Abstract
Introduction:
Osteonecrosis of the jaw (ONJ) is an adverse event that requires association of both systemic risk factors, such as powerful anti-resorptives (pARs; e.g. zoledronic acid [ZOL]), and local oral risk factors (e.g. tooth extraction, periodontitis). Whereas optimal oral health prior to initiate pARs is recognized as critically important for minimizing ONJ risk, the efficacy of preventive/maintenance measures in patients who are taking pARs is understudied. Rice rats fed a standard diet (STD), rich in insoluble fiber, develop localized periodontitis. STD-rats with localized periodontitis treated with ZOL for 18–24 wk develop ONJ. Hence, we hypothesized that controlling/preventing localized periodontitis in the ZOL-treated rats, reduces ONJ occurrence.
Methods:
We used two approaches to attempt reducing periodontitis prevalence: 1) periodontal cleaning (PC); and 2) replacing the STD-diet with a nutritionally-equivalent diet high in soluble fiber (SF). 75 four-week-old male rats were weight-randomized into five groups (n = 15) in a 24-week experiment. Three groups ate the STD-diet and two the high SF-diet. STD-diet groups received intravenous (IV) vehicle (VEH) q4wks (STD + VEH), 80 μg/kg ZOL q4wks IV (STD + ZOL), or ZOL plus PC q2wks (STD + ZOL + PC). The SF-diet groups received VEH (SF + VEH) or ZOL (SF + ZOL). Jaws were processed for histopathology and evaluated for ONJ prevalence and tissue-level periodontitis.
Results:
1) 40% of STD + VEH rats developed maxillary localized periodontitis with no ONJ; 2) 50% of STD + ZOL rats developed ONJ; 3) 7% of STD + ZOL + PC rats developed ONJ (p < 0.01 vs. STD + ZOL); and 4) one SF + ZOL rat developed localized periodontitis, and no SF + VEH or SF + ZOL rats developed ONJ (p < 0.001 vs. STD + ZOL).
Conclusions:
1) Periodontal cleaning in ZOL-treated rats decreases localized periodontitis severity and reduces ONJ prevalence; and 2) feeding a SF-diet to ZOL-treated rats reduces both incidence of localized periodontitis and ONJ. Our data indicates strong oral microbial community shifts according to oral health condition and trends in the shifts associated with diet.
Keywords: Prevention, Controlling, ONJ, Periodontitis, Oral microbiome, Rice rats
1. Introduction
Osteonecrosis of the jaw (ONJ) is a potentially severe adverse event defined as exposed bone, or bone that can be probed through an intraoral or extraoral fistula, in the maxillofacial region for more than eight weeks, in patients with no history of radiation therapy or metastatic disease in the jaws, who have been treated with powerful antiresorptives (pARs), including nitrogen-containing bisphosphonates (N-BPs, e.g. zoledronic acid [ZOL]) [1,2] and anti-RANKL antibodies (e.g. denosumab) [1-4], or angiogenesis inhibitors [1,2,5-9]. Though pAR-related ONJ is more common in patients with cancer (1.8–5% incidence) than with osteoporosis (0.01–0.03% incidence) [1,2,10,11], all data suggest a similar general ONJ pathophysiology in the two patient populations [1,2,12].
Both clinical and preclinical data suggest that most ONJ cases require concurrent systemic risk factors (e.g. pARs or angiogenesis inhibitors) and local oral risk factors, which include tooth extraction [1,2,12-17], inflammatory dental disease (e.g., periodontitis, periapical infection), and ill-fitting removable partial prostheses [1,2,12-15,18-23].
Once patients taking pARs develop ONJ, the clinical management can be challenging and the outcomes are not only difficult to predict, but also often problematic [24-26]. The American Association of Oral and Maxillofacial Surgeons (AAOMS) guidelines for preventing ONJ in patients about to begin pAR therapy advise a two-phase approach: 1) dental assessment with completion of any procedures recommended to remedy all inflammatory dental disease; and 2) oral hygiene instruction and initiation of appropriate dental care going forward [2]. Though the incidence of ONJ has declined as these measures have been implemented [27], it remains a significant problem [12,28,29]. Importantly, the efficacy of applying appropriate dental care measures in patients with cancer who are already taking pARs (patients at risk), has been the subject of limited formal investigation [28,29].
Refinement of established animal models for ONJ to better understand ONJ pathophysiology is highly desirable. ONJ models that use standard laboratory rodents (Rattus and Mus), which are resistant to developing periodontitis [30], require experimental means to induce periodontitis, that can include application of a ligature around a molar in the presence/absence of pARs administration [18,31]. On the other hand, ONJ models that use the rice rat (Oryzomys palustris), which is naturally prone to develop generalized [32-35] or localized periodontitis [36], do not require additional means to induce the condition. Thus, the challenge for standard laboratory rodents is to induce enough periodontitis, which when combined with pARs, induces ONJ. In contrast, since rice rats are naturally prone to periodontitis, the challenge for this species is to create a negative model for periodontitis, for which the effects of pAR treatment of the natural periodontitis-positive environment (positive model) can be accurately validated. Impaction of dietary insoluble fiber in the gingival sulcus is a risk factor for localized periodontitis in the rice rat [36], and rice rats with localized periodontitis develop a high prevalence of ONJ lesions when receiving clinically-relevant doses of ZOL [22]. We hypothesized that controlling or preventing localized periodontitis in ZOL-treated rice rats, reduces the occurrence of ONJ. To test our hypothesis, we made use of the established ONJ model in rice rats with localized periodontitis [22], combined with two innovative approaches: 1) implementing periodontal maintenance measures to control localized periodontitis by periodic periodontal cleaning of the impacted dietary material in the gingival sulcus of rice rats; and 2) preventing the development of localized periodontitis (negative model) by replacing the insoluble fiber of the STD diet (cellulose) with high levels of soluble fiber (SF diet) [37,38] in a nutritionally-similar diet to reduce the likelihood of food impaction.
Diet composition affects the oral microbiota in humans and laboratory rodents [39-42]. Furthermore, the physical and chemical properties of the dietary chow often influence periodontal health status. Specifically, we observed that localized periodontitis is often present in rice rats fed a diet high in insoluble fiber (STD diet) [36] and that generalized periodontitis often occurs in rice rats fed a high sucrose and casein diet (HSC) [32-34]. To further characterize the ONJ model in rice rats with periodontitis [22], we analyzed the oral microbiota to determine the effects of diet and health status of the periodontium on the relative abundance of microbial communities in the gingiva of rice rats.
2. Materials and methods
2.1. Animal care and management
Rice rats were generated in-house using a monogamous continuous breeding system [43]. Care and management were undertaken as before [21,22]. Justifications for using the localized periodontitis model [22,36,44] and the high SF diet are detailed in Supplemental material.
2.2. Study design
Seventy five clinically healthy, male rice rats (age 4 wks, body weight [BW] ≥30 g, and body condition scores ≥3.0) were randomized into five groups (N = 15/group): 1) standard diet (STD) + vehicle (VEH) (STD + VEH); 2) STD diet + ZOL (STD + ZOL); 3) STD diet + ZOL + periodontal cleaning (PC) (STD + ZOL + PC); 4) high soluble fiber (SF) diet + VEH (SF + VEH); and 5) high SF diet + ZOL (SF + ZOL) (Supplemental Fig. 1A). The standard rodent chow (Envigo Teklad LM-485 [irradiated 7912], Indianapolis, IN, USA) used in the STD groups induces a high prevalence of localized periodontitis [22,36,44]. The high SF diet (Research Diets Inc.; Product # D15073101, New Brunswick, NJ, USA) used in the SF groups is nutritionally-similar and replaces the insoluble fiber of the STD diet with 7.5% inulin and 10% fructo-oligosaccharides that represent prebiotic soluble fiber [37,38,45] (see Supplemental material for details). VEH rats were injected intravenously (IV) with sterile saline solution every 4 wks. ZOL rats received an oncology equivalent dose of ZOL (80 μg/kg BW) IV every 4 wks [19,21,22,46]. ZOL, provided by Novartis Pharma AG (Basel, Switzerland), was dissolved in sterile saline (pH 7.2, 0.2 mg/mL) and injected at 0.4 mL/100 g BW into the tail vein of each rat placed in a rodent restrainer. Bi-weekly oral exams, BW measurements, and injections started at weaning (age 4 wks) and continued for 24 wks. Bi-weekly periodontal cleaning of localized periodontitis lesions at interdental maxillary molars (M)2M3 spaces was performed as needed in STD + ZOL + PC rats, simultaneously with the bi-weekly oral exam, starting at the second week of treatments (age 6-wks) and continuing throughout the study (see below for details). Rats with BW loss or body condition score deterioration were monitored daily and offered diet gel and supplemental fluids. All rats were necropsied after 24 weeks at age 28-wks.
The current study is part of a larger investigation [44]. Following the principle of reduction, as one of the 3Rs principles applied to animal research [47,48], we utilized the same concurrent STD + VEH and STD + ZOL groups as previously reported [44].
2.3. In vivo oral exams
Oral exams were performed bi-weekly under anesthesia in all rats to evaluate the prevalence and severity of maxillary periodontal lesions, particularly at interdental M2M3, where localized periodontitis lesions usually develop [44]. Rats were presented in random order to calibrated investigators (EJC, JGM) who were blinded to the treatment group of the rat. Each maxillary quadrant was assigned a gross quadrant grade (GQG) indicative of lesion severity (Table 1) [22,44].
Table 1.
Criteria for gross quadrant grades (GQG).
| GQG | Degree | Gross lesion description |
|---|---|---|
| 0 | Absence | None |
| 0.5 | Incipient | Hair strands and food particles in an interdental space with no soft tissue inflammation. |
| 1 | Slight | Discrete lesion in an interdental space; minimal gingival recession; impacted materials (hair, food debris); inflammation at margin of impacted materials. |
| 2 | Mild | Single open wound lesion in an interdental space with ulceration/ recession of lingual gingiva along 1/2 to 2/3 the lingual gingival margin of one involved molar; limited extension into the lingual mucosa; inflammation/swelling/redness at margin of impacted material; or presence of two separate GQG = 1 lesions (one always lingual, the other lingual or buccal) in the same quadrant. |
| 3 | Moderate | Open wound involving gingival recession/ulceration along entire lingual gingival margin of two molars; ulceration extending into lingual mucosa towards midline in maxilla or onto lingual plate (mandible); inflammation/ swelling/redness at margin of impacted material; possible involvement of buccal mucosa. |
| 4 | Severe | Open wound involving gingival recession/ulceration around all three molars; ulceration extending into lingual mucosa towards the midline in maxilla or onto lingual plate (mandible) and onto the buccal mucosa; marked gingival inflammation/swelling/ redness along lingual gingival margin of all molars; possible tooth migration or loss. |
2.4. Periodontal cleaning procedure
The periodontal cleaning procedure is intended to simulate a treatment that patients receive during periodontal maintenance and could be considered “appropriate ongoing dental care” [2]. All rats in the STD + ZOL + PC group were first orally examined and assigned a GQG. When impacted material was observed at the palatal aspect of any maxillary M2M3 interdental space during an oral exam, periodontal cleaning was immediately done using dental loupes (4× magnification) with #23 explorer and bent forceps until no impacted material was visible. Care was taken to minimize trauma to the gingival tissues. This protocol continued every 2 wks throughout study in the STD + ZOL + PC group. The periodontal cleaning procedure is in line with periodontal maintenance therapy in humans, which targets debriding accretions from sites afflicted by periodontitis.
