Abstract
Background
Chronic kidney disease (CKD) is an independent risk factor for osteoporosis and is more prevalent among people with CKD than among people who do not have CKD. Although several drugs have been used to effectively treat osteoporosis in the general population, it is unclear whether they are also effective and safe for people with CKD, who have altered systemic mineral and bone metabolism.
Objectives
To assess the efficacy and safety of pharmacological interventions for osteoporosis in patients with CKD stages 3‐5, and those undergoing dialysis (5D).
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 25 January 2021 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
Randomised controlled trials comparing any anti‐osteoporotic drugs with a placebo, no treatment or usual care in patients with osteoporosis and CKD stages 3 to 5D were included.
Data collection and analysis
Two review authors independently selected studies, assessed their quality using the risk of bias tool, and extracted data. The main outcomes were the incidence of fracture at any sites; mean change in the bone mineral density (BMD; measured using dual‐energy radiographic absorptiometry (DXA)) of the femoral neck, total hip, lumbar spine, and distal radius; death from all causes; incidence of adverse events; and quality of life (QoL). Summary estimates of effect were obtained using a random‐effects model, and results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes, and mean difference (MD) for continuous outcomes. Confidence in the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results
Seven studies involving 9164 randomised participants with osteoporosis and CKD stages 3 to 5D met the inclusion criteria; all participants were postmenopausal women. Five studies included patients with CKD stages 3‐4, and two studies included patients with CKD stages 5 or 5D. Five pharmacological interventions were identified (abaloparatide, alendronate, denosumab, raloxifene, and teriparatide). All studies were judged to be at an overall high risk of bias.
Among patients with CKD stages 3‐4, anti‐osteoporotic drugs may reduce the risk of vertebral fracture (RR 0.52, 95% CI 0.39 to 0.69; low certainty evidence). Anti‐osteoporotic drugs probably makes little or no difference to the risk of clinical fracture (RR 0.91, 95% CI 0.79 to 1.05; moderate certainty evidence) and adverse events (RR 0.99, 95% CI 0.98 to 1.00; moderate certainty evidence). We were unable to incorporate studies into the meta‐analyses for BMD at the femoral neck, lumbar spine and total hip as they only reported the percentage change in the BMD in the intervention group.
Among patients with severe CKD stages 5 or 5D, it is uncertain whether anti‐osteoporotic drug reduces the risk of clinical fracture (RR 0.33, 95% CI 0.01 to 7.87; very low certainty evidence). It is uncertain whether anti‐osteoporotic drug improves the BMD at the femoral neck because the certainty of this evidence is very low (MD 0.01, 95% CI 0.00 to 0.02). Anti‐osteoporotic drug may slightly improve the BMD at the lumbar spine (MD 0.03, 95% CI 0.03 to 0.04, low certainty evidence). No adverse events were reported in the included studies. It is uncertain whether anti‐osteoporotic drug reduces the risk of death (RR 1.00, 95% CI 0.22 to 4.56; very low certainty evidence).
Authors' conclusions
Among patients with CKD stages 3‐4, anti‐osteoporotic drugs may reduce the risk of vertebral fracture in low certainty evidence. Anti‐osteoporotic drugs make little or no difference to the risk of clinical fracture and adverse events in moderate certainty evidence. Among patients with CKD stages 5 and 5D, it is uncertain whether anti‐osteoporotic drug reduces the risk of clinical fracture and death because the certainty of this evidence is very low. Anti‐osteoporotic drug may slightly improve the BMD at the lumbar spine in low certainty evidence. It is uncertain whether anti‐osteoporotic drug improves the BMD at the femoral neck because the certainty of this evidence is very low. Larger studies including men, paediatric patients or individuals with unstable CKD‐mineral and bone disorder are required to assess the effect of each anti‐osteoporotic drug at each stage of CKD.
Plain language summary
Pharmacological treatments for osteoporosis in patients with chronic kidney disease
What is the issue? Patients with chronic kidney disease (CKD) have an increased risk of osteoporosis (weakened bone strength), which can often lead to bone fracture. Several drugs are available for the treatment of osteoporosis; however, it is unknown whether these drugs are equally effective and safe in patients with CKD because bone strength impairment in these patients occurs via a different mechanism.
What did we do? Data were collected from studies including patients with osteoporosis and CKD stages 3‐5, and those undergoing dialysis (stage 5D) with data available on fracture, change in the bone mineral density (BMD; a bone strength index), and adverse events. We included seven studies with available evidence up to 25 January 2021, comparing anti‐osteoporotic drugs (abaloparatide, alendronate, denosumab, raloxifene, and teriparatide) with placebo (a dummy drug), in 9,164 postmenopausal women. We performed a meta‐analysis to assess the effects of these anti‐osteoporotic drugs.
What did we find? In postmenopausal women with CKD stages 3‐4, anti‐osteoporotic drugs may reduce vertebral fracture in low certainty evidence. Anti‐osteoporotic drugs probably make little or no difference to clinical fracture and adverse events in moderate certainty evidence. In postmenopausal with CKD stages 5 or 5D, it is uncertain whether anti‐osteoporotic drug reduces the risk of clinical fracture and death, and anti‐osteoporotic drug may slightly improve BMD at the lumbar spine in low certainty evidence. It is uncertain whether anti‐osteoporotic drug improve BMD at the femoral neck.
Conclusions Among postmenopausal women with CKD stages 3‐4, anti‐osteoporotic drugs may reduce the risk of vertebral fracture. Among patients with CKD stages 5 and 5D, anti‐osteoporotic drug may slightly improve bone strength. However, these conclusions are based on limited data and therefore uncertain.
Summary of findings
Background
Description of the condition
The World Health Organization (WHO) defines osteoporosis as ‘a disease characterised by low bone mass and microarchitectural deterioration of bone tissue, leading to enhanced bone fragility and a consequent increase in fracture risk’ (WHO 1994). Thereafter, the National Institutes of Health (NIH) defines osteoporosis mechanistically as ‘a skeletal disorder characterised by compromised bone strength predisposing to a higher risk of fracture. Bone strength reflects the integration of two main features: bone quantity and bone quality’ (NIH 2001). The clinical diagnosis of osteoporosis is broadly based on bone mineral density (BMD) measurements. BMD is converted into a T‐score, which indicates the number of standard deviations (SDs) above or below the mean BMD for young adults. Osteoporosis is diagnosed when the T‐scores are < ‐2.5 SD (WHO 1994). Osteoporosis dose not manifest clinically manifestations until a fracture develops. These osteoporotic fractures are a global healthcare burden. An estimated 9.0 million osteoporotic fractures were reported worldwide in 2000 (Johnell 2006), with an estimated annual cost of 19 billion USD in the USA (Burge 2007) and 1.8 billion GBP in the UK (Burge 2001). The ability to perform activities of daily living deteriorates after a fracture, along with the quality of life (QoL). Furthermore, morbidity and death are markedly increased in patients following a major bone fracture (Browner 1996; Keene 1993). Therefore, preventive interventions are therefore needed to reduce or prevent fractures in patients with osteoporosis. Current national osteoporosis guidelines recommend pharmacological interventions with anti‐osteoporotic drugs in addition to non‐pharmacological interventions that include modifying nutrition, ceasing smoking, performing weight‐bearing exercises, and moderating alcohol intake (Eastell 2019; Kanis 2019; Naranjo Hernandez 2018; NOGG 2017; Qaseem 2017).
The number of patients with chronic kidney disease (CKD) is increasing. In 2015, CKD was ranked the 10th most common cause of death globally, with an age‐standardised annual death rate of 19.2 per 100,000 of the population (GBD 2016). Thus, CKD is a major healthcare problem. Osteoporosis is an important comorbidity in patients with CKD. The National Health and Nutrition Examination Survey indicated that osteoporosis is two times more common in patients with moderate‐to‐severe CKD than in the general population (Nickolas 2006). Furthermore, the prevalence of osteopenia in patients undergoing dialysis is up to 20% in skeletal structures clinically associated with fracture (Stein 1996). Fractures have been reported to occur 2 to 100 times more frequently in patients with CKD than in age‐matched individuals without CKD (Alem 2000; Nickolas 2006). Patients with CKD who have fractures also develop other serious problems. Major bone fractures are associated with high rates of hospitalisation and death (Kim 2016; Tentori 2013). Healthcare–associated costs after fractures exceeded $600 million in 2010 in the USA (Kim 2016).
Conditions associated with CKD make the diagnosis and treatment of osteoporosis difficult (Cunningham 2004). Impairment of skeletal strength in patients with CKD occurs via a different mechanism. Kidney Disease: Improving Global Outcomes (KDIGO) defines CKD‐mineral and bone disorder (MBD) as a systemic condition of mineral and bone metabolism resulting from CKD (KDIGO 2009). The disorder is characterised by the following: 1) abnormalities in calcium, phosphorus, parathyroid hormone (PTH), or vitamin D metabolism; 2) bone turnover, mineralization, volume linear growth, or strength abnormalities; or 3) vascular or another soft‐tissue calcification (KDIGO 2009). The initial onset of CKD‐MBD occurs in early‐stage CKD (Levin 2007). Bone disorder caused by CKD‐MBD is termed renal osteodystrophy (ROD); it is a form of osteoporosis and a complex heterogeneous disorder of bone quality and density. ROD is traditionally classified as follows: hyperparathyroid bone disease, mild hyperparathyroid bone disease, mixed osteodystrophy, low turnover/adynamic bone disease, and osteomalacia (Llach 2000). Although bone biopsy is the gold standard diagnostic tool of ROD, access is limited, and it is not suitable for repeated evaluations. Alternatively, bone turnover markers such as intact PTH and alkaline phosphatase, are used clinically by nephrologists; however, their predictive values for bone turnover is limited (Khairallah 2018a; Sprague 2016). The state of bone turnover should be evaluated when an anti‐osteoporotic drug is used, because drug use may lead to adynamic bone disease in patients with CKD (Amerling 2010). A single cross‐sectional study of 13 patients with CKD stages 2‐4 suggested that the use of bisphosphonates was associated with adynamic bone disease in these patients (Amerling 2010). Although that study did not demonstrate that bisphosphonates caused adynamic bone disease, no large‐scale clinical safety data are available for patients with moderate‐to‐severe CKD treated with bisphosphonates. In addition, the key drug used in patients with osteoporosis is contraindicated for those with severe CKD (Nitta 2017). Based on this, the CKD‐MBD KDIGO guidelines were revised in 2017 to recommend the use of BMD measurements to assess fracture risk. In addition, they emphasised the importance of managing CKD‐MBD by controlling of vitamin D deficiency, hyperphosphataemia, and hyperparathyroidism before initiating anti‐osteoporotic drugs for CKD‐associated osteoporosis (KDIGO 2017).
Description of the intervention
A number of agents are effective for the treatment of osteoporosis in the general population, including bisphosphonates, denosumab, selective oestrogen receptor modulators (SERMs), and teriparatide (Crandall 2014). In addition, abaloparatide and romosozumab, which have been recently introduced, and strontium ranelate are used to treat osteoporosis (Reginster 2019).
Bisphosphonates
Bisphosphonates are analogues of inorganic pyrophosphates that inhibit osteoclast function. They are typically administered orally in pill form, although intravenous (IV) bisphosphonates are also available. Oral regimes involve daily or weekly administration, whereas IV bisphosphonates are administered monthly or yearly. The first‐line treatment for osteoporosis is usually bisphosphonates when pharmacological intervention is recommended. However, the use of bisphosphonates may lead to bisphosphonate‐related osteonecrosis of the jaw (Ruggiero 2004) and atypical femoral fracture (Donnelly 2012). In addition, oral bisphosphonates may cause erosive oesophagitis when patients fail to maintain an upright posture for approximately 30 minutes after taking the medicine with a glass of water (De Groen 1996).
Denosumab
Denosumab is a fully humanised monoclonal antibody specific to the receptor activator of nuclear factor kappa B ligand (RANKL), which mainly regulates osteoclasts. The recommended dosage of denosumab is 60 mg administered by subcutaneous(SC) injection by every 6 months (Bone 2008). The discontinuation of denosumab may lead to a rebound in bone turnover and rapid BMD loss and increased risk of fracture (Miller 2008). This is an important difference from bisphosphonates. Conversely, treatment adherence and patient preferences may be better with denosumab than with bisphosphonates (Eliasaf 2016; Morizio 2018). Major adverse effects associated with denosumab include cellulitis, urinary tract infections, hypocalcaemia, osteonecrosis of the jaw, and atypical femoral fracture. Denosumab does not depend on kidney clearance for its metabolism and excretion. However, the low kidney function is associated with more frequent hypocalcaemia (Block 2012; Dave 2015).
selective oestrogen receptor modulators (SERMs)
SERMs (bazedoxifene, raloxifene) are synthetic non‐steroidal compounds that interact with oestrogen receptors. Differing from oestrogen, these medicines act as either receptor agonists or antagonists in the target sites. They are associated with a lower cancer risk than oestrogen, and they have beneficial effects on the bone. SERMs have been shown to reduce the risk of only vertebral fractures (Crandall 2014). They are typically administered orally in pill form once a day. Serious adverse effects associated with SERMs include deep venous thrombosis (DVT), pulmonary embolism, and stroke (Adomaityte 2008; Barrett‐Connor 2006).
Teriparatide
Teriparatide is a recombinant human PTH (1‐34). It is administered by SC injection, either daily or weekly. PTH generally stimulates osteoclast activity to release more ionic calcium into the blood, subsequently elevating the serum calcium levels. Teriparatide has anabolic effects on the skeleton, with the most pronounced effects on cancellous bone. The adverse effects include temporary elevation of the serum calcium levels, postural hypotension, dizziness, headache, and nausea (Neer 2001).
Abaloparatide
Abaloparatide is an analogue of a PTH; it was approved by the United States Food and Drug Administration (US FDA) to treat osteoporosis in 2017. It has a relatively greater affinity for PTH/PTHrP receptor type 1 (or PTHR1) in the transient state and is an anabolic agent. The recommended dose of abaloparatide is 80 μg, administered via SC injection once a day. Adverse effects include hypercalciuria, dizziness, nausea, headache, palpitations, fatigue, upper abdominal pain, and vertigo (Miller 2016).
Romosozumab
Romosozumab is a humanised monoclonal antibody that binds and inhibits the activity of the protein sclerostin. It has a dual effects on the bone; it increases bone formation and decreases bone breakdown. The recommended dose of romosozumab is 210 mg, administered via SC injection once a month; it should be limited to 12 doses. Serious adverse effects are cardiac death, heart attack, and stroke (Saag 2017). Other adverse effects include headache, joint pain, and pain at the injection site (Cosman 2016). The approval of romosozumab was on hold owing to its serious adverse effects, but it was finally approved by the US FDA for the treatment of osteoporosis in 2019 with a black box warning.
Strontium ranelate
Strontium ranelate consists of two divalent cation atoms. Strontium has pharmacological actions and its structure is closely related to calcium, an active component of the bone. This agent has been suggested to decrease bone resorption and stimulate bone formation. The recommended oral daily dose of strontium ranelate is 2 g (Meunier 2004). Adverse effects include nausea and diarrhoea. The use of this agent may lead to DVT, heart attack, and severe allergic reaction (Abrahamsen 2014; Osborne 2010).
How the intervention might work
Available anti‐osteoporotic drugs are antiresorptive and/or anabolic agents. In the general population, these drugs improve the BMD and reduce the risk of some fractures. A systematic review reported that bisphosphonates, denosumab, and teriparatide reduced the risk of fractures compared with the placebo in postmenopausal women with osteoporosis. These interventions were found to reduce vertebral fractures (relative risk (RR) reduction range: 0.40 to 0.60) and nonvertebral fractures (RR reduction range: 0.60 to 0.80). Raloxifene, which is a SERMs, reduced the risks of only vertebral fractures (Crandall 2014). A more recent systematic review indicated that abaloparatide, romosozumab, and strontium ranelate also reduce the incidence of fractures compared with placebo in postmenopausal women with osteoporosis. This review showed that abaloparatide, romosozumab, and strontium ranelate reduces the incidence of vertebral fractures (RR: 0.13 (95% credible interval (CrI) 0.04 to 0.34); 0.31 (95% CrI 0.2 to 0.37); and 0.71 (95% CrI 0.63 to 0.80), respectively) and nonvertebral fractures (RR: 0.50 (95% CrI 0.28 to 0.85); 0.64 (95% CrI 0.49 to 0.81); and 0.87 (95% CrI 0.76 to 0.99), respectively) (Reginster 2019). Anti‐osteoporotic drugs may also be indicated for patients with CKD who have a stable CKD‐MBD and undergoing bone turnover assessment.
Why it is important to do this review
According to the WHO, the elderly population is continuing to grow globally at an unprecedented rate (He 2016). Clinical and epidemiological evidence indicates that ageing is a major factor associated with the incidence of CKD and osteoporosis (Glassock 2012; Kanis 2005). Osteoporotic fractures are highly co‐prevalent with CKD in the elderly population (Klawansky 2003). Treatment for osteoporosis in patients with CKD is therefore an area of high unmet need. The publication of the CKD‐MBD KDIGO guidelines in 2017 represented a dramatic change in the previous paradigm regarding the diagnosis and treatment of osteoporosis in patients with CKD. These guidelines have changed the view of the nephrologists in terms of the management of osteoporosis and its treatment in patients with CKD. However, it remains unclear how nephrologists should manage their patients (Khairallah 2018a; Khairallah 2018b). Cochrane systematic review has evaluated interventions for bone disease in only kidney transplant recipients (Palmer 2019). Only one other systematic review has evaluated interventions for osteoporosis in patients with CKD, and this study was not comprehensive (Wilson 2017). Wilson 2017 searched only published studies in PubMed and the Cochrane Central Register of Controlled Studies (CENTRAL). Meta‐analyses that exclude unpublished studies and outcomes are likely to overestimate the effects of the evaluated interventions or miss important adverse events, as reflected in Chapters 8.14.1 and 10.2.1 of the Cochrane Handbook (Higgins 2011). Additionally, limiting the review to only English‐language articles may introduce a bias.
Objectives
To assess the efficacy and safety of pharmacological interventions for osteoporosis in patients with CKD stages 3‐5, and those undergoing dialysis (5D).
Methods
Criteria for considering studies for this review
Types of studies
We included all published, unpublished, and ongoing randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which treatment allocation was determined by alternation, use of alternate medical records, date of birth, or other predictable methods).
Types of participants
Participants of any age with CKD stages 3–5D as defined by the K/DOQI (Levey 2003) or the KDIGO guidelines (Eknoyan 2013) were considered for inclusion. The review excluded patients who had a functioning kidney transplant or were those treated with corticosteroids, because corticosteroids strongly contribute to the progression of osteoporosis. Additionally, two Cochrane reviews have already evaluated these populations (Allen 2016; Palmer 2019). The target population included patients with evidence of severe osteopenia or osteoporosis according to WHO criteria (T score < −2.0 SD). The International Society for Clinical Densitometry suggests that the diagnosis of osteoporosis in children and adolescents should not be made based on densitometric criteria alone (ISCD 2019). Thus, based on a previous study (Ward 2007), children who had at least one low‐trauma fracture and/or reduced BMD were included.
Types of interventions
Patients receiving anti‐osteoporotic drugs were compared with individuals receiving a placebo, no treatment, or usual care. The primary intervention was treatment with anti‐osteoporotic drugs, including the following:
Bisphosphonates (etidronate, clodronate, tiludronate, alendronate, risedronate, ibandronate, pamidronate, zoledronate)
Denosumab
SERMs (bazedoxifene, raloxifene)
Teriparatide
Abaloparatide
Romosozumab
Strontium ranelate
Other treatments (e.g., vitamin D, phosphate binders, calcium supplements, calcimimetics, dialysate calcium adjustment, and dietary calcium or phosphate manipulation) were excluded from primary comparisons but were listed as co‐interventions. This approach was used as these interventions were included in three previous Cochrane reviews (Palmer 2007b; Palmer 2009; Ruospo 2018). We did not place any restrictions on the doses of therapy. All studies had a follow‐up period of at least six months.
Types of outcome measures
Primary outcomes
The primary outcomes at final follow‐up were as follows.
Incidence of fracture at any sites (clinical or radiographic)
Mean change in the BMD measured using dual‐energy radiographic absorptiometry (DXA) at the femoral neck, total hip, lumbar spine, or distal radius.
Adverse events: osteonecrosis of the jaw that delays dental healing, atypical femoral fracture, any gastroesophageal disorder (oesophagitis, oesophageal ulcer, oesophageal stricture, oesophageal erosions, dysphagia, gastric bleeding, duodenitis, or ulceration), nausea, diarrhoea, any musculoskeletal disorders (bone pain, arthralgia, myalgia, and muscle cramps), fever, hypersensitivity reactions, cellulitis, venous thromboembolism, stroke, oedema, hot flushes, acute kidney injury (AKI), histological osteomalacia or low‐bone turnover renal osteodystrophy, urinary tract infections (UTI), sepsis, and any other complication that may occur.
Secondary outcomes
The secondary outcomes at maximal follow‐up were as follows:
-
SONG core outcomes: the SONG core outcomes, as specified by the Standardised Outcomes in Nephrology initiative (SONG 2017). We evaluated the following:
Death (any cause, including cardiovascular)
Cardiovascular and cerebrovascular morbidity
Life participation (only in participants undergoing peritoneal dialysis (PD))
Fatigue score (only in participants undergoing haemodialysis (HD))
Vascular access failure (only in participants undergoing HD)
PD‐related infections (only in participants undergoing PD)
PD failure (only in participants undergoing PD)
QoL as reported in individual studies
Serum levels of intact PTH, calcium, phosphorus, and alkaline phosphatase (total or bone‐specific).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 25 January 2021 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources:
Monthly searches of CENTRAL
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register were identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website under CKT Register of Studies.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies, and clinical practice guidelines
Experts/organisations in the field seeking information about unpublished or incomplete studies
Grey literature sources (e.g., abstracts, dissertations, and theses), in addition to those already included in the Cochrane Kidney and Transplant Register of Studies
Data collection and analysis
Selection of studies
Two review authors independently screened the titles and abstracts from search results and coded them as ‘retrieve’ (eligible or potentially eligible/unclear) or ‘do not retrieve’. We retrieved the full text of study reports or publications, which were then independently assessed by two review authors for inclusion. The reason for excluding ineligible studies was recorded. We resolved any disagreement through discussion or, if required, by consultation with a third author. We identified and excluded duplicates studies and collated multiple reports of the same study; this enabled each study rather than each report, to act as a unit of interest in the review. The selection process was recorded in sufficient detail to the enable completion of the PRISMA flow diagram and to generate a table detailing the ‘Characteristics of excluded studies’ (Moher 2009).
Data extraction and management
A data collection form for was used to document study characteristics and outcome data; this form was piloted on at least one study included in the review. The data extraction form included the following items.
Methods: study design, total duration of study, number of study centres and location, study setting, withdrawals, and date of study
Participants: number (N), mean age, age range, sex, baseline CKD stage, diagnostic criteria, follow‐up duration, inclusion criteria, and exclusion criteria
Interventions: intervention, comparison, concomitant medications, and intervention dosage
Outcomes: primary and secondary outcomes specified and collected, and time points reported
Notes: funding for studies and notable conflicts of interest reported by study authors, and any other necessary information
Two review authors independently extracted outcome data from the included studies. In the ‘Characteristics of included studies’, we noted if the study authors did not report outcome data in a usable way. We resolved disagreements by consensus or by involving a third author. One review author transferred data into Review Manager. Double data entry was used to confirm that the data were entered correctly data were entered correctly. A second review author spot‐checked study characteristics for accuracy against study reports.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Were reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
We judged each potential source of bias as high, low, or unclear, and included text from the study report and justification for our judgement in the risk of bias table. We summarised the risk of bias judgements across studies for each of the domains listed. Blinding was considered for different key outcomes where necessary (e.g., for unblinded outcome assessment, the risk of bias for death (any cause) may be differ from that for a participant‐reported health‐related QoL scale). We contacted study authors for additional information to clarify any risk of bias when the study reports did not provide enough detail to allow for a clear judgement. We considered the risk of bias for studies that contribute to a particular outcome when considering treatment effects.
We assessed the overall risk of bias based on the following bias domains: allocation concealment, blinding of outcome assessors, and incomplete outcome data.
Low risk of bias: all the above domains are at a low risk of bias
High risk of bias: one or more of the above domains are at a high or unclear risk of bias
Measures of treatment effect
We analysed dichotomous outcomes as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment, the mean difference (MD) or the standardised mean difference (SMD) were used if different scales were used. For outcomes provided as rates, the results were expressed as rate ratios with 95% CIs. If studies included a mixture of change‐from‐baseline and final value scores, we used the (unstandardised) MD method in RevMan according to Chapter 9.4.5.2 of the Cochrane handbook (Higgins 2011). However, the change‐from‐baseline and final value scores were not combined as the SMD, as the SD would reflect the differences in the reliability of the measurements rather than the differences in the measurement scale (Higgins 2011). If a study reported outcomes at multiple time points, we used the last time point recorded. We performed meta‐analyses only when this approach was meaningful; that is, if treatments, participants, and the underlying clinical questions were sufficiently similar to allow for pooling. Skewed data were to be presented descriptively (for example, as medians and interquartile ranges for each group).
Unit of analysis issues
We did not anticipate the inclusion of studies with non‐standard designs, such as cross‐over studies and cluster‐RCTs, in the review. However, studies with multiple arms could be identified and included. In such cases, all intervention groups that were relevant to the review were included. To avoid double counting of the comparator, the number of patients in the comparator group was divided across the number of eligible intervention arms.
Dealing with missing data
Any further information required from the original author was requested in writing by (e.g. emailing the corresponding author), and any relevant information obtained in this manner was included in the review. Important numerical data, such as the number of screened and randomised patients, as well as the intention‐to‐treat (ITT), as‐treated, and per‐protocol populations, were carefully evaluated. Attrition rates, including dropouts, losses to follow‐up, and withdrawals were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
Assessment of heterogeneity
Heterogeneity was initially assessed by the visual inspection of the forest plots. Thereafter, statistical heterogeneity was quantified using the I² statistic, which described the percentage of total variation across studies that was due to heterogeneity rather than sampling error (Higgins 2003). I² values can be interpreted as follows:
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed I² value depended on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi² test, or a CI for I²) (Higgins 2011).
Assessment of reporting biases
If possible, funnel plots were used to assess for the potential existence of small study bias (Higgins 2011).
Data synthesis
Different CKD patient populations (patients with CKD stage 3‐5, and patients undergoing dialysis (5D)) were analysed separately. When the selected relevant studies were sufficiently similar, a meta‐analysis was performed. Considering substantial heterogeneity between studies, we used the random‐effects model. If substantial or considerable heterogeneity (I²> 60%) was present, we did not perform a meta‐analysis (see Assessment of heterogeneity).
Subgroup analysis and investigation of heterogeneity
If sufficient data were available, we conducted the following subgroup analyses for the primary outcomes.
Age (< 18 years and ≥ 18 years)
Sex
Types of interventions
intact PTH (< 50, 50 to 300, and > 300 pg/mL)
Concomitant use of vitamin D
Sensitivity analysis
Sensitivity analyses, defined a priori, were performed to assess the robustness of our conclusions. We performed the following sensitivity analyses for the primary outcomes.
Excluding studies judged to be at a high overall risk of bias
Excluding studies judged to be at a high or unclear risk of bias for at least one of the overall risk of bias domains.
Summary of findings and assessment of the certainty of the evidence
The main results of the review are presented in the ‘Summary of findings’ tables. These tables presented key information related to the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2011a). The ‘Summary of findings’ tables also included an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). This approach defined the quality of a body of evidence as the extent to which one could be confident that an estimate of effect or association was close to the true quantity of specific interest. The quality of a body of evidence involved consideration of the within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias (Schunemann 2011b). We planned to present the following outcomes in the ‘Summary of findings’ tables.
Incidence of fracture at any sites
Mean change in the BMD measured using DXA at the femoral neck, total hip, lumbar spine, and distal radius
Death (any cause)
Incidence of adverse events
QoL.
Results
Description of studies
Detailed descriptions of the studies covered in this review are provided in the following tables: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
After searching the Specialised Register, contacting pharmaceutical companies, an additional web search, and removing duplicates, a total of 84 records were identified. After title and abstract screening and full‐text review, seven studies (48 records) were included (ACTIVE 2016; FIT 1993; FREEDOM 2009; FTP 2001; Haghverdi 2014; Hernandez 2003; MORE 1999), and 23 studies (33 records) were excluded. Three ongoing studies were identified (NCT02792413; IRCT20180506039549N1; NCT02440581) and these will be assessed in a future update of this review (Figure 1).
1.
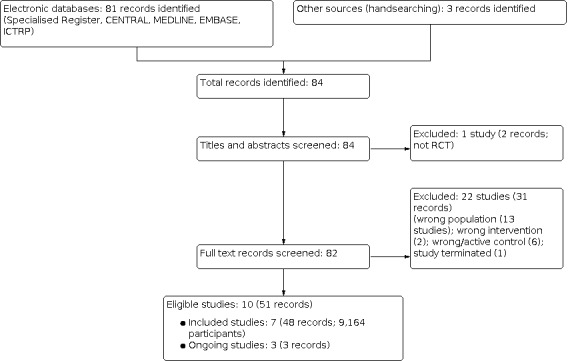
Flow diagram.
Included studies
The seven studies (48 records) included in this systematic review are summarised in the Characteristics of included studies.
Study design
All studies were parallel RCTs.
Sample size
A total of 9,164 randomised participants were included in this review. The sample sizes ranged from 50 to 4,973. Five studies were included in the subgroup of large studies (ACTIVE 2016; FIT 1993; FREEDOM 2009; FTP 2001; MORE 1999).
Setting
We included four multinational studies (ACTIVE 2016; FREEDOM 2009; FTP 2001; MORE 1999), two single‐country multicentre studies (FIT 1993; Hernandez 2003), and one single‐centre study (Haghverdi 2014).
Participants
All studies were conducted in postmenopausal women. The mean age ranged from 62.5 to 77.6 years. Five studies (ACTIVE 2016; FIT 1993; FREEDOM 2009; FTP 2001; MORE 1999) included patients with CKD stages 3a‐4. Two studies (Haghverdi 2014; Hernandez 2003) included patients undergoing HD or with CKD stage 5 not yet receiving dialysis. Patients receiving PD were not included. FREEDOM 2009 reported data separately for CKD stages 3 and 4, and MORE 1999 reported data for stages 3a and 3b‐4.
Interventions
Five agents were identified: abaloparatide, alendronate, denosumab, raloxifene, and teriparatide. One study compared abaloparatide with placebo and the active control teriparatide (ACTIVE 2016); one study compared alendronate with placebo (FIT 1993); one study compared denosumab with placebo (FREEDOM 2009); one study compared teriparatide with placebo (FTP 2001); and three studies compared raloxifene with placebo (Haghverdi 2014; Hernandez 2003; MORE 1999).
Outcomes
The duration of follow‐up ranged from 8 to 54 months. The following reported outcomes included data based on paired comparisons.
Fracture was reported in six studies (9,114 participants) (ACTIVE 2016; FIT 1993; FREEDOM 2009; FTP 2001; Haghverdi 2014; MORE 1999). Five studies assessed radiographic vertebral fracture assessed by a blinded, independent assessor (ACTIVE 2016; FIT 1993; FREEDOM 2009; FTP 2001; MORE 1999). Five studies assessed non‐vertebral or clinical fracture (FIT 1993; FREEDOM 2009; FTP 2001; Haghverdi 2014; MORE 1999).
-
BMD was assessed in all studies.
BMD at femoral neck: ACTIVE 2016; FIT 1993; Haghverdi 2014; Hernandez 2003; MORE 1999
BMD at lumbar spine: ACTIVE 2016; FIT 1993; FREEDOM 2009; FTP 2001; Haghverdi 2014; Hernandez 2003; MORE 1999
BMD at total hip: ACTIVE 2016; FIT 1993; FREEDOM 2009
Adverse events were reported in all studies
-
SONG outcomes
Death was reported in four studies (5,664 participants) (FIT 1993; Haghverdi 2014; Hernandez 2003; MORE 1999)
Cardiovascular and cerebrovascular morbidity were reported in two studies (3,471 participants) reported (FIT 1993; FREEDOM 2009)
Life participation was not reported
Fatigue score was not reported
Vascular access failure was reported in two studies (110 participants) (Haghverdi 2014; Hernandez 2003)
PD‐related infections were not reported
PD failure was not reported
QoL was not reported
Serum levels of intact PTH, calcium, phosphorus, and alkaline phosphatase (total) were reported by one study (60 participants) (Haghverdi 2014).
Excluded studies
The characteristics of the excluded studies based on full‐text assessment are shown in the Characteristics of excluded studies table.
Risk of bias in included studies
The results of risk of bias assessment of the seven included studies are summarised in Figure 2 and Figure 3. Two authors independently assessed the included studies for each checklist item as having a high, low, or unclear risk of bias.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Two studies (ACTIVE 2016; FIT 1993) used a computer‐generated random sequence. The remaining five studies (FREEDOM 2009; FTP 2001; Haghverdi 2014; Hernandez 2003; MORE 1999) did not indicate the randomisation methods and were therefore categorised as unclear.
We used a subset of data from these five studies (ACTIVE 2016; FIT 1993; FREEDOM 2009; FTP 2001; MORE 1999), which also enrolled postmenopausal women with normal kidney function. The number of patients with CKD in each study was as follows.
ACTIVE 2016: 527/2,463 (21.4%)
FIT 1993: 581/2,027 (28.7%)
FREEDOM 2009: 2,890/7,868 (36.7%)
FTP 2001: 83/1,637 (5.1%)
MORE 1999: 4,973/7,705 (64.5%).
ACTIVE 2016 and FIT 1993 determined that the balance of the allocated groups was maintained because the number of extracted participants from each study was acceptable.
Allocation concealment
Two studies (ACTIVE 2016; FIT 1993) used central randomisation, whereas no information was included in the other five studies, which were categorised as unclear.
Blinding
Performance bias
All studies reported adequate double‐blinding procedures (ACTIVE 2016; FIT 1993; FREEDOM 2009; FTP 2001; Haghverdi 2014; Hernandez 2003; MORE 1999).
Detection bias
Outcome assessors were blinded in five studies (ACTIVE 2016; FIT 1993; FREEDOM 2009; FTP 2001; MORE 1999). Other studies (Haghverdi 2014; Hernandez 2003) included no description and were categorised as unclear.
Incomplete outcome data
For the primary efficacy outcome of fracture events, four studies (ACTIVE 2016; FIT 1993; FREEDOM 2009; MORE 1999) reported results of the ITT analyses. Two studies (FTP 2001; MORE 1999) were classified as high risk because missing outcome data did not balance the numbers across intervention groups, and these studies excluded more than 10% of participants from the final analysis. The other five studies (ACTIVE 2016; FIT 1993; FREEDOM 2009; Haghverdi 2014; Hernandez 2003) were classified as unclear because there was insufficient information for judgement.
Selective reporting
Two studies (ACTIVE 2016; FIT 1993) defined the primary efficacy and safety outcomes in the protocols associated with the published manuscripts. Protocols were not available for the other five studies (FREEDOM 2009; FTP 2001; Haghverdi 2014; Hernandez 2003; MORE 1999). Only the percent improvement in the treatment groups (no control group data) were reported in four studies for death (FIT 1993; MORE 1999) and BMD (ACTIVE 2016; FIT 1993; FREEDOM 2009) Four studies were judged to be at high risk of reporting bias (ACTIVE 2016; FIT 1993; FREEDOM 2009; MORE 1999) and three studies were judged to have unclear risk of bias (FTP 2001; Haghverdi 2014; Hernandez 2003).
Other potential sources of bias
Six studies were sponsored by pharmaceutical companies (ACTIVE 2016; FIT 1993; FREEDOM 2009; FTP 2001; Hernandez 2003; MORE 1999).
Effects of interventions
Summary of findings 1. Any anti‐osteoporotic drugs versus placebo in postmenopausal women with osteoporosis and CKD stages 3‐4.
| Any anti‐osteoporotic drugs versus placebo in postmenopausal womenwith osteoporosis and CKD stages 3‐4 | |||||
|
Patient or population: postmenopausal women with osteoporosis and CKD stages 3‐4 Settings: multinational; outpatients Intervention: any anti‐osteoporotic drugs (abaloparatide, alendronate, denosumab, raloxifene, teriparatide) Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Any anti‐osteoporotic drugs | ||||
|
Vertebral fracture by radiography Follow up: range 19 to 54 months |
73 per 1000 | 38 per 1000 (28 to 50) |
RR 0.52 (0.39 to 0.69) |
9,054 (5) | ⊕⊕⊝⊝ low1, 2 |
|
Clinical fracture Follow up: range 24 to 54 months |
54 per 1000 | 49 per 1000 (43 to 57) |
RR 0.91 (0.79 to 1.05) |
5,827 (4) | ⊕⊕⊕⊝ moderate1 |
|
Mean change in BMD of the femoral neck Follow up: range 19 to 54 months |
Included studies only reported the percentage change in the BMD in the intervention group. In the three studies the mean change in BMD of the femoral neck was reported to improve by approximately 0.5% to 5% in the intervention group. | ‐ | 6,081 (3) | ⊕⊝⊝⊝ very low1,3,4 | |
|
Mean change in BMD of the lumbar spine Follow up: range 19 to 54 months |
Included studies only reported the percentage change in the BMD in the intervention group. In the five studies the mean change in BMD of the lumbar spine was reported to improve by approximately 1% to 15% in the intervention group. | ‐ | 9,054 (5) | ⊕⊝⊝⊝ very low1,3,4 | |
|
Mean change in BMD of the total hip Follow up: range 19 to 54 months |
Included studies only reported the percentage change in the BMD in the intervention group. In the three studies the mean change in BMD of the total hip was reported to improve by approximately 5% to 6% in the intervention group. | ‐ | 3,998 (3) | ⊕⊝⊝⊝ very low1,3,4 | |
| Mean change in BMD of the distal radius | Not reported | ‐ | ‐ | ‐ | |
|
Adverse events Follow up: range 19 to 54 months |
946 per 1000 | 937 per 1000 (927 to 946) |
RR 0.99 (0.98 to 1.00) |
9,054 (5) | ⊕⊕⊕⊝ moderate1 |
|
Death Follow up: range 36 to 54 months |
Included studies only reported total death. Death ranged from 0.7% to 1.6% | Not estimable | 4,973 (2) | ⊕⊕⊝⊝ low1, 3 | |
| QoL | Not reported | ‐ | ‐ | ‐ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CKD: chronic kidney disease; CI: Confidence interval; RR: Risk Ratio; BMD: bone mineral density; QoL: quality of life | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | |||||
1 Downgraded one level due to a serious risk of bias: all studies had a high overall risk of bias 2 Downgraded one level due to inconsistency: there was substantial heterogeneity
3Downgraded one level due to a publication bias: there were high risk of reporting bias
4Downgraded one level due to indirectness: surrogate endpoint was evaluated
Summary of findings 2. Raloxifene versus placebo for postmenopausal women with osteoporosis and CKD stages 5 and 5D.
| Raloxifene versus placebo for postmenopausal women with osteoporosis and CKD stages 5 and 5D | |||||
|
Patient or population: postmenopausal women with osteoporosis and CKD stages 5 and 5D Settings: Iran, Venezuela; in‐ and outpatients Intervention: any anti‐osteoporotic drugs (raloxifene) Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Raloxifene | ||||
| Vertebral fracture by radiography | Not reported | ‐ | ‐ | ‐ | |
|
Clinical fracture Follow up: 8 months |
33 per 1000 | 11 per 1000 (0 to 260) |
RR 0.33 (0.01 to 7.87) |
60 (1) | ⊕⊝⊝⊝ very low1, 2 |
| Mean change in BMD of the femoral neck Follow up: range 8 to 12 months | The mean change in BMD of the femoral neck was 0.01 g/cm² higher with raloxifene than placebo (95% CI 0.00 to 0.02) (mean change in BMD of the femoral neck in placebo group was ‐0.009 to ‐0.002 g/cm²) |
MD 0.01 (0.00 to 0.02) |
110 (2) | ⊕⊝⊝⊝ very low1, 3, 4 | |
| Mean change in BMD of the lumbar spine Follow up: range 8 to 12 months | The mean change in BMD of the lumbar spine was 0.03 g/cm² higher with raloxifene than placebo (95% CI 0.03 to 0.04) (mean change BMD of the lumbar in placebo group was ‐0.019 to ‐0.003 g/cm²) |
MD 0.03 (0.03 to 0.04) |
110 (2) | ⊕⊕⊝⊝ low1, 4 | |
| Mean change in BMD of the total hip | Not reported | ‐ | ‐ | ‐ | |
| Mean change in BMD of the distal radius | Not reported | ‐ | ‐ | ‐ | |
|
Adverse events Follow up: range 8 to 12 months |
No adverse events were observed in the included studies | Not estimable | 110 (2) | ⊕⊝⊝⊝ very low1, 2 | |
|
Death Follow up: range 8 to 12 months |
50 per 1000 | 50 per 1000 (11 to 228) |
RR 1.00 (0.22 ‐ 4.56) |
110 (2) | ⊕⊝⊝⊝ very low1, 2 |
| QoL | Not reported | ‐ | ‐ | ‐ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CKD: chronic kidney disease; CI: Confidence interval; RR: Risk Ratio; MD: Mean Difference; BMD: bone mineral density; QoL: quality of life | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded one level due to a serious risk of bias: all studies had a high overall risk of bias 2Downgraded two levels due to serious imprecision: there were very few/no events and the CIs encompass both considerable benefit and considerable harm 3Downgraded one level due to inconsistency: there was considerable heterogeneity 4Downgraded one level due to indirectness: surrogate endpoint was evaluated
We were unable to perform the qualitative analysis as planned for the following reasons:
We could not obtain sufficient information on each CKD stage, despite contacting the corresponding authors.
Most of the studies reported both vertebral and non‐vertebral or clinical fracture.
These were handled as follows:
-
Stage of CKD was divided into 2 groups: 1) stages 3‐4, and 2) stages 5 and 5D, based on the study's description/definition.
FREEDOM 2009 reported data separately for stages 3 and 4
MORE 1999 reported data separately for stages 3a and 3b‐4.
We divided “the Fracture at any sites” into “Vertebral fracture by radiography” and “Clinical fracture”. Clinical fracture was defined as any site fractures with fracture‐related symptoms( FIT 1993).
1. Patients with osteoporosis and CKD stages 3‐4
Five studies were eligible (ACTIVE 2016; FIT 1993; FTP 2001; FREEDOM 2009; MORE 1999). The anti‐osteoporotic drugs included were abaloparatide, alendronate, denosumab, teriparatide, and raloxifene.
Fracture: vertebral fracture by radiography
In the meta‐analysis using the inverse variance random‐effects model, anti‐osteoporotic drugs may reduce the risk of vertebral fracture (Analysis 1.1 (5 studies): RR 0.52, 95% CI 0.39 to 0.69; low certainty evidence). Heterogeneity was moderate (I² = 40%).
1.1. Analysis.

