Abstract
Proper placental development and function relies on hormone receptors and signaling pathways that make the placenta susceptible to disruption by endocrine disrupting chemicals, such as phthalates. Here, we review relevant research on the associations between phthalate exposures and dysfunctions of the development and function of the placenta, including morphology, physiology, and genetic and epigenetic effects. This review covers in vitro studies, in vivo studies in mammals, and studies in humans. We also discuss important gaps in the literature. Overall, the evidence indicates that toxicity to the placental and maternal-fetal interface is associated with exposure to phthalates. Further studies are needed to better elucidate the mechanisms through which phthalates act in the placenta as well as additional human studies that assess placental disruption through pregnancy with larger sample sizes.
Keywords: Phthalate, endocrine disrupting chemicals, placenta, placenta toxicology, developmental origins of health and disease
1. INTRODUCTION
Quality of life has been significantly increased since the Industrial Revolution by technical innovations in industrial and agricultural processes [1]. Many of these advances rely on synthetic chemical production, which has led to widespread environmental contamination due to inappropriate disposal and poor environmental stewardship [2]. Many of these environmental contaminants are considered endocrine disrupting chemicals (EDCs) as they have been shown to interfere with proper endocrine function [3].
EDCs are defined as “exogenous chemicals, or mixtures of chemicals, that can interfere with any aspect of hormone action” [2]. These chemicals are known to display nonmonotonic dose-response curves because hormones interact with and activate their receptors in a nonlinear fashion. The intersection of non-linear dose response curves and multiple possible mechanisms of action can lead to U-shaped or inverted U-shaped dose response curves [3,4]. EDCs are found in a wide range of industrial and consumer products, including plastics, personal care products, pesticides, disinfectant products, solvents, and pharmaceuticals [3,5]. As a result, humans are constantly exposed to low doses of mixtures of EDCs on a daily basis by ingestion, inhalation, and dermal contact and are at risk of detrimental effects on hormonal and physiological health [3,4]. Particularly concerning are exposures that occur during pregnancy, which may impair maternal health or normal placental function, and lead to abnormal fetal development and future disease [6].
During mammalian embryonic development, proper placental function plays a critical role in organogenesis and tissue differentiation. The placenta is the principal modulator of nutrient supply to the growing embryo during gestation. This period is tightly regulated to result in normal fetal organ structure and body formation [7,8]. Previous studies have reported that toxicant exposures during gestation can cause adverse outcomes in exposed children, such as thalidomide and limb malformations and methyl mercury and Minamata disease [9]. Embryogenesis is a critical window of development, characterized by heightened sensitivity to environmental factors that may interfere with the fetal reprogramming process. Thus, the developmental origins of health and disease (DOHaD) hypothesis posits that an adverse environment experienced during development can increase the risk of disease later in life [10–12]. Human and other mammalian experimental pregnancy models have reported abnormalities as a result of irregular maternal diet, exposure to synthetic hormones, and inadvertent exposures to environmental chemicals including EDCs [13–15].
During pregnancy, women and fetuses are exposed to mixtures of chemicals including EDCs [13,16,17]. For example, chemicals used in plastics, including phthalates, have been detected in high levels in maternal blood or urine, umbilical cord blood, and amniotic fluid [13,14,18,19]. Phthalates are synthetic chemicals with ubiquitous human exposure that are known to have complex EDC actions [20,21]. Thus, the purpose of this minireview is to review recent research on the impact of phthalates on placental morphophysiology. We focus on in vitro and in vivo studies, from both human and animal models, of phthalate exposure during fetal development. Furthermore, we review the effects of various phthalates on placental morphology and physiology and placental factors required for normal fetal development.
1.1. PHTHALATES
Phthalates and phthalate esters are a large group of compounds used as non-covalently bound plasticizers and solvents found in a wide range of products including polyvinyl chloride plastics, coatings, cosmetics, personal care products, medical tubing, building materials, food processing equipment, and children’s toys [22,23]. Phthalates have been identified as contaminants in indoor air and household dust [24]. Humans are regularly exposed to multiple phthalates via dermal exposure from personal care and household products, parenteral exposure from medications, intravenous exposure from blood transfusions or pharmacological treatment, oral exposure from drinking and eating food that was processed with plastic tubing or stored in cans lined with epoxy resins, and environmental exposure from dust in the air [25–27]. Phthalates exhibit structure-activity relationships that determine their uses; low molecular weight (LMW) phthalates, including diethyl phthalate (DEP), dibutyl phthalate (DBP), and diisobutyl phthalate (DiBP), are typically used in fragrances in personal care products, such as perfumes and nail polish as well as pharmaceutical formulations, whereas high molecular weight (HMW) phthalates, including di(2-ethylhexyl) phthalate (DEHP), benzyl butyl phthalate (BzBP), and di-isononyl phthalate (DiNP), are used as plasticizers and additives in industrial products including adhesives, flooring, plastic toys, and paints [22,24,28] (Figure 1). Thus, with this widespread use, over 18 billion pounds of phthalates are produced annually and phthalates can be found in the environment, wildlife, and human tissues, justifying public health concern and the importance of understanding their toxicologic effects [22,24,29,30].
Figure 1:
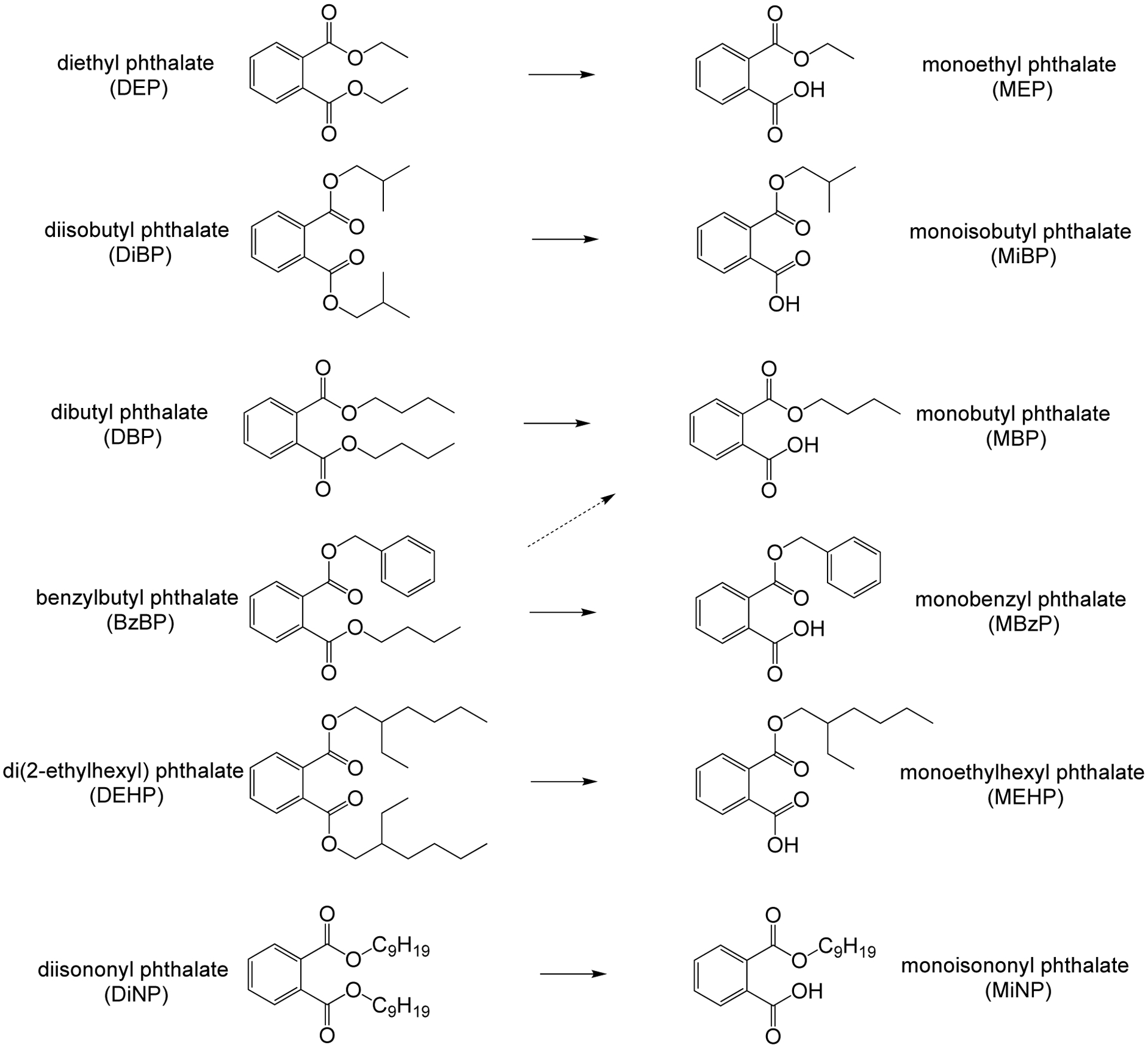
Six of the most common phthalates, arranged by increasing molecular weight, and their primary monoester metabolites.
The United States Environmental Protection Agency suggests a safety level of 0.22 mg/kg/d for DEHP, one of the most widely used phthalates, whereas the Health Canada-European Medicines Agency suggests the tolerable daily intake as 0.4 mg/kg/d [31,32]. These levels are based on traditional regulatory toxicology assessments, which have revealed high no observed adverse effect levels for phthalates and do not consider endocrine disruption. In the European Union, DEHP, DBP, BBP and DIBP are considered substances of very high concern (SVHCs) and are restricted through the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation.
Based on studies performed in the US and Germany, the estimated range of daily human exposure to DEHP is ~3 to 30 μg/kg/d, DEP is 2.32 to 12 μg/kg/d, BzBP is 0.26 to 0.88 μg/kg/d, DBP is 0.84 to 5.22 μg/kg/d, and DiBP is 0.12 to 1.4 μg/kg/d [33,34]. DiNP human occupational exposure levels reach up to 26 μg/kg/d, whereas exposure in infants can reach levels of up to 120 μg/kg/d [35,36]. Furthermore, occupational exposure to DEHP has been estimated to be between 143 and 286 μg/kg/d [33,34]. Even though estimated human exposure to phthalates is low compared with safety doses from regulatory agencies, significant numbers of studies report that phthalate exposure comparable to human exposure levels is associated with significant endocrine disruption effects in human and animal studies [3]. Biomonitoring studies of phthalate metabolites in pregnant women’s urine indicate that exposure to phthalates in pregnant women in the US and around the world is similar to non-pregnant women [13,37–39].
Upon oral exposure, phthalates are initially metabolized in the saliva and gastrointestinal tract, where esterases and lipases transform the parent compounds into monoester metabolites (Figure 1) [40,41]. Importantly, toxicological effects of phthalates are caused by these monoester metabolites, not the original parent compounds [42–47]. Several previous studies have detected phthalates in different human matrices, including urine, blood, breast milk, semen, ovarian follicular fluid, and saliva [48–51]. In addition, the half-life of DEHP in human body is on the order of hours, with about 50% of an initial dose metabolized and excreted in urine after 44 h as monoesters [52]. Phthalate metabolites have been found in maternal and cord blood, placenta tissues, and amniotic fluid, justifying their toxicologic effects and health concern during pregnancy and fetal development [19,53]. Pregnant women are a particularly vulnerable group considering that various reports have suggested that in utero exposure to these chemicals impairs fetal programming [54].
Although phthalates have short biological half-lives, exposure is continuous and has been shown to disrupt normal biological function. Like persistent organic pollutants, phthalates are lipophilic, but quick metabolism reduces their bioaccumulation [55]. Phthalates have been found to act by genomic, non-genomic, and epigenetic mechanisms of action to alter gene expression, cell proliferation, and apoptosis in mammalian tissues [56]. Phthalates act as ligands for intracellular receptors, such as estrogen and androgen receptors (ER and AR), as well as interfere with peroxisome proliferator-activated receptor (PPAR) signaling [57]. Phthalate and metabolite interaction with AR, ERs or PPAR can lead to the impaired action of endogenous signal molecules on hormone-dependent tissue [58,59]. For example, their interaction with PPARγ signaling impairs placental function [58,60], which can lead to adverse pregnancy consequences, such as timing of delivery and spontaneous miscarriage occurrence. In addition, similar to other EDCs, exposure to phthalates and their metabolites can disrupt steroidogenesis, suppressing or stimulating expression or activity of steroidogenic enzymes in gonadal or other steroidogenic tissue in mammals of both sexes [20,61].
1.2. PLACENTA
The placenta is a transient, multifunctional organ that forms the interface between the mother and developing embryos/fetuses present in female eutherian mammals throughout gestation [62,63]. The placenta plays critical roles in gestation, including anchoring the developing embryo/fetus to the uterine wall, mediating maternal immune tolerance to nidation, providing immune protection by maternal antibodies, mediating gas exchange for respiration, providing synthesis and transport of nutrients for the fetus, and removing waste products during embryonic development until parturition [64]. In addition, the placenta produces/releases a variety of steroids, hormones (such as growth hormone, prolactin, and placental lactogens), and cytokines and expresses several receptors including steroid receptors and glucose transporters that modulate proper fetal development [65].
Placenta development and morphology are varied among mammals. Differences include features such as gross shape, histology of the maternal-fetal interface, and type of maternal-fetal interdigitation [62,66]. After ovulation, the endometrium of the uterus of mammals responds to high progesterone levels from the corpora lutea with changes in the functional layer, such as accumulating glycogen and glycoproteins inside of the endometrial glands [67]. With fecundation, the production and accumulation of secretory products in the uterine glands contribute to the pre-implantation environment of the blastocyst [68]. These hormonal and morphological changes in the uterine endometrium are known as decidualization [69].
The placenta is a maternal-fetal organ that develops when the outer layer, or trophectoderm, of the blastocyst attaches to the endometrial epithelium of the mother. Once adhered, trophectoderm cells called trophoblast stem cells (TSCs) differentiate into cytotrophoblasts (CTBs), which initiate the invasion process (syncytialization) into the underlying endometrium layer. As pregnancy progresses, the CTBs further differentiate into either syncytiotrophoblasts (STBs) or extravillous cytotrophoblasts (EVTs). The endometrial cells concomitantly undergo differentiation during pregnancy and are transformed into a specialized tissue known as decidua [70,71]. During the first trimester of pregnancy, CTBs rapidly proliferate to form primary villi, which consist of a CTB core with an outer layer of STB. Soon afterwards, primary villi mature into secondary and tertiary villi, which are characterized by the invasion of extraembryonic mesenchymal cells, villous branching, and vascularization of the uterine wall. During this process, CTBs start to differentiate into EVTs, which migrate into the maternal decidua and myometrium to anchor the placenta to the uterus and reach spiral arteries that will supply maternal blood to the placenta. EVTs are characterized by their invasiveness and angiogenic activity because they produce prostaglandins, metalloproteinases, and angiogenic factors [70,72,73].
