Abstract
Objective
To investigate the efficacy, acceptability, and safety of muscle relaxants for low back pain.
Design
Systematic review and meta-analysis of randomised controlled trials.
Data sources
Medline, Embase, CINAHL, CENTRAL, ClinicalTrials.gov, clinicialtrialsregister.eu, and WHO ICTRP from inception to 23 February 2021.
Eligibility criteria for study selection
Randomised controlled trials of muscle relaxants compared with placebo, usual care, waiting list, or no treatment in adults (≥18 years) reporting non-specific low back pain.
Data extraction and synthesis
Two reviewers independently identified studies, extracted data, and assessed the risk of bias and certainty of the evidence using the Cochrane risk-of-bias tool and Grading of Recommendations, Assessment, Development and Evaluations, respectively. Random effects meta-analytical models through restricted maximum likelihood estimation were used to estimate pooled effects and corresponding 95% confidence intervals. Outcomes included pain intensity (measured on a 0-100 point scale), disability (0-100 point scale), acceptability (discontinuation of the drug for any reason during treatment), and safety (adverse events, serious adverse events, and number of participants who withdrew from the trial because of an adverse event).
Results
49 trials were included in the review, of which 31, sampling 6505 participants, were quantitatively analysed. For acute low back pain, very low certainty evidence showed that at two weeks or less non-benzodiazepine antispasmodics were associated with a reduction in pain intensity compared with control (mean difference −7.7, 95% confidence interval−12.1 to−3.3) but not a reduction in disability (−3.3, −7.3 to 0.7). Low and very low certainty evidence showed that non-benzodiazepine antispasmodics might increase the risk of an adverse event (relative risk 1.6, 1.2 to 2.0) and might have little to no effect on acceptability (0.8, 0.6 to 1.1) compared with control for acute low back pain, respectively. The number of trials investigating other muscle relaxants and different durations of low back pain were small and the certainty of evidence was reduced because most trials were at high risk of bias.
Conclusions
Considerable uncertainty exists about the clinical efficacy and safety of muscle relaxants. Very low and low certainty evidence shows that non-benzodiazepine antispasmodics might provide small but not clinically important reductions in pain intensity at or before two weeks and might increase the risk of an adverse event in acute low back pain, respectively. Large, high quality, placebo controlled trials are urgently needed to resolve uncertainty.
Systematic review registration
PROSPERO CRD42019126820 and Open Science Framework https://osf.io/mu2f5/.
Introduction
Low back pain is a major global public health problem, placing a burden on individuals, healthcare, and society, and has been the leading cause of disability worldwide for the past 30 years.1 In the United States, low back pain is responsible for the highest total expenditure on healthcare—in 2016 estimated to be $134.5bn (£95.0bn; €110.5bn) (95% confidence interval $122.4bn to $146.9bn).2 Low back pain is a common reason to visit a general practitioner,3 4 when patients are often prescribed analgesics to manage their symptoms.5 6 7
Muscle relaxants, a broad class of drugs that include non-benzodiazepine antispasmodics and antispastics (table 1), are frequently prescribed in the UK and US. In 2020, prescriptions in England exceeded 1.3 million,11 and in the US more than 30 million prescriptions of muscle relaxants were recorded for ambulatory care visits in 2016.12 Muscle relaxants are the third most commonly prescribed drug for low back pain.5 7 9 12 Recommendations for the use of muscle relaxants have, however, conflicted between international clinical practice guidelines for low back pain.11 13 For example, the US guideline recommends non-benzodiazepine antispasmodics as the drug of choice for acute low back pain,14 the Belgian guideline discourages such use,15 and the UK guideline does not make a recommendation.16
Table 1.
Overview of muscle relaxants* grouped according to clinical utility
| Group | Clinical utility | Examples of drugs included in this review |
|---|---|---|
| Antispastics | To reduce heightened muscle tone (spasticity) commonly associated with cerebral palsy, multiple sclerosis, and spinal cord injuries | Baclofen, dantrolene |
| Non-benzodiazepine antispasmodics | To reduce acute muscle spasm commonly associated with muscle injury. These drugs also have a strong sedative action | Carisoprodol, cyclobenzaprine, metaxalone, methocarbamol, thiocolchicoside, tizanidine, tolperisone, orphenadrine |
| Benzodiazepines | To reduce acute muscle spasm commonly associated with muscle injury. These drugs also have a strong sedative action as well as anxiolytic, hypnotic, and anticonvulsant actions | Diazepam |
| Miscellaneous | Although less commonly classified as muscle relaxants, several other drugs are prescribed for their ability to reduce muscle spasm or muscle tone (spasticity), or both. These include botulinum toxins and non-benzodiazepine hypnotics | Botulinum toxin, eszopiclone |
Muscle relaxants are generally prescribed to reduce muscle spasm or muscle tone (spasticity), or both. The term muscle relaxant is broad and includes many different chemically unrelated drugs with different clinical utility and mechanisms of action.8 The choice of muscle relaxant and frequency of prescription by a doctor varies between countries,9 10 with considerable clinical uncertainty in preferencing one muscle relaxant over another.
A systematic review that included five randomised controlled trials (n=497 participants) published up to end of October 2015 provides the most recent evidence that muscle relaxants produce a clinically meaningful reduction in pain intensity for people with acute low back pain (mean difference −21.3, 95% confidence interval −29.0 to −13.5).17 Several large randomised controlled trials have since been published. Furthermore, this systematic review did not include evidence from randomised controlled trials in clinical trial registries, which might lead to an overestimation of the effect.18 To address this knowledge gap, we systematically reviewed the evidence to estimate the efficacy, acceptability, and safety of muscle relaxants compared with placebo, usual care, or no treatment in adults with low back pain. We evaluated the certainty of the evidence supporting the findings using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach19 20 and discuss its clinical relevance.
Methods
We followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) reporting guideline.21 Supplementary file 1 shows the protocol deviations.
Data sources and searches
We searched Medline, Embase, CINAHL, the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Back and Neck Group’s trial register (through CENTRAL), ClinicalTrials.gov, the EU Clinical Trials Register, and the World Health Organization’s International Clinical Trial Registry Platform from inception to 23 February 2021. The search strategies were developed and piloted by the review team for bibliographic databases and clinical trial registries using medical subject headings or Emtree and text words for “low back pain”, “randomised controlled trials”, and “spasmolytic muscle relaxant medicines” (see supplementary files 2 and 3). We searched the reference lists from retrieved full text articles and previous systematic reviews. Searches were also done through PROSPERO for any ongoing or recently completed systematic reviews.
Eligibility criteria
Records of randomised controlled trials were included that allocated adults (≥18 years) with non-specific low back pain22 to receive a systemically administered dose of a spasmolytic muscle relaxant8 compared with a non-active control group (sham (placebo) drug, continuation of usual care, waiting list, or no treatment). We also included randomised controlled trials that investigated the combination of two drugs compared with one drug alone (eg, tizanidine and ibuprofen versus ibuprofen). Drugs had to be classified as muscle relaxants, listed on the WHO Anatomical Therapeutic Chemical (ATC) classification system,23 and licensed in the US (Food and Drug Administration24), Europe (European Medicines Agency25), or Australia (Australian Register of Therapeutic Goods26) as at 29 March 2019 (see supplementary file 4). Trials reported in English, Italian, Portuguese, Spanish, German, and Dutch were included. We did not restrict the inclusion of trials by the duration of low back pain reported, trial publication status, outcomes reported, or instrument used to assess outcomes. Trials were excluded that investigated suspended muscle relaxants or those not currently licensed in the US, Europe, or Australia, reported in other languages, and sampled participants with specific spinal conditions (eg, infection, neoplasm, inflammatory disease, or fracture)22 or with sciatica.27 We excluded trials that sampled multiple health conditions unless separate data were available for the participants with non-specific low back pain.
Study selection
The review team independently screened the titles and abstracts of all identified records in duplicate. We retrieved full length records of those deemed eligible and screened these again to confirm inclusion. The full length record of a trial registration was defined as the primary web page and all subsidiary pages and files located on the trial registry. Disagreements were resolved through discussion (AGC, MAW, MDJ, MCF, HBL, RRNR) or, when necessary, consultation of a third independent reviewer (MKB or JHM). When further information was required to confirm eligibility, we contacted authors up to three times within a six week period.
In instances where a trial was linked to multiple record sources, we used an established hierarchy, giving preference to the main published trial report, followed by other published records of the trial (eg, conference abstracts), and, lastly, the trial registry record. When no evidence of publication was found, we classified the trial registry record as the primary record.
