Abstract
The COVID-19 pandemic caused by the novel SARS-CoV-2 virus has caused havoc across the entire world. Even though several COVID-19 vaccines are currently in distribution worldwide, with others in the pipeline, treatment modalities lag behind. Accordingly, researchers have been working hard to understand the nature of the virus, its mutant strains, and the pathogenesis of the disease in order to uncover possible drug targets and effective therapeutic agents. As the research continues, we now know the genome structure, epidemiological and clinical features, and pathogenic mechanism of SARS-CoV-2. Here, we summarized the potential therapeutic targets involved in the life cycle of the virus. On the basis of these targets, small-molecule prophylactic and therapeutic agents have been or are being developed for prevention and treatment of SARS-CoV-2 infection.
Key words: SARS-CoV-2, COVID-19, Therapeutic, Prophylactic, Small-molecule inhibitors
Graphical abstract
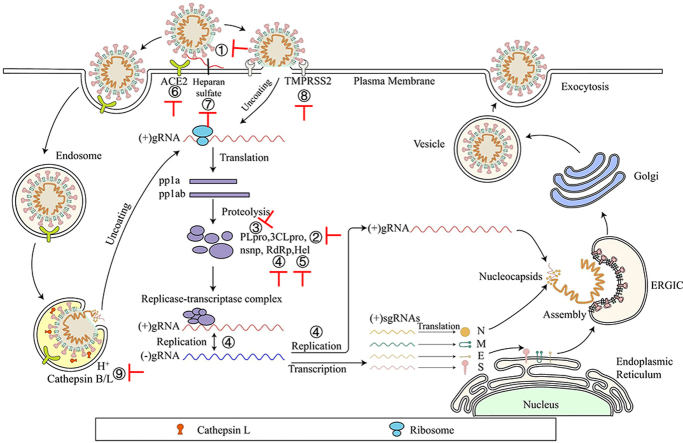
SARS-CoV-2 enters the cell by recognizing the host cell receptor through the S protein and uses the cell's system for genome and protein synthesis and viral particle assembly, each of these steps can serve as a target for identification of inhibitors against SARS-CoV-2 infection.
1. Introduction
In 2019, a new infectious respiratory disease emerged. A novel coronavirus was identified as the pathogen causing the outbreak of atypical pneumonia1,2 and given a nomenclature of 2019 novel coronavirus (2019-nCoV) by the World Health Organization (WHO). The International Committee on Taxonomy of Viruses (ICTV) renamed the virus as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and the disease as coronavirus disease 2019 (COVID-19)7. Some virologists suggested changing the name to human coronavirus 2019 (HCoV-19) to avoid confusion with SARS-CoV that emerged in 20023.
SARS-CoV-2 belongs to the genus betacoronavirus, together with SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV, with 82% and 50% homology, respectively)4, 5, 6. The main symptoms of COVID-19 include fever, fatigue, dry cough, upper chest discomfort and dyspnea. Severe cases are reported to show sepsis, secondary infections and organ failure. By 22 June 2021, COVID-19 had spread to more than 223 countries with more than 178,360,849 confirmed cases reported globally, including more than 3,869,384 deaths (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). On 30 January 2020, WHO declared the COVID-19 outbreak to be a global public health emergency, and on 11 March 2020, WHO characterized COVID-19 as a pandemic1. While several COVID-19 vaccines have been approved for general or emergency use, no specific antiviral drugs are now available for prophylaxis or treatment of SARS-CoV-219, 244. Therefore, it is still urgently needed to develop effective prophylactics and therapeutics against SARS-CoV-2 infection. Here, we summarized the potential therapeutic targets involved in the life cycle of the virus, treatments now in clinical use, the progress of candidate drugs, and potential prophylactic and therapeutic drugs based on these targets to treat COVID-19 in the future.
2. Viral structure
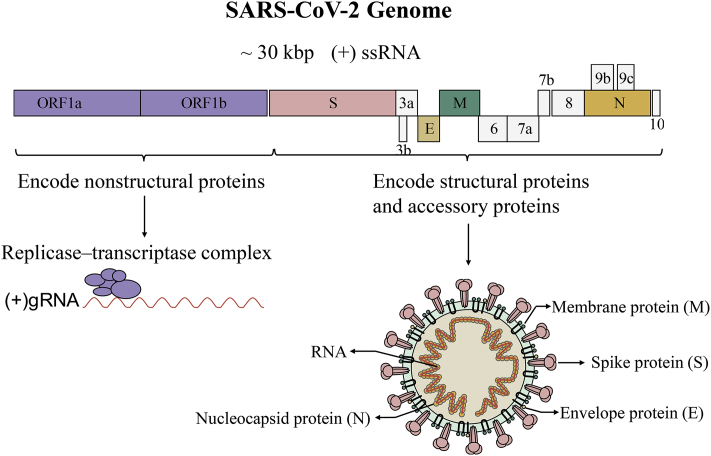
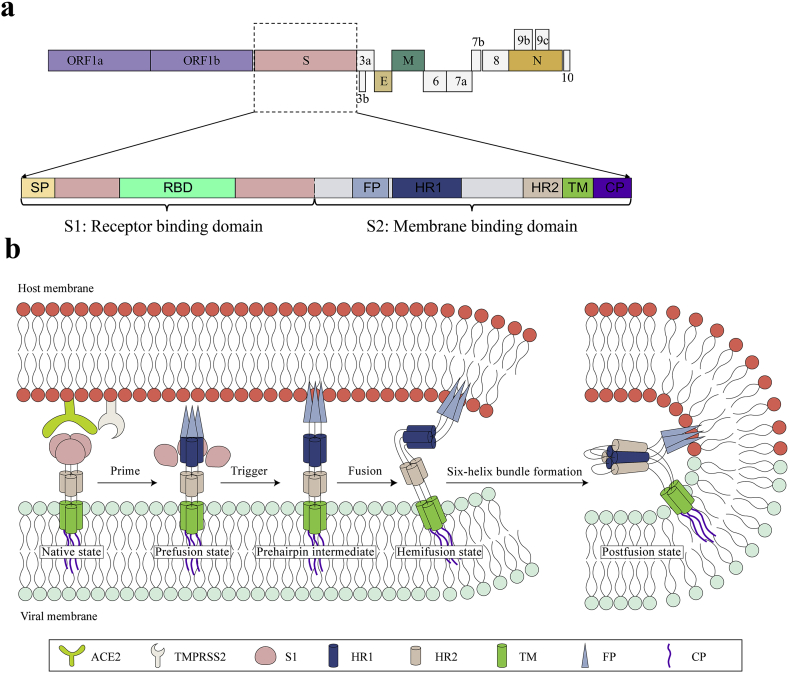
SARS-CoV-2 is an enveloped virus with a positive-sense, single-stranded RNA [(+) ssRNA] genome of ~30 kb8 (Fig. 1). Upon cell entry, genomic RNA is translated as either ORF1a or ORF1ab owing to a frame shift, which is then cleaved into nonstructural proteins (nsps) by viral proteinases. These nsps are mainly responsible for the replication and transcription of genomic RNA. A series of subgenomic RNAs (sgRNAs) are discontinuously transcribed and finally translated into structural proteins [spike protein (S), envelope protein (E), membrane protein (M), and nucleocapsid protein (N)], and several accessory proteins (3a, 3b, 6, 7a, 7 b, 8, 9b, 9c and 10)8,9. A lipid bilayer comprising the S protein, the M protein and the E protein cloaks the helical nucleocapsids, consisting of the N protein that is associated with the viral RNA (Fig. 1).
Figure 1.
Schematic diagram of genome composition and particle structure of SARS-CoV-2. SARS-CoV-2 genome consists of open reading frames (ORFs) expressing structural proteins including spike protein (S), envelope protein (E), membrane protein (M), and nucleocapsid protein (N), and ORFs1a, 1 b, etc. Expressing non-structural and accessory proteins. Among them, N protein and (+) ssRNA form nucleocapsids with three other structural proteins to form mature viral particles.
3. SARS-CoV-2 life cycle and potential targets for the development of small-molecule inhibitors against SARS-CoV-2 infection
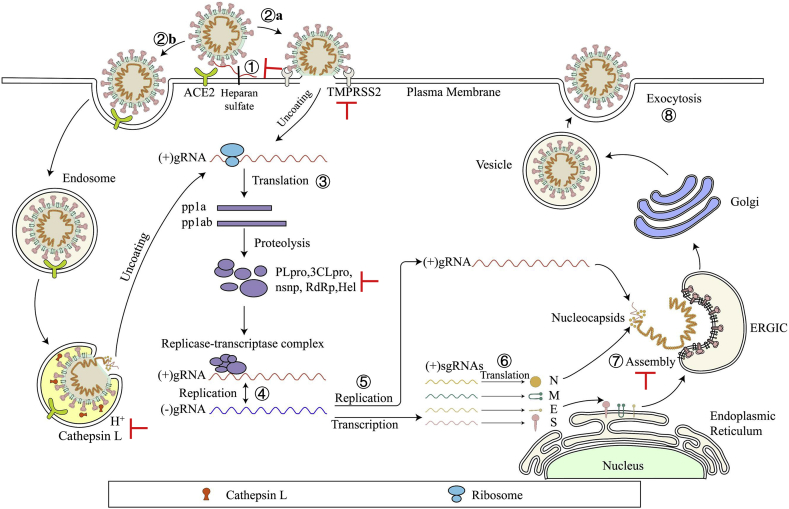
SARS-CoV-2 enters the target cell through two different ways, either plasma or endosomal membrane fusion (Fig. 2). The S protein of SARS-CoV-2 mediates the attachment of virus to the membrane of the host cell through its interaction with angiotensin-converting enzyme 2 (ACE2) and cellular heparan sulfate as the entry receptors, respectively10,11. For plasma membrane fusion, the S protein can be activated by transmembrane protease serine 2 (TMPRSS2) in close proximity to the ACE2 receptor, which initiates fusion between the viral membrane and the plasma membrane. In the absence of TMPRSS2, SARS-CoV-2 can be internalized via endocytosis10. After the virus enters the cell via the endocytic pathway, a lysosome-mediated drop in pH occurs in the endosome. The low pH environment activates endosomal proteinases, such as cathepsin B/L, which activate the S protein and prepares the virus for subsequent steps of fusion10,12.
Figure 2.
Life cycle of SARS-CoV-2. SARS-CoV-2 first binds, via its S protein, to the receptor ACE2 on the target cell (①). Then, the virus must gain access to the host cell cytosol through plasma (②a) or endosomal membrane fusion (②b). This is assisted by activation of S protein by TMPRRS2 (②a) or cathepsin B/L (②b), followed by fusion of the viral and cellular membranes. The viral genome is released, uncoated and translated into viral replicase polyproteins pp1a and 1 ab (③), which are then cleaved into nonstructural proteins (nsps) by viral proteinases as papain-like protease (PLpro) and 3C-like protease (3CLpro). Many of these nsps as RNA-dependent RNA polymerase (RdRp) or Helicase (Hel) then assemble into the replicase–transcriptase complex which replicates the (+)-sense genomic RNA ((+) gRNA). (−)-sense genomic RNA ((−) gRNA) is synthesized and used as a template to form (+) gRNA) and subgenomic RNAs (sgRNAs) (⑤). The viral structural proteins, S, E, and M are translated from sgRNAs (⑥) and inserted into the endoplasmic reticulum (ER), from where they are transported to the ER–Golgi intermediate compartment (ERGIC) to interact with the (+) gRNA-encapsidated N proteins and assemble into viral particles (⑦). The budded vesicles containing mature viral particles are then transported to the cell surface for release after maturation in the Golgi bodies (⑧). Possible targets for inhibitors are marked in red.
After membrane fusion, either with the host cell membrane or the endosome membrane, the viral (+)-sense genomic RNA [(+) gRNA] is released into the cytoplasm to allow translation of the two polyproteins, pp1a and 1 ab. Autoproteolytic cleavage of polyproteins produces more than a dozen nsps13, including papain-like protease (PLpro), 3C-like protease (3CLpro), RNA-dependent RNA polymerase (RdRp) and helicase. Of these, PLpro and 3CLpro are responsible for polyprotein cleavage. And some nsps use (+) gRNA as a template to form a replicase–transcriptase complex.
After generating (−)-sense genomic RNA [(−) gRNA], it can serve as the template for the synthesis of (+) gRNA, which becomes the genome of the new virus particle13,14. Transmembrane structural proteins (S, M and E) undergo a series of steps in the endoplasmic reticulum (ER) including being synthesized, inserted and folded, and finally transported to the ER-Golgi intermediate compartment (ERGIC)15. The N proteins are translated in the cytoplasm and encapsulate (+) gRNA to form nucleocapsids16. Virion assembly occurs in the ERGIC, and virions are then transported from the ERGIC through the Golgi apparatus to the cell surface via small vesicles16.
Potential small-molecule anti-SARS-CoV-2 drugs can be divided into two categories, depending on the targets. The first class of inhibitors target the viral proteins, such as S protein, viral enzymes (PLpro, 3CLpro, RdRp and helicase)17, 18, 19, 20, and some structural proteins21,22 (Fig. 2 and Table 2). The second class of inhibitors can interact with the host proteins, such as the receptor ACE2 or heparan sulfate, the serine protease TMPRSS2, or the endosomal acid protease cathepsin L to prevent viral entry10,23,24, as well as some regulators of the human immune system signaling pathways required for virus replication and infection25,26 (Fig. 2 and Table 3).
Table 2.
Small-molecule SARS-CoV-2 inhibitors targeting viral proteins.
| Number of chemical structures in Fig. | Inhibitor | Testing model | Activity IC50 (μmol/L) | Toxicity CC50 (μmol/L) | Clinical information | Ref. |
|---|---|---|---|---|---|---|
| Entry inhibitor | ||||||
| Fig. 4 (1) | Salvianolic acid C (Sal-C) | In vitro | 3.41 | ≥100 | Preclinical | 58 |
| Fig. 4 (2) | Arbidol | In vitro | 4.11 | 31.79 | ChiCTR2000029573 NCT04252885 |
60,64 |
| Fig. 4 (3) | DRI-C23041 | In vitro (pseudovirus) | 5.6 | ≥135 | Preclinical | 65 |
| Fig. 4 (4) | Cepharanthine | In vitro | 1.41 | 11.22 | Preclinical | 66 |
| In vitro | 0.13 | – | Preclinical | 78 | ||
| In vitro | 2.8 | 12.9 | Preclinical | 94 | ||
| Fig. 4 (5) | Abemaciclib | In vitro | 3.16 | 7.08 | Preclinical | 66 |
| Fig. 4 (6) | Osimertinib | In vitro | 3.98 | 10.00 | Preclinical | 66 |
| Fig. 4 (7) | Trimipramine | In vitro | 20.52 | ≥20 | Preclinical | 66 |
| In vitro | 1.5 | – | Preclinical | 78 | ||
| Fig. 4 (8) | Colforsin | In vitro | 23.06 | 25.2 | Preclinical | 66 |
| Fig. 4 (9) | Ingenol | In vitro | 0.06 | ≥20 | Preclinical | 66 |
| Fig. 4 (10) | Clofazimine | In vitro | 0.31 | – | Preclinical | 68 |
| Replication inhibitors | ||||||
| Target 3CLpro | ||||||
| Fig. 5 (11) | Tafenoquine | In vitro | 2.5 | – | Preclinical | 72 |
| In vitro | ~ 2.6 | ≥50 | Preclinical | 73 | ||
| Fig. 5 (12) | 12 | In vitro | 0.25 ± 0.15 | ≥100 | Preclinical | 74 |
| Fig. 5 (13) | 13 | In vitro | 0.15 ± 0.14 | 63.3 ± 2.3 | Preclinical | 74 |
| Fig. 5 (14) | 14 | In vitro | 0.9 ± 0.8 | ≥100 | Preclinical | 74 |
| Fig. 5 (15) | 15 | In vitro | 0.8 ± 0.7 | ≥100 | Preclinical | 74 |
| Fig. 5 (16) | Baicalin | In vitro | 10.27 | ≥200 | Preclinical | 75 |
| Fig. 5 (17) | Baicalein | In vitro | 1.69 | ≥200 | Preclinical | 75 |
| In vitro | 10 | ≥100 | Preclinical | 76 | ||
| In vitro | 2.9 | >500 | Preclinical | 77 | ||
| Fig. 5 (18) | Masitinib | In vitro | 3.2 | – | Preclinical | 78 |
| Fig. 5 (19) | Ebselen | In vitro | 4.67 ± 0.80 | – | Preclinical | 79 |
| Fig. 5 (20) | N3 | In vitro | 16.77 ± 1.70 | – | Preclinical | 79 |
| Fig. 5 (21) | Cinanserin | In vitro | 20.61 ± 0.97 | ≥200 | Preclinical | 79 |
| Fig. 5 (22) | 22 | In vitro | 0.53 ± 0.01 | ≥100 | Preclinical | 82 |
| Fig. 5 (23) | 23 | In vitro | 0.72 ± 0.09 | ≥100 | Preclinical | 82 |
| Fig. 5 (24) | 24 | In vitro | 4–5 | – | Preclinical | 83 |
| Fig. 5 (25) | GC373 | In vitro | 1.5 | ≥200 | Preclinical | 84 |
| Fig. 5 (26) | GC376 | In vitro | 0.92 | ≥200 | Preclinical | 84 |
| In vitro | 3.37 ± 1.68 | ≥100 | Preclinical | 86 | ||
| In vitro | 0.70 | ≥200 | Preclinical | 87 | ||
| In vitro | 2.189 ± 0.092 | ≥100 | Preclinical | 85 | ||
| Fig. 5 (27) | 27 | In vitro | 2.883 ± 0.227 | ≥100 | Preclinical | 85 |
| Fig. 5 (28) | Boceprevir | In vitro | 1.31 ± 0.58 | ≥100 | Preclinical | 86 |
| In vitro | 15.57 | ≥200 | Preclinical | 87 | ||
| In vitro | 50.1 | >10 | Preclinical | 94 | ||
| Fig. 5 (29) | Calpain inhibitors II | In vitro | 2.07 ± 0.76 | ≥100 | Preclinical | 86 |
| Fig. 5 (30) | Calpain inhibitors XII | In vitro | 0.49 ± 0.18 | ≥100 | Preclinical | 86 |
| Fig. 5 (31) | Bepridil | In vitro | 0.86 | >25 | Preclinical | 92 |
| Fig. 5 (32) | MPI5 | In vitro | 2.5–5 | – | Preclinical | 93 |
| Fig. 5 (33) | MPI8 | In vitro | 1.25–2.5 | – | Preclinical | 93 |
| Fig. 5 (34) | Nelfinavir mesylate | In vivo | 3.3 | 12.3 | Preclinical | 94 |
| In vitro | 1.13 | 24.32 | Preclinical | 152 | ||
| Fig. 5 (35) | Z-FA-FMK | In vitro | 0.13 | ≥50 | Preclinical | 95 |
| Fig. 5 (36) | DA-3003-1 | In vitro | 4.47 | 7.74 | Preclinical | 95 |
| Fig. 5 (37) | MG-115 | In vitro | 0.023 | 1.13 | Preclinical | 95 |
| Fig. 5 (38) | MK0893 | In vitro | 3.16 | 12.59 | Preclinical | 95 |
| Fig. 5 (39) | Suramin | In vitro | ~20 | ≥5000 | Preclinical | 96 |
| Fig. 5 (40) | Lopinavir | In vitro | 26.63 | 49.75 | Phase 2 (NCT04276688, NCT04315948) | 99,104,124 |
| Fig. 5 (41) | Ritonavir | In vitro | ≥100 | 48.91 | Phase 2 NCT04276688 |
99,104 |
| In vitro | 8.63 | 74.11 | 152 | |||
| Fig. 5 (42) | Dipyridamole (DIP) | In vitro | 0.1 | – | Preclinical | 106 |
| Fig. 5 (43) | GRL-0920 | In vitro | 2.8 | ≥100 | Preclinical | 107 |
| Fig. 5 (44) | MI-09 | In vivo | 0.86 | – | Preclinical | 108 |
| Fig. 5 (45) | MI-30 | In vivo | 0.54 | – | Preclinical | 108 |
| Target PLpro | ||||||
| Fig. 5 (46) | Dasatinib | – | – | – | Clinical cases | 114 |
| Fig. 5 (47) | Dronedarone | In vitro | 4.5 | 12.1 | Preclinical | 94 |
| Fig. 5 (48) | 48 | In vitro | 21.0 | ≥80 | Preclinical | 109 |
| Fig. 5 (49) | GRL-0617 | In vitro | 2.1 | ≥100 | Preclinical | 117 |
| Target RdRp | ||||||
| Fig. 5 (50) | Remdesivir | In vivo | 0.77 | ≥100 | Phase 4 (NCT04252664, NCT04257656, NCT04315948, | 19,124,119,125 |
| Fig. 5 (51) | Favipiravir | In vitro | 61.88 | >400 | ChiCTR2000029600 ChiCTR2000030254 |
19 |
| Fig. 5 (52) | β-d-N4-Hydroxycytidine (EIDD-1931) | In vitro | 0.3 | >10 | Preclinical | 120 |
| Fig. 5 (53) | Dolutegravir | In vitro | 22.04 | ≥40 | Preclinical | 146 |
| Other small-molecule inhibitor | ||||||
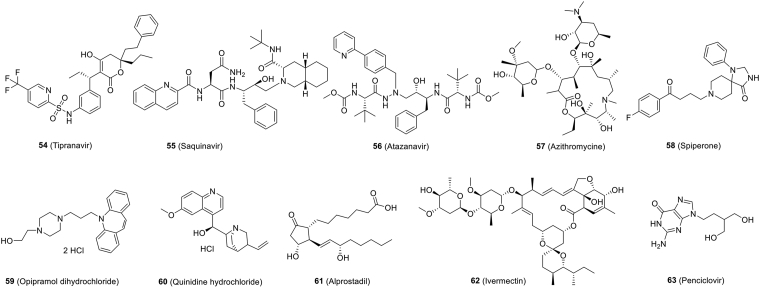
| Fig. 6 (54) | Tipranavir | In vitro | 13.34 | 76.80 | Preclinical | 152 |
| Fig. 6 (55) | Saquinavir | In vitro | 8.83 | 44.43 | Preclinical | 152 |
| Fig. 6 (56) | Atazanavir | In vitro | 9.36 | >81 | Preclinical | 152 |
| Fig. 6 (57) | Azithromycin | In vitro | 2.12 | >40 | Phase 2 NCT04329832 |
146 |
| Fig. 6 (58) | Spiperone | In vitro | 2.49 | >40 | Preclinical | 146 |
| Fig. 6 (59) | Opipramol dihydrochloride | In vitro | 5.05 | >40 | Preclinical | 146 |
| Fig. 6 (60) | Quinidine hydrochloride | In vitro | 5.11 | >40 | Preclinical | 146 |
| Fig. 6 (61) | Alprostadil | In vitro | 5.39 | >40 | Preclinical | 146 |
| Fig. 6 (62) | Ivermectin | In vitro | ~2 | – | Preclinical | 157 |
| In vitro | 4.1 | 13.2 | Preclinical | 94 | ||
| Fig. 6 (63) | Penciclovir | In vitro | 95.96 | >400 | Preclinical | 19 |
Table 3.
