Abstract
Developing efficient and low-cost urea oxidation reaction (UOR) catalysts is a promising but still challenging task for environment and energy conversion technologies such as wastewater remediation and urea electrolysis. In this work, NiO nanoparticles that incorporated graphene as the NiO@Graphene composite were constructed to study the UOR process in terms of density functional theory. The single-atom model, which differed from the previous heterojunction model, was employed for the adsorption/desorption of urea and CO2 in the alkaline media. As demonstrated from the calculated results, NiO@Graphene prefers to adsorb the hydroxyl group than urea in the initial stage due to the stronger adsorption energy of the hydroxyl group. After NiOOH@Graphene was formed in the alkaline electrolyte, it presents excellent desorption energy of CO2 in the rate-determining step. Electronic density difference and the d band center diagram further confirmed that the Ni(III) species is the most favorable site for urea oxidation while facilitating charge transfer between urea and NiO@Graphene. Moreover, graphene provides a large surface for the incorporation of NiO nanoparticles, enhancing the electron transfer between NiOOH and graphene and promoting the mass transport in the alkaline electrolyte. Notably, this work provides theoretical guidance for the electrochemical urea oxidation work.
Introduction
The continuous increase in the energy demand needs to pursuit a clean and renewable energy source because non-renewable energy sources such as traditional fossil fuels are limited and lead to global warming.1,2 It is necessary to bridge the gap between academia and the industry with extensive research and their practical applications. Although hydrogen and oxygen evolution reactions (HER and OER, respectively) have been considered as revolutionary fuel cell designs that utilize water-splitting technology, they suffer from efficiency drawbacks and can be realized in limited pristine fresh water and with the use of noble metal catalysts.3−5 Urea oxidation reaction (UOR) is a fundamental step in fulfilling the need for practical green energy because it does not need a high-voltage supply and also does not release both O2 and H2 gases simultaneously, encountered during water splitting.6,7 Furthermore, urea is an abundant component of human and animal waste, which can result in the production of problematic ammonia under normal degradation or standard hydrolysis practices.8 More importantly, the UOR process could provide an opportunity for waste disposal and green hydrogen production.9
In a typical UOR, urea in an alkali electrolyte is oxidized to the production of N2 and CO2 at the anode and H2 on the cathode from water electrolysis.10 This process is depicted in eqs 1–3, respectively.
| 1 |
| 2 |
| 3 |
The UOR process is slow and inefficient under normal conditions due to the six-electron transfer process from the anode to the cathode.11,12 Thus, it is necessary to modify the working electrode using a catalyst. Nickel-based materials are considered as one of the most promising groups of materials for catalysis in UOR owing to their low cost, easy synthesis route, and abundance in nature.13,14 For example, Tammam and Saleh developed a NiO-modified electrode for electrocatalytic urea oxidation in the alkaline media and confirmed that the UOR process is a completely irreversible diffusion-controlled route.15 To improve the conductivity of Ni-based materials while maintaining the compounds’ catalytic performance, several effective strategies were applied, including the introduction of conductive support,16 elemental doping,17 high valence Ni-based materials,18 and defect engineering.19 Many of these effective strategies were developed to realize the commercial implementation of Ni-catalyst driven UOR. Nevertheless, the in-depth theoretical-fundamental understanding on the UOR was not studied due to its complicated multistep gas adsorption and desorption.
Moreover, one major theoretical drawback found by density functional theory calculations is the rate-limiting intermediate step of CO2 desorption during the reaction on the anode.20 In our previous work, we investigated the use of nano-NiO supported on eggshell membrane-derived carbon for a Ni-catalyzed UOR, and the periodic heterojunction model was selected to illustrate the impact between porous carbon and NiO nanoparticles.16 NiO also possesses many merits, including easy-to-obtain, low-cost, and exchangeable valence states.7,16 Meanwhile, graphene was employed as the alternative porous carbon for simulations. It is important to add theoretical simulation to understand the UOR process using different models, especially for the existence of a single-atom model due to attractive findings.21,22 The in-depth mechanism of UOR in NiO@Graphene is still not clear. Consequently, it is useful to employ the single-atom model to investigate the role of NiO@Graphene in the UOR process.
In this work, a single-atom model was built to understand the relationship of the NiO@Graphene composite and its urea oxidation behavior. The single-atom model, which differs from the previous theoretical model, was applied to illustrate the influence between graphene and NiO nanoparticles. Meanwhile, this work also served as an important theoretical supplement for the previous research. Prior to the investigation of UOR, the adsorption of the hydroxyl group and urea on NiO@Graphene was compared. Then, the adsorption of urea and CO2 on NiO and NiOOH with graphene was calculated and compared. The electron density difference map was also used to study the electron transfer of the NiO@Graphene composite.
