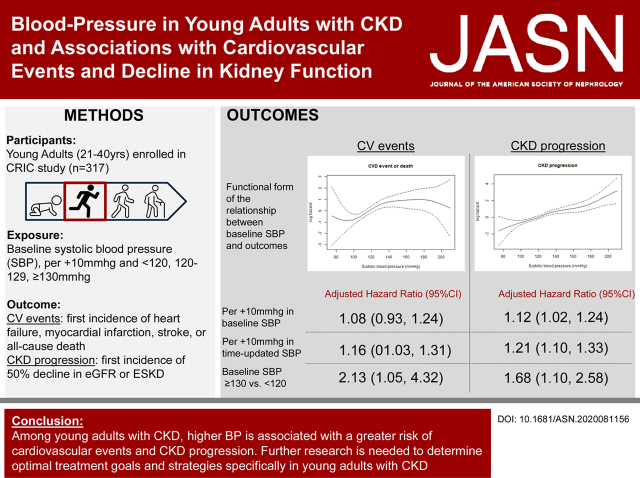
Significance Statement
Although young adults (aged 18–40 years) with CKD are at risk for poor cardiovascular and renal outcomes, with hypertension an important and potentially modifiable risk factor, they are largely absent from observational studies and clinical trials of BP in patients with CKD. To address this knowledge gap, this observational study provides a description of BP and its relation to outcomes specifically in young adults with CKD. It demonstrates that among young adults with CKD, higher BP is associated with cardiovascular events (particularly heart failure) and CKD progression. The study’s findings may provide a foundation for future work to develop best practices for BP management in young adults with CKD and improve outcomes.
Keywords: blood pressure, chronic kidney disease, cardiovascular disease, outcomes
Visual Abstract
Abstract
Background
BP is an important modifiable risk factor for cardiovascular events and CKD progression in middle-aged or older adults with CKD. However, studies describing the relationship between BP with outcomes in young adults with CKD are limited.
Methods
In an observational study, we focused on 317 young adults (aged 21–40 years) with mild to moderate CKD enrolled in the Chronic Renal Insufficiency Cohort (CRIC) Study. Exposures included baseline systolic BP evaluated continuously (per 10 mm Hg increase) and in categories (<120, 120–129, and ≥130 mm Hg). Primary outcomes included cardiovascular events (heart failure, myocardial infarction, stroke, or all-cause death) and CKD progression (50% decline of eGFR or ESKD). We used Cox proportional hazard models to test associations between baseline systolic BP with cardiovascular events and CKD progression.
Results
Cardiovascular events occurred in 52 participants and 161 had CKD progression during median follow-up times of 11.3 years and 4.1 years, respectively. Among those with baseline systolic BP ≥130 mm Hg, 3%/yr developed heart failure, 20%/yr had CKD progression, and 2%/yr died. In fully adjusted models, baseline systolic BP ≥130 mm Hg (versus systolic BP<120 mm Hg) was significantly associated with cardiovascular events or death (hazard ratio [HR], 2.13; 95% confidence interval [95% CI], 1.05 to 4.32) and CKD progression (HR, 1.68; 95% CI, 1.10 to 2.58).
Conclusions
Among young adults with CKD, higher systolic BP is significantly associated with a greater risk of cardiovascular events and CKD progression. Trials of BP management are needed to test targets and treatment strategies specifically in young adults with CKD.
Young adults (aged approximately 18–40 years) with CKD are an important, and largely understudied, CKD subpopulation. Similar to older adults, young adults with CKD are at risk for poor cardiovascular (CV) and kidney outcomes, with hypertension acting as an important, potentially modifiable risk factor.1–3 Although young adults may represent a small proportion of adults living with CKD, the increasing rates of obesity, hypertension, and diabetes in younger Americans means the number of young adults with CKD is likely to increase.4–6 However, there are limited data from observational studies or clinical trials specifically in young-adult patients with CKD to inform optimal treatment of hypertension. BP management decisions for young adults with CKD largely rely on extrapolating concepts from research using either pediatric patients (<18 years) or older adults (>50 years) with CKD.
A growing body of research has looked at the association between BP and outcomes for young adults in the general, non-CKD population. Studies demonstrate significant associations between higher BPs in young adults with the development of atherosclerosis, cardiac dysfunction, and CV events later in life.7–10 Other studies suggest that higher BP in adolescence or young adulthood is associated with future kidney dysfunction and ESKD.11–13 Many of these studies use a follow-up period of 20–30 years, in part because the short-term incidence rate is low, and to best capture the effect of cumulative exposure to small elevations in BP. Although the number of young adults with CKD included in these studies is not always specified, on the basis of findings from prior epidemiologic research, we could assume a relatively low prevalence (approximately 2%–5%).14
We therefore performed an observational study to test the association of baseline and time-updated BP with CV events and CKD progression in young adults with CKD.
Methods
Study Population
We studied men and women aged 21–40 years with mild to moderate CKD enrolled in the Chronic Renal Insufficiency Cohort (CRIC) study. In total, the CRIC study enrolled 3939 participants ≥21 years of age between June 2003 and August 2008 at seven clinical centers across the United States. Patients with eGFR of 20–70 ml/min per 1.73 m2, using the Modification of Diet in Renal Disease study equation, were eligible for enrollment, and patients with New York Heart Association class III or IV heart failure (HF) were ineligible. Additional details on study design, inclusion, and exclusion criteria, and baseline characteristics of the participants have been previously published.15,16 For this study, we excluded all participants >40 years of age (excluded n=3622), leaving a study population of n=317 adults with CKD between the ages of 21 and 40 years. All study participants provided written informed consent, and institutional review boards at each of the participating sites approved the study protocol.
Exposure: BP
For our analysis we used the mean systolic BP (SBP) value from the baseline visit and time updated SBP values using data from subsequent annual visits. Baseline SBP was evaluated continuously (per 10 mm Hg increase) and in categories (<120, 120–129, or ≥130 mm Hg). These values were selected to remain consistent with the definitions for normal BP, elevated BP, and hypertensive according to the 2017 American College of Cardiology/American Heart Association BP guidelines.17 Secondary analyses evaluated diastolic BP (DBP) also measured at the baseline visit.
Per the CRIC protocol, BP was measured by centrally trained staff using a standardized method in a quiet, standardized setting.18 Participants abstained from caffeine, smoking, and exercise at least half an hour before and until completion of the BP measurement. The Welch-Allyn Tycos Classic Hand Aneroid sphygmomanometer was the standard equipment for all BP measurements at CRIC clinical visits. The mean of three seated resting BP readings was used to define the SBP value for each visit.
