Abstract
Introduction:
Despite the great promise for therapies using antisense oligonucleotides (ASOs), their adverse effects, which include pro-inflammatory effects and thrombocytopenia, have limited their use. Previously, these effects have been linked to the phosphorothioate (PS) backbone necessary to prevent rapid ASO degradation in plasma. The main aim of this study was to assess the impact of the nucleic acid portion of an ASO-type drug on platelets and determine if it may contribute to thrombosis or thrombocytopenia.
Methods:
Platelets were isolated from healthy donors and men with advanced prostate cancer. Effects of antisense oligonucleotides (ASO), oligonucleotides, gDNA, and microRNA on platelet activation and aggregation were evaluated. A mouse model of lung thrombosis was used to confirm the effects of PS-modified oligonucleotides in vivo.
Results:
Platelet exposure to gDNA, miRNA, and oligonucleotides longer than 16-mer at a concentration above 8 mM resulted in the formation of hypersensitive platelets, characterized by an increased sensitivity to low-dose thrombin (0.1 nM) and increase in p-Selectin expression (6–8 fold greater than control; p < 0.001). The observed nucleic acid (NA) effects on platelets were toll-like receptor (TLR) -7 subfamily dependent. Injection of a p-Selectin inhibitor significantly (p = 0.02) reduced the formation of oligonucleotide-associated pulmonary microthrombosis in vivo.
Conclusion:
Our results suggest that platelet exposure to nucleic acids independent of the presence of a PS modification leads to a generation of hypersensitive platelets and requires TLR-7 subfamily receptors. ASO studies conducted in cancer patients may benefit from testing the ASO effects on platelets ex vivo before initiation of patient treatment.
Keywords: Platelets, Antisense oligonucleotides, Toll-like receptors, Prostate Cancer
1. Introduction
Over the past decade, antisense oligonucleotides (ASOs) have emerged as a promising therapeutic tool for disease intervention [1-3].
ASOs are composed of single-strand DNA that is 15–25 nucleotides long and complementary to target mRNA [4,5]. Since the initial discovery in 1978 as a tool to silence viral nucleic acid in cell culture, synthetic oligonucleotides have been tested as gene modulating drugs in a variety of diseases, including cancer [5-7]. Despite numerous clinical trials over the years, only a limited number of oligonucleotide-based drugs have been approved for clinical use [7]. Toxicity remains a fundamental concern with the clinical application of most ASOs, and off-target effects as a result of ASO therapeutics are fairly common [4,8]. Improved understanding of the mechanisms of ASO-associated toxicity is important since several promising ASO-based therapeutics have recently failed safety trials [4,9]. In one human trial, up to 20% of the trial participants who received the ASO based therapy developed a pulmonary embolism [9], while a phase III human trial of two other ASOs resulted in severe thrombocytopenic events halting any further human testing [10]. Interestingly, a dose-dependent decline in platelet numbers has been frequently observed not only in humans but also in animal studies including those conducted in monkeys and primates [11].
Previous studies investigating the causes of ASO associated adverse effects on platelets have phosphorothioate (PS) backbone, that provides drug’s resistance to nucleases, may be the cause of some of the ASO-associated toxicities [12-15]. An investigation into the causes of oligonucleotide-based drug-associated thrombocytopenia often observed in human and animal trials [16,17], demonstrated that exposure of nuclease resistant ASO to platelets in vitro and in vivo resulted in dose-dependent platelet activation that was concluded to be also caused by the presence of a PS modification [12,13].
To improve the safety of the oligonucleotide-based drugs, a 2′-O-methoxyethyl (2′-MOE) modifications that similarly resist the nuclease degradation were introduced in the second generation ASOs [10]. Although some of the adverse effects were reduced with the use of the second generation ASOs, thrombocytopenia remained a major concern [4,18].
Interestingly, platelet exposure to DNA and RNA molecules in vitro was previously shown to lead to platelet activation [19-21]. Platelet expression of toll-like receptors (TLR) responsible for nucleic acid recognition is well known [22,23]. Activation of platelet TLRs by foreign nucleic acids (e.g. viral, bacterial) triggers several hemostatic and inflammatory responses, including the formation of platelet-leukocyte aggregates and thrombin generation [24,25]. Therefore, a nucleic acid-based drug, such as an ASO, may promote platelet activation if the drug comes in direct contact with platelets.
In the present study, we aimed to investigate the roles of platelet TLR-7,-8,-9 in platelet activation by nucleic acids and to assess if the increased risk of thrombocytopenia or thrombosis observed in vivo and human trials for ASO-type drugs may be associated with platelet-activating actions by the nucleic acid portion of the drug.
