Abstract
Purpose
This Stay Active after Rehabilitation (STAR) study examined the effects of a pedometer-based behavioral intervention for individuals with COPD during three weeks of inpatient pulmonary rehabilitation (PR) on patients’ physical activity levels six weeks and six months after PR, including steps (primary outcome), moderate-intensity physical activity, and sedentary time as well as patient quality of life, symptoms, and other psychological and clinical variables.
Patients and Methods
Rehabilitation patients with COPD wore a triaxial accelerometer (ActiGraph wGT3X) for seven days two weeks before (T0) as well as six weeks (T3) and six months (T4) after PR. In addition to the three-week inpatient PR (control group, CG), the randomly allocated intervention group (IG) received a brief pedometer-based behavioral intervention with the application of the following behavior-change techniques: performing the behavior, individual goal-setting, self-monitoring, and feedback. The effects were analyzed using analysis of covariance with an intention-to-treat approach.
Results
A total of 327 patients (69% male, age: 58 years, FEV1 (%): 53.5, six-minute walk distance: 447.8 m) were randomly allocated to either the IG (n = 167) or CG (n = 160). Although both groups increased their daily steps after PR (IG: MT3-T0 = 1152, CG: MT3-T0 = 745; IG: MT4-T0 = 795, CG: MT4-T0 = 300), the slightly higher increases in daily steps in the IG compared to the CG at T3 (Δ388 steps, d = 0.16) and T4 (Δ458 steps, d = 0.15) were not statistically significant (p > 0.05 for all). Patients in both groups showed moderate to high pre-post-changes in terms of secondary outcomes, but no advantage favoring the IG was found.
Conclusion
The results show that adding a pedometer-based behavioral intervention to standard German three-week inpatient PR for COPD patients did not result in more physical activity in terms of steps and moderate-intensity physical activity or less sedentary time. However, both groups (IG and CG) showed remarkably enhanced physical activity levels six weeks and six months after PR, as well as improvements in other secondary outcomes (eg, quality of life).
Keywords: physical activity, exercise, physical activity promotion, behavior change, long-term follow-up
Plain Language Summary
Pulmonary rehabilitation programs aim to increase the physical activity levels in COPD patients, as low physical activity is related to poor health outcomes (eg, higher mortality rates). However, patients with COPD often find it difficult to stay physically active after rehabilitation. This study examines whether adding a pedometer to German inpatient rehabilitation programs helps COPD patients stay active after completing the rehabilitation period. Pedometers are small, lightweight, portable devices that measure the user’s number of steps; pedometers are useful in providing feedback to the user on activity levels and they help users set daily goals for being physically active (eg, 10,000 steps a day). In this study, all COPD rehabilitants—those with pedometers and those without pedometers—showed a significant increase both in their daily steps and their moderate-intensity physical activity in the six weeks and six months of follow-up. Adding the pedometer to the three-week inpatient rehabilitation program did not result in any additional physical activity six weeks or six months after the rehabilitation program.
Introduction
Physical activity (PA) plays a crucial role in the treatment of non-communicable diseases,1 especially in chronic obstructive pulmonary disease (COPD). Clinical evidence demonstrates that, in COPD patients, PA leads to substantial health effects, including increased exercise capacity,2 reduced dyspnoea,3 improved quality of life,4 and lower mortality rates.5 Nevertheless, patients with COPD often show significantly reduced PA levels when compared to healthy controls or even with other patients with non-communicable diseases.6 Accordingly, the promotion of PA is a key therapeutic goal in COPD patients in general and in pulmonary rehabilitation (PR).7 PR provides proven short-term benefits in terms of exercise capacity, dyspnea, anxiety, depression, and quality of life;8 although, in individuals with COPD, PR programs often fail to substantially enhance long-term PA.9,10 Therefore, for optimized patient treatment during PR, effective strategies to improve PA are needed.10
Despite a wide interest among scientists and practitioners in interventions and strategies to promote PA in COPD patients,11,12 Burge et al13 conclude that there is limited evidence for improvements in PA resulting from these strategies, which include exercise training, counselling, and pharmacological treatment. In general, the use of a pedometer to monitor one’s PA is regarded as one of the most effective intervention methods for promoting PA.14 Pedometers are small, lightweight, portable devices that measure the user’s number of steps; they are particularly effective when combined with specific behavior change techniques (BCTs), such as documenting one’s PA behavior, setting PA goals, and monitoring PA changes.15–17 In a recent meta-analysis specific to COPD patients, pedometer-based interventions achieved similar PA promotion effects when used either as stand-alone interventions or in addition to PR.16 Adding pedometers to PR resulted in heterogeneous effects, with an average PA increase of approximately 2,050 steps per day, whereas standard PR achieved only a small PA increase of approximately 300 steps per day (d = 0.51).16 All seven primary studies included in this review were conducted in the setting of outpatient rehabilitation. This means that the patients were not hospitalized overnight but visited a hospital, clinic, or associated facility for treatment, and rehabilitation was applied over a relatively long duration (8–52 weeks). In contrast, in Germany, PR is usually delivered as an inpatient treatment, which means that patients stay overnight in a specialized rehabilitation clinic for a significantly shorter intervention period (three to four weeks) where they receive lodging, food, and treatment.18 To date, no findings are available on the long-term effects on COPD patients’ PA behavior after the application of pedometer-based interventions during German inpatient PR.
Purpose of This Study and Hypothesis
The overarching goal of the Stay Active after Rehabilitation (STAR) study (Trial Registry: Clinicaltrials.gov; ID: NCT02966561) was to gain a better understanding of PA in patients with COPD before and after PR.19 The pre-specified main research question examined whether the integration of a pedometer-based intervention in inpatient PR for COPD patients leads to a sustained improvement in PA levels six weeks and six months after PR.19 Our primary hypothesis was that, in patients with COPD, inpatient PR (standard rehabilitation in Germany) along with a pedometer-based behavior-change intervention would result in significantly higher PA levels (eg, a greater number of steps, more moderate-intensity PA, less sedentary time) six weeks and six months after rehabilitation when compared with inpatient PR along with an equal duration of PA-related patient education.