Small-molecule SARS-CoV-2 inhibitors targeting host cell proteins.
| Number of chemical structures in Fig. | Inhibitor | Testing model | Activity IC50 (μmol/L) | Toxicity CC50 (μmol/L) | Clinical information | Ref. |
|---|---|---|---|---|---|---|
| Target ACE2 | ||||||
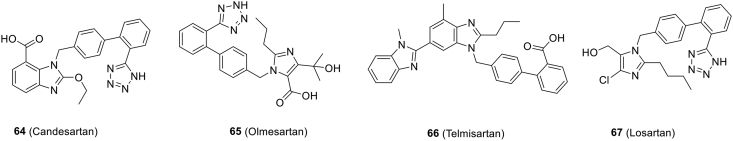
| Fig. 7 (64) | Candesartan | In vitro | – | – | Preclinical | 160 |
| Fig. 7 (65) | Olmesartan | In vitro | – | – | Preclinical | 159 |
| Fig. 7 (66) | Telmisartan | – | – | – | Phase 3 NCT04356495 |
|
| Fig. 7 (67) | Losartan | – | – | – | Phase 1 (NCT04312009, NCT04311177, NCT04328012, NCT04335123) | 162, 163, 164 |
| Target TMPRSS2 | ||||||
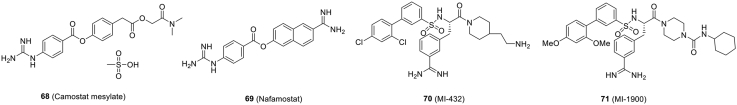
| Fig. 8 (68) | Camostat mesylate | In vitro (pseudovirus) | ~1 | ≥500 | Phase 2a/4 (NCT04321096, NCT04338906) | 10 |
| Fig. 8 (69) | Nafamostat | In vitro | 22.50 | ≥100 | NCT04352400 | 19 |
| 0.01 | – | 177 | ||||
| Fig. 8 (70) | MI-432 | In vitro | ≤10 | ≥50 | Preclinical | 180 |
| Fig. 8 (71) | MI-1900 | In vitro | ≤50 | ≥50 | Preclinical | 180 |
| Target cathepsin B/L | ||||||
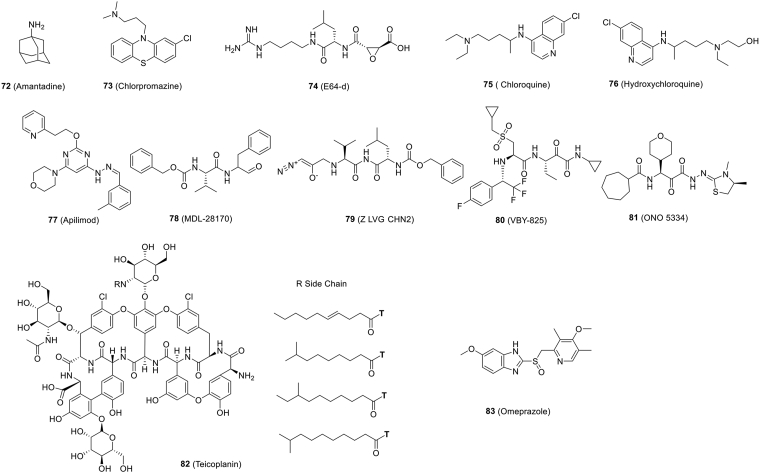
| Fig. 9 (72) | Amantadine | In vitro | ≥100 | ≥100 | Preclinical | 187 |
| Fig. 9 (73) | Chlorpromazine | In vitro | ≥100 | ≥100 | Preclinical | 187 |
| Fig. 9 (74) | E64-d | In vitro | ~4.487 | ≥0 | Preclinical | 187 |
| Fig. 9 (75) | Chloroquine | In vitro | 2.71 | 273.20 | Several clinical trials (ChiCTR2000029609 et al.) | 97,188,189 |
| In vitro | 2.01 | >30 | 212 | |||
| Fig. 9 (76) | Hydroxychloroquine | In vivo (macaques) | 4.51 | 249.50 | Several clinical trials (ChiCTR2000029803, ChiCTR2000029868, ChiCTR2000029898, ChiCTR2000029899, ChiCTR2000029992, ChiCTR2000030054, NCT04315948) | 124,146,188,189,191 |
| In vitro | 4.47 | >30 | 212 | |||
| Fig. 9 (77) | Apilimod | In vitro | 0.023 | – | Preclinical | 68 |
| In vitro | ~0.01 | – | Preclinical | 197 | ||
| Fig. 9 (78) | MDL-28170 | In vitro | 0.22 | – | Preclinical | 68 |
| Fig. 9 (79) | Z LVG CHN2 | In vitro | 0.19 | – | Preclinical | 68 |
| Fig. 9 (80) | VBY-825 | In vitro | 0.3 | – | Preclinical | 68 |
| Fig. 9 (81) | ONO 5334 | In vitro | 0.41 | – | Preclinical | 68 |
| Fig. 9 (82) | Teicoplanin | In vitro | 1.66 | – | Preclinical | 201 |
| Fig. 9 (83) | Omeprazole | In vitro | 17.06 | ≥40 | Preclinical | 146 |
| Dual inhibitors: Target cathepsin L and 3CLpro | ||||||
| Fig. 5 (29) | Calpain inhibitors II | In vitro | 2.07 ± 0.76 | ≥100 | Preclinical | 86,88 |
| Fig. 5 (30) | Calpain inhibitors XII | In vitro | 0.49 ± 0.18 | ≥100 | Preclinical | 86,88 |
| Other small-molecule inhibitor | ||||||
| Fig. 10 (84) | Thalidomide | – | – | – | Phase 2 (NCT04273581, NCT04273529) | 205 |
| Fig. 10 (85) | Fingolimod | – | – | – | NCT04280588 | 205,207 |
| Fig. 10 (86) | Teriflunomide | In vitro | 6 | – | Small patient cohort | 208 |
| In vitro | 26.06 | 850.5 | 210 | |||
| Fig. 10 (87) | Leflunomide | In vitro | 41.49 | 879.0 | Preclinical | 210 |
| Fig. 10 (88) | Brequinar | In vitro | 0.123 | 231.3 | Preclinical | 210 |
| Fig. 10 (89) | S312 | In vitro | 1.56 | 158.2 | Preclinical | 210 |
| Fig. 10 (90) | S416 | In vitro | 0.017 | 178.6 | Preclinical | 210 |
| Fig. 10 (91) | ROC-325 | In vitro | 3.28 ± 0.57 | >30 | Preclinical | 212 |
| Fig. 10 (92) | Clomipramine | In vitro | 13.6 ± 2.96 | >30 | Preclinical | 212 |
| Fig. 10 (93) | Hycanthone | In vitro | 5.79 ± 0.26 | 14.2 | Preclinical | 212 |
| Fig. 10 (94) | Mefloquine | In vitro | 3.85 ± 0.24 | 8.78 | Preclinical | 212 |
| In vitro | 8.06 | 18.53 | Preclinical | 244 | ||
| In vivo | 3.2 | ≥10 | Preclinical | 94 | ||
| Fig. 10 (95) | Dithioerythritol thiosulfonate 16 | In vitro | 50 | ≥500 | Preclinical | 214 |
| GNS561 | In vitro | 0.006 for USA-WA1/2020; 0.03 for IHU MI6 | 2.0 for USA-WA1/2020; 6.7 for IHU MI6 | Preclinical | 215 | |
| Fig. 10 (96) | VPS34-IN1 | In vitro | 0.55 | ≥50 | Preclinical | 218 |
| Fig. 10 (97) | PIK-III | In vitro | 0.12 | ≥50 | Preclinical | 218 |
| Fig. 10 (98) | Orlistat | In vitro | 21.25 | ≥1000 | Preclinical | 218 |
| Fig. 10 (99) | Triacsin C | In vitro | 0.04 | ≥50 | Preclinical | 218 |
| Fig. 10 (100) | MI-1851 | In vitro | ≤10 | ≥50 | Preclinical | 180 |
| Fig. 10 (101) | Decanoyl-RVKR-chloromethylketone (dec-RVKR-cmk) | In vitro | 0.057 | 318.2 | Preclinical | 221 |
| Fig. 10 (102) | Homoharringtonine | In vitro | 2.55 | 59.75 | Preclinical | 99 |
| Fig. 10 (103) | Emetine | In vitro | 0.46 | 56.46 | Preclinical | 99 |
| In vitro | 0.0004 | >10 | Preclinical | 94 | ||
| Fig. 10 (104) | 2-Deoxy-d-glucose (2-DG) | In vitro | 9090 | – | Preclinical | 228,229 |
| Fig. 10 (105) | Pladienolide B | In vitro | 0.007 | – | Preclinical | 228 |
| Fig. 10 (106) | Ribavirin | In vitro | 70 | – | NCT04356677 | 228 |
| Fig. 10 (107) | NMS-873 | In vitro | 0.025 | – | Preclinical | 140,228 |
| Fig. 10 (108) | Cycloheximide | In vitro | 0.17 | – | Preclinical | 228 |
| Fig. 10 (109) | Baricitinib | – | – | – | Phase 2,3,4 (2020-001854-23, 2020-001354-22, NCT04358614) | 232 |
| Fig. 10 (110) | Nitazoxanide | In vitro | 2.12 | >35.53 | Phase 2, 3 (NCT04341493, NCT01056380, NCT04348409) | 19,238 |
| In vitro | 4.90 | >300 | 237 | |||
| Fig. 10 (111) | JIB-04 | In vitro | 0.695 | >300 | Preclinical | 237 |
| Fig. 10 (112) | Fenofibrate | In vitro | 20 | >100 | Preclinical | 187 |
| Fig. 10 (113) | Plitidepsin | In vitro | 0.70 nmol/L for Vero E6; 0.73 nmol/L for hACE2-293 T; 1.62 nmol/L for human lung cells | 1.99 nmol/L in Vero E6; ≥200 nmol/L for hACE2-293 T; 65.43 nmol/L for human lung cells | Phase 1/2 (NCT04382066) | 187,239 |
| Fig. 10 (114) | Clemizole hydrochloride | In vitro | 23.94 | ≥40 | Preclinical | 146 |
| Fig. 10 (115) | Benztropine mesylate | In vitro | 17.79 | >>50 | Preclinical | 244 |
| In vitro | 1.8 | – | Preclinical | 78 | ||
| Fig. 10 (116) | Fluphenazine dihydrochloride | In vitro | 8.98 | 20.02 | Preclinical | 244 |
| Fig. 10 (117) | Amodiaquine hydrochloride | In vitro | 5.64 | >38.63 | Preclinical | 244 |
| Fig. 10 (118) | Amodiaquine dihydrochloride dihydrate | In vitro | 4.94 | 34.42 | Preclinical | 244 |
| Fig. 10 (119) | Thiethylperazine maleate | In vitro | 8.02 | 18.37 | Preclinical | 244 |
| Fig. 10 (120) | Triparanol | In vitro | 6.41 | 21.21 | Preclinical | 244 |
| Fig. 10 (121) | Terconazole | In vitro | 16.14 | 41.46 | Preclinical | 244 |
| Fig. 10 (122) | Fluspirilene | In vitro | 5.32 | 30.33 | Preclinical | 244 |
| Fig. 10 (123) | Clomipramine hydrochloride | In vitro | 7.59 | >29.68 | Preclinical | 244 |
| Fig. 10 (124) | Promethazine hydrochloride | In vitro | 10.44 | >42.59 | Preclinical | 244 |
| Fig. 10 (125) | Toremifene citrate | In vitro | 11.3 | 20.51 | Preclinical | 244 |
| Fig. 10 (126) | Tamoxifen citrate | In vitro | 8.98 | 37.96 | Preclinical | 244 |
| Fig. 10 (127) | Imatinib mesylate | In vitro | 5.32 | >30.86 | Preclinical | 244 |
| In vivo (humanized mice carrying hPSC-derived lung xenografts) | 2.15 | – | Preclinical | 27 | ||
| Fig. 10 (128) | Bruceine A | In vitro | 0.011 | 31.4 | Preclinical | 246 |
| Fig. 10 (129) | Bufalin | In vitro | 0.018 | >40 | Preclinical | 246 |
| Fig. 10 (130) | Cinobufagin | In vitro | 0.018 | >40 | Preclinical | 246 |
| Fig. 10 (131) | Bufotaline | In vitro | 0.0259 | >40 | Preclinical | 246 |
| Fig. 10 (132) | Periplocoside | In vitro | 0.0657 | >40 | Preclinical | 246 |
| Fig. 10 (133) | Brusatol | In vitro | 0.0492 | 19 | Preclinical | 246 |
| Fig. 10 (134) | Digoxin | In vitro | 0.1541 | >40 | Preclinical | 246 |
| Fig. 10 (135) | Veratridine | In vitro | 2.376 | >100 | Preclinical | 246 |
| Fig. 10 (136) | Oridonin | In vitro | 1.462 | >40 | Preclinical | 246 |
| Fig. 10 (137) | Isoalantolactone | In vitro | 1.483 | >40 | Preclinical | 246 |
| Fig. 10 (138) | Isoliensinine | In vitro | 1.615 | 40 | Preclinical | 246 |
| Fig. 10 (139) | Alantolactone | In vitro | 1.724 | 36.7 | Preclinical | 246 |
| Fig. 10 (140) | Cryptotanshinone | In vitro | 5.024 | >100 | Preclinical | 246 |
| Fig. 10 (141) | Dehydrocostus lactone | In vitro | 2.322 | 36.2 | Preclinical | 246 |
| Fig. 10 (142) | Momordinic | In vitro | 3.529 | >40 | Preclinical | 246 |
| Fig. 10 (143) | Liensinine | In vitro | 2.537 | 25.4 | Preclinical | 246 |
| Fig. 10 (144) | Dehydrodiisoeugenol | In vitro | 10.29 | ≥100 | Preclinical | 246 |
| Fig. 10 (145) | Cornuside | In vitro | 5.262 | ≥40 | Preclinical | 246 |
| Fig. 10 (146) | Roburicacid | In vitro | 5.267 | ≥40 | Preclinical | 246 |
| Fig. 10 (147) | Coniferylaldehyde | In vitro | 11.03 | ≥40 | Preclinical | 246 |
| Fig. 10 (148) | Panduratin A | In vitro | 0.81 | 14.71 | Preclinical | 247 |
| Fig. 10 (149) | Thioguanine | In vitro | 1.7 | 25.4 | Preclinical | 94 |
| Fig. 10 (150) | Moxidectin | In vitro | 3.1 | 6.9 | Preclinical | 94 |
| Fig. 10 (151) | Ivacaftor | In vitro | 3.7 | 12.9 | Preclinical | 94 |
| Fig. 10 (152) | Azelnidipine | In vitro | 5.3 | 12.9 | Preclinical | 94 |
| Fig. 10 (153) | Penfluridol | In vitro | 2.4 | 12.9 | Preclinical | 94 |
| Fig. 10 (154) | Salinomycin | In vitro | 0.00048 | 13.1 | Preclinical | 94 |
| Fig. 10 (155) | Monensin | In vitro | 6.4 | 6.6 | Preclinical | 94 |
| Fig. 10 (156) | Maduramicin | In vitro | 1.3 | 3.4 | Preclinical | 94 |
| Fig. 10 (157) | Tilorone | In vitro | 0.18 | – | Preclinical | 248 |
| Fig. 10 (158) | Pyronaridine | In vitro | 0.198 | – | Preclinical | 248 |
| Fig. 10 (159) | Mycophenolic acid | In vivo (humanized mice carrying hPSC-derived lung xenografts) | 0.9 | – | Preclinical | 27 |
| Fig. 10 (160) | Quinacrine dihydrochloride | In vivo (humanized mice carrying hPSC-derived lung xenografts) | 0.84 | – | Preclinical | 27 |
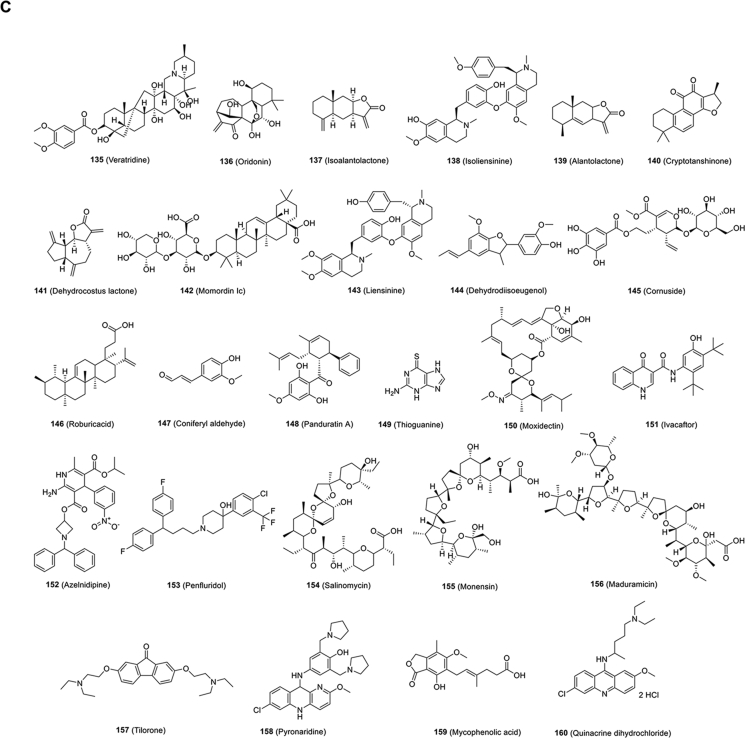
4. Strategies for developing small-molecule SARS-CoV-2 inhibitors
Based on individual approaches used for the discovery of anti-SARS-CoV-2 agents, strategies can be divided into three major categories. The first one is virtual screening. Cryo-electron microscopy, X-ray crystallography, as well as homology modeling, could provide various efficacious protein structures, promoting molecular docking for rapid identification of hit or lead compounds through screening free or commercially accessible databases, such as ZINC, DrugBank, or ChemDiv. This approach is effective, economical, and time-saving. The second approach is the experiment-based high-throughput screening (HTS). Similar to virtual screening, the first objective of HTS is to identify active small molecules within compound libraries, including approved drugs, clinical trial drug candidates and even in-house compound databases. This method, which screens a large number of compounds against a known drug target, is also a time-consuming and expensive process with no guarantee of success. The third strategy is to repurpose the application of clinical and preclinical drugs. Given the available knowledge on their safety profiles, these methods could be easily implemented to rapidly identify effective drugs for use in clinics or clinical trials to treat patients based on emergency use authorization.
Other methods of computer-aided drug design are used much less frequently than virtual screening. Structure- and fragment-based drug designs are iterative processes for designing new small-molecule inhibitors. For example, molecular docking, de novo drug design, pharmacophore modeling and quantitative structure–activity relationship models are commonly used for guiding inhibitor screening and optimization. Compared with repurposing old drugs for new indications, other strategies mentioned above are generally limited by long processing time and high cost, making them unsuited for the development of drugs for emergency use, such as COVID-19. However, we propose that computer-aided, small-molecule drug discovery will be one of the most important strategies for research and development of COVD-19 therapeutics and prophylactics in the future.
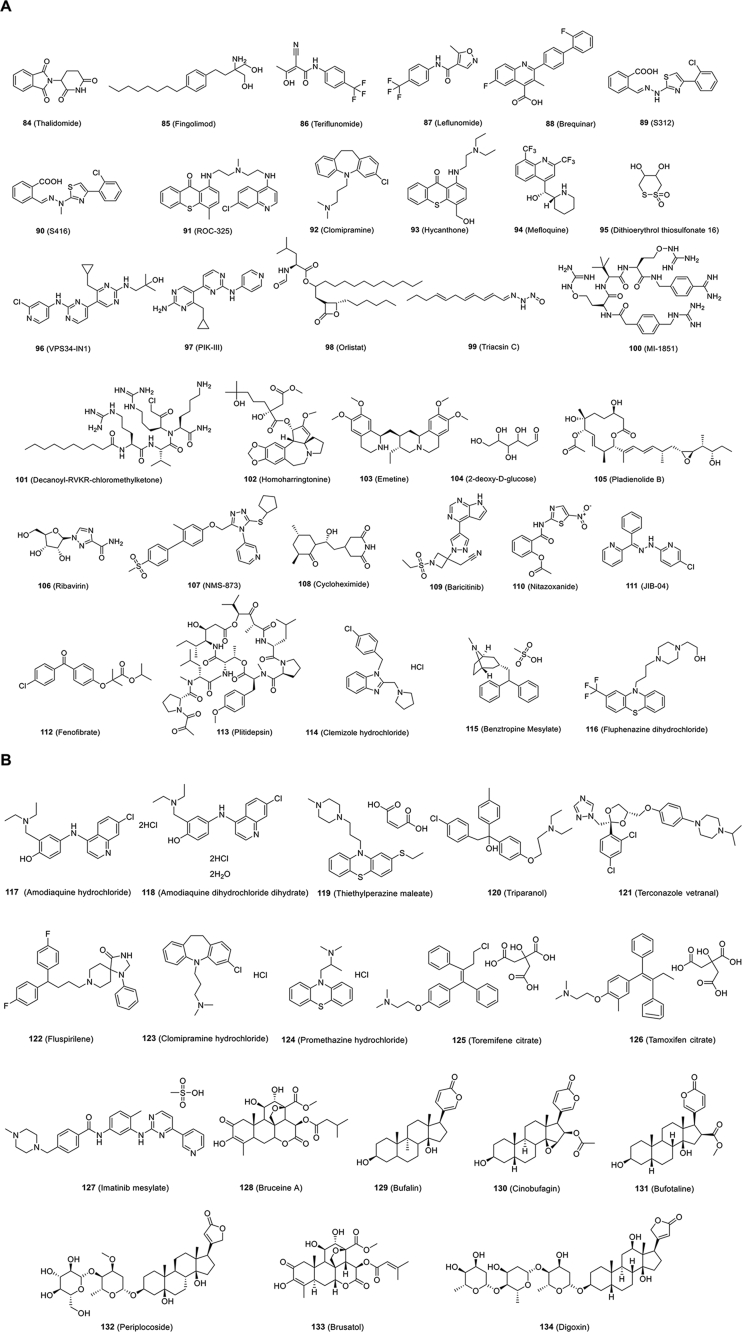
The development of antiviral agents against SARS-CoV-2 infection calls for the use of human disease-related cells to create novel models to study the biological characteristics of SARS-CoV-2 and to promote drug screening. Given that SARS-CoV-2 mainly infects the respiratory tract, researchers have used human pluripotent stem cells (hPSCs) to develop a lung organoid model (hPSC-LO) for SARS-CoV-2 infection27. hPSC-LOs, especially alveolar type II-like cells, could be easily infected by SARS-CoV-2, displaying strong induction of chemokines upon SARS-CoV-2 infection, consistent with the phenomenon observed in COVID-19 patients. At the same time, as a supplement to hPSC-LOs, these researchers also used hPSCs to construct colon organoids (hPSC-COs) to explore the response of colon cells to SARS-CoV-2 infection. Using hPSC-LOs, they conducted HTS of drugs approved by FDA and identified several SARS-CoV-2 entry inhibitors, including imatinib mesylate [Fig. 10B (127)], mycophenolic acid and quinacrine dihydrochloride [Fig. 10C (159, 160). These three drugs were proven to be blockers of SARS-CoV-2 infection in a toxicity-independent manner in Vero E6 cells with half maximal inhibitory concentrations (IC50s) of 2.15, 0.9 and 0.84 μmol/L, respectively (Table 3)27. Taken together, these data demonstrate that cell disease models provide valuable tools for drug screening to identify COVID-19 therapeutic candidates.
Figure 10.
Chemical structures of other small-molecule inhibitors targeting host.
Traditional Chinese medicine (TCM) has been widely applied in clinics in China for COVID-19 patients. Ni et al.28 have reported that after three patients with COVID-19 treated with Shuanghuanglian Oral Liquid, their symptoms improved, and the patients finally recovered without any adverse reactions. In addition, Lianhuaqingwen was proven to be effective in inhibiting SARS-CoV-2 infection in Vero E6 cells and attenuating the production of proinflammatory cytokines, suggesting that Lianhuaqingwen may have a potential inhibitory effect on the cytokine storm induced by SARS-CoV-2 infection29. From the above experience, researchers should also make some headway to screen and develop promising TCM compounds or extracts as efficacious COVID-19 therapeutics.
