Abstract
Objectives:
The current tools for the management of diabetes mellitus (DM) do not prevent its development or complications, so investigations into promising new treatments, for example, honey-royal jelly (H-RJ) mixtures, are needed. This study was conducted to evaluate the effect of royal jelly on DM by measuring the biochemical parameters (fasting blood glucose and glycated hemoglobin [HbA1c]) accompanying streptozotocin (i.p. 75 mg/kg once)-induced type 1 DM (T1DM) in rats. Other objectives were to evaluate the effects of H-RJ on lipid parameters.
Methods:
Ten healthy control male Sprague-Dawley rats (120–150 g) were compared to untreated T1DM (n = 10), metformin-treated T1DM (n = 10), and H-RJ-treated T1DM rats (n = 10) on plasma and whole-blood glycemic control indices (fasting blood glucose, HbA1c %, insulin, and insulin resistance) and plasma lipid profile (triglycerides [TGs] and total, low-density lipoprotein -, high-density lipoprotein -, and very low-density lipoprotein [VLDL]-cholesterol). Diabetes was induced by a single intraperitoneal injection of streptozotocin (SZT) at 75 mg/kg body weight (BW). The T1DM-H-RJ rat group received daily RJ (100 mg/kg BW). Parametric data are presented as mean ± SD and were analyzed for comparison using one-way ANOVA in SPSS software.
Results:
H-RJ normalized glycemic control indices, but its effect on lipid parameters was variable. H-RJ and metformin had comparable effects. The H-RJ treatment caused a significant reduction in plasma VLDL-C content in comparison to the control treatment and metformin. The limitation of this study is that it was restricted to T1DM and did not involve type 2 DM. In addition, the study was limited to male Sprague-Dawley rats, with no females.
Conclusion:
The H-RJ mixture is a promising antidiabetic alternative medicine. It effectively reduces VLDL-C and TG in diabetic rats.
Keywords: Blood sugar, experimental diabetes, glycated hemoglobin, honey, insulin, lipid profile, rats, royal jelly
Introduction
Diabetes mellitus (DM) is currently considered a public health issue, and its prevalence is gradually increasing; soon, it may threaten society as an epidemic.[1] The Kingdom of Saudi Arabia leads the Middle East and North Africa in the prevalence of metabolic disorders, including diabetes, which is on the rise.[2,3] DM affects people’s quality of life due to its complications (retinopathy, renal impairment, and cardiovascular disease), which lead to an increase in the mortality rate.[4] DM is not caused by a single factor but by multiple factors (genetic, social, and environmental) that can be managed by a combination of a healthy diet, physical activity, and medication, but it requires lifelong management.[5] No medication is considered a cure for DM, and this has led some diabetic patients to use alternative and complementary medicine (acupuncture, chiropractic, and herbal medicine).[6] Some studies have reported that honey ameliorates hyperglycemia and oxidative damage caused by DM.[7,8] Moreover, honey was reported to increase high-density lipoprotein (HDL) and protect the liver by reducing hepatic transaminases.[9]
Few studies have measured the effect of honey on glycated hemoglobin (HbA1c). A study conducted in 2009 by Bahrami et al. revealed that the use of honey in type 2 diabetic patients for a duration of 8 weeks led to an increase in the value of HbA1c.[10] Other studies have shown that the combination of honey with antidiabetic drugs can increase antioxidant processes and improve glycemic control.[11,12] According to a study published by Bobiş et al. in 2018, honey may be considered beneficial for the management of DM, as it controls hyperglycemia and limits the occurrence of other metabolic disorders.[13] Honey has also been used in alternative and complementary medicine to treat some diseases, including abdominal pain, wounds, asthma, and burns, and has become a subject of new research interest during the past few years.[12] Experimental studies have shown that RJ has antioxidant, antihypercholesterolemic, and hypoglycemic properties.[14-16] Honey is mentioned in the Quran, the Holy Book of Islam: “And your Lord inspired the bees, by saying: Take you habitations in the mountains and in the trees and in what they erect. Then, eat of all fruits, and follow the ways of your Lord made easy (for you). There comes forth from their bellies, a drink of varying color wherein is healing for people. Verily, in this is indeed a sign for people who think.” Thus, honey has therapeutic value against many diseases and needs to be investigated. Nevertheless, few studies have been performed to evaluate the effect of RJ on type 1 DM (T1DM). Therefore, this study aimed mainly to evaluate the efficacy of oral administration of a honey-royal jelly (H-RJ) mixture on the biochemical and histopathological changes accompanying streptozotocin-induced T1DM in rats. Another objective was to evaluate the effect of H-RJ on fasting blood sugar and HbA1c in comparison to metformin. Finally, we looked into the effect of the H-RJ mixture on complications due to DM.