CV and Kidney Outcomes
Our primary outcomes were time to CV events and CKD progression. CV events was a composite measure that included the following events: HF, myocardial infarction (MI), cerebrovascular accident (CVA), and all-cause mortality. The composite CKD progression variable consisted of 50% decline in eGFR from baseline value or ESKD.
To identify HF, MI, and CVA, participants were queried every 6 months by alternating in-person and telephone visits. At each of these encounters, they were asked whether they were hospitalized, experienced a possible CV event, or underwent specific set of tests and procedures. Relevant medical records were retrieved for review by at least two physicians to ascertain events of HF, MI, and CVA for all admissions.2,19,20 Death was identified through report from next of kin, review of hospital records, retrieval of death certificates or obituaries, and linkage with the Social Security Mortality Master File through 2018.
Kidney function was assessed by centrally measured serum creatinine values using the kinetic Jaffe method at the baseline and yearly visits. eGFR was calculated using the CKD Epidemiology Collaboration equation.21 eGFR by serum creatinine was calculated at each annual visit, and participants were defined as having a 50% decline in eGFR at the first visit with an eGFR that was ≤50% of the baseline value. ESKD, defined as receiving dialysis or kidney transplant, was determined by participant self-report/local clinical center ascertainment, or crosslinkage with the United States Renal Data System. We also performed a secondary analysis with the outcomes of continuous decline in eGFR using all available eGFR measures in follow-up.
Covariates
All covariates used within the study analysis were collected at the baseline visit, concurrently with baseline SBP values. Demographic and medical history variables (history of diabetes and prior CV disease) were collected by participant interviews. Medication use was ascertained by patient self-report. Urine albumin-creatinine ratios reflect values from 24-hour urine collections.
Statistical Analyses
Participant characteristics were reported as mean and SD for normally distributed variables or as median and interquartile range for skewed variables. Baseline characteristics were described in the analytic cohort overall and stratified by baseline SBP category.
For both the CV and CKD progression outcomes, a participant was assigned to have experienced that event at a certain time point with the first incidence of any of the component events. We evaluated CV events and CKD progression independently of one another, such that patients were not censored from one outcome if they met criteria for the other. Participant follow-up in this study was censored either at the time of death (for CKD progression only), withdrawal, lost to follow-up, or at the end of the follow-up period in 2018.
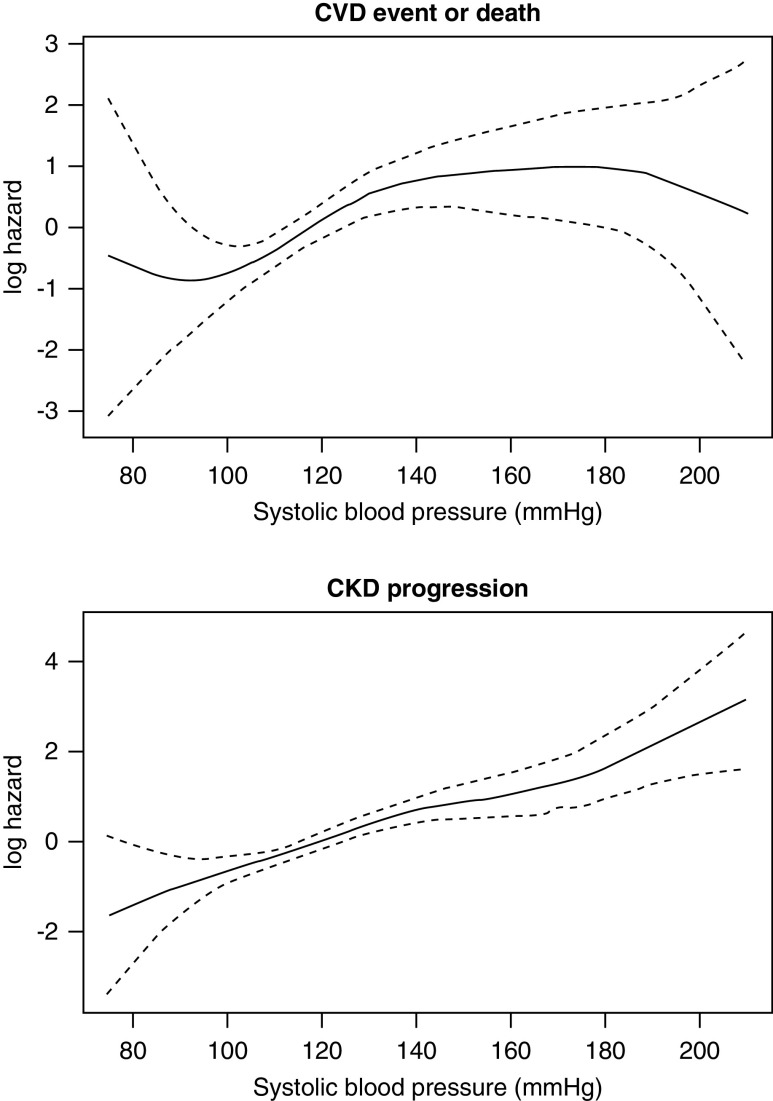
We calculated incidence rates of each outcome across SBP categories. We used Kaplan-Meier estimates to generate survival curves to estimate the probability of event-free survival for both CV events and CKD progression, assessed independently of each other. The curves were stratified by baseline SBP category. We evaluated the functional form of the association of SBP with each event type using penalized spline bases for SBP with four degrees of freedom.
We investigated the association between baseline SBP with CV events and CKD progression by applying a series of nested Cox proportional-hazards models, using baseline SBP as a continuous predictor. For both our CV events and CKD progression outcomes, Model 1 adjusted for age, race/ethnicity, and baseline eGFR. Model 2 adjusted for Model 1+ diabetes and prevalent CV disease (history of HF, MI or need for coronary revascularization, and CVA) and urine albumin-creatinine ratio. Because the urine albumin-creatinine ratio was missing 7.5% of young-adult participants, multiple imputation was utilized for missing values. We repeated the analyses using categorical SBP. From the Cox models, we calculated point estimates for hazard ratios (HR) and 95% confidence intervals (95% CI). P values were calculated using the Wald test for continuous SBP and the likelihood ratio test with two degrees of freedom for categorical SBP.
We performed two secondary analyses. In the first, we evaluated eGFR slope as a continuous outcome using linear mixed models, centering SBP at 120 mm Hg, and adjusting for covariates as above. In the second, secondary analysis, we repeated the above analysis using baseline DBP as the primary exposure. Baseline DBP was also assessed continuously per 10 mm Hg increase, using a binary definition of <90 mm Hg or ≥90 mm Hg.