2.5. Euthanasia and tissue collection
After 24 weeks, rats were euthanized by CO2 inhalation, followed by cervical dislocation. Blood was collected by cardiac puncture and the serum separated and stored at −20 °C. Jaws were excised and trimmed and high resolution photographs from maxillae and mandibles were taken as previously described [22]. Quadrants were fixed at 4 °C in 4% paraformaldehyde for 48 h and then transferred to 70% ethanol. Microcomputed tomography (MicroCT) scans of selected quadrants were obtained, followed by histopathologic and immunohistochemistry analyses.
2.6. Gross quadrant grade (GQG) scoring
To assess the severity of gross jaw lesions and guide selection of quadrants for MicroCT and histopathologic examination, a GQG scoring system was applied [22,44] (Table 1). In vivo GQGs were assigned many times to the maxillary quadrants only during oral exams. Ex vivo GQGs were assigned once to both maxillary and mandibular quadrants by examining the high resolution photographs. All photographs were presented for GQG scoring in a randomized order to treatment-blinded investigators (EJC; JIA; DBK) [44]. The in vivo GQG for a rat represents the higher GQG of its two maxillary quadrants. The ex vivo GQG for a rat represents the highest GQG among its four quadrants.
2.7. Quantification of maxillary gross oral lesion area
Gross maxillary oral lesion area (Le.Ar, mm2) and total maxillary area (Tt.Mx.Ar, mm2) in quadrants with GQG ≥ 1 were measured using high resolution photographs with the outline tool of the AxioVision SE64 Rel software version 4.9.1 (Carl Zeiss, Germany) as before [22]. Le.Ar was expressed as a percentage of Tt.MxAr (=100 * Le.Ar / Tt.Mx.Ar).
2.8. MicroCT
MicroCT scanning was performed on a subset of 36 maxillary quadrants based on GQG as follows: 1) STD + VEH with GQG = 0 (n = 6) and STD + VEH with GQG ≥ 1 (n = 6); 2) STD + ZOL with GQG ≥ 1 (n = 6); 3) STD + ZOL + PC with GQG ≥ 1 (n = 6); 4) SF + VEH with GQG = 0 (n = 6); and 5) SF + ZOL with GQG = 0 (n = 6). Images were acquired using the following parameters: 80 kVP/120 μA, 0.5 mm aluminum filter, 2 k camera resolution, 10 μm voxel size, 0.5° rotation step, and 360° tomographic rotation. Cross-sectional images were reconstructed using a filtered back-projection algorithm (Bruker Skyscan, NRecon, Kontich, Belgium) as previously described [49] and per recommendations of the American Society of Bone and Mineral Research [50]. The 2D images were aligned identically in the mesiodistal and axial planes with all molars simultaneously visible [44]. Quantitative volumetric analysis of alveolar bone is described briefly in Supplemental material and depicted in Supplemental Fig. 2A and B.
2.9. Histopathologic analysis
2.9.1. Specimen selection, preparation, and examination of jaw quadrant sections
All maxillary and mandibular quadrants with GQG ≥ 1 underwent histopathologic examination. In GQG = 0 rats, the left maxilla and mandible were examined. Quadrants were decalcified in 5% formic acid [22,32]. Jaw tissues were paraffin-embedded and serially sectioned in the mesiodistal plane at 4-μm thickness at five to six different intraquadrant levels separated by about 250 μm. A multi-level examination of each quadrant was conducted on palatal (one or two levels), interdental (M1M2, M2M3; three levels), and buccal regions (one level). Paraffin sections were stained with hematoxylin and eosin (H&E) and then analyzed by one observer (EJC), who was blinded to both GQG and treatment.
2.9.2. Definition of ONJ and quantification of necrotic bone
For rats with two or more GQG ≥ 1 quadrants, the quadrant with the higher GQG from each arch was initially analyzed. If this quadrant was free of ONJ, we evaluated the quadrant with the next highest GQG ≥ 1, until the rat was either diagnosed as ONJ-positive or ONJ-free. A rat with ONJ in one or more quadrants was considered ONJ-positive.
We used a histopathologic approach to diagnose ONJ (Table 2). Exposed bone was identified by the absence of overlying gingival epithelium and lamina propria [19,21,22]. Necrotic bone was identified using: 1) a pattern recognition approach [51-53], and 2) specific criteria from preclinical ONJ studies that require bone matrix containing ≥10 adjacent lacunae that were either empty or contained pyknotic osteocyte nuclei or cellular debris [54,55]. Two observers (EJC, JIA) surveyed all levels independently. When their diagnoses differed, agreement was reached by reviewing relevant slides together.
Table 2.
Criteria for histopathologic PD Score.
| PD Score |
Degree | Description |
|---|---|---|
| 0 | Absence | None |
| 1 | Slight | Gingivitis: slight hyperplasia of the gingival epithelium, intraepithelial inflammatory cell infiltration. Occasional modest bacterial plaque accumulation. Normal lamina propria, periodontal ligament and alveolar bone. |
| 2 | Mild | Periodontitis: gingival hyperplasia and slight migration of junctional epithelium at interdental space. Moderate accumulation of bacterial plaque and hair/food debris. Limited buccolingual extension of lesion. Inflammatory cell infiltration of the gingival epithelium, lamina propria, and periodontal ligament. Mild disruption of periodontal ligament; mild alveolar bone loss. |
| 3 | Moderate | Gingival hyperplasia, marked apical migration of junctional epithelium at interdental region. Marked accumulation of bacterial plaque and hair/food debris. Buccolingual extension of lesion. Moderate inflammatory cell infiltration of lamina propria, disruption of periodontal ligament; moderate alveolar bone loss. |
| 4 | Severe | Marked apical migration of junctional epithelium with near full root exposure or exposure of bifurcation. Marked accumulation of bacterial plaque and hair/food debris. Marked buccolingual and mesiodistal extension of lesion. Marked inflammatory cell infiltration of lamina propria, disruption of periodontal ligament; severe alveolar bone loss |
| ONJ | Exposed, necrotic bone; frequent bacterial colonies directly on bone surfaces. Lack of overlying gingival epithelium and lamina propria. Severe destruction of the periodontium, fibrosis and inflammatory cell infiltration of soft oral tissues. One or more fields of 10 or more contiguous empty osteocyte lacunae. |
Data collection for osteocyte lacunae was performed at 200× magnification in sections within a 0.15–0.25 mm2 region of interest that contained localized periodontitis. Bone area and total area in the region of interest were measured (Osteomeasure; Decatur, GA, USA). The total number of osteocyte lacunae and the number of empty lacunae were counted, and the percentage of empty lacunae and the number of empty lacunae per bone area (#/mm2) were calculated [21,22,44]. For analysis, the data for each group were divided into GQG = 0 and GQG ≥ 1 subsets to allow for comparison of rats with and without gross lesions by treatment (VEH or ZOL).
2.9.3. Assessment of periodontitis
2.9.3.1. PD Score.
The severity of periodontal lesions was assessed histopathologically [19,21,44] (Table 2). H&E-stained sections of each quadrant were analyzed by one observer (EJC). A PD Score was assigned for each quadrant to each rat. The PD Score for a rat is the greatest PD Score found among its quadrants. Quadrants and rats with PD Score = 1 were considered positive for gingivitis, while quadrants and rats with PD Score ≥ 2 were considered positive for periodontitis.
2.9.3.2. Alveolar bone loss.
Alveolar bone loss was determined by measuring alveolar bone height at interdental regions of M1M2 and M2M3. It is defined as the distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) in mesiodistal plane sections of maxillae and mandibles (Supplemental Figs. 3B and 4G) [32,35]. Measurements were made by a blinded observer (EJC) on tissues randomized by GQG and treatment. Interdental spaces showing a complete absence of alveolar bone were assigned a default value of 1500 μm [32,35].
2.9.4. Immunohistochemical detection of TRAPc5b+ (osteoclasts), CD 15+(neutrophils), and CD 68+ (monocyte lineage) cells
A rabbit polyclonal anti-TRAPc5b antibody (ab192780, Abcam; Cambridge, UK; 1.6 μg/mL), a rabbit monoclonal anti-CD15 (FUT4/1478R) antibody (NBP2–53367, Novus Biologicals, Centennial, CO; 35 μg/mL) and a mouse monoclonal anti-CD68 antibody (ab31630, Abcam, Cam bridge, MA; 3.3 μg/mL) were used to characterize the inflammatory response of periodontal tissues to treatments by IHC as previously described [21]. TRAPc5b is a marker for osteoclasts [44]. CD15 (FUT4/1478R) is a marker for neutrophils [56], and CD68 is a marker for cells in the monocyte lineage including macrophages, pericytes, and osteoclasts [57]. The region of interest began 300 μm mesial to the M1 mesial root and ended 300 μm distal to the M3 distal root. The region of interest was bounded by the CEJ on the coronal aspect and the superior border of the maxillae on the apical aspect. Bone perimeter (B.Pm; mm) and osteoclast number in the region of interest were counted. The number of TRAcP5b+ cells per mm B.Pm was calculated (#/mm).
2.9.5. Analyses of the oral microbiome
Diet composition affects not only the gut [58-61] but also the oral microbiota [39-42] in humans and laboratory rodents. Furthermore, the physical and chemical properties of the dietary chow often influence periodontal health status. We hypothesized that in rice rats, the oral microbiota differs by diet and oral health status. The aims of the oral microbiome analysis were to compare the composition of microbial communities in: 1) gingiva of rice rats fed the STD, HSC, and high SF diets; and 2) gingiva of rice rats fed the STD diet with: a) healthy periodontal tissues (no periodontitis); b) periodontitis; and c) ONJ.
2.9.5.1. Tissue collection.
We used gingival samples from rice rats of the current study and from archived samples of a previous study that included rice rats fed the HSC diet for 24 weeks [21,22,44]. Each group contained 7–8 samples from rats of both genders, age 22–40 wks. Sample material was collected from: 1) gingiva from the maxillary M2M3 interdental space from rice rats receiving STD, HSC, and high SF diets, and 2) gingiva from rats fed the STD diet with the following features: a) healthy periodontal tissues (no localized periodontitis); b) localized periodontitis, and c) ONJ lesions. Gingiva were excised with a sterile scalpel blade and forceps at necropsy. Gingival samples were placed in DNA extraction buffer, flash frozen in liquid nitrogen, and stored at −80 °C until the time of extraction.
2.9.5.2. DNA extractions and sequencing.
Genomic DNA was extracted (DNEasy Powersoil Pro Kit; Qiagen Co.; Hilden, DE) per manufacturer instructions. The extracted DNA was submitted to the Medical University of South Carolina Genomics Center for 16S rRNA gene DNA amplification. Bacterial 16S rRNA gene amplicons were sequenced utilizing the standard Illumina protocol (Illumina, Inc.; San Diego, CA) (see Supplemental material for details).
2.9.6. Statistics
We used the chi-square test to determine whether diet/treatments were related to prevalence of gross lesions (in vivo). Fisher Exact test was used to examine the association between diet/treatments and prevalence of rats with gross lesions (ex vivo) and histopathologic oral lesions. Differences in body weight and oral exam GQG (in vivo) were assessed with two-way ANOVA on repeated measures, with treatment and treatment duration as the main effects. Ex vivo maxillary lesions by GQG, alveolar bone loss, empty osteocyte lacunae, TRAP5b+, CD15+ and CD68+ cells were evaluated by two-way ANOVA. Significant differences among groups were determined by Holm-Sidak post hoc analysis. MicroCT was assessed with one-way ANOVA followed by Holm-Sidak post hoc analysis. The statistical analyses of the oral microbiota are described in Supplemental material. Data are expressed as Mean ± SD, except when otherwise indicated. P values less than 0.05 were considered statistically significant.