Results and Discussion
Competitive Adsorption of the Urea/Hydroxyl Group on NiO@Graphene
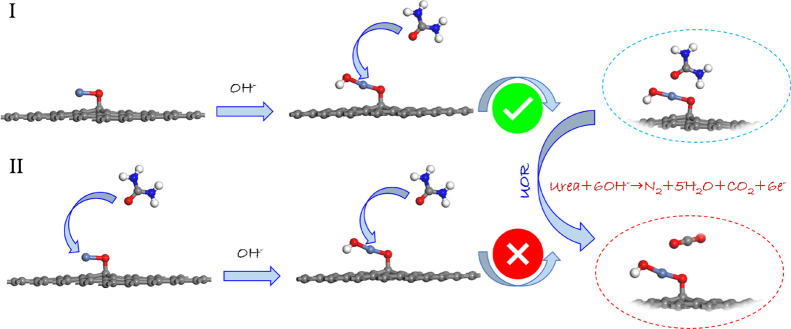
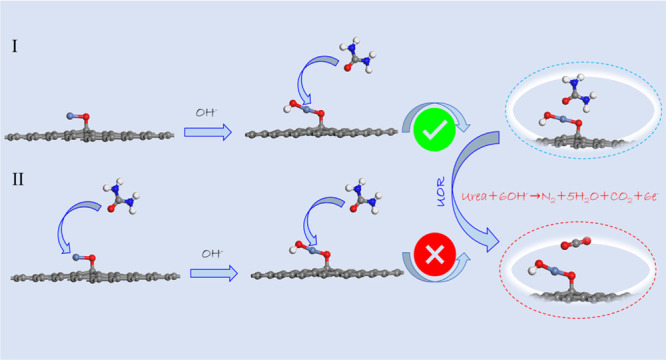
In an earlier literature of UOR toward NiO and its derivatives, most of the theoretical studies have been performed by considering the adsorption/desorption of CO2 in the gas–liquid interface, which matched well with the formed *COO species.16 However, the competitive adsorption of urea and the hydroxyl group on the surface of the NiO-based electrocatalyst cannot be ignored before urea oxidation. Thus, to understand the competitive adsorption, both the adsorption energy of urea and the hydroxyl group were calculated. As shown in Scheme 1, the adsorption energy of the hydroxyl group (route I) on NiO@Graphene is calculated to be −3.49 eV, which is higher than that of urea (route II). It means that NiOOH@Graphene was formed in the alkaline media without the disturbance of urea adsorption under the applied potential.
Scheme 1. Schematic Illustration of the Adsorption Route of Urea/Hydroxyl on NiO@Graphene.
(I) Adsorption of the hydroxyl group first; (II) adsorption of urea first (gray for C, red for O, blue for N, white for H, and light blue for Ni atoms).
Theoretical Analysis on NiO@Graphene and NiOOH@Graphene
To get an understanding into the electrocatalytic urea oxidation mechanism of NiO@Graphene, DFT calculations were utilized based on the single-atom model. Generally, NiO(II) nanoparticles will be oxidized into NiOOH(III) in the alkaline environment, which is due to the redox reaction (eq 4) occurring at the NiO(II) nanoparticles, as shown in Scheme 1.
| 4 |
| 5 |
In a typical cyclic voltammetry diagram of pure NiO in the presence of the alkaline electrolyte, the oxidation peak around 0.35 V corresponds to the transformation of Ni(II) to Ni(III), and the reduction peak around 0.15 V corresponds to the transformation of Ni(III) to Ni(II).23 The formed Ni(III) species was regarded as the active site for the UOR process. Before the research on nickel oxide, nickel hydroxide was first electrooxidized to NiOOH species in alkaline media (eq 5), and then urea molecules adsorbed on the NiOOH species via bridging coordination, whereby a Ni atom interacts with a C atom (urea). It means that the onset potential for the UOR has the potential for the formation of NiOOH via Ni electrooxidation. The next dissociation of urea on NiOOH is multiple processes, producing a variety of intermediate species, such as the typical reaction pathway that was proposed as *CO(NH2)2 → *CO(·HNNH2) → *CO(·HNNH) → *CO(·HNN) → *CO(N·N) → *CO(OH) → *CO(NH2)2 → *CO(OH·OH) → *COO. By analyzing the Gibbs energy and the resistance of each step (Table S1), they uncovered that the rate-determining step (RDS) is the desorption of CO2 from NiOOH species. Thus, to simplify the DFT calculation, the adsorption of CO2 on the given composite was used to simulate the RDS process of urea oxidation.
