SUMMARY
Seven mobile oxazolidinone resistance genes, including cfr, cfr(B), cfr(C), cfr(D), cfr(E), optrA, and poxtA, have been identified to date. The cfr genes code for 23S rRNA methylases, which confer a multiresistance phenotype that includes resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A compounds. The optrA and poxtA genes code for ABC-F proteins that protect the bacterial ribosomes from the inhibitory effects of oxazolidinones. The optrA gene confers resistance to oxazolidinones and phenicols, while the poxtA gene confers elevated MICs or resistance to oxazolidinones, phenicols, and tetracycline. These oxazolidinone resistance genes are most frequently found on plasmids, but they are also located on transposons, integrative and conjugative elements (ICEs), genomic islands, and prophages. In these mobile genetic elements (MGEs), insertion sequences (IS) most often flanked the cfr, optrA, and poxtA genes and were able to generate translocatable units (TUs) that comprise the oxazolidinone resistance genes and occasionally also other genes. MGEs and TUs play an important role in the dissemination of oxazolidinone resistance genes across strain, species, and genus boundaries. Most frequently, these MGEs also harbor genes that mediate resistance not only to antimicrobial agents of other classes, but also to metals and biocides. Direct selection pressure by the use of antimicrobial agents to which the oxazolidinone resistance genes confer resistance, but also indirect selection pressure by the use of antimicrobial agents, metals, or biocides (the respective resistance genes against which are colocated on cfr-, optrA-, or poxtA-carrying MGEs) may play a role in the coselection and persistence of oxazolidinone resistance genes.
KEYWORDS: oxazolidinones, cfr, optrA, poxtA, horizontal transfer, plasmid, transposon, genomic island, integrative and conjugative element, prophage, mobile genetic element
INTRODUCTION
In 1987, scientists at E. I. DuPont de Nemours & Co. described a new class of synthetic antibacterial agents, the oxazolidinones (1). These are heterocyclic molecules with an oxygen and a nitrogen in a five-membered ring bridged with a carbonyl group. These agents are active in vitro, mainly against streptococci, enterococci, and staphylococci (1). Their in vivo activity against these bacteria has been confirmed in the respective animal models (1). Although the lead substance, DuP 721, showed promising antibacterial activities, serious toxicity problems were noted, which finally led not only to the dropping of the development of DuP 721 as a potential antimicrobial agent, but also to the cessation of the entire work on oxazolidinones by DuPont. Scientists at Pharmacia & Upjohn, however, believed that oxazolidinones might represent valuable antimicrobial agents for the therapy of infections caused by the aforementioned Gram-positive pathogens. They developed analogue molecules, including the two novel oxazolidinones U-100592 and U-100766, and tested them for their antimicrobial activity and toxicity in clinical trials (2). Linezolid (formerly known as U-100766) showed a remarkable spectrum of activity, as it proved to be active against vancomycin-resistant Enterococcus faecalis and Enterococcus faecium (3–7), methicillin-resistant Staphylococcus aureus (3–8), and penicillin-resistant Streptococcus pneumoniae (3–6, 9), and thereby represented a most valuable agent against multiresistant Gram-positive pathogens. Linezolid also showed modest activity against several Gram-negative bacteria, including Moraxella catarrhalis, Bordetella pertussis, and Pasteurella multocida (3, 10–12). Moreover, linezolid also displayed modest activity against several Gram-positive and Gram-negative anaerobic bacteria, including Clostridioides difficile, Clostridium perfringens, Bacteroides fragilis, Peptostreptococcus spp., and Fusobacterium spp., as well against Prevotella spp. (3, 13–16). Finally, linezolid also showed substantial activity against Mycobacterium tuberculosis, the Mycobacterium avium complex, and some rapidly growing mycobacteria (3, 17, 18). Oxazolidinones act in a mainly bacteriostatic manner against staphylococci and enterococci, as confirmed by time-kill experiments (3, 8, 19). However, bactericidal activity has been observed not only against S. pneumoniae, but also against Streptococcus pyogenes, C. perfringens, and B. fragilis (3).
Linezolid was the first oxazolidinone approved exclusively for human use in April 2000 under the trade name Zyvox (https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021130s022lbl.pdf). According to the product label, linezolid is approved for the treatment of (i) uncomplicated skin and skin structure infections, (ii) complicated skin and skin structure infections, including diabetic foot infections, without concomitant osteomyelitis, (iii) nosocomial pneumonia, and (iv) community-acquired pneumonia, including concurrent bacteremia. Linezolid is also one of the few treatment options for infections caused by vancomycin-resistant enterococci, such as E. faecalis and E. faecium, including cases with concurrent bacteremia. In June 2014, the expanded-spectrum oxazolidinone tedizolid was approved, also only for use in humans, under the trade name Sivextro (https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205435s000lbl.pdf). Tedizolid is indicated for the treatment of acute bacterial skin and skin structure infections caused by susceptible isolates of S. aureus (including methicillin-resistant [MRSA] and methicillin-susceptible [MSSA] isolates), S. pyogenes, Streptococcus agalactiae, the Streptococcus anginosus group, and E. faecalis. Although not approved for veterinary use, linezolid and tedizolid may be used in non-food-producing animals, such as dogs and cats, via the Animal Medicinal Drug Use Clarification Act of 1994 (AMDUCA) in the United States or via similar cascade regulations in other countries. This extralabel use by veterinarians requires proof—usually via an antibiogram—that there is no other antimicrobial agent approved for veterinary use that is efficacious against the causative bacterial pathogen in this specific case. Due to the high treatment costs, oxazolidinones have rarely been used to treat dogs and cats (20). The future will show whether this will change, since linezolid has recently become a generic drug.
MODE OF ACTION OF OXAZOLIDINONES
The oxazolidinones act by a novel mode of action different from those of all other antimicrobial agents. Several studies have shown that oxazolidinones inhibit bacterial protein biosynthesis. However, oxazolidinones inhibit neither the elongation reaction nor the binding of either N-formylmethionyl-tRNA or mRNA to the 30S ribosomal subunit. Oxazolidinones also do not prevent the formation of a binary complex between the initiation factor IF2 and N-formylmethionyl-tRNA. Instead, oxazolidinones bind to the 23S portion of the 50S subunit and prevent it from forming a complex with the 30S ribosomal subunit, N-formylmethionine-tRNA, GTP, mRNA, and the initiation factors IF1 to IF3. Thus, a functional 70S initiation complex cannot be formed and mRNA cannot be translated (Fig. 1). Oxazolidinones thus inhibit bacterial protein biosynthesis at a very early stage, i.e., before it has started (19, 21–24). This mode of action is different from those of other protein biosynthesis inhibitors, such as aminoglycosides (inhibition of translation and misreading), chloramphenicol (blocking of peptide bond formation by inhibition of the peptidyl transferase), macrolides (inhibition of peptide chain elongation and blocking of the assembly of the 50S subunit), streptogramins (inhibition of peptide chain elongation), and tetracyclines (interfering with the binding of incoming aminoacyl-tRNA to the A-site) (25, 26). Due to this novel mode of action and the observation that linezolid is active against Gram-positive pathogens that carry a wide range of resistance genes that code for either modifying enzymes, efflux mechanisms, or proteins that modify/protect the target sites of other antimicrobial agents, cross-resistance between the oxazolidinones and other antimicrobial agents has not been expected (27).
FIG 1.
Schematic presentation of the mode of action of oxazolidinones. Oxazolidinones inhibit protein biosynthesis by preventing the formation of a functional 70S initiation complex composed of the 30S ribosomal subunit, initiation factors, mRNA, formylmethionyl-tRNA (fMET-tRNA), and the 50S ribosomal subunit. (Based on data from reference 26.)
MECHANISMS OF OXAZOLIDINONE RESISTANCE
Zurenko and colleagues investigated the ability of the reference strain S. aureus ATCC 29213 to generate spontaneous linezolid-resistant mutants in the presence of 2-, 4-, and 8-fold the MIC. No linezolid-resistant colonies were detected at any of these concentrations, which corresponded to a spontaneous mutation frequency of S. aureus ATCC 29213 of less than 8 × 10−11 (3). Similar experiments with four strains each of MRSA, MSSA, and also methicillin-resistant and methicillin-susceptible Staphylococcus epidermidis were conducted by Kaatz and Seo (8). They found that the development of spontaneous linezolid resistance at 2-fold the MIC was for all 12 isolates below the detection limit, i.e., less than 1 × 10−9 (8). In vitro selection experiments with 10 clinical vancomycin-resistant enterococci (four E. faecalis, five E. faecium, and one Enterococcus gallinarum isolate) and the reference strain E. faecalis ATCC 29212 yielded resistant mutants with distinctly elevated linezolid MICs that had single-point mutations at various positions in the central loop of domain V of the 23S rRNA, including C2610G, G2576U, G2513U, G2512U, and G2505A (28). This locus has previously been shown to be the preferential area for oxazolidinone resistance-mediating mutations (29, 30). Studies on E. faecalis also showed that recombination proficiency has an impact on the frequency and the locus of mutations conferring linezolid resistance (31) (Table 1).
TABLE 1.
Mechanisms of oxazolidinone resistance
| Gene | Resistance mechanism(s) | Associated resistance phenotypeb | Location(s)f | Reference(s) |
|---|---|---|---|---|
| 23S rRNA | Point mutation | LZD | Chromosomal | 32–35, 39, 40 |
| rplC | Point mutation, deletion, insertion | LZDc | Chromosomal | 34–40 |
| rplD | Point mutation, deletion, insertion | LZDc | Chromosomal | 34–42 |
| cfr | rRNA methylase | PHE, LIN, LZD, PLM, STA | Plasmid and chromosomal | 45, 46 |
| cfr(B) | rRNA methylase | PHE, LIN, LZD, PLM, STA | Plasmid and chromosomal | 48, 49, 53 |
| cfr(C) | rRNA methylasea | PHE, LIN, LZD, PLM, STA | Plasmid and chromosomal | 50 |
| cfr(D) | rRNA methylasea | PHE, LIN, LZD, PLM, STA | Plasmid and chromosomal | 51 |
| cfr(E) | rRNA methylasea | (PHE, LIN, LZD, PLM, STA)d | Chromosomal | 52 |
| optrA | ABC-F protein | PHE, LZD, TZDe | Plasmid and chromosomal | 54 |
| poxtA | ABC-F protein | PHE, LZDc, TETc | Plasmid and chromosomal | 56 |
Assumed based on structural comparisons.
LIN, lincosamides; LZD, linezolid; PHE, phenicols; PLM, pleuromutilins; STA, streptogramin A; TET, tetracycline; TZD, tedizolid. Depending on the bacterium in which this gene was detected, the resistance phenotype conferred by the gene may overlap with intrinsic resistance properties of the host bacterium.
Confers only a minor increase in the respective MICs.
To be confirmed.
Certain OptrA proteins are known to confer elevated MICs to LZD and TZD.
“Chromosomal” includes chromosomally integrated transposons, integrative and conjugative elements (ICEs), and prophages; “plasmid” also includes transposons integrated into plasmids.
The first clinical linezolid-resistant S. aureus isolate was identified in a patient who was treated with linezolid. Further analysis showed that this isolate had the aforementioned G2576U mutation in the 23S rRNA (32). A linezolid-resistant E. faecium isolate that also exhibited the G2576U mutation, was obtained from a patient without prior exposure to oxazolidinones in 2001 as well (33). Over the following years, numerous strains with linezolid resistance-mediating mutations in the 23S rRNA have been described. Reviews by Long and Vester (34) and by Stefani et al. (35) summarize the linezolid resistance-mediating mutations seen in the 23S rRNA, including the bacteria in which they have been found. In addition, information concerning whether the respective mutations originated from in vitro selection experiments or were detected in clinical isolates was also provided (34). Among all mutations listed, G2576U is most widespread (34, 35).
Besides mutations in the 23S rRNA, linezolid resistance may also be due to modifications in the genes coding for the ribosomal proteins L3 (rplC) and L4 (rplD) (Table 1). These modifications include point mutations that result in single or multiple amino acid exchanges and deletions of variable length, but also insertions (34–38). Mutations in the L3 and L4 proteins were often found together with modifications in the 23S rRNA (34–36, 39, 40). Studies on S. aureus mutants that carried only modifications in the rplC or rplD genes (36) suggested that these modifications only slightly increased the linezolid MICs, whereas high linezolid MICs were mainly due to 23S rRNA modifications (36). An intermediate status to linezolid, combined with macrolide and chloramphenicol resistance, was found to be due to 6-bp deletions in the rplD gene of S. pneumoniae (41). A mutation in the rplD gene that led to the amino acid exchange G71D was also described in C. perfringens (42). Although mutations in the rplV gene coding for the ribosomal protein L22 have been observed in coagulase-negative staphylococci (CoNS) (43, 44), they most likely do not play a role in linezolid resistance. All of the aforementioned modifications in the 23S rRNA and in the rplC and rplD genes were chromosomally fixed and not horizontally transferable. Thus, bacteria carrying these mutations were disseminated by clonal expansion or developed de novo from susceptible strains.
This situation changed in 2000, when the first transferable oxazolidinone resistance gene, cfr, was identified in a bovine Staphylococcus sciuri isolate from Germany (45). This plasmid-borne gene was initially described as a novel chloramphenicol-florfenicol resistance gene. It took another 5 years until the mechanism of resistance was identified (46). The Cfr protein represents an RNA methyltransferase of the radical S-adenosylmethionine (SAM) superfamily, which targets the adenine residue at position 2503 in the 23S rRNA and thereby causes resistance (46). This adenine residue, however, is located exactly in the overlapping binding area for five chemically distinct classes of antimicrobial agents that inhibit bacterial protein biosynthesis, namely the phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A antibiotics (47). The additional methylation at A2503 prevents the binding of the aforementioned antimicrobial agents to the ribosome, thereby conferring resistance to all of them. The corresponding multiresistance phenotype has been termed PhLOPSA (47). More recently, variants of the cfr gene, termed cfr(B) (48, 49), cfr(C) (50), cfr(D) (51), and cfr(E) (52) have been described (Table 1). For cfr(B), it was shown that this gene confers multiple antimicrobial resistance by the same mechanisms as the cfr gene (53). The Cfr(B), Cfr(C), Cfr(D), and Cfr(E) proteins shared 74%, 64%, 55%, and 51% amino acid identity with Cfr.
In 2015, the oxazolidinone resistance gene optrA was identified on a plasmid in an E. faecalis isolate of human origin in China (54). Cloning and expression of this gene in E. faecalis JH2-2 and S. aureus RN4220 showed that optrA conferred resistance not only to both oxazolidinones, linezolid and tedizolid, but also to fluorinated and nonfluorinated phenicols (54). The optrA gene codes for an ABC-F protein which confers resistance by ribosome protection (55) (Table 1).
The gene poxtA was detected in a MRSA isolate of clinical origin in Italy in 2018 (56). In the recipient strains E. faecalis JH2-2 and S. aureus RN4220, the cloned poxtA gene conferred only 2-fold increases of the MICs to linezolid, tedizolid, chloramphenicol, tetracycline, and doxycycline, all of which are below the Clinical and Laboratory Standards Institute (CLSI)-approved breakpoints for the “resistant” category. Solely for florfenicol, an 8-fold increase in the MICs of both host strains was observed. Thus, it appears a bit questionable to consider poxtA to be a phenicol-oxazolidinone-tetracycline resistance gene. The PoxtA protein is also a member of the ABC-F family, but it is only 32% identical to OptrA (56) (Table 1).
Recently, Hua and colleagues performed comparative transcriptome analyses of a low-level linezolid resistant E. faecalis isolate and two susceptible E. faecalis isolates, including the reference strain ATCC 29212. Among the differentially expressed genes, three genes were significantly upregulated and were predicted to be associated with drug resistance through active efflux pumps and biofilm formation. Whether these genes play a role in the development of low-level linezolid resistance remains to be clarified (57).
MONITORING OF LINEZOLID RESISTANCE
Clinical Breakpoints and Quality Control Ranges
The monitoring of linezolid and tedizolid susceptibility requires approved clinical breakpoints and quality control ranges. The two main organizations worldwide, the Clinical and Laboratory Standards Institute (CLSI) (http://em100.edaptivedocs.net/dashboard.aspx) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (https://www.eucast.org/clinical_breakpoints/), have both set clinical breakpoints for linezolid and tedizolid that are applicable to staphylococci, enterococci, and streptococci, which are freely accessible from the respective websites. The breakpoints set by these two organizations are similar, but not identical, and they occasionally also differ in the target bacteria for which they have been approved. For agar disk diffusion assays, CLSI recommends the use of 30-μg linezolid disks, whereas EUCAST recommends the use of 10-μg linezolid disks. CLSI provides no approved tedizolid breakpoints for agar disk diffusion and also recommends that bacteria identified as linezolid resistant by agar disk diffusion should be confirmed by a MIC method. This suggests that broth microdilution is the recommended method to determine oxazolidinone susceptibility in routine diagnostics.
The quality control (QC) ranges set for the reference strains also differ in part between CLSI and EUCAST. The main differences are that (i) CLSI uses specific staphylococcal reference strains for disk diffusion (S. aureus ATCC 25923) and MIC determination (S. aureus ATCC 29213), whereas EUCAST uses the same strain (S. aureus ATCC 29213) for both antimicrobial susceptibility testing (AST) methods, and (ii) that the aforementioned disks with different linezolid contents are recommended by CLSI and EUCAST, respectively. Thus, the use of different clinical breakpoints and different QC parameters can result in slight differences between the monitoring results when following CLSI or EUCAST methodologies.
Monitoring Programs for Linezolid Susceptibility
In 2002, the worldwide Zyvox Annual Appraisal of Potency and Spectrum (ZAAPS) program was initiated to monitor trends in the linezolid susceptibility patterns of six groups of bacterial pathogens (58). These include S. aureus, CoNS, Enterococcus spp., S. pneumoniae, streptococci of the beta-hemolytic group, and streptococci of the viridans group. In the initial study, a total of 7,971 bacterial isolates were investigated for their susceptibility to linezolid and 21 other antimicrobial agents by broth microdilution according to the CLSI recommendations. The isolates originated from North America (two nations, 30 sites), South America (two nations, five sites), Europe (six nations, 16 sites), and the Asia-Pacific region (two nations, three sites) and comprised 3,687 S. aureus isolates, 870 CoNS, 1,070 enterococci, 1,770 S. pneumoniae isolates, 387 beta-hemolytic streptococci, and 187 viridans group streptococci (58). In total, four linezolid-resistant isolates with linezolid MICs of ≥8 μg/ml, one each of S. aureus, S. epidermidis, E. faecium, and the viridans group Streptococcus, were identified. All of these isolates had the mutation G2576U in their 23S rRNA (58).
Over the next 10 consecutive years, the ZAAPS program was continued and expanded (59–68). An excellent addition to the pure phenotypic monitoring is that all isolates identified as linezolid resistant were investigated for the underlying resistance mechanisms. After a break in 2013, the program continued for the following years (69–71). The last report dates from 2018 and reports the susceptibility data from 2016 (71). Here, 8,325 bacterial isolates were obtained from 76 medical centers in 42 countries (excluding the United States). Among them, 17 isolates were confirmed as linezolid resistant. A single S. aureus isolate from Panama carried the cfr gene, and eight E. faecalis isolates from Europe, Latin America, and Southeast Asia, as well as one Streptococcus gallolyticus isolate from Thailand, harbored the optrA gene. The remaining resistant isolates, including four S. epidermidis isolates from Germany and Italy, two E. faecium isolates from Italy, and one Streptococcus mitis group isolate from Slovenia, had the 23S rRNA mutation G2576U and occasionally insertions or exchanges in the genes coding for the ribosomal proteins L3 or L4 (71).
Figure 2 shows the percentages of linezolid susceptibility of the three groups of bacteria, S. aureus, CoNS, and Enterococcus spp. Streptococci were—with a few exceptions—completely susceptible over the entire monitoring period from 2002 to 2016. In general, the susceptibility rates for S. aureus ranged between 99.93 and 100%, for CoNS between 98.81 and 100%, and for Enterococcus spp. between 98.83 and 100%. Even though resistant target bacteria were identified occasionally, these data confirmed that after more than 15 years of clinical use, there is no trend toward increasing resistance visible worldwide.
FIG 2.
Percentages of linezolid-susceptible S. aureus, coagulase-negative staphyloccoci (CoNS), and Enterococcus isolates as determined in the worldwide Zyvox Annual Appraisal of Potency and Spectrum (ZAAPS) program during the years 2002 to 2016 (58–66, 69–71).
In addition to the ZAAPS program, the Linezolid Experience and Accurate Determination of Resistance (LEADER) surveillance program was started in 2004 (72). This program is focused exclusively on the situation in the United States, monitors the susceptibility of virtually the same set of target bacteria as ZAAPS, and also performs AST by broth microdilution according to CLSI recommendations. From 2004 on, isolates from the United States were excluded from the ZAAPS program. The initial LEADER study investigated 4,414 isolates provided by 50 medical centers from 34 states in the United States, including Washington, DC (72). Over the following years, results from the LEADER program have been published (43, 44, 73–79). The latest data are from 2015. A summary of the results from 2011 to 2015 showed very low rates of nonsusceptible S. aureus (<0.1 to 0.1%), CoNS (0.5 to 1.2%), Enterococcus spp. (0.3 to 0.7%), and viridans group streptococci (0.0 to 0.7%), whereas all S. pneumoniae and beta-hemolytic streptococci were susceptible to linezolid (79). A comparison of the susceptibility percentages between the ZAAPS and LEADER programs has been published for the years 2004 to 2012 and for S. aureus, CoNS, and Enterococcus spp. In general, the susceptibility rates for all three groups of bacteria from the United States were equal or lower than those of the international strain collections (80).
In addition to these linezolid-specific monitoring programs, detection of resistance to linezolid among bacteria of the genera Enterococcus, Staphylococcus, and Streptococcus of human origin is included in various country-specific monitoring programs, such as NethMap from the Netherlands (https://www.rivm.nl/bibliotheek/rapporten/2020-0065.pdf), the Danish Integrated Antimicrobial Resistance Monitoring and Research Program (DANMAP) (https://www.danmap.org/reports/2019), the Swedish Antibiotic Sales and Resistance in Human Medicine (SWEDRES) and the Swedish Veterinary Antibiotic Resistance Monitoring (SVARM) (https://www.sva.se/media/jzdlctnk/rapport_swedres-svarm_2018.pdf), the Norwegian Surveillance System for Antimicrobial Drug Resistance NORM/NORM-Vet (https://www.vetinst.no/overvaking/antibiotikaresistens-norm-vet), the British Society for Antimicrobial Chemotherapy (BSAC) Resistance Surveillance Programme for the UK and Ireland (https://bsacsurv.org/), and the PanEuropean EARS-Net annual surveillance reports on antimicrobial resistance published by the European Centre for Disease Prevention and Control (ECDC) (https://www.ecdc.europa.eu/en/antimicrobial-resistance/surveillance-and-disease-data/report), as well as the China Antimicrobial Surveillance Network (CHINET) (www.chinets.com) and the China Antimicrobial Resistance Surveillance System (CARSS) (http://carss.cn/). Similarly to ZAAPS and LEADER, all of these monitoring and surveillance programs reported very low percentages of linezolid-resistant target bacteria. However, they used in part different methodologies and interpretive criteria, which renders their results not directly comparable.
In the veterinary sector, only DANMAP, NORM-Vet, and the German national resistance monitoring program for veterinary pathogens, GERM-Vet (https://www.bvl.bund.de/DE/Arbeitsbereiche/05_Tierarzneimittel/01_Aufgaben/05_AufgAntibiotikaResistenz/05_GERMvet/GERMvet_node.html), monitor linezolid resistance of selected Gram-positive pathogens from animals. Although all three programs use different standards for performing AST and interpreting the results, no linezolid-resistant target bacteria have been identified in DANMAP 2019 and GERM-Vet 2018, while a single Streptococcus canis isolate from a clinical infection of a dog and three E. faecalis isolates from raw dog food samples were identified in NORM-Vet 2019.
THE MOBILE OXAZOLIDINONE RESISTANCE GENE cfr
Geographical Distribution and Host Bacteria of the cfr Gene
A database search that included PubMed (https://pubmed.ncbi.nlm.nih.gov/) and NCBI Nucleotide (https://www.ncbi.nlm.nih.gov/nucleotide/) databases was done for all currently known mobile oxazolidinone resistance genes. It identified the gene cfr to be present in 25 countries in five continents (Fig. 3). It is important to understand that this geographical distribution reflects the participation in monitoring programs, such as ZAAPS and LEADER, and the areas in which research groups were particularly active in the search for oxazolidinone resistance genes and the analysis of linezolid-resistant bacteria, such as China, Germany, Ireland, Italy, and Spain. The host bacteria in which the cfr gene has been identified include the Gram-positive genera Staphylococcus, Streptococcus, Enterococcus, Bacillus, Jeotgalicoccus, Macrococcus, and Mammaliicoccus, as well as the Gram-negative genera Escherichia, Proteus, Providencia, Morganella, and Pasteurella (81–84). These cfr-carrying bacteria originated from humans, cattle, pigs, horses, dogs, cats, chickens, turkeys, ducks, or geese, but also from chicken meat, beef, and pork, as well as from environmental samples.
FIG 3.
Geographical distribution of cfr-carrying bacteria. The countries in blue are those from which the occurrence of cfr-carrying bacteria has been reported.
Occurrence of the cfr gene in Staphylococcus spp.
The staphylococcal species Staphylococcus sciuri, Staphylococcus lentus, Staphylococcus vitulinus, Staphylococcus fleurettii, and Staphylococcus stepanovicii have recently been reclassified as new species within the genus Mammaliicoccus (85). Members of two of these species, S. sciuri and S. lentus, carry mobile oxazolidinone resistance genes. Since all references referring to the corresponding publications and database entries list these two species as members of the genus Staphylococcus, we decided to keep the former genus assignment here for the sake of convenience.
Although initially identified in an S. sciuri isolate of bovine origin in Germany about 20 years ago (45), this gene has since been found in a total of 19 staphylococcal species, including coagulase-positive, coagulase-variable, and coagulase-negative species. Some of these cfr-carrying staphylococci occur exclusively in humans, such as Staphylococcus capitis and Staphylococcus hominis. cfr-carrying S. capitis isolates have been identified in China (86–88) and in the United States (74), whereas S. hominis isolates carrying the cfr gene have been detected in Italy (89) and the United States (79).
Another group of cfr-carrying staphylococci has so far only been identified in a specific staphylococcal species from one defined animal host in a specific country; these include Staphylococcus pseudintermedius from a dog in Portugal (90), Staphylococcus rostri from ducks in China (91, 92), Staphylococcus warneri and Staphylococcus hyicus from pigs in Denmark (93), and Staphylococcus auricularis, Staphylococcus chromogenes, and Staphylococcus kloosii from calves in Germany (94).
A third group of cfr-positive staphylococci has been described in both human and animal hosts. These include S. aureus, Staphylococcus haemolyticus, Staphylococcus saprophyticus, Staphylococcus cohnii, and Staphylococcus arlettae. S. aureus isolates (including MRSA isolates) have been seen in humans from Brazil (95), China (96–98), Colombia (99, 100), Ecuador (GenBank accession no. KY448337), Ireland (101, 102), Pakistan (103), Panama (71), Spain (104–111), the United Arab Emirates (112), and the United States (74, 79, 113–116). However, such isolates have also been obtained from a horse in Germany (117), beef in Egypt (118), and pigs in Belgium (119), China (98, 120–122), and South Korea (123, 124). S. epidermidis isolates of human origin containing the cfr gene have been found in Brazil (125), China (126, 127), France (65, 128, 129), Germany (130, 131), Ireland (132, 133), Italy (65, 67, 68, 70, 89, 134–137), Mexico (65, 67), Poland (138), Spain (65, 139–142), and the United States (74, 79, 113, 143–146). In addition, a single porcine cfr-positive S. epidermidis isolate was recently detected in China (147). cfr-carrying S. haemolyticus strains have been found in humans from Brazil (125), China (97, 147), Egypt (148), India (149–152), Mexico (65), Spain (111), and Vietnam (40). Porcine and bovine cfr-carrying S. haemolyticus isolates have only been detected in China (91, 92, 147). S. saprophyticus isolates have been found in pigs from China (122, 153), in turkeys from Egypt (154), and in humans and calves from Germany (94). S. cohnii isolates have been obtained from humans in China (88, 97, 147, 155), Germany (94), Mexico (67), India (151), and Vietnam (40). They have also been found in pork and chicken meat (156), as well as in pigs (122, 153) and chickens (91, 92) from China, calves from Germany (94), and environmental samples from Spain (157). S. arlettae isolates have been found in humans from India (151), turkeys from Egypt (154), pigs (122), ducks (91, 92), and chickens (158) from China, as well as in samples from a swine farm environment in Spain (157).
The fourth group of cfr-carrying staphylococci includes the CoNS species Staphylococcus equorum, Staphylococcus lentus, S. sciuri, and Staphylococcus simulans, which have been exclusively found in animals, food of animal origin, and/or environmental samples. S. equorum isolates have been detected in pork, chicken meat, and chickens from China (147, 156) and in environmental samples from Spain (157). S. lentus isolates have been found in cattle from Belgium (159, 160) and Germany (94, 117), turkeys from Egypt (154), chickens from China (91, 92, 147), and pigs from China (153) and Germany (117), as well as samples from a pig farm environment in Germany (161). S. sciuri isolates were present in cattle from Germany (45, 94, 117), Belgium (159, 160), and China (147), and in pigs (91, 92, 122, 153, 162, 163), ducks and chickens (91, 92, 147), chicken meat (156), dogs (153, 164), and cats (153) from China, as well as in turkeys from Egypt (154). Finally, cfr-carrying S. simulans isolates have been identified in pigs (91, 92), pork, and chicken meat (156) from China, cattle in Germany (94, 117), and pigs in Denmark (93).
Occurrence of the cfr gene in Enterococcus spp.
In contrast to the widespread occurrence of the gene cfr in staphylococci, this gene has comparatively rarely been detected among members of the genus Enterococcus. So far, it has been found in the six species E. faecalis, E. faecium, Enterococcus thailandicus, Enterococcus casseliflavus, E. gallinarum, Enterococcus hirae, and Enterococcus avium. cfr-carrying E. faecalis strains have been identified not only in humans from China (165), Thailand (166), and the United States (113, 114, 167), but also among pigs from Brazil (168, 169), China (170–173), and Italy (174), as well as in sewage from a pig farm in China (175). In contrast, cfr-positive E. faecium strains have mainly been isolated from humans. The corresponding reports were from Canada (176), China (177), Germany (178), Ireland (132), Italy (179, 180), Poland (181), and the United Kingdom (182). Thus far, the only report about the gene cfr in E. faecium from an animal is from the United States, where such an isolate had been detected in cattle (183). Reports about cfr-carrying E. thailandicus from pigs (174, 175) and sewage from a pig farm (175), as well as E. casseliflavus (184), E. avium (174), E. hirae (GenBank accession no. MK798156), and E. gallinarum from pigs (174, 184) all originated from China and/or Italy.
Occurrence of the cfr gene in other Gram-positive bacteria.
There are only a few reports of the presence of the cfr gene in other Gram-positive bacteria. The cfr gene was found in Streptococcus suis (82, 185) and in Bacillus spp. (186–188), as well as in Macrococcus caseolyticus and Jeotgalicoccus pinnipedialis (189), all from pigs in China.
Occurrence of the cfr gene in Gram-negative bacteria.
Despite the fact that many Gram-negative bacteria, especially those of the order Enterobacterales, are intrinsically resistant to some of the antimicrobial classes to which the gene cfr confers resistance, such as lincosamides, oxazolidinones, and streptogramin A antibiotics, this multiresistance gene has been found occasionally in the species Escherichia coli, Proteus vulgaris, Proteus mirabilis, Proteus cibarius, Providencia rettgeri, Morganella morganii, and Pasteurella multocida, all from food-producing animals in China. The cfr-carrying E. coli isolates were from pigs (190–196) or chickens (197), whereas the corresponding P. vulgaris isolates were all from pigs (198–200). One report described the presence of cfr in a porcine P. mirabilis isolate (201) while another report mentioned it in a Proteus isolate of not further specified food animal origin (202). All P. cibarius isolates originated from geese (203). A cfr-carrying plasmid (GenBank accession no. CP060728) was identified in a P. rettgeri isolate of duck origin. The cfr-positive M. morganii isolate was of pig origin (83) and the P. multocida isolate of duck origin (84).
Mobile Genetic Elements That Are Involved in the Dissemination of the cfr Gene
The cfr gene has most frequently been located on mobile genetic elements (MGEs). Among these, plasmids are the preferred location. Plasmids have also been shown to act as vectors for cfr-carrying transposons and translocatable units (TUs). In addition, chromosomally located cfr-carrying transposons, TUs, and integrative and conjugative elements (ICEs) have also been described. Numerous plasmids harboring the cfr gene have been detected in various Gram-positive and Gram-negative genera. In the following descriptions of cfr-carrying plasmids, focus is put on those plasmids for which complete plasmid sequences are available. These plasmids differ in size, structure, and organization, as well as in the numbers and types of colocated antimicrobial, heavy metal, or biocide resistance genes (Tables 2 and 3).
TABLE 2.
Characteristics of completely sequenced cfr-carrying plasmids in Gram-positive bacteria
| Plasmid(s) | Origin | Size (bp) | Colocated resistance gene(s) | GenBank accession no. |
|---|---|---|---|---|
| pSA8589 | S. aureus, human, USA | 6,962 | KC561137 | |
| pMSA16 | S. aureus, cattle, China | 7,054 | erm(A) | JQ246438 |
| pSS-03 | S. cohnii, pig, China | 7,057 | erm(C) | JQ219851 |
| pSS-03 | S. arlettae, pig, China | 7,122 | erm(C) | JF834911 |
| pSAM13-0451 | S. epidermidis, human, Ireland | 8,558 | erm(T), Δlsa(B) | KY579373 |
| pHNCR35 | S. simulans, human, China | 9,880 | fexA | KF861983 |
| pK8D55P-cfr | S. sciuri, duck, China | 12,724 | erm(C), aadD, tet(L) | CP065963 |
| pSS-01 | S. cohnii, pig, China | 15,703 | fexA, aacA-aphD | JF834909 |
| pERGB | S. aureus, human, Spain | 15,259 | aadD, tet(L), dfrK | JN970906 |
| pSCFS1 | S. sciuri, cattle, Germany | 17,108 | erm(33), lsa(B), spc | NC_005076 |
| pH8C110P-cfr | S. sciuri, animal feed, China | 24,103 | erm(B), aadD, tet(L) | CP065796 |
| pSAM13-0401 | S. aureus, human, Ireland | 27,502 | lsa(B) | KU510528 |
| p12-00322 | S. epidermidis, human, Germany | 36,754 | lsa(B) | KM521836 |
| Unnamed 1 | S. aureus, pig, China | 37,510 | fexA | CP065195 |
| p12-02300 | S. epidermidis, human, Germany | 38,864 | fexA | KM521837 |
| pY96A | S. aureus, pig, China | 39,212 | fexA | CP065516 |
| p14-01514 | S. epidermidis, human, Germany | 39,243 | fexA | KX520649 |
| pSA737, pSEPI8573 | S. aureus, human, USA | 39,287 | fexA | KC206006, KC222021 |
| pSR01 | S. aureus, human, China | 39,500 | aacA-aphD | CP048644 |
| pLRSA417 | S. aureus, human, China | 39,504 | aacA-aphD | KJ922127 |
| pSX01 | S. xylosus, pig, China | 39,969 | aacA-aphD | KP890694 |
| pY8P168P-cfr | S. saprophyticus, pig, China | 41,503 | fexA, aacA-aphD | CP065798 |
| pSAM12-0145 | S. aureus, human, Ireland | 41,590 | fexA | KU521355 |
| pGMI17-006 | S. aureus, human, Denmark | 45,885 | fexA, lsa(B) | CP028164 |
| pH29-46 | S. lentus, chicken, China | 46,167 | fexA, aacA-aphD | CP059680 |
| pk8D6P-cfr | S. sciuri, duck, China | 53,742 | fexA, aacA-aphD, aadD, ble | CP065793 |
| pWo27-9 | S. sciuri, pig, China | 55,724 | optrA, aadD, ble | KX982169 |
| pWo28-1 | S. sciuri, pig, China | 60,565 | optrA, fexA, aadD, aacA-aphD, ble | KX982171 |
| pWo28-3 | S. sciuri, pig, China | 60,563 | optrA, fexA, aadD, aacA-aphD, ble | KT601170 |
| pSA-01 | S. arlettae, chicken, China | 63,558 | fexB, erm(B), erm(C), erm(T), aadD, aacA-aphD, tet(L), fosD | KX274135 |
| pSP01 | S. epidermidis, human, Italy | 76,991 | lsa(B), blaZ, msr(A), cop | KR230047 |
| Unnamed | E. faecalis, cattle, China | 11,940 | CP028840 | |
| pCPPF5 | E. faecalis, pig, China | 12,270 | KC954773 | |
| pE30 | E. faecalis, unknown, China | 12,270 | KT717888 | |
| pFSIS1608820 | E. faecium, cattle, USA | 28,222 | optrA, fexA, erm(A), erm(B), aphA3, spc | CP028728 |
| pEF-01 | E. faecalis, cattle, China | 32,388 | fexA | CP002208 |
| pE35048-oc | E. faecium, human, Italy | 41,816 | optrA, erm(B), Δlnu(E) | MF580438 |
| pF120805 | E. faecium, human, Ireland | 72,924 | optrA, erm(A), erm(B), aphA3, aadE, lnu(A), lnu(B) | KY579372 |
| pFas4-2 | E. hirae, pig, China | 85,629 | fexA, lsa(B), ars operon | MK798156 |
| p4 | E. faecalis, pig, China | 95,693 | erm(B), aacA-aphD, aphA3, ble | MH830362 |
| pBS-03 | Bacillus sp., pig, China | 7,446 | aadY | JQ394981 |
| pBS-01 | Bacillus sp., pig, China | 16,492 | erm(B) | NC_013963 |
| pBS-02 | Bacillus sp., pig, China | 16,543 | NC_014557 |
TABLE 3.
