SUMMARY
Echinococcosis is considered a cosmopolitan zoonosis caused by different species of small taeniid tapeworms of the genus Echinococcus and is regarded as a neglected zoonosis. Cystic and alveolar echinococcoses are endemic diseases of Tibetan, Pamir, and Iranian plateaus. All of the countries within the Iranian plateau are affected by echinococcosis. Pakistan, Turkey, and Iran are the three most populous countries of the region, in which echinococcosis is highly endemic. The three neighboring countries share strong cultural and socioeconomic ties. The present study aimed to provide a broad review of the status of cystic and alveolar echinococcosis, summarizing the current knowledge about geographical distribution, molecular epidemiology, and transmission dynamics of Echinococcus granulosus sensu lato and Echinococcus multilocularis in this region. Additionally, we aimed to understand disease burden and risk factors as basic requirements for establishing a surveillance system and planning prevention and control programs. A considerable body of information is available on different aspects of echinococcosis in this region; however, several information and research gaps need to be filled before planning control programs. None of the countries in the region have an elaborate echinococcosis control program. Effective control programs require multi/intersectoral coordination within a One Health approach with a long-term political and administrative commitment and enhanced international collaboration among the three countries.
KEYWORDS: alveolar echinococcosis, cystic echinococcosis, epidemiology, hydatid disease, Pakistan, Turkey, Iran, transmission, genotypes
INTRODUCTION
Echinococcoses are different diseases caused by the different species of small taeniid tapeworms of the genus Echinococcus infecting a wide range of mammals all over the world. The two main types of echinococcoses are cystic and alveolar echinococcosis, caused by Echinococcus granulosus sensu lato and Echinococcus multilocularis, respectively. Cystic echinococcosis (CE) is considered a prevalent zoonotic disease of humans and herbivorous animals in most parts of the world. Most causative agents of CE are transmitted between dogs as primary definitive hosts and different livestock species as the intermediate hosts. The infection results from the accidental ingestion of infective eggs of E. granulosus sensu lato through contact with feces, fur of infected dogs, and contaminated water and food (1–4). Alveolar echinococcosis (AE) as an emerging disease that originates from the larval stage of E. multilocularis (5). The infection results from the accidental ingestion of infective eggs of the adult tapeworm through contact with feces/fur of infected carnivores, mainly foxes, and/or contaminated fruits or vegetables. AE is transmitted between foxes as the primary definitive hosts and different rodent species as the main intermediate hosts (5) and mainly occurs in temperate to cold regions of the Northern Hemisphere.
CE develops mostly in the liver and/or lungs and rarely in other organs of infected animals and humans and can cause various degrees of signs and symptoms. In livestock animals, clinical symptoms are usually minimal, and infections are normally detected during routine meat inspection (6). CE in humans is often considered a chronic disease, and a large proportion of infected patients are asymptomatic, resulting in the underestimation of the total number of infected individuals (7). Echinococcus granulosus sensu lato is a cluster of cryptic species that are markedly different in their host specificity, pathogenicity, habitat distribution, and transmission dynamics as well as their zoonotic potential (8, 9). At least nine different genotypes/species have been identified within Echinococcus granulosus sensu lato, including three genotypes within E. granulosus sensu stricto (genotypes G1 to G3), E. equinus (G4 genotype), E. ortleppi (G5 genotype), E. felidis, E. intermedius (genotypes G6 and G7), and E. canadensis (G8 and G10) (10, 11).
The marked differences among the various taxa have been attributed to adaptations to different predator-prey systems in different regions and environments that are reflected in Echinococcus species life cycles (5).
The endemicity of CE has been attributed to various biotic and abiotic factors. CE transmission in humans can be influenced by a set of behavioral and socioeconomic factors (e.g., farming activities, including traditional sheep raising and agricultural practice, contact with dogs, geophagy, outdoor activities, or contaminated matrices) which may facilitate acquiring a high egg concentration of E. granulosus sensu lato (12).
CE is highly endemic and widespread in many parts of the world, especially in specific rural settings, e.g., pastoral and rural communities where humans and animals live in close proximity in most parts of Africa, the Middle East, Mediterranean Europe, Central Asia, South America, and western China (13). The Middle East is considered a hot spot of CE, where E. granulosus sensu lato has coincided with sheep for almost 10,000 to 12,000 years (14). A large part of the general population is at risk of CE in Central Asia, including Afghanistan, Pakistan, Kazakhstan, Kyrgyzstan, Uzbekistan, Tajikistan, and Turkmenistan (15). Iran and Turkey constitute nearly one-third of the Middle East surface and half of its population. They are among the most highly affected countries with livestock and human CE. Both countries are considered a zone of endemicity of CE, where high prevalence rates of infection with various species and genotypes of E. granulosus sensu lato have been reported in different livestock animals. Pakistan is one of the most populous countries in the world located in South Asia. CE is endemic in some regions of Pakistan (15); however, geographical distribution of the disease in the intermediate and definitive hosts is unclear. The incidence of the disease appears to be generally low in humans, and this is more likely to be underestimated, as a substantial portion of human cases are probably undiagnosed. CE also imposes a substantial economic loss to livestock husbandry and humans in terms of direct and indirect costs associated with human surgical and other treatment costs and livestock-related losses (16–18). The annual monetary burden of human and animal CE has been estimated at $232 million in Iran and $89 million for livestock in Turkey. Thus, the economic burden of CE could be substantial (16, 19). Based on the current evidence, it should be taken into consideration that CE imposes large socioeconomic burdens on the nations’ economies in the three countries (16), though it remains a neglected zoonotic disease (20, 21).
On the other hand, AE is widely distributed in the northern and central regions of Asia (i.e., most of the Russian Federation and central Asian states) extending into western China and northern Japan. Southwards, distribution of AE becomes increasingly patchy, but it can be highly endemic in climatically favorable areas in Turkey and Iran. There are isolated human AE cases known from the northern parts of South Asia, but the situation in Pakistan, Afghanistan, India, Bhutan, and Nepal is data deficient (13). Generally, the presence and spread of E. multilocularis are less well known than those of the CE agents, because the life cycle does not include livestock where the Echinococcus cysts are easily observed. Rather, E. multilocularis is transmitted by wildlife (foxes and small mammals), which is rarely investigated, and differential diagnosis of human AE with other space-occupying lesions of the liver is not trivial in regions where the parasite is not suspected to occur.
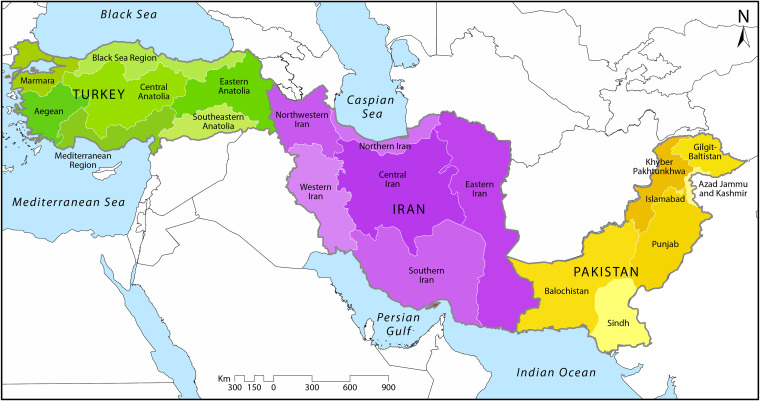
In-depth understanding of the epidemiology of echinococcosis in humans and animals can be valuable for developing control programs in a cost-effective way in order to reduce the transmission of the parasite to humans. In the present study, a broad review was performed by a comprehensive review of the literature to assess the current situation of E. granulosus sensu lato and E. multilocularis in Iran, Turkey, and Pakistan (Fig. 1) and to provide a framework for expanding what is known about echinococcoses, including the causative agents and life cycles, epidemiological and molecular data, and transmission dynamics among different host settings as well as potential risk factors.
FIG 1.
Geographical districts of Iran, Pakistan, and Turkey.
ECHINOCOCCUS SPECIES LIFE CYCLES IN SOUTHWESTERN ASIA
E. granulosus sensu lato is predominantly transmitted in various synanthropic cycles between a wide range of livestock species (as intermediate hosts) and dogs (as the definitive host). Moreover, sylvatic/wild cycles of E. granulosus sensu lato also occur between wild carnivores such as foxes, jackals, and wolves and wild ruminants such as wild sheep (Ovis orientalis), goitered gazelle (Gazella subgutturosa), and mouflon (Ovis gmelinii anatolica) acting as intermediate hosts (22–24), indicating the coexistence of the synanthropic and sylvatic cycles. The overlap of both pastoral and sylvatic cycles of E. granulosus sensu lato has also been reported in different areas, but usually it is not clear if wildlife infection occurs independently from the domestic transmission or if it represents a spillover from infection of livestock and dogs. Echinococcus granulosus sensu lato and E. multilocularis have been found in wild carnivores such as foxes, jackals, and wolves through a sylvatic cycle (23, 24), indicating a sympatric occurrence of the two species in some places. Nonetheless, there is uncertainty on the Echinococcus species or genotypes that infect wildlife, e.g., in Iran (13). A cycle involving donkeys (Equus asinus) has been reported to exist in Iran and Turkey (25–27). Furthermore, an important desert cycle occurs between dogs and camels in Iran and Pakistan. A link between domestic and wild cycles may be facilitated by human activities in areas where raw infected offal of slaughtered livestock is accessible to stray dogs, jackals, or foxes or where sheep or other livestock carcasses are scavenged by wild carnivores (28, 29).
Alveolar echinococcosis (AE) caused by E. multilocularis is predominantly transmitted between wild carnivores, mainly red foxes as definitive hosts, although domestic dogs may be involved in the transmission in some areas. Arvicoline rodents and some other small mammals act as intermediate hosts (4, 5). The disease is endemic in Eastern Anatolia of Turkey and northwestern Iran (30). Recently, an active focus of AE has been described in northeastern Iran in Khorasan Razavi Province (31). It has been suggested that in Iran, domestic dogs may contribute to the life cycle of E. multilocularis through hunting of rodents in rural areas (13).
HUMAN CYSTIC ECHINOCOCCOSIS IN IRAN
Data on the epidemiology of human cystic echinococcosis (CE) are mainly categorized in three sections, i.e., surgical incidence based on hospital records, seroprevalence surveys, and community-based ultrasound studies.
Human CE in Iran
Nourjah et al. (32) carried out the first comprehensive study on human CE incidence based on hospital records of surgically confirmed patients in 1980. The study was conducted across Iran on a total number of 4,850 patients operated on in government hospitals, with an overall annual incidence rate of 1.45 cases per 100,000 persons. The highest and lowest incidences of 4.45 and 1.0 per 100,000 population were recorded in Khorasan and Hormozgan provinces, respectively (32). A further study on 2,083 CE cases operated between 2001 and 2005 indicated an annual incidence of 0.72/100,000 human population across Iran, which appeared to decrease to 0.54/100,000 people at the end of the study period in 2005 (33). Human CE is distributed all over the country, with an incidence rate of 0.6 to 1.2 per 100,000 individuals (34). Fasihi Harandi et al. (16) reported that between 2000 and 2009, there was an average annual number of 1,295 surgical CE operations in Iran. In that study, the incidence rate of CE was estimated at 1.27/100,000 population, and the number of asymptomatic people with CE infection living in the country was estimated at 635,232 (16).
Another hospital-based study reported 318 CE cases (183 females and 135 males) in Tabriz, East Azerbaijan (Northwest Iran), over a 10-year period (2001 to 2012), showing a more frequent infection in females (35). Also, in a retrospective hospital-based study, 294 cases were reported in Urmia, West Azerbaijan, with an average of 29.4 cases of CE per year leading to surgical operations and an incidence rate of 0.98/100,000 population (36), indicating an increasing trend in recent years compared to that in a previous study in this city (37).
Other hospital-based studies indicated an increase in surgical operations of CE in Hamadan, Zanjan, and Kermanshah provinces in Iran. In Hamadan Province, a total of 55 cases were reported between 1982 and 1992 (10 years) (38), whereas 179 cases were reported between 1992 and 2006 (14 years), with an annual incidence rate of surgical operations estimated at 1.33 cases/100,000 population. Housewives and farmers were at increased risk of contracting the disease (39). In Zanjan Province, a total of 56 operated CE cases were reported between 1984 and 1993. Based on the data presented in the mentioned study, there was a constant trend in the incidence of CE in Zanjan until 1991. However, from 1992 to 1993, an increase of CE numbers was noted (40). Moreover, 12 operated cases per year (0.75/100,000) were reported in Kermanshah during 1986 to 1993, whereas between 2003 and 2008, 48.5 cases per year were documented in medical records (2.55/100,000) (41).
Northeastern Iran is considered a focus of CE endemicity, with an incidence rate of up to 3.27 cases per 100,000 individuals, as highlighted by a study on the spatial analysis of surgically managed CE cases (42) as well as other reports (38, 43). In the southern province of Fars, 501 surgical cases of CE were studied in two major referral hospitals in Shiraz over a 15-year period (2004 to 2018), and an estimated incidence of 0.74/100,000 population was reported (44).
Overall, it is not clear if the rising CE numbers are due to increasing infection risk or to improvements of facilities (e.g., documentation, health facilities, and new diagnostic techniques) leading to better and more frequent diagnosis of CE in the country.
The true prevalence and incidence of CE may be even higher than reported, since many asymptomatic cases are never diagnosed, leading to underestimation. This is confirmed by frequent previous reports of incidental diagnosis of CE during imaging screening. Therefore, the use of community-based ultrasonography and seroepidemiological studies in mass population screening can be valuable in determining the true situation of CE.
The national CE surveillance system of the Ministry of Health and Medical Education collected data on CE in Iran from 1995 to 2014, in which a total of 8,518 CE cases were included. The incidence rate was lowest in 2007 (0.35/100,000), whereas the highest rate was registered in 2011, with 0.88/100,000 (45). These data indicate the significant burden of CE on the Iranian health system. It should be noted that the surveillance system for zoonotic diseases is usually hampered by underreporting. Surveillance programs are intensified at certain periods of time in the country, with health authorities exerting pressure on public hospitals to report data, but in other periods, data may not be appropriately reported when this pressure is relaxed.
Extreme caution should be made on using serology as the sole diagnostic test for the surveillance of human populations (46). Several seroepidemiological studies have been undertaken in Iran (seroprevalence rates ranged from 1% to 21.4%); however, because of the unreliability of serology, the results are not interpretable in terms of the prevalence of the disease in the population (19, 47–51). Serological tests suffer from very poor sensitivity and specificity; hence, the predictive values are extremely low. Seroprevalence may be affected by various factors, including cyst stage, cyst localization, the number and size of the cysts, parasite genotype and other endemic diseases in the region, type of serological method, and antigens used in the tests (52–55).
Primary serological screening has not been considered a robust strategy for the serodiagnosis of CE due to several pitfalls related to sensitivity and specificity (46, 53). Imaging workup including ultrasonography and other imaging techniques such as computed tomography (CT) scan and magnetic resonance imaging (MRI) (56) along with the confirmative use of the serological assessment can be potentially capable of establishing a reliable diagnosis for CE; however, extreme caution should be made in the interpretation of the results from subjects who are negative on imaging but are seropositive (52, 57). The use of the WHO’s international ultrasonography classification of stage-specific cystic images is highly recommended for CE diagnosis (58).
The community prevalence of ultrasound-diagnosed CE has been estimated to be 0.2% to 1.8% in Iran, compared to the annual surgical incidence of 1.27 per 100,000. Asymptomatic and/or non-health care-seeking individuals may be underestimated in Iran. The estimation may depend on limited accessibility to medical imaging centers and/or health-seeking behavior of individuals (16) as well as probable differences in pathogenicity or infectivity among E. granulosus sensu lato species and genotypes (59).
Ultrasound prevalence of CE has been estimated to be 1.8% for nomadic tribes of Fars in southern Iran (60) and 0.2% of 1,140 subjects for rural communities of Kerman (19). In this regard, regular community-based ultrasound surveys are needed in Iran to obtain more accurate estimation of CE status and to provide sound epidemiological and surveillance data. However, it should be taken into consideration that ultrasound is highly sensitive to detect abdominal CE, but extra-abdominal lesions, e.g., thoracic cysts, could be overlooked in such surveys. In addition, it is difficult to detect very small cysts in the early stages by ultrasound (53). Nonetheless, mass population screening incorporating community-based ultrasound with serology is considered suitable for providing solid background data of the CE status in the population (19).
Detailed epidemiological information on CE in children is limited. Current evidence indicates that the disease is relatively frequent in children in Iran. From March 1996 to March 2010, 100 CE patients under 14 years of age were referred to Mofid Children’s Hospital in Tehran (61). Furthermore, 31 CE patients were referred to the Children’s Medical Center Hospital in Tehran between 1995 and 2005 (62). Also, 40 CE patients under the age of 15 were hospitalized in Imam Khomeini medical center in Ahwaz between 1994 and 2000 (63). Twenty-three CE cases were also reported over a period of 5 years (2001 to 2006) from Tabriz Children’s hospital in the eastern Azarbaijan province in the northwest of Iran. Between 2001 and 2011, 59 CE cases among children were reported from Tabriz Children Hospital, with most of the patients being residents of East Azerbaijan and Ardabil provinces (64, 65). Sixteen pediatric patients were reported in a recent study from a pediatric hospital in Babol, northern Iran. A high frequency of multiorgan involvement, including the involvement of liver, lung, spleen, kidney, and pelvis, was noted in 5 of 16 children (31%) (66). The total number of pediatric CE cases is likely underreported. The incidence of CE in children is indicative of active transmission cycles of the parasite, and it demonstrates the success of CE control programs in regions of endemicity.
Molecular Epidemiology of CE in Humans in Iran
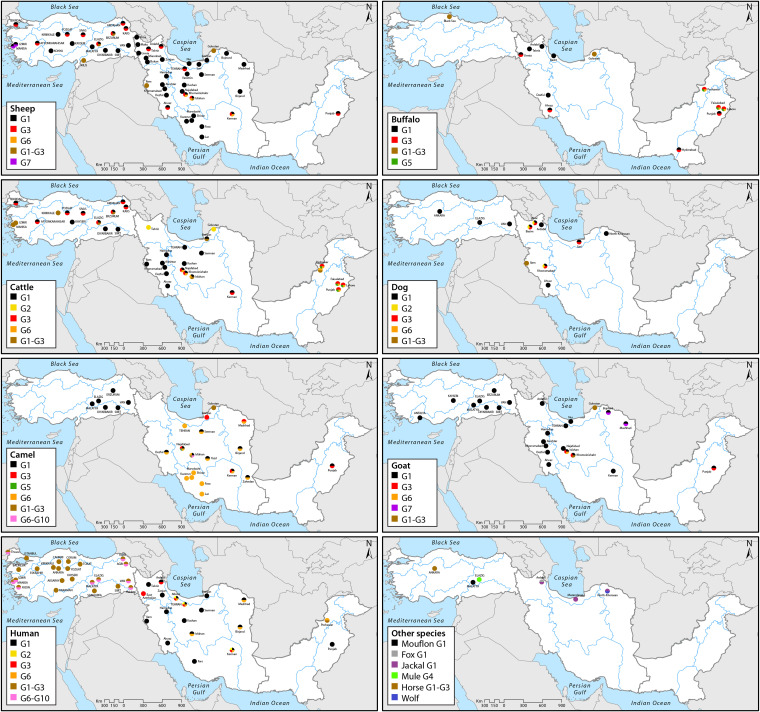
The characteristics of human CE genotypes have been summarized in Fig. 2. E. granulosus sensu stricto (G1 to G3) has been reported as the most frequent species responsible for human CE in Iran (67–76). The first molecular report in Iran on E. granulosus genotypes in 1998 by Zhang et al. confirmed the isolation of the G1 genotype from all four examined human samples (77). The G1 genotype is the most common genotype responsible for human CE cases across Iran. Recently, E. granulosus sensu stricto (G2 genotype) was reported in one human CE isolate in Kerman Province in the southeastern part of Iran for the first time (78), but this genotype is now considered a microvariant of G3 (79). Human infections with the G3 genotype (buffalo strain) were first reported by Pezeshki et al. in Ardabil Province in northwest Iran. This genotype was responsible for 2 of 9 isolates of human CE cases (72). Moreover, Nikmanesh et al. reported CE infections caused by the G3 genotype in 3 human isolates (out of 30 isolates, 10%) from patients undergoing surgery in two hospitals in Tehran during 2010 to 2012 (80). Five of 125 formalin-fixed paraffin-embedded human tissues were identified as having G3 in Iran (78). G1 and G3 genotypes have been found to contribute to the transmission cycle of CE in neighboring areas, East Azerbaijan Province of Iran and Van Province of Turkey (81).
FIG 2.
Distributions of the genotypes of E. granulosus sensu lato in human, livestock, and carnivorous species in Iran, Pakistan, and Turkey based on molecular identification (for more details, please refer to the main text).
According to Alvarez Rojas et al., more than 11% of human CE cases worldwide have been attributed to G6/G7 genotypes (82). The G6 genotype of E. intermedius was previously reported as the second most common genotype responsible for 9.1% to 88.9% of human CE in Iran (68, 83, 84), and it is emerging as a significant genotype worldwide. Rostami et al. reported higher prevalence of the G6 genotype in Iran from pathology specimens of 125 CE patients (40.8%) in comparison with previous studies in Iran (78). In the studies conducted in southeastern areas of the country, the G6 genotype was found in 8/9 (88%) and 24/42 (57%) of human samples in South Khorasan and Kerman provinces, respectively (83, 84). This may indicate probable differences of the genotypes’ infectivity or pathogenicity to humans, potential host-related genetic characteristics, possible differences in fecundity and resistance among Echinococcus species/genotypes to the environmental conditions, and abundance of camels more adapted to the genotypes perpetuating in those areas (84).