Materials and Methods
The STAR study was conducted in accordance with the Declaration of Helsinki, and all subjects provided written informed consent. The ethics commission of the University of Erlangen-Nürnberg approved the study in 2015 (Re. No. 321_15B). The study protocol was registered at Clinicaltrials.gov in November 2016 (ID: NCT02966561) and published in 2017.19
Study Design and Treatments
The STAR study took place within the German rehabilitation system. In Germany, all insured persons can apply for rehabilitation. Taking into account the assessment of the individuals’ general practitioner, an expert decided whether this application could be approved. The approved measures are usually carried out through an three-week inpatient rehabilitation program at a specialized clinic.
The study used a single-center, randomized controlled trial (RCT) design with two parallel groups and five measurement points: T0 = two weeks before the start of rehabilitation, T1 = the start of rehabilitation, T2 = the end of rehabilitation, T3 = six weeks after rehabilitation, and T4 = six months after rehabilitation.
All of the study participants in both the intervention group (IG) and control group (CG) received an intensive, interprofessional three-week inpatient PR (standard care; see the study protocol for details on the intervention components).19 This rehabilitation program followed current international and national guidelines for the diagnosis, therapy, and rehabilitation of COPD patients.7,20 In addition to the standard rehabilitation program, the IG received a pedometer-based PA promotion intervention (two 45-minute sessions), while the CG received a revision of PA-related patient education of an equal duration with no pedometer. For both groups, the first lesson was situated at the end of the second week of PR, and the second lesson took place in the middle of the third week. At the beginning of their PR, the IG participants received a pedometer, which they were advised to wear throughout the PR and take home afterward. The intervention for the IG used the following behavior change techniques (BCTs), which are numbered according to Michie et al21 shaping knowledge (instructions on how to perform the behavior [4.1]), goals and planning (individual goal-setting for PA behavior [1.1]), feedback and monitoring (self-monitoring of one’s PA behavior using a pedometer [2.3] and documentation of one’s PA in a diary [2.3]), and feedback on PA behavior (2.2) during PR. The detailed procedure of the IG behavioral intervention is described in Supplementary Table S6. Only the CG received repeated information regarding PA during patient education sessions and warm-up exercises.
Patients were assigned to the two arms after T0 using a central random allocation sequence. The randomization, which drew on a randomization list provided externally by researchers at the University of Erlangen-Nürnberg, was carried out by a study assistant after the initial medical examination. The block size for the randomization was six participants per block. The randomization was stratified according to the four prognostic dichotomous variables (gender: male vs female; COPD stage: GOLD stage 1–2 vs stage 3–4; age: < 60 years vs ≥ 60 years; and type of rehabilitation: PR in patients with stable COPD vs PR after acute care hospitalization due to COPD).
The participants were blinded in relation to the study group allocations. They were informed that the effectiveness of the two exercise therapy programs was compared, that both programs met the current scientific standards, and that they were suitable for improving the health status of COPD patients. Blinding of the therapists was not feasible due to their involvement in the same clinical rehabilitation team.
Eligibility Criteria
We recruited COPD patients with an approved three-week inpatient PR and an assignment to start their rehabilitation at the Rehabilitation Clinic of Bad Reichenhall, Germany. We included patients with COPD, that is, all GOLD symptoms and risk groups A–D or all COPD stages 1–4.22 The diagnosis of COPD, which had initially been made by a medical practitioner in each patient’s hometown, was confirmed by a pulmonologist from the rehabilitation clinic at the start of the PR; this confirmation included a lung function test (Tiffeneau index FEV1/VC after short-acting beta agonists (SABA) ≤ 0.70).
The exclusion criteria included serious comorbidities, for example, malignancies that were not curatively treated or severe cardiac, orthopedic, or severe psychiatric comorbidities; a considerable reduction in vision or hearing; and the inability to speak German. At the beginning of the PR, the admitting physician checked the inclusion and exclusion criteria.
Outcomes and Assessments
The primary outcome was the difference in the number of objectively measured daily steps between the IG and CG at T4. The secondary outcomes related to PA were the differences between groups in steps at T3, as well as moderate-intensity PA and sedentary time at T3 and T4. Furthermore, differences between IG and CG in terms of other secondary outcomes (see below) were also examined. In addition, changes in all outcomes from T0 to T3 and T4 (or T1 to T2 and T4) were examined in both groups.
For all three measurement points, the PA of the patients was measured on seven consecutive days using the validated tri-axial accelerometer ActiGraph (Pensacola, Florida) wGT3X-BT, which is explicitly recommended for COPD.23–25 Based on the raw accelerometer output, we calculated the number of participants’ daily steps, the amount of time spent in moderate-intensity PA, and the amount of time spent sedentary. The detailed method used for validating and processing the accelerometers’ raw data and calculating light-intensity and moderate-intensity PA was described previously.26
In addition, the following secondary outcomes were assessed: quality of life via the Saint George’s Respiratory Questionnaire (SGRQ)27 and the COPD Assessment Test (CAT),28 depression using the Patient Health Questionnaire (PHQ-9),29 COPD-related anxiety,30 fear avoidance in COPD31 and dyspnea (numerical rating scale).19,32 Furthermore, demographic characteristics (eg, sex, age, height, weight, and work-status), 6 Minute Walk Distance (6MWD),33 acute exacerbations of COPD (AECOPD), smoking status, diagnosis and co-morbidities assessed at T1 will be reported.