3. Results
3.1. General observations
Almost all rats completed the study uneventfully except for three SF + VEH rats and one STD + ZOL rat, which were found dead in their cages without preceding clinical signs of disease. Body weight did not differ at any time among the groups (Supplemental Fig. 1B).
3.2. Gross analysis of oral lesions
3.2.1. In vivo exams
40–60% of the STD-diet groups developed persistent maxillary lesions (GQG ≥ 0.5) by Week 7 (age 11 wks), with no significant differences among the STD-diet groups in prevalence (Fig. 1A). Rats in the SF + VEH and SF + ZOL groups had 0 and 7% prevalence of gross maxillary lesions throughout the study, respectively (Fig. 1A), with a significantly lower prevalence compared to the STD-diet groups through Wks 7–23 (p < 0.001) (Fig. 1A).
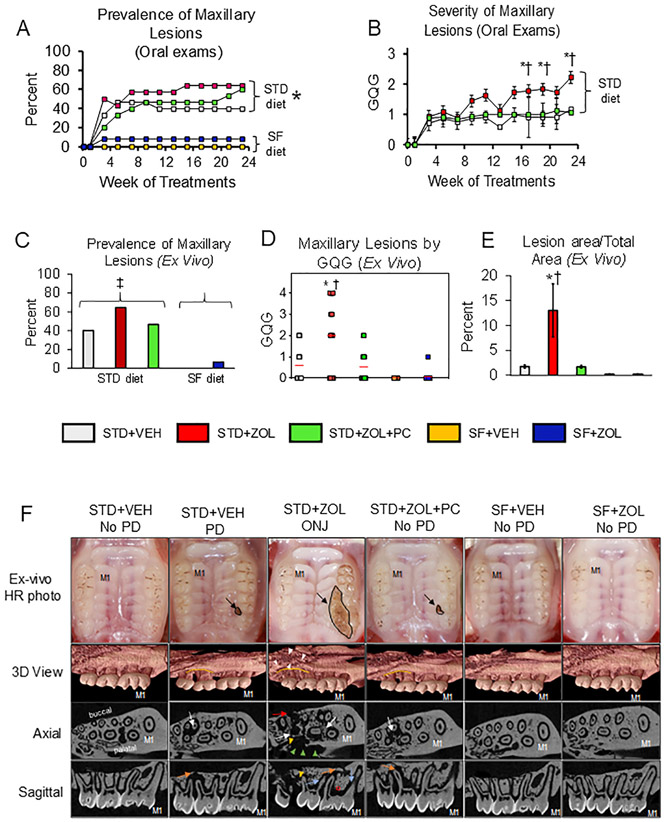
Fig. 1. Prevalence and characteristics of gross oral lesions and by MicroCT.
A. Prevalence of maxillary lesions (GQG ≥ 0.5) rose quickly during Weeks 3–7 in groups receiving the STD diet and remained relatively stable thereafter. In contrast, rats receiving the high SF diet had an extremely low prevalence of such lesions. STD diet rats had a significantly greater prevalence during wks 7 to 23 compared to rats fed the SF diet (*p < 0.001) B. Severity of maxillary lesions in STD + ZOL rats was significantly greater at Weeks 17, 19, and 23 compared to both STD + VEH (*p < 0.005) and STD + ZOL + PC (†p < 0.005) rats; Mean ± SD. C. STD diet groups had a significantly greater prevalence of ex vivo maxillary lesions compared to the SF group (‡p < 0.001). There were no differences in prevalence among the three groups of STD diet fed rats or between the two groups of SF diet fed rats. D. Whereas ex vivo maxillary lesions with GQG ≥ 3 occurred only in the STD + ZOL group, every maxillary lesion in the STD + VEH and STD + ZOL + PC groups had GQG ≤ 2. Severity of maxillary lesions was significantly greater in STD + ZOL compared to STD + ZOL + PC and STD + VEH rats (†p = 0.0034 and *p = 0.009, respectively); Bars indicate Mean ± SD. E. Maxillary lesions were significantly larger in STD + ZOL rats compared to STD + ZOL + PC and STD + VEH rats, (†p = 0.031 and *p = 0.038, respectively). F. Representative high-resolution photographs and microCT images from each group are shown. Small discrete lesions (GQG = 1) restricted to the maxillary M2M3 interdental space are seen in STD + VEH and STD + ZOL + PC rats, while more severe lesions (up to GQG = 4) are seen only in the STD + ZOL group (black arrow). Note a large open wound involving gingival recession/ulceration around all three molars extending into the palatal mucosa towards the midline and buccal migration of M3. 3D MicroCT scans show that STD + VEH rats with PD have greater alveolar bone loss (demarcated in yellow) at the M2M3 interdental space compared to the M1M2 interdental space. STD + ZOL rats with ONJ have more severe alveolar bone loss, with a mottled appearance, at the M2M3 interdental space (white arrowheads), and extending onto the hard palate. STD + ZOL + PC rats show alveolar bone loss at the M2M3 interdental space and minimal bone loss at M1M2, similar to what is observed in STD + VEH rats with PD. Maxillae from rats in the STD + VEH, SF + VEH and SF + ZOL groups with no PD show minimal bone loss at both the M1M2 and M2M3 interdental spaces. Axial and sagittal slices show marked osteolysis (white arrows) at the M1M2 and M2M3 interdental regions of STD + ZOL rats with ONJ. Osteosclerosis (red Ω), erosion of the cortical borders (red arrow) and a scooped out or crater like defect (green arrow heads) with sequestered bone fragments are also seen is STD + ZOL rats with ONJ. Widening of the periodontal ligament (blue arrows) at both interdental regions, sequestered bone (yellow arrowhead) at the M2M3 interdental region, and increased number of bone marrow spaces are seen in STD + ZOL rats with ONJ. In contrast, STD + ZOL + PC and STD + VEH rats with PD show localized alveolar bone loss (orange arrows) constricted to the M2M3 region with no sequestrum present. STD + VEH, SF + VEH and SF + ZOL rats with no PD have normal PDL spaces with absence of osteolysis and sequestrum.
Severity of maxillary lesions in the STD + VEH group approximated GQG = 1 after Week 3 (Fig. 1B). In contrast, severity reached 1.7 by Week 9 and rose to 2.2 by Week 23 in the STD + ZOL group and was significantly higher than in the STD + VEH group at weeks 17, 19, and 23 (p < 0.05) (Fig. 1B). A meaningful calculation of severity cannot be made for the SF-diet groups (SF + VEH and SF + ZOL), because only one SF-diet rats had a lesion.
Localized periodontitis lesions developed in 11 of the 15 rats in the STD + ZOL + PC group at one time or another. Four of the 11 rats with oral lesions developed bilateral maxillary localized periodontitis and periodontal cleaning procedures were performed on both sides. The other four rats of this group never developed any localized periodontitis. A total of 139 periodontal cleaning procedures were performed in the 11 rats with localized periodontitis, an average of 12.6 procedures per rat during the 24 wks. It was not uncommon for cleaned spots to regress to GQG = 0 for several weeks after a cleaning and then flare again. Severity in STD + ZOL + PC rats was lower compared to STD + ZOL rats at weeks 17, 19, and 23 (p < 0.05), and did not differ from that in STD + VEH rats (p > 0.05) (Fig. 1B).
3.2.2. Ex vivo exams
Though in vivo oral exams were useful for early detection and serial tracking of gross features of localized maxillary periodontitis, most oral cavity regions (e.g., buccal aspect of maxillary molars, all aspects of mandible) offered such poor visual access that evaluation and GQG assignment in vivo of non-palatal sites was not possible, requiring a complementary post-mortem ex vivo oral exam.
The ex vivo exam of maxillae showed very similar data to the last in vivo oral exam (Fig. 1A), with lower prevalence of maxillary lesions in the SF-diet groups compared to the STD-diet groups (p < 0.001) (Fig. 1C). Furthermore, as with the in vivo oral exams (Fig. 1B), maxillary lesion severity was higher in STD + ZOL rats compared to rats in STD + VEH (p = 0.009) and STD + ZOL + PC (p = 0.034) (Fig. 1D). Maxillary lesions with GQG ≥3 were found only in the STD + ZOL group (Fig. 1D). Maxillary GQGs in SF + ZOL were also less than in STD + ZOL (Fig. 1D). Size of maxillary lesions was greater in STD + ZOL compared to STD + ZOL + PC (p = 0.031) and STD + VEH (p = 0.038), respectively (Fig. 1E).
MicroCT images of STD + VEH and STD + ZOL + PC rats with PD showed localized alveolar bone loss at the M2M3 interdental region, the location where gross lesions are first seen (Fig. 1F). STD + ZOL rats with ONJ had substantial alveolar osteolysis and destruction at the M2M3 interdental space, extending towards the M1M2 interdental space. Furthermore, we observed widening of the periodontal ligament, presence of sequestra, erosion of the cortical plate, crater like defects, and osteosclerosis in rats of the STD + ZOL group, features commonly seen in ONJ patients treated with pARs (Fig. 1F). The occurrence of these radiographic features in ZOL-treated rats with ONJ is presented in Table 3. STD + VEH rats with no PD, SF + VEH rats, and SF + ZOL rats had none to minimal alveolar bone loss.
Table 3.
Occurrence of radiographic features of ONJ in ZOL-treated rice rats that developed ONJ.
| Group | Sequestration | Trabecular sclerosis |
Periodontal ligament widening |
Cortical erosion |
Crater- like defects |
|---|---|---|---|---|---|
| STD + ZOLa | 83.3% (5/6) | 100% (6/6) | 100% (6/6) | 100% (6/6) | 50% (3/6) |
| STD + ZOL + PC | 1/1 | 0/1 | 1/1 | 1/1 | 0/1 |
6 of 7 rats in the STD + ZOL group were scanned by MicroCT.
Volumetric MicroCT analysis showed a greater amount of BV and BV/TV in STD + ZOL + PC rats compared to STD + VEH rats with and without PD (p = 0.001; p = 0.001, respectively). In contrast, no significant differences in these parameters were found between STD + ZOL rats with ONJ compared to STD + VEH rats with or without PD (Supplemental Fig. 2C &D). Mandibles showed near complete absence of gross oral lesions in all rats (Supplemental Fig. 4A & B).
