Abstract
Background: Studies have shown that the prevalence of children born with high birth weight or large for gestational age (LGA) is increasing. This is true for spontaneous pregnancies; however, children born after frozen embryo transfer (FET) as part of assisted reproductive technology (ART) also have an elevated risk. In recent years, the practice of FET has increased rapidly and while the perinatal and obstetric risks are well-studied, less is known about the long-term health consequences.
Objective: The aim of this systematic review was to describe the association between high birth weight and LGA on long-term child outcomes.
Data Sources: PubMed, Scopus, and Web of Science were searched up to January 2021. Exposure included high birth weight and LGA. Long-term outcome variables included malignancies, psychiatric disorders, cardiovascular disease, and diabetes.
Study Selection: Original studies published in English or Scandinavian languages were included. Studies with a control group were included while studies published as abstracts and case reports were excluded.
Data Extraction: The methodological quality, in terms of risk of bias, was assessed by pairs of reviewers. Robins-I (www.methods.cochrane.org) was used for risk of bias assessment in original articles. For systematic reviews, AMSTAR (www.amstar.ca) was used. For certainty of evidence, we used the GRADE system. The systematic review followed PRISMA guidelines. When possible, meta-analyses were performed.
Results: The search included 11,767 articles out of which 173 met the inclusion criteria and were included in the qualitative analysis, while 63 were included in quantitative synthesis (meta-analyses). High birth weight and/or LGA was associated with low to moderately elevated risks for certain malignancies in childhood, breast cancer, several psychiatric disorders, hypertension in childhood, and type 1 and 2 diabetes.
Conclusions: Although the increased risks for adverse outcome in offspring associated with high birth weight and LGA represent serious health effects in childhood and in adulthood, the size of these effects seems moderate. The identified risk association should, however, be taken into account in decisions concerning fresh and frozen ART cycles and is of general importance in view of the increasing prevalence in high birthweight babies.
Keywords: assisted reproduction, frozen embryo transfer, large for gestational age, high birth weight, long-term morbidity, cancer, diabetes
Introduction
The association between preterm birth (PTB), low birth weight (LBW), and small for gestational age (SGA) and neonatal and long-term outcomes is well-described and suggests higher risks for cardiovascular diseases, diabetes, hypertension, and stroke later in life according to the Barker hypothesis (1). Less attention has been paid to high birthweight children and children born large for gestational age (LGA), particularly the long-term outcomes. The prevalence of high birthweight and LGA babies is increasing (2, 3), in parallel with the worldwide rise in obesity, also among women of childbearing age (3). In assisted reproduction, several studies have shown that children born after transfer of frozen/thawed embryos (FET) have a lower risk of preterm birth, low birth weight, and SGA compared with singletons born after fresh transfer but also a higher risk of being born with a high birth weight and LGA (4–6). Due to high success rates, FET of vitrified/warmed blastocysts has increased dramatically in recent years, including the “freeze all” technique where all available embryos of good quality are cryopreserved for later use in a natural or programmed cycle (7–11). The perinatal outcomes for babies of high birth weight and being LGA are mainly associated with difficulties at delivery such as asphyxia, shoulder dystocia, hypoglycemia, respiratory problems, cesarean section, and obstetric injuries (12, 13). For long-term outcomes, an association has been found between high birth weight and child malignancies, breast cancer, psychiatric disorders, and cardiometabolic diseases (14–19).
The aim of this systematic review and meta-analysis is to summarize the present knowledge on long-term outcomes for children born with a high birth weight or being LGA.
Methods
We searched PubMed, Scopus, and Web of Science databases up to January 2021. Exposures were large for gestational age and high birth weight. Long-term morbidity outcomes studied were cancer, metabolic disease, cardiovascular disease, and psychiatric disorders. Cancer was focused on breast cancer, child malignancies in the central nervous system (CNS), hematological malignancies, and Wilm's tumor. Metabolic diseases were focused on diabetes type 1 and type 2. Cardiovascular disease was focused on hypertension and other cardiovascular disorders. Psychiatric disorders were focused on schizophrenia/psychosis and cognitive disorders. Some of these outcomes, when appropriate, were used for meta-analysis.
Systematic Search for Evidence
The terms used in the searches are listed below:
LGA[tiab] OR large for gestational age[tiab] OR large-for-gestational age[tiab] OR HBW[tiab] OR high birth weight*[tiab] OR higher birth weight*[tiab] OR highest birth weight*[tiab] OR high birthweight*[tiab] OR higher birthweight*[tiab] OR highest birthweight*[tiab] OR macrosomia[tiab]. Because of large heterogenecity in the nomenclature of diseases and to avoid missing any important morbidity, we decided not to include any specific disease or morbidity terms in the search.
We also manually searched reference lists of identified articles for additional references. Guidelines for meta-analysis and systematic reviews (SR) of observational studies were followed (20). The literature search was performed by two researchers (Å.M. and C.B.) and one librarian. Screening of abstracts and of full papers for inclusion was done by pairs of reviewers. Differences of opinion in the team were solved by discussion until consensus was achieved.
The last literature search was performed January 14, 2021.
Inclusion and Exclusion of Studies
Original studies published in English or Scandinavian languages were included. In the case of double publication, the latest study was included. Studies with a control group were included. Studies published only as abstracts and case reports were excluded.
Definitions
High birth weight was defined by each author but usually ≥4,000 or ≥4,500 or occasionally >5 g. LGA was defined by each author.
Appraisal of Certainty of Evidence
The methodological quality of original studies, in terms of risk of bias, was assessed by pairs of reviewers by the tool Robins-I (http://www.methods.cochrane.org). For systematic reviews, we used AMSTAR (http://www.amstar.ca). For certainty of evidence, we used the GRADE system (21). The systematic review followed PRISMA guidelines (22).
Data Synthesis
Outcomes are given in odds ratio (OR), adjusted odds ratio (AOR), hazard ratio (HR), adjusted hazard ratio (AHR), relative risk (RR), adjusted relative risk (ARR), incidence rate ratio (IRR), adjusted incidence rate ratio (AIRR), standardized incidence ratio (SIR), or random-effects odds ratio (REOR) with 95% CIs. Meta-analyses were performed despite significant heterogeneity in reference groups and despite the fact that outcomes were given in AOR, ARR, or ROR. However, studies reporting estimates as HR, AHR, AIRR, and SIR were not mixed with the RR- and OR-based outcomes. The HR- and IR-based outcomes were also too few to be included in a separate meta-analysis. A random-effects meta-analysis using the Der Simonian and Laird method, with the estimate of heterogeneity being taken from the Mantel–Haenszel model, was used in the analysis (command metan in Stata 15).
Results
The search strategy identified a total of 11,767 abstracts, of which 173 were selected for inclusion in the systematic review and 63 for inclusion in quantitative synthesis (meta-analysis) (Figure 1). No papers, particularly focusing in children with high birth weight born after FET, were identified.
Figure 1.
PRISMA flow chart. From Moher et al. (22). For more information, visit www.prisma-statement.org.
Among the studies included were 19 meta-analyses, 73 cohort studies, 74 case–control studies, and seven cross-sectional studies (tables, characteristics of included studies and excluded studies, with reasons for exclusion, are presented in Supplementary Tables 1.1–1.4, 2.1–2.4).
A quality assessment of the cohort, case–control, and cross-sectional studies included is presented in Supplementary Tables 3.1–3.4 and for systematic reviews in Supplementary Table 4. Of the selected cohort, case–control, and cross-sectional studies, 28 articles had low, 79 had moderate, 47 had serious, and two had critical risk of bias. Of the systematic reviews, 10 were of high, five of medium, and four were of low quality. Summary of findings (SoF) is presented in Supplementary Table 5.
Malignancies
Outcomes are listed in Table 1.1.
Table 1.1.
LGA and high birth weight and long-term outcomes—malignancies.
| Author, year, country | Study design | Cases | Outcomes (risk estimates) | Reference group (weight) | Comments/adjustments | Risk of bias | Directness | Precision |
|---|---|---|---|---|---|---|---|---|
| Breast cancer Systematic reviews/meta-analyses n = 3 | ||||||||
| Michels and Xue (2006), USA (21) | • Meta-analysis • Cohort n = 11 • Case–control n = 16 |
12,301 | • Birth weight >4,000 g (one study >3,000 g) • Cohort studies OR/HR/SIR 1.24 (95% CI 1.10–1.40) • Case–control studies OR/HR/SIR 1.21 (95% CI 1.06–1.38) • Total RR 1.23 (95% CI 1.13–1.24) |
<2,500 g | Partly overlap with Xue (24) | |||
| Xue and Michels (2007), USA (23) | • Cohort n = 14 Case–control n = 18 • Systematic review, meta-analysis |
21,845 | RR with increased birth weights 1.15 (1.09–1.21) | • Partly overlap (23) • The association disappeared after adjustment for birth length |
||||
| Zhou et al. (2020), China (24) | • Case/control n = 16 • Systematic review, meta-analysis |
16,000 | • RR per 500 g increase in birth weight • All ages: 1.02 (95% CI 1.01–1.03) • Pre-menopausal RR 1.09 (95% CI 1.04–1.15) |
|||||
| Breast cancer Original articles n = 19 | ||||||||
| Andersson et al. (2001), Sweden (25) • All cancers |
Cohort n = 1,080 | 62 | Birth weight 4,000–5,500 g RR 1.57 (95% CI 0.67–3.64) | 1,600–3,000 g | Adjusted for cohort membership, gestational age | Serious | Good | Poor |
| Ahlgren et al. (2003), Denmark (26) | Cohort n = 106,504 | 2,334 | • Risk increase 8% per 1,000 g increase in birth weight (95% CI 1–16%) • Birth weight >5,000 g RR 1.2 |
3,000–3,399 g | Adjustments for age and calendar period | Moderate | Good | Good |
| Ahlgren et al. (2004), Denmark (27) | Cohort n = 117,415 | 3,340 | • Weight category 4,000 g (median) • RR 1.17 (95% CI 1.02–1.33) |
2,500 g (median) | Adjustments for attained age, calendar period, age of first childbirth and parity | Moderate | Good | Good |
| Ahlgren et al. (2007), Denmark (28) | Cohort >200,000 men and women | 3,066 | RR for trend 1.05 (95% CI 0.98–1.12) | 3,000–3,499 g | Adjustment for age and calendar period | Moderate | Good | Good |
| Barber et al. (2019), USA (29) | Cohort n = 20,959 | 601 | Birth weight >4,000 g HR 1.26 (95% CI 0.97–1.63) | 2,500–3,999 g | Adjustments for time period, age, parity, age at first birth and family history of breast cancer | Serious | Good | Fair |
| dos Santos et al. (2004), UK (30) | Cohort n = 2,176 | 59 | Birth weight≥4,000 g ARR 1.57 (95% CI 0.60–4.13) | <3,000 g | Adjusted for age | Moderate | Good | Poor |
| Innes et al. (2000), USA (14) | Case–control | 484 | Birth weight >4,500 g AOR 3.10 (95% CI 1.18–7.97) | 2,500–3,499 g | Adjustments for gestational age, preeclampsia, abruptio placentae, multiple gestation, parity (birth rank), number of previous births, maternal age, paternal age, and race | Serious | Good | Poor |
| Lahmann et al. (2004), Sweden (35) | Case–control | 89 | Birth weight >4,000 g AOR 2.66 (95% CI 0.96–7.41) | <3,000 g | Adjustments for gestational age, birth year, pre-eclampsia, parental occupation, adult BMI, and educational attainment | Serious | Good | Poor |
| McCormack et al. (2003), Sweden (31) | Cohort n = 5,358 | 359 | • Birth weight >4,000 g Premenopausal (<50 years) RR 3.48 (95% CI 1.29–9.38) • Postmenopausal (>50 years) RR 0.87 (95% CI 0.56–1.36) |
<3,000 g | Adjustments for gestational age, marital status, children in home, age at first marriage, level of education, occupation, car possession | Low | Good | Fair |
| Mellemkjær et al. (2003), Denmark (36) | Case–control | 881 | Birth weight ≥4,000 g AOR 1.25 (95% CI 1.00–1.55) | 3,000–3,499 g | Adjustments for marital status, birth order, maternal age at birth | Moderate | Good | Good |
| Michels et al. (1996), USA (37) | Case–control | 582 | Lower birth categories had significantly lower OR. Example 3,000–3,499 AOR 0.68 (95% CI 0.48–0.97) | >4,000 | Adjustments for age, parity, cohort, age at first birth, age at menarche, BMI and family history of breast cancer | Serious | Good | Good |
| Michels and Xue (2006), USA, (21) | • Longitudinal cohort • n = 152,608 |
3,140 | Lower weight categories had significantly lower HR. Example HR 0.66 (95% CI 0.47–0.93) if <2,495 g | >3,815 g | Adjustments for age, premature birth, age at menarche, BMI at age 18, current BMI, family history of breast cancer, history of benign breast disease, age at first birth, oral contraceptive use, physical activity, and alcohol consumption | Low | Good | Good |
| Mogren et al. (1999), Sweden (33) | • Cohort • n = 248,701 |
57 | • High birth weight, >4,500 g • SIR 7.35 (95% CI 0.10–40.87) |
Sex, age, calendar-specific person-year | Low | Good | Poor | |
| Sanderson et al. (2002), USA (38) | Case–control | 288 | • High birth weight ≥4,000 g • AOR 0.7 (95% CI 0.4–1.4) |
2,500–2,999 g | • Total 1,459 breast cancer, premenopausal interviewed, n = 288/296 • Adjusted for age, income, family history of breast cancer, history of fibroid adenoma, age at menarche, parity, age at first live birth |
Moderate | Fair | Fair |
| Troisi et al. (2013), Sweden, Norway, Denmark (39) | Case–control | 1,419 | • Birth weight ≥4,000 g RR 1.14 (95% CI 0.98–1.34) • Continuous per 500 g RR 1.07 (95% CI 1.02–1.13) |
2,500–3,999 g | Adjusted for gestational length | Low | Good | Good |
| Titus-Ernstoff et al. (2002), USA (40) | Case–control | 5,659 | Birth weight ≥4,500 g OR 1.18 (95% CI 0.92–1.51) | 3,000–3,499 g | Adjustments for BMI at reference date, Jewish/non-Jewish, family history of breast cancer, age at first birth, parity, age at menopause | Serious | Good | Fair |
| Vatten et al. (2002), Norway (41) | Case–control | 373 | Birth weight >3,730 g OR 1.4 (95% CI 1.1–1.9) | <3,090 g | Adjustments for age at first birth and parity | Low | Fair | Fair |
| Vatten et al. (2005), Norway (34) | • Cohort • n = 16,016 |
312 | Birth weight >3,840 g RR 1.5 (95% CI 1.0–2.2) | <3,040 g | Adjustments for year of birth, gestational length, marital status, socioeconomic status, maternal age, and birth order | Moderate | Good | Fair |
| Wu et al. (2011), USA (42) | Case–control | 2,259 | Birth weight ≥4,000 g OR 1.97 (95% CI 1.15–3.39) | <2,500 g | Adjustment for age, age at menarche, parity, adult BMI, Asian ethnicity, interviewer, years in USA, menopausal status, age at menopause, total calories, physical activity, and family history of breast cancer | Serious | Poor | Fair |
| • CNS tumors • Systematic reviews/meta-analyses • n = 4 | ||||||||
| Dahlhaus et al. (2016), Germany (43) | • Systematic review • Cohort n = 3 • Case–control n = 11 |
18,845 | • >4,000 g • Astrocytoma REOR 1.60 (96% CI 1.23–2.09) • Ependymoma REOR 1.18 (95% CI 0.97–1.43) • Medulloblastoma REOR 1.31 (95% CI 1.08–1.58) |
<4,000 g | Different adjustments in different studies | |||
| Georgakis et al. (2017), Greece (45) | • Systematic review and MA • Cohort n = 9 • Case–control n = 32 |
53,167 | • CNS tumors overall • >4,000 g OR 1.14 (95% CI 1.08–1.20) • LGA OR 1.12 (95% CI 1.03–1.22) |
<4,000 g AGA | Only child cases n = 22,330 I meta-analyses | |||
| Harder et al. (2008), Germany (44) | • Meta-analysis • Cohort n = 2 • Case–control n = 6 |
3,665 | • >4,000 g • Astrocytoma OR 1.38 (95% CI 1.07–1.79) • Medulloblastoma OR 1.27 (95% CI 1.02–1.60) |
<4,000 g | ||||
| Harder et al. (2010), Germany (47) | • Meta-analysis • Cohort n = 1 • Case–control n = 10 |
3,004 | • >4,000 g OR 1.19 (95% CI 1.04–1.36) | <4,000 g | ||||
| CNS tumors Original articles n = 18 | ||||||||
| Crump et al. (2015), Sweden (46) | • Cohort • n = 3,571,574 |
2,809 | • Birth weight ≥4,000 g • IRR 1.13 (95% CI 1.03–1.25) |
2,500–3,999 g | Adjusted for year of birth both continuous and categorical, gender, fetal growth, parental country of birth, maternal education, familiar history of brain tumor in parents or siblings | Low | Good | Good |
| Emerson et al. (1991), USA (186) | Case–control | 157 | • Birth weight >4,000 g All histologies • AOR 1.4 (95% CI 1.0–2.0) |
<4,000 g | Adjustments for matching variables; county of birth and birth year | Moderate | Good | Fair |
| Greenop et al. (2014), Australia (180) | Case–control | 319 | • Birth weight >4,000 g AOR 0.9 (95% CI 0.8–1.0) • LGA AOR 0.8 (95% CI 0.5–1.2) |
2,500–3,999 g AGA | Adjusted for maternal age, year of birth, ethnicity, maternal folate supplementation | Serious | Good | Fair |
| Johnson et al. (2016), USA (190) | Cross-sectional | 184 | • Birth weight >3,915–5,815 g • HR 1.38 (95% CI 0.85–2.26) |
<3,020 g | Adjusted for gestational age category | Moderate | Poor | Poor |
| Kitahara et al. (2014), Denmark (48) | • Cohort • n = 320,425 |
608 | HR 1.13 (95% CI 1.04–1.24) per 0.5 kg increase in birth weight | No adjustments | Low | Good | Good | |
| Mallol-Mesnard et al. (2008), France (183) | Case–control | 209 | Birth weight >4,000 g AOR 1.0 (95% CI 0.5–1.7) | 2,500–4,000 g | Matched for age and sex | Moderate | Good | Fair |
| McLaughlin et al. (2009), USA (181) | Case–control | 529 | Birth weight ≥4,000 g RR1.4 (95% CI 0.7–2.5) | 2,500–3,499 g | Adjustments for birth year, region, gender, race and birth weight | Moderate | Good | Poor |
| Oksuzyan et al. (2013), USA (184) | Case–control | 3,308 | • Birth weight >4,000 g AOR 1.12 (95% CI 0.91–1.38) • LGA AOR 1.09 (95% CI 0.89–1.27) |