2. Material and methods
2.1. Human platelet isolation
Written informed consent in accordance with the Declaration of Helsinki was received from all participants before inclusion in the study. All patient samples were obtained under an institutional review board–approved protocol at the University of Michigan following informed consent. Whole blood was collected using BD Vacutainer Plus Citrate Tubes (BD cat# 363083). PRP was obtained from whole blood by centrifugation (200 rcf for 20 min at room temperature). PRP was then transferred into a separate tube containing acid citrate dextrose (ACD) (2.5%) for an additional centrifugation step (200 ×g for 7 min) to remove any contaminating blood cells (e.g. lymphocytes). PRP was then transferred into a separate tube and platelets were precipitated by centrifugation (800 rcf for 10 min) in the presence of apyrase (0.02 U/mL) and washed once in wash buffer (140 mM NaCl, 5 mM KCl, 12 mM trisodium citrate, 10 mM glucose, 12.5 mM sucrose, pH 6.0). Harvested platelets were counted using HEMAVET 950 FS (The Americas Drew Scientific Inc.) and resuspended in Tyrode’s solution (137 mmol/L NaCl, 2 mmol/L KCl, 12 mmol/L NaHCO3, 0.3 mmol/L NaH2PO4, 1 mmol/L MgCl2, 2 mmol/L CaCl2, 5.5 mmol/L glucose, and 5 mmol/L HEPES, pH 7.3, containing 0.35% BSA) before use.
2.2. Fluorescent oligonucleotide and oligonucleotide sequences
Non-targeting Phosphorothioate-modified (PS) ASO: NC5 PS 5′-G*C*G* A*C*T* A*T*A* C*G*C* G*C*A* A*T*A* T*G-3′.; non-targeting 2-O-methoxyethyl (MOE)-modified PS-ASO (PS/ME): NC5 2 O Me/PS 5′-mG*mC*mG* mA*mC*T* A*T*A* C*G*C* G*C*A* mA*mU*mA* mU*mG-3′ (* indicates PS chain presence, m indicates 2′ O-Methyl RNA base); non-targeting fluorescently labeled oligo P-TGA GGT AGT AGG TTG TAT AGT T-C6-A647; GCG ACT ATA CGC GCA ATA TG; GCG ACT ATA CGC GCA ATA TGG CGC GCG CAAT; were all from Integrated DNA Technologies, Inc. (Coralville, Iowa, cat# 259509703 and cat#259509704).
2.3. Nucleic acid preparation
Genomic DNA was purified from cultured PC-3 cells using the Gentra Puregene Cell Kit (QIAGEN, Cat. No. 158767) and DNA concentration was measured using the spectrophotometer NanoDrop 2000 (Thermo Scientific). Isolated DNA was stored in 1.5 mL DNA LoBind Tubes (Eppendorf, Cat No. 022431021) at −20 °C. To generate shorter length DNA 50 mers, DNA fragmentation was achieved with dsDNA Fragmentase (NEBioLabs, cat: MO348A) by incubating gDNA with the enzyme for 35 min at 37C per manufacturer’s protocol. The four cancer-associated precursor-microRNAs (pre-miRNA) were ordered from Ambion by Life Technologies (Product No. AM17100): hsa-pre-miR-132-3p (Product ID PM10166), hsa-pre-miR-134-5p (Product ID PM10341), hsa-pre-miR-141-3p (Product ID PM10860), and hsa-pre-miR-181c-5p (Product ID PM10181). The miRNAs were resuspended in nuclease-free water to a concentration of 50 μM/μl and stored at −20 °C until use.
2.4. Cell culture
Human prostate cancer cell lines PC-3 were obtained from the ATCC (Manassas, VA). All cells were authenticated by the University of Michigan DNA Sequencing Core using short tandem repeat DNA fingerprinting; all cells were mycoplasma negative. All cells were maintained in RPMI 1640 medium (Gibco; Life Technologies) supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine, 100 U/ml penicillin G sodium.
2.5. Platelet aggregation
Washed human platelets were adjusted to a concentration of 3 × 108 platelets/ml. Prior to aggregation experiments platelets were incubated with PS- or PS/ME-ASOs, PC-3 cancer cell-derived genomic DNA, or pre-miRNAs for 30 min at 37 °C under static conditions. Platelet incubations with ASOs, gDNA, pre-miRNA, or proteins (thrombospondin-1 or Epidermal Growth Factor) in all experiments from here on were performed using 1.5 ml LoBind Tubes (Eppendorf, cat# 022431021). The aggregation response to human α-thrombin (2700 NIH units/mg; i.e., units obtained by direct comparison with a National Institutes of Health Thrombin reference standard), purchased from Enzyme Research Laboratories (South Bend, IN), PS-ASO (24 mM) or gDNA (24 mM) isolated from prostate cancer cell line (PC-3) cells was measured using a lumi-aggregometer (Model 700D, Chronolog Corp) with stirring at 1200 rpm at 37 °C.
2.6. Flow cytometry sample preparation
Washed platelets were adjusted to a concentration of 6 × 108 platelets/ml before incubation with gDNA, PS-ASO, miRNA, or proteins (thrombospondin-1 or Epidermal Growth Factor) at an appropriate concentration. Platelet p-Selectin expression was established using CD62P PE Ab (BD Bioscience, Cat: 555524). Samples were diluted 50 fold before being analyzed using a Bio-RAD ZE5 cell analyzer. Data were analyzed using FlowJo (version 10) flow cytometry software (FlowJo LLC, Ashland, OR).
2.7. TLR-7,-8,-9 inhibition
For TLR-7/8/9 inhibition washed platelets (6 × 108 platelets/ml) were incubated in the dark with TLR7/8/9 antagonist at 2 μg/ml (ODN 2088, Miltenyi Biotec Inc.) for 30 min at 37 °C.
