Abstract
Objective:
Farnesoid-X-activated receptors (FXR) are key modulators of liver regeneration. Milk thistle and Chicory are known as potent protective remedies in several liver disorders. The objective of this work was to examine the role of FXR in the hepato-healing properties of milk thistle (MTE) and chicory extracts (CE) in a rat model of acetaminophen-induced hepatotoxicity.
Materials and Methods:
Male Wistar rats were randomly divided into seven groups including control, vehicle, acetaminophen (500 mg/kg/day, oral), acetaminophen plus oral MTE 200 and 400 mg/kg/day, and acetaminophen plus oral CE 500 and 1000 /kg/day for 28 days. Liver function and histology as well as the pattern of hepatic FXR expression were assessed after 4 weeks.
Results:
Administration of acetaminophen was associated with a significant elevation of liver transaminase along with the architectural injuries. In contrast, chronic concomitant administration of both MTE and CE significantly restored the liver function and structural abnormality. The main molecular findings of the study revealed that the lower doses of both MTE and CE led to a marked upregulation of hepatic FXR expression.
Conclusion:
Discovery of the involvement of the nuclear modulating pathways in hepatoprotective activity of the extracts, providesa new mechanistic insight which needs further investigations.
Key Words: Farnesoid X receptors (FXR), Acetaminophen, Milk thistle, Chicory, Necrosis
Introduction
Several kinds of chemicals and diseases may threaten the liver which depends on the severity of the effect on the hepatic architecture and/or function. In spite of dramatic advances in drug development technology, there is no specific approved drug for chronic and intensive liver damages. It is probably because liver regeneration is not easily possible in advanced progressive failure stages. Some pathological pathways such as oxidative stress and inflammation have a pivotal role in the development and progression of the liver diseases (Jassim, 2013 ▶; Del Campo et al., 2018 ▶). Although the exact mechanistic pathways of liver diseases are not fully understood, several molecular mechanisms have been documented. Farnesoid-X activated receptor, known as FXR, is one of the metabolic nuclear receptors with high hepatic expression which is a master regulator of the bile acid synthesis, conjugation, and enterohepatic circulation (Pathak et al., 2017 ▶), as well as lipid and glucose metabolism (Jiang et al., 2015 ▶; Taoka et al., 2016 ▶). Nowadays, this ligand-gated nuclear receptorsis also considered a key regulator in liver regeneration (Li and Guo, 2015 ▶). It is endogenously activated by bile acids particularly chenodeoxycholic acid (CDCA) (Akhondzadeh et al., 2005 ▶). Several clinical and experimental studies indicated that dysregulation of the hepatic FXR gene expression and/or activity is strongly correlated with the development of chronic liver damages (Zhang et al., 2009 ▶; Lee et al., 2010 ▶).
According to the long-time traditional medicine experiences and experimental and clinical studies, some medicinal herbs are considered potent liver protecting agents. Two medicinal herbs of the Asteraceae family, including milk thistles (Silybum marianum) and chicory (Cichorium intybus), are pharmacologically effective in prevention and even treatment of liver diseases (Kailash and Swatantra Kumar, 2016 ▶; Sadat Sharifi and Bakhshaei, 2017 ▶). Based on this documented hepatoprotective activity and the protective role of FXR in several liver diseases, the present study was designed to find out the possible role of nuclear receptors of FXR in the hepato-healing properties of the two herbs.
Materials and Methods
Chemicals
Acetaminophen powder was obtained from DarouPakhsh Pharmaceutical Manufacturing Company (Temad Co., Karaj, Iran). Ketamine and Xylazine were purchased from Alfasan (Woerden, Holland). Chicory root (C.intybus L.) and milk thistle seed (S.marianum L.) were purchased from Tehran botanical market and authenticated by the Herbarium of the School of Traditional Medicine, ShahidBeheshti University of Medical Sciences (voucher No. HMS-516 and HMS-517 for S.marianum and C.intybus, respectively).
Preparation of the extracts
Chicory extract preparation
To prepare the aqueous extract of chicory root, after washing the roots with cold water, we left them to dry in air at room temperature. Then, the roots were crushed and extracted by 60°C water via percolation method (water/dry root ratio 8:1; extraction time: 10 hr). The extract was filtered and dried using aspray dryer. Feed flow rate was 20 l/hr with inlet and outlet air temperature of 190±2 and 75±2°C, respectively.
Milk thistle extract preparation
The methanolic extract of milk thistle seeds was prepared by percolation method using 99.99% (v/v) methanol. The extract was concentrated using avacuum rotary evaporator (Heidolph, Germany) and left to dry in a desiccator. The extraction yield (w/w) of both herbal extracts was calculated as the weight of dry extract/weight of dry starting material×100.
Quantification of active ingredients in the extracts
The main active constituents of the aqueous extract of C.intybus (as inulin) and methanolic extract of S.marianum (Silymarin: as silibinin) was determined by the quantitative high-performance liquid chromatographic (HPLC: Knauer, Germany) and UV-spectrophotometry (Spectro UV-VIS double beam pc scanning spectrophotometer UVD 2960) methods, respectively.
Animals
Eight-week-old male Wistar rats, weighing 200–250g, were obtained from Royan Animal Breeding Center, Karaj, Iran. They were kept under standard conditions (12 hr light/dark cycle at 20–24°C and 50±5% relative humidity). Animals had free access to food and water during the study. The animal care and experimentation were performed according to the national guidelines and protocols approved by the Research Ethics Committee of Alborz University of Medical Sciences in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No.85-23, revised 1996).
