Abstract
Background
Hand‐foot skin reaction (HFSR) is the most common regorafenib‐induced adverse event and is in need of effective prevention and palliation.
Materials and Methods
The Regorafenib Dose Optimization Study (ReDOS), a four‐arm, previously published trial with a 1:1:1:1 randomization scheme, was analyzed in a manner in keeping with the original protocol to assess whether clobetasol 0.05% cream (a corticosteroid) applied to the palms and soles twice per day for 8 weeks was more effective when prescribed preemptively (before the development of HFSR) versus reactively (after the development of HFSR). Patients were assessed during the first two cycles of regorafenib.
Results
Sixty‐one patients received preemptive clobetasol, and 55 received reactive clobetasol. Groups were balanced on demographics. Over the first two cycles, no evidence of HFSR occurred in 30% with preemptive clobetasol versus 13% with reactive clobetasol (p = .03). During the first cycle, 54% and 45% of patients had no HFSR with preemptive and reactive clobetasol, respectively (p = .35). During the second cycle, 33% and 15% had no HFSR with preemptive and reactive clobetasol, respectively (p = .02). During the second cycle, rates of grade 1, 2, and 3 HFSR were 30%, 8%, and 3%, respectively, with preemptive clobetasol and 43%, 18%, and 7%, respectively, with reactive clobetasol (p = .12). Patient‐reported outcomes showed HFSR compromised nearly all activities of daily living with worse quality of life in patients who received reactive versus preemptive clobetasol. No clobetasol‐induced adverse events were reported.
Conclusion
Preemptive clobetasol might lessen regorafenib‐induced hand‐foot reactions compared with reactive therapy. Further confirmatory studies are needed in a larger patient cohort.
Implications for Practice
Regorafenib causes hand‐foot skin reactions. Preemptive clobetasol, a high‐potency topical corticosteroid, appears to lessen the severity of this adverse event. Although further study is needed, the favorable adverse event profile of this intervention might prompt clinicians to discuss this option with their patients.
Keywords: Hand‐foot skin reaction, Palmer‐plantar erythrodysesthesia, Skin toxicity, Regorafenib
Short abstract
Hand‐foot skin reaction is a common regorafenib‐induced adverse event. This article reports on the use of topical clobetasol and its role when administered preventively, before a skin reaction occurs.
Introduction
Hand‐foot skin reaction (HFSR) is the most common regorafenib‐induced adverse event [1, 2]. Occurring in more than 50% of patients within 6 weeks, HFSR manifests as palmar and plantar redness, pain, hyperkeratosis, and desquamation. Severe grade 3+ reactions arise in 10%–15% of patients [1, 2]. Even when mild, HFSR negatively impacts quality of life [3].
To date, strategies have sought to palliate sorafenib‐ and other drug‐induced HSFR with little focus on regorafenib‐induced HFSR, although structural similarities between sorafenib and regorafenib suggest the possibility of overlapping success. These strategies have included keratolytics, moisturizers, phototherapy, and corticosteroids [4, 5]. Anecdotes suggest that topical corticosteroids palliate drug‐induced HFSR and therefore merit further study [5].
We report here on HFSR from the previously published ReDOS trial [6]. We report results on topical clobetasol (a high‐potency corticosteroid used for a variety of skin conditions) and its role when administered preemptively, or before the development of HFSR, versus reactively, or after a skin reaction has already occurred. This report is aimed at preventing/palliating this common, distressing drug‐induced adverse event.
Materials and Methods
Overview
ReDOS was conducted within the Academic and Community Cancer Research United network, a multi‐institutional, Mayo Clinic–supported organization. ReDOS showed that dose escalation of regorafenib for colorectal cancer improves clinical outcomes (NCT02368886) [6]. Medical centers participated after institutional review board approval.
Eligibility
Eligibility criteria are as follows [6]: (a) age ≥ 18 years; (b) histological/cytological colorectal cancer; (c) incurable metastatic cancer with progression on previous therapy and measurable or nonmeasurable disease; (d) Eastern Cooperative Oncology Group (ECOG) performance score of 1 or better; (e) life expectancy of 3+ months; (f) acceptable hemogram parameters, liver function tests, serum creatinine, and albumin; (g) negative pregnancy test, if relevant; (h) patient able to provide informed consent and complete questionnaires independently or with assistance; and (i) patient willing to provide blood for correlative studies (results to be reported later). Exclusion criteria are as follows: (a) prior therapy with regorafenib or ongoing clobetasol propionate; (b) major surgery or similar event ≤28 days before randomization; (c) heart issues, including congestive heart failure of New York Heart Association class greater than 2, unstable angina, or cardiac arrhythmias that required antiarrhythmic drug therapy other than beta blockers or digoxin; (d) uncontrolled hypertension; (e) symptomatic brain metastases; (f) thrombotic event ≤6 months of randomization, hemorrhage, or history of bleeding diathesis; (g) persistent proteinuria (grade 3+); (h) inability to swallow/absorb oral medication; (i) medical conditions such as ongoing infection, chronic hepatitis B or C, history of pheochromocytoma, history of organ allograft, renal failure requiring dialysis, nonhealing wound, dehydration, interstitial lung disease, prior substance abuse, or ongoing toxicity grade 2+ from prior cancer therapy; (j) concurrent cancer therapy other than that prescribed in the trial or use of herbal interventions; or (k) known study drug intolerance.