3.2.3. Histopathologic analysis of oral lesions
3.2.3.1. Prevalence of ONJ: evaluation of exposed necrotic bone.
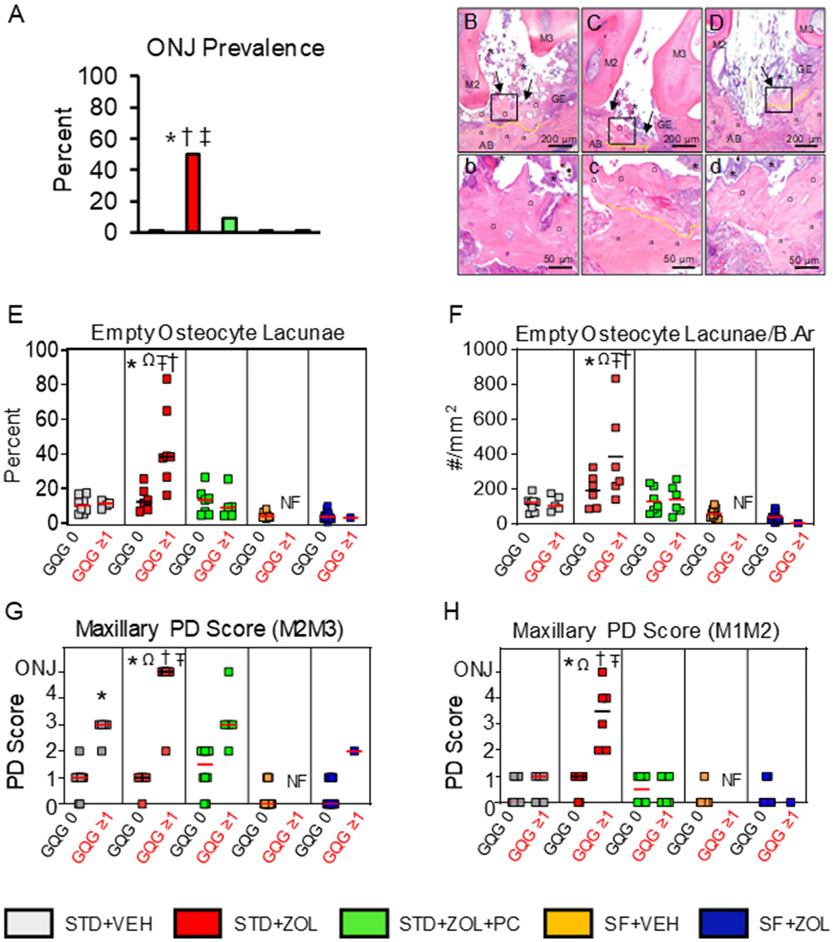
We found no ONJ in STD + VEH rats (Fig. 2A). In contrast, 50% (7/14) of STD + ZOL rats had ONJ. Only 7% (1/15) of STD + ZOL + PC rats developed ONJ (Fig. 2A). Thus, the prevalence of ONJ was higher in STD + ZOL rats compared to both STD + ZOL + PC (p = 0.012) and STD + VEH (p = 0.02) rats, respectively (Fig. 2A). None of the rats in the SF-diet groups developed ONJ. Hence, ONJ prevalence in STD + ZOL rats was also higher than SF + ZOL (p = 0.002) and SF + VEH rats (p = 0.04), respectively (Fig. 2A). Photomicrographs of the maxillary M2M3 interdental region of STD + ZOL rats depict multiple areas of exposed necrotic bone at maxillary palatal and buccal surfaces (Fig. 2B-D).
Fig. 2. Histopathologic assessment of necrotic exposed bone and other features.
A. STD + ZOL rats had greater prevalence of ONJ compared to STD + VEH (*p = 0.02), STD + ZOL + PC (†p = 0.012) and both SF groups (‡p < 0.001). 50% of STD + ZOL rats developed ONJ compared to 7% and 0% of STD + ZOL + PC and STD + VEH rats, respectively. Both SF + VEH and SF + ZOL groups had 0% prevalence of ONJ. B–D. Photomicrographs showing features of ONJ found in the STD + ZOL group. b–d depict areas outlined in black in B–D, at a four-fold higher magnification. Maxillary oral lesions are shown at the M2M3 interdental space, in proximity to the palatal (B–C) and buccal (D) surfaces of STD + ZOL rats with ONJ. Ulcerated gingival epithelium (GE) accompanied by adherent bacterial colonies (*) and exposed, necrotic bone (black arrows) is present at the M2M3 interdental space. b–d. Exposed necrotic bone (Ω) with 10+ confluent empty osteocyte lacunae or osteocytes with pyknotic nuclei is seen next to vital bone (α) which has lacunae occupied by osteocytes with basophilic nuclei (demarcated in yellow). Severe inflammatory cell infiltration and fibrosis was often observed. E. The percentage of empty osteocyte lacunae was significantly greater in STD + ZOL rats with GQG≥1 lesions compared to the STD + ZOL subgroup with GQG = 0 lesions (Ωp < 0.001). STD + ZOL rats with GQG≥1 also had greater percentage of empty osteocyte lacunae compared to STD + VEH (*p = 0.001), STD + ZOL + PC (†p = 0.002), and both SF groups (‡p < 0.05). There are no significant differences in percentage of empty osteocyte lacunae among STD + VEH, STD + ZOL + PC, SF + VEH, and SF + ZOL. F. The number of empty osteocyte lacunae/bone area (B.Ar, mm2) was higher in STD + ZOL rats with GQG≥1 compared to STD + ZOL rats with GQG = 0 (Ωp = 0.005). STD + ZOL rats with GQG≥1 also had a greater number of empty osteocyte lacunae/bone area compared to STD + VEH (*p = 0.001) and STD + ZOL + PC rats with GQG≥1 (†p = 0.001) as well as SF + VEH and SF + ZOL rats with GQG = 0 (‡p < 0.05). G. Maxillary PD Score at the M2M3 interdental space. PD Scores ranged from gingivitis (PD Score 1) to mild periodontitis (PD Score 2) in the STD diet group subsets with GQG = 0. STD + VEH rats with GQG≥1 had mild to moderate periodontitis (PD Score 2–3) with a median score of 3. STD + ZOL rats had greater prevalence of periodontitis lesions compared to STD + VEH (*p = 0.002) and STD + ZOL + PC (†p = 0.005) rats with GQG = 0. STD + ZOL rats also had greater prevalence of periodontitis than SF + ZOL rats (‡p < 0.05). STD + VEH rats with GQG≥1 had greater prevalence of PD lesions compared to STD + VEH rats with GQG = 0 (*p > 0.05). Two-way ANOVA showed an effect in diet in which SF rats had less severe periodontitis lesions compared to STD rats (*p < 0.05). H. Maxillary PD Score at the M1M2 interdental space. There was a significantly higher prevalence and severity of periodontitis lesions in STD + ZOL rats with GQG≥1 compared to STD + ZOL rats with GQG = 0 (Ωp < 0.005). STD + ZOL also had a higher prevalence and severity of periodontitis lesions compared to STD + VEH (*p = 0.006), STD + ZOL + PC (†P = 0.006), SF + VEH (Ŧp = 0.017), and SF + ZOL (‡p = 0.016). Red or black transverse lines in each group of the graphs depict median values.
We found no differences in the percentage of empty osteocyte lacunae (Fig. 2E) and empty osteocyte lacunae/bone area (Fig. 2F) in STD + VEH rats with GQG ≥ 1 compared to STD + VEH rats with GQG = 0 (p = 0.955). In contrast, these parameters were greater in STD + ZOL rats with GQG ≥ 1 (mainly ONJ cases) compared to STD + ZOL rats with GQG = 0 (p < 0.001; p = 0.005 respectively) (Fig. 2E & F). STD + ZOL rats with GQG ≥ 1 (mainly ONJ cases) also had a higher percentage of empty osteocyte lacunae and empty osteocyte lacunae/bone area compared to STD + VEH (p = 0.001; p = 0.002), STD + ZOL + PC rats (p = 0.002; p = 0.003), SF + VEH rats (p < 0.05), and SF + ZOL rats (p < 0.05), respectively (Fig. 2E & F), regardless of GQG.
3.2.3.2. PD Score of maxillary lesions.
Two-way ANOVA indicated a diet effect on the severity of maxillary oral lesions at M2M3, with lower PD Scores in SF-diet rats (SF + VEH and SF + ZOL) compared to STD-diet rats (STD + VEH, STD + ZOL, and STD + ZOL + PC) (p < 0.05), respectively (Fig. 2G). STD + VEH rats with GQG = 0 usually had histologic PD Scores <2, with a median value of 1 (Fig. 2G). STD + VEH rats with GQG ≥ 1 always had histologic PD Scores ≥2, with a median value of 3. The STD + ZOL group had a 50% prevalence of ONJ (Fig. 2A). However, when the STD + ZOL group was divided into subsets that had no gross lesions (GQG = 0) and gross lesions (GQG ≥ 1), the prevalence for ONJ was 0% and 88%, respectively (Fig. 2G). Furthermore, STD + ZOL rats with GQG ≥1 had greater PD Scores compared to the STD + ZOL group with GQG = 0 (Fig. 2G). The subset of rats in the STD + ZOL + PC group with GQG ≥ 1 had one rat (7%) with ONJ, and a median histologic PD Score of 3 (Fig. 2A & G). Representative photomicrographs of the histopathologic findings at maxillary M2M3 in rats of the different experimental groups are shown in Supplemental Fig. 3A.
At maxillary interdental M1M2, the STD + ZOL subset of rats with GQG ≥ 1 had greater PD Scores compared to STD + ZOL rats with GQG = 0 (p < 0.05). In addition, STD + ZOL rats with GQG ≥ 1 also had greater PD Scores compared to STD + ZOL + PC (p = 0.03) and STD + VEH rats (p = 0.03), respectively (Fig. 2H).
Consistent with their GQGs (Supplemental Fig. 4A & B), the mandibles had low PD Scores, with median values of 0 or 1 at M1M2 and M2M3, and no intergroup differences (Supplemental Fig. 4C & D).
3.2.3.3. Alveolar bone loss.
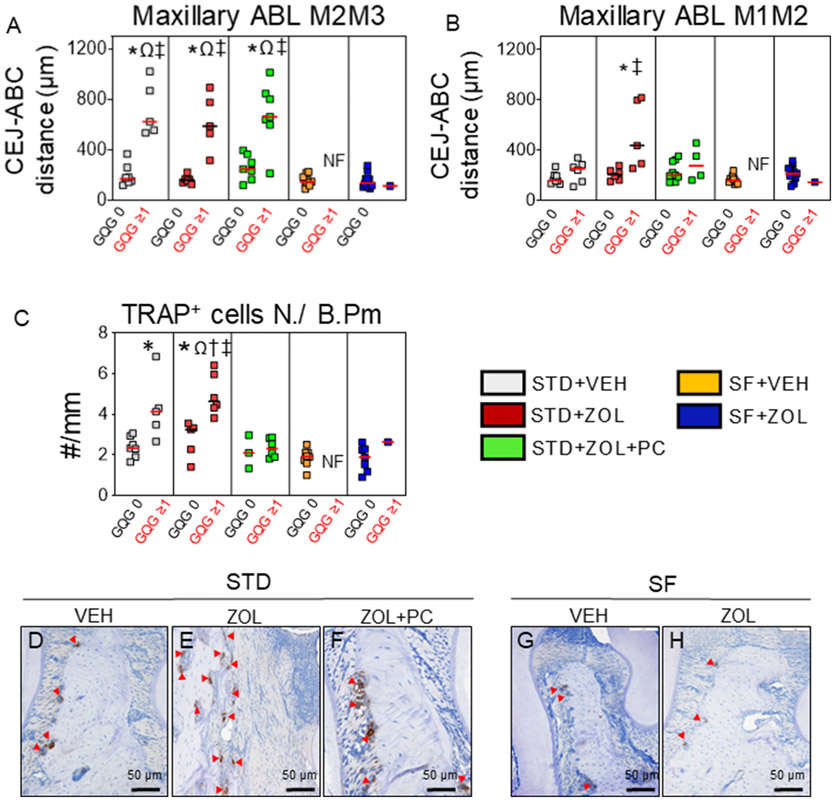
Photomicrographs of representative rats from each group displaying alveolar bone loss at maxillary M2M3 and M1M2 are shown in Supplemental Fig. 3B. At maxillary M2M3, STD + VEH rats with GQG ≥ 1 had greater alveolar bone loss than STD + VEH rats with GQG = 0 (p < 0.001) (Fig. 3A). STD + ZOL rats with GQG ≥ 1 also had greater alveolar bone loss than STD + ZOL rats with GQG = 0 (p < 0.001) (Fig. 3A). STD + ZOL + PC rats with GQG ≥ 1 had greater alveolar bone loss than STD + ZOL + PC rats with GQG = 0 (p < 0.001) (Fig. 3A), but no differences compared to STD + ZOL rats with GQG ≥ 1 (p > 0.05). Both STD + VEH and STD + ZOL rats with GQG ≥ 1 had greater alveolar bone loss than SF + VEH and SF + ZOL rats (p < 0.001), respectively (Fig. 3A).
