Abstract
While anti-viral antibodies generally confer protective functions, antibodies against dengue virus (DENV) are associated with enhanced disease susceptibility. Antibodies can mediate DENV infection of leukocytes via Fcγ receptors, likely contributing to dengue disease pathogenesis. To determine if this mechanism accounts for variable disease severity, we examined Fab and Fc structures of anti-DENV antibodies from patients pre- and post-infection and with variable disease outcomes. Neither antibody titers nor neutralizing activity correlated with disease severity in DENV-infected populations. Rather, DENV infection induced a specific increase in IgG1 afucosylation and the levels of afucosylated IgG1 were predictive of dengue disease severity. Thus, the IgG1 fucosylation status represents a robust prognostic tool for dengue disease, highlighting the key role of the Fc glycan structure in dengue pathogenesis.
One Sentence Summary:
The antibody Fc glycan structure during secondary infection determines susceptibility to severe dengue disease.
Immune status to dengue virus (DENV) currently represents the greatest risk factor for hospitalization after a bite from a DENV-infected mosquito (1). Depending on the infecting DENV serotype, primary infection commonly leads to inapparent infection, but secondary infection can produce life-threatening symptoms (2, 3). A mismatch between the infecting serotype and the memory adaptive immunity is hypothesized to lead to exacerbated immune responses. Disease enhancement has been proposed to be mediated by pre-existing DENV-reactive IgG antibodies, which at subneutralizing levels promote infection of leukocytes. This phenomenon, termed antibody-dependent enhancement (ADE), is dependent on the interactions of the IgG Fc domain with Fcγ receptors (FcγRs) expressed on leukocytes (4).
Consistent with a pathogenic role for IgG antibodies in dengue, epidemiologic studies support that pre-existing anti-DENV titers are a key determinant for susceptibility to symptomatic disease (5, 6). Additional susceptibility factors likely exist, as <5% of patients with pre-existing anti-DENV IgGs develop severe disease. Given the dependence of DENV ADE on Fc–FcγR interactions, disease susceptibility may be determined by the affinity of these antibodies for specific FcγRs, the abundance of FcγR+-leukocytes, FcγR expression levels on leukocytes, and FcγR alleles (7–9). The affinity of the IgG molecule for the various FcγR types is dynamically regulated during an immune response and determined by the Fc domain protein sequence and the composition of the