Outcomes
The choice of outcomes was based on the core outcome domains for clinical trials in low back pain28 and those of other reviews of analgesics for low back pain.29 30 31 The primary outcomes were pain intensity and acceptability (satisfaction with the treatment regimen measured by the number of patients who discontinued treatment for any reason). Secondary outcomes were disability, adverse events (as defined by each study), serious adverse events (as defined by each study), and withdrawal from treatment because of adverse effects (tolerability).
Data extraction
Using a standardised, piloted form, two reviewers independently extracted data on the trial characteristics, participants, interventions, comparisons, and outcomes from each trial. In the absence of data, we transformed or estimated measures of variance using the recommendations from section 6.5.2 in the Cochrane Handbook for Systematic Reviews of Interventions.32 Briefly, we transformed standard errors or 95% confidence intervals for group level estimates to standard deviations using equations from section 6.5.2.2.32 If studies reported mean differences between groups and P values, we calculated the between group standard error using equations from section 6.5.2.3, and used the between group estimates in the meta-analysis.32 When no measure of variance was reported, a conservative standard deviation of 30 was imputed. We resolved disagreements for data extraction through discussion, or with arbitration by a third reviewer if necessary. When data were not reported in the trial, we contacted authors up to three times over a period of six weeks.
Risk of bias and certainty of evidence
Two independent reviewers appraised study level risk of bias using the Cochrane risk of bias tool33 and recommendations from Furlan et al.34 Thirteen criteria were assessed across the risk of bias domains selection, performance, attrition, detection, reporting, and other sources of bias.34 Because recommendations were not available from Furlan et al,34 we used previously published criteria to determine overall study risk of bias for each trial.35 Two reviewers independently determined certainty of the evidence for each analysis using the GRADE system.19 20 Certainty of the evidence is best considered as the certainty that the true effect lies within a particular range.36 We downgraded the certainty of evidence if a serious flaw was present in the domains of risk of bias, inconsistency, imprecision, and publication bias (see supplementary file 5). The certainty of evidence was initially classified as high then as moderate, low, or very low certainty. High certainty meant that we were very confident that the true effect was close to that of the estimate of the effect. Moderate certainty meant that we were moderately confident in the effect estimate; the true effect was likely to be close to the estimate of the effect but with a possibility of being substantially different. Low certainty meant that we had limited confidence in the effect estimate; the true effect might be substantially different from the estimate of the effect. Very low certainty meant that we had very little confidence in the effect estimate; the true effect was likely to be substantially different from the estimate of effect.19 Disagreements between appraisals of risk of bias and certainty of evidence were resolved through discussion, or, when required, by arbitration with a third reviewer.
Data synthesis and analysis
We conducted meta-analyses of trials for each outcome using the available data for immediate term (≤2 weeks post-randomisation) and short term (3-13 weeks post-randomisation) follow-up. When data for multiple time points were available for short term follow-up, we chose the time closest to six weeks. All analyses were stratified by the clinical utility of the muscle relaxant (antispastic, non-benzodiazepine antispasmodic, benzodiazepine, and miscellaneous) and the duration of low back pain observed in the included trials; acute (0-6 weeks), subacute (6-12 weeks), chronic (>12 weeks), and mixed (participants with multiple durations for symptoms). To incorporate trials with multiple comparisons, we followed guidance,32 dividing the control group sample size by the number of trial arms. As benchmarks for clinically important effects are usually expressed on a 0-100 scale,37 38 and to facilitate clinical interpretation of results,39 we converted aggregate outcome data (measure of central tendency and dispersion) for pain and disability to a common 0 (no pain or disability) to 100 (worst pain or disability) scale. We divided the mean and variability measures by the top number of scale and multiplied by 100—for example, 0-24 Roland-Morris Disability Questionnaire score divided by 24 and multiplied by 100. Supplementary files 6 and 7 provide details on the pain and disability measures used by the studies, and conversion procedures. We considered a difference in favour of muscle relaxants of at least 10 points for pain and disability to be the minimal clinically important effect.39 40 This threshold has been used in other reviews of analgesics for low back pain.29 31 41
Random effects meta-analytic models were fit through restricted maximum likelihood estimation, using dmetar in R (version 3.6.1).42 We expressed effects for continuous outcomes with the mean between group difference and accompanying 95% confidence intervals, and effects for binary outcomes using the relative risk and accompanying 95% confidence intervals. The Q statistic and the between study variance (τ2) were estimated from each analysis and these values were used to calculate 95% prediction intervals for the pooled effect and I2 values. We used these measures to form judgments about heterogeneity in conjunction with visual inspection of the distribution of effect sizes in the forest plots. We formed judgments about publication bias for each meta-analysis by visually inspecting funnel plots and considering the proportion of trials included from trial registry records for that outcome.
Planned investigation of heterogeneity
We conducted a planned subgroup analysis to explore whether heterogeneity varied by prescribed dose. The dose comprised three levels: standard dose, more than standard dose, or less than standard dose, according to the Prescribers Digital Reference,43 Monthly Index of Medical Specialties,44 or Australian Medicines Handbook.45
Sensitivity analyses
We conducted sensitivity analyses to assess the influence on effect estimates of trials with unclear definitions of non-specific low back pain, trials where measures of variance were imputed, trials at high risk of bias, trials reported as trial registry records, trials without a placebo comparison, and trials investigating muscle relaxants less commonly prescribed, including carisoprodol and thiocolchicoside. This was done by repeating the main analyses without the relevant trials included.
Patient and public involvement
No patients or members of the public were directly involved in this study because of a lack of funding, although we did speak to patients about the study and we asked a member of the public to read our manuscript after submission. We plan to disseminate the results of this review to the relevant patient organisations.
Results
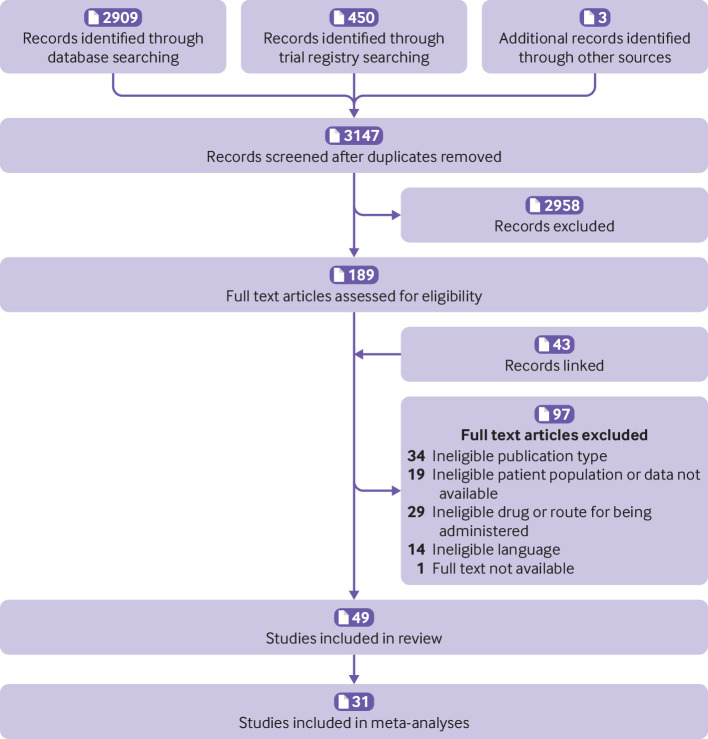
Overall, 3362 records were identified, 215 duplicates removed, and 3147 records screened during title and abstract screening. Forty nine trials were included in the review: 35 were peer reviewed journal articles,46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 two were conference abstracts,57 74 and 12 were trial registry records83 84 85 86 87 88 89 90 91 92 93 94 (fig 1 and supplementary file 8). Thirty one trials (two trial registry records and one conference abstract), including a total of 6505 participants, contributed data to the meta-analyses.
Fig 1.
Flow of record selection process
Study characteristics
The included trials investigated 18 different muscle relaxants, most commonly non-benzodiazepine antispasmodics (n=29), miscellaneous (n=11), antispastics (n=5), and benzodiazepines (n=4). The muscle relaxants investigated were administered orally in 35 trials, by intramuscular injection in 10 trials, and by intravenous injection in one trial. In three trials the drugs were administered in a mixed manner, by intramuscular injection and subsequent oral doses. Most trials (n=32) compared a muscle relaxant with placebo.
Thirty five trials sampled participants with acute low back pain, two trials sampled participants with subacute low back pain, and eight trials sampled participants with chronic low back pain. Two trials investigated participants with both acute and subacute low back pain (mixed duration sample) and two trials did not report the duration of low back pain.