Some researchers have provided a novel drug discovery strategy to manage COVID-19 by systematically studying the molecular details of SARS-CoV-230. They first successfully cloned, labeled, and expressed 26 of 29 viral proteins in human cells, and then applied affinity purification mass spectrometry (AP-MS) for identification of human proteins, which could physically interact with each viral protein. In the end, they identified 332 high-confidence SARS-CoV-2-human protein–protein interactions. Sixty-six druggable human proteins, or host factors targeted by 69 existing FDA-approved drugs, are reported. The efficacy in live SARS-CoV-2 infection assays of 69 compounds is currently being evaluated30. Host-dependent factors that mediate viral infection may become effective molecular targets for the development of a broadly effective antiviral therapy against SARS-CoV-2 infection.
5. Small-molecule SARS-CoV-2 inhibitors targeting viral proteins
5.1. Entry inhibitors
Like SARS-CoV, SARS-CoV-2 uses a glycosylated, homotrimeric class I fusion S protein to gain entry into host cells31, 32, 33. The SARS-CoV-2 S gene denotes the functional components: signal peptide (SP) and receptor-binding domain (RBD) in the S1 subunit and fusion peptide (FP), heptad repeat 1 (HR1), heptad repeat 2 (HR2), transmembrane (TM) and cytoplasm (CP) in the S2 subunit (Fig. 3A). Class I fusion proteins catalyze membrane fusion reaction through a sequence of states: (1) native state, (2) prefusion state, (3) prehairpin intermediate state, (4) hemifusion state, and (5) postfusion state (Fig. 3B). The S protein is comprised of S1 and S2 subunits and exists in a metastable prefusion conformation. Binding between the RBD of S1 and the receptor ACE2 triggers a conformational change of the S2 subunit, which destabilizes the prefusion trimer, and this results in shedding of the S1 subunit and activating the fusogenic activity of the S2 subunit34, 35, 36. During the fusion process, the FP is exposed and inserts into the host cell membrane, triggering the transient formation of a prehairpin intermediate that bridges the viral and cell membranes. Then, the HR1 and HR2 associate with each other to form a six-helix bundle (6-HB), drawing both viral and target cell membranes into close proximity in a manner that results in fusion between the viral and host cell membranes15.
Figure 3.
Structural regions and fusion mechanism of SARS-CoV-2 S protein. (A) The functional regions in SARS-CoV-2 S protein include SP (signal peptide, light yellow), RBD (receptor-binding domain; light green), FP (fusion peptide; light blue), HR1 (heptad repeat 1; gray-blue), HR2 (heptad repeat 2; flesh), TM (transmembrane; grass green), and CP (cytoplasmic; purple). (B) SARS-CoV-2 S protein fusion pathway base on class I fusion protein. The S protein starts in the native state and undergoes priming of the S1 subunit by relevant proteases to achieve the prefusion state. Subsequent triggering by relevant proteases will enable the FP to insert in the host membrane and allow the S protein to form the prehairpin intermediate. The prehairpin begins to fold back on itself due to HR1 and HR2 interactions forming the 6-HB, and eventual postfusion stable states. During the S protein foldback, the two membranes will approach each other until the outer leaflets merge (hemifusion) and eventually the inner leaflets merge.
5.1.1. Peptides
Peptides derived from the HR1 and HR2 domains in the class I viral fusion protein block the viral 6-HB formation by binding to the pre-hairpin intermediate, thus showing antiviral activity37. This activity has been reported for emerging CoVs, including SARS-CoV and MERS-CoV35,38, 39, 40. In response to the outbreak of SARS-CoV, a group of HR2-based peptides that could effectively inhibit viral infection were developed35,40, 41, 42, 43. A pan-CoV fusion inhibitor, designated EK1, was designed, which could inhibit the fusion of diverse HCoVs, including SARS-CoV, MERS-CoV, HCoV-229 E, HCoV-NL63, and HCoV-OC4344. More recent studies showed that EK1 is an effective peptide inhibitor against SARS-CoV-2 S protein-mediated membrane fusion and pseudovirus infection in a dose-dependent manner (Table 1)45. Recent studies have shown that conjugation of a lipid group to a peptide is a feasible strategy to enhance the antiviral activity and in vivo stability of the lipopeptide viral fusion inhibitor46, 47, 48. The lipopeptide EK1C4 derived from EK1 was found to be an effective inhibitor against S protein-mediated membrane fusion and pseudotyped SARS-CoV-2 infection, with IC50s of 1.3 and 15.8 nmol/L, respectively, which are about 241- and 149-fold more potent than that of the unmodified peptide EK1, respectively (Table 1)45. Similarly, Zhu et al.49 designed an HR2 sequence-based lipopeptide fusion inhibitor, termed IPB02, which exhibited highly potent activity in inhibiting SARS-CoV-2 S protein-mediated cell–cell fusion and pseudovirus infection (Table 1).
Table 1.
Summary of peptide-based SARS-CoV-2 inhibitors.
| Peptide | Sequence | Testing model | Activity IC50 (μmol/L) | Toxicity CC50 (μmol/L) | Clinical information | Ref. |
|---|---|---|---|---|---|---|
| EK1 | SLDQINVTFLDLEYEMKKLEEAIKKLEESYIDLKEL | In vitro | 2.468 | – | Preclinical | 45 |
| EK1C4 | SLDQINVTFLDLEYEMKKLEEAIKKLEESYIDLKELGSGSG-PEG4 (cholesterol) | In vitro | 0.0365 | ≥5 | Preclinical | 45 |
| IPB02 | ISGINASVVNIQKEIDRLNEVAKNLNESLIDLQELK (cholesterol) | In vitro (pseudovirus) | 0.08 ± 0.017 | – | Preclinical | 49 |
| SARS-CoV-2-HR2P (aa 1168–1203) | DISGINASVVNIQKEIDRLNEVAKNLNESLIDLQEL | In vitro (pseudovirus) | 0.98 | – | Preclinical | 50 |
| [SARSHRC-PEG4]2-chol | [DISGINASWNIQKEIDRLNEVAKNLNESLIDLQEL-PEG4]2-chol | In vivo | ~0.005 | >100 | Preclinical | 51 |
| SBP1 | IEEQAKTFLDKFNHEAEDLFYQS | – | – | – | Preclinical | 52 |
| AHB1 | DEDLEELERLYRKAEEVAKEAKDASRRGDDERAKEQMERAMRLFDQVFELAQELQEKQTDGNRQKATHLDKAVKEAADELYQRVRELEEQVMHVLDQVSELAHELLHKLTGEELERAAYFNWWATEMMLELIKSDDEREIREIEEEARRILEHLEELARK | In vitro | 0.035 | – | Preclinical | 53 |
| AHB2 | ELEEQVMHVLDQVSELAHELLHKLTGEELERAAYFNWWATEMMLELIKSDDEREIREIEEEARRILEHLEELARK | In vitro | 0.016 | – | Preclinical | 53 |
| LCB1 | DKEWILQKIYEIMRLLDELGHAEASMRVSDLIYEFMKKGDERLLEEAERLLEEVER | In vitro | 0.000024 | – | Preclinical | 53 |
| LCB3 | NDDELHMLMTDLVYEALHFAKDEEIKKRVFQLFELADKAYKNNDRQKLEKVVEELKELLERLLS | In vitro | 0.000048 | – | Preclinical | 53 |
| ATN-161 | Ac-PHSCN-NH2 | In vitro | 3.16 | >1000 | Preclinical | 54 |
| SARS-BLOCK Peptide 5 | Unknown | In vitro (pseudovirus) | Sub-micromolar | <20 | Preclinical | 57 |
| P9 | NGAICWGPCPTAFRQIGNCGHFKVRCCKIR | In vitro | 2.4 μg/mL | – | Preclinical | 184 |
| P9R | NGAICWGPCPTAFRQIGNCGRFRVRCCRIR | In vitro | 0.9 μg/mL | ≥300 μg/mL | Preclinical | 184 |
| 8P9R | (NGAICWGPCPTAFRQIGNCGRFRVRCCRIR)∗8 | In vivo | 0.3 μg/mL | ≥200 μg/mL | Preclinical | 185 |
The sequence alignment has shown that the S2 subunits of SARS-CoV-2 and SARS-CoV are highly conserved, and the overall identity of the HR1 and HR2 domains is 92.6% and 100%, respectively, while in the HR1 core region, eight of 21 residues showed mutations (about 38% difference)50. Therefore, it is necessary to design fusion inhibitory peptides based on the amino acid sequences of SARS-CoV-2 HR1 and HR2. Unlike EK1, SARS-CoV-2-HR1P (aa924‒965) and SARS-CoV-2-HR2P (aa1168‒1203) are derived from the HR1 and HR2 of SARS-CoV-250. Results reveal that SARS-CoV-2-HR2P showed potent fusion–inhibitory activity with an IC50 of 0.18 μmol/L, whereas SARS-CoV-2-HR1P exhibited no significant inhibition at concentrations up to 40 μmol/L (Table 1)50.
de Vries et al.51 designed a dimeric lipopeptide fusion inhibitor, [SARSHRC-PEG4]2-chol, varying from SARS-CoV-2-HRP2 only in a single amino acid50. [SARSHRC-PEG4]2-chol inhibited SARS-CoV-2 entry with an IC50 of ~5 nmol/L in TMPRSS2-positive Vero E6 cells. While toxicity of [SARSHRC-PEG4]2-chol in human airway epithelium was minimal, even at the high concentrations tested (< 20% at 100 μmol/L, Table 1). It is worth noting that daily intranasal administration to SARS-CoV-2 ferrets can completely prevent SARS-CoV-2 direct-contact transmission51.
Peptides to disrupt SARS-CoV-2-RBD binding to ACE2 can also inhibit the virus which target the stage of viral attachment to prevent entry to host cells, a new modality for COVID-19 therapeutic intervention. For example, SBP1 (a 23-mer peptide fragment) consisting of amino acids in the α1 helix of the ACE2 peptidase domain (PD) was synthesized. The results of bio-layer interferometry revealed that SBP1 could specifically bind with SARS-CoV-2-RBD in low nanomolar concentration (Table 1)52, and block the interaction between SARS-CoV-2 S protein and ACE2, thereby preventing the virus from entering host cells and providing a new treatment and diagnostic strategy against COVID-1952.
To inhibit the viral attachment between S protein and ACE2, the peptides designed by Cao et al.53 using two de novo design approaches, also known as minibinders, were either built around an ACE2 helix or based on RBD-binding motifs. AHB1 and AHB2 (Table 1), followed the first approach, exhibited strongly neutralization SARS-CoV-2 with IC50s of 35 and 15.5 nmol/L, respectively. Using the second approach, LCB1 and LCB3 neutralized SARS-CoV-2 with IC50s of 23.54 and 48.1 pmol/L, respectively (Table 1). These hyperstable minibinders provide new approaches for SARS-CoV-2 therapeutics.
Beddingfield and colleagues identified ATN-161, the fibronectin-derived anticancer peptide, that inhibited SARS-CoV-2 attachment through a hypothesized α5β1 integrin-based mechanism and indicated that ATN-161 could reduce SARS-CoV-2 infection with an IC50 of 3.16 μmol/L54 (Table 1). Integrins have been shown to bind to ACE2 and SARS-CoV-2 S protein55,56. The results of Beddingfield et al.54 suggest that inhibiting S protein interaction with α5β1 integrin and the interaction between α5β1 integrin and ACE2 using ATN-161 represents a promising approach to treat COVID-19.
Watson et al.57 designed peptides, SARS-BLOCK™, by mimicking the SARS-CoV-2 RBD that target the stage of viral attachment. They designed, simulated, synthesized, modeled epitopes, predicted peptide folding, and characterized behavior of synthetic peptides. Among of them, peptides 1, 4, 5 and 6 blocked SARS-CoV-2 pseudotyped virus infection in ACE2-HEK293 cells. And peptide 5 showed inhibitory activity with an IC50 in the sub-micromolar concentrations and an IC95 of ~2.22 μmol/L (Table 1).
5.1.2. Small-molecule compounds
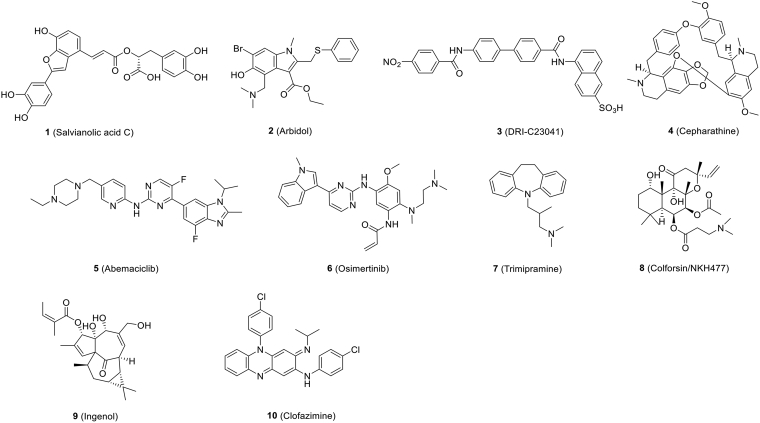
The SARS-CoV-2 S protein plays a key role in recognizing receptor and mediating virus-cell membrane fusion showing itself to be an efficient mediator of viral entry. The S protein is not only an important binding site for neutralizing antibodies, but it is also a major target for therapeutic drug development. Yang et al.58 report that salvianolic acid C [Sal-C, Fig. 4 (1)], a hydrophilic compound from Danshen, a TCM, potent to inhibit SARS-CoV-2 infection in blocking the formation of 6-HB core of S protein. And Sal-C exhibited potent antiviral activity against authentic SARS-CoV-2 with an IC50 of 3.41 μmol/L (Table 2)58. Their study advances a potential use of Sal-C for COVID-19 therapy or prophylaxis and provides a basis for the development of fusion inhibitors against SARS-CoV-2 infection.
Figure 4.
Chemical structures of small-molecule inhibitors that inhibit SARS-CoV-2 entry.
Arbidol [umifenpvor, Fig. 4 (2)], an anti-influenza drug approved in China and Russia, targets the SARS-CoV-2 S protein to impede S protein-mediated membrane fusion and, hence, the entry of virus into host cells59. It showed satisfactory activity against SARS-CoV-2 in vitro60. IC50 and the 50% cytotoxic concentration (CC50) of Arbidol in cell-based assays were 4.11 and 31.79 μmol/L, respectively, and the selectivity index (SI = CC50/IC50) was 7.73 (Table 2)60. Several clinical trials were evaluated for the treatment of COVID-19, including arbidol monotherapy and arbidol combined with lopinavir/ritonavir61,62. According to clinical trials, post-exposure prophylaxis using arbidol could reduce infection exposed to confirmed cases of COVID-1963. Compared to a supportive care group, arbidol monotherapy presented little effect for patients hospitalized with mild and moderate COVID-1964.
Novel drug-like compounds, DRI-C23041, DRI-C91005, which targeted the viral attachment stage, inhibited the interaction of hACE2 with SARS-CoV-2 S protein in cell-free ELISA-type assays65. DRI-C23041 [Fig. 4 (3)] inhibited SARS-CoV-2-S pseudovirus with IC50 of 5.6 μmol/L (Table 2)65.
Using the SARS-S and MERS-S pseudovirus infection assays, six compounds (cepharanthine, abemaciclib, osimertinib, trimipramine, colforsin, and ingenol) [Fig. 4 (4–9)], were identified as cell entry inhibitors from a HTS in approved drug libraries. The molecular mechanism of action of these small-molecule entry inhibitors has not been fully characterized. These inhibitors have been further confirmed to reduce (> 30%) cytopathic effect (CPE) caused by SARS-CoV-2 infection in Vero E6 cells with IC50s of 1.41, 3.16, 3.98, 20.52, 23.06 and 0.06 μmol/L, respectively (Table 2)66.
Clofazimine [Fig. 4 (10)], an FDA-approved molecule, was found to be an anti-tuberculosis drug, which was later used to treat leprosy67 and then showed antiviral activity against SARS-CoV-2 with an IC50 of 310 nmol/L in vitro68. Clofazimine, which has recently been identified as a broad-spectrum inhibitor of coronaviruses, may be a promising candidate for coronaviruses that have emerged and may emerge in the future. Because of its comparatively low manufacturing cost, clofazimine could significantly reduce the health burden, particularly in developing countries. In antiviral assays, clofazimine exhibited excellent antiviral activity against SARS-CoV-2 in vitro and in vivo. In addition, when combined with remdesivir, antiviral synergy was demonstrated against SARS-CoV-2 in vitro and in vivo. In mechanistic studies, clofazimine was shown to inhibit cell fusion between effector cells expressing SARS-CoV-2 S protein and Vero cells69.
5.2. Replication inhibitors
5.2.1. 3C-like protease inhibitors
Two large polyproteins, pp1a and pp1ab, are inactive until cleavage by virally encoded cysteine proteases, namely, 3CLpro and PLpro70, into different numbers of nsps in replication of SARS-CoV-2. The processing of pp1a and pp1ab is indispensable for viral life cycles. Thus, inhibition of cysteine proteases, is an effective therapeutic strategy for COVID-19.
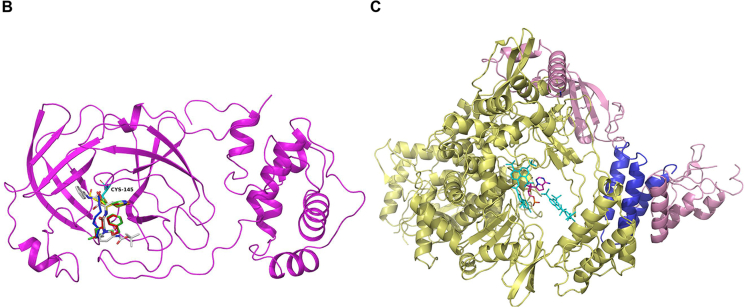
3CLpro represents the most attractive target for the discovery of SARS-CoV-2 inhibitors. Previous studies demonstrate that amino residue Cys145 in the catalytic pocket of 3CLpro is an effective site for the development of covalent inhibitors against SARS-CoV and other coronaviruses71.
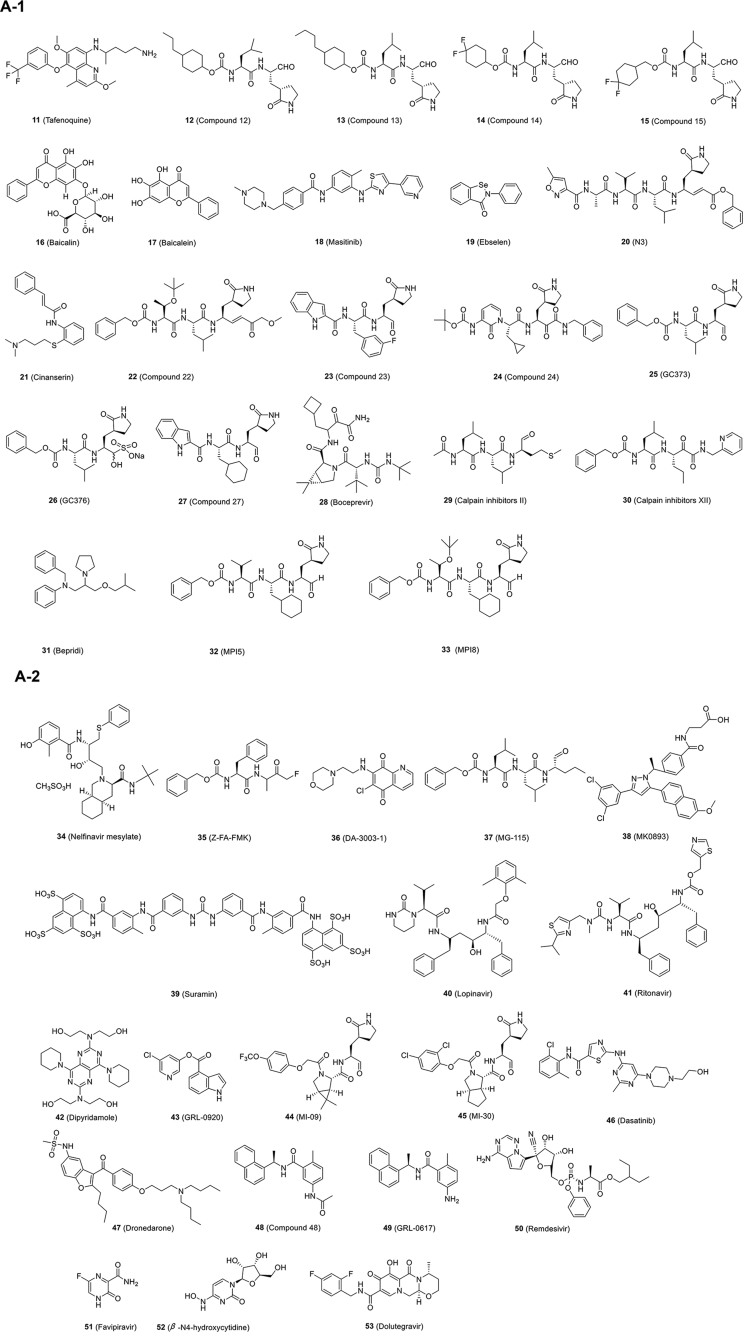
Chen and his colleagues72 established a HTS platform based on fluorescence resonance energy transfer (FRET) to identify drugs targeting the SARS-CoV-2 3CLpro from compound libraries, especially FDA-approved drugs, to be used immediately to treat patients with COVID-19. Their findings indicate that the 8-aminoquinoline antimalarial drug tafenoquine [Fig. 5A-1 (11)] induced significant conformational change in SARS-CoV-2 3CLpro, exposing some hydrophobic residues and ultimately leading to protein aggregation, diminishing its protease activity. Moreover, tafenoquine significantly repressed the yield of SARS-CoV-2 RNA in a cell culture system with an IC50 of around 2.5 μmol/L (Table 2). In Vero E6 cells, another research team reported that tafenoquine has an IC50 of ~2.6 μmol/L for SARS-CoV-2 (Table 2), which is four times more potent than hydroxychloroquine73. Time-of-addition experiment is consistent with the different mechanism for tafenoquine versus hydroxychloroquine. Physiologically based pharmacokinetic models indicate that the unbound concentration of tafenoquine may exceed EC90 for at least 8 weeks after administration in the lungs of COVID-19 patients.
Figure 5.
SARS-CoV-2 replication inhibitors. (A) Chemical structures of SARS-CoV-2 replication inhibitors. (B) Overall views of the 3CLpro-N3 (yellow) complex overlapped with carmofur (blue), 13 b (silver), GC373 (red) and GC376 (green) (PDB ID: 7BQY, 7BUY, 6Y2F, 6WTJ and 6WTK), and amino residue Cys145 was shown as cyan. (C) Overall views of the RdRp-suramin (cyan) complex overlapped with the remdesivir (magenta)-bound RdRp structure (PDB ID: 7D4F and 7BV2). nsp 12 was shown as yellow and accessary subunits nsp 7 and nsp 8 were shown as blue and pink.
Some 3CLpro inhibitors synthesized by Rathnayake et al.74 show activity against multiple coronaviruses in enzyme- and cell-based assays. Among compounds 12–15 (6c, 6e, 6h and 6j in Ref. 74) [Fig. 5A-1 (12–15)], 13 showed the most potent antiviral activity against SARS-CoV-2 3CLpro in both fluorescence resonance energy transfer enzyme assay (IC50, 0.17 μmol/L) and cell-based assay (IC50, 0.15 μmol/L, Table 2).