Fc-associated glycan (10) (Fig. 1A). Severe dengue patients are characterized by increased abundance of afucosylated IgG1 glycoforms, which exhibit higher affinity for the activating FcγRIIIa (11). However, whether afucosylated anti-DENV IgG is the result of secondary DENV infection, or their increased abundance truly represents a prognostic factor for susceptibility to severe dengue disease remains unknown.
Fig. 1. Hospitalized dengue disease cases exhibit elevated levels of afucosylated IgG1 glycoforms.
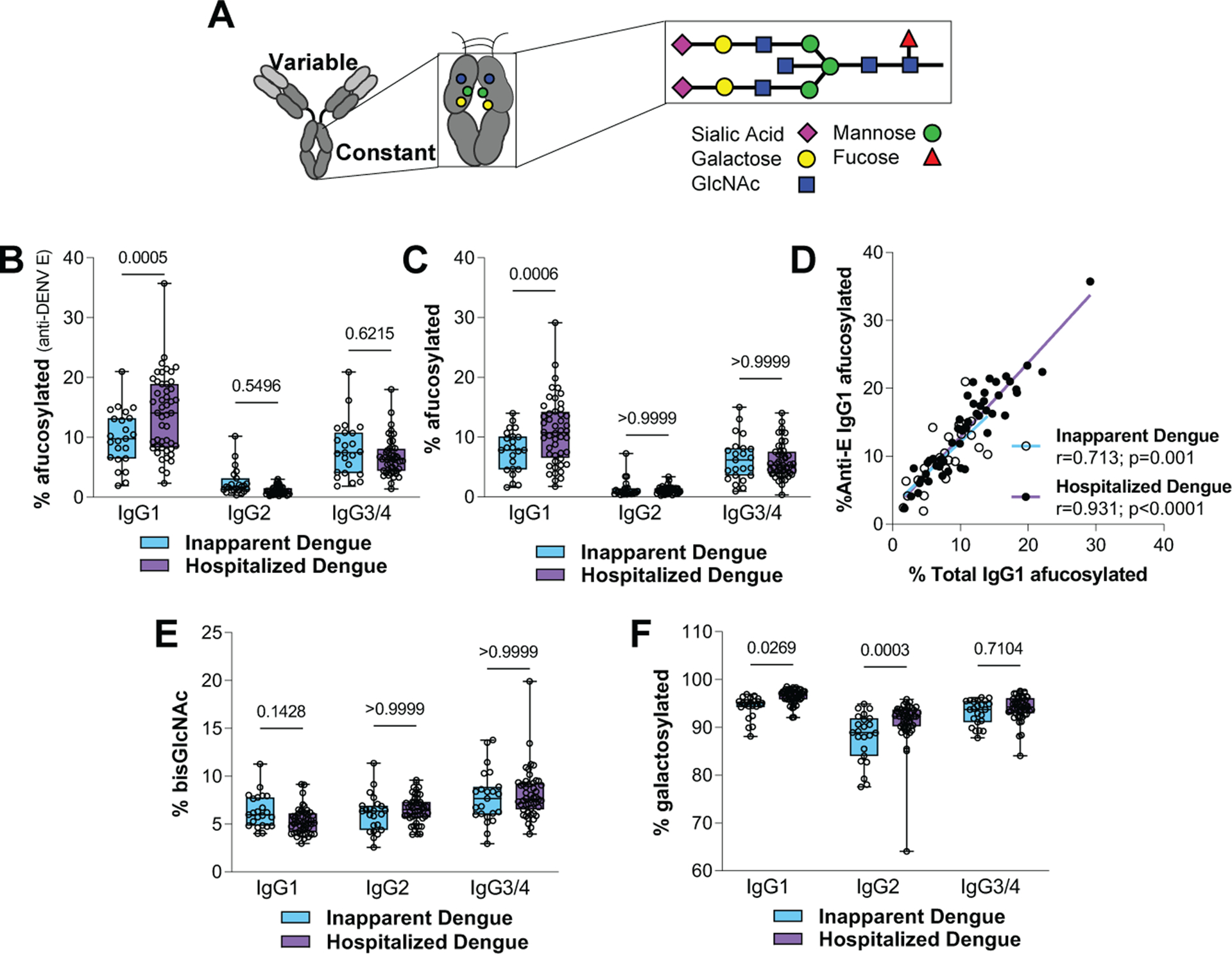
(A) Overview of the Fc-associated glycan. (B to C) Analysis of the levels of afucosylated IgG1glycoforms for (B) anti-DENV E-specific and (C) total IgGs from inapparent and hospitalized cases. (D) Correlation of the abundance of afucosylated IgG1 levels of total with DENV E-specific IgGs. (E) Bisecting GlcNAc and (F) galactosylated Fc glycoforms of IgGs from inapparent and hospitalized cases. One-way ANOVA/Bonferroni post-hoc for B, C, E, and F; Pearson correlation analysis for D.
To investigate the contribution of immune status and IgG Fc glycoforms to the development of severe disease, we analyzed the distribution of IgG subclasses and Fc-associated glycoforms from individuals with variable dengue disease severity (Fig. 1, fig. S1, and table S1). Hospitalized cases exhibited a global elevation in plasma levels of afucosylated IgG1 Fc glycoforms for both antigen-specific (anti-DENV E) and total IgGs (Fig. 1, B to D). Elevated levels of IgG1 afucosylation were also observed in hospitalized patients at the time of admission, confirming that these effects were not related to differences in sample timing and not induced in response to clinical management (Fig. S1A). No differences in afucosylated Fc levels were observed for the other IgG subclasses, suggesting the existence of subclass-specific regulatory mechanisms for Fc fucosylation, likely associated with the conditions that drive IgG class switch (Fig. 1, B and C). Hospitalized cases also exhibited elevated levels of IgG1 and IgG2 galactosylation (Fig. 1, E and F), which are expected to have limited biological significance (12). The global increase in IgG1 afucosylation raises the possibility of competition effects by non-antigen-specific IgGs, which may limit the Fc function of anti-DENV IgGs. However, such effects are expected to be minimal, as FcγRIIIa has low affinity for monomeric IgG1 (10). Indeed, in a model of mAb-mediated thrombocytopenia, the presence of excess, non-antigen-specific afucosylated IgG had no impact on cytotoxic anti-platelet mAb activity (fig. S2).