Risk of bias
Of the 39 completed trials assessed for overall risk of bias, eight were assessed at low risk, three at moderate risk, and 28 at high risk. The most common reasons for being at high risk were from attrition bias (failure to report intention-to-treat effects (n=8)), performance bias (inadequate blinding of participants (n=6) or care providers (n=7)), and detection bias (inadequate blinding of outcome assessors (n=6)). There was also unclear risk of selection bias from inadequate reporting of allocation concealment (n=33) and random sequence generation (n=25) (see supplementary file 9).
Qualitative synthesis for primary outcome pain intensity (≤2 weeks)
A total of 25 included trials were unsuitable for meta-analysis of the primary outcome pain intensity. Eight trial registry records83 84 85 86 87 91 92 93 provided no data and 17 peer reviewed journal articles48 49 50 56 60 61 63 65 66 70 71 73 74 79 80 81 82 did not provide data for relevant treatment effects. Supplementary file 10 provides full details of these trials. For the trials that did not report relevant treatment effects for meta-analysis, 11 sampled participants with acute low back pain, five with chronic low back pain, and one did not report the duration of low back pain.
Five trials concluded that non-benzodiazepine antispasmodics were superior to control for acute low back pain,48 49 56 63 74 whereas two trials found no difference.73 79 Two trials concluded that antispastics were superior to control.80 82 One trial concluded that benzodiazepines were superior to control,66 whereas one trial found no difference.60
Four trials50 61 65 81 concluded that miscellaneous muscle relaxants (ie, botulinum toxin) were superior to control for chronic low back pain, and one trial71 concluded that benzodiazepines were superior to control for pain intensity in the supine position but not while sitting.
Finally, one trial70 that did not report the duration of low back pain concluded that antispastics were not superior to control.
Efficacy and acceptability
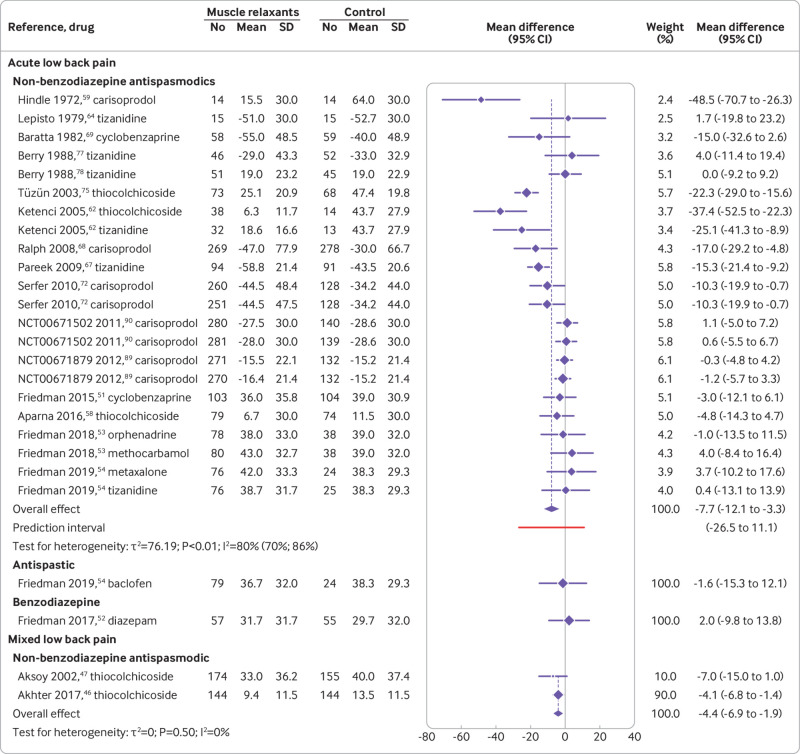
Acute low back pain—Seventeen trials (24 comparisons) determined the efficacy of muscle relaxants for acute low back pain at immediate (≤2 weeks) follow-up and four trials (seven comparisons) at short term (3-13 weeks) follow-up. Thirteen trials (16 comparisons) determined the acceptability of muscle relaxants for acute low back pain. Non-benzodiazepine antispasmodics were associated with a reduction in pain intensity at two weeks or less compared with control (mean difference −7.7, 95% confidence interval −12.1 to −3.3; 16 trials, 4546 participants; very low certainty evidence) (fig 2 and table 2). Non-benzodiazepine antispasmodics were not associated with a reduction in pain intensity compared with control at 3-13 weeks (0.6, −4.5 to 5.7; 3 trials, 612 participants; moderate certainty evidence) or disability at two weeks or less (−3.3, −7.3 to 0.7; 7 trials, 2438 participants; very low certainty evidence) and 3-13 weeks (4.3, −1.4 to 10.1; 2 trials, 422 participants; moderate certainty evidence) (table 2, table 3, and supplementary files 11-13). No difference was found in the acceptability of non-benzodiazepine antispasmodics compared with control (relative risk 0.8, 95% confidence interval 0.6 to 1.1; 13 trials, 2834 participants; very low certainty evidence) (table 4 and supplementary file 14). Evidence ranging from moderate to low certainty showed no benefit of antispastic and benzodiazepine drugs compared with control for pain and disability at immediate (≤2 weeks) and short term (3-13 weeks) follow-up, except for reduction in disability associated with benzodiazepines at 3-13 weeks (mean difference −6.9, 95% confidence interval −12.1 to −1.7; 1 trial, 103 participants; moderate certainty evidence) (table 2, table 3, and supplementary files 11-13).
Fig 2.
Effect of muscle relaxants compared with control on pain intensity (0-100 scale) at immediate term (≤2 weeks) post-randomisation for adults with low back pain. Negative values for mean differences indicate that effects favour muscle relaxants compared with control, whereas negative values for trial observations indicate change from baseline
Table 2.
Summary of findings and certainty of evidence for low back pain in association with muscle relaxants
| Summary of findings | Certainty of evidence | Overall certainty of evidence | ||||||
|---|---|---|---|---|---|---|---|---|
| No of participants (No of trials) | Mean difference (95% CI), 0-100 | Risk of bias | Inconsistency | Imprecision | Publication bias | |||
| Acute low back pain | ||||||||
| Non-benzodiazepine antispasmodics: | ||||||||
| ≤2 weeks | 4546 (16) | −7.7 (−12.1 to −3.3) | Downgraded* | Downgraded† | Downgraded‡ | Not downgraded | Very low | |
| 3-13 weeks | 612 (3) | 0.6 (−4.5 to 5.7) | Not downgraded | Not downgraded | Downgraded‡ | Not downgraded | Moderate | |
| Antispastics: | ||||||||
| ≤2 weeks | 103 (1) | −1.6 (−15.3 to 12.1) | Not downgraded | Not downgraded¶ | Downgraded§e | Not downgraded¶ | Low | |
| 3-13 weeks | 99 (1) | 4.0 (−7.7 to 15.7) | Not downgraded | Not downgraded¶ | Downgraded‡ | Not downgraded¶ | Moderate | |
| Benzodiazepines: | ||||||||
| ≤2 weeks | 112 (1) | 2.0 (−9.8 to 13.8) | Not downgraded | Not downgraded¶ | Downgraded‡ | Not downgraded¶ | Moderate | |
| 3-13 weeks | 103 (1) | −1.0 (−10.4 to 8.4) | Not downgraded | Not downgraded¶ | Downgraded§ | Not downgraded¶ | Low | |
| Subacute low back pain | ||||||||
| Miscellaneous: | ||||||||
| 3-13 weeks | 28 (1) | −19.0 (−41.9 to 3.9) | Downgraded* | Not downgraded¶ | Downgraded§ | Not downgraded¶ | Very low | |
| Chronic low back pain | ||||||||
| Antispastics: | ||||||||
| 3-13 weeks | 80 (1) | −5.4 (−13.7 to 2.9) | Downgraded* | Not downgraded | Downgraded§ | Not downgraded¶ | Very low | |
| Miscellaneous: | ||||||||
| 3-13 weeks | 52 (1) | −19.9 (−31.5 to −8.3) | Not downgraded | Not downgraded¶ | Downgraded‡ | Not downgraded¶ | Moderate | |
| Mixed low back pain | ||||||||
| Non-benzodiazepine antispasmodics: | ||||||||
| ≤2 weeks | 617 (2) | −4.4 (−6.9 to −1.9) | Downgraded* | Not downgraded | Not downgraded | Not downgraded | Low | |
| 3-13 weeks | 329 (1) | −5.8 (−13.8 to 2.2) | Downgraded* | Not downgraded¶ | Downgraded§ | Not downgraded¶ | Very low | |
Data are mean differences for pain intensity on a 0 to 100 scale. Negative values for mean differences indicate that effects favour muscle relaxant medicines compared to control.
