Abstract
Use of electronic cigarettes is rapidly increasing among youth and young adults, but little is known regarding the long-term cardiopulmonary health impacts of these nicotine-containing devices. Our group has previously demonstrated that chronic, inhaled nicotine induces pulmonary hypertension (PH) and right ventricular (RV) remodeling in mice. These changes were associated with upregulated RV angiotensin-converting enzyme (ACE). Angiotensin II receptor blockers (ARBs) have been shown to reverse cigarette smoking-induced PH in rats. ACE inhibitor and ARB use in a large retrospective cohort of patients with PH is associated with improved survival. Here, we utilized losartan (an ARB specific for angiotensin II type 1 receptor) to further explore nicotine-induced PH. Male C57BL/6 mice received nicotine vapor for 12 h/day, and exposure was assessed using serum cotinine to achieve levels comparable to human smokers or electronic cigarette users. Mice were exposed to nicotine for 8 wk and a subset was treated with losartan via an osmotic minipump. Cardiac function was assessed using echocardiography and catheterization. Although nicotine exposure increased angiotensin II in the RV and lung, this finding was nonsignificant. Chronic, inhaled nicotine significantly increased RV systolic pressure and RV free wall thickness versus air control. These parameters were significantly lower in mice receiving both nicotine and losartan. Nicotine significantly increased RV internal diameter, with no differences seen between the nicotine and nicotine-losartan group. Neither nicotine nor losartan affected left ventricular structure or function. These findings provide the first evidence that antagonism of the angiotensin II type 1 receptor can ameliorate chronic, inhaled nicotine-induced PH and RV remodeling.
NEW & NOTEWORTHY Chronic, inhaled nicotine causes pulmonary hypertension and right ventricular remodeling in mice. Treatment with losartan, an angiotensin II type 1 receptor antagonist, ameliorates nicotine-induced pulmonary hypertension and right ventricular remodeling. This novel finding provides preclinical evidence for the use of renin-angiotensin system-based therapies in the treatment of pulmonary hypertension, particularly in patients with a history of tobacco-product use.
Keywords: angiotensin, heart, inhalation, losartan, nicotine
INTRODUCTION
Cigarette smoking in the United States, while declining, contributes to nearly 500,000 deaths per year and is the major cause of cardiopulmonary disease (1). Increased electronic cigarette use, however, threatens to undo decades-long progress toward reducing global cigarette smoking (2, 3). Surveys between 2017 and 2018 show that 20.8% of high school students and 7.6% of young adults in the United States are actively using electronic cigarettes (4, 5). These statistics are particularly concerning considering the paucity of knowledge regarding the long-term cardiopulmonary health implications of electronic cigarette use (6).
Nicotine, an addictive agonist of the nicotinic cholinergic receptor family, is of particular interest as the principle biologically active constituent of electronic cigarette vapor. Despite this, the effects of inhaled nicotine in isolation have been poorly characterized. Previously, we have shown that exposure to chronic, inhaled nicotine causes pulmonary hypertension (PH) and right ventricular (RV) remodeling in mice (7). This novel finding parallels previous literature connecting cigarette smoking to the development of PH in multiple animal models (8–11). Pathogenesis of both nicotine- and cigarette smoking-induced PH appears to be associated with dysregulation of the renin-angiotensin system (7, 8, 11).
The effects of nicotine on the renin-angiotensin system are well-studied. Nicotine upregulates the prohypertensive, prohypertrophic, and proinflammatory components of the renin-angiotensin system including angiotensin II, angiotensin-converting enzyme (ACE), and angiotensin II type 1 receptor (AT1R); nicotine downregulates the counter-regulatory components of the renin-angiotensin system including angiotensin II type 2 receptor (AT2R) and ACE2 (12). Treatment of rats with losartan, a specific antagonist of the AT1R ameliorated cigarette smoking-induced PH (8, 11). Furthermore, a retrospective study of over 24,000 patients with PH in the Veterans Affairs system demonstrated significantly improved survival in patients receiving an ACE inhibitor or angiotensin II receptor blocker (13). This study aims to assess the effects of AT1R blockade on chronic, inhaled nicotine-induced PH and RV remodeling using a mouse model.
MATERIALS AND METHODS
Animals
Adult, male C57BL6/J mice (8–12-wk-old) were purchased from Jackson Laboratory (Bar Harbor, ME) for use in all experiments. Mice were housed in a temperature- and humidity-controlled facility under a 12-h/12-h light-dark cycle, fed standard mouse chow (iOS Teklab Extruded Rodent Diet 2019S; Envigo, Huntingdon, United Kingdom) and water ad libitum. The sodium content of the mouse chow was 0.1%. All procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Louisiana State University Health Sciences Center Institutional Animal Care and Use Committee (Protocol No. 3674).
Losartan Infusion
Before nicotine or air exposure, a subset of mice was surgically implanted with subcutaneous osmotic minipumps (Model 1004; Alzet, Cupertino, CA) containing losartan potassium dissolved in saline (MilliporeSigma, Burlington, MA). Following 4 wk of infusion, the procedure was repeated and old pumps were replaced with new, identically-prepared pumps. Mice were anesthetized with 2.5% isoflurane and preoperatively injected with 50 µL of subcutaneous Buprenorphine-SR (ZooPharm, Windsor, CO). Losartan dosage during weeks 1 through 4 was ∼6.5 mg/kg/day (based on a body weight of 23–25 g). Losartan dosage during weeks 5 through 8 was ∼5.0 mg/kg/day (based on a body weight of 30 g).
Radio-Telemetry Implantation and Blood Pressure Measurement
A subset of mice (n = 6/treatment group) was implanted with radio-telemetry probes (PA-C10 or HD-X10; DSI, St. Paul, MN) before nicotine exposure as previously reported (14, 15). After a 1-wk recovery period, mice underwent baseline recording of conscious blood pressure for 24 h. Twenty-four-hour blood pressure was recorded again at 8 wk. The areas above and below the systolic blood pressure traces were calculated to establish normotensive and hypertensive zones. A systolic blood pressure of 130 mmHg was used as a threshold for these calculations.