In humans, the placenta is functionally mature by 10–12 weeks of gestation. Placental growth precedes fetal growth such that the placenta is larger than the fetus until 15–16 weeks (full term at 40 weeks gestation) [74]. In mice, placentation begins just before mid-gestation, the definitive placenta is established at embryonic day 11, and the maximum placental volume is reached by day 16.5, as determined by stereology analysis (parturition at day 19–20) [75,76]. Before proper placenta maturation, secretions of the uterus support embryonic development [77,78]. The placentas of rodents and humans present a similar discoid aspect and hemochorial maternal-fetal interface (Figure 2). However, rodent placentas lack the well-defined villous structures of human placentas [68,79]. Instead, in rodents, maternal blood bathes branching structures in a region called the placental labyrinth where most nutrient and gas exchange occur [68,79]. The rodent structures analogous to human chorionic villi have a trichorial arrangement with two layers of syncytiotrophoblasts in contact with the fetal endothelium and a cytotrophoblast cell layer in contact with maternal blood [7,75]. The rodent placenta also has a junctional zone which serves an endocrine function, comprised of spongiotrophoblasts and glycogen cells [68]. Thus, the rodent placenta is composed of the labyrinth zone, the basal zone, the decidua, and the metrial gland [80–82]. The labyrinth zone separates the maternal blood from the fetal blood vessels, containing a layer of cytotrophoblasts (outer trophectoderm) and layers of syncytiotrophoblasts. The basal zone is under the labyrinth zone and contains three types of differentiated cells: spongiotrophoblasts, trophoblastic giant cells, and glycogen cells. The decidua, formed by endometrial modification during pregnancy, plays an essential role in the development of the vascularized decidual-placental interface. The metrial gland is located in the mesometrial triangle of the uterus beyond the decidua on the maternal side and is composed of stromal cells, inflammatory cells, spinal-shaped arteries, trophoblasts, and fibroblasts [83,84].
Figure 2:
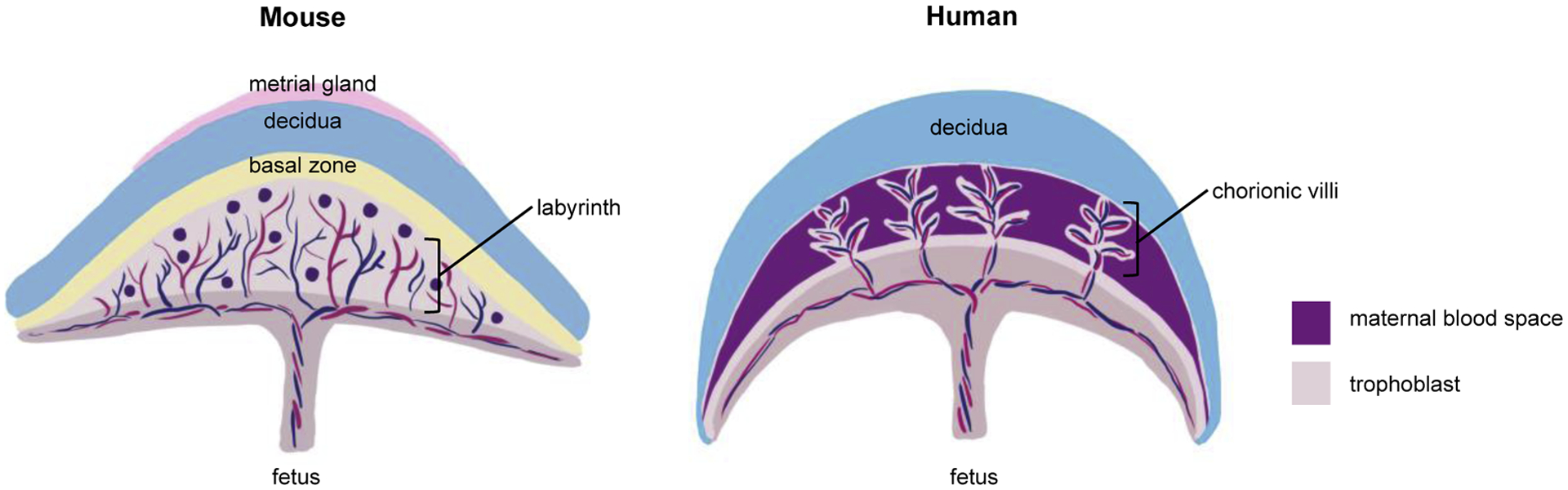
Comparison of rodent and human placental physiology.
1.3. PLACENTAL DYSFUNCTION
Environmental chemical disruption of placental development and function during pregnancy is associated with negative health outcomes in both the fetus and mother, including preterm birth, low birth weight, preeclampsia, and gestational diabetes [3,85]. The placenta is an endocrine organ with important hormone signaling function and numerous steroid hormone receptors [65], making it especially vulnerable to endocrine disruption effects. Disruptions of placental steroidogenesis and receptor expression are impacts of chemical exposure that may be observed in vitro or in animal studies. For example, PPARγ is expressed in the placenta and activates the expression of downstream genes involved in nutritional supply, hormonal secretion, and inflammation and are important in successful pregnancy [86,87]. Phthalates and their metabolites are known to interact with PPARs in various tissues [88,89] and have been shown to activate PPAR signaling, especially PPARγ [90,91]. Disruption of placental function can also be observed physiologically. Placental weight and thickness and the ratio of body weight to placental weight (BW:PW) are studied as markers of placental efficiency and health [92]. However, these are crude measurements that are best complemented with additional studies at the molecular levels.
Placental epigenetics is a growing area of interest for understanding the impact of environmental chemicals on later life health and disease [93]. Epigenetic modifications affect gene transcription without altering the underlying DNA code. Epigenetic effects of environmental chemicals, including phthalates, on fetal development and pregnancy health may involve changes such as DNA methylation and alteration of the expression of noncoding RNAs, including long noncoding RNAs (lncRNAs) and micro RNAs (miRNAs). Epigenetic changes are very responsive to environmental conditions such as exposure to EDCs [94]. DNA methylation is the best-characterized mechanism of epigenetic regulation [95,96]. Methylation in mammals involves the addition of methyl groups to cytosine to form 5-methylcytosine that can be measured in specific genes or repetitive DNA sequences such as long interspersed nuclear elements (LINEs) and short interspersed nuclear elements (SINEs). Imprinting is a genetic process that silences one parental allele, resulting in monoallelic expression of a subset of genes. Most imprinted genes are located in clusters of differentially methylated regions including imprinting control regions [97]. Paternally expressed genes can promote fetal growth, whereas maternally expressed genes can suppress growth, and the placenta is especially susceptible to these parental influences [98]. The placental gene cluster IGF2/H19 links imprinting status to altered nutrient allocation and poor fetal growth [99]. The fetal epigenome may also be affected by noncoding RNAs that act as epigenetic regulators of gene expression such as miRNAs and lncRNAs. MicroRNAs are approximately 22 nucleotides in length and can regulate gene expression post-transcriptionally by alterations in complementarity with a target mRNA, inhibiting protein synthesis [100]. Many miRNA clusters within the placenta have been shown to regulate placental development and function [99]. Long non-coding RNAs are a heterogeneous class of nonprotein coding transcripts longer than 200 nucleotides with important roles that may also be involved in the outcomes of EDC toxicity [101].
In the following sections, we discuss the literature on the effects of phthalates on the placenta in cell culture, animal studies, and human studies on physiological and molecular endpoints, including placenta size and shape, hormone signaling, gene expression, and epigenetics.
2. MATERIAL AND METHODS
Articles were selected using PubMed and Google Scholar without restricting publication year. Search terms included “phthalates placenta,” “human placenta phthalate exposure,” “endocrine disrupting chemicals placenta,” “phthalates placenta fetus,” and variations on these terms with the individual phthalates DEHP, DBP, DiBP, DEP, DiNP, and BzBP. The list of identified studies was cross checked with the reference lists of previously published reviews on endocrine disrupting chemicals and the placenta [102,103]. Inclusion criteria were in vitro and in vivo studies carried out in all mammals, including humans, showing the effects of phthalate exposure on the placenta specifically. Epidemiological studies were included if they were cohort, case-control, or cross-sectional. Articles were sought with details regarding the sex of the exposed animal (if there was maternal or paternal exposure); the type of exposure; fetal sex; the phthalate specification and design. No papers were excluded based on a positive or negative effect or association with phthalate exposure. Papers with exposure to mixtures of different EDCs were excluded unless they had separate analyses for phthalates. Articles not written in English and conference papers were also excluded from the review.
3. EFFECTS OF PHTHALATES ON THE PLACENTA
3.1. IN VITRO STUDIES
In vitro studies are important for investigating the mechanism through which phthalates alter placental development and function and can be performed with immortalized cell lines or primary cell cultures. Although convenient, cell lines are less representative of in vivo physiology than primary cell cultures [103]. Phthalates are generally not cytotoxic to placenta cells at concentrations of 500 μM or less in culture, well above levels of phthalates metabolites measured in human samples [104]. Few studies have treated placental cells with environmentally relevant doses of phthalates and most used only DEHP and/or its major metabolite monoethylhexyl phthalates (MEHP) (Table 1). The majority of studies use a short culture period, typically 24 hours.
Table 1:
In Vitro Studies of the Effects of Phthalate Exposure on Placenta Cells
| Phthalate | Dose | Model | Placental Outcomes | Reference |
|---|---|---|---|---|
| DEHP, MEHP, EHA | 25, 50, 100, 200 μM | HRP-1 rat trophoblastic cells |
|
(Yan Xu et al., 2006) |
| MEHP | 11.25, 22.5, 45, 90, or 180 μM | Human placental cell line HTR-8/SVneo |
|
(Tetz et al., 2013) |
| MEHP | 10, 45, 90 or 180 μM | Human placental macrophages (term), human macrophage-like cell line THP-1 |
|
(Tetz et al., 2015) |
| MEHP | 10, 25, 50, 100, and 180 μM | Human placental cell line HTR-8/SVneo |
|
(Meruvu, Zhang, Bedi, et al., 2016) |
| MEHP | 25, 50, 100, and 180 μM | Human placental cell line HTR-8/SVneo |
|
(Meruvu, Zhang, & Choudhury, 2016) |
| MEHP | 1–500 μM | Primary cytotrophoblasts (term) |
|
(X. K. Wang et al., 2016) |
| 11 diesters and 3 monoesters | 100 μM | Mammalian COS1 and human JEG-3 cells |
|
(R. Xu et al., 2016) |
| MBP MBzP MEHP MEP |
MBP: 200 nM MBzP: 3 μM MEHP: 700 nM MEP: 1.5 μM Each individually and all four in one dose |
Human trophoblast progenitor cells (first trimster), villous cytotrophoblast cells (second trimester) |
|
(Adibi et al., 2017) |
| BzBP, DBP, DEHP, DMP | 5–500 μM | JEG-3 cells |
|
(Pérez-Albaladejo et al., 2017) |
| MEHP | 1, 10, 100, 200 μM | Human placental cell line HTR-8/SVneo |
|
(Gao et al., 2017) |
| DEHP | 5 μM 4 weeks of treatment |
Rhesus monkey trophoblast stem cells 119-T |
|
(Midic et al., 2018) |
| DEHP, MEHP | DEHP: 1–50 μM MEHP: 1–25 μM |
Human JEG-3 cells |
|
(Petit et al., 2018) |
| MEHP | 0.1, 1, and 10 μM | Primary villous cytotrophoblasts (term) |
|
(Shoaito et al., 2019) |
| DEHP, MEHP | 20, 200, 500 μM | Human JEG-3 cells |
|
(Zhang et al., 2020) |
| DEHP | 4, 40, 100, 400 μM | Human placental cell line HTR-8/SVneo; human JEG-3 cells |
|
(Du et al., 2020) |
3.1.1. Studies in cell lines
Commonly used human placental cell lines include JEG-3, composed of trophoblast cells, and HTR-8/SVneo, composed of a mixed population of cells [105], as well as rodent and primate derived cell lines. Numerous studies have investigated the effects of DEHP and/or its primary metabolite MEHP on placental cell lines, but few studies have considered other phthalates (Table 1).
Multiple studies have identified placental steroidogenesis as a target of phthalate action. DEHP and MEHP treatments (20–500 μM) of JEG-3 cells have been shown to disrupt steroidogenesis by altering mRNA and protein levels of steroidogenic enzymes and increasing progesterone and estrogen levels in culture media compared to controls [106]. BzBP and DBP have been shown to disrupt steroidogenesis by decreasing aromatase activity in JEG-3 cells [104]. Diesters with six or fewer carbons, including dicyclohexyl phthalate (DCHP) and bis(2-butoxyethyl) phthalate (BBOP), have also been shown to inhibit the activity of multiple steroidogenic enzymes in JEG-3 cells [107]. A recent study on thyroid hormones showed that HTR-8/SVneo cells treated with DEHP (400 μM) showed reduced consumption of thyroid hormones in culture media and downregulation and inhibition of the thyroid hormone transport protein transthyretin [108]. Studies by Tetz et al. in HTR-8/SVneo cells and human macrophage-like THP-1 cells indicate that MEHP disrupts prostaglandin synthesis and release, which are necessary for the initiation of labor, suggesting a mechanism through which MEHP may increase risk of preterm birth [109,110].
In HRP-1 rat trophoblast cells, DEHP and MEHP (25–200 μM) have been shown to alter expression of PPARγ receptors and fatty acid binding proteins, suggesting that phthalates alter fatty acid homeostasis in the placenta [111]. Another study of MEHP (10–200 μM) in HTR-8/SVneo cells found that MEHP inhibited extravillous trophoblast invasion and the activity of an important regulator of invasion, matrix metallo-proteinase-9 (MMP-9) [58]. This effect was rescued by inhibition of PPARγ, suggesting that MEHP acts on MMP-9 via PPAR signaling [58]. DEHP and MEHP exposures have also been shown to lead to lipid dysregulation in JEG-3 cells [112] and oxidative stress in HTR-8/SVneo cells [109,113,114].