Hospitalized cases exhibited differential platelet and hematocrit (Hct) levels (Fig. 2, A and B) and were classified according to disease severity (13). Compared to dengue fever (DF), hemorrhagic fever (DHF) and shock syndrome (DSS) cases exhibited elevated levels of afucosylated IgG1 glycoforms (Fig. 2C), but no major differences were noted in the abundance of other Fc glycoforms (fig. S1, B to I). Among all hospitalized cases, the abundance of afucosylated IgG1 levels correlated with platelet levels and Hct (Fig. 2, D and E). To determine whether the increase in afucosylation truly represents a prognostic factor of disease severity or is the outcome of severe disease, we analyzed IgG samples from hospitalized patients obtained at the time of admission. Patients that developed DHF or DSS had significantly higher levels of afucosylated IgG1 glycoforms at admission compared to DF patients (Fig. 2F). ROC analysis also confirmed that IgG1 afucosylation levels at hospital admission are predictive of severe dengue disease (Fig. 2G).
Fig. 2. Afucosylation is associated with dengue disease severity and correlates with biological features of severe disease.
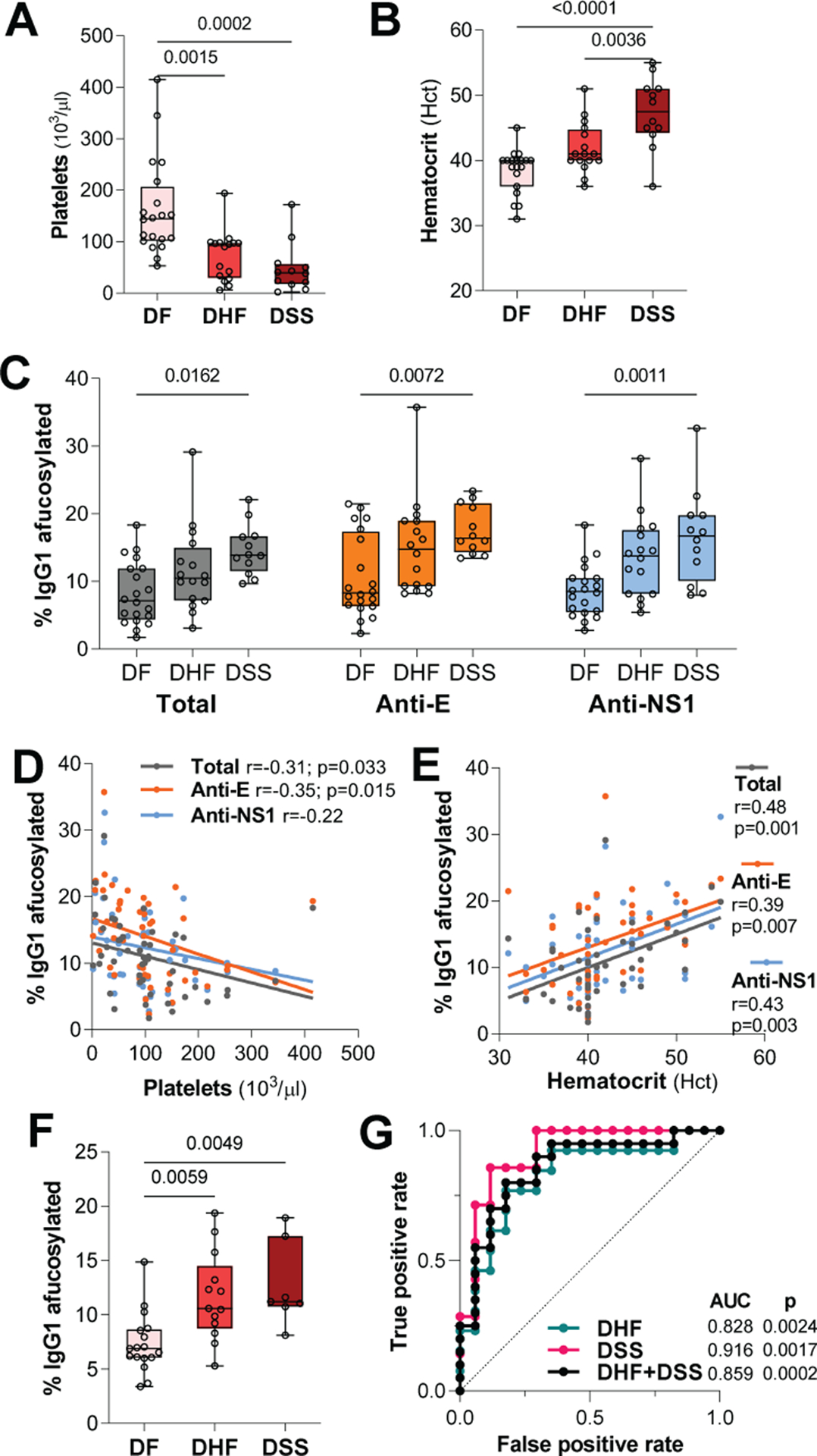
(A) Platelet counts and (B) Hct in hospitalized dengue patients with variable clinical classification. (C) Afucosylation levels for total, anti-E, and anti-NS1 IgG1 from dengue patients with variable disease severity. (D to E) Correlation of afucosylated IgG1 levels with platelets and Hct among hospitalized cases. (F) Abundance of afucosylated IgG1 glycoforms in plasma samples from hospitalized patients obtained at the time of admission. (G) ROC analysis assessing the predictive value of the levels of IgG1 afucosylation at admission for severe dengue disease. One-way ANOVA/Bonferroni post-hoc for A to C and F; Pearson correlation analysis for D to E.