Chronic Nicotine Inhalation Model
Nicotine-exposed mice were housed in a nicotine inhalation chamber (La Jolla Alcohol Research, La Jolla, CA) as previously described (7). Briefly, nicotine vapor was produced by bubbling air at a flow rate of 20 L/min through a gas-washing bottle containing a pure nicotine solution (free base; Sigma Aldrich, St. Louis, MO). The highly concentrated nicotine vapor was diluted through the addition of 60 L/min of clean air and was homogeneously distributed between chambers at a flow rate of 7 to 8 L/min. Air flow rate was adjusted to produce nicotine vapor concentrations which resulted in blood cotinine (a more stable metabolite of nicotine) levels comparable to human smokers. Exposure to nicotine followed a 12 h-on/12 h-off schedule with the nicotine exposure (9:00 PM to 9:00 AM) overlapping with the dark cycle (6:00 PM to 6:00 AM). Blood cotinine levels were measured using ELISA (Calbiotech, El Cajon, CA). Blood samples were collected via submandibular vein puncture within 1 h of the nicotine-exposure period ending. Mice in the air-exposed group were housed outside of the chamber, but in the same room.
Echocardiography
Echocardiography was performed at baseline and after 8 wk of exposure using the Vevo 3100 Imaging System with a 30-MHz probe (VisualSonics, Toronto, Canada). Measurements were recorded under 1%–1.5% isoflurane anesthesia with mice placed on a heated pad. Heart rates were maintained between 400 and 550 beats/min. Short-axis and long-axis views were captured using B-mode images, and M-mode recordings were collected using a two-dimensional reference sector for left ventricular (LV) dimensions. Right ventricular (RV) dimensions were derived from B-mode images. Ultrasound image processing was performed using the leading-edge method with VisualSonics software. All measurements were performed on a minimum of 3 cardiac cycles, and group averages were calculated for each time point.
RV Pressure Measurement
Following 8 wk of exposure, mice underwent right heart catheterization. Mice were anesthetized with 2%–3% isoflurane and placed on a heated pad. After dissection to expose the right jugular vein, a pressure transducer (SPR-1000; Millar, Houston, TX) was inserted into the RV through the right jugular vein and right atrium. RV pressure was recorded and analyzed by the PowerLab 8/35 acquisition system (ADInstuments, Colorado Springs, CO).
Plasma and Tissue Collection
Mice under anesthesia were euthanized by decapitation at the conclusion of the 8-wk study. Trunk blood was collected immediately into microtubes containing 40 μL of protease inhibitor cocktail concentrate (Attoquant Diagnostics, Vienna, Austria) and placed on ice. Heart and lung tissues were collected and snap-frozen in liquid nitrogen for further analysis. In the heart tissue, RV was separated from LV and interventricular septum and individually weighed. Tissue was stored at −80°C.
Angiotensin Peptide Analysis
Blood treated with protease inhibitor cocktail was centrifuged at 3,000 g for 10 min at 4°C. Plasma was collected, transferred to a new microtube, snap-frozen in liquid nitrogen, and stored at −80°C. Frozen plasma, RV, LV, and lung tissue were shipped on dry ice to Attoquant Diagnostics (Vienna, Austria) for the analysis of 6 angiotensin (ANG) metabolites (ANG I, ANG II, ANG III, ANG IV, ANG 1–7, and ANG 1–5) via liquid chromatography–mass spectrometry.
Statistical Analysis
Data were expressed as means ± SE. Findings were analyzed, where appropriate, by Student’s t test or two-way ANOVA followed by Tukey–Kramer post hoc test for multiple comparisons between means using GraphPad Prism 8 (GraphPad Software, San Diego, CA). P < 0.05 was considered statistically significant.
RESULTS
Chronic, Inhaled Nicotine Exposure in Mice Produces Serum Cotinine Concentrations Comparable to Human Smokers
Our study sought to examine the effects of angiotensin II type 1 receptor (AT1R) blockade on pulmonary hypertension (PH) and right ventricular (RV) remodeling in mice exposed to chronic, inhaled nicotine. To achieve this, male, C57BL6/J mice (aged 10–12 wk) were housed for 8 wk in room air or in an inhalation chamber receiving daily, 12 h on/12 h off exposure to vapor nicotine. Nicotine exposure was assessed via measurement of serum cotinine levels (a more stable metabolite of nicotine) due to the short half-life of nicotine in mice (16). Air-exposed mice, housed outside of the nicotine inhalation chamber, had undetectable serum cotinine levels. Serum cotinine in mice receiving nicotine was 559 ± 57 ng/mL (Fig. 1). This concentration is comparable to those found within human smokers and our previously published study using this exposure model in mice (614 ± 39 ng/mL) (7, 16). Plasma cotinine levels in humans following electronic cigarette use are similar to those found following cigarette smoking (17).
Figure 1.
Group-averaged serum cotinine levels in air- and nicotine-exposed mice measured by ELISA. n = 12/group. Data are displayed as means ± SE. ****P < 0.0001 (two-tailed t test).
Chronic, Inhaled Nicotine Does Not Significantly Increase Angiotensin II in the Lung, RV, or LV
The renin-angiotensin system (RAS), through its actions on the AT1R, is implicated in the pathogenesis of both PH and cardiac remodeling (12, 18). In addition, we have previously shown increased expression of angiotensin-converting enzyme (ACE) and RAS-associated signaling molecules in the RV, but not the left ventricle (LV), of nicotine-exposed mice (7). Samples of plasma, lung, RV, and LV from nicotine- and air-exposed mice (n = 8/group) were assessed by liquid chromatography–mass spectrometry for quantification of six angiotensin (ANG) peptides. Eight weeks of chronic, inhaled nicotine resulted in significantly elevated levels of plasma ANG 1–7 (P < 0.5; Table 1). There were nonsignificant elevations of ANG II in the lung (P = 0.077), RV (P = 0.064), and LV (P = 0.097) following nicotine exposure (Table 1). Expression of ANG I, ANG III, ANG IV, and ANG 1–5 was unchanged by nicotine exposure in plasma and the three tissues. ANG III and ANG 1–7 are metabolites of ANG II which exert counterregulatory effects via preferential activation of the ANG II receptor type 2 (AT2R) and Mas receptor. These actions include reducing inflammation, inducing natriuresis, and attenuating PH (24–27). ANG 1–5, a metabolite of ANG 1–7, may have similar effects at the AT2R and Mas receptor (28). ANG IV is a metabolite of ANG III which may have hypertensive effects via action on the AT1R (29).