We performed several sensitivity analyses. In the first, we tested the association between time-updated SBP with risk of CVD and CKD progression, adjusting for time-updated eGFR. In a second sensitivity analysis, we repeated our primary analysis testing the association between baseline SBP with CV events and CKD progression adding number of antihypertensive medications at baseline as a covariate. In a third sensitivity analysis, we repeated the primary analysis, excluding all participants without antihypertensive medication use at baseline. Lastly, in a fourth sensitivity analysis, we performed Fine-Gray competing risk-weighted Cox models for CKD progression to account for censoring secondary to dropout and death.
Analyses describing the 4-year trajectories of SBP and antihypertensive use were also performed. Participants were stratified according to baseline SBP category, and mean SBP values and 95% CIs were calculated for each annual visit. We created scatter plots to describe the relationship between change in SBP value and antihypertensive use over 4 years. To account for intervisit variability, we calculated linear trends for change in SBP and antihypertensive use for each participant. The absolute change over 4 years on the basis of the calculated linear trend was used to create the final scatterplots.
For all analyses, P values <0.05 were considered as significant. All statistical analyses were conducted using SPSS Statistics version 26.0 (IBM Corp, Armonk, NY) or R, version 3.6.2 (R Core Team, 2019).
Results
Baseline Characteristics
The study population included 317 young-adult participants with CKD (Table 1). Overall, 175 (55%) of participants had SBP <120 mm Hg, 63 (20%) had SBP between 120 and 129 mm Hg, and 79 (25%) had SBP ≥130 mm Hg at the baseline visit. At baseline, 19% of young adults in CRIC were not on any antihypertensive therapy. The percentage of participants on >1 antihypertensive medication was highest in the SBP ≥130 mm Hg group (77%), followed by the SBP 120–129 mm Hg (59%) and SBP <120 mm Hg (35%) groups. Age was similar across SBP groups. At each higher SBP strata, there was a higher proportion of participants of Black race (30% versus 41% versus 51%), Hispanic ethnicity (13% versus 16% versus 29%), and with prevalent CVD. The mean eGFR was highest in the SBP <120 mm Hg group (mean eGFR: 55 ml/min per 1.73 m2) and lowest in the ≥130 mm Hg group (mean eGFR: 46 ml/min per 1.73 m2). Patients with a baseline SBP ≥130 mm Hg had the highest proportion with diabetes.
Table 1.
Baseline characteristics of CRIC study participants under the age of 40 yr
| Characteristic | CRIC Age <40 Yr | |||
|---|---|---|---|---|
| All (n=317) | SBP <120 (n=175) | SBP 120–129 (n=63) | SBP ≥130 (n=79) | |
| Demographics | ||||
| Age, yr, mean (SD) | 33 (±5) | 33 (±4) | 34 (±5) | 32 (±5) |
| Female sex, n (%) | 144 (45) | 86 (49.1) | 26 (41.3) | 32 (40.5) |
| White, non-Hispanic, n (%) | 123 (39) | 86 (49.1) | 25 (39.7) | 12 (15.2) |
| Black, n (%) | 118 (37) | 52 (29.7) | 26 (41.3) | 40 (50.6) |
| Hispanic, n (%) | 56 (18) | 23 (13.1) | 10 (15.9) | 23 (29.1) |
| Other, n (%) | 20 (6) | 14 (8.0) | 2 (3.2) | 4 (5.1) |
| Cause of kidney disease, n (%) | ||||
| Diabetes | 75 (23.7) | 28 (16.0) | 16 (25.4) | 31 (39.2) |
| Hypertension | 49 (15.5) | 21 (12.0) | 13 (20.6) | 15 (19.0) |
| Other | 88 (27.8) | 65 (37.1) | 11 (17.5) | 12 (15.2) |
| Unknown | 105 (33.1) | 61 (34.9) | 23 (36.5) | 21 (26.6) |
| Any history of CV disease, n (%) | 20 (6) | 11 (6.3) | 2 (3.2) | 7 (8.9) |
| Diabetes, n (%) | 97 (31) | 35 (20) | 21 (33.3) | 41 (51.9) |
| LDL, mg/dl, mean (SD) | 109 (±39) | 106 (±35) | 101 (±42) | 116.4 (±43) |
| SBP, mm Hg, mean (SD) | 120 (±20) | 107 (±9) | 125 (±3) | 148 (±19) |
| DBP, mm Hg | 76.6 (13.5) | 69.1 (9.6) | 80.3 (8.2) | 90.4 (12.0) |
| BMI, kg/m2, mean (SD) | 30.5 (±8.4) | 29.3 (±7.8) | 31.6 (±7.6) | 32.1 (±9.9) |
| Number of antihypertensives, n (%) | ||||
| 0 | 61 (19.4) | 51 (29.1) | 5 (7.9) | 5 (6.5) |
| 1 | 96 (30.5) | 62 (35.4) | 21 (33.3) | 13 (16.9) |
| >1 | 158 (50.2) | 62 (35.4) | 37 (58.7) | 59 (76.6) |
| ACE/ARB, n (%) | 199 (63.2) | 108 (61.7) | 44 (69.8) | 47 (61.0) |
| Beta blocker, n (%) | 82 (26.0) | 33 (18.9) | 19 (30.2) | 30 (39.0) |
| Calcium channel inhibitor, n (%) | 77 (24.4) | 25 (14.3) | 22 (34.9) | 30 (39.0) |
| Diuretic, n (%) | 110 (34.9) | 42 (24.0) | 26 (41.3) | 42 (54.5) |
| eGFR, ml/min per 1.73 m2, mean (SD) | 51 (±19) | 55 (±19) | 48 (±16) | 46 (±19) |
| 24 h albumin-creatinine ratio, mg/g, median (IQR) | 335.2 (38.6–1396.1) | 115.8 (10.7–419.6) | 512.8 (141.4–1547.2) | 1774.3 (524.1–3497.5) |
All values listed as: mean (SD), median (interquartile range [IQR]), number (% of cohort/quartile). LDL, low density lipoprotein; BMI, body mass index.
Incidence Rates for CV Events and CKD Progression Across BP Groups
The median follow-up times for CV events and CKD progression were 11.3 and 4.1 years, respectively. The rates for CV events and CKD progression are illustrated in Figure 1 and Supplemental Table 1. Aside from CVA, the incidence rates were greater across higher baseline BP categories. Of the CV events, the highest rate was seen for HF; the rate was 28.2/1000 person-years (95% CI, 17.3 to 43.5) for participants with SBP ≥130 mm Hg. Participants with a baseline SBP ≥130 mm Hg had a higher incidence rate of CKD progression (incidence rate, 196.6/1000 person-years; 95% CI, 150.4 to 252.8) compared with those with an SBP 120–129 mm Hg and SBP <120 mm Hg.