Consistent with prior reports (6, 14), we also observed increased anti-DENV IgG levels (Fig. 3A) and frequency of secondary DENV infection (Fig. 3B) in hospitalized, compared to inapparent cases. When patients were stratified based on immune history, the elevated anti-DENV titers of hospitalized cases were found to be due to the higher frequency of secondary DENV infection in these patients. Secondary DENV infection was characterized by comparable anti-DENV IgG titers between inapparent and hospitalized cases, suggesting that neither the anti-DENV IgG titers nor the immune history alone can sufficiently predict dengue disease susceptibility (Fig. 3C). By contrast, IgG1 afucosylation was specifically elevated only in hospitalized, but not in inapparent cases with prior history of DENV infection (Fig. 3D and fig. S3, A to C). Likewise, IgG1 afucosylation among hospitalized patients was associated with platelet levels and Hct (Fig. 2, D and E), but no such association was observed for anti-DENV IgG titers (Fig. 3, E and F). Thus, IgG1 afucosylation, when combined with DENV immune status, represents a more sensitive and accurate determinant for dengue disease susceptibility and is associated with clinical severity of symptomatic dengue disease.
Fig. 3. Afucosylation, but not pre-existing IgG titers, are associated with dengue disease susceptibility and severity.
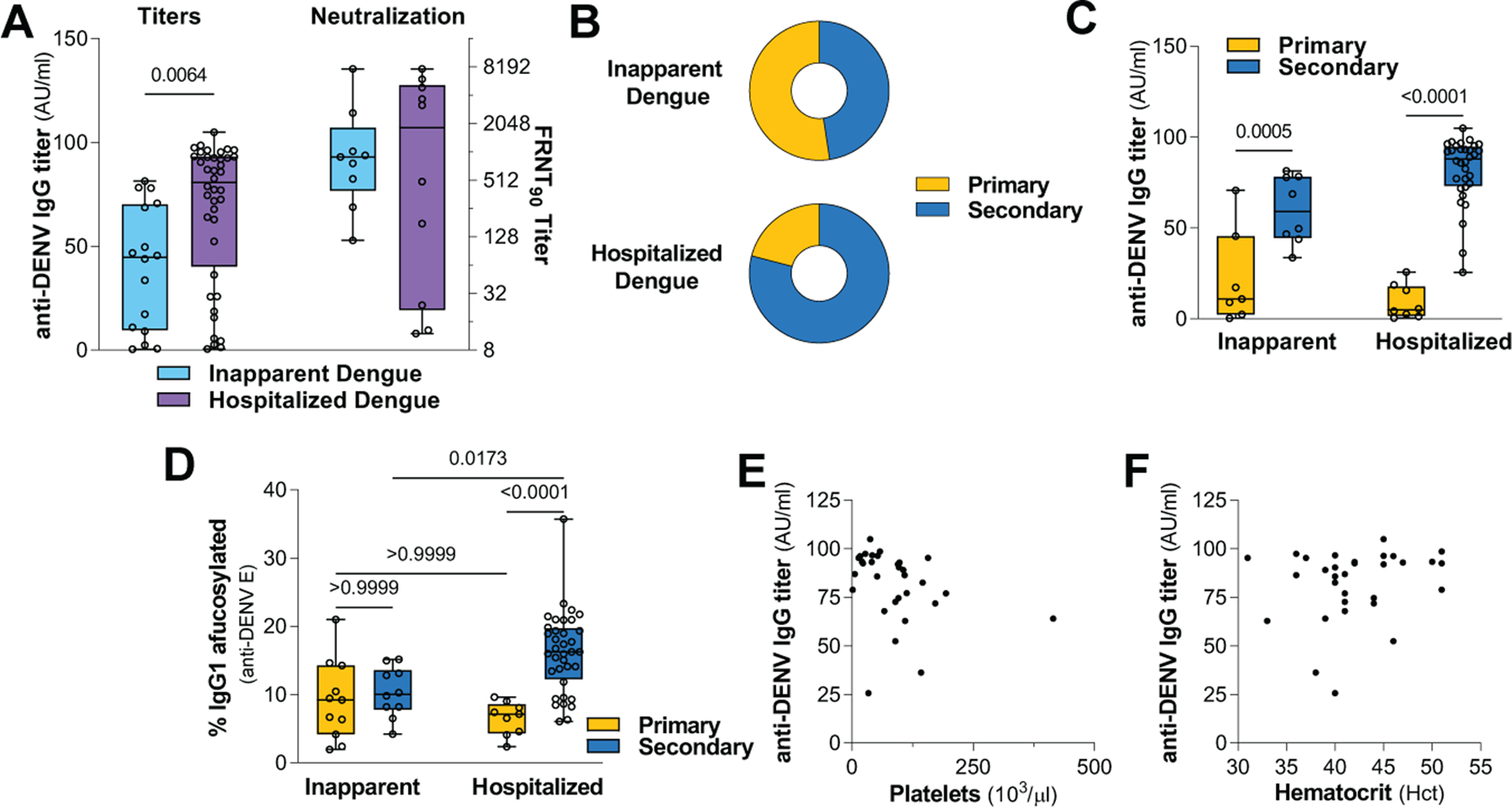
(A) Anti-DENV binding and neutralizing titers (against DENV-1 and DENV-2) and (B) DENV immune status of inapparent and hospitalized dengue cases. Unpaired two-tailed t test. (C) Anti-DENV IgG titers and (D) abundance of afucosylated anti-DENV E IgG1 in dengue cases stratified based on DENV immune status. One-way ANOVA/Bonferroni post-hoc. Correlation of anti-DENV IgG titers with (E) platelet levels and (F) Hct.
To assess whether it is the severity of the disease that is inducing higher afucosylation or afucosylated IgG antibodies are elicited upon secondary DENV exposure, we compared the levels of IgG1 afucosylation among patients with identical clinical classification (DF) but differential DENV immune history. DF patients with prior DENV exposure exhibited elevated IgG1 afucosylation levels, suggesting that immune history, rather disease severity, determines the IgG1 fucosylation status (Fig. 4A). Likewise, primary DF cases exhibited elevated levels of afucosylated IgG1 glycoforms at convalescence compared to the acute phase of infection. This effect was not observed in secondary cases, which had persistently high levels of IgG1 afucosylation both at the acute and at the convalescent phase (Fig. 4B and fig. S3D).
Fig. 4. DENV infection specifically modulates IgG Fc fucosylation.