2,500–4,000 g | Adjusted for race, gestational age, birth order, maternal age, father's education, and source of payment for delivery | Moderate | Good | Fair |
| O'Neill et al. (2015), USA+UK (50) | Case–control | 3,561, 5,702 | • Birth weight per 0.5 kg increase • AOR 1.05 (95% CI 1.01–1.08) • AOR 1.07 (95% CI 1.04–1.10) • Birth weight ≥4,000 g • AOR 1.18 (95% CI 1.06–1.32) • AOR 1.14 (95% CI 0.98–1.34) |
Per 500-g increase, 3,000–3,490 g | Adjusted for maternal age, plurality, gender, state and year of birth, birth order, maternal ethnicity | Moderate | Good | Good |
| Savitz and Ananth (1994), USA (64) | Case–control | 47 | Birth weight > 4,000 g OR 2.3 (95% CI 0.9–6.0) | 2,500–4,000 g | Adjusted for year of diagnosis | Serious | Good | Poor |
| Schüz et al. (2001), Germany (81) | Case–control | 466 | • Birth weight >4,000 g • OR 1.31 (95% CI 0.97–1.78) |
2,500–4,000 g | Adjustments for gender, age group of 1 year, year of birth, degree of urbanization and socioeconomic status | Serious | Good | Fair |
| Schüz and Forman (2007), Germany (65) | Case–control | 389 | • Birth weight >4,000 g • AOR 1.34 (95% CI 0.97–1.85) • LGA AOR 1.18 (95% CI 0.80–1.72) |
2,500–4,000 g | Stratified for gender and age, adjusted for urbanization and socioeconomy | Serious | Good | Fair |
| Spix et al. (2009), Germany (196) | Case–control | • Leukemia • Cases = 229 • Controls = 557 • CNS • Cases = 88 • Controls = 204 |
• Birth weight >4,000 g Leukemia AOR 1.96 (95% CI 1.12–3.41) • CNS tumors AOR 3.55 (95% CI 0.81–15.62) <2,500 g |
2,500–4,000 | • Matching criteria, sex, age, and year of diagnosis • Response rate cases 78.1% and controls 61.4% |
Serious | Good | Poor |
| Tettamanti et al. (2016), Sweden (49) | Cohort n = 2,032,727 | 758 | • LGA • Glioma ARR 1.11 (95% CI 0.82–1.49) • Meningioma ARR 0.92 (95% CI 0.50–1.68)?? • Neuroma ARR 1.31 (95% CI 0.62–2.80) • Birth weight 4,000–6,000 g • Glioma ARR 1.12 (95% CI 0.86–1.47) • Meningioma ARR 0.71 (95% CI 0.40–1.28) • Neuroma ARR 0.99 (95% CI 0.49–2.01) |
AGA 2,500–3,999 g | Adjustments for sex, maternal and paternal age, maternal birthplace, birth cohort, parental socioeconomic index at birth, birth weight by gestational age, head circumference, and birth length | Low | Good | Fair |
| Tran et al. (2017), USA (195) | Case–control | 72 | • Birth weight >4,000 g • AOR 2.5 (95% CI 1.2–5.2) • >4,000 g + LGA • AOR 2.7 (95% CI 1.1–6.2) |
2,500–4,000 g AGA | Adjustments for sex, ethnicity, year of birth, age at diagnosis, gestational age, maternal age, and DOE sites | Moderate | Good | Poor |
| Urayama et al. (2007), USA (185) | Case–control | 508 | Birth weight >4,000 g AOR 1.22 (95% CI 0.90–1.66) | 2,500–3,999 g | Adjustment for age, race, ethnicity, gestational age, birth order, abnormalities, socioeconomic factors, type of delivery | Moderate | Good | Fair |
| Von Behren and Reynolds (2003), USA (179) | Case–control | 746 | Birth weight ≥4,000 g OR 1.05 (95% CI 0.7–1.35) | 2,500–3,999 g | Adjustments for birth date and sex | Moderate | Good | Fair |
| Yaezel et al. (1997), USA, Australia, Canada (66) | Case–control | 252 | Birth weight >4,000 g AOR 1.2 (95% CI 0.7–1.8) | <4,000 g | Adjusted for maternal age, birth order, gestational age, sex, maternal race, maternal/paternal education, income, age at diagnosis | Moderate | Good | Good |
| • Hematologic malignancies • Systematic reviews n = 2 | ||||||||
| Caughey and Michels (2009), USA (192) | SR and MA 28 case–control and 4 cohort studies | 16,501 | • Birth weight >4,000 g All leukemias • AOR 1.35 (96% CI 1.24–1.48) |
Differs between 2,500–2,999 and <4,000 g | Different adjustments in different studies | |||
| Hjalgrim et al. (2003), Denmark (191) | SR and MA 18 case–control studies | 10,282 | Birth weight >4,000 g AOR for ALL and leukemia combined OR 1.26 (95% CI 1.17–1.37) | Different adjustments in different studies | ||||
| • Hematologic malignancies • Original articles n = 29 | ||||||||
| Cnattingus et al. (1995), Sweden (77) | Case–control | 613 | • LL Birth weight >4,000 g • AOR 1.7 (95% CI 1.1–2.7) |
3,000–3,499 g | Matched by sex and month and year of birth | Moderate | Good | Fair |
| Crump et al. (2015), Sweden (193) | • Cohort • n = 3,569,333 |
1,960 | • ALL LGA • AIRR 1.22 (95% CI 1.06–1.40) • Birth weight >4,000 g • AIRR 1.19 (95% CI 1.06–1.32) |
AGA 2,500–3,999 g | Adjusted for sex, birth year, fetal growth, parental country of birth, ALL in parent or sibling, | Low | Good | Good |
| Groves et al. (2018), USA (59) | Case–control | 633 | • ALL Birth weight >4,000 g • AOR 1.28 (95% CI 1.01–1.61) |
2,500–4,000 g | Adjusted for age, sex, ethnicity, county of residence and day of birth | Moderate | Good | Good |
| Hjalgrim et al. (2004), Denmark, Sweden, Norway Iceland (52) | Case–control | 2,204 | • Birth weight ≥4,500 g • ALL AOR 1.19 (95% CI 0.09–1.58) • Trend per kg increase 1.26 (95% CI 1.13–1.41) • AML AOR 0.95 (95% CI 0.45–2.04) • Trend per kg increase 1.09 (95% CI 0.82–1.45) |
3,500–3,999 g | • Matched for sex, year and month of birth • Trend adjusted for birth order, gestational age, parental age |
Moderate | Good | Poor |
| Kaatsch et al. (1998), Tyskland (67) | Case–control | 2,356 | • Birth weight >4,000 g Leukemia AOR 1.64 (95% CI 1.16–2.32) • No statistics on lymphoma |
2,500–4,000 g | • Matched for age, sex and place of residence at diagnosis • 81% response for cases and 67% for controls |
Serious | Good | Fair |
| Koifman et al. (2008), Brazil (194) | Case–control | 201 | Birth weight >4,000 g Infant leukemia AOR 1.20 (95% CI 1.02–1.43) | 2,500–2,999 g | Adjusted for sex, income, maternal age, pesticide exposure, hormonal intake during pregnancy | Serious | Good | Fair |
| Ma et al. (2005), USA (78) | Case–control | • 313 ALL • 53 AML |
• Birth weight > 4,000 g ALL AOR 1.04 (95% CI 0.52–2.10) • AML AOR 1.60 (95% CI 0.13–19.9) |
<2,500 g | Adjusted for household income, maternal education | Moderate | Good | Poor |
| McLaughlin et al. (2006), USA (189) | Case–control | 1,070 | • Birth weight ≥4,500 g • ALL AOR 1.10 (95% CI 0.67–1.73) • AML AOR 3.89 (95% CI 1.63–8.26) |
3,000–3,499 g | Matched for year of birth Adjustments for year of birth, race, gender, ethnicity, maternal age, gestational age | Moderate | Good | Fair |
| Mogren et al. (1999), Sweden (33) | Cohort n = 248,701 | 97 | • High birth weight, >4,500 g • SIR 4.29 (95% CI 1.56–9.33) |
Sex, age, calendar-specific person-year | Low | Good | Fair | |
| Okcu et al. (2002), USA (53) | Case–control | 104 total leukemia83 ALL | • Leukemia total birth weight >4,000 g AOR 1.7 (95% CI 0.9–3.0) • ALL AOR 2.2 (95% CI 1.2–4.1) |
2,500–4,000 g | Adjusted for year of birth, sex, gestational age, maternal age, tobacco use, parity and race | Low | Good | Moderate |
| O'Neill et al. (2015), USA+UK (50) | Case–control | 5,561, 7,826 | • Birth weight per 500 g increase • AOR 1.05 (95% CI 1.01–1.08) • AOR 1.07 (95% CI 1.04–1.10) • Birth weight ≥4,000 g • AOR 1.20 (95% CI 1.10–1.32) • AOR 1.10 (95% CI 0.96–1.26) |
• Per 500 g increase • 3,000–3,490 g |
Adjusted for maternal age, plurality, gender, state and year of birth, birth order, maternal ethnicity | Moderate | Good | Good |
| Paltiel et al. (2015), Multinational (51) | • Cohort • n = 112,781 |
• Leukemia, n = 115 • ALL, n = 98 |
• Birth weight >4,000 g • OR 1.31 (95% CI 0.97–1.78) |
<4,000 g | Adjusted for sex, maternal age, pregnancy weight gain, BMI, first born, maternal smoking | Low | Good | Fair |
| Peckham-Gregory et al. (2017), USA (63) | Case–control | 374 cases in total of which 89 cases with Burkitt's lymphoma | If LGA Subgroup analysis Burkitt lymphoma AOR 2.0 (95% CI 1.10–3.65) | Non-LGA | Adjusted for sex, maternal race, maternal ethnicity, year of birth, maternal education | Moderate | Poor | Poor |
| Petridou et al. (1997), Greece (54) | Case–control | 153 | Childhood leukemia AOR per 500 g increase in birth weight 1.36 (95% CI 1.04–1.77) | No ref | Matched for gender, age ±6 months, urban area | Serious | Good | Fair |
| Petridou et al. (2015), Sweden (62) | • Cohort • n = 3,444,136 |
684 | • LGA • Non-Hodgkin lymphoma AHR 1.83 (95% CI 1.20–2.79) • Hodgkin lymphoma AHR 0.7 (95% CI 0.22–2.2) • Birth weight ≥4,000 g • Non-Hodgkin lymphoma AHR 1.10 (95% CI 0.88–1.38) • Hodgkin lymphoma AHR 1.14 (95% CI 0.78–1.67) |
• 2,500–3,999 g AGA | Adjusted for sex, maternal age, maternal education, gestational age, birth order | Low | Good | Fair |
| Podvin et al. (2006), USA (55) | Case–control | • 376 ALL • 85 AML |
• >4,000 g ALL AOR 1.6 (95% CI 1.2–2.1) • AML AOR 1.2 (95% CI 0.7–2.1) |
2,500–3,999 g | Adjusted for mother's age | Moderate | Good | Good |
| Rangel et al. (2010), Brazil (68) | Case–control | Eligible number of cases 544. Included number of cases 410 | • Birth weight ≥4,000 g • Non-Hodgkin lymphoma OR 1.99 (95% CI 1.08–3.69) • Leukemia OR 1.86 (95% CI 1.04–3.30) |
<4,000 g | • Matched for gender and age • <50% responders among cases |
Critical | Good | Poor |
| Reynolds et al. (2002), USA (56) | Case–control | • 307 ALL <2 years • 1,100 ALL 2–4 years • 240 AML |
• Birth weight >4,000 g • AML OR 0.7 (95% CI 0.42–1.19) • ALL <2 years OR 0.93 (95% CI 0.63–1.39) • ALL 2–4 years OR 1.14 (95% CI 0.91–1.41) |
2,500–3,999 g | No adjustments | Moderate | Good | Moderate |
| Robinson et al. (1987), USA (57) | Case–control | 521 cases, 219 cases available for analysis | Birth weight >4,000 g ALL Relative Odds Ratio 0.73 Subgroup analysis >3,800 g and diagnosis <4 years of age OR 2.09 (95% CI 1.18–3.70) | <4,000 g | • Control group 1. Matched for date of birth and county of birth • Control group 2: year of birth • 4:1 • <50% of eligible cases identified |
Serious | Good | Poor |
| Roman et al. (2013), USA, Germany, and UK (58) | Case–control pooled | 3,922 | • Weight centile >90. Boys AOR 1.2 (95% CI 1.1–1.5). Girls 1.3 (95% CI 1.1–1.6) • Per kilo increase boys 1.2 (95% CI 1.1–1.3) Girls 1.2 (95% CI 1.1–1.4) • Birth weight >4,500 g AOR 1.8 (95% CI 1.2–2.6) |
3,000–3,999 g | • Controls matched for age at diagnosis • Adjusted for country, gestational age, sex, age at diagnosis • *Adjusted for sex and diagnosis • 58% of eligible controls participate |
Moderate | Good | Fair |
| Savitz and Ananth (1994), USA (64) | Case–control | • 71 ALL • 26 lymphoma |
• Birth weight > 4,000 g ALL OR 0.7 (95% CI 0.2–2.3) • Lymphoma OR 3.3 (95% CI 1.0–11.1) |
2,500–4,000 g | Adjusted for year of diagnosis and maternal smoking | Serious | Good | Poor |
| Schüz and Forman (2007), Germany (65) | Case–control | • ALL, n = 621 • AML, n = 94 • Non-Hodgkin lymphoma, n = 164 |
• Birth weight >4,000 g • ALL AOR 1.41 (95% CI 1.08–1.84) • AML AOR 1.56 (95% CI 0.88–2.79) • Non-Hodgkin lymphoma AOR 0.94 (95% CI 0.54–1.63) • LGA • ALL AOR 1.45 (95% CI 1.07–1.97) • AML AOR 1.45 (95% CI 0.75–2.83) • Non-Hodgkin lymphoma AOR 1.40 (95% CI 0.81–2.43) |
2,500–4,000 g | Stratified for gender and age, adjusted for urbanization, and socioeconomic factors | Serious | Good | Fair |
| Smith et al. (2009), UK (60) | Case–control | 1,632 | Birth weight >4,000 g AOR 1.2 (95% CI 1.02–1.43) | 2,500–4,000 g | Matched for sex, month, and year of birth, area of residence | Moderate | Good | Fair |
| Spix et al. (2009), Germany (196) | Case–control | • Leukemia • Cases = 229 • Controls = 557 • CNS • Cases = 88 • Controls = 204 |
• Birth weight >4,000 g Leukemia AOR 1.96 (95% CI 1.12–3.41) • CNS tumors AOR 3.55 (95% CI 0.81–15.62) <2,500 g |
2,500–4,000 g | • Matching criteria, sex, age, and year of diagnosis • Response rate cases 78.1% and controls 61.4% |
Serious | Good | Poor |
| Tran et al. (2017), USA (195) | Case–control | 207 | • Birth weight >4,000 g • Leukemia AOR 1.4 (95% CI 0,7–2.6) • >4,000 g+LGA AOR 1.7 (95% CI 0.8–3.7) |
• 2,500–4,000 g • AGA |
Matched for year of birth, county of residence, sex, ethnicity, maternal age. Adjusted for sex, ethnicity, year of birth, age at diagnosis, gestational age, maternal age | Moderate | Good | Poor |
| Triebwasser et al. (2016), USA (16) | Case–control | 1,216 | Birth weight ≥4,000 g AOR 1.23 (95% CI 1.02–1.48) | 2,500–3,999 g | Matched for month and year of birth, sex and ethnicity | Moderate | Good | Good |
| Westergaard et al. (1997), Denmark (76) | Cohort | • 704 ALL • 114 AML |
• Birth weight 4,010–4,509 g ALL ARR 1.59 (95% CI 1.17–2.17) • AML ARR 1.66 (95% CI 0.83–3.31) |
3,010–3,509 g | Adjusted for age, sex, calendar period, maternal age at birth, birth order | Low | Good | Good |
| Yaezel et al. (1997), USA, Australia, Canada (66) | Case–control | • ALL 1,284 • AML 185 • Non-Hodgkin lymphoma 190 |
• Birth weight >4,000 g ALL AOR 1.5 (95% CI 1.1–1.9) • AML AOR 1.5 (95% CI 1.0–2.4) • Non-Hodgkin lymphoma 1.5 (95% CI 1.0–2.4) |
<4,000 g | Adjusted for maternal age, birth order, gestational age, sex, maternal race, maternal/paternal education, income, age at diagnosis | Moderate | Good | Good |
| Zack et al. (1991), Sweden (61) | Case–control | 411 | • Per 100-g increase in birth weight • OR 1.0 (95% CI 1.0–1.0) |
Matched for sex, month, and year of birth | Moderate | Good | NA | |
| • Wilm's tumor • Systematic reviews, n = 1 | ||||||||
| Chu et al. (2010), Canada (69) | • Systematic review, • 12 studies, cohort n = 3, case–control n = 7 and case–cohort n = 2 |
>6,000 cases | • Birth weight >4,000 g, OR 1.36 (95% CI 1.12–1.64) • LGA vs. AGA: OR 1.51 (95% CI 1.25–1.83) |
2,500–4,000 g | • Case–control studies: matched for sex, year of birth, and/or year of diagnosis • Cohort studies adjusted at least for sex, year of birth. Some also adjusted for birth order, maternal age, residence., maternal education, socioeconomy |
|||
| • Wilm's tumor • Original articles n = 14 | ||||||||
| Crump et al. (2014), Sweden (70) | • Cohort • 3,571,574 |
443 | • ≥4,000 g, girls, AHR 2.22 (95% CI 1.63–3.029) • Boys AHR 1.44 (95% CI 1.06–1.96) |
2,500–3,999 g | Adjusted for age, fetal growth, gestational age at birth, birth order, maternal age, maternal education | Low | Good | Good |
| Daniels et al. (2008), USA (72) | Case–control | 521 | • ≥4,500 g, OR 1.7 (95% CI 0.9–3.3) Subgroup analysis (nephrogenic rests) • >4,000 g OR 21.1 (95% CI 1.2–3.9) |
2,500– <4,000 g | Matched for child's age, geographic area | Serious | Good | Fair |
| Heck et al. (2019), Denmark (73) | Case–control | 217 | • >4,000 g, OR 1.57 (95% CI 1.11–2.22) • LGA or 1.79 (95% CI 1.08–2.96) |
2,500– <4,000 g | Matched for sex and year of birth | Low | Good | Fair |
| Heuch et al. (1996), Norway (71) | Cohort | 199 | Birth weight >4,000 g IRR 1.19 (96% CI 0.72–1.98) | 3,001–3,500 g | Adjusted for age and sex | Moderate | Good | Fair |
| Jepsen et al. (2004), Denmark (74) | Case–control | 126 | Birth weight 4,000–4,499 g OR 0.88 (95% CI 0.44–1.62) | <3,500 g | No adjustments | Moderate | Good | Poor |
| Lindblad et al. (1992), Sweden (75) | Case–control | 110 | >4,000 g, OR 1.2 (95% CI 0.7–2.0) | <4,000 g | Matched or sex and date of birth | Moderate | Good | Poor |
| Olshan et al. (1993), USA (79) | Case–control | 612 | • Birth weight 4,001–4,500 g • AOR 1.27 (95% CI 0.65–2.51) |
3,001–3,500 g | Adjusted for household income and father's education | Serous | Poor | Poor |
| O'Neill (2015), USA, UK (50) | Case–control | 1,129, 1,515 | • Birth weight per 0.5-kg increase • AOR 1.17 (95% CI 1.10–1.24) • AOR 1.12 (95% CI 1.05–1.18) • Birth weight ≥4,000 g • AOR 1.55 (95% CI 1.29–1.87) • AOR 1.31 (95% CI 0.98–1.77) |
Per 0.5-kg increase, 3,000–3,490 g | Adjusted for maternal age, plurality, gender, state and year of birth, birth order, maternal ethnicity | Moderate | Good | Good |
| Puumala et al. (2008), USA (80) | Case–control | 138 | Birth weight >4,000 g AHR 1.54 (95% CI 0.99–2.40) | Adjusted for sex and year of birth | Moderate | Good | Fair | |
| Rangel et al. (2010), Brazil (68) | Case–control | Eligible number of cases 544. Included number of cases 410 | • Birth weight ≥4,000 g • OR 4.76 (2.72–8.28) g |
<4,000 g | • Matched for gender and age • <50% responders among cases |
Critical | Good | Poor |
| Schyz (90), Germany | Case–control | 177 | >4,000 g, OR 1.58 (95% CI 1.01–2.48) | 2,500– <4,000 g | Stratified by gender, age and year of birth and adjusted for socioeconomy and degree of urbanization | Serious | Fair | Poor |
| Schyz (91), Denmark, Sweden, Finland, Norway | Case–control | 690 | • >4,500 g, OR 1.90 (95% CI 1.29–2.81) • LGA OR 1.76 (95% CI 1.21–2.57) |
• 3,000–3,500 g • AGA |
Matched by birth month and year, sex and country | Low | Good | Good |
| Smulevich et al. (1999), Russia (83) | Case–control | 48 | Birth weight >4,000 g OR 5.1 (95% CI 1.6–16.4) | 2,500–4,000 g | No adjustments | Moderate | Fair | Poor |
| Yaezel et al. (1997), USA (66) | Case–control | 169 | Birth weight >4,000 g AOR 2.1 (95% CI 1.4–3.4) | <4,000 g | Adjusted for maternal age, birth order, gestational age, sex, maternal race, maternal/paternal education, income, age at diagnosis | Moderate | Good | Good |
OR, odds ratio; AOR, adjusted odds ratio; HR, hazard ratio; AHR, adjusted hazard ratio; SIR, standard incidence ratio; REOR, random-effects odds ratio; RR, relative risk; ARR, adjusted relative risk; IRR, incidence risk ratio; AIRR, adjusted incidence risk ratio.
Breast Cancer
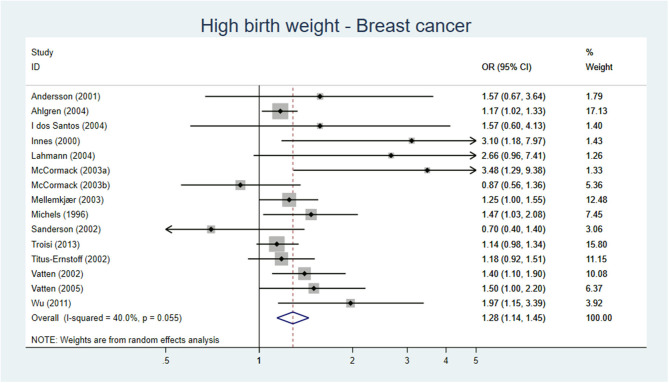
Three SR/meta-analyses (23–25), 10 cohort studies (26–35), and nine case–control studies (14, 36–43) investigated the association between high birth weight and the risk of breast cancer. The three SR, one of high and two of low quality, reported an increase of breast cancer per 500 g increase in birth weight [RR 1.02 (95% CI 1.01–1.03)] (25) and if birth weight was >4,000 g [RR 1.23 (95% CI 1.13–1.24) and RR 1.15 (1.09–1.21)] (23, 24). Among the 10 cohort studies, five out of nine studies with low to moderate risk of bias (27–29, 31–35, 39), found an association between high birth weight and later development of breast cancer. Three out of four case–control studies with low to moderate risk of bias also found an association (37, 40, 42). When only evaluating studies with low risk of bias (32, 33, 40, 42), three studies found an association. Our meta-analysis including 15 original studies showed a pooled AOR of 1.24 (95% 1.11–1.39) for development of breast cancer, when comparing birth weight >4,000 or >4,500 g vs. birth weight of <4,000 g (Figure 2).
Figure 2.
Forest plot describing the association between high birth weight and breast cancer.
Conclusion: High birth weight is probably associated with a moderate increase in breast cancer, moderate certainty of evidence (GRADE ⊕⊕⊕O).