2.8. In vivo thrombosis model
All animal use protocols complied with the Principles of Laboratory and Animal Care established by the National Society for Medical Research and were approved by the University of Michigan Committee on Use and Care of Animals. Briefly, 8–10 week old male and female NOD-SCID or wild type C57Bl/6 J (from Jackson laboratory) weighing 26–39 g were used. PS- or ME/PS-ASO at appropriate doses was administered intravenously (i.e. via tail vein) in a fixed volume of 100 μl. To establish the effect of p-Selectin inhibitor (Santa Cruz BioTech, Cat: sc-220688), 10 mg/kg resuspended in 100 ul of PBS was administered intravenously for 10 min before the transfusion of PS-ASO. The total duration of the experiment was 16 min, and all animals were euthanized by exposure to isoflurane vapors, followed by cervical dislocation.
2.9. Transmission electron microscopy (TEM)
Washed platelets were adjusted to a concentration of 6 × 108 platelets/ml, left untreated, or co-incubated with either PS-ASO or DNA for 20 min. Platelets were then added onto the Thermanox Plastic coverslips (Nunc, Rochester NY) and centrifuged at 2200 RPM for 10 min. The slides were then fixed in 2.5% Glutaraldehyde in 0.1 M Cacodylate Buffer, pH 7.2 at 4 °C overnight before being transferred to the University of Michigan for analyses. The full protocol for sample preparation is available at (https://brcf.medicine.umich.edu/wp-content/uploads/2019/12/Conv_TEM_Cells_Protocol_WJC.pdf). Images were acquired using JEOL JEM 1400 PLUS TEM.
2.10. Fluorescent microscopy
Washed platelets were adjusted to a concentration of 1 × 108 platelets/ml and then co-incubated with 1 mm of fluorescently labeled single-stranded oligonucleotide (P-TGA GGT AGT AGG TTG TAT AGT T-C6-A647) for 20 min. Platelets were then added onto the Thermanox Plastic coverslips (Nunc, Rochester NY) and centrifuged at 1000 RPM for 5 min. The slides were then fixed in 4% formaldehyde for 20 min. Platelets were then stained with WGA-Fluor488 (ThermoFisher cat# W11261) per the manufacturer’s protocol. Images were taken with Olympus TE 2000 BX41 microscope equipped with an Olympus UPlanFL N 100×/1.3 FN26.5 objective and an Olympus U-HGLGPS lamp. Images were acquired with an Olympus DP73 CCD camera. Electronic shutters and image acquisition were under the control of Olympus cellSens Standard 1.7 software. Images were acquired by fluorescence microscopy with an image capture time of 200 to 500 ms.
2.11. Lung histology
Euthanized animals were perfused with PBS and lungs were perfused additionally with PBS through the trachea. Lungs were then fixed in 10% formalin for at least 24 h, and embedded in paraffin. Several sections were cut and stained with H&E. Hematoxylin and eosin (H&E) stained slides were reviewed under light microscopy by a board-certified surgical pathologist (A.M.U.), who was blinded to other experimental data. Essentially as described previously [26], for ten non-overlapping representative 400× fields, the number of vessels with aggregated platelets and/or fibrin plugs was counted and divided by the number of total vessels to determine the percentage of vessels with aggregated platelets and/or fibrin plugs.
2.12. Statistical analysis
Student’s t-test was used to evaluate significance between pairs of groups. For comparison among multiple groups, data were assessed by using one-way ANOVA. P value <0.05 was considered significant. Analyses were performed using GraphPad Prism software and presented as mean ± standard error (SE).
Statistical analysis comparing the number of occluded vessels was performed using Negative Binomial regression to account for overdispersion (p = 0.003). Groups means are reported with 95% confidence intervals constructed by exponentiating the corresponding Wald-type intervals on the log-scale. Negative binomials regressions were fit in R using the package MASS. Overdispersion was found to be present using the odTest function from the pscl package which implements a likelihood ratio test.