Statistical Analysis
Sample size calculations with dropout rate estimates revealed that 502 patients needed to be recruited to provide 351 patients; this number of patients will statistically support a small effect size of d = 0.3 with an alpha error of 5% and a power of 80%.19 To analyze the differences between the IG and CG at T3 and T4, we performed multiple analyses of covariances (ANCOVAs) for each outcome and measurement occasion (T3, T4) separately. Group assignment (IG vs CG) was included as a predictor variable, and gender, age, progression of COPD (GOLD stage 1–2 vs stage 3–4), type of rehabilitation (PR in patients with stable COPD vs PR after hospitalization), and the baseline value of the respective outcomes were included as covariates. The adjusted mean differences (AMD), including a 95% confidence interval and the effect size Cohen’s d, were also reported.34 We had examined the distributions of the residuals (after ANCOVA) graphically with respect to violations of the normal distribution, violations of heteroscedasticity, and the influence of outliers.
In addition, the mean values (M) and standard deviations (SD) for the IG and CG were reported for all measurement points. To estimate the changes during PR, the mean changes between the baseline and T3 or T4 (or between T1 and T2/T4) and the standardized effect size (SES) were reported.35 To calculate the SES, the mean change values were divided by the SD of the baseline values. The values of 0.2, 0.5, and 0.8 for the standardized measures (Cohen’s d, SES) were rated as low, medium, and high, respectively.34
We included all randomized participants in the analyses (employing intention-to-treat analysis). To deal with missing data, we used multiple imputation36 to generate 10 complete data sets. The pooled results are reported. For the multiple imputation, we used the MICE37 R package. In all of the analyses, the alpha level was set at 5%. Data management was performed in IBM SPSS version 25, while the statistical environment R (http://www.r-project.org/index.html) was used to conduct the statistical analyses.
Results
Participant Flow
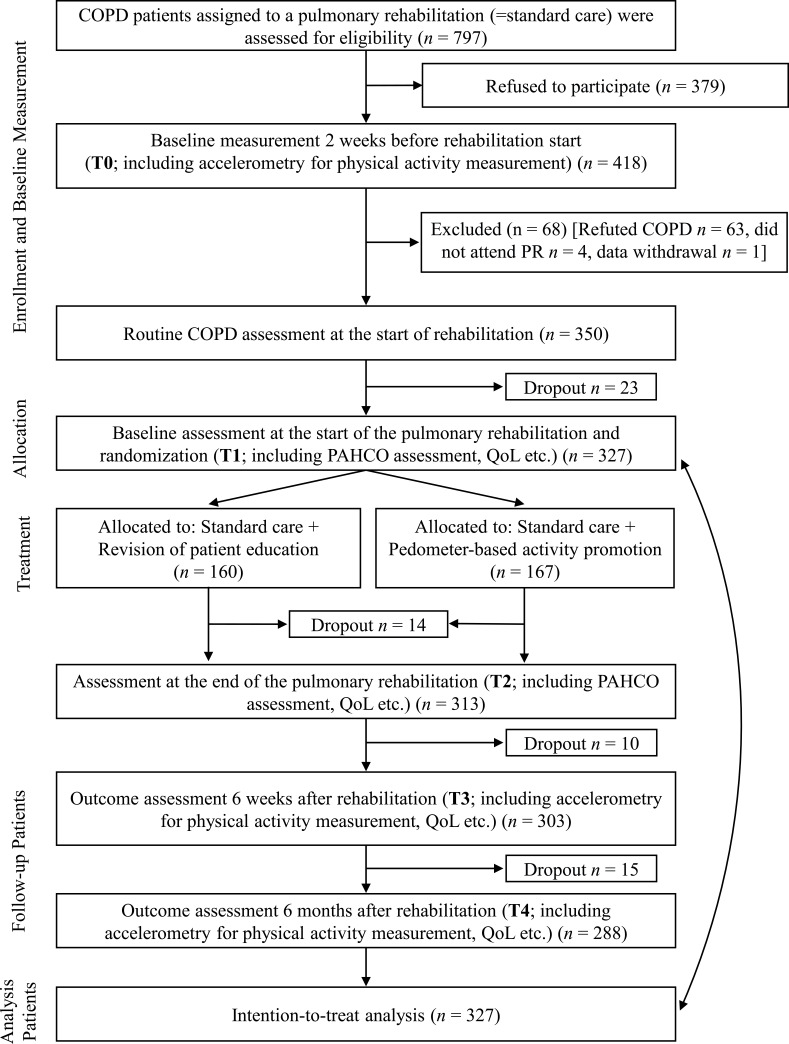
We recruited 418 patients who were initially diagnosed with COPD by a general practitioner; 63 patients had to be excluded because their COPD diagnosis could not be confirmed by a lung function test (Tiffeneau index FEV1/VC not ≤ 0.70), which was regularly performed at the beginning of the PR.
At randomization, the STAR study comprised 327 COPD patients at T1 (IG = 167, CG = 160) with a mean age of 58.02 ± 5.43 years; 226 (69%) patients were male. Participants were recruited between June 2016 and June 2018, and follow-up was finished in December 2019. The baseline demographic and clinical characteristics of the IG and the CG are displayed in Table 1. Figure 1 shows the CONSORT flowchart for the study participants.38
Table 1.