Figure 1.
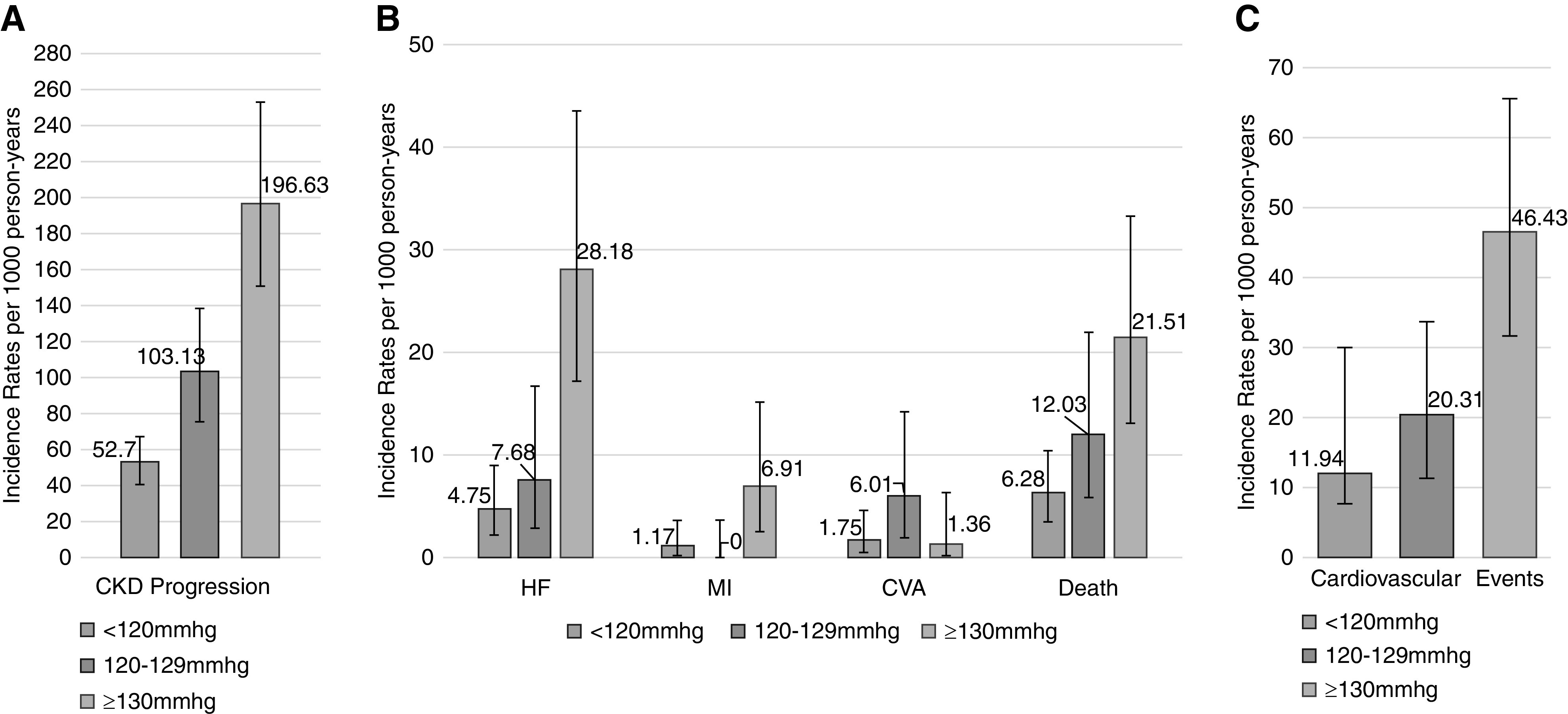
Incidence rates for CKD progression. Defined by (A) 50% decline in eGFR or ESKD, (B) CV outcomes, and (C) CV events, defined by first incidence of MI, congestive HF, stroke, or any-cause death, stratified by baseline BP group (per 1000 person-years).
Kaplan-Meier curves demonstrated those with an SBP ≥130 mm Hg had the lowest event-free survival of CV events and CKD progression, whereas those with a baseline SBP <120 mm Hg had the highest (Supplemental Figure 1).
Association of BP with CV Outcomes and Mortality
Using an unadjusted spline analysis, there was a positive linear relationship between higher SBPs and CV events (Figure 2A). In Cox models, every 10 mm Hg higher baseline SBP was associated with a 21% higher hazard for CV events in the unadjusted model (Table 2). This association was attenuated with adjustment. When using the exposure of baseline SBP category, the SBP ≥130 mm Hg group was significantly associated with CV events and death in the unadjusted and adjusted models compared with those with an SBP <120 mm Hg (adjusted HR, 2.13; 95% CI, 1.05 to 4.32).
Figure 2.
Log hazard spline by continuous baseline SBP values. (A) CV events defined by first incidence of MI, congestive HF, stroke, or any-cause death. (B) CKD progression defined by 50% decline in eGFR or ESKD.
Table 2.
Association between SBP and CV events (defined by first incidence of MI, congestive HF, stroke, or any-cause death using Cox proportional-hazards modeling
| Baseline SBP (Cohort n=317) | HR (95% CI) for Cardiovascular Events | ||
|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | |
| Per +10 mm Hg in SBP | 1.21 (1.10 to 1.33) | 1.18 (1.06 to 1.31) | 1.08 (0.93 to 1.24) |
| <120 mm Hg | 1.0 ref | 1.0 ref | 1.0 ref |
| 120–129mm Hg | 1.70 (0.85 to 3.43) | 1.59 (0.78 to 3.24) | 1.47 (0.70 to 3.11) |
| ≥130 mm Hg | 3.90 (2.20 to 6.90) | 3.52 (1.91 to 6.48) | 2.13 (1.05 to 4.32) |
Model 1 adjusted for age, race/ethnicity, and eGFR. Model 2 adjusted for Model 1+ diabetes, urine albumin to creatinine ratio, and history of CV disease including HF, MI, or stroke.
Association of BP with CKD Progression
Of the 161 participants classified as having CKD progression, eight met criteria by ESKD and 153 met criteria by having a 50% decrease in eGFR. By conclusion of the follow-up period, 134 participants had progressed to ESKD (Supplemental Figure 2). There was a positive linear relationship between higher SBPs and CKD progression (Figure 2B) in spline analyses. In Cox models, every 10 mm Hg higher baseline SBP was associated significantly with CKD progression in unadjusted and adjusted models (adjusted HR, 1.12; 95% CI, 1.02 to 1.24). Across SBP categories, in unadjusted models, both 120–129 mm Hg and ≥130 mm Hg SBP groups were associated with greater risk of CKD progression, as compared with participants with SBP <120 mm Hg. Only the ≥130 mm Hg baseline SBP group was significantly associated with CKD progression in the adjusted models (HR, 1.68; 95% CI, 1.10 to 2.58) (Table 3). In a sensitivity analysis, the association between baseline SBP with CKD progression was similar in Fine-Gray weighted models accounting for competing risk of death (adjusted HR baseline SBP ≥130, 1.68; 95% CI, 1.10 to 2.58).