3. Results
3.1. PS-ASO and nucleic acids generate prothrombotic and highly reactive platelets
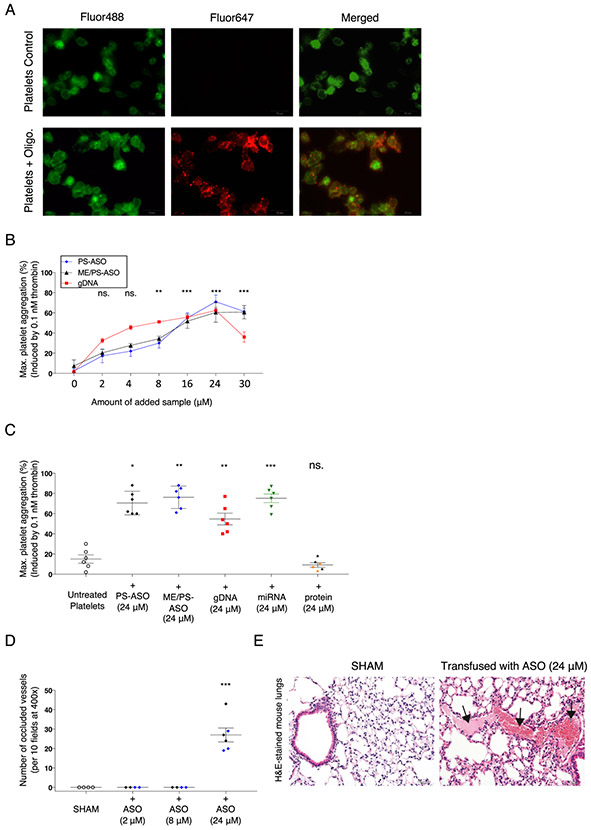
Most ASO drugs are typically 8–50 base pairs in length; hence, we initially evaluated whether platelets are able to internalize a relatively short fluorescently labeled oligonucleotide. We observed that incubation of human platelets with a fluorescently labeled nucleic acid (NA) sequence (20-mer) lead to oligonucleotide accumulation on the surface of platelets (Fig. 1A). Previous studies have observed the direct effect of NAs (DNA and RNA) and ASOs with phosphorothioate (PS) modifications on platelets. Therefore, we investigated if similar effects are observed using gDNA from prostate cancer cells (PC-3) and the two non-targeted PS- and ME/PS-modified oligonucleotide sequences (PS-ASO) chosen for our studies. We assessed platelet aggregation in response to low-dose thrombin following exposure to PS-modified ASOs and found that platelet exposure to gDNA or to oligonucleotide sequence with stabilizing modifications (PS or ME/PS) dramatically increased platelet aggregation in a dose-dependent manner, with the highest response for platelets incubated with 24 μM of ASO (Fig. 1B and Supplemental Fig. 1).
Fig. 1.
PS- and ME/PS- ASO and nucleic acids generate prothrombotic and highly reactive platelets. A. Representative fluorescence microscopy image of platelets stained with WGA-Fluor488 (green) after co-incubation with a CY5-labeled non-targeted oligonucleotide (20-mer) (red); scale bar = 10 μm. Right: A magnified section showing oligonucleotide (red) co-localization with platelets (green). B. An increase in platelet sensitivity to thrombin was observed following incubation with ASO-2′O-ME/PS or PS-ASO at various concentrations (0–30 μM). The data are presented as the average of 3 independent experiments showing the maximum fold increase of aggregation to thrombin (0.1 nM) after the addition of increasing amounts of PS- or ME/PS-modified nucleic acid sequence (ASO). The error bars indicate standard deviations. Platelets from 3 different healthy donors were used. C. An increase in platelet sensitivity to thrombin (0.1 nM) following incubation with oligo, PS-ASO (n = 3), ME/PS-ASO (n = 3), gDNA (n = 6) from PC-3 cells, mixture of four pre-miRNAs (Supp. Fig. 1, n = 6) or proteins (thrombospondin-1) (n = 3, orange) or epidermal growth factor (n = 3, black), was observed. Maximum aggregations after 3 min are shown. The error bars indicate standard deviations. *p = 0.02, **p < 0.01, ***p < 0.001, ns - not significant. D. Histological quantification of occluded vessels (normalized to the total number of vessels) in the lungs of mice injected (IV) with ASO (ME/PS-ASO (blue), or PS-ASO (black)) at various concentrations (2 μM (n = 4), 8 μM (n = 4), 24 μM (n = 6)). The vessels were counted under light microscopy at 400× magnification. The number of clotted vessels was recorded per 10 fields. ***p < 0.001. E. Representative images of H&E-stained lung tissues from animals transfused with PS-ASO (24 μM) or ME/PS-ASO (24 μM) revealed intravascular fibrin precipitation and thrombus formation (black arrows). Lungs from mice following transfusion of PS-ASO (24 μM). (H&E, 200× magnification, scale bar = 500 μm).
We then assessed whether the generation of the hypersensitive platelets [27] may be a result of exposure to random nucleic acid molecules too. We performed aggregation studies using the same high dose of PS and ME/PS – modified ASOs, gDNA from prostate cancer cells (PC-3), a mixture of four single-stranded miRNAs, and two different platelet proteins (Thrombospondin-1 and Epidermal Growth Factor). We found that all nucleic acids used in our aggregation study promoted the formation of platelets with increased sensitivity to low-dose thrombin (Fig. 1C). Importantly, we found that the NA effect on platelets was not unique to cell origin (Supplemental Fig. 2) and only exposure to exogenous nucleic acids, not proteins, generated hypersensitive platelets.
Next, to establish the impact of PS- modified ASOs in vivo, mice were injected (via tail vein) with saline (SHAM) or with PS- or ME/PS modified sequence at varying concentrations (2 mM, 8 mM, and 24 mM). Histologic analysis performed after the modified sequence exposure revealed the presence of pulmonary microvascular thrombosis only in the lungs of animals exposed to the highest dose (24 mM) of PS- or ME/PS-ASOs (Fig. 1D and E). Notably, in human ASO trials where thromboembolic events were observed [9], the administered dose was 900 mg/kg and 1150 mg/kg, both significantly greater than the 30 mg/kg (equivalent to 24 mM) used in our in vivo study.