Comparison 1: Any anti‐osteoporotic drug versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 1: Vertebral fracture by radiography
Fracture: clinical fracture
In the meta‐analysis using the inverse variance random‐effects model, anti‐osteoporotic drugs probably makes little or no difference to the risk of clinical fracture (Analysis 1.2 (4 studies): RR 0.91, 95% CI 0.79 to 1.05; moderate certainty evidence). However, we could not incorporate the study of FTP 2001 into the meta‐analysis because no clinical fractures occurred in either the treatment and placebo groups. Heterogeneity was low (I² = 0%).
1.2. Analysis.

Comparison 1: Any anti‐osteoporotic drug versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 2: Clinical fracture
Mean change in BMD
Femoral neck
Three studies (ACTIVE 2016; FIT 1993; MORE 1999) (6,081 patients) described the assessment of the BMD at the femoral neck. However, we were unable to incorporate these studies into the meta‐analysis because only the percentage change in the BMD in the treatment group was reported. In the three studies the mean change in BMD of the femoral neck was reported to improve by approximately 0.5% to 5% in the intervention group. The certainty of evidence was very low.
Lumbar spine
All five studies (9,054 patients) described the assessment of the BMD at the lumbar spine. However, we were unable to incorporate these studies into the meta‐analysis because only the percentage change in the BMD in the treatment group was reported. In the five studies the mean change in BMD of the lumbar spine was reported to improve by approximately 1% to 15% in the intervention group. The certainty of evidence was very low.
Total hip
Three studies (ACTIVE 2016; FIT 1993; FREEDOM 2009) (3,998 patients) described the assessment of the BMD at the total hip. However, these studies could not be incorporated into the meta‐analysis because only the percentage change in the BMD in the treatment group was reported. In the three studies the mean change in BMD of the total hip was reported to improve by approximately 5% to 6% in the intervention group. The certainty of evidence was very low.
Radius
This outcome was not reported by the included studies.
Adverse events
In the meta‐analysis using the inverse variance random‐effects model, the use of anti‐osteoporotic drug probably makes little or no difference to adverse events (Analysis 1.3 (4 studies): RR 0.99, 95% CI 0.98 to 1.00; moderate certainty evidence). Heterogeneity was low (I² = 2%). FTP 2001 could not be incorporated into the meta‐analysis but reported adverse events were observed in 99.1% of all study patients (576/581).
1.3. Analysis.