Methods
Animals: Standard rat meal and 40 male Sprague Dawley rats weighing 120–130 g were purchased from the Animal Unit of the Faculty of Pharmacy, King Saud University, Saudi Arabia. The rats were caged individually and were kept at 25°C, 56% humidity (40–70%) and a 12 h light: dark cycle with free access to food and tap water. The standard diet had 2850.0 kcal/kg energy in the form of 64.0% carbohydrates, 20% crude protein, 4% crude fat, 3.5% crude fiber, and 6% ash. It also contained 0.5% salt, 1% calcium, 0.6% phosphorous, and Vitamin A (20.0 IU/g), D (2.20 IU/g), and E (70.0 IU/kg), and trace elements (cobalt, copper, iodine, iron, manganese, selenium, and zinc). The experiment was conducted according to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Natural royal jelly manufactured by Royal Power (2000 mg – 30 capsules) was obtained from authorized pharmacies in Saudi Arabia. The analysis of H-RJ was performed in the Food Safety and Quality Control Laboratory, Faculty of Agriculture, Cairo University, Egypt. H-RJ contained the following items (protein (g/100 g) 1.64, fats (g/100 g) 0.01, water (g/100 g) 38.09, ash (g/100 g) 0.15, fructose g/100 g 17.53, glucose g/100 g 16.83, sucrose g/100 g 25.75, total phenols (mg/kg) 310.81, total flavonoids (mg/kg) 23.66, sodium (mg/kg) 629.38, potassium (mg/kg) 938.46, iron (mg/kg) 6.43, phosphorus (mg/kg) 1105.95, calcium (mg/kg) 147.64, zinc (mg/kg) 10.86, chromium (mg/kg) 0.53, copper (mg/kg) 2.04, manganese (mg/kg) 0.56, and magnesium (mg/kg) 163.01.
Determination of chemical compounds
Determination of moisture content, hydroxymethyl furfural (HMF), protein, and fat in the H-RJ sample was performed according to methods given by the Association of Official Analytical Chemists[17] and harmonized methods of the International Honey Commission.[18] Moisture is an important parameter in determining honey quality. Too high a moisture content often causes fermentation of honey, leading to a low shelf life and unpleasant flavor.[19]
The level of HMF indicates the freshness of honey and is affected by the storage conditions/periods and the extent to which it is heated. High HMF levels in a honey sample suggest the possibility of adulteration, overheating, or overly long storage.[20]
Total phenols were determined by colorimetry with the Folin–Ciocalteu reagent according to the method described by Singleton and Rossi.[21] Total flavonoids were determined by the aluminum chloride colorimetric method described by Ranganna (1986).[22]
Experimental design
After excluding the rats that died and rats with low glucose levels, the net number of non-diabetic or diabetic rats eligible for the study was 28 out of 40 (seven non-diabetic and 21 diabetic), which were subsequently distributed into four groups. All rats in this study received a standard diet during the trial, and all suggested treatments were administered through oral gavage once daily. All rats were weighed before the experiment, according to which the diabetic rats were distributed into the experimental groups as follows: In the non-diabetic group (seven rats), normal rats were orally injected with distilled water (0.5 ml/rat) once daily; in the untreated diabetic group (seven rats), diabetic rats were given distilled water (0.5 ml/rat) through oral gavage once daily; the metformin diabetic group (seven rats) was orally injected with 1 ml/day metformin drug solution (dose = 100 mg/kg); and royal jelly diabetic group (R): Seven diabetic rats were orally administered RJ at 100 mg/kg body weight for 28 days. The dose of royal jelly was taken from a previous study on the effects of royal jelly on histopathological changes in the testes of diabetic rats.[23]
Ethical approval
All procedures were performed according to the NIH Guiding Principles in the Care and Use of Animals and were approved by the Ethics Committee for Medical Research, Deanship of Scientific Research, Qassim University (Approval No. 19-09-02, Friday, April 10, 2020). Study Design: This was an experimental study.