CNS Tumors
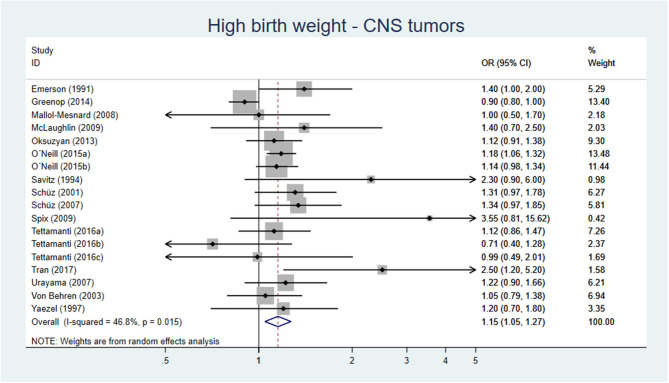
Four SR/meta-analyses, three cohort studies, 14 case–control studies, and one cross-sectional study reported on the association between high birth weight and CNS tumors. Two SRs, of medium and high quality, found an association between birth weight >4,000 g and astrocytoma [OR 1.38 (95% CI 1.07–1.79) and REOR 1.60 (95% CI 1.23–2.09)] and medulloblastoma [OR 1.27 (95% CI 1.02–1.60) and REOR 1.31(95% CI 1.08–1.58)] compared with <4,000 g (44, 45). A meta-analysis of medium quality (46) found for neuroblastoma, an OR of 1.19 (95% CI 1.04–1.36) for birth weight >4,000 g compared with <4,000 g. The SR/meta-analysis (high quality) by Georgakis and co-workers in 2017 (47) reporting on all CNS tumors, found an OR of 1.14 (95% CI 1.08–1.20) for high birth weight and an OR of 1.12 (95% CI 1.03–1.22) for LGA. Two cohort studies, both with low risk of bias, found an association between high birth weight and CNS tumors (48, 49), while one cohort study, with low risk of bias, found no association between LGA and CNS tumors (50). Nine out of 14 case–control studies had moderate risk of bias, where three studies (45, 51, 52) found an association between birth weight >4,000 g and CNS tumors, while six case–control studies, with moderate risk of bias, and one cross-sectional study (53) found no association.
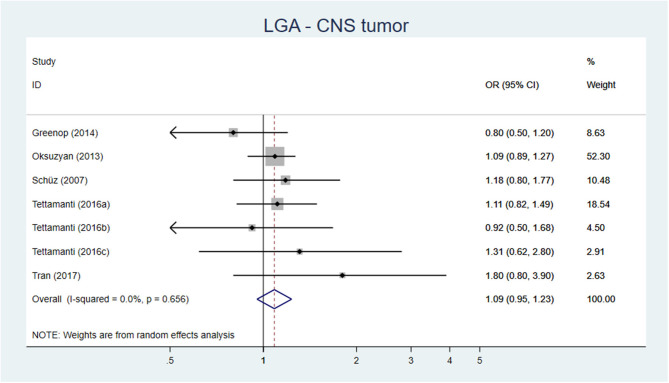
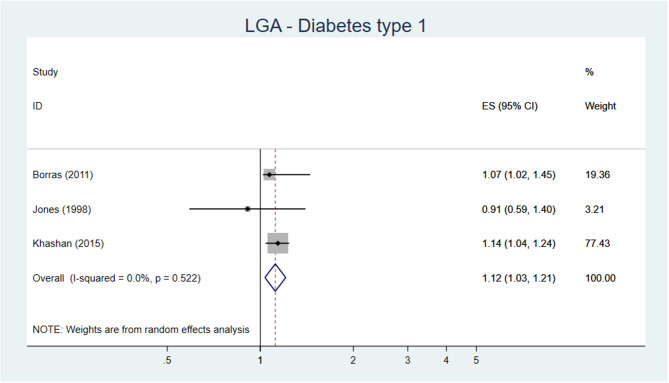
Our meta-analysis, including 15 original studies, showed a pooled AOR of 1.15 (95% CI 1.05–1.27) for development of CNS tumors, when comparing birth weight >4,000 or >4,500 g vs. birth weight of <4,000 g (Figure 3). For LGA vs. AGA, the corresponding figure was AOR 1.09 (95% CI 0.95–1.23) (Figure 4).
Figure 3.
Forest plot describing the association between high birth weight and CNS tumors.
Figure 4.
Forest plot describing the association between LGA and CNS tumor.
Conclusion: High birth weight is probably associated with a slight increase of CNS tumors, moderate certainty of evidence (GRADE ⊕⊕⊕O).
Hematological Malignancies
Two systematic reviews (54, 55), four cohort studies (34, 56–58) and 17 case–control studies (51, 52, 59–73) investigated the association between high birth weight and leukemia, one cohort study (74), and two case–control studies (16, 75) reported on lymphoma and five case–control studies (76–80) had investigated the impact of high birth weight on both leukemia and lymphoma.
Leukemia
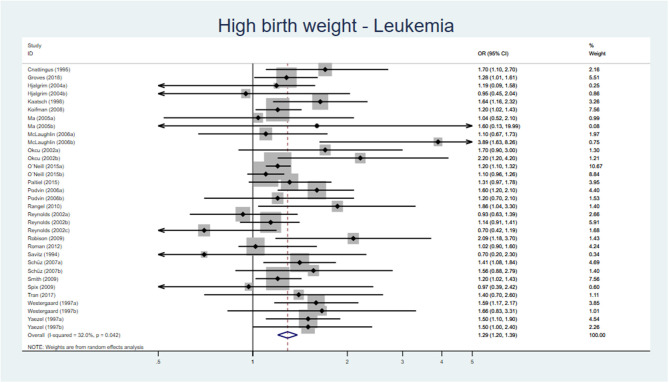
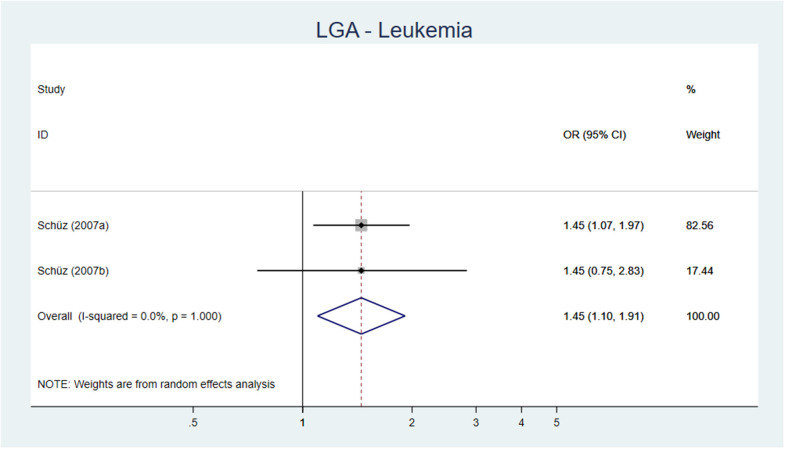
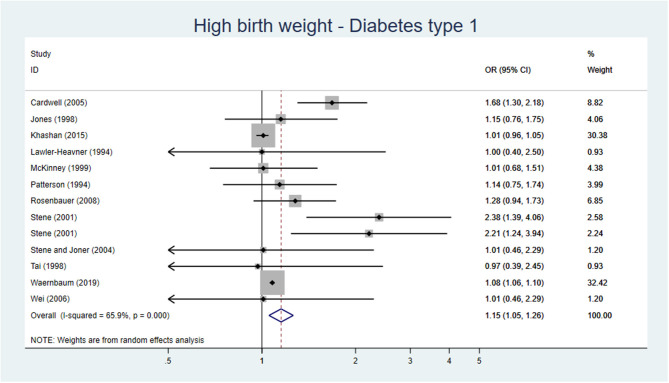
Both SR, of high and low quality, respectively, reported an association between birth weight >4,000 g and leukemia [OR 1.25 (95% CI 1.17–1.37) and AOR 1.35 (95% CI 1.24–1.48)] (54, 55). Two out of three cohort studies (56–58), all with low risk of bias, found an association between birth weight >4,000 g and acute lymphatic leukemia (ALL) (56, 58) and between LGA and ALL (56). Fourteen of the 22 case–control studies investigating the association between high birth weight and leukemia had a low to moderate risk of bias, and of these, 10 showed an increased risk if birth weight ≥4,000 or ≥4,500 g. The results from 22 original studies reporting on leukemia and high birth weight were pooled in a meta-analysis showing an AOR of 1.29 (95% CI 1.20–1.39) (Figure 5) and for LGA an AOR of 1.45 (95% CI 1.10–1.91) (Figure 6).
Figure 5.
Forest plot describing the association between high birth weight and leukemia.
Figure 6.
Forest plot describing the association between LGA and leukemia.
Lymphoma
One cohort and seven case–control studies reported on lymphoma. The cohort study by Petridou et al. (74) (low risk of bias) reported an increased risk for non-Hodgkin lymphoma when the child was born LGA while no significant increased risk was found for high birth weight. Two case–control studies with moderate risk of bias (16, 78), comparing >4,000 g as exposure to the reference <4,000 g, reported an association between high birth weight and Hodgkin/non-Hodgkin lymphoma. One case–control study, with moderate risk of bias reported an association between LGA and risk of Burkitt's lymphoma but no increased risk for other lymphomas (75).
Conclusion: High birth weight is probably associated with a moderate increase in leukemia, moderate certainty of evidence (GRADE ⊕⊕⊕O). LGA may be associated with a moderate increase in non-Hodgkin lymphoma, low certainty of evidence (GRADE ⊕⊕OO).
Wilm's Tumor
One SR (81), two cohort studies (82, 83), and 12 case–control studies (51, 78, 80, 84–92) reported on Wilm's tumor in childhood. The SR being of medium quality reported an increased risk for Wilm's tumor if birth weight >4,000 g as well as for LGA [OR 1.36 (95% CI 1.12–1.64) and OR 1.51 (95% CI 1.25–1.83)] (81).
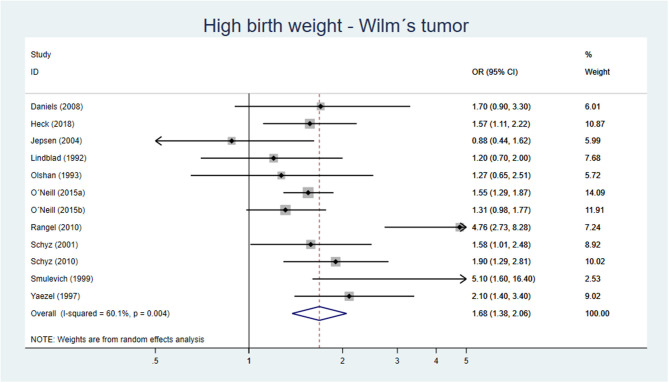
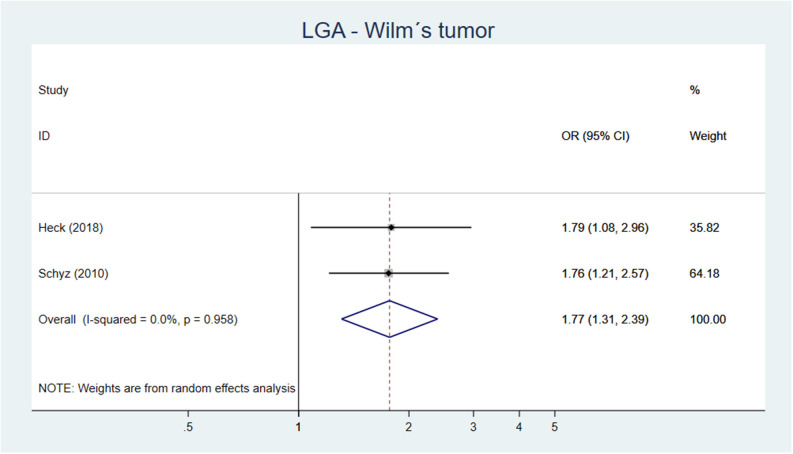
One out of two cohort studies with low-moderate risk of bias (82, 83) showed an association between high birth weight and Wilm's tumor (82). Five out of eight case–control studies, being of low to moderate risk of bias showed an increased risk of Wilm's tumor if birth weight >4,000 g or if LGA. Our meta-analysis including 11 original studies showed a pooled AOR of 1.68 (95% CI 1.38–2.06) for Wilm's tumor, when comparing birth weight >4,000 g vs. birth weight of <4,000 g (Figure 7). For LGA vs. AGA, the corresponding figure was AOR 1.77 (95% CI 1.31–2.39) (Figure 8).
Figure 7.
Forest plot describing the association between high birth weight and Wilm's tumor.
Figure 8.
Forest plot describing the association between LGA and Wilm's tumor.
Conclusion: High birth weight and/or LGA is probably associated with a moderate increase in Wilm's tumor, moderate certainty of evidence (GRADE ⊕⊕⊕O).
Psychiatric Disorders
Outcomes are listed in Table 1.2a.
Table 1.2a.
LGA, high birth weight, and long-term outcomes—psychiatric disorders.
| Author, year, country | Study design | Cases | Outcomes (risk estimates) | Reference group (weight) | Comments/adjustments | Risk of bias | Directness | Precision |
|---|---|---|---|---|---|---|---|---|
| • Psychiatric disorders • Systematic reviews n = 1 | ||||||||
| Davies (100), UK | Systematic review, meta-analysis | Not reported | • Birth weight >4,000 g • OR 0.86 (95% CI 0.80–0.92) |
Not stated | No adjustments performed | |||
| • Psychiatric disorders • Original articles n = 10 | ||||||||
| Gunnell et al. (2003), Sweden (17) | Cohort 334,577 | • 80 with schizophrenia • 124 with non-affective, non-schizophrenic psychosis |
• Schizophrenia: • Birth weight >4,000 g • HR 3.37 (95% CI 1.68–6.74) • Non-affective psychosis: • HR 1.24 (95% CI 0.75–2.05) |
3,501–4,000 g | Adjustments: gestational age, birth weight, birth length, ponderal index, head circumference, season of birth, urbanicity of residence at birth, age of mother, Apgar score at 1 minute, maternal parity, delivery by cesarean section, congenital malformation, uterine atony/prolonged labor, parental education | Moderate | Good | Good |
| Herva et al. (2008), Finland* (90) | • Cohort • 4,007 men and 4,332 women |
1,026 (current), 315 (self-reported physician-diagnosed) depression | • Likelihood for current depression 4,500–4,999 g • men OR 1.21 (95% CI 0.72–2.03; women OR 2.02 (95% CI 1.20–3.39) • Likelihood for self-reported physician-diagnosed depression 4,500 g: men OR 1.30 (95% CI 0.50–3.40), women OR 0.46 (95% CI 0.11–1.90) |
3,000–3,499 g | Adjustments: father's social class, mother's depression during pregnancy, mother's smoking during pregnancy, parity, mother's education, gestational age, mother's age at child's birth, mother's BMI before pregnancy | Moderate | Good | Good |
| Keskinen et al. (2013), Finland (87) | • Cohort • 10,526 |
150 | • Schizophrenia • Birth weight >4,500 g • HR 2.0 (95% CI 1.0–4.0) • In the group without parental psychosis HR 1.5 (95% CI 0.7–3.4) • In the group with parental psychosis HR 11.4 (95% CI 3.3–39.7) • Birth weight >4,500 g in relation to gestational age and the risk of schizophrenia. HR 1.2 (95% CI 0.7–1.9), p = 0.46 • In the group without parental psychosis HR 1.0 (95% CI 0.6–1.7), p = 0.99 • In the group with parental psychosis HR 3.2 (95% CI 1.2–9.0), p = 0.03 |
2,500–4,500 g | The results were reported as gender-adjusted HRs with 95% CIs. The association between parental gender, gestational age, psychosis, and birth weight was adjusted for maternal BMI (continuous variable) | Low | Good | Good |
| Lahti et al. (2015), Finland (92) | Cohort 12,597 | 1,660 | • Risk of any mental disorder (all subjects) LGA HR 1.03 (95% CI 0.75–1.41) • Risk of psychotic disorder (women) LGA HR 2.43 (95% CI 1.19–4.96) |
AGA = between −2 and +2 SD of that predicted by gestational age | Stratified for sex and year of birth, and adjusted for gestational age, socioeconomic position in childhood and mothers' marital status at childbirth | Low | Good | Good |
| Liuhanen et al. (2018), Finland (88) | • Cohort 4,223, • Family study • 256 |
256 | • Schizophrenia: Birth weight >4,000 g and high genetic risk OR 2.7 (95% CI 1.2–6.0) p = 0.013 • For women OR 7.6 (95% CI 2.8–20.5) • In fully adjusted model, there was no interaction between birth weight and genetic risk of social anhedonia (p = 0.61), or schizophrenia diagnosis (p = 0.24) |
Those with low genetic risk and birth weight ≤4,000 g | Adjustments: sex, gestational age, mother's BMI, and 3 principal component analyses | Low | Good | Fair |
| Moilanen et al. (2010), Finland (84) | Cohort 10,934 | 111 | • Risk of schizophrenia: Birth weight ≥4,500 g OR 2.4 (95% CI 1.1–4.9) • Large babies (>2 SD) for “corrected” gestational age • OR 2.1 (95% CI 1.0–5.1) |
2,500–4,499 g | Adjusted for gestational age, parental history of psychosis, sex | Low | Good | Fair |
| Perquier et al. (2014), France (89) | Cohort 41,144 | 2,601 with new onset, 3,734 with recurrent depression | • Risk of depression • Birth weight >4,000 g • New-onset OR 1.16 (95% CI 1.01–1.34), Recurrent OR 1.11 (95% CI 0.99–1.26) |
2,500–4,000 g | Adjustments: age; time since menopause; age at menarche; physical activity; energy intake; marital status; educational level; World War II food deprivation; psychological difficulties at work; alcohol intake; tobacco status; menstrual cycle length; number of children; type of menopause; history of cancer, type 2 diabetes, or vascular diseases; sleep duration; menopausal hormone therapy use | Low | Good | Good |
| Van Lieshout et al. (2020), Canada (93) | • Cohort • 2,151 |
628 | • Birth weight >4,000 g • Conduct disorder, OR 3.19 (95% CI 1.37–7.43) • Oppositional defiant disorder (ODD), OR 1.79 (95% CI 1.11–2.91), • ADHD OR 1.77 (95% CI 1.21–2.80) • Birth weight >4,000 g and socioeconomic disadvantage • ODD OR 5.86 (95% CI 2.60–13.25) • Major depressive disorder • OR 4.24 (95% CI 1.69–10.66), Generalized anxiety disorder OR 3.85 (95% CI 1.64–9.08) compared with those with higher socioeconomic status |
2,500–4,000 g | Adjusted for participant age, sex, socioeconomic status of the family, parental mental health, and gestational DM | Moderate | Fair | Good |
| Wegelius et al. (2011), Finland (85) | • Cohort • 1,051 |
360 | • Schizophrenia • Birth weight >4,000 g • HRR 1.68 (95% CI 1.13–2.50), p = 0.010 • Risk of primary psychotic disorder • Birth weight >4,000 g • HRR 1.18 (95% CI 0.84–1.65), p = 0.35 |
3,000–4,000 g | Adjustments: sex, maternal and paternal history of psychotic disorder | Moderate | Good | Fair |
| Wegelius et al. (2013), Finland (86) | Cohort 1,051 | 282 | High birth weight (>4,000 g) was associated with more severe symptoms of bizarre behavior, as reflected by the statistically significant quadratic term (βLinear = −3.92, SE = 0.76, p < 0.001; βQuadratic = 0.57, SE = 0.12, p < 0.001) | 3,000–4,000 g | Adjusted for sex, place of birth and year of birth | Moderate | Good | Fair |
ADHD, attention deficit hyperactivity disorder; AGA, appropriate for gestational age; BMI, body mass index; CI, confidence interval; HR, hazard ratio; HRR, hazard rate ratio; LGA, large for gestational age; NA, not available; ODD, oppositional defiant disorder; OR, odds ratio.
Schizophrenia
Four out of six cohort studies, with low to moderate risk of bias, found an association between high birth weight and/or LGA and schizophrenia (17, 93–95). All studies but one (17) included both males and females and were adjusted by sex. High birth weight also increased the risk of schizophrenia considerably in families with parental psychosis (94, 96). However, two studies found no association in adjusted models (96, 97).
Depression
Two cohort studies, one with low and one with moderate risk of bias reported on depression. In these studies, women born with high birth weight had increased risk for new-onset depression (98) and current depression (98, 99). In men, no association was found (99).
Psychiatric Disorders in General
According to a recent systematic review and meta-analysis, high birth weight >4,000 g was a protective factor for different types of psychotic disorders (OR 0.86, 95% CI 0.80–0.92) (100). In our search, we found three cohort studies investigating the association between several mental or psychotic disorders and high birth weight with contradictory results. According to two Finnish studies, no general increased risk of any mental disorder (substance use, psychotic, mood, anxiety, personality disorders, suicides, suicide attempts) or any primary psychotic disorder was observed in individuals born LGA (95, 101). However, Van Lieshout et al. (102) reported higher odds of some psychiatric disorders [oppositional defiant disorder, conduct disorder, attention deficit hyperactivity disorder (ADHD)] in 12–17-year-old children born macrosomic (102). Participants exposed to macrosomia and socioeconomic disadvantage were more susceptible to major depressive disorders, and generalized anxiety disorders, compared with those with higher socioeconomic status (102).
Conclusion: High birth weight and/or LGA may be associated with a moderate increase in schizophrenia and an increase in depression, low certainty of evidence (GRADE ⊕⊕OO).
It is uncertain whether high birth weight is associated with psychiatric disorders in general, very low certainty of evidence (GRADE ⊕OOO).
Cognitive Function
Outcomes are listed in Table 1.2b.
Table 1.2b.