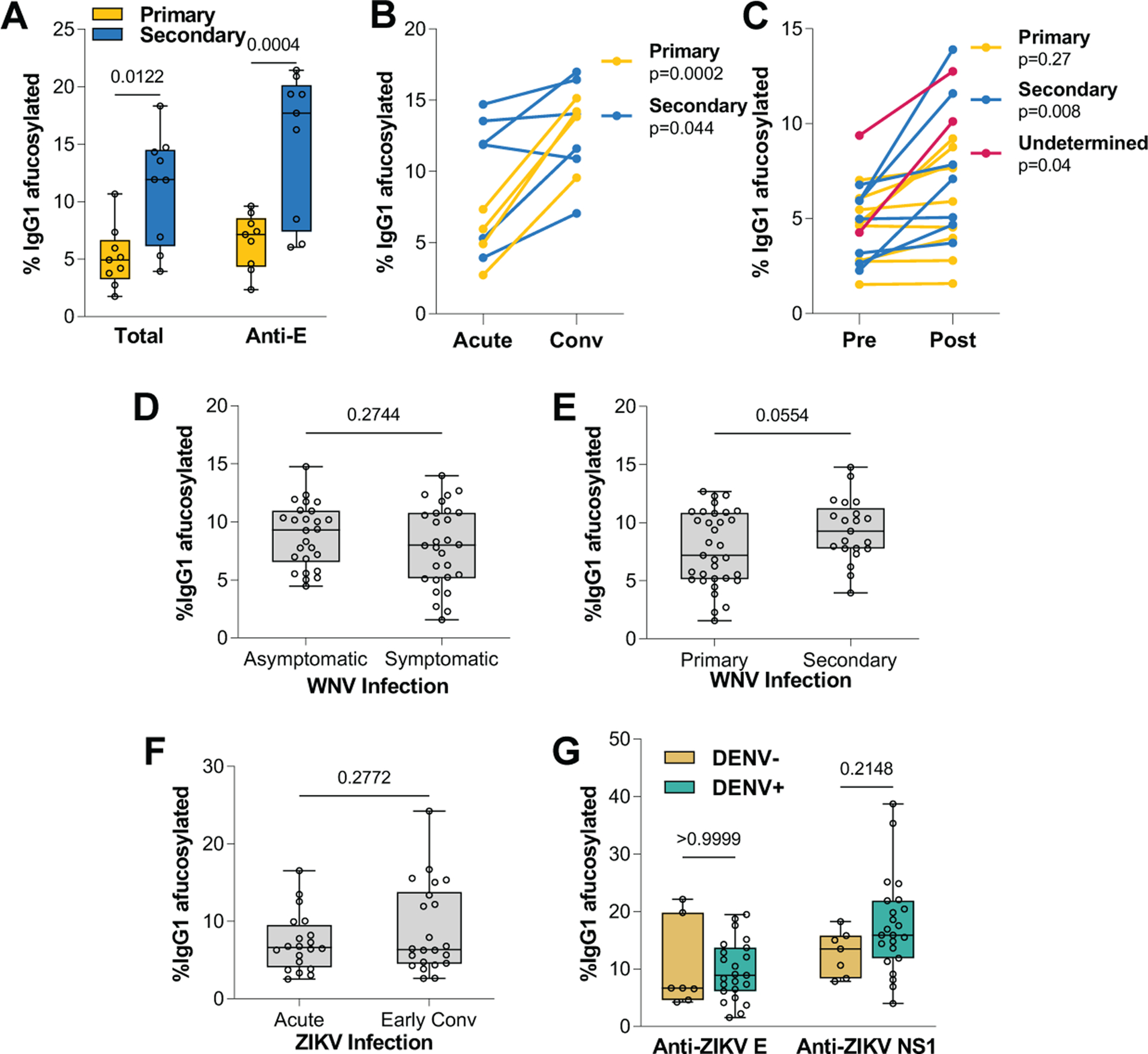
(A) Abundance of afucosylated IgG1 glycoforms in patients with identical disease classification (DF). (B) Levels of IgG1 afucosylation in DF patients during acute infection and at convalescence. (C) Afucosylation of total IgG1 in matched plasma samples (pre- and post-DENV infection). Patients were stratified based on DENV immune status. Afucosylated IgG1 levels in WNV patients with differential (D) disease severity and (E) WNV immune status. (F) Analysis of IgG1 afucosylation in ZIKV patients at the acute phase of infection or at early convalescence. (G) Levels of afucosylated IgG1 glycoforms of anti-ZIKV E and anti-ZIKV NS1 IgGs in ZIKV patients with differential DENV immune history. One-way ANOVA/Bonferroni post-hoc for A and G; two-tailed t test (B to C: paired; D to F: unpaired).
Although the determinants that regulate Fc fucosylation are poorly characterized, the observed elevation in the levels of IgG1 afucosylation in secondary cases may reflect specific modulation by DENV infection of the pathways that regulate Fc fucosylation. We therefore included in our study individuals with matched blood samples obtained before and after DENV infection (table S1). We observed that DENV infection specifically induced an increase in IgG1 afucosylation (Fig. 4C). By contrast, no changes were noted in the levels of afucosylation of other IgG subclasses, and in other glycan modifications (fig. S3, E to L). To determine whether these effects on IgG1 afucosylation extend to other flaviviruses, we analyzed the Fc glycan of IgGs from asymptomatic or symptomatic West Nile (WNV) patients with differential immune status (fig. S4, A to D, and table S2). In contrast to DENV, no differences in IgG1 afucosylation levels were observed among WNV patients with different disease severity (Fig. 4D) or immune status (Fig. 4E). We also assessed the IgG Fc glycan in plasma samples obtained from Zika virus (ZIKV)-infected patients at the acute infection phase and at early convalescence (table S3). Comparable IgG1 afucosylation levels were observed at acute phase and convalescence, suggesting that in contrast to DENV, ZIKV infection has no impact on Fc fucosylation (Fig. 4F and fig. S4, E to H).