One study has examined the effects of phthalates on placental cells using a low dose and long window of exposure to most accurately represent human exposure conditions. Trophoblast stem cells (line 119-T) from rhesus monkeys were exposed to DEHP (5 μM) for four weeks, resulting in decreased expression of genes related to trophoblast development, implantation, and immunomodulation [115]. The trophoblast stem cells were more significantly disrupted by DEHP and other EDCs than embryonic stem cells from the same species under the same conditions, suggesting that trophoblast invasion is more susceptible to endocrine disruption than embryonic development [116]. More studies of the effects of phthalates on placental cells are needed at low doses and over longer periods of exposure.
3.1.2. Studies in primary cells
A few studies have used primary cultures of human placental cells from term placentas or terminated pregnancies. Term primary cytotrophoblasts exposed to MEHP (100–150 μM) had altered mRNA and protein levels for important regulators of parturition through the non-canonical NF-kB (RelB/p52) signaling pathway [117]. RelB/p52 association with genes that promote labor was upregulated in the presence of MEHP, but this effect was blocked by silencing the NF-kB signaling pathway, suggesting a potential mechanism for MEHP in preterm birth [117]. Another study of term primary cytotrophoblasts exposed to low doses of MEHP (0.1–10 μM) resulted in disruption of PPARγ activity, altered lipid composition, and decreased human chorionic gonadotropin (hCG) production [60]. The effects of MEHP on PPARγ activity had U-shaped dose response curves, indicative of a non-monotonic dose response at environmentally relevant levels of exposure [60].
In first trimester trophoblast progenitor cells and second trimester villous cytotrophoblast cells exposed to environmentally relevant doses of four phthalate metabolites (MBP: 200 nM, MBzP: 3 μM, MEHP: 700 nM, MEP: 1.5 μM) individually and as a mixture, the phthalates had different effects on gene expression, PPARγ levels, and hCG levels [118]. The effects differed significantly by the sex of the cells, emphasizing the need to consider sex differences in placental studies [118].
3.2. IN VIVO STUDIES
Rodents are popular models for animal studies of placental development and function due to their small size and quick development as well as the opportunity to study sex-specific effects as each pup develops with its own placenta [103]. Similar to the in vitro studies, the majority of rodent studies of the effects of phthalates on the placenta have focused on DEHP (Table 2). Importantly, no studies identified for this review used environmentally relevant doses of phthalates and the majority employed gavage as the method of dosing, which is stressful for the animals and less representative of human exposure compared to other methods of oral dosing [119]. Additionally, few studies examined sex differences. Thus, significant opportunities for investigators interested in environmentally relevant (μg/kg/day) levels of exposure via oral dosing exist to examine sex differences in placental effects.
Table 2:
In Vivo Studies of the Effects of Phthalate on the Placenta
| Phthalate | Exposure window | Dose / Exposure | Model | Placenta Outcomes | Reference |
|---|---|---|---|---|---|
| DEHP | Prenatal GD 0 to GD19 | 750 mg/kg 1500 mg/kg Oral dose |
Sprague-Dawley rats |
|
(Y. Xu et al., 2008) |
| DIHP DEHP DEHA |
Oral dose GD8 to GD15 or single IP dose on GD 14, sacrifice on GD16 | 100 mg/kg, oral or IP | INS7 ER-luc mice |
|
(ter Veld et al., 2009) |
| DEHP | GD8.5 to 12.5, sacrifice on GD13.5 | 750 mg/kg, gavage | JF1/OG2 mice |
|
(Kang et al., 2011) |
| DEHP | GD1–13, sacrifice on GD9 or 13 | 125 mg/kg 250 mg/kg 500 mg/kg gavage |
CD1 mice |
|
(Zong et al., 2015) |
| di-n-hexyl phthalate (DHP) dicyclohexyl phthalate (DCHP) | GD6–19 | 20 mg/kg 100 mg/kg 500 mg/kg gavage |
Wistar albino rats |
|
(Ahbab et al., 2017) |
| DBP | GD6–18 Sacrifice at GD19 or cross to form F2 and F3 generations | 500 mg/kg gavage |
Wistar rats |
|
(Mahaboob Basha & Radha, 2017) |
| DEHP | GD0–6, 7–12, or 13–17, sacrifice on GD18 | 50 mg/kg 200 mg/kg gavage |
ICR mice |
|
(Shen et al., 2017) |
| DEHP | GD0–17, sacrifice on GD15 or 18 | 50 mg/kg 200 mg/kg gavage |
ICR mice |
|
(Yu et al., 2018) |
| DEHP | GD0–20, sacrifice GD 10 or 20 | 100 mg/kg gavage |
Albino rats |
|
(Saadeldin et al., 2018) |
| DEHP | GD6.5–14.5, sacrifice GD 15.5 | 500 mg/kg gavage |
C57BL mice |
|
(Tang et al., 2019) |
| DEHP | GD7–12, sacrifice on GD20 | 500 mg/kg 1000 mg/kg gavage |
Wistar rats |
|
(W. Xu et al., 2020) |
| DEHP | GD0–14, sacrifice on GD15 | 50 mg/kg 200 mg/kg gavage |
ICR mice |
|
(Zhang et al., 2020) |
Prenatal exposure to high doses of DEHP (50–1000 mg/kg/day) generally resulted in intrauterine growth restriction (IUGR) in the pups, disruption of placental development, and fetal defects [120–124]. Similar gross embryo-fetal toxic effects were observed from DBP exposure (500 mg/kg/day) across three generations [125] as well as di-n-hexyl phthalate (DHP) and DCHP (20–500 mg/kg/day) exposure [126]. DEHP exposure also resulted in disruptions in placental steroidogenesis [106,124,127] and thyroid hormone signaling [128] as well as PPAR signaling and fatty acid homeostasis [129]. More studies at lower doses are necessary to determine whether similar placental endpoints are affected at environmentally relevant doses. In addition, rodent studies provide an excellent opportunity to study timing of exposure in relation to placental disruption. Future studies should consider preconception exposures, paternal exposures, and key periods of exposure during development to provide insight that cannot be gleaned from human studies, where exposure is unavoidable.
3.3. HUMAN STUDIES
Human studies on the associations between prenatal (and preconception) exposures to phthalates and health outcomes are mainly cohort and case-control studies involving exposure analysis by measurement of phthalate levels in parental urine, predominantly maternal urine during pregnancy, and tissues of the placenta and cord blood. Studies vary in their method of sampling, which may impact results, as the placenta is a heterogeneous organ with differences in physiology between the maternal and fetal sides as well as proximity to the umbilical cord. In addition, single urine spot samples are less representative of phthalate exposure than repeated pooled samples during pregnancy [130]. This review covers both morphological and molecular placental outcomes, as described below (Table 3).
Table 3:
Human Studies on the Associations Between Placental Disruption and Phthalate Exposure
| Phthalate | Exposure window | Dose/measurement time, sample | Model | Effect on placenta | Conclusions | Reference |
|---|---|---|---|---|---|---|
| MMP, MEP, MBP, MBzP MEHP, MEHHP, MEOHP | Maternal prenatal | 0.40–92.07 ng/L/1st trimester, urine 0.32–74.10 ng/L/2nd trimester, urine 0.20–51.99 ng/L/3rd trimester, urine |
2,725 pregnant women (1399 boys’ and 1326 girls’ pregnancies) |
|
Exposure to phthalates may cause the placenta to become thicker and more circular in the last two gestational trimesters, sexually dimorphic | (Zhu et al., 2018) |
| MCPP, MBP, MiBP, MBzP, MEP, MCNP, MCOP MEHP, MEHHP, MEOHP, MECPP | Maternal prenatal | 0.2–0.6 μg/L/between 23- and 29-weeks’ gestation, urine | 473 pregnant women (boys’ pregnancies) |
|
There are possible associations between phthalates exposure and placental weight and PFR | (Philippat et al., 2019) |
| MEP, MBP, MiBP, MBzP, MEHP, MEHHP, MEOHP, MECPP, MCPP, MCOP, MCNP | Paternal and maternal preconception, and maternal prenatal | 2.81 ng/ml (MEHP) to 39.4 ng/ml (MEP)/paternal preconception, urine 2.45 ng/ml (MEHP) to 48.5 ng/ml (MEP)/maternal preconception, urine 2.55 ng/ml (MEHP) to 39.4 ng/ml (ΣDEHP)/maternal prenatal-6-, 21- and 35-weeks’ gestation, urine |
132 mothers and 68 fathers (65 couples) |
|
Paternal and maternal urinary phthalate metabolites may affect placental weight and the BW:PW regardless of fetal sex | (Mustieles et al., 2019) |
| MEHP, MEOHP, MEHHP, MECPP, MnBP, MiBP, MBzP. | Maternal prenatal | 279.8 nm/L (ΣDEHP)/early 3rd trimester, urine | 54 placentas |
|
There is an association with lower expression of genes involved in trophoblast differentiation. Results are less consistent for genes that control steroidogenesis pathway | (Adibi et al., 2010) |
| MnBP, MBzP, MEHP, MEP, MiBP, MEOHP, MEHHP, MECPP, and MCPP | Maternal prenatal | 18nM (MEHP) to 1.3 μM (MEP) /34 weeks’ gestation, urine | 180 placentas |
|
Prenatal exposure to phthalates is modestly associated with molecular changes in placental tissue during pregnancy. Associations are stronger in male vs. female placentas, and with MnBP and MiBP | (Adibi et al., 2017) |
| DIBP, DBP, DEHP | Maternal prenatal | 0.08 g/L-4498.53 g/L (DEHP), 0.19 g/L-461.12 g/L (DBP), 0.18 g/L-281.36 g/L (DIBP)/ at delivery, cord blood | 207 placentas |
|
Phthalates might not only induce PPARγ activation by inducing peroxisome proliferation and binding to PPARγ directly, but also by increasing their protein expression in the placenta | (Huang et al., 2018) |
| BBP, DMP, DEP, DEHP, DNOP | Maternal prenatal | 3.09 μg/L (DMP) to 648.59 μg/L (DEHP) (high exposed group) 3 μg/L (DMP) to 492.76 μg/L (DMP) (low exposed group)/at delivery, cord blood |
187 pregnant women (127 from Chenghai-high exposed group and 60 from Haojiang-low exposed group) |
|
Neonatal exposure to phthalates could overexpress the MT isoforms. Different phthalates cause distinct effects, sexually dimorphic | (Li et al., 2016) |
| MBP, MMP, MEHP, MEOHP, MEHHP | Maternal prenatal | 33.2, 9.2, 5.7, 11.4, and 4.6 ng/mL to MBP, MMP MEHP, MEHHP, and MEOHP, respectively/3rd trimester, urine | 119 placentas (55 FGR cases and 64 normal controls) |
|
There is a link between changes in placental LINE-1 methylation and prenatal phthalate exposure | (Zhao et al., 2015) |
| 23 phthalates | Maternal prenatal | 231 ng/ml/1st trimester, urine | 49 pregnant women |
|
Placental EGFR hypermethylation and decreased expression occur in women with high total phthalate exposure, suggesting that this gene specifically may be a target for endocrine disruption consequences by phthalates exposure | (Grindler et al., 2018) |
| MnBP, MBzP, MCNP, MCOP, MCPP, MECPP, MEHHP, MEHP, MEOHP, MEP, MiBP | Maternal prenatal | Log (level) ranged between 0.523 (MEOHP) to −0.056 (MCNP)/ 1st trimester, urine | 196 pregnant women |
|
Prenatal exposure to phthalates perturbs methylation of the imprinting genes H19 and IGF2 in the placenta, sexually dimorphic | (LaRocca et al., 2014) |
| MBP, MMP, MEHP, MEOHP, MEHHP | Maternal prenatal | 3.8 ng/mL (MEHP) to 25.7 ng/mL (MPB)/ 3rd trimester, urine | Placenta of 181 mother-newborn pairs (80 FGR newborns, 101 normal newborns) |
|
Changes in placental DNA methylation may represent an underlying biological pathway linking prenatal phthalate exposure and IUGR | (Zhao et al., 2016) |
| MiBP, MCPP, MCNP, MCOP, MECPP, MEHHP, MEOHP, MBzP, MnBP, MHiBP, MiBP, MEHP, MNP, MMP, MEP MHiNCH, MCOCH | Maternal prenatal | Mean of 158.7 ng/ml (MEP -metabolite with the highest average concentration)/ near delivery date, urine | 10 women with uncomplicated dichorionic diamniotic twin pregnancies at term |
|
There is a link between lncRNA and the genomic imprinting and there are correlations between phthalate exposures and a panel of lncRNAs | (Machtinger et al., 2018) |
| MnBP, MBzP, MCNP, MCOP, MCPP, MECPP, MEHHP, MEHP, MEOHP, MEP, MiBP | Maternal prenatal | Log (level) ranged between 0.523 (MEOHP) to −0.056 (MCNP)/ 1st trimester, urine | 179 pregnant women |
|
Prenatal phthalate exposure is associated with abnormal miRNA expression in placenta, suggesting a potential molecular target of EDC toxicity | (LaRocca et al., 2016) |
| MiBP, MCOP, MECCP, MEHHP, MEOHP, MBzP, MHiBP, MEHP, MEP | Maternal prenatal | 86.9% (range: 50%–100%))/ near delivery date, urine | 10 women with twin pregnancies |
|
Prenatal phthalate exposure is associated with abnormal profiles of circulating placenta-derived EV-miRNAs | (Zhong et al., 2019) |
| MMP, MEP, MBP, MBzP, MEHP, MEHHP, MEOHP | Maternal prenatal | 50.092 × 103 ng L−1 (MBP), 13.311 × 103 ng L−1 (MMP), 8.786 × 103 ng L−1 (MEP), 7.380 × 103 ng L−1 (MEOHP) / 1st trimester, urine | 2,469 placentas |
|
Maternal phthalate exposure is associated with inflammatory variations in placental tissues, with associations stronger in placentae of male than of female fetuses | (J. Q. Wang et al., 2020) |
3.3.1. Morphological Outcomes
Placental morphology (size, shape, and weight) is strongly associated with fetal outcome and is thus an important marker of health effects [131–133]. Since exposure to phthalates is associated with changes in placental morphology, it likely influences fetal development. In this section, the major associations of phthalates and their metabolites on placental morphology are described.