Comparison 1: Any anti‐osteoporotic drug versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 3: Adverse events
Death (any cause)
FIT 1993 and MORE 1999 (5,554 patients) reported death, however they could not be incorporated into the meta‐analysis because only the total number of deaths was reported. The death in these studies ranged from 0.7% to 1.6%. The certainty of evidence was low.
Cardiovascular and cerebrovascular morbidity
FIT 1993 and FREEDOM 2009 (3,471 patients) assessed cardiovascular and cerebrovascular morbidity. However, FIT 1993 could not be incorporated into the meta‐analysis because it only reported total cardiovascular or cerebrovascular events (cardiovascular events 2.6% and cerebrovascular events 2.2%). Denosumab probably makes little or no difference to cardiovascular and cerebrovascular morbidity (Analysis 1.4 (1 study, 8,281 participants): RR 1.00, 95% CI 0.75 to 1.32; moderate certainty evidence).
1.4. Analysis.

Comparison 1: Any anti‐osteoporotic drug versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 4: Cardiovascular and cerebrovascular morbidity
Quality of life
QoL was not reported by the included studies.
Bone markers
Bone markers were not reported by the included studies.
2. Patients with osteoporosis and CKD stages 5 and 5D
Two eligible studies were identified (Haghverdi 2014; Hernandez 2003), both evaluated raloxifene.
Fracture: vertebral fracture evidenced by radiography
Vertebral fracture identified by radiography was not reported by the included studies.
Fracture: clinical fracture
Haghverdi 2014 reported it is uncertain whether raloxifene reduces the risk of clinical fracture (Analysis 2.1: RR 0.33, 95% CI 0.01 to 7.87; very low certainty evidence).
2.1. Analysis.

Comparison 2: Raloxifene versus placebo for patients with osteoporosis and CKD stage 5D, Outcome 1: Clinical fracture
Mean change in the BMD
Femoral neck
It is uncertain whether raloxifene improves the BMD at the femoral neck (Analysis 2.2 (2 studies, 110 participants): MD 0.01, 95% CI 0.00 to 0.02; very low certainty evidence). Heterogeneity was high (I² = 91%).
2.2. Analysis.

Comparison 2: Raloxifene versus placebo for patients with osteoporosis and CKD stage 5D, Outcome 2: Mean change in femoral neck BMD (DXA)
Lumbar spine
Raloxifene may increase the BMD at the lumbar spine (Analysis 2.3 (2 studies, 110 participants): MD 0.03, 95% CI 0.03 to 0.04; low certainty evidence). Heterogeneity was low (I² = 0%).
2.3. Analysis.

Comparison 2: Raloxifene versus placebo for patients with osteoporosis and CKD stage 5D, Outcome 3: Mean change in lumbar spine BMD (DXA)
Total hip
BMD in the total hip was not reported by the included studies.
Radius
BMD in the radius was not reported by the included studies.
Adverse events
Both studies (Haghverdi 2014; Hernandez 2003) reported no adverse events. The certainty of evidence was very low.
Death
It is uncertain whether raloxifene reduces the risk of death (Analysis 2.5 (2 studies, 110 participants): RR 1.00, 95% CI 0.22 to 4.56; very low certainty evidence).
2.5. Analysis.

Comparison 2: Raloxifene versus placebo for patients with osteoporosis and CKD stage 5D, Outcome 5: Death
Vascular access failure
Vascular access failure was not reported by the included studies.
Life participation, fatigue score, PD‐related infections, or PD failure
Life participation, fatigue scores, PD‐related infections, or PD failure were not by the included studies.
Quality of life
QoL was not reported by the included studies.
Bone markers
Haghverdi 2014 reported some differences in bone markers between raloxifene and placebo (Analysis 2.7; Analysis 2.8; Analysis 2.9; Analysis 2.10). However, the baseline data were not balanced, and no marked changes were observed between the treatment and placebo groups.
2.7. Analysis.

Comparison 2: Raloxifene versus placebo for patients with osteoporosis and CKD stage 5D, Outcome 7: Serum intact PTH
2.8. Analysis.

Comparison 2: Raloxifene versus placebo for patients with osteoporosis and CKD stage 5D, Outcome 8: Serum calcium
2.9. Analysis.

Comparison 2: Raloxifene versus placebo for patients with osteoporosis and CKD stage 5D, Outcome 9: Serum phosphorus
2.10. Analysis.

Comparison 2: Raloxifene versus placebo for patients with osteoporosis and CKD stage 5D, Outcome 10: Serum alkaline phosphatase (total)
Subgroup analyses
Subgroup analyses based on age (< 18 years and ≥ 18 years) and sex were not possible as all patients were postmenopausal women.
Types of interventions
Five drugs were identified (abaloparatide, alendronate, denosumab, teriparatide, and raloxifene). Meta‐analysis could be only conducted about teriparatide. The results are provided below.
Abaloparatide
ACTIVE 2016 reported it is uncertain whether abaloparatide reduces the risk of vertebral fracture because the certainty of this evidence is very low (Analysis 3.1: RR 0.25, 95% CI 0.03 to 2.20) and abaloparatide probably makes little or no difference to adverse events (Analysis 3.5: RR 1.01, 95% CI 0.94 to 1.10; moderate certainty evidence).
3.1. Analysis.

Comparison 3: Abaloparatide versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 1: Vertebral fracture by radiography
3.5. Analysis.

Comparison 3: Abaloparatide versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 5: Adverse events
Alendronate
FIT 1993 reported alendronate may make little or no difference to the risk of vertebral fracture (Analysis 4.1: RR 0.74, 95% CI 0.33 to 1.66; low certainty evidence) and the risk of clinical fracture (Analysis 4.2: RR 0.79, 0.52 to 1.20; low certainty evidence).
4.1. Analysis.

Comparison 4: Alendronate versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 1: Vertebral fracture by radiography
4.2. Analysis.

Comparison 4: Alendronate versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 2: Clinical fracture
Denosumab
FREEDOM 2009 reported denosumab probably reduces the risk of vertebral fracture (Analysis 5.1: RR 0.41, 95% CI 0.28 to 0.58; moderate certainty evidence), may make little or no difference to the risk of clinical fracture (Analysis 5.2: RR 0.86, 95%CI 0.66 to 1.12; low certainty evidence), and probably makes little or no difference to adverse events (Analysis 5.6: RR 0.99, 95% CI 0.97 to 1.01; moderate certainty evidence), and cardiovascular and cerebrovascular morbidity (Analysis 5.7: RR 1.00, 95% CI 0.75 to 1.32; moderate certainty evidence).
5.1. Analysis.

Comparison 5: Denosumab versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 1: Vertebral fracture by radiography
5.2. Analysis.

Comparison 5: Denosumab versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 2: Clinical fracture
5.6. Analysis.

Comparison 5: Denosumab versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 6: Adverse events
5.7. Analysis.

Comparison 5: Denosumab versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 7: Cardiovascular and cerebrovascular morbidity
Teriparatide
Teriparatide probably reduces the risk of vertebral fracture (Analysis 6.1: RR 0.31, 95% CI 0.10 to 0.90; moderate certainty evidence). Heterogeneity was low (I² = 0%). Teriparatide may make little or no difference to adverse events (Analysis 6.5: RR 0.95, 95% CI 0.74 to 1.14; low certainty evidence). Heterogeneity was high (I² = 79%).
6.1. Analysis.

Comparison 6: Teriparatide versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 1: Vertebral fracture by radiography
6.5. Analysis.

Comparison 6: Teriparatide versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 5: Adverse events
Raloxifene
MORE 1999 reported that raloxifene probably reduces the risk of vertebral fracture (Analysis 7.1: RR 0.60, 95% CI 0.36 to 1.00; moderate certainty evidence), may make little or no difference to the risk of clinical fracture (Analysis 7.2: RR 0.96, 95% CI 0.80 to 1.16; low certainty evidence) and probably makes little or no difference to adverse events (Analysis 7.5: RR 0.99, 95% CI 0.98 to 1.00; moderate certainty evidence).
7.1. Analysis.

Comparison 7: Raloxifene versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 1: Vertebral fracture by radiography
7.2. Analysis.

Comparison 7: Raloxifene versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 2: Clinical fracture
7.5. Analysis.

Comparison 7: Raloxifene versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 5: Adverse events
Intact PTH level (< 50, 50‐300, and > 300 pg/mL)
Most eligible patients were likely to have stable intact PTH levels. We contacted the relevant study authors to request further details; however, they were unable to provide further information.
Concomitant use of vitamin D
Most eligible patients were likely to use vitamin D. We contacted the relevant study authors to request further details; however, they were unable to provide further information.
Sensitivity analyses
All eligible studies were considered to be at a high risk for bias. In the sensitivity analysis, we excluded studies judged to be at a high risk of bias for at least one of the overall risk of bias domains. FTP 2001 and MORE 1999 were excluded from this analysis (Analysis 8.1; Analysis 8.2; Analysis 8.3). The results were similar to Analysis 1.1, Analysis 1.2, and Analysis 1.3.
8.1. Analysis.