Study Design
This preplanned analysis assessed clobetasol for regorafenib‐induced HFSR. In the original ReDOS trial, patients were randomly assigned to one of four arms, with the intervention administered over two cycles of regorafenib (cycle length was 28 days): (1) preemptive clobetasol + regorafenib (starting dose 80 mg/day with potential escalation to 160 mg/day); (2) reactive clobetasol + regorafenib (starting dose 80 mg/day with potential dose escalation to 160 mg/day); (3) preemptive clobetasol + regorafenib (160 mg starting dose); (4) reactive clobetasol + regorafenib (160 mg starting dose). This report describes this four‐arm study from the vantage point of topical clobetasol for HFSR within the first two cycles of regorafenib. Thus, the primary analyses described here compressed the four‐arm trial into two to assess preemptive versus reactive clobetasol based on an a priori goal outlined in the original study protocol [7]. In preplanned analyses, arms 1 and 3 were consolidated into the preemptive clobetasol group, and arms 2 and 4 into the reactive clobetasol group.
Clobetasol Intervention and Supportive Care
Clobetasol was self‐administered as a thin layer of 0.05% cream to the palms and soles twice per day over two cycles of regorafenib. Patients avoided washing their hands and feet for 1 hour after clobetasol administration. Patients applied the clobetasol in either a preemptive or reactive manner. Preemptive clobetasol meant prior to HSFR. Reactive meant after HSFR.
Patients were allowed other topical interventions for HSFR 1 hour after clobetasol and were advised to check their hands and feet frequently, consider a pedicure/manicure, use a pumice stone prior to regorafenib, wear well‐padded footwear, avoid pressure points or hand/foot abrasion, and soak their hands and feet [4].
Monitoring, Questionnaires, and Dose Reductions
Patients were assessed weekly (days 1, 8, 15, and 22) during the first two cycles of regorafenib (cycle length 28 days), although, to maintain parsimony, mostly only end of cycle results are reported. Weekly visits entailed a history and physical examination; adverse event assessment (per the National Cancer Institute's Common Terminology Criteria for Adverse Events [CTCAE], version 4.0); medication diaries for clobetasol adherence (as appropriate) and regorafenib; and questionnaires collection. The latter included the validated 14‐item Hand‐Foot Syndrome Questionnaire completed on day 14 of cycle 1 and on days 1, 14, and 28 of cycle 2 [8].
The protocol outlined only regorafenib dose reductions and provided definitions of grade 1, 2, and 3 HFSR, per CTCAE, version 4. Regorafenib dose reductions were not to be instituted for grade 1 HFSR. For first occurrence, grade 2 skin toxicity, a regorafenib dose reduction was defined and followed by a minimum 7‐day interruption in therapy, if needed. Resumption of regorafenib was dependent upon skin toxicity resolution to grade 1 or better with a drop in regorafenib dose upon restarting. If HFSR resulted in a fourth occurrence of grade 2 or worse toxicity, regorafenib was to be permanently discontinued. Similar but more stringent dose reductions were outlined for grade 3 adverse events; regorafenib was to be held immediately for a minimum of 7 days and permanently after the third occurrence of grade 3 HFSR.
Endpoints
This report describes the percentage of patients with no HFSR based on preemptive versus reactive clobetasol. Quality of life is also reported. Overall survival/progression‐free survival (PFS) based on HSFR is also reported [9, 10, 11, 12, 13]. Overall survival (OS) is defined as time from day after cycle 1 end date to death due to any cause. PFS is defined as time from day after cycle 1 end date to disease progression (per RECIST 1.1) or death due to any cause, whichever occurred first.
Statistical Analysis
Patients who were eligible, consented, and received protocol treatment were evaluable. Given the absence of a significant interaction (p = .45) between the two interventions (regorafenib dosing strategy and clobetasol usage) using the outcome no HFSR (vs. any HFSR) in the first two cycles as the response variable, we decided to pool the data for the dose escalation and standard dose treatment with regorafenib and compare the two clobetasol usage strategies (preemptive vs. reactive). Descriptive statistics are presented, and comparisons between the two groups (preemptive vs. reactive) were carried out with Chi‐square tests for categorical variables and Wilcoxon rank‐sum test for continuous variables. For calculating the percentage of no HFSR in cycles 1 and 2, the patients who were off protocol treatment are included in the group of “any hand‐foot reaction” to maintain a consistent denominator. The odds of remaining free of hand‐foot toxicity in the first two cycles were examined using logistic regression models in specific subgroups of interest; interaction between the clobetasol strategy and specific variable of interest were included in the model. For patient‐reported HFSR, data imputation was undertaken with CTCAE. Specifically, if the questionnaire was completed (i.e., the summary score can be calculated), we used the patient‐reported summary score; if the questionnaire was missing (i.e., the summary score cannot be calculated) but expected (i.e., the patient was still on protocol treatment at the questionnaire time point) and had CTCAE grade equal to 0, then the questionnaire summary score was set to 0 (best possible score); otherwise, if the questionnaire was expected but incomplete or missing and the CTCAE grade was greater than 0, then the summary score was set to 100 (worst possible score). A mixed‐effects model was then used to compare skin toxicity based on all time points. Compound symmetry covariance structure was used to take into account within‐patient correlation. Sensitivity analyses using the same mixed model with no imputation were also conducted. A landmark analysis was used to examine the relationship between HFSR in cycle 1 and OS/PFS. The landmark time is the day after the end of cycle 1 treatment. Patients who remained event‐free after cycle 1 were included in the analysis. Kaplan‐Meier curves were constructed for OS/PFS based on whether HFSR occurred in cycle 1 per CTCAE criteria; a log‐rank test was used to compare survival. All analyses were performed with SAS version 9.4 software (SAS Institute Inc., Cary, NC), and a p value <.05 was considered statistically significant.