3.2. Platelet uptake of nucleic acids is dependent on TLR-7/8/9 and results in increased P-selectin expression
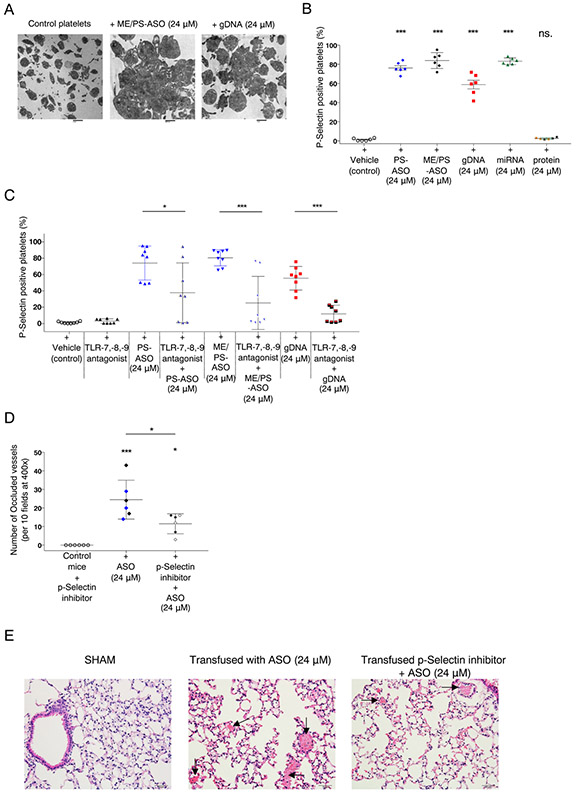
Since previous studies suggested that PS-ASO exposure may increase platelet consumption [13], we examined platelets incubated with PS-ASOs using electron microscopy and noted platelet aggregate formation following exposure to ME/PS-ASOs and gDNA (Fig. 2A). To confirm platelet ability to form aggregates, we performed platelet aggregation after incubations with PS-ASOs or gDNA in the absence of agonists. We found that although platelet spontaneous aggregation was very weak, platelets exposed to NA were significantly more prone to aggregation than the vehicle treated controls (Supplemental Fig. 3). Generation of hyperreactive platelets following nucleic acid exposure encouraged us to examine the effect of PS-ASO and NAs on platelet activation. Flow cytometry analysis of platelets exposed to both PS- and ME/PS- modified oligonucleotides, nucleic acids (gDNA and pre-miRNAs), and proteins (TSP1 or EGF) demonstrated a significant increase in platelet activation, indicated by an increase in p-Selectin-positive platelets after exposure to NAs, but not proteins (Fig. 2B).
Fig. 2.
Platelet uptake of ASOs and nucleic acids is dependent on TLR-7/8/9 and results in increased P-selectin expression. A. Representative electron microscopy image of healthy donor platelets (scale bar = 2 μm) and platelet aggregates after platelet incubation with ASO-2′O-ME/PS (24 μM). Scale bar = 600 nm. Platelet aggregates after platelet incubation with gDNA (24 μM). (scale bar = 1 μm). B. Representative histogram showing flow cytometric analysis of healthy donor platelet p-Selectin expression following platelet co-incubation with PS-ASO (24 μM, n = 6), gDNA (24 μM, n = 6), pre-miRNA (Supp. Fig. 1, 24 μM, n = 6) and protein (TSP1 or EGF). C. Summary of multiple flow cytometry experiments examining p-Selectin expression following co-incubation of platelets from healthy donors with the TLR-7,-8,-9 antagonist PS-ASO (24 μM, n = 8) or gDNA (24 μM, n = 8), alone or in the presence of the TLR-7,-8,-9 antagonist. D. Histological quantification of occluded vessels (normalized to the total number of vessels) in the lungs of mice injected (IV) with the p-Selectin inhibitor (n = 6) PS (black, n = 3) - or ME/PS-ASO (24 μM) (blue, n = 3) or with a p-Selectin inhibitor followed by injection of the modified ASO (24 μM)(n = 6). The vessels were counted under light microscopy at 400× magnification. The number of clotted vessels was recorded per 10 fields. *p = 0.027 (p-Selectin inhibitor vs. p-Selectin inhibitor + ASO), *p = 0.012 (ASO vs. p-Selectin inhibitor + ASO), E. Representative images of H&E-stained lung tissues from animals transfused with PS- or ME/PS-ASO (24 μM) or with p-Selectin inhibitor, followed by PS- or ME/PS-ASO (24 μM). (H&E, 200× magnification, scale bar = 500 μm).
To determine the mechanism of NA-induced activation of platelets, we assayed the functional role of platelet Toll-like receptors (TLRs) -7,-8,-9, shown to be activated by exogenous nucleic acids [23,24]. Given that deletion of Tlr9 in mice leads to impaired thrombosis [28], we aimed to investigate the impact of TLR function on NA-induced platelet activation. Hence, we evaluated platelet activation marker p-Selectin expression following platelet exposure to PS- and ME/PS-ASO or gDNA in the presence of a TLR-7,-8,-9 antagonist [29]. The inhibition of TLR-7,-8,-9 was sufficient to inhibit platelet activation, diminished generation of p-Selectin-positive platelets (Fig. 2C), and prevented platelet aggregation in response to low dose thrombin (Supplemental Fig. 4). A previous study showed the ability to inhibit platelet aggregate formation with p-Selectin monoclonal antibodies [30]. To assess if the observed effects of PS- and ME/PS-ASOs in vivo may be impeded by interfering with p-Selectin function, we injected mice with a p-Selectin inhibitor followed by transfusion with PS-ASO (24 μM). Although both groups of animals transfused with PS- or ME/PS-ASO or the combination of the p-Selectin inhibitor and PS- or ME/PS-ASO showed the presence of pulmonary microvessel thrombosis, overall, there was a significant decrease in the number of occluded vessels within the group of animals treated with the p-Selectin inhibitor prior to modified oligonucleotide treatment (Fig. 2D and E).