Baseline Characteristics
| Total (n = 327) | Intervention Group (n = 167) | Control Group (n = 160) | |
|---|---|---|---|
| Male, % | 69.0 | 68.7 | 69.4 |
| Age, years | 58.02 (5.43) | 58.01 (5.51) | 58.03 (5.47) |
| BMI, kg/m2 | 27.59 (6.71) | 27.29 (6.13) | 27.90 (7.27) |
| FEV1 (%) | 53.51 (18.47) | 53.05 (18.39) | 54.00 (18.61) |
| GOLD stage (I/II/III/IV), % | 9.0/45.2/35.3/9.3 | 7.3/46.3/36.6/9.8 | 10.8/43.9/36.3/8.9 |
| GOLD group (A/B/C/D), % | 1.6/43.6/0/54.8 | 1.3/42.1/0/56.6 | 2.0/45.1/0/52.9 |
| CAT | 23.39 (6.67) | 23.22 (6.61) | 23.57 (6.76) |
| No. comorbidities | 4.74 (2.43) | 4.71 (2.47) | 4.77 (2.40) |
| Current smoker, % | 47.0 | 47.2 | 46.8 |
| Employed, % | 75.3 | 72.3 | 78.3 |
| AECOPD, % | 16.0 | 17.1 | 14.9 |
| 6MWD, m | 447.86 (103.71) | 444.83 (101.75) | 450.98 (105.95) |
| SGRQ* | 52.60 (17.74) | 52.74 (17.40) | 52.46 (18.14) |
| PA and sedentary time | |||
| - At least moderate-intensity PA, min/day | 29.92 (23.29) | 29.11 (22.04) | 30.74 (24.54) |
| - Steps per day | 5891 (3027) | 5816 (2939) | 5986 (3124) |
| - Sedentary behavior, min/day | 560.56 (92.52) | 562.67 (87.86) | 558.41 (97.29) |
Notes: Data presented as mean values (standard deviation) unless otherwise stated.
Abbreviations: BMI, body mass index; SGRQ, Saint George’s Respiratory Questionnaire; CAT, COPD Assessment Test; PA, physical activity; 6MWD, six–minute walk distance; AECOPD, Pulmonary rehabilitation directly after hospitalization due to acute exacerbation of chronic obstructive pulmonary disease (AECOPD) versus stable COPD
Figure 1.
Consort flow chart.
Abbreviations: SGRQ, Saint George’s Respiratory Questionnaire; QoL, Quality of Life; PAHCO, Physical activity-related Health Competence.
Physical Activity: Steps (Primary Outcome), Moderate-Intensity Physical Activity, and Sedentary Time
Changes from T0 to T3/T4
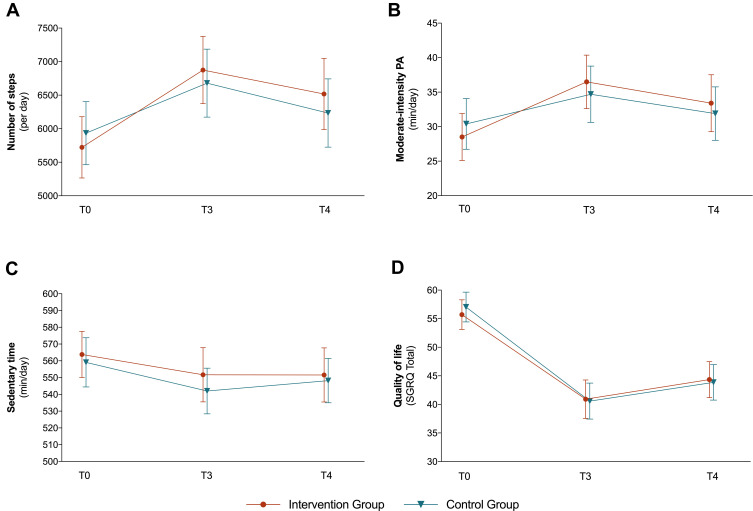
Both the IG and the CG showed a significant increase in daily steps and moderate-intensity PA from T0 to T3. The mean changes from T0 to T3 were slightly higher for both outcomes in the IG (+1153 steps; +8.0 min moderate-intensity PA) than in the CG (+745 steps; +4.3 min moderate-intensity PA). The results and patterns for the step changes from T0 to T4 are quite similar (IG: +795; CG: +299) to those from T0 to T3 while the moderate-intensity PA levels at T4 are almost similar to the T0 baseline values. Both groups showed small reductions in their daily sedentary time from the baseline to T3 (IG: −12.1 min; CG: −17.1 min) and T4 (IG: −12.2 min; CG: −10.9 min) (see Table 2 and Figure 2).
Table 2.
Mean Values (M) and Standard Deviations (SD) for Steps/Day (Primary Outcome), Moderate-Intensity Physical Activity, Sedentary Time, and Quality of Life (SGRQ Total) at Baseline (T0), Six Weeks (T3), and Six Months After Rehabilitation (T4)
| Outcome | Group | T0 | T3 | T4 | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||
| Steps/day | IG | 5722.4 | 2948.6 | 6875.0 | 3229.5 | 6517.7 | 3427.8 |
| CG | 5934.5 | 3101.0 | 6679.5 | 3337.4 | 6234.0 | 3357.6 | |
| Moderate-intensity physical activity (min/day) | IG | 28.5 | 22.0 | 36.5 | 25.0 | 33.4 | 26.6 |
| CG | 30.4 | 24.2 | 34.7 | 26.9 | 31.9 | 25.5 | |
| Sedentary time (min/day) | IG | 563.8 | 88.7 | 551.7 | 104.2 | 551.6 | 104.0 |
| CG | 559.1 | 96.8 | 542.0 | 89.7 | 548.2 | 87.2 | |
| SGRQ total | IG | 55.5 | 16.8 | 40.9 | 21.6 | 44.3 | 19.8 |
| CG | 57.0 | 17.1 | 40.4 | 20.7 | 43.6 | 20.5 | |
Abbreviations: SGRQ, Saint George’s Respiratory Questionnaire; IG, intervention group; CG, control group.
Figure 2.
Changes in primary and secondary outcomes presented separately for the intervention group (IG) and control group (CG).
Notes: Vertical bars denote 95% confidence intervals; higher scores in the SGRQ indicate more limitations.
Abbreviations: PA, physical activity; SGRQ, Saint George´s Respiratory Questionnaire; T0, before the rehabilitation; T3, 6 weeks after rehabilitation; T4, 6 months after rehabilitation.