Results
Baseline Characteristics
A total of 116 patients from 39 medical centers are the focus of this report. Sixty‐one received preemptive therapy with clobetasol, and 55 received reactive therapy. Seven from the original cohort of 123 were nonevaluable and not included (Fig. 1).
Figure 1.
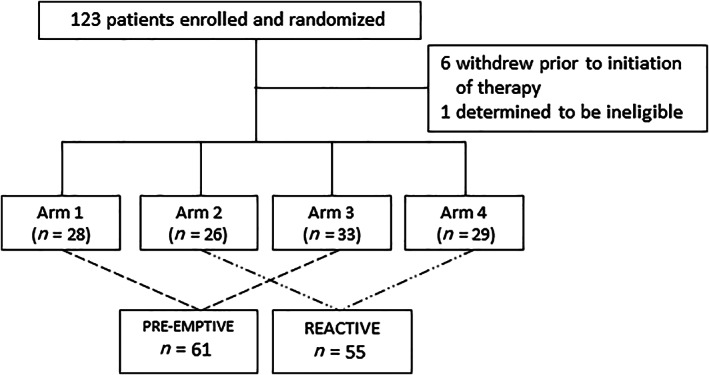
Consort diagram shows the selection of patients who were included in the analyses reported here. The four original arms included the following: (1) clobetasol + regorafenib (starting dose 80 mg per day with a potential dose escalation to 160 mg per day); (2) reactive clobetasol + regorafenib (starting dose 80 mg per day with a potential dose escalation to 160 mg per day); (3) preemptive clobetasol + regorafenib (160 mg starting dose); and (4) reactive clobetasol + regorafenib (160 mg starting dose).
Groups were balanced on age, gender, performance score, and body mass index (weight in kilograms/height in meters2; Table 1). Baseline characteristics showed no statistically significant group differences.
Table 1.
Baseline demographics (n = 116)
| Characteristic | Total (n = 116) | Preemptive clobetasol (n = 61) | Reactive clobetasol (n = 55) | p value a |
|---|---|---|---|---|
| Age, median (IQR), yr | 61 (53, 68) | 63 (53, 68) | 61 (53, 69) | .43 |
| Gender | .37 | |||
| Male | 71 (61) | 35 (57) | 36 (66) | |
| Female | 45 (39) | 26 (43) | 19 (35) | |
| Performance score | .59 | |||
| 0 | 43 (37) | 24 (39) | 19 (35) | |
| 1 | 73 (63) | 37 (61) | 36 (67) | |
| Body mass index, median (IQR), kg/m2 | 28 (24, 32) | 28 (24, 32) | 27 (24, 32) | .95 |
| Number of metastatic sites | .14 | |||
| 1 | 8 (7) | 5 (8) | 3 (6) | |
| 2 | 30 (26) | 20 (31) | 10 (18) | |
| 3+ | 78 (67) | 36 (59) | 42 (76) | |
| BRAF testing | .98 | |||
| Mutated | 2 (2) | 1 (2) | 1 (2) | |
| Wild type | 37 (32) | 20 (33) | 17 (31) | |
| Unknown | 77 (66) | 40 (66) | 37 (67) | |
| KRAS testing | .94 | |||
| Mutated | 55 (47) | 29 (48) | 26 (47) | |
| Wild type | 58 (50) | 31 (61) | 27 (49) |
Data are presented as n (%) unless otherwise indicated.
Chi‐square test for categorical variables and Wilcoxon rank‐sum test for continuous variables.
Abbreviation: IQR, interquartile range.
HFSR, per CTCAE, and Patient‐Reported Outcomes
Rates of no HFSR over the first two regorafenib cycles showed that no toxicity occurred in 30% (of 61 total patients) with preemptive versus 13% (of 55 total patients) with reactive therapy (p = .03). During the first cycle of regorafenib, the percentages of patients who did not develop HFSR, as per CTCAE, were 54% and 45% with preemptive and with reactive clobetasol, respectively (p = .35; Table 2). During the second cycle, the percentage of patients who did not develop HFSR were 33% and 15%, respectively, with preemptive and reactive therapy (p = .02; Table 2).
Table 2.
Rates of HSFR, as per CTCAE, among regorafenib‐treated patients
| Cycle 1 regorafenib | Cycle 2 regorafenib | |||||
|---|---|---|---|---|---|---|
| HSFR outcome | Preemptive clobetasol (n = 61) | Reactive clobetasol (n = 55) | p value | Preemptive clobetasol (n = 61) | Reactive clobetasol (n = 55) | p value |
| No HSFR | 33 (54) | 25 (45) | .35 | 20 (33) | 8 (15) | .02 |
| Any HFSR a | 28 (46) | 30 (55) | 41 (67) | 47 (85) | ||
| HSFR by grade | ||||||
| 0 | 33 (54) | 25 (45) | 20 (33) | 8 (15) | ||
| 1 | 11 (18) | 8 (15) | .35 | 18 (30) | 18 (43) | .12 |
| 2 | 11 (18) | 13 (24) | 5 (8) | 10 (18) | ||
| 3 | 6 (10) | 6 (11) | 2 (3) | 4 (7) | ||
| Missing | 0 (0) | 3 (5) | 16 (26) b | 15 (27) b | ||
Data are presented as n (%).
This row includes all patients who had HFSR as well as patients with missing data.
Twenty‐eight patients stopped regorafenib by cycle 2, resulting in missing data.
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; HFSR, hand‐foot skin reaction.