Induction of T1DM
After 7 days of acclimatization, rats were weighed. Ten rats were randomly assigned to the healthy normal control group. We induced T1DM in the remaining rats by intraperitoneal injection of 75 mg/kg streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO, USA) dissolved in 0.5 mL sterile sodium citrate buffer (pH 7.4) with 10% sucrose dissolved in drinking water to increase the metabolic stress on pancreatic β-cells. After 72 h, a morning fasting tail blood droplet was taken to estimate blood glucose using the OneTouch® Select Plus, LifeScan, Switzerland. Twenty-one rats with a blood glucose level >250 mg/dL were considered diabetic and were enrolled in the experimental T1DM group.[24] A similar test was done for the control rats to exclude spontaneous diabetes. Diabetic rats were subcutaneously injected weekly with 2 IU of human insulin (Glargine, Lantus) to guard against their death during the experiment.
Treatment and sampling
All the rats (40) were divided into four groups, one as a healthy control group and three groups of diabetic rats. T1DM rats were divided into three subgroups (n = 10 each): Untreated diabetic group, 100 mg/kg/daily metformin-treated group (by gavage; dissolved in 1 mL water) and H-RRJ-treated group (by gavage; 2 g/daily diluted 1:1 in distilled water). A 98% honey-2% royal jelly mixture that was readymade and commercially available was used (Buram GmbH, Berlin, Germany). After 28 days of continuous treatment, fasting morning blood samples were collected into heparin tubes from the jugular vein of all animals before they were sacrificed under light diethyl ether anesthesia. A 100 mL aliquot of whole blood was separated for HbA1c assay, and the remaining sample was centrifuged for 10 min at 5000 rpm and 4°C to recover the plasma, which was aliquoted and frozen at −40°C. Plasma was used to measure glucose, HbA1c, insulin, triglycerides (TGs), and total HDL- (HDL-C), very low-density lipoprotein–cholesterol (VLDL-C), and low-density lipoprotein-cholesterol (LDL-C).
Statistical analysis
All data are presented as mean ± SD and were statistically analyzed for significance of differences between groups using one-way ANOVA and the LSD test at a confidence level of 95% and P < 0.05 using SPSS for Windows V.20 (SPSS Inc., Chicago, IL, USA).
Results
The data in Table 1 show the significant normalization of fasting plasma glucose and whole-blood HbA1c in H-RJ-treated rats compared to untreated experimental diabetic rats (P < 0.001 for each). The treated, healthy control and control metformin-treated groups had non-significant differences in these two parameters.
Table 1.
Effect of H-RJ-T on glycemic control indices (fasting plasma glucose (mg/dL) and whole-blood HbA1c[%]) after 28 days of treatment in streptozotocin-induced T1DM rats compared to metformin-treated (M-T) and healthy control rats (H-Cs). Data shown are means of differences (MoDs) and P values. I = index group
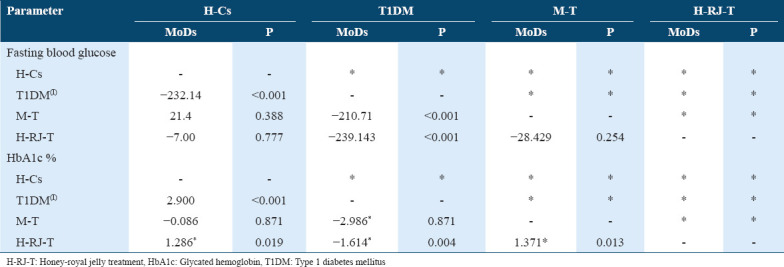
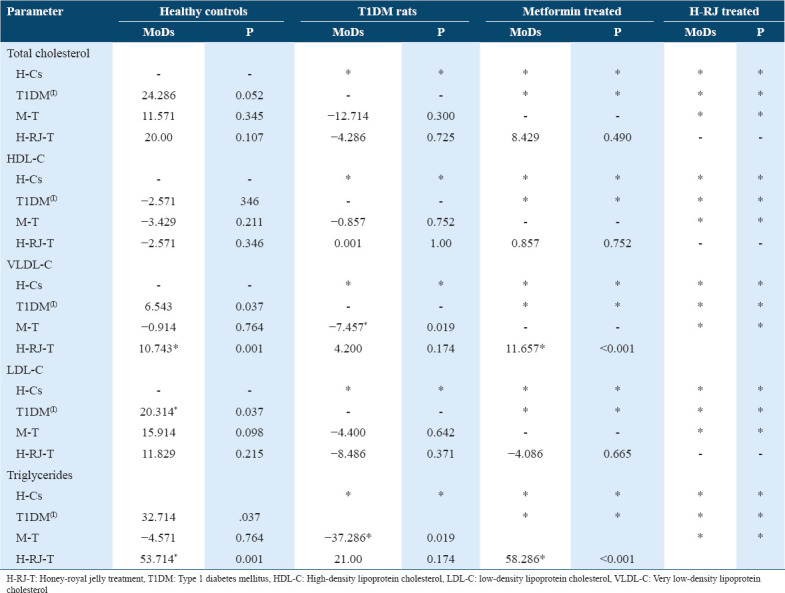
Table 2 shows that H-RJ treatment caused a significant reduction in plasma VLDL-C content in comparison to the control treatment and metformin in diabetic rats. H-RJ treatment had no clear effect on plasma total or LDL-C content. H-RJ treatment caused a significant reduction in plasma TG in comparison with metformin.
