Abstract
Intestinal failure requires the placement and maintenance of a long-term central venous catheter for the provision of fluids and/or nutrients. Complications associated with this access contribute to significant morbidity and mortality, while the loss of access is an increasingly common reason for intestinal transplant referral. As more emphasis has been placed on the prevention of central line-associated bloodstream infections and new technologies have developed, care for central lines has improved; however, because care has evolved independently in local centers, care of central venous access varies significantly in this vulnerable population. The present position paper from the Intestinal Failure Special Interest Group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) reviews current evidence and provides recommendations for central line management in children with intestinal failure.
Keywords: catheter-related thrombosis, central line-associated bloodstream infection, central venous catheter, intestinal rehabilitation program, parenteral nutrition
Pediatric intestinal failure (IF) is characterized by the inability of the gastrointestinal tract to absorb adequate nutrition and fluid to maintain hydration and support growth resulting in the need for long-term parenteral nutrition (PN) delivered via a central venous catheter (CVC) (1). Long-term central venous access is required while working toward enteral autonomy, the ultimate goal of intestinal rehabilitation. Even with advances in the care of central venous access and decreases in complication rates, CVC related complications pose potentially life-threatening risks and remain a key factor in long-term outcomes 9 (Tables 1 and 2). IF patients often require repeated vascular interventions and commonly develop central line-associated complications, which can result in loss of access (2). With the decline in IF associated liver disease, the common indications for intestinal transplant have shifted with loss of central access playing a major role (3). Loss of access above the diaphragm, in fact, can be a contraindication for multivisceral transplant. The recent establishment of multidisciplinary intestinal rehabilitation programs (IRP) with advances in infection prevention, anticoagulation, and monitoring have been shown to decrease these risks (4,5). With the aim of improving care and outcomes for all children with a prolonged need for central access the Venous Access: National Guideline and Registry Development (VANGUARD) Initiative recently published guidelines for the preservation of central venous access in children (6). The objective of the present position paper is to review the current literature and provide recommendations regarding important aspects of CVC management for children with IF.
TABLE 1.
Central venous catheter complication rates in pediatric intestinal failure: infection and dysfunction
| Type | Tunneled CVC (rate/1000 catheter days [citation]) |
PICC* (rate/1000 catheter days [citation]) | |
|---|---|---|---|
| Infection/CLABSI | Pre | Post | |
| Ethanol | 13.9 | 1.6 [61] | 3.95 [7] |
| 4.74 | 0.36 [88] | 2.6 [12] | |
| 5.5 | 3.1 [87] | 5.3 [11] | |
| Taurolidine | 3.7 [80] | ||
| 1.5 [58] | |||
| 2.8 [7] | |||
| 4.2 [12] | |||
| Care bundle† | |||
| 4.16 | 0.25 [72] | ||
| 12.7 | 4.3 [73] | ||
| Anticoagulation‡ | 8.6 | 1.1 [74] | |
| 4.8 | 2.9 [27] | ||
| 1.41 | 0.4 [22] | ||
| 7.9 | 4.4 [90] | ||
| Dysfunction | Pre | Post | |
| Fracture or breakage | 2.76 [7] | ||
| Ethanol | 0.48 | 4.15 [88] | 1.56 [12] |
| 1.8 | 1.53 [87] | 0.2 [11] | |
| 5.6 [80] | |||
| 3.68 [7] | |||
| 0.26 [12] | |||
| Occlusion | 3.95 [7] | ||
| 0.82 | 2.94 [88] | 7 [12] | |
| Ethanol | 0.6 | 0.3 [87] | 1 [11] |
| 6 [80] | |||
| 2.21 [7] | |||
| 5.5 [12] | |||
CLABSI = central line-associated blood stream infection; CVC = central venous catheter; PICC = peripherally inserted central catheter.
Reported baseline PICC rates without evaluation of intervention.
Ethanol usage not reported.
All patients had a catheter-related thrombus, ethanol usage not reported.
TABLE 2.
Central venous catheter complication rates in pediatric intestinal failure: venous injury and malposition
| Type | Tunneled CVC (rate/1000 catheter days [citation]) | PICC Rate (rate/1000 catheter days [citation]) |
|---|---|---|
| Venous injury | ||
| Stenosis | 0.59 [7] | 0.87 [7] |
| Thrombosis | 0.15 [7] | 0.55 [7], 0.2 [11] |
| Malposition | ||
| Malposition or | 0.15 [7] | 1.9 [7] |
| Dislodgement | 0.44 [7] | 1.66 [7] |
| 2.4 [80] |
CVC = central venous catheter; PICC = peripherally inserted central catheter.
METHODS
Relevant literature was reviewed using PubMed/MEDLINE databases applying the following terms: intestinal failure, short bowel syndrome, central venous catheter, central venous access, parenteral nutrition, central line-associated bloodstream infection, and catheter-related thrombus. Literature searches were conducted through January 31, 2020. Non-English literature was excluded. Sections were completed by individual authors with review and editing of the drafts by the coauthors. The available literature and expert opinion were used by the authors to formulate recommendations for each section. These recommendations were modified and ultimately agreed upon by the Intestinal Rehabilitation Special Interest Group members via electronic and phone communication. A grading method was not used for the recommendations secondary to the limited quantity and quality of the pediatric IF data available. An image library was compiled to show both normal and abnormal findings associated with central venous access (see Supplemental Digital Content Figure 1, http://links.lww.com/MPG/C152).
TYPES OF CENTRAL VENOUS ACCESS
Options for central venous access include tunneled CVC, peripherally inserted central catheter (PICC), and implanted central venous access devices or port, each with their own risks and benefits. Tunneled CVC are the most common type of long-term central access for IF patients owing to their stability, durability, longevity, accessibility, and potentially decreased risk of infection (7,8). Traditionally used for short-term central access, PICC lines have been associated with lower rates of central venous thrombosis and with increased risk of peripheral clots, venous stenosis, line breakage, and site infection or phlebitis (8–10). Evidence of infection rates comparing PICC lines and tunneled CVCs is conflicting, with two pediatric IF studies showing decreased or equivalent infection rates and an adult study reporting an increased rate and shorter time to first infection with PICC lines (8,11,12). Cuffed PICC lines carry lower rates of infection, malposition, and thrombus formation compared with uncuffed PICC lines (13). Despite a lower infection risk, ports are typically reserved for patients who require infrequent access as accessing a port requires a needle stick, and over time, with repeated punctures, the membrane or septum that the needle passes through can breakdown (14). The benefits of a port are nullified when it has to be accessed continuously for the daily administration of PN.
To prevent complications, it is best practice to use the smallest diameter and least number of lumens that meet the patient’s clinical needs (6). Most children with IF only require a single-lumen catheter. Catheter diameter varies by line type and is fit to the size of the patient, although no standard recommendation exists to determine the optimal line size. Large diameter catheters can cause a venous obstruction and resulting thrombosis, while multilumen catheters are associated with infection, thrombosis, and malfunction (14–16). Silicone catheters are preferred to permit the use of ethanol locks. Line material composition will be further discussed in the section addressing central line-associated bloodstream infections (CLABSI).
Subclavian or internal jugular vein access is typically preferred to femoral vein access to improve comfort and reduce risk of thrombosis and infection from stool or enteric tube output (17,18). Although most tunneled CVC exit the skin on the chest, subcutaneous tunneling to achieve the desired exit site, such as the back, is possible.
Alternative line sites and complex endovascular procedures have been used to address limited central access. Case reports have been published with transhepatic, translumbar, azygos, and direct atrial insertion of central lines, and stenoses dilated or stented and clots traversed to reestablish access (19,20). Because of the risk of morbidity and mortality, these procedures should only be attempted by experienced interventionalists in conjunction with an intestinal transplant center when transplant evaluation has been or will be sought.
1. Recommendations for venous access:
Tunneled, single lumen, cuffed silicone catheters should be used for children with IF.
Upper extremity access is the preferred location when available.
Alternative line sites and endovascular procedures should only be done by experienced interventionalists in discussion with an intestinal transplant center when transplant evaluation has been or will be sought.
ROUTINE CARE OF CENTRAL VENOUS CATHETERS
The steps involved in CVC maintenance and routine care, performed by various caregivers in ambulatory or inpatient settings, directly impact complication rates and preservation of long-term venous access. They are often documented in care bundles which have been used to decrease unnecessary variation, resulting in improved CLABSI and line replacement rates (21–25). Published guidelines offer comprehensive recommendations on details of care management (6,14,26).
Care Technique and Caregiver Training
With the burden and risk of care for pediatric patients with IF falling on caregivers, education for this group is imperative (27). It is recommended that CVC management rest solely on caregivers or staff members trained specifically to care for venous access devices. The educational process for caregivers starts while children are initially admitted to the hospital and typically requires multiple sessions, potentially utilizing simulation, to ensure comfort and competence, and not infrequently re-training (28). Some families and IRP find it useful to have parents ‘‘room in’’ so that the person who will be doing the care at home has a chance to practice these skills in the hospital setting.
External Site Care: Skin Preparation and Dressings
Catheter care should reinforce the importance of proper hand hygiene with manipulation of site, tubing, or catheter connections. This single, evidence-based intervention has clearly demonstrated a reduction in CLABSI rates in patients including neonates (29). Aseptic technique should be maintained for all manipulations of the CVC, and clean or sterile gloves should be worn for dressing changes (14).