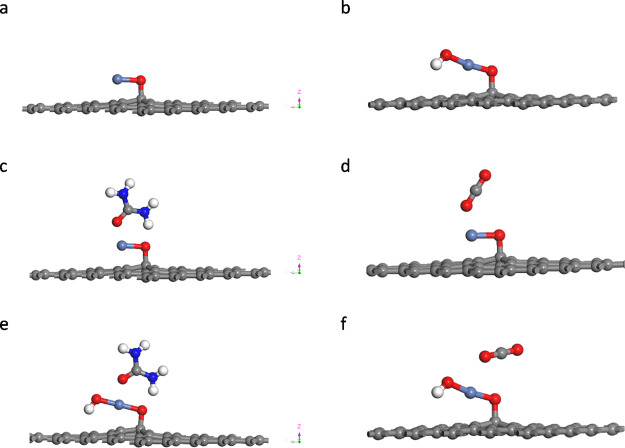
Graphene was an alternative instead of porous carbon in our previous work with many advantages of high conductivity and stable support.16 It is noted that graphene preferentially anchors NiO nanoparticles via electrostatic attraction due to its electronegativity.23 Hence, we considered that the connection of NiO on the given graphene substrate was achieved by the Ni–O–C bond. The optimized configurations are listed in Figure 1, and the corresponding adsorption energies of urea and CO2 molecules are listed in Table 1 and Table S2.
Figure 1.
Optimized structure of (a) NiO@Graphene and (b) NiOOH@Graphene. The adsorption of urea (c) and CO2 (d) on the surface of NiO@Graphene and the adsorption of urea (e) and CO2 (f) on the surface of NiOOH@Graphene (red for O, white for H, gray for C, blue for N, and light blue for Ni atoms).
Table 1. DFT Data for the Adsorption of CO2 and Urea on the Surface of NiO@Graphene and NiOOH@Graphene, Respectively.
| species | optimized energy (eV) | adsorption energy (eV) | the shortest distance between CO2 and the catalyst (Å) | the shortest distance between urea and the catalyst (Å) |
|---|---|---|---|---|
| NiO@Graphene-Urea | –11619.6959 | –1.377778046 | Ni–O: 1.920 | |
| O–H: 1.744 | ||||
| NiOOH@Graphene-Urea | –12072.8731 | –0.77729845 | Ni–O: 1.920 | |
| O–H: 1.744 | ||||
| NiO@Graphene-CO2 | –10569.1883 | –0.628792601 | Ni–O: 2.040 | |
| NiOOH@Graphene-CO2 | –11022.4195 | –0.082424595 | Ni–O: 2.569 |
A weaker adsorption energy generally corresponds to a more stable system. NiO nanoparticles reacted with the hydroxyl group in alkaline to form NiOOH followed by linking the graphene substrate with the Ni–O–C bond. In the case of the NiO@Graphene composite, Ni(II) species were supposed to be the active sites, and urea molecules were attracted on the given composite. The most active site of the NiO@Graphene composite adsorption is the Ni(II) species with an adsorption energy of −1.37 eV (Figure 2a). This interaction may originate from the interaction (Ni–O:urea) between Ni 3d and O 2p orbital electrons of the urea molecule. However, the adsorption energy of NiOOH@Graphene toward the urea molecule is −0.77 eV. Ni(III) species played as active sites for urea oxidation, and the lower urea adsorption energy of NiOOH@Graphene suggests that it is difficult to adsorb urea compared to the performance of NiO@Graphene. The possible reason for this phenomenon is that the Ni–O:urea bond between NiOOH@Graphene and urea is affected from the around group (i.e., OH–). To verify this point, the adsorption energy of NiOOH@Graphene over the hydroxyl group was calculated to be −2.32 eV, which is a strong interaction. This result further confirms the above explanation and also illustrates that urea adsorbed on the surface of the catalyst can accelerate the electrochemical process to some extent.
Figure 2.