Although the two provinces are considered important areas for camel breeding, sheep breeding is also relatively common in the area (83, 85). Sadjjadi et al. analyzed 8 specimens of human cerebral CE, and all isolates were found to be the G6 genotype, whereas all eight isolates of hepatic cysts were identified as the G1 genotype (86). The authors suggested that the G6 genotype may have a particular tendency to settle in the brain. Further investigations are needed for a better understanding of the probable affinity of the G6 genotype to the central nervous system (CNS).
Risk Factors for Human CE
Risk factor analysis identified several factors influencing human CE epidemiology in Iran. It should be noted that no sound experimental evidence has been generated in recent time to document specific routes of transmission of echinococcosis to people living in rural and urban areas. Current data have been mostly derived from epidemiological and/or exposure analyses performed in regions of endemicity. Figure 3 illustrates the situations in which CE transmission has occurred in the three countries. The results of a recent meta-analysis support the hypothesis that dog contact, drinking contaminated water, and, probably, food are major routes of CE transmission in areas of endemicity (2). However, the evidence for the comparative significance of food and water to CE transmission is weak, and further in-depth studies are required on this topic. Free-roaming dogs could contaminate surface water as well as farm produce intended for human consumption. Therefore, eating contaminated food and raw vegetables can be one of the major risk factors of human CE in Iran. A global multicriteria-based ranking of foodborne parasites conducted by the Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO) ranked E. granulosus as second among the list of 24 food-transmitted parasites, confirming the importance of foods and vegetables in the transmission of echinococcoses (87). Contaminated water has been recently considered an important risk factor of echinococcosis and is suspected to be one of the significant routes of transmission (88, 89). However, little information is available regarding the presence of E. granulosus eggs in water, and more environmental studies are required on this topic in the region. In the northern province of Mazandaran, 2 of 989 water samples (0.2%) were found contaminated with taeniid eggs by using conventional parasitological techniques (90).
FIG 3.
Epidemiology of cystic echinococcosis in Iran, Pakistan, and Turkey. (A) Uncontrolled population of free-roaming dogs in urban areas in Iran. (B) Unregulated abattoir in Turkey. (C) Home slaughter butcher’s shop and stray dog in Pakistan. (D) Free-roaming dogs feeding on a dead livestock body left around an abattoir in Iran. (E and F) Traditional animal husbandry in the region: a shepherd with his dogs and children herding livestock with a sheep dog in Iran. (G) Unfenced vegetable fields in Pakistan and the dogs roaming freely in the farms. (H) Community-based ultrasound survey on cystic echinococcosis in Iran.
Several studies have been conducted in Iran on the presence of taeniid eggs in vegetables by using microscopic techniques; however, no molecular evidence has been presented to document E. granulosus eggs in vegetables. Findings of a recent study performed in northwestern Iran revealed that 7.8% of 2,757 vegetable samples were contaminated with taeniid eggs, and lettuce was found as the most contaminated vegetable species. Interestingly, the frequency of taeniid eggs on the vegetable samples grown in unfenced fields was 2.7 times more than that of those grown in fenced vegetable fields (91). In the southern city of Shiraz, taeniid eggs were detected in 2.5% (2/80) and 4.1% (6/144) of vegetable samples collected from markets and farms, respectively (92). In Qazvin Province, 1.8% of 218 vegetable samples were found contaminated with Taenia/Echinococcus eggs (93).
Frequent human contact with dogs or sheep has been reported in many studies in Iran. The presence of sheep in the vicinity of a place where there are infected dogs is an important factor for the contamination of sheep wool with parasite eggs. Sheep shearing by nomads and sheep markets in rural areas could potentially result in human infection (38). However, it should be noted that no experimentally sound evidence has been available to document specific routes of CE transmission. Current data have been mostly derived from epidemiological and/or exposure analyses performed in regions of endemicity. Echinococcus/Taenia eggs have been frequently recovered from soil/dirt, dog feces, and vegetables (91); therefore, it is usually believed that the CE transmission is most probably initiated from these sources. Studies have shown a higher prevalence rate of CE in females than in males (1.05:1.0 to 3.0:1.0). This may be explained by the fact that housewives, particularly in rural areas, have more frequent contacts with potential infection sources due to their activities such as washing vegetables, animal care, milking, and cleaning the home environment and the yard. Women have a key role in crop and livestock sectors in the region. The female contribution in the agricultural labor force in the Middle East has increased from 34% during 1990 to 1995% to 45% in 2011 (94, 95). In Pakistan, 80% of milk production activities at small farms are conducted by rural women (96). Geophagy, the tendency to eat soil by pregnant women, especially in rural areas, may also be responsible for some cases (38). However, the significance of gender difference has been variable in different regions, and we should be cautious about the significance of higher CE frequency in women due to several factors, particularly the gender selection bias in sampling (7, 19). The disease is more prevalent in the 20- to 40-years age group; however, it should be noted that the disease is chronic, and it may take a long time for a cyst to grow to a symptomatic stage. Also, the fact that 20- to 40-year-old women might be more frequently tested by ultrasound for pregnancy could partially explain the age and gender distribution of CE in the region.
People living in urban areas of Iran are more likely to present as CE cases to health centers. It is believed that CE is gradually being urbanized, and it can therefore no longer be regarded as a rural disease (16). Urban areas in Iran have challenges with the free-roaming dog population. It has been estimated that the total dog population in Iran is approximately 3.5 to 11.5 million dogs; however, no official data and/or specific studies are available in the country (19). As in many developing countries, municipal abattoirs are usually substandard, and home slaughter is prevailing (13). Moreover, the increase in outdoor activities such as camping and recreational activities of the people living in the cities has also contributed to the increasing rate of CE in urban areas. The changing population demography over the last 3 decades and migrations of rural people to the urban and periurban areas may contribute to the apparent rise of urban CE cases (16, 39, 97). Therefore, a large population of free-roaming dogs, low-quality substandard abattoirs, unlicensed/unregulated abattoirs, practice of home slaughter, increased outdoor activities, and migration to urban regions are among the major factors of CE prevalence in urban areas.
CE IN LIVESTOCK SPECIES IN IRAN
In Iran, all ungulate livestock species can act as intermediate hosts of E. granulosus, including sheep, goats, cattle, buffaloes, camels, and donkeys. Moreover, Echinococcus cysts have been described in wild ruminants, including wild sheep (Ovis orientalis) and goitered gazelle (Gazella subgutturosa) (22).
Prevalence of CE in Livestock Species in Iran
Livestock CE is widespread in many areas of Iran; however, in some areas of the country, epidemiological data are not available, inadequate, and/or in need of updating. A substantial amount of information has come from retrospective abattoir-based studies (postmortem examinations). We tried to use confirmed data obtained from research studies for estimating the prevalence of CE, because official data reported by the abattoirs are not considered reliable, as described before (29). False-negative diagnosis may be made by overlooking small intraparenchymal cysts (98). On the other hand, there may be false-positive results due to unspecific granulomas, pseudotuberculosis, emphysema, abscesses, fatty degeneration, and cysticerus tenuicollis (Taenia hydatigena) that could be mistaken as CE (29, 99, 100).
It should be taken into account that the prevalence of CE tends to be underestimated in slaughterhouse-based surveys. Given the low rate of cyst growth and the marked age dependency of prevalence in livestock, preferred slaughtering of young animal in abattoirs will result in a gross underestimate of the true prevalence. Such can only be achieved if data from abattoir studies are age stratified (101). Further age-specific studies on CE in livestock are required in Iran as well as other countries.
The origin of livestock arriving at the slaughterhouse should also be considered in this regard, particularly when the catchment region of the abattoir includes both high- and low-prevalence areas.
Differences in the prevalence of CE in livestock species based on abattoir studies may result from a number of factors such as the differences in geographical areas, different parasite species/genotypes existing in the livestock (102), animal husbandry systems such as traditional free grazing versus commercial animal production, differences in infection pressure and transmission dynamics (103), the sociocultural behavior of livestock owners, attitude toward dogs (104, 105), and environmental factors that are involved in the perpetuation of the parasite (106). Furthermore, it has been reported that seasonal difference in the prevalence of livestock CE may be associated with differences in the age of animals sent to the abattoir (104, 107). In fact, seasonal patterns of CE prevalence among livestock cannot be accurately determined if the abattoir studies are not age stratified, but any reports of such seasonal fluctuations are likely a result of sampling bias given the long development time of hydatid cysts (108).
Prevalence studies of CE in livestock in Iran since 1994 have indicated that sheep have high rates of infection in most regions compared to that for other livestock. Based on available data, the prevalence of livestock CE in Iran ranges from 1.3% to 74.4% in sheep, 0.4% to 37.8% in goats, 4.3% to 31.9% in buffaloes, 1.3% to 40.1% in cattle, 8.8% to 40.4% in camels, and 2% in donkeys (13, 27, 109, 110).
There is evidence of a higher prevalence rate of CE among livestock in the northwestern regions of Iran than in semidesert zones of the central and eastern regions (104, 107, 111–114).
According to existing data, sheep are the key intermediate hosts in the transmission of CE in most parts of Iran, with fertility rates that may exceed 88% (38). Sheep are mostly infected with E. granulosus sensu stricto according to genotyping studies. Camels with fertility rates ranging from 67% to 70% are also important intermediate hosts (115, 116) as well as goats with fertility rates ranging from 61% to 66% (115, 117). Based on the data in the literature, cysts of cattle and buffaloes have markedly lower fertility than those of other livestock species in Iran (38, 116, 118, 119). This can be linked to different host infectivity of different Echinococcus species and genotypes but also to different age at slaughter (e.g., camels versus sheep), exposure of the offal or carcasses to dogs (animal husbandry), and possibly the livestock breeds (5).
Molecular Epidemiology of CE in Livestock in Iran
The various species and genotypes of E. granulosus sensu lato show considerable differences of adaptation to different livestock hosts (29). To assess the genetic diversity of E. granulosus sensu lato among livestock species in Iran, data on molecular epidemiological surveys that have been conducted to date have been summarized in Fig. 2. Evidence shows that the two species that most frequently infect livestock in Iran are E. granulosus sensu stricto (G1 and G3 genotypes) and E. intermedius (E. canadensis; G6 genotype).
There is ample evidence for the presence of the dog-sheep cycle of CE in most regions of Iran with the G1 genotype as the most prevalent variant. The coexistence of E. granulosus sensu stricto and E. intermedius in livestock has also been documented. The first reports of the G2 genotype (now considered a variant of G3) were documented from goat in Isfahan Province (120). The first confirmation of the G2 genotype in cattle was reported from Golestan Province, Iran (121). The G3 genotype was isolated from camel in the central part of Iran (122). Other studies found the G3 genotype in buffaloes, cattle, sheep, and camels originating from different regions of the country (123). However, this genotype was not found in buffaloes in southwestern Iran (72, 115, 121, 124), although it is known to occur in buffaloes in the east and southeastern parts of Turkey (125), which share borders with the West Azerbaijan province of Iran.
A study reported the isolation of E. granulosus sensu stricto from Iranian wild sheep (Ovis orientalis) from the Khojir National Park near Tehran, indicating the probability of interaction between the domestic and wild cycles of the parasite (126).
The presence of G6 genotype of E. intermedius has been confirmed in several studies in Iran, and current evidence demonstrated the presence of this parasite cycle in sheep, goats, cattle, and camels. It is particularly well adapted to camels and, possibly, goats but seems to have a secondary role in the transmission via sheep (16, 68).
Coexistence of G1 and G3 was found in sheep in Iran (127). Moreover, the G1 and G3 genotypes have been isolated from infected goats in Iran in the ranges of 63% to 100% and 0% to 25% of isolates, respectively. A previous study reported E. intermedius with a G6 genotype frequency of 12% in infected goats (128). Furthermore, there was evidence of the presence of the E. intermedius G7 genotype in six isolates of goats in the northeastern province of North Khorasan in Iran (129). Further research will be needed to confirm the presence of the G7 genotype in animals and humans in the country. In any case, both genotypes are closely related and can be considered microvariants (130, 131).
According to molecular epidemiological studies, E. granulosus sensu stricto (both G1 and G3 genotypes) is considered a major cause of bovine CE in Iran. Approximately 64% to 100% of bovine CE cases were caused by the G1 genotype, whereas the G3 genotype was responsible for 5% to 28% of the infections. The presence of E. intermedius (G6 genotype) has also been documented among cattle in Iran, with a genotype frequency of 6% to 36% (68, 70). In Iranian buffaloes, E. granulosus sensu stricto has frequently been reported with prevalence rates of 92% to 100% and 8% for G1 and G3 genotypes, respectively (124, 132). In camels, both the G1 and G3 genotypes of E. granulosus sensu stricto were detected with frequencies of 25% to 88% and 22%, respectively. It has been demonstrated that 12% to 100% of camels are infected by other species of Echinococcus, including E. intermedius (G6 genotype) (132, 133).
Echinococcus ortleppi (G5 genotype) was recently reported from Iran; however, the dynamics of the parasite transmission are unknown. Molecular studies are required to confirm the transmission of this genotype between different intermediate (e.g., cattle, camel, and human) and definitive hosts. G5 presence in Iran is based on two studies reporting E. ortleppi from a camel in central Iran (134) and from 5 of 45 cattle from the northern province of Gilan (135). A recent study from Mazandaran Province indicates the dominance of the G1-G3 genotype in sheep and cattle in Caspian littoral regions (136). Evidence indicates that, like humans, cattle and camels are capable of harboring multiple genotypes, suggesting their importance for maintaining the CE life cycle in various parts of the country, with camels being largely restricted to the central, southeastern, and eastern arid ecosystems (84).
E. GRANULOSUS SENSU LATO OF CARNIVORES IN IRAN
E. granulosus sensu lato has been isolated from dogs as well as wild carnivores, including wolf, jackal, and red fox, indicating the presence of both domestic and wild cycles in Iran (23, 24, 137–139).
Echinococcus granulosus sensu lato in definitive hosts has been reported in various parts of Iran. However, the coverage and quality of data differ across Iran, and in some parts of the country, such data do not exist, are inadequate, or need updating. More surveys are required to focus on the biological and ecological aspects as well as the epidemiological and geographical patterns of E. granulosus sensu lato in Iran.
Based on data available, domestic dogs are by far the most important definitive hosts both by population size and prevalence. The dog population in Iran has been estimated to range from 3.5 to 11.5 million, of which 70% to 90% were stray dogs (19). Reported prevalence estimates range from approximately 7% to 64% in stray dogs and 3% to 63% in owned dogs, depending on the geographical area and the type of specimen examined. Unless stated otherwise, the following data were determined by necropsy and visual inspection of worms.
In the northern Caspian coastal provinces, the prevalence of canine echinococcosis was reported to be 21.1% to 46.7% in Mazandaran Province and 25% in Gilan province (140–144).
A comprehensive study conducted in 13 provinces of Iran reported 27.2% prevalence in sheep dogs. The percentage of infection was between 3.3% in Sistan, southeast Iran, and 63.3% in Isfahan, central Iran (142). Also, the number of worms detected in each sample varied extensively from a single worm to approximately 2,000 worms; however, most dogs harbored fewer than 50 worms (142). In five western provinces of Iran (West Azarbaijan, Kordestan, Kermanshah, Ilam, and Lorestan), the prevalence of E. granulosus sensu lato was reported at 9% to 44%, classified as medium to high prevalence (24, 28, 48, 145, 146); worm burden was determined to range from 3 to 2,000 worms (24).
In the northwestern province of East Azerbaijan, 20% of stray dogs were found to be infected in four different areas, including Ahar, Basmenj, Anakhatoun, and Sarizamin (147). Using fecal samples for PCR (copro-PCR), a prevalence of 23.7% was found in domestic dogs of Moghan Plain in the northwestern province of Ardabil (138). In this region, Zare-Bidaki et al. also estimated the prevalence in owned dogs at 27.1% based on copro-antigen enzyme-linked immunosorbent assay (ELISA) (148).
In the northeastern part of the country, depending on the technique used, the prevalence of E. granulosus sensu lato in dogs in different counties was as follows: Chenaran, 17% to 21.6%; Mashhad, 22% to 38%; and Bojnurd, 62% (23, 149–151). In the study conducted in Mashhad, 81% of infected dogs harbored 1 to 100 worms, 13% had 100 to 1,000 worms, and heavy worm burdens (>1,000 worms) were reported in 4.5% of infected dogs (151). In the central regions of the country, available prevalence estimates are 12.3% to 63.3% in Isfahan and 55.7% in Kashan (113, 142, 152). In the southern parts of the country, studies resulted in prevalences of 34.4% to 36.2% in Shiraz, 6.6% to 6.8% in Kerman, and 4.2% to 20% in Khuzestan (142, 153–157).
Some studies conducted in Iran have indicated that there is no association between prevalence rates and age and sex of the dogs (151, 158); however, these findings need to be interpreted cautiously because of the small sample size.
There are few studies on wild carnivore species as hosts of E. granulosus sensu lato and E. multilocularis. Data have to be regarded as preliminary due to small sample sizes and limited geographical coverage in most of the studies. Beiromvand et al. (23), using a necropsy method, found mixed infections with both E. multilocularis and E. granulosus sensu lato in two golden jackals (Canis aureus) and one wolf (Canis lupus) and infection with only E. multilocularis in three jackals in Chenaran, Khorasan Razavi Province, in northeastern Iran. These results indicate the sympatric transmission of different Echinococcus species by wild animals in the region. In the same area, E. granulosus sensu lato was detected in fecal specimens of 16.9% of 77 dogs and 66.7% of nine jackals as well as all of three red foxes (Vulpes vulpes), one wolf, and one hyena (Hyaena hyaena) (23).
Echinococcus multilocularis was first reported in the early 1970s in 10% of the red foxes of Moghan plain of the northwestern parts of the country, where both E. granulosus sensu lato and E. multilocularis are endemic in carnivores (139). Further studies revealed prevalence rates of 22.9% in red foxes and 16% in jackals (159). Using specific copro-PCR, Mobedi et al. found E. granulosus sensu lato infection in 10 (11.9%) red foxes and a golden jackal in Moghan Plain in northwestern Iran (138). However, no evidence of E. multilocularis infection was found in wild carnivores in the study area.
The frequency and intensity of canine echinococcosis may be influenced by a number of factors, including access to uncooked offal, dogs freely roaming in and around slaughterhouses, home slaughtering of livestock, particularly in the rural areas, dog occupation and ownership status, and age and sex of the dogs. Home slaughtering mainly due to traditional activities and religious ceremonies can increase the risk of transmission of infection to dogs. Socioeconomic factors related to dog ownership, including regular anthelmintic treatments of dogs, knowledge of dog owners concerning echinococcosis, and cultural and economic background of dog owners also contribute to the distribution and intensity of the infection (160–163).
Echinococcus granulosus sensu stricto (G1 genotype) appears to be the most common species in canines. A genotyping study in western Iran by Parsa et al. (146) indicated the existence of three E. granulosus sensu stricto genotypes in dogs from Lorestan Province. G1 was the most abundant genotype (75.0%), followed by G3 (15.0%) and G2 (10.0%) (146). Other studies in this province have also reported the presence of the G1 genotype in livestock species (Fig. 2). Different species and genotypes of E. granulosus sensu lato (G1, G3, and G6) have also been found in northwestern Iranian stray dogs (147). Previously, E. granulosus sensu stricto (G1-G3 complex) was found to be the predominant species transmitted in Ilam Province in the west of Iran (145). In the Sothern Caspian Sea, northern Iran, E. granulosus G1 and G3 genotypes have been reported in dogs and G1 in jackals (140). Another study reported G1 genotype from four dogs, two jackals, and one wolf in North Khorasan Province, northeastern Iran (164). It is quite possible to see spillover events between domestic and sylvatic transmission cycles of different genotypes. Further clarification of Echinococcus epidemiology in dogs requires in-depth understanding of the transmission dynamics of different species and genotypes in the country.
ALVEOLAR ECHINOCOCCOSIS AND E. MULTILOCULARIS IN IRAN
Human alveolar echinococcosis (AE) is considered underreported and/or underestimated in Iran, and only sporadic data are available for the disease. Cases of human AE have been reported in the northwestern, southern, and eastern parts Iran. The estimated annual number of AE cases in Iran is 11 cases, but this number should be interpreted with caution. Cases of human AE were first documented between 1948 and 1993 in the northwestern province of Azerbaijan, which involved 37 patients (<1 case per year), including farmers, shepherds, workers, housewives, and hunters (159, 165). Owing to the fact that AE is highly endemic in the neighboring provinces in Turkey, the disease is likely underreported in Iran. Further comprehensive studies are needed to clarify the endemicity status for human AE in Iran.
Recently, some human AE cases were reported from Ardabil, Khorasan Razavi, Khuzestan, and Tehran provinces. The geographical spread of the human cases approximately correlates with the records of E. multilocularis in animals, and so there is substantial likelihood that the human cases are autochthonous.
In Iran, no E. multilocularis metacestode was found in any of the 5,000 examined rodents (I. Mobedi, unpublished) in the course of epidemiological studies conducted in 1971 in the Moghan Plain in the northwestern part of the country (139). In a later study in Ardabil Province performed in 1992, no metacestodes were found in 2,505 rodents examined (159), but adult E. multilocularis organisms were detected in foxes and jackals, indicating an ongoing parasite life cycle in wildlife (145). Metacestodes have been isolated in several small mammal species acting as intermediate hosts in Khorasan Razavi Province, including Microtus transcaspicus (Transcaspian vole), Ochotona rufescens (Afghan pika), Crocidura gmelini, Mus musculus, and Apodemus witherbyi (31). M. transcaspicus had the highest frequency of infection (>40% of trapped Transcaspian voles were infected), suggesting an important role in the life cycle of E. multilocularis in this area. Overwhelming evidence suggests autochthonous transmission of E. multilocularis in the region. Despite the identification of various intermediate host species, far too little data are currently available on the impact of each species in the life cycle. Moreover, the fertility of the metacestodes of E. multilocularis remains unclear. The involvement of other susceptible rodents should not be excluded from investigations due to lack of evidence supporting their role (5).