In routine line care, skin preparation involves thorough cleansing to immediately decontaminate and inhibit pathogen growth (30). Choices in antiseptic solutions for skin preparation include isopropyl alcohol, hydrogen peroxide, povidone–iodine, acetone, and chlorhexidine gluconate (CHG). A 2% CHG solution has been demonstrated to be more effective than povidone–iodine solutions with respect to skin decontamination and CLABSI prevention, and a combined CHG–alcohol solution is widely used in pediatric and adult patients (29,31). Povidone–iodine may be preferred if there is skin irritation associated with the CHG solution, or in neonates (14). With regards to the neonatal population specifically, there has not been any data that clearly describes safety and efficacy of CHG use in infants <2 months, although trends including a US National Survey suggest that many NICUs do use CHG (32).
The CVC dressing is an external barrier to bacteria, fungi, and moisture, that aids in line securement. The CVC insertion site should be inspected daily for localized signs of infection. Therefore, transparent, semipermeable or permeable polyurethane dressings are most commonly used. These dressings should be changed every 7 days, and only more frequently if the dressing is not occlusive or if the site is compromised. When a patient has oozing or bleeding at the site, significant perspiration/moisture, or a local reaction to the polyurethane or adhesive, a gauze dressing is suitable. Gauze dressings should be changed every 24 h to facilitate observation of the insertion site, or more frequently as needed. There is insufficient evidence of the superiority of gauze or transparent dressing with respect to preventing infection or other catheterrelated outcomes (33,34). In addition to the central line dressing, many young children and those with developmental disabilities benefit from a physical barrier covering the CVC such as close-fitting clothing or commercially available central line wrap to help keep the end of the central line covered when not in use and prevent the patient from pulling or picking at the line and dressing.
The routine use of antimicrobial ointments may promote fungal infections or antimicrobial resistance, therefore is not advised (14). If a localized area of discharge or suspected skin infection is present, limited use of an antibiotic ointment may be considered with daily dressing changes for close site surveillance. Before initiating antibiotics, the site should be cultured to guide treatment. A temporary transition to a gauze dressing may prevent moisture and facilitate regular inspection. If the suspected infection worsens or does not respond to topical treatments intravenous antibiotics must be considered.
CHG impregnated disks, sponges, or dressing placed at the catheter site provide an additional antimicrobial barrier and have been used to reduce the risk of infection. Only limited pediatric data have been published that support universal use. A randomized, controlled study of 705 neonates comparing CHG impregnated patch to standard dressing demonstrated a reduced rate of catheter colonization (defined by semi-quantitative colony count following the culture of distal 5 cm of the catheter tip postremoval) but no difference in CLABSI (14,35). Contact dermatitis has been reported after use in infants <2 months of age (34). In general, CHG impregnated patches should be considered in all patients requiring long term CVC access.
Administration Set-Up, Access, De-Access, and Other Line Cares
The administration set, including tubing and connectors, along with the CVC cap placed at the catheter hub, establish a sterile, closed infusion system. The cap should be accessed only following a thorough cleansing technique using an antiseptic solution (‘‘scrub the hub’’). Furthering this principle of decontaminating the catheter entry site, antiseptic barrier caps have been adopted into practice guidelines at many centers. A recent meta-analysis suggests that these devices reduce CLABSI rates, although the safety for routine use in neonates has not been established (34,36). Administration sets, including tubing, should be changed at least every 24 h in patients receiving lipids, but may be changed less frequently in patients not receiving lipids (ie, every 3–4 days) (29). When IV lipid emulsions are administered separately, there are evolving considerations regarding administration set changes as frequently as every 12 h, although the practicality of this recommendation as compared to cost-effectiveness and risk of multiple system entries warrants further evaluation (37).
Locking and flushing the CVC at set intervals with an anticoagulant solution (heparin or saline) helps to maintain catheter patency, although heparin should not be used with ethanol locks (38). Recommended locking volumes are influenced by catheter and tubing fill volume and patient size (see Table 1 for internal volumes of central venous catheters, Supplemental Digital Content, http://links.lww.com/MPG/C154). Flushing prevents mixing of incompatible medications and solutions. It should occur before and following PN administration, intravenous medication administration, laboratory draws, and blood transfusions. To maintain patency, dormant catheter lumens should be flushed with normal saline and locked at least daily, and ports should be accessed and flushed monthly.
Radiographic Surveillance of CVC
Catheter imaging may be required to evaluate line placement, survey venous patency, and assess for complications such as catheter-related thrombosis (CRT). A chest radiograph may be used to monitor or evaluate CVC position. The catheter is in a central location when the tip approximates the cavoatrial junction. There is variation in the frequency of monitoring; some institutions evaluate PICC lines as frequently as monthly and tunneled CVCs every 6–12 months. When necessary, fluoroscopy may provide direct evidence of catheter location, patency, presence of fibrin sheath, or internal fracture of the line (39).
Accurate knowledge of central venous patency is important for IF care and risk assessment. Venous ultrasonography, although widely used, may not be sensitive enough for surveillance or diagnosis of vascular occlusions or subtle intravascular sequelae of indwelling catheters; however, ultrasonography can be used to follow established lesions over time (6). Venography is considered the diagnostic standard for the accurate evaluation of venous patency. Limitations include the relative invasiveness of the study, radiation and intravenous contrast exposure, the requirement for vascular interventional expertise, and the need for the targeted study of the anatomic area of interest (6). Additional imaging studies such as CT and MR venography offer comprehensive information regarding venous obstruction; however, considerations regarding radiation exposure (CT), anesthesia (MR), and center experience must be taken into account. For tip-associated thrombi, an echocardiogram can also be used (40). In practice, many centers choose ultrasonography as the first-line imaging modality to evaluate for occlusion and reserve CT, MR, or traditional venography when further delineation of access is required. When used as a screening tool for vascular access among patients off anticoagulation, ultrasonography can be considered. Multidisciplinary central venous access team formation should be considered in centers managing pediatric patients with IF, with the goal of monitoring catheter events, incorporating radiographic surveillance modalities, and, when necessary, offering the intervention to maintain venous patency (6).
2. Recommendations pertaining to routine CVC care:
Proper technique and hygiene surrounding CVC care are of paramount importance in preventing CVC-associated complications. Caregivers should receive directed education regarding CVC care before initial discharge, with subsequent reinforcement education as needed.
CHG impregnated supplies (disk, sponge, or dressing) should be considered for central line dressing in pediatric IF patients.
Routine surveillance of central venous access should be performed by US. MR, CT, or traditional venography should be reserved for when further delineation of access is required.
ADDITIONAL CONSIDERATIONS FOR CARE
The development and maintenance of a therapeutic relation and the nurturing of childhood activities are essential to the care of children with complex, chronic disease. These components are particularly necessary in the setting of a CVC, which may be in place for more than a decade without major complication (41); however, the vigorous pursuit of this goal can, at times, be counterproductive and obstruct rational, values-based discussions between the family and care team. In many circumstances, decisions regarding ‘‘real world’’ questions relating to the CVC cannot be informed by evidence due to a paucity of data. In these situations, the medical team’s role is to clearly articulate known and suspected risk, while maintaining a therapeutic relation and providing guidance on risk mitigation.
School
Many children requiring CVC into childhood will regularly attend school, thus requiring an awareness of special needs, common problems, and reasons for concern by the education team (42). State and local regulations, availability, privileges and comfort of the school nurse, and the medical plan of the individual patient preclude a dogmatic approach. Rather, they support the necessity of an Individual Health Plan and ongoing communication between parents or guardians of the child and the education team (43). The role of the IRP is to stimulate such communication, reasonably consider the impact of the medical and nutrition plan on school attendance and performance, and to assist in providing the school with guidance regarding safe device care and indications for care escalation. Some programs supply schools, and families in general, with a kit along with specific instructions which can be used in case of emergency (44). Potential items include gloves, surgical mask, alcohol wipes, an extra dressing, clamp, sterile gauze, and a roll of tape. Unless a nurse trained in CVC care is with the child or at the school, any issues with the line should be immediately directed to the parent with the supplies in the kit only acting as a temporizing measure if needed. In general, no one at school should be manipulating the CVC.
Sports
Sports participation is an important physical and emotional component of childhood supporting wellness and quality of life (45). Physical limitations vary by patient, but all patients with IF must guard medical devices from physical injury or from being pulled during play. We recommend avoidance of contact sports and use of an abdominal binder, ace wrap, or tight-fitting shirt to keep lines and tubes close to the body, decreasing the risk of injury. Parents should be encouraged to assess for dressing occlusiveness before and after a child engages in sport and should notify their medical team of recurrent problems with excessive moisture or rash due to sweating. Additionally, the risk of heat injury and excessive fluid losses should be assessed and planned for with the provision of ‘‘as needed’’ fluid boluses.
Recreational Swimming
Swimming introduces an incompletely defined but potentially severe risk to those requiring chronic central venous access. Contamination of various chlorine-treated (swimming pools), stagnant (lakes and ponds), and flowing (oceans and rivers) bodies of water with human pathogens has been well documented, though proper maintenance may minimize outbreaks. The potentially fatal risk of such contaminants gaining access to central circulation via the CVC is unclear, but many IRP readily share anecdotes of infection from organisms such as Pseudomonas aeruginosa. Dry-suits specifically designed for patients with CVC are available, but at substantial cost and have not been studied. Parents seeking guidance are confronted by mixed messaging from support programs, online resources and blogs, and even IRP. These conflicting recommendations and practices reflect the paucity of data to guide a safe and clear approach for swimming with a central line. A recent survey of 16 pediatric home PN programs found inconsistency of recommendations, ranging from strict swimming avoidance to permission for ocean swimming (46). All programs permitting swimming in low-risk situations recommended immediate site cleaning and dressing change following water exposure and avoidance of submersion for 4–6 weeks after CVC placement. Ultimately, the decision to permit children with IF to swim lies with the parent or guardian.