A large cohort study involving 2,725 pregnant women (with 1399 male fetuses and 1326 female fetuses) showed significant associations between prenatal maternal exposure (in the three gestational trimesters) to seven phthalate metabolites, monomethyl phthalate (MMP), monoethyl phthalate (MEP), monobutyl phthalate (MBP), monobenzyl phthalate (MBzP) and the DEHP metabolites MEHP, mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), and mono-2-ethyl-5-oxohexyl phthalate (MEOHP), and placental size and shape at birth, depending on the fetal sex and the trimester of exposure (Zhu et al. (2018). Urine levels of phthalate metabolites were determined throughout pregnancy, with concentrations in the first, second, and third trimesters of 0.40–92.07 ng/L, 0.32–74.10 ng/L, and 0.20–51.99 ng/L, respectively. MBP concentration was positively associated with both placental breadth and surface area, whereas the difference between placental length and breadth was negatively associated with MMP, MBP, and LMW phthalates in the first trimester. In the 2nd trimester, phthalate exposure was associated only with placental thickness in MMP, MBP, MEOHP, MEHHP, and LMW and HMW phthalates. In the last trimester, the placental thickness was positively associated with MBP and MEHP. Analysis of sex differences showed that the associations were more significant in pregnancies with male fetuses compared to female fetuses; MEHP, MEHHP, and LMW phthalate exposure was associated with increased placental thickness in both second and third trimesters in pregnancies with males, whereas these associations were null in pregnancies with females. Overall, the study suggests that exposure to certain phthalates may be associated with the placenta becoming thicker and more circular in the last two gestational trimesters, with some dependence on fetal sex [134].
Another cohort study of 473 pregnant women analyzed whether maternal exposure to 11 phthalate metabolites, including mono 3-carboxypropyl phthalate (MCPP), MBP, mono-isobutyl phthalate (MiBP), MBzP, MEP, monocarboxy-isononyl phthalate (MCNP), monocarboxy-isooctyl phthalate (MCOP) and 4 metabolites of DEHP (MEHP, MEHHP, MEOHP, and mono 2-ethyl-5-carboxypentyl phthalate (MECPP)), measured in spot urine samples collected between weeks 23 and 29 of gestation was associated with changes in placental weight at birth and BW:PW. [135]. This study showed a negative association between MCNP and placental weight and between MCNP and MCOP and birth weight to placental weight ratio. Unlike the Zhu et al. (2018) study that showed an association between some phthalates and placental size, this study identified no association between MBP and DEHP levels and placental weight. This study restricted the analysis to only pregnancies with male fetuses.
In a prospective analysis, 132 mothers and 68 fathers (65 couples) and their singleton pregnancies were analyzed for associations between maternal and paternal urinary phthalate metabolite concentrations, placental weight, and BW:PW [136]. Eleven phthalate metabolites (MEP, MBP, MiBP, MBzP, MEHP, MEHHP, MEOHP, MECPP, MCPP, MCOP, and MCNP) were measured and averaged in multiple paternal (n=196) and maternal (n=596) preconception and maternal prenatal (n=328) samples. The mean urinary phthalate levels ranged from 2.81 ng/ml (MEHP) to 39.4 ng/ml (MEP) in the paternal preconception window from 2.45 ng/ml (MEHP) to 48.5 ng/ml (MEP) in the maternal preconception window; and from 2.55 ng/ml (MEHP) to 39.4 ng/ml (molar sum of DEHP metabolites, ΣDEHP) in the maternal prenatal window. This study identified a negative association between paternal urinary concentrations of MECPP and ΣDEHP metabolites with placental weight, an inverse association between maternal preconception MEP concentrations and BW:PW, and a negative association between prenatal MEP concentrations and placental weight. Prenatal DEHP metabolite concentrations showed suggestive associations towards a lower BW:PW. These results suggest that some paternal and maternal urinary phthalate metabolites may be associated with altered placental weight and BW:PW.
To date, few studies have examined the associations between prenatal or preconception exposure to phthalates and placental size, shape, and weight. The morphological effects of phthalate exposure on human placental development remains understudied. In addition, size, shape, and weight of the placenta are crude measures of placenta function; future studies should include additional endpoints of analysis, such as gene expression, discussed below.
3.3.2. Gene Expression Endpoints
Phthalates and their metabolites are known to activate PPARγ in the placenta as well as other singaling pathways. Gene expression analyses of the assocations between PPARγ and other mRNAs important to fetal and gestational health from human studies are described below.
A 2010 study measured the expression of genes involved in steroidogenesis, including aromatase (CYP19A1), P450 cholesterol side-chain cleavage enzyme (CYP11A1), 17beta hydroxysteroid dehydrogenase type 1 (17BHSD1), and cytochrome P450 1B1 (CYP1B1), and trophoblast differentiation, including PPARγ, aryl hydrocarbon receptor (AHR), and human chorionic gonadotropin (hCG) in 54 term placentas [137]. Gene expression changes were assessed in association with maternal prenatal urinary phthalate levels of MEHP, MEOHP, MEHHP, MECPP, MBP, MiBP, and MBzP. Higher urinary concentrations of DEHP metabolites were associated with lower expression of genes involved in trophoblast differentiation. Results were less consistent for genes in the placental steroidogenesis pathway.
A second study by the same authors assessed sex-specific associations between maternal phthalate exposure (MBP, MBzP, MEHP, MEP, MiBP, MEOHP, MEHHP, MECPP, and MCPP) and placental expressions of single genes involved in hCG and other placental hormone synthesis and regulation (chorionic gonadotropin alpha (CGA), CYP19A1, and CYP11A1), adipogenesis and metabolic programming (PPARγ), xenobiotic sensing (AHR), trophoblast differentiation (PPARγ, CGA), lipid transport (PPARγ, and 17BHSD1), the fatty acid transport protein 4 (SLC27A4) and a 204 bp transcript for COX2 (PTGS20) [138]. Maternal urine samples were collected at a mean gestational age of 34 weeks. The authors showed that mRNA levels (HSD17B1, CYP19A1, CGA, and PPARγ) were higher in male placentas than female placentas at the lowest quartile of phthalate levels and those differences were either lost or reversed over the range of phthalates. Levels of mRNA were inversely correlated with phthalates in male placentas. Associations in female placentas were positive in high MBzP concentrations, all quartiles of MnBP, and the third quartiles of MiBP. MCPP concentrations were associated with decreased levels of placental PPARγ in male placentas. Male PPARγ mRNA was also lower in large for gestational age cases compared to non-cases. This study is another example of the importance of studying the effects of phthalates differentially between fetal sex.
Another study assessed the associations between three phthalates (DIBP, DBP, and DEHP) and PPARγ expression in the placentas of 207 healthy pregnant Chinese women without family or personal history of occupational exposure to phthalates ([139]. Phthalate concentrations measured in cord blood ranged from 0.08–4498.53 μg/L for DEHP, 0.19–461.12 μg/L for DBP, and 0.18–281.36 μg/L for DiBP. Natural log transformed phthalate levels were positively associated with PPARγ protein expression. The authors suggest that phthalates might activate PPARγ by inducing peroxisome proliferation and binding to PPARγ directly, increasing the protein levels in the placenta and leading to placental abnormalities.
Alterations in placental metallothioneins (MTs), fatty acid transport protein 1 (FATP1), and heart fatty acid-binding protein (HFABP) mRNA can adversely influence fetal health [140]. Metallothioneins are an important metal regulators involved in the micronutrient homeostasis and heavy metal detoxification [141], whereas the other two genes are involved in the transfer of essential fatty acid between the mother and the fetus [142]. One study analyzed the association between concentrations of BzBP, dimethyl phthalate (DMP), DEP, DEHP, and di-n-octyl phthalate (DNOP) in cord blood and the expression of mRNA of MTs, FATP1, and HFABP in the placenta [140]. The study involved 187 pregnant women (127 from Chenghai representing a high exposure group and 60 from Haojiang representing a low exposure group) and showed that MT-1A mRNA was higher in the low exposure group compared with the high exposure group. Additionally, the expression of FATP1 and HFABP mRNA in the placentas from the high exposure group was higher than in the low exposure group. In the high exposure group, DEHP was positively associated with MT-1A mRNA and DNOP was negatively correlated with both MT and MT-2A. FATP1 and HFABP mRNAs levels were increased with increasing DEP levels. The authors divided the placental samples into male and female and showed that DMP induced the expression of MT and MT-2A in male and female; further, a positive correlation was observed between DEHP and MT, as well as DEHP and MT-2A in females, and DEP was positively correlated with HFABP in males and MT-1A and FATP1 in females. These results emphasize the importance of studying sex-specific effects of phthalates on placental development and function. The associations found in this study suggest that neonatal exposure to some phthalates could overexpress MT isoforms, which may affect fetal growth and placental essential fatty acid homeostasis.
The effects of phthalates on mRNAs expressed in the placenta still need further investigation. Studies already indicate that changes in gene expression relevant to fetal development occur in association with exposure to phthalates measured in maternal or maternal-fetal samples throughout pregnancy and parturition.
3.3.3. Epigenetic Outcomes
Human studies on the epigenetic changes associated with phthalate exposure have investigated methylation changes, disruption of imprinting, altered expression of noncoding RNAs in maternal urine and placenta samples. These studies are described below.
3.3.3.1. Methylation
A case-control study analyzed associations between maternal prenatal phthalate exposure to two LMW phthalates (MBP and MMP) and three HMW phthalates (MEHP, MEOHP, and MEHHP), infant growth, and global DNA methylation (LINE-1 methylation) in 119 human placenta samples representing 55 IUGR cases and 64 normal controls [143]. Prenatal phthalate exposure was assessed by measuring maternal urinary concentrations in the third trimester. The median values were 33.2, 9.2, 5.7, 11.4, and 4.6 ng/mL of MBP, MMP MEHP, MEHHP, and MEOHP, respectively. DEHP metabolites were higher in FGR cases than those in normal controls. Placental LINE-1 methylation was positively associated with fetal birth weight and negatively associated with urinary phthalate metabolites concentrations (MEHHP and ΣDEHP). Every natural-log unit increase in urinary concentrations of MEHHP and ΣDEHP was associated with a decrease in birth weight mediated through LINE-1 methylation. These findings suggest a link between changes in placental LINE-1 methylation and prenatal phthalate exposure.
A recent study measured the maternal levels of 23 phthalates in the first trimester of gestation and associated them with the methylome and the consequent transcriptome of placental genes in early pregnancy [144]. The mean total maternal phthalate concentration was 231 ng/mL and the results showed 282 differentially methylated regions corresponding to 245 unique genes in the early human placenta for high compared to low total phthalate exposure. In the gene expression analysis, the authors found 39 significant methylation-gene expression correlations, which correspond to 23 unique gene symbols, with most of these relationships inversely correlated (29 out of 39). Pathway molecular analysis of the list of genes identified from methylation-gene expression identified the ErbB signaling pathway as the top pathway involved and the epidermal growth factor receptor was present in 18/51 pathways identified. This signaling pathway includes receptor tyrosine kinases that activate signaling cascades that regulate many cellular events including proliferation, survival, migration/invasion, or differentiation [145] and includes the epidermal growth factor receptor and other receptor tyrosine kinases that are important for cell cycle progression in placental trophoblasts [146]. The authors identified placental EGFR hypermethylation and decreased expression in women with high total phthalate exposure, suggesting that this gene specifically may be a target for endocrine disruption consequences by phthalate exposure. These studies indicate that EDCs such as phthalates are risk factors for adverse outcomes caused by changes in DNA methylation, but the biological mechanism for the interference of phthalates with placental DNA methylation remains unclear.
3.3.3.2. Imprinting
LaRocca et al. (2014) studied the association between the concentrations of 11 phthalates (MnBP, MBzP, MCNP, MCOP, MCPP, MECPP, MEHHP, MEHP, MEOHP, MEP, and MiBP) measured in urine in the first trimester of 196 women and differentially methylated regions of the paternally expressed gene insulin-like growth factor 2 (IGF-2) and the maternally expressed non-coding gene H19. The authors found a decrease in H19 methylation, which was associated with high levels of the sum of all phthalate metabolites and metabolites of LMW phthalates. Inverse associations were observed between the sum of all phthalate metabolites and LMW phthalate concentrations and IGF-2 methylation. In addition, the variation in methylation was not associated with changes in allele-specific expression. However, an increased deviation of allele-specific expression of H19 was associated with ΣDEHP metabolites and HMW phthalates. In addition, prenatal exposures to HMW phthalates and DEHP metabolites were associated with aberrant imprinting of H19 in male newborns, suggesting that DNA methylation alterations following prenatal phthalate exposure may be sexually dimorphic.
Another important study examined associations between exposure to phthalates and altered DNA methylation of growth-related genes in the human placenta [148]. This study found that urinary maternal MEHHP and MEOHP were inversely associated with placental IGF2 DNA methylation. The associations were found in growth-restricted infants, suggesting that changes in placental DNA methylation represent an underlying biological pathway linking prenatal phthalate exposure and intrauterine growth restriction.
A third study showed that maternal urinary concentrations of MEHP, MEHHP, MECPP, and MEOHP were positively correlated with H19 and IGF2 expressed in placental samples after adjustment for in vitro fertilization and sex [101]. As IGF2 and H19 are among the most studied imprinted genes, the authors pointed out the link between lncRNA and the genomic imprinting [149]. It is still unclear if the methylome of imprinted genes affects fetal and gestational outcomes in response to phthalate exposure, but these studies suggest that phthalates may be associated with disruption of placental DNA methylation.
3.3.3.3. Noncoding RNAs
Expanding on their previous study, LaRocca et al. (2016) studied the relationship between prenatal exposure to 11 phthalates measured in 179 maternal urine samples collected in the first trimester and miRNA expression in human placenta samples. The authors found an association between phthalate levels and the expression of 3 miRNAs: miR-142-3p, miR15a-5p, and miR-185. The gene enrichment analysis performed revealed biological processes including: a) regulation of protein serine/threonine kinase activity, b) cellular response to insulin stimulus, c) insulin-like growth factor receptor signaling pathway, and d) positive regulation of protein insertion into mitochondrial membrane involved in apoptotic signaling pathway associated with the potential mRNA targets of these 3 miRNAs.