Differences Between IG/CG at T3/T4
Descriptively, the IG showed higher mean values than the CG in terms of steps and moderate-intensity PA at T3 and T4 (see Table 3). However, none of these differences (steps in the IG compared to the CG at T3: AMD = 388 steps, d = 0.16 and T4: AMD = 458 steps, d = 0.15; moderate-intensity PA at T3: AMD = 3.5 min, d = 0.17 and T4: AMD = 3.0 min, d = 0.14) were statistically significant (p > 0.05 for all). Additionally, the differences between the IG and CG for sedentary time at T3 (AMD = 6.3 min, d = 0.08) and T4 (Δ0.1 min, d = 0.00) were also not statistically significant.
Table 3.
Adjusted Mean Differences (AMD) with 95% Confidence Intervals (95% CI), p-values and Cohen’s d Between Intervention Group and Control Group in Steps/Day (Primary Outcome), moderate-intensity Physical Activity, Sedentary Time and Quality of Life (SGRQ Total)
| Outcome | Measurement Point | AMD [95% CI] | p | Cohen’s d |
|---|---|---|---|---|
| Steps/day | T3 | 387.8 [−140.6; 916.2] | 0.153 | 0.16 |
| T4 | 458.3 [−191.9; 1108.4] | 0.171 | 0.15 | |
| Moderate-intensity physical activity (min/day) | T3 | 3.5 [−1.0; 8.0] | 0.136 | 0.17 |
| T4 | 3.0 [−1.8; 7.8] | 0.225 | 0.14 | |
| Sedentary time (min/day) | T3 | 6.3 [−10.8; 23.5] | 0.470 | 0.080 |
| T4 | 0.1 [−18;2 18.5] | 0.988 | 0.002 | |
| SGQR total | T3 | 0.51 [−3.3; 4.3] | 0.794 | 0.029 |
| T4 | 0.65 [−2.9; 4.2] | 0.718 | 0.040 |
Abbreviations: SGRQ, St. George’s Respiratory Questionnaire; AMD, adjusted mean difference; T3, six weeks after rehabilitation; T4, six months after rehabilitation.
Other Secondary Outcomes
Both groups showed a medium-to-high improvement in terms of health-related quality of life (SGRQ total) from T0 to T3 (IG: −14.6; CG: −16.7), and medium improvement from T0 to T4 (IG: −11.2; CG: −13.4). Similar to the PA-related outcomes, IG and CG showed no significant differences in terms of quality of life at T3 (AMD = 0.51, d = 0.03) and T4 (AMD = 0.65, d = 0.04). In addition to the SGRQ total score, Supplementary Table S1 includes the SGRQ domains.
The other secondary outcomes (eg, CAT, COPD-related anxiety, fear avoidance in COPD, PHQ-9) showed similar patterns: improved values from T0 to T3 and from T0 to T4 with no differences between CG and IG at both follow-ups (see Supplementary Tables S1-S5).
Discussion
To the best of our knowledge, the current STAR study is the first to examine the long-term effects of the addition of a pedometer-based intervention to German inpatient PR on COPD patients’ PA levels six weeks and six months after PR. In both trial groups, our RCT showed improved PA levels (increased daily steps and moderate-intensity PA, less sedentary time) in COPD patients six weeks and six months after three weeks of inpatient PR, which is standard in Germany. However, contrary to our primary hypothesis, the addition of a brief pedometer-based behavioral intervention to PR did not result in additional improvements in PA (measured in daily steps, moderate-intensity PA, and sedentary time).
Comparison with Other Studies
Compared to other studies, the pedometer intervention in our study demonstrated no additional effects on the primary outcome of steps nor on the secondary outcomes. A review from Armstrong et al16 identified seven studies from which six found an additional PA-enhancing effect for a pedometer intervention in the context of outpatient PR, and reported increases of between 680 and 2,600 steps. Our IG increased their PA volume when compared to the CG by only 388 steps (statistically insignificant) at the six-week follow-up and 458 steps at the six-month follow-up. Descriptively, the IG performed a greater number of steps and more time in moderate-intensity PA post-PR, but the effects were smaller (dT3 = 0.15; dT4 = 0.16) than initially assumed (d = 0.30) and smaller than reported in international studies (d = 0.51).16 The descriptive effects of the pedometer-based intervention on sedentary time were even smaller and were also statistically insignificant. These results may have several causes.
First, we had a challenging CG that also received a revision of PA-related patient education along with standard inpatient PR, which is an intensive, interprofessional, and guideline-based intervention in a specialized rehabilitation clinic.39 In Germany, the current practice of three weeks’ for inpatient PR (with the possibility to request an extension, if necessary) is based on individual initial assessments that culminate in a combination of patient-tailored therapies totaling more than 20 hours per week. The PR program is implemented by an interdisciplinary rehabilitation team and includes several interventional components that also address the promotion of PA (see the study protocol19 for program details). This means the quality of this program, in terms of PA promotion, is already high. Within German rehabilitation, around half of the exercise therapy departments emphasize PA promotion.40 The cooperating clinic for this study is one such clinic, which means that the exercise/physical therapists are very familiar with the concept of behavioral exercise therapy41 and thus apply BCTs; this includes instructions on how to perform PA behavior after PR (see study protocol; Additional File 1).19 PA changes in COPD patients after PR without pedometers (=usual care) in other studies show an average increase of 250 steps per day.16 Compared to this, our results revealed that the three-week inpatient PR program employed in the STAR study (with and without pedometers) was associated with substantially greater increases in PA after PR. Other studies of mostly outpatient PR without additional pedometers showed effects between −187 and +892 steps.16 With an increase at T3 of 797 steps for the non-pedometer group and 1,107 for the pedometer group, the pre-post changes in the STAR study lie above most of the pre-post changes reported in other studies, most of which had shorter follow-ups.
Second, the pedometer-based intervention in the IG may have been too short in terms of duration. The two 45-minute sessions may have been insufficiently long for some COPD patients to ensure the effective use of the pedometers and the PA diary, which is where patients recorded and monitored their daily steps after PR.