Pets
The human–animal bond provides a profound therapeutic opportunity in the care of those with chronic illness, providing documented mental, social, and physiological benefits (47). Even in situations of immunocompromise, animal companions may provide these benefits without substantial risk of zoonotic disease (47). Steps should be taken to promote line integrity in the presence of pets. Particularly in the setting of pets that may attempt to chew or play with tubing, adequate physical protection of the insertion site and catheter itself is recommended. Any line or tubing puncture by an animal should prompt immediate evaluation. Family awareness of zoonotic disease risk and advocacy of handwashing before and after animal care should be made clear.
Travel
A paucity of medical literature has not prevented the development and sharing of robust, lay guidance for travel with a CVC. The most important advice for those intending to travel is of thoughtful and organized preparation. Notification of needs should be made of the travel destination well in advance, and new needs such as of additional hydration in warm climates, should be considered. Details of medical and surgical history, current medications and PN prescription, and 24-h contact information for a patient’s managing provider can be packed in electronic and paper form. When possible, emergency planning is recommended through a priori identification of a local pharmacy, urgent care center, and durable goods supplier. Evaluating the cost and benefit of various types of travel insurance (eg, travel cancellation, medical treatment, evacuation for medical emergency) in advance of travel is important, as unexpected hospitalizations or emergent needs may arise. Some home care companies assist in this planning and may even ship PN and other supplies to the family’s destination. In situations that demand it, normal saline or standard dextrose-containing fluids may be used in place of PN to maintain hydration. Finally, the US Transportation Security Administration (TSA) disability office is reachable by phone or email. The assistance of a TSA Passenger Support Specialist should be requested at least 72 hours before travel, particularly as ‘‘checking’’ PN and supplies risks greatly compounding the inconvenience of lost luggage. See the TSA Web site for more information about traveling with medical conditions (https://www.tsa.gov/news/press/releases/2016/11/15/tsa-shares-tips-travelers-disabilities-medical-devices-medical).
Emergency Preparedness
Children with IF count themselves among the estimated 13.7 million American children with special health care needs, a vulnerable population dependent on life-sustaining health care technology (48). This places them at grave danger in the event of a natural or manmade disaster (49). The development of a disaster plan and disaster kit, including dextrose-containing solution, has been advocated for all families, though a recent report found the majority of patients followed in an IRP are unprepared (50). Needs of the child with IF include timely PN delivery, PN refrigeration, electrically powered infusion pumps, access to clean dressing supplies, and frequent laboratory assessment. Variability of disaster risk and individual needs of the patient and family make a generic, prescriptive approach unhelpful. Yet, completion and maintenance of an ‘‘Emergency Information Form’’ or standardized emergency letter in multiple formats (eg, digital, laminated physical copy, and so on), creation of an emergency supply kit including an additional power source, and thoughtful consideration of other aspects of family planning may improve confidence and the chance of event-free survival in even the unlikeliest of disasters (44,49). Emergency preparedness for children with IF has been tested due to the recent COVID-19 global pandemic. IRP have reported shortages of personal protective equipment required for in-home central line care, disruption of home nursing visits, decreased frequency of in-person clinic visits, and delayed procedures all of which have potential effects on central venous access (51).
3. Recommendations regarding general considerations—sports, travel, and emergencies:
All children with IF should be provided with an emergency letter that details the specific needs of the individual child in case of an emergency.
Discuss with families the risks of swimming and sports participation with strategies to protect the dressing and central line.
All travel plans should be discussed with the intestinal rehabilitation team well in advance of travel to facilitate discussion of a plan of care in case of emergency.
COMPLICATIONS
Central Line-Associated Blood Stream Infections
As sepsis remains a major source of mortality in the IF population, timely recognition and proper management of CLABSI is critical (52). Most commonly, the presentation involves fever (temperature ≥ 100.48F/38.0ºC) with variable clinical appearance. Rates of bacteremia may be as high as 70% in PN dependent children with CVC who present with fever, regardless of additional characteristics (53,54). Therefore, any child with a CVC and fever should be evaluated for bacteremia, even in the setting of other localizing symptoms.
Initial treatment of these children should begin with empiric antimicrobial therapy infused through the CVC while the appropriate diagnostic evaluation proceeds. Fluid resuscitation is an integral part of the initial management in cases of cardiovascular compromise due to sepsis. In these scenarios, early intensivist involvement may allow for vasoactive medications to support cardiovascular status. Care can be aided by protocols integrated into the health record at referral centers (55,56); however, many children rely on initial stabilization at a local hospital before transfer to their primary IRP center. In these scenarios, standardized emergency letters and timely communication can aid in expediting the care that they receive. IF emergency letters can be incorporated into the electronic medical record (currently available for all centers in the foundation system of the EPIC as ‘‘Emergent Care Letter for Pediatric Intestinal Failure’’) (Fig. 1).
FIGURE 1.

Standardized emergency letter for children with intestinal failure.
Primary diagnostic evaluation involves at a minimum blood culture of all lumens of the CVC with many IRP also obtaining a peripheral culture. Additional evaluation for an alternative fever source should be considered on a case-by-case basis, such as urine testing, seasonal viral testing, or chest radiography to evaluate respiratory symptoms. At this time, validated models to predict bacteremia based on clinical characteristics remain in development (57).
Empiric antimicrobial therapy should target a broad range of common pathogens involved in CLABSI. Typical regimens include antipseudomonal penicillin, third- or fourth-generation cephalosporin, or carbapenem to provide broad Gram-negative coverage including Pseudomonas. Vancomycin is often added to this regimen to provide coverage against Staphylococcal species, including methicillin-resistant staphylococci and coagulase-negative species that must be treated as true pathogens in this population. In addition to the typical pathogens, a recent study of pediatric home PN patients also highlighted the importance of focusing on institutional-specific susceptibilities (58). In patients with repeated antimicrobial exposure, it may become necessary to tailor antimicrobial regimens to individual pathogen history, especially as multidrug resistant bacteria become more prevalent; however, because repeat infections with identical species are uncommon, targeting antimicrobial therapy should not delay timely antibiotic administration. Fungal pathogens make up a small but significant portion of CLABSI, especially in those with repeated antibiotic exposure (53). In the well-appearing child, antifungal therapy can often be deferred at the time of presentation; however, fungal coverage with a fungicidal drug such as caspofungin should be strongly considered in the ill-appearing child or if clinical concern persists despite adequate antibacterial coverage.
Children with suspected CLABSI should be observed in the hospital on empiric antibiotic therapy infused through the CVC regardless of other infectious sources found. The optimal length of observation has long been considered 48 h, although more recent work suggests that 24 h may be sufficient (59). If bacteremia is identified, repeat CVC cultures should be obtained every 24 h until the bacteremia clears. Patients should be observed until full antimicrobial sensitivity is available to tailor the antimicrobial regimen to the individual pathogen. It is important to note the high rate of polymicrobial bacteremia in this population (53,54). As such, the identification of a single pathogen should not lead to narrowing of antibiotic coverage until at least 48 h after the initial culture was obtained, at which point it is safe to assume that no other pathogens are present.
With the concern for long-term vascular access, most CLABSI in children with IF can be treated through the central line to clear the infection and salvage the line (60,61). CVC removal should be reserved for persistently positive blood cultures (3 days of positive cultures) despite appropriate antimicrobial therapy, recurrence of infection with the same organism suggesting biofilm formation within the CVC, or in the setting of severe clinical compromise. In this setting, negative blood cultures and a period free of central access are ideal before line reinsertion at a new site. It was believed that CLABSI was an indication for removing a CVC; however, the success of treating through and clearing these infections and the increased application of antimicrobial lock therapy, both therapeutically and prophylactically, has limited line removal to only refractory or severe infections. In fact, the majority may continue to receive PN via the CVC while being treated for a CLABSI.
Ethanol locks are used for treatment and prevention of CLABSI. Most studies have examined the effectiveness of daily 70% ethanol, although promising results were seen in a recent pilot study of 30% ethanol–2.8% citrate locks (62). A meta-analysis in pediatric IF suggests that prophylactic use of 70% ethanol locks significantly reduces the rate of CLABSI compared to heparin locks (63). In these studies, ethanol locks were used anywhere from weekly to daily with at least 2 h dwell time. Ethanol locks have also decreased the need for line removal when used as an adjunct to standard antimicrobial therapy in the treatment of CLABSI (64,65). Concerns exist around effects of ethanol locks on CVC integrity. Initial studies reported a detrimental effect of ethanol treatment on polyurethane catheter integrity, leading most centers to use ethanol locks exclusively in silicone catheters (66). Further in vitro work has shown a negligible effect on the mechanical properties of both silicone and polyurethane catheters (67); however, in both instances, the findings were not determined to be clinically relevant by the authors. Despite a few studies showing higher rates of thrombosis, catheter occlusion, and line repairs in those receiving ethanol locks, the clear benefits of decreasing infection rate lead to an overall decrease in line replacements and favor their use (63).
