Abstract
According to the National Cancer Institute in 2020 there will be an estimated 21,750 new ovarian cancer cases and 276,480 new breast cancer cases. Both breast and ovarian cancer are hormone dependent cancers, meaning they cannot grow without the presence of hormones. The two most studied hormones in these two cancers are estrogen and progesterone, which are also involved in the modulation of pain. The incidence of pain in breast and ovarian cancer is very high. Research about mechanisms involved in modulation of pain by hormones are still being debated, as some studies find estrogen to be anti-nociceptive and others pro-nociceptive in pain studies. Moreover, analgesic treatments for breast and ovarian cancer-associated pain are limited and often ineffective. In this review, we will focus on estrogen and progesterone mechanisms of action in modulation of pain and cancer. We will also discuss new treatment options for these types of cancer and associated-pain.
Keywords: ovarian; breast; cancer; estrogen, progesterone; pain; cannabinoids
1. Introduction
The societal and economic impact of cancer is tremendous, accounting for an estimated 1.7 million new diagnoses and 600,000 deaths in 2018 (American Cancer Society). Breast and ovarian cancer are among the leading causes of death for women in the United States. In 2020, breast cancer will be accounted for 276,480 new cases and 42,170 deaths whereas ovarian cancer will be responsible for 21,750 new cases and 13,940 deaths (NCI, 2020). These types of cancer are considered hormone dependent, meaning that the cancer is dependent on the hormone for development and survival (Jeon et al., 2016, Subramani et al., 2017). The full extent to which ovarian hormones contribute to the development of cancer has yet to be determined, but estrogen has been demonstrated to be a key component. Furthermore, ovarian hormones have also been demonstrated to contribute to pain sensitivity, which may result from the cancers themselves (depending on the location of the tumor; such as on nerves, in bones, abdominal cavity or spinal cord), or the treatment of the cancers. Indeed, the beneficial effect of chemotherapeutic agents on tumor suppression comes with major unwanted side effects such as weight and hair loss, nausea and vomiting and neuropathic pain (Gaskin and Richard, 2012). Standard treatment for cancer-associated pain is opioids, but due to unwanted side effects such as constipation and risk of respiratory depression, new drugs are needed and being developed to combat pain. This includes the emerging field of cannabinoids and the endogenous cannabinoid system. In this review, we will discuss the contribution of ovarian hormones in the development of hormone-dependent cancer, chemotherapy-induced pain associated with hormone-dependent cancer, and response to analgesics.
2. Evolving definition of pain
Pain is defined by the International Association of the Study of Pain (IASP) in 2020 as, “an unpleasant sensory and emotional experience associated with, or resembling that associated with actual or potential tissue damage” (Raja et al., 2020). This definition has changed over the years from the ancient Greeks associated pain with emotions or appetites to Descartes (1664) who conceived of the pain system as a straight-through channel from the skin (burn) to the brain. Several pain theories followed after that such as Max von Frey identifying the theory of cutaneous senses in 1894 and 1895, followed by Pavlov (1927) associating pain with presentation of food in dogs and several others such as the gate-control theory postulating that perception of pain is determined by the interactions between different types of fibers, both small-diameter pain transmitting and large-diameter nonpain transmitting fibers (Melczak and Wall, 1965). This theory revolutionize the field of pain by asserting that activation of large diameter (fast-conducting) nonpain transmitting fibers could indirectly inhibit signals from small diameter (slow-conducting) pain transmitting fibers and block the transmission and perception of pain (Melczak and Wall, 1965). Melzack refined its former theory to integrate the neurophysiology of phantom limb pain (Melczak, 1990, 1999) by proposing the body-self matrix theory. This theory is associated with a genetically built-in matrix of neurons for the whole body comprising widely distributed network that incorporates somatosensory, limbic and thalamocortical components. These components contain smaller parallel networks that contribute to sensory-discriminative, affective-motivational and evaluative-cognitive dimensions of pain experience as the neuromatrix (Melzack, 1990, 1999). The conceptualization of pain is constantly evolving over time through advance in our understanding of pain transmission and modulation and breakthrough discoveries (see review by Borsook et al., 2018 Guindon and Hohmann, 2009). In the following sections, we will discuss the three components of pain, the different types of pain experienced and associated or/not with chemotherapy-induced pain in breast and ovarian cancers. We will also review the animal pain models used to improve our understanding of pain pathways in these two cancers and the different mechanisms involved in the modulation of pain and the role of hormones in breast and ovarian cancers.
3. Three components of pain
The current predominant theory of pain is that it comprises three components: sensory-discriminative, affective-motivational and evaluative-cognitive (Figure 1). The sensory component correspond to the intensity of the pain or also referred as sensory (how it feels), including the spatial (location) and temporal characteristics and the quality of pain (Talbot et al., 2019; Guindon and Hohmann, 2009) (Figure 1). Secondly, the affective-motivational component is simply identified as the “unpleasantness” or associated with the affective part that captures how bad or how unpleasant the pain and what are the emotions associated to this pain and the motivational part associated with the aspect that induce a protective action toward the pain (Talbot et al., 2019; Guindon and Hohmann, 2009)(Figure 1). Thirdly, the evaluative-cognitive component is the evaluation of the pain and situation engaging cognitive functions to address the situation (Talbot et al., 2019; Guindon et al., 2006) (Figure 1). However, pain remains a personal and complex multidimensional experience influenced by social, cultural, environmental, neurophysiological, psychophysical, psychological or neuropsychological aspects (Melzack and Katz, 1999; Price, 1999) (Figure 1). Indeed, ovarian and breast cancer will be associated with different types of pain which can be correlated to different types of cancer (chemotherapeutic agents, radiation, surgical removal, hormonal therapy) treatments at different stages of cancer progression (such as metastasis to bone, spinal cord or bowel obstruction). For example, local pain experienced by a tumor compressing a nerve in the breast, post-surgical pain felt after removal of the breast, visceral pain attributed to advanced tumors in the ovaries and potentially abdominal metastasis and finally chemotherapy-induced peripheral neuropathy generated by chemotherapeutic agents given as treatment to stop breast and ovarian cancers growth.
Figure 1. Three components of pain and influencing factors.
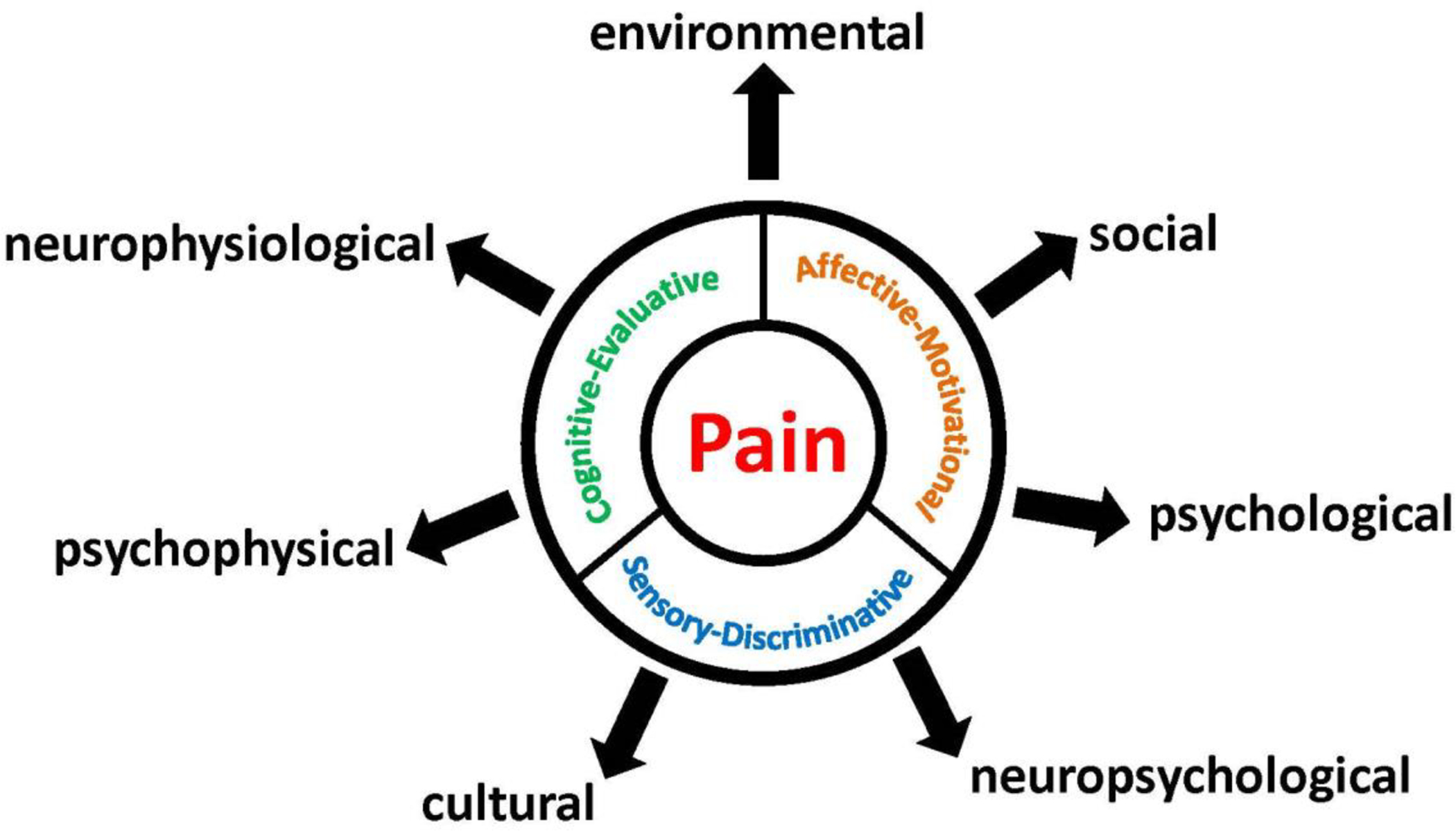
Pain comprises three components: sensory-discriminative, affective-motivational and evaluative-cognitive. Several factors influence individual’s experience and perception of pain.