Table 1.
Angiotensin peptide quantification via liquid chromatography–mass spectrometry in air- and nicotine-exposed mouse tissues
| ANG I (1–10) | ANG II (1–8) | ANG III (2–8) | ANG IV (3–8) | ANG 1–7 | ANG 1–5 | |
|---|---|---|---|---|---|---|
| Tissue | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) |
| Plasma, pmol/L | ||||||
| Air | 133.9 (70.7 to 197.0) | 73.5 (30.7 to 116.3) | 70.8 (38.4 to 103.3) | 10.7 (7.4 to 14.0) | 6.4 (2.6 to 10.4) | 13.1 (6.5 to 19.7) |
| Nicotine | 208.5 (109.4 to 307.7) | 104.1 (45.9 to 162.2) | 105.2 (70.3 to 140.1) | 16.3 (7.1 to 24.6) | 26.3* (6.4 to 46.2) | 23.1 (10.7 to 35.6) |
| RV, fmol/L | ||||||
| Air | 18.8 (13.9 to 23.6) | 147.4 (78.39 to 216.5) | 10.5 (9.3 to 11.7) | 5.9 (4.9 to 6.9) | 15.0 (15.0 to 15.0) | 6.1 (4.3 to 7.9) |
| Nicotine | 26.2 (15.0 to 37.4) | 241.8 (155.1 to 328.4) | 10.2 (9.7 to 10.6) | 5.7 (4.3 to 7.1) | 15.0 (15.0 to 15.0) | 7.5 (4.5 to 10.5) |
| LV, fmol/L | ||||||
| Air | 25.7 (18.1 to 33.3) | 209.0 (110.9 to 307.1) | 10.43 (9.4 to 11.4) | 6.4 (4.9 to 8.0) | 23.0 (16.8 to 29.1) | 9.5 (6.5 to 12.5) |
| Nicotine | 34.3 (25.4 to 43.3) | 320.8 (209.1 to 432.4) | 11.4 (9.1 to 13.8) | 6.5 (5.1 to 7.9) | 28.6 (20.2 to 37.1) | 11.6 (7.7 to 15.5) |
| Lung, fmol/L | ||||||
| Air | 36.6 (8.0 to 65.3) | 95.6 (52.4 to 138.8) | 18.4 (8.4 to 28.4) | 7.2 (3.7 to 10.7) | 26.3 (7.2 to 45.4) | 16.2 (5.3 to 27.0) |
| Nicotine | 22.6 (16.9 to 28.3) | 153.0 (96.4 to 209.5) | 35.1 (12.0 to 58.2) | 7.9 (4.6 to 11.2) | 18.5 (11.8 to 25.1) | 12.8 (10.7 to 15.0) |
Chronic, inhaled nicotine increases ANG 1–7 in plasma. There were nonsignificant elevations of ANG II in the RV (P = 0.064) and lung (P = 0.077) of nicotine-exposed mice. These entries are indicated in bold. n = 8/group. *P < 0.05 (two-tailed t test). LV, left ventricular; RV, right ventricular.
Systemic Blood Pressure is Unaffected by Angiotensin II Type 1 Receptor Blockade and Nicotine
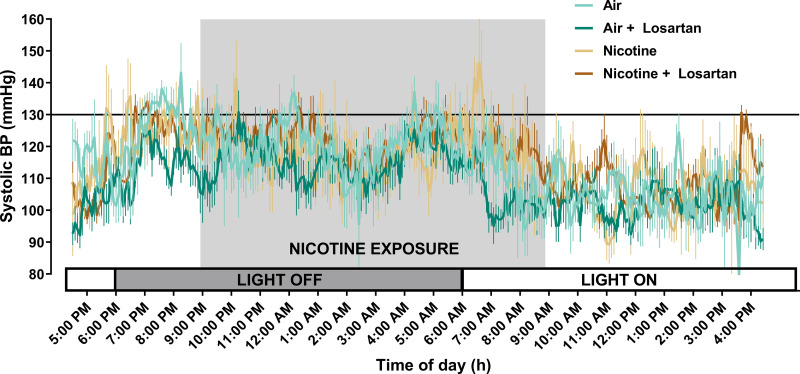
We have previously shown that inhaled nicotine increases systemic blood pressure during the first 3 wk of exposure, with systemic blood pressure returning to normal in subsequent weeks of exposure (7). In the current study, no differences were noted in systolic blood pressure following 8 wk of exposure to losartan, nicotine, or both (Fig. 2).
Figure 2.
Average 24-h systolic blood pressure at 8 wk in mice exposed to air (light teal, n = 6), air and losartan (dark teal, n = 6), nicotine (light brown, n = 6), and nicotine and losartan (dark brown, n = 6). The shaded area corresponds to time of nicotine exposure; the solid line indicates the threshold for normotensive and hypertensive zones (130 mmHg). Data are displayed as means ± SE. No significant differences were measured (two-way ANOVA with Tukey–Kramer post hoc test).