Comparison 8: Sensitivity analysis: any anti‐osteoporotic drugs versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 1: Vertebral fracture by radiography
8.2. Analysis.

Comparison 8: Sensitivity analysis: any anti‐osteoporotic drugs versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 2: Clinical fracture
8.3. Analysis.

Comparison 8: Sensitivity analysis: any anti‐osteoporotic drugs versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 3: Adverse events
Funnel plots
No funnel plots were generated to evaluate potential publication bias because less than 10 eligible RCTs were available for each pooled analysis.
Discussion
Summary of main results
Seven studies randomising 9,164 patients were included in our meta‐analyses of the main outcomes. All participants were postmenopausal women. Five studies included patients with CKD stages 3‐4, and two studies included patients with CKD stages 5 or 5D. Five anti‐osteoporotic agents were identified: abaloparatide, alendronate, denosumab, raloxifene, and teriparatide.
Among patients with CKD stages 3‐4, anti‐osteoporotic drugs may reduce the risk of vertebral fracture. Anti‐osteoporotic drugs probably makes little or no difference to the risk of clinical fracture or adverse events. The efficacy and safety of anti‐osteoporotic drugs were similar during sensitivity analysis, which excluded studies with a high risk of bias for at least one of the overall risk of bias domains.
Among patients with CKD stages 5 and 5D, it is uncertain whether raloxifene reduces the risk of clinical fracture and death. Raloxifene may slightly improve the BMD at the lumbar spine, and uncertain effects on BMD at the femoral neck.
We could not perform a meta‐analysis of each type of intervention because each drug was assessed in individual studies.
Overall completeness and applicability of evidence
Both published and unpublished data were included in this review. We contacted the relevant corresponding authors to acquire the data that were not reported in the published articles. However, the information obtained was insufficient. All study participants were postmenopausal women; therefore, the evidence obtained cannot be directly applied to men and paediatric patients. Importantly, the CKD‐BMD of all study participants was stable at baseline; therefore, the evidence cannot be applied to patients with insufficient control of CKD‐BMD.
Quality of the evidence
The certainty of the evidence was graded using the GRADE approach (GRADE 2008). As shown in the Table 1, among patients with CKD stages 3‐4, vertebral fracture was assessed to be of low certainty owing to concerns of serious risks of bias and inconsistency. We assessed clinical fracture and adverse events to be of moderate certainty owing to concerns of serious risks of bias.
As shown in the Table 2, among patients with CKD stages 5 and 5D, we assessed clinical fracture and death to be of very low certainty owing to concerns of serious risks of bias and serious imprecision. We assessed the mean change in the BMD at the lumbar spine to be of low certainty owing to concerns of serious risks of bias and indirectness.
We assessed the mean change in the BMD at the femoral neck to be of very low certainty owing to concerns of serious risks of bias, inconsistency, and indirectness.
Potential biases in the review process
We performed a comprehensive search using several different databases; however, we cannot rule out the possibility that smaller studies were missed. In addition, although we contacted the corresponding authors and conducted web searches to collect additional data, we were unable to obtain sufficient information. There might be a potential bias due to data availability or publication status.
Agreements and disagreements with other studies or reviews
This review differed from the review by Wilson 2017 in several aspects. Toussaint 2010 was excluded because most of the patients did not have osteoporosis, and the ACTIVE 2016 study was newly included. In addition, this review demonstrated an effect that included all drug subtypes. The primary results of this review were consistent with those of Wilson 2017. However, the evidence is limited to patients with CKD stages 3‐5D.
The effects of each drug were consistent with findings in the general population (Crandall 2014; Reginster 2019). Regarding the five large studies identified (ACTIVE 2016; FIT 1993; FREEDOM 2009; FTP 2001; MORE 1999), we used a subset of data from these five studies in our meta‐analysis. The each subset result was consistent with the overall results of each study. However, the 95% CIs for the estimates were wide owing to the smaller sample sizes.
Authors' conclusions
Implications for practice.
Among patients with CKD stages 3‐4, anti‐osteoporotic drugs may reduce the risk of vertebral fracture in low certainty evidence. Anti‐osteoporotic drugs probably make little or no difference to the risk of clinical fracture and adverse events in moderate certainty evidence.
In low certainty evidence, patients with CKD stages 5 and 5D, it is uncertain whether anti‐osteoporotic drug reduces the risk of clinical fracture and death because the certainty of this evidence is very low. Anti‐osteoporotic drug may slightly improve the BMD at the lumbar spine in low certainty evidence. It is uncertain whether anti‐osteoporotic drug improves the BMD at the femoral neck because the certainty of this evidence was very low.
Implications for research.
Several concerns remain, and future studies should address the following points.
This review could not assess the effectiveness or safety of anti‐osteoporotic drugs in patients with unstable CKD‐MBD; it is important to establish the recommendations for these patients.
This review could not assess the effectiveness or safety of anti‐osteoporotic drugs in men or paediatric patients; it is also important to establish the recommendations for these patients.
We could not sufficiently assess the effectiveness or safety of anti‐osteoporotic drugs in patients with each CKD stage. Therefore, these analyses should be repeated when more data become available.
We could not sufficiently assess the effect of each anti‐osteoporotic drug. Therefore, these analyses should be repeated after the publication of more data.
Future studies should compare the subtypes of anti‐osteoporotic drugs in order to provide clinicians with information on the comparative effectiveness of available therapies.
History
Protocol first published: Issue 9, 2019
Acknowledgements
We wish to thank Gail Higgins at the Cochrane Kidney and Transplant Group for designing our search strategy, and Narelle Willis for editorial support with the review.
The authors are grateful to the following peer reviewers for their time and comments: Dr Pablo Antonio URENA TORRES (Chief of Dialysis Services at the AURA Nord Saint Ouen, Paris, France), Tim Cundy (University of Auckland Aotearoa‐New Zealand), and Katherine Wesseling Perry.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Any anti‐osteoporotic drug versus placebo for patients with osteoporosis and CKD stages 3‐4.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Vertebral fracture by radiography | 5 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.39, 0.69] | |
| 1.2 Clinical fracture | 4 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.79, 1.05] | |
| 1.3 Adverse events | 4 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.98, 1.00] | |
| 1.4 Cardiovascular and cerebrovascular morbidity | 1 | 2890 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.75, 1.32] |
Comparison 2. Raloxifene versus placebo for patients with osteoporosis and CKD stage 5D.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Clinical fracture | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 2.2 Mean change in femoral neck BMD (DXA) | 2 | 110 | Mean Difference (IV, Random, 95% CI) | 0.01 [0.00, 0.02] |
| 2.3 Mean change in lumbar spine BMD (DXA) | 2 | 110 | Mean Difference (IV, Random, 95% CI) | 0.03 [0.03, 0.04] |
| 2.4 Adverse events | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.5 Death | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.22, 4.56] |
| 2.6 Vascular access failure | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.7 Serum intact PTH | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 80.50 [‐82.73, 243.73] |
| 2.8 Serum calcium | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.81, ‐0.19] |
| 2.9 Serum phosphorus | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.51, 0.91] |
| 2.10 Serum alkaline phosphatase (total) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 213.70 [‐170.98, 598.38] |
2.4. Analysis.

Comparison 2: Raloxifene versus placebo for patients with osteoporosis and CKD stage 5D, Outcome 4: Adverse events
2.6. Analysis.

Comparison 2: Raloxifene versus placebo for patients with osteoporosis and CKD stage 5D, Outcome 6: Vascular access failure
Comparison 3. Abaloparatide versus placebo for patients with osteoporosis and CKD stages 3‐4.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Vertebral fracture by radiography | 1 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.20] |
| 3.2 Mean change in femoral neck BMD (DXA) | 1 | 335 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 3.3 Mean change in lumbar spine BMD (DXA) | 1 | 335 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 3.4 Mean change in total hip BMD (DXA) | 1 | 335 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 3.5 Adverse events | 1 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.94, 1.10] |
3.2. Analysis.

Comparison 3: Abaloparatide versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 2: Mean change in femoral neck BMD (DXA)
3.3. Analysis.

Comparison 3: Abaloparatide versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 3: Mean change in lumbar spine BMD (DXA)
3.4. Analysis.

Comparison 3: Abaloparatide versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 4: Mean change in total hip BMD (DXA)
Comparison 4. Alendronate versus placebo for patients with osteoporosis and CKD stages 3‐4.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Vertebral fracture by radiography | 1 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.33, 1.66] | |
| 4.2 Clinical fracture | 1 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.52, 1.20] |
Comparison 5. Denosumab versus placebo for patients with osteoporosis and CKD stages 3‐4.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Vertebral fracture by radiography | 1 | 2890 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.28, 0.58] |
| 5.2 Clinical fracture | 1 | 2890 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.66, 1.12] |
| 5.3 Mean change in femoral neck BMD (DXA) | 1 | 2890 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 5.4 Mean change in lumbar spine BMD (DXA) | 1 | 2890 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 5.5 Mean change in total hip BMD (DXA) | 1 | 2890 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 5.6 Adverse events | 1 | 2890 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.97, 1.01] |
| 5.7 Cardiovascular and cerebrovascular morbidity | 1 | 2890 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.75, 1.32] |
5.3. Analysis.

Comparison 5: Denosumab versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 3: Mean change in femoral neck BMD (DXA)
5.4. Analysis.

Comparison 5: Denosumab versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 4: Mean change in lumbar spine BMD (DXA)
5.5. Analysis.

Comparison 5: Denosumab versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 5: Mean change in total hip BMD (DXA)
Comparison 6. Teriparatide versus placebo for patients with osteoporosis and CKD stages 3‐4.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Vertebral fracture by radiography | 2 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.10, 0.90] |
| 6.2 Clinical fracture | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.3 Mean change in femoral neck BMD (DXA) | 2 | 442 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 6.4 Mean change in lumbar spine BMD (DXA) | 2 | 442 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 6.5 Adverse events | 2 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.79, 1.14] |
6.2. Analysis.

Comparison 6: Teriparatide versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 2: Clinical fracture
6.3. Analysis.

Comparison 6: Teriparatide versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 3: Mean change in femoral neck BMD (DXA)
6.4. Analysis.

Comparison 6: Teriparatide versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 4: Mean change in lumbar spine BMD (DXA)
Comparison 7. Raloxifene versus placebo for patients with osteoporosis and CKD stages 3‐4.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Vertebral fracture by radiography | 1 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.36, 1.00] | |
| 7.2 Clinical fracture | 1 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.80, 1.16] | |
| 7.3 Mean change in femoral neck BMD (DXA) | 1 | 4973 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 7.4 Mean change in lumbar spine BMD (DXA) | 1 | 4973 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 7.5 Adverse events | 1 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.98, 1.00] |
7.3. Analysis.

Comparison 7: Raloxifene versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 3: Mean change in femoral neck BMD (DXA)
7.4. Analysis.