Characteristics of completely sequenced cfr-carrying plasmids in Gram-negative bacteria
| Plasmid | Origin | Size (bp) | Colocated resistance gene(s) | GenBank accession no. |
|---|---|---|---|---|
| Unnamed 4 | E. coli, pig, China | 28,519 | CP037908 | |
| pFSEC-01 | E. coli, pig, China | 33,885 | KR779901 | |
| pHNEP129 | E. coli, pig, China | 35,336 | mcr-1.1 | MT667261 |
| pSD11 | E. coli, pig, China | 37,672 | KM212169 | |
| pEC14cfr | E. coli, pig, China | 37,663 | KY865319 | |
| pGXEC6 | E. coli, pig, China | 38,405 | KM580533 | |
| pGXEC3 | E. coli, pig, China | 41,646 | blaCTX-M-14b | KM580532 |
| pFT130-1 | E. coli, migratory bird, China | 52,088 | floR, aphA3, tet(A), blaTEM-176 | CP040091 |
| pHNEP124 | E. coli, pig, China | 60,430 | blaTEM-1, mcr-1.1 | NZ_MT667260 |
| pEC295cfr | E. coli, pig, China | 67,077 | erm(B) | KY865320 |
| pEC12 | E. coli, pig, China | 70,158 | MG677985 | |
| pHNFP671 | E. coli, pig, China | 82,807 | KP324830 | |
| pHNEP28_cfr | E. coli, livestock farm, China | 108,837 | tet(M), qnrS1, blaTEM-1 | KT845955 |
| pSCEC2 | E. coli, pig, China | 135,615 | floR, strA, strB, tet(A), sul2 | KF152885 |
| pYPR25-2 | P. rettgeri, duck, China | 35,276 | CP060728 | |
| plas1.1.1 | P. mirabilis, pig, China | 12,795 | CP047113 | |
| pJPM35-2 | P. mirabilis, duck, China | 35,276 | CP053900 | |
| pG11-51 (p52) | P. cibarius, goose, China | 51,644 | ble | CP047287 |
| pG32-51 | P. cibarius, goose, China | 51, 686 | ble | CP053373 |
| pZF1-cfr | P. cibarius, pig, China | 59,168 | ble | CP047341 |
| pZF2-cfr | P. cibarius, pig, China | 59,167 | ble | CP045009 |
| pZN3-cfr-121kb | P. vulgaris, pig, China | 121,294 | floR, msr(E), mph(E), lnu(F), aadA2, aacC4, hph, aphA1, tet(B), sul1, sul2, dfrA12, ble, qacEΔ1 | CP047346 |
| pPvSC3 | P. vulgaris, chicken/pig, China | 284,528 | floR, catB3, aadA1, strA, strB, tet(B), sul1, sul2, blaOXA-10, qacEΔ1, ars operon, mer operon | CP034667 |
Plasmids carrying the cfr gene in Staphylococcus spp.
The completely sequenced cfr-carrying plasmids among staphylococci ranged in size between 6,962 and 76,991 bp. All of them—except plasmid pSA8589—harbored one to eight additional resistance genes.
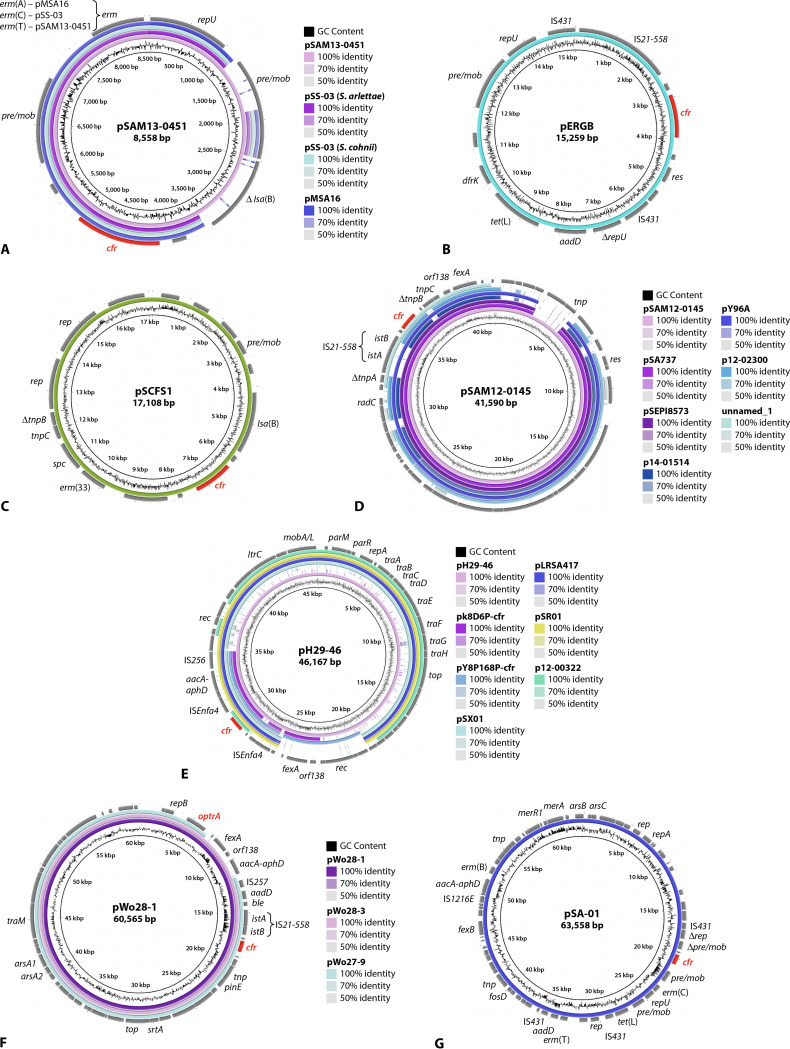
Plasmid pSA8589 from human S. aureus isolate 1900 in the United States is the smallest currently known cfr-carrying plasmid in staphylococci. It is composed only of the cfr gene, a plasmid replication gene (rep), a recombinase gene, a plasmid recombination/mobilization gene (pre/mob), and a truncated tnpB gene for a Tn554-associated transposase (116, 204). The four slightly larger plasmids—pMSA16 from bovine S. aureus in China, pSS-03 from porcine S. cohnii and S. arlettae in China, and pSAM13-0451 from human S. aureus in Ireland—share a common structure comprising the cfr gene, a repU gene for plasmid replication, a pre/mob gene, and different erm genes [erm(A) in pMSA16, erm(C) in pSS-03, and erm(T) in pSAM13-0451] for combined resistance to macrolides, lincosamides, and streptogramin B (MLSB) antibiotics (Table 2). Plasmid pSAM13-0451 is about 1.5 kbp larger than the other plasmids and harbors a truncated lsa(B) gene and a second complete pre/mob gene, of which only remnants are present in the other three plasmids (Fig. 4A).
FIG 4.
Structural comparison of cfr-carrying staphylococcal plasmids (constructed by BLAST Ring Image Generator [BRIG]). Relevant genes with known functions and insertion elements are indicated for the respective reference plasmid in the outer ring. The cfr and the optrA genes are indicated in red. The innermost circle provides a size scale, while the next innermost circle shows the GC content. Other plasmids used for comparison (if available) are indicated by color-coded rings, with the reference plasmid representing the innermost colored ring. (A) pSAM13-0451, pSS-03 (Staphylococcus arlettae), pSS-03 (Staphylococcus cohnii), and pMSA16, (B) pERGB, (C) pSCFS1, (D) pSAM12-0145, pSA737, pSEPI8573, p14-01514, pY96A, p12-02300, and unnamed_1, (E) pH29-46, pk8D6P-cfr, pY8P168P-cfr, pSX01, pLRSA417, pSR01, and p12-00322, (F) and pWo28-1, pWo28-3, and pWo27-9, as well as (G) pSA-01.
Another five plasmids—pK8D55P-cfr, pERGB, pSS-01, pSCFS1, and pH8C110P-cfr—range in size between 12,724 and 24,103 bp and display largely different organizations, which is also reflected by the different colocated resistance genes (Table 2). The three plasmids, pK8D55P-cfr from an S. sciuri isolate of duck origin in China, pERGB from a human S. aureus isolate in Spain (106) (Fig. 4B), and pH8C110P-cfr from an S. sciuri isolate of animal feed origin in China, share the resistance genes aadD and tet(L), which have also been found on other staphylococcal plasmids, such as the multiresistance plasmid pAFS11 (205), but they differ in the additional resistance genes erm(C), dfrK, and erm(B), respectively. Plasmid pSS-01 from a porcine S. cohnii isolate in China harbored the aminoglycoside resistance gene aacA-aphD on a Tn4401-like nonconjugative transposon and the phenicol exporter gene fexA on a Tn558 transposon, in addition to cfr (122). Plasmid pSCFS1 from a bovine S. sciuri in Germany was the first completely sequenced cfr-carrying plasmid (206). This plasmid carries the additional resistance gene lsa(B) for elevated MICs to lincosamides, the spectinomycin resistance gene spc, and the MLSB resistance gene erm(33) (206) (Fig. 4C). The gene erm(33) is a naturally occurring fusion product between erm(A) and erm(C) (207).
Seven plasmids, ranging in size between 37 and 41 kbp and including pSAM12-0145 from a human S. aureus isolate in Ireland (102), p12-02300 and p14-01514 from human S. epidermidis in Germany (130), and the two identical plasmids pSA737 and pSEPI8573 from human S. aureus isolates in the United States (204), as well as an unnamed plasmid and plasmid pY96A, both from S. aureus of porcine origin, shared large portions of similarity, including the cfr region. All seven plasmids harbored only the fexA gene as an additional resistance gene. Large parts of these plasmids exhibit reading frames for proteins with unknown functions. Plasmid pSAM13-0401 from a human S. aureus isolate in Ireland (102) shared the IS21-558-cfr part of this region with several of the aforementioned plasmids (Fig. 4D).
Another five cfr-carrying plasmids, namely p12-00322 from a human S. epidermidis isolate in Germany (130), pSR01 (GenBank accession no. CP048644) and pLRSA417 (208) from human S. aureus isolates in China, pSX01 from a porcine S. xylosus isolate in China (GenBank accession no. KP890694), and pH29-46 from a chicken S. lentus isolate in China (GenBank accession no. CP059680) ranged between 36 and 46 kbp and were related in their structure and organization. The plasmids pY8P168P-cfr (GenBank accession no. CP065798) from a porcine S. saprophyticus isolate and pk8D6P-cfr (GenBank accession no. CP065793) from an S. sciuri isolate of duck origin, both from China, shared the region comprising the genes cfr and aacA-aphD with several of the other plasmids and the fexA region with plasmid pH29-46 (Fig. 4E). Four of these plasmids harbored only the gene aacA-aphD or lsa(B) as an additional resistance gene, while the remaining plasmids carried the genes aacA-aphD and fexA or aacA-aphD, fexA, aadD, and ble in addition to cfr (Table 2). The 45,885-bp plasmid pGMI17-006 (GenBank accession no. CP028164) from a human S. aureus isolate in Denmark carried only the resistance genes fexA and lsa(B) and differed in its structure from the aforementioned plasmids.
The three staphylococcal plasmids that harbored the cfr gene and the oxazolidinone/phenicol resistance gene optrA all originated from porcine S. sciuri isolates in China. They were similar in size and structure (Fig. 4F). While the two larger plasmids, pWo28-1 and pWo28-3, harbored the same set of additional resistance genes, i.e., ble, aadD, aacA-aphD, fexA, and optrA (162, 163), the approximately 5-kbp-smaller plasmid pWo27-9 (GenBank accession no. KX982169) lacked the resistance genes aacA-aphD and fexA.
The cfr-carrying plasmid pSA-01 from a chicken S. arlettae isolate in China had a size of 63,558 bp and a unique structure and carried eight additional resistance genes, including aacA-aphD, aadD, erm(B), erm(C), erm(T), fexB, tet(L), and fosD (158) (Fig. 4G). The largest completely sequenced cfr-carrying plasmid in staphylococci known thus far is plasmid pSP01 from a human S. epidermidis isolate in Italy (135). This conjugative plasmid harbored not only the additional antimicrobial resistance genes lsa(B), blaZ, and msr(A), but also a gene for copper resistance. The lsa(B)-IS21-558-cfr region of plasmid pSP01 shared 99% identity with that of plasmid p12-00322 from a human S. epidermidis isolate in Germany (130, 135).
Partial sequences of numerous cfr-carrying plasmids have been deposited in databases. These sequences range in size from 2,570 to 37,848 bp. The shorter sequences of <10 kbp usually comprise only the cfr gene and its immediate flanking regions, as present in plasmids pSCFS4 from a bovine S. simulans isolate in Germany (2,570 bp; GenBank accession no. AM086400), pSCFS7-like from a human S. epidermidis isolate in Spain (3,824 bp; GenBank accession no. KP229554), pSCFS7 from a human S. aureus isolate in Ireland (4,043 bp; GenBank accession no. FR675942), pHNZT2 from a porcine S. simulans isolate in China (5,086 bp; GenBank accession no. KF861985), pMHZ from a human S. capitis isolate in China (5,247 bp; GenBank accession no. JX232067), pRM01 from a human S. cohnii isolate in China (5,247 bp; GenBank accession no. KC820815); pHNLKJC2 from an S. sciuri isolate from raw pork in China (5,635 bp; GenBank accession no. KF751701), p7LC from a human S. epidermidis isolate in the United States (5,882 bp; GenBank accession no. JX910899), p1128105 from a human S. aureus isolate in the United States (7,020 bp; GenBank accession no. KJ866414), pHNKF3 from a porcine S. simulans isolate in China (7,320; GenBank accession no. KF861984), pHNTLD18 from an S. equorum isolate from retail meat in China (8,510 bp; GenBank accession no. KF751702), and pSS-02 from a porcine S. saprophyticus isolate in China (8,850 bp; GenBank accession no. JF834910), as well as pSCFS3 from a bovine S. aureus isolate in Germany (9,497 bp; GenBank accession no. AM086211). These short plasmid segments occasionally included—besides cfr—another antimicrobial resistance gene, such as aacA-aphD (p7LC, pHNKF3), erm(C) (pHNLKJC2), or fexA (pSCFS4, pSS-02, pHNTLD18, and pSCFS3). Larger plasmid sequences were available from plasmids pWo48-2 from a porcine S. sciuri isolate in China (13,244 bp; GenBank accession no. KX982175), pSS-02 from a human S. haemolyticus isolate in China (13,976 bp; GenBank accession no. JX827253), pJP1-like from a chicken S. lentus isolate in China (14,318 bp; GenBank accession no. KF129408), pSS-01 from a porcine S. cohnii isolate in China (15,703 bp; GenBank accession no. JF834909), p45547X from a human S. aureus isolate in Brazil (16,848 bp; GenBank accession no. KJ192337), pJP2 from an S. rostri isolate of duck origin in China (18,065 bp; GenBank accession no. KC989517), pSS-04 from a porcine S. sciuri isolate in China (18,496 bp; GenBank accession no. KF129410), pSCFS6 from a porcine S. warneri isolate in Denmark (22,010 bp; GenBank accession no. AM408573), and pHK01 from a human S. cohnii isolate in China (37,848 bp; GenBank accession no. KC820816). All of these larger sequences contained one or more additional resistance genes, such as fexA (pSS-02 and pHK01), fexA and aacA-aphD (pSS-01), fexA and lnu(B) (pSCFS6), aacA-aphD and aadD (pWo48-2), fexA, aacA-aphD, and erm(B) (pSS-04), fexA, aacA-aphD, aadD, and ble (pJP1-like), aadY-like, aadD, erm(B), and tet(L) (p45547X), as well as fexA, aacA-aphD, aadD, erm(B), ble, and fosD (pJP2).
Plasmids carrying the cfr gene in Enterococcus spp.
The completely sequenced cfr-carrying plasmids from E. faecalis deposited in the databases are all from food-producing animals in China (Table 3). Three plasmids, ranging in size between 11,940 and 12,270 bp, were structurally closely related to one another and to the corresponding region of the larger plasmid pEF-01 (Fig. 5A) (170). They include an unnamed plasmid from bovine E. faecalis (GenBank accession no. CP028840), plasmid pCPPF5 from a porcine E. faecalis isolate (171), and plasmid pE30 from a not further specified food-producing animal (GenBank accession no. KT717888). None of these plasmids harbored additional resistance genes. It should be noted that plasmid pCPPF5 was unable to confer the PhLOPSA phenotype in E. faecalis, but when the respective cfr gene was cloned in E. coli, it conferred elevated MICs to chloramphenicol and florfenicol (171). Plasmid pEF-01 was the first completely sequenced cfr-carrying plasmid in enterococci (170). This plasmid originated from bovine E. faecalis, had a size of 32,388 bp, and carried the additional resistance gene fexA (170) (Fig. 5A). The largest completely sequenced cfr-carrying plasmid in E. faecalis to date is plasmid p4 from a porcine E. faecalis isolate (GenBank accession no. MH830362). This plasmid had a size of 95,693 bp and harbored the additional resistance genes erm(B), aacA-aphD, aphA3, and ble (Fig. 5B). Partial sequences of cfr-carrying plasmids deposited in GenBank include pHOU-cfr from a human E. faecalis isolate in China (3,494 bp; GenBank accession no. JQ660368) and pW9-2 from a porcine E. faecalis isolate in China (25,761 bp; GenBank accession no. JQ911741). In the latter sequence, the MLSB gene erm(B) was also present.
FIG 5.
Structural comparison of cfr-carrying enterococcal plasmids (constructed by BRIG). Relevant genes with known functions and insertion elements are indicated for the respective reference plasmid in the outer ring. The cfr gene is indicated in red. The innermost circle provides a size scale, while the next innermost circle shows the GC content. Other plasmids used for comparison (if available) are indicated by color-coded rings, with the reference plasmid representing the innermost colored ring. (A) pEF-01, pCPPF5, pE30, and unnamed, (B) p4, pF120805 and pE35048-oc, (C) pFSIS1608820, and (D) pFas4-2.
Three completely sequenced cfr-carrying plasmids have been identified so far in E. faecium (Table 2). The smallest plasmid, pFSIS1608820 from bovine E. faecium in the United States, has a size of 28,222 bp. It harbored the additional resistance genes aphA3, erm(A), erm(B), fexA, optrA, and spc (183) (Fig. 5C). The 41,816-bp plasmid pE35048-oc originated from a human E. faecium isolate in Italy. It carried the additional resistance genes erm(B) and optrA, as well as a truncated lnu(E) gene (180). This plasmid shared large portions of similarity, including the cfr region, with the 72,924-bp plasmid pF120805 of a human E. faecium isolate from Ireland (132) (Fig. 5B). Plasmid pF120805 harbored seven additional resistance genes, including aadE, aphA3, erm(A), erm(B), lnu(A), lnu(B), and optrA. Plasmids pE35048-oc and pF120805 from E. faecium were in part related to each other and to plasmid p4 from E. faecalis (Fig. 5B).
The only completely sequenced cfr-carrying plasmid from E. hirae known thus far is the 85,629-bp plasmid pFas4-2 (209). This plasmid carried the additional antimicrobial resistance genes fexA and lsa(B), as well as an ars operon for arsenic resistance (Fig. 5D).
Incomplete sequences of the plasmids p3-38 (21,116 bp; GenBank accession no. JQ911740) and pW3 (27,360 bp; GenBank accession no. JQ911739), both from porcine E. thailandicus isolates in China, and plasmid pEn24cfr from a porcine E. casseliflavus isolate in China (13,614 bp; GenBank accession no. KF792823) are available. No additional resistance genes were detected in any of these three sequences.
Plasmids carrying the cfr gene in Bacillus spp.
So far, three complete cfr-carrying plasmids have been reported from not further specified Bacillus spp. of porcine origin in China (Table 2) (186–188). Two of the plasmids, pBS-01 and pBS-02, were similar in size (∼16.5 kbp) and shared about 10 kbp of their sequences, including the cfr upstream region. Plasmid pBS-01 carried a complete transposon Tn917 with the MLSB resistance gene erm(B) (186). In contrast, plasmid pBS-02 did not harbor additional resistance genes and also showed a cfr downstream part that differed from the genetic context in plasmid pBS-01 (187). The distinctly smaller plasmid pBS-03 (∼7.5 kbp) shared only the cfr gene and its upstream-located Δpre/mob gene with the other two plasmids (188). However, plasmid pBS-03 harbored a novel streptomycin resistance gene, designated aadY (188).
Plasmids carrying the cfr gene in other Gram-positive bacteria.
Only incomplete sequences of the cfr-carrying plasmid pStrcfr from porcine S. suis in China are available. One sequence describes the cfr region (8,762 bp; GenBank accession no. KC844836) and showed the presence of the cfr gene bracketed by ISEnfa5 elements and inserted into the lnu(E) reading frame (82). The other sequence (13,837 bp; GenBank accession no. KF129409) describes the colocated fexA-carrying transposon Tn558 on pStrcfr.
The incomplete sequence of the ca. 53-kbp plasmid pJP1 from a porcine J. pinnipedialis isolate in China (8,896 bp; GenBank accession no. JQ320084) shows the cfr gene upstream of the resistance genes aadD and ble, as well as the insertion sequence IS21-558. Further PCR screening identified the additional resistance genes aacA-aphD and erm(C) on plasmid pJP1. A plasmid indistinguishable from pJP1 was also identified in porcine M. caseolyticus from China (189).
Plasmids carrying the cfr gene in Escherichia coli.
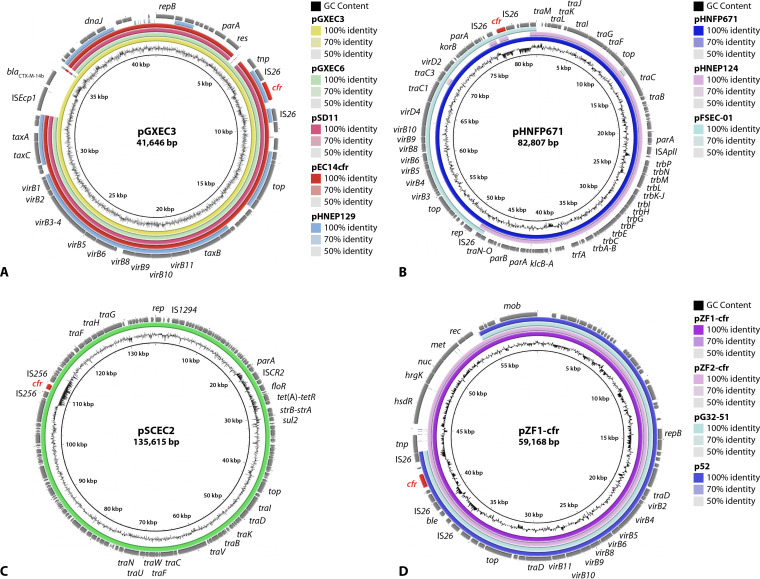
Completely sequenced cfr-carrying plasmids from E. coli, as deposited in the databases, differ distinctly in size and structure (Table 3). All of these plasmids originated from E. coli isolates of animal origin. An unnamed plasmid with a size of 28,519 bp (GenBank accession no. CP037908) is to date the smallest completely sequenced cfr-carrying plasmid from E. coli. This plasmid was of pig origin and did not exhibit additional resistance genes. The five plasmids pHNEP129 (GenBank accession no. MT667261), pSD11 (210), pEC14cfr (196), pGXEC6 (194), and pGXEC3 (194) were all from porcine E. coli isolates in China, ranged in size from 35,336 to 41,646 bp, and were closely related in their structure and organization (Fig. 6A). In contrast to the other plasmids, which did not harbor additional antimicrobial resistance genes, pHNEP129 carried a mcr-1.1 gene for colistin resistance and pGXEC3 had a blaCTX-M-14b gene for an extended-spectrum β-lactamase (ESBL) (194). The 82,807-bp plasmid pHNFP671 from porcine E. coli in China closely resembled in part the 33,885-bp plasmid pFSEC-01 and the 60,430-bp plasmid pHNEP124, both also from porcine E. coli isolates in China. The comparison of the maps of all three plasmids showed that there are overlapping areas between pFSEC-01 and pHNEP124 and suggested that the large plasmid pHNFP671 may have developed from a recombination between the two smaller plasmids (Fig. 6B), during which the additional antimicrobial resistance genes on plasmid pHNEP124—mcr-1.1 and blaTEM-1—were lost. A detailed analysis of the sequence of plasmid pFSEC-01 showed that the cfr gene, bracketed by two IS26 elements in the same orientation, was inserted into a plasmid closely related to pEA3 from the plant pathogen Erwinia amylovora. This observation suggests that plasmid pFSEC-01 may have been transferred between different bacterial genera of both animal and plant origins (193).
FIG 6.
Structural comparison of cfr-carrying plasmids in E. coli and Proteus spp. (constructed by BRIG). Relevant genes with known functions and insertion elements are indicated for the respective reference plasmid in the outer ring. The cfr gene is indicated in red. The innermost circle provides a size scale, while the next innermost circle shows the GC content. Other plasmids used for comparison (if available) are indicated by color-coded rings, with the reference plasmid representing the innermost colored ring. (A) pGXEC3, pGXEC6, pSD11, pEC14cfr, and pHNEP129 (all E. coli), (B) pHNFP671, pHNEP124, and pFSEC-01 (all E. coli), (C) pSCEC2 (E. coli), and (D) pZF1-cfr, pZF2-cfr, pGE32-51, and p52 (all P. cibarius).
The remaining five plasmids included the plasmid pFT130-1 (52,088 bp; GenBank accession no. CP040091) from an E. coli isolate from a migratory bird in China, as well as the plasmids pHNEP28_cfr (108,837 bp) (211), pEC295cfr (67,077 bp) (196), pEC12 (70,158 bp; GenBank accession no. MG677985) (196), and pSCEC2 (135,615 bp) (191), all from porcine E. coli isolates in China. While plasmid pEC12 did not harbor additional antimicrobial resistance genes, plasmid pEC295cfr carried an erm(B) gene. The remaining three plasmids had three to five additional antimicrobial resistance genes, such as qnrS1, tet(M), and blaTEM-1 (pHNEP28_cfr) (211), aphA3, blaTEM-176, floR, and tet(A) (pFT130-1), or floR, strA, strB, sul2, and tet(A) (pSCEC2) (Fig. 6C) (191).
In addition, the 12,390-bp segment of the ca. 110-kbp plasmid pEC-01 from a porcine E. coli isolate in China showed that the cfr gene was bracketed by IS26 elements in the same orientation (190). Plasmid pEC-01 was the first cfr-carrying plasmid described in E. coli.
Plasmids carrying the cfr gene in Proteus and Providencia spp.
Members of the three species P. mirabilis, P. cibarius, and P. vulgaris have been identified as carriers of plasmid-borne cfr genes. The respective isolates were all of food animal origin in China (Table 3). The 12,795-bp plasmid plas1.1.1 (GenBank accession no. CP047113) from a porcine P. mirabilis isolate is the smallest cfr-carrying plasmid within the genus Proteus. Another two plasmids of 35,276 bp, pJPM35-2 (GenBank accession no. CP053900) from P. mirabilis and pYPR25-2 (GenBank accession no. CP060728) from P. rettgeri, both of duck origin, were identical in their nucleotide sequences. None of these three plasmids harbored additional antimicrobial resistance genes.
The complete sequences of four cfr-carrying plasmids from P. cibarius have been deposited in databases. All four plasmids also carried the bleomycin resistance gene ble. The two smaller plasmids, pG11-51 (p52) (51,644 bp) (203) and pG32-51 (51,686 bp) (212), from P. cibarius of goose origin, were almost identical in their structure and organization, as were the two larger plasmids pZF1-cfr (59,168 bp) and pZF2-cfr (59,167 bp) (213). A comparison of the four plasmids showed that the two smaller plasmids shared large parts of their sequences with those of the two larger plasmids (Fig. 6D). This also included the cfr region, which revealed the cfr gene being bracketed by IS26 elements in the same orientation (213). It should be noted that plasmid pG32-51 is described in the database entry as originating from P. cibarius, but in the associated publication to be from P. vulgaris (213). Moreover, the presence of an intact erm(C) gene in plasmid pG11-51 (p52) is indicated in the publication (203), whereas only a truncated erm(B) gene is present in the database entry (GenBank accession no. CP047287).
The two cfr-carrying plasmids from P. vulgaris were distinctly larger than the aforementioned other plasmids from members of the genus Proteus. In addition, they harbored a large number of additional antimicrobial resistance genes. The 121,294-bp plasmid pZN3-cfr-121kbp (GenBank accession no. CP047346) originated from a porcine P. vulgaris isolate and carried the additional antimicrobial resistance genes aacC4 [aac(3)-IV], aadA2, hph [aph(4)-Ia], aphA1 [aph(3′)-Ia], ble, dfrA12, floR, lnu(F), mph(E), msr(E), sul1, sul2, and tet(B), as well as the biocide resistance gene qacEΔ1. While the database entry states that plasmid pPvSC3 originated from a P. vulgaris isolate of chicken origin, it is referred to as originating from a pig in the corresponding publication. This conjugative plasmid was 284,528 bp in size and harbored the additional antimicrobial resistance genes aadA1, blaOXA-10, catB3, floR, strA, strB, sul1, sul2, and tet(B), and the biocide resistance gene qacEΔ1, as well as an ars operon for arsenic resistance and a mer operon for mercury resistance (199) (Table 3).
Transposons and integrative and conjugative elements carrying the cfr gene.
So far, only three cfr-carrying transposons have been described, one in the Gram-negative genus Morganella (83) and the other two in the Gram-positive genus Staphylococcus (214).
The cfr-carrying transposon Tn6451 was recently identified in a porcine M. morganii isolate in China (83). Tn6451 has a size of 111,238 bp. As a derivative of transposon Tn7, it contains a typical Tn7 transposition module comprising the five genes tnsABCDE (83, 215). Tn6451 is inserted into the chromosomal attTn7 site, which is located in the transcriptional terminator of the gene glmS, and produces 5-bp direct repeats at the integration site (5′-AGATA-3′) (83). Usually, Tn7 transposons utilize a “cut-and-paste” transposition mechanism (215, 216), although Tn6451 apparently was not able to excise from its chromosomal location, as no excision product was detected by PCR (83). The cfr gene in Tn6451 was located in a novel genetic structure (IS26-cfr-ΔTn554 tnpB-ΔTn3 tnpA-IS26), which was bracketed by two IS26 copies in the same orientation (83). Recombination of the two IS26 copies resulted in the formation of a TU, which consisted of the gene cfr, ΔTn554 tnpB, ΔTn3 tnpA, and one copy of IS26 (83), as shown by PCR and sequence analysis. In addition to cfr, Tn6451 harbored another 14 antimicrobial resistance genes, including aac(6′)-Ib-cr, aacC4, aadA1, arr-3, blaOXA-1, catB3, dfrA1, dfrA27, floR, hph, sat2, sul1, sul2, tet(B), and the biocide resistance gene qacEΔ1 (83).
Tn6349 is a composite transposon of 48,350 bp recently described in a clinical sequence type 5 (ST5)-MRSA-II strain in Italy (214). Tn6349 was bounded by two copies of IS1216 in the same orientation. It inserted into a ΦN315-like prophage present in the chromosome of the ST5-MRSA-II strain and created 8-bp direct repeats (5′-AAACAAAT-3′) at the integration site (214). The Tn6349 transposon displayed a mosaic structure, which was possibly generated from the recombination between a pE35048-oc-like plasmid (180) and the novel poxtA- and fexB-carrying transposon Tn6657 (214). Concerning the transferability of Tn6349, the formation of a Tn6349-associated TU, most likely resulting from the recombination of the terminal IS1216, was shown. However, neither the transfer of this TU to either E. faecalis or S. aureus by electrotransformation or conjugation nor the activation of the ΦN315-like prophage was observed (214). Most recently, a review was published in which a novel view was proposed about structures that were bounded by members of the IS26 family orientated in the same direction. Such structures were not considered true composite transposons and should be termed as “pseudo-compound transposons” (PCTs) (217). Based on this new nomenclature, Tn6349 should be also classified as a PCT, as the insertion sequence IS1216 bounding Tn6349 belongs to the IS26 family.
Within Tn6349, another cfr-carrying composite transposon, Tn6644, was identified (214). The 5,091-bp transposon Tn6644 was bounded by identical ISEnfa5 copies in the same orientation. Tn6644 was inserted into the lincomycin resistance gene lnu(E) and bracketed by 3-bp direct repeats (5′-GAT-3′) (214). This structure has already been described in plasmids or chromosomal DNA of S. suis (218), E. casseliflavus (184), and E. faecium (180), but only recently received the designation Tn6644 (214). A TU of 3.4 kbp which comprised the cfr gene and one copy of ISEnfa5 was demonstrated by PCR (214).
To date, only two cfr-harboring ICEs, namely ICEPmiChnBCP11 (201) and ICEPvuChnBC22 (200), were identified in P. mirabilis and P. vulgaris, respectively. Both strains were isolated from fecal swabs of diarrheal pigs in China (200, 201). ICEPmiChnBCP1 was 139,487 bp in size and carried—in addition to cfr—19 other antimicrobial resistance genes, including aac(6′)-Ib-cr, aacC4, aadA2, aphA1, arr-3, blaCTX-M-65, blaOXA-1, catB3, dfrA32, ereA, fosA3, floR, hph, strA, strB, sul1, sul2 (two copies), and tet(C). ICEPvuChnBC22 was 148,751 bp in size and harbored the additional resistance genes aac(3)-IV (two copies), aac(6′)-lb-cr (two copies), aadA2, aphA1, arr-3 (two copies), blaDHA-1, blaNDM-1, blaOXA-1, bleMBL, catB4, dfrA32, ereA, hph (two copies), sul1 (three copies), qacEΔ1 (three copies), and tet(A). Both ICEPmiChnBCP11 and ICEPvuChnBC22, belong to the SXT/R391 family, which is one of the largest ICE families (219). Similarly to other members of the SXT/R391 family, the two cfr-carrying ICEs also integrate into the 5′ end of the prfC gene, which codes for the peptide chain release factor 3 (219). In both cfr-bearing ICEs, the cfr gene was surrounded by two copies of IS26, which is widespread among Gram-negative bacteria (217). This means that IS26 may play a crucial role in the integration of the cfr gene into these ICEs. As expected for functionally active ICEs, both ICEs could successfully transfer to the recipient E. coli EC600 strain via conjugation (200, 201). According to the currently published literature, transposons and ICEs—in comparison to plasmids—appear to play a less prominent role in the dissemination of the cfr gene within and beyond species and genera.
Insertion sequences generating cfr-carrying translocatable units.
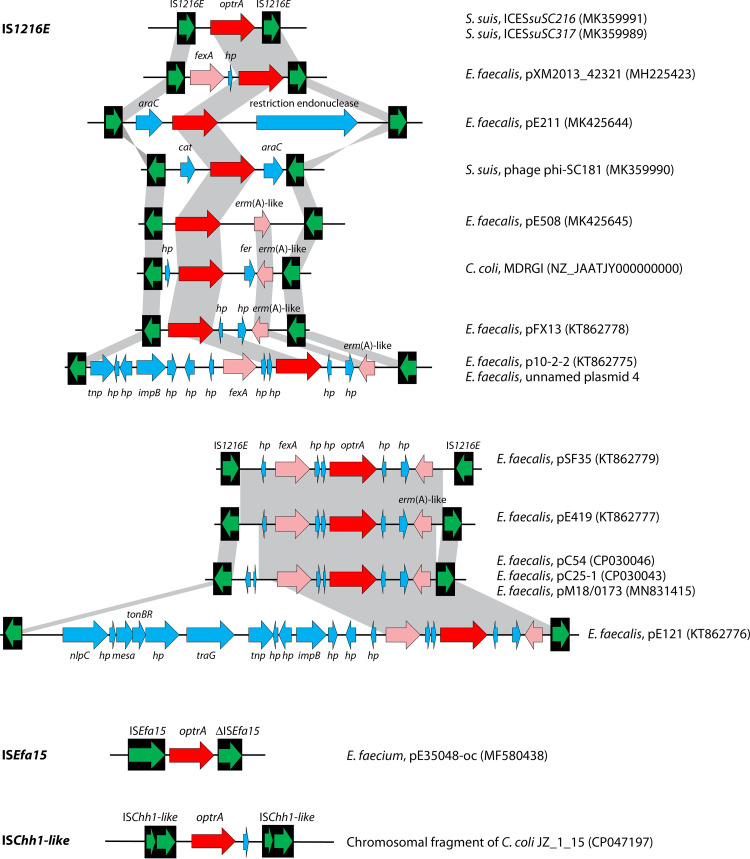
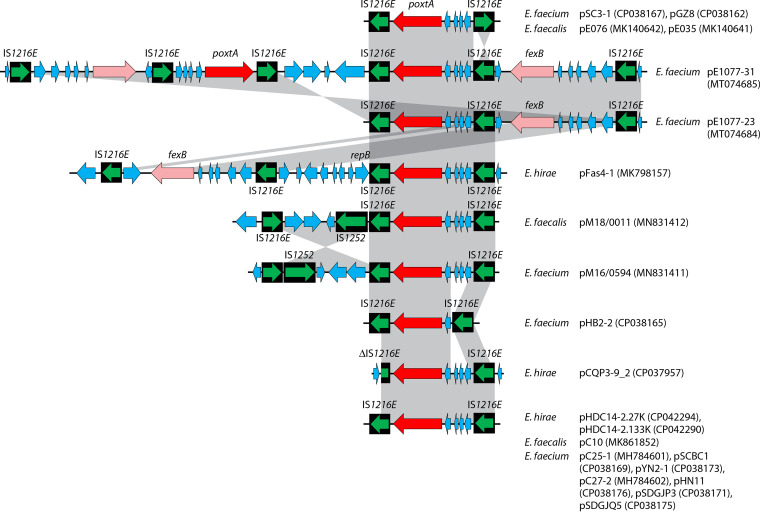
Insertion sequences (IS) are the simplest mobile genetic elements in the bacterial genomes (220). They typically consist of at least one reading frame that codes for the transposase required for mobility and a pair of terminal inverted repeats at both ends (221). IS elements play a vital role in the transfer and spread of antimicrobial resistance genes, since two identical or closely related copies of the IS elements flanking one or more resistance genes can form a “composite transposon”-like structure (222), many of which are now referred to as PCTs (217). Such structures are capable of moving a variety of antimicrobial resistance genes, thereby fostering the dissemination of antimicrobial resistance. So far, seven different IS elements have been identified to bracket the cfr gene and generate such structures, including IS256, IS21-558, IS431, IS1216E, ISEnfa4, ISEnfa5, and IS26 (Fig. 7).