Fig. 3. Maxillary alveolar bone loss and in situ analysis of TRAPc5b+ osteoclasts.
A. Alveolar bone loss was greater at the M2M3 interdental space in rats with GQG≥1 compared to rats with GQG = 0 of STD + VEH, STD + ZOL, and STD + ZOL + PC groups in each respective group (Ωp < 0.001). Alveolar bone loss was also significantly greater in STD rats with GQG≥1 compared to SF rats with GQG = 0 (‡p < 0.001). B. Alveolar bone loss was greater at the M1M2 interdental space in STD + ZOL rats with GQG≥1 compared to STD + VEH and SF + VEH groups (*p = 0.044; ‡p = 0.003, respectively). There were more TRAPc5b+ cells/B.Pm in STD + ZOL rats with GQG≥1 compared to STD + ZOL rats with GQG = 0 (Ωp < 0.001). STD + ZOL rats with GQG≥1 also had more TRAPc5b+ cells/B.Pm than STD + VEH (*p < 0.001), STD + ZOL + PC (†p < 0.001), SF + VEH, and SF + ZOL groups (‡p < 0.001). TRAPc5b+ cells/B.Pm was also greater in STD + VEH rats with GQG≥1 compared to STD + VEH rats with GQG = 0 (*p < 0.05) (C). Red or black transverse lines in each group of the graphs depict median values. Representative photomicrographs of TRAPc5b+ cells (red arrow heads) at alveolar bone surfaces of maxillae from STD + VEH (D), STD + ZOL (E), STD + ZOL + PC (F), SF + VEH (G), and SF + ZOL (H).
At maxillary M1M2, STD + ZOL rats with GQG ≥ 1 had more alveolar bone loss compared to all groups, in particular to STD + VEH (p = 0.044) and SF + VEH and SF + ZOL rats with GQG = 0 (P = 0.003), respectively (Fig. 3B). Furthermore, and consistent with low PD Scores found in mandibles (Supplemental Fig. 4C & D), we found no significant alveolar bone loss at mandibular M1M2 and M2M3 interdental regions of all groups (Supplemental Fig. 4E & F). Photomicrographs of representative rats from each group displaying the mandibular alveolar bone condition and histopathologic findings at M1M2 and M2M3 are shown (Supplemental Fig. 4G & H).
3.2.4. Immunohistochemical detection of TRAPc5b+ (osteoclasts), CD 15+(neutrophils), and CD 68+ (monocyte lineage) cells
Two-way ANOVA revealed an effect of diet on the number of osteoclasts, with SF-diet groups having fewer TRAcP5b+ cells/B.Pm at maxillary M2M3 alveolar bone surfaces compared to STD-diet groups (p < 0.001). STD + ZOL rats with GQG ≥1 had more TRAcP5b+ cells than STD + ZOL rats with GQG = 0 (p < 0.001) (Fig. 3C). STD + ZOL rats with GQG ≥ 1 also had more TRAcP5b + cells than STD + ZOL + PC rats with GQG ≥ 1, STD + VEH rats with GQG = 0, and both SF diet groups (p < 0.001) (Fig. 3C). Fig. 3D-H depict photomicrographs of representative maxillae from rats of each group with TRAcP5b+ cells at alveolar bone surfaces at the M2M3 interdental space. We also found more CD15+ cells (neutrophils) in STD + VEH rats with GQG ≥ 1 compared to STD + VEH rats with GQG = 0 (p = 0.024). However, no differences were found in CD15+ cells among STD + VEH, STD + ZOL and STD + ZOL + PC groups, respectively (Supplemental Fig. 5A). Furthermore, more CD 68+ cells were found in STD + ZOL rats with ONJ (GQG ≥ 1) compared to STD + VEH rats with GQG = 0 and GQG ≥ 1 (p = 0.001), respectively. In addition, STD + ZOL rats with ONJ had more CD68+ cells compared to STD + ZOL rats with GQG = 0 (p = 0.003) and STD + ZOL + PC rats with GQG ≥ 1 (p < 0.001) (Supplemental Fig. 5B).
3.2.5. Oral microbiome
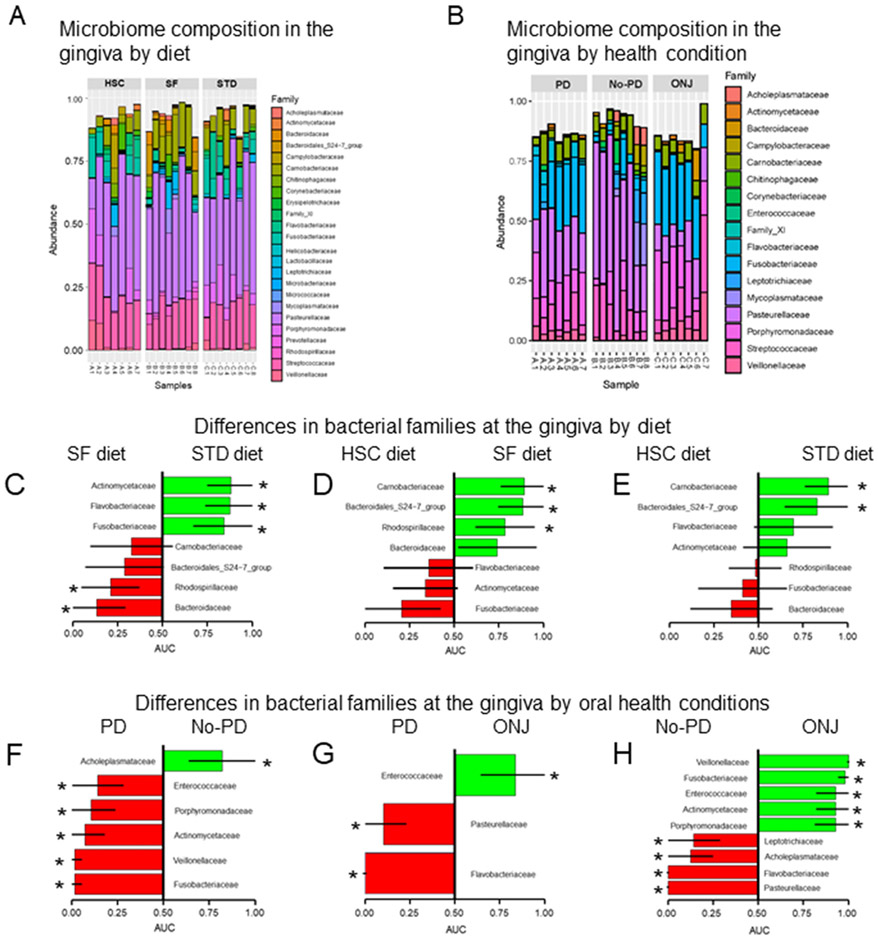
No statistical differences were found in the bacterial family composition at the gingiva of rice rats across diets after adjustment for multiple comparisons using false discovery rate at q = 0.1. However, seven families were nominally significant (Kruskal-Wallis ANOVA, p < 0.05). In post-hoc pairwise comparisons, STD diet rats had greater abundance (Mann-Whitney rank sum test, p < 0.05) of Actinomycetaceae, Flavobacteriaceae and Fusobacteriaceae and lower abundance of Rhodospirillaceae and Bacteriodaceae compared to SF-diet rats (p < 0.05) (Fig. 4C). SF-diet rats had greater abundance (p < 0.05) of Carnobacteriaceae, Bacteroidales, and Rhodospirillaceae compared to HSC-diet rats (Fig. 4D). Furthermore, STD-diet rats had greater abundance of Carnobacteriaceae and Bacteroidales compared to HSC-diet rats (Fig. 4E).
Fig. 4. Oral microbiome composition and differences in bacterial families by diet and oral health condition after 24 wks of treatments.
Bar plots summarizing (A) the gingiva microbiome composition by diet; high sucrose and casein (HSC), high soluble fiber (SF) and standard diet (STD) and (B) the gingiva microbiome composition at family levels by oral health condition; maxillary localized periodontitis (PD), No-PD (healthy) and ONJ. Differences in bacterial families at the gingiva between: (C) rats fed the SF diet compared to rats fed the STD diet; (D) rats fed the HSC diet compared to rats fed the SF diet; and (E) rats fed the HSC diet compared to rats fed the STD diet. Differences in bacterial families at the gingiva between quadrants with: (F) PD and No-PD lesions; (G) PD and ONJ; and (H) No-PD and ONJ. AUC: area under the curve. Asterisk (*) depict significant differences (Kruskal-Wallis, p < 0.05).
3.2.5.1. Oral health condition effect on the oral microbiome.
We observed significant differences in the bacterial family composition at the gingiva of rats across the different health conditions after adjustment for multiple comparison using false discovery rate at q = 0.1: nine bacterial families differed in the gingiva based on the oral health condition status (Kruskal-Wallis ANOVA, p < 0.05). STD-diet rats with maxillary localized periodontitis or ONJ had a greater abundance of Actinomycetaceae, Fusobacteriaceae, Phorphyromonadaceae, Enterobacteriaceae, and Veillonellaceae compared to STD-diet rats with no periodontitis (Fig. 4F & H). STD-diet rats with ONJ had a greater abundance of Enterobacteriaceae, but lower abundance of Flavobacteriaceae and Pasteurellaceae, compared to STD-diet rats with periodontitis (Fig. 4G).
4. Discussion
Most ONJ cases in humans are the consequence of coincident systemic risk factors (pARs or angiogenesis inhibitors) and local risk factors that include tooth extraction and periodontitis. We report two efficacious methods for controlling/preventing periodontitis that reduce ONJ prevalence. The first is a translational approach with potential clinical relevance, while the second is a significant refinement to an established small animal model of ONJ [22]. Altogether these data confirm that localized periodontitis represents an oral risk factor for ONJ in ZOL-treated rice rats [22,44], and further support a role for periodontitis in the pathophysiology of ONJ [15,62,63].
Having formal steps proven to reduce the incidence of ONJ in patients taking ZOL for oncology purposes would be helpful for both patients and the medical and dental communities. This study made a prospective, pre-clinical investigation of an understudied clinical scenario in which dental care measures are implemented in patients with cancer who are already taking pARs [28,29]. ZOL-treated rats with localized periodontitis that received periodic periodontal cleaning of the localized lesions, had significantly lower prevalence of ONJ than similar ZOL-treated rats that received no periodontal cleaning. This periodontal cleaning procedure repeatedly dislodged impacted hair and fiber at the palatal aspect of the maxillary M2M3 interdental space from individual rats over a 24 wks period. One can easily conceive of something similar being done by a dental professional during periodontal maintenance or even by a patient during routine home care that involves flossing, waterpicking, or an interdental brush. The AAOMS recommendation concerning “appropriate dental care” is now based on universally-agreed upon best general dental practices for maintaining good oral hygiene and dental health. These preclinical data may establish a basis for clinical research that specifically compares patients in good dental health, with and without ongoing periodontal maintenance, who take ZOL for oncology purposes. The idea would be to formally prove prospectively in patients that periodontal maintenance reduces the incidence of ONJ in patients taking ZOL. If periodontal maintenance reduced ONJ incidence in this type of trial, the case supporting the AAOMS recommendation for oncology patients taking ZOL to participate in periodontal maintenance programs would be strengthened.