Angiotensin II Type 1 Receptor Blockade Ameliorates Nicotine-Induced Pulmonary Hypertension
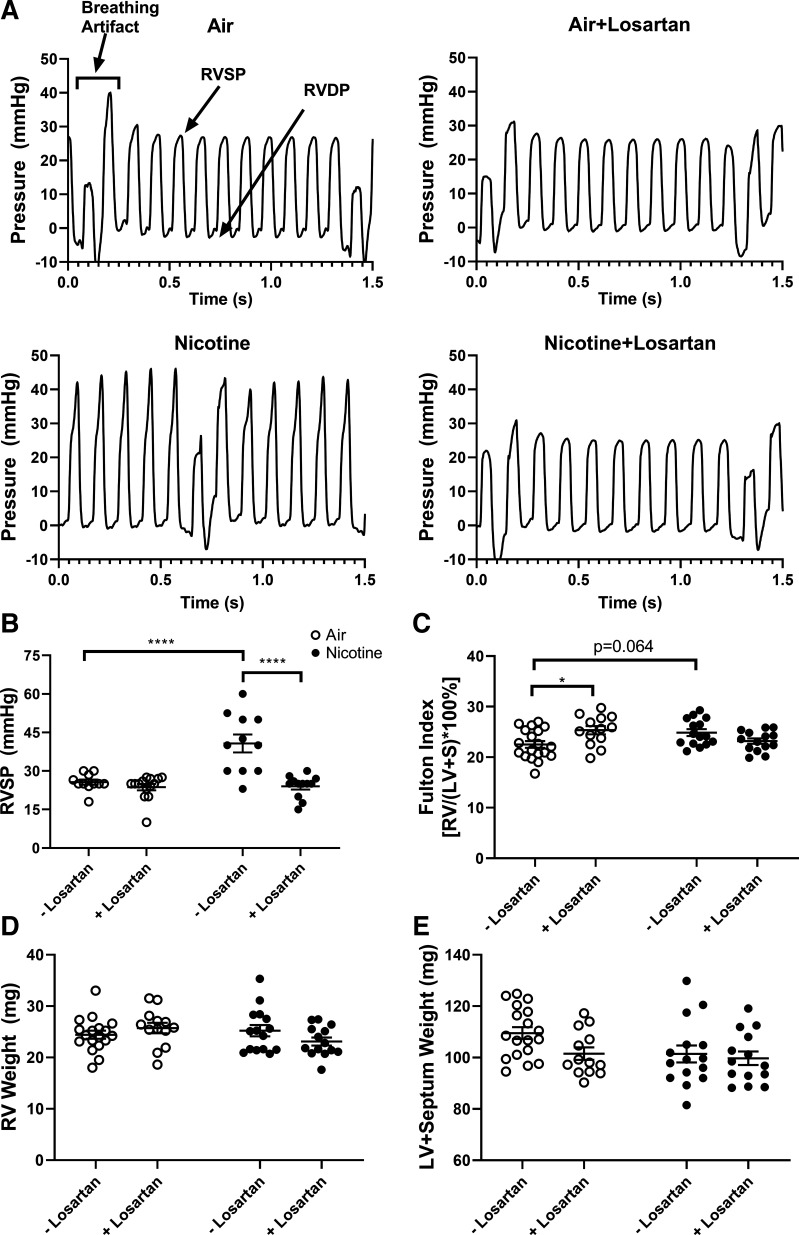
At the conclusion of the 8-wk study, mice underwent right heart catheterization for measurement of RV systolic pressure (RVSP), allowing estimation of systolic pressure in the pulmonary artery (Fig. 3, A and B). Chronic, inhaled nicotine significantly increased RVSP (40.7 ± 3.5 mmHg, n = 11) versus air exposure (25.6 ± 1.0 mmHg, n = 11, P < 0.0001). Mice receiving both losartan and nicotine had significantly reduced RVSP (24.0 ± 1.3 mmHg, n = 12, P < 0.0001) versus nicotine alone. There was no difference in RVSP between air-exposed mice and either losartan-infused group (air-losartan: 23.7 ± 1.2 mmHg, n = 14).
Figure 3.
Losartan treatment ameliorates chronic, inhaled nicotine-induced pulmonary hypertension measured by cardiac catheterization. A: representative right ventricular (RV) pressure tracings for mice exposed to air (upper left), air and losartan (upper right), nicotine (lower left), and nicotine and losartan (lower right). B: RV systolic pressure (RVSP) in air- and nicotine-exposed mice with or without losartan treatment. n = 11–14/group. C: Fulton index in air- and nicotine-exposed mice with or without losartan treatment. n = 13–19/group. D and E: RV weight and left ventricular (LV) and interventricular septum (S) weight. n = 13–19/group. Data are displayed as means ± SE. *P < 0.05, ****P < 0.0001 (two-way ANOVA with Tukey–Kramer post hoc test).
Fulton index (RV weight divided by the sum of the weight of the LV and interventricular septum, LV + S) was used to assess RV hypertrophy associated with PH (Fig. 3C) (30). There was a nonsignificant increase in Fulton index in chronic, inhaled nicotine exposure (24.9 ± 0.7, n = 15) versus air exposure (22.5 ± 0.7, n = 19, P = 0.064). Fulton index between air-exposed mice receiving losartan (25.3 ± 0.8, n = 13) and nicotine-exposed mice receiving losartan (23.2 ± 0.5, n = 14) was not significantly different. Surprisingly, Fulton index in losartan-infused, air-exposed mice was significantly increased versus mice receiving air without treatment (P < 0.05). Examination of individual heart weights suggests that this finding was driven by reduced LV + S weight (air-losartan: 101.5 ± 2.4, n = 13 vs. air: 109.5 ± 2.3, n = 18), rather than increased RV weight. The differences in RV and LV + S weights were not significant (Fig. 3, D and E).
Angiotensin II Type 1 Receptor Blockade Ameliorates Nicotine-Induced Right Ventricular Remodeling
Hypertrophic remodeling of the RV leading to dysfunction and failure is a hallmark of mortality in PH (31). Echocardiography was used to assess the structure of the right side of the heart following chronic, inhaled nicotine exposure and losartan treatment (Fig. 4). Reflective of the observed elevation in RVSP, RV free wall thickness during diastole (RV FWT;d) was increased in nicotine mice (0.44 ± 0.01 mm, n = 12) versus air mice (0.31 ± 0.01 mm, n = 10, P < 0.0001). Losartan infusion of nicotine-exposed mice resulted in significantly decreased RV FWT;d (0.34 ± 0.01 mm, n = 8, P < 0.0001) versus nicotine alone. There was no difference in RV FWT;d between air mice and the air-losartan group (0.317 ± 0.01 mm, n = 7). Chronic, inhaled nicotine increased RV internal diameter during diastole (RVID;d; 1.56 ± 0.04 mm, n = 12) versus air mice (1.24 ± 0.07 mm, n = 10, P < 0.0001), suggesting dilation of the chamber. We have previously shown a nonsignificant trend towards increased RVID;d in nicotine-exposed mice (7). RVID;d between air-exposed mice receiving losartan (1.23 ± 0.04, n = 7) and nicotine-exposed mice receiving losartan (1.44 ± 0.03, n = 8) was not significantly different.
Figure 4.