FIG 7.
Insertion sequences flanking the cfr gene. The insertion sequences IS256, ISEnfa5, IS21-558, IS431, IS1216E, ISEnfa4, and IS26 are displayed as black boxes, with the green arrow(s) inside symbolizing the respective transposase gene(s). The cfr gene is shown as a red arrow. Additional genes are shown as blue arrows. In all cases, the arrowhead indicates the direction of transcription. Whenever direct repeats were identified at the termini of the IS elements that flank the cfr region, they are indicated in boxes. The gray-shaded area indicates >99% nucleotide sequence identity. For each specific IS-cfr-IS arrangement, the bacterial species, the location (plasmid/chromosomal DNA) and the database accession number (in brackets) are given on the righthand side.
(i) IS256-cfr-IS256.
IS256, first detected in S. aureus, is composed of a single open reading frame (ORF) that codes for a transposase flanked by imperfect inverted repeats. The IS256 element typically creates 8- or 9-bp target site duplications (TSDs) upon transposition (223). The element is widespread in both chromosomal DNA and plasmids among Gram-positive bacteria, such as Staphylococcus spp. and Enterococcus spp., and is rarely reported in Enterobacteriaceae (190). The cfr gene bracketed by IS256 in the same orientation was initially detected on the IncA/C plasmid pSCEC2 from a porcine E. coli isolate in China (190). The IS256-flanked structure was not stable, as the cfr-carrying central region plus one IS256 could form a TU via IS256-mediated recombination (190). Shortly after this report was published, an identical structure bounded by IS256 was also described in the chromosomal DNA of chicken S. lentus LQQ24, LQW5, and LQW12, as well as on plasmid pSS-04 from porcine S. sciuri GN5-1 in China (92). It should be noted that on plasmid pSS-04, the ORF of the righthand IS256 element (designated here “IS256-like”) exhibited only 95% (1,115/1,173) nucleotide sequence identity to that of the IS256 first identified in S. aureus, and no direct repeats were detected (92). Moreover, a BLASTN search revealed that the “IS256-cfr-IS256” structure was also found in chromosomal DNA of S. lentus H29 of chicken origin in China (Fig. 7). In S. lentus LQQ24, S. lentus H29, and plasmid pSCEC2, the characteristic 8-bp TSDs were observed immediately downstream of the left IS256 and upstream of the right IS256; however, their nucleotide sequences are distinct from each other (Fig. 7). In S. lentus LQW12, it is not possible to determine whether there are direct repeats at both ends of the IS256-cfr-IS256 structure, since the complete sequence of the right-hand IS256 element was not obtained (92). Recently, an IS256-flanked structure that comprised the resistance genes cfr and aacA-aphD, as well as a merR-like transcriptional regulator gene, was detected on the S. saprophyticus plasmid pY8P168P-cfr.
(ii) IS21-558-cfr-IS21-558.
IS21-558, also called ISSau9, is a member of the IS21 family. The IS21-558 element was originally identified on plasmid pSCFS3 recovered from a S. aureus strain of pig origin in Germany (117). It consists of two overlapping ORFs, istAS and istBS, encoding 445-amino-acid (aa) and 250-aa proteins, respectively (93). The cfr-harboring segment bounded by two directly oriented copies of IS21-558 was initially described in a variant of the transposon Tn558 that was located on the ca. 43-kbp plasmid pSCFS6 from both porcine S. warneri and S. simulans isolates in Denmark (93). The clindamycin resistance gene lsa(B) was also present in the ∼4.7-kbp cfr-carrying central region and was located immediately downstream of the cfr gene. A 6-bp TSD (5′-TACGTC-3′) was found at both ends of the IS21-558-cfr-IS21-558 structure (93). In addition, a BLASTN search showed that the same cfr-carrying structure was also present on the ca. 46-kbp plasmid pGMI17-006 from S. aureus strain CFSAN064038 in Denmark (Fig. 7). This observation suggests that the IS21-558-cfr-IS21-558 structure has the ability to spread between different staphylococcal species.
(iii) IS431-cfr-IS431.
IS431, also termed IS257, is found in Staphylococcus species. For many years, the two names IS431 and IS257 have been used to refer to the same or closely related IS elements (224). To date, two cfr-positive plasmids in which the cfr-carrying structure bracketed by IS431 orientated in the same direction is present, p12-00322 from human S. epidermidis in Germany and pSA-01 from chicken S. arlettae in China, have been identified (130, 158). On p12-00322, the cfr-carrying central region, surrounded by IS431 in the same orientation, contained one copy of the IS21-558 element and the gene lsa(B) conferring low-level clindamycin resistance (130). Whether the IS431-cfr-IS431 structure in p12-00322 can form a TU remains to be tested. On pSA-01, an approximately 11.5-kbp segment surrounded by IS431 comprises, besides the cfr gene, the MLSB resistance gene erm(C), the tetracycline resistance gene tet(L), two intact copies of the recombination/mobilization gene pre/mob, a truncated Δpre/mob gene, and two replication-associated genes, namely the plasmid replication gene repU and a truncated Δrep gene (158). Moreover, the structure flanked by IS431 on pSA-01 has been confirmed to be unstable, as the presence of a 12,481-bp circular intermediate was confirmed by PCR (158). On both plasmids, no direct repeats are found at either end of the IS431-cfr-IS431 structure (Fig. 7). Additional resistance genes are colocated in the IS431-cfr-IS431 structure in both cfr-positive plasmids. Coselection and cotransfer of the cfr gene possibly may occur under the selective pressure imposed by the use of the respective antimicrobial agents.
(iv) IS1216E-cfr-IS1216E.
The IS1216E element with a size of 808 bp was first found in E. faecium and belongs to the IS6 family. The involvement of IS1216E in the mobility of the gene cfr was first described in the nonconjugative plasmid pEF-01 from E. faecalis strain EF-01, which was isolated from bovine feces in China (170). On plasmid pEF-01, two directly oriented copies of the IS1216E element flanked a 12.4-kbp segment that carried the cfr gene. However, no direct repeats were observed at both ends of the IS1216E-cfr-IS1216E structure (170). This structure was regarded as an active TU due to the presence of a circular form containing one intact IS1216E element and the sequence between the two copies of IS1216E (170). However, there are to date no other reports about the IS1216E-cfr-IS1216E structure apart from plasmid pEF-01. The definitive role of IS1216E elements in spreading the oxazolidinone resistance gene cfr remains to be confirmed.
(v) ISEnfa4-cfr-ISEnfa4.
The IS256 family element ISEnfa4 encodes a single transposase of 390 aa and harbors imperfect 26-bp terminal inverted repeats (175). Originally, the ISEnfa4 element (initially also designated IS256-like) was found in close proximity to cfr on plasmid pSS-01 from porcine S. cohnii (122). On plasmid pSS-01, an approximately 8.5-kbp segment harboring a Tn4001-like transposon, cfr, orf1, and ISEnfa4 was flanked by 8-bp direct repeats (5′-TGTTCGAG-3′) at its ends (122). This identical structure was also present on plasmid pSX01 from S. xylosus (Fig. 7). The same cfr-harboring segment, flanked by other direct repeats (5′-GAAAATCA-3′), was observed on several other plasmids from Staphylococcus, including pLRSA417 and pSR01 from S. aureus, and pH29-46 from S. lentus (208). A similar genetic context flanking the cfr gene was found on another three plasmids with partial sequence, including pMHZ from S. capitis, pRM01 from S. cohnii, and pHNKF3 from S. simulans (208). In addition, the “ISEnfa4-cfr-ISEnfa4” structure was also described on three conjugative plasmids of different sizes from porcine Enterococcus isolates, namely, pW9-2 from E. faecalis, as well as p3-38 and pW3, both from E. thailandicus (175). On the three plasmids, a 4,447-bp cfr-bearing region bounded by two ISEnfa4 elements in the same orientation revealed the presence of 8-bp TSDs. Two different 8-bp TSDs were observed in the three plasmids (175). A free circular intermediate form containing the cfr gene region and one intact ISEnfa4 element could be obtained via PCR assays (175). The partial sequence of the cfr-harboring plasmid pHOU-cfr from human clinical E. faecalis isolate 603-50427X in Thailand (166) shared high homology with the ISEnfa4-cfr-ISEnfa4 structure on the aforementioned three plasmids (Fig. 7).
(vi) ISEnfa5-cfr-ISEnfa5.
ISEnfa5, a member of the IS3 family, was originally identified on plasmid pStrcfr from a porcine S. suis isolate in China (82). It is composed of two overlapping reading frames encoding proteins of 224 aa and 299 aa, respectively (82). The ISEnfa5 element has imperfect terminal inverted repeats of 39 bp (right inverted repeat [IRR]) and 40 bp (left inverted repeat [IRL]) at its termini (82). On the plasmid pStrcfr, the gene cfr was initially found to be flanked by two copies of ISEnfa5 in the same orientation, and 3-bp direct repeats (5′-GAT-3′) were present immediately upstream and downstream of the ISEnfa5-cfr-ISEnfa5 structure (82). Subsequently, the same genetic structure was also reported on plasmids or in the chromosomal DNA of enterococci and staphylococci of different origin, such as human S. aureus AOUC-0915 in Italy (225), porcine E. casseliflavus En83 and En77 in China (184), plasmid pE35048-oc from an E. faecium isolate of human origin in Italy (180), plasmid p4 of a porcine E. faecalis (GenBank accession no. MH830362), and plasmid pF120805 from an E. faecium isolate of human origin in Ireland (132) (Fig. 7). In addition, another 3-bp direct repeat (5′-ACA-3′) flanking the ISEnfa5-cfr-ISEnfa5 structure was found in the chromosomal DNA of S. lentus LQQ9 of chicken origin in China (92). The chicken S. lentus isolates LQW11, LQW6, LQQ37, and LQQ27-2, although not sequenced, also showed this structure in their chromosomal DNA (92). The ISEnfa5-cfr-ISEnfa5 structure is functionally active, as shown by the presence of a TU containing the gene cfr, one copy of ISEnfa5, and the sequences between cfr and the two ISEnfa5 copies (82). This may in part explain the wide distribution of this structure.
(vii) IS26-cfr-IS26.
The insertion sequence IS26 is 820 bp in size and consists of a 705-bp reading frame that encodes a single transposase. IS26 was originally assigned to the IS6 family. However, recently the IS6 family has been redefined as the IS26 family (226). The IS26 element is most commonly found associated with antimicrobial resistance genes in Gram-negative bacteria (227, 228). The involvement of IS26 elements in the movement of the gene cfr was first identified in porcine P. vulgaris strain PV-01 in China in 2011 (198). An approximately 7-kbp cfr-containing fragment bracketed by two IS26 copies in the same orientation was integrated into the chromosomal fimD gene of PV-01 (198). Some other genes were also identified in the cfr-containing fragment, including a recombination/mobilization gene (pre/mob), a truncated Tn554 transposase B gene (ΔtnpB), a recombinase gene (rec), and two truncated plasmid replication-associated genes (Δrep) (198). Direct repeats (8 bp; 5′-GTTGATAC-3′) were detected immediately upstream and downstream of the inserted region (198). During the following years, another six cfr-carrying fragments with distinct structures bounded by IS26 have been identified in the chromosomal DNA or on plasmids of Proteus spp. and E. coli (Fig. 7). The second type of IS26-cfr-IS26 structure was described on a ca. 284-kbp plasmid pPvSC3 from a P. vulgaris isolate of pig origin in China (199). The cfr-bearing central region on pPvSC3 displayed partial homology to that in P. vulgaris PV-01. In pPvSC3, the rec gene was disrupted into two parts by another IS26 element, and the IS26 elements were oriented in the opposite direction compared to the gene cfr (199). Similarly to the situation in P. vulgaris PV-01, a cfr-carrying segment plus an IS26 element could also be looped out via IS26-mediated recombination (199). The third type of IS26-cfr-IS26 was reported on plasmid pEC295cfr from the porcine E. coli isolate LN310P in China (196). In addition to the genes rec and pre/mob, the IS26-flanked region on pEC295cfr also contained a transcriptional activator gene, ramA, and an ORF for a putative inner membrane protein. It should be mentioned that on plasmid pEC295cfr, the two IS26 copies were in the opposite orientation (196). The fourth type of IS26-cfr-IS26 structure was first found on the conjugative plasmid pFSEC-01 from porcine E. coli in China (193). Unlike the first three types, the ORFs between the gene cfr and the righthand IS26 element were missing, and a reading frame encoding a 342-aa truncated Rep protein was present immediately upstream of the cfr gene on pFSEC-01. No cfr-carrying intermediate circular forms were detected on pFSEC-01 (190). The same cfr-harboring structure identified on pFSEC-01 was also found on the 82,807-bp plasmid pHNFP671 (GenBank accession no. KP324830) from E. coli of pig origin in China. The fifth type of IS26-cfr-IS26 structure was detected on plasmid pJPM35-2 (GenBank accession no. CP053900) from P. mirabilis and pYPR25-2 (GenBank accession no. CP060728) from P. rettgeri, both of duck origin in China. In addition to the cfr gene, only the rec gene exists in the IS26-flanked segment (Fig. 7). The sixth type of IS26-cfr-IS26 structure was found on the two plasmids pHNEP129 (GenBank accession no. MT667261) and pHNEP124 (GenBank accession no. MT667260), both of which were obtained from porcine E. coli isolates in China. To our knowledge, the IS26-cfr-IS26 structure on pHNEP129 and pHNEP124 is the simplest type, and no other ORFs were in the IS26-bracketed central region except the cfr gene (Fig. 7). The seventh type of IS26-cfr-IS26 structure, most commonly found in E. coli, consists of a 1,545-bp cfr-carrying region surrounded by IS26. Since it was first reported on plasmid pEC-01 (190), this structure has been successively identified on other plasmids, all of which were derived from E. coli, including pSD11 (210), pEC14cfr (196), pHNEP28_cfr (211), pFT130-1 (GenBank accession no. CP040091), pGXEC3, and pGXEC6 (194). In addition, this structure was confirmed to be unstable, as the cfr-carrying central region plus one IS26 copy can easily be excised (190, 210). It is worth pointing out that, except for in PV-01, no TSDs were found immediately upstream and downstream of the IS26-cfr-IS26 structure. Recently, a novel mode of IS26 movement was described to explain the formation of this structure (228). A TU, composed of an IS26 element and an adjacent DNA fragment, preferentially targets another copy of IS26 via a conservative process without duplication of the IS and of the target site (228).
Chromosomal cfr Genes
Plasmids, TUs, transposons, and ICEs carrying cfr genes can integrate in whole or in part into the chromosomal DNA of bacteria. In the previous subchapters, multiple cfr genes associated with the aforementioned MGEs have been described. In this subchapter, only a few examples of chromosomally located cfr genes are depicted in more detail.
The MRSA isolate CM05, which originated from a linezolid-treated patient in Colombia, was the first clinical cfr-carrying staphylococcal isolate (229). The cfr gene in this isolate was located within a 15,511-bp region of most likely plasmidic origin that was inserted between bases 1256 and 1257 of the 23S rRNA allele 4 (100). Two regions of 1,546 bp, which included the erm(B) gene and were located in the same orientation, flanked the cfr gene and its downstream IS21-588 element. Recombination between these two identical regions resulted in the formation of a 5,987-bp TU, which comprised the cfr gene, the IS21-558 element, and one copy of the erm(B) region. The loss of this TU yielded a CM05 deletion variant which was linezolid susceptible but had a fitness advantage over CM05 in the absence of a selective pressure (100).
The gene cfr was also found in the chromosomal DNA of a porcine MRSA ST9 isolate from China (120). Detailed analysis of the integration site revealed that a 5,334-bp segment had inserted downstream of the ccr genes into the staphylococcal cassette chromosome mec element (SCCmec) type IVb. This segment comprised the cfr gene bracketed by an IS256 element in the downstream part and an ISEnfa4 element in the upstream part, both in opposite orientations (120). Bearing in mind that oxazolidinones are important for the treatment of infections caused by MRSA, the finding of the methicillin resistance gene mecA together with the gene cfr located in the same SCCmec element is alarming. This strain has been reported in 2014; since then, no other MRSA isolates with this particular SCCmec element have been reported, suggesting that this strain with its SCCmec element has not further disseminated.
The analysis of cfr being bracketed by IS elements showed that such structures—PCTs or true transposons—are not only present on plasmids, but also in the chromosomal DNA. Examples for such chromosomal IS-bounded cfr genes are found in (i) an S. lentus isolate from a chicken in China (92), where cfr was bracketed by IS256, (ii) various S. lentus isolates from chickens in China (92), an S. aureus isolate from a human in Italy (214) and E. casseliflavus isolates from pigs in China (184), where cfr was bracketed by ISEnfa5, and (iii) P. vulgaris from a pig in China (198), where a larger cfr-carrying segment was bracketed by IS26.
In porcine S. sciuri from China, the cfr gene has been detected in close vicinity to a chromosomal optrA gene in a different genetic environment (163). Here, both oxazolidinone resistance genes were located next to each other but in different orientations. The insertion sequence ISEnfa5 was located upstream of cfr, whereas upstream of optrA, the transcriptional regulator gene araC and either complete or partially truncated Tn558 transposons were found (163). In porcine S. cohnii and S. sciuri from China, the cfr gene and its associated IS21-558 element were located in the chromosomal DNA, and two different genetic environments were detected (153). In the S. cohnii isolate, the IS21-558-cfr segment was inserted into a Tn558 transposon, thereby deleting both the tnpA and tnpB genes of this transposon. In the S. sciuri isolate, the resistance genes aadD and ble were found upstream of the IS21-558-cfr segment, while in the downstream region, genes for a resolvase and a transposase, as well as a complete Tn558, were found. Interestingly, exactly the same cfr region was found on a plasmid from porcine S. sciuri, suggesting that the chromosomal location resulted from the partial or complete integration of a former cfr-carrying plasmid (153).
In S. suis from pigs in China, the cfr gene was found in a 57,542-bp chromosomal antibiotic-resistance-associated genomic island, designated ARGI4 (185). The cfr gene was bracketed by a complete IS1216E in the upstream part and a truncated ISEnfa5 and a complete ISTeha2 in the downstream part. The ARGI4 proved to be not transferable by conjugation (185).
MOBILE OXAZOLIDINONE RESISTANCE GENE cfr(B)
Geographical Distribution and Host Bacteria of the cfr(B) Gene
The gene cfr(B) has so far only been detected in two genera, Clostridioides and Enterococcus. All cfr(B)-carrying isolates known thus far are from humans. cfr(B)-positive Clostridioides difficile (formerly known as Clostridium difficile or Peptoclostridium difficile) isolates have been identified in China (GenBank accession no. CP003939), Spain (49), Greece (230), Sweden (GenBank accession no. MPDX01000657), and the United Kingdom (GenBank accession no. HG002396 and HG002389), as well as in Honduras and Chile (52). E. faecalis isolates harboring the cfr(B) gene were found in Japan (231), as well as in Panama and the United States (167), whereas the corresponding E. faecium isolates were detected in Germany (232), the Netherlands (GenBank accession no. NXPC01000098 and NXPD01000081), and the United States (48, 79). In available information, all cfr(B)-carrying bacteria exhibited linezolid MICs above the clinical breakpoint for resistance.
Mobile Genetic Elements That Are Involved in the Dissemination of the cfr(B) Gene
Plasmids carrying the cfr(B) gene.
To the best of our knowledge, the sequence of a single complete plasmid that harbors the cfr(B) gene has been deposited in GenBank and associated databases (GenBank accession no. LR135358). This plasmid, designated plasmid 2, is 293,851 bp in size, originates from an E. faecium isolate of human origin in the Netherlands, and—besides cfr(B)—does not carry additional resistance genes (Table 4). Large plasmids of ≥200 kbp that carry the gene cfr(B) in variants of Tn6218 were described in E. faecium isolates of human origin in Germany (232).
TABLE 4.
Characteristics of completely sequenced plasmids carrying cfr(B), cfr(C), or cfr(D) genes
| cfr gene | Plasmid | Origin | Size (bp) | Colocated resistance gene(s) | GenBank accession no. |
|---|---|---|---|---|---|
| cfr(B) | Plasmid 2 | E. faecium, human, Netherlands | 293,851 | LR135358 | |
| cfr(C) | pCd13_cfrC | C. difficile, human, Greece | 6,961 | aphA3 | MH229772 |
| pTx-40 | C. coli, cattle, USA | 48,048 | aphA3, ΔaadE, hph, tet(O) | KX686749 | |
| pN61925F | C. coli, cattle, USA | 48,049 | aphA3, ΔaadE, hph, tet(O) | MK541989 | |
| pN61740F | C. coli, cattle, USA | 48,049 | aphA3, ΔaadE, hph, tet(O) | MK541988 | |
| pN46788F | C. coli, cattle, USA | 50,413 | aphA3, ΔaadE, hph, tet(O) | MK541987 | |
| pSH89 | C. coli, pig, China | 57,366 | aphA3, tet(O) | CP047217 | |
| pJZ_1_79 | C. coli, pig, China | 62,417 | aphA3, tet(O) | CP047213 | |
| cfr(D) | p15-307-1_02 | E. faecium, human, France | 103,074 | erm(A)-like, erm(B), optrA | CP044318 |
| pM17/0314 | E. faecium, human, Ireland | 103,600 | erm(A)-like, erm(B), optrA | MN831413 | |
| pBP5067_P1 | E. faecium, human, India | 122,126 | optrA, erm(B), vanA gene cluster (vanRSHAXYZ) | CP059807 | |
| pBA17124_P1 | E. faecium, human, India | 130,516 | optrA, erm(B), vanA gene cluster (vanRSHAXYZ) | CP059785 | |
Transposons carrying the cfr(B) gene.
So far, the gene cfr(B) has been associated with the nonconjugative transposon Tn6218 (48, 233). This transposon or variants thereof have been detected at various positions in the chromosomal DNA of C. difficile, as well as in E. faecium and E. faecalis (48, 232, 233). The prototype Tn6218 from C. difficile strain Ox2167 (GenBank accession no. HG002396) is 8,495 bp in size and originated from the United Kingdom (233). A slightly smaller Tn6218 variant of 8,407 bp (GenBank accession no. KR610408) has been identified in clinical E. faecium isolates from the United States (48). This variant differed distinctly from the other Tn6218 variants in its structure and composition (232). In general, the Tn6218 elements found in E. faecium and C. difficile were essentially the same, except for the integrase genes, whose gene products showed only 86% identity (48). Bender and coworkers found among five German clinical E. faecium isolates Tn6218 elements that were highly similar or even identical to the original Tn6218 (232). The cfr(B) genes in Greek C. difficile isolates were located on chromosomal Tn6218 elements (230). The cfr(B) genes of C. difficile isolates from Honduras were also located on Tn6218-like elements in the chromosomal DNA (52). A Japanese E. faecalis isolate carried a Tn6218 element that was closely related (98.62 to 99.97%) to the corresponding elements from C. difficile isolates in the United Kingdom and China, as well as from E. faecium isolates from Germany and the Netherlands (231). The cfr(B) genes in C. difficile from Chile were located in a not further described chromosomal genetic structure that contained transposase and integrase genes (52).
A comparison of the Cfr(B) proteins found in E. faecalis, E. faecium, and C. difficile revealed 99.7 to 100% identity (48). Similar results by comparing the cfr(B) nucleotide sequences were seen by Kuroda and coworkers, who identified only single-nucleotide polymorphisms (SNPs) at four defined positions between all cfr(B) genes (231).
MOBILE OXAZOLIDINONE RESISTANCE GENE cfr(C)
Geographical Distribution and Host Bacteria of the cfr(C) Gene
The gene cfr(C) has been identified in only three species to date—C. difficile, Clostridium bolteae, and Campylobacter coli. While the cfr(C)-carrying C. difficile isolates were from humans in Belgium (234), France (234), Germany (234), Italy (234), Greece (230), Honduras (52), and Costa Rica (52), the C. bolteae isolate originated from a human in France (234). In contrast, all cfr(C)-harboring C. coli isolates were of animal origin, i.e., from cattle in the United States (50, 235) and from pigs in China (236, 237).
Mobile Genetic Elements That Are Involved in the Dissemination of the cfr(C) Gene
Plasmids carrying the cfr(C) gene.
The smallest plasmid carrying the gene cfr(C) was identified in a C. difficile strain of human origin in Greece. This plasmid of 6,961 bp, designated pCd13_cfrC, comprised a plasmid replication gene, a plasmid recombination gene, and the aphA3 gene for resistance to kanamycin, neomycin, and amikacin besides cfr(C). Distinctly larger plasmids carrying the cfr(C) gene have been detected in C. coli (Table 4). In bovine C. coli from the United States, the cfr(C) gene was found on the conjugative 48,048-bp plasmid pTx-40 (50). The tetracycline resistance gene tet(O), the kanamycin/neomycin/amikacin resistance gene aphA3, the hygromycin resistance gene hph and a truncated streptomycin resistance gene aadE were detected in the vicinity of the cfr(C) gene (50). Two plasmids, pN61925F and pN61740F, which closely resembled pTx-40 in size (48,049 bp) and structure and also carried the same set of antimicrobial resistance genes, were identified in bovine C. coli in 2014 as part of the U.S. National Antimicrobial Resistance Monitoring System (NARMS) program (235). A slightly larger plasmid from C. coli in the United States, pN46788F with a size of 50,413 bp, closely resembled the aforementioned plasmids (Fig. 8). In C. coli from pigs in China, two conjugative plasmids of 62,417 bp (pJZ_1_79) and 57,366 bp (pSH89) were found. Both of them harbored the tet(O) gene as well as the genes cfr(C) and aphA3, albeit with the insertion sequence IS607* upstream of cfr(C) (237).
FIG 8.
Structural comparison of cfr(C)-carrying plasmids in C. coli (constructed by BRIG). Relevant genes with known functions and insertion elements are indicated for the respective reference plasmid in the outer ring. The cfr(C) gene is indicated in red. The innermost circle provides a size scale, while the next innermost circle shows the GC content. Other plasmids used for comparison are indicated by color-coded rings, with the reference plasmid representing the innermost colored ring. The plasmids used for this comparison are pN46788F, pN61740F, pN61925F, and pTx-40.
Integrative and conjugative elements carrying the cfr(C) gene.
Candela and coworkers described three different types of ICE-like elements in C. difficile, designated ICEDA275, ICEF548, and ICEDA203. Partial structures have been reported for all three ICEs, and a size of 24,150 bp has been indicated for ICEDA203 (234). Unfortunately, the sequences of these three ICEs have not been deposited in any databases. According to the authors, GenBank searches identified the ICE90B3, which differed from ICEDA203 by one base pair exchange, in C. bolteae (234). PCR assay with primers located at the termini of ICE90B3 and directed toward the flanking regions showed that a TU, which points toward the mobility of the element, was formed in C. bolteae (234). The cfr(C) genes in C. difficile from Honduras and Chile have also been reported to be located on ICEF548-like elements (52).
Chromosomal cfr(C) Genes
Liu and coworkers identified the gene cfr(C) in the chromosomal DNA of four C. coli isolates from pigs in China (236). Detailed sequence analysis revealed that the cfr(C)-carrying genomic regions represented three novel multidrug resistance genomic islands (MDRGIs) of different sizes. MDRGI1 was 17,277 bp in size and was inserted into a gene for a hypothetical protein (236). It harbored the resistance genes Δtet(O), lnu(C), aac, aacA-aphD, aadE, Δsat4, aphA3, cfr(C), and tet(O). MDRGI2 was 20,074 bp in size and was inserted between two genes for hypothetical proteins (236). It carried the resistance genes tet(O), lnu(C), spc, aphA7, ΔaadE, sat4, aphA3, cfr(C), aadE, and Δtet(O). Part of the resistance gene region in MDRGI2 was flanked by 629-bp direct repeats. PCR assays confirmed the presence of a TU of 5,815 bp, which was generated by recombination between the direct repeats. This TU comprises the resistance genes ΔaadE, sat4, aphA3, and cfr(C) in addition to another four genes (236). The formation of such TUs might explain why the same (or a very similar) set of resistance genes is found in cfr(C)-carrying C. coli. MDRGI3 was inserted into the CRISPR-associated gene. The available partial sequence revealed that MDRGI3 included at least the resistance genes ΔaadE, sat4, aphA3, and cfr(C) (236).
In another study, five different chromosomal regions in which the cfr(C) gene had been inserted were identified among porcine C. coli isolates from China. In all genetic environments, the cfr(C) gene was located in close proximity to the aphA3 gene. The insertion sequence IS607* was located upstream of cfr(C)-aphA3 in one of the five regions, while the insertion sequence ISCco7 was found downstream of cfr(C) in two regions. Whether or not these insertion sequences play a role in the mobility of cfr(C) remains to be answered. Further antimicrobial resistance genes present in the five chromosomal regions included tet(O) in all but one region, as well as sat4, aadE, and aph(2″), which were found in two of the five environments in one or two copies (237).
MOBILE OXAZOLIDINONE RESISTANCE GENE cfr(D)
Geographical Distribution and Host Bacteria of the cfr(D) Gene
Comparatively little information is currently available about the gene cfr(D). It has been identified only in E. faecium and E. faecalis isolates from humans. The corresponding E. faecium isolates originated from Australia (51), France (238), Ireland (239), and the Netherlands (GenBank accession no. LR135354). The cfr(D) gene was identified in E. faecalis isolates from Italy and Spain (174, 240). In all cfr(D)-carrying isolates, the phenicol/oxazolidinone resistance gene optrA was also present.
Mobile Genetic Elements That Are Involved in the Dissemination of the cfr(D) Gene
Plasmids carrying the cfr(D) gene.
Only a few completely sequenced plasmids carrying cfr(D) have been described. The 103,074-bp plasmid p15-307-1_02 originated from a French E. faecium isolate (238) and the 103,600-bp plasmid pM17/0314 (239) from an Irish E. faecium (Table 4). Both plasmids also carried the resistance genes erm(A)-like, optrA, and erm(B). The 122,126-bp plasmid pBP5067_P1 (GenBank accession no. CP059807) and the 130,516-bp plasmid pBA17124_P1 (GenBank accession no. CP059785) were from human E. faecium isolates from India. These plasmids were related in their structures and carried the additional resistance genes optrA and erm(B), as well as the vanA gene cluster (vanRSHAXYZ) (Table 4). A partial sequence, which comprises the cfr(D) region, is available for plasmid 4 of the Dutch E. faecium isolate E8014. In the three plasmids pM17/0314, p15-307-1_02, and plasmid 4, the cfr(D) gene and a complete or a truncated guaA gene, which encodes a glutamine-hydrolyzing GMP synthase, are flanked by IS1216 elements in the same orientation (238). In the two Indian plasmids, the guaA gene is missing, but the IS1216 elements are present. In the Australian E. faecium isolate E637001, the cfr(D) contig exhibited 100% nucleotide sequence identity with the corresponding region of plasmid 2 from France (51). The Spanish E. faecalis isolate X528 carried the cfr(D) gene on a 4,545-bp contig, which was identical to the respective region of the plasmid 4 from the Dutch E. faecium E8014. Whether or not the cfr(D) gene in the Spanish E. faecalis isolate is plasmid-borne remains to be answered, as conjugation assays failed to show the transferability of cfr(D) (240).
MOBILE OXAZOLIDINONE RESISTANCE GENE cfr(E)
Geographical Distribution and Host Bacteria of the cfr(E) Gene
The gene cfr(E) is the youngest member in the cfr family. It has so far only been described in a single C. difficile isolate of human origin from Mexico (52).
Mobile Genetic Elements Associated with the cfr(E) Gene
The cfr(E) gene is potentially part of a mobile genetic element, since genes for a DNA invertase, a recombinase, an ATP binding protein, a transcriptional regulator, and two hypothetical proteins have been detected in the close vicinity to cfr(E). The entire segment has been reported to be inserted into the chromosomal gene adeC, which codes for an adenine deaminase (52).
MOBILE OXAZOLIDINONE RESISTANCE GENE optrA
Geographical Distribution and Host Bacteria of the optrA Gene
According to the PubMed and NCBI Nucleotide databases, the gene optrA is present in 29 countries/regions of six continents (Fig. 9), 18 of which were also positive for cfr-carrying bacteria. The host bacteria carrying the optrA gene are mainly Enterococcus spp., although several studies have also identified optrA in the genera Staphylococcus, Streptococcus, Clostridium, and Campylobacter. In addition, optrA genes have been identified in the genomes of members of the genera Fusobacterium, Listeria, and Salmonella. Similarly to the situation with the cfr gene, optrA-positive bacteria also originated from humans, various animals (cattle, pigs, chickens, turkeys, ducks, dogs, and cats), and food of animal origin (eggs, pork, beef, and chicken and turkey meat), as well as from environmental sources. In addition, optrA-positive enterococci also originated from vegetables, such as caraway seeds, cucumber, and onion.
FIG 9.
Geographical distribution of optrA-carrying bacteria. The countries in blue are those from which the occurrence of optrA-carrying bacteria has been reported.
Occurrence of the optrA gene in Enterococcus spp.
Initially identified in E. faecalis and E. faecium from humans, pigs, and chickens in China in 2015 (54), the optrA gene is widespread in Enterococcus spp., especially in E. faecalis and E. faecium. optrA-carrying E. faecalis strains have been detected in humans from Australia (51), Austria (241), Bangladesh (242), Belgium (243), China, including Taiwan (54, 71, 165, 167, 244–251), Colombia (252), Denmark (253), Ecuador (167), Egypt (254), France (167, 255), Germany (256), Greece (257), Guatemala (71, 167), Ireland (69, 70, 167, 239), Japan (231), South Korea (258, 259), Malaysia (69, 167), Mexico (71, 167), Panama (167), Poland (260), Spain (240, 261, 262), Sweden (167), Thailand (167), Tunisia (263), Turkey (264), and the United States (79, 264). E. faecalis isolates carrying the optrA gene were also found in various animals, including pigs from Brazil (169, 265), China (173, 246, 266–270), Italy (174), South Korea (271), and the United States (183), chickens from China (246), Colombia (272), South Korea (271), and Tunisia (273, 274), cattle from the United States (183), and dogs from China (164). In addition, optrA-positive E. faecalis isolates have also been obtained from animal food (beef, chicken meat, pork, and egg) and vegetables (caraway seeds and cucumber) in China (164), beef in Denmark (275), and chicken meat in South Korea (276, 277), as well as from wastewater in Tunisia (278). In China, optrA-positive E. faecalis isolates have also been identified on shared bicycles (279).
Similarly, optrA-positive E. faecium isolates were also widely identified in humans from Australia (51), Belgium (243), China (245, 248–250), France (255), Germany (256), Greece (280), Ireland (132, 239), Italy (180), South Korea (259), Pakistan (281), Spain (262), Turkey (264), and the United States (264). The optrA-positive E. faecium isolates were also isolated from pigs in China (266, 267) and Italy (174), cattle in South Korea (271) and the United States (183), chickens (271), ducks (282) and chicken meat (276) in South Korea, and turkey meat in Denmark (275), as well as from environmental samples in Spain (283). Apart from E. faecalis and E. faecium, the optrA gene has also been detected in E. casseliflavus isolates from onions, beef, and chicken meat (164), in E. gallinarum from a pig (284), and in E. avium, E. hirae, E. thailandicus, E. gallinarum, and not further specified Enterococcus isolates of human origin, all from China (248, 249). A recent study described the presence of optrA-positive E. avium, E. thailandicus, and E. gallinarum isolates from pigs in Italy (174).
Occurrence of the optrA gene in Staphylococcus spp.
Unlike the widespread occurrence of the gene optrA in the genus Enterococcus, this gene has been rarely reported in the genus Staphylococcus. optrA-carrying MRSA isolates were found both in humans and animals (pigs, chickens, and ducks) from China (98). However, the optrA-positive S. sciuri isolates occurred exclusively in animals, such as dogs (153, 164), pigs (153, 162, 163), and cats (153) from China and turkeys from Egypt (154). Another group of optrA-carrying CoNS species, including S. xylosus, S. lentus, S. saprophyticus, and S. epidermidis, has so far only been found in turkeys from Egypt (154).
Occurrence of the optrA gene in other Gram-positive bacteria.
There are several reports on the presence of the optrA gene in the genus Streptococcus, including S. agalactiae, S. gallolyticus, and S. suis. The optrA-positive S. agalactiae and S. gallolyticus were found exclusively in humans from China (285) and Thailand (71), respectively, whereas the corresponding S. suis isolates were present only in pigs from China (185, 286–288). Another species of optrA-carrying bacteria is Clostridium perfringens, so far only identified in chickens from China (289). Moreover, whole-genome sequences of several Listeria monocytogenes isolates (GenBank accession no. AARQTE010000003, AARQTE010000015, AARQTG010000014, AARQTK010000001, AARQTK010000013, and AARQTG010000006) from environmental swabs in the United States revealed the presence of an optrA gene.
Occurrence of the optrA gene in Gram-negative bacteria.
So far, there are only two published reports about the identification of the optrA gene in Gram-negative bacteria, both in the genus Campylobacter. The respective optrA-positive C. coli originated either from chickens and ducks (290) or from pigs (291) in China. However, complete optrA genes have also been identified in the whole-genome sequences of Campylobacter jejuni from duck meat in China (GenBank accession no. CP048771), Fusobacterium sp. from a human fecal sample in China (GenBank accession no. CP060637), and Salmonella sp. from a cloacal swab of a chicken in China (GenBank accession no. QFLJ01000014).