Table 3.
Association between BP and CKD progression (defined by 50% decline in eGFR or ESKD) using Cox proportional-hazards modeling
| Baseline SBP Variables (Cohort n=317) | HR (95% CI) for CKD Progression | ||
|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | |
| Per +10 mm Hg in SBP | 1.36 (1.26 to 1.46) | 1.27 (1.17 to 1.38) | 1.12 (1.02 to 1.24) |
| <120 mm Hg | 1.0 ref | 1.0 ref | 1.0 ref |
| 120–129 mm Hg | 1.94 (1.31 to 2.87) | 1.64 (1.10 to 2.45) | 1.20 (0.78 to 1.86) |
| ≥130mm Hg | 3.68 (2.56 to 5.29) | 2.63 (1.80 to 3.83) | 1.68 (1.10 to 2.58) |
Model 1 adjusted for age, race/ethnicity, and eGFR. Model 2 adjusted for Model 1+ diabetes, urine albumin-creatinine ratio, and history of CV disease including HF, MI, or stroke.
In a secondary analysis, we found a significant association between higher SBP and decline in eGFR modeling continuously. In unadjusted models, every 10 mm Hg higher SBP above 120 mm Hg was associated with an annual decline of 0.8% (95% CI, 0.6 to 1.0) (Supplemental Table 2). In adjusted models, this association remained statistically significant; every 10 mm Hg higher SBP was associated with an annual decline of 0.9% (95% CI, 0.6 to 1.1) in eGFR.
Changes in SBP Over Time and Associations with CV Events and CKD Progression
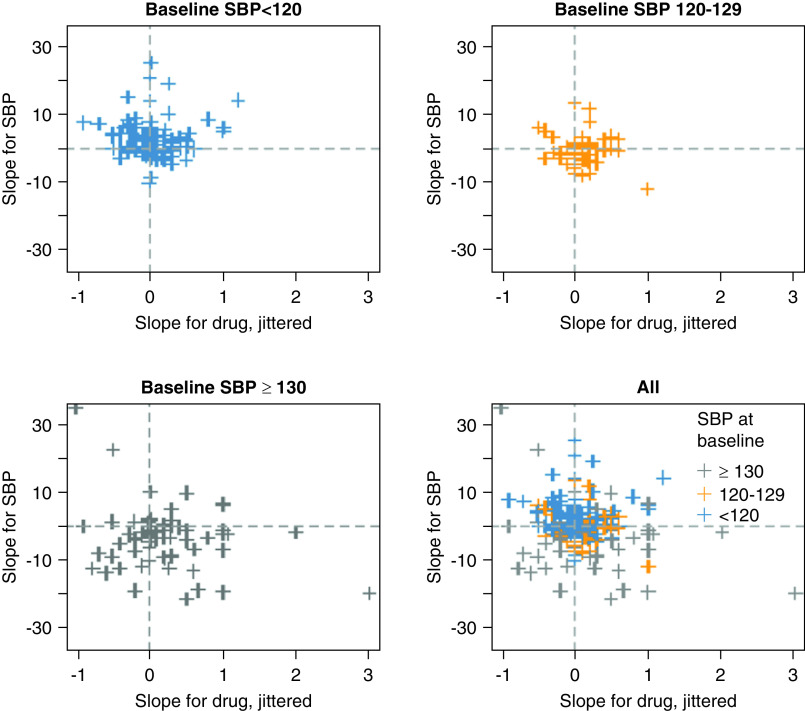
Changes in SBP relative to changes in antihypertensive use were plotted for each participant and are presented in Figure 3. The relationship was heterogenous overall as evidenced by the bottom-right scatterplot. Subjectively, participants with a baseline SBP ≥130 mm Hg had a negative SBP slope over 4 years, and there was less change in SBP over time in participants with baseline SBP <120 mm Hg or 120–129 mm Hg.
Figure 3.
Scatterplots, stratified by baseline BP category, comparing linear trend for change in SBP and number of antihypertensives over 4 years on a participant level.
As a sensitivity analysis, we examined the association of time-updated SBP with outcome measures (Supplemental Table 3). In adjusted models (including adjustment for time-updated eGFR), every 10 mm Hg higher time updated SBP was associated with higher risk of CV events (HR, 1.16; 95% CI, 1.03 to 1.31) and CKD progression (HR, 1.21; 95% CI, 1.10 to 1.33); these findings were stronger than those observed for baseline SBP.
Sensitivity Analyses: Accounting for Antihypertensive Medication Use
Sensitivity analyses adding the number of baseline antihypertensive medications as a covariable to the fully adjusted models did not change the association between baseline SBP with CV events and CKD progression (Supplemental Table 4). When we repeated our analyses only using study participants who had use of ≥1 antihypertensive medication at baseline (n=256), the results were similar to our primary analysis of all participants (Supplemental Table 5).
Secondary Analysis: Association between DBP with CV Events and CKD Progression
The mean baseline DBP was 77 mm Hg, and 14% of participants had a DBP ≥90 mm Hg. Supplemental Figure 3 depicts the functional form of the association between baseline DBP with CV events and CKD progression, respectively, and demonstrates a linear positive association of higher DBP with risk of CV events and CKD progression. In adjusted models, baseline DBP on a continuous scale was not associated with CV events or CKD progression. Likewise, in adjusted models, a baseline DBP ≥90 mm Hg compared with <90 mm Hg was not significantly associated with CV events or CKD progression Supplemental Table 6.
Discussion
In this study of 317 young-adult CKD participants aged 21–40 years old, we observed a graded, linear association of higher SBP, with a greater risk of CV events and faster CKD progression. Among those with a baseline SBP ≥130 mm Hg, 3%/yr developed HF, 20%/yr had CKD progression, and 2%/yr died. In adjusted models, a baseline SBP ≥130 mm Hg was associated with two- to three-fold higher risk of CV events and CKD progression. Moreover, there appeared to be a stronger association when time-updated SBP was used as the exposure. The results from this study contribute to our understanding of the association of BP and risk of CV disease and CKD progression in the young-adult patient with CKD, on whom existing literature is sparse.