Table 2.
Effect of H-RJ-T on the fasting plasma lipid profile after 28 days of treatment in streptozotocin-induced T1DM rats compared to metformin-treated (M-T) and healthy control rats (H-Cs). Data shown are means of differences (MoDs) and P value. I = index group
Discussion
The royal jelly-honey that was used in this study contained hydroxymethylfurfural 2.03 (mg/kg), fructose 17.52 (g/100 g), glucose 16.82 (g/100 g), and sucrose 25.75 (g/100 g).
Fructose is monosaccharide that is absorbed from the gastrointestinal tract more slowly than glucose. Therefore, its metabolism is largely independent of insulin, and it is quickly removed by the liver, so its ingestion leads to only a mild increase in blood sugar and can be used as a sweetener for diabetic patients with type 2 diabetes. Natural honey has many therapeutic properties, such as antibacterial, hepatoprotective, hypoglycemic, antihypertensive, and reproductive properties, and improves oxidative stress in the kidneys and pancreas of diabetic rats induced by STZ.[25] Streptozotocin can lead to DM in experimental animals through its cytotoxic action on β-cells in the islets of Langerhans.[26] In our study, metformin improved fasting blood sugar and HbA1c, but in the study done by Nasrolahi et al. in 2012, metformin did not improve the blood sugar of diabetic rats.[12] The results of this study showed that royal jelly-honey and metformin significantly (P < 0.01) promoted good control of FBS compared to the control treatment in diabetic rats. Our study showed that HbA1C was significantly controlled with H-RJ treatment, while metformin did not significantly affect HbA1C in diabetic rats.
However, the diabetic rats on royal jelly showed significant decreases in VLDL-C and TGs compared to diabetic control rats. The difference between our study and the study by Nasrolahi et al.[12] was that our study showed an effect of metformin on reducing VLDL-C and TG, while no such effect was seen by Nasrolahi et al.[12] Our findings on the effect of royal jelly on blood lipid levels are in line with Nasrolahi et al. and Erejuwa et al. (2011) which showed reduced levels of TG and VLDL cholesterol in diabetic rats on natural honey compared to diabetic control rats.[12,25] In our study, there were no effects of metformin or royal jelly on LDL or total cholesterol, while in Nasrolahi et al. (2012) and Erejuwa et al. (2011), the levels of LDL and total cholesterol were decreased by natural honey, which may be explained by the variety of honey used.[12,25] HDL, in our study, was not affected by royal jelly, while Erejuwa et al. showed that the level of HDL cholesterol was increased by natural honey compared to metformin alone in diabetic rats.[25] In view of these findings, it may be concluded that phenolic compounds such as flavonoids are potent antioxidants that are able to prevent and/or inhibit diabetes-induced derangements.[25] This conclusion is supported by Yaman et al. (2016) who concluded that “natural honey has a hepatoprotective and nephroprotective effect in rats with experimental aflatoxicosis due to its antioxidant activity.”[27] Metformin, in our study, showed more protective effect on the kidney as compared to royal jelly-honey. Honey is considered an antioxidant because it contains phenolic groups, the amounts of which depend on the variety of honey.[28] Regarding eye complications, the H-RJ-treated and metformin-treated groups both had fewer eye complications than the control diabetic rats. There was little difference between the two treatment groups in eye complications, as both of them showed only a mild change in edema in the stroma of the cornea and vacuolation of granular cells of the retina. In our H-RJ mixture, the total phenol content was 310.81 mg/kg, and the total flavonoid content was 23.66 mg/kg, while in the study done by Daniela Pauliuc et al. (2020), thyme honey contained the lowest flavonoid content of the honeys tested (17.45 mg), while raspberry honey had the highest (33.58 mg).[29]
Conclusion
Royal jelly-honey can effectively control the blood sugar of diabetic rats (fasting blood sugar and HbA1c). It can also effectively reduce the lipids VLDL-C and TG. Royal jelly-honey can be considered a nephroprotective and antioxidant. These findings on honey are expected to stimulate further research, especially on the possibility of managing DM through interventions that look to control DM and reduce oxidative damage. The beneficial effects of honey on diabetes might not be limited to controlling glycemia but might also extend to improving the associated metabolic disruptions in this complicated metabolic disorder. There is no doubt that more well-designed, rigorously conducted randomized controlled studies are necessary to further validate our findings related to the effects of royal jelly-honey.