4. Different types of pain in breast and ovarian cancers
Different types of pain are involved in breast and ovarian cancer: local, post-operative/post-surgical, visceral, chemotherapy-induced peripheral neuropathic (CIPN) and bone metastatic pain. Local pain also referred as localized pain felt in one part of your body, it is localized type of pain that could be felt, for example, in one specific area of the breast (Guindon and Hohmann, 2009; Smith et al., 2019) (Figure 2). Post-operative/post-surgical pain will be felt after breast cancer surgical removal such as pain at the incision site and from removal of breast cancer tissues (Bovbjerg et al., 2019; Cheng et al., 2018; Ilfeld et al., 2016)(Figure 2). For ovarian cancer, the abdominal incision can caused post-operative/post-surgical pain that will be felt at the site of incision (Kay et al., 2020; Lemoine et al., 2019). Visceral pain is defined as pain emanating from the internal thoracic, pelvic or abdominal organs (Guindon and Hohmann, 2009)(Figure 2). In general, this type of pain will be vague and poorly localized since it is characterized by the hypersensitivity of organ distension (Guindon and Hohmann, 2009). Ovarian cancer patients will experience visceral pain such as abdominal bloating prior and after diagnosis (Smith, 2006). In breast cancer patients, visceral pain is also felt when metastasis of abdominal organs occurred in metastatic breast cancer (Heo et al., 2019; Wood et al., 2017). Chemotherapy-induced peripheral neuropathy (CIPN) is occurring in cancer patients following treatment with the chemotherapeutic agents which are comprised of three classes (platinums, taxanes and vinca alkaloids) (Bao et al., 2017; Jin et al., 2020; Nyrop et al., 2019; Rivera et al., 2018). CIPN commonly manifests in the hands and feet, with symptoms favoring sensory over motor deficits (Figure 2). Sensory deficits may present as a loss of sensation (numbing), a heightened response to pain (hyperalgesia) of painful response to ordinarily innocuous stimulation (allodynia), leading sometimes to chronic pain in cancer patients (Blanton et al., 2019; Hamood et al., 2018; Staff et al., 2018). Symptoms of CIPN may not abate after discontinuation of the chemotherapy, a phenomenon referred clinically as “coasting” (Windebank et al., 2008). The prevalence of CIPN in cancer patients is estimated between 19 and 85 % (Fallon, 2013). Breast (Brook et al., 2018; Salvador et al., 2019; Yin et al., 2005) and ovarian (Kumar et al., 1992; Tiwari et al., 2007; Zhang et al., 2013) cancers can induce bone metastasis that will induce bone pain. In fact, pain from bone metastasis is describe by patients as slowly increasing over a period of time until it becomes unbearable (Tiwari et al., 2007; Zhang et al., 2013). Spinal cord metastasis is generating pain and discomfort that will be worst at night or during inactivity (bed rest). However, long bones metastasis (arms and legs) cause pain while during activity or movement, so, in this case, rest will alleviate pain (Tiwari et al., 2007; Zhang et al., 2012).
Figure 2. Schematic representation of the different types of pain and treatment options for cancer related pain.
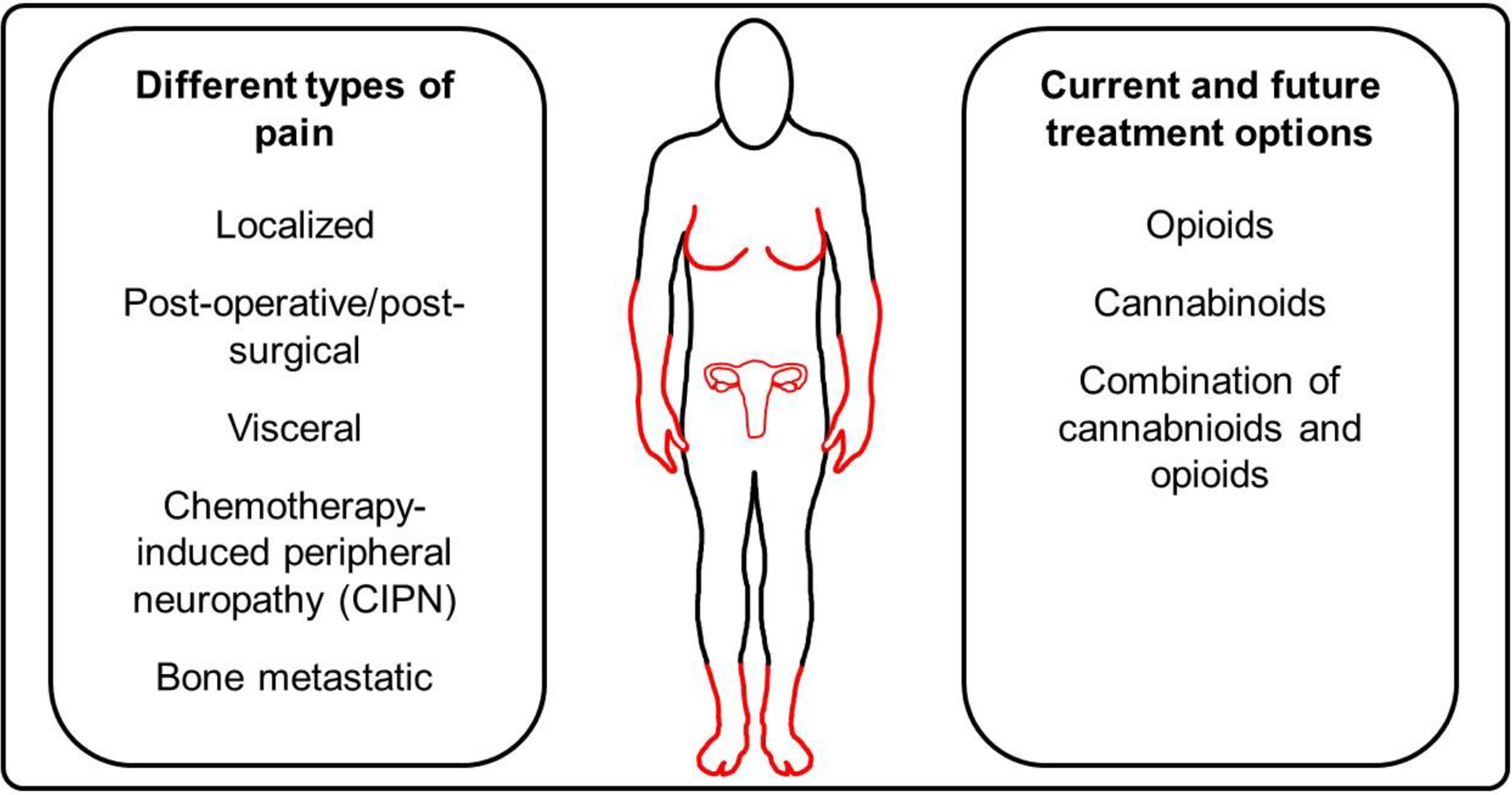
The different types of pain experienced in breast and ovarian cancer are illustrated as well as the current and future treatment options for cancer-related pain.
5. Sex hormones and their role in breast and ovarian cancer
There are two hormones that are mainly associated with breast and ovarian cancers: estrogen and progesterone. These two sex steroid hormones contribute to tumorigenesis, metastasis and cancer prognosis. Estrogen and progesterone also contribute heavily to normal breast, ovarian and uterine development. In this section we will discuss the role of estrogen, progesterone and their role in both breast and ovarian cancers. However, it is important to first understand the developmental role of these hormones in female reproduction and how they influence development in reproductive tissues.
5.1. Role of estrogen and progesterone in female reproduction
Breast development involves three hormonal dependent stages (Briskin et al., 2015; Zolfaroli et al., 2018). First, during puberty, oestrogen acts to form the terminal end buds and elongates the ducts (Briskin et al., 2015; Subramani et al., 2017). Second, during puberty and young adulthood, progesterone continues to elongate the mammary ducts and begins the side branching (Lange et al., 2008; Valadez-Cosmes et al., 2016). Finally, during pregnancy, prolactin stimulates alveolar genesis and lactogenic development (Briskin et al., 2015).
The estrogen receptors are nuclear receptors involved in the formation of breast, ovaries and uterine tissues more specifically defined as estrogen receptors (ER) alpha (α) and beta (β). ERα and ERβ are found in the epithelium of the breast, ovaries and uterine tissues (Cheng et al., 2018). ERα are the main source of estrogen in the breast and are associated with the luminal type of breast cancer (Zolfaroli et al., 2018). Estrogen receptors α also play a crucial role in the development of mammary glands. Indeed, preclinical studies as demonstrated that deletion of ERα in the mammary glands of female mice leads to the underdeveloped mammary glands (Hilton et al., 2018).
The two isoforms of nuclear progesterone receptors: progesterone receptors A (PRA) and progesterone receptors B (PRB) (Lange et al., 2008) are involved in mammary gland, uterine and ovarian development (Valadez-Cosmes et al., 2016). PRA and PRB are found in the epithelium of breast, ovaries and uterine tissues (Taraborrelli, 2015). PRA and PRB are from the same gene however, they have two different promoters which are nearly identical except that PRB has 164 more amino acids than PRA (Zolfaroli et al., 2018). Functionally, it has been demonstrated in preclinical studies that deletion of PRB induce abnormal mammary formation, while deletion of PRA gene cause abnormal uterine development, but normal development of mammary glands (Hilton et al., 2018).