Another study analyzed the expression of miRNAs contained in extracellular vesicles (EVs) that are released by the placenta into the maternal circulation and their associations with maternal prenatal exposure to 13 phthalates measured in urine near delivery time [151]. Placenta-derived EV-miRNAs are released throughout pregnancy and seem to be involved as endocrine-like mediators contributing to pregnancy and fetal growth [152]. The expression of miR-518e was highest among women with high urinary levels of MBzP. These results reveal that prenatal exposure to EDCs is associated with altered profiles of circulating placenta-derived EV-miRNAs; however, the study focused only on twin pregnancies, so more comprehensive studies are needed.
The miRNA called miR-518 was associated with exposure to phthalates in the two studies above [151,152]. This noncoding RNA is a member of the C19MC family, which has increased expression in the 1st trimester and is exclusive to the placenta and the reproductive system. Higher expression of miR-518 has been associated in other studies with pregnancy complications, including preeclampsia and other abnormalities [153,154].
Another study analyzed the association between phthalates in maternal urinary samples collected near delivery date and the expression of 87 lncRNAs in 10 human placenta samples [101]. Fifteen phthalate metabolites were measured (MiBP, MCPP, MCNP, MCOP, MECPP, MEHHP, MEOHP, MBzP, MnBP, mono-hydroxyisobutyl phthalate (MHiBP), MiBP, MEHP, mono-isononyl phthalate (MNP), MMP, and MEP), as well as two metabolites of the phthalate alternative di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH), cyclohexane-1,2-dicarboxylic acid monohydroxyisononyl ester (MHiNCH) and cyclohexane-1,2-dicarboxylic acid monocarboxyisoctyl ester (MCOCH). MEP had the highest average concentration, with a mean of 158.7 ng/ml. MCNP concentrations also correlated with most lncRNAs. Overall, a number of lncRNAs were strongly correlated with multiple phthalate metabolites. Most lncRNAs demonstrated similar response patterns to maternal urinary phthalate metabolites, with the most relevant upregulations associated with MCNP. Overall, these studies suggest that noncoding RNA regulation is a potentially significant mechanism indicating prenatal phthalate toxicity and warrants future investigations.
1. LIMITATIONS AND STRENGTHS
This review reveals a number of limitations in the available body of research on phthalates and placental function. For the in vitro studies, many experiments used doses much higher than human exposure levels. In addition, these studies evaluated types of placental cells individually, which may eliminate or distort signaling and microenvironment structure differences. For the in vivo studies, rodents are the only model used to date; inclusion of other mammalian species could improve translational to humans. The in vivo studies described in this review have mostly focused on one phthalate and employed levels and methods of dosing that are not representative of human exposure. Low dose studies are sorely lacking. Further, in vivo studies that analyze preconception exposure or paternal exposures are limited. In the human studies, many studies used one single urine sample during gestation to determine phthalate exposure, which does not represent long-term exposure as well as multiple pooled samples would since phthalate metabolites have short half-lives [130]. In addition, many studies have analyzed a single point of the placenta at a single timepoint, which may not represent the entire placenta and may limit the comparability of studies. Few studies measured phthalate concentrations directly in placenta samples, which could reveal whether phthalates accumulate. Many studies, of all kinds, did not evaluate the difference in effect between fetal sexes (“placental sex”). Finally, many studies had small sample numbers, which makes it difficult to statistically evaluate the effects between variables such as placental sex. These gaps leave room for further study to effectively understand the damage that phthalates can cause to placental function. The main strength of this review is that, to our knowledge, it covers all studies published before January 2021 of all relevant types (in vitro, rodent, human) concerning the morphological, physiological, and molecular effects of phthalates on the placenta to provide an integrated overview of the state of the science. Further, this review emphasizes areas for future study.
2. FUTURE NEEDS
This review reveals some important gaps in the literature that are necessary to fill to effectively assess the effects of phthalates on the placenta and their biological mechanisms involved. Here, we describe these gaps. The majority of in vitro studies are carried out with commercial cell lines, which may not be consistent with the real specificities of placental cells. In addition, most in vitro studies use cells from male placentas or cells whose sex is unknown. Thus, future in vitro studies should develop cell lines more consistent with the characteristics (including the microenvironment) of placental cells and develop advanced models for the same purpose. In addition, sex-specific analyses should be performed, and doses should be used to mimic human exposure. Some options for alternative in vitro models such as the use of chips and 3D models are already available [155,156] and have been described in a recent review [102]. Further development of organoid models of the placenta would improve the translational potential of cell culture models.
As discussed above, in vivo studies in rodents are significantly lacking in dose amount and method of exposure that are relevant to human exposure. In addition, studies on other mammals with translational application to humans are needed. A recent review of animal models of the placenta describes the advantages and limitations of models such as guinea pigs, sheep, and non-human primates and points out the need for studies to pay attention to temporality throughout pregnancy, complications with pregnancies, hormonal aspects related to the placenta, and other particularities that must be taken into account when choosing a study animal for human translation [157]. The results of animal studies should furthermore be compared to human epidemiology studies and human in vitro models to assess their translational value.
For human studies, we observe the need for more studies that analyze preconception exposures to phthalates, paternal exposure to phthalates, and the whole pregnancy, including measures of phthalates in urine across all trimesters, measurements throughout the whole placenta, and assessment of placental endpoints at various times throughout pregnancy. In addition, every study should observe differences between the placental sexes. The preconception period is also relevant and needs to be studied more deeply [136], as well as paternal exposure to phthalates [158,159]. Multiple studies reported an increase in methylation across gestation [160,161] and changes in imprinting across gestational trimesters [162], emphasizing the importance of gestational time as a relevant factor in the assessment of the effects of phthalates on the placenta. The need to collect samples at several points across the placenta and at different times was illustrated a recent study that identified large within-placenta variability of transcripts, dependent of the time and placental location of collection [163]. Future studies encompassing these aspects may better elucidate placental dysfunctions resulting from exposure to phthalates. In addition to all the above factors, the literature would be enriched by more studies with larger sample size.
6. CONCLUSIONS
Increasing concern regarding the adverse effects of phthalates on gestation and fetal heath has driven research investigating the potential biological mechanisms of action and physiological damages behind phthalate exposure. This review compiles studies that inform understanding of the effects of phthalate exposure in the context of placental dysfunction. Despite gaps in the literature, the studies described herein strongly suggest that exposure to phthalates causes damage to placental health and fetal development. The biological mechanisms and the specific translation to human fetal and gestation health are poorly understood, but future molecular studies will be the key to better understanding. Thus, additional research is needed to understand how environmental exposure to phthalates can drive human placental disruption. Specifically, well-designed animal studies and human studies will be necessary to understand how phthalates and their metabolites interact with placental receptors to disrupt downstream molecular signaling and how phthalates change the placental microenvironment to lead to dysfunction.
Highlights.
The placenta is a sensitive endocrine organ
Phthalates may disrupt placenta development and function
In vitro experiments show that phthalates are toxic to placenta cells
Phthalate exposure is associated with morphological changes in placenta size and shape
Phthalate exposure is associated with placental changes in gene expression and epigenetic alterations
Funding:
This research was supported by CNPq (#304724/2017-3/ N° 12/2017 to JBG) and FAPES/CNPq (PRONEX N° 24/2018, #572/2018 to JBG). It was also supported by National Institutes of Health (R01 ES028661 to JAF and K99 ES031150 and T32 ES007326 to GRW)
Abbreviations
- AR
androgen receptor
- BBOP
bis(2-butoxyethyl) phthalate
- BW:PW
body weight to placental weight
- BzBP
benzyl butyl phthalate
- CTBs
cytotrophoblasts
- DBP
dibutyl phthalate
- DCHP
dicyclohexyl phthalate
- DEHP
di(2-ethylhexyl) phthalate
- DEP
diethyl phthalate
- DHP
di-n-hexyl phthalate
- DiBP
diisobutyl phthalate
- DINCH
di(isononyl)cyclohexane-1,2-dicarboxylate
- DiNP
di-isononyl phthalate
- DMP
dimethyl phthalate
- DNOP
di-n-octyl phthalate
- DOHaD
developmental origins of health and disease
- EDCs
endocrine disrupting chemicals
- ER
estrogen receptor
- EV
extracellular vesicle
- EVTs
extravillous cytotrophoblasts
- hCG
human chorionic gonadotropin
- HMW
high molecular weight
- IUGR
intrauterine growth restriction
- LINEs
long interspersed nuclear elements
- LMW
low molecular weight
- lncRNAs
long noncoding RNAs
- MBP
monobutyl phthalate
- MBzP
monobenzyl phthalate
- MCNP
monocarboxy-isononyl phthalate
- MCOCH
cyclohexane-1,2-dicarboxylic acid monocarboxyisoctyl ester
- MCOP
monocarboxy-isooctyl phthalate
- MCPP
mono 3-carboxypropyl phthalate
- MECPP
mono 2-ethyl-5-carboxypentyl phthalate
- MEHHP
mono-2-ethyl-5-hydroxyhexyl phthalate
- MEHP
monoethylhexyl phthalates
- MEOHP
mono-2-ethyl-5-oxohexyl phthalate
- MEP
monoethyl phthalate
- MHiBP
mono-hydroxyisobutyl phthalate
- MiBP
mono-isobutyl phthalate
- MINCH
cyclohexane-1,2-dicarboxylic acid monohydroxyisononyl ester
- miRNAs
micro RNAs
- MMP-9
matrix metallo-proteinase-9
- MMP
monomethyl phthalate
- PPAR
peroxisome proliferator-activated receptor
- SINEs
short interspersed nuclear elements
- STBs
syncytiotrophoblasts
- TSCs
trophoblast stem cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors do not have any conflicts of interest or potential conflicts of interest to disclose.
Disclosure statement: The authors have nothing to disclose.
REFERENCES
- [1].Casals-Casas C, Desvergne B, Endocrine disruptors: From endocrine to metabolic disruption, Annu. Rev. Physiol 73 (2011) 135–162. 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- [2].Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, Vom Saal FS, Endocrine-disrupting chemicals and public health protection: A statement of principles from the Endocrine Society, Endocrinology. 153 (2012) 4097–4110. 10.1210/en.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT, EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals, Endocr. Rev 36 (2015) 1–150. 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vandenberg LN, Hauser R, Marcus M, Olea N, V Welshons W, Human exposure to bisphenol A (BPA)., Reprod. Toxicol 24 (2007) 139–77. 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- [5].Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC, Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement, Endocr. Rev 30 (2009) 293–342. 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Heindel JJ, Vandenberg LN, Developmental Origins of Health and Disease: A Paradigm for Understanding Disease Etiology and Prevention, Curr Opin Pediatr. 27 (2015) 248–253. 10.1097/MOP.0000000000000191.Developmental. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rossant J, Cross JC, Lunenfeld S, Rossant 2001, 2 (2001) 538–548. [Google Scholar]
- [8].Woods L, Perez-Garcia V, Hemberger M, Regulation of Placental Development and Its Impact on Fetal Growth—New Insights From Mouse Models, Front. Endocrinol. (Lausanne) 9 (2018) 1–18. 10.3389/fendo.2018.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Grandjean P, Late insights into early origins of disease, Basic Clin Pharmacol Toxicol 102 (2008) 94–99. 10.1111/j.1742-7843.2007.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barker DJP, Clark PM, Fetal undernutrition and disease in later life, Rev. Reprod 2 (1997) 105–112. 10.1530/ror.0.0020105. [DOI] [PubMed] [Google Scholar]