LGA, high birth weight, and long-term outcomes—cognitive performance.
| Author, year, country | Study design | Cases | Outcomes (risk estimates) | Reference group (weight) | Comments/adjustments | Risk of bias | Directness | Precision |
|---|---|---|---|---|---|---|---|---|
| Original articles n = 21 | ||||||||
| Alati et al. (2009), Australia (98) | • Cohort • 4,971 |
• Social problems Quintile 5 (highest birth weight): OR 1.57 (95% CI 1.12–2.20) • Anxious/depressive symptoms Quintile 5: OR 1.1 (95% CI 0.80–1.51) |
Quintile 3 | Adjustments: parity and child age, socio-economic position, maternal alcohol and tobacco use, maternal anxiety and depression in pregnancy | Moderate | Good | Good | |
| Bergvall et al. (2006), Sweden (108) | • Cohort • 357,768 |
35,821 | Risk of low intellectual performance: birth weight (SDS) more than 2: OR 0.98 (95% CI 0.90–1.06) | Birth weight (SDS) −2 to +2 | Adjustments: gestational age, mothers age and parity, socioeconomic factors (household socioeconomic status, education, family structure) | Moderate | Good | Good |
| Buschgens (2009), The Netherlands (97) | • Cohort • 2,230 |
• Birth weight >4,500 g • Inattention (TCP**p < 0.01); • Hyperactivity/impulsivity (TCP p < 0.01) • Aggression (CBCL*** <0.05; TCP < 0.01) • Delinquency (TCP < 0.01) |
2,500–4,500 g | Multiple linear regression analyses, for each separate (standardized) variable | Low | Good | Good | |
| Dawes et al. (2015), UK (114) | • UK Biobank resource • 18,819 |
For hearing, vision, reaction time and IQ, the middle category had significantly better performance than both the low and high categories (both p < 0.001) | The top and bottom 3% by birth weight were compared with the middle 3% (centered on the 50th percentile) | An ANOVA model was applied, hearing, vision, and cognition as the dependent variable and group (bottom, middle, or top 3% of the distribution) as the independent variable in the model, with the covariates age, sex, Townsend deprivation index quintile, educational level, smoking, diabetes, cardiovascular disease, hypertension, high cholesterol, and maternal smoking | Serious | Poor | Fair | |
| Duffy et al. (2020), USA (113) | • Cohort • 108,348 |
• Children born LGA • Did not meet proficiency on mathematics ARR 0.96 (95% CI 0.92–0.99) • Did not meet proficiency on English language or arts ARR 0.97 (95% CI 0.95–0.99) • Referred for special education ARR 0.98 (95% CI 0.94–1.03) |
AGA | Adjustments: maternal ethnicity, age, education, nativity, marital status, Medicaid status, parity, maternal obesity, pre-gestational or gestational diabetes, tobacco, alcohol, or drug during pregnancy, excessive weight gain during pregnancy, infant gender, and year of birth | Moderate | Good | Good | |
| Eide et al. (2007), Norway (109) | Cohort 317,761 | 4,912 | Large infants (z-score birth weight >3.00) had a slightly elevated risk of low intelligence score (OR 1.22, 95% CI 1.00–1.48) | z-score −0.49 to 0.50 | Adjustments: maternal age, maternal education, parity, adult height, BMI The gestational age–specific z-score (SD above or below the mean of birth weight was calculated using Norwegian population standards) | Moderate | Good | Good |
| Flensborg-Madsen and Mortensen (2017), Denmark (112) | Cohort 4,696 | • Standardized intelligence score • Birth weight >4,000 g • At the age 19 years • mean difference 1.35 (95% CI −0.83 to 3.52), 28 years −0.03 (−4.05 to 4.00), 50 years 2.90 (−0.35 to 6.14) |
3,001–3,500 g | Adjustments: infant sex, infant socioeconomic status, mother's age at birth, birth order, mother's smoking in last trimester, gestational age | Moderate | Good | Good | |
| Haglund and Källen (2011), Sweden (94) | • Case–control • 68,964 |
250 | • Both autism and Asperger: LGA vs. adequate weight for gestational age OR 0.3 (95% CI 0–1.9) • Any obstetrical risk factor (prematurity, low Apgar scores, growth restriction, or macrosomia) • Autism with mental retardation, AOR 1.3 (95% CI 0.3–2.2) • Autism without cognitive impairment AOR 3.1 (95% CI 1.7–5.7) |
2,500–4,000 g | Adjusted for year of birth, maternal age 40 years or older, primiparity, maternal birth outside Sweden, and gender | Moderate | Fair | Good |
| Kristensen et al. (2014), Norway (111) | • Cohort • 217,746 |
• The crude mean IQ score • Birth weights of ≥5,000 g was 1.2 points (95% CI 0.3–2.2) lower |
4,000–4,499 g | In the multivariable analysis included gestational age, year of birth, birth order, sibship size, mother's and father's ages at child's birth, mother's marital status, highest parental educational level, father's income level. Mean sibship birth weight, maximum sibship birth weight, and fraternal relatedness were added to the random-effects model | Moderate | Good | Good | |
| Leonard et al. (2008), Australia (95) | Cohort 219,877 | 2,625 | • Mild-moderate ID (>4,500 g) OR 1.10 (95% CI 0.75–1.61) • Severe ID: OR 1.29 (95% CI 0.40–4.10); ID with autism spectrum disorder: OR 1.66 (95% CI 0.60–4.56) • Caucasian infants with excess intrauterine growth (percentage of optimal birth weight 124) were more likely to be diagnosed with ID associated with autism spectrum disorder OR 2.36 (95% CI 0.93–6.03) |
3,000–3,499 g | Adjustments: marital status, maternal country at birth, health insurance status, paternal occupation, geographic remoteness, socioeconomic well-being | Moderate | Good | Good |
| Lundgren et al. (2003), Sweden (110) | Cohort 620,834 | • Risk for subnormal intellectual performance: • High birth weight (>2 SDS) according to the BMI groups at young adulthood: normal BMI (18.5–24.9) OR 0.92 (95% CI 0.87–0.98), BMI 25–29.9 OR 1.33 (95% CI 1.20–1.48), BMI >30 OR 1.86 (1.58–2.19) |
Subjects born at term with normal birth weight | Adjusted for gestational age, low Apgar score, head circumference SDS at birth, height SDS at conscription and parental education | Moderate | Good | Good | |
| Moore et al. (2012), USA (96) | Cohort 5,979,605 | 20,206 | • Risk of autism: • Term LGA (95th percentile) infants 39–41 weeks AOR, 1.16 (95% CI 1.08–1.26) Preterm LGA infants 23–31 weeks AOR, 0.45 (95% CI 0.21–0.95) |
Subjects born with birth weight AGA | Adjusted for maternal age, race, hypertension, pre-eclampsia, diabetes, birth order, twin gestation, and months since last live birth | Moderate | Good | Good |
| Power et al. (2006), UK (107) | • Cohort • 13,980 |
• For 1 kg increase in birth weight, 7-year mathematics z-score increased 0.23 (0.19 adjusted for parental interest in child's progress) and adult qualifications increased 0.22 (on a 5-point scale) • Mean z-scores for math (>4,000): • boys 0.10, girls 0.14 |
Adjustments for gender, gestational age (32–44 weeks), exact age of test and for parental interest in child's progress | Moderate | Good | Good | ||
| Record et al. (1969), UK (103) | Cohort 41,543 | • Mean verbal reasoning scores of first-born children (40–41 weeks of gestation) • Birth weight 2,000–2,400: 96.9–98.9 • Birth weight 3,000–3,400: 102.1–104.2 • Birth weight 4,000–4,400: 104.3–105.3 |
Results reported according to sex, duration of gestation, birth order | Moderate | Poor | Good | ||
| Richards et al. (2001), UK (105) | Cohort 3,900 | • Birth weight was associated with cognitive ability at age 8 (with an estimated SD score of 0.44 (95% CI 0.28–0.59)) between the lowest and highest birth weight categories • At age 43 high birth weight (4,010–5,000) vs. normal birth weight • Standardized cognitive score: • Verbal memory −0.17 (−0.31 to −0.04) • Search accuracy 0.02 (−0.11 to 0.16) • Search speed −0.07 (−0.21 to 0.07) |
3,010–3,500 g | Adjusted for sex, father's social class, mother's education, birth order, and mother's age. From age 11 to age 43, each cognitive score was further adjusted for the score of previous age | Moderate | Good | Good | |
| Räikkonen et al. (2013), Finland (106) | Cohort 931 | The whole cohort | Men who were born larger were more likely to perform better in the Finnish Defense Forces Basic Intellectual Ability Test over time [1.22–1.43 increase in odds to remain in the top relative to the lower two thirds in ability over time per each SD increase in body size (95% CI 1.04–1.79)] | • No specific mention of birth weight categories • Adjustments: gestational age, mother's age, height and parity; social class in childhood; history of breast feeding; education; diagnosis of diseases |
Low | Good | Good | |
| Sörensen et al. (1997), Denmark (104) | • Cohort • 4,300 |
• The Boerge Piren test (validated intelligence test) increased from 39.9 at a birth weight of ≥2,500 g to 44.6 at a birth weight of 4,200 g. • Above a birth weight of 4,200 g the test score decreased slightly |
Adjusted for gestational age, length at birth, maternal age and parity, marital status, and employment | Moderate | Good | Good | ||
| Tamai (2020), Japan (101) | Cohort 36,321 | • At 2.5 years: • Unable to walk ARR 7.1 (95% CI 1.0–5.9) • Unable to say meaningful words ARR 10 (95% CI 3.8–26) • Unable to compose two-phrase sentence ARR 3.5 (95% CI 1.9–6.3) • Unable to say his/her name ARR 1.9 (95% CI 1.2–3) • Unable to use a spoon ARR 4.8 (95% CI 1.9–12.3) • All differences disappeared at 5.5 years of age • However not for LGA >3 SD |
• −1.28 to 1.28 SD • Normal birth weight |
Adjustments: parity, singleton, gender, maternal age, maternal smoking, maternal and paternal education level | Moderate | Good | Fair | |
| van Mil et al. (2015), The Netherlands (100) | • Cohort • 6,015 |
• Risk of attention problems in children born with high birth weight percentile β (95% CI): • The attention problems subscale of the CBCL/1.5–5*** • >90th percentile 0.05 (−0.02 to 0.12) p value 0.17 • >80th percentile 0.01 (−0.07 to 0.04), p = 0.61 |
Subjects born with birth weight AGA | Adjusted for Apgar score 1 minute after birth, mode of delivery, maternal age, national origin, educational level, parity, BMI, psychological symptoms, smoking, alcohol use, folic acid supplementation use, gestational diabetes, pre-eclampsia | Moderate | Good | Good | |
| Yang et al. (2019), China (99) | Cohort 9,295 | 724 | • Behavioral problems • Macrosomia (n = 268) OR 1.61 (95% CI 1.16–2.22) |
Normal and low birth weight | Adjustments: age, sex | Serious | Poor | Good |
| Zhang et al. (2020), China (102) | Cohort | 4,026 | • Gross motor DQ ARC 0.49 (95% CI 0.36–0.63) • Fine motor DQ ARC −2.73 (95% CI −2.87 to −2.59) • Adaptability DQ ARC −1.19 (95% CI −1.33 to −1.05) • Language DQ ARC 0.43 (95% CI 0.29–0.57) • Social behavior DQ ARC 1.10 (95% CI 0.95–1.24) • Overall no clear differences |
Normal birth weight | Adjustments: maternal smoking, gender of infant, mode of delivery, neonatal asphyxia, birth length, gestational week, educational level of parent | Moderate | Fair | Fair |
Teacher's Checklist of Psychopathology.
Child Behavior Checklist.
AGA, appropriate for gestational age; AOR, adjusted odds ratio; ARC, adjusted regression coefficient; ARR, adjusted relative risk; BMI, body mass index; CBCL, The Child Behavior Checklist; DQ, development quotient; ID, intellectual disability; IQ, intelligence quotient; LGA, large for gestational age; NA, not available; OR, odds ratio; SDS, standard deviation score; TCP, The Teacher's Checklist of Psychopathology.
Autism
One case–control study with moderate risk of bias reported no association of LGA with autism or Asperger syndrome (103). Two cohort studies with moderate risk of bias reported a slightly increased risk for autism in children born LGA (104, 105).
Behavioral Problems
Four cohort studies reported results on associations between high birth weight/LGA and behavior/attention problems among children and adolescents aged 6–16 years, of which three reported an association between LGA and behavioral problems (106–108).
In a study with low risk of bias, a higher risk for externalizing behaviors (inattention, hyperactivity/impulsivity, aggression, delinquency) was found in high birthweight children (106). In another study with moderate risk of bias, an association between birth weight and social problems was observed in babies at the higher end of the birth weight distribution (107). In contrast, one study (109) found that high birthweight children had no increased risk of attention problems. In a study from Japan, the relation between LGA and neurodevelopment was U-shaped, with mild LGA having the lowest risk and severe LGA (>3 SD) was associated with higher risk of unfavorable behavioral development (110), while another study found no association (111).
Cognitive Development
In five cohort studies with low or moderate risk of bias, high birth weight was associated with high cognitive ability (112–115) and 7-year math score (116).
Intellectual Performance
Eight cohort studies investigated the association between high birth weight and intellectual performance, seven with moderate and one with serious risk of bias. Five of these studies consisted of a study population of Nordic conscripts (117–121), one was a large cohort study of children born in Western Australia (104) and one study was from the USA (122). In five studies, no clear association was found between high birth weight and intellectual performance, risk of intellectual disability, or low IQ score (104, 117–119, 121). However, in one study the crude mean IQ score was 1.2 points lower for those with the extreme birth weight (≥5,000 g) (120). The major part of the apparent association between high birth weight and low IQ score was caused by confounding family factors (120). Of note, the risk for subnormal intellectual performance was dependent on a BMI at young adulthood BMI >30 OR 1.86 (1.58–2.19) (119). In the recently published study from the USA, a slightly decreased risk of poor academic performance was noticed for LGA children (122). In addition, one study from UK Biobank, the middle birth weight category showed better performance for hearing, vision, reaction time, and IQ than the highest category (123).
Conclusion: High birth weight and/or LGA may be associated with a slight increase in autism and behavioral problems, low certainty of evidence (GRADE ⊕⊕OO). High birth weight may be positively associated with cognitive ability, low certainty of evidence (GRADE ⊕⊕OO). No association was found between high birth weight and/or LGA and intellectual performance, moderate certainty of evidence (GRADE ⊕⊕⊕O).
Cardiovascular Health
Outcomes are listed in Table 1.3. Two SR/meta-analyses of high quality, one on hypertension and blood pressure (19) and one on coronary heart disease (CHD) (124), were included, together with 27 original articles.
Table 1.3.
LGA and high birth weight and long-term outcomes—cardio-vascular diseases.
| Author, year, country | Study design | Cases | Outcomes (risk estimates) | Reference group (weight) | Comments/adjustments | Risk of bias | Directness | Precision |
|---|---|---|---|---|---|---|---|---|
| Cardio-vascular | ||||||||
| Systematic review/meta-analysis, n = 2 | ||||||||
| Zhang et al. (2013), China (19) | • SR meta-analysis • 31 studies |
NA for hypertension | • Overall weighted mean differences (WMD) (all age groups) • SBP: −0.25 mmHg (95% CI −0.92 to 0.42) • DBP: 0.20 mmHg (95% CI −0.23 to 0.62) • Hypertension: • RR: 1.00 (95% CI 0.93–1.06) • SBP, DBP, and risk of hypertension are higher among individuals with HBW during childhood but lower during adulthood |
• NBW 2,500–4,000 g or the 10–90th percentile for GA • NBW n = 559,979 |
Not specified | |||
| Wang et al. (2014), China (115) | SR+ Meta-analysis | • Cases with CHD: • n = 11,218 • – |
• CHD in HBW vs. NBW • Pooled OR (random-effects model) • OR 0.89 (95% CI 0.79–1.01) |
NBW 2,500–4,000 g | Non-Adjusted | |||
| CVD, Original articles, n = 21 | ||||||||
| Blood pressure/hypertension, n = 14 | ||||||||
| Azadbakht et al. (2014), Iran (116) | • Cohort • n = 5,528 • n = 2,726 girls • n = 2,802 boys |
• HBW • High SBP AOR 0.6 (95% CI 0.3–1.2) • High DBP AOR 0.8 (95% CI 0.4–1.6) |
2,500–4,000 g | Adjustments: Age, sex, SES, parent's income, parent's education, birth order, family history of chronic disease, breast feeding during, type complementary food, sedentary lifestyle, BMI | Serious | Fair | Fair | |
| Dong (2017), China (117) | • Cross sectional • High birth weight n = 4,981 • Normal birth weight n = 4,981 |
• High blood pressure • Boys n = 2,144 • Girls n = 1,086 |
• High blood pressure • Boys: AOR 0.96 (95% CI 0.77–1.20) • Girls: AOR 0.91 (95% CI 0.68–1.22) |
2,500–3,999 g | • Matched age, sex, province • Adjusted: Parental education, delivery, breast feeding, family history of disease, food intake and physical activities, BMI |
Serious | Poor | Good |
| Espineira (2011), Brazil (118) | • Cohort • n = 515 |
Continuous outcome | LGA had higher BP than controls (p < 0.05) | AGA | • Gender matched • Adjusted: Gender, waist circumference and height |
Serious | Fair | Poor |
| Ferreira (2018), Brazil (119) | • Cross-sectional • School based • n = 829 |
• High BP • *OBP 8.5% n = 70 • **HoBP 3.8% • n = 32 |
Each increase of 100 g in birth weight did not influence office or home BP | BW | Simple linear regression analysis | Serious | Fair | Fair |
| Gunnarsdottir et al. (2002), Iceland (120) | • Cohort • n = 4,601 total • n = 2,337 men • n = 2,264 women |
• Hypertension • 40–47% of women • 59–61% of men • Numbers NA |
• Risk for hypertension • Women, AOR (95% CI): • ≤ 3.45 kg • 1.4 (95% CI 1.1–1.8) • >3.45 to ≤3.75 kg • (95% CI 0.8–1.3) • >4.0 kg • 0.9 (95% CI 0.7–1.2) • P for trend* <0.001 • P for trend** <0.001 • Men, AOR (95% CI): • ≤ 3.45 kg • (95% CI 0.8–1.3) • >3.45 to ≤3.75 kg • (95% CI 0.8–1.2) • >4.0 kg • 0.8 (95% CI 0.7–1.1) • P for trend* <0.051 • P for trend** <0.004 • Inverse association between size at birth and adult hypertension, strongest among women born small who were overweight in adulthood and for those without a family history of hypertension |
3,750–4,000 g | • Adjusted for adult BMI, education, smoking habits, physical activity or family history of hypertension • Adjusted for trend: • *age, year of birth • ** age, year of birth, BMI |
Moderate | Good | Good |
| Kuciene et al. (2018), Lithuania (121) | • Cross-sectional • Singleton, adolescents n = 4,598 • Boys n = 2,103 • Girls n = 2,495 |
• High blood pressure • n = 1,178 |
• Risk for high blood pressure • >4,000 g AOR 1.34 (95% CI 1.11–1.63)* • LGA AOR 1.44 (95% CI 1.16–1.79)* • >4,000 g and normal weight in adolescence: • AOR 1.37 (95% CI 1.11–1.70)** • 2,500–4,000 g and overweight/obesity • AOR 3.63 (95% CI 2.99–4.41)** • >4,000 g and overweight/obesity • AOR 4.36 (95% CI 3.04–6.26)** • LGA and normal weight in adolescence: • AOR 1.40 (95% CI 1.10–1.80)** • AGA and overweight/obesity • AOR 3.39 (95% I 2.79–4.13)** • LGA and overweight/obesity • AOR 5.03(95% CI 3.33–7.60)** |
• 2,500–4,000 g • AGA |
• *Adjustments in multivariable logistic regression analysis: • age, sex, and BMI • ** Adjustments in multivariable logistic regression analysis: • age and sex |
Moderate | Good | Fair |
| Launer et al. (1993), Netherlands (122) | • Cohort • n = 374 |
Continuous outcome | Relation between SBP and birth weight appeared U-shaped in 4-year-old children | Birth weight | Adjusted for sex, gestational age, birth length, BP at 1 week (mmHg), blood pressure at 3 months (mmHg), current weight (kg) | Serious | Fair | Poor |
| Ledo et al. (2018), Brazil (123) | • Cross-sectional • n = 719 |
• SBP >90th • percentile • n = 22 • DBP >90th • Percentile • n = 36 |
HBW was not associated with high blood pressure | 2,500–3,999 g | Adjusted for sex | Moderate | Fair | Poor |
| Li et al. (2006), USA (124) | • Longitudinal cohort • n = 98 |
• NA • Continuous outcome |
• Birth weight was inversely associated with SBP in children in pre-pubertal stage but was not statistically significant in early or late puberty (r = −0.23 (SD 1.1), p < 0.05) • SBP significantly increased from pre-puberty to early or late puberty (sexual maturation) among children with HBW |
<4,000 g | Adjusted for gender, race, age, pubertal status, BMI percentile | Serious | Poor | Fair |
| Li et al. (2013), China (125) | • Cohort • Childhood • n = 1,415 • Adolescence n = 1,112 |
Continuous outcome | • Childhood SBP and DBP: • No statistically significant difference • Adolescence • SBP • Cases: 110.83 ± 9.43 mmHg • Controls: 109.33 ± 9.26 mmHg • P = 0.0002 • DBP • Cases:72.10 ± 6.39 mmHg • Controls: 71.58 ± 6.47 mmHg • P = 0.055 • Similar results after adjustment in multi-mixed model |
2,500–4,000 g | • Controls matched by sex and birth date • Adjustment in multi-linear analysis: • Repeated measures, birth year, sex, mother's occupation, age of delivery and adding weight during pregnancy, hypertension during delivery, gestational age, parity, and picky eating in childhood |