Third, it could be that the high baseline PA levels in our two groups (>5800 steps per day) reduced the effectiveness of the intervention and that the pedometer-based interventions would have been more successful when applied to individuals with a lower baseline PA level.
We decided to report the secondary outcome quality of life for two reasons. First, quality of life plays a prominent role for patients with COPD, and second, we were able to show in a previous analysis of the STAR data that quality of life is directly related to physical activity.42 The descriptive effects of the pedometer-based intervention on quality of life were even smaller and statistically insignificant. However, the improvement in both groups (IG and CG) indicates a positive effect of the PR program here as well.
In summary, the high-quality standard rehabilitation program employed for both the IG and the CG led to a considerable PA-promoting effect, which was difficult to increase via the—perhaps too short—pedometer intervention.
Strengths and Limitations
Our study has several strengths. The design of the STAR study is suitable for overcoming restrictions in previous empirical findings, as it features PA after PR as the primary outcome, includes an appropriately powered sample size, employs objective measures of PA and a long-term follow-up, and collects baseline PA data before PR.
Nevertheless, this study has some limitations. First, the effective sample size at T4 (n = 288) was slightly smaller than the pre-calculated number (n = 351). However, irrespective of the fact that the study false inclusion rate (due to initial misdiagnosis by the general practitioners) and dropout rate were higher than expected, the effects found were also significantly smaller than hypothesized,19 meaning that even a slightly larger sample would probably not have led to different conclusions. Second, even though the use of accelerometers has been acknowledged as a high scientific standard, and due to the fact that this study followed recent data-processing recommendations, accelerometry has some restrictions: water-based activities (eg, swimming) were not recorded, the valid daily wear time of accelerometers of at least 10 hours did not cover the entire day, and the method of accelerometry under-detected non-ambulatory PA (eg, exercise of the upper limbs). Third, only 418 of the 797 individuals contacted were willing to participate. The included participants may represent those with a greater interest in PA. Fourth, the study population differs from most other COPD populations by having a relatively young average age of only 58 years. Moreover, these patients were recruited from a single country and, importantly, from a single clinic with a specific—though typical for Germany—inpatient setting, which reduces the generalizability of the present findings. Fifth, as the IG and CG received their PR at the same clinic, it cannot be ruled out that the two groups exchanged information on pedometers with a possible unblinding effect regarding study group allocation.
Implications and Future Research
Our results suggest that the addition of a brief pedometer-based intervention does not substantially enhance the long-term PA-promoting effect of inpatient PR in COPD and should therefore not be implemented into the clinical practice of PR in this brief duration.
To improve the PA-promoting effect of pedometer-based interventions in COPD patients, it appears that longer intervention periods are more promising. Longer intervention periods are especially helpful to form the users’ habits regarding their use of the pedometer and self-controlling daily activity levels based on the pedometer-measured steps, and the entry of those steps into their PA diary, which may facilitate realistic PA-related goal setting in a next step.
Our study detected significant increases in PA levels following PR; however, not all patients showed better PA levels at T3, and not all patients with increased PA levels at T3 could maintain these improvements at T4. In addition, the large 95% confidence intervals for the PA changes indicate high interpersonal heterogeneity. Therefore, future research should identify responders and non-responders regarding PA improvements and accurately describe them using important disease-related (eg, disease severity) and behavioral criteria (eg, stage of change).43 Furthermore, sub-group analyses should identify those with initial PA improvements and declines in PA levels in the long term. These analyses could profit from a simultaneous consideration of disease-related parameters (eg, exercise capacity)44 and important psychological determinants of PA (motivation, self-regulation; see for example Geidl et al).41 Such findings could lead to a better understanding of PA changes in COPD patients and support the development of tailored PA promotion strategies.
Even the physically active participants in our study had very long sedentary times prior to the PR,26 and our results showed that PR did not substantially affect this behavior. This result supports previous research that demonstrated that interventions to promote PA and reduce sedentary time do not follow the same logic; rather, each must be addressed specifically.45 Currently, the topic of sedentarism is only barely considered in clinical guidelines.46 Given that sedentary time in COPD is an independent predictor of poor prognosis47 and mortality,48 future clinical guidelines and PR programs should not only address PA promotion but also the reduction in sedentary time.
Conclusion
The results of this RCT indicate that the addition of a brief pedometer-based behavioral intervention to inpatient PR did not result in more PA in terms of steps and moderate-intensity PA or less sedentary time six weeks and six months after PR. The intervention also had no additional effect on other secondary outcomes, such as quality of life. Nevertheless, both groups of COPD patients, the IG and CG, showed remarkably enhanced PA levels and other secondary outcomes six weeks and six months following standard German inpatient PR.
Acknowledgments
The authors thank German Pension Insurance, Section Bavaria South (Deutsche Rentenversicherung Bayern Süd), which sponsors and supports this study. We thank study nurses Stephanie Häusl, Andrea Klotz, Maike Messerschmidt, and Berta Obermaier as well as the recruiting physicians and the involved therapeutical team of the Bad Reichenhall Clinic for their outstanding engagement. We would like to thank Sam Cassar, Lorena Miranda, Samuel Tonne, Anna Ryan, and Tyler Prinkey for their support in implementing the study as student research assistants.
Funding Statement
This study is funded by German Pension Insurance, Section Bavaria South (Deutsche Rentenversicherung Bayern Süd; Abteilung Rehabilitation und Sozialmedizin, Am Alten Viehmarkt 2, 84028 Landshut, Germany; reference number: 5.011-6.031.115), which was not involved in the design of the study; in the collection, management, analysis, or interpretation of the data; in writing the manuscript; or in the decision to submit the report for publication.
Data Sharing Statement
Data is available upon reasonable request from Dr. Wolfgang Geidl; e-mail: wolfgang.geidl@fau.de.