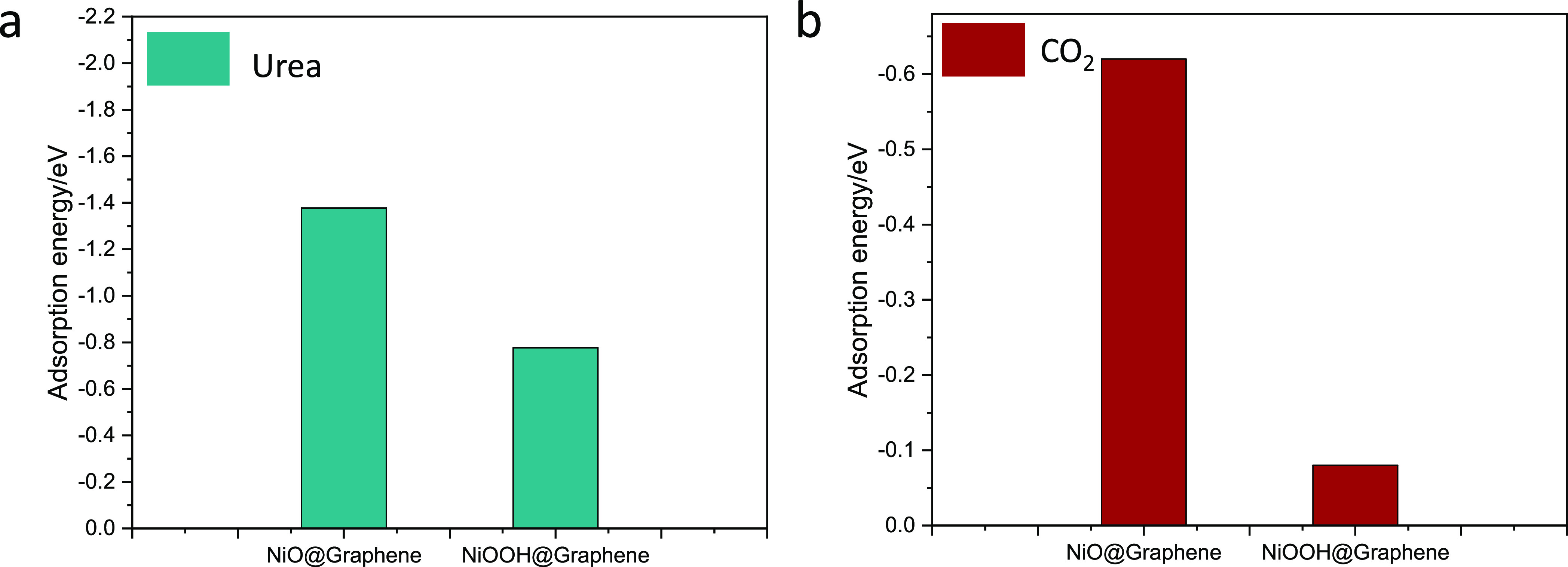
Adsorption energies of (a) urea and (b) CO2 molecules on NiO@Graphene and NiOOH@Graphene.
Moreover, the desorption step of CO2 from the Ni species is regarded as the rate-determining step for urea oxidation on the Ni-based electrocatalysts. Therefore, the adsorption energies of CO2 on NiO@Graphene and NiOOH@Graphene were calculated (Figure 2b). The CO2 adsorption of NiO@Graphene is −0.62 eV, which is higher than that of NiOOH@Graphene (−0.08 eV). It may be due to the molecular interaction between CO2 and the catalyst. So, the distance between CO2 and the catalyst is an important parameter to explain this phenomenon. Consequently, the shortest distance between CO2 and NiO@Graphene is 2.040 Å, which is slightly smaller than that of NiOOH@Graphene (Figures S1 and S2). This result further confirms that NiOOH@Graphene was easily desorbed on the CO2 molecule during urea oxidation. Moreover, Ni(III) species play the active sites for efficient urea oxidation. This result also is further explained by the electron density difference diagrams.
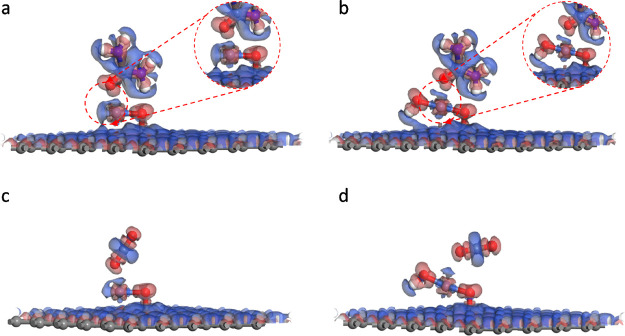
The electron density difference map is calculated to investigate the electron transfer between the given sample and urea/CO2 molecules intuitively. It can be observed from Figure 3a,b that electrons transfer from the center Ni atom and the adjacent O atom of urea to the intermediate region between NiO@Graphene or NiOOH@Graphene and urea. There is a significant charge aggregation between Ni and O atoms (Figure 3a,b), suggesting the potential formation of Ni–O covalent bonds. This is due to the charge transfer and redistribution that will lead to the hybridization of the Ni 3d and O 2p orbitals. Considering the effect of graphene, the control experiments were also carried out under the same conditions. Graphene does not have any contributions to urea/CO2 adsorption (Figure S3). In the viewpoint of electron configuration, Ni(II) species ([Ar]3d8) with two unsaturated d orbitals could be filled by O ([He]2s22p4) well compared to those of Ni(III) species ([Ar]3d7). Generally, the stronger the hybridization of Ni-O bond, the stronger the adsorption toward the urea molecule. NiO@Graphene presents the larger overlapping area of charge density and with the stronger covalent interaction (Figure S4). As for CO2 adsorption, the NiO@Graphene composite presents a similar adsorption behavior. In the whole electrochemical urea oxidation process, the adsorption/desorption of CO2 is regarded as the key descriptor for the UOR in an alkaline environment. So, the NiOOH@Graphene composite performs a better desorption behavior than NiO@Graphene (Figure S5).