During the first cycle of regorafenib, rates of grade 1, 2, and 3 HFSR were 18%, 18%, and 10% with preemptive clobetasol versus 15%, 24%, and 11% with reactive clobetasol (p = .35). During the second cycle, the respective rates of grade 1, 2, and 3 HFSR were 30%, 8%, and 3% with preemptive therapy and 43%, 18%, and 7% with reactive therapy (p = .12).
When examining factors associated with absence of HFSR per CTCAE criteria during the entire first two cycles of regorafenib, regorafenib dosing strategy (escalating dose vs. standard dose), gender, age, and ECOG performance score were not predictive of toxicity (Fig. 2).
Figure 2.
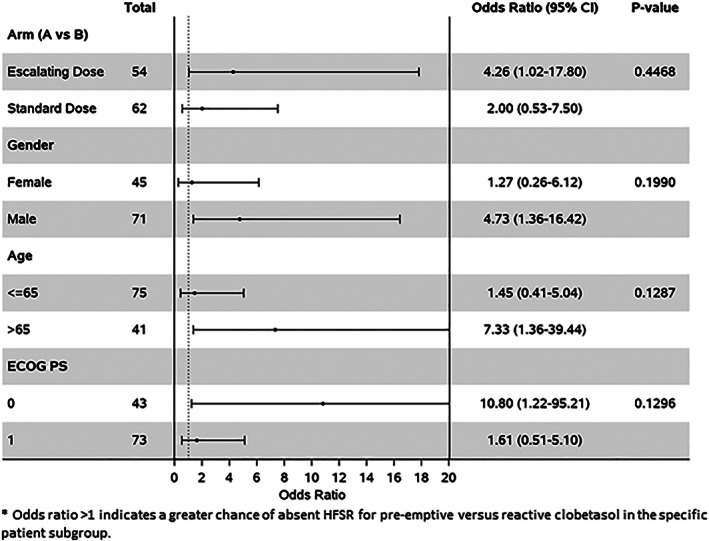
A forest plot shows that an ECOG PS of 0 was a predictor of no HFSR. Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance score; HFSR, hand‐foot skin reaction.
Rates of missing patient‐reported data were notable (approximately 40%–50% at various time points). The mixed model that used patient‐reported data after data imputation and that relied on CTCAE version 4 showed trends of lesser HFSR during cycle 2 in the preemptive clobetasol group; sensitivity analyses with no imputation provided confirmatory conclusions, as shown in Table 3.
Table 3.
Patient‐reported hand‐foot reactions
| With imputation | Without imputation | |||||
|---|---|---|---|---|---|---|
| Cycle/day | Preemptive clobetasol hand‐foot score | Reactive clobetasol hand‐foot score | p value | Preemptive clobetasol hand‐foot score | Reactive clobetasol hand‐foot score | p value |
| 1/14 | 28 | 33 | .50 | 25 | 37 | .27 |
| 2/1 | 28 | 33 | .57 | 20 | 23 | .77 |
| 2/14 | 50 | 62 | .13 | 17 | 43 | .02 |
| 2/28 | 46 | 67 | .01 | 22 | 35 | .25 |
Results of a mixed‐effects model with imputation of data from CTCAE and with only raw patient‐reported data. A lower score indicates a better quality of life.
Abbreviation: CTCAE, Common Terminology Criteria for Adverse Events.
Quality of Life
Patient‐reported quality of life appears in Table 4. Nearly all activities—from opening a door to meal preparation to managing personal hygiene to getting dressed to putting on shoes to walking to driving—were challenging with HFSR. With few exceptions, quality of life was reported to be worse by the end of the second regorafenib cycle among patients on reactive therapy (only descriptive data reported). In exploring quality of life within all four study arms, no differences between arms were observed (supplemental online Fig. 1).
Table 4.
Quality of life at the end of each chemotherapy cycle based on presence or absence of HFSR and on other factors
| Preemptive clobetasol | Reactive clobetasol | |||||||
|---|---|---|---|---|---|---|---|---|
| Week 4 | Week 8 | Week 4 | Week 8 | |||||
| Statement and response | No HFSR (n = 25) | Any HFSR (n = 20) | No HFSR (n = 16) | Any HFSR (n = 22) | No HFSR (n = 19) | Any HFSR (n = 23) | No HFSR (n = 7) | Any HFSR (n = 30) |
| Area affected by HFSR | ||||||||
| Hands | 2 (13) | 0 | 0 | 6 (29) | 2 (22) | 4 (27) | 1 (50) | 3 (17) |
| Feet | 1 (7) | 11 (55) | 1 (25) | 2 (1)) | 1 (11) | 5 (33) | 0 | 2 (11) |