Disclosure
Wolfgang Geidl reports grants from German Pension Insurance, Section Bavaria South (DRV Bayern Süd), during the conduct of the study. Michael Schuler reports grants from Deutsche Rentenversicherung Bayern Nord (German Pension Insurance North Bavaria), during the conduct of the study. Eriselda Mino reports grants from German Pension Insurance, Section Bavaria South, during the conduct of the study. The authors declare no other conflicts of interest in this work.
References
- 1.WHO. Global action plan for the prevention and control of noncommunicable diseases 2013–2020. Available from: https://apps.who.int/iris/bitstream/handle/10665/94384/9789241506236_eng.pdf;jsessionid=589456D20F240ADB306C33DCC306E846?sequence=1. Accessed September19, 2020.
- 2.Zwerink M, van der Palen J, van der Valk P, Brusse-Keizer M, Effing T. Relationship between daily physical activity and exercise capacity in patients with COPD. Respir Med. 2013;107(2):242–248. doi: 10.1016/j.rmed.2012.09.018 [DOI] [PubMed] [Google Scholar]
- 3.Meshe OF, Claydon LS, Bungay H, Andrew S. The relationship between physical activity and health status in patients with chronic obstructive pulmonary disease following pulmonary rehabilitation. Disabil Rehabil. 2017;39(8):746–756. doi: 10.3109/09638288.2016.1161842 [DOI] [PubMed] [Google Scholar]
- 4.Esteban C, Quintana JM, Aburto M, et al. Impact of changes in physical activity on health-related quality of life among patients with COPD. Eur Respir J. 2010;36(2):292–300. doi: 10.1183/09031936.00021409 [DOI] [PubMed] [Google Scholar]
- 5.Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331–342. doi: 10.1378/chest.10-2521 [DOI] [PubMed] [Google Scholar]
- 6.Barker J, Smith Byrne K, Doherty A, et al. Physical activity of UK adults with chronic disease: cross-sectional analysis of accelerometer-measured physical activity in 96 706 UK Biobank participants. Int J Epidemiol. 2019;48(4):1167–1174. doi: 10.1093/ije/dyy294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):64. doi: 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 8.McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793. doi: 10.1002/14651858.CD003793.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busby AK, Reese RL, Simon SR. Pulmonary rehabilitation maintenance interventions: a systematic review. Am J Health Behav. 2014;38(3):321–330. doi: 10.5993/AJHB.38.3.1 [DOI] [PubMed] [Google Scholar]
- 10.Spruit MA, Pitta F, McAuley E, Zuwallack RL, Nici L. Pulmonary rehabilitation and physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(8):924–933. doi: 10.1164/rccm.201505-0929CI [DOI] [PubMed] [Google Scholar]
- 11.Mantoani LC, Rubio N, McKinstry B, MacNee W, Rabinovich RA. Interventions to modify physical activity in patients with COPD: a systematic review. Eur Respir J. 2016;48(1):69–81. doi: 10.1183/13993003.01744-2015 [DOI] [PubMed] [Google Scholar]
- 12.Troosters T, Blondeel A, Rodrigues FM, Janssens W, Demeyer H. Strategies to increase physical activity in chronic respiratory diseases. Clin Chest Med. 2019;40(2):397–404. doi: 10.1016/j.ccm.2019.02.017 [DOI] [PubMed] [Google Scholar]
- 13.Burge AT, Cox NS, Abramson MJ, Holland AE. Interventions for promoting physical activity in people with chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2020;4:CD012626. doi: 10.1002/14651858.CD012626.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheid JL, West SL. Opportunities of wearable technology to increase physical activity in individuals with chronic disease: an editorial. Int J Environ Res Public Health. 2019;16:17. doi: 10.3390/ijerph16173124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goode AP, Hall KS, Batch BC, et al. The Impact of interventions that integrate accelerometers on physical activity and weight loss: a systematic review. Ann Behav Med. 2017;51(1):79–93. doi: 10.1007/s12160-016-9829-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong M, Winnard A, Chynkiamis N, Boyle S, Burtin C, Vogiatzis I. Use of pedometers as a tool to promote daily physical activity levels in patients with COPD: a systematic review and meta-analysis. Eur Respir Rev. 2019;28:154. doi: 10.1183/16000617.0039-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King AC, Whitt-Glover MC, Marquez DX, et al. Physical activity promotion: highlights from the 2018 physical activity guidelines advisory committee systematic review. Med Sci Sports Exerc. 2019;51(6):1340–1353. doi: 10.1249/MSS.0000000000001945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lingner H, Ernst S, Groβhennig A, et al. Asthma control and health-related quality of life one year after inpatient pulmonary rehabilitation: the ProKAR Study. J Asthma. 2015;52(6):614–621. doi: 10.3109/02770903.2014.996650 [DOI] [PubMed] [Google Scholar]
- 19.Geidl W, Semrau J, Streber R, et al. Effects of a brief, pedometer-based behavioral intervention for individuals with COPD during inpatient pulmonary rehabilitation on 6-week and 6-month objectively measured physical activity: study protocol for a randomized controlled trial. Trials. 2017;18(1):396. doi: 10.1186/s13063-017-2124-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogelmeier C, Buhl R, Burghuber O, et al. Leitlinie zur Diagnostik und Therapie von Patienten mit chronisch obstruktiver Bronchitis und Lungenemphysem (COPD) [Guideline for the Diagnosis and Treatment of COPD Patients]. Pneumologie. 2018;72(4):253–308. doi: 10.1055/s-0043-125031 [DOI] [PubMed] [Google Scholar]
- 21.Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95. doi: 10.1007/s12160-013-9486-6 [DOI] [PubMed] [Google Scholar]