Moghan Plateau of Ardabil Province is a well-known area of endemicity for E. multilocularis. In the early 1970s, Mobedi and Sadighian reported E. multilocularis in 10% of red foxes in the Moghan Plain in northwestern Iran (139). E. multilocularis was isolated from 22.9% of red foxes and 16% of jackals as well as from two wild cats in Ardabil Province in the northwestern part of Iran (159). Further studies conducted by Mobedi et al. confirmed the presence of E. multilocularis in wild carnivores of Moghan Plain using specific copro-PCR. However, the differences in diagnostic procedures or ecological changes in recent years such as population growth, immigration, establishment of new villages and towns around local rivers, and the construction of new dams, new water reservoirs, and irrigation systems should be noted when comparing old and new data (138).
Beiromvand et al. (23) have reported a focus of E. multilocularis in Khorasan Razavi Province in the northeast of Iran. Infection in five jackals (two of them as coinfections with E. granulosus sensu lato) and a wolf in Chenaran region showed that both parasites may be sympatric in this region (23). In Chenaran, a higher frequency of E. multilocularis was found in wild carnivores (100%) than in domestic and stray dogs (6.5%). The burden of infection was found as high (>1,000 worms) in the jackals and low (1 to 100 worms) in the wolf. Moreover, in a copro-PCR-based investigation, 6.5% of dogs (5/77 dogs) and all the wild carnivores (nine jackals, three foxes, one wolf, and one hyena) involved in the study were found to be infected with E. multilocularis (23). The occurrence of E. multilocularis in wolves and golden jackals in Iran is considered sporadic (23). Another study in North Khorasan Province reported the presence of E. multilocularis by molecular evaluation (4%; eight jackals and two foxes) (164). It is noteworthy that the high prevalence of E. multilocularis in carnivores in Khorasan Razavi Province indicates a potential autochthonous transmission of this parasite, and it is suggestive of its potential risk to human population. Chenaran County is located near the Iran-Turkmenistan border. Fragmentary data on E. multilocularis in Turkmenistan indicate that red foxes, corsac foxes (Vulpes corsac), and golden jackals may be involved in the persistence of the life cycle (166). Therefore, transboundary transmission of E. multilocularis needs to be considered in the epidemiology of AE in the region.
ECHINOCOCCOSES IN PAKISTAN
Transmission of E. granulosus sensu lato between sheep and dogs plays a key role in the domestic life cycle of the parasite in Pakistan. No abattoirs generally exist in the rural areas, and home slaughter of animals is likely to be the major risk factor of CE in the country (18). Due to lack of comprehensive data on echinococcosis, further studies are needed to clarify the importance of this disease in Pakistan.
Human and Animal CE in Pakistan
Human CE has not been extensively investigated in Pakistan. So far, no community-based ultrasound survey has been performed in Pakistan, and only a limited number of hospital-based studies or case reports are available for different provinces of the country, mainly from Khyber Pakhtunkhwa, Punjab, Baluchistan, and Sindh, which are likely to cover only a fraction of the total CE cases in the country (167–180). A 10-year period study by Fatimi et al. (180) reported 49 human pulmonary CE patients in Karachi and Punjab. Refugees from Afghanistan accounted for 30% of these patients; the remaining 70% were Pakistani citizens (180).
A hospital-based study performed in five major metropolitan cities of Pakistan reported 188 surgically confirmed CE patients from 2008 to 2018, with a higher occurrence of the disease in females (61.7%) (181). A total of 198 CE cases were reported by three hospitals in the northeastern region of Lahore in the Punjab province during 2012 to 2017: 41.4% male and 58.6% female (182). A total of 228 cases have been reported from three regions of Islamabad, Rawalpindi, and Peshawar, where most records concern young adults (>20 to 30 years; 22.8%). Farmers (21.9%) and butchers (11.4%) were the most important occupations associated with CE. Occurrence of CE has been attributed to well-known risk factors such as poor sanitation and unhygienic living conditions, rural lifestyle, home slaughtering, and substandard slaughterhouses, which all create a suitable environment for the transmission of CE (183).
In total, 1,611 cases of CE have been recorded in Pakistan between 1990 and 2018. This is assumed to be a gross underestimate of the true situation, and there is need for community-based ultrasound screening, seroepidemiological surveys, and a CE registry system in the region to provide up-to-date knowledge on the socioeconomic burden of the disease in Pakistan (184). There is an urgent need of developing and implementing an effective national surveillance system for monitoring the incidence of CE and AE in Pakistan. Also, improving the hospital information system is essential for conducting large-scale high-quality studies in the future. Latif et al. reported the presence of the G1 genotype of E. granulosus sensu stricto (two human cysts) in the Punjab province of Pakistan using an mt-CO1 gene marker (18) (Fig. 2). Subsequent studies confirmed the dominant presence of E. granulosus sensu stricto (G1-G3) in humans; however, E. intermedius (G6 genotype) was also reported from humans in Pakistan (179, 185, 186). Based on cox1, cytb, and nad1 sequences of 38 human Echinococcus cyst samples, the relative frequencies of E. granulosus sensu stricto, E. canadensis (G6/G7), and E. multilocularis in Punjab Province were 92.0%, 5.3%, and 2.7%, respectively (187). A recent study revealed the high rate of E. granulosus sensu stricto in humans (35 cases), followed by E. canadensis (G6/G7; 2 cases), revealing camel-dog and pig-dog cycle interactions (187).
Between 1989 and 2018, the reported prevalence rates of livestock CE in Pakistan were 2.2% to 15.8% in sheep, 1.7% to 6.2% in goats, 2.4% to 35% in cattle, 5.2% to 24.4% in buffaloes, and 17.3% to 77.5% in camels. Herbivores such as donkeys can contribute to the life cycle in Pakistan (18, 179, 188–200).
In a study conducted in abattoirs of the Punjab area in Pakistan, CE was diagnosed in 17.3% of 590 camels (95% fertility), 7.5% of 15,857 sheep (86.4% fertility), 7.2% of 5,300 buffaloes (84.3% fertility), 5.5% of 15,001 goats (79.1% fertility), and 5.2% of 2,990 cattle (75.2% fertility) (18). A recent study by Mehmood et al. indicated prevalence rate of livestock in seven major cities of Pakistan from 2016 to 2018, where higher prevalence belonged to buffaloes (11.9%) and sheep (11.5%), followed by cattle (8.9%) and goats (3.6%) (195). Like other countries in the region, limited age-specific data are available for livestock CE in Pakistan.
Two genotypes of E. granulosus sensu stricto (G1 and G3) have been identified in goats, camels, and cattle in a district of Punjab (18, 201). The G1 genotype was detected in cattle, buffaloes, sheep, and goats in three districts of Punjab, including Okara, Jhang, and Lahore (201). Another study identified different genotypes in sheep (G1 and G3), goat (G1 and G3), cattle (G1), buffalo (G3), and camel (G1) (18, 202). Findings of a recent study indicate a significant frequency of G3 genotype in sheep (77.1%) and buffaloes (93.3%), whereas most of the goat isolates (65.4%) were identified as the G1 genotype. Considering the large population of buffaloes, it seems the buffalo-dog cycle perpetuates the G3 genotype in Pakistan (202).
The existence of E. granulosus sensu stricto (G1-G3; 16 cattle isolates) and G6 genotype (one cattle isolate) has been confirmed in Khyber Pakhtunkhwa (179). Moreover, the G1-G3 complex has been identified in buffaloes in Hyderabad, Sindh Province, southeastern Pakistan (192). In a recent study in Pakistan, the G3 genotype was reported to be prevalent in bovines (38/60 cattle and buffalo) in Lahore, Faisalabad, and Peshawar, followed by G1 (14/60) and G5 (8/60) (203). Based on current (insufficient) knowledge, E. granulosus sensu stricto is by far the most frequent species of Echinococcus in Pakistan (Fig. 2).
Human and Animal AE in Pakistan
Little is known about human AE in Pakistan. Records of E. multilocularis are limited to human cases. AE incidence is believed to be very low, as the estimated number of AE cases per year is reported as <1 (165). Human AE has been found, based on three isolates, in Khyber Pakhtunkhwa, Pakistan, while a record from cattle in the same area needs confirmation (179). Our knowledge is very limited about the occurrence of AE in the definitive and intermediate hosts in Pakistan. Until recently, no studies had been conducted on animals in Pakistan. A very recent publication indicates the occurrence of E. multilocularis from three foxes (3/68 [4.4%]) in Kaghan and Siran regions of northern Pakistan (204). The first molecular confirmation of human AE has been reported from one patient in Punjab Province (187). To determine the current situation of AE in Pakistan, further comprehensive epidemiological assessments are required.
ECHINOCOCCOSES IN TURKEY
As in most parts of western Asia, the transmission of CE in Turkey is based on the “classical” domestic sheep-dog cycle, where feeding of raw offal to dogs is considered to be the most common way for spreading the parasite (5, 13). In addition, records from wild animals indicate the presence of E. granulosus sensu stricto in wild sheep (Ovis gmelinii anatolica); however, little is known about wild definitive and intermediate hosts involved in the sylvatic cycle of the parasites in Turkey (205).
HUMAN CE IN TURKEY
CE has been regularly reported in different regions of Turkey. Based on the official reports of the Ministry of Health, a total of 52,000 patients (3,257 people/year) underwent surgery for CE between 1990 and 2005 in different geographical regions. The annual incidence of CE in Turkey ranged from 0.8 per 100,000 to 2 per 100,000 (206), with foci of elevated incidence (6.4/100.000) in some parts of the country (207). A total of 14,789 surgical patients were documented between 2001 and 2005, most of whom have been reported from central Anatolia (38.6% of the total number of patients), followed by the Aegean (16.94%), Mediterranean (16.09%), Black Sea (5.70%), and southeastern Anatolian regions (208).
Seroprevalence estimates of human CE range from 2.7% to 14.6% in different regions of Turkey (208–213). However, as mentioned earlier, serological surveys in human populations should be interpreted with caution due to the limitations and pitfalls associated with this type of study. Meaningful results can be obtained in epidemiological studies through using multiple imaging as well as immunodiagnostic techniques (46).
In several recent studies, the prevalence of CE in humans in Turkey was determined using abdominal ultrasound screening. Six population-based studies have been reported in the Eastern and Central Anatolia and Aegean regions of Turkey with limited age and geographical ranges and high endemicity (208, 210, 211, 214–216). In a large-scale ultrasound study in six areas of endemicity in Turkey, hydatid cysts were found in 53 of 8,618 (0.6%) participants (217). The prevalence of CE was found to be 0.3% in a screening survey on children in Manisa Province of Turkey, where 8.9% and 10.1% seroprevalence by ELISA and indirect hemagglutination assay (IHA) had been reported in the same subjects. Other community-based ultrasound studies found 0.2% prevalence among 2,500 primary school children from Elazig, 0.15% prevalence among primary school children of Manisa in 2007, and 0.47% prevalence among 7- to 88-year-old individuals from four villages of Aydin in 2012 (211, 214, 215). A cross-sectional ultrasound study detected abdominal CE in 53 of 8,618 (0.61%; 95% confidence interval [CI], 0.2% to 1.9%) people screened in various rural populations of Turkey from 2014 to 2015, with an age- and sex-adjusted prevalence of 0.59% (95% CI, 0.19% to 1.85%). No lung cysts were diagnosed in 7 individuals who underwent additional chest radiography (217).
Molecular Epidemiology of Human CE in Turkey
As in other regions of endemicity of Western Asia and the Middle East, the most frequent genotype affecting humans in Turkey is the G1 genotype, followed by the G3, G6, and G7 genotypes. Very recently, a single human patient was found infected with the G4 genotype of E. equinus (218).
As summarized in Fig. 2, different DNA-based molecular studies in Turkey have reported E. granulosus sensu stricto (G1 and G3 genotypes) as the dominant species responsible for human CE in Turkey. Utuk et al. reported the G1 genotype infecting humans from Elazig in Eastern Anatolia (219). Another study indicated that all of the human CE cases belonged to genotypes G1-G3 in Istanbul in the Marmara region of northwestern Turkey (220). The presence of the G1/G3 cluster has been confirmed in human formalin-fixed paraffin-embedded tissues in Elazig (221). Moreover, the G1 genotype has been found to affect humans in Edirne in the Thrace region bordering Greece and Bulgaria (222). Echinococcus granulosus sensu stricto was demonstrated in eastern provinces of Turkey among pediatric CE cases (223). Another study in central Anatolia found the majority (12 of 16) of human isolates to belong to the G1 genotype, while three cases belonged to the G3 genotype and the remaining isolate was E. canadensis G7 genotype (224).
The G6 (“camel strain”) and G7 (“pig strain”) genotypes are closely related and often not discriminated in molecular studies. Members of this genotypic cluster were confirmed in human patients as shown in Fig. 2 (209, 221, 222, 225). The G7 genotype has been reported from humans in western Turkey as well as in central Anatolia (209, 222, 224). The occurrence of the G6 genotype was reported from two human isolates (221, 226). Recently, five human cysts of the G6/7 cluster were identified in Aydin Province of the Aegean region (225).
CE OF LIVESTOCK IN TURKEY
Numerous studies reported the prevalence of livestock CE in Turkey over the past years, with overall prevalence estimates in different regions ranging from 3.5% to 58.6% (206, 227–235). Stratified for different animal species, prevalence ranged from 3.5% to 70.9% in sheep, 1.6% to 25.1% in goats, 3% to 46.4% in cattle, and 10.2% to 41.1% in buffaloes. The results of several studies published in Turkish language were also considered here. A wild cycle in Turkish mouflon (Ovis gmelinii anatolica) has been revealed by molecular confirmation (205).
The molecular epidemiology of CE in livestock species is summarized in Fig. 2. A large proportion of genotyping studies, especially in livestock, have shown that E. granulosus sensu stricto (G1 and G3 genotypes) is the dominant species responsible for sheep, cattle, goat, and camel infection in Turkey (125, 209, 219, 222, 226, 233, 236). Echinococcus intermedius (both G6 and G7 genotypes) was found less frequently in sheep (209, 237). Additionally, E. granulosus sensu stricto (G1) and E. equinus (G4) have been confirmed in horses and donkeys (27, 178, 206). As E. equinus is a rather specific parasite of equids, a transmission pattern involving stray dogs and donkey/horse seems to exist in Turkey (26, 238). Further support for the autochthonous transmission of E. equinus in Turkey was presented very recently in the report of a human patient confirmed to be infected by this species in Konya, Inner Anatolia (218).
PREVALENCE AND MOLECULAR EPIDEMIOLOGY OF CANINE ECHINOCOCCOSIS IN TURKEY
Studies in Turkey have reported prevalences of canine infection from 0.8% to 40.5% depending on the geographical region and diagnostic technique (239–244). Copro-antigen ELISA diagnosed echinococcosis in 8.9% of owned dogs in Antakya, a southeastern city near the border with Syria (242).
As shown in Fig. 2, there are few molecular epidemiological studies on canine echinococcosis using different techniques. A fecal prevalence of 14% was found in dogs (owned, guard and sheep dogs) from Ankara in the central Anatolia region based on copro-PCR studies (245). In Aydın Province, one of 100 surveyed dogs was found to be positive (244). Mitochondrial CO1 gene sequence analyses revealed that E. granulosus sensu stricto (G1) is also the dominant species responsible for stray dog infection in the eastern province of Van, where four dogs (40% [4/10]) were found to be infected (246). An isolate from one dog showed the G1 genotype in Elazig in Eastern Anatolia (219).
AE AND E. MULTILOCULARIS IN TURKEY
The central Asian countries and the Caucasus region as well as Turkey are considered areas of endemicity for E. multilocularis and human AE. The first AE case was reported in Turkey by Mutlu in 1939 (240). Since then, a total of approximately 500 human cases of AE have been reported in more than 60 investigations (206, 240, 247–250). Two hundred six cases of AE in Turkey have been reported from eastern, southeastern, and central Anatolia and the Black Sea region. During 1980 to 2000, an incidence of 0.4 cases/100,000 was recorded in southeastern Anatolia, including Diyarbakır, Sanliurfa, and Batman provinces (248). In addition, 162 human cases of AE were reported between 2000 and 2010, mostly from eastern and southeastern Anatolia (250), especially Erzurum Province (83 cases) (250).
Between 1994 and 1999, 219 cases of intracranial echinococcosis were documented in Turkey (251), of which 16 were found to be AE (two cases had CNS involvement). Another study reported four AE cases with cerebral involvement in eastern Turkey over a period of 27 months (252). Estimates arrive at an annual incidence of at least 100 new hepatic AE cases in Turkey (165). However, further hospital- as well as population-based studies are required to demonstrate the extent of the problem.
In the past 2 decades, E. multilocularis expanded its distribution in Europe, correlated with rising red fox populations, increasing urbanization of foxes, anthropogenic climate changes, a growing human population at risk, and the involvement of domestic dogs (253–258). Most of these developments are observed in Turkey, but hard data are not available. As in most countries of endemicity, human cases of AE in Turkey are likely underreported, but in the absence of reliable surveys, a shift to an increase in AE incidence has not yet been noted. AE has been more frequently diagnosed in females, and this is likely due to the involvement of women in farming activities in the country (240, 259). However, the difference in AE rates between men and women is not significant, and fragmentary data cannot be reasonably assessed because sound epidemiological investigations are absent. Since 1980, AE case series have documented age ranges from 7 to 70 years in Turkey (240, 259, 260). AE has been reported from the liver, but other organs were also involved, including brain, lung, kidney, and pancreas.
Many parts of Turkey are considered endemic for E. multilocularis. Despite the large number of human cases, there are very few data on the infection of animal hosts, including rodents. There is only one study on the infection of rodents with E. multilocularis conducted in Eastern Anatolia (Erzurum). Among 498 trapped small mammals (Microtus spp., Apodemus spp., Mesocricetus spp., and Crocidura spp.), 49 showed suspected lesions on the livers, and 5 Microtus irani exhibited fertile metacestodes of E. multilocularis, with a prevalence of 1.3% via molecular assessment (261). No further data exist from rodents; however, AE has been reported in a wild boar from Bingöl Province in eastern Anatolia (262).
Only one infected fox has been reported from the European part of the country, in 1963, based on microscopic identification (263). A very recent study performed in 20 counties of Erzurum, Eastern Anatolia, found E. multilocularis infection in 42% of foxes (21/50 carcasses) using sedimentation and counting techniques. Also in this study, 63 of 600 fox fecal samples (10.5%) were positive for E. multilocularis using a sequential sieving and flotation method followed by PCR sequencing (264). Another recent study reported E. multilocularis infection in 8 of 405 (1.9%) fecal samples from red foxes in the central Anatolia (Kayseri and Nevşehir) and the European parts of the Marmara region (Kırklareli, Edirne, and Tekirdağ) using flotation and sieving followed by multiplex PCR (265). In the Eastern Anatolian province of Erzurum, E. multilocularis infection was found in 1 of 10 red foxes based on microscopy and molecular confirmation (266). More recently, a Eurasian lynx (Lynx lynx) was found infected with E. multilocularis in Erzurum, where the largest number of human AE has been reported (267). Some foxes have infiltrated into the cities, mostly feeding on foods in household waste or rodents. This presents a potential risk of AE transmission in urban areas (255). Further in-depth studies on the distribution of infection in definitive and intermediate hosts are required in this region and other parts of the country. It is yet unclear whether wolves, jackals, dogs, and other definitive hosts contribute to the life cycle or the parasite cycle depends on foxes as definitive hosts in most parts of the parasite’s geographical range in the Northern Hemisphere (5).
BURDEN OF CE IN IRAN, PAKISTAN, AND TURKEY
Several studies on the monetary cost of human and animal CE have been conducted in Iran, Turkey, and Pakistan. However, disability-adjusted life years (DALY) as a well-recognized index of the nonmonetary burden of CE has not yet been calculated in these countries. Global DALYs for echinococcoses have been estimated at 871,000 by the WHO Foodborne Disease Burden Epidemiology Reference Group (FERG) (CE, 183,573 DALYs; AE, 687,823 DALYs). The far higher DALY for AE (despite the lower number of cases) results from the severity of the disease (higher mortality and fewer treatment options), particularly in regions with less-developed medical systems (268, 269).
The estimated average annual cost of CE in Iran is more than $236.7 million per year (both direct and indirect costs). The annual cost of CE in livestock is estimated at $138.1 million, including direct ($23.7 million) and indirect costs ($114.4 million). Human CE is estimated to cost more than $98.6 million (16). Between 2000 and 2009, the average annual number of surgical CE operations was estimated as 1,295 cases, although, in the absence of a reliable surveillance system at the time, this number is certainly an underestimate. Productivity loss for asymptomatic/non-health care-seeking people was estimated to be approximately $100 million, based on two community-based ultrasound investigations conducted by Saberi-Firouzi et al. (60) and Harandi et al. (16, 19), with an estimated number of 635,000 asymptomatic cases in the country. The average cost of surgical treatment of a patient with hepatic or pulmonary echinococcosis in a public hospital in Iran has been estimated at $1,027 (16).
In ruminants, the total annual economic loss due to CE has been estimated as $459,660 in a municipal abattoir of the southwestern city of Ahwaz. This was calculated based on the market prices at the time of publication for the direct (cost of the condemned organs) as well as the indirect costs (livestock production losses, e.g., reductions in milk, hide, wool, etc.) incurred by CE in livestock (104, 110). This indicates that CE causes a substantial loss for the animal industry in Iran. In North Khorasan Province, northeastern Iran, a 5-year retrospective study estimated the cost of food lost due to condemnation in livestock at $421,826 between 2005 and 2010, most of which was caused by CE found in carcasses (52.2%) (270). In Khuzestan Province in southwestern Iran, the economic importance of parasite-related condemnation in livestock has been estimated at $1,148,181, and CE was responsible for 29.2% of all economic losses associated with condemnation (271). Such data are valuable for the development of cost-benefit analyses as a basis for the economic rationale of CE control programs.