5.2. Estrogen and progesterone in breast cancer
Preclinical studies have demonstrated that PRA and PRB can be expressed by ERα transcription events, but transcription can also happen independently of ERα (Lange et al., 2008). However, ERα is necessary to induce expression of either progesterone receptor in estrogen receptor positive cells (Jeon et al., 2016). Progesterone and estrogen receptors are usually only found in luminal epithelial cells that are non-dividing (Cheng et al., 2018). However, these non-dividing cells are adjacent to proliferating stem cells, showing that estrogen and progesterone can affect cells in a paracrine or autocrine fashion (Tian et al., 2018). Nonetheless, these cells are adjacent to proliferating cells, indicating that these receptors may be growth promoting to the nearby cells using a paracrine pathway (Briksen et al., 2015). Progesterone activated genes in breast cancer are pro-tumor progression and are aggressive (Lange et al., 2008). In vitro, it has been shown that after the initial burst of proliferation (after treatment of breast cancer cells with progesterone), progesterone proliferative effects are stopped (Lange et al., 2008). Indeed, after additional treatment with progesterone, breast cancer cells did not proliferate, but have shown longer survival (Tian et al., 2018). Lastly, in vitro studies have demonstrated that PRB increases cell motility leading to cancer progression and metastasis (Lange et al., 2008).
Not surprisingly, estrogen has been shown to regulate breast cancer cell growth via GREB1, which is an early estrogen response gene (Cheng et al., 2018). Moreover, estrogen receptors are able to regulate GREB1 expression which is directly linked to the amount of estrogen in the plasma (Cheng et al., 2018). More evidence have shown that breast cancer patients before menopause (higher estrogen concentration) have a much higher GREB1 expression relative to post-menopausal patients (Ho et al., 2003). Preclinical studies have demonstrated that GREB1 acts as a regulator in the transcription of estrogen receptor target genes (Cheng et al., 2018). Indeed, when immortalized breast cancer cells (MCF-7 cells) do not express GREB1, most of the target genes are no longer expressed (Cheng et al., 2018). GREB1 is an estrogen receptor target gene allowing cancer cells to form colonies and when GREB1 is no longer expressed the cancer cells have impaired colony formation (Cheng et al., 2018).
Preclinical studies have shown that estrogen and progesterone have a proliferative effect on breast cancer cells by inducing tumorigenesis and metastasis (Tian et al., 2018). These sex steroid hormones accomplish the effect by inducing cyclin G1 (Tian et al., 2018). Cyclin G1 is the target of p53 (tumor suppression gene) and is responsible for the regulatory action of cell cycle and apoptosis. Studies have used MCF-7 cells to demonstrate that estrogen and progesterone play a role in inducing cyclin G1 by their administration in a dose-dependent manner (Tian et al., 2018). They found increased cell survival, viability and number of cells in the G2/M phase of the cell cycle as well as an increase in G1 mRNA and protein (Tian et al., 2018). Furthermore, the induction of cyclin G1 causes the cell cycle to be unregulated which is a key condition for tumor growth (Tian et al., 2018). Progesterone induced cell proliferation through cyclin D1 (Jeon et al., 2016). Cyclin D1 is a molecule that blocks cells in the G1 phase of proliferation (Jeon et al., 2016).
Moreover, receptor activator of nuclear factor (RANK) kappa B (NF-κB) and its ligand (RANKL) are critical for the development of human breast (Briskin et al., 2015). Recent studies suggest that RANKL is a key paracrine mediator of progesterone mitogenic signal (Fernandez-Valdivia et al., 2012). Indeed, induction of RANKL elicit proliferative and pro-survival signals that are similar and necessary for mammary morphogenesis and tumorigenesis underlying a dual role of RANKL (Fernandez-Valdivia et al., 2012; Tanos et al., 2013).
5.3. Different type of breast cancers
In this section, the different types of breast cancers are briefly discussed. Hormone dependent breast cancers are classified according to the presence or absence of hormones and their receptors (Hilton et al., 2018). Indeed, the HER-2 (human growth factor receptor-2) is a type of breast cancer with a very poor prognosis and carry an extra copy of the HER-2 gene (Subramani, et al., 2017). Luminal type A breast cancer is a low-grade cancer, slowly growing identify as an estrogen receptor positive cancer with the best prognosis (Hilton et al., 2018). However, luminal type B is also estrogen receptor positive, but has a very poor prognosis as it grows rapidly (Zolfaroli et al., 2018). Finally, the last type of breast cancer is basal. This type has the worst prognosis as it is triple negative, it has no hormonal receptors and characterized by its poor prognosis (Briskin et al., 2015).
5.4. Breast cancers treatments
It is commonly known that the standard treatments for breast cancer include: surgery, chemotherapy, radiation therapy and hormone therapy (Maughan et al., 2010). Surgical intervention is usually a mastectomy partial or complete, which is the total removal of breast tissue (Diaby et al., 2015). Sometimes nearby lymph nodes are removed in the eventuality that the tumors have metastasized into these tissues (Carmocan et al., 2017). Along with surgery, chemotherapy is used to kill cancer cells and reduce tumors size (Shufelt et al., 2018). As previously mentioned, chemotherapy drugs are highly associated with the induction of pain (chemotherapy-induced neuropathic pain) which has been discussed previously (section 4). Radiation therapy is used after surgery and chemotherapy and generally the entire breast area is radiated (Maughan et al., 2010). Hormonal therapy is given during the aforementioned treatments and even after these treatments. It is important to know that active adjuvant hormonal therapy is mandatory for all hormonal receptor positive breast cancers (Carmocan et al., 2017). The first line of defense is tamoxifen, which is an aromatase inhibitors and selective estrogen receptor modulator (Zalfaroli et al., 2018). Aromatase inhibitors block endogenous estrogen production, thus lowering the burden of disease progression (Hilton et al., 2018). Tamoxifen is given to both pre and post-menopausal women. If resistance to tamoxifen occurs there are other options for aromatase inhibitors that are effective, such as: anastrozol, letrozol, exemestan and fluvestrant (Carmocan et al., 2017).
There two paths of hormonal therapy, the first is to block estrogen production by ovarian inhibition (Diaby et al., 2015). This can be accomplished by surgical intervention, radiation, chemical (LH-RH analogues), and aromatase inhibitors (Maughan et al., 2010). The second path of hormonal therapy is blocking estrogen in tumor cells, by SERMs and SERDs (Zolfaroli et al., 2018; Shufelt et al., 2018). SERMs can act as both estrogen agonists and antagonists depending on the drug targets (Carmocan et al., 2017). SERDs inhibit estrogen receptor activity (fluvestrant) by antagonistic activity (Francis et al., 2015). It should also be mentioned that along with hormone therapy, tocopherols (such as vitamin E) have shown to inhibit estrogen induced stem cell activity (Bak et al., 2018).
5.5. Estrogen and progesterone in ovarian cancer
Estrogen and progesterone have very different effects on ovarian cancer (Ho et al., 2003). Indeed, estrogen stimulated ovarian cell proliferation and enhances epithelial mesenchymal transition (Jeon et al., 2016). Mesenchymal transition is the process in which cells lose polarity, cell-adhesion and induces invasive process leading to metastasis (Cheng et al., 2018). Both estrogen and progesterone are implicated in ovarian carcinogenesis (Ho et al., 2003). Estrogen favors the neoplastic formation of ovarian surface epithelium while progesterone offers protection against ovarian carcinoma (Ho et al., 2003). However, it has been difficult to fully understand their mechanisms of action on the tumorigenic process (Ho et al., 2003). Ovarian cancers are usually not inherited but can rely on BCRA1 and 2 genes (Diep et al., 2015). These genes have shown an increased risk for ovarian cancer (Ponce et al., 2020). Protective effects against ovarian cancer such as pregnancy and breast feeding has been demonstrated (Francis et al., 2015). Studies have shown that estrogen-based contraceptives reduce the risk of ovarian cancer (Jeon et al., 2016) through reduction of the number of ovarian cycles (Tian et al., 2018).
5.6. Ovarian cancer treatments
Despite tremendous research involving ovarian cancer, it remains among the deadliest types of cancers in women (Chandara et al., 2019). The treatment of ovarian cancer includes surgical removal of tumors, chemotherapy (platinum analogs and taxanes) and radiation treatment (Roett et al., 2009). Surgery treatment usually involves a total hysterectomy, as well as the removal of the pelvic and para-aortic lymph nodes and the omentum (Lheureux et al., 2019; Roett et al., 2009). If metastasis to the appendix is present, appendectomy is performed after the initial surgery (Roett et al., 2009). The stage of cancer will also determine whether chemotherapy is given before or after surgery (Orr et al., 2018). However, in advanced stages of ovarian cancer, chemotherapy is given before and after surgery (Orr et al., 2018). Studies have showed that the combination of surgery and chemotherapy is more effective at preventing a relapse of cancer than chemotherapy or surgery alone (Pignata et al., 2017). Immuno-therapeutics and other alternatives to chemotherapy have shown a great deal of promise clinically and pre-clinically in the treatment of ovarian cancer (Chandara et al., 2019). The drug bevacizumab is a monoclonal antibody that targets vascular endothelial growth factor (present in high concentrations in late stage of ovarian cancer) and increases survival in ovarian cancer patients (Lheureux et al., 2019).
6. Estrogen and progesterone in the modulation of pain
The different types of pain involved in breast and ovarian cancer: local, post-operative/post-surgical, visceral, chemotherapy-induced peripheral neuropathic (CIPN) and bone metastatic pain. Limited studies have evaluated the influence of sex (estrogen and progesterone) hormones in the modulation of pain in cancer conditions. Although sex hormones and their relation to pain has been studied more extensively in the past decades, we are still at an early stage and further studies are needed to better understands the role of sex hormones in pain modulation.