- 22.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49:3. doi: 10.1183/13993003.00214-2017 [DOI] [PubMed] [Google Scholar]
- 23.Rabinovich RA, Louvaris Z, Raste Y, et al. Validity of physical activity monitors during daily life in patients with COPD. Eur Respir J. 2013;42(5):1205–1215. doi: 10.1183/09031936.00134312 [DOI] [PubMed] [Google Scholar]
- 24.van Remoortel H, Raste Y, Louvaris Z, et al. Validity of six activity monitors in chronic obstructive pulmonary disease: a comparison with indirect calorimetry. PLoS One. 2012;7:6. doi: 10.1371/journal.pone.0039198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strath SJ, Pfeiffer KA, Whitt-Glover MC. Accelerometer use with children, older adults, and adults with functional limitations. Med Sci Sports Exerc. 2012;44(Suppl 1):S77–S85. doi: 10.1249/MSS.0b013e3182399eb1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geidl W, Carl J, Cassar S, et al. Physical activity and sedentary behaviour patterns in 326 persons with COPD before starting a pulmonary rehabilitation: a cluster analysis. JCM. 2019;8(9):1346. doi: 10.3390/jcm8091346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. [DOI] [PubMed] [Google Scholar]
- 28.Ardelean DL, Iulia L, Popescu R, et al. Evaluation of COPD patients using CAT-COPD assessment test. Pneumologia. 2012;61(4):221–229. [PubMed] [Google Scholar]
- 29.Löwe B, Spitzer R, Zipfel S, Herzog W. Gesundheitsfragebogen Für Patienten (PHQ D). Komplettversion Und Kurzform. Testmappe Mit Manual, Fragebögen, Schablonen. 2. Auflage. Karlsruhe: Pfizer; 2002. [Google Scholar]
- 30.Kühl K, Kuhn C, Kenn K, Der RW. COPD-Angst-Fragebogen (CAF): ein neues Instrument zur Erfassung krankheitsspezifischer Ängste bei COPD-Patienten. Psychother Psychosom Med Psychol. 2011;61(01):e1–e9. doi: 10.1055/s-0030-1248281 [DOI] [PubMed] [Google Scholar]
- 31.Stenzel N, Rief W, Kenn K. Fear Avoidance - Eine Bedeutsame Aktivitätsbremse Bei Chronisch Obstruktiver Lungenerkrankung (COPD)? Vortrag Präsentiert Auf Dem 54. Hannover: Kongress der Deutschen Gesellschaft für Pneumologie und Beatmungsmedizin (DGP); 2013. [Google Scholar]
- 32.Janssens T, Geidl W, Carl J, et al. Reliability and validity of dyspnea numeric rating scales as a patient-reported outcome in pulmonary rehabilitation of COPD [abstract]. Eur Respir J. 2019;54(Suppl 63):OA3810. doi: 10.1183/13993003.congress-2019.OA3810 [DOI] [Google Scholar]
- 33.American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 34.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 35.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care. 1989;27(3):S178–S189. [DOI] [PubMed] [Google Scholar]
- 36.Enders CK. Analyzing longitudinal data with missing values. Rehabil Psychol. 2011;56(4):267–288. doi: 10.1037/a0025579 [DOI] [PubMed] [Google Scholar]
- 37.van Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:3. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 38.Schulz KF, Altman DG, Moher D, the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18. doi: 10.1186/1741-7015-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz K, Jelusic D, Wittmann M, et al. Inspiratory muscle training does not improve clinical outcomes in 3-week COPD rehabilitation: results from a randomised controlled trial. Eur Respir J. 2018;51:1. [DOI] [PubMed] [Google Scholar]
- 40.Sudeck G, Geidl W, Deprins J, Pfeifer K. The role of physical activity promotion in typical exercise therapy concepts: a latent class analysis based on a national survey in German rehabilitation settings. Disabil Rehabil. 2019;57:1–11. doi: 10.1080/09638288.2019.1608322 [DOI] [PubMed] [Google Scholar]
- 41.Geidl W, Semrau J, Pfeifer K. Health behaviour change theories: contributions to an ICF-based behavioural exercise therapy for individuals with chronic diseases. Disabil Rehabil. 2014;36(24):2091–2100. doi: 10.3109/09638288.2014.891056 [DOI] [PubMed] [Google Scholar]
- 42.Carl J, Geidl W, Schuler M, et al. Towards a better understanding of physical activity in people with COPD: predicting physical activity after pulmonary rehabilitation using an integrative competence model. Chron Respir Dis. in print. doi: 10.1177/1479973121994781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mesquita R, Meijer K, Pitta F, et al. Changes in physical activity and sedentary behaviour following pulmonary rehabilitation in patients with COPD. Respir Med. 2017;126:122–129. doi: 10.1016/j.rmed.2017.03.029 [DOI] [PubMed] [Google Scholar]
- 44.Ramon MA, Ter Riet G, Carsin A-E, et al. The dyspnoea-inactivity vicious circle in COPD: development and external validation of a conceptual model. Eur Respir J. 2018;52(3). doi: 10.1183/13993003.00079-2018 [DOI] [PubMed] [Google Scholar]
- 45.Rutten GM, Savelberg HH, Biddle SJH, Kremers SPJ. Interrupting long periods of sitting: good STUFF. Int J Behav Nutr Phys Act. 2013;10:1. doi: 10.1186/1479-5868-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewthwaite H, Effing TW, Olds T, Williams MT. Physical activity, sedentary behaviour and sleep in COPD guidelines: a systematic review. Chron Respir Dis. 2017;14(3):231–244. doi: 10.1177/1479972316687224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hill K, Gardiner PA, Cavalheri V, Jenkins SC, Healy GN. Physical activity and sedentary behaviour: applying lessons to chronic obstructive pulmonary disease. Intern Med J. 2015;45(5):474–482. doi: 10.1111/imj.12570 [DOI] [PubMed] [Google Scholar]
- 48.Furlanetto KC, Donaria L, Schneider LP, et al. Sedentary behavior is an independent predictor of mortality in subjects with COPD. Respir Care. 2017;62(5):579–587. doi: 10.4187/respcare.05306 [DOI] [PubMed] [Google Scholar]