Economic losses caused by livestock CE are presumably high also in Pakistan. CE-related losses have been estimated at $276 per 100 infected sheep and goats, whereas CE-related losses were estimated at $165 per 100 infected buffaloes, cattle, and camels (18).
No data are available on the monetary cost of human CE in Turkey; therefore, systematic studies on human-related CE costs are required (17). According to Sariözkan and Yalçin (17), there is a considerable economic impact of livestock CE on the Turkish animal industry, with an estimated $89 million (12% to 13% of the market values) annual production loss. Calculated per species of livestock, this amounts to $54 million for sheep, $2.7 million for goats, and $32.4 million for cattle. According to a study conducted on cattle slaughtered in two abattoirs in Erzurum Province between December 2008 and April 2009, the direct economic losses due to hydatid cysts was calculated as TL 3.320 (227).
PROSPECTS FOR CE CONTROL IN THE REGION
Present Status of Control Programs
Despite the implementation of at least 18 echinococcosis control programs in different regions of the world, e.g., Iceland, New Zealand, Tasmania, Uruguay, Cyprus, China, Chile, and Spain, no integrated control program has yet been implemented across Iran, Turkey, and Pakistan, where echinococcoses remain a major health problem in the 21st century (272).
Only one pilot control study has been conducted in rural and urban areas of the city of Kerman in southeastern Iran. The project, run between 1991 and 1994, was mainly based on culling stray dogs and biannual treatment of owned dogs with praziquantel. The mean infection rate of dogs was 5% in 1993 and dropped to 1.5% in 1994, probably due to the impact of the project. Consequently, the rate of CE in slaughtered animals dropped in 1995 compared to that in 1994 (5.4% in April 1994 and 0.5% in March 1995) (273).
Targets, Approaches, and Tools for Implementation of Control Programs
Dog dosing, dog population management, meat inspection, and livestock vaccination.
Targeting the definitive hosts, particularly dogs, can be considered the first line of the control strategies. Praziquantel (PZQ) has been shown to be very effective in the control of E. granulosus sensu lato in owned dogs, stray dogs, and foxes (5 mg/kg body weight, 4 to 8 times per year) (272). There are numerous studies where the dog dosing component of the control program has been evaluated. More recently, a second pillar of control has been proposed: the immunization of intermediate hosts with the EG95 vaccine to reduce the number of cysts available to definitive hosts. While the efficacy of the vaccine and its potential role in the control programs are well documented, further field trials are required in different regions of endemicity of the world (274). Drawbacks of the EG95 livestock vaccine (Providean Hidatil EG95), which is currently provided by Tecnovax Sanidad Animal (Buenos Aires, Argentina), include potential reduced performance of the vaccine for certain E. granulosus genotypes such as G6 (remains to be investigated in livestock vaccination trials) and the relatively high price ($1.80 to $1.90 per dose), which may prevent its application in resource-poor countries (82, 272). An integrated control program consisting of an appropriate combination of PZQ dosing and EG95 livestock vaccination (with free-roaming dog population control where necessary) should be effective in reducing or eliminating CE transmission in different regions of endemicity. Moreover, incorporation of other control strategies, including the improvement of slaughterhouse facilities and practices, control of home slaughter, and educational programs, are also advised (272, 275).
Dog population management (DPM) methods, including catch, neuter, and release (CNR) and culling, have been applied in combination with other strategies such as dog dosing (276, 277). However, ethical concerns have been raised regarding culling. Effective DPM needs cooperation of various organizations such as municipalities, veterinary and health authorities, education stakeholders, and the media. Dog culling as a method of zoonoses prevention used to be carried out in an irregular manner in many parts of the region under review but is now largely abandoned for ethical concerns. Sheltering stray dogs has been suggested but appears to be feasible only in some areas. No vaccine is currently available for prevention of canine echinococcosis.
Slaughterhouses are officially supervised by the veterinary sector. However, some municipal slaughterhouses may not have appropriate facilities for destroying the condemned organs, leading to the offal being available for dogs. Improvement of carcass inspection and slaughter facilities in general are urgently needed as an important component for CE control. Illegal slaughtering, home slaughter practices during cultural and religious events, and lack of knowledge of the disease by animal owners also play key roles in CE transmission and need to be addressed (193). It seems that integrated PZQ dosing, EG95 vaccination, and health education should be considered an appropriate strategy compared to other control options.
Health education and science communication.
School children can be targeted for health education and promotion of knowledge about the disease and its prevention. However, the education approach has not been proven to be effective as a stand-alone measure anywhere (272). It is rather considered to be a complementary activity to win acceptance of the local people for control and prevention activities. Apart from the role of academic institutions in promoting health education, the media, including radio, television, newspapers, and different platforms of social media, can be used to provide substantive insights into the promotion of public health through science communication frameworks. Radio and television are capable of spreading information to a large population at a low cost per individual.
The use of media requires an in-depth understanding of the importance of the One Health approach in the transmission of infectious diseases, including echinococcoses. Unfortunately, the importance of this issue is still not well embraced by the relevant authorities in most countries of high endemicity. It is therefore recommended that governments encourage all relevant authorities to deliver training programs enabling local media to provide public education on zoonotic diseases (278).
To promote community health, interactive health talks can be organized discussing the origin of various diseases and why public health may be at risk (279, 280). Science communication can also be considered in other contexts, including science exhibitions, media production, etc. Crucial changes are required in the care policy and educational system in the context of science communication for raising the scientific literacy of the public. Several drawbacks are associated with the use of social media, including the spreading of inappropriate or inaccurate scientific data and erroneous contents (281).
The Iranian National Parasitology Museum, founded in 2006 and affiliated with the Faculty of Veterinary Medicine, University of Tehran, not only has tried to be involved in academic activities but also is engaged in enhancing science literacy among the general public, especially school students, through the promotion of science communication programs across different disciplines of parasitology (e.g., echinococcosis). Freely available collections of the museum provide people with an opportunity to appreciate nature and to enjoy scientific activities.
Mathematical modeling.
Due to the diverse conditions and complexities of echinococcosis, a one-size-fits-all strategy will not be successful for interrupting transmission in all areas of endemicity. Therefore, mathematical models have attracted the attention of researchers over the last 5 decades. Models are needed to incorporate the effects of a number of factors, including the demographics of the host populations (i.e., age-associated differences such as age prevalence and intensity), seasonality (i.e., intraseasonal variations), density-dependent variations, and spatial aggregation due to overdispersion of the parasite in a specific population as well as natural immunity and the association with disease transmission dynamics (282–290).
Modeling can be applied in targeting parasite transmission at high and low infection pressures, thereby paving the way for targeted and cost-effective control measures. Overall, mathematical models are useful in providing valuable information for predicting infection pressure and its variations in a group of hosts over time (i.e., human and animal) in different spatial contexts by demonstrating the dynamics of echinococcosis transmission. This can lead to the implementation of effective control strategies (284, 291).
To the best of our knowledge, no mathematical models have ever been applied in the three countries under review, although a mathematical model has been developed to explain transmission dynamics and treatment of E. multilocularis in foxes via quantitative approach of Petri net (PN), where forecasting the thresholds and network analysis could be helpfully effective for designing efficient experimental settings under spatial and temporal heterogeneities (292).
One Health approach.
Due to the complexity of CE transmission, control of this zoonosis requires a true One Health (OH) approach in policies, legislation, programs, and research projects for achieving successful control. In-depth understanding of Echinococcus transmission and the achievement of appropriate control need the incorporation of multiple sectors, including public health, veterinary, and agricultural sectors, as well as other disciplines, including epidemiology, ecology, parasitology, geography, economics, modeling, biomedical engineering, anthropology, and sociology (293–295). A high tendency for interdisciplinary and interinstitutional cooperation is required to bring us closer in order to establish an integrated OH approach for the control and surveillance of echinococcoses, especially in the low- and middle-income settings.
Combining academic expertise with experience in the field will be valuable in addressing the challenges of an OH approach through knowledge cocreation as described by Ribeiro et al. (296). The success of this approach depends not only on such efforts but also on providing rapid communication between disciplines, creating interdisciplinary educational programs, and raising the scientific literacy of the public. On the other hand, there may be challenges and friction associated with the inclusion of a wide range of expertise and stakeholders with diverging priorities and interests.
Joint sessions can be held for both health services (veterinary and medical) and the public in an open discussion forum on issues regarding integrated surveillance and monitoring systems for both humans and animal CE. This can be facilitated by forming a national OH commission and/or OH joint steering committee.
Further development of integrated epidemiological studies will require systematic understanding of the hosts, agents, and environmental factors that underlie the transmission of zoonotic infections, including CE, in order to improve control strategies and management of the diseases.
Surveillance.
It is noteworthy that there is a critical challenge for proper surveillance of both echinococcoses (CE and AE), particularly regarding the prevalence and incidence of both human and animal CE as well as canine infection with adult stages. In many developing countries, including Pakistan, Iran, and Turkey, an effective reliable surveillance system in not in place, and it has been shown that official/governmental surveillance data significantly underreport. In Pakistan, no surveillance system is yet available under the umbrella of health authorities, reporting human and animal CE data on a national level. Official surveillance data from Iran are available, but it should be noted that the data suffer from underreporting and lack of sensitivity (297, 298). According to the communicable disease surveillance system in Turkey, most diseases are expected to be notified only by clinicians and hospital staff; however, considerable underreporting of communicable diseases has been documented in Iran and Turkey (297, 298). Appropriate surveillance data would be needed for embarking on programs and evaluating their impacts on a quarterly basis in the beginning and over an annual period during the implementation of control programs (272). In this regard, surgical and treatment data for human CE need to be registered in a standardized and accessible format, and an appropriate national CE registry system can be of great benefit in regions of endemicity. Accordingly, Iran joined the European Register of Cystic Echinococcosis (ERCE) in 2015 (299, 300), and the Ministry of Health and Medical Education supported the Research Center for Hydatid Disease in Iran to establish a national CE registry system. As mentioned earlier, regular and active mass screening surveys using portable ultrasound devices and hospital registry records should be appropriately applied by the health care system to assess the status of CE in areas of endemicity, especially in rural and nomadic/seminomadic communities. This will pave the way for active and effective CE surveillance (301).
For CE surveillance in the definitive hosts, copro-antigen ELISA could be the screening method of choice, with a better ratio between sensitivity and specificity and better applicability than the other techniques such as necropsy (time consuming, high laboratory standard needed, and only applicable to dead animals) and arecoline testing (hazardous and low sensitivity) (101, 285).
Abattoir surveillance of CE in livestock provides valuable data for the assessment of the overall transmission risk and monitoring of a control scheme. Passive livestock CE surveillance constitutes continuous examination of offal in each abattoir, regularly reported to veterinary authorities. However, there are serious concerns on the value of this kind of data, as prevalence data are only useful when accompanied by data on the precise origin of the animals and their age class, which are often not available, e.g., due to unrecorded animal transport across the country. Trained staff and careful supervision are needed for establishing a vigilant reporting system. Therefore, active surveillance through abattoir-based studies provides more reliable information on the dynamics of livestock CE transmission in areas of endemicity (101). However, again, most of the abattoir studies lack age-stratified data, resulting in a substantial underestimation of the actual CE rate in livestock due to decreased CE prevalence at young and marketable ages (101, 285) Therefore, the recording of age groups and comparison of similar age groups at various time points provide more valuable information on CE status (272).
A reliable serological test is not available for livestock animals, and mass ultrasound screening is difficult to perform for diagnosis in sheep and goats in Iran, although its efficacy was previously established in Tunisia and Italy (302, 303). Additionally, sentinel lambs can be screened by this method for detecting continuing transmission of the parasite (304).
Cost-Benefit Analysis of Control Programs
Before any cost-benefit analyses of control strategies can be performed, reliable and comprehensive estimates have to be established of the overall cost of CE, including human CE (e.g., cost of surgery, productivity losses of patients and of undiagnosed cases, etc.) as well as CE in livestock (i.e., direct and indirect costs). Additionally, a complementary analysis of the nonmonetary burden of CE, measured using disability-adjusted life years (DALYs), should enter the calculation.
For policy makers, cost-benefit analyses are useful for choosing appropriate long-term control strategies. The benefits of a specific intervention measure(s) to reduce disease burden (including averted DALYs) can be presented to decision makers, allowing appropriate resource allocation. The averted DALYs linked to the interventions are considered an approach for evaluating the cost effectiveness of control strategies. Therefore, DALYs averted by control programs need to be calculated. (305, 306). Based on the proportional benefit of control measures in Shiqu County, Tibetan Plateau (costs to both public health and agricultural sectors), the cost per averted DALY was estimated at less than $150 (307).
CONCLUDING REMARKS
Echinococcoses have been endemic in the region for a long time, and targeted control programs are required for each area of endemicity. This requires detailed baseline data on different epidemiological settings including human disease as well as infection in the animal hosts. There is much work to be done in the near future to improve our understanding of the epidemiology of echinococcosis in Iran, Pakistan, and Turkey. Establishing an effective and reliable surveillance system and disease registry is a key measure for providing detailed information about the echinococcosis epidemiology in the region. Community-based ultrasound screening of the population living in different areas of endemicity is required to increase our knowledge of the epidemiology and risk factors of human echinococcosis in the region. Improved diagnosis and management of the disease can be achieved through the development of national and international clinical guidelines based on the WHO ultrasound classification of echinococcosis (308).
A major knowledge gap is the lack of hard experimental evidences for different routes of echinococcosis transmission. Food and vegetables, soil, water, and animal body surfaces are among the suspected vehicles for the transmission of echinococcosis; however, very few studies have been performed to fill this important knowledge gap. Despite the remarkable bulk of information available for human and animal echinococcosis from different regions of endemicity, age-specific data have been generally absent from most of the studies. Estimating disability-adjusted life years (DALY) and monetary burden of echinococcosis as well as cost-benefit analyses of different scenarios of control programs are required in each country of endemicity.
Science communications as well as public and professional education (for the general public, physicians, veterinarians, people working in agriculture, dog owners, hunters, children, etc.) are crucial for the successful implementation of any control program. Control measures are multidisciplinary programs and require multi/intersectoral coordination with a clear and comprehensive One Health approach and long-term administrative commitment in Iran, Turkey, and Pakistan.
ACKNOWLEDGMENTS
We thank all the people who have investigated echinococcoses in Turkey, Iran, and Pakistan. They remarkably improved our understanding of the epidemiology of echinococcosis and biology of Echinococcus.
Research reported in this publication was supported by Elite Researcher Grant Committee under award number 958680 from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran.
This study was conducted in collaboration with the Iranian National Parasitology Museum, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran (https://inpm.ut.ac.ir/en/).
We declare no conflicts of interest.
Biographies

Mehdi Borhani, Ph.D., completed his Ph.D. at the Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran. Currently, he is working as a research fellow in the Research Center for Hydatid Disease in Iran (RCHD), Kerman University of Medical Sciences, Kerman, Iran. He previously studied the effects of helminth antigens in a murine model of experimental allergic encephalomyelitis and has participated in field research conditions in slaughterhouses, the veterinary reference lab in the veterinary organization, as well as medical diagnostic laboratories in various clinical settings affiliated with the Iranian Ministry of Health and Medical Education.

Saeid Fathi, Ph.D., completed his Ph.D. in veterinary parasitology at the Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran. He is currently a research fellow at the University of Tehran, working on immunomodulatory peptides of Fasciola in a murine model of multiple sclerosis.

Enayat Darabi, Ph.D., is a Ph.D. graduate of medical parasitology from the School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.

Fatemeh Jalousian, Ph.D., completed her Ph.D. at Tarbiat Modares University. She is currently working as an assistant professor in the Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran. She is experienced in teaching parasitology and has published numerous articles on parasitology.

Sami Simsek, Ph.D., is a Professor of Veterinary Parasitology at the Department of Parasitology, Faculty of Veterinary Medicine, University of Firat, Elazig, Turkey. He has authored more than 140 articles on parasitic zoonoses, including cystic echinococcosis.

Haroon Ahmed, Ph.D., has been working as an associate professor at the Department of Biosciences, COMSATS University Islamabad (CUI), Islamabad, Pakistan, since 2011. He has 8 years of teaching experience. He has authored/coauthored more than 70 publications on the neglected tropical diseases in international journals. His research works are related to zoonotic diseases of public health importance such as echinococcosis, gastrointestinal nematodes, toxoplasmosis, and brucellosis in humans and animals using microscopic, pathological, serological, and molecular techniques. His principal interests are animal sciences, parasitology/microbiology, parasite ecology, vector-borne/neglected tropical diseases, and tropical biomedicine.

Harun Kaya Kesik, Ph.D., received a Bachelor of Veterinary Science (B.V.Sc.) degree from the Faculty of Veterinary Medicine in Firat University, Elazig, Turkey, and a master’s degree (M.V.Sc.) and Ph.D. in Veterinary Parasitology from the Department of Veterinary Parasitology in the University of Firat, Elazig, Turkey. Dr. Kesik is currently working as an Assistant Professor at the Department of Parasitology, Faculty of Veterinary Medicine, Bingol University, Bingol, Turkey. His current research interests focus on the molecular characterization and genotyping of Echinococcus species, molecular phylogeny, gene cloning and expression in helminth parasites, in silico analyses, epidemiology, and control strategies of parasitic infections in livestock.

Seyed Hossein Hosseini, D.V.M., Ph.D., is a Professor of Veterinary Parasitology and past Dean of the Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran. He has nearly 30 years of experience in research and training in parasitology and zoonotic infections, including echinococcosis. He has written extensively on veterinary parasitology, particularly echinococcosis/hydatid diseases.

Thomas Romig, Ph.D., is a Professor in the Parasitology Unit, University of Hohenheim, Stuttgart, Germany. He has more than 30 years of experience of research and training in the field of parasite biology, especially parasitic zoonoses, including echinococcosis and hydatid disease. He has been very active in the research on zoonotic diseases in sub-Saharan Africa. Since 2009, Dr. Romig has been involved in the Cystic Echinococcosis in Sub-Saharan Africa Research Initiative (CESSARi), funded by a German research agency (DFG), to coordinate and focus research capacities in various African countries. Dr. Romig has published more than 130 articles in international peer-reviewed journals.

Majid Fasihi Harandi, M.S.P.H., Ph.D., is a Professor of Medical Parasitology at the School of Medicine, Kerman University of Medical Sciences, Kerman, Iran. He is the founding director of the Research Center for Hydatid Disease in Iran. During the past 25 years, his activities have been focused on research and training in parasitic diseases, particularly echinococcosis and hydatid disease. He has authored more than 100 articles on cystic echinococcosis and other parasitic zoonoses. He is the Asian Secretary of the World Association of Echinococcosis.

Iraj Mobedi, D.V.M., Ph.D., is an Emeritus Professor of Parasitology at the School of Public Health, Tehran University of Medical Sciences, Tehran, Iran. He is the cofounder of the Iranian National Parasitology Museum in the Faculty of Veterinary Medicine, University of Tehran. For more than half a century, Professor Mobedi has trained several generations of parasitologists across the country. During most of his career, Professor Mobedi focused his research activities on the taxonomy and identification of parasitic fauna of animals in Iran. He has authored several books and more than 120 articles in the field of parasitology, with special interest in zoonotic parasitic infections.