In this translational study, periodontal cleaning did not reduce the prevalence of periodontitis. However, the severity and size of the gross lesions were lower in the ZOL-treated periodontal cleaning group compared to the ZOL-treated group that did not receive periodontal cleaning, and was the same as in the VEH group. ZOL-treated rats that underwent periodontal cleaning still experienced alveolar bone loss at the maxillary M2M3 interdental space, but had fewer osteoclasts and CD68+ cells compared to ZOL-treated rats that underwent no periodontal cleaning. CD68 is highly expressed by cells in the monocyte lineage including macrophages, pericytes, and osteoclasts [64]. Studies have shown that a reduction of CD68+ cells in inflammatory conditions affecting different organs correlates with a positive response to treatment and tissue healing [65,66]. Thus, our findings suggest the possibility that periodic periodontal cleaning reduces the development of ONJ by ameliorating the inflammatory response at the periodontium and controlling the progression of periodontitis. However, it was not sufficient to promote gingival healing and prevent the loss of alveolar bone at M2M3. The number of neutrophils was not affected by periodontal cleaning in ZOL-treated rats. Further studies are required to define the role of these cells in rice rat periodontitis.
We have previously shown that there is an association between the severity of periodontitis and the development of ONJ in ZOL-treated rice rats and that ZOL can also exacerbate pre-existing periodontitis [22]. Localized periodontitis is associated with local production and accumulation of pro-inflammatory cytokines in periodontal tissues, which ultimately enhance RANKL-driven inflammatory resorption of alveolar bone. The severity of periodontitis generally reflects the degree of the host inflammatory response and the pathological changes present in periodontal soft and hard tissues. Thus, the greater the severity of periodontitis, the more inflammatory molecules would be released in the periodontal microenvironment, causing greater hard and soft tissue destruction. Alveolar bone in periodontal lesions is simply resorbed by inflammatory-bone resorption. However, a plausible explanation for the sequence of events in the pathogenesis of pAR-related ONJ is that with the simultaneous presence of pARs (ZOL) and oral risk factors (periodontitis), bone resorption is slowed by the action of pARs, causing alveolar bone that would have been resorbed to persist, giving inflammatory molecules in the periodontium sufficient time to cause osteocyte cell death in the persisting bone. Dying osteocytes release large amounts of damage-associated molecular patterns (DAMPs) [67,68]. DAMPS released into the canaliculi reach bone surfaces and vascular canals in the bone, initiating inflammatory responses by binding to various pattern recognition receptors (PRRs), which are expressed by osteoclasts and macrophages, as well as activating targeted bone resorption by osteoclasts [69-73]. It is possible that in the context of impaired bone resorption, as occurs in patients receiving antiresorptives, a greater severity of periodontal lesions would induce higher level of osteocyte cell death, and consequently greater amount of DAMPs accumulating in the necrotic bone and activation of PRRs, triggering robust immune responses, inflammation and necrosis, and ultimately inducing ONJ lesions.
Our finding is somewhat similar to another preclinical study that showed a reduction in the occurrence of tooth-extraction-related ONJ when local preventive measures are applied [74]. Furthermore, removing a ligature that had induced experimental periodontitis around a molar two weeks before its extraction decreased the incidence of ONJ in mice [31]. Other ligature-induced experimental periodontitis studies were conducted in Wistar rats that received either VEH or 5-fluorouracil, a chemotherapy agent that aggravates periodontitis and alveolar bone loss. Subsequently, the ligature was removed and the rats were subjected to scaling and root planning (SRP), with or without administration of adjuvant therapies. SRP alone was not sufficient to reduce alveolar bone loss [75-77]. However, when SRP was combined with systemic probiotic administration [76] or laser therapy [77], SRP reduced inflammation and favored periodontal tissue repair. Importantly, our finding parallels periodontal maintenance therapy outcomes in patients [78-80], in that regular periodontal maintenance therapy, which includes mechanical debridement as needed at sites afflicted by periodontitis, stops the progression of, but does not reverse alveolar bone loss. The main benefit of periodontal cleaning in this setting is reducing ONJ prevalence.
The study also shows that feeding ZOL-treated rats a nutritionally-similar pelleted rodent chow that replaces the insoluble fiber of the STD diet with high levels of soluble fiber (SF diet), prevented the development of both localized periodontitis lesions and ONJ. The SF diet is a potentially important step in refining the rice rat model of ONJ. In contrast to traditional laboratory rodents (Rattus, Mus), which are resistant to naturally-occurring periodontitis [30], rice rats (Oryzomys palustris) somewhat resemble humans in that they are naturally prone to develop periodontitis [32-36,81]. However, a powerful aspect of preclinical models of ONJ is their ability to support experiments in the presence and absence of known risk factors. ONJ-related studies in the presence and absence of experimental periodontitis in Rattus and Mus are straightforward [18,31]. At the current time, working with rice rats is less straightforward for this important feature because making periodontitis-free rice rats has proven extremely difficult. Rice rats develop two distinctive types of periodontitis without mechanical intervention: 1) a localized form that affects the maxilla, occurring in rats fed STD rodent chow [36]; and 2) a generalized form, affecting both jaws, occurring in rats fed the HSC diet [32-35]. The defining feature of localized periodontitis is impacted material that causes a localized chronic inflammatory response, which results in irreversible loss of periodontal attachment and bone [36]. Without a readily-accessible “periodontitis-free” rice rat model that parallels what is natively found in Rattus and Mus, the rice rat model has an acknowledged shortcoming.
In the current study, we observed a strong diet effect. Rice rats fed the SF diet rarely developed gross or histopathologic periodontitis in any quadrant through age 28 wks. Perhaps most notably, ZOL-treated rice rats fed the SF diet never developed ONJ. The main mechanism of the high SF diet in reducing localized periodontitis appears to lie in circumventing the initial mechanical trauma-induced inflammation from the accumulated cellulose, hemicellulose and lignin (insoluble fiber) of the STD diet, by replacing it with soluble fiber (7.5% inulin and 10% fructo-oligosaccharides).
Though, no statistical differences were found in the bacterial family composition of the rice rat gingiva across diets after multiple comparison correction, seven families were nominally significant. In post-hoc pairwise comparisons, STD diet rats had greater abundance of Actinomycetaceae, Flavobacteriaceae and Fusobacteriaceae compared to SF-diet rats, whereas SF-diet rats had greater abundance of Rhodospirillaceae and Bacteriodaceae compared to STD-diet rats. SF-diet rats had greater abundance of Carnobacteriaceae, Bacteroidales, and Rhodospirillaceae compared to HSC-diet rats. Furthermore, STD-diet rats had greater abundance of Carnobacteriaceae and Bacteroidales compared to HSC-diet rats. Lack of statistical significance for the changes in the gingival microbiota according to dietary interventions maybe due to a fairly small effect size relative to the number of samples, leading to limited power to detect such effects.
Comparison of microbiota according to health condition indicates a strong association of Actinomycetaceae, Fusobacteriaceae, Phorphyromonadaceae, Enterobacteriaceae, and Veillonellaceae in gingiva with localized periodontitis and ONJ lesions compared to healthy gingiva. Porphyromonas, Fusobacteria and Proteobacteria are associated with human periodontal disease [82]. In line with our findings, species of the families Fusobacteriaceae, Actinomycetaceae and Veillonellaceae are also associated with human ONJ [83,84]. Further studies will be required to specifically determine the genera and species of bacteria found in periodontitis and ONJ lesions of rice rats. Most importantly the mechanism leading to abundance increases and possible mediatory effects of microbiome composition shifts in the gingiva need to be interrogated in the future.
Connecting the interesting microbiome observations according to diet and significant difference in disease status, we notice that Actinomycetaceae and Fusobacteriaceae are both decreased with SF diet, compared to the STD and HSC diets, and also increased in periodontitis and ONJ, indicating potential protective effect of the SF diet. Fusobacteriaceae is further increased in gingiva of HSC diet mice compared to both STD and more so with SF, suggesting a potentially enhancing pathogenic effect of the HSC diet.
This study presents few weaknesses that we are aware of. One of these, is the lack of a STD + VEH + PC control group for STD + ZOL + PC rats. This shortcoming made it difficult to learn about any specific effect of ZOL on the periodontal tissue responses following the periodontal cleaning of localized periodontitis lesions. Another weakness is that the in vivo periodic oral exams performed in the rats permit adequate visualization of maxillary palatal oral lesions but not of the buccal lesions. Thus, we were unable to determine the time of occurrence and progression of buccal oral lesions during the experimental time period.
5. Conclusion
This study shows that: 1) periodontal cleaning in ZOL-treated rats with localized periodontitis reduces ONJ prevalence; and 2) feeding a high SF diet to ZOL-treated rats prevents localized periodontitis and ONJ.
Supplementary Material
Acknowledgments
This research was supported by NIH grant R01DE023783-01A from the National Institute of Dental and Craniofacial Research (NIDCR) and, in-part, with resources provided by the North Florida/South Georgia Veterans Health System. The work reported herein does not represent the views of the US Department of Veterans Affairs or the US Government. We are thankful to Dr. Juerg Gasser and Novartis Pharma AG (Basel, Switzerland) for providing zoledronic acid.
Funding
This research was supported by the National Institute of Dental and Craniofacial Research (NIDCR), R01DE023783-01A. AVA was supported by NIH/NLM R01 LM012517 and NIH/NCATS UL1 TR001450. CMN was supported by NIH/NIDCR K08 DE025447 and NIH/NIGMS P20 GM130457-01A1 7720.
Abbreviations:
- ONJ
osteonecrosis of the jaw
- ZOL
zoledronic acid
- pARs
powerful antiresorptives
- RANKL
receptor activator of nuclear factor-κB ligand
- PC
periodontal cleaning
- M
molar
- CEJ
cemento-enamel junction
- STD
standard
- SF
soluble fiber
- VEH
vehicle
- GQG
gross quadrant grade
- B.Pm
bone perimeter
- Le. Ar
lesion area
- Tt.Mx.Ar
total maxillary area
Footnotes
Declaration of competing interest
The authors have no conflicts of interest. AVA is a scientific advisory board member for Second Genome, Inc., which has not contributed to this research.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bone.2021.115866.