Mobile Genetic Elements That Are Involved in the Dissemination of the optrA Gene
Numerous plasmids carrying the gene optrA have been described in various Gram-positive bacteria. As done for the cfr-carrying plasmids, we focused the description of the optrA-harboring plasmids on their size, structure, and organization, as well as the colocated additional genes that conferred resistance to antimicrobial agents, heavy metals, or biocides (Table 5).
TABLE 5.
Characteristics of completely sequenced optrA-carrying plasmids in bacteria
| Plasmid | Origin | Size (bp) | Colocated resistance gene(s) | GenBank accession no. |
|---|---|---|---|---|
| pKUB3007-4 | E. faecalis, human, Japan | 36,331 | fexA | AP018547 |
| pKUB3006-4 | E. faecalis, human, Japan | 36,331 | fexA | AP018542 |
| pM17/0149 | E. faecalis, human, Ireland | 36,331 | fexA | MN831410 |
| p6742_1 | E. faecalis, human, Poland | 36,331 | fexA | KY513280 |
| pEFs17-1 | E. faecalis, animal, South Korea | 36,331 | fexA | MT223178 |
| pN48037F-3 | E. faecalis, pig, USA | 40,269 | fexA | CP028723 |
| pN60443F-2 | E. faecalis, cattle, USA | 41,597 | fexA, erm(B) | CP028725 |
| pC25-1 | E. faecalis, pig, China | 45,581 | fexA, erm(A) | CP030043 |
| pAF379 | E. faecalis, urban wastewater, Tunisia | 45,603 | erm(A), spc | NHNF01000009 |
| pEF10748 | E. faecalis, human, China | 53,178 | fexA | MK993385 |
| pL9 | E. faecalis, pig, Brazil | 58,593 | fexA, tet(S) | CP041776 |
| pC54 | E. faecalis, pig, China | 64,500 | fexA, erm(A) | CP030046 |
| pS7316optrA | E. faecalis, human, Japan | 68,368 | fexA, erm(B), tet(L), tet(M) | LC499744 |
| p1 | E. faecalis, pig, China | 74,536 | fexA, erm(A), spc, copper resistance operon | MH830363 |
| pE211 | E. faecalis, pig, China | 77,562 | fexA | MK425644 |
| pEF123 | E. faecalis, chicken, China | 79,682 | fexA, catA, erm(A), erm(B), aphA3, str, tet(M), tet(L), sat4, bcrABR, dfrG | KX579977 |
| pL15 | E. faecalis, pig, Brazil | 82,898 | catA, erm(A), erm(B), tet(M), tet(L), spc, tcr operon, copper resistance operon | CP042214 |
| pE508 | E. faecalis, pig, China | 84,468 | fexA, erm(A), aacA-aphD, tet(L), tet(O/W/32/O) | MK425645 |
| pE211-2 | E. faecalis, pig, China | 87,785 | fexA, erm(A), erm(B), aacA-aphD, aadE, aphA3, tet(L), tet(M), sat4, lnu(B), lsa(E), spw, dfrG | MK784777 |
| pL8 | E. faecalis, pig, Brazil | 91,525 | erm(A), erm(B), lnu(C), spc | CP042217 |
| pE035 | E. faecalis, pig, China | 121,524 | poxtA, fexB, erm(A), erm(B), aacA-aphD, lnu(G), bcrABDR, dfrG | MK140641 |
| pFSIS1608820 | E. faecium, cattle, USA | 28,222 | cfr, fexA, erm(A), erm(B), aphA3, spc | CP028728 |
| pE35048-oc | E. faecium, human, Italy | 41,816 | cfr, erm(B), Δlnu(E) | MF580438 |
| pEfmO_03 | E. faecium, human, Ireland | 58,684 | fexA | MT261365 |
| pF120805 | E. faecium, human, Ireland | 72,924 | cfr, erm(A), erm(B), aphA3, aadE, lnu(A), lnu(B) | KY579372 |
| p15-307-1_02 | E. faecium, human, France | 103,074 | cfr(D), erm(A), erm(B) | CP044318 |
| pM17/0314 | E. faecium, human, Ireland | 103,600 | cfr(D), erm(A), erm(B) | MN831413 |
| pBP5067_P1 | E. faecium, human, India | 122,126 | cfr(D), erm(B), vanA gene cluster (vanRSHAXYZ) | CP059807 |
| pBA17124_P1 | E. faecium, human, India | 130,516 | cfr(D), erm(B), vanA gene cluster (vanRSHAXYZ) | CP059785 |
| Unnamed | E. faecium, human, India | 142,820 | erm(B), aadE, aphA3, tet(S), sat4, vanA gene cluster (vanRSHAXYZ) | CP040238 |
| pWo27-9 | S. sciuri, pig, China | 55,724 | cfr, aadD, ble | KX982169 |
| pWo28-3 | S. sciuri, pig, China | 60,563 | cfr, fexA, aadD, aacA-aphD, ble | KT601170 |
| pWo28-1 | S. sciuri, pig, China | 60,565 | cfr, fexA, aadD, aacA-aphD, ble | KX982171 |
| p2C45 | C. perfringens, chicken, China | 148,618 | fexA, erm(A), lnu(P) | NZ_JAAQTM010000004 |
Plasmids carrying the optrA gene in Enterococcus spp.
The completely sequenced optrA-carrying plasmids in Enterococcus spp. varied in size from 28,222 bp to 142,820 bp (Table 5). All of these plasmids carried 1 to 13 additional resistance genes. The five identical plasmids with a size of 36,311 bp—pKUB3007-4 and pKUB3006-4 (231) from human E. faecalis isolates in Japan, pM17/0149 (239) and p6742_1 (260) from human E. faecalis isolates in Ireland and Poland, respectively, and pEFs17-1 (GenBank accession no. MT223178) from an E. faecalis isolate of not further specified animal origin in South Korea—showed large portions of similarity with pN48037F-3 and pN60443F-2 from porcine and bovine E. faecalis isolates in the United States (183). These latter plasmids are about 3.9 and 5.3 kbp larger than the aforementioned five plasmids. All of these plasmids harbored only the fexA gene as additional resistance gene, except for pN60443F-2, which also harbored an erm(B) gene (Fig. 10A).
FIG 10.
Structural comparison of optrA-carrying plasmids in enterococci (constructed by BRIG). Relevant genes with known functions and insertion elements are indicated for the respective reference plasmid in the outer ring. The optrA gene is indicated in red. The innermost circle provides a size scale, while the next innermost circle shows the GC content. Other plasmids used for comparison are indicated by color-coded rings, with the reference plasmid representing the innermost colored ring. (A) pN60443F-2, pN48037F-3, pEFs17-1, p6742_1, pM17/0149, pKUB3006-4, and pKUB3007-4, and (B) pC54 and pC25-1, as well as (C) unnamed, pBA17124_P1, pBP5067_P1, pM17/0314, and p15-307-1_02.
The 45,581-bp plasmid pC25-1 shared large portions of similarity with the 64,500-bp plasmid pC54 (266). Both plasmids were from porcine E. faecalis in China and carried the additional resistance genes fexA and erm(A) (266) (Fig. 10B). The 45,603-bp plasmid pAF379 from E. faecalis isolated from urban wastewater in Tunisia is so far the only completely sequenced optrA-carrying plasmid from environmental samples. This plasmid showed a distinct structure compared to the other completely sequenced plasmids and carried the Tn554-associated resistance genes erm(A) and spc genes as well (278). Similarly, the 58,593-bp plasmid pL9 (GenBank accession no. CP041776) from a porcine E. faecalis in Brazil had a unique structure and harbored the additional resistance genes fexA and tet(S).
A group of five E. faecalis plasmids, ranging in size between 68,368 and 87,785 bp, included the plasmids pS7316optrA from a human in Japan, pEF123 from a chicken in China, p1 and pE211-2 from pigs in China, and pL15 from a pig in Brazil. These five plasmids showed limited sequence similarity to one another and carried 3 to 13 additional genes, which conferred resistance to phenicols, MLSB antibiotics, tetracyclines, aminoglycosides, trimethoprim, pleuromutilins, and/or bacitracin (Table 5). Two of these plasmids, p1 and pL15, also had copper resistance genes. Another group of plasmids from E. faecalis, which shared limited nucleotide sequence similarity, ranged in size from 53,178 bp to 121,524 bp and included pEF10748 from a human in China, pE211, pE508, and pE035 from pigs in China, and pL8 from a pig in Brazil. Plasmids pEF10748 and pE211 carried only fexA as an additional resistance gene, while the other three larger plasmids harbored four to eight additional resistance genes, including lnu(C), erm(B), erm(A), and spc in pL8 (GenBank accession no. CP042217), aacA-aphD, fexA, tet(L), tet(O/W/32/O), and erm(A) in pE508 (269), and erm(B), aacA-aphD, bcrABDR, erm(A), lnu(G), dfrG, fexB, and poxtA in pE035 (174).
The smallest completely sequenced optrA-harboring plasmid from E. faecium to date is the 28,222-bp plasmid pFSIS1608820 (183). This plasmid originated from cattle in the United States and carried the additional antimicrobial resistance genes erm(B), fexA, erm(A), spc, aphA3, and cfr. Three plasmids from human E. faecium isolates, ranging in size from 41,816 bp to 72,924 bp, differed in their structure and organization. These three plasmids carried distinct additional resistance genes. Plasmid pE35048-oc from Italy also harbored Δlnu(E), cfr, and erm(B) (180); pEfmO_03 from Ireland only harbored fexA (292), while another Irish plasmid, pF120805, carried the seven additional resistance genes cfr, lnu(A), erm(A), erm(B), aphA3, aadE, and lnu(B) (132).
Four optrA-carrying plasmids from human E. faecium, that also harbored the oxazolidinone resistance gene cfr(D) and ranged in size from 103,074 bp to 130,516 bp, included plasmids pM17/0314 from Ireland (239), pBP5067_P1 (GenBank accession no. CP059807) and pBA17124_P1 (GenBank accession no. CP059785) from India, and p15-307-1_02 from France (255). They displayed a similar structure to that of a larger cfr(D)-lacking unnamed plasmid (142,820 bp; GenBank accession no. CP040238) from human E. faecium in India (Table 5). Apart from optrA, all of these five plasmids had three or more additional resistance genes, namely, erm(A), erm(B), and cfr(D) in pM17/0314 and p15-307-1_02, the vanA gene cluster (vanZYXAHSR), erm(B), and cfr(D) in pBA17124_P1 and pBP5067_P1, and the vanA gene cluster (vanZYXAHSR), erm(B), aphA3, sat4, aadE, and tet(S) in the unnamed plasmid (Fig. 10C).
Numerous partial sequences of optrA-carrying plasmids from Enterococcus spp. were found by database search. They ranged in size from 2,452 to 91,477 bp, and almost all were from E. faecalis isolates. Four of the 19 shorter sequences of <10 kbp comprised only the optrA gene, including plasmids p751258 (2,452 bp; GenBank accession no. MF443378) from a human E. faecalis isolate in Ecuador, p539673 (3,880 bp; GenBank accession no. MF443371) and p532444 (4,026 bp; GenBank accession no. MF443370) from human E. faecalis isolates in China, and pL14 (7,644 bp; GenBank accession no. CP043725) from a porcine E. faecalis isolate in Brazil, as well as the two larger incompletely sequenced plasmids, p_optrA 15–307-1_NODE_07 (10,411 bp; GenBank accession no. PHLC01000010) and p599799 (14,437 bp; GenBank accession no. MF443373), both from human E. faecalis isolates in France and Thailand, respectively. In the remaining 15 shorter sequences of <10 kbp, one or two additional resistance genes were detected, such as fexA in p719171 (4,550 bp; GenBank accession no. MF443375) and p898246 (6,171 bp; GenBank accession no. MF443382) from human E. faecalis isolates in Ireland, as well as in pXM2013_42321 (6,372 bp; GenBank accession no. MH225423), p570347 (6,499 bp; GenBank accession no. MF443372) and pWHXH (6,772 bp; GenBank accession no. MH225422), all from human E. faecalis isolates in China. The truncated or intact erm(A)-like gene was present in pFX13 (6,656 bp; GenBank accession no. KT862778) and p529360 (6,399 bp; GenBank accession no. MF443369) from porcine and human E. faecalis isolates in China, respectively; fexA and erm(A) occurred in six plasmids, all from human E. faecalis isolates in China, including pXM2013_71028 (8,128 bp; GenBank accession no. MH225424), p1207_26W003 (8,128 bp; GenBank accession no. MH225416), p19677 (8,138 bp; GenBank accession no. MH225418), pZJ11066 (8,817 bp; GenBank accession no. MH225425), pSZ21494 (8,875 bp; GenBank accession no. MH225420), and p1203_10W003 (9,146 bp; GenBank accession no. MH225415). fexA and truncated erm(A)-like genes were also present in pE419 (9,676 bp; GenBank accession no. KT862777) and pM18/0173 (9,742 bp; GenBank accession no. MN831415) from human E. faecalis isolates in China and Ireland. Some other larger sequences of >10 kbp also comprise these two genes, fexA and erm(A), separately or simultaneously. The gene erm(A) was present in p_optrA 13–014_NODE_03 (10,835 bp; GenBank accession no. PHKZ01000005) from a human E. faecalis isolate in France, while the fexA gene was present in pSF35 (10,130 bp; GenBank accession no. KT862779) from a chicken E. faecalis isolate in China, pXY17 (11,036 bp; GenBank accession no. KT862780) from porcine E. faecalis isolates in China, and 10 plasmids from human E. faecalis isolates, namely, pM17/0240 (10,551 bp; GenBank accession no. MN831414), pM18/0497 (12,562 bp; GenBank accession no. MN831419) and p839260 (15,795 bp; GenBank accession no. MF443381) from Ireland, p29462 (21,568 bp; GenBank accession no. MH225419), p1202_21W014 (21,568 bp; GenBank accession no. MH225414), pE394 (36,331 bp; GenBank accession no. KP399637) and p452115 (36,458 bp; GenBank accession no. MF443368) from China, p_optrA 16–196_NODE_02 (35,057 bp; GenBank accession no. PHLE01000003) and p973450 (72,835 bp; GenBank accession no. MF443385) from France, and p441341 (35,059 bp; GenBank accession no. MF443367) from Sweden. Both genes, fexA and erm(A), were simultaneously detected in six plasmids from human E. faecalis isolates, namely, p838523 (10,006 bp; GenBank accession no. MF443380) from Malaysia, p986223 (12,051 bp; GenBank accession no. MF443387) and p986247 (13,157 bp; GenBank accession no. MF443388) from Mexico, p824270 (13,262 bp; GenBank accession no. MF443379) and p912300 (13,265 bp; GenBank accession no. MF443383) from the United States, and p739884 (13,262 bp; GenBank accession no. MF443376) from China. The gene fexA, accompanied by a truncated erm(A)-like gene, was present in plasmid pM18/0906 (11,697 bp; GenBank accession no. MN831417) from human E. faecalis in Ireland. The plasmids pE121 (22,854 bp; GenBank accession no. KT862776) and p10-2-2 (14,349 bp; GenBank accession no. KT862775), as well as p981649 (42,438 bp; GenBank accession no. MF443386) and p743142 (68,959 bp; GenBank accession no. MF443377), from porcine and human E. faecalis isolates in China (including Taiwan) harbored the fexA gene and a truncated erm(A)-like gene, as well as the spectinomycin resistance gene spc together with fexA and erm(A), respectively. In addition, the aminoglycoside resistance gene aph(2″)-IIIa was identified in p687671 (41,890 bp; GenBank accession no. MF443374) from a human E. faecalis isolate in Panama. The genes fexA, erm(A), and erm(B) were detected in p956343 (91,477 bp; GenBank accession no. MF443384) from a human E. faecalis isolate in Guatemala. Four additional resistance genes, erm(B), aacA-aphD, spw, and erm(A), were present in the incompletely sequenced plasmid p_optrA 16–164-1_NODE_01 (16,208 bp; GenBank accession no. PHLD01000003).
Bender and coworkers described diverse optrA genetic environments among E. faecalis and E. faecium isolates from humans in Germany. They most frequently found the fexA gene upstream of optrA, whereas in one isolate, an erm(B) gene was detected at this position. In several isolates, an erm(A) gene was detected downstream of optrA. One isolate harbored an aadE-sat4-aphA3 resistance gene cluster in the vicinity of optrA (256). A study by Deshpande and coworkers described the genetic environment of mostly plasmid-borne optrA genes in human E. faecalis isolates from different countries/regions (167). They found 15 in part strikingly different optrA regions among 23 incompletely sequenced plasmids. Most frequently the genes fexA and/or araC were detected in the vicinity of optrA. Single copies of the insertion sequences IS1216E or ISEnfa1 were detected in only four isolates each (167). Similar results were obtained for E. faecalis isolates from humans and various food-producing animals in China (246).
Plasmids carrying the optrA gene in Staphylococcus spp.
To date, the sequences of three completely sequenced optrA-carrying plasmids from staphylococci have been deposited in the databases (Table 5). All three plasmids, pWo27-9 (163), pWo28-1 (163), and pWo28-3 (162), originated from porcine S. sciuri isolates in China and carried the gene cfr as well (Fig. 4F). In addition to the genes optrA and cfr, the additional resistance genes ble and aadD (pWo27-9), as well as ble, aadD, aacA-aphD, and fexA (pWo28-1 and pWo28-3) were present (Fig. 4F). The genetic environment of the plasmid-borne optrA and cfr genes was very similar to that found in the incompletely sequenced plasmid pWo35-20 (GenBank accession no. KX982166), also from a porcine S. sciuri isolate in China (163). Several other plasmids from porcine methicillin-resistant S. sciuri isolates in China were identified as carrying the optrA gene (163).
Plasmids carrying the optrA gene in other Gram-positive bacteria.
Only one optrA-carrying plasmid, p2C45, from C. perfringens was found in the databases (Table 5). This plasmid had a size of 148,618 bp, originated from chicken in China, and carried the additional resistance genes fexA, erm(A), and lnu(P) (289). The segment carrying the optrA gene, with its downstream erm(A), the ferredoxin-encoding gene fer, a gene for a hypothetical protein, and IS1216E elements at both termini, displayed 99.9% nucleotide sequence identity to the corresponding region in the aforementioned plasmid pE508 from porcine E. faecalis isolates in China (289).
Transposons, integrative and conjugative elements, and prophages carrying the optrA gene.
The 16,350-bp transposon Tn6823 from S. aureus isolates of chicken origin in China is a variant of transposon Tn558 and consists of the three transposase genes tnpA, tnpB, and tnpC, orf138, and the phenicol resistance gene fexA. An additional eight genes were inserted into the Tn558 backbone, namely, four genes for hypothetical proteins hp1 to hp4, the topoisomerase gene top, the mobilization gene mob, the transcriptional regulator gene araC and the optrA gene (293). Almost identical Tn6823 sequences were present in the chromosomal DNA of a porcine S. sciuri isolate (GenBank accession no. KX447572) and a human E. avium isolate (GenBank accession no. MH018573), both from China. All of these Tn6823 elements were integrated into the chromosomal radC gene coding for a DNA repair protein (293).
Another novel chromosome-borne optrA-carrying transposon, designated Tn6674, was found in a porcine E. faecalis isolate in China (270). Tn6674 has a size of 12,932 bp (GenBank accession no. MK737778). As a Tn554 derivative, it carries the transposase genes tnpA, tnpB, and tnpC and the Tn554-associated resistance genes spc and erm(A). In addition, Tn6674 also harbored the resistance genes fexA and optrA. Like Tn6823, Tn6674 was also inserted into the chromosomal radC gene (270). Circular forms of Tn6674 were detected by PCR, suggesting that this transposon is functionally active (270). Transposon Tn6674 was also found in the chromosomal DNA of the E. faecalis isolates A101 (GenBank accession no. MH018572), TZ2 (GenBank accession no. MH225421), EF294 (GenBank accession no. QDDM01000007), 33710 (GenBank accession no. QNHF01000012), and 743142 (GenBank accession no. MF443377) from humans in China (including Taiwan), and in E. faecalis Efl-952 from a human isolate in Greece (257).
The sequence of a third optrA-carrying transposon, designated Tn6261, from porcine E. faecalis in China is only available as a database entry (GenBank accession no. KU354267). This transposon is 8,886 bp in size and harbors Tn558-like tnpA and tnpB genes, the erm(A) gene, and a gene coding for an SAM-dependent methyltransferase, in addition to optrA. Tn6261 was also integrated in the chromosomal radC gene of E. faecalis.
Two ICEs of the ICESa2603 family that carry the optrA gene have so far been identified in porcine S. suis isolates from China (287). ICESsuSC216 had a size of 53,020 bp and carried the additional antimicrobial resistance genes aadD, erm(B) (two copies), and tet(O). In contrast, the tandem ICESsuSC317 was 103,324 bp in size and harbored the tetracycline resistance genes tet(L) and tet(O), in addition to optrA (287).
The optrA-bearing prophage ΦSC181 had a size of 54,771 bp and carried the additional antimicrobial resistance genes mef(A), aacA-aphD, and cat. It also originated from a porcine S. suis isolate in China (287).
Insertion sequences generating optrA-carrying translocatable units.
In contrast to the situation with the cfr gene, only three insertion sequences, namely IS1216E, ISEfa15, and ISChh1-like, have been identified to bracket the gene optrA (Fig. 11).
FIG 11.
Insertion sequences flanking the optrA gene. The insertion sequences IS1216E, ISEfa15, and ISChh1-like are displayed as black boxes with the green arrow(s) inside symbolizing the respective transposase gene(s). The optrA gene is shown as a red arrow. Additional resistance genes, such as fexA and erm(A)-like, are displayed as rose arrows, while other genes are shown as blue arrows. In all cases, the arrowhead indicates the direction of transcription. Whenever direct repeats were identified at the termini of the IS elements that flank the cfr region, they are indicated in boxes. The gray-shaded area indicates >99% nucleotide sequence identity. For each specific IS-cfr-IS arrangement, the bacterial species, the location (plasmid/integrative and conjugative element [ICE]/chromosomal multidrug resistance genomic island [MDRGI]) and the database accession number (in brackets) are given on the righthand side.
(i) IS1216E-optrA-IS1216E.
Most frequently, the gene optrA, with or without additional genes, was found to be bracketed by two identical IS1216E copies in the same orientation. When these IS1216E copies recombine, a TU is generated, which then can integrate into a plasmid, an ICE, or at different chromosomal sites. If integrated into a conjugative plasmid or an ICE, this may result in the dissemination of the optrA gene across strain, species, or even genus boundaries.
The simplest version of insertion sequences bracketing the optrA gene, in which only the optrA gene was located between the two IS1216E elements, was found in the ICEs ICESsuSC216 (GenBank accession no. MK359991) and ICESsuSC317 (GenBank accession no. MK359989), both from porcine S. suis isolates in China (287). However, more complex arrangements usually carried two or more genes in addition to optrA and the two IS1216E elements. These genes included the resistance genes erm(A)-like, cat, and fexA; the transposase gene tnp; the DNA-directed DNA polymerase gene impB; the transcriptional regulator gene araC; the tyrosine kinase gene fer; and hp genes for hypothetical proteins. In plasmid pE508 (GenBank accession no. MK425645) from porcine E. faecalis in China, the array IS1216E-optrA-erm(A)-like-IS1216E was detected, whereas the array IS1216E-fexA-hp-optrA-IS1216E was present in plasmid pXM2013_42321 (GenBank accession no. MH225423) from a human E. faecalis isolate in China. The same arrangement was seen in three incompletely sequenced plasmids from canine E. faecalis isolates in China (164). In other incompletely sequenced plasmids from E. faecalis originating from dogs, pork, or vegetables in China, a central region comprising the genes hp-fexA-hp-optrA-hp-hp-hp-erm(A) was bracketed by IS1216E elements in the same and in opposite orientations (164). The array IS1216E-cat-optrA-araC-IS1216E was identified in the prophage ΦSC181 from S. suis (287). Other arrays included IS1216E-araC-optrA-Eco57I-IS1216E, which also contained the gene for a restriction endonuclease, in plasmid pE211 (GenBank accession no. MK425644) and IS1216E-optrA-hp-hp-erm(A)-like-IS1216E in pFX13 (GenBank accession no. KT862778), both from porcine E. faecalis isolates in China. In a chromosomal MDRGI from C. coli (GenBank accession no. NZ_JAATJY000000000), the optrA gene was found to be embedded into the array IS1216E-hp-optrA-fer-erm(A)-like-IS1216E (290). The most complex plasmid-borne array, IS1216E-tnp-hp-hp-impB-hp-hp-hp-fexA-hp-hp-optrA-hp-hp-erm(A)-like-IS1216E, was present in plasmid p10-2-2 (GenBank accession no. KT862775) from a porcine E. faecalis isolate in China and in an unnamed plasmid of E. faecalis isolate 4 from a human in Spain (261), while a similar array, IS1216E-tnp-hp-hp-impB-hp-hp-hp-fexA-hp-hp-optrA-hp-hp-erm(A)-like-IS1216E, was found on a not further specified plasmid from a human E. faecalis isolate in Mexico (167). In C. coli, the array IS1216E-tnp-hp-fexA-hp-optrA-IS1216E was detected in the chromosomal DNA, and the formation of a TU, that comprised one IS1216E and the genes located between the two IS1216E copies, was confirmed (291).
There are also some arrangements in which the optrA gene with additional genes was bracketed by two identical IS1216E copies in opposite orientations. All of these arrays contained the segment fexA-hp-hp-optrA-hp-hp-erm(A)-like. Moreover, the arrays in plasmids pE419 (GenBank accession no. KT862777) from human E. faecalis and pSF35 (GenBank accession no. KT862779) from chicken E. faecalis isolates in China were closely related, except for the opposite orientation of IS1216E (246). The array IS1216E-hp-hp-fexA-hp-hp-optrA-hp-hp-erm(A)-like-IS1216E showed a very high similarity in plasmids pC54 (GenBank accession no. CP030046) and pC25-1 (GenBank accession no. CP030043) from porcine E. faecalis in China (266) and pM18/0173 (GenBank accession no. MN831415) from human E. faecalis in Ireland (239). The most complex array, IS1216E-nlpC-hp-mesa-tonBR-hp-traG-tnp-hp-hp-impB-hp-hp-hp-fexA-hp-hp-optrA-hp-hp-erm(A)-like-IS1216E, was present in plasmid pE121 (GenBank accession no. KT862776) from a human E. faecalis isolate in China (246).
(ii) ISEfa15-optrA-ΔISEfa15.
Apart from IS1216E, the insertion sequence ISEfa15 was also shown to bracket the optrA gene in the array ISEfa15-optrA-ΔISEfa15 in plasmid pE35048-oc (GenBank accession no. MF580438) from a human E. faecium isolate in Italy (180). In the corresponding study, the formation of a TU comprising optrA and the ΔISEfa15 was confirmed (180). It should be noted that this ISEfa15-optrA-ΔISEfa15 segment has also been referred to as Tn6628 (214).
(iii) ISChh1-like-optrA-ISChh1-like.
Insertion sequences, designated ISChh1-like, have been found to bracket the optrA gene in porcine C. coli isolates from China. The sizes of the ISChh1-like flanked structures in different C. coli isolates varied between 6,802 and 6,807 bp (291). The formation of a TU, which might arise from the recombination of the ISChh1-like elements upstream and downstream of optrA, could not be confirmed (291).
Chromosomal optrA Genes
The aforementioned optrA-carrying transposons, ICEs, and prophages are all integrated into the chromosomal DNA of the corresponding Enterococcus or Staphylococcus isolates. All three transposons had integrated into the radC gene. The ICESsuSC216 was inserted at the rplL locus. Upon integration, it generated perfect 15-bp direct TSDs at its termini (5′-TTATTTAAGAGTAAC-3′). The integration site for ICESsuSC317 was at the rumA locus. This ICE produced imperfect 14-bp direct TSDs at both termini (5′-CACATAGAAGTTGT-3′ [right terminus] and 5′-CACGTGGAGACGGT-3′ [left terminus]) (287). In S. suis, both loci—rplL and rumA—are well-known insertion hot spots of MGEs, including prophages and ICEs (294, 295). The optrA-carrying prophage ΦSC181 was also identified in the chromosomal DNA of a porcine S. suis isolate in China (287). It was located at the rumA locus and produced imperfect 14-bp direct TSDs upon integration (5′-CACATAGAAGTTGT-3′ [right terminus] and 5′-CACGTGGAGTGTGT-3′ [left terminus]).
Several database entries (GenBank accession no. RXOX01000014, RHVS01000013, RHWF01000013, and RHVZ01000013, among others) identified the optrA gene in the chromosomal DNA of E. faecalis isolates from different sites in hospitals in Pakistan, including washroom sinks, bedside lights, nurse call button, and bedside rails. In all of these sequences, which ranged from 55,162 bp to 57,753 bp, only the fexA gene was present as an additional resistance gene. Moreover, the optrA gene was also found in the chromosomal DNA of eight E. faecalis isolates from animals and humans in China (246). The contigs harboring the optrA gene ranged in size from 6,088 bp to 29,141 bp. All but one (contig of isolate E079) of these contigs also harbored one complete additional antimicrobial resistance gene in the vicinity of the optrA gene. The four contigs from human isolates E147 and E381, as well as porcine isolates 5-7 and 10-120, carried the additional resistance gene fexA, while the contig from the chicken isolate LY4 carried the erm(A)-like gene. The remaining contigs from the porcine isolate G20 and the human isolate E016 harbored a truncated erm(A)-like gene and a complete Tn558 transposon that included the fexA gene (246). In E. faecalis isolates from dogs and raw food (egg, beef, pork, and chicken meat) in China, the chromosomal optrA region occasionally identified the erm(A) gene downstream of optrA, whereas the complete fex(A)-carrying transposon Tn558 was commonly found upstream of optrA (164). In a few cases, only the fexA gene without the remaining parts of Tn558 was present, and a complete Tn554 that included the erm(A) and spc genes was present upstream of the optrA and fexA genes (164). In E. casseliflavus isolates from beef and chicken meat in China, a complete Tn558 was found in the vicinity of the optrA gene (164). In the latter two studies (164, 246), plasmid-borne optrA genes were also investigated for their genetic environment. The examples presented clearly showed that the genetic environments of the chromosomal optrA genes in E. faecalis differed distinctly from those on plasmids (164, 246).
The chromosomal optrA genes in S. sciuri and S. simulans isolates from dogs, cats, and pigs in China often showed the optrA gene with its upstream araC gene in close proximity to complete or truncated Tn558 elements (164).
In C. coli isolates from ducks and chickens in China, the optrA genes were located within chromosomal MDRGIs (290). One MDRGI, with a size of 14,592 bp (GenBank accession no. NZ_JAATKE000000000), was inserted into the C. coli housekeeping gene YSU_03710, which codes for an acetyltransferase, and carried the additional resistance genes fexA, tet(O), tet(L), spc, and aadE. The other MDRGI (GenBank accession no. NZ_JAATJY000000000), which is about 3 kbp larger, had been inserted between the gene YSU_02690 for a SAM-dependent methyltransferase and the gene YSU_02685 for a hypothetical protein. This MDRGI harbored the additional resistance genes tet(O), tet(L), catA, and erm(A)-like (290). In a porcine C. coli isolate from China, the optrA gene was located in a chromosomal MDRGI composed of the IS1216E-optrA-fexA-tnp-IS1216E segment and the additional resistance genes aadE, sat4, and aphA3 (291).
Variants of OptrA
The wild-type optrA gene, as identified in E. faecalis and E. faecium, is widely spread among E. faecalis, E. faecium, and S. suis isolates. However, since its first description, at least 69 variants of the optrA gene, which differed by 1 to 20 aa in their deduced OptrA sequences, have been detected (Table 6). This corresponds to an amino acid identity of 97.1 to 99.8% compared with the wild-type OptrA. Most frequently, amino acid substitutions at positions 176 (Y176D), 393 (G393D), 3 (K3E), and 40 (G40D) were observed (Table 6). In some studies, the MIC values for linezolid (and tedizolid) of the isolates carrying the different OptrA variants were determined (153, 164, 248, 249, 256). The comparison of the MIC values with the associated OptrA variants suggested that the different OptrA variants might have an impact on the relative oxazolidinone susceptibility/resistance of the respective isolates. Thus, the variants D, EDP, KD, KLDP, RD, RDK, and RDKP, as well as the wild-type OptrA, were commonly found in isolates that exhibited high linezolid MIC values of ≥8 mg/liter, whereas the variants DDTD, EYDM, EYDDK, EYDNDM, and KDTP have usually been found in isolates that exhibited low linezolid MICs of ≤2 mg/liter (153, 164, 248, 249, 256). However, several OptrA variants were also found to be associated with variable linezolid MICs (153, 164, 248, 249), which suggests that not only the OptrA protein but also additional factors may account for the linezolid MIC.
TABLE 6.
Comprehensive presentation of the OptrA variants identified to date
| OptrA variant | Amino acid substitution(s)a | Species | GenBank accession no. or referenceb |
|---|---|---|---|
| Wild type | E. faecalis | WP_063854496.1 | |
| S. suis | WP_063854496.1 | ||
| E. faecium | WP_063854496.1 | ||
| D_1 | Y176D | S. suis | WP_099810410.1 |
| C. jejuni | WP_099810410.1 | ||
| C. coli | WP_099810410.1 | ||
| E. faecalis | WP_099810410.1 | ||
| D_2 | G40D, R239- | S. suis | WP_105150713.1 |
| DC | Y176D, Y601C | S. suis | WP_105141008.1 |
| DD_1 | G40D, Y176D | S. suis | WP_105114403.1 |
| DD_2 | G40D, G393D | S. suis | WP_136628908.1 |
| DD_3 | Y176D, G393D | S. suis | WP_094467217.1 |
| E. faecium | WP_094467217.1 | ||
| E. faecalis | WP_094467217.1 | ||
| E. casseliflavus | WP_094467217.1 | ||
| S. sciuri | 164 | ||
| DD_4 | Y176D, G394D | S. suis | WP_105209901.1 |
| DK | Y176D, E256K | E. faecalis | AON96411.1 |
| DM | Y176D, I622M | E. faecalis | 164 |
| E. casseliflavus | 164 | ||
| DP | G40D, T481P | S. suis | WP_105138726.1 |
| DP_2 | Y176D, T481P | S. suis | WP_099809080.1 |
| E. faecalis | WP_099809080.1 | ||
| Enterococcaceae | QBA99765.1 | ||
| DS | Y176D, G394S | E. faecalis | 248 |
| DDD | G40D, Y176D, G393D | S. suis | WP_050572105.1 |
| E. faecium | WP_050572105.1 | ||
| DDM | Y176D, G393D, I622M | E. faecium | WP_002360182.1 |
| DDP | G40D, Y176D, T481P | S. suis | WP_105116489.1 |
| DDP_2 | G40D, G393D, T481P | E. faecalis | WP_002415370.1 |
| S. suis | WP_002415370.1 | ||
| DGP | Y176D, S411G, T481P | S. suis | WP_105129307.1 |
| DVD | Y176D, I235V, G393D | S. suis | QEM40870.1 |
| DVD_2 | Y176D, A350V, G393D | E. faecalis | WP_141422915.1 |
| E. faecium | WP_141422915.1 | ||
| DDKD | G40D, Y176D, I287K, G393D | S. suis | WP_170243993.1 |
| DDTD | G40D, Y176D, P179T, G393D | E. faecalis | 248 |
| DNDM | Y176D, D247N, G393D, I622M | S. sciuri | 164 |
| DRDK | G40D, I104R, Y176D, E256K | S. suis | WP_105157283.1 |
| DDKDP | G40D, Y176D, E290K, G393D, T481P | S. suis | WP_105095882.1 |
| E | K3E | E. faecalis | QCC21367.1 |
| E_2 | D401E | E. faecalis | WP_172694219.1 |
| ED | K3E, Y176D | E. faecalis | WP_078122664.1 |
| E. faecium | NTR32945.1 | ||
| ED_2 | K3E, G393D | Enterococcaceae | QBA99711.1 |
| EDD | K3E, Y176D, G393D | E. faecalis | WP_078122475.1 |
| E. gallinarum | WP_078122475.1 | ||
| S. sciuri | AOQ25869.1 | ||
| E. faecium | QCX35246.1 | ||
| EYD | K3E, N12Y, Y176D | S. suis | WP_050571857.1 |
| EDM | K3E, Y176D, I622M | E. faecalis | WP_089202004.1 |
| E. faecium | WP_089202004.1 | ||
| EDP | K3E, Y176D, T481P | E. faecium | WP_128704351.1 |
| E. faecalis | RXF20311.1 | ||
| EDDD | K3E, G40D, Y176D, G393D | S. suis | WP_050571447.1 |
| EDDD_2 | K3E, G87D, Y176D, G393D | E. faecalis | NSO88909.1 |
| EDDM | K3E, Y176D, G393D, I622M | E. faecalis | WP_153246992.1 |
| EDVD | K3E, Y176D, I235V, G393D | E. faecium | 248 |
| EYDD | K3E, N12Y, Y176D, G393D | S. suis | WP_129406995.1 |
| S. sciuri | WP_129406995.1 | ||
| Salmonella sp. | RXY94784.1 | ||
| EYDE | K3E, N12Y, G40D, Y176E | S. suis | WP_105142857.1 |
| EYDM | K3E, N12Y, Y176D, I622M | E. faecium | 248 |
| EYDP | K3E, N12Y, G40D, T481P | S. suis | WP_105096713.1 |
| EYDDD | K3E, N12Y, G40D, Y176D, G393D | E. faecium | WP_131648058.1 |
| S. suis phage ΦSC181 | QEM40833.1 | ||
| EYDDK | K3E, N12Y, Y176D, G393D, E583K | S. sciuri | 153 |
| EYDND | K3E, N12Y, Y176D, D247N, G393D | S. simulans | AVE17190.1 |
| EYDRC | K3E, N12Y, G40D, K130R, Y135C | S. suis | WP_105119002.1 |
| EYDNDM | K3E, N12Y, Y176D, D247N, G393D, I622M | S. aureus | WP_159314661.1 |
| S. sciuri | WP_159314661.1 | ||
| E. faecalis | AON96416.1 | ||
| E. avium | AXM43510.1 | ||
| EYDVDM | K3E, N12Y, Y176D, I235V, G393D, I622M | S. suis | WP_105182874.1 |
| EYDNKDM | K3E, N12Y, Y176D, D247N, Q310K, G393D, I622M | L. monocytogenes | EEX0182872.1 |
| EYDDNDGPM | K3E, N12Y, G40D, Y176D, D247N, G393D, S411G, T481P, I622M | E. faecalis | WP_131639407.1 |
| EDELYNKQLEIG | K3E, Y176D, Q541E, M552L, N560Y, K562N, Q565K, E614Q, I627L, D633E, N640I, R650G | E. faecium | WP_125276231.1 |
| EYKKCDVASKELYNKQLEIG | K3E, N12Y, E37K, N122K, Y135C, Y176D, A350V, V395A, A396S, Q509K, Q541E, M552L, N560Y, K562N, Q565K, E614Q, I627L, D633E, N640I, R650G | E. faecalis | 256 |
| EYKWDVKELYNKQLEIG | K3E, N12Y, N122K, Y135W, Y176D, A350V, Q509K, Q541E, M552L, N560Y, K562N, Q565K, E614Q, I627L, D633E, N640I, R650G | E. faecium | WP_181727040.1 |
| EYKWDVDASKELYNKQLEIG | K3E, N12Y, N122K, Y135W, Y176D, A350V, G393D, V395A, A396S, Q509K, Q541E, M552L, N560Y, K562N, Q565K, E614Q, I627L, D633E, N640I, R650G | E. faecium | WP_173495098.1 |
| H | Q219H | E. faecalis | WP_138807048.1 |
| I | T572I | E. faecalis | AWH59008.1 |
| K | I287K | S. suis | WP_105126734.1 |
| KD | T112K, Y176D | E. faecalis | WP_080477306.1 |
| S. suis | WP_080477306.1 | ||
| KDP | T112K, Y176D, T481P | E. faecalis | WP_126267515.1 |
| KDTP | T112K, Y176D, P179T, T481P | E. faecalis | 248 |
| KLDP | T112K, S147L, Y176D, T481P | E. faecium | 248 |
| KDDGP | T112K, Y176D, G393D, S411G, T481P | S. suis | WP_105120738.1 |
| KDKGP | T112K, Y176D, E290K, S411G, T481P | Fusobacterium sp. | WP_187422904.1 |
| P | T481P | C. perfringens | WP_170876513.1 |
| C. coli | WP_170876513.1 | ||
| RD | I104R, Y176D | S. suis | WP_105145462.1 |
| E. faecalis | 164 | ||
| RDK | I104R, Y176D, E256K | E. faecalis | WP_105108188.1 |
| E. faecium | WP_105108188.1 | ||
| S. suis | WP_105108188.1 | ||
| RDKP | I104R, Y176D, E256K, T481P | S. suis | WP_105134398.1 |
| RDKGP | I104R, Y176D, E256K, S411G, T481P | S. suis | WP_105110522.1 |
| SDDP | A27S, G40D, G393D, T481P | S. gallolyticus subsp. pasteurianus | ATM29806.1 |
| T | A13T | S. suis | WP_099876735.1 |
| YDD | N12Y, Y176D, G393D | S. sciuri | 164 |
Substituted amino acids are shown in bold and underlined; the hyphen in variant D_2 indicates that the respective amino acid is deleted.