REFERENCES
- 1.Thompson RCA. 2017. Biology and systematics of Echinococcus. Adv Parasitol 95:65–109. 10.1016/bs.apar.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Torgerson PR, Robertson LJ, Enemark HL, Foehr J, van der Giessen JWB, Kapel CMO, Klun I, Trevisan C. 2020. Source attribution of human echinococcosis: a systematic review and meta-analysis. PLoS Negl Trop Dis 14:e0008382. 10.1371/journal.pntd.0008382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higuita N, Iván A, Brunetti E, McCloskey C. 2016. Cystic echinococcosis. J Clin Microbiol 54:518–523. 10.1128/JCM.02420-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen H, Vuitton L, Tuxun T, Li J, Vuitton DA, Zhang W, McManus DP. 2019. Echinococcosis: advances in the 21st century. Clin Microbiol Rev 32:e00075-18. 10.1128/CMR.00075-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romig T, Deplazes P, Jenkins D, Giraudoux P, Massolo A, Craig PS, Wassermann M, Takahashi K, De La Rue M. 2017. Ecology and life cycle patterns of Echinococcus species. Adv Parasitol 95:213–314. 10.1016/bs.apar.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Abdulhameed M, Habib I, Al-Azizz S, Robertson I. 2018. Knowledge, awareness and practices regarding cystic echinococcosis among livestock farmers in Basrah Province, Iraq. Vet Sci 5:17. 10.3390/vetsci5010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Possenti A, Manzano-Román R, Sánchez-Ovejero C, Boufana B, La Torre G, Siles-Lucas M, Casulli A. 2016. Potential risk factors associated with human cystic echinococcosis: systematic review and meta-analysis. PLoS Negl Trop Dis 10:e0005114. 10.1371/journal.pntd.0005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McManus DP. 2013. Current status of the genetics and molecular taxonomy of Echinococcus species. Parasitology 140:1617–1623. 10.1017/S0031182013000802. [DOI] [PubMed] [Google Scholar]
- 9.Carmena D, Cardona GA. 2014. Echinococcosis in wild carnivorous species: epidemiology, genotypic diversity, and implications for veterinary public health. Vet Parasitol 202:69–94. 10.1016/j.vetpar.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Thompson RC. 2020. The molecular epidemiology of echinococcus infections. Pathogens 9:453. 10.3390/pathogens9060453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romig T, Ebi D, Wassermann M. 2015. Taxonomy and molecular epidemiology of Echinococcus granulosus sensu lato. Vet Parasitol 213:76–84. 10.1016/j.vetpar.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 12.Chaâbane-Banaoues R, Oudni-M’Rad M, Cabaret J, M’Rad S, Mezhoud H, Babba H. 2015. Infection of dogs with Echinococcus granulosus: causes and consequences in an hyperendemic area. Parasit Vectors 8:231. 10.1186/s13071-015-0832-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deplazes P, Rinaldi L, Alvarez Rojas CA, Torgerson PR, Harandi MF, Romig T, Antolova D, Schurer JM, Lahmar S, Cringoli G, Magambo J, Thompson RCA, Jenkins EJ. 2017. Global distribution of alveolar and cystic echinococcosis. Adv Parasitol 95:315–493. 10.1016/bs.apar.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Yanagida T, Mohammadzadeh T, Kamhawi S, Nakao M, Sadjjadi SM, Hijjawi N, Abdel-Hafez SK, Sako Y, Okamoto M, Ito A. 2012. Genetic polymorphisms of Echinococcus granulosus sensu stricto in the Middle East. Parasitol Int 61:599–603. 10.1016/j.parint.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Zhang Z, Wu W, Shi B, Li J, Zhou X, Wen H, McManus DP. 2015. Epidemiology and control of echinococcosis in central Asia, with particular reference to the People’s Republic of China. Acta Trop 141:235–243. 10.1016/j.actatropica.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Fasihi Harandi M, Budke CM, Rostami S. 2012. The monetary burden of cystic echinococcosis in Iran. PLoS Negl Trop Dis 6:e1915. 10.1371/journal.pntd.0001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarıözkan S, Yalçın C. 2009. Estimating the production losses due to cystic echinococcosis in ruminants in Turkey. Vet Parasitol 163:330–334. 10.1016/j.vetpar.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 18.Latif AA, Tanveer A, Maqbool A, Siddiqi N, Kyaw-Tanner M, Traub RJ. 2010. Morphological and molecular characterisation of Echinococcus granulosus in livestock and humans in Punjab, Pakistan. Vet Parasitol 170:44–49. 10.1016/j.vetpar.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Harandi MF, Moazezi SS, Saba M, Grimm F, Kamyabi H, Sheikhzadeh F, Sharifi I, Deplazes P. 2011. Sonographical and serological survey of human cystic echinococcosis and analysis of risk factors associated with seroconversion in rural communities of Kerman, Iran. Zoonoses Public Health 58:582–588. 10.1111/j.1863-2378.2011.01407.x. [DOI] [PubMed] [Google Scholar]
- 20.Borhani M, Fathi S, Lahmar S, Ahmed H, Abdulhameed MF, Fasihi Harandi M. 2020. Cystic echinococcosis in the Eastern Mediterranean region: neglected and prevailing! PLoS Negl Trop Dis 14:e0008114. 10.1371/journal.pntd.0008114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig PS, McManus DP, Lightowlers MW, Chabalgoity JA, Garcia HH, Gavidia CM, Gilman RH, Gonzalez AE, Lorca M, Naquira C, Nieto A, Schantz PM. 2007. Prevention and control of cystic echinococcosis. Lancet Infect Dis 7:385–394. 10.1016/S1473-3099(07)70134-2. [DOI] [PubMed] [Google Scholar]
- 22.Elsami A, Rahbari S, Meydani M. 1981. Cestodes and trematodes of wild sheep, Ovis ammon orientalis, and goitered gazelle, Gazella subgutturosa, in Iran. Vet Parasitol 8:99–101. 10.1016/0304-4017(81)90023-6. [DOI] [Google Scholar]
- 23.Beiromvand M, Akhlaghi L, Massom SH, Mobedi I, Meamar AR, Oormazdi H, Motevalian A, Razmjou E. 2011. Detection of Echinococcus multilocularis in carnivores in Razavi Khorasan Province, Iran using mitochondrial DNA. PLoS Negl Trop Dis 5:e1376. 10.1371/journal.pntd.0001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalimi A, Motamedi G, Hosseini M, Mohammadian B, Malaki H, Ghamari Z, Far FG. 2002. Echinococcosis/hydatidosis in western Iran. Vet Parasitol 105:161–171. 10.1016/S0304-4017(02)00005-5. [DOI] [PubMed] [Google Scholar]
- 25.Kesik HK, Kilinc SG, Simsek S, Gul A. 2019. Occurrence of liver hydatid cysts in a donkey and molecular characterization of Echinococcus equinus. J Parasitol 105:442–445. 10.1645/19-3. [DOI] [PubMed] [Google Scholar]
- 26.Simsek S, Roinioti E, Eroksuz H. 2015. First report of Echinococcus equinus in a donkey in Turkey. Korean J Parasitol 53:731–735. 10.3347/kjp.2015.53.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eslami A, Shayan P, Bokaei S. 2014. Morphological and genetic characteristics of the liver hydatid cyst of a donkey with Iran origin. Iran J Parasitol 9:302–310. [PMC free article] [PubMed] [Google Scholar]
- 28.Dalimi A, Sattari A, Motamedi G. 2006. A study on intestinal helminthes of dogs, foxes and jackals in the western part of Iran. Vet Parasitol 142:129–133. 10.1016/j.vetpar.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 29.Cardona GA, Carmena D. 2013. A review of the global prevalence, molecular epidemiology and economics of cystic echinococcosis in production animals. Vet Parasitol 192:10–32. 10.1016/j.vetpar.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 30.Mahmoudi S, Mamishi S, Banar M, Pourakbari B, Keshavarz H. 2019. Epidemiology of echinococcosis in Iran: a systematic review and meta-analysis. BMC Infect Dis 19:929. 10.1186/s12879-019-4458-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beiromvand M, Akhlaghi L, Massom S, Meamar A, Darvish J, Razmjou E. 2013. Molecular identification of Echinococcus multilocularis infection in small mammals from northeast, Iran. PLoS Negl Trop Dis 7:e2313. 10.1371/journal.pntd.0002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nourjah N, Sahba GH, Baniardalani M, Chavshin A. 2004. Study of 4850 operated hydatidosis cases in Iran. Southeast Asian J Trop Med Public Health 35:218–222. [Google Scholar]
- 33.Eftekhar M, Athari A. 2007. Five years survey of human hydatidosis in Iran, p 38. Proceedings of the National Congress of Hydatid Cyst, National Congress of Hydatid Cyst, Yasuj, Iran. [Google Scholar]
- 34.Rokni MB. 2008. The present status of human helminthic diseases in Iran. Ann Trop Med Parasitol 102:283–295. 10.1179/136485908X300805. [DOI] [PubMed] [Google Scholar]
- 35.Vahedi MA, Vahedi ML. 2012. Demographics of patients with surgical and nonsurgical cystic echinococcosis in east Azerbaijan from 2001 to 2012. Pak J Biol Sci 15:186–191. 10.3923/pjbs.2012.186.191. [DOI] [PubMed] [Google Scholar]
- 36.Mohammadzadeh HH, Bozorgomid A, Alinia T, Hazrati TK, Mahmodlou R. 2013. Human cystic echinococcosis in west Azerbaijan, northwest Iran: a retrospective hospital based survey from 2000 to 2009. Iran J Parasitol 8:323–326. [PMC free article] [PubMed] [Google Scholar]
- 37.Mousavi J, Hazrati TKH, Mehryar A, Nikbin R. 2003. Study on the frequency of human hydatid cyst in the clinical centers of Urmia between the years of 1991-2001. J Urmia Univ Med Sci 14:151–152. [Google Scholar]
- 38.Rokni MB. 2009. Echinococcosis/hydatidosis in Iran. Iran J Parasitol 4:1–16. [Google Scholar]
- 39.Ahmadi NAA, Hamidi M. 2008. A retrospective analysis of human cystic echinococcosis in Hamedan Province, an endemic region of Iran. Ann Trop Med Parasitol 102:603–609. 10.1179/136485908X337517. [DOI] [PubMed] [Google Scholar]
- 40.Nourian A, Zargham D, Nourizadeh H. 1993. A survey on the surgical cases of hydatid cyst in the shafieieh hospital of Zanjan city, 1984-1993. J Zanjan Univ Med Sci 16:22–30. [Google Scholar]
- 41.Hamzavi Y, Vejdani M, Nazari N, Mikaeili A. 2011. The trend of hydatidosis in Kermanshah Province, Western Iran (1986-2008). Iran J Parasitol 6:33–40. [PMC free article] [PubMed] [Google Scholar]
- 42.Ebrahimipour M, Budke CM, Najjari M, Cassini R, Asmarian N. 2016. Bayesian spatial analysis of the surgical incidence rate of human cystic echinococcosis in north-eastern Iran. Acta Trop 163:80–86. 10.1016/j.actatropica.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Khazaei SS, Rezaeian S, Khazaei Z, Goodarzi E, Khazaei SS, Mohammadian M, Salehiniya H, Ayubi E, Mohammadian-Hafshejani A. 2016. Epidemiological and clinical characteristics of patients with hydatid cysts in Khorasan Razavi Province, from 2011 to 2014. Iran J Parasitol 11:364–370. [PMC free article] [PubMed] [Google Scholar]
- 44.Shahriarirad R, Erfani A, Eskandarisani M, Rastegarian M, Taghizadeh H, Sarkari B. 2020. Human cystic echinococcosis in southwest Iran: a 15-year retrospective epidemiological study of hospitalized cases. Trop Med Health 48:49. 10.1186/s41182-020-00238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeinali M, Mohebali M, Shirzadi MR, Rahimi EB, Erfani H, Pourmozafari J, Ghanbari M. 2017. Human cystic echinococcosis in different geographical zones of Iran: an observational study during 1995–2014. Iran J Public Health 46:1623–1631. [PMC free article] [PubMed] [Google Scholar]
- 46.Torgerson PR, Deplazes P. 2009. Echinococcosis: diagnosis and diagnostic interpretation in population studies. Trends Parasitol 25:164–170. 10.1016/j.pt.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Rakhshanpour A, Harandi MF, Moazezi SS, Rahimi MT, Mohebali M, Mowlavi G, Babaei Z, Ariaeipour M, Heidari Z, Rokni MB. 2012. Seroprevalence of human hydatidosis using ELISA method in Qom Province, central Iran. Iran J Parasitol 7:10–15. [PMC free article] [PubMed] [Google Scholar]
- 48.Abdi J, Taherikalani M, Asadolahi K, Emaneini M. 2013. Echinococcosis/hydatidosis in Ilam Province, western Iran. Iran J Parasitol 8:417–422. [PMC free article] [PubMed] [Google Scholar]
- 49.Zibaei M, Azargoon A, Ataie-Khorasgani M, Ghanadi K, Sadjjadi SM. 2013. The serological study of cystic echinococcosis and assessment of surgical cases during 5 years (2007-2011) in Khorram Abad, Iran. Niger J Clin Pract 16:221–225. 10.4103/1119-3077.110156. [DOI] [PubMed] [Google Scholar]
- 50.Sarkari B, Hosseini F, Abdolahi Khabisi S, Sedaghat F. 2017. Seroprevalence of cystic echinococcosis in blood donors in Fars Province, southern Iran. Parasite Epidemiol Control 2:8–12. 10.1016/j.parepi.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fotoohi S, Hashemi Tabar GR, Borji H. 2013. Serodiagnosis of human hydatidosis with an ELISA developed based on antigens derived from sheep hydatid cysts and comparison with a commercial human ELISA kit. Asian Pac J Trop Med 6:723–727. 10.1016/S1995-7645(13)60126-1. [DOI] [PubMed] [Google Scholar]
- 52.Siles-Lucas M, Casulli A, Conraths FJ, Müller N. 2017. Laboratory diagnosis of Echinococcus spp. in human patients and infected animals. Adv Parasitol 96:159–257. 10.1016/bs.apar.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Rogan MT. 2002. Immunological approaches for transmission and epidemiological studies in cestode zoonoses-the role of serology in human infection, p 135–145. In Craig P, Pawlowski Z (ed), Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands. [Google Scholar]
- 54.Li T, Ito A, Pengcuo R, Sako Y, Chen X, Qiu D, Xiao N, Craig PS. 2011. Post-treatment follow-up study of abdominal cystic echinococcosis in Tibetan communities of northwest Sichuan Province, China. PLoS Negl Trop Dis 5:e1364. 10.1371/journal.pntd.0001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fathi S, Jalousian F, Hosseini SH, Parsa H, Kordafshari S. 2016. A study of cross-reactivity between recombinant EPC1 antigen of Echinococcus granulosus in serum from patients with confirmed cystic echinococcosis infection and other parasitic infections. Am J Trop Med Hyg 94:1313–1317. 10.4269/ajtmh.15-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turgut AT, Altin L, Topçu S, Kiliçoğlu B, Aliinok T, Kaptanoğlu E, Karademir A, Koşar U. 2007. Unusual imaging characteristics of complicated hydatid disease. Eur J Radiol 63:84–93. 10.1016/j.ejrad.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 57.Feng X, Wen H, Zhang Z, Chen X, Ma X, Zhang J, Qi X, Bradshaw H, Vuitton D, Craig PS. 2010. Dot immunogold filtration assay (DIGFA) with multiple native antigens for rapid serodiagnosis of human cystic and alveolar echinococcosis. Acta Trop 113:114–120. 10.1016/j.actatropica.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 58.Brunetti E, Kern P, Vuitton DA, Writing Panel for the WHO-IWGE . 2010. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 114:1–16. 10.1016/j.actatropica.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 59.Thompson RCA. 2008. The taxonomy, phylogeny and transmission of Echinococcus. Exp Parasitol 119:439–446. 10.1016/j.exppara.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 60.Saberi-Firouzi M, Kaffashian F, Hayati E, Ghaderi AA, Keshavarz H, Arshadi S, Arshadi C, Sotudehmaram MSE, Massarrat MS, Ghalambor MA. 1998. Prevalence of hydatidosis in nomadic tribes of southern Iran. Med J Islam Repub Iran 12:113–118. [Google Scholar]
- 61.Mirshemirani A, Khaleghnejad A, Kouranloo J, Sadeghian N, Rouzrokh M, Hasas-Yeganeh S. 2011. Liver hydatid cyst in children (a 14-year review). Iran J Pediatr 21:385–389. [PMC free article] [PubMed] [Google Scholar]
- 62.Mamishi S, Sagheb S, Pourakbari B. 2007. Hydatid disease in Iranian children. J Microbiol Immunol Infect 40:428–431. [PubMed] [Google Scholar]
- 63.Talaiezadeh AH, Maraghi S. 2006. Hydatid disease in children: a different pattern than adults. Pak J Med Sci 22:329–332. [Google Scholar]
- 64.Hosseinpour SR. 2007. The clinical and epidemiological features of hydatid disease in children in Tabriz, Iran. Pak Pediatr J 31:75–79. [Google Scholar]
- 65.Aslanabadi S, Zarrintan S, Abdoli-Oskouei S, Salehpour F, Zarrintan A, Beheshtirouy S, Abdollahi H, Badebarin D. 2013. Hydatid cyst in children: a 10-year experience from Iran. Afr J Paediatr Surg 10:140–144. 10.4103/0189-6725.115040. [DOI] [PubMed] [Google Scholar]
- 66.Mohammadi M, Mamishi S, Pourakbari B, Faraz Z, Khodabandeh M, Mahmoudi S. 2021. Cystic echinococcosis in children: high frequency of multiple organ involvement in North of Iran. Infect Disord Drug Targets 21:125–129. 10.2174/1871526520666200228104316. [DOI] [PubMed] [Google Scholar]
- 67.Yousefi E, Rafiei A, Rashidi I, Khademvatan S, Foroutan M. 2019. Molecular characterization of Echinococcus granulosus in paraffin-embedded human tissues from Southwest Iran. Asian Pac J Trop Med 12:507–511. 10.4103/1995-7645.271290. [DOI] [Google Scholar]
- 68.Harandi MF, Hobbs RP, Adams PJ, Mobedi I, Morgan-Ryan UM, Thompson RCA. 2002. Molecular and morphological characterization of Echinococcus granulosus of human and animal origin in Iran. Parasitology 125:367–373. 10.1017/s0031182002002172. [DOI] [PubMed] [Google Scholar]
- 69.Ahmadi N, Dalimi A. 2006. Characterization of Echinococcus granulosus isolates from human, sheep and camel in Iran. Infect Genet Evol 6:85–90. 10.1016/j.meegid.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 70.Shahnazi M, Hejazi H, Salehi M, Andalib AR. 2011. Molecular characterization of human and animal Echinococcus granulosus isolates in Isfahan, Iran. Acta Trop 117:47–50. 10.1016/j.actatropica.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 71.Gholami S, Sosari M, Fakhar M, Sharif M, Daryani A, Hashemi MB, Vahadi M. 2012. Molecular characterization of Echinococcus granulosus from hydatid cysts isolated from human and animals in Golestan Province, north of Iran. Iran J Parasitol 7:8–16. [PMC free article] [PubMed] [Google Scholar]
- 72.Pezeshki A, Akhlaghi L, Sharbatkhori M, Razmjou E, Oormazdi H, Mohebali M, Meamar AR. 2013. Genotyping of Echinococcus granulosus from domestic animals and humans from Ardabil Province, northwest Iran. J Helminthol 87:387–391. 10.1017/S0022149X1200051X. [DOI] [PubMed] [Google Scholar]
- 73.Farhadi M, Fazaeli A, Haniloo A. 2015. Genetic characterization of livestock and human hydatid cyst isolates from northwest Iran, using the mitochondrial cox1 gene sequence. Parasitol Res 114:4363–4370. 10.1007/s00436-015-4673-y. [DOI] [PubMed] [Google Scholar]
- 74.Jafari R, Sanei B, Baradaran A, Spotin A, Bagherpour B, Yousofi Darani H. 2018. Genetic characterization of Echinococcus granulosus strains isolated from humans based on nad1 and cox1 gene analysis in Isfahan, central Iran. J Helminthol 92:696–702. 10.1017/S0022149X17000967. [DOI] [PubMed] [Google Scholar]
- 75.Khademvatan S, Yousefi E, Rafiei A, Rahdar M, Saki J. 2013. Molecular characterization of livestock and human isolates of Echinococcus granulosus from south-west Iran. J Helminthol 87:240–244. 10.1017/S0022149X12000296. [DOI] [PubMed] [Google Scholar]
- 76.Kinkar L, Laurimäe T, Balkaya I, Casulli A, Zait H, Irshadullah M, Sharbatkhori M, Mirhendi H, Rostami-Nejad M, Ponce-Gordo F, Rehbein S, Kia EB, Simsek S, Šnábel V, Umhang G, Varcasia A, Saarma U. 2018. Genetic diversity and phylogeography of the elusive, but epidemiologically important Echinococcus granulosus sensu stricto genotype G3. Parasitology 145:1613–1622. 10.1017/S0031182018000549. [DOI] [PubMed] [Google Scholar]
- 77.Zhang L, Eslami A, Hosseini SH, McManus DP. 1998. Indication of the presence of two distinct strains of Echinococcus granulosus in Iran by mitochondrial DNA markers. Am J Trop Med Hyg 59:171–174. 10.4269/ajtmh.1998.59.171. [DOI] [PubMed] [Google Scholar]
- 78.Rostami S, Torbaghan SS, Dabiri S, Babaei Z, Mohammadi MA, Sharbatkhori M, Fasihi Harandi M, Harandi MF. 2015. Genetic characterization of Echinococcus granulosus from a large number of formalin-fixed, paraffin-embedded tissue samples of human isolates in Iran. Am J Trop Med Hyg 92:588–594. 10.4269/ajtmh.14-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kinkar L, Laurimäe T, Sharbatkhori M, Mirhendi H, Kia EB, Ponce-Gordo F, Andresiuk V, Simsek S, Lavikainen A, Irshadullah M, Umhang G, Oudni-M'rad M, Acosta-Jamett G, Rehbein S, Saarma U. 2017. New mitogenome and nuclear evidence on the phylogeny and taxonomy of the highly zoonotic tapeworm Echinococcus granulosus sensu stricto. Infect Genet Evol 52:52–58. 10.1016/j.meegid.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 80.Nikmanesh B, Mirhendi H, Ghalavand Z, Alebouyeh M, Sharbatkhori M, Kia EB, Mohebali M, Eghbali M, Rokni MB. 2014. Genotyping of Echinococcus granulosus isolates from human clinical samples based on sequencing of mitochondrial genes in Iran. Iran J Parasitol 9:20–27. [PMC free article] [PubMed] [Google Scholar]
- 81.Barazesh A, Sarkari B, Shahabi S, Halidi AG, Ekici A, Aydemir S, Mahami-Oskouei M. 2020. Genetic diversity of Echinococcus granulosus isolated from humans: a comparative study in two cystic echinococcosis endemic areas, Turkey and Iran. Biomed Res Int 2020:3054195–3054197. 10.1155/2020/3054195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alvarez Rojas CA, Gauci CG, Lightowlers MW. 2013. Antigenic differences between the EG95‐related proteins from Echinococcus granulosus G1 and G6 genotypes: implications for vaccination. Parasite Immunol 35:99–102. 10.1111/pim.12009. [DOI] [PubMed] [Google Scholar]
- 83.Karamian M, Haghighi F, Hemmati M, Taylor WR, Salehabadi A, Ghatee MA. 2017. Heterogeneity of Echinococcus canadensis genotype 6 − the main causative agent of cystic echinococcosis in Birjand, Eastern Iran. Vet Parasitol 245:78–85. 10.1016/j.vetpar.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 84.Lashkarizadeh MR, Hooshmand N, Nasibi S, Mohammadi MA, Shamsaddini S, Kamyabi H, Rostami S, Fasihi Harandi M. 2019. Genetic profile of hydatid cysts in patients with multi-organ involvement: mixed infections by different strains. Vector Borne Zoonotic Dis 19:724–730. 10.1089/vbz.2018.2427. [DOI] [PubMed] [Google Scholar]
- 85.Ebadzadeh H, Ahmadi K, Mohammadnia-Afrozi S, Abbas-Taghani R, Moradi-Eslami A, Abbasi M, Yari S. 2016. Agricultural statistics. Ministry of Agriculture, Tehran, Iran. [Google Scholar]
- 86.Sadjjadi SM, Mikaeili F, Karamian M, Maraghi S, Sadjjadi FS, Shariat-Torbaghan S, Kia EB. 2013. Evidence that the Echinococcus granulosus G6 genotype has an affinity for the brain in humans. Int J Parasitol 43:875–877. 10.1016/j.ijpara.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 87.Devleesschauwer B, Bouwknegt M, Dorny P, Gabriël S, Havelaar AH, Quoilin S, Robertson LJ, Speybroeck N, Torgerson PR, van der Giessen JWB, Trevisan C. 2017. Risk ranking of foodborne parasites: state of the art. Food Waterborne Parasitol 8-9:1–13. 10.1016/j.fawpar.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lass A, Szostakowska B, Kontogeorgos I, Korzeniewski K, Karamon J, Sulima M, Karanis P. 2019. First detection of Echinococcus multilocularis in environmental water sources in endemic areas using capsule filtration and molecular detection methods. Water Res 160:466–474. 10.1016/j.watres.2019.05.050. [DOI] [PubMed] [Google Scholar]
- 89.Lass A, Ma L, Kontogeorgos I, Xueyong Z, Li X, Karanis P. 2020. Contamination of wastewater with Echinococcus multilocularis–possible implications for drinking water resources in the QTP China. Water Res 170:115334. 10.1016/j.watres.2019.115334. [DOI] [PubMed] [Google Scholar]
- 90.Yousefi Z, Enayati A, Mohammadpoor R. 2009. Parasitic contamination of wells drinking water in Mazandaran Province. J Environ Health Sci Eng 6:241–246. [Google Scholar]
- 91.Hajipour N, Soltani M, Ketzis J, Hassanzadeh P. 2021. Zoonotic parasitic organisms on vegetables: impact of production system characteristics on presence, prevalence on vegetables in northwestern Iran and washing methods for removal. Food Microbiol 95:103704. 10.1016/j.fm.2020.103704. [DOI] [PubMed] [Google Scholar]
- 92.Asadpour M, Malekpour H, Jafari A, Bahrami S. 2016. Diversity of parasitic contamination in raw vegetables commonly consumed in Shiraz, southwest of Iran. Asian Pacific J Trop Dis 6:160–162. 10.1016/S2222-1808(15)61004-0. [DOI] [Google Scholar]
- 93.Shahnazi M, Jafari-Sabet M. 2010. Prevalence of parasitic contamination of raw vegetables in villages of Qazvin Province, Iran. Foodborne Pathog Dis 7:1025–1030. 10.1089/fpd.2009.0477. [DOI] [PubMed] [Google Scholar]
- 94.Dadvar-Khani F, Choobchian S. 2015. Women and rural development in Iran: analysis of the effect of socioeconomic changes on women’ work in agriculture. Cience Nat 37:36–50. 10.5902/2179460X19427. [DOI] [Google Scholar]
- 95.Abdelali-Martini M. 2011. Empowering women in the rural labor force with a focus on agricultural employment in the Middle East and North Africa (MENA). https://www.un.org/womenwatch/daw/csw/csw56/egm/Martini-EP-9-EGM-RW-Sep-2011.pdf.