Downgraded two levels: >50% of participants were from studies at high risk of bias.
Downgraded one level: heterogeneity (I2) was >50%.
Downgraded one level: limits of the 95% confidence interval crossed the minimally clinically important difference or the null.
Downgraded two levels: limits of the 95% confidence interval crossed the minimally clinically important difference and the null.
Not downgraded: could not be determined with one study.
Table 3.
Summary of findings and certainty of evidence for disability from low back pain in association with muscle relaxants
| Summary of findings | Certainty of evidence | Overall certainty of evidence | ||||||
|---|---|---|---|---|---|---|---|---|
| No of participants (No of trials) | Mean difference (95% CI), 0-100 | Risk of bias | Inconsistency | Imprecision | Publication bias | |||
| Acute low back pain | ||||||||
| Non-benzodiazepine antispasmodics: | ||||||||
| ≤2 weeks | 2438 (7) | −3.3 (−7.3 to 0.7) | Downgraded* | Downgraded† | Downgraded‡ | Not downgraded | Very low | |
| 3-13 weeks | 422 (2) | 4.3 (−1.4 to 10.1) | Not downgraded | Not downgraded | Downgraded‡ | Not downgraded | Moderate | |
| Antispastics: | ||||||||
| ≤2 weeks | 103 (1) | 2 (−15.6 to 19.6) | Not downgraded | Not downgraded§ | Downgraded¶ | Not downgraded§ | Low | |
| Benzodiazepines: | ||||||||
| ≤2 weeks | 112 (1) | 0 (−13.2 to 13.2) | Not downgraded | Not downgraded§ | Downgraded¶ | Not downgraded§ | Low | |
| 3-13 weeks | 103 (1) | −6.9 (−12.1 to −1.7) | Not downgraded | Not downgraded§ | Downgraded‡ | Not downgraded§ | Moderate | |
| Chronic low back pain | ||||||||
| Antispastics: | ||||||||
| 3-13 weeks | 80 (1) | −3.2 (−8.3 to 1.8) | Downgraded* | Not downgraded | Downgraded‡ | Not downgraded§ | Very low | |
| Miscellaneous: | ||||||||
| 3-13 weeks | 52 (1) | −5.6 (−20.6 to 9.4) | Not downgraded | Not downgraded§ | Downgraded¶ | Not downgraded§ | Low | |
| Mixed low back pain | ||||||||
| Non-benzodiazepine antispasmodics: | ||||||||
| ≤2 weeks | 329 (1) | −19.2 (−27.7 to −10.7) | Downgraded* | Not downgraded§ | Not downgraded | Not downgraded§ | Low | |
Data are mean differences for disability on a 0 to 100 scale. Negative values for mean differences indicate that effects favour muscle relaxant medicines compared to control.
Downgraded two levels: >50% of participants were from studies at high risk of bias.
Downgraded one level: heterogeneity (I2) was >50%.
Downgraded one level: limits of the 95% confidence interval crossed the minimally clinically important difference or the null.
Downgraded two levels: limits of the 95% confidence interval crossed the minimally clinically important difference and the null.
Not downgraded: could not be determined with one study.
Table 4.
Summary of findings and certainty of evidence for acceptability of muscle relaxants for low back pain
| Summary of findings | Certainty of evidence | Overall certainty of evidence | ||||||
|---|---|---|---|---|---|---|---|---|
| No of participants (No of trials) | Relative risk* (95% CI) | Risk of bias | Inconsistency | Imprecision | Publication bias | |||
| Acute low back pain | ||||||||
| Non-benzodiazepine antispasmodics | 2834 (13) | 0.8 (0.6 to 1.1) | Downgraded† | Not downgraded | Downgraded‡ | Not downgraded | Very low | |
| Chronic low back pain | ||||||||
| Antispastics | 84 (1) | 1.6 (0.2 to 12.9) | Downgraded† | Not downgraded | Downgraded‡ | Not downgraded§ | Very low | |
| Miscellaneous | 101 (2) | 0.6 (0.2 to 1.7) | Not downgraded | Not downgraded | Downgraded‡ | Not downgraded | Moderate | |
Data are relative risk for acceptability. A relative risk <1 indicates that effects favour muscle relaxants compared with control.
Downgraded two levels: >50% of participants were from studies at high risk of bias.
Downgraded one level: limits of the 95% confidence interval crossed the null.
Not downgraded: could not be determined with one study.
Subacute low back pain—One trial determined the efficacy of muscle relaxants on pain intensity at short term follow-up for subacute low back pain. Miscellaneous muscle relaxants (botulinum toxin) were not associated with a reduction in pain intensity compared with control (−19.0, −41.9 to 3.9; one trial, 28 participants; very low certainty evidence) (table 2 and supplementary file 11).
Chronic low back pain—Two trials (three comparisons) determined the efficacy of muscle relaxants on pain intensity at short term follow-up for chronic low back pain. Three trials (four comparisons) determined the acceptability of muscle relaxants for chronic low back pain. Evidence ranging from moderate to very low certainty showed no benefit compared with control for antispastic and miscellaneous muscle relaxants for pain, disability, and acceptability except for pain intensity at 3-13 weeks with miscellaneous muscle relaxants (eszoplicone) (−19.9, −31.5 to −8.3; 1 trial, 52 participants; moderate certainty evidence) (table 2, table 3, table 4, and supplementary files 11, 13, and 14).
Mixed low back pain—Two trials (two comparisons) determined the efficacy of muscle relaxants at immediate term follow-up and one trial at short term follow-up for mixed low back pain. Non-benzodiazepine antispasmodics were associated with a reduction in pain intensity at two weeks or less compared with control (−4.4, −6.9 to −1.9; 2 trials, 617 participants; low certainty) but not at 3-13 weeks (−5.8, −13.8 to 2.2; 1 trial, 329 participants; very low certainty evidence) (fig 2, table 2, and supplementary file 11). Non-benzodiazepine antispasmodics were associated with a reduction in disability at two weeks or less compared with control (−19.2, −27.7 to −10.7; 1 trial, 329 participants; low certainty evidence) (table 3 and supplementary file 12).
Safety
Acute low back pain—Twenty two trials (28 comparisons) determined the safety of muscle relaxants for acute low back pain. The type and reporting of adverse and serious adverse events varied across trials. Compared with control, non-benzodiazepine antispasmodics were associated with an increase in the risk of an adverse event (relative risk 1.6, 95% confidence interval 1.2 to 2.0; 16 trials, 3404 participants; low certainty evidence) but not a serious adverse event (2.3, 0.3 to 20.8; 2 trials, 830 participants; very low certainty evidence) (table 5 and supplementary files 15 and 16). Antispastic drugs were also associated with an increase in the risk of an adverse event (2.0, 1.1 to 3.8; 2 trials, 290 participants; moderate certainty evidence) whereas benzodiazepines were not (1.8, 0.9 to 3.6; 2 trials, 159 participants; low certainty evidence) (table 5 and supplementary file 15). Participants receiving antispastics were more likely to discontinue treatment owing to an adverse event (34.6, 2.1 to 568.0; 1 trial, 195 participants; very low certainty evidence) whereas participants receiving non-benzodiazepine antispasmodics were not (1.5, 0.6 to 3.5; 5 trials, 1641 participants; very low certainty evidence) (table 5 and supplementary file 17).
Table 5.