Figure 3.
Electron density difference of the urea molecule adsorbed on (a) NiO@Graphene and (b) NiOOH@Graphene and CO2 adsorbed on (c) NiO@Graphene and (d) NiOOH@Graphene. The red hooded face means the enrichment of electrons, while the blue one means the deficiency of electrons.
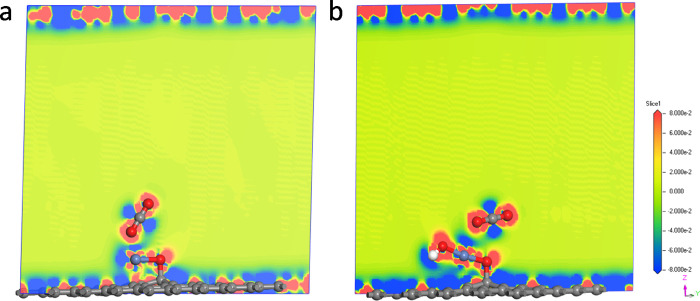
To further confirm this conclusion, the slight electron density difference in Figure 4 shows little bit more electrons from the Ni atom to the O atom of urea on the NiOOH@Graphene composite than those on the NiO@Graphene composite, which indicates that the Ni(III) species presented its favorable active sites in the key step of UOR. This is kept in line with the adsorption energy results where the NiO@Graphene composite shows favorable adsorption toward urea, and the NiOOH@Graphene composite shows better CO2 desorption performance in the key reaction.
Figure 4.
Slice images of the adsorption of the CO2 molecule on the surface of (a) NiO@Graphene and (b) NiOOH@Graphene and the corresponding slice of the electron density difference. The contour around the atoms represents electron accumulation (red) or electron deletion (blue).
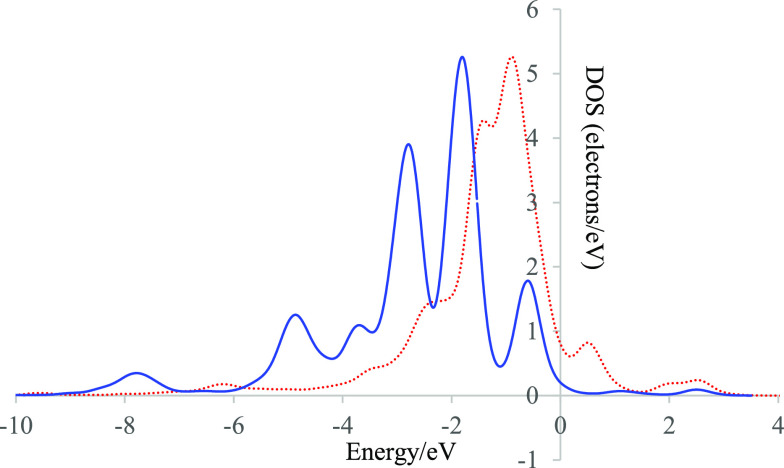
The above results demonstrate that the NiO@Graphene composite can effectively adsorb urea and then form a Ni–O covalent bond with urea, which is well related with the adsorption/desorption properties and surface charge density of molecules. After NiOOH@Graphene is formed in the alkaline media, Ni 3d orbitals bond to the O 2p orbital of urea near the Fermi level, suggesting the charge transfer between Ni and O atoms. Furthermore, based on the DOS diagram in NiO@Graphene and NiOOH@Graphene, the peaks of NiOOH@Graphene become tightened (Figure 5). The peaks of Ni 3d in NiOOH@Graphene shift lower energy near the Fermi level compared to those of NiO@Graphene. The center of the Ni 3d orbital peak moves from −1.61 eV (NiO@Graphene) to −2.42 eV (NiOOH@Graphene). Additionally, more electrons flow from the Ni atom to the O atom to establish a strong Ni–O bond, resulting in C–Oads with a weakened bond energy, thus facilitating the *COO desorption from urea. Generally, the Fermi level can expose the ability of electron transfer on the electrocatalyst surface. The larger the Fermi level, the higher the electron transfer capability. Compared to the Fermi level of NiO@Graphene (1.61 eV), the much larger Fermi level of NiOOH@Graphene (2.42 eV) suggests that the Ni(III) sample can significantly improve the electron transfer ability of NiOOH@Graphene. In addition, the lower d band center leads to a weaker adsorption for CO2. Based on the above DFT analysis, we conclude that the active electron density of NiOOH@Graphene was effectively upshifted in the UOR process.