There a several regions involved in pain responses including the spinal cord and dorsal root ganglion. Both the spinal cord and dorsal root ganglion contain estrogen and progesterone receptors (Palmeira et al., 2011). It has been shown that changes in estrogen levels due to normal menstrual cycles or hormone replacement therapy has been shown to cause recurrent or chronic pain in women (Pieretti et al., 2011). Estrogen is involved in the modulation of pain via the central nervous system (Vincent et al., 2008). This has been postulated as one of the reasons why women have increased sensitivity to neuropathic pain and why long term effects of CIPN are prominent in ovarian and breast cancer survivors (Hou et al., 2018; Aloisi, 2017).
Preclinical studies investigating the impact of female sex hormones on pain and anti-nociceptive response have largely focused on estradiol (E2), and to a far lesser extent, progesterone (P4). To address this question, studies commonly employ a surgical ovariectomy model, removing the ovaries, followed by exogenous hormone replacement. Using this approach, it is possible to evaluate differences between normally cycling females versus non-cycling females, as well as the effect of specific hormones, on baseline nociceptive responses, as well as response to various anti-nociceptive compounds. Using this approach estradiol (E2) has been demonstrated to mediate both pro- and anti-nociceptive responses.
6.1. Anti-nociceptive responses
Estradiol may mediate anti-nociceptive responses through modulation of neuronal, glial, and immune-system processes. Activation of ERβ by synthetic ERβ-selective agonists has been demonstrated to be antinociceptive in rodent models of visceral pain (Cao et al., 2012) and neuropathic pain; both surgical and chemotherapy-induced (Ma et al., 2016). Estradiol has also been found to reduce cell excitability via calcium influx through voltage-gated calcium channels (VGCCs) (Chaban et al., 2003) and activity at the ATP receptor P2X on DRG neurons (Chaban and Micevych, 2005). Inhibition of VGCCs by estradiol was demonstrated to occur via modulation of metabotropic glutamate receptors mGLUR2/3 (Chaban et al., 2011). Co-administration of estradiol also was found to antagonize the nociceptive response elicited by subcutaneous injection of the P2XR agonist α,β-me-ATP (Lu et al., 2013).
Expression of markers associated with pain have also been found to be reduced by estradiol. In ovariectomized rats, estradiol replacement produced an anti-nociceptive response and a decrease in pERK expression, which was upregulated in the pain model (Stinson et al., 2019). Moreover, another study found ovariectomy produced allodynia and hyperalgesia in rats, which was reversed by estradiol and corresponded to a decrease in substance P in DRG neurons (Sarajari and Oblinger, 2010). Estradiol has been demonstrated to modulate neuro-immune and peripheral immune system process, which contribute to sensitization and recovery from pain. Estradiol relieved inflammatory pain and mechanical allodynia in a neuropathic pain model in rats, which corresponded to reduced astrocyte and microglia activation and levels of pro-inflammatory cytokines in the dorsal horn of the spinal cord (Shivers et al., 2015; Lee et al., 2018). Expression of pro-inflammatory markers were also reduced in B-cells when treated with estradiol (Canellada et al., 2008).
6.2. Pro-nociceptive responses
Estrogen has also been demonstrated to produce pro-nociceptive actions through mediation of various G-protein coupled receptors, ligand-gated ion channels, and lipid signaling molecules. Activation of the G-protein coupled estrogen receptor, GPER (formerly GPR30) has been demonstrated to produce both spontaneous pain behaviors (Deliu et al., 2020) as well as potentiate pain responses (An et al., 2014; Alvarez et al., 2014b) (Figure 3). Activation of GPER in trigeminal ganglion neurons results in increased intracellular calcium, promoting nociceptive signaling (Fehrenbacher et al., 2009). GPER also co-localizes with the ionotropic 5HT3A serotonin receptor in DRG neurons, and activation is associated with a potentiation of visceral pain responses (Lu et al., 2009) (Figure 3). Estradiol may also promote pro-nociceptive activity via modulation of glutamatergic signaling. Estradiol has been found to increase expression of NMDA receptors in the hippocampus (Weiland et al., 1992; Foy et al., 1999). Estradiol was found to promote increased NMDA receptor activity and long term potentiation in the formalin inflammatory pain model (Xiao et al., 2013) (Figure 3). Estradiol may also promote nociception through increased expression of the ligand gated cation channels TRPV1 and TRPA1, which were found to be elevated in the peritoneum of women with endometriosis (Greaves et al., 2014), and further confirmed in rat studies to be mediated by estrogen receptor activation (Pohoczky et al., 2016) (Figure 3).
Figure 3. Hypothetical mechanisms of action of estrogen.
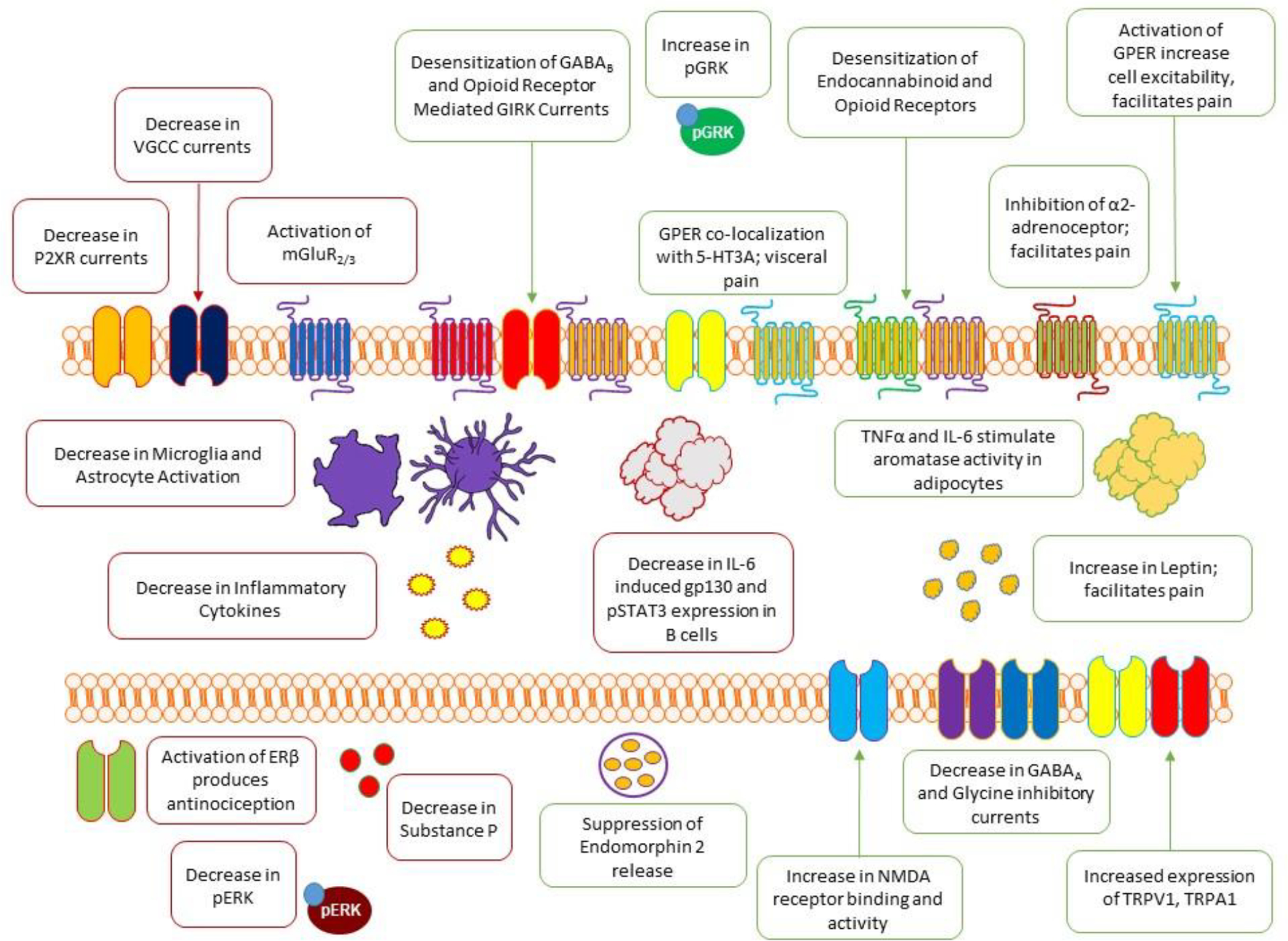
Preclinical studies has demonstrated that estradiol can modulate pain in terms of pro- and anti-nociceptive responses as explained extensively in section 6.
In addition to facilitating excitatory neurotransmission, estrogen may also negatively regulate inhibitory neurotransmission. Estrogen has been demonstrated to negatively regulate expression and activity of both ionotropic and metabotropic receptors involved in antinociceptive neurotransmission. Estradiol was found to decrease inhibitory glycine (Jiang et al., 2009) and GABAA (Rudrick et al., 2001) currents (Figure 3). Inhibition of potassium currents by estrogen may also render nociceptors more excitable, and estradiol has been demonstrated to negatively regulate GIRK currents coupled to μ opioid and GABAB receptor activation (Lagrange et al., 1996; Lagrange et al., 1997) (Figure 3). Estradiol has been found to negatively regulate expression and activity of the α2-adrenoceptor (Karkanias et al., 1997). Activation of α2-adrenoceptors produces antinociceptive effects, and is suppressed by estradiol (Nag and Mohka, 2004; Nag et al., 2016). Estradiol may promote nociception through activities in fat cells. Estradiol was shown to increase pain via positive regulation of leptin production (Alvarez et al., 2014a). Moreover, pro-inflammatory cytokines may also stimulate aromatase activity in adipocytes, leading to increased estradiol production (Velasco et al., 2006) (Figure 3). Finally, estradiol can negatively regulate two key antinociceptive systems; the endogenous opioid system and the endocannabinoid system. Estradiol negatively regulates cannabinoid CB1 receptor density in the amygdala, a key region for pain modulation (Castelli et al., 2014) (Figure 3). The release of endogenous opioids, its activity and opioid receptors are also negatively modulated by estradiol. Estradiol has been found to suppress endomorphin 2 release (Kumar et al., 2015), and desensitize opioid receptors (Kelly et al., 1999), possibly through increased activity of G-protein coupled receptor kinases (GRKs) which can desensitize both opioid and cannabinoid receptors (Ansonoff et al., 2001; Abraham et al., 2018; Melief et al., 2010; Daigle et al., 2009) (Figure 3).