Summary of findings and certainty of evidence for safety of muscle relaxants for low back pain
| Summary of findings | Certainty of evidence | Overall certainty of evidence | ||||||
|---|---|---|---|---|---|---|---|---|
| No of participants (No of trials) | Relative risk* (95% CI) | Risk of bias | Inconsistency | Imprecision | Publication bias | |||
| Adverse events | ||||||||
| Acute low back pain: | ||||||||
| Non-benzodiazepine antispasmodics | 3404 (16) | 1.6 (1.2 to 2.0) | Downgraded† | Not downgraded | Not downgraded | Not downgraded | Low | |
| Antispastics | 290 (2) | 2.0 (1.1 to 3.8) | Downgraded‡ | Not downgraded | Not downgraded | Not downgraded | Moderate | |
| Benzodiazepines | 159 (2) | 1.8 (0.9 to 3.6) | Downgraded‡ | Not downgraded | Downgraded§ | Not downgraded | Low | |
| Chronic low back pain: | ||||||||
| Miscellaneous | 95 (2) | 1.5 (0.4 to 5.7) | Not Downgraded | Not downgraded | Downgraded§ | Not downgraded | Moderate | |
| Mixed low back pain: | ||||||||
| Non-benzodiazepine antispasmodics | 329 (1) | 1.6 (0.6 to 4.3) | Downgraded† | Not downgraded¶ | Downgraded§ | Not downgraded¶ | Very low | |
| Serious adverse events | ||||||||
| Acute low back pain: | ||||||||
| Non-benzodiazepine antispasmodics | 830 (2) | 2.3 (0.3 to 20.8) | Downgraded† | Not downgraded | Downgraded§ | Not downgraded | Very low | |
| Tolerability | ||||||||
| Acute low back pain: | ||||||||
| Non-benzodiazepine antispasmodics | 1641 (5) | 1.5 (0.6 to 3.5) | Downgraded† | Not downgraded | Downgraded§ | Not downgraded | Very low | |
| Antispastics | 195 (1) | 34.6 (2.1 to 568.0) | Downgraded† | Not downgraded¶ | Downgraded§ | Not downgraded | Very low | |
Data are relative risk for adverse and serious adverse events. A relative risk <1 indicates that effects favour muscle relaxants compared with control.
Downgraded two levels: >50% of participants were from studies at high risk of bias.
Downgraded one level: >25% but <50% of participants were from studies at high risk of bias.
Downgraded one level: limits of the 95% confidence interval crossed the null.
Not downgraded: could not be determined with one study.
Chronic low back pain—Two trials (two comparisons) determined the safety of muscle relaxants for chronic low back pain. Compared with control, no difference was found in the risk of experiencing an adverse event with miscellaneous muscle relaxants (1.5, 0.4 to 5.7; 2 trials, 95 participants; moderate certainty evidence) (table 5 and supplementary file 15).
Mixed low back pain—One trial determined the safety of muscle relaxants for mixed low back pain. Compared with control, no difference was found in the risk of experiencing an adverse event with non-benzodiazepine antispasmodics (1.6, 0.6 to 4.3; 1 trial, 329 participants; very low certainty evidence) (table 5 and supplementary file 15).
Subgroup and sensitivity analyses
Supplementary files 18-20 present detailed results for the subgroup, funnel plot, and sensitivity analyses, respectively. Owing to a lack of data, sensitivity analyses were only conducted for non-benzodiazepine antispasmodics for acute low back pain. The sensitivity analyses did not explain heterogeneity in the disability (≤2 weeks), acceptability, adverse events, and tolerability outcomes. When trials from clinical trial registries (mean difference −10.2, 95% confidence interval −15.6 to −4.7) or trials without a placebo comparator (−11, −17 to −5.1) were excluded, the estimated effect for pain intensity (≤2 weeks) changed to within the minimum clinically important difference. When excluding trials at high risk of bias, however, the effect decreased to zero (0.2, −4.9 to 5.4).
Discussion
We found very low certainty evidence that non-benzodiazepine antispasmodic drugs might reduce pain intensity at two weeks or less for patients with acute low back pain. This effect is small—less than 8 points on a 0–100 point scale—and does not meet common thresholds to be clinically meaningful. Non-benzodiazepine antispasmodics might have little to no effect on pain intensity at 3-13 weeks or on disability at all follow-up time points; however, the certainty of evidence ranged from moderate to very low. No trials evaluated the effect of muscle relaxants on long term outcomes. Low and very low certainty evidence showed that non-benzodiazepine antispasmodics might increase the risk of adverse events and might have little to no effect on treatment discontinuation, respectively. The number of trials investigating other muscle relaxants was small. The certainty of evidence was reduced because a large number of trials were at high risk of bias.
Strengths and weaknesses of this review
This systematic review was prospectively registered and reported in line with PRISMA.21 We included a broad scope of licensed muscle relaxants evaluated in randomised controlled trials as they provide the best evidence on the efficacy and safety of currently used muscle relaxants in clinical practice. We included findings from 49 trials of muscle relaxants for low back pain published up until 23 February 2021. To assess study level risk of bias we used the Cochrane risk of bias tool and published recommendations from the Cochrane Back and Neck Group34 and we evaluated the certainty of the evidence using the GRADE system.20 Finally, unlike with previous reviews,17 95 we searched clinical trial registries for relevant trials.
Inadequate reporting and authors’ failure to respond to data requests meant some relevant trials were not included in each meta-analysis. We restricted the inclusion of studies based on publication language. Although we included trials published in English, Italian, Portuguese, Spanish, German, and Dutch, we could have missed some relevant trials. We relied on the definition of adverse and serious adverse events as reported from the included trials, therefore definitions might have varied between trials. We included trials in which participants received co-administered additional analgesics. Although most included trials compared drugs with placebo, the inclusion of other analgesics could have influenced the interpretation of findings. Finally, interpretation of the outcome acceptability has limitations because participants could discontinue treatment for any reason, including recovery.
Evidence update
Our review updates the evidence for use of muscle relaxants in adults with low back pain. For example, we included 31 trials (6505 participants) in the quantitative analysis, compared with 15 trials (3362 participants) in the most recent systematic review.17 Although the previous systematic reviews17 95 and three clinical practice guidelines13 endorse non-benzodiazepine antispasmodics as an effective treatment for acute low back pain, we found considerable uncertainty in their clinical effectiveness and safety.
For this review we pooled data for all non-benzodiazepine antispasmodics according to the shared clinical utility in managing acute low back pain.8 The choice of muscle relaxant and frequency of prescription by a GP varies between countries.9 10 For example carisoprodol, commonly prescribed in the US,12 is no longer marketed in several Europeans countries96 or the UK97 because of an increased risk of misuse and dependency. We repeated the primary analyses after exclusion of trials that investigated carisoprodol and found that effects were comparable.
Meaning of the study
Although the observed effect of non-benzodiazepine antispasmodics in reducing pain compared with control at two weeks or less was statistically significant, the magnitude of the effect was too small to be considered clinically important. The upper limits of the confidence interval do not, however, exclude a clinically meaningful effect on pain intensity. The modest overall effect is reported at group level, which could still mean that some, but not all, individuals gain a worthwhile benefit.98
We identified important heterogeneity in the effect of non-benzodiazepine antispasmodics on pain, with the prediction interval spanning −26.5 to 11.1. Heterogeneity might have increased because of the inclusion of trials reporting unusually large effects, such as that of Hindle et al published in 197259 (−48.5, −70.7 to −26.3), where the placebo comparison showed no change from baseline, which is atypical of the clinical course of recovery for patients with acute low back pain.99 The subgroup and sensitivity analyses were unable to explain the heterogeneity observed in the pooled effect for pain. Restricting the analysis to a comparison of non-benzodiazepine antispasmodics with placebo showed a statistically significant effect greater than the threshold for minimally clinical important difference. As the removal of high risk of bias trials reduced the effect to zero, however, we advise caution when interpreting these findings.
Implications for clinical practice and policy
International clinical practice guidelines provide conflicting recommendations for the use of muscle relaxants11; of 15 clinical practice guidelines, six recommend muscle relaxants to manage low back pain, five do not recommend them, and four do not offer a recommendation.13 Our review shows uncertainty of evidence for the efficacy and safety of muscle relaxants. Although non-benzodiazepine antispasmodics might reduce pain intensity at two weeks or less for acute low back pain, the effect is unlikely to be considered clinically important. In addition, non-benzodiazepine antispasmodics could increase the risk of an adverse event being reported (commonly, dizziness, drowsiness, headache, and nausea100), but might have little to no effect on treatment discontinuation, suggesting the treatment and increased risk of adverse events are acceptable. The low to very low certainty of evidence does not, however, allow any firm recommendations. We would encourage clinicians to discuss this uncertainty in the efficacy and safety of muscle relaxants with patients, sharing information about the possibility for a worthwhile benefit in pain reduction but increased risk of experiencing a non-serious adverse event, to allow them to make informed treatment decisions.
Unanswered questions and future research
Large, definitive, placebo controlled trials are urgently needed to evaluate the efficacy and safety of muscle relaxants. New trials should follow the core outcome set for non-specific low back pain101 and the recommendations of the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials,102 to improve the pooling of results and comparability between trials. Future trials should also endeavour to adhere to methodological safeguards to reduce bias and transparently report results following the Consolidated Standards of Reporting Trials statement.103
Although muscle relaxants are typically prescribed for short term use, the effects of long term use are not known. High quality data are required to evaluate the efficacy and safety of long term use. This is particularly important when considering that the risk of dependency and misuse associated with muscle relaxants has been observed from indirect evidence.95 100
Conclusions
This systematic review found very low certainty evidence that non-benzodiazepine antispasmodics for the treatment of acute low back pain might provide a small and not clinically meaningful improvement in pain intensity at two weeks or less. The risk of adverse events but not serious adverse events might be increased with use of non-benzodiazepine antispasmodics, although the evidence ranges from low to very low certainty. Large, high quality, placebo controlled trials are urgently needed to resolve uncertainties about the efficacy and safety of muscle relaxants for low back pain.