| Both | 12 (80) | 9 (45) | 3 (75) | 13 (62) | 6 (67) | 6 (40) | 1 (50) | 13 (72) |
| Would you say your hand‐foot syndrome tends to be | ||||||||
| Not painful | 15 (75) | 6 (30) | 10 (91) | 5 (24) | 7 (58) | 7 (41) | 4 (100) | 5 (25) |
| Moderately painful | 4 (20) | 9 (45) | 0 | 13 (62) | 5 (42) | 9 (53) | 0 | 12 (60) |
| Very painful | 1 (5) | 5 (25) | 1 (9) | 3 (14) | 0 | 1 (6) | 0 | 3 (15) |
| I find it hard to turn the key in my door because of my hand‐foot syndrome | ||||||||
| No, never | 21 (91) | 16 (80) | 13 (100) | 12 (55) | 12 (86) | 12 (71) | 4 (100) | 10 (48) |
| Yes, from time to time | 1 (4) | 4 (20) | 0 | 8 (36) | 1 (7) | 5 (29) | 0 | 9 (43) |
| Yes, always | 1 (4) | 0 | 0 | 2 (9) | 1 (7) | 0 | 0 | 2 (10) |
| I find it hard to prepare my meals because of my hand‐foot syndrome | ||||||||
| No, never | 22 (96) | 13 (65) | 13 (100) | 14 (64) | 12 (86) | 12 (71) | 4 (100) | 11 (52) |
| Yes, from time to time | 1 (4) | 5 (25) | 0 | 6 (27) | 2 (14) | 3 (18) | 0 | 8 (38) |
| Yes, always | 0 | 2 (10) | 0 | 2 (9) | 0 | 2 (12) | 0 | 2 (10) |
| I have difficulty performing everyday actions because of my hand‐foot syndrome | ||||||||
| No, never | 21 (91) | 10 (50) | 12 (92) | 10 (46) | 11 (79) | 8 (47) | 4 (100) | 8 (38) |
| Yes, from time to time | 2 (9) | 7 (35) | 1 (8) | 10 (46) | 3 (21) | 8 (47) | 0 | 11 (52) |
| Yes, always | 0 | 3 (15) | 0 | 2 (9) | 0 | 1 (6) | 0 | 2 (10) |
| I have difficulty washing myself, putting on makeup, or shaving because of my hand‐foot syndrome | ||||||||
| No, never | 22 (96) | 16 (80) | 13 (100) | 16 (73) | 14 (100) | 12 (71) | 4 (100) | 11 (52) |
| Yes, from time to time | 0 | 3 (15) | 0 | 4 (18) | 0 | 5 (29) | 0 | 8 (38) |
| Yes, always | 1 (4) | 1 (5) | 0 | 2 (9) | 0 | 0 | 0 | 2 (10) |
| I find it hard to drive my car because of my hand‐foot syndrome | ||||||||
| No, never | 21 (96) | 17 (85) | 13 (100) | 18 (82) | 13 (93) | 15 (88) | 4 (100) | 12 (57) |
| Yes, from time to time | 0 | 3 (15) | 0 | 2 (9) | 1 (7) | 1 (6) | 0 | 8 (38) |
| Yes, always | 1 (5) | 0 | 0 | 2 (9) | 0 | 1 (6) | 0 | 1 (5) |
| I find it hard to put on stockings/tights (or my socks) because of my hand‐foot syndrome | ||||||||
| No, never | 21 (91) | 11 (55) | 13 (100) | 14 (64) | 12 (86) | 11 (65) | 4 (100) | 11 (52) |
| Yes, from time to time | 1 (4) | 9 (45) | 0 | 7 (32) | 1 (7) | 6 (35) | 0 | 8 (38) |
| Yes, always | 1 (4) | 0 | 0 | 1 (5) | 1 (7) | 0 | 0 | 2 (10) |
| I take longer than usual to get dressed because of my hand‐foot syndrome | ||||||||
| No, never | 20 (87) | 11 (55) | 12 (92) | 11 (50) | 11 (79) | 10 (59) | 4 (100) | 10 (48) |
| Yes, from time to time | 2 (9) | 7 (35) | 1 (8) | 10 (46) | 3 (21) | 6 (35) | 0 | 10 (48) |
| Yes, always | 1 (4) | 2 (10) | 0 | 1 (5) | 0 | 1 (6) | 0 | 1 (5) |
| I have difficulty putting on my shoes because of my hand‐foot syndrome | ||||||||
| No, never | 20 (87) | 11 (55) | 13 (100) | 14 (64) | 11 (79) | 9 (53) | 4 (100) | 9 (43) |
| Yes, from time to time | 2 (9) | 6 (30) | 0 | 7 (32) | 3 (21) | 6 (35) | 0 | 9 (43) |
| Yes, always | 1 (4) | 3 (15) | 0 | 1 (5) | 0 | 2 (12) | 0 | 3 (14) |