- 96.Ahmad TI. 2013. The role of rural women in livestock management: socio-economic evidences from diverse geographical locations of Punjab (Pakistan). Doctoral dissertation. Université Toulouse le Mirail, Toulouse, France. [Google Scholar]
- 97.Ahmadi NA, Badi F. 2011. Human hydatidosis in Tehran, Iran: a retrospective epidemiological study of surgical cases between 1999 and 2009 at two university medical centers. Trop Biomed 28:450–456. [PubMed] [Google Scholar]
- 98.Larrieu E, Costa MT, Cantoni G, Alvarez R, Cavagion L, Labanchi JL, Bigatti R, Araya D, Herrero E, Alvarez E, Mancini S, Cabrera P. 2001. Ovine Echinococcus granulosus transmission dynamics in the province of Rio Negro, Argentina, 1980–1999. Vet Parasitol 98:263–272. 10.1016/S0304-4017(01)00442-3. [DOI] [PubMed] [Google Scholar]
- 99.Bardonnet K, Piarroux R, Dia L, Schneegans F, Beurdeley A, Godot V, Vuitton DA. 2002. Combined eco-epidemiological and molecular biology approaches to assess Echinococcus granulosus transmission to humans in Mauritania: occurrence of the ‘camel’ strain and human cystic echinococcosis. Trans R Soc Trop Med Hyg 96:383–386. 10.1016/s0035-9203(02)90369-x. [DOI] [PubMed] [Google Scholar]
- 100.Gatti A, Alvarez AR, Araya D, Mancini S, Herrero E, Santillan G, Larrieu E. 2007. Ovine echinococcosis: I. Immunological diagnosis by enzyme immunoassay. Vet Parasitol 143:112–121. 10.1016/j.vetpar.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 101.Craig P, Mastin A, van Kesteren F, Boufana B. 2015. Echinococcus granulosus: epidemiology and state-of-the-art of diagnostics in animals. Vet Parasitol 213:132–148. 10.1016/j.vetpar.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 102.McManus DP. 2006. Molecular discrimination of taeniid cestodes. Parasitol Int 55:31–37. [DOI] [PubMed] [Google Scholar]
- 103.Erbeto K, Zewde G, Kumsa B. 2010. Hydatidosis of sheep and goats slaughtered at Addis Ababa Abattoir: prevalence and risk factors. Trop Anim Health Prod 42:803–805. 10.1007/s11250-009-9495-4. [DOI] [PubMed] [Google Scholar]
- 104.Daryani A, Alaei R, Arab R, Sharif M, Dehghan MH, Ziaei H. 2007. The prevalence, intensity and viability of hydatid cysts in slaughtered animals in the Ardabil province of Northwest Iran. J Helminthol 81:13–17. 10.1017/S0022149X0720731X. [DOI] [PubMed] [Google Scholar]
- 105.Kebede N, Abuhay A, Tilahun G, Wossene A. 2009. Financial loss estimation, prevalence and characterization of hydatidosis of cattle slaughtered at Debre Markos municipality abattoir, Ethiopia. Trop Anim Health Prod 41:1787–1789. 10.1007/s11250-009-9356-1. [DOI] [PubMed] [Google Scholar]
- 106.Ibrahim MM. 2010. Study of cystic echinococcosis in slaughtered animals in Al Baha region, Saudi Arabia: interaction between some biotic and abiotic factors. Acta Trop 113:26–33. 10.1016/j.actatropica.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 107.Ansari-Lari M. 2005. A retrospective survey of hydatidosis in livestock in Shiraz, Iran, based on abattoir data during 1999-2004. Vet Parasitol 133:119–123. 10.1016/j.vetpar.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 108.Berhe G. 2009. Abattoir survey on cattle hydatidosis in Tigray region of Ethiopia. Trop Anim Health Prod 41:1347–1352. 10.1007/s11250-009-9320-0. [DOI] [PubMed] [Google Scholar]
- 109.Ahmadi NA. 2005. Hydatidosis in camels (Camelus dromedarius) and their potential role in the epidemiology of Echinococcus granulosus in Iran. J Helminthol 79:119–125. 10.1079/joh2005279. [DOI] [PubMed] [Google Scholar]
- 110.Ahmadi NA, Meshkehkar M. 2011. An abattoir-based study on the prevalence and economic losses due to cystic echinococcosis in slaughtered herbivores in Ahwaz, south-western Iran. J Helminthol 85:33–39. 10.1017/S0022149X10000234. [DOI] [PubMed] [Google Scholar]
- 111.Ziaei H, Fakhar M, Armat S. 2011. Epidemiological aspects of cystic echinococcosis in slaughtered herbivores in Sari abattoir, North of Iran. J Parasit Dis 35:215–218. 10.1007/s12639-011-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mansoorlakooraj H, Saadati D, Javadi R, Heydari S, Torki E, Gholami H, Fard RMN. 2011. A survey on hydatidosis in livestock in northern Iran based on data collected from slaughterhouses from 2004 to 2008. Vet Parasitol 182:364–367. 10.1016/j.vetpar.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 113.Arbabi M, Hooshyar H. 2006. Survey of echinococcosis and hydatidosis in Kashan region, central Iran. Iran J Public Health 35:75–81. [Google Scholar]
- 114.Fakhar M, Sadjjadi SM. 2007. Prevalence of hydatidosis in slaughtered herbivores in Qom Province, central part of Iran. Vet Res Commun 31:993–997. 10.1007/s11259-007-0017-4. [DOI] [PubMed] [Google Scholar]
- 115.Hajialilo E, Harandi MF, Sharbatkhori M, Mirhendi H, Rostami S. 2012. Genetic characterization of Echinococcus granulosus in camels, cattle and sheep from the south-east of Iran indicates the presence of the G3 genotype. J Helminthol 86:263–270. 10.1017/S0022149X11000320. [DOI] [PubMed] [Google Scholar]
- 116.Hosseini SH, Eslami A. 1998. Morphological and developmental characteristics of Echinococcus granulosus derived from sheep, cattle and camels in Iran. J Helminthol 72:337–341. 10.1017/s0022149x00016709. [DOI] [PubMed] [Google Scholar]
- 117.Rajabloo M, Hosseini SH, Jalousian F. 2012. Morphological and molecular characterisation of Echinococcus granulosus from goat isolates in Iran. Acta Trop 123:67–71. 10.1016/j.actatropica.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 118.Roostaei M, Fallah M, Maghsood AH, Saidijam M, Matini M. 2017. Prevalence and fertility survey of hydatid cyst in slaughtered livestock in Hamadan abattoir, western Iran, 2015 - 2016. Avicenna J Clin Microbiol Infect 4:43361–43368. 10.5812/ajcmi.43361. [DOI] [Google Scholar]
- 119.Amin Pour A, Hosseini SH, Shayan P. 2012. The prevalence and fertility of hydatid cysts in buffaloes from Iran. J Helminthol 86:373–377. 10.1017/S0022149X11000514. [DOI] [PubMed] [Google Scholar]
- 120.Hosseini-Safa A, Mohagegh MA. 2016. First report of Tasmanian sheep strain (G2) genotype isolated from Iranian goat using the high resolution melting (HRM) analysis. Gastroenterol Hepatol Bed Bench 9:S70–S74. [PMC free article] [PubMed] [Google Scholar]
- 121.Sharbatkhori M, Tanzifi A, Rostami S, Rostami M, Harandi MF. 2016. Echinococcus granulosus sensu lato genotypes in domestic livestock and humans in Golestan Province. Rev Inst Med Trop Sao Paulo 58:38. 10.1590/S1678-9946201658038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sharbatkhori M, Harandi MF, Mirhendi H, Hajialilo E, Kia EB. 2011. Sequence analysis of cox1 and nad1 genes in Echinococcus granulosus G3 genotype in camels (Camelus dromedarius) from central Iran. Parasitol Res 108:521–527. 10.1007/s00436-010-2092-7. [DOI] [PubMed] [Google Scholar]
- 123.Abedi B, Maghsood AH, Khansarinejad B, Fallah M, Matini M, Gholami S, Pagheh AS, Ghasemikhah R. 2019. Genotyping of Echinococcus granulosus isolates from livestock based on mitochondrial cox1 gene, in the Markazi Province, Iran. J Parasit Dis 43:592–596. 10.1007/s12639-019-01132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pour AA, Hosseini SH, Shayan P. 2011. Comparative genotyping of Echinococcus granulosus infecting buffalo in Iran using cox1 gene. Parasitol Res 108:1229–1234. 10.1007/s00436-010-2170-x. [DOI] [PubMed] [Google Scholar]
- 125.Vural G, Baca AU, Gauci CG, Bagci O, Gicik Y, Lightowlers MW. 2008. Variability in the Echinococcus granulosus cytochrome c oxidase 1 mitochondrial gene sequence from livestock in Turkey and a re-appraisal of the G1-3 genotype cluster. Vet Parasitol 154:347–350. 10.1016/j.vetpar.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 126.Eslami A, Meshgi B, Jalousian F, Rahmani S, Salari MA. 2016. Genotype and phenotype of Echinococcus granulosus derived from wild sheep (Ovis orientalis) in Iran. Korean J Parasitol 54:55–60. 10.3347/kjp.2016.54.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sharbatkhori M, Mirhendi H, Jex AR, Pangasa A, Campbell BE, Kia EB, Eshraghian MR, Harandi MF, Gasser RB. 2009. Genetic categorization of Echinococcus granulosus from humans and herbivorous hosts in Iran using an integrated mutation scanning‐phylogenetic approach. Electrophoresis 30:2648–2655. 10.1002/elps.200900145. [DOI] [PubMed] [Google Scholar]
- 128.Pestechian N, Safa AH, Tajedini M, Rostami-Nejad M, Mousavi M, Yousofi H, Javanmard SH. 2014. Genetic diversity of Echinococcus granulosus in center of Iran. Korean J Parasitol 52:413–418. 10.3347/kjp.2014.52.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fadakar B, Tabatabaei N, Borji H, Naghibi A. 2015. Genotyping of Echinococcus granulosus from goats and sheep indicating G7 genotype in goats in the Northeast of Iran. Vet Parasitol 214:204–207. 10.1016/j.vetpar.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 130.Addy F, Wassermann M, Kagendo D, Ebi D, Zeyhle E, Elmahdi IE, Umhang G, Casulli A, Harandi MF, Aschenborn O, Kern P, Mackenstedt U, Romig T. 2017. Genetic differentiation of the G6/7 cluster of Echinococcus canadensis based on mitochondrial marker genes. Int J Parasitol 47:923–931. 10.1016/j.ijpara.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 131.Laurimäe T, Kinkar L, Romig T, Omer RA, Casulli A, Umhang G, Gasser RB, Jabbar A, Sharbatkhori M, Mirhendi H, Ponce-Gordo F, Lazzarini LE, Soriano SV, Varcasia A, Rostami Nejad M, Andresiuk V, Maravilla P, González LM, Dybicz M, Gawor J, Šarkūnas M, Šnábel V, Kuzmina T, Saarma U. 2018. The benefits of analysing complete mitochondrial genomes: deep insights into the phylogeny and population structure of Echinococcus granulosus sensu lato genotypes G6 and G7. Infect Genet Evol 64:85–94. 10.1016/j.meegid.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 132.Nejad MR, Taghipour N, Nochi Z, Mojarad EN, Mohebbi SR, Harandi MF, Zali MR. 2012. Molecular identification of animal isolates of Echinococcus granulosus from Iran using four mitochondrial genes. J Helminthol 86:485–492. 10.1017/S0022149X1100071X. [DOI] [PubMed] [Google Scholar]
- 133.Karimi A, Dianatpour R. 2008. Genotypic and phenotypic characterization of Echinococcus granulosus of Iran. Biotechnology (Faisalabad) 7:757–762. 10.3923/biotech.2008.757.762. [DOI] [Google Scholar]
- 134.Ebrahimipour M, Sadjjadi SM, Yousofi Darani H, Najjari M. 2017. Molecular studies on cystic echinococcosis of Camel (Camelus dromedarius) and report of Echinococcus ortleppi in Iran. Iran J Parasitol 12:323–331. [PMC free article] [PubMed] [Google Scholar]
- 135.Nematdoost K, Ashrafi K, Majidi-Shad B, Kia EB, Zeinali A, Sharifdini M. 20 October 2020. Genetic characterization of Echinococcus granulosus sensu lato in livestock and human isolates from north of Iran indicates the presence of E. ortleppi in cattle. Acta Parasitol 10.1007/s11686-020-00293-0. [DOI] [PubMed] [Google Scholar]
- 136.Gorgani-Firouzjaee T, Kalantrai N, Ghaffari S, Alipour J, Siadati S. 2019. Genotype characterization of livestock and human cystic echinococcosis in Mazandaran Province, Iran. J Helminthol 93:255–259. 10.1017/S0022149X1800010X. [DOI] [PubMed] [Google Scholar]
- 137.Sadjjadi SM. 2006. Present situation of echinococcosis in the Middle East and Arabic North Africa. Parasitol Int 55:S197–S202. 10.1016/j.parint.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 138.Mobedi I, Zare-Bidaki M, Siavashi MR, Naddaf SR, Kia EB, Mahmoudi M. 2013. Differential detection of Echinococcus spp. copro-DNA by nested-PCR in domestic and wild definitive hosts in Moghan Plain, Iran. Iran J Parasitol 8:107–113. [PMC free article] [PubMed] [Google Scholar]
- 139.Mobedi I, Sadighian A. 1971. Echinococcus multilocularis Leuckart, 1863, in red foxes, Vulpes vulpes Linn., in Moghan, Azerbaijan Province, northwest of Iran. J Parasitol 57:493. 10.2307/3277900. [DOI] [PubMed] [Google Scholar]
- 140.Gholami S, Jahandar H, Abastabar M, Pagheh A, Mobedi I, Sharbatkhori M. 2016. Echinococcus granulosus sensu stricto in dogs and jackals from Caspian Sea region, northern Iran. Iran J Parasitol 11:186–194. [PMC free article] [PubMed] [Google Scholar]
- 141.Sadighian A. 1969. Helminth parasites of stray dogs and jackals in Shahsavar area, Caspian region, Iran. J Parasitol 55:372–374. 10.2307/3277413. [DOI] [PubMed] [Google Scholar]
- 142.Eslami A, Hosseini SH. 1998. Echinococcus granulosus infection of farm dogs of Iran. Parasitol Res 84:205–207. 10.1007/s004360050383. [DOI] [PubMed] [Google Scholar]
- 143.Aho MB. 2013. Investigation of parasitic infections of stray dogs in Qazvin, Gilan and Mazandaran provinces, Iran. University of Tehran, Tehran, Iran. [Google Scholar]
- 144.Gholami SH, Mobedi E, Ziaee H, Sharif M. 1999. Intestinal helminth parasites in dog and jackal in inferent areas of Sari in the years 1371-72. J Maz Univ Med Sci 9:1–12. [Google Scholar]
- 145.Dalimi A, Shamsi M, Khosravi A, Ghaf-Farifar F, Ghaffarifar F. 2017. Genotyping Echinococcus granulosus from canine isolates in Ilam province, west of Iran. Iran J Parasitol 12:614–621. [PMC free article] [PubMed] [Google Scholar]
- 146.Parsa F, Fasihi Harandi M, Rostami S, Sharbatkhori M. 2012. Genotyping Echinococcus granulosus from dogs from Western Iran. Exp Parasitol 132:308–312. 10.1016/j.exppara.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 147.Shariatzadeh SA, Spotin A, Gholami S, Fallah E, Hazratian T, Mahami-Oskouei M, Montazeri F, Moslemzadeh HR, Shahbazi A. 2015. The first morphometric and phylogenetic perspective on molecular epidemiology of Echinococcus granulosus sensu lato in stray dogs in a hyperendemic Middle East focus, northwestern Iran. Parasit Vectors 8:409. 10.1186/s13071-015-1025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zare-Bidaki M, Mobedi I, Naddaf SR, Kia EB, Mahmoudi M, Piazak N, Nekouie H, Ahari SS, Habibzadeh S, Siavashi M. 2009. Prevalence of Echinococcus spp. infection using coproantigen ELISA among canids of Moghan Plain, Iran. Iran J Public Health 38:112–118. [Google Scholar]
- 149.Emamapour SR, Borji H, Nagibi A. 2015. An epidemiological survey on intestinal helminths of stray dogs in Mashhad, north-east of Iran. J Parasit Dis 39:266–271. 10.1007/s12639-013-0319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Arzamani K, Rouhani S, Mousazadeh-Mojarrad A, Sedeghi S, Rostami M, Raeghi S. 2016. Identification of zoonotic parasites isolated from stray dogs in Bojnurd County located in north-east of Iran. Nov Biomed 4:185–188. [Google Scholar]
- 151.Kamrani AR, Sardari K, Razmi GR. 2006. Prevalence of Echinococcus granulosus and other intestinal helminths of stray dogs in Mashhad area, Iran. Arch Razi Inst 61:143–189. [Google Scholar]
- 152.Hejazei S, Pestechian N, Abdi J. 2004. Survey of cestodes in stray dogs of Isfahan. J Isfahan Med Sch 22:50–53. [Google Scholar]
- 153.Hoghoughi N, Jalayer T. 1967. The prevalence of Echinococcus granulosus in dogs in Shiraz, Iran. Ann Trop Med Parasitol 61:437–438. 10.1080/00034983.1967.11686512. [DOI] [PubMed] [Google Scholar]
- 154.Mehrabani D, Oryan A, Sadjjadi SM. 1999. Prevalence of Echinococcus granulosus infection in stray dogs and herbivores in Shiraz, Iran. Vet Parasitol 86:217–220. 10.1016/s0304-4017(99)00151-x. [DOI] [PubMed] [Google Scholar]
- 155.Sharifi I, Zia-Ali N. 1996. The present status and intensity of Echinococcus granulosus infection in 391 stray dogs in rural and urban areas of the city of Kerman, Iran. Iran J Public Health 25:13–20. [Google Scholar]
- 156.Mirbadie SR, Kamyabi H, Mohammadi MA, Shamsaddini S, Harandi MF. 2018. Copro-PCR prevalence of Echinococcus granulosus infection in dogs in Kerman, south-eastern Iran. J Helminthol 92:17–21. 10.1017/S0022149X17000074. [DOI] [PubMed] [Google Scholar]
- 157.Beiromvand M, Rafiei A, Razmjou E, Maraghi S. 2018. Multiple zoonotic helminth infections in domestic dogs in a rural area of Khuzestan Province in Iran. BMC Vet Res 14:224. 10.1186/s12917-018-1529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maleky F, Moradkhan M. 2000. Echinococcosis in the stray dogs of Tehran, Iran. Ann Trop Med Parasitol 94:329–331. 10.1080/00034983.2000.11813547. [DOI] [PubMed] [Google Scholar]
- 159.Zariffard MR. 1998. Study of Echinococcus granulosus and Echinococcus multilocularis infections in Canidae in Ardabile Province of Iran. Arch Inst Razi 48:47–52. [Google Scholar]
- 160.Huang Y, Heath DD, Yang W, Qiu JM, Chen XW, Yang Y, Wang Q, Li TY, Xiao YF, Qiu DC, Xiao N, Chen FX, Ge S, Se D. 2008. Epidemiology and risk factor analysis for canine echinococcosis in a Tibetan pastoral area of Sichuan. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 26:245–252. [PubMed] [Google Scholar]
- 161.Otero-Abad B, Torgerson PR. 2013. A systematic review of the epidemiology of echinococcosis in domestic and wild animals. PLoS Negl Trop Dis 7:e2249. 10.1371/journal.pntd.0002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Buishi I, Walters T, Guildea Z, Craig P, Palmer S. 2005. Reemergence of canine Echinococcus granulosus infection, Wales. Emerg Infect Dis 11:568–571. 10.3201/eid1104.040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Acosta-Jamett G, Cleaveland S, Barend M, Cunningham AA, Bradshaw H, Craig PS. 2010. Echinococcus granulosus infection in domestic dogs in urban and rural areas of the Coquimbo region, north-central Chile. Vet Parasitol 169:117–122. 10.1016/j.vetpar.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 164.Heidari Z, Sharbatkhori M, Mobedi I, Mirhendi SH, Nikmanesh B, Sharifdini M, Mohebali M, Zarei Z, Arzamani K, Kia EB. 2019. Echinococcus multilocularis and Echinococcus granulosus in canines in North-Khorasan Province, northeastern Iran, identified using morphology and genetic characterization of mitochondrial DNA. Parasit Vectors 12:606. 10.1186/s13071-019-3859-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Torgerson PR, Keller K, Magnotta M, Ragland N. 2010. The global burden of alveolar echinococcosis. PLoS Negl Trop Dis 4:e722. 10.1371/journal.pntd.0000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shaikenov BS. 2006. Distribution and ecology of Echinococcus multilocularis in Central Asia. Parasitol Int 55:S213–S219. 10.1016/j.parint.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 167.Qudsia R, Aisha KH. Demographics of cystic echinococcosis patients treated surgically in Lahore, Pakistan: a single centre study from 2007-2018. Helminthologia, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Khan A, Ahmed H, Naz K, Gul S, Ishaque SM, Zaidi SSA, Afzal MS, Ali MS, Bokari SA, Budke CM. 2020. Surgically confirmed cases of cystic echinococcosis from Baluchistan Province, Pakistan for the years 2011–2018. Acta Trop 205:105354. 10.1016/j.actatropica.2020.105354. [DOI] [PubMed] [Google Scholar]