Comparison 7: Raloxifene versus placebo for patients with osteoporosis and CKD stages 3‐4, Outcome 4: Mean change in lumbar spine BMD (DXA)
Comparison 8. Sensitivity analysis: any anti‐osteoporotic drugs versus placebo for patients with osteoporosis and CKD stages 3‐4.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Vertebral fracture by radiography | 3 | Risk Ratio (IV, Random, 95% CI) | 0.44 [0.32, 0.61] | |
| 8.2 Clinical fracture | 2 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.67, 1.05] | |
| 8.3 Adverse events | 2 | 3417 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.97, 1.01] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ACTIVE 2016.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2 (active comparator)
Co‐interventions
|
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to each treatment groups by means of a central, interactive, automated telephone system |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed because participants were assigned to each group using a central, interactive, automated telephone system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active comparator (teriparatide) could not be repackaged and blinded. However, the comparison between abaloparatide and placebo, which was main purpose of this study, could be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Efficacy and safety outcomes were assessed by blinded and independent assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing outcome data were balanced in numbers across intervention groups; however, the reasons for missing data were not stated |
| Selective reporting (reporting bias) | High risk | All predefined efficacy and safety outcomes were reported, however BMD was reported incompletely so that these data could not be entered in a meta‐analysis (no control group data) |
| Other bias | High risk | The study was funded by Radius Health |
FIT 1993.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to each treatment group by means of a central system by computer‐generated codes |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed because participants were assigned to each group using a central system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Triple‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Efficacy and safety outcomes were assessed by blinded and independent assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | All predefined efficacy and safety outcomes were reported however BMD and death were reported incompletely so that these data could not be entered in a meta‐analysis (no control group data) |
| Other bias | High risk | The study was funded by Merck Research Laboratories |
FREEDOM 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐intervention
|
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised; method of randomisation was not reported; however "Randomization was stratified according to 5‐year age group" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Triple‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Efficacy and safety outcomes were assessed by blinded and independent assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing outcome data balanced between intervention groups; reasons for missing data were not reported |
| Selective reporting (reporting bias) | High risk | BMD was reported incompletely so that these data could not be entered in a meta‐analysis (no control group data) |
| Other bias | High risk | The study was funded by Amgen |
FTP 2001.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Co‐intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised; method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Triple‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Efficacy and safety outcomes were assessed by blinded and independent assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing outcome data did not balance in numbers across intervention groups, and reasons for missing data was not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement: The protocol was not available |
| Other bias | High risk | The study was funded by Eli Lilly |
Haghverdi 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was described as randomised; method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing outcome data balanced in numbers across intervention groups, and reasons for missing data was stated. However, insufficient information to permit judgement because of not mention about ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement: the protocol was not available |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Hernandez 2003.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised; method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants were followed up. However, insufficient information to permit judgement because of not mention about ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement: The protocol was not available |
| Other bias | High risk | The study was supported by Eli Lilly |
MORE 1999.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Co‐interventions
|
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised; method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Triple‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Efficacy and safety outcomes were assessed by blinded and independent assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing outcome data did not balance in numbers across intervention groups, and reasons for missing data was not reported |
| Selective reporting (reporting bias) | High risk | The protocol was not available. BMD and death were reported incompletely so that these data could not be entered in a meta‐analysis (no control group data) |
| Other bias | High risk | The study was funded by Eli Lilly |
BMD ‐ bone mineral density; BMI ‐ body mass index; CKD ‐ chronic kidney disease; (e)GFR ‐ (estimated) glomerular filtration rate; HD ‐ haemodialysis; PTH ‐ parathyroid hormone; RCT ‐ randomised controlled trial; SC ‐ subcutaneous
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ariyoshi 2006 | Wrong population: most patients had normal bone condition |
| DIVINE 2011 | Wrong control: compared to an active control (ibandronate versus alendronate) |
| Fukunaga 2002 | Wrong control: compared to an active control (risedronate versus etidronate) |
| Grotz 1998 | Wrong population: kidney transplant recipients |
| Hagino 2014 | Wrong control: compared to an active control (75 mg risedronate/month versus 2.5 mg risedronate/day) |
| Hashiba 2004 | Wrong population: most patients had normal bone condition |
| Hashiba 2006 | Wrong population: most patients had normal bone condition |
| Iseri 2019 | Wrong control: compared to an active control (denosumab versus alendronate) |
| JPRN‐C000000390 | Not RCT |
| Kishimoto 2006 | Wrong control: compared to an active control (17.5 mg risedronate/week versus 2.5 mg risedronate/day) |
| Kleinstueck 2001 | Wrong population: most the patients were not osteoporotic |
| Kushida 2006 | Wrong control: compared to an active control (risedronate versus etidronate) |
| NCT00261625 | Wrong population: no mention of the patients' bone condition |
| NCT00299572 | wrong population: no mention of the patients' bone condition |
| Omidvar 2011 | wrong population: kidney transplant recipients |
| Ruzhytska 2015 | Wrong population: inclusion of patients with CKD stages 1 and 2. Further information was requested, but no response was received |
| Saito 2012 | Wrong population: patients' bone condition was not sufficiently evaluated |
| Sirsat 2010 | Wrong population: kidney transplant recipients |
| Toussaint 2010 | Wrong population: most patients were not osteoporotic |
| UMIN00001829 | This study was not terminated prior to commencement |
| Wang 2008b | Wrong intervention: salmon calcitonin |
| Wang 2008c | Wrong intervention: salmon calcitonin |
| Wetmore 2005 | Wrong population: most patients were not osteoporotic |
RCT ‐ randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
IRCT20180506039549N1.
| Study name | Effect of alendronate in patents with osteoporosis and chronic kidney disease |
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention
Control
|
| Outcomes |
|
| Starting date | August 6, 2018 |
| Contact information | Shokouh Shayanpour Golestan St, Ahvaz 61357‐12794 Ahvaz Iran (Islamic Republic of) +98 61 3333 7077 shayanpor.sh@ajums.ac.ir Ahvaz University of Medical Sciences |
| Notes | Further information was requested, but no response was received. |
NCT02440581.
| Study name | Renal osteodystrophy: A fresh approach |
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention group
Control
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | July, 2015 |
| Contact information | Hartmut Malluche, MD University of Kentucky Lexington, Kentucky, United States, 40536 859‐323‐5049 hhmall@uky.edu |
| Notes | Further information was requested. The corresponding author responded that the study is in the final year and the first results will be available at the end of this year |
NCT02792413.
| Study name | Randomised controlled study evaluating the effect of a biotherapy treatment (Anti‐RANKL ligand antibody: Denosumab) on bone and vascular metabolism in osteoporotic chronic kidney disease |
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention
Control
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | November 19, 2018 |
| Contact information | Prof Jean‐Paul CRISTOL CHU Lapeyronie, Department of Biochemistry and Hormonology, Montpellier, FRANCE +33(0)4 67 33 83 15 jp‐cristol@chu‐montpellier.fr |
| Notes | Recruitment may be ongoing or complete |
BMD ‐ bone mineral density; CKD ‐ chronic kidney disease; HD ‐ haemodialysis; PTH ‐ parathyroid hormone; SC ‐ subcutaneous
Differences between protocol and review
We could not obtain sufficient information on each CKD stage, despite contacting the corresponding authors.
Most of the studies reported both vertebral and non‐vertebral or clinical fracture.
These were handled as follows:
Stage of CKD was divided into 2 groups: 1) stages 3‐4 and 2) stages 5 and 5D.
We divided “the Fracture at any sites” into “Vertebral fracture by radiography” and “Clinical fracture”. Clinical fracture was defined as any site fractures with fracture‐related symptoms (FIT 1993).
Contributions of authors
Draft the protocol: TH, NW
Study selection: TH, YH, YM
Extract data from studies: TH, YH, YM, NW
Enter data into RevMan: TH, YH, YM
Carry out the analysis: TH, NW
Interpret the analysis: TH, NW
Draft the final review: TH, NW
Disagreement resolution: TH, YH, YM, NW
Update the review: TH
Sources of support
Internal sources
New Source of support, Other
External sources
New Source of support, Other
Declarations of interest
TH: no known conflicts of interest
YH: no known conflicts of interest
YM: no known conflicts of interest
NW: no known conflicts of interest.
New
References
References to studies included in this review
ACTIVE 2016 {published data only}
- Bilezikian JP, Fitzpatrick LA, Williams GC, Hu MY, Hattersley G, Rizzoli R. Bone mineral density and bone turnover marker changes with sequential abaloparatide/alendronate: Results of ACTIVExtend [abstract no: MO0655]. Journal of Bone & Mineral Research 2017;32(1):S377. [EMBASE: 620203679] [Google Scholar]
- Bilezikian JP, Hattersley G, Mitlak BH, Hu MY, Fitzpatrick LA, Dabrowski C, et al. Abaloparatide in patients with mild or moderate renal impairment: results from the ACTIVE phase 3 trial. Current Medical Research & Opinion 2019;35(12):2097-102. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bilezikian JP, Hattersley G, Williams G, Hu MY, Fitzpatrick LA, Papapoulos S. Abaloparatide-SC has minimal effects in subjects with mild or moderate renal impairment: Results from the ACTIVE trial [abstract no: SU0282]. Journal of Bone & Mineral Research 2016;31(Suppl 1):S264. [EMBASE: 620694016] [Google Scholar]
- Cosman F, Hattersley G, Hu MY, Williams GC, Fitzpatrick LA, Black DM. Effects of abaloparatide-sc on fractures and bone mineral density in subgroups of postmenopausal women with osteoporosis and varying baseline risk factors. Journal of Bone & Mineral Research 2017;32(1):17-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial [Erratum in: JAMA. 2017 Jan 24;317(4):442; PMID: 28118431]. JAMA 2016;316(7):722-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Moreira CA, Fitzpatrick LA, Wang Y, Recker RR. Effects of abaloparatide-SC (BA058) on bone histology and histomorphometry: the ACTIVE phase 3 trial. Bone 2017;97:314-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
FIT 1993 {published data only}
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996;348(9041):1535-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Black DM, Reiss TF, Nevitt MC, Cauley J, Karpf D, Cummings SR. Design of the Fracture Intervention Trial. Osteoporosis International 1993;3 Suppl 3:S29-39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998;280(24):2077-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Barrett-Connor EL, Schwartz A, Santora AC, Bauer DC, Suryawanshi S, et al. Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension. Journal of Bone & Mineral Research 2004;19(8):1259-69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jamal SA, Bauer DC, Ensrud KE, Cauley JA, Hochberg M, Ishani A, et al. Alendronate treatment in women with normal to severely impaired renal function: an analysis of the fracture intervention trial. Journal of Bone & Mineral Research 2007;22(4):503-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
FREEDOM 2009 {published data only}
- Broadwell A, Ebeling PR, Franek E, Goemaere S, Wagman RB, Yin X, et al. Safety and efficacy of denosumab among subjects with mild-to-moderate chronic kidney disease (CKD) in the fracture reduction evaluation of denosumab in osteoporosis every 6 months. Extension study [abstract no: 1889]. Arthritis & Rheumatology 2017;69:Suppl 10. [EMBASE: 618911821] [Google Scholar]
- Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis [Erratum in: N Engl J Med. 2009 Nov 5;361(19):1914]. New England Journal of Medicine 2009;361(8):756-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Egbuna OI, Cheung AM, Siddhanti S, Wang A, Daizadeh N, Anthony M, et al. Treatment of osteoporosis by RANKL inhibition with denosumab in women at high cardiovascular risk and with renal impairment does not accelerate vascular calcification [abstract no: SA-PO2319]. Journal of the American Society of Nephrology 2010;21(Abstract Suppl):640A. [Google Scholar]
- Jamal SA, Ljunggren O, Stehman-Breen, C, Cummings S, McClung M, Goemaere S, et al. The effects of denosumab on bone mineral density (BMD) and fracture by level of renal function [abstract no: PP355]. Bone 2010;47(Suppl 1):S207-8. [EMBASE: 71732799] [Google Scholar]
- Jamal SA, Ljunggren O, Stehman-Breen C, Cummings SR, McClung MR, Goemaere S, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. Journal of Bone & Mineral Research 2011;26(8):1829-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
FTP 2001 {published data only}