References
- [1].Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O’Ryan F, Reid IR, Ruggiero SL, Taguchi A, Tetradis S, Watts NB, Brandi ML, Peters E, Guise T, Eastell R, Cheung AM, Morin SN, Masri B, Cooper C, Morgan SL, Obermayer-Pietsch B, Langdahl BL, Al Dabagh R, Davison KS, Kendler DL, Sandor GK, Josse RG, Bhandari M, El Rabbany M, Pierroz DD, Sulimani R, Saunders DP, Brown JP, Compston J, J. International Task Force on Osteonecrosis of the, Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus, J Bone Miner Res 30 (1) (2015) 3–23. [DOI] [PubMed] [Google Scholar]
- [2].Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, O’Ryan F, American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update, J. Oral Maxillofac. Surg 72 (10) (2014) 1938–1956. [DOI] [PubMed] [Google Scholar]
- [3].Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA, Viniegra M, Fan M, Jiang Q, Dansey R, Jun S, Braun A, Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, doubleblind study, J. Clin. Oncol 28 (35) (2010) 5132–5139. [DOI] [PubMed] [Google Scholar]
- [4].Van den Wyngaert T, Wouters K, Huizing MT, Vermorken JB, RANK ligand inhibition in bone metastatic cancer and risk of osteonecrosis of the jaw (ONJ): non bis in idem? Support Care Cancer 19 (12) (2011) 2035–2040. [DOI] [PubMed] [Google Scholar]
- [5].Nicolatou-Galitis O, Kouri M, Papadopoulou E, Vardas E, Galiti D, Epstein JB, Elad S, Campisi G, Tsoukalas N, Bektas-Kayhan K, Tan W, Body JJ, Migliorati C, Lalla RV, M.B.S. Group, Osteonecrosis of the jaw related to non-antiresorptive medications: a systematic review, Support Care Cancer 27 (2) (2019) 383–394. [DOI] [PubMed] [Google Scholar]
- [6].Pimolbutr K, Porter S, Fedele S, Osteonecrosis of the jaw associated with antiangiogenics in antiresorptive-naive patient: a comprehensive review of the literature, Biomed. Res. Int 2018 (2018) 8071579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Estilo CL, Fornier M, Farooki A, Carlson D, Bohle G III, Huryn JM, Osteonecrosis of the jaw related to bevacizumab, J. Clin. Oncol 26 (24) (2008) 4037–4038. [DOI] [PubMed] [Google Scholar]
- [8].Guarneri V, Miles D, Robert N, Dieras V, Glaspy J, Smith I, Thomssen C, Biganzoli L, Taran T, Conte P, Bevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer, Breast Cancer Res. Treat 122 (1) (2010) 181–188. [DOI] [PubMed] [Google Scholar]
- [9].Koch FP, Walter C, Hansen T, Jager E, Wagner W, Osteonecrosis of the jaw related to sunitinib, Oral Maxillofac Surg 15 (1) (2011) 63–66. [DOI] [PubMed] [Google Scholar]
- [10].Rugani P, Walter C, Kirnbauer B, Acham S, Begus-Nahrman Y, Jakse N, Prevalence of medication-related osteonecrosis of the jaw in patients with breast cancer, prostate cancer, and multiple myeloma, Dent J (Basel) 4 (4) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Coleman RE, Collinson M, Gregory W, Marshall H, Bell R, Dodwell D, Keane M, Gil M, Barrett-Lee P, Ritchie D, Bowman A, Liversedge V, De Boer RH, Passos-Coelho JL, O’Reilly S, Bertelli G, Joffe J, Brown JE, Wilson C, Tercero JC, Jean-Mairet J, Gomis R, Cameron D, Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04), J Bone Oncol 13 (2018) 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wan JT, Sheeley DM, Somerman MJ, Lee JS, Mitigating osteonecrosis of the jaw (ONJ) through preventive dental care and understanding of risk factors, Bone Res 8 (2020) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Soundia A, Hadaya D, Esfandi N, de Molon RS, Bezouglaia O, Dry SM, Pirih FQ, Aghaloo T, Tetradis S, Osteonecrosis of the jaws (ONJ) in mice after extraction of teeth with periradicular disease, Bone 90 (2016) 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Song M, Alshaikh A, Kim T, Kim S, Dang M, Mehrazarin S, Shin KH, Kang M, Park NH, Kim RH, Preexisting periapical inflammatory condition exacerbates tooth extraction-induced bisphosphonate-related osteonecrosis of the jaw lesions in mice, J. Endod 42 (11) (2016) 1641–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Katsarelis H, Shah NP, Dhariwal DK, Pazianas M, Infection and medication-related osteonecrosis of the jaw, J. Dent. Res 94 (4) (2015) 534–539. [DOI] [PubMed] [Google Scholar]
- [16].Kikuiri T, Kim I, Yamaza T, Akiyama K, Zhang Q, Li Y, Chen C, Chen W, Wang S, Le AD, Shi S, Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice, J. Bone Miner. Res 25 (7) (2010) 1668–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hasegawa T, Hayashida S, Kondo E, Takeda Y, Miyamoto H, Kawaoka Y, Ueda N, Iwata E, Nakahara H, Kobayashi M, Soutome S, Yamada SI, Tojyo I, Kojima Y, Umeda M, Fujita S, Kurita H, Shibuya Y, Kirita T, Komori T, Japanese M Study Group of Co-operative Dentistry with, Medication-related osteonecrosis of the jaw after tooth extraction in cancer patients: a multicenter retrospective study, Osteoporos Int 30 (1) (2019) 231–239. [DOI] [PubMed] [Google Scholar]
- [18].Aghaloo TL, Kang B, Sung EC, Shoff M, Ronconi M, Gotcher JE, Bezouglaia O, Dry SM, Tetradis S, Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat, J. Bone Miner. Res 26 (8) (2011) 1871–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Aguirre JI, Akhter MP, Kimmel DB, Pingel JE, Williams A, Jorgensen M, Kesavalu L, Wronski TJ, Oncologic doses of zoledronic acid induce osteonecrosis of the jaw-like lesions in rice rats (Oryzomys palustris) with periodontitis, J. Bone Miner. Res 27 (10) (2012) 2130–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].de Molon RS, Cheong S, Bezouglaia O, Dry SM, Pirih F, Cirelli JA, Aghaloo TL, Tetradis S, Spontaneous osteonecrosis of the jaws in the maxilla of mice on antiresorptive treatment: a novel ONJ mouse model, Bone 68 (2014) 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Messer JG, Jiron JM, Mendieta Calle JL, Castillo EJ, Israel R, Phillips EG, Yarrow JF, Van Poznak C, Kesavalu L, Kimmel DB, Aguirre JI, Zoledronate treatment duration is linked to bisphosphonate-related ONJ prevalence in rice rats with generalized periodontitis, Oral Dis. 25 (4) (2019) 1116–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Messer JG, Mendieta Calle JL, Jiron JM, Castillo EJ, Van Poznak C, Bhattacharyya N, Kimmel DB, Aguirre JI, Zoledronic acid increases the prevalence of medication-related osteonecrosis of the jaw in a dose dependent manner in rice rats (Oryzomys palustris) with localized periodontitis, Bone 108 (2018) 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kang B, Cheong S, Chaichanasakul T, Bezouglaia O, Atti E, Dry SM, Pirih FQ, Aghaloo TL, Tetradis S, Periapical disease and bisphosphonates induce osteonecrosis of the jaws in mice, J. Bone Miner. Res 28 (7) (2013) 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Migliorati CA, Woo SB, Hewson I, Barasch A, Elting LS, Spijkervet FK, Brennan MT, A systematic review of bisphosphonate osteonecrosis (BON) in cancer, Support Care Cancer 18 (8) (2010) 1099–1106. [DOI] [PubMed] [Google Scholar]
- [25].Kuhl S, Walter C, Acham S, Pfeffer R, Lambrecht JT, Bisphosphonate-related osteonecrosis of the jaws - a review, Oral Oncol. 48 (10) (2012) 938–947. [DOI] [PubMed] [Google Scholar]
- [26].Ruggiero SL, Emerging concepts in the management and treatment of osteonecrosis of the jaw, Oral and Maxillofacial Surgery Clinics of North America 25 (1) (2013) 11–20. [DOI] [PubMed] [Google Scholar]
- [27].Sim Ie W, Sanders KM, Borromeo GL, Seymour JF, Ebeling PR, Declining incidence of medication-related osteonecrosis of the jaw in patients with cancer, J. Clin. Endocrinol. Metab 100 (10) (2015) 3887–3893. [DOI] [PubMed] [Google Scholar]
- [28].Iuliis FDE, Taglieri L, Amoroso L, Vendittozzi S, Blasi L, Salerno G, Lanza R, Scarpa S, Prevention of osteonecrosis of the jaw in patients with bone metastases treated with bisphosphonates, Anticancer Res. 34 (5) (2014) 2477–2480. [PubMed] [Google Scholar]
- [29].Vandone AM, Donadio M, Mozzati M, Ardine M, Polimeni MA, Beatrice S, Ciuffreda L, Scoletta M, Impact of dental care in the prevention of bisphosphonate-associated osteonecrosis of the jaw: a single-center clinical experience, Ann. Oncol 23 (1) (2012) 193–200. [DOI] [PubMed] [Google Scholar]
- [30].Struillou X, Boutigny H, Soueidan A, Layrolle P, Experimental animal models in periodontology: a review, Open Dent. J 4 (2010) 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim T, Kim S, Song M, Lee C, Yagita H, Williams DW, Sung EC, Hong C, Shin K-H, Kang MK, Park N-H, Kim RH, Removal of pre-existing periodontal inflammatory condition before tooth extraction ameliorates medication-related osteonecrosis of the jaw-like lesion in mice, Am. J. Pathol 188 (10) (2018) 2318–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Aguirre JI, Akhter M, Kimmel D, Pingel J, Xia X, Williams A, Jorgensen M, Edmonds K, Lee J, Reinhard M, Battles A, Kesavalu L, Wronski TJ, Enhanced alveolar bone loss in a model of non-invasive periodontitis in rice rats, Oral Dis. 18 (5) (2012) 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gotcher JE, Jee WS, The progress of the periodontal syndrome in the rice rat, I. Morphometric and autoradiographic studies, J Periodontal Res 16 (3) (1981) 275–291. [DOI] [PubMed] [Google Scholar]
- [34].Gupta O, Shaw J, Periodontal disease in the rice rat. I. Anatomic and histopathologic findings, Oral Surg. Oral Med Oral Pathol 9 (6) (1956) 592–603. [DOI] [PubMed] [Google Scholar]
- [35].Aguirre JI, Akhter MP, Neuville KG, Trcalek CR, Leeper AM, Williams AA, Rivera M, Kesavalu L, Ke HZ, Liu M, Kimmel DB, Age-related periodontitis and alveolar bone loss in rice rats, Arch. Oral Biol 73 (2017) 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Messer JG, Jiron JM, Chen HY, Castillo EJ, Mendieta Calle JL, Reinhard MK, Kimmel DB, Aguirre JI, Prevalence of food impaction-induced periodontitis in conventionally housed marsh rice rats (Oryzomys palustris), Comp Med 67 (1) (2017) 43–50. [PMC free article] [PubMed] [Google Scholar]
- [37].Benyacoub J, Rochat F, Saudan KY, Rochat I, Antille N, Cherbut C, von der Weid T, Schiffrin EJ, Blum S, Feeding a diet containing a fructooligosaccharide mix can enhance Salmonella vaccine efficacy in mice, J. Nutr 138 (1) (2008) 123–129. [DOI] [PubMed] [Google Scholar]
- [38].Ten Bruggencate SJ, Bovee-Oudenhoven IM, Lettink-Wissink ML, Katan MB, Van Der Meer R, Dietary fructo-oligosaccharides and inulin decrease resistance of rats to salmonella: protective role of calcium, Gut 53 (4) (2004) 530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Adler CJ, Dobney K, Weyrich LS, Kaidonis J, Walker AW, Haak W, Bradshaw CJ, Townsend G, Soltysiak A, Alt KW, Parkhill J, Cooper A, Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions, Nat Genet 45 (4) (2013), 450–5, 455e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sedghi L, Byron C, Jennings R, Chlipala GE, Green SJ, Silo-Suh L, Effect of dietary fiber on the composition of the murine dental microbiome, Dent J (Basel) 7 (2) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhu H, Willcox MD, Green RM, Knox KW, Effect of different diets on oral bacteria and caries activity in Sprague-Dawley rats, Microbios 91 (367) (1997) 105–120. [PubMed] [Google Scholar]