For every species in which the respective OptrA variant was detected, only one representative protein sequence is indicated. In cases where no OptrA sequences have been deposited in the databases, the publication that describes the respective OptrA variant is given.
MOBILE OXAZOLIDINONE RESISTANCE GENE poxtA
Geographical Distribution and Host Bacteria of the poxtA Gene
According to the PubMed and NCBI Nucleotide databases, the gene poxtA is present in 11 countries on four continents (Fig. 12). In most of these countries cfr- and/or optrA-carrying bacteria have also been detected. The host bacteria carrying the poxtA gene are so far exclusively Enterococcus spp. and Staphylococcus spp.
FIG 12.
Geographical distribution of poxtA-carrying bacteria. The countries in blue are those from which the occurrence of poxtA-carrying bacteria has been reported.
The poxtA gene was first described in a MRSA isolate of clinical origin in Italy in 2018 (56, 214). However, this gene was more frequently reported in E. faecium isolates obtained from humans in Greece (280), Ireland (239), Pakistan (281), Portugal (296), Spain (262, 296), Turkey (264), and the United States (264, 281), from pigs in China (297) and Italy (174, 298), from cattle in Spain (296), from air samples of a pig farm in Spain (283), from cattle, chicken, and ducks in South Korea (299), and from milk, retail meat, and food-producing animals in Tunisia (273, 296). Moreover, the poxtA gene was detected in E. faecalis isolates from humans in Ireland (239) and Spain (262), and also from chickens and ducks in South Korea (299). In addition, studies in China and Italy reported that the poxtA gene was detected in E. hirae isolates from pigs (174, 209, 300). Database searches also identified the poxtA gene in the whole-genome sequence of the Pediococcus acidilactici isolate BCC1, which was obtained from a chicken cecum sample in China (GenBank accession no. CP018763).
Mobile Genetic Elements That Are Involved in the Dissemination of the poxtA Gene
Plasmids carrying the poxtA gene in Enterococcus spp.
Plasmids seem to play an important role in the dissemination of poxtA among enterococci. So far, poxtA-carrying plasmids have only been described in the three enterococcal species E. faecalis, E. faecium, and E. hirae (Table 7). Based on the transfer characteristics of the plasmids in enterococci, they are usually classified as (i) pheromone-responding plasmids, (ii) the pMG1 family, (iii) the Inc18 family, or (iv) the mobilizable plasmids (301). Except for the pMG1 family, poxtA-carrying plasmids have been detected in the following three types of plasmids: pheromone-responding plasmids (e.g., pE035), Inc18 family plasmids (e.g., pC27-2), and mobilizable plasmids (e.g., pE1077-23).
TABLE 7.
Characteristics of completely sequenced poxtA-carrying plasmids in enterococci
| Plasmid | Origin | Size (bp) | Colocated resistance gene(s) | GenBank accession no. |
|---|---|---|---|---|
| pM18/0011 | E. faecalis, human, Ireland | 18,280 | MN831412 | |
| pE076 | E. faecalis, pig, China | 19,832 | fexB | MK140642 |
| pC10 | E. faecalis, pig, China | 37,990 | fexB, tet(M), tet(L), cat | MK861852 |
| pE035 | E. faecalis, pig, China | 121,524 | erm(B), aacA-aphD, bcrABDR, erm(A), lnu(G), dfrG, fexB, optrA | MK140641 |
| pM16/0594 | E. faecium, human, Ireland | 21,849 | tet(M), tet(L) | MN831411 |
| pE1077-23 | E. faecium, pig, China | 23,710 | MT074684 | |
| pSDGJQ5 | E. faecium, chicken, China | 30,457 | CP038175 | |
| pT-E1077-31 | E. faecium, pig, China | 31,742 | MT074685 | |
| pHB2-2 | E. faecium, chicken, China | 32,169 | tet(M), tet(L) | CP038165 |
| pGZ8 | E. faecium, pig, China | 36,911 | tet(M), tet(L) | CP038162 |
| pSC3-1 | E. faecium, chicken, China | 36,802 | tet(M), tet(L) | CP038167 |
| pSCBC1 | E. faecium, pig, China | 41,082 | tet(M), tet(L) | CP038169 |
| pYN2-1 | E. faecium, pig, China | 41,394 | tet(M), tet(L) | CP038173 |
| pSDGJP3 | E. faecium, pig, China | 51,661 | tet(M), tet(L), dfrG | CP038171 |
| pC27-2 | E. faecium, pig, China | 62,386 | fexB, erm(B), aphA3, Δsat4, aadE, tet(M), tet(L), czcD, dfrG | MH784602 |
| pC25-1 | E. faecium, pig, China | 67,678 | fexB, erm(B), aphA3, Δsat4, aadE, tet(M), tet(L), czcD, dfrG | MH784601 |
| pHN11 | E. faecium, chicken, China | 69,757 | fexB, erm(B), aphA3, Δsat4, aadE, tet(M), tet(L), czcD, dfrG | CP038176 |
| pHDC14-2.27K | E. hirae, pig, China | 27,303 | CP042294 | |
| pCQP3-9_2 | E. hirae, pig, China | 33,132 | erm(B), tet(M), tet(L) | CP037957 |
| pFas4-1 | E. hirae, pig, China | 57,267 | fexB, erm(B), tet(M), tet(L), dfrG, vat(E) | MK798157 |
| pHDC14-2.133K | E. hirae, pig, China | 133,362 | erm(B), tet(M), tet(L), catA8, dfr, aacA-aphD, spw, lsa(E), lnu(B), aphA3, sat4, aadE | CP042290 |
| pY80 | S. haemolyticus, pig, China | 55,758 | tet(L), aadD, fexB, czcD | CP063444 |
In E. faecalis, the complete sequences of four poxtA-carrying plasmids have been deposited in the databases (Table 7). Their sizes ranged from 18,280 bp to 121,524 bp. The two smaller plasmids pM18/0011 (18,280 bp) and pE076 (19,832 bp) were from E. faecalis isolates of human origin in Ireland (239) and from porcine E. faecalis isolates (268) in China. Plasmid pM18/0011 did not harbor additional resistance genes, whereas plasmid pE076 carried a fexB gene. The 37,990-bp plasmid pC10 was found in a porcine E. faecalis isolate from China and carried the additional resistance genes fexB, tet(M), tet(L), and cat. The pheromone-responding conjugative plasmid pE035 is 121,524 bp in size and harbored the three florfenicol resistance genes poxtA, optrA, and fexB. In addition, it also carried the MLSB resistance genes erm(A) and erm(B), the bifunctional aminoglycoside resistance gene aacA-aphD, the lincosamide resistance gene lnu(G), the trimethoprim resistance gene dfrG, and the bacitracin resistance operon bcrABDR. It proved to be transferable, with high transfer frequencies of 4.5 ×10−3 ± 0.3 × 10−3. Three mobile loci, including a circularizable structure containing aacA-aphD, a mobile bcrABDR locus, and a mobile dfrG locus, were found on this plasmid, and all proved to be active. The presence of the three mobile loci on a poxtA-carrying multiresistance plasmid renders this plasmid flexible. In addition, these three loci will aid in the persistence and dissemination of this plasmid among enterococci and putatively also among other Gram-positive bacteria (268).
In E. faecium, the sizes of the poxtA-carrying plasmids ranged from 21,849 to 69,757 bp. Except for the smallest plasmid, pM16/0594, which was found in an isolate of human origin in Ireland (239), all other poxtA-carrying plasmids were found in isolates from pigs and chickens in China (266, 267, 297). Among them, plasmids carrying the rep2_pRE25 replication gene, such as plasmids pC27-2 and pC25-1 (Table 7), were commonly identified in food-producing animals in China (266, 267, 297). The rep2_pRE25 gene is associated with the Inc18 broad-host-range plasmid family, which seems to be involved in the dissemination of poxtA across different Gram-positive bacterial genera and species (301). Plasmid pM16/0594 had a size of 21,849 bp and carried the additional tetracycline resistance gene tet(M). Another three poxtA-carrying plasmids, pE1077-23, pSDGJQ5, and pT-E1077-31, which ranged in size from 23,710 bp to 31,742 bp and did not harbor additional resistance genes, were detected in E. faecium isolates from pigs and chickens in China. Among them, the 23,710-bp mobilizable poxtA-carrying plasmid pE1077-23 (302) was most likely generated by the integration of a staphylococcal Tn6657-like transposon into a 9,317-bp plasmid, most closely related (99.9%) to the 9,312-bp enterococcal plasmid pISMMS_VER4_p6 (GenBank accession no. CP012453). Coinciding with replicative transposition, a characteristic 8-bp duplication of the sequence 5′-TTTGATAC-3′ was formed at the target site in the plasmid. Conjugation experiments revealed that pE1077-23 can be mobilized by pE1077-217, a 217,661-bp conjugative plasmid present in the same E. faecium isolate (302). The six plasmids pHB2-2, pGZ8, pSC3-1, pSCBC1, pYN2-1, and pSDGJP3 ranged in size from 32,169 bp to 51,661 bp. All of them carried the additional tetracycline resistance genes tet(M) and tet(L), with pSDGJP3 also harboring the trimethoprim resistance gene dfrG. They had been detected in E. faecium isolates from pigs and chickens in China. The five smaller plasmids shared substantial similarity with the larger plasmid pSDGJP3, which also comprised the region carrying the resistance genes poxtA, tet(L), and tet(M) (Fig. 13A). The last group of poxtA-carrying plasmids from E. faecium included the three plasmids pC27-2, pC25-1, and pHN11. These plasmids again originated from E. faecium isolates of chicken or pig origin in China, and their sizes varied between 62,386 bp and 69,757 bp. They were structurally related and had the additional antimicrobial resistance genes fexB, erm(B), aphA3, Δsat4, aadE, tet(M), tet(L), and dfrG, as well as the cobalt/zinc/cadmium resistance gene czcD, in common (Fig. 13B).
FIG 13.
Structural comparison of poxtA-carrying plasmids in enterococci (constructed by BRIG). Relevant genes with known functions and insertion elements are indicated for the respective reference plasmid in the outer ring. The poxtA gene is indicated in red. The innermost circle provides a size scale, while the next innermost circle shows the GC content. Other plasmids used for comparison are indicated by color-coded rings, with the reference plasmid representing the innermost colored ring. (A) pSDGJP3, pYN2-1, pSCBC1, pSC3-1, pGZ8, and pHB2-2, and (B) pHN11, pC25-1, and pC27-2, as well as (C) pFas4-2, pCQP3-9_2, pC10, and pM16/0594.
In E. hirae, poxtA-carrying plasmids have only been detected in porcine isolates from China (209, 300) (Table 7). The sizes of the plasmids ranged from 27,303 bp to 133,362 bp. Among them, plasmid pHDC14-2.27K was the smallest and did not carry additional antimicrobial resistance genes. In contrast, plasmid pFas4-1 was 57,267 bp in size and harbored—besides poxtA—not only the resistance genes fexB, tet(M), and tet(L), but also the streptogramin A resistance gene vat(E), the MLSB resistance gene erm(B), and the trimethoprim resistance gene dfrG (209). Three mobile loci, including a mobile poxtA locus, a mobile fexB locus, and a mobile tet(M)-tet(L) locus, were identified in plasmid pFas4-1 (209). Plasmid pFas4-1 from a porcine E. hirae isolate shared large regions with the smaller plasmids pCQP3-9_2 (also from porcine E. hirae), pC10 from porcine E. faecalis, and pM16/0594 from human E. faecium. The common regions within all four plasmids included the IS1216E-poxtA-IS1216E segment, as well as the IS1216E-bounded tet(L)- and tet(M)-containing segment. Moreover, the IS1216E-bounded fexB segment was found in plasmids pFas4-2, pCQP3-9_2, and pC10 (Fig. 13C). With a size of 133,362 bp, plasmid pHDC14-2.133K was the largest poxtA-carrying plasmid from E. hirae detected thus far. This plasmid harbored the additional antimicrobial resistance genes erm(B), tet(M), tet(L), catA8, aacA-aphD, spw, lsa(E), lnu(B), aphA3, sat4, aadE, and a not further specified dfr gene.
Plasmids carrying the poxtA gene in Staphylococcus spp.
The 55,758-bp plasmid pY80, obtained from a porcine S. haemolyticus isolate in China, carried the genes czcD, fexB, tet(L), and aadD along with poxtA (Table 7).
Transposons carrying the poxtA gene.
Three transposons carrying the poxtA gene have been described so far. The mosaic transposon Tn6349 from S. aureus carries the poxtA and cfr genes along with other resistance genes (214). The small poxtA- and fexB-carrying transposon Tn6657 was located within Tn6349. Both transposons are described in detail in the section dealing with cfr-carrying transposons. In addition, the poxtA gene was located together with the gene fexB in an IS1216-flanked Tn6246-like element in E. faecium from cow milk in Tunisia (296). Whether or not this structure with the composition IS1216-poxtA-IS1216-hp-hp-fexB-IS1216 (with all genes in the same orientation) is a real transposon or a PCT needs to be clarified. Hybridization with poxtA and fexB probes suggested a location of this structure on plasmids of approximately 30 and 100 kb, of which the 30-kb plasmid could be transferred by conjugation into E. faecium BM4105RF (296).
Insertion sequences generating poxtA-carrying translocatable units.
As previously seen with the gene optrA, IS1216E elements are the insertion sequences that bracket the poxtA gene. Numerous different contexts have been identified and are shown in Fig. 14. The most frequently observed context shows the poxtA gene and four small ORFs for hypothetical proteins bracketed by IS1216E elements in the same orientation. This arrangement was seen in all poxtA-carrying plasmids listed in Table 7, except in the E. faecium plasmid pHB2-2, where only one ORF for a hypothetical protein was present, and in the E. hirae plasmid pCQP3-9_2, where the IS1216E downstream of poxtA was truncated. This IS1216E-poxtA-hp-hp-hp-hp-IS1216E arrangement was also part of larger poxtA genetic environments, where IS1216E-bounded fexB genes were identified upstream (pE1077-23 from E. faecium) or downstream (pFas4-1 from E. hirae) of the poxtA region. In plasmid pT-E1077-31 from porcine E. faecium isolates, the entire poxtA-fexB region was duplicated and present in opposite orientations. In four of the plasmids, namely, pSC3-1 and pGZ8 from E. faecium and pE076 and pE035 from E. faecalis, the IS1216E-poxtA-hp-hp-hp-hp-IS1216E was also present, albeit with the two IS1216E elements in opposite orientations (Fig. 14). It should also be noted that the poxtA gene located in the whole-genome sequence of P. acidilactici BCC1 was bracketed by IS1216E elements.
FIG 14.
Insertion sequences flanking the poxtA gene. The insertion sequences IS1216E and IS1252 are displayed as black boxes, with the green arrow inside symbolizing the respective transposase gene. The poxtA gene is shown as a red arrow. The additional resistance gene fexB is displayed as a rose arrow, while other genes are shown as blue arrows. In all cases, the arrowhead indicates the direction of transcription. The gray-shaded area indicates >99% nucleotide sequence identity. For each specific IS-cfr-IS arrangement, the bacterial species, the plasmid on which it is located, and the database accession number (in brackets) are given on the righthand side.
The formation of a TU by the recombination of the IS1216E elements was confirmed for plasmid pFas4-1. Here, a TU of 3,321 bp that included the gene poxtA and one copy of IS1216E was detected (209). During conjugation experiments using E. faecium isolate E1077 as a donor, a novel 31,742-bp plasmid, designated pT-E1077-31, was identified in a transconjugant. Sequence analysis indicated that pT-E1077-31 was formed by the integration of a Tn6657-derived, IS1216E-based fexB- and poxtA-carrying TU into a copy of plasmid pE1077-23 (302). These observations suggested that IS1216E might play a relevant role in the persistence and the dissemination of the poxtA gene among enterococci.
Chromosomal poxtA Genes
Although plasmids are the predominant poxtA gene carriers in enterococci, this gene has also been identified in the chromosomal DNA of the MRSA isolate AOUC-0915 of human origin in Italy (214). In this MRSA isolate, the poxtA gene was part of the 48-kbp transposon Tn6349, which was inserted into a chromosomal ΦN315-like prophage (214).
COLOCATED RESISTANCE GENES AND THEIR ROLE IN THE DISSEMINATION OF OXAZOLIDINONE RESISTANCE GENES
Since oxazolidinones are exclusively approved for therapeutic use in humans and are strictly forbidden for use in food-producing animals, the direct selection pressure imposed by the use of oxazolidinones in animals is negligible. However, all three groups of mobile oxazolidinone resistance genes, including the various cfr genes, optrA, and poxtA, also confer phenicol resistance. Chloramphenicol has been banned since 1994 from use in food-producing animals in the European Union (303). Other countries followed this example, and according to the FAO, most countries had banned chloramphenicol for use in food animal production by 2002 (http://www.fao.org/asiapacific/news/detail-events/fr/c/47419/). The reason for this ban was to protect consumers from possible adverse effects caused by chloramphenicol residues in food animal carcasses or products, as chloramphenicol is able to provoke an irreversible, dose-independent aplastic anemia in humans (303). In 1995, florfenicol, a fluorinated thiamphenicol derivative which does not have this side effect, was approved in the European Union for use in cattle (303). In 2000, it was also approved for use in pigs (303). In other countries, including China, florfenicol was also approved for use in fish and poultry. Thus far, florfenicol or derivatives thereof have not been approved for use in human medicine worldwide. The widespread use of florfenicol in farm animals may select for florfenicol-resistant bacteria, which also include those that carry cfr genes, optrA, and/or poxtA. A recent study from China showed that the presence of florfenicol residues is associated with the abundance of oxazolidinone resistance genes in livestock manures (304). Interestingly, the antimicrobial resistance genes, frequently colocated with either cfr genes, optrA, or poxtA, are the phenicol exporter genes fexA in staphylococci, fexB in enterococci, and floR in Gram-negative bacteria. The presence of two phenicol resistance genes may account for higher phenicol MICs, especially when both genes are located on the same plasmid, as shown for cfr and fexA (117).
In Tables 2 to 5 and Table 7, resistance genes that can colocate with cfr genes, optrA, or poxtA on plasmids are listed. These tables clearly showed that, besides phenicol resistance genes, genes coding for resistance to other frequently used classes of antimicrobial agents are also often present on plasmids carrying oxazolidinone resistance genes. In Gram-positive bacteria, these genes include (i) the MLSB resistance genes erm(A), erm(B), erm(C), erm(T), and erm(33), (ii) the aminoglycoside resistance genes aacA-aphD, aadD, aphA3, aadE, and aadY, (iii) the tetracycline resistance genes tet(L), tet(M), tet(S), and tet(O/W/32/O), (iv) the spectinomycin resistance genes spc and spw, (v) the lincosamide resistance genes lnu(A), lnu(B), lnu(G), lnu(P), and lsa(E), (vi) the macrolide resistance genes msr(A) and mef(E), (vii) the streptothricin resistance gene sat4 and the bacitracin resistance operon bcrABR, (viii) the vancomycin resistance vanA gene cluster, (ix) the bleomycin resistance gene ble, (x) the trimethoprim resistance gene dfrG, and (xi) the streptogramin A resistance gene vat(E). In addition, a copper resistance operon or the gene czcD for resistance to cobalt, zinc, and cadmium was occasionally detected on cfr-, optrA-, or poxtA-carrying plasmids. In Gram-negative bacteria, the colocated resistance genes included, besides floR, (i) the β-lactam resistance genes blaCTX-M-14b, blaOXA-10, blaTEM-1, and blaTEM-176, (ii) the tetracycline resistance genes tet(A), tet(B), and tet(M), (iii) the aminoglycoside resistance genes aacC4, aadA1, aadA2, aphA1, hph, strA, and strB, (iv) the colistin resistance gene mcr-1.1, (v) the macrolide resistance genes msr(E) and mph(E), (vi) the lincosamide resistance gene lnu(F), (vii) the MLSB resistance gene erm(B), (viii) the sulfonamide resistance genes sul1 and sul2, (ix) the trimethoprim resistance gene dfrA12, (x) the chloramphenicol resistance gene catB3, (xi) the bleomycin resistance gene ble, and (xii) the quinolone resistance gene qnrS1. Occasionally, the quaternary ammonium compound resistance gene qacEΔ1, the mercury resistance operon mer, and the arsenic resistance operon ars were also detected.
The listing of all these colocated antimicrobial, biocide, and heavy metal resistance genes shows that there are manifold options for coselection of the oxazolidinone resistance genes. Several of these antimicrobial classes, such as the tetracyclines, penicillins, macrolides, sulfonamides, and trimethoprim, as well as aminoglycosides, are approved for and are widely used in veterinary medicine worldwide. The examples presented (Tables 2 to 5 and Table 7) showed that oxazolidinone resistance genes are often colocated with genes conferring resistance to antimicrobial agents, biocides and metals on the same plasmid and that cfr-, optrA-, and poxtA-carrying multiresistance plasmids are widespread among bacteria of animal and human origin. The same is true for transposons, ICEs, and prophages that carry oxazolidinone resistance genes. When such multiresistance MGEs are transferred to new host bacteria, all of their colocated resistance genes are transferred, too. It is important to understand that the selective pressure by the use of one selecting agent is sufficient to ensure that the bacterium does not lose the respective multiresistance MGE (305).
In summary, not only the direct selection pressure imposed by the use of florfenicol in animals and oxazolidinones in humans, but also the indirect selection pressure imposed by the use of any of the other aforementioned non-oxazolidinone antimicrobial agents in humans and animals as well as the use of heavy metals or biocides play important roles in the coselection and persistence of mobile oxazolidinone resistance genes. The concept of indirect selective pressure imposed by the use of non-oxazolidinone antibiotics is important in our understanding of how oxazolidinone resistance genes disseminate and finally end up in bacterial lineages of humans and animals. The results of monitoring and surveillance studies in combination with a detailed analysis of the respective bacteria and individual antimicrobial consumption data will tell whether the prevalence of oxazolidinone resistance genes in clinical isolates will increase in the coming years due to coselection.
MOLECULAR AND PHENOTYPIC DETECTION OF OXAZOLIDINONE RESISTANCE GENES
When a new mobile oxazolidinone resistance gene was identified, a PCR assay to specifically detect this gene was usually described as well. This was true for cfr (117), cfr(B) (48), cfr(C) (50), cfr(D) (238), optrA (54), and poxtA (298). In the meantime, numerous other PCR primers and conditions have been described for most of the aforementioned oxazolidinone resistance genes (e.g., see references 212, 259, 262, 281, 296, 298, 306, 307). Bender and coworkers developed a multiplex PCR to simultaneously detect the mobile oxazolidinone resistance genes cfr, optrA, and poxtA in enterococcal isolates of clinical origin (308). Hasman and colleagues developed a web tool, LRE-Finder (where LRE stands for linezolid-resistant enterococci), for the detection of the most common 23S rRNA mutations, G2576T and G2505A, and the mobile oxazolidinone resistance genes optrA, cfr, cfr(B), and poxtA in whole-genome sequences from enterococci (309). The LRE-Finder tool was validated against 21 LRE isolates and 1,473 non-LRE isolates. It showed 100% agreement with the results of phenotypic susceptibility testing (309). LRE-Finder version 1.0 is available at https://cge.cbs.dtu.dk/services/LRE-finder/.
Mobile oxazolidinone resistance genes can also be identified in whole-genome or whole-plasmid sequences when referring to the five most common antimicrobial resistance databases, including ResFinder (https://cge.cbs.dtu.dk/services/ResFinder/) (310), the Comprehensive Antibiotic Resistance Database (CARD; https://card.mcmaster.ca/) (311), AMRFinder at the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/pathogens/antimicrobial-resistance/AMRFinder/) (312), ARG-ANNOT (http://backup.mediterranee-infection.com/article.php?laref=282&titre=arg-annot) (313), and MEGARes (https://megares.meglab.org/) (314). All seven mobile oxazolidinone resistance genes known to date could only be identified using the ResFinder and the AMRFinder tools. CARD did not identify cfr(C) and cfr(E), while ARG-ANNOT and MEGARes missed the genes cfr(C), cfr(D), and cfr(E).
For phenotypic detection of linezolid- or tedizolid-resistant staphylococci, streptococci, enterococci, and corynebacteria, the clinical breakpoints from CLSI or EUCAST might be used. However, mobile oxazolidinone resistance genes also occurred in other bacteria, including the genera Bacillus, Campylobacter, Clostridioides, Clostridium, Escherichia, Listeria, Morganella, Pediococcus, Providencia, and Proteus, among others. Thus far, no clinical breakpoints have been available to reliably assess resistance or susceptibility to linezolid and tedizolid in these bacteria in phenotypic assays.
Dejoies and coworkers comparatively investigated 20 E. faecalis and 80 E. faecium isolates (including one optrA/poxtA-, 17 poxtA-, and 20 optrA-carrying isolates) for their linezolid susceptibility by broth microdilution and seven commercial methods (agar disc diffusion [Bio-Rad] and Etest [bioMérieux], two nonautomated broth assays [Sensititre and UMIC], and three automated broth assays [MicroScan WalkAway, Phoenix, and Vitek 2]) (315). Results were read after 18 and 42 h and interpreted according to CLSI and EUCAST breakpoints. Substantial variations in the results obtained with the different AST methods were found; in particular, the automated systems Phoenix and Vitek 2 did not detect several isolates classified as resistant by broth microdilution. In general, the nonautomated methods (UMIC and Sensititre) and, to a lesser extent, Etest exhibited an acceptable correlation with the broth microdilution reference method for the detection of isolates with low linezolid MICs after expanded incubation. Another two comparative studies for the detection of linezolid susceptibility provided, in part, different results (316, 317).
In a study from South Korea, 27 MRSA isolates from 14 patients were investigated for their linezolid susceptibility using the automated system Vitek 2 (bioMérieux, Marcy-l’Étoile, France) and broth microdilution according to CLSI (318). Only four isolates from the same patient were identified as resistant (MICs ≥ 8 μg/ml) by both methods, while the remaining 23 isolates were classified as resistant by Vitek 2 and as susceptible by broth microdilution (318). Molecular analysis of the 27 isolates identified the 23S rRNA mutations (T2500A in two of the five rRNA operons) only in the four resistant isolates, while none of the isolates carried the genes cfr, cfr(B), or optrA (318).
As screening of bacterial isolates for linezolid resistance becomes increasingly important in microbiological diagnostic laboratories, Werner and colleagues validated a screening agar for the detection of linezolid-resistant enterococci (319). The authors recommended the use of an enterococcal selective agar (e.g., Enterococcosel agar) supplemented with 2 mg/liter linezolid and an incubation period of 48 h. SuperLinezolid agar was developed and validated by Nordmann and coworkers (320). This selective culture medium was intended to screen for linezolid-resistant Gram-positive bacteria of the genera Staphylococcus and Enterococcus and contains 1.5 mg/liter linezolid as the threshold concentration. This medium can accurately detect linezolid-resistant staphylococci and enterococci after 24 h of incubation.
REVERSAL AND INHIBITION OF OXAZOLIDINONE RESISTANCE GENES
The development of inhibitors of resistance-mediating proteins, which are often small-molecular compounds that by binding to the targets destroy or block the activity of resistance-mediating proteins, is a promising approach to reverse the efficacy of antimicrobial agents in the treatment of infections caused by multidrug-resistant bacteria. For instance, the inhibitors for β-lactamases, clavulanic acid and sulbactam, could significantly prolong the life span and extend the application of β-lactams (321, 322). However, no inhibitor has been used to reverse the resistance to oxazolidinones conferred by Cfr or OptrA. Mechanism explorations, especially structural and biochemical studies, can provide hundreds of new targets and opportunities for future drug discovery. Given that ATP hydrolysis is a characteristic requirement for ABC-F proteins, including OptrA and PoxtA, to confer resistance, inhibitors targeting ATP hydrolysis at nucleotide binding domains should be considered (323). Recently, Zhong et al. found a novel and specific inhibitor of OptrA, CP1, which suppressed the ATPase activity of OptrA in vitro by 30% (324). A hydrogen bond formed between the 8-position phenylcyclic cyano group in CP1, and the amino acid residue Lys-271 allows CP1 to form a stable complex with the OptrA protein (324), which impaired the ribosome protection function of OptrA. This study provided a theoretical basis for the further optimization of the inhibitor structure to obtain inhibitors with higher efficiencies. Besides, it also provided a possibility to develop inhibitors that target the ATPase centers of either ABC-F proteins or ABC efflux pumps to counteract antimicrobial resistance conferred by them (324). To date, there is no report about an inhibitor for Cfr-mediated oxazolidinone resistance, but several studies found that some E. faecalis (171, 172) and S. haemolyticus (153) isolates, were, for yet unknown reasons, susceptible to linezolid despite the fact that they harbored a complete cfr gene including its promoter (153, 171, 172). In one case, the authors could also show that the Cfr protein was produced and was detectable by Western blotting. In addition, the Cfr-specific methylation of A2503 was also shown, suggesting that there must be factors that are responsible for the non-PhLOPSA phenotype (171). In another study, a cfr(B) gene from either C. difficile or E. faecium was unable to confer linezolid resistance when cloned and transferred into different E. faecalis recipient strains (232). Thus, unraveling and using the mechanism of Cfr and Cfr(B) failure to confer resistance to linezolid could be an opportunity to overcome the Cfr-/Cfr(B)-mediated oxazolidinone resistance.
CONCLUSIONS AND OUTLOOK
Oxazolidinones are important antimicrobial agents for the treatment of infections caused by multidrug-resistant Gram-positive bacteria. Thus, it is of utmost relevance to preserve their efficacy for the future. During the first 20 years after introduction of linezolid into clinical use, only very low numbers of resistant bacteria have been identified in respective monitoring programs. However, despite this overall very favorable situation, resistant bacteria have been identified occasionally in samples from humans and animals. Even worse, some of these resistant bacteria harbor transferable oxazolidinone resistance genes, of which at least seven different ones have been identified. They are spread all over the world and have been identified in numerous Gram-positive, but also Gram-negative, bacteria. Surprisingly, all oxazolidinone resistance genes known so far not only confer resistance to oxazolidinones, but also to phenicols (cfr and its variants, as well as optrA and poxtA) and tetracyclines (poxtA) or lincosamides, pleuromutilins, and streptogramin A antibiotics (cfr and its variants). This situation offers manifold opportunities for coselection by the use of the respective antimicrobial agents. Oxazolidinones are used in human medicine, but only very rarely in companion animals and not at all in food-producing animals. In contrast, phenicols, such as chloramphenicol, play only a minor role in human medicine, whereas florfenicol is widely and exclusively used for therapeutic purposes in livestock animals worldwide. Moreover, tetracyclines are the most and second most frequently used group of antimicrobial agents—after β-lactams—in human and veterinary medicine, respectively (325, 326). As the aforementioned oxazolidinone resistance genes are circulating among and between bacteria of human, animal, environmental, and food origin, a One Health approach is needed for monitoring the emergence and transmission of these genes and the bacteria which harbor them.
In addition, the oxazolidinone resistance genes have been found on a variety of MGEs, including plasmids, transposons, ICEs, prophages, and genomic islands, in various bacteria. These MGEs, but also IS-mediated, oxazolidinone resistance gene-carrying TUs, which can integrate into the aforementioned MGEs, play an important role in the spread of cfr, optrA, and poxtA genes across not only strain, species, and genus, but also family and order borders. In addition, these MGEs often carry additional resistance genes which support the coselection and persistence of the oxazolidinone resistance genes. The most important measure to reduce the dissemination of resistant bacteria is to decrease the selection pressure. This is usually achieved by a reduced application of the respective selecting or coselecting antimicrobial agents. However, due to the numerous colocated antimicrobial resistance genes (as visible from Tables 2 to 5 and Table 7), it will be a difficult task to avoid the coselection of oxazolidinone resistance genes in the different bacteria. Encouragingly, the Chinese Ministry of Agriculture and Rural Affairs (CMARA) has issued a pilot project entitled “Action of Reduction of Antimicrobial Agents used in Veterinary Practice,” which aims at maintaining zero increase in the use of antimicrobial agents in farm animals over the period from 2018 to 2021 (http://www.moa.gov.cn/govpublic/SYJ/201804/t20180420_6140711.htm). Moreover, the CMARA further issued a strict withdrawal policy which included the instruction that all antimicrobial agents were to be prohibited as growth promoters from 1 July 2020 onward (http://www.moa.gov.cn/govpublic/xmsyj/201907/t20190710_6320678.htm). In other countries/regions of the world, similar regulations are in place. In the European Union, the use of antimicrobial growth promoters has been prohibited since 2006 (https://ec.europa.eu/commission/presscorner/detail/en/IP_05_1687), and numerous countries have started attempts to decrease the use of antimicrobial agents in human and veterinary medicine. As an example, the sales figures of veterinary antimicrobial agents in Germany have dropped by more than 60% between 2011 and 2019 (https://www.bft-online.de/fileadmin/bft/publikationen/Blickpunkt/BP_94/Blickpunkt_94.pdf). The future will show whether the reduction of the selective pressure by a lesser use of coselecting antimicrobial agents (e.g., tetracyclines, phenicols, lincosamides, and pleuromutilins) will have a positive impact on the dissemination of oxazolidinone resistance genes in bacteria of both animal and human origin.
The costs of antimicrobial agents can also regulate, in a way, the quantity of use. The original linezolid-containing Zyvox was a high-cost antimicrobial agent that was only prescribed and used when there was no less expensive option. In 2017, linezolid has become a generic drug that is no longer protected by a patent. As a consequence, several generic linezolid-containing medicinal products have become available, and their costs are distinctly lower than that of Zyvox. The future will show whether these lower costs will lead to an increased use of linezolid, accompanied by a higher selection pressure and an increase in the frequency of resistant isolates. In this regard, monitoring and surveillance programs need to include oxazolidinones (if not done yet) to early detect a rise in oxazolidinone resistance and—if one is detected—to rapidly implement counteractive measures. In addition, newly developed approaches such as inhibitors of linezolid resistance determinants, phages/phage lysins, and also compounds of traditional Chinese medicine are promising weapons and alternative ways to combat oxazolidinone-resistant pathogens.
ACKNOWLEDGMENTS
We thank Carola Giersch for preparing Fig. 1 and Jennifer K. Bender for helpful discussions.
This work was in part funded by the National Natural Science Foundation of China (grants 31761133022 and 81861138051 to C.W. and Y.W.), the National Key Research and Development Program of China (grant 2017YFD0500102 to W.Z.), the Program for Innovative Research Team (in Science and Technology) in University of Henan Province (grants 18IRTSTHN020 and 19IRTSTHN007 to X.-D.D.), the German Research Foundation (grant SCHW382/11-1 to H.K., A.T.F., and S.S.), and the German Federal Ministry of Education and Research (BMBF) under project numbers 01KI1727D and 01KI2009D as part of the Research Network Zoonotic Infectious Diseases (A.T.F. and S.S.).