Experimental design and protocol
Forty-nine animals were randomly divided into 7 groups (n=7 rats in each) including a control group, a vehicle group assigned to received 0.3% Carboxy methyl cellulose (CMC)as vehicle, an acetaminophen group assigned to receive acetaminophen at 500 mg/kg/day (oral, suspended in 0.3% CMC ), two groups assigned to receive acetaminophen (500 mg/kg/day, oral) concomitant with chicory extract (CE; 500 and 1000 mg/kg/day,oral), and two groups assigned to receive acetaminophen (500 mg/kg/day, oral) concomitant with Milk Thistle extract (MTE, suspended in 0.3% CMC at 200 and 400 mg/kg/day, oral).
During the study period (28 days), all solutions were prepared freshly just prior to daily administration and given once a day at the same time. In all extract-treated groups, animals received the assigned dose 1 hrafter administration of acetaminophen. For detection of any sign of morbidity and/or mortality, the animals were observed twice a day. At the end of the study, under deep surgical anesthesia using intraperitoneal injections of ketamine (60 mg/kg) and xylazine (8 mg/kg), bilateral thoracotomy was performed, and blood samples were obtained gently from the right ventricle.
Determination of Serum biochemical parameters
Blood samples were collected to determine the serum levels of some biochemical markers including albumin (ALB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) lactate dehydrogenase (LDH) Gamma-glutamyltransferase (GGT), and total and direct bilirubin using Pars Azmun commercial kits (Pars Azmun Co, INC, Karaj, Iran), according to the manufacturer’s guidelines.
Histopathological assessments
For histopathological assessments, the largest right lobe of each liver was removed and immediately fixed in a 10% formalin solution. After dehydration and clearance, the samples were embedded in paraffin wax and sectioned into a 5-μm thickness. Tissue staining using Hematoxylin and Eosin (H&E), Masson's Trichrome, and Reticulin was performed for detecting any pathological signs of toxicity, fibrotic scars, or necrotic lesion, respectively.
Hepatic FXR gene expression using real time RT-PCR technique
Preparation of the samples to identify the expression of the hepatic FXR was done according to the protocol (Safari et al., 2014 ▶). Briefly, about 50 mg of the hepatic tissue was homogenized using a polytron tissue homogenizer (DAIHAN-brand Homogenizing Stirrer, HS-30E; Korea). RNA was then extracted using Trizol (Qiagen) based on the manufacturer’s instructions. Then, the cDNA synthesis was performed using Reverse Transcriptase cDNA synthesis kit (Fermentas), based on the protocol. Expression of FXR was measured by Real-Time PCR using SYBR GREEN (TAKARA). The experiments were performed in duplicates as follows: denaturation at 95°C for 10 min followed by 45 cycles at 95°C for 10 sec and 60°C for 10 sec and 72°C for 10 sec. The expression level of FXR was normalized to
that of GAPDH gene and expressed as fold-change ratio. The exact nucleotide sequences of the FXR and GAPDH primers are shown in Table 1.
Table 1.
The exact nucleotide sequences of the FXR and GAPDH primers
| Genes | Forward | Reverse |
|---|---|---|
| FXR | TGGGAATGTTGGCTGAATG | CCTGTGGCATTCTCTGTTTG |
| GAPDH | GCCTTCTCTTGTGACAAAGTG | CTTCCCATTCTCAGCCTTG |
Statistical analysis
Data presented as Mean±SEM were analyzed using One-way analysis of variance (ANOVA) followed by Duncan's multiple range test for between groups comparisons. A P-value of <0.05 was considered statistically significant. The quantification of gene expressions was analyzed and plotted using REST 2009 (Technical University Munich, Germany) and GraphPad Prism 8.0.2 GraphPad Software Inc., San Diego, CA, USA) software, respectively.
Results
Analytical assessment of MTE and CE
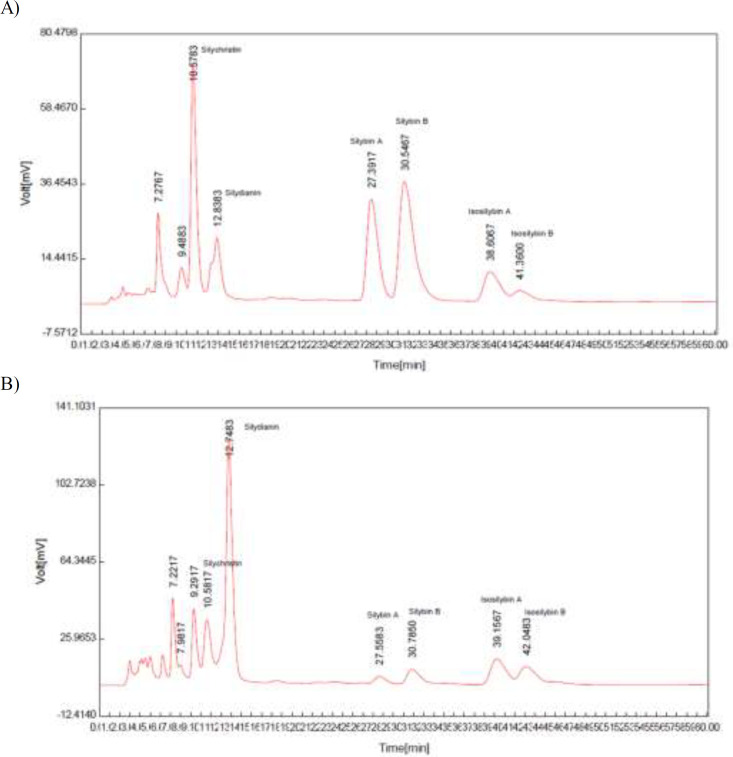
The HPLC chromatograms of standard silymarin and MTE sample are shown in Figure 1. The total amount of active ingredients as silibinin was 50.2% (W/W%) in the MTE sample. Moreover, spectrophotometric UV-Vis analysis of CE revealed that the inulin content of the CE was 43.58% (W/W%).