7. Treatment for Breast and Ovarian Cancer Related Pain
The advancement of diagnosis and treatments (surgical removal, chemotherapeutic agents, radiation, hormonal therapy) of cancer has resulted in a large proportion of cancer patients left with chronic cancer pain alone or associated with chronic non cancer pain (Manchikanti et al., 2018). Traditionally, treatment of breast and ovarian cancer pain consisted mostly of opioids. However, their undesirable side effects such as nausea and vomiting, constipation, development of addiction and analgesic tolerance have led to the development of novel therapeutic strategies to alleviate pain in cancer patients (Blanton et al., 2019; Manchikanti et al., 2018). Indeed, endogenous cannabinoid system has shown great promise and efficacy in alleviating cancer-related pain such as CIPN (Blanton et al., 2019). In this section, we will discuss antinociceptive treatment with opioids and cannabinoids.
7.1. Opioids
Opioids are drugs that interact with endogenous opioid receptors and result in pain relief (Paredes et al., 2019). Morphine, oxycodone, transdermal fentanyl and transdermal buprenorphine are the four strong opioids commonly used to alleviate breast and ovarian cancer related pain (Wiffen et al., 2017; Bennett et al., 2017). These drugs are effective pain relievers in cancer patients by reducing from 3 points on a 0–10 pain rating scale (Bennett et al., 2017). Despite efficacy in relieving breast and ovarian cancer pain, the long-term use of opioids is associated with recognized side effects such as constipation, nausea, vomiting and addiction (Blake et al., 2017; Wiffen et al., 2017). It is important to note that there is also unrecognized adverse side effects caused by chronic opioids in cancer patients such as endocrinopathy, neurotoxicity, sleep-disordered breathing and in some circumstances misuse and/or abuse (Bennett et al., 2017). Chronic cancer pain is a complex biopsychosocial phenomenon with a prevalence ranging between 19 and 85 % of cancer patients (Fallon, 2013). Therefore, the need to target novel therapies with long-term efficacy in alleviating chronic cancer pain is necessary.
7.2. Cannabinoids
Cannabinoids have shown excellent therapeutic potential in preclinical studies (Blanton et al., 2019). The endocannaboid system plays a crucial role in pain processing and modulation (Guindon and Hohmann, 2009; Blanton et al., 2019). Indeed, cannabis and cannabinoid-based pharmacotherapies have generated great amount of enthusiasm in the past decade as a novel treatment for cancer associated-pain such as CIPN (Blanton et al., 2019). Canada and 34 states in the United States have introduced laws to allow medical use of cannabis to treat disease or alleviate symptoms (Cyr et al., 2018; Ware et al., 2015). Different types of products are found in the cannabinoid class ranging from whole-plant cannabis flower or cannabis extracts, such as nabiximols (Sativex), to synthetic capsules (nabilone and dronabinol) (Hazekamp et al., 2013). Strong evidence are supporting the use of cannabinoids for the treatment of chronic pain in cancer patients (Blanton et al., 2019; Ware et al., 2015). Moreover, the National Academies of Sciences, Engineering, and Medicine published a report in January 2017 outlining the health effects of cannabinoids and cannabis as medicine in a variety of conditions including chronic pain (The National Academies of Sciences, Engineering and Medicine, 2017). It is obvious that compounds targeting the endocannabinoid system show great promise as a novel class of medications particularly in alleviating pain. In a time of novel treatment being desperately needed, the cannabinoid compounds show great promise in reducing cancer pain.
7.3. Cannabinoid-Opioid interaction
Cannabinoid CB1 and opioid receptors are both associated with analgesia and both share similar mechanisms of action including inhibitory effect on G protein coupling and presynaptic expression that underlie a neuromodulatory role as inhibitors of neurotransmitter release (Blanton et al., 2019). Whether co-expression of cannabinoid and opioid receptors is required for the analgesic effects of either class of compounds appears to vary based by ligand, dose, and receptor profile, as well as types of pain. Preclinical studies have found that the CB1 receptor antagonist AM251 blocked mu-opioid-mediated analgesia in the tail flick test (Da Fonseca Pacheco et al., 2008). Moreover, the antinociceptive effects of CB2-specific agonist AM1241 were absent in mu opioid knockout mice and inhibited by intraplantar administration of naloxone or anti-serum to β-endorphin (Ibrahim et al., 2005). Therefore, the use of cannabinoid medications either as replacement therapy or opioid-sparing adjuvant therapy is an interesting possibility. Clinical studies also suggest that cannabinoid compounds could be useful in combination with opioids and, therefore, reduce opioid doses, leading to lower side effects (Abrams et al., 2011; Johnson et al., 2013). In addition, several studies suggest that either oral Δ9-THC (Narang et al., 2008) or smoked cannabis (Abrams et al., 2011) can elicit additive beneficial effects on chronic pain when used in combination with either morphine or oxycodone.
8. Conclusions
It is obvious that breast and ovarian cancer are the leading causes of death in women and have a devastating social and economic impact. However, less is known about the hormonal influence on breast and ovarian cancer-associated pain following cancer treatments. In this review, we address the role of estrogen and progesterone in the context of pain modulation. This is a new field of study since for years the influence of hormones on pain perception were left unnoticed. Recently, studies are evaluating the impact of hormones on the modulation of pain and therefore research findings are still being debated. In our review, we found evidence showing that estrogen can lead to pro or anti-nociceptive mechanisms of action in response to cancer conditions. Future new studies will shed the light on the influence of sex hormones in the modulation of pain in cancer. We also address the importance of multimodal approaches in alleviating cancer-related pain during cancer treatment as well as chronic pain persisting months after cancer treatment. Opioids are used to alleviate pain in cancer although chronic use is associated with several side effects. There is a great need for novel, highly effective therapeutic agents for cancer-related/chronic pain and the endocannabinoid system is proving to be one of the most promising system. The broad neuromodulatory role of cannabinoids and the diverse library of both natural and synthetic compounds offer a wealth of possible future treatments for cancer-related/cancer pain. Moreover, new preclinical and clinical studies are suggesting that cannabinoids and opioids mechanism of action interacts to alleviate chronic pain. Further studies are needed to better understand the interaction between the opioid and cannabinoid system. This new exciting avenue will lead to improve treatment in our quest to alleviate pain in cancer patients.
Acknowledgments
This work has been supported by the National Institute on Drug Abuse DA044999-01A1 (JG) and Texas Tech University Health Sciences Center School of Medicine 121035 (JG) grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Authors do not have any conflict of interest.