| It is hard for me to stand because of my hand‐foot syndrome | ||||||||
| No, never | 21 (91) | 8 (40) | 12 (92) | 14 (64) | 12 (86) | 8 (47) | 4 (100) | 10 (48) |
| Yes, from time to time | 2 (9) | 10 (50) | 1 (8) | 7 (32) | 2 (14) | 8 (47) | 0 | 9 (43) |
| Yes, always | 0 | 2 (10) | 0 | 1 (5) | 0 | 1 (6) | 0 | 2 (10) |
| I have difficulty walking, even over quite short distances, because of my hand‐foot syndrome | ||||||||
| No, never | 20 (87) | 9 (45) | 12 (92) | 11 (50) | 12 (86) | 8 (47) | 4 (100) | 10 (48) |
| Yes, from time to time | 2 (9) | 9 (45) | 1 (8) | 9 (41) | 2 (14) | 8 (47) | 0 | 9 (43) |
| Yes, always | 1 (4) | 2 (10) | 0 | 2 (9) | 0 | 1 (6) | 0 | 2 (10) |
| I tend to stay seated or lying down because of my hand‐foot syndrome | ||||||||
| No, never | 20 (87) | 9 (45) | 12 (92) | 13 (59) | 12 (86) | 10 (59) | 4 (100) | 10 (48) |
| Yes, from time to time | 2 (9) | 9 (45) | 1 (8) | 8 (36) | 2 (14) | 7 (41) | 0 | 10 (48) |
| Yes, always | 1 (4) | 2 (10) | 0 | 1 (5) | 0 | 0 | 0 | 1 (5) |
| I find it hard to fall asleep because of my hand‐foot syndrome | ||||||||
| No, never | 22 (96) | 15 (75) | 12 (100) | 17 (77) | 11 (79) | 14 (82) | 4 (100) | 16 (76) |
| Yes, from time to time | 0 | 5 (25) | 0 | 5 (23) | 3 (21) | 3 (18) | 0 | 5 (24) |
| Yes, always | 1 (4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| My work is suffering because of my hand‐foot syndrome | ||||||||
| No, never | 22 (96) | 16 (80) | 12 (92) | 17 (77) | 14 (100) | 15 (88) | 4 (100) | 15 (71) |
| Yes, from time to time | 1 (4) | 3 (15) | 1 (8) | 2 (9) | 0 | 2 (12) | 0 | 4 (19) |
| Yes, always | 0 | 1 (5) | 0 | 3 (14) | 0 | 0 | 0 | 2 (10) |
| My relationships with others are less amicable because of my hand‐foot syndrome | ||||||||
| No, never | 21 (91) | 15 (75) | 11 (92) | 18 (82) | 13 (93) | 14 (82.) | 4 (100) | 17 (81) |
| Yes, from time to time | 1 (4) | 5 (25) | 1 (8) | 3 (14) | 1 (7) | 3 (18) | 0 | 4 (19) |
| Yes, always | 1 (4) | 0 | 0 | 1 (5) | 0 | 0 | 0 | 0 |
| Indicate the level of your pain | ||||||||
| Not painful | 19 (86) | 6 (30) | 9 (75) | 9 (41) | 9 (64) | 6 (38) | 3 (100) | 7 (35) |
| Moderately painful | 0 | 10 (50) | 0 | 11 (50) | 5 (36) | 8 (50) | 0 | 12 (60) |
| Very painful | 3 (14) | 4 (20) | 3 (25) | 2 (9) | 0 | 2 (13) | 0 | 1 (5) |
Missing data are not denoted.
Abbreviation: HFSR, hand‐foot skin reaction.
Survival Outcomes and HFSR
For absent and present HFSR, the median PFS was 1.6 months (95% confidence interval [CI]: 1.0, 4.1) versus 1.1 months (0.9, 2.7; p = .92). For absent versus present HFSR, the median overall survival was 6.9 months (95% CI: 4.8, 10.6) versus 8.8 months (6.3, 11.3; p = .67), respectively (Fig. 3A, 3B).
Figure 3.
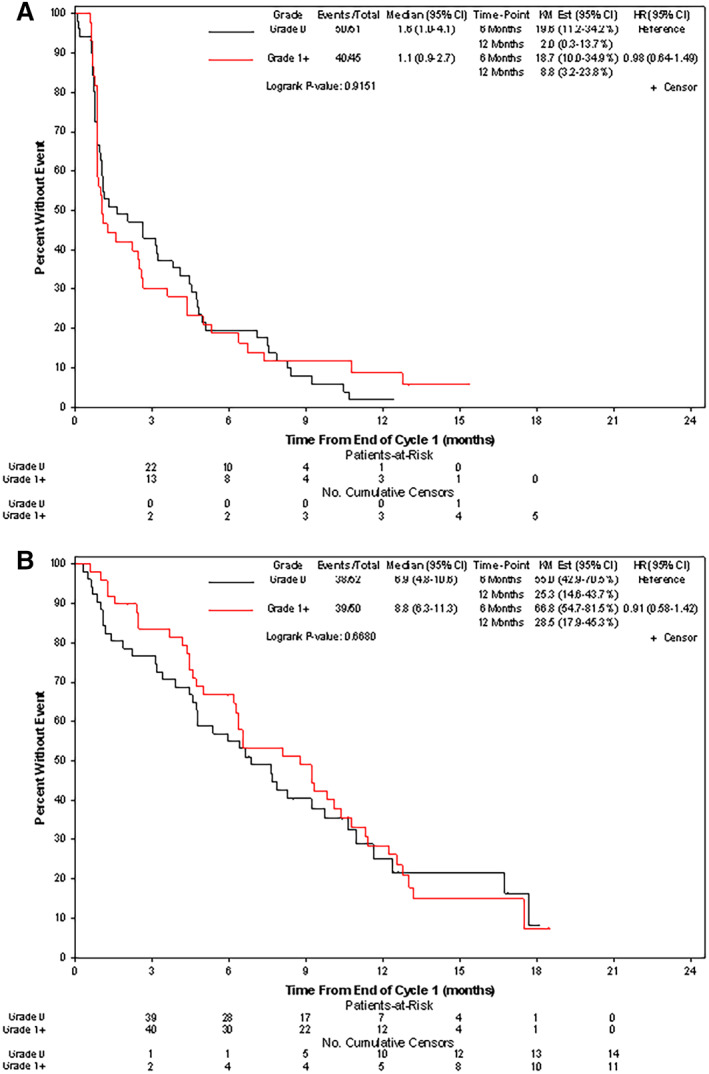
(A): Median progression‐free survival was 1.6 months (95% CI: 1.0, 4.1) versus 1.1 months (0.9, 2.7; p = .92) for absent versus present HFSR. (B): Median overall survival was 6.9 months (95% CI: 4.8, 10.6) versus 8.8 months (6.3, 11.3; p = .67) for absent and present HFSR, respectively. Abbreviations: CI, confidence interval; HFSR, hand‐foot skin reaction; HR, hazard ratio; KM, Kaplan‐Meier.