- [42].Messer JG, La S, Kipp DE, Castillo EJ, Yarrow JF, Jorgensen M, Wnek RD, Kimmel DB, Aguirre JI, Diet-induced generalized periodontitis in Lewis rats, Comp Med 69 (5) (2019) 384–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Aguirre JI, Edmonds K, Zamora B, Pingel J, Thomas L, Cancel D, Schneider L, Reinhard MK, Battles AH, Akhter MP, Kimmel DB, Wronski TJ, Breeding, husbandry, veterinary care, and hematology of marsh rice rats (Oryzomys palustris), a small animal model for periodontitis, J. Am. Assoc. Lab. Anim. Sci 54 (1) (2015) 51–58. [PMC free article] [PubMed] [Google Scholar]
- [44].Messer JG, Castillo EJ, Abraham AM, Jiron JM, Israel R, Yarrow JF, Thomas S, Reynolds MC, Wnek RD, Jorgensen M, Wanionok N, Van Poznak C, Bhattacharyya I, Kimmel DB, Aguirre JI, Anti-vascular endothelial growth factor antibody monotherapy causes destructive advanced periodontitis in rice rats (Oryzomys palustris), Bone 130 (2020) 115141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Niness KR, Inulin and oligofructose: what are they? J. Nutr 129 (7 Suppl) (1999) 1402S–1406S. [DOI] [PubMed] [Google Scholar]
- [46].Gasser JA, Ingold P, Venturiere A, Shen V, Green JR, Long-term protective effects of zoledronic acid on cancellous and cortical bone in the ovariectomized rat, J. Bone Miner. Res 23 (4) (2008) 544–551. [DOI] [PubMed] [Google Scholar]
- [47].Schechtman LM, Implementation of the 3Rs (refinement, reduction, and replacement): validation and regulatory acceptance considerations for alternative toxicological test methods, ILAR J. 43 (Suppl) (2002) S85–S94. [DOI] [PubMed] [Google Scholar]
- [48].Richmond J, Refinement, reduction, and replacement of animal use for regulatory testing: future improvements and implementation within the regulatory framework, ILAR J. 43 (Suppl) (2002) S63–S68. [DOI] [PubMed] [Google Scholar]
- [49].Yarrow JF, Conover CF, Beggs LA, Beck DT, Otzel DM, Balaez A, Combs SM, Miller JR, Ye F, Aguirre JI, Neuville KG, Williams AA, Conrad BP, Gregory CM, Wronski TJ, Bose PK, Borst SE, Testosterone dose dependently prevents bone and muscle loss in rodents after spinal cord injury, J. Neurotrauma 31 (9) (2014) 834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bouxsein M, Boyd SK, Christiansen BA, Guldberg R, Jepsen KJ, Muller R, Guidelines for assessment of bone microstructure in rodents using micro-computed tomography, J. Bone Miner. Res 25 (7) (2010) 1468–1486. [DOI] [PubMed] [Google Scholar]
- [51].Franco-Pretto E, Pacheco M, Moreno A, Messa O, Gnecco J, Bisphosphonate-induced osteonecrosis of the jaws: clinical, imaging, and histopathology findings, Oral Surg. Oral Med. Oral Pathol. Oral Radiol 118 (4) (2014) 408–417. [DOI] [PubMed] [Google Scholar]
- [52].Zheng LZ, Wang JL, Kong L, Huang L, Tian L, Pang QQ, Wang XL, Qin L, Steroid-associated osteonecrosis animal model in rats, J Orthop Translat 13 (2018) 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yang L, Boyd K, Kaste SC, Kamdem Kamdem L, Rahija RJ, Relling MV, A mouse model for glucocorticoid-induced osteonecrosis: effect of a steroid holiday, J. Orthop. Res 27 (2) (2009) 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kuroshima S, Yamashita J, Chemotherapeutic and antiresorptive combination therapy suppressed lymphangiogenesis and induced osteonecrosis of the jaw-like lesions in mice, Bone 56 (1) (2013) 101–109. [DOI] [PubMed] [Google Scholar]
- [55].Yamashita J, Koi K, Yang DY, McCauley LK, Effect of zoledronate on oral wound healing in rats, Clin. Cancer Res 17 (6) (2011) 1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Curtiellas-Piñol V, Ventura-Juárez J, Ruiz-Baca E, Romo-Lozano Y, Morphological changes and phagocytic activity during the interaction of human neutrophils with Sporothrix schenckii: an in vitro model, Microb. Pathog 129 (2019) 56–63. [DOI] [PubMed] [Google Scholar]
- [57].Holness CL, Simmons DL, Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins, Blood 81 (6) (1993) 1607–1613. [PubMed] [Google Scholar]
- [58].Queipo-Ortuno MI, Seoane LM, Murri M, Pardo M, Gomez-Zumaquero JM, Cardona F, Casanueva F, Tinahones FJ, Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels, PLoS One 8 (5) (2013), e65465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kolodziejczyk AA, Zheng D, Elinav E, Diet-microbiota interactions and personalized nutrition, Nat Rev Microbiol 17 (12) (2019) 742–753. [DOI] [PubMed] [Google Scholar]
- [60].Lecomte V, Kaakoush NO, Maloney CA, Raipuria M, Huinao KD, Mitchell HM, Morris MJ, Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters, PLoS One 10 (5) (2015), e0126931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Leeming ER, Johnson AJ, Spector TD, Le Roy CI, Effect of diet on the gut microbiota: rethinking intervention duration, Nutrients 11 (12) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Thumbigere-Math V, Michalowicz BS, Hodges JS, Tsai ML, Swenson KK, Rockwell L, Gopalakrishnan R, Periodontal disease as a risk factor for bisphosphonate-related osteonecrosis of the jaw, J. Periodontol 85 (2) (2014) 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B, American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update, J. Oral Maxillofac. Surg 67 (5 Suppl) (2009) 2–12. [DOI] [PubMed] [Google Scholar]
- [64].Chistiakov DA, Killingsworth MC, Myasoedova VA, Orekhov AN, Bobryshev YV, CD68/macrosialin: not just a histochemical marker, Lab. Investig 97 (1) (2017) 4–13. [DOI] [PubMed] [Google Scholar]
- [65].Cascao R, Vidal B, Lopes IP, Paisana E, Rino J, Moita LF, Fonseca JE, Decrease of CD68 synovial macrophages in celastrol treated arthritic rats, PLoS One 10 (12) (2015), e0142448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Caprioli F, Bose F, Rossi RL, Petti L, Vigano C, Ciafardini C, Raeli L, Basilisco G, Ferrero S, Pagani M, Conte D, Altomare G, Monteleone G, Abrignani S, Reali E, Reduction of CD68+ macrophages and decreased IL-17 expression in intestinal mucosa of patients with inflammatory bowel disease strongly correlate with endoscopic response and mucosal healing following infliximab therapy, Inflamm. Bowel Dis 19 (4) (2013) 729–739. [DOI] [PubMed] [Google Scholar]
- [67].Komori T, Functions of the osteocyte network in the regulation of bone mass, Cell Tissue Res. 352 (2) (2013) 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Andreev D, Liu M, Weidner D, Kachler K, Faas M, Gruneboom A, Schlotzer-Schrehardt U, Munoz LE, Steffen U, Grotsch B, Killy B, Kronke G, Luebke AM, Niemeier A, Wehrhan F, Lang R, Schett G, Bozec A, Osteocyte necrosis triggers osteoclast-mediated bone loss through macrophage-inducible C-type lectin, J. Clin. Invest 130 (9) (2020) 4811–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Liu-Bryan R, Terkeltaub R, Emerging regulators of the inflammatory process in osteoarthritis, Nat. Rev. Rheumatol 11 (1) (2015) 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Takeuchi O, Akira S, Pattern recognition receptors and inflammation, Cell 140 (6) (2010) 805–820. [DOI] [PubMed] [Google Scholar]
- [71].Ibrahim ZA, Armour CL, Phipps S, Sukkar MB, RAGE and TLRs: relatives, friends or neighbours? Mol. Immunol 56 (4) (2013) 739–744. [DOI] [PubMed] [Google Scholar]
- [72].Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E, Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein, J. Biol. Chem 279 (9) (2004) 7370–7377. [DOI] [PubMed] [Google Scholar]
- [73].Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T, Mincle is an ITAM-coupled activating receptor that senses damaged cells, Nat. Immunol 9 (10) (2008) 1179–1188. [DOI] [PubMed] [Google Scholar]
- [74].Toro LF, de Mello-Neto JM, Santos F.F.V.d., Ferreira LC, Statkievicz C, Cintra LTÂ, Issa JPM, Dornelles RCM, de Almeida JM, Nagata MJH, Garcia VG, Theodoro LH, Casatti CA, Ervolino E, Application of autologous platelet-rich plasma on tooth extraction site prevents occurence of medication-related osteonecrosis of the jaws in rats, Scientific Reports 9 (1) (2019) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Swerts AA, Santos BFE, Bruzadelli SR, Brigagao M, Lima DC, Fernandes LA, Treatment of experimental periodontal disease by laser therapy in simvastatin-modified rats, J. Appl. Oral Sci 25 (4) (2017) 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Miessi DMJ, Garcia VG, Ervolino E, Scalet V, Nuernberg MAA, Dos Santos Neto OM, da Rocha TE, Theodoro LH, Lactobacillus reuteri associated with scaling and root planing in the treatment of periodontitis in rats submitted to chemotherapy, Arch. Oral Biol 117 (2020) 104825. [DOI] [PubMed] [Google Scholar]
- [77].Theodoro LH, Caiado RC, Longo M, Novaes VC, Zanini NA, Ervolino E, de Almeida JM, Garcia VG, Effectiveness of the diode laser in the treatment of ligature-induced periodontitis in rats: a histopathological, histometric, and immunohistochemical study, Lasers Med. Sci 30 (4) (2015) 1209–1218. [DOI] [PubMed] [Google Scholar]
- [78].Plessas A, Nonsurgical periodontal treatment: review of the evidence, Oral Health Dent Manag 13 (1) (2014) 71–80. [PubMed] [Google Scholar]
- [79].Slot DE, Dorfer CE, Van der Weijden GA, The efficacy of interdental brushes on plaque and parameters of periodontal inflammation: a systematic review, Int. J. Dent. Hyg 6 (4) (2008) 253–264. [DOI] [PubMed] [Google Scholar]
- [80].Slot DE, Valkenburg C, Van der Weijden GAF, Mechanical plaque removal of periodontal maintenance patients: a systematic review and network meta-analysis, J. Clin. Periodontol 47 (Suppl. 22) (2020) 107–124. [DOI] [PubMed] [Google Scholar]
- [81].Ryder MI, Histological and ultrastructural characteristics of the periodontal syndrome in the rice rat, I. General light microscopic observations and ultrastructural observations of initial inflammatory changes, J Periodontal Res 15 (5) (1980) 502–515. [DOI] [PubMed] [Google Scholar]
- [82].Perez-Chaparro PJ, Goncalves C, Figueiredo LC, Faveri M, Lobao E, Tamashiro N, Duarte P, Feres M, Newly identified pathogens associated with periodontitis: a systematic review, J. Dent. Res 93 (9) (2014) 846–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wei X, Pushalkar S, Estilo C, Wong C, Farooki A, Fornier M, Bohle G, Huryn J, Li Y, Doty S, Saxena D, Molecular profiling of oral microbiota in jawbone samples of bisphosphonate-related osteonecrosis of the jaw, Oral Dis. 18 (6) (2012) 602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Pushalkar S, Li X, Kurago Z, Ramanathapuram LV, Matsumura S, Fleisher KE, Glickman R, Yan W, Li Y, Saxena D, Oral microbiota and host innate immune [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.