What is already known on this topic
Muscle relaxants are the third most frequently prescribed drugs for low back pain
Clinical practice guidelines provide conflicting recommendations for the use of muscle relaxants to treat low back pain
What this study adds
Very low certainty evidence shows that non-benzodiazepine antispasmodics might offer a small, non-clinically important reduction in pain intensity at two weeks or less for acute low back pain
Low and very low certainty evidence shows non-benzodiazepine antispasmodics might increase the risk of adverse events and might have little to no effect on treatment discontinuation compared with control, respectively
Large, definitive, placebo controlled trials are urgently needed to resolve uncertainties about the efficacy and safety of muscle relaxants
Web extra.
Extra material supplied by authors
Supplementary information: files 1-20
Contributors: TF, MKB, and JHM conceived the study. TF, MKB, MAW, MCF, SMS, SMG, RD, and JHM contributed to the study design and protocol development. TF conducted the search. AGC, TF, MKB, MDJ, MAW, MCF, RRNR, and HBL selected the studies, extracted data, and did the quality appraisal. AGC and MAW analysed the data. AGC, MAW, and JHM had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. AGC wrote the first draft of the manuscript. All authors provided substantive feedback on the manuscript and have read and approved the final version. AGC is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. AGC is supported by the University of New South Wales (UNSW) Prince of Wales Clinical School postgraduate research scholarship and a Neuroscience Research Australia (NeuRA) PhD candidature supplementary scholarship. MKB is supported by a NeuRA PhD candidature scholarship and supplementary scholarship and was additionally funded during this work by an Australian government research training programme scholarship and a UNSW research excellence award. MAW is supported by a university postgraduate award and School of Medical Sciences top-up scholarship from UNSW, and a postgraduate scholarship from the National Health and Medical Research Council of Australia. MCF is supported by an Australian medical research future fund grant (GNTID1170205). HBL is supported by an Australian government postgraduate award. RRNR is supported by UNSW School of Medical Sciences postgraduate research scholarship and a NeuRA PhD candidature supplementary scholarship. SMS receives salary support from the National Health and Medical Research Council of Australia (#1105040). SMG is supported by the Rebecca L Cooper Medical Research Foundation. JHM receives project funding support from the National Health and Medical Research Council and the Medical Research Future Fund of Australia.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author (AGC) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: We will disseminate our findings to patient organisations and through traditional media and social media outlets.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Ethical approval: Not required.
Data availability statement
Data sharing: The dataset used and analysed during this study and the accompanying code are available from the corresponding author on reasonable request.
References
- 1. James SL, Abate D, Abate KH, et al. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-858. 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dieleman JL, Cao J, Chapin A, et al. US Health Care Spending by Payer and Health Condition, 1996-2016. JAMA 2020;323:863-84. 10.1001/jama.2020.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deyo RA, Weinstein JN. Low back pain. N Engl J Med 2001;344:363-70. 10.1056/NEJM200102013440508. [DOI] [PubMed] [Google Scholar]
- 4. Bernstein IA, Malik Q, Carville S, Ward S. Low back pain and sciatica: summary of NICE guidance. BMJ 2017;356:i6748. 10.1136/bmj.i6748. [DOI] [PubMed] [Google Scholar]
- 5. Gore M, Tai KS, Sadosky A, Leslie D, Stacey BR. Use and costs of prescription medications and alternative treatments in patients with osteoarthritis and chronic low back pain in community-based settings. Pain Pract 2012;12:550-60. 10.1111/j.1533-2500.2012.00532.x. [DOI] [PubMed] [Google Scholar]
- 6. Hart OR, Uden RM, McMullan JE, Ritchie MS, Williams TD, Smith BH. A study of National Health Service management of chronic osteoarthritis and low back pain. Prim Health Care Res Dev 2015;16:157-66. 10.1017/S1463423614000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ivanova JI, Birnbaum HG, Schiller M, Kantor E, Johnstone BM, Swindle RW. Real-world practice patterns, health-care utilization, and costs in patients with low back pain: the long road to guideline-concordant care. Spine J 2011;11:622-32. 10.1016/j.spinee.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Trevor A. Katzung BG, Knuidering-Hall M. Skeletal Muscle Relaxants. In: Katzung & Trevor’s Pharmacology: Examination & Board Review. McGraw-Hill Education, 2018. [Google Scholar]
- 9. Mafi JN, McCarthy EP, Davis RB, Landon BE. Worsening trends in the management and treatment of back pain. JAMA Intern Med 2013;173:1573-81. 10.1001/jamainternmed.2013.8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Michaleff ZA, Harrison C, Britt H, Lin CW, Maher CG. Ten-year survey reveals differences in GP management of neck and back pain. Eur Spine J 2012;21:1283-9. 10.1007/s00586-011-2135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schreijenberg M, Koes BW, Lin CC. Guideline recommendations on the pharmacological management of non-specific low back pain in primary care - is there a need to change? Expert Rev Clin Pharmacol 2019;12:145-57. 10.1080/17512433.2019.1565992. [DOI] [PubMed] [Google Scholar]
- 12. Soprano SE, Hennessy S, Bilker WB, Leonard CE. Assessment of Physician Prescribing of Muscle Relaxants in the United States, 2005-2016. JAMA Netw Open 2020;3:e207664. 10.1001/jamanetworkopen.2020.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oliveira CB, Maher CG, Pinto RZ, et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J 2018;27:2791-803. 10.1007/s00586-018-5673-2. [DOI] [PubMed] [Google Scholar]
- 14. Qaseem A, Wilt TJ, McLean RM, Forciea MA, Clinical Guidelines Committee of the American College of Physicians . Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med 2017;166:514-30. 10.7326/M16-2367. [DOI] [PubMed] [Google Scholar]
- 15.Van Wambeke P, Desomer A, Ailliet L, et al. Low back pain and radicular pain: assessment and management. KCE Report 287. Belgian Health Care Knowledge Centre, 2017. https://kce.fgov.be/sites/default/files/atoms/files/KCE_287_Low_back_pain_Report.pdf
- 16.National Institute for Health and Care Excellence. Low Back Pain and Sciatica in Over 16s: Assessment and Management. NICE, 2016. https://www.nice.org.uk/guidance/ng59 [PubMed]
- 17. Abdel Shaheed C, Maher CG, Williams KA, McLachlan AJ. Efficacy and tolerability of muscle relaxants for low back pain: Systematic review and meta-analysis. Eur J Pain 2017;21:228-37. 10.1002/ejp.907. [DOI] [PubMed] [Google Scholar]
- 18. Bagg MK, O’Hagan E, Zahara P, et al. Systematic reviews that include only published data may overestimate the effectiveness of analgesic medicines for low back pain: a systematic review and meta-analysis. J Clin Epidemiol 2020;124:149-59. 10.1016/j.jclinepi.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 19. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401-6. 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 20. Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet 2017;389:736-47. 10.1016/S0140-6736(16)30970-9. [DOI] [PubMed] [Google Scholar]
- 23.WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index. 2020. https://www.whocc.no/atc_ddd_index/
- 24.U.S. Food and Drug Administration. Drugs@FDA. 2020. https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases
- 25. European Medicines Agency . Medicines. 2020. https://www.ema.europa.eu/en/medicines