Our description of the association between higher BPs and increased risk of CV events is consistent with existing studies of older adults with CKD.3,22–24 In a study of the African American Study of Kidney Disease and Hypertension (AASK) trial, higher baseline clinic SBP, and SBP by 24-hour ambulatory monitor, were associated with CV events.25 In a subgroup analysis of the 2646 participants with CKD included in the Systolic Blood Pressure Intervention (SPRINT) study, there was a significant decrease in CV events in those randomized to intensive SBP management (<120 mm Hg) compared with those with a standard SBP goal (<140 mm Hg).22 The findings from these studies are similar to our observation that high SBP was associated with increased risk for CV events. However, the average age of African American Study of Kidney Disease and Hypertension trial participants was 60 years, and the SPRINT trial only included individuals >50 years. Our study provides information on a population largely excluded from previous studies of BP and CVD in patients with CKD, and thus, addresses a knowledge gap in the literature. Moving forward, inclusion of young adults with CKD in clinical trials of BP management will be important to identify optimal treatment for hypertension, because it may differ from older patients with CKD.
Of the subtypes of CV disease, we found that the rates of HF were highest for young adults with CKD, particularly for those with a baseline SBP ≥130 mm Hg. Directly comparable data in young adults from the general population is limited. One analysis of 3667 young adults from the Coronary Artery Risk Development in Adolescents study found an association between cumulative BP and risk for HF in middle age.26 A recent analysis of the Study of High Blood Pressure in Pediatrics: Adult Hypertension Onset in Youth study (participants were adolescents with prehypertension and without CKD) demonstrated that BP levels below those considered hypertensive were associated with increased left ventricular mass index and cardiac remodeling.27 The SPRINT trial found that BP lowering led to the greatest reduction in risk of HF (compared with other CV events) in older adults.28 Additionally, our findings share many similarities with the pediatric CKD literature. Several studies within the CKD in Children cohort demonstrated an association between higher SBP with left ventricular hypertrophy in children with mild to moderate CKD.29–31 Efforts to reduce BP in young adults with CKD may have beneficial effects on lifelong risk of HF and HF complications.
We found a significant association between higher baseline SBP and CKD progression, both when CKD progression was defined as a 50% decline in eGFR/ESKD and as continuous decline in eGFR. The association between SBP and CKD progression has been observed in several studies of pediatric patients with CKD.31–34 The Strict Blood-Pressure Control and Progression of Renal Failure in Children trial found that intensive BP control, defined as 24-hour mean arterial pressure <50th percentile on 24-hour ambulatory BP monitoring, resulted in a significant reduction in rate of eGFR decline in pediatric patients with CKD.35 Extrapolating these findings to young adults, along with our observation of a linear relationship between baseline SBP and outcomes, leads us to hypothesize that BP targets for young adults may be different and/or lower than 130 mm Hg; further trials are needed to identify specific BP targets in this population.
The relationship between BP and eGFR decline in the adult CKD literature is more uncertain. Lower BP has been associated with reduced eGFR decline in some observational trials.36 A subgroup analysis of participants with CKD enrolled in the SPRINT trial, suggested that intensive BP control may be associated with declining kidney function.22 One possibility is the physiologic environment of the young-adult kidney is more akin to that of a pediatric patient than an older adult, whose physiology may reflect a lifetime of vascular injury and maladaptation. Observational data suggest the association between SBP and incident ESKD varies over a lifetime.37
We were able to gather some insights about use of antihypertensive medications in this population. No distinct trend was apparent to describe the relationship between changes in antihypertensive medications and SBP slope. We did not find that adjusting for antihypertensive medications or excluding participants not taking antihypertensive medications affected our findings.
We noted a stronger association between SBP with CV events and CKD progression when using time-updated, rather than baseline, values. This is similar to a recent study of the CKD in Children cohort that identified an association between time-updated SBP and CKD progression.32 Additionally, compared with baseline SBP, time-updated SBP was found to have a stronger association with CKD progression in a study of the entire CRIC cohort.36 Our findings suggest frequent annual monitoring of BP in young adults with CKD may be important. More research is needed to address the complexity, and possible shortcomings, inherent with the use of time-updated models before any definitive interpretation can be made.38
We also observed some characteristics that may be unique to young adults. The functional form of the association between DBP and CV events appeared linear. This is in contrast to studies in primarily older adults with CKD that have found a U- or J-shaped association.39,40 Although in fully adjusted models, higher baseline DBP was not significantly associated with CV events or CKD progression. This is comparable to prior studies in patients with CKD.19,41,42
Understanding how young adults with CKD fit into existing paradigms established by research in pediatric and older adults is challenging. In pediatric patients with CKD, optimal BP management for a patient aged <18 years is age, sex, and height dependent.43 In contrast, for patients ≥18 years of age, the BP goal consists of remaining below a single set value regardless of those factors.17,43 More research is needed to best understand the relationship between BP and outcomes as it pertains to young adults rather than to apply results of studies conducted in other populations to this distinct subgroup. This approach is consistent with recent efforts for precision medicine in nephrology. By themselves, the results of our analysis are insufficient to support changing BP targets for young adults with CKD. Nevertheless, our findings may form the basis of future trials to test specific SBP targets (and potentially even lower SBP targets) and approaches to treatment of elevated SBP. Lastly, more research is needed to further understand how the association between BP with CV events and CKD progression varies by age.
Relevant strengths of our analysis include using participants from a large, well-characterized CKD cohort. All BP data were collected using standardized procedures, reducing the risk for measurement error. Identification of CVD outcomes was protocolized and were adjudicated by two, independent physician reviews. All eGFR measures were obtained at annual research study visits and not for clinical purposes. However, we recognize some limitations as well. The limited number of participants, low absolute incidence rates, and relatively short duration of follow-up may have reduced the power of our analysis to identify a significant association between SBP and CV events. We were not able to ascertain the cause of CKD in the majority of this population. We attempted to minimize confounding by using potentially relevant covariables; however, as with all observational studies, we could not eliminate the risk for unmeasured confounding. This was an observational study, so causality cannot be determined; we note that causality is particularly challenging to understand in terms of SBP and CKD progression. Nonetheless, adjusting for time-updated eGFR did not reduce the significance of the relationship between higher SBP and CKD progression. Due to data limitations, we were unable to ascertain duration of antihypertensive use before baseline. Finally, the study sample consisted of research volunteers closely followed in nephrology clinics, possibly limiting the generalizability of our results to the broader CKD population.