3.3. PS- and ME/PS- modified oligonucleotides decrease platelet concentration in vitro
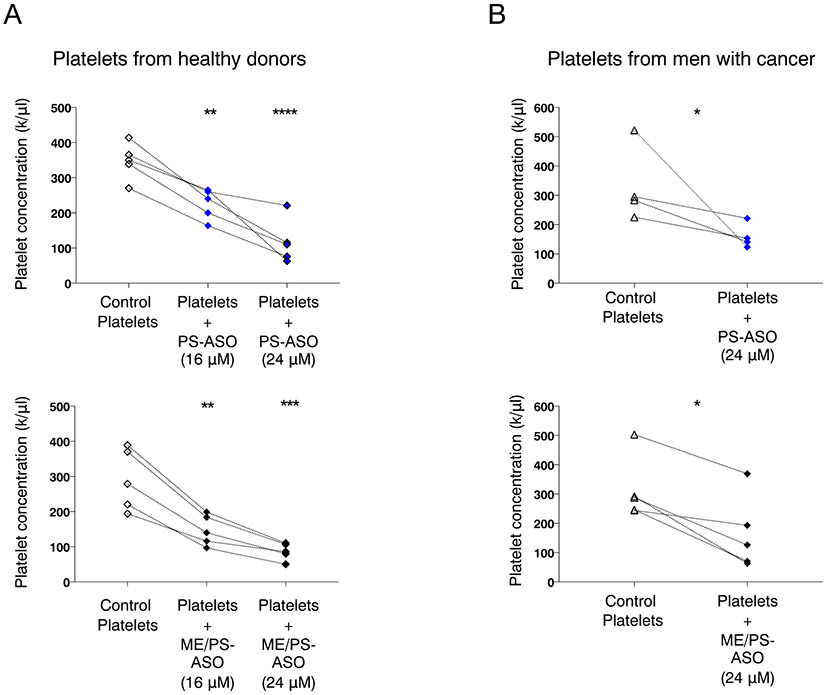
Because it is not possible to accurately assess whether the non-targeting PS- and ME/PS modified oligonucleotides used in our experiments may induce thrombocytopenia in healthy donors, we measured the platelet concentration before and after the incubation of platelets with PS- or ME/PS-ASOs in vitro. For these experiments, we exposed platelets from healthy donors (n = 9) to two doses of modified oligonucleotides (16 and 24 μM). We observed a significant decrease in platelet concentrations following incubation with 16 μM PS- or ME/PS-ASO and an even more significant decrease after platelets were exposed to a higher dose (24 μM) of oligonucleotide (Fig. 3A). Similarly, we then analyzed the effect of PS- an ME/PS-ASOs on platelet concentration from patients with advanced cancer (n = 9 patients with CRPC) not on antiplatelet drugs (e.g. aspirin, clopidogrel). We found that modified oligonucleotide incubation with platelets from men with metastatic prostate cancer resulted in a similar decrease in platelet concentration (Fig. 3B).
Fig. 3.
Platelet exposure to ASOs leads to decrease in platelet numbers ex vivo. A. Platelet concentration of healthy donor platelets (n = 10, line connects the same donor across experiments) before and after exposure to PS-ASO (16 μM or 24 μM) (upper panel) or to or ME/PS-ASO (16 μM or 24 μM) (lower panel). A significant decrease in the number of platelets was observed after the addition of an ASO-type molecules. **p = 0.004, **p = ***p < 0.001 B. Platelet concentrations in patients with prostate cancer (n = 9) before and after exposure to PS-ASO (24 μM) (upper panel), or ME/PS-ASO (24 μM) (lower panel). A significant decrease in the number of platelets was observed after the addition of ASO-type molecules; PS-ASO *p = 0.041, ME/PS-ASO *p = 0.015.
4. Discussion
The most common stabilizing modification for oligonucleotide-based drugs is the PS chain that prevents nuclease degradation in blood. Previous studies investigating the effects of PS chain on platelet biology observed dose-, sequence- and length-dependent platelet activation [12,13]. The same studies also concluded that the platelet activation by PS-modified oligonucleotides did not involve TLR9, a receptor whose agonist sequence was used in their studies. Meanwhile, activation of platelet TLR9 receptor by various factors including nucleic acid has been previously demonstrated to be associated with platelet hyperreactivity and activation [19,31,32]. Exposure of platelets to influenza, a single-stranded RNA virus, demonstrated a TLR7 dependent platelet activation with subsequent upregulation of platelet activation marker p-Selectin [33]. Together these studies indicate that nucleic acid-induced platelet activation may be associated with platelet TLRs.