Baicalin and baicalein [Fig. 5A-1 (16, 17)], key components in TCM Scutellaria B., were reported as the first noncovalent, nonpeptidomimetic inhibitors of SARS-CoV-2 3CLpro, which exhibited potent antiviral activity with IC50s of 10.27 and 1.69 μmol/L, respectively, in a cell-based system (Table 2)75. Crystal structure of 3CLpro in complex with baicalein shows that baicalein occupies the core of the substrate-binding pocket through interacting with the crucial S1/S2 subsites and the oxyanion loop, blocking substrates from approaching the active site75. This unique mode of action can be regarded as a completely different type of 3CLpro inhibitor. Another team reported that infection of SARS-CoV-2 and VSV were potently inhibited by baicalein with IC50s around 10 and 15 μmol/L, respectively76. Mechanistically, baicalein inhibits mitochondrial OXPHOS, which is reversibly related to mPTP activity in host cells. The virus changes mitochondrial metabolism by inactivating mPTP to promote its production, and the inhibition of OXPHOS attenuates viral replication. A recent research team also reported that the ethanol extract of Scutellaria baicalensis and baicalein, the major component, showed inhibition of SARS-CoV-2 replication in Vero cells with IC50s of 0.74 and 2.9 μmol/L, respectively77. Ethanol extract inhibits virus entry, whereas baicalein mainly acts on the post-entry stage of the virus.
To inhibit the replication of HCoV-OC43, Drayman's team78 screened a library of 1900 clinically safe drugs, identified 26 top hits and further tested their antiviral activity against SARS-CoV-2. Of the 26 drugs tested, the compounds with the best antiviral activity against SARS-CoV-2 were cepharanthine (IC50 = 0.13 μmol/L), flupenthixol (IC50 = 0.56 μmol/L), desloratadine (IC50 = 0.9 μmol/L), trimipramine (IC50 = 1.5 μmol/L), lapatinib (IC50 = 1.6 μmol/L), benztropine (IC50 = 1.8 μmol/L), bafetinib (IC50 = 2.2 μmol/L), azelastine (IC50 = 2.4 μmol/L) and masitinib [Fig. 5A-1 (18)] (IC50 = 3.2 μmol/L, Table 2). By studying the mechanism of action, they found that masitinib, a cancer treatment drug developed as a tyrosine-kinase inhibitor, inhibited the activity of the SARS-CoV-2 3CLpro.
To identify drug candidates for clinical trials, Jin and coworkers79 initiated multiple strategies that combining structure-assisted drug design, virtual drug screening and HTS could rapidly discover novel lead compounds. This strategy resulted in the development of a FRET assay to test more than 10,000 compounds as inhibitors of 3CLpro. First, they identified a mechanism-based inhibitor, N3, by computer-aided drug design and then determined that N3 bound with 3CLpro of SARS-CoV-2 at a resolution of 2.1 Å (PDB code 7BQY) by measuring the crystal structure of 3CLpro in complex with N3. Finally, they found that seven FDA-approved or clinical drugs (ebselen, disulfiram, TDZD-8, tideglusib, carmofur, shikonin and PX-12) could inhibit 3CLpro using an enzymatic inhibition assay. However, it should be pointed out that the mechanism of action of six of them (ebselen, disulfiram, tideglusib, carmofur, shikonin and PX-12) remains to be elucidated. Ma et al.80 proved that these six inhibitors were nonspecific inhibitors of 3CLpro. Among these compounds, ebselen and N3 [Fig. 5A-1 (19, 20)] showed the strongest inhibition against SARS-CoV-2 in a plaque-reduction assay with IC50s of 4.67 and 16.77 μmol/L, respectively (Table 2). They also identified cinanserin [Fig. 5A-1 (21)], a well-characterized serotonin antagonist, which displayed moderate inhibition against SARS-CoV-2 with an IC50 value of 20.61 μmol/L (Table 2)79. Later, Yang and coworkers81 presented the X-ray crystal structure of SARS-CoV-2 3CLpro in complex with carmofur at a resolution of 1.6 Å (PDB code 7BUY). The Yang and Liu groups82 also co-published X-ray crystal structures of SARS-CoV-2 3CLpro in complex with peptidomimetic aldehyde compounds 22 and 23 (11a and 11b in Ref. 82) [Fig. 5A-1 (22, 23)]. Both 22 and 23 exhibited good anti-SARS-CoV-2-infection activities in cells with IC50s of 0.53 ± 0.01 and 0.72 ± 0.09 μmol/L, respectively, using a plaque-reduction assay (Table 2). Cytotoxicity and pharmacokinetic experiments were carried out later, suggesting that the two compounds were promising drug candidates for further clinical studies82.
Zhang et al.83 synthesized a series of peptidomimetic α-ketoamides as broad-spectrum inhibitors of 3CLpro of alphacoronaviruses, betacoronaviruses and enteroviruses. Recently, they determined the crystal structure of 3CLpro of SARS-CoV-2 at 1.75 Å resolution (PDB code 6Y2E) and co-crystal structures bound with compound 24 (13b in Ref. 83) [PDB code 6Y2F, Fig. 5A-1 (24)] with α-ketoamide as the warhead. 24 exhibited inhibition against SARS-CoV-2 infection in human Calu 3 cells with an IC50 of 4–5 μmol/L (Table 2). The pharmacokinetic studies of 24, given these favorable results, provide a promising framework for the development of new 3CLpro inhibitors for COVID-19.
Similarly, the dipeptide-based protease inhibitors GC373 and GC376 [Fig. 5A-1 (25, 26)] were effective inhibitors against 3CLpro of both SARS-CoV and SARS-CoV-2. GC373 is a peptide aldehyde metabolite of GC376.The binding mode of the inhibitors on SARS-CoV-2 3CLpro showed a covalent modification of the amino residue Cys145 in the catalytic site (PDB code 6WTK and 6WTJ). More importantly, both GC373 and GC376 were found to be potent inhibitors of SARS-CoV-2 replication in cells with IC50s near 1 μmol/L with little to no toxicity (Table 2), making them potent drug candidates for the treatment of COVID-1984. Structural comparison of reported 3CLpro‒inhibitor complex reveals that all of the covalent inhibitor connected to the sulphur atom of amino residue Cys145 (Fig. 5B).
Iketani et al.85 described three structurally diverse compounds–27 (4 in Ref. 85) [Fig. 5A-1 (27)], GC376, and MAC-5576–with inhibitory activity against the SARS-CoV-2 3CLpro. Next, they tested these compounds for inhibition of SARS-CoV-2 viral replication. They found that 27 and GC376 could block SARS-CoV-2 infection with IC50 values of 2.883 ± 0.227 and 2.189 ± 0.092 μmol/L (Table 2), respectively, whereas MAC-5576 did not.
Recently, four inhibitors targeting the 3CLpro of SARS-CoV-2, namely GC376, boceprevir, and calpain inhibitors II and XII [Fig. 5A-1 (26, 28–30)], were identified with IC50s ranging from 0.45 to 4.13 μmol/L in the enzymatic assay86. Significantly, the four compounds exhibited inhibition against SARS-CoV-2 replication in cells with IC50s ranging from 0.49 to 3.37 μmol/L (Table 2). Especially, boceprevir and calpain inhibitors II and XII represent novel chemotypes, providing a starting point for the development of novel SARS-CoV-2 inhibitors86. Another article also reported that boceprevir and GC376 showed inhibitory effects against SARS-CoV-2 in Vero cells [multiplicity of infection (MOI) = 0.01] with IC50 values of 15.57 and 0.70 μmol/L, respectively (Table 2)87. Moreover, combination of GC376 with remdesivir had a sterilizing additive effect87. In addition, some researchers found that the anti-3CLpro activity of calpain inhibitors II and XII was weaker than that of another 3CLpro inhibitor, GC376, in the SARS-CoV-2 3CLpro enzyme inhibition assay88. However, calpain inhibitors II and XII actually performed better than GC376 in reducing the replication of SARS-CoV-2 in cell culture86. They recently discovered that calpain inhibitors II and XII are also have inhibitory activity against human cathepsin L, which is a host-protease responsible for viral entry88. Calpain inhibitors II and XII are dual inhibitors that efficiently target viral protease 3CLpro and human protease cathepsin L, which may explain the excellent antiviral activity of them, despite having inferior affinity for 3CLpro when compared to the specific inhibitor GC376. Dual inhibitors can potentially inhibit drug resistance. Even if the viral protein changes, this type of inhibitor remains effective against unchanged human host proteins. In addition, Hu et al.89 found that boceprevir, calpain inhibitors II and XII, and GC-376 showed broad-spectrum antiviral activity against SARS-CoV-2, SARS-CoV and MERS-CoV infection, as well as human coronaviruses (CoVs) 229 E, OC43, and NL63. In addition, Cáceres et al.90 has reported that GC376 is effective in inhibiting SARS-CoV-2 infection in vivo. Treatment of SARS-CoV-2-infected K18-hACE2 mice with GC376 resulted in decreased viral loads and reduced inflammation. Recently, Shi et al.91 reported that application of low-dose GC376 in combination with GS441524, a parent nucleotide analog of remdesivir, that targets the coronavirus RdRp, via intranasal or intranasal and intramuscular administration could effectively protect mice against challenge of mouse-adapted SARS-CoV-2.
Vatansever et al.92 have reported that 6 small-molecule drugs (pimozide, ebastine, rupintrivir, bepridil, sertaconazole, and rimonabant) exhibited 50% inhibition of 3CLpro activity at concentration below 100 μmol/L and that bepridil [Fig. 5A-1 (31)] was the basic molecule that potentiates dual functions by raising endosomal pH to interfere with SARS-CoV-2 entry into the host cell, thereby inhibiting 3CLpro activity in infected cells. Their results revealed that bepridil inhibited CPE induced by SARS-CoV-2 infection in Vero E6 and A549/ACE2 cells with IC50 value of 0.86 and 0.46 μmol/L, respectively.
Based on the previous medicinal chemistry studies about 3CLpro of SARS-CoV, Yang et al.93 have designed and synthesized a series of SARS-CoV-2 3CLpro inhibitors that contain β-(S-2-oxopyrrolidin-3-yl)-alaninal (Opal) for the formation of a reversible covalent bond with the cysteine C145 in SARS-CoV-2 3CLpro active site. Among them, MPI5 and MPI8 [Fig. 5A-1 (32, 33)] completely inhibited CPE induced by SARS-CoV-2 infection in Vero E6 cells at 2.5–5 μmol/L and A549 cells at 0.16–0.31 μmol/L. In preclinical and clinical studies on COVID-19 treatment, their inhibitory potency was remarkably higher than that of some existing molecules.
Jan et al.94 identified that boceprevir and nelfinavir mesylate [Fig. 5A (28, 34)] can inhibit SARS-CoV-2 infection and replication with IC50s of 50.1 and 3.3 μmol/L, respectively, by measuring viral-induced CPE (Table 2). Through target-based assay, they found that nelfinavir mesylate and boceprevir showed inhibitory activity against 3CL pro. Also, nelfinavir mesylate was selected to evaluate its anti-infective efficacy in female golden Syrian hamsters. Nelfinavir mesylate showed good antiviral effects in vivo, and the viral load in hamster lungs was significantly reduced.
Walrycin B, hydroxocobalamin, suramin sodium, Z-DEVD-FMK, LLL-12, and Z-FA-FMK were identified as the most potent 3CLpro inhibitors among 23 hits in a SARS-CoV-2 3CLpro enzyme assay95. The protease inhibitor Z-FA-FMK [Fig. 5A-2 (35)] inhibited CPE induced by SARS-CoV-2 infection with an IC50 of 0.13 μmol/L with no apparent cytotoxicity (Table 2). However, hydroxocobalamin, suramin sodium, and Z-DEVP-FMK were invalidated in the CPE assay. Walrycin B and LLL-12 showed apparent toxicity to Vero E6 cells. Other compounds identified, including DA-3003-1, MG-115 and MK0893 [Fig. 5A-2 (36–38)], all exhibited antiviral activity, as well, with more or less cytotoxicity to Vero E6 cells (Table 2). Another research team reported that an antiparasitic drug suramin [Fig. 5A-2 (39)] inhibits SARS-CoV-2 replication and SARS-CoV-2 infection in Vero E6 cells with an IC50 of ~20 μmol/L96 (Table 2). And suramin could reduce the viral load by two–three logs in Vero E6 cells or Calu-3 2B4 cells (the human lung epithelial cell line). Analysis of time-of-addition and plaque reduction assays performed on Vero E6 cells indicated that suramin acts in the early stages of the replication cycle and may prevent virus binding or entry.
Lopinavir/ritonavir [LPV/r, Fig. 5A-2 (40, 41)], a protease inhibitor used for HIV infection, showed inhibitory activity against the replication of SARS-CoV, MERS-CoV and SARS-CoV-2 in vitro (Table 2)97, 98, 99. Although these drugs were initially thought to inhibit SARS-CoV and MERS-CoV 3CLpro18,100, 101, it should be pointed out that lopinavir and ritonavir failed to inhibit the activity of SARS-CoV-2 3CLpro86. In Korea, clinical administration of LPV/r reduced SARS-CoV-2 viral load rapidly, but no placebo-controlled trial was carried out, and the sample of patients was limited102. On the other hand, Cao et al.103 carried out a randomized, controlled, open-label clinical trial for the treatment of severe COVID-19 with LPV/r (400 and 100 mg, respectively). The two sets of clinical results showed that LPV/r offered little clinical improvement beyond the standard of care. Further, Hung et al.104 carried out a multicenter, prospective, open-label, randomized phase two trial in COVID-19 adult patients admitted to six hospitals in Hong Kong. Early triple antiviral therapy (combination of lopinavir 400 mg and ritonavir 100 mg every 12 h, ribavirin 400 mg every 12 h, and three doses of eight million international units of interferon β-1b on alternate days) was safe and superior to lopinavir–ritonavir alone (lopinavir 400 mg and ritonavir 100 mg every 12 h) in alleviating symptoms and shortening the time of viral elimination and length of hospitalization in patients with mild to moderate COVID-19104. Dual antiviral therapy with interferon β-1b as the backbone will be conducted in clinical studies in the future. Most recently, WHO reported the latest solidarity trial interim results of lopinavir, which, unlike the previously reported results, appeared to have little or no effect on hospitalized COVID-19 patients105.
The Luo group106 screened the U.S. FDA-approved drug library and found that the anticoagulation agent dipyridamole [DIP, Fig. 5A-2 (42)] might bind to the SARS-CoV-2 protease 3CLpro, suppressing more than 50% of SARS-CoV-2 replication at a concentration of 100 nmol/L in Vero E6 cells (Table 2). Indeed, after two weeks of DIP adjunctive treatment, all eight severe patients showed remarkably positive outcomes.
Hattori et al.107 reported that one indole-chloropyridinyl-ester derivative, GRL-0920 [Fig. 5A-2 (43)], targeting 3CLpro of SARS-CoV-2, exerted potent activity against SARS-CoV-2 (IC50 = 2.8 μmol/L) in cell-based assays performed using Vero E6 cells without significant toxicity, as examined with immunocytochemistry (Table 2).
As mentioned above, although some SARS-CoV-2 3CLpro inhibitors have been reported, the previous literature on SARS-CoV-2 3CLpro inhibitors has not included data from the experiments using SARS-CoV-2-infected animal models. Qiao et al.108 designed and synthesized 32 new bicycloproline-containing 3CLpro inhibitors, all of which derived from boceprevir or telaprevir. All compounds inhibited the activity of SARS-CoV-2 3CLpro with IC50s of 7.6–748.5 nmol/L in vitro. Two compounds, MI-09 and MI-30, [Fig. 5A-2 (44, 45)] showed excellent antiviral activity against SARS-CoV-2 with IC50s of 0.86 and 0.54 μmol/L (MOI of 0.1, Vero E6 cells), respectively in cell-based assays (Table 2). In a transgenic mouse model, MI-09 or MI-30 could significantly reduce lung viral load and lung lesions. Also, MI-09 or MI-30 showed good pharmacokinetic properties and safety in rats.
5.2.2. Papain-like protease inhibitors
PLpro is the other crucial viral protease spurring the discovery of anti-SARS-CoV-2 drugs, and its crystal structure has recently been resolved (PDB code 6W9C). PLpro is also reported to drive virus evasion of host innate immune defenses by reversing host ubiquitination and ISGylation events109. Thus, PLpro inhibitors may not only directly inhibit SARS-CoV-2 replication, but also perform a complementary function by normalizing the body's immune response against virus invasion. Previous studies identified that thiopurine (6-mercaptopurine and 6-thioguanine, antitumor drugs) showed inhibitory activity against SARS-CoV and MERS-CoV PLpro110, so repurposing these candidates for treating COVID-19 seems to be a reasonable approach.
Some noncovalent small-molecule inhibitors, rac3j, rac3k and rac5c, against SARS-CoV PLpro111 also target SARS-CoV-2 PLpro, preventing the self-processing of nsp 3 in cells and reducing SARS-CoV-2-induced CPE under high (33 μmol/L) concentrations. For rac5c, it is worth mentioning that treatment at 11 μmol/L in 0.1% DMSO continued to show a clear reduction of CPE, indicating effective antiviral activity. For rac3j and rac3k, CPE reduction diminished at lower concentrations112.
Referring to the crystal structure of SARS-CoV-2 PLpro, Kouznetsova et al.113 performed useful data mining of the conformation of FDA-approved drugs. 147 compounds were identified as potential inhibitors of SARS-CoV-2 PLpro. Among them, dasatinib [Fig. 5A-2 (46), Table 2] showed antiviral activity against SARS-CoV-2 in clinical cases114, but it was unclear whether dasatinib directly interacts with PLpro. A patient with chronic myeloid leukemia and COVID-19 was treated with dasatinib (100 mg/day) in combination with antibiotics for 11 days, resulting in the disappearance of fever. Two weeks later, two consecutive swab tests were negative for SARS-CoV-2 RNA. Dronedarone [Fig. 5A-2 (47)], which was reported by Jan et al.94, is effective in inhibiting the activity of PLpro. And dronedarone showed antiviral activity against CPE induced by SARS-CoV-2 infection with an IC50 of 4.5 μmol/L (CC50 of 12.1 μmol/L) in Vero E6 cells (Table 2), which is an ion channel modulator.
Mirza et al.115 reported compound Z93 as a potential human ubiquitin-specific protease 2 (USP2) inhibitor through integrated in silico efforts. USP2 inhibitors, such as thiopurine analogs, have been reported to inhibit SARS-CoV PLpro. However, based on the above results, it can only be speculated that Z93 might be a potential chemical lead targeting SARS-CoV-2 PLpro, thus warranting further evaluation in vitro. Additionally, the Pegan group109 declared that naphthalene-based inhibitors [48 (6 in Ref. 109) and GRL-0617, Fig. 5A-2 (48, 49)] showed inhibitory activity against SARS-CoV-2 PLpro and antiviral activity with IC50s of 21.0 and 27.6 μmol/L, respectively (Table 2). Gao et al.116 showed that GRL-0617 was effective in inhibiting SARS-CoV-2 PLpro activity with an IC50 of 2.2 ± 0.3 μmol/L and that its mechanism of action was not limited to occupying the substrate pockets, but rather extended to sealing the entrance to the substrate binding cleft, thereby preventing the binding of the substrate. Another team reported that GRL-0617 showed a promising inhibitory activity against SARS-CoV-2 PLpro in vitro with an IC50 of 2.1 μmol/L and effective antiviral inhibition of SARS-CoV-2 in cell-based assays. No apparent cytotoxicity of GRL-0617 on Vero E6 cells was observed with concentrations up to 100 μmol/L117.
5.2.3. RNA-dependent RNA polymerase (RdRp) inhibitors
RdRp, a nsp, also known as nsp 12, catalyzes the synthesis of viral genome, which plays a central role in coronaviral replication. Thus, it is considered as an excellent drug target for antiviral inhibitors. The Rao group118 solved a cryo-electron microscopy structure of full-length RdRp in complex with nsp 7 and nsp 8 at a resolution of 2.9 Å and speculated the binding mode of nucleotide analogs remdesivir and favipiravir to explain the mechanisms of inhibition. The elegant results provide novel insight and lay a solid foundation for structure-based antiviral inhibitor design.
Remdesivir [GS-5734, Fig. 5A-2 (50)], a nucleotide analogue, was originally developed for Ebola treatment119. Since its triphosphate form resembles adenosine triphosphate (ATP), it was used as a substrate for viral RdRp, and it performed broad-spectrum activity against coronaviruses120,121. Remdesivir was first suggested by Morse et al.6 as a COVID-19 therapeutic for treatment of patients infected by SARS-CoV-2. Remdesivir (IC50 = 0.77 μmol/L; CC50 > 100 μmol/L; SI > 129.87) potently blocked SARS-CoV-2 infection in Vero E6 cells at a MOI of 0.05 and showed high SI (Table 2)19. Recently, Beigel et al.122 reported the results of the ACTT-1 clinical trial of remdesivir. The ACTT-1 clinical trial is a double-blind, randomized, placebo-controlled global phase III clinical trial. Remdesivir was superior to placebo in shortening recovery time and reducing lower respiratory tract infections in adult hospitalized patients with COVID-19122. On 1 May 2020, remdesivir was made available under the U.S. FDA Emergency Use Authorization (EUA) for the treatment of severely ill hospitalized patients with COVID-19. The research group also pointed out that combining remdesivir with other treatments or antiviral drugs to improve the prognosis of COVID-19 patients should be evaluated in the future122. Despite this, a randomized, double-blind, placebo-controlled study showed that 237 enrolled patients showed no association between remdesivir use and statistically significant clinical benefits123. This study had to be terminated early owing to the adverse events observed in patients. WHO published the mid-term results of the Solidarity Trial of Remdesivir using for COVID-19 patients on 15 October 2020. Unlike earlier expectations, remdesivir appeared to have little, or no, effect on hospitalized COVID-19 patients in terms of overall mortality, initiation of ventilation and duration of hospital stay124. Yin et al.126 have reported that suramin, a 100-year-old drug, and its derivatives are at least 20-fold more potent than remdesivir in inhibiting SARS-CoV-2 infection by targeting RdRp. The crystal structure of RdRp in complex with suramin has revealed two binding sites in RdRp (Fig. 5C). As a non-nucleotide inhibitor, suramin could ultimately aid in structure-based drug development for COVID-19126.
Favipiravir [Fig. 5A-2 (51), Table 2, IC50 = 61.88 μmol/L, CC50 > 400 μmol/L, SI > 6.46] had low anti-SARS-CoV-2 activity in cell culture assays19. However, favipiravir has been shown to completely protect mice against Ebola virus challenge and has an IC50 value of 67 μmol/L in Vero E6 cells127, suggesting that further in vivo studies should be undertaken to assess the efficacy of this antiviral nucleoside in the treatment of COVID-19. In Shenzhen, a clinical trial of favipiravir on COVID-19 patients was conducted (ChiCTR2000029600), and it showed that 35 patients in the favipiravir arm had significantly shorter viral clearance duration in contrast with the control arm containing 45 patients128. In another multi-centric randomized study (ChiCTR200030254), treatment with favipiravir of COVID-19 patients led to an improved recovery at the 7th day129.
β-d-N4-Hydroxycytidine [EIDD-1931, Fig. 5A-2 (52)] is an orally available ribonucleoside analogue with broad-spectrum antiviral activity against RNA viruses, including influenza, Ebola, CoV, and Venezuelan equine encephalitis (VEE) virus130, 131, 132, 133. Sheahan et al.120 reported that EIDD-1931 showed antiviral activity against SARS-CoV-2 in Vero cells with an IC50 of 0.3 μmol/L (Table 2). Both prophylactic and therapeutic administration of EIDD-2801, an orally available EIDD-1931-prodrug, in mice infected with SARS-CoV or MERS-CoV, could improve pulmonary function and reduce virus titer and body weight loss120. The mechanism of action of EIDD-2801 is different from that of remdesivir. Remdesivir is a chain terminator134, while EIDD-2801 causes mutagenesis in the viral RNA. In addition, EIDD-2801 is active against remdesivir-resistant mutants and has a higher genetic barrier to drug resistance than remdesivir120. For VEE and influenza viruses, compound EIDD-2801 inhibited RdRp to exert its antiviral functions, but the mechanism of action against coronaviruses was not well documented135.