Figure 5.
d density of states of Ni in NiO (dotted red line) and NiOOH (blue line). The Fermi level is set to zero, and the vertical lines represent the d band center.
Comparison of the Heterojunction Model and Single-Atom Model
In our previous work, the heterojunction model based on NiO nanoparticles and graphene was constructed to simplify the theoretical work. The heterojunction structure is a general model to simulate the multicomponent composite. DFT calculations with the CASTEP package were employed to reveal the effect of biomass-derived porous carbon on the UOR performance of the NiO@C nanocomposite and uncover the Ni(III) species working as active sites for UOR. In this process, the NiO@C nanocomposite just played the intermediate role. It turned into the NiOOH@C nanocomposite with the assistance of the alkaline media; then, Ni(III) species in NiOOH@C acted as active sites for efficient urea oxidation. However, the above model just qualitatively revealed the influence of the porous structure on the electronic structure of NiO nanoparticles and the synergistic effect between NiO nanoparticles and porous carbon. From the observed SEM and TEM images, it is also applicable for the single-atom model considering the Ni species playing as active sites and the ideal carbon substrate in the UOR. Furthermore, DFT calculations with the Dmol3 package were used in this study to investigate the role of Ni(III) species in the UOR. Impressively, a different phenomenon was observed where NiO@Graphene has the favorable adsorption of urea. It indicates that NiO@Graphene turned into NiOOH@Graphene in the alkaline electrolyte first, and then urea absorbed on NiO@Graphene.
Based on the above analysis, it can be demonstrated that NiO@Graphene shows favorable adsorption of the hydroxyl group in the first stage and then turns into NiOOH@Graphene under the alkaline conditions for efficient urea oxidation. The presence of Ni(III) species and excellent electrical conductivity of NiOOH@Graphene show better desorption of CO2. Moreover, benefiting from the excellent conductivity of graphene, electrons transferred from urea to NiOOH@Graphene through the Ni–O:urea bond easier. Graphene also provides a function for facilitating alkaline electrolyte diffusion, ensuring the formation of Ni(III) species and promoting mass transfer effectively. Such a composite structure has the above merits to guarantee the stability and efficiency of NiO@Graphene as an efficient UOR electrocatalyst.
Conclusions
In this work, the single-atom model where NiO nanoparticles were bonded with graphene as the NiO@Graphene composite was constructed for the electrochemical urea oxidation in terms of theoretical view. DFT calculations showed that NiO nanoparticles dispersed on graphene provide strong adsorption with the hydroxyl group; then, NiOOH@Graphene was formed after NiO@Graphene reacted with the hydroxyl group. Compared to NiO@Graphene, NiOOH@Graphene presents a higher desorption energy of CO2 molecules in the key rate-determining step. Notably, the Ni(III) species in NiOOH@Graphene is the most favorable site for the urea oxidation reaction. Moreover, NiOOH@Graphene not only guarantees the stability of NiOOH and graphene but also promotes the electron transfer between NiOOH and graphene. Benefiting from the coupling effect between the Ni(III) species and graphene, NiO@Graphene can reach excellent electrocatalytic urea oxidation theoretically. These studies provide theoretical guidance that NiO@Graphene played the intermediate role in the urea oxidation process before Ni(III) species formed in the alkaline electrolyte. NiOOH@Graphene also facilitates the desorption of CO2 from the catalyst surface for UOR catalysis. More experimental investigations based on the NiO nanoparticles and graphene will be verified in the future.
Computational Methods
To understand the origin of the electrocatalytic urea oxidation mechanism of the NiO@Graphene composite, density functional theory (DFT) calculations were conducted using the Dmol3 package with the Perdew–Burke–Ernzerhof (PBE) formulation of the generalized gradient approximation (GGA) program.24 The adsorption of urea and CO2 on NiO@Graphene was investigated compared to that on NiOOH@Graphene. The single-atom structure was selected in this investigation. The core electrons were treated by DFT semi-core pseudopotentials.25 The DNP basis set was chosen as it can provide more precision for hydrogen-involved calculations. The convergence thresholds for energy change, maximum force, and maximum displacement are set to be 2 × 10–5 Hartree, 0.004 Hartree Å–1, and 0.005 Å, respectively. A vacuum layer of 15 Å thick was employed along the z direction to eliminate the interactions between different surfaces. In this work, the adsorption energy of urea or CO2 is an important reference point for determining the activity and stability of a urea electrocatalyst. Therefore, the adsorption energies of urea or CO2 over NiO@Graphene and NiOOH@Graphene are calculated according to eq 6.
| 6 |
where Eads is the adsorption energy, Etotal is the total energy for the adsorption state, Eslab is the energy of the optimized surface of C@NiO or C@NiOOH, and Eadsorbate is the energy of a single urea or CO2 molecule. So, a more negative Eads in eq 6 implies that the adsorption is thermodynamically more favorable.