Moderate | Fair | Fair |
| Schooling et al. (2010), China (126) | • Longitudinal cohort study • Men n = 5,051 • Women • n = 13,907 |
• High blood pressure • Men 55.9% (n = 2,824) • Women 47.2% (n = 6,564) |
• Risk of HBP • per birth weight SD: • All: AOR 0.94 (95% CI 0.91–0.97) |
Birth weight | Adjusted for study phase, age and sex, SES, number of offspring, height, BMI, WHR | Serious | Poor | Good |
| Strufaldi et al. (2009), Brazil (127) | • Cross-sectional • n = 739 |
Continuous outcome | • Inverse association between birth weight and BP • SBP and DBP was negatively associated with BW • Adjusted SBP: • Q1: 105.3 (95% CI 103–107.5) • Q2: 94.8 (95% CI 92.7–96.9) • Q3: 95.5 (95% CI 93.4–97.6) • Q4: 95.7 (95% CI 93.6–97.8) |
• BW quartiles. • Q1: ≤2.9 kg • Q2: 2.91–3.20 kg • Q3: 3.21–3.58 kg • Q4: >3.58 kg |
Adjusted for gender, prematurity, BMI | Serious | Fair | Fair |
| Tan et al. (2018), China (128) | • Cohort • n = 49,357 |
• High SBP • n = 7,654High DBP • n = 4,787Hypertension • n = 9,479 |
• High birth weight • Adjusted OR of hypertension • AOR 0.84 (95% CI 0.77–0.92) • High SBP • AOR 0.89 (95% CI 0.80–1.00) • High DBP • AOR 0.82 (95% CI 0.75–0.90) • BW had a negative association with BP across the whole BP range |
2.5–4.0 kg | Adjusted for age, gender, height, BW/gestational age, family history of hypertension, parental educational level, family income, region, BMI | Serious | Fair | Good |
| Yiu et al. (1998), USA (129) | • Cohort • n = 2,958 |
Continuous outcome | • HBW >4,500 g (97th percentile) • Significant inverse relationship between birth weight and SBP. For every 1-kg decrease in BW in term infants, SBP increased by 1.3 mmHg and DBP by 0.6 mmHg |
AGA (3rd−97th percentile) | Adjusted for gestational age, race, sex, follow-up height, follow-up weight | Serious | Poor | Poor |
| Coronary heart disease (CHD), n = 1 | ||||||||
| Rashid et al. (2019), USA (130) | • Cohort • n = 9,820 |
• Incident heart failure • n = 432 |
• HBW compared with medium BW: • Incident heart failure: • AHR 1.27 (95% CI 1.05–1.54) • No significant association with all-cause mortality or myocardial infarction |
2,500–4,000 g | Adjusted for age, sex, BMI, current and former smoking, ethanol intake, hypertension, diabetes mellitus, left ventricular hypertrophy, income, systolic BP, and high-density lipoprotein | Serious | Fair | Fair |
| Atrial fibrillation/other cardio-vascular risk factors, n = 6 | ||||||||
| Conen et al. (2010), USA (131) | • Longitudinal prospective cohort • n = 27,982 |
• Cases AF • n = 735 |
• Risk of AF in BW categories • Adjusted HR • >4,500 vs. <2,500 g • *AHR 1.63 (95% CI 1.07–2.50) • Fully adjusted HR • **AHR 1.29 (95% CI 0.84–1.98) • P-linear trend 0.23 |
<2,500 g | • *Age, hypercholesterolemia, smoking, exercise, alcohol consumption, education, race, HRT therapy, BMI, SBP, DBP, diabetes • **All above plus adult height, body weight between 18 and 30 years |
Serious | Fair | Fair |
| Johnsson et al. (2018), Sweden (133) | • Cohort, matched • n = 644, • only 54 participated |
Continuous outcome | • No differences regarding blood pressure, lipid profiles, apolipoproteins, high-sensitivity CRP, or common carotid artery (CCA) wall dimension • Cases: 37% higher intima thickness in radial artery (RA-IT) (p < 0.01) and 44% difference in radial intima/media ratio (RA-I:M ratio) (p < 0.01) |
3,140–3,950 g | RA-IT and RA-I: M adjusted for gender, gestational age, smoking, BMI, systolic and diastolic blood pressure, CRP, and apolipoprotein B/A1 ratio | Critical | Poor | Poor |
| Larsson et al. (2015), Sweden (132) | • Cohort • n = 29,551 men • n = 23,454 women |
• Cases AF • n = 2,711 men • n = 1,491 = women |
• Risk for atrial fibrillation • Relative risk (RR)+95% CI 4,000–4,999 g • Men • ARR 1.03 (95% CI 0.94–1.15)* • ARR 0.89 (95% CI 0.80–0.99)** • Women • ARR 1.07 (95% CI 0.91–1.27)* • ARR 0.96 (95% CI 0.81–1.14)** • ≥5,000 g • Men • ARR 1.29 (95% CI 1.05–1.58)* • ARR 1.06 (95% CI 0.86–1.30)** • Women • ARR 1.50 (95% CI 1.01–2.24) • ARR 1.21 (95% CI 0.81–1.81)** |
2,500–3,999 g | • Adjustments in multivariable logistic regression analysis: • *Age, preterm birth, • **Plus education, smoking status and pack year of smoking, family history of myocardial infarction before 60 years and age, history of coronary heart disease or heart failure, history of hypertension, history of diabetes, BMI, and height |
Moderate | Good | Fair |
| Perkiömäki et al. (2016), Finland (135) | • Cohort • rMSSD: • n = 1,799 men • n = 2,279 women • BRS: • n = 902 men • n = 1,020 women |
Continuous outcome | • In men higher birth weight was independently associated with poorer cardiac autonomic function [seated (r = −0.058, p = 0.014) and standing rMSSD (r = −0.090, p < 0.001), standing BRS (r = −0.092, p = 0.006)]. Multivariate analysis p < 0.05 for all. • Same association was not seen in women |
Birth weight | • Vagally mediated heart rate variability (rMSSD, sitting or standing) • Spontaneous baroreflex sensitivity (BRS) at age 46 • Adjusted for: • Continuous adult variables: BMI, height, SBP, DBP, waist–hip ratio, glucose, glycated hemoglobin, total cholesterol, high density cholesterol, triglycerides • Categorized adult variables: current smoking, sitting time, alcohol consumption, sufficiency of sleep, physical activity |
Moderate | Good | Good |
| Skilton et al. (2014), Finland (134) | Cohort | n = 696Continuous outcome | • Mean carotid intima thickness: • Adj. beta-coefficient: • 0.022 (95% CI 0.007–0.036) (p = 0.003) • No difference in brachial flow mediated dilation, BP between LGA and normal BW |
Normal birth weight 50–75th percentile | Adjusted for age, sex, study center, SES, marital status, cardiovascular risk factors, BMI | Moderate | Good | Fair |
| Timpka et al. (2019), UK (136) | • Longitudinal cohort study • n = 1,964 |
Continuous outcome | Higher BW z-scores were associated with small differences of diastolic function in adolescence | Z-scores between 10th and 90th percentiles | • Adjusted for maternal pre-pregnancy BMI, age, level of education and smoking during pregnancy • Final model additionally adjusted for factors in adolescence; BMI, SBP, heart rate |
Moderate | Fair | Fair |
BW, birth weight; HBW, high birth weight; NBW, normal birth weight; GA, gestational age; LGA, large for gestational age; AGA, appropriate for gestational age; SGA, small for gestational age; BMI, body mass index; HBP, high blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; SES, socioeconomic status; CHD, coronary heart disease; AF, atrial fibrillation; OBP, office blood pressure; HoBP, home blood pressure; AOR, adjusted odds ratio; AHR, adjusted hazard ratio.
Blood Pressure and Hypertension
The SR and meta-analysis by Zhang et al. (19), including 31 studies on the association between high birth weight or LGA and blood pressure or hypertension, showed that high birth weight in younger children (6–12 years) was associated with a higher systolic and diastolic blood pressure, while in older adults (41–60 years) the reverse association was found. The same pattern was seen for the relative risk of hypertension. The authors describe the phenomenon as a “catch-down” effect in the elevation of blood pressure that is observed in subjects with high birth weight as they grow older (19). Hence, older individuals with high birth weight are less likely to develop hypertension than those with normal birth weight (19).
Fourteen original studies (125–138), not included in the review by Zhang et al. (19) were found. Four studies, all with serious risk of bias, showed an inverse relation between high birth weight/LGA and blood pressure, but the mean age of the individuals included in the studies varied tremendously ranging from 6–9 to >50 years of age. Six studies, four with serious and two with moderate risk of bias, showed no association between high birth weight/LGA and blood pressure/hypertension. The two studies with moderate risk of bias included individuals with age ranging from 6–18 years (126) to 33–65 years (129). Finally, four studies, one with moderate risk of bias and three with serious risk of bias, showed that high birth weight/LGA was positively associated with high blood pressure/hypertension. The study with moderate risk of bias included individuals with age 12–15 years (130).
Conclusion: There may be an association between high birth weight and hypertension in childhood, low certainty of evidence (GRADE ⊕⊕OO).
There may be an inverse association between high birth weight and hypertension in adulthood, low certainty of evidence (GRADE ⊕⊕OO).
Coronary Heart Disease
One SR of high quality including 27 articles on birth weight and CHD in adults was identified (124). A meta-analysis based on six prospective cohort studies on CHD exploring the risk of CHD in high birthweight children found no difference in the risk of CHD in children with high birth weight [OR 0.89 (95% CI 0.79–1.01)] (124). Furthermore, the meta-analysis showed that a 1-kg increase in birth weight is associated with a lower risk of CHD [OR 0.83 (95% CI 0.80–0.86)].
Only one original study (139) from the USA was identified which was not included in the SR.
Conclusion: There is probably no difference in the risk of CHD in men and women born with high birth weight compared with adults born with normal birth weight, moderate certainty of evidence (GRADE ⊕⊕⊕O).
Atrial Fibrillation and Other Cardiovascular Outcomes
Two studies with serious (140) and moderate risk of bias (141) explored the association between high birth weight and atrial fibrillation in adulthood and found no association.
Two studies found higher thickness of the radial artery intima (142) and the carotid artery intima (143) in adults of high birth weight or LGA while other cardiovascular risk factors and arterial function did not differ. In a Finnish study with moderate risk of bias, men with higher birth weight had a higher risk of poor cardiac autonomic function while the same association was not seen in women (144). Finally, higher BW z-scores were associated with small differences in diastolic function in adolescence in a study with moderate risk of bias (145).
Conclusion: It is uncertain if there is an association between high birth weight or LGA and altered cardiovascular function in adulthood, very low certainty of evidence (GRADE ⊕OOO).
Diabetes
Outcomes are listed in Table 1.4.
Table 1.4.
LGA and high birth weight and long-term outcomes—type 1 and type 2 diabetes.
| Author, year, country | Study design | Cases | Outcomes (risk estimates) | Reference group (weight) | Comments/adjustments | Risk of bias | Directness | Precision |
|---|---|---|---|---|---|---|---|---|
| • Type 1 and type 2 diabetes • Systematic review/meta-analysis n = 6 | ||||||||
| Cardwell et al. (2010), UK (137) | • Type 1 diabetes • Meta-analysis • Cohort n = 5 case–control n = 20 • 30 populations |
12,087 | • Birth weight >4,000 g: • OR (cohort studies) 1.15 (95% CI 1.05–1.26) • OR (case–control studies) 1.05 (95% CI 0.95–1.17) • AOR (all studies) 1.11 (95% CI 1.03–1.20) |
3,000–3,500 g | All ages included in risk estimates not only children/adolescents <18 years | |||
| Harder et al. (2007), Germany (158) | • Type 2 diabetes • Meta-analysis • cohort n = 10 • case–control n = 3 |
6,901 | • Birth weight >4,000 g: • 1OR 1.27 (95% CI 1.01–1.59) • 2OR 1.36 (95% CI 1.07–1.73) |
• 1 ≤ 4,000 g • 22,500 g • 4,000 g |
No separate OR calculated for children/adolescents <18 years | |||
| Harder et al. (2009), Germany (18) | • Type 1 diabetes • Meta-analysis • cohort n = 2 • case–control n = 10 |
7,491 | • Birth weight >4,000 g: • OR. 1.17 (95% CI 1.09–1.26) • AOR 1.43 (95% CI 1.11–1.85) |
<4,000 g | Adjusted for confounders in seven of the included studies and wide difference in the number of confounders ranging from 2 to 14 | |||
| Knop et al. (2018), China (160) | • Type 2 diabetes • Systematic review, meta-analysis • 49 studies • Cohort n = 36 • Case–control n = 8 • Cross-sectional n = 5 • (for high birth weight 32 studies) |
43,549 | • Birth weight >4,500 g: • OR 1.19 (95% CI 1.04–1.36) |
4,000–4,500 g | Adult only (>18 years) | |||
| Whincup et al. (2008), UK (159) | Type 2 diabetes systematic review, meta-analysis | 6,260 | • Per 1,000-g increase: • OR 0.80 (95% CI 0.72–0.89) • Birth weight >4,000 g: • OR 1.35 (95% CI 0.67–2.72) |
<4,000 g | Adults | |||
| Zhao et al. (2018), China (161) | • Type 2 diabetes • Meta-analysis, • Cohort n = 16 • Case–control n = 5 |
22,341 | • Birth weight >4,000 g: • OR was calculated for all ages: • OR 1.11 (95% CI 1.00–1.24) |
2,500–4,000 g | Only 2 studies were limited to children/adolescents less than 18 years, both were case–control studies. No separate calculated OR for children/adolescents separately | |||
| Original articles | ||||||||
| • Type 1 diabetes • Original articles n =20 | ||||||||
| Bock et al. (1994), Denmark (144) | Case–control | 837 | • No statistical differences in mean birth weight between the cases and controls: • 3,381, SD 536 g vs. 3,351, SD 602 g |
• Exclusion criteria: mother with IDDM at the time of birth • No risk estimates |
Serious | Good | Fair | |
| Borras et al. (2011), Spain (145) | Case–control | 306 | • LGA >90 percentile • OR for diabetes 1.45 (95% CI 1.02–2.07) |
10–90th percentile | • No adjustment • 43 of originally 349 cases excluded due to missing data on birth weight |
Serious | Good | Fair |
| Cardwell et al. (2005), UK (138) | Cohort study | 991 | • Birth weight >4,000 g: • ARR 1.68 (95% CI 1.30–2.18) • Birth weight 3,500–3,999 g: • ARR 1.48 (95% CI 1.20–1.83) |
<3,000 g | • Adjusted for maternal age, birth order, year of birth, gestational age • Missing data 8% |
Moderate | Good | Good |
| Goldacre (2017), UK (139) | Cohort study | 2,969 | • Birth weight 4,000–5,499 g: • AHR 1.12 (95% CI 0.99–1.27) • Birth weight 3,500–3,999 g: • AHR 1.11 (95% CI 1.02–1.22) |
3,000–3,499 g | Adjusted for infant sex, gestational age, maternal type 1 diabetes, maternal obesity, deprivation quintile, and caesarean section | Moderate | Good | Good |
| Haynes et al. (2007), Australia (146) | Cohort | 840 | • Birth weight ≥4,000 g: • IRR 1.19 (95% CI 0.95–1.49) • Birth weight 3,500–3,999 g: • IRR 1.09 (95% CI 0.92–1.28) |
3,000–3,499 g | Adjusted for maternal age, gestational age, birth order, and year of birth | Moderate | Good | Good |
| Levins et al. (2007), UK (140) | Cohort | 518 | • Estimated rate of diabetes (<15 years) in birth weight categories: • 3,500–3,999: Rate 1.55 (95% CI 1.28–1.86) • ≥4,000: Rate 1.65 (95% CI 1.17–2.26) |
No ref group | Adjusted for year of birth, Rates only per 1,000 individuals presented. No difference between birth categories | Serious | Good | Fair |
| Jones et al. (1999), UK (147) | Case–control study | 315 | • Birth weight 3,500–3,900 g: • ARR 1.00 (95% CI 0.74–1.36) • Birth weight ≥4,000 g: • ARR 1.15 (95% CI 0.76–1.75) |
3,000–3,499 g | Adjusted for maternal age, parity, birth weight for gestational age, gestational age and year of birth. Data included in Ievins (1997) and more restricted data material | Moderate | Good | Fair |
| Khashan et al. (2015), Sweden (141) | Cohort study | 13,944 | • Birth weight 4,000–5,500 g: • ARR 1.01 (95% CI 0.96–1.05) • LGA (+2 SD above mean) vs. AGA • RR 1.14 (95% CI 1.04–1.24) |
3,000–3,999 g | Adjusted for offspring age as a time-dependent variable, year of birth, maternal age, education, BMI, country of origin, pre-gestational diabetes, gestational diabetes and infant sex | Low | Good | Good |
| Kuchlbauer et al. (2014), Germany (142) | Cohort study | 1,117 | No risk estimate available. cases with type 1 diabetes had higher birth weight measured as SDS (0.15 vs. 0.03) than the newborn in the control SDS (z-scores) are calculated from birth weights based on population reference values | No adjustment. No risk estimates | Critical | Good | Fair | |
| Lawler-Heavner et al. (1994), USA (148) | Case–control study | 221 | • Birth weight 3,500–3,999 g: • AOR 0.9 (95% CI 0.5–1.7) • Birth weight ≥4,000 g: • AOR 1.0 (95% CI 0.4–2.5) |
<3,000 g | Adjusted for sex, age and birth in Colorado | Serious | Good | Fair |
| McKinney et al. (1999), UK (149) | Case–control study | 196 | • Birth weight ≥3,500 g: • OR 1.01 (95% CI 0.68–1.51) |
2,500–3,000 g | Uncertain whether the results are adjusted or not | Serious | Good | Fair |
| Metcalfe and Baum (1992), UK (150) | Case–control study | 952 | • Results given according to proportions in three categories of birth weight: • <2,500: insulin-dependent diabetes mellitus (IDDM) 65 (7%), Office of Population Censuses and Surveys (OPCS) 32,779 (6%) • 2,500–3,999: IDDM 783(82%), OPCS 509707 (86%) • ≥4,000: IDDM 104 (11%), OPCS 46012 (8%) |
No adjustments. No risk estimates. No conclusions drawn | Serious | Good | Fair | |
| Patterson et al. (1994), UK (151) | Case–control study | 529 | • Birth weight ≥4,000 g; • OR 1.14 (95% CI 0.75–1.74) |
2,500–3,999 g | No adjustments | Serious | Good | Fair |
| Rosenbauer et al. (2008), Germany (152) | • Case–control • Nationwide hospital-based surveillance (ESPED) |
• 760 • 719 cases in birthweight analysis |
• Birth weight ≥4,000 g: • AOR 1.28 (95% CI 0.94–1.73) |
3,000–3,999 g | Probably adjusted for familiar type 1 diabetes, social status, maternal age, number of siblings and change of residency | Moderate | Good | Fair |
| Stene et al. (2001), Norway (143) | Cohort study | 1,824 | • 3,500–3,999 g: RR 2.11 (95% CI 1.24–3.58) • 4,000–4,499 g: RR 2.38 (95% CI 1.39–4,06) • ≥4,500 g: RR 2.21 (95% CI 1.24–3.94) |
<2,000 g | Adjusted for sex, maternal age, plurality, birth weight, gestational age, caesarean section, pre-eclampsia, year of birth | Low | Good | Fair |
| Stene and Joner (2004), Norway (153) | Case–control study | 545 | • 3,500–3,999 g: AOR 0.94 (95% CI 0.44–1.99) • ≥4,000 g: AOR 1.01 (95% CI 0.46–2.29) |
<2,500 g | Adjusted for sex, maternal age, plurality, birth weight, gestational age, caesarean section, pre-eclampsia, duration of breast feeding, maternal education, atopic eczema, allergic rhino-conjunctivitis and asthma | Low | Good | Fair |
| Tai et al. (1998), Taiwan (154) | Case–control | 117 | • Birth weight ≥4,000 g: • AOR 0.97 (95% CI 0.39–2.45) |
<3,000 g | Adjusted for age, sex | Critical | Poor | Poor |
| Wadsworth et al. (1997), UK (155) | Case–control | • 281 • 218 cases included in the analysis |
• No significant association with birth weight analyzed as a continuous variable • Unadjusted OR per kg increase in birth weight 0.94 (95% CI 0.65–1.35) |
Unadjusted | Serious | Good | Poor | |
| Waernbaum et al. (2019), Sweden (156) | Case–control study | 14,949 | AOR 1.08 (95% CI 1.06–1.10) | Birth weight z-score category with the interval 0–1 as reference | Adjusted for urinary tract infection, PROM, maternal age, PTB, maternal BMI | Low | Good | Good |
| Wei et al. (2006), Taiwan (157) | Case–control study | 277 | ≥4,000 g: AOR 1.01 (95% CI 0.46–2.29) | <2,600 g | Adjusted for age, sex, socioeconomy, family history of diabetes„ delivery order, breast feeding, BMI, and GDM | Moderate | Fair | Fair |
| Type 2 diabetes | ||||||||
| Hu et al. (2020), China (163) | Cohort | 48,118 | ≥4,000 g: AOR 1.20 (95% CI 1.07–1.34) | 2,500–3,499 g | Adjustments: age, gender, smoking, drinking, education, physical activity, diet habits, systolic blood pressure, dyslipidemia, BMI | Moderate | Fair | Good |
| Zhu et al. (2013), China (164) | Cross-sectional survey | • 903 children with overweight • 2 with type 2 diabetes • 6 with impaired fasting glucose • 16 with impaired glucose tolerance • 2 with impaired fasting glucose + impaired glucose intolerance |
• Birth weight ≥4,000 g: • AOR 1.92 (95% CI 1.06–3.49) • Subgroup of girls analyzed separately: • AOR 4.38 (95% CI 1.21–15.85) |
2,500–3,999 g | Adjusted for age, gender, parental education. Only few children with type 2 diabetes or impaired fasting glucose | Moderate | Fair | Fair |
LGA, large-for-gestational-age; AGA, appropriate-for-gestational-age; HOMA-IR, homeostasis model assessment-insulin resistance; MS, metabolic syndrome; GDM, gestational diabetes mellitus; LBW, low birth weight; HBW, high birth weight; NBW, normal birth weight.
Type 1 Diabetes
Two SR and meta-analyses (18, 146) (moderate and low quality), six cohort studies (147–152), and 14 case–control studies (153–166) reported on the association between high birth weight or LGA and type 1 diabetes. Both SR/meta-analyses reported an association between high birth weight and childhood-onset type 1 diabetes [AOR of 1.43 (95% CI 1.11–1.85) and AOR 1.11 (95% CI 1.03–1.20)] (18, 146).
Of the 20 original studies, four were assessed being of low, six of moderate, and the rest of critical or serious risk of bias.