5.2.4. Helicase inhibitors
Helicase, a motor protein, is responsible for separation and/or rearrangement of viral nucleic acid duplexes before transcription or replication136. The helicase known as nsp 13 consists of three major domains—a putative N-terminal metal-binding domain (MBD), a hinge domain, and a helicase domain. And the N-terminal forms a Zn-binding domain, while the C-terminal forms a helicase domain with a conserved motif and participates in both unravelling double-stranded (ds) DNA and capping of viral RNA. Studies have shown that nsp13-dependent disintegration was an essential process for the replication, transcription, and translation of SARS-CoV-2 genome137. Therefore, helicases are potential targets for antiviral therapies of COVID-19 and inhibitors of helicases, such as bananins, 5-hydroxychromone derivatives, ADKs, and SSYA10-001, and are expected to be used in the treatment of COVID-19138, 139, 140, 141. In addition, clofazimine was shown to inhibit SARS-CoV-2 replication by interfering with the function of helicase69. The biggest challenge in targeting helicase is the relatively low selectivity of helicase inhibitors. Right now, no antiviral targeting helicase has moved beyond preclinical development.
5.2.5. 2′-O-Ribose methyltransferase (2ʹ-O-MTase) inhibitors
The nsp 16, or 2′-O-MTase, is another crucial protein responsible for SARS-CoV replication142, 143, 144 by catalyzing 5′-terminal caps structures (m7GpppN) of mRNA for methylation, thereby preventing recognition and activation of host immune responses144,145.
By using model docking and molecular dynamics simulation, Khan et al.144 established dolutegravir [Fig. 5A-2 (53), detaG = −9.4 kcal/mol] as an excellent lead candidate for the crucial protein 2′-O-Mtase. Dolutegravir, an integrase strand-transfer inhibitor, with inhibitory activity against human immunodeficiency virus type 1 (HIV-1) infection, could suppress SARS-CoV-2 replication with IC50 of 22.04 μmol/L and CC50 > 40 μmol/L in Vero E6 cells (Table 2)146.
5.2.6. RNA-binding N-terminal domain inhibitors
N protein, usually located inside the virions, is an abundant coronavirus protein that binds with the viral genome to form the ribonucleoprotein. It plays a critical role in viral RNA transcription and replication147, making it a potential antiviral drug target. Recent studies showed that N protein is a multifunctional protein responsible for binding to the viral RNA genome and packing it into a long helical nucleocapsid structure148. It is also reported to regulate host–pathogen interaction and induce protective immune responses149.
The Medhi group22 identified two potential hit compounds, ZINC00003118440 and ZINC0000146942, both of which might bind the RNA-binding N-terminal domain of SARS-CoV-2 N protein, which were theophylline and pyrimidone derivatives, respectively. Thereafter, Kang et al.150, for the first time, resolved the X-ray crystal structure of SARS-CoV-2 N protein at a resolution of 2.7 Å, revealing the specific surface charge distributions that facilitates the drug discovery specific to ribonucleotide binding domain of SARS-CoV-2 N protein.
5.3. Others
Studies have shown that the genome sequence of SARS-CoV-2 is very similar to that of SARS-CoV. Several SARS-CoV-2 proteins with > 90% sequence similarity, such as S protein, 3CLpro, PLpro, RdRp and 2′-O-MTase, could be used as drug targets. Meanwhile, many small molecules have been described as potential drug candidates for treatment of COVID-19, but their targets have not been identified. Some of these small-molecule drugs, are summarized below.
5.3.1. Broad-spectrum antiviral compounds
Early in the COVID-19 pandemic, some anti-flu drugs (for example, oseltamivir) were applied to treat COVID-19 patients151. Yamamoto et al.152 reproted that nine approved HIV-1 protease inhibitors exhibited antiviral activity against SARS-CoV-2 in vitro. Among these inhibitors, tipranavir [Fig. 6 (54), IC50 = 13.34 μmol/L, CC50 = 76.80 μmol/L, SI = 5.76], ritonavir [Fig. 5A-2 (41), IC50 = 8.63 μmol/L, CC50 = 74.11 μmol/L, SI = 8.59], saquinavir [Fig. 6 (55), IC50 = 8.83 μmol/L, CC50 = 44.43 μmol/L, SI = 5.03], atazanavir [Fig. 6 (56), IC50 = 9.36 μmol/L, CC50 > 81 μmol/L, SI > 8.65] and nelfinavir [Fig. 5A-2 (34), IC50 = 1.13 μmol/L, CC50 = 24.32 μmol/L, SI = 21.52] all exhibited antiviral activity (Table 2). Notably, nelfinavir effectively inhibited SARS-CoV-2 replication at a low concentration and exhibited the high SI among of them152, Indicated that nelfinavir is a potential drug candidate for the treatment of COVID-19 and should therefore be evaluated in patients with SARS-CoV-2 infection.
Figure 6.
Chemical structures of other small-molecule inhibitors against SARS-CoV-2.
5.3.2. Screening of FDA-approved drugs for the prevention of SARS-CoV-2
Repurposing of approved drugs is a time-saving strategy for drug development. In this way, Touret et al.146 screened the Prestwick Chemical Library composed of 1520 approved drugs in an SARS-CoV-2-infected cell-based assay. The results showed that 15 molecules exhibited inhibition of SARS-CoV-2 replication in vitro. Among of them, 11 compounds exhibited antiviral potency with 2 < IC50 ≤ 20 μmol/L. Two of them with the highest antiviral activity were obtained from azithromycin [Fig. 6 (57), IC50 = 2.12 μmol/L, SI > 19] and hydroxychloroquine [Fig. 9 (76), IC50 = 4.17 μmol/L, SI > 10] and were therefore selected for clinical trials (Table 2)153. Among of them, spiperone [Fig. 6 (58)], a dopaminergic D2 antagonist, which was already identified as an antiviral molecule against the human pathogenic polyomaviruses154, showed most potent antiviral activity against SARS-CoV-2 infection with an IC50 of 2.49 μmol/L and SI value of 16 (Table 2). The next three most efficient drugs were opipramol dihydrochloride [Fig. 6 (59), IC50 = 5.05 μmol/L, SI > 7.9], a tricyclic antidepressant, quinidine hydrochloride [Fig. 6 (60), IC50 = 5.11 μmol/L, SI > 7.8], an antiarrhythmic drug, and alprostadil [Fig. 6 (61), IC50 = 5.39 μmol/L, SI > 7.4], a prostaglandin known as a cardiovascular drug. The remaining nine drugs out of 15 showed 1.0 < SI < 5.3 (Table 2)146. Some of these candidates may provide information to guide downstream experiments in small animal models, discover more effective derivatives, or evaluate drug combinations in vitro with potential enhancement of efficacy.
Figure 9.
Chemical structures of small-molecule inhibitors targeting cathepsin B/L.
Ivermectin [Fig. 6 (62)], an FDA-approved antiparasitic agent with a broad spectrum of activity, high efficacy and a broad safety profile, is reported to have potent antiviral activity against HIV-1 and dengue virus by inhibiting protein nuclear import155,156. Caly et al.157 found that ivermectin could inhibit SARS-CoV-2 replication with an IC50 of ~2 μmol/L in vitro (Table 2), demonstrating that it is worthy of further research to treat COVID-19.
The repurposed drug anakinra in a phase III randomized clinical trial was able to reduce the requirement of invasive mechanical ventilation and mortality rate in severe COVID-19 cases without serious side effects158.
Five FDA-approved drugs, including penciclovir [Fig. 6 (63)], were reevaluated for their effects on cytotoxicity, viral production, and infection rates of SARS-CoV-2 by using standard methods19. The results showed that penciclovir inhibited viral infection with an IC50 value of 95.96 μmol/L (Table 2), which was far from satisfactory compared to other compounds tested at the same time. However, some data indicated that compounds with high IC50 values may have surprising antiviral activity in vivo127. Therefore, it is necessary to detect the antiviral activity of these compounds in vivo.
6. Small-molecule SARS-CoV-2 inhibitors targeting host proteins
Based on existing treatments, new SARS-CoV-2 mutations are likely to be resistant to drugs. Therefore, an initial suggestion is to use host-targeting therapeutic approach to reduce the aggressiveness and mortality resulted from SARS-CoV-2 infections.
6.1. Inhibitors targeting ACE2 to block the interaction between S protein and ACE2
Many studies have shown that host ACE2 is the specific receptor for SARS-CoV-2 S protein5,10. Inhibitors that block the binding between S protein and ACE2 have been considered to use for treatment of COVID-19 by preventing virus entry into the host cells. Candesartan and olmesartan [Fig. 7 (64, 65)], which are angiotensin II receptor blockers (ARBs), as reported, play a key role in virus entry23,159,160. Clinical trials will also be conducted on another ARB derivative, telmisartan [Fig. 7 (66), Clinical Trials gov, NCT04356495]161. In addition, blockers of angiotensin receptor one, such as losartan [Fig. 7 (67)], as inhibitors of the renin-angiotensin system, could be a useful therapeutic option in reducing lung inflammation and pneumonia in COVID-19 patients162,163. Currently on the clinical trial website, losartan is being used to study its effect on suppress the pathologic damage in inpatients and outpatients with COVID-19 and its safety to the COVID-19 patients with respiratory failure (Table 3)164,165.
Figure 7.
Chemical structures of small-molecule inhibitors targeting ACE2.
An anthraquinone compound, emodin, which derived from genus Rheum and Polygonum, can interfere with the interactions between S protein and ACE2 by competing with ACE2 and inhibiting the 3a ion channel of coronavirus166,167. Promazine is an anti-psychotic drug that shares a similar structure with emodin and presents comparable inhibitory effect on the replication of SARS-CoV with a similar mechanism of action167. Based on the similarity of S protein sequences between SARS-CoV-2 and SARS-CoV, we could conclude that emodin and promazine may inhibit SARS-CoV-2 infection by blocking the binding between S protein and ACE2, and both of them are considered as potential drugs for the treatment of COVID-19168, 169, 170.
Nicotianamine, a unique secondary metabolite in soybean, is a potent inhibitor of ACE2171, and it is considered as a potential drug candidate for the treatment of COVID-19172,173. Similarly, flavonoids were also a class of natural products which possess antioxidant, anti-inflammatory and antiviral functions. Lead flavonoids (e.g., hesperidin, naringin, ECGC and quercetin) screened out by molecular docking might serve as cell entry inhibitors by targeting S protein or ACE2174.
6.2. TMPRSS2 inhibitors
SARS-CoV-2 attaches to the ACE2 receptors via S protein, which is subsequently cleaved by TMPRSS2, a host serine protease that has been exploited as therapeutic targets. Hoffmann and colleagues demonstrated that the TMPRSS2 inhibitors proved useful in blocking virus entry10.
Camostat mesylate [Fig. 8 (68)], a TMPRSS2 inhibitor in clinical, significantly reduced SARS-CoV-2 pseudovirus entry into Calu-3 cells with an IC50 of ~1 μmol/L with no cytotoxic effects (CC50 > 500 μmol/L, Table 3)10. Similarly, camostat mesylate was effective significantly in reducing Calu-3 authentic SARS-CoV-2 infection in Calu-3 cells and SARS-CoV-2 pseudovirus infection in primary human lung cells10. A randomized phase 2a clinical trial (Clinical Trials gov, NCT04321096) and double-blinded, randomized, placebo-controlled phase four trials in (Clinical Trials gov, NCT04338906) were performed to evaluate the activity of camostat mesylate as a treatment for SARS-CoV-2 infection. Bromhexine, a generic mucolytic, is a TMPRSS2 inhibitor175, which is currently being evaluated clinically as a treatment for COVID-19 (Clinical Trials gov, NCT04273763). In order to evaluate the impact of bromhexine on COVID-19 treatment, a larger phase one clinical trial with 140 participants (Clinical Trials gov, NCT04340349) was performed, including treatment with bromhexine alone or in combination with hydroxychloroquine sulfate. A broad-spectrum serine protease inhibitor, nafamostat [Fig. 8 (69)], could inhibit the activity of TMPRSS2. Experiments have shown that nafamostat mesylate can inhibit SARS-CoV-2 infection to human lung cells176. Standard assays were also carried out by Wang et al.19 to test the effects of nafamostat on the cytotoxicity, virus yield and infection rates of SARS-CoV-2, and the results demonstrate that the IC50 value of nafamostat is 22.50 μmol/L with cytotoxicity CC50 > 100 mmol/L (Table 3). In another article, nafamostat mesylate inhibited SARS-CoV-2 infection CPE in Calu-3 cells with an IC50 of around 10 nmol/L177. Furthermore, Asakura and Ogawa178 claimed that the combination of nafamostat and heparin may be effective against COVID-19 from the perspectives of antiviral and anti-DIC (disseminated intravascular coagulation) with enhanced fibrinolysis symptoms for COVID-19 patients. In addition, a randomized clinical trial was conducted to the evaluated ability of nafamostat to slow down lung disease for adult COVID-19 patients (Clinical Trials gov, NCT04352400).
Figure 8.
Chemical structures of small-molecule inhibitors targeting TMPRSS2.
MI-432179 and MI-1900 [Fig. 8 (70, 71)] are two TMPRSS2 inhibitors, which are prospective peptide mimetic inhibitors180. The inhibitor MI-1900 is the less polar analog of MI-432. Recently, MI-432 showed inhibition against SARS-CoV-2 infection in Calu-3 cells, which reduced by 75-fold virus titer at a concentration of 10 μmol/L, and MI-1900 exhibited strong inhibition of SARS-CoV-2 replication at 50 μmol/L and reduced by 35- to 280-fold viral titers (Table 3)180.
6.3. Cathepsin B/L
In addition to TMPRSS2, cathepsin B and cathepsin L, being active in the early and late endosome, respectively, can also trigger viral S protein cleavage and promote viral fusion181,182.
P9, derived from mouse β-defensin-4, is a peptide that interfere cathepsin L activity, which showed antiviral activity against SARS-CoV, MERS-CoV, and influenza viruses through the inhibition of endosomal acidification thus indirectly interfering cathepsin L activity183. The researchers optimized P9 by replacing arginine with the weakly positively charged amino acids (histidine and lysine) to generate P9R, which showed significantly higher potency against SARS-CoV-2 infection than P9, as determined by a plaque reduction assay in Vero E6 cells (0.9 vs. 2.4 μg/mL, Table 1). And the CC50 of P9R was >300 μg/mL in MDCK, Vero E6 and A549 cells184. Further, the authors describe an eight-branched derivative, 8P9R, that showed more potent antiviral activity (IC50 = 0.3 μg/mL) in high salt condition (PBS) than that of P9R in Vero E6 cells. The cytotoxicity assay indicated that CC50 of 8P9R was higher than 200 μg/mL in Vero E6 cells (Table 1). The 8P9R can inhibit the two entry pathways of SARS-CoV-2 in cells including endocytic pathway and TMPRSS2-mediated surface pathway by aggregating viral particles. In addition, 8P9R, or the combination of repurposed drugs, arbidol, chloroquine and camostat could significantly suppress SARS-CoV-2 replication in hamsters and SARS-CoV in mice185.
Amantadine and chlorpromazine [Fig. 9 (72, 73)], which were previously used to abrogate viral entry via clathrin-mediated endocytosis24,186, proved to have no prominent antiviral efficacy against SARS-CoV-2 and only a partial inhibition at 100 μmol/L for amantadine in Vero E6 cells (Table 3)187. E64-d [Fig. 9 (74)], a broad cathepsin B/L inhibitor, showed inhibitory activity against SARS-CoV-2 pseudovirus infection with an IC50 of ~4.487 μmol/L in Vero E6 cells (Table 3).
Chloroquine [Fig. 9 (75)] prevents viral infection by increasing the endosomal pH, which, in turn, inhibits hydrolytic activity of cathepsin L19. In in vitro experiments, chloroquine showed strong inhibitory activity against SARS-CoV-2 with an EC50 of 1.13 μmol/L, CC50 > 100 μmol/L and SI > 88.50 (Table 3) in Vero E6 cells (MOI = 0.05)19. Hydroxychloroquine [Fig. 9 (76)] is a derivative of chloroquine and is used to treat autoimmune diseases. The Chinese Clinical Trials Registry has documented several clinical trials using chloroquine or hydroxychloroquine for COVID-19188,189. Consequently, some research groups compared hydroxychloroquine with chloroquine for inhibitory against SARS-CoV-2 in vitro. The results showed that hydroxychloroquine had lower antiviral activity compared to chloroquine, at least at certain MOIs (Table 3)189. Although it was proven that chloroquine could inhibit SARS-CoV-2 infection in Vero cells, it does not block SARS-CoV-2 infection in the TMPRSS2-expressing Calu-3 cells190. The Maisonnasse group191 tested different treatment strategies for hydroxychloroquine in rhesus macaques, including comparison with placebo treatment alone or in combination with azithromycin in macaques. Neither hydroxychloroquine nor the combination of hydroxychloroquine and azithromycin showed a significant effect on viral load in any of the analyzed tissues. The U.S. FDA has withdrawn its EUA status given to chloroquine and hydroxychloroquine in view of these recent developments. The latest clinical results of hydroxychloroquine were also published by WHO in the interim results of the Solidarity Trial of antiviral drugs for the treatment of COVID-19. The results showed that hydroxychloroquine cannot significantly reduce mortality of COVID-19 hospitalized patients. At the same time, it cannot reduce the rate of mechanical ventilation (intubation) and duration of hospital stay124.
To identify candidate therapeutics for COVID-19, Riva et al.68 screened a drugs library consisting of approximately 12,000 clinical-stage or FDA-approved small molecules. They reported the identification of 21 molecules that inhibit SARS-CoV-2 infection in a dose-dependent manner. Five of the most potent compounds, apilimod [Fig. 9 (77)] and the cysteine protease inhibitors MDL-28170, Z LVG CHN2, VBY-825 and ONO 5334 [Fig. 9 (78–81)], were effective against SARS-CoV-2 infection with IC50s of 0.023, 0.22, 0.19, 0.3 and 0.41 μmol/L, respectively, in Vero E6 cells (Table 3). As reported, MDL-28170, a cathepsin B inhibitor, could impairs infection by SARS-CoV and Ebola virus192,193, ONO 5334 is a cathepsin K inhibitor194, and VBY-825 acts as a reversible cathepsin protease inhibitor195. Apilimod, a specific PIKfyve kinase inhibitor, was shown to be effective in inhibiting virus entry, which is in agreement with the report that PIKfyve is predominately presented in early endosomes and plays an important role in maintaining endomembrane homeostasis196. Another study reported that apilimod inhibits infection with authentic SARS-CoV-2 strain 2019-nCoV/USA-WA1/2020 virus in Vero E6 cells with an IC50 of ~10 nmol/L (Table 3)197. Z LVG CHN2 probably acts as inhibitor of an endosomal protease198. Notably, MDL-28170, ONO 5334 and apilimod exhibited inhibitory activity against viral replication in human pneumocyte-like cells that derived from induced pluripotent stem cells, and apilimod displayed antiviral activity in a primary human lung explant model68.
Teicoplanin [Fig. 9 (82)], a currently used antibiotic in the treatment of Gram-positive bacterial infection, showed to be active against SARS-CoV, MERS-CoV and Ebola virus in vitro199,200. In the reports of Zhang et al.201, teicoplanin acts on the early step of coronavirus viral life cycle through directly inhibiting the enzymatic activity of cathepsin L. The concentration of teicoplanin required to inhibit 50% of SARS-CoV-2 pseudoviruses into the cytoplasm was only 1.66 μmol/L (Table 3) in HEK293T cells, which is much lower than the commonly used 8.78 μmol/L dose to inhibit Gram-positive bacteria201. Baron and coworkers encourage further investigation of teicoplanin for the treatment of COVID-19199. Dalbavancin, a homolog of teicoplanin, also showed inhibitory activity against SARS-CoV-2 entry in a dose-dependent manner201.
Touret et al.146 found that omeprazole [Fig. 9 (83)] can inhibit SARS-CoV-2 with IC50 of 17.06 μmol/L and CC50 > 40 μmol/L in Vero E6 cells (Table 3). Omeprazole, a proton pump inhibitor used as an antiulcer agent, has been demonstrated to increase the pH of endosomal/Golgi pathway either by inhibiting ATPase proton pomp, or by buffering the pH. This endosomal pH modification will limit the processing of S protein by endosomal proteases, thereby blocking the entry of viruses mediated by the membrane fusion process146.
The 3CLpro calpain inhibitors II/XII are also reported to be active against human cathepsin L88. Calpain inhibitors II/XII inhibit the activity of cathepsin L with IC50s of 0.41 and 1.62 nmol/L, respectively88. One of the advantages of calpain inhibitors II and XII, as dual inhibitors, is their ability to target the viral protease 3CLpro and the human protease cathepsin L, thus showing better drug effects at lower doses. Another advantage is that they can inhibit drug resistance.
Cathepsin B/L are crucial elements of the lysosomal pathway, and disruption of these host cell proteases offers potential for COVID-19 therapies. Smieszek et al.202 conducted a HTS to identify compounds that could downregulate the expression of cathepsin L/cathepsin B. Amantadine (10 μmol/L) can downregulate the expression of the cathepsin L gene, which appears to further disrupt the lysosomal pathway. Based on this, researchers believe that amantadine can reduce the viral load in SARS-CoV-2-positive patients and that it may be used as an effective treatment to reduce virus replication and infectivity, which may lead to better clinical outcomes.
6.4. Others
Innate immune response plays roles in combating coronavirus infection, while interferon can enhance the immune responses25. In human cells, blockage of the signal pathway required for viral replication is expected to exhibit some antiviral effect.
In patients infected with SARS-CoV-2, histological examination showed a strong cytokine storm and inflammatory response, and with the release of large amounts of interleukin (IL)-6, excessive inflammation and further lung damage resulted26,203. Thalidomide [Fig. 10A (84)] has anti-inflammatory activity owing to its ability to accelerate the degradation of messenger RNA in blood cells, thereby reducing reduce tumor necrosis factor-α. In addition, thalidomide can increase the secretion of interleukins, such as IL-12, and activate natural killer cells204. Thalidomide was one of many drugs redirected after the COVID-19 outbreak and used in clinical trials to treat COVID-19 patients (Table 3)205. It is a treatment for cancer and inflammatory diseases, but it is currently being used in two phase two clinical trials in combination with low-dose hormone therapy and adjuvant therapy for COVID-19.
Multiple sclerosis (MS) is an immune-mediated neurological disease that requires long-term immunotherapy and has been shown to increase the risk of SARS-CoV-2 infection206. Fingolimod [Fig. 10A (85)], a sphingosine-1-phosphate receptor immunomodulator, which is effective in the treatment of MS and is currently being tested as a treatment for COVID-19-associated acute respiratory distress syndrome (Table 3)205,207. A patient with MS infected with SARS-CoV-2 was treated with fingolimod and had a favorable outcome206.
In one study, Hu et al.208 evaluated leflunomide for COVID-19 treatment with a small cohort of patients, while the active metabolite of leflunomide, teriflunomide [Fig. 10A (86)], as an approved DHODH inhibitor, has been approved for treating autoimmune diseases209. They also proved that teriflunomide conferred a profound antiviral efficacy of IC50 = 6 μmol/L in SARS-CoV-2-infected cells at MOI of 0.03 (Table 3). Clinical data show that patients treated with leflunomide had shorter viral shedding time (median of 5 days) than that of the controls (median of 11 days). The C-reactive protein levels of patients given leflunomide also decreased significantly, indicating that immunopathological inflammation was well controlled. In leflunomide-treated patients, no obvious adverse reactions were observed208. This small-scale preliminary study on the compassionate use of leflunomide provides data basis for leflunomide as a potential therapeutic for COVID-19 in the future.
By virtual screening, Xiong et al.210 identified two potent human DHODH inhibitors with the same novel scaffold, S312 and S416, which is obviously different from those of leflunomide/teriflunomide208 or brequinar211. All compounds of teriflunomide, leflunomide, brequinar, S312, and S416 [Fig. 10A (86–90)] showed inhibitory effects against SARS-CoV-2 (at MOI = 0.05), with IC50s of 26.06, 41.49, 0.123, 1.56 and 0.017 μmol/L and CC50s of 850.5, 879.0, 231.3, 158.2 and 178.6 μmol/L, respectively210 (Table 3). These results indicate that both S312/S416 and leflunomide/teriflunomide, which have dual actions of antiviral and immunoregulation, may have the clinical potential to cure SARS-CoV-2.