Biographies

Stefan Schwarz is a Professor for Microbiology and Epizootics, as well as the managing director of the Institute of Microbiology and Epizootics in the Centre of Infection Medicine, within the Department of Veterinary Medicine at the Freie Universität Berlin, Berlin, Germany. Since the late 1980s, his main research interests are the molecular genetics of resistance to antimicrobial agents, biocides, and antimicrobial peptides in various Gram-positive and Gram-negative bacteria. A particular focus of his research activities is on the mobile genetic elements that carry resistance genes. As a cat lover, he supports an animal shelter in his free time.

Wanjiang Zhang is a Professor in the Division of Bacterial Diseases of Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Harbin, China. His current research interests focus on emerging antimicrobial resistance in bacteria of animal and environmental origin, especially the prevalence, dissemination, and control of resistance to carbapenems and oxazolidinones. Screening of new antibacterial compounds from traditional Chinese medicine or natural plants is another main point of his research activities.

Xiang-Dang Du is a professor for Veterinary Pharmacology in the College of Veterinary Medicine at Henan Agricultural University, Zhengzhou, China. He serves as a member for the Veterinary Drug Evaluation Committee of the Ministry of Agriculture and for the Commission of Chinese Veterinary Pharmacopoeia. Since 2000, his main research interests have focused on the identification of novel resistance genes in various Gram-positive and Gram-negative bacteria of animal origin and the mobile genetic elements associated with antimicrobial resistance genes.

Henrike Krüger is a veterinarian by training and currently a Ph.D. student in the Institute of Microbiology and Epizootics in the Centre of Infection Medicine within the Department of Veterinary Medicine at the Freie Universität Berlin, Berlin, Germany. She is particularly interested in emerging antimicrobial resistances within the One Health context. The focus of her current research activities is on resistance to last-resort antimicrobial agents among veterinary pathogens and comparative phenotypic and genotypic studies on livestock-associated MRSA from pigs in Germany and China.

Andrea T. Feßler is a postdoctoral research fellow at the Institute of Microbiology and Epizootics in the Centre of Infection Medicine within the Department of Veterinary Medicine at the Freie Universität Berlin, Berlin, Germany. Since 2009, she has worked in the field of bacterial resistance to antimicrobial agents and biocides. Her main research interests are the characterization of novel resistance genes and the development of antimicrobial and biocide susceptibility testing methods, quality control ranges, and clinical breakpoints for various antimicrobial agents and bacterial species.

Shizhen Ma is a doctor in the College of Veterinary Medicine at China Agricultural University. She has worked intensively on the transmission of antibiotic resistant bacteria, especially oxazolidinone-resistant staphylococci and extended-spectrum β-lactamase (ESBL)-producing E. coli from humans and household animals in rural China.

Yao Zhu is a postdoctoral researcher at the Harbin Veterinary Research Institute, CAAS. He obtained his Ph.D. from the same institution in June 2019. His Ph.D. study focused on the prevalence and dissemination of mobile carbapenem and colistin resistance genes in Enterobacteriaceae. His research currently focuses on emerging mechanisms of resistance to carbapenems and oxazolidinones in bacteria of animal origin, as well as the discovery of novel antibacterial peptides.

Congming Wu is a Professor for Veterinary Microbiology and the Director of the Department of Basic Veterinary Medicine in the College of Veterinary Medicine at China Agricultural University. In the last two decades, he focused his research activities on the emergence and transmission of antibiotic-resistant bacteria, such as multiresistant Staphylococcus, E. coli, Salmonella, and Campylobacter. His research results have played an important role in the regulation and management of veterinary antibiotics in China.

Jianzhong Shen is an academician of the Chinese Academy of Engineering and Dean and Professor of the College of Veterinary Medicine, China Agricultural University. During the last two decades, he has accumulated extensive knowledge on the emergence and the transmission of antimicrobial resistance through the One Health approach, by discovering several novel antimicrobial resistance genes (e.g., optrA and mcr-1), which are highly critical to public health, and identifying potential transmission routes of resistance genes in the food supply chain.

Yang Wang is a Professor in the College of Veterinary Medicine at China Agricultural University. He has worked intensively on antimicrobial resistance in bacteria of animal, human and environmental origin. Since 2006, he has been involved in research on the emergence, transmission, and control of extensively drug-resistant Gram-positive and Gram-negative bacteria. His results have provided important scientific evidence for controlling the spread of antibiotic resistance and issuing key regulations and policies.
REFERENCES
- 1.Slee AM, Wuonola MA, McRipley RJ, Zajac I, Zawada MJ, Bartholomew PT, Gregory WA, Forbes M. 1987. Oxazolidinones, a new class of synthetic antibacterial agents: in vitro and in vivo activities of DuP 105 and DuP 721. Antimicrob Agents Chemother 31:1791–1797. 10.1128/aac.31.11.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brickner SJ, Hutchinson DK, Barbachyn MR, Manninen PR, Ulanowicz DA, Garmon SA, Grega KC, Hendges SK, Toops DS, Ford CW, Zurenko GE. 1996. Synthesis and antibacterial activity of U-100592 and U-100766, two oxazolidinone antibacterial agents for the potential treatment of multidrug-resistant Gram-positive bacterial infections. J Med Chem 39:673–679. 10.1021/jm9509556. [DOI] [PubMed] [Google Scholar]
- 3.Zurenko GE, Yagi BH, Schaadt RD, Allison JW, Kilburn JO, Glickman SE, Hutchinson DK, Barbachyn MR, Brickner SJ. 1996. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob Agents Chemother 40:839–845. 10.1128/AAC.40.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bain KT, Wittbrodt ET. 2001. Linezolid for the treatment of resistant Gram-positive cocci. Ann Pharmacother 35:566–575. 10.1345/aph.10276. [DOI] [PubMed] [Google Scholar]
- 5.Tsuji BT, Kaatz GW, Ryback MJ. 1999. –2021. Linezolid and other oxazolidinones. In Antimicrobial therapy and vaccines volume II: antimicrobial agents. Antimicrobe, Pittsburgh, PA. http://www.antimicrobe.org/d13.asp. Accessed 12 Jan 2021. [Google Scholar]
- 6.Noskin GA, Siddiqui F, Stosor V, Hacek D, Peterson LR. 1999. In vitro activities of linezolid against important Gram-positive bacterial pathogens including vancomycin-resistant enterococci. Antimicrob Agents Chemother 43:2059–2062. 10.1128/AAC.43.8.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rybak MJ, Hershberger E, Moldovan T, Grucz RG. 2000. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against staphylococci and enterococci, including vancomycin-intermediate and -resistant strains. Antimicrob Agents Chemother 44:1062–1066. 10.1128/aac.44.4.1062-1066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaatz GW, Seo SM. 1996. In vitro activities of oxazolidinone compounds U100592 and U100766 against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother 40:799–801. 10.1128/AAC.40.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baum SE, Crawford SA, McElmeel ML, Whitney CG, Jorgensen JH. 2002. Comparative activities of the oxazolidinone AZD2563 and linezolid against selected recent North American isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother 46:3094–3095. 10.1128/aac.46.9.3094-3095.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein EJC, Citron DM, Merriam CV. 1999. Linezolid activity compared to those of selected macrolides and other agents against aerobic and anaerobic pathogens isolated from soft tissue bite infections in humans. Antimicrob Agents Chemother 43:1469–1474. 10.1128/AAC.43.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoppe JE. 1999. In vitro susceptibilities of Bordetella pertussis and Bordetella parapertussis to the novel oxazolidinones eperezolid (PNU-100592) and linezolid (PNU-100766). J Chemother 11:220–221. 10.1179/joc.1999.11.3.220. [DOI] [PubMed] [Google Scholar]
- 12.Zhanel GG, Karlowsky JA, Low DE, Hoban DJ, Group T. 2000. Antibiotic resistance in respiratory tract isolates of Haemophilus influenzae and Moraxella catarrhalis collected from across Canada in 1997–1998. J Antimicrob Chemother 45:655–662. 10.1093/jac/45.5.655. [DOI] [PubMed] [Google Scholar]
- 13.Citron DM, Merriam CV, Tyrrell KL, Warren YA, Fernandez H, Goldstein EJC. 2003. In vitro activities of ramoplanin, teicoplanin, vancomycin, linezolid, bacitracin, and four other antimicrobials against intestinal anaerobic bacteria. Antimicrob Agents Chemother 47:2334–2338. 10.1128/AAC.47.7.2334-2338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein EJC, Citron DM, Merriam CV, Warren YA, Tyrrell KL, Fernandez HT. 2003. In vitro activities of daptomycin, vancomycin, quinupristin- dalfopristin, linezolid, and five other antimicrobials against 307 Gram-positive anaerobic and 31 Corynebacterium clinical isolates. Antimicrob Agents Chemother 47:337–341. 10.1128/AAC.47.1.337-341.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peláez T, Alonso R, Pérez C, Alcalá L, Cuevas O, Bouza E. 2002. In vitro activity of linezolid against Clostridium difficile. Antimicrob Agents Chemother 46:1617–1618. 10.1128/AAC.46.5.1617-1618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips OA, Rotimi VO, Jamal WY, Shahin M, Verghese TL. 2003. Comparative in vitro activity of PH-027 versus linezolid and other anti-anaerobic antimicrobials against clinical isolates of Clostridium difficile and other anaerobic bacteria. J Chemother 15:113–117. 10.1179/joc.2003.15.2.113. [DOI] [PubMed] [Google Scholar]
- 17.Wallace RJ, Jr, Brown-Elliott BA, Ward SC, Crist CJ, Mann LB, Wilson RW. 2001. Activities of linezolid against rapidly growing mycobacteria. Antimicrob Agents Chemother 45:764–767. 10.1128/AAC.45.3.764-767.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters J, Kondo KL, Lee RK, Lin CK, Inderlied CB. 1995. In-vitro activity of oxazolidinones against Mycobacterium avium Complex. J Antimicrob Chemother 35:675–679. 10.1093/jac/35.5.675. [DOI] [PubMed] [Google Scholar]
- 19.Daly JS, Eliopoulos GM, Reiszner E, Moellering RC, Jr.. 1988. Activity and mechanism of action of DuP 105 and DuP 721, new oxazolidinone compounds. J Antimicrob Chemother 21:721–730. 10.1093/jac/21.6.721. [DOI] [PubMed] [Google Scholar]
- 20.Papich MG. 2013. Antibiotic treatment of resistant infections in small animals. Vet Clin North Am Small Anim Pract 43:1091–1107. 10.1016/j.cvsm.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Diekema DJ, Jones RN. 2000. Oxazolidinones. Drugs 59:7–16. 10.2165/00003495-200059010-00002. [DOI] [PubMed] [Google Scholar]
- 22.Eustice DC, Feldman PA, Zajac I, Slee AM. 1988. Mechanism of action of DuP 721: inhibition of an early event during initiation of protein synthesis. Antimicrob Agents Chemother 32:1218–1222. 10.1128/aac.32.8.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin AH, Murray RW, Vidmar TJ, Marotti KR. 1997. The oxazolidinone eperezolid binds to the 50S ribosomal subunit and competes with binding of chloramphenicol and lincomycin. Antimicrob Agents Chemother 41:2127–2131. 10.1128/AAC.41.10.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swaney SM, Aoki H, Ganoza MC, Shinabarger DL. 1998. The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria. Antimicrob Agents Chemother 42:3251–3255. 10.1128/AAC.42.12.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franklin TJ, Snow GA. 2005. Biochemistry and molecular biology of antimicrobial drug action, 6th ed. Springer, New York, NY. [Google Scholar]
- 26.Livermore DM. 2003. Linezolid in vitro: mechanism and antibacterial spectrum. J Antimicrob Chemother 51:ii9–ii16. 10.1093/jac/dkg249. [DOI] [PubMed] [Google Scholar]
- 27.Fines M, Leclercq R. 2000. Activity of linezolid against Gram-positive cocci possessing genes conferring resistance to protein synthesis inhibitors. J Antimicrob Chemother 45:797–802. 10.1093/jac/45.6.797. [DOI] [PubMed] [Google Scholar]
- 28.Prystowsky J, Siddiqui F, Chosay J, Shinabarger DL, Millichap J, Peterson LR, Noskin GA. 2001. Resistance to linezolid: characterization of mutations in rRNA and comparison of their occurrences in vancomycin-resistant enterococci. Antimicrob Agents Chemother 45:2154–2156. 10.1128/AAC.45.7.2154-2156.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kloss P, Xiong L, Shinabarger DL, Mankin AS. 1999. Resistance mutations in 23 S rRNA identify the site of action of the protein synthesis inhibitor linezolid in the ribosomal peptidyl transferase center. J Mol Biol 294:93–101. 10.1006/jmbi.1999.3247. [DOI] [PubMed] [Google Scholar]
- 30.Xiong L, Kloss P, Douthwaite S, Andersen NM, Swaney S, Shinabarger DL, Mankin AS. 2000. Oxazolidinone resistance mutations in 23S rRNA of Escherichia coli reveal the central region of domain V as the primary site of drug action. J Bacteriol 182:5325–5331. 10.1128/jb.182.19.5325-5331.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lobritz M, Hutton-Thomas R, Marshall S, Rice LB. 2003. Recombination proficiency influences frequency and locus of mutational resistance to linezolid in Enterococcus faecalis. Antimicrob Agents Chemother 47:3318–3320. 10.1128/aac.47.10.3318-3320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsiodras S, Gold HS, Sakoulas G, Eliopoulos GM, Wennersten C, Venkataraman L, Moellering RC, Jr, Ferraro MJ. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207–208. 10.1016/S0140-6736(01)05410-1. [DOI] [PubMed] [Google Scholar]
- 33.Jones RN, Della-Latta P, Lee LV, Biedenbach DJ. 2002. Linezolid-resistant Enterococcus faecium isolated from a patient without prior exposure to an oxazolidinone: report from the SENTRY antimicrobial surveillance program. Diagn Microbiol Infect Dis 42:137–139. 10.1016/S0732-8893(01)00333-9. [DOI] [PubMed] [Google Scholar]
- 34.Long KS, Vester B. 2012. Resistance to linezolid caused by modifications at its binding site on the ribosome. Antimicrob Agents Chemother 56:603–612. 10.1128/AAC.05702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stefani S, Bongiorno D, Mongelli G, Campanile F. 2010. Linezolid resistance in staphylococci. Pharmaceuticals (Basel) 3:1988–2006. 10.3390/ph3071988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Locke JB, Hilgers M, Shaw KJ. 2009. Novel Ribosomal Mutations in Staphylococcus aureus Strains Identified through Selection with the Oxazolidinones Linezolid and Torezolid (TR-700). Antimicrob Agents Chemother 53:5265–5274. 10.1128/AAC.00871-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Locke JB, Hilgers M, Shaw KJ. 2009. Mutations in ribosomal protein L3 are associated with oxazolidinone resistance in staphylococci of clinical origin. Antimicrob Agents Chemother 53:5275–5278. 10.1128/AAC.01032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Almeida LM, de Araújo MRE, Sacramento AG, Pavez M, de Souza AG, Rodrigues F, Gales AC, Lincopan N, Sampaio JLM, Mamizuka EM. 2013. Linezolid resistance in Brazilian Staphylococcus hominis strains is associated with L3 and 23S rRNA ribosomal mutations. Antimicrob Agents Chemother 57:4082–4083. 10.1128/AAC.00437-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bongiorno D, Campanile F, Mongelli G, Baldi MT, Provenzani R, Reali S, Lo Russo C, Santagati M, Stefani S. 2010. DNA methylase modifications and other linezolid resistance mutations in coagulase-negative staphylococci in Italy. J Antimicrob Chemother 65:2336–2340. 10.1093/jac/dkq344. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen LTT, Nguyen KNT, Le PNTA, Cafini F, Pascoe B, Sheppard SK, Nguyen TB, Nguyen TPH, Nguyen TV, Pham TTK, Morikawa K, Nguyen DQ, Duong HX. 2020. The emergence of plasmid-borne cfr-mediated linezolid resistant-staphylococci in Vietnam. J Glob Antimicrob Resist 22:462–465. 10.1016/j.jgar.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Wolter N, Smith AM, Farrell DJ, Schaffner W, Moore M, Whitney CG, Jorgensen JH, Klugman KP. 2005. Novel mechanism of resistance to oxazolidinones, macrolides, and chloramphenicol in ribosomal protein L4 of the pneumococcus. Antimicrob Agents Chemother 49:3554–3557. 10.1128/AAC.49.8.3554-3557.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hölzel CS, Harms KS, Schwaiger K, Bauer J. 2010. Resistance to linezolid in a porcine Clostridium perfringens strain carrying a mutation in the rplD gene encoding the ribosomal protein L4. Antimicrob Agents Chemother 54:1351–1353. 10.1128/AAC.01208-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flamm RK, Farrell DJ, Mendes RE, Ross JE, Sader HS, Jones RN. 2012. LEADER surveillance program results for 2010: an activity and spectrum analysis of linezolid using 6801 clinical isolates from the United States (61 medical centers). Diagn Microbiol Infect Dis 74:54–61. 10.1016/j.diagmicrobio.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 44.Flamm RK, Mendes RE, Hogan PA, Ross JE, Farrell DJ, Jones RN. 2015. In vitro activity of linezolid as assessed through the 2013 LEADER surveillance program. Diagn Microbiol Infect Dis 81:283–289. 10.1016/j.diagmicrobio.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Schwarz S, Werckenthin C, Kehrenberg C. 2000. Identification of a plasmid-borne chloramphenicol-florfenicol resistance gene in Staphylococcus sciuri. Antimicrob Agents Chemother 44:2530–2533. 10.1128/aac.44.9.2530-2533.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kehrenberg C, Schwarz S, Jacobsen L, Hansen LH, Vester B. 2005. A new mechanism for chloramphenicol, florfenicol and clindamycin resistance: methylation of 23S ribosomal RNA at A2503. Mol Microbiol 57:1064–1073. 10.1111/j.1365-2958.2005.04754.x. [DOI] [PubMed] [Google Scholar]
- 47.Long KS, Poehlsgaard J, Kehrenberg C, Schwarz S, Vester B. 2006. The Cfr rRNA methyltransferase confers resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A antibiotics. Antimicrob Agents Chemother 50:2500–2505. 10.1128/AAC.00131-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deshpande LM, Ashcraft DS, Kahn HP, Pankey G, Jones RN, Farrell DJ, Mendes RE. 2015. Detection of a new cfr-like gene, cfr(B), in Enterococcus faecium isolates recovered from human specimens in the United States as part of the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother 59:6256–6261. 10.1128/AAC.01473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marín M, Martín A, Alcalá L, Cercenado E, Iglesias C, Reigadas E, Bouza E. 2015. Clostridium difficile isolates with high linezolid MICs harbor the multiresistance gene cfr. Antimicrob Agents Chemother 59:586–589. 10.1128/AAC.04082-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang Y, Dai L, Sahin O, Wu Z, Liu M, Zhang Q. 2017. Emergence of a plasmid-borne multidrug resistance gene cfr(C) in foodborne pathogen Campylobacter. J Antimicrob Chemother 72:1581–1588. 10.1093/jac/dkx023. [DOI] [PubMed] [Google Scholar]
- 51.Pang S, Boan P, Lee T, Gangatharan S, Tan SJ, Daley D, Lee YT, Coombs GW. 2020. Linezolid-resistant ST872 Enteroccocus faecium harbouring optrA and cfr(D) oxazolidinone resistance genes. Int J Antimicrob Agents 55:105831. 10.1016/j.ijantimicag.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 52.Stojković V, Ulate MF, Hidalgo-Villeda F, Aguilar E, Monge-Cascante C, Pizarro-Guajardo M, Tsai K, Tzoc E, Camorlinga M, Paredes-Sabja D, Quesada-Gómez C, Fujimori DG, Rodríguez C. 2019. cfr(B), cfr(C), and a new cfr-like gene, cfr(E), in Clostridium difficile strains recovered across Latin America. Antimicrob Agents Chemother 64:e01074-19. 10.1128/AAC.01074-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hansen LH, Vester B. 2015. A cfr-like gene from Clostridium difficile confers multiple antibiotic resistance by the same mechanism as the cfr gene. Antimicrob Agents Chemother 59:5841–5843. 10.1128/AAC.01274-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Lv Y, Cai J, Schwarz S, Cui L, Hu Z, Zhang R, Li J, Zhao Q, He T, Wang D, Wang Z, Shen Y, Li Y, Feßler AT, Wu C, Yu H, Deng X, Xia X, Shen J. 2015. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother 70:2182–2190. 10.1093/jac/dkv116. [DOI] [PubMed] [Google Scholar]
- 55.Sharkey LKR, Edwards TA, O’Neill AJ. 2016. ABC-F proteins mediate antibiotic resistance through ribosomal protection. mBio 7:e01975-15. 10.1128/mBio.01975-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antonelli A, D'Andrea MM, Brenciani A, Galeotti CL, Morroni G, Pollini S, Varaldo PE, Rossolini GM. 2018. Characterization of poxtA, a novel phenicol–oxazolidinone–tetracycline resistance gene from an MRSA of clinical origin. J Antimicrob Chemother 73:1763–1769. 10.1093/jac/dky088. [DOI] [PubMed] [Google Scholar]
- 57.Hua R, Xia Y, Wu W, Yan J, Yang M. 2018. Whole transcriptome analysis reveals potential novel mechanisms of low-level linezolid resistance in Enterococcus faecalis. Gene 647:143–149. 10.1016/j.gene.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 58.Ross JE, Anderegg TR, Sader HS, Fritsche TR, Jones RN. 2005. Trends in linezolid susceptibility patterns in 2002: report from the worldwide Zyvox Annual Appraisal of Potency and Spectrum program. Diagn Microbiol Infect Dis 52:53–58. 10.1016/j.diagmicrobio.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 59.Anderegg TR, Sader HS, Fritsche TR, Ross JE, Jones RN. 2005. Trends in linezolid susceptibility patterns: report from the 2002–2003 worldwide Zyvox Annual Appraisal of Potency and Spectrum (ZAAPS) Program. Int J Antimicrob Agents 26:13–21. 10.1016/j.ijantimicag.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 60.Jones RN, Ross JE, Fritsche TR, Sader HS. 2006. Oxazolidinone susceptibility patterns in 2004: report from the Zyvox® Annual Appraisal of Potency and Spectrum (ZAAPS) Program assessing isolates from 16 nations. J Antimicrob Chemother 57:279–287. 10.1093/jac/dki437. [DOI] [PubMed] [Google Scholar]
- 61.Ross JE, Fritsche TR, Sader HS, Jones RN. 2007. Oxazolidinone susceptibility patterns for 2005: international report from the Zyvox® Annual Appraisal of Potency and Spectrum study. Int J Antimicrob Agents 29:295–301. 10.1016/j.ijantimicag.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 62.Jones RN, Kohno S, Ono Y, Ross JE, Yanagihara K. 2009. ZAAPS International Surveillance Program (2007) for linezolid resistance: results from 5591 Gram-positive clinical isolates in 23 countries. Diagn Microbiol Infect Dis 64:191–201. 10.1016/j.diagmicrobio.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 63.Ross JE, Farrell DJ, Mendes RE, Sader HS, Jones RN. 2011. Eight-year (2002–2009) Summary of the Linezolid (Zyvox® Annual Appraisal of potency and Spectrum; ZAAPS) Program in European Countries. J Chemother 23:71–76. 10.1179/joc.2011.23.2.71. [DOI] [PubMed] [Google Scholar]
- 64.Flamm RK, Farrell DJ, Mendes RE, Ross JE, Sader HS, Jones RN. 2012. ZAAPS Program results for 2010: an activity and spectrum analysis of linezolid using clinical isolates from 75 medical centres in 24 countries. J Chemother 24:328–337. 10.1179/1973947812Y.0000000039. [DOI] [PubMed] [Google Scholar]
- 65.Flamm RK, Mendes RE, Ross JE, Sader HS, Jones RN. 2013. An international activity and spectrum analysis of linezolid: ZAAPS Program results for 2011. Diagn Microbiol Infect Dis 76:206–213. 10.1016/j.diagmicrobio.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 66.Mendes RE, Hogan PA, Streit JM, Jones RN, Flamm RK. 2014. Zyvox® Annual Appraisal of Potency and Spectrum (ZAAPS) program: report of linezolid activity over 9 years (2004–12). J Antimicrob Chemother 69:1582–1588. 10.1093/jac/dkt541. [DOI] [PubMed] [Google Scholar]
- 67.Biedenbach DJ, Farrell DJ, Mendes RE, Ross JE, Jones RN. 2010. Stability of linezolid activity in an era of mobile oxazolidinone resistance determinants: results from the 2009 Zyvox® Annual Appraisal of Potency and Spectrum program. Diagn Microbiol Infect Dis 68:459–467. 10.1016/j.diagmicrobio.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 68.Jones RN, Ross JE, Bell JM, Utsuki U, Fumiaki I, Kobayashi I, Turnidge JD. 2009. Zyvox® Annual Appraisal of Potency and Spectrum program: linezolid surveillance program results for 2008. Diagn Microbiol Infect Dis 65:404–413. 10.1016/j.diagmicrobio.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 69.Mendes RE, Hogan PA, Jones RN, Sader HS, Flamm RK. 2016. Surveillance for linezolid resistance via the Zyvox® Annual Appraisal of Potency and Spectrum (ZAAPS) programme (2014): evolving resistance mechanisms with stable susceptibility rates. J Antimicrob Chemother 71:1860–1865. 10.1093/jac/dkw052. [DOI] [PubMed] [Google Scholar]
- 70.Pfaller MA, Mendes RE, Streit JM, Hogan PA, Flamm RK. 2017. ZAAPS Program results for 2015: an activity and spectrum analysis of linezolid using clinical isolates from medical centres in 32 countries. J Antimicrob Chemother 72:3093–3099. 10.1093/jac/dkx251. [DOI] [PubMed] [Google Scholar]
- 71.Mendes RE, Deshpande L, Streit JM, Sader HS, Castanheira M, Hogan PA, Flamm RK. 2018. ZAAPS programme results for 2016: an activity and spectrum analysis of linezolid using clinical isolates from medical centres in 42 countries. J Antimicrob Chemother 73:1880–1887. 10.1093/jac/dky099. [DOI] [PubMed] [Google Scholar]
- 72.Draghi DC, Sheehan DJ, Hogan P, Sahm DF. 2005. In vitro activity of linezolid against key Gram-positive organisms isolated in the United States: results of the LEADER 2004 surveillance program. Antimicrob Agents Chemother 49:5024–5032. 10.1128/AAC.49.12.5024-5032.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones RN, Fritsche TR, Sader HS, Ross JE. 2007. LEADER surveillance program results for 2006: an activity and spectrum analysis of linezolid using clinical isolates from the United States (50 medical centers). Diagn Microbiol Infect Dis 59:309–317. 10.1016/j.diagmicrobio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 74.Farrell DJ, Mendes RE, Ross JE, Sader HS, Jones RN. 2011. LEADER Program results for 2009: an activity and spectrum analysis of linezolid using 6,414 clinical isolates from 56 medical centers in the United States. Antimicrob Agents Chemother 55:3684–3690. 10.1128/AAC.01729-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jones RN, Ross JE, Castanheira M, Mendes RE. 2008. United States resistance surveillance results for linezolid (LEADER Program for 2007). Diagn Microbiol Infect Dis 62:416–426. 10.1016/j.diagmicrobio.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 76.Farrell DJ, Mendes RE, Ross JE, Jones RN. 2009. Linezolid surveillance program results for 2008 (LEADER Program for 2008). Diagn Microbiol Infect Dis 65:392–403. 10.1016/j.diagmicrobio.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 77.Mendes RE, Flamm RK, Hogan PA, Ross JE, Jones RN. 2014. Summary of linezolid activity and resistance mechanisms detected during the 2012 LEADER surveillance program for the United States. Antimicrob Agents Chemother 58:1243–1247. 10.1128/AAC.02112-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flamm RK, Mendes RE, Hogan PA, Streit JM, Ross JE, Jones RN. 2016. Linezolid surveillance results for the United States (LEADER surveillance program 2014). Antimicrob Agents Chemother 60:2273–2280. 10.1128/AAC.02803-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pfaller MA, Mendes RE, Streit JM, Hogan PA, Flamm RK. 2017. Five-year summary of in vitro activity and resistance mechanisms of linezolid against clinically important Gram-positive cocci in the United States from the LEADER surveillance program (2011 to 2015). Antimicrob Agents Chemother 61:e00609-17. 10.1128/AAC.00609-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mendes RE, Deshpande LM, Jones RN. 2014. Linezolid update: stable in vitro activity following more than a decade of clinical use and summary of associated resistance mechanisms. Drug Resist Updat 17:1–12. 10.1016/j.drup.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 81.Shen J, Wang Y, Schwarz S. 2013. Presence and dissemination of the multiresistance gene cfr in Gram-positive and Gram-negative bacteria. J Antimicrob Chemother 68:1697–1706. 10.1093/jac/dkt092. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Li D, Song L, Liu Y, He T, Liu H, Wu C, Schwarz S, Shen J. 2013. First report of the multiresistance gene cfr in Streptococcus suis. Antimicrob Agents Chemother 57:4061–4063. 10.1128/AAC.00713-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Y, Lei C, Zuo L, Kong L, Kang Z, Zeng J, Zhang X, Wang H. 2019. A novel cfr-carrying Tn7 transposon derivative characterized in Morganella morganii of swine origin in China. J Antimicrob Chemother 74:603–606. 10.1093/jac/dky494. [DOI] [PubMed] [Google Scholar]
- 84.Chen H, Deng H, Cheng L, Liu R, Fu G, Shi S, Wan C, Fu Q, Huang Y, Huang X. 2020. First report of the multiresistance gene cfr in Pasteurella multocida strains of avian origin from China. J Glob Antimicrob Resist 23:251–255. 10.1016/j.jgar.2020.09.018. [DOI] [PubMed] [Google Scholar]
- 85.Madhaiyan M, Wirth JS, Saravanan VS. 2020. Phylogenomic analyses of the Staphylococcaceae family suggest the reclassification of five species within the genus Staphylococcus as heterotypic synonyms, the promotion of five subspecies to novel species, the taxonomic reassignment of five Staphylococcus species to Mammaliicoccus gen. nov., and the formal assignment of Nosocomiicoccus to the family Staphylococcaceae. Int J Syst Evol Microbiol 70:5926–5936. 10.1099/ijsem.0.004498. [DOI] [PubMed] [Google Scholar]
- 86.Cai JC, Hu YY, Zhang R, Zhou HW, Chen G-X. 2012. Linezolid-resistant clinical isolates of meticillin-resistant coagulase-negative staphylococci and Enterococcus faecium from China. J Med Microbiol 61:1568–1573. 10.1099/jmm.0.043729-0. [DOI] [PubMed] [Google Scholar]
- 87.Zhou W, Niu D, Cao X, Ning M, Zhang Z, Shen H, Zhang K. 2015. Clonal dissemination of linezolid-resistant Staphylococcus capitis with G2603T mutation in domain V of the 23S rRNA and the cfr gene at a tertiary care hospital in China. BMC Infect Dis 15:97. 10.1186/s12879-015-0841-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Song Y, Lv Y, Cui L, Li Y, Ke Q, Zhao Y. 2017. cfr-mediated linezolid-resistant clinical isolates of methicillin-resistant coagulase-negative staphylococci from China. J Glob Antimicrob Resist 8:1–5. 10.1016/j.jgar.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 89.Campanile F, Mongelli G, Bongiorno D, Adembri C, Ballardini M, Falcone M, Menichetti F, Repetto A, Sabia C, Sartor A, Scarparo C, Tascini C, Venditti M, Zoppi F, Stefani S. 2013. Worrisome Trend of New Multiple Mechanisms of Linezolid Resistance in Staphylococcal Clones Diffused in Italy. J Clin Microbiol 51:1256–1259. 10.1128/JCM.00098-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Couto N, Belas A, Rodrigues C, Schwarz S, Pomba C. 2016. Acquisition of the fexA and cfr genes in Staphylococcus pseudintermedius during florfenicol treatment of canine pyoderma. J Glob Antimicrob Resist 7:126–127. 10.1016/j.jgar.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y, He T, Schwarz S, Zhao Q, Shen Z, Wu C, Shen J. 2013. Multidrug resistance gene cfr in methicillin-resistant coagulase-negative staphylococci from chickens, ducks, and pigs in China. Int J Med Microbiol 303:84–87. 10.1016/j.ijmm.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 92.He T, Wang Y, Schwarz S, Zhao Q, Shen J, Wu C. 2014. Genetic environment of the multi-resistance gene cfr in methicillin-resistant coagulase-negative staphylococci from chickens, ducks, and pigs in China. Int J Med Microbiol 304:257–261. 10.1016/j.ijmm.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 93.Kehrenberg C, Aarestrup FM, Schwarz S. 2007. IS21-558 Insertion sequences are involved in the mobility of the multiresistance gene cfr. Antimicrob Agents Chemother 51:483–487. 10.1128/AAC.01340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cuny C, Arnold P, Hermes J, Eckmanns T, Mehraj J, Schoenfelder S, Ziebuhr W, Zhao Q, Wang Y, Feßler AT, Krause G, Schwarz S, Witte W. 2017. Occurrence of cfr-mediated multiresistance in staphylococci from veal calves and pigs, from humans at the corresponding farms, and from veterinarians and their family members. Vet Microbiol 200:88–94. 10.1016/j.vetmic.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 95.Gales AC, Deshpande LM, de Souza AG, Pignatari ACC, Mendes RE. 2015. MSSA ST398/t034 carrying a plasmid-mediated Cfr and Erm(B) in Brazil. J Antimicrob Chemother 70:303–305. 10.1093/jac/dku366. [DOI] [PubMed] [Google Scholar]
- 96.Wu D, Wang H, Zhu F, Jiang S, Sun L, Zhao F, Yu Y, Chen Y. 2020. Characterization of an ST5-SCCmec II-t311 methicillin-resistant Staphylococcus aureus strain with a widespread cfr-positive plasmid. J Infect Chemother 26:699–705. 10.1016/j.jiac.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 97.Jian J, Chen L, Xie Z, Zhang M. 2018. Dissemination of cfr-mediated linezolid resistance among Staphylococcus species isolated from a teaching hospital in Beijing, China. J Int Med Res 46:3884–3889. 10.1177/0300060518781636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li S-M, Zhou Y-F, Li L, Fang L-X, Duan J-H, Liu F-R, Liang H-Q, Wu Y-T, Gu W-Q, Liao X-P, Sun J, Xiong Y-Q, Liu Y-H. 2018. Characterization of the multi-drug resistance gene cfr in methicillin-resistant Staphylococcus aureus (MRSA) strains isolated from animals and humans in China. Front Microbiol 9:2925. 10.3389/fmicb.2018.02925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arias CA, Vallejo M, Reyes J, Panesso D, Moreno J, Castañeda E, Villegas MV, Murray BE, Quinn JP. 2008. Clinical and microbiological aspects of linezolid resistance mediated by the cfr gene encoding a 23S rRNA methyltransferase. J Clin Microbiol 46:892–896. 10.1128/JCM.01886-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Locke JB, Rahawi S, LaMarre J, Mankin AS, Shaw KJ. 2012. Genetic environment and stability of cfr in methicillin-resistant Staphylococcus aureus CM05. Antimicrob Agents Chemother 56:332–340. 10.1128/AAC.05420-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shore AC, Brennan OM, Ehricht R, Monecke S, Schwarz S, Slickers P, Coleman DC. 2010. Identification and characterization of the multidrug resistance gene cfr in a Panton-Valentine leukocidin-positive sequence type 8 methicillin-resistant Staphylococcus aureus IVa (USA300) isolate. Antimicrob Agents Chemother 54:4978–4984. 10.1128/AAC.01113-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shore AC, Lazaris A, Kinnevey PM, Brennan OM, Brennan GI, Connell B, Feßler AT, Schwarz S, Coleman DC. 2016. First report of cfr-carrying plasmids in the pandemic sequence type 22 methicillin-resistant Staphylococcus aureus staphylococcal cassette chromosome mec type IV clone. Antimicrob Agents Chemother 60:3007–3015. 10.1128/AAC.02949-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Azhar A, Rasool S, Haque A, Shan S, Saeed M, Ehsan B, Haque A. 2017. Detection of high levels of resistance to linezolid and vancomycin in Staphylococcus aureus. J Med Microbiol 66:1328–1331. 10.1099/jmm.0.000566. [DOI] [PubMed] [Google Scholar]