This observational study of young adults with CKD provides novel data of the strong, linear association between SBP with CV disease and CKD progression. As we progress into an era of precision medicine, applying treatment strategies to young adults developed in primarily populations who are not young adults is not ideal. The assumed longevity of the participants in our study, along with the relatively high rate of adverse outcomes associated with higher SBP, emphasizes the risk of suboptimal treatment might result in a significant personal and societal burden. The results from this study may provide insights into future work, including clinical trials and best practices for BP management in young-adults with CKD.
Disclosures
J. Flynn reports receiving personal fees from Silvergate Pharmaceuticals, Springer, Up to Date and grant support from the American Heart Association and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) unrelated to this work; reports being a scientific advisor or member as Editor-in-Chief of Pediatric Nephrology, editorial board member of the Journal of Pediatrics, BP Monitoring, Hypertension, and board member of Renal Physicians Association. N. Bansal reports being a scientific advisor or membership with CJASN and American Journal of Kidney Diseases. All remaining authors have nothing to disclose.
Funding
This study was supported by the National Institutes of Health grants R01 DK103612 (N. Bansal) and T32DK997662 (Hingorani) and Seattle Children’s Hospital/University of Washington. The CRIC study was conducted by the CRIC Study Investigators and supported by the NIDDK.
Data Sharing Statement
All data and materials have been made publicly available at the NIDDK Central Repository and can be accessed through request at https://repository.niddk.nih.gov/studies/cric/.
Supplementary Material
Acknowledgments
The data from the CRIC reported here were supplied by the NIDDK Central Repositories. This manuscript was not prepared in collaboration with the investigators of the CRIC study and does not necessarily reflect the opinions or views of the CRIC study, the NIDDK Central Repositories, or the NIDDK. Dr. Alexander J. Kula and Dr. Nisha Bansal designed the study, Dr. Alexander J. Kula and Dr. David K. Prince performed study analysis, Dr. Alexander J. Kula, Dr. David K. Prince, Dr. Joseph T. Flynn, and Dr. Nisha Bansal analyzed study results, Dr. Alexander J. Kula and Dr. David K. Prince made all tables and figures, Dr. Alexander J. Kula, Dr. David K. Prince, Dr. Joseph T. Flynn, and Dr. Nisha Bansal drafted and revised the paper. All authors approved the final version of this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020081156/-/DCSupplemental.
Supplemental Table 1. Incidence rates of cardiovascular events and CKD progression by baseline BP category.
Supplemental Table 2. Association of baseline SBP with continuous eGFR slope using linear mixed models. eGFR slope represents yearly percent change in eGFR relative to baseline.
Supplemental Table 3. Association between time-updated BP and cardiovascular events and CKD progression using Cox proportional-hazards modeling.
Supplemental Table 4. Sensitivity analysis adding number of antihypertensive medications at baseline to the fully adjusted models describing the association of baseline SBP with CV events and CKD progression using Cox proportional-hazards modeling.
Supplemental Table 5. Association between baseline SBP with CV events and CKD progression, excluding participants without antihypertensive medication use at baseline
Supplemental Table 6. Association between DBP with cardiovascular (CV) events (defined by first incidence of MI, congestive HF, stroke, or any-cause death) and CKD progression (defined by 50% decline in eGFR or ESKD) using Cox proportional-hazards modeling.
Supplemental Figure 1. Survival curves for event-free survival from CV events or CKD progression stratified by baseline BP category.
Supplemental Figure 2. Time to CKD progression (50% decline in GFR) and ESKD for participants observed to have both during follow-up period (n=134).
Supplemental Figure 3. Unadjusted log hazard spline by continuous baseline DBP values. Cardiovascular events (A) defined by first incidence of MI, congestive HF, stroke, or any-cause death. CKD progression (B) defined by 50% decline in eGFR or ESKD.
References
- 1. Palit S, Chonchol M, Cheung AK, Kaufman J, Smits G, Kendrick J: Association of BP with death, cardiovascular events, and progression to chronic dialysis in patients with advanced kidney Disease. Clin J Am Soc Nephrol 10: 934–940, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bansal N, McCulloch CE, Rahman M, Kusek JW, Anderson AH, Xie D, et al.; CRIC Study Investigators: Blood pressure and risk of all-cause mortality in advanced chronic kidney disease and hemodialysis: The chronic renal insufficiency cohort study. Hypertension 65: 93–100, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aggarwal R, Petrie B, Bala W, Chiu N: Mortality outcomes with intensive blood pressure targets in chronic kidney disease patients. Hypertension 73: 1275–1282, 2019. [DOI] [PubMed] [Google Scholar]
- 4. Rosner B, Cook NR, Daniels S, Falkner B: Childhood blood pressure trends and risk factors for high blood pressure: The NHANES experience 1988-2008. Hypertension 62: 247–254, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al.: Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA 315: 2292–2299, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al.; SEARCH for Diabetes in Youth Study: Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 376: 1419–1429, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allen NB, Siddique J, Wilkins JT, Shay C, Lewis CE, Goff DC, et al.: Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA 311: 490–497, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yano Y, Reis JP, Colangelo LA, Shimbo D, Viera AJ, Allen NB, et al.: Association of blood pressure classification in young adults using the 2017 American college of cardiology/American heart association blood pressure guideline with cardiovascular events later in life. JAMA 320: 1774–1782, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kishi S, Teixido-Tura G, Ning H, Venkatesh BA, Wu C, Almeida A, et al.: Cumulative blood pressure in early adulthood and cardiac Dysfunction in middle age: The CARDIA study. J Am Coll Cardiol 65: 2679–2687, 2015. [DOI] [PubMed] [Google Scholar]
- 10. Lee H, Yano Y, Cho SMJ, Park JH, Park S, Lloyd-Jones DM, et al.: Cardiovascular risk of isolated systolic or diastolic hypertension in young adults. Circulation 141: 1778–1786, 2020. [DOI] [PubMed] [Google Scholar]
- 11. Zheng W, Mu J, Chu C, Hu J, Yan Y, Ma Q, et al.: Association of blood pressure trajectories in early life with subclinical renal damage in middle age. J Am Soc Nephrol 29: 2835–2846, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leiba A, Fishman B, Twig G, Gilad D, Derazne E, Shamiss A, et al.: Association of adolescent hypertension with future end-stage renal disease. JAMA Intern Med 179: 517–523, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blowey DL, Flynn JT, Warady BA: Are there consequences of adolescent blood pressure on kidney function in adulthood? Am J Kidney Dis 74: 567–569, 2019. [DOI] [PubMed] [Google Scholar]