In our work, we observed that exposure of platelets to several types of nucleic acids even in the absence of PS chain lead to platelet increased expression of activation marker p-Selectin, and this effect could be blocked by pretreatment of platelets with TLR-7,-8,-9 antagonist. The electron microscopy studies of platelet TLR9 showed increased expression on platelets following platelet exposure to type IV collagen [32], suggesting that TLR9 platelet surface expression may be affected by external factors.
In addition to the direct effects of nucleic acid effects on platelets, several studies have described nucleic acid associated changes in coagulation as well [19,26]. In our study, we did not explore the effect of PS-modified or -unmodified sequences on coagulation, and additional studies are needed to investigate whether ASO-type drug-associated hematologic complications may also be a result of changes in coagulation profiles. Our findings do, however, suggest that that interference with the functions of platelet TLR-7,-8,-9 or p-Selectin may be sufficient to mitigate some of the experimental prothrombotic PS-ASO effects.
5. Conclusion
In this study, we investigated and characterized the biological effects of the nuclease-resistant oligonucleotides, gDNA and miRNA on human platelet function. We show that platelet uptake of PS-modified and unmodified nucleic acids via TLR-7,-8,-9 results in the generation of hypersensitive platelets that are prone to aggregation. Our studies also suggest that NA-associated platelet hypercoagulability may be minimized by the addition of a p-Selectin inhibitor. Future research is needed to assess if the generation of hypercoagulable platelets may be associated with the uptake of cell-free DNA in cancer patients and if those patients, in particular, might be at a higher risk to develop thrombotic complications associated with ASO treatments. The results from the present work indicate that ASO studies conducted in cancer patients may benefit from initially testing the ASO effects on platelets ex vivo before initiation of treatment.
Supplementary Material
Acknowledgments
Generous support for this study was provided by Mr. John Glasnak and the Deborah F. and Richard J. Haller Family to A.Z. and G.S.P. The study was also funded in part by the following grants to M.H.: HL114405, GM131835 and GM105671; and in part by the Prostate Cancer Foundation, A. Alfred Taubman Medical Research Institute, and a Department of Defense Physician Research Training Award (W81XWH-14-1-0287) to T.M.M.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.thromres.2021.01.006.
References
- [1].Stein CA, Castanotto D, FDA-approved oligonucleotide therapies in 2017, Mol. Ther 25 (5) (2017) 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schoch KM, Miller TM, Antisense oligonucleotides: translation from mouse models to human neurodegenerative diseases, Neuron. 94 (6) (2017) 1056–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Le BT, Raguraman P, Kosbar TR, Fletcher S, Wilton SD, Veedu RN, Antisense oligonucleotides targeting angiogenic factors as potential cancer therapeutics, Mol. Ther. Nucleic Acids 14 (2019) 142–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chi X, Gatti P, Papoian T, Safety of antisense oligonucleotide, and siRNA-based therapeutics, Drug Discov. Today 22 (5) (2017) 823–833. [DOI] [PubMed] [Google Scholar]
- [5].Verma A, Recent advances in antisense oligonucleotide therapy in genetic neuromuscular diseases, Ann. Indian Acad. Neurol 21 (1) (2018) 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zamecnik PC, Stephenson ML, Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide, Proc. Natl. Acad. Sci. U. S. A 75 (1) (1978) 280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Roberts TC, Langer R, Wood MJA, Advances in oligonucleotide drug delivery, Nat. Rev. Drug Discov 19 (10) (2020) 673–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jason TL, Koropatnick J, Berg RW, Toxicology of antisense therapeutics, Toxicol. Appl. Pharmacol 201 (1) (2004) 66–83. [DOI] [PubMed] [Google Scholar]
- [9].Chowdhury S, Burris III HA, Patel M, Infante JR, Jones SF, Voskoboynik M, et al. , A phase I dose escalation, safety and pharmacokinetic (PK) study of AZD5312 (IONIS-ARRx), a first-in-class Generation 2.5 antisense oligonucleotide targeting the androgen receptor (AR), Eur. J. Cancer 69 (2016) S145. [Google Scholar]
- [10].Crooke ST, Baker BF, Witztum JL, Kwoh TJ, Pham NC, Salgado N, et al. , The effects of 2′-O-methoxyethyl containing antisense oligonucleotides on platelets in human clinical trials, Nucleic Acid Ther. 27 (3) (2017) 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Narayanan P, Shen L, Curtis BR, Bourdon MA, Nolan JP, Gupta S, et al. , Investigation into the mechanism(s) that leads to platelet decreases in cynomolgus monkeys during administration of ISIS 104838, a 2′-MOE-modified antisense oligonucleotide, Toxicol. Sci 164 (2) (2018) 613–626. [DOI] [PubMed] [Google Scholar]