Chloroquine and hydroxychloroquine, which are widely studied as clinical drugs for COVID-19, have a variety of cellular effects, including alkalizing lysosomes and blocking autophagy. Therefore, Gorshkov et al.212 evaluated additional lysosomotropic compounds to identify drugs that inhibit SARS-CoV-2. Six compounds (ROC-325, clomipramine, hycanthone, chloroquine, hydroxychloroquine and mefloquine) [Fig. 10A (91–93), Fig. 9 (75, 76), Fig. 10A (94)] showed inhibition of SARS-CoV-2 infection in Vero E6 cells with IC50s ranging from 2.0 to 13 μmol/L (Table 3) and SIs ranging from 1.5- to > 10-fold. In the EpiAirway 3D tissue model, these compounds exhibited a variety of functions, including prevention of lysosome function and autophagy and the entry of pseudotyped particles, increase of the pH of the lysosome, and reduction of (ROC-325) viral titers.
In order to enter and infect cells, viruses use a series of strategies to take advantage of the cellular and biochemical properties of cell membranes. As demonstrated, one of the entry mechanisms used by HIV was thiol-mediated uptake213. Cheng et al.214 systematically screened many effective inhibitors using fluorescent cyclic oligochalcogenides that enter cells by thiol-mediated uptake. Preliminary results on SARS-CoV-2 pseudovirus infection showed that the most potent activities were found for dithioerythritol thiosulfonate 16 [Fig. 10A (95)] with an IC50 of around 50 μmol/L, while toxicity was detected only at 500 μmol/L214. This study revealed that thiol-mediated uptake may be an interesting research direction for the development of future anti-SARS-CoV-2 drugs.
Halfon et al.215 reported that GNS561 showed most potent antiviral effect against two SARS-CoV-2 strains (IC50 = 0.006 μmol/L for USA-WA1/2020 and IC50 = 0.03 μmol/L for IHU MI6; MOI = 0.1) compared to chloroquine and remdesivir (Table 3). GNS561, located in LAMP2-positive lysosomes, together with SARS-CoV-2, blocked autophagy by increasing the size of LC3-II spots and increasing the volume of autophagic vacuoles in the cytoplasm with the presence of multilamellar bodies characteristic of a complexed autophagy215.
VPS34 is a multifunctional protein involved in autophagy, endocytosis and other processes. Two well-characterized VPS34 inhibitors, VPS34-IN1 [Fig. 10A (96)]216 and PIK-III [Fig. 10A (97)]217, showed anti-SARS-CoV-2 effects at a MOI of 0.01 with IC50s of 0.29 and 0.202 μmol/L in Vero E6 cells, respectively. However, both VPS34-IN1 and PIK-III exhibited strong cytotoxicity at 50 and 16.67 μmol/L in Vero E6 cells, respectively (Table 3)218. Orlistat and triacsin C [Fig. 10A (98, 99)] inhibit fatty acid metabolism, which have exhibited antiviral activity219,220. Both compounds also exhibited antiviral activity against SARS-CoV-2 with IC50s of 422.3 and 19.5 μmol/L in Vero E6 cells, respectively (Table 3) and did not induce obvious cytotoxicity, even at the high concentrations of 50 and 500 μmol/L, respectively. Time-of-addition studies show that these four inhibitors act at the post-entry step in the virus life cycle. Next, the researchers explored if these inhibitors were effective in Calu-3 by directly measuring production of infectious SARS-CoV-2 and cytotoxicity. IC50s of 0.55 μmol/L (VPS34-IN1), 0.12 μmol/L (PIK-III), 21.25 μmol/L (orlistat), and 0.04 μmol/L (triacsin C), and CC50s of > 50 μmol/L (VPS34-IN1), > 50 μmol/L (PIK-III), > 1 mmol/L (orlistat), and > 50 μmol/L (oriacsin C) were reported at the same time (Table 3)218.
MI-1851 [Fig. 10A (100)], a furin inhibitor, efficiently inhibited SARS-CoV-2 multiplication in Calu-3 cells, which produced a 30- to 190-fold reduction in virus titers at 10 μmol/L (Table 3). Combining various TMPRSS2 inhibitors (MI-432 or MI-1900) with furin inhibitor MI-1851 exhibited more potent antiviral activity against SARS-CoV-2 than any TMPRSS2 or furin inhibitor in an equimolar amount180. In addition, decanoyl-RVKR-chloromethylketone, a peptidomimetic furin inhibitor [Fig. 10A (101)] was shown to block SARS-CoV-2 S processing and inhibit SARS-CoV-2 infection with an IC50 of 0.057 μmol/L by plaque reduction assay221 (Table 3).
Homoharringtonine and emetine [Fig. 10A (102, 103)] showed antiviral activity against SARS-CoV-2 with IC50s of 2.55 and 0.46 μmol/L in Vero E6 cells, respectively (Table 3)99. Homoharringtonine has been reported to have antitumor activity by inhibiting protein transcription via its binding the site for ribosomal A222,223. Homoharringtonine has shown effective antiviral activity against herpes virus, coronavirus, rhabdoviruses and other viruses222,224,225. Emetine is a protein synthesis inhibitor, inhibits malaria through binding to the ribosomal E site of Plasmodium falciparum226,227. However, potential cardiotoxicity has limited its clinical use in recent years.
Bojkova et al.228 have identified SARS-CoV-2-modulated host cell pathways. Inhibition of these pathways could suppress SARS-CoV infection in human cells. A human cell-culture model infected with a clinical isolate of SARS-CoV-2 was established to analyze the infection profile of SARS-CoV-2 by translatome and proteome proteomics. Their results showed that SARS-CoV-2 reshaped the central cellular pathways, such as translation, splicing, carbon metabolism, protein homeostasis and nucleic acid metabolism. Small-molecule inhibitors that target these pathways prevent the virus from replicating in cells. Indeed, inhibition of glycolysis by 2-deoxy-d-glucose [2-DG, Fig. 10A (104)], an inhibitor of hexokinase (i.e., glycolysis), was previously shown to suppress rhinovirus infection in mice229. Blocking glycolysis with nontoxic concentrations of 2-DG inhibited SARS-CoV-2 replication in Caco-2 cells (Table 3)228. Pladienolide B [Fig. 10A (105)], a spliceosome inhibitor, targets the splicing factor SF3B1, which inhibited SARS-CoV-2 replication with an IC50 of 0.007 μmol/L that were not toxic to human Caco-2 cells (Table 3)228. Next, the team performed gene ontology analysis and identified a major cluster of metabolic pathways consisting of diverse nucleic acid metabolism sub-pathways upon SARS-CoV-2 infection. The researchers further confirmed that the replication of coronavirus depends on availability of cellular nucleotide pools230. Compounds interfering with nucleic acid metabolism, such as ribavirin [Fig. 10A (106)], could inhibit SARS-CoV-2 replication at low micromolar (IC50 = 0.07 mmol/L) and clinically achievable concentrations (Table 3)228,231. A clinical trial for ribavirin was recently initiated (Clinical Trials gov; NCT04356677). At the same time, NMS-873 [Fig. 10A (107)], a small-molecule inhibitor of ATP P97, could effectively inhibit SARS-CoV-2 replication at low nanomolar concentrations (IC50 = 0.025 μmol/L, Table 3)140,228, revealing that these two types of inhibitors can be used as potential treatment options for SARS-CoV-2. Cycloheximide [Fig. 10A (108)], an inhibitor of translation elongation, significantly inhibited SARS-CoV-2 replication with an IC50 of 0.17 μmol/L that are not toxic to human Caco-2 cells (Table 3)228.
AP-2-associated protein kinase 1 (AAK1) is a host kinase that regulates clathrin-mediated endocytosis232. Baricitinib [Fig. 10A (109)], a janus kinase inhibitor, is an AAK1-binding drug, which was supposed as a suitable drug candidate for COVID-19 because it can inhibit viral assembly by preventing AAK1-mediated endocytosis232. According to the EU Clinical Trials Register phases two and 3 (2020-001854-23), as well as phase-4 (2020-001354-22), clinical trials are now using baricitinib on COVID-19 patients (Table 3).
Nitazoxanide [Fig. 10A (110)] is an FDA-approved antibiotic for the treatment of diarrhea caused by Giardia parvum and Giardia lamblia and has broad-spectrum antiviral activity233. Nitazoxanide, which targets host-regulated processes involved in viral replication233, has shown in vitro activity against MERS-CoV and other animal coronaviruses234,235. The recent experimental result showed that nitazoxanide also had inhibitory activity against SARS-CoV-2 in vitro (IC50 = 2.12 μmol/L; CC50 > 35.53 μmol/L; SI > 16.76) (Table 3)19. In another study, nitazoxanide and JIB-04 [Fig. 10A (111)] had IC50 values of 4.90 μmol/L and 695 nmol/L in Vero E6 cells (Table 3), respectively. JIB-04 is a pan-Jumonji histone demethylase inhibitor236. Neither drug induced cytotoxicity at 300 μmol/L and thus had excellent selectivity index (SI > 150)237. A recent review evaluated nine clinical trials of nitazoxanide for assessing the safety, cost and potential use of this drug for COVID-19238.
Rodon et al.187 screened drugs that can combat SARS-CoV-2-induced CPE and virus replication in vitro from existing drugs approved for use in humans. Among of them, Fenofibrate [Fig. 10A (112)] is clinically used to treat dyslipidemia via activation of PPARa, and it also inhibited the CPE exerted by SARS-CoV-2 on Vero E6 cells at 20 μmol/L (Table 3). Plitidepsin [Fig. 10A (113)], the most potent one, targets eukaryotic elongation factor 1A2 and has been previously used for the treatment of multiple myeloma187. White and colleagues also reported that plitidepsin exhibits antiviral activity against SARS-CoV-2239. Based on immunofluorescence technology, they tested the inhibitory effects of plitidepsin on SARS-CoV-2 replication in Vero E6 cells, hACE2-293 T cells and human lung cells with IC50s of 0.70, 0.73 and 1.62 nmol/L with limited toxicity in cell culture (Table 3). Meanwhile, they demonstrated the in vivo antiviral activity of plitidepsin in two SARS-CoV-2 infected mouse models with reduced viral replication in the lungs. Plitidepsin has also successfully completed a phase 1/2 clinical study for the treatment of COVID-19 (Clinical Trials gov, NCT04382066).
Nitric oxide (NO) is a gas with various biological activities produced by arginine through NO synthase. NO inhalation is beneficial for most patients with severe ARDS240. Inhalation of NO triggers the relaxation of smooth muscles in pulmonary blood vessels, leading to increased blood flow and adequate ventilation in the lungs241. NO showed inhibition of the synthesis of viral RNA and proteins242. Therefore, NO inhalation may be an effective method for the treatment of patients with severe COVID-19169,241.
Clemizole hydrochloride [Fig. 10A (114)], a potent inhibitor of transient receptor potential channel TRPC5 and an orally bioavailable histamine H1 antagonist with potential antitumor and anti-allergic activities243, could prevent SARS-CoV-2 replication with an IC50 of 23.94 μmol/L and CC50 > 40 μmol/L in Vero E6 cells (Table 3)146.
Benztropine Mesylate [Fig. 10A (115)], an anticholinergic that works by blocking a certain natural substance (acetylcholine) is used to treat symptoms of Parkinson's disease or involuntary movement. It could inhibit SARS-CoV-2 replication with an IC50 of 13.8 μmol/L (MOI = 0.004) or 17.79 μmol/L (MOI = 0.01) and CC50 of >> 50 μmol/L in Vero E6 cells by CellTiter-Glo assays (Table 3)244.
The Weston group presented data on the antiviral activity of several FDA-approved drugs against SARS-CoV-2, including mefloquine hydrochloride [Fig. 10A (94)], fluphenazine dihydrochloride, amodiaquine hydrochloride, amodiaquine dihydrochloride dihydrate, thiethylperazine maleate, triparanol, terconazole vetranal, fluspirilene, clomipramine hydrochloride, promethazine hydrochloride, toremifene citrate, tamoxifen citrate and imatinib mesylate [Fig. 10A and B (116–127)], and the IC50 values of these drugs are at non-cytotoxic concentrations244. The IC50s at MOI = 0.01 and CC50s in Vero E6 cells of these drugs are shown in Table 3. Also, another team reported that toremifene and tamoxifen, as selective estrogen receptor modulators (SERMs), can affect ACE2 expression and offer a potential therapeutic approach for SARS-CoV-2170,245.
Zhang et al.246 established a CPE-based HTS assay in Vero-E6 cells that are permissive to SARS-CoV-2 infection to screen for inhibitors aiming for the entire viral life cycle. They screened a library collection of natural compounds containing 1058 compounds to identify potential inhibitors of SARS-CoV-2 in cell culture. After the primary screening, 30 hits with > 50% protection from CPE were identified. Among them, 17 drugs are newly discovered inhibitors of SARS-CoV-2. They next evaluated the effects of 17 newly discovered anti-SARS-CoV-2 compounds (bruceine A, cinobufagin, bufotaline, periplocoside, brusatol, veratridine, oridonin, isoalantolactone, isoliensinine, alantolactone, dehydrocostus lactone, momordinic, liensinine, dehydrodiisoeugenol, cornuside, roburicacid, coniferylaldehyde) and three previously reported coronaviruses inhibitors (bufalin, digoxin, and cryptotanshinone) [Fig. 10B (128–147)] by measuring the alterations of viral genome levels. All tested compounds showed inhibitory effects on virus propagation in a dose-dependent manner with the IC50 values ranging from 0.011 to 11.03 μmol/L (Table 3). According to the results of CCK-8 assay, the CC50 values of these compounds were also calculated (Table 3).
Kanjanasirirat et al.247 have used fluorescence-based SARS-CoV-2 nucleoprotein detection and plaque reduction assays to screen for antiviral candidates. Among 122 Thai natural products, they found that panduratin A [Fig. 10B (148)] could significantly inhibit SARS-CoV-2 infection in Vero E6 cells with IC50 of 0.81 μmol/L (CC50 = 14.71 μmol/L, Table 3). Their study also reported that treatment with panduratin A was able to inhibit viral infectivity in human airway epithelial cells.
Using cell-based infection assays, Jan et al.94 have screened more than 3000 agents that have been applied in humans and animals, including 2855 small molecules and 190 traditional herbal medicines. They have identified 15 active small molecules in concentrations ranging from 0.1 nmol/L to 50 μmol/L (Table 3), including nelfinavir [Fig. 5A-2 (34)], boceprevir [Fig. 5A-1 (28)], thioguanine [Fig. 10B (149)], cepharanthine [Fig. 4 (4)], emetine [Fig. 10A (103)], ivermectin [Fig. 6 (62)], moxidectin [Fig. 10B (150)], mefloquine [Fig. 10A (94)], ivacaftor, azelnidipine, penfluridol [Fig. 10B (151–153)], dronedarone [Fig. 5A-2 (44)], salinomycin, monensin and maduramicin [Fig. 10B (154–156)]. Mefloquine identified protected hamster disease models against challenge with SARS-CoV-2.
Puhl et al.248 found that some drugs shown to have in vitro activity against Ebola also showed activity against SARS-CoV-2. Therefore, they tested three small-molecule drugs active against Ebola virus in various cell lines (VeroE6, Vero 76, Caco-2, Calu-3, A549-ACE2, HUH-7 and monocytes) infected with SARS-CoV-2248. The compilation of these results suggests that there is considerable variability in antiviral activity observed in different cell lines. They found that tilorone and pyronaridine [Fig. 10B (157, 158)] inhibited virus replication with IC50s of 180 and 198 nmol/L in A549-ACE2 cells, respectively (Table 3).
Hopefully, clofazimine was verified to inhibit SARS-CoV-2 by its interference with viral membrane fusion and the function of helicase, as well as upregulated gene expression of innate immune-related pathways in cells69.
7. Conclusions and perspectives
Despite tremendous global efforts, COVID-19 remains a serious concern. Although many clinical trials of the repurposed drugs, immune-based therapies and investigational antivirals have been conducted, there is still no highly effective therapeutic for treatment of COVID-19 available. The mutation and pandemic of SARS-CoV-2 make vaccine and drug discovery more uncertain. Accordingly, developing specific or broad-spectrum inhibitors for SARS-CoV-2 virus entry, replication or prevention is urgently needed.
Continuous worldwide surveillance of the SARS-CoV-2 genome and continued efforts for quick screening of small-molecule databases will allow us to produce effective lead compounds or drug candidates, facilitating further in vitro, in vivo and clinical trials with which to determine their efficacy in the management of COVID-19. Computer-based drug design can accelerate the drug development process, but new interventions are still likely to require months to years to develop. Considering the case fatality rate of COVID-19, the quick screening of therapeutic agents for the repurposing of FDA-approved and clinical trial drugs may be a more practical approach. Repurposing old drugs for new indications may potentially lower development costs and shorten development timelines. Indeed, several clinical drugs and drug combinations are under repositioning in the treatment of COVID-19 patients. However, none of the clinical trials has resulted in completely satisfactory results, except for experiments still ongoing without final results.
In the long-term perspective, much basic work needs to be done in terms of generating effective small-molecule drugs. Like SARS-CoV and MERS-CoV, SARS-CoV-2 genome encodes nsps (3CLpro, PLpro, helicase and RdRp), structural proteins (S glycoprotein) and accessory proteins. S proteins and nsps described above are identified as attractive targets for antiviral agent development. So far, hundreds of active compounds have been screened for inhibitors against SARS-CoV-2 infection; however, many of these studies have not been rigorously conducted. Thus, it is essential for researchers to fully assess promiscuous compounds, avoiding the false positive activity readouts. In many cases, hit compounds containing Michael receptor, phenolic and quinone substructures or other unstable chemical bonds are actually nondruggable, now recognized as pan assay interference compounds (PAINS). Furthermore, protein structural analyses suggest that active sites in viral proteins are probably conserved among SARS-CoV, MERS-CoV and SARS-CoV-2. Therefore, we have enough relevant experience to design coronavirus inhibitors. Continuous efforts toward the accurate crystal structure of verified drug targets, preclinical evaluation of drug candidates, gathering of clinical evidence, as well as artificial intelligence, are all necessary for the successful identification of anti-SARS-CoV-2 drugs. Furthermore, host-targeted agents able to stimulate innate antiviral responses, or modulate virus–host interactions, are also options for COVID-19 treatment. With the ongoing efforts to prevent gradually increasing cases around the globe, the outbreak of COVID-19 has highlighted the importance for the development of broad-spectrum antiviral agents to combat future coronaviruses.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81974302 and 82041025), the Program for “333 Talents Project” of Hebei Province (A202002003, China), and Science and Technology Project of Hebei Education Department (QN2021071, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Yaning Gao, Email: gyn9225@bjmu.edu.cn.
Fei Yu, Email: shmyf@hebau.edu.cn.
Shibo Jiang, Email: shibojiang@fudan.edu.cn.