- Boonen S, Marin F, Mellstrom D, Xie L, Desaiah D, Krege JH, et al. Safety and efficacy of teriparatide in elderly women with established osteoporosis: bone anabolic therapy from a geriatric perspective. Journal of the American Geriatrics Society 2006;54(5):782-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chen P, Miller PD, Delmas PD, Misurski DA, Krege JH. Change in lumbar spine BMD and vertebral fracture risk reduction in teriparatide-treated postmenopausal women with osteoporosis. Journal of Bone & Mineral Research 2006;21(11):1785-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Crans GG, Silverman SL, Genant HK, Glass EV, Krege JH. Association of severe vertebral fractures with reduced quality of life: reduction in the incidence of severe vertebral fractures by teriparatide. Arthritis & Rheumatism 2004;50(12):4028-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Chen P, Krege JH. Response to teriparatide in patients with baseline 25-hydroxyvitamin D insufficiency or sufficiency. Journal of Clinical Endocrinology & Metabolism 2007;92(12):4630-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Delmas PD, Licata AA, Reginster JY, Crans GG, Chen P, Misurski DA, et al. Fracture risk reduction during treatment with teriparatide is independent of pretreatment bone turnover. Bone 2006;39(2):237-43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Genant HK, Crans GG, Vargas SJ, Krege JH. Teriparatide reduces the fracture risk associated with increasing number and severity of osteoporotic fractures. Journal of Clinical Endocrinology & Metabolism 2005;90(3):1583-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Genant HK, Halse J, Briney WG, Xie L, Glass EV, Krege JH. The effects of teriparatide on the incidence of back pain in postmenopausal women with osteoporosis. Current Medical Research & Opinion 2005;21(7):1027-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Genant HK, Siris E, Crans GG, Desaiah D, Krege JH. Reduction in vertebral fracture risk in teriparatide-treated postmenopausal women as assessed by spinal deformity index. Bone 2005;37(2):170-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. Journal of Bone & Mineral Research 2003;18(11):1932-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Krege JH, Wan X. Teriparatide and the risk of nonvertebral fractures in women with postmenopausal osteoporosis. Bone 2012;50(1):161-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Miller PD, Schwartz EN, Chen P, Misurski DA, Krege JH. Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporosis International 2007;18(1):59-68. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. New England Journal Medicine 2001;344(19):1434-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Paschalis EP, Glass EV, Donley DW, Eriksen EF. Bone mineral and collagen quality in iliac crest biopsies of patients given teriparatide: new results from the Fracture Prevention Trial. Journal of Clinical Endocrinology & Metabolism 2005;90(8):4644-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Satterwhite J, Heathman M, Miller PD, Marin F, Glass EV, Dobnig H. Pharmacokinetics of teriparatide (rhPTH[1-34]) and calcium pharmacodynamics in postmenopausal women with osteoporosis. Calcified Tissue International 2010;87(6):485-92. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman SL, Piziak VK, Chen P, Misurski DA, Wagman RB. Relationship of health related quality of life to prevalent and new or worsening back pain in postmenopausal women with osteoporosis. Journal of Rheumatology 2005;32(12):2405-9. [MEDLINE: ] [PubMed] [Google Scholar]
- Uusi-Rasi K, Semanick LM, Zanchetta JR, Bogado CE, Eriksen EF, Sato M, et al. Effects of teriparatide [rhPTH (1-34)] treatment on structural geometry of the proximal femur in elderly osteoporotic women. Bone 2005;36(6):948-58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Watts NB, Miller PD, Kohlmeier LA, Sebba A, Chen P, Wong M, et al. Vertebral fracture risk is reduced in women who lose femoral neck BMD with teriparatide treatment. Journal of Bone & Mineral Research 2009;24(6):1125-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haghverdi 2014 {published data only}
- Haghverdi F, Farbodara T, Mortaji S, Soltani P, Saidi N. Effect of raloxifene on parathyroid hormone in osteopenic and osteoporotic postmenopausal women with chronic kidney disease stage 5 [Erratum in: Iran J Kidney Dis. 2015 Jan;9(1):76; PMID: 25599744]. Iranian Journal of Kidney Diseases 2014;8(6):461-6. [MEDLINE: ] [PubMed] [Google Scholar]
Hernandez 2003 {published data only}
- Heilberg IP, Hernandez E, Alonzo E, Valera R, Ferreira LG, Gomes SA, et al. Estrogen receptor (ER) gene polymorphism may predict the bone mineral density response to raloxifene in postmenopausal women on chronic hemodialysis. Renal Failure 2005;27(2):155-61. [MEDLINE: ] [PubMed] [Google Scholar]
- Hernandez E, Valera R, Alonzo E, Bajares-Lilue M, Carlini R, Capriles F, et al. Effects of raloxifene on bone metabolism and serum lipids in postmenopausal women on chronic hemodialysis. Kidney International 2003;63(6):2269-74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hernandez E, Valera R, Teran J, Bajares-Lilue M, Capriiles F, Alonzo E, et al. The effect of raloxifene, a selective estrogen receptor modulator (SERM), on bone resorption markers and serum lipids in postmenopausal women on chronic hemodialysis (HD) [abstract no: A2974]. Journal of the American Society of Nephrology 2000;11(Sept):563-4A. [CENTRAL: CN-00550530] [Google Scholar]
- Hernandez E, Valera R, Teran J, Bajares-Lilue M, Capriles F, Alonzo E, et al. One year controlled study on the effect of raloxifene, a selective estrogen receptor modulator (SERM), on bone metabolism and serum lipids on postmenopausal women on chronic hemodialysis (HD) [abstract no: A3879]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):743A. [CENTRAL: CN-00550533] [Google Scholar]
- Weisinger, JR, Heilberg IP, Hernandez E, Carlini R, Bellorin-Font E. Selective estrogen receptor modulators in chronic renal failure. Kidney International - Supplement 2003;63(85):S62-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MORE 1999 {published data only}
- Borgstrom F, Johnell O, Kanis JA, Oden A, Sykes D, Jonsson B. Cost effectiveness of raloxifene in the treatment of osteoporosis in Sweden: an economic evaluation based on the MORE study. Pharmacoeconomics 2004;22(17):1153-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators [Erratum in: JAMA 1999 Dec 8;282(22):2124]. JAMA 1999;282(7):637-45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ishani A, Blackwell T, Jamal SA, Cummings SR, Ensrud KE, MORE Investigators. The effect of raloxifene treatment in postmenopausal women with CKD. Journal of the American Society of Nephrology 2008;19(7):1430-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnell O, Kanis JA, Black DM, Balogh A, Poor G, Sarkar S, et al. Associations between baseline risk factors and vertebral fracture risk in the Multiple Outcomes of Raloxifene Evaluation (MORE) Study. Journal of Bone & Mineral Research 2004;19(5):764-72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kanis JA, Borgstrom F, Johnell O, Oden A, Sykes D, Jonsson B. Cost-effectiveness of raloxifene in the UK: an economic evaluation based on the MORE study. Osteoporosis International 2005;16(1):15-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Melamed M, Neugarten J, Blackwell T, Arnsten J, Ensrud K, Ishani A, et al. The protective effects on kidney function of 4 years of treatment with raloxifene, a selective estrogen receptor modulator: results from the MORE trial [abstract no: SA-PO915]. Journal of the American Society of Nephrology 2007;18(Abstracts):544A. [Google Scholar]
- Silverman SL, Shen W, Minshall ME, Xie S, Moses KH. Prevalence of depressive symptoms in postmenopausal women with low bone mineral density and/or prevalent vertebral fracture: results from the Multiple Outcomes of Raloxifene Evaluation (MORE) study. Journal of Rheumatology 2007;34(1):140-4. [MEDLINE: ] [PubMed] [Google Scholar]
- Siris E, Adachi JD, Lu Y, Fuerst T, Crans GG, Wong M, et al. Effects of raloxifene on fracture severity in postmenopausal women with osteoporosis: results from the MORE study. Multiple Outcomes of Raloxifene Evaluation. Osteoporosis International 2002;13(11):907-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Uusi-Rasi K, Beck TJ, Semanick LM, Daphtary MM, Crans GG, Desaiah D, et al. Structural effects of raloxifene on the proximal femur: results from the Multiple Outcomes of Raloxifene Evaluation trial. Osteoporosis International 2006;17(4):575-86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ariyoshi 2006 {published data only}
- Ariyoshi T, Eishi K, Sakamoto I, Matsukuma S, Odate T. Effect of etidronic acid on arterial calcification in dialysis patients. Clinical Drug Investigation 2006;26(4):215-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DIVINE 2011 {published data only}
- Miller P, Eis SR, Mautalen C, Ramirez F, Jonkanski I. Effects of intravenous ibandronate injection on renal function in postmenopausal women with osteoporosis at high risk for renal disease compared with ibandronate infusion or oral alendronate-the DIVINE study [abstract no: MO0382]. Journal of Bone & Mineral Research 2010;25(Suppl 1):S472. [EMBASE: 71500190] [Google Scholar]
- Miller PD, Ragi-Eis S, Mautalen C, Ramirez F, Jonkanski I. Effects of intravenous ibandronate injection on renal function in women with postmenopausal osteoporosis at high risk for renal disease--the DIVINE study. Bone 2011;49(6):1317-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fukunaga 2002 {published data only}
- Fukunaga M, Kushida K, Kishimoto H, Shiraki M, Taketani Y, Minaguchi H, et al. A comparison of the effect of risedronate and etidronate on lumbar bone mineral density in Japanese patients with osteoporosis: a randomized controlled trial. Osteoporosis International 2002;13(12):971-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shigematsu T, Muraoka R, Sugimoto T, Nishizawa Y. Risedronate therapy in patients with mild-to-moderate chronic kidney disease with osteoporosis: post-hoc analysis of data from the risedronate phase III clinical trials. BMC Nephrology 2017;18(1):66. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grotz 1998 {published data only}
- Grotz W, Rump JA, Niessen A, Schmidt-Gayk H, Schollmeyer P. Treatment of bone pain after kidney transplantation. Transplantation Proceedings 1998;30(5):2114-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grotz WH, Rump LC, Niessen A, Schmidt-Gayk H, Reichelt A, Kirste G, et al. Treatment of osteopenia and osteoporosis after kidney transplantation. Transplantation 1998;66(8):1004-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hagino 2014 {published data only}
- Hagino H, Kishimoto H, Ohishi H, Horii S, Nakamura T. Efficacy, tolerability and safety of once-monthly administration of 75mg risedronate in Japanese patients with involutional osteoporosis: a comparison with a 2.5mg once-daily dosage regimen. Bone 2014;59:44-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Inoue D, Maehara M, Oikawa I, Shigematsu T, Nishizawa Y. Efficacy and safety of once-monthly risedronate in osteoporosis subjects with mild-to-moderate chronic kidney disease: a post hoc subgroup analysis of a phase III trial in Japan. Journal of Bone & Mineral Metabolism 2019;37(4):730-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hashiba 2004 {published data only}
- Hashiba H, Aizawa S, Tamura K, Shigematsu T, Kogo H. Inhibitory effects of etidronate on the progression of vascular calcification in hemodialysis patients. Therapeutic Apheresis & Dialysis 2004;8(3):241-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hashiba 2006 {published data only}
- Hashiba H, Aizawa S, Tamura K, Kogo H. Inhibition of the progression of aortic calcification by etidronate treatment in hemodialysis patients: long-term effects. Therapeutic Apheresis & Dialysis 2006;10(1):59-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Iseri 2019 {published data only}
- Iseri K, Watanabe M, Yoshikawa H, Mitsui H, Endo T, Yamamoto Y, et al. Effects of denosumab and alendronate on bone health and vascular function in hemodialysis patients: a randomized, controlled trial. Journal of Bone & Mineral Research 2019;34(6):1014-24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
JPRN‐C000000390 {published data only}
- Hamano T, Fujii N, Mikami S, Ito T, Katayama M, Obi H, et al. The effect of low dose hormone replacement therapy or SERM on bone metabolism in hemodialysis patients. Osteoporosis (Japan) 2000;14(2):83-6. [Google Scholar]
- Ito T. The effect of low dose hormone replacement therapy or raloxifene on bone and lipid metabolism in hemodialysis patients. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000000480 (first received 1 April 2006).
Kishimoto 2006 {published data only}
- Kishimoto H, Fukunaga M, Kushida K, Shiraki M, Itabashi A, Nawata H, et al. Efficacy and tolerability of once-weekly administration of 17.5 mg risedronate in Japanese patients with involutional osteoporosis: a comparison with 2.5-mg once-daily dosage regimen. Journal of Bone & Mineral Metabolism 2006;24(5):405-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shigematsu T, Muraoka R, Sugimoto T, Nishizawa Y. Risedronate therapy in patients with mild-to-moderate chronic kidney disease with osteoporosis: post-hoc analysis of data from the risedronate phase III clinical trials. BMC Nephrology 2017;18(1):66. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kleinstueck 2001 {published data only}
- Kleinstueck DB, Santiestiban H, Benet LZ, Frassetto LA. Effects of short-term alendronate on bone mineral density in end stage renal disease [abstract no: A1842]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):358A. [CENTRAL: CN-00446117] [Google Scholar]
Kushida 2006 {published data only}
- Kushida K, Fukunaga M, Kishimoto H, Shiraki M, Itabashi A, Inoue T, et al. A comparison of incidences of vertebral fracture in Japanese patients with involutional osteoporosis treated with risedronate and etidronate: a randomized, double-masked trial. Journal of Bone & Mineral Metabolism 2006;22(5):469-78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shigematsu T, Muraoka R, Sugimoto T, Nishizawa Y. Risedronate therapy in patients with mild-to-moderate chronic kidney disease with osteoporosis: post-hoc analysis of data from the risedronate phase III clinical trials. BMC Nephrology 2017;18(1):66. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00261625 {published data only}
- Kao TW. Can alendronate suppress calcification and improve bone density in chronic peritoneal dialysis patients? [Can alendronate suppress aortic and coronary artery calcification and improve bone mineral density in chronic peritoneal dialysis patients?]. www.clinicaltrials.gov/show/NCT00261625 (first received 5 December 2005).
NCT00299572 {published data only}
- Lai CF. Alendronate for vascular calcification in peritoneal dialysis patients? [Can alendronate suppress aortic and coronary artery calcification and improve bone mineral density in chronic peritoneal dialysis patients?]. www.clinicaltrials.gov/show/NCT00299572 (first received 7 March 2006).
Omidvar 2011 {published data only}
- Omidvar B, Ghorbani A, Shahbazian H, Beladi Mousavi SS, Shariat Nabavi SJ, Alasti M. Comparison of alendronate and pamidronate on bone loss in kidney transplant patients for the first 6 months of transplantation [Erratum in: Iran J Kidney Dis. 2012 Jul;6(4):321]. Iranian Journal of Kidney Diseases 2011;5(6):420-4. [MEDLINE: ] [PubMed] [Google Scholar]
- Omidvar B, Ghorbani A, Shahbazian H, Mousavi SS, Hayati F, Nabavi SJ. Comparison the effect of alendronate and pamidronate on early BMD changes in kidney transplant patients in the first 6 months of transplantation [abstract no: 0740]. International Journal of Rheumatic Diseases 2010;13(Suppl 1):170. [EMBASE: 70198173] [Google Scholar]
Ruzhytska 2015 {published data only}
- Ruzhytska O, Martynyuk L. Effects on bone tissue of 12 months' combined treatment with alfacalcidol and strontium ranelate in patients with mild to moderate chronic renal failure [abstract no: FP435]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii216. [EMBASE: 72206855] [Google Scholar]
Saito 2012 {published data only}
- Saito O, Saito T, Asakura S, Akimoto T, Inoue M, Ando Y, et al. Effects of raloxifene on bone metabolism in hemodialysis patients with type 2 diabetes. International Journal of Endocrinology & Metabolism 2012;10(2):464-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sirsat 2010 {published data only}
- Sirsat RA, Puri VS, Holkar SD, Almeida AF, Langote AC, Kothari JP, et al. Is pamidronate more effective than alendronate in adult patients for the prevention of bone loss after kidney transplantation? [abstract no: PUB116]. Journal of the American Society of Nephrology 2010;21(Abstract Suppl):836A. [Google Scholar]
Toussaint 2010 {published data only}
- Toussaint ND, Lau KK, Polkinghorne KR, Kerr PG. Randomised placebo-controlled trial of alendronate on vascular calcification in patients with chronic kidney disease [abstract no: 008]. Nephrology 2009;14(Suppl 1):A2-3. [CENTRAL: CN-00775976] [Google Scholar]
- Toussaint ND, Lau KK, Polkinghorne KR, Kerr PG. Randomised placebo-controlled trial of alendronate on vascular calcification in patients with chronic kidney disease [abstract no: SA-FC345]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):81A. [Google Scholar]
- Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG. Effect of alendronate on vascular calcification in CKD stages 3 and 4: a pilot randomized controlled trial. American Journal of Kidney Diseases 2010;56(1):57-68. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
UMIN00001829 {published data only}
- Mori K. Impact of raloxifene, eldecalcitol and their combination therapy on bone indices in postmenopausal subjects with osteoporosis and chronic kidney disease stage 3: re Bone Study. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000021054 (first received 13 July 2015).
Wang 2008b {published data only}