Anecdotally, families occasionally describe difficulty withdrawing the ethanol, especially in smaller diameter catheters. With the low surface tension of ethanol, drawing back slowly and gently may improve the success of removal. These difficulties have led to differences in protocols between flushing or aspirating following the dwell. There have been retrospective studies that suggest the use of ethanol locks can cause mild systemic symptoms regardless of flushing or drawing back, although notably no evidence of toxicity was reported in a prospective study in infants using a flushing protocol (68,69).
Ethanol lock shortages have occurred and were associated with increased CLABSI rates when locks were used on less of a frequent basis (70). Alternatives to ethanol exist for both treatment and prophylaxis. With the unpredictability of the ethanol supply and concerns for catheter effects, access to these alternative antimicrobial lock therapies is needed. Antibiotic lock therapy effectively prevents bacteremia from sensitive organisms, although use has been restricted due to the risk of resistance (71). A small pilot study showed that adding amphotericin B based lock therapy to the treatment of Candida spp. can treat persistent fungemia and potentially lead to line clearance (72). Taurolidine, although approval has not been pursued in the United States, is an effective prophylactic lock with a significant decrease in the incidence of CLABSI and no reported adverse events (73–75). Sodium EDTA locks are gaining favor in Canada, however, published evidence in the dialysis population suggests an increase in thrombotic events despite the decrease in CLABSI rate (76).
Exit site infections can be identified when erythema and purulent drainage are found at the catheter exit site. The Infectious Disease Society of America recommends obtaining a swab of any drainage for culture to target topical antibiotic therapy to an identified organism (77). When children do not respond to topical antibiotics, systemic antibiotics should be considered even in the absence of systemic symptoms such as fever (77). In resistant cases, line replacement should be considered. Tunnel and pocket infections occur when the exit site infection extends into the poorly vascularized subcutaneous space occupied by a tunneled catheter or fully implanted central venous access device respectively. Both tunnel and pocket infections are considered absolute indications for replacement of central venous access due to their poor response to systemic antimicrobial therapy (77).
4. Recommendations regarding central line-associated bloodstream infections:
All children with IF and CVC who develop a fever (≥38.0ºC) should be admitted to the hospital and assessed for bacteremia with central and peripheral blood cultures while receiving broad-spectrum empiric antibiotics through the CVC for at least 48 h, awaiting culture results regardless of other infectious sources.
If clinically stable, discuss with the patient’s IRP before line removal for CLABSI.
Prophylactic lock therapy with ethanol or other nonantibiotic locks should be strongly considered in all children with IF who have had at least one central line-associated bloodstream infection or are at high risk for infection.
MECHANICAL COMPLICATIONS
Mechanical CVC complications seen in patients with IF include line breaks, line occlusions, and vascular thromboses. According to recent American Society of Parenteral and Enteral Nutrition (ASPEN) CVC guidelines for adult home PN patients, there is no difference in the type of CVC and mechanical complication (polyurethane vs silicone, PICC vs tunneled, implanted ports vs tunneled) (78). Mechanical CVC complications can result in a sudden loss of line function, resulting in abrupt discontinuation of PN and rebound hypoglycemia (79). Emergency care may need to be sought for the reestablishment of access depending on the amount of parenteral support needed and ability of the patient to tolerate enteral feeds.
Efforts should be made to prevent and treat mechanical complications to avoid premature CVC removal. As with treatment for CLABSI, ‘‘save the line’’ should be the prevailing dogma in managing mechanical CVC complications in IF patients, reserving line replacement for circumstances in which the line cannot be salvaged.
Line Breaks and Repairs
Breakage of the external portion of the catheter is a frequent complication in pediatric patients with rates reported from 0.04 to 5.6/1000 catheter days (80,81). This is due to line clamping, direct trauma, material wear, repeated kinking, and attempts to unclog catheters. These issues can be exacerbated by inappropriate central line dressing leaving the thin portion of the CVC exposed and susceptible to breakage. Standard of care in pediatric IF patients is to repair the catheter to avoid CVC replacement and preserve vascular access. While all centers repair tunneled CVC when possible, some IRP also repair PICC lines.
There is a theoretical risk of bacteremia due to the exposed CVC lumen and catheter manipulation during repair. While concerns were raised by Lungdren et al about the increased risk of CLABSI after catheter repair in children, more recent retrospective studies specific to pediatric IF patients have shown CVC repair to be highly successful with a low risk of infection (80,82,83). McNiven et al found no catheter-repair associated CLABSIs, while Chan et al reported a CLABSI in only one repair (1%) in an immunocompromised transplant patient (82,83). As the integrity of the CVC may be compromised with each repair, some IRP limit the number of repairs allowed before replacement.
There are no studies to date examining postrepair prophylactic antibiotic use. Evidence does not support routine blood culture and empiric antibiotic treatment after CVC repair, unless the fever is also present. Patients should be instructed to present immediately for any CVC breakage, and timely repair should be attempted whenever possible.
CVC Occlusions
Catheter occlusions and CRT are common complications in patients with long-term CVC. These can increase morbidity, interrupt treatment, and require CVC removal. Within 1–2 years of catheter placement, 14–36% of patients will experience a CVC occlusion (84). Multiple lumens, a distal CVC tip, and tunneled CVC are at higher risk of developing occlusions which can be partial (inability to aspirate blood but the ability to flush or infuse through the catheter) or complete (inability to aspirate, flush or infuse via the catheter) (85). Potential etiologies include mechanical or postural factors, precipitate (medication or PN), catheter malposition, or thrombosis. Treatment requires an accurate diagnosis of the cause of catheter occlusion. In the approach to CVC occlusion, mechanical obstruction and malposition should be ruled out first, followed by evaluation for precipitate and thrombus. If the clinical assessment does not yield the etiology, radiography or a trial of tissue plasminogen activator (tPA) should be pursued (Fig. 2).
FIGURE 2.
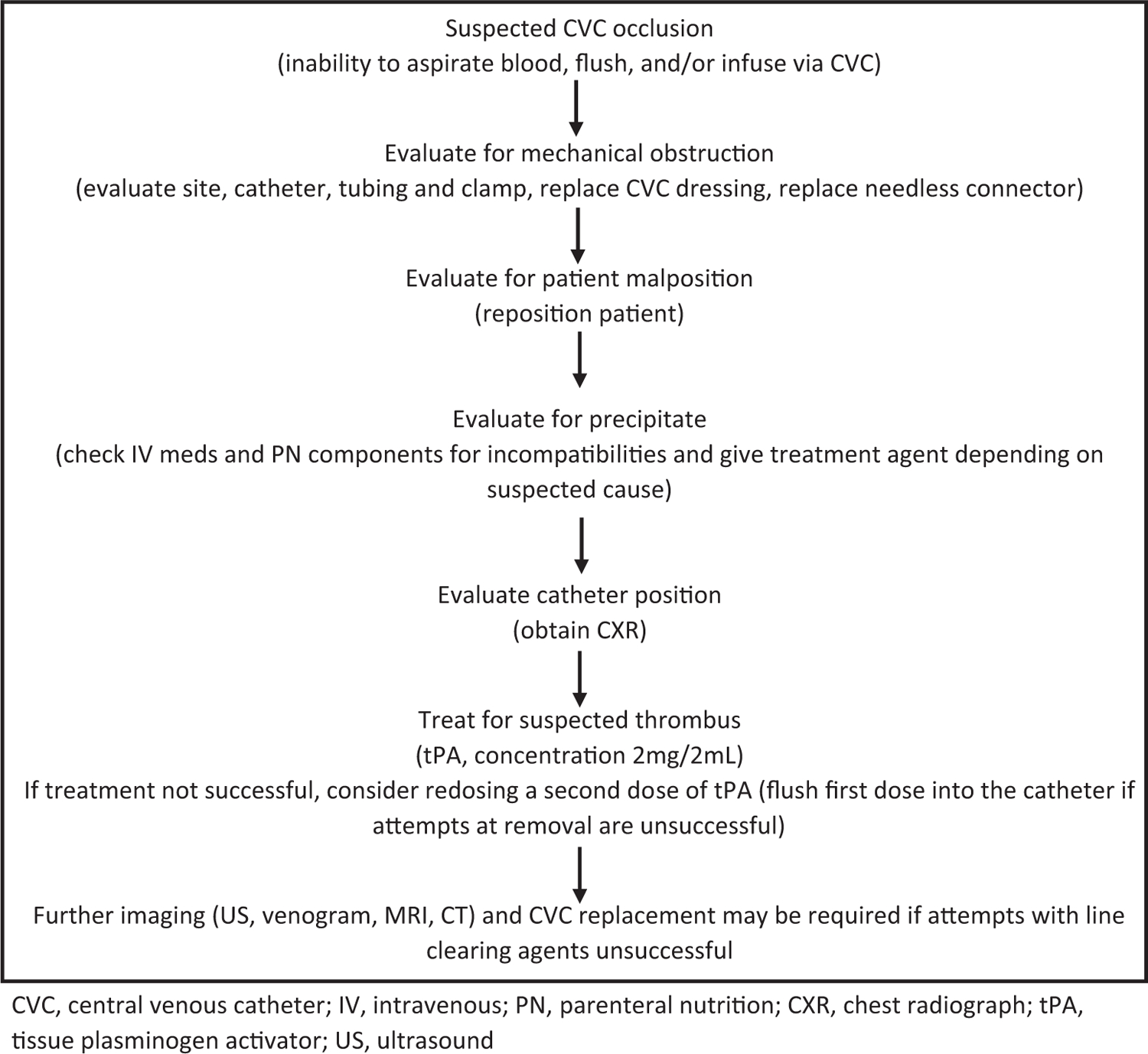
Suspected central venous catheter occlusion algorithm.