- [11].Barker DJP, The Developmental Origins of Adult Disease, J. Am. Coll. Nutr 23 (2004) 588S–595S. 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- [12].Schug TT, Janesick A, Blumberg B, Heindel JJ, Endocrine disrupting chemicals and disease susceptibility, J. Steroid Biochem. Mol. Biol 127 (2011) 204–215. 10.1016/j.jsbmb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Woodruff TJ, Zota AR, Schwartz JM, Environmental chemicals in pregnant women in the united states: NHANES 2003–2004, Environ. Health Perspect 119 (2011) 878–885. 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Johns LE, Ferguson KK, Cantonwine DE, McElrath TF, Mukherjee B, Meeker JD, Urinary BPA and phthalate metabolite concentrations and plasma vitamin D levels in pregnant women: A repeated measures analysis, Environ. Health Perspect 125 (2017) 1–9. 10.1289/EHP1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Argyraki M, Damdimopoulou P, Chatzimeletiou K, Grimbizis GF, Tarlatzis BC, Syrrou M, Lambropoulos A, In-utero stress and mode of conception: Impact on regulation of imprinted genes, fetal development and future health, Hum. Reprod. Update 25 (2019) 777–801. 10.1093/humupd/dmz025. [DOI] [PubMed] [Google Scholar]
- [16].Fisher M, Arbuckle TE, Liang CL, Leblanc A, Gaudreau E, Foster WG, Haines D, Davis K, Fraser WD, Concentrations of persistent organic pollutants in maternal and cord blood from the maternal-infant research on environmental chemicals (MIREC) cohort study, Environ. Heal. A Glob. Access Sci. Source 15 (2016) 1–14. 10.1186/s12940-016-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li LX, Chen L, Meng XZ, Chen BH, Chen SQ, Zhao Y, Zhao LF, Liang Y, Zhang YH, Exposure Levels of Environmental Endocrine Disruptors in Mother-Newborn Pairs in China and Their Placental Transfer Characteristics, PLoS One. 8 (2013) 1–9. 10.1371/journal.pone.0062526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cobellis L, Latini G, DeFelice C, Razzi S, Paris I, Ruggieri F, Mazzeo P, Petraglia F, High plasma concentrations of di-(2-ethylhexyl)-phthalate in women with endometriosis, Hum. Reprod 18 (2003) 1512–1515. 10.1093/humrep/deg254. [DOI] [PubMed] [Google Scholar]
- [19].Silva MJ, a Reidy J, Herbert a R, Preau JL, Needham LL, Calafat a M., Detection of phthalate metabolites in human amniotic fluid., Bull. Environ. Contam. Toxicol 72 (2004) 1226–1231. 10.1007/s00128-004-0374-4. [DOI] [PubMed] [Google Scholar]
- [20].Hannon PR, Flaws JA, The effects of phthalates on the ovary, Front. Endocrinol. (Lausanne) 6 (2015) 1–19. 10.3389/fendo.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee D-H, Shioda T, Soto AM, Vom Saal FS, V Welshons W, Zoeller RT, Myers JP, Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses., Endocr. Rev 33 (2012) 1–78. 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schettler T, Skakkebæk NE, De Kretser D, Leffers H, Human exposure to phthalates via consumer products, Int. J. Androl 29 (2006) 134–139. 10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- [23].Brehm E, Flaws JA, Transgenerational effects of endocrine-disrupting chemicals on Male and female reproduction, Endocrinology. 160 (2019) 1421–1435. 10.1210/en.2019-00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG, Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust, Environ. Sci. Technol 37 (2003) 4543–4553. 10.1021/es0264596. [DOI] [PubMed] [Google Scholar]
- [25].Buckley JP, Kim H, Wong E, Rebholz C, Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014, Env. Int 131 (2019) 105057. 10.1016/j.envint.2019.105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jeon S, Kim KT, Choi K, Migration of DEHP and DINP into dust from PVC flooring products at different surface temperature, Sci. Total Environ 547 (2016) 441–446. 10.1016/j.scitotenv.2015.12.135. [DOI] [PubMed] [Google Scholar]
- [27].N.I. of H. NIH, Tox Town ENVIRONMENTAL HEALTH CONCERNS AND TOXIC CHEMICALS WHERE YOU LIVE, WORK, AND PLAY, NIH, Natl. Institutes Heal. (2017). [Google Scholar]
- [28].Hauser R, Calafat AM, Phthalates and human health, Occup. Environ. Med 62 (2005) 806–818. 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Blount BC, Milgram KE, Silva MJ, Malek NA, Reidy JA, Needham LL, Brock JW, Quantitative Detection of Eight Phthalate Metabolites in Human Urine Using HPLC–APCI-MS/MS, Anal. Chem 72 (2000) 4127–4134. 10.1021/ac000422r. [DOI] [PubMed] [Google Scholar]
- [30].Braun JM, Sathyanarayana S, Hauser R, Phthalate exposure and children’s health, Curr. Opin. Pediatr 25 (2013) 247–254. 10.1097/MOP.0b013e32835e1eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].EPA, Guidelines for Reproductive Toxicity Risk Assessment These guidelines replace two proposed guidelines: Proposed Guidelines for Female Reproductive Risk and Proposed Guidelines for Male Reproductive Risk, Fed. Regist 61 (1996) 56274–56322. [Google Scholar]
- [32].Doull J, Cattley R, Elcombe C, Lake BG, Swenberg J, Wilkinson C, Williams G, Van Gemert M, A cancer risk assessment of di(2-ethylhexyl)phthalate: Application of the new U.S. EPA risk assessment guidelines, Regul. Toxicol. Pharmacol 29 (1999) 327–357. 10.1006/rtph.1999.1296. [DOI] [PubMed] [Google Scholar]
- [33].Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, Tabacova S, Tyl R, Williams P, Zacharewski T, NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate, Reprod. Toxicol 16 (2002) 529–653. 10.1016/S0890-6238(02)00032-1. [DOI] [PubMed] [Google Scholar]
- [34].Koch HM, Calafat AM, Human body burdens of chemicals used in plastic manufacture, Philos. Trans. R. Soc. B Biol. Sci 364 (2009) 2063–2078. 10.1098/rstb.2008.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Babich MA, Chen SB, Greene MA, Kiss CT, Porter WK, Smith TP, Wind ML, Zamula WW, Risk assessment of oral exposure to diisononyl phthalate from children’s products, Regul. Toxicol. Pharmacol 40 (2004) 151–167. 10.1016/j.yrtph.2004.06.005. [DOI] [PubMed] [Google Scholar]
- [36].Hines CJ, Hopf NB, Deddens JA, Silva MJ, Calafat AM, Occupational exposure to diisononyl phthalate (DiNP) in polyvinyl chloride processing operations, Int. Arch. Occup. Environ. Health 85 (2012) 317–325. 10.1007/s00420-011-0674-z. [DOI] [PubMed] [Google Scholar]
- [37].Machtinger R, Berman T, Adir M, Mansur A, Baccarelli AA, Racowsky C, Calafat AM, Hauser R, Nahum R, Urinary concentrations of phthalate metabolites, bisphenols and personal care product chemical biomarkers in pregnant women in Israel, Environ. Int 116 (2018) 319–325. 10.1016/j.envint.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Meeker H Hu DE, Cantonwine H, Lamadrid-Figueroa AM, Calafat AS, Ettinger M, Hernandez-Avila R, Loch-Caruso MM, Téllez-Rojo, Urinary phthalate metabolites in relation to preterm birth in Mexico City, Environ. Health Perspect 117 (2009) 1587–1592. 10.1289/ehp.0800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yazdy MM, Coull BA, Gardiner JC, Aguiar A, Calafat AM, Ye X, Schantz SL, Korrick SA, A possible approach to improving the reproducibility of urinary concentrations of phthalate metabolites and phenols during pregnancy, J. Expo. Sci. Environ. Epidemiol 28 (2018) 448–460. 10.1038/s41370-018-0050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Frederiksen H, Skakkebæk NE, Andersson A-M, Metabolism of phthalates in humans, Nutr Food Res. 51 (2007) 889–911. 10.1002/mnfr.200600243. [DOI] [PubMed] [Google Scholar]
- [41].Hanioka N, Kinashi Y, Tanaka-Kagawa T, Isobe T, Jinno H, Glucuronidation of mono(2-ethylhexyl) phthalate in humans: roles of hepatic and intestinal UDP-glucuronosyltransferases, Arch. Toxicol 91 (2017) 689–698. 10.1007/s00204-016-1708-9. [DOI] [PubMed] [Google Scholar]
- [42].Davis BJ, Weaver R, Gaines LJ, Heindel JJ, Mono-(2-ethylhexyl) Phthalate Suppresses Estradiol Production Independent of FSH-cAMP Stimulation in Rat Granulosa Cells, Toxicol Appl Pharmacol. 128 (1994) 224–228. [DOI] [PubMed] [Google Scholar]
- [43].Lovekamp TN, Davis BJ, Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells, Toxicol. Appl. Pharmacol 172 (2001) 217–224. 10.1006/taap.2001.9156. [DOI] [PubMed] [Google Scholar]
- [44].Lovekamp-Swan T, Davis BJ, Mechanisms of phthalate ester toxicity in the female reproductive system, Environ. Health Perspect 111 (2003) 139–145. 10.1289/ehp.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang W, Craig ZR, Basavarajappa MS, Hafner KS, Flaws JA, Mono-(2-Ethylhexyl) Phthalate Induces Oxidative Stress and Inhibits Growth of Mouse Ovarian Antral Follicles1, Biol. Reprod 87 (2012) 1–10. 10.1095/biolreprod.112.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hannon PR, Brannick KE, Wang W, Flaws JA, Mono(2-Ethylhexyl) Phthalate Accelerates Early Folliculogenesis and Inhibits Steroidogenesis in Cultured Mouse Whole Ovaries and Antral Follicles, Biol. Reprod 92 (2015) 120–120. 10.1095/biolreprod.115.129148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lake BG, Gray T.J. b., Lewis D.F. v., Beamand JA, Hodder KD, Purchase R, Gangolli SD, Structure-activity relationships for induction of peroxisomal enzyme activities by phthalate monoesters in primary rat hepatocyte cultures, Toxicol. Ind. Health 3 (1987) 165–183. 10.1177/074823378700300212. [DOI] [PubMed] [Google Scholar]
- [48].Latini G, De Felice C, Verrotti A, Plasticizers, infant nutrition and reproductive health, Reprod. Toxicol 19 (2004) 27–33. 10.1016/j.reprotox.2004.05.011. [DOI] [PubMed] [Google Scholar]
- [49].Silva MJ, Reidy JA, Samandar E, Herbert AR, Needham LL, Calafat AM, Detection of phthalate metabolites in human saliva, Arch. Toxicol 79 (2005) 647–652. 10.1007/s00204-005-0674-4. [DOI] [PubMed] [Google Scholar]
- [50].Krotz SP, Carson SA, Tomey C, Buster JE, Phthalates and bisphenol do not accumulate in human follicular fluid, J. Assist. Reprod. Genet 29 (2012) 773–777. 10.1007/s10815-012-9775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Du YY, Fang YL, Wang YX, Zeng Q, Guo N, Zhao H, Li YF, Follicular fluid and urinary concentrations of phthalate metabolites among infertile women and associations with in vitro fertilization parameters, Reprod. Toxicol 61 (2016) 142–150. 10.1016/j.reprotox.2016.04.005. [DOI] [PubMed] [Google Scholar]
- [52].Koch HM, Bolt HM, Angerer J, Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP, Arch. Toxicol 78 (2004) 123–130. 10.1007/s00204-003-0522-3. [DOI] [PubMed] [Google Scholar]
- [53].Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P, Exposure to Di(2-ethylhexyl)phthalate in humans during pregnancy: A preliminary report, Biol. Neonate 83 (2003) 22–24. 10.1159/000067012. [DOI] [PubMed] [Google Scholar]
- [54].Rolfo A, Nuzzo AM, De Amicis R, Moretti L, Bertoli S, Leone A, Fetal–maternal exposure to endocrine disruptors: Correlation with diet intake and pregnancy outcomes, Nutrients. 12 (2020) 1–19. 10.3390/nu12061744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Genuis SJ, Beesoon S, Lobo RA, Birkholz D, Human Elimination of Phthalate Compounds: Blood, Urine, and Sweat (BUS) Study, Sci. World J 2012 (2012) 1–10. 10.1100/2012/615068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hlisníková H, Petrovičová I, Kolena B, Šidlovská M, Sirotkin A, Effects and mechanisms of phthalates’ action on reproductive processes and reproductive health: A literature review, 2020. 10.3390/ijerph17186811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Takeuchi S, Iida M, Kobayashi S, Jin K, Matsuda T, Kojima H, Differential effects of phthalate esters on transcriptional activities via human estrogen receptors α and β, and androgen receptor, Toxicology. 210 (2005) 223–233. 10.1016/j.tox.2005.02.002. [DOI] [PubMed] [Google Scholar]
- [58].Gao F, Hu W, Li Y, Shen H, Hu J, Mono-2-ethylhexyl phthalate inhibits human extravillous trophoblast invasion via the PPARγ pathway, Toxicol. Appl. Pharmacol 327 (2017) 23–29. 10.1016/j.taap.2017.04.014. [DOI] [PubMed] [Google Scholar]
- [59].Kambia NK, Séverin I, Farce A, Moreau E, Dahbi L, Duval C, Dine T, Sautou V, Chagnon MC, In vitro and in silico hormonal activity studies of di-(2-ethylhexyl)terephthalate, a di-(2-ethylhexyl)phthalate substitute used in medical devices, and its metabolites, J. Appl. Toxicol 39 (2019) 1043–1056. 10.1002/jat.3792. [DOI] [PubMed] [Google Scholar]
- [60].Shoaito H, Petit J, Chissey A, Auzeil N, Guibourdenche J, Gil S, Laprévote O, Fournier T, Degrelle SA, The Role of Peroxisome Proliferator–Activated Receptor Gamma (PPARγ) in Mono(2-ethylhexyl) Phthalate (MEHP)-Mediated Cytotrophoblast Differentiation, Environ. Health Perspect 127 (2019) 027003. 10.1289/EHP3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Barakat R, Seymore T, Lin PCP, Park CJ, Ko CMJ, Prenatal exposure to an environmentally relevant phthalate mixture disrupts testicular steroidogenesis in adult male mice, Environ. Res 172 (2019) 194–201. 10.1016/j.envres.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Furukawa S, Kuroda Y, Sugiyama A, A comparison of the histological structure of the placenta in experimental animals, J. Toxicol. Pathol 27 (2014) 11–18. 10.1293/tox.2013-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Aplin JD, Myers JE, Timms K, Westwood M, Tracking placental development in health and disease, Nat. Rev. Endocrinol 16 (2020) 479–494. 10.1038/s41574-020-0372-6. [DOI] [PubMed] [Google Scholar]
- [64].Bauer MK, Harding JE, Bassett NS, Breier BH, Oliver MH, Gallaher BH, Evans PC, Woodall SM, Gluckman PD, Fetal growth and placental function, Mol. Cell. Endocrinol 140 (1998) 115–120. 10.1016/S0303-7207(98)00039-2. [DOI] [PubMed] [Google Scholar]