- 26.Australian Government Department of Health Therapeutic Goods Administration. Australian Register of Therapeutic Goods. 2020. https://www.tga.gov.au/australian-register-therapeutic-goods
- 27. Koes BW, van Tulder MW, Peul WC. Diagnosis and treatment of sciatica. BMJ 2007;334:1313-7. 10.1136/bmj.39223.428495.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chiarotto A, Deyo RA, Terwee CB, et al. Core outcome domains for clinical trials in non-specific low back pain. Eur Spine J 2015;24:1127-42. 10.1007/s00586-015-3892-3. [DOI] [PubMed] [Google Scholar]
- 29. Machado GC, Maher CG, Ferreira PH, Day RO, Pinheiro MB, Ferreira ML. Non-steroidal anti-inflammatory drugs for spinal pain: a systematic review and meta-analysis. Ann Rheum Dis 2017;76:1269-78. 10.1136/annrheumdis-2016-210597. [DOI] [PubMed] [Google Scholar]
- 30. Abdel Shaheed C, Maher CG, Williams KA, Day R, McLachlan AJ. Efficacy, Tolerability, and Dose-Dependent Effects of Opioid Analgesics for Low Back Pain: A Systematic Review and Meta-analysis. JAMA Intern Med 2016;176:958-68. 10.1001/jamainternmed.2016.1251. [DOI] [PubMed] [Google Scholar]
- 31. Ferreira GE, McLachlan AJ, Lin CC, et al. Efficacy and safety of antidepressants for the treatment of back pain and osteoarthritis: systematic review and meta-analysis. BMJ 2021;372:m4825. 10.1136/bmj.m4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Wiley, 2019, https://training.cochrane.org/handbook/current 10.1002/9781119536604. [DOI] [Google Scholar]
- 33. Higgins JPT, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group. Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Furlan AD, Malmivaara A, Chou R, et al. Editorial Board of the Cochrane Back, Neck Group . 2015 updated method guideline for systematic reviews in the Cochrane Back and Neck Group. Spine (Phila Pa 1976) 2015;40:1660-73. 10.1097/BRS.0000000000001061. [DOI] [PubMed] [Google Scholar]
- 35. Furukawa TA, Salanti G, Atkinson LZ, et al. Comparative efficacy and acceptability of first-generation and second-generation antidepressants in the acute treatment of major depression: protocol for a network meta-analysis. BMJ Open 2016;6:e010919. 10.1136/bmjopen-2015-010919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hultcrantz M, Rind D, Akl EA, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol 2017;87:4-13. 10.1016/j.jclinepi.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105-21. 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 38. Ostelo RWJG, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976) 2008;33:90-4. 10.1097/BRS.0b013e31815e3a10. [DOI] [PubMed] [Google Scholar]
- 39. Busse JW, Bartlett SJ, Dougados M, et al. Optimal strategies for reporting pain in clinical trials and systematic reviews: Recommendations from an OMERACT 12 workshop. J Rheumatol 2015;42:1962-70. 10.3899/jrheum.141440. [DOI] [PubMed] [Google Scholar]
- 40. Ferreira ML, Herbert RD, Ferreira PH, et al. The smallest worthwhile effect of nonsteroidal anti-inflammatory drugs and physiotherapy for chronic low back pain: a benefit-harm trade-off study. J Clin Epidemiol 2013;66:1397-404. 10.1016/j.jclinepi.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 41. Chou R, Deyo R, Friedly J, et al. Systemic pharmacologic therapies for low back pain: A systematic review for an American College of physicians clinical practice guideline. Ann Intern Med 2017;166:480-92. 10.7326/M16-2458. [DOI] [PubMed] [Google Scholar]
- 42. Harrer M, Cuijpers P, Furukawa TA, et al. Doing meta-analysis in R: A hands-on guide. Prot Lab Erlangen, 2019. [Google Scholar]
- 43. Prescriber’s Digital Reference . PDR LLC. 2020. https://www.pdr.net
- 44.MIMS. Monthly Index of Medical Specialities. 2020. https://www.mims.co.uk/
- 45.Australian Medicines Handbook. 2020 (online). Adelaide: Australian Medicines Handbook. 2020. https://amhonline.amh.net.au/
- 46. Akhter N, Siddiq MZ. Comparative efficacy of diclofenac sodium alone and in combination with thiocolchicoside in patients with low back pain. Med Forum 2017;28:93-6. [Google Scholar]
- 47. Aksoy C, Karan A, Diraçoǧlu D. Low back pain: Results of an open clinical trial comparing the standard treatment alone to the combination of standard treatment and thiocolchicoside. J Orthop Traumatol 2002;3:103-8. 10.1007/s101950200036. [DOI] [Google Scholar]
- 48. Emrich OMD, Milachowski KA, Strohmeier M. Methocarbamol bei akuten Rückenschmerzen: Eine randomisierte, doppelblinde, placebokontrollierte Studie. [Methocarbamol in acute low back pain. A randomized double-blind controlled study.] MMW Fortschr Med 2015;157(Suppl 5):9-16. 10.1007/s15006-015-3307-x. [DOI] [PubMed] [Google Scholar]
- 49. Fathie K. A second look at skeletal muscle relaxant: a double-blind study with metaxalone. Curr Ther Res Clin Exp 1964;6:677-83. [PubMed] [Google Scholar]
- 50. Foster L, Clapp L, Erickson M, Jabbari B. Botulinum toxin A and chronic low back pain: a randomized, double-blind study. Neurology 2001;56:1290-3. 10.1212/WNL.56.10.1290. [DOI] [PubMed] [Google Scholar]
- 51. Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: A randomized clinical trial. JAMA 2015;314:1572-80. 10.1001/jama.2015.13043. [DOI] [PubMed] [Google Scholar]
- 52. Friedman BW, Irizarry E, Solorzano C, et al. Diazepam Is No Better Than Placebo When Added to Naproxen for Acute Low Back Pain. Ann Emerg Med 2017;70:169-176.e1. 10.1016/j.annemergmed.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Friedman BW, Cisewski D, Irizarry E, et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Naproxen With or Without Orphenadrine or Methocarbamol for Acute Low Back Pain. Ann Emerg Med 2018;71:348-356.e5. 10.1016/j.annemergmed.2017.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Friedman BW, Irizarry E, Solorzano C, et al. A Randomized, Placebo-Controlled Trial of Ibuprofen Plus Metaxalone, Tizanidine, or Baclofen for Acute Low Back Pain. Ann Emerg Med 2019;74:512-20. 10.1016/j.annemergmed.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goforth HW, Preud’homme XA, Krystal ADA. A randomized, double-blind, placebo-controlled trial of eszopiclone for the treatment of insomnia in patients with chronic low back pain. Sleep 2014;37:1053-60. 10.5665/sleep.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gold RH. Orphenadrine citrate: sedative or muscle relaxant? Clin Ther 1978;1:451-3. [Google Scholar]
- 57. Herskowitz A. BOTOX (Botulinum Toxin Type A) treatment of patients with sub-acute low back pain: A randomized, double blind, placebo-controlled study. J Pain 2004;5:S62. 10.1016/j.jpain.2004.02.214. [DOI] [Google Scholar]
- 58. Aparna P, Geetha P, Shanmugasundaram P. Comparison of aceclofenac and combination (Aceclofenac + thiocolchicoside) therapy in acute low back pain patients. Res J Pharm Technol 2016;9:1927-9. 10.5958/0974-360X.2016.00394.2. [DOI] [Google Scholar]
- 59. Hindle TH, 3rd, Palma L. Comparison of carisoprodol, butabarbital, and placebo in treatment of the low back syndrome. Calif Med 1972;117:7-11. [PMC free article] [PubMed] [Google Scholar]
- 60. Hingorani K. Diazepam in backache. A double-blind controlled trial. Ann Phys Med 1966;8:303-6. [PubMed] [Google Scholar]
- 61. Jazayeri SM, Ashraf A, Fini HM, Karimian H, Nasab MV. Efficacy of botulinum toxin type a for treating chronic low back pain. Anesth Pain Med 2011;1:77-80. 10.5812/aapm.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ketenci A, Ozcan E, Karamursel S. Assessment of efficacy and psychomotor performances of thiocolchicoside and tizanidine in patients with acute low back pain. Int J Clin Pract 2005;59:764-70. 10.1111/j.1742-1241.2004.00454.x. [DOI] [PubMed] [Google Scholar]
- 63. Klinger N, Wilson R, Kanniainen C, et al. Intravenous oprhenadrine for the treatment of lumbar paravertebral muscle strain. Curr Ther Res Clin Exp 1988;43:247-54. [Google Scholar]
- 64. Lepisto P. A Comparative Trial of DS 103-282 and Placebo in the Treatment of Acute Skeletal Muscle Spasms Due to Disorders of the Back. Curr Ther Res Clin Exp 1979;26:454-9. [Google Scholar]