- 14. Hallan SI, Matsushita K, Sang Y, Mahmoodi BK, Black C, Ishani A, et al.; Chronic Kidney Disease Prognosis Consortium: Age and association of kidney measures with mortality and end-stage renal disease. JAMA 308: 2349–2360, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, et al.; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: The Chronic Renal Insufficiency Cohort (CRIC) study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003. [DOI] [PubMed] [Google Scholar]
- 16. Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, et al.; Chronic Renal Insufficiency Cohort (CRIC) Study Group: Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Himmelfarb C, et al.: 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines [published correction appears in J Am Coll Cardiol 71: 2275–2279, 2018 10.1016/j.jacc.2018.03.016]. J Am Coll Cardiol 71: e127–e248, 2018. 29146535 [Google Scholar]
- 18. Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, et al. ; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: Hypertension awareness, treatment, and control in adults with CKD: Results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 55: 441–451, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bansal N, McCulloch CE, Lin F, Alper A, Anderson AH, Cuevas M, et al.: Blood pressure and risk of cardiovascular events in patients on chronic hemodialysis: The CRIC study (Chronic Renal Insufficiency Cohort). Hypertension 70: 435–443, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mills KT, Chen J, Yang W, Appel LJ, Kusek JW, Alper A, et al.; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney Disease. JAMA 315: 2200–2210, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheung AK, Rahman M, Reboussin DM, Craven TE, Greene T, Kimmel PL, et al.; SPRINT Research Group: Effects of intensive BP control in CKD. J Am Soc Nephrol 28: 2812–2823, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheung AK, Chang TI, Cushman WC, Furth SL, Ix JH, Pecoits-Filho R, et al.; Conference Participants: Blood pressure in chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 95: 1027–1036, 2019. [DOI] [PubMed] [Google Scholar]
- 24. Malhotra R, Nguyen HA, Benavente O, Mete M, Howard BV, Mant J, et al.: Association between more intensive vs less intensive blood pressure lowering and risk of mortality in chronic kidney disease stages 3 to 5: A systematic review and meta-analysis. JAMA Intern Med 177: 1498–1505, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gabbai FB, Rahman M, Hu B, Appel LJ, Charleston J, Contreras G, et al.; African American Study of Kidney Disease and Hypertension (AASK) Study Group: Relationship between ambulatory BP and clinical outcomes in patients with hypertensive CKD. Clin J Am Soc Nephrol 7: 1770–1776, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nwabuo CC, Appiah D, Moreira HT, Vasconcellos HD, Yano Y, Reis JP, et al.: Long-term cumulative blood pressure in young adults and incident heart failure, coronary heart disease, stroke, and cardiovascular disease: The CARDIA study. Eur J Prev Cardiol, 2020. 10.1177/2047487320915342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Urbina EM, Mendizábal B, Becker RC, Daniels SR, Falkner BE, Hamdani G, et al.: Association of blood pressure level with left ventricular mass in adolescents. Hypertension 74: 590–596, 2019. [DOI] [PubMed] [Google Scholar]
- 28. Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al.; SPRINT Research Group: A randomized trial of intensive versus standard blood-pressure control [published correction appears in N Engl J Med 377: 2506, 2017 10.1056/NEJMx170008]. N Engl J Med 373: 2103–2116, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sinha MD, Tibby SM, Rasmussen P, Rawlins D, Turner C, Dalton RN, et al.: Blood pressure control and left ventricular mass in children with chronic kidney disease. Clin J Am Soc Nephrol 6: 543–551, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mitsnefes M, Flynn J, Cohn S, Samuels J, Blydt-Hansen T, Saland J, et al.; CKiD Study Group: Masked hypertension associates with left ventricular hypertrophy in children with CKD. J Am Soc Nephrol 21: 137–144, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilson AC, Flynn JT: Blood pressure in children with chronic kidney disease: Lessons learned from the Chronic Kidney Disease in Children cohort study. Pediatr Nephrol 35: 1203–1209, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reynolds BC, Roem JL, Ng DKS, Matsuda-Abedini M, Flynn JT, Furth SL, et al.: Association of time-varying blood pressure with chronic kidney disease progression in children. JAMA Netw Open 3: e1921213, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fathallah-Shaykh SA, Flynn JT, Pierce CB, Abraham AG, Blydt-Hansen TD, Massengill SF, et al.: Progression of pediatric CKD of nonglomerular origin in the CKiD cohort. Clin J Am Soc Nephrol 10: 571–577, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ku E, McCulloch CE, Warady BA, Furth SL, Grimes BA, Mitsnefes MM: Twenty-four-hour ambulatory blood pressure versus clinic blood pressure measurements and risk of adverse outcomes in children with CKD. Clin J Am Soc Nephrol 13: 422–428, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, et al.; ESCAPE Trial Group: Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361: 1639–1650, 2009. [DOI] [PubMed] [Google Scholar]
- 36. Anderson AH, Yang W, Townsend RR, Pan Q, Chertow GM, Kusek JW, et al.; Chronic Renal Insufficiency Cohort Study Investigators: Time-updated systolic blood pressure and the progression of chronic kidney disease: A cohort study. Ann Intern Med 162: 258–265, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kovesdy CP, Alrifai A, Gosmanova EO, Lu JL, Canada RB, Wall BM, et al.: Age and outcomes associated with BP in patients with incident CKD. Clin J Am Soc Nephrol 11: 821–831, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xie D, Yang W, Jepson C, Roy J, Hsu JY, Shou H, et al.; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: Statistical methods for modeling time-updated exposures in cohort studies of chronic kidney disease. Clin J Am Soc Nephrol 12: 1892–1899, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rifkin DE, Katz R, Chonchol M, Shlipak MG, Sarnak MJ, Fried LF, et al.: Blood pressure components and decline in kidney function in community-living older adults: The Cardiovascular Health Study. Am J Hypertens 26: 1037–1044, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Navaneethan SD, Schold JD, Jolly SE, Arrigain S, Blum MF, Winkelmayer WC, et al. : Blood pressure parameters are associated with all-cause and cause-specific mortality in chronic kidney disease. Kidney Int 92: 1272–1281, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haider AW, Larson MG, Franklin SS, Levy D; Framingham Heart Study: Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med 138: 10–16, 2003. [DOI] [PubMed] [Google Scholar]
- 42. Lee TC, Cavalcanti RB, McDonald EG, Pilote L, Brophy JM: Diastolic hypotension may attenuate benefits from intensive systolic targets: Secondary analysis of a randomized controlled trial. Am J Med 131: 1228–1233.e1, 2018. [DOI] [PubMed] [Google Scholar]
- 43. Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al.; Subcommittee on Screening and Management of High Blood Pressure in Children: Clinical practice guideline for screening and management of high blood pressure in children and adolescents [published correction appears in Pediatrics 140: e20173035, 2017 10.1542/peds.2017-3035]. Pediatrics 140: e20171904, 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.