- [65].Fowden AL, Forhead AJ, Sferruzzi-Perri AN, Burton GJ, Vaughan OR, Review: Endocrine regulation of placental phenotype, Placenta. 36 (2015) S50–S59. 10.1016/j.placenta.2014.11.018. [DOI] [PubMed] [Google Scholar]
- [66].Leiser R, Kaufmann P, Placental structure: In a comparative aspect, Exp. Clin. Endocrinol. Diabetes 102 (1994) 122–134. 10.1055/s-0029-1211275. [DOI] [PubMed] [Google Scholar]
- [67].Burton GJ, Jauniaux E, The cytotrophoblastic shell and complications of pregnancy, Placenta. 60 (2017) 134–139. 10.1016/j.placenta.2017.06.007. [DOI] [PubMed] [Google Scholar]
- [68].Dilworth MR, Sibley CP, Review: Transport across the placenta of mice and women, Placenta. 34 (2013) 1–6. 10.1016/j.placenta.2012.10.011. [DOI] [PubMed] [Google Scholar]
- [69].Aplin JD, Uterus-endometrium, in: Skinner MK(Ed.), Encycl. Reprod, Amsterdam: Elsevier, 2018: pp. 326–332. [Google Scholar]
- [70].James JL, Carter AM, Chamley LW, Human placentation from nidation to 5 weeks of gestation. Part I: What do we know about formative placental development following implantation?, Placenta. 33 (2012) 327–334. 10.1016/j.placenta.2012.01.020. [DOI] [PubMed] [Google Scholar]
- [71].Pollheimer J, Vondra S, Baltayeva J, Beristain AG, Knöfler M, Regulation of placental extravillous trophoblasts by the maternal uterine environment, Front. Immunol 9 (2018) 1–18. 10.3389/fimmu.2018.02597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Suman P, Gupta SK, Comparative analysis of the invasion-associated genes expression pattern in first trimester trophoblastic (HTR-8/SVneo) and JEG-3 choriocarcinoma cells, Placenta. 33 (2012) 874–877. 10.1016/j.placenta.2012.06.017. [DOI] [PubMed] [Google Scholar]
- [73].Knöfler M, Pollheimer J, Human placental trophoblast invasion and differentiation: A particular focus on Wnt signaling, Front. Genet 4 (2013) 1–14. 10.3389/fgene.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Blackburn S, Maternal, fetal, and neonatal physiology: a clinical perspective, 3 rd, Saunders, Philadelphia, 2007. [Google Scholar]
- [75].Silva JF, Serakides R, Intrauterine trophoblast migration: A comparative view of humans and rodents, Cell Adhes. Migr 10 (2016) 88–110. 10.1080/19336918.2015.1120397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Coan PM, Ferguson-Smith AC, Burton GJ, Developmental dynamics of the definitive mouse placenta assessed by stereology, Biol. Reprod 70 (2004) 1806–1813. 10.1095/biolreprod.103.024166. [DOI] [PubMed] [Google Scholar]
- [77].Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E, Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy, J. Clin. Endocrinol. Metab 87 (2002) 2954–2959. 10.1210/jcem.87.6.8563. [DOI] [PubMed] [Google Scholar]
- [78].Jones CJP, Choudhury RH, Aplin JD, Tracking nutrient transfer at the human maternofetal interface from 4 weeks to term, Placenta. 36 (2015) 372–380. 10.1016/j.placenta.2015.01.002. [DOI] [PubMed] [Google Scholar]
- [79].Sandovici I, Hoelle K, Angiolini E, Constância M, Placental adaptations to the maternal-fetal environment: Implications for fetal growth and developmental programming, Reprod. Biomed. Online 25 (2012) 68–89. 10.1016/j.rbmo.2012.03.017. [DOI] [PubMed] [Google Scholar]
- [80].Cline JM, Dixon D, Ernerudh J, Faas MM, Göhner C, Häger JD, Markert UR, Pfarrer C, Svensson-Arvelund J, Buse E, The Placenta in Toxicology. Part III:Pathologic Assessment of the Placenta, Toxicol. Pathol 42 (2014) 339–344. 10.1177/0192623313482207. [DOI] [PubMed] [Google Scholar]
- [81].Soares MJ, Chakraborty D, Rumi MAK, Konno T, Renaud SJ, Investigating the Hemochorial Maternal-Fetal, 33 (2013) 233–243. 10.1016/j.placenta.2011.11.026.RAT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Furukawa S, Tsuji N, Sugiyama A, Morphology and physiology of rat placenta for toxicological evaluation, J. Toxicol. Pathol 32 (2019) 1–17. 10.1293/TOX.2018-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Furukawa S, Hayashi S, Abe M, Hagio S, Irie K, Kuroda Y, Ogawa I, Sugiyama A, Background data on developmental parameters during the gestation period in rats, J. Toxicol. Pathol 26 (2013) 83–88. 10.1293/tox.26.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Peel S, Granulated metrial gland cells, Adv Anat Embryol Cell Biol. 115 (1989) 1–112. 10.1007/978-3-642-74170-8. [DOI] [PubMed] [Google Scholar]
- [85].Varshavsky J, Smith A, Wang A, Hom E, Izano M, Huang H, Padula A, Woodruff TJ, Heightened susceptibility: A review of how pregnancy and chemical exposures influence maternal health, Reprod. Toxicol 92 (2020) 14–56. 10.1016/j.reprotox.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Holdsworth-Carson SJ, Permezel M, Rice GE, Lappas M, Preterm and infection-driven preterm labor: The role of peroxisome proliferator-activated receptors and retinoid X receptor, Reproduction. 137 (2009) 1007–1015. 10.1530/REP-08-0496. [DOI] [PubMed] [Google Scholar]
- [87].Hu W, Gao F, Zhang H, Hiromori Y, Arakawa S, Nagase H, Nakanishi T, Hu J, Activation of Peroxisome Proliferator-Activated Receptor Gamma and Disruption of Progesterone Synthesis of 2-Ethylhexyl Diphenyl Phosphate in Human Placental Choriocarcinoma Cells: Comparison with Triphenyl Phosphate, Environ. Sci. Technol 51 (2017) 4061–4068. 10.1021/acs.est.7b00872. [DOI] [PubMed] [Google Scholar]
- [88].Lapinskas PJ, Brown S, Leesnitzer LM, Blanchard S, Swanson C, Cattley RC, Corton JC, Role of PPARα in mediating the effects of phthalates and metabolites in the liver, Toxicology. 207 (2005) 149–163. 10.1016/j.tox.2004.09.008. [DOI] [PubMed] [Google Scholar]
- [89].Corton JC, Lapinskas PJ, Peroxisome proliferator-activated receptors: Mediators of phthalate ester-induced effects in the male reproductive tract?, Toxicol. Sci 83 (2005) 4–17. 10.1093/toxsci/kfi011. [DOI] [PubMed] [Google Scholar]
- [90].Kambia N, Renault N, Dilly S, Farce A, Dine T, Gressier B, Luyckx M, Brunet C, Chavatte P, Molecular modelling of phthalates - PPARs interactions, J. Enzyme Inhib. Med. Chem 23 (2008) 611–616. 10.1080/14756360802205059. [DOI] [PubMed] [Google Scholar]
- [91].Taxvig C, Dreisig K, Boberg J, Nellemann C, Schelde AB, Pedersen D, Boergesen M, Mandrup S, Vinggaard AM, Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARγ activation, Mol. Cell. Endocrinol 361 (2012) 106–115. 10.1016/j.mce.2012.03.021. [DOI] [PubMed] [Google Scholar]
- [92].Hayward CE, Lean S, Sibley CP, Jones RL, Wareing M, Greenwood SL, Dilworth MR, Placental Adaptation: What Can We Learn from Birthweight:Placental Weight Ratio?, Front. Physiol 7 (2016) 1–13. 10.3389/fphys.2016.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Marsit CJ, Placental Epigenetics in Children’s Environmental Health, Semin. Reprod. Med 34 (2015) 36–41. 10.1055/s-0035-1570028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Alegría-Torres JA, Baccarelli A, Bollati V, Epigenetics and lifestyle, Epigenomics. 3 (2011) 267–277. 10.2217/epi.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, Fitzhugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, Levine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Hong ML, Dubois J, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, De La Bastide M, Dedhia N, Blöcker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JGR, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AFA, Stupka E, Szustakowki J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Raymond C, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Patrinos A, Morgan MJ, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S, Chen YJ, Erratum: Initial sequencing and analysis of the human genome: International Human Genome Sequencing Consortium (Nature (2001) 409 (860–921)), Nature. 412 (2001) 565–566. 10.1038/35087627. [DOI] [PubMed] [Google Scholar]
- [96].Bird A, DNA methylation patterns and epigenetic memory, Genes Dev. 16 (2002) 16–21. 10.1101/gad.947102.6. [DOI] [PubMed] [Google Scholar]
- [97].Reik W, Walter J, Genomic imprinting: Parental influence on the genome, Nat. Rev. Genet 2 (2001) 21–32. 10.1038/35047554. [DOI] [PubMed] [Google Scholar]
- [98].Piedrahita JA, The role of imprinted genes in fetal growth abnormalities, Birth Defects Res. Part A - Clin. Mol. Teratol 91 (2011) 682–692. 10.1002/bdra.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Mouillet J-F, Ouyang Y, Coyne C, Sadovsky Y, MicroRNAs in placental health and disease, Am J Obs. Gynecol 213 (2016) S163–S172. 10.1016/j.ajog.2015.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Ambros V, The functions of animal microRNAs, Nature. 431 (2004) 350–355. 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- [101].Machtinger R, Zhong J, Mansur A, Adir M, Racowsky C, Hauser R, Brennan K, Karlsson O, Baccarelli AA, Placental lncRNA expression is associated with prenatal phthalate exposure, Toxicol. Sci 163 (2018) 116–122. 10.1093/toxsci/kfy013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Gingrich J, Ticiani E, Veiga-Lopez A, Placenta Disrupted: Endocrine Disrupting Chemicals and Pregnancy, Trends Endocrinol. Metab 31 (2020) 508–524. 10.1016/j.tem.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Strakovsky RS, Schantz SL, Using experimental models to assess effects of Bisphenol A (BPA) and phthalates on the placenta: Challenges and perspectives, Toxicol. Sci 166 (2018) 250–268. 10.1093/toxsci/kfy224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Pérez-Albaladejo E, Fernandes D, Lacorte S, Porte C, Comparative toxicity, oxidative stress and endocrine disruption potential of plasticizers in JEG-3 human placental cells, Toxicol. Vitr 38 (2017) 41–48. 10.1016/j.tiv.2016.11.003. [DOI] [PubMed] [Google Scholar]
- [105].Abou-Kheir W, Barrak J, Hadadeh O, Daoud G, HTR-8/SVneo cell line contains a mixed population of cells, Placenta. 50 (2017) 1–7. 10.1016/j.placenta.2016.12.007. [DOI] [PubMed] [Google Scholar]
- [106].Zhang S, Sun C, Zhao S, Wang B, Wang H, Zhang J, Wang Y, Cheng H, Zhu L, Shen R, Sun M, Xu T, Zhao L, Exposure to DEHP or its metabolite MEHP promotes progesterone secretion and inhibits proliferation in mouse placenta or JEG-3 cells, Environ. Pollut 257 (2020) 113593. 10.1016/j.envpol.2019.113593. [DOI] [PubMed] [Google Scholar]
- [107].Xu R, Mao B, Li S, Liu J, Li X, Li H, Su Y, Hu G, Lian Q-Q, Ge R-S, Structure-activity relationships of phthalates in inhibition of human placental 3β-hydroxysteroid dehydrogenase 1 and aromatase, Reprod. Toxicol 61 (2016) 151–161. 10.1016/j.reprotox.2016.04.004. [DOI] [PubMed] [Google Scholar]
- [108].Du ZP, Feng S, Li YL, Li R, Lv J, Ren WQ, Feng QW, Liu P, Wang QN, Di-(2-ethylhexyl) phthalate inhibits expression and internalization of transthyretin in human placental trophoblastic cells, Toxicol. Appl. Pharmacol 394 (2020) 114960. 10.1016/j.taap.2020.114960. [DOI] [PubMed] [Google Scholar]
- [109].Tetz LM, Cheng AA, Korte CS, Giese RW, Wang P, Harris C, Meeker JD, Loch-Caruso R, Mono-2-ethylhexyl phthalate induces oxidative stress responses in human placental cells in vitro, Toxicol. Appl. Pharmacol 268 (2013) 47–54. 10.1016/j.taap.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Tetz LM, Aronoff DM, Loch-Caruso R, Mono-ethylhexyl phthalate stimulates prostaglandin secretion in human placental macrophages and THP-1 cells, Reprod. Biol. Endocrinol 13 (2015). 10.1186/s12958-015-0046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Xu Y, Knipp GT, Cook TJ, Effects of di-(2-ethylhexyl)-phthalate and its metabolites on the lipid profiling in rat HRP-1 trophoblast cells, Arch. Toxicol 80 (2006) 293–298. 10.1007/s00204-005-0047-z. [DOI] [PubMed] [Google Scholar]
- [112].Petit J, Wakx A, Gil S, Fournier T, Auzeil N, Rat P, Laprévote O, Lipidome-wide disturbances of human placental JEG-3 cells by the presence of MEHP, Biochimie. 149 (2018) 1–8. 10.1016/j.biochi.2018.03.002. [DOI] [PubMed] [Google Scholar]
- [113].Meruvu S, Zhang J, Bedi YS, Choudhury M, Mono-(2-ethylhexyl) phthalate induces apoptosis through miR-16 in human first trimester placental cell line HTR-8/SVneo, Toxicol. Vitr 31 (2016) 35–42. 10.1016/j.tiv.2015.11.010. [DOI] [PubMed] [Google Scholar]
- [114].Meruvu S, Zhang J, Choudhury M, Mono-(2-ethylhexyl) Phthalate Increases Oxidative Stress Responsive miRNAs in First Trimester Placental Cell Line HTR8/SVneo, Chem. Res. Toxicol 29 (2016) 430–435. 10.1021/acs.chemrestox.6b00038. [DOI] [PubMed] [Google Scholar]
- [115].Midic U, Goheen B, Vincent KA, VandeVoort CA, Latham KE, Changes in gene expression following long-term in vitro exposure of Macaca mulatta trophoblast stem cells to biologically relevant levels of endocrine disruptors, Reprod. Toxicol 77 (2018) 154–165. 10.1016/j.reprotox.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Midic U, Vincent KA, VandeVoort CA, Latham KE, Effects of long-term endocrine disrupting compound exposure on Macaca mulatta embryonic stem cells, Reprod. Toxicol 65 (2016) 382–393. 10.1016/j.reprotox.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wang XK, Agarwal M, Parobchak N, Rosen A, Vetrano AM, Srinivasan A, Wang B, Rosen T, Mono-(2-ethylhexyl) phthalate promotes pro-labor gene expression in the human placenta, PLoS One. 11 (2016). 10.1371/journal.pone.0147013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Adibi JJ, Zhao Y, Zhan LV, Kapidzic M, Larocque N, Koistinen H, Huhtaniemi IT, Stenman UH, An investigation of the single and combined phthalate metabolite effects on human chorionic gonadotropin expression in placental cells, Environ. Health Perspect 125 (2017) 1–12. 10.1289/EHP1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Vandenberg LN, Welshons WV, Saal FSV, Toutain PL, Myers JP, Should oral gavage be abandoned in toxicity testing of endocrine disruptors?, Environ. Heal. A Glob. Access Sci. Source 13 (2014) 1–7. 10.1186/1476-069X-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Zong T, Lai L, Hu J, Guo M, Li M, Zhang L, Zhong C, Yang B, Wu L, Zhang D, Tang M, Kuang H, Maternal exposure to di-(2-ethylhexyl) phthalate disrupts placental growth and development in pregnant mice, J. Hazard. Mater 297 (2015) 25–33. 10.1016/j.jhazmat.2015.04.065. [DOI] [PubMed] [Google Scholar]