References
- Abrams DI, Couey P, Shade SB, Kelly ME, Benowitz NL, 2011. Cannabinoid-opioid interaction in chronic pain. Clin. Pharmacol. Ther 90, 844–851. 10.1038/clpt.2011.188 [DOI] [PubMed] [Google Scholar]
- Abraham AD, Schattauer SS, Reichard KL, Cohen JH, Fontaine HM, Song AJ, Johnson SD, Land BB, Chavkin C, 2018. Estrogen regulation of GRK2 inactivates Kappa opioid receptor signaling mediating analgesia, but not aversion. J. Neurosci 38, 8031–8043. 10.1523/JNEUROSCI.0653-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloisi AM, 2017. Why We Still Need To Speak About Sex Differences and Sex Hormones in Pain. Pain Ther 6, 111–114. 10.1007/s40122-017-0084-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Bogen O, Chen X, Giudice LC, Levine JD, 2014a. Ectopic endometrium-derived leptin produces estrogen-dependent chronic pain in a rat model of endometriosis. Neuroscience 258, 111–120. 10.1016/j.neuroscience.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Bogen O, Levine JD, 2014b. Role of nociceptor estrogen receptor GPR30 in a rat model of endometriosis pain. Pain 155, 2680–2686. 10.1016/j.pain.2014.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Soceity. Cancer Facts and figures https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2018.html (Accessed November 24, 2020)
- An G, Li W, Yan T, Li S, 2014. Estrogen rapidly enhances incisional pain of ovariectomized rats primarily through the G protein-coupled estrogen receptor. Int. J. Mol. Sci 15, 10479–10491. 10.3390/ijms150610479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansonoff MA, Etgen AM, 2001. Estrogen increases G protein coupled receptor kinase 2 in the cortex of female rats. Brain Res 898, 186–189. 10.1016/S0006-8993(01)02161-8 [DOI] [PubMed] [Google Scholar]
- Bak MJ, Furmanski P, Shan NL, Lee HJ, Bao C, Lin Y, Shih WJ, Yang CS, Suh N, 2018. Tocopherols inhibit estrogen-induced cancer stemness and OCT4 signaling in breast cancer. Carcinogenesis 39, 1045–1055. 10.1093/carcin/bgy071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao T, Basal C, Seluzicki C, Li SQ, Seidman AD, Mao J, Kettering S, Kettering S, 2017. Bao T, Basal C, Seluzicki C, Li SQ, Seidman AD, Mao JJ. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat 2016;159(2):327–33 159, 327–333. 10.1007/s10549-016-3939-0.Long-term [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M, Paice JA, Wallace M, 2017. Pain and Opioids in Cancer Care: Benefits, Risks, and Alternatives. Am. Soc. Clin. Oncol. Annu. Meet 37, 705–713. [DOI] [PubMed] [Google Scholar]
- Blake A, Wan BA, Malek L, DeAngelis C, Diaz P, Lao N, Chow E, O’Hearn S, 2017. A selective review of medical cannabis in cancer pain management. Ann. Palliat. Med 6, S215–S222. 10.21037/apm.2017.08.05 [DOI] [PubMed] [Google Scholar]
- Blanton HL, Brelsfoard J, DeTurk N, Pruitt K, Narasimhan M, Morgan DJ, Guindon J, 2019. Cannabinoids: Current and Future Options to Treat Chronic and Chemotherapy-Induced Neuropathic Pain. Drugs 79, 969–995. 10.1007/s40265-019-01132-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Youssef AM, Simons L, Elman I, Hospitals M, Alto P, 2018. When pain gets stuck: the evolution of pain chronification and treatment resistance. Pain 159. 10.1097/j.pain.0000000000001401.When [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovbjerg DH, Keefe FJ, Soo MS, Manculich J, Van A, Zuley ML, Ahrendt GM, Skinner CS, Sara N, Shelby RA, 2019. Persistent breast pain in post-surgery breast cancer survivors and women with no his. Acta Oncol 58, 763–768. 10.1080/0284186X.2019.1574023.Persistent [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisken C, Hess K, Jeitziner R, 2015. Progesterone and overlooked endocrine pathways in breast cancer pathogenesis. Endocrinology 156, 3442–3450. 10.1210/en.2015-1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook N, Brook E, Dharmarajan A, Dass CR, Chan A, 2018. International Journal of Biochemistry and Cell Biology Breast cancer bone metastases : pathogenesis and therapeutic targets. Int. J. Biochem. Cell Biol 96, 63–78. 10.1016/j.biocel.2018.01.003 [DOI] [PubMed] [Google Scholar]
- Canellada A, Alvarez I, Berod L, Gentile T, 2008. Estrogen and progesterone regulate the IL-6 signal transduction pathway in antibody secreting cells. J. Steroid Biochem. Mol. Biol 111, 255–261. 10.1016/j.jsbmb.2008.06.009 [DOI] [PubMed] [Google Scholar]
- Cao D-Y, Ji Y, Tang B, Traub RJ, 2012. Estrogen Receptor β Activation Is Antinociceptive in a Model of Visceral Pain in the Rat. J. Pain 13, 685–694. 10.1016/j.jpain.2012.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmocan C, Drãgãnescu M, 2017. Hormone Therapy in Breast Cancer. Chirurgia (Bucur) 112, 413–417. 10.21614/chirurgia.112.4.413 [DOI] [PubMed] [Google Scholar]
- Castelli M, Fadda P, Casu A, Spano M, Casti A, Fratta W, Fattore L, 2014. Male and female rats differ in brain cannabinoid CB1 receptor density and function and in behavioural traits predisposing to drug addiction: effect of ovarian hormones. Curr. Pharm. Des 20, 2100–13. 10.2174/13816128113199990430 [DOI] [PubMed] [Google Scholar]
- Chaban V, Li J, McDonald JS, Rapkin A, Micevych P, 2011. Estradiol attenuates the adenosine triphosphate-induced increase of intracellular calcium through group II metabotropic glutamate receptors in rat dorsal root ganglion neurons. J. Neurosci. Res 89, 1707–1710. 10.1002/jnr.22718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban VV, Mayer EA, Ennes HS, Micevych PE, 2003. Estradiol inhibits ATP-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience 118, 941–948. 10.1016/S0306-4522(02)00915-6 [DOI] [PubMed] [Google Scholar]
- Chaban VV, Micevych PE, 2005. Estrogen receptor-α mediates estradiol attenuation of ATP-induced Ca2+ signaling in mouse dorsal root ganglion neurons. J. Neurosci. Res 81, 31–37. 10.1002/jnr.20524 [DOI] [PubMed] [Google Scholar]
- Chandra A, Vishwanatha JK, Ahmad S, 2019. Ovarian cancer : Current status and strategies for improving therapeutic outcomes. Cancer Med 7018–7031. 10.1002/cam4.2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M, Michalski S, Kommagani R, 2018. Role for growth regulation by Estrogen in breast cancer 1 (GREB1) in hormone-dependent cancers. Int. J. Mol. Sci 19, 1–14. 10.3390/ijms19092543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr C, Arboleda MF, Aggarwal SK, Balneaves LG, Daeninck P, Néron A, Prosk E, Vigano A, 2018. Cannabis in palliative care: Current challenges and practical recommendations. Ann. Palliat. Med 7, 463–477. 10.21037/apm.2018.06.04 [DOI] [PubMed] [Google Scholar]
- Da Fonseca Pacheco D, Klein A, De Castro Perez A, Da Fonseca Pacheco CM, De Francischi JN, Duarte IDG, 2008. The μ-opioid receptor agonist morphine, but not agonists at δ- Or κ-opioid receptors, induces peripheral antinociception mediated by cannabinoid receptors. Br. J. Pharmacol 154, 1143–1149. 10.1038/bjp.2008.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle TL, Kearn CS, Mackie K, 2009. Rapid CB1 cannabinoid receptor desensitization defines the timecourse of ERK1/2 MAP kinase signaling. Neuropharmacology 54, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliu E, Brailoiu GC, Arterburn JB, Oprea TI, Benamar K, Dun NJ, Brailoiu E, 2020. Mechanisms of G Protein-Coupled Estrogen Receptor-Mediated Spinal Nociception. J. Pain 13, 742–754. 10.1016/j.jpain.2012.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descartes R, 1664. L’homme translated by M. Foster in 1901. Lectures on the history of physiology during 16th, 17th, and 18th centuries. Cambridge: Cambridge University Press. [Google Scholar]
- Diaby V, Tawk R, Sanogo V, Xiao H, Montero A, 2015. A review of systematic reviews of the cost-effectiveness of hormone therapy, chemotherapy, and targeted therapy for breast cancer. Cancer Reserach Treat 151, 27–40. 10.1007/s10549-015-3383-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep C, Daniel A, Mauro L, Knuston T, Lange C, 2015. Progesterone action in breast, uterine, and ovarian cancers Caroline. J. Mol. Endocrinol 112. 10.1530/jme-14-0252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon MT, 2013. Neuropathic pain in cancer. Br. J. Anaesth 111, 105–111. 10.1093/bja/aet208 [DOI] [PubMed] [Google Scholar]
- Fehrenbacher JC, LoVerme J, Clarke W, Hargreaves KM, Piomelli D, Taylor BK, 2009. Rapid pain modulation with nuclear receptor ligands. Brain Res. Rev 60, 114–124. 10.1016/j.brainresrev.2008.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-valdivia R, Lydon JP, 2012. Molecular and Cellular Endocrinology From the ranks of mammary progesterone mediators, RANKL takes the spotlight. Mol. Cell. Endocrinol 357, 91–100. 10.1016/j.mce.2011.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW, 1999. 17β-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J. Neurophysiol 81, 925–929. 10.1152/jn.1999.81.2.925 [DOI] [PubMed] [Google Scholar]
- Francis P, Ciruelos E, Bellet M, Bonnefoi HR, Climent MA, Davidson NE, Jr CEG, Walley BA, Kerbrat P, Buchholz S, Ingle JN, Winer EP, Rabaglio-poretti M, Maibach R, 2015. Adjuvant Ovarian Suppression in Premenopausal Breast Cancer. N. Engl. J. Med 372, 436–446. 10.1056/NEJMoa1412379.Adjuvant [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin DJ, Richard P, 2012. The Economic Costs of Pain in the United States. J. Pain 13, 715–724. 10.1016/j.jpain.2012.03.009 [DOI] [PubMed] [Google Scholar]