- 169.Murtaza LG, Shah MG, Merani AH, Khan MS, Khushk SM, Khokhar AM. 2013. Study of human cystic echinococcosis in Hyderabad, Pakistan. Pak J Sci Ind Res Biol Sci 56:53–55. [Google Scholar]
- 170.Amin U, Mahmood R, Manzoor S, Ahmad S. 2009. Hydatid cysts in abdominal wall and ovary in a case of diffuse abdominal hydatidosis: imaging and pathological correlation. J Radiol Case Rep 3:25–31. 10.3941/jrcr.v3i5.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Masroor I, Azeemuddin M, Khan S, Barakzai A. 2010. Hydatid disease of the breast. Singapore Med J 51:72–75. [PubMed] [Google Scholar]
- 172.Ali M, Mahmood K, Khan P. 2009. Hydatid cysts of the brain. J Ayub Med Coll Abbottabad 21:152–154. [PubMed] [Google Scholar]
- 173.Arjumand AK, Baig IM, Gazozai S, Akram S, Kehar SI. 2011. Prevalence of hydatid disease in Jinnah post graduate medical center, Karachi. Int J Pathol 9:79–80. [Google Scholar]
- 174.Nadeem M, Biyabani SR, Pervez S. 2013. Renal failure: unusual clinical presentation of an isolated intrarenal hydatid cyst. Case Rep 2013:bcr2013200616. 10.1136/bcr-2013-200616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nadeem N, Khan H, Fatimi S, Ahmad MN. 2006. Giant multiple intra-abdominal hydatid cysts. J Ayub Med Coll Abbottabad 18:71–73. [PubMed] [Google Scholar]
- 176.Shamim M. 2010. Mammary and femoral hydatid cysts. J Pak Med Assoc 60:687–688. [PubMed] [Google Scholar]
- 177.Biyabani SR, Abbas F, Ghaffar S, Talati J. 2000. Unusual presentations of hydatid disease of the urinary tract. J Urol 163:896–898. 10.1016/S0022-5347(05)67830-5. [DOI] [PubMed] [Google Scholar]
- 178.Ahmed H, Ali S, Afzal MS, Khan AA, Raza H, Shah ZH, Simsek S. 2017. Why more research needs to be done on echinococcosis in Pakistan. Infect Dis Poverty 6:90. 10.1186/s40249-017-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ali I, Panni MK, Iqbal A, Munir I, Ahmad S, Ali A. 2015. Molecular characterization of Echinococcus species in Khyber Pakhtunkhwa, Pakistan. Acta Sci Vet 43:1277. [Google Scholar]
- 180.Fatimi SH, Sajjad N, Hanif HM, Muzaffar M. 2010. Ruptured hydatid cyst presenting as pneumothorax. J Infect Dev Ctries 4:256–258. 10.3855/jidc.538. [DOI] [PubMed] [Google Scholar]
- 181.Muqaddas H, Arshad M, Ahmed H, Mehmood N, Khan A, Simsek S. 2019. Retrospective study of cystic echinococcosis (CE) based on hospital record from five major metropolitan cities of Pakistan. Acta Parasitol 64:866–872. 10.2478/s11686-019-00109-w. [DOI] [PubMed] [Google Scholar]
- 182.Khan A, Zahoor S, Ahmed H, Malik U, Butt RA, Muzam MS, Kilinc SG, Noor N, Zahoor S, Afzal MS, Mansur H, Irum S, Simsek S. 2018. A retrospective analysis on the cystic echinococcosis cases occurred in northeastern Punjab Province, Pakistan. Korean J Parasitol 56:385–390. 10.3347/kjp.2018.56.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Khan A, Ahmed H, Simsek S, Gondal MA, Afzal MS, Irum S, Muhammad I, Mansur H, Fatima A, Ali MS, Riaz N, Akbar A, Weiping W, Yayi G. 2019. Poverty-associated emerging infection of cystic echinococcosis in population of Northern Pakistan: a hospital based study. Trop Biomed 36:324–334. [PubMed] [Google Scholar]
- 184.Khan A, Ahmed H, Budke CM. 2019. Echinococcosis in Pakistan: a call for research. Lancet Infect Dis 19:581. 10.1016/S1473-3099(19)30221-X. [DOI] [PubMed] [Google Scholar]
- 185.Muqaddas H, Mehmood N, Arshad M. 2020. Genetic variability and diversity of Echinococcus granulosus sensu lato in human isolates of Pakistan based on cox1 mt-DNA sequences (366bp). Acta Trop 207:105470. 10.1016/j.actatropica.2020.105470. [DOI] [PubMed] [Google Scholar]
- 186.Khan A, Ahmed H, Khan H, Simsek S, Kilinc SG, Kesik HK, Yayi G, Celik F, Afzal MS, Budke CM. 2020. First report of Echinococcus canadensis (G6/G7) by sequence analysis from the Khyber Pakhtunkhwa province of Pakistan. Acta Trop 209:105559. 10.1016/j.actatropica.2020.105559. [DOI] [PubMed] [Google Scholar]
- 187.Khan A, Ahmed H, Simsek S, Liu H, Yin J, Wang Y, Shen Y, Cao J. 2020. Molecular characterization of human Echinococcus isolates and the first report of E. canadensis (G6/G7) and E. multilocularis from the Punjab Province of Pakistan using sequence analysis. BMC Infect Dis 20:262. 10.1186/s12879-020-04989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mustafa I, Shahbaz M, Asif S, Khan MR, Saeed U, Sadiq F, Mehmood T, Ahmed H, Simsek S. 2015. Availability, cyst characteristics and hook morphology of Echinococcus granulosus isolates from livestock (cattle, sheep and goats) in Central Punjab, Pakistan. Kafkas Univ Vet Fak Derg 21:849–854. [Google Scholar]
- 189.Anwar AH, Khan MN. 1998. Parasitic fauna of camel in Pakistan, p 69–76. Proceedings of the Third Annual Meeting for Animal Production under Arid Conditions, vol 2. ISOCARD, Al-Ain, United Arab Emirates. [Google Scholar]
- 190.Khan A, Farooq H, Simsek S, Gondal MA, Ahmed H. 2018. Prevalence of hydatidosis in livestocks in Chakwal District of Pakistan. Asian Pac J Trop Med 11:34. 10.4103/1995-7645.243101. [DOI] [Google Scholar]
- 191.Mirani AH, Akhtar N, Brohe MA, Bughio S, Oad FC. 2000. Hydatidosis in buffaloes at Larkana slaughter house (Pakistan). Pak J Biol Sci 3:1311–1312. 10.3923/pjbs.2000.1311.1312. [DOI] [Google Scholar]
- 192.Ehsan M, Akhter N, Bhutto B, Arijo A, Ali Gadahi J. 2017. Prevalence and genotypic characterization of bovine Echinococcus granulosus isolates by using cytochrome oxidase 1 (Co1) gene in Hyderabad, Pakistan. Vet Parasitol 239:80–85. 10.1016/j.vetpar.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 193.Khan A, Ahmed H, Simsek S, Afzal MS, Cao J. 2020. Spread of cystic echinococcosis in Pakistan due to stray dogs and livestock slaughtering habits: research priorities and public health importance. Front Public Heal 7:412. 10.3389/fpubh.2019.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Khan A, Ahmed H, Khan H, Saleem S, Simsek S, Brunetti E, Afzal MS, Manciulli T, Budke CM. 2020. Cystic echinococcosis in Pakistan: a review of reported cases, diagnosis, and management. Acta Trop 212:105709. 10.1016/j.actatropica.2020.105709. [DOI] [PubMed] [Google Scholar]
- 195.Mehmood N, Arshad M, Ahmed H, Simsek S, Muqaddas H. 2020. Comprehensive account on prevalence and characteristics of hydatid cysts in livestock from Pakistan. Korean J Parasitol 58:121–127. 10.3347/kjp.2020.58.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Haleem S, Niaz S, Qureshi NA, Ullah R, Alsaid MS, Alqahtani AS, Shahat AA. 2018. Incidence, risk factors, and epidemiology of cystic echinococcosis: a complex socioecological emerging infectious disease in Khyber Pakhtunkhwa, province of Pakistan. Biomed Res Int 2018:5042430. 10.1155/2018/5042430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Khan MA, Tanveer A, Younus M, Shafiq M, Saeed K, Ammara HT, Gill TJ. 2010. Prevalence, organ specificity and economic impact of hydatidosis in the cattle slaughtered in the Lahore Abattoir. Int J Agro Vet Med Sci 4:38–40. [Google Scholar]
- 198.Tasawar Z, Naz F, Lashari MH. 2015. The prevalence of hydatidosis in sheep and buffaloes at Multan, Punjab, Pakistan. Glob Vet 12:332–335. [Google Scholar]
- 199.Iqbal HJ, Maqbool A, Lateef M, Khan MA, Riaz A, Mahmood A, Atif FA, Ali Z, Ahmad MS. 2012. Studies on hydatidosis in sheep and goats at Lahore, Pakistan. J Anim Plant Sci 22:894–897. [Google Scholar]
- 200.Anwar A, Shamim H, Rana H, Khan M, Qudoos A. 2000. Hydatidosis: prevalence and biometrical studies in cattle (Bob Indicub). Pak J Agric Sci 37:29–32. [Google Scholar]
- 201.Shahzad W, Abbas A, Munir R, Khan MS, Avais M, Ahmad J, Rana MY, Mehmood F. 2014. A PCR analysis of prevalence of Echinococcus granulosus genotype G1 in small and large ruminants in three districts of Punjab, Pakistan. Pak J Zool 46:1541–1544. [Google Scholar]
- 202.Mehmood N, Muqaddas H, Arshad M, Ullah MI, Khan ZI. 2020. Comprehensive study based on mtDNA signature (nad1) providing insights on Echinococcus granulosus ss genotypes from Pakistan and potential role of buffalo-dog cycle. Infect Genet Evol 81:104271. 10.1016/j.meegid.2020.104271. [DOI] [PubMed] [Google Scholar]
- 203.Alvi MA, Ohiolei JA, Saqib M, Li L, Tayyab MH, Alvi AA, Wu Y-T, Fu B-Q, Yan H-B, Jia W-Z. 2020. Echinococcus granulosus (sensu stricto) (G1, G3) and E. ortleppi (G5) in Pakistan: phylogeny, genetic diversity and population structural analysis based on mitochondrial DNA. Parasit Vectors 13:347. 10.1186/s13071-020-04199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Khan A, Umhang G, Ullah Z, Boué F, Bastid V, Ullah I, Mahmood S, Afzal MS, Ahmed H. 2021. Investigation of Echinococcus multilocularis in foxes and dogs in Pakistan by detection of copro-DNA. Parasitol Res 120:731–737. 10.1007/s00436-020-07001-x. [DOI] [PubMed] [Google Scholar]
- 205.Simsek S, Eroksuz Y. 2009. Occurrence and molecular characterization of Echinococcus granulosus in Turkish mouflon (Ovis gmelinii anatolica). Acta Trop 109:167–169. 10.1016/j.actatropica.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 206.Altintas N. 2008. Parasitic zoonotic diseases in Turkey. Vet Ital 44:633–646. [PubMed] [Google Scholar]
- 207.Gonlugur U, Ozcelik S, Gonlugur TE, Arici S, Celiksoz A, Elagoz S, Cevit R. 2009. The retrospective annual surgical incidence of cystic echinococcosis in Sivas, Turkey. Zoonoses Public Health 56:209–214. 10.1111/j.1863-2378.2008.01186.x. [DOI] [PubMed] [Google Scholar]
- 208.Yazar S, Yaman O, Cetinkaya F, Sahin I. 2006. Cystic echinococcosis in central Anatolia, Turkey. Saudi Med J 27:205–209. [PubMed] [Google Scholar]
- 209.Snábel V, Altintas N, D'Amelio S, Nakao M, Romig T, Yolasigmaz A, Gunes K, Turk M, Busi M, Hüttner M, Sevcová D, Ito A, Altintas N, Dubinský P. 2009. Cystic echinococcosis in Turkey: genetic variability and first record of the pig strain (G7) in the country. Parasitol Res 105:145–154. 10.1007/s00436-009-1376-2. [DOI] [PubMed] [Google Scholar]
- 210.Kilimcioğlu AA, Özkol M, Bayındır P, Girginkardeşler N, Östan İ, Ok ÜZ. 2006. The value of ultrasonography alone in screening surveys of cystic echinococcosis in children in Turkey. Parasitol Int 55:273–275. 10.1016/j.parint.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 211.Ertabaklar H, Dayanır Y, Ertuğ S. 2012. Research to investigate the human cystic echinococcosis with ultrasound and serologic methods and educational studies in different provinces in Aydin/Turkey. Turkiye Parazitol Derg 36:142–146. 10.5152/tpd.2012.34. [DOI] [PubMed] [Google Scholar]
- 212.Akalin S, Kutlu SSS, Caylak SD, Onal O, Kaya SS, Bozkurt AIİ. 2014. Seroprevalence of human cystic echinococcosis and risk factors in animal breeders in rural communities in Denizli, Turkey. J Infect Dev Ctries 8:1188–1194. 10.3855/jidc.4343. [DOI] [PubMed] [Google Scholar]
- 213.Arda B, Pullukcu H, Yamazhan T, Sipahi OR, Tamsel S, Demirpolat G, Korkmaz M. 2009. Prevalence of Echinococcus granulosus detected using enzyme immunoassay and abdominal ultrasonography in a group of students staying in a state dormitory in Turkey. Turk J Med Sci 39:791–794. [Google Scholar]
- 214.Ok UZ, Ozkol M, Kilimcioğlu AA, Dinç G, Bayindir P, Ostan I, Pabuşçu Y, Ozcan C, Korkmaz M, Coşkun S, Yüksel H, Girginkardeşler N. 2007. A province-based study using sampling method to investigate the prevalence of cystic echinococcosis among primary school children in Manisa, Turkey. Acta Trop 103:116–122. 10.1016/j.actatropica.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 215.Bakal U, Kazez A, Akyol M, Kocakoc E, Simsek S. 2012. A portable ultrasound based screening study on the prevalence and risk factors of cystic echinococcosis in primary school children in East Turkey. Acta Trop 123:91–95. 10.1016/j.actatropica.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 216.Kilimcioğlu AA, Girginkardeşler N, Korkmaz M, Özkol M, Düzgün F, Östan İ, Pabuşcu Y, Dinç G, Ok ÜZ. 2013. A mass screening survey of cystic echinococcosis by ultrasonography, Western blotting, and ELISA among university students in Manisa, Turkey. Acta Trop 128:578–583. 10.1016/j.actatropica.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 217.Tamarozzi F, Akhan O, Cretu CM, Vutova K, Akinci D, Chipeva R, Ciftci T, Constantin CM, Fabiani M, Golemanov B, Janta D, Mihailescu P, Muhtarov M, Orsten S, Petrutescu M, Pezzotti P, Popa AC, Popa LG, Popa MI, Velev V, Siles-Lucas M, Brunetti E, Casulli A. 2018. Prevalence of abdominal cystic echinococcosis in rural Bulgaria, Romania, and Turkey: a cross-sectional, ultrasound-based, population study from the HERACLES project. Lancet Infect Dis 18:769–778. 10.1016/S1473-3099(18)30221-4. [DOI] [PubMed] [Google Scholar]
- 218.Macin S, Orsten S, Samadzade R, Colak B, Cebeci H, Fındık D. 2021. Human and animal cystic echinococcosis in Konya, Turkey: molecular identification and the first report of E. equinus from human host in Turkey. Parasitol Res 120:563–568. 10.1007/s00436-021-07050-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Utuk AE, Simsek S, Koroglu E, McManus DP. 2008. Molecular genetic characterization of different isolates of Echinococcus granulosus in east and southeast regions of Turkey. Acta Trop 107:192–194. 10.1016/j.actatropica.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 220.Ergin S, Saribas S, Yuksel P, Zengin K, Midilli K, Adas G, Arikan S, Aslan M, Uysal H, Caliskan R. 2010. Genotypic characterisation of Echinococcus granulosus isolated from human in Turkey. Afr J Microbiol Res 4:551–555. [Google Scholar]
- 221.Simsek S, Kaplan M, Ozercan IH. 2011. A comprehensive molecular survey of Echinococcus granulosus in formalin-fixed paraffin-embedded tissues in human isolates in Turkey. Parasitol Res 109:411–416. 10.1007/s00436-011-2269-8. [DOI] [PubMed] [Google Scholar]
- 222.Eryıldız C, Sakru N. 2012. Molecular characterization of human and animal isolates of Echinococcus granulosus in the Thrace Region, Turkey. Balkan Med J 29:261–267. 10.5152/balkanmedj.2012.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bakal U, Simsek S, Kazez A. 2015. Surgical and molecular evaluation of pediatric hydatid cyst cases in eastern Turkey. Korean J Parasitol 53:785–788. 10.3347/kjp.2015.53.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Orsten S, Çiftçi T, Yüce G, Ünal E, Doğrul AB, Azizova A, Akıncı D, Akhan O. 2019. Investigation of genetic diversity of Echinococcus granulosus from human host and the first report of Echinococcus canadensis from Central Anatolia Turkey. Int J Infect Dis 79:119. 10.1016/j.ijid.2018.11.293. [DOI] [Google Scholar]
- 225.Oral AB, Soyder A, Malatyalı E, Ertuğ S, Ertabaklar H. 2018. Genotyping of Echinococcus granulosus isolates by sequencing of mitochondrial cytochrome c oxidase subunit 1 (cox1) gene in Aydin. Mikrobiyol Bul 52:198–205. (In Turkish.) 10.5578/mb.66711. [DOI] [PubMed] [Google Scholar]
- 226.Simsek S, Balkaya I, Ciftci AT, Utuk AE. 2011. Molecular discrimination of sheep and cattle isolates of Echinococcus granulosus by SSCP and conventional PCR in Turkey. Vet Parasitol 178:367–369. 10.1016/j.vetpar.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 227.Balkaya İ, Şimşek S. 2010. Prevalence and economic importance of hydatidosis and fasciolosis in slaughtered cattle in Erzurum province of Turkey. Kafkas Univ Vet Fak Derg 16:793–797. [Google Scholar]
- 228.Umur S. 2003. Prevalence and economic importance of cystic echinococcosis in slaughtered ruminants in Burdur, Turkey. J Vet Med B 50:247–252. 10.1046/j.1439-0450.2003.00667.x. [DOI] [PubMed] [Google Scholar]
- 229.Gicik Y, Arslan MÖ, Kara M, Kose M. 2004. The prevalence of cystic echinococcosis in cattle and sheep slaughtered in the Kars Province. Türkiye Parazitoloji Derg 28:136–139. [Google Scholar]
- 230.Esatgil MU, Tüzer E. 2007. Prevalence of hydatidosis in slaughtered animals in Thrace, Turkey. Türkiye Parazitol Derg 31:41–45. [PubMed] [Google Scholar]
- 231.Kara M, Gicik Y, Sari B, Bulut H, Arslan MO. 2009. A slaughterhouse study on prevalence of some helminths of cattle and sheep in Malatya province, Turkey. J Anim Vet Adv 8:2200–2205. [Google Scholar]
- 232.Oguz B, Deger S. 2013. Cystic echinococcosis and cysticerci of Taenia hydatigena in cattle and sheep slaughtered in a van local slaughterhouse. Turkiye Parazitol Derg 37:186–189. 10.5152/tpd.2013.41. [DOI] [PubMed] [Google Scholar]
- 233.Simsek S, Balkaya I, Koroglu E. 2010. Epidemiological survey and molecular characterization of Echinococcus granulosus in cattle in an endemic area of eastern Turkey. Vet Parasitol 172:347–349. 10.1016/j.vetpar.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 234.Beyhan YE, Umur Ş. 2011. Molecular characterization and prevalence of cystic echinococcosis in slaughtered water buffaloes in Turkey. Vet Parasitol 181:174–179. 10.1016/j.vetpar.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 235.Köse M, Sevimli FK. 2008. Prevalence of cystic echinococcosis in slaughtered cattle in Afyonkarahisar. Turkiye Parazitol Derg 32:27–30. [PubMed] [Google Scholar]
- 236.Cengiz G, Gonenc B. 2020. Comparison of molecular and morphological characterization and haplotype analysis of cattle and sheep isolates of cystic echinococcosis. Vet Parasitol 282:109132. 10.1016/j.vetpar.2020.109132. [DOI] [PubMed] [Google Scholar]