Acknowledgments
This work is supported by the Foundation and Frontier Research Project of Chongqing of China (no. cstc2018jcyjAX0513) and the Science and Technology Research Program of Chongqing Municipal Education Commission (no. KJQN201801125), NASA/EPSCoR (no. NNX16AQ98A), and NSF/EPSCoR (no. OIA-1849206, South Dakota 2D Materials for Biofilm Engineering, Science and Technology Center (2DBEST)). In addition, S. Lu acknowledges Yuehui Wang's care during his Ph.D. period.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01758.
Images of the shortest distance between urea/CO2 and NiO@Graphene/NiOOH@Graphene; the electron density difference of urea/CO2 on NiO@Graphene and NiOOH@Graphene, respectively; Gibbs energies (ΔG) for the possible steps on NiOOH; and the optimized energy of all appeared molecules based on the single-atom model (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Chen Z.; Wei W.; Ni B.-J. Cost-effective catalysts for renewable hydrogen production via electrochemical water splitting: Recent advances. Curr. Opin. Green Sustainable Chem. 2021, 27, 100398. 10.1016/j.cogsc.2020.100398. [DOI] [Google Scholar]
- Lu S.; Hummel M.; Gu Z.; Gu Y.; Cen Z.; Wei L.; Zhou Y.; Zhang C.; Yang C. Trash to treasure: A novel chemical route to synthesis of NiO/C for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 16144–16153. 10.1016/j.ijhydene.2019.04.191. [DOI] [Google Scholar]
- Tahir M.; Pan L.; Idrees F.; Zhang X.; Wang L.; Zou J.-J.; Wang Z. L. Electrocatalytic oxygen evolution reaction for energy conversion and storage: a comprehensive review. Nano Energy 2017, 37, 136–157. 10.1016/j.nanoen.2017.05.022. [DOI] [Google Scholar]
- Li Y.; Sun Y.; Qin Y.; Zhang W.; Wang L.; Luo M.; Yang H.; Guo S. Recent advances on water-splitting electrocatalysis mediated by noble-metal-based nanostructured materials. Adv. Energy Mater. 2020, 10, 1903120. 10.1002/aenm.201903120. [DOI] [Google Scholar]
- Nie M.; Du S.; Li Q.; Hummel M.; Gu Z.; Lu S. Tungsten carbide as supports for trimetallic AuPdPt electrocatalysts for methanol oxidation. J. Electrochem. Soc. 2020, 167, 044510 10.1149/1945-7111/ab754d. [DOI] [Google Scholar]
- Sayed E. T.; Eisa T.; Mohamed H. O.; Abdelkareem M. A.; Allagui A.; Alawadhi H.; Chae K.-J. Direct urea fuel cells: Challenges and opportunities. J. Power Sources 2019, 417, 159–175. 10.1016/j.jpowsour.2018.12.024. [DOI] [Google Scholar]
- Lu S.; Gu Z.; Hummel M.; Zhou Y.; Wang K.; Xu B. B.; Wang Y.; Li Y.; Qi X.; Liu X. Nickel oxide immobilized on the carbonized eggshell membrane for electrochemical detection of urea. J. Electrochem. Soc. 2020, 167, 106509. 10.1149/1945-7111/ab9c80. [DOI] [Google Scholar]
- Sigurdarson J. J.; Svane S.; Karring H. The molecular processes of urea hydrolysis in relation to ammonia emissions from agriculture. Rev. Environ. Sci. Biotechnol. 2018, 17, 241–258. 10.1007/s11157-018-9466-1. [DOI] [Google Scholar]
- Ke K.; Wang G.; Cao D.; Wang G. Recent Advances in the Electro-Oxidation of Urea for Direct Urea Fuel Cell and Urea Electrolysis. Electrocatalysis 2020, 41–78. 10.1007/978-3-030-43294-2_2. [DOI] [PubMed] [Google Scholar]
- Singh R. K.; Schechter A. Electrochemical investigation of urea oxidation reaction on β-Ni(OH)2 and Ni/Ni (OH)2. Electrochim. Acta 2018, 278, 405–411. 10.1016/j.electacta.2018.05.049. [DOI] [Google Scholar]
- Xiong P.; Ao X.; Chen J.; Li J.-G.; Lv L.; Li Z.; Zondode M.; Xue X.; Lan Y.; Wang C. Nickel diselenide nanoflakes give superior urea electrocatalytic conversion. Electrochim. Acta 2019, 297, 833–841. 10.1016/j.electacta.2018.12.043. [DOI] [Google Scholar]