CVC, central venous catheter; IV, intravenous; PN, parenteral nutrition; CXR, chest radiograph; tPA, tissue plasminogen activator; US, ultrasound
Mechanical Obstruction
Mechanical obstruction can occur at the CVC site, along the tubing, or as a result of a blood vessel wall occluding the catheter tip. The pinch-off syndrome is a rare but potentially life-threatening mechanical obstruction, in which the catheter passes through the narrow angle between the first rib and the lateral portion of the clavicle (called the pinch-off area). This puts the catheter at risk for compression or fracture, which can lead to embolization of the catheter tip into the central vascular system (84).
To evaluate for external mechanical obstructions, the site should be checked to ensure that tight sutures are not pinching the catheter and that the line does not appear to have been displaced or dislodged. The catheter and external tubing should be checked for a kink and to ensure the clamp is open. The patient should then be repositioned to rule out catheter tip occlusion by the blood vessel wall. Repositioning can be accomplished by raising the patient’s ipsilateral arm, moving the patient to a standing or sitting position, or rolling the patient onto their side. If there are no obvious site issues and repositioning maneuvers fail, a chest radiograph should be obtained to check the catheter position and to look for an internal kink in the catheter. The latter may not be seen on routine chest radiograph, and a dye study might be needed for diagnosis (84). If positional CVC dysfunction continues, the pinch-off syndrome should be ruled out by fluoroscopy.
Precipitate
Medications and PN components can precipitate in the line, leading to increasingly sluggish flow through the catheter and eventual occlusion. Incompatible mixtures or inappropriate medication concentration can cause precipitation (86). Common causes of precipitates include calcium phosphate crystals, lipid residue from PN, inappropriate pH of infusions, and heparin/ethanol lock incompatibility. An IRP or home care company pharmacist should regularly review intravenous medications and PN components for incompatibilities.
Treatment of a precipitate may include fibrinolytic and nonfibrinolytic agents depending on the suspected cause. Nonfibrinolytic agents aim to increase precipitate solubility by changing the pH in the catheter lumen. Treatments include 70% ethanol for lipid residue and sodium bicarbonate or sodium hydroxide for basic medications. Acidic medications and calcium phosphate crystals have been treated with 0.1% hydrochloric acid. This practice has been largely discontinued due to concerns about damaging the CVC (84,87). To help prevent medication-precipitate occlusion, the CVC should be flushed with normal saline between each medication dose and incompatible agents should not be given at the same time. Mineral precipitates should be prevented by careful review of PN components, as maintaining appropriate calcium and phosphorus ratios avoid precipitation of calcium phosphate crystals. To prevent ethanol/heparin precipitation, the use of heparin is discouraged with concurrent use of ethanol locks (88). If heparin must be used, it is recommended that the ethanol lock be removed, the line flushed with normal saline, and heparin then instilled into the CVC.
Thrombotic Occlusion
A thrombotic process, such as the development of a fibrin sheath around the catheter tip, a clot within the CVC, or CRT can lead to CVC occlusion. Fibrin sheaths can occur within 24 h to 2 weeks after CVC placement. While catheter function is usually unaffected, a fibrin sheath can lead to a partial occlusion when withdrawing from the catheter. Occasionally, occlusion due to a fibrin sheath can be released by flushing or infusing the catheter. Intraluminal thrombus within the CVC causes 5–25% of all catheter occlusions and may result in complete occlusion (84). Most studies in adults and children have shown no difference between saline or heparin flushes in terms of intraluminal CVC clot prevention, although heparin flushes are more expensive and have the potential for heparin-induced thrombocytopenia (38). Randomized studies are needed to determine the ideal flush solution, concentration, frequency, efficacy, and cost-effectiveness of prophylactic thrombolytic flushes. Although increased catheter thrombosis rates have been reported with the use of ethanol lock therapy, larger studies are needed to assess the effect of dwell time, frequency and concentration of ethanol locks on intraluminal CVC thrombosis rates (78,89).
Suspected intraluminal CVC thrombus can be empirically treated with tPA, the only FDA-approved thrombolytic. tPA initiates fibrinolysis by catalyzing the conversion of plasminogen to plasmin (84). Although there are no studies that look at the use of tPA at home versus in the emergency department, it is often administered at home by a registered nurse. Large studies have confirmed its efficacy in children (83–95%) with no adverse events reported and major hemorrhage reported in only 0.3% of adult patients (84,85). Any tPA released into circulation is rapidly metabolized by the liver (plasma half-life < 5 min), making systemic complications such as bleeding unlikely, although tPA should be used with caution in patients at high risk of bleeding or embolic complications (38).
If tPA fails to clear the catheter, a guidewire can be inserted through the catheter lumen to dislodge a thrombus at the tip of the CVC. Fibrin sheath stripping has also been used for CVC occlusion that fails medical management. Although effective if done by experienced interventional radiologists, these procedures are more invasive and only used as a last resort in patients with limited vascular access (84).
Children with IF are at risk of CRT secondary to endothelial damage, disrupted blood flow from the CVC, and hypercoagulable states such as CLABSI, high PN dextrose or calcium concentration, and inherited thrombophilic conditions (90). A mural thrombus (a blood clot that adheres to the vessel wall) can occlude the tip of the catheter and cause partial occlusion or can progress into a deep venous thrombosis that leads to complete occlusion. Complications associated with CRT include increased risk of CLABSI, pulmonary embolism, postthrombotic syndrome, and superior vena cava (SVC) syndrome (38,91).
Up to 50% of children with long-term CVCs develop CRT, although 50% of those are asymptomatic (84). Only 12% present with pain, erythema, warmth, swelling, tenderness to palpation, and development of collateral vessels in the surrounding area (84). In a review of 30 pediatric IF patients, 40% had >2 thrombosed central veins, which may affect long-term survival or transplantation requirement (92). See the Radiographic surveillance of CVC section for CRT evaluation and monitoring recommendations.
Although the data is weak, treatment of newly formed CRT with low molecular weight heparin is generally recommended for 6 weeks to 3 months, dependent on the extent of the thrombus, initial response to therapy, and whether thrombophilic factors persist (93). Hematology consultation is also helpful to evaluate these factors and aid in anticoagulation management. Initial anticoagulant therapy aims to prevent thrombus extension and subsequent pulmonary embolization (38). Some centers discontinue anticoagulation after the treatment period if there are no signs of chronic thrombus. Given the high risk of recurrence, many continue prophylactic anticoagulation until the CVC is removed (94). Pediatric guidelines from the American College of Chest Physicians recommend prophylactic anticoagulation for long-term home PN in general, although these recommendations are not based on pediatric IF patients (95). Although more data is needed, there is some suggestion that newer intravenous lipid emulsions containing fish oil may be associated with decreased CRT (96,97).
With improvements in intestinal rehabilitation, the criteria for intestinal transplant referral have changed over the years, although CRT and the resulting loss of central access have continued to be an important indication. While previous criteria included loss of two of four discrete upper body central veins (left and right subclavian and internal jugular veins), a recent revision of the listing criteria modified that recommendation to thrombosis of three of four sites or occlusion of a brachiocephalic vein in children (3). These recommendations were supported by a single-center study by Burghardt et al that showed significantly improved prediction of need for intestinal transplant with loss of three rather than two CVC sites (2). Patients who have lost multiple central venous access sites should be referred to an intestinal transplant center for evaluation and management before access is so limited that an intestinal transplant is no longer an option.
Line Replacement
Efforts to prevent and treat mechanical failures and CLABSI to avoid premature removal of a CVC are crucial to preserve vascular access for pediatric IF patients whose survival is dependent on longterm PN; however, line replacement should be considered in serious infections (persistent bacteremia/fungemia, life-threatening sepsis, tunnel infection), malfunction (precipitate, thrombus, breakage that cannot be cleared/repaired), malposition, displacement, internal kinking, SVC syndrome, and pinch-off syndrome (77). Although lines replaced for infectious causes require placement in a different location, lines removed due to malpositioning or breaks can often be replaced in the same location using a guidewire to preserve the site of access (98). In general, the benefits of CVC removal must be weighed against the difficulty of obtaining alternate access.
5. Recommendations pertaining to central line mechanical complications:
In children with IF, CVC should be repaired whenever possible to preserve central venous access.
Children with IF and a newly identified CRT should be treated with low molecular weight heparin for at least 6 weeks with guidance from a hematologist.
Children with IF who have persistence of at least one chronic thrombus should be maintained on prophylactic anticoagulation with low molecular weight heparin.
Children who have lost multiple sites of central venous access should be considered for referral to an intestinal transplant center for evaluation and management.
CENTRAL VENOUS ACCESS PROGRAM MANAGEMENT
To create guidelines and policies surrounding long-term central venous access, widespread, collaborative data collection is needed (6,99). To achieve this goal, standardized data collection is needed at individual centers taking care of patients with IF. Recent guidelines published by the VANGUARD Task Force outline data elements of a venous access record and recommend an individualized vascular plan of care for all patients requiring prolonged central venous access (6). The vascular record and plan should be included in the medical record where it is accessible by all central venous access stakeholders (6). See Fig. 2 for a sample data element list, Supplemental Digital Content, http://links.lww.com/MPG/C153.
No national benchmarks exist that are specific to central venous access in IF patients, although inpatient pediatric CLABSI rates have been tracked by The Children’s Hospitals’ Solutions for Patient Safety with a rate of 1.3 infections/1000 catheter days in all pediatric patients with CVC (100). With the introduction of CVC care bundles, including ethanol lock therapy, many IRP have successfully decreased overall CLABSI rates in IF patients to near or below these levels despite significant CLABSI risk factors (21,22).