Since ZIKV and DENV co-circulate in endemic areas, dysregulated IgG1 afucosylation induced upon DENV exposure might result in higher abundance of afucosylated Fc glycoforms. To investigate the impact of pre-existing anti-DENV immunity on the Fc glycosylation of IgGs elicited upon ZIKV infection, we analyzed the Fc glycan structure of ZIKV-infected patients with differential DENV immune history of DENV infection (table S4). Anti-ZIKV E and NS1 IgGs from DENV-naïve or experienced ZIKV patients exhibited comparable levels of afucosylated IgG1 (Fig. 4G) and of other Fc glycoforms (Fig. S5, A to D).
As recently demonstrated for other enveloped viruses (15), DENV could modulate IgG1 afucosylation either by eliciting unique inflammatory cues to B cells (16), or via direct infection of B cells (17, 18). This may modulate Fc fucosylation through inappropriate activation of cellular antiviral responses and/or dysregulated B cell function. Irrespective of the mechanism, we observed persistently high levels of IgG1 afucosylation at convalescence, suggesting that DENV infection has lasting consequences on the IgG Fc glycan structure. Given the well-established link between autoimmunity and Fc afucosylation (19), this may put dengue patients at risk for developing autoimmune pathologies, as has been observed in population-wide studies (20, 21).
Although substantial evidence supports ADE mechanisms in dengue disease pathogenesis, the role of pre-existing IgG is less clear in shaping disease susceptibility to other flaviviruses like WNV or ZIKV (22, 23). Increased IgG1 afucosylation was evident only in DENV-, but not in ZIKV- or WNV-infected patients, suggesting that aberrant Fc fucosylation likely represents an immune evasion mechanism that uniquely drives dengue disease pathogenesis through modulation of the anti-DENV IgG–FcγRIIIa interaction.
In summary, our findings support that DENV infection causes a specific increase in afucosylated IgG1 glycoforms. In contrast to DENV immune status, IgG1 afucosylation levels are not only associated with susceptibility to symptomatic disease, but also correlate with the specific clinical manifestations of severe dengue disease. Thus, the IgG1 afucosylation status represents a robust prognostic tool to predict susceptibility to symptomatic dengue disease, confirming the role of Fc–FcγR interactions in mediating ADE of dengue disease.
Supplementary Material
Acknowledgments:
We thank the participating patients, and the doctors and nurses of the three hospitals in Kampong Cham province for patient enrollment and sample collection, and H. Rekol from the National Dengue Control Program. Plasma samples from ZIKV- and WNV-infected individuals were obtained from BEI resources and the NHLBI Biologic Specimen and Data Repository Information Coordinating Center, respectively. We thank E. Lam, R. Francis (Rockefeller University), and R. Sherwood (Cornell University) for excellent technical support.
Funding:
We acknowledge support from the HHMI/Wellcome Trust (208710/Z/17/Z to T.C.), the NIAID (R01AI137276 to S.B.; U19AI111825 to J.V.R.), and the Rockefeller University. Sample collection from DENV patients was supported by the EU Seventh Framework Program (FP7/2007–2011). This manuscript was prepared using samples from ZIKV-infected individuals provided by Blood Systems Research Institute (BSRI) from studies funded in whole or in part by NHLBI (HHSN268201100001I); Roche Molecular Systems, Inc.; and the Department of Health and Human Services, Biomedical Advanced Research and Development Authority (HHSO100201600010C). The content is solely the responsibility of the authors and does not necessarily represent the official views of BSRI or the NIH.
Footnotes
Competing interests: A provisional patent application related to this work has been filed.
Data and materials availability: All data are available in the main text or the supplementary materials. All materials, except for clinical specimens, are available on request after completion of a Materials Transfer Agreement.