Authors’ Declaration Statements
Ethics approval and consent to participate
All procedures were performed according to the NIH Guiding Principles in the Care and Use of Animals and were approved by the Ethics Committee for Medical Research, Deanship of Scientific Research, Qassim University (Approval No. 19-09-02.
Availability of data and material
The data used in this study are available and will be provided by the corresponding author on a reasonable request.
References
- 1.Lam DW, LeRoith D. The worldwide diabetes epidemic. Curr Opin Endocrinol Diabetes Obes. 2012;19:93–6. doi: 10.1097/MED.0b013e328350583a. [DOI] [PubMed] [Google Scholar]
- 2.Abdullah A, Hasan H, Raigangar V, Bani-Issa W. C-peptide versus insulin:Relationships with risk biomarkers of cardiovascular disease in metabolic syndrome in young Arab females. Int J Endocrinol. 2012;2012:420792. doi: 10.1155/2012/420792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert AA, Al-Dawish A, Mujammami M, Dawish MA. Type 1 diabetes mellitus in Saudi Arabia:A soaring epidemic. Int J Pediatr. 2018;2018:9408370. doi: 10.1155/2018/9408370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Diabetes Federation. IDF Diabetes Atlas. Brussels: International Diabetes Federation; 2015. [Google Scholar]
- 5.Imam K. Clinical features, diagnostic criteria and pathogenesis of diabetes mellitus. Adv Exp Med Biol. 2012;771:340–55. doi: 10.1007/978-1-4614-5441-0_25. [DOI] [PubMed] [Google Scholar]
- 6.Nahas R, Moher M. Complementary and alternative medicine for the treatment of Type 2 diabetes. Can Family Physician. 2009;55:591–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Beretta G, Orioli M, Facino RM. Antioxidant and radical scavenging activity of honey in endothelial cell cultures (EA. hy926) Planta Med. 2007;73:1182–9. doi: 10.1055/s-2007-981598. [DOI] [PubMed] [Google Scholar]
- 8.Erejuwa OO, Sulaiman SA, Wahab MS, Sirajudeen KN, Salleh MM, Gurtu S. Antioxidant protection of Malaysian tualang honey in pancreas of normal and streptozotocin-induced diabetic rats. Ann Endocrinol. 2010;71:291–6. doi: 10.1016/j.ando.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Erejuwa OO, Sulaiman SA, Wahab MS, Sirajudeen KN, Salleh MM, Gurtu S. Hepatoprotective effect of tualang honey supplementation in streptozotocin-induced diabetic rats. Int J Appl Res Nat Prod. 2012;4:37–41. [Google Scholar]
- 10.Bahrami M, Ataie-Jafari A, Hosseini S, Foruzanfar MH, Rahmani M, Pajouhi M. Effects of natural honey consumption in diabetic patients:An 8-week randomized clinical trial. Int J Food Sci Nutr. 2009;60:618–26. doi: 10.3109/09637480801990389. [DOI] [PubMed] [Google Scholar]
- 11.Erejuwa OO, Sulaiman SA, Ab Wahab MS, Sirajudeen KN, Salleh MS, Gurtu S. Glibenclamide or metformin combined with honey improves glycemic control in streptozotocin-induced diabetic rats. Int J Biol Sci. 2011;7:244. doi: 10.7150/ijbs.7.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasrolahi O, Heidari R, Rahmani F, Farokhi F. Effect of natural honey from Ilam and metformin for improving glycemic control in streptozotocin-induced diabetic rats. Avicenna J Phytomed. 2012;2:212. [PMC free article] [PubMed] [Google Scholar]
- 13.Bobiş O, Dezmirean DS, Moise AR. Honey and diabetes:The importance of natural simple sugars in diet for preventing and treating different type of diabetes. Oxid Med Cell Longev. 2018;2018:4757893. doi: 10.1155/2018/4757893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagai T, Inoue R. Preparation and the functional properties of water extract and alkaline extract from royal jelly. Food Chem. 2004;84:181–6. [Google Scholar]