- [12].Flierl U, Nero TL, Lim B, Arthur JF, Yao Y, Jung SM, et al. , Phosphorothioate backbone modifications of nucleotide-based drugs are potent platelet activators, J. Exp. Med 212 (2) (2015) 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sewing S, Roth AB, Winter M, Dieckmann A, Bertinetti-Lapatki C, Tessier Y, et al. , Assessing single-stranded oligonucleotide drug-induced effects in vitro reveals key risk factors for thrombocytopenia, PLoS One 12 (11) (2017), e0187574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Levin AA, A review of the issues in the pharmacokinetics and toxicology of phosphorothioate antisense oligonucleotides, Biochim. Biophys. Acta 1489 (1) (1999) 69–84. [DOI] [PubMed] [Google Scholar]
- [15].Shen W, De Hoyos CL, Migawa MT, Vickers TA, Sun H, Low A, et al. , Chemical modification of PS-ASO therapeutics reduces cellular protein-binding and improves the therapeutic index, Nat. Biotechnol 37 (6) (2019) 640–650. [DOI] [PubMed] [Google Scholar]
- [16].Henry S, Kim T, Kramer-Sticklund K, Zanardi T, Fey R, Levin A, Antisense Drug Technology. Principles, Strategies, and Applications, 2008, pp. 327–364. [Google Scholar]
- [17].Faqi AS, A Comprehensive Guide to Toxicology in Preclinical Drug Development, Academic Press, 2012. [Google Scholar]
- [18].Chi KN, Zoubeidi A, Gleave ME, Custirsen (OGX-011): a second-generation antisense inhibitor of clusterin for the treatment of cancer, Expert Opin. Investig. Drugs 17 (12) (2008) 1955–1962. [DOI] [PubMed] [Google Scholar]
- [19].Bhagirath VC, Dwivedi DJ, Liaw PC, Comparison of the proinflammatory and procoagulant properties of nuclear, mitochondrial, and bacterial DNA, Shock. 44 (3) (2015) 265–271. [DOI] [PubMed] [Google Scholar]
- [20].Fiedel BA, Schoenberger JS, Gewurz H, Modulation of platelet activation by native DNA, J. Immunol 123 (6) (1979) 2479–2483. [PubMed] [Google Scholar]
- [21].Zakharian RA, Rukhkian LA, The mechanism of the proaggregant action on the thrombocytes of RNA and DNA molecules, Nauchnye Doki Vyss Shkoly Biol Nauki (2) (1990) 31–35. [PubMed] [Google Scholar]
- [22].Hally KE, La Flamme AC, Larsen PD, Harding SA, Platelet Toll-like receptor (TLR) expression and TLR-mediated platelet activation in acute myocardial infarction, Thromb. Res 158 (2017) 8–15. [DOI] [PubMed] [Google Scholar]
- [23].Semple JW, Italiano JE Jr., Freedman J, Platelets and the immune continuum, Nat. Rev. Immunol 11 (4) (2011) 264–274. [DOI] [PubMed] [Google Scholar]
- [24].Cognasse F, Nguyen KA, Damien P, McNicol A, Pozzetto B, Hamzeh-Cognasse H, et al. , The inflammatory role of platelets via their TLRs and Siglec receptors, Front. Immunol 6 (2015) 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].LP DA, Schattner M, Platelet toll-like receptors in thromboinflammation, Front. Biosci. (Landmark Ed) 22 (2017) 1867–1883. [DOI] [PubMed] [Google Scholar]
- [26].Gresele P, Momi S, Berrettini M, Nenci GG, Schwarz HP, Semeraro N, et al. , Activated human protein C prevents thrombin-induced thromboembolism in mice. Evidence that activated protein c reduces intravascular fibrin accumulation through the inhibition of additional thrombin generation, J. Clin. Invest 101 (3) (1998) 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Packham MA, Methods for detection of hypersensitive platelets. Philadelphia, June 1977, Thromb. Haemost 40 (1) (1978) 175–195. [PubMed] [Google Scholar]
- [28].El-Sayed OM, Dewyer NA, Luke CE, Elfline M, Laser A, Hogaboam C, et al. , Intact Toll-like receptor 9 signaling in neutrophils modulates normal thrombogenesis in mice, J. Vasc. Surg 64 (5) (2016) 1450–1458 (e1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hackstein H, Knoche A, Nockher A, Poeling J, Kubin T, Jurk M, et al. , The TLR7/8 ligand resiquimod targets monocyte-derived dendritic cell differentiation via TLR8 and augments functional dendritic cell generation, Cell. Immunol 271 (2) (2011) 401–412. [DOI] [PubMed] [Google Scholar]
- [30].Merten M, Thiagarajan P, P-selectin expression on platelets determines size and stability of platelet aggregates, Circulation. 102 (16) (2000) 1931–1936. [DOI] [PubMed] [Google Scholar]
- [31].Panigrahi S, Ma Y, Hong L, Gao D, West XZ, Salomon RG, et al. , Engagement of platelet toll-like receptor 9 by novel endogenous ligands promotes platelet hyperreactivity and thrombosis, Circ. Res 112 (1) (2013) 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Thon JN, Peters CG, Machlus KR, Aslam R, Rowley J, Macleod H, et al. , T granules in human platelets function in TLR9 organization and signaling, J. Cell Biol 198 (4) (2012) 561–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Koupenova M, Vitseva O, MacKay CR, Beaulieu LM, Benjamin EJ, Mick E, et al. , Platelet-TLR7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis, Blood. 124 (5) (2014) 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.