Our meta-analysis, including 13 studies, found a pooled OR of 1.15 (95% CI 1.05–1.26) for type 1 diabetes when comparing birth weight >4,000 g with <4,000 g (Figure 9). For LGA vs. AGA, the OR was 1.1 (95% CI 1.03–1.21) (Figure 10). All but one study (163) included children below 18 years of age. Two of the eight studies not included in the meta-analysis had moderate risks of bias and these studies found no significant association between high birth weight and type 1 diabetes. Other studies not included in the meta-analysis were of serious or critical risk of bias.
Figure 9.
Forest plot describing the association between high birth weight and Diabetes type 1.
Figure 10.
Forest plot describing the association between LGA and Diabetes type 1.
Conclusion: High birth weight and/or LGA is probably associated with a slight increase in type 1 diabetes, moderate certainty of evidence (GRADE ⊕⊕⊕O).
Type 2 Diabetes
Four SR investigated the association between birth weight/high birth weight and type 2 diabetes (167–170). Three of these SR were considered being of high quality (168–170). The literature search identified few additional studies (171, 172). The SR by (168, 170) only included adults while the SR by (167, 169) also included children; however, only in a few studies. The SR by Knop et al. (169) reported a slight increase in type 2 diabetes if birth weight is above 4,500 g, OR 1.19 (95% CI 1.04–1.36), while the SR by Zhao et al. (170) found no increase, OR 1.11 (95% CI 1.00–1.24) for birth weight above 4,000 g. The SR by Knop et al. (169) pointed out the J-shaped association with a higher risk, particularly at low and to a less extent at high birth weight.
Conclusion: High birth weight may be associated with a slight increase in type 2 diabetes, low certainty of evidence (GRADE ⊕⊕ OO).
Discussion
In this systematic review and meta-analysis, we have summarized the evidence for an association between high birth weight and/or LGA and some severe long-term outcomes for the children (Supplementary Table 5). The outcomes included are malignancies in childhood and breast cancer, cardiovascular diseases, psychiatric disorders, and diabetes type 1 and 2. To clarify if such associations exist and if so, the magnitude of these associations is of high importance for children born after spontaneous conception in view of the dramatic increase in obesity among women of childbearing age and the associated rise in high birth weight babies. In ART, these findings are important due to the increase in frozen/thawed cycles in ART and the recent findings of higher risks of high birth weight and LGA in offspring from FET cycles.
The systematic literature search identified a huge number of articles which were scrutinized and 173 of these publications were selected for this review.
The choice of the selected types of malignancies was based on the number of publications. Thus, our SR does not include all types of malignancies, but the ones where most publications were identified. The metabolic part was limited to diabetes type 1 and 2. Cardiovascular and psychiatric diseases were selected due to being common in the population and having a high impact on human health.
Malignancies
We found a small to moderately increased risk for all types of malignancies studied, with estimates of OR between 1.19 and 1.69. The most pronounced association was found for Wilm's tumor. The biological mechanism linking fetal growth and cancer is largely unknown (51). The observation in children with overgrowth disorders, such as Beckwith–Wiedemann syndrome (BWS), supports a theory that the number, size, and proliferative potential of muscle stem cells (173) which correlate with birth weight are involved. These cells are particularly susceptible to oncogenic mutations and thus a faster growing fetus may involve an increased cancer risk. BWS children, characterized by increased fetal growth, are prone to a wide range of cancers, including Wilm's tumor and leukemia (174). BWS is caused by overexpression of insulin-like factor 2 (IGF-2) gene. Furthermore, several cancers in adults are associated with increased levels of IGFs. Since IGF levels also are increased in heavy babies without these syndromes, there may be a more general association between levels of IGF in newborns and risk of childhood cancer (175). Further support for the IGF-1 theory comes from a study on children with congenital IGF-1 deficiency who seems to be protected against the risk of developing cancer (176). Other suggested mechanisms include exposure of fast-growing babies to elevated levels of estrogen in utero and/or epigenetic mechanisms, both associated with fetal birth weight and cancer risk (177).
Psychiatric Disorders
Four out of six cohort studies on high birth weight and/or LGA and schizophrenia reported an increased risk of developing schizophrenia in the offspring while no association was found in two studies in adjusted models. All these studies were performed in the Nordic countries and the limit for being born with high birth weight varied being >4,000 and >4,500 g. The mechanisms underlying the association between high birth weight and schizophrenia are unclear. It has been suggested that potential fetal exposure to gestational diabetes may play a role, as an association between maternal diabetes and schizophrenia among offspring has been found (94, 178). Furthermore, gestational diabetes may lead to macrosomic babies, who are at increased risk of delivery complications such as shoulder dystocia and asphyxia which also, per se, may increase a risk to later psychiatric problems (12, 94). Interestingly, in a study using self-report questionnaires, high birth weight increased the risk for depression only in women (99).
Interestingly, a recent systematic review suggested that high birth weight was protective of psychotic disorders in general (100). It was, however, unclear which studies were included in this review and no quality assessment was presented.
In many studies, several types of psychiatric disturbances were investigated and even combined. This may explain the contradictory results concerning high birth weight/LGA and psychiatric disorders. Environmental and socioeconomic status probably play an important role in a person's susceptibility for a psychiatric disease making those with higher socioeconomic status less vulnerable (102).
There might be an association between high birth weight/LGA and negative behaviors in adolescence. The reasons for this connection are largely unknown. Family and genetic factors certainly are important in the tendency of developing behavior problems, but the neurobiological mechanisms underlying interactions to high birth weight are unclear (106). Due to delivery complications, the macrosomic infants have an increased risk of birth trauma and asphyxia (12, 176). Such adverse perinatal outcomes are, per se, associated with later behavioral problems (179).
Most of the studies about intellectual performance and high birth weight have been carried out on male conscripts generally excluding women and part of the most vulnerable men. A reassuring notice was that no association was found between high birth weight/LGA and risk of intellectual disability, or low IQ score. However, according to Lundgren et al. (119) high BMI in adulthood had a negative effect on IQ level.
Cognitive performance was positively related to high birth weight at least up to the birth weight of 4,200 g (113). This association is thought to be mediated by optimal prenatal factors and healthy nutrition both pre- and postnatally. Such findings related to mental development emphasize the importance of maternal care during pregnancy (113).
Cardiovascular Diseases
Based on the current evidence, there may be an age-related association between high birth weight/LGA and high blood pressure in childhood while the opposite is found in adulthood. For CHD or cardiovascular function in adults, there was no obvious association with high birth weight or LGA. In the study by Wang et al. (124) the focus was on the relation between birth weight and CHD over the full birth weight range from low to high birth weight, and they found a consistent inverse relation between birth weight and CHD.
In general, individuals with high birth weight are taller and heavier later in life than subjects with normal birth weight (180). However, their metabolic health seems to be better later in life as they have less adipose tissue than lean mass (181).
Contradictory to the findings of a lower risk of CHD in children born with high birth weight, babies are more likely to be born large-for-gestational age in mothers with diabetes, increasing the risk of diabetes and CHD later in the children's life (18). High birth weight could be a result of gestational diabetes in the mother thus, hypothetically, high birth weight may be a potential risk factor of CHD in the offspring (182, 183).
Type 1 Diabetes
In our meta-analysis, high birth weight was associated with a slightly higher risk of type 1 diabetes in line with previous meta-analyses (18, 146).
The mechanism between high birth weight and type 1 diabetes seems unknown. It may be other factors besides the birth weight per se that are responsible for this association. Gestational diabetes and maternal overweight during pregnancy are risk factors for increased birth weight (184, 185). It has been suggested that maternal and/or fetal hyperglycemia also may predispose to an increased susceptibility of the overstimulated fetal pancreatic beta cells to processes causing type 1 diabetes (186, 187). Furthermore, a rapid postnatal growth during the first year of life also seem to be associated with a later risk of developing type 1 diabetes (18). Other triggering factors of the genetic predisposition may also be related to the association between high birth weight and type 1 diabetes (188).
Type 2 Diabetes
For type 2 diabetes, recently performed meta-analyses of high quality found some divergent results. Knop et al. (169) identified a small but significant increase in risk of type 2 diabetes at birth weight above 4,500 g while the meta-analysis by Zhao et al. (170), could not identify any increased risk; however, the estimate was of borderline significance. The biological mechanism behind such an association, if it exists, is a matter of debate. According to the fetal programming hypothesis, also small changes in organ maturation during the fetal period might result in altered growth and disordered endocrine function in adulthood (169).
Strengths and Limitations
The major strength of this systematic review is the comprehensive literature search, identifying a considerable number of relevant articles. The ability to present meta-analyses, either of high quality and recently published or new meta-analyses performed for the purpose of this SR, makes interpretation of the summarized literature easier to capture. The main limitation is that all data are based on observational studies, both cohort studies being of higher quality but also case–control studies with their inborn risk of selection bias. Our conclusions are, however, based mainly on meta-analyses and/or on studies with low risk of bias.
In conclusion, this systematic review and meta-analysis, investigating high birth weight and LGA as risk factors for adverse outcome in offspring, found elevated risks for certain malignancies in childhood, breast cancer, several psychiatric disorders, hypertension in childhood, although not in adulthood, and type 1 and type 2 diabetes. Although these risks represent serious health effects, both in childhood and in adulthood, the size of these effects seems moderate. The results are important for the overall implications of increasing birth weight and will contribute to the ongoing discussion of the pros and cons of fresh or frozen embryo transfer cycles in ART.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Author Contributions
ÅM, HL, AL, NO, AP, LR, VS-A, and CB contributed to conception and design of the study. ÅM and CB search databases. Screening of abstracts and of full papers for inclusion was done by pairs of reviewers by ÅM, HL, AL, NO, AP, LR, VS-A, and CB. MP performed the statistical analysis. ÅM, HL, AL, NO, AP, LR, VS-A, MP, and CB wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Gedeon Richter for providing travel, accommodation, and working facilities to the Nordic Collaboration Group. Furthermore, we thank librarian Helen Sjöblom for excellent help with literature search.
Footnotes
Funding. This work was supported in part by grants from the Swedish state under the agreement between the Swedish government and the county councils the ALF-agreement (ALFGBG-70940), the Hjalmar Svensson's foundation, and the Research Council of Norway through its Centres of Excellence funding scheme, Project Number 262700.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.675775/full#supplementary-material
References
- 1.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. (1993) 36:62–7. 10.1007/BF00399095 [DOI] [PubMed] [Google Scholar]
- 2.Surkan PJ, Hsieh CC, Johansson AL, Dickman PW, Cnattingius S. Reasons for increasing trends in large for gestational age births. Obstet Gynecol. (2004) 104:720–6. 10.1097/01.AOG.0000141442.59573.cd [DOI] [PubMed] [Google Scholar]
- 3.Poston L, Caleyachetty R, Cnattingius S, Corvalán C, Uauy R, Herring S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. (2016) 4:1025–36. 10.1016/S2213-8587(16)30217-0 [DOI] [PubMed] [Google Scholar]
- 4.Wennerholm UB, Henningsen AK, Romundstad, Bergh C, Pinborg A, Skjaerven R, et al. Perinatal outcomes of children born after frozen-thawed embryo transfer: a Nordic cohort study from the CoNARTaS group. Hum Reprod. (2013) 28:2545–53. 10.1093/humrep/det272 [DOI] [PubMed] [Google Scholar]
- 5.Pinborg A, Loft A, Aaris Henningsen A-K, Rasmussen S, Andersen AN. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995-2006. Fertil Steril. (2010) 94:1320–7. 10.1016/j.fertnstert.2009.05.091 [DOI] [PubMed] [Google Scholar]
- 6.Maheshwari A, Pandey S, Amalraj Raja E, Shetty A, Hamilton M, Bhattacharya S. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum Reprod Update. (2018) 24:35–58. 10.1093/humupd/dmx031 [DOI] [PubMed] [Google Scholar]
- 7.Chen Z-J, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus Frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med. (2016) 375:523–33. 10.1056/NEJMoa1513873 [DOI] [PubMed] [Google Scholar]
- 8.Vuong LN, Dang VQ, Ho TM, Huynh BG, Ha DT, et al. IVF transfer of fresh or Frozen embryos in women without polycystic ovaries. N Engl J Med. (2018) 378:137–47. 10.1056/NEJMoa1703768 [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Sun Y, Hao C, Zhnag H, Wei D, Zhang Y, et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med. (2018) 378:126–36. 10.1056/NEJMoa1705334 [DOI] [PubMed] [Google Scholar]
- 10.Wei D, Liu JY, Sun Y, Shi Y, Zhang B, Liu JQ, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet. (2019) 393:1310–8. 10.1016/S0140-6736(18)32843-5 [DOI] [PubMed] [Google Scholar]
- 11.Stormlund S, Sopa N, Zedeler A, Bogstad J, Praetorius L, Nielsen HS, et al. Freeze-all versus fresh blastocyst transfer strategy during in vitro fertilization in women with regular menstrual cycles: multicentre randomised trial. BMJ. (2020) 370:m2519. 10.1136/bmj.m2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henriksen T. The macrosomic fetus: a challenge in current obstetrics. Acta Obstet Gynecol Scand. (2008) 87:134–45. 10.1080/00016340801899289 [DOI] [PubMed] [Google Scholar]
- 13.Beta J, Khan N, Khalil A, Fiolna M, Ramadan G, Akolekar R. Maternal and neonatal complications of fetal macrosomia: systematic review and meta-analysis. Ultrasound Obstet Gynecol. (2019) 54:308–18. 10.1002/uog.20279 [DOI] [PubMed] [Google Scholar]
- 14.Innes K, Byers T, Schymura M. Birth characteristics and subsequent risk for breast cancer in very young women. Am J Epidemiol. (2000) 152:1121–8. 10.1093/aje/152.12.1121 [DOI] [PubMed] [Google Scholar]
- 15.Gu S, An X, Fang L, Zhang X, Zhang C, Wang J, et al. Risk factors and long-term health consequences of macrosomia: a prospective study in Jiangsu Province, China. J Biomed Res. (2012) 26:235–40. 10.7555/JBR.26.20120037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Triebwasser C, Wang R, DeWan A, Metayer C, Morimoto L, Wiemels J, et al. Birth weight and risk of paediatric Hodgkin lymphoma: findings from a population-based record linkage study in California. Eur J Cancer. (2016) 69:19–27. 10.1016/j.ejca.2016.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunnell D, Rasmussen F, Fouskakis D, Tynelius P, Harrison G. Patterns of fetal and childhood growth and the development of psychosis in young males: a cohort study. Am J Epidemiol. (2003) 158:291–300. 10.1093/aje/kwg118 [DOI] [PubMed] [Google Scholar]
- 18.Harder T, Roepke K, Diller N, Stechling Y, Dudenhausen JW, Plagemann A. Birth weight, early weight gain, and subsequent risk of type 1 diabetes: systematic review and meta-analysis. Am J Epidemiol. (2009) 169:1428–36. 10.1093/aje/kwp065 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Li H, Liu S-J, Fu GJ, Zhao Y, Xie YJ, et al. The associations of high birth weight with blood pressure and hypertension in later life: a systematic review and meta-analysis. Hypertens Res. (2013) 36:725–35. 10.1038/hr.2013.33 [DOI] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 21.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck Ytter Y, Alonso Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group . Preferred reporting items for systematic reviews and meta analyses: the PRISMA statement. PLoS Med. 6:e1000097. 10.1371/journal.pmed1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michels KB, Xue F. Role of birth weight in the etiology of breast cancer. Int J Cancer. (2006) 119:2007–25. 10.1002/ijc.22004 [DOI] [PubMed] [Google Scholar]
- 24.Xue F, Michels KB. Intrauterine factors and risk of breast cancer: a systematic review and meta-analysis of current evidence. Lancet Oncol. (2007) 8:1088–100. 10.1016/S1470-2045(07)70377-7 [DOI] [PubMed] [Google Scholar]
- 25.Zhou W, Chen X, Huang H, Liu S, Wie A, Lan L. Birth weight and incidence of breast cancer: dose-response meta-analysis of prospective studies. Clin Breast Cancer. (2020) 5:e555–68. 10.1016/j.clbc.2020.04.011 [DOI] [PubMed] [Google Scholar]
- 26.Andersson SW, Bengtsson C, Hallberg L, Lapidus L, Niklasson A, Wallgren A, et al. Cancer risk I Swedish women: the relation to size at birth. Br J Cancer. (2001) 84:1193–8. 10.1054/bjoc.2000.1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahlgren M, Sørensen T, Wohlfahrt J, Haflidadóttir Á, Holst C, Melbye M. Birth weight and risk of breast cancer in a cohort of 105,504 women. Int J Cancer. (2003) 107:997–1000. 10.1002/ijc.11481 [DOI] [PubMed] [Google Scholar]
- 28.Ahlgren M, Melbye M, Wohjfahrt J, Sørensen T. Growth patterns and the risk of breast cancer in women. N Engl J Med. (2004) 351:1619–26. 10.1056/NEJMoa040576 [DOI] [PubMed] [Google Scholar]
- 29.Ahlgren M, Wohlfahrt J, Olsen L, Sørensen T, Melbye M. Birth weight and risk of breast cancer. Cancer. (2007) 110:412–9. 10.1002/cncr.22773 [DOI] [PubMed] [Google Scholar]
- 30.Barber L, Bertrand K, Rosenberg L, Battaglia T, Palmer J. Pre- and perinatal factors and incidence of breast cancer in the Black Women's Health Study. Cancer Causes Control. (2019) 30:87–95. 10.1007/s10552-018-1103-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.dos Santos Silva I, De Stavola BL, Hardy J, Kuh DJ, McCormack VA, Wadsworth J. Is the association of birth weight with premenopausal breast cancer risk mediated through childhood growth? Br J Cancer. (2004) 91:519–24. 10.1038/sj.bjc.6601972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCormack VA, dos Santos Silva I, De Stavola BL, Mohsen R, Leon DA, Lithell HO. Fetal growth and subsequent risk of breast cancer: results from long term follow up of Swedish cohort. BMJ. (2003) 326:248. 10.1136/bmj.326.7383.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michels KB, Xue F, Terry KL, Willett WC. Longitudinal study of birth weight and the incidence of breast cancer in adulthood. Carcinogenesis. (2006) 27:2464–8. 10.1093/carcin/bgl105 [DOI] [PubMed] [Google Scholar]
- 34.Mogren I, Damber L, Tavelin B, Högberg U. Characteristics of pregnancy and birth and malignancy in the offspring (Sweden). Cancer Cause Control. (1999) 10:85–94. 10.1023/A:1008813701634 [DOI] [PubMed] [Google Scholar]