Author contributions
Shibo Jiang and Fei Yu designed the work. Fei Yu, Yaning Gao, Rong Xiang, Zhengsen Yu, Yang Wang, and Lili Wang collected data and wrote the manuscript. Shanshan Huo, Yanbai Li, Ruiying Liang and Qinghong Hao designed and regenerated the conceptual pictures. Shibo Jiang, Fei Yu and Tianlei Ying revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., et al. Author correction: a new coronavirus associated with human respiratory disease in China. Nature. 2020;580 doi: 10.1038/s41586-020-2202-3. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang S., Shi Z., Shu Y., Song J., Gao G.F., Tan W., et al. A distinct name is needed for the new coronavirus. Lancet. 2020;395:949. doi: 10.1016/S0140-6736(20)30419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J.M., Chung Y.S., Jo H.J., Lee N.J., Kim M.S., Woo S.H., et al. Identification of coronavirus isolated from a patient in Korea with COVID-19. Osong Public Health Res Perspect. 2020;11:3–7. doi: 10.24171/j.phrp.2020.11.1.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem. 2020;21:730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coronaviridae Study Group of the International Committee on Taxonomy of V The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim D., Lee J.Y., Yang J.S., Kim J.W., Kim V.N., Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181:914–921. doi: 10.1016/j.cell.2020.04.011. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S., et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microb Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clausen T.M., Sandoval D.R., Spliid C.B., Pihl J., Perrett H.R., Painter C.D., et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183:1043–1057. doi: 10.1016/j.cell.2020.09.033. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham R.L., Sparks J.S., Eckerle L.D., Sims A.C., Denison M.R. SARS coronavirus replicase proteins in pathogenesis. Virus Res. 2008;133:88–100. doi: 10.1016/j.virusres.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masters P.S. The molecular biology of coronaviruses. Adv Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stertz S., Reichelt M., Spiegel M., Kuri T., Martinez-Sobrido L., Garcia-Sastre A., et al. The intracellular sites of early replication and budding of SARS-coronavirus. Virology. 2007;361:304–315. doi: 10.1016/j.virol.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin M.H., Moses D.C., Hsieh C.H., Cheng S.C., Chen Y.H., Sun C.Y., et al. Disulfiram can inhibit MERS and SARS coronavirus papain-like proteases via different modes. Antivir Res. 2018;150:155–163. doi: 10.1016/j.antiviral.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheahan T.P., Sims A.C., Leist S.R., Schafer A., Won J., Brown A.J., et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaher N.H., Mostafa M.I., Altaher A.Y. Design, synthesis and molecular docking of novel triazole derivatives as potential CoV helicase inhibitors. Acta Pharm. 2020;70:145–159. doi: 10.2478/acph-2020-0024. [DOI] [PubMed] [Google Scholar]
- 21.O'Keefe B.R., Giomarelli B., Barnard D.L., Shenoy S.R., Chan P.K., McMahon J.B., et al. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J Virol. 2010;84:2511–2521. doi: 10.1128/JVI.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarma P., Shekhar N., Prajapat M., Avti P., Kaur H., Kumar S., et al. In-silico homology assisted identification of inhibitor of RNA binding against 2019-nCoV N-protein (N terminal domain) J Biomol Struct Dyn. 2020;39:1–9. doi: 10.1080/07391102.2020.1753580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 24.Phonphok Y., Rosenthal K.S. Stabilization of clathrin coated vesicles by amantadine, tromantadine and other hydrophobic amines. FEBS Lett. 1991;281:188–190. doi: 10.1016/0014-5793(91)80390-o. [DOI] [PubMed] [Google Scholar]
- 25.Omrani A.S., Saad M.M., Baig K., Bahloul A., Abdul-Matin M., Alaidaroos A.Y., et al. Ribavirin and interferon alfa-2a for severe middle east respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X., Han M., Li T., Sun W., Wang D., Fu B., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han Y., Duan X., Yang L., Nilsson-Payant B.E., Wang P., Duan F., et al. Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature. 2021;589:270–275. doi: 10.1038/s41586-020-2901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni L., Zhou L., Zhou M., Zhao J., Wang D.W. Combination of western medicine and Chinese traditional patent medicine in treating a family case of COVID-19. Front Med. 2020;14:210–214. doi: 10.1007/s11684-020-0757-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li R., Hou Y., Huang J., Pan W., Ma Q., Shi Y., et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol Res. 2020;156:104761. doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94 doi: 10.1128/JVI.00127-20. e00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walls A.C., Tortorici M.A., Snijder J., Xiong X., Bosch B.J., Rey F.A., et al. Tectonic conformational changes of a coronavirus spike glycoprotein promote membrane fusion. Proc Natl Acad Sci U S A. 2017;114:11157–11162. doi: 10.1073/pnas.1708727114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S., Xiao G., Chen Y., He Y., Niu J., Escalante C.R., et al. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet. 2004;363:938–947. doi: 10.1016/S0140-6736(04)15788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bosch B.J., van der Zee R., de Haan C.A., Rottier P.J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He Y. Synthesized peptide inhibitors of HIV-1 gp41-dependent membrane fusion. Curr Pharmaceut Des. 2013;19:1800–1809. doi: 10.2174/1381612811319100004. [DOI] [PubMed] [Google Scholar]
- 38.Lu L., Liu Q., Zhu Y., Chan K.H., Qin L., Li Y., et al. Structure-based discovery of middle east respiratory syndrome coronavirus fusion inhibitor. Nat Commun. 2014;5:3067. doi: 10.1038/ncomms4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C., Xia S., Zhang P., Zhang T., Wang W., Tian Y., et al. Discovery of hydrocarbon-stapled short alpha-helical peptides as promising middle east respiratory syndrome coronavirus (MERS-CoV) fusion inhibitors. J Med Chem. 2018;61:2018–2026. doi: 10.1021/acs.jmedchem.7b01732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bosch B.J., Martina B.E., Van Der Zee R., Lepault J., Haijema B.J., Versluis C., et al. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc Natl Acad Sci U S A. 2004;101:8455–8460. doi: 10.1073/pnas.0400576101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ujike M., Nishikawa H., Otaka A., Yamamoto N., Yamamoto N., Matsuoka M., et al. Heptad repeat-derived peptides block protease-mediated direct entry from the cell surface of severe acute respiratory syndrome coronavirus but not entry via the endosomal pathway. J Virol. 2008;82:588–592. doi: 10.1128/JVI.01697-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu I.J., Kao C.L., Hsieh S.C., Wey M.T., Kan L.S., Wang W.K. Identification of a minimal peptide derived from heptad repeat (HR) 2 of spike protein of SARS-CoV and combination of HR1-derived peptides as fusion inhibitors. Antivir Res. 2009;81:82–87. doi: 10.1016/j.antiviral.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aydin H., Al-Khooly D., Lee J.E. Influence of hydrophobic and electrostatic residues on SARS-coronavirus S2 protein stability: insights into mechanisms of general viral fusion and inhibitor design. Protein Sci. 2014;23:603–617. doi: 10.1002/pro.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia S., Yan L., Xu W., Agrawal A.S., Algaissi A., Tseng C.K., et al. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci Adv. 2019;5 doi: 10.1126/sciadv.aav4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Y., Chong H., Yu D., Guo Y., Zhou Y., He Y. Design and characterization of cholesterylated peptide HIV-1/2 fusion inhibitors with extremely potent and long-lasting antiviral activity. J Virol. 2019;93:e02312–e02318. doi: 10.1128/JVI.02312-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chong H., Xue J., Zhu Y., Cong Z., Chen T., Wei Q., et al. Monotherapy with a low-dose lipopeptide HIV fusion inhibitor maintains long-term viral suppression in rhesus macaques. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu Y., Zhang X., Ding X., Chong H., Cui S., He J., et al. Exceptional potency and structural basis of a T1249-derived lipopeptide fusion inhibitor against HIV-1, HIV-2, and simian immunodeficiency virus. J Biol Chem. 2018;293:5323–5334. doi: 10.1074/jbc.RA118.001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Y., Yu D., Yan H., Chong H., He Y. Design of potent membrane fusion inhibitors against SARS-CoV-2, an emerging coronavirus with high fusogenic activity. J Virol. 2020;94 doi: 10.1128/JVI.00635-20. e00635-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia S., Zhu Y., Liu M., Lan Q., Xu W., Wu Y., et al. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell Mol Immunol. 2020;17:765–767. doi: 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Vries R.D., Schmitz K.S., Bovier F.T., Predella C., Khao J., Noack D., et al. Intranasal fusion inhibitory lipopeptide prevents direct-contact SARS-CoV-2 transmission in ferrets. Science. 2021;371:1379–1382. doi: 10.1126/science.abf4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang G., Pomplun S., Loftis R., Tan X., Loas A., Pentelute B.L. Investigation of ACE2 N-terminal fragments binding to SARS-CoV-2 Spike RBD. bioRxiv. 2020 https://www.biorxiv.org/content/10.1101/2020.03.19.999318v2 Available from: [Google Scholar]
- 53.Cao L., Goreshnik I., Coventry B., Case J.B., Miller L., Kozodoy L., et al. De novo design of picomolar SARS-CoV-2 miniprotein inhibitors. Science. 2020;370:426–431. doi: 10.1126/science.abd9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beddingfield B.J., Iwanaga N., Chapagain P., Zheng W., Roy J., Hu T.Y., et al. The integrin binding peptide, ATN-161, as a novel therapy for SARS-CoV-2 infection. JACC Basic Transl Sci. 2021;6:1–8. doi: 10.1016/j.jacbts.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Clercq E. New developments in anti-HIV chemotherapy. Biochim Biophys Acta. 2002;1587:258–275. doi: 10.1016/s0925-4439(02)00089-3. [DOI] [PubMed] [Google Scholar]
- 56.Han Y., Kral P. Computational design of ACE2-based peptide inhibitors of SARS-CoV-2. ACS Nano. 2020;14:5143–5147. doi: 10.1021/acsnano.0c02857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watson A., Ferreira L., Hwang P., Xu J., Stroud R. Peptide antidotes to SARS-CoV-2 (COVID-19) bioRxiv. 2020 https://www.biorxiv.org/content/10.1101/2020.08.06.238915v2 Available from: [Google Scholar]
- 58.Yang C., Pan X., Xu X., Cheng C., Huang Y., Li L., et al. Salvianolic acid C potently inhibits SARS-CoV-2 infection by blocking the formation of six-helix bundle core of spike protein. Signal Transduct Target Ther. 2020;5:220. doi: 10.1038/s41392-020-00325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vankadari N. Arbidol: a potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein. Int J Antimicrob Agents. 2020;56:105998. doi: 10.1016/j.ijantimicag.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X., Cao R., Zhang H., Liu J., Xu M., Hu H., et al. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov. 2020;6:28. doi: 10.1038/s41421-020-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H., et al. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J Infect. 2020;81:e1–e5. doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lian N., Xie H., Lin S., Huang J., Zhao J., Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect. 2020;26:917–921. doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J.N., Wang W.J., Peng B., Peng W., Zhang Y.S., Wang Y.L., et al. Potential of arbidol for post-exposure prophylaxis of COVID-19 transmission: a preliminary report of a retrospective cohort study. Curr Med Sci. 2020;40:480–485. doi: 10.1007/s11596-020-2203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Y., Xie Z., Lin W., Cai W., Wen C., Guan Y., et al. Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: an exploratory randomized controlled trial. Med (N Y) 2020;1:105–113. doi: 10.1016/j.medj.2020.04.001. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bojadzic D., Alcazar O., Chen J., Chuang S.T., Condor C.J.M., Shehadeh L.A., et al. Small-molecule inhibitors of the coronavirus spike: ACE2 protein‒protein interaction as blockers of viral attachment and entry for SARS-CoV-2. ACS Infect Dis. 2021;7:1519–1534. doi: 10.1021/acsinfecdis.1c00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen C.Z., Xu M., Pradhan M., Gorshkov K., Petersen J.D., Straus M.R., et al. Identifying SARS-CoV-2 entry inhibitors through drug repurposing screens of SARS-S and MERS-S pseudotyped particles. ACS Pharmacol Transl Sci. 2020;3:1165–1175. doi: 10.1021/acsptsci.0c00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gopal M., Padayatchi N., Metcalfe J.Z., O'Donnell M.R. Systematic review of clofazimine for the treatment of drug-resistant tuberculosis. Int J Tubercul Lung Dis. 2013;17:1001–1007. doi: 10.5588/ijtld.12.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riva L., Yuan S., Yin X., Martin-Sancho L., Matsunaga N., Pache L., et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020;586:113–119. doi: 10.1038/s41586-020-2577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan S., Yin X., Meng X., Chan J.F., Ye Z.W., Riva L., et al. Clofazimine broadly inhibits coronaviruses including SARS-CoV-2. Nature. 2021;593:418–423. doi: 10.1038/s41586-021-03431-4. [DOI] [PubMed] [Google Scholar]
- 70.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu S., Zheng Q., Wang Z. Potential covalent drugs targeting the main protease of the SARS-CoV-2 coronavirus. Bioinformatics. 2020;36:3295–3298. doi: 10.1093/bioinformatics/btaa224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Y., Yang W.H., Huang L.M., Wang Y.C., Yang C.S., Yi L.L., Hou M.H., et al. Inhibition of severe acute respiratory syndrome coronavirus 2 main protease by tafenoquine invitro. bioRxiv. 2020 https://www.biorxiv.org/content/10.1101/2020.08.14.250258v1 Available from: [Google Scholar]
- 73.Dow G.S., Luttick A., Fenner J., Wesche D., Yeo K.R., Rayner C. Tafenoquine inhibits replication of SARS-Cov-2 at pharmacologically relevant concentrations in vitro. bioRxiv. 2020 https://www.biorxiv.org/content/10.1101/2020.07.12.199059v1 Available from: [Google Scholar]
- 74.Rathnayake A.D., Zheng J., Kim Y., Perera K.D., Mackin S., Meyerholz D.K., et al. 3C-like protease inhibitors block coronavirus replication in vitro and improve survival in MERS-CoV-infected mice. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abc5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Su H., Yao S., Zhao W., Li M., Liu J., Shang W., et al. Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS-CoV-2 3CL protease in vitro. bioRxiv. 2020 https://www.biorxiv.org/content/10.1101/2020.04.13.038687v1 Available from: [Google Scholar]
- 76.Huang S., Liu Y., Zhang Y., Zhang R., Zhu C., Fan L., et al. Baicalein inhibits SARS-CoV-2/VSV replication with interfering mitochondrial oxidative phosphorylation in a mPTP dependent manner. Signal Transduct Target Ther. 2020;5:266. doi: 10.1038/s41392-020-00353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu H., Ye F., Sun Q., Liang H., Li C., Li S., et al. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J Enzym Inhib Med Chem. 2021;36:497–503. doi: 10.1080/14756366.2021.1873977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Drayman N., Jones K.A., Azizi S.A., Froggatt H.M., Tan K., Maltseva N.I., et al. Drug repurposing screen identifies masitinib as a 3CLpro inhibitor that blocks replication of SARS-CoV-2 in vitro. bioRxiv. 2020 https://www.biorxiv.org/content/10.1101/2020.08.31.274639v1 Available from: [Google Scholar]
- 79.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., et al. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 80.Ma C., Hu Y., Townsend J.A., Lagarias P.I., Marty M.T., Kolocouris A., et al. Ebselen, disulfiram, carmofur, PX-12, tideglusib, and shikonin are nonspecific promiscuous SARS-CoV-2 main protease inhibitors. ACS Pharmacol Transl Sci. 2020;3:1265–1277. doi: 10.1021/acsptsci.0c00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jin Z., Zhao Y., Sun Y., Zhang B., Wang H., Wu Y., et al. Structural basis for the inhibition of SARS-CoV-2 main protease by antineoplastic drug carmofur. Nat Struct Mol Biol. 2020;27:529–532. doi: 10.1038/s41594-020-0440-6. [DOI] [PubMed] [Google Scholar]
- 82.Dai W., Zhang B., Jiang X.M., Su H., Li J., Zhao Y., et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved alpha-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vuong W., Khan M.B., Fischer C., Arutyunova E., Lamer T., Shields J., et al. Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication. Nat Commun. 2020;11:4282. doi: 10.1038/s41467-020-18096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iketani S., Forouhar F., Liu H., Hong S.J., Lin F.Y., Nair M.S., et al. Lead compounds for the development of SARS-CoV-2 3CL protease inhibitors. Nat Commun. 2021;12:2016. doi: 10.1038/s41467-021-22362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma C., Sacco M.D., Hurst B., Townsend J.A., Hu Y., Szeto T., et al. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 2020;30:678–692. doi: 10.1038/s41422-020-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fu L., Ye F., Feng Y., Yu F., Wang Q., Wu Y., et al. Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease. Nat Commun. 2020;11:4417. doi: 10.1038/s41467-020-18233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sacco M.D., Ma C., Lagarias P., Gao A., Townsend J.A., Meng X., et al. Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against M(pro) and cathepsin L. Sci Adv. 2020;6 doi: 10.1126/sciadv.abe0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu Y., Ma C., Szeto T., Hurst B., Tarbet B., Wang J. Boceprevir, calpain inhibitors II and XII, and GC-376 have broad-spectrum antiviral activity against coronaviruses. ACS Infect Dis. 2021;7:586–597. doi: 10.1021/acsinfecdis.0c00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Caceres C.J., Cardenas-Garcia S., Carnaccini S., Seibert B., Rajao D.S., Wang J., et al. Efficacy of GC-376 against SARS-CoV-2 virus infection in the K18 hACE2 transgenic mouse model. Sci Rep. 2021;11:9609. doi: 10.1038/s41598-021-89013-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi Y., Shuai L., Wen Z., Wang C., Yan Y., Jiao Z., et al. The preclinical inhibitor GS441524 in combination with GC376 efficaciously inhibited the proliferation of SARS-CoV-2 in the mouse respiratory tract. Emerg Microb Infect. 2021;10:481–492. doi: 10.1080/22221751.2021.1899770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vatansever E.C., Yang K.S., Drelich A.K., Kratch K.C., Cho C.C., Kempaiah K.R., et al. Bepridil is potent against SARS-CoV-2 in vitro. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2012201118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang K.S., Ma X.R., Ma Y., Alugubelli Y.R., Scott D.A., Vatansever E.C., et al. A quick route to multiple highly potent SARS-CoV-2 main protease inhibitors. ChemMedChem. 2021;16:942–948. doi: 10.1002/cmdc.202000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jan J.T., Cheng T.R., Juang Y.P., Ma H.H., Wu Y.T., Yang W.B., et al. Identification of existing pharmaceuticals and herbal medicines as inhibitors of SARS-CoV-2 infection. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2021579118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu W., Xu M., Chen C.Z., Guo H., Shen M., Hu X., et al. Identification of SARS-CoV-2 3CL protease inhibitors by a quantitative high-throughput screening. ACS Pharmacol Transl Sci. 2020;3:1008–1016. doi: 10.1021/acsptsci.0c00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Salgado-Benvindo C., Thaler M., Tas A., Ogando N.S., Bredenbeek P.J., Ninaber D.K., et al. Suramin inhibits SARS-CoV-2 infection in cell culture by interfering with early steps of the replication cycle. Antimicrob Agents Chemother. 2020;64:e00900–e00920. doi: 10.1128/AAC.00900-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S., et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chan J.F., Yao Y., Yeung M.L., Deng W., Bao L., Jia L., et al. Treatment with lopinavir/ritonavir or interferon-beta1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Choy K.T., Wong A.Y., Kaewpreedee P., Sia S.F., Chen D., Hui K.P.Y., et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir Res. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nukoolkarn V., Lee V.S., Malaisree M., Aruksakulwong O., Hannongbua S. Molecular dynamic simulations analysis of ritonavir and lopinavir as SARS-CoV 3CL(pro) inhibitors. J Theor Biol. 2008;254:861–867. doi: 10.1016/j.jtbi.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu C.Y., Jan J.T., Ma S.H., Kuo C.J., Juan H.F., Cheng Y.S., et al. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc Natl Acad Sci U S A. 2004;101:10012–10017. doi: 10.1073/pnas.0403596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lim J., Jeon S., Shin H.Y., Kim M.J., Seong Y.M., Lee W.J., et al. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Kor Med Sci. 2020;35:e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M.Y., et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.WHO R&D Blueprint . World Health Organization; 2020. Informal consultation on prioritization of candidate therapeutic agents for use in novel coronavirus 2019 infection.https://www.who.int/teams/blueprint/covid-19 Available from: [Google Scholar]
- 106.Liu X., Li Z., Liu S., Sun J., Chen Z., Jiang M., et al. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta Pharm Sin B. 2020;10:1205–1215. doi: 10.1016/j.apsb.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hattori S.I., Higshi-Kuwata N., Raghavaiah J., Das D., Bulut H., Davis D.A., et al. GRL-0920, an indole chloropyridinyl ester, completely blocks SARS-CoV-2 infection. mBio. 2020;11 doi: 10.1128/mBio.01833-20. e01833-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qiao J., Li Y.S., Zeng R., Liu F.L., Luo R.H., Huang C., et al. SARS-CoV-2 M(pro) inhibitors with antiviral activity in a transgenic mouse model. Science. 2021;371:1374–1378. doi: 10.1126/science.abf1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Freitas B.T., Durie I.A., Murray J., Longo J.E., Miller H.C., Crich D., et al. Characterization and noncovalent inhibition of the deubiquitinase and deISGylase activity of SARS-CoV-2 papain-like protease. ACS Infect Dis. 2020;6:2099–2109. doi: 10.1021/acsinfecdis.0c00168. [DOI] [PubMed] [Google Scholar]
- 110.Chen X., Chou C.Y., Chang G.G. Thiopurine analogue inhibitors of severe acute respiratory syndrome-coronavirus papain-like protease, a deubiquitinating and deISGylating enzyme. Antivir Chem Chemother. 2009;19:151–156. doi: 10.1177/095632020901900402. [DOI] [PubMed] [Google Scholar]
- 111.Baez-Santos Y.M., Barraza S.J., Wilson M.W., Agius M.P., Mielech A.M., Davis N.M., et al. X-ray structural and biological evaluation of a series of potent and highly selective inhibitors of human coronavirus papain-like proteases. J Med Chem. 2014;57:2393–2412. doi: 10.1021/jm401712t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klemm T., Ebert G., Calleja D.J., Allison C.C., Richardson L.W., Bernardini J.P., et al. Mechanism and inhibition of the papain-like protease, PLpro, of SARS-CoV-2. EMBO J. 2020;39 doi: 10.15252/embj.2020106275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kouznetsova V.L., Zhang A., Tatineni M., Miller M.A., Tsigelny I.F. Potential COVID-19 papain-like protease PL(pro) inhibitors: repurposing FDA-approved drugs. PeerJ. 2020;8 doi: 10.7717/peerj.9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abruzzese E., Luciano L., D'Agostino F., Trawinska M.M., Pane F., De Fabritiis P. SARS-CoV-2 (COVID-19) and chronic myeloid leukemia (CML): a case report and review of ABL kinase involvement in viral infection. Mediterr J Hematol Infect Dis. 2020;12 doi: 10.4084/MJHID.2020.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mirza M.U., Ahmad S., Abdullah I., Froeyen M. Identification of novel human USP2 inhibitor and its putative role in treatment of COVID-19 by inhibiting SARS-CoV-2 papain-like (PLpro) protease. Comput Biol Chem. 2020;89:107376. doi: 10.1016/j.compbiolchem.2020.107376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gao X., Qin B., Chen P., Zhu K., Hou P., Wojdyla J.A., et al. Crystal structure of SARS-CoV-2 papain-like protease. Acta Pharm Sin B. 2021;11:237–245. doi: 10.1016/j.apsb.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fu Z., Huang B., Tang J., Liu S., Liu M., Ye Y., et al. The complex structure of GRL0617 and SARS-CoV-2 PLpro reveals a hot spot for antiviral drug discovery. Nat Commun. 2021;12:488. doi: 10.1038/s41467-020-20718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gao Y., Yan L., Huang Y., Liu F., Zhao Y., Cao L., et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368:779–782. doi: 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eastman R.T., Roth J.S., Brimacombe K.R., Simeonov A., Shen M., Patnaik S., et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci. 2020;6:672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sheahan T.P., Sims A.C., Zhou S., Graham R.L., Pruijssers A.J., Agostini M.L., et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9 doi: 10.1128/mBio.00221-18. e00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of COVID-19–final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V., Abdool Karim Q., et al. Repurposed antiviral drugs for COVID-19–interim WHO solidarity trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Elfiky A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yin W., Luan X., Li Z., Zhou Z., Wang Q., Gao M., et al. Structural basis for inhibition of the SARS-CoV-2 RNA polymerase by suramin. Nat Struct Mol Biol. 2021;28:319–325. doi: 10.1038/s41594-021-00570-0. [DOI] [PubMed] [Google Scholar]
- 127.Oestereich L., Ludtke A., Wurr S., Rieger T., Munoz-Fontela C., Gunther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antivir Res. 2014;105:17–21. doi: 10.1016/j.antiviral.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 128.Coomes E.A., Haghbayan H. Favipiravir, an antiviral for COVID-19? J Antimicrob Chemother. 2020;75:2013–2014. doi: 10.1093/jac/dkaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Du Y.X., Chen X.P. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin Pharmacol Ther. 2020;108:242–247. doi: 10.1002/cpt.1844. [DOI] [PubMed] [Google Scholar]