Figure 1.
Typical HPLC Chromatograms of A) standard silymarin and B) methanolic extract of Milk Thistle seeds (as sample). HPLC instrument (Knauer, Germany) equipped with an Agilent Knauer- UV K2501diode array detector, Knauer- K1001 pump, Agilent Eclipse-XDB-C18 analytical column (125 mm, 4.6 mm, 5μm). The aqueous mobile phase A: phosphoric acid R, methanol R, water R (0.5:35:65 V/V/V) mobile phase B: phosphoric acid R, methanol R, water R (0.5:50:50 V/V/V), mobile phase flow rate 0.8 ml/min, injection volume 20μl
Histopathological examination
Histopathological changes in the liver following acetaminophen treatment
As H&E (Figure 2), Masson's trichrome (Figure 3) and reticulin (Figure 4) staining showed, administration of vehicle did not show any sign of histological alteration compared to the control group. Chronic administration of acetaminophen, however, led to remarkable hepatotoxicity which was characterized by significant congestion, sinusoidal dilation, vacuolization, and necrosis. With lower degrees, other signs of liver toxicity such as inflammatory infiltration, Kupffercell hyperplasia, bile stasis and plugs as well as pyknosis were seen. Although Masson's trichrome staining did not detect any signs of fibrotic bundles following acetaminophen treatment (Figure 3), a dense network of thick reticulin fibers was developed in acetaminophen-treated group (Table 1and Figure 4).
Figure 2.
Hepatic Hematoxylin-Eosin (H&E)-stained sections (X400) in the (A) Control, (B) Vehicle, (C) Acetaminophen, (D and E) Milk Thistle extract (MTE 200 and 400 mg/kg/day) and (F and G) Chicory extract (CE 500 and 1000 mg/kg/day) groups after 28 days
Figure 3.
Hepatic Masson’s trichrome stained sections (X400) in the (A) Control, (B) Vehicle, (C) Acetaminophen, (D and E) Milk Thistle extract (MTE 200 and 400 mg/kg/day) and (F and G) Chicory extract (CE 500 and 1000 mg/kg/day) groups after 28 days
Figure 4.
Hepatic Reticulin stained sections (X400) in the (A) Control, (B) Vehicle, (C) Acetaminophen, (D and E) Milk Thistle extract (MTE 200 and 400 mg/kg/day) and (F and G) Chicory extract (CE 500 and 1000 mg/kg/day) groups after 28 days
Histopathological changes in the liver following CE treatment
According to H&E staining (Figure 2 and Table 1), co-administration of CE with acetaminophen, significantly reversed the signs of acetaminophen-induced liver injury. The observed hepatoprotective effects of both 500 and 1000 mg/kg/day of CE were relatively similar (Table 1). Moreover, thick reticulin fibers markedly disappeared due to CE administration (500 and 1000 mg/kg/kg) (Figure 4).
Histopathological changes in the liver following MTE treatment
As shown in Figure 2 and Table 1, concomitant administration of MTE and acetaminophen was also accompanied bymarked improvement of histopathological injuries induced by chronic administration of acetaminophen.
There was no significant difference in the healing properties of the two doses of MTE (200 and 400 mg/kg/day). In addition, administration of MTE at both doses markedly removed the thick reticulin fibers (Figure 4).
Serum biomarker assessment results
The effects of chronic administration of vehicle or acetaminophen
Compared to the control group, administration of the vehicle was not associated with remarkable changes in the serum levels of ALT, AST, GGT, LDH, ALP and ALB. However, chronic administration of acetaminophen led to a significant increase in ALT (p<0.05), AST (p<0.01) and LDH (p<0.01), but not GGT, ALP, ALB, or total and direct bilirubin serum levels (Table 2).
Table 2.