- 15.Gou H, Sagia A, Sato M, Miyazawa I, Shibata M, Takahata Y, et al. Royal jelly supplementation improve lipoprotein metabolism in humans. J Nutr Sci Vitaminol (Tokyo) 2007;53:354–8. doi: 10.3177/jnsv.53.345. [DOI] [PubMed] [Google Scholar]
- 16.Munstedt K, Baregello M, Hauenschild A. Royal jelly reduces the serum glucose levels in healthy subjects. J Med Food. 2009;12:1–3. doi: 10.1089/jmf.2008.0289. [DOI] [PubMed] [Google Scholar]
- 17.AOAC. Official Methods of Analysis, Association of Official Analytical Chemists. 15th ed. Arlington, VA, USA: AOAC; 1990. [Google Scholar]
- 18.Bogdanov S. Harmonized Methods of the International Honey Commission. Bee Hexagon Issue. 2009. [Last accessed on 2021 May 15]. pp. 2–61. Available from: https://www.ihc-platform.net/ihcmethods2009.pdf .
- 19.Bogdanov S, Martin P. Honey authenticity mitteilungen aus dem Gebiete der. Lebensmitteluntersuchung Hyg. 2002;93:232–54. [Google Scholar]
- 20.Castro-Vázquez L, Díaz-Maroto MC, González-Viñas MA, de la Fuente E, Pérez-Coello MS. Influence of storage conditions on chemical composition and sensory properties of citrus honey. J Agric Food Chem. 2008;56:1999–2006. doi: 10.1021/jf072227k. [DOI] [PubMed] [Google Scholar]
- 21.Singleton VL, Rossi JA. Colorimetry of total phenols with phosphomolybdic- phosphtungstic acid reagents. Am J Enology Viticult. 1965;16:144–58. [Google Scholar]
- 22.Ranganna S. Handbook of Analyses and Quality Control for Fruits and Vegetables Products. New Delhi, India: Tata Mc Grow Hill Publishing Co Ltd; 1986. [Google Scholar]
- 23.Ghanbari E, Nejati V, Khazaei M. Antioxidant and protective effects of Royal jelly on histopathological changes in testis of diabetic rats. Int J Reprod Biomed. 2016;14:519. [PMC free article] [PubMed] [Google Scholar]
- 24.Abdel-Salam AM, Ismail MS, Farahna MM, Mousa HM. Protective effects of whey protein mixed with Garcinia kola and olive leaves extract against alloxan-induced oxidative stress and diabetes in rats. Bull Natl Res Centre. 2018;42:1–7. [Google Scholar]
- 25.Erejuwa OO, Sulaiman SA, Ab Wahab MS. Honey:A novel antioxidant. Molecules. 2012;17:4400–23. doi: 10.3390/molecules17044400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenna Ø, Qvigstad G, Brenna E, Waldum HL. Cytotoxicity of streptozotocin on neuroendocrine cells of the pancreas and the gut. Dig Dis Sci. 2003;48:906–10. doi: 10.1023/a:1023043411483. [DOI] [PubMed] [Google Scholar]
- 27.Yaman T, Yener Z, Celik I. Histopathological and biochemical investigations of protective role of honey in rats with experimental aflatoxicosis. BMC Complement Altern Med. 2016;16:232. doi: 10.1186/s12906-016-1217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosa A, Tuberoso CI, Atzeri A, Melis MP, Bifulco E, Dessì MA. Antioxidant profile of strawberry tree honey and its marker homogentisic acid in several models of oxidative stress. Food Chem. 2011;129:1045–53. doi: 10.1016/j.foodchem.2011.05.072. [DOI] [PubMed] [Google Scholar]
- 29.Pauliuc D, Dranca F, Oroian M. Antioxidant activity, total phenolic content, individual phenolics and physicochemical parameters suitability for Romanian honey authentication. Foods. 2020;9:306. doi: 10.3390/foods9030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are available and will be provided by the corresponding author on a reasonable request.