Clobetasol and Regorafenib Adherence
With preemptive clobetasol, the percentage of days this topical corticosteroid was applied, as in cycle 1, was 86% (mean number of days applied [SD]: 24 [7]); in cycle 2, it was 90% (25 [6]). With reactive therapy, in cycle 1, the mean number of days (SD) of clobetasol application was 12 (7); in cycle 2, it was 19 (9).
Patients received from 75% to 100% of the intended dose of regorafenib with percentages well over 90% in the study groups for the majority of patients during the first cycle. During the second cycle, the percentages of intended dose of regorafenib dropped with rates per week during the second cycle of 90%, 89%, and 78% and of 86%, 82%, and 74% in preemptively and reactively treated patients, respectively.
Clobetasol Adverse Events
No clobetasol‐related adverse events were reported.
Discussion
This study found that preemptive therapy with clobetasol, a high‐potency topical corticosteroid, is associated with lower rates of regorafenib‐induced HFSR by the second cycle of regorafenib. By the end of the second cycle, the rate of freedom from HFSR was increased to 30% (compared with 13% with reactive therapy). The severity of reactions was less, patient‐reported quality of life more favorable, and adverse events directly attributable to the clobetasol absent. Although these findings might be viewed as modest and preliminary, particularly in view of patient dropout by the second cycle, they are important and worthy of further study [4].
An important message is regorafenib‐induced HFSR is associated with compromise in the performance of daily activities. Patients struggled with opening doors, washing themselves, putting on makeup or shaving, putting on their socks and shoes, and even standing or walking [14]. These data should provide the impetus to find other interventions, perhaps in combination with topical clobetasol, to help patients—and particularly those with borderline to poor performance status—either sidestep or manage this cutaneous toxicity.
Interestingly, although skin toxicity from other agents, such as epidermal growth factor receptor inhibitors, has been directly associated with improved survival outcomes, this association has received less attention with regorafenib and related agents [10, 15, 16]. For example, in a retrospective study of only 40 patients, Ochi and others reported a longer median survival among patients who developed HFSR [16]. Admittedly, in the study reported here, this analysis was post hoc and exploratory in nature. Nonetheless, the current study is larger and prospective and showed absent associations. Regardless of associations between HFSR and survival outcomes, it is important to find ways to prevent and palliate this drug‐induced adverse event.
The current study has its limitations. First, it was devoid of a translational component—such as, for example, the assessment of inflammatory biomarkers within the skin—which might have solidified the role of topical corticosteroids in palliating HFSR. A translational component might have been of value for developing other agents with an even higher degree of palliative efficacy. Second, the current report is a secondary—albeit preplanned—analysis of a phase II trial, and, generally, practice‐changing studies rely on primary endpoints from robust phase III trials. Third, this was not a placebo‐controlled trial, a design that would have strengthened conclusions. Fourth, we used a cream in the current study, and some have suggested that other formulations might provide greater efficacy. Nonetheless, topical clobetasol, as prescribed here, provides a clear pathway for future investigation.
Conclusion
Finally, are the data from this study strong enough now to prescribe topical clobetasol, or a similar corticosteroid, preemptively with regorafenib? Although further data are needed to draw definitive conclusions, health care providers might begin to discuss this preemptive strategy with patients, particularly as this approach seems well tolerated.
Author Contributions
Conception/design: Aminah Jatoi, Tanios Bekaii‐Saab
Provision of study material or patients: Aminah Jatoi, Fang‐Shu Ou, Daniel H. Ahn, Tyler J. Zemla, Jennifer G. Le‐Rademacher, Patrick Boland, Kristen K. Ciombor, Nisha L. Jacobs, Boris Pasche, James M. Cleary, Jeannine S. McCune, Katrina S. Pedersen, Afsaneh Barzi, E. Gabriela Chiorean, Erica N. Heying, Heinz‐Josef Lenz, Jeff A. Sloan, Axel Grothey, Mario E. Lacouture, Tanios Bekaii‐Saab
Collection and/or assembly of data: Aminah Jatoi, Fang‐Shu Ou, Daniel H. Ahn, Tyler J. Zemla, Jennifer G. Le‐Rademacher, Patrick Boland, Kristen K. Ciombor, Nisha L. Jacobs, Boris Pasche, James M. Cleary, Jeannine S. McCune, Katrina S. Pedersen, Afsaneh Barzi, E. Gabriela Chiorean, Erica N. Heying, Heinz‐Josef Lenz, Jeff A. Sloan, Axel Grothey, Mario E. Lacouture, Tanios Bekaii‐Saab
Data analysis and interpretation: Fang‐Shu Ou
Manuscript writing: Aminah Jatoi, Tanios Bekaii‐Saab
Final approval of manuscript: Aminah Jatoi, Fang‐Shu Ou, Daniel H. Ahn, Tyler J. Zemla, Jennifer G. Le‐Rademacher, Patrick Boland, Kristen K. Ciombor, Nisha L. Jacobs, Boris Pasche, James M. Cleary, Jeannine S. McCune, Katrina S. Pedersen, Afsaneh Barzi, E. Gabriela Chiorean, Erica N. Heying, Heinz‐Josef Lenz, Jeff A. Sloan, Axel Grothey, Mario E. Lacouture, Tanios Bekaii‐Saab