- 104.de Dios Caballero J, Pastor MD, Vindel A, Máiz L, Yagüe G, Salvador C, Cobo M, Morosini M-I, del Campo R, Cantón R, the GEIFQ Study Group . 2015. Emergence of cfr-mediated linezolid resistance in a methicillin-resistant Staphylococcus aureus epidemic clone isolated from patients with cystic fibrosis. Antimicrob Agents Chemother 60:1878–1882. 10.1128/AAC.02067-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sierra JM, Camoez M, Tubau F, Gasch O, Pujol M, Martin R, Domínguez MA. 2013. Low prevalence of cfr-mediated linezolid resistance among methicillin-resistant Staphylococcus aureus in a Spanish hospital: case report on linezolid resistance acquired during linezolid therapy. PLoS One 8:e59215. 10.1371/journal.pone.0059215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ruiz de Gopegui E, Juan C, Zamorano L, Pérez JL, Oliver A. 2012. Transferable multidrug resistance plasmid carrying cfr associated with tet(L), ant(4′)-Ia, and dfrK genes from a clinical methicillin-resistant Staphylococcus aureus ST125 strain. Antimicrob Agents Chemother 56:2139–2142. 10.1128/AAC.06042-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sánchez García M, De la Torre MÁ, Morales G, Peláez B, Tolón MJ, Domingo S, Candel FJ, Andrade R, Arribi A, García N, Martínez Sagasti F, Fereres J, Picazo J. 2010. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA 303:2260–2264. 10.1001/jama.2010.757. [DOI] [PubMed] [Google Scholar]
- 108.Morales G, Picazo JJ, Baos E, Candel FJ, Arribi A, Peláez B, Andrade R, de la Torre M-Á, Fereres J, Sánchez-García M. 2010. Resistance to linezolid is mediated by the cfr gene in the first report of an outbreak of linezolid-resistant Staphylococcus aureus. Clin Infect Dis 50:821–825. 10.1086/650574. [DOI] [PubMed] [Google Scholar]
- 109.Ruiz-Ripa L, Feßler AT, Hanke D, Eichhorn I, Azcona-Gutiérrez JM, Alonso CA, Pérez-Moreno MO, Aspiroz C, Bellés A, Schwarz S, Torres C. 2020. Mechanisms of linezolid resistance among clinical Staphylococcus spp. in Spain: spread of methicillin- and linezolid-resistant S. epidermidis ST2. Microb Drug Resist 27:145–153. 10.1089/mdr.2020.0122. [DOI] [PubMed] [Google Scholar]
- 110.Ruiz-Ripa L, Bellés A, García M, Torres C. 2020. Detection of a cfr-positive MRSA CC398 strain in a pig farmer in Spain. Enferm Infecc Microbiol Clin 39:139–141. 10.1016/j.eimc.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 111.Feßler AT, Calvo N, Gutiérrez N, Muñoz Bellido JL, Fajardo M, Garduño E, Monecke S, Ehricht R, Kadlec K, Schwarz S. 2014. Cfr-mediated linezolid resistance in methicillin-resistant Staphylococcus aureus and Staphylococcus haemolyticus associated with clinical infections in humans: two case reports. J Antimicrob Chemother 69:268–270. 10.1093/jac/dkt331. [DOI] [PubMed] [Google Scholar]
- 112.Senok A, Nassar R, Celiloglu H, Nabi A, Alfaresi M, Weber S, Rizvi I, Müller E, Reissig A, Gawlik D, Monecke S, Ehricht R. 2020. Genotyping of methicillin resistant Staphylococcus aureus from the United Arab Emirates. Sci Rep 10:18551. 10.1038/s41598-020-75565-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mendes RE, Deshpande LM, Castanheira M, DiPersio J, Saubolle MA, Jones RN. 2008. First report of cfr-mediated resistance to linezolid in human staphylococcal clinical isolates recovered in the United States. Antimicrob Agents Chemother 52:2244–2246. 10.1128/AAC.00231-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sahm DF, Deane J, Bien PA, Locke JB, Zuill DE, Shaw KJ, Bartizal KF. 2015. Results of the surveillance of tedizolid activity and resistance program: in vitro susceptibility of Gram-positive pathogens collected in 2011 and 2012 from the United States and Europe. Diagn Microbiol Infect Dis 81:112–118. 10.1016/j.diagmicrobio.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 115.Locke JB, Zuill DE, Scharn CR, Deane J, Sahm DF, Denys GA, Goering RV, Shaw KJ. 2014. Linezolid-resistant Staphylococcus aureus strain 1128105, the first known clinical isolate possessing the cfr multidrug resistance gene. Antimicrob Agents Chemother 58:6592–6598. 10.1128/AAC.03493-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Locke JB, Zuill DE, Scharn CR, Deane J, Sahm DF, Goering RV, Jenkins SG, Shaw KJ. 2014. Identification and characterization of linezolid-resistant cfr-positive Staphylococcus aureus USA300 isolates from a New York City Medical Center. Antimicrob Agents Chemother 58:6949–6952. 10.1128/AAC.03380-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kehrenberg C, Schwarz S. 2006. Distribution of Florfenicol Resistance Genes fexA and cfr among Chloramphenicol-Resistant Staphylococcus Isolates. Antimicrob Agents Chemother 50:1156–1163. 10.1128/AAC.50.4.1156-1163.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Osman KM, Amer AM, Badr JM, Helmy NM, Elhelw RA, Orabi A, Bakry M, Saad ASA. 2016. Antimicrobial resistance, biofilm formation and mecA characterization of methicillin-susceptible S. aureus and non-S. aureus of beef meat origin in Egypt. Front Microbiol 7:222. 10.3389/fmicb.2016.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peeters LEJ, Argudín MA, Azadikhah S, Butaye P. 2015. Antimicrobial resistance and population structure of Staphylococcus aureus recovered from pigs farms. Vet Microbiol 180:151–156. 10.1016/j.vetmic.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 120.Li D, Wu C, Wang Y, Fan R, Schwarz S, Zhang S. 2015. Identification of multiresistance gene cfr in methicillin-resistant Staphylococcus aureus from pigs: plasmid location and integration into a staphylococcal cassette chromosome mec complex. Antimicrob Agents Chemother 59:3641–3644. 10.1128/AAC.00500-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guo D, Liu Y, Han C, Chen Z, Ye X. 2018. Phenotypic and molecular characteristics of methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolated from pigs: implication for livestock-association markers and vaccine strategies. Infect Drug Resist 11:1299–1307. 10.2147/IDR.S173624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Y, Zhang W, Wang J, Wu C, Shen Z, Fu X, Yan Y, Zhang Q, Schwarz S, Shen J. 2012. Distribution of the multidrug resistance gene cfr in Staphylococcus species isolates from swine farms in China. Antimicrob Agents Chemother 56:1485–1490. 10.1128/AAC.05827-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Moon DC, Tamang MD, Nam H-M, Jeong J-H, Jang G-C, Jung S-C, Park Y-H, Lim S-K. 2015. Identification of livestock-associated methicillin-resistant Staphylococcus aureus isolates in Korea and molecular comparison between isolates from animal carcasses and slaughterhouse workers. Foodborne Pathog Dis 12:327–334. 10.1089/fpd.2014.1868. [DOI] [PubMed] [Google Scholar]
- 124.Kang HY, Moon DC, Mechesso AF, Choi J-H, Kim S-J, Song H-J, Kim MH, Yoon S-S, Lim S-K. 2020. Emergence of cfr-mediated linezolid resistance in Staphylococcus aureus isolated from pig carcasses. Antibiotics 9:769. 10.3390/antibiotics9110769. [DOI] [Google Scholar]
- 125.Martínez-Meléndez A, Morfín-Otero R, Villarreal-Treviño L, Camacho-Ortíz A, González-González G, Llaca-Díaz J, Rodríguez-Noriega E, Garza-González E. 2016. Molecular epidemiology of coagulase-negative bloodstream isolates: detection of Staphylococcus epidermidis ST2, ST7 and linezolid-resistant ST23. Braz J Infect Dis 20:419–428. 10.1016/j.bjid.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu C, Chen C, Ye Y, Li X, Sun J, Xu L, Ming L. 2020. The emergence of Staphylococcus epidermidis simultaneously nonsusceptible to linezolid and teicoplanin in China. Diagn Microbiol Infect Dis 96:114956. 10.1016/j.diagmicrobio.2019.114956. [DOI] [PubMed] [Google Scholar]
- 127.Cui L, Wang Y, Li Y, He T, Schwarz S, Ding Y, Shen J, Lv Y. 2013. Cfr-mediated linezolid-resistance among methicillin-resistant coagulase-negative staphylococci from infections of humans. PLoS One 8:e57096. 10.1371/journal.pone.0057096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dortet L, Glaser P, Kassis-Chikhani N, Girlich D, Ichai P, Boudon M, Samuel D, Creton E, Imanci D, Bonnin R, Fortineau N, Naas T. 2018. Long-lasting successful dissemination of resistance to oxazolidinones in MDR Staphylococcus epidermidis clinical isolates in a tertiary care hospital in France. J Antimicrob Chemother 73:41–51. 10.1093/jac/dkx370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Decousser J-W, Desroches M, Bourgeois-Nicolaos N, Potier J, Jehl F, Lina G, Cattoir V, Vandenesh F, Doucet-Populaire F, Microbs Study Group . 2015. Susceptibility trends including emergence of linezolid resistance among coagulase-negative staphylococci and meticillin-resistant Staphylococcus aureus from invasive infections. Int J Antimicrob Agents 46:622–630. 10.1016/j.ijantimicag.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 130.Bender J, Strommenger B, Steglich M, Zimmermann O, Fenner I, Lensing C, Dagwadordsch U, Kekulé AS, Werner G, Layer F. 2015. Linezolid resistance in clinical isolates of Staphylococcus epidermidis from German hospitals and characterization of two cfr-carrying plasmids. J Antimicrob Chemother 70:1630–1638. 10.1093/jac/dkv025. [DOI] [PubMed] [Google Scholar]
- 131.Weßels C, Strommenger B, Klare I, Bender J, Messler S, Mattner F, Krakau M, Werner G, Layer F. 2018. Emergence and control of linezolid-resistant Staphylococcus epidermidis in an ICU of a German hospital. J Antimicrob Chemother 73:1185–1193. 10.1093/jac/dky010. [DOI] [PubMed] [Google Scholar]
- 132.Lazaris A, Coleman DC, Kearns AM, Pichon B, Kinnevey PM, Earls MR, Boyle B, O’Connell B, Brennan GI, Shore AC. 2017. Novel multiresistance cfr plasmids in linezolid-resistant methicillin-resistant Staphylococcus epidermidis and vancomycin-resistant Enterococcus faecium (VRE) from a hospital outbreak: co-location of cfr and optrA in VRE. J Antimicrob Chemother 72:3252–3257. 10.1093/jac/dkx292. [DOI] [PubMed] [Google Scholar]
- 133.Gabriel EM, Fitzgibbon S, Clair J, Coffey A, O’Mahony JM. 2015. Characterisation of clinical meticillin-resistant Staphylococcus epidermidis demonstrating high levels of linezolid resistance (>256 μg/ml) resulting from transmissible and mutational mechanisms. J Infect Chemother 21:547–549. 10.1016/j.jiac.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 134.Mendes RE, Deshpande LM, Farrell DJ, Spanu T, Fadda G, Jones RN. 2010. Assessment of linezolid resistance mechanisms among Staphylococcus epidermidis causing bacteraemia in Rome, Italy. J Antimicrob Chemother 65:2329–2335. 10.1093/jac/dkq331. [DOI] [PubMed] [Google Scholar]
- 135.Brenciani A, Morroni G, Pollini S, Tiberi E, Mingoia M, Varaldo PE, Rossolini GM, Giovanetti E. 2016. Characterization of novel conjugative multiresistance plasmids carrying cfr from linezolid-resistant Staphylococcus epidermidis clinical isolates from Italy. J Antimicrob Chemother 71:307–313. 10.1093/jac/dkv341. [DOI] [PubMed] [Google Scholar]
- 136.Russo A, Campanile F, Falcone M, Tascini C, Bassetti M, Goldoni P, Trancassini M, Della Siega P, Menichetti F, Stefani S, Venditti M. 2015. Linezolid-resistant staphylococcal bacteraemia: a multicentre case–case–control study in Italy. Int J Antimicrob Agents 45:255–261. 10.1016/j.ijantimicag.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 137.Brenciani A, Morroni G, Mingoia M, Varaldo PE, Giovanetti E. 2016. Stability of the cargo regions of the cfr-carrying, multiresistance plasmid pSP01 from Staphylococcus epidermidis. Int J Med Microbiol 306:717–721. 10.1016/j.ijmm.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 138.Kosecka-Strojek M, Sadowy E, Gawryszewska I, Klepacka J, Tomasik T, Michalik M, Hryniewicz W, Miedzobrodzki J. 2020. Emergence of linezolid-resistant Staphylococcus epidermidis in the tertiary children’s hospital in Cracow, Poland. Eur J Clin Microbiol Infect Dis 39:1717–1725. 10.1007/s10096-020-03893-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lozano C, Ruiz-García M, Gómez-Sanz E, López-García P, Royo-García G, Zarazaga M, Torres C. 2012. Characterization of a cfr-positive methicillin-resistant Staphylococcus epidermidis strain of the lineage ST22 implicated in a life-threatening human infection. Diagn Microbiol Infect Dis 73:380–382. 10.1016/j.diagmicrobio.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 140.Rodríguez-Lucas C, Rodicio MR, Càmara J, Domínguez MÁ, Alaguero M, Fernández J. 2020. Long-term endemic situation caused by a linezolid- and meticillin-resistant clone of Staphylococcus epidermidis in a tertiary hospital. J Hosp Infect 105:64–69. 10.1016/j.jhin.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 141.Cafini F, Nguyen LTT, Higashide M, Román F, Prieto J, Morikawa K. 2016. Horizontal gene transmission of the cfr gene to MRSA and Enterococcus: role of Staphylococcus epidermidis as a reservoir and alternative pathway for the spread of linezolid resistance. J Antimicrob Chemother 71:587–592. 10.1093/jac/dkv391. [DOI] [PubMed] [Google Scholar]
- 142.Baos E, Candel FJ, Merino P, Pena I, Picazo JJ. 2013. Characterization and monitoring of linezolid-resistant clinical isolates of Staphylococcus epidermidis in an intensive care unit 4 years after an outbreak of infection by cfr-mediated linezolid-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis 76:325–329. 10.1016/j.diagmicrobio.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 143.Li X, Arias CA, Aitken SL, Galloway Peña J, Panesso D, Chang M, Diaz L, Rios R, Numan Y, Ghaoui S, DebRoy S, Bhatti MM, Simmons DE, Raad I, Hachem R, Folan SA, Sahasarabhojane P, Kalia A, Shelburne SA. 2018. Clonal emergence of invasive multidrug-resistant Staphylococcus epidermidis deconvoluted via a combination of whole-genome sequencing and microbiome analyses. Clin Infect Dis 67:398–406. 10.1093/cid/ciy089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bonilla H, Huband MD, Seidel J, Schmidt H, Lescoe M, McCurdy SP, Lemmon MM, Brennan LA, Tait-Kamradt A, Puzniak L, Quinn JP. 2010. Multicity outbreak of linezolid-resistant Staphylococcus epidermidis Associated with clonal spread of a cfr-containing strain. Clin Infect Dis 51:796–800. 10.1086/656281. [DOI] [PubMed] [Google Scholar]
- 145.LaMarre J, Mendes RE, Szal T, Schwarz S, Jones RN, Mankin AS. 2013. The genetic environment of the cfr gene and the presence of other mechanisms account for the very high linezolid resistance of Staphylococcus epidermidis isolate 426-3147L. Antimicrob Agents Chemother 57:1173–1179. 10.1128/AAC.02047-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Doern CD, Park JY, Gallegos M, Alspaugh D, Burnham C-AD. 2016. Investigation of linezolid resistance in staphylococci and enterococci. J Clin Microbiol 54:1289–1294. 10.1128/JCM.01929-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu C, Zhang X, Liang J, Li Q, Lin H, Lin C, Liu H, Zhou D, Lu W, Sun Z, Lin X, Zhang H, Li K, Xu T, Bao Q, Lu J. 2021. Characterization of florfenicol resistance genes in the coagulase-negative Staphylococcus (CoNS) isolates and genomic features of a multidrug-resistant Staphylococcus lentus strain H29. Antimicrob Resist Infect Control 10:9. 10.1186/s13756-020-00869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Maarouf L, Omar H, El-Nakeeb M, Abouelfetouh A. 2020. Prevalence and mechanisms of linezolid resistance among staphylococcal clinical isolates from Egypt. Eur J Clin Microbiol Infect Dis 40:815–823. 10.1007/s10096-020-04045-w. [DOI] [PubMed] [Google Scholar]
- 149.Manoharan M, Sistla S, Ray P. 2020. Prevalence and molecular determinants of antimicrobial resistance in clinical isolates of Staphylococcus haemolyticus from India. Microb Drug Resist 10.1089/mdr.2019.0395. [DOI] [PubMed] [Google Scholar]
- 150.Bakthavatchalam YD, Vasudevan K, Neeravi A, Perumal R, Veeraraghavan B. 2020. First draft genome sequence of linezolid and rifampicin resistant Staphylococcus haemolyticus. Jpn J Infect Dis 73:296–299. 10.7883/yoken.JJID.2019.081. [DOI] [PubMed] [Google Scholar]
- 151.Mittal G, Bhandari V, Gaind R, Rani V, Chopra S, Dawar R, Sardana R, Verma PK. 2019. Linezolid resistant coagulase negative staphylococci (LRCoNS) with novel mutations causing blood stream infections (BSI) in India. BMC Infect Dis 19:717. 10.1186/s12879-019-4368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rajan V, Kumar VGS, Gopal S. 2014. A cfr-positive clinical staphylococcal isolate from India with multiple mechanisms of linezolid-resistance. Indian J Med Res 139:463–467. [PMC free article] [PubMed] [Google Scholar]
- 153.Sun C, Zhang P, Ji X, Fan R, Chen B, Wang Y, Schwarz S, Wu C. 2018. Presence and molecular characteristics of oxazolidinone resistance in staphylococci from household animals in rural China. J Antimicrob Chemother 73:1194–1200. 10.1093/jac/dky009. [DOI] [PubMed] [Google Scholar]
- 154.Moawad AA, Hotzel H, Awad O, Roesler U, Hafez HM, Tomaso H, Neubauer H, El-Adawy H. 2019. Evolution of antibiotic resistance of coagulase-negative staphylococci isolated from healthy turkeys in Egypt: first report of linezolid resistance. Microorganisms 7:476. 10.3390/microorganisms7100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen H, Wu W, Ni M, Liu Y, Zhang J, Xia F, He W, Wang Q, Wang Z, Cao B, Wang H. 2013. Linezolid-resistant clinical isolates of enterococci and Staphylococcus cohnii from a multicentre study in China: molecular epidemiology and resistance mechanisms. Int J Antimicrob Agents 42:317–321. 10.1016/j.ijantimicag.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 156.Zeng Z-L, Wei H-K, Wang J, Lin D-C, Liu X-Q, Liu J-H. 2014. High prevalence of Cfr-producing Staphylococcus species in retail meat in Guangzhou, China. BMC Microbiol 14:151. 10.1186/1471-2180-14-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ruiz-Ripa L, Feßler AT, Hanke D, Sanz S, Olarte C, Mama OM, Eichhorn I, Schwarz S, Torres C. 2020. Coagulase-negative staphylococci carrying cfr and PVL genes, and MRSA/MSSA-CC398 in the swine farm environment. Vet Microbiol 243:108631. 10.1016/j.vetmic.2020.108631. [DOI] [PubMed] [Google Scholar]
- 158.Liu B-H, Lei C-W, Zhang A-Y, Pan Y, Kong L-H, Xiang R, Wang Y-X, Yang Y-X, Wang H-N. 2017. Colocation of the multiresistance gene cfr and the fosfomycin resistance gene fosD on a novel plasmid in Staphylococcus arlettae from a chicken farm. Antimicrob Agents Chemother 61:e01388-17. 10.1128/AAC.01388-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Argudín MA, Vanderhaeghen W, Butaye P. 2015. Diversity of antimicrobial resistance and virulence genes in methicillin-resistant non-Staphylococcus aureus staphylococci from veal calves. Res Vet Sci 99:10–16. 10.1016/j.rvsc.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 160.Vanderhaeghen W, Vandendriessche S, Crombé F, Nemeghaire S, Dispas M, Denis O, Hermans K, Haesebrouck F, Butaye P. 2013. Characterization of methicillin-resistant non-Staphylococcus aureus staphylococci carriage isolates from different bovine populations. J Antimicrob Chemother 68:300–307. 10.1093/jac/dks403. [DOI] [PubMed] [Google Scholar]
- 161.Schoenfelder SMK, Dong Y, Feßler AT, Schwarz S, Schoen C, Köck R, Ziebuhr W. 2017. Antibiotic resistance profiles of coagulase-negative staphylococci in livestock environments. Vet Microbiol 200:79–87. 10.1016/j.vetmic.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 162.Li D, Wang Y, Schwarz S, Cai J, Fan R, Li J, Feßler AT, Zhang R, Wu C, Shen J. 2016. Co-location of the oxazolidinone resistance genes optrA and cfr on a multiresistance plasmid from Staphylococcus sciuri. J Antimicrob Chemother 71:1474–1478. 10.1093/jac/dkw040. [DOI] [PubMed] [Google Scholar]
- 163.Fan R, Li D, Feßler AT, Wu C, Schwarz S, Wang Y. 2017. Distribution of optrA and cfr in florfenicol-resistant Staphylococcus sciuri of pig origin. Vet Microbiol 210:43–48. 10.1016/j.vetmic.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 164.Wu Y, Fan R, Wang Y, Lei L, Feßler AT, Wang Z, Wu C, Schwarz S, Wang Y. 2019. Analysis of combined resistance to oxazolidinones and phenicols among bacteria from dogs fed with raw meat/vegetables and the respective food items. Sci Rep 9:15500. 10.1038/s41598-019-51918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen Q, Yin D, Li P, Guo Y, Ming D, Lin Y, Yan X, Zhang Z, Hu F. 2020. First report Cfr and OptrA co-harboring linezolid-resistant Enterococcus faecalis in China. Infect Drug Resist 13:3919–3922. 10.2147/IDR.S270701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Diaz L, Kiratisin P, Mendes RE, Panesso D, Singh KV, Arias CA. 2012. Transferable plasmid-mediated resistance to linezolid due to cfr in a human clinical isolate of Enterococcus faecalis. Antimicrob Agents Chemother 56:3917–3922. 10.1128/AAC.00419-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Deshpande LM, Castanheira M, Flamm RK, Mendes RE. 2018. Evolving oxazolidinone resistance mechanisms in a worldwide collection of enterococcal clinical isolates: results from the SENTRY Antimicrobial Surveillance Program. J Antimicrob Chemother 73:2314–2322. 10.1093/jac/dky188. [DOI] [PubMed] [Google Scholar]
- 168.Filsner PHLN, de Almeida LM, Moreno M, Moreno LZ, Matajira CEC, Silva KC, Pires C, Cerdeira LT, Sacramento AG, Mamizuka EM, Lincopan N, Moreno AM. 2017. Identification of the cfr methyltransferase gene in Enterococcus faecalis isolated from swine: first report in Brazil. J Glob Antimicrob Resist 8:192–193. 10.1016/j.jgar.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 169.Almeida LM, Gaca A, Bispo PM, Lebreton F, Saavedra JT, Silva RA, Basílio-Júnior ID, Zorzi FM, Filsner PH, Moreno AM, Gilmore MS. 2020. Coexistence of the oxazolidinone resistance–associated genes cfr and optrA in Enterococcus faecalis from a healthy piglet in Brazil. Front Public Health 8:518. 10.3389/fpubh.2020.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu Y, Wang Y, Wu C, Shen Z, Schwarz S, Du X-D, Dai L, Zhang W, Zhang Q, Shen J. 2012. First report of the multidrug resistance gene cfr in Enterococcus faecalis of animal origin. Antimicrob Agents Chemother 56:1650–1654. 10.1128/AAC.06091-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu Y, Wang Y, Schwarz S, Wang S, Chen L, Wu C, Shen J. 2014. Investigation of a multiresistance gene cfr that fails to mediate resistance to phenicols and oxazolidinones in Enterococcus faecalis. J Antimicrob Chemother 69:892–898. 10.1093/jac/dkt459. [DOI] [PubMed] [Google Scholar]
- 172.Fang L-X, Duan J-H, Chen M-Y, Deng H, Liang H-Q, Xiong YQ, Sun J, Liu Y-H, Liao X-P. 2018. Prevalence of cfr in Enterococcus faecalis strains isolated from swine farms in China: predominated cfr-carrying pCPPF5-like plasmids conferring “non-linezolid resistance” phenotype. Infect Genet Evol 62:188–192. 10.1016/j.meegid.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 173.Chen L, Han D, Tang Z, Hao J, Xiong W, Zeng Z. 2020. Co-existence of the oxazolidinone resistance genes cfr and optrA on two transferable multi-resistance plasmids in one Enterococcus faecalis isolate from swine. Int J Antimicrob Agents 56:105993. 10.1016/j.ijantimicag.2020.105993. [DOI] [PubMed] [Google Scholar]
- 174.Fioriti S, Morroni G, Coccitto SN, Brenciani A, Antonelli A, Di Pilato V, Baccani I, Pollini S, Cucco L, Morelli A, Paniccià M, Magistrali CF, Rossolini GM, Giovanetti E. 2020. Detection of oxazolidinone resistance genes and characterization of genetic environments in enterococci of swine origin, Italy. Microorganisms 8:2021. 10.3390/microorganisms8122021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu Y, Wang Y, Schwarz S, Li Y, Shen Z, Zhang Q, Wu C, Shen J. 2013. Transferable multiresistance plasmids carrying cfr in Enterococcus spp. from swine and farm environment. Antimicrob Agents Chemother 57:42–48. 10.1128/AAC.01605-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Patel SN, Memari N, Shahinas D, Toye B, Jamieson FB, Farrell DJ. 2013. Linezolid resistance in Enterococcus faecium isolated in Ontario, Canada. Diagn Microbiol Infect Dis 77:350–353. 10.1016/j.diagmicrobio.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 177.Xu Z, Wei Y, Wang Y, Xu G, Cheng H, Chen J, Yu Z, Chen Z, Zheng J. 2020. In vitro activity of radezolid against Enterococcus faecium and compared with linezolid. J Antibiot (Tokyo) 73:845–851. 10.1038/s41429-020-0345-y. [DOI] [PubMed] [Google Scholar]
- 178.Klare I, Fleige C, Geringer U, Thürmer A, Bender J, Mutters NT, Mischnik A, Werner G. 2015. Increased frequency of linezolid resistance among clinical Enterococcus faecium isolates from German hospital patients. J Glob Antimicrob Resist 3:128–131. 10.1016/j.jgar.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 179.Brenciani A, Morroni G, Vincenzi C, Manso E, Mingoia M, Giovanetti E, Varaldo PE. 2016. Detection in Italy of two clinical Enterococcus faecium isolates carrying both the oxazolidinone and phenicol resistance gene optrA and a silent multiresistance gene cfr. J Antimicrob Chemother 71:1118–1119. 10.1093/jac/dkv438. [DOI] [PubMed] [Google Scholar]
- 180.Morroni G, Brenciani A, Antonelli A, D’Andrea MM, Di Pilato V, Fioriti S, Mingoia M, Vignaroli C, Cirioni O, Biavasco F, Varaldo PE, Rossolini GM, Giovanetti E. 2018. Characterization of a multiresistance plasmid carrying the optrA and cfr resistance genes from an Enterococcus faecium clinical isolate. Front Microbiol 9:2189. 10.3389/fmicb.2018.02189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Krawczyk B, Wysocka M, Kotłowski R, Bronk M, Michalik M, Samet A. 2020. Linezolid-resistant Enterococcus faecium strains isolated from one hospital in Poland—commensals or hospital-adapted pathogens? PLoS One 15:e0233504. 10.1371/journal.pone.0233504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Inkster T, Coia J, Meunier D, Doumith M, Martin K, Pike R, Imrie L, Kane H, Hay M, Wiuff C, Wilson J, Deighan C, Hopkins KL, Woodford N, Hill R. 2017. First outbreak of colonization by linezolid- and glycopeptide-resistant Enterococcus faecium harbouring the cfr gene in a UK nephrology unit. J Hosp Infect 97:397–402. 10.1016/j.jhin.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 183.Tyson GH, Sabo JL, Hoffmann M, Hsu C-H, Mukherjee S, Hernandez J, Tillman G, Wasilenko JL, Haro J, Simmons M, Wilson EW, White PL, Dessai U, McDermott PF. 2018. Novel linezolid resistance plasmids in Enterococcus from food animals in the USA. J Antimicrob Chemother 73:3254–3258. 10.1093/jac/dky369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu Y, Wang Y, Dai L, Wu C, Shen J. 2014. First report of multiresistance gene cfr in Enterococcus species casseliflavus and gallinarum of swine origin. Vet Microbiol 170:352–357. 10.1016/j.vetmic.2014.02.037. [DOI] [PubMed] [Google Scholar]
- 185.Huang J, Sun J, Wu Y, Chen L, Duan D, Lv X, Wang L. 2019. Identification and pathogenicity of an XDR Streptococcus suis isolate that harbours the phenicol-oxazolidinone resistance genes optrA and cfr, and the bacitracin resistance locus bcrABDR. Int J Antimicrob Agents 54:43–48. 10.1016/j.ijantimicag.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 186.Dai L, Wu C-M, Wang M-G, Wang Y, Wang Y, Huang S-Y, Xia L-N, Li B-B, Shen J-Z. 2010. First report of the multidrug resistance gene cfr and the phenicol resistance gene fexA in a Bacillus strain from swine feces. Antimicrob Agents Chemother 54:3953–3955. 10.1128/AAC.00169-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang W-J, Wu C-M, Wang Y, Shen Z-Q, Dai L, Han J, Foley SL, Shen J-Z, Zhang Q. 2011. The new genetic environment of cfr on plasmid pBS-02 in a Bacillus strain. J Antimicrob Chemother 66:1174–1175. 10.1093/jac/dkr037. [DOI] [PubMed] [Google Scholar]
- 188.Wang Y, Schwarz S, Shen Z, Zhang W, Qi J, Liu Y, He T, Shen J, Wu C. 2012. Co-location of the multiresistance gene cfr and the novel streptomycin resistance gene aadY on a small plasmid in a porcine Bacillus strain. J Antimicrob Chemother 67:1547–1549. 10.1093/jac/dks075. [DOI] [PubMed] [Google Scholar]
- 189.Wang Y, Wang Y, Schwarz S, Shen Z, Zhou N, Lin J, Wu C, Shen J. 2012. Detection of the staphylococcal multiresistance gene cfr in Macrococcus caseolyticus and Jeotgalicoccus pinnipedialis. J Antimicrob Chemother 67:1824–1827. 10.1093/jac/dks163. [DOI] [PubMed] [Google Scholar]
- 190.Wang Y, He T, Schwarz S, Zhou D, Shen Z, Wu C, Wang Y, Ma L, Zhang Q, Shen J. 2012. Detection of the staphylococcal multiresistance gene cfr in Escherichia coli of domestic-animal origin. J Antimicrob Chemother 67:1094–1098. 10.1093/jac/dks020. [DOI] [PubMed] [Google Scholar]
- 191.Zhang W-J, Xu X-R, Schwarz S, Wang X-M, Dai L, Zheng H-J, Liu S. 2014. Characterization of the IncA/C plasmid pSCEC2 from Escherichia coli of swine origin that harbours the multiresistance gene cfr. J Antimicrob Chemother 69:385–389. 10.1093/jac/dkt355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Deng H, Sun J, Ma J, Li L, Fang L-X, Zhang Q, Liu Y-H, Liao X-P. 2014. Identification of the multi-resistance gene cfr in Escherichia coli isolates of animal origin. PLoS One 9:e102378. 10.1371/journal.pone.0102378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang R, Sun B, Wang Y, Lei L, Schwarz S, Wu C. 2016. Characterization of a cfr-carrying plasmid from porcine Escherichia coli that closely resembles plasmid pEA3 from the plant pathogen Erwinia amylovora. Antimicrob Agents Chemother 60:658–661. 10.1128/AAC.02114-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang W-J, Wang X-M, Dai L, Hua X, Dong Z, Schwarz S, Liu S. 2015. Novel conjugative plasmid from Escherichia coli of swine origin that coharbors the multiresistance gene cfr and the extended-spectrum-β-lactamase gene blaCTX-M-14b. Antimicrob Agents Chemother 59:1337–1340. 10.1128/AAC.04631-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang H, Zhao X, Wang X, Chang W. 2017. Prevalence and antimicrobial resistance profiles of Escherichia coli isolated from free-range pigs. J Infect Dev Ctries 11:652–655. 10.3855/jidc.9269. [DOI] [PubMed] [Google Scholar]
- 196.Wang X, Zhu Y, Hua X, Chen F, Wang C, Zhang Y, Liu S, Zhang W. 2018. F14:A−:B− and IncX4 Inc group cfr-positive plasmids circulating in Escherichia coli of animal origin in Northeast China. Vet Microbiol 217:53–57. 10.1016/j.vetmic.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 197.Wang M, Chen SY, Zhang JX, He XX, Xiong WG, Sun YX. 2018. Variations of antibiotic resistance profiles in chickens during administration of amoxicillin, chlortetracycline and florfenicol. J Appl Microbiol 125:1692–1701. 10.1111/jam.14065. [DOI] [PubMed] [Google Scholar]
- 198.Wang Y, Wang Y, Wu C-M, Schwarz S, Shen Z, Zhang W, Zhang Q, Shen J-Z. 2011. Detection of the staphylococcal multiresistance gene cfr in Proteus vulgaris of food animal origin. J Antimicrob Chemother 66:2521–2526. 10.1093/jac/dkr322. [DOI] [PubMed] [Google Scholar]
- 199.Zhang Y, Lei C-W, Wang H-N. 2019. Identification of a novel conjugative plasmid carrying the multiresistance gene cfr in Proteus vulgaris isolated from swine origin in China. Plasmid 105:102440. 10.1016/j.plasmid.2019.102440. [DOI] [PubMed] [Google Scholar]
- 200.Kong L-H, Xiang R, Wang Y-L, Wu S-K, Lei C-W, Kang Z-Z, Chen Y-P, Ye X-L, Lai Y, Wang H-N. 2020. Integration of the blaNDM-1 carbapenemase gene into a novel SXT/R391 integrative and conjugative element in Proteus vulgaris. J Antimicrob Chemother 75:1439–1442. 10.1093/jac/dkaa068. [DOI] [PubMed] [Google Scholar]
- 201.Lei C-W, Chen Y-P, Kang Z-Z, Kong L-H, Wang H-N. 2018. Characterization of a novel SXT/R391 integrative and conjugative element carrying cfr, blaCTX-M-65, fosA3, and aac(6′)-Ib-cr in Proteus mirabilis. Antimicrob Agents Chemother 62:e00849-18. 10.1128/AAC.00849-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ying Y, Wu F, Wu C, Jiang Y, Yin M, Zhou W, Zhu X, Cheng C, Zhu L, Li K, Lu J, Xu T, Bao Q. 2019. Florfenicol resistance in Enterobacteriaceae and whole-genome sequence analysis of florfenicol-resistant Leclercia adecarboxylata strain R25. Int J Genomics 2019:9828504. 10.1155/2019/9828504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhu T, Liu S, Ying Y, Xu L, Liu Y, Jin J, Ying J, Lu J, Lin X, Li K, Xu T, Bao Q, Li P. 2020. Genomic and functional characterization of fecal sample strains of Proteus cibarius carrying two floR antibiotic resistance genes and a multiresistance plasmid-encoded cfr gene. Comp Immunol Microbiol Infect Dis 69:101427. 10.1016/j.cimid.2020.101427. [DOI] [PubMed] [Google Scholar]
- 204.Mendes RE, Deshpande LM, Bonilla HF, Schwarz S, Huband MD, Jones RN, Quinn JP. 2013. Dissemination of a pSCFS3-Like cfr-carrying plasmid in Staphylococcus aureus and Staphylococcus epidermidis clinical isolates recovered from hospitals in Ohio. Antimicrob Agents Chemother 57:2923–2928. 10.1128/AAC.00071-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Feßler AT, Zhao Q, Schoenfelder S, Kadlec K, Brenner Michael G, Wang Y, Ziebuhr W, Shen J, Schwarz S. 2017. Complete sequence of a plasmid from a bovine methicillin-resistant Staphylococcus aureus harbouring a novel ica-like gene cluster in addition to antimicrobial and heavy metal resistance genes. Vet Microbiol 200:95–100. 10.1016/j.vetmic.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 206.Kehrenberg C, Ojo KK, Schwarz S. 2004. Nucleotide sequence and organization of the multiresistance plasmid pSCFS1 from Staphylococcus sciuri. J Antimicrob Chemother 54:936–939. 10.1093/jac/dkh457. [DOI] [PubMed] [Google Scholar]
- 207.Schwarz S, Kehrenberg C, Ojo KK. 2002. Staphylococcus sciuri gene erm(33), encoding inducible resistance to macrolides, lincosamides, and streptogramin B antibiotics, is a product of recombination between erm(C) and erm(A). Antimicrob Agents Chemother 46:3621–3623. 10.1128/aac.46.11.3621-3623.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cai JC, Hu YY, Zhou HW, Chen G-X, Zhang R. 2015. Dissemination of the same cfr-carrying plasmid among methicillin-resistant Staphylococcus aureus and coagulase-negative staphylococcal isolates in China. Antimicrob Agents Chemother 59:3669–3671. 10.1128/AAC.04580-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Li D, Cheng Y, Schwarz S, Yang M, Du X-D. 2020. Identification of a poxtA- and cfr-carrying multiresistant Enterococcus hirae strain. J Antimicrob Chemother 75:482–484. 10.1093/jac/dkz449. [DOI] [PubMed] [Google Scholar]