Histological characteristics of H&E and Trichrome stained liver sections in the control, vehicle-treated, acetaminophen-treated (500 mg/kg/day), Milk Thistle Extract treated (MTE 200 and 400 mg/kg/day) and chicory extract treated groups (CE500 and 1000 mg/kg/day) groups after 28 days
| Groups | Control | Vehicle | Acet.500 | MTE200 | MTE400 | CE500 | CE1000 | |
|---|---|---|---|---|---|---|---|---|
| Number | H&Estaining | |||||||
| 1 | Glycogen depletion | 0 | 0 | + | + | 0 | 0 | 0 |
| 2 | Hemorrhage | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | Congestion | 0 | 0 | +++ | ++ | ++ | ++ | ++ |
| 4 | Sinusoidal dilation | 0 | 0 | ++ | + | + | + | + |
| 5 | Edema | 0 | 0 | ++ | 0 | + | + | + |
| 6 | Inflammatory infiltration (Lymphocytic infiltration) |
0 | 0 | + | + | + | + | 0 |
| 7 | Vacuolization | 0 | 0 | ++++ | +++ | ++ | ++ | + |
| 8 | Bile stasis | 0 | 0 | + | + | 0 | 0 | 0 |
| 9 | Bile plugs | 0 | 0 | + | + | 0 | 0 | 0 |
| 10 | Kupffer cell hyperplasia | 0 | 0 | + | + | + | + | ++ |
| 11 | Pyknosis | 0 | 0 | + | + | 0 | 0 | 0 |
| 12 | Necrosis | 0 | 0 | ++ | ++ | 0 | 0 | 0 |
| Masson's Trichrome staining | ||||||||
| Fibrosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Reticulin staining | ||||||||
| Necrosis | 0 | 0 | ++ | 0 | 0 | 0 | 0 | |
Histological changes were scored as none (0), active damage less than 25% (+), active damage less than 50% (++), active damage less than 75% (+++) damage and active damage more than 75% (++++).
Histological changes were scored as none (0), active damage less than 25% (+), active damage less than 50% (++), active damage less than 75% (+++) damage and active damage more than 75% (++++).
The effects of chronic administration of CE (500 and 1000 mg/kg/day)
As shown in Table 2, chronic administration of CE500 was associated with a significant reduction in ALT (p<0.001), AST (p<0.001), LDH (p<0.01) and ALP (p<0.001) serum levels compared to the acetaminophen-treated group. The LDH decline was obviously more pronounced at the dose of 1000 mg/kg/day compared to 500mg/kg (p<0.05). In contrast, the ALP reduction at 500 mg/kg/day was more significant compared to 1000 mg/kg/day (p<0.05). The serum levels of GGT, ALB, total and direct bilirubin did not show a significant change among the experimental groups.
The effects of chronic administration of MTE (200 and 400 mg/kg/day)
According to Table 2 and compared to the acetaminophen-treated group, serum levels of ALT (p<0.01), AST (p<0.001) and ALP (p<0.01) were significantly reduced in the MTE200 and 400 groups. Moreover, compared to the acetaminophen-treated group, administration of MTE400 was associated with a significant LDH level reduction (p<0.05). The serum levels of GGT, ALB, and total and direct bilirubin did not show significant changes among the experimental groups.
Real time RT-PCR gene expression results
Alteration in the hepatic FXR mRNA expression due to vehicle or acetaminophen treatment
The findings of the real time RT-PCR method revealed that, compared to the control group, chronic administration of the vehicle was not associated with significant changes in the expression of hepatic FXR gene. Hepatic expression of FXR in the acetaminophen-treated group was also accompanied bya non-statistically significant reduction in comparison to the control one (Figure 5).
Figure 5.
Effect of chronic administration of acetaminophen, chicory (A) and milk thistle (B) extracts on the expression of hepatic FXRgene. Experimental groups including Control, Vehicle (0.3% CMC), Acetaminophen (500 mg/kg/day, oral), Milk Thistle extract 200 mg/kg/day, oral (MTE200), 400 mg/kg/day, oral (MTE400), Chicory extract 500 mg/kg/day, oral (CE500) and 1000 mg/kg/day, oral (CE1000). The duration of the study was 4 weeks. Data are presented as mean±SEM (n=7 in each). *p<0.05 and****p<0.0001
Alteration in the hepatic FXR mRNA expression due to CE treatment
The hepatic expression of FXR showed a dose-reversal pattern (Figure 5A). In comparison to the higher dose, administration of the lower dose of CE significantly increased the expression of hepatic FXR; the observed up-regulation of the FXR following 500 mg/kg/day of CE was 8.53 (p<0.0001), 13.22 (p<0.0001) and 6.85 (p<0.001) folds when compared to the control, acetaminophen alone and the high dose of CE, respectively.
Table 3.
The levels (mean±SEM, n=7) of serum biochemical markers including aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH) and gamma-glutamyltransferase (GGT), alkaline phosphatase (ALP), albumin (ALB), total (Total Bil) and direct bilirubin (Direct Bil) in the control, vehicle-treated, acetaminophen-treated (500 mg/kg/day), Milk Thistle Extract treated (MTE 200 and 400 mg/kg/day) and chicory Extract treated (CE500 and 1000 mg/kg/day) groups after 28 days
| AST | ALT | LDH | GGT | ALP | Alb | Total Bil | Direct Bil | |
|---|---|---|---|---|---|---|---|---|
| Control | 104.0±1.51 | 70.0±2.50 | 695.0±76.88 | 2.5±0.64 | 837.6±48 | 3.78±0.07 | 0.20±0.03 | 0.10±0.00 |
| Vehicle | 110.3±3.60 | 73.16±2.15 | 759.8±39.88 | 3.7±0.54 | 767.5±32.2 | 3.68±0.08 | 0.20±0.00 | 0.13±0.01 |
| Acet. | 169.0±15.67** | 148.2±13.76* | 1119.4±136.8** | 2.71±0.96 | 850.0±56.52 | 3.64±0.06 | 0.25±0.02 | 0.11±0.01 |
| MTE200 | 104.5±6.6†† | 65.7±6.0††† | 898.0±58.3* | 2.5±0.5 | 636.3±45††* | 3.65±0.11 | 0.22±0.03 | 0.10±0.01 |
| MTE400 | 118.0±6.5†† | 72.5±5.5††† | 787.5±47.8† | 3.5±0.95 | 640.2±43.3††* | 3.68±0.05 | 0.24±0.02 | 0.11±0.03 |
| CE500 | 92.2±2.5††† | 63.5±7.1††† | 733.6±43.8†† | 2.2±0.47 | 616.1±46.4**†† | 3.80±0.05 | 0.22±0.02 | 0.12±0.02 |
| CE1000 | 98.8±3.9†† | 65.0±2.00††† | 594.2±41.6††● | 3.0±0.22 | 803.5±23.7● | 3.82±0.05 | 0.21±0.03 | 0.10±.02 |
*Significant difference compared tothe control group (*p<0.05 and**p<0.01); †significant difference compared tothe acetaminophen-treated group (†p<0.05, ††p<0.01 and †††p<0.001); ●significant difference compared to the CE500-treated group (●p<0.05)
In contrast, administration of 1000 mg/kg/day of CE, was not accompanied bysignificant alterations in the gene expression in comparison to the control or acetaminophen groups (1.24 vs. 1). As a clear finding, in comparison to lower dose of CE, the higher dose of CE significantly reduced the relative gene expression of the hepatic FXR (1 vs. 0.14, p<0.0001).