Losartan treatment ameliorates chronic, inhaled nicotine-induced right ventricular (RV) remodeling assessed by echocardiography. RV free wall thickness in diastole (RV FWT;d) (A) and RV internal diameter in diastole (RVID;d) (B) in air- and nicotine-exposed mice with or without losartan treatment. Heart rate was maintained between 400 and 550 beats/min. Data are displayed as means ± SE. n = 7–12/group; *P < 0.05, ****P < 0.0001 (two-way ANOVA with Tukey–Kramer post hoc test).
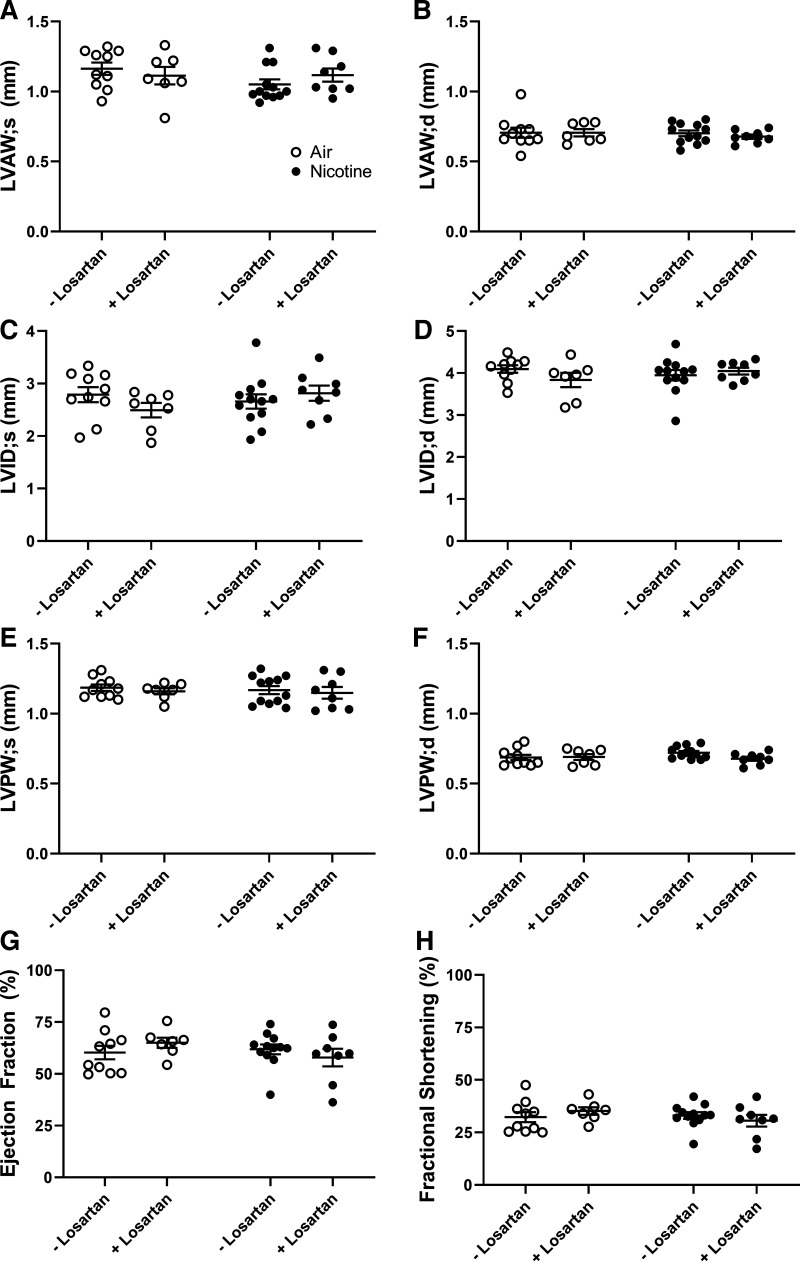
Despite significant nicotine-induced changes to the RV which were ameliorated by losartan infusion, we observed no changes in any parameter of LV structure or function (Fig. 5). LV anterior wall thickness in systole and diastole (LVAW;s and LVAW;d), LV internal diameter during systole and diastole (LVID;s and LVID;d), LV posterior wall thickness in systole and diastole (LVPW;s and LVPW;d), ejection fraction (EF), and fractional shortening (FS) were all unchanged by nicotine-exposure or losartan treatment. We found no significant difference in systemic blood pressure between air-losartan mice and nicotine-losartan mice via radio-telemetry monitoring (Fig. 2). These findings support our previously published results showing transient systolic blood pressure elevations with no LV remodeling or dysfunction following chronic inhaled nicotine (7).
Figure 5.
Left ventricular (LV) structure and function, assessed by echocardiography, are unaffected by nicotine and losartan. A and B: LV anterior wall thickness in systole and diastole (LVAW;s and LVAW;d); (C and D): LV internal diameter in systole and diastole (LVID;s and LVID;d); (E and F): LV posterior wall thickness in systole and diastole (LVPW;s and LVPW;d); ejection fraction (G) and fractional shortening (H) in air- and nicotine-exposed mice with or without losartan treatment. Heart rate was maintained at 400 to 550 beats/min. Data are displayed as means ± SE. n = 7–12/group. No significant differences were measured (two-way ANOVA with Tukey–Kramer post hoc test).
DISCUSSION
The current study assessed the effects of AT1R blockade on chronic, inhaled nicotine-induced PH and RV remodeling using a murine model. Eight weeks of inhaled nicotine exposure appears to upregulate expression of angiotensin II in the RV and lung, but this finding was nonsignificant (P = 0.064 and P = 0.077, respectively). This provides support for our previous findings of upregulated ACE expression and RAS-associated signaling in the RV of nicotine-exposed mice (7). This study shows, for the first time, that losartan treatment ameliorates nicotine-induced increases in RVSP and RV wall thickness.
PH is a complex disease state that encompasses five major groups: pulmonary arterial hypertension (group 1), PH due to left heart dysfunction (group 2), PH due to lung disease (group 3), chronic thromboembolic PH (group 4), and multifactorial PH (group 5) (31). Cigarette smoking has been associated with group 1 and group 4 PH in a large, international case-control study (32). The role of cigarette smoking in the development of LV dysfunction, pulmonary disease, and multisystem pathology further suggests involvement in the pathogenesis of group 2, group 3, and group 5 PH (19, 33, 34). The classification of inhaled nicotine-induced PH is currently unknown. LV remodeling and dysfunction were absent in both the current study and our previously published study (7); a group 2 PH classification is, therefore, unlikely. Alternative methods of nicotine delivery do, however, lead to LV changes. One month of daily 0.6 mg/kg intraperitoneal nicotine injections in rats causes LV remodeling and dysfunction; these changes are attenuated by injection of the AT1R antagonist, irbesartan (35).