Disclosures
Katrina S. Pedersen: Pfizer, UpToDate, MedScape (C/A), Array Research (SAB [institutional]), AbbVie, Array, BiolineRx, Boston Biomedical/Sumitomo Dainippon, Bristol‐Myers Squibb, Daiichi Sankyo, Ipsen, MedImmune, Nouscom, Novartis, Pfizer, Pierre Fabre, Rafael, Roche/Genentech, Natera (SAB), NCCN (Colorectal Guidelines Committee, unpaid), Beigene (travel, investigator meeting), Nouscom (travel, investigator meeting); Mario Lacouture: Bayer (H, C/A); Daniel Ahn: Exelixis, Genentech, Eisai, Ipsen (C/A), Bayer, AstraZeneca (RF); Kristin Ciombor: Foundation Medicine, Natera, Merck (C/A), Array (SAB), Bristol‐Myers Squibb, Pfizer, Calithera, Incyte, Array, Daiichi Sankyo, Nucana, Merck (RF); James Cleary: AbbVie, Merus, Roche, Bristol‐Myers Squibb (RF [institutional]), Merck, AstraZeneca, Esperas Pharma, Tesaro (RF); Bristol‐Myers Squibb (C/A, travel; E. Gabriela Chiorean: Boehringer Ingelheim, Celgene, Roche/Ignyta, Incyte, Merck, MacroGenics, Halozyme, Stemline, Eli Lilly and Company, Fibrogen, Bristol‐Myers Squibb, Rafael, Clovis (RF), Array, AstraZeneca, Eisai, Celgene, Ipsen, Legend, Sobi (SAB), Noxxon (C/A); Heinz‐Josef Lenz: Bayer (C/A, SAB); Axel Grothey: Bayer (RF, SAB). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
Appendix S1. Supporting Information.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Grothey A, Van Cutsem E, Sobrero A et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet 2013;381:303–312. [DOI] [PubMed] [Google Scholar]
- 2. Demetri GD, Reichardt P, Kang YK et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet 2013;381:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nardone B, Hensley JR, Kulik L et al. The effect of hand‐foot skin reaction associated with the multikinase inhibitors sorafenib and sunitinib on health‐related quality of life. J Drugs Dermatol 2012;11:e61–e65. [PubMed] [Google Scholar]
- 4. Ren Z, Zhu K, Kang H et al. Randomized controlled trial of the prophylactic effect of urea‐based cream on sorafenib‐associated hand‐foot skin reactions in patients with advanced hepatocellular carcinoma. J Clin Oncol 2015;33:894–900. [DOI] [PubMed] [Google Scholar]
- 5. Nagore E, Insa A, Sanmartín O. Antineoplastic therapy‐induced palmar plantar erythrodysesthesia ('hand‐foot') syndrome. Incidence, recognition and management. Am J Clin Dermatol 2000;1:225–234. [DOI] [PubMed] [Google Scholar]
- 6. Gressett SM, Stanford BL, Hardwicke F. Management of hand‐foot syndrome induced by capecitabine. J Oncol Pharm Pract 2006;12:131–141. [DOI] [PubMed] [Google Scholar]
- 7. Bekaii‐Saab TS, Ou FS, Ahn DH et al. Regorafenib dose‐optimisation in patients with refractory metastatic colorectal cancer (ReDOS): A randomised, multicentre, open‐label, phase 2 study. Lancet Oncol 2019;20:1070–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sibaud V, Dalenc F, Chevreau C et al. HFS‐14, a specific quality of life scale developed for patients suffering from hand‐foot syndrome. The Oncologist 2011;16:1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shomura M, Kagawa T, Shiraishi K et al. Skin toxicity predicts efficacy to sorafenib in patients with advanced hepatocellular carcinoma. World J Hepatol 2014;6:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brøndum L, Alsner J, Sørensen BS et al. Associations between skin rash, treatment outcome, and single nucleotide polymorphisms in head and neck cancer patients receiving the EGFR‐inhibitor zalutumumab: Results from the DAHANCA 19 trial. Acta Oncol 2018;57:1159–1164. [DOI] [PubMed] [Google Scholar]
- 11. Nasu S, Suzuki H, Shiroyama T et al. Skin rash can be a useful marker for afatinib efficacy. Anticancer Res 2018;38:1783–1788. [DOI] [PubMed] [Google Scholar]
- 12. Wei F, Shin D, Cai X. Incidence, risk and prognostic role of anti‐epidermal growth factor receptor‐induced skin rash in biliary cancer: A meta‐analysis. Int J Clin Oncol 2018;23:443–451. [DOI] [PubMed] [Google Scholar]
- 13. Pérez‐Soler R, Chachoua A, Hammond LA et al. Determinants of tumor response and survival with erlotinib in patients with non–small‐cell lung cancer. J Clin Oncol 2004;22:3238–3247. [DOI] [PubMed] [Google Scholar]
- 14. Dranitsaris G, Vincent MD, Yu J et al. Development and validation of a prediction index for hand‐foot skin reaction in cancer patients receiving sorafenib. Ann Oncol 2012;23:2103–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brøndum L, Alsner J, Sørensen BS et al. Associations between skin rash, treatment outcome, and single nucleotide polymorphisms in head and neck cancer patients receiving the EGFR‐inhibitor zalutumumab: Results from the DAHANCA 19 trial. Acta Oncol 2018;57:1159–1164. [DOI] [PubMed] [Google Scholar]
- 16. Abdel‐Rahman O, Fouad M. Correlation of cetuximab‐induced skin rash and outcomes of solid tumor patients treated with cetuximab: A systematic review and meta‐analysis. Crit Rev Oncol Hematol 2015;93:127–135. [DOI] [PubMed] [Google Scholar]
- 17. Ochi M, Kamoshida T, Ohkawara A et al. Multikinase inhibitor‐associated hand‐foot skin reaction as a predictor of outcomes in patients with hepatocellular carcinoma treated with sorafenib. World J Gastroenterol 2018;24:3155–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