- Wang SX, Li H. Effects of salmon calcitonin in treatment of osteoporosis in patients undergoing dialysis. Chung-Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2008;88(6):387-90. [MEDLINE: ] [PubMed] [Google Scholar]
Wang 2008c {published data only}
- Wang SX, Li H. Salmon calcitonin in prevention of osteoporosis in maintenance dialysis patients. Chinese Medical Journal 2008;121(14):1280-4. [MEDLINE: ] [PubMed] [Google Scholar]
Wetmore 2005 {published data only}
- Wetmore JB, Benet LZ, Kleinstuck D, Frassetto L. Effects of short-term alendronate on bone mineral density in haemodialysis patients. Nephrology 2005;10(4):393-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
IRCT20180506039549N1 {published data only}
- Masmou B. Effect of alendronate in patents with osteoporosis and chronic kidney disease [Effect of alendronate on bone densitometry in osteoporetic chronic kidney disease stage 3a-3b]. en.irct.ir/trial/33312 (first received 22 October 2018).
NCT02440581 {published data only}
- Malluche H. Renal osteodystrophy: an individual management approach [Renal osteodystrophy: a fresh approach]. www.clinicaltrials.gov/show/NCT02440581 (first received 12 May 2015).
NCT02792413 {published data only}
- Cristol JP. Study evaluating denosumab on bone and vascular metabolism in osteoporotic chronic kidney disease (HDENO) [Randomized controlled study evaluating the effect of a biotherapy treatment (anti-RANKL ligand antibody: denosumab) on bone and vascular metabolism in osteoporotic chronic kidney disease]. www.clinicaltrials.gov/show/NCT02792413 (first received 7 June 2016).
Additional references
Abrahamsen 2014
- Abrahamsen B, Grove EL, Vestergaard P. Nationwide registry-based analysis of cardiovascular risk factors and adverse outcomes in patients treated with strontium ranelate. Osteoporosis International 2014;25(2):757-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Adomaityte 2008
- Adomaityte J, Farooq M, Qayyum R. Effect of raloxifene therapy on venous thromboembolism in postmenopausal women. A meta-analysis. Thrombosis & Haemostasis 2008;99(2):338-42. [MEDLINE: ] [PubMed] [Google Scholar]
Alem 2000
- Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney International 2000;58(1):396-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Allen 2016
- Allen CS, Yeung JH, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database of Systematic Reviews 2016, Issue 10. Art. No: CD001347. [DOI: 10.1002/14651858.CD001347.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Amerling 2010
- Amerling R, Harbord NB, Pullman J, Feinfeld DA. Bisphosphonate use in chronic kidney disease: association with adynamic bone disease in a bone histology series. Blood Purification 2010;29(3):293-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Barrett‐Connor 2006
- Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. New England Journal of Medicine 2006;355(2):125-37. [PMID: ] [DOI] [PubMed] [Google Scholar]
Block 2012
- Block GA, Bone HG, Fang L, Lee E, Padhi D. A single-dose study of denosumab in patients with various degrees of renal impairment. Journal of Bone & Mineral Research 2012;27(7):1471-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bone 2008
- Bone HG, Bolognese MA, Yuen CK, Kendler DL, Wang H, Liu Y, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. Journal of Clinical Endocrinology & Metabolism 2008;93(6):2149-57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Browner 1996
- Browner WS, Pressman AR, Nevitt MC, Cummings SR. Mortality following fractures in older women: the study of osteoporotic fractures. Archives of Internal Medicine 1996;156(14):1521-5. [MEDLINE: ] [PubMed] [Google Scholar]
Burge 2001
- Burge RT, Worley D, Johansen A, Bhattacharyya S, Bose U. The cost of osteoporotic fractures in the UK: Projections for 2000-2020. Journal of Medical Economics 2001;4:51–62. [EMBASE: 32537253] [Google Scholar]
Burge 2007
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. Journal of Bone & Mineral Research 2007;22(3):465-75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cosman 2016
- Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, et al. Romosozumab treatment in postmenopausal women with osteoporosis. New England Journal of Medicine 2016;375(16):1532-43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Crandall 2014
- Crandall CJ, Newberry SJ, Diamant A, Lim YW, Gellad WF, Booth MJ, et al. Comparative effectiveness of pharmacologic treatments to prevent fractures: an updated systematic review. Annals of Internal Medicine 2014;161(10):711-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cunningham 2004
- Cunningham J, Sprague SM, Cannata-Andia J, Coco M, Cohen-Solal M, Fitzpatrick L, et al. Osteoporosis in chronic kidney disease. American Journal of Kidney Diseases 2004;43(3):566-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dave 2015
- Dave V, Chiang CY, Booth J, Mount PF. Hypocalcemia post denosumab in patients with chronic kidney disease stage 4-5. American Journal of Nephrology 2015;41(2):129-37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
De Groen 1996
- De Groen PC, Lubbe DF, Hirsch LJ, Daifotis A, Stephenson W, Freedholm D, et al. Esophagitis associated with the use of alendronate. New England Journal of Medicine 1996;335(14):1016-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Donnelly 2012
- Donnelly E, Saleh A, Unnanuntana A, Lane JM. Atypical femoral fractures: epidemiology, etiology, and patient management. Current Opinion in Supportive & Palliative Care 2012;6(3):348-54. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eastell 2019
- Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. Journal of Clinical Endocrinology & Metabolism 2019;104(5):1595-622. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eknoyan 2013
- Eknoyan G, Lameire N, Eckardt KU, Kasiske BL, Wheeler DC, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD: summary of recommendation statements. Kidney International Supplements 2013;3(1):5-14. [DOI: 10.1053/j.ajkd.2014.01.416] [DOI] [Google Scholar]
Eliasaf 2016
- Eliasaf A, Amitai A, Maram Edry M, Yosselson Superstine S, Rotman Pikielny P. Compliance, persistence, and preferences regarding osteoporosis treatment during active therapy or drug holiday. Journal of Clinical Pharmacology 2016;56(11):1416-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GBD 2016
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015 [Erratum in: Lancet. 2017 Jan 7;389(10064):e1; PMID: 28091379]. Lancet 2016;388(10053):1459-544. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glassock 2012
- Glassock RJ, Rule AD. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney International 2012;82(3):270-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
He 2016
- He W, Goodkind D, Kowal P. An Aging World: 2015. www.census.gov/content/dam/Census/library/publications/2016/demo/p95-16-1.pdf (accessed 27 May 2021).
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
ISCD 2019
- International Society for Clinical Densitometry (ISCD). Skeletal health assessment In children from infancy to adolescence. https://iscd.org/learn/official-positions/pediatric-positions (last accessed 5 July 2021).
Johnell 2006
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporosis International 2006;17(12):1726-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kanis 2005
- Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, et al. Assessment of fracture risk. Osteoporosis International 2005;16(6):581-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kanis 2019
- Kanis JA, Cooper C, Rizzoli R, Reginster JY, Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women [Erratum in: Osteoporos Int. 2020 Jan;31(1):209; PMID: 31673731] [Erratum in: Osteoporos Int. 2020 Apr;31(4):801; PMID: 32072205]. Osteoporosis International 2019;30(1):3-44. [MEDLINE: 30324412] [Google Scholar]
KDIGO 2009
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney International - Supplement 2009;Aug(113):S1-130. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDIGO 2017
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) [Erratum in: Kidney Int Suppl (2011). 2017 Dec;7(3):e1; PMID: 30681074]. Kidney International Supplements 2017;7(1):1-59. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keene 1993
- Keene GS, Parker MJ, Pryor GA. Mortality and morbidity after hip fractures. BMJ 1993;307(6914):1248-50. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khairallah 2018a
- Khairallah P, Nickolas TL. Updates in CKD-associated osteoporosis. Current Osteoporosis Reports 2018;16(6):712-23. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khairallah 2018b
- Khairallah P, Nickolas TL. Management of osteoporosis in CKD. Clinical Journal of The American Society of Nephrology: CJASN 2018;13(6):962-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2016
- Kim SM, Long J, Montez-Rath M, Leonard M, Chertow GM. Hip fracture in patients with non-dialysis-requiring chronic kidney disease. Journal of Bone & Mineral Research 2016;31(10):1803-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Klawansky 2003
- Klawansky S, Komaroff E, Cavanaugh PF Jr, Mitchell DY, Gordon MJ, Connelly JE, et al. Relationship between age, renal function and bone mineral density in the US population. Osteoporosis International 2003;14(7):570-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levey 2003
- Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification [Erratum in: Ann Intern Med. 2003 Oct 7;139(7):605]. Annals of Internal Medicine 2003;139(2):137-47. [PMID: ] [DOI] [PubMed] [Google Scholar]
Levin 2007
- Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease [Erratum in: Kidney Int. 2009 Jun 1;75(11):1237; PMID: 30036915]. Kidney International 2007;71(1):31-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Llach 2000
- Llach F, Bover, J. Brenner and Rector's The Kidney. 6th edition. Vol. 2. Philadelphia: WB Saunders Company, 2000. [ISBN: 978-0721679983] [Google Scholar]
Meunier 2004
- Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. New England Journal of Medicine 2004;350(5):459-68. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Miller 2008
- Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 2008;43(2):222-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Miller 2016
- Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, et al. Effect of Abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial [Erratum in: JAMA. 2017 Jan 24;317(4):442; PMID: 28118431]. JAMA 2016;316(7):722-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morizio 2018
- Morizio P, Burkhart JI, Ozawa S. Denosumab: a unique perspective on adherence and cost-effectiveness compared with oral bisphosphonates in osteoporosis patients. Annals of Pharmacotherapy 2018;52(10):1031-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Naranjo Hernandez 2018
- Naranjo Hernández A, Díaz Del Campo Fontecha P, Aguado Acín MP, Arboleya Rodríguez L, Casado Burgos E, Castañeda S, et al. Recommendations by the Spanish Society of Rheumatology on Osteoporosis [Recomendaciones de la Sociedad Espanola de Reumatologia sobre osteoporosis]. Reumatología Clínica 2019;15(4):188-210. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Neer 2001
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. New England Journal of Medicine 2001;344(9):1434-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nickolas 2006
- Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. Journal of the American Society of Nephrology 2006;17(11):3223-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NIH 2001
- NIH Consensus Development Panel. NIH Consensus Development Panel on Osteoporosis Prevention. Diagnosis, and Therapy, March 7-29, 2000: highlights of the conference. Southern Medical Journal 2001;94(6):569–73. [MEDLINE: ] [PubMed] [Google Scholar]
Nitta 2017
- Nitta K, Yajima A, Tsuchiya K. Management of osteoporosis in chronic kidney disease. Internal Medicine 2017;56(24):3271-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NOGG 2017
- National Osteoporosis Guideline Group. NOGG 2017: Clinical guideline for the prevention and treatment of osteoporosis. www.sheffield.ac.uk/NOGG/NOGG%20Guideline%202017.pdf (accessed 27 May 2021).
Osborne 2010
- Osborne V, Layton D, Perrio M, Wilton L, Shakir SA. Incidence of venous thromboembolism in users of strontium ranelate: an analysis of data from a prescription-event monitoring study in England. Drug Safety 2010;33(7):579-91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Palmer 2007b
- Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GFM. Vitamin D compounds for people with chronic kidney disease not requiring dialysis. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD008175. [DOI: 10.1002/14651858.CD008175] [DOI] [PubMed] [Google Scholar]
Palmer 2009
- Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF. Vitamin D compounds for people with chronic kidney disease requiring dialysis. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD005633. [DOI: 10.1002/14651858.CD005633.pub2] [DOI] [PubMed] [Google Scholar]
Palmer 2019
- Palmer SC, Chung EY, McGregor DO, Bachmann F, Strippoli GF. Interventions for preventing bone disease in kidney transplant recipients. Cochrane Database of Systematic Reviews 2019, Issue 10. Art. No: CD005015. [DOI: 10.1002/14651858.CD005015.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Qaseem 2017
- Qaseem A, Forciea MA, McLean RM, Denberg TD, Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians [Erratum in: Ann Intern Med. 2017 Sep 19;167(6):448; PMID: 28975328]. Annals of Internal Medicine 2017;166(11):818-39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reginster 2019
- Reginster JY, Bianic F, Campbell R, Martin M, Williams SA, Fitzpatrick LA. Abaloparatide for risk reduction of nonvertebral and vertebral fractures in postmenopausal women with osteoporosis: a network meta-analysis. Osteoporosis International 2019;30(7):1465-73. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruggiero 2004
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. Journal of Oral & Maxillofacial Surgery 2004;62(5):527-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ruospo 2018
- Ruospo M, Palmer SC, Natale P, Craig JC, Vecchio M, Elder GJ, et al. Phosphate binders for preventing and treating chronic kidney disease-mineral and bone disorder (CKD-MBD). Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD006023. [DOI: 10.1002/14651858.CD006023.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saag 2017
- Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. New England Journal of Medicine 2017;377(15):1417-27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
SONG 2017
- SONG Initiative. The SONG Handbook Version 1.0. www.songinitiative.org/reports-and-publications/ 2017.
Sprague 2016
- Sprague SM, Bellorin-Font E, Jorgetti V, Carvalho AB, Malluche HH, Ferreira A, et al. Diagnostic accuracy of bone turnover markers and bone histology in patients with CKD treated by dialysis. American Journal of Kidney Diseases 2016;67(4):559-66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stein 1996
- Stein MS, Packham DK, Ebeling PR, Wark JD, Becker GJ. Prevalence and risk factors for osteopenia in dialysis patients. American Journal of Kidney Diseases 1996;28(4):515-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tentori 2013
- Tentori F, McCullough K, Kilpatrick RD, Bradbury BD, Robinson BM, Kerr PG, et al. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney International 2013;85(1):166-73. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2007
- Ward L, Tricco AC, Phuong P, Cranney A, Barrowman N, Gaboury I, et al. Bisphosphonate therapy for children and adolescents with secondary osteoporosis. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD005324. [DOI: 10.1002/14651858.CD005324.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1994
- World Health Organization. Assessment of fracture risk and its implication to screening for postmenopausal osteoporosis: Technical report series 843. apps.who.int/iris/handle/10665/39142 (accessed 27 May 2021).
Wilson 2017
- Wilson LM, Rebholz CM, Jirru E, Liu MC, Zhang A, Gayleard J, et al. Benefits and harms of osteoporosis medications in patients with chronic kidney disease. Annals of Internal Medicine 2017;166(9):649-58. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hara 2019
- Hara T, Hijikata Y, Matsubara Y, Watanabe N. Pharmacological interventions for osteoporosis in people with chronic kidney disease stages 3‐5D. Cochrane Database of Systematic Reviews 2019, Issue 9. Art. No: CD013424. [DOI: 10.1002/14651858.CD013424] [DOI] [PMC free article] [PubMed] [Google Scholar]