6. Recommendations for central venous access program management:
All centers following children with IF should, at a minimum, track the number of outpatient CLABSI per 1000 catheter days.
CONCLUSIONS
With the improvement in care and survival associated with the development of specialized multidisciplinary intestinal rehabilitation centers, children with IF are living longer and requiring specialized management of their central access. Loss of access can necessitate intestinal transplantation, whereas the total loss of access could preclude this potentially life-saving option. With careful management, central venous access in children with IF can be preserved while avoiding the potential complications and improving quality of life (Table 3).
TABLE 3.
Summary of recommendations for management of central venous access in pediatric intestinal failure
| Types of central venous acces 1. Tunneled, single lumen, cuffed silicone catheters should be used for children with intestinal failure. 2. Upper extremity access is the preferred location when available. 3. Alternative line sites and endovascular procedures should only be done by experienced interventionalists in discussion with an intestinal transplant center when transplant evaluation has been or will be sought. Routine care of central venous catheter 1. Proper technique and hygiene surrounding central venous catheter care are of paramount importance in preventing central venous catheter-associated complications. Caregivers should receive directed education regarding central venous catheter cares before initial discharge, with subsequent reinforcement education as needed. 2. Chlorhexidine gluconate impregnated supplies (disk, sponge, or dressing) should be considered for central line dressing in pediatric intestinal failure patients. 3. Routine surveillance of central venous access should be performed by ultrasonography. MR, CT, or traditional venography should be reserved for assessment for when further delineation of access is required. Additional considerations for care 1. All children with intestinal failure should be provided with an emergency letter that details specific needs of the individual child in case of an emergency. 2. Discuss with families the risks of swimming and sports participation with strategies to protect the dressing and central line. 3. All travel plans should be discussed with the intestinal rehabilitation team well in advance of travel to facilitate discussion of a plan of care in case of emergency. Central line-associated bloodstream infections 1. All children with intestinal failure and central venous catheter who develop a fever (≥38.0ºC) should be admitted to the hospital and assessed for bacteremia with central and peripheral blood cultures while receiving broad-spectrum empiric antibiotics through the central venous catheter for at least 48 h, awaiting culture results regardless of other infectious sources. 2. If clinically stable, discuss with the patient’s intestinal rehabilitation program before line removal for central line-associated bloodstream infection. 3. Prophylactic lock therapy with ethanol or other nonantibiotic locks should be strongly considered in all children with intestinal failure who have had at least one central line-associated bloodstream infection or are at high risk for infection. Mechanical complications 1. In children with intestinal failure, central venous catheters should be repaired whenever possible to preserve central venous access. 2. Children with intestinal failure and a newly identified catheter-related thrombus should be treated with low molecular weight heparin for at least 6 weeks with guidance from a hematologist. 3. Children with intestinal failure who have persistence of at least one chronic thrombus should be maintained on prophylactic anticoagulation with low molecular weight heparin. 4. Children who have lost multiple sites of central venous access should be considered for referral to an intestinal transplant center for evaluation and management. Central venous access program management 1. All centers following children with IF should track the number of outpatient central line-associated bloodstream infections per 1000 catheter days. |
Supplementary Material
What Is Known
Pediatric patients with intestinal failure require long-term central venous access for parenteral nutrition.
Loss of central venous access is a common indication for an intestinal transplant.
What Is New
The present position paper recommends general principles to optimize central venous access management of children with intestinal failure.
Acknowledgments:
The authors gratefully acknowledge the assistance of the following Intestinal Failure Special Interest Group members who supplied images included in the Central Venous Access Image Library available as Supplemental Digital Content: Dr John Thompson, Dr Joanne Lai, and Dr Catherine Larson-Nath. Kelly Reichert MS, RN, FNP-C, CCRN and Sara J Fidanza, MS, RN, CNS-BC, CPNP-PC also significantly contributed to the table of internal volumes of central venous catheters found in the Supplemental Digital Content.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
The authors report no conflicts of interest.
Publisher's Disclaimer: Disclaimers: The NASPGHAN practice guidelines and position papers are evidence-based decision-making tools for managing health conditions. They are authorized by the NASPGHAN Executive Council, peer reviewed, and periodically updated. They are not to be construed as standards of care and should not be construed as establishing a legal standard of care or as encouraging, advocating, requiring, or discouraging any particular treatment. All decisions regarding the care of a patient should be made by the health care team, patient, and family in consideration of all aspects of the individual patient’s specific medical circumstances. While NASPGHAN makes every effort to present accurate and reliable information, these guidelines are provided ‘‘as is’’ without any warranty of accuracy, reliability, or otherwise, either express or implied. NASPGHAN does not guarantee, warrant, or endorse the products or services of any firm, organization, or person. Neither NASPGHAN nor its officers, directors, members, employees, or agents will be liable for any loss, damage, or claim with respect to any liabilities, including direct, special, indirect, nor consequential damages, incurred in connection with the guidelines or reliance on the information presented.
REFERENCES
- 1.Duggan CP, Jaksic T. Pediatric intestinal failure. N Engl J Med 2017;377:666–75. [DOI] [PubMed] [Google Scholar]
- 2.Burghardt KM, Wales PW, de Silva N, et al. Pediatric intestinal transplant listing criteria – a call for a change in the new era of intestinal failure outcomes. Am J Transplant 2015;15:1674–81. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman SS, Avitzur Y, Beath SV, et al. New insights into the indications for intestinal transplantation: consensus in the year 2019. Transplantation 2020;104:937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond IR, de Silva N, Pencharz PB, et al. Neonatal short bowel syndrome outcomes after the establishment of the first Canadian multidisciplinary intestinal rehabilitation program: preliminary experience. J Pediatr Surg 2007;42:806–11. [DOI] [PubMed] [Google Scholar]
- 5.Merras-Salmio L, Pakarinen MP. Refined multidisciplinary protocol-based approach to short bowel syndrome improves outcomes. J Pediatr Gastroenterol Nutr 2015;61:24–9. [DOI] [PubMed] [Google Scholar]
- 6.Baskin KM, Mermel LA, Saad TF, et al. Evidence-based strategies and recommendations for preservation of central venous access in children. JPEN J Parenter Enteral Nutr 2019;43:591–614. [DOI] [PubMed] [Google Scholar]
- 7.LaRusso K, Schaack G, Fung T, et al. Should you pick the PICC? Prolonged use of peripherally inserted central venous catheters in children with intestinal failure. J Pediatr Surg 2019;54:999–1004. [DOI] [PubMed] [Google Scholar]
- 8.Christensen LD, Holst M, Bech LF, et al. Comparison of complications associated with peripherally inserted central catheters and Hickman™ catheters in patients with intestinal failure receiving home parenteral nutrition. six-year follow up study. Clin Nutr 2016;35:912–7. [DOI] [PubMed] [Google Scholar]
- 9.Shin HS, Towbin AJ, Zhang B, et al. Venous thrombosis and stenosis after peripherally inserted central catheter placement in children. Pediatr Radiol 2017;47:1670–5. [DOI] [PubMed] [Google Scholar]
- 10.Allen AW, Megargell JL, Brown DB, et al. Venous thrombosis associated with the placement of peripherally inserted central catheters. J Vasc Interv Radiol 2000;11:1309–14. [DOI] [PubMed] [Google Scholar]
- 11.Piper HG, de Silva NT, Amaral JG, et al. Peripherally inserted central catheters for long-term parenteral nutrition in infants with intestinal failure. J Pediatr Gastroenterol Nutr 2013;56:578–81. [DOI] [PubMed] [Google Scholar]
- 12.Blotte C, Styers J, Zhu H, et al. A comparison of broviac((R)) and peripherally inserted central catheters in children with intestinal failure. J Pediatr Surg 2017;52:768–71. [DOI] [PubMed] [Google Scholar]
- 13.Toh LMHW, Mavili E, Moineddin R, et al. Are cuffed peripherally inserted central catheters superior to uncuffed peripherally inserted central catheters? A retrospective review in a tertiary pediatric center. J Vasc Interv Radiol 2013;24:1316–22. [DOI] [PubMed] [Google Scholar]
- 14.O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control 2011;39(Suppl):S1–34. [DOI] [PubMed] [Google Scholar]