- 35.Vatten LJ, Lund Nilsen TI, Tretl S, Trichopoulos D, Romundstad PR. Size at birth and risk of breast cancer: prospective population-based study. Int J Cancer. (2005) 114:461–4. 10.1002/ijc.20726 [DOI] [PubMed] [Google Scholar]
- 36.Lahmann PH, Gullber B, Olsson H, Boeing H, Berglund G, Lissner L. Birth weight is associated with postmenopausal breast cancer risk in Swedish women. Br J Cancer. (2004) 91:1666–8. 10.1038/sj.bjc.6602203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mellemkjær L, Olsen ML, Sørensen HT, Thulstrup AM, Olsen J, Olsen JH. Birth weight and risk of early-onset breast cancer (Denmark). Cancer Causes Control. (2003) 14:61–4. 10.1023/A:1022570305704 [DOI] [PubMed] [Google Scholar]
- 38.Michels K, Trichopoulos D, Robins JM, Rosner BA, Manson JE, Hunter DJ, et al. Birthweight as a risk factor for breast cancer. Lancet. (1996) 348:1542–6. 10.1016/S0140-6736(96)03102-9 [DOI] [PubMed] [Google Scholar]
- 39.Sanderson M, Shu XO, Jin F, Dai Q, Ruan Z, Gao Y-T, et al. Weight at birth and adolescence and premenopausal breast cancer risk in a low-risk population. Br J Cancer. (2002) 86:84–8. 10.1038/sj.bjc.6600009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Troisi R, Grotmol T, Jacobsen J, Tretli S, Toft-Sørensen H, Gissler M, et al. Perinatal characteristics and breast cancer risk in daughters; a Scandinavian population-based study. J Dev Orig Health Dis. (2013) 4:35–41. 10.1017/S2040174412000645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Titus-Ernstoff L, Egan KM, Newcomb PA, Ding J, Trentham-Dietz A, Greenberg FR, et al. Early life factors in relation to breast cancer risk in postmenopausal women. Cancer Epidemiol Biomarker Prev. (2002) 11:207–10. [PubMed] [Google Scholar]
- 42.Vatten LJ, Mæhle BO, Lund Nilsen TI, Tretl S, Hsieh CC, Trichopoulos D, et al. Birth weight as a predictor of breast cancer, a case–control study in Norway. Br J Cancer. (2002) 86:89–92. 10.1038/sj.bjc.6600011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu AH, McKean-Cowdin R, Tseng C-C. Birth weight and other perinatal factors and risk of breast cancer in Asian-Americans. Breast Cancer Res Treat. (2011) 130:917–25. 10.1007/s10549-011-1640-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dahlhaus A, Prengel P, Spector L, Pieper D. Birthweight and subsequent risk of childhood primary brain tumors: an updated meta-analysis. Pediatr Blood Cancer. (2016) 64:e26299. 10.1002/pbc.26299 [DOI] [PubMed] [Google Scholar]
- 45.Harder T, Plagemann A, Harder A. Birth weight and subsequent risk of childhood primary brain tumors: a meta-analysis. Am J Epidemiol. (2008) 168:366–73. 10.1093/aje/kwn144 [DOI] [PubMed] [Google Scholar]
- 46.Harder T, Plagemann A, Harder A. Birth weight and risk of neuroblastoma: a meta-analysis. Int J Epidemiol. (2010) 39:746–56. 10.1093/ije/dyq040 [DOI] [PubMed] [Google Scholar]
- 47.Georgakis MK, Kalogirou EI, Liaskas A, Karalexi MA, Papathoma P, Ladopoulou K, et al. Anthropometrics at birth and risk of a primary central nervous system tumour: a systematic review and meta-analysis. Eur J Cancer. (2017) 75:117–31. 10.1016/j.ejca.2016.12.033 [DOI] [PubMed] [Google Scholar]
- 48.Crump C, Sundquist J, Sieh W, Winkleby M, Sundquist K. Perinatal and familial risk factors for brain tumors in childhood through young adulthood. Cancer Res. (2015) 75:576–83. 10.1158/0008-5472.CAN-14-2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitahara CM, Gamborg M, Rajaraman P, Sørensen TIA, Baker JL. A prospective study of height and body mass index in childhood, birth weight, and risk of adult glioma over 40 years of follow-up. Am J Epidemiol. (2014) 180:821–9. 10.1093/aje/kwu203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tettamanti G, Ljung R, Mathiesen T, Schwartzbaum J, Feychting M. Birth size characteristics and risk of brain tumors in early adulthood. Results from a Swedish Cohort Study. Cancer Epidemiol Biomarker Prev. (2016) 25:678–85. 10.1158/1055-9965.EPI-15-1096 [DOI] [PubMed] [Google Scholar]
- 51.O'Neill KA, Murphy MFG, Bunch KJ, Puumala SE, Carozza SE, Chow EJ, et al. Infant birtweight and risk of childhood cancer: international population-bases case control studies of 40 000 cases. Int J of Epidemiol. (2015) 44:153–68. 10.1093/ije/dyu265 [DOI] [PubMed] [Google Scholar]
- 52.Tran LT, Lai HTM, Kotiyama C, Uwatoko F, Akiba S. The association between high birth weight and the risk of childhood CNS tumors and leukemia: an analysis of a US case–control study in an epidemiological database. BMC Cancer. (2017) 17:687. 10.1186/s12885-017-3681-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson KJ, Zoellner NL, Gutmann DH. Peri-gestational risk factors for pediatric brain tumors in neurofibromatosis type 1. Cancer Epidemiol. (2016) 42:53–9. 10.1016/j.canep.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caughey RW, Michels KM. Birth weight and childhood leukemia: a meta-analysis and review of the current evidence. Int J Cancer. (2009) 124:2658–70. 10.1002/ijc.24225 [DOI] [PubMed] [Google Scholar]
- 55.Hjalgrim LL, Westergaard T, Rostgaard K, Schmiegelow K, Melbye M, Hjalgrim H, et al. Birth weight as a risk factor for childhood leukemia: a meta-analysis of 18 epidemiologic studies. Am J Epidemiol. (2003) 158:724–35. 10.1093/aje/kwg210 [DOI] [PubMed] [Google Scholar]
- 56.Crump C, Sundquist J, Sieh W, Winkleby MA, Sundquist K. Perinatal and familial risk factors for acute lymphoblastic Leukemia in a Swedish National Cohort. Cancer. (2015) 121:1040–7. 10.1002/cncr.29172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paltiel O, Tikelis G, Linet M, Golding J, Lemeshow S, Philips G, et al. Birth weight and childhood cancer: primary findings from the International Childhood Cancer Cohort Consortium (14C). Paediatr Perinat Epidemiol. (2015) 29:335–45. 10.1111/ppe.12193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westergaard T, Andersen PK, Pedersen JB, Olsen JH, Frisch M, Sørensen HT, et al. Birth characteristics, sibling patterns and acute leukemia risk in childhood: a population-based Cohort study. J Natl Cancer Inst. (1997) 89:939–47. 10.1093/jnci/89.13.939 [DOI] [PubMed] [Google Scholar]
- 59.Spix C, Schulze-Rath R, Kaatsch P, Blettner M. Case–control study on risk factors for leukaemia and brain tumors in children under 5 years in Germany. Klin Pediatr. (2009) 221:362–8. 10.1055/s-0029-1239531 [DOI] [PubMed] [Google Scholar]
- 60.Cnattingus S, Zack MM, Ekbom A, Gunnarskog J, Kreuger A, Linet M, et al. Prenatal and neonatal risk factors for childhood lymphatic leukemia. J Natl Canc Inst. (1995) 87:908–14. 10.1093/jnci/87.12.908 [DOI] [PubMed] [Google Scholar]
- 61.Groves FD, Watkins BT, Roberts DJ, Tucker TC, Shen T, Flood TJ. Birth weight and risk of childhood acute lymphoblasic leukemia in arizona, illinois and kentucky. South Med J. (2018) 111:579–84. 10.14423/SMJ.0000000000000873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hjalgrim LL, Rostgaard K, Hjalgrim H, Westergaard T, Thomassen H, Forestier E, et al. Birth weight and risk for childhood leukemia in Denmark, Sweden, Norway and Iceland. J Natl Canc Inst. (2004). 96:1549–56. 10.1093/jnci/djh287 [DOI] [PubMed] [Google Scholar]
- 63.Koifman S, Pombo-de-Oliveira MS. The Brazilian collaborative study group of infant acute leukemia. Br J Cancer. (2008) 98:664–7. 10.1038/sj.bjc.6604202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma X, Metayer C, Does MB, Buffler PA. Maternal pregnancy loss, birth characteristics and childhood leukemia (Unites States). Cancer Cause Control. (2005) 16:1075–83. 10.1007/s10552-005-0356-9 [DOI] [PubMed] [Google Scholar]
- 65.McLaughlin CC, Baptiste MS, Schymura MJ, Nasca PC, Zdeb MS. Birth weight, maternal weight and childhood leukaemia. Br J Cancer. (2006) 94:1738–44. 10.1038/sj.bjc.6603173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okcu MF, Goodman KJ, Carozza S, Weiss N, Burau KC, Bleyer A, et al. Birth weight, ethnicity and occurrence of cancer in children: a population-based incident case–control study in the State of Texas, USA. Cancer Causes Control. (2002) 13:595–602. 10.1023/A:1019555912243 [DOI] [PubMed] [Google Scholar]
- 67.Petridou E, Trichopoulos D, Kalpothaki V, Pourtsidis A, Kogevinas M, Kalamanti M, et al. The risk of childhood leukaemia in Greece: a nationwide case–control study. Br J Cancer. (1997) 76:1241–7. 10.1038/bjc.1997.541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Podvin D, Kuehn C, Mueller B, Williams M. Maternal and birth characteristics in relation to childhood leukaemia. Paediatr Perinat Epidemiol. (2006) 20:312–22. 10.1111/j.1365-3016.2006.00731.x [DOI] [PubMed] [Google Scholar]
- 69.Reynolds P, Von Behren J, Elkin EP. Birth characteristics and Leukemia in young children. Am J Epidemiol. (2002) 155:603–13. 10.1093/aje/155.7.603 [DOI] [PubMed] [Google Scholar]
- 70.Robinson LL, Codd M, Gunderson P, Neglia JP, Smithson WA, King FL. Birth weight as a risk factor for childhood acute lymphoblastic leukemia. Pediatr Hematol Oncol. (1987) 4:63–72. 10.3109/08880018709141250 [DOI] [PubMed] [Google Scholar]
- 71.Roman E, Lightfoot T, Smith AG, Forman MR, Linet MS, Robinson L, et al. Childhood acute lymphoblastic leukaemia and birthweight: insights from a pooled analysis of case–control data from Germany, the United Kingdom and the United States. Eur J Cancer. (2013) 49:1437–47. 10.1016/j.ejca.2012.11.017 [DOI] [PubMed] [Google Scholar]
- 72.Smith A Lightfoot T Simpson J Roman E on behalf of the UKCCS investigators . Birth weight, sex and childhood cancer: a report from the United Kingdom Childhood Cancer Study. Cancer Epidemiol. (2009) 33:363–7. 10.1016/j.canep.2009.10.012 [DOI] [PubMed] [Google Scholar]
- 73.Zack M, Adami H-O, Ericson A. Maternal and perinatal risk factors for childhood leukemia. Cancer Res. (1991) 5:3696–701. [PubMed] [Google Scholar]
- 74.Petridou ET, Sergentanis TN, Skalkidou A, Antonopoulos CN, Dessypris N, Svensson T, et al. Maternal and birth anthropometric characteristics in relation to the risk of childhood lymphomas: a Swedish nationwide cohort study. Eur J Cancer. (2015) 4:535–41. 10.1097/CEJ.0000000000000122 [DOI] [PubMed] [Google Scholar]
- 75.Peckham-Gregory EC, Danysh HE, Brown AL, Eckstein O, Grimes A, Chakraborty R, et al. Evaluation of maternal and perinatal characteristics on childhood lymphoma risk: a population-based case–control study. Pediatr Blood Cancer. (2017) 64:e26321. 10.1002/pbc.26321 [DOI] [PubMed] [Google Scholar]
- 76.Savitz DA, Ananth CV. Birth characteristics of childhood cancer cases, controls and their siblings. Pediatr Hematol Oncol. (1994) 11:587–99. 10.3109/08880019409141806 [DOI] [PubMed] [Google Scholar]
- 77.Schüz J, Forman MR. Birthweight by gestational age and childhood cancer. Cancer Causes Control. (2007) 18:655–63. 10.1007/s10552-007-9011-y [DOI] [PubMed] [Google Scholar]
- 78.Yaezel MW, Ross JA, Buckley JD, Woods WG, Ruccione K, Robison LL. High birth weight and risk of specific childhood cancers: a report from the Children's Cancer Group. J Pediatr. (1997) 131:671–7. 10.1016/S0022-3476(97)70091-X [DOI] [PubMed] [Google Scholar]
- 79.Kaatsch P, Kaleysch U, Meinert R, Meisner A, Hoisl M, Shüz J, et al. German case control study on childhood leukaemia-Basic considerations, methodology and summary of the results. Klin Pädiatr. (1998) 201:185–91. 10.1055/s-2008-1043877 [DOI] [PubMed] [Google Scholar]
- 80.Rangel M, Cypriano M, de Martino Lee ML, Luisi FAV, Petrilli AS, et al. Leukemia, non-Hodgkin's lymphoma and Wilm's tumor in childhood: the role of birth weight. Eur J Pediatr. (2010) 169:875–81. 10.1007/s00431-010-1139-1 [DOI] [PubMed] [Google Scholar]
- 81.Chu A, Heck JE, Ribeiro KB, Brennan P, Bofetta P, Buffer P, et al. Wilm's tumour: a systematic review of risk factors and meta-analysis. Paediatr Perinat Epidemiol. (2010) 24:449–69. 10.1111/j.1365-3016.2010.01133.x [DOI] [PubMed] [Google Scholar]
- 82.Crump C, Sundquist J, Sieh W, Winklby M, Sundquist K. Perinatal risk factors for Wilm's tumor in a Swedish national cohort. Eur J Epidemiol. (2014) 29:191–7. 10.1007/s10654-014-9880-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heuch JM, Heuch I, Kvåle G. Birth characteristics and risk of Wilm's tumour: a nationwide prospective study in Norway. Br J Cancer. (1996) 74:1148–51. 10.1038/bjc.1996.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Daniels JL, Pan IJ, Olshan AF, Breslow NE, Bunin GR, Ross JA. Obstetric history and birth characteristics and Wilms tumor: a report from the Children's Oncology Group. Cancer Causes Control. (2008) 19:1102–10. 10.1007/s10552-008-9174-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heck JE, He D, Janzen C, Federman N, Olsen J, Ritz B, et al. Fetal programming and Wilms tumor. Pediatr Blood Cancer. (2019) 66:e27461. 10.1002/pbc.27461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jepsen P, Olsen ML, Mellemkjær L, Olsen JH, Sørensen HT. A registry-based study of gender, fetal growth and risk of Wilms tumor. Pediatr Hematol Oncol. (2004) 21:435–9. 10.1080/08880010490457213 [DOI] [PubMed] [Google Scholar]
- 87.Lindblad P, Zack M, Adami H-O, Ericson A. Maternal and perinatal risk factors for Wilm's tumor: a nationwide nested case–control study in Sweden. Int J Cancer. (1992) 51:38–41. 10.1002/ijc.2910510108 [DOI] [PubMed] [Google Scholar]
- 88.Olshan AF, Breslow NE, Faletta JM, Grufferman S, Pendergrass T, Robison LL, et al. Risk factors for wilms tumor. Cancer. (1993) 72:938–44. [DOI] [PubMed] [Google Scholar]
- 89.Puumala SE, Soler JT, Johnson KJ, Spector LG. Birth characteristics and Wilms tumor in Minnesota. Int J Cancer. (2008) 122:1368–73. 10.1002/ijc.23275 [DOI] [PubMed] [Google Scholar]
- 90.Schüz J, Kaletsch U, Meinert R, Kaatsch P, Michaelis J. High-birth weight and other risk factors for Wilms tumour: results of a population-based case-control study. Eur J Pediatr. (2001) 160:333–8. 10.1007/pl00008443 [DOI] [PubMed] [Google Scholar]
- 91.Schüz J, Schmidt LS, Kogner P, Lähteenmäki PM, Pal N, Stokland T, et al. Birth characteristics and Wilms tumors in children in the Nordic countries: a register-based case-control study. Int J Cancer. (2011) 128:2166–73. 10.1002/ijc.25541 [DOI] [PubMed] [Google Scholar]
- 92.Smulevich VB, Solinova LG, Belyakova SV. Parental occupation and other factors and cancer risk in children: I. Study methodology and non-occupational factors. Int J Cancer. (1999) 83:712–7. [DOI] [PubMed] [Google Scholar]
- 93.Moilanen K, Jokelainen J, Jones PB, Hartikainen AL, Järvelin MR, Isohanni M. Deviant intrauterine growth and risk of schizophrenia: a 34-year follow-up of the Northern Finland 1966 Birth Cohort. Schizophr Res. (2010) 124:223–30. 10.1016/j.schres.2010.09.006 [DOI] [PubMed] [Google Scholar]
- 94.Wegelius A, Tuulio-Henriksson A, Pankakoski M, Haukka J, Lehto U, Paunio T, et al. An association between high birth weight and schizophrenia in a Finnish schizophrenia family study sample. Psychiatry Res. (2011) 190:181–6. 10.1016/j.psychres.2011.05.035 [DOI] [PubMed] [Google Scholar]
- 95.Wegelius A, Pankakoski M, Lehto U, Suokas J, Häkkinen L, Tuulio-Henriksson A, et al. An association between both low and high birth weight and increased disorganized and negative symptom severity in schizophrenia and other psychoses. Psychiatry Res. (2013) 205:18–24. 10.1016/j.psychres.2012.08.026 [DOI] [PubMed] [Google Scholar]
- 96.Keskinen E, Miettunen J, Koivumaa-Honkanen H, Mäki P, Isohanni M, Jääskeläinen E. Interaction between parental psychosis and risk factors during pregnancy and birth for schizophrenia - the Northern Finland 1966 Birth Cohort study. Schizophr Res. (2013) 145:56–62. 10.1016/j.schres.2012.12.033 [DOI] [PubMed] [Google Scholar]
- 97.Liuhanen J, Suvisaari J, Kajantie E, Miettunen J, Sarin AP, Järvelin MR, et al. Interaction between compound genetic risk for schizophrenia and high birth weight contributes to social anhedonia and schizophrenia in women. Psychiatry Res. (2018) 259:148–53. 10.1016/j.psychres.2017.10.020 [DOI] [PubMed] [Google Scholar]
- 98.Perquier F, Lasfargues A, Mesrine S, Clavel-Chapelon F, Fagherazzi G. Body-size throughout life and risk of depression in postmenopausal women: findings from the E3N cohort. Obesity. (2014) 22:1926–34. 10.1002/oby.20799 [DOI] [PubMed] [Google Scholar]
- 99.Herva A, Pouta A, Hakko H, Läksy K, Joukamaa M, Veijola J. Birth measures and depression at age 31 years: the Northern Finland 1966 Birth Cohort Study. Psychiatry Res. (2008) 160:263–70. 10.1016/j.psychres.2007.07.020 [DOI] [PubMed] [Google Scholar]
- 100.Davies C, Segre G, Estradé A, Radua J, De Micheli A, Provenzani U, et al. Prenatal and perinatal risk and protective factors for psychosis: a systematic review and meta-analysis. Lancet Psychiatry. (2020) 7:399–410. 10.1016/S2215-0366(20)30057-2 [DOI] [PubMed] [Google Scholar]
- 101.Lahti M, Eriksson JG, Heinonen K, Kajantie E, Lahti J, Wahlbeck K, et al. Late preterm birth, post-term birth, and abnormal fetal growth as risk factors for severe mental disorders from early to late adulthood. Psychol Med. (2015) 4:985–99. 10.1017/S0033291714001998 [DOI] [PubMed] [Google Scholar]
- 102.Van Lieshout RJ, Savoy CD, Ferro MA, Krzeczkowski JE, Colman I. Macrosomia and psychiatric risk in adolescence. Eur Child Adolesc Psychiatry. (2020). 29:5137–45. 10.1007/s00787-019-01466-7 [DOI] [PubMed] [Google Scholar]
- 103.Haglund NG, Källén KB. Risk factors for autism and Asperger syndrome. Perinatal factors and migration. Autism. (2011) 15:163–83. 10.1177/1362361309353614 [DOI] [PubMed] [Google Scholar]
- 104.Leonard H, Nassar N, Bourke J, Blair E, Mulroy S, de Klerk N, et al. Relation between intrauterine growth and subsequent intellectual disability in a ten-year population cohort of children in Western Australia. Am J Epidemiol. (2008) 167:103–11. 10.1093/aje/kwm245 [DOI] [PubMed] [Google Scholar]
- 105.Moore GS, Kneitel AW, Walker CK, Gilbert WM, Xing G. Autism risk in small and large-for-gestational-age infants. Am J Obstet Gynecol. (2012) 206:314.e1–9. 10.1016/j.ajog.2012.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Buschgens CJ, Swinkels SH, van Aken MA, Ormel J, Verhulst FC, Buitelaar JK. Externalizing behaviors in preadolescents: familial risk to externalizing behaviors, prenatal and perinatal risks, and their interactions. Eur Child Adolesc Psychiatry. (2009) 18:65–74. 10.1007/s00787-008-0704-x [DOI] [PubMed] [Google Scholar]
- 107.Alati R, Najman JM, O'Callaghan M, Bor W, Williams GM, Clavarino A. Fetal growth and behaviour problems in early adolescence: findings from the Mater University Study of Pregnancy. Int J Epidemiol. (2009) 38:1390–400. 10.1093/ije/dyp252 [DOI] [PubMed] [Google Scholar]
- 108.Yang Y, Qi Y, Cui Y, Li B, Zhang Z, Zhou Y, et al. Emotional and behavioral problems, social competence and risk factors in 6-16-year-old students in Beijing, China. PLoS ONE. (2019) 14:e0223970. 10.1371/journal.pone.0223970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Mil NH, Steegers-Theunissen RP, Motazedi E, Jansen PW, Jaddoe VW, Steegers EA, et al. Low and high birth weight and the risk of child attention problems. J Pediatr. (2015) 166:862–9.e1–3. 10.1016/j.jpeds.2014.12.075 [DOI] [PubMed] [Google Scholar]
- 110.Tamai K, Yorifuji T, Takeuchi A, Fukushima Y, Nakamura M, Matsumoto N, et al. Associations of birth weight with child health and neurodevelopment among term infants: a nationwide Japanese population-based study. J Pediatr. (2020). 10.1016/j.jpeds.2020.06.075 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 111.Zhang M, Gazimbi M, Chen Z, Zhang B, Chen Y, Yu Y, et al. Association between birth weight and neurodevelopment at age 1-6 months: results from the Wuhan Healthy Baby Cohort. BMJ Open. (2020) 10:e031916. 10.1136/bmjopen-2019-031916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Record RG, McKeown T, Edwards JH. The relation of measured intelligence to birth weight and duration of gestation. Ann Hum Genet. (1969) 33:71–9. 10.1111/j.1469-1809.1969.tb01631.x [DOI] [PubMed] [Google Scholar]