- Greaves E, Grieve K, Horne AW, Saunders PTK, 2014. Elevated peritoneal expression and estrogen regulation of nociceptive ion channels in endometriosis. J. Clin. Endocrinol. Metab 99, E1738–E1743. 10.1210/jc.2014-2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Beaulieu P, Hohmann AG, 2006. Pharmocology of the Cannbinoid System, in: Pharmocology of Pain IASP Press. [Google Scholar]
- Guindon J, Hohmann AG, 2009. Pain: Mechanisms and Measurement. Handb. Neurosci. Behav. Sci 1–24. 10.1002/9780470478509.neubb002033 [DOI] [Google Scholar]
- Hamood R, Hamood H, Merhasin I, Boker-Keinan L, 2018. Chronic pain and other symptoms among breast cancer survivors: prevalence, predictors, and effects on quality of life. Breast Cacncer Res Treat 167, 157–169. 10.1007/s10549-017-4485-0 [DOI] [PubMed] [Google Scholar]
- Hazekamp A, Heerdink E, 2013. The prevalance and incidence of medicinal cannabis on prescription in The Netherlands. Eur. J. Clin. Pharmacol 59, 1575–1580. [DOI] [PubMed] [Google Scholar]
- Heo MH, Kim HK, Lee H, Kim J, Ahn J, Im Y, 2019. Clinical activity of fulvestrant in metastatic breast cancer previously treated with endocrine therapy and / or chemotherapy. Korean J Intern Med 34, 1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton HN, Clarke CL, Graham JD, 2018. Estrogen and progesterone signalling in the normal breast and its implications for cancer development. Mol. Cell. Endocrinol 466, 2–14. 10.1016/j.mce.2017.08.011 [DOI] [PubMed] [Google Scholar]
- Ho SM, 2003. Estrogen, progesterone and epithelial ovarian cancer. Reprod. Biol. Endocrinol 1, 1–8. 10.1186/1477-7827-1-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Huh B, Kim HK, Kim KH, Abdi S, 2018. Treatment of chemotherapy-induced peripheral neuropathy: Systematic review and recommendations. Pain Physician 21, 571–592. 10.36076/ppj.2018.6.571 [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, Malan TP, 2005. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. PNAS 102, 3093–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilfeld BM, Gloria S, Brian M, Cheng GS, 2016. Pain Management A review of postoperative analgesia for breast cancer surgery. Pain Manag 6. 10.2217/pmt-2015-0008 [DOI] [PubMed] [Google Scholar]
- Jeon SY, Hwang KA, Choi KC, 2016. Effect of steroid hormones, estrogen and progesterone, on epithelial mesenchymal transition in ovarian cancer development. J. Steroid Biochem. Mol. Biol 158, 1–8. 10.1016/j.jsbmb.2016.02.005 [DOI] [PubMed] [Google Scholar]
- Jiang P, Kong Y, Zhang XB, Wang W, Liu CF, Le Xu T, 2009. Glycine receptor in rat hippocampal and spinal cord neurons as a molecular target for rapid actions of 17-β-estradiol. Mol. Pain 5, 1–14. 10.1186/1744-8069-5-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Zhang Y, Wenqing Y, 2020. Chemotherapy-induced peripheral neuropathy among patients with ovarian cancer. Int J Gynaecol Obs 149, 303–308. 10.1002/ijgo.13137 [DOI] [PubMed] [Google Scholar]
- Johnson JR, Chb MB, 2013. An Open-Label Extension Study to Investigate the Long-Term Safety and Tolerability of THC / CBD Oromucosal Spray and Oromucosal THC Spray in Patients With Terminal Cancer-Related Pain Refractory to Strong Opioid Analgesics. J. Pain Symptom Manage 46, 207–218. 10.1016/j.jpainsymman.2012.07.014 [DOI] [PubMed] [Google Scholar]
- Karkanias GB, Li CS, Etgen AM, 1997. Estradiol reduction of α2-adrenoceptor binding in female rat cortex is correlated with decreases in α(2A/D)-adrenoceptor messenger RNA. Neuroscience 81, 593–597. 10.1016/S0306-4522(97)00359-X [DOI] [PubMed] [Google Scholar]
- Kay A, Venn M, Urban R, Gray H, Goff B, 2020. Postoperative narcotic use in patients with ovarian cancer on an Enhanced Recovery After Surgery (ERAS) pathway. Gynecol Oncol 156, 634–628. 10.1016/j.ygyno.2019.12.018 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Lagrange AH, Wagner EJ, Rønnekleiv OK, 1999. Rapid effects of estrogen to modulate G protein-coupled receptors via activation of protein kinase A and protein kinase C pathways. Steroids 64, 64–75. 10.1016/S0039-128X(98)00095-6 [DOI] [PubMed] [Google Scholar]
- Kumar A, Storman EM, Liu NJ, Gintzler AR, 2015. Estrogens suppress spinal endomorphin 2 release in female rats in phase with the estrous cycle. Neuroendocrinology 102, 33–43. 10.1159/000430817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar L, Bhargava VL, Rao RC, Rath GK, Kataria SP, 1992. Bone metastasis in ovarian cancer. Asia-Oceania J. Obstet. Gynaecol 18, 309–13. 10.1111/j.1447-0756.1992.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ, 1997. Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol. Pharmacol 51, 605–612. 10.1124/mol.51.4.605 [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Wagner EJ, RØnnekleiv OK, Kelly MJ, 1996. Estrogen Rapidly Attenuates a GABAB Response in Hypothalamic Neurons. Neuroendocrinology 64, 114–123. 10.1159/000127106 [DOI] [PubMed] [Google Scholar]
- Lange CA, Yee D, 2008. Progesterone and breast cancer. Women’s Heal 4, 151–162. 10.2217/17455057.4.2.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Choi HY, Ju BG, Yune TY, 2018. Estrogen alleviates neuropathic pain induced after spinal cord injury by inhibiting microglia and astrocyte activation. Biochim. Biophys. Acta - Mol. Basis Dis 1864, 2472–2480. 10.1016/j.bbadis.2018.04.006 [DOI] [PubMed] [Google Scholar]
- Lemoine A, Lambaudie E, Bonnet F, Leblanc F, Alfonsi P, 2019. Perioperative care of epithelial ovarian cancer: Article drafted from the French Guidelines in oncology entitled “Initial management of patients with epithelial ovarian cancer” developed by FRANCOGYN, CNGOF, SFOG, GINECO-ARCAGY under the aegis of CNGOF an. Gynecol Obs. Fertil Senol 47, 187–196. 10.1016/j.gofs.2018.12.005 [DOI] [PubMed] [Google Scholar]
- Lheureux S, Braunstein M, 2019. Epithelial Ovarian Cancer : Evolution of Management in the Era of Precision Medicine. A Cancer J. Clin 69. 10.3322/caac.21559 [DOI] [PubMed] [Google Scholar]
- Lu CL, Hsieh JC, Dun NJ, Oprea TI, Wang PS, Luo JC, Lin HC, Chang FY, Lee SD, 2009. Estrogen Rapidly Modulates 5-Hydroxytrytophan-Induced Visceral Hypersensitivity via GPR30 in Rats. Gastroenterology 137, 1040–1050. 10.1053/j.gastro.2009.03.047 [DOI] [PubMed] [Google Scholar]
- Lu Y, Jiang Q, Yu L, Lu ZY, Meng SP, Su D, Burnstock G, Ma B, 2013. 17β-estradiol rapidly attenuates P2X3 receptor-mediated peripheral pain signal transduction via ERα and GPR30. Endocrinology 154, 2421–2433. 10.1210/en.2012-2119 [DOI] [PubMed] [Google Scholar]
- Ma JN, McFarland K, Olsson R, Burstein ES, 2016. Estrogen Receptor Beta Selective Agonists as Agents to Treat Chemotherapeutic-Induced Neuropathic Pain. ACS Chem. Neurosci 7, 1180–1187. 10.1021/acschemneuro.6b00183 [DOI] [PubMed] [Google Scholar]
- Manchikanti L, Manchikanti K, Kaye Alan, Kaye Adam, Hirsch J, 2018. Challenges and concerns of persistent opioid use in cancer patients. Expert Rev. Anticancer Ther 18, 705–718. 10.1080/14737140.2018.1474103 [DOI] [PubMed] [Google Scholar]
- Maughan KL, Lutterbie MA, Ham PS, 2010. Treatment of breast cancer. Am. Fam. Physician 81, 1339–1346. 10.1056/nejm199810013391407 [DOI] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Bruchas MR, Chavkin C, 2010. Ligand-directed c-Jun N-terminal kinase activationdisrupts opioid receptor signaling. PNAS 2010, 1–6. 10.1073/pnas.1000751107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, 1999. From the gate to the neuromatrix. Pain Suppl 6. 10.1016/s0304-3959(99)00145-1 [DOI] [PubMed] [Google Scholar]
- Melzack R, 1990. Phantom limbs and the concept of a neuromatrix. Trends Neurosci 13, 88–92. 10.1016/0166-2236(90)90179-e [DOI] [PubMed] [Google Scholar]
- Melzack R, Katz J, 1999. Pain measurement in persons in pain. In Wall PD & Melzack R (Eds.), Textbook of pain (4th ed., pp. 409–426). London: Churchill Livingstone. 10.1016/S0039-6109(05)70381-9 [DOI] [Google Scholar]
- Melzack R, Wall PD, 1965. Pain mechanisms: a new theory. Science (80-.) 150. 10.1126/science.150.3699.971 [DOI] [PubMed] [Google Scholar]
- Nag S, Mokha SS, 2016. Activation of the trigeminal α 2 -adrenoceptor produces sex-specific, estrogen dependent thermal antinociception and antihyperalgesia using an operant pain assay in the rat. Behav. Brain Res 314, 152–158. 10.1016/j.bbr.2016.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S, Mokha SS, 2004. SHORT COMMUNICATION Estrogen attenuates antinociception produced ¨ lliker – Fuse nucleus in the rat by stimulation of Ko. Eur. J. Neurosci 20, 3203–3207. 10.1111/j.1460-9568.2004.03775.x [DOI] [PubMed] [Google Scholar]
- Narang S, Gibson D, Wasan A, Michna E, Nedelijkovic S, Jamison R, 2008. Efficacy of Dronabinol as an Adjuvant Treatment for Chronic Pain Patients on Opiod Therapy. J. Pain 9. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute, NIH. Cancer Stat Facts: Breast Cancer https://seer.cancer.gov/statfacts/html/breast.html (accessed 18 November 2020)
- National Cancer Institute, NIH. Cancer Stat Facts: Ovarian Cancer https://seer.cancer.gov/statfacts/html/ovary.html (accessed 18 November 2020)
- Nyrop K, Deal A, Reeder-Hayes K, Shachar S, Reeve B, Basch E, Choi S, Lee J, Wood W, Anders C, Carey L, Dees E, Jolly T, Kimmick G, Karuturi M, Reinbolt R, Speca J, Hyman M, 2019. Patient-Reported and Clinician-Reported Chemotherapy-Induced Peripheral Neuropathy in Patients With Early Breast Cancer : Current Clinical Practice. Cancer 125, 2945–2954. 10.1002/cncr.32175 [DOI] [PubMed] [Google Scholar]
- Orr B, Edwards RP, 2018. Diagnosis and Treatment of Ovarian C ancer. Hematol. Clin. NA 32, 943–964. 10.1016/j.hoc.2018.07.010 [DOI] [PubMed] [Google Scholar]
- de A. Palmeira CC, Ashmawi HA, de P. Posso I, 2011. Sex and Pain Perception and Analgesia. Rev. Bras. Anestesiol 61, 814–828. 10.1016/S0034-7094(11)70091-5 [DOI] [PubMed] [Google Scholar]
- Paredes S, Cantillo S, Candido KD, Knezevic NN, 2019. An association of serotonin with pain disorders and its modulation by estrogens. Int. J. Mol. Sci 20. 10.3390/ijms20225729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP, 1927. Conditioned reflexes Oxford: Humphrey Milford. [Google Scholar]
- Pieretti S, Gianuario A, Goivannadrea R, Marzoli F, Piccaro G, Minosi P, Aloisi AM, 2011. Gender differences in pain and its relief. Ann. Ist. Super. Sanita 52. 10.4415/ANN_16_02_09 [DOI] [PubMed] [Google Scholar]
- Pignata S, Cecere SC, Du Bois A, Harter P, Heitz F, 2017. Treatment of recurrent ovarian cancer. Ann. Oncol 28, viii51–viii56. 10.1093/annonc/mdx441 [DOI] [PubMed] [Google Scholar]
- Pohóczky K, Kun J, Szalontai B, Szöke É, Sághy É, Payrits M, Kajtár B, Kovács K, Környei JL, Garai J, Garami A, Perkecz A, Czeglédi L, Helyes Z, 2016. Estrogen-dependent up-regulation of TRPA1 and TRPV1 receptor proteins in the rat endometrium. J. Mol. Endocrinol 56, 135–149. 10.1530/JME-15-0184 [DOI] [PubMed] [Google Scholar]
- Ponce J, Fernandez- S, Feliubadaló L, Teulé A, Lázaro C, Brunet JM, Candás- B, 2020. Assessment of ovarian reserve and reproductive outcomes in BRCA1 or BRCA2 mutation carriers. Int. J. Gynecol. cancer Off. J. Int. Gynecol 30, 83–88. 10.1136/ijgc-2019-000626 [DOI] [PubMed] [Google Scholar]
- Price D, 1999. Psychological mechanisms of pain and analgesia: Progresss in pain research and managment IASP Press, Seattle. [Google Scholar]
- Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, Song X-J, Stevens B, Sullivan MD, Tutelman PR, Ushida T, Vader K, 2020. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 161, 1976–1982. 10.1097/j.pain.0000000000001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera DR, Ganz PA, Weyrich MS, Bandos H, Melnikow J, 2018. Chemotherapy-Associated Peripheral Neuropathy in Patients With Early-Stage Breast Cancer : A Systematic Review. J. Natl. Cancer Inst 110. 10.1093/jnci/djx140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roett MA, Evans P, 2009. Ovarian Cancer : An Overview. Am. Fam. Physician 80. [PubMed] [Google Scholar]
- Rudick CN, Woolley CS, 2001. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J. Neurosci 21, 6532–6543. 10.1523/jneurosci.21-17-06532.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador F, Llorente A, Gomis RR, 2019. From latency to overt bone metastasis in breast cancer : potential for treatment and prevention. J. Pathol 249, 6–18. 10.1002/path.5292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarajari S, Oblinger MM, 2010. Estrogen effects on pain sensitivity and neuropeptide expression in rat sensory neurons. Exp. Neurol 224, 163–169. 10.1016/j.expneurol.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivers K-Y, Amador N, Abrams L, Hunter D, Jenab S, Quiñones-Jenab V, 2015. Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic–pituitary–adrenal axis activity. Cytokine 72, 121–129. 10.1016/j.cyto.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shufelt C, Merz N, Pettinger M, Choi L, Chlebowski R, Crandall C, Liu S, Dorothy L, Prentice R, Manson J, 2018. Estrogen-Alone Therapy and Invasive Breast Cancer Incidence by Dose, Formulation, and Route of Delivery. Menopause 25, 985–991. 10.1097/gme.0000000000001115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LH, 2006. Early clinical detection of ovarian cancer: a review of the evidence. Expert Rev. Anticancer Ther 6, 1045–52. 10.1586/14737140.6.7.1045 [DOI] [PubMed] [Google Scholar]
- Smith TG, Troeschel AN, Castro KM, Arora NK, Stein K, 2019. original report abstract Perceptions of Patients With Breast and Colon Cancer of the Management of Cancer-Related Pain, Fatigue, and Emotional Distress in Community Oncology. J. Clin. Oncol 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff NP, Grisold A, Grisold W, Windebank AJ, Grisolod W, Windebank AJ, 2018. Chemotherapy-Induced Peripheral Neuropathy: A Current Review. Ann. Neurol 81, 772–781. 10.1002/ana.24951.Chemotherapy-Induced [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson C, Logan SM, Bellinger LL, Rao M, Kinchington PR, Kramer PR, 2019. Estradiol Acts in Lateral Thalamic Region to Attenuate Varicella Zoster Virus Associated Affective Pain. Neuroscience 414, 99–111. 10.1016/j.neuroscience.2019.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani R, Nandy SB, Pedroza DA, Lakshmanaswamy R, 2017. Role of growth hormone in breast cancer. Endocrinology 158, 1543–1555. 10.1210/en.2016-1928 [DOI] [PubMed] [Google Scholar]
- Talbot K, Madden VJ, Jones SL, Moseley GL, Town C, Africa S, 2019. The sensory and affective components of pain : are they differentially modifiable dimensions or inseparable aspects of a unitary experience ? A systematic review. Br. J. Anaesth 123, e263–e272. 10.1016/j.bja.2019.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanos T, Sflomos G, Echeverria P, Ayyanan A, Gutierrez M, Delaloye J-F, Raffoul W, Fiche M, Dougall W, Schneider P, Yalcin-Ozuysal O, Brisken C, 2013. Progesterone/RANKL is a major regulatory axis in the human breast. Sci. Transl. Med 5. 10.1126/scitranslmed.3005654 [DOI] [PubMed] [Google Scholar]
- Taraborrelli S, 2015. Physiology, production and action of progesterone. Mol. Cell. Endocrinol 94, 8–16. 10.1111/aogs.12771 [DOI] [PubMed] [Google Scholar]
- The National Academies of Sciences, Engineering and Medicine. The health effects of cannabis and cannabinoids. The current state of evidence and recommendations for research 2017. https://www.nationalacademies.org. 10.17226/24625. https://www.ncbi.nlm.nih.gov/books/NBK423845/. [DOI] [PubMed]
- Tian JM, Ran B, Zhang CL, Yan DM, Li XH, 2018. Estrogen and progesterone promote breast cancer cell proliferation by inducing cyclin G1 expression. Brazilian J. Med. Biol. Res 51, 1–7. 10.1590/1414-431X20175612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A, Kumar N, Bajpai R, Lal P, 2007. Bone metastasis from ovarian cancer. J. Cancer Res. Ther 3. 10.4103/0973-1482.31969 [DOI] [PubMed] [Google Scholar]
- Valadez-cosmes P, Cerb M, V ER, Camacho-arroyo I, 2016. Molecular and Cellular Endocrinology Membrane progesterone receptors in reproduction and cancer. Mol. Cell. Endocrinol 434, 2008–2017. 10.1016/j.mce.2016.06.027 [DOI] [PubMed] [Google Scholar]
- Velasco I, Rueda J, Acién P, 2006. Aromatase expression in endometriotic tissues and cell cultures of patients with endometriosis. Mol. Hum. Reprod 12, 377–381. 10.1093/molehr/gal041 [DOI] [PubMed] [Google Scholar]
- Vincent K, Tracey I, 2008. Hormones and Their Interaction with the Pain Experience. Rev. Pain 2, 20–24. 10.1177/204946370800200206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware MA, Wang T, Shapiro S, Collet JP, 2015. Cannabis for the Management of Pain: Assessment of Safety Study (COMPASS). J. Pain 16, 1233–1242. 10.1016/j.jpain.2015.07.014 [DOI] [PubMed] [Google Scholar]
- Weiland NG, 1992. Estradiol selectively regulates agonist binding sites on the N-methyl-D-aspartate receptor complex in the ca1 region of the hippocampus. Endocrinology 131, 662–668. 10.1210/endo.131.2.1353442 [DOI] [PubMed] [Google Scholar]
- Wiffen PJ, Wee B, Derry S, Bell RF, Moore RA, 2017. Opioids for cancer pain - an overview of Cochrane reviews. Cochrane Database Syst. Rev 2017. 10.1002/14651858.CD012592.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windebank A, Grisold W, 2008. Chemotherapy-induced neuropathy. J. Peripher. Nerv. Syst 13, 27–46. 10.1111/j.1529-8027.2008.00156.x [DOI] [PubMed] [Google Scholar]
- Wood R, Mitra D, De Courcy J, Iyer S, 2017. Patient-reported pain severity, pain interference and health status in HR + / HER2 − advanced / metastatic breast cancer. ESMO open 2, 1–7. 10.1136/esmoopen-2017-000227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Yang Y, Zhang Yan, Zhang X, Zhao Z, Zhang Yu-qiu, 2013. Estrogen in the Anterior Cingulate Cortex Contributes to Pain-Related Aversion. Cereb. cortex 23, 2190–2203. 10.1093/cercor/bhs201 [DOI] [PubMed] [Google Scholar]
- Yin J, Pollock C, Kelly K, 2005. Mechanisms of cancer metastasis to the bone. Cell Res 15, 57–62. 10.1038/sj.cr.7290266 [DOI] [PubMed] [Google Scholar]
- Zhang M, Sun J, 2013. Bone metastasis from ovarian cancer. Clinical analysis of 26 cases. Saudi Med. J 34, 1270–3. [PubMed] [Google Scholar]
- Zhang Y, Lü N, Zhao ZQ, Zhang YQ, 2012. Involvement of estrogen in rapid pain modulation in the rat spinal cord. Neurochem. Res 37, 2697–2705. 10.1007/s11064-012-0859-1 [DOI] [PubMed] [Google Scholar]
- Zolfaroli I, Tarín JJ, Cano A, 2018. The action of estrogens and progestogens in the young female breast. Eur. J. Obstet. Gynecol. Reprod. Biol 230, 204–207. 10.1016/j.ejogrb.2018.03.057 [DOI] [PubMed] [Google Scholar]