- [121].Shen R, Zhao LL, Yu Z, Zhang C, Chen YH, Wang H, Zhang ZH, Xu DX, Maternal di-(2-ethylhexyl) phthalate exposure during pregnancy causes fetal growth restriction in a stage-specific but gender-independent manner, Reprod. Toxicol 67 (2017) 117–124. 10.1016/j.reprotox.2016.12.003. [DOI] [PubMed] [Google Scholar]
- [122].Du YY, Guo N, Wang YX, Hua X, Deng TR, Teng XM, Yao YC, Li YF, Urinary phthalate metabolites in relation to serum anti-Müllerian hormone and inhibin B levels among women from a fertility center: A retrospective analysis, Reprod. Health 15 (2018) 1–12. 10.1186/s12978-018-0469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Tang C, Luo C, Hua Y, Zhou K, Duan H, Ma F, Zhang Y, Li Y, Qiu D, Wang C, Placental P-glycoprotein inhibition enhances susceptibility to Di-(2-ethylhexyl)-phthalate induced cardiac malformations in mice: A possibly promising target for congenital heart defects prevention, PLoS One. 14 (2019) 1–14. 10.1371/journal.pone.0214873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Xu W, Wu H, Shang L, Gene expression in rat placenta after exposure to di(2-ethylhexyl) phthalate, Hum. Exp. Toxicol (2020). 10.1177/0960327120954259. [DOI] [PubMed] [Google Scholar]
- [125].Mahaboob Basha P, Radha MJ, Gestational di-n-butyl phthalate exposure induced developmental and teratogenic anomalies in rats: a multigenerational assessment, Environ. Sci. Pollut. Res 24 (2017) 4537–4551. 10.1007/s11356-016-8196-6. [DOI] [PubMed] [Google Scholar]
- [126].Ahbab MA, Güven C, Koçkaya EA, Barlas N, Comparative developmental toxicity evaluation of di- n -hexyl phthalate and dicyclohexyl phthalate in rats, Toxicol. Ind. Health 33 (2017) 696–716. 10.1177/0748233717711868. [DOI] [PubMed] [Google Scholar]
- [127].Saadeldin IM, Hussein MA, Suleiman AH, Abohassan MG, Ahmed MM, Moustafa AA, Moumen AF, Abdel-Aziz Swelum A, Ameliorative effect of ginseng extract on phthalate and bisphenol A reprotoxicity during pregnancy in rats, Environ. Sci. Pollut. Res 25 (2018) 21205–21215. 10.1007/s11356-018-2299-1. [DOI] [PubMed] [Google Scholar]
- [128].Yu Z, Han Y, Shen R, Huang K, yuan Xu Y, nan Wang Q, shan Zhou S, xiang Xu D, biao Tao F, Gestational di-(2-ethylhexyl) phthalate exposure causes fetal intrauterine growth restriction through disturbing placental thyroid hormone receptor signaling, Toxicol. Lett 294 (2018) 1–10. 10.1016/j.toxlet.2018.05.013. [DOI] [PubMed] [Google Scholar]
- [129].Xu Y, Agrawal S, Cook TJ, Knipp GT, Maternal Di-(2-ethylhexyl)-phthalate Exposure Influences Essential Fatty Acid Homeostasis in Rat Placenta, Placenta. 29 (2008) 962–969. 10.1016/j.placenta.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Shin HM, Bennett DH, Barkoski J, Ye X, Calafat AM, Tancredi D, Hertz-Picciotto I, Variability of urinary concentrations of phthalate metabolites during pregnancy in first morning voids and pooled samples, Environ. Int 122 (2019) 222–230. 10.1016/j.envint.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Alwasel SH, Harrath AH, Aljarallah JS, Abotalib Z, Osmond C, Al Omar SY, Thornburg K, Barker DJP, The velocity of fetal growth is associated with the breadth of the placental surface, but not with the length, Am. J. Hum. Biol 25 (2013) 534–537. 10.1002/ajhb.22405. [DOI] [PubMed] [Google Scholar]
- [132].Eriksson JG, Kajantie E, Thornburg KL, Osmond C, Barker DJP, Mothers body size and placental size predict coronary heart disease in men, Eur. Heart J 32 (2011) 2297–2303. 10.1093/eurheartj/ehr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Barker DJP, Thornburg KL, Osmond C, Kajantie E, Eriksson JG, The surface area of the placenta and hypertension in the offspring in later life, Int J Dev Biol 54 (2010) 525–530. 10.1387/ijdb.082760db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].duo Zhu Y, Gao H, Huang K, wei Zhang Y, xiu Cai X, yuan Yao H, jing Mao L, Ge X, shan Zhou S, yuan Xu Y, xiu Jin Z, Sheng J, qin Yan S, jun Pan W, hu Hao J, Zhu P, biao Tao F, Prenatal phthalate exposure and placental size and shape at birth: A birth cohort study, Environ. Res 160 (2018) 239–246. 10.1016/j.envres.2017.09.012. [DOI] [PubMed] [Google Scholar]
- [135].Philippat C, Heude B, Botton J, Alfaidy N, Calafat AM, Slama R, Annesi-Maesano I, Bernard J, Botton J, Charles MA, Dargent-Molina P, De Lauzon-Guillian B, Ducimetière P, De Agostini M, Foliguet B, Forhan A, Fritel X, Germa A, Goua V, Hankard R, Heude B, Kaminski M, Larroque B, Lelong N, Lepeule J, Pierre F, Marchand L, Nabet C, Slama R, Saurel-Cubizolles MJ, Schweitzer M, Thiebaugeorge O, Prenatal exposure to select phthalates and phenols and associations with fetal and placental weight among male births in the EDEN Cohort (France), Environ. Health Perspect 127 (2019) 1–8. 10.1289/EHP3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Mustieles V, Minguez-Alarcón L, Christou G, Ford JB, Dimitriads I, Hauser R, Souter I, Messrlian C, E. ans R.H.S.T. EARTH, Placental weight in relation to maternal and paternal preconception and prenatal urinary phthalate metabolite concentrations among subfertile couples, Env. Res 169 (2019) 272–279. 10.1016/j.envres.2018.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Adibi JJ, Whyatt RM, Hauser R, Bhat HK, Davis BJ, Calafat AM, Hoepner LA, Perera FP, Tang D, Williams PL, Transcriptional biomarkers of steroidogenesis and trophoblast differentiation in the placenta in relation to prenatal phthalate exposure, Environ. Health Perspect 118 (2010) 291–296. 10.1289/ehp.0900788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Adibi JJ, Buckley JP, Lee MK, Williams PL, Just AC, Zhao Y, Bhat HK, Whyatt RM, Maternal urinary phthalates and sex-specific placental mRNA levels in an urban birth cohort, Environ. Heal. A Glob. Access Sci. Source 16 (2017) 1–14. 10.1186/s12940-017-0241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Huang Y, Garcia JM, Shu W, Rong H, Zhang L, Wang Y, Tan Y, Lin H, Zeng H, an Chen J, Peroxisome proliferator activated receptor gamma in human placenta may mediate the adverse effects of phthalates exposure in pregnancy, Reprod. Toxicol 75 (2018) 121–126. 10.1016/j.reprotox.2017.10.001. [DOI] [PubMed] [Google Scholar]
- [140].Li B, Xu X, Zhu Y, Cao J, Zhang Y, Huo X, Neonatal phthalate ester exposure induced placental MTs, FATP1 and HFABP mRNA expression in two districts of southeast China, Sci. Rep 6 (2016) 1–7. 10.1038/srep21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Feng W, Cai J, Pierce WM, Franklin RB, Maret W, Benz FW, Kang YJ, Metallothionein transfers zinc to mitochondrial aconitase through a direct interaction in mouse hearts, Biochem. Biophys. Res. Commun 332 (2005) 853–858. 10.1016/j.bbrc.2005.04.170. [DOI] [PubMed] [Google Scholar]
- [142].Knipp GT, Audus KL, Soares MJ, Nutrient transport across the placenta, Adv. Drug Deliv. Rev 38 (1999) 41–58. 10.1016/S0169-409X(99)00005-8. [DOI] [PubMed] [Google Scholar]
- [143].Zhao Y, Shi H, Xie C, Chen J, Laue H, Zhang Y, Prenatal Phthalate Exposure, Infant Growth, and Global DNAMethylation of Human Placenta Yan, Environ. Mol. Mutagen 56 (2015) 286–292. 10.1002/em.21916. [DOI] [PubMed] [Google Scholar]
- [144].Grindler NM, Vanderlinden L, Karthikraj R, Kannan K, Teal S, Polotsky AJ, Powell TL, Yang IV, Jansson T, Exposure to Phthalate, an Endocrine Disrupting Chemical, Alters the First Trimester Placental Methylome and Transcriptome in Women, Sci. Rep 8 (2018) 1–9. 10.1038/s41598-018-24505-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Citri A, Yarden Y, EGF-ERBB signalling: Towards the systems level, Nat. Rev. Mol. Cell Biol 7 (2006) 505–516. 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- [146].Fock V, Plessl K, Fuchs R, Dekan S, Milla SK, Haider S, Fiala C, Knöfler M, Pollheimer J, Trophoblast subtype-specific EGFR/ERBB4 expression correlates with cell cycle progression and hyperplasia in complete hydatidiform moles, Hum. Reprod 30 (2015) 789–799. 10.1093/humrep/dev027. [DOI] [PubMed] [Google Scholar]
- [147].LaRocca J, Binder AM, McElrath TF, Michels KB, The impact of first trimester phthalate and phenol exposure on IGF2/H19 genomic imprinting and birth outcomes, Environ. Res 133 (2014) 396–406. 10.1016/j.envres.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Zhao Y, Chen J, Wang X, Song Q, Xu HH, Zhang YH, Third trimester phthalate exposure is associated with DNA methylation of growth-related genes in human placenta, Sci. Rep 6 (2016) 1–8. 10.1038/srep33449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Fatima R, Akhade VS, Pal D, Rao SM, Long noncoding RNAs in development and cancer: potential biomarkers and therapeutic targets, Mol. Cell. Ther 3 (2015). 10.1186/s40591-015-0042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].LaRocca J, Binder AM, McElrath TF, Michels KB, First-Trimester Urine Concentrations of Phthalate Metabolites and Phenols and Placenta miRNA Expression in a Cohort of U.S. Women, Environ. Health Perspect 124 (2016) 380–387. 10.1289/ehp.1408409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Zhong J, Baccarelli AA, Mansur A, Adir M, Nahum R, Hauser R, Bollati V, Racowsky C, Machtinger R, Maternal Phthalate and Personal Care Products Exposure Alters Extracellular Placental miRNA Profile in Twin Pregnancies, Reprod. Sci 26 (2019) 289–294. 10.1177/1933719118770550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Mitchell MD, Peiris HN, Kobayashi M, Koh YQ, Duncombe G, Illanes SE, Rice GE, Salomon C, Placental exosomes in normal and complicated pregnancy, Am. J. Obstet. Gynecol 213 (2015) S173–S181. 10.1016/j.ajog.2015.07.001. [DOI] [PubMed] [Google Scholar]
- [153].Molares-Prieto DM, Ospina-Prieto S, Ospina-Prieto M, Stephanie Chaiwangyen, Wittaya Schoenleben, U.R. Markert, Pregnancy-associated miRNA-clusters, J. Reprod. Immunol 97 (2013) 51–61. 10.1016/j.jri.2012.11.001. [DOI] [PubMed] [Google Scholar]
- [154].Morales-Prieto W, Diana M Chaiwangyena, U. Ospina-Prieto, Stephanie Schneider, J. Herrmann, B. Gruhn, U. Markert, MicroRNA expression profiles of trophoblastic cells, Placenta. 33 (2012) 725–734. 10.1016/j.placenta.2012.05.009. [DOI] [PubMed] [Google Scholar]
- [155].Blundell C, Yi YS, Ma L, Tess ER, Farrell MJ, Georgescu A, Aleksunes LM, Huh D, Placental Drug Transport-on-a-Chip: A Microengineered In Vitro Model of Transporter-Mediated Drug Efflux in the Human Placental Barrier, Adv. Healthc. Mater 7 (2018) 1–9. 10.1002/adhm.201700786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Fry RC, Bangma J, Szilagyi J, Rager JE, Developing novel in vitro methods for the risk assessment of developmental and placental toxicants in the environment, Toxicol. Appl. Pharmacol 378 (2019) 114635. 10.1016/j.taap.2019.114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Grigsby PL, Animal Models to Study Placental Development and Function throughout Normal and Dysfunctional Human Pregnancy Peta, Semin Reprod Med. 34 (2016) 11–16. 10.1055/s-0035-1570031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Wu H, Ashcraft L, Whitcomb BW, Rahil T, Tougias E, Sites CK, Pilsner JR, Parental contributions to early embryo development: Influences of urinary phthalate and phthalate alternatives among couples undergoing IVF treatment, Hum. Reprod 32 (2017) 65–75. 10.1093/humrep/dew301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Wu H, Estill MS, Shershebnev A, Suvorov A, Krawetz SA, Whitcomb BW, Dinnie H, Rahil T, Sites CK, Pilsner JR, Preconception urinary phthalate concentrations and sperm DNA methylation profiles among men undergoing IVF treatment: A cross-sectional study, Hum. Reprod 32 (2017) 2159–2169. 10.1093/humrep/dex283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Yuen RKC, Pẽaherrera MS, Von Dadelszen P, McFadden DE, Robinson WP, DNA methylation profiling of human placentas reveals promoter hypomethylation of multiple genes in early-onset preeclampsia, Eur. J. Hum. Genet 18 (2010) 1006–1012. 10.1038/ejhg.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Novakovic B, Yuen RK, Gordon L, Penaherrera MS, Sharkey A, Moffett A, Craig JM, Robinson WP, Saffery R, Evidence for widespread changes in promoter methylation profile in human placenta in response to increasing gestational age and environmental/stochastic factors, BMC Genomics. 12 (2011) 529. 10.1186/1471-2164-12-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Pozharny Y, Lambertini L, Ma Y, Ferrara L, Litton CG, Diplas A, Jacobs AR, Chen J, Stone JL, Wetmur J, Lee MJ, Genomic loss of imprinting in first-trimester human placenta, Am. J. Obstet. Gynecol 202 (2010) 391.e1–391.e8. 10.1016/j.ajog.2010.01.039. [DOI] [PubMed] [Google Scholar]
- [163].Adibi JJ, Hauser R, Williams PL, Whyatt RM, Thaker HM, Nelson H, Herrick R, Bhat HK, Placental biomarkers of phthalate effects on mRNA transcription: Application in epidemiologic research, Environ. Heal. A Glob. Access Sci. Source 8 (2009) 1–11. 10.1186/1476-069X-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]