- 130.Reynard O., Nguyen X.N., Alazard-Dany N., Barateau V., Cimarelli A., Volchkov V.E. Identification of a new ribonucleoside inhibitor of Ebola virus replication. Viruses. 2015;7:6233–6240. doi: 10.3390/v7122934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Urakova N., Kuznetsova V., Crossman D.K., Sokratian A., Guthrie D.B., Kolykhalov A.A., et al. β-d-N4-Hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome. J Virol. 2018;92 doi: 10.1128/JVI.01965-17. e01965-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Toots M., Yoon J.J., Cox R.M., Hart M., Sticher Z.M., Makhsous N., et al. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aax5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Agostini M.L., Pruijssers A.J., Chappell J.D., Gribble J., Lu X., Andres E.L., et al. Small-molecule antiviral β-d-N4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J Virol. 2019;93 doi: 10.1128/JVI.01348-19. e01348-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Gotte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from middle east respiratory syndrome coronavirus. J Biol Chem. 2020;295:4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mestres J. The target landscape of N4-hydroxycytidine based on its chemical neighborhood. bioRxiv. 2020 https://www.biorxiv.org/content/10.1101/2020.03.30.016485v1 Available from: [Google Scholar]
- 136.Kwong A.D., Rao B.G., Jeang K.T. Viral and cellular RNA helicases as antiviral targets. Nat Rev Drug Discov. 2005;4:845–853. doi: 10.1038/nrd1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Frick D.N., Lam A.M. Understanding helicases as a means of virus control. Curr Pharmaceut Des. 2006;12:1315–1338. doi: 10.2174/138161206776361147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tanner J.A., Zheng B.J., Zhou J., Watt R.M., Jiang J.Q., Wong K.L., et al. The adamantane-derived bananins are potent inhibitors of the helicase activities and replication of SARS coronavirus. Chem Biol. 2005;12:303–311. doi: 10.1016/j.chembiol.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim M.K., Yu M.S., Park H.R., Kim K.B., Lee C., Cho S.Y., et al. 2,6-Bis-arylmethyloxy-5-hydroxychromones with antiviral activity against both hepatitis C virus (HCV) and SARS-associated coronavirus (SCV) Eur J Med Chem. 2011;46:5698–5704. doi: 10.1016/j.ejmech.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee C., Lee J.M., Lee N.R., Jin B.S., Jang K.J., Kim D.E., et al. Aryl diketoacids (ADK) selectively inhibit duplex DNA-unwinding activity of SARS coronavirus NTPase/helicase. Bioorg Med Chem Lett. 2009;19:1636–1638. doi: 10.1016/j.bmcl.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Adedeji A.O., Singh K., Calcaterra N.E., DeDiego M.L., Enjuanes L., Weiss S., et al. Severe acute respiratory syndrome coronavirus replication inhibitor that interferes with the nucleic acid unwinding of the viral helicase. Antimicrob Agents Chemother. 2012;56:4718–4728. doi: 10.1128/AAC.00957-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Decroly E., Debarnot C., Ferron F., Bouvet M., Coutard B., Imbert I., et al. Crystal structure and functional analysis of the SARS-coronavirus RNA cap 2'-O-methyltransferase nsp 10/nsp 16 complex. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Menachery V.D., Debbink K., Baric R.S. Coronavirus non-structural protein 16: evasion, attenuation, and possible treatments. Virus Res. 2014;194:191–199. doi: 10.1016/j.virusres.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Khan R.J., Jha R.K., Amera G.M., Jain M., Singh E., Pathak A., et al. Targeting SARS-CoV-2: a systematic drug repurposing approach to identify promising inhibitors against 3C-like proteinase and 2'-O-ribose methyltransferase. J Biomol Struct Dyn. 2020;39:2679–2692. doi: 10.1080/07391102.2020.1753577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Encinar J.A., Menendez J.A. Potential drugs targeting early innate immune evasion of SARS-coronavirus 2 via 2’-O-methylation of viral RNA. Viruses. 2020;12:525. doi: 10.3390/v12050525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Touret F., Gilles M., Barral K., Nougairede A., van Helden J., Decroly E., et al. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci Rep. 2020;10:13093. doi: 10.1038/s41598-020-70143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cong Y., Ulasli M., Schepers H., Mauthe M., V'Kovski P., Kriegenburg F., et al. Nucleocapsid protein recruitment to replication-transcription complexes plays a crucial role in coronaviral life cycle. J Virol. 2020;94 doi: 10.1128/JVI.01925-19. e01925-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McBride R., van Zyl M., Fielding B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014;6:2991–3018. doi: 10.3390/v6082991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ahmed S.F., Quadeer A.A., McKay M.R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12:254. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kang S., Yang M., Hong Z., Zhang L., Huang Z., Chen X., et al. Crystal structure of SARS-CoV-2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta Pharm Sin B. 2020;10:1228–1238. doi: 10.1016/j.apsb.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yamamoto N., Matsuyama S., Hoshino T., Yamamoto N. Nelfinavir inhibits replication of severe acute respiratory syndrome coronavirus 2 in vitro. bioRxiv. 2020 https://www.biorxiv.org/content/10.1101/2020.04.06.026476v1 Available from: [Google Scholar]
- 153.Brown S.M., Peltan I.D., Webb B., Kumar N., Starr N., Grissom C., et al. Hydroxychloroquine versus azithromycin for hospitalized patients with suspected or confirmed COVID-19 (HAHPS). protocol for a pragmatic, open-label, active comparator trial. Ann Am Thorac Soc. 2020;17:1008–1015. doi: 10.1513/AnnalsATS.202004-309SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Goodwin E.C., Atwood W.J., DiMaio D. High-throughput cell-based screen for chemicals that inhibit infection by simian virus 40 and human polyomaviruses. J Virol. 2009;83:5630–5639. doi: 10.1128/JVI.00203-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gonzalez C.A., Sahagun P.A.M., Diez Liebana M.J., Fernandez M.N., Sierra V.M., Garcia V.J.J. The pharmacokinetics and interactions of ivermectin in humans–a mini-review. AAPS J. 2008;10:42–46. doi: 10.1208/s12248-007-9000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wagstaff K.M., Sivakumaran H., Heaton S.M., Harrich D., Jans D.A. Ivermectin is a specific inhibitor of importin alpha/beta-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. 2012;443:851–856. doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Huet T., Beaussier H., Voisin O., Jouveshomme S., Dauriat G., Lazareth I., et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2:e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kuster G.M., Pfister O., Burkard T., Zhou Q., Twerenbold R., Haaf P., et al. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur Heart J. 2020;41:1801–1803. doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Callera G.E., Antunes T.T., Correa J.W., Moorman D., Gutsol A., He Y., et al. Differential renal effects of candesartan at high and ultra-high doses in diabetic mice-potential role of the ACE2/AT2R/Mas axis. Biosci Rep. 2016;36 doi: 10.1042/BSR20160344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Deppe S., Boger R.H., Weiss J., Benndorf R.A. Telmisartan: a review of its pharmacodynamic and pharmacokinetic properties. Expet Opin Drug Metabol Toxicol. 2010;6:863–871. doi: 10.1517/17425255.2010.494597. [DOI] [PubMed] [Google Scholar]
- 162.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020;81:537–540. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sun M.L., Yang J.M., Sun Y.P., Su G.H. Inhibitors of RAS might be a good choice for the therapy of COVID-19 pneumonia. Zhonghua Jiehe He Huxi Zazhi. 2020;43:E014. doi: 10.3760/cma.j.issn.1001-0939.2020.0014. [DOI] [PubMed] [Google Scholar]
- 164.Speth R.C. Response to recent commentaries regarding the involvement of angiotensin-converting enzyme 2 (ACE2) and renin-angiotensin system blockers in SARS-CoV-2 infections. Drug Dev Res. 2020;81:643–646. doi: 10.1002/ddr.21672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Talreja H., Tan J., Dawes M., Supershad S., Rabindranath K., Fisher J., et al. A consensus statement on the use of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in relation to COVID-19 (corona virus disease 2019) N Z Med J. 2020;133:85–87. [PubMed] [Google Scholar]
- 166.Schwarz S., Wang K., Yu W., Sun B., Schwarz W. Emodin inhibits current through SARS-associated coronavirus 3a protein. Antivir Res. 2011;90:64–69. doi: 10.1016/j.antiviral.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ho T.Y., Wu S.L., Chen J.C., Li C.C., Hsiang C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir Res. 2007;74:92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sanklecha V. Novel coronavirus COVID-19 and its diagnosis and treatments. Int J. 2020;8:39–43. [Google Scholar]
- 169.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Takahashi S., Yoshiya T., Yoshizawa-Kumagaye K., Sugiyama T. Nicotianamine is a novel angiotensin-converting enzyme 2 inhibitor in soybean. Biomed Res. 2015;36:219–224. doi: 10.2220/biomedres.36.219. [DOI] [PubMed] [Google Scholar]
- 172.Chen H., Du Q. Potential natural compounds for preventing SARS-CoV-2 (2019-nCoV) infection. Preprints. 2020. https://www.preprints.org/manuscript/202001.0358/v2# Preprints. Available from: [Google Scholar]
- 173.Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bhowmik D., Nandi R., Prakash A., Kumar D. Evaluation of flavonoids as 2019-nCoV cell entry inhibitor through molecular docking and pharmacological analysis. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lucas J.M., Heinlein C., Kim T., Hernandez S.A., Malik M.S., True L.D., et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4:1310–1325. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hoffmann M., Schroeder S., Kleine-Weber H., Muller M.A., Drosten C., Pohlmann S. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.00754-20. e00754-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yamamoto M., Kiso M., Sakai-Tagawa Y., Iwatsuki-Horimoto K., Imai M., Takeda M., et al. The anticoagulant nafamostat potently inhibits SARS-CoV-2 S protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-type-dependent manner. Viruses. 2020;12:629. doi: 10.3390/v12060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Asakura H., Ogawa H. Potential of heparin and nafamostat combination therapy for COVID-19. J Thromb Haemostasis. 2020;18:1521–1522. doi: 10.1111/jth.14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hammami M., Rühmann E., Maurer E., Heine A., Gütschow M., Klebe G., et al. New 3-amidinophenylalanine-derived inhibitors of matriptase. MedChemComm. 2012;3:807–813. [Google Scholar]
- 180.Bestle D., Heindl M.R., Limburg H., Van Lam van T., Pilgram O., Moulton H., et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci Alliance. 2020;3 doi: 10.26508/lsa.202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Qiu Z., Hingley S.T., Simmons G., Yu C., Das Sarma J., Bates P., et al. Endosomal proteolysis by cathepsins is necessary for murine coronavirus mouse hepatitis virus type 2 spike-mediated entry. J Virol. 2006;80:5768–5776. doi: 10.1128/JVI.00442-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Regan A.D., Shraybman R., Cohen R.D., Whittaker G.R. Differential role for low pH and cathepsin-mediated cleavage of the viral spike protein during entry of serotype II feline coronaviruses. Vet Microbiol. 2008;132:235–248. doi: 10.1016/j.vetmic.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhao H., Zhou J., Zhang K., Chu H., Liu D., Poon V.K., et al. A novel peptide with potent and broad-spectrum antiviral activities against multiple respiratory viruses. Sci Rep. 2016;6:22008. doi: 10.1038/srep22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhao H., To K.K.W., Sze K.H., Yung T.T., Bian M., Lam H., et al. A broad-spectrum virus- and host-targeting peptide against respiratory viruses including influenza virus and SARS-CoV-2. Nat Commun. 2020;11:4252. doi: 10.1038/s41467-020-17986-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhao H., To K.K.W., Lam H., Zhou X., Chan J.F., Peng Z., et al. Cross-linking peptide and repurposed drugs inhibit both entry pathways of SARS-CoV-2. Nat Commun. 2021;12:1517. doi: 10.1038/s41467-021-21825-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang L.H., Rothberg K.G., Anderson R.G. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rodon J., Munoz-Basagoiti J., Perez-Zsolt D., Noguera-Julian M., Paredes R., Mateu L., et al. Identification of plitidepsin as potent inhibitor of SARS-CoV-2-induced cytopathic effect after a drug repurposing screen. Front Pharmacol. 2021;12:646676. doi: 10.3389/fphar.2021.646676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 190.Hoffmann M., Mosbauer K., Hofmann-Winkler H., Kaul A., Kleine-Weber H., Kruger N., et al. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature. 2020;585:588–590. doi: 10.1038/s41586-020-2575-3. [DOI] [PubMed] [Google Scholar]
- 191.Maisonnasse P., Guedj J., Contreras V., Behillil S., Solas C., Marlin R., et al. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020;585:584–587. doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- 192.Schneider M., Ackermann K., Stuart M., Wex C., Protzer U., Schatzl H.M., et al. Severe acute respiratory syndrome coronavirus replication is severely impaired by MG132 due to proteasome-independent inhibition of M-calpain. J Virol. 2012;86:10112–10122. doi: 10.1128/JVI.01001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhou Y., Simmons G. Development of novel entry inhibitors targeting emerging viruses. Expert Rev Anti Infect Ther. 2012;10:1129–1138. doi: 10.1586/eri.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yamada H., Mori H., Nakanishi Y., Nishikawa S., Hashimoto Y., Ochi Y., et al. Effects of the cathepsin K inhibitor ONO-5334 and concomitant use of ONO-5334 with methotrexate on collagen-induced arthritis in cynomolgus monkeys. Int J Rheumatol. 2019;2019:5710340. doi: 10.1155/2019/5710340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Elie B.T., Gocheva V., Shree T., Dalrymple S.A., Holsinger L.J., Joyce J.A. Identification and pre-clinical testing of a reversible cathepsin protease inhibitor reveals anti-tumor efficacy in a pancreatic cancer model. Biochimie. 2010;92:1618–1624. doi: 10.1016/j.biochi.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rutherford A.C., Traer C., Wassmer T., Pattni K., Bujny M.V., Carlton J.G., et al. The mammalian phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) regulates endosome-to-TGN retrograde transport. J Cell Sci. 2006;119:3944–3957. doi: 10.1242/jcs.03153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kang Y.L., Chou Y.Y., Rothlauf P.W., Liu Z., Soh T.K., Cureton D., et al. Inhibition of PIKfyve kinase prevents infection by Zaire ebolavirus and SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117:20803–20813. doi: 10.1073/pnas.2007837117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bjorck L., Grubb A., Kjellen L. Cystatin C, a human proteinase inhibitor, blocks replication of herpes simplex virus. J Virol. 1990;64:941–943. doi: 10.1128/jvi.64.2.941-943.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Baron S.A., Devaux C., Colson P., Raoult D., Rolain J.M. Teicoplanin: an alternative drug for the treatment of COVID-19? Int J Antimicrob Agents. 2020;55:105944. doi: 10.1016/j.ijantimicag.2020.105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Colson P., Raoult D. Fighting viruses with antibiotics: an overlooked path. Int J Antimicrob Agents. 2016;48:349–352. doi: 10.1016/j.ijantimicag.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang J., Ma X., Yu F., Liu J., Zou F., Pan T., et al. Teicoplanin potently blocks the cell entry of 2019-nCoV. bioRxiv. 2020 https://www.biorxiv.org/content/10.1101/2020.02.05.935387v1 Available from: [Google Scholar]
- 202.Smieszek S.P., Przychodzen B.P., Polymeropoulos M.H. Amantadine disrupts lysosomal gene expression: a hypothesis for COVID19 treatment. Int J Antimicrob Agents. 2020;55:106004. doi: 10.1016/j.ijantimicag.2020.106004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Barlow A., Landolf K.M., Barlow B., Yeung S.Y.A., Heavner J.J., Claassen C.W., et al. Review of emerging pharmacotherapy for the treatment of coronavirus disease 2019. Pharmacotherapy. 2020;40:416–437. doi: 10.1002/phar.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Newfield C. New medical indications for thalidomide and its derivatives. The Science Journal of the Lander College of Arts and Sciences. 2018;12:3. [Google Scholar]
- 205.Rosa S.G.V., Santos W.C. Clinical trials on drug repositioning for COVID-19 treatment. Rev Panam Salud Públic. 2020;44:e40. doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Foerch C., Friedauer L., Bauer B., Wolf T., Adam E.H. Severe COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Mult Scler Relat Disord. 2020;42:102180. doi: 10.1016/j.msard.2020.102180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Giovannoni G., Hawkes C., Lechner-Scott J., Levy M., Waubant E., Gold J. The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult Scler Relat Disord. 2020;39:102073. doi: 10.1016/j.msard.2020.102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hu K., Wang M., Zhao Y., Zhang Y., Wang T., Zheng Z., et al. A small-scale medication of leflunomide as a treatment of COVID-19 in an open-label blank-controlled clinical trial. Virol Sin. 2020;35:725–733. doi: 10.1007/s12250-020-00258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fragoso Y.D., Brooks J.B. Leflunomide and teriflunomide: altering the metabolism of pyrimidines for the treatment of autoimmune diseases. Expet Rev Clin Pharmacol. 2015;8:315–320. doi: 10.1586/17512433.2015.1019343. [DOI] [PubMed] [Google Scholar]
- 210.Xiong R., Zhang L., Li S., Sun Y., Ding M., Wang Y., et al. Novel and potent inhibitors targeting DHODH are broad-spectrum antivirals against RNA viruses including newly-emerged coronavirus SARS-CoV-2. Protein Cell. 2020;11:723–739. doi: 10.1007/s13238-020-00768-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen S.F., Perrella F.W., Behrens D.L., Papp L.M. Inhibition of dihydroorotate dehydrogenase activity by brequinar sodium. Cancer Res. 1992;52:3521–3527. [PubMed] [Google Scholar]
- 212.Gorshkov K., Chen C.Z., Bostwick R., Rasmussen L., Tran B.N., Cheng Y.S., et al. The SARS-CoV-2 cytopathic effect is blocked by lysosome alkalizing small molecules. ACS Infect Dis. 2020;7:1389–1408. doi: 10.1021/acsinfecdis.0c00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ryser H.J., Fluckiger R. Progress in targeting HIV-1 entry. Drug Discov Today. 2005;10:1085–1094. doi: 10.1016/S1359-6446(05)03550-6. [DOI] [PubMed] [Google Scholar]
- 214.Cheng Y., Pham A.T., Kato T., Lim B., Moreau D., López-Andarias J., et al. Inhibitors of thiol-mediated uptake. Chem Sci. 2021;12:626–631. doi: 10.1039/d0sc05447j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Halfon P., Bestion E., Zandi K., Andreani J., Baudoin J.P., Scola B.L., et al. GNS561 exhibits potent in vitro antiviral activity against SARS-CoV-2 through autophagy inhibition. bioRxiv. 2020 doi: 10.3390/v14010132. https://www.biorxiv.org/content/10.1101/2020.10.06.327635v1 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bago R., Malik N., Munson M.J., Prescott A.R., Davies P., Sommer E., et al. Characterization of VPS34-IN1, a selective inhibitor of Vps34, reveals that the phosphatidylinositol 3-phosphate-binding SGK3 protein kinase is a downstream target of class III phosphoinositide 3-kinase. Biochem J. 2014;463:413–427. doi: 10.1042/BJ20140889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Dowdle W.E., Nyfeler B., Nagel J., Elling R.A., Liu S., Triantafellow E., et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat Cell Biol. 2014;16:1069–1079. doi: 10.1038/ncb3053. [DOI] [PubMed] [Google Scholar]
- 218.Silvas J.A., Jureka A.S., Nicolini A.M., Chvatal S.A., Basler C.F. Inhibitors of VPS34 and lipid metabolism suppress SARS-CoV-2 replication. bioRxiv. 2020 doi: 10.1016/j.celrep.2021.109479. https://www.biorxiv.org/content/10.1101/2020.07.18.210211v1 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ammer E., Nietzsche S., Rien C., Kuhnl A., Mader T., Heller R., et al. The anti-obesity drug orlistat reveals anti-viral activity. Med Microbiol Immunol. 2015;204:635–645. doi: 10.1007/s00430-015-0391-4. [DOI] [PubMed] [Google Scholar]
- 220.Nasheri N., Joyce M., Rouleau Y., Yang P., Yao S., Tyrrell D.L., et al. Modulation of fatty acid synthase enzyme activity and expression during hepatitis C virus replication. Chem Biol. 2013;20:570–582. doi: 10.1016/j.chembiol.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 221.Cheng Y.W., Chao T.L., Li C.L., Chiu M.F., Kao H.C., Wang S.H., et al. Furin inhibitors block SARS-CoV-2 spike protein cleavage to suppress virus production and cytopathic effects. Cell Rep. 2020;33:108254. doi: 10.1016/j.celrep.2020.108254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Dong H.J., Wang Z.H., Meng W., Li C.C., Hu Y.X., Zhou L., et al. The natural compound homoharringtonine presents broad antiviral activity in vitro and in vivo. Viruses. 2018;10:601. doi: 10.3390/v10110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lu S., Wang J. Homoharringtonine and omacetaxine for myeloid hematological malignancies. J Hematol Oncol. 2014;7:2. doi: 10.1186/1756-8722-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Dyall J., Coleman C.M., Hart B.J., Venkataraman T., Holbrook M.R., Kindrachuk J., et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014;58:4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Andersen P.I., Krpina K., Ianevski A., Shtaida N., Jo E., Yang J., et al. Novel antiviral activities of obatoclax, emetine, niclosamide, brequinar, and homoharringtonine. Viruses. 2019;11:964. doi: 10.3390/v11100964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Grollman A.P. Structural basis for inhibition of protein synthesis by emetine and cycloheximide based on an analogy between ipecac alkaloids and glutarimide antibiotics. Proc Natl Acad Sci U S A. 1966;56:1867–1874. doi: 10.1073/pnas.56.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wong W., Bai X.C., Brown A., Fernandez I.S., Hanssen E., Condron M., et al. Cryo-EM structure of the plasmodium falciparum 80S ribosome bound to the anti-protozoan drug emetine. Elife. 2014;3 doi: 10.7554/eLife.03080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bojkova D., Klann K., Koch B., Widera M., Krause D., Ciesek S., et al. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583:469–472. doi: 10.1038/s41586-020-2332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gualdoni G.A., Mayer K.A., Kapsch A.M., Kreuzberg K., Puck A., Kienzl P., et al. Rhinovirus induces an anabolic reprogramming in host cell metabolism essential for viral replication. Proc Natl Acad Sci U S A. 2018;115:E7158–E7165. doi: 10.1073/pnas.1800525115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pruijssers A.J., Denison M.R. Nucleoside analogues for the treatment of coronavirus infections. Curr Opin Virol. 2019;35:57–62. doi: 10.1016/j.coviro.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wu L.S., Rower J.E., Burton J.R., Jr., Anderson P.L., Hammond K.P., Baouchi-Mokrane F., et al. Population pharmacokinetic modeling of plasma and intracellular ribavirin concentrations in patients with chronic hepatitis C virus infection. Antimicrob Agents Chemother. 2015;59:2179–2188. doi: 10.1128/AAC.04618-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rossignol J.F. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antivir Res. 2014;110:94–103. doi: 10.1016/j.antiviral.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rossignol J.F. Nitazoxanide, a new drug candidate for the treatment of middle east respiratory syndrome coronavirus. J Infect Public Health. 2016;9:227–230. doi: 10.1016/j.jiph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cao J., Forrest J.C., Zhang X. A screen of the NIH clinical collection small molecule library identifies potential anti-coronavirus drugs. Antivir Res. 2015;114:1–10. doi: 10.1016/j.antiviral.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wang L., Chang J., Varghese D., Dellinger M., Kumar S., Best A.M., et al. A small molecule modulates Jumonji histone demethylase activity and selectively inhibits cancer growth. Nat Commun. 2013;4:2035. doi: 10.1038/ncomms3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Son J., Huang S., Zeng Q., Bricker T.L., Case J.B., Zhou J., et al. Nitazoxanide and JIB-04 have broad-spectrum antiviral activity and inhibit SARS-CoV-2 replication in cell culture and coronavirus pathogenesis in a pig model. bioRxiv. 2020 doi: 10.1128/mbio.03377-21. https://www.biorxiv.org/content/10.1101/2020.09.24.312165v1 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pepperrell T., Pilkington V., Owen A., Wang J., Hill A.M. Review of safety and minimum pricing of nitazoxanide for potential treatment of COVID-19. J Virus Erad. 2020;6:52–60. doi: 10.1016/S2055-6640(20)30017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.White K.M., Rosales R., Yildiz S., Kehrer T., Miorin L., Moreno E., et al. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science. 2021;371:926–931. doi: 10.1126/science.abf4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rossaint R., Gerlach H., Schmidt-Ruhnke H., Pappert D., Lewandowski K., Steudel W., et al. Efficacy of inhaled nitric oxide in patients with severe ARDS. Chest. 1995;107:1107–1115. doi: 10.1378/chest.107.4.1107. [DOI] [PubMed] [Google Scholar]
- 241.Berhes M., Fabian A., Laszlo I., Vegh T., Molnar C., Fulesdi B., et al. Organ replacement therapy and life-supporting treatment modalities in critically ill COVID-19 patients. Orv Hetil. 2020;161:704–709. doi: 10.1556/650.2020.31813. [DOI] [PubMed] [Google Scholar]
- 242.Hui D.S. An overview on severe acute respiratory syndrome (SARS) Monaldi Arch Chest Dis. 2005;63:149–157. doi: 10.4081/monaldi.2005.632. [DOI] [PubMed] [Google Scholar]
- 243.Richter J.M., Schaefer M., Hill K. Clemizole hydrochloride is a novel and potent inhibitor of transient receptor potential channel TRPC5. Mol Pharmacol. 2014;86:514–521. doi: 10.1124/mol.114.093229. [DOI] [PubMed] [Google Scholar]
- 244.Weston S., Coleman C.M., Haupt R., Logue J., Matthews K., Li Y., et al. Broad anti-coronavirus activity of food and drug administration-approved drugs against SARS-CoV-2 in vitro and SARS-CoV in vivo. J Virol. 2020;94:e01218–e01220. doi: 10.1128/JVI.01218-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lu L., Su S., Yang H., Jiang S. Antivirals with common targets against highly pathogenic viruses. Cell. 2021;184:1604–1620. doi: 10.1016/j.cell.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 246.Zhang Z.R., Zhang Y.N., Li X.D., Zhang H.Q., Xiao S.Q., Deng F., et al. A cell-based large-scale screening of natural compounds for inhibitors of SARS-CoV-2. Signal Transduct Target Ther. 2020;5:218. doi: 10.1038/s41392-020-00343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kanjanasirirat P., Suksatu A., Manopwisedjaroen S., Munyoo B., Tuchinda P., Jearawuttanakul K., et al. High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component panduratin A as anti-SARS-CoV-2 agents. Sci Rep. 2020;10:19963. doi: 10.1038/s41598-020-77003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Puhl A.C., Fritch E.J., Lane T.R., Tse L.V., Yount B.L., Sacramento C.Q., et al. Repurposing the ebola and marburg virus inhibitors tilorone, quinacrine, and pyronaridine: in vitro activity against SARS-CoV-2 and potential mechanisms. ACS Omega. 2021;6:7454–7468. doi: 10.1021/acsomega.0c05996. [DOI] [PMC free article] [PubMed] [Google Scholar]