Treatment for patients with PH comprises supportive therapies (diuretics, oxygen, and anticoagulants), calcium channel blockers, and targeted therapies (phosphodiesterase-5 inhibitors, prostacyclin analogues and receptor agonists, and endothelin receptor antagonists) (31). Although not currently standard, the Veterans Affairs cohort provides compelling evidence for the addition of ACE inhibitors and AT1R blockers to the PH treatment regimen (13). Our studies of nicotine-induced PH in mice and others’ studies of cigarette smoking-induced PH in rats further support the plausibility of RAS-based therapy in PH patients, particularly those with a history of electronic or combustible cigarette use (7, 8, 11).
Previous studies have implicated both nicotine and the AT1R in cardiac remodeling (reviewed extensively in Ref. 12). Cardiac fibroblasts exposed to nicotine in vitro have increased proliferation and collagen production mediated by the protein kinase C signaling pathway (36). Protein kinase C signaling also mediates RV hypertrophy and fibrosis in PH rats. These protein kinase C-mediated findings can be replicated in vitro via treatment of healthy cardiac fibroblasts with ANG II (22). In our study, we demonstrate a nonsignificant, nicotine-induced increase in ANG II concentration in the RV and lungs of mice leading to elevated RVSP and RV remodeling. There were no changes in circulating ANG I or ANG II, suggesting potential involvement of local RAS in the heart and lungs (23, 37). Serum ANG 1–7 was significantly elevated, but the importance of this finding is unclear. ANG 1–7 opposes the hypertrophic effects of ANG II and could be upregulated in these mice via a compensatory mechanism (38, 39). Sampling and storage of plasma and tissue for the analysis of ANG peptides was standardized, but it is not possible to rule out contamination with blood or peptides generated ex vivo. The effect of losartan to reduce RVSP and RV wall thickness was robust, but losartan effects on RV chamber dilation were subtle.
We found no significant difference between RV chamber diameter in air-losartan mice and nicotine-losartan mice. There was, however, no significant reduction in RV chamber diameter between nicotine mice and nicotine-losartan mice. RV dilation leading to failure is a major cause of mortality in patients with PH (31). Combination therapy with AT1R blockade and thiazide diuretics, commonly available as losartan-hydrochlorothiazide, may provide greater amelioration of the observed nicotine-induced RV dilation (31, 42). In heart failure patients, chlorthalidone (a long-acting thiazide-like diuretic, which also inhibits the sodium-chloride symporter) is preferred over hydrochlorothiazide (short-acting) (40); combined losartan-chlorthalidone therapy has previously been shown to be well-tolerated and efficacious in a randomized clinical trial (41). Future studies of nicotine-induced PH could explore this dual therapeutic approach.
Our study is not without limitations. The chronic, nicotine inhalation model used here achieves serum cotinine levels which compare favorably to those found in human cigarette smokers. Despite comparable nicotine delivery, our model exposes mice continuously over a 12-h period each day in contrast to the intermittent exposure of a typical electronic cigarette user. Future investigations should employ an intermittent nicotine delivery method to more closely replicate human behavior. An intermittent approach could include 15 min of nicotine exposure followed by 15 min air exposure throughout a 12-h period. In addition, the dose-effect of nicotine on PH and RV remodeling is currently unknown and should be examined in future work. Previous studies of nicotine’s cardiovascular effects (heart rate, blood pressure, and skin temperature) suggest that maximal effects are achieved at moderate nicotine doses, with a flattening of the dose-response curve at higher exposure levels (21).
Nicotine is the principal, but not the only, biologically active inhalant found in electronic cigarette vapor. Nicotine-containing electronic cigarettes cause transient elevations in heart rate and blood pressure in patients that were not seen in patients receiving inhaled nicotine or nicotine-free electronic cigarettes (20). This synergistic interaction between nicotine and other electronic cigarette-associated inhalants warrants further exploration. PH development and the efficacy of losartan treatment may differ in mice exposed to various combinations of nicotine, flavoring agents, and electronic cigarette humectants.
GRANTS
This study was supported by the National Heart, Lung, and Blood Institute Grant R01HL135635 (to E.L, X.Y., and J.D.G).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.L., X.Y., and J.D.G. conceived and designed research; N.D.F., T.M.M., A.W., E.L., X.Y., and J.D.G. performed experiments; N.D.F., T.M.M., A.W., E.L., X.Y., and J.D.G. analyzed data; N.D.F., T.M.M., E.L., X.Y., and J.D.G. interpreted results of experiments; N.D.F., T.M.M., E.L., X.Y., and J.D.G. prepared figures; N.D.F. drafted manuscript; N.D.F., E.L., X.Y., and J.D.G. edited and revised manuscript; N.D.F., T.M.M., A.W., E.L., X.Y., and J.D.G. approved final version of manuscript.