- 15.Janik JE, Conlon SJ, Janik JS. Percutaneous central access in patients younger than 5 years: size does matter. J Pediatr Surg 2004;39: 1252–6. [DOI] [PubMed] [Google Scholar]
- 16.Early TF, Gregory RT, Wheeler JR, et al. Increased infection rate in double-lumen versus single-lumen Hickman catheters in cancer patients. South Med J 1990;83:34–6. [DOI] [PubMed] [Google Scholar]
- 17.Parienti JJ, Mongardon N, Megarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med 2015;373:1220–9. [DOI] [PubMed] [Google Scholar]
- 18.Ge X, Cavallazzi R, Li C, et al. Central venous access sites for the prevention of venous thrombosis, stenosis and infection. Cochrane Database Syst Rev 2012;3:CD004084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigues AF, van Mourik ID, Sharif K, et al. Management of end-stage central venous access in children referred for possible small bowel transplantation. J Pediatr Gastroenterol Nutr 2006;42:427–33. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan PM, Merritt R, Pelayo JC, et al. Recanalization of occluded central veins in a parenteral nutrition-dependent child with no access. Pediatrics 2018;141(Suppl 5):S416–20. [DOI] [PubMed] [Google Scholar]
- 21.Ardura MI, Lewis J, Tansmore JL, et al. Central catheter-associated bloodstream infection reduction with ethanol lock prophylaxis in pediatric intestinal failure: Broadening quality improvement initiatives from hospital to home. JAMA Pediatr 2015;169:324–31. [DOI] [PubMed] [Google Scholar]
- 22.Ormsby JA, Bukoye B, Lajoie D, et al. Enhanced central venous catheter bundle for pediatric parenteral-dependent intestinal failure. Am J Infect Control 2018;46:1284–9. [DOI] [PubMed] [Google Scholar]
- 23.Schulman J, Stricof R, Stevens TP, et al. Statewide NICU central-line-associated bloodstream infection rates decline after bundles and checklists. Pediatrics 2011;127:436–44. [DOI] [PubMed] [Google Scholar]
- 24.Rinke ML, Bundy DG, Chen AR, et al. Central line maintenance bundles and CLABSIs in ambulatory oncology patients. Pediatrics 2013;132:e1403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller MR, Niedner MF, Huskins WC, et al. Reducing PICU central line-associated bloodstream infections: 3-year results. Pediatrics 2011;128:e1077–83. [DOI] [PubMed] [Google Scholar]
- 26.Pittiruti M, Hamilton H, Biffi R, et al. ESPEN guidelines on parenteral nutrition: central venous catheters (access, care, diagnosis and therapy of complications). Clin Nutr 2009;28:365–77. [DOI] [PubMed] [Google Scholar]
- 27.Drews B, Macaluso M, Piper H, et al. Caregiver education reduces the incidence of community-acquired CLABSIs in the pediatric patient with intestinal failure. Gastroenterol Nurs 2017;40:458–62. [DOI] [PubMed] [Google Scholar]
- 28.Raphael BP, Takvorian-Bene M, Gallotto M, et al. Learning gaps and family experience, nurse-facilitated home parenteral nutrition simulation-based discharge training: proof-of-concept study. Nutr Clin Pract 2019. Advance online publication. [DOI] [PubMed] [Google Scholar]
- 29.Chesshyre E, Goff Z, Bowen A, et al. The prevention, diagnosis and management of central venous line infections in children. J Infect 2015;71(Suppl 1):S59–75. [DOI] [PubMed] [Google Scholar]
- 30.Gibbons S, Richardson DS. Central venous catheter care. In: Duggan CP, Jaksic T, Gura KM, eds. Clinical Management of Intestinal Failure 1st ed Boca Raton, FL: CRC Press; 2011:331–58. [Google Scholar]
- 31.Chaiyakunapruk N, Veenstra DL, Lipsky BA, et al. Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: a meta-analysis. Ann Intern Med 2002;136:792–801. [DOI] [PubMed] [Google Scholar]
- 32.Johnson J, Bracken R, Tamma PD, et al. Trends in chlorhexidine use in US neonatal intensive care units: results from a follow-up national survey. Infect Control Hosp Epidemiol 2016;37:1116–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann KK, Weber DJ, Samsa GP, et al. Transparent polyurethane film as an intravenous catheter dressing. A meta-analysis of the infection risks. JAMA 1992;267:2072–6. [PubMed] [Google Scholar]
- 34.Duesing LA, Fawley JA, Wagner AJ. Central venous access in the pediatric population with emphasis on complications and prevention strategies. Nutr Clin Pract 2016;31:490–501. [DOI] [PubMed] [Google Scholar]
- 35.Garland JS, Alex CP, Mueller CD, et al. A randomized trial comparing povidone-iodine to a chlorhexidine gluconate-impregnated dressing for prevention of central venous catheter infections in neonates. Pediatrics 2001;107:1431–6. [DOI] [PubMed] [Google Scholar]
- 36.Voor In’t Holt AF, Helder OK, Vos MC, et al. Antiseptic barrier cap effective in reducing central line-associated bloodstream infections: a systematic review and meta-analysis. Int J Nurs Stud 2017;69:34–40. [DOI] [PubMed] [Google Scholar]
- 37.Boullata JI, Gilbert K, Sacks G, et al. A.S.P.E.N. clinical guidelines: parenteral nutrition ordering, order review, compounding, labeling, and dispensing. JPEN J Parenter Enteral Nutr 2014;38:334–77. [DOI] [PubMed] [Google Scholar]
- 38.van Ommen CH, Tabbers MM. Catheter-related thrombosis in children with intestinal failure and long-term parenteral nutrition: how to treat and to prevent? Thromb Res 2010;126:465–70. [DOI] [PubMed] [Google Scholar]
- 39.Tan PL, Gibson M. Central venous catheters: the role of radiology. Clin Radiol 2006;61:13–22. [DOI] [PubMed] [Google Scholar]
- 40.Chick JF, Reddy SN, Bhatt RD, et al. Significance of echocardiographically detected central venous catheter tip-associated thrombi. J Vasc Interv Radiol 2016;27:1872–7. [DOI] [PubMed] [Google Scholar]
- 41.Moukarzel AA, Haddad I, Ament ME, et al. 230 patient years of experience with home long-term parenteral nutrition in childhood: natural history and life of central venous catheters. J Pediatr Surg 1994;29:1323–7. [DOI] [PubMed] [Google Scholar]
- 42.Corrigan ML, Huang S, Weaver A, et al. Resources for the provision of nutrition support to children in educational environments. Nutr Clin Pract 2017;32:834–43. [DOI] [PubMed] [Google Scholar]
- 43.Lyon L. School assessment form for students with special health care needs. NASN Sch Nurse 2012;27:288–92. [DOI] [PubMed] [Google Scholar]
- 44.Toor KT, Burke RV, Demeter NE, et al. Improving disaster preparedness of families with a parenteral nutrition-dependent child. J Pediatr Gastroenterol Nutr 2018;67:237–41. [DOI] [PubMed] [Google Scholar]
- 45.Coleman N, Nemeth BA, LeBlanc CMA. Increasing wellness through physical activity in children with chronic disease and disability. Curr Sports Med Rep 2018;17:425–32. [DOI] [PubMed] [Google Scholar]
- 46.Miller J, Dalton MK, Duggan C, et al. Going with the flow or swimming against the tide: should children with central venous catheters swim? Nutr Clin Pract 2014;29:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedmann E, Son H. The human-companion animal bond: how humans benefit. Vet Clin North Am Small Anim Pract 2009;39:293–326. [DOI] [PubMed] [Google Scholar]
- 48.Child and adolescent health measurement initiative. 2016–2017 national survey of children’s health (NSCH) data query Data resource center for child and adolescent health supported by cooperative agreement U59MC27866 from the U.S. Department of Health and Human Services, Health Resources and Services Administration’s Maternal and Child Health Bureau (HRSA MCHB). www.cahmi.org. Accessed March 1, 2019. [Google Scholar]
- 49.American Academy of Pediatrics, Committee on Pediatric Emergency Medicine and Council on Clinical Information Technology, American College of Emergency Physicians, Pediatric Emergency Medicine Committee. Policy statement—emergency information forms and emergency preparedness for children with special health care needs. Pediatrics 2010;125:829–37. [DOI] [PubMed] [Google Scholar]
- 50.Goodhue CJ, Demeter NE, Burke RV, et al. Mixed-methods pilot study: disaster preparedness of families with children followed in an intestinal rehabilitation clinic. Nutr Clin Pract 2016;31:257–65. [DOI] [PubMed] [Google Scholar]
- 51.Galloway DP, Mathis MS, Wilkinson LT, et al. The effect of the COVID-19 pandemic on pediatric intestinal failure healthcare delivery. JPEN J Parenter Enteral Nutr 2020. Advance online publication. [DOI] [PubMed] [Google Scholar]
- 52.Pierret ACS, Wilkinson JT, Zilbauer M, et al. Clinical outcomes in pediatric intestinal failure: a meta-analysis and meta-regression. Am J Clin Nutr 2019;110:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szydlowski EG, Rudolph JA, Vitale MA, et al. Bloodstream infections in patients with intestinal failure presenting to a pediatric emergency department with fever and a central line. Pediatr Emerg Care 2017;33:e140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eisenberg M, Monuteaux MC, Fell G, et al. Central line-associated bloodstream infection among children with intestinal failure presenting to the emergency department with fever. J Pediatr 2018;196:237.e1–43.e1. [DOI] [PubMed] [Google Scholar]
- 55.Hariharan S, Mezoff EA, Dandoy CE, et al. A quality improvement initiative to decrease time to antibiotics for children with intestinal failure, fever, and a central line. Pediatr Qual Saf 2018;3:e090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hudgins JD, Goldberg V, Fell GL, et al. Reducing time to antibiotics in children with intestinal failure, central venous line, and fever. Pediatrics 2017;140:e20171201. [DOI] [PubMed] [Google Scholar]