- 113.Sørensen HT, Sabroe S, Olsen J, Rothman KJ, Gillman MW, Fischer P. Birth weight and cognitive function in young adult life: historical cohort study. BMJ. (1997) 315:401–3. 10.1136/bmj.315.7105.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Richards M, Hardy R, Kuh D, Wadsworth ME. Birth weight and cognitive function in the British 1946 birth cohort: longitudinal population based study. BMJ. (2001) 322:199–203. 10.1136/bmj.322.7280.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Räikkonen K, Kajantie E, Pesonen AK, Heinonen K, Alastalo H, Leskinen JT, et al. Early life origins cognitive decline: findings in elderly men in the Helsinki Birth Cohort Study. PLoS ONE. (2013) 8:e54707. 10.1371/journal.pone.0054707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Power C, Jefferis BJ, Manor O, Hertzman C. The influence of birth weight and socioeconomic position on cognitive development: does the early home and learning environment modify their effects? J Pediatr. (2006) 148:54–61. 10.1016/j.jpeds.2005.07.028 [DOI] [PubMed] [Google Scholar]
- 117.Bergvall N, Iliadou A, Tuvemo T, Cnattingius S. Birth characteristics and risk of low intellectual performance in early adulthood: are the associations confounded by socioeconomic factors in adolescence or familial effects? Pediatrics. (2006) 117:714–21. 10.1542/peds.2005-0735 [DOI] [PubMed] [Google Scholar]
- 118.Eide MG, Oyen N, Skjaerven R, Bjerkedal T. Associations of birth size, gestational age, and adult size with intellectual performance: evidence from a cohort of Norwegian men. Pediatr Res. (2007) 62:636–42. 10.1203/PDR.0b013e31815586e9 [DOI] [PubMed] [Google Scholar]
- 119.Lundgren EM, Cnattingius S, Jonsson B, Tuvemo T. Birth characteristics and different dimensions of intellectual performance in young males: a nationwide population-based study. Acta Paediatr. (2003) 92:1138–43. 10.1111/j.1651-2227.2003.tb02473.x [DOI] [PubMed] [Google Scholar]
- 120.Kristensen P Susser E Irgens LM Mehlum IS Corbett K Bjerkedal T . The association of high birth weight with intelligence in young adulthood: a cohort study of male siblings. Am J Epidemiol. (2014) 180:876–84. 10.1093/aje/kwu241 [DOI] [PubMed] [Google Scholar]
- 121.Flensborg-Madsen T, Mortensen EL. Birth weight and intelligence in young adulthood and midlife. Pediatrics. (2017) 139:e20163161. 10.1542/peds.2016-3161 [DOI] [PubMed] [Google Scholar]
- 122.Duffy K, McVeigh K, Lipkind H, Kershaw T, Ickovics J. Large for gestational age and risk for academic delays and learning disabilities: assessing modification by maternal obesity and diabetes. Int J Environ Res Public Health. (2020) 15:5473. 10.3390/ijerph17155473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dawes P, Cruickshanks KJ, Moore DR, Fortnum H, Edmondson-Jones M, McCormack A, et al. The effect of prenatal and childhood development on hearing, vision and cognition in adulthood. PLoS ONE. (2015) 10:e0136590. 10.1371/journal.pone.0136590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang S-F, Shu L, Sheng J, Mu M, Wang S, Tao X-Y, et al. Birth weight and risk of coronary heart disease in adults: a meta-analysis of prospective cohort studies. J Dev Origins Health Dis. (2014) 5:408–19. 10.1017/S2040174414000440 [DOI] [PubMed] [Google Scholar]
- 125.Azadbakht L, Kelishadi R, Saraf-Bank S, Qorbani M, Ardalan G, Heshmat R, et al. The association of birth weight with cardiovascular risk factors and mental problems among Iranian school-aged children: the CASPIAN-III study. Nutrition. (2014) 30:150–8. 10.1016/j.nut.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 126.Dong YH, Zou ZY, Yang ZP, Wang ZH, Jing J, Luo JY, et al. Association between high birth weight and hypertension in children and adolescents: a cross-sectional study in China. J Human Hypertens. (2017) 31:737–43. 10.1038/jhh.2017.22 [DOI] [PubMed] [Google Scholar]
- 127.Espineira AR, Fernandes-Rosa FL, Bueno AC, de Souza RM, dio Moreira AC, de Castro M, et al. Postnatal growth and cardiometabolic profile in young adults born large for gestational age. Clin Endocrinol. (2011) 75:335–41. 10.1111/j.1365-2265.2011.04054.x [DOI] [PubMed] [Google Scholar]
- 128.Ferreira VR, Jardim TV, Póvoa TR, Mendonc KL, Nascente FN, Carneiro CS, et al. Birth weight and its association with blood pressure and nutritional status in adolescents. J Pediatr. (2018) 94:184–91. 10.1016/j.jped.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 129.Gunnarsdottir I, Birgisdottir BE, Thorsdottir I, Gudnason V, Benediktsson R. Size at birth and coronary artery disease in a population with high birth weight. Am J Clin Nutr. (2002) 76:1290–4. 10.1093/ajcn/76.6.1290 [DOI] [PubMed] [Google Scholar]
- 130.Kuciene R, Dulskiene V, Medzioniene J. Associations between high birth weight, being large for gestational age, and high blood pressure among adolescents: a cross-sectional study. Eur J Nutr. (2018) 57:373–81. 10.1007/s00394-016-1372-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Launer LJ, Hofman A, Grobbee DE. Relation between birth weight and blood pressure: longitudinal study of infants and children. BMJ. (1993) 307:1451–4. 10.1136/bmj.307.6917.1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ledo DL, Suano-Souza FI, Franco MDCP, Strufaldi MWL. Body mass index and cardiovascular risk factors in children and adolescents with high birth weight. Ann Nutr Metab. (2018) 72:272–8. 10.1159/000488595 [DOI] [PubMed] [Google Scholar]
- 133.Li C, Huang T-K, Cruz ML, Goran MI. Birth weight, puberty, and systolic blood pressure in children and adolescents: a longitudinal analysis. J Human Hypertens. (2006) 20:444–50. 10.1038/sj.jhh.1002021 [DOI] [PubMed] [Google Scholar]
- 134.Li Y, Wu J, Yu J, Gao E, Meads C, Afnan M, et al. EBM-CONNECT Collaboration. Is fetal macrosomia related to blood pressure among adolescents? A birth cohort study in China. J Hum Hypertens. (2013) 11:686–92. 10.1038/jhh.2013.31 [DOI] [PubMed] [Google Scholar]
- 135.Schooling CM, Jiang CQ, Lam TH, Cowling BJ, Au Yeung L, Zhang WS, et al. Estimated birth weight and adult cardiovascular risk factors in a developing southern Chinese population: a cross sectional study. BMC Public Health. (2010) 10:270. 10.1186/1471-2458-10-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Strufaldi MW, Silva EM, Franco MC, Puccini RF. Blood pressure levels in childhood: probing the relative importance of birth weight and current size. Eur J Pediatr. (2009) 168:619–24. 10.1007/s00431-008-0813-z [DOI] [PubMed] [Google Scholar]
- 137.Tan M, Cai L, Ma J, Jing J, Ma Y, Chen Y. The association of gestational age and birth weight with blood pressure among children: a Chinese national study. J Hum Hypertens. (2018) 32:651–9. 10.1038/s41371-018-0084-8 [DOI] [PubMed] [Google Scholar]
- 138.Yiu V, Buka S, Zurakowski D, McCormick M, Brenner B, Jabs K. Relationship between birthweight and blood pressure in childhood. Am J Kidney Dis. (1999) 33:253–60. 10.1016/S0272-6386(99)70297-0 [DOI] [PubMed] [Google Scholar]
- 139.Rashid A, Agarwala A, Novak E, Brown DL. Association of high birth weight with incident heart failure in the ARIC study. J Am Heart Assoc. (2019) 8:e011524. 10.1161/JAHA.118.011524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Conen D, Tedrow UB, Cook NR, Buring JE, Albert CM. Birth weight is a significant risk factor for incident atrial fibrillation. Circulation. (2010) 122:764–70. 10.1161/CIRCULATIONAHA.110.947978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Larsson SC, Drca N, Jensen-Urstad M, Wolk A. Incidence of atrial fibrillation in relation to birth weight and preterm birth. Int J Cardiol. (2015) 178:149–52. 10.1016/j.ijcard.2014.10.138 [DOI] [PubMed] [Google Scholar]
- 142.Johnsson IW, Naessén T, Ahlsson F, Gustafsson J. High birth weight was associated with increased radial artery intima thickness but not with other investigated cardiovascular risk factors in adulthood. Acta Paediatr. (2018) 107:2152–7. 10.1111/apa.14414 [DOI] [PubMed] [Google Scholar]
- 143.Skilton MR, Siitonen N, Würtz P, Viikari JS, Juonala M, Seppälä I, et al. High birth weight is associated with obesity and increased carotid wall thickness in young adults: the cardiovascular risk in young Finns study. Arterioscler Thromb Vasc Biol. (2014) 34:1064–8. 10.1161/ATVBAHA.113.302934 [DOI] [PubMed] [Google Scholar]
- 144.Perkiömäki N, Auvinen J, Tulppo MP, Hautala AJ, Perkiömäki J, Karhunen V, et al. Association between birth characteristics and cardiovascular autonomic function at mid-life. PLoS ONE. (2016) 11:e0161604. 10.1371/journal.pone.0161604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Timpka S, Hughes AD, Chaturvedi N, Franks PW, Lawlor DA, Rich-Edwards JW, et al. Birth weight and cardiac function assessed by echocardiography in adolescence: Avon longitudinal study of parents and children. Ultrasound Obstet Gynecol. (2019) 54:225–31. 10.1002/uog.20128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cardwell CR, Stene LC, Joner G, Davis EA, Sinek O, Rosenbaurer J, et al. Birthweight and the risk of childhood-onset type 1 diabetes: a meta-analysis of observational studies using individual patient data. Diabetologia. (2010) 53:641–51. 10.1007/s00125-009-1648-5 [DOI] [PubMed] [Google Scholar]
- 147.Cardwell C, Carson D, Patterson C. Parental age at delivery, birth order, birth weight and gestational age are associated with the risk of childhood Type 1 diabetes: a UK regional retrospective cohort study. Diabetic Med. (2005) 22:200–6. 10.1111/j.1464-5491.2005.01369.x [DOI] [PubMed] [Google Scholar]
- 148.Goldacre R. Associations between birthweight, gestational age at birth and subsequent type 1 diabetes in children under 12: a retrospective cohort study in England, 1998-2012. Diabetologia. (2017) 61:616–25. 10.1007/s00125-017-4493-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Levins R, Roberts SE, Goldacre MJ. Perinatal factors associated with subsequent diabetes mellitus in the child: record linkage study. Diabetic Med. (2007) 24:664–70. 10.1111/j.1464-5491.2007.02147.x [DOI] [PubMed] [Google Scholar]
- 150.Khashan AS, Kenny LC, Lundholm C, Kearney PN, Gong T, Mc Namee R, et al. Gestational age and birth weight and the risk of childhood type 1 diabetes: a population-based cohort and sibling design study. Diabetes Care. (2015) 38:2308–15. 10.2337/dc15-0897 [DOI] [PubMed] [Google Scholar]
- 151.Kuchlbauer V, Vogel M, Gausche R, Kapellen T, Rothe U, Vogel C, et al. High birth weights but not excessive weight gain prior to manifestation are related to earlier onset of diabetes in childhood: ‘accelerator hypothesis‘ revisited. Pediatric Diabetes. (2014) 15:428–35. 10.1111/pedi.12107 [DOI] [PubMed] [Google Scholar]
- 152.Stene LC, Magnus P, Lie RP, Søvik O, Joner G. Birth weight and childhood onset type 1 diabetes: a population-based cohort study. BMJ. (2001) 332:889–92. 10.1136/bmj.322.7291.889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bock T, Pedersen CR, Vølund A, Pallesen CS, Buschard K. Perinatal determinants among children who later develop IDDM. Diabetic Care. (1994) 17:1154–7. 10.2337/diacare.17.10.1154 [DOI] [PubMed] [Google Scholar]
- 154.Borras V, Freitas A, Castell C, Gisbert R, Jane M. Type 1 diabetes and perinatal factors in Catalonia (Spain). Pediatric Diabetes. (2011) 12:419–23. 10.1111/j.1399-5448.2010.00711.x [DOI] [PubMed] [Google Scholar]
- 155.Haynes A, Bower C, Bulsara MK, Finns J, Jones TW, Davis EA. Perinatal risk factors for childhood Type 1 diabetes in Western-Australia – a population-based study (1980-2002). Diabetic Med. (2007) 24:564–70. 10.1111/j.1464-5491.2007.02149.x [DOI] [PubMed] [Google Scholar]
- 156.Jones M, Swerdlow A, Gill L, Goldacre MJ. Pre-natal and early life risk factors for childhood onset diabetes mellitus: a record linkage study. Int J Epidemiol. (1999) 27:444–9. 10.1093/ije/27.3.444 [DOI] [PubMed] [Google Scholar]
- 157.Lawler-Heavner J, Cruickshanks KJ, Hay WW, Gay EC, Hamman RF. Birth size and risk of insulin-dependent diabetes mellitus (IDDM). Diabetes Res Clin Prac. (1994) 24:153–9. 10.1016/0168-8227(94)90110-4 [DOI] [PubMed] [Google Scholar]
- 158.McKinney P, Parslow R, Gurney K, Law GR, Bodansky HJ, Williams R. Perinatal and neonatal determinants of childhood type 1 diabetes. Diabetes Care. (1999) 22:928–31. 10.2337/diacare.22.6.928 [DOI] [PubMed] [Google Scholar]
- 159.Metcalfe MA, Baum JD. Family characteristics and insulin dependent diabetes. Arch Disease Childhood. (1992) 67:731–6. 10.1136/adc.67.6.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Patterson CC, Carson DJ, Hadden DR, Waugh NR, Cole SK, et al. A case-control investigation of perinatal risk factors for childhood IDDM in Northern Ireland and Scotland. Diabetes Care. (1994) 17:376–81. 10.2337/diacare.17.5.376 [DOI] [PubMed] [Google Scholar]
- 161.Rosenbauer J, Herzig P, Giani G. Early infant feeding and risk of type 1 diabetes mellitus – a nationwide population-based case – control study in pre-school children. Diabetes Metab Res Rev. (2008) 24:211–22. 10.1002/dmrr.791 [DOI] [PubMed] [Google Scholar]
- 162.Stene LC, Joner G. Atopic disorders and risk of childhood-onset type 1 diabetes in individuals. Clin Exp Allergy. (2004) 34:201–6. 10.1111/j.1365-2222.2004.01864.x [DOI] [PubMed] [Google Scholar]
- 163.Tai TY, Wang CY, Lin LL, Lee LT, Tsai ST, Chen CJ. A case–control study on risk factors for type 1 diabetes in Taipei city. Diabetes Res Clin Prac. (1998) 42:197–203. 10.1016/S0168-8227(98)00105-3 [DOI] [PubMed] [Google Scholar]
- 164.Wadsworth E, Shield JP, Hunt LP, Baum JD. A case–control study of environmental factors associated with diabetes in the under 5s. Diabetic Med. (1997) 14:390–6. [DOI] [PubMed] [Google Scholar]
- 165.Waernbaum I, Dahlquist G, Lund T. Perinatal risk factors for type 1 diabetes revisited: a population-based register study. Diabetologia. (2019) 62:1173–84. 10.1007/s00125-019-4874-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wei JN, Li HY, Chang CH, Sung FC, Li CY, Lin CC, et al. Birth weight and type 1 diabetes among schoolchildren in Taiwan – a population-based case–controlled study. Diabetes Res Clin Prac. (2006) 74:309–15. 10.1016/j.diabres.2006.04.018 [DOI] [PubMed] [Google Scholar]
- 167.Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol. (2007) 165:849–57. 10.1093/aje/kwk071 [DOI] [PubMed] [Google Scholar]
- 168.Whincup PH, Kaye SK, Owen CG, Huxley R, Cook DG, Anazawa S, et al. Birth weight and risk of type 2 diabetes a systematic review. JAMA. (2008) 300:2886–97. 10.1001/jama.2008.886 [DOI] [PubMed] [Google Scholar]
- 169.Knop MR, Geng TT, Gorny AW, Ding R, Li C, Ley SH, et al. Birth weight and risk of type 2 diabetes mellitus, cardiovascular disease, and hypertension in adults: a meta-analysis of 7 646 267 participants from 135 studies. J Am Heart Assoc. (2018) 7:e008870. 10.1161/JAHA.118.008870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhao H, Song A, Zhang Y, Zhen Y, Song G, Ma H. The association between birth weight and the risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Endocrine J. (2018) 65:923–33. 10.1507/endocrj.EJ18-0072 [DOI] [PubMed] [Google Scholar]
- 171.Zhu H, Zhang X, Li MZ, Xie J, Yang XL. Prevalence of type 2 diabetes and pre-diabetes among overweight or obese children in Tianjin, China. Diabetic Medicine. (2013) 30:1457–65. 10.1111/dme.12269 [DOI] [PubMed] [Google Scholar]
- 172.Hu C, Mu Y, Wan Q, Hu R, Shi L, Su Q, et al. Association between birth weight and diabetes: role of body mass index and lifestyle later in life. J Diabetes. (2020) 12:10–20. 10.1111/1753-0407.12960 [DOI] [PubMed] [Google Scholar]
- 173.Capittini C, Bergamaschi P, De Silvestri A, Marchesi A, Genovese V, Romano B, et al. Birth weight as a risk factor for cancer in adulthood: the stem cell perspective. Maturitas. (2011) 69:91–3. 10.1016/j.maturitas.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 174.Rahman N. Mechanisms predisposing to childhood overgrowth and cancer. Curr Opin Genet Dev. (2005) 15:227–33. 10.1016/j.gde.2005.04.007 [DOI] [PubMed] [Google Scholar]
- 175.Callan AC, Milne E. Involvement of the IGF system in fetal growth and childhood cancer: an overview of potential mechanisms. Cancer Causes Control. (2009) 20:1783–98. 10.1007/s10552-009-9378-z [DOI] [PubMed] [Google Scholar]
- 176.Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency trends to confer protection against post-natal development of malignancies. Eur J Endocrinol. (2011) 164:485–9. 10.1530/EJE-10-0859 [DOI] [PubMed] [Google Scholar]
- 177.O'Neill KA, Bunch KJ, Murphy MF. Intrauterine growth and childhood leukemia and lymphoma risk. Expert Rev Hematol. (2012) 5:59–76. 10.1586/ehm.12.39 [DOI] [PubMed] [Google Scholar]
- 178.Kandhal P, Miller BJ. Shared early life risk factors for schizophrenia and diabetes. Minerva Psichiatr. (2013) 54:197−210. [Google Scholar]
- 179.Ben Amor L, Grizenko N, Schwartz G, Lageix P, Baron C, Ter-Stepanian M, et al. Perinatal complications in children with attention-deficit hyperactivity disorder and their unaffected siblings. J Psychiatry Neurosci. (2005) 30:120–6. [PMC free article] [PubMed] [Google Scholar]
- 180.Cnattingius S, Villamor E, Lagerros YT, Wikström A-K, Granath F. High birth weight and obesity-a vicious circle across generations. Int J Obes. (2011) 36:1320–4. 10.1038/ijo.2011.248 [DOI] [PubMed] [Google Scholar]
- 181.Bouhours-Nouet N, Dufresne S, de Casson FB, Mathieu E, Douay O, Gatelais F, et al. High birth weight and early postnatal weight gain protect obese children and adolescents from truncal adiposity and insulin resistance: metabolically healthy but obese subjects? Diab Care. (2008) 31:1031–6. 10.2337/dc07-1647 [DOI] [PubMed] [Google Scholar]
- 182.Curhan A, Curhan GC, Chertow GM, Willett WC, Spiegelman D, Colditz GA, et al. Birth weight and adult hypertension and obesity in women. Circulation. (1996) 94:1310–5. 10.1161/01.CIR.94.6.1310 [DOI] [PubMed] [Google Scholar]
- 183.Curhan GC, Willett WC, Rimm EB, Spiegelman D. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. (1996) 94:3246–50. 10.1161/01.CIR.94.12.3246 [DOI] [PubMed] [Google Scholar]
- 184.Silverman BL, Landsberg L, Metzger BE. Fetal hyperinsulinism in offspring of diabetic mothers. Association with the subsequent development of childhood obesity. Ann NY Acad Sci. (1993) 699:36–45. 10.1111/j.1749-6632.1993.tb18835.x [DOI] [PubMed] [Google Scholar]
- 185.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol. (2003) 189:1698–704. 10.1016/S0002-9378(03)00828-7 [DOI] [PubMed] [Google Scholar]
- 186.Dörner G, Plagemann A. Perinatal hyperinsulinism as possible predisposing factor for diabetes mellitus, obesity and enhanced cardiovascular risk in later life. Horm Metab Res. (1994) 26:213–21. 10.1055/s-2007-1001668 [DOI] [PubMed] [Google Scholar]
- 187.Dörner G, Plagemann A, Neu A, Rosenbauer J. Gestational diabetes as possible risk factor for Type I childhood-onset diabetes in the offspring. Neuro Endocrinol Lett. (2000) 21:355–9. [PubMed] [Google Scholar]
- 188.Mehers KL, Gillespie KM. The genetic basis for type 1 diabetes. Br Med Bull. (2008) 88:115–29. 10.1093/bmb/ldn045 [DOI] [PubMed] [Google Scholar]
- 189.Von Behren J, Reynolds P. Birth characteristics and brain cancers in young children. Int J Epidemiol. (2003) 32:248–56. 10.1093/ije/dyg057 [DOI] [PubMed] [Google Scholar]
- 190.Greenop KR, Blair EM, Bower C, Armstrong BK, Milne E. Factors relating to pregnancy and birth and the risk of childhood brain tumors: results from an Australian case–control study. Pediatr Blood Cancer. (2014) 61:493–8. 10.1002/pbc.24751 [DOI] [PubMed] [Google Scholar]
- 191.McLaughlin CC, Baptiste MS, Schymura M, Zdeb MS, Nasca P. Perinatal risk factors for neuroblastoma. Cancer Causes Control. (2009) 20:289–301. 10.1007/s10552-008-9243-5 [DOI] [PubMed] [Google Scholar]
- 192.Mallol-Mesnard N, Menegaux F, Lacour B, Hartmann O, Frappaz D, Doz F, et al. Birth characteristics and childhood malignant central nervous system tumors: the ESCALE study (French Society for Childhood Cancer). Cancer Detect Prev. (2008) 32:79–86. 10.1016/j.cdp.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 193.Oksuzyan S, Crespi CM, Cockburn M, Mezei G, Kheifets L. Birth weight and other perinatal factors and childhood CNS tumors: a case–control study in California. Cancer Epidemiol. (2013) 37:402–9. 10.1016/j.canep.2013.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Urayama K, Von Behren J, Reynolds P. Birth characteristics and risk of neuroblastoma in young children. Am J Epidemiol. (2007) 165:486–95. 10.1093/aje/kwk041 [DOI] [PubMed] [Google Scholar]
- 195.Emerson JC, Malone KE, Daling JR, Starzyk P. Childhood brain tumor risk in relation to birth characteristics. J Clin Epidemiol. (1991) 44:1159–66. 10.1016/0895-4356(91)90148-3 [DOI] [PubMed] [Google Scholar]
- 196.Schüz J, Kaletsch U, Kaatsch P, Meinert R, Michaelis J. Risk factors for pediatric tumors of the central nervous system: results from a german population-based case-control study. Med Pediatr Oncol. (2001) 36:274–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.