REFERENCES
- 1.Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, Neff LJ. Current cigarette smoking among adults - United States, 2016. Morb Mortal Wkly Rep 67: 53–59, 2018. doi: 10.15585/mmwr.mm6702a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman SJ, Mammone J, Van Katwyk SR, Sritharan L, Tran M, Al-Khateeb S, Grjibovski A, Gunn E, Kamali-Anaraki S, Li B, Mahendren M, Mansoor Y, Natt N, Nwokoro E, Randhawa H, Song MY, Vercammen K, Wang C, Woo J, Poirier MJP. Cigarette consumption estimates for 71 countries from 1970 to 2015: systematic collection of comparable data to facilitate quasi-experimental evaluations of national and global tobacco control interventions. BMJ 365: l2231, 2019. doi: 10.1136/bmj.l2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMillen RC, Gottlieb MA, Whitmore Shaefer RM, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res 17: 1195–1202, 2015. doi: 10.1093/ntr/ntu213. [DOI] [PubMed] [Google Scholar]
- 4.Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students — United States, 2011–2018. Morb Mortal Wkly Rep 67: 1276–1277, 2018. doi: 10.15585/mmwr.mm6745a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai H, Leventhal AM. Prevalence of e-cigarette use among adults in the United States, 2014-2018. JAMA 322: 1824–1827, 2019. doi: 10.1001/jama.2019.15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDonald A, Middlekauff HR. Electronic cigarettes and cardiovascular health: what do we know so far? Vasc Health Risk Manag 15: 159–174, 2019. doi: 10.2147/VHRM.S175970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oakes JM, Xu J, Morris TM, Fried ND, Pearson CS, Lobell TD, Gilpin NW, Lazartigues E, Gardner JD, Yue X. Effects of chronic nicotine inhalation on systemic and pulmonary blood pressure and right ventricular remodeling in mice. Hypertension 75: 1305–1314, 2020. doi: 10.1161/HYPERTENSIONAHA.119.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han S-X, He G-M, Wang T, Chen L, Ning Y-Y, Luo F, An J, Yang T, Dong J-J, Liao Z-L, Xu D, Wen F-Q. Losartan attenuates chronic cigarette smoke exposure induced pulmonary arterial hypertension in rats: possible involvement of angiotensin-converting enzyme-2. Toxicol Appl Pharmacol 245: 100–107, 2010. doi: 10.1016/j.taap.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weissmann N, Lobo B, Pichl A, Parajuli N, Seimetz M, Puig-Pey R, Ferrer E, Peinado VI, Domínguez-Fandos D, Fysikopoulos A, Stasch JP, Ghofrani HA, Coll-Bonfill N, Frey R, Schermuly RT, Garcia-Lucio J, Blanco I, Bednorz M, Tura-Ceide O, Tadele E, Brandes RP, Grimminger J, Klepetko W, Jaksch P, Rodriguez-Roisin R, Seeger W, Grimminger F, Barbera J. Stimulation of soluble guanylate cyclase prevents cigarette smoke-induced pulmonary hypertension and emphysema. Am J Respir Crit Care Med 189: 1359–1373, 2014. doi: 10.1164/rccm.201311-2037OC. [DOI] [PubMed] [Google Scholar]
- 10.Wright JL, Tai H, Churg A. Vasoactive mediators and pulmonary hypertension after cigarette smoke exposure in the guinea pig. J Appl Physiol (1985) 100: 672–678, 2006. doi: 10.1152/japplphysiol.00274.2005. [DOI] [PubMed] [Google Scholar]
- 11.Yuan YM, Luo L, Guo Z, Yang M, Ye RS, Luo C. Activation of renin angiotensin- aldosterone system (RAAS) in the lung of smoking-induced pulmonary arterial hypertension (PAH) rats. J Renin Angiotensin Aldosterone Syst 16: 249–253, 2015. doi: 10.1177/1470320315576256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oakes JM, Fuchs RM, Gardner JD, Lazartigues E, Yue X. Nicotine and the renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 315: R895–R906, 2018. doi: 10.1152/ajpregu.00099.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahm T, Hess E, Barón AE, Maddox TM, Plomondon ME, Choudhary G, Maron BA, Zamanian RT, Leary PJ. Renin-angiotensin-aldosterone system inhibitor use and mortality in pulmonary hypertension: insights from the Veterans Affairs CART Database. Chest S0012-3692: 34851–34860, 2020. doi: 10.1016/j.chest.2020.09.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia H, Sriramula S, Chhabra KH, Lazartigues E. Brain angiotensin converting enzyme type 2 shedding contributes to the development of neurogenic hypertension. Circ Res, 113: 1087–1096, 2013. doi: 10.1161/CIRCRESAHA.113.301811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Sriramula S, Xia H, Moreno-Walton L, Culicchia F, Domenig O, Poglitsch M, Lazartigues E. Clinical relevance and role of neuronal AT1 receptors in ADAM17-mediated ACE2 shedding in neurogenic hypertension. Circ Res. 121: 43–55, 2017. doi: 10.1161/CIRCRESAHA.116.310509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 190: 269–319, 2007. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- 17.Marsot A, Simon N. Nicotine and cotinine levels with electronic cigarette: a review. Int J Toxicol 35: 179–185, 2016. doi: 10.1177/1091581815618935. [DOI] [PubMed] [Google Scholar]
- 18.Maron BA, Leopold JA. The role of the renin-angiotensin-aldosterone system in the pathobiology of pulmonary arterial hypertension (2013 Grover Conference Series). Pulm Circ 4: 200–210, 2014. doi: 10.1086/675984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aaron CP, Hoffman EA, Lima JAC, Kawut SM, Bertoni AG, Vogel-Claussen J, Habibi M, Hueper K, Jacobs DR Jr, Kalhan R, Michos ED, Post WS, Prince MR, Smith BM, Ambale-Venktesh B, Liu CY, Zemrak F, Watson KE, Budoff M, Bluemke DA, Barr RG. Pulmonary vascular volume, impaired left ventricular filling and dyspnea: The MESA Lung Study. PLoS ONE 12: e0176180, 2017. doi: 10.1371/journal.pone.0176180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arastoo S, Haptonstall KP, Choroomi Y, Moheimani R, Nguyen K, Tran E, Gornbein J, Middlekauff HR. Acute and chronic sympathomimetic effects of e-cigarette and tobacco cigarette smoking: role of nicotine and non-nicotine constituents. Am J Physiol Heart Circ Physiol 319: H262–H270, 2020. doi: 10.1152/ajpheart.00192.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benowitz NL, Jacob P, Herrera B. Nicotine intake and dose response when smoking reduced–nicotine content cigarettes. Clin Pharmacol Ther 80: 703–714, 2006. doi: 10.1016/j.clpt.