- 237.Mehmood S, Simsek S, Celik F, Kesik HK, Kilinc SG, Ahmed H. 2020. Molecular survey on cattle and sheep hydatidosis and first detection of Echinococcus canadensis (G6/G7) in sheep in Turkey. Parasitology 147:1055–1062. 10.1017/S0031182020000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Simsek S, Cevik A. 2014. First detection and molecular characterization of Echinococcus equinus in a Mule in Turkey. Acta Parasitol 59:773–777. 10.2478/s11686-014-0308-1. [DOI] [PubMed] [Google Scholar]
- 239.Umur S, Arslan MO. 1998. The prevalence of helminth species in stray dogs in Kars district. Acta Parasitol Turc 22:188–193. (In Turkish.) [Google Scholar]
- 240.Altintas N. 2003. Past to present: echinococcosis in Turkey. Acta Trop 85:105–112. 10.1016/s0001-706x(02)00213-9. [DOI] [PubMed] [Google Scholar]
- 241.Demirkazık M, Koltaş İS, Aktaş H, Kocaçiftçi İ. 2007. Adana ili sokak köpekleri dışkısında Echinococcus granulosus antijen varlığının araştırılması. Ulus Parazitoloji Kongresi 2007:18–23. [Google Scholar]
- 242.Guzel M, Yaman M, Koltas IS, Demirkazik M, Aktas H. 2008. Detection of Echinococcus granulosus coproantigens in dogs from Antakya Province, Turkey. Helminthologia 45:150–153. 10.2478/s11687-008-0030-3. [DOI] [Google Scholar]
- 243.Öter K, Bilgin Z, Tınar R, Tüzer E. 2011. Tapeworm infections in stray dogs and cats in İstanbul, Turkey. Kafkas Univ Vet Fak Derg 17:595–599. [Google Scholar]
- 244.Kuru BB, Aypak S, Aysul N. 2013. Prevalence of Echinococcus granulosus determined with polymerase chain reaction in dogs in Aydin district. Turkiye Parazitol Derg 37:78–83. (In Turkish.) 10.5152/tpd.2013.20. [DOI] [PubMed] [Google Scholar]
- 245.Öge H, Öge S, Gönenç B, Sarımehmetoğlu O, Özbakış G. 2017. Coprodiagnosis of Echinococcus granulosus infection in dogs from Ankara, Turkey. Vet Parasitol 242:44–46. 10.1016/j.vetpar.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 246.Oguz B, Ozdal N, Kilinc OO, Deger MS. 2018. Preliminary studies on the prevalence and genotyping of Echinococcus granulosus infection in stray dogs in Van Province, Turkey. J Vet Res 62:497–502. 10.2478/jvetres-2018-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Uysal V, Paksoy N. 1986. Echinococcosis multilocularis in Turkey. J Trop Med Hyg 89:249–255. [PubMed] [Google Scholar]
- 248.Uzunlar AK, Yilmaz F, Bitiren M. 2003. Echinococcosis multilocularis in south-eastern Anatolia, Turkey. East Afr Med J 800:395–397. [PubMed] [Google Scholar]
- 249.Altintas N. 1995. The pathology of the echinococcosis (50 cases) and the current echinococcus problem in Turkey. Türkiye Ektopatoloji Derg 1:55–58. [Google Scholar]
- 250.Miman Ö, Yazar S. 2012. Alveolar echinococcosis in Turkey: in the light of the literature. Turkiye Parazitol Derg 36:116–120. (In Turkish.) 10.5152/tpd.2012.28. [DOI] [PubMed] [Google Scholar]
- 251.Altinörs N, Bavbek M, Caner HH, Erdoğan B. 2000. Central nervous system hydatidosis in Turkey: a cooperative study and literature survey analysis of 458 cases. J Neurosurg 93:1–8. 10.3171/jns.2000.93.1.0001. [DOI] [PubMed] [Google Scholar]
- 252.Aydin Y, Barlas O, Yolaş C, Aydin IH, Ceviz A, Aladağ A, Oren D, Akdemir D. 1986. Alveolar hydatid disease of the brain. Report of four cases. J Neurosurg 65:115–119. 10.3171/jns.1986.65.1.0115. [DOI] [PubMed] [Google Scholar]
- 253.Combes B, Comte S, Raton V, Raoul F, Boué F, Umhang G, Favier S, Dunoyer C, Woronoff N, Giraudoux P. 2012. Westward spread of Echinococcus multilocularis in foxes, France, 2005–2010. Emerg Infect Dis 18:2059–2062. 10.3201/eid1812.120219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Dubinský P, Malczewski A, Miterpakova M, Gawor J, Reiterova K. 2006. Echinococcus multilocularis in the red fox Vulpes vulpes from the East Carpathian region of Poland and the Slovak Republic. J Helminthol 80:243–247. [PubMed] [Google Scholar]
- 255.Deplazes P, Hegglin D, Gloor S, Romig T. 2004. Wilderness in the city: the urbanization of Echinococcus multilocularis. Trends Parasitol 20:77–84. 10.1016/j.pt.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 256.Antolova D, Reiterova K, Miterpakova M, Dinkel A, Dubinský P. 2009. The first finding of Echinococcus multilocularis in dogs in Slovakia: an emerging risk for spreading of infection. Zoonoses Public Health 56:53–58. 10.1111/j.1863-2378.2008.01154.x. [DOI] [PubMed] [Google Scholar]
- 257.Bruzinskaite R, Sarkūnas M, Torgerson PR, Mathis A, Deplazes P. 2009. Echinococcosis in pigs and intestinal infection with Echinococcus spp. in dogs in southwestern Lithuania. Vet Parasitol 160:237–241. 10.1016/j.vetpar.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 258.Vuitton DA, Demonmerot F, Knapp J, Richou C, Grenouillet F, Chauchet A, Vuitton L, Bresson-Hadni S, Millon L. 2015. Clinical epidemiology of human AE in Europe. Vet Parasitol 213:110–120. 10.1016/j.vetpar.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 259.Altintaş N. 1998. Cystic and alveolar echinococcosis in Turkey. Ann Trop Med Parasitol 92:637–642. 10.1080/00034983.1998.11813323. [DOI] [PubMed] [Google Scholar]
- 260.Gürler AT, Bölükbaş CS, Açıcı M, Umur Ş. 2019. Overview of Echinococcus multilocularis in Turkey and in the World. Turkiye Parazitol Derg 43:18–35. 10.4274/tpd.galenos.2019.6300. [DOI] [PubMed] [Google Scholar]
- 261.Avcioglu H, Guven E, Balkaya I, Kirman R, Bia MM, Gulbeyen H, Kurt A, Yaya S, Demirtas S. 2017. First detection of Echinococcus multilocularis in rodent intermediate hosts in Turkey. Parasitology 144:1821–1827. 10.1017/S0031182017001226. [DOI] [PubMed] [Google Scholar]
- 262.Kesik HK, Kilinc SG, Celik F, Simsek S, Ahmed H. 2020. A case-study of the molecular diagnosis of Echinococcus multilocularis in wild boar with comments on its public health significance in Turkey. J Parasitol 106:730–734. 10.1645/19-196. [DOI] [PubMed] [Google Scholar]
- 263.Merdivenci A. 1963. Türkiyede tilki (Vulpes vulpes) lerde ilk helmintolojik arastirma ve ilk Echinococcus multilocularis (Leuckart, 1864) Vogel, 1955 olayi. Turk Vet Hek Dern Derg 33:290–296. [Google Scholar]
- 264.Avcioglu H, Guven E, Balkaya I, Kirman R, Akyuz M, Bia MM, Gulbeyen H, Yaya S. 2021. Echinococcus multilocularis in Red Foxes in Turkey: increasing risk in urban. Acta Trop 216:105826. 10.1016/j.actatropica.2021.105826. [DOI] [PubMed] [Google Scholar]
- 265.Gürler AT, Gori F, Umur Ş, Açıcı M, Deplazes P. 2018. Investigation of Echinococcus multilocularis in environmental definitive host feces in the Asian and the European parts of Turkey. Front Vet Sci 5:48. 10.3389/fvets.2018.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Avcioglu H, Guven E, Balkaya I, Kirman R, Bia MM, Gulbeyen H. 2016. First molecular characterization of Echinococcus multilocularis in Turkey. Vector Borne Zoonotic Dis 16:627–629. 10.1089/vbz.2016.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Avcioglu H, Guven E, Balkaya I, Kirman R. 2018. Echinococcus multilocularis in a Eurasian lynx (Lynx lynx) in Turkey. Parasitology 145:1147–1150. 10.1017/S0031182018000082. [DOI] [PubMed] [Google Scholar]
- 268.Lötsch F, Budke CM, Auer H, Kaczirek K, Waneck F, Lagler H, Ramharter M. 2019. Evaluation of direct costs associated with alveolar and cystic echinococcosis in Austria. PLoS Negl Trop Dis 13:e0007110. 10.1371/journal.pntd.0007110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Torgerson PR, Devleesschauwer B, Praet N, Speybroeck N, Willingham AL, Kasuga F, Rokni MB, Zhou X-N, Fèvre EM, Sripa B, Gargouri N, Fürst T, Budke CM, Carabin H, Kirk MD, Angulo FJ, Havelaar A, de Silva N. 2015. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med 12:e1001920. 10.1371/journal.pmed.1001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Borji H, Parandeh S. 2010. The abattoir condemnation of meat because of parasitic infection, and its economic importance: results of a retrospective study in north-eastern Iran. Ann Trop Med Parasitol 104:641–647. 10.1179/136485910X12851868780261. [DOI] [PubMed] [Google Scholar]
- 271.Borji H, Azizzadeh M, Kamelli M. 2012. A retrospective study of abattoir condemnation due to parasitic infections: economic importance in Ahwaz, southwestern Iran. J Parasitol 98:954–957. 10.1645/GE-2988.1. [DOI] [PubMed] [Google Scholar]
- 272.Craig PS, Hegglin D, Lightowlers MW, Torgerson PR, Wang Q. 2017. Echinococcosis: control and prevention. Adv Parasitol 96:55–158. 10.1016/bs.apar.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 273.Sharifi I, Daneshvar H, Ziaali N, Nikian Y, Ebrahimi AM, Keshavarz H, Masoud J. 1996. Evaluation of a control program on hydatid cyst in the city of Kerman. J Kerman Univ Med Sci 3:168–174. [Google Scholar]
- 274.Larrieu E, Mujica G, Araya D, Labanchi JL, Arezo M, Herrero E, Santillán G, Vizcaychipi K, Uchiumi L, Salvitti JC, Grizmado C, Calabro A, Talmon G, Sepulveda L, Galvan JM, Cabrera M, Seleiman M, Crowley P, Cespedes G, García Cachau M, Gino L, Molina L, Daffner J, Gauci CG, Donadeu M, Lightowlers MW. 2019. Pilot field trial of the EG95 vaccine against ovine cystic echinococcosis in Rio Negro, Argentina: 8 years of work. Acta Trop 191:1–7. 10.1016/j.actatropica.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 275.Eckert J, Gemmel MA, Meslin FX, Pawlowski ZS. (ed). 2001. Chapter 3. Echinococcosis in animals: clinical aspects, diagnosis and treatment, p 72–99. WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris, France. [Google Scholar]
- 276.Larrieu E, Zanini F. 2012. Critical analysis of cystic echinococcosis control programs and praziquantel use in South America, 1974-2010. Rev Panam Salud Publica 31:81–87. 10.1590/s1020-49892012000100012. [DOI] [PubMed] [Google Scholar]
- 277.Lembo T, Craig PS, Miles MA, Hampson KR, Meslin FX. 2013. Chapter 12. Zoonoses prevention, control, and elimination in dogs, p 205–258. In Macpherson C, Meslin FX, Wandeler A. (ed), Dogs, zoonoses and public health. CABI Publishing, Wallingford, United Kingdom. [Google Scholar]
- 278.Wakefield MA, Loken B, Hornik RC. 2010. Use of mass media campaigns to change health behaviour. Lancet 376:1261–1271. 10.1016/S0140-6736(10)60809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.O'Brien MC, McConnon A, Hollywood LE, Cuskelly GJ, Barnett J, Raats M, Dean M. 2015. Let’s talk about health: shoppers’ discourse regarding health while food shopping. Public Health Nutr 18:1001–1010. 10.1017/S1368980014001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Sugimoto CR, Thelwall M, Larivière V, Tsou A, Mongeon P, Macaluso B. 2013. Scientists popularizing science: characteristics and impact of TED talk presenters. PLoS One 8:e62403. 10.1371/journal.pone.0062403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Tsubokura M, Onoue Y, Torii HA, Suda S, Mori K, Nishikawa Y, Ozaki A, Uno K. 2018. Twitter use in scientific communication revealed by visualization of information spreading by influencers within half a year after the Fukushima Daiichi nuclear power plant accident. PLoS One 13:e0203594. 10.1371/journal.pone.0203594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Gemmell MA, Roberts MG. 1995. Modelling Echinococcus life cycles, p 333–354. In Lymbery AJ, Thompson RCA. (ed), Echinococcus and hydatid disease. CAB International, Wallingford, United Kingdom. [Google Scholar]
- 283.Roberts M, Lawson J, Gemmell M. 1987. Population dynamics in echinococcosis and cysticercosis: mathematical model of the life-cycles of Taenia hydatigena and T. ovis. Parasitology 94:181–197. 10.1017/S0031182000053555. [DOI] [PubMed] [Google Scholar]
- 284.Atkinson JAM, Williams GM, Yakob L, Clements ACA, Barnes TS, McManus DP, Yang YR, Gray DJ. 2013. Synthesising 30 years of mathematical modelling of Echinococcus TRansmission. PLoS Negl Trop Dis 7:e2386. 10.1371/journal.pntd.0002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Torgerson PR, Heath DD. 2003. Transmission dynamics and control options for Echinococcus granulosus. Parasitology 127:S143–S158. 10.1017/S0031182003003810. [DOI] [PubMed] [Google Scholar]
- 286.Torgerson PR, Shaikenov BS, Rysmukhambetova AT, Ussenbayev AE, Abdybekova AM, Burtisurnov KK. 2003. Modelling the transmission dynamics of Echinococcus granulosus in dogs in rural Kazakhstan. Parasitology 126:417–424. 10.1017/s0031182003002932. [DOI] [PubMed] [Google Scholar]
- 287.Burlet P, Deplazes P, Hegglin D. 2011. Age, season and spatio-temporal factors affecting the prevalence of Echinococcus multilocularis and Taenia taeniaeformis in Arvicola terrestris. Parasit Vectors 4:6. 10.1186/1756-3305-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Heinzmann D, Barbour AD, Torgerson p.R. 2011. A mechanistic individual-based two-host interaction model for the transmission of a parasitic disease. Int J Biomath 04:443–460. 10.1142/S1793524511001313. [DOI] [Google Scholar]
- 289.Lloyd AL. 2004. Estimating variability in models for recurrent epidemics: assessing the use of moment closure techniques. Theor Popul Biol 65:49–65. 10.1016/j.tpb.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 290.Hansen F, Jeltsch F, Tackmann K, Staubach C, Thulke HH. 2004. Processes leading to a spatial aggregation of Echinococcus multilocularis in its natural intermediate host Microtus arvalis. Int J Parasitol 34:37–44. 10.1016/j.ijpara.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 291.White LA, Forester JD, Craft ME. 2018. Dynamic, spatial models of parasite transmission in wildlife: their structure, applications and remaining challenges. J Anim Ecol 87:559–580. 10.1111/1365-2656.12761. [DOI] [PubMed] [Google Scholar]
- 292.Khan A, Ahmed H, Sohail A, Alam F, Simsek S. 2020. A mathematical modelling approach for treatment and control of echinococcus multilocularis. Parasitology 147:376–381. 10.1017/S0031182019001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Rabinowitz PM, Kock R, Kachani M, Kunkel R, Thomas J, Gilbert J, Wallace R, Blackmore C, Wong D, Karesh W, Natterson B, Dugas R, Rubin C, Stone Mountain One Health Proof of Concept Working Group . 2013. Toward proof of concept of a one health approach to disease prediction and control. Emerg Infect Dis 19:e130265. 10.3201/eid1912.130265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zinsstag J, Schelling E, Waltner-Toews D, Tanner M. 2011. From “one medicine” to “one health” and systemic approaches to health and well-being. Prev Vet Med 101:148–156. 10.1016/j.prevetmed.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kardjadj M, Ben-Mahdi MH. 2019. Epidemiology of dog-mediated zoonotic diseases in Algeria: a One Health control approach. New Microbes New Infect 28:17–20. 10.1016/j.nmni.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ribeiro CDS, van de Burgwal LHM, Regeer BJ. 2019. Overcoming challenges for designing and implementing the One Health approach: a systematic review of the literature. One Health 7:100085. 10.1016/j.onehlt.2019.100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Durusoy R, Karababa AO. 2010. Completeness of hepatitis, brucellosis, syphilis, measles and HIV/AIDS surveillance in Izmir, Turkey. BMC Public Health 10:71. 10.1186/1471-2458-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Kazerooni PA, Fararouei M, Nejat M, Akbarpoor M, Sedaghat Z. 2018. Under-ascertainment, under-reporting and timeliness of Iranian communicable disease surveillance system for zoonotic diseases. Public Health 154:130–135. 10.1016/j.puhe.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 299.Rossi P, Tamarozzi F, Galati F, Akhan O, Cretu CM, Vutova K, Siles-Lucas M, Brunetti E, Casulli A. 2020. The European Register of Cystic Echinococcosis, ERCE: state-of-the-art five years after its launch. Parasit Vectors 13:236. 10.1186/s13071-020-04101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Rossi P, Tamarozzi F, Galati F, Pozio E, Akhan O, Cretu CM, Vutova K, Siles-Lucas M, Brunetti E, Casulli A, HERACLES extended network . 2016. The first meeting of the European Register of Cystic Echinococcosis (ERCE). Parasit Vectors 9:243. 10.1186/s13071-016-1532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Macpherson CNL. 2005. Human behaviour and the epidemiology of parasitic zoonoses. Int J Parasitol 35:1319–1331. 10.1016/j.ijpara.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 302.Dore F, Varcasia A, Pipia AP, Sanna G, Pinna Parpaglia ML, Corda A, Romig T, Scala A. 2014. Ultrasound as a monitoring tool for cystic echinococcosis in sheep. Vet Parasitol 203:59–64. 10.1016/j.vetpar.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 303.Lahmar S, Chéhida FB, Pétavy AF, Hammou A, Lahmar J, Ghannay A, Gharbi HA, Sarciron ME. 2007. Ultrasonographic screening for cystic echinococcosis in sheep in Tunisia. Vet Parasitol 143:42–49. 10.1016/j.vetpar.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 304.Cabrera PA, Lloyd S, Haran G, Pineyro L, Parietti S, Gemmell MA, Correa O, Morana A, Valledor S. 2002. Control of Echinococcus granulosus in Uruguay: evaluation of different treatment intervals for dogs. Vet Parasitol 103:333–340. 10.1016/s0304-4017(01)00603-3. [DOI] [PubMed] [Google Scholar]
- 305.Neumann PJ, Thorat T, Zhong Y, Anderson J, Farquhar M, Salem M, Sandberg E, Saret CJ, Wilkinson C, Cohen JT. 2016. A systematic review of cost-effectiveness studies reporting cost-per-DALY averted. PLoS One 11:e0168512. 10.1371/journal.pone.0168512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA, Salomon JA, Sculpher MJ, Trikalinos TA, Russell LB, Siegel JE, Ganiats TG. 2016. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 316:1093–1103. 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 307.Budke CM, Jiamin Q, Qian W, Torgerson PR. 2005. Economic effects of echinococcosis in a disease-endemic region of the Tibetan Plateau. Am J Trop Med Hyg 73:2–10. 10.4269/ajtmh.2005.73.2. [DOI] [PubMed] [Google Scholar]
- 308.Ebrahimipour M, Budke CM, Harandi MF. 2020. Control of cystic echinococcosis in Iran: where do we stand? Trends Parasitol 36:578–581. 10.1016/j.pt.2020.04.007. [DOI] [PubMed] [Google Scholar]