References and Notes:
- 1.Bhatt S et al. , The global distribution and burden of dengue. Nature 496, 504–507 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaughn DW et al. , Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 181, 2–9 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Halstead SB, Nimmannitya S, Cohen SN, Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J Biol Med 42, 311–328 (1970). [PMC free article] [PubMed] [Google Scholar]
- 4.Bournazos S, Gupta A, Ravetch JV, The role of IgG Fc receptors in antibody-dependent enhancement. Nat Rev Immunol 20, 633–643 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salje H et al. , Reconstruction of antibody dynamics and infection histories to evaluate dengue risk. Nature 557, 719–723 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katzelnick LC et al. , Antibody-dependent enhancement of severe dengue disease in humans. Science 358, 929–932 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguilar-Briseño JA et al. , TLR2 on blood monocytes senses dengue virus infection and its expression correlates with disease pathogenesis. Nat Commun 11, 3177 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noecker CA, Amaya-Larios IY, Galeana-Hernández M, Ramos-Castañeda J, Martínez-Vega RA, Contrasting associations of polymorphisms in FcγRIIa and DC-SIGN with the clinical presentation of dengue infection in a Mexican population. Acta Trop 138, 15–22 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Mohsin SN et al. , Association of FcγRIIa polymorphism with clinical outcome of dengue infection: first insight from Pakistan. Am J Trop Med Hyg 93, 691–696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bournazos S, Wang TT, Dahan R, Maamary J, Ravetch JV, Signaling by antibodies: recent progress. Annu Rev Immunol 35, 285–311 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang TT et al. , IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science 355, 395–398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borghi S et al. , FcRn, but not FcγRs, drives maternal-fetal transplacental transport of human IgG antibodies. Proc Natl Acad Sci U S A 117, 12943–12951 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization, Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. (2nd Edition, 1997). [Google Scholar]
- 14.Simon-Loriere E et al. , Increased adaptive immune responses and proper feedback regulation protect against clinical dengue. Sci Transl Med 9, (2017). [DOI] [PubMed] [Google Scholar]
- 15.Larsen MD et al. , Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science 371, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Upasani V et al. , Impaired antibody-independent immune response of B cells in patients with acute dengue infection. Front Immunol 10, 2500 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanini F et al. , Virus-inclusive single-cell RNA sequencing reveals the molecular signature of progression to severe dengue. Proc Natl Acad Sci U S A 115, E12363–E12369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Upasani V et al. , Direct infection of B cells by dengue virus modulates B cell responses in a Cambodian pediatric cohort. Front Immunol 11, 594813 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang TT, IgG Fc Glycosylation in human immunity. Curr Top Microbiol Immunol 423, 63–75 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li HM, Huang YK, Su YC, Kao CH, Increased risk of autoimmune diseases in dengue patients: a population-based cohort study. J Infect 77, 212–219 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Vo HTM et al. , Autoantibody profiling in plasma of dengue virus-infected individuals. Pathogens 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pantoja P et al. , Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat Commun 8, 15674 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terzian ACB et al. , Viral load and cytokine response profile does not support antibody-dependent enhancement in dengue-primed Zika virus-infected patients. Clin Infect Dis 65, 1260–1265 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ly S et al. , Asymptomatic dengue virus infections, Cambodia, 2012–2013. Emerg Infect Dis 25, 1354–1362 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Štambuk J et al. , Global variability of the human IgG glycome. Aging (Albany NY) 12, 15222–15259 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hue KD et al. , Validation of an internally controlled one-step real-time multiplex RT-PCR assay for the detection and quantitation of dengue virus RNA in plasma. J Virol Methods 177, 168–173 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke DH, Casals J, Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg 7, 561–573 (1958). [DOI] [PubMed] [Google Scholar]
- 28.Ramos HJ et al. , IL-1β signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog 8, e1003039 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Auerswald H et al. , Broad and long-lasting immune protection against various Chikungunya genotypes demonstrated by participants in a cross-sectional study in a Cambodian rural community. Emerg Microbes Infect 7, 13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang TT et al. , Anti-HA glycoforms drive B cell affinity selection and determine influenza vaccine efficacy. Cell 162, 160–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bournazos S, Gazumyan A, Seaman MS, Nussenzweig MC, Ravetch JV, Bispecific anti-HIV-1 antibodies with enhanced breadth and potency. Cell 165, 1609–1620 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okeley NM et al. , Development of orally active inhibitors of protein and cellular fucosylation. Proc Natl Acad Sci U S A 110, 5404–5409 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li T et al. , Modulating IgG effector function by Fc glycan engineering. Proc Natl Acad Sci U S A 114, 3485–3490 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith P, DiLillo DJ, Bournazos S, Li F, Ravetch JV, Mouse model recapitulating human Fcγ receptor structural and functional diversity. Proc Natl Acad Sci U S A 109, 6181–6186 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