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Chichger H, Vang A, O'Connell KA, Zhang P, Mende U, Harrington EO, Choudhary G. PKC δ and βII regulate angiotensin II-mediated fibrosis through p38: a mechanism of RV fibrosis in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 308: L827–L836, 2015. doi: 10.1152/ajplung.00184.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Mello WC. Local renin angiotensin aldosterone systems and cardiovascular diseases. Med Clin North Am 101: 117–127, 2017. doi: 10.1016/j.mcna.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 24.El-Hashim AZ, Renno WM, Raghupathy R, Abduo HT, Akhtar S, Benter IF. Angiotensin-(1–7) inhibits allergic inflammation, via the MAS1 receptor, through suppression of ERK1/2- and NF-kB-dependent pathways. Br J Pharmacol 166: 1964–1976, 2012. doi: 10.1111/j.1476-5381.2012.01905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hampl V, Herget J, Bíbová J, Baňasová A, Husková Z, Vaňourková Z, Jíchová Š, Kujal P, Vernerová Z, Sadowski J, Červenka L. Intrapulmonary activation of the angiotensin-converting enzyme type 2/angiotensin 1-7/G-protein-coupled Mas receptor axis attenuates pulmonary hypertension in Ren-2 transgenic rats exposed to chronic hypoxia. Physiol Res 64: 25–38, 2015. doi: 10.33549/physiolres.932861. [DOI] [PubMed] [Google Scholar]
- 26.Ocaranza MP, Riquelme JA, Garcia L, Jalil JE, Chiong M, Santos RAS, Lavandero S. Counter- regulatory renin–angiotensin system in cardiovascular disease. Nat Rev Cardiol 17: 116–129, 2020. doi: 10.1038/s41569-019-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padia SH, Kemp BA, Howell NL, Fournie-Zaluski M, Roques BP, Carey RM. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor–mediated natriuresis in rats. Hypertension 51: 460–465, 2008. doi: 10.1161/HYPERTENSIONAHA.107.103242. [DOI] [PubMed] [Google Scholar]
- 28.Yu L, Kuichang Y, Phuong HTA, Byung MP, Kim SH. Angiotensin-(1-5), an active mediator of renin-angiotensin system, stimulates ANP secretion via Mas receptor. Peptides 86: 33–41, 2016. doi: 10.1016/j.peptides.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Lochard N, Thibault G, Silversides DW, Touyz RM, Reudelhuber TL. Chronic production of angiotensin IV in the brain leads to hypertension that is reversible with an angiotensin II AT1 receptor antagonist. Circ Res 94: 1451–1457, 2004. doi: 10.1161/01.RES.0000130654.56599.40. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Schreier DA, Hacker TA, Chesler NC. Progressive right ventricular functional and structural changes in a mouse model of pulmonary arterial hypertension. Physiol Rep 1: e00184, 2013. doi: 10.1002/phy2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lan NSH, Massam BD, Kulkarni SS, Lang CC. Pulmonary arterial hypertension: pathophysiology and treatment. Diseases 6: e38, 2018. doi: 10.3390/diseases6020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keusch S, Hildenbrand FF, Bollmann T, Halank M, Held M, Kaiser R, Kovacs G, Lange TJ, Seyfarth HJ, Speich R, Ulrich S. Tobacco smoke exposure in pulmonary arterial and thromboembolic pulmonary hypertension. Respiration 88: 38–45, 2014. doi: 10.1159/000359972. [DOI] [PubMed] [Google Scholar]
- 33.Kamimura D, Cain LR, Mentz RJ, White WB, Blaha MJ, DeFilippis AP, Fox ER, Rodriguez CJ, Keith RJ, Benjamin EJ, Butler J, Bhatnagar A, Robertson RM, Winniford MD, Correa A, Hall ME. Cigarette smoking and incident heart failure: insights from the Jackson Heart Study. Circulation 137: 2572–2582, 2018. doi: 10.1161/CIRCULATIONAHA.117.031912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 370: 765–773, 2007. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 35.Ramalingam A, Budin SB, Mohd. Fauzi N, Ritchie RH, Zainalabidin S. Angiotensin II type I receptor antagonism attenuates nicotine-induced cardiac remodeling, dysfunction, and aggravation of myocardial ischemia- reperfusion injury in rats. Front Pharmacol 10: 1493, 2019. doi: 10.3389/fphar.2019.01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vang A, Clements RT, Chichger H, Kue N, Allawzi A, O'Connell K, Jeong E-M, Dudley SC, Sakhatskyy P, Lu Q, Zhang P, Rounds S, Choudhary G. Effect of α7 nicotinic acetylcholine receptor activation on cardiac fibroblasts: a mechanism underlying RV fibrosis associated with cigarette smoke exposure. Am J Physiol Lung Cell Mol Physiol 312: L748–L759, 2017. doi: 10.1152/ajplung.00393.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall RP. The pulmonary renin-angiotensin system. Curr Pharm Des 9: 715–722, 2003. doi: 10.2174/1381612033455431. [DOI] [PubMed] [Google Scholar]
- 38.Iwata M, Cowling RT, Yeo SJ, Greenberg B. Targeting the ACE2–Ang-(1–7) pathway in cardiac fibroblasts to treat cardiac remodeling and heart failure. J Mol Cell Cardiol 51: 542–547, 2011. doi: 10.1016/j.yjmcc.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santos RA. Angiotensin-(1-7). Hypertension 63: 1138–1147, 2014. doi: 10.1161/HYPERTENSIONAHA.113.01274. [DOI] [PubMed] [Google Scholar]
- 40.Messerli FH, Rimoldi SF, Bangalore S. The transition from hypertension to heart failure contemporary update. JACC Heart Fail 5: 543–551, 2017. doi: 10.1016/j.jchf.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Pareek A, Basavanagowdappa H, Zawar S, Kumar A, Chandurkar N. A randomized, comparative study evaluating the efficacy and tolerability of losartan-low dose chlorthalidone (6.25 mg) combination with losartan-hydrochlorothiazide (12.5 mg) combination in Indian patients with mild-to-moderate essential hypertension. Expert Opin Pharmacother. 10: 1529–1536, 2009. doi: 10.1517/14656560902991514. [DOI] [PubMed] [Google Scholar]
- 42.Konstam MA, Kiernan MS, Bernstein D, Bozkurt B, Jacob M, Kapur NK, Kociol RD, Lewis EF, Mehra MR, Pagani FD, Raval AN, Ward C, American Heart Association Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; and Council on Cardiovascular Surgery and Anesthesia. Evaluation and management of right-sided heart failure: a scientific statement from the American Heart Association. Circulation 137: e578–e622, 2018. doi: 10.1161/CIR.0000000000000560. [DOI] [PubMed] [Google Scholar]