- 57.Figueroa-Phillips LM, Bonafide CP, Coffin SE, et al. Development of a clinical prediction model for central line-associated bloodstream infection in children presenting to the emergency department. Pediatr Emerg Care 2020;36:e600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raphael BP, Fournier G, McLaughlin SR, et al. Antibiotic susceptibility and therapy in central line infections in pediatric home parenteral nutrition patients. J Pediatr Gastroenterol Nutr 2020;70:59–63. [DOI] [PubMed] [Google Scholar]
- 59.Chang MI, Carlson SJ, Nandivada P, et al. Challenging the 48-hour rule-out for central line-associated bloodstream infections in the pediatric intestinal failure population: a retrospective pilot study. JPEN J Parenter Enteral Nutr 2016;40:567–73. [DOI] [PubMed] [Google Scholar]
- 60.Robinson JL, Casey LM, Huynh HQ, et al. Prospective cohort study of the outcome of and risk factors for intravascular catheter-related bloodstream infections in children with intestinal failure. JPEN J Parenter Enteral Nutr 2014;38:625–30. [DOI] [PubMed] [Google Scholar]
- 61.Bond A, Chadwick P, Smith TR, et al. Diagnosis and management of catheter-related bloodstream infections in patients on home parenteral nutrition. Frontline Gastroenterol 2020;11:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanegas Calderon O, Rahhal R. 30% ethanol locks are effective in preventing central line-associated bloodstream infections in pediatric intestinal failure: a pilot study. Nutr Clin Pract 2020. Advance online publication. [DOI] [PubMed] [Google Scholar]
- 63.Rahhal R, Abu-El-Haija MA, Fei L, et al. Systematic review and meta-analysis of the utilization of ethanol locks in pediatric patients with intestinal failure. JPEN J Parenter Enteral Nutr 2018;42:690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blackwood RA, Issa M, Klein K, et al. Ethanol lock therapy for the treatment of intravenous catheter infections that have failed standard treatment. J Pediatric Infect Dis Soc 2017;6:94–7. [DOI] [PubMed] [Google Scholar]
- 65.Onland W, Shin CE, Fustar S, et al. Ethanol-lock technique for persistent bacteremia of long-term intravascular devices in pediatric patients. Arch Pediatr Adolesc Med 2006;160:1049–53. [DOI] [PubMed] [Google Scholar]
- 66.Bell AL, Jayaraman R, Vercaigne LM. Effect of ethanol/trisodium citrate lock on the mechanical properties of carbothane hemodialysis catheters. Clin Nephrol 2006;65:342–8. [DOI] [PubMed] [Google Scholar]
- 67.Crnich CJ, Halfmann JA, Crone WC, et al. The effects of prolonged ethanol exposure on the mechanical properties of polyurethane and silicone catheters used for intravascular access. Infect Control Hosp Epidemiol 2005;26:708–14. [DOI] [PubMed] [Google Scholar]
- 68.Chhim RF, Crill CM, Collier HK, et al. Ethanol lock therapy: a pilot infusion study in infants. Ann Pharmacother 2015;49:431–6. [DOI] [PubMed] [Google Scholar]
- 69.Mermel LA, Alang N. Adverse effects associated with ethanol catheter lock solutions: a systematic review. J Antimicrob Chemother 2014;69:2611–9. [DOI] [PubMed] [Google Scholar]
- 70.Ralls MW, Blackwood RA, Arnold MA, et al. Drug shortage-associated increase in catheter-related blood stream infection in children. Pediatrics 2012;130:e1369–73. [DOI] [PubMed] [Google Scholar]
- 71.van de Wetering MD, van Woensel JB, Lawrie TA. Prophylactic antibiotics for preventing gram positive infections associated with long-term central venous catheters in oncology patients. Cochrane Database Syst Rev 2013;2013:CD003295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGhee W, Michaels MG, Martin JM, et al. Antifungal lock therapy with liposomal amphotericin B: a prospective trial. J Pediatric Infect Dis Soc 2016;5:80–4. [DOI] [PubMed] [Google Scholar]
- 73.Lambe C, Poisson C, Talbotec C, et al. Strategies to reduce catheter-related bloodstream infections in pediatric patients receiving home parenteral nutrition: The efficacy of taurolidine-citrate prophylactic-locking. JPEN J Parenter Enteral Nutr 2018;42:1017–25. [DOI] [PubMed] [Google Scholar]
- 74.Hulshof EC, Hanff LM, Olieman J, et al. Taurolidine in pediatric home parenteral nutrition patients. Pediatr Infect Dis J 2017;36:233–5. [DOI] [PubMed] [Google Scholar]
- 75.Chu HP, Brind J, Tomar R, et al. Significant reduction in central venous catheter-related bloodstream infections in children on HPN after starting treatment with taurolidine line lock. J Pediatr Gastroenterol Nutr 2012;55:403–7. [DOI] [PubMed] [Google Scholar]
- 76.Kanaa M, Wright MJ, Akbani H, et al. Cathasept line lock and microbial colonization of tunneled hemodialysis catheters: a multicenter randomized controlled trial. Am J Kidney Dis 2015;66:1015–23. [DOI] [PubMed] [Google Scholar]
- 77.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Aociety of America. Clin Infect Dis 2009;49:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kovacevich DS, Corrigan M, Ross VM, et al. American Society for Parenteral and Enteral Nutrition Guidelines for the Selection and Care of Central Venous Access Devices for Adult Home Parenteral Nutrition Administration. JPEN J Parenter Enteral Nutr 2019;43:15–31. [DOI] [PubMed] [Google Scholar]
- 79.Bendorf K, Friesen CA, Roberts CC. Glucose response to discontinuation of parenteral nutrition in patients less than 3 years of age. JPEN J Parenter Enteral Nutr 1996;20:120–2. [DOI] [PubMed] [Google Scholar]
- 80.Lundgren IS, Zhou C, Malone FR, et al. Central venous catheter repair is associated with an increased risk of bacteremia and central line-associated bloodstream infection in pediatric patients. Pediatr Infect Dis J 2012;31:337–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anderson KT, Bartz-Kurycki MA, Martin R, et al. Tunneled central venous catheters in pediatric intestinal failure: a single-center experience. J Surg Res 2018;231:346–51. [DOI] [PubMed] [Google Scholar]
- 82.McNiven C, Switzer N, Wood M, et al. Central venous catheter repair is not associated with an increased risk of central line infection or colonization in intestinal failure pediatric patients. J Pediatr Surg 2016;51:395–7. [DOI] [PubMed] [Google Scholar]
- 83.Chan AP, Baldivia PS, Reyen LE, et al. Central venous catheter repair is highly successful in children with intestinal failure. J Pediatr Surg 2019;54:517–20. [DOI] [PubMed] [Google Scholar]
- 84.Baskin JL, Pui C, Reiss U, et al. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 2009;374:159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baskin JL, Reiss U, Wilimas JA, et al. Thrombolytic therapy for central venous catheter occlusion. Haematologica 2012;97:641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robinson CA, Sawyer JE. Y-site compatibility of medications with parenteral nutrition. J Pediatr Pharmacol Ther 2009;14:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Werlin SL, Lausten T, Jessen S, et al. Treatment of central venous catheter occlusions with ethanol and hydrochloric acid. JPEN J Parenter Enteral Nutr 1995;19:416–8. [DOI] [PubMed] [Google Scholar]
- 88.Mezoff EA, Fei L, Troutt M, et al. Ethanol lock efficacy and associated complications in children with intestinal failure. JPEN J Parenter Enteral Nutr 2016;40:815–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mokha JS, Davidovics ZH, Samela K, et al. Effects of ethanol lock therapy on central line infections and mechanical problems in children with intestinal failure. JPEN J Parenter Enteral Nutr 2017;41:625–31. [DOI] [PubMed] [Google Scholar]
- 90.Journeycake JM, Buchanan GR. Thrombotic complications of central venous catheters in children. Curr Opin Hematol 2003;10:369–74. [DOI] [PubMed] [Google Scholar]
- 91.McLaughlin CM, Bennett M, Channabasappa N, et al. Anticoagulation results in increased line salvage for children with intestinal failure and central venous thrombosis. J Pediatr Surg 2018;53:1052–5. [DOI] [PubMed] [Google Scholar]
- 92.Gonzalez-Hernandez J, Daoud Y, Styers J, et al. Central venous thrombosis in children with intestinal failure on long-term parenteral nutrition. J Pediatr Surg 2016;51:790–3. [DOI] [PubMed] [Google Scholar]
- 93.Smith R, Jones S, Newall F. Six weeks versus 3 months of anticoagulant treatment for pediatric central venous catheter-related venous thromboembolism. J Pediatr Hematol Oncol 2017;39:518–23. [DOI] [PubMed] [Google Scholar]
- 94.Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133(Suppl):454S–545S. [DOI] [PubMed] [Google Scholar]
- 95.Monagle P, Chan AKC, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e737S–801S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jami MM, Bhardwaj V, Merritt RJ. Intravenous fish oil lipid emulsion prevents catheter-related thromboses in pediatric patients with intestinal failure. J Pediatr 2018;198:301–3. [DOI] [PubMed] [Google Scholar]
- 97.Pichler J, Biassoni L, Easty M, et al. Reduced risk of pulmonary emboli in children treated with long-term parenteral nutrition. Clin Nutr 2016;35:1406–13. [DOI] [PubMed] [Google Scholar]
- 98.Cook D, Randolph A, Kernerman P, et al. Central venous catheter replacement strategies: a systematic review of the literature. Crit Care Med 1997;25:1417–24. [DOI] [PubMed] [Google Scholar]
- 99.Baskin KM, Durack JC, Abu-Elmagd K, et al. Chronic central venous access: From research consensus panel to national multistakeholder initiative. J Vasc Interv Radiol 2018;29:461–9. [DOI] [PubMed] [Google Scholar]
- 100.Children’s hospitals’ solutions for patient safety: our results https://www.solutionsforpatientsafety.org/our-results/, Updated 2019. Accessed May 29, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