- 65. Machado D, Kumar A, Jabbari B. Abobotulinum toxin A in the treatment of chronic low back pain. Toxins (Basel) 2016;8:E374. 10.3390/toxins8120374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Moll W. Zur Therapie akuter lumbovertebraler Syndrome durch optimale medikamentöse Muskelrelaxation mittels Diazepam [Therapy of acute lumbovertebral syndromes through optimal muscle relaxation using diazepam. Results of a double-blind study on 68 cases]. Med Welt 1973;24:1747-51. [PubMed] [Google Scholar]
- 67. Pareek A, Chandurkar N, Chandanwale AS, Ambade R, Gupta A, Bartakke G. Aceclofenac-tizanidine in the treatment of acute low back pain: a double-blind, double-dummy, randomized, multicentric, comparative study against aceclofenac alone. Eur Spine J 2009;18:1836-42. 10.1007/s00586-009-1019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ralph L, Look M, Wheeler W, Sacks H. Double-blind, placebo-controlled trial of carisoprodol 250-mg tablets in the treatment of acute lower-back spasm. Curr Med Res Opin 2008;24:551-8. 10.1185/030079908X261014. [DOI] [PubMed] [Google Scholar]
- 69. Baratta RR. A double-blind study of cyclobenzaprine and placebo in the treatment of acute musculoskeletal conditions of the low back. Curr Ther Res Clin Exp 1982;32:646-52. [Google Scholar]
- 70. Salvini S, Antonelli S, De Micheli G, et al. Dantrolene sodium in low back pain and cervico brachialgia treatment: a controlled study. Curr Ther Res Clin Exp 1986;39:172-7. [Google Scholar]
- 71. Schliessbach J, Vuilleumier PH, Siegenthaler A, et al. Analgesic effect of clobazam in chronic low-back pain but not in experimentally induced pain. Eur J Pain 2017;21:1336-45. 10.1002/ejp.1032. [DOI] [PubMed] [Google Scholar]
- 72. Serfer GT, Wheeler WJ, Sacks HJ. Randomized, double-blind trial of carisoprodol 250 mg compared with placebo and carisoprodol 350 mg for the treatment of low back spasm. Curr Med Res Opin 2010;26:91-9. 10.1185/03007990903382428. [DOI] [PubMed] [Google Scholar]
- 73. Tervo T, Petaja L, Lepisto P. A controlled clinical trial of a muscle relaxant analgesic combination in the treatment of acute lumbago. Br J Clin Pract 1976;30:62-4. [PubMed] [Google Scholar]
- 74.Thompson M, Kennedy G. Treatment of acute low back pain: compartive trial of two muscle relaxants, tizanidine and chlormezanone with placebo. Scandinavian Journal of Rheumatology. 2009;4-40. https://www.tandfonline.com/doi/abs/10.3109/03009748309118006
- 75. Tüzün F, Ünalan H, Öner N, et al. Multicenter, randomized, double-blinded, placebo-controlled trial of thiocolchicoside in acute low back pain. Joint Bone Spine 2003;70:356-61. 10.1016/S1297-319X(03)00075-7. [DOI] [PubMed] [Google Scholar]
- 76. Zaringhalam J, Manaheji H, Rastqar A, Zaringhalam M. Reduction of chronic non-specific low back pain: a randomised controlled clinical trial on acupuncture and baclofen. Chin Med 2010;5:15. 10.1186/1749-8546-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Berry H, Hutchinson DR. A multicentre placebo-controlled study in general practice to evaluate the efficacy and safety of tizanidine in acute low-back pain. J Int Med Res 1988;16:75-82. 10.1177/030006058801600201. [DOI] [PubMed] [Google Scholar]
- 78. Berry H, Hutchinson DR. Tizanidine and ibuprofen in acute low-back pain: results of a double-blind multicentre study in general practice. J Int Med Res 1988;16:83-91. 10.1177/030006058801600202. [DOI] [PubMed] [Google Scholar]
- 79. Borenstein DG, Lacks S, Wiesel SW. Cyclobenzaprine and naproxen versus naproxen alone in the treatment of acute low back pain and muscle spasm. Clin Ther 1990;12:125-31. [PubMed] [Google Scholar]
- 80. Casale R. Acute low back pain: Symptomatic treatment with a muscle relaxant drug. Clin J Pain 1988;4:81-8 10.1097/00002508-198806000-00004. [DOI] [Google Scholar]
- 81. Cogné M, Petit H, Creuzé A, Liguoro D, de Seze M. Are paraspinous intramuscular injections of botulinum toxin a (BoNT-A) efficient in the treatment of chronic low-back pain? A randomised, double-blinded crossover trial. BMC Musculoskelet Disord 2017;18:454. 10.1186/s12891-017-1816-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dapas F, Hartman SF, Martinez L, et al. Baclofen for the treatment of acute low-back syndrome. A double-blind comparison with placebo. Spine (Phila Pa 1976) 1985;10:345-9. 10.1097/00007632-198505000-00010. [DOI] [PubMed] [Google Scholar]
- 83. ACTRN12616000017426 . A randomised controlled feasibility study of managing sleep with Zopiclone in participants with acute low back pain and sleep disturbances. Australia New Zealand Clinical Trials Registry, 2016. [Google Scholar]
- 84.EUCTR2017-004530-29. No Title. Clinicaltrialsregister.eu. 2017.
- 85.NCT02887534. Evaluation of Efficacy and Safety of SPARC1401 in Acute Low Back Pain. ClinicalTrials.gov. 2016.
- 86.NCT01587508. Study Comparing A New Drug Containing The Combination Meloxicam And Cyclobenzaprine In The Treatment Of Acute Lumbago. ClinicalTrials.gov. 2012.
- 87.EUCTR2019-001885-14. No Title. Clinicaltrialsregister.eu. 2019.
- 88.IRCT20111109008035N4. The study of efficacy of melatonin, and zolpidem on the sleep quality, and severity of pain in the patients with chronic non-specific low back pain. ISRCTN. 2017.
- 89.NCT00671879. Study to Evaluate Two Formulations of Carisoprodol in Subjects With Musculoskeletal Spasm of the Lower Back. ClinicalTrials.gov. 2008.
- 90.NCT00671502. A Study to Evaluate Two Formulations of Carisoprodol in Subjects With Musculoskeletal Spasm of the Lower Back. ClinicalTrials.gov. 2008.
- 91.NCT00817986. A Study to Evaluate the Safety and Tolerability of Arbaclofen Placarbil (XP19986) in Subjects With Acute Back Spasms. ClinicalTrials.gov. 2009.
- 92.NCT00404417. Botulinum Toxin A for the Treatment of Chronic Lumbar Back Pain. ClinicalTrials.gov. 2006.
- 93.NCT00384579. Pilot Study to Assess the Efficacy of Botulinum Toxin B on Pain and Disability in Subjects With Acute Low Back Pain. ClinicalTrials.gov. 2006.
- 94.NCT00384371. Pilot Study to Assess the Efficacy of Botulinum Toxin A Treatments on Pain and Disability in Sub-Acute Low Back Pain. ClinicalTrials.gov. 2006.
- 95. van Tulder MW, Touray T, Furlan AD, Solway S, Bouter LM. Muscle relaxants for non-specific low back pain. Cochrane Database Syst Rev 2003;2017:CD004252. 10.1002/14651858.CD004252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bramness JG, Buajordet I, Skurtveit S. The role of pharmacoepidemiological studies in the market withdrawal of carisoprodol (Somadril®) in Europe. Nor Epidemiol 2008;18:167-72. 10.5324/nje.v18i2.29. [DOI] [Google Scholar]
- 97.Medicines and Health products Regulatory Agency. Carisoprodol and meprobamate: risks outweigh benefits. GOV.UK. 2014. https://www.gov.uk/drug-safety-update/carisoprodol-and-meprobamate-risks-outweigh-benefits
- 98. Underwood M, Tysall C. Antidepressants for musculoskeletal pain. BMJ 2021;372:n80. 10.1136/bmj.n80. [DOI] [PubMed] [Google Scholar]
- 99. Henschke N, Maher CG, Refshauge KM, et al. Prognosis in patients with recent onset low back pain in Australian primary care: inception cohort study. BMJ 2008;337:a171-171. 10.1136/bmj.a171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Witenko C, Moorman-Li R, Motycka C, et al. Considerations for the appropriate use of skeletal muscle relaxants for the management of acute low back pain. P T 2014;39:427-35. [PMC free article] [PubMed] [Google Scholar]
- 101. Chiarotto A, Deyo RA, Terwee CB, et al. Core outcome domains for clinical trials in non-specific low back pain. Eur Spine J 2015;24:1127-42. 10.1007/s00586-015-3892-3. [DOI] [PubMed] [Google Scholar]
- 102. Dworkin RH, Turk DC, Farrar JT, et al. IMMPACT . Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9-19. 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 103. Schulz KF, Altman DG, Moher D, CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: files 1-20
Data Availability Statement
Data sharing: The dataset used and analysed during this study and the accompanying code are available from the corresponding author on reasonable request.