- Bao C.; Niu Q.; Chen Z.-A.; Cao X.; Wang H.; Lu W. Ultrathin nickel-metal-organic framework nanobelt based electrochemical sensor for the determination of urea in human body fluids. RSC Adv. 2019, 9, 29474–29481. 10.1039/C9RA05716A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayler J. D.; Leahy D. K.; Simmons E. M. A pharmaceutical industry perspective on sustainable metal catalysis. Organometallics 2018, 38, 36–46. [Google Scholar]
- Kaushik M.; Moores A. New trends in sustainable nanocatalysis: Emerging use of earth abundant metals. Curr. Opin. Green Sustainable Chem. 2017, 7, 39–45. 10.1016/j.cogsc.2017.07.002. [DOI] [Google Scholar]
- Tammam R. H.; Saleh M. M. On the electrocatalytic urea oxidation on nickel oxide nanoparticles modified glassy carbon electrode. J. Electroanal. Chem. 2017, 794, 189–196. 10.1016/j.jelechem.2017.04.023. [DOI] [Google Scholar]
- Lu S.; Hummel M.; Gu Z.; Wang Y.; Wang K.; Pathak R.; Zhou Y.; Jia H.; Qi X.; Zhao X.; Xu B. B.; Liu X. Highly efficient urea oxidation via nesting nano-nickel oxide in eggshell membrane-derived carbon. ACS Sustainable Chem. Eng. 2021, 9, 1703–1713. 10.1021/acssuschemeng.0c07614. [DOI] [Google Scholar]
- Xie J.; Liu W.; Lei F.; Zhang X.; Qu H.; Gao L.; Hao P.; Tang B.; Xie Y. Iron-incorporated α-Ni(OH)2hierarchical nanosheet arrays for electrocatalytic urea oxidation. Chem. – Eur. J. 2018, 24, 18408–18412. 10.1002/chem.201803718. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Wang L.; Lin H.; Liu Y.; Ye J.; Wen Y.; Chen A.; Wang L.; Ni F.; Zhou Z.; Sun S.; Li Y.; Zhang B.; Peng H. A lattice-oxygen-involved reaction pathway to boost urea oxidation. Angew. Chem., Int. Ed. 2019, 58, 16820–16825. 10.1002/anie.201909832. [DOI] [PubMed] [Google Scholar]
- Tong Y.; Chen P.; Zhang M.; Zhou T.; Zhang L.; Chu W.; Wu C.; Xie Y. Oxygen vacancies confined in nickel molybdenum oxide porous nanosheets for promoted electrocatalytic urea oxidation. ACS Catal. 2018, 8, 1–7. 10.1021/acscatal.7b03177. [DOI] [Google Scholar]
- Daramola D. A.; Singh D.; Botte G. G. Dissociation rates of urea in the presence of NiOOH catalyst: a DFT analysis. J. Phys. Chem. A. 2010, 114, 11513–11521. 10.1021/jp105159t. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Gao R.; Ji S.; Li H.; Tang K.; Jiang P.; Hu H.; Zhang Z.; Hao H.; Qu Q.; Liang X.; Chen W.; Dong J.; Wang D.; Li Y. Atomic-level modulation of electronic density at cobalt single-atom sites derived from metal-organic frameworks: enhanced oxygen reduction performance. Angew. Chem., Int. Ed. 2021, 60, 3212–3221. 10.1002/anie.202012798. [DOI] [PubMed] [Google Scholar]
- Ban J.; Wen X.; Xu H.; Wang Z.; Liu X.; Cao G.; Shao G.; Hu J. Dual evolution in defect and morphology of single-atom dispersed carbon based oxygen electrocatalyst. Adv. Funct. Mater. 2021, 31, 2010472. 10.1002/adfm.202010472. [DOI] [Google Scholar]
- Lu Y.; Su L.; Qi J.; Lei S.; Liu B.; Zang Q.; Shi S.; Yan X. A combined DFT and experimental study on the nucleation mechanism of NiO nanodots on graphene. J. Mater. Chem. A 2018, 6, 13717–13724. 10.1039/C8TA03451F. [DOI] [Google Scholar]
- Perdew J. P.; Burke K.; Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- Kresse G.; Hafner J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J. Phys.: Condens. Matter 1994, 6, 8245. 10.1088/0953-8984/6/40/015. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.