- 210.Sun J, Deng H, Li L, Chen M-Y, Fang L-X, Yang Q-E, Liu Y-H, Liao X-P. 2015. Complete nucleotide sequence of cfr-carrying IncX4 plasmid pSD11 from Escherichia coli. Antimicrob Agents Chemother 59:738–741. 10.1128/AAC.04388-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liu X-Q, Wang J, Li W, Zhao L-Q, Lu Y, Liu J-H, Zeng Z-L. 2017. Distribution of cfr in Staphylococcus spp. and Escherichia coli strains from pig farms in China and characterization of a novel cfr-carrying F43:A−:B− plasmid. Front Microbiol 8:329. 10.3389/fmicb.2017.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Li P, Zhu T, Zhou D, Lu W, Liu H, Sun Z, Ying J, Lu J, Lin X, Li K, Ying J, Bao Q, Xu T. 2020. Analysis of resistance to florfenicol and the related mechanism of dissemination in different animal-derived bacteria. Front Cell Infect Microbiol 10:369. 10.3389/fcimb.2020.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Peng K, Li R, He T, Liu Y, Wang Z. 2020. Characterization of a porcine Proteus cibarius strain co-harbouring tet(X6) and cfr. J Antimicrob Chemother 75:1652–1654. 10.1093/jac/dkaa047. [DOI] [PubMed] [Google Scholar]
- 214.D'Andrea MM, Antonelli A, Brenciani A, Di Pilato V, Morroni G, Pollini S, Fioriti S, Giovanetti E, Rossolini GM. 2019. Characterization of Tn6349, a novel mosaic transposon carrying poxtA, cfr and other resistance determinants, inserted in the chromosome of an ST5-MRSA-II strain of clinical origin. J Antimicrob Chemother 74:2870–2875. 10.1093/jac/dkz278. [DOI] [PubMed] [Google Scholar]
- 215.Peters JE. 2014. Tn7. Microbiol Spectr 2. 10.1128/microbiolspec.MDNA3-0010-2014. [DOI] [PubMed] [Google Scholar]
- 216.Parks AR, Peters JE. 2007. Transposon Tn7 is widespread in diverse bacteria and forms genomic islands. J Bacteriol 189:2170–2173. 10.1128/JB.01536-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Harmer CJ, Pong CH, Hall RM. 2020. Structures bounded by directly-oriented members of the IS26 family are pseudo-compound transposons. Plasmid 111:102530. 10.1016/j.plasmid.2020.102530. [DOI] [PubMed] [Google Scholar]
- 218.Zhao Q, Wendlandt S, Li H, Li J, Wu C, Shen J, Schwarz S, Wang Y. 2014. Identification of the novel lincosamide resistance gene lnu(E) truncated by ISEnfa5-cfr-ISEnfa5 insertion in Streptococcus suis: de novo synthesis and confirmation of functional activity in Staphylococcus aureus. Antimicrob Agents Chemother 58:1785–1788. 10.1128/AAC.02007-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Carraro N, Burrus V. 2014. Biology of three ICE families: SXT/R391, ICEBs1, and ICESt1/ICESt3. Microbiol Spectr 2. 10.1128/microbiolspec.MDNA3-0008-2014. [DOI] [PubMed] [Google Scholar]
- 220.Iranzo J, Gómez MJ, López de Saro FJ, Manrubia S. 2014. Large-scale genomic analysis suggests a neutral punctuated dynamics of transposable elements in bacterial genomes. PLoS Comput Biol 10:e1003680. 10.1371/journal.pcbi.1003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mahillon J, Chandler M. 1998. Insertion sequences. Microbiol Mol Biol Rev 62:725–774. 10.1128/MMBR.62.3.725-774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Razavi M, Kristiansson E, Flach C-F, Larsson DGJ. 2020. The association between insertion sequences and antibiotic resistance genes. mSphere 5:e00418-20. 10.1128/mSphere.00418-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hennig S, Ziebuhr W. 2010. Characterization of the transposase encoded by IS256, the prototype of a major family of bacterial insertion sequence elements. J Bacteriol 192:4153–4163. 10.1128/JB.00226-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Harmer CJ, Hall RM. 2020. IS26 family members IS257 and IS1216 also form cointegrates by copy-in and targeted conservative routes. mSphere 5:e00811-19. 10.1128/mSphere.00811-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Antonelli A, D’Andrea MM, Galano A, Borchi B, Brenciani A, Vaggelli G, Cavallo A, Bartoloni A, Giovanetti E, Rossolini GM. 2016. Linezolid-resistant cfr-positive MRSA, Italy. J Antimicrob Chemother 71:2349–2351. 10.1093/jac/dkw108. [DOI] [PubMed] [Google Scholar]
- 226.Harmer CJ, Hall RM. 2019. An analysis of the IS6/IS26 family of insertion sequences: is it a single family? Microb Genom 5:e000291. 10.1099/mgen.0.000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Harmer CJ, Hall RM. 2016. IS26-mediated formation of transposons carrying antibiotic resistance genes. mSphere 1:e00038-16. 10.1128/mSphere.00038-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Harmer CJ, Moran RA, Hall RM. 2014. Movement of IS26-associated antibiotic resistance genes occurs via a translocatable unit that includes a single IS26 and preferentially inserts adjacent to another IS26. mBio 5:e01801-14–e01814. 10.1128/mBio.01801-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Toh SM, Xiong L, Arias CA, Villegas MV, Lolans K, Quinn J, Mankin AS. 2007. Acquisition of a natural resistance gene renders a clinical strain of methicillin-resistant Staphylococcus aureus resistant to the synthetic antibiotic linezolid. Mol Microbiol 64:1506–1514. 10.1111/j.1365-2958.2007.05744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chatedaki C, Voulgaridi I, Kachrimanidou M, Hrabak J, Papagiannitsis CC, Petinaki E. 2019. Antimicrobial susceptibility and mechanisms of resistance of Greek Clostridium difficile clinical isolates. J Glob Antimicrob Resist 16:53–58. 10.1016/j.jgar.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 231.Kuroda M, Sekizuka T, Matsui H, Suzuki K, Seki H, Saito M, Hanaki H. 2018. Complete genome sequence and characterization of linezolid-resistant Enterococcus faecalis clinical isolate KUB3006 carrying a cfr(B)-transposon on its chromosome and optrA-plasmid. Front Microbiol 9:2576. 10.3389/fmicb.2018.02576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bender JK, Fleige C, Klare I, Fiedler S, Mischnik A, Mutters NT, Dingle KE, Werner G. 2016. Detection of a cfr(B) variant in German Enterococcus faecium clinical isolates and the impact on linezolid resistance in Enterococcus spp. PLoS One 11:e0167042. 10.1371/journal.pone.0167042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Dingle KE, Elliott B, Robinson E, Griffiths D, Eyre DW, Stoesser N, Vaughan A, Golubchik T, Fawley WN, Wilcox MH, Peto TE, Walker AS, Riley TV, Crook DW, Didelot X. 2014. Evolutionary history of the Clostridium difficile pathogenicity locus. Genome Biol Evol 6:36–52. 10.1093/gbe/evt204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Candela T, Marvaud J-C, Nguyen TK, Lambert T. 2017. A cfr-like gene cfr(C) conferring linezolid resistance is common in Clostridium difficile. Int J Antimicrob Agents 50:496–500. 10.1016/j.ijantimicag.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 235.Zhao S, Mukherjee S, Hsu C-H, Young S, Li C, Tate H, Morales CA, Haro J, Thitaram S, Tillman GE, Dessai U, McDermott P. 2019. Genomic analysis of emerging florfenicol-resistant Campylobacter coli isolated from the cecal contents of cattle in the United States. mSphere 4:e00367-19. 10.1128/mSphere.00367-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Liu D, Li X, Liu W, Yao H, Liu Z, Feßler AT, He J, Zhou Y, Shen Z, Wu Z, Schwarz S, Zhang Q, Wang Y. 2019. Characterization of multiresistance gene cfr(C) variants in Campylobacter from China. J Antimicrob Chemother 74:2166–2170. 10.1093/jac/dkz197. [DOI] [PubMed] [Google Scholar]
- 237.Tang Y, Lai Y, Yang X, Cao X, Hu Y, Wang X, Wang H. 2020. Genetic environments and related transposable elements of novel cfr(C) variants in Campylobacter coli isolates of swine origin. Vet Microbiol 247:108792. 10.1016/j.vetmic.2020.108792. [DOI] [PubMed] [Google Scholar]
- 238.Guerin F, Sassi M, Dejoies L, Zouari A, Schutz S, Potrel S, Auzou M, Collet A, Lecointe D, Auger G, Cattoir V. 2020. Molecular and functional analysis of the novel cfr(D) linezolid resistance gene identified in Enterococcus faecium. J Antimicrob Chemother 75:1699–1703. 10.1093/jac/dkaa125. [DOI] [PubMed] [Google Scholar]
- 239.Egan SA, Shore AC, O’Connell B, Brennan GI, Coleman DC. 2020. Linezolid resistance in Enterococcus faecium and Enterococcus faecalis from hospitalized patients in Ireland: high prevalence of the MDR genes optrA and poxtA in isolates with diverse genetic backgrounds. J Antimicrob Chemother 75:1704–1711. 10.1093/jac/dkaa075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ruiz-Ripa L, Feßler AT, Hanke D, Eichhorn I, Azcona-Gutiérrez JM, Pérez-Moreno MO, Seral C, Aspiroz C, Alonso CA, Torres L, Alós J-I, Schwarz S, Torres C. 2020. Mechanisms of linezolid resistance among enterococci of clinical origin in Spain—detection of optrA- and cfr(D)-carrying E. faecalis. Microorganisms 8:1155. 10.3390/microorganisms8081155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kerschner H, Rosel AC, Hartl R, Hyden P, Stoeger A, Ruppitsch W, Allerberger F, Apfalter P. 2020. Oxazolidinone resistance mediated by optrA in clinical Enterococcus faecalis isolates in upper Austria: first report and characterization by whole genome sequencing. Microb Drug Resist 10.1089/mdr.2020.0098. [DOI] [PubMed] [Google Scholar]
- 242.Roy S, Aung MS, Paul SK, Ahmed S, Haque N, Khan ER, Barman TK, Islam A, Abedin S, Sultana C, Paul A, Hossain MA, Urushibara N, Kawaguchiya M, Sumi A, Kobayashi N. 2020. Drug resistance determinants in clinical isolates of Enterococcus faecalis in Bangladesh: identification of oxazolidinone resistance gene optrA in ST59 and ST902 lineages. Microorganisms 8:1240. 10.3390/microorganisms8081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Argudín MA, Youzaga S, Dodémont M, Heinrichs A, Roisin S, Deplano A, Nonhoff C, Hallin M. 2019. Detection of optrA-positive enterococci clinical isolates in Belgium. Eur J Clin Microbiol Infect Dis 38:985–987. 10.1007/s10096-019-03504-3. [DOI] [PubMed] [Google Scholar]
- 244.Chen H, Wang X, Yin Y, Li S, Zhang Y, Wang Q, Wang H. 2019. Molecular characteristics of oxazolidinone resistance in enterococci from a multicenter study in China. BMC Microbiol 19:162. 10.1186/s12866-019-1537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhou W, Gao S, Xu H, Zhang Z, Chen F, Shen H, Zhang C. 2019. Distribution of the optrA gene in Enterococcus isolates at a tertiary care hospital in China. J Glob Antimicrob Resist 17:180–186. 10.1016/j.jgar.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 246.He T, Shen Y, Schwarz S, Cai J, Lv Y, Li J, Feßler AT, Zhang R, Wu C, Shen J, Wang Y. 2016. Genetic environment of the transferable oxazolidinone/phenicol resistance gene optrA in Enterococcus faecalis isolates of human and animal origin. J Antimicrob Chemother 71:1466–1473. 10.1093/jac/dkw016. [DOI] [PubMed] [Google Scholar]
- 247.Chien J-Y, Mendes RE, Deshpande LM, Hsueh P-R. 2017. Empyema thoracis caused by an optrA-positive and linezolid-intermediate Enterococcus faecalis strain. J Infect 75:182–184. 10.1016/j.jinf.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 248.Cai J, Schwarz S, Chi D, Wang Z, Zhang R, Wang Y. 2019. Faecal carriage of optrA-positive enterococci in asymptomatic healthy humans in Hangzhou, China. Clin Microbiol Infect 25:630.e1-630–e6. 10.1016/j.cmi.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 249.Cai J, Wang Y, Schwarz S, Lv H, Li Y, Liao K, Yu S, Zhao K, Gu D, Wang X, Zhang R, Shen J. 2015. Enterococcal isolates carrying the novel oxazolidinone resistance gene optrA from hospitals in Zhejiang, Guangdong, and Henan, China, 2010–2014. Clin Microbiol Infect 21:1095.e1-1095–e4. 10.1016/j.cmi.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 250.Cui L, Wang Y, Lv Y, Wang S, Song Y, Li Y, Liu J, Xue F, Yang W, Zhang J. 2016. Nationwide surveillance of novel oxazolidinone resistance gene optrA in Enterococcus isolates in China from 2004 to 2014. Antimicrob Agents Chemother 60:7490–7493. 10.1128/AAC.01256-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zou J, Xia Y. 2020. Molecular characteristics and risk factors associated with linezolid-resistant Enterococcus faecalis infection in Southwest China. J Glob Antimicrob Resist 22:504–510. 10.1016/j.jgar.2020.03.027. [DOI] [PubMed] [Google Scholar]
- 252.Saavedra SY, Bernal JF, Montilla-Escudero E, Torres G, Rodríguez MK, Hidalgo AM, Ovalle MV, Rivera S, Perez-Gutierrez E, Duarte C. 2020. [National surveillance of clinical isolates of Enterococcus faecalis resistant to linezolid carrying the optrA gene in Colombia, 2014–2019. Rev Panam Salud Publica 44:e104. 10.26633/RPSP.2020.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Vorobieva V, Roer L, Justesen US, Hansen F, Frimodt-Møller N, Hasman H, Hammerum AM. 2017. Detection of the optrA gene in a clinical ST16 Enterococcus faecalis isolate in Denmark. J Glob Antimicrob Resist 10:12–13. 10.1016/j.jgar.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 254.Said HS, Abdelmegeed ES. 2019. Emergence of multidrug resistance and extensive drug resistance among enterococcal clinical isolates in Egypt. Infect Drug Resist 12:1113–1125. 10.2147/IDR.S189341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sassi M, Guérin F, Zouari A, Beyrouthy R, Auzou M, Fines-Guyon M, Potrel S, Dejoies L, Collet A, Boukthir S, Auger G, Bonnet R, Cattoir V. 2019. Emergence of optrA-mediated linezolid resistance in enterococci from France, 2006–16. J Antimicrob Chemother 74:1469–1472. 10.1093/jac/dkz097. [DOI] [PubMed] [Google Scholar]
- 256.Bender JK, Fleige C, Lange D, Klare I, Werner G. 2018. Rapid emergence of highly variable and transferable oxazolidinone and phenicol resistance gene optrA in German Enterococcus spp. clinical isolates. Int J Antimicrob Agents 52:819–827. 10.1016/j.ijantimicag.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 257.Tsilipounidaki K, Gerontopoulos A, Papagiannitsis C, Petinaki E. 2019. First detection of an optrA-positive, linezolid-resistant ST16 Enterococcus faecalis from human in Greece. New Microbes New Infect 29:100515. 10.1016/j.nmni.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Park K, Jeong YS, Chang J, Sung H, Kim M-N. 2020. Emergence of optrA-mediated linezolid-nonsusceptible Enterococcus faecalis in a tertiary care hospital. Ann Lab Med 40:321–325. 10.3343/alm.2020.40.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lee S-M, Huh HJ, Song DJ, Shim HJ, Park KS, Kang C-I, Ki C-S, Lee NY. 2017. Resistance mechanisms of linezolid-nonsusceptible enterococci in Korea: low rate of 23S rRNA mutations in Enterococcus faecium. J Med Microbiol 66:1730–1735. 10.1099/jmm.0.000637. [DOI] [PubMed] [Google Scholar]
- 260.Gawryszewska I, Żabicka D, Hryniewicz W, Sadowy E. 2017. Linezolid-resistant enterococci in Polish hospitals: species, clonality and determinants of linezolid resistance. Eur J Clin Microbiol Infect Dis 36:1279–1286. 10.1007/s10096-017-2934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Càmara J, Camoez M, Tubau F, Pujol M, Ayats J, Ardanuy C, Domínguez MÁ. 2019. Detection of the novel optrA gene among linezolid-resistant enterococci in Barcelona, Spain. Microb Drug Resist 25:87–93. 10.1089/mdr.2018.0028. [DOI] [PubMed] [Google Scholar]
- 262.Moure Z, Lara N, Marín M, Sola-Campoy PJ, Bautista V, Gómez-Bertomeu F, Gómez-Dominguez C, Pérez-Vázquez M, Aracil B, Campos J, Cercenado E, Oteo-Iglesias J, Spanish Linezolid-Resistant Enterococci Collaborating Group . 2020. Interregional spread in Spain of linezolid-resistant Enterococcus spp. isolates carrying the optrA and poxtA genes. Int J Antimicrob Agents 55:105977. 10.1016/j.ijantimicag.2020.105977. [DOI] [PubMed] [Google Scholar]
- 263.Raddaoui A, Chebbi Y, Tanfous FB, Mabrouk A, Achour W. 2020. First description of clinical linezolid resistant Enterococcus sp. in North Africa. J Glob Antimicrob Resist 21:169–170. 10.1016/j.jgar.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 264.Carvalhaes CG, Sader HS, Flamm RK, Streit JM, Mendes RE. 2020. Assessment of tedizolid in vitro activity and resistance mechanisms against a collection of Enterococcus spp. causing invasive infections, including isolates requiring an optimized dosing strategy for daptomycin from U.S. and European Medical Centers, 2016 to 2018. Antimicrob Agents Chemother 64:e00175-20. 10.1128/AAC.00175-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Almeida LM, Lebreton F, Gaca A, Bispo PM, Saavedra JT, Calumby RN, Grillo LM, Nascimento TG, Filsner PH, Moreno AM, Gilmore MS. 2020. Transferable resistance gene optrA in Enterococcus faecalis from swine in Brazil. Antimicrob Agents Chemother 64:e00142-20. 10.1128/AAC.00142-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kang Z-Z, Lei C-W, Kong L-H, Wang Y-L, Ye X-L, Ma B-H, Wang X-C, Li C, Zhang Y, Wang H-N. 2019. Detection of transferable oxazolidinone resistance determinants in Enterococcus faecalis and Enterococcus faecium of swine origin in Sichuan Province, China. J Glob Antimicrob Resist 19:333–337. 10.1016/j.jgar.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 267.Lei C-W, Kang Z-Z, Wu S-K, Chen Y-P, Kong L-H, Wang H-N. 2019. Detection of the phenicol–oxazolidinone–tetracycline resistance gene poxtA in Enterococcus faecium and Enterococcus faecalis of food-producing animal origin in China. J Antimicrob Chemother 74:2459–2461. 10.1093/jac/dkz198. [DOI] [PubMed] [Google Scholar]
- 268.Hao W, Shan X, Li D, Schwarz S, Zhang S-M, Li X-S, Du X-D. 2019. Analysis of a poxtA- and optrA-co-carrying conjugative multiresistance plasmid from Enterococcus faecalis. J Antimicrob Chemother 74:1771–1775. 10.1093/jac/dkz109. [DOI] [PubMed] [Google Scholar]
- 269.Shang Y, Li D, Shan X, Schwarz S, Zhang SM, Chen YX, Ouyang W, Du XD. 2019. Analysis of two pheromone-responsive conjugative multiresistance plasmids carrying the novel mobile optrA locus from Enterococcus faecalis. Infect Drug Resist 12:2355–2362. 10.2147/IDR.S206295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Li D, Li X-Y, Schwarz S, Yang M, Zhang S-M, Hao W, Du X-D. 2019. Tn6674 is a novel enterococcal optrA-carrying multiresistance transposon of the Tn554 family. Antimicrob Agents Chemother 63:e00809-19. 10.1128/AAC.00809-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Tamang MD, Moon DC, Kim S-R, Kang HY, Lee K, Nam H-M, Jang G-C, Lee H-S, Jung S-C, Lim S-K. 2017. Detection of novel oxazolidinone and phenicol resistance gene optrA in enterococcal isolates from food animals and animal carcasses. Vet Microbiol 201:252–256. 10.1016/j.vetmic.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 272.Cavaco LM, Bernal JF, Zankari E, Léon M, Hendriksen RS, Perez-Gutierrez E, Aarestrup FM, Donado-Godoy P. 2017. Detection of linezolid resistance due to the optrA gene in Enterococcus faecalis from poultry meat from the American continent (Colombia). J Antimicrob Chemother 72:678–683. 10.1093/jac/dkw490. [DOI] [PubMed] [Google Scholar]
- 273.Elghaieb H, Freitas AR, Abbassi MS, Novais C, Zouari M, Hassen A, Peixe L. 2019. Dispersal of linezolid-resistant enterococci carrying poxtA or optrA in retail meat and food-producing animals from Tunisia. J Antimicrob Chemother 74:2865–2869. 10.1093/jac/dkz263. [DOI] [PubMed] [Google Scholar]
- 274.Elghaieb H, Tedim AP, Abbassi MS, Novais C, Duarte B, Hassen A, Peixe L, Freitas AR. 2020. From farm to fork: identical clones and Tn6674-like elements in linezolid-resistant Enterococcus faecalis from food-producing animals and retail meat. J Antimicrob Chemother 75:30–35. 10.1093/jac/dkz419. [DOI] [PubMed] [Google Scholar]
- 275.Cavaco LM, Korsgaard H, Kaas RS, Seyfarth AM, Leekitcharoenphon P, Hendriksen RS. 2017. First detection of linezolid resistance due to the optrA gene in enterococci isolated from food products in Denmark. J Glob Antimicrob Resist 9:128–129. 10.1016/j.jgar.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 276.Kim YB, Seo KW, Son SH, Noh EB, Lee YJ. 2019. Genetic characterization of high-level aminoglycoside-resistant Enterococcus faecalis and Enterococcus faecium isolated from retail chicken meat. Poult Sci 98:5981–5988. 10.3382/ps/pez403. [DOI] [PubMed] [Google Scholar]
- 277.Yoon S, Son SH, Kim YB, Seo KW, Lee YJ. 2020. Molecular characteristics of optrA-carrying Enterococcus faecalis from chicken meat in South Korea. Poult Sci 99:6990–6996. 10.1016/j.psj.2020.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Freitas AR, Elghaieb H, León-Sampedro R, Abbassi MS, Novais C, Coque TM, Hassen A, Peixe L. 2017. Detection of optrA in the African continent (Tunisia) within a mosaic Enterococcus faecalis plasmid from urban wastewaters. J Antimicrob Chemother 72:3245–3251. 10.1093/jac/dkx321. [DOI] [PubMed] [Google Scholar]
- 279.Gu J, Xie X-J, Liu J-X, Shui J-R, Zhang H-Y, Feng G-Y, Liu X-Y, Li L-C, Lan Q-W, Jin Q-H, Li R, Peng L, Lei C-W, Zhang A-Y. 2020. Prevalence and transmission of antimicrobial-resistant Staphylococci and Enterococci from shared bicycles in Chengdu, China. Sci Total Environ 738:139735. 10.1016/j.scitotenv.2020.139735. [DOI] [PubMed] [Google Scholar]
- 280.Papagiannitsis CC, Tsilipounidaki K, Malli E, Petinaki E. 2019. Detection in Greece of a clinical Enterococcus faecium isolate carrying the novel oxazolidinone resistance gene poxtA. J Antimicrob Chemother 74:2461–2462. 10.1093/jac/dkz155. [DOI] [PubMed] [Google Scholar]
- 281.Wardenburg KE, Potter RF, D'Souza AW, Hussain T, Wallace MA, Andleeb S, Burnham C-AD, Dantas G. 2019. Phenotypic and genotypic characterization of linezolid-resistant Enterococcus faecium from the USA and Pakistan. J Antimicrob Chemother 74:3445–3452. 10.1093/jac/dkz367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Na SH, Moon DC, Choi M-J, Oh S-J, Jung D-Y, Kang HY, Hyun B-H, Lim S-K. 2019. Detection of oxazolidinone and phenicol resistant enterococcal isolates from duck feces and carcasses. Int J Food Microbiol 293:53–59. 10.1016/j.ijfoodmicro.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 283.Ruiz-Ripa L, Feßler AT, Hanke D, Sanz S, Olarte C, Eichhorn I, Schwarz S, Torres C. 2020. Detection of poxtA- and optrA-carrying E. faecium isolates in air samples of a Spanish swine farm. J Glob Antimicrob Resist 22:28–31. 10.1016/j.jgar.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 284.Yao T-G, Li B-Y, Luan R-D, Wang H-N, Lei C-W. 2020. Whole genome sequence of Enterococcus gallinarum EG81, a porcine strain harbouring the oxazolidinone-phenicol resistance gene optrA with chromosomal and plasmid location. J Glob Antimicrob Resist 22:598–600. 10.1016/j.jgar.2020.06.012. [DOI] [PubMed] [Google Scholar]
- 285.Zheng J-x, Chen Z, Xu Z-c, Chen J-w, Xu G-j, Sun X, Yu Z-j, Qu D. 2020. In vitro evaluation of the antibacterial activities of radezolid and linezolid for Streptococcus agalactiae. Microb Pathog 139:103866. 10.1016/j.micpath.2019.103866. [DOI] [PubMed] [Google Scholar]
- 286.Du F, Lv X, Duan D, Wang L, Huang J. 2019. Characterization of a linezolid- and vancomycin-resistant Streptococcus suis isolate that harbors optrA and vanG operons. Front Microbiol 10:2026. 10.3389/fmicb.2019.02026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Shang Y, Li D, Hao W, Schwarz S, Shan X, Liu B, Zhang S-M, Li X-S, Du X-D. 2019. A prophage and two ICESa2603-family integrative and conjugative elements (ICEs) carrying optrA in Streptococcus suis. J Antimicrob Chemother 74:2876–2879. 10.1093/jac/dkz309. [DOI] [PubMed] [Google Scholar]
- 288.Zhang C, Zhang P, Wang Y, Fu L, Liu L, Xu D, Hou Y, Li Y, Fu M, Wang X, Wang S, Ding S, Shen Z. 2020. Capsular serotypes, antimicrobial susceptibility, and the presence of transferable oxazolidinone resistance genes in Streptococcus suis isolated from healthy pigs in China. Vet Microbiol 247:108750. 10.1016/j.vetmic.2020.108750. [DOI] [PubMed] [Google Scholar]
- 289.Zhou Y, Li J, Schwarz S, Zhang S, Tao J, Fan R, Walsh TR, Wu C, Wang Y. 2020. Mobile oxazolidinone/phenicol resistance gene optrA in chicken Clostridium perfringens. J Antimicrob Chemother 75:3067–3069. 10.1093/jac/dkaa236. [DOI] [PubMed] [Google Scholar]
- 290.Liu D, Yang D, Liu X, Li X, Feßler AT, Shen Z, Shen J, Schwarz S, Wang Y. 2020. Detection of the enterococcal oxazolidinone/phenicol resistance gene optrA in Campylobacter coli. Vet Microbiol 246:108731. 10.1016/j.vetmic.2020.108731. [DOI] [PubMed] [Google Scholar]
- 291.Tang Y, Lai Y, Wang X, Lei C, Li C, Kong L, Wang Y, Wang H. 2020. Novel insertion sequence ISChh1-like mediating acquisition of optrA gene in foodborne pathogen Campylobacter coli of swine origin. Vet Microbiol 252:108934. 10.1016/j.vetmic.2020.108934. [DOI] [PubMed] [Google Scholar]
- 292.Egan SA, Corcoran S, McDermott H, Fitzpatrick M, Hoyne A, McCormack O, Cullen A, Brennan GI, O’Connell B, Coleman DC. 2020. Hospital outbreak of linezolid-resistant and vancomycin-resistant ST80 Enterococcus faecium harbouring an optrA-encoding conjugative plasmid investigated by whole-genome sequencing. J Hosp Infect 105:726–735. 10.1016/j.jhin.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 293.Zhu Y, Zhang W, Wang C, Liu W, Yang Q, Luan T, Wang L, Schwarz S, Liu S. 2020. Identification of a novel optrA-harbouring transposon, Tn6823, in Staphylococcus aureus. J Antimicrob Chemother 75:3395–3397. 10.1093/jac/dkaa323. [DOI] [PubMed] [Google Scholar]
- 294.Palmieri C, Princivalli MS, Brenciani A, Varaldo PE, Facinelli B. 2011. Different genetic elements carrying the tet(W) gene in two human clinical isolates of Streptococcus suis. Antimicrob Agents Chemother 55:631–636. 10.1128/AAC.00965-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Huang J, Liang Y, Guo D, Shang K, Ge L, Kashif J, Wang L. 2016. Comparative genomic analysis of the ICESa2603 family ICEs and spread of erm(B)- and tet(O)-carrying transferable 89K-subtype ICEs in swine and bovine isolates in China. Front Microbiol 7:55. 10.3389/fmicb.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Freitas AR, Tedim AP, Duarte B, Elghaieb H, Abbassi MS, Hassen A, Read A, Alves V, Novais C, Peixe L. 2020. Linezolid-resistant (Tn6246::fexB-poxtA) Enterococcus faecium strains colonizing humans and bovines on different continents: similarity without epidemiological link. J Antimicrob Chemother 75:2416–2423. 10.1093/jac/dkaa227. [DOI] [PubMed] [Google Scholar]
- 297.Huang J, Wang M, Gao Y, Chen L, Wang L. 2019. Emergence of plasmid-mediated oxazolidinone resistance gene poxtA from CC17 Enterococcus faecium of pig origin. J Antimicrob Chemother 74:2524–2530. 10.1093/jac/dkz250. [DOI] [PubMed] [Google Scholar]
- 298.Brenciani A, Fioriti S, Morroni G, Cucco L, Morelli A, Pezzotti G, Paniccià M, Antonelli A, Magistrali CF, Rossolini GM, Giovanetti E. 2019. Detection in Italy of a porcine Enterococcus faecium isolate carrying the novel phenicol-oxazolidinone-tetracycline resistance gene poxtA. J Antimicrob Chemother 74:817–818. 10.1093/jac/dky505. [DOI] [PubMed] [Google Scholar]
- 299.Na S-H, Moon D-C, Kim M-H, Kang H-Y, Kim S-J, Choi J-H, Mechesso A-F, Yoon S-S, Lim S-K. 2020. Detection of the phenicol–oxazolidinone resistance Gene poxtA in Enterococcus faecium and Enterococcus faecalis from food-producing animals during 2008–2018 in Korea. Microorganisms 8:1839. 10.3390/microorganisms8111839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Kang Z-Z, Lei C-W, Yao T-G, Zhang Y, Wang Y-L, Ye X-L, Wang X-C, Gao Y-F, Wang H-N. 2019. Whole-genome sequencing of Enterococcus hirae CQP3-9, a strain carrying the phenicol–oxazolidinone–tetracycline resistance gene poxtA of swine origin in China. J Glob Antimicrob Resist 18:71–73. 10.1016/j.jgar.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 301.Clewell DB, Weaver KE, Dunny GM, Coque TM, Francia MV, Hayes F. 2014. Extrachromosomal and mobile elements in enterococci: transmission, maintenance, and epidemiology. In Gilmore MS, Clewell DB, Ike Y, Shankar N (ed), Enterococci: from commensals to leading causes of drug resistant infection [Internet]. Massachusetts Eye and Ear Infirmary, Boston, Massachussetts. [PubMed] [Google Scholar]
- 302.Shan X, Li X-S, Wang N, Schwarz S, Zhang S-M, Li D, Du X-D. 2020. Studies on the role of IS1216E in the formation and dissemination of poxtA-carrying plasmids in an Enterococcus faecium clade A1 isolate. J Antimicrob Chemother 75:3126–3130. 10.1093/jac/dkaa325. [DOI] [PubMed] [Google Scholar]
- 303.Schwarz S, Kehrenberg C, Doublet B, Cloeckaert A. 2004. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol Rev 28:519–542. 10.1016/j.femsre.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 304.Wang Y, Li X, Fu Y, Chen Y, Wang Y, Ye D, Wang C, Hu X, Zhou L, Du J, Shen J, Xia X. 2020. Association of florfenicol residues with the abundance of oxazolidinone resistance genes in livestock manures. J Hazard Mater 399:123059. 10.1016/j.jhazmat.2020.123059. [DOI] [PubMed] [Google Scholar]
- 305.Schwarz S, Loeffler A, Kadlec K. 2017. Bacterial resistance to antimicrobial agents and its impact on veterinary and human medicine. Vet Dermatol 28:82–e19. 10.1111/vde.12362. [DOI] [PubMed] [Google Scholar]
- 306.Li P, Yang Y, Ding L, Xu X, Lin D. 2020. Molecular investigations of linezolid resistance in enterococci OptrA variants from a hospital in Shanghai. Infect Drug Resist 13:2711–2716. 10.2147/IDR.S251490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Ding L, Li P, Yang Y, Lin D, Xu X. 2020. The epidemiology and molecular characteristics of linezolid-resistant Staphylococcus capitis in Huashan Hospital, Shanghai. J Med Microbiol 69:1079–1088. 10.1099/jmm.0.001234. [DOI] [PubMed] [Google Scholar]
- 308.Bender JK, Fleige C, Klare I, Werner G. 2019. Development of a multiplex-PCR to simultaneously detect acquired linezolid resistance genes cfr, optrA and poxtA in enterococci of clinical origin. J Microbiol Methods 160:101–103. 10.1016/j.mimet.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 309.Hasman H, Clausen PTLC, Kaya H, Hansen F, Knudsen JD, Wang M, Holzknecht BJ, Samulioniené J, Røder BL, Frimodt-Møller N, Lund O, Hammerum AM. 2019. LRE-Finder, a Web tool for detection of the 23S rRNA mutations and the optrA, cfr, cfr(B) and poxtA genes encoding linezolid resistance in enterococci from whole-genome sequences. J Antimicrob Chemother 74:1473–1476. 10.1093/jac/dkz092. [DOI] [PubMed] [Google Scholar]
- 310.Bortolaia V, Kaas RS, Ruppe E, Roberts MC, Schwarz S, Cattoir V, Philippon A, Allesoe RL, Rebelo AR, Florensa AF, Fagelhauer L, Chakraborty T, Neumann B, Werner G, Bender JK, Stingl K, Nguyen M, Coppens J, Xavier BB, Malhotra-Kumar S, Westh H, Pinholt M, Anjum MF, Duggett NA, Kempf I, Nykäsenoja S, Olkkola S, Wieczorek K, Amaro A, Clemente L, Mossong J, Losch S, Ragimbeau C, Lund O, Aarestrup FM. 2020. ResFinder 4.0 for predictions of phenotypes from genotypes. J Antimicrob Chemother 75:3491–3500. 10.1093/jac/dkaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Alcock BP, Raphenya AR, Lau TTY, Tsang KK, Bouchard M, Edalatmand A, Huynh W, Nguyen A-LV, Cheng AA, Liu S, Min SY, Miroshnichenko A, Tran H-K, Werfalli RE, Nasir JA, Oloni M, Speicher DJ, Florescu A, Singh B, Faltyn M, Hernandez-Koutoucheva A, Sharma AN, Bordeleau E, Pawlowski AC, Zubyk HL, Dooley D, Griffiths E, Maguire F, Winsor GL, Beiko RG, Brinkman FSL, Hsiao WWL, Domselaar GV, McArthur AG. 2020. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res 48:D517–D525. 10.1093/nar/gkz935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Feldgarden M, Brover V, Haft DH, Prasad AB, Slotta DJ, Tolstoy I, Tyson GH, Zhao S, Hsu C-H, McDermott PF, Tadesse DA, Morales C, Simmons M, Tillman G, Wasilenko J, Folster JP, Klimke W. 2019. Validating the AMRFinder tool and resistance gene database by using antimicrobial resistance genotype-phenotype correlations in a collection of isolates. Antimicrob Agents Chemother 63:e00483-19. 10.1128/AAC.00483-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Gupta SK, Padmanabhan BR, Diene SM, Lopez-Rojas R, Kempf M, Landraud L, Rolain J-M. 2014. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob Agents Chemother 58:212–220. 10.1128/AAC.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Doster E, Lakin SM, Dean CJ, Wolfe C, Young JG, Boucher C, Belk KE, Noyes NR, Morley PS. 2020. MEGARes 2.0: a database for classification of antimicrobial drug, biocide and metal resistance determinants in metagenomic sequence data. Nucleic Acids Res 48:D561–D569. 10.1093/nar/gkz1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Dejoies L, Boukthir S, Péan de Ponfilly G, Le Guen R, Zouari A, Potrel S, Collet A, Auger G, Jacquier H, Fihman V, Dortet L, Cattoir V. 2020. Performance of commercial methods for linezolid susceptibility testing of Enterococcus faecium and Enterococcus faecalis. J Antimicrob Chemother 75:2587–2593. 10.1093/jac/dkaa180. [DOI] [PubMed] [Google Scholar]
- 316.Qi C, Zheng X, Obias A, Scheetz MH, Malczynski M, Warren JR. 2006. Comparison of testing methods for detection of decreased linezolid susceptibility due to G2576T mutation of the 23S rRNA gene in Enterococcus faecium and Enterococcus faecalis. J Clin Microbiol 44:1098–1100. 10.1128/JCM.44.3.1098-1100.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Tenover FC, Williams PP, Stocker S, Thompson A, Clark LA, Limbago B, Carey RB, Poppe SM, Shinabarger D, McGowan JE. 2007. Accuracy of six antimicrobial susceptibility methods for testing linezolid against staphylococci and enterococci. J Clin Microbiol 45:2917–2922. 10.1128/JCM.00913-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Yoo IY, Kang O-K, Shim HJ, Huh HJ, Lee NY. 2020. Linezolid resistance in methicillin-resistant Staphylococcus aureus in Korea: high rate of false resistance to linezolid by the VITEK 2 system. Ann Lab Med 40:57–62. 10.3343/alm.2020.40.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Werner G, Fleige C, Klare I, Weber RE, Bender JK. 2019. Validating a screening agar for linezolid-resistant enterococci. BMC Infect Dis 19:1078. 10.1186/s12879-019-4711-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Nordmann P, Rodríguez-Villodres A, Poirel L. 2019. A selective culture medium for screening linezolid-resistant gram-positive bacteria. Diagn Microbiol Infect Dis 95:1–4. 10.1016/j.diagmicrobio.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 321.Ball AP, Davey PG, Geddes AM, Farrell ID, Brookes GR. 1980. Clavulanic acid and amoxycillin: a clinical, bacteriological, and pharmacological study. Lancet 315:620–623. 10.1016/S0140-6736(80)91118-6. [DOI] [PubMed] [Google Scholar]
- 322.Bush K. 1988. Beta-lactamase inhibitors from laboratory to clinic. Clin Microbiol Rev 1:109–123. 10.1128/cmr.1.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Ero R, Kumar V, Su W, Gao Y-G. 2019. Ribosome protection by ABC-F proteins—molecular mechanism and potential drug design. Protein Sci 28:684–693. 10.1002/pro.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Zhong X, Xiang H, Wang T, Zhong L, Ming D, Nie L, Cao F, Li B, Cao J, Mu D, Ruan K, Wang L, Wang D. 2018. A novel inhibitor of the new antibiotic resistance protein OptrA. Chem Biol Drug Des 92:1458–1467. 10.1111/cbdd.13311. [DOI] [PubMed] [Google Scholar]
- 325.World Health Organization (WHO). 2018. Report on surveillance of antibiotic consumption 2016–2018: early implementation. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 326.World Organisation for Animal Health (OIE). 2020. OIE annual report on antimicrobial agents intended for use in animals: better understanding of the global situation: fourth report. World Organisation for Animal Health, Paris, France. [Google Scholar]