Alteration in the hepatic FXR mRNA expression due to MTE treatment
As shown in Figure 5B, the expression level of hepatic FXR gene was negatively correlated with the administered dosage of MTE. Compared to the controls, chronic administration of 200mg/kg/day led to a 6.48-fold (p<0.001) increase in the FXR mRNA expression. Moreover, the expression of FXR was 10.04 (p<0.0001) and 4.48 (p<0.0001) times higher in the 200 mg/kg/day group compared to the acetaminophen alone and 400 mg/kg/day groups, respectively. There was no change in the expression of FXR following administration of MTE at 400mg/kg/day although a2.24-fold up-regulation was seen compared to the acetaminophen-treated group (p<0.04). Generally, compared to the lower dose of MTE, administration of the higher dose was accompanied by a significant FXR mRNA down-regulation (0.22 vs. 1, p<0.0001).
Discussion
The present study was designed to investigate the role of Farnesoid-X-activated receptors in the hepatoprotective effect of Milk Thistle and Chicory extracts. As the main findings of the experiment, different doses of the extracts exhibited a different FXR expression pattern; lower doses in either MTE or CE were associated with marked up-regulation in hepatic FXR gene expression, whereas the dose increment in both groups led to considerable down-regulation of the gene. Indeed, a negative correlation was observed between the FXR gene expression and the level of the dose. Biochemical and histological findings were also similar to those of other previous studies and confirmed the hepatoprotective roles of the extracts for both administered doses. In the present study, however, the chronic administration of acetaminophen was not associated with marked reductions in the expression of hepatic FXR mRNA level.
Acetaminophen-induced hepatic injury is one the practical experimental models of hepatotoxicity characterized by serum abnormality along with histopathological deformity. In line with previous reports (Adil et al., 2016 ▶; Mazraati and Minaiyan, 2018 ▶), administration of acetaminophen in the present study was associated with marked alteration ofthe serum levels of liver enzymes besides obvious histopathological injury. In contrast to our data, Adil et al. findings showed that administration of acetaminophen at 700 mg/kg/day for 14 days was accompanied with a significant down-regulation of hepatic FXR (Adil et al., 2016 ▶). Although the studies were not completely conducted underthe same conditions, it seems that the effect of acetaminophen on the expression of hepatic FXR is more dose-dependent than time-dependent.
Acute and chronic liver diseases are one of leading causes of mortality and morbidity all around the world. The etiology and pathophysiology of these major disorders are not fully known, but there is emerging evidence which uncovers some underlying molecular mechanisms involved in the disease state, in which the role of some specific nuclear receptors, such as farnesoid receptor, is identified. The FXR physiologically plays a major role in modulating the bile acid synthesis and hemostasis in the body (Li and Chiang, 2013 ▶; Jacinto and Fang, 2014 ▶). In addition, it has an important role in glucose and lipid regulatory pathways (Li and Guo, 2015 ▶; Hylemon et al., 2017 ▶). Several clinical and experimental studies support the concept that there is a negative correlation between the hepatic FXR gene expression and development or worsening of the liver disease state (Zhang et al., 2009 ▶; Lee et al., 2010 ▶). As Adil et al. showed, acetaminophen-induced hepatotoxicity was associated with marked down-regulation of hepatic FXR mRNA level (Adil et al., 2016 ▶). Moreover, decrease in bile acid receptor expression in experimental models of liver fibrosis, is another evidence which indicates the crucial role of FXR in the liver pathology. According to Verbeke et al. findings, using a potent selective FXR agonist, obeticholic acid (INT-747), in two models of cirrhotic rats, including bile duct ligation (BDL) and thioacetamide-induced toxicity, led to marked improvement ofendothelial vasodilation via activation of intrahepatic eNOS (endothelial-derived nitric oxide synthase) pathway (Verbeke et al., 2014 ▶). In addition, some experimental models of FXR-deficient animals also confirmeda protective role of these types of nuclear receptors in the liver function and/or architecture (Su et al., 2012 ▶; Kong et al., 2016 ▶). In contrast, there is some evidence showing a significant up-regulation of hepatic FXR expression in some liver disorders (Aguilar-Olivos et al., 2015 ▶). There are also several reports indicating the anti-inflammatory and anti-fibrotic roles of FXR activation in experimental animal models (Shaik et al., 2014 ▶; Massafra et al., 2016 ▶). As Verbeke et al. showed, 4 week administration of obeticholic acid in thioacetamide-induced cirrhotic rats accompanied by marked reduction of pro-inflammatory cytokines and pro-fibrotic markers during thioacetamide-administration, strongly reversed the established cirrhosis (Verbeke et al., 2016 ▶). Despite the above points, there are some discrepancies regarding the protective role of FXR up-regulation in hepatic disorders. A more recent study revealed that using obeticholic acid in reversible bile duct ligated rats for 7 days, was associated with biliary injury exacerbation which was secondary due to up-regulation of the bile salt export pump (van Golen et al., 2018 ▶).
Several species of medicinal plants have historically been considered therapeutic targets in the prevention, palliation and/or treatment of liver disease signs and symptoms. In this regard, two medicinal herbs from the Asteraceae family, including milk thistle (S.marianum) and chicory (C.intybus), are well-known for their hepato-healing properties. Milk Thistle standard extract obtained from seeds of S.marianum, known as silymarin, is composed of 7 flavonolignans and polyphenols, in which, silibinin is considered the main active ingredient (Bijak, 2017 ▶). There are several clinical and experimental investigations which confirm the hepatoprotective role of the extracts and/or their active components. A marked reduction in the plasma levels of liver enzymes such as ALT, AST and ALP by silymarin (de Avelar et al., 2017 ▶) has been frequently reported. Moreover, administration of silymarin in liver disease with different etiologies, led to significant restoration of histopathological and structural abnormality of the liver (Surai, 2015 ▶; de Avelar et al., 2017 ▶). Despite extensive studies, the exact mechanism of the action of silymarin and/or its active component is not fully understood. In this regard, considerable attention has been paid to its anti-oxidative (Stiuso et al., 2014 ▶) and anti-inflammatory properties (Verbeke et al., 2016 ▶; Tsaroucha et al., 2018 ▶). This appears to occur in different ways including direct free radical scavenging activity, inhibition of reactive radical species formation, and mitochondrial function restoration (Surai, 2015 ▶). In addition, according to other related studies, the potent anti-inflammatory property of the extract is due to itsinhibitory effect on the main transcriptional factor of NF-κB (Stevenson and Hurst, 2007 ▶; Gupta et al., 2014 ▶; Surai, 2015 ▶). The latter is involved in several key processes such as inflammatory response, cell differentiation, and apoptosis (Surai, 2015 ▶). Recently, a valuable literature survey has shown that the FXR plays a key modulator role in several metabolic and inflammatory processes (Shaik et al., 2015 ▶). The hepatoprotective action of chicory extract in the literature is also attributed to its prominent antioxidant (Li et al., 2014 ▶; Soliman et al., 2016 ▶) and anti-inflammatory activity (Cavin et al., 2005 ▶).
Given the protective role of FXR which was documented in several lines of evidence, the present study focused on the role of this type of nuclear receptors in the pharmacological effects of MTE and CE. Although biochemical or histopathological findings did not show any negative dose-response relationship, the observed reverse correlation between the dose (for both MTE and CE) and hepatic FXR expression was a considerable point. As compared to the lower doses, the higher ones were surprisingly associated with considerable down-regulation of hepatic FXR. Recently, Adil and his colleagues evaluated the protective effects of naringin against acetaminophen-induced hepatic and renal toxicity (Adil et al., 2016 ▶). They showed that naringin pretreatment markedly restored the hepatic FXR mRNA expression which was damaged by chronic administration of acetaminophen. The study also used silymarin as the positive control at a single daily dose (25 mg/kg/day for 2 weeks), and at the administered dose, it corrected the mRNA expression of the hepatic FXR(Adil et al., 2016 ▶). According to the present findings, it is not clear why administration of higher doses of MTE or CE was associated with obvious reduction ofthe hepatic expression of FXR. The observed down-regulation was the same as the control ones and was not a pathological down-regulation. Interestingly, toxicological findings of chicory showed that compared to the lower dose, higher doses of chicory extract increased the CCL4-induced cytotoxicity inisolated hepatocytes (Jamshidzadeh et al., 2010 ▶). They also showed that chicory extract at higher doses did not protect the liver against CCL4-induced hepatotoxicity (Jamshidzadeh et al., 2010 ▶). In spite of the present findings, there is some evidence indicating that toxicological evaluation of chicory root extract did not show any sign of obvious toxicity in both chronic and acute toxicological assessment tests (Schmidt et al., 2007 ▶; Conforti et al., 2008 ▶). Therefore, such a dose-independent expression pattern of hepatic FXR might be due to participation of other regulatory signaling pathways.
The main limitation of this study was lack of information on alteration of glutathione modulating pathway and its relationship with the FXR gene expression. Further specific molecular investigations are recommended to be conducted to elucidate the exact mechanism(s) of the observed dose-reversal relationship and answer the question whether they are foe or friend at higher doses.
Acknowledgment
This work was supported by the Vice Chancellor for Research Affairs, Alborz University of Medical Sciences.
Conflicts of interest
The authors have declared that there is no conflict of interest.
References
- Adil M, Kandhare AD, Ghosh P, Venkata S, Raygude KS, Bodhankar SL. Ameliorative effect of naringin in acetaminophen-induced hepatic and renal toxicity in laboratory rats: role of FXR and KIM-1. Ren Fail. 2016;38:1007–1020. doi: 10.3109/0886022X.2016.1163998. [DOI] [PubMed] [Google Scholar]
- Aguilar-Olivos NE, Carrillo-Córdova D, Oria-Hernández J, Sánchez-Valle V, Ponciano-Rodríguez G, Ramírez-Jaramillo M, Chablé-Montero F, Chávez-Tapia NC, Uribe M, Méndez-Sánchez N. The nuclear receptor FXR, but not LXR, up-regulates bile acid transporter expression in non-alcoholic fatty liver disease. Ann Hepatol. 2015;14:487–493. [PubMed] [Google Scholar]
- Akhondzadeh S, Tahmacebi‐Pour N, Noorbala AA, Amini H, Fallah‐Pour H, Jamshidi AH, Khani M. Crocus sativus L in the treatment of mild to moderate depression: a double‐blind randomized and placebo‐controlled trial. Phytother Res. 2005;19:148–151. doi: 10.1002/ptr.1647. [DOI] [PubMed] [Google Scholar]
- Bijak M. a Major Bioactive Component of Milk Thistle (Silybum marianum L Gaernt.)-Chemistry, Bioavailability, and Metabolism. Molecules. 2017;22:1942. doi: 10.3390/molecules22111942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavin C, Delannoy M, Malnoe A, Debefve E, Touché A, Courtois D, Schilter B. Inhibition of the expression and activity of cyclooxygenase-2 by chicory extract. Biochem. Biophys Res Commun. 2005;327:742–749. doi: 10.1016/j.bbrc.2004.12.061. [DOI] [PubMed] [Google Scholar]
- Conforti F, Ioele G, Statti GA, Marrelli M, Ragno G, Menichini F. Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food Chem Toxicol. 2008;46:3325–3332. doi: 10.1016/j.fct.2008.08.004. [DOI] [PubMed] [Google Scholar]
- de Avelar CR, Pereira EM, de Farias Costa PR, de Jesus RP, de Oliveira LPM. Effect of silymarin on biochemical indicators in patients with liver disease: Systematic review with meta-analysis. World J Gastroenterol. 2017;23:5004–5017. doi: 10.3748/wjg.v23.i27.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campo JA, Gallego P, Grande L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J Hepatol. 2018;10:1–7. doi: 10.4254/wjh.v10.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Tyagi AK, Deshmukh-Taskar P, Hinojosa M, Prasad S, Aggarwal BB. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch Biochem Biophys. 2014;559:91–99. doi: 10.1016/j.abb.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Hylemon PB, Takabe K, Dozmorov M, Nagahashi M, Zhou H. Bile acids as global regulators of hepatic nutrient metabolism. Liver Research. 2017;1:10–16. doi: 10.1016/j.livres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto S, Fang S. Essential roles of bile acid receptors FXR and TGR5 as metabolic regulators. Animal Cells and Systems. 2014;18:359–364. [Google Scholar]
- Jamshidzadeh A, Khoshnood MJ, Dehghani Z, Niknahad H. Hepatoprotective Activity of Cichorium intybus L Leaves Extract Against Carbon Tetrachloride Induced Toxicity. IJPR. 2010;5:41–46. [Google Scholar]
- Jassim AM. Protective Effect of Petroselinum crispum (parsley) extract on histopathological changes in liver, kidney and pancreas induced by Sodium Valproate-In male Rats. Kufa J Vet Sci. 2013;4:20–27. [Google Scholar]
- Jiang Q, Peng J, Liu SN, Shen ZF. Farnesoid X receptor regulates glucose and lipid metabolisms. 2015;Yao Xue Xue Bao 50:245–251. [PubMed] [Google Scholar]
- Kailash C, Swatantra Kumar J. Therapeutic potential of cichorium intybus in lifestyle disorders: a review. Asian J Pharm Clin Res. 2016;9:20–25. [Google Scholar]
- Kong B, Zhu Y, Li G, Williams JA, Buckley K, Tawfik O, Luyendyk JP, Guo GL. Mice with hepatocyte-specific FXR deficiency are resistant to spontaneous but susceptible to cholic acid-induced hepatocarcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2016;310:G295–G302. doi: 10.1152/ajpgi.00134.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FY, de Aguiar Vallim TQ, Chong HK, Zhang Y, Liu Y, Jones SA, Osborne TF, Edwards PA. Activation of the farnesoid X receptor provides protection against acetaminophen-induced hepatic toxicity. Mol Endocrinol. 2010;24:1626–1636. doi: 10.1210/me.2010-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G-Y, Gao H-Y, Huang J, Lu J, Gu J-K, Wang J-H. Hepatoprotective effect of Cichorium intybus L a traditional Uighur medicine, against carbon tetrachloride-induced hepatic fibrosis in rats. World J Gastroenterol. 2014;20:4753–4760. doi: 10.3748/wjg.v20.i16.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Guo L. Farnesoid X receptor, the bile acid sensing nuclear receptor, in liver regeneration. Acta Pharm Sin B. 2015;5:93–98. doi: 10.1016/j.apsb.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Chiang JYL. Nuclear receptors in bile acid metabolism. Drug Metab Rev. 2013;45:145–155. doi: 10.3109/03602532.2012.740048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massafra V, Ijssennagger N, Plantinga M, Milona A, Ramos Pittol JM, Boes M, van Mil SWC. Splenic dendritic cell involvement in FXR-mediated amelioration of DSS colitis. BBA Mol Basis Dis. 2016;1862:166–173. doi: 10.1016/j.bbadis.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Mazraati P, Minaiyan M. Hepatoprotective Effect of Metadoxine on Acetaminophen-induced Liver Toxicity in Mice. ABR. 2018;7:67–67. doi: 10.4103/abr.abr_142_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak P, Liu H, Boehme S, Xie C, Krausz KW, Gonzalez F, Chiang JYL. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J Biol Chem. 2017;292:11055–11069. doi: 10.1074/jbc.M117.784322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadat Sharifi, Bakhshaei Pharmacological effect of seven medicinal plants as a traditional preparation. IIOABJ. 2017;8:8–12. [Google Scholar]
- Safari F, Bayat G, Shekarforoush S, Hekmatimoghaddam S, Anvari Z, Moghadam MF, Sohrab Hajizadeh. Expressional profile of cardiac uncoupling protein-2 following myocardial ischemia reperfusion in losartan- and ramiprilat-treated rats. Journal of the renin-angiotensin-aldosterone system. JRAAS. 2014;15:209–217. doi: 10.1177/1470320312474050. [DOI] [PubMed] [Google Scholar]
- Schmidt BM, Ilic N, Poulev A, Raskin I. Toxicological evaluation of a chicory root extract. Food Chem Toxicol. 2007;45:1131–1139. doi: 10.1016/j.fct.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaik FB, Prasad DV, Narala VR. Role of farnesoid X receptor in inflammation, and resolution. Inflamm Res . 2015;64:9–20. doi: 10.1007/s00011-014-0780-y. [DOI] [PubMed] [Google Scholar]
- Soliman HA, El-Desouky MA, Hozayen WG, Ahmed RR, Khaliefa AK. Hepatoprotective effects of parsley, basil, and chicory aqueous extracts against dexamethasone-induced in experimental rats. JCMR. 2016;5:65–71. doi: 10.5455/jice.20160124113555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson DE, Hurst RD. Polyphenolic phytochemicals--just antioxidants or much more? Cell Mol Life Sci. 2007;64:2900–2916. doi: 10.1007/s00018-007-7237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiuso P, Scognamiglio I, Murolo M, Ferranti P, De Simone C, Rizzo MR, Tuccillo C, Caraglia M, Loguercio C, Federico A. Serum oxidative stress markers and lipidomic profile to detect NASH patients responsive to an antioxidant treatment: a pilot study. Oxid Med Cell Longev. 2014;2014 doi: 10.1155/2014/169216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Ma C, Liu J, Li N, Gao M, Huang A, Wang X, Huang W, Huang X. Downregulation of nuclear receptor FXR is associated with multiple malignant clinicopathological characteristics in human hepatocellular carcinoma. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1245–1253. doi: 10.1152/ajpgi.00439.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai PF. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants (Basel) 2015;4:204–247. doi: 10.3390/antiox4010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoka H, Yokoyama Y, Morimoto K, Kitamura N, Tanigaki T, Takashina Y, Tsubota K, Watanabe M. Role of bile acids in the regulation of the metabolic pathways. World J Diabetes. 2016;7:260–270. doi: 10.4239/wjd.v7.i13.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaroucha AK, Valsami G, Kostomitsopoulos N, Lambropoulou M, Anagnostopoulos C, Christodoulou E, Falidas E, Betsou A, Pitiakoudis M, Simopoulos CE. Silibinin Effect on Fas/FasL, HMGB1, and CD45 Expressions in a Rat Model Subjected to Liver Ischemia-Reperfusion Injury. J Invest Surg. 2018;31:1–12. doi: 10.1080/08941939.2017.1360416. [DOI] [PubMed] [Google Scholar]
- van Golen RF, Olthof PB, Lionarons DA, Reiniers MJ, Alles LK, Uz Z, de Haan L, Ergin B, de Waart DR, Maas A, JoanneVerheij , Peter L. Olde Damink, FrankG. Schaap. Jansen, StevenW;Thomas M. vanGulik & Michal Heger. 2018. FXR agonist obeticholic acid induces liver growth but exacerbates biliary injury in rats with obstructive cholestasis. Sci rep, 8 doi: 10.1038/s41598-018-33070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke L, Farre R, Trebicka J, Komuta M, Roskams T, Klein S, Elst IV, Windmolders P, Vanuytsel T, Nevens F, Laleman W. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59:2286–2298. doi: 10.1002/hep.26939. [DOI] [PubMed] [Google Scholar]
- Verbeke L, Mannaerts I, Schierwagen R, Govaere O, Klein S, Vander Elst I, Windmolders P, Farre R, Wenes M, Mazzone M, Nevens F, van Grunsven LA, Trebicka J, Laleman W. FXR agonist obeticholic acid reduces hepatic inflammation and fibrosis in a rat model of toxic cirrhosis. Sci Rep. 2016;6 doi: 10.1038/srep33453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang J, Liu Q, Harnish DC. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J Hepatol. 2009;51:380–388. doi: 10.1016/j.jhep.2009.03.025. [DOI] [